Introduction
Transporters are membrane proteins that facilitate
the movement of various substances such as nutrients,
neurotransmitters, ions, metabolites and drugs, and are involved in
important biological processes including the regulation of cell
signaling and the organization of cellular organelles (1). Originally, these membrane proteins
were categorized as ATP-independent transporter proteins, but in
2004 they were classified into two major superfamilies: The
ATP-binding cassette (ABC) and solute carrier (SLC) families
(2-4).
Membrane transporters SLCs, which are more numerous
than ABCs, play a crucial role in facilitating communication
between the cell and its environment. Genetic variants in the SLC
family have been associated with various diseases, including
neurological or metabolic disorders and cancer (5,6).
Despite their biological importance, SLCs are among the most
understudied class of proteins, with >455 membrane-bound
proteins classified into 66 families, for this reason, numerous
aspects of their biology remain unknown (7).
There have been significant advances in the
structural biology of membrane proteins, which have greatly
improved the understanding of molecular-level transport (6). SLC transporters are an extremely
diverse family of membrane proteins. The most common structural
classes in human SLCs are the LeuT-like fold leucine transporter
(such as SLC6) and the Major Facilitator Superfamily (such as SLC2)
(7-10). The diversity of SLC proteins is
determined by the specificity of the substrate, as well as the
different regulatory properties and tissue- and cell-type-specific
metabolic requirements (11-13).
Ovarian cancer (OC) is a gynecological pathology
with a high mortality rate, often diagnosed at an advanced stage,
leading to a poor prognosis (14). The main response at the onset of
the disease is instrumental screening followed by surgical
ablation. However, therapeutic options are limited, especially in
relapses, which often become resistant to chemotherapy drugs
(15,16). The complexity and heterogeneity
of OC can result from the uncontrolled proliferation of epithelial,
germ or stromal cells, leading to the development of malignant
tumors with differences in epidemiology, clinical characteristics,
response to chemotherapy and prognosis (16).
In recent decades, it has been widely demonstrated
that hereditary or acquired genetic alterations have an important
role in the etiology of OC. For instance, BRCA1 and
BRCA2 mutations have long been associated with an increased
risk of developing breast cancer or OC (16-18). Additionally, genetic variants in
other genes such as RAD51C, RAD51D and PALB2,
as well as in MLH1, MSH2 and MSH6 genes, have
been identified in 15-20% of OC cases (19,20). This knowledge allows us to
identify and screen individuals with a greater probability of
developing certain tumor syndromes and to activate counseling and
tumor surveillance, particularly when the risk assessment is
correlated with a previous family history (15).
Recent studies have shown that changes in gene
expression levels can significantly impact patient survival and
their response to chemotherapy. Additionally, identifying the
molecular pathways and biomarkers involved in tumor growth,
proliferation and migration in OC is crucial in fighting this type
of tumor. Transcription factors that modulate regulatory genes
involved in epithelial-mesenchymal transition (EMT) have been
recently identified (21-23).
Chen et al (23)
demonstrated that upregulation of RUNX family transcription factor
1 (RUNX1) is linked to tumor progression and overall survival (OS),
while its knockdown showed a significant decrease in the capacity
for proliferation and invasion in OC cell lines. Additionally,
RUNX1 knockdown reduces EMT through the EGFR/AKT/STAT3 pathway and
promotes apoptosis via the FOXO1-Bcl2 axis in OC cell lines.
Furthermore, lower expression of RUNX1 improves sensitivity to
chemotherapeutics in patients, as observed in short hairpin-RUNX1
ovarian cell lines, suggesting a synergistic effect (23). Moreover, not only genes but also
mutation types can play a role in different sensitivities to
chemotherapy treatments, as shown by certain studies reporting a
different sensitivity to PARP inhibitors, depending on the type and
location of BRCA1/2 mutations or in other genes (24).
An increasing body of information has been obtained
regarding the role played by ABC and SLC transporters in the
development of multidrug resistance (MDR). This information has
been gathered from gene expression analysis in OC cell lines and
human primary tumors using microarray techniques (25,26). These analyses have highlighted
changes in the expression patterns of transporters and their
involvement in tumor progression and the development of resistance
to chemotherapy drugs (27).
Teng et al (28) have
demonstrated that ABCC1 or ABCG2 overexpression compromised the
drug response in OC cell lines, decreasing their cytotoxic
capacity. It was also observed that the knockout of the singular
genes or competition by specific inhibitors reversed the resistance
process since they significantly reduced the efflux of the
anticancer drug from the cells (28).
In cancer, including OC, SLC transporters are
dysregulated. This allows tumors to obtain more energy and
nutrients giving them an advantage in supporting their metabolic
needs (29,30). Additionally, some SLCs can
contribute to drug resistance by interfering with the cell death
processes and various signaling pathways that influence
proliferative capacity and tumor progression (3,31). Therefore, the present review aims
to summarize the current knowledge regarding the involvement of
SLCs in OC and how they may impact the pharmacological
response.
SLCs expressed in OC
SLC transporters are differentially expressed in
various cell types and tissues. Dysregulation of these transporters
is linked to metabolic diseases and tumorigenesis. While a number
of studies have explored the role of SLCs in different types of
tumors, there has been insufficient research focusing on their
involvement in OC (4-6). The SLCs associated with this form
of cancer exhibit different transport mechanisms (Fig. 1). The localization and further
information on the transporters reported in the present review are
listed in Table I.
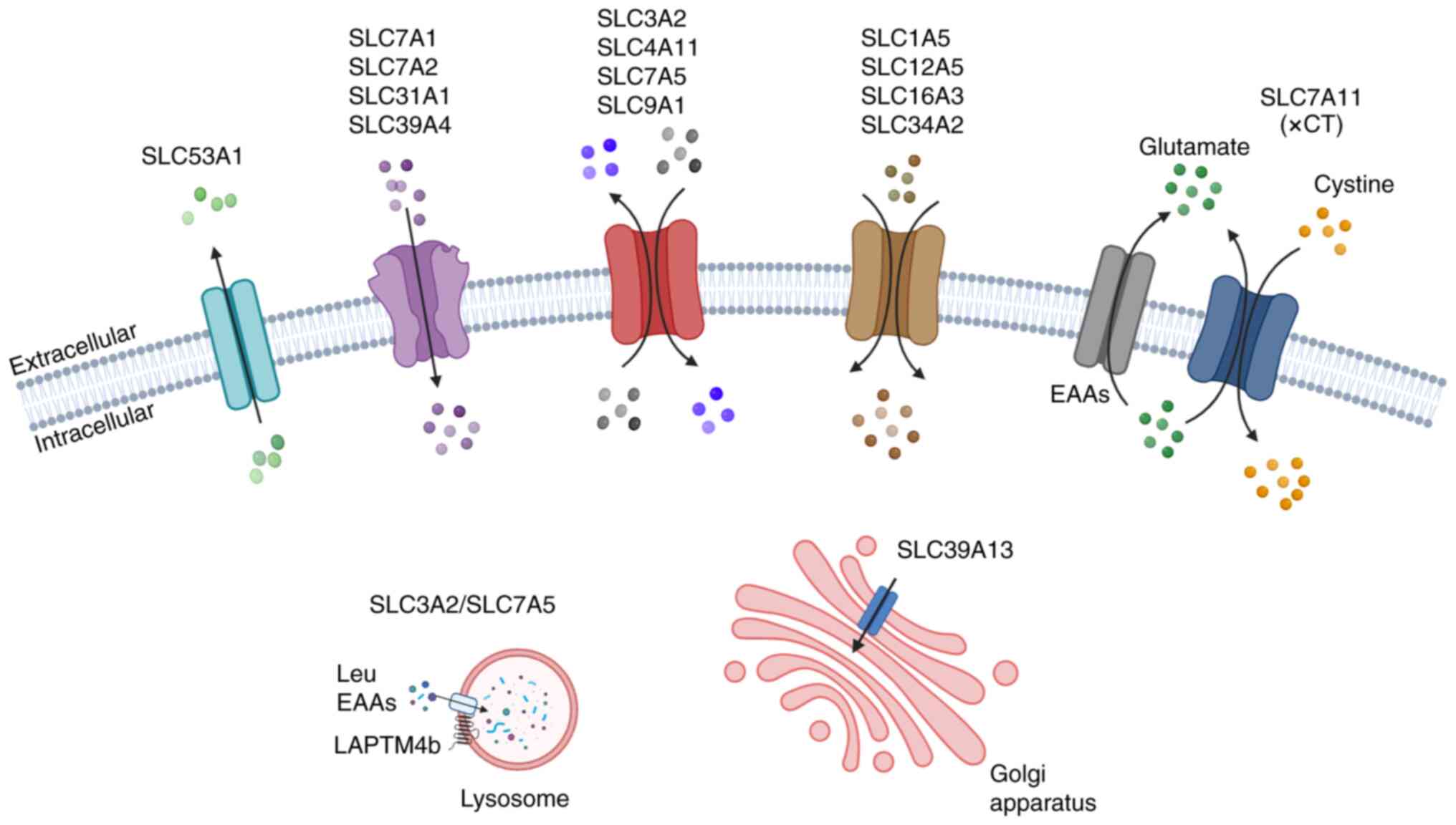 | Figure 1Schematic representation of SLCs
responsible for transporting various substances across the cell
membrane in OC. The figure illustrates examples of SLCs
demonstrating the transporter types and their specific localization
in OC. Uniporters: SLC53A1 (efflux); SLC7A1, SLC7A2, SLC31A1,
SLC39A4, SLC32A2/SLC7A5 and SLC39A13 (influx). Antiporters: SLC3A2,
SLC4A11, SLC7A and SLC9A1. Symporters: SLC1A5, SLC12A5, SLC16A3 and
SLC34A2. The xCT system involving SLC7A11 in the exchange of
L-Cystine/L-Glu across the plasma membrane. EAAs, essential amino
acids; LAPTM4b, lysosome associated protein transmembrane 4B; SLC,
solute carrier. Created with BioRender software. |
 | Table ITransport type and location of SLCs
in ovarian cancer. |
Table I
Transport type and location of SLCs
in ovarian cancer.
| SLCs | Alias | RefSeq ID | Transport type | Subcellular
location | (Refs.) |
|---|
| SLC1A5 | ASCT2, AAT | NM_005628 |
Na+/neutral AA
cotransporter | Plasma
membrane | (32,33) |
| SLC3A2 | 4F2hc, CD98 | NM_001012662.3 | AA exchanger
(Cys/Gln), uptake Leu | Plasma and
lysosomal membrane | (37) |
| SLC4A11 | BTR1, NaBC1 | NM_001174090.2 |
Na+/OH− and
NH4+ transporter | Plasma
membrane | (43) |
| SLC7A1 | ATRC1, CAT-1 | NM_003045.5 | CAT-facilitated
transporter | Plasma
membrane | (3,49) |
| SLC7A2 | ATRC2, CAT-2 | NM_003046.6 | CAT-facilitated
transporter | Plasma
membrane | (3) |
| SLC7A5 | LAT1 | NM_003486 | Uptake Leu | Plasma and
lysosomal membrane | (38) |
| SLC7A6 | LAT2 | NM_001076785.3 | LAT
transporter | Plasma
membrane | (3) |
| SLC7A11 | xCT | NM_014331.4 | Cystine/Glu
antiporter | Plasma
membrane | (54) |
| SLC9A1 | NHE-1 | NM_003047.5 |
Na+/H+
exchanger-1 | Plasma
membrane | (81,83) |
| SLC12A5 | KCC2 | NM_001134771.2 |
K+/Cl−
cotransporter | Plasma
membrane | (89) |
| SLC16A3 | MCT4 | NM_001206950.2 | Lactic acid, ketone
bodies and pyruvate transport | Plasma
membrane | (59,90) |
| SLC31A1 | CTR1 | NM_001859.4 | Copper
transporter | Plasma
membrane | (98) |
| SLC34A2 | NaPi2b | NM_006424.3 | Na+/Pi
cotransporter | Plasma
membrane | (101) |
| SLC39A4 | ZIP4 | NM_017767.3 | Uptake Zn | Plasma
membrane | (111) |
| SLC39A13 | ZIP13 | NM_001128225.3 | Zn transporter | Golgi
apparatus | (113) |
| SLC53A1 | XPR1 | NM_004736.4 | Pi transporter | Plasma
membrane | (102) |
SLC1A5
The expression levels of SLC proteins differ between
healthy and cancer cells. Amino acid transporters, such as SLC1A5
(also known as ASCT2), play a crucial role in cancer metabolism by
supporting the increased energy demand for rapid cellular growth.
SLC1A5 is involved in the uptake of amino acids (Ala, Ser, Cys and
Gln), and downregulation of Gln metabolism has been found to
inhibit cell proliferation in various tumors, including OC
(3,32,33). In OC tissues, SLC1A5 is
significantly upregulated and has been linked to clinical factors
and prognosis (32,33). In epithelial OC (EOC), high
expression of both SLC1A5 and phosphorylated (p-)mTOR has been
observed, and the mTOR signaling pathway is known to promote tumor
cell proliferation through Gln metabolism. Furthermore, the
co-expression of SLC1A5 and p-mTOR has been associated with poor
OS, indicating a synergistic effect on the growth and development
of EOC (33). Recent studies
have also identified specific microRNAs (miRNAs) that can modulate
SLC1A5 gene expression in OC. For instance, upregulation of
miR-122-5p has been shown to regulate SLC1A5 expression by
downregulating circular RNA (circ)_0072995, thereby affecting cell
growth, apoptosis and invasion. This study reported the role of the
cir_0072995/miR-122-5p/SLC1A5 axis in OC tumorigenesis (34). Another study highlighted a
similar mechanism of SLC1A5 regulation through the
circ_0025033/hsa_miR-370-3p axis (35). Additionally, a new axis has been
identified between claudin-4, SLC1A5 and SLC7A5. Claudin-4
is a gene that encodes a tight junctional protein involved in
modulating genomic instability and is associated with worse patient
outcomes in OC (36). This axis
plays a critical role in amino acid transport through the plasma
membrane, contributing to increased OC aggressiveness (36).
SLC3A2
The SLC3A2 gene, also known as 4F2hc or CD98,
encodes for a type II transmembrane glycoprotein that can bind to
SLC7A5, SLC7A6, SLC7A7, SLC7A8, SLC7A10 and SLC7A11, forming
heterodimeric transporters expressed in different tissues (37,38). In particular, the interaction
between SLC3A2 and SLC7A11 (also known as xCT) or SLC7A5 [also
known as L-type amino acid transporter (LAT) 1] is involved in an
exchange that imports Cystine and essential amino acids (EAAs) and
exports Glu and Gln, respectively (37). In addition to the cell membrane,
the heterodimeric complex formed by SLC3A2 and SLC7A5 is present in
the lysosomal membrane, where SLC3A2/SLC7A5 binds to lysosome
associated protein transmembrane 4B (LAPTM4b) promoting Leu and
other EAAs to influx into lysosomes, which is required for mTORC1
activation via V-ATPase (38).
Several studies have demonstrated that SLC3A2
expression and its partners are dysregulated in a number of cancer
types, where this protein is involved in different stages of tumor
development (39-41). In OC, SLC3A2 upregulation
supports chemotherapy treatment and decreases tumor masses
(40,41). A recent bioinformatics analysis
study demonstrated the association of the SLC3A2-CD147 complex as a
potential risk factor in patients with OC (42).
SLC4A11
The SLC4 family includes 10 proteins involved in the
homeostasis control of intracellular pH (pHi) that mediate
Cl−/HCO3− and
Na+/HCO3− membrane cotransport. A
divergent role has been shown for the SLC4A11 protein that instead
mediates the Na+/OH− and NH4+
exchange (43). In OC cells, the
metabolic changes typical of the neoplastic environment induce
upregulation of H+ transporters with consequent
extracellular acidification, supporting tumor invasion and
metastasis (43-46). It has been demonstrated that
SLC4A11 upregulation is more evident in OC tissues than in normal
tissues, particularly in patients with metastasis vs. those without
metastasis. Moreover, higher SLC4A11 expression has been linked to
poor OS. Dataset analysis of the SLC4A11 gene regulation
highlighted that regulation depends on methylation and DNA
amplification processes (43).
SLC7 family
The SLC7 family genes mediate amino acid
transport, and their dysregulation is linked to a number of human
diseases, different stages of tumor development and the drug
resistance of different cancer types (47,48). The SLC7 family is divided into
two subfamilies, namely the cationic amino acid (CAT) and LAT
transporter families. The human CAT subfamily includes SLC7A1,
SLC7A2, SLC7A3 and SLC7A4, while the LAT family comprises six
proteins, SLC7A5, SLC7A6, SLC7A7, SLC7A8, SLC7A10 and SLC7A11
(3,49). To date, few studies have examined
the role of SLCs in OC. However, some proteins of the SLC7 family,
SLC7A2, SLC7A5 and SLC7A11, have been recognized to play critical
roles in OC (38,47,50-54).
Different expression of SLC7 members was revealed in
OC compared with normal tissue using the GEPIA dataset, which
showed the upregulation of SLC7A1, SLC7A4 and SLC7A7, and the
downregulation of SLC7A2 and SLC7A8 (47). A recent study by Gong et
al (47) showed that SLC7A1
upregulation was correlated with poor OS in OC, as was reported in
different tumor types where high SLC7A1 expression was also
involved in the tumor-infiltrating immune microenvironment
(48). In addition, patients
with OC and high SLC7A1 levels develop drug resistance and a higher
probability of recurrence (47,49). Another recent study by Circle-seq
revealed that some upregulated genes, including SLC7A1, were
associated with OC prognosis and were able to influence the cell
adhesion and extracellular matrix-receptor interaction pathways
(55).
Unlike SLC7A1, SLC7A2 is downregulated in various
tumor types and induces tumor proliferation and resistance to
chemotherapy drugs (48).
Moreover, in an OC datasets analysis, Sun et al (50) observed that the SLC7A2 expression
levels were significantly lower in younger individuals compared
with patients ≥60 years old. In addition, this study also
demonstrated the role of SLC7A2 in tumor progression by functional
experiments in cancer cell lines. The results highlighted that
SLC7A2 interferes with apoptosis, signaling pathways and drug
resistance. Further SLC7A2 knockdown experiments showed an
increased capacity for cell invasion and migration as well as
elevated levels of EMT protein markers, such as N-cadherin and
vimentin, in OC cell lines (50).
Numerous studies have shown that SLC7A5, also known
as LAT1, is upregulated in several OC cell lines and primary
tumors. In patients with OC, elevated levels of LAT1 have been
correlated with tumor growth, angiogenesis and poor survival rates
(32,56-58). A recent study using
immunohistochemical analysis demonstrated that SLC7A5 upregulation
is associated with certain histological subtypes, such as ovarian
clear cell carcinoma (OCCC) (59). It was also previously shown that
high SLC7A5 expression is correlated with chemoresistance only in
CCC histological sub-types (58). SLC7A5 interacts with SLC3A2 to
form a heterodimeric amino acid transporter and is involved in Leu
uptake into lysosomes, mediating the interaction with LAPTM4b to
activate the mTORC1 complex, as aforementioned (38). A recent study of OCCC
demonstrated that inhibiting SLC7A5, which suppresses Leu entry,
reduced cellular growth via the mTOR pathway (60). As aforementioned, the
claudin-4/SLC1A5/SLC7A5 axis plays a critical role in decreasing
patient survival, contributing to increased tumor aggressiveness
(36).
The role of SLC7A6 has been investigated in the
A2780 and A2780/cisplatin (CDDP) EOC cell lines using dataset
analysis. This analysis highlighted an increased expression of
CircSLC7A6 in A2780/CDDP cells, which was correlated with SLC7A6
upregulation and miR-2682-5p downregulation. Moreover, the
CircSLC7A6/miR-26825p/SLC7A6 axis has been confirmed through
CircSLC7A6 silencing experiments, revealing a direct decrease of
SLC7A6 and an increased expression of miR-2682-5p (61). In addition, Li et al
(61) reported a synergistic
anti-proliferative and pro-apoptotic capacity of CDDP and baicalein
when CircSLC7A6 was knocked down in A2780/CDDP cells.
SLC7A11 is the functional subunit of the Xc-system,
which targets the exchange of L-Cystine and L-Glu across the plasma
membrane (48). Numerous studies
have highlighted the role of SLC7A11 in cancer biology (48,62-64). Altered expression of the
SLC7A11 gene can regulate cell apoptosis, ferroptosis and
autophagy in different types of cancer (54,65,66). The Cystine/Glu transport mediated
by SLC7A11 promotes glutathione (GSH) biosynthesis, decreases
reactive oxygen species (ROS) levels and protects cells from lipid
peroxidation, as well as playing a role in metabolism, cell
proliferation and drug resistance (67). In OC, the regulation of SLC7A11
has been the subject of much research and has sparked some
controversy. One study indicated that high levels of SLC7A11 in
patients with OC were linked to a favorable prognosis, while
another study suggested that SLC7A11 was a poor prognostic factor
and a potential therapeutic target associated with platinum
resistance (53,68). Additionally, low expression of
SLC7A11 inhibited the process of disulfidptosis and was associated
with a poor prognosis. In this research, database analysis was
conducted on a cohort of patients divided into two groups (worse
and improved prognosis) and it was found that high expression of a
gene set, which included SLC7A11, was correlated with the
group showing improved prognosis (69). Previous dataset analyses have
reported that the downregulation of SLC7A11 in drug-resistant OC
tissues and paclitaxel-resistant cell lines negatively modulated
autophagy genes (STX17, UVRAG and RAB33B)
through competing endogenous RNA interactions (54,70,71).
Numerous studies have shown that SLC7A11 is
regulated by various factors and is involved in cell death
processes, making it a therapeutic target in tumor progression
(54,65). The high expression levels of
SLC7A11, determined through CCAAT enhancer binding protein γ
(CEBPG)-mediated transcriptional control, inhibited ferroptosis and
promoted ovarian tumor growth in in vivo experiments. These
results were confirmed by CEBPG knockdown, which reduced ovarian
tumor cell proliferation both in vitro and in vivo.
Additionally, upregulation of CEBPG and SLC7A11 is associated with
poor outcomes in patients with OC (54,72). Similarly, Ogiwara et al
(73) demonstrated that, in
AT-rich interaction domain 1A-deficient OC cell lines, decreased
SLC7A11 protein expression led to low GSH levels, inducing cell
vulnerability to drugs targeting glutamate-cysteine ligase
synthetase catalytic subunit (GCLC). The inhibition of GSH/GCLC
leads to apoptosis by increasing ROS levels (73). SLC7A11 is also involved in
ferroptosis through silencing STEAP3, which reduces the expression
levels of this Cystine/Glu transporter and inhibits the tumor
growth of OC cells via the p53/SLC7A11 pathway (74). Another study showed that SNAI
family transcriptional repressor 2 binds to the SLC7A11 gene
promoter, decreasing its expression and inhibiting cell apoptosis
and ferroptosis in OC cell lines (54,75). Additionally, the interaction
between HRD1 and SLC7A11 induces the degradation of the transporter
and suppresses tumorigenesis, promoting ferroptosis in OC (76).
Long non-coding (lnc)RNA and miRNA are important
regulators of gene expression (77). Evidence indicates their
involvement in both promoting and suppressing cancer in different
tumor types, including OC. A recent study highlighted that lncRNA
ADAMTS9-AS1 was upregulated in OC cells. Knocking down this lncRNA
promoted ferroptosis, inhibiting cancer cell proliferation and
migration. These effects were achieved via the miR587/SLC7A11 axis,
suggesting that lncRNA ADAMTS9-AS1 plays a critical role in SLC7A11
expression (78).
In recent years, SLC7A11 has been identified as a
biomarker involved in the mechanism of ferroptosis and in the
alteration of a series of signaling pathways that can influence
proliferative capacity and tumor progression (79,80). Moreover, bioinformatics analyses
have been conducted to explore the tumor expression of SLC7A11 and
evaluate its association with patient prognosis and survival in OC.
This indicates SLC7A11 as an important factor in prognostic
assessment (48,53,54,69).
SLC9A1
SLC9A1, also known as Na+/H+
exchanger 1 (NHE1), is a ubiquitous membrane protein involved in
pHi control. In tumor cells, the metabolic switch leads to a
decrease in pHi due to lactate production, which releases
H+ ions in anaerobic conditions (46). Thus, to prevent
hyper-acidification in the OC cell environment, transporters
excluding protons from across the plasma membrane are upregulated
to regulate the cellular pH (81,82). Increased NHE1 levels have been
observed in EOC cell lines and tissues. Moreover, NHE1 upregulation
has been correlated with shorter OS compared with individuals with
lower NHE1 levels in patients with EOC (83). Through in vivo
experiments, Szadvari et al (82) have reported that overexpression
of the NHE1-Na+/Ca2+ exchanger 1 complex
leads to alkalinization of pHi and prevents intracellular
Na+ overload. However, alterations in NHE1 function,
such as internalization or inhibition, result in cell
hyper-acidification that induces apoptosis, which plays a critical
role in cancer growth (46,82).
SLC12A5
The SLC12A5 gene, which encodes a potassium
chloride cotransporter, is significantly expressed in various human
cancer types and promotes the progression of prostate, bladder
urothelial, hepatocellular and colorectal carcinoma (84-87), as well as other tumor types.
There is an association between SLC12A5 and methyltransferases or
DNA repair proteins (88).
Research conducted by Yang et al (89) demonstrated the prognostic value
of SLC12A5 in OC, where increased expression was associated
with poor prognosis and survival. The authors also found a positive
correlation between SLC12A5 protein upregulation and a more
aggressive or invasive tumor phenotype. Gene amplification of
SLC12A5 was detected in ~10.3% of OC cases, while no
upregulation was observed in normal ovarian tissues.
SLC16A3
The SLC16A gene family consists of
transporter proteins termed monocarboxylate transporters (MCTs),
which are involved in metabolic processes and pH balance. This
family includes SLCA16A1 (MCT1), SLCA16A7 (MCT2),
SLCA16A8 (MCT3) and SLCA16A3 (MCT4) (90). Metabolic reprogramming and
epigenetic modifications are well-known hallmarks of cancer, and
they play a significant role in the uncontrolled growth and
proliferation of tumor cells (91,92). Upregulation of SLC16A1 and
SLC16A3 has been well-documented in the context of the tumor
environment, due to their role in maximizing the capacity of
lactate exporters, which helps prevent intracellular hyper-acidosis
(93-95). RNA-sequencing (RNA-Seq) analysis
revealed that SLC16A1 and SLC16A3 are upregulated in
OC tissues compared with normal tissues. Additionally, SLC16A3
expression was found to be elevated in metastatic tissue and
correlated with poor prognosis, suggesting it could be a potential
therapeutic target (95).
In a previous study, it was found that certain SLC
proteins, such as SLC16A3, can impact how cells respond to
chemotherapy in both OC cell lines and tissues. These proteins can
interfere with the movement of drugs across cell membranes. High
expression of SLC16A3 was positively correlated with the MDR1
marker (96).
Furthermore, an analysis using Affymetrix Human
Genome U219 microarrays in OC cell lines revealed the dysregulated
expression of 32 SLCs. Specifically, 17 genes showed increased
expression (such as SLC16A3, SLC2A9, SLC16A14,
SLC38A4 and SLC39A8), while 15 genes showed decreased
expression (such as SLC2A14, SLC6A15, SLC8A1
and SLC27A2). The study demonstrated that the significant
upregulation of SLC16A3 contributed to drug resistance in cancer
cells (97).
SLC31A1
SLC31A1, also known as CTR1, regulates copper
homeostasis and acts as a transporter for platinum-based drugs
(98). Regarding drug delivery,
a study has linked SLC31A1 to the development of CDDP resistance in
patients with OC (99). Various
mechanisms including epigenetic changes, protein expression and
post-translational modifications can influence drug resistance
(25). Specifically, the
transcriptional regulation of SLC31A1 in patients with
CDDP-resistant EOC has been studied (100). Researchers using a CRISPR
CAPTURE approach followed by mass spectrometry demonstrated that
the transcription factor, ZNF711, targets the SCL31A1
promoter and recruits the demethylase, JHDM2A, in OC cell lines.
This mechanism leads to increased activation of SLC31A1
transcription by removing the repressive transcriptional marker,
H3K9me2. Additionally, the downregulation of this transcription
factor has been linked to enhanced resistance to CDDP in patients
with EOC by suppressing SLC31A1 transcription (100).
SLC34A2
The sodium-dependent phosphate transporter type 2b
(NaPi2b; also known as SLC34A2 and NPT2) is a member of the SLC34
family, which also includes secondary transporters (such as NaPi2a
and NaPi2c). The SLC34A2 gene encodes a protein involved in
uptake control and in maintaining inorganic phosphate balance and
is typically expressed in tissues under physiological conditions.
However, upregulation of this protein has been observed in certain
tumors, such as OC, leading to toxic accumulation of intracellular
phosphate (101). Genome-scale
CRISPR/Cas9 loss-of-function analysis in human cancer cell lines
has revealed that inhibiting xenotropic and polytropic retrovirus
receptor 1 (XPR1)-dependent phosphate efflux in
SLC34A2-overexpressing cell lines can induce cancer cell death by
disrupting inorganic phosphate balance (102). Analysis of datasets has shown
high SLC34A2 expression in ovarian tumor tissues, which is
correlated with reduced life expectancy (103).
SLC39 family
The availability of Zn2+ in cells depends
on various physiological factors, including uptake and efflux
facilitated by specific transporters with different tissue
localizations. Changes in transporter expression and Zn
availability are considered to be linked to certain diseases and
can pose an additional risk factor for tumor development. The
transporter families, SLC39 (ZIP) and SLC30 (ZnT), are responsible
for the uptake and excretion of zinc ions, respectively. The
storage of this ion is regulated by metallothioneins. ZIP
transporters consist of four subfamilies with 14 different isoforms
(ZIP1-14), characterized by 8 highly conserved transmembrane
domains (104,105). ZnT transporters are divided
into four groups with 6 transmembrane helices and a conserved
zinc-binding site between helices II and V, where specific amino
acids play a crucial role in determining metal specificity
(105). Several studies have
demonstrated an aberrant expression of SLC39A4 (ZIP4) in various
types of tumors, including breast, pancreatic, ovarian carcinoma
and hepatocarcinoma (106-109). RNA-seq data analyses have
confirmed upregulation of ZIP4 in EOC tissues compared with
normal tissues. This zinc transporter, activated by the
lysophosphatic acid (LPA)/PPARγ axis, is upregulated in mice with
more aggressive EOC, leading to spheroid formation and promoting
cancer stem cell (CSC) activity and drug resistance to commonly
used drugs such as CDDP or doxorubicin (DOX) (110). In high-grade serous ovary
carcinoma, ZIP4 is upregulated compared with normal human tissues
(111). Upregulation of this
transporter mediates CSC-related cellular functions including
tumor-forming capacity, the ability to increase cancer
proliferation and invasion as well as conferring resistance to CDDP
and DOX. ZIP4 is particularly associated with increased expression
of CSC markers, such as aldehyde dehydrogenase 1 family member A1,
SOX9, OCT4 and NOTCH3 (108).
SLC39A13 (ZIP13) is involved in Zn release from the Golgi apparatus
and vesicles, and its dysfunction is correlated with connective
tissue disorders (104).
Dataset analysis has shown a significant correlation between ZIP13
expression and poor OS and progression-free Survival (PFS) in human
OC. Additionally, ZIP13 knockdown significantly reduced the
migratory and invasive abilities of OC cells in vitro
(112). In a metastasis model
using BALB/c nude mice, OC cells with depleted ZIP13 via
CRISPR/Cas9 technology, showed significantly decreased metastasis
both in terms of tumor number and size compared with the control
groups. This reduction in metastasis is considered to be due to the
inhibition of the Src/focal adhesion kinase (FAK) signaling pathway
(112).
SLC53A1
SLC53A1, also known as XPR1, is a gene
involved in the efflux of inorganic phosphate. XPR1 variants
determine the intracellular phosphate accumulation, leading to the
formation of calcium phosphate precipitates (102). Recent research has shown high
expression of XPR1 in OCCC cell lines. Experiments conducted in
vitro and in mouse xenograft models using small interfering
RNA-mediated knockdown of XPR1 in EOC cell lines have revealed its
significant role in cellular proliferation and tumorigenicity in OC
(113). Furthermore, as
aforementioned, XPR1 plays a role in controlling phosphate
homeostasis. Experiments in SLC34A2-overexpressing cell lines have
shown that the loss of the XPR1 phosphate exporter inhibits cancer
cell viability (102).
Therapeutic drugs and target genes
OC treatment options are determined by the stage of
the disease. A number of studies have aimed to understand how to
overcome drug resistance mechanisms (25,114). Various biological processes,
including epigenetic changes, modifications in plasma membrane
transport with drug accumulation and dysregulation of signaling
pathways can lead to chemotherapeutic drugs resistance in OC
(25). Recently, SLC
transporters have gained recognition for their role in maintaining
substrate availability and facilitating the influx or efflux of
drugs across plasma membrane. Increasing knowledge underscores the
importance of SLC transporters, such as SLC3A2 and the SLC7A
family, in anticancer drug resistance (Table II) (38,41,49,50,60,61).
 | Table IIPharmaceuticals targeting SLCs and
signaling pathways in ovarian cancer. |
Table II
Pharmaceuticals targeting SLCs and
signaling pathways in ovarian cancer.
| SLCs | Expression |
Pharmaceuticals | Targets | Effect | (Refs.) |
|---|
| SLC1A5 | ↑ | - | ↑p-mTOR | ↑Tumorigenesis | (33) |
| ↓ | - |
↑miR122-5p/↓circ_0072995 | ↓Tumorigenesis | (34) |
| ↓ | - |
↑miR370-3p/↓circ_0025033 | ↓Tumorigenesis | (35) |
| ↑ | - |
↑claudin-4/SLC7A5 | ↑Tumorigenesis | (36) |
| SLC3A2 | ↑ | CDDP | ↑ZEB1 | ↑Drug
sensitivity | (41) |
| ↑ | | ↑mTORC1 | ↑Cancer
proliferation | (38) |
| SLC7A1 | ↑ | CDDP | - | ↓Drug
sensitivity | (49) |
| SLC7A2 | ↓ | CDDP | ↑EMT markers | ↑Tumorigenesis | (50) |
| SLC7A5 | ↑ | Chemotherapy
drugs | ↑mTORC1 | ↑Tumorigenesis | (38,58) |
| ↑ | |
↑claudin4/SLC1A5 | ↑Tumorigenesis | (36) |
| ↑ | | ↑mTORC1 | ↑Cancer
proliferation | (38) |
| ↓ | | ↑mTOR | ↓Tumorigenesis | (60) |
| SLC7A6 | ↓ |
Cisplatin-baicalein |
↓CircSLC7A6/↑miR-26825p | ↓Tumor
growth/↑Apoptosis | (61) |
| SLC7A11 | ↓ | - | ↓Disulfide
bonds |
↓Disulfidptosis/↑Poor prognosis | (69) |
| ↓ | - | ↓STEAP3 via
p53 | ↓Tumor growth | (74) |
| ↓ | MESA | ↑NRF2
↓SLC7A11/GPX4 | ↑Ferroptosis | (79) |
| ↓ | PtQ | ↓SLC7A11/GPX4 | ↑Ferroptosis | (80) |
| SLC9A1 | ↑ | Zoniporide | - | ↓Cancer
proliferation | (46) |
| ↑ | HMA | - | ↓Cancer
proliferation | |
| SLC31A1 | ↑ | CDDP |
↑ZNF711/↑JHDM2A | ↑Drug
sensitivity | (100) |
| ↑ | BIX-01294 | ↓H3K9me2 | ↑Drug
sensitivity | |
| SLC34A2 | ↓ | LIFA | - | ↑PFS, ↑OS | (101) |
| ↓ | UpRi | - | ↑PFS, ↑OS | (101,105) |
| SLC39A4 | ↑ | CDDP-DOX | ↑LPA/PPARγ | ↑Tumorigenesis | (111) |
| ↑ | CDDP-DOX | ↑ALDH1, ↑SOX9,
↑OCT4, ↑NOTCH3 | ↑Tumorigenesis | (109) |
| SLC39A13 | ↓ | - | ↓Src/FAK | ↓Tumorigenesis | (113) |
Most chemotherapeutic drugs function by inducing
apoptotic processes in tumor cells. SLC7A11 is involved in various
molecular pathways that are key in treating drug resistance in
several tumors, including OC. A number of studies have suggested
that the involvement of SLC7A11 can restore sensitivity to drugs
and overcome chemoresistance to different antineoplastic molecules
(54). Recent studies have shown
that SLC7A11 can influence either cell proliferation or tumor
progression (79,80). Treatment with the morpholine
derivative, N-(4-morpholinomethylene) ethanesulfonamide (MESA), or
the quinoline derivative Pt(II)-based complex, PtQ, in OC cells
induced ferroptosis and inhibited the SLC7A11/glutathione
peroxidase 4 (GPX4) signaling pathway. Specifically, treatment with
MESA in OC cell lines led to cell death by increasing nuclear
factor erythroid 2-related factor 2 (NRF2) expression and affecting
ferroptosis-related signaling pathways (79,80).
Current advances in drugs design have introduced
new inhibitory molecules representing a valid approach for
regulating target genes. SLC9A1 plays a critical role in cancer
growth, and a previous study in OC cells have shown that SLC9A1
inhibitors (such as Zoniporide and 5-N,N-hexamethylene amiloride)
could support chemotherapeutic treatment to reduce proliferative
capacity (46). As
aforementioned, drug delivery experiments have shown that
suppressing SLC31A1 expression impaired CDDP resistance in patients
with EOC. Treatment with BIX-01294 (a diazepin-quinazolinamine
derivative), a histone methyltransferase inhibitor, has been shown
to increase the sensitivity of EOC cells to CDDP by removing the
repressive transcriptional effects of SLC31A1 mediated by ZNF711
transcription factor and the demethylase, JHDM2A (100).
Targeted therapies have revolutionized the
landscape of OC using highly selective monoclonal antibodies, and
studies with a specific antibody-drug conjugates (ADCs) are
underway (115).
SLC34A2-targeting ADCs, LIFA (lifastuzumab vedotin) or UpRi
(Upifitamab rilsodotin), have been used as a treatment for
gynecological tumors. This approach combines the tumor-targeting
ability of monoclonal antibodies with chemotherapy agents.
Promising trials are underway in OC to improve health-related
quality of life and treatment efficacy, particularly in terms of
PFS, OS and other measures (101,116).
Altered expression of Zn transporters and their
availability have been linked to various solid tumors and represent
an additional risk factor for disease progression (104,105). Research has shown that
upregulation of SLC39A4 is activated by the LPA/PPARγ axis,
inducing resistance to drugs such as CDDP or DOX (110). SLC39A13 knockdown reduces
migratory and invasive abilities of OC cells. In a BALB/c nude mice
model injected with ZIP13-depleted OC cells, significantly
decreased tumorigenesis through inhibition of the Src/FAK signaling
pathway was observed (112).
Table II reports the expression
of known SLCs in OC correlated to target genes associated with
cancer proliferation, survival and resistance to chemotherapy.
Therefore, studying the different ways in which SLC transporters
impact cancer cells and assessing the activation or inhibition of
signaling pathways could be a crucial step in expediting the
development of drugs to treat OC.
Conclusions
The present review discussed the involvement of SLC
transporter proteins in OC and summarized the existing evidence
regarding their role. While a number of SLCs are extensively
studied in various types of cancer, their role in OC has not yet
been fully explored. Various SLCs play a crucial role in tumor
cells by supporting rapid growth and modifying the cellular
microenvironment. Recent bioinformatics analysis of ovarian tumor
tissues has revealed different expression levels of SLCs,
highlighting their involvement in cancer progression and modifying
drug sensitivity. The heterogeneity of SLC expression in various
diseases and tumors including OC, and their dysregulation is also
associated with tumor progression. However, as aforementioned,
numerous SLCs in OC are still uncharacterized and poorly
understood, leading to limited options for improving cancer cell
response to chemotherapy drugs. Furthermore, early-stage OC
diagnosis requires the identification of new potential biomarkers
to predict response to chemotherapy drugs and improve the OC
prognosis. Moreover, it is important to consider the long-term
impact on the quality of life, as it may influence therapeutic
treatment.
New strategies targeting SLCs through innovative
immunotherapy may increase the therapeutic opportunities and
improve the response to chemotherapeutic drugs for treating OC. In
the last decade, multi-omics data analysis has provided valuable
information that can support the understanding of clinical aspects
(such as PFS and OS) and the expression of SLCs. Therefore, more
focused studies are needed to identify a subset of genes, including
SLC transporters, that are prognostically relevant. This is crucial
to bridge the information gap between the dysregulation of
molecular pathways, immunotherapy response and drug resistance
linked to poor outcomes in OC.
Availability of data and materials
Not applicable.
Authors' contributions
BQ, MCF and MM conceived and designed the review.
SS was responsible for acquisition and interpretation of the data.
BQ, SS, MCF and MM drafted and edited the manuscript for
publication and reviewed the literature. All authors read and
approved the final version of the manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Barbara Quaresima: https://orcid.org/0000-0003-3462-624x; Stefania
Scicchitano: https://orcid.org/0000-0002-3566-7214; Maria Concetta
Faniello: https://orcid.org/0000-0001-6938-2754; Maria
Mesuraca: https://orcid.org/0000-0002-5455-168X.
Acknowledgements
Not applicable.
Funding
This work was supported by the Next Generation EU - Italian
NRRP, Mission 4, Component 2, Investment 1.5, call for the creation
and strengthening of 'Innovation Ecosystems', building 'Territorial
R&D Leaders' (Directorial Decree n. 2021/3277) - project
Tech4You - Technologies for climate change adaptation and quality
of life improvement (project no. ECS0000009).
References
|
1
|
César-Razquin A, Snijder B,
Frappier-Brinton T, Isserlin R, Gyimesi G, Bai X, Reithmeier RA,
Hepworth D, Hediger MA, Edwards AM and Superti-Furga G: A call for
systematic research on solute carriers. Cell. 162:478–87. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hediger MA, Romero MF, Peng JB, Rolfs A,
Takanaga H and Bruford EA: The ABCs of solute carriers:
Physiological, pathological and therapeutic implications of human
membrane transport proteinsIntroduction. Pflugers Arch.
447:465–468. 2004. View Article : Google Scholar
|
|
3
|
Xia R, Peng HF, Zhang X and Zhang HS:
Comprehensive review of amino acid transporters as therapeutic
targets. Int J Biol Macromol. 260(Pt 2): 1296462024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nwosu ZC, Song MG, di Magliano MP,
Lyssiotis CA and Kim SE: Nutrient transporters: connecting cancer
metabolism to therapeutic opportunities. Oncogene. 42:711–724.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: the metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin L, Yee SW, Kim RB and Giacomini KM:
SLC transporters as therapeutic targets: emerging opportunities.
Nat Rev Drug Discov. 14:543–560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schlessinger A, Zatorski N, Hutchinson K
and Colas C: Targeting SLC transporters: Small molecules as
modulators and therapeutic opportunities. Trends Biochem Sci.
48:801–814. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dvorak V and Superti-Furga G: Structural
and functional annotation of solute carrier transporters:
Implication for drug discovery. Expert Opin Drug Discov.
18:1099–1115. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie T, Chi X, Huang B, Ye F, Zhou Q and
Huang J: Rational exploration of fold atlas for human solute
carrier proteins. Structure. 30:1321–1330.e5. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perland E and Fredriksson R:
Classification systems of secondary active transporters. Trends
Pharmacol Sci. 38:305–315. 2017. View Article : Google Scholar
|
|
11
|
Nishimura M and Naito S: Tissue-specific
mRNA expression profiles of human solute carrier transporter
superfamilies. Drug Metab Pharmacokinet. 23:22–44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morioka S, Perry JSA, Raymond MH, Medina
CB, Zhu Y, Zhao L, Serbulea V, Onengut-Gumuscu S, Leitinger N,
Kucenas S, et al: Efferocytosis induces a novel SLC program to
promote glucose uptake and lactate release. Nature. 563:714–718.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Hagan S, Wright Muelas M, Day PJ,
Lundberg E and Kell DB: GeneGini: Assessment via the Gini
Coefficient of Reference 'Housekeeping' genes and diverse human
transporter expression profiles. Cell Syst. 6:230–244.e1. 2018.
View Article : Google Scholar
|
|
14
|
Li J, Li J and Jiang W: Effects of
different surgical extents on prognosis of patients with malignant
ovarian sex cord-stromal tumors: A retrospective cohort study. Sci
Rep. 14:226302024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kostov S, Watrowski R, Kornovski Y,
Dzhenkov D, Slavchev S, Ivanova Y and Yordanov A: Hereditary
gynecologic cancer syndromes-A narrative review. Onco Targets Ther.
15:381–405. 2022. View Article : Google Scholar :
|
|
16
|
González-Martín A, Harter P, Leary A,
Lorusso D, Miller RE, Pothuri B, Ray-Coquard I, Tan DSP, Bellet E,
Oaknin A, et al: Newly diagnosed and relapsed epithelial ovarian
cancer: ESMO Clinical Practice Guideline for diagnosis, treatment
and follow-up. Ann Oncol. 34:833–848. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Quaresima B, Romeo F, Faniello MC, Di
Sanzo M, Liu CG, Lavecchia A, Taccioli C, Gaudio E, Baudi F,
Trapasso F, et al: BRCA1 5083del19 mutant allele selectively
up-regulates periostin expression in vitro and in vivo. Clin Cancer
Res. 14:6797–6803. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Crugliano T, Quaresima B, Gaspari M,
Faniello MC, Romeo F, Baudi F, Cuda G, Costanzo F and Venuta S:
Specific changes in the proteomic pattern produced by the
BRCA1-Ser1841Asn missense mutation. Int J Biochem Cell Biol.
39:220–226. 2007. View Article : Google Scholar
|
|
19
|
Wei X, Sun L, Slade E, Fierheller CT,
Oxley S, Kalra A, Sia J, Sideris M, McCluggage WG, Bromham N, et
al: Cost-Effectiveness of Gene-Specific Prevention Strategies for
Ovarian and Breast Cancer. JAMA Netw Open. 7:e23553242024.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanson H, Kulkarni A, Loong L, Kavanaugh
G, Torr B, Allen S, Ahmed M, Antoniou AC, Cleaver R, Dabir T, et
al: UK consensus recommendations for clinical management of cancer
risk for women with germline pathogenic variants in cancer
predisposition genes: RAD51C, RAD51D, BRIP1 and PALB2. J Med Genet.
60:417–429. 2023. View Article : Google Scholar
|
|
21
|
Scicchitano S, Faniello MC and Mesuraca M:
Zinc Finger 521 Modulates the Nrf2-notch signaling pathway in human
ovarian carcinoma. Int J Mol Sci. 24:147552023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scicchitano S, Montalcini Y, Lucchino V,
Melocchi V, Gigantino V, Chiarella E, Bianchi F, Weisz A and
Mesuraca M: Enhanced ZNF521 expression induces an aggressive
phenotype in human ovarian carcinoma cell lines. PLoS One.
17:e02747852022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Y, He Z, Yang S, Chen C, Xiong W, He
Y and Liu S: RUNX1 knockdown induced apoptosis and impaired EMT in
high-grade serous ovarian cancer cells. J Transl Med. 21:8862023.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Collet L, Hanvic B, Turinetto M, Treilleux
I, Chopin N, Le Saux O and Ray-Coquard I: BRCA1/2 alterations and
reversion mutations in the area of PARP inhibitors in high grade
ovarian cancer: State of the art and forthcoming challenges. Front
Oncol. 14:13544272024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Wang X, Zhu X, Zhong L, Jiang Q,
Wang Y, Tang Q, Li Q, Zhang C, Wang H and Zou D: Drug resistance in
ovarian cancer: From mechanism to clinical trial. Mol Cancer.
23:662024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marjamaa A, Gibbs B, Kotrba C and Masamha
CP: The role and impact of alternative polyadenylation and miRNA
regulation on the expression of the multidrug resistance-associated
protein 1 (MRP-1/ABCC1) in epithelial ovarian cancer. Sci Rep.
13:174762023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elsnerova K, Bartakova A, Tihlarik J,
Bouda J, Rob L, Skapa P, Hruda M, Gut I, Mohelnikova-Duchonova B,
Soucek P and Vaclavikova R: Gene expression profiling reveals novel
candidate markers of ovarian carcinoma intraperitoneal metastasis.
J Cancer. 8:3598–3606. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Teng QX, Lei ZN, Wang JQ, Yang Y, Wu ZX,
Acharekar ND, Zhang W, Yoganathan S, Pan Y, Wurpel J, et al:
Overexpression of ABCC1 and ABCG2 confers resistance to talazoparib
a poly (ADP-Ribose) polymerase inhibitor. Drug Resist Updat.
73:1010282024. View Article : Google Scholar
|
|
29
|
Sniegowski T, Korac K, Bhutia YD and
Ganapathy V: SLC6A14 and SLC38A5 Drive the Glutaminolysis and
Serine-Glycine-One-Carbon Pathways in Cancer. Pharmaceuticals.
14:2162021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chiarella E, Aloisio A, Scicchitano S,
Todoerti K, Cosentino EG, Lico D, Neri A, Amodio N, Bond HM and
Mesuraca M: ZNF521 Enhances MLL-AF9-Dependent hematopoietic stem
cell transformation in acute myeloid leukemias by altering the gene
expression landscape. Int J Mol Sci. 22:108142021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bharadwaj R, Jaiswal S, Velarde de la Cruz
EE and Thakare RP: Targeting solute carrier transporters (SLCs) as
a therapeutic target in different cancers. Diseases. 12:632024.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kaira K, Nakamura K, Hirakawa T, Imai H,
Tominaga H, Oriuchi N, Nagamori S, Kanai Y, Tsukamoto N, Oyama T,
et al: Prognostic significance of L-type amino acid transporter 1
(LAT1) expression in patients with ovarian tumors. Am J Transl Res.
7:1161–1171. 2015.PubMed/NCBI
|
|
33
|
Guo H, Xu Y, Wang F, Shen Z, Tuo X, Qian
H, Wang H and Wang K: Clinical associations between ASCT2 and
p-mTOR in the pathogenesis and prognosis of epithelial ovarian
cancer. Oncol Rep. 40:3725–3733. 2018.PubMed/NCBI
|
|
34
|
Huang X, Luo Y and Li X: Circ_0072995
promotes ovarian cancer progression through regulating
miR-122-5p/SLC1A5 Axis. Biochem Genet. 60:153–172. 2022. View Article : Google Scholar
|
|
35
|
Ma H, Qu S, Zhai Y and Yang X:
circ_0025033 promotes ovarian cancer development via regulating the
hsa_miR-370-3p/SLC1A5 axis. Cell Mol Biol Lett. 27:942022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Villagomez FR, Lang J, Rosario FJ,
Nunez-Avellaneda D, Webb P, Neville M, Woodruff ER and Bitler BG:
Claudin-4 Modulates Autophagy via SLC1A5/LAT1 as a Mechanism to
Regulate Micronuclei. Cancer Res Commun. 4:1625–1642. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang C, Shafaq-Zadah M, Pawling J,
Hesketh GG, Dransart E, Pacholczyk K, Longo J, Gingras AC, Penn LZ,
Johannes L and Dennis JW: SLC3A2 N-glycosylation and Golgi
remodeling regulate SLC7A amino acid exchangers and stress
mitigation. J Biol Chem. 299:1054162023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Milkereit R, Persaud A, Vanoaica L, Guetg
A, Verrey F and Rotin D: LAPTM4b recruits the LAT1-4F2hc Leu
transporter to lysosomes and promotes mTORC1 activation. Nat
Commun. 6:72502015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park E, Kim H, Yoon S and Jang B: The role
of CD98 heavy chain in cancer development. Histol Histopathol.
16:187492024.
|
|
40
|
He J, Liu D, Liu M, Tang R and Zhang D:
Characterizing the role of SLC3A2 in the molecular landscape and
immune microenvironment across human tumors. Front Mol Biosci.
9:9614102022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cui Y, Qin L, Tian D, Wang T, Fan L, Zhang
P and Wang Z: ZEB1 Promotes Chemoresistance to Cisplatin in Ovarian
Cancer Cells by Suppressing SLC3A2. Chemotherapy. 63:262–271. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou XY, Li JY, Tan JT, HuangLi YL, Nie XC
and Xia P: Clinical significance of the CD98hc-CD147 complex in
ovarian cancer: A bioinformatics analysis. J Obstet Gynaecol.
43:21880852023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qin L, Li T and Liu Y: High SLC4A11
expression is an independent predictor for poor overall survival in
grade 3/4 serous ovarian cancer. PLoS One. 12:e01873852017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Estrella V, Chen T, Lloyd M, Wojtkowiak J,
Cornnell HH, Ibrahim-Hashim A, Bailey K, Balagurunathan Y, Rothberg
JM, Sloane BF, et al: Acidity generated by the tumor
microenvironment drives local invasion. Cancer Res. 73:1524–1535.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gatenby RA, Gawlinski ET, Gmitro AF,
Kaylor B and Gillies RJ: Acid-mediated tumor invasion: A
multidisciplinary study. Cancer Res. 66:5216–5223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sanhueza C, Araos J, Naranjo L, Toledo F,
Beltrán AR, Ramírez MA, Gutiérrez J, Pardo F, Leiva A and Sobrevia
L: Sodium/proton exchanger isoform 1 regulates intracellular pH and
cell proliferation in human ovarian cancer. Biochim Biophys Acta
Mol Basis Dis. 1863:81–91. 2017. View Article : Google Scholar
|
|
47
|
Gong W, Chen Y and Zhang Y: Prognostic and
clinical significance of Solute Carrier Family 7 Member 1 in
ovarian cancer. Transl Cancer Res. 10:602–612. 2021. View Article : Google Scholar
|
|
48
|
Hushmandi K, Einollahi B, Saadat SH, Lee
EHC, Farani MR, Okina E, Huh YS, Nabavi N, Salimimoghadam S and
Kumar AP: Amino acid transporters within the solute carrier
superfamily: Underappreciated proteins and novel opportunities for
cancer therapy. Mol Metab. 84:1019522024. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
You S, Zhu X, Yang Y, Du X, Song K, Zheng
Q, Zeng P and Yao Q: SLC7A1 overexpression is involved in energy
metabolism reprogramming to induce tumor progression in epithelial
ovarian cancer and is associated with immune-infiltrating cells. J
Oncol. 2022:58648262022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sun T, Bi F, Liu Z and Yang Q: SLC7A2
serves as a potential biomarker and therapeutic target for ovarian
cancer. Aging (Albany NY). 12:13281–13296. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jeckelmann JM, Zaugg J, Morozova V, Müller
J, Kantipudi S, Schroeder M, Graff J, Albrecht C, Altmann KH,
Gertsch J and Fotiadis D: Structure, Function and Pharmacology of
SLC7 Family Members and Homologues. Chimia (Aarau). 76:1011–1018.
2022. View Article : Google Scholar
|
|
52
|
Jiang S, Zou J, Dong J, Shi H, Chen J, Li
Y, Duan X and Li W: Lower SLC7A2 expression is associated with
enhanced multidrug resistance, less immune infiltrates and worse
prognosis of NSCLC. Cell Commun Signal. 21:92023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu X, Shen S, Qin J, Fei W, Fan F, Gu J,
Shen T, Zhang T and Cheng X: High co-expression of SLC7A11 and GPX4
as a predictor of platinum resistance and poor prognosis in
patients with epithelial ovarian cancer. BJOG. 129(Suppl 2):
S40–S49. 2022. View Article : Google Scholar
|
|
54
|
Fantone S, Piani F, Olivieri F, Rippo MR,
Sirico A, Di Simone N, Marzioni D and Tossetta G: Role of
SLC7A11/xCT in Ovarian Cancer. Int J Mol Sci. 25:5872024.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang Y, Dong K, Jia X, Du S, Wang D, Wang
L, Qu H, Zhu S, Wang Y, Wang Z, et al: A novel extrachromosomal
circular DNA related genes signature for overall survival
prediction in patients with ovarian cancer. BMC Med Genomics.
16:1402023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fan X, Ross DD, Arakawa H, Ganapathy V,
Tamai I and Nakanishi T: Impact of system L amino acid transporter
1 (LAT1) on proliferation of human ovarian cancer cells: A possible
target for combination therapy with anti-proliferative
aminopeptidase inhibitors. Biochem Pharmacol. 80:811–818. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kaji M, Kabir-Salmani M, Anzai N, Jin CJ,
Akimoto Y, Horita A, Sakamoto A, Kanai Y, Sakurai H and Iwashita M:
Properties of L-type amino acid transporter 1 in epidermal ovarian
cancer. Int J Gynecol Cancer. 20:329–336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sato K, Miyamoto M, Takano M, Furuya K and
Tsuda H: Significant relationship between the LAT1 expression
pattern and chemoresistance in ovarian clear cell carcinoma.
Virchows Arch. 474:701–710. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Baczewska M, Supruniuk E, Bojczuk K, Guzik
P, Milewska P, Konończuk K, Dobroch J, Chabowski A and Knapp P:
Energy substrate transporters in high-grade ovarian cancer: Gene
expression and clinical implications. Int J Mol Sci. 23:89682022.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sekine M, Koh I, Nakamoto K, Nosaka S,
Tomono K, Sugimoto J and Kudo Y: Selective inhibition of L-type
amino acid transporter 1 suppresses cell proliferation in ovarian
clear cell carcinoma. Anticancer Res. 43:2509–2517. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li S, Yi Z, Li M and Zhu Z: Baicalein
improves the chemoresistance of ovarian cancer through regulation
of CirSLC7A6. J Ovarian Res. 16:2122023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang X, Chen Y, Wang X, Tian H, Wang Y,
Jin J, Shan Z, Liu Y, Cai Z, Tong X, et al: Stem Cell Factor SOX2
Confers Ferroptosis Resistance in Lung Cancer via Upregulation of
SLC7A11. Cancer Res. 81:5217–5229. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang J, Zhou Y, Xie S, Wang J, Li Z, Chen
L, Mao M, Chen C, Huang A, Chen Y, et al: Metformin induces
Ferroptosis by inhibiting UFMylation of SLC7A11 in breast cancer. J
Exp Clin Cancer Res. 40:2062021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cao N, Zhang F, Yin J, Zhang J, Bian X,
Zheng G, Li N, Lin Y and Luo L: LPCAT2 inhibits colorectal cancer
progression via the PRMT1/SLC7A11 axis. Oncogene. 43:1714–1725.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lee J and Roh JL: SLC7A11 as a gateway of
metabolic perturbation and ferroptosis vulnerability in cancer.
Antioxidants (Basel). 11:24442022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Škubník J, Svobodová Pavlíčková V, Ruml T
and Rimpelová S: Autophagy in cancer resistance to paclitaxel:
Development of combination strategies. Biomed Pharmacother.
161:1144582023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Jyotsana N, Ta KT and DelGiorno KE: The
Role of Cystine/Glutamate Antiporter SLC7A11/xCT in the
Pathophysiology of Cancer. Front Oncol. 12:8584622022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yang J, Wang C, Cheng S, Zhang Y, Jin Y,
Zhang N and Wang Y: Construction and validation of a novel
ferroptosis-related signature for evaluating prognosis and immune
microenvironment in ovarian cancer. Front Genet. 13:10944742023.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cong Y, Cai G, Ding C, Zhang H, Chen J,
Luo S and Liu J: Disulfidptosis-related signature elucidates the
prognostic, immunologic, and therapeutic characteristics in ovarian
cancer. Front Genet. 15:13789072024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yin F, Yi S, Wei L, Zhao B, Li J, Cai X,
Dong C and Liu X: Microarray-based identification of genes
associated with prognosis and drug resistance in ovarian cancer. J
Cell Biochem. 120:6057–6070. 2019. View Article : Google Scholar
|
|
71
|
Ke Y, Chen X, Su Y, Chen C, Lei S, Xia L,
Wei D, Zhang H, Dong C, Liu X and Yin F: Low Expression of SLC7A11
Confers Drug Resistance and Worse Survival in Ovarian Cancer via
Inhibition of Cell Autophagy as a Competing Endogenous RNA. Front
Oncol. 11:7449402021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang X, Zheng X, Ying X, Xie W, Yin Y and
Wang X: CEBPG suppresses ferroptosis through transcriptional
control of SLC7A11 in ovarian cancer. J Transl Med. 21:3342023.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ogiwara H, Takahashi K, Sasaki M, Kuroda
T, Yoshida H, Watanabe R, Maruyama A, Makinoshima H, Chiwaki F,
Sasaki H, et al: Targeting the Vulnerability of Glutathione
Metabolism in ARID1A-Deficient Cancers. Cancer Cell. 35:177–190.e8.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Han Y, Fu L, Kong Y, Jiang C, Huang L and
Zhang H: STEAP3 Affects Ovarian Cancer Progression by Regulating
Ferroptosis through the p53/SLC7A11 Pathway. Mediators Inflamm.
2024:40485272024. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jin Y, Chen L, Li L, Huang G, Huang H and
Tang C: SNAI2 promotes the development of ovarian cancer through
regulating ferroptosis. Bioengineered. 13:6451–6463. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang Y, Wang S and Zhang W: HRD1 functions
as a tumor suppressor in ovarian cancer by facilitating
ubiquitination-dependent SLC7A11 degradation. Cell Cycle.
22:1116–1126. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chiarella E, Aloisio A, Scicchitano S,
Bond HM and Mesuraca M: Regulatory Role of microRNAs Targeting the
Transcription Co-Factor ZNF521 in Normal Tissues and Cancers. Int J
Mol Sci. 22:84612021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Cai L, Hu X, Ye L, Bai P, Jie Y and Shu K:
Long non-coding RNA ADAMTS9-AS1 attenuates ferroptosis by Targeting
microRNA-587/solute carrier family 7 member 11 axis in epithelial
ovarian cancer. Bioengineered. 13:8226–8239. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Sun B, Zhang L, Wu B and Luo X: A
Morpholine Derivative N-(4-Morpholinomethylene) ethanesulfonamide
induces ferroptosis in tumor cells by targeting NRF2. Biol Pharm
Bull. 47:417–426. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Shen X, Peng Y, Zhou H, Ye X, Han Z and
Shi X: A Pt(II) complex bearing N-heterocycle ring induced
ferroptotic cell death in ovarian cancer. J Inorg Biochem.
253:1125022024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang W, Liu T, Jiang L, Chen J, Li Q and
Wang J: Immunogenic cell death-related gene landscape predicts the
overall survival and immune infiltration status of ovarian cancer.
Front Genet. 13:10012392022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Szadvari I, Hudecova S, Chovancova B,
Matuskova M, Cholujova D, Lencesova L, Valerian D, Ondrias K,
Babula P and Krizanova O: Sodium/calcium exchanger is involved in
apoptosis induced by H2S in tumor cells through
decreased levels of intracellular pH. Nitric Oxide. 87:1–9. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang H, Long X, Wang D, Lou M, Zou D, Chen
R, Nian W and Zhou Q: Increased expression of Na+/H+ exchanger
isoform 1 predicts tumor aggressiveness and unfavorable prognosis
in epithelial ovarian cancer. Oncol Lett. 16:6713–6720.
2018.PubMed/NCBI
|
|
84
|
Yuan S, He SH, Li LY, Xi S, Weng H, Zhang
JH, Wang DQ, Guo MM, Zhang H, Wang SY, et al: A potassium-chloride
co-transporter promotes tumor progression and castration resistance
of prostate cancer through m6A reader YTHDC1. Cell Death Dis.
14:72023. View Article : Google Scholar :
|
|
85
|
Liu JY, Dai YB, Li X, Cao K, Xie D, Tong
ZT, Long Z, Xiao H, Chen MK, Ye YL, et al: Solute carrier family 12
member 5 promotes tumor invasion/metastasis of bladder urothelial
carcinoma by enhancing NF-κB/MMP-7 signaling pathway. Cell Death
Dis Mar. 8:e26912017. View Article : Google Scholar
|
|
86
|
Tong Q, Qin W, Li ZH, Liu C, Wang ZC, Chu
Y and Xu XD: SLC12A5 promotes hepatocellular carcinoma growth and
ferroptosis resistance by inducing ER stress and cystine transport
changes. Cancer Med. 12:8526–8541. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Xu L, Li X, Cai M, Chen J, Li X, Wu WK,
Kang W, Tong J, To KF, Guan XY, et al: Increased expression of
Solute carrier family 12 member 5 via gene amplification
contributes to tumour progression and metastasis and associates
with poor survival in colorectal cancer. Gut. 65:635–646. 2016.
View Article : Google Scholar
|
|
88
|
Jiang Y, Liao HL and Chen LY: A Pan-Cancer
Analysis of SLC12A5 Reveals Its Correlations with Tumor Immunity.
Dis Markers. 2021:30626062021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yang GP, He WP, Tan JF, Yang ZX, Fan RR,
Ma NF, Wang FW, Chen L, Li Y, Shen HW, et al: Overexpression of
SLC12A5 is associated with tumor progression and poor survival in
ovarian carcinoma. Int J Gynecol Cancer. 29:1280–1284. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Fisel P, Schaeffeler E and Schwab M:
Clinical and functional relevance of the Monocarboxylate
transporter family in disease pathophysiology and drug therapy.
Clin Transl Sci. 11:352–364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Hanahan D: Hallmarks of Cancer: New
Dimensions. Cancer Discov. 12:31–46. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Navarro C, Ortega Á, Santeliz R, Garrido
B, Chacín M, Galban N, Vera I, De Sanctis JB and Bermúdez V:
Metabolic Reprogramming in Cancer Cells: Emerging molecular
mechanisms and novel therapeutic approaches. pharmaceutics.
14:13032022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Latif A, Chadwick AL, Kitson SJ, Gregson
HJ, Sivalingam VN, Bolton J, McVey RJ, Roberts SA, Marshall KM,
Williams KJ, et al: Monocarboxylate transporter 1 (MCT1) is an
independent prognostic biomarker in endometrial cancer. BMC Clin
Pathol. 17:272017. View Article : Google Scholar
|
|
94
|
Sohrabi E, Moslemi M, Rezaie E, Nafissi N,
Khaledi M, Afkhami H, Fathi J and Zekri A: The tissue expression of
MCT3, MCT8, and MCT9 genes in women with breast cancer. Genes
Genomics. 43:1065–1077. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chatterjee P, Bhowmik D and Roy SS: A
systemic analysis of monocarboxylate transporters in ovarian cancer
and possible therapeutic interventions. Channels (Austin).
17:22730082023. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Cheng L, Lu W, Kulkarni B, Pejovic T, Yan
X, Chiang JH, Hood L, Odunsi K and Lin B: Analysis of chemotherapy
response programs in ovarian cancers by the next-generation
sequencing technologies. Gynecol Oncol. 11:159–169. 2010.
View Article : Google Scholar
|
|
97
|
Januchowski R, Zawierucha P, Ruciński M,
Andrzejewska M, Wojtowicz K, Nowicki M and Zabel M: Drug
transporter expression profiling in chemoresistant variants of the
A2780 ovarian cancer cell line. Biomed Pharmacother. 68:447–453.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Lee J, Peña MM, Nose Y and Thiele DJ:
Biochemical characterization of the human copper transporter Ctr1.
J Biol Chem. 277:4380–4387. 2002. View Article : Google Scholar
|
|
99
|
Puris E, Fricker G and Gynther M: The role
of solute carrier transporters in efficient anticancer drug
delivery and therapy. Pharmaceutics. 15:3642023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wu G, Peng H, Tang M, Yang M, Wang J, Hu
Y, Li Z, Li J, Li Z and Song L: ZNF711 down-regulation promotes
CISPLATIN resistance in epithelial ovarian cancer via interacting
with JHDM2A and suppressing SLC31A1 expression. EBioMedicine.
71:1035582021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Banerjee S, Drapkin R, Richardson DL and
Birrer M: Targeting NaPi2b in ovarian cancer. Cancer Treat Rev.
112:1024892023. View Article : Google Scholar
|
|
102
|
Bondeson DP, Paolella BR, Asfaw A,
Rothberg MV, Skipper TA, Langan C, Mesa G, Gonzalez A, Surface LE,
Ito K, et al: Phosphate dysregulation via the XPR1-KIDINS220
protein complex is a therapeutic vulnerability in ovarian cancer.
Nat Cancer. 3:681–695. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Vlasenkova R, Nurgalieva A, Akberova N,
Bogdanov M and Kiyamova R: Characterization of SLC34A2 as a
potential prognostic marker of oncological diseases. Biomolecules.
11:18782021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Stiles LI, Ferrao K and Mehta KJ: Role of
zinc in health and disease. Clin Exp Med. 24:382024. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Chen B, Yu P, Chan WN, Xie F, Zhang Y,
Liang L, Leung KT, Lo KW, Yu J, Tse GMK, et al: Cellular zinc
metabolism and zinc signaling: from biological functions to
diseases and therapeutic targets. Signal Transduct Target Ther.
9:62024. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Vogel-González M, Musa-Afaneh D, Rivera
Gil P and Vicente R: Zinc Favors Triple-negative breast cancer's
microenvironment modulation and cell plasticity. Int J Mol Sci.
22:91882021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Liu M, Yang J, Zhang Y, Zhou Z, Cui X,
Zhang L, Fung KM, Zheng W, Allard FD, Yee EU, et al: ZIP4 Promotes
Pancreatic Cancer Progression by Repressing ZO-1 and Claudin-1
through a ZEB1-Dependent Transcriptional Mechanism. Clin Cancer
Res. 24:3186–3196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Fan Q, Zhang W, Emerson RE and Xu Y: ZIP4
is a novel cancer stem cell marker in high-grade serous ovarian
cancer. Cancers (Basel). 12:36922020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Scheiter A, Evert K, Reibenspies L,
Cigliano A, Annweiler K, Müller K, Pöhmerer LM, Xu H, Cui G, Itzel
T, et al: RASSF1A independence and early galectin-1 upregulation in
PIK3CA-induced hepatocarcinogenesis: New therapeutic venues. Mol
Oncol. 16:1091–1118. 2022. View Article : Google Scholar
|
|
110
|
Fan Q, Cai Q, Li P, Wang W, Wang J, Gerry
E, Wang TL, Shih IM, Nephew KP and Xu Y: The novel ZIP4 regulation
and its role in ovarian cancer. Oncotarget. 8:90090–90107. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Cai Q, Fan Q, Buechlein A, Miller D,
Nephew KP, Liu S, Wan J and Xu Y: Changes in mRNA/protein
expression and signaling pathways in in vivo passaged mouse ovarian
cancer cells. PLoS One. 13:e01974042018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Cheng X, Wang J, Liu C, Jiang T, Yang N,
Liu D, Zhao H and Xu Z: Zinc transporter SLC39A13/ZIP13 facilitates
the metastasis of human ovarian cancer cells via activating Src/FAK
signaling pathway. J Exp Clin Cancer Res. 40:1992021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Akasu-Nagayoshi Y, Hayashi T, Kawabata A,
Shimizu N, Yamada A, Yokota N, Nakato R, Shirahige K, Okamoto A and
Akiyama T: PHOSPHATE exporter XPR1/SLC53A1 is required for the
tumorigenicity of epithelial ovarian cancer. Cancer Sci.
113:2034–2043. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chandra A, Pius C, Nabeel M, Nair M,
Vishwanatha JK, Ahmad S and Basha R: Ovarian cancer: Current status
and strategies for improving therapeutic outcomes. Cancer Med.
8:7018–7031. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Sato S, Shoji T, Jo A, Otsuka H, Abe M,
Tatsuki S, Chiba Y, Takatori E, Kaido Y, Nagasawa T, et al:
Antibody-Drug Conjugates: The new treatment approaches for ovarian
cancer. Cancers (Basel). 16:25452024. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Karpel HC, Powell SS and Pothuri B:
Antibody-Drug Conjugates in Gynecologic Cancer. Am Soc Clin Oncol
Educ Book. 43:e3907722023. View Article : Google Scholar : PubMed/NCBI
|















