Introduction
Esophageal cancer (EC) is among the top 10 leading
causes of cancer-related mortality worldwide, particularly among
Asian males (1). Of note, there
were 0.51 million new cases of EC and 0.44 million related deaths
worldwide in 2022, respectively (1). EC primarily manifests as two
subtypes: Esophageal squamous cell carcinoma (ESCC) and esophageal
adenocarcinoma (EAC) (2). ESCC
accounts for ~90% of global EC cases and incidence and mortality
rates associated with ESCC are expected to increase in 2030 and
2040 compared to 2020 (3). Major
risk factors for ESCC include alcohol consumption and tobacco use
(4). Although alcohol is often
identified as the primary risk factor, combining smoking with
alcohol consumption can have a synergistic effect, significantly
elevating the relative risk (4).
For example, the relative risk for patients who heavily use both
tobacco and alcohol is 35.4 for Caucasian males and 149.2 for males
of African origin, compared with that of non-smokers or to those
who do not consume alcohol of the same ethnicity (5). The pathogenesis appears to be
associated with inflammation of the squamous epithelium, leading to
dysplasia and malignant changes in situ (6). Observational studies indicate a
40-50% reduction in the risk of developing ESCC and EAC with
aspirin or non-steroidal anti-inflammatory drug treatment (7,8).
EC is a common malignant tumor worldwide, often presenting clinical
symptoms of dysphagia when the disease has advanced, causing
obstruction or metastasis in the esophageal cavity. Treatment of EC
remains contentious, primarily due to high incidence of distant
metastasis in ≥50% of patients (9). Achieving a favorable therapeutic
outcome is challenging, whether through surgery, radiation therapy
or chemotherapy. Following surgical intervention, the overall
5-year survival rate for patients with EC is <20% (9), suggesting that ESCC cells may
develop survival mechanisms to survive under conditions of stress,
such as hypoxia and use of anticancer drugs (10). Therefore, identification of novel
biomarkers and potential therapeutic targets for ESCC is key.
Neuregulins (NRGs) belong to the group of
transmembrane proteins encoded by four genes (NRG1-4). Among these,
NRG1 is upregulated in several types of cancer, such as prostate,
lung cancer, pancreas cancer, suggesting NRG1 is essential for cell
proliferation and differentiation. NRG1 encompasses six distinct
protein types (I-VI) and ≥31 isoforms (11-13). All NRG1 protein types share a
conserved EGF-like domain that distinguishes them from other EGF
family members. This EGF-like domain is sufficient to induce
biological activity (14-17).
The EGF-like domain of NRG1 can bind human epidermal growth factor
receptor (HER) 3, inducing HER2-HER3 heterodimer formation and
subsequent activation of the PI3K/AKT and RAS/RAF signaling
pathways (12). NRG1 expression
is increased in patients with head and neck squamous cell carcinoma
(HNSCC) (18). NRG1 expression
is induced in patients with breast cancer with diabetes,
potentially through epigenetic regulation of hyperglycemia on the
NRG1 enhancer region (19). NRG1
is upregulated in patients with gastric cancer (20). Although HER3 is not associated
with poor prognosis, NRG1 serves as an independent prognostic
marker in gastric cancer (20).
Moreover, NRG1 is a potential tumor promoter, either through being
a target of chromosome translocations or via activation by fusion
or promoter insertion in breast cancer (21-25). The Cancer Genome Atlas (TCGA,
cancer.gov/ccg/research/genome-sequencing/tcga)
and MSK-IMPACT (cbioportal.org/study/summary?id=heme_
msk_impact_2022) databases have identified NRG1 rearrangements with
novel fusion partners in multiple types of cancer, including
breast, head and neck, renal, lung, ovarian, pancreatic, prostate
and uterine cancer (25). To the
best of our knowledge, no monoclonal antibodies or inhibitors have
yet been developed to directly target NRG1. Current therapies
targeting NRG1 primarily focus on creating monoclonal antibodies
against its binding receptors, HER2 and HER3 (26). Afatinib, an irreversible pan-HER
inhibitor, inhibits cell proliferation and metastatic features of
ESCC cells (27). Based on the
oncogenesis role of NRG1 signaling, Kim et al (28) investigated the effects of
zenocutuzumab, a bispecific antibody for HER2 and HER3, in patients
with cancer that contain NRG1 gene fusions (trial no.
NCT02912949).
Autophagy is a key cellular survival mechanism
essential for various biological functions, including development,
maintaining cellular equilibrium and immune responses (29). The dysregulation of autophagy
leads to a range of diseases, such as cancer, neurodegenerative
diseases, cardiovascular disorder, diabetes, autoimmune disease and
aging (30). Autophagy involves
cellular self-digestion, allowing cells to break down damaged
organelles and misfolded proteins, particularly in response to
nutrients and oxygen-deprivation conditions, such as starvation and
hypoxia, which are common features in tumors prior to angiogenesis
(31). The role of autophagy in
cancer is complex (32).
Elevated levels of autophagy have been observed in tumor cells;
autophagy is also induced in tumor metastasis and during cancer
treatments (33). Autophagy
activators or inhibitors modulate epithelial-mesenchymal
transformation-associated proteins, inhibiting cancer cell
migration and invasion (34).
Moreover, targeting autophagy-associated proteins enhances cancer
suppressive effects of anti-cancer drugs (35-39). Conversely, sorafenib, a clinical
drug used in treatment of hepatocellular carcinoma, induces
excessive autophagy, triggering autophagic cell death in renal
cancer cells (38). These
results suggest that the role of autophagy in cancers depends on
cancer types, stages and treatment.
The principal regulator of autophagy is mTOR and its
activity is negatively modulated by downstream signals from PI3K
and AKT (40). mTOR is a central
regulatory factor governing cell proliferation and metabolism
(40). During periods of
nutrient scarcity, mTOR is inhibited, thereby activating autophagy
(41). In the context of
autophagy, two notable markers are ubiquitin-binding protein p62
and LC3-II (31). p62 functions
as an autophagic receptor, binding LC3-II to facilitate the
delivery of ubiquitinated proteins to autophagosome and lysosome
for degradation (42). As
autophagy is activated, there is an increase in p62 protein
degradation (43). However, the
role of NRG1 in autophagy and regulation in ESCC remains unclear.
Thus, the present study aimed to investigate the role of NRG1
signaling in modulating biological functions of cancer cells, such
as cell proliferation, mobility and survival, as well as the
potential association between NRG1 and its downstream signaling
components with clinical outcomes in patients diagnosed with
ESCC.
Materials and methods
Cell culture
The human EC cell lines CE48T/VGH (cat. no. 60165),
CE81T/VGH (cat. no. 60166) and CE146T/VGH (cat. no. 60167), derived
from well-differentiated ESCC, were obtained from the Bioresource
Collection and Research Center. DMEM (cat. no. 12100-046, Thermo
Fisher Scientific, Inc.) supplemented with 10% (v/v) FBS (cat. no.
SH30071.03, Cytiva), 100 U/ml penicillin, 100 mg/ml streptomycin
and non-essential amino acids (cat. no. 11140-050, Thermo Fisher
Scientific, Inc.) was used to culture ESCC cell lines as reported
previously (39,44) in a humidified atmosphere
containing 5% CO2 at 37°C.
Cell viability assay
Pooled small interfering (si)RNA was obtained from
Dharmacon, consisting of 3-4 individual siRNAs with chemical
modifications, which has been shown to achieve stable gene
silencing in vivo for ≥1 week (45). ESCC cells were seeded in a
96-well plate (cat. no. 655083, Greiner Bio-One International GmbH)
and transfected at 37°C for 72 h with 5 nM scrambled siRNA (cat.
no. D-001810-10-05) or a pool of siRNA targeting NRG1(cat. no.
L-004608-02-0005, both Dharmacon) using RNAiMAX (cat. no.
13778-150; Thermo Fisher Scientific, Inc.). Target sequences for
scramble siRNA were 5′-UGG UUU ACA UGU CGA CUA A-3′, 5′-UGG UUU ACA
UGU UGU GUG A-3′, 5′-UGG UUU ACA UGU UUU CUG A-3′ and 5′-UGG UUU
ACA UGU UUU CCU A-3′. The target sequences for siNRG1 were 5′-UUU
CAA ACC CCU CGA GAU A-3′, 5′-UUG UAA AAU GUG CGG AGA A-3′, 5′-GGG
GAG UGC UUC AUG GUG A-3′ and 5′-ACAU CCA CCA CUG GGA CAA-3′.
CellTiter-Glo (cat. no. G7573, Promega Corporation) was added to
the cells and luminescence was quantified using a Fluoroskan Ascent
FL reader (Thermo Fisher Scientific, Inc.). ATP levels were
considered to indicate viability. Furthermore, cell viability was
monitored with an impedance-based instrument system (iCELLigence,
ACEA Biosciences, Inc.) for live cells. In brief, ESCC cells
(2×104 cells/well) were transfected with 5 nM scrambled
siRNA or pooled siNRG1 in electronic plates (E-Plates L8, ACEA
Biosciences, Inc.) containing 400 µl DMEM with 10% FBS. The
cellular impedance was measured every 15 min for 96 h.
Alternatively, cells were fixed in 70% ethanol at -20°C overnight
and stained with propidium iodide (50 µg/ml, MilliporeSigma)
at room temperature in the dark for 30 min. The stained cells were
analyzed and quantified with NovoExpress flow cytometry software
(version 1.6.2) in an NovoCyte benchtop flow cytometer system
(Agilent Technologies, Inc.; version 1.6.2).
Western blot analysis
Transfected cells were lysed in RIPA buffer [1%
NP40, 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.25% sodium
deoxycholate, 1% SDS, protease inhibitor cocktail and phosphatase
inhibitor]. The proteins were quantified with bicinchoninic acid
assay and separated by 10-12% SDS-PAGE (20 µg of protein
loaded per lane) and transferred onto nitrocellulose membranes. The
membranes were blocked with 5% BSA (cat. no. A5611; Sigma-Aldrich;
Merck KGaA) at room temperature for 3 h and incubated with
1,000-fold diluted primary antibodies against NRG1 (cat. no.
ab191139, Abcam), phosphorylated (p-)AKT (cat. no. 4060), AKT (cat.
no. 4691), p-cellular rapidly accelerated fibrosarcoma (p-cRAF;
cat. no. 9427), cRAF (cat. no. 53745), β-actin (cat. no. 3700; all
Cell Signaling Technology, Inc.), LC3B (cat. no. ARG55799) and p62
(cat. no. ARG55040; both Arigo Biolaboratories Corp.) at 4°C
overnight. The proteins were probed with 1:5,000 HRP-conjugated
secondary antibody (cat. nos. sc-2004 and sc-2005, Santa Cruz
Biotechnology, Inc.) at room temperature for 1 h and the proteins
on the membranes were visualized by enhanced chemiluminescent (ECL)
kit (TB-ECL-250 ECL, TOPBIO) using Multi-Function Gel Image System
(cat. no. MGIS-21-C2-6M, TOPBIO). The protein levels were
quantified using ImageJ software (National Institutes of
Health).
Autophagy flux assays
LC3B-II turnover in cells were calculated with
Western blot. Briefly, the cells were treated with or without
chloroquine (CQ, 20 µM) at 37°C incubator for 3 h prior
harvesting. The proteins were extracted and used to measure
autophagic flux as previously reported (46). Alternatively, autophagosome dye
(DAP, 0.1 µM) or autolysosome dye (DAL, 0.5 µM) were
used to stain cells in the culture medium at 37°C for 30 min to
monitor autophagy activity, respectively, as previously reported
(47). The autophagosomes and
autolysosomes were observed by confocal microscopy.
Clonogenic assay
The cells were plated in 12-well plates at a density
of 5×103 cells/well and transfected with scramble siRNA
or siRNA against NRG1. Subsequently, cells were cultured in DMEM
(cat. no. 12100-046, Thermo Fisher Scientific, Inc.) supplemented
with 10% (v/v) FBS (cat. no. SH30071.03, Cytiva), 100 U/ml
penicillin, 100 mg/ml streptomycin and non-essential amino acids
(cat. no. 11140-050, Thermo Fisher Scientific, Inc.) at 37°C, which
was refreshed every 3 days for 2 weeks. The cell colonies were
fixed with 2% paraformaldehyde at room temperature for 15 min and
stained with 20% ethanol containing 0.25% crystal violet (cat. no.
C0775, Merck KGaA.) at room temperature for 20 min. The stained
cells were washed with PBS three times and colonies >1 mm in
diameter were counted and quantified with Image J software (version
1.54; National Institutes of Health) in ≥3 independent
experiments.
Clinical samples and reverse
transcription-quantitative PCR
Human ESCC and normal adjacent (distance, >2 cm)
tissue was obtained from 120 patients who underwent esophageal
resection at the Department of Surgery of Kaohsiung Veterans
General Hospital (Kaohsiung, Taiwan) between October 2002 and
October 2018. The age range of participants was 35 and 75 years
old. The sex distribution was predominantly male, with over 90% of
patients being male. Patients who had received neoadjuvant
treatment were excluded from the study. Only those who underwent
esophagectomy with gastric conduit reconstruction with/without
adjuvant treatment were included. The present study was approved by
the institutional review board of Kaohsiung Veterans General
Hospital (approval nos. VGHKS 95-CT3-21 and VGHKS 15-CT12-10).
Written informed consent was obtained from all subjects. Total RNA
from the 120 paired tissues was extracted using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.). RNA was precipitated
using 0.5 ml isopropanol. The concentration, purity and quantity of
total RNA were assessed using a Nanodrop 1000 spectrophotometer
(Nanodrop Technologies, Inc.). A total of 2 µg RNA was
extracted by RNA extraction kit (Invitrogen; Thermo Fisher
Scientific, Inc.), then reverse-transcribed with oligo-dT primers
and SuperScript III Reverse Transcriptase according to the
manufacturer's instructions (Invitrogen; Thermo Fisher Scientific,
Inc.) at 50°C for 50 min, followed by enzyme inactivation at 85°C
for 5 min. The resulting cDNA was used for quantitative PCR
analysis with gene-specific primers as follows: NRG1 forward,
5′-CCA CTG GGA CAA GCC ATC TT-3′ and reverse, 5′-TTC ACC ATG AAG
CAC TCC CC-3′ and β-actin forward, 5-′AGC GAG CAT CCC CCA AAG TT-3′
and reverse, 5′-GGG CAC GAA GGC TCA TCA TT-3′. Thermocycling
conditions were as follows: Initial denaturation at 3 min at 95°C,
followed by 40 cycles of 15 sec at 95°C and 1 min at 60°C. The gene
expression was detected using SYBR Green I assay (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The relative abundance
of NRG1 mRNA was assessed using the StepOnePlus system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The 2−ΔΔCq
method was used for quantification of relative changes in gene
expression (48).
Invasion and migration assay
For the invasion assay, 3×105 ESCC cells
were seeded into the upper chamber, which was pre-coated with 50
µl 0.5% Matrigel in DMEM containing 1% FBS at 37°C for 30
min (47,48). The lower chamber was supplemented
with 500 µl DMEM containing 10% FBS. Cells were allowed to
pass through the Matrigel-coated chamber at 37°C for 24 h, followed
by fixation in 2% paraformaldehyde at room temperature for 15 min
and staining with 0.25% crystal violet at room temperature for 30
min. The images were captured using a light inverted microscope
(magnification, ×10). The migration assay was conducted using
Culture-Insert 2 Wells (Ibidi) designed for 24-well plates. A total
of 1×106 cells were seeded with 70 µl DMEM
containing 10% FBS at 37°C for overnight, after which the culture
insert was removed for 24 h. The cells were fixed with 2%
paraformaldehyde at room temperature for 15 min to measure the open
area and images were captured using a light inverted microscope
(magnification, ×10).
Tumor sphere viability
ESCC cells were plated at a density of
4×103 cells/well in an ultra-low attachment 96-well
plate (Costar®; Corning, Inc.) using DMEM with 10% FBS
and cultivated at 37°C for 7 days to promote formation of spheroid
cells. Viability of these spheroid cells was assessed by staining
with Calcein AM (1 µM) and ethidium homodimer-1 (EthD-1, 2
µM) using the LIVE/DEAD® Viability/Cytotoxicity
kit (Thermo Fisher Scientific, Inc.) at 37°C for 30 min.
Fluorescence microscopy (magnification, ×10) was used to capture
images of live (green) and dead (red) spheroid cells, which were
quantitatively analyzed with a Fluoroskan Ascent FL reader (Thermo
Fisher Scientific, Inc.) at excitation and emission wavelengths of
485 and 530 nm for Calcein AM and 645 nm for EthD-1,
respectively.
Immunohistochemistry (IHC)
Tissue microarray (TMA; cat no. ES701) purchased
from SuperBiochips was analyzed through IHC staining of protein of
the TMA blocks (50,51). The blocks were immersed in sodium
citrate buffer (10 mM, pH 6.0), boiled at 125°C in a pressure
boiler for 10 min for antigen retrieval and then blocked with 3%
hydrogen peroxide at room temperature for 30 min. The tissue
sections were stained with anti-NRG1 (1:100, cat. no. ab191139,
Abcam) at 4°C overnight, Following the TBS-T (0.5% Tween-20)
washes, staining was carried out with 1:3000 diluted secondary
antibody conjugated with HRP) polymer at room temperature for 30
min using the Epredia UltraVision™ Quanto Detection System
(TA-125-QHDX, Thermo Fisher Scientific, Inc.). The washed slides
were stained with hematoxylin (Sigma-Aldrich; Merck KGaA) at room
temperature for 5 sec. The slides were allowed to dry, then mounted
with a coverslip, subsequently examined under a light microscope at
the appropriate magnification (×20). Intensity and percentage of
NRG1 staining was scored. The score was calculated as the total
value of staining intensity (0, negative; 1, weak expression; and
2, moderate expression) and percentage of cells staining at each
intensity level [0 (<5%), 1 (5-25%), 2 (26-50%), 3 (51-75%), and
4 (>75%)] (49).
Statistical analysis
The TCGA (The Cancer Genome Atlas) dataset for gene
expression levels and clinical outcome of ESCC patients were
downloaded from Xena platform (xenabrowser.net/). The gene and protein levels of NRG1
in tumor and adjacent normal tissue were evaluated by SPSS
Statistics 28.0 (IBM Corporation) using Wilcoxon signed-rank test.
The cutoff value is for high and low levels of NRG1 protein
(3) depends on the receiver
operating characteristic (ROC) curve. The cutoff values of NRG1
gene expression levels were 10.8322 for overall survival,
progression-free interval, disease-specific survival, and 9.15815
for disease-free interval. To assess the association between NRG1
and clinicopathological factors, Student's unpaired t-test,
Mann-Whitney U test and Kruskal-Wallis were applied. Cumulative
survival rates were assessed using Kaplan-Meier curves, with
significance determined using log-rank test. The association
between protein levels and survival outcomes, including overall,
progression-free interval, disease-specific and disease-free
interval survival, was adjusted for cell differentiation (moderate
+ poor vs. well) and American Joint Committee on Cancer
pathological stage (stage III + IV vs. stage I + II) using a
multivariate Cox regression model. P<0.05 (two-sided) was
considered to indicate a statistically significant difference. For
cell culture experiments, results were derived from ≥3 independent
replicates, with significance assessed using a non-parametric
two-tailed Student's unpaired t-test. One-way ANOVA followed by
Tukey's post hoc test was used for comparisons between >2
groups. The data were analyzed by GraphPad Prism 5.0 (Dotmatics).
Data are expressed as the mean ± SD.
Results
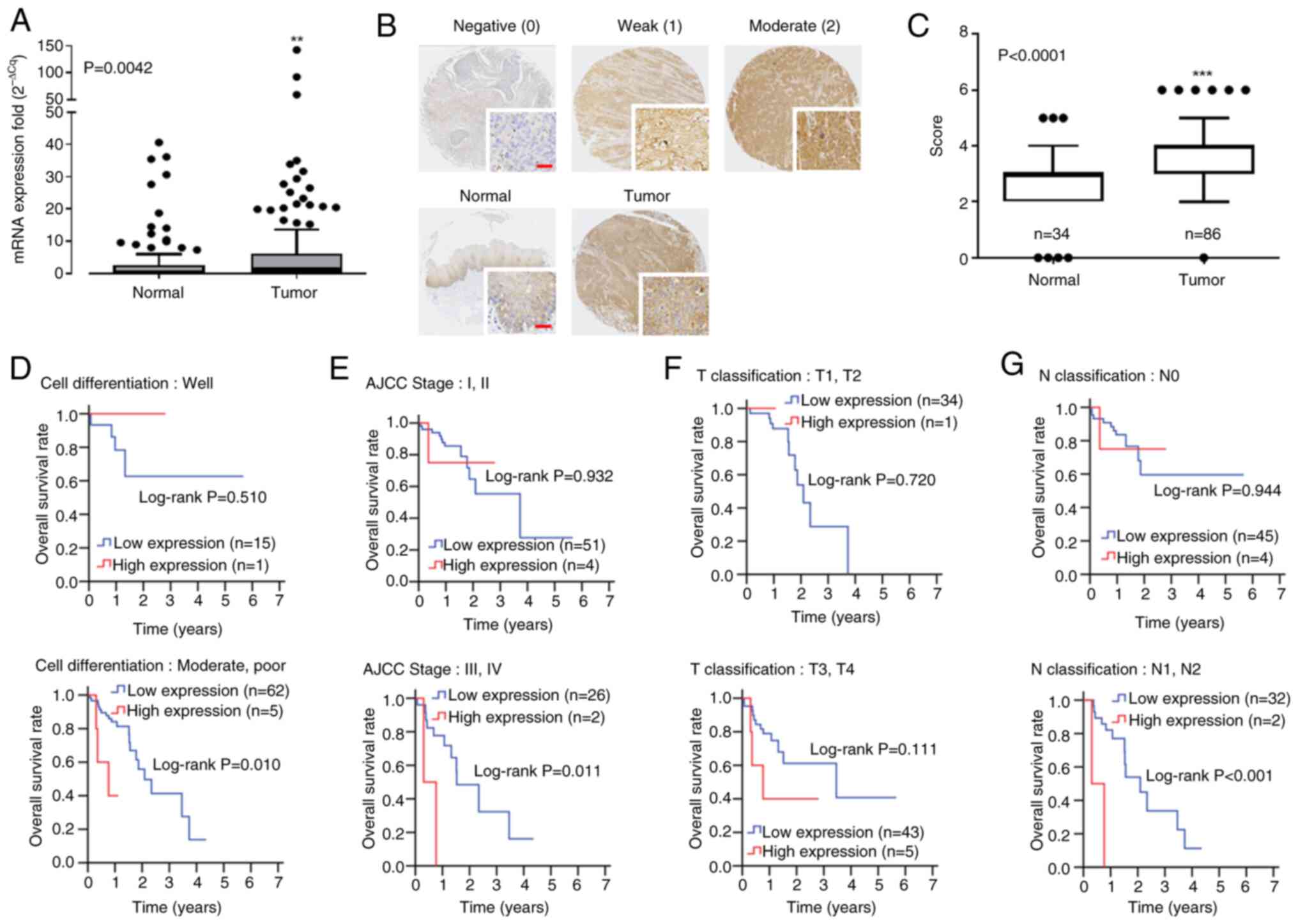
NRG1 expression is elevated in tumor
tissue and associated with poor prognosis of patients with
ESCC
To investigate the potential association between
NRG1 and patient survival, analysis of NRG1 mRNA expression was
conducted in individuals with ESCC. Quantitative PCR was employed
to assess NRG1 expression in 120 paired primary ESCC and adjacent
normal tissue samples from Kaohsiung Veterans General Hospital.
There was significantly elevated NRG1 gene expression in tumor
tissue of patients with ESCC compared with adjacent normal tissue
(9.36±24.90 vs. 3.27±7.32; Table
IA; Fig. 1A). To determine
NRG1 expression at the protein level, IHC was employed (Fig. 1B). NRG1 protein levels were
significantly higher in ESCC compared with corresponding
tumor-adjacent normal tissue cores (Fig. 1C). NRG1 levels were higher in
tumor compared with those in adjacent normal tissue (Table IB). However, the association
between NRG1 protein expression and overall and disease-specific
survival was not significant (Tables SI and SII). To monitor the
association between clinicopathological characteristics and NRG1,
TCGA database was used to analyze the effect of high and low
expression of NRG1 using the receiver operating characteristics
curve (Fig. 1D-G). Higher
expression of NRG1 was associated with unfavorable overall survival
of patients with poor differentiation (Fig. 1D), advanced stage (AJCC stage III
and IV; Fig. 1E) and lymph node
invasion (Fig. 1G). Following
adjustments for cell differentiation and AJCC pathological stage
for adjusted hazard ratio (AHR) with multiple Cox regression
analysis, mortality risk was significantly higher in patients with
high expression of NRG1, particularly in male patients (AHR, 4.98;
95% CI, 1.34-18.46, Table II)
or patients with poor differentiation of ESCC (AHR, 5.03; 95% CI,
1.37-18.54), advanced stage of disease (stage III and IV; AHR,
7.00, 95% CI, 1.32-37.17) and poor nodal status (N1-2 vs. N0; AHR,
12.02; 95% CI, 1.99-72.60). Therefore, it was hypothesized that
NRG1 functions as an oncogene.
 | Table INeuregulin-1 expression in tumor and
adjacent normal tissue from patients with esophageal squamous cell
carcinoma. |
Table I
Neuregulin-1 expression in tumor and
adjacent normal tissue from patients with esophageal squamous cell
carcinoma.
A, mRNA
|
|---|
Adjacent normal
(n=120)
| Tumor (n=120)
| P-value |
|---|
| Mean
expression | Median
expression | Mean
expression | Median
expression |
|---|
| 3.27±7.32 | 0.41 | 9.36±24.90 | 1.46 | 0.004 |
|
| B.
Proteina |
|
Adjacent normal
(n=120)
| Tumor (n=120)
| P-value |
| Mean
expression | Median
expression | Mean
expression | Median
expression |
|
| 2.68±1.32 | 3.00 | 3.66±1.14 | 4.00 | <0.001 |
 | Table IIImpact of NRG1 expression on overall
survival of patients with esophageal squamous cell carcinoma. |
Table II
Impact of NRG1 expression on overall
survival of patients with esophageal squamous cell carcinoma.
| Variable | NRG1
expression | n (%) | CHR (95% CI) | P-valuea | AHR (95% CI) | P-valueb |
|---|
| Sex | | | | | | |
| Female | Low | 10 (90.9) | 1.00 | | 1.00 | |
| High | 1 (9.1) | 0.04
(0.00-4.58×1011) | 0.837 | 1.00
(0.00-2.67×1030) | 1.000c,d |
| Male | Low | 67 (93.1) | 1.00 | | 1.00 | |
| High | 5 (6.9) | 4.29
(1.20-15.32) | 0.025 | 4.98
(1.34-18.46) | 0.016c,d |
| Age, years | | | | | | |
| ≤60 | Low | 48 (92.3) | 1.00 | | 1.00 | |
| High | 4 (7.7) | 2.33
(0.52-10.46) | 0.270 | 3.59
(0.73-17.68) | 0.117c,d |
| >60 | Low | 29 (93.5) | 1.00 | | 1.00 | |
| High | 2 (6.5) | 2.63
(0.31-22.00) | 0.373 | 3.64
(0.37-35.43) | 0.266c,d |
| Cell
differentiation | | | | | | |
| Well | Low | 15 (93.8) | 1.00 | | 1.00 | |
| High | 1 (6.3) | 0.41
(0.00-1.01×105) | 0.671 | Incalculable | 0.995d |
| Moderate,
poor | Low | 62 (92.5) | 1.00 | | 1.00 | |
| High | 5 (7.5) | 4.73
(1.29-17.30) | 0.019 | 5.03
(1.37-18.54) | 0.015d |
| AJCC pathological
stage | | | | | | |
| I, II | Low | 51 (92.7) | 1.00 | | 1.00 | |
| High | 4 (7.3) | 1.09
(0.14-8.51) | 0.932 | 1.25
(0.16-10.01) | 0.836c |
| III, IV | Low | 26 (92.9) | 1.00 | | 1.00 | |
| High | 2 (7.1) | 6.49
(1.25-33.78) | 0.026 | 7.00
(1.32-37.17) | 0.022c |
| T
classification | | | | | | |
| T1, T2 | Low | 34 (97.1) | 1.00 | | 1.00 | |
| High | 1 (2.9) | 0.05
(0.00-3.98×109) | 0.812 | Incalculable | 0.992c,e |
| T3, T4 | Low | 43 (89.6) | 1.00 | | 1.00 | |
| High | 5 (10.4) | 2.71
(0.76-9.76) | 0.126 | 3.28
(0.87-12.38) | 0.079c,e |
| N
classification | | | | | | |
| N0 | Low | 45 (91.8) | 1.00 | | 1.00 | |
| High | 4 (8.2) | 1.08
(0.14-8.43) | 0.944 | 1.10
(0.13-8.99) | 0.930c,f |
| N1, N2 | Low | 32 (94.1) | 1.00 | | 1.00 | |
| High | 2 (5.9) | 11.62
(2.10-64.17) | 0.005 | 12.02
(1.99-72.60) | 0.007c,f |
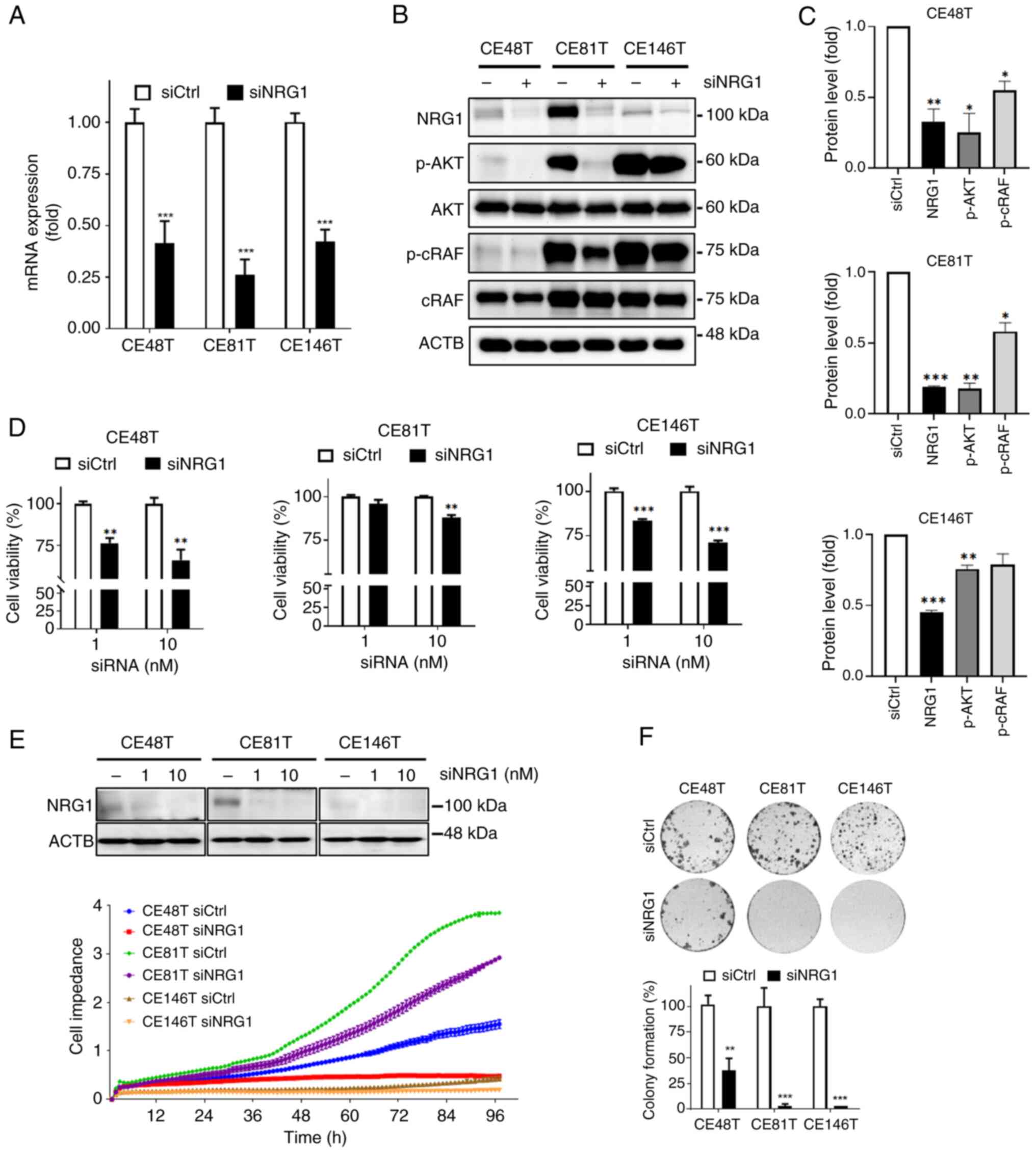
Silencing NRG1 decreases cancer cell
proliferation
To determine the role of NRG1 in ESCC, siRNA was
used to knock down NRG1 in CE48T, CE81T and CE146T cells. To
minimize off-target effects of siRNA, a siRNA pool was used. NRG1
mRNA levels exhibited a decrease in the presence of siNRG1
(Fig. 2A), accompanied by a
corresponding attenuation in protein expression (Fig. 2B and C). The phosphorylation
levels of downstream regulators in NRG1/HER signaling, AKT and
cRAF, were consistently decreased (Fig. 2B and C). To validate the effect
of siNRG1 on cell viability, cellular ATP levels were used.
Viability of ESCC cells decreased with increasing concentrations of
siNRG1 (Fig. 2D). The use of 10
nM siRNA against NRG1 significantly suppressed viability of three
ESCC cell lines, whereas 1 nM siRNA significantly deceased cell
viability only in CE48T and CE146T (Fig. 2D). ESCC cell lines were cultured
in electronic plates to monitor cell viability, with impedance
plots showing cell indexes (CI), revealing a significant decrease
in CI in NRG1-silencing compared with control cells (Fig. 2E). Colony formation assay was
used to evaluate the effect of siNRG1 on anchorage-independent
proliferation of ESCC cells (51). There was a significant decrease
in number of colonies of ESCC cells (CE48T, CE81T, and CE146T)
following 1-week treatment with siNRG1 (10 nM; Fig. 2F), suggesting a decrease in
proliferative capacity. Thus, siNRG1 exhibited cytotoxicity against
ESCC cells, effectively decreasing cell viability and
proliferation.
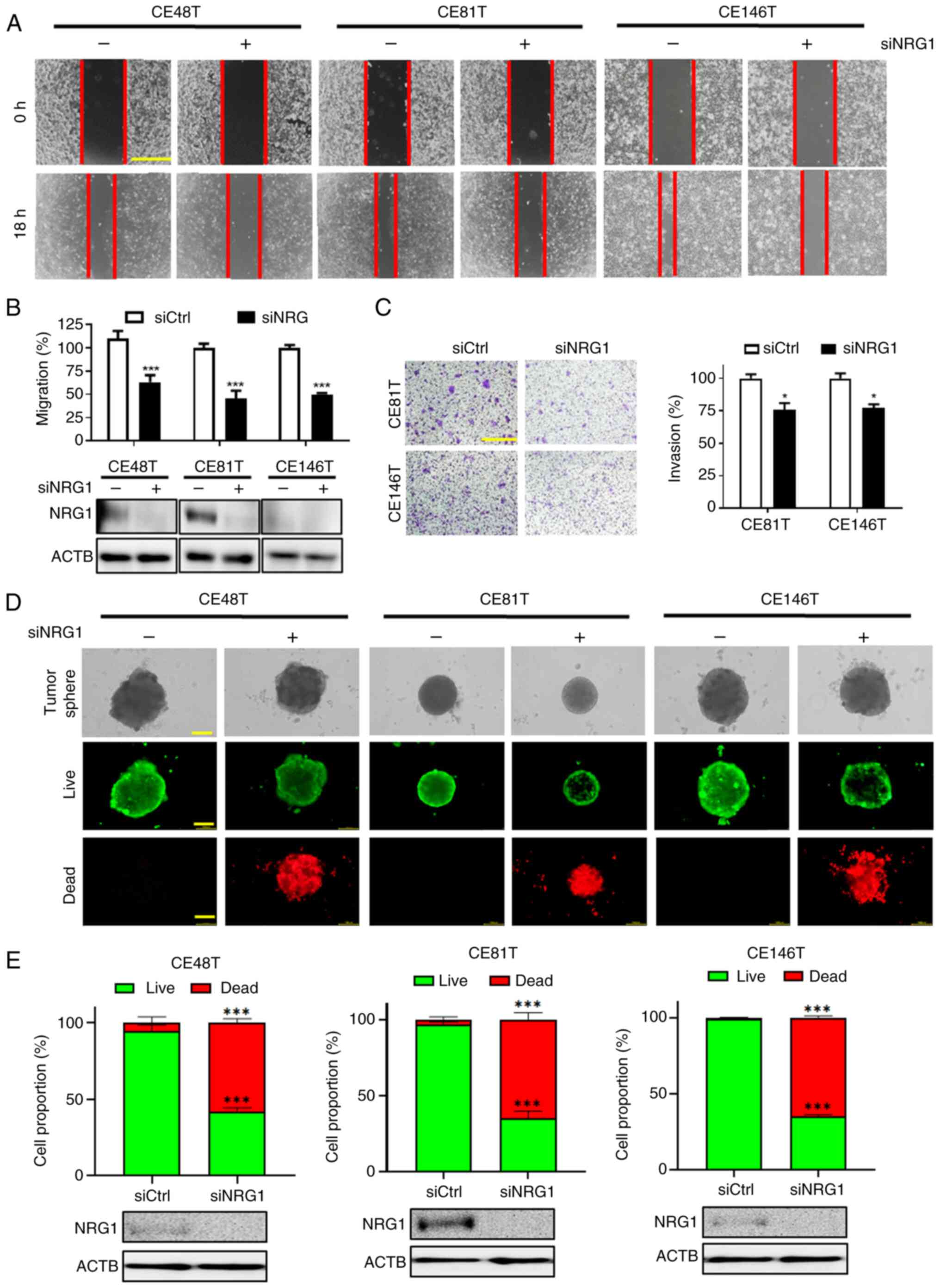
Silencing NRG1 decreases cancer cell
mobility and viability of tumor sphere
The present study explored the effect of NRG1 on
cell migration and invasion. Silencing NRG1 with 1 nM siRNA
inhibited migration in all three ESCC cell lines (Fig. 3A and B). Additionally, silencing
NRG1 significantly diminished the invasive ability of CE81T and
CE146T cells (Fig. 3C).
Furthermore, the sphere is a three-dimensional structure that
possesses fewer nutrients and oxygen supply within its core
compared with surface cells (52). This characteristic mimics the
growth conditions of cancer cells within a tumor. Sphere formation
assay indicated that transfection with siNRG1 led to smaller sphere
volumes of ESCC cells compared with control (Fig. 3D). Live (green)/dead (red)
staining was employed to determine whether silencing NRG1 affected
the ratio of live and dead cells within tumor sphere. The results
demonstrated a significant decrease in number of live and an
increase in that of dead cells following transfection with siNRG1
(Fig. 3E). Therefore, siNRG1
inhibited sphere formation ability of ESCC cells and increased the
proportion of dead cells.
Co-expression of NRG1 and its signaling
molecules are associated with poor prognosis of ESCC
The expression of NRG1 and its downstream signaling
molecules, cRAF and AKT, was associated with enhanced migratory and
invasive capabilities in ESCC cells, which are associated with
metastasis and cancer aggressiveness (54). The present study examined the
prognostic value of NRG1, cRAF and AKT expression in patients with
ESCC (Fig. 4). Kaplan-Meier
survival analysis demonstrated that patients with high
co-expression of NRG1 and cRAF had significantly shorter
progression-free interval survival (Fig. 4B), although no significant
differences were observed in overall (Fig. 4A), disease-specific or
disease-free survival (Fig. 4C and
D) compared with those with low NRG1 and cRAF co-expression.
Additionally, high co-expression of NRG1 and AKT was significantly
associated with worse overall survival (Fig. 4F) and shorter disease-free
interval survival (Fig. 4H) but
did not show a significant association with progression-free
interval (Fig. 4E) or
disease-specific survival (Fig.
4G).
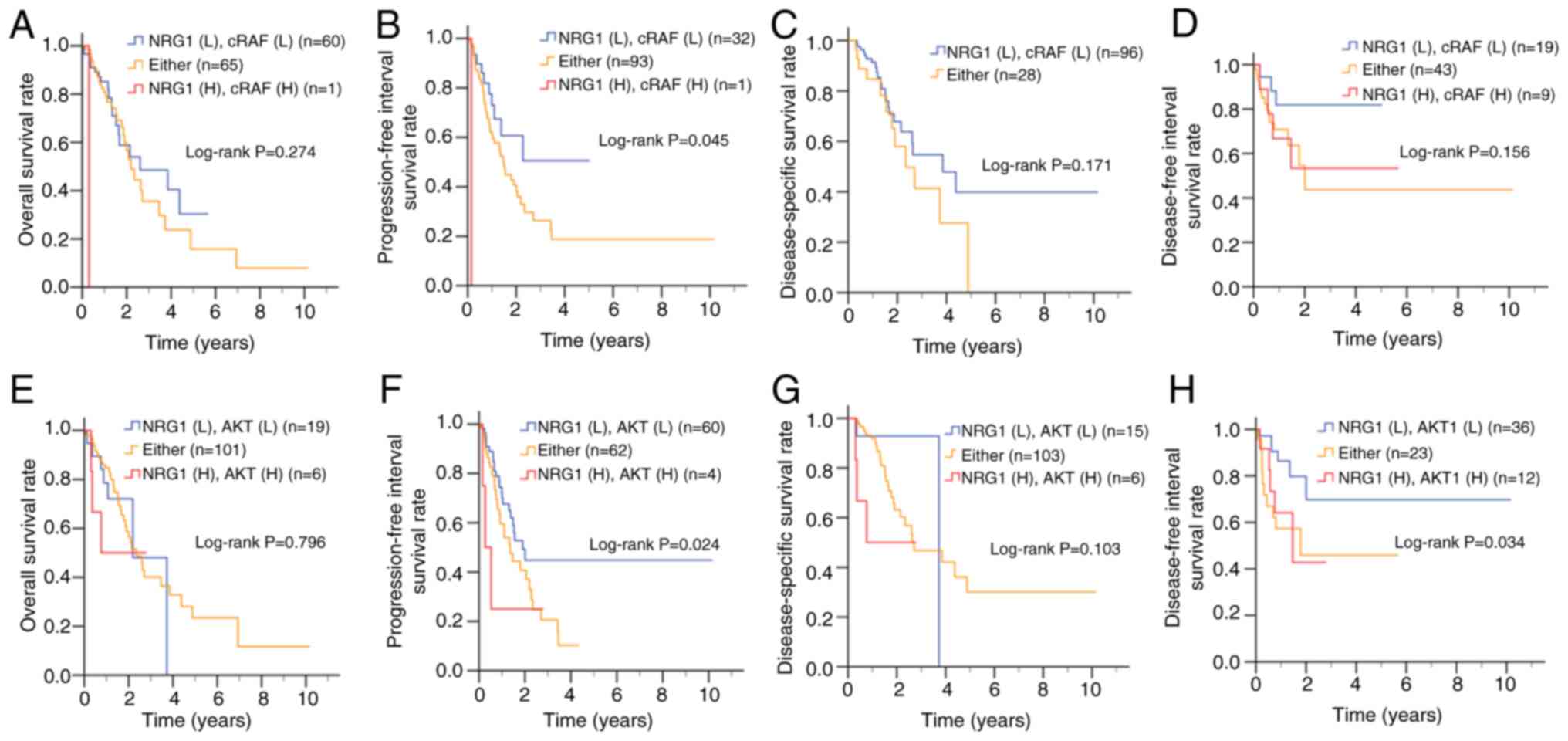 | Figure 4Clinical association between gene
expression of NRG1, cRAF and AKT in patients with ESCC. Based on an
ESCC dataset from The Cancer Genome Atlas, Kaplan-Meier plots were
used to analyze the association between NRG1 and cRAF expression of
patients with ESCC and (A) overall, (B) progression-free interval,
(C) disease-specific and (D) disease-free interval survival.
Association between NRG1 and AKT gene expression and (E) overall
(F) progression-free interval, (G) disease-specific and (H)
disease-free interval survival. NRG, neuregulin-1; ESCC, esophageal
squamous cell carcinoma; cRAF, cellular rapidly accelerated
fibrosarcoma; L, low; H, high expression; Either, NRG1(H) cRAF(L)
or NRG1(L)/cRAF(H); yrs, years. |
To account for variations in cell differentiation
and AJCC pathological stage, multivariate Cox proportional hazard
model was used to evaluate association between survival outcomes
and NRG1 expression alone or in combination with cRAF expression
(Table III). ESCC patients
with high co-expression of NRG1 and cRAF exhibited a markedly
increased risk of mortality compared with those with low
co-expression. These patients had significantly higher risks for
both overall (AHR, 44.72, CI, 4.54-440.89, Table III) and progression-free
interval survival (AHR, 93.44, CI,7.93-1101.57). Although patients
with high expression levels of NRG1 and AKT showed poorer outcomes
in progression-free interval, disease-specific survival, and
disease-free survival, these associations were not significant
(Table IV). These results
highlight the complex nature of the effects of NRG1on the ESCC
prognosis.
 | Table IIIEffect of NRG1/cRAF co-expression on
survival of patients with esophageal squamous cell carcinoma. |
Table III
Effect of NRG1/cRAF co-expression on
survival of patients with esophageal squamous cell carcinoma.
A, Overall survival
|
|---|
| Variable | Expression | n (%) | CHR (95% CI) | P-value | AHR (95% CI) | P-value |
|---|
| NRG1 | Low | 120 (95.2) | 1.00 | | 1.00 | |
| High | 6 (4.8) | 2.01
(0.62-6.52) | 0.244a | 3.40
(0.99-11.71) | 0.052b |
| cRAF | Low | 65 (52.4) | 1.00 | | 1.00 | |
| High | 61 (47.6) | 1.22
(0.69-2.15) | 0.501a | 1.07
(0.60-1.91) | 0.809b |
| NRG1 (L), cRAF
(L) | - | 60 (47.6) | 1.00 | | 1.00 | |
| Either | - | 65 (51.6) | 1.17
(0.66-2.07) | 0.591a | 1.23
(0.69-2.20) | 0.489c |
| NRG1 (H), cRAF
(H) | - | 1 (0.8) | 39.89
(4.15-383.56) | 0.001a | 44.72
(4.54-440.89) | 0.001c |
|
| B, Progression-free
interval survival |
|
| Variable | ROC | n (%) | CHR (95% CI) | P-value | AHR (95% CI) | P-value |
|
| NRG1 | Low | 120 (95.2) | 1.00 | | 1.00 | |
| High | 6 (4.8) | 2.93
(1.16-7.38) | 0.023a | 5.11
(1.90-13.72) | 0.001b |
| cRAF | Low | 37 (29.4) | 1.00 | | 1.00 | |
| High | 89 (70.6) | 1.38
(0.76-2.50) | 0.297a | 1.32
(0.73-2.41) | 0.360b |
| NRG1 (L), cRAF
(L) | - | 32 (25.4) | 1.00 | | 1.00 | |
| Either | - | 93 (73.8) | 1.61
(0.84-3.09) | 0.155a | 1.77
(0.90-3.49) | 0.101c |
| NRG1 (H), cRAF
(H) | - | 1 (0.8) | 59.49
(5.39-656.09) | 0.001a | 93.44
(7.93-1101.57) | <0.001c |
|
| C, Disease-specific
survival |
|
| Variable | ROC | n (%) | CHR (95% CI) | P-value | AHR (95% CI) | P-value |
|
| NRG1 | Low | 118 (95.2) | 1.00 | | 1.00 | |
| High | 6 (4.8) | 3.17
(0.96-10.53) | 0.059a | 7.76
(2.04-29.53) | 0.003b |
| cRAF | Low | 102 (82.3) | 1.00 | | 1.00 | |
| High | 22 (17.7) | 1.27
(0.59-2.73) | 0.542a | 1.31
(0.60-2.85) | 0.497b |
| NRG1 (L), cRAF
(L) | - | 96 (77.4) | 1.00 | | 1.00 | |
| Either | - | 28 (22.6) | 1.63
(0.80-3.31) | 0.175a | 1.63
(0.80-3.31) | 0.175c |
| NRG1 (H), cRAF
(H) | - | 0 (0.0) | - | - | - | - |
|
| D, Disease-free
interval survival |
|
| Variable | ROC | n (%) | CHR (95% CI) | P-value | AHR (95% CI) | P-value |
|
| NRG1 | Low | 52 (73.2) | 1.00 | | 1.00 | |
| High | 19 (26.8) | 1.29
(0.52-3.20) | 0.580a | 1.97
(0.75-5.16) | 0.169b |
| cRAF | Low | 29 (40.8) | 1.00 | | 1.00 | |
| High | 42 (59.2) | 1.94
(0.75-4.99) | 0.172a | 2.03
(0.79-5.26) | 0.144b |
| NRG1 (L), cRAF
(L) | - | 19 (26.8) | 1.00 | | 1.00 | |
| Either | - | 43 (60.6) | 1.74
(0.70-4.34) | 0.234a | 2.72
(0.78-9.49) | 0.117c |
| NRG1 (H), cRAF
(H) | - | 9 (12.7) | 1.30
(0.44-3.89) | 0.636a | 2.70
(0.60-12.10) | 0.194c |
 | Table IVEffect of NRG1 and AKT co-expression
on patients with esophageal squamous cell carcinoma. |
Table IV
Effect of NRG1 and AKT co-expression
on patients with esophageal squamous cell carcinoma.
A, Overall survival
|
|---|
| Variable | Expression | n (%) | CHR (95% CI) | P-value | AHR (95% CI) | P-value |
|---|
| NRG1 | Low | 120 (95.2) | 1.00 | | 1.00 | |
| High | 6 (4.8) | 2.01
(0.62-6.52) | 0.244a | 3.40
(0.99-11.71) | 0.052b |
| AKT | Low | 19 (15.1) | 1.00 | | 1.00 | |
| High | 107 (84.9) | 0.88
(0.39-1.96) | 0.746a | 0.75
(0.33-1.71) | 0.500b |
| NRG1 (L), AKT
(L) | - | 19 (15.1) | 1.00 | | 1.00 | |
| Either | - | 101 (80.2) | 0.73
(0.36-1.48) | 0.387a | 0.84
(0.37-1.89) | 0.671c |
| NRG1 (H), AKT
(H) | - | 6 (4.8) | 2.01
(0.62-6.52) | 0.244a | 1.74
(0.45-6.73) | 0.426c |
|
| B, Progression-free
interval survival |
|
| Variable | ROC | n (%) | CHR (95% CI) | P-value | AHR (95% CI) | P-value |
|
| NRG1 | Low | 120 (95.2) | 1.00 | | 1.00 | |
| High | 6 (4.8) | 2.93
(1.16-7.38) | 0.023a | 5.11
(1.90-13.72) | 0.001b |
| AKT | Low | 62 (49.2) | 1.00 | | 1.00 | |
| High | 64 (50.8) | 1.53
(0.92-2.55) | 0.103a | 1.62
(0.97-2.71) | 0.066b |
| NRG1 (L), AKT
(L) | - | 60 (47.6) | 1.00 | | 1.00 | |
| Either | - | 62 (49.2) | 1.53
(0.92-2.55) | 0.101a | 1.66
(0.98-2.81) | 0.061c |
| NRG1 (H), AKT
(H) | - | 4 (3.2) | 2.18
(0.68-7.03) | 0.192a | 2.87
(0.85-9.66) | 0.088c |
|
| C, Disease-specific
survival |
|
| Variable | ROC | n (%) | CHR (95% CI) | P-value | AHR (95% CI) | P-value |
|
| NRG1 | Low | 118 (95.2) | 1.00 | | 1.00 | |
| High | 6 (4.8) | 3.17
(0.96-10.53) | 0.059a | 7.76
(2.04-29.53) | 0.003b |
| AKT | Low | 15 (12.1) | 1.00 | | 1.00 | |
| High | 109 (87.9) | 1.77
(0.42-7.42) | 0.436a | 1.44
(0.34-6.16) | 0.621b |
| NRG1 (L), AKT
(L) | - | 15 (12.1) | 1.00 | | 1.00 | |
| Either | - | 103 (83.1) | 0.86
(0.33-2.24) | 0.753a | 1.64
(0.39-6.93) | 0.499c |
| NRG1 (H), AKT
(H) | - | 6 (4.8) | 3.17
(0.96-10.53) | 0.059a | 4.97
(0.83-29.88) | 0.080c |
|
| D, Disease-free
interval survival |
|
| Variable | ROC | n (%) | CHR (95% CI) | P-value | AHR (95% CI) | P-value |
|
| NRG1 | Low | 52 (73.2) | 1.00 | | 1.00 | |
| High | 19 (26.8) | 1.29
(0.52-3.20) | 0.580a | 1.97
(0.75-5.16) | 0.169b |
| AKT | Low | 43 (60.6) | 1.00 | | 1.00 | |
| High | 28 (39.4) | 3.42
(1.40-8.35) | 0.007a | 4.24
(1.69-10.66) | 0.002b |
| NRG1 (L), AKT
(L) | - | 36 (50.7) | 1.00 | | 1.00 | |
| Either | - | 23 (32.4) | 2.25
(0.95-5.30) | 0.064a | 3.21
(1.16-8.84) | 0.024c |
| NRG1 (H), AKT
(H) | - | 12 (16.9) | 1.65
(0.60-4.54) | 0.329a | 2.91
(0.88-9.60) | 0.079c |
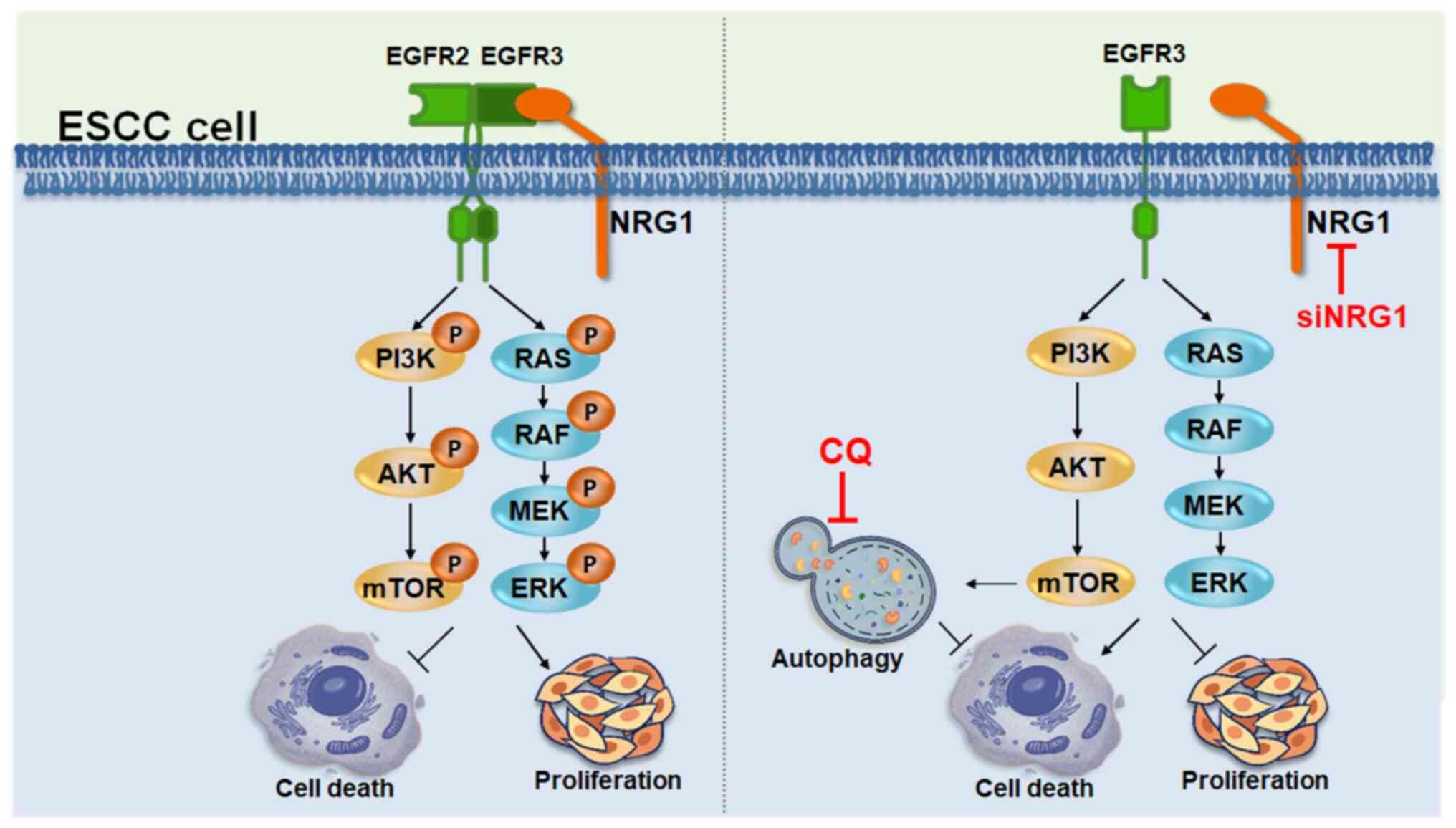
Silencing NRG1 induces cytoprotective
autophagy in ESCC cells
Downstream signals of NRG1, namely p-AKT and p-cRAF,
were inhibited in ESCC cells in which NRG1 was silenced. The
inactivation of AKT decreases activity of its substrate protein,
mTOR, which is a negative regulator of autophagy (53). RAF inhibitors activate
cytoprotective autophagy to facilitate resistance to stressed
conditions (54). Therefore, it
was hypothesized that autophagy is activated in ESCC cells in
response to downregulation of NRG1. To confirm whether siNRG1
promotes autophagy in ESCC cells, a fluorescence assay was used to
observe the changes in autophagosomes. ESCC cells were transfected
with siNRG1 and treated with EBSS and CQ as controls for autophagy
inducer and inhibitor, respectively (Fig. 5A-C). More autophagosome (DAP) and
autolysosomes (DAL) puncta were observed in the siNRG1 and EBSS
groups compared with the control group, indicating activation of
autophagy. Conversely, fluorescence of DAL decreased in the CQ
group, indicating inhabitation of autophagy (Fig. 5A-C). To investigate the effects
of silencing NRG1 on autophagic activity, expression of autophagy
marker LC3B-II and p62 was determined using western blot analysis
(Fig. 5D and E). The results
demonstrated decreased expression levels of p62 and LC3B-II in ESCC
cells following transfection with siNRG1 (Fig. 5D and E). CQ was added to examine
the effect of siNRG1 on LC3B-II turnover, indicative of autophagic
flux. The silencing NRG1 increased net LC3B-II protein levels in
ESCC cells treated with or without autophagy inhibitor CQ (Fig. 5F and G). Autophagic flux was
higher in ESCC cells in which NRG1 was silenced compared with
control. Therefore, silencing NRG1 increased autophagosome and
autolysosome formation and promoted autophagic activity in ESCC
cells.
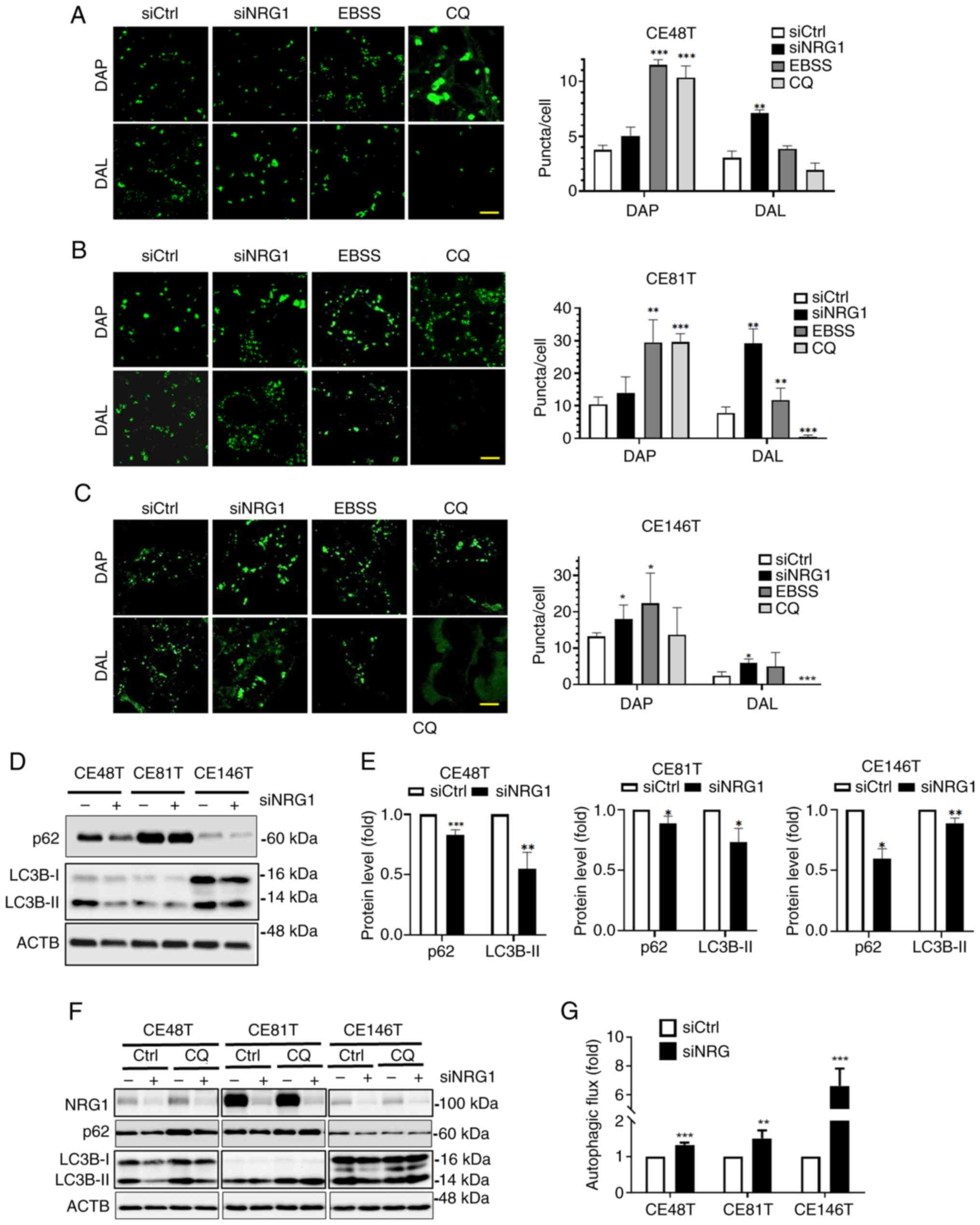 | Figure 5Effect of NRG1 on autophagy activity
of ESCC cells. (A) Transfected CE48T, (B) CE81T and (C) CE146T ESCC
cells were stained with autophagosome (DAP, 0.1 µM) or
autolysosome dye (DAL, 0.5 µM). ESCC cells were treated with
CQ for autophagy induction and inhibition, respectively. The
autophagosome and autolysosome puncta were observed under a
confocal microscope. Scale bar, 10 µm. (D) Protein levels of
NRG1, p62 and LC3B following silencing were (E) quantified. (F)
Transfected ESCC cells were treated with CQ (20 µM) to
examine LC3B-II turnover using immunoblotting. (G) LC3B-II was used
to determine autophagic flux. *P<0.05,
**P<0.01, ***P<0.001 vs. siCtrl. NRG,
neuregulin-1; ESCC, esophageal squamous cell carcinoma; DAP, dye of
autophagosome; DAL, dye of autolysosome; EBSS, Earle's Balanced
Salt Solution; CQ, chloroquine; si, small interfering; Ctrl,
control; ACTB, β-actin. |
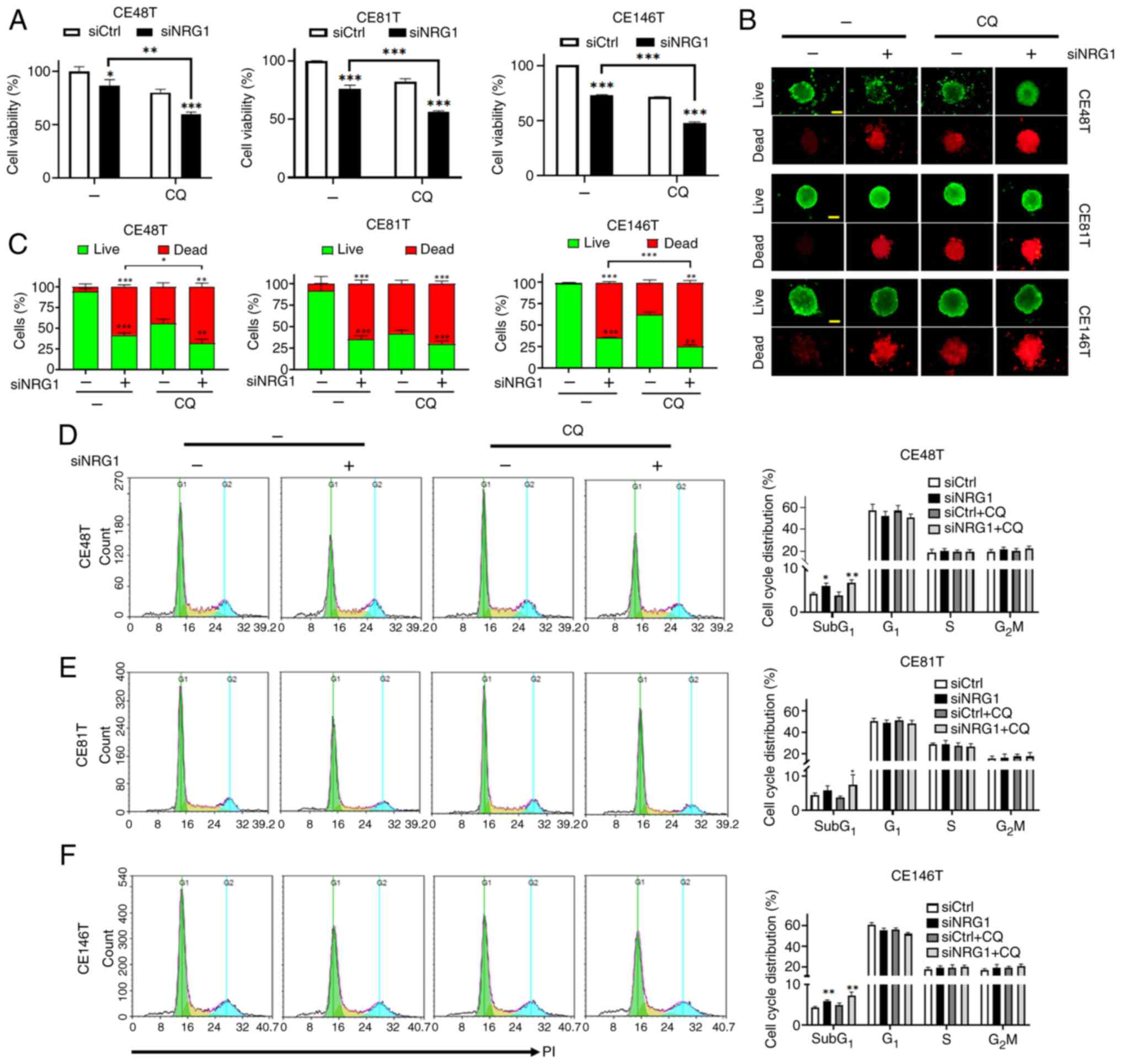
Autophagy functions as a detrimental or survival
pathway in cells in response to stress (31,55). Cells treated with siNRG1 and CQ
displayed a significant decrease in cell viability compared with
siNRG1-alone (Fig. 6A). In
addition, sphere formation assay and live (green)/dead (red)
staining were employed to assess whether combined siNRG1 and CQ
affected the ratio of live and dead cells within an ESCC sphere
(Fig. 6B and C). ESCC cells
transfected with siNRG1 and treated with CQ displayed a significant
decrease in live and a significant increase in dead cells within
spheres (Fig. 6B and C).
Compared with siNRG1, siNRG1 + CQ increased the proportion of dead
cells. Therefore, the effect on proliferation or death of ESCC
cells was assessed. ESCC cells transfected with siNRG1 with or
without CQ exhibited an increase the numbers of cells in
subG1 phase (Fig.
6D-F), indicating cell cycle arrest was not induced in a
specific phase. Therefore, the combined use of siNRG1 and CQ may
not primarily affect proliferation of ESCC cells by regulating the
cell cycle progression.
Co-expression of NRG1 and LC3B is
associated with unfavorable prognosis of patients with ESCC
Based on the aforementioned results that NRG1 and
autophagy may contribute to survival pathways and silencing NRG1
decreases LC3B levels in ESCC cell lines, the present study
analyzed data from TCGA to explore the association between NRG1 and
LC3B in patients with ESCC (Fig.
7; Table V). LC3B has two
isoforms, differing by a single amino acid (C113 vs. Y113)
(56); therefore, LC3B1 and
LC3B2 were included in the analysis. Kaplan-Meier survival curves
revealed that patients with high co-expression of NRG1 and LC3B1
had significantly shorter overall, progression-free interval and
disease-specific survival (Fig.
7A-C) compared with those with low co-expression of NRG1 and
LC3B1. However, no significant difference was observed in
disease-free interval survival (Fig.
7D). Similarly, high co-expression of NRG1 and LC3B2 was
associated with poorer overall, progression-free interval,
disease-specific (Fig. 7E-G) and
disease-free interval survival (Fig.
7H).
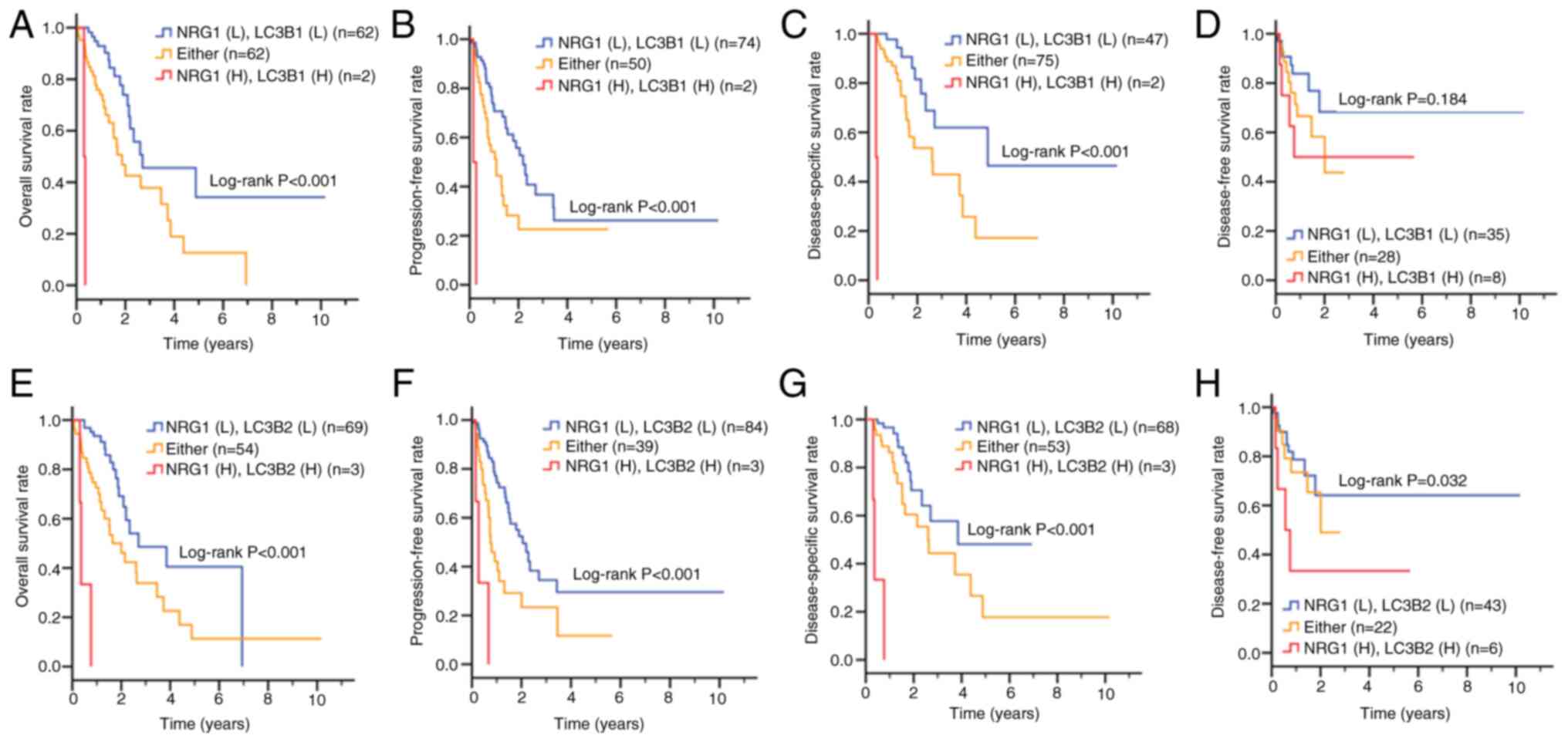 | Figure 7Clinical association between gene
expression of NRG1 and LC3B in patients with ESCC. Based on an ESCC
dataset from The Cancer Genome Atlas, Kaplan-Meier plots were used
to analyze the association between NRG1 and LC3B1 expression of
patients with ESCC and (A) overall, (B) progression-free interval,
(C) disease-specific and (D) disease-free interval survival.
Association between NRG1 and LC3B2 gene expression and (E) overall,
(F) progression-free interval, (G) disease-specific and (H)
disease-free interval survival. NRG1, neuregulin-1; ESCC,
esophageal squamous cell carcinoma; L, low expression; H, high
expression; Either, NRG1(H)cRAF(L) or NRG1(L)/cRAF(H); yrs,
years. |
 | Table VEffect of NRG1 and LC3B1
co-expression on survival of patients with esophageal squamous cell
carcinoma. |
Table V
Effect of NRG1 and LC3B1
co-expression on survival of patients with esophageal squamous cell
carcinoma.
A, Overall survival
|
|---|
| Variable | Expression | n (%) | CHR (95% CI) | P-value | AHR (95% CI) | P-value |
|---|
| NRG1 | Low | 120 (95.2) | 1.00 | | 1.00 | |
| High | 6 (4.8) | 2.01
(0.62-6.52) | 0.244a | 3.40
(0.99-11.71) | 0.052b |
| LC3B1 | Low | 66 (52.4) | 1.00 | | 1.00 | |
| High | 60 (47.6) | 2.56
(1.43-4.57) | 0.001a | 2.17
(1.21-3.91) | 0.010b |
| NRG1 (L), LC3B1
(L) | - | 62 (49.2) | 1.00 | | 1.00 | |
| Either | - | 62 (49.2) | 2.10
(1.18-3.72) | 0.011a | 2.36
(1.30-4.27) | 0.005c |
| NRG1 (H), LC3B1
(H) | - | 2 (1.6) | 30.50
(5.82-159.70) | <0.001a | 50.98
(9.25-280.88) | <0.001c |
|
| B, Progression-free
interval survival |
|
| Variable | ROC | n (%) | CHR (95% CI) | P-value | AHR (95% CI) | P-value |
|
| NRG1 | Low | 120 (95.2) | 1.00 | | 1.00 | |
| High | 6 (4.8) | 2.93
(1.16-7.38) | 0.023a | 5.11
(1.90-13.72) | 0.001b |
| LC3B1 | Low | 78 (61.9) | 1.00 | | 1.00 | |
| High | 48 (38.1) | 1.94
(1.16-3.24) | 0.012a | 1.90
(1.09-3.31) | 0.024b |
| NRG1 (L), LC3B1
(L) | - | 74 (58.7) | 1.00 | | 1.00 | |
| Either | - | 50 (39.7) | 1.81
(1.09-3.02) | 0.022a | 1.95
(1.16-3.27) | 0.012c |
| NRG1 (H), LC3B1
(H) | - | 2 (1.6) | 25.06
(5.14-122.25) | <0.001a | 34.31
(6.86-171.71) | <0.001c |
|
| C, Disease-specific
survival |
|
| Variable | ROC | n (%) | CHR (95% CI) | P-value | AHR (95% CI) | P-value |
|
| NRG1 | Low | 118 (95.2) | 1.00 | | 1.00 | |
| High | 6 (4.8) | 3.17
(0.96-10.53) | 0.059a | 7.76
(2.04-29.53) | 0.003b |
| LC3B1 | Low | 51 (41.1) | 1.00 | | 1.00 | |
| High | 73 (58.9) | 2.78
(1.32-5.84) | 0.007a | 2.42
(1.14-5.16) | 0.022b |
| NRG1 (L), LC3B1
(L) | - | 47 (37.9) | 1.00 | | 1.00 | |
| Either | - | 75 (60.5) | 2.16
(1.05-4.46) | 0.037a | 2.71
(1.25-5.89) | 0.012c |
| NRG1 (H), LC3B1
(H) | - | 2 (1.6) | 177.84
(15.73-2010.96) | <0.001a | 360.05
(29.44-4403.07) | <0.001c |
|
| D, Disease-free
interval survival |
|
| Variable | ROC | n (%) | CHR (95% CI) | P-value | AHR (95% CI) | P-value |
|
| NRG1 | Low | 52 (73.2) | 1.00 | | 1.00 | |
| High | 19 (26.8) | 1.29
(0.52-3.20) | 0.580a | 1.97
(0.75-5.16) | 0.169b |
| LC3B1 | Low | 46 (64.8) | 1.00 | | 1.00 | |
| High | 25 (35.2) | 2.53
(1.07-5.98) | 0.035a | 3.24
(1.34-7.84) | 0.009b |
| NRG1 (L), LC3B1
(L) | - | 35 (49.3) | 1.00 | | 1.00 | |
| Either | - | 28 (39.4) | 1.47
(0.63-3.47) | 0.375a | 1.94
(0.74-5.10) | 0.179c |
| NRG1 (H), LC3B1
(H) | - | 8 (11.3) | 2.10
(0.70-6.24) | 0.184a | 2.94
(0.86-10.05) | 0.087c |
To account for variations in cell differentiation
and AJCC pathological stage, multivariate Cox proportional hazard
model was used to evaluate the association between survival
outcomes and NRG1 expression alone or in combination with LC3B1
(Table V). Patients with high
co-expression of NRG1 and LC3B1 showed significantly increased risk
of mortality compared with those with NRG1(low)/LC3B1(low).
Patients with high co-expression of NRG1 and LC3B1 had higher risk
for overall (AHR, 50.98; CI, 9.25-280.88), progression-free
interval (AHR, 34.31, CI, 6.86-171.71) and disease-specific
survival (AHR, 360.05, CI, 29.44-4403.07; Table V). Likewise, patients with high
co-expression of NRG1 and LC3B2 had significantly worse overall
(AHR, 23.11, CI, 6.19-86.25), progression-free interval (AHR,
12.65, CI, 3.68-43.47), disease-specific (AHR, 48.50, CI,
11.53-204.10) and disease-free interval survival (AHR, 3.71, CI,
1.16-11.87; Table VI). These
findings suggest that high co-expression of NRG1 and LC3B may
contribute to tumor progression and relapse in ESCC.
 | Table VIEffect of NRG1 and LC3B2
co-expression on survival of patients with esophageal squamous cell
carcinoma. |
Table VI
Effect of NRG1 and LC3B2
co-expression on survival of patients with esophageal squamous cell
carcinoma.
A, Overall survival
|
|---|
| Variable | Expression | n (%) | CHR (95% CI) | P-value | AHR (95% CI) | P-value |
|---|
| NRG1 | Low | 120 (95.2) | 1.00 | | 1.00 | |
| High | 6 (4.8) | 2.01
(0.62-6.52) | 0.244a | 3.40
(0.99-11.71) | 0.052b |
| LC3B2 | Low | 72 (57.1) | 1.00 | | 1.00 | |
| High | 54 (42.9) | 2.79
(1.56-4.98) | 0.001a | 2.39
(1.32-4.34) | 0.004b |
| NRG1 (L), LC3B2
(L) | - | 69 (54.8) | 1.00 | | 1.00 | |
| Either | - | 54 (42.9) | 1.98
(1.12-3.47) | 0.018a | 2.29
(1.27-4.14) | 0.006c |
| NRG1 (H), LC3B2
(H) | - | 3 (2.4) | 14.94
(4.22-52.93) | <0.001a | 23.11
(6.19-86.25) | <0.001c |
|
| B, Progression-free
interval survival |
|
| Variable | ROC | n (%) | CHR (95% CI) | P-value | AHR (95% CI) | P-value |
|
| NRG1 | Low | 120 (95.2) | 1.00 | | 1.00 | |
| High | 6 (4.8) | 2.93
(1.16-7.38) | 0.023a | 5.11
(1.90-13.72) | 0.001b |
| LC3B2 | Low | 87 (69.0) | 1.00 | | 1.00 | |
| High | 39 (31.0) | 2.53
(1.51-4.26) | <0.001a | 2.33
(1.37-3.98) | 0.002b |
| NRG1 (L), LC3B2
(L) | - | 84 (66.7) | 1.00 | | 1.00 | |
| Either | - | 39 (31.0) | 2.11
(1.25-3.55) | 0.005a | 2.30
(1.36-3.92) | 0.002c |
| NRG1 (H), LC3B2
(H) | - | 3 (2.4) | 9.34
(2.78-31.35) | <0.001a | 12.65
(3.68-43.47) | <0.001c |
|
| C, Disease-specific
survival |
|
| Variable | ROC | n (%) | CHR (95% CI) | P-value | AHR (95% CI) | P-value |
|
| NRG1 | Low | 118 (95.2) | 1.00 | | 1.00 | |
| High | 6 (4.8) | 3.17
(0.96-10.53) | 0.059a | 7.76
(2.04-29.53) | 0.003b |
| LC3B2 | Low | 71 (57.3) | 1.00 | | 1.00 | |
| High | 53 (42.7) | 2.66
(1.33-5.34) | 0.006a | 2.15
(1.06-4.38) | 0.034b |
| NRG1 (L), LC3B2
(L) | - | 68 (54.8) | 1.00 | | 1.00 | |
| Either | - | 53 (42.7) | 1.68
(0.85-3.30) | 0.136a | 2.06
(1.01-4.24) | 0.048c |
| NRG1 (H), LC3B2
(H) | - | 3 (2.4) | 33.66
(8.53-132.90) | <0.001a | 48.50
(11.53-204.10) | <0.001c |
|
| D, Disease-free
interval survival |
|
| Variable | ROC | n (%) | CHR (95% CI) | P-value | AHR (95% CI) | P-value |
|
| NRG1 | Low | 52 (73.2) | 1.00 | | 1.00 | |
| High | 19 (26.8) | 1.29
(0.52-3.20) | 0.580a | 1.97
(0.75-5.16) | 0.169b |
| LC3B2 | Low | 56 (78.9) | 1.00 | | 1.00 | |
| High | 15 (21.1) | 3.00
(1.23-7.27) | 0.015a | 3.87
(1.55-9.69) | 0.004b |
| NRG1 (L), LC3B2
(L) | - | 43 (60.6) | 1.00 | | 1.00 | |
| Either | - | 22 (31.0) | 1.08
(0.43-2.67) | 0.876a | 1.36
(0.52-3.58) | 0.537c |
| NRG1 (H), LC3B2
(H) | - | 6 (8.5) | 3.31
(1.11-9.89) | 0.032a | 3.71
(1.16-11.87) | 0.027c |
Discussion
NRG1 serves a dual role in cancer development;
however, its specific role in ESCC remains unclear. Here, NRG1 gene
and protein levels were elevated in tumor tissue and associated
with poor outcomes in patients with ESCC (Fig. 8). Silencing NRG1 led to cancer
cell death and decreased tumor sphere formation, accompanied by
decreased phosphorylation of AKT and cRAF. Co-expression of NRG1
and cRAF increased mortality risk of overall and progression-free
survival. Silencing NRG1 triggered cytoprotective autophagy,
evidenced by increased autophagosome/autolysosome formation and
autophagic flux. CQ enhanced cancer cell death in NRG1-deficient
ESCC cells. Patients with high co-expression of NRG1 and LC3B1 or
LC3B2 had worse prognosis compared with those with low
co-expression. Given the poor prognosis and treatment outcomes for
ESCC, the present findings suggested that NRG1 may serve as a
promising biomarker and therapeutic target. Combination of siNRG1
and CQ, which showed an enhanced inhibitory effect, highlights its
potential for use as a viable treatment strategy for ESCC.
NRG1, a member of the NRG family, is a ligand for
the HER3 receptor associated with aspects of tumor progression in
numerous types of human cancer, such as lung cancer, breast cancer
and prostate cancer (57-59).
These aspects include cell proliferation, differentiation,
angiogenesis and metastasis. NRG1 is overexpressed in various types
of cancer (57-59) and activates downstream signaling
pathways such as MAPK and PI3K by binding members of the HER family
(60). In non-small cell lung
cancer, blocking the NRG1 signaling pathway may inhibit tumor
growth and enhance response to chemotherapy (61). These findings indicate that NRG1
serves as an oncogene in cancer development. Conversely, other
studies have reported decreased NRG1 expression in breast cancer
cell lines due to gene methylation; loss of NRG1 gene can lead to
chromosomal abnormalities in breast and colon cancer (62,63). NRG1 may serve a suppressor role
in the development of lung adenocarcinoma and may be associated
with AKT and ERK1/2 pathways (64). Hence, NRG1 may serve a dual role
in tumors, functioning as both an oncogene and tumor suppressor
gene depending on the type of cancer. Despite elevated expression
of NRG1 in numerous types of cancer (57-59), its role in ESCC remains unclear.
Here, the upregulation of mRNA and protein levels of NRG1 was
observed in ESCC specimens. The high expression of NRG1 was
associated with worse survival in patients with ESCC with poorly
differentiated tumors and advanced AJCC stage and lymph node
invasion. The present results demonstrated that silencing NRG1
leads to a significant decrease in viability, colony formation,
migration and invasion of ESCC cell lines.
NRG1 isoforms are predominantly expressed in
different organs, serving a key role in proliferation, survival,
migration and differentiation of various types of cell, including
epithelial, nerve, cardiac and skeletal muscle cells (65). NRG1 mediates activation of
downstream signaling pathways associated with malignancy. Several
gene fusions associated with NRG1 have been identified in lung
cancer, including CD47-NRG1, Syndecan-4-NRG1, RNA binding protein
with multiple splicing -NRG1, Werner syndrome protein (WRN)-NRG1
and Solute carrier family 3 member 2 (SLC3A2)-NRG1 (20,66,67). NRG1 is abnormally expressed in
various types of tumor and is associated with aspects of tumor
progression, such as cell proliferation, differentiation, invasion
and metastasis (58,59). The molecular weight of NRG1
observed in SDS-PAGE is 25% higher than expected, which is due to
protein modification glycosylation (68). The present study indicated that
NRG1 was highly expressed in patients with ESCC and associated with
poor prognosis. However, the role of specific isoforms, gene
translocation or post-translation modification of NRG1 in ESCC
remains unclear; thus, further investigations are required to
determine the association between NRG1 isoforms/localization and
post-translation modification with prognosis in patients with ESCC.
The investigation of these isoforms and modifications may lead to
identification of therapeutic biomarkers for ESCC and facilitate
development of treatment strategies.
siRNA-mediated NRG1 silencing experiments in CE48T,
CE81T and CE146T cell lines revealed a decrease in downstream
signaling molecules, including p-AKT and p-cRAF, following NRG1
silencing, thereby influencing the associated MAPK and PI3K
pathway. Both MAPK and PI3K pathways are required for cell
proliferation and mobility (40). ESCC cell lines silenced with
siNRG1 exhibited decreased cell proliferation, migration, viability
and spheroid formation, confirming the key role of NRG1 as an
oncogene in ESCC. Moreover, AKT and cRAF negatively regulate
autophagy (53,54), which allows cancer cell survive
in stressed conditions, such as hypoxia, suspension growth and
chemotherapeutic stress. Induction of autophagy was evident
following NRG1 silencing. Using DAP and DAL, the present study
observed a significant increase in numbers of autophagosomes and
autolysosomes following NRG1 silencing. p62 and LC3-II protein
levels decreased following NRG1 silencing. Silencing NRG1 increased
LC3-II flux when co-treated with autophagy inhibitor. These
findings suggested that NRG1 silencing may inactivate AKT and cRAF
to enhance autophagy in ESCC cells. Notably, combination of NRG1
silencing and autophagy inhibition, as demonstrated by live/dead
staining following treatment with CQ, resulted in increased
cytotoxicity against ESCC cells. Following NRG1 silencing,
autophagy was activated to allow cancer cells to survive,
suggesting that a potential treatment strategy for ESCC may involve
autophagy inhibitors. Moreover, NRG1 and autophagy serve key roles
on survival of ECSS cells. Patients with ESCC with high
co-expression of NRG1 and LC3B had higher mortality risk compared
with those with low co-expression of NRG1 and LC3B. Although
further studies with a greater number of cases and different
cohorts are required to determine the association between NRG1 and
autophagy markers in ESCC, combining siNRG1 and autophagy
inhibitors may be an alternative treatment strategy to improve
outcomes for patients with ESCC.
Taken together, the present study demonstrated that
elevated levels of NRG1 were associated with tumor progression of
ESCC. Silencing NRG1 inhibited proliferation and migration of ESCC
cells. Co-expression of NRG1 and cRAF may be key for malignancy and
prognosis of patients with ESCC. Furthermore, autophagy may serve
as a survival mechanism in ESCC cells in which NRG1 is silenced.
While siRNA-based results of the present study support the
oncogenic role of NRG1 in ESCC cells, further investigations
involving overexpression of NRG1 in ESCC cells with low NRG1
expression are necessary to confirm whether NRG1-mediated
downstream factors contribute to cell proliferation and
mobility.
Supplementary Data
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
CWS and YGG conceived the study, confirm the
authenticity of all the raw data and reviewed the manuscript. HWC,
CHL and CCC analyzed data. YRC, WHY, CWS, HWC, CCC, YCT and PFL
performed experiments and interpreted data. PFL, YCT and CWS
designed the experiments. CWS and YCT wrote the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
This project was approved by the Ethics Committee of
the Kaohsiung Veterans General Hospital (approval nos. VGHKS
95-CT3-21 and VGHKS 15-CT12-10). Written informed consent was
obtained from all subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Paul Morgan
(Icahn School of Medicine at Mount Sinai, New York, USA) for
English editing.
Funding
The present study was supported by National Science and
Technology Council (grant nos. 113-2320-B-037-029-MY3 and
113-2320-B-110-002-MY3), Zuoying Armed Forces General Hospital
(grant nos. KAFGH-ZY-A-112003 and 111002), National Sun Yat-sen
University (grant no. KSVNSU112-006), National Sun Yat-sen
University and Kaohsiung Medical University Joint Research Project
(grant nos. 112-P06, 113-P11 and 113-P14) and Kaohsiung Medical
University Research Center Grant (grant no. KMU-TC112A04).
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar
|
|
2
|
Rustgi AK and El-Serag HB: Esophageal
carcinoma. N Engl J Med. 371:2499–509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu CQ, Ma YL, Qin Q, Wang PH, Luo Y, Xu
PF and Cui Y: Epidemiology of esophageal cancer in 2020 and
projections to 2030 and 2040. Thorac Cancer. 14:3–11. 2023.
View Article : Google Scholar :
|
|
4
|
Napier KJ, Scheerer M and Misra S:
Esophageal cancer: A Review of epidemiology, pathogenesis, staging
workup and treatment modalities. World J Gastrointest Oncol.
6:112–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brown LM, Hoover RN, Greenberg RS,
Schoenberg JB, Schwartz AG, Swanson GM, Liff JM, Silverman DT,
Hayes RB and Pottern LM: Are racial differences in squamous cell
esophageal cancer explained by alcohol and tobacco use? J Natl
Cancer Inst. 86:1340–1345. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mao WM, Zheng WH and Ling ZQ:
Epidemiologic risk factors for esophageal cancer development. Asian
Pac J Cancer Prev. 12:2461–2466. 2011.
|
|
7
|
Liao HY, Wang GP, Gu LJ, Huang SH, Chen
XL, Li Y and Cai SW: HIF-1α siRNA and cisplatin in combination
suppress tumor growth in a nude mice model of esophageal squamous
cell carcinoma. Asian Pac J Cancer Prev. 13:473–477. 2012.
View Article : Google Scholar
|
|
8
|
Sun L and Yu S: Meta-analysis:
Non-steroidal anti-inflammatory drug use and the risk of esophageal
squamous cell carcinoma. Dis Esophagus. 24:544–549. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang JC, Chan D, Chung PY, Liu Y, Lam AK,
Law S, Huang W, Chan AS, Lam KH and Zhou Y: Downregulation of
chemokine (C-C motif) ligand 5 induced by a novel
8-hydroxyquinoline derivative (91b1) suppresses tumor invasiveness
in esophageal carcinoma. Int J Mol Med. 54:1112024. View Article : Google Scholar
|
|
11
|
Fernandez-Cuesta L and Thomas RK:
Molecular pathways: Targeting NRG1 fusions in lung cancer. Clin
Cancer Res. 21:1989–1994. 2015. View Article : Google Scholar
|
|
12
|
Meyer D and Birchmeier C: Multiple
essential functions of neuregulin in development. Nature.
378:386–390. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Riaz IB, Naqvi SAA, He H, Asghar N,
Siddiqi R, Liu H, Singh P, Childs DS, Ravi P, Hussain SA, et al:
First-line systemic treatment options for metastatic
castration-sensitive prostate cancer: A living systematic review
and network Meta-analysis. JAMA Oncol. 9:635–645. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Laskin J, Liu SV, Tolba K, Heining C,
Schlenk RF, Cheema P, Cadranel J, Jones MR, Drilon A, Cseh A, et
al: NRG1 fusion-driven tumors: Biology, detection, and the
therapeutic role of afatinib and other ErbB-targeting agents. Ann
Oncol. 31:1693–1703. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rimer M, Cohen I, Lømo T, Burden SJ and
McMahan UJ: Neuregulins and erbB receptors at neuromuscular
junctions and at agrin-induced postsynaptic-like apparatus in
skeletal muscle. Mol Cell Neurosci. 12:1–15. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Telesco SE, Shih AJ, Jia F and
Radhakrishnan R: A multiscale modeling approach to investigate
molecular mechanisms of pseudokinase activation and drug resistance
in the HER3/ErbB3 receptor tyrosine kinase signaling network. Mol
Biosyst. 7:2066–2080. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wen D, Peles E, Cupples R, Suggs SV, Bacus
SS, Luo Y, Trail G, Hu S, Silbiger SM, Levy RB, et al: Neu
differentiation factor: A transmembrane glycoprotein containing an
EGF domain and an immunoglobulin homology unit. Cell. 69:559–572.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alvarado D, Ligon GF, Lillquist JS, Seibel
SB, Wallweber G, Neumeister VM, Rimm DL, McMahon G and LaVallee TM:
ErbB activation signatures as potential biomarkers for anti-ErbB3
treatment in HNSCC. PLoS One. 12:e01813562017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee C, Kim M, Park C, Jo W, Seo JK, Kim S,
Oh J, Kim CS, Ryu HS, Lee KH and Park J: Epigenetic regulation of
Neuregulin 1 promotes breast cancer progression associated to
hyperglycemia. Nat Commun. 14:4392023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yun S, Koh J, Nam SK, Park JO, Lee SM, Lee
K, Lee KS, Ahn SH, Park DJ, Kim HH, et al: Clinical significance of
overexpression of NRG1 and its receptors, HER3 and HER4, in gastric
cancer patients. Gastric Cancer. 21:225–236. 2018. View Article : Google Scholar
|
|
21
|
Adélaïde J, Huang HE, Murati A, Alsop AE,
Orsetti B, Mozziconacci MJ, Popovici C, Ginestier C, Letessier A,
Basset C, et al: A recurrent chromosome translocation breakpoint in
breast and pancreatic cancer cell lines targets the neuregulin/NRG1
gene. Genes Chromosomes Cancer. 37:333–345. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang HE, Chin SF, Ginestier C, Bardou VJ,
Adélaïde J, Iyer NG, Garcia MJ, Pole JC, Callagy GM, Hewitt SM, et
al: A recurrent chromosome breakpoint in breast cancer at the
NRG1/neuregulin 1/heregulin gene. Cancer Res. 64:6840–6844. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prentice LM, Shadeo A, Lestou VS, Miller
MA, deLeeuw RJ, Makretsov N, Turbin D, Brown LA, Macpherson N,
Yorida E, et al: NRG1 gene rearrangements in clinical breast
cancer: Identification of an adjacent novel amplicon associated
with poor prognosis. Oncogene. 24:7281–7289. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shin DH, Lee D, Hong DW, Hong SH, Hwang
JA, Lee BI, You HJ, Lee GK, Kim IH, Lee YS and Han JY: Oncogenic
function and clinical implications of SLC3A2-NRG1 fusion in
invasive mucinous adenocarcinoma of the lung. Oncotarget.
7:69450–69465. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Drilon A, Somwar R, Mangatt BP, Edgren H,
Desmeules P, Ruusulehto A, Smith RS, Delasos L, Vojnic M,
Plodkowski AJ, et al: Response to ERBB3-directed targeted therapy
in NRG1-rearranged cancers. Cancer Discov. 8:686–695. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shin DH, Jo JY and Han JY: Dual Targeting
of ERBB2/ERBB3 for the treatment of SLC3A2-NRG1-mediated lung
cancer. Mol Cancer Ther. 17:2024–2033. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hou G, Niu T, Jia A, Zhang Y, Chen X, Wei
H, Jia Y, Xu Y, Li Y, Wang P and Chatterjee A: NRG1 promotes
tumorigenesis and metastasis and afatinib treatment efficiency is
enhanced by NRG1 inhibition in esophageal squamous cell carcinoma.
Biochem Pharmacol. 218:1159202023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim DW, Schram AM, Hollebecque A, Nishino
K, Macarulla T, Rha SY, Duruisseaux M, Liu SV, Al Hallak MN,
Umemoto K, et al: The phase I/II eNRGy trial: Zenocutuzumab in
patients with cancers harboring NRG1 gene fusions. Future Oncol.
20:1057–1067. 2024. View Article : Google Scholar
|
|
29
|
Yamamoto H, Zhang S and Mizushima N:
Autophagy genes in biology and disease. Nat Rev Genet. 24:382–400.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ichimiya T, Yamakawa T, Hirano T, Yokoyama
Y, Hayashi Y, Hirayama D, Wagatsuma K, Itoi T and Nakase H:
Autophagy and autophagy-related diseases: A Review. Int J Mol Sci.
21:89742020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu PF, Farooqi AA, Peng SY, Yu TJ, Dahms
HU, Lee CH, Tang JY, Wang SC, Shu CW and Chang HW: Regulatory
effects of noncoding RNAs on the interplay of oxidative stress and
autophagy in cancer malignancy and therapy. Semin Cancer Biol.
83:269–282. 2022. View Article : Google Scholar
|
|
32
|
Li Z, Zhang Y, Lei J and Wu Y: Autophagy
in oral cancer: Promises and challenges (Review). Int J Mol Med.
54:1162024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Debnath J, Gammoh N and Ryan KM: Autophagy
and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol.
24:560–575. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen HT, Liu H, Mao MJ, Tan Y, Mo XQ, Meng
XJ, Cao MT, Zhong CY, Liu Y, Shan H and Jiang GM: Crosstalk between
autophagy and epithelial-mesenchymal transition and its application
in cancer therapy. Mol Cancer. 18:1012019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu TJ, Shiau JP, Tang JY, Yen CH, Hou MF,
Cheng YB, Shu CW and Chang HW: Physapruin a induces reactive oxygen
species to trigger cytoprotective autophagy of breast cancer cells.
Antioxidants (Basel). 11:13522022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu PF, Tsai KL, Hsu CJ, Tsai WL, Cheng
JS, Chang HW, Shiau CW, Goan YG, Tseng HH and Wu CH: Drug
repurposing screening identifies tioconazole as an ATG4 inhibitor
that suppresses autophagy and sensitizes cancer cells to
chemotherapy. Theranostics. 8:830–845. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu PF, Chang HW, Cheng JS, Lee HP, Yen
CY, Tsai WL, Cheng JT, Li YJ, Huang WC, Lee CH, et al: Map1lc3b and
sqstm1 modulated autophagy for tumorigenesis and prognosis in
certain subsites of oral squamous cell carcinoma. J Clin Med.
7:4782018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Serrano-Oviedo L, Ortega-Muelas M,
Garcia-Cano J, Valero ML, Cimas FJ, Pascual-Serra R,
Fernandez-Aroca DM, Roche O, Ruiz-Hidalgo MJ, Belandia B, et al:
Autophagic cell death associated to Sorafenib in renal cell
carcinoma is mediated through Akt inhibition in an ERK1/2
independent fashion. PLoS One. 13:e02008782018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang CY, Lee CH, Tu CC, Wu CH, Huang MT,
Wei PL and Chang YJ: Glucose-regulated protein 94 mediates
progression and metastasis of esophageal squamous cell carcinoma
via mitochondrial function and the NF-kB/COX-2/VEGF axis.
Oncotarget. 9:9425–9441. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bahar ME, Kim HJ and Kim DR: Targeting the
RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical
studies. Signal Transduct Target Ther. 8:4552023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim YC and Guan KL: mTOR: A pharmacologic
target for autophagy regulation. J Clin Invest. 125:25–32. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pankiv S, Clausen TH, Lamark T, Brech A,
Bruun JA, Outzen H, Øvervatn A, Bjørkøy G and Johansen T:
p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of
ubiquitinated protein aggregates by autophagy. J Biol Chem.
282:24131–24145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu WJ, Ye L, Huang WF, Guo LJ, Xu ZG, Wu
HL, Yang C and Liu HF: p62 links the autophagy pathway and the
ubiqutin-proteasome system upon ubiquitinated protein degradation.
Cell Mol Biol Lett. 21:292016. View Article : Google Scholar
|
|
44
|
Tsai ST, Wang PJ, Liou NJ, Lin PS, Chen CH
and Chang WC: ICAM1 is a potential cancer stem cell marker of
esophageal squamous cell carcinoma. PLoS One. 10:e01428342015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hickerson RP, Vlassov AV, Wang Q, Leake D,
Ilves H, Gonzalez-Gonzalez E, Contag CH, Johnston BH and Kaspar RL:
Stability study of unmodified siRNA and relevance to clinical use.
Oligonucleotides. 18:345–354. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cheng JS, Tsai WL, Liu PF, Goan YG, Lin
CW, Tseng HH, Lee CH and Shu CW: The MAP3K7-mTOR axis promotes the
proliferation and malignancy of hepatocellular carcinoma cells.
Front Oncol. 9:4742019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu PF, Chen CF, Ger LP, Tsai WL, Tseng
HH, Lee CH, Yang WH and Shu CW: MAP3K11 facilitates autophagy
activity and is correlated with malignancy of oral squamous cell
carcinoma. J Cell Physiol. 237:4275–4291. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
49
|
Liu PF, Hu YC, Kang BH, Tseng YK, Wu PC,
Liang CC, Hou YY, Fu TY, Liou HH, Hsieh IC, et al: Expression
levels of cleaved caspase-3 and caspase-3 in tumorigenesis and
prognosis of oral tongue squamous cell carcinoma. PLoS One.
12:e01806202017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu PF, Chen HC, Cheng JS, Tsai WL, Lee
HP, Wang SC, Peng WH, Lee CH, Ger LP and Shu CW: Association of
ATG4B and phosphorylated ATG4B proteins with tumorigenesis and
prognosis in oral squamous cell carcinoma. Cancers (Basel).
11:18542019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rajendran V and Jain MV: In vitro
tumorigenic assay: Colony forming assay for cancer stem cells.
Methods Mol Biol. 1692:89–95. 2018. View Article : Google Scholar
|
|
52
|
Singh SK, Abbas S, Saxena AK, Tiwari S,
Sharma LK and Tiwari M: Critical role of three-dimensional
tumorsphere size on experimental outcome. Biotechniques.
69:333–338. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xu Z, Han X, Ou D, Liu T, Li Z, Jiang G,
Liu J and Zhang J: Targeting PI3K/AKT/mTOR-mediated autophagy for
tumor therapy. Appl Microbiol Biotechnol. 104:575–587. 2020.
View Article : Google Scholar
|
|
54
|
Huang Y, Zhen Y, Chen Y, Sui S and Zhang
L: Unraveling the interplay between RAS/RAF/MEK/ERK signaling
pathway and autophagy in cancer: From molecular mechanisms to
targeted therapy. Biochem Pharmacol. 217:1158422023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chang KC, Liu PF, Chang CH, Lin YC, Chen
YJ and Shu CW: The interplay of autophagy and oxidative stress in
the pathogenesis and therapy of retinal degenerative diseases. Cell
Biosci. 12:12022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jiang TX, Zou JB, Zhu QQ, Liu CH, Wang GF,
Du TT, Luo ZY, Guo F, Zhou LM, Liu JJ, et al: SIP/CacyBP promotes
autophagy by regulating levels of BRUCE/Apollon, which stimulates
LC3-I degradation. Proc Natl Acad Sci USA. 116:13404–13413. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu J and Kern JA: Neuregulin-1 activates
the JAK-STAT pathway and regulates lung epithelial cell
proliferation. Am J Respir Cell Mol Biol. 27:306–313. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Meetze K, Vincent S, Tyler S, Mazsa EK,
Delpero AR, Bottega S, McIntosh D, Nicoletti R, Winston WM, Weiler
S, et al: Neuregulin 1 expression is a predictive biomarker for
response to AV-203, an ERBB3 inhibitory antibody, in human tumor
models. Clin Cancer Res. 21:1106–1114. 2015. View Article : Google Scholar
|
|
59
|
Montero JC, Rodríguez-Barrueco R, Ocaña A,
Díaz-Rodríguez E, Esparís-Ogando A and Pandiella A: Neuregulins and
cancer. Clin Cancer Res. 14:3237–3241. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Krivosheya D, Tapia L, Levinson JN, Huang
K, Kang Y, Hines R, Ting AK, Craig AM, Mei L, Bamji SX and
El-Husseini A: ErbB4-neuregulin signaling modulates synapse
development and dendritic arborization through distinct mechanisms.
J Biol Chem. 283:32944–32956. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hegde GV, de la Cruz CC, Chiu C, Alag N,
Schaefer G, Crocker L, Ross S, Goldenberg D, Merchant M, Tien J, et
al: Blocking NRG1 and other ligand-mediated Her4 signaling enhances
the magnitude and duration of the chemotherapeutic response of
non-small cell lung cancer. Sci Transl Med. 5:171ra182013.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chua YL, Ito Y, Pole JC, Newman S, Chin
SF, Stein RC, Ellis IO, Caldas C, O'Hare MJ, Murrell A and Edwards
PA: The NRG1 gene is frequently silenced by methylation in breast
cancers and is a strong candidate for the 8p tumour suppressor
gene. Oncogene. 28:4041–452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Pole JC, Courtay-Cahen C, Garcia MJ, Blood
KA, Cooke SL, Alsop AE, Tse DM, Caldas C and Edwards PA:
High-resolution analysis of chromosome rearrangements on 8p in
breast, colon and pancreatic cancer reveals a complex pattern of
loss, gain and translocation. Oncogene. 25:5693–5706. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang Y, Ning Z, Zhou X, Yang Z, Tang H, Xu
M, Wang X, Zhao J and Bai Y: Neuregulin1 acts as a suppressor in
human lung adenocarcinoma via AKT and ERK1/2 pathway. J Thorac Dis.
10:3166–3179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Adashek JJ, Pandya C, Maragakis NJ, De P,
Cohen PR, Kato S and Kurzrock R: Neuregulin-1 and ALS19 (ERBB4): At
the crossroads of amyotrophic lateral sclerosis and cancer. BMC
Med. 22:742024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dhanasekaran SM, Balbin OA, Chen G, Nadal
E, Kalyana-Sundaram S, Pan J, Veeneman B, Cao X, Malik R, Vats P,
et al: Transcriptome meta-analysis of lung cancer reveals recurrent
aberrations in NRG1 and Hippo pathway genes. Nat Commun.
5:58932014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Duruisseaux M, McLeer-Florin A, Antoine M,
Alavizadeh S, Poulot V, Lacave R, Rabbe N, Cadranel J and Wislez M:
NRG1 fusion in a French cohort of invasive mucinous lung
adenocarcinoma. Cancer Med. 5:3579–3585. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Shamir A and Buonanno A: Molecular and
cellular characterization of Neuregulin-1 type IV isoforms. J
Neurochem. 113:1163–1176. 2010. View Article : Google Scholar : PubMed/NCBI
|