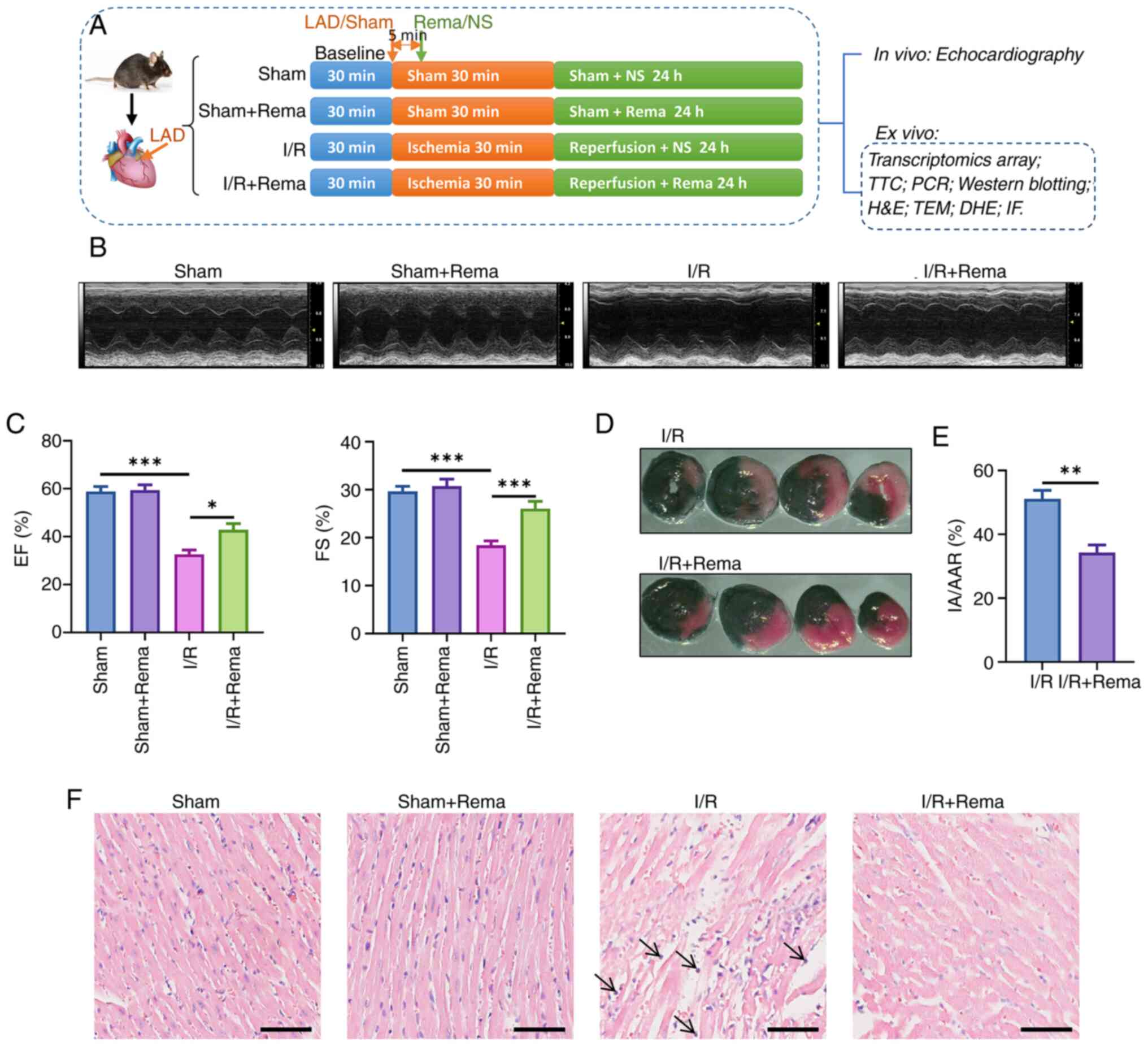
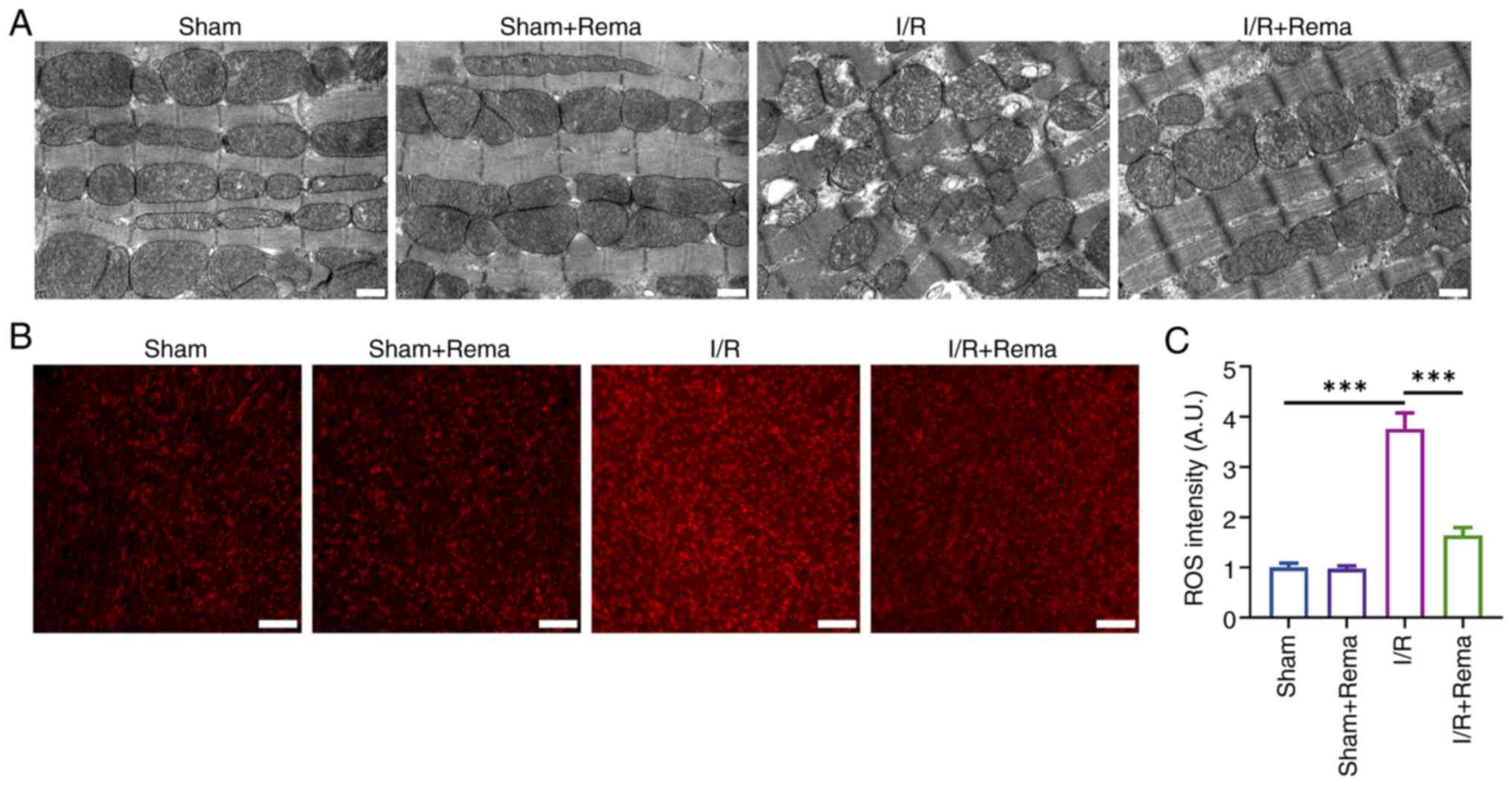
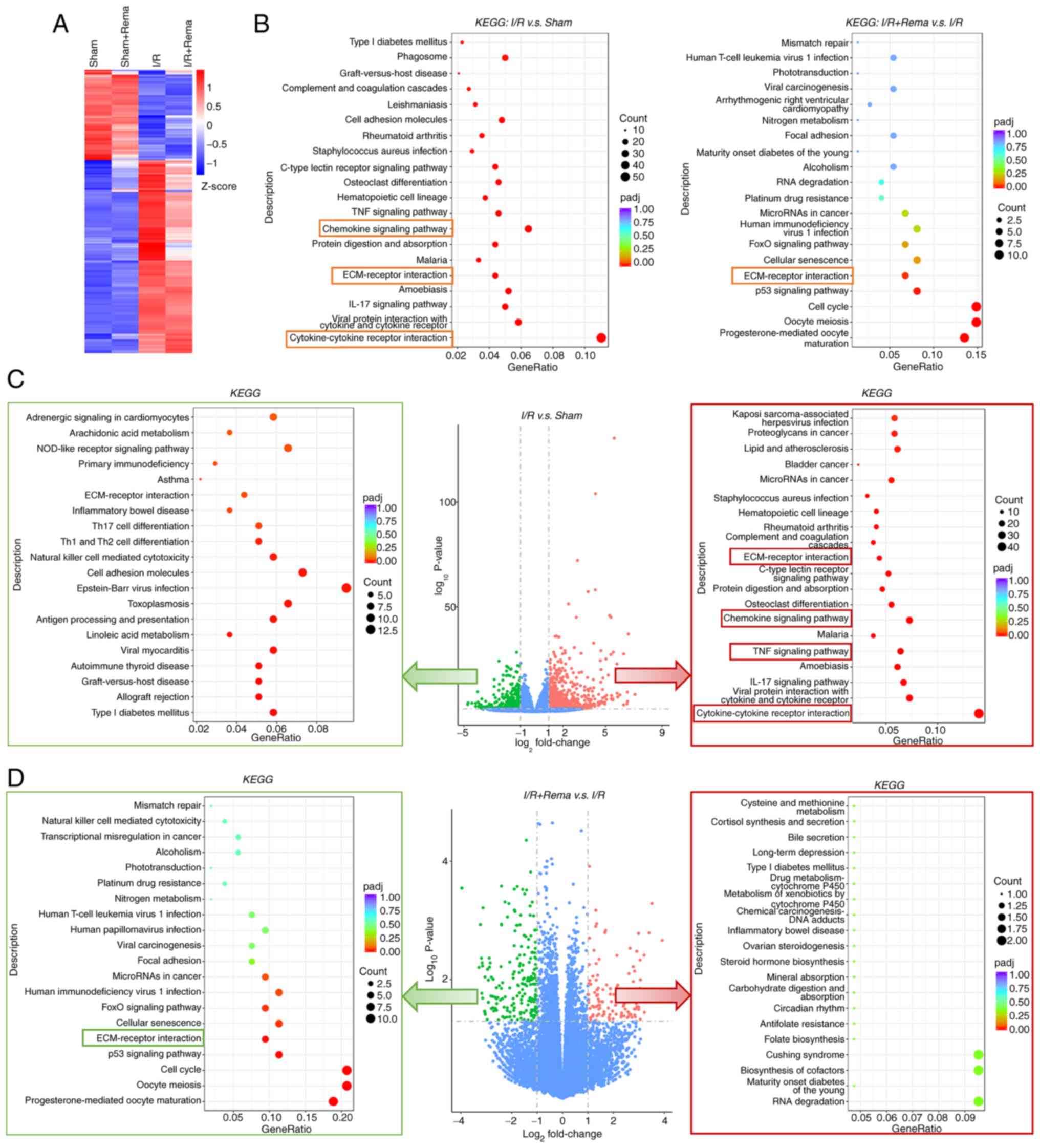
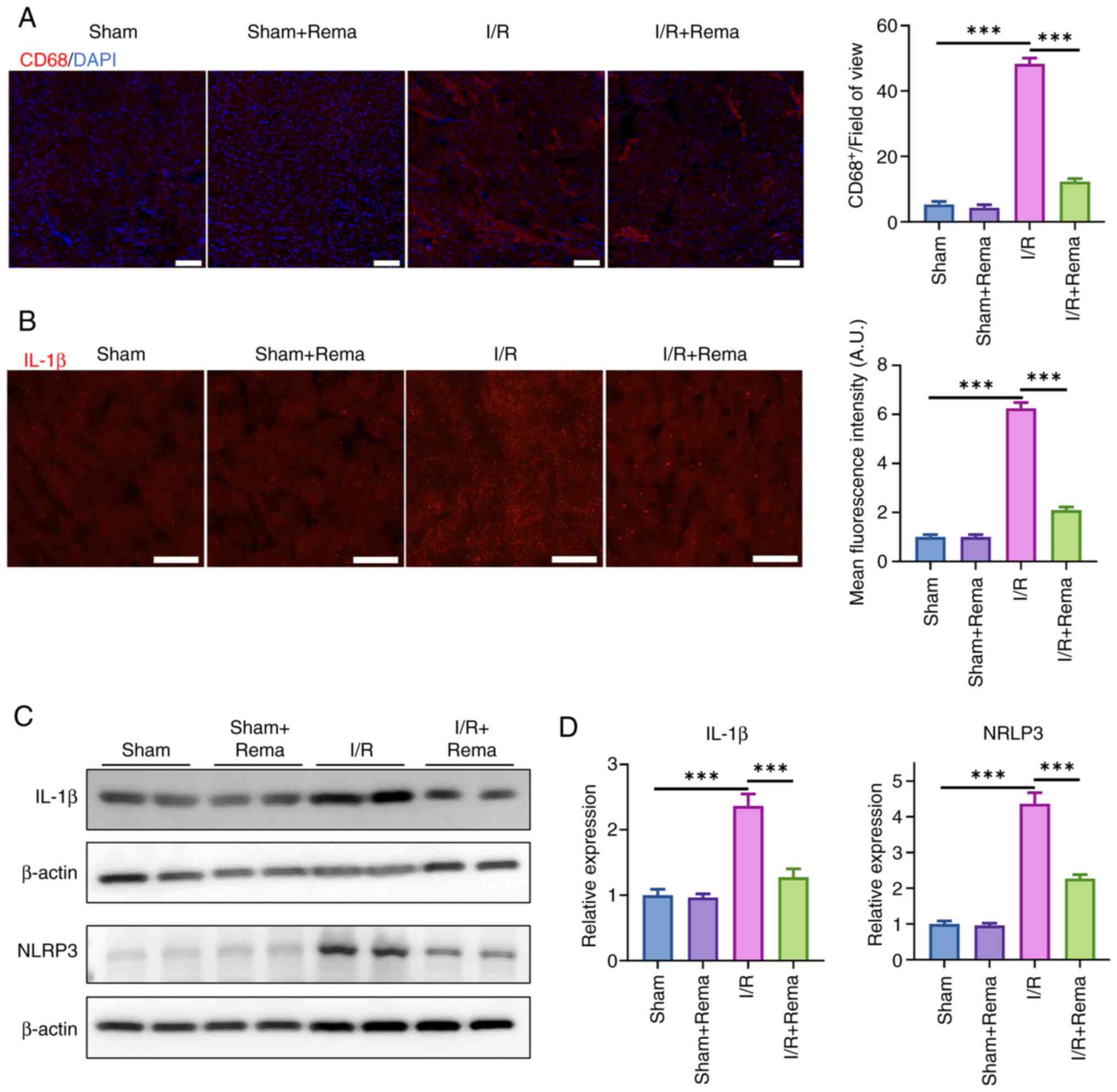
|
1
|
Kim SH and Fechner J: Remimazolam-current
knowledge on a new intravenous benzodiazepine anesthetic agent.
Korean J Anesthesiol. 75:307–315. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sneyd JR and Rigby-Jones AE: Remimazolam
for anaesthesia or sedation. Curr Opin Anaesthesiol. 33:506–511.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu K, Wei S, Ling W, Wei Y, Ran X, Huang
H, Wang M, Wei N, Liao Y, Qin Z, et al: Remimazolam versus propofol
for deep sedation/anaesthesia in upper gastrointestinal endoscopy
in elderly patients: A multicenter, randomized controlled trial. J
Clin Pharm Ther. 47:2230–2236. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Hert S and Moerman A: Myocardial injury
and protection related to cardiopulmonary bypass. Best Pract Res
Clin Anaesthesiol. 29:137–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cherry AD: Mitochondrial dysfunction in
cardiac surgery. Anesthesiol Clin. 37:769–785. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Francisco J and Del Re DP: Inflammation in
myocardial ischemia/reperfusion injury: Underlying mechanisms and
therapeutic potential. Antioxidants (Basel). 12:19442023.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ong SB, Hernández-Reséndiz S,
Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA and
Hausenloy DJ: Inflammation following acute myocardial infarction:
Multiple players, dynamic roles, and novel therapeutic
opportunities. Pharmacol Ther. 186:73–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Li L, Wang Z, Zhang J and Zhou Z:
Myocardial ischemia-reperfusion injury; molecular mechanisms and
prevention. Microvasc Res. 149:1045652023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burgoyne JR, Mongue-Din H, Eaton P and
Shah AM: Redox signaling in cardiac physiology and pathology. Circ
Res. 111:1091–1106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ngo DH and Vo TS: An updated review on
pharmaceutical properties of gamma-aminobutyric acid. Molecules.
24:26782019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen C, Zhou X, He J, Xie Z, Xia S and Lu
G: The roles of GABA in ischemia-reperfusion injury in the central
nervous system and peripheral organs. Oxid Med Cell Longev.
2019:40283942019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian J and Kaufman DL: The GABA and
GABA-receptor system in inflammation, anti-tumor immune responses,
and COVID-19. Biomedicines. 11:2542023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Zhang H, Yuan H, Chen S, Yu Y,
Zhang X, Gao Z, Du H, Li W, Wang Y, et al: Prophylactic
supplementation with lactobacillus reuteri or its metabolite GABA
protects against acute ischemic cardiac injury. Adv Sci (Weinh).
11:e23072332024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu T, Lai T, Chen J, Lu Y, He F, Chen Y
and Xie Y: Effect of remimazolam induction on hemodynamics in
patients undergoing valve replacement surgery: A randomized,
double-blind, controlled trial. Pharmacol Res Perspect.
9:e008512021. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang F, Yi JM, Gong HY, Lu ZY, Chen J,
Fang B, Chen C and Liu ZY: Remimazolam benzenesulfonate anesthesia
effectiveness in cardiac surgery patients under general anesthesia.
World J Clin Cases. 9:10595–10603. 2021. View Article : Google Scholar
|
|
16
|
Wang Z, Huang S, Sheng Y, Peng X, Liu H,
Jin N, Cai J, Shu Y, Li T, Li P, et al: Topiramate modulates
post-infarction inflammation primarily by targeting monocytes or
macrophages. Cardiovasc Res. 113:475–487. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai W, Liu L, Shi X, Liu Y, Wang J, Fang
X, Chen Z, Ai D, Zhu Y and Zhang X: Alox15/15-HpETE aggravates
myocardial ischemia-reperfusion injury by promoting cardiomyocyte
ferroptosis. Circulation. 147:1444–1460. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao E, Lei YH, Shang X, Huang ZM, Zuo L,
Boucher M, Fan Q, Chuprun JK, Ma XL and Koch WJ: A novel and
efficient model of coronary artery ligation and myocardial
infarction in the mouse. Circ Res. 107:1445–1453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tan XQ, Cheng XL, Zhang L, Wu BW, Liu QH,
Meng J, Xu HY and Cao JM: Multi-walled carbon nanotubes impair
Kv4.2/4.3 channel activities, delay membrane repolarization and
induce bradyarrhythmias in the rat. PLoS One. 9:e1015452014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang L, Sim I, Moqbel S and Wu L:
Dapansutrile ameliorated chondrocyte inflammation and
osteoarthritis through suppression of MAPK signaling pathway. Hum
Exp Toxicol. 41:96032712211454012022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Chen R, Zhang H, Tang B, Luo Y, Yang Y,
Zhong X, Chen S, Xu X, Huang S and Liu C: Macrophages in
cardiovascular diseases: Molecular mechanisms and therapeutic
targets. Signal Transduct Target Ther. 9:1302024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chong AJ, Pohlman TH, Hampton CR,
Shimamoto A, Mackman N and Verrier ED: Tissue factor and thrombin
mediate myocardial ischemia-reperfusion injury. Ann Thorac Surg.
75:S649–S655. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Toldo S and Abbate A: The NLRP3
inflammasome in acute myocardial infarction. Nat Rev Cardiol.
15:203–214. 2018. View Article : Google Scholar
|
|
25
|
Hu Q, Liu X, Wen C, Li D and Lei X:
Remimazolam: An updated review of a new sedative and anaesthetic.
Drug Des Devel Ther. 16:3957–3974. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Antonik LJ, Goldwater DR, Kilpatrick GJ,
Tilbrook GS and Borkett KM: A placebo- and midazolam-controlled
phase I single ascending-dose study evaluating the safety,
pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056):
Part I. Safety, efficacy, and basic pharmacokinetics. Anesth Analg.
115:274–283. 2012. View Article : Google Scholar
|
|
27
|
Kilpatrick GJ: Remimazolam: Non-clinical
and clinical profile of a new sedative/anesthetic agent. Front
Pharmacol. 12:6908752021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He J, Liu D, Zhao L, Zhou D, Rong J, Zhang
L and Xia Z: Myocardial ischemia/reperfusion injury: Mechanisms of
injury and implications for management (Review). Exp Ther Med.
23:4302022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heusch G: Myocardial ischemia/reperfusion:
Translational pathophysiology of ischemic heart disease. Med.
5:10–31. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gunata M and Parlakpinar H: A review of
myocardial ischaemia/reperfusion injury: Pathophysiology,
experimental models, biomarkers, genetics and pharmacological
treatment. Cell Biochem Funct. 39:190–217. 2021. View Article : Google Scholar
|
|
31
|
Wu L, Zhao H, Wang T, Pac-Soo C and Ma D:
Cellular signaling pathways and molecular mechanisms involving
inhalational anesthetics-induced organoprotection. J Anesth.
28:740–758. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ferrando C, Soro M and Belda FJ:
Protection strategies during cardiopulmonary bypass: Ventilation,
anesthetics and oxygen. Curr Opin Anaesthesiol. 28:73–80. 2015.
View Article : Google Scholar
|
|
33
|
Li S, Lei Z, Yang X, Zhao M, Hou Y, Wang
D, Tang S, Li J and Yu J: Propofol protects myocardium from
ischemia/reperfusion injury by inhibiting ferroptosis through the
AKT/p53 signaling pathway. Front Pharmacol. 13:8414102022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eroglu A: The effect of intravenous
anesthetics on ischemia-reperfusion injury. Biomed Res Int.
2014:8215132014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang GR, Peng CM, Liu ZZ and Leng YF: The
effect of dexmedetomidine on myocardial ischemia/reperfusion injury
in patients undergoing cardiac surgery with cardiopulmonary bypass:
A meta-analysis. Eur Rev Med Pharmacol Sci. 25:7409–7417.
2021.PubMed/NCBI
|
|
36
|
Sneyd JR, Absalom AR, Barends CRM and
Jones JB: Hypotension during propofol sedation for colonoscopy: A
retrospective exploratory analysis and meta-analysis. Br J Anaesth.
128:610–622. 2022. View Article : Google Scholar :
|
|
37
|
Kim KM: Remimazolam: Pharmacological
characteristics and clinical applications in anesthesiology. Anesth
Pain Med (Seoul). 17:1–11. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Algoet M, Janssens S, Himmelreich U, Gsell
W, Pusovnik M, Van den Eynde J and Oosterlinck W: Myocardial
ischemia-reperfusion injury and the influence of inflammation.
Trends Cardiovasc Med. 33:357–366. 2023. View Article : Google Scholar
|
|
39
|
Xiang M, Lu Y, Xin L, Gao J, Shang C,
Jiang Z, Lin H, Fang X, Qu Y, Wang Y, et al: Role of oxidative
stress in reperfusion following myocardial ischemia and its
treatments. Oxid Med Cell Longev. 2021:66140092021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Li X, Sun T, Li T and Li Q:
Pyroptosis in myocardial ischemia/reperfusion and its therapeutic
implications. Eur J Pharmacol. 971:1764642024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Toldo S, Mauro AG, Cutter Z and Abbate A:
Inflammasome, pyroptosis, and cytokines in myocardial
ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol.
315:H1553–H1568. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xia Y, He F, Wu X, Tan B, Chen S, Liao Y,
Qi M, Chen S, Peng Y, Yin Y and Ren W: GABA transporter sustains
IL-1β production in macrophages. Sci Adv. 7:eabe92742021.
View Article : Google Scholar
|
|
43
|
Bu J, Huang S, Wang J, Xia T, Liu H, You
Y, Wang Z and Liu K: The GABAA receptor influences
pressure overload-induced heart failure by modulating macrophages
in mice. Front Immunol. 12:6701532021. View Article : Google Scholar
|
|
44
|
Yi S, Tao X, Wang Y, Cao Q, Zhou Z and
Wang S: Effects of propofol on macrophage activation and function
in diseases. Front Pharmacol. 13:9647712022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu XR, Cao L, Li T, Chen LL, Yu YY, Huang
WJ, Liu L and Tan XQ: Propofol attenuates
H2O2-induced oxidative stress and apoptosis
via the mitochondria- and ER-medicated pathways in neonatal rat
cardiomyocytes. Apoptosis. 22:639–646. 2017. View Article : Google Scholar : PubMed/NCBI
|