Introduction
Aging is closely associated with the occurrence and
progression of senile diseases (1). Previous studies have shown that
stem cell senescence is one of the key factors for the aging and
functional decline of tissues and organs, which is also a key
target for the prevention and treatment of senile diseases
(2,3). With the acceleration of global
population aging and the increase in life expectancy, research on
the biology of aging, prevention and treatment of aging-related
diseases is correspondingly increasing in importance (4,5).
The concept of stem cell aging is among the most recent and
significant concepts in the study of human aging. Stem cells should
not be considered 'immortal', but they rather undergo gradual aging
as the body ages (6). This aging
process in stem cells results in a progressive decline in their
self-renewal and multipotent differentiation capacities, which may
lead to uncontrolled proliferation and differentiation. Such
changes are key initiating factors for the deterioration of
structural integrity, functional decline and the emergence of
irreparable damage, subsequently contributing to the onset and
progression of related age-related diseases (7-9).
Additionally, the decline in bone marrow function is another
contributing factor to the development of age-related hematopoietic
disorders (10,11).
Mesenchymal stem cells (MSCs) are attractive targets
in the emerging field of stem cell aging research (12,13). Due to their low immunogenicity
and unique biological properties, bone marrow MSCs have been widely
utilized in clinical applications, including hematopoietic stem
cell transplantation, tissue repair, treatment of autoimmune
diseases and as vectors for gene therapy (14,15). The relationship between the bone
marrow hematopoietic microenvironment and hematopoietic stem cells
(HSCs) has been frequently likened to a 'soil and seed' analogy
(16,17). Bone marrow stromal cells and
their precursors, bone marrow MSCs, are essential components of the
hematopoietic microenvironment, interacting with hematopoietic
progenitor cells that rely on this environment for proliferation,
where a dynamic balance is maintained for hematopoiesis (18-20). MSCs serve an important regulatory
role in the self-renewal and multidirectional differentiation of
HSCs (21,22). Clinical studies have previously
shown that reduced or abnormal hematopoietic function is relatively
common in the elderly population, presenting as age-related anemia,
leukemia and immune deficiencies (23-25). However, the mechanism underlying
age-related hematopoietic dysfunction remain unclear, warranting
further investigation into whether the aging of bone marrow MSCs is
associated with this phenomenon.
Age-gradient-associated differences in MSCs are
hypothesized to be key to analyzing hematopoietic decline in the
elderly. Previous studies on the dynamics of bone marrow aging have
identified significant differences between the hematopoietic
microenvironments of the elderly and the younger population
(26,27). Previous studies have demonstrated
that aging of bone marrow hematopoiesis is closely associated with
a decrease in IGF1 in the bone marrow microenvironment, and that
Kitl and Igf1 expression are coregulated and variable between
individual mice at the middle age and expression of these factors
is predictive of HSC activation and lymphoid commitment (11,20). To further elucidate these
differences and the microenvironmental theory underlying the
decline in hematopoietic function in older individuals, the
potential impact of the bone marrow microenvironment on
hematopoietic function was investigated in the present study by
examining the in vitro changes in bone marrow MSCs.
Additionally, the present study seeks to establish a theoretical
foundation for understanding the aging hematopoietic
microenvironment, emphasizing that the fate of bone marrow
hematopoietic cells is influenced not by intrinsic properties but
by their external surroundings. This approach also offers novel
therapeutic insights for the prevention and treatment of
hematopoietic dysfunction in the elderly.
Materials and methods
Sample collection and categorization
Bone marrow samples were originated from the First
Hospital of Chongqing Medical University (Chongqing, China;
collection lasted from 1/1/2024 to 1/6/2024). Volunteers (of either
sex) without hematologic malignant diseases who had only symptoms
of iron-deficiency anemia (IDA) were eligible for collection. The
bone marrow samples were then categorized by age, designating
volunteers aged ≤25 years as the young group (Y group), those aged
45-55 years as the middle-aged group (M group) and those aged ≥65
years as the elderly group (O group). Sex and age distribution of
patients is included in Table I.
The present study was approved (approval no. 2023092) by The Ethics
Committee of Chongqing Medical University (Chongqing, China).
Written informed consent was obtained from all participants prior
to publication of the present study.
 | Table IDemographic characteristics of the
volunteers. |
Table I
Demographic characteristics of the
volunteers.
| Variable | Total | Y | M | O | χ2 | P-value |
|---|
| n=184 | n=49 | n=51 | n=84 |
|---|
| Female, n (%) | 96 (52.17) | 27 (55.10) | 29 (56.86) | 40 (47.62) | 1.316 | 0.518 |
| Male, n (%) | 88 (47.83) | 22 (44.90) | 22 (43.14) | 44 (52.38) | | |
Bone marrow puncture
The posterior superior iliac spine was selected to
be the site for bone marrow puncture. Specifically, 0.2 ml bone
marrow fluid was extracted for the preparation of bone marrow
smears, followed by Wright's staining (cat. no. S0217; BIOSS) to
observe the morphological characteristics of bone marrow cells. A
total of 3 ml bone marrow was collected from each volunteer for
bone marrow culture.
Bone marrow cytological examination
Qualified bone marrow smears were prepared using
Wright's staining technique. In total, 0.5-0.8 ml Wright's Giemsa A
solution was added to the smear, ensuring that the staining
solution covers the entire specimen for 1 min. Subsequently,
Wright's Giemsa B solution was added to the A solution, with a
volume that is 2-3X that of the A solution to facilitate thorough
mixing, followed by a staining period of 3-10 min. After washing
with water and allowing to dry, the proportions and counts of
various hematopoietic cells in the bone marrow were observed under
a light microscope (Olympus CKX41; Olympus Corporation) to assess
whether the bone marrow sample exhibits signs of hematological
disorders or malignant lesions.
ELISA
The supernatant from the bone marrow samples and the
co-culture system were isolated before the capture antibody
solution was added, which took place in a 96-well plate (cat. no.
FCP962; Beyotime Institute of Biotechnology) and was incubated
overnight at 4°C. Subsequently, the plate was washed five times
with a washing solution to ensure drying. Plasma was then diluted
with the sample diluent to concentrations of 1:5, 1:10, 1:50, 1:100
and 1:2,000 to ascertain the optimal concentration. Finally, the
concentrations of stem cell factor (SCF; cat. no. QZ-10611),
granulocyte macrophage (GM) colony stimulating factor (CSF; cat.
no. QZ-10908), IL-3 (cat. no. QZ-10502) and erythropoietin (EPO;
cat. no. QZ-10444; all from Quanzhou Jiubang Biotechnology Co.,
Ltd.) were measured through antigen-antibody interactions, before
the absorbance (450 nm) of the cytokines was quantified using a
microplate reader (Rayto RT-6100; Rayto Life and Analytical
Sciences Co., Ltd.) in conjunction with a standard curve.
In vitro culture
Human bone marrow mononuclear cells (MNCs) were
isolated through red blood cell lysis (cat. no. BL503A; Biosharp
Life Sciences), lymphocyte separation (cat. no. LTS1077; Tianjin
Haoyang Biological Products Technology Co., Ltd.) and density
gradient centrifugation (room temperature; 5 min; 16,020 × g). The
isolated MNCs were then counted and evenly distributed into 60-mm
culture dishes for in vitro culture. During the initial
stages of cell culture, the medium DMEM F12 (Gibco; Thermo Fisher
Scientific, Inc.) supplemented 10% fetal bovine serum (FBS; cat.
no. FSP500; Shanghai ExCell Biology, Inc.) was partially replaced
every 3 days until adherent-dependent cells emerged. Subsequently,
a complete medium exchange and cell passaging procedures were
performed to separate and purify the adherent-dependent cells.
Furthermore, during the early phases of the in vitro culture
of bone marrow MNCs, the supernatant cells were collected for
additional in vitro culture. The supernatant cells were
treated with a magnetic bead sorting kits (cat. nos. 130-092-263
and 130-100-453; Miltenyi Biotec GmbH), and these cells were
successively treated with CD38- and CD34-labeled magnetic beads,
respectively; and CD34-positive and CD38-negative HSCs were
collected for co-culture operation under the action of a magnetic
field by the negative and positive sorting methods,
respectively.
Flow cytometry
After counting (1×106),
adherent-dependent cells and suspended cells were allocated to
Eppendorf tubes separately and treated with 5% BSA (cat. no.
SW3015; Beijing Solarbio Science & Technology Co., Ltd.)
blocking buffer under light conditions for 30 min. The adherent
cells were stained with specific surface marker antibodies
(dilution ratio 1:100) at 4°C for 30 min in the dark, including
CD73 (cat. no. 561258), CD90 (cat. no. 555595), CD105 (cat. no.
561443), CD14 (cat. no. 557153), CD34 (cat. no. 555821), CD45 (cat.
no. 555482) and human leukocyte antigen (HLA-DR; cat. no. 555811),
whilst the suspended cells were stained with the appropriate
antibodies against CD34 (cat. no. 555821) and CD38 (cat. no.
567147) (all from BD Biosciences) before identification using flow
cytometry. Following washing with PBS (cat. no. G4250-500ML; Wuhan
Servicebio Technology Co., Ltd.), the isolated and purified
adherent cell pellet was collected, resuspended in 100 μl
PBS and mixed gently with 900 μl of pre-chilled 75% ethanol
to ensure complete homogenization for subsequent cell cycle
assessment. After counting, these cells were also used for
apoptosis measurement and subsequently resuspended in 500 μl
PBS. Finally, the results of the cell cycle and apoptosis analyses
were obtained through flow cytometry (BD FACSAria™ Fusion; BD
FACSDiva™ Software; BD Biosciences).
Co-culture
The present study used multiple co-culture systems.
The first system utilized bone marrow plasma from groups Y and O to
intervene in the proliferation of bone marrow MSCs across the
respective groups. The second system leveraged the suspended
proliferative characteristics of HSCs and the adherent
proliferative characteristics of bone marrow MSCs. In this system,
Y group HSCs were selected as the experimental subjects to
investigate their hematopoietic activity, with bone marrow MSCs
from each group serving as intervention factors to establish an
in vitro hematopoietic co-culture system. The third system
involved co-culturing the bone marrow MSCs from group Y with the
differentiated adherent bone marrow cells from group O.
Subsequently, the pre-treated hematopoietic cells were collected
for in vitro hematopoiesis assays and assessment of colony
forming unit (CFU)-erythroid (E), burst forming unit (BFU)-E,
CFU-GM and CFU-granulocytes/erythroids/macrophages/megakaryocytes
(GEMM) formation.
Humanized hematopoietic colony formation
assay
Y group hematopoietic stem progenitor cells were
collected from the hematopoietic co-culture system, added to
MethoCult® medium (cat. no. 04034; STEMCELL
Technologies, Inc.), shaken well and then plated into culture
dishes for incubation at 37°C with 5% CO2 for 14 days.
The types and counts of colonies were assessed using an inverted
microscope (Olympus CKX41; Olympus Corporation). (A single CFU-E is
formed by 8-200 erythrocytes; a single BFU-E is formed by >200
erythrocytes; a single CFU-GM is formed by at least 20 granulocytes
and macrophages; a single CFU-GEMM is formed by >500 cells
containing erythrocytes, granulocytes and macrophages).
EdU incorporation
Following intervention with bone marrow plasma,
equal amounts of Y group bone marrow MSCs were seeded into culture
dishes to restore normal proliferation. An equal volume of
pre-warmed EdU (cat. no. C0078S; Beyotime Institute of
Biotechnology) working solution (final concentration of EdU at 10
μM) and culture medium was then added to the dishes,
followed by an additional 5-h incubation at 37°C with 5%
CO2. After EdU labeling was completed, cells were
treated with fixation and permeabilization solutions for 15 min,
incubated with Click reaction solution in the dark at room
temperature for 30 min and then stained for nuclei (cat. no. C1005;
Beyotime Institute of Biotechnology). Finally, proliferation was
analyzed using fluorescence detection.
Immunofluorescence
After intervention with bone marrow plasma, equal
amounts of M group bone marrow MSCs were seeded into culture dishes
to restore normal proliferation. The cells were fixed with 4%
paraformaldehyde (room temperature; 15 min) and permeabilized with
Triton X-100 (room temperature; 15 min). Subsequently, they were
incubated with antibodies for senescence-associated proteins p53
(cat. no. BF8013; Affinity Biosciences) (dilution ratio 1:500) and
p21 (cat. no. AF6290; Affinity Biosciences) (dilution ratio 1:500)
at room temperature for 1 h, followed by blocking (cat. no. P0260;
Beyotime Institute of Biotechnology) (room temperature; 10 min) and
incubation with secondary antibodies (DyLight 488, Goat Anti-Mouse
IgG; cat. no. A23210; and Dylight 549, Goat Anti-Rabbit IgG; cat.
no. A23320; both from Abbkine Scientific Co., Ltd.) (dilution ratio
1:100) at room temperature for 1 h in the dark to form
antigen-antibody complexes. Fluorescence was then observed and
quantified using a confocal microscope (AX/AX R with NSPARC; Nikon
Corporation).
Osteogenesis and adipogenesis
Equal amounts of O group bone marrow MSCs, following
intervention with bone marrow plasma, were seeded into culture
dishes to restore normal proliferation. Upon reaching 70%
confluence, the culture medium was removed, before 2 ml
OriCell® Human BMMSC Osteogenic Differentiation Medium
(cat. no. HUXMA-90021; Cyagen Biosciences, Inc.) was added into
each dish, with the medium replaced every 3 days. After 2-4 weeks
of induction, alkaline phosphatase staining (cat. no. G1481;
Beijing Solarbio Science & Technology Co., Ltd.) was performed
to assess osteogenic differentiation. Similarly, when the bone
marrow MSCs attained 70% confluence, the upper culture medium was
removed and 2 ml OriCell® Human BMMSC Adipogenic
Differentiation Medium A (cat. no. HUXMA-90031; Cyagen Biosciences,
Inc.) was added to each dish. Following 3 days of induction, the
medium was aspirated and 2 ml OriCell® Human BMMSC
Adipogenic Differentiation Medium B (cat. no. HUXMA-90031; Cyagen
Biosciences, Inc.) was introduced. After 1 day, medium B was
discarded and medium A was reinstated. The two media were
thereafter alternated in this manner whilst regularly monitoring
cell status. Finally, Oil Red O staining (cat. no. G1262; Beijing
Solarbio Science & Technology Co., Ltd.) was used to evaluate
the adipogenic differentiation of the cells.
Cell counting kit-8 (CCK-8)
Bone marrow MSCs were seeded into 96-well plates,
with 100 μl (containing 2,000 cells) added to each well.
Following this, 10 μl CCK-8 (cat. no. C0038; Beyotime
Institute of Biotechology) solution was added to each well and the
cells were incubated in a culture incubator (cat. no. BB150-2TCS-L;
Thermo Fisher Scientific, Inc.) at 37°C with 5% CO2 for
6 h. Absorbance was measured at 450 nm and cell viability was
analyzed based on the results.
Transmission electron microscopy
Bone marrow MSCs were digested with trypsin (cat.
no. 25200072; Gibco; Thermo Fisher Scientific, Inc.), counted and
the cell pellet (≥106) was collected. Next, 3%
paraformaldehyde fixative was gently added along the wall of the
tube for at least 2 h at room temperature. Cell precipitates were
stained (room temperature; 3 h) with heavy metal salts and embedded
(room temperature; 1 h) in epoxy resin. After making ultrathin
sections, (70-100 nm) they were used for observation. The prepared
samples were subsequently sent to the Electron Microscopy
Laboratory of Chongqing Medical University for observation.
β-galactosidase staining
Bone marrow MSCs were cultured to 70% confluency and
then the upper layer of medium was removed. Following a wash with
PBS, β-galactosidase fixation solution was added and the cells were
fixed at room temperature for 15 min. After fixation, PBS was used
to wash away the fixative and the cells were incubated overnight at
37°C with β-galactosidase staining solution (cat. no. C0602;
Beyotime Institute of Biotechnology). For suspended hematopoietic
stem progenitor cells, these cells were collected by centrifugation
and stained using the same method. The stained cells were then
placed on a glass slide for observation under an optical
microscope.
Transcriptome sequencing (RNA-Seq)
The isolated and purified Y group and O group bone
marrow MSCs were sent to the Beijing Genomics Institute for RNA-Seq
(BGI Genomics Co., Ltd.). The sequences and expression information
of the transcripts from both groups were analyzed (https://biosys.bgi.com/#/report/login)
using Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene
Ontology (GO) enrichment methods. The sequencing results were
processed using the STRING database (https://cn.string-db.org/) and Cytoscape software
(Cytoscape 3.7.2; https://cytoscape.org/download.html) to construct a
protein-protein interaction (PPI) analysis of differentially
expressed genes.
Reverse transcription-quantitative PCR
(RT-qPCR)
Under enzyme-free conditions, RNA was extracted from
human bone marrow MSCs using an RNA extraction kit (cat. no.
R0018M; Beyotime Institute of Biotechnology). Primer sequences for
the genes to be validated are included in Table SI. Reverse transcription was
performed using RT kit (cat. no. RR037A; Takara Bio, Inc.)
according to the manufacturer's protocol, followed by qPCR using
SYBR Green qPCR Master Mix (cat. no. 639676; Takara Bio, Inc.) on
the circulator real-time detection system (Bio-Rad). Actin was used
as the reference gene. (Thermal cycle conditions: the first step of
pre-denaturation 95°C for 30 sec, the second step 95°C for 10 sec
and 60°C for 30 sec, cycle 40 times). The quantification method was
the ΔΔCq method (28).
Statistical analysis
All experiments were performed in triplicate at a
minimum. Statistical analysis was conducted using GraphPad Prism
9.5 (Dotmatics). Measurement data were expressed as the mean ± SD.
Comparisons between two groups of data that met the criteria of
normal distribution and homogeneity of variance were performed
using an unpaired t-test. For comparisons among multiple groups,
one-way analysis of variance was employed, followed by Tukey's post
hoc test. For comparisons of data between groups at different time
points, repeated measures ANOVA was utilized, with Bonferroni
correction for post hoc analysis. Pearson correlation analysis was
used to assess the relationships among various indicators.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Analysis of age-related differences in
bone marrow function
For the present study examining the causes of
diminished bone marrow function in the elderly, 184 anemia samples
were collected using random sampling methodology (Table SII). Bone marrow smears
indicated that the proportions of myeloid, erythroid and lymphoid
lineages, in addition to the counts of megakaryocytes, were normal
across all samples, suggesting the absence of hematological
malignancies (Fig. 1A-C).
Statistical analysis revealed that the O group comprised the
highest proportion of samples, suggesting a significant degradation
of hematopoietic function in the elderly. Furthermore, there were
no sex differences among the groups, indicating that the decline in
hematopoietic function did not vary between elderly male and female
population (Table I).
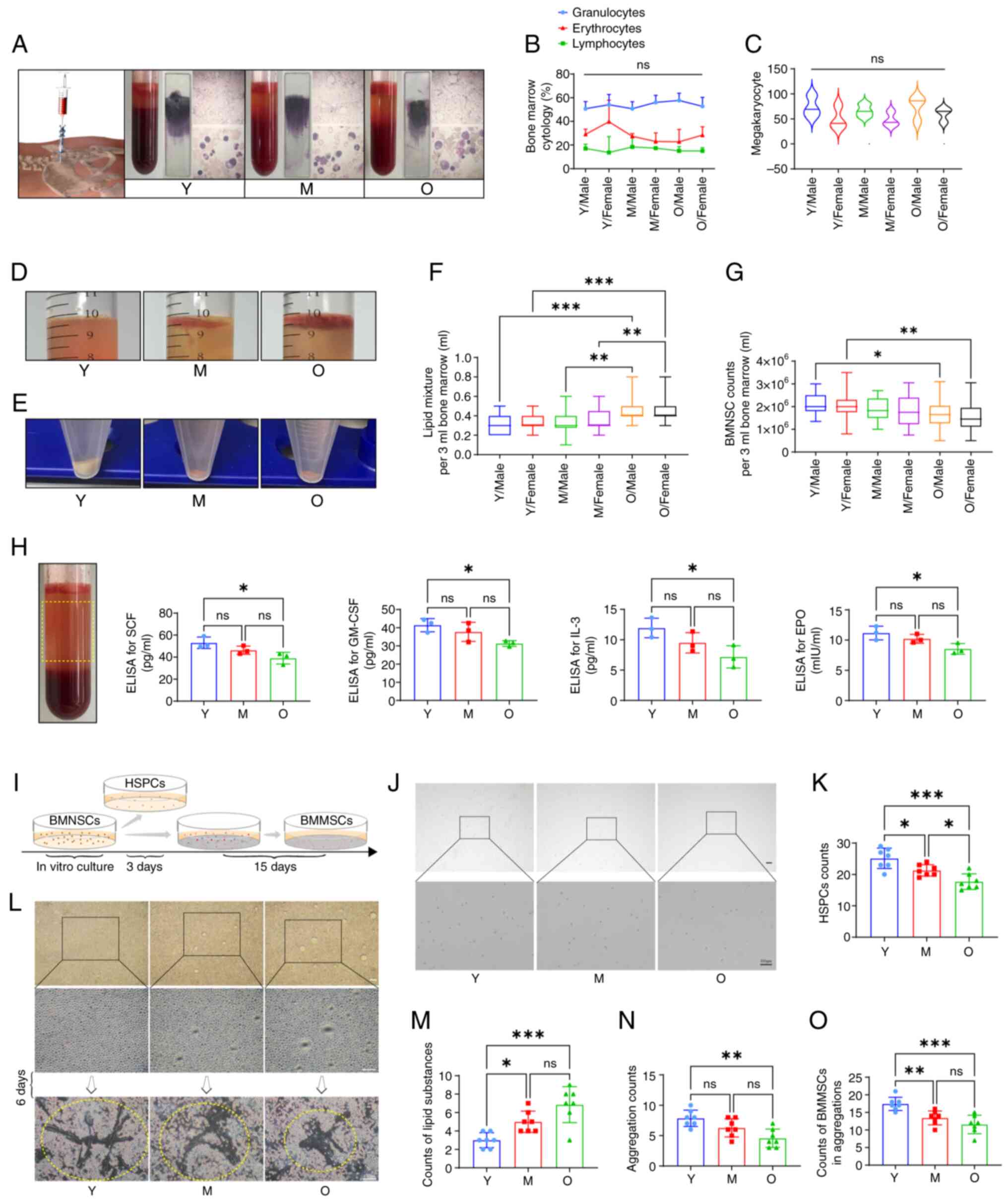 | Figure 1Analysis of age-related differences
in bone marrow associated with senile anemia. (A) Collection of
bone marrow samples from the posterior superior iliac spine. (B)
Clinical examination of bone marrow cytology for the samples
(n=184). (C) Assessment of megakaryocyte lineage in bone marrow
samples (n=184). (D) Variations in marrow adiposity. (E)
Differences in the extraction methods for MNCs from the bone
marrow. (F) Statistical analysis of lipid mixture content in each
bone marrow sample (n=184). (G) Statistical analysis of the number
of MNCs extracted from each bone marrow sample (n=184). (H)
Examination of hematopoietic-related factors SCF, GM-CSF, IL-3 and
EPO in the bone marrow (n=3). (I) Schematic representation of bone
marrow cell culture. (J) In vitro culture and observation of
hematopoietic stem progenitor cells following magnetic bead
sorting. (K) Statistical analysis of the number of hematopoietic
stem progenitor cells across various age groups (n=7). (L) In
vitro culture and early proliferation observation of MNCs from
bone marrow across different age groups, alongside bone marrow
MSCs. (M) Statistical analysis of the production of suspended
lipids during the in vitro culture of MNCs from bone marrow
(n=7). (N) Statistical analysis of aggregation regions during the
early adherent growth phase of bone marrow MSCs (n=7). (O)
Statistical analysis of variations in the number of bone marrow
MSCs in the early aggregation regions (n=7). *P<0.05,
**P<0.01 and ***P<0.001. MNCs,
mononuclear cells; SCF, stem cell factor; GM-CSF, granulocyte
colony stimulating factor; EPO, erythropoietin; MSCs, mesenchymal
stem cells; ns, no significance. |
The lipid composition in bone marrow samples
exhibited differential levels based on age, with the O group
showing the highest lipid content (Fig. 1D and F). MNCs were obtained from
the bone marrow samples using density gradient centrifugation,
where the data indicated a gradual decline in the number of MNCs as
age increased (Fig. 1E and G).
Assessments of hematopoietic-related factors in bone marrow plasma
showed reduced levels of SCF, GM-CSF, IL-3 and EPO in the O group
(Fig. 1H). In vitro
cultured, magnetically-sorted bone marrow hematopoietic stem
progenitor cells (Fig. 1I and J)
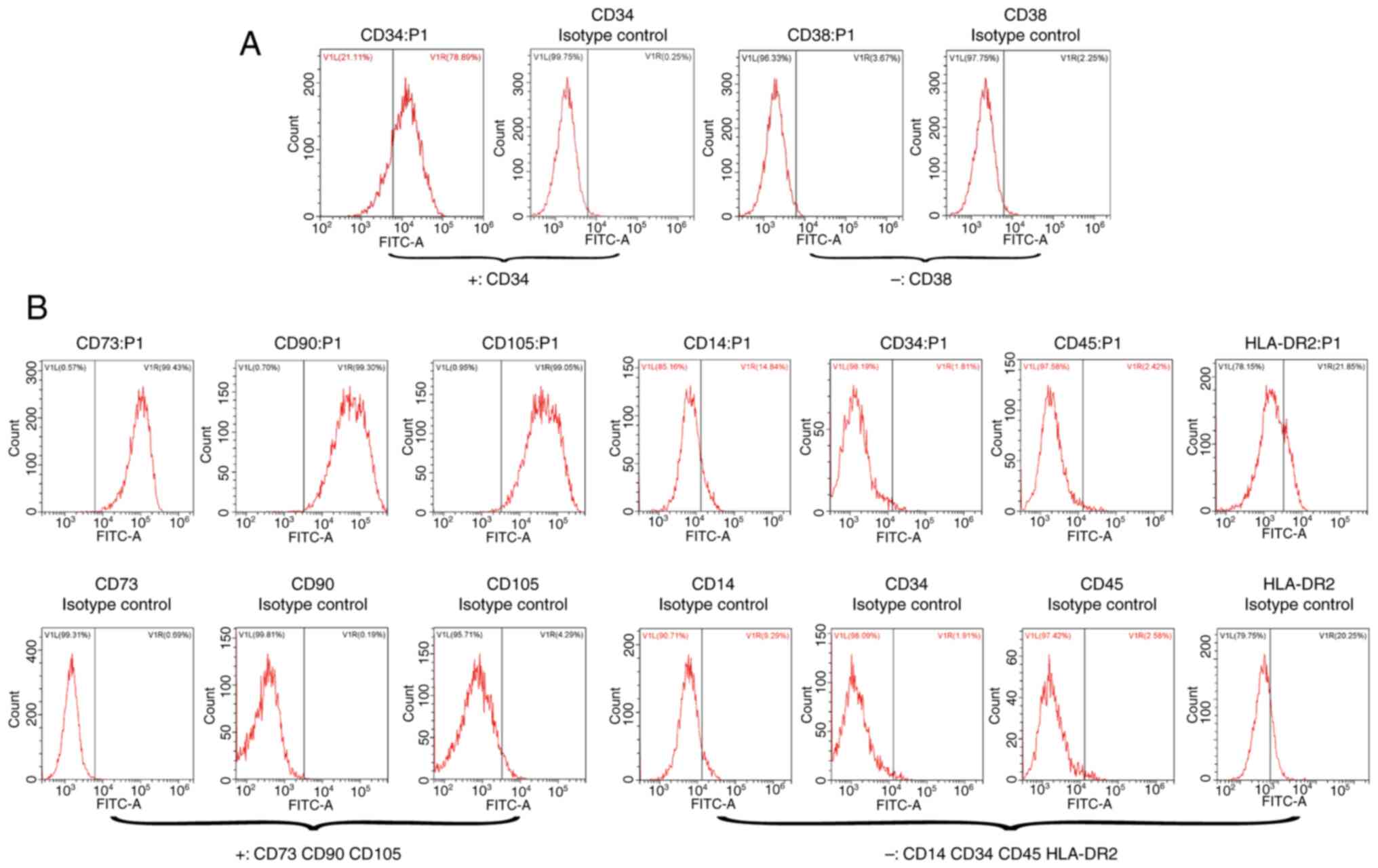
were subsequently analyzed for the expression of surface markers
using flow cytometry, revealing that CD34 was positive and CD38 was
negative, consistent with the definition of bone marrow HSCs
(Fig. 2A). Statistical analysis
indicated that the content of HSCs sourced from the O group was the
lowest, whilst that from the Y group was the highest (Fig. 1K). In the early stages of the
in vitro culture of bone marrow MNCs (Fig. 1L), the upper culture medium of
the O group exhibited the highest lipid production (Fig. 1M), although this phenomenon was
not observed in subsequent culture and passaging stages. On day 6
of in vitro culture, bone marrow MSCs displayed trends of
adherent aggregative proliferation under microscopy. Flow cytometry
for the expression of surface markers demonstrated that CD73, CD90
and CD105 were positive, whilst CD14, CD34, CD45 and HLA-DR2 were
negative, aligning with the definition of bone marrow MSCs
(Fig. 2B). Compared with the
other groups, the O group exhibited the smallest clustered area of
bone marrow MSCs and the fewest number of cells forming clusters
(Fig. 1N and O). These results
suggested that the decline in bone marrow function among the
elderly population is attributed to a reduction in the number of
stem cells and a deterioration of their functional capacity, which
is closely associated with the bone marrow microenvironment.
However, the causal relationship remains to be validated.
Changes in the bone marrow
microenvironment lead to a decrease in MSC numbers and
function
To further investigate the effects of alterations in
the bone marrow microenvironment on bone marrow MSCs, the in
vitro proliferation of MSCs derived from bone marrow plasma
samples of the Y and O groups across age gradients was next
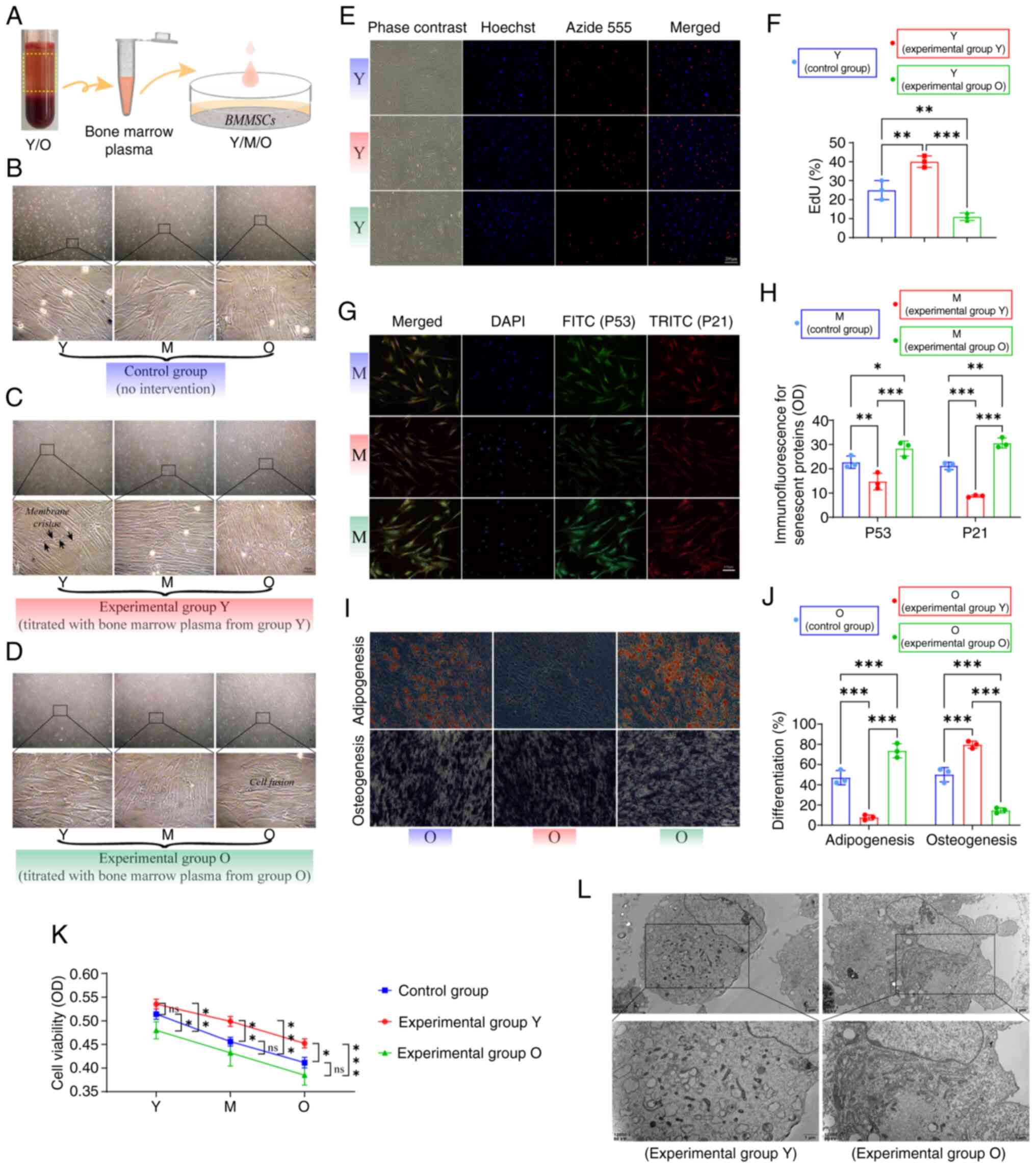
assessed (Fig. 3A). Control
group results (no intervention) exhibited distinctively dense
contact phenomena in MSCs during extensive proliferation (Fig. 3B). Microscopic observations
revealed that MSCs in the experimental Y group exhibited a regular
and organized growth pattern, forming membrane ridges at points of
contact (Fig. 3C). Under
identical culture conditions, MSCs in the experimental O group
displayed fused growth characteristics, with unclear cell
boundaries and varied morphologies (Fig. 3D). EdU assays demonstrated a
reduced proliferative capacity in Y group MSCs in the O group
compared with that in the control group (Fig. 3E and F). Immunofluorescence
results indicated that Y group bone marrow plasma could decrease
the expression of senescence-associated proteins p53 and p21 in M
group MSCs, whereas M group MSCs exhibited increased levels of p53
and p21 following intervention with O group bone marrow plasma
(Fig. 3G and H). Regarding the
multidirectional differentiation capacity of bone marrow MSCs, a
complementary trend toward osteogenic and adipogenic
differentiation was observed (29). Differentiation results suggested
that, compared with those in the control group, Y group bone marrow
plasma enhanced the osteogenic potential of O group MSCs whilst
diminishing their adipogenic capacity (Fig. 3I and J). During the in
vitro culture phase, Y group bone marrow plasma significantly
increased the viability of MSCs across all groups, whilst O group
plasma led to decreased cell viability in all groups (Fig. 3K). Electron microscopy results
revealed that MSCs from the experimental Y group contained abundant
intracellular materials and active organelles, most notably the
endoplasmic reticulum. By contrast, MSCs from the experimental O
group exhibited irregular membrane folds, enlarged cell bodies,
increased translucent intracellular materials and significant cell
differentiation (Fig. 3L). These
findings suggested that bone marrow MSCs are influenced by their
growth environment, where modulating such bone marrow
microenvironment will likely effectively alter the stemness of
these cells (30,31).
Changes in MSCs lead to a decrease in
hematopoietic capacity of HSCs
To investigate the direct relationship between
age-related changes in bone marrow stem cells and hematopoiesis in
the elderly population, bone marrow MSCs from various age groups
were co-cultured with HSCs from the Y group using in vitro
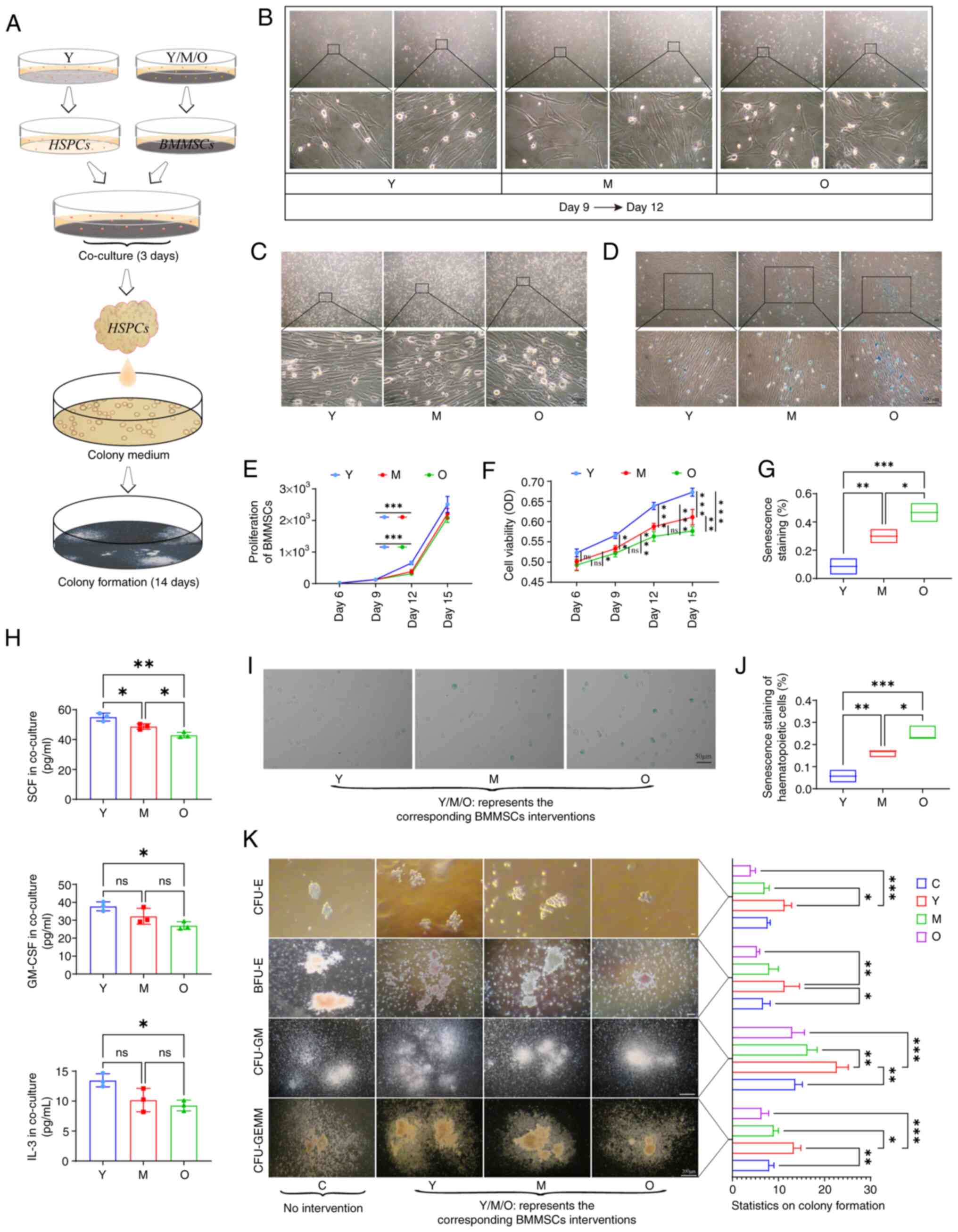
modeling (Fig. 4A). The MSCs
co-cultured for this experiment exhibited robust proliferative
capabilities in vitro (Fig.
4B). Statistical analysis indicated that during the early
stages of proliferation in vitro, Y group MSCs displayed the
highest proliferation capacity, whilst there was no statistically
significant difference in cell numbers among the groups as culture
time extended (Fig. 4E). Cell
viability assessments revealed that primary cultured MSCs
maintained robust cell viability at all stages (Fig. 4F). The proliferation of stem
cells is closely associated with cell contact, where prolonged
in vitro culture time may reduce the differences in stemness
among the stem cells (32-35). Microscopic observations indicated
that MSCs predominantly exhibited a spindle shape, where after
extensive proliferation, a fusion growth phenomenon was observed,
particularly pronounced in the O group (Fig. 4C). β-galactosidase staining for
cellular aging indicated a significant presence of
senescence-positive MSCs at areas of severe cell fusion growth
(Fig. 4D and G). The levels of
hematopoietic-related factors in different co-culture system
supernatants were next measured and it was found that O group MSCs,
as intervention factors, exhibited the lowest expression of SCF,
GM-CSF and IL-3 when co-cultured with Y group HSCs (Fig. 4H). Compared with that in other
systems, co-culturing with O group MSCs resulted in the significant
aging of Y group HSCs (Fig. 4I),
as evidenced by the increase in the proportion of
senescence-positive HSCs (Fig.
4J). In human bone marrow colony formation assays, CFU-E and
BFU-E colonies formed earliest, where statistical analysis of
colony numbers indicated age-related differences among the
experimental groups, with the O group exhibiting the poorest colony
formation capability. In terms of CFU-GM and CFU-GEMM formation,
the number of colonies and the density of cells within colonies
differed significantly. Y group HSCs co-cultured with Y group MSCs
formed a greater number of colonies with higher cell density,
whilst Y group HSCs co-cultured with O group MSCs produced the
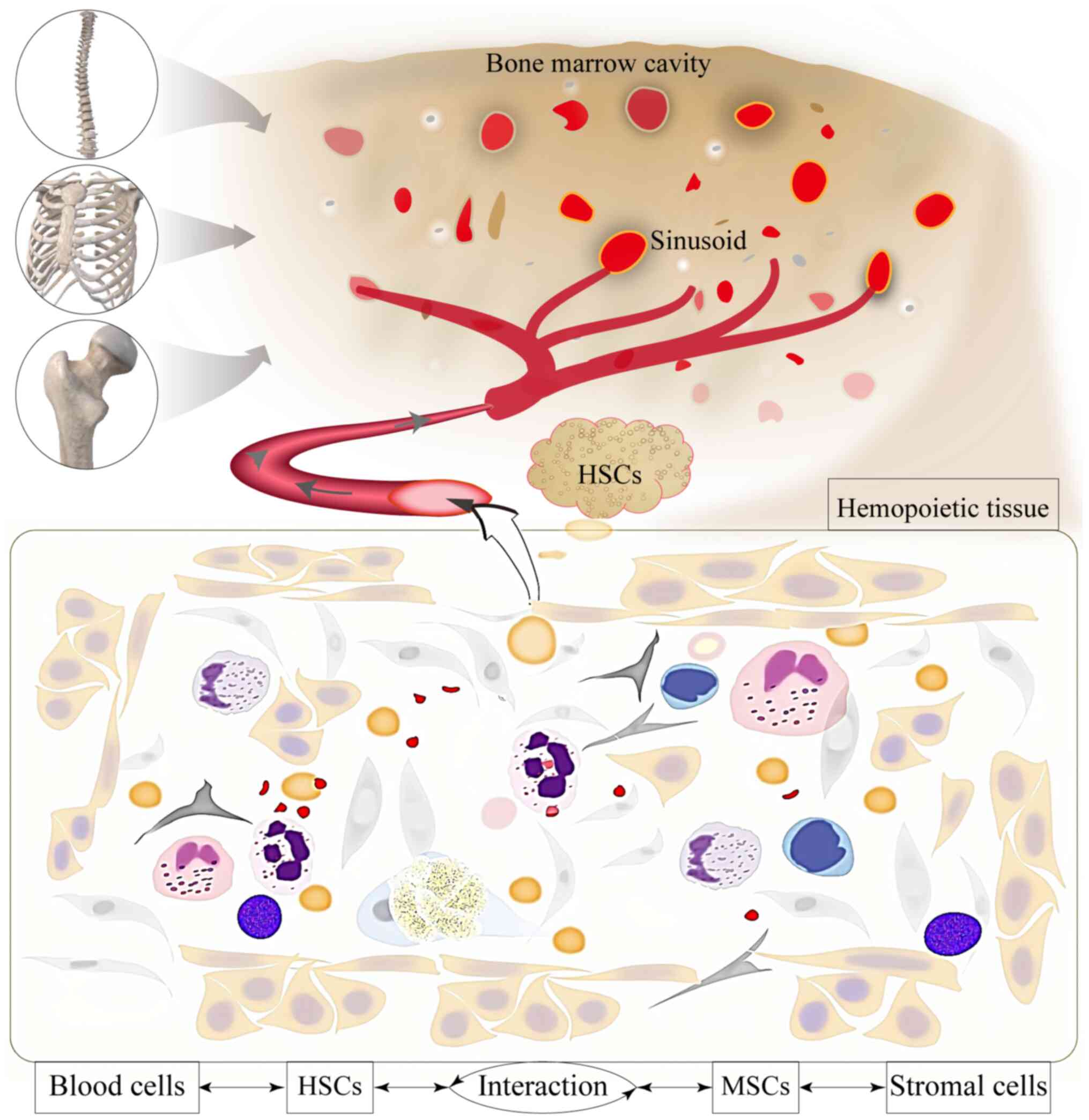
fewest colonies with the lowest cell density (Fig. 4K). These results suggested that
the state of MSCs coexisting with HSCs in the bone marrow will
likely affect the hematopoietic capacity of HSCs (Fig. 5).
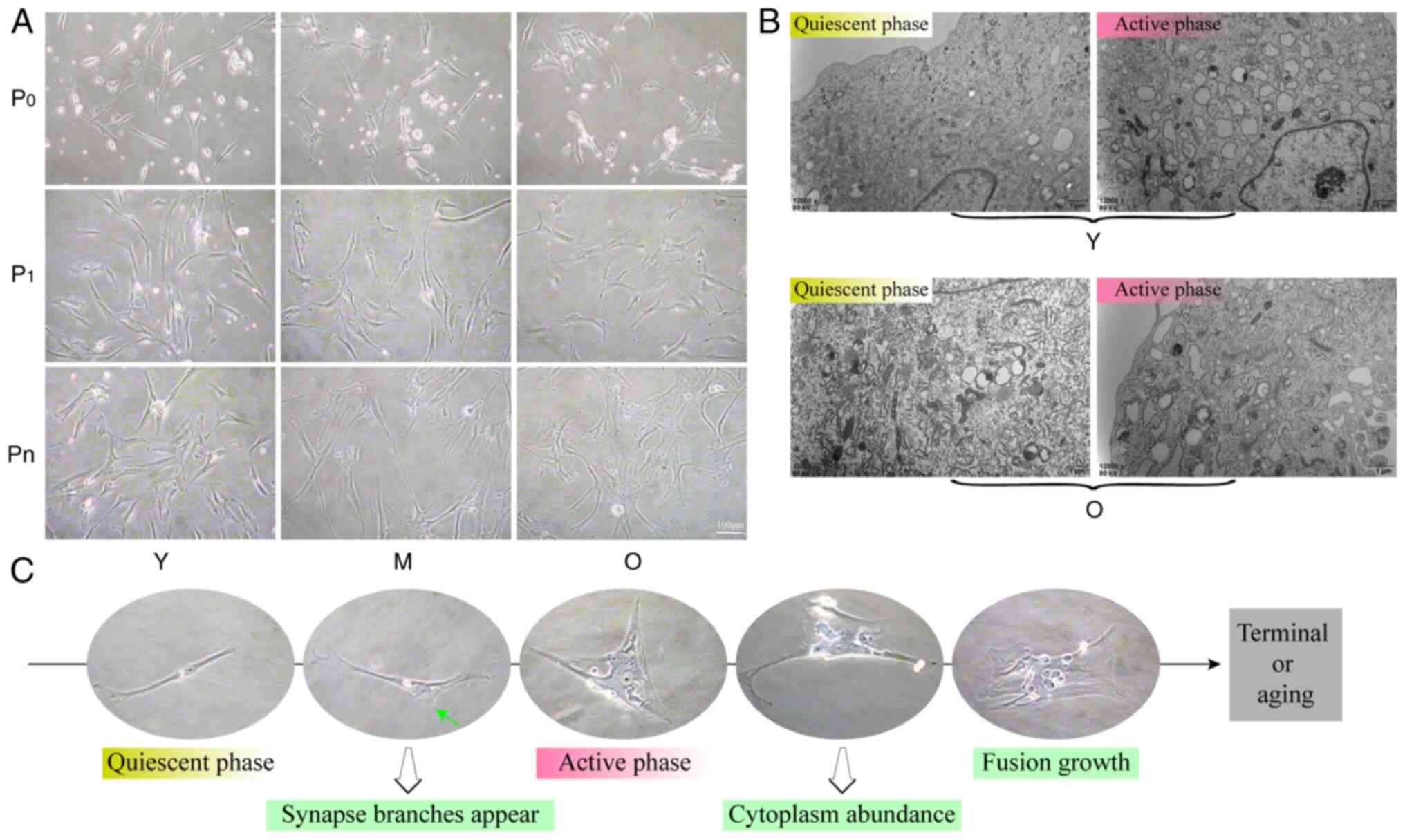
Loss of stemness in MSCs during in vitro
culture
Bone marrow MSCs serve an important role in bone
marrow function. To investigate the growth characteristics of MSCs,
their morphological features were analyzed at various stages of
in vitro culture. During the early primary culture of Y
group MSCs, cells exhibited a spindle shape, characterized by
relatively large nucleoplasm and a small number of protruding
branches at the ends. As age increased or as the in vitro
culture progressed, the intracellular content of MSCs became more
abundant, where the nucleoplasm decreased and the spindle
characteristics gradually diminished, resulting in a transformation
to polygonal or irregular shapes until differentiation into
terminal cells. This morphology was commonly observed in the
long-term in vitro culture of O group MSCs (Fig. 6A and C). Culturing results
indicated that the various morphologies of MSCs coexisted in the
different age groups, each with specific proportions and patterns.
Electron microscopy observations revealed that MSCs primarily
existed in the following two states: A quiescent phase and an
active phase. Compared with quiescent MSCs, those in the active
phase exhibited increased organelle function, particularly in the
endoplasmic reticulum, along with an increase in vesicular
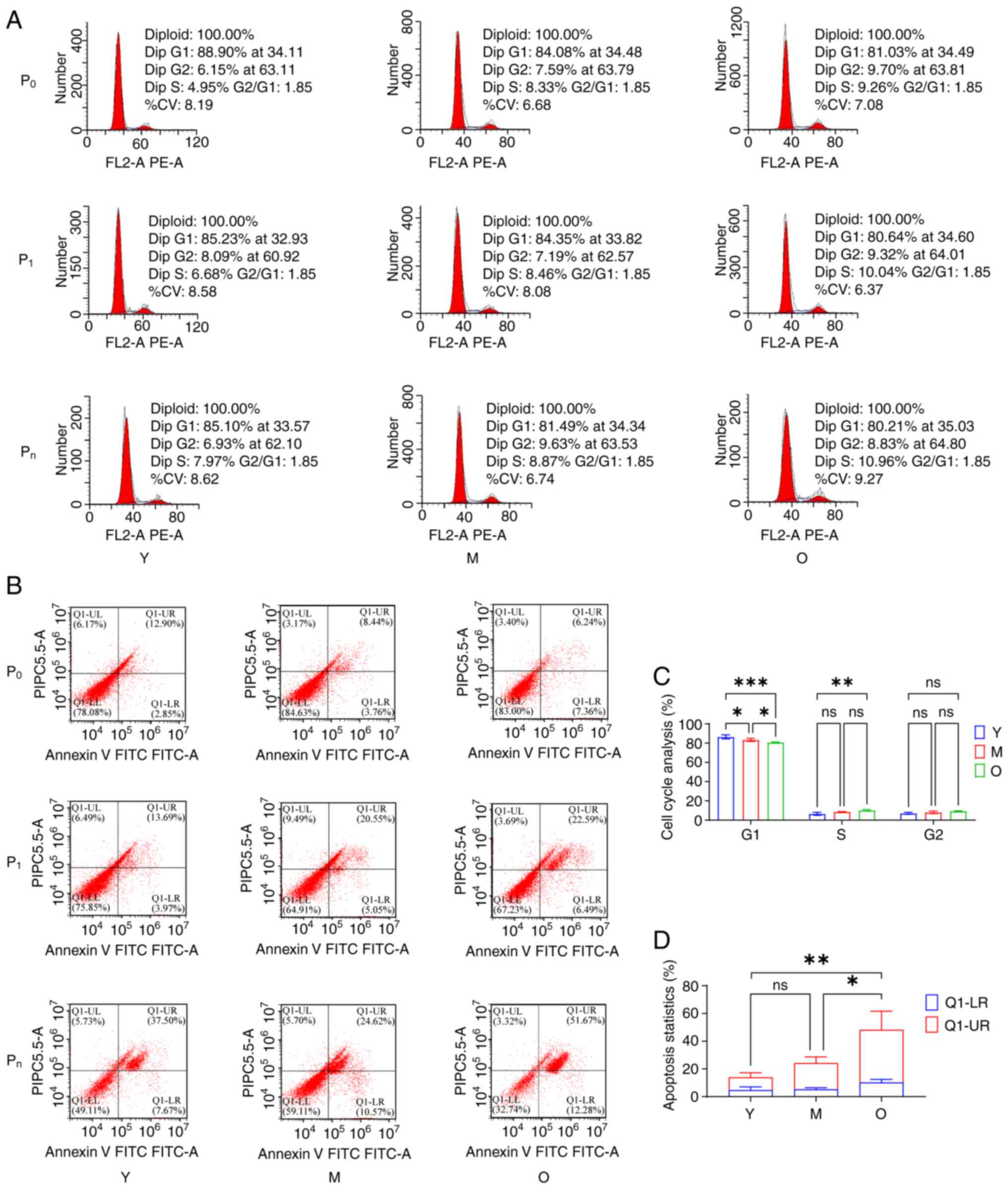
structures (Fig. 6B). Cell cycle
analysis showed that MSCs predominantly remained in the
G1 phase during in vitro culture, with
age-related differences based on the culture stage. In the primary
culture (P0 stage) of Y group MSCs, the proportion of cells in the
G1 phase was the highest, whilst in the passaged culture
(Pn, n≥3) of O group MSCs, the proportion of cells in the
G1 phase was the lowest (Fig. 7A and C). Apoptosis results
indicated that as age increased and culture time extended, the
proportion of apoptotic MSCs was also increased, with a
particularly rapid rise in late-stage apoptosis (Fig. 7B and D). These findings suggested
that the stemness of MSCs evolves in relation to differences in
origin and culture stage, highlighting the importance of the in
vitro culture phase as a critical factor for intervening in
their stemness.
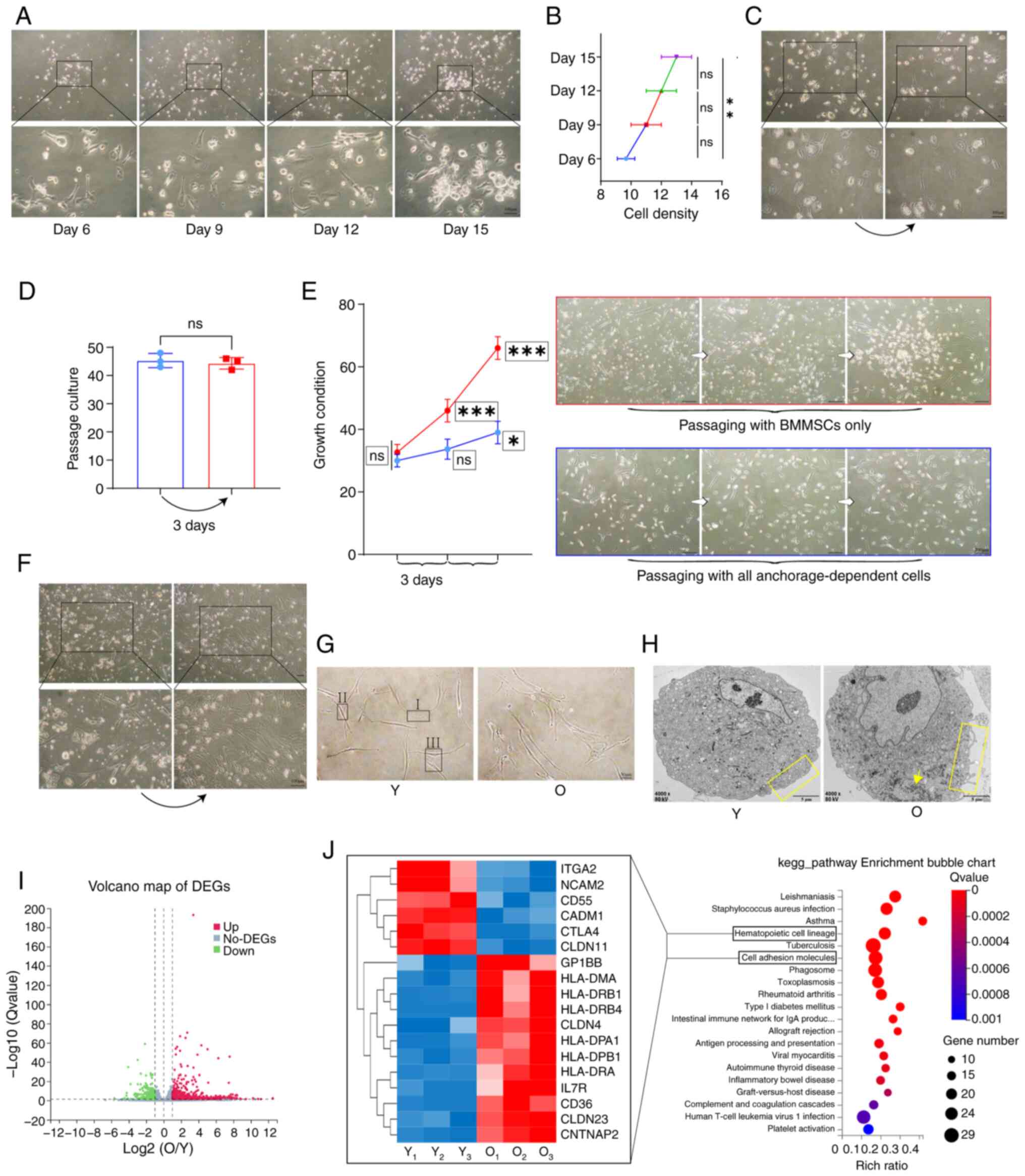
Identifying key causes of MSC stemness
loss through transcriptomics analysis
In the investigation of the key factors influencing
the differences in stemness among bone marrow MSCs, early in the
primary culture of the O group, certain bone marrow materials were
found to exhibit substratum adherence, resembling the behavior of
MSCs, primarily consisting of morphologically diverse bone marrow
stromal cells, alongside the generation of highly refractive bone
marrow terminal substances. Under these conditions, MSCs
demonstrated reduced proliferation (Fig. 8A and B). Passage of these bone
marrow materials showed no significant changes (Fig. 8C and D). The MSCs were then
isolated from these materials for passage culture, where the
results indicated that MSCs could effectively proliferate when the
influence of the bone marrow materials was removed (Fig. 8E). When the efficiently
proliferating Y group MSCs were co-cultured with these bone marrow
materials, the morphology of the Y group MSCs transitioned from
distinctly spindle-shaped to irregular polygonal forms,
characterized by fusion growth (Fig.
8F). These results suggested that changes in the stemness of
MSCs are influenced by their growth microenvironment.
To analyze this variability in MSC proliferation, Y
group and O group MSCs, which were isolated and purified, were
observed using both optical and electron microscopy. Y group MSCs
primarily communicated through three types of contact:
'Synapse-to-synapse', 'synapse-to-nuclear zone' and 'nuclear
zone-to-nuclear zone'. By contrast, O group MSCs exhibited more
complex modes of contact due to increased synaptic branching and
morphological diversity, with these three forms of cell contact
being more pronounced among O group MSCs (Fig. 8G). Electron microscopy results
indicated that Y group MSCs had smooth plasma membranes, uniform
cell shapes and equal cell body sizes, whilst O group MSCs
displayed irregular membrane protrusions, diverse cell morphologies
and increased accumulation of translucent intracellular materials
and glycogen (Fig. 8H).
In the transcriptomic analysis, KEGG enrichment
analysis of differential genes between Y group and O group MSCs
indicated significant differences in hematopoiesis and cell
adhesion. Cluster heat maps revealed distinctions in genes related
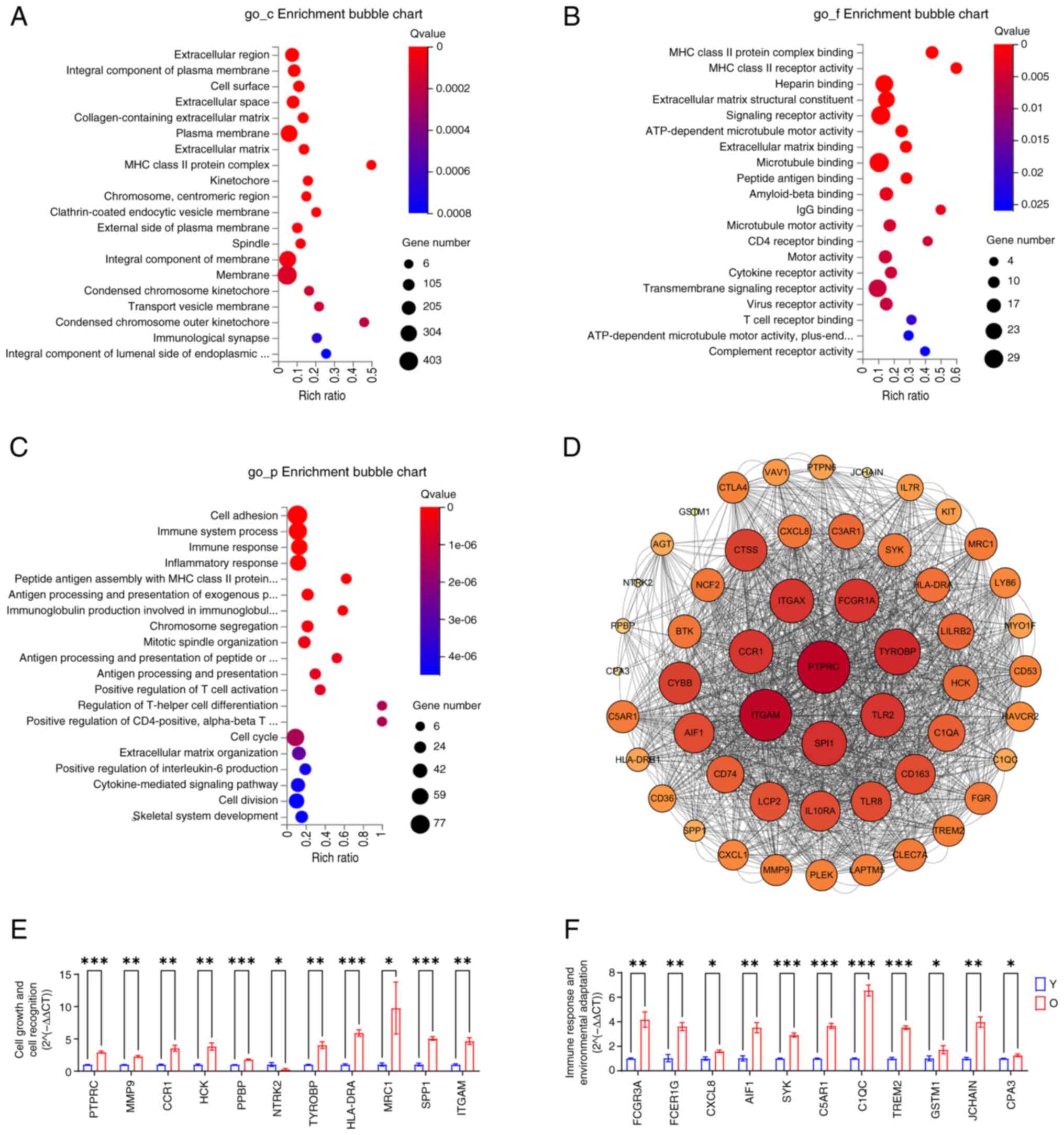
to hematopoiesis and cell adhesion between the two groups (Fig. 8I and J). GO_cellular component
enrichment results suggested that the differences between Y group
and O group MSCs are reflected in spatial changes of the plasma
membrane and cell membrane transport systems (Fig. 9A). GO_molecular function
enrichment results highlighted that the differences in membrane
components predominantly manifested in the expression of cell
recognition-related receptors and signaling molecules on membranes
(Fig. 9B). GO_biological process
enrichment results indicated that these differences are closely
associated cell proliferation, adhesion, immune recognition and
regulation of bone marrow MSCs (Fig.
9C). PPI analysis displayed the associations of the top 53
differential genes with the greatest variation (Fig. 9D). Finally, RT-qPCR validation
results demonstrated substantial differences in cell growth and
communication between Y group and O group MSCs (Fig. 9E), with differential validation
also observed in genes associated with signaling recognition and
immune response (Fig. 9F). These
findings suggested that bone marrow MSCs exhibit varying functional
stress responses to changes in the bone marrow microenvironment,
ultimately adapting their stemness to meet the organism's
demands.
Discussion
Hematopoietic stem cell transplantation is one of
the primary modalities for treating hematologic malignancies
(36,37). Transplanting HSCs in conjunction
with bone marrow MSCs can enhance transplantation success rates and
promote postoperative hematopoietic reconstruction (38). However, not all transplantation
outcomes in clinical settings are favorable. Therefore, in addition
to selecting appropriate donors, further investigation into bone
marrow MSCs is essential for analyzing strategies to optimally
reconstruct the hematopoietic niche (39,40).
Exosomes have emerged as a focal point in the study
of bone marrow MSCs (41). The
therapeutic effects of exosomes derived from bone marrow MSCs
primarily result from their ability to mitigate inflammatory damage
(42,43). This phenomenon is closely
associated with the low immunogenicity of bone marrow MSCs
(44). These attributes make
MSCs widely used in regenerative medicine (45,46). This is likely to be one of the
key functional manifestations of these cells within the bone marrow
microenvironment and a significant factor for successful bone
marrow transplantation. Experimental results indicate that the
decline in bone marrow function in the elderly is not solely
attributable to hematopoietic stem progenitor cells. The reduction
in bone marrow MSCs signifies a dysfunction within the bone marrow
of older individuals, whilst the formation of numerous terminal
bone marrow cells serves as a compensatory mechanism for sustaining
bone marrow function (47). The
biological differences observed in isolated and purified bone
marrow MSCs at various stages of in vitro culture are both a
consequence of and a contributor to variations in the bone marrow
microenvironment (48). Bone
marrow MSCs achieve this adaptation through morphological and
functional changes. Modifications in cell contact mechanisms and
plasma membrane composition allow these cells to efficiently sense
the requirements of the bone marrow. The heightened activity of
organelles, primarily the endoplasmic reticulum and the increased
presence of vesicular materials, suggest that bone marrow MSCs can
effectively process signals from the bone marrow microenvironment.
Compared with that in the Y group, O group bone marrow MSCs
exhibited elevated expression of numerous immune-related genes,
with receptors and pathways associated with immune function being
activated within the membrane system (49). It has been previously reported
that aging hematopoiesis tends to favor myeloid differentiation
(50). Therefore, during the
aging process, bone marrow MSCs will likely coordinate changes in
the bone marrow microenvironment with the hematopoietic functions
of hematopoietic stem progenitor cells (51).
The present study examined the relationship among
the bone marrow hematopoietic microenvironment, hematopoietic stem
progenitor cells and aging, to elucidate the factors contributing
to the decline in hematopoietic function in the elderly. The
accumulation of 'abnormalities' within the bone marrow
microenvironment during cellular activities diminishes the stemness
of stem cells and alters the expression of stemness-related genes.
The fate of bone marrow stem cells is influenced not only by the
cells themselves but also by their surrounding environment. In
treating hematologic diseases in the elderly, the interactions
among bone marrow stem cells merit further investigation,
particularly compared with the changes resulting from the intrinsic
aging of hematopoietic stem progenitor cells (52,53).
Overall, MSCs can assume an important role in bone
marrow transplantation therapy and the treatment of hematopoietic
disorders, which may provide ideas for novel therapeutic or
adjuvant approaches (54).
Because of the invasive procedures and ethical requirements of bone
marrow aspiration, it was chosen to collect bone marrow from
volunteers presenting with symptoms of IDA, in order to exclude the
interference of hematologic malignant diseases on the one hand, and
mainly because these individuals showed normal results on review
after non-radiochemotherapy. Although the anemic samples may be
representative of the general population, our approach minimizes
the study limitations. The present study only dissected the
differential changes in MSCs in young and old individuals from the
perspective of an age gradient. Changes in the bone marrow
microenvironment are highly complex with multifactorial outcomes.
Other perspectives are needed to fully resolve the causes
underlying differences in bone marrow function. Differences in the
recruitment and migration capacity of MSCs can be performed in
future studies, combined with self-renewal capacity assays and
multidirectional differentiation capacity assays to assess
capability of MSCs in different age groups. In clinical treatment,
more effective bone marrow functional recovery can be achieved not
only by screening the source of MSCs, but also by interventions to
improve the function of the recipient's own MSCs, in addition to by
enhancing the stemness of MSCs in transplantation therapy (55,56).
In conclusion, the findings of the present study
provide novel theoretical support in utilizing MSCs for the
treatment of hematological diseases, especially in bone marrow
transplantation. On this basis, the stemness changes of MSCs were
analyzed and are expected to establish more effective screening
methods and interventions.
Supplementary Data
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The data generated in the
present study may be found in the National Center for Biotechnology
Information under accession number PRJNA1183324 or at the following
URL: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1183324.
Authors' contributions
CW and ZY performed all the experimental assays. LW,
ZW, JH and YW designed the study. KD and JS analyzed the data. All
authors read and approved the final version of the manuscript. CW
and YW confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved (approval no.
2023092) by The Ethics Committee of Chongqing Medical University
(Chongqing, China). Written informed consent was obtained from all
participants prior to publication of the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81873103 and 81673748).
References
|
1
|
Khaltourina D, Matveyev Y, Alekseev A,
Cortese F and Ioviţă A: Aging fits the disease criteria of the
international classification of diseases. Mech Ageing Dev.
189:1112302020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hakimizadeh E, Tadayon S, Zamanian MY,
Soltani A, Giménez-Llort L, Hassanipour M and Fatemi I:
Gemfibrozil, a lipid-lowering drug, improves hepatorenal damages in
a mouse model of aging. Fundam Clin Pharmacol. 37:599–605. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hakimizadeh E, Zamanian MY, Borisov VV,
Giménez-Llort L, Ehsani V, Kaeidi A, Hassanshahi J, Khajehasani F,
Movahedinia S and Fatemi I: Gemfibrozil, a lipid-lowering drug,
reduces anxiety, enhances memory, and improves brain oxidative
stress in d-galactose-induced aging mice. Fundam Clin Pharmacol.
36:501–508. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cai Y, Song W, Li J, Jing Y, Liang C,
Zhang L, Zhang X, Zhang W, Liu B, An Y, et al: The landscape of
aging. Sci China Life Sci. 65:2354–2454. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tchkonia T, Palmer AK and Kirkland JL: New
horizons: Novel approaches to enhance healthspan through targeting
cellular senescence and related aging mechanisms. J Clin Endocrinol
Metab. 106:e1481–e1487. 2021. View Article : Google Scholar :
|
|
6
|
Rudolph KL: Stem cell aging. Mech Ageing
Dev. 193:1113942021. View Article : Google Scholar
|
|
7
|
Liu B, Qu J, Zhang W, Izpisua Belmonte JC
and Liu GH: A stem cell aging framework, from mechanisms to
interventions. Cell Rep. 41:1114512022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
López-Otín C, Pietrocola F, Roiz-Valle D,
Galluzzi L and Kroemer G: Meta-hallmarks of aging and cancer. Cell
Metab. 35:12–35. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Plakhova N, Panagopoulos V, Vandyke K,
Zannettino ACW and Mrozik KM: Mesenchymal stromal cell senescence
in haematological malignancies. Cancer Metastasis Rev. 42:277–296.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aguilar-Navarro AG, Meza-León B,
Gratzinger D, Juárez-Aguilar FG, Chang Q, Ornatsky O, Tsui H,
Esquivel-Gómez R, Hernández-Ramírez A, Xie SZ, et al: Human aging
alters the spatial organization between CD34+ hematopoietic cells
and adipocytes in bone marrow. Stem Cell Reports. 15:317–325. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Young K, Eudy E, Bell R, Loberg MA,
Stearns T, Sharma D, Velten L, Haas S, Filippi MD and Trowbridge
JJ: Decline in IGF1 in the bone marrow microenvironment initiates
hematopoietic stem cell aging. Cell Stem Cell. 28:1473–1482.e7.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weng Z, Wang Y, Ouchi T, Liu H, Qiao X, Wu
C, Zhao Z, Li L and Li B: Mesenchymal stem/stromal cell senescence:
Hallmarks, mechanisms, and combating strategies. Stem Cells Transl
Med. 11:356–371. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Al-Azab M, Safi M, Idiiatullina E,
Al-Shaebi F and Zaky MY: Aging of mesenchymal stem cell: Machinery,
markers, and strategies of fighting. Cell Mol Biol Lett. 27:692022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu Y, Ge J, Huang C, Liu H and Jiang H:
Application of mesenchymal stem cell therapy for aging frailty:
From mechanisms to therapeutics. Theranostics. 11:5675–5685. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burnham AJ, Daley-Bauer LP and Horwitz EM:
Mesenchymal stromal cells in hematopoietic cell transplantation.
Blood Adv. 4:5877–5887. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ambrosi TH and Chan CKF: A seed-and-soil
theory for blood ageing. Nat Cell Biol. 25:9–11. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shannon K and Link DC: Soil and Seed:
Coconspirators in therapy-induced myeloid neoplasms. Blood Cancer
Discov. 1:10–12. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deniz IA, Karbanová J, Wobus M, Bornhäuser
M, Wimberger P, Kuhlmann JD and Corbeil D: Mesenchymal stromal
cell-associated migrasomes: A new source of chemoattractant for
cells of hematopoietic origin. Cell Commun Signal. 21:362023.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nachmias B, Zimran E and Avni B:
Mesenchymal stroma/stem cells: Haematologists' friend or foe? Br J
Haematol. 199:175–189. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Young KA, Telpoukhovskaia MA, Hofmann J,
Mistry JJ, Kokkaliaris KD and Trowbridge JJ: Variation in
mesenchymal KITL/SCF and IGF1 expression in middle age underlies
steady-state hematopoietic stem cell aging. Blood. 144:378–391.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou Y, Cai X, Zhang X, Dong Y, Pan X, Lai
M, Zhang Y, Chen Y, Li X, Li X, et al: Mesenchymal stem/stromal
cells from human pluripotent stem cell-derived brain organoid
enhance the ex vivo expansion and maintenance of hematopoietic
stem/progenitor cells. Stem Cell Res Ther. 15:682024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang A, Strat AN, Rahman M, Zhang H, Bao
W, Liu Y, Shi D, An X, Manwani D, Shi P, et al: Murine bone marrow
mesenchymal stromal cells have reduced hematopoietic maintenance
ability in sickle cell disease. Blood. 138:2570–2582. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rossi M, Meggendorfer M, Zampini M,
Tettamanti M, Riva E, Travaglino E, Bersanelli M, Mandelli S,
Antonella Galbussera A, Mosca E, et al: Clinical relevance of
clonal hematopoiesis in persons aged ≥80 years. Blood.
138:2093–2105. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stauder R, Valent P and Theurl I: Anemia
at older age: Etiologies, clinical implications, and management.
Blood. 131:505–514. 2018. View Article : Google Scholar
|
|
25
|
Colom Díaz PA, Mistry JJ and Trowbridge
JJ: Hematopoietic stem cell aging and leukemia transformation.
Blood. 142:533–542. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Guan Q, Wang Z, Feng J, Zou J and
Gao B: Consequences of aging on bone. Aging Dis. 15:2417–2452.
2023.PubMed/NCBI
|
|
27
|
Kumar N, Saraber P, Ding Z and Kusumbe AP:
Diversity of vascular niches in bones and joints during
homeostasis, ageing, and diseases. Front Immunol. 12:7982112021.
View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Chen Q, Shou P, Zheng C, Jiang M, Cao G,
Yang Q, Cao J, Xie N, Velletri T, Zhang X, et al: Fate decision of
mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death
Differ. 23:1128–1139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Redondo J, Bailey S, Kemp KC, Scolding NJ
and Rice CM: The bone marrow microenvironment in immune-mediated
inflammatory diseases: Implications for mesenchymal stromal
cell-based therapies. Stem Cells Transl Med. 13:219–229. 2024.
View Article : Google Scholar :
|
|
31
|
Tan L, Liu X, Dou H and Hou Y:
Characteristics and regulation of mesenchymal stem cell plasticity
by the microenvironment-specific factors involved in the regulation
of MSC plasticity. Genes Dis. 9:296–309. 2020. View Article : Google Scholar
|
|
32
|
Xie Y, Tang C, Huang Z, Zhou S, Yang Y,
Yin Z, Heng BC, Chen W, Chen X and Shen W: Extracellular matrix
remodeling in stem cell culture: A potential target for regulating
stem cell function. Tissue Eng Part B Rev. 28:542–554. 2022.
View Article : Google Scholar
|
|
33
|
Samal JRK, Rangasami VK, Samanta S,
Varghese OP and Oommen OP: Discrepancies on the role of oxygen
gradient and culture condition on mesenchymal stem cell fate. Adv
Healthc Mater. 10:e20020582021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kann AP, Hung M and Krauss RS: Cell-cell
contact and signaling in the muscle stem cell niche. Curr Opin Cell
Biol. 73:78–83. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Le Saux G, Wu MC, Toledo E, Chen YQ, Fan
YJ, Kuo JC and Schvartzman M: Cell-cell adhesion-driven contact
guidance and its effect on human mesenchymal stem cell
differentiation. ACS Appl Mater Interfaces. 12:22399–22409. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang X, Huang R and Zhang X and Zhang X:
Current status and prospects of hematopoietic stem cell
transplantation in China. Chin Med J (Engl). 135:1394–1403. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chang YJ, Pei XY and Huang XJ:
Haematopoietic stem-cell transplantation in China in the era of
targeted therapies: Current advances, challenges, and future
directions. Lancet Haematol. 9:e919–e929. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li T, Luo C, Zhang J, Wei L, Sun W, Xie Q,
Liu Y, Zhao Y, Xu S and Wang L: Efficacy and safety of mesenchymal
stem cells co-infusion in allogeneic hematopoietic stem cell
transplantation: A systematic review and meta-analysis. Stem Cell
Res Ther. 12:2462021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Katzerke C, Schaffrath J, Lützkendorf J,
Janssen M, Merbach AK, Nerger K, Binder M, Baum C, Lauer K, Rohde
C, et al: Reduced proliferation of bone marrow MSC after allogeneic
stem cell transplantation is associated with clinical outcome.
Blood Adv. 7:2811–2824. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Comazzetto S, Shen B and Morrison SJ:
Niches that regulate stem cells and hematopoiesis in adult bone
marrow. Dev Cell. 56:1848–1860. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lotfy A, AboQuella NM and Wang H:
Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical
trials. Stem Cell Res Ther. 14:662023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang S, Lei B, Zhang E, Gong P, Gu J, He
L, Han L and Yuan Z: Targeted therapy for inflammatory diseases
with mesenchymal stem cells and their derived exosomes: From basic
to clinics. Int J Nanomedicine. 17:1757–1781. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Regmi S, Raut PK, Pathak S, Shrestha P,
Park PH and Jeong JH: Enhanced viability and function of
mesenchymal stromal cell spheroids is mediated via autophagy
induction. Autophagy. 17:2991–3010. 2021. View Article : Google Scholar :
|
|
44
|
Song N, Scholtemeijer M and Shah K:
Mesenchymal stem cell immunomodulation: Mechanisms and therapeutic
potential. Trends Pharmacol Sci. 41:653–664. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hoang DM, Pham PT, Bach TQ, Ngo ATL,
Nguyen QT, Phan TTK, Nguyen GH, Le PTT, Hoang VT, Forsyth NR, et
al: Stem cell-based therapy for human diseases. Signal Transduct
Target Ther. 7:2722022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tsiapalis D and O'Driscoll L: Mesenchymal
stem cell derived extracellular vesicles for tissue engineering and
regenerative medicine applications. Cells. 9:9912020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang X, Chen D, Long H and Zhu B: The
mechanisms of pathological extramedullary hematopoiesis in
diseases. Cell Mol Life Sci. 77:2723–2738. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sanmartin MC, Borzone FR, Giorello MB,
Pacienza N, Yannarelli G and Chasseing NA: Bone marrow/bone
pre-metastatic niche for breast cancer cells colonization: The role
of mesenchymal stromal cells. Crit Rev Oncol Hematol.
164:1034162021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Massaro F, Corrillon F, Stamatopoulos B,
Dubois N, Ruer A, Meuleman N, Bron D and Lagneaux L: Age-related
changes in human bone marrow mesenchymal stromal cells: Morphology,
gene expression profile, immunomodulatory activity and miRNA
expression. Front Immunol. 14:12675502023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kwack KH, Zhang L, Kramer ED, Thiyagarajan
R, Lamb NA, Arao Y, Bard JE, Seldeen KL, Troen BR, Blackshear PJ,
et al: Tristetraprolin limits age-related expansion of
myeloid-derived suppressor cells. Front Immunol. 13:10021632022.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
da Silva Gonçalves CE and Fock RA:
Semaphorins and the bone marrow microenvironment: New candidates
that influence the hematopoietic system. Cytokine Growth Factor
Rev. 76:22–29. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kokkaliaris KD and Scadden DT: Cell
interactions in the bone marrow microenvironment affecting myeloid
malignancies. Blood Adv. 4:3795–3803. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hofmann J and Kokkaliaris KD: Bone marrow
niches for hematopoietic stem cells: Life span dynamics and
adaptation to acute stress. Blood. 144:21–34. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mohseni R, Mahdavi Sharif P, Behfar M,
Modaresi MR, Shirzadi R, Mardani M, Jafari L, Jafari F, Nikfetrat Z
and Hamidieh AA: Evaluation of safety and efficacy of allogeneic
adipose tissue-derived mesenchymal stem cells in pediatric
bronchiolitis obliterans syndrome (BoS) after allogeneic
hematopoietic stem cell transplantation (allo-HSCT). Stem Cell Res
Ther. 14:2562023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Si YC, Li Q, Xie CE, Niu X, Xia XH and Yu
CY: Chinese herbs and their active ingredients for activating xue
(blood) promote the proliferation and differentiation of neural
stem cells and mesenchymal stem cells. Chin Med. 9:132014.
View Article : Google Scholar
|
|
56
|
Wang Z, Wang L, Jiang R, Li C, Chen X,
Xiao H, Hou J, Hu L, Huang C and Wang Y: Ginsenoside Rg1 prevents
bone marrow mesenchymal stem cell senescence via NRF2 and PI3K/Akt
signaling. Free Radic Biol Med. 174:182–194. 2021. View Article : Google Scholar : PubMed/NCBI
|