Introduction
Due to improved living standards, life pattern
changes and the aging of the society, the incidence of diabetes
mellitus (DM) continues to escalate annually (1). On the basis of the International
Diabetes Federation Global Diabetes Map 2021, there are currently
537 million patients with diabetes (range, 20-79 years old)
worldwide, accounting for 10.5% of the total global population
(2). It has been reported that
20-40% of individuals with diabetes develop diabetic nephropathy
(DN), a microvascular complication of DM (3). Although previous studies indicated
that renin-angiotensin system blockers could delay the onset of DN,
they failed to halt the progression of the end-stage renal disease
(ESRD). Therefore, emphasis should be placed on the early detection
and interventions for DN. Additionally, elucidating the early
mechanism of DN development for preventing and delaying disease
progression is of great importance.
Glomerular podocyte loss is recognized as a critical
element in the development of proteinuria, glomerulosclerosis and
deterioration of renal function in DN (4). Mitochondria play a crucial role in
cellular energy production and several cellular processes,
including antioxidant defense, calcium homeostasis and apoptosis
(5). Most importantly, oxidative
phosphorylation (OXPHOS) in mitochondria is associated with the
synthesis of adenosine triphosphate (ATP), thus providing energy
for different cellular functions (6,7).
Mitochondria are organelles that are constantly undergoing dynamic
changes. Therefore, maintaining the normal mitochondrial morphology
to maximize ATP production is of great significance. Dynamic
changes in fission, fusion and autophagy substantially depend on
mitochondrial structure and morphology, which subsequently govern
mitochondrial dynamics (8). A
previous study demonstrated that OXPHOS was increased in
mitochondrial fusion and decreased in mitochondrial fission
(9). Additionally, enhanced
fission and reduced fusion, causing podocyte mitochondrial
breakage, were significantly associated with the development of DN
(10). Therefore, maintaining
dynamic mitochondrial homeostasis in podocytes could be an
effective therapeutic strategy for DN (3).
Over the past few years, the application of
Traditional Chinese Medicine (TCM) in the field of medical science
has been gradually recognized by both domestic and foreign
counterparts (11). Emerging
evidence has supported the unique advantages of TCM in preventing
DM progression (12,13). Therefore, it is meaningful to
investigate the effective constituents of TCM for managing DM. A
large randomized clinical trial (FOCUS) revealed that Jinlida
granules (JLD) reduced the risk of diabetes in subjects with
metabolic abnormalities (14).
JLD is composed of 17 Chinese herbs, including Ginseng,
Polygonum multiflorum, Salvia miltiorrhiza,
Sophora flavescens, Rehmannia glutinosa, Epimedium,
Rhizoma coptidis, Atractylodes lancea preparata,
Radix ophiopogonis, Polygonum multiflorum, Cornus
officinalis, Polyporia cocos, Eupatorium
fortunei, Rhizoma anemarrhenae, Pachyrhizua
angulatus, Semen litchi and Cortex lycii radices,
with known therapeutic effects. JLD, an innovative TCM formulation,
is developed based on empirical knowledge guided by the theory of
'collateral disease'. This compound can effectively strengthen the
spleen and against phlegm and moisture overload (15) Previous studies revealed that JLD
exerted a positive effect on improving insulin resistance and
regulating disruptions in glucose and lipid metabolism (15,16). However, the particular mechanism
underlying the effects of JLD on DN remains unclear. Therefore, the
current study aimed to investigate the therapeutic effects of JLD
on DN and its relative molecular mechanism, thus highlighting the
possible key role of JLD in podocyte apoptosis via the adenosine
monophosphate-activated protein kinase (AMPK)/peroxisome
proliferator-activated receptor-γ co-activator-1α (PGC-1α) pathway
to ameliorate mitochondrial dysfunction.
Materials and methods
Reagents
JLD was obtained from Shijiazhuang Yiling
Pharmaceutical Co., Ltd. and was pulverized into ultrafine powder.
Valsartan (Val) was purchased from Nova Beijing Pharmaceutical Co.,
Ltd., while insulin glargine (Gla) from Sanofi Beijing
Pharmaceutical Co., Ltd. JLD and Val were individually dissolved in
0.5% sodium carboxymethyl cellulose (CMC) for intragastric
administration. Gla was administered by subcutaneous injection.
Animals
In the present study, animal experiments were
ethically approved by the Animal Ethical Committee of the Tianjin
Medical University Chu Hsien-I Memorial Hospital (approval no.
2022084; Tianjin, China). SPF grade male db/m and db/db mice (age,
7 weeks-old; weight, 35-40 g) were purchased from Gempharmatech
Co., Ltd. and maintained in the Laboratory Animal Center. Mice were
maintained at a temperature of 23±3°C, humidity of 45±15%, and a
12/12-h light/dark cycle with free access to food and water.
Following acclimatization for two weeks, mice were then allocated
into the following six groups: control (db/m), model (db/db), Val
(db/db + 10 mg/kg/d Val), Gla (db/db + 3 U/kg/d Gla), JLD-L (db/db
+ 1.75 g/kg/d JLD) and JLD-H (db/db + 3.5 g/kg/d JLD) groups (n=10
mice/group). At the age of nine weeks, mice were daily treated with
JLD, Val or an equal volume of CMC for eight weeks. After 12 h of
fasting, blood from the tail tip of the mice was collected and
fasting blood glucose (FBG) was measured using a glucometer. FBG
and body weight (BW) levels were recorded weekly throughout the
experimental period. At the end of administration, mice entered the
metabolic system for metabolic data monitoring and urine were
collected by metabolic cage. Blood and kidneys were collected
following mice euthanasia by 30-70% vol/min CO2
absorption. Following euthanasia, mice were examined for cardiac
arrest, respiratory arrest, body rigidity and dilated pupils to
confirm animal death. If the mice were not already dead, cervical
dislocation followed.
Detection of renal function
indicators
Urinary albumin content (cat. no. JL20493-96T) was
measured using the corresponding ELISA kit. In addition, creatinine
(Cr, cat. no. JL-T0928-96) and blood urea nitrogen (BUN, cat. no.
JL-T1014-96) kits were provided by Jianglai Biotechnology
(Jianglaibio) Co., Ltd.
Histopathological staining of kidney
tissue sections
Following fixed with 4% formaldehyde for 24 h at
4°C, the kidney tissues were cut into 4-μm thick slices and
embedded in paraffin. Subsequently, the slices were stained with
hematoxylin and eosin (H&E) and observed under a light
microscope (Olympus Corporation).
Transmission electron microscopy
(TEM)
Fresh renal cortex tissues were collected and fixed
with 2% glutaraldehyde at 4°C overnight. The tissues were inserted
into the pure EMBed 812 (cat. no. 90529-77-4; SPI), and then kept
at 37°C overnight. A total of 2% uranium acetate saturated alcohol
solution (avoiding light) was used for staining for 8 min and 2.6%
lead citrate (avoiding CO2) was used for staining for 8
min at room temperature. Following tissue section staining, images
of the foot process and mitochondria were captured under TEM
(Hitachi High-Technologies Corporation).
Immunofluorescence staining
Renal tissue paraffin sections were placed in xylene
and dewaxed in water using descending ethanol series, and then
antigenically repaired. Subsequently, the tissue sections were
sealed with 1% BSA (Beijing Solarbio Science & Technology Co.,
Ltd.). and incubated with a primary antibody at 4°C overnight. The
primary antibodies used were the following: Anti-NPHS2 (1:200; cat.
no. ab229037; Abcam), anti-SYNPO (1:200; cat. no. 21064-1-AP;
Proteintech Group, Inc.). Following tissue incubation with the
corresponding FITC-labeled secondary antibody (1:200; cat. no.
S2003; Simubiotech) for 1 h at 37°C, the sections were blocked
using an anti-fluorescence quenching blocking solution containing
DAPI (cat. no. S2110; Beijing Solarbio Science & Technology
Co., Ltd.) for 10 min at 4°C. Images of the stained tissues were
captured under a laser scanning confocal microscope (Carl Zeiss AG)
or standard microscope (Olympus Corporation). The images were
analyzed using ImageJ 1.53v software (National Institutes of
Health).
TUNEL assay
Apoptotic cells in renal tissue sections were
assessed using a TUNEL reagent for 15 min at 37°C (Beyotime
Institute of Biotechnology). Cell nuclei were stained with DAPI
(cat. no. S2110; Beijing Solarbio Science & Technology Co.,
Ltd.) for 10 min at 4 °C. The apoptotic cells indicated by red
fluorescence were observed under a fluorescent microscope.
Cell culture
Conditional permanent MPC5 cells were provided by
Professor Mingzhen Li (Tianjin Medical University Chu Hsien-I
Memorial Hospital). Cells were cultured in RPMI-1640 medium
supplemented with 11.1 mM glucose (Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin, 1% streptomycin (100 μg/ml) and
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at
37°C in an incubator with 5% CO2. After reaching 80%
confluence, MPC5 cells were exposed to glucose (MilliporeSigma) or
mannitol (MA), as control. MPC5 cells were then treated with 50
(JLD-L), 100 (JLD-M) and 200 μg/ml (JLD-H) JLD or Val (2
μmol/l; cat. no. HY-18204; MedChemExpress) or AICAR (1
mmol/l; cat. no. HY-13417; MedChemExpress) or Compound C (5
μmol/l; cat. no. HY-13418A; MedChemExpress) in a high
glucose (HG; 25 mmol/l) environment. MPC5 cells were then allocated
into the following groups: The normal glucose, MA, HG, HG + Val, HG
+ JLD-L, HG + JLD-M, HG + JLD-H, HG + AICAR, HG + AICAR + JLD, HG +
Compound C and HG + Compound C + JLD groups.
Cell viability assay
A Cell Counting Kit-8 (CCK-8) assay (Invigentech,
Inc.) was carried out to assess the viability of cells. Briefly,
MPC5 cells were cultured for 24 h at 37°C with 5% CO2.
When the cell density reached 80%, cells were treated with 50, 100,
200, 400 and 800 μg/ml JLD or 11.1, 16.7, 25, 33.3 and 50
mmol/l glucose solution for an additional 24 h. Each well was then
supplemented with 10 μl CCK-8 reagent and MPC5 cells were
cultured for 1 h at 37°C in an incubator. Finally, the absorbance
at a wavelength of 450 nm was measured by an enzyme marker (Bio-Tek
Instruments, Inc.).
Reactive oxygen species (ROS) and MitoSOX
assay
ROS content in cells was determined using
fluorescent probe dihydroethidium (DHE; US Everbright Inc.)
staining and cell fluorescence was observed under a fluorescent
microscope (Olympus Corporation). In addition, superoxide levels in
mitochondria of living cells were monitored by MitoSOX (ABclonal
Biotech Co., Ltd.) assay. Red fluorescence was observed under a
fluorescent microscope (Olympus Corporation).
Mitochondrial membrane potential
(MMP)
MMP was assessed using a corresponding kit (cat. no.
M8650; Beijing Solarbio Science & Technology Co., Ltd.).
Briefly, MPC5 cells were cultured in confocal dishes and following
cell staining with JC-1 for 20 min at 37°C, and living cells were
observed under a confocal microscope.
Assessment of mitochondrial DNA (mtDNA)
copy numbers
Total DNA was extracted from kidney tissues and MPC5
cells using a DNA extraction kit (cat. no. D1700; Beijing Solarbio
Science & Technology Co., Ltd.). Using gene-specific PCR
primers synthesized by Tsingke Biotechnology Co., Ltd.,
quantitative PCR was employed to determine mtDNA copy number.
Enzyme activation at 93°C (10 min), denaturation at 93°C (15 sec),
annealing at 55°C (30 sec) and extension at 72°C (30 sec) followed
by 40 cycles. The primer sequences used were as follows: mtND1
forward, 5′-ACCATTTGCAGACGCCATAA-3′ and reverse,
5′-TGAAATTGTTTGGGCTACGG-3′; and β-globin forward,
5′-GAAGCGATTCTAGGGAGCAG-3′ and reverse,
5′-GGAGCAGCGATTCTGAGTAGA-3′. The relative mtDNA content was
ascertained via normalizing mtDNA expression to that of
β-globin.
MitoTracker assay
A MitoTracker assay (Cell Signaling Technology,
Inc.) was used to locate mitochondria in living MPC5 cells.
Mitochondrial morphology was observed under a confocal
microscope.
Flow cytometry
According to the manufacturer's protocol, the
Annexin V-FITC apoptosis detection kit (cat. no. BB-4101-50T;
BestBio Biotechnologies Co., Ltd.) was employed to stain MPC5 cells
(1×105 cells/hole). Subsequently, apoptotic cells were
identified using flow cytometry (model, BD FACSVerse; BD
biosciences) and the data were analyzed using FlowJo 10.8.1
software (FlowJo LLC).
Cell transfection
For PGC-1α silencing, MPC5 cells were transfected
with specific small interfering RNAs (siRNAs) targeting PGC-1α
(concentration, 20 μmol/l; sense,
5′-GCCAAACCAACAACUUUAUTT-3′ and antisense,
5′-AUAAAGUUGUUGGUUUGGCTT-3′) or normal control siRNAs
(concentration, 20 μmol/l; sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′) using the Lipofectamine®
2000 transfection reagent. After cell transfection for 6 h at 37°C,
the medium was changed, and the next 24 h after which the cells
were intervened. Both siRNA sequences were provided by Shanghai
GenePharma Co., Ltd. Transfected MPC5 cells were then treated with
25 mmol/l HG or 200 μg/ml JLD for the subsequent
experiments.
Western blot analysis
Proteins were extracted from kidney tissues or MPC5
cells using a RIPA cracking buffer (cat. no. R0020; Beijing
Solarbio Science & Technology Co., Ltd.), supplemented with
phosphatase inhibitor (Calyculin A) and phenylmethylsulfonyl
fluoride. Protein concentration was measured by the BCA method. The
same quantity of protein extracts (20 μg) was separated by
10% SDS-PAGE and the proteins were then transferred onto
nitrocellulose membranes. Following sealing in Tris-buffered
solution (pH, 7.2-7.4), containing 10% Tween-20 and 5% skim milk,
for 1 h, the membranes were incubated with primary antibodies at
4°C overnight. The following day, the membrane was incubated with
the Goat Anti-Rabbit HRP-conjugated secondary antibody (1:5,000;
cat. no. BS13278; Bioworld Technology, Inc.) for 1 h. The
immunoreactive protein bands were visualized using an enhanced
chemiluminescence kit (Beijing Solarbio Science & Technology
Co., Ltd.) and analyzed using ImageJ software. The antibodies used
were the following: Anti-NPHS2 (1:1,000; cat. no. ab229037; Abcam),
anti-phosphorylated (p)- dynamin-related peptide 1
(DRP1S616; 1:1,000; cat. no. 3455S; Cell Signaling
Technology, Inc.), anti-p-DRP1S637(1:1,000; cat. no.
ab193216; Abcam), anti-PGC-1α (1:1,000; cat. no. ab54481; Abcam),
anti-p-AMPK (1:1,000; cat. no. ab13448; Abcam) and anti-AMPK
(1:1,000; cat. no. ab32047; Abcam); anti-optic atrophy protein 1
(OPA1; 1:1,000; cat. no. 880471S; Cell Signaling Technology, Inc.),
anti-DRP1 (1:1,000; cat. no. 8570S; Cell Signaling Technology,
Inc.) and anti-mitofusin 2 (MFN2; 1:1,000; cat. no. 9482S; Cell
Signaling Technology, Inc.)); anti-synaptopodin (SYNPO; 1:1,000;
cat. no. 21064-1-AP; Proteintech Group, Inc.), anti-BAX (1:2,000;
cat. no. 50599-2-IG; Proteintech Group, Inc.), anti-BCL2 (1:2,000;
cat. no. 26593-1-AP; Proteintech Group, Inc.) and anti-β-actin
(1:2,000; cat. no. 20536-1-AP; Proteintech Group, Inc.); and
anti-cleaved caspase 3 (1:1,000; cat. no. A2156; ABclonal Biotech
Co., Ltd.).
RNA-sequencing
RNA sequencing was performed on fresh kidney tissues
derived from mice in the db/m, db/db and db/db + JLD-H groups.
Total RNA was extracted from kidney tissues using TRIzol reagent.
Sample quality verification was performed using Fragment Analyzer
(model, 5300; Agilent Technologies, Inc.). The library was prepared
using the Optimal Dual-mode mRNA Library Prep Kit (cat. no.
LR00R96; BGI, Inc.). Fragment Analyzer measured library loading
concentrations at least 5 ng/μl. mRNA was enriched by oligo
(dT)-attached magnetic beads and reversed transcribed into cDNA.
Following cDNA end repairment, an A nucleotide was added to the
blunt fragments. The single-stranded cyclized DNA products were
replicated to construct DNA nanoballs (DNBs). DNBs were then loaded
into the patterned nanoarray and paired end of 150 base reads were
performed on the T7 platform (BGI, Inc.). Data were analyzed,
visualized and mined using the 'Dr. Tom' system (https://biosys.bgi.com). The differentially expressed
genes were identified using the DESeq2 v1.4.5 package, with Q≤0.05.
Kyoto Encyclopedia of Genes and Genomes (KEGG; https://www.kegg.jp/) and Gene Ontology (GO;
http://www.geneontology.org/) enrichment
analyses were performed on differentially expressed genes with a Q
value of ≤0.05. RNA-sequencing and analysis of results were
performed by BGI Genomics Co., Ltd.
Statistical analyses
Graphical representations were created using
GraphPad Prism 9.0 software (Dotmatics). All results are expressed
as the mean ± SEM. The differences among multiple groups were
compared by one-way ANOVA followed by Tukey's post hoc test, while
those between two groups by unpaired Student's t-tests. P<0.05
was considered to indicate a statistically significant
difference.
Results
JLD restores the kidney function of db/db
mice
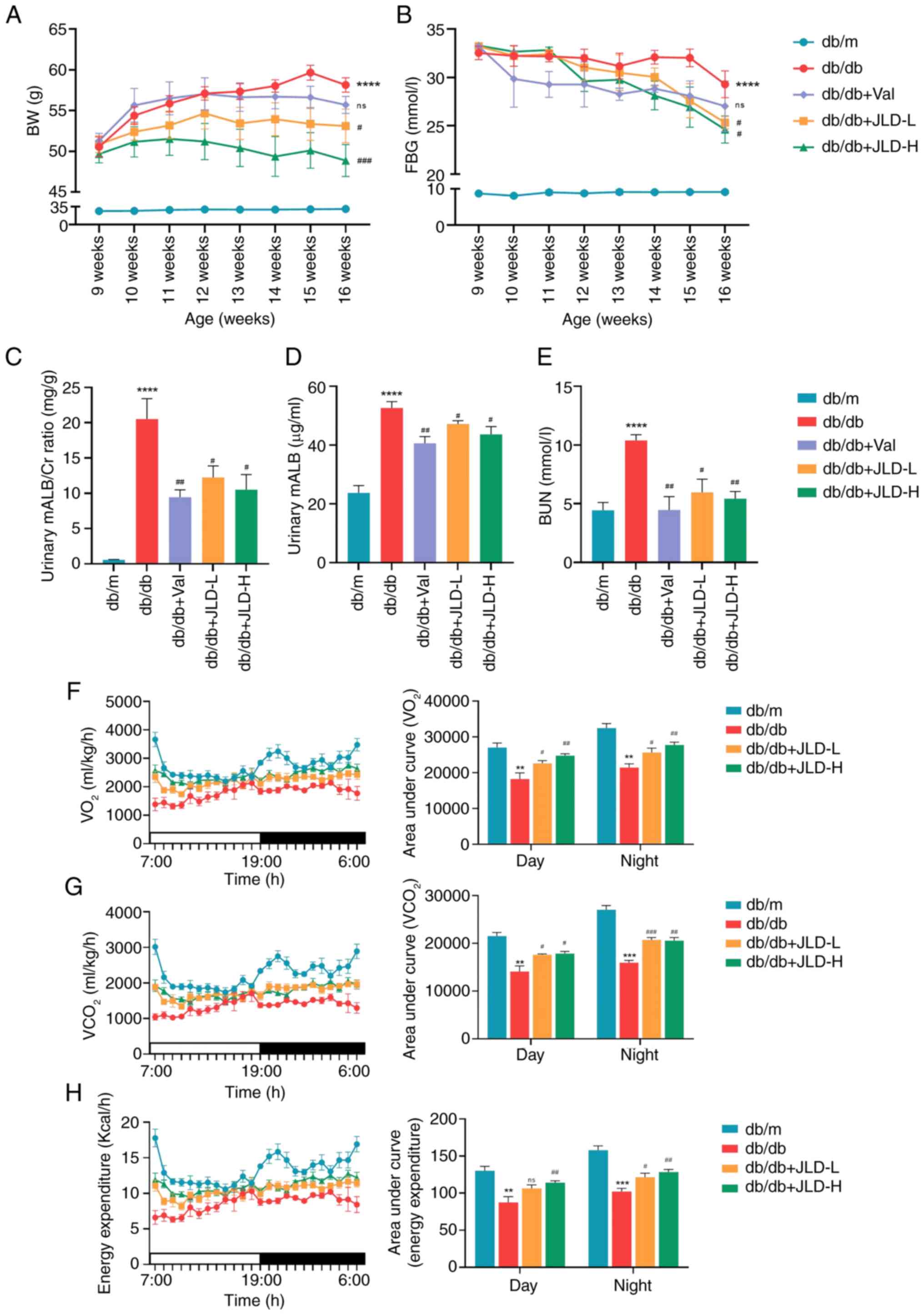
The results demonstrated that FBG and BW levels were
significantly increased in db/db mice compared with db/m mice. Mice
in JLD groups showed decreased FBG levels and BW compared with
those in db/db group, thus supporting the hypoglycemic and weight
loss effects of JLD (Fig. 1A and
B). Furthermore, the urinary microalbumin/Creatinine (mALB/Cr)
rate, and mALB and BUN levels were dose-dependently reduced in the
JLD groups compared with the db/db group (Fig. 1C-E). Additionally, the renal
function indicators were significantly improved in Val group
(Fig. 1C-E). In addition, FBG
levels, but not renal function, were also significantly improved in
the Gla group (Fig. S1A-C). At
the same time, energy metabolism was enhanced in db/db mice after
JLD intervention (Fig. 1F-H).
Overall, these findings indicated that JLD could effectively
restore kidney function in the db/db group, possibly independently
of its hypoglycemic effect.
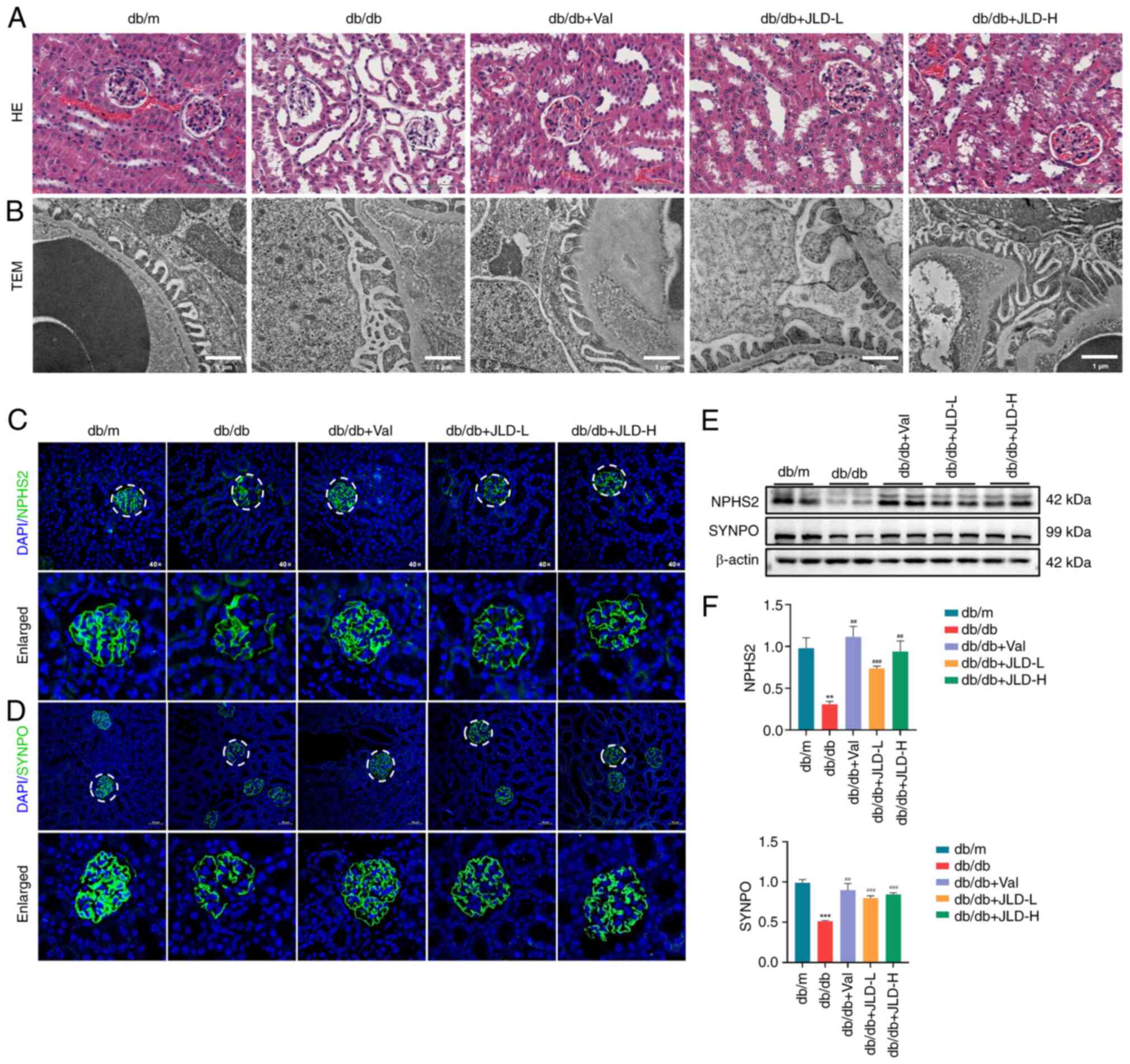
JLD improves glomerular morphology and
podocyte injury in db/db mice
As illustrated in Fig. 2A, the renal pathology of db/db
mice was characterized by tubule dilatation and glomerular atrophy.
TEM showed that the glomerular basement membrane thickened and the
foot process was fused in db/db mice (Fig. 2B). However, glomerular morphology
was improved in the JLD groups. Podocyte injury is generally
considered as a critical step in the development of DKD. Therefore,
in the present study, to assess podocyte injury, the expression
levels of the functional- and podocyte-specific indicators NPHS2
and SYNPO were detected. Immunofluorescence and western blot
results revealed that both NPHS2 and SYNPO were downregulated in
db/db mice and their expression levels were restored following mice
treatment with JLD (Fig. 2C-F).
The aforementioned results demonstrated that JLD could
significantly improve glomerular morphology and podocyte
injury.
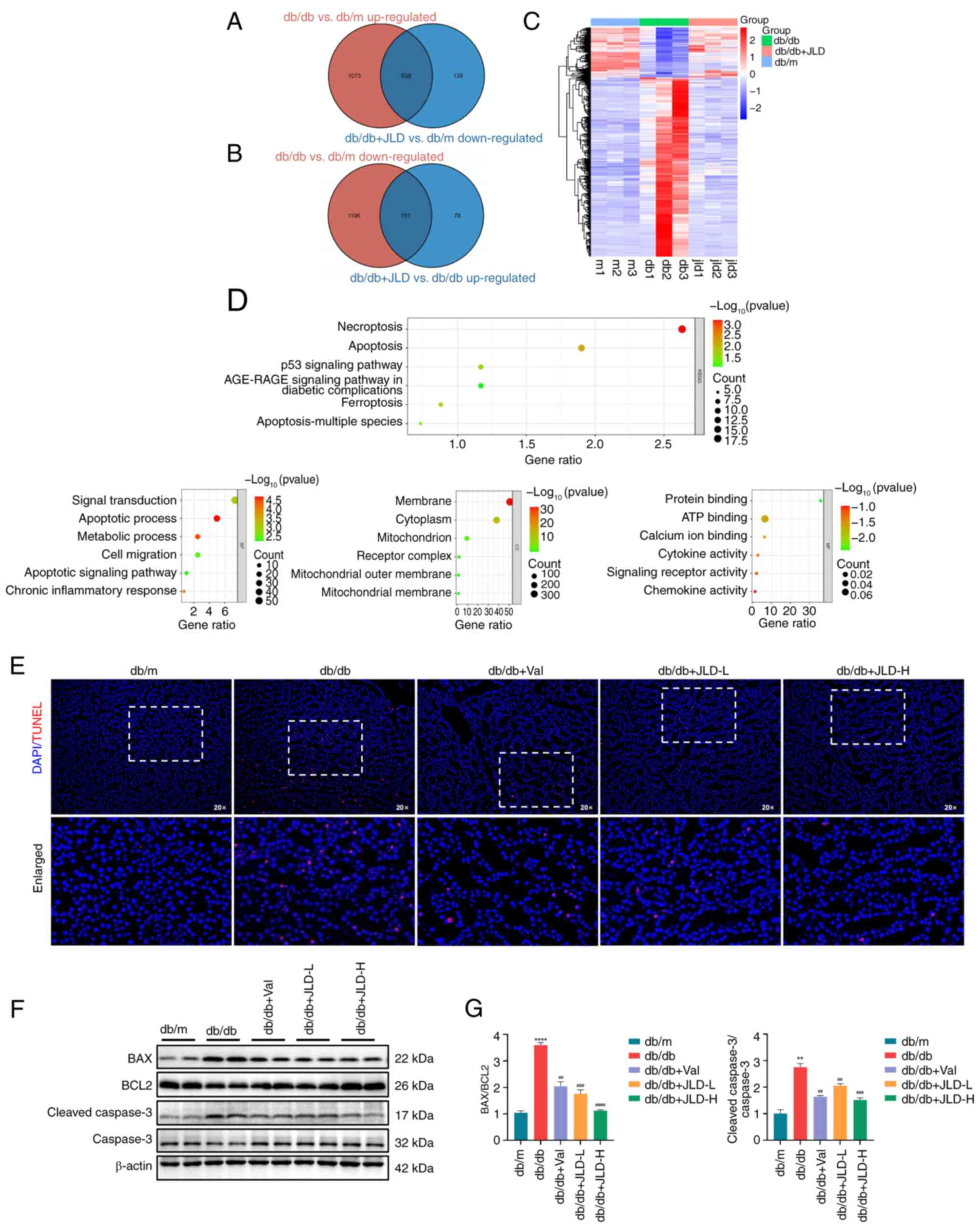
JLD inhibits cell apoptosis in db/db
mice
To explore the molecular mechanism underlying the
effect of JLD on improving renal function and podocyte injury, RNA
sequencing and KEGG enrichment analyses were performed in the
kidney tissues of mice. In the aforementioned animal experiments,
the results demonstrated that the general condition and kidney
injury in mice in the JLD-H group (db/db + 3.5 g/kg/d JLD) were
improved more significantly. Therefore, the kidney tissues isolated
from JLD-H group were selected for RNA sequencing. The analysis
revealed that a total of 539 factors were upregulated in db/db
group and downregulated in JLD group. Similarly, 151 factors were
downregulated in db/db group and upregulated in JLD group (Fig. 3A-C). Furthermore, KEGG and GO
enrichment analysis revealed that the differentially expressed
genes were mainly clustered in apoptotic pathways (Fig. 3D). In addition, western blot
analysis was performed to detect the apoptosis-related proteins,
namely BAX, BCL2 and cleaved caspase 3. Therefore, cell apoptosis
was enhanced in db/db mice compared with db/m mice, while it was
restored in JLD groups (Fig. 3F and
G). Consistently, TUNEL staining assays revealed that the
number of apoptotic cells was increased in db/db group and reduced
in JLD groups (Fig. 3E).
Overall, the aforementioned findings indicated that JLD could
markedly inhibit cell apoptosis in db/db mice.
JLD inhibits DRP1-mediated mitochondrial
fission and alleviates mitochondrial dysfunction in db/db mice
In previous studies, excessive mitochondrial fission
and mitochondrial dysfunction were observed in HG-induced MPC5
cells, thus resulting in enhanced ROS production and cell apoptosis
(17,18). Consistently, in the present
study, mitochondrial morphology and function were assessed. The
results revealed that the morphology of mitochondria in the db/db
group was characterized by abnormal structure, accompanied by
swelling, rupture and mitochondrial ridge reduction. However, the
morphology of mitochondria was restored in mice in the JLD group
(Fig. 4A). In db/db mice,
p-DRP1S616 was upregulated and p-DRP1S637 was
downregulated, while the protein expression levels of the
mitochondrial fusion-related proteins, OPA1 and MFN2, were also
reduced, thus suggesting that diabetic mice were characterized by
enhanced mitochondrial division and decreased podocyte fusion
(Fig. 4C and D). In addition,
attenuated mitochondrial fission, decreased ROS levels and
increased copy numbers of mtDNA were observed in mice in the JLD
groups (Fig. 4B and E). These
findings suggested that JLD could significantly inhibit
mitochondrial division and alleviate mitochondrial dysfunction.
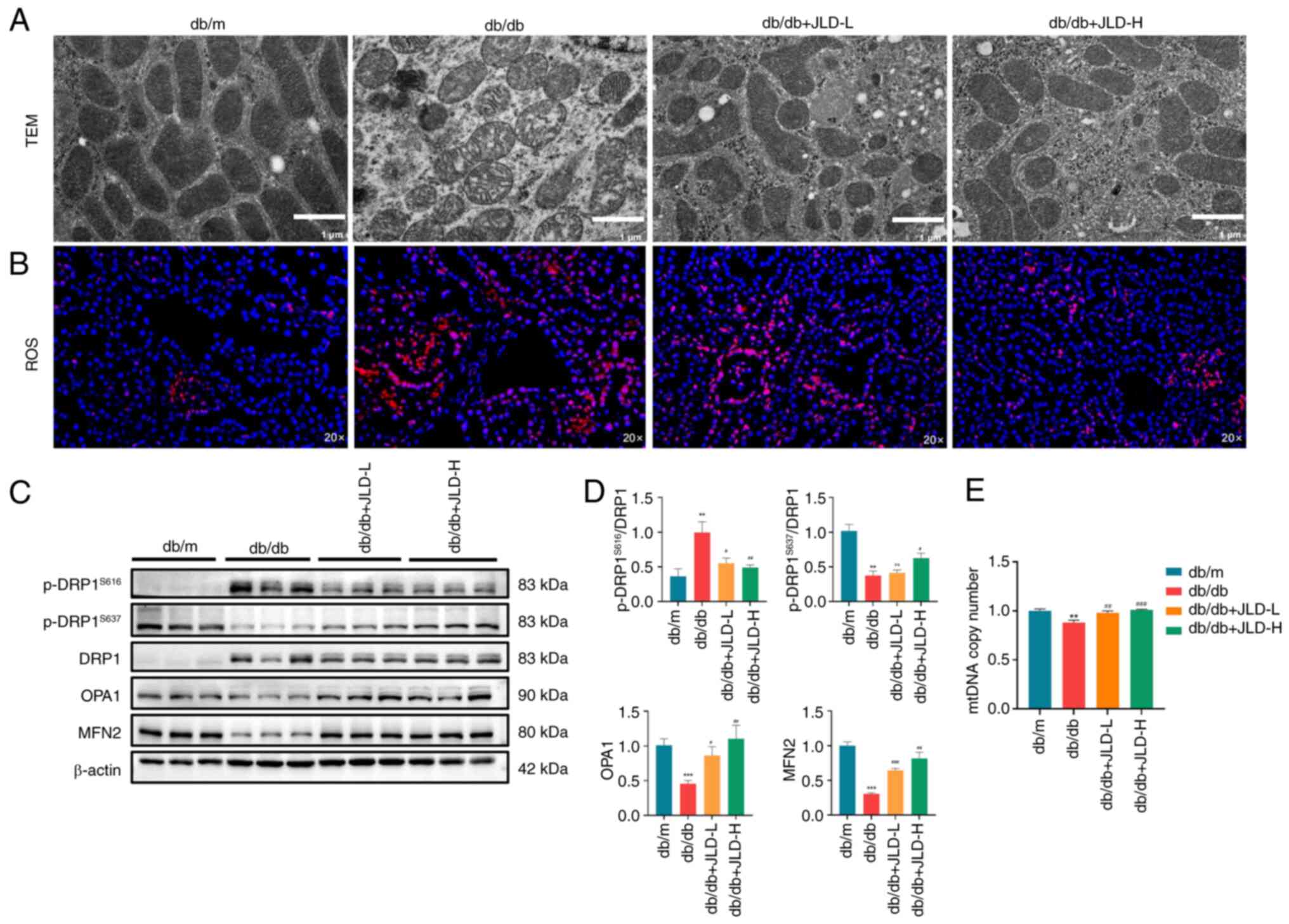 | Figure 4JLD inhibits DRP1-mediated
mitochondrial fission and alleviates mitochondrial dysfunction of
db/db mice. (A) TEM for mitochondria (scale bar, 1 μm). (B)
ROS staining (magnification, ×20). (C and D) Relative protein
expression of p-DRP1S616, p-DRP1S637, DRP1,
OPA1 and MFN2 in mice. (E) mtDNA copy number in each group.
**P<0.01 and ***P<0.001 vs. db/m group;
#P<0.05, ##P<0.01 and
###P<0.001 vs. db/db group. JLD, Jinlida granules;
DRP1, dynamin-related peptide 1; TEM, transmission electron
microscopy; ROS, reactive oxygen species; p-, phosphorylated; OPA1,
optic atrophy protein 1; MFN, mitofusin; mtDNA, mitochondrial
DNA. |
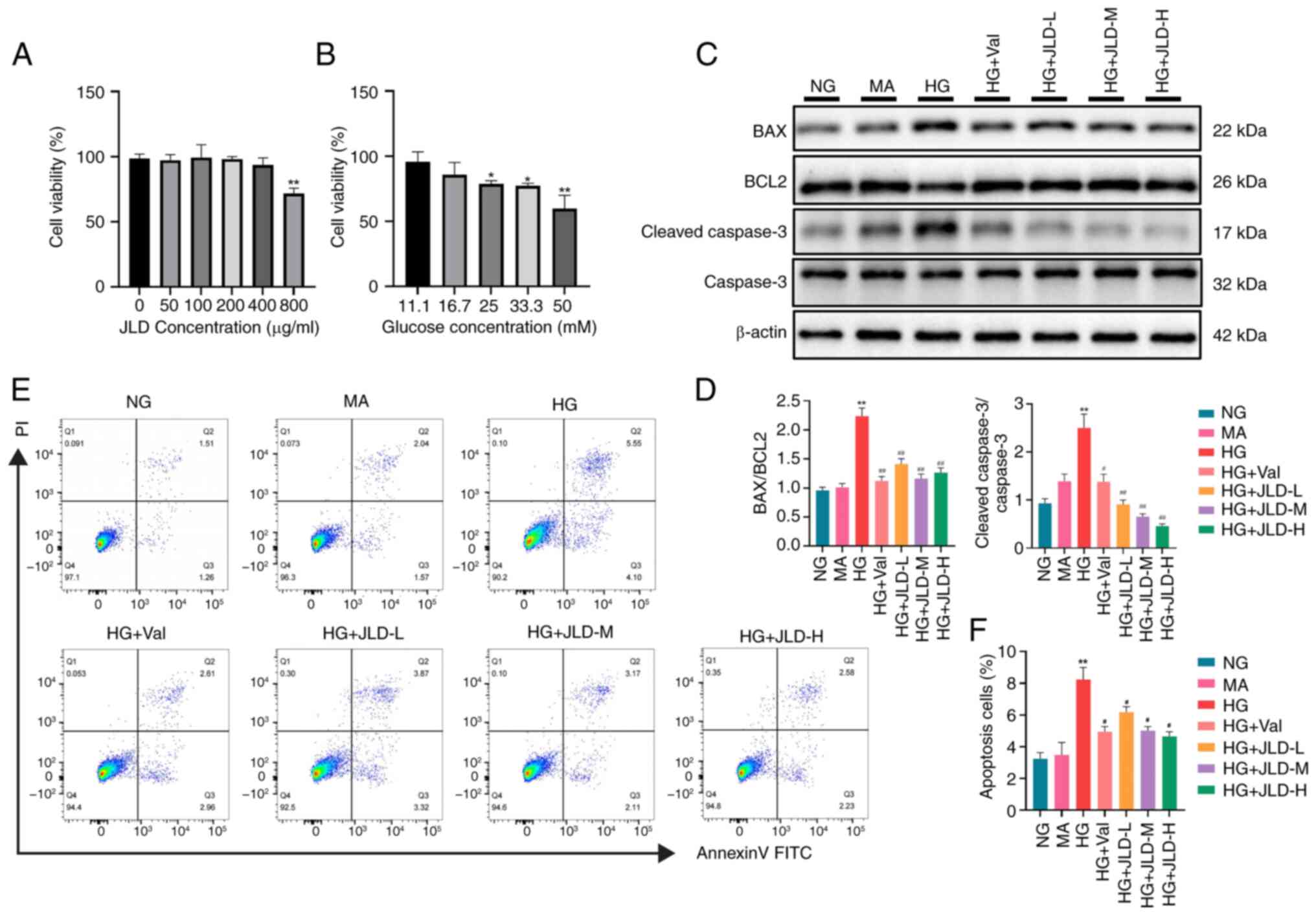
JLD alleviates HG-induced MPC5 cell
apoptosis
To investigate the possible mechanisms by which JLD
could improve cell apoptosis and mitochondrial dysfunction,
hyperglycemia was simulated in vitro by treating MPC5 cells
with HG. For CCK-8 assays, cells were co-treated with increasing
concentrations of JLD (50, 100 and 200 μg/ml) and 25 mmol/l
HG (Fig. 5A and B). In
HG-treated MPC5 cells, apoptosis was increased, as evidenced by
cleaved caspase 3 and BAX upregulation and BCL2 downregulation
(Fig. 5C and D). Flow cytometry
revealed that cell apoptosis was enhanced in podocytes induced by
HG. However, this effect was reversed by JLD (Fig. 5E and F). These results verified
that JLD could inhibit HG-induced podocyte apoptosis.
JLD alleviates mitochondrial fission and
mitochondrial dysfunction in MPC5 cells
Consistent with the in vivo results,
MitoTracker staining revealed mitochondrial fragmentation in
HG-induced MPC5 cells (Fig. 6B).
MMP upregulation and elevated mitochondrial ROS production are
closely associated with mitochondrial dysfunction. Therefore, the
results demonstrated that MMP was downregulated in HG-induced MPC5
cells, while JC-1 staining revealed mitochondrial depolarization.
The aforementioned effects were reversed by JLD administration
(Fig. 6A). In addition, MitoSOX
staining demonstrated that ROS production was increased in
HG-induced podocytes, and it was significantly reduced by JLD
(Fig. 6C). Furthermore, the
western blot analysis results indicated that HG-induced MPC5 cells
displayed excessive mitochondrial division and decreased
mitochondrial fusion, which were reversed following treatment with
JLD (Fig. 6D and E). Finally,
the RT-qPCR results identified that JLD could restore the reduced
copy number of mtDNA in HG-induced podocytes (Fig. 6F). Overall, these findings
verified that JLD could significantly inhibit mitochondrial
division and improve mitochondrial dysfunction.
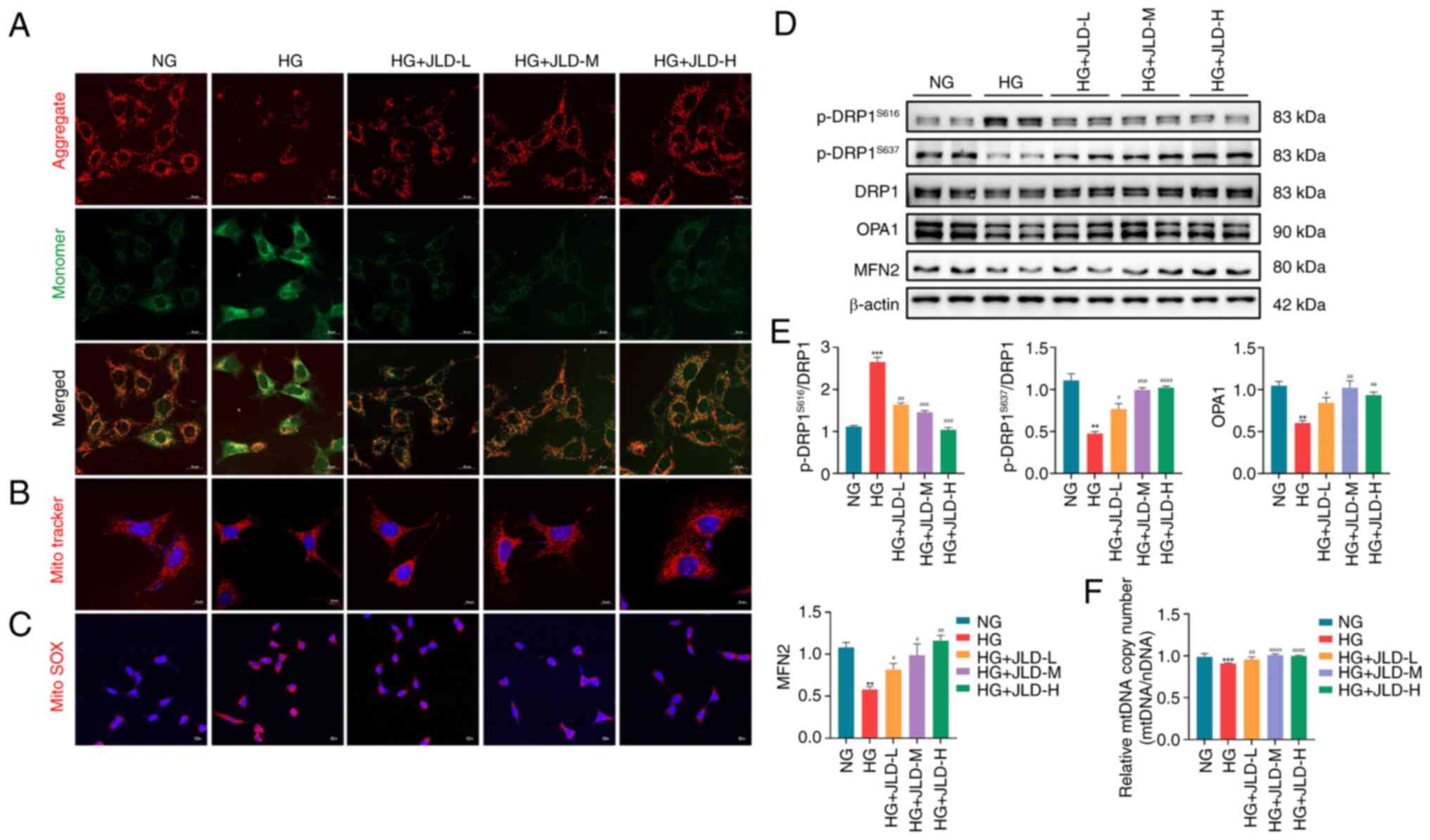 | Figure 6JLD alleviates mitochondrial fission
and mitochondrial dysfunction induced by HG in MPC5 cells. (A) JC-1
staining (scale bar, 20 μm). (B) Mito Tracker staining
(scale bar, 20 μm). (C) MitoSOX staining (magnification,
×10). (D and E) Relative protein expression of
p-DRP1S616, p-DRP1S637, DRP1, OPA1 and MFN2
in MPC5. (F) mtDNA copy number in each group.
**P<0.01 and ***P<0.001 vs. NG group;
#P<0.05, ##P<0.01,
###P<0.001 and ####P<0.0001 vs. HG
group. JLD, Jinlida granules; HG, high glucose; p-, phosphorylated;
DRP1, dynamin-related peptide 1; OPA1, optic atrophy protein 1;
MFN, mitofusin; mtDNA, mitochondrial DNA; NG, normal glucose. |
JLD alleviates mitochondrial division and
apoptosis via activating the AMPK/PGC-1α pathway
AMPK and PGC-1α serve critical roles in the
maintenance of mitochondrial homeostasis. Therefore, dysfunction of
this pathway has been related to the pathogenesis of several
metabolic diseases, such as diabetes (19,20). To explore whether the AMPK/PGC-1α
pathway participates in the JLD-mediated podocyte apoptosis and
mitochondrial dysfunction improvement, the in vivo and in
vitro protein expression levels of AMPK/PGC-1α were detected.
The analysis showed that AMPK phosphorylation and PGC-1α expression
were reduced in the db/db group, while they were significantly
enhanced in JLD groups (Fig. 7A and
B). Consistent with the in vivo results, the
phosphorylation levels of AMPK/PGC-1α expression were decreased in
HG-induced podocytes, which were reversed after JLD intervention
(Fig. 7C and D). Therefore, it
was hypothesized that JLD could ameliorate podocyte apoptosis and
mitochondrial dysfunction via the AMPK/PGC-1α pathway. The
aforementioned finding was verified following MPC5 cell treatment
with AICAR, an AMPK activator, or its inhibitor, compound C.
Therefore, p-AMPK and PGC-1α were downregulated in HG- and compound
C-intervened MPC5 cells, thus promoting excessive mitochondrial
division and apoptosis. However, HG-induced MPC5 cell treatment
with JLD and AICAR displayed the opposite effect (Fig. 7E, G-I). To further support that
PGC-1α could be considered as a critical factor in JLD action to
ameliorate mitochondrial dysfunction and apoptosis in podocytes,
MPC5 cells were transfected with siRNA clones targeting PGC-1α
(Fig. S2). As expected, PGC-1α
knockdown abrogated the effects of JLD on ameliorating excessive
mitochondrial division and apoptosis in podocytes (Fig. 7F and G). To summarize, the
aforementioned results confirmed that PGC-1α could be considered as
a key effector of JLD in ameliorating apoptosis and mitochondrial
dysfunction in podocytes through the AMPK pathway.
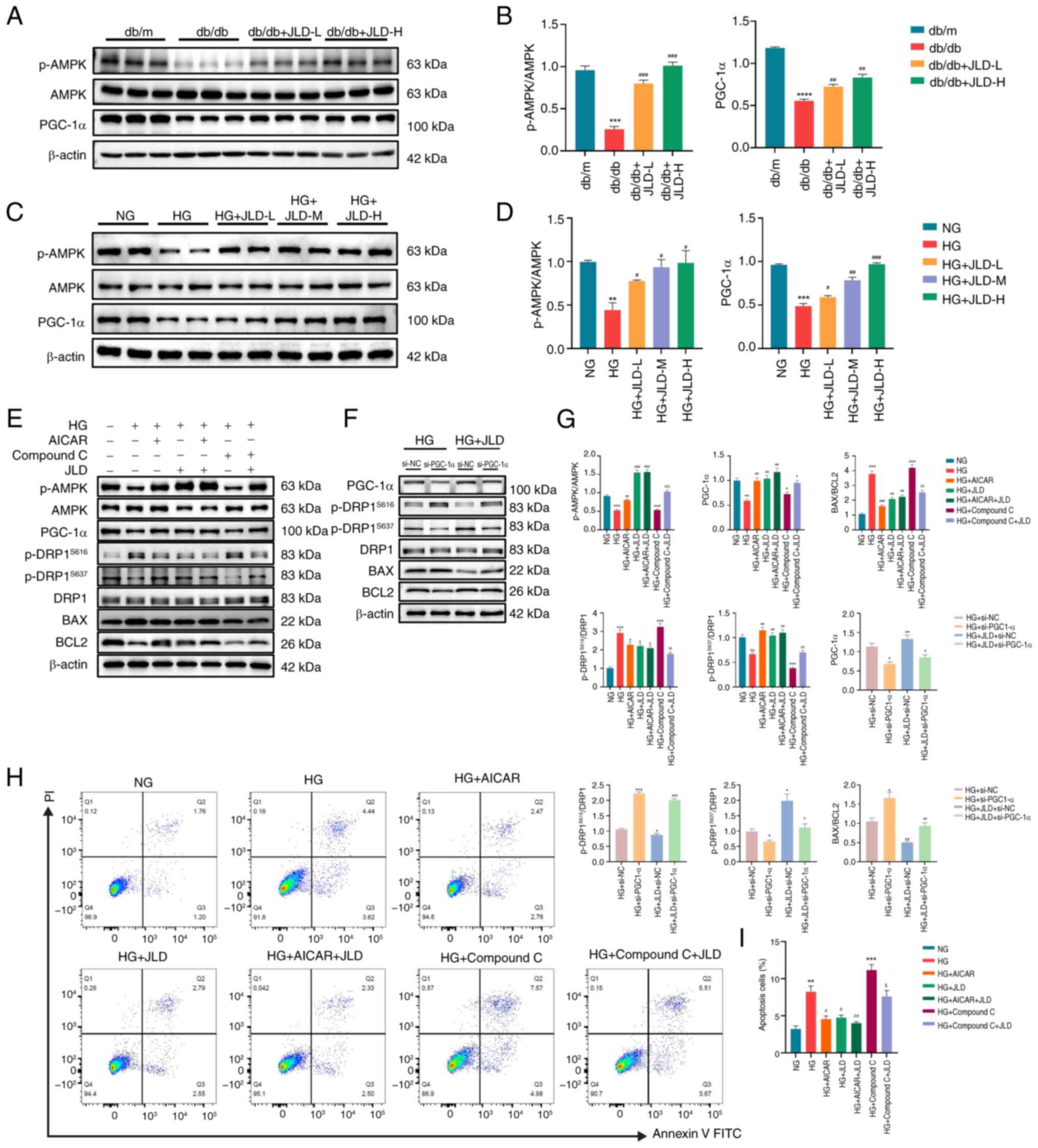 | Figure 7JLD alleviates mitochondrial division
and apoptosis by activating the AMPK/PGC-1α pathway. (A and B)
Relative protein expression of p-AMPK and PGC-1α in mice. (C and D)
Relative protein expression of p-AMPK and PGC-1α in MPC5 cells.
(E-G, H, I) MPC5 cells were treated with or without JLD (200
μg/ml), with or without AICAR (1 mmol/l), and with or
without Compound C (5 μmol/l). Relative protein expression
of p-AMPK, PGC-1α, p-DRP1S616, p-DRP1S637,
DRP1, BAX and BCL2 in MPC5 cells. The Annexin V/PI staining of
apoptotic rate in each group. (F and G) MPC5 were transfected with
si-NC or PGC-1α siRNA (si-PGC-1α) and then treated with or without
JLD (200 μg/ml). Relative protein expression of PGC-1α,
p-DRP1S616, p-DRP1S637, DRP1, BAX and BCL2 in
MPC5 cells. $P<0.05, $$P<0.01 and
$$$P<0.001 vs. HG + Compound C group;
*P<0.05, **P<0.01 and
***P<0.001 vs. HG + si-NC group;
#P<0.05, ##P<0.01 and
###P<0.001 vs. HG + JLD + si-NC group. JLD, Jinlida
granules; AMPK, adenosine monophosphate-activated protein kinase;
PGC-1α, peroxisome proliferator-activated receptor-γ
co-activator-1α; p-, phosphorylated; DRP1, dynamin-related peptide
1; siRNA, small interfering RNA; NC, negative control; HG, high
glucose; NG, normal glucose. |
Discussion
Diffuse thickening of the glomerular basement
membrane, dilatation of the tethered membranes and nodular
sclerosis (Kimmelstiel-Wilson nodules) are hallmark
histopathological features of DN, which accounts for ~50% of all
cases of chronic kidney disease (CKD) (21,22). As a complication of DM, DN is
significantly involved in ESRD. Despite the prevalence and severity
of DN, the benefits of the current therapeutic interventions remain
limited. The results of the present study showed that JLD could
protect against DN and effectively restore renal function.
Lower glomerular filtration rate and persistent
proteinuria are hallmarks of DN. In patients with DN, podocyte
damage and peduncle fusion are the primary manifestations of
proteinuria. Since podocytes are terminally differentiated cells,
DN progresses irreversibly if >20% of them are lost (23). In the present study, RNA
sequencing and in vitro/in vivo experiments showed
that the protective effect of JLD on podocytes was mediated via
diminishing podocyte injury and apoptosis. It has been reported
that podocytes can maintain their complex structure via regulating
the cytoskeleton and extracellular matrix, which require
substantial mitochondrial energy supply (24). Mitochondria are the major source
of cellular energy and their dysfunction is notably associated with
podocyte injury in patients with DN. Their dysfunction is affected
by mitochondrial biogenesis, fusion and fission (25). Mitochondrial fission and fusion
are mutually complementary processes that reinforce each other to
maintain mitochondrial function and morphology. The aforementioned
two processes can be achieved by cleavage and junction of the inner
and outer mitochondrial membrane. Fusion is fundamental for
homogenization of mitochondrial dynamics across the cellular
mitochondrial network and enhances the efficiency of mitochondrial
reactions (26). By contrast,
mitochondrial fission is generally related to metabolic stress,
thus severely contributing to mitochondrial degradation and
apoptosis (25). Mitochondrial
fusion is facilitated by MFN1 and MFN2, located in the outer
mitochondrial membrane, and OPA1, located in the inner
mitochondrial membrane (27).
Fission is dominantly regulated by DRP1 and it can be accelerated
by either increased DRP1 activity or DRP1 translocation to the
outer mitochondrial membrane. Post-translational changes could
affect DRP1 activity and translocation (28). For example, phosphorylating DRP1
at Ser-616 could promote fission, while DRP1 phosphorylation at
Ser-637 could typically suppress this process (29,30). Herein, podocyte treatment with
JLD reduced mitochondrial fission and improved mitochondrial
dysfunction.
The regulation of cell growth, proliferation,
differentiation, metabolism and survival is largely dependent on
AMPK, which promotes the physiological development of various
organs in the human body. The progression of DN and CKD can be
facilitated by AMPK dysregulation (31,32). AMPK activity was reduced in
HG-induced podocytes, thus altering cleft septa and cytoskeletal
dysfunction. By contrast, regulating AMPK activity could affect
actin dynamics and improve the architecture of foot process
(33). In addition to AMPK
inactivation, HG stimulation could also reactivate mammalian target
of rapamycin pathway and induce apoptosis in podocytes (34). A previous study from our
laboratory showed that JLD activated AMPK and enhanced autophagy in
NIT-1 pancreatic β cells (15).
Consistently, in the present study, JLD acted as an activator of
AMPK, which not only ameliorated the structure of foot process but
also attenuated podocyte apoptosis.
PGC-1α is a transcriptional coactivator that acts
via responding to mitochondrial biogenesis and energy consumption
(35,36). Apart from that, PGC-1α regulates
mitochondrial dysfunction and mitochondrial quality control
mechanisms (37). A previous
research revealed a strong association between type 2 DM and
genetic variations in PGC-1α (38). Furthermore, PGC-1α expression was
markedly reduced in DN and CKD (39,40). Other studies verified that PGC-1α
expression was primarily regulated by AMPK in diverse nephrotic
diseases, including DN (41,42). The results revealed that JLD
could possibly exert its pharmacological effect via activating AMPK
to upregulate PGC-1α, which in turn could ameliorate mitochondrial
dysfunction. However, further investigation is needed to explore
the mechanism by which JLD activates the phosphorylation of
AMPK.
Another study suggested that that quercetin,
luteolin and baicalin were the main active components of JLD, which
acted against DM (43). Among
them, quercetin is considered to have a protective role in podocyte
apoptosis in DN (44). Several
related bioactive compounds of JLD, such as puerarin and
ginsenosides, have been considered to exhibit therapeutic effects
against DN (45,46). To develop novel therapeutic
strategies for DN, it is essential to find out the potential
bioactive components of JLD and clarify how they affect
mitochondrial homeostasis. The present study supported that JLD, a
complex comprising multiple active ingredients, displayed
therapeutic benefits in DN. JLD ameliorated podocyte apoptosis and
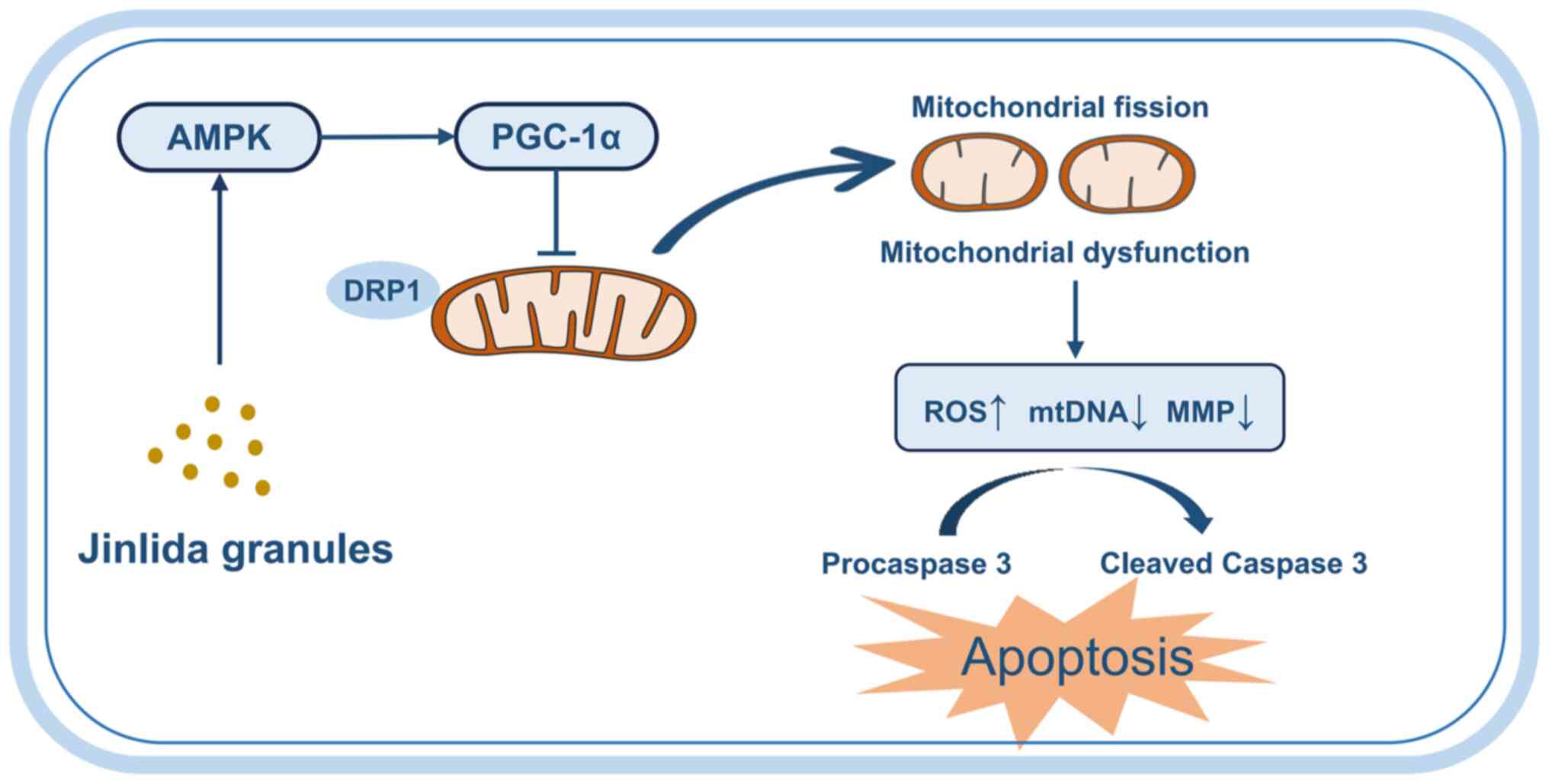
mitochondrial dysfunction via the AMPK/PGC-1α pathway (Fig. 8).
As summarized, the current study described in detail
the protective effects of JLD on podocytes from the perspective of
mitochondrial function. The results indicated that JLD could be a
potential activator of AMPK, which in turn mediated downstream
PGC-1α factor to suppress podocyte apoptosis and mitochondrial
fission. The aforementioned findings supported that JLD could serve
as an effective therapeutic measure against DN and offer a novel
insight into the molecular mechanisms of JLD in the treatment of
this disease.
Supplementary Data
Availability of data and materials
The data generated in the present study may be found
in the Genome Sequence Archive under accession number CRA019949 or
at the following URL: https://ngdc.cncb.ac.cn/gsa/search?searchTerm=CRA019949.
Authors' contributions
SS conceptualized the study, wrote the original
draft and conducted project administration. SY and YC developed
methodology and performed formal analysis. TF performed software
and formal analysis. JQ conducted investigation and data curation.
LT developed methodology and conducted investigation. MZ validated
data and conducted investigation. SW performed data validation and
formal analysis. BS wrote, reviewed and edited the manuscript and
conducted project administration. LC wrote, reviewed and edited the
manuscript, conducted project administration and provided
resources. SS, BS and LC confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Experimental procedures conducted in the present
study were approved by the Animal Ethical Committee of Tianjin
Medical University Chu HsienI Memorial Hospital (approval no.
2022084; Tianjin, China).
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
AMPK
|
adenosine monophosphate-activated
protein kinase
|
|
ATP
|
adenosine triphosphate
|
|
BW
|
body weight
|
|
BUN
|
blood urea nitrogen
|
|
CCK-8
|
Cell Counting Kit-8
|
|
CKD
|
chronic kidney disease
|
|
CMC
|
carboxymethyl cellulose
|
|
Cr
|
creatinine
|
|
DM
|
diabetes mellitus
|
|
DN
|
diabetic nephropathy
|
|
ESRD
|
end-stage renal disease
|
|
FBG
|
fasting blood glucose
|
|
Gla
|
glargine
|
|
HG
|
high glucose
|
|
mALB
|
microalbumin
|
|
MFN
|
mitofusin
|
|
MMP
|
mitochondrial membrane potential
|
|
OPA1
|
optic atrophy protein 1
|
|
PGC-1α
|
peroxisome proliferator-activated
receptor-γ co-activator-1α
|
|
TCM
|
Traditional Chinese Medicine
|
|
TEM
|
transmission electron microscopy
|
Acknowledgements
Not applicable.
Funding
The present study was supported by the project of Tianjin Health
Committee Traditional Chinese Medicine and Integrated Traditional
Chinese and Western Medicine Project (grant no. 2023152) and the
Tianjin Key Medical Discipline (Specialty) Construction Project
(grant no. TJYXZDXK-032A).
References
|
1
|
Tuttle KR, Agarwal R, Alpers CE, Bakris
GL, Brosius FC, Kolkhof P and Uribarri J: Molecular mechanisms and
therapeutic targets for diabetic kidney disease. Kidney Int.
102:248–260. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Magliano DJ and Boyko EJ: IDF Diabetes
Atlas 10th edition Scientific Committee: Idf Diabetes Atlas.
International Diabetes Federation © International Diabetes
Federation; Brussels: 2021
|
|
3
|
Cleveland KH and Schnellmann RG:
Pharmacological targeting of mitochondria in diabetic kidney
disease. Pharmacol Rev. 75:250–262. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petrazzuolo A, Sabiu G, Assi E, Maestroni
A, Pastore I, Lunati ME, Montefusco L, Loretelli C, Rossi G, Nasr
MB, et al: Broadening horizons in mechanisms, management, and
treatment of diabetic kidney disease. Pharmacol Res.
190:1067102023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dimmer KS and Scorrano L: (De)constructing
mitochondria: What for? Physiology (Bethesda). 21:233–241.
2006.PubMed/NCBI
|
|
6
|
Bhargava P and Schnellmann RG:
Mitochondrial energetics in the kidney. Nat Rev Nephrol.
13:629–646. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Flemming N, Pernoud L, Forbes J and Gallo
L: Mitochondrial dysfunction in individuals with diabetic kidney
disease: A systematic review. Cells. 11:24812022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meyer JN, Leuthner TC and Luz AL:
Mitochondrial fusion, fission, and mitochondrial toxicity.
Toxicology. 391:42–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mishra P, Carelli V, Manfredi G and Chan
DC: Proteolytic cleavage of Opa1 stimulates mitochondrial inner
membrane fusion and couples fusion to oxidative phosphorylation.
Cell Metab. 19:630–641. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Forbes JM and Thorburn DR: Mitochondrial
dysfunction in diabetic kidney disease. Nat Rev Nephrol.
14:291–312. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X, Jiang L, Liu XQ, Huang YB, Wang
AL, Zeng HX, Gao L, Zhu QJ, Xia LL and Wu YG: Paeoniflorin binds to
VEGFR2 to restore autophagy and inhibit apoptosis for podocyte
protection in diabetic kidney disease through PI3K-AKT signaling
pathway. Phytomedicine. 106:1544002022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gong M, Guo Y, Dong H, Wu F, He Q, Gong J
and Lu F: Modified Hu-lu-ba-wan protects diabetic glomerular
podocytes via promoting PKM2-mediated mitochondrial dynamic
homeostasis. Phytomedicine. 123:1552472024. View Article : Google Scholar
|
|
13
|
Shen Z, Cui T, Liu Y, Wu S, Han C and Li
J: Astragalus membranaceus and Salvia miltiorrhiza ameliorate
diabetic kidney disease via the 'gut-kidney axis'. Phytomedicine.
121:1551292023. View Article : Google Scholar
|
|
14
|
Ji H, Zhao X, Chen X, Fang H, Gao H, Wei
G, Zhang M, Kuang H, Yang B, Cai X, et al: Jinlida for diabetes
prevention in impaired glucose tolerance and multiple metabolic
abnormalities: The FOCUS randomized clinical trial. JAMA Intern
Med. 184:727–735. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang D, Tian M, Qi Y, Chen G, Xu L, Zou X,
Wang K, Dong H and Lu F: Jinlida granule inhibits palmitic acid
induced-intracellular lipid accumulation and enhances autophagy in
NIT-1 pancreatic β cells through AMPK activation. J Ethnopharmacol.
161:99–107. 2015. View Article : Google Scholar
|
|
16
|
Zhang H, Hao Y, Wei C, Yao B, Liu S, Zhou
H, Huang D, Zhang C and Wu Y: Chinese medicine Jinlida granules
improve high-fat-diet induced metabolic disorders via activation of
brown adipose tissue in mice. Biomed Pharmacother. 114:1087812019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Susztak K, Raff AC, Schiffer M and
Böttinger EP: Glucose-induced reactive oxygen species cause
apoptosis of podocytes and podocyte depletion at the onset of
diabetic nephropathy. Diabetes. 55:225–233. 2006. View Article : Google Scholar
|
|
18
|
Qin X, Zhao Y, Gong J, Huang W, Su H, Yuan
F, Fang K, Wang D, Li J, Zou X, et al: Berberine protects
glomerular podocytes via inhibiting Drp1-mediated mitochondrial
fission and dysfunction. Theranostics. 9:1698–1713. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Steinberg GR and Hardie DG: New insights
into activation and function of the AMPK. Nat Rev Mol Cell Biol.
24:255–272. 2023. View Article : Google Scholar
|
|
20
|
Handschin C and Spiegelman BM: Peroxisome
proliferator-activated receptor gamma coactivator 1 coactivators,
energy homeostasis, and metabolism. Endocr Rev. 27:728–735. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mohandes S, Doke T, Hu H, Mukhi D, Dhillon
P and Susztak K: Molecular pathways that drive diabetic kidney
disease. J Clin Invest. 133:e1656542023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tervaert TW, Mooyaart AL, Amann K, Cohen
AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer
E, et al: Pathologic classification of diabetic nephropathy. J Am
Soc Nephrol. 21:556–563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mukhi D and Susztak K: The transcriptomic
signature of the aging podocyte. Kidney Int. 98:1079–1081. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu S, Yuan Y, Xue Y, Xing C and Zhang B:
Podocyte injury in diabetic kidney disease: A focus on
mitochondrial dysfunction. Front Cell Dev Biol. 10:8328872022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Audzeyenka I, Bierżyńska A and Lay AC:
Podocyte bioenergetics in the development of diabetic nephropathy:
The role of mitochondria. Endocrinology. 163:bqab2342022.
View Article : Google Scholar
|
|
26
|
Haroon S and Vermulst M: Linking
mitochondrial dynamics to mitochondrial protein quality control.
Curr Opin Genet Dev. 38:68–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song M and Dorn GW II: Mitoconfusion:
Noncanonical functioning of dynamism factors in static mitochondria
of the heart. Cell Metab. 21:195–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sabouny R and Shutt TE: Reciprocal
regulation of mitochondrial fission and fusion. Trends Biochem Sci.
45:564–577. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu Q, Zhang H, Cortés NG, Wu D, Wang P,
Zhang J, Mattison JA, Smith E, Bettcher LF, Wang M, et al:
Increased Drp1 acetylation by lipid overload induces cardiomyocyte
death and heart dysfunction. Circ Res. 126:456–470. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tilokani L, Nagashima S, Paupe V and
Prudent J: Mitochondrial dynamics: Overview of molecular
mechanisms. Essays Biochem. 62:341–360. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huynh C, Ryu J, Lee J, Inoki A and Inoki
K: Nutrient-sensing mTORC1 and AMPK pathways in chronic kidney
diseases. Nat Rev Nephrol. 19:102–122. 2023. View Article : Google Scholar
|
|
32
|
Juszczak F, Caron N, Mathew AV and
Declèves AE: Critical role for AMPK in metabolic disease-induced
chronic kidney disease. Int J Mol Sci. 21:79942020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rogacka D, Audzeyenka I and Piwkowska A:
Regulation of podocytes function by AMP-activated protein kinase.
Arch Biochem Biophys. 692:1085412020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eid AA, Ford BM, Bhandary B, de Cassia
Cavaglieri R, Block K, Barnes JL, Gorin Y, Choudhury GG and Abboud
HE: Mammalian target of rapamycin regulates Nox4-mediated podocyte
depletion in diabetic renal injury. Diabetes. 62:2935–2947. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li SY and Susztak K: The role of
peroxisome proliferator-activated receptor γ coactivator 1α
(PGC-1α) in kidney disease. Semin Nephrol. 38:121–126. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fontecha-Barriuso M, Lopez-Diaz AM,
Guerrero-Mauvecin J, Miguel V, Ramos AM, Sanchez-Niño MD,
Ruiz-Ortega M, Ortiz A and Sanz AB: Tubular mitochondrial
dysfunction, oxidative stress, and progression of chronic kidney
disease. Antioxidants (Basel). 11:13562022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Halling JF and Pilegaard H:
PGC-1α-mediated regulation of mitochondrial function and
physiological implications. Appl Physiol Nutr Metab. 45:927–936.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu H, Deng X, Shi Y, Su Y, Wei J and Duan
H: PGC-1α, glucose metabolism and type 2 diabetes mellitus. J
Endocrinol. 229:R99–R115. 2016. View Article : Google Scholar
|
|
39
|
Ji JL, Li JY, Liang JX, Zhou Y, Liu CC,
Zhang Y, Zhang AQ, Liu H, Ma RX and Li ZL: Tubular TMEM16A promotes
tubulointerstitial fibrosis by suppressing PGC-1α-mediated
mitochondrial homeostasis in diabetic kidney disease. Cell Mol Life
Sci. 80:3472023. View Article : Google Scholar
|
|
40
|
Fontecha-Barriuso M, Martin-Sanchez D,
Martinez-Moreno JM, Monsalve M, Ramos AM, Sanchez-Niño MD,
Ruiz-Ortega M, Ortiz A and Sanz AB: The role of PGC-1α and
mitochondrial biogenesis in kidney diseases. Biomolecules.
10:3472020. View Article : Google Scholar
|
|
41
|
Hou S, Zhang T, Li Y, Guo F and Jin X:
Glycyrrhizic acid prevents diabetic nephropathy by activating
AMPK/SIRT1/PGC-1α signaling in db/db Mice. J Diabetes Res.
2017:28659122017. View Article : Google Scholar
|
|
42
|
Dugan LL, You YH, Ali SS, Diamond-Stanic
M, Miyamoto S, DeCleves AE, Andreyev A, Quach T, Ly S, Shekhtman G,
et al: AMPK dysregulation promotes diabetes-related reduction of
superoxide and mitochondrial function. J Clin Invest.
123:4888–4899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gu H, Zhong L, Zhang Y, Sun J, Liu L and
Liu Z: Exploring the mechanism of Jinlida granules against type 2
diabetes mellitus by an integrative pharmacology strategy. Sci Rep.
14:102862024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu Y, Li Y, Xu L, Shi J, Yu X, Wang X, Li
X, Jiang H, Yang T, Yin X, et al: Quercetin attenuates podocyte
apoptosis of diabetic nephropathy through targeting EGFR signaling.
Front Pharmacol. 12:7927772022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
He JY, Hong Q, Chen BX, Cui SY, Liu R, Cai
GY, Guo J and Chen XM: Ginsenoside Rb1 alleviates diabetic kidney
podocyte injury by inhibiting aldose reductase activity. Acta
Pharmacol Sin. 43:342–353. 2022. View Article : Google Scholar :
|
|
46
|
Li X, Wang J, Yan J, He JC, Li Y and Zhong
Y: Additive renal protective effects between arctigenin and
puerarin in diabetic kidney disease. Biomed Pharmacother.
171:1161072024. View Article : Google Scholar : PubMed/NCBI
|