In total, >100 RNA modifications (termed the
epitranscriptome) are currently known in living organisms, and are
involved in various biological processes that are associated with
multiple functions, including adipogenesis, stem cell
differentiation and the heat shock response (1,2).
Methylation of the N6 position of adenosine was first
identified in eukaryotic mRNAs in the 1970s. This modified base is
referred to as N6-methyladenosine (m6A)
(3,4). m6A has been found to be
a prevalent and abundant internal modification of a wide range of
RNAs, including but not restricted to mRNAs, transfer RNAs,
ribosomal RNAs, microRNAs (miRNAs/miRs) and long non-coding RNAs
(lncRNAs) (5).
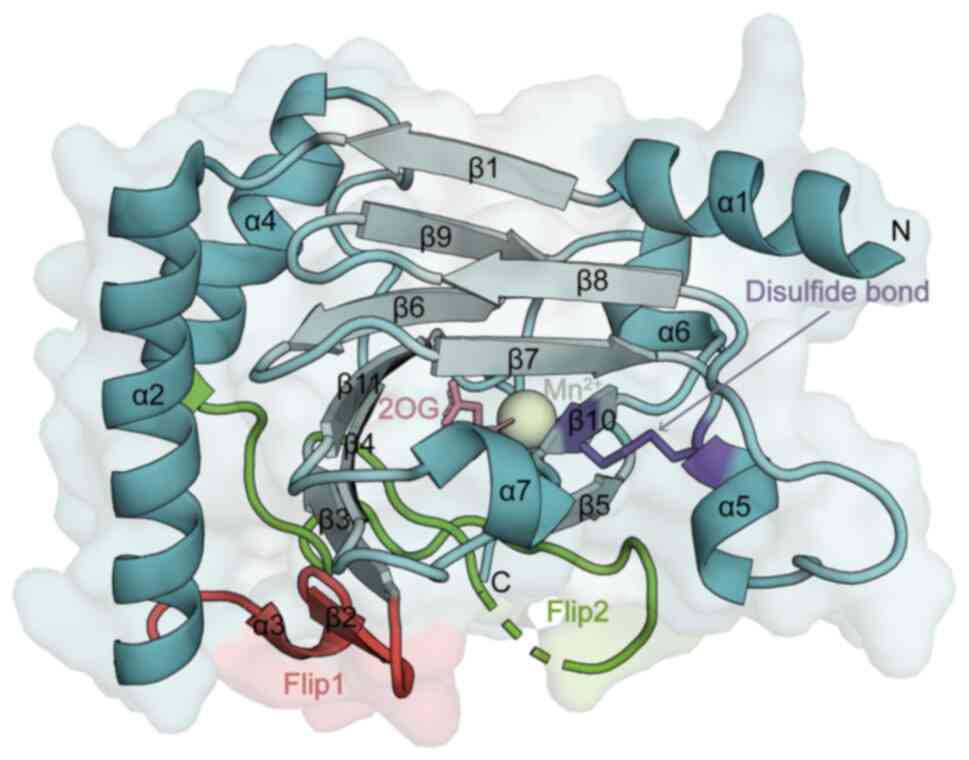
ALKBH5 belongs to the non-heme Fe(II)/α-KG-dependent
dioxygenase AlkB family of proteins, and the human AlkB family has
nine members, including ALKBH1-8 and FTO (44). α-KG-dependent dioxygenases are an
extended superfamily of enzymes that catalyse a wide range of
oxidative reactions; these enzymes share the same double-stranded
β-helical core fold (DSBH fold), although they act on different
substrates and have conserved 2-oxoglutarate (2OG; also known as
α-KG) and Fe2+ binding sites (45,46). ALKBH5, similar to other AlkB
family proteins, binds 2OG and a metal cofactor (Fe2+)
in a conserved manner (35,42). The core of the ALKBH5 catalytic
structural domain consists of the typical conserved DSBH fold,
which consists of a total of 11 β-strands and 7 α-helices (42) (Fig. 2). The different substrate
specificities of different AlkB proteins are determined by the
composition of the external secondary structural elements of the
conserved DSBH fold, with the most notable structural difference
being the so-called 'nucleotide recognition lid', which is unique
to the AlkB protein subfamily (47). The cap region is further
classified into two sections, Flip1 (residues 117-129) and Flip2
(residues 136-165) (48).
Compared with those of ALKBH2 and FTO, the Flip1 region of ALKBH5
has an uncovered and relatively large space in the active site, and
the Flip2 region is highly flexible (42). The unique composition and
conformation of these regions may confer unique substrate-selective
features to ALKBH5 (40). In
addition, the unique disulfide bond structure (between residues
Cys-230 and Cys-267) of ALKBH5 is highly conserved only among
different species of ALKBH5 proteins, which prevents
double-stranded DNA and double-stranded RNA from accessing the
active site of ALKBH5 and determines the binding preference of
ALKBH5 for single-stranded substrates (40,42).
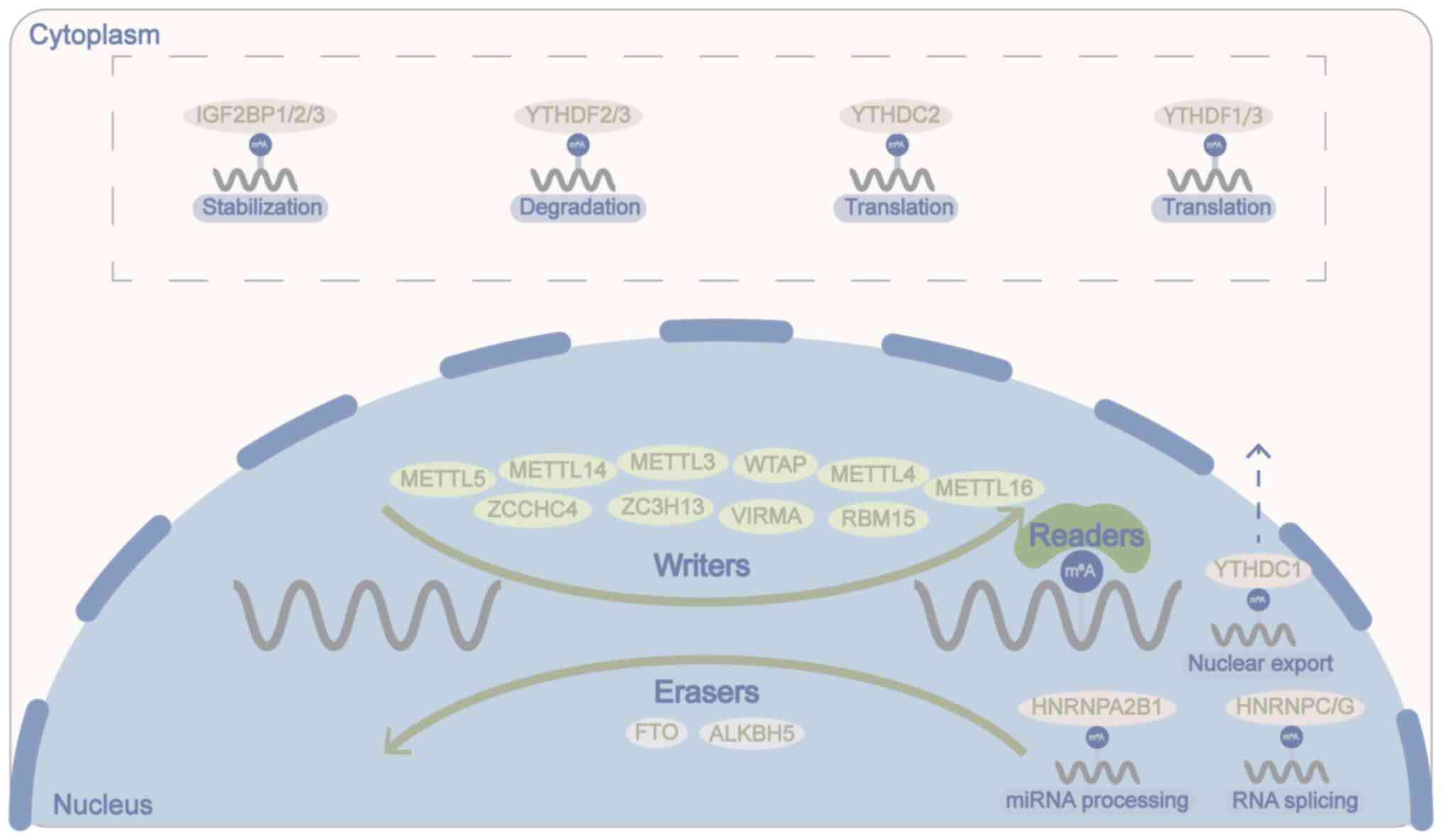
Endogenous ALKBH5 is predominantly found in the
nucleus, where it colocalizes with nuclear speckles, and is less
abundant in the cytoplasm (34).
Nuclear speckles are integrally regulated nuclear bodies that
promote gene expression, and >30 nuclear speckled proteins serve
crucial roles in both transcription and splicing (49). The demethylation activity of
ALKBH5 affects mRNA export, RNA splicing, mRNA stability, gene
expression and the assembly of mRNA processing factors in nuclear
speckles (34,50). Under normal physiological
conditions, ALKBH5 is highly expressed in the lungs, followed by
the testis, pancreas, spleen and ovaries (51). ALKBH5 serves a key role in
meiosis, and has been found to participate in spermatogenesis
(34), skeletal muscle
development (52), learning and
memory (53), and other
biological behaviours. ALKBH5 is involved in osteoarthritis
(54), pulmonary fibrosis
(55), stroke (56), systemic lupus erythematosus
(57) and diabetes (58) as an important biomolecule. In
addition, ALKBH5 is closely associated with a variety of
tumours.
ALKBH5 has been extensively studied in a variety of
tumours, such as glioblastoma (GBM) (59), head and neck squamous cell
carcinoma (60), colorectal
cancer (CRC) (61), ovarian
carcinoma (62), cervical cancer
(63), acute myeloid leukaemia
(AML) (64), breast cancer
(65), melanoma (66), hepatocellular carcinoma (HCC)
(67), and lung cancer (68), and serves a role in tumour growth
(69), tumour metastasis
(70), tumour immunity (71), tumour drug resistance (62) and tumour metabolism (72). ALKBH5 has been shown to act
through several important pathways, including the PI3K/AKT
(73), Janus kinase 1
(JAK1)/STAT3 (74), Wnt
(75), NF-κB (76) and mTOR (77) pathways. However, the potential
mechanism of ALKBH5 in cancer has not been fully investigated and
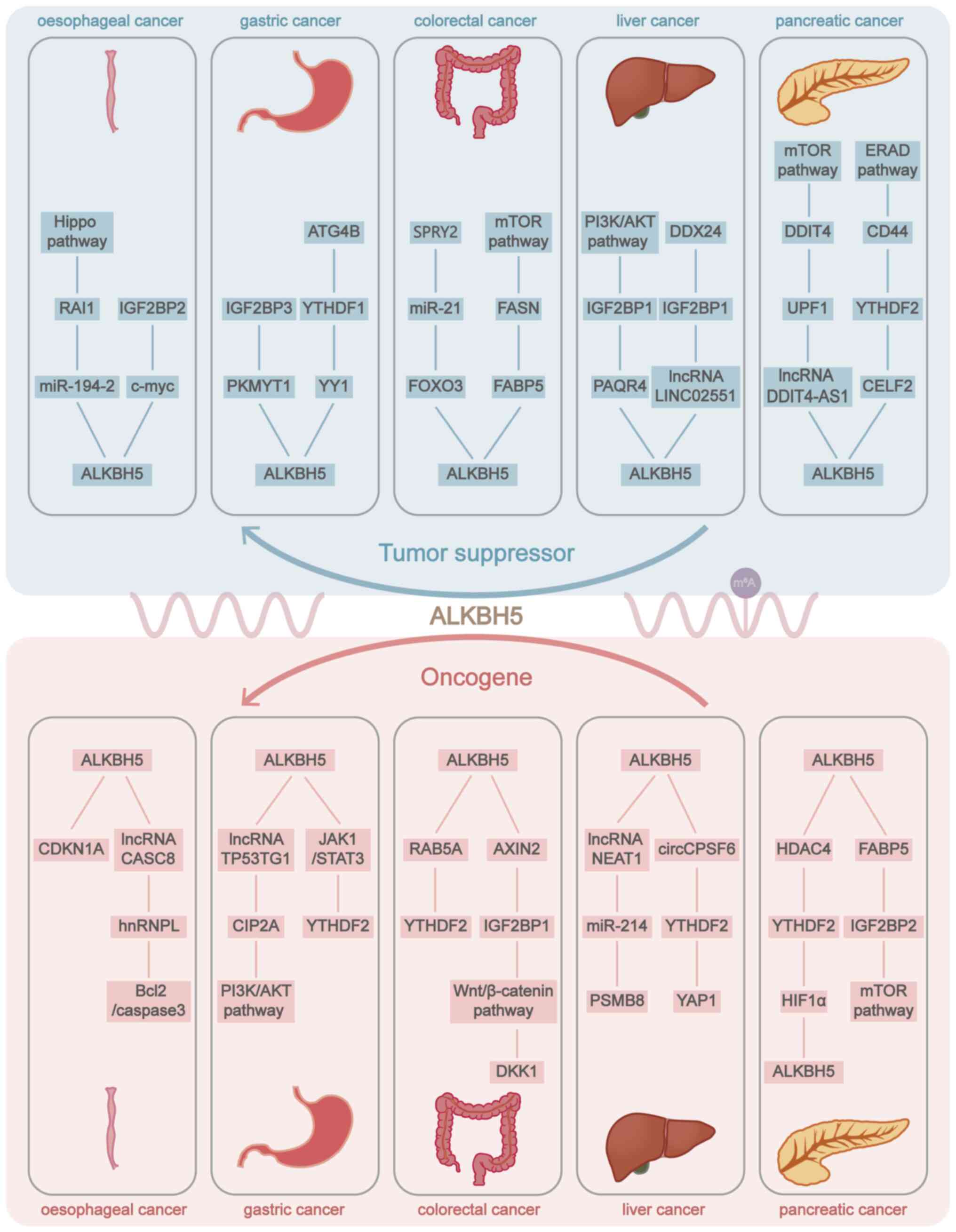
is controversial. ALKBH5 serves dual roles in different types of
cancer as either a tumour promoter or a tumour suppressor (78,79) and occasionally serves both pro-
and antigenic roles in the same type of cancer (67,80) (Fig. 3; Table SI). The molecular mechanism of
ALKBH5 needs to be further investigated.
The present review focuses on the research progress
regarding the role of ALKBH5 in digestive system tumours, paying
attention to the regulatory roles of different targets of ALKBH5,
as well as the current research status of small-molecule inhibitors
targeting ALKBH5.
OC is a highly malignant tumour of the digestive
system, and most cases are diagnosed at advanced stages, resulting
in a high recurrence rate and poor prognosis (81). OC is the eighth most commonly
diagnosed cancer and the sixth most common cause of cancer-related
deaths worldwide (82). The
incidence and mortality rates of OC vary by sex, and it is more
common in male patients than in female patients (82). The major histologic subtypes of
OC include oesophageal squamous cell carcinoma (OSCC) and
oesophageal adenocarcinoma, of which OSCC is the most common
squamous-cell carcinoma (83).
Currently, the biological role and regulatory mechanism of ALKBH5
in OC have been less well studied than those in other digestive
system cancers, and research progress is relatively limited
(73,84-90).
Reduced ALKBH5 expression, which is frequently
detected in OC tissues, accelerates OC cell proliferation,
migration and invasion (91).
Chen et al (92) reported
that ALKBH5 inhibited miR-194-2 through m6A
demethylation, promoted RAI1 expression, activated the Hippo
signalling pathway and inhibited Yes associated protein/tafazzin
function to prevent the malignant behaviour of OC. Xue et al
(93) reported a positive
feedback loop between miR-193a-3p and ALKBH5, which decreased the
expression of ALKBH5 in OC, and promoted the growth and metastasis
of OC. Qiao et al (94)
reported that serine hydroxymethyltransferase 2 regulated the
stability of c-myc mRNA through METTL3/FTO/ALKBH5/IGF2BP2-mediated
m6A modification, thereby promoting the malignant
progression of OC with immune escape.
Some researchers have proposed the opposite,
suggesting that ALKBH5 promotes the progression of OC and acts as a
tumour-promoting factor (95,96). Nagaki et al (95) reported that ALKBH5 downregulated
p21 expression and regulated the cell cycle to promote the
proliferation of OC cells. At present, the mechanisms of
m6A modification and regulation of lncRNAs in tumours
are as follows: m6A can cause structural remodelling of
lncRNAs, thus affecting the interaction of lncRNAs with proteins
regulating RNA-protein interactions (97); m6A promotes
transcriptional repression induced by lncRNAs (25); lncRNAs act as competing
endogenous RNAs (ceRNAs) under m6A modification
regulation, regulating miRNA expression and biological functions
(98); and m6A is
involved in regulating the stability of lncRNA transcripts
(96). Wu et al (96) reported that ALKBH5 stabilized the
lncRNA CASC8 transcript and induced the upregulation of the lncRNA
CASC8, which activated the Bcl2/caspase3 pathway by stabilizing the
expression of heterogeneous nuclear ribonucleoprotein L, thus
promoting the progression of OC and reducing the sensitivity of OC
cells to cisplatin.
GC is one of the most important malignant tumours
globally and is characterized by high lethality (99). GC ranks fifth in global incidence
and fourth in mortality among all cancer types, with an incidence
rate in men that is twice as high as that in women (82,100). The World Health Organization
histologic classification of GC includes stomach adenocarcinoma,
gastric squamous cell carcinoma, gastric adenosquamous carcinoma,
gastric undifferentiated carcinoma, gastroblastoma and gastric
neuroendocrine neoplasms, with the vast majority of GCs being
adenocarcinomas (83). Emerging
studies have reported the important role of m6A
modifications in GC, and the molecular mechanisms of dysregulated
m6A modifications and abnormalities in GC have received
increasing attention; however, the function of ALKBH5 in GC remains
controversial (70,84,85,101).
ALKBH5 is commonly highly expressed in GC, and high
ALKBH5 expression promotes the proliferation, invasion and
migration of GC cells both in vivo and in vitro
(78,84,102). High ALKBH5 expression is
related to a poor prognosis in patients with GC (74). Bioinformatics analysis revealed
that ALKBH5 may be a key gene influencing the immune
microenvironment of GC and regulating the progression of GC
(103). Specifically, with
respect to the molecular mechanism, the upregulation of ALKBH5
expression in GC is involved in the invasion and migration of GC
cells through the regulation of the lncRNA NEAT1, which acts as a
scaffold and affects the expression of downstream genes by
regulating enhancer of zeste 2 polycomb repressive complex 2
subunit (84). Wang et al
(85) showed that the lncRNA
NRON interacts with ALKBH5 to increase the mRNA stability and
expression of Nanog, which serves an oncogenic role in GC. Fang
et al (104) found that
ALKBH5 suppressed the m6A modification of the lncRNA
TP53TG1, reduced lncRNA TP53TG1 stability, inhibited the binding of
the lncRNA TP53TG1 to cellular inhibitor of PP2A and its
ubiquitination-mediated degradation, and promoted the activation of
the PI3K/AKT pathway, thereby promoting GC progression. ALKBH5,
which is regulated by long-integrated non-coding (LINC)00659,
upregulates JAK1 expression, activates the JAK1/STAT3 pathway, and
promotes the proliferation and migration of GC cells (74). Chen et al (78) found that ALKBH5 downregulated
ChaC glutathione specific gamma-glutamylcyclotransferase 1
expression by erasing the m6A modification, disrupted
reactive oxygen species (ROS) homeostasis in GC, attenuated the
chemosensitivity of GC cells, and promoted GC development and
metastasis. Exposure to the environmental carcinogen N-nitroso
compounds activates ALKBH5-zinc finger with KRAB and SCAN domains
3-YTHDF2-VEGFA signalling, promoting malignant progression of GC
(102). Upregulation of ALKBH5
expression enhances circFOXP1 expression by mediating the
m6A modification level of circFOXP1 in GC, thereby
regulating SOX4 expression and miR-338-3p to promote the
progression of GC (105). Suo
et al (106) noted that
the heat shock protein family A (Hsp70) member 4/ALKBH5/CD58 axis
reduced cytotoxicity of CD8+T cell, inducing immune
escape in GC cells.
However, it has also been reported that ALKBH5
expression is decreased in GC and that high ALKBH5 expression can
predict a favourable prognosis, inhibit GC carcinogenesis and
metastasis, and act as a tumour suppressor (70,101,107). Bioinformatics analysis revealed
that ALKBH5 serves an antitumour role in GC and is a potential
prognostic marker and immunotherapy target for GC (101). Hu et al (70) found that ALKBH5-IGF2BP3
upregulated the expression of PKMYT1 in an m6A-dependent
manner, inhibiting the invasion and migration of GC cells. ALKBH5
negatively regulates YY1, thereby inhibiting the activation of the
autophagy related 4B cysteine peptidase-dependent autophagy pathway
and serving a protective role in GC development (107).
CRC is the third most prevalent cancer worldwide and
the second leading cause of cancer-related deaths (82). It is the second most common type
of cancer diagnosed in women and the third most common type of
cancer in men (82). The onset
of CRC is insidious, with symptoms often not presenting until it
reaches an advanced stage, and effective cancer screening measures
can reduce the incidence and mortality of CRC (108). Although research on CRC has
made great progress, the specific molecular mechanisms that
influence tumorigenesis and development remain elusive. Numerous
studies have reported that ALKBH5-mediated m6A
modification serves a role in CRC progression (61,69,109,110).
Histologic subtypes of CRC include colorectal
adenocarcinoma and neuroendocrine tumours, with colon
adenocarcinoma (COAD) being the most common type (83). Bioinformatics analysis revealed
that ALKBH5 expression is downregulated in CRC, downregulation of
ALKBH5 is associated with distant metastasis and the
clinicopathological features of CRC and high ALKBH5 expression
enhances immune infiltration (111). ALKBH5, which acts as a tumour
suppressor in CRC (111,112),
has been suggested to be independently associated with prognosis.
ALKBH5 can suppress the growth and metastasis of CRC through
various pathways and molecular mechanisms. For example, Zhang et
al (109) reported that
ALKBH5 targeted the PHD finger protein 20 (PHF20) m6A
modification, downregulated PHF20 expression and inhibited CRC
progression. Luo et al (110) found that ALKBH5 removed the
m6A modification of solute carrier family 7 member 11
mRNA and suppressed its transcription, and that upregulation of
ALKBH5 expression promoted ROS release and ferroptosis in CRC.
ALKBH5 can also promote CD8+ T-cell infiltration in the
CRC tumour microenvironment via the NF-κB-C-C motif chemokine
ligand 5 axis, attenuating the malignant behaviour of CRC (113). Furthermore, Wu et al
(114) reported that ALKBH5
inhibited the proliferation and migration of CRC cells, and exerted
antitumour effects by regulating the FOXO3-miR-21-sprouty RTK
signaling antagonist 2 axis. Yan et al (115) revealed that ALKBH5 and YTHDF1
might affect the immune architecture of COAD and that high ALKBH5
expression with low YTHDF expression might enhance the infiltration
of immune cells and improve the immunotherapeutic efficacy of
patients with colorectal adenocarcinoma. ALKBH5 can also serve a
tumour suppressor role in CRC by regulating the tumour glycolysis
pathway, downregulating the expression of jumonji domain containing
8 in an m6A-dependent manner, and inhibiting glycolysis
by suppressing the enzymatic activity of pyruvate kinase M1/2
(86). Ye et al (116) demonstrated that in obese
patients with CRC, the downregulation of ALKBH5 and FTO
synergistically negatively regulated the expression of hexokinase 2
(HK2), a key enzyme in glycolysis, in an
m6A-IGF2BP2-dependent manner, activating the FOXO
signalling pathway and promoting glucose metabolism, which in turn
increased the proliferative capacity of CRC cells. Ye et al
(87) demonstrated that the
ALKBH5-fatty acid binding protein 5 (FABP5)-fatty acid
synthase-mTOR axis inhibited tumour progression by affecting lipid
metabolism and autophagy in CRC. Shao et al (117) revealed that ALKBH5 increased
the sensitivity of CRC cells to radiotherapy and that the
ALKBH5-circAFF2-YTHDF2-cullin associated and neddylation
dissociated 1-Cullin1/NEDD8 axis was a potential chemotherapeutic
target for CRC. ALKBH5 is downregulated in mutant p53-induced CRC
and promotes CRC progression through the p53-ALKBH5-lncRNA
CARMN-YTHDF2/YTHDF3-miR-5683-fibroblast growth factor 2-Akt/mTOR
pathway (118). Feng et
al (119) reported that
ALKBH5 could affect the immunosuppressive function of
myeloid-derived suppressor cells (MDSCs) in CRC by regulating
arginase 1 expression, and could inhibit the progression of
CRC.
Primary liver cancer is one of the most common
malignancies of the digestive system, the sixth most common type of
cancer and the third leading cause of cancer-related deaths
worldwide (82). The incidence
of liver cancer differs between sexes. The incidence and mortality
rates in men are 2 to 3-fold higher than those in women. In men,
liver cancer ranks fifth in terms of incidence rate and second in
terms of mortality rate among tumours (82). Primary liver cancer includes HCC
and intrahepatic cholangiocarcinoma (ICC), as well as other rare
types. HCC is the most common primary malignancy of the liver,
accounting for >80% of all liver cancer cases, and has a high
mortality rate (129). An
increasing number of studies have reported the dysregulation of the
m6A modification in HCC and the regulatory mechanisms of
m6A-related molecules, revealing the role of
m6A in HCC (67,73,88,130).
ALKBH5 has been identified as a tumour suppressor
in HCC, suppresses tumour growth and metastasis in HCC, and is a
prognostic factor in HCC, predicting favourable clinical outcomes
(67,73). Chen et al (67) reported that
ALKBH5-IGF2BP1-mediated m6A modification led to
post-transcriptional repression of LY6/PLAUR domain containing 1
(LYPD1) and that dysregulation of the ALKBH5/LYPD1 axis promoted
the malignant behaviour of HCC cells. ALKBH5, together with
IGF2BP1, downregulates the expression of progestin and AdipoQ
Receptor family member 4 in an m6A-dependent manner and
inhibits the activation of the PI3K/AKT pathway, thereby
suppressing the malignant progression of HCC cells (73). Zhang et al (131) reported that ALKBH5 in HCC
regulated the decay of lncRNA LINC02551 mediated by IGF2BP1
recognition in an m6A-dependent manner, reduced the
expression of the lncRNA LINC02551 and facilitated the degradation
of DEAD-box helicase 24, thereby inhibiting epithelial-mesenchymal
transition (EMT) in HCC. With the global increase in type 2
diabetes mellitus and obesity, non-alcoholic fatty liver disease
(NAFLD) is becoming an increasingly significant risk factor for HCC
(132). ALKBH5 promotes the
expression of the lncRNA LINC01468, which mediates lipid
metabolism, and promotes chemoresistance and tumour progression in
NAFLD-associated HCC (133).
ALKBH5, as a tumour suppressor, regulates ubiquitin protein ligase
E3 component n-recognin 7 (UBR7) in an m6A-dependent
manner, and UBR7 inhibits aerobic glycolysis in HCC cells by
regulating the Kelch-like ECH-associated protein 1-nuclear factor
erythroid 2-related factor 2-BTB domain and CNC homolog 1-HK2 axi
(134).
Hepatitis B virus (HBV) infection is considered to
be an important risk factor for HCC (140), and the clarification of the
molecular mechanisms of HBV-associated HCC (HBV-HCC) and
identification of potential biomarkers are crucial for clinical
diagnosis and accurate treatment. A recent study has shown that
ALKBH5 expression is increased in HBV-positive HCC cells, and that
HBV promotes the maintenance of HBV-HCC stemness and immune escape
by stabilizing snail family transcriptional repressor 2 (SNAI2)
transcripts and increasing the number of ligands for the immune
checkpoint CD155 through the ALKBH5-SNAI2-YTHDF2 axis (141). The HBV X protein (HBx) induces
HBV-associated aberrant epigenetic modifications that promote
hepato-carcinogenesis (142).
Qu et al (143) reported
that high ALKBH5 expression predicted a poor prognosis in patients
with HBV-HCC and that the HBx-ALKBH5 positive feedback loop through
the HBx-WD repeat domain 5-trimethylated H3 lysine 4-ALKBH5 axis
was involved in the development of HBV-HCC.
ICC is the second most common primary malignancy of
the liver and has a markedly higher recurrence rate than HCC
(144). Several studies have
reported that high ALKBH5 expression in patients with ICC is
closely related to poor outcomes (145,146). Gao et al (145) reported that ALKBH5 serves a
role in maintaining the stemness and progression of ICC via BUB1
mitotic checkpoint serine/threonine kinase B. Qiu et al
(146) suggested that
ALKBH5-YTHDF2 specifically modulated PD-L1 expression and
suppressed T-cell-mediated antitumour immunity in ICC.
GBC is the most common malignant tumour of the
biliary tract, and early diagnosis and detection of GBC can improve
the survival rate of patients with GBC (147). Chronic inflammation has been
shown to be a driving factor of GBC, with cholelithiasis being the
most important risk factor (148). ALKBH5 in GBC has been
investigated in relatively few studies; only a single study has
shown that TGFβ1 negatively regulates the translation efficiency of
forkhead box A1 (FOXA1) by inhibiting the binding ability of ALKBH5
to the FOXA1 coding sequence region, thereby promoting metastasis
in GBC with EMT (149).
PC is a highly malignant gastrointestinal tumour
that is difficult to diagnose and treat and has poor survival
outcomes. PC is the seventh leading cause of cancer-related
mortality worldwide, and due to its poor prognosis, PC accounts for
almost as many deaths (466,000) as cases (496,000) (82). However, to the best of our
knowledge, the molecular mechanisms underlying the high mortality
rate of PC have not yet been clarified. Thus, identifying the key
molecular mechanisms that influence the progression of PC is
important in the subsequent selection of therapeutic strategies for
PC and in improving the survival of patients with PC.
The histologic classifications of PC include
pancreatic ductal adenocarcinoma (PDAC), pancreatic neuroendocrine
tumours (pNETs) and solid pseudopapillary tumours of the pancreas,
and PDAC is the most common type of PC (83). Gemcitabine (GEM) is the
first-line chemotherapy for patients with PC, and adjuvant
chemotherapy with GEM has been proven to improve the overall
survival (OS) and disease-free survival (DFS) of patients with PC
(150). However, GEM resistance
is an impediment to first-line chemotherapy for PDAC (151), and investigating the mechanisms
underlying GEM resistance and increasing the sensitivity of PC
cells to GEM is crucial. Emerging studies have demonstrated that
ALKBH5 can increase the sensitivity of PC cells to GEM chemotherapy
and suppress the progression of PC (75,77,89). ALKBH5 was downregulated in a
GEM-treated patient-derived xenograft model, and upregulation of
ALKBH5 expression inhibited Wnt signalling by downregulating the
m6A modification of WNT inhibitory factor 1, which
sensitized PDAC to chemotherapy (75). In addition, low ALKBH5 expression
increased the m6A modification of lncRNA SH3BP5-AS1,
promoted the recognition of lncRNA SH3BP5-AS1 by IGF2BP1, improved
the stability and expression of lncRNA SH3BP5-AS1, activated the
Wnt signalling pathway, and upregulated the expression of
C-terminal binding protein 1, which led to GEM resistance and
promoted the invasion, migration and stemness of PC cells (89). The stability of the lncRNA
DDIT4-AS1 is maintained by the m6A modification site and
ALKBH5 mediates the demethylation of the lncRNA DDIT4-AS1,
downregulates the level of the lncRNA DDIT4-AS1, and inhibits the
phosphorylation of UPF1, the degradation of DDIT4 mRNA and the
activation of the mTOR pathway, thereby inhibiting the stemness
characteristics of PDAC cells and increasing the chemosensitivity
of tumour cells to GEM (77).
Bioinformatics predictions have revealed that
ALKBH5 expression is strongly associated with the infiltration of
immune cells (152), OS and DFS
(153) in PC. Guo et al
(79) reported that low ALKBH5
expression was related to a poor overall prognosis and
clinicopathological features in patients with PC, and the specific
mechanism was that the lack of ALKBH5 expression inhibited the
activation of ALKBH5-period circadian regulator 1-ATM-checkpoint
kinase 2-P53/cell division cycle 25C signalling in a manner
dependent on m6A-YTHDF2, which promoted the migration
and invasion of PC cells. It has been reported that ALKBH5
suppresses PC cell motility by demethylating the lncRNA KCNK15-AS1
(154). The lncRNA KCNK15-AS1
also inhibits the malignant behaviour of PC cells by modulating
KCNK15 and PTEN to activate the AKT pathway in PC cells (90). Lai et al (155) pointed out that ALKBH5
positively regulates CUGBP Elav-like family member 2 (CELF2) in PC,
and CELF2 mediates CD44 alternative splicing to affect the
endoplasmic reticulum-associated degradation signalling pathway,
which regulates the stemness and apoptosis of PC cells, and
inhibits their proliferation and metastasis. Huang et al
(156) showed that ALKBH5 can
also target iron metabolism regulators through the ALKBH5-F-box and
leucine rich repeat protein 5-iron-regulatory protein 2
(IRP2)/SNAI1 axis in PC. In addition, PC is considered the most
hypoxic tumour among solid tumours (157), and under hypoxic conditions,
the ALKBH5-histone deacetylase 4-hypoxia-inducible factor-1α
positive feedback loop promotes glycolysis and migration in PC
cells (72).
pNETs are the second most common malignancy of the
pancreas, with increasing incidence (158); however, the role of ALKBH5 in
pNETs is largely undefined. Chen et al (159) demonstrated that upregulated
ALKBH5 increased FABP5 expression in an m6A
modification-dependent manner, which activated the PI3K/AKT/mTOR
signalling pathway, thus promoting lipid metabolism and malignant
behaviour in pNETs.
Several 2OG analogues have been found to exhibit
suppressive effects on ALKBH5. Takahashi et al (168) screened two novel compounds,
Ena15 (IC50, 18.3±1.8 μM) and Ena21
(IC50, 15.7±1.0 μM), which specifically inhibited
ALKBH5 according to high-throughput screening. Of these, Ena15 was
the first reported non-competitive inhibitor that targets not only
ALKBH5 but also other AlkB family proteins, while Ena21 is a more
common 2OG competitive inhibitor, and both Ena15 and Ena21
inhibited GBM growth and arrested the cell cycle at the
G0/G1 phase (168). In a drug repurposing study,
Malacrida et al (171)
performed proteomics and found that
2-methyl-3-propyl-5H-imidazo[1,2-c][1,3] benzoxazin-5-thione
(MV1035) could target and inhibit ALKBH5, negatively regulate the
expression of CD73, and markedly suppress the migration and
invasion of GBM cells. 5-carboxy-8-hydroxyquinoline (IOX1)
(IC50, 2.9±0.3 μM), a broad-spectrum 2OG
inhibitor, also competitively suppresses the activity of ALKBH5
(172). Tang et al
(173) found that IOX1 delayed
GBM tumour growth and increased the efficacy of anti-PD-1 therapy
by inhibiting ALKBH5. Li et al (170) found that the ALKBH5-specific
inhibitor ALK-04 inhibited melanoma growth and enhanced the
response of melanoma to tumour immunotherapy.
In addition, several research groups have
identified ALKBH5 inhibitors that are not 2OG analogues (176,177). 8539-0746 has moderate ALKBH5
demethylase inhibitory activity, while 4-(Trifluoromethyl)benzyl
4-(6-(2,6-dihydroxy-3-nitrobenzoyl)pyrazolo[1,5-a]pyrimidin-2-yl)benzoate
(DDO-2728) (IC50, 2.97 μM) is an optimized
inhibitor based on 8539-0746, as a novel and highly selective
inhibitor of ALKBH5 with a 2OG-independent mechanism, selectively
inhibiting the demethylase activity of ALKBH5 but not FTO (176). DDO-2728 targets the
ALKBH5-transforming acidic coiled-coil containing protein 3 axis to
induce the apoptosis and cell cycle arrest of AML cells, thereby
inhibiting tumour progression (176). Komal et al (177) found that two ZINC15 compounds
(ZINC78774792 and ZINC00546946), which bind to the ALKBH5 active
site with the lowest binding energy, are potential ALKBH5
inhibitors that might have therapeutic value in the context of
heart failure.
Increasing evidence has shown that epigenetic
regulation serves a role in cancer. m6A modification is
the most vital and prevalent type of modification in human
eukaryotic mRNAs. m6A modification and its regulatory
factors are closely associated with tumorigenesis and the
development of tumours. With an increasing number of studies on
m6A, the m6A demethylase ALKBH5 has received
increasing attention. Previous studies on ALKBH5 have focused on
its role in lung cancer, GBM and malignant haematological diseases
(59,64,68,178-180), and emerging studies have shown
that ALKBH5 is also extensively involved in various organic
processes in digestive tract malignancies (67,75,84,91,109).
The current review indicates that the expression
pattern and function of ALKBH5 in gastrointestinal tract cancer are
controversial and that ALKBH5 has dual regulatory effects. ALKBH5
mainly serves as a cancer suppressor in OC, CRC, GBC and PC,
suppresses the proliferation, migration and invasion of tumour
cells, and regulates tumorigenesis, tumour progression, tumour
metastasis, tumour drug resistance, tumour immunity and tumour
metabolism. In GC, ICC and HBV-HCC, it functions as an oncogene and
promotes tumour development. However, some studies have reported
the opposite results. But in HCC, ALKBH5 is dually regulated in
HCC, and the number of studies on inhibiting and promoting the
development of HCC is about the same. For example, in HCC, ALKBH5
inhibits the malignant biological properties of HCC, and low ALKBH5
expression is related to an unfavourable prognosis. ALKBH5 can also
promote the proliferation, migration and invasion of HCC cells
through the ALKBH5-MAP3K8 axis (73,88). Mechanistically, the role of
ALKBH5 in gastrointestinal tract cancer is also complex, and ALKBH5
is associated with multiple target genes and molecular mechanisms
through its m6A methylation eraser function. For
example, it is involved in the regulation of non-coding RNAs
(ncRNAs), the coregulation of multiple m6A readers and
the maintenance of the stemness of tumour stem cells.
Given the functional importance of ALKBH5 as an
independent prognostic marker for a variety of digestive tract
tumours, there is great potential for the clinical application of
ALKBH5 as a novel treatment target for digestive malignancies.
Considering the different roles of ALKBH5 in different digestive
system tumours, the therapeutic strategies of ALKBH5 in different
tumour function will be discussed separately. One is as an
oncogenic factor that is often highly expressed in cancer. One
study has mentioned that vesicle-encapsulated ALKBH5 siRNA can be
used in combination with anti-PD-1 treatment and can improve the
efficacy of immunotherapy in CRC (61). To the best of our knowledge,
inhibitors of ALKBH5 have only been studied in GBM, melanoma and
hematologic tumours, but have not been observed in digestive system
tumours. Further research on ALKBH5 inhibitors is expected in the
future to achieve more efficient treatment. The other is as a
tumour suppressor that is often downregulated in cancer. It has
been mentioned in the literature that it is possible to treat
tumours using nanomaterials with ALKBH5 mRNA to enhance ectopic
ALKBH5 expression (86). Some
tumours with low expression of ALKBH5 can be suitably treated with
an ALKBH5 agonist to improve the antitumour efficacy (181); however, no studies on ALKBH5
agonists have been mentioned. Further research investigating ALKBH5
agonists should be conducted in the future.
Although numerous studies have revealed the
function and mechanism of ALKBH5 in digestive system tumours, there
are still some problems to be explored. First, as a potential
biomarker for the clinical diagnosis, prognosis and treatment of
digestive system tumours, the research investigating ALKBH5 in
digestive system tumours is limited to clinical samples,
bioinformatics data and animal models. Large-scale, multicenter,
prospective clinical studies are needed to further improve the
accuracy and specificity of ALKBH5 as a biomarker in digestive
tumours. Second, there are still some unclear mechanisms of ALKBH5
regulating digestive system tumours, such as the regulation of
ALKBH5 and ncRNAs, the function of ALKBH5 in cancer stem cells, and
the role of ALKBH5 in GBC. Some studies have only focused on the
role of ALKBH5 in altering the RNA levels of downstream genes but
have not addressed whether ALKBH5 regulates downstream genes by
remodelling downstream structures, altering the stability of
downstream transcripts or acting as ceRNAs. ALKBH5 is related to
the maintenance of stemness of cancer stem cells and is involved in
tumour progression and tumour drug resistance; however, there are
few studies on ALKBH5 in cancer stem cells. In GBC, few studies
have been conducted on ALKBH5, and further studies should examine
the function of ALKBH5 in GBC. Third, although ALKBH5 has been
shown to be a promising therapeutic target, at present, inhibitors
of ALKBH5 have only been studied in animal experiments in some
tumours and lack clinical practice. In addition, agonists targeting
ALKBH5 as well as liposomes and nanomaterials loaded with ALKBH5
drugs also need to be further developed.
In conclusion, the expression, function and
regulatory mechanisms of ALKBH5 in gastrointestinal tumours are
summarized, suggesting that ALKBH5 serves a key role in the
tumorigenesis and progression of gastrointestinal tumours. This
means that ALKBH5 is a potential diagnostic, prognostic and
therapeutic biomarker in digestive tract tumours. However, there
are still some problems to be explored. The research of ALKBH5
lacks large-scale clinical data to verify the accuracy and
specificity of ALKBH5 as a biomarker, the specific regulatory
mechanism of ALKBH5 still needs further elucidation, and the
specific targeted drug development of ALKBH5 is insufficient and
clinical practice is lacking. More large-scale, multicenter data
and prospective clinical studies are required in the future to
further validate the accuracy of ALKBH5 as a biomarker and promote
the application of personalized medicine for gastrointestinal
cancer in clinical practice.
Not applicable.
LZ and MJ designed and wrote the original draft. QS
and XY acquired the data. LZ, MJ, YO, YP, WY and YF edited and
revised the manuscript. All authors read and approved the final
version of the manuscript and agreed to accountable for all aspects
of the work. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant no. 81974383) and the Natural Science
Foundation of Hubei (grant no. 2023AFD044).
|
1
|
Boccaletto P, Machnicka MA, Purta E,
Piatkowski P, Baginski B, Wirecki TK, de Crécy-Lagard V, Ross R,
Limbach PA, Kotter A, et al: MODOMICS: A database of RNA
modification pathways. 2017 Update. Nucleic Acids Res.
46:D303–D307. 2018. View Article : Google Scholar :
|
|
2
|
Lewis CJT, Pan T and Kalsotra A: RNA
modifications and structures cooperate to guide RNA-protein
interactions. Nat Rev Mol Cell Biol. 18:202–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Desrosiers R, Friderici K and Rottman F:
Identification of methylated nucleosides in messenger RNA from
Novikoff hepatoma cells. Proc Natl Acad Sci USA. 71:3971–3975.
1974. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adams JM and Cory S: Modified nucleosides
and bizarre 5′-termini in mouse myeloma mRNA. Nature. 255:28–33.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wei W, Ji X, Guo X and Ji S: Regulatory
Role of N6 -methyladenosine (m6A) methylation
in rna processing and human diseases. J Cell Biochem.
118:2534–2543. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ivanova I, Much C, Di Giacomo M, Azzi C,
Morgan M, Moreira PN, Monahan J, Carrieri C, Enright AJ and
O'Carroll D: The RNA m6A reader YTHDF2 is essential for
the posttranscriptional regulation of the maternal transcriptome
and oocyte competence. Mol Cell. 67:1059–1067.e4. 2017. View Article : Google Scholar
|
|
7
|
Xiang Y, Laurent B, Hsu CH, Nachtergaele
S, Lu Z, Sheng W, Xu C, Chen H, Ouyang J, Wang S, et al: RNA
m6A methylation regulates the ultraviolet-induced DNA
damage response. Nature. 543:573–576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu Y, Xie L, Wang M, Xiong Q, Guo Y, Liang
Y, Li J, Sheng R, Deng P, Wang Y, et al: Mettl3-mediated
m6A RNA methylation regulates the fate of bone marrow
mesenchymal stem cells and osteoporosis. Nat Commun. 9:47722018.
View Article : Google Scholar
|
|
9
|
Christiansen J, Kolte AM, Hansen TVO and
Nielsen FC: IGF2 mRNA-binding protein 2: Biological function and
putative role in type 2 diabetes. J Mol Endocrinol. 43:187–195.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Li L, Li J, Zhao B, Huang G, Li X,
Xie Z and Zhou Z: The emerging role of m6A modification in
regulating the immune system and autoimmune diseases. Front Cell
Dev Biol. 9:7556912021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu J, Chai P, Xie M, Ge S, Ruan J, Fan X
and Jia R: Histone lactylation drives oncogenesis by facilitating
m6A reader protein YTHDF2 expression in ocular melanoma.
Genome Biol. 22:852021. View Article : Google Scholar
|
|
12
|
Zhao BS, Roundtree IA and He C:
Post-transcriptional gene regulation by mRNA modifications. Nat Rev
Mol Cell Biol. 18:31–42. 2017. View Article : Google Scholar
|
|
13
|
Hastings MH: m(6)A mRNA methylation: A new
circadian pacesetter. Cell. 155:740–741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu N, Zhou KI, Parisien M, Dai Q,
Diatchenko L and Pan T: N6-methyladenosine alters RNA structure to
regulate binding of a low-complexity protein. Nucleic Acids Res.
45:6051–6063. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han
D, Fu Y, Parisien M, Dai Q, Jia G, et al:
N6-methyladenosine-dependent regulation of messenger RNA stability.
Nature. 505:117–120. 2014. View Article : Google Scholar
|
|
16
|
Alarcón CR, Lee H, Goodarzi H, Halberg N
and Tavazoie SF: N6-methyladenosine marks primary microRNAs for
processing. Nature. 519:482–485. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang M, Cui X, Sun Q, Wang Y, Liu J, Sun
Z, Ren J, Sun Y, Han L and Li W: Lnc-PLCB1 is stabilized by METTL14
induced m6A modification and inhibits Helicobacter pylori mediated
gastric cancer by destabilizing DDX21. Cancer Lett. 588:2167462024.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu P, Fang X, Liu Y, Tang Y, Wang W, Li X
and Fan Y: N6-methyladenosine modification of circCUX1 confers
radioresistance of hypopharyngeal squamous cell carcinoma through
caspase1 pathway. Cell Death Dis. 12:2982021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang X, Liu B, Nie Z, Duan L, Xiong Q,
Jin Z, Yang C and Chen Y: The role of m6A modification in the
biological functions and diseases. Signal Transduct Target Ther.
6:742021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ping XL, Sun BF, Wang L, Xiao W, Yang X,
Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS, et al: Mammalian WTAP is
a regulatory subunit of the RNA N6-methyladenosine
methyltransferase. Cell Res. 24:177–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang
L, Jia G, Yu M, Lu Z, Deng X, et al: A METTL3-METTL14 complex
mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem
Biol. 10:93–95. 2014. View Article : Google Scholar :
|
|
22
|
Pendleton KE, Chen B, Liu K, Hunter OV,
Xie Y, Tu BP and Conrad NK: The U6 snRNA m6A
methyltransferase METTL16 regulates SAM synthetase intron
retention. Cell. 169:824–835.e14. 2017. View Article : Google Scholar
|
|
23
|
Chen H, Gu L, Orellana EA, Wang Y, Guo J,
Liu Q, Wang L, Shen Z, Wu H, Gregory RI, et al: METTL4 is an snRNA
m6Am methyltransferase that regulates RNA splicing. Cell
Res. 30:544–547. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng H, Chen B, Wei W, Guo S, Han H, Yang
C, Ma J, Wang L, Peng S, Kuang M and Lin S:
N6-methyladenosine (m6A) in 18S rRNA promotes
fatty acid metabolism and oncogenic transformation. Nat Metab.
4:1041–1054. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Patil DP, Chen CK, Pickering BF, Chow A,
Jackson C, Guttman M and Jaffrey SR: m(6)A RNA methylation promotes
XIST-mediated transcriptional repression. Nature. 537:369–373.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wen J, Lv R, Ma H, Shen H, He C, Wang J,
Jiao F, Liu H, Yang P, Tan L, et al: Zc3h13 regulates nuclear RNA
m6A methylation and mouse embryonic stem cell
self-renewal. Mol Cell. 69:1028–1038.e6. 2018. View Article : Google Scholar
|
|
27
|
Ren W, Lu J, Huang M, Gao L, Li D, Wang GG
and Song J: Structure and regulation of ZCCHC4 in
m6A-methylation of 28S rRNA. Nat Commun. 10:50422019.
View Article : Google Scholar
|
|
28
|
Zhang X, Li MJ, Xia L and Zhang H: The
biological function of m6A methyltransferase KIAA1429 and its role
in human disease. PeerJ. 10:e143342022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liao S, Sun H and Xu C: YTH domain: A
family of N6-methyladenosine (m6A) readers.
Genomics Proteomics Bioinformatics. 16:99–107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alarcón CR, Goodarzi H, Lee H, Liu X,
Tavazoie S and Tavazoie SF: hnrnpa2b1 is a mediator of
m(6)A-dependent nuclear RNA processing events. Cell. 162:1299–1308.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang H, Weng H, Sun W, Qin X, Shi H, Wu
H, Zhao BS, Mesquita A, Liu C, Yuan CL, et al: Recognition of RNA
N6-methyladenosine by IGF2BP proteins enhances mRNA
stability and translation. Nat Cell Biol. 20:285–295. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang
Y, Yi C, Lindahl T, Pan T, Yang YG and He C: N6-methyladenosine in
nuclear RNA is a major substrate of the obesity-associated FTO. Nat
Chem Biol. 7:885–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bartosovic M, Molares HC, Gregorova P,
Hrossova D, Kudla G and Vanacova S: N6-methyladenosine demethylase
FTO targets pre-mRNAs and regulates alternative splicing and 3′-end
processing. Nucleic Acids Res. 45:11356–11370. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng G, Dahl JA, Niu Y, Fedorcsak P,
Huang CM, Li CJ, Vågbø CB, Shi Y, Wang WL, Song SH, et al: ALKBH5
is a mammalian RNA demethylase that impacts RNA metabolism and
mouse fertility. Mol Cell. 49:18–29. 2013. View Article : Google Scholar :
|
|
35
|
Aik W, Scotti JS, Choi H, Gong L,
Demetriades M, Schofield CJ and McDonough MA: Structure of human
RNA N6-methyladenine demethylase ALKBH5 provides
insights into its mechanisms of nucleic acid recognition and
demethylation. Nucleic Acids Res. 42:4741–4754. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou B and Han Z: Crystallization and
preliminary X-ray diffraction of the RNA demethylase ALKBH5. Acta
Crystallogr Sect F Struct Biol Cryst Commun. 69:1231–1234. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zou S, Toh JDW, Wong KHQ, Gao YG, Hong W
and Woon ECY: N(6)-Methyladenosine: A conformational marker that
regulates the substrate specificity of human demethylases FTO and
ALKBH5. Sci Rep. 6:256772016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jia G, Yang CG, Yang S, Jian X, Yi C, Zhou
Z and He C: Oxidative demethylation of 3-methylthymine and
3-methyluracil in single-stranded DNA and RNA by mouse and human
FTO. FEBS Lett. 582:3313–3319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang X, Wei LH, Wang Y, Xiao Y, Liu J,
Zhang W, Yan N, Amu G, Tang X, Zhang L and Jia G: Structural
insights into FTO's catalytic mechanism for the demethylation of
multiple RNA substrates. Proc Natl Acad Sci USA. 116:2919–2924.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Feng C, Liu Y, Wang G, Deng Z, Zhang Q, Wu
W, Tong Y, Cheng C and Chen Z: Crystal structures of the human RNA
demethylase Alkbh5 reveal basis for substrate recognition. J Biol
Chem. 289:11571–11583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen W, Zhang L, Zheng G, Fu Y, Ji Q, Liu
F, Chen H and He C: Crystal structure of the RNA demethylase ALKBH5
from zebrafish. FEBS Lett. 588:892–898. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu C, Liu K, Tempel W, Demetriades M, Aik
W, Schofield CJ and Min J: Structures of human ALKBH5 demethylase
reveal a unique binding mode for specific single-stranded
N6-methyladenosine RNA demethylation. J Biol Chem. 289:17299–17311.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Purslow JA, Nguyen TT, Egner TK, Dotas RR,
Khatiwada B and Venditti V: Active site breathing of human Alkbh5
revealed by solution NMR and accelerated molecular dynamics.
Biophys J. 115:1895–1905. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ougland R, Rognes T, Klungland A and
Larsen E: Non-homologous functions of the AlkB homologs. J Mol Cell
Biol. 7:494–504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Clifton IJ, McDonough MA, Ehrismann D,
Kershaw NJ, Granatino N and Schofield CJ: Structural studies on
2-oxoglutarate oxygenases and related double-stranded beta-helix
fold proteins. J Inorg Biochem. 100:644–669. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Aik W, McDonough MA, Thalhammer A,
Chowdhury R and Schofield CJ: Role of the jelly-roll fold in
substrate binding by 2-oxoglutarate oxygenases. Curr Opin Struct
Biol. 22:691–700. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sundheim O, Talstad VA, Vågbø CB,
Slupphaug G and Krokan HE: AlkB demethylases flip out in different
ways. DNA Repair (Amst). 7:1916–1923. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yu B, Edstrom WC, Benach J, Hamuro Y,
Weber PC, Gibney BR and Hunt JF: Crystal structures of catalytic
complexes of the oxidative DNA/RNA repair enzyme AlkB. Nature.
439:879–884. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Galganski L, Urbanek MO and Krzyzosiak WJ:
Nuclear speckles: Molecular organization, biological function and
role in disease. Nucleic Acids Res. 45:10350–10368. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tang C, Klukovich R, Peng H, Wang Z, Yu T,
Zhang Y, Zheng H, Klungland A and Yan W: ALKBH5-dependent m6A
demethylation controls splicing and stability of long 3′-UTR mRNAs
in male germ cells. Proc Natl Acad Sci USA. 115:E325–E333. 2018.
View Article : Google Scholar
|
|
51
|
Tsujikawa K, Koike K, Kitae K, Shinkawa A,
Arima H, Suzuki T, Tsuchiya M, Makino Y, Furukawa T, Konishi N and
Yamamoto H: Expression and sub-cellular localization of human ABH
family molecules. J Cell Mol Med. 11:1105–1116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang X, Mei C, Ma X, Du J, Wang J and Zan
L: m6A methylases regulate myoblast proliferation,
apoptosis and differentiation. Animals (Basel). 12:7732022.
View Article : Google Scholar
|
|
53
|
Lv Z, Xu T, Li R, Zheng D, Li Y, Li W,
Yang Y and Hao Y: Downregulation of m6A methyltransferase in the
hippocampus of tyrobp −/− Mice and implications for
learning and memory deficits. Front Neurosci. 16:7392012022.
View Article : Google Scholar
|
|
54
|
Ye G, Li J, Yu W, Xie Z, Zheng G, Liu W,
Wang S, Cao Q, Lin J, Su Z, et al: ALKBH5 facilitates CYP1B1 mRNA
degradation via m6A demethylation to alleviate MSC senescence and
osteoarthritis progression. Exp Mol Med. 55:1743–1756. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang Y, Liu Q, Ning J, Jiang T, Kang A,
Li L, Pang Y, Zhang B, Huang X, Wang Q, et al: The
proteasome-dependent degradation of ALKBH5 regulates ECM deposition
in PM2.5 exposure-induced pulmonary fibrosis of mice. J
Hazard Mater. 432:1286552022. View Article : Google Scholar
|
|
56
|
Liu C, Chen H, Tao X, Li C, Li A and Wu W:
ALKBH5 protects against stroke by reducing endoplasmic reticulum
stress-dependent inflammation injury via the STAT5/PERK/EIF2α/CHOP
signaling pathway in an m6A-YTHDF1-dependent manner. Exp Neurol.
372:1146292024. View Article : Google Scholar
|
|
57
|
Deng LJ, Fang XY, Wu J, Li QR, Mao YM,
Leng RX, Fan YG and Ye DQ: ALKBH5 expression could affect the
function of t cells in systemic lupus erythematosus patients: A
case-control study. Curr Pharm Des. 28:2270–2278. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Qu M, Zuo L, Zhang M, Cheng P, Guo Z, Yang
J, Li C and Wu J: High glucose induces tau hyperphosphorylation in
hippocampal neurons via inhibition of ALKBH5-mediated Dgkh
m6A demethylation: A potential mechanism for diabetic
cognitive dysfunction. Cell Death Dis. 14:3852023. View Article : Google Scholar
|
|
59
|
Zhang S, Zhao BS, Zhou A, Lin K, Zheng S,
Lu Z, Chen Y, Sulman EP, Xie K, Bögler O, et al: m6A
demethylase ALKBH5 maintains tumorigenicity of glioblastoma
stem-like cells by sustaining FOXM1 expression and cell
proliferation program. Cancer Cell. 31:591–606.e6. 2017. View Article : Google Scholar
|
|
60
|
Jin S, Li M, Chang H, Wang R, Zhang Z,
Zhang J, He Y and Ma H: The m6A demethylase ALKBH5 promotes tumor
progression by inhibiting RIG-I expression and interferon alpha
production through the IKKε/TBK1/IRF3 pathway in head and neck
squamous cell carcinoma. Mol Cancer. 21:972022. View Article : Google Scholar
|
|
61
|
Zhai J, Chen H, Wong CC, Peng Y, Gou H,
Zhang J, Pan Y, Chen D, Lin Y, Wang S, et al: ALKBH5 drives immune
suppression via targeting AXIN2 to promote colorectal cancer and is
a target for boosting immunotherapy. Gastroenterology. 165:445–462.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nie S, Zhang L, Liu J, Wan Y, Jiang Y,
Yang J, Sun R, Ma X, Sun G, Meng H, et al: ALKBH5-HOXA10
loop-mediated JAK2 m6A demethylation and cisplatin resistance in
epithelial ovarian cancer. J Exp Clin Cancer Res. 40:2842021.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liang L, Zhu Y, Li J, Zeng J and Wu L:
ALKBH5-mediated m6A modification of circCCDC134 facilitates
cervical cancer metastasis by enhancing HIF1A transcription. J Exp
Clin Cancer Res. 41:2612022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang J, Li Y, Wang P, Han G, Zhang T,
Chang J, Yin R, Shan Y, Wen J, Xie X, et al: Leukemogenic chromatin
alterations promote AML leukemia stem cells via a KDM4C-ALKBH5-AXL
signaling axis. Cell Stem Cell. 27:81–97.e8. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu H, Lyu H, Jiang G, Chen D, Ruan S, Liu
S, Zhou L, Yang M, Zeng S, He Z, et al: ALKBH5-mediated m6A
demethylation of GLUT4 mRNA promotes glycolysis and resistance to
HER2-targeted therapy in breast cancer. Cancer Res. 82:39742022.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang H, Zhao S, Liu H, Liu Y, Zhang Z,
Zhou Z, Wang P, Qi S and Xie J: ALKBH5 facilitates the progression
of skin cutaneous melanoma via mediating ABCA1 demethylation and
modulating autophagy in an m6A-dependent manner. Int J
Biol Sci. 20:1729–1743. 2024. View Article : Google Scholar :
|
|
67
|
Chen Y, Zhao Y, Chen J, Peng C, Zhang Y,
Tong R, Cheng Q, Yang B, Feng X, Lu Y, et al: ALKBH5 suppresses
malignancy of hepatocellular carcinoma via m6A-guided
epigenetic inhibition of LYPD1. Mol Cancer. 19:1232020. View Article : Google Scholar
|
|
68
|
Jin D, Guo J, Wu Y, Yang L, Wang X, Du J,
Dai J, Chen W, Gong K, Miao S, et al: m6A demethylase
ALKBH5 inhibits tumor growth and metastasis by reducing
YTHDFs-mediated YAP expression and inhibiting
miR-107/LATS2-mediated YAP activity in NSCLC. Mol Cancer.
19:402020. View Article : Google Scholar
|
|
69
|
Shen D, Lin J, Xie Y, Zhuang Z, Xu G, Peng
S, Tang G, Bai L, Zhu M, Zhang Y, et al: RNA demethylase ALKBH5
promotes colorectal cancer progression by posttranscriptional
activation of RAB5A in an m6A-YTHDF2-dependent manner. Clin Transl
Med. 13:e12792023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hu Y, Gong C, Li Z, Liu J, Chen Y, Huang
Y, Luo Q, Wang S, Hou Y, Yang S and Xiao Y: Demethylase ALKBH5
suppresses invasion of gastric cancer via PKMYT1 m6A modification.
Mol Cancer. 21:342022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Dong F, Qin X, Wang B, Li Q, Hu J, Cheng
X, Guo D, Cheng F, Fang C, Tan Y, et al: ALKBH5 facilitates
hypoxia-induced paraspeckle assembly and IL8 secretion to generate
an immunosuppressive tumor microenvironment. Cancer Res.
81:5876–5888. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Liu X, Feng M, Hao X, Gao Z, Wu Z, Wang Y,
Du L and Wang C: m6A methylation regulates hypoxia-induced
pancreatic cancer glycolytic metabolism through ALKBH5-HDAC4-HIF1α
positive feedback loop. Oncogene. 42:2047–2060. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang W, Huang Q, Liao Z, Zhang H, Liu Y,
Liu F, Chen X, Zhang B, Chen Y and Zhu P: ALKBH5 prevents
hepatocellular carcinoma progression by post-transcriptional
inhibition of PAQR4 in an m6A dependent manner. Exp Hematol Oncol.
12:12023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Fang Y, Wu X, Gu Y, Shi R, Yu T, Pan Y,
Zhang J, Jing X, Ma P and Shu Y: LINC00659 cooperated with ALKBH5
to accelerate gastric cancer progression by stabilising JAK1 mRNA
in an m6 A-YTHDF2-dependent manner. Clin Transl Med.
13:e12052023. View Article : Google Scholar
|
|
75
|
Tang B, Yang Y, Kang M, Wang Y, Wang Y, Bi
Y, He S and Shimamoto F: m6A demethylase ALKBH5 inhibits
pancreatic cancer tumorigenesis by decreasing WIF-1 RNA methylation
and mediating Wnt signaling. Mol Cancer. 19:32020. View Article : Google Scholar
|
|
76
|
Jiang Y, Wan Y, Gong M, Zhou S, Qiu J and
Cheng W: RNA demethylase ALKBH5 promotes ovarian carcinogenesis in
a simulated tumour microenvironment through stimulating NF-κB
pathway. J Cell Mol Med. 24:6137–6148. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang Y, Liu X, Wang Y, Lai S, Wang Z,
Yang Y, Liu W, Wang H and Tang B: The m6A demethylase
ALKBH5-mediated upregulation of DDIT4-AS1 maintains pancreatic
cancer stemness and suppresses chemosensitivity by activating the
mTOR pathway. Mol Cancer. 21:1742022. View Article : Google Scholar
|
|
78
|
Chen C, Zhai E, Liu Y, Qian Y, Zhao R, Ma
Y, Liu J, Huang Z, Chen J and Cai S: ALKBH5-mediated CHAC1
depletion promotes malignant progression and decreases
cisplatin-induced oxidative stress in gastric cancer. Cancer Cell
Int. 23:2932023. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Guo X, Li K, Jiang W, Hu Y, Xiao W, Huang
Y, Feng Y, Pan Q and Wan R: RNA demethylase ALKBH5 prevents
pancreatic cancer progression by posttranscriptional activation of
PER1 in an m6A-YTHDF2-dependent manner. Mol Cancer. 19:912020.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chen F, Li M and Wang L: LncRNA CASC11
promotes hepatocellular carcinoma progression via upregulation of
UBE2T in a m6A-dependent manner. Front Oncol.
11:7726712021. View Article : Google Scholar
|
|
81
|
Lagergren J and Lagergren P: Oesophageal
cancer. BMJ. 341:c62802010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Nagtegaal ID, Odze RD, Klimstra D, Paradis
V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA;
WHO Classification of Tumours Editorial Board: The 2019 WHO
classification of tumours of the digestive system. Histopathology.
76:182–188. 2020. View Article : Google Scholar :
|
|
84
|
Zhang J, Guo S, Piao HY, Wang Y, Wu Y,
Meng XY, Yang D, Zheng ZC and Zhao Y: ALKBH5 promotes invasion and
metastasis of gastric cancer by decreasing methylation of the
lncRNA NEAT1. J Physiol Biochem. 75:379–389. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang S, Wang Y, Zhang Z, Zhu C, Wang C, Yu
F and Zhao E: Long non-coding RNA NRON promotes tumor proliferation
by regulating ALKBH5 and nanog in gastric cancer. J Cancer.
12:6861–6872. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wu S, Yun J, Tang W, Familiari G,
Relucenti M, Wu J, Li X, Chen H and Chen R: Therapeutic
m6A eraser ALKBH5 mRNA-loaded exosome-liposome hybrid
nanoparticles inhibit progression of colorectal cancer in
preclinical tumor models. ACS Nano. 17:11838–11854. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ye M, Hu C, Chen T, Yu P, Chen J, Lu F, Xu
L, Zhong Y, Yan L, Kan J, et al: FABP5 suppresses colorectal cancer
progression via mTOR-mediated autophagy by decreasing FASN
expression. Int J Biol Sci. 19:3115–3127. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
You Y, Wen D, Zeng L, Lu J, Xiao X, Chen
Y, Song H and Liu Z: ALKBH5/MAP3K8 axis regulates PD-L1+ macrophage
infiltration and promotes hepatocellular carcinoma progression. Int
J Biol Sci. 18:5001–5018. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lin C, Wang Y, Dong Y, Lai S, Wang L, Weng
S and Zhang X: N6-methyladenosine-mediated SH3BP5-AS1 upregulation
promotes GEM chemoresistance in pancreatic cancer by activating the
Wnt signaling pathway. Biol Direct. 17:332022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
He Y, Yue H, Cheng Y, Ding Z, Xu Z, Lv C,
Wang Z, Wang J, Yin C, Hao H and Chen C: ALKBH5-mediated
m6A demethylation of KCNK15-AS1 inhibits pancreatic
cancer progression via regulating KCNK15 and PTEN/AKT signaling.
Cell Death Dis. 12:11212021. View Article : Google Scholar
|
|
91
|
Xiao D, Fang TX, Lei Y, Xiao SJ, Xia JW,
Lin TY, Li YL, Zhai JX, Li XY, Huang SH, et al: m6A
demethylase ALKBH5 suppression contributes to esophageal squamous
cell carcinoma progression. Aging (Albany NY). 13:21497–21512.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chen P, Li S, Zhang K, Zhao R, Cui J, Zhou
W, Liu Y, Zhang L and Cheng Y: N6-methyladenosine
demethylase ALKBH5 suppresses malignancy of esophageal cancer by
regulating microRNA biogenesis and RAI1 expression. Oncogene.
40:5600–5612. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Xue J, Xiao P, Yu X and Zhang X: A
positive feedback loop between AlkB homolog 5 and miR-193a-3p
promotes growth and metastasis in esophageal squamous cell
carcinoma. Hum Cell. 34:502–514. 2021. View Article : Google Scholar
|
|
94
|
Qiao Z, Li Y, Cheng Y, Li S and Liu S:
SHMT2 regulates esophageal cancer cell progression and immune
Escape by mediating m6A modification of c-myc. Cell Biosci.
13:2032023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Nagaki Y, Motoyama S, Yamaguchi T,
Hoshizaki M, Sato Y, Sato T, Koizumi Y, Wakita A, Kawakita Y, Imai
K, et al: m6 A demethylase ALKBH5 promotes proliferation of
esophageal squamous cell carcinoma associated with poor prognosis.
Genes Cells. 25:547–561. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wu Q, Zhang H, Yang D, Min Q, Wang Y,
Zhang W and Zhan Q: The m6A-induced lncRNA CASC8 promotes
proliferation and chemoresistance via upregulation of hnRNPL in
esophageal squamous cell carcinoma. Int J Biol Sci. 18:4824–4836.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Liu N, Dai Q, Zheng G, He C, Parisien M
and Pan T: N(6)-methyladenosine-dependent RNA structural switches
regulate RNA-protein interactions. Nature. 518:560–564. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
López MJ, Carbajal J, Alfaro AL, Saravia
LG, Zanabria D, Araujo JM, Quispe L, Zevallos A, Buleje JL, Cho CE,
et al: Characteristics of gastric cancer around the world. Crit Rev
Oncol Hematol. 181:1038412023. View Article : Google Scholar
|
|
101
|
Ji T, Gao X, Li D, Huai S, Chi Y, An X, Ji
W, Yang S and Li J: Identification and validation of signature for
prognosis and immune microenvironment in gastric cancer based on
m6A demethylase ALKBH5. Front Oncol. 12:10794022023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wang Q, Huang Y, Jiang M, Tang Y, Wang Q,
Bai L, Yu C, Yang X, Ding K, Wang W, et al: The demethylase ALKBH5
mediates ZKSCAN3 expression through the m6A modification
to activate VEGFA transcription and thus participates in
MNNG-induced gastric cancer progression. J Hazard Mater.
473:1346902024. View Article : Google Scholar
|
|
103
|
Xu X, Zhou E, Zheng J, Zhang C, Zou Y, Lin
J and Yu J: Prognostic and predictive value of m6a 'eraser' related
gene signature in gastric cancer. Front Oncol. 11:6318032021.
View Article : Google Scholar
|
|
104
|
Fang D, Ou X, Sun K, Zhou X, Li Y, Shi P,
Zhao Z, He Y, Peng J and Xu J: m6A modification-mediated lncRNA
TP53TG1 inhibits gastric cancer progression by regulating CIP2A
stability. Cancer Sci. 113:4135–4150. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wang S, Zhu X, Hao Y, Su TT and Shi W:
ALKBH5-mediated m6A modification of circFOXP1 promotes gastric
cancer progression by regulating SOX4 expression and sponging
miR-338-3p. Commun Biol. 7:5652024. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Suo D, Gao X, Chen Q, Zeng T, Zhan J, Li
G, Zheng Y, Zhu S, Yun J, Guan XY and Li Y: HSPA4 upregulation
induces immune evasion via ALKBH5/CD58 axis in gastric cancer. J
Exp Clin Cancer Res. 43:1062024. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wang S, Nie J, Xu K, Liu Y, Tong W, Li A,
Zuo W, Liu Z and Yang F: YY1 is regulated by ALKBH5-mediated m6A
modification and promotes autophagy and cancer progression through
targeting ATG4B. Aging (Albany NY). 15:9590–9613. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhang Z, Wang L, Zhao L, Wang Q, Yang C,
Zhang M, Wang B, Jiang K, Ye Y, Wang S and Shen Z:
N6-methyladenosine demethylase ALKBH5 suppresses colorectal cancer
progression potentially by decreasing PHF20 mRNA methylation. Clin
Transl Med. 12:e9402022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Luo J, Yu H, Yuan Z, Ye T and Hu B: ALKBH5
decreases SLC7A11 expression by erasing m6A modification and
promotes the ferroptosis of colorectal cancer cells. Clin Transl
Oncol. 25:2265–2276. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Ji J, Liu S, Liang Y and Zheng G:
Comprehensive analysis of m6A regulators and relationship with
tumor microenvironment, immunotherapy strategies in colorectal
adenocarcinoma. BMC Genom Data. 24:442023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Ji L, Chen S, Gu L and Zhang X:
Exploration of potential Roles of m6A regulators in colorectal
cancer prognosis. Front Oncol. 10:7682020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ge J, Liu SL, Zheng JX, Shi Y, Shao Y,
Duan YJ, Huang R, Yang LJ and Yang T: RNA demethylase ALKBH5
suppresses tumorigenesis via inhibiting proliferation and invasion
and promoting CD8+ T cell infiltration in colorectal cancer. Transl
Oncol. 34:1016832023. View Article : Google Scholar :
|
|
114
|
Wu X, Dai M, Li J, Cai J, Zuo Z, Ni S,
Zhang Q and Zhou Z: m(6) A demethylase ALKBH5 inhibits cell
proliferation and the metastasis of colorectal cancer by regulating
the FOXO3/miR-21/SPRY2 axis. Am J Transl Res. 13:11209–11222.
2021.
|
|
115
|
Yan G, An Y, Xu B, Wang N, Sun X and Sun
M: Potential impact of ALKBH5 and YTHDF1 on tumor immunity in colon
adenocarcinoma. Front Oncol. 11:6704902021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ye M, Chen J, Lu F, Zhao M, Wu S, Hu C, Yu
P, Kan J, Bai J, Tian Y and Tang Q: Down-regulated FTO and ALKBH5
co-operatively activates FOXO signaling through m6A methylation
modification in HK2 mRNA mediated by IGF2BP2 to enhance glycolysis
in colorectal cancer. Cell Biosci. 13:1482023. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Shao Y, Liu Z, Song X, Sun R, Zhou Y,
Zhang D, Sun H, Huang J, Wu C, Gu W, et al: ALKBH5/YTHDF2-mediated
m6A modification of circAFF2 enhances radiosensitivity of
colorectal cancer by inhibiting Cullin neddylation. Clin Transl
Med. 13:e13182023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Liu N, Jiang X, Zhang G, Long S, Li J,
Jiang M, Jia G, Sun R, Zhang L and Zhang Y: LncRNA CARMN m6A
demethylation by ALKBH5 inhibits mutant p53-driven tumour
progression through miR-5683/FGF2. Clin Transl Med. 14:e17772024.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Feng L, Li M, Ma J, Wang W, Wang S, Mao Z
and Zhang Y: ALKBH5 regulates arginase 1 expression in MDSCs and
their immunosuppressive activity in tumor-bearing host. Noncoding
RNA Res. 9:913–920. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Guo T, Liu DF, Peng SH and Xu AM: ALKBH5
promotes colon cancer progression by decreasing methylation of the
lncRNA NEAT1. Am J Transl Res. 12:4542–4549. 2020.PubMed/NCBI
|
|
121
|
Yue S, Li J, Lee SY, Lee HJ, Shao T, Song
B, Cheng L, Masterson TA, Liu X, Ratliff TL and Cheng JX:
Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT
activation underlies human prostate cancer aggressiveness. Cell
Metab. 19:393–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Lewis CA, Brault C, Peck B, Bensaad K,
Griffiths B, Mitter R, Chakravarty P, East P, Dankworth B, Alibhai
D, et al: SREBP maintains lipid biosynthesis and viability of
cancer cells under lipid- and oxygen-deprived conditions and
defines a gene signature associated with poor survival in
glioblastoma multiforme. Oncogene. 34:5128–5140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Nieman KM, Kenny HA, Penicka CV, Ladanyi
A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB,
Hotamisligil GS, et al: Adipocytes promote ovarian cancer
metastasis and provide energy for rapid tumor growth. Nat Med.
17:1498–1503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Jiang L, Xiao L, Sugiura H, Huang X, Ali
A, Kuro-o M, Deberardinis RJ and Boothman DA: Metabolic
reprogramming during TGFβ1-induced epithelial-to-mesenchymal
transition. Oncogene. 34:3908–3916. 2015. View Article : Google Scholar
|
|
125
|
Howe LR, Chang SH, Tolle KC, Dillon R,
Young LJ, Cardiff RD, Newman RA, Yang P, Thaler HT, Muller WJ, et
al: HER2/neu-induced mammary tumorigenesis and angiogenesis are
reduced in cyclooxygenase-2 knockout mice. Cancer Res.
65:10113–10119. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Ma C, Kesarwala AH, Eggert T,
Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, Terabe M, Kapoor
V, ElGindi M, et al: NAFLD causes selective CD4(+) T lymphocyte
loss and promotes hepatocarcinogenesis. Nature. 531:253–257. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Sun M, Yue Y, Wang X, Feng H, Qin Y, Chen
M, Wang Y and Yan S: ALKBH5-mediated upregulation of CPT1A promotes
macrophage fatty acid metabolism and M2 macrophage polarization,
facilitating malignant progression of colorectal cancer. Exp Cell
Res. 437:1139942024. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Li S, Du M, Xu K, Ben S, Zhu T, Guo M, Xin
J, Zhu L, Gu D, Zhang Z and Wang M: Genetic modulation of BET1L
confers colorectal cancer susceptibility by reducing miRNA binding
and m6A modification. Cancer Res. 83:2142–2154. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Chang Q, Zhou X, Mao H, Feng J, Wu X,
Zhang Z and Hu Z: ALKBH5 promotes hepatocellular carcinoma cell
proliferation, migration and invasion by regulating TTI1
expression. Biomol Biomed. 24:1216–1230. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zhang H, Liu Y, Wang W, Liu F, Wang W, Su
C, Zhu H, Liao Z, Zhang B and Chen X: ALKBH5-mediated
m6A modification of lincRNA LINC02551 enhances the
stability of DDX24 to promote hepatocellular carcinoma growth and
metastasis. Cell Death Dis. 13:9262022. View Article : Google Scholar
|
|
132
|
Sarin SK, Kumar M, Eslam M, George J, Al
Mahtab M, Akbar SMF, Jia J, Tian Q, Aggarwal R, Muljono DH, et al:
Liver diseases in the Asia-Pacific region: A lancet
gastroenterology & hepatology commission. Lancet Gastroenterol
Hepatol. 5:167–228. 2020. View Article : Google Scholar
|
|
133
|
Wang H, Wang Y, Lai S, Zhao L, Liu W, Liu
S, Chen H, Wang J, Du G and Tang B: LINC01468 drives NAFLD-HCC
progression through CUL4A-linked degradation of SHIP2. Cell Death
Discov. 8:4492022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Zhao L, Kang M, Liu X, Wang Z, Wang Y,
Chen H, Liu W, Liu S, Li B, Li C, et al: UBR7 inhibits HCC
tumorigenesis by targeting Keap1/Nrf2/Bach1/HK2 and glycolysis. J
Exp Clin Cancer Res. 41:3302022. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Liu Z, Wang Q, Wang X, Xu Z, Wei X and Li
J: Circular RNA cIARS regulates ferroptosis in HCC cells through
interacting with RNA binding protein ALKBH5. Cell Death Discov.
6:722020. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Adjibade P, Di-Marco S, Gallouzi IE and
Mazroui R: The RNA demethylases ALKBH5 and FTO regulate the
translation of ATF4 mRNA in sorafenib-treated hepatocarcinoma
cells. Biomolecules. 14:9322024. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Yeermaike A, Gu P, Liu D and Nadire T:
LncRNA NEAT1 sponges miR-214 to promoted tumor growth in
hepatocellular carcinoma. Mamm Genome. 33:525–533. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Liu H, Jiang Y, Lu J, Peng C, Ling Z, Chen
Y, Chen D, Tong R, Zheng S and Wu J: m6A-modification
regulated circ-CCT3 acts as the sponge of miR-378a-3p to promote
hepatocellular carcinoma progression. Epigenetics. 18:22047722023.
View Article : Google Scholar
|
|
139
|
Chen Y, Ling Z, Cai X, Xu Y, Lv Z, Man D,
Ge J, Yu C, Zhang D, Zhang Y, et al: Activation of YAP1 by
N6-methyladenosine-modified circcpsf6 drives malignancy in
hepatocellular carcinoma. Cancer Res. 82:599–614. 2022. View Article : Google Scholar
|
|
140
|
Levrero M and Zucman-Rossi J: Mechanisms
of HBV-induced hepatocellular carcinoma. J Hepatol. 64(Suppl 1):
S84–S101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Meng Y, Shu Z, Wang X, Hong L, Wang B,
Jiang J, He K, Cao Q, Shi F, Wang H, et al: Hepatitis B
virus-mediated m6A demethylation increases hepatocellular carcinoma
stemness and immune escape. Mol Cancer Res. 22:642–655. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Tian Y, Yang W, Song J, Wu Y and Ni B:
Hepatitis B virus X protein-induced aberrant epigenetic
modifications contributing to human hepatocellular carcinoma
pathogenesis. Mol Cell Biol. 33:2810–2816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Qu S, Jin L, Huang H, Lin J, Gao W and
Zeng Z: A positive-feedback loop between HBx and ALKBH5 promotes
hepatocellular carcinogenesis. BMC Cancer. 21:6862021. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Razumilava N and Gores GJ:
Cholangiocarcinoma. Lancet. 383:2168–2179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Gao Y, Yu M, Liu Z, Liu Y, Kong Z, Zhu C,
Qin X, Li Y and Tang L: m6A demethylase ALKBH5 maintains
stemness of intrahepatic cholangiocarcinoma by sustaining BUB1B
expression and cell proliferation. Transl Oncol. 41:1018582024.
View Article : Google Scholar
|
|
146
|
Qiu X, Yang S, Wang S, Wu J, Zheng B, Wang
K, Shen S, Jeong S, Li Z, Zhu Y, et al: M6A demethylase
ALKBH5 regulates PD-L1 expression and tumor immunoenvironment in
intrahepatic cholangiocarcinoma. Cancer Res. 81:4778–4793. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Roa JC, García P, Kapoor VK, Maithel SK,
Javle M and Koshiol J: Gallbladder cancer. Nat Rev Dis Primers.
8:692022. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Jiang D, Wu Y, Liu L, Shen Y, Li T, Lu Y,
Wang P, Sun C, Wang K, Wang K and Ye H: Burden of gastrointestinal
tumors in Asian countries, 1990-2021: An analysis for the global
burden of disease study 2021. Clin Epidemiol. 16:587–601. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Wu Z, Ke Q, Jiang L, Hong H, Pan W, Chen
W, Abudukeremu X, She F and Chen Y: TGF-β1 facilitates gallbladder
carcinoma metastasis by regulating FOXA1 translation efficiency
through m6A modification. Cell Death Dis. 15:4222024.
View Article : Google Scholar
|
|
150
|
Oettle H, Neuhaus P, Hochhaus A, Hartmann
JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J,
Arning MB, et al: Adjuvant chemotherapy with gemcitabine and
long-term outcomes among patients with resected pancreatic cancer:
The CONKO-001 randomized trial. JAMA. 310:1473–1481. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Zeng S, Pöttler M, Lan B, Grützmann R,
Pilarsky C and Yang H: Chemoresistance in pancreatic cancer. Int J
Mol Sci. 20:45042019. View Article : Google Scholar :
|
|
152
|
Zhang T, Sheng P and Jiang Y: m6A
regulators are differently expressed and correlated with immune
response of pancreatic adenocarcinoma. J Cancer Res Clin Oncol.
149:2805–2822. 2023. View Article : Google Scholar
|
|
153
|
Cho SH, Ha M, Cho YH, Ryu JH, Yang K, Lee
KH, Han ME, Oh SO and Kim YH: ALKBH5 gene is a novel biomarker that
predicts the prognosis of pancreatic cancer: A retrospective
multicohort study. Ann Hepatobiliary Pancreat Surg. 22:305–309.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
He Y, Hu H, Wang Y, Yuan H, Lu Z, Wu P,
Liu D, Tian L, Yin J, Jiang K and Miao Y: ALKBH5 inhibits
pancreatic cancer motility by decreasing long non-coding RNA
KCNK15-AS1 methylation. Cell Physiol Biochem. 48:838–846. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Lai S, Wang Y, Li T, Dong Y, Lin Y, Wang
L, Weng S, Zhang X and Lin C: N6-methyladenosine-mediated CELF2
regulates CD44 alternative splicing affecting tumorigenesis via
ERAD pathway in pancreatic cancer. Cell Biosci. 12:1252022.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Huang R, Yang L, Zhang Z, Liu X, Fei Y,
Tong WM, Niu Y and Liang Z: RNA m6A demethylase ALKBH5
protects against pancreatic ductal adenocarcinoma via targeting
regulators of iron metabolism. Front Cell Dev Biol. 9:7242822021.
View Article : Google Scholar
|
|
157
|
Tan Z, Xu J, Zhang B, Shi S, Yu X and
Liang C: Hypoxia: A barricade to conquer the pancreatic cancer.
Cell Mol Life Sci. 77:3077–3083. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Williams JK, Schwarz JL and Keutgen XM:
Surgery for metastatic pancreatic neuroendocrine tumors: A
narrative review. Hepatobiliary Surg Nutr. 12:69–83. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Chen J, Ye M, Bai J, Gong Z, Yan L, Gu D,
Hu C, Lu F, Yu P, Xu L, et al: ALKBH5 enhances lipid metabolism
reprogramming by increasing stability of FABP5 to promote
pancreatic neuroendocrine neoplasms progression in an
m6A-IGF2BP2-dependent manner. J Transl Med. 21:7412023. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Guo H, Wang B, Xu K, Nie L, Fu Y, Wang Z,
Wang Q, Wang S and Zou X: m6A reader HNRNPA2B1 promotes
esophageal cancer progression via up-regulation of ACLY and ACC1.
Front Oncol. 10:5530452020. View Article : Google Scholar
|
|
161
|
Xu LC, Pan JX and Pan H: Construction and
validation of an m6A RNA methylation regulators-based prognostic
signature for esophageal cancer. Cancer Manag Res. 12:5385–5394.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Xie J, Huang Z, Jiang P, Wu R, Jiang H,
Luo C, Hong H and Yin H: Elevated N6-methyladenosine RNA levels in
peripheral blood immune cells: A novel predictive biomarker and
therapeutic target for colorectal cancer. Front Immunol.
12:7607472021. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Liu X, Liu L, Dong Z, Li J, Yu Y, Chen X,
Ren F, Cui G and Sun R: Expression patterns and prognostic value of
m6A-related genes in colorectal cancer. Am J Transl Res.
11:3972–3991. 2019.
|
|
164
|
Nakagawa N, Tanaka K, Sonohara F,
Kandimalla R, Sunagawa Y, Inokawa Y, Takami H, Hayashi M, Yamada S,
Kanda M, et al: Novel prognostic implications of methylated RNA and
demethylases in resected HCC and background liver tissue.
Anticancer Res. 40:6665–6676. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Yao Y, Luo L, Xiang G, Xiong J, Ke N, Tan
C, Chen Y and Liu X: The expression of m6A regulators
correlated with the immune microenvironment plays an important role
in the prognosis of pancreatic ductal adenocarcinoma. Gland Surg.
11:147–165. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Lin B, Pan Y, Yu D, Dai S, Sun H, Chen S,
Zhang J, Xiang Y and Huang C: Screening and identifying m6A
regulators as an independent prognostic biomarker in pancreatic
cancer based on the cancer genome atlas database. Biomed Res Int.
2021:55736282021. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Xu F, Zhang Z, Yuan M, Zhao Y, Zhou Y, Pei
H and Bai L: M6A regulatory genes play an important role in the
prognosis, progression and immune microenvironment of pancreatic
adenocarcinoma. Cancer Invest. 39:39–54. 2021. View Article : Google Scholar
|
|
168
|
Takahashi H, Hase H, Yoshida T, Tashiro J,
Hirade Y, Kitae K and Tsujikawa K: Discovery of two novel ALKBH5
selective inhibitors that exhibit uncompetitive or competitive type
and suppress the growth activity of glioblastoma multiforme. Chem
Biol Drug Des. 100:1–12. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Selberg S, Seli N, Kankuri E and Karelson
M: Rational design of novel anticancer small-molecule RNA m6A
demethylase ALKBH5 inhibitors. ACS Omega. 6:13310–13320. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Li N, Kang Y, Wang L, Huff S, Tang R, Hui
H, Agrawal K, Gonzalez GM, Wang Y, Patel SP and Rana TM: ALKBH5
regulates anti-PD-1 therapy response by modulating lactate and
suppressive immune cell accumulation in tumor microenvironment.
Proc Natl Acad Sci USA. 117:20159–20170. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Malacrida A, Rivara M, Di Domizio A,
Cislaghi G, Miloso M, Zuliani V and Nicolini G: 3D proteome-wide
scale screening and activity evaluation of a new ALKBH5 inhibitor
in U87 glioblastoma cell line. Bioorg Med Chem. 28:1153002020.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Li F, Kennedy S, Hajian T, Gibson E,
Seitova A, Xu C, Arrowsmith CH and Vedadi M: A radioactivity-based
assay for screening human m6A-RNA methyltransferase, METTL3-METTL14
complex, and demethylase ALKBH5. J Biomol Screen. 21:290–297. 2016.
View Article : Google Scholar
|
|
173
|
Tang W, Xu N, Zhou J, He Z, Lenahan C,
Wang C, Ji H, Liu B, Zou Y, Zeng H and Guo H: ALKBH5 promotes
PD-L1-mediated immune escape through m6A modification of ZDHHC3 in
glioma. Cell Death Discov. 8:4972022. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Fang Z, Mu B, Liu Y, Guo N, Xiong L, Guo
Y, Xia A, Zhang R, Zhang H, Yao R, et al: Discovery of a potent,
selective and cell active inhibitor of m6A demethylase
ALKBH5. Eur J Med Chem. 238:1144462022. View Article : Google Scholar
|
|
175
|
You Y, Fu Y, Huang M, Shen D, Zhao B, Liu
H, Zheng Y and Huang L: Recent advances of m6A demethylases
inhibitors and their biological functions in human diseases. Int J
Mol Sci. 23:58152022. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Wang YZ, Li HY, Zhang Y, Jiang RX, Xu J,
Gu J, Jiang Z, Jiang ZY, You QD and Guo XK: Discovery of
Pyrazolo[1,5-a] pyrimidine Derivative as a novel and Selective
ALKBH5 inhibitor for the treatment of AML. J Med Chem.
66:15944–15959. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Komal S, Gohar A, Althobaiti S, Ahmad Khan
I, Cui LG, Zhang LR, Han SN and Shakeel M: ALKBH5 inhibitors as a
potential treatment strategy in heart failure-inferences from gene
expression profiling. Front Cardiovasc Med. 10:11943112023.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Chang G, Xie GS, Ma L, Li P, Li L and
Richard HT: USP36 promotes tumorigenesis and drug sensitivity of
glioblastoma by deubiquitinating and stabilizing ALKBH5. Neuro
Oncol. 25:841–853. 2023. View Article : Google Scholar :
|
|
179
|
Cheng C, Wang P, Yang Y, Du X, Xia H, Liu
J, Lu L, Wu H and Liu Q: Smoking-induced M2-TAMs, via circEML4 in
EVs, promote the progression of NSCLC through ALKBH5-regulated m6A
modification of SOCS2 in NSCLC cells. Adv Sci (Weinh).
10:e23009532023. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Shen C, Sheng Y, Zhu AC, Robinson S, Jiang
X, Dong L, Chen H, Su R, Yin Z, Li W, et al: RNA demethylase ALKBH5
selectively promotes tumorigenesis and cancer stem cell
self-renewal in acute myeloid leukemia. Cell Stem Cell.
27:64–80.e9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Zhang J, Zhang Y, Qu B, Yang H, Hu S and
Dong X: If small molecules immunotherapy comes can the prime be far
behind? Eur J Med Chem. 218:1133562021. View Article : Google Scholar
|
|
182
|
Schrödinger, LLC: The PyMOL molecular
graphics system version 2.6.0a0. 2010.
|