|
1
|
Bagcchi S: WHO's global tuberculosis
report 2022. Lancet Microbe. 4:e202023. View Article : Google Scholar
|
|
2
|
Asadi L, Croxen M, Heffernan C, Dhillon M,
Paulsen C, Egedahl ML, Tyrrell G, Doroshenko A and Long R: How much
do smear-negative patients really contribute to tuberculosis
transmissions? Re-examining an old question with new tools.
EClinicalMedicine. 43:1012502022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meriki HD, Wung NH, Tufon KA, Tony NJ,
Ane-Anyangwe I and Cho-Ngwa F: Evaluation of the performance of an
in-house duplex PCR assay targeting the IS6110 and rpoB genes for
tuberculosis diagnosis in Cameroon. BMC Infect Dis. 20:7912020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Natarajan S, Ranganathan M, Hanna LE and
Tripathy S: Transcriptional profiling and deriving a seven-gene
signature that discriminates active and latent tuberculosis: An
integrative bioinformatics approach. Genes (Basel). 13:6162022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Molloy A, Harrison J, McGrath JS, Owen Z,
Smith C, Liu X, Li X and Cox JAG: Microfluidics as a novel
technique for tuberculosis: From diagnostics to drug discovery.
Microorganisms. 9:23302021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meier JP, Möbus S, Heigl F,
Asbach-Nitzsche A, Niller HH, Plentz A, Avsar K, Heiß-Neumann M,
Schaaf B, Cassens U, et al: Performance of T-Track® TB,
a novel dual marker RT-qPCR-based whole-blood test for improved
detection of active tuberculosis. Diagnostics (Basel). 13:7582023.
View Article : Google Scholar
|
|
7
|
Çiftci İH and Karakeçe E: Comparative
evaluation of TK SLC-L, a rapid liquid mycobacterial culture
medium, with the MGIT system. BMC Infect Dis. 14:1302014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
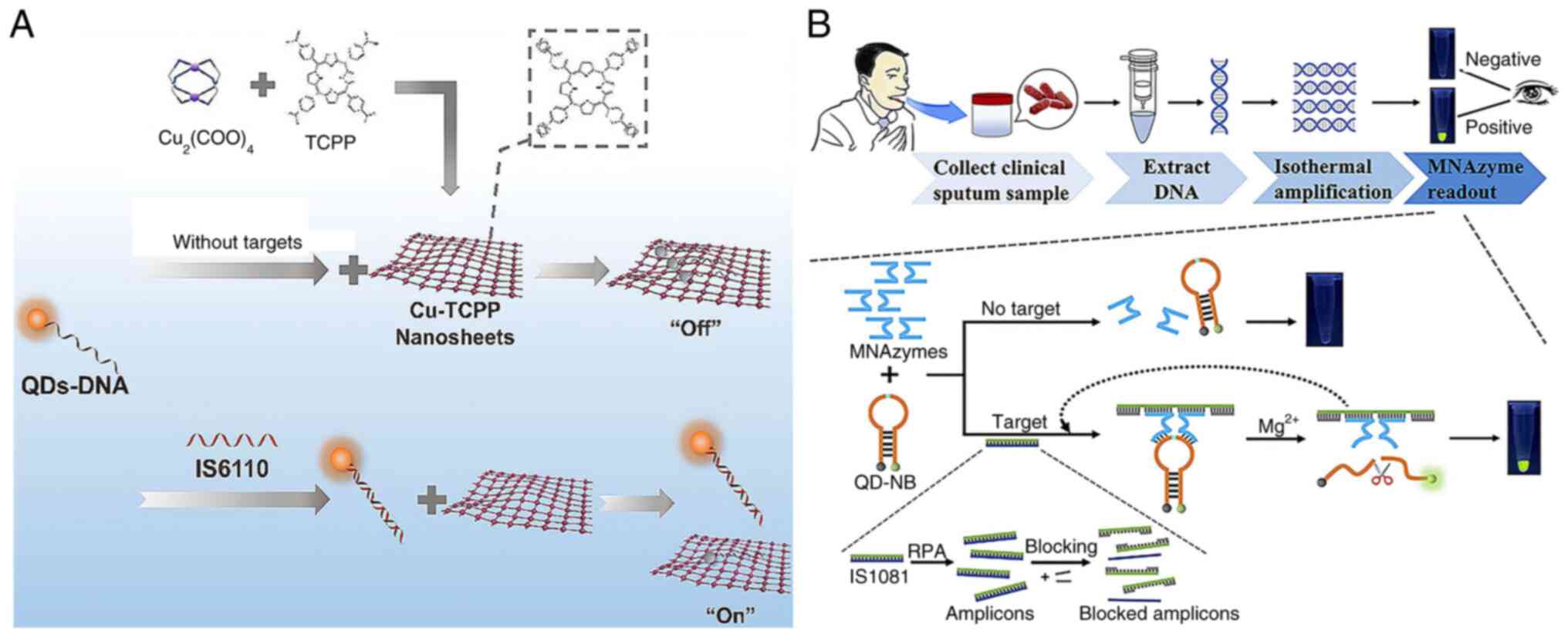
|
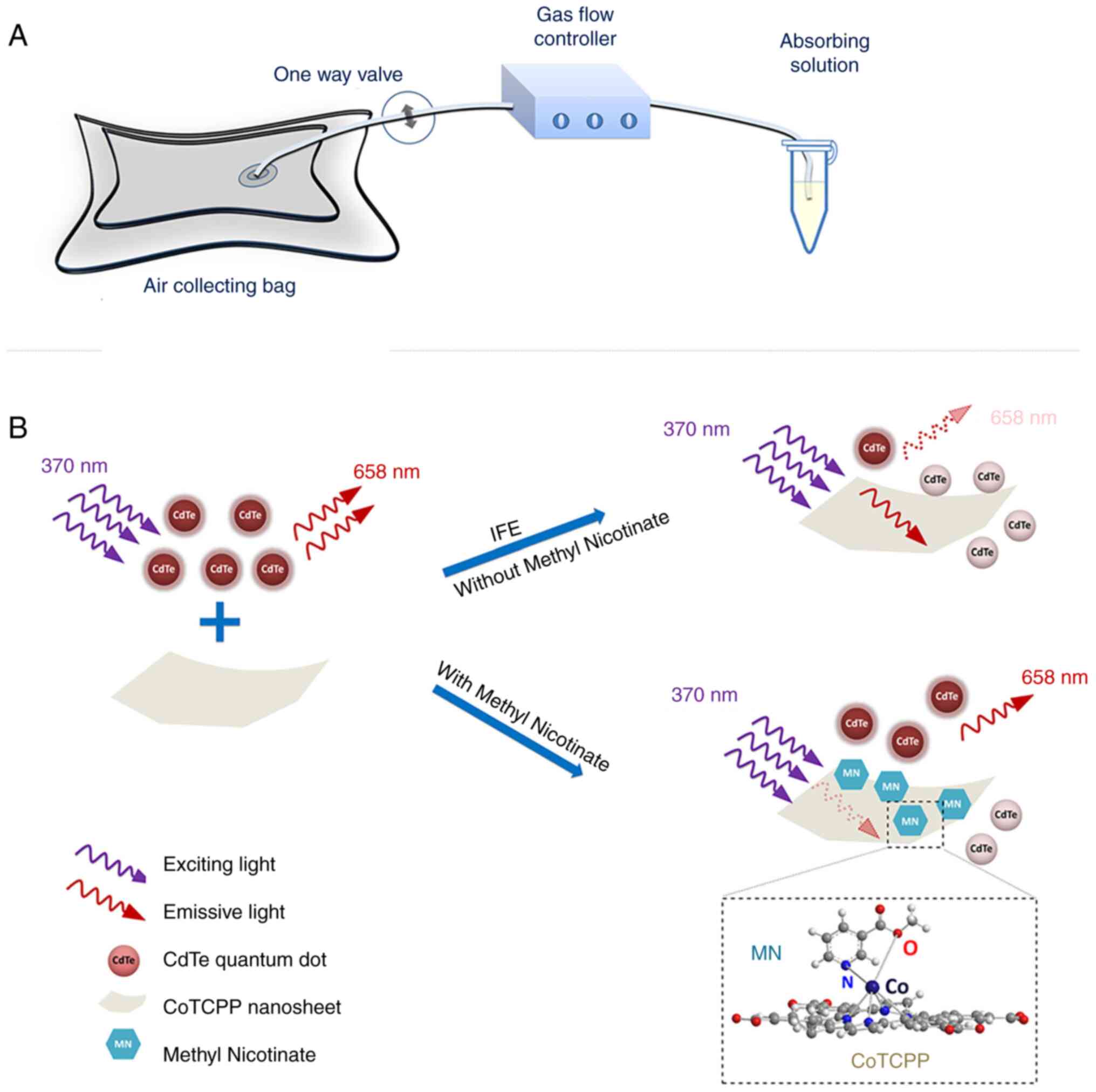
Okoi C anderson STB, Antonio M, Mulwa SN,
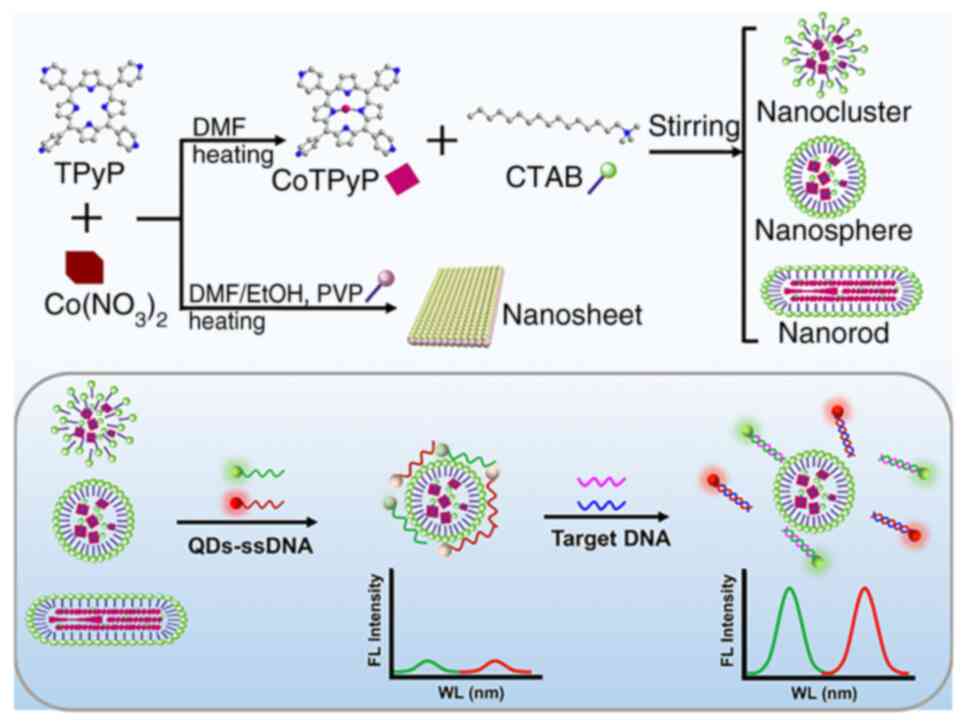
Gehre F and Adetifa IMO: Non-tuberculous mycobacteria isolated from
pulmonary samples in sub-Saharan Africa-a systematic review and
meta analyses. Sci Rep. 7:120022017. View Article : Google Scholar
|
|
9
|
Reed JL, Walker ZJ, Basu D, Allen V, Nicol
MP, Kelso DM and McFall SM: Highly sensitive sequence specific qPCR
detection of Mycobacterium tuberculosis complex in respiratory
specimens. Tuberculosis (Edinb). 101:114–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang X, Fan S, Ma Y, Chen H, Xu JF, Pi J,
Wang W and Chen G: Current progress of functional nanobiosensors
for potential tuberculosis diagnosis: The novel way for TB control?
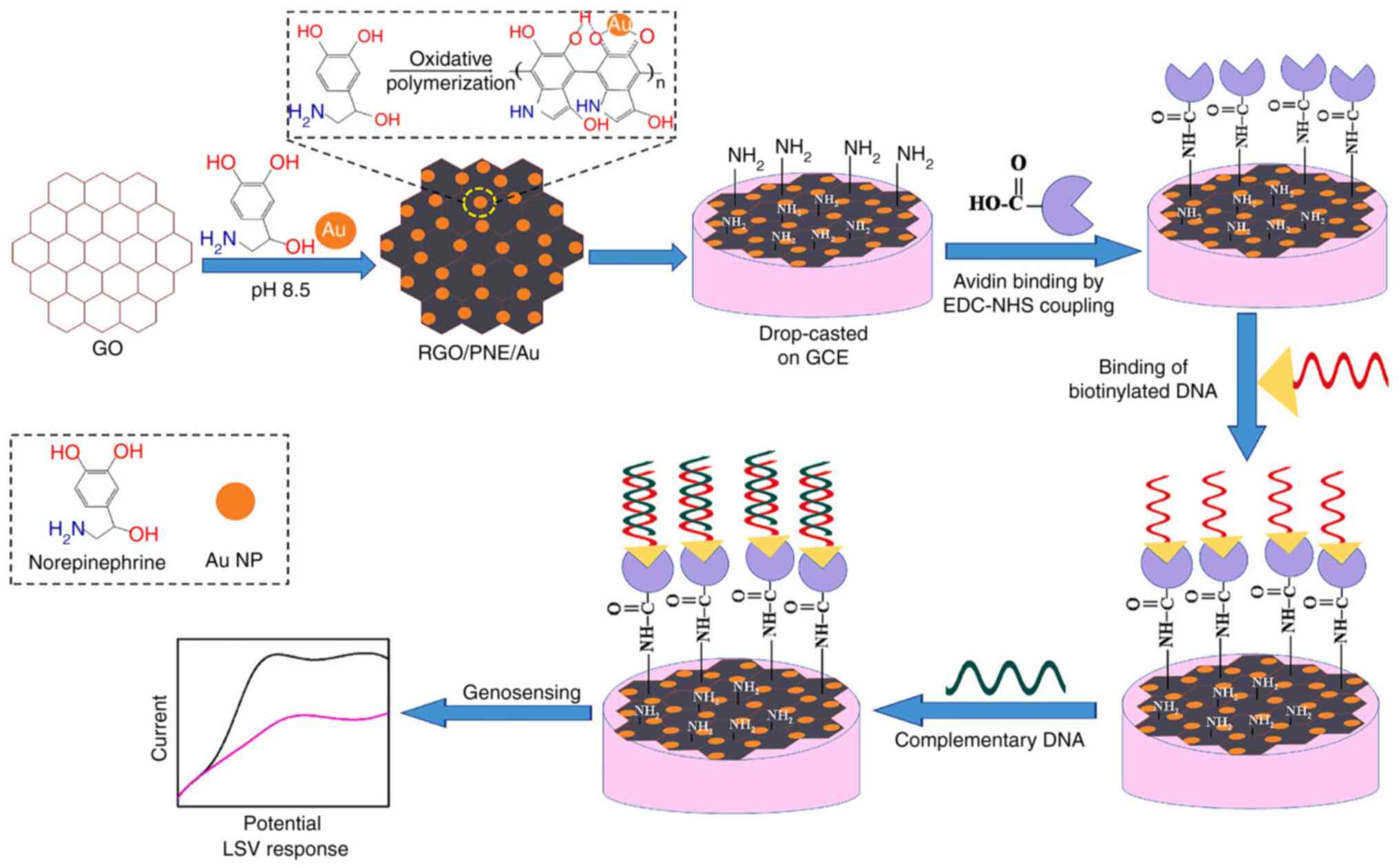
Front Bioeng Biotechnol. 10:10366782022. View Article : Google Scholar :
|
|
11
|
Lyu M, Zhou J, Zhou Y, Chong W, Xu W, Lai
H, Niu L, Hai Y, Yao X, Gong S, et al: From tuberculosis bedside to
bench: UBE2B splicing as a potential biomarker and its regulatory
mechanism. Signal Transduct Target Ther. 8:822023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Metcalf T, Soria J, Montano SM, Ticona E,
Evans CA, Huaroto L, Kasper M, Ramos ES, Mori N, Jittamala P, et
al: Evaluation of the GeneXpert MTB/RIF in patients with
presumptive tuberculous meningitis. PLoS One. 13:e01986952018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tu Phan LM, Tufa LT, Kim HJ, Lee J and
Park TJ: Trends in diagnosis for active tuberculosis using
nanomaterials. Curr Med Chem. 26:1946–1959. 2019. View Article : Google Scholar
|
|
14
|
Joshi H, Kandari D, Maitra SS and
Bhatnagar R: Biosensors for the detection of Mycobacterium
tuberculosis: A comprehensive overview. Crit Rev Microbiol.
48:784–812. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pourakbari R, Shadjou N, Yousefi H,
Isildak I, Yousefi M, Rashidi MR and Khalilzadeh B: Recent progress
in nanomaterial-based electrochemical biosensors for pathogenic
bacteria. Mikrochim Acta. 186:8202019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uhuo OV, Waryo TT, Douman SF, Januarie KC,
Nwambaekwe KC, Ndipingwi MM, Ekwere P and Iwuoha EI: Bioanalytical
methods encompassing label-free and labeled tuberculosis
aptasensors: A review. Anal Chim Acta. 1234:3403262022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu K, Liang ZC, Ding X, Hu H, Liu S,
Nurmik M, Bi S, Hu F, Ji Z, Ren J, et al: Nanomaterials in the
prevention, diagnosis, and treatment of Mycobacterium tuberculosis
infections. Adv Healthc Mater. 7:17005092018. View Article : Google Scholar
|
|
18
|
Tan P, Li H, Wang J and Gopinath SCB:
Silver nanoparticle in biosensor and bioimaging: Clinical
perspectives. Biotechnol Appl Biochem. 68:1236–1242. 2021.
|
|
19
|
Muthukrishnan L: Multidrug resistant
tuberculosis-diagnostic challenges and its conquering by
nanotechnology approach-an overview. Chem Biol Interact.
337:1093972021. View Article : Google Scholar
|
|
20
|
Zhou B, Zhu M, Hao Y and Yang P:
Potential-resolved electrochemiluminescence for simultaneous
determination of triple latent tuberculosis infection markers. ACS
Appl Mater Interfaces. 9:30536–30542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dykman L and Khlebtsov N: Gold
nanoparticles in biomedical applications: Recent advances and
perspectives. Chem Soc Rev. 41:2256–2282. 2012. View Article : Google Scholar
|
|
22
|
Sapsford KE, Algar WR, Berti L, Gemmill
KB, Casey BJ, Oh E, Stewart MH and Medintz IL: Functionalizing
nanoparticles with biological molecules: Developing chemistries
that facilitate nanotechnology. Chem Rev. 113:1904–2074. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Drain PK, Bajema KL, Dowdy D, Dheda K,
Naidoo K, Schumacher SG, Ma S, Meermeier E, Lewinsohn DM and
Sherman DR: Incipient and subclinical tuberculosis: A clinical
review of early stages and progression of infection. Clin Microbiol
Rev. 31:e00021–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rosi NL and Mirkin CA: Nanostructures in
biodiagnostics. Chem Rev. 105:1547–1562. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Singh V and Chibale K: Strategies to
combat multi-drug resistance in tuberculosis. Acc Chem Res.
54:2361–2376. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Golichenari B, Nosrati R, Farokhi-Fard A,
Abnous K, Vaziri F and Behravan J: Nano-biosensing approaches on
tuberculosis: Defy of aptamers. Biosens Bioelectron. 117:319–331.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eivazzadeh-Keihan R, Saadatidizaji Z,
Mahdavi M, Maleki A, Irani M and Zare I: Recent advances in gold
nanoparticles-based biosensors for tuberculosis determination.
Talanta. 275:1260992024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Golichenari B, Nosrati R, Farokhi-Fard A,
Faal Maleki M, Gheibi Hayat SM, Ghazvini K, Vaziri F and Behravan
J: Electrochemical-based biosensors for detection of Mycobacterium
tuberculosis and tuberculosis biomarkers. Crit Rev Biotechnol.
39:1056–1077. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Seele PP, Dyan B, Skepu A, Maserumule C
and Sibuyi NRS: Development of gold-nanoparticle-based lateral flow
immunoassays for rapid detection of TB ESAT-6 and CFP-10.
Biosensors (Basel). 13:3542023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kamra E, Prasad T, Rais A, Dahiya B,
Sheoran A, Soni A, Sharma S and Mehta PK: Diagnosis of
genitourinary tuberculosis: Detection of mycobacterial
lipoarabinomannan and MPT-64 biomarkers within urine extracellular
vesicles by nano-based immuno-PCR assay. Sci Rep. 13:115602023.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dahiya B, Prasad T, Rais A, Sheoran A,
Kamra E, Mor P, Soni A, Sharma S and Mehta PK: Quantification of
mycobacterial proteins in extrapulmonary tuberculosis cases by
nano-based real-time immuno-PCR. Future Microbiol. 18:771–783.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tripathi A, Jain R and Dandekar P: Rapid
visual detection of Mycobacterium tuberculosis DNA using gold
nanoparticles. Anal Methods. 15:2497–2504. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang H, Chen Y, Zuo J, Deng C, Fan J, Bai
L and Guo S: MXene-incorporated C60NPs and Au@Pt with
dual-electric signal outputs for accurate detection of
Mycobacterium tuberculosis ESAT-6 antigen. Biosens Bioelectron.
242:1157342023. View Article : Google Scholar
|
|
34
|
Patnaik N and Dey RJ: Label-free
citrate-stabilized silver nanoparticles-based, highly sensitive,
cost-effective, and rapid visual method for the differential
detection of Mycobacterium tuberculosis and mycobacterium bovis.
ACS Infect Dis. 10:426–435. 2024. View Article : Google Scholar
|
|
35
|
Pei X, Hong H, Liu S and Li N: Nucleic
acids detection for Mycobacterium tuberculosis based on gold
nanoparticles counting and rolling-circle amplification. Biosensors
(Basel). 12:4482022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
León-Janampa N, Shinkaruk S, Gilman RH,
Kirwan DE, Fouquet E, Szlosek M, Sheen P and Zimic M:
Biorecognition and detection of antigens from Mycobacterium
tuberculosis using a sandwich ELISA associated with magnetic
nanoparticles. J Pharm Biomed Anal. 215:1147492022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J and He F: Mycobacterium
tuberculosis piezoelectric sensor based on AuNPs-mediated enzyme
assisted signal amplification. Talanta. 236:1229022022. View Article : Google Scholar
|
|
38
|
Xie J, Mu Z, Yan B, Wang J, Zhou J and Bai
L: An electrochemical aptasensor for Mycobacterium tuberculosis
ESAT-6 antigen detection using bimetallic organic framework.
Mikrochim Acta. 188:4042021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Prabowo BA, Purwidyantri A, Liu B, Lai HC
and Liu KC: Gold nanoparticle-assisted plasmonic enhancement for
DNA detection on a graphene-based portable surface plasmon
resonance sensor. Nanotechnology. 32:0955032021. View Article : Google Scholar
|
|
40
|
Tai MJY, Perumal V, Gopinath SCB, Raja PB,
Ibrahim MNM, Jantan IN, Suhaimi NSH and Liu WW: Laser-scribed
graphene nanofiber decorated with oil palm lignin capped silver
nanoparticles: A green biosensor. Sci Rep. 11:54752021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mohd Azmi UZ, Yusof NA, Abdullah J, Alang
Ahmad SA, Mohd Faudzi FN, Ahmad Raston NH, Suraiya S, Ong PS,
Krishnan D and Sahar NK: Portable electrochemical immunosensor for
detection of Mycobacterium tuberculosis secreted protein
CFP10-ESAT6 in clinical sputum samples. Mikrochim Acta. 188:202021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gupta S, Bhatter P and Kakkar V:
Point-of-care detection of tuberculosis using magnetoresistive
biosensing chip. Tuberculosis (Edinb). 127:1020552021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
León-Janampa N, Zimic M, Shinkaruk S,
Quispe-Marcatoma J, Gutarra A, Le Bourdon G, Gayot M, Changanaqui
K, Gilman RH, Fouquet E, et al: Synthesis, characterization and
bio-functionalization of magnetic nanoparticles to improve the
diagnosis of tuberculosis. Nanotechnology. 31:1751012020.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Terefinko D, Dzimitrowicz A,
Bielawska-Pohl A, Klimczak A, Pohl P and Jamroz P: The influence of
cold atmospheric pressure plasma-treated media on the cell
viability, motility, and induction of apoptosis in in human
non-metastatic (MCF7) and metastatic (MDA-MB-231) breast cancer
cell lines. Int J Mol Sci. 22:38552021. View Article : Google Scholar
|
|
45
|
Gupta AK, Singh A and Singh S: Diagnosis
of Tuberculosis: Nanodiagnostics Approaches. Saxena S and Khurana
S: NanoBioMedicine. Springer; Singapore: pp. 261–283. 2020,
View Article : Google Scholar
|
|
46
|
Cordeiro M, Ferreira Carlos F, Pedrosa P,
Lopez A and Baptista PV: Gold nanoparticles for diagnostics:
Advances towards points of care. Diagnostics (Basel). 6:432016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang Y, Yu L, Kong X and Sun L:
Application of nanodiagnostics in point-of-care tests for
infectious diseases. Int J Nanomedicine. 12:4789–4803. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chowdhury NK, Choudhury R, Gogoi B, Chang
CM and Pandey RP: Microbial synthesis of gold nanoparticles and
their application. Curr Drug Targets. 23:752–760. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lopes TS, Alves GG, Pereira MR, Granjeiro
JM and Leite PEC: Advances and potential application of gold
nanoparticles in nanomedicine. J Cell Biochem. 120:16370–16378.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Anker JN, Hall WP, Lyandres O, Shah NC,
Zhao J and Van Duyne RP: Biosensing with plasmonic nanosensors. Nat
Mater. 7:442–453. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Datta M, Desai D and Kumar A: Gene
specific DNA sensors for diagnosis of pathogenic infections. Indian
J Microbiol. 57:139–147. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mi X, He F, Xiang M, Lian Y and Yi S:
Novel phage amplified multichannel series piezoelectric quartz
crystal sensor for rapid and sensitive detection of Mycobacterium
tuberculosis. Anal Chem. 84:939–946. 2012. View Article : Google Scholar
|
|
53
|
Zhang X, Feng Y, Duan S, Su L, Zhang J and
He F: Mycobacterium tuberculosis strain H37Rv electrochemical
sensor mediated by aptamer and AuNPs-DNA. ACS Sens. 4:849–855.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Teengam P, Siangproh W, Tuantranont A,
Vilaivan T, Chailapakul O and Henry CS: Multiplex paper-based
colorimetric DNA sensor using pyrrolidinyl peptide nucleic
acid-induced AgNPs aggregation for detecting MERS-CoV, MTB, and HPV
oligonucleotides. Anal Chem. 89:5428–5435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pascu B, Negrea A, Ciopec M, Duteanu N,
Negrea P, Bumm LA, Grad mBuriac O, Nemeş NS, Mihalcea C and
Duda-Seiman DM: Silver nanoparticle synthesis via photochemical
reduction with sodium citrate. Int J Mol Sci. 24:2552022.
View Article : Google Scholar
|
|
56
|
Iravani S, Korbekandi H, Mirmohammadi SV
and Zolfaghari B: Synthesis of silver nanoparticles: Chemical,
physical and biological methods. Res Pharm Sci. 9:385–406.
2014.
|
|
57
|
Salvador M, Marqués-Fernandez JL,
Martinez-Garcia JC, Fiorani D, Arosio P, Avolio M, Brero F,
Balanean F, Guerrini A, Sangregorio C, et al: Double-layer fatty
acid nanoparticles as a multiplatform for diagnostics and therapy.
Nanomaterials (Basel). 12:2052022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cheon HJ, Lee SM, Kim SR, Shin HY, Seo YH,
Cho YK, Lee SP and Kim MI: Colorimetric detection of MPT64 antibody
based on an aptamer adsorbed magnetic nanoparticles for diagnosis
of tuberculosis. J Nanosci Nanotechnol. 19:622–626. 2019.
View Article : Google Scholar
|
|
59
|
Yan Z, Gan N, Zhang H, Wang D, Qiao L, Cao
Y, Li T and Hu F: A sandwich-hybridization assay for simultaneous
determination of HIV and tuberculosis DNA targets based on signal
amplification by quantum dots-PowerVision™ polymer coding
nanotracers. Biosens Bioelectron. 71:207–213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen P, Meng Y, Liu T, Peng W, Gao Y, He
Y, Qu R, Zhang C, Hu W and Ying B: Sensitive urine immunoassay for
visualization of lipoarabinomannan for noninvasive tuberculosis
diagnosis. ACS Nano. 17:6998–7006. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hu O, Li Z, Wu J, Tan Y, Chen Z and Tong
Y: A multicomponent nucleic acid enzyme-cleavable quantum dot
nanobeacon for highly sensitive diagnosis of tuberculosis with the
naked eye. ACS Sens. 8:254–262. 2023. View Article : Google Scholar
|
|
62
|
He Q, Cai S, Wu J, Hu O, Liang L and Chen
Z: Determination of tuberculosis-related volatile organic biomarker
methyl nicotinate in vapor using fluorescent assay based on quantum
dots and cobalt-containing porphyrin nanosheets. Mikrochim Acta.
189:1082022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hu O, Li Z, He Q, Tong Y, Tan Y and Chen
Z: Fluorescence biosensor for one-step simultaneous detection of
Mycobacterium tuberculosis multidrug-resistant genes using
nanoCoTPyP and double quantum dots. Anal Chem. 94:7918–7927. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kabwe KP, Nsibande SA, Lemmer Y, Pilcher
LA and Forbes PBC: Synthesis and characterisation of quantum dots
coupled to mycolic acids as a water-soluble fluorescent probe for
potential lateral flow detection of antibodies and diagnosis of
tuberculosis. Luminescence. 37:278–289. 2022. View Article : Google Scholar
|
|
65
|
Shi T, Jiang P, Peng W, Meng Y, Ying B and
Chen P: Nucleic acid and nanomaterial synergistic amplification
enables dual targets of ultrasensitive fluorescence quantification
to improve the efficacy of clinical tuberculosis diagnosis. ACS
Appl Mater Interfaces. 16:14510–14519. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kabwe KP, Nsibande SA, Pilcher LA and
Forbes PBC: Development of a mycolic acid-graphene quantum dot
probe as a potential tuberculosis biosensor. Luminescence.
37:1881–1890. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liang L, Chen M, Tong Y, Tan W and Chen Z:
Detection of Mycobacterium tuberculosis IS6110 gene fragment by
fluorescent biosensor based on FRET between two-dimensional
metal-organic framework and quantum dots-labeled DNA probe. Anal
Chim Acta. 1186:3390902021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Mohd Bakhori N, Yusof NA, Abdullah J,
Wasoh H, Ab Rahman SK and Abd Rahman SF: Surface enhanced CdSe/ZnS
QD/SiNP electrochemical immunosensor for the detection of
Mycobacterium tuberculosis by combination of CFP10-ESAT6 for better
diagnostic specificity. Materials (Basel). 13:1492019. View Article : Google Scholar
|
|
69
|
Qian J, Cui H, Lu X, Wang C, An K, Hao N
and Wang K: Bi-color FRET from two nano-donors to a single
nano-acceptor: A universal aptasensing platform for simultaneous
determination of dual targets. Chem Eng J. 401:1260172020.
View Article : Google Scholar
|
|
70
|
Zhang LM, Li R, Zhao XC, Zhang Q and Luo
XL: Increased transfusion of fresh frozen plasma is associated with
mortality or worse functional outcomes after severe traumatic brain
injury: A retrospective study. World Neurosurg. 104:381–389. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang X, Hu Y, Yang X, Tang Y, Han S, Kang
A, Deng H, Chi Y, Zhu D and Lu Y: FÖrster resonance energy transfer
(FRET)-based biosensors for biological applications. Biosens
Bioelectron. 138:1113142019. View Article : Google Scholar
|
|
72
|
Chen S, Yu YL and Wang JH: Inner filter
effect-based fluorescent sensing systems: A review. Anal Chim Acta.
999:13–26. 2018. View Article : Google Scholar
|
|
73
|
Afsari HS, Cardoso Dos Santos M, Lindén S,
Chen T, Qiu X, van Bergen En Henegouwen PM, Jennings TL, Susumu K,
Medintz IL, Hildebrandt N and Miller LW: Time-gated FRET
nanoassemblies for rapid and sensitive intra- and extracellular
fluorescence imaging. Sci Adv. 2:e16002652016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gliddon HD, Howes PD, Kaforou M, Levin M
and Stevens MM: A nucleic acid strand displacement system for the
multiplexed detection of tuberculosis-specific mRNA using quantum
dots. Nanoscale. 8:10087–10095. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Futane A, Narayanamurthy V, Jadhav P and
Srinivasan A: Aptamer-based rapid diagnosis for point-of-care
application. Microfluid Nanofluidics. 27:152023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kumar S, Wang Z, Zhang W, Liu X, Li M, Li
G, Zhang B and Singh R: Optically active nanomaterials and its
biosensing applications-a review. Biosensors (Basel). 13:852023.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sharifi S, Vahed SZ, Ahmadian E, Dizaj SM,
Eftekhari A, Khalilov R, Ahmadi M, Hamidi-Asl E and Labib M:
Detection of pathogenic bacteria via nanomaterials-modified
aptasensors. Biosens Bioelectron. 150:1119332020. View Article : Google Scholar
|
|
78
|
Pornprom T, Phusi N, Thongdee P, Pakamwong
B, Sangswan J, Kamsri P, Punkvang A, Suttisintong K,
Leanpolchareanchai J, Hongmanee P, et al: Toward the early
diagnosis of tuberculosis: A gold particle-decorated
graphene-modified paper-based electrochemical biosensor for Hsp16.3
detection. Talanta. 267:1252102024. View Article : Google Scholar
|
|
79
|
Wang J, Shao W, Liu Z, Kesavan G, Zeng Z,
Shurin MR and Star A: Diagnostics of tuberculosis with
single-walled carbon nanotube-based field-effect transistors. ACS
Sens. 9:1957–1966. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Le TN, Descanzo MJN, Hsiao WWW, Soo PC,
Peng WP and Chang HC: Fluorescent nanodiamond immunosensors for
clinical diagnostics of tuberculosis. J Mater Chem B. 12:3533–3542.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bisht N, Patel M, Dwivedi N, Kumar P,
Mondal DP, Srivastava AK and Dhand C: Bio-inspired
polynorepinephrine based nanocoatings for reduced graphene
oxide/gold nanoparticles composite for high-performance biosensing
of Mycobacterium tuberculosis. Environ Res. 227:1156842023.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Seo G, Lee G, Kim W, An I, Choi M, Jang S,
Park YJ, Lee JO, Cho D and Park EC: Ultrasensitive biosensing
platform for Mycobacterium tuberculosis detection based on
functionalized graphene devices. Front Bioeng Biotechnol.
11:13134942023. View Article : Google Scholar
|
|
83
|
Mogha NK, Sahu V, Sharma RK and Masram DT:
Reduced graphene oxide nanoribbon immobilized gold nanoparticle
based electrochemical DNA biosensor for the detection of
Mycobacterium tuberculosis. J Mater Chem B. 6:5181–5187. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Li Y, Peng D, Guo S, Yang B, Zhou J, Zhou
J, Zhang Q and Bai L: Aptasensor for Mycobacterium tuberculosis
antigen MPT64 detection using anthraquinone derivative confined in
ordered mesoporous carbon as a new redox nanoprobe.
Bioelectrochemistry. 147:1082092022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Rizi KS, Hatamluyi B, Rezayi M, Meshkat Z,
Sankian M, Ghazvini K, Farsiani H and Aryan E: Response surface
methodology optimized electrochemical DNA biosensor based on
HAPNPTs/PPY/MWCNTs nanocomposite for detecting Mycobacterium
tuberculosis. Talanta. 226:1220992021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Javed A, Abbas SR, Hashmi MU, Babar NUA
and Hussain I: Graphene oxide based electrochemical genosensor for
label free detection of mycobacterium tuberculosis from raw
clinical samples. Int J Nanomedicine. 16:7339–7352. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Omar RA, Verma N and Arora PK: Development
of ESAT-6 based immunosensor for the detection of mycobacterium
tuberculosis. Front Immunol. 12:6538532021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Jaroenram W, Kampeera J, Arunrut N,
Karuwan C, Sappat A, Khumwan P, Jaitrong S, Boonnak K, Prammananan
T, Chaiprasert A, et al: Graphene-based electrochemical genosensor
incorporated loop-mediated isothermal amplification for rapid
on-site detection of Mycobacterium tuberculosis. J Pharm Biomed
Anal. 186:1133332020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kahng SJ, Soelberg SD, Fondjo F, Kim JH,
Furlong CE and Chung JH: Carbon nanotube-based thin-film resistive
sensor for point-of-care screening of tuberculosis. Biomed
Microdevices. 22:502020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hidayah NMS, Liu WW, Lai CW, Noriman NZ,
Khe CS, Hashim U and Lee HC: Comparison on graphite, graphene oxide
and reduced graphene oxide: Synthesis and characterization. AIP
Conf Proc. 1892:1500022017. View Article : Google Scholar
|
|
91
|
Ping J, Zhou Y, Wu Y, Papper V, Boujday S,
Marks RS and Steele TW: Recent advances in aptasensors based on
graphene and graphene-like nanomaterials. Biosens Bioelectron.
64:373–385. 2015. View Article : Google Scholar
|
|
92
|
Raccichini R, Varzi A, Passerini S and
Scrosati B: The role of graphene for electrochemical energy
storage. Nat Mater. 14:271–279. 2015. View Article : Google Scholar
|
|
93
|
Yan Q, Zhi N, Yang L, Xu G, Feng Q, Zhang
Q and Sun S: A highly sensitive uric acid electrochemical biosensor
based on a nano-cube cuprous oxide/ferrocene/uricase modified
glassy carbon electrode. Sci Rep. 10:106072020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Barra A, Nunes C, Ruiz-Hitzky E and
Ferreira P: Green carbon nanostructures for functional composite
materials. Int J Mol Sci. 23:18482022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chaturvedi M, Patel M, Bisht N, Shruti,
Das Mukherjee M, Tiwari A, Mondal DP, Srivastava AK, Dwivedi N and
Dhand C: Reduced graphene oxide-polydopamine-gold nanoparticles: A
ternary nanocomposite-based electrochemical genosensor for rapid
and early Mycobacterium tuberculosis detection. Biosensors (Basel).
13:3422023. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Tian J, Deng SY, Li DL, Shan D, He W,
Zhang XJ and Shi Y: Bioinspired polydopamine as the scaffold for
the active AuNPs anchoring and the chemical simultaneously reduced
graphene oxide: Characterization and the enhanced biosensing
application. Biosens Bioelectron. 49:466–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Li Y, Shi S, Cao H, Zhao Z, Su C and Wen
H: Improvement of the antifouling performance and stability of an
anion exchange membrane by surface modification with graphene oxide
(GO) and polydopamine (PDA). J Memb Sci. 566:44–53. 2018.
View Article : Google Scholar
|
|
98
|
Xia L, Vemuri B, Gadhamshetty V and
Kilduff J: Poly (ether sulfone) membrane surface modification using
norepinephrine to mitigate fouling. J Memb Sci. 598:1176572020.
View Article : Google Scholar
|
|
99
|
Dhand C, Ong ST, Dwivedi N, Diaz SM,
Venugopal JR, Navaneethan B, Fazil MH, Liu S, Seitz V, Wintermantel
E, et al: Bio-inspired in situ crosslinking and mineralization of
electrospun collagen scaffolds for bone tissue engineering.
Biomaterials. 104:323–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Teengam P, Siangproh W, Tuantranont A,
Vilaivan T, Chailapakul O and Henry CS: Electrochemical
impedance-based DNA sensor using pyrrolidinyl peptide nucleic acids
for tuberculosis detection. Anal Chim Acta. 1044:102–109. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Thangamuthu M, Hsieh KY, Kumar PV and Chen
GY: Graphene- and graphene oxide-based nanocomposite platforms for
electrochemical biosensing applications. Int J Mol Sci.
20:29752019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Vu CA and Chen WY: Field-effect transistor
biosensors for biomedical applications: Recent advances and future
prospects. Sensors (Basel). 19:42142019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Chen S and Bashir R: Advances in
field-effect biosensors towards point-of-use. Nanotechnology.
34:4920022023. View Article : Google Scholar :
|
|
104
|
Szunerits S, Rodrigues T, Bagale R, Happy
H, Boukherroub R and Knoll W: Graphene-based field-effect
transistors for biosensing: Where is the field heading to? Anal
Bioanal Chem. 416:2137–2150. 2024. View Article : Google Scholar
|
|
105
|
Krishnan SK, Nataraj N, Meyyappan M and
Pal U: Graphene-based field-effect transistors in biosensing and
neural interfacing applications: Recent advances and prospects.
Anal Chem. 95:2590–2622. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gong X, Shuai L, Beingessner RL, Yamazaki
T, Shen J, Kuehne M, Jones K, Fenniri H and Strano MS: Size
selective corona interactions from self-assembled rosette and
single-walled carbon nanotubes. Small. 18:e21049512022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kumar THV, Rajendran J, Atchudan R, Arya
S, Govindasamy M, Habila MA and Sundramoorthy AK: Cobalt
ferrite/semiconducting single-walled carbon nanotubes based
field-effect transistor for determination of carbamate pesticides.
Environ Res. 238:1171932023. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Liu H, Liu F, Sun Z, Cai X, Sun H, Kai Y,
Chen L and Jiang C: Single layer aligned semiconducting
single-walled carbon nanotube array with high linear density.
Nanotechnology. 33:3753012022. View Article : Google Scholar
|
|
109
|
Wang Y, Liu D, Zhang H, Wang J, Du R, Li
TT, Qian J, Hu Y and Huang S: Methylation-induced reversible
metallic-semiconducting transition of single-walled carbon nanotube
arrays for high-performance field-effect transistors. Nano Lett.
20:496–501. 2020. View Article : Google Scholar
|
|
110
|
Tran TT, Clark K, Ma W and Mulchandani A:
Detection of a secreted protein biomarker for citrus Huanglongbing
using a single-walled carbon nanotubes-based chemiresistive
biosensor. Biosens Bioelectron. 147:1117662020. View Article : Google Scholar
|
|
111
|
Shao W, Shurin MR, Wheeler SE, He X and
Star A: Rapid detection of SARS-CoV-2 Antigens using high-purity
semiconducting single-walled carbon nanotube-based field-effect
transistors. ACS Appl Mater Interfaces. 13:10321–10327. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Li T, Liang Y, Li J, Yu Y, Xiao MM, Ni W,
Zhang Z and Zhang GJ: Carbon nanotube field-effect transistor
biosensor for ultrasensitive and label-free detection of breast
cancer exosomal miRNA21. Anal Chem. 93:15501–15507. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Chen H, Xiao M, He J, Zhang Y, Liang Y,
Liu H and Zhang Z: Aptamer-functionalized carbon nanotube
field-effect transistor biosensors for Alzheimer's disease serum
biomarker detection. ACS Sens. 7:2075–2083. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Hui YY, Chen OJ, Lin HH, Su YK, Chen KY,
Wang CY, Hsiao WW and Chang HC: Magnetically modulated fluorescence
of nitrogen-vacancy centers in nanodiamonds for ultrasensitive
biomedical analysis. Anal Chem. 93:7140–7147. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Boruah A and Saikia BK: Synthesis,
characterization, properties and novel applications of fluorescent
nanodiamonds. J Fluoresc. 32:863–885. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Mzyk A, Sigaeva A and Schirhagl R:
Relaxometry with nitrogen vacancy (NV) centers in diamond. Acc Chem
Res. 55:3572–3580. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Daniel MC and Astruc D: Gold
nanoparticles: Assembly, supramolecular chemistry,
quantum-size-related properties, and applications toward biology,
catalysis, and nanotechnology. Chem Rev. 104:293–346. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Medintz IL, Uyeda HT, Goldman ER and
Mattoussi H: Quantum dot bioconjugates for imaging, labelling and
sensing. Nat Mater. 4:435–446. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wei Y and Yang R: Nanomechanics of
graphene. Natl Sci Rev. 6:324–348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Eckhardt S, Brunetto PS, Gagnon J, Priebe
M, Giese B and Fromm KM: Nanobio silver: Its interactions with
peptides and bacteria, and its uses in medicine. Chem Rev.
113:4708–4754. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zhao P, Xu Q, Tao J, Jin Z, Pan Y, Yu C
and Yu Z: Near infrared quantum dots in biomedical applications:
Current status and future perspective. Wiley Interdiscip Rev
Nanomed Nanobiotechnol. 10:e14832018. View Article : Google Scholar
|
|
122
|
Laurent S, Bridot JL, Elst LV and Muller
RN: Magnetic iron oxide nanoparticles for biomedical applications.
Future Med Chem. 2:427–449. 2010. View Article : Google Scholar
|
|
123
|
Haiss W, Thanh NT, Aveyard J and Fernig
DG: Determination of size and concentration of gold nanoparticles
from UV-vis spectra. Anal Chem. 79:4215–4221. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kim D, Shin K, Kwon SG and Hyeon T:
Synthesis and biomedical applications of multifunctional
nanoparticles. Adv Mater. 30:e18023092018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Sobhanan J, Anas A and Biju V:
Nanomaterials for fluorescence and multimodal bioimaging. Chem Rec.
23:e2022002532023. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Katz E and Willner I: Integrated
nanoparticle-biomolecule hybrid systems: Synthesis, properties, and
applications. Angew Chem Int Ed Engl. 43:6042–6108. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Li B, Wang W, Zhao L, Wu Y, Li X, Yan D,
Gao Q, Yan Y, Zhang J, Feng Y, et al: Photothermal therapy of
tuberculosis using targeting pre-activated macrophage
membrane-coated nanoparticles. Nat Nanotechnol. 19:834–845. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Nair A, Greeny A, Nandan A, Sah RK, Jose
A, Dyawanapelly S, Junnuthula V, K V A and Sadanandan P: Advanced
drug delivery and therapeutic strategies for tuberculosis
treatment. J Nanobiotechnology. 21:4142023. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
El-Samadony H, Althani A, Tageldin MA and
Azzazy HME: Nanodiagnostics for tuberculosis detection. Expert Rev
Mol Diagn. 17:427–443. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Li M, Singh R, Wang Y, Marques C, Zhang B
and Kumar S: Advances in novel nanomaterial-based optical fiber
biosensors-a review. Biosensors (Basel). 12:8432022. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Vu CQ and Arai S: Quantitative imaging of
genetically encoded fluorescence lifetime biosensors. Biosensors
(Basel). 13:9392023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Hemmerová E and Homola J: Combining
plasmonic and electrochemical biosensing methods. Biosens
Bioelectron. 251:1160982024. View Article : Google Scholar : PubMed/NCBI
|