Acute lung injury (ALI) is characterized by a marked
decrease in lung function due to non-cardiac inflammatory injuries
(1). Its more severe form, acute
respiratory distress syndrome (ARDS), is pathologically
characterized by widespread alveolar damage, increased lung
vascular permeability and diffuse alveolar edema (2). These changes lead to substantial
alveolar flooding and subsequent hypoxemia, compromising oxygen
exchange (3,4). In clinical practice, accurately
diagnosing ALI/ARDS and determining its severity can be
challenging, especially due to individual variability between
patients. To improve treatment outcomes, standardizing assessments
for pulmonary and extrapulmonary involvement in ARDS is key,
ensuring that each patient receives the most suitable and effective
treatment (5). The Berlin
Definition provides key diagnostic criteria for ARDS, including
timing (onset within 1 week of a known clinical insult or new or
worsening respiratory symptoms), chest imaging (bilateral opacities
on imaging that are not fully explained by effusion, lobar/lung
collapse or nodules), origin of edema (respiratory failure not
fully explained by cardiac issues or fluid overload; objective
assessments such as echocardiography may be required to rule out
hydrostatic edema if no ADRS risk factor is present) and
oxygenation (6). In addition to
these tests, imaging is widely applied for diagnosis. In the middle
and late stages of ARDS, chest radiographs detect blurred edges,
bilateral patchy appearance and fusion into large patchy ground
glass or solid infiltrating shadows. Pulmonary interstitial
fibrosis is typically visualized at a later stage. Computed
tomography (CT) technology can further improve the accuracy of
examination, however the application of CT is limited by the high
doses of radiation and risk of transport of critically ill
patients. Meanwhile, chest radiography and CT have limited ability
to identify early lung lesions. Lung ultrasonography can be
employed for the auxiliary examination of ARDS. This technique is
widely used owing to its convenience, safety and ability to
dynamically evaluate lesions. However, the relatively low
specificity and technical requirements increases the probability of
a false positive. In addition, Swan-Ganz catheterization, as an
invasive adjunct, is used for the diagnosis of ARDS (7,8).
However, due to damage incurred to patients and further
complications caused by this technology, it is presently not used
as a routine examination method (9). Diagnostic criteria for ARDS, such
as increased pulmonary vascular permeability and diffuse alveolar
injury, are difficult to determine in clinical practice. Meanwhile,
it is often a challenge for clinicians to diagnose ALI/ARDS and
assess the severity of the condition due to issues such as
individual heterogeneity of patients (10). Therefore, there is need to
standardize the assessment of pulmonary and extrapulmonary
involvement in ARDS to ensure patients can receive appropriate and
effective individualized treatment (5). At the pathophysiological level,
alveolar-capillary barrier destruction, abnormal pulmonary
inflammation, oxidative stress and microcirculation disturbance
occur during ALI/ARDS (11-13). These hallmarks of pathogenesis
reveal the changes of the molecular structure. In addition, the
biomarkers found in the pathological process are summarized in
Table I. Combining insights from
biomarkers and hallmarks provides a more individualized and
adaptive approach to disease treatment, yielding better outcomes
and minimal side effects.
In the United States, the mortality rate of ALI/ARDS
38.5-41.1%, and nearly 80% in individuals aged ≥80 years (24-26). Currently, the standard treatment
approach focuses on mechanical ventilation with lung-protective
strategies and addressing underlying causes (27). In the absence of targeted
therapy, ventilation strategies emphasize low tidal volumes (6
ml/kg) and the lung-protective ventilation strategy, which involves
maintaining low airway pressure (<30 cm H2O),
applying moderate positive end-expiratory pressure and allowing a
controlled increase in Partial Pressure of CO2) to
enhance alveolar ventilation and oxygenation (28). However, high airway pressures can
lead to complications such as pneumothorax, interstitial emphysema
and ventilator-induced lung injury due to barotrauma from excessive
pressure to the lungs during mechanical ventilation (29,30). Moreover, in the advanced stages
of ALI, where lung tissue becomes more consolidated, mechanical
ventilation may lose its effectiveness. Beyond the immediate
clinical challenges of ALI/ARDS, long-term impacts include
psychological trauma and financial strain on patients and families
(31). Therefore, an early
intervention is required to prevent and slow the progression of
ALI/ARDS and new techniques are needed to improve diagnostic
biomarker sensitivity and identify novel therapeutic targets.
Continuous research yields large amounts of data
arising from diverse sources. To meet the increasing demand for
personalized data processing, artificial intelligence (AI) is being
developed and applied as an effective and reliable solution.
Optimized data visualization functionality facilitates analysis and
is time-saving (44). In
addition, by integrating data from various sources, a more
comprehensive understanding of disease complexity and novel
therapeutic targets and strategies may be achieved. However,
medical data mining continues to face challenges, such as disease
diversity, heterogeneity of treatments and outcomes and complexity
in collecting, processing, and interpreting data (45).
Studies employing omics methods have identified
racial- and sex-based genetic differences in various diseases
(46,47), highlighting disparities in
disease susceptibility and prevalence. These genetic variations
contribute to differences in conditions such as essential
hypertension (48), ovarian
cancer (49) and pulmonary
nodules (50). A study analyzing
data from nearly 40 million ARDS-associated deaths demonstrated the
influence of genetic variations on ARDS mortality rates, with
African American males experiencing the highest mortality (51). Moreover, through a comprehensive
literature review, Flores et al (52) observed a positive association
between genetic variations and susceptibility to ALI/ARDS, as well
as its outcomes, primarily through association studies. These
findings emphasize that genetic differences play a crucial role in
the clinical management and diagnosis of ALI/ARDS, offering new
insights into the underlying pathophysiology. Thus, ALI/ARDS
results from complex interactions between genetic and non-genetic
factors. This condition exhibits high heterogeneity, influenced by
individual genetic differences, cell-cell interaction and
fluctuations in internal and external environments. These factors
regulate lung inflammation and affect the incidence and progression
of ALI/ARDS (53). Advancements
in multi-omics, especially single-cell multi-omics, provide more
detailed understanding of the regulatory and causal associations
between genetic factors and cellular functions in the development
and progression of ALI/ARDS.
From the perspective of multi-omics technology, the
present review systematically summarizes and reviews the
pathological mechanism and targets of ALI/ARDS. To the best of our
knowledge, the present study is the first to review
single-cell-based ALI/ARDS (such as the detection of heterogeneous
genes) and multi-omics disease research and comprehensively
summarize integration technology of multi-omics, as well as the
association between these genetic susceptibility targets and
ALI/ARDS pathogenesis (Table
II), providing a foundation for further investigation into the
mechanisms underlying this condition.
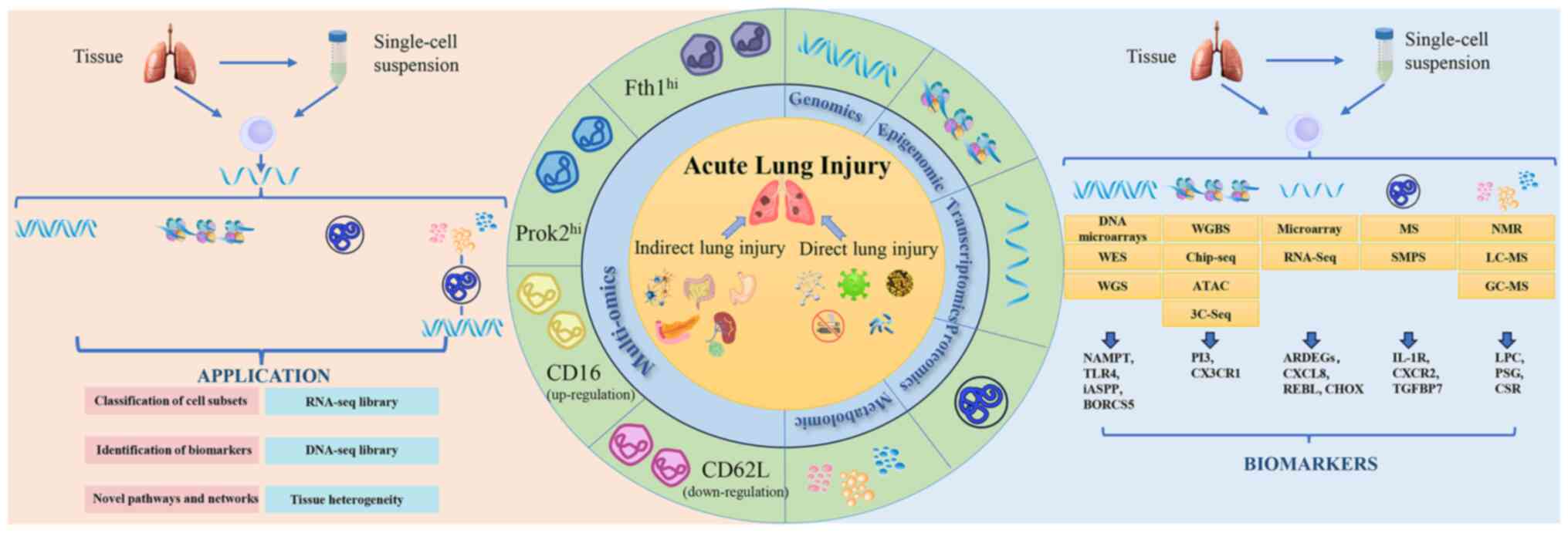
Numerous biomarkers and therapeutic targets have
been used in diagnosis and treatment of ARDS (Table I). Current studies typically use
samples of serum and lung tissues from biopsy and bronchoalveolar
lavage fluid (BALF) to identify molecular biomarkers for ALI/ARDS
(82,83). Lung tissue biopsy induces
potential complications and increases the infection rate, hence
liquid samples are used for serial sampling over time. Certain
biomarkers tailored to specific clinical needs have shown potential
for future clinical application, as shown in Table I, while high-throughput
technologies minimize selection bias and offer more comprehensive
assessment of biomarkers.
Genomics, as the earliest and most established omics
technology, serves a foundational role in the omics field. Genetic
susceptibility of diseases and large-scale data analysis facilitate
disease detection. With continuous advancements in genomics
technology and understanding of the pathogenesis of ALI/ARDS,
numerous genes associated with risk and severity of ALI/ARDS have
been identified (84), such as
angiopoietin-2 (85) and
pre-elafin (PI3) (86). These
genes, which are associated with susceptibility to ARDS, provide
understanding of its pathogenesis and contribute to the
identification of new biomarkers and therapeutic targets.
Gene-based therapy is becoming more prominent in clinical practice
(87-89). In recent years, studies have
implicated ferroptosis in the pathogenesis of ALI/ARDS by promoting
the accumulation of reactive oxygen species (90,91). Studies (54,59) have reported genetic targets using
both candidate gene and genome-wide association study (GWAS)
approaches, including nicotinamide phosphoribosyl transferase,
toll-like receptor 4 (TLR4), myosin light-chain kinase (MYLK) and
macrophage migration inhibitory factor (59). Genomic methods such as DNA
microarray and reverse transcription (RT)-PCR confirmed that the
inhibitor of apoptosis-stimulating protein of p53 protects against
ALI by inhibiting ferroptosis, thereby mitigating oxidative stress
via the nuclear factor erythroid 2-related factor 2
(Nrf2)/hypoxia-inducible factor α/transferrin signaling pathway
(54). These studies (67,92) offer insights and potential
therapeutic avenues for clinical treatment (). Furthermore, through
meta-analysis and GWAS of ARDS, Guo et al (67) identified a novel susceptibility
locus with two annotated genes BLOC-1 Related Complex Subunit 5 and
Dual specificity phosphatase 16. These genes are involved in
immune-inflammatory processes and serve as functional genetic
predictors of susceptibility to ARDS.
In addition, the mechanism by which microRNAs
(miRNAs or miRs) contained in exosomes participate in intercellular
communication and serve an immunomodulatory role in ALI/ARDS has
investigated: Shen et al (93) found that miR-125b-5p is enriched
in exosomes of adipose-derived stem cells (ADSCs) through miRNA
microarrays and analysis of databases such as miRDB, miRtarbase,
starBase and TargetScan (93).
In Cecum Ligation And Puncture (CLP)-induced sepsis models (Six-to
eight-week-old male healthy BALB/C mice), ADSC exosomes can
alleviate lung tissue damage and reduce mortality rates by
significantly increasing expression of Nrf2 and glutathione
Peroxidase 4 (GPX4) (94,95).
miR-125b-5p in ADSC exosomes can alleviate inflammation-induced
pulmonary microvascular endothelial cell(PMVEC) ferroptosis by
regulating the Keap1/Nrf2/GPX4 pathway, thereby improving ALI in
sepsis (93). Consistently, Ma
et al (96) synthesized a
novel engineered exosome (N-exo) and found that N-exo containing
miRNA 182-5p significantly improved ALI in vivo and in
vitro by targeting the NADPH oxidase 4/Dynamin-related protein
1/NLRP3 signaling pathway (96).
Additionally, Fan et al (97) used Illumina HiSeq 2500 to
sequence benzo(a)pyrene (BaP)-associated miRNAs in plasma samples
and screened differentially expressed miRNAs; hsa-miR-122-5p/Tumor
protein 53 (TP53) axis regulates WNT5A through the non-canonical
Wnt signaling pathway, thereby participating in BaP-induced lung
epithelial injury (97). Wang
et al identified and validated gene expression changes
associated with pathogenesis of hemorrhagic shock-induced ALI/ARDS,
including mRNA, miRNA, long non-coding (lnc)RNA and circular
(circ)RNA, through high-throughput sequencing analysis and
RT-quantitative (q)PCR; regulatory networks of lncRNA-miRNA-mRNA
and circRNA-miRNA-mRNA may serve a key role in pathological
processes, such as NOD-like receptor signaling pathway, JAK/STAT
signaling pathway, cytokine/cytokine receptor interaction and so on
(98). Whole exon sequencing
refers to sequencing of all exons of a gene, although the exon
region only accounts for ~1% of the whole genome and contains 85%
of the disease-causing mutations. Therefore, whole exon sequencing
has contributed toward understanding individual genomes to
facilitate development of personalized treatment and prevention
strategies. Compared with whole genome sequencing, its cost and
technical requirements are lower (48). There have been recent reports on
clinical cases using this technique to verify the metastatic origin
of lymph node cancer cells in multiple types of primary lung cancer
(99,100). In addition, Zhang et al
(101) used whole exon
sequencing to reveal diverse enriched pathways (such as
p53/SLC7A11-cystine uptake-ferroptosis pathway and embryonic
development pathway related to p53 and Mdm2 in normal lung tissue
and pre-invasive and invasive adenocarcinoma (101). Whole exon sequencing is
primarily employed in genetic diseases and rarely used in ALI/ARDS
(50). However, with the
maturity and popularity of this technology as well as combination
of emerging technologies such as data mining, whole exon sequencing
to provide personalized treatment for ALI/ARDS patients may be a
possible application to facilitate future development of
gene-targeted treatment.
Genomics facilitates understanding of the genetic
heterogeneity and diversity of cells in patients with ALI/ARDS. It
has been hypothesized that the ALI/ARDS susceptibility gene or
promoter loci obtained by genomics can be inhibited or activated to
prevent the occurrence and development of disease (51), suggesting a potential method to
prevent or cure ALI/ARDS. However, the clinical and diagnostic
applications of these identified genes (such as angiopoietin-2 gene
(85) and PI3 (86)) a have insufficient specificities
and sensitivities. Single nucleotide polymorphisms (SNPs) or single
nucleotide structural variations have not been fully studied.
When compared with genomics, epigenomics provides a
deeper understanding of the genetic mechanisms and processes of
gene expression in ALI/ARDS. Epigenomics refers to investigation of
gene activity regulation and expression changes that are not
reliant on the DNA sequence (102). In ARDS, expression of
cell-specific genes is distinguished based on their epigenomic
features, such as DNA methylation, histone modification and local
chromatin configuration (103).
Epigenomics can reveal reversible and dynamic genetic changes and
analyze epigenetic marks in different cell types to provide targets
for the treatment of ALI/ARDS.
A growing number of studies are exploring epigenetic
changes in ARDS, moving beyond traditional examination of the
genome or transcriptome (69,104,105). DNA methylation, a key
epigenetic modification, serves a pivotal role in ARDS pathogenesis
by directly inhibiting transcription and expression by preventing
specific transcription factors from binding their target sequences
on candidate genes, thereby altering gene activity (106,107). DNA methylation variations may
serve as specific diagnostic biomarkers for ARDS. Zhang et
al (60) identified
hypermethylation sites in peptidase inhibitor 3 (PI3) and
microglial fractalkine receptor (CX3CR1) genes, which were
associated with downregulated gene expression in ARDS, suggesting
their involvement in pathophysiology. Additionally, a methylation
profile study of patients with coronavirus disease 2019 (COVID)-19
and ARDS found hypomethylated probes linked to COVID-19 predictors,
including genes participating in interferon regulation and viral
response (70). This indicated
increased gene expression during severe infection, highlighting
potential biomarkers for ARDS in the context of COVID-19 (70). Further supporting the role of DNA
methylation in ARDS, an integrative omics study identified the
rs7967111 genetic variant at 12p13.2, which was significantly
associated with increased risk of ARDS (38). Sensitivity analysis and
pleiotropy evaluation suggest that this locus might act as a
pathogenic ARDS mutation, interfering with the cascade of DNA
methylation and histone modification (38). Moreover, in a lipopolysaccharide
(LPS)-induced ALI mouse model, increased DNA methyltransferase
levels were observed, along with increased 5-methylcytosine levels,
indicating higher DNA methylation levels (17). Genome-wide epigenomic analysis of
lung tissue has revealed that several inflammation-associated genes
(such as IL-10, IL-1β and CXCR2/5/6) (108) are downregulated through
methylation, further supporting the role of epigenetic alteration
in the disease process.
Given the relative ease of DNA isolation and
stability of DNA methylation marks, epigenetic signatures hold
promise as biomarkers for ARDS. As it has been confirmed that DNA
methylation or histone modification at certain sites is related to
the degree of ALI/ARDS, artificial DNA methylation regulation in
the lung may have an inhibitory effect on progression of ALI/ARDS
(104). Currently, epigenomics
often only focuses on the effects of a single gene or pathway,
while the disease mechanism of ALI/ARDS involves multi-factor
interaction, which requires validation studies in this field
(112-114). Occurrence and development of
ALI/ARDS is a dynamic and rapid process. Although epigenetic
modifications have different effects at different stages, dynamic
and long-term follow-up studies lack explanation of the dynamic
characteristics. In addition, the limited sample size may prevent
reflection of the pathological process. Therefore, large-scale
studies and long-term follow-up are required.
Transcriptomics aims to capture both coding and
ncRNA and quantify gene expression heterogeneity among cells, lung
tissue and organs. RNAs are messengers that function between DNA
and proteins; RNAs in single cells convert genetic information
stored in DNA into proteins (115). The transcriptome can serve as
an indirect indicator of protein expression, suggesting genomic
activity in real-time (116).
Transcriptomics analysis involves comprehensive examination of
modifications in gene expression, offering insight into the
regulatory mechanisms governing key genes and pathways in disease
progression.. Transcriptional profile of various types of cell
(such as endothelial cells and epithelial cells) in ALI/ARDS can
determine the prognosis and outcome of ALI/ARDS (117,118).
Through comparative gene expression analysis between
patients with ALI/ARDS and healthy controls, several differentially
expressed genes (DEGs) that are closely associated with the disease
have been identified (76).
Among these markers, certain genes and transcription factors (such
as cytoskeleton associated protein 2 Gene (CKAP2), purinergic
receptor P2Y, G-protein coupled, 14 (P2RY14), Retinol Binding
Protein 2 (RBP2) and Thymidylate Synthetase (TYMS)) serve key roles
in disease onset and progression; others serve as molecular markers
associated with disease prognosis (76). These markers enhance
understanding of ALI/ARDS pathogenesis and offer new avenues for
early diagnosis, disease monitoring and the development of
personalized treatments.
Transcriptomic technology has highlighted major
alterations in immune dysfunction-associated gene expression in
lung parenchyma. C-reactive protein and serum and amyloid A1 are
among the most upregulated genes at both transcriptional and
translational levels, making them potential predictive markers for
diseases such as COVID-19 (66).
Similarly, in patients with ALI/ARDS, the regulation of immune
responses plays a key role in disease severity. Decreased number
and proportion of regulatory T cells are associated with worsened
disease outcome, while elevated levels of pro-inflammatory
cytokines contribute to disease exacerbation. If the regulation
process of transcription and expression of pro-inflammatory
cytokines is inhibited, the severity of ALI/ARDS may be decreased,
and imbalance of inflammatory response may be regulated to a
balanced state so as to slow progress of ALI/ARDS (119,120). Furthermore, transcriptomics
holds considerable promise for developing novel therapeutic
strategies for ALI/ARDS. For example, Ma et al (90) used the Gene Expression Omnibus
database to analyze ALI/ARDS models and identified immune-mediated
ferroptosis genes involved in oxidative stress and metabolic
processes, suggesting potential targets for immunotherapy aimed at
modulating underlying pathophysiology. Transcriptome studies in
lung tissue have also revealed that furosemide mitigates
inflammatory responses and oxidative stress by modulating the
thioredoxin-interacting protein/NOD-like receptor thermal protein
domain-associated protein 3 (NLRP3)/gasdermin D pathway,
influencing inflammasome activation and pyroptosis (121-123). Wang et al performed
high-throughput analysis of hemorrhagic shock-induced ALI/ARDS,
identifying a network of lncRNA-miRNA-mRNA (including 12 lncRNAs, 5
miRNAs and 15 mRNAs) and circRNA-miRNA-mRNA interactions (including
16 miRNAs and 39 mRNAs and 10 circRNAs) associated with ALI/ARDS
(98). The discovery of these
regulatory changes facilitates development of novel treatments
targeting the metabolic processes in ALI/ARDS. miRNA has become one
of the therapeutic strategies of choice. Downregulated the
aforementioned miRNAs (such as MAP2K3, Copine 1 and
Cortactin(Map2k3, Cpne1, and Cttn)) may serve as a treatment of
ALI/ARDS.
The large amount of data involved in transcriptomic
techniques presents difficulties in processing and analysis. The
standardization of data processing and analysis is Standardization
of data processing and analysis is challenging due to the need to
apply different experimental methods. Therefore, development of
efficient data processing tools and algorithms and the exploration
of novel data integration and standardization methods are required.
Advancing the integration of transcriptomic techniques and other
omics techniques to understand the function and regulatory networks
of biological systems can facilitate further research. Moreover,
the clinical implementation of transcriptomic markers necessitates
further validation and optimization to ensure accuracy and
reliability.
Compared with traditional cell population analysis,
transcriptomic data offer a novel perspective on understanding gene
expression, revealing regulatory mechanisms of key genes and
pathways involved in disease processes (124). Advances in cell capture,
phenotyping, molecular biology and bioinformatics continue to
expand the potential for transcriptomics in biological and medical
applications (125). Further
technological progress and research may identify clinically
relevant transcriptomic markers for ALI/ARDS to redefine treatment
approaches, improving patient outcomes and quality of life.
Proteins are the primary building blocks of life and
maintain most cellular functions, including replicating DNA,
facilitating transduction of genetic information, catalyzing
metabolic reactions and driving cellular motility (126). Because protein profiles reflect
organ-specific information more accurately than DNA or RNA alone,
proteomics may reveal mechanisms that cannot be identified at the
genomic level and are directly associated with clinical phenomena.
Deep proteome profiling has identified biomarkers linked to
ALI/ARDS progression, such as caveolin-1 (127), MMPs (128), vascular endothelial growth
factor (VEGF) (129), receptor
of advanced glycation end products (130), tumor necrosis factor (TNF-α)
and IL-8 (131), providing
insights into diagnosis, prognosis and mechanistic understanding of
the disease at all stages (132). In 2003, proteomics was proposed
to analyze altered protein expression comprehensively, allowing
early biomarker discovery, refined therapeutic strategies and
accelerated drug development (133). Increasingly, proteomics studies
use diverse sample types, such as BALF (134), lung tissue (135), blood and exhaled breath
condensate (136), offering
flexibility in identification of key proteins and pathways
associated with ALI/ARDS. This approach enhances understanding of
inflammatory and repair signaling pathways and specific proteins
relevant to ALI/ARDS pathology with potential clinical applications
as biomarkers.
ALI/ARDS triggers complex changes in lung protein
expression; >100 protein biomarkers have emerged as potential
therapeutic targets (137). In
2004, Bowler et al (138) profiled proteins in plasma and
pulmonary edema fluid of patients with ALI, identifying >300
distinct proteins (138). These
biomarkers, analyzed through methods such as protein-protein
interaction networks, data-independent acquisition proteomics and
parallel-reaction monitoring, demonstrate promising diagnostic and
prognostic potential (19).
Studies have highlighted the involvement of proteins such as
caveolin-1 (127), MMPs
(128), vascular endothelial
growth factor (VEGF) (129),
receptor of advanced glycation end products (130), tumor necrosis factor (TNF-α)
and IL-8 (131). These proteins
are primarily expressed by alveolar epithelial or endothelial
cells. Therefore, regulation of protein expression in epithelial or
endothelial cells during ALI/ARDS may be a strategy for future
treatment. However, a holistic approach is needed to capture the
dynamic, multifaceted changes that characterize ALI.
Recent advances in mass spectrometry (MS) technology
have enabled high-throughput protein sequencing, leading to broader
application of proteomics in ALI/ARDS research (139,140). MS-based proteomics techniques
include isotope-encoded affinity tagging, stable isotope labeling
of amino acids in cell culture, isobaric tagging for relative and
absolute quantification (iTRAQ), gas chromatography and liquid
chromatography (LC). Xu et al (56) used iTRAQ to identify proteins
differentially expressed in endotoxin-induced porcine ARDS models,
noting that IL-1 receptor antagonist protein, α-trypsin-interacting
inhibitor heavy chain H4, MMP-1 and MMP-10 are highly expressed
after LPS-induced ARDS and decreased following hemadsorption (HA)
treatment. This indicates HA treatment could mitigate ARDS by
curbing cytokine storms, improving alveolar barrier integrity and
restoring proteomic balance in the exudative phase. Clinical
verification is required to determine whether HA can improve
disease progression of patients with ALI/ARDS (56). Adrover et al (61) used LC-MS to reveal that a CXCR2-
and circadian-regulated program in neutrophils decreases
inflammation by modulating proteome profiles. This depletes granule
content and extracellular trap formation, leading to a reduction in
inflammatory proteins (61).
Dong et al (68)
conducted proteomic assay and Mendelian randomization to identify
plasma insulin-like growth factor binding protein 7 as a novel
biomarker involved in platelet function in ARDS pathology,
providing a promising target for further experimental and clinical
research.
Proteomics methods are relatively complex and
certain proteins may exhibit instability, which limits their
application. Thus, optimizing proteomic analysis methods may be a
future research focus. Additionally, proteomics can be integrated
with other omics techniques to mitigate its limitations, thereby
advancing understanding of complex biological systems such as
ALI/ARDS.
The application of proteomics has identified protein
biomarkers for ALI/ARDS diagnosis and prognosis (such as fatty
acid-binding protein 5 (67),
Chromosome Segregation 1 Like) (141). However, the human proteome is
dynamic and certain protein biomarkers exhibit structural diversity
after modification. PTMs are chemical modifications that are
primarily catalyzed by enzymes that play key roles in the
functional proteome and are the key mechanism underlying increased
proteomic diversity (142).
PTMs are commonly involved in complex and dynamic cellular
processes by regulating cellular activity, localization and
interactions with other cell molecules such as proteins, nucleic
acids, lipids and cofactors (143). PTMs include phosphorylation,
glycosylation, ubiquitination, methylation, acetylation, lipidation
and protein hydrolysis, and are involved in almost all aspects of
normal cell biology and pathogenesis (144). Moreover, there is a growing
body of evidence implicating PTMs in a number of human disease
mechanisms, which makes the utilization and understanding of PTMs
key in the discovery of molecular mechanisms and therapeutic
targets for ALI/ARDS (144,145).
Protein phosphorylation is one of the most studied
and common PTMs, occurring primarily on threonine, serine, and
tyrosine residues (146-148).
Protein phosphorylation serves a major role in regulating the cell
cycle, division, apoptosis and signal transduction as a key
mechanism of cellular signal transduction (149). Zhao et al (150) demonstrated the importance of
MAPK and the JAK/STAT pathway by enriching the transcriptome of
lung tissue, followed by protein immunoblotting to demonstrate that
Xie-Bai-San (XBS) inhibits phosphorylation of extracellular
signal-regulated kinases (ERKs) and STAT3 to combat ALI (150). PTMs occur on different amino
acid side chains or peptide bonds and are usually mediated by
enzymatic activity (146). Li
et al (151) found that
histone deacetylase, an important epigenetic modifying enzyme, can
promote ALI by upregulating the phosphorylation of Rho-associated
protein kinase 1 (151).
In conclusion, protein phosphorylation and
dephosphorylation are the most prevalent key regulatory mechanisms
that regulate and control protein viability and function. Protein
phosphorylation plays an important role in the pathological
mechanisms of ALI/ARDS and may serve as a biomarker and potential
therapeutic target for ALI/ARDS. However, most of the biomarkers in
phosphoproteomics are still in the preclinical stage and there are
only few methods available to detect protein phosphorylation with
low specificity, such as mass spectrometry and quantitative
phosphoproteomics (152).
Therefore, there is need for larger prospective cohort studies on
phosphoproteomics, as well as development of assays and reduction
of assay cost to facilitate their application in clinical
practices.
With the development of PTMs in recent years,
lactate proteolysis has become a focus of research (146). Lactate is a key metabolite of
glycolysis, and lactate proteolysis is crucial for lactate
functioning, which is involved in glycolysis-related cellular
function and macrophage polarization (153). Lactate proteolysis is a protein
modification induced by lactate accumulation, which can affect gene
transcription by altering spatial conformation of histones and
thereby regulating the expression of associated genes (154). Increased production of lactate
under hypoxia and mitochondrial dysfunction and decreased clearance
due to renal and hepatic injury facilitate the accumulation of
lactate in septic patients; therefore, high levels of serum lactate
are a key biomarker of sepsis prognosis (155).
Ubiquitination is a key PTM mediated by three
enzymes (E1, E2 and E3) that are involved in the degradation of
proteins as well as in the regulation of cellular functions, such
as cell cycle, proliferation, apoptosis, differentiation,
metastasis, gene expression, transcriptional regulation, signaling,
repair of damage and inflammatory immunity (161). Ubiquitination serves an
important role in the pathogenesis of ALI and other types of lung
diseases, and the expressions of various inflammatory and
anti-inflammatory factors are regulated by ubiquitination. For
example, the deubiquitinating enzyme Ubiquitin Specific Peptidase
13 (USP13) stabilizes the anti-inflammatory receptor immunoglobin
interleukin-1-related receptor (IL-1R8/Sigirr) to inhibit lung
inflammation (162). Qian et
al (163) found E3
ubiquitin ligase, tripartite motif-containing 47 (TRIM47), is
highly expressed in vascular endothelial cells and the knockdown of
TRIM47 inhibits the transcription of pro-inflammatory cytokines,
thereby suppressing LPS-induced lung inflammation and ALI (163). Li et al (164) found that inflammatory cytokine
secretion is decreased by inhibiting Nrf2 ubiquitinated proteasomal
degradation, which attenuates LPS-induced ALI (164). There is growing evidence of the
involvement of ubiquitination in development of ALI/ARDS, but
studies of ALI/ARDS based on ubiquitination proteomics are not
comprehensive (165,166). Optimization of ubiquitination
proteomics methodology and may identify ubiquitination
biomarkers.
Protein phosphorylation, endocytosis and
ubiquitination are the most prevalent key mechanisms that regulate
and control protein viability and functions. Protein
phosphorylation, endocytosis and ubiquitination serve key roles in
the pathological mechanisms of ALI/ARDS and may serve as biomarkers
and potential therapeutic targets. However, most biomarkers for
modified proteomics are still in the preclinical stage, with few
assays and low specificity. Therefore, there is need for larger
prospective cohort studies of modified proteomics, as well as
development of additional assays and decreased costs to facilitate
application in clinical practice.
Metabolomics involves qualitative and quantitative
analyses of low-molecular weight metabolites or small molecule
chemicals involved in metabolism and present in an organism or cell
to reveal the association between dynamic metabolic changes and
pathophysiological processes influenced by internal and external
factors (167). Metabolomics
uses information modeling and system integration to evaluate
indicators, thereby offering insights into dynamic metabolic
changes in organisms. Metabolomics applications involve techniques
to elucidate large differences in polarity, charge and size of
multiple metabolites, and the most common techniques are based on
nuclear magnetic resonance spectroscopy and MS (168). Metabolomics can analyze changes
in cell metabolic state in real-time and reveal the metabolic
pathways in development of ALI/ARDS to facilitate the discovery of
novel biomarkers for early diagnosis and prognosis evaluation.
Moreover, advancements in MS technology have
enhanced the speed of small molecule analysis in cell metabolomics,
enabling high-throughput metabolomics and novel routes for systems
biology, functional genomics, drug discovery and personalized
medicine (169,170). For example, using an LC-MS
platform, Evans et al (171) analyzed BALF samples from
patients with ARDS and healthy controls, identifying 37 biomarkers
primarily affecting amino acid metabolism, glycolysis,
gluconeogenesis, fatty acid biosynthesis and phospholipid and
purine metabolism. Similarly, Nan et al (71) used ultra-high-performance
LC-MS/MS to examine the metabolomic profiles of patients with
community-acquired pneumonia (CAP). The aforementioned study
distinguished acute and remission phases of CAP and identified
myristoyl lysophosphatidylcholine (LPC) in patient plasma, a
metabolite inversely associated with disease severity, suggesting
its potential as a biomarker and therapeutic target for pneumonia.
Additionally, Fan et al (172) used metabolomics to
differentiate and diagnose ALI in patients with acute aortic
dissection, identifying β-hydroxybutyrate and TNF-α as independent
risk factors for ALI. The aforementioned study also highlighted
elevated levels of pyruvate, alanine, malondialdehyde and lactic
acid, along with changes in amino acid profiles, providing valuable
insight into ALI mechanisms and potential biomarkers for diagnosis
and treatment (172).
Large-scale metabolomics research is increasingly
used to explore individual responses to environmental stimuli based
on genetic background and time-dependent variations (173,174). Beyond observing metabolic
shifts following drug administration, metabolomics offers a
detailed and accurate view of drug mechanisms and therapeutic
efficacy. Li et al (62)
employed Ultra Performance Liquid Chromatography (UPLC-triple-time
of flight (TOF)/MS to analyze the metabolic effects of Ganoderma
atrum polysaccharide (PSG) on rats; significant alterations in
histidine, nitrogen, tryptophan and glycerol phospholipid
metabolism were observed, which supported the protective effect of
PSG against ALI, preserving lung histology and decreasing
pro-inflammatory cytokines and NO in serum and lung tissue.
Similarly, using Ultra Performance Liquid Chromatography Quadrupole
TOF-MS (UPLC-QTOF-MS), Hu et al (63) examined plasma samples from
LPS-induced ALI rats treated with crude Scutellariae radix
(CSR) and wine-processed S. radix (WSR) and found 16
biomarkers in LPS-induced rat plasma; CSR influenced ALI by
regulating abnormal sphingolipid metabolic pathways, whereas
WSR-treated samples primarily showed adjustments in retinol and
tryptophan metabolism, indicating mechanisms of these
treatments.
However, metabolomics research has problems. For
example, small sample sizes may prevent detection of significant
differences. Differences in the metabolome of patients with
ALI/ARDS caused by different diseases may affect the further report
of metabolomics. For metabolomics to become a reliable tool in
intensive care, issues associated with sample collection and
processing, multivariate data analysis and patient selection must
be addressed (175). For
example, patients tend to choose traditional clinical application
techniques and there is instability of metabolic markers (176). Future research should improve
speed and precision of metabolomics in detecting metabolites across
large samples. Developing methods for accurately analyzing small
samples is key for expanding metabolomic clinical utility.
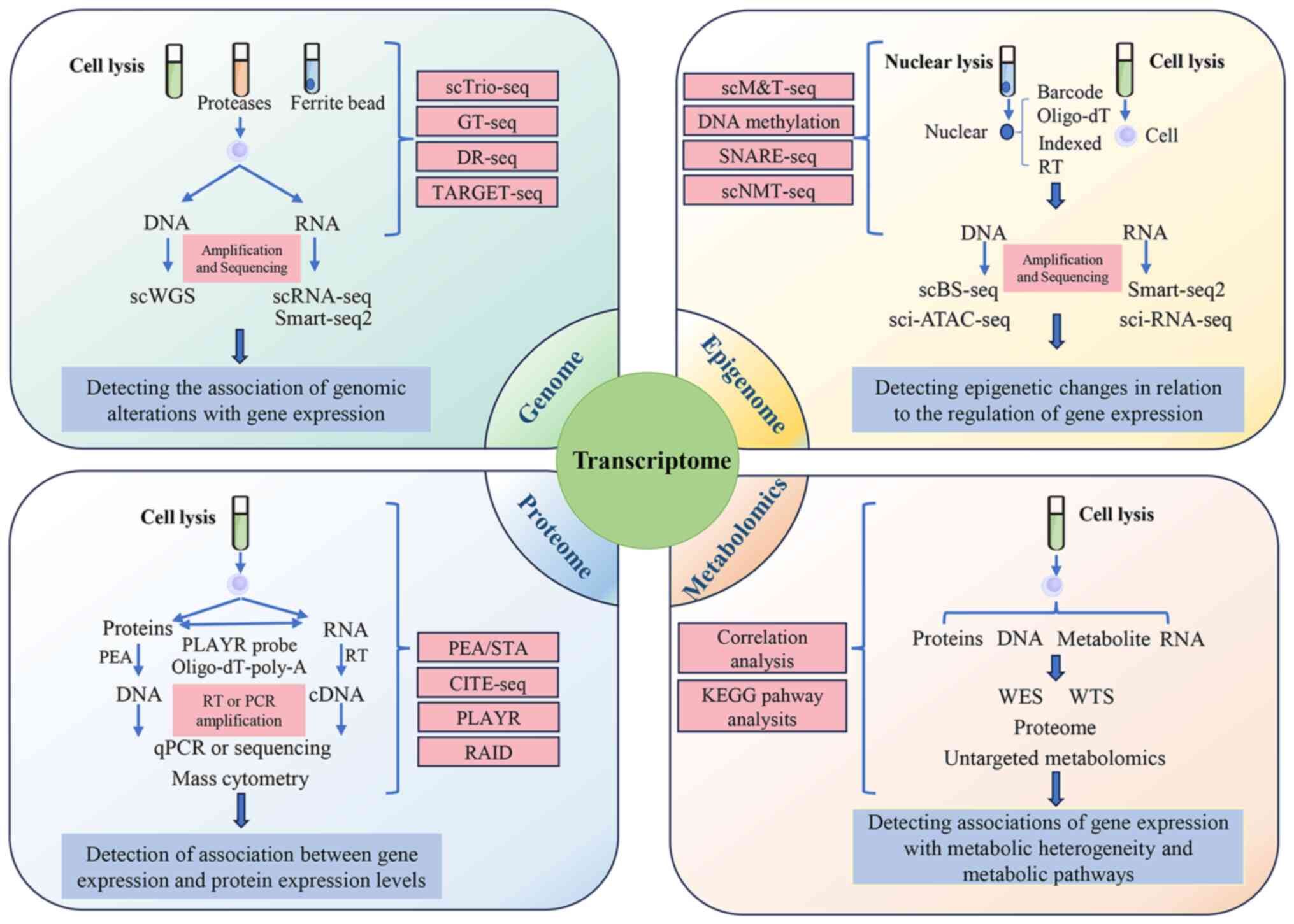
The development of single-cell multi-omics
technology facilitates analysis of genomics, proteomics,
transcriptomics and chromatin accessibility at an individual cell
level (Fig. 2) (177). This integrative approach is key
in studying the immune response dynamics in patients with ARDS and
animal models. Single-cell multi-omics not only helps identify
biomarkers associated with causes and treatment of ARDS but also
provides key insights into patient-specific disease progression and
outcomes.
Gene expression profiling through genomic or
transcriptomic sequencing serves a central role in understanding
disease genetics (178,179). However, relying solely on
genomics or transcriptomics limits insight due to individual
variations in DEGs across populations and lack of clear
gene-disease associations. Integrating genome and transcriptome
data provides a more comprehensive approach to investigating
complex diseases, bridging genetic variations and disease
phenotypes.
Combining genomics and transcriptomics has advanced
understanding of the molecular mechanisms driving ALI/ARDS,
especially pathways involved in inflammation (180). Furthermore, by examining gene
expression dynamics, integrated genomics and transcriptomics offer
valuable insight into potential targets for disease treatment.
Compared to using one of these technologies alone, transcriptomics
and genomics are more likely to reveal responses and expression
pathways of specific cell populations at the cell level. The
increased understanding of inflammatory pathways at the cellular
level is more conducive to implementation of therapeutic strategies
for specific cell types, such as lymphocytes, monocytes and
endothelial cells (181). Jiang
et al (65) used
single-cell RNA sequencing (scRNA-seq) and differential gene
analysis to compare peripheral blood mononuclear cells from
patients with pneumonia and sepsis with early ARDS with those
without ARDS; downregulated cytokine signaling 3 (SOCS3) expression
in monocytes from patients with ARDS was observed, alongside
increased expression of IFN-induced pro-inflammatory genes (such as
Ras-related protein in brain 11A (RAB11A)), which may inhibit
exocytosis of neutrophils, particularly in CD16+ cells,
suggesting that monocytes in patients with ARDS exhibit
dysregulated gene expression patterns beyond
inflammation-associated genes. This finding may suggest that
improving the monocyte intrinsic regulatory system can alleviate
ALI/ARDS (65). Other studies
have demonstrated the use of integrated genomics and
transcriptomics in understanding cell-specific responses (57,75,80,89,182). Lai et al (75) performed scRNA-Seq to study
expression of the protein arginine methyltransferase 4 (PRMT4)
gene, known to mediate lymphocyte apoptosis, in activated T
lymphocytes, finding its upregulation induces caspase 3-mediated
cell death signaling. Short hairpin (sh)RNA-mediated PRMT4
knockdown inhibits LPS-induced caspase 3 activation, suggesting it
as a promising therapeutic target. Huang et al (57), through multi-network analysis,
explored how Kruppel-like factor 2 (KLF2) regulates endothelial
integrity in ALI. Overexpressed KLF1, a regulator of
ARDS-associated genes associated with pulmonary microvascular
endothelial cytokine storm, oxidation and coagulation, was found to
improve LPS-induced ALI. Conversely, expression of pulmonary KLF2,
a key ARDS-associated gene regulator, decreased. KLF2 improves
endothelial barrier function and activates the Ras-related C3
botulinum toxin substrate 1 (Rac1) pathway in human microvascular
endothelial cells via the Rap guanine nucleotide exchange factor
3/exchange protein directly activated by cAMP 1 pathway.
Furthermore, DEG analysis and scRNA-Seq in a mouse
malaria-associated ARDS model revealed an increase in endothelial
cell number during disease regression. Therefore, promoting the
proliferation of endothelial cells may be an interesting novel
treatment for malaria-associated ARDS in combination with
antimalarial drugs (80).
MultiNicheNet interaction analysis has unveiled substantial
modifications in critical ligand-receptor interactions during ARDS
remission, offering potential targets for novel therapeutic
strategies (80). Clinical
studies using mRNA and miRNA genomics and transcriptomics show T
cell dysfunction in patients with ARDS, highlighting the
therapeutic importance of regulating immune pathways (81,182).
Combined analysis of transcriptomics and genomics
enhances ability to diagnose and treat ALI/ARDS. Nonetheless,
further exploration of specific molecular mechanisms is needed to
realize this potential.
Integrating transcriptome and epigenome data is
valuable for understanding the association between ALI/ARDS and
genetic polymorphisms. The identification of genetic polymorphisms
can identify increased risk of disease within a certain group.
Tejera et al (188)
investigated the association between genetic variants and ARDS
susceptibility in a cohort of 449 patients with ARDS and 1,031
at-risk patients who did not meet the criteria for ARDS during
hospitalization and genotyped NPs, particularly the variant
rs2664581; ELISA of plasma PI3 levels revealed that rs2664581 (or
associated SNPs in linkage disequilibrium) may impact PI3 capacity
to inhibit heightened human neutrophil Elastase activity, a genetic
variant linked to ARDS risk. Notably, different rs2664581 genotypes
resulted in varying plasma PI3 levels, with the Hap2 (TTC)
haplotype, containing the variant allele rs2664581, identified as a
risk factor for ARDS. Moreover, plasma PI3 level was significantly
lower in patients with ARDS with a allele homozygosity than in
those with the C allele variant. These findings underscore the
association between PI3 gene polymorphisms, ARDS susceptibility and
circulating plasma PI3 levels, thereby emphasizing the role of
genetic and epigenetic factors in disease risk and highlighting PI3
as a potential biomarker for targeted therapeutic intervention.
Integration of transcriptome and epigenome analysis
offers deeper understanding of ARDS pathogenesis to unveil new
potential inflammatory targets for its prevention and treatment.
Sun et al (189)
expanded on previous findings (190) linking variants of MYLK gene to
severe asthma susceptibility by investigating the role of the
non-muscle (nm) MYLK promoter region in ARDS. The 2512-bp DNA of
this region was synthesized based on the NM_053025 sequence.
Site-directed mutagenesis was used to modify the nmMYLK DNA
sequence, which was sequenced to examine its function in ARDS. The
aforementioned study also conducted electrophoretic mobility shift
assays to detect TF binding to nmMYLK and tested promoter
demethylation using 5-aza-2′-deoxycytidine in an LPS-induced ALI
mouse model to verify the role nmMYLK. The aforementioned study
revealed that exogenous and endogenous inflammatory factors, such
as cytokines, mechanical stress, hypoxia and DNA demethylation, can
significantly increase nmMYLK promoter activity, influencing
ARDS-associated SNPs by altering TF binding and increasing nmMLYK
expression, potentially affecting inflammatory severity in ARDS
(189). Increasing activity of
this site may serve a crucial role in suppression of inflammation
in clinical ALI/ARDS cases.
Integrating transcriptome and epigenome data helps
clarify the role of plasma cytokine levels. Kovacs-Kasa et
al (191) observed elevated
plasma cytokine levels in severe acute respiratory syndrome
coronavirus-2 (SARS-CoV-2)-infected patients but these cytokines
alone did not increase pulmonary microvascular endothelial
permeability directly. The plasma factors causing pulmonary
microvascular injury and increased endothelial permeability remain
unknown. Electric cell-substrate impedance sensing assessment of
plasma factors demonstrates that thrombin, angiopoietin 2, VEGF,
complement factors C3a and C5a and spike protein contributed to
increased endothelial permeability, although SARS-CoV-2 plasma
induces milder, shorter-lived endothelial injury compared with
plasma from patients with unconfirmed SARS-CoV2 infection. Among 15
cytokines analyzed, plasma levels of the pro-inflammatory cytokines
IL-1b, IL-2, IL-6, IL-8 and IL-17 are elevated in patients with
novel CoV, with IL-10 exhibiting the greatest increase compared
with the normal control group (191). Pretreatment with thrombin
inhibitors, neutralizing antibodies against cytokines, C3a and C5a
receptor antagonists or angiotensin-converting enzyme 2 (ACE2)
antibodies alone or in combination does not significantly decrease
SARS-CoV-2-induced endothelial permeability, underscoring the
complexity of SARS-CoV-2-associated endothelial injury (192).
Integrating transcriptome and epigenome analyses is
instrumental in identifying drug targets and underlying mechanisms.
Xian et al (193)
combined shRNA lentivirus silencing, RNA isolation, qPCR,
immunofluorescence, confocal microscopy and chromatin
immunoprecipitation to investigate the effect of metformin on ARDS;
the primary molecular target, Electron Transport Chain Complex I
(ETCCI), exerts an inhibitory effect by decreasing ATP production.
Metformin prevents NLRP3 inflammasome activation, macrophage
infiltration and collagen deposition by inhibiting TLR-induced
mitochondrial DNA (mtDNA) synthesis (193). These results indicate that
metformin, an affordable and well-tolerated drug, may be repurposed
to prevent and alleviate LPS-induced ARDS, highlighting it as a
potential therapeutic agent.
In summary, integrating transcriptomics and
epigenomics facilitates understanding of the association between
gene polymorphisms, changes in plasma cytokine levels and gene
modifications in ARDS. These insights are key for the future of
disease diagnosis, treatment and prognosis assessment. However,
challenges remain, including the need for advanced technology and
equipment for data acquisition and processing, ongoing optimization
and updating of bioinformatics methods and the translation of
research findings into applications for clinical practice.
Transcriptomics involves comprehensive analysis of
the entire transcriptional activity within cells at specific
developmental or physiological stages, allowing for the detection
and quantification of specific nucleic acid sequences (194). By contrast, proteomics is the
systematic study of all proteins expressed within a genome to
unravel the molecular networks governing complex biological
processes at the protein level (139). Integrating transcriptomic and
proteomic data offers holistic understanding of biological samples,
providing critical insight into physiological processes and disease
mechanisms. In ALI/ARDS research, this combined approach has aided
in understanding disease pathogenesis and identifying potential
therapeutic targets.
As a key reaction in ALI/ARDS, inflammatory
response plays an important role in progression and prognosis:
Activation of inflammatory responses aggravates ALI/ARDS severity
(9,77,195). Understanding the molecular
mechanisms underlying the inflammatory response holds promise for
improving diagnosis and treatment of ALI/ARDS. Considering the
heterogeneity and limited research findings (196), DNA microarray analysis and
genotyping have been integrated with proteomics to study ALI
pathogenesis. Tang et al (197) recently analyzed the
inflammatory response characteristics of ALI/ARDS in obesity by
targeting the transcriptional profile of lung tissue in
high-fat-induced obese ALI mice. The biological functions of DEGs
and expression characteristics of protein were explored through
transcription profiling, Gene Ontology and Kyoto Encyclopedia of
Genes and Genomes analysis. The comprehensive transcriptome and
proteomics verified that lncFirre is an effective therapeutic
target for obesity-associated ALI and revealed its inflammatory
signaling mechanism in ALI. Moreover, inflammation-associated
lncRNAs played a crucial role in the inflammatory pathway of ALI,
which provides precise targets for drug development (198). Furthermore, by merging
proteomic with transcriptomic data, studies have identified key
tissue and circulating biomarkers that predict disease progression
or deterioration in patients with idiopathic pulmonary fibrosis and
ALI (199-201). Using a combination of RNA-seq
and SomaLogic proteome assay, significant increases in pathways
such as mast cell chemokines, T helper-2 (Th-2) axis and Wnt
signaling were identified, providing valuable diagnostic insights
for ALI/ARDS (199). Sinha
et al (200) employed
scRNA-Seq and plasma proteomics to investigate pathological
mechanisms underlying ARDS. The aforementioned study characterized
a COVID-19-enriched neutrophil state, its mechanism of action and
dexamethasone-mediated modulation of neutrophil function to support
the development of targeted immunotherapies for severe COVID-19. It
remains unclear whether dexamethasone improves ARDS caused by
COVID-19 in a manner similar to that in patients with ARDS from
other causes. Moreover, by applying scRNA-Seq and unbiased serum
proteomics, Ramaswamy et al (201) investigated multisystem
inflammatory syndrome in children and COVID-19 in adults and
healthy individuals, revealing specific alterations, such as
heightened cytotoxic gene expression in natural killer and
CD8+ T cells, which could aid in early disease diagnosis
and prognosis assessment. Thus, transcriptomics and proteomics are
pivotal in examining immune response-associated characteristics and
mechanisms in ARDS progression.
Oxidative stress, a key factor in ALI/ARDS
pathogenesis, has been studied using transcriptomic and proteomic
tools. Application of quantitative redox proteomics has revealed
that S-glutathione promotes interaction between fatty acid binding
protein 5 and peroxisome proliferator-activated receptor (PPAR)β/δ,
activating PPARβ/δ target genes and suppressing LPS-induced
macrophage inflammation (67).
Additionally, a comparison of ALI mouse models underscores the key
role of S-glutathione in cellular antioxidant defense during ALI
(67). MMPs serve as key
mediators and effectors of alveolar-capillary membrane damage and
repair due to oxidative stress in ALI/ARDS. They contribute to
degradation of non-structural extracellular matrix (ECM) components
in experimental lung injury, including
syndecan-1/keratinocyte-derived chemokine and macrophage
inflammatory protein-1α (128).
Further studies have highlighted Cav-1 as a regulatory factor in
apoptotic pathways in different stages of lung injury (202,203). Cav-1 interacts with or is
upregulated by death receptor agonists such as Fas ligands, TNF and
TNF-related Apoptosis Inducing Ligand. Under normal conditions, it
interacts with the key autophagy marker protein light chain 3B, a
relationship that is disrupted by reactive oxygen species (ROS).
This suggests Cav-1 is involved in regulating autophagy-mediated
cell death during ALI/ARDS (204-206). In summary, a combination of
transcriptomics and proteomics can assess the extent of oxidative
stress. Targets of oxidative stress are expected to provide a new
way for the treatment of ALI/ARDS.
The potential of single-cell multi-omics technology
in diagnosing, assessing treatment strategies, predicting prognosis
and outcomes of ALI/ARDS continues to expand (207). Integrating metabolomics with
other omics data has become essential in understanding diseases and
developing effective treatments (172,175,208). Analyzing the association
between DEGs obtained from transcriptome data with differential
metabolites can examine organismal variations at causal and
effectual levels. This combined approach allows identification of
pivotal genes, metabolites, metabolic pathways and regulatory
networks that underlie complex disease mechanisms. Additionally,
genomics aids in identifying ALI/ARDS susceptibility-associated
genetic targets (209,210). Single-cell multi-omics
techniques may overcome the inherent limitations of individual
omics approaches, providing more precise, reliable data for
comprehensive understanding of disease mechanisms.
Single-cell multi-omics technology has recently
made notable contributions to the study of ALI/ARDS and other
diseases, especially in elucidating cellular and organelle-level
molecular mechanisms in ALI/ARDS (207,211). Zhang et al (212) used such as glucose uptake and
mitochondrial calcium measurement, fluorescence staining, protein
blot analysis, apoptosis assay, ROS flow cytometry analysis, small
interfering RNA duplex transfection, total RNA isolation and
real-time RT-PCR to explore the mitochondrial pathway involved in
hyperoxia-induced lung injury, which revealed a novel
TLR4/stanniocalcin 1 (STC1)-mediated mitochondrial pathway that
serves both homeostatic and oxidant-induced cytoprotective roles in
lung endothelial cells. Similarly, Wang et al (213) explored the potential molecular
mechanism of AU-rich binding factor 1 (AUF1, in regulating iron
homeostasis in sepsis-induced ALI: Co- and RNA immunoprecipitation
and measurements of cell viability, lipid peroxidation, iron
accumulation and total glutathione levels demonstrated that
activating the AUF1 pathway may alleviate sepsis-induced ALI.
Single-cell multi-omics has demonstrated elevated iron and
Malondialdehyde levels in lung tissue, along with marked NRF2
downregulation and activating transcription factor 3 (ATF3)
upregulation, further validating AUF1 therapeutic potential in
alleviating CLP-induced ALI.
Despite their advantages, omics techniques
currently require cell isolation from tissue, which can disrupt
intercellular interactions. However, advancements in spatial
multi-omics hold promise for overcoming these limitations by
allowing spatially resolved understanding of molecular interactions
within tissues (207).
With continuous advancements in biotechnology,
multi-omics technology may provide more precise characterization of
the heterogeneity and prognosis of disease. Multi-omics technology
and AI algorithms promote the development of precision medicine
(116). Large-scale data from
multi-omics technology is difficult to integrate (219). Poirion et al (220) proposed a unique computational
modeling framework, DeepProg, which uses patient survival as the
target model to predict novel patient survival risk. DeepProg is
run by processing multiple types of omics dataset through a
combination of deep learning and machine learning algorithms. This
approach has advantages of high sensitivity and high specificity in
cancer classification (221),
survival characteristics (222)
and treatment planning (223).
The high predictability based on deep learning is primarily
attributed to its ability to automatically capture non-linearities
and perform dimensionality reduction and hierarchical
representation. In addition, Cui et al (224) constructed a basic model, scGPT,
that can reveal complex biological interactions in the single-cell
domain by harnessing the power of pretrained transformers on a
large amount of single-cell data. The data entered in the model in
advance by the developer can then be transferred to downstream
tasks through fine-tuning, such as cell type annotation,
perturbation prediction and multi-batch and multi-group
integration. The pretrained model demonstrates strong extrapolation
capability to present meaningful clustering patterns on unseen
datasets (138).
In summary, AI-based data integration studies of
multi-omics data are primarily classified into the following four
categories: i) Feature selection and dimensionality reduction, ii)
prediction of clinical outcomes; iii) clustering for subtype
discovery (225).
High-dimensional multi-omics techniques analyze data integration
through algorithms to observe molecular-molecular interactions and
biological phenomena in living organisms. To the best of our
knowledge, however, there are relatively few studies on AI
algorithms in ALI/ARDS disease prediction and cell subtype
classification (204,205). The use of high-accuracy
prediction and drug utilization models may improve disease
prognosis and drug selection for ALI/ARDS as well as the
development of precision medicine.
There is extensive research on the application of
multi-omics technology in ALI/ARDS (37,226,227). Application of multi-omics
techniques facilitate diagnosis of diseases, such as through the
upregulation or downregulation of serum markers mentioned above. In
addition, multi-omics technology can clarify the disease mechanism
of ALI/ARDS, such as the upstream and downstream communication
between inflammatory response and reaction processes of oxidative
stress (228). To translate
current research into the clinical setting, biomarkers must be
validated. The analysis of potential biomarkers by high-throughput
omics technology requires collection of biological samples from
patients at different stages, followed by verification of
feasibility and effectiveness in clinical trials. The genomic data
of patients should be integrated and analyzed to construct a
patient information database platform for ALI/ARDS, identify
multi-omics data associated with therapeutic drugs, and adjust
personalized treatment plans for patients at different stages. A
good feedback mechanism should be established. When feasible
treatment schemes or diagnostic methods in research are applied to
clinical practice, timely feedback to researchers and continuous
exploration and development of basic experimental mechanisms is key
for development of long-term and effective clinical solutions.
Unbiased analysis of preclinical and clinical samples should be
performed. Unbiased analysis should involve careful selection of
data, ensuring that the sample is representative of the population,
and that the analysis does not overemphasize certain subsets of
data that may lead to skewed or inaccurate results. While
researchers conduct animal experiments to complete preclinical
research, the use of clinical ALI/ARDS samples to correct the
problems which found in animal experiments can promote clinical
research progress. Combination of AI and multi-omics can determine
new solutions to clinical problems through secondary analysis of
datasets. Classification and regression tree and artificial neural
networks have been applied in individualized treatment of ARDS and
the integration of algorithms can be more helpful than existing
single algorithmic approach in clinical treatment (229).
Advancements in multi-omics techniques such as
genomics, transcriptomics and proteomics are increasingly applied
at the single-cell level, providing a detailed view of cell states
and disease pathobiology (39,40,230). scRNA-Seis technology enables
identification of rare and complex cell populations, as well as
revealing gene regulatory networks and developmental trajectories
in cell lineages (231). For
example, Montoro et al (58) discovered several novel cell
types, including a pulmonary ionocyte, by characterizing
transcriptional profiles in mouse lung epithelial cells. The
function of the ionocyte is yet to be fully understood (58). The complex lung tissue
architecture, with its diverse specialized cell types, presents
challenges for traditional analytical methods in accurately
characterizing specific cell subpopulations and their roles,
especially in common respiratory diseases such as ALI/ARDS.
scRNA-Seq offers single-cell resolution in genomics, providing a
tool to accurately capture cellular states (232,233), pinpoint targets for targeted
therapies and analyze pathophysiological mechanisms in various
types of tissues and cell (72).
Recent studies using scRNA-seq have expanded
understanding of cellular subpopulations in ALI (137,234,235). Wang et al (73) identified distinct neutrophil
subpopulations in ALI mouse lung through scRNA-seq: Highly Ferritin
heavy chain (Fth1)- and Prokineticin 2 (Prok2)-expressing
neutrophils. These subpopulations serve specific roles in ALI
pathology and exhibit that the Fth1hi Neu population may
promote the pathological development in the lung of patients with
ARDS, highlighting their potential as biomarkers for disease
prognosis. Similarly, by conducting single-cell sequencing and
spatial transcriptomics, Boyd et al (64) classified lung fibroblasts into
three subpopulations: Resting, ECM-synthesizing and inflammatory
(64). They found that a subset
of fibroblasts remodels the lung microenvironment and promotes
immune cell infiltration by expressing ECM proteases such as a
disintegrin and metalloproteinase with thrombospondin motifs 4
(ADAMTS4), ultimately impairing lung function (64). Additionally, Tang et al
(74) demonstrated that RUNX1
protein enhances mitochondrial autophagy and decreases lung
inflammation during ALI through RNA-Seq of RUNX1-silenced alveolar
epithelial cells, identifying a key regulatory pathway in ALI
response.
Single-cell sequencing allows precise definition of
lung cell types and states and presents new avenues for developing
biomarkers, diagnostic tools and targeted therapies for ALI and
ARDS (55). However, there are
inherent challenges. Lung specimens used in single-cell studies are
often small and prone to degradation, making it challenging to draw
robust conclusions on cellular activity. Furthermore, scRNA-Seq
tends to capture high-expression genes, leading to
underrepresentation of low-expression genes in its output (236).
The rapid advancement of scRNA-seq, particularly
with spatial-based single-cell technology, holds potential to
deepen understanding of cellular interactions and spatial
organization within lung tissue, thereby improving knowledge of
lung health and disease mechanisms (237). While single-cell sequencing has
already provided insights into complex biological systems (238), continued research leveraging
emerging single-cell technologies is key to determine molecular
mechanisms and pathophysiology underlying ALI/ARDS and advance
therapeutic strategies for these diseases.
While single-cell multi-omics technology has
facilitated research on ALI and ARDS, several challenges remain.
First, sensitivity and resolution need improvement to accurately
detect low-abundance biomolecules and rare cell subpopulations.
Second, enhanced data processing methods are essential to handle
the high dimensionality and complexity of single-cell data
effectively. Furthermore, interpreting single-cell data within
complex biological systems and translating them into clinically
relevant information is challenging.
Current application of multi-omics technology in
ALI/ARDS research is limited due to large amounts of data and the
difficulty of cross-omics integration. Interpretation algorithms
are often limited by differences in methodology and platform
technology. This may be resolved by adopting more advanced
algorithms to integrate multi-omics data and promoting
standardization of omics technology. Other technical limitations of
this technology include low single-cell resolution, challenges in
sample separation, cell damage and data generation. Using
single-cell RNA sequencing, separating individual cells from a
heterogeneous sample is a major problem without
cross-contamination, which may introduce variability, impacting the
accuracy of results. No matter which kind of techniques such as
microfluidics, laser capture, or even standard mechanical isolation
methods, all these methods could introduce mechanical stress or
damage to cells, which can alter cellular behavior, leading to
biased or inaccurate data, especially when studying sensitive or
rare cell types. The molecular mechanism of ALI/ARDS involves
dynamic cell responses and time-dependent processes, while omics
techniques often only capture biological information at a single
time point. Thus, complete understanding of dynamic processes may
require multiple sampling and analytical tools with higher temporal
resolution. Moreover, high cost and large databases limit the
development of this technology. Extracting clinically relevant
results from large datasets poses a challenge. Patient and
cell-to-cell differences are often influenced by multiple factors
and increasing heterogeneity increases the sample size requirement.
However, difficulty of sample extraction limits the number of
clinical samples available.. Therefore, the development of
non-invasive sampling methods for longitudinal studies in
critically ill patients may better capture the dynamics of the
disease.
Despite the aforementioned challenges, advancement
of single-cell multi-omics holds promise for biomedicine,
particularly in understanding the pathogenesis of complex diseases
such as ALI and ARDS. This technology enables precise, detailed
analysis of cell heterogeneity throughout disease progression.
Capturing multiple molecular layers, such as the genome,
transcriptome, proteome and metabolome, within individual cells can
unravel the intricate networks driving disease onset and
development. In ALI and ARDS, single-cell multi-omics deepens
knowledge of essential processes such as pulmonary inflammation,
immune response and apoptosis and offers specific disease
biomarkers for early diagnosis, prognosis and treatment.
Machine learning is a technique that enables
computer systems to use data to continuously improve performance.
It belongs to a branch of AI whose main purpose is to allow
computers to learn from data to recognize patterns and make
predictions. With the increase in available biological data,
machine learning can automate analytical processes and improve
efficiency and accuracy of data processing. Because biological data
often contain a large number of features and complex patterns, it
presents a challenge to human analysts. Machine learning can
identify patterns in these complex datasets. Machine learning can
also be used to build predictive models for predicting changes in
gene expression and disease development. These models can
facilitate predictions and guide future research, especially when
experimental data is lacking (239). As aforementioned, ARDS is a
syndrome rather than a specific pathological entity and is
currently identified based on clinical criteria (9). Machine learning models can predict
the presence or absence of disease based on patient clinical
characteristics and laboratory test results, which can improve the
accuracy of diagnosis. Machine learning can predict development of
diseases and the prognosis of patients, aiding personalized and
precision medicine practices (240). The predictive accuracy of
machine learning models is affected by factors such as missing
values and uncertainties involved with medical data. In addition,
protecting patient privacy is also challenging for development of
machine learning (241).
In future, single-cell multi-omics technologies may
elucidate pathogenesis of ALI and ARDS by precisely detecting and
analyzing biomolecular changes in individual cells, integrating
data from different sources to gain a more comprehensive
understanding of disease complexity as well as new therapeutic
targets and strategies. Additionally, interdisciplinary
collaboration and integration with other technologies, such as AI
and data analysis, are expected to progress disease research.
Further refinement of single-cell multi-omics technology are
anticipated, improving sensitivity, resolution and data analysis
methods to allow more accurate detection of biomolecular changes
within cells. Moreover, establishing a robust single-cell
multi-omics database and resource-sharing platform may facilitate
data sharing and support advancements in disease research and
development. Future studies should identify novel therapeutic
targets and drug mechanisms by applying single-cell
multi-omics.
Not applicable.
ZZ, XQ, JY, JK and WH wrote the manuscript. ZZ, XQ
and JY constructed the figures. JQ, GT, XF and FM revised the
manuscript. XF conceived and supervised the study. All authors have
read and approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare they have no competing
interests.
Not applicable.
The present study was supported by National Natural Science
Foundation of China (grant no. 82073911), Taishan Scholars Program
(grant no. Tsqn202211220), Shandong Traditional Chinese Medicine
Science and Technology Project (grant no. M-2022261), Joint
Innovation Team for Clinical & Basic Research (grant no.
202401) and Natural Science Foundation of Shandong Province, China
(grant no. ZR2022QB149).
|
1
|
Bateman RM, Sharpe MD, Jagger JE, Ellis
CG, Solé-Violán J, López-Rodríguez M, Herrera-Ramos E,
Ruíz-Hernández J, Borderías L, Horcajada J, et al: 36th
International symposium on intensive care and emergency medicine:
Brussels, Belgium. 15-18 march 2016. Crit Care. 20(Suppl 2):
S942016. View Article : Google Scholar
|
|
2
|
Hussain M, Khurram Syed S, Fatima M,
Shaukat S, Saadullah M, Alqahtani AM, Alqahtani T, Bin Emran T,
Alamri AH, Barkat MQ and Wu X: Acute respiratory distress syndrome
and COVID-19: A literature review. J Inflamm Res. 14:7225–7242.
2021. View Article : Google Scholar
|
|
3
|
Schuster DP: What is acute lung injury?
What is ARDS? Chest. 107:1721–1726. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thompson BT, Chambers RC and Liu KD: Acute
respiratory distress syndrome. N Engl J Med. 377:562–572. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Villar J, Szakmany T, Grasselli G and
Camporota L: Redefining ARDS: A paradigm shift. Crit Care.
27:4162023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
ARDS Definition Task Force; Ranieri VM,
Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E,
Camporota L and Slutsky AS: Acute respiratory distress syndrome:
The berlin definition. JAMA. 307:2526–2533. 2012.PubMed/NCBI
|
|
7
|
Matthay MA, Arabi Y, Arroliga AC, Bernard
G, Bersten AD, Brochard LJ, Calfee CS, Combes A, Daniel BM,
Ferguson ND, et al: A new global definition of acute respiratory
distress syndrome. Am J Respir Crit Care Med. 209:37–47. 2024.
View Article : Google Scholar :
|
|
8
|
Gorman EA, O'Kane CM and McAuley DF: Acute
respiratory distress syndrome in adults: Diagnosis, outcomes,
long-term sequelae, and management. Lancet. 400:1157–1170. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meyer NJ, Gattinoni L and Calfee CS: Acute
respiratory distress syndrome. Lancet. 398:622–637. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stanski NL and Wong HR: Prognostic and
predictive enrichment in sepsis. Nat Rev Nephrol. 16:20–31. 2020.
View Article : Google Scholar :
|
|
11
|
Janga H, Cassidy L, Wang F, Spengler D,
Oestern-Fitschen S, Krause MF, Seekamp A, Tholey A and Fuchs S:
Site-specific and endothelial-mediated dysfunction of the
alveolar-capillary barrier in response to lipopolysaccharides. J
Cell Mol Med. 22:982–998. 2018. View Article : Google Scholar
|
|
12
|
Kumar V: Pulmonary innate immune response
determines the outcome of inflammation during pneumonia and
sepsis-associated acute lung injury. Front Immunol. 11:17222020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pokharel MD, Garcia-Flores A, Marciano D,
Franco MC, Fineman JR, Aggarwal S, Wang T and Black SM:
Mitochondrial network dynamics in pulmonary disease: Bridging the
gap between inflammation, oxidative stress, and bioenergetics.
Redox Biol. 70:1030492024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aisiku IP, Yamal JM, Doshi P, Benoit JS,
Gopinath S, Goodman JC and Robertson CS: Plasma cytokines IL-6,
IL-8, and IL-10 are associated with the development of acute
respiratory distress syndrome in patients with severe traumatic
brain injury. Crit Care. 20:2882016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang N, Yang Y, Xie Y, Yang G, Wang Q, Li
C, Liu Z and Huang JA: CD274 (PD-L1) negatively regulates M1
macrophage polarization in ALI/ARDS. Front Immunol. 15:13448052024.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao J, Zhen N, Zhou Q, Lou J, Cui W,
Zhang G and Tian B: NETs promote inflammatory injury by activating
cGAS-STING pathway in acute lung injury. Int J Mol Sci.
24:51252023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui Y, Wang X, Lin F, Li W, Zhao Y, Zhu F,
Yang H, Rao M, Li Y, Liang H, et al: MiR-29a-3p improves acute lung
injury by reducing alveolar epithelial cell PANoptosis. Aging Dis.
13:899–909. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang SW, Zhang Q, Lu D, Fang YC, Yan XC,
Chen J, Xia ZK, Yuan QT, Chen LH, Zhang YM, et al: GPR84 regulates
pulmonary inflammation by modulating neutrophil functions. Acta
Pharmacol Sin. 44:1665–1675. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong R, Luo H, Long G, Xu J, Huang C, Zhou
X, Shang Y and Zhang D: Integrative proteomic profiling of lung
tissues and blood in acute respiratory distress syndrome. Front
Immunol. 14:11589512023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Korde A, Haslip M, Pednekar P, Khan A,
Chioccioli M, Mehta S, Lopez-Giraldez F, Bermejo S, Rojas M, Dela
Cruz C, et al: MicroRNA-1 protects the endothelium in acute lung
injury. JCI Insight. 8:e1648162023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L, Tang Y, Tang J, Liu X, Zi S, Li S,
Chen H, Liu A, Huang W, Xie J, et al: Endothelial cell-derived
extracellular vesicles expressing surface VCAM1 promote
sepsis-related acute lung injury by targeting and reprogramming
monocytes. J Extracell Vesicles. 13:e124232024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Helou DG, Quach C, Hurrell BP, Li X, Li M,
Akbari A, Shen S, Shafiei-Jahani P and Akbari O: LAIR-1 limits
macrophage activation in acute inflammatory lung injury. Mucosal
Immunol. 16:788–800. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park S, Kim M, Park M, Jin Y, Lee SJ and
Lee H: Specific upregulation of extracellular miR-6238 in
particulate matter-induced acute lung injury and its
immunomodulation. J Hazard Mater. 445:1304662023. View Article : Google Scholar
|
|
24
|
Blank R and Napolitano LM: Epidemiology of
ARDS and ALI. Crit Care Clin. 27:439–458. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Katzenstein AL, Bloor CM and Leibow AA:
Diffuse alveolar damage-the role of oxygen, shock, and related
factors. A review. Am J Pathol. 85:209–228. 1976.PubMed/NCBI
|
|
26
|
Razzaque MS, Nazneen A and Taguchi T:
Immunolocalization of collagen and collagen-binding heat shock
protein 47 in fibrotic lung diseases. Mod Pathol. 11:1183–1188.
1998.
|
|
27
|
Raghavendran K and Napolitano LM:
Definition of ALI/ARDS. Crit Care Clin. 27:429–437. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Laffey JG and Kavanagh BP: Ventilation
with lower tidal volumes as compared with traditional tidal volumes
for acute lung injury. N Engl J Med. 343:812author reply 813-4.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gattinoni L, Marini JJ, Collino F, Maiolo
G, Rapetti F, Tonetti T, Vasques F and Quintel M: The future of
mechanical ventilation: Lessons from the present and the past. Crit
Care. 21:1832017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matthay MA, Ware LB and Zimmerman GA: The
acute respiratory distress syndrome. J Clin Invest. 122:2731–2740.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rubenfeld GD and Herridge MS: Epidemiology
and outcomes of acute lung injury. Chest. 131:554–562. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xia K, Sun HX, Li J, Li J, Zhao Y, Chen L,
Qin C, Chen R, Chen Z, Liu G, et al: The single-cell stereo-seq
reveals region-specific cell subtypes and transcriptome profiling
in Arabidopsis leaves. Dev Cell. 57:1299–1310 e4. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu SQ, Gao ZJ, Wu J, Zheng HM, Li B, Sun
S, Meng XY and Wu Q: Single-cell and spatially resolved analysis
uncovers cell heterogeneity of breast cancer. J Hematol Oncol.
15:192022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen P, Tang S, Li M, Wang D, Chen C, Qiu
Y, Fang Z, Zhang H, Gao H, Weng H, et al: Single-cell and spatial
transcriptomics decodes wharton's jelly-derived mesenchymal stem
cells heterogeneity and a subpopulation with wound repair
signatures. Adv Sci (Weinh). 10:e22047862023. View Article : Google Scholar
|
|
35
|
Ji AL, Rubin AJ, Thrane K, Jiang S,
Reynolds DL, Meyers RM, Guo MG, George BM, Mollbrink A,
Bergenstråhle J, et al: Multimodal analysis of composition and
spatial architecture in human squamous cell carcinoma. Cell.
182:497–514 e22. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bhargava M and Wendt CH: Biomarkers in
acute lung injury. Transl Res. 159:205–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang G, Ma X, Huang W, Wang S, Lou A, Wang
J, Tu Y, Cui W, Zhou W, Zhang W, et al: Macrophage biomimetic
nanoparticle-targeted functional extracellular vesicle micro-RNAs
revealed via multiomics analysis alleviate sepsis-induced acute
lung injury. J Nanobiotechnology. 22:3622024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Du M, Garcia JGN, Christie JD, Xin J, Cai
G, Meyer NJ, Zhu Z, Yuan Q, Zhang Z, Su L, et al: Integrative omics
provide biological and clinical insights into acute respiratory
distress syndrome. Intensive Care Med. 47:761–771. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun D, Guan X, Moran AE, Wu LY, Qian DZ,
Schedin P, Dai MS, Danilov AV, Alumkal JJ, Adey AC, et al:
Identifying phenotype-associated subpopulations by integrating bulk
and single-cell sequencing data. Nat Biotechnol. 40:527–538. 2022.
View Article : Google Scholar :
|
|
40
|
Wu Y, Hu SS, Zhang R, Goplen NP, Gao X,
Narasimhan H, Shi A, Chen Y, Li Y, Zang C, et al: Single cell RNA
sequencing unravels mechanisms underlying senescence-like
phenotypes of alveolar macrophages. iScience. 26:1071972023.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Long E, Yin J, Shin JH, Li Y, Li B, Kane
A, Patel H, Sun X, Wang C, Luong T, et al: Context-aware
single-cell multiomics approach identifies cell-type-specific lung
cancer susceptibility genes. Nat Commun. 15:79952024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bertrams W, Jung AL and Schmeck B:
Modeling of Pneumonia and Acute Lung Injury: Bioinformatics,
Systems Medicine, and Artificial Intelligence. Systems Med.
2:573–580. 2021. View Article : Google Scholar
|
|
43
|
Lam E and dos Santos CC: Advances in
molecular acute lung injury/acute respiratory distress syndrome and
ventilator-induced lung injury: The role of genomics, proteomics,
bioinformatics and translational biology. Curr Opin Crit Care.
14:3–10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen C, Wu Y, Li J, Wang X, Zeng Z, Xu J,
Liu Y, Feng J, Chen H, He Y and Xia R: TBtools-II: A 'one for all,
all for one' bioinformatics platform for biological big-data
mining. Mol Plant. 16:1733–1742. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang J, Li Y, Liu Q, Li L, Feng A, Wang T,
Zheng S, Xu A and Lyu J: Brief introduction of medical database and
data mining technology in big data era. J Evid Based Med. 13:57–69.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Grumet FC, Coukell A, Bodmer JG, Bodmer WF
and McDevitt HO: Histocompatibility (HL-A) antigens associated with
systemic lupus erythematosus. A possible genetic predisposition to
disease. N Engl J Med. 285:193–196. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bajwa EK: The genetics of acute lung
injury: Looking back and pointing the way forward. Crit Care.
13:1082009. View
Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ergul A: Hypertension in black patients:
An emerging role of the endothelin system in salt-sensitive
hypertension. Hypertension. 36:62–67. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shukla P and Singh KK: Uncovering
mitochondrial determinants of racial disparities in ovarian cancer.
Trends Cancer. 7:93–97. 2021. View Article : Google Scholar
|
|
50
|
Mirsaeidi M, Machado RF, Schraufnagel D,
Sweiss NJ and Baughman RP: Racial difference in sarcoidosis
mortality in the United States. Chest. 147:438–449. 2015.
View Article : Google Scholar :
|
|
51
|
Moss M and Mannino DM: Race and gender
differences in acute respiratory distress syndrome deaths in the
United States: An analysis of multiple-cause mortality data
(1979-1996). Crit Care Med. 30:1679–1685. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Flores C, Pino-Yanes Mdel M and Villar J:
A quality assessment of genetic association studies supporting
susceptibility and outcome in acute lung injury. Crit Care.
12:R1302008. View
Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhou H, Fan EK and Fan J: Cell-cell
interaction mechanisms in acute lung injury. Shock. 55:167–176.
2021. View Article : Google Scholar :
|
|
54
|
Jiang L, Kon N, Li T, Wang SJ, Su T,
Hibshoosh H, Baer R and Gu W: Ferroptosis as a p53-mediated
activity during tumour suppression. Nature. 520:57–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Klein AM, Mazutis L, Akartuna I,
Tallapragada N, Veres A, Li V, Peshkin L, Weitz DA and Kirschner
MW: Droplet barcoding for single-cell transcriptomics applied to
embryonic stem cells. Cell. 161:1187–1201. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xu X, Jia C, Luo S, Li Y, Xiao F, Dai H
and Wang C: Effect of HA330 resin-directed hemoadsorption on a
porcine acute respiratory distress syndrome model. Ann Intensive
Care. 7:842017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Huang RT, Wu D, Meliton A, Oh MJ, Krause
M, Lloyd JA, Nigdelioglu R, Hamanaka RB, Jain MK, Birukova A, et
al: Experimental lung injury reduces kruppel-like factor 2 to
increase endothelial permeability via regulation of RAPGEF3-Rac1
signaling. Am J Respir Crit Care Med. 195:639–651. 2017. View Article : Google Scholar :
|
|
58
|
Montoro DT, Haber AL, Biton M, Vinarsky V,
Lin B, Birket SE, Yuan F, Chen S, Leung HM, Villoria J, et al: A
revised airway epithelial hierarchy includes CFTR-expressing
ionocytes. Nature. 560:319–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lynn H, Sun X, Casanova N, Gonzales-Garay
M, Bime C and Garcia JGN: Genomic and genetic approaches to
deciphering acute respiratory distress syndrome risk and mortality.
Antioxid Redox Signal. 31:1027–1052. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang S, Wu Z, Xie J, Yang Y, Wang L and
Qiu H: DNA methylation exploration for ARDS: A multi-omics and
multi-microarray interrelated analysis. J Transl Med. 17:3452019.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Adrover JM, Aroca-Crevillén A, Crainiciuc
G, Ostos F, Rojas-Vega Y, Rubio-Ponce A, Cilloniz C,
Bonzón-Kulichenko E, Calvo E, Rico D, et al: Programmed 'disarming'
of the neutrophil proteome reduces the magnitude of inflammation.
Nat Immunol. 21:135–144. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li L, Fu WW, Wu RT, Song YH, Wu WY, Yin
SH, Li WJ and Xie MY: Protective effect of Ganoderma atrum
polysaccharides in acute lung injury rats and its metabolomics. Int
J Biol Macromol. 142:693–704. 2020. View Article : Google Scholar
|
|
63
|
Hu L, Wang Y, Sun H, Xiong Y, Zhong L, Wu
Z and Yang M: An untargeted metabolomics approach to investigate
the wine-processed mechanism of Scutellariae radix in acute lung
injury. J Ethnopharmacol. 253:1126652020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Boyd DF, Allen EK, Randolph AG, Guo XJ,
Weng Y, Sanders CJ, Bajracharya R, Lee NK, Guy CS, Vogel P, et al:
Exuberant fibroblast activity compromises lung function via
ADAMTS4. Nature. 587:466–471. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Jiang Y, Rosborough BR, Chen J, Das S,
Kitsios GD, McVerry BJ, Mallampalli RK, Lee JS, Ray A, Chen W and
Ray P: Single cell RNA sequencing identifies an early monocyte gene
signature in acute respiratory distress syndrome. JCI Insight.
5:e1356782020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang S, Yao X, Ma S, Ping Y, Fan Y, Sun S,
He Z, Shi Y, Sun L, Xiao S, et al: A single-cell transcriptomic
landscape of the lungs of patients with COVID-19. Nat Cell Biol.
23:1314–1328. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Guo Y, Liu Y, Zhao S, Xu W, Li Y, Zhao P,
Wang D, Cheng H, Ke Y and Zhang X: Oxidative stress-induced FABP5
S-glutathionylation protects against acute lung injury by
suppressing inflammation in macrophages. Nat Commun. 12:70942021.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Dong X, Zhu Z, Wei Y, Ngo D, Zhang R, Du
M, Huang H, Lin L, Tejera P, Su L, et al: Plasma insulin-like
growth factor binding protein 7 contributes causally to ARDS 28-day
mortality: Evidence from multistage mendelian randomization. Chest.
159:1007–1018. 2021. View Article : Google Scholar :
|
|
69
|
Wendisch D, Dietrich O, Mari T, von
Stillfried S, Ibarra IL, Mittermaier M, Mache C, Chua RL, Knoll R,
Timm S, et al: SARS-CoV-2 infection triggers profibrotic macrophage
responses and lung fibrosis. Cell. 184:6243–6261 e27. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Bradic M, Taleb S, Thomas B, Chidiac O,
Robay A, Hassan N, Malek J, Ait Hssain A and Abi Khalil C: DNA
methylation predicts the outcome of COVID-19 patients with acute
respiratory distress syndrome. J Transl Med. 20:5262022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Nan W, Xiong F, Zheng H, Li C, Lou C, Lei
X, Wu H, Gao H and Li Y: Myristoyl lysophosphatidylcholine is a
biomarker and potential therapeutic target for community-acquired
pneumonia. Redox Biol. 58:1025562022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Whitney JE, Lee IH, Lee JW and Kong SW:
Evolution of multiple omics approaches to define pathophysiology of
pediatric acute respiratory distress syndrome. Elife.
11:e774052022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang K, Wang M, Liao X, Gao S, Hua J, Wu
X, Guo Q, Xu W, Sun J, He Y, et al: Locally organised and activated
Fth1(hi) neutrophils aggravate inflammation of acute lung injury in
an IL-10-dependent manner. Nat Commun. 13:77032022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tang X, Zhong L, Tian X, Zou Y, Hu S, Liu
J, Li P, Zhu M, Luo F and Wan H: RUNX1 promotes mitophagy and
alleviates pulmonary inflammation during acute lung injury. Signal
Transduct Target Ther. 8:2882023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lai Y, Li X, Li T, Li X, Nyunoya T, Chen
K, Kitsios G, Nouraie M, Zhang Y, McVerry BJ, et al: Protein
arginine N-methyltransferase 4 (PRMT4) contributes to lymphopenia
in experimental sepsis. Thorax. 78:383–393. 2023. View Article : Google Scholar
|
|
76
|
Li G, Yan K, Zhang W, Pan H and Guo P:
ARDS and aging: TYMS emerges as a promising biomarker and
therapeutic target. Front Immunol. 15:13652062024. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gong T, Zhang X, Liu X, Ye Y, Tian Z, Yin
S, Zhang M, Tang J and Liu Y: Exosomal Tenascin-C primes macrophage
pyroptosis amplifying aberrant inflammation during sepsis-induced
acute lung injury. Transl Res. 270:66–80. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Xia LX, Xiao YY, Jiang WJ, Yang XY, Tao H,
Mandukhail SR, Qin JF, Pan QR, Zhu YG, Zhao LX, et al: Exosomes
derived from induced cardiopulmonary progenitor cells alleviate
acute lung injury in mice. Acta Pharmacol Sin. 45:1644–1659. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liu P, Yang S, Shao X, Li C, Wang Z, Dai H
and Wang C: Mesenchymal stem cells-derived exosomes alleviate acute
lung injury by inhibiting alveolar macrophage pyroptosis. Stem
Cells Transl Med. 13:371–386. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Pollenus E, Possemiers H, Knoops S, Prenen
F, Vandermosten L, Thienpont C, Abdurahiman S, Demeyer S, Cools J,
Matteoli G, et al: Single cell RNA sequencing reveals endothelial
cell killing and resolution pathways in experimental
malaria-associated acute respiratory distress syndrome. PLoS
Pathog. 20:e10119292024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Mitsuyama Y, Matsumoto H, Togami Y, Oda S,
Onishi S, Yoshimura J, Murtatsu A, Ito H, Ogura H, Okuzaki D and
Oda J: T cell dysfunction in elderly ARDS patients based on miRNA
and mRNA integration analysis. Front Immunol. 15:13684462024.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zheng L, Liu C, Wang H, Zhang J, Mao L,
Dong X, Hu S, Li N, Pi D, Qiu J, et al: Intact lung tissue and
bronchoalveolar lavage fluid are both suitable for the evaluation
of murine lung microbiome in acute lung injury. Microbiome.
12:562024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wu G, Sun Y, Wang K, Chen Z, Wang X, Chang
F, Li T, Feng P and Xia Z: Relationship between elevated soluble
CD74 and severity of experimental and clinical ALI/ARDS. Sci Rep.
6:300672016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Meyer NJ: Beyond single-nucleotide
polymorphisms: Genetics, genomics, and other 'omic approaches to
acute respiratory distress syndrome. Clin Chest Med. 35:673–684.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Reilly JP, Wang F, Jones TK, Palakshappa
JA, Anderson BJ, Shashaty MGS, Dunn TG, Johansson ED, Riley TR, Lim
B, et al: Plasma angiopoietin-2 as a potential causal marker in
sepsis-associated ARDS development: Evidence from Mendelian
randomization and mediation analysis. Intensive Care Med.
44:1849–1858. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wang Z, Beach D, Su L, Zhai R and
Christiani DC: A genome-wide expression analysis in blood
identifies pre-elafin as a biomarker in ARDS. Am J Respir Cell Mol
Biol. 38:724–732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Devaney J, Contreras M and Laffey JG:
Clinical review: Gene-based therapies for ALI/ARDS: Where are we
now? Crit Care. 15:2242011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Tromp TR, Stroes ESG and Hovingh GK:
Gene-based therapy in lipid management: The winding road from
promise to practice. Expert Opin Investig Drugs. 29:483–493. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ramsey LB, Brown JT, Vear SI, Bishop JR
and Van Driest SL: Gene-based dose optimization in children. Annu
Rev Pharmacol Toxicol. 60:311–331. 2020. View Article : Google Scholar
|
|
90
|
Ma A, Feng Z, Li Y, Wu Q, Xiong H, Dong M,
Cheng J, Wang Z, Yang J and Kang Y: Ferroptosis-related signature
and immune infiltration characterization in acute lung injury/acute
respiratory distress syndrome. Respir Res. 24:1542023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Cheng H, Wang X, Yao J, Yang C and Liu J:
Mitophagy and ferroptosis in sepsis-induced ALI/ARDS: Molecular
mechanisms, interactions and therapeutic prospects of medicinal
plants. J Inflamm Res. 17:7819–7835. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li Y, Cao Y, Xiao J, Shang J, Tan Q, Ping
F, Huang W, Wu F, Zhang H and Zhang X: Inhibitor of
apoptosis-stimulating protein of p53 inhibits ferroptosis and
alleviates intestinal ischemia/reperfusion-induced acute lung
injury. Cell Death Differ. 27:2635–2650. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Shen K, Wang X, Wang Y, Jia Y, Zhang Y,
Wang K, Luo L, Cai W, Li J, Li S, et al: miR-125b-5p in adipose
derived stem cells exosome alleviates pulmonary microvascular
endothelial cells ferroptosis via Keap1/Nrf2/GPX4 in sepsis lung
injury. Redox Biol. 62:1026552023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Dodson M, Castro-Portuguez R and Zhang DD:
NRF2 plays a critical role in mitigating lipid peroxidation and
ferroptosis. Redox Biol. 23:1011072019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Stockwell BR: Ferroptosis turns 10:
Emerging mechanisms, physiological functions, and therapeutic
applications. Cell. 185:2401–2421. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ma C, Liu K, Wang F, Fei X, Niu C, Li T
and Liu L: Neutrophil membrane-engineered Panax ginseng
root-derived exosomes loaded miRNA 182-5p targets NOX4/Drp-1/NLRP3
signal pathway to alleviate acute lung injury in sepsis:
Experimental studies. Int J Surg. 110:72–86. 2024.
|
|
97
|
Fan L, Bin Wang, Ma J, Ye Z, Nie X, Cheng
M, Xie Y, Gu P, Zhang Y, You X, et al: Role and mechanism of WNT5A
in benzo(a)pyrene-induced acute lung injury and lung function
decline. J Hazard Mater. 460:1323912023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wang Z, Chang P, Ye J, Ma W, Zhou J, Zhang
P, Chen X, Jia B, Zheng M, Huang W and Wang T: Genome-wide
landscape of mRNAs, microRNAs, lncRNAs, and circRNAs in hemorrhagic
shock-induced ALI/ARDS in rats. J Trauma Acute Care Surg.
90:827–837. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Bai G, Li Y, Ji Y, Peng Y, Yang Z and Zhao
L: Treatments and whole exon sequencing of a case with multiple
primary lung cancer. J Cardiothorac Surg. 18:582023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Guo W, Zhou B, Bie F, Huai Q, Xue X, Guo
L, Tan F, Xue Q, Zhao L and Gao S: Single-cell RNA sequencing
analysis reveals transcriptional heterogeneity of multiple primary
lung cancer. Clin Transl Med. 13:e14532023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhang Y, Fu F, Zhang Q, Li L, Liu H, Deng
C, Xue Q, Zhao Y, Sun W, Han H, et al: Evolutionary proteogenomic
landscape from pre-invasive to invasive lung adenocarcinoma. Cell
Rep Med. 5:1013582024. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Egger G, Liang G, Aparicio A and Jones PA:
Epigenetics in human disease and prospects for epigenetic therapy.
Nature. 429:457–463. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Liao SY, Casanova NG, Bime C, Camp SM,
Lynn H and Garcia JGN: Identification of early and intermediate
biomarkers for ARDS mortality by multi-omic approaches. Sci Rep.
11:188742021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Binnie A, Tsang JLY, Hu P, Carrasqueiro G,
Castelo-Branco P and Dos Santos CC: Epigenetics of sepsis. Crit
Care Med. 48:745–756. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Ahmad S, Manzoor S, Siddiqui S, Mariappan
N, Zafar I and Ahmad A and Ahmad A: Epigenetic underpinnings of
inflammation: Connecting the dots between pulmonary diseases, lung
cancer and COVID-19. Semin Cancer Biol. 83:384–398. 2022.
View Article : Google Scholar
|
|
106
|
Yang IV, Lozupone CA and Schwartz DA: The
environment, epigenome, and asthma. J Allergy Clin Immunol.
140:14–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Scheinecker C, Goschl L and Bonelli M:
Treg cells in health and autoimmune diseases: New insights from
single cell analysis. J Autoimmun. 110:1023762020. View Article : Google Scholar
|
|
108
|
Balnis J, Madrid A, Hogan KJ, Drake LA,
Chieng HC, Tiwari A, Vincent CE, Chopra A, Vincent PA, Robek MD, et
al: Blood DNA methylation and COVID-19 outcomes. Clin Epigenetics.
13:1182021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhang Y, Jurkowska R, Soeroes S, Rajavelu
A, Dhayalan A, Bock I, Rathert P, Brandt O, Reinhardt R, Fischle W
and Jeltsch A: Chromatin methylation activity of Dnmt3a and
Dnmt3a/3L is guided by interaction of the ADD domain with the
histone H3 tail. Nucleic Acids Res. 38:4246–4253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Goldberg AD, Allis CD and Bernstein E:
Epigenetics: A landscape takes shape. Cell. 128:635–638. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wu D, Spencer CB, Ortoga L, Zhang H and
Miao C: Histone lactylation-regulated METTL3 promotes ferroptosis
via m6A-modification on ACSL4 in sepsis-associated lung injury.
Redox Biol. 74:1031942024. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Rombauts A, Bódalo Torruella M,
Abelenda-Alonso G, Perera-Bel J, Ferrer-Salvador A, Acedo-Terrades
A, Gabarrós-Subirà M, Oriol I, Gudiol C, Nonell L and Carratalà J:
Dynamics of gene expression profiling and identification of
high-risk patients for severe COVID-19. Biomedicines. 11:13482023.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Bejaoui Y, Humaira Amanullah F, Saad M,
Taleb S, Bradic M, Megarbane A, Ait Hssain A, Abi Khalil C and El
Hajj N: Epigenetic age acceleration in surviving versus deceased
COVID-19 patients with acute respiratory distress syndrome
following hospitalization. Clin Epigenetics. 15:1862023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Singer BD, Mock JR, Aggarwal NR, Garibaldi
BT, Sidhaye VK, Florez MA, Chau E, Gibbs KW, Mandke P, Tripathi A,
et al: Regulatory T cell DNA methyltransferase inhibition
accelerates resolution of lung inflammation. Am J Respir Cell Mol
Biol. 52:641–652. 2015. View Article : Google Scholar :
|
|
115
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
He X, Liu X, Zuo F, Shi H and Jing J:
Artificial intelligence-based multi-omics analysis fuels cancer
precision medicine. Semin Cancer Biol. 88:187–200. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Xiong S, Hong Z, Huang LS, Tsukasaki Y,
Nepal S, Di A, Zhong M, Wu W, Ye Z, Gao X, et al: IL-1β suppression
of VE-cadherin transcription underlies sepsis-induced inflammatory
lung injury. J Clin Invest. 130:3684–3698. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Qian G and Fang H, Chen A, Sun Z, Huang M,
Luo M, Cheng E, Zhang S, Wang X and Fang H: A hub gene signature as
a therapeutic target and biomarker for sepsis and geriatric
sepsis-induced ARDS concomitant with COVID-19 infection. Front
Immunol. 14:12578342023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zhang ZT, Xie K, Luo RJ, Zhang DY, He ZW,
Li KF, Lin SH and Xu F: Dexmedetomidine alleviates acute lung
injury by promoting Tregs differentiation via activation of
AMPK/SIRT1 pathway. Inflammopharmacology. 31:423–438. 2023.
View Article : Google Scholar
|
|
120
|
Xu Z, Jiang X, Dai X and Li B: The dynamic
role of FOXP3(+) tregs and their potential therapeutic applications
during SARS-CoV-2 infection. Front Immunol. 13:9164112022.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
He G, Chen K, Wang H, Li X, Li W, Liu L,
Chen J, Yang D, Hu J, Xu D, et al: Fudosteine attenuates acute lung
injury in septic mice by inhibiting pyroptosis via the
TXNIP/NLRP3/GSDMD pathway. Eur J Pharmacol. 926:1750472022.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Tsubaki H, Tooyama I and Walker DG:
Thioredoxin-interacting protein (TXNIP) with focus on brain and
neurodegenerative diseases. Int J Mol Sci. 21:93572020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Lee S, Nakahira K, Dalli J, Siempos II,
Norris PC, Colas RA, Moon JS, Shinohara M, Hisata S, Howrylak JA,
et al: NLRP3 inflammasome deficiency protects against microbial
sepsis via increased lipoxin B4 synthesis. Am J Respir Crit Care
Med. 196:713–726. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Conesa A, Madrigal P, Tarazona S,
Gomez-Cabrero D, Cervera A, McPherson A, Szcześniak MW, Gaffney DJ,
Elo LL, Zhang X and Mortazavi A: A survey of best practices for
RNA-seq data analysis. Genome Biol. 17:132016. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Reilly JP, Christie JD and Meyer NJ: Fifty
years of research in ARDS. genomic contributions and opportunities.
Am J Respir Crit Care Med. 196:1113–1121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Restrepo-Pérez L, Joo C and Dekker C:
Paving the way to single-molecule protein sequencing. Nat
Nanotechnol. 13:786–796. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Jin Y, Lee SJ, Minshall RD and Choi AM:
Caveolin-1: A critical regulator of lung injury. Am J Physiol Lung
Cell Mol Physiol. 300:L151–L160. 2011. View Article : Google Scholar
|
|
128
|
Davey A, McAuley DF and O'Kane CM: Matrix
metalloproteinases in acute lung injury: Mediators of injury and
drivers of repair. Eur Respir J. 38:959–970. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Mura M, Han B, Andrade CF, Seth R, Hwang
D, Waddell TK, Keshavjee S and Liu M: The early responses of VEGF
and its receptors during acute lung injury: Implication of VEGF in
alveolar epithelial cell survival. Crit Care. 10:R1302006.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Uchida T, Shirasawa M, Ware LB, Kojima K,
Hata Y, Makita K, Mednick G, Matthay ZA and Matthay MA: Receptor
for advanced glycation end-products is a marker of type I cell
injury in acute lung injury. Am J Respir Crit Care Med.
173:1008–1015. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Fremont RD, Koyama T, Calfee CS, Wu W,
Dossett LA, Bossert FR, Mitchell D, Wickersham N, Bernard GR,
Matthay MA, et al: Acute lung injury in patients with traumatic
injuries: Utility of a panel of biomarkers for diagnosis and
pathogenesis. J Trauma. 68:1121–1127. 2010.
|
|
132
|
Ding Z, Wang N, Ji N and Chen ZS:
Proteomics technologies for cancer liquid biopsies. Mol Cancer.
21:532022. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Hanash S: Disease proteomics. Nature.
422:226–232. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Rao Z, Liu S, Li Z, Wang Q, Gao F, Peng H,
Ren D, Zang Y, Li H, Li Y, et al: Alarmin-loaded extracellular
lipid droplets induce airway neutrophil infiltration during type 2
inflammation. Immunity. 57:2514–2529.e7. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhou Y, Huang X, Yu H, Shi H, Chen M, Song
J, Tang W, Teng F, Li C, Yi L, et al: TMT-based quantitative
proteomics revealed protective efficacy of Icariside II against
airway inflammation and remodeling via inhibiting LAMP2, CTSD and
CTSS expression in OVA-induced chronic asthma mice. Phytomedicine.
118:1549412023. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Bowler RP, Ellison MC and Reisdorph N:
Proteomics in pulmonary medicine. Chest. 130:567–574. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Zi SF, Wu XJ, Tang Y, Liang YP, Liu X,
Wang L, Li SL, Wu CD, Xu JY, Liu T, et al: Endothelial cell-derived
extracellular vesicles promote aberrant neutrophil trafficking and
subsequent remote lung injury. Adv Sci (Weinh). 11:e24006472024.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Bowler RP, Duda B, Chan ED, Enghild JJ,
Ware LB, Matthay MA and Duncan MW: Proteomic analysis of pulmonary
edema fluid and plasma in patients with acute lung injury. Am J
Physiol Lung Cell Mol Physiol. 286:L1095–L1104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Rozanova S, Barkovits K, Nikolov M,
Schmidt C, Urlaub H and Marcus K: Quantitative mass
spectrometry-based proteomics: An overview. Methods Mol Biol.
2228:85–116. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Shuken SR: An introduction to mass
spectrometry-based proteomics. J Proteome Res. 22:2151–2171. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Gao CL, Song JQ, Yang ZN, Wang H, Wu XY,
Shao C, Dai HX, Chen K, Guo YW, Pang T and Li XW: Chemoproteomics
of marine natural product naamidine J unveils CSE1L as a
therapeutic target in acute lung injury. J Am Chem Soc. Sep
26–2024.Epub ahead of print. View Article : Google Scholar
|
|
142
|
Hsu CY, Fu SH, Chien MW, Liu YW, Chen SJ
and Sytwu HK: Post-translational modifications of transcription
factors harnessing the etiology and pathophysiology in colonic
diseases. Int J Mol Sci. 21:32072020. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Zhu G, Jin L, Sun W, Wang S and Liu N:
Proteomics of post-translational modifications in colorectal
cancer: Discovery of new biomarkers. Biochim Biophys Acta Rev
Cancer. 1877:1887352022. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Li Y, Huang Y and Li T: PTM-X: Prediction
of post-translational modification crosstalk within and across
proteins. Methods Mol Biol. 2499:275–283. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Ran H, Li C, Zhang M, Zhong J and Wang H:
Neglected PTM in animal adipogenesis: E3-mediated ubiquitination.
Gene. 878:1475742023. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Ebert T, Tran N, Schurgers L, Stenvinkel P
and Shiels PG: Ageing-oxidative stress, PTMs and disease. Mol
Aspects Med. 86:1010992022. View Article : Google Scholar
|
|
147
|
Zhang WJ, Zhou Y, Zhang Y, Su YH and Xu T:
Protein phosphorylation: A molecular switch in plant signaling.
Cell Rep. 42:1127292023. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Shindo S, Kakizaki S, Sakaki T, Kawasaki
Y, Sakuma T, Negishi M and Shizu R: Phosphorylation of nuclear
receptors: Novelty and therapeutic implications. Pharmacol Ther.
248:1084772023. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Paulo JA and Schweppe DK: Advances in
quantitative high-throughput phosphoproteomics with sample
multiplexing. Proteomics. 21:e20001402021. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Zhao A, Guo C, Wang L, Chen S, Xu Q, Cheng
J, Zhang J, Jiang J, Di J, Zhang H, et al: Xiebai San alleviates
acute lung injury by inhibiting the phosphorylation of the
ERK/Stat3 pathway and regulating multiple metabolisms.
Phytomedicine. 128:1553972024. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Li N, Liu B, Xiong R, Li G, Wang B and
Geng Q: HDAC3 deficiency protects against acute lung injury by
maintaining epithelial barrier integrity through preserving
mitochondrial quality control. Redox Biol. 63:1027462023.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Zeng X, Lan Y, Xiao J, Hu L, Tan L, Liang
M, Wang X, Lu S, Peng T and Long F: Advances in phosphoproteomics
and its application to COPD. Expert Rev Proteomics. 19:311–324.
2022. View Article : Google Scholar
|
|
153
|
Chen L, Huang L, Gu Y, Cang W, Sun P and
Xiang Y: Lactate-lactylation hands between metabolic reprogramming
and immunosuppression. Int J Mol Sci. 23:119432022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Jing F, Zhang J, Zhang H and Li T:
Unlocking the multifaceted molecular functions and diverse disease
implications of lactylation. Biol Rev Camb Philos Soc. Sep
16–2024.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Garcia-Alvarez M, Marik P and Bellomo R:
Sepsis-associated hyperlactatemia. Crit Care. 18:5032014.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Fan M, Yang K, Wang X, Zhang X, Xu J, Tu
F, Gill PS, Ha T, Williams DL and Li C: Lactate impairs vascular
permeability by inhibiting HSPA12B expression via GPR81-dependent
signaling in sepsis. Shock. 58:304–312. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Yao G and Yang Z: Glypican-3 knockdown
inhibits the cell growth, stemness, and glycolysis development of
hepatocellular carcinoma cells under hypoxic microenvironment
through lactylation. Arch Physiol Biochem. 130:546–554. 2024.
|
|
158
|
Wang J, Yang P, Yu T, Gao M, Liu D, Zhang
J, Lu C, Chen X, Zhang X and Liu Y: Lactylation of PKM2 suppresses
inflammatory metabolic adaptation in pro-inflammatory macrophages.
Int J Biol Sci. 18:6210–6225. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Dichtl S, Lindenthal L, Zeitler L, Behnke
K, Schlösser D, Strobl B, Scheller J, El Kasmi KC and Murray PJ:
Lactate and IL6 define separable paths of inflammatory metabolic
adaptation. Sci Adv. 7:eabg35052021. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Wang L, Li S, Luo H, Lu Q and Yu S: PCSK9
promotes the progression and metastasis of colon cancer cells
through regulation of EMT and PI3K/AKT signaling in tumor cells and
phenotypic polarization of macrophages. J Exp Clin Cancer Res.
41:3032022. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Cockram PE, Kist M, Prakash S, Chen SH,
Wertz IE and Vucic D: Ubiquitination in the regulation of
inflammatory cell death and cancer. Cell Death Differ. 28:591–605.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Li L, Wei J, Li S, Jacko AM, Weathington
NM, Mallampalli RK, Zhao J and Zhao Y: The deubiquitinase USP13
stabilizes the anti-inflammatory receptor IL-1R8/Sigirr to suppress
lung inflammation. EBioMedicine. 45:553–562. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Qian Y, Wang Z, Lin H, Lei T, Zhou Z,
Huang W, Wu X, Zuo L, Wu J, Liu Y, et al: TRIM47 is a novel
endothelial activation factor that aggravates
lipopolysaccharide-induced acute lung injury in mice via K63-linked
ubiquitination of TRAF2. Signal Transduct Target Ther. 7:1482022.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Li J, Deng SH, Li J, Li L, Zhang F, Zou Y,
Wu DM and Xu Y: Obacunone alleviates ferroptosis during
lipopolysaccharide-induced acute lung injury by upregulating
Nrf2-dependent antioxidant responses. Cell Mol Biol Lett.
27:292022. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Gao L, Zhang W, Shi XH, Chang X, Han Y,
Liu C, Jiang Z and Yang X: The mechanism of linear ubiquitination
in regulating cell death and correlative diseases. Cell Death Dis.
14:6592023. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
De Silva ARI and Page RC: Ubiquitination
detection techniques. Exp Biol Med (Maywood). 248:1333–1346. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Wishart DS: Metabolomics for investigating
physiological and pathophysiological processes. Physiol Rev.
99:1819–1875. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
McGarrah RW, Crown SB, Zhang GF, Shah SH
and Newgard CB: Cardiovascular metabolomics. Circ Res.
122:1238–1258. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Zampieri M, Sekar K, Zamboni N and Sauer
U: Frontiers of high-throughput metabolomics. Curr Opin Chem Biol.
36:15–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Paglia G and Astarita G: Metabolomics and
lipidomics using traveling-wave ion mobility mass spectrometry. Nat
Protoc. 12:797–813. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Evans CR, Karnovsky A, Kovach MA,
Standiford TJ, Burant CF and Stringer KA: Untargeted LC-MS
metabolomics of bronchoalveolar lavage fluid differentiates acute
respiratory distress syndrome from health. J Proteome Res.
13:640–649. 2014. View Article : Google Scholar :
|
|
172
|
Fan L, Meng K, Meng F, Wu Y and Lin L:
Metabolomic characterization benefits the identification of acute
lung injury in patients with type A acute aortic dissection. Front
Mol Biosci. 10:12221332023. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Tzanakis K, Nattkemper TW, Niehaus K and
Albaum SP: MetHoS: A platform for large-scale processing, storage
and analysis of metabolomics data. BMC Bioinformatics. 23:2672022.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Lind L, Fall T, Ärnlöv J, Elmståhl S and
Sundström J: Large-Scale Metabolomics and the Incidence of
Cardiovascular Disease. J Am Heart Assoc. 12:e0268852023.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Serkova NJ, Standiford TJ and Stringer KA:
The emerging field of quantitative blood metabolomics for biomarker
discovery in critical illnesses. Am J Respir Crit Care Med.
184:647–655. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Muthubharathi BC, Gowripriya T and
Balamurugan K: Metabolomics: Small molecules that matter more. Mol
Omics. 17:210–229. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Ingelfinger F, Beltrán E, Gerdes LA and
Becher B: Single-cell multiomics in neuroinflammation. Curr Opin
Immunol. 76:1021802022. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Zormpas E, Queen R, Comber A and Cockell
SJ: Mapping the transcriptome: Realizing the full potential of
spatial data analysis. Cell. 186:5677–5689. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Fatumo S, Chikowore T, Choudhury A, Ayub
M, Martin AR and Kuchenbaecker K: A roadmap to increase diversity
in genomic studies. Nat Med. 28:243–250. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Kan M, Shumyatcher M and Himes BE: Using
omics approaches to understand pulmonary diseases. Respir Res.
18:1492017. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Fagerberg L, Hallström BM, Oksvold P,
Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S,
Danielsson A, Edlund K, et al: Analysis of the human
tissue-specific expression by genome-wide integration of
transcriptomics and antibody-based proteomics. Mol Cell Proteomics.
13:397–406. 2014. View Article : Google Scholar :
|
|
182
|
Robles-Remacho A, Sanchez-Martin RM and
Diaz-Mochon JJ: Spatial transcriptomics: Emerging technologies in
tissue gene expression profiling. Anal Chem. 95:15450–15460. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Banavasi H, Nguyen P, Osman H and Soubani
AO: Management of ARDS - what works and what does not. Am J Med
Sci. 362:13–23. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Li H, Li D, Ledru N, Xuanyuan Q, Wu H,
Asthana A, Byers LN, Tullius SG, Orlando G, Waikar SS and Humphreys
BD: Transcriptomic, epigenomic, and spatial metabolomic cell
profiling redefines regional human kidney anatomy. Cell Metab.
36:1105–1125.e10. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Xiong X, James BT, Boix CA, Park YP,
Galani K, Victor MB, Sun N, Hou L, Ho LL, Mantero J, et al:
Epigenomic dissection of Alzheimer's disease pinpoints causal
variants and reveals epigenome erosion. Cell. 186:4422–4437.e21.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Ozsolak F and Milos PM: RNA sequencing:
Advances, challenges and opportunities. Nat Rev Genet. 12:87–98.
2011. View Article : Google Scholar :
|
|
187
|
Marand AP, Chen Z, Gallavotti A and
Schmitz RJ: A cis-regulatory atlas in maize at single-cell
resolution. Cell. 184:3041–3055.e21. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Tejera P, Wang Z, Zhai R, Su L, Sheu CC,
Taylor DM, Chen F, Gong MN, Thompson BT and Christiani DC: Genetic
polymorphisms of peptidase inhibitor 3 (elafin) are associated with
acute respiratory distress syndrome. Am J Respir Cell Mol Biol.
41:696–704. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Sun X, Sun BL, Sammani S, Bermudez T,
Dudek SM, Camp SM and Garcia JGN: Genetic and epigenetic regulation
of the non-muscle myosin light chain kinase isoform by lung
inflammatory factors and mechanical stress. Clin Sci (Lond).
35:963–977. 2021. View Article : Google Scholar
|
|
190
|
Adyshev DM, Moldobaeva N, Mapes B,
Elangovan V and Garcia JG: MicroRNA regulation of nonmuscle myosin
light chain kinase expression in human lung endothelium. Am J
Respir Cell Mol Biol. 49:58–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Kovacs-Kasa A, Zaied AA, Leanhart S,
Koseoglu M, Sridhar S, Lucas R, Fulton DJ, Vazquez JA and Annex BH:
Elevated cytokine levels in plasma of patients with SARS-CoV-2 do
not contribute to pulmonary microvascular endothelial permeability.
Microbiol Spectr. 10:e01671212022. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Albini A, Calabrone L, Carlini V,
Benedetto N, Lombardo M, Bruno A and Noonan DM: Preliminary
evidence for IL-10-induced ACE2 mRNA expression in lung-derived and
endothelial cells: Implications for SARS-Cov-2 ARDS pathogenesis.
Front Immunol. 12:7181362021. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Xian H, Liu Y, Rundberg Nilsson A,
Gatchalian R, Crother TR, Tourtellotte WG, Zhang Y, Aleman-Muench
GR, Lewis G, Chen W, et al: Metformin inhibition of mitochondrial
ATP and DNA synthesis abrogates NLRP3 inflammasome activation and
pulmonary inflammation. Immunity. 54:1463–1477.e11. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Wang Y, Liu B, Zhao G, Lee Y, Buzdin A, Mu
X, Zhao J, Chen H and Li X: Spatial transcriptomics: Technologies,
applications and experimental considerations. Genomics.
115:1106712023. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Han J, Zhang X, Cai M, Tian F, Xu Y, Chen
H, He W, Zhang J and Tian H: TSPO deficiency exacerbates acute lung
injury via NLRP3 inflammasome-mediated pyroptosis. Chin Med J
(Engl). 137:1592–1602. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Xu H, Sheng S, Luo W, Xu X and Zhang Z:
Acute respiratory distress syndrome heterogeneity and the septic
ARDS subgroup. Front Immunol. 14:12771612023. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Tang W, Wu S, Tang Y, Ma J, Ao Y, Liu L
and Wei K: Microarray analysis identifies lncFirre as a potential
regulator of obesity-related acute lung injury. Life Sci.
340:1224592024. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Zou L, Yu Q, Zhang L, Yuan X, Fang F and
Xu F: Identification of inflammation related lncRNAs and Gm33647 as
a potential regulator in septic acute lung injury. Life Sci.
282:1198142021. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Sivakumar P, Ammar R, Thompson JR, Luo Y,
Streltsov D, Porteous M, McCoubrey C, Cantu E III, Beers MF, Jarai
G and Christie JD: Integrated plasma proteomics and lung
transcriptomics reveal novel biomarkers in idiopathic pulmonary
fibrosis. Respir Res. 22:2732021. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Sinha S, Rosin NL, Arora R, Labit E,
Jaffer A, Cao L, Farias R, Nguyen AP, de Almeida LGN, Dufour A, et
al: Dexamethasone modulates immature neutrophils and interferon
programming in severe COVID-19. Nat Med. 28:201–211. 2022.
View Article : Google Scholar :
|
|
201
|
Ramaswamy A, Brodsky NN, Sumida TS, Comi
M, Asashima H, Hoehn KB, Li N, Liu Y, Shah A, Ravindra NG, et al:
Immune dysregulation and autoreactivity correlate with disease
severity in SARS-CoV-2-associated multisystem inflammatory syndrome
in children. Immunity. 54:1083–1095.e7. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Tong F, Shen W, Zhao J, Hu Y, Zhao Q, Lv
H, Liu F, Meng Z and Liu J: Silencing information regulator 1
ameliorates lipopolysaccharide-induced acute lung injury in rats
via the upregulation of caveolin-1. Biomed Pharmacother.
165:1150182023. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Ren Y, Li L, Wang MM, Cao LP, Sun ZR, Yang
ZZ, Zhang W, Zhang P and Nie SN: Pravastatin attenuates
sepsis-induced acute lung injury through decreasing pulmonary
microvascular permeability via inhibition of Cav-1/eNOS pathway.
Int Immunopharmacol. 100:1080772021. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Liang X, Wei SQ, Lee SJ, Fung JK, Zhang M,
Tanaka A, Choi AM and Jin Y: p62 sequestosome 1/light chain 3b
complex confers cytoprotection on lung epithelial cells after
hyperoxia. Am J Respir Cell Mol Biol. 48:489–496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Tanaka A, Jin Y, Lee SJ, Zhang M, Kim HP,
Stolz DB, Ryter SW and Choi AM: Hyperoxia-induced LC3B interacts
with the Fas apoptotic pathway in epithelial cell death. Am J
Respir Cell Mol Biol. 46:507–514. 2012. View Article : Google Scholar :
|
|
206
|
Chen ZH, Lam HC, Jin Y, Kim HP, Cao J, Lee
SJ, Ifedigbo E, Parameswaran H, Ryter SW and Choi AM: Autophagy
protein microtubule-associated protein 1 light chain-3B (LC3B)
activates extrinsic apoptosis during cigarette smoke-induced
emphysema. Proc Natl Acad Sci USA. 107:18880–18885. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Baysoy A, Bai Z, Satija R and Fan R: The
technological landscape and applications of single-cell
multi-omics. Nat Rev Mol Cell Biol. 24:695–713. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Wong HR, Cvijanovich NZ, Allen GL, Thomas
NJ, Freishtat RJ, Anas N, Meyer K, Checchia PA, Lin R, Shanley TP,
et al: Validation of a gene expression-based subclassification
strategy for pediatric septic shock. Crit Care Med. 39:2511–2517.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Rao A, Barkley D, França GS and Yanai I:
Exploring tissue architecture using spatial transcriptomics.
Nature. 596:211–220. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Computational Pan-Genomics Consortium:
Computational pan-genomics: Status, promises and challenges. Brief
Bioinform. 19:118–135. 2018.
|
|
211
|
Vandereyken K, Sifrim A, Thienpont B and
Voet T: Methods and applications for single-cell and spatial
multi-omics. Nat Rev Genet. 24:494–515. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Zhang Y, Shan P, Srivastava A, Li Z and
Lee PJ: Endothelial stanniocalcin 1 maintains mitochondrial
bioenergetics and prevents oxidant-induced lung injury via
toll-like receptor 4. Antioxid Redox Signal. 30:1775–1796. 2019.
View Article : Google Scholar :
|
|
213
|
Wang Y, Chen D, Xie H, Jia M, Sun X, Peng
F, Guo F and Tang D: AUF1 protects against ferroptosis to alleviate
sepsis-induced acute lung injury by regulating NRF2 and ATF3. Cell
Mol Life Sci. 79:2282022. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Ghiasi M, Kheirandish Zarandi P, Dayani A,
Salimi A and Shokri E: Potential therapeutic effects and nano-based
delivery systems of mesenchymal stem cells and their isolated
exosomes to alleviate acute respiratory distress syndrome caused by
COVID-19. Regen Ther. 27:319–328. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Deng H, Zhu L, Zhang Y, Zheng L, Hu S,
Zhou W, Zhang T, Xu W, Chen Y, Zhou H, et al: Differential lung
protective capacity of exosomes derived from human adipose tissue,
bone marrow, and umbilical cord mesenchymal stem cells in
sepsis-induced acute lung injury. Oxid Med Cell Longev.
2022:78378372022. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Xian H, Watari K, Sanchez-Lopez E,
Offenberger J, Onyuru J, Sampath H, Ying W, Hoffman HM, Shadel GS
and Karin M: Oxidized DNA fragments exit mitochondria via mPTP- and
VDAC-dependent channels to activate NLRP3 inflammasome and
interferon signaling. Immunity. 55:1370–1385.e8. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Li J, Bai Y, Tang Y, Wang X, Cavagnaro MJ,
Li L, Li Z, Zhang Y and Shi J: A 4-benzene-indol derivative
alleviates LPS-induced acute lung injury through inhibiting the
NLRP3 inflammasome. Front Immunol. 13:8121642022. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Xie H, Liu X, Zhou Q, Huang T, Zhang L,
Gao J, Wang Y, Liu Y, Yan T, Zhang S and Wang CY: DNA Methylation
Modulates Aging Process in Adipocytes. Aging Dis. 13:433–446. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Picard M, Scott-Boyer MP, Bodein A, Périn
O and Droit A: Integration strategies of multi-omics data for
machine learning analysis. Comput Struct Biotechnol J.
19:3735–3746. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Poirion OB, Jing Z, Chaudhary K, Huang S
and Garmire LX: DeepProg: An ensemble of deep-learning and
machine-learning models for prognosis prediction using multi-omics
data. Genome Med. 13:1122021. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Ravindran U and Gunavathi C: A survey on
gene expression data analysis using deep learning methods for
cancer diagnosis. Prog Biophys Mol Biol. 177:1–13. 2023. View Article : Google Scholar
|
|
222
|
P D and G C: A systematic review on
machine learning and deep learning techniques in cancer survival
prediction. Prog Biophys Mol Biol. 174:62–71. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Schmidt-Erfurth U, Sadeghipour A, Gerendas
BS, Waldstein SM and Bogunović H: Artificial intelligence in
retina. Prog Retin Eye Res. 67:1–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Cui H, Wang C, Maan H, Pang K, Luo F, Duan
N and Wang B: mscGPT: Toward building a foundation model for
single-cell multi-omics using generative AI. Nat Methods.
21:1470–1480. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Kang M, Ko E and Mersha TB: A roadmap for
multi-omics data integration using deep learning. Brief Bioinform.
23:bbab4542022. View Article : Google Scholar :
|
|
226
|
Lin M, Xu F, Sun J, Song J, Shen Y, Lu S,
Ding H, Lan L, Chen C, Ma W, et al: Integrative multi-omics
analysis unravels the host response landscape and reveals a serum
protein panel for early prognosis prediction for ARDS. Crit Care.
28:2132024. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Zhang S, Huang W, Wu X, Chen H, Wang L,
Chao J, Xie J and Qiu H: IBR1, a novel endogenous IFIH1-binding
dsRNA, governs IFIH1 activation and M1 macrophage polarisation in
ARDS. Clin Transl Med. 14:e700272024. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Fang X, Bai C and Wang X: Bioinformatics
insights into acute lung injury/acute respiratory distress
syndrome. Clin Transl Med. 1:92012. View Article : Google Scholar
|
|
229
|
Zhang Z, Navarese EP, Zheng B, Meng Q, Liu
N, Ge H, Pan Q, Yu Y and Ma X: Analytics with artificial
intelligence to advance the treatment of acute respiratory distress
syndrome. J Evid Based Med. 13:301–312. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Ober-Reynolds B, Wang C, Ko JM, Rios EJ,
Aasi SZ, Davis MM, Oro AE and Greenleaf WJ: Integrated single-cell
chromatin and transcriptomic analyses of human scalp identify
gene-regulatory programs and critical cell types for hair and skin
diseases. Nat Genet. 55:1288–1300. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Hwang B, Lee JH and Bang D: Single-cell
RNA sequencing technologies and bioinformatic pipelines. Exp Mol
Med. 50:1–14. 2018. View Article : Google Scholar
|
|
232
|
Wang Y and Navin NE: Advances and
applications of single-cell sequencing technologies. Mol Cell.
58:598–609. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Gawad C, Koh W and Quake SR: Single-cell
genome sequencing: Current state of the science. Nat Rev Genet.
17:175–188. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Yang R, Zheng T, Xiang H, Liu M and Hu K:
Lung single-cell RNA profiling reveals response of pulmonary
capillary to sepsis-induced acute lung injury. Front Immunol.
15:13089152024. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Kang ZY, Huang QY, Zhen NX, Xuan NX, Zhou
QC, Zhao J, Cui W, Zhang ZC and Tian BP: Heterogeneity of immune
cells and their communications unveiled by transcriptome profiling
in acute inflammatory lung injury. Front Immunol. 15:13824492024.
View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Suvà ML and Tirosh I: Single-Cell RNA
sequencing in cancer: Lessons learned and emerging challenges. Mol
Cell. 75:7–12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Halpern KB, Shenhav R, Matcovitch-Natan O,
Toth B, Lemze D, Golan M, Massasa EE, Baydatch S, Landen S, Moor
AE, et al: Single-cell spatial reconstruction reveals global
division of labour in the mammalian liver. Nature. 542:352–356.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Van de Sande B, Lee JS, Mutasa-Gottgens E,
Naughton B, Bacon W, Manning J, Wang Y, Pollard J, Mendez M, Hill
J, et al: Applications of single-cell RNA sequencing in drug
discovery and development. Nat Rev Drug Discov. 22:496–520. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Greener JG, Kandathil SM, Moffat L and
Jones DT: A guide to machine learning for biologists. Nat Rev Mol
Cell Biol. 23:40–55. 2022. View Article : Google Scholar
|
|
240
|
Handelman GS, Kok HK, Chandra RV, Razavi
AH, Lee MJ and Asadi H: eDoctor: Machine learning and the future of
medicine. J Intern Med. 284:603–619. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Deo RC: Machine learning in medicine.
Circulation. 132:1920–1930. 2015. View Article : Google Scholar : PubMed/NCBI
|