Introduction
Remimazolam (Rema) is a novel intravenous anesthetic
agent that activates the γ-aminobutyric acid (GABA) receptor
(GABAR), with a fast onset and recovery, and minimal inhibition of
cardiopulmonary function (1,2).
Rema may exhibit potential in critically ill patients due to the
mild cardiac inhibitory effect (3). Notably, anesthesiologists face
challenges in protecting vital organs during perioperative periods.
Therefore, further investigations are required to explore the
pharmacological effects of Rema on organs and tissues other than
the brain to elucidate the underlying mechanism, especially in
cardiac protection during perioperative periods.
Myocardial ischemia/reperfusion (I/R) injury is a
complex pathophysiological process that affects postoperative
cardiac function recovery in patients who have undergone
cardiopulmonary bypass (CPB) (4,5).
When cardiomyocytes are ischemic and damaged, reactive oxygen
species (ROS) are activated, and the release of inflammatory
cytokines and chemokines such as TNF-α, IL-1, IL-6, C-C chemokine
receptor 2 (CCR2) and C-X-C motif chemokine ligands (CXCLs) is
promoted (6). These factors
activate neutrophils and mononuclear macrophages in the blood,
infiltrate damaged myocardial cells, and cause microvascular
damage, myocardial cell death, extracellular matrix (ECM)
degradation and myocardial remodeling, and further promote
inflammatory reactions (7).
Conversely, the activated inflammatory response further exacerbates
oxidative stress, and increases myocardial injury and functional
impairment (8). Therefore,
inflammation and oxidative stress are key factors in myocardial I/R
injury, and may act as key intervention targets for alleviating
myocardial I/R injury (9).
Further investigations are required for the development of novel
treatment options for myocardial I/R injury and the reduction of
perioperative cardiovascular complications.
GABA is a central inhibitory neurotransmitter and
bioactive small molecule substance that is widely present in
peripheral tissue and cells (10). The results of a previous study
suggested that GABA may serve a key role in alleviating I/R injury
and promoting tissue repair (11). In addition, the results of a
previous study suggested that activation of GABAR may inhibit
pro-inflammatory activities and promote anti-inflammatory
phenotypes in rodent immune cells (12). However, studies focused on the
role of GABA or GABAR in myocardial I/R are limited. Wang et
al (13) previously reported
that GABA secreted by the gut microbiome exhibited a cardiac
protective effect against I/R injury. A recent clinical study
demonstrated that Rema alleviated the surgical stress response in
cardiac surgery and adverse cardiac reactions; however, the
specific underlying mechanisms are yet to be fully understood
(14,15). A previous study has highlighted
that GABARs are expressed in macrophages, and GABA/GABAR may
exhibit potential as a target for oxidative stress and inflammation
regulation (16). Therefore,
regulation of GABAR function in macrophages may provide a novel
approach for the treatment of cardiac I/R injury.
The present study examined whether the GABAR agonist
Rema improves cardiac I/R injury through activation of GABARs in
the heart, subsequently inhibiting inflammation and oxidative
stress, using a mouse cardiac I/R model and cultured Raw264.7
macrophages. The present study may provide a novel theoretical
basis for the use of Rema as an anesthetic in clinical
practice.
Materials and methods
Mouse model
Adult male C57BL/6 mice (age, 8-10 weeks; weight,
25.61±1.25 g) were obtained from the Experimental Animal Center at
Southwest Medical University (Luzhou, China) for the animal
experiments. The present study was approved by the Institutional
Animals Ethics Committee at Southwest Medical University, China
(ethics approval no. 20221117-011; Luzhou, China) and followed the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals. All mice used in the present study were
maintained at a specific pathogen-free animal center at Southwest
Medical University (Luzhou, China) at a constant temperature
(25-27°C) and humidity (45-50%), with a 12 h/light/dark cycle. Mice
had free access to sterilized drinking water and food pellets. Mice
were anesthetized with 2-5% isoflurane (induction, 5%; maintenance,
2-3%) using a gas anesthesia machine (RWD Life Science Co., Ltd.).
The classical method for ligating the left anterior descending
coronary artery (LAD) establishes 30-min ischemia and 24-h
reperfusion to create a mouse model of left ventricular anterior
wall myocardial I/R injury (Fig.
1A) (17). Sham mice
underwent thoracotomy without ligation of the LAD. Mice were
randomly divided into four groups: i) Sham (n=12); ii) sham + Rema
(n=12); iii) I/R (n=18); and iv) I/R + Rema (n=18). Mice were
intraperitoneally injected with 15 mg/kg Rema (Jiangsu Hengrui
Pharmaceutical Co., Ltd.) following ligation of the LAD for 5 min.
Furthermore, 0.9% normal saline was used as the control in the sham
and I/R groups. Mice were anesthetized and euthanized immediately
after 24 h of reperfusion or sham treatment. Mice were anesthetized
using 5% isoflurane followed by cervical dislocation to obtain the
heart. A total of 60 mice were used at the beginning in the present
experiment. However, there were 4 mice in the I/R and I/R + Rema
groups that died unexpectedly during construction of the cardiac
I/R model. Finally, 56 mice [Sham (n=12), sham + Rema (n=12), I/R
(n=16) and I/R + Rema (n=16)] were used in the final analysis. It
is difficult to achieve a 100% survival rate for the cardiac I/R
model, even with a skilled laboratory technician (18). The cause of death was
pneumothorax or cardiac arrest due to myocardial ischemia.
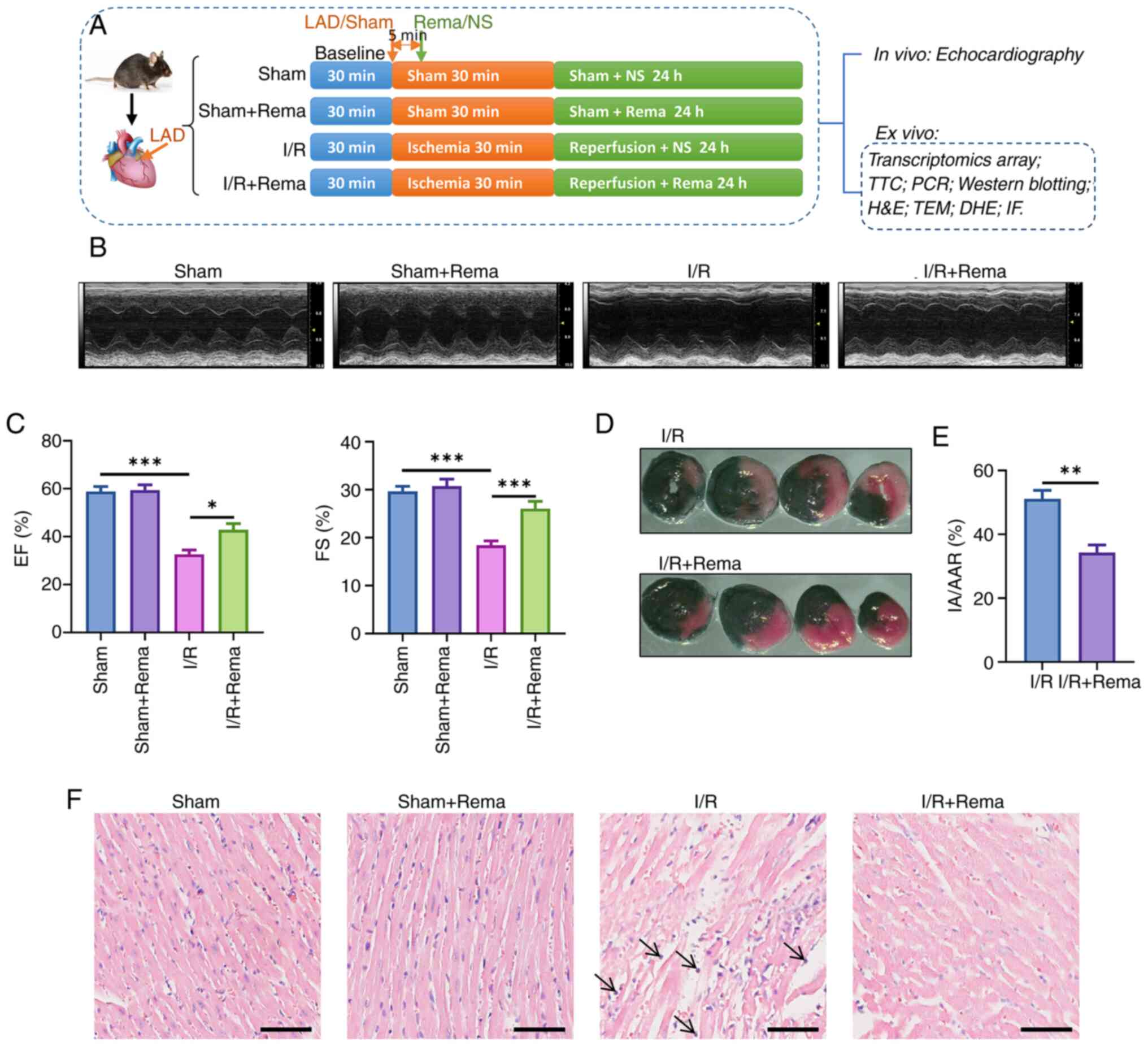 | Figure 1Rema alleviates I/R-induced cardiac
injury and dysfunction. (A) Schematic of the experimental design.
The I/R model was conducted by ligation of the LAD for 30 min and
reperfusion for 24 h. Rema or NS as the control was administered 5
min after LAD. (B) Typical recording of M-type echo in each group.
(C) Rema inhibited the I/R-induced decrease of EF and FS values.
n=5 mice in the sham and sham + Rema groups; n=8 mice in the I/R
and I/R + Rema groups. (D) TTC staining and (E) statistical graph
showing that Rema alleviated I/R-induced cardiac infarction by
decreasing the cardiac infarction area (n=5). (F) H&E staining
showing that Rema alleviated I/R-induced cardiac tissue
inflammation. Arrows indicate neutrophil infiltration. Scale bar,
50 μm. *P<0.01; **P<0.01;
***P<0.001. DHE, dihydroethidium; EF, ejection
fraction; FS, fractional shortening; IA/AAR, ratio of the infarct
area to the area at risk; IF, immunofluorescence; I/R,
ischemia/reperfusion; LAD, left anterior descending artery; NS,
0.9% NaCl solution; Rema, remimazolam; TEM, transmission electron
microscopy; TTC, 2, 3, 5-triphenyl tetrazolium chloride. |
Echocardiography analysis of intact
hearts
Mice were terminally anesthetized using 2-5%
isoflurane (induction, 5%; maintenance, 2-3%). Transthoracic M-mode
echocardiographic recordings were obtained using the
Vevo® 3100 micro-ultrasound imaging system (FUJIFLIM
Wako Pure Chemical Corporation) according to the manufacturer's
instructions. The ejection fraction (EF) and fractional shortening
(FS) were determined using the recorded measurements.
2,3,5-triphenyl tetrazolium chloride
(TTC) staining and measurement of the myocardial infarct size
Following 24 h of reperfusion, mice were quickly
anesthetized using 5% isoflurane and sacrificed by cervical
dislocation, and the injured hearts were obtained. The thoracic
cavity of the mice was accessed and 0.5% Evans blue (Sigma-Aldrich;
Merck KGaA) was injected into the cardiac apex through reverse
perfusion of the aortic root at room temperature, which was kept at
−20°C for 1 h. The heart was cut into 1-mm thickness along the
short axis. Heart sections were infiltrated with PBS and
subsequently incubated in 1% TTC for 40 min at 37°C to
differentiate the infarcted heart regions. Notably, different
colors were indicative of ischemic and non-ischemic areas of the
heart. Slices were fixed with 4% polyformaldehyde for 30 min at
room temperature. ImagePro Plus 6.0 (Media Cybernetics, Inc.) was
used to measure the areas at risk and infarct size.
Transcriptomics array
The transcriptomics array was conducted by Novogene
Co., Ltd., according to the manufacturer's instructions. Heart
tissues were acquired from each group (sham, 5 mice; sham + Rema, 5
mice; I/R, 9 mice; and I/R + Rema, 8 mice). Total RNA was obtained
from each heart tissue with TRNzol Universal Reagent (cat. no:
DP424; Tiangen Biotech Co., Ltd.) and the RNA integrity was
assessed using the RNA Nano 6000 Assay Kit of the Bio-analyzer 2100
system (cat. no. 5067-1511; Agilent Technologies, Inc.). First
strand cDNA was synthesized using random hexamer primers (10 min at
25°C; 15 min at 42°C; 15 min at 70°C; hold at 4°C) and a library
was prepared for transcriptome sequencing with the
NEBNext® Ultra™ II RNA Library Prep Kit for Illumina
(cat. no. NEB#E7775L; New England BioLabs, Inc.). Clustering of the
index-coded samples was performed on a cBot Cluster Generation
System using a TruSeq PE Cluster Kit v3-cBot-HS (cat. no.
FC-401-3001; Illumina, Inc.). The library preparations [>1.5 nM;
measured by reverse transcription-quantitative PCR (RT-qPCR)] were
sequenced on an Illumina Novaseq platform (Illumina, Inc.) with the
NovaSeq 6000 S4 Reagent Kit v1.5 (300 cycles; cat. no. 20028312;
Illumina, Inc.) and 150 base pair paired-end reads were generated.
Feature Counts (version 1.5.0; https://subread.sourceforge.net/) was used to count
the read numbers mapped to each gene. Data were analyzed with R
software (version 3.5.0; https://www.r-project.org/) and Kyoto Encyclopedia of
Genes and Genomes (KEGG; https://www.kegg.jp/kegg/pathway.html) for enrichment
analysis.
Transmission electron microscopy
(TEM)
TEM was used to investigate the subcellular
structure of heart tissue following different I/R or Rema
treatments according to a previously described protocol (19). Briefly, heart tissues were fixed
with 3% glutaraldehyde in phosphoric buffer overnight at 4°C and
subsequently fixed with 1% osmate for 1-2 h at 4°C. Tissues were
dehydrated with gradient alcohol from 30 to 100%, infiltrated with
a solution of epoxy resin at room temperature and acetone-embedded
in epoxy resin for 24 h at 60°C. Ultra-thin sections (thickness, 50
nm) were cut and mounted on copper grids, and stained with uranyl
acetate for 15 min at room temperature and lead citrate for 2 min
at room temperature in the dark. Ultrastructural images were
obtained using TEM (JEM-1400PLUS; Japan Electronics Co., Ltd.) at
80 kV.
H&E staining
Hearts from different groups were fixed with 4%
polyformaldehyde for 24 h at 4°C. Cardiac slices were sectioned
with 4-μm thickness along the short axis. Routine H&E
staining (G1076; Wuhan Servicebio Technology Co., Ltd.) was
performed to visualize the overall morphology and structure of
myocardial cells at room temperature. The cardiac slices were moved
into hematoxylin and stained for 10 min. Subsequently, the slices
were moved into water and the hematoxylin and floating color were
washed away for 2 min. The differentiation fluid (1% hydrochloric
acid alcohol) was added to tissues for differentiation for 30 sec.
The slices were moved into water for washing. Eosin was added to
the tissues and tissues were stained for 5 min, and then the slices
were washed with water. The slices were placed into 95% alcohol for
differentiation, and then in anhydrous alcohol for dehydration.
Slices were sealed with xylene. Images were acquired using a BX63
automated microscope (Olympus Corporation) under a bright field
with a ×20 objective lens automatically.
ROS measurement with dihydroethidium
(DHE) staining
The superoxide-sensitive dye DHE (cat. no. HY-D0079;
MedChemExpress) was used for ROS measurements. Fresh cardiac
tissues obtained from the four experimental groups were embedded in
Tissue-Tek OCT (Thermo Fisher Scientific, Inc.). Cardiac
cross-sections (thickness, 10 μm) were incubated with DHE
(10 μM in 0.01% DMSO) at 37°C for 30 min in a humidified
dark chamber. Red DHE fluorescence was detected using an Olympus
IX83 fluorescence microscope (Olympus Corporation) at room
temperature and analyzed with ImageJ (v1.47; National Institutes of
Health).
Immunofluorescence staining
Fresh cardiac tissues from different treatments were
embedded in Tissue-Tek OCT (Thermo Fisher Scientific, Inc.).
Cardiac cross-sections (thickness, 10 μm) from each group
were acquired along the short axis and fixed with 4%
polyformaldehyde for 30 min at room temperature. Cardiac sections
were treated with 0.1% Triton X-100 for permeabilization for 10 min
and blocked with 5% goat serum (G1208; Wuhan Servicebio Technology
Co., Ltd.) for 1 h at room temperature. Sections were incubated
with primary rabbit anti-CD68 antibody (1:50; cat. no. ab283654;
Abcam) or rabbit anti-IL-1β antibody (1:50; cat. no. ab234437;
Abcam) at 4°C overnight. After washing three times (5 min each)
with PBS, cardiac sections were incubated with the secondary
antibody Goat Anti-Rabbit IgG conjugated with Alexa
Fluor® 594 (1:300; cat. no. ab150080; Abcam) for 1 h at
room temperature. The nucleus was stained and cardiac cross-section
were mounted with undiluted Fluoroshield Mounting Medium with DAPI
(cat. no. ab104139; Abcam). Images were acquired on a BX63
automated microscope (Olympus Corporation) with fluorescence
(excitation, 590 nm; emission, 617 nm) to scan the whole film under
a ×20 objective lens automatically and were quantified using
ImageJ.
Cell culture
Raw264.7 cells (cat. no. TIB-71; American Type
Culture Collection) were cultured in 6-well plates at a density of
1-2×105 cells/well for 24 h in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin (Gibco; Thermo
Fisher Scientific, Inc.). These cells were cultured in a
CO2 incubator with 95% air and 5% CO2 at
37°C. Cells were cultured in DMEM without FBS and pre-treated with
100 μg/ml Rema (diluted in DMEM) for 20 min, and
subsequently treated with lipopolysaccharide (LPS; 0.5 μg/ml
diluted in DMEM; cat. no. HY-D1056H, MedChemExpress) for 24 h in
incubator at 37°C. Cells were cultured in only DMEM without Rema
and LPS as the control. After different treatments for 24 h, cells
were harvested for RT-qPCR. The culture medium supernatant was
collected for ELISAs. In addition, the NOD-like receptor thermal
protein domain associated protein 3 (NLRP3) inhibitor dapansutrile
(20) (DAPA; 5 μM; cat.
no. HY-17629; MedChemExpress) was used with LPS for treatment for
24 h in an incubator at 37°C.
ELISA
Mouse Uncoated ELISA kits (Shanghai Jianglai
Biotechnology Co., Ltd.) were used to determine the protein levels
of TNF-α using the Mouse TNF-α ELISA Kit (cat. no. 10484), IL-6
using the Mouse IL-6 ELISA Kit (cat. no. 20268) and IL-1β using the
Mouse IL-1β ELISA Kit (cat. no. 18442) in culture medium
supernatant from cultured Raw264.7 cells with different treatments
using a standard curve according to the manufacturer's
instructions. To avoid the influence of cell numbers, the same
number of Raw264.7 cells was cultured in each group.
RT-qPCR
Total RNA was isolated from heart tissues or
Raw264.7 cells using the RNA Easy Fast Tissue/Cell Kit (cat. no.
DP451; Tiangen Biotech Co., Ltd.). Subsequently, total RNA was
reverse transcribed into cDNA using ReverTra® Ace qPCR
RT Master Mix (cat. no. FSQ-201; Toyobo Life Science) with the
following temperature protocol: 37°C for 15 min, 50°C for 5 min,
98°C for 5 min and 4°C hold. qPCR was performed using Taq Pro
Universal SYBR qPCR Master Mix (cat. no. Q712; Vazyme Biotech Co.,
Ltd.) with an initial denaturation at 95°C for 30 sec and the
following thermocycling conditions: 95°C for 10 sec, 60°C for 25
sec and 72°C for 30 sec for 40 cycles. mRNA expression levels of
target genes were normalized to those of GAPDH using the
2−ΔΔCq method (21).
The primers used in the present study are shown in Table I.
 | Table IPrimers for reverse
transcription-quantitative PCR analysis of different genes. |
Table I
Primers for reverse
transcription-quantitative PCR analysis of different genes.
| Gene | Primer sequence
(5′-3′) |
|---|
| IL-1β | F:
GCAACTGTTCCTGAACTCAACT |
| R:
ATCTTTTGGGGTCCGTCAACT |
| IL-6 | F:
TAGTCCTTCCTACCCCAATTTCC |
| R:
TTGGTCCTTAGCCACTCCTTC |
| CCR2 | F:
ATCCACGGCATACTATCAACATC |
| R:
CAAGGCTCACCATCATCGTAG |
| CXCL1 | F:
AGACTCCAGCCACACTCCAA |
| R:
TGACAGCGCAGCTCATTG |
| CXCL5 | F:
CCTGGTCCGGGATCTTGT |
| R:
CATGAATGGCGAGATGGAA |
| CCL12 | F:
ATTTCCACACTTCTATGCCTCCT |
| R:
ATCCAGTATGGTCCTGAAGATCA |
| TNF-α | F:
CCCTCACACTCAGATCATCTTCT |
| R:
GCTACGACGTGGGCTACAG |
| GAPDH |
F:CATCACTGCCACCCAGAAGACTG |
| R:
ATGCCAGTGAGCTTCCCGTTCAG |
Western blot analysis
Total protein was extracted from heart tissues using
RIPA lysis buffer (Beyotime Institute of Biotechnology). Total
protein was quantified using a BCA Protein Assay Kit (Pierce;
Thermo Fisher Scientific, Inc.), and 20 μg protein/lane was
separated by SDS-PAGE on a 5% stacking gel and 10% separation gel.
Separated proteins were subsequently transferred to a PVDF membrane
(Millipore, Sigma), which was blocked in Protein Free Rapid
Blocking Buffer (1X; cat. no. PS108P, Epizyme; Ipsen Pharma) for 2
h at room temperature. Membranes were incubated with primary
antibodies against IL-1β (1:1,000; cat. no. ab234437; Abcam), NLRP3
(1:1,000; cat. no. ab270449; Abcam) and β-actin (1:1,000; cat. no.
AF0003; Beyotime Institute of Biotechnology) overnight at 4°C.
Following primary antibody incubation, membranes were incubated
with HRP-conjugated goat anti-rabbit IgG antibody (1:3,000; cat.
no. D110058; Sangon Biotech Co., Ltd.) or HRP-conjugated goat
anti-mouse IgG antibody (1:3,000; cat. no. D110087; Sangon Biotech
Co., Ltd.) for 1 h at room temperature. Membranes were incubated
with chemiluminescent HRP substrate (MilliporeSigma) at room
temperature for 30 sec. Images were obtained using the Universal
Hood II System (Bio-Rad Laboratories, Inc.) and analyzed using
ImageJ.
Statistical analysis
Continuous data are presented as the mean ± SEM and
were analyzed using two-way ANOVA followed by the Sidak test for
further multiple group comparisons (GraphPad Prism 9.0; Dotmatics).
For two groups, differences were evaluated using an unpaired
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Rema alleviates I/R-induced cardiac
dysfunction
In the present study, cardiac function was evaluated
by echocardiography in vivo (Fig. 1B and C). The results demonstrated
that EF values decreased from 58.84±2.09% (sham group) to
32.65±1.67% (I/R group), highlighting that I/R significantly
inhibited cardiac function. In addition, EF values increased to
42.90±2.39% (I/R + Rema group) following treatment with Rema,
highlighting that Rema significantly inhibited I/R-induced cardiac
dysfunction. Notably, the impact of Rema on FS was comparable
(Fig. 1B), with levels
increasing from 18.42±0.84% to 26.05±1.44% (n=8; P<0.05).
The results of the TTC staining assay revealed
minimal areas of infarction in the sham and sham + Rema groups
(Fig. S1); however, the cardiac
infarct area (IA) in the I/R group was increased (Fig. 1D). Notably, treatment with Rema
markedly decreased the infarction area compared with that in the
I/R group, as the ratio of the IA to the area at risk shifted from
51.11±2.66% to 34.29±2.36% (n=5; P<0.01; Fig. 1D and E).
The results of the H&E staining assay revealed
that, in the I/R group, cardiomyocytes and myocardial fibers were
arranged in a disordered manner (Fig. 1F). In addition, some myocardial
fibers showed interstitial edema and inflammatory neutrophil
infiltration, which are indicated by black arrows. Following
treatment with Rema, structural damage and inflammatory cell
infiltration of myocardial tissues were alleviated. Notably,
treatment with Rema alone did not affect the myocardial
structure.
Rema alleviates I/R-induced mitochondrial
structural disruption and oxidative stress
Inflammation and oxidative stress are key
pathological and physiological processes in cardiac I/R injury
(6,7). Thus, the effects of Rema on
I/R-induced subcellular structural disruption of myocardial tissues
were evaluated using TEM and ROS levels were evaluated using DHE
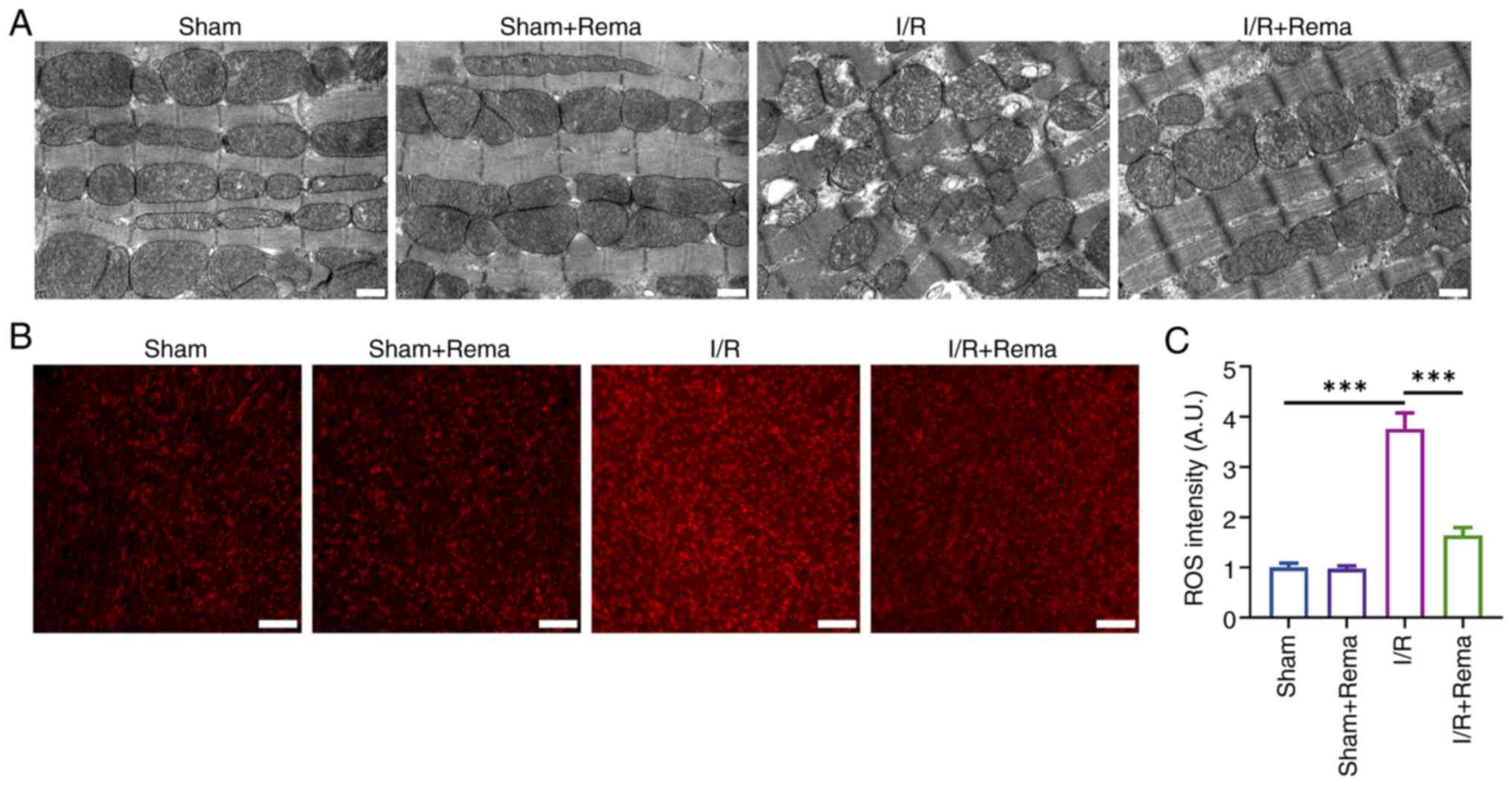
staining (Fig. 2). Notably,
mitochondria are a key source of oxidative stress and ROS
production (8). The results of
the present study demonstrated that I/R injury caused disordered
arrangement of the cardiac cell structure, including swelling of
the mitochondrial structure and vacuoles, while treatment with Rema
improved the I/R-induced structural abnormalities of cardiomyocytes
(Fig. 2A). DHE staining
demonstrated that I/R significantly induced ROS production, while
Rema significantly alleviated I/R-induced ROS production (n=5;
Fig. 2B and C). Notably, the
mitochondrial structure and ROS levels in the sham group were not
impacted following treatment with Rema.
Transcriptomics arrays may highlight the
underlying signaling pathway
To further explore the mechanisms underlying the
alleviation of cardiac I/R injury following treatment with Rema, a
transcriptomics array was carried out using heart tissues (Fig. 3). A heatmap of gene clusters in
each group is shown in Fig. 3A.
The results of the present study revealed that I/R injury induced
alterations in gene clusters, while treatment with Rema partially
reversed these I/R-induced changes in gene cluster. KEGG enrichment
analysis of differentially expressed genes (DEGs; P<0.05 and
log2 fold change >1) demonstrated that DEGs were
enriched in pathways such as 'cytokine-cytokine receptor
interaction' and 'ECM-receptor interaction' (Fig. 3B). Following treatment with Rema,
DEGs were enriched in pathways such as 'cell cycle' and
'ECM-receptor interaction'. DEGs that were notably upregulated or
downregulated were further analyzed, and the results revealed that
I/R induced upregulation of 820 DEGs and downregulation of 431
DEGs. Notably, the 820 upregulated DEG were enriched in
inflammatory pathways, including the 'TNF signaling pathway' and
'chemokine signaling pathway', and in 'cytokine-cytokine receptor
interaction' and 'ECM-receptor interaction'. Following treatment
with Rema, 108 DEGs were upregulated and 195 DEGs were
downregulated. Notably, the 195 downregulated DEGs were enriched in
'ECM-receptor interaction'.
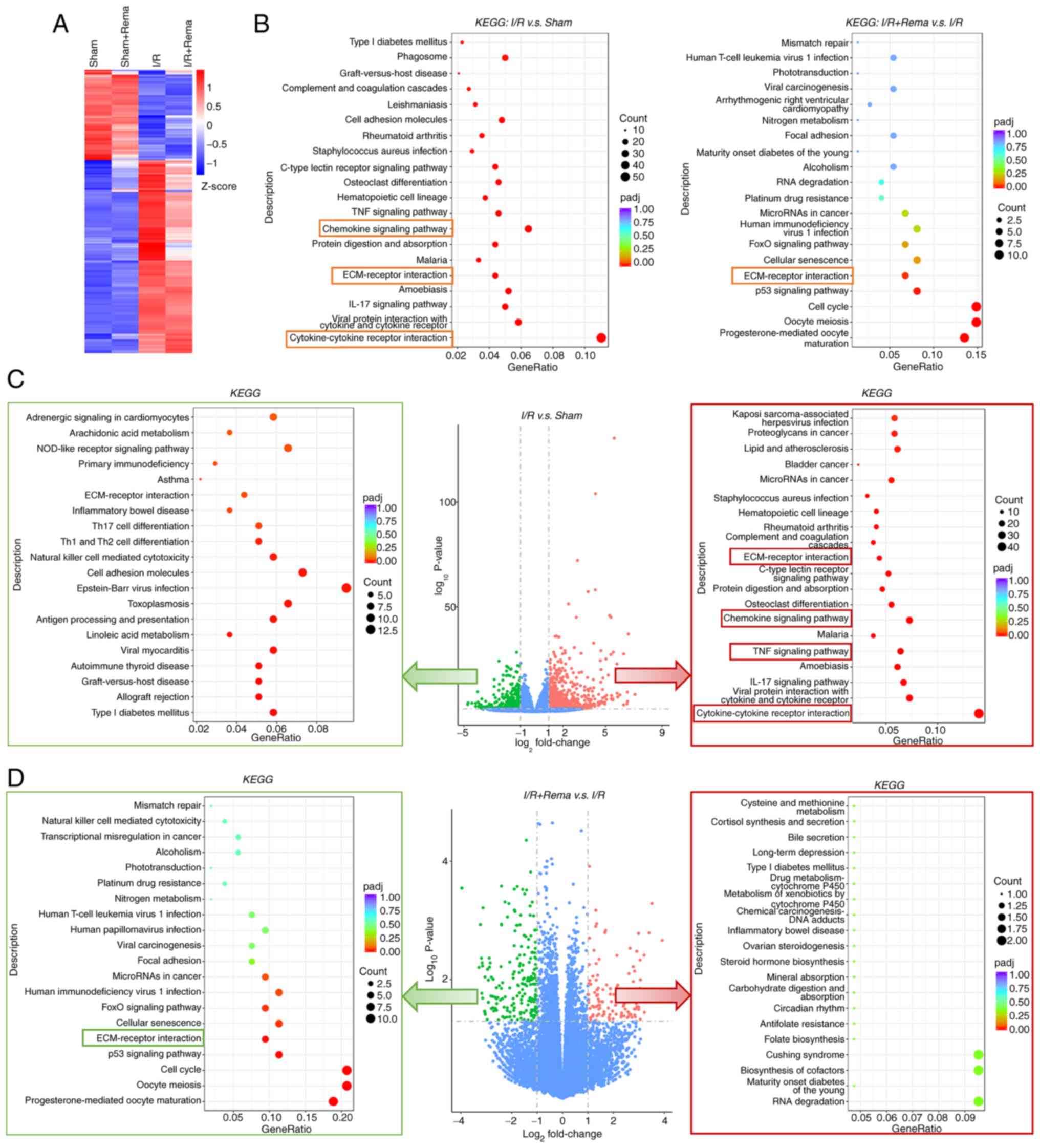 | Figure 3Transcriptomics arrays showing that
Rema exerts effects on I/R-induced cardiac injury via specific
signaling pathways. (A) Heatmap of gene clusters in each group. I/R
treatment induced changes in gene clusters, while Rema partially
reversed the I/R-induced changes. The level of gene expression was
standardized using the Z-score. (B) KEGG enrichment analysis of
DEGs (P<0.05 and log2 fold change >1) for I/R vs.
sham and I/R + Rema vs. I/R showing the top 20 pathways. (C)
Volcano plot (middle panel) revealing that I/R induced the
upregulation of 820 DEGs and the downregulation of 431 DEGs. The
820 upregulated DEGs were enriched in inflammatory pathways,
including the 'TNF signaling pathway' and 'chemokine signaling
pathway', and in 'cytokine-cytokine receptor interaction' and
'ECM-receptor interaction' (right panel with red frame). (D)
Volcano plot (middle panel) revealing that Rema induced the
upregulation of 108 DEGs and the downregulation of 195 DEGs. The
195 downregulated DEGs were enriched in 'ECM-receptor interaction'
(left panel with green frame). Pathways highlighted with red and
green boxes are inflammatory and inflammation-related pathways. n=5
in the sham and sham + Rema groups; n=9 in the I/R group; n=8 in
the I/R + Rema group. Ctrl, control; DEGs, differentially expressed
genes; ECM, extracellular matrix; I/R, ischemia/reperfusion; KEGG,
Kyoto Encyclopedia of Genes and Genomes; padj, adjusted P-value;
Rema, remimazolam. |
Collectively, these results suggested that Rema may
exert effects on I/R injury via the inflammatory pathway. Thus, the
heatmap showed the expression levels of the main genes involved in
inflammatory, cytokine or chemokine pathways in Fig. 4A, and these were analyzed using
RT-qPCR (Fig. 4B and C). The
I/R-induced increases of the expression levels of CXCL1, CXCL5,
CCR2, CC motif chemokine ligand 12 (CCL12), IL-1β, IL-6 and TNF-α
were reduced following treatment with Rema (n=5). These results
further demonstrated that Rema may exert protective effects on I/R
injury via the regulation of genes involved in inflammatory,
cytokine or chemokine pathways.
Rema protects against I/R-induced cardiac
injury via NLRP3/IL-1β
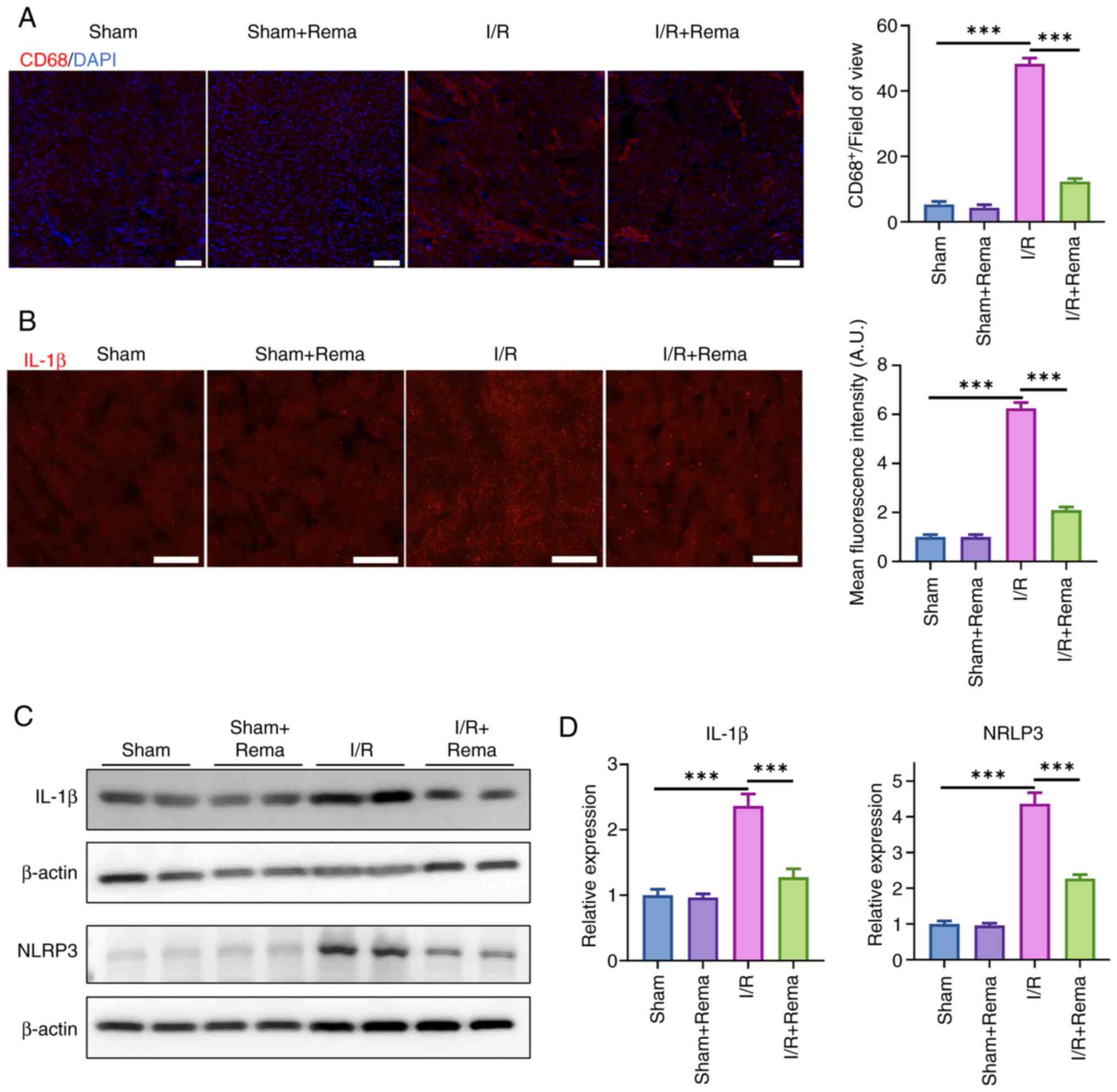
The activation of macrophages is the key
pathophysiological process in cardiac I/R injury (22). I/R causes an inflammatory
response and activated macrophages are recruited to myocardial
tissue (23). Thus, the
activation of macrophages was determined in the present study using
immunofluorescence staining of CD68, which is expressed in
macrophages (Fig. 5A). Notably,
the results of the present study revealed that I/R injury
significantly increased the number of CD68+ cells, while
treatment with Rema inhibited I/R-induced CD68+ cell
proliferation. As CD68 is expressed in macrophages and is widely
found in circulating blood, the present study showed that there was
some overlap between the red fluorescence signal and cardiomyocytes
(Fig. 5A). This may be due to
CD68 staining with red fluorescence overlapping with cardiomyocytes
as a few macrophages infiltrated the myocardial tissue.
NLRP3, a key inflammatory regulatory factor, serves
a role in myocardial cell inflammation through the activation of
downstream inflammatory factor release, thereby causing myocardial
damage. Thus, NLRP3 is a key treatment target in I/R injury
(24). In the present study, the
expression levels of IL-1β were examined using immunofluorescence
staining, and NLRP3 and IL-1β expression was evaluated using
western blotting (Fig. 5). The
results of the present study demonstrated that I/R injury
significantly increased the fluorescence intensity of IL-1β, while
treatment with Rema inhibited I/R-induced IL-1β expression (n=3;
P<0.01; Fig. 5B). In
addition, the protein expression levels of NLRP3 and IL-1β were
increased following I/R, while treatment with Rema reversed the
I/R-induced alterations in NLRP3 and IL-1β expression (n=4;
Fig. 5C and D). Notably,
treatment with Rema alone did not impact NLRP3 and IL-1β
expression. Collectively, these data suggested that Rema may
protect against I/R-induced cardiac injury via NLRP3/IL-1β.
Rema inhibits the LPS-induced
inflammatory response in RAW264.7 cells
In the present study, the effects of Rema (100
μg/ml) on the LPS-mediated expression of inflammatory
factors were evaluated using cultured RAW264.7 cells (Fig. 6). The release of IL-1β, IL-6 and
TNF-α in the cell supernatant was detected using ELISAs. LPS
increased the expression levels of IL-1β, IL-6 and TNF-α to
1,668.66±89.35, 490.57±12.49 and 2,904.05±194.51 pg/ml,
respectively. Following treatment with Rema, the expression levels
of IL-1β, IL-6 and TNF-α were reduced to 773.04±28.19, 177.25±8.62
and 756.64±48.08 pg/ml, respectively (n=3; Fig. 6B). Furthermore, RT-qPCR analysis
revealed that LPS treatment increased the relative expression
levels of IL-1β, IL-6 and TNF-α 232.89±8.16, 71.99±7.75 and
17.57±1.63 times compared with the control group, respectively.
Notably, treatment with Rema reduced the relative expression levels
of IL-1β, IL-6 and TNF-α 51.51±3.02, 13.12±0.63 and 2.77±0.23 times
compared with the LPS group, respectively (n=3; Fig. 6C). In addition, Rema treatment
did not significantly affect the effects of the NLRP3 inhibitor
DAPA (5 μM) on LPS-induced release of IL-1β, IL-6 and TNF-α
in the Raw264.7 cell supernatant (n=3; Fig. 6D).
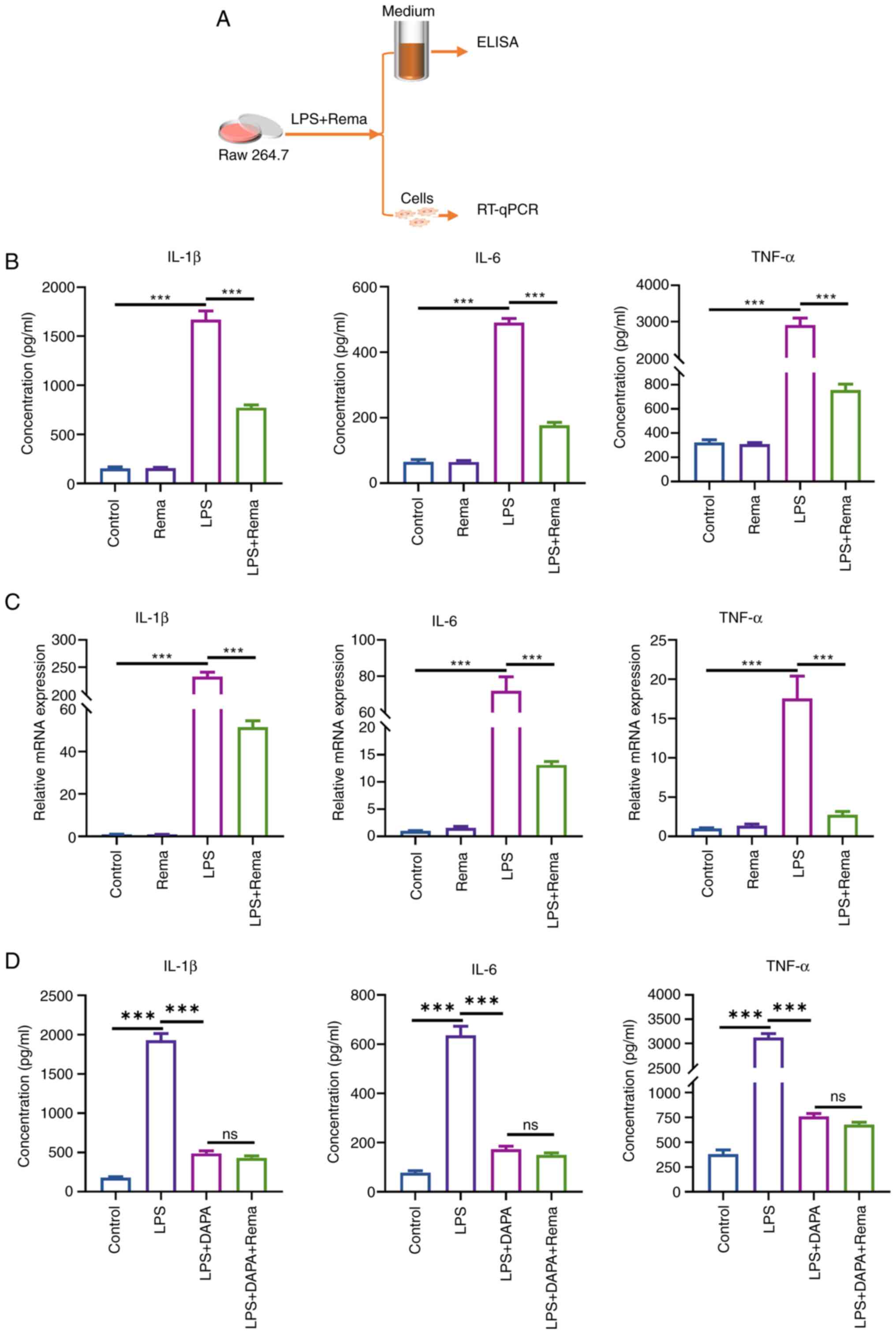 | Figure 6Rema alleviates LPS-induced release
and increased expression levels of IL-1β, IL-6 and TNF-α in
cultured Raw264.7 cells. (A) Experimental protocol using cultured
Raw264.7 macrophages. (B) LPS induced alterations in the release of
IL-1β, IL-6 and TNF-α in Raw264.7 cells, while treatment with Rema
inhibited these LPS-mediated changes (n=3). (C) LPS induced
alterations in the gene expression levels of IL-1β, IL-6 and TNF-α
in Raw264.7 cells, while treatment with Rema inhibited these
effects (n=3). (D) DAPA inhibited the LPS-induced release of IL-1β,
IL-6 and TNF-α in Raw264.7 cells, while treatment with Rema did not
affect the alterations induced by DAPA (n=3).
***P<0.001. DAPA, dapansutrile; LPS,
lipopolysaccharide; ns, not significant; Rema, remimazolam;
RT-qPCR, reverse transcription-quantitative PCR. |
Discussion
As a novel intravenous anesthetic agent, Rema
differs from alternate conventional anesthetics, with a rapid
onset, recovery and metabolism (2). According to the metabolic pathways,
Rema is rapidly metabolized by tissue esterase to an inactive
carboxy acid metabolite, and the related clinical research also
showed that Rema has no accumulation effects following long-term
use (25,26). Rema has been used in clinical
practice for several years since 2020 (27). More clinical studies and clinical
cases regarding addictive behaviors or unpredicted mental effects,
especially in long-term use of Rema, should be performed and
reported. The results of previous clinical studies have revealed
that Rema exhibits stable hemodynamics in critically ill patients,
without causing a dose-dependent blood pressure reduction (3,14,15). Thus, the present study focused on
the impact of Rema on cardiac I/R injury. The results first
demonstrated that Rema inhibited cardiac I/R injury by attenuating
I/R-induced inflammation and oxidative stress via the NLRP3/IL-1β
pathway. Therefore, the present study provides a novel theoretical
basis for the use of Rema in clinical practice, particularly in
cardiac surgery.
I/R injury is the main pathophysiological process
leading to cardiac injury in ischemic heart diseases, and one of
the main causes of postoperative cardiac dysfunction and death in
critically patients who have undergone cardiac surgery (28-30). Notably, anesthetic agents that
exert cardioprotective effects in patients may be more beneficial
in this context (31,32). The results of previous studies
have revealed that numerous intravenous anesthetic agents,
including propofol, dexmedetomidine and etomidate, exert
cardioprotective effects (33-35). However, these agents may also
cause adverse effects in patients, including inhibition of the
circulatory system, leading to hypotension (36). Thus, the development of novel
anesthetic agents with few adverse effects is required. Notably,
Rema exerts similar anesthetic effects as propofol. Rema acts on
GABARs in the central nervous system; however, the involved
metabolic pathways differ from those required by propofol (37). The results of the present study
demonstrated that Rema inhibited I/R-induced myocardial injury in
mice, highlighted by improved cardiac function and decreased
myocardial infarction. Thus, the cardiac protective effects of Rema
may facilitate its use in clinical practice.
Inflammation and oxidative stress are key mechanisms
underlying I/R injury (38,39). The results of the present study
revealed that Rema effectively suppressed I/R-induced elevation of
inflammation levels and oxidative stress. Further transcriptomics
analysis and RT-qPCR demonstrated that Rema exerted
cardioprotective effects through cytokines, chemokines and the ECM.
In addition, the NLRP3 inflammasome serves a role in myocardial I/R
injury (40,41), exhibiting potential as a
treatment target (24,42). Thus, the effects of Rema on NLRP3
inflammasome activation and the downstream secretion of IL-1β were
examined in the present study. The results demonstrated that Rema
significantly inhibited the I/R-induced increase in the expression
levels of NLRP3 and IL-1β. The results of the transcriptomics
analysis may also provide a basis for understanding the mechanisms
underlying the additional effects of Rema in cardiac I/R
injury.
Macrophages are key cells involved in the
inflammatory response of the heart and are key intervention targets
for cardiovascular diseases (22). Previous studies have revealed
that GABARs were not expressed in cardiomyocytes; however, these
were expressed in macrophages (16,43). The results of the present study
revealed that treatment with Rema significantly inhibited the
increase in CD68+ cells induced by I/R, indicating that
macrophages may be involved in the protective effects of Rema
against I/R injury. In addition, Rema significantly inhibited
LPS-induced increases in IL-1β, IL-6 and TNF-α expression and
secretion. Collectively, these results may indicate that Rema
inhibited the LPS-induced inflammatory phenotype of macrophages,
leading to reduced cardiac inflammation during I/R injury. The
results of a previous study have revealed that propofol, a similar
intravenous anesthetic activating GABAR, regulated inflammation by
modulating macrophage migration, recruitment, polarization and
pyroptosis (44). However,
research regarding macrophages in the effects of propofol on
cardiac I/R injury is also limited.
The present study has limitations. The present study
revealed that macrophages may serve important roles in the cardiac
protective effects of Rema against I/R injury. However, the direct
effect of Rema on cardiomyocytes was not investigated in the
present study. The results of previous studies have revealed that
propofol exerted a direct cardioprotective effect in cultured
neonatal myocardial cells treated with H2O2
(33,45). Although GABAR is not expressed in
cardiomyocytes, Rema may affect cardiomyocytes directly through
other mechanisms. Thus, whether Rema also exerts a direct effect on
cardiomyocytes and alleviates oxidative stress-induced myocardial
cell damage independent of GABARs also remains unknown. Further
investigations should focus on the use of cell co-culturing to
further investigate the direct and indirect effects of Rema in
cardiomyocytes. Further investigations may reveal the cellular and
molecular mechanisms underlying the effects of Rema in the heart.
These investigations will further expand the understanding of
cardiac protective effects of Rema and other anesthetics acting
through GABAR.
In conclusion, the present study systematically
investigated the effects of Rema on I/R-induced cardiac injury and
the specific underlying mechanisms. The results of the present
study revealed that Rema may inhibit I/R-induced inflammation and
oxidative stress. The present study provides a novel theoretical
basis for the use of Rema in clinical practice, with potential for
beneficial outcomes in patients who experience cardiac I/R injury
during CPB.
Supplementary Data
Availability of data and materials
The RNA sequencing data generated in the present
study may be found in the BioProject database of the National
Library of Medicine under accession number PRJNA1173328 or at the
following URL: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1173328.
The other data generated in the present study may be requested from
the corresponding author.
Authors' contributions
XBW, XRL and TL designed the present study. XRL,
GJS, YW, TTC, PZ, TL and LL performed the experiments and acquired
the data. XRL, TL, CHL and XBW analyzed the data. XRL and XBW
drafted the manuscript. CHL, LL and TL revised the manuscript. XBW
and XRL provided final approval of the manuscript. XRL and XBW
confirmed the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental designs and protocols involving
animals were approved by the Institutional Animals Ethics Committee
at Southwest Medical University, China (approval no. 20221117-011;
Luzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 31900813), Sichuan Science and
Technology Program, China (grant nos. 2022YFS0632, 2022YFS0627,
2023ZYD0093 and 2024NSFSC0305) and Bureau of Science and Technology
of Luzhou (grant no. 2024JYJ006).
References
|
1
|
Kim SH and Fechner J: Remimazolam-current
knowledge on a new intravenous benzodiazepine anesthetic agent.
Korean J Anesthesiol. 75:307–315. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sneyd JR and Rigby-Jones AE: Remimazolam
for anaesthesia or sedation. Curr Opin Anaesthesiol. 33:506–511.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu K, Wei S, Ling W, Wei Y, Ran X, Huang
H, Wang M, Wei N, Liao Y, Qin Z, et al: Remimazolam versus propofol
for deep sedation/anaesthesia in upper gastrointestinal endoscopy
in elderly patients: A multicenter, randomized controlled trial. J
Clin Pharm Ther. 47:2230–2236. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Hert S and Moerman A: Myocardial injury
and protection related to cardiopulmonary bypass. Best Pract Res
Clin Anaesthesiol. 29:137–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cherry AD: Mitochondrial dysfunction in
cardiac surgery. Anesthesiol Clin. 37:769–785. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Francisco J and Del Re DP: Inflammation in
myocardial ischemia/reperfusion injury: Underlying mechanisms and
therapeutic potential. Antioxidants (Basel). 12:19442023.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ong SB, Hernández-Reséndiz S,
Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA and
Hausenloy DJ: Inflammation following acute myocardial infarction:
Multiple players, dynamic roles, and novel therapeutic
opportunities. Pharmacol Ther. 186:73–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Li L, Wang Z, Zhang J and Zhou Z:
Myocardial ischemia-reperfusion injury; molecular mechanisms and
prevention. Microvasc Res. 149:1045652023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burgoyne JR, Mongue-Din H, Eaton P and
Shah AM: Redox signaling in cardiac physiology and pathology. Circ
Res. 111:1091–1106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ngo DH and Vo TS: An updated review on
pharmaceutical properties of gamma-aminobutyric acid. Molecules.
24:26782019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen C, Zhou X, He J, Xie Z, Xia S and Lu
G: The roles of GABA in ischemia-reperfusion injury in the central
nervous system and peripheral organs. Oxid Med Cell Longev.
2019:40283942019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian J and Kaufman DL: The GABA and
GABA-receptor system in inflammation, anti-tumor immune responses,
and COVID-19. Biomedicines. 11:2542023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Zhang H, Yuan H, Chen S, Yu Y,
Zhang X, Gao Z, Du H, Li W, Wang Y, et al: Prophylactic
supplementation with lactobacillus reuteri or its metabolite GABA
protects against acute ischemic cardiac injury. Adv Sci (Weinh).
11:e23072332024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu T, Lai T, Chen J, Lu Y, He F, Chen Y
and Xie Y: Effect of remimazolam induction on hemodynamics in
patients undergoing valve replacement surgery: A randomized,
double-blind, controlled trial. Pharmacol Res Perspect.
9:e008512021. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang F, Yi JM, Gong HY, Lu ZY, Chen J,
Fang B, Chen C and Liu ZY: Remimazolam benzenesulfonate anesthesia
effectiveness in cardiac surgery patients under general anesthesia.
World J Clin Cases. 9:10595–10603. 2021. View Article : Google Scholar
|
|
16
|
Wang Z, Huang S, Sheng Y, Peng X, Liu H,
Jin N, Cai J, Shu Y, Li T, Li P, et al: Topiramate modulates
post-infarction inflammation primarily by targeting monocytes or
macrophages. Cardiovasc Res. 113:475–487. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai W, Liu L, Shi X, Liu Y, Wang J, Fang
X, Chen Z, Ai D, Zhu Y and Zhang X: Alox15/15-HpETE aggravates
myocardial ischemia-reperfusion injury by promoting cardiomyocyte
ferroptosis. Circulation. 147:1444–1460. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao E, Lei YH, Shang X, Huang ZM, Zuo L,
Boucher M, Fan Q, Chuprun JK, Ma XL and Koch WJ: A novel and
efficient model of coronary artery ligation and myocardial
infarction in the mouse. Circ Res. 107:1445–1453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tan XQ, Cheng XL, Zhang L, Wu BW, Liu QH,
Meng J, Xu HY and Cao JM: Multi-walled carbon nanotubes impair
Kv4.2/4.3 channel activities, delay membrane repolarization and
induce bradyarrhythmias in the rat. PLoS One. 9:e1015452014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang L, Sim I, Moqbel S and Wu L:
Dapansutrile ameliorated chondrocyte inflammation and
osteoarthritis through suppression of MAPK signaling pathway. Hum
Exp Toxicol. 41:96032712211454012022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Chen R, Zhang H, Tang B, Luo Y, Yang Y,
Zhong X, Chen S, Xu X, Huang S and Liu C: Macrophages in
cardiovascular diseases: Molecular mechanisms and therapeutic
targets. Signal Transduct Target Ther. 9:1302024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chong AJ, Pohlman TH, Hampton CR,
Shimamoto A, Mackman N and Verrier ED: Tissue factor and thrombin
mediate myocardial ischemia-reperfusion injury. Ann Thorac Surg.
75:S649–S655. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Toldo S and Abbate A: The NLRP3
inflammasome in acute myocardial infarction. Nat Rev Cardiol.
15:203–214. 2018. View Article : Google Scholar
|
|
25
|
Hu Q, Liu X, Wen C, Li D and Lei X:
Remimazolam: An updated review of a new sedative and anaesthetic.
Drug Des Devel Ther. 16:3957–3974. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Antonik LJ, Goldwater DR, Kilpatrick GJ,
Tilbrook GS and Borkett KM: A placebo- and midazolam-controlled
phase I single ascending-dose study evaluating the safety,
pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056):
Part I. Safety, efficacy, and basic pharmacokinetics. Anesth Analg.
115:274–283. 2012. View Article : Google Scholar
|
|
27
|
Kilpatrick GJ: Remimazolam: Non-clinical
and clinical profile of a new sedative/anesthetic agent. Front
Pharmacol. 12:6908752021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He J, Liu D, Zhao L, Zhou D, Rong J, Zhang
L and Xia Z: Myocardial ischemia/reperfusion injury: Mechanisms of
injury and implications for management (Review). Exp Ther Med.
23:4302022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heusch G: Myocardial ischemia/reperfusion:
Translational pathophysiology of ischemic heart disease. Med.
5:10–31. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gunata M and Parlakpinar H: A review of
myocardial ischaemia/reperfusion injury: Pathophysiology,
experimental models, biomarkers, genetics and pharmacological
treatment. Cell Biochem Funct. 39:190–217. 2021. View Article : Google Scholar
|
|
31
|
Wu L, Zhao H, Wang T, Pac-Soo C and Ma D:
Cellular signaling pathways and molecular mechanisms involving
inhalational anesthetics-induced organoprotection. J Anesth.
28:740–758. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ferrando C, Soro M and Belda FJ:
Protection strategies during cardiopulmonary bypass: Ventilation,
anesthetics and oxygen. Curr Opin Anaesthesiol. 28:73–80. 2015.
View Article : Google Scholar
|
|
33
|
Li S, Lei Z, Yang X, Zhao M, Hou Y, Wang
D, Tang S, Li J and Yu J: Propofol protects myocardium from
ischemia/reperfusion injury by inhibiting ferroptosis through the
AKT/p53 signaling pathway. Front Pharmacol. 13:8414102022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eroglu A: The effect of intravenous
anesthetics on ischemia-reperfusion injury. Biomed Res Int.
2014:8215132014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang GR, Peng CM, Liu ZZ and Leng YF: The
effect of dexmedetomidine on myocardial ischemia/reperfusion injury
in patients undergoing cardiac surgery with cardiopulmonary bypass:
A meta-analysis. Eur Rev Med Pharmacol Sci. 25:7409–7417.
2021.PubMed/NCBI
|
|
36
|
Sneyd JR, Absalom AR, Barends CRM and
Jones JB: Hypotension during propofol sedation for colonoscopy: A
retrospective exploratory analysis and meta-analysis. Br J Anaesth.
128:610–622. 2022. View Article : Google Scholar :
|
|
37
|
Kim KM: Remimazolam: Pharmacological
characteristics and clinical applications in anesthesiology. Anesth
Pain Med (Seoul). 17:1–11. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Algoet M, Janssens S, Himmelreich U, Gsell
W, Pusovnik M, Van den Eynde J and Oosterlinck W: Myocardial
ischemia-reperfusion injury and the influence of inflammation.
Trends Cardiovasc Med. 33:357–366. 2023. View Article : Google Scholar
|
|
39
|
Xiang M, Lu Y, Xin L, Gao J, Shang C,
Jiang Z, Lin H, Fang X, Qu Y, Wang Y, et al: Role of oxidative
stress in reperfusion following myocardial ischemia and its
treatments. Oxid Med Cell Longev. 2021:66140092021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Li X, Sun T, Li T and Li Q:
Pyroptosis in myocardial ischemia/reperfusion and its therapeutic
implications. Eur J Pharmacol. 971:1764642024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Toldo S, Mauro AG, Cutter Z and Abbate A:
Inflammasome, pyroptosis, and cytokines in myocardial
ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol.
315:H1553–H1568. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xia Y, He F, Wu X, Tan B, Chen S, Liao Y,
Qi M, Chen S, Peng Y, Yin Y and Ren W: GABA transporter sustains
IL-1β production in macrophages. Sci Adv. 7:eabe92742021.
View Article : Google Scholar
|
|
43
|
Bu J, Huang S, Wang J, Xia T, Liu H, You
Y, Wang Z and Liu K: The GABAA receptor influences
pressure overload-induced heart failure by modulating macrophages
in mice. Front Immunol. 12:6701532021. View Article : Google Scholar
|
|
44
|
Yi S, Tao X, Wang Y, Cao Q, Zhou Z and
Wang S: Effects of propofol on macrophage activation and function
in diseases. Front Pharmacol. 13:9647712022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu XR, Cao L, Li T, Chen LL, Yu YY, Huang
WJ, Liu L and Tan XQ: Propofol attenuates
H2O2-induced oxidative stress and apoptosis
via the mitochondria- and ER-medicated pathways in neonatal rat
cardiomyocytes. Apoptosis. 22:639–646. 2017. View Article : Google Scholar : PubMed/NCBI
|