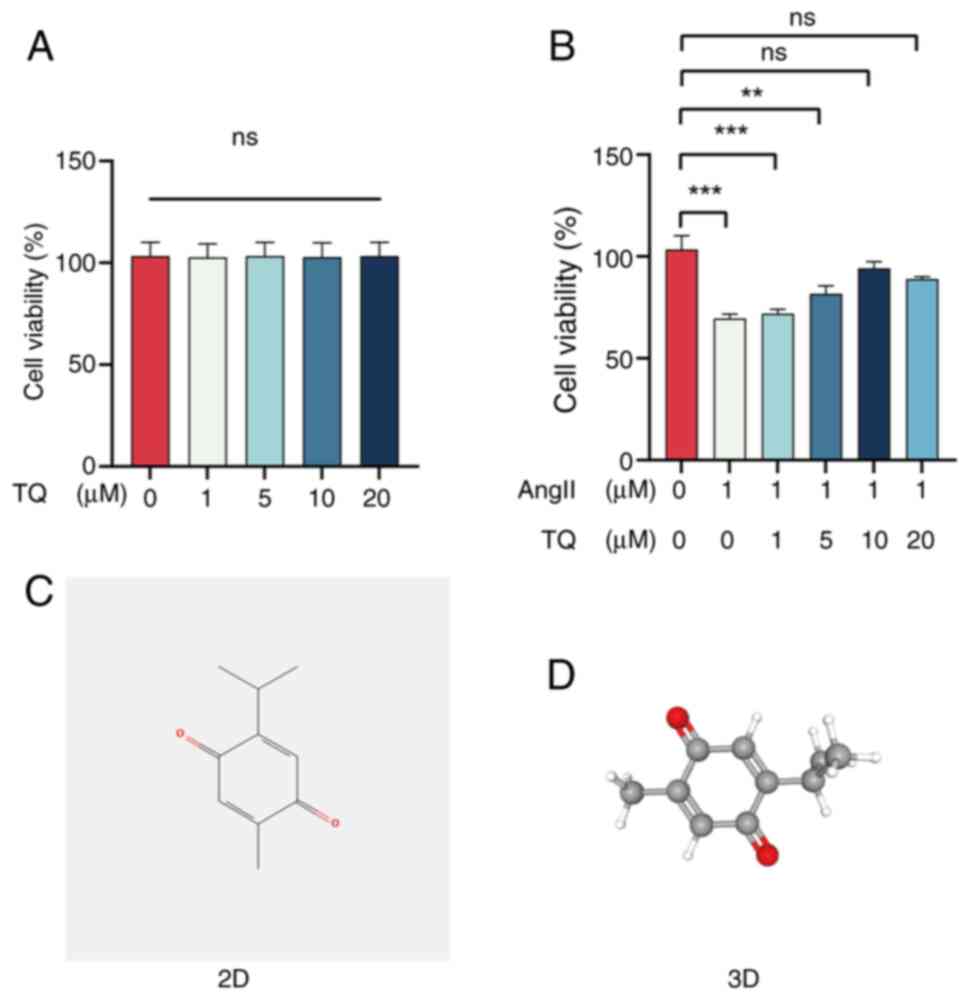
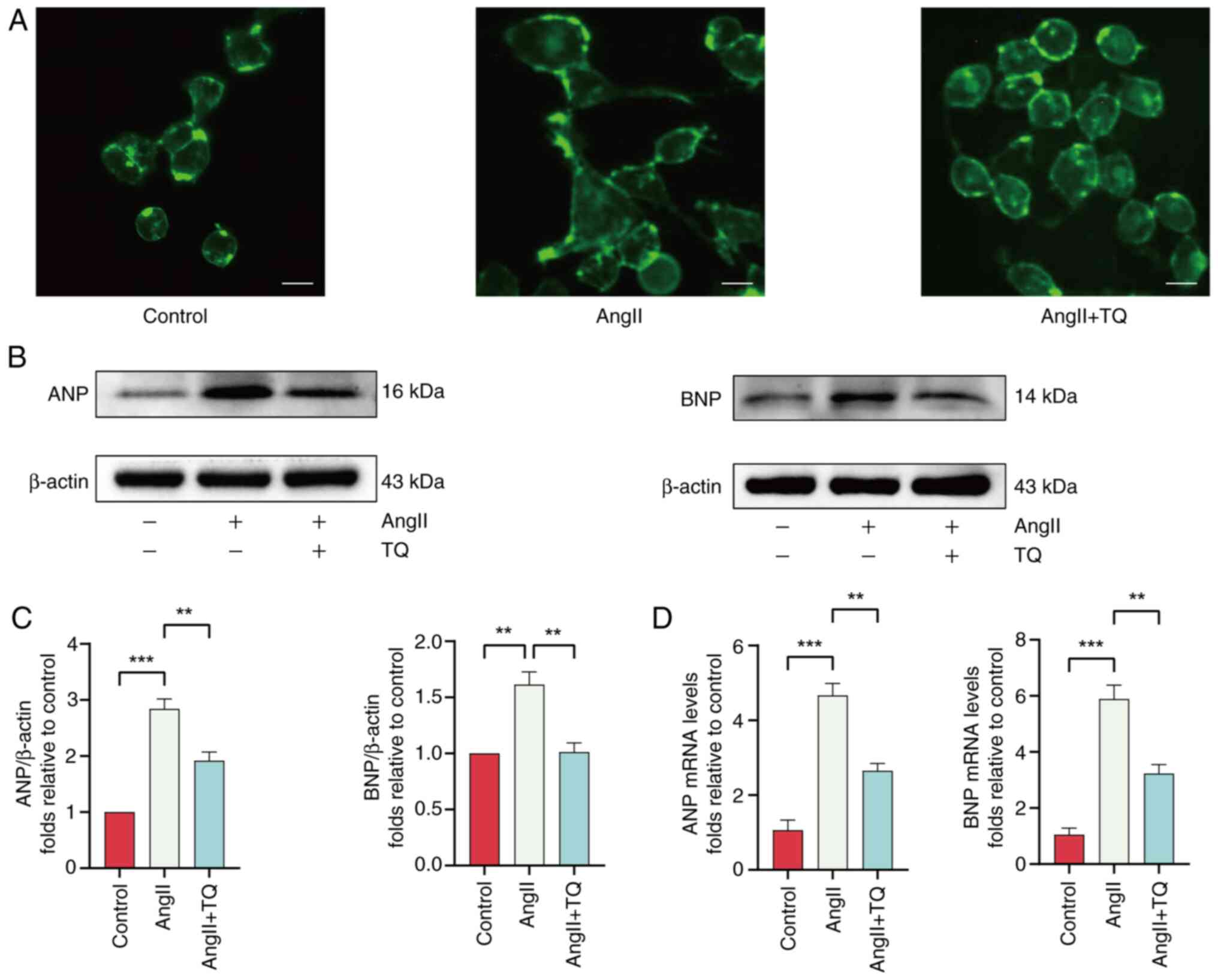
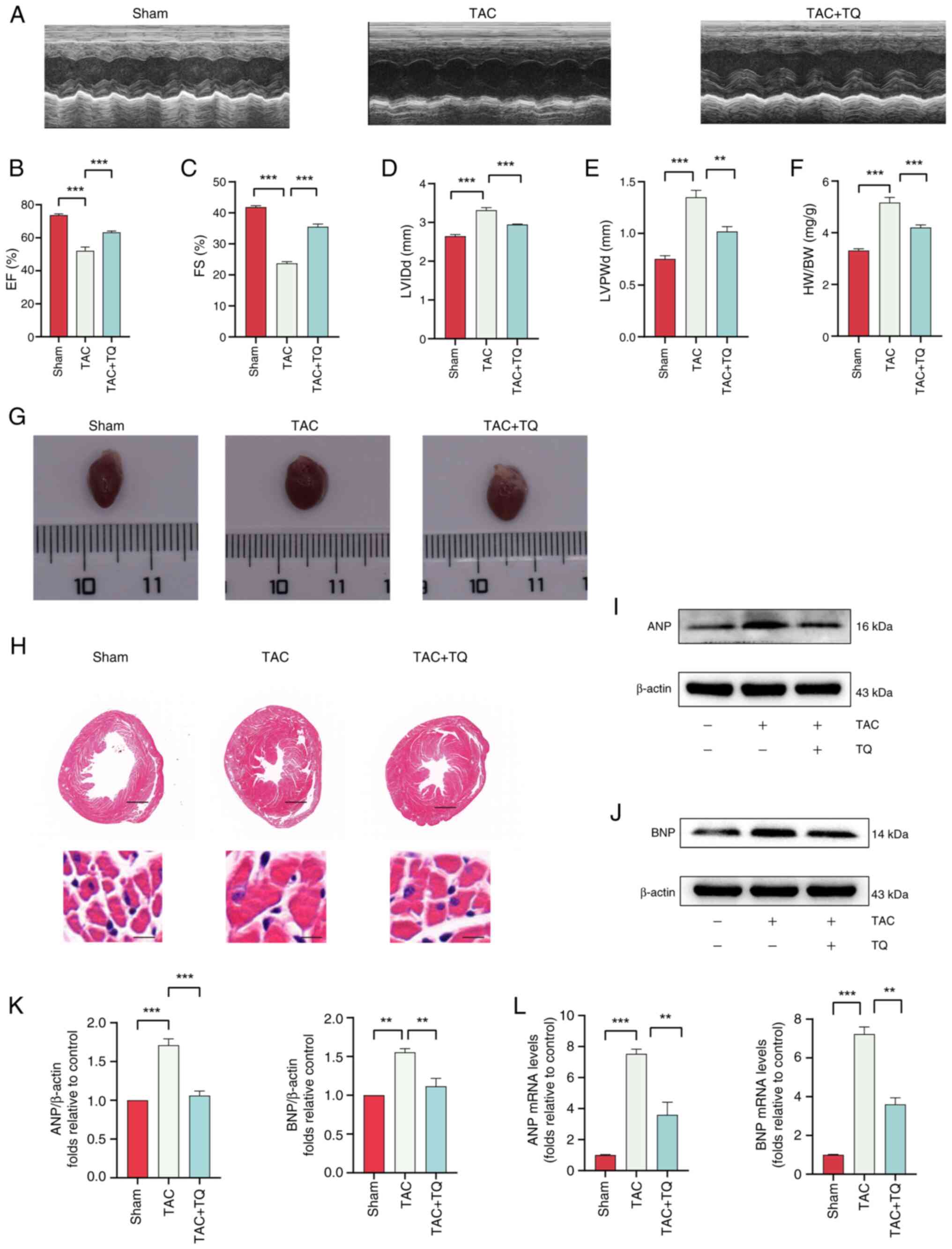
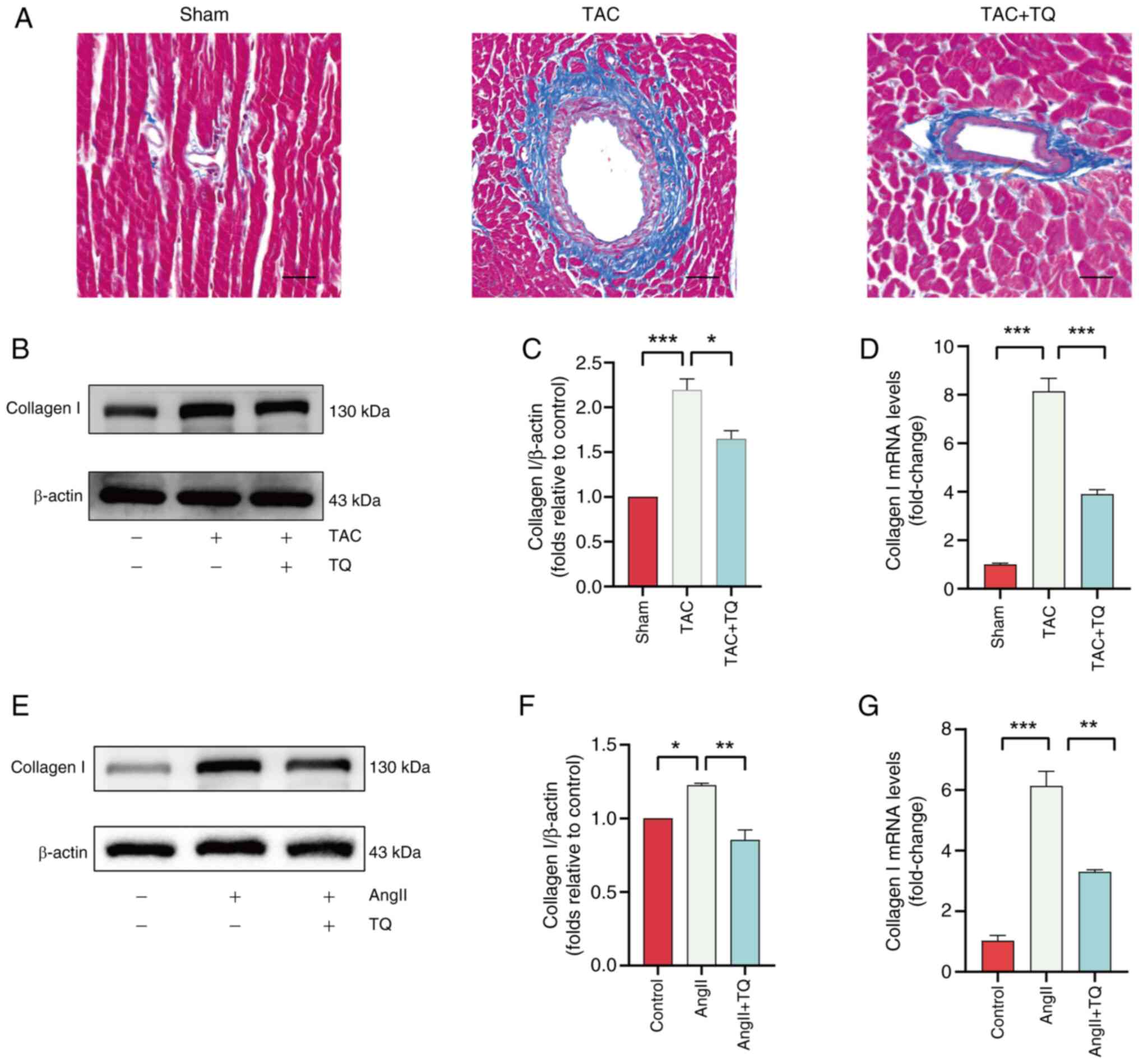
|
1
|
Frey N and Olson EN: Cardiac hypertrophy:
The good, the bad, and the ugly. Annu Rev Physiol. 65:45–79. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu M, Wan CX, Huang SH, Wang HB, Fan D, Wu
HM, Wu QQ, Ma ZG, Deng W and Tang QZ: Oridonin protects against
cardiac hypertrophy by promoting P21-related autophagy. Cell Death
Dis. 10:4032019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakai A, Yamaguchi O, Takeda T, Higuchi Y,
Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, et
al: The role of autophagy in cardiomyocytes in the basal state and
in response to hemodynamic stress. Nat Med. 13:619–624. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ritterhoff J and Tian R: Metabolic
mechanisms in physiological and pathological cardiac hypertrophy:
New paradigms and challenges. Nat Rev Cardiol. 20:812–829. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakamura M and Sadoshima J: Mechanisms of
physiological and pathological cardiac hypertrophy. Nat Rev
Cardiol. 15:387–407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maillet M, Van Berlo JH and Molkentin JD:
Molecular basis of physiological heart growth: Fundamental concepts
and new players. Nat Rev Mol Cell Biol. 14:38–48. 2013. View Article : Google Scholar
|
|
7
|
Gali-Muhtasib H, Roessner A and
Schneider-Stock R: Thymoquinone: A promising anti-cancer drug from
natural sources. Int J Biochem Cell Biol. 38:1249–1253. 2006.
View Article : Google Scholar
|
|
8
|
Darakhshan S, Bidmeshki Pour A,
Hosseinzadeh Colagar A and Sisakhtnezhad S: Thymoquinone and its
therapeutic potentials. Pharmacol Res. 95-96:138–158. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Woo CC, Kumar AP, Sethi G and Tan KH:
Thymoquinone: Potential cure for inflammatory disorders and cancer.
Biochem Pharmacol. 83:443–451. 2012. View Article : Google Scholar
|
|
10
|
Mialet-Perez J and Vindis C: Autophagy in
health and disease: Focus on the cardiovascular system. Essays
Biochem. 61:721–732. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xue R, Zeng J, Chen Y, Chen C, Tan W, Zhao
J, Dong B, Sun Y, Dong Y and Liu C: Sestrin 1 ameliorates cardiac
hypertrophy via autophagy activation. J Cell Mol Med. 21:1193–1205.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang K, Long Q, Saja K, Huang F, Pogwizd
SM, Zhou L, Yoshida M and Yang Q: Knockout of the ATPase inhibitory
factor 1 protects the heart from pressure overload-induced cardiac
hypertrophy. Sci Rep. 7:105012017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kelly DP: PPARs of the heart: Three is a
crowd. Circ Res. 92:482–484. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Majdalawieh A and Ro HS: PPARgamma1 and
LXRalpha face a new regulator of macrophage cholesterol homeostasis
and inflammatory responsiveness, AEBP1. Nucl Recept Signal.
8:e0042010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kung J and Henry RR: Thiazolidinedione
safety. Expert Opin Drug Saf. 11:565–579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng Y, Wang L, Zhang Z, He X, Fan Q,
Cheng X, Qiao Y, Huang H, Lai S, Wan Q, et al: Puerarin activates
adaptive autophagy and protects the myocardium against
doxorubicin-induced cardiotoxicity via the 14-3-3γ/PKCε pathway.
Biomed Pharmacother. 153:1134032022. View Article : Google Scholar
|
|
17
|
Cheng Y, Shen A, Wu X, Shen Z, Chen X, Li
J, Liu L, Lin X, Wu M, Chen Y, et al: Qingda granule attenuates
angiotensin II-induced cardiac hypertrophy and apoptosis and
modulates the PI3K/AKT pathway. Biomed Pharmacother.
133:1110222021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He H, Wang L, Qiao Y, Yang B, Yin D and He
M: Epigallocatechin-3-gallate pretreatment alleviates
doxorubicin-induced ferroptosis and cardiotoxicity by upregulating
AMPKα2 and activating adaptive autophagy. Redox Biol.
48:1021852021. View Article : Google Scholar
|
|
19
|
Lu Q, Hu S, Guo P, Zhu X, Ren Z, Wu Q and
Wang X: PPAR-γ with its anti-fibrotic action could serve as an
effective therapeutic target in T-2 toxin-induced cardiac fibrosis
of rats. Food Chem Toxicol. 152:1121832021. View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Rockman HA, Ross RS, Harris AN, Knowlton
KU, Steinhelper ME, Field LJ, Ross J Jr and Chien KR: Segregation
of atrial-specific and inducible expression of an atrial
natriuretic factor transgene in an in vivo murine model of cardiac
hypertrophy. Proc Natl Acad Sci USA. 88:8277–8281. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen H, Zhuo C, Zu A, Yuan S, Zhang H,
Zhao J and Zheng L: Thymoquinone ameliorates pressure
overload-induced cardiac hypertrophy by activating the AMPK
signalling pathway. J Cell Mol Med. 26:855–867. 2022. View Article : Google Scholar
|
|
23
|
Ji YX, Zhang P, Zhang XJ, Zhao YC, Deng
KQ, Jiang X, Wang PX, Huang Z and Li H: The ubiquitin E3 ligase
TRAF6 exacerbates pathological cardiac hypertrophy via
TAK1-dependent signalling. Nat Commun. 7:112672016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li HH, Kedar V, Zhang C, McDonough H, Arya
R, Wang DZ and Patterson C: Atrogin-1/muscle atrophy F-box inhibits
calcineurin-dependent cardiac hypertrophy by participating in an
SCF ubiquitin ligase complex. J Clin Invest. 114:1058–1071. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie X, Bi HL, Lai S, Zhang YL, Li N, Cao
HJ, Han L, Wang HX and Li HH: The immunoproteasome catalytic β5i
subunit regulates cardiac hypertrophy by targeting the autophagy
protein ATG5 for degradation. Sci Adv. 5:eaau04952019. View Article : Google Scholar
|
|
26
|
Gyöngyösi M, Winkler J, Ramos I, Do QT,
Firat H, McDonald K, González A, Thum T, Díez J, Jaisser F, et al:
Myocardial fibrosis: Biomedical research from bench to bedside. Eur
J Heart Fail. 19:177–191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bacmeister L, Schwarzl M, Warnke S,
Stoffers B, Blankenberg S, Westermann D and Lindner D: Inflammation
and fibrosis in murine models of heart failure. Basic Res Cardiol.
114:192019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Doroszko A, Dobrowolski P,
Radziwon-Balicka A and Skomro R: New insights into the role of
oxidative stress in onset of cardiovascular disease. Oxid Med Cell
Longev. 2018:95638312018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Z, Song Y, Liu L, Hou N, An X, Zhan D,
Li Y, Zhou L, Li P, Yu L, et al: miR-199a impairs autophagy and
induces cardiac hypertrophy through mTOR activation. Cell Death
Differ. 24:1205–1213. 2017. View Article : Google Scholar :
|
|
30
|
Simonson B, Subramanya V, Chan MC, Zhang
A, Franchino H, Ottaviano F, Mishra MK, Knight AC, Hunt D, Ghiran
I, et al: DDiT4L promotes autophagy and inhibits pathological
cardiac hypertrophy in response to stress. Sci Signal.
10:eaaf59672017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Obsil T and Obsilova V: Structural basis
of 14-3-3 protein functions. Semin Cell Dev Biol. 22:663–672. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu W, Yang B, Qiao Y, Zhou Q, He H and He
M: Kaempferol protects mitochondria and alleviates damages against
endotheliotoxicity induced by doxorubicin. Biomed Pharmacother.
126:1100402020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Diebold I, Hennigs JK, Miyagawa K, Li CG,
Nickel NP, Kaschwich M, Cao A, Wang L, Reddy S, Chen PI, et al:
BMPR2 preserves mitochondrial function and DNA during reoxygenation
to promote endothelial cell survival and reverse pulmonary
hypertension. Cell Metab. 21:596–608. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Caglayan E, Stauber B, Collins AR, Lyon
CJ, Yin F, Liu J, Rosenkranz S, Erdmann E, Peterson LE, Ross RS, et
al: Differential roles of cardiomyocyte and macrophage peroxisome
proliferator-activated receptor gamma in cardiac fibrosis.
Diabetes. 57:2470–2479. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Duan SZ, Ivashchenko CY, Russell MW,
Milstone DS and Mortensen RM: Cardiomyocyte-specific knockout and
agonist of peroxisome proliferator-activated receptor-gamma both
induce cardiac hypertrophy in mice. Circ Res. 97:372–379. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li T, Guo R, Zong Q and Ling G:
Application of molecular docking in elaborating molecular
mechanisms and interactions of supramolecular cyclodextrin.
Carbohydr Polym. 276:1186442022. View Article : Google Scholar
|
|
37
|
Oka T, Akazawa H, Naito AT and Komuro I:
Angiogenesis and cardiac hypertrophy: Maintenance of cardiac
function and causative roles in heart failure. Circ Res.
114:565–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang C, Liu J, Pan H, Yang X and Bian K:
Mitochondrial dysfunction induced by excessive ROS/RNS-metabolic
cardiovascular disease and traditional Chinese medicines
intervention. Zhongguo Zhong Yao Za Zhi. 36:2423–2428. 2011.In
Chinese. PubMed/NCBI
|
|
39
|
Liu BY, Li L, Liu GL, Ding W, Chang WG, Xu
T, Ji XY, Zheng XX, Zhang J and Wang JX: Baicalein attenuates
cardiac hypertrophy in mice via suppressing oxidative stress and
activating autophagy in cardiomyocytes. Acta Pharmacol Sin.
42:701–714. 2021. View Article : Google Scholar :
|
|
40
|
Gao M, Hu F, Hu M, Hu Y, Shi H, Zhao GJ,
Jian C, Ji YX, Zhang XJ, She ZG, et al: Sophoricoside ameliorates
cardiac hypertrophy by activating AMPK/mTORC1-mediated autophagy.
Biosci Rep. 40:BSR202006612020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li MH, Zhang YJ, Yu YH, Yang SH, Iqbal J,
Mi QY, Li B, Wang ZM, Mao WX, Xie HG and Chen SL: Berberine
improves pressure overload-induced cardiac hypertrophy and
dysfunction through enhanced autophagy. Eur J Pharmacol. 728:67–76.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Togliatto G, Lombardo G and Brizzi MF: The
future challenge of reactive oxygen species (ROS) in hypertension:
From bench to bed side. Int J Mol Sci. 18:19882017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dhalla AK, Hill MF and Singal PK: Role of
oxidative stress in transition of hypertrophy to heart failure. J
Am Coll Cardiol. 28:506–514. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Qin F, Lennon-Edwards S, Lancel S, Biolo
A, Siwik DA, Pimentel DR, Dorn GW, Kang YJ and Colucci WS:
Cardiac-specific overexpression of catalase identifies hydrogen
peroxide-dependent and -independent phases of myocardial remodeling
and prevents the progression to overt heart failure in
G(alpha)q-overexpressing transgenic mice. Circ Heart Fail.
3:306–313. 2010. View Article : Google Scholar
|
|
45
|
Liu C, Wu QQ, Cai ZL, Xie SY, Duan MX, Xie
QW, Yuan Y, Deng W and Tang QZ: Zingerone attenuates aortic
banding-induced cardiac remodelling via activating the eNOS/Nrf2
pathway. J Cell Mol Med. 23:6466–6478. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang Z and Klionsky DJ: Eaten alive: A
history of macroautophagy. Nat Cell Biol. 12:814–822. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hansen M, Rubinsztein DC and Walker DW:
Autophagy as a promoter of longevity: Insights from model
organisms. Nat Rev Mol Cell Biol. 19:579–593. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sun M, Ouzounian M, de Couto G, Chen M,
Yan R, Fukuoka M, Li G, Moon M, Liu Y, Gramolini A, et al:
Cathepsin-L ameliorates cardiac hypertrophy through activation of
the autophagy-lysosomal dependent protein processing pathways. J Am
Heart Assoc. 2:e0001912013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Drosatos K, Khan RS, Trent CM, Jiang H,
Son NH, Blaner WS, Homma S, Schulze PC and Goldberg IJ: Peroxisome
proliferator-activated receptor-γ activation prevents
sepsis-related cardiac dysfunction and mortality in mice. Circ
Heart Fail. 6:550–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Desvergne B and Wahli W: Peroxisome
proliferator-activated receptors: Nuclear control of metabolism.
Endocr Rev. 20:649–688. 1999.PubMed/NCBI
|
|
52
|
Herrmann JE, Heale J, Bieraugel M, Ramos
M, Fisher RL and Vickers AE: Isoproterenol effects evaluated in
heart slices of human and rat in comparison to rat heart in vivo.
Toxicol Appl Pharmacol. 274:302–312. 2014. View Article : Google Scholar
|
|
53
|
Yang J, Liu Y, Fan X, Li Z and Cheng Y: A
pathway and network review on beta-adrenoceptor signaling and beta
blockers in cardiac remodeling. Heart Fail Rev. 19:799–814. 2014.
View Article : Google Scholar
|
|
54
|
Yamaguchi O: Autophagy in the heart. Circ
J. 83:697–704. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
He H, Luo Y, Qiao Y, Zhang Z, Yin D, Yao
J, You J and He M: Curcumin attenuates doxorubicin-induced
cardiotoxicity via suppressing oxidative stress and preventing
mitochondrial dysfunction mediated by 14-3-3γ. Food Funct.
9:4404–4418. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen X, Peng X, Luo Y, You J, Yin D, Xu Q,
He H and He M: Quercetin protects cardiomyocytes against
doxorubicin-induced toxicity by suppressing oxidative stress and
improving mitochondrial function via 14-3-3γ. Toxicol Mech Methods.
29:344–354. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen H, Tan H, Wan J, Zeng Y, Wang J, Wang
H and Lu X: PPAR-γ signaling in nonalcoholic fatty liver disease:
Pathogenesis and therapeutic targets. Pharmacol Ther.
245:1083912023. View Article : Google Scholar
|
|
58
|
Botta M, Audano M, Sahebkar A, Sirtori CR,
Mitro N and Ruscica M: PPAR agonists and metabolic syndrome: An
established role? Int J Mol Sci. 19:11972018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
NiX X, Li XY, Wang Q and Hua J: Regulation
of peroxisome proliferator-activated receptor-gamma activity
affects the hepatic stellate cell activation and the progression of
NASH via TGF-β1/Smad signaling pathway. J Physiol Biochem.
77:35–45. 2021. View Article : Google Scholar
|
|
60
|
Deng W, Meng Z, Sun A and Yang Z:
Pioglitazone suppresses inflammation and fibrosis in nonalcoholic
fatty liver disease by down-regulating PDGF and TIMP-2: Evidence
from in vitro study. Cancer Biomark. 20:411–415. 2017. View Article : Google Scholar
|