Mycobacterium tuberculosis (MTB) infection causes
tuberculosis (TB), which affects 25% of the world's population. In
2022, the World Health Organization reported 10.6 million new TB
infections, 133 per 100,000 persons and 1.3 million mortalities
(1). TB is the second most
deadly infectious disease worldwide, surpassed only by COVID-19,
with its mortality rate nearly double that of HIV/AIDS (2). Early and accurate TB diagnosis is
crucial for its control and management (3). Early discovery improves therapy,
limiting illness progression and serious consequences (4). Accurate identification of TB cases
can reduce the spread of MTB, especially in densely populated and
resource-limited areas, thereby reducing pressure on the healthcare
system (5).
Immunological, radiographic and bacteriological
methods are used to diagnose TB (6). The tuberculin skin test and INF-γ
release assay are simple immunological assays that detect TB within
72 h. However, the window time of the disease, the immune system
and experimental methods can cause false positives and negatives
(7). Chest X-rays and
computerized tomography scans can detect lung abnormalities and
track illness progression, but they are less sensitive and specific
and cannot distinguish TB from other lung infectious disorders
(8,9). Antacid smear microscopy and sputum
culture are the main bacterial tests; however, smear microscopy has
just 30% sensitivity and mycobacterial culture, the gold standard,
takes 2 weeks to provide positive results, during which MTB will
spread in the population (10,11). Automated Nucleic Acid
Amplification (PCR) Assay System (GeneXpert) can detect MTB and
rifampicin resistance in 2 h (12); however, due to the expensive cost
of instruments and reagents and the strict environmental
requirements of the assay, GeneXpert is challenging to apply in
distant and impoverished locations and its sensitivity is still
limited for sputum specimens with low bacterial loads (13). Thus, rapid, cost-effective,
precise and sensitive TB diagnostic methods are needed.
Due to the efficiency and simplicity of
nanotechnology, diagnostic devices based on nanomaterials are
typically compact and easy to operate, making them suitable for
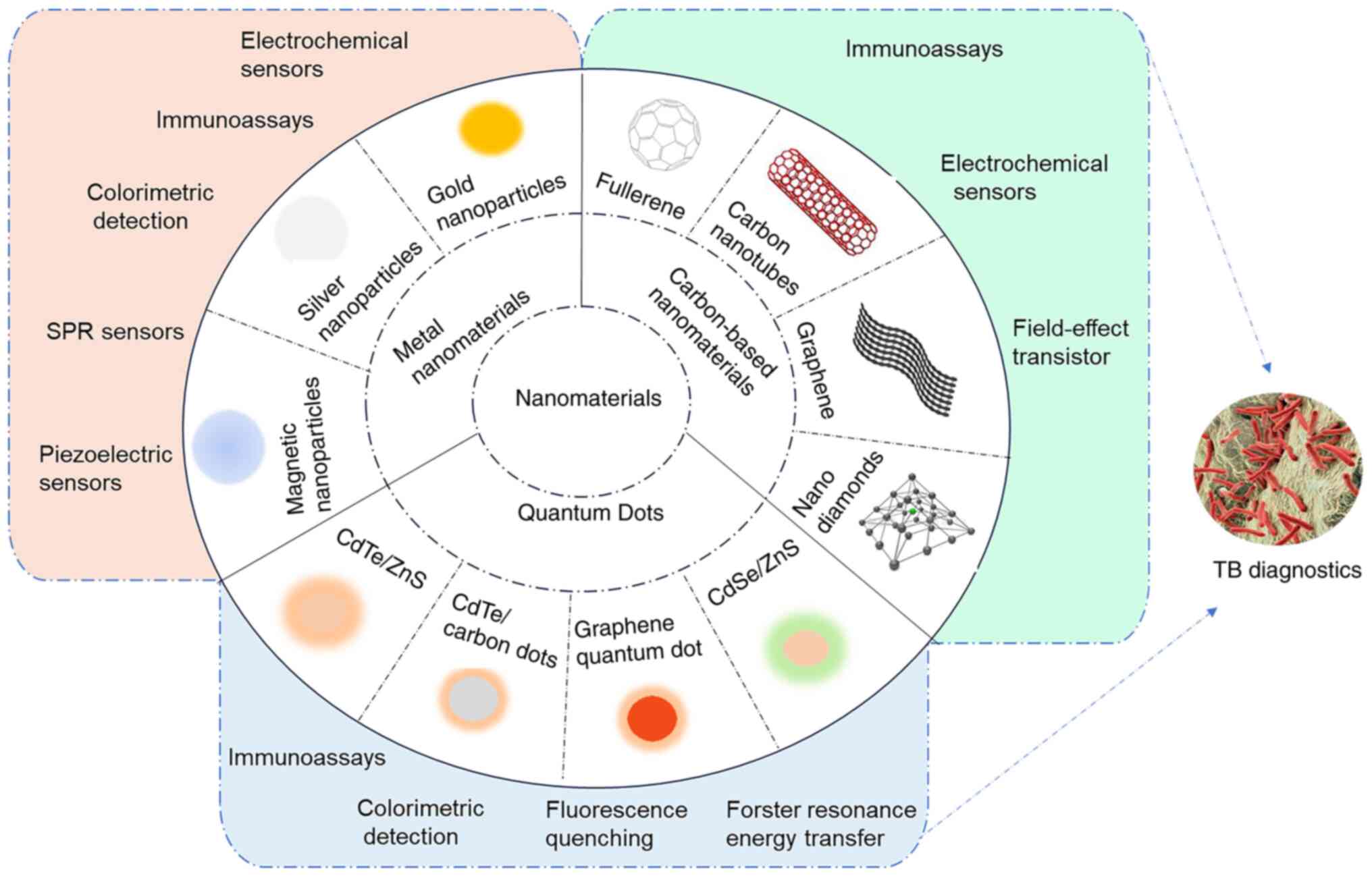
on-site testing and resource-limited settings (26). Fig. 1 presents the nanomaterials and
corresponding biosensors for TB diagnosis. The current article
reviewed the latest research advances in TB diagnostics using
nanomaterials, including their mechanisms and functions and
analyzes the structure and performance of various novel nano
biosensors. The limitations and challenges of nanomaterials in TB
diagnosis are also discussed, along with strategies to overcome
them. The present review aims to provide researchers with insights
for developing safe, rapid and effective TB diagnostic methods.
Metal nanoparticles improve TB diagnosis by
overcoming some traditional drawbacks. Table I presents recent advances in
metal nanomaterials-based diagnostics for TB. Due to their optical,
electronic and magnetic characteristics, they can be modified with
various ligands and detect TB biomarkers at low concentrations
(13,27). Moreover, they can be designed to
be portable and user-friendly for convenient use at the medical
treatment site. This allows for the adoption of decentralized
testing in areas with limited resources, making diagnostic assays
potentially more affordable (28).
AuNPs can attach multiple diagnostic probe molecules
due to their high surface-to-volume ratio (44). They can be used for long-term
diagnostics since they are chemically stable and air- and
water-resistant (18). The first
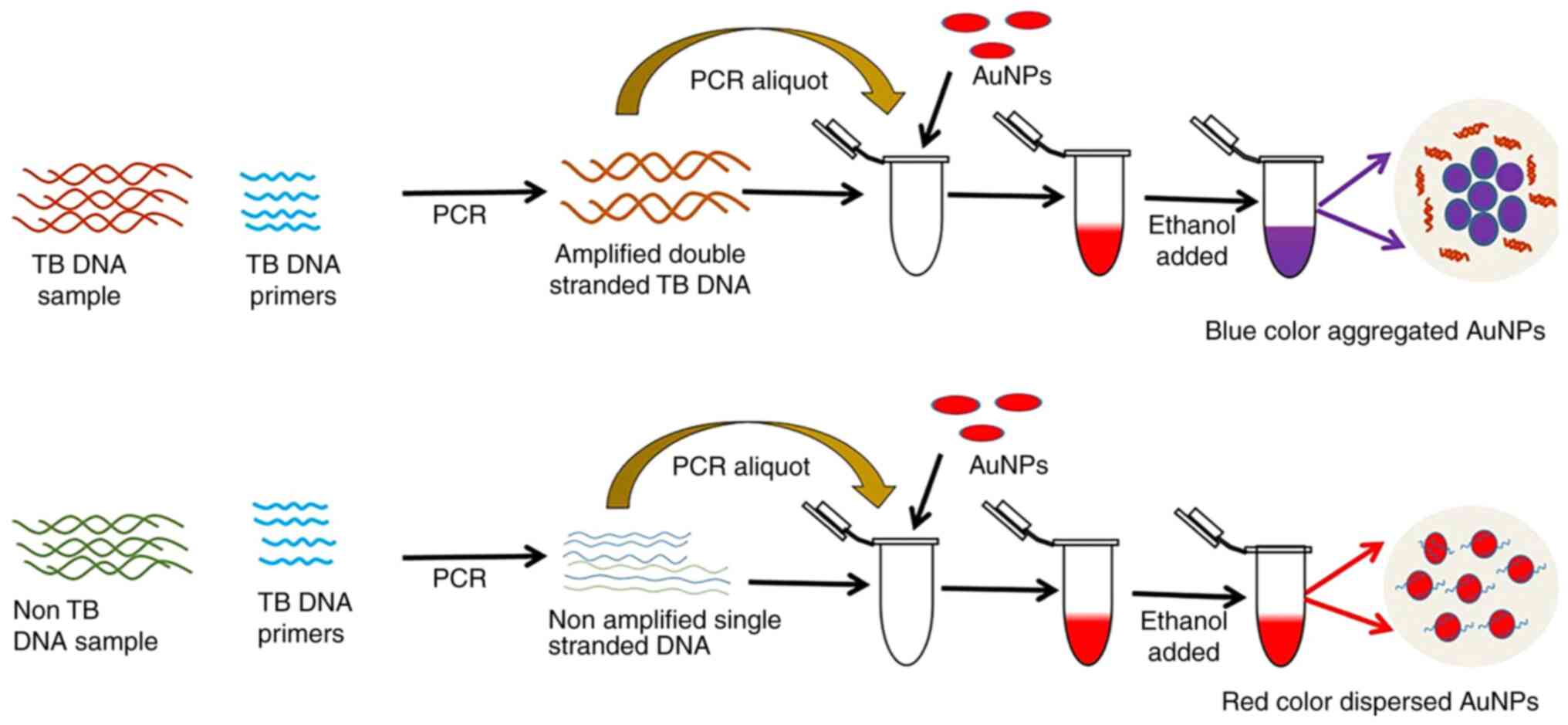
reported application of AuNPs in TB diagnosis was a colorimetric
assay developed by Gupta et al (45). In that study, oligonucleotides of
the Mycobacterium tuberculosis RNA polymerase subunit gene sequence
were first extracted and then combined with AuNPs and, at a
wavelength of 526 nm, the gold nanoprobe solution stayed pink in
the presence of the complementary DNA. By contrast, the solution
turned purple without the complementary DNA. The assay takes only
15 min per test, with minimal contamination, as it is performed in
a separate tube and allows visualization of the results. Follow-up
studies showed that this approach detected TB more precisely and
sensitively when compared with the automated liquid culture system
and semi-nested PCR (46,47).
The local electric field enhancement effect induced
by surface plasmon resonance (SPR) can enhance the optical activity
near the surface of metal nanoparticles, such as surface-enhanced
Raman scattering and fluorescence enhancement. The plasma-enhanced
effect of AuNPs has several applications in fields such as
biomarkers, sensors, photocatalysis and optoelectronics (48,49). Plasma coupling is related to the
size, shape, structure and spatial arrangement of NPs (50). Research in this area can help to
optimize biosensor structures. Prabowo et al (39) studied the effect of AuNP shapes
on plasmonic enhancement for DNA detection. They bound TB's
designed single-stranded probe DNA (ssDNA) with gold nano-urchins
and nanorods. Then, both mixtures were adsorbent onto a
graphene-coated SPR sensor due to the π-π interactions. During the
construction of the SPR sensor, annealing the Au layer increased
the sensor's graphene coverage and DNA probe load. In experimental
plasmonic activity comparison, gold nano-urchins showed the best
amplification, detecting DNA hybridization at fM levels. They
conclude that gold nano-urchin-assisted DNA detection offers the
possibility of early screening for TB using portable sensors.
Due to their affordable cost, simple structure and
easy operation, piezoelectric sensors are becoming a TB detection
research hotspot (51). A
piezoelectric sensor generates electricity from pressure,
acceleration and force. Quartz, Rochelle salt and some ceramics
generate an electrical charge when subject to mechanical stress, a
phenomenon known as the piezoelectric effect (52). Exploiting the special physical
and chemical properties of Au at the nanoscale, AuNPs can markedly
enhance the performance of piezoelectric sensors for detecting MTB
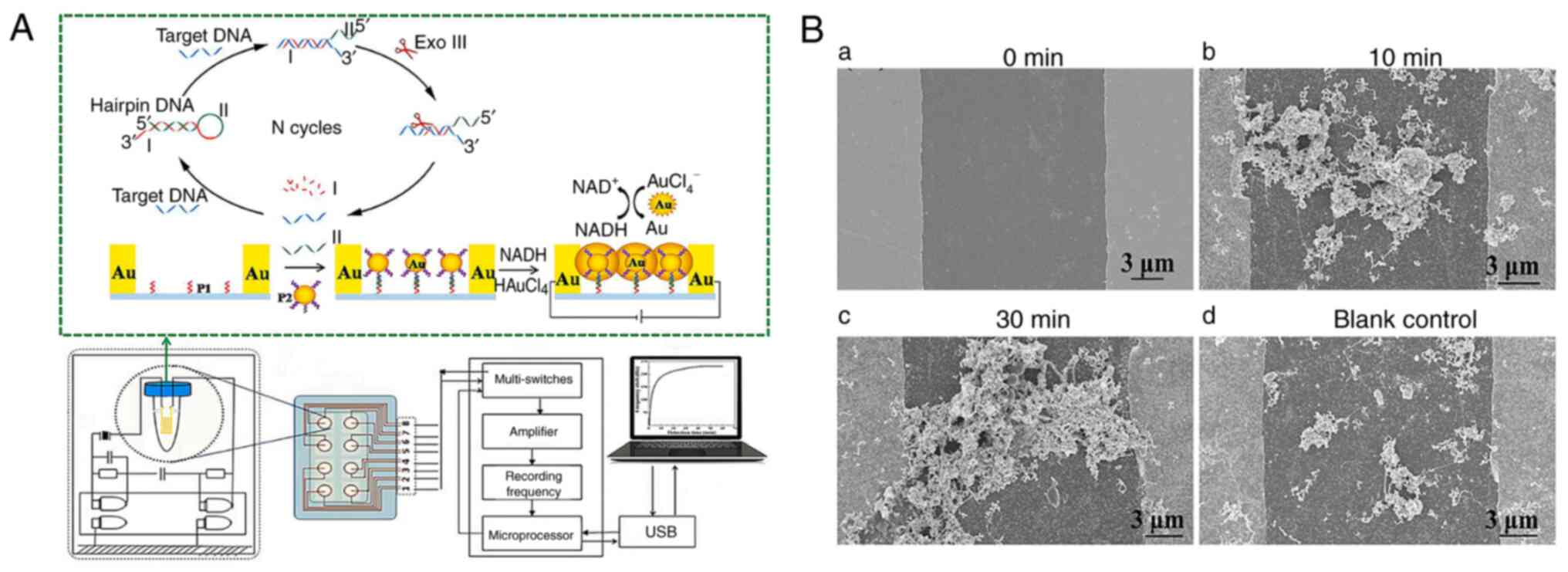
(53). Zhang et al
(37) developed a novel
piezoelectric sensor based on AuNPs-mediated enzyme-assisted signal
amplification for TB diagnosis (Fig.
3A). The biomarker was the 16S rDNA variable region of TB.
AuNPs were coupled to the hybridized detecting probe and grown in
HAuCl4 and NADH solutions to transmit electricity
between electrode gaps (Fig.
3B). The piezoelectric system detects TB rapidly and
sensitively thanks to AuNPs-mediated signal amplification. The
process is simple, fast and suited for developing compact portable
equipment.
AgNPs, like AuNPs, are chemically stable,
electrically conductive and can possess catalytic activity. Their
electron transfer efficiency is superior to that of AuNPs, which
have more prominent extinction bands (18). Recent advances have seen the use
of charge-neutral peptide nucleic acids (PNAs) as hybridization
agents in AgNP-based colorimetric DNA assays, enhancing the process
by causing nanoparticles to cluster more rapidly in solution
without immobilization, thus boosting DNA hybridization
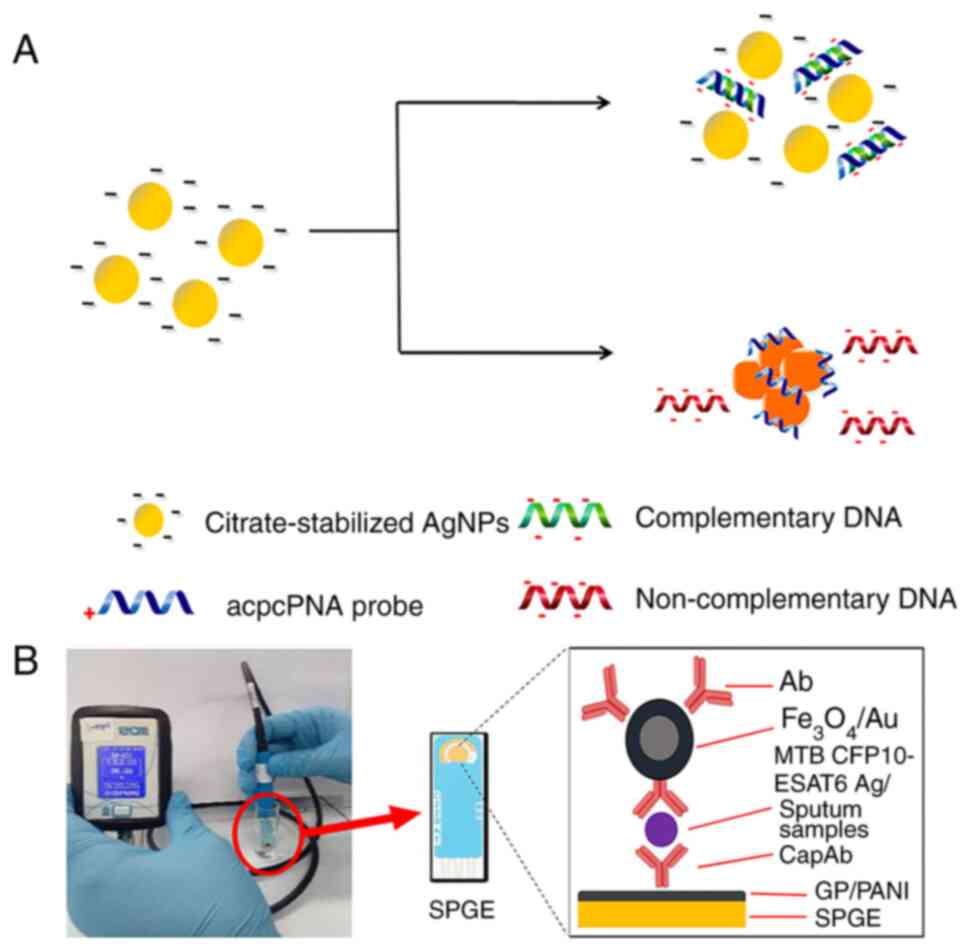
effectiveness. Teengam et al (54) developed a colorimetric DNA
detection sensor based on PNA-induced AgNP aggregation (Fig. 4A). They designed a detection
probe from PNA with a positively charged lysine modification at its
C-terminus (acpcPNA), leading to the aggregation of negatively
charged AgNPs and a subsequent swift shift in color. This sensor
effectively detected TB oligonucleotides, demonstrating a low
detection limit of 1.27 nM, showcasing fast, selective and
sensitive DNA detection capability.
Conventional methods for producing AgNPs typically
involve the use of reducing agents such as sodium citrate,
NaBH4 and hydrazine. While effective in controlling
nanoparticle size, these agents pose significant environmental
risks (55,56), prompting the pursuit of greener
alternatives. Tai et al (40) devised a method to synthesize
AgNPs using oil palm lignin, which is rich in phenolic hydroxyl
groups and offers an environmentally friendly and cost-efficient
solution for AgNP production. These lignin-coated AgNPs were
subsequently bonded to laser-etched graphene nanofibers, enabling
the direct linkage of single-stranded DNA to form a TB
bioelectrode. To assess the performance of the sensor, they
analyzed the ability of DNA samples attached to AgNPs to bind to
the target DNA by selective hybridization and mismatch assessment.
Electrochemical impedance spectroscopy further substantiated the
ability of the sensor to detect concentrations as low as 1 fM,
achieving a detection limit of 10−15M based on a
signal-to-noise ratio (S/N=3:1) with a signal-to-noise ratio of
3:1. The researchers highlighted that this TB detection method is
sensitive and ecologically friendly.
MNPs are generally composed of iron, nickel, cobalt
and oxides. MNPs feature a high surface-to-volume ratio, excellent
dispersibility and strong interactions with biological molecules
(57). Gupta et al
(42) developed a giant
magnetoresistance (GMR) biosensor to detect TB-specific early
secreted antigenic target-6 (ESAT-6) protein. This GMR biosensing
assay labels monoclonal antibodies against ESAT-6 antigen with
MNPs. In the presence of ESAT-6, MNPs bind to the GMR sensor
proportionally to protein concentration, altering its electrical
resistance. Simulations of the GMR biosensor have shown that it can
detect ESAT-6 at pg/ml levels. Cheon et al (58) developed a colorimetric biosensing
system to detect MTB 64 protein (MPT64) using nucleic acid
aptamer-modified MNPs. The aptamer on the surface of the MNP
initially inhibits its catalase activity. Upon binding with MPT64
in the sample, the aptamer releases, thereby restoring the enzyme
activity of the MNP. TB can subsequently be detected within 70 min
by measuring the enzyme-substrate fluorescence spectra.
QDs are nanoscale semiconductor particles with
size-tunable fluorescence, meaning smaller dots emit blue light
while larger ones emit red light (19). As fluorescent probes, QDs can
mark MTB nucleic acid and are more photostable and less prone to
photobleaching than organic dyes (59). The surface of QDs can be modified
with various functional groups or nanomaterials to improve their
solubility, stability and biocompatibility (60). Table II presents recent advances in
quantum dots (QDs) based diagnostics for TB.
In fluorescence resonance energy transfer
(FRET)-based systems, QDs can provide energy and bind acceptors.
When these probes attach to target nucleic acids such as MTB RNA or
DNA, structural alterations influence energy transfer efficiency,
resulting in quenching or fluorescence changes, allowing
quantitative analysis (69,70). This QD quenching technology-based
biosensor serves as a fast, sensitive and easy-to-use diagnostic
tool (71). Liang et al
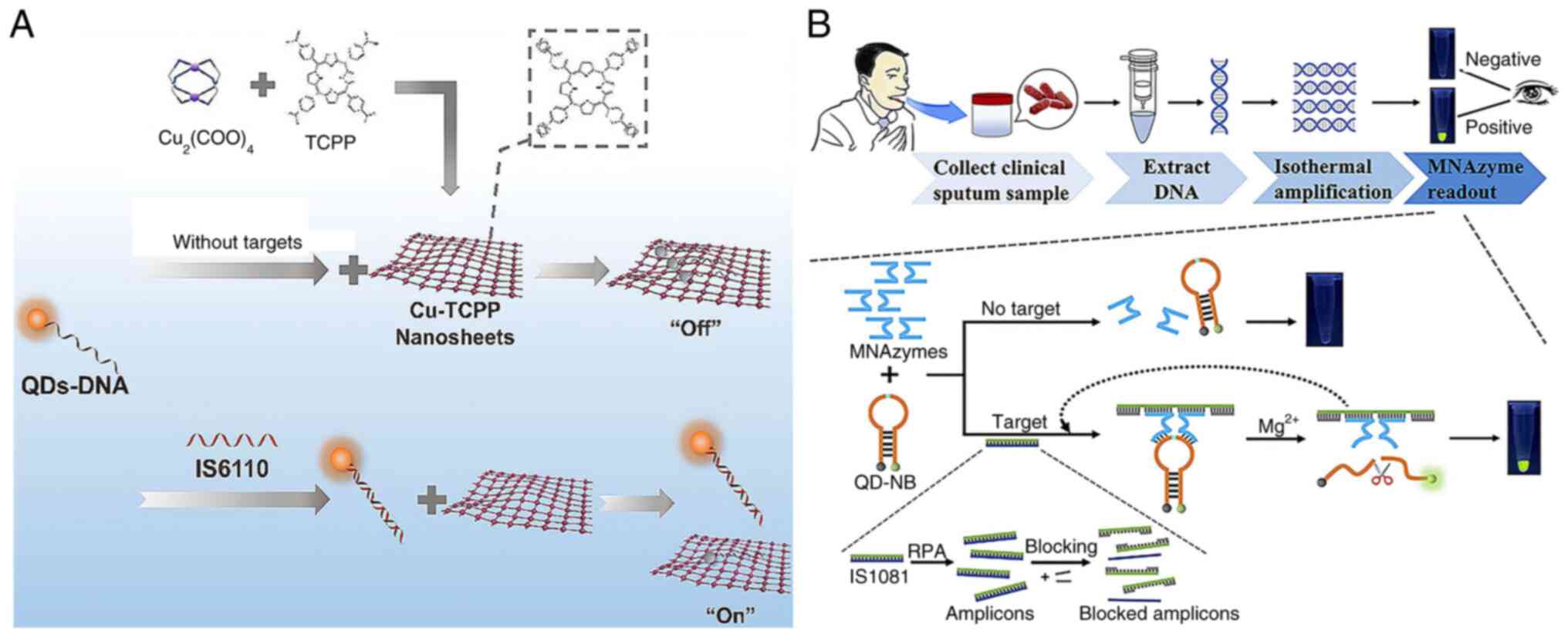
(67) used carboxyl-modified
CdTe QDs to label single-stranded DNA (QDs-DNA) as a fluorescence
donor. In their approach, Cu-TCPP (a two-dimensional metal-organic
framework) nanosheets were used as the fluorescence acceptor for
QDs-DNA. QDs-DNA attached to Cu-TCPP, resulting in fluorescence
quenching in the absence of targets. However, when the target
nucleic acids were present, QDs-DNA formed with them a dsDNA
complex, preserving strong fluorescence (Fig. 5A). The sensor exhibited a linear
response from 0.05 to 1.0 nM and a 35 pM detection limit. This
QD-based fluorescent technology for clinical sputum analysis
achieved high sensitivity and specificity.
The primary inner filter effect (IFE) is the
absorption of excitation light by various chromophores in solution
or matrix, while the secondary inner filter effect refers to the
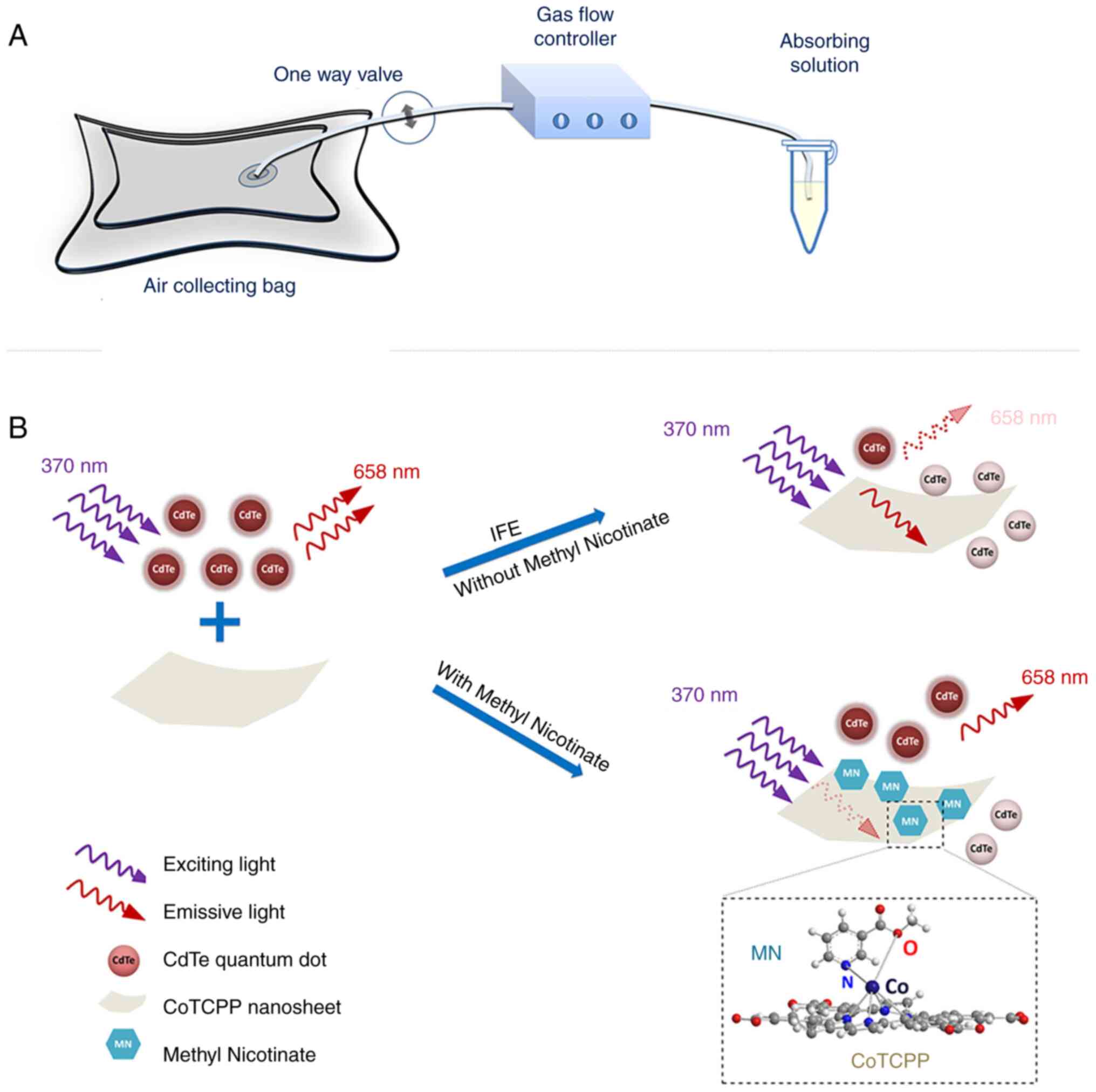
absorption of emission radiation (72). He et al (62) found that cobalt-metalized
tetrakis (4-carboxyphenyl) porphyrin (CoTCPP) could modulate the
fluorescence emission and quenching of QDs through the inner filter
effect. Thus, they developed a fluorescent probe based on CdTe QDs
and CoTCPP nanosheets to analyze methyl nicotinate in vapor samples
of MTB (Fig. 6). CoTCPP and QDs
cannot become close enough to access FRET due to electrostatic
repulsion. By contrast, the IFE affects QD fluorescence quenching.
They used red-emitting QDs as fluorescent signal switches whose
fluorescent are quenched by CoTCPP but restored by methyl nicotine.
The platform effectively detects methyl nicotinate with a relative
standard deviation <3.33%, the detection time was only 4 min and
it was linear in the range of 1-100 μM with a detection
limit of 0.59 μM.
With single excitation and multiple emission, QDs
could identify numerous MTB markers simultaneously. This
multiplexing capacity simplifies the instrumentation and
experimental setup and comprehensively explains the infection's
presence and severity (73,74). Zhou et al (20) developed an immunosensor to
measure latent tuberculosis infection biomarkers (IFN-γ, TNF-α and
IL-2) by embedding carbon and CdS QDs on AuNPs and magnetic beads.
Then three antibody1-labeled markers were immobilized at three
electrode positions to capture the corresponding antigens and
simultaneously detected with antibody2 and QD functionalized
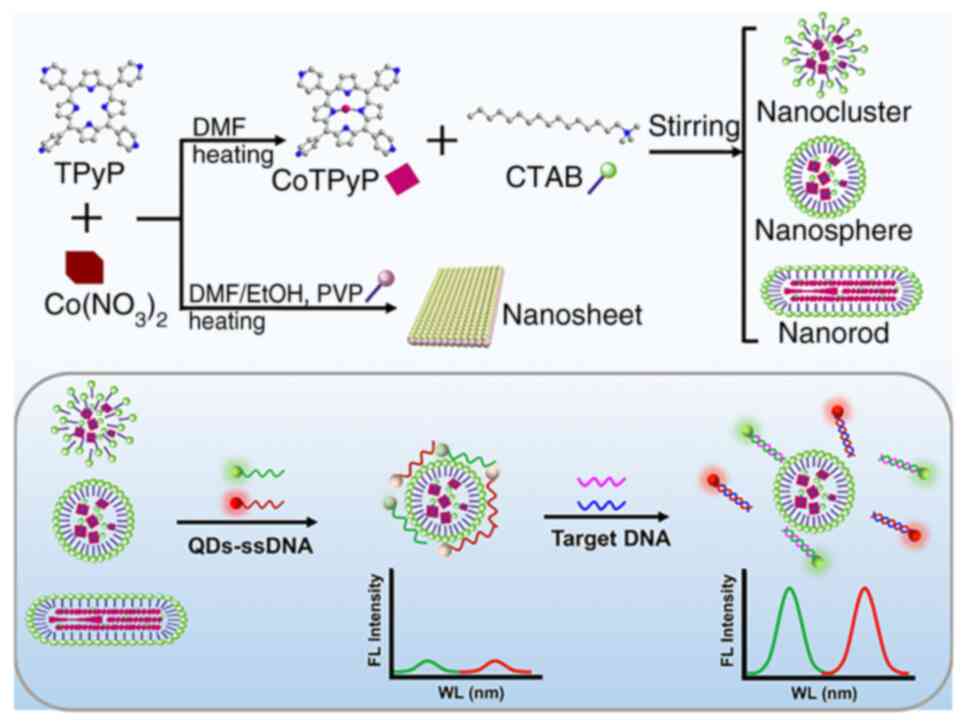
nanoprobes. Hu et al (63) developed a novel fluorescence
biosensor that uses nanocobalt 5,10,15,20-tetra (4-pyridyl)-21H,23H
porphine (nanoCoTPyP) and dual QDs to simultaneously detect two
drug-resistant genes of MTB, specifically rpoB531 and katG315
(Fig. 7). The green and red QDs
were linked to the single strand (ss)DNA probes ssDNA1 and ssDNA2
and combined to form QD-ssDNA probes. These probes interact with
nanoCoTPyP through electrostatic forces, π-π stacking and hydrogen
bonding, resulting in fluorescence quenching through FRET and
photoinduced electron transfer. This biosensor enables the
concurrent quantification of the two genes in one test using the
distinct emission wavelengths of the dual QDs. Notably, this
approach allows for the simultaneous identification of two
mutations in the PCR products of multi-drug resistant tuberculosis
within a 95-min timeframe.
Carbon-based nanomaterials, such as fullerene,
carbon nanotubes, nanodiamonds and graphene, show great potential
for TB diagnosis (75,76). These materials can be engineered
to detect specific TB biomarkers, even at very low concentrations
(77). Recent advances in the
use of carbon-based nanomaterials for TB diagnostics are summarized
in Table III. Additionally,
carbon nanomaterial-based point-of-care testing devices can be
portable and easily used, which is beneficial in low-resource
TB-endemic areas. making them particularly advantageous in
low-resource, TB-endemic regions.
Graphene is often employed in sensors designed as
reduced graphene oxide (rGO), a cost-effective form produced via
chemical and hydrothermal reduction of graphene oxide (83). rGO is favored in biosensor design
for its high current density, exceptional electrocatalytic
properties, extensive surface area, excellent thermal conductivity
and numerous electroactive sites (90,91). However, due to van der Waals
forces and its inherent laminar structure, rGO tends to aggregate,
leading to a decrease in surface area and thus reducing its sensing
ability (92,93). The commonly used reducing agents
for rGO, such as hydrazine and NaBH4, are highly toxic
and hazardous (94). Chaturvedi
et al (95) addressed
this issue by reducing GO to rGO and coating it with a
biocompatible, nanometer-thick polydopamine (PDA) layer. PDA is
rich in functional groups such as amines, imines and catechols,
facilitating dense covalent attachment of biomolecules and
providing binding sites for metal nanoparticles. Consequently, they
engineered a nanocomposite of rGO, PDA and AuNPs and applied it to
carbon electrodes to enhance the electroactive surface area and
electron transport. Electrochemical analysis using cyclic
voltammetry and linear sweep voltammetry revealed a sensitivity of
2.12×10−3 mA μM−1 and a response time
of 5 sec for target DNA detection at 0.1×10−7 mM.
PDA thin coatings improve the antifouling properties
and cytocompatibility of carbon nanomaterials (96). The adhesive properties of PDA
facilitate the attachment of biomolecules to biosensor transducers
through physical interactions (97). Polynorepinephrine (PNE), a
compound closely related to PDA, possesses additional -OH groups
and superior coating uniformity; however, it has rarely been
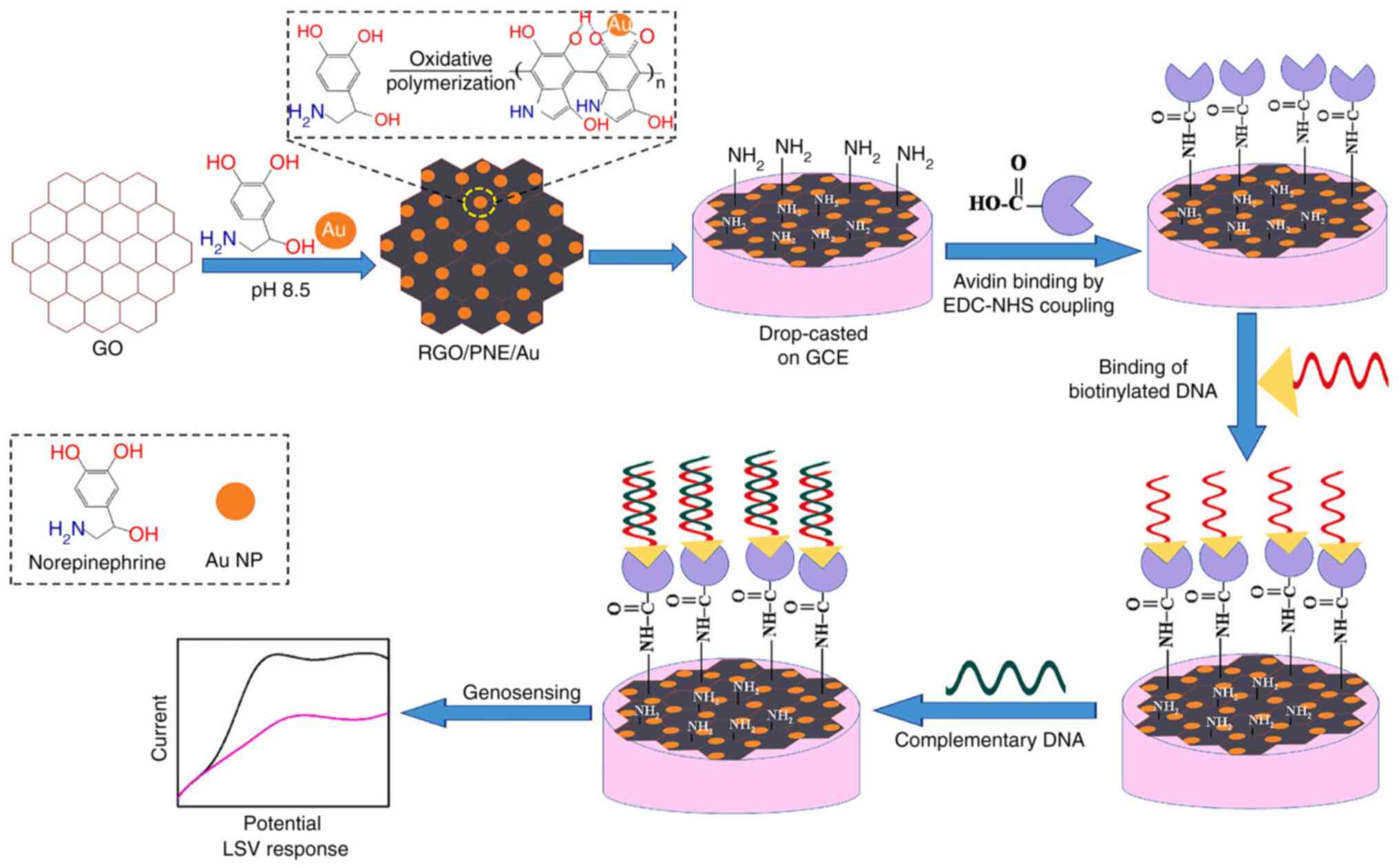
investigated in TB biosensors. (98,99). Bisht et al (81) researched PNE as a coating for rGO
and AuNPs in the development of an electrochemical nanobiosensor
targeting MTB (Fig. 8). The
active rGO, coupled with the reactive quinone groups and AuNPs,
synergistically forms a high-performance biosensing platform that
facilitates substantial biomolecule loading and delivers an
exceptional electrochemical response. The study demonstrated that
the PNE-modified system (rGO/PNE/Au) outperforms the PDA-modified
counterpart (rGO/PDA/Au) for the development of electrochemical
biosensors. The PNE-modified system achieves a markedly higher
electrochemical response and offers a surface richer in functional
groups, enhancing the loading capacity for biomolecules such as
probe DNA. The biosensor demonstrated high sensitivity
(2.3×10−3 mA μM−1), a low detection
limit (0.1×10−7 μM) and a quick response time of
5 sec.
Paper-based analytical devices (PADs) require
minimal training and are highly portable, which is crucial for
field testing and point-of-care TB diagnostics (100). Graphene nanomaterials have a
large surface area, which provides more active sites for
biomolecule adsorption, enhancing the electrochemical properties of
sensors (101). They boost the
sensitivity and specificity of PADs, allowing for the detection of
TB biomarkers at low concentrations. Pornprom et al
(78) introduced a PAD biosensor
using AuNP-decorated carboxyl graphene (GCOOH) to detect heat shock
protein (Hsp16.3), a key TB infection biomarker. The AuNPs enhance
the electrochemical properties of the sensor, while the GCOOH, with
its numerous binding sites, facilitates direct antibody
immobilization through carboxyl groups and primary amines. The PAD
sensor specifically recognizes Hsp16.3, requiring only 5 μl
sample volume, performed effectively with a detection limit of 0.01
ng/ml and quickly detected TB-infected clinical samples within 20
min.
Unlike other electrochemical sensors, field-effect
transistor (FET) biosensors involve semiconductor manufacturing
(102). This enables the
large-scale production of these sensors, making them ideal for
widespread use in assessing infection status, which is the purpose
of point-of-care testing (103). Graphene-based field-effect
transistors (GFETs) have a low on/off ratio compared with other
semiconductor materials because they lack a bandgap. However, the
low noise characteristic of GFETs can compensate for this
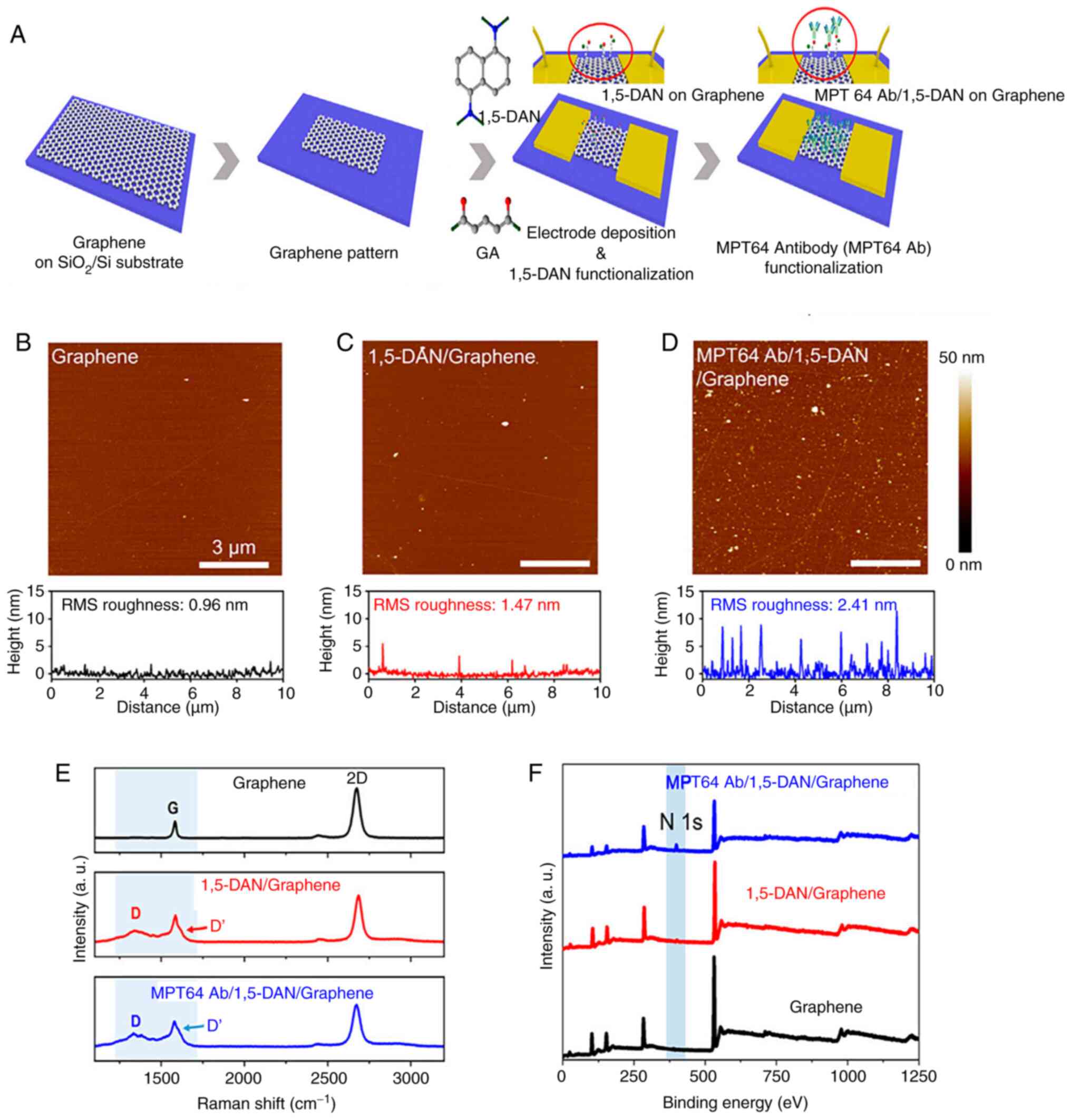
limitation, enhancing their overall performance (104,105). Seo et al (82) designed a GFET biosensor for MTB
MPT64 protein detection to construct an effective point-of-care TB
testing platform. To efficiently conjugate antibodies, the graphene
channels of the GFET were functionalized by immobilizing
1,5-diaminonaphthalene (1,5-DAN) and glutaraldehyde linker
molecules. Atomic force microscopy was used to investigate the
surface roughness of graphene after functionalization with MPT64 Ab
and 1,5-DAN. As shown in Fig. 9,
Raman spectroscopy and X-ray photoelectron spectroscopy validated
the successful and uniform immobilization of linker molecules on
the graphene surface and the subsequent antibody conjugation. The
MPT64 antibody-functionalized GFET achieved a detection limit of 1
fg/ml in real-time and demonstrated greater sensitivity and faster
detection compared with ELISA.
Single-walled carbon nanotubes (SWCNTs), another
popular carbon nanomaterial, are similar in size to biomolecules
and have an average diameter of 1 nm (106). They possess low charge-carrier
density and high intrinsic carrier mobility, making them ideal for
detecting electrostatic interactions and charge transfer during
biological processes (107).
Since 1998, SWCNTs have been used to fabricate FETs, demonstrating
exceptional performance in biosensing due to their distinctive
physical characteristics (108). SWCNTs have advantages over
graphene, silicon nitride and silicon nanowires as FET functional
nanomaterials. Their tiny diameter helps reduce gate leakage and
exhibit high conductivity, biocompatibility, charge mobility and
stability (109,110). Researchers have constructed
SWCNT-based FET biosensors to detect SARS antigens (111), cancer exosomal miRNA (112) and Alzheimer's disease
biomarkers (113). The limit of
detection of these biosensors is equivalent to advanced techniques
such as nucleic acid amplification tests and ELISA.
It is essential to compare the manipulation,
production, stability and adaptation of these materials when
selecting them for TB biosensing applications.
AuNPs are stable for long-term use, while AgNPs are
more susceptible to oxidation (123). QDs are photostable and MNPs
remain stable in different conditions. Graphene and SWCNTs are
stable but prone to aggregation, needing functionalization for
improved dispersion (124).
AuNPs and AgNPs easily integrate into biosensors.
QDs, despite toxicity concerns, offer tunable fluorescence in
biosensing. MNPs are ideal for magnetic separation in assays
(125). Carbon nanomaterials
are adaptable for electronic and electrochemical biosensors but may
require miniaturization for point-of-care use (126).
In summary, the choice of nanomaterial for TB
diagnostics is application-specific, balancing manipulation ease,
synthesis complexity, stability and adaptability to achieve
sensitive, specific and cost-effective biosensors.
While nanomaterial-based sensing systems have shown
significant advances in the detection of MTB, several limitations
and challenges must be addressed to fully realize their potential
in TB diagnostics.
Nanomaterials can degrade over time, leading to
reduced sensitivity and reliability of the biosensors. Factors such
as environmental conditions, storage methods and interaction with
biological fluids can affect their stability. Surface modification
techniques, such as coating with stabilizing agents such as
polyethylene glycol or thiol groups, can enhance the stability of
nanomaterials. Additionally, rigorous quality control measures
during manufacturing and storage can help maintain the integrity of
the nanomaterials (127).
Some nanomaterials, particularly MNPs and QDs, can
exhibit toxicity when introduced into biological systems. This can
lead to adverse effects on cells and tissues, limiting their use in
in vivo diagnostics. Surface functionalization with
biocompatible polymers or targeting ligands can reduce toxicity and
improve cell uptake. Furthermore, developing biodegradable
nanomaterials can mitigate long-term health risks (128).
Biological and chemical components in patient
samples can interfere with the detection process, leading to false
positives or negatives. Common interferents include proteins,
lipids and other biomolecules Advanced sample preparation
techniques, such as pre-concentration and purification, can reduce
interference. Additionally, designing nanomaterials with specific
recognition elements, such as antibodies or aptamers, can enhance
selectivity and reduce cross-reactivity (129).
The synthesis and functionalization of nanomaterials
can be costly and technically challenging, particularly for
large-scale production. High costs can limit the accessibility of
these technologies in resource-limited settings. Developing
cost-effective synthesis methods, such as green chemistry
approaches and scalable manufacturing processes, can reduce
production costs. Additionally, optimizing the use of nanomaterials
to achieve the desired performance with minimal material usage can
help make these technologies more affordable (24,129).
In addition to the properties of nanomaterials,
response detection technology plays a crucial role in the
analytical performance of biosensors for TB diagnostics. Optical
assays, such as those using AuNPs, offer simplicity and
cost-effectiveness but may have limited sensitivity and be prone to
interference from complex sample matrices (130). Fluorescence assays, often
employing QDs, provide high sensitivity and specificity due to
their unique optical properties, yet they require specialized
equipment and can suffer from photobleaching (131). Electrochemical assays, enhanced
by carbon-based nanomaterials such as graphene, are known for their
high sensitivity, rapid response and low cost, but are susceptible
to electrode fouling and necessitate careful handling (132). Each detection technology
presents distinct advantages and challenges and the optimal choice
for TB biosensors depends on the balance between sensitivity,
specificity, cost and operational simplicity. The development of
future biosensors should aim to integrate the strengths of these
detection methods to enhance diagnostic reliability and
practicality.
In conclusion, nanomaterials for MTB detection may
revolutionize TB diagnostics by addressing the inadequacies of
clinical approaches. Metal nanoparticles, such as gold and silver,
have been employed in colorimetric and electrochemical biosensors
to speed up detection. QD-based platforms, such as the QD-NB-based
MNAzyme colorimetric assay and the double QDs-ssDNA probe, can
detect different TB markers simultaneously and are ultra-sensitive.
Carbon-based nanomaterials, such as the graphene-based PAD, can
quickly detect MTB in trace specimens. In serum, SWCNT FETs rapidly
distinguish TB-positive from negative samples.
Despite the promising research progress reviewed
here, limitations and problems remain. For instance, biosensor
stability, biocompatibility and long-term performance need
improvement. New diagnostic procedures also need substantial
clinical validation to assure safety, efficacy and regulatory
compliance. In practice, biological and chemical components can
interfere with sensors; thus, their anti-interference capabilities
must be strengthened. Researchers should improve sensor design,
nanomaterial fabrication and data interpretation to overcome such
challenges.
Not applicable.
LC conducted the overall planning of the review,
carried out the literature search and selection process. JZ drafted
the core content. HW analyzed and discussed the literature in
depth, offering valuable insights and assisting in refining the
text. LC performed supplementary literature searches and
validations, enhancing the comprehensiveness and accuracy of the
review. Furthermore, JZ and LC jointly verified the authenticity of
the relevant data points sourced from the reviewed literature. Data
authentication is not applicable. All authors read and approved the
final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
No funding was received.
|
1
|
Bagcchi S: WHO's global tuberculosis
report 2022. Lancet Microbe. 4:e202023. View Article : Google Scholar
|
|
2
|
Asadi L, Croxen M, Heffernan C, Dhillon M,
Paulsen C, Egedahl ML, Tyrrell G, Doroshenko A and Long R: How much
do smear-negative patients really contribute to tuberculosis
transmissions? Re-examining an old question with new tools.
EClinicalMedicine. 43:1012502022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meriki HD, Wung NH, Tufon KA, Tony NJ,
Ane-Anyangwe I and Cho-Ngwa F: Evaluation of the performance of an
in-house duplex PCR assay targeting the IS6110 and rpoB genes for
tuberculosis diagnosis in Cameroon. BMC Infect Dis. 20:7912020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Natarajan S, Ranganathan M, Hanna LE and
Tripathy S: Transcriptional profiling and deriving a seven-gene
signature that discriminates active and latent tuberculosis: An
integrative bioinformatics approach. Genes (Basel). 13:6162022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Molloy A, Harrison J, McGrath JS, Owen Z,
Smith C, Liu X, Li X and Cox JAG: Microfluidics as a novel
technique for tuberculosis: From diagnostics to drug discovery.
Microorganisms. 9:23302021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meier JP, Möbus S, Heigl F,
Asbach-Nitzsche A, Niller HH, Plentz A, Avsar K, Heiß-Neumann M,
Schaaf B, Cassens U, et al: Performance of T-Track® TB,
a novel dual marker RT-qPCR-based whole-blood test for improved
detection of active tuberculosis. Diagnostics (Basel). 13:7582023.
View Article : Google Scholar
|
|
7
|
Çiftci İH and Karakeçe E: Comparative
evaluation of TK SLC-L, a rapid liquid mycobacterial culture
medium, with the MGIT system. BMC Infect Dis. 14:1302014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okoi C anderson STB, Antonio M, Mulwa SN,
Gehre F and Adetifa IMO: Non-tuberculous mycobacteria isolated from
pulmonary samples in sub-Saharan Africa-a systematic review and
meta analyses. Sci Rep. 7:120022017. View Article : Google Scholar
|
|
9
|
Reed JL, Walker ZJ, Basu D, Allen V, Nicol
MP, Kelso DM and McFall SM: Highly sensitive sequence specific qPCR
detection of Mycobacterium tuberculosis complex in respiratory
specimens. Tuberculosis (Edinb). 101:114–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang X, Fan S, Ma Y, Chen H, Xu JF, Pi J,
Wang W and Chen G: Current progress of functional nanobiosensors
for potential tuberculosis diagnosis: The novel way for TB control?
Front Bioeng Biotechnol. 10:10366782022. View Article : Google Scholar :
|
|
11
|
Lyu M, Zhou J, Zhou Y, Chong W, Xu W, Lai
H, Niu L, Hai Y, Yao X, Gong S, et al: From tuberculosis bedside to
bench: UBE2B splicing as a potential biomarker and its regulatory
mechanism. Signal Transduct Target Ther. 8:822023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Metcalf T, Soria J, Montano SM, Ticona E,
Evans CA, Huaroto L, Kasper M, Ramos ES, Mori N, Jittamala P, et
al: Evaluation of the GeneXpert MTB/RIF in patients with
presumptive tuberculous meningitis. PLoS One. 13:e01986952018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tu Phan LM, Tufa LT, Kim HJ, Lee J and
Park TJ: Trends in diagnosis for active tuberculosis using
nanomaterials. Curr Med Chem. 26:1946–1959. 2019. View Article : Google Scholar
|
|
14
|
Joshi H, Kandari D, Maitra SS and
Bhatnagar R: Biosensors for the detection of Mycobacterium
tuberculosis: A comprehensive overview. Crit Rev Microbiol.
48:784–812. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pourakbari R, Shadjou N, Yousefi H,
Isildak I, Yousefi M, Rashidi MR and Khalilzadeh B: Recent progress
in nanomaterial-based electrochemical biosensors for pathogenic
bacteria. Mikrochim Acta. 186:8202019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uhuo OV, Waryo TT, Douman SF, Januarie KC,
Nwambaekwe KC, Ndipingwi MM, Ekwere P and Iwuoha EI: Bioanalytical
methods encompassing label-free and labeled tuberculosis
aptasensors: A review. Anal Chim Acta. 1234:3403262022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu K, Liang ZC, Ding X, Hu H, Liu S,
Nurmik M, Bi S, Hu F, Ji Z, Ren J, et al: Nanomaterials in the
prevention, diagnosis, and treatment of Mycobacterium tuberculosis
infections. Adv Healthc Mater. 7:17005092018. View Article : Google Scholar
|
|
18
|
Tan P, Li H, Wang J and Gopinath SCB:
Silver nanoparticle in biosensor and bioimaging: Clinical
perspectives. Biotechnol Appl Biochem. 68:1236–1242. 2021.
|
|
19
|
Muthukrishnan L: Multidrug resistant
tuberculosis-diagnostic challenges and its conquering by
nanotechnology approach-an overview. Chem Biol Interact.
337:1093972021. View Article : Google Scholar
|
|
20
|
Zhou B, Zhu M, Hao Y and Yang P:
Potential-resolved electrochemiluminescence for simultaneous
determination of triple latent tuberculosis infection markers. ACS
Appl Mater Interfaces. 9:30536–30542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dykman L and Khlebtsov N: Gold
nanoparticles in biomedical applications: Recent advances and
perspectives. Chem Soc Rev. 41:2256–2282. 2012. View Article : Google Scholar
|
|
22
|
Sapsford KE, Algar WR, Berti L, Gemmill
KB, Casey BJ, Oh E, Stewart MH and Medintz IL: Functionalizing
nanoparticles with biological molecules: Developing chemistries
that facilitate nanotechnology. Chem Rev. 113:1904–2074. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Drain PK, Bajema KL, Dowdy D, Dheda K,
Naidoo K, Schumacher SG, Ma S, Meermeier E, Lewinsohn DM and
Sherman DR: Incipient and subclinical tuberculosis: A clinical
review of early stages and progression of infection. Clin Microbiol
Rev. 31:e00021–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rosi NL and Mirkin CA: Nanostructures in
biodiagnostics. Chem Rev. 105:1547–1562. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Singh V and Chibale K: Strategies to
combat multi-drug resistance in tuberculosis. Acc Chem Res.
54:2361–2376. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Golichenari B, Nosrati R, Farokhi-Fard A,
Abnous K, Vaziri F and Behravan J: Nano-biosensing approaches on
tuberculosis: Defy of aptamers. Biosens Bioelectron. 117:319–331.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eivazzadeh-Keihan R, Saadatidizaji Z,
Mahdavi M, Maleki A, Irani M and Zare I: Recent advances in gold
nanoparticles-based biosensors for tuberculosis determination.
Talanta. 275:1260992024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Golichenari B, Nosrati R, Farokhi-Fard A,
Faal Maleki M, Gheibi Hayat SM, Ghazvini K, Vaziri F and Behravan
J: Electrochemical-based biosensors for detection of Mycobacterium
tuberculosis and tuberculosis biomarkers. Crit Rev Biotechnol.
39:1056–1077. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Seele PP, Dyan B, Skepu A, Maserumule C
and Sibuyi NRS: Development of gold-nanoparticle-based lateral flow
immunoassays for rapid detection of TB ESAT-6 and CFP-10.
Biosensors (Basel). 13:3542023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kamra E, Prasad T, Rais A, Dahiya B,
Sheoran A, Soni A, Sharma S and Mehta PK: Diagnosis of
genitourinary tuberculosis: Detection of mycobacterial
lipoarabinomannan and MPT-64 biomarkers within urine extracellular
vesicles by nano-based immuno-PCR assay. Sci Rep. 13:115602023.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dahiya B, Prasad T, Rais A, Sheoran A,
Kamra E, Mor P, Soni A, Sharma S and Mehta PK: Quantification of
mycobacterial proteins in extrapulmonary tuberculosis cases by
nano-based real-time immuno-PCR. Future Microbiol. 18:771–783.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tripathi A, Jain R and Dandekar P: Rapid
visual detection of Mycobacterium tuberculosis DNA using gold
nanoparticles. Anal Methods. 15:2497–2504. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang H, Chen Y, Zuo J, Deng C, Fan J, Bai
L and Guo S: MXene-incorporated C60NPs and Au@Pt with
dual-electric signal outputs for accurate detection of
Mycobacterium tuberculosis ESAT-6 antigen. Biosens Bioelectron.
242:1157342023. View Article : Google Scholar
|
|
34
|
Patnaik N and Dey RJ: Label-free
citrate-stabilized silver nanoparticles-based, highly sensitive,
cost-effective, and rapid visual method for the differential
detection of Mycobacterium tuberculosis and mycobacterium bovis.
ACS Infect Dis. 10:426–435. 2024. View Article : Google Scholar
|
|
35
|
Pei X, Hong H, Liu S and Li N: Nucleic
acids detection for Mycobacterium tuberculosis based on gold
nanoparticles counting and rolling-circle amplification. Biosensors
(Basel). 12:4482022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
León-Janampa N, Shinkaruk S, Gilman RH,
Kirwan DE, Fouquet E, Szlosek M, Sheen P and Zimic M:
Biorecognition and detection of antigens from Mycobacterium
tuberculosis using a sandwich ELISA associated with magnetic
nanoparticles. J Pharm Biomed Anal. 215:1147492022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J and He F: Mycobacterium
tuberculosis piezoelectric sensor based on AuNPs-mediated enzyme
assisted signal amplification. Talanta. 236:1229022022. View Article : Google Scholar
|
|
38
|
Xie J, Mu Z, Yan B, Wang J, Zhou J and Bai
L: An electrochemical aptasensor for Mycobacterium tuberculosis
ESAT-6 antigen detection using bimetallic organic framework.
Mikrochim Acta. 188:4042021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Prabowo BA, Purwidyantri A, Liu B, Lai HC
and Liu KC: Gold nanoparticle-assisted plasmonic enhancement for
DNA detection on a graphene-based portable surface plasmon
resonance sensor. Nanotechnology. 32:0955032021. View Article : Google Scholar
|
|
40
|
Tai MJY, Perumal V, Gopinath SCB, Raja PB,
Ibrahim MNM, Jantan IN, Suhaimi NSH and Liu WW: Laser-scribed
graphene nanofiber decorated with oil palm lignin capped silver
nanoparticles: A green biosensor. Sci Rep. 11:54752021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mohd Azmi UZ, Yusof NA, Abdullah J, Alang
Ahmad SA, Mohd Faudzi FN, Ahmad Raston NH, Suraiya S, Ong PS,
Krishnan D and Sahar NK: Portable electrochemical immunosensor for
detection of Mycobacterium tuberculosis secreted protein
CFP10-ESAT6 in clinical sputum samples. Mikrochim Acta. 188:202021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gupta S, Bhatter P and Kakkar V:
Point-of-care detection of tuberculosis using magnetoresistive
biosensing chip. Tuberculosis (Edinb). 127:1020552021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
León-Janampa N, Zimic M, Shinkaruk S,
Quispe-Marcatoma J, Gutarra A, Le Bourdon G, Gayot M, Changanaqui
K, Gilman RH, Fouquet E, et al: Synthesis, characterization and
bio-functionalization of magnetic nanoparticles to improve the
diagnosis of tuberculosis. Nanotechnology. 31:1751012020.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Terefinko D, Dzimitrowicz A,
Bielawska-Pohl A, Klimczak A, Pohl P and Jamroz P: The influence of
cold atmospheric pressure plasma-treated media on the cell
viability, motility, and induction of apoptosis in in human
non-metastatic (MCF7) and metastatic (MDA-MB-231) breast cancer
cell lines. Int J Mol Sci. 22:38552021. View Article : Google Scholar
|
|
45
|
Gupta AK, Singh A and Singh S: Diagnosis
of Tuberculosis: Nanodiagnostics Approaches. Saxena S and Khurana
S: NanoBioMedicine. Springer; Singapore: pp. 261–283. 2020,
View Article : Google Scholar
|
|
46
|
Cordeiro M, Ferreira Carlos F, Pedrosa P,
Lopez A and Baptista PV: Gold nanoparticles for diagnostics:
Advances towards points of care. Diagnostics (Basel). 6:432016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang Y, Yu L, Kong X and Sun L:
Application of nanodiagnostics in point-of-care tests for
infectious diseases. Int J Nanomedicine. 12:4789–4803. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chowdhury NK, Choudhury R, Gogoi B, Chang
CM and Pandey RP: Microbial synthesis of gold nanoparticles and
their application. Curr Drug Targets. 23:752–760. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lopes TS, Alves GG, Pereira MR, Granjeiro
JM and Leite PEC: Advances and potential application of gold
nanoparticles in nanomedicine. J Cell Biochem. 120:16370–16378.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Anker JN, Hall WP, Lyandres O, Shah NC,
Zhao J and Van Duyne RP: Biosensing with plasmonic nanosensors. Nat
Mater. 7:442–453. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Datta M, Desai D and Kumar A: Gene
specific DNA sensors for diagnosis of pathogenic infections. Indian
J Microbiol. 57:139–147. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mi X, He F, Xiang M, Lian Y and Yi S:
Novel phage amplified multichannel series piezoelectric quartz
crystal sensor for rapid and sensitive detection of Mycobacterium
tuberculosis. Anal Chem. 84:939–946. 2012. View Article : Google Scholar
|
|
53
|
Zhang X, Feng Y, Duan S, Su L, Zhang J and
He F: Mycobacterium tuberculosis strain H37Rv electrochemical
sensor mediated by aptamer and AuNPs-DNA. ACS Sens. 4:849–855.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Teengam P, Siangproh W, Tuantranont A,
Vilaivan T, Chailapakul O and Henry CS: Multiplex paper-based
colorimetric DNA sensor using pyrrolidinyl peptide nucleic
acid-induced AgNPs aggregation for detecting MERS-CoV, MTB, and HPV
oligonucleotides. Anal Chem. 89:5428–5435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pascu B, Negrea A, Ciopec M, Duteanu N,
Negrea P, Bumm LA, Grad mBuriac O, Nemeş NS, Mihalcea C and
Duda-Seiman DM: Silver nanoparticle synthesis via photochemical
reduction with sodium citrate. Int J Mol Sci. 24:2552022.
View Article : Google Scholar
|
|
56
|
Iravani S, Korbekandi H, Mirmohammadi SV
and Zolfaghari B: Synthesis of silver nanoparticles: Chemical,
physical and biological methods. Res Pharm Sci. 9:385–406.
2014.
|
|
57
|
Salvador M, Marqués-Fernandez JL,
Martinez-Garcia JC, Fiorani D, Arosio P, Avolio M, Brero F,
Balanean F, Guerrini A, Sangregorio C, et al: Double-layer fatty
acid nanoparticles as a multiplatform for diagnostics and therapy.
Nanomaterials (Basel). 12:2052022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cheon HJ, Lee SM, Kim SR, Shin HY, Seo YH,
Cho YK, Lee SP and Kim MI: Colorimetric detection of MPT64 antibody
based on an aptamer adsorbed magnetic nanoparticles for diagnosis
of tuberculosis. J Nanosci Nanotechnol. 19:622–626. 2019.
View Article : Google Scholar
|
|
59
|
Yan Z, Gan N, Zhang H, Wang D, Qiao L, Cao
Y, Li T and Hu F: A sandwich-hybridization assay for simultaneous
determination of HIV and tuberculosis DNA targets based on signal
amplification by quantum dots-PowerVision™ polymer coding
nanotracers. Biosens Bioelectron. 71:207–213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen P, Meng Y, Liu T, Peng W, Gao Y, He
Y, Qu R, Zhang C, Hu W and Ying B: Sensitive urine immunoassay for
visualization of lipoarabinomannan for noninvasive tuberculosis
diagnosis. ACS Nano. 17:6998–7006. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hu O, Li Z, Wu J, Tan Y, Chen Z and Tong
Y: A multicomponent nucleic acid enzyme-cleavable quantum dot
nanobeacon for highly sensitive diagnosis of tuberculosis with the
naked eye. ACS Sens. 8:254–262. 2023. View Article : Google Scholar
|
|
62
|
He Q, Cai S, Wu J, Hu O, Liang L and Chen
Z: Determination of tuberculosis-related volatile organic biomarker
methyl nicotinate in vapor using fluorescent assay based on quantum
dots and cobalt-containing porphyrin nanosheets. Mikrochim Acta.
189:1082022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hu O, Li Z, He Q, Tong Y, Tan Y and Chen
Z: Fluorescence biosensor for one-step simultaneous detection of
Mycobacterium tuberculosis multidrug-resistant genes using
nanoCoTPyP and double quantum dots. Anal Chem. 94:7918–7927. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kabwe KP, Nsibande SA, Lemmer Y, Pilcher
LA and Forbes PBC: Synthesis and characterisation of quantum dots
coupled to mycolic acids as a water-soluble fluorescent probe for
potential lateral flow detection of antibodies and diagnosis of
tuberculosis. Luminescence. 37:278–289. 2022. View Article : Google Scholar
|
|
65
|
Shi T, Jiang P, Peng W, Meng Y, Ying B and
Chen P: Nucleic acid and nanomaterial synergistic amplification
enables dual targets of ultrasensitive fluorescence quantification
to improve the efficacy of clinical tuberculosis diagnosis. ACS
Appl Mater Interfaces. 16:14510–14519. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kabwe KP, Nsibande SA, Pilcher LA and
Forbes PBC: Development of a mycolic acid-graphene quantum dot
probe as a potential tuberculosis biosensor. Luminescence.
37:1881–1890. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liang L, Chen M, Tong Y, Tan W and Chen Z:
Detection of Mycobacterium tuberculosis IS6110 gene fragment by
fluorescent biosensor based on FRET between two-dimensional
metal-organic framework and quantum dots-labeled DNA probe. Anal
Chim Acta. 1186:3390902021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Mohd Bakhori N, Yusof NA, Abdullah J,
Wasoh H, Ab Rahman SK and Abd Rahman SF: Surface enhanced CdSe/ZnS
QD/SiNP electrochemical immunosensor for the detection of
Mycobacterium tuberculosis by combination of CFP10-ESAT6 for better
diagnostic specificity. Materials (Basel). 13:1492019. View Article : Google Scholar
|
|
69
|
Qian J, Cui H, Lu X, Wang C, An K, Hao N
and Wang K: Bi-color FRET from two nano-donors to a single
nano-acceptor: A universal aptasensing platform for simultaneous
determination of dual targets. Chem Eng J. 401:1260172020.
View Article : Google Scholar
|
|
70
|
Zhang LM, Li R, Zhao XC, Zhang Q and Luo
XL: Increased transfusion of fresh frozen plasma is associated with
mortality or worse functional outcomes after severe traumatic brain
injury: A retrospective study. World Neurosurg. 104:381–389. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang X, Hu Y, Yang X, Tang Y, Han S, Kang
A, Deng H, Chi Y, Zhu D and Lu Y: FÖrster resonance energy transfer
(FRET)-based biosensors for biological applications. Biosens
Bioelectron. 138:1113142019. View Article : Google Scholar
|
|
72
|
Chen S, Yu YL and Wang JH: Inner filter
effect-based fluorescent sensing systems: A review. Anal Chim Acta.
999:13–26. 2018. View Article : Google Scholar
|
|
73
|
Afsari HS, Cardoso Dos Santos M, Lindén S,
Chen T, Qiu X, van Bergen En Henegouwen PM, Jennings TL, Susumu K,
Medintz IL, Hildebrandt N and Miller LW: Time-gated FRET
nanoassemblies for rapid and sensitive intra- and extracellular
fluorescence imaging. Sci Adv. 2:e16002652016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gliddon HD, Howes PD, Kaforou M, Levin M
and Stevens MM: A nucleic acid strand displacement system for the
multiplexed detection of tuberculosis-specific mRNA using quantum
dots. Nanoscale. 8:10087–10095. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Futane A, Narayanamurthy V, Jadhav P and
Srinivasan A: Aptamer-based rapid diagnosis for point-of-care
application. Microfluid Nanofluidics. 27:152023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kumar S, Wang Z, Zhang W, Liu X, Li M, Li
G, Zhang B and Singh R: Optically active nanomaterials and its
biosensing applications-a review. Biosensors (Basel). 13:852023.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sharifi S, Vahed SZ, Ahmadian E, Dizaj SM,
Eftekhari A, Khalilov R, Ahmadi M, Hamidi-Asl E and Labib M:
Detection of pathogenic bacteria via nanomaterials-modified
aptasensors. Biosens Bioelectron. 150:1119332020. View Article : Google Scholar
|
|
78
|
Pornprom T, Phusi N, Thongdee P, Pakamwong
B, Sangswan J, Kamsri P, Punkvang A, Suttisintong K,
Leanpolchareanchai J, Hongmanee P, et al: Toward the early
diagnosis of tuberculosis: A gold particle-decorated
graphene-modified paper-based electrochemical biosensor for Hsp16.3
detection. Talanta. 267:1252102024. View Article : Google Scholar
|
|
79
|
Wang J, Shao W, Liu Z, Kesavan G, Zeng Z,
Shurin MR and Star A: Diagnostics of tuberculosis with
single-walled carbon nanotube-based field-effect transistors. ACS
Sens. 9:1957–1966. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Le TN, Descanzo MJN, Hsiao WWW, Soo PC,
Peng WP and Chang HC: Fluorescent nanodiamond immunosensors for
clinical diagnostics of tuberculosis. J Mater Chem B. 12:3533–3542.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bisht N, Patel M, Dwivedi N, Kumar P,
Mondal DP, Srivastava AK and Dhand C: Bio-inspired
polynorepinephrine based nanocoatings for reduced graphene
oxide/gold nanoparticles composite for high-performance biosensing
of Mycobacterium tuberculosis. Environ Res. 227:1156842023.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Seo G, Lee G, Kim W, An I, Choi M, Jang S,
Park YJ, Lee JO, Cho D and Park EC: Ultrasensitive biosensing
platform for Mycobacterium tuberculosis detection based on
functionalized graphene devices. Front Bioeng Biotechnol.
11:13134942023. View Article : Google Scholar
|
|
83
|
Mogha NK, Sahu V, Sharma RK and Masram DT:
Reduced graphene oxide nanoribbon immobilized gold nanoparticle
based electrochemical DNA biosensor for the detection of
Mycobacterium tuberculosis. J Mater Chem B. 6:5181–5187. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Li Y, Peng D, Guo S, Yang B, Zhou J, Zhou
J, Zhang Q and Bai L: Aptasensor for Mycobacterium tuberculosis
antigen MPT64 detection using anthraquinone derivative confined in
ordered mesoporous carbon as a new redox nanoprobe.
Bioelectrochemistry. 147:1082092022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Rizi KS, Hatamluyi B, Rezayi M, Meshkat Z,
Sankian M, Ghazvini K, Farsiani H and Aryan E: Response surface
methodology optimized electrochemical DNA biosensor based on
HAPNPTs/PPY/MWCNTs nanocomposite for detecting Mycobacterium
tuberculosis. Talanta. 226:1220992021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Javed A, Abbas SR, Hashmi MU, Babar NUA
and Hussain I: Graphene oxide based electrochemical genosensor for
label free detection of mycobacterium tuberculosis from raw
clinical samples. Int J Nanomedicine. 16:7339–7352. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Omar RA, Verma N and Arora PK: Development
of ESAT-6 based immunosensor for the detection of mycobacterium
tuberculosis. Front Immunol. 12:6538532021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Jaroenram W, Kampeera J, Arunrut N,
Karuwan C, Sappat A, Khumwan P, Jaitrong S, Boonnak K, Prammananan
T, Chaiprasert A, et al: Graphene-based electrochemical genosensor
incorporated loop-mediated isothermal amplification for rapid
on-site detection of Mycobacterium tuberculosis. J Pharm Biomed
Anal. 186:1133332020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kahng SJ, Soelberg SD, Fondjo F, Kim JH,
Furlong CE and Chung JH: Carbon nanotube-based thin-film resistive
sensor for point-of-care screening of tuberculosis. Biomed
Microdevices. 22:502020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hidayah NMS, Liu WW, Lai CW, Noriman NZ,
Khe CS, Hashim U and Lee HC: Comparison on graphite, graphene oxide
and reduced graphene oxide: Synthesis and characterization. AIP
Conf Proc. 1892:1500022017. View Article : Google Scholar
|
|
91
|
Ping J, Zhou Y, Wu Y, Papper V, Boujday S,
Marks RS and Steele TW: Recent advances in aptasensors based on
graphene and graphene-like nanomaterials. Biosens Bioelectron.
64:373–385. 2015. View Article : Google Scholar
|
|
92
|
Raccichini R, Varzi A, Passerini S and
Scrosati B: The role of graphene for electrochemical energy
storage. Nat Mater. 14:271–279. 2015. View Article : Google Scholar
|
|
93
|
Yan Q, Zhi N, Yang L, Xu G, Feng Q, Zhang
Q and Sun S: A highly sensitive uric acid electrochemical biosensor
based on a nano-cube cuprous oxide/ferrocene/uricase modified
glassy carbon electrode. Sci Rep. 10:106072020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Barra A, Nunes C, Ruiz-Hitzky E and
Ferreira P: Green carbon nanostructures for functional composite
materials. Int J Mol Sci. 23:18482022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chaturvedi M, Patel M, Bisht N, Shruti,
Das Mukherjee M, Tiwari A, Mondal DP, Srivastava AK, Dwivedi N and
Dhand C: Reduced graphene oxide-polydopamine-gold nanoparticles: A
ternary nanocomposite-based electrochemical genosensor for rapid
and early Mycobacterium tuberculosis detection. Biosensors (Basel).
13:3422023. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Tian J, Deng SY, Li DL, Shan D, He W,
Zhang XJ and Shi Y: Bioinspired polydopamine as the scaffold for
the active AuNPs anchoring and the chemical simultaneously reduced
graphene oxide: Characterization and the enhanced biosensing
application. Biosens Bioelectron. 49:466–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Li Y, Shi S, Cao H, Zhao Z, Su C and Wen
H: Improvement of the antifouling performance and stability of an
anion exchange membrane by surface modification with graphene oxide
(GO) and polydopamine (PDA). J Memb Sci. 566:44–53. 2018.
View Article : Google Scholar
|
|
98
|
Xia L, Vemuri B, Gadhamshetty V and
Kilduff J: Poly (ether sulfone) membrane surface modification using
norepinephrine to mitigate fouling. J Memb Sci. 598:1176572020.
View Article : Google Scholar
|
|
99
|
Dhand C, Ong ST, Dwivedi N, Diaz SM,
Venugopal JR, Navaneethan B, Fazil MH, Liu S, Seitz V, Wintermantel
E, et al: Bio-inspired in situ crosslinking and mineralization of
electrospun collagen scaffolds for bone tissue engineering.
Biomaterials. 104:323–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Teengam P, Siangproh W, Tuantranont A,
Vilaivan T, Chailapakul O and Henry CS: Electrochemical
impedance-based DNA sensor using pyrrolidinyl peptide nucleic acids
for tuberculosis detection. Anal Chim Acta. 1044:102–109. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Thangamuthu M, Hsieh KY, Kumar PV and Chen
GY: Graphene- and graphene oxide-based nanocomposite platforms for
electrochemical biosensing applications. Int J Mol Sci.
20:29752019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Vu CA and Chen WY: Field-effect transistor
biosensors for biomedical applications: Recent advances and future
prospects. Sensors (Basel). 19:42142019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Chen S and Bashir R: Advances in
field-effect biosensors towards point-of-use. Nanotechnology.
34:4920022023. View Article : Google Scholar :
|
|
104
|
Szunerits S, Rodrigues T, Bagale R, Happy
H, Boukherroub R and Knoll W: Graphene-based field-effect
transistors for biosensing: Where is the field heading to? Anal
Bioanal Chem. 416:2137–2150. 2024. View Article : Google Scholar
|
|
105
|
Krishnan SK, Nataraj N, Meyyappan M and
Pal U: Graphene-based field-effect transistors in biosensing and
neural interfacing applications: Recent advances and prospects.
Anal Chem. 95:2590–2622. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gong X, Shuai L, Beingessner RL, Yamazaki
T, Shen J, Kuehne M, Jones K, Fenniri H and Strano MS: Size
selective corona interactions from self-assembled rosette and
single-walled carbon nanotubes. Small. 18:e21049512022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kumar THV, Rajendran J, Atchudan R, Arya
S, Govindasamy M, Habila MA and Sundramoorthy AK: Cobalt
ferrite/semiconducting single-walled carbon nanotubes based
field-effect transistor for determination of carbamate pesticides.
Environ Res. 238:1171932023. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Liu H, Liu F, Sun Z, Cai X, Sun H, Kai Y,
Chen L and Jiang C: Single layer aligned semiconducting
single-walled carbon nanotube array with high linear density.
Nanotechnology. 33:3753012022. View Article : Google Scholar
|
|
109
|
Wang Y, Liu D, Zhang H, Wang J, Du R, Li
TT, Qian J, Hu Y and Huang S: Methylation-induced reversible
metallic-semiconducting transition of single-walled carbon nanotube
arrays for high-performance field-effect transistors. Nano Lett.
20:496–501. 2020. View Article : Google Scholar
|
|
110
|
Tran TT, Clark K, Ma W and Mulchandani A:
Detection of a secreted protein biomarker for citrus Huanglongbing
using a single-walled carbon nanotubes-based chemiresistive
biosensor. Biosens Bioelectron. 147:1117662020. View Article : Google Scholar
|
|
111
|
Shao W, Shurin MR, Wheeler SE, He X and
Star A: Rapid detection of SARS-CoV-2 Antigens using high-purity
semiconducting single-walled carbon nanotube-based field-effect
transistors. ACS Appl Mater Interfaces. 13:10321–10327. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Li T, Liang Y, Li J, Yu Y, Xiao MM, Ni W,
Zhang Z and Zhang GJ: Carbon nanotube field-effect transistor
biosensor for ultrasensitive and label-free detection of breast
cancer exosomal miRNA21. Anal Chem. 93:15501–15507. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Chen H, Xiao M, He J, Zhang Y, Liang Y,
Liu H and Zhang Z: Aptamer-functionalized carbon nanotube
field-effect transistor biosensors for Alzheimer's disease serum
biomarker detection. ACS Sens. 7:2075–2083. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Hui YY, Chen OJ, Lin HH, Su YK, Chen KY,
Wang CY, Hsiao WW and Chang HC: Magnetically modulated fluorescence
of nitrogen-vacancy centers in nanodiamonds for ultrasensitive
biomedical analysis. Anal Chem. 93:7140–7147. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Boruah A and Saikia BK: Synthesis,
characterization, properties and novel applications of fluorescent
nanodiamonds. J Fluoresc. 32:863–885. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Mzyk A, Sigaeva A and Schirhagl R:
Relaxometry with nitrogen vacancy (NV) centers in diamond. Acc Chem
Res. 55:3572–3580. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Daniel MC and Astruc D: Gold
nanoparticles: Assembly, supramolecular chemistry,
quantum-size-related properties, and applications toward biology,
catalysis, and nanotechnology. Chem Rev. 104:293–346. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Medintz IL, Uyeda HT, Goldman ER and
Mattoussi H: Quantum dot bioconjugates for imaging, labelling and
sensing. Nat Mater. 4:435–446. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wei Y and Yang R: Nanomechanics of
graphene. Natl Sci Rev. 6:324–348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Eckhardt S, Brunetto PS, Gagnon J, Priebe
M, Giese B and Fromm KM: Nanobio silver: Its interactions with
peptides and bacteria, and its uses in medicine. Chem Rev.
113:4708–4754. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zhao P, Xu Q, Tao J, Jin Z, Pan Y, Yu C
and Yu Z: Near infrared quantum dots in biomedical applications:
Current status and future perspective. Wiley Interdiscip Rev
Nanomed Nanobiotechnol. 10:e14832018. View Article : Google Scholar
|
|
122
|
Laurent S, Bridot JL, Elst LV and Muller
RN: Magnetic iron oxide nanoparticles for biomedical applications.
Future Med Chem. 2:427–449. 2010. View Article : Google Scholar
|
|
123
|
Haiss W, Thanh NT, Aveyard J and Fernig
DG: Determination of size and concentration of gold nanoparticles
from UV-vis spectra. Anal Chem. 79:4215–4221. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kim D, Shin K, Kwon SG and Hyeon T:
Synthesis and biomedical applications of multifunctional
nanoparticles. Adv Mater. 30:e18023092018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Sobhanan J, Anas A and Biju V:
Nanomaterials for fluorescence and multimodal bioimaging. Chem Rec.
23:e2022002532023. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Katz E and Willner I: Integrated
nanoparticle-biomolecule hybrid systems: Synthesis, properties, and
applications. Angew Chem Int Ed Engl. 43:6042–6108. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Li B, Wang W, Zhao L, Wu Y, Li X, Yan D,
Gao Q, Yan Y, Zhang J, Feng Y, et al: Photothermal therapy of
tuberculosis using targeting pre-activated macrophage
membrane-coated nanoparticles. Nat Nanotechnol. 19:834–845. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Nair A, Greeny A, Nandan A, Sah RK, Jose
A, Dyawanapelly S, Junnuthula V, K V A and Sadanandan P: Advanced
drug delivery and therapeutic strategies for tuberculosis
treatment. J Nanobiotechnology. 21:4142023. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
El-Samadony H, Althani A, Tageldin MA and
Azzazy HME: Nanodiagnostics for tuberculosis detection. Expert Rev
Mol Diagn. 17:427–443. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Li M, Singh R, Wang Y, Marques C, Zhang B
and Kumar S: Advances in novel nanomaterial-based optical fiber
biosensors-a review. Biosensors (Basel). 12:8432022. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Vu CQ and Arai S: Quantitative imaging of
genetically encoded fluorescence lifetime biosensors. Biosensors
(Basel). 13:9392023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Hemmerová E and Homola J: Combining
plasmonic and electrochemical biosensing methods. Biosens
Bioelectron. 251:1160982024. View Article : Google Scholar : PubMed/NCBI
|