Introduction
Glaucoma is a group of progressive optic
neuropathies and is currently the most prevalent ocular disease
worldwide, second only to cataracts in terms of blindness (1). Current estimates indicate a global
prevalence of 3.5% among individuals aged 40 to 80 years, with
projections suggesting a staggering 111.8 million individuals will
suffer from blindness attributable to glaucoma by the year 2040
(2,3). This condition, characterized by its
prevalence and impact on vision, primarily stems from the
deterioration of retinal ganglion cell (RGC) axons as they traverse
the optic nerve head (ONH) upon exiting the eye (4). This degenerative process results in
persistent impairment of the retinal nerve fiber layer (5,6),
with exacerbation often observed due to deformation of the lamina
cribrosa (LC) (7). Previous
investigations have established a positive correlation between
elevated intraocular pressure (IOP) and high-pressure glaucoma
(8), underscoring its pivotal
role in inducing damage to RGC axons and subsequent cell demise
(9), thereby emerging as a
significant risk factor for the development of glaucoma (10). Consequently, interventions aimed
at reducing IOP, through pharmacological, laser, or surgical
modalities, have proven efficacious in attenuating glaucoma
progression and preserving visual function (3). Nonetheless, certain presentations
of glaucoma, such as those featuring normal IOP, pose challenges as
they diverge from the typical paradigm (11,12). Additionally, the heterogeneity of
glaucomatous subtypes, coupled with the limited effectiveness of
conventional therapies, underscores the complexity inherent to
managing this multifaceted condition (13). Glaucoma poses a significant
challenge in clinical management, which is exacerbated by the
limited efficacy of conventional treatments in reversing the optic
nerve damage associated with the condition (3). Thus, a comprehensive understanding
of the underlying mechanisms and pathophysiology governing glaucoma
pathogenesis is required. This entails elucidating the complex
signaling pathways implicated in pathogenesis to identify novel
targets for interventions aimed at optic nerve protection and
regeneration at the molecular level. Previous studies have
highlighted the degeneration of RGCs and loss of axons, along with
damage and remodeling of the LC, as pivotal events in glaucoma
pathogenesis (Fig. 1) (5). Moreover, in our research (data not
shown), it was found that there appears to be a certain correlation
among the above-mentioned pivotal events-Caveolin-1 (Cav-1). Cav-1
cannot only prevent the degeneration of RGCs and optic nerve damage
in glaucoma by positively or negatively mediating cell signaling
pathways and neurotrophic factors, but also regulate the
physiological and pathological changes of the LC by influencing the
metabolism of the extracellular matrix (ECM), thus playing an
important role in the occurrence and prevention of glaucoma
(14-16). The signaling pathways activated
by genetic and environmental factors constitute a complex and
multifaceted system characterized by extensive crosstalk between
downstream signaling molecules. Consequently, these pathways are
interlinked and overlapping, thereby preventing their complete
isolation. Nevertheless, the regulation of proapoptotic genes,
neuroprotective factors and regenerative factors are subject to
complex modulation within these pathways, ultimately culminating in
glaucoma development (13). The
regulatory effects within these pathways are not unidimensional but
emerge from the complex interplay and mutual influence of multiple
factors. In light of the structural framework of this discourse,
the present review delves into the pathophysiological mechanisms of
RGCs apoptosis, optic nerve safeguarding and regeneration, and LC
damage and remodeling; the specific molecular mechanisms and the
interrelationships of pathways are shown in Fig. 2. This categorization is based on
the most extensively researched segments of pathways, with the aim
of offering deeper insights into cellular processes and furnishing
vital clues and targets for the treatment and pharmacological
management of glaucoma.
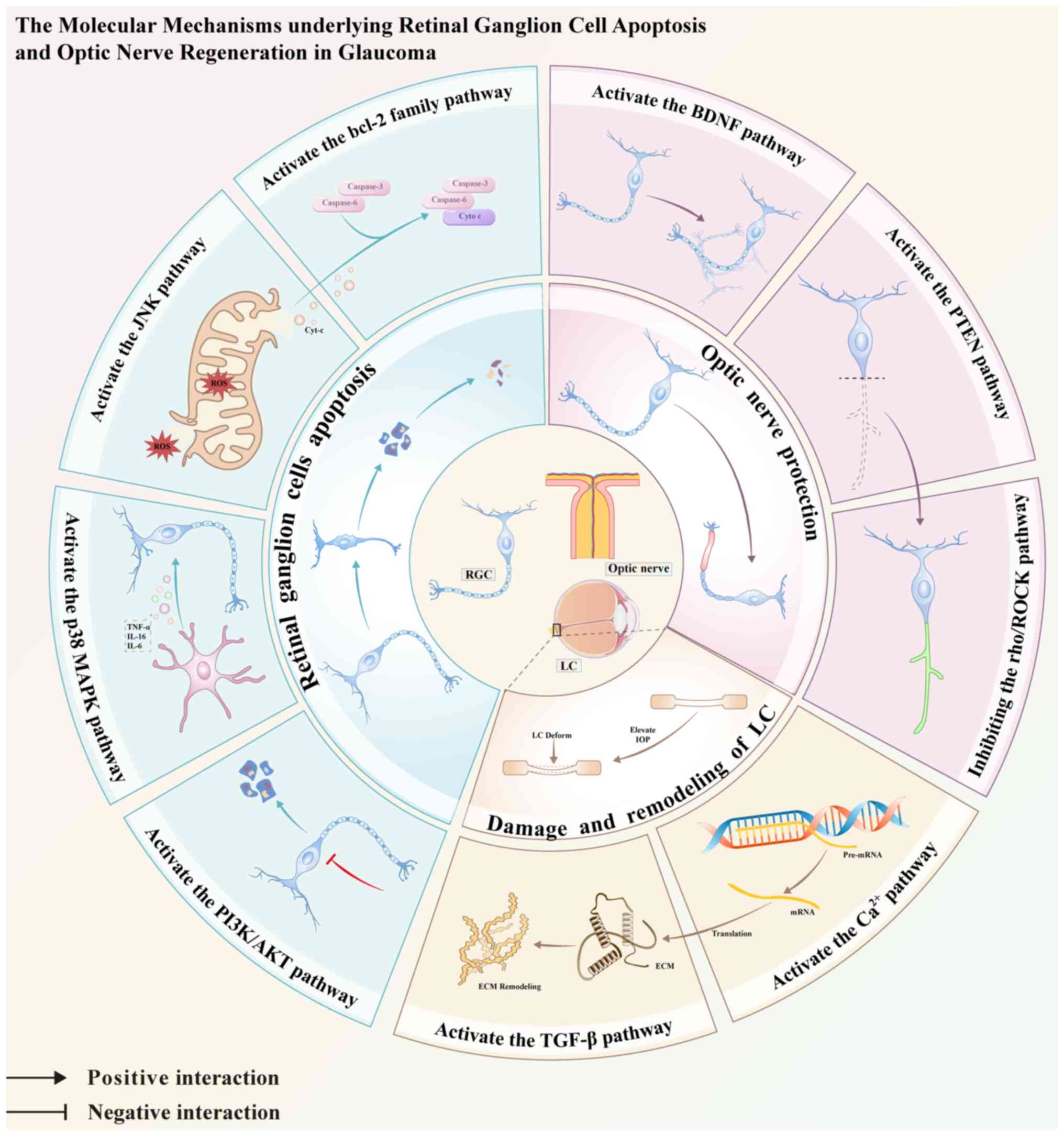 | Figure 1Summary of the role of signaling
pathways in the mechanisms of glaucoma in retinal ganglion cells
apoptosis, optical nerve protection and regeneration, and the LC
damage and remodeling. TNF-α, tumor necrosis factor-alpha; IL,
interleukin; ROS, reactive oxygen species; cyt-c, cytochrome c;
caspase, cysteinyl aspartate-specific proteinase; LC, lamina
cribrosa; ECM, extracellular matrix; IOP, intraocular pressure. |
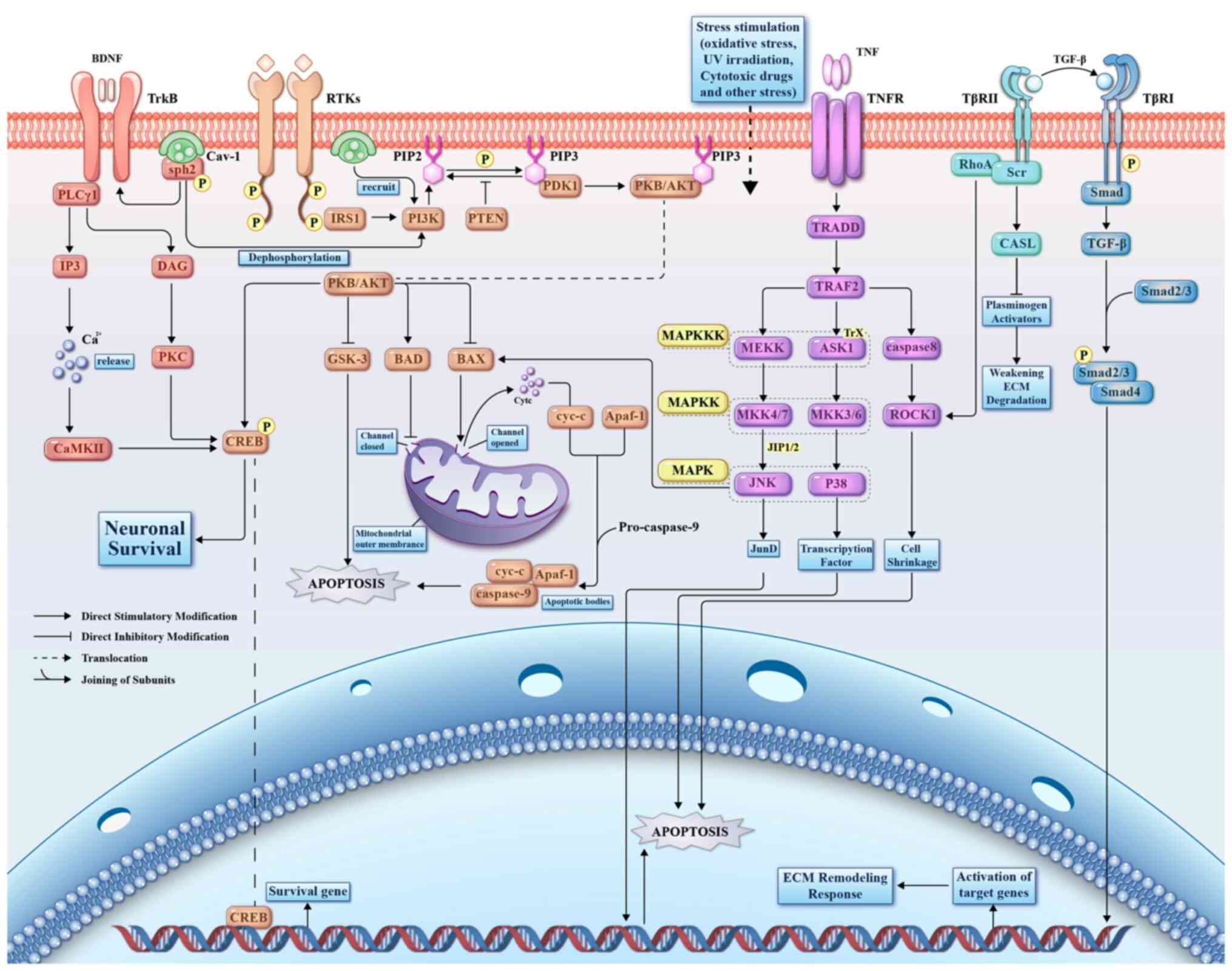 | Figure 2Review of glaucoma related signaling
pathways and their mechanisms. BDNF, brain-derived neurotrophic
factor; TrkB, tropomyosin receptor kinase B; PLCγ1,
1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase γ-1;
CaMKII, Ca2+-calmodulin protein kinase II; DAG,
diacylglycerol; PKC, protein kinase C; CREB, cAMP-response element
binding protein; Shp2, tyrosine phosphatase 2; Cav-1, caveolin-1;
RTKs, receptor tyrosine kinases; IRS1, insulin receptor substrate
1; PI3K, phosphoinositide 3-kinase; PKB, protein kinase B; GSK-3,
glycogen synthase kinase 3; BAD, Bcl-2-associated death promoter;
BAX, Bcl-2 Associated X Protein; cyt-c, cytochrome c; Apaf-1,
apoptotic protease activating factor 1; caspase, cysteinyl
aspartate specific proteinase; PTEN, phosphatase and tensin
homolog; PDK1, 3-phosphoinositide-dependent protein kinase 1; TNF,
tumor necrosis factor; TNFR, TNF receptor; TRADD, TNFR type
1-associated death domain protein; TRAF2, TNFR associated factor 2;
Trx, thioredoxin; MAPK, mitogen-activated protein kinase; MAPKKK,
MAPK kinase kinase; ASK1, apoptosis signal-regulating kinase 1;
MAPKK, MAPK kinase; MKK4/7, MAPK kinase 4/7; MKK3/6, MAPKkinase
3/6; ROCK1, Rho-associated coiled-coil forming protein kinase 1;
JIP1/2, JNK-interacting protein 1; JNK, c-Jun N-terminal kinase;
TGF-β, Transforming growth factor-beta; TβRI, TGF-β type I
receptor; TβRII, TGF-β type II receptor; RhoA, Ras homolog family
member A; ECM, extracellular matrix; Src, non-receptor tyrosine
kinase; CASL, Src scaffolding protein; PIP2,
phosphatidylinositol-4,5-bisphosphate; PIP3,
phosphatidylinositol-3,4,5-bisphosphate. |
Molecular signaling pathways and apoptosis
in RGCs
Apoptosis, also known as programmed cell death, is
widely considered the principal contributor to the loss of RGCs in
glaucoma (17). Numerous
mechanisms capable of triggering apoptosis in RGCs have been
identified, including increased IOP, ischemia and reperfusion
events, oxidative stress and neuroinflammatory responses (18). The complicated involvement of
multiple molecular and signaling pathways in these pathological
changes is of paramount importance, with particular emphasis on the
phosphoinositide 3-kinase (PI3K)/AKT, mitogen-activated protein
kinase (MAPK) and Bcl-2 family pathways (19,20). A comprehensive understanding of
these complex signaling pathways holds great promise for
intervention at the molecular level during the early stages of
glaucoma, with the goal of impeding apoptotic pathways of RGCs.
These interventions have the potential to significantly slow or
even halt glaucoma progression.
PI3K/AKT signaling pathway
The PI3K/AKT signaling pathway represents a pivotal
intracellular cascade that governs various cellular processes, such
as inhibition of apoptosis, facilitation of proliferation (19) and safeguarding against
injury-induced loss of RGCs in the context of glaucoma (21,22). Its complex orchestration involves
several key molecular components, including receptor tyrosine
kinases (RTKs), PI3K, phosphatidylinositol-4,5-bisphosphate (PIP2),
phosphatidylinositol-3,4,5-bisphosphate (PIP3) and protein kinase B
(PKB)/AKT (23-25). RTKs serve as a principal upstream
regulator of the PI3K/AKT signaling cascade, instigated by
extracellular growth factors to initiate signaling events and
activate PI3K (25,26). Within the cell membrane, PIP2 and
PIP3 are minor phospholipid constituents. In PI3K/AKT-mediated
apoptosis, PIP2 is converted to PIP3, which accumulates as a
pivotal docking phospholipid within the plasma membrane.
Subsequently, PIP3 binds to specific structural domains,
facilitating the recruitment of AKT to its kinase PDK1, thereby
activating PKB/AKT (27). This
activation event culminates in the attenuation of RGCs apoptosis,
thereby impeding glaucoma (28).
Ultimately, this complex regulatory network contributes to a
reduction in RGCs apoptosis and a subsequent delay in the onset and
progression of glaucoma pathology (Fig. 2).
In addition, previous studies have shown that
Caveolins play a significant role in regulating several signal
transduction pathways in ocular diseases including glaucoma
(29,30). There are three subtypes of
Caveolins in vertebrates, namely Cav-1, Cav-2 and Cav-3 (31). Among them, Cav-1 exhibits strong
immunoreactivity in the RGC layer, and has been identified as a
gene locus associated with glaucoma in genome-wide association
studies (15). Experiments have
found that the expression of Cav-1 is downregulated in the induced
glaucoma mouse model, and the mice showed ocular hypertension,
further indicating the protective role of Cav-1 in glaucoma
(32). It has been found that
Cav-1 has been proven to play a positive role in the PI3K/AKT
signaling pathway, and can inhibit apoptosis, inflammation and
oxidative stress by upregulating the PI3K/AKT signal transduction
pathway (33-35), thereby preventing the induced
degeneration of RGCs in glaucoma. Moreover, studies have suggested
that acteoside, the main ingredient of Yunnan purple taro, and
baicalin, the dry root of the Chinese herbal medicine
Scutellaria baicalensis, have significant effects in
preventing and treating glaucoma, reducing the loss, autophagy and
oxidative stress of RGCs, and thus preventing the occurrence and
development of glaucoma (36-38). Experiments have demonstrated that
Acteoside can prevent the autophagic apoptosis of RGCs and delay
the optic nerve atrophy induced by glaucoma by activating the
PI3K/AKT signaling pathway (16,38). A recent study found that the
protective effect of Acteoside on glaucoma is affected by Cav-1
(16). Cav-1-deficient mice not
only reverse the effects of Acteoside on the oxidative stress and
autophagy of RGCs, but also reverse the inhibitory effect of
Acteoside on RGCs loss and its activation effect on the PI3K/AKT
signaling pathway. This indicates that Acteoside relies on the
positive regulation of Cav-1 to activate the PI3K/AKT pro-survival
signaling pathway, thereby preventing the deterioration of glaucoma
(16). Furthermore, in a study
by Zhao et al (39), RGCs
were stimulated with N-methyl-D-aspartate (NMDA) to establish an
in vitro glaucoma model. Methyl-thiazolyl-tetrazolium assay
indicated a decline in RGCs' viability and an increase in apoptotic
rates with increasing NMDA concentrations. Concurrently, there was
a decrease in the expression of phosphorylated (p-)AKT and p-PI3K
in RGCs. Subsequent administration of baicalin, a compound found to
counteract the promoting effect of NMDA on RGCs apoptosis, restored
the diminished levels of p-AKT and p-PI3K proteins, further
corroborating the involvement of the PI3K/AKT pathway in
alleviating apoptosis and injury in NMDA-treated RGCs.
Additionally, treatment with LY294002, a PI3K inhibitor, reversed
the beneficial effects of baicalein on viability and expression of
RGCs, underscoring the crucial role of the PI3K/AKT signaling
pathway in modulating survival and function of RGCs. Collectively,
these findings highlight the significant association between the
PI3K/AKT pathway and RGCs, highlighting its potential as a
therapeutic target for impeding RGCs' apoptosis and delaying
glaucoma progression.
MAPK signaling pathway
MAPK constitutes a group of serine-threonine kinases
that serve as pivotal elements in intracellular signal transduction
mechanisms and orchestrate a diverse array of cellular processes,
including differentiation, proliferation, apoptosis, inflammation
and responses to various stress stimuli (40). The MAPK signaling cascade
delineates a sequential series of kinase events involving MAP3K,
MAP2K and MAPK. Initially, MAP3K, a Ser/Thr protein kinase, is
activated through phosphorylation, which subsequently catalyzes the
activation of MAP2K at its activation site. Consequently, MAP2K
increases MAPK activity by affecting dual phosphorylation events at
Thr and Tyr residues situated within specific motifs (41). The MAPK pathway, in its complex
network, diverges into three principal routes: Extracellular
signal-regulated kinase (ERK), p38 MAPK and c-Jun N-terminal kinase
(JNK) (40) (Fig. 2). Activation of ERK promotes cell
survival, whereas the activation of p38 MAPK and JNK plays a
critical role in mediating apoptosis in RGCs (41,42).
p38 MAPK signaling pathway
The apoptosis of RGCs has been associated with the
activation of microglia and the subsequent release of inflammatory
mediators such as tumor necrosis factor-alpha (TNF-α), interleukin
(IL)-16 and IL-6 (5,43). Moreover, canonical inducers of
p38 MAPK activation encompass inflammatory cytokines such as TNF-α,
IL-6 and IL-1β (44).
Osteopontin (OPN) serves not only as a phosphorylated glycoprotein
but also as a pro-inflammatory cytokine in various tissues and
cellular contexts (45-47). Its involvement extends to the
pathogenesis of numerous neurodegenerative disorders and is closely
associated with the regulation of autophagy and its ramifications
on neuronal function (48). In
the retina, knockout of OPN in mice revealed that microglia showed
no signs of activation, such as migration from the inner retina to
the subretinal space, or morphological changes in the amoeboid
microglia (49). It was deduced
that OPN may promote the transition of microglia to the amoeboid
phenotype and activate microglia (50). A study conducted by Yu et
al (51) established a rat
model characterized by chronic ocular hypertension (COH), revealing
microglial activation, upregulated expression of microglia-derived
OPN and changes in inflammatory cytokine levels (TNF-α, IL-1β and
IL-6). Treatment with an inhibitor targeting the p38 MAPK pathway
in activated microglia led to diminished production of TNF-α, IL-1β
and IL-6. Additionally, intravitreal administration of anti-OPN
antibodies in COH-afflicted rats resulted in a significant
reduction in p38 expression compared with that in the untreated COH
cohort. These findings highlight the potential of OPN to induce
apoptosis of RGCs via microglial activation, with the p38 MAPK
pathway playing a pivotal role in this complex cascade of
events.
JNK signaling pathway
The JNK pathway plays a pivotal role in the
pathogenesis of diverse diseases, including Alzheimer's disease,
Parkinson's disease and glaucoma (52-54). Its significance as a prospective
target for neuroprotective intervention has attracted considerable
attention (55). Among the
proteins implicated in JNK activation and the regulation of
neuronal apoptosis, JNK interacting protein 1 (JIP1) emerges as a
noteworthy scaffolding protein (42). In the specific context of
glaucoma, the complex interplay between mitochondrial dysfunction,
oxidative stress and apoptosis in RGCs is well-established
(56). Notably, Rotenone, a
lipid-soluble environmental toxin, induces RGCs' apoptosis by
impeding mitochondrial complex I (57). In a study conducted by Liu et
al (42), the suppression of
JIP1 in mice exposed to rotenone led to diminished JNK activation,
reduced caspase-3 cleavage, and a decrease in TUNEL-positive RGCs
within the retina. This attenuation of rotenone-induced RGCs
electrophysiological dysfunction highlights the potential
therapeutic relevance of the JIP1-JNK signaling axis in alleviating
RGCs degeneration. Furthermore, thioredoxin 1 (Trx1), a 12 kDa
oxidoreductase, assumes a critical role in antioxidant and
anti-apoptotic processes during periods of oxidative stress
(58,59). Melatonin, renowned for its
protective effects against H2O2-induced
apoptosis and oxidative stress, functions by preserving the
expression of Trx1 and thioredoxin reductase 1 (TrxR1), and the
activity of TrxR1 in RGC-5 cells. The significance of Trx1 in
melatonin-mediated protection against oxidative stress-induced
apoptosis is highlighted by observations indicating compromised
protective effects of Trx1 knockdown. Intriguingly, the alleviating
effect of Trx1 silencing in RGC-5 cells was partially counteracted
by the administration of a JNK signaling inhibitor (60). This suggests a complex
interaction between JNK signaling and Trx1 in modulating apoptosis
and oxidative damage in RGC-5 cells, implicating the JNK signaling
pathway in safeguarding RGC-5 cells against detrimental
effects.
Indirect traumatic optic neuropathy (ITON) shares a
pathological resemblance with glaucoma, characterized by apoptosis
of RGCs and subsequent optic nerve atrophy (61). Experimental induction of an ITON
model coupled with the activation of JNK/c-Jun signaling revealed
concurrent microglial and NLRP3 inflammasome activation.
Conversely, inhibition of JNK/c-Jun signaling was found to
forestall NLRP3 inflammasome activation in microglia, thereby
protecting against RGCs' death and axonal degeneration (62). This elucidated the complex
interaction among JNK signaling, microglial activation and RGCs'
survival pathways, suggesting a potential approach for therapeutic
intervention in conditions characterized by optic nerve damage.
Bcl-2 family/caspase
Apoptosis typically occurs via two distinct
pathways: The intrinsic pathway, which is mediated by mitochondria,
and the extrinsic pathway, which is mediated by death receptors.
The Bcl-2 family of proteins plays a critical role (63). In RGCs, mitochondrial dysfunction
and oxidative stress are closely associated with apoptosis,
suggesting the involvement of the Bcl-2 family of proteins in RGCs'
death (64). This family is
comprised of three main types: Pro-apoptotic BH3 proteins (BIM,
BID, PUMA, BMF, NOXA, BIK, BAD and HRK), pro-survival proteins
(BCL-2, BCL-XL, BCL-W, MCL-1 and A1/BFL-1), and apoptotic effectors
(BAX, BAK and BOK) (65-67). Notably, BAX and BAK play pivotal
roles in triggering apoptosis and exhibit a strong affinity for BH3
structural domains (67). Under
conditions such as nutrient deprivation, lack of growth factors,
oxidative stress, exposure to γ-irradiation, or treatment with
cytotoxic drugs, BH3 activator proteins selectively bind to the BH3
structural domain-binding groove in BAX/BAK, prompting their
activation through structural changes. This triggers the formation
of BAX and BAK oligomers in the outer mitochondrial membrane,
leading to the creation of pores that permeabilize the membrane.
Consequently, cytochrome, an apoptotic factor residing within the
mitochondria, is released, which subsequently activates caspase-9
and caspase-3. Activated caspases initiate the breakdown and
elimination of RGCs (66-68)
(Fig. 2).
In cases of glaucoma resulting from damage to the
optic nerve, there is a significant increase in the levels of genes
that promote cell death, such as BAX and BAD, in both the affected
retina and optic nerve (20).
This increase led to the widespread death of RGCs. Extensive
research has revealed that activating the cAMP-response element
binding protein (CREB)/BCL-2 pathway can prevent mitochondrial cell
death. Conversely, the disruption of CREB function tends to worsen
cell death, resulting in a decreased BCL-2/BAX ratio, ultimately
leading to the loss of mitochondrial function (69). Pituitary adenylate
cyclase-activating polypeptide (PACAP) has emerged as a powerful
protector of nerve cells, exhibiting significant neuroprotective
properties (70). PACAP is
important in preventing RGCs' death, as well documented in various
animal models of retinal damage (71). After optic nerve injury, there is
a significant increase in PACAP and its receptor PAC1R in the layer
of RGCs, indicating the prevention of cell death mediated by
caspase-3 in RGCs. This series of events is further characterized
by an increase in the activation of cAMP-responsive CREB and an
elevation in the levels of BCL-2 (70). Altogether, these findings
highlight the crucial role of the CREB/BCL-2 pathway in reducing
cell death in RGCs and emphasize its importance in maintaining
retinal health and function.
Molecular signaling pathways and optic nerve
protection and regeneration
Degeneration of RGC axons is a pivotal element in
the pathogenesis of glaucomatous neurodegeneration, as postulated
in scholarly discourse (6).
Ocular hypertension, which is frequently correlated with elevated
IOP, poses a significant risk of structural impairment of the ONH,
manifesting as retinal rim thinning, augmented cup/disc ratio, and
optic disc cupping in severe manifestations. Such pathological
alterations can cause irreversible damage to the optic nerve in
patients with glaucoma (72).
Although reduction of IOP can mitigate disease progression, it does
not adequately address the fundamental issue of optic nerve
degeneration (73). Hence, there
is a compelling impetus to explore the complexities of the
signaling pathways associated with the safeguarding and
rejuvenation of the optic nerve, presenting promising broad for
impeding or reversing the process of optic nerve damage in
glaucoma.
Brain-derived neurotrophic factor (BDNF)
signaling pathway
BDNF constitutes a critical neurotrophic element
pivotal in the processes of neuronal development, differentiation
and survival (74). Its
principal synthesis occurs in the brain and retina, where it exerts
considerable neuroprotective effects in conjunction with other
neurotrophic factors. This protective mechanism operates by
alleviating the loss of RGCs following optic nerve trauma through
its interaction with receptor tyrosine kinases (Trk family) located
in the ONH (75). Specifically,
the binding of nerve growth factor to TrkA, BDNF to TrkB, and
neurotrophic factor-3 to TrkC has been established (74). Among them, furthermore, the
BDNF/TrkB signaling pathway plays a crucial role in the health of
RGCs and the prevention of apoptosis (14). BDNF administration has been
proven to delay the apoptosis of RGCs and extend the survival of
neurons under various stress conditions (76). As aforementioned, Cav-1
participates in signaling pathways, and its deficiency will impair
retinal function. However, a recent study proposed that Cav-1
negatively regulates the BDNF/TrkB signal transduction in RGCs
through its interaction with the tyrosine phosphatase 2 (Shp2)
(14). It has been reported that
the dysregulation of Shp2 is related to neurodegenerative diseases
in the brain and eyes (77,78). Abbasi et al (15) demonstrated through experiments
that the silencing of Shp2 has a protective effect on retinal
function under chronic experimental glaucoma conditions, and this
protective effect depends on Cav-1 in the retina. Cav-1 may use
raft microdomains as a platform to recruit Shp2, and then transfer
Shp2 to the vicinity of its target TrkB receptor through this
platform for interaction (79).
TrkB is a high-affinity receptor for BDNF and can support the
long-term survival of RGCs (75). Under stress conditions, Cav-1
will be hyperphosphorylated in RGCs, and the number of its bindings
with Shp2 will increase significantly, leading to the activation of
Shp2 and then the negative phosphorylation of the TrkB receptor
(80). The long-term
dephosphorylation of TrkB will result in the loss of axonal
integrity and hinder the axonal regeneration and other
neuroprotective effects of BDNF and neurotrophic factor-4 (NT-4)
(14). Therefore, in-depth
exploration of the complex interactions among BDNF, TrkB, Shp2 and
Cav-1 is of great significance for the treatment of glaucoma.
Targeting this pathway is expected to increase the survival
probability of neurons, protect the optic nerve and relieve vision
problems, bringing new hope for the clinical treatment of
glaucoma.
Activation of the PI3K/AKT signaling cascade and
ERK, accompanied by CREB phosphorylation (Fig. 2), plays a pivotal role in
fostering cellular survival and thwarting mitochondrial apoptosis,
thereby conferring neuroprotection (81). In glaucoma, noteworthy deviations
in the functional dynamics and expression patterns of BDNF and TrkB
have been observed, particularly within the retinal milieu. In this
context, the interaction between BDNF and TrkB elicits the
activation of the PI3K/AKT and ERK pathways, culminating in CREB
phosphorylation and consequently enhancing retinal resilience
(75,82). Furthermore, engagement of the p75
neurotrophic factor receptor (p75NTR), acting as a BDNF receptor,
with the brain-derived neurotrophic factor precursor (pro-BDNF)
induces apoptosis, thus presenting a counterpoint to the effects
induced by TrkB binding (75,83). Consequently, strategic
interventions targeting the proBDNF/p75NTR signaling axis have
emerged as a promising approach for increasing neuronal sustenance
and proliferation.
A previous study has revealed the capacity of
lithium to increase the population density of RGCs in a
dose-dependent manner, concomitant with the upregulation of BDNF
observed at both the mRNA and protein tiers (84). The protein Dock3, belonging to
the Dock family and known for its involvement in cellular adhesion
and neurite elongation, has been delineated as a facilitator of
axonal regeneration and neuroprotection in vivo (85,86). Notably, the signaling cascade
mediated by Dock3 is activated by BDNF, and conversely, Dock3
reciprocally enhances the stimulatory influence of BDNF on neurite
extension (74).
Furthermore, BDNF levels consistently decreased in
patients with glaucoma having ONH damage (81). Patients with primary open-angle
glaucoma (POAG) and normotensive glaucoma exhibit significantly
lower serum BDNF levels than controls, with even lower levels
observed in early-stage glaucoma than in moderate glaucoma. These
findings suggest that BDNF can potentially serve as a biomarker for
glaucoma.
Phosphatase and tensin homolog (PTEN)
signaling pathway
PTEN is a phosphatase that acts on lipids and
proteins and has been implicated in the pathogenesis of
neurodegenerative diseases. Notably, in murine models with a PTEN
knockout, substantial enhancement of axonal regeneration following
optic nerve injury has been observed (87). This phenomenon underscores the
pivotal role of PTEN in the modulation of cellular responses to
injury. Mechanistically, PTEN exerts its inhibitory effect on cell
proliferation by impeding the phosphorylation of PIP2 to PIP3,
thereby thwarting the activation of the PI3K pathway and its
downstream effectors, including AKT and the mTOR cascade (88) (Fig. 2). Conversely, the downregulation
of PTEN promotes the activation of the PI3K/AKT/mTOR axis, which
promotes axon regeneration in the optic nerve and augments the
survival of RGCs post-injury (89). Furthermore, the suppressor of
cytokine signaling 3 (SOCS3) is a pivotal regulator of signaling
pathways associated with fundamental cellular processes such as
proliferation, survival, migration and genomic stability (90). Comparative analyses revealed
markedly reduced RGCs' survival in murine models featuring either
pure optic nerve injury or SOCS3 knockout, compared with those with
PTEN deficiency alone or in combination with SOCS3 (91). Additionally, investigations of
optic nerve compression have revealed dendrite and axon retention
and regeneration (91).
The miR-200 family plays a critical role in
regulating cellular proliferation and apoptosis (92). PTEN has been identified as a
co-target of the miR-200 family, indicating a regulatory interplay
between them (93,94). Current investigations on POAG
have shifted attention toward the involvement of trabecular
meshwork (TM) cells and apoptosis of RGCs. Shen et al
(93) elucidated the interaction
between PTEN and miR-200c in TM cells, with oxidative
stress-inducing decreased expression of miR-200c-3p, consequently
leading to elevated PTEN levels and heightened cellular apoptosis
(95). Additionally, miR-200c-3p
has been shown to negatively regulate PTEN expression, cleaved
caspase-3 and Bax, while concurrently enhancing the expression of
p-AKT, AKT and mTOR, thereby promoting cell proliferation and
inhibiting apoptosis, which can be reversed by PTEN (93).
Furthermore, genes associated with mitochondrial
function, such as Dynlt1a and Lars2, have been implicated in
facilitating axon regeneration, and PTEN inhibition upregulates
their expression (96).
Moreover, PTEN inhibition was found to induce the dedifferentiation
of intrinsically light-sensitive RGCs, thereby activating the
intrinsic peripheral axon regeneration capacity (96).
In summary, PTEN has emerged as a pivotal regulator
in both the upstream and downstream pathways governing optic nerve
axons and survival and regeneration of RGCs. Consequently, PTEN
inhibition has emerged as a promising therapeutic target for optic
nerve protection and regeneration in patients with glaucoma.
Rho/ROCK signaling pathways
Rho, a constituent of the cytoplasmic Rho family of
small GTP-binding proteins, belongs to the Ras superfamily of
GTPases and encompasses three distinct isoforms: RhoA, RhoB and
RhoC (97). Notably, RhoA
exhibits a marked increase in the ONH of individuals with glaucoma
(98). Acting as a pivotal
downstream effector of Rho GTPases, ROCK (Rho-associated protein
kinase) represents a serine/threonine kinase, existing in two
isoforms, namely ROCK1 and ROCK2 (98,99). Pertinently, microglia,
acknowledged for their significance in the pathogenesis of
glaucoma, are implicated in neurotoxicity under the influence of
activated ROCK, thereby contributing to optic neuropathy (100,101). Significantly, the
administration of ROCK inhibitors, including Y-27632, Y-39983,
netarsudil and ripasudil, inhibits ROCK activation, consequently
facilitating the axonal regeneration of RGCs (102-105). Hence, the use of ROCK
inhibitors has emerged as a promising approach for the advancement
of optical neuroprotective strategies in glaucoma.
ROCK inhibitors have been proposed to slow down
optic nerve damage by lowering IOP in glaucoma (99), ROCK can improve cell
proliferation, inhibit apoptosis, and lower IOP by blocking the Rho
kinase cascade activation response (106). Moreover, ROCK inhibitors can
provide optic neuroprotection by targeting the cytoskeleton in the
TM and Schlemm's canal (SC) cells, increasing aqueous humor (AH)
outflow and reducing IOP (99).
In glaucoma, ischemia leads to progressive damage to retinal tissue
and optic nerves (107). The
Rho/ROCK signaling pathway is present in vascular smooth muscle and
promotes relaxation, suggesting that ROCK inhibitors could offer
neuroprotection by inducing vasodilation to improve blood flow to
the retina and optic nerve, thereby supporting axonal regeneration
and survival of RGCs (108).
Shaw et al (104)
discovered that RGCs survival and optic nerve axon regeneration
were significantly higher in mice treated topically with a
Rock/norepinephrine transporter protein (Net) inhibitor than in
mice administered a placebo. This topical treatment also resulted
in reduced ROCK target protein phosphorylation in the retina and
proximal optic nerve. RGCs play a crucial role in optic nerve
damage in glaucoma, with the expression of Rho-kinase significantly
increased in apoptotic RGCs. Liu et al (109) conducted in vitro
experiments by treating RGCs with siRhoA, and found that this
treatment effectively downregulated RhoA expression, protecting
cells from H2O2-induced damage. They also
observed a reduction in the expression of ROCK1, the ROCK2 receptor
and Casepase-3, along with an elevation in Bcl-2 expression at both
the mRNA and protein levels. In conclusion, blocking the Rho/ROCK
signaling pathway could be a promising approach for developing new
strategies for optic nerve protection and axon regeneration in
glaucoma treatment.
Molecular signaling pathways and LC damage
and remodeling
The LC is a reticular sieve-like structure located
in the sclera, from which the axons of RGCs converge for the optic
nerve to penetrate (110); this
is the initial site where damage to ganglion cells and axons occurs
in glaucoma (111). The
aforementioned study found that RGCs are the initial sites of
damage to ganglion cells and axons in glaucoma. It has been found
that the LC depth is generally more posterior, and the LC
morphology is more distorted in patients with glaucoma than in
healthy eyes, which blocks axonal transport and reduces the
diffusion of nutrients from capillaries within the laminar bundles
to neighboring axons, promoting damage to axons and their cell
bodies (112,113). In addition, LC cells can pass
through laminar bundles to adjacent axons. In addition, LC cells
can exhibit negative effects on the environment in which RGC axons
traverse through ECM remodeling (114). The current study of the
signaling pathways involved in the damage and remodeling of the LC
could help identify potential therapeutic targets to stop the
damage and remodeling of the LC and restore the normal morphology
and function of the LC to slow down the glaucomatous process.
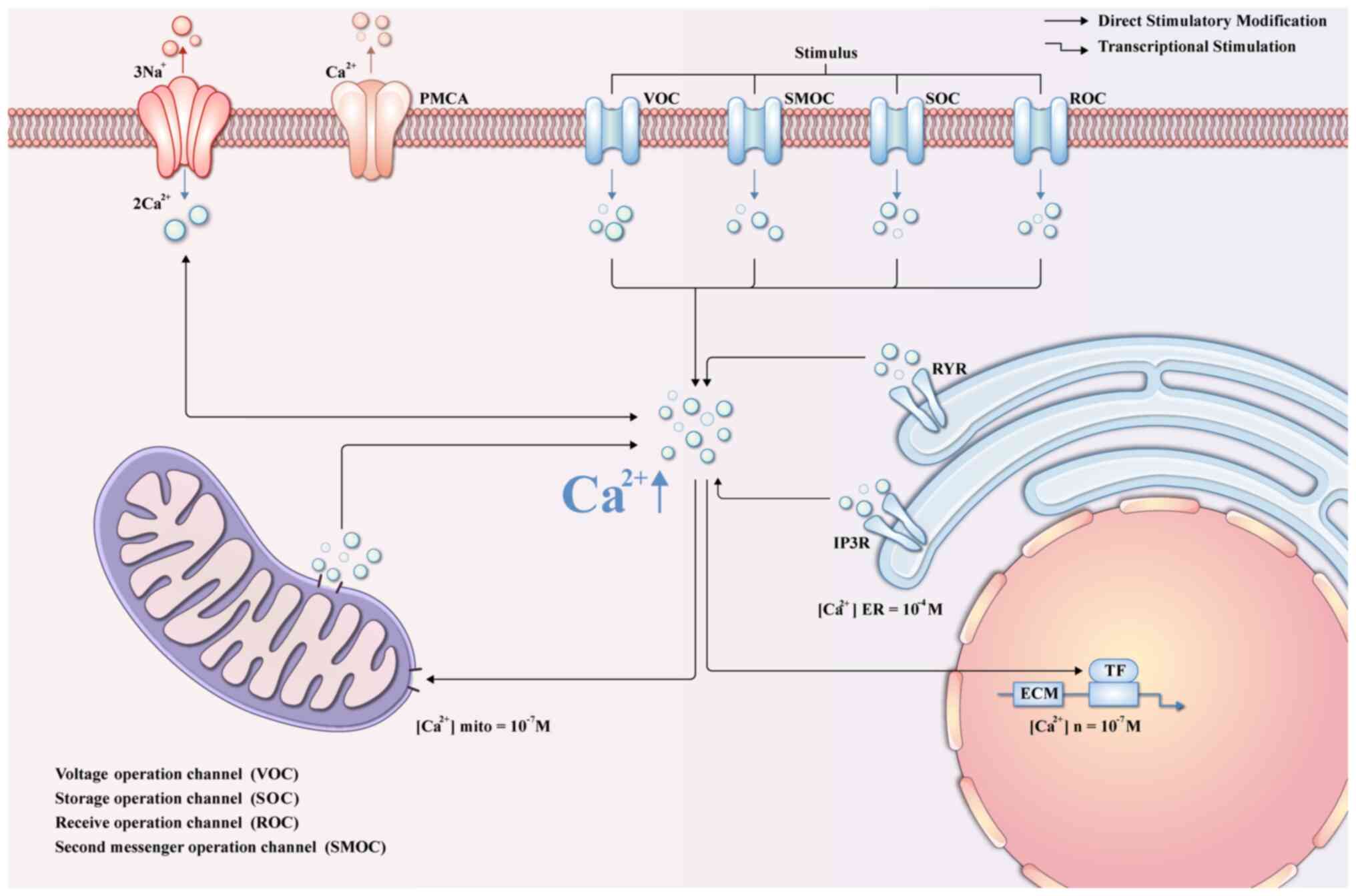
Calcium ions (Ca2+) signaling
pathways
Ca2+ enter cells and interact with
various Ca2+-binding proteins, serving as pivotal
signaling entities in the regulation of numerous physiological
processes (115). Perturbation
of Ca2+ homeostasis has been implicated in various
pathological conditions, including oxidative stress-induced cell
death in glaucoma (116).
Notably, cytoplasmic Ca2+ levels are aberrantly elevated
in LC cells in models of oxidative stress-induced glaucoma
(117). This elevation
comprises two distinct components: The direct influx of
extracellular calcium into the cytoplasm in response to the stress
stimulus, and release from intracellular stores, namely the
endoplasmic reticulum and sarcoplasmic reticulum, mediated by the
Inositol 1,4,5-trisphosphate receptor (IP3R) and ryanodine receptor
(118,119). The increase in intracellular
Ca2+ in glaucomatous LC cells promotes the expression of
ECM genes, thereby instigating excessive ECM deposition, as shown
in Fig. 3. Consequently,
fibroblasts undergo phenotypic alterations and differentiate into
myofibroblasts, driving connective tissue fibrosis, and
exacerbating glaucomatous optic neuropathy (120). Additionally, other studies have
also demonstrated that the synthesis and secretion processes of
ECM-related molecules are also regulated by Cav-1 (121,122). The decrease in Cav-1 expression
contributes to the disruption of ECM remodeling, thus protecting
the microenvironment around RGCs and maintaining the integrity of
the structure and normal physiological functions of ocular tissues
(122).
In the context of fibrosis, protein kinase C (PKC),
p38, p42/44-MAPK and the PI3K/mTOR axis were proposed as pivotal
signaling transduction mediators downstream of Ca2+
signaling (120). Experimental
simulation of glaucoma through hypo-osmotic-induced cell swelling
has revealed notable upregulation in the expression and activity of
PKCα, p38 and p42/p44-MAPKs (120). Additionally, increased mRNA
expression of PI3K, IP3R, mTOR and Ca2+-calmodulin
protein kinase II was observed in LC under such conditions. The
calcium-modulated phosphatase-NFAT signaling pathway plays a
significant role in promoting fibroblast proliferation, activation
and contraction. Notably, Cyclosporin A, an inhibitor of NFAT
activity, has shown significant suppression of NFATc3 expression
induced by oxidative stress, as well as elevation of pro-fibrotic
ECM genes in both normal and glaucomatous LC cells (123). Hence, targeting PKCα, p38,
p42/p44-MAPKs, PI3K/mTOR and the calmodulin phosphatase-NFATc3
signaling pathways emerges as a potential therapeutic strategy for
addressing glaucoma-associated LC fibrosis.
Besides, L-type Ca2+ channels are
increased in glaucomatous LC cells, and the administration of
verapamil, a blocker of L-type Ca2+ channels, reduces
the mechanical strain-induced ECM gene response in human LC cells
(124). Blocking
Ca2+ signaling has been hypothesized to attenuate the
fibrotic response in glaucomatous optic neuropathy. It has been
found that voltage-independent, stretch-activated cation channels
and transient receptor potentials typical of TRPC1 and TRPC6 are
highly expressed in glaucomatous LC cells and are also involved in
abnormally high levels of Ca2+ in glaucomatous LC cells
(125). Hu et al
(126) established a mouse
model of COH and found that the increase in ATP levels and
Ca2+ endocytosis in response to hydrostatic pressure was
accompanied by upregulation of Transient Receptor Potential
Vanilloid 1 (TRPV1, encoded by the TRPV1 gene) and
1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase γ-1
(PLCγ1, encoded by the PLCG1 gene) expression. In the same study,
knockdown of TRPV1 and PLCG1 genes resulted in a significant
reduction in ATP levels and intracellular Ca2+, as well
as a significant attenuation of apoptosis and autophagy in RGCs. In
addition, the expression and activity of the
Ca2+-dependent potassium channel maxi-K were
significantly elevated in glaucomatous LC cells (127). In summary, Ca2+
homeostasis is a critical factor for cell function and survival,
and the maintenance of Ca2+ homeostasis helps attenuate
LC cell injury and ECM remodeling, providing a potential target for
the treatment of persistent optic neuropathy (128).
Transforming growth factor-beta (TGF-β)
signaling pathway
TGF-β belongs to the dimeric peptide growth factor
family and plays pivotal roles in various signaling cascades
associated with cellular processes such as differentiation,
proliferation, chemotaxis and fibrosis. It encompasses three
primary isoforms, namely TGF-β1, TGF-β2 and TGF-β3, among which
TGF-β2 exhibits a particularly close association with glaucoma
(88,129). TGF-β orchestrates ECM
remodeling, which contributes to the deposition of fibrotic
material in the retina, consequently instigating glaucomatous
conditions (130). Elevated
levels of TGF-β have been detected in glaucomatous AH (131), underscoring its involvement in
the pathogenesis of glaucoma. Additionally, TGF-β2 has been shown
to activate Src signaling by upregulating the Src scaffolding
protein CasL, thereby impeding the expression of tissue plasminogen
activator and attenuating ECM degradation (132). It has been proposed that TGF-β
exerts its influence on ECM generation and remodeling in tandem
with the classical Smad pathway (88). Within this pathway, TGF-β binds
to TGF-β type II receptor (TβRII), initiating a cascade wherein the
TβRII phosphorylates TGF-β type I receptor (133,134), leading to downstream
phosphorylation of Smad2 and Smad3. These phosphorylated Smads form
a complex with Smad4, translocate to the nucleus, and modulate gene
transcription, thereby promoting ECM production within the ONH and
precipitating glaucoma (88,135). Moreover, members of the TGF-β
superfamily can induce glaucoma development by activating PI3K/AKT
and MAPK signaling pathways (134). The above content is clearly
presented in Fig. 2.
Recent investigations have revealed a significant
elevation of TGF-β2 levels in glaucomatous ONH and LC, as evidenced
in a non-human primate glaucoma model, highlighting the involvement
of TGF-β2 in glaucoma pathogenesis (136). TGF-β signaling exerts a direct
influence on the expression of microRNAs (miRNAs), which in turn
regulate protein translation. Specifically, dysregulation of
miRNA-29 family expression, attributed to TGF-β2 signaling, may
contribute to glaucomatous pathology by modulating ECM deposition
and remodeling within the LC (137). Furthermore, TGF-β signaling has
been implicated in the differentiation of myoblasts into
myofibroblasts through Smad3-mediated downregulation of miRNA-29
expression (138). Aberrant
miRNA-29 expression, which results in dysregulated TGF-β2
signaling, may promote glaucoma by exacerbating ECM deposition and
remodeling within the LC (137). Additionally, upregulation of
the long non-coding RNA (lncRNA) series NR_003923 has been observed
in glaucomatous tissues, with knockdown experiments revealing
inhibition of TGF-β signaling and reduced cell migration, fibrosis
and autophagy (139). To
summarize, targeting TGF-β signaling emerges as a promising
therapeutic approach for the treatment of glaucoma.
Other mechanisms related to signaling
pathways
In recent years, notable advances in molecular
biology and cytogenetics have unveiled novel mechanisms, along with
conventional molecular signal transduction pathways. Oxidative
stress, inflammation and glutamate have emerged as pivotal factors
in the pathogenesis of glaucoma (140-142). Previous studies have
demonstrated that the dysregulation of the WNT/β-catenin pathway
induces glutamate excitotoxicity, leading to increased inflammation
and oxidative stress, and then participates in the occurrence and
development of glaucoma (143).
In the retina, Wnt/β-catenin signal transduction can maintain
neuronal homeostasis and regulate neuronal regeneration as well as
provide neuroprotection against mutations, oxidative stress, laser
and light damage (144,145). The Wnt/β-catenin pathway has
two states, namely 'on' and 'off' (146). In the off state, adenomatous
polyposis coli, glycogen synthase kinase 3 (GSK3), axin and casein
kinase form a β-catenin protein destruction complex, which mediates
its phosphorylation and inhibits the transcription of Wnt/β-catenin
target genes. When the pathway is turned on, Wnt binds to the
Frizzled receptor and forms a complex with LRP5/6, phosphorylates
and activates the Dishevelled (Dvl) protein. Dvl inhibits the
β-catenin protein destruction complex, causing β-catenin to be
retained in the cytoplasm and translocated to the nucleus. There,
it forms a complex with the T cell factor family/lymphocyte
enhancer-binding factor to activate the transcription of downstream
target genes such as c-Myc, c-Jun and cyclin D1 (Fig. 4), thereby regulating the cell
cycle and playing a regulatory role in cell survival and
proliferation (147,148). In addition, experimental
studies have found that the activation of the Wnt/β-catenin signal
pathway can lead to axonal regeneration in a mouse model of optic
nerve injury (149). In the
same experimental context, it was also found that Wnt signal
transduction is active in RGCs, and Wnt3a can also increase the
survival rate of RGCs after injury. Moreover, Wnt signal
transduction can induce the expression of genes with
pro-regenerative properties (such as STAT3 and CNTF) (145,150,151), while being inhibited by genes
that suppress regeneration (such as KLF4 and ephrin) (145,152,153). It has been previously shown
that the WNT/β-catenin pathway is also involved in the
pathophysiology of TM cells (154). The activation of this pathway
can promote the survival of TM cells and may participate in the
metabolic regulation of the ECM. When this signal pathway becomes
abnormal, the structure and function of the TM will be affected,
which may lead to the obstruction of AH outflow, increased IOP, and
is closely related to the occurrence of ocular diseases such as
glaucoma (143). In conclusion,
the Wnt/β-catenin signaling pathway plays an important regulatory
role in multiple aspects of the pathogenesis of glaucoma, including
its impact on the survival of RGCs, axonal regeneration, and cell
behaviors and tissue microenvironment related to the maintenance of
IOP homeostasis. Therefore, it can be regarded as a highly
promising target for the treatment of glaucoma.
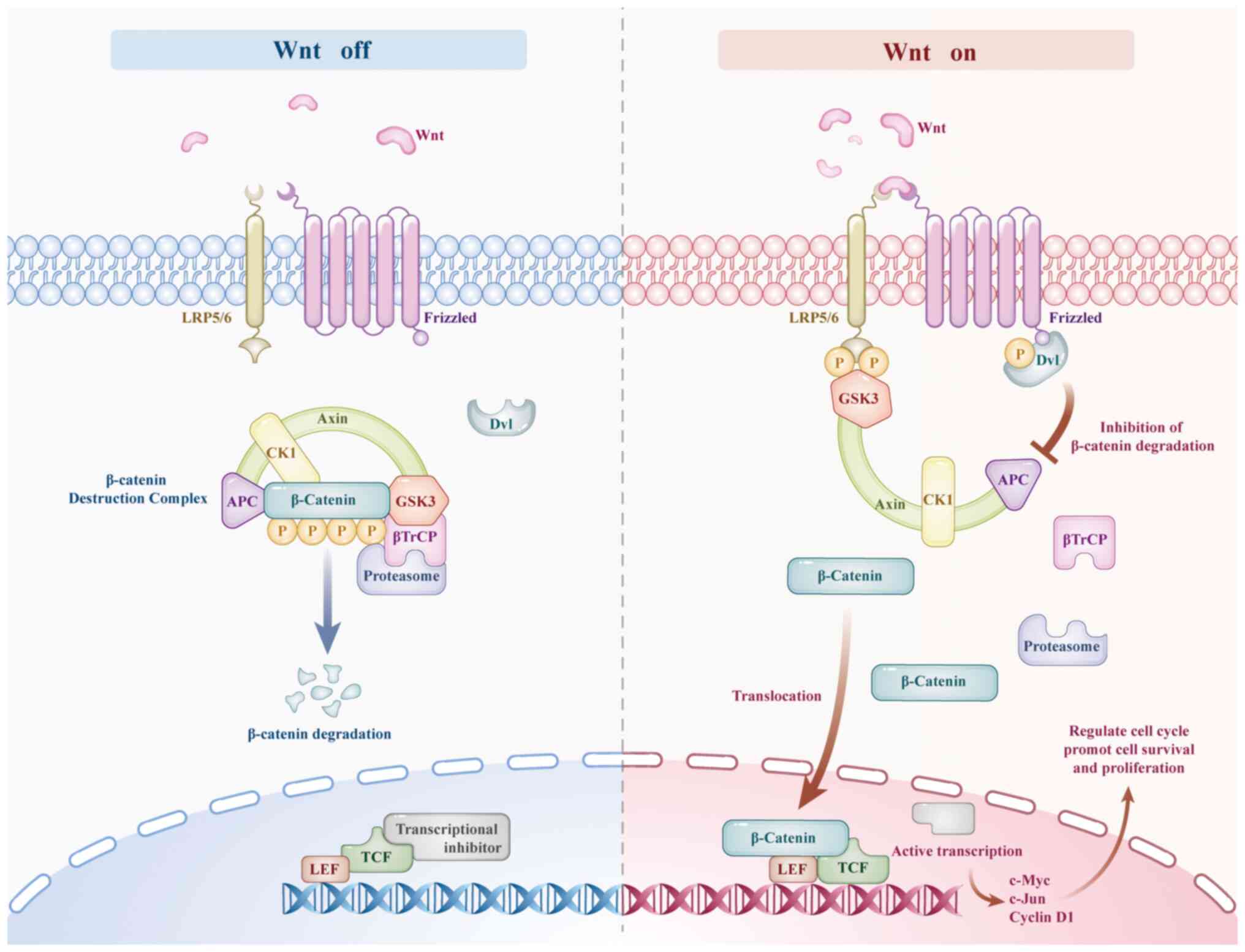 | Figure 4Regulation of the Wnt/β-catenin
signaling pathway. LRP5/6, low-density lipoprotein receptor-related
protein 5/6; frizzled, frizzled receptor; axin, axis inhibition
protein; Dvl, dishevelled protein; CK1, casein kinase 1; GSK-3,
glycogen synthase kinase 3; βTrCP, beta-transducin
repeat-containing protein; TCF, T cell factor; LEF, lymphocyte
enhancer-binding factor; APC, adenomatous polyposis coli protein;
c-Myc, cellular myelocytomatosis virus oncogene homolog; c-Jun,
proto-oncogene c-Jun. |
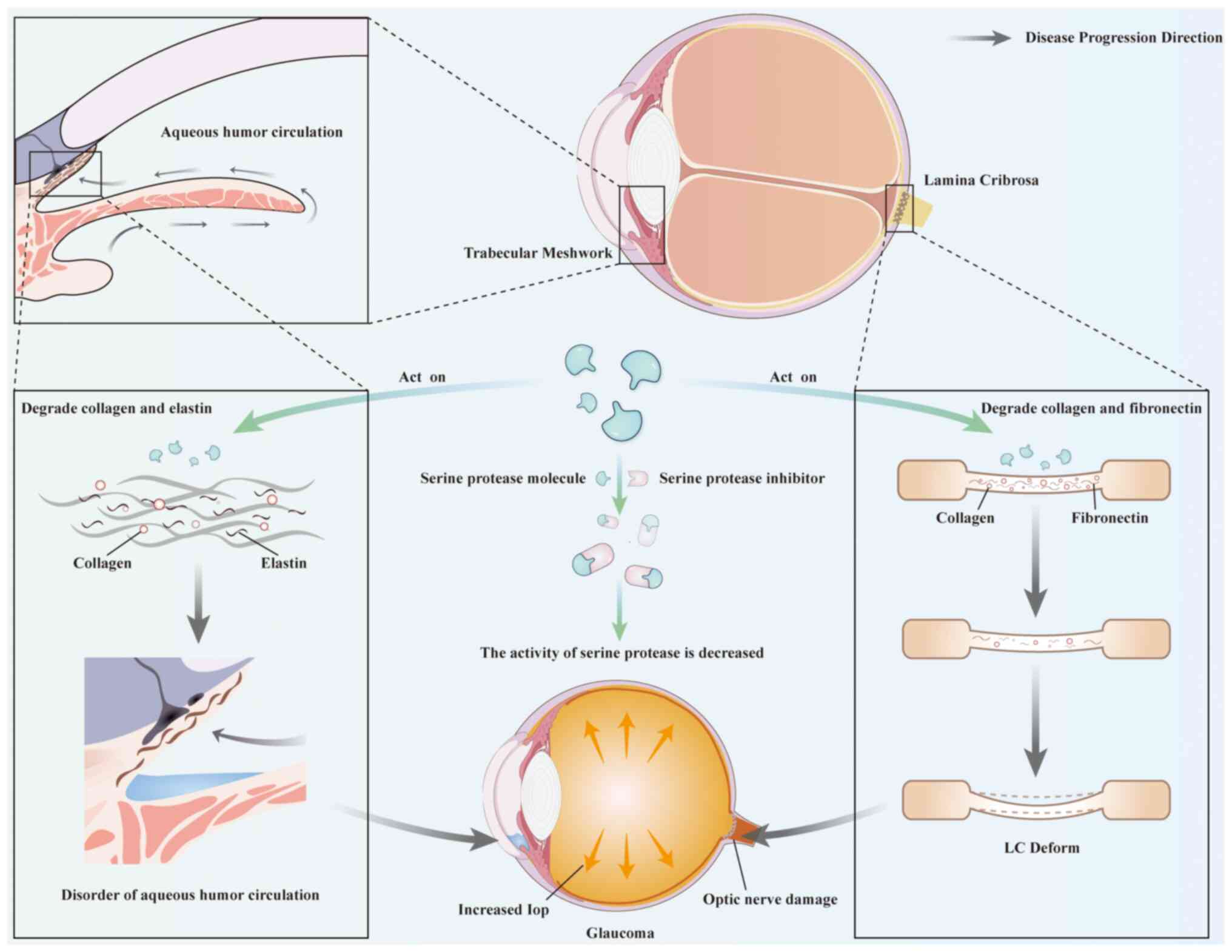
Besides, in the research on the pathogenesis of
glaucoma, serine proteases and their inhibitors have also become
relatively new focuses. In glaucoma, serine proteases (such as
plasmin) can participate in the remodeling of the ECM and affect
the survival of RGCs (155,156). Under pathological conditions,
when excessive serine proteases act on the ECM in the LC, they may
overly degrade the components in the ECM, making the structure of
the LC loose or causing local defects, which will cause compression
and damage to the optic nerve (124,157) (Fig. 5). When serine proteases act on
the TM, the disorder of ECM homeostasis leads to an increase in the
resistance to AH outflow and an increase in IOP (Fig. 5), thus contributing to the
occurrence and development of glaucoma (158). Furthermore, it has been
revealed that while the proteolytic activity of serine proteases in
glaucoma increases, the protease inhibitory activity of nerve
serine protease inhibitors decreases in chronic glaucoma animal
models (156). SerpinA1 and
SerpinA3 are important members of the serine protease inhibitor
family. Elevated SerpinA1 levels have been proven to indirectly
affect Wnt signaling by regulating the AKT pathway through
increasing the synthesis of GSK-3β, resulting in a decrease in
β-catenin levels and delaying glaucoma to a certain extent
(159). Several research
studies have put forward that gene therapy or the external
application of nerve serine protease inhibitors, and likewise in
transgenic mice with overexpressed nerve serine protease
inhibitors, is capable of demonstrating a protective impact on the
functions of RGCs (160-162).
This protection encompasses the restoration of autophagy,
microglial and synaptic functions in the context of glaucoma
(163). In summary, serine
proteases may damage intraocular tissues due to excessive
hydrolysis in glaucoma, while their inhibitors can inhibit the
harmful effects of proteases by regulating relevant signaling
pathways and other means, thus playing an inhibitory role in the
development of glaucoma. They are expected to become new targets
for the treatment of glaucoma.
Research findings show that insights into the role
of the protease cysteinyl asparaginase in the apoptotic pathway
have emerged, with evidence suggesting that the absence of
caspase-8 in astrocytes can shield RGCs from inflammatory injury
driven by glial cells (163).
Conversely, the inhibition of caspase-8 cleavage suppressed
apoptosis in RGCs. Moreover, mice lacking cysteine 7 exhibited
greater retention of RGCs following an optic nerve crush than
wild-type mice, indicating the potential neuroprotective effect of
blocking cysteine (164).
Although histone and DNA modifications, miRNAs and lncRNAs have
been implicated in glaucoma development (88), their epigenetic nature has not
been extensively examined.
Conclusions
Glaucoma has emerged as a significant threat to
human vision and is characterized by complex and diverse
pathogenesis. By investigating the signaling pathways involved in
RGCs apoptosis, optic nerve protection and regeneration, as well as
damage and remodeling of the LC, it was identified that these
signaling pathway molecules can interact with various signal
cascades. These factors cover signaling pathways including
PI3K/AKT, p38 MAPK, JNK, Bcl-2 family/caspase, BDNF, PTEN,
Rho/ROCK, Ca2+ and TGF-β. They regulate the activity and
expression of transcription factors through interactive coupling,
and then directly act on the level of gene expression, assuming a
crucial regulatory function throughout the whole process from the
onset to the progression of glaucoma and profoundly influencing the
disease progression of glaucoma. Cav-1 also has a critical
significance in the pathophysiological process of glaucoma. It has
complex interactions with the numerous signaling pathways
aforementioned, which is essential for in-depth exploration of the
pathogenesis of glaucoma. In addition, the WNT/β-catenin pathway,
caspase, together with the biological effects of serine proteases
and their inhibitors, have all been confirmed to be closely
associated with the occurrence and development of glaucoma and play
an indispensable role in the disease evolution process of glaucoma.
In recent years, increasing experimental evidence suggests that
intervening or regulating interactions between signaling pathways
could potentially treat or alleviate symptoms associated with
glaucoma. At present, numerous drug experiments have developed
targeted drugs associated with the aforementioned signaling
pathways that exhibit promising effects on RGCs' apoptosis while
promoting optic nerve regeneration and preventing LC remodeling;
however, most of them remain at the stage of animal experimentation
or clinical research. Further extensive and comprehensive studies
are required to achieve large-scale clinical translation of these
findings. Additionally, the present review did not adequately
address some emerging mechanisms and non-classical signaling
pathways, which will be explored further in future studies.
Understanding and harnessing these signaling pathways presents
immense potential for reversing RGCs apoptosis and alleviating
optic nerve damage caused by glaucoma.
Availability of data and materials
Not applicable.
Authors' contributions
XW collected literature, created images, wrote and
revised the manuscript. LS collected literature and wrote the
manuscript. XH designed the study and edited the manuscript. ZL, XC
and RX created images and revised the manuscript. YX and YS
reviewed and edited the manuscript. GW designed the study, reviewed
and edited the manuscript. PZ designed the study, reviewed and
revised the manuscript. All authors read and approved the final
version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present review was supported by the National Natural Youth
Science Foundation (grant no. 82205198), the China Postdoctoral
Science Foundation project (grant no. 2022M711984), the Shandong
Provincial Natural Science Foundation General Program (grant no.
ZR2020MH393), the Postdoctoral Innovation Project of Shandong
(grant no. 202101012) and the China Postdoctoral Foundation General
Program (grant no. 2020M672127).
References
|
1
|
Jayaram H, Kolko M, Friedman DS and
Gazzard G: Glaucoma: Now and beyond. Lancet. 402:1788–1801. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tham YC, Li X, Wong TY, Quigley HA, Aung T
and Cheng CY: Global prevalence of glaucoma and projections of
glaucoma burden through 2040: A systematic review and
meta-analysis. Ophthalmology. 121:2081–2090. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kang JM and Tanna AP: Glaucoma. Med Clin
North Am. 105:493–510. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Downs JC and Girkin CA: Lamina cribrosa in
glaucoma. Curr Opin Ophthalmol. 28:113–119. 2017. View Article : Google Scholar :
|
|
5
|
Fernández-Albarral JA, Ramírez AI, de Hoz
R, Matamoros JA, Salobrar-García E, Elvira-Hurtado L, López-Cuenca
I, Sánchez-Puebla L, Salazar JJ and Ramírez JM: Glaucoma: From
pathogenic mechanisms to retinal glial cell response to damage.
Front Cell Neurosci. 18:13545692024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Syc-Mazurek SB and Libby RT: Axon injury
signaling and compartmentalized injury response in glaucoma. Prog
Retin Eye Res. 73:1007692019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li L and Song F: Biomechanical research
into lamina cribrosa in glaucoma. Natl Sci Rev. 7:1277–1279. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Crupi L, Capra AP, Paterniti I, Lanza M,
Calapai F, Cuzzocrea S, Ardizzone A and Esposito E: Evaluation of
the nutraceutical Palmitoylethanolamide in reducing intraocular
pressure (IOP) in patients with glaucoma or ocular hypertension: A
systematic review and meta-analysis. Nat Prod Res. 1–20. 2024.
View Article : Google Scholar
|
|
9
|
Keuthan CJ, Schaub JA, Wei M, Fang W,
Quillen S, Kimball E, Johnson TV, Ji H, Zack DJ and Quigley HA:
Regional gene expression in the retina, optic nerve head, and optic
nerve of mice with optic nerve crush and experimental glaucoma. Int
J Mol Sci. 24:137192023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu L and Yang X, Zhang J, Jiang W, Hou T,
Zong Y, Bai H, Yang K and Yang X: Long non-coding RNA SNHG11
regulates the Wnt/β-catenin signaling pathway through rho/ROCK in
trabecular meshwork cells. FASEB J. 37:e228732023. View Article : Google Scholar
|
|
11
|
Tsai T, Reinehr S, Deppe L, Strubbe A,
Kluge N, Dick HB and Joachim SC: Glaucoma animal models beyond
chronic IOP increase. Int Mol Sci. 25:9062024. View Article : Google Scholar
|
|
12
|
Leung DYL and Tham CC: Normal-tension
glaucoma: Current concepts and approaches-A review. Clin Exp
Ophthalmol. 50:247–259. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang W and Wang H: Understanding the
complex genetics and molecular mechanisms underlying glaucoma. Mol
Aspects Med. 94:1012202023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abbasi M, Gupta V, Chitranshi N,
Moustardas P, Ranjbaran R and Graham SL: Molecular mechanisms of
glaucoma pathogenesis with implications to caveolin adaptor protein
and Caveolin-Shp2 axis. Aging Dis. 15:2051–2068. 2024. View Article : Google Scholar :
|
|
15
|
Abbasi M, Gupta VK, Chitranshi N, Gupta V,
Ranjbaran R, Rajput R, Pushpitha K, Kb D, You Y, Salekdeh GH, et
al: Inner retinal injury in experimental glaucoma is prevented upon
AAV mediated Shp2 silencing in a caveolin dependent manner.
Theranostics. 11:6154–6172. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xi X, Chen Q, Ma J, Wang X, Xia Y, Wen X,
Cai B and Li Y: Acteoside protects retinal ganglion cells from
experimental glaucoma by activating the PI3K/AKT signaling pathway
via caveolin 1 upregulation. Ann Transl Med. 10:3122022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang JH, Wang MJ, Tan YT, Luo J and Wang
SC: A bibliometric analysis of apoptosis in glaucoma. Front
Neurosci. 17:11051582023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Erichev VP, Khachatryan GK and Khomchik
OV: Current trends in studying pathogenesis of glaucoma. Vestn
Oftalmol. 137:268–274. 2021.In Russian. View Article : Google Scholar
|
|
19
|
Xu F, Na L, Li Y and Chen L: Roles of the
PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and
tumours. Cell Biosci. 10:542020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Levkovitch-Verbin H: Retinal ganglion cell
apoptotic pathway in glaucoma: Initiating and downstream
mechanisms. Prog Brain Res. 220:37–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nie XG, Fan DS, Huang YX, He YY, Dong BL
and Gao F: Downregulation of microRNA-149 in retinal ganglion cells
suppresses apoptosis through activation of the PI3K/Akt signaling
pathway in mice with glaucoma. Am J Physiol Cell Physiol.
315:C839–C849. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Husain S, Ahmad A, Singh S, Peterseim C,
Abdul Y and Nutaitis MJ: PI3K/Akt pathway: A role in δ-opioid
receptor-mediated RGC Neuroprotection. Invest Ophthalmol Vis Sci.
58:6489–6499. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bilanges B, Posor Y and Vanhaesebroeck B:
PI3K isoforms in cell signalling and vesicle trafficking. Nat Rev
Mol Cell Biol. 20:515–534. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jafari M, Ghadami E, Dadkhah T and
Akhavan-Niaki H: PI3k/AKT signaling pathway: Erythropoiesis and
beyond. J Cell Physiol. 234:2373–2385. 2019. View Article : Google Scholar
|
|
25
|
Sánchez-Alegría K, Flores-León M,
Avila-Muñoz E, Rodríguez-Corona N and Arias C: PI3K signaling in
neurons: A central node for the control of multiple functions. Int
J Mol Sci. 19:37252018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haddadi N, Lin Y, Travis G, Simpson AM,
Nassif NT and McGowan EM: PTEN/PTENP1: 'Regulating the regulator of
RTK-dependent PI3K/Akt signalling', new targets for cancer therapy.
Mol Cancer. 17:372018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yudushkin I: Getting the Akt Together:
Guiding Intracellular Akt Activity by PI3K. Biomolecules. 9:672019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu K, Li S, Yang Q, Zhou Z, Fu M, Yang X,
Hao K, Liu Y and Ji H: MicroRNA-145-5p targeting of TRIM2 mediates
the apoptosis of retinal ganglion cells via the PI3K/AKT signaling
pathway in glaucoma. J Gene Med. 23:e33782021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ariotti N and Parton RG: SnapShot:
Caveolae, caveolins, and cavins. Cell. 154:704–704.e1. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Parton RG and Collins BM: The structure of
caveolin finally takes shape. Sci Adv. 8:eabq69852022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Surguchov A: Caveolin: A new link between
diabetes and AD. Cell Mol Neurobiol. 40:1059–1066. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Elliott MH, Ashpole NE, Gu X, Herrnberger
L, McClellan ME, Griffith GL, Reagan AM, Boyce TM, Tanito M, Tamm
ER and Stamer WD: Caveolin-1 modulates intraocular pressure:
Implications for caveolae mechanoprotection in glaucoma. Sci Rep.
6:371272016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu Q, Shi W, Lv P, Meng W, Mao G, Gong C,
Chen Y, Wei Y, He X, Zhao J, et al: Critical role of caveolin-1 in
aflatoxin B1-induced hepatotoxicity via the regulation of oxidation
and autophagy. Cell Death Dis. 11:62020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
De Almeida CJG: Caveolin-1 and Caveolin-2
can be antagonistic partners in inflammation and beyond. Front
Immunol. 8:15302017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kang Q, Xiang Y, Li D, Liang J, Zhang X,
Zhou F, Qiao M, Nie Y, He Y, Cheng J, et al: MiR-124-3p attenuates
hyperphosphorylation of Tau protein-induced apoptosis via
caveolin-1-PI3K/Akt/GSK3β pathway in N2a/APP695swe cells.
Oncotarget. 8:24314–24326. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu H, Chen B and Ren Q: Baicalin relieves
hypoxia-aroused H9c2 cell apoptosis by activating
Nrf2/HO-1-mediated HIF1α/BNIP3 pathway. Artif Cells Nanomed
Biotechnol. 47:3657–3663. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xiao JR, Do CW and To CH: Potential
therapeutic effects of baicalein, baicalin, and wogonin in ocular
disorders. J Ocul Pharmacol Ther. 30:605–614. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Q, Xi X, Zeng Y, He Z, Zhao J and Li
Y: Acteoside inhibits autophagic apoptosis of retinal ganglion
cells to rescue glaucoma-induced optic atrophy. J Cell Biochem.
120:13133–13140. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao N, Shi J, Xu H, Luo Q, Li Q and Liu
M: Baicalin suppresses glaucoma pathogenesis by regulating the
PI3K/AKT signaling in vitro and in vivo. Bioengineered.
12:10187–10198. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim EK and Choi EJ: Compromised MAPK
signaling in human diseases: An update. Arch Toxicol. 89:867–882.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Moustardas P, Aberdam D and Lagali N: MAPK
pathways in ocular pathophysiology: Potential therapeutic drugs and
challenges. Cells. 12:6172023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu W, Li X, Chen X, Zhang J, Luo L, Hu Q,
Zhou J, Yan J, Lin S and Ye J: JIP1 deficiency protects retinal
ganglion cells from apoptosis in a Rotenone-induced injury model.
Front Cell Dev Biol. 7:2252019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Silverman SM and Wong WT: Microglia in the
retina: Roles in development, maturity, and disease. Annu Rev Vis
Sci. 4:45–77. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Canovas B and Nebreda AR: Diversity and
versatility of p38 kinase signalling in health and disease. Nat Rev
Mol Cell Biol. 22:346–366. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mazaheri N, Peymani M, Galehdari H, Ghaedi
K, Ghoochani A, Kiani-Esfahani A and Nasr-Esfahani MH: Ameliorating
Effect of Osteopontin on H2O2-Induced apoptosis of human
oligodendrocyte progenitor cells. Cell Mol Neurobiol. 38:891–899.
2018. View Article : Google Scholar
|
|
46
|
Sun CM, Enkhjargal B, Reis C, Zhou KR, Xie
ZY, Wu LY, Zhang TY, Zhu QQ, Tang JP, Jiang XD and Zhang JH:
Osteopontin attenuates early brain injury through regulating
autophagy-apoptosis interaction after subarachnoid hemorrhage in
rats. CNS Neurosci Ther. 25:1162–1172. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huang RH, Quan YJ, Chen JH, Wang TF, Xu M,
Ye M, Yuan H, Zhang CJ, Liu XJ and Min ZJ: Osteopontin promotes
cell migration and invasion, and inhibits apoptosis and autophagy
in colorectal cancer by activating the p38 MAPK signaling pathway.
Cell Physiol Biochem. 41:1851–1864. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ulland TK, Song WM, Huang SC, Ulrich JD,
Sergushichev A, Beatty WL, Loboda AA, Zhou Y, Cairns NJ, Kambal A,
et al: TREM2 maintains microglial metabolic fitness in Alzheimer's
disease. Cell. 170:649–663.e13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ruzafa N, Pereiro X, Aspichueta P, Araiz J
and Vecino E: The retina of osteopontin deficient mice in aging.
Mol Neurobiol. 55:213–221. 2018. View Article : Google Scholar :
|
|
50
|
Lin EY, Xi W, Aggarwal N and Shinohara ML:
Osteopontin (OPN)/SPP1: From its biochemistry to biological
functions in the innate immune system and the central nervous
system (CNS). Int Immunol. 35:171–180. 2023. View Article : Google Scholar :
|
|
51
|
Yu H, Zhong H, Li N, Chen K, Chen J, Sun
J, Xu L, Wang J, Zhang M, Liu X, et al: Osteopontin activates
retinal microglia causing retinal ganglion cells loss via p38 MAPK
signaling pathway in glaucoma. FASEB J. 35:e214052021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ando K, Uemura K, Kuzuya A, Maesako M,
Asada-Utsugi M, Kubota M, Aoyagi N, Yoshioka K, Okawa K, Inoue H,
et al: N-cadherin regulates p38 MAPK signaling via association with
JNK-associated leucine zipper protein: Implications for
neurodegeneration in Alzheimer disease. J Biol Chem. 286:7619–7628.
2011. View Article : Google Scholar :
|
|
53
|
Spigolon G, Cavaccini A, Trusel M, Tonini
R and Fisone G: cJun N-terminal kinase (JNK) mediates
cortico-striatal signaling in a model of Parkinson's disease.
Neurobiol Dis. 110:37–46. 2018. View Article : Google Scholar
|
|
54
|
Mammone T, Chidlow G, Casson RJ and Wood
JPM: Expression and activation of mitogen-activated protein kinases
in the optic nerve head in a rat model of ocular hypertension. Mol
Cell Neurosci. 88:270–291. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kim BJ, Silverman SM, Liu Y, Wordinger RJ,
Pang IH and Clark AF: In vitro and in vivo neuroprotective effects
of cJun N-terminal kinase inhibitors on retinal ganglion cells. Mol
Neurodegener. 11:302016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kang EY, Liu PK, Wen YT, Quinn PMJ, Levi
SR, Wang NK and Tsai RK: Role of oxidative stress in ocular
diseases associated with retinal ganglion cells degeneration.
Antioxidants (Basel). 10:19482021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu S, Chen S, Ren J, Li B and Qin B:
Ghrelin protects retinal ganglion cells against rotenone via
inhibiting apoptosis, restoring mitochondrial function, and
activating AKT-mTOR signaling. Neuropeptides. 67:63–70. 2018.
View Article : Google Scholar
|
|
58
|
Yeo EJ, Eum WS, Yeo HJ, Choi YJ, Sohn EJ,
Kwon HJ, Kim DW, Kim DS, Cho SW, Park J, et al: Protective role of
transduced Tat-thioredoxin1 (Trx1) against oxidative stress-induced
neuronal cell death via ASK1-MAPK signal pathway. Biomol Ther
(Seoul). 29:321–330. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hu J, Liu J, Chen S, Zhang C, Shen L, Yao
K and Yu Y: Thioredoxin-1 regulates the autophagy induced by
oxidative stress through LC3-II in human lens epithelial cells.
Clin Exp Pharmacol Physiol. 50:476–485. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gao S, Cheng Q, Hu Y, Fan X, Liang C, Niu
C, Kang Q and Wei T: Correction to: Melatonin antagonizes oxidative
stress-induced apoptosis in retinal ganglion cells through
activating the thioredoxin-1 pathway. Mol Cell Biochem.
479:13172024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bernardo-Colón A, Vest V, Cooper ML,
Naguib SA, Calkins DJ and Rex TS: Progression and pathology of
traumatic optic neuropathy from repeated primary blast exposure.
Front Neurosci. 13:7192019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chu X, Wang C, Wu Z, Fan L, Tao C, Lin J,
Chen S, Lin Y and Ge Y: JNK/c-Jun-driven NLRP3 inflammasome
activation in microglia contributed to retinal ganglion cells
degeneration induced by indirect traumatic optic neuropathy. Exp
Eye Res. 202:1083352021. View Article : Google Scholar
|
|
63
|
Glab JA, Cao Z and Puthalakath H: Bcl-2
family proteins, beyond the veil. Int Rev Cell Mol Biol. 351:1–22.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Maes ME, Schlamp CL and Nickells RW: BAX
to basics: How the BCL2 gene family controls the death of retinal
ganglion cells. Prog Retin Eye Res. 57:1–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Singh R, Letai A and Sarosiek K:
Regulation of apoptosis in health and disease: The balancing act of
BCL-2 family proteins. Nat Rev Mol Cell Biol. 20:175–193. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kaloni D, Diepstraten ST, Strasser A and
Kelly GL: BCL-2 protein family: Attractive targets for cancer
therapy. Apoptosis. 28:20–38. 2023. View Article : Google Scholar :
|
|
67
|
Aniogo EC, George BPA and Abrahamse H:
Role of Bcl-2 family proteins in photodynamic therapy mediated cell
survival and regulation. Molecules. 25:53082020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tsuji T, Murase T, Konishi Y and Inatani
M: Optic nerve injury enhanced mitochondrial fission and increased
mitochondrial density without altering the uniform mitochondrial
distribution in the unmyelinated axons of retinal ganglion cells in
a mouse model. Int J Mol Sci. 24:43562023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Guo KM, Li W, Wang ZH, He LC, Feng Y and
Liu HS: Low-dose aspirin inhibits trophoblast cell apoptosis by
activating the CREB/Bcl-2 pathway in pre-eclampsia. Cell Cycle.
21:2223–2238. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ye D, Shi Y, Xu Y and Huang J: PACAP
attenuates optic nerve Crush-induced retinal ganglion cell
apoptosis via activation of the CREB-Bcl-2 pathway. J Mol Neurosci.
68:475–484. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ye D, Yang Y, Lu X, Xu Y, Shi Y, Chen H
and Huang J: Spatiotemporal expression changes of PACAP and its
receptors in retinal ganglion cells after optic nerve crush. J Mol
Neurosci. 68:465–474. 2019. View Article : Google Scholar
|
|
72
|
Michelessi M, Lucenteforte E, Oddone F,
Brazzelli M, Parravano M, Franchi S, Ng SM and Virgili G: Optic
nerve head and fibre layer imaging for diagnosing glaucoma.
Cochrane Database Syst Rev. 2015:CD0088032015.PubMed/NCBI
|
|
73
|
Hakim A, Guido B, Narsineni L, Chen DW and
Foldvari M: Gene therapy strategies for glaucoma from IOP reduction
to retinal neuroprotection: Progress towards non-viral systems. Adv
Drug Deliv Rev. 196:1147812023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kimura A, Namekata K, Guo X, Harada C and
Harada T: Neuroprotection, growth factors and BDNF-TrkB signalling
in retinal degeneration. Int J Mol Sci. 17:15842016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Mysona BA, Zhao J and Bollinger KE: Role
of BDNF/TrkB pathway in the visual system: Therapeutic implications
for glaucoma. Expert Rev Ophthalmol. 12:69–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Dheer Y, Chitranshi N and Gupta V, Abbasi
M, Mirzaei M, You Y, Chung R, Graham SL and Gupta V: Bexarotene
modulates Retinoid-X-Receptor expression and is protective against
neurotoxic endoplasmic reticulum stress response and apoptotic
pathway activation. Mol Neurobiol. 55:9043–9056. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gupta VK, Rajala A and Rajala RV: Insulin
receptor regulates photoreceptor CNG channel activity. Am J Physiol
Endocrinol Metab. 303:E1363–E1372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Gómez del Rio MA, Sánchez-Reus MI,
Iglesias I, Pozo MA, García-Arencibia M, Fernández-Ruiz J,
García-García L, Delgado M and Benedí J: Neuroprotective properties
of standardized extracts of hypericum perforatum on rotenone model
of Parkinson's disease. CNS Neurol Disord Drug Targets. 12:665–679.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kim HY, Park EJ, Joe EH and Jou I:
Curcumin suppresses Janus kinase-STAT inflammatory signaling
through activation of Src homology 2 domain-containing tyrosine
phosphatase 2 in brain microglia. J Immunol. 171:6072–6079. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Gupta VK, You Y, Klistorner A and Graham
SL: Shp-2 regulates the TrkB receptor activity in the retinal
ganglion cells under glaucomatous stress. Biochim Biophys Acta.
1822:1643–1649. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gupta V, You Y, Li J, Gupta V, Golzan M,
Klistorner A, van den Buuse M and Graham S: BDNF impairment is
associated with age-related changes in the inner retina and
exacerbates experimental glaucoma. Biochim Biophys Acta.
1842:1567–1578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Osborne A, Khatib TZ, Songra L, Barber AC,
Hall K, Kong GYX, Widdowson PS and Martin KR: Neuroprotection of
retinal ganglion cells by a novel gene therapy construct that
achieves sustained enhancement of brain-derived neurotrophic
factor/tropomyosin-related kinase receptor-B signaling. Cell Death
Dis. 9:10072018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang X, Ma W, Wang T, Yang J, Wu Z, Liu K,
Dai Y, Zang C, Liu W, Liu J, et al: BDNF-TrkB and
proBDNF-p75NTR/Sortilin signaling pathways are involved in
Mitochondria-mediated neuronal apoptosis in dorsal root ganglia
after sciatic nerve transection. CNS Neurol Disord Drug Targets.
19:66–82. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wu MM, Zhu TT, Wang P, Kuang F, Hao DJ,
You SW and Li YY: Dose-dependent protective effect of lithium
chloride on retinal ganglion cells is interrelated with an
upregulated intraretinal BDNF after optic nerve transection in
adult rats. Int J Mol Sci. 15:13550–13563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Alexander MS and Velinov M:
DOCK3-Associated neurodevelopmental Disorder-clinical features and
molecular basis. Genes (Basel). 14:19402023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Namekata K, Tsuji N, Guo X, Nishijima E,
Honda S, Kitamura Y, Yamasaki A, Kishida M, Takeyama J, Ishikawa H,
et al: Neuroprotection and axon regeneration by novel
low-molecular-weight compounds through the modification of DOCK3
conformation. Cell Death Discov. 9:1662023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Li L, Fang F, Feng X, Zhuang P, Huang H,
Liu P, Liu L, Xu AZ, Qi LS, Cong L and Hu Y: Single-cell
transcriptome analysis of regenerating RGCs reveals potent glaucoma
neural repair genes. Neuron. 110:2646–2663.e6. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Gauthier AC and Liu J: Epigenetics and
signaling pathways in glaucoma. Biomed Res Int. 2017:57123412017.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Chen AM, Azar SS, Har ris A, Brecha NC and
Pérez de Sevilla Müller L: PTEN expression regulates gap junction
connectivity in the retina. Front Neuroanat. 15:6292442021.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Xu K, Yu L, Wang Z, Lin P, Zhang N, Xing Y
and Yang N: Use of gene therapy for optic nerve protection: Current
concepts. Front Neurosci. 17:11580302023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Mak HK, Ng SH, Ren T, Ye C and Leung CK:
Impact of PTEN/SOCS3 deletion on amelioration of dendritic
shrinkage of retinal ganglion cells after optic nerve injury. Exp
Eye Res. 192:1079382020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Saleeb R, Kim SS, Ding Q, Scorilas A, Lin
S, Khella HW, Boulos C, Ibrahim G and Yousef GM: The miR-200 family
as prognostic markers in clear cell renal cell carcinoma. Urol
Oncol. 37:955–963. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Shen Y, Zhu Y and Rong F: miR-200c-3p
regulates the proliferation and apoptosis of human trabecular
meshwork cells by targeting PTEN. Mol Med Rep. 22:1605–1612. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Li XY, Wang SS, Han Z, Han F, Chang YP,
Yang Y, Xue M, Sun B and Chen LM: Triptolide restores autophagy to
alleviate diabetic renal fibrosis through the
miR-141-3p/PTEN/Akt/mTOR pathway. Mol Ther Nucleic Acids. 9:48–56.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wang Y, Niu L, Zhao J, Wang M, Li K and
Zheng Y: An update: Mechanisms of microRNA in primary open-angle
glaucoma. Brief Funct Genomics. 20:19–27. 2021. View Article : Google Scholar
|
|
96
|
Rheaume BA, Xing J, Lukomska A, Theune WC,
Damania A, Sjogren G and Trakhtenberg EF: Pten inhibition
dedifferentiates long-distance axon-regenerating intrinsically
photosensitive retinal ganglion cells and upregulates
mitochondria-associated Dynlt1a and Lars2. Development.
150:dev2016442023. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Van de Velde S, De Groef L, Stalmans I,
Moons L and Van Hove I: Towards axonal regeneration and
neuroprotection in glaucoma: Rho kinase inhibitors as promising
therapeutics. Prog Neurobiol. 131:105–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wang J, Liu X and Zhong Y:
Rho/Rho-associated kinase pathway in glaucoma (Review). Int J
Oncol. 43:1357–1367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Al-Humimat G, Marashdeh I, Daradkeh D and
Kooner K: Investigational rho kinase inhibitors for the treatment
of glaucoma. J Exp Pharmacol. 13:197–212. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ahmad I and Subramani M: Microglia:
Friends or foes in glaucoma? A Developmental Perspective. Stem
Cells Transl Med. 11:1210–1218. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Sato K, Ohno-Oishi M, Yoshida M, Sato T,
Aizawa T, Sasaki Y, Maekawa S, Ishikawa M, Omodaka K, Kawano C, et
al: The GPR84 molecule is a mediator of a subpopulation of retinal
microglia that promote TNF/IL-1α expression via the rho-ROCK
pathway after optic nerve injury. Glia. 71:2609–2622. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Sagawa H, Terasaki H, Nakamura M, Ichikawa
M, Yata T, Tokita Y and Watanabe M: A novel ROCK inhibitor,
Y-39983, promotes regeneration of crushed axons of retinal ganglion
cells into the optic nerve of adult cats. Exp Neurol. 205:230–240.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lingor P, Tönges L, Pieper N, Bermel C,
Barski E, Planchamp V and Bähr M: ROCK inhibition and CNTF interact
on intrinsic signalling pathways and differentially regulate
survival and regeneration in retinal ganglion cells. Brain.
131:250–263. 2008. View Article : Google Scholar
|
|
104
|
Shaw PX, Sang A, Wang Y, Ho D, Douglas C,
Dia L and Goldberg JL: Topical administration of a Rock/Net
inhibitor promotes retinal ganglion cell survival and axon
regeneration after optic nerve injury. Exp Eye Res. 158:33–42.
2017. View Article : Google Scholar
|
|
105
|
Nishijima E, Namekata K, Kimura A, Guo X,
Harada C, Noro T, Nakano T and Harada T: Topical ripasudil
stimulates neuroprotection and axon regeneration in adult mice
following optic nerve injury. Sci Rep. 10:157092020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Pagano L, Lee JW, Posarelli M, Giannaccare
G, Kaye S and Borgia A: ROCK inhibitors in corneal diseases and
Glaucoma-A comprehensive review of these emerging drugs. J Clin
Med. 12:67362023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Palmhof M, Wagner N, Nagel C, Biert N,
Stute G, Dick HB and Joachim SC: Retinal ischemia triggers early
microglia activation in the optic nerve followed by neurofilament
degeneration. Exp Eye Res. 198:1081332020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Tokushige H, Waki M, Takayama Y and
Tanihara H: Effects of Y-39983, a selective Rho-associated protein
kinase inhibitor, on blood flow in optic nerve head in rabbits and
axonal regeneration of retinal ganglion cells in rats. Curr Eye
Res. 36:964–970. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Liu Q, Liu C and Lei B: siRNA mediated
downregulation of RhoA expression reduces oxidative induced
apoptosis in retinal ganglion cells. Curr Mol Med. 24:630–636.
2024. View Article : Google Scholar
|
|
110
|
Tan NY, Koh V, Girard MJ and Cheng CY:
Imaging of the lamina cribrosa and its role in glaucoma: A review.
Clin Exp Ophthalmol. 46:177–188. 2018. View Article : Google Scholar
|
|
111
|
Liu XY and Fan N: Lamina cribrosa defect
and progress of glaucoma. Zhonghua Yan Ke Za Zhi. 56:17–20. 2020.In
Chinese. PubMed/NCBI
|
|
112
|
Kim YW, Jeoung JW, Kim DW, Girard MJ, Mari
JM, Park KH and Kim DM: Clinical assessment of lamina cribrosa
curvature in eyes with primary Open-angle glaucoma. PLoS One.
11:e01502602016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Kim JA, Lee SH, Son DH, Kim TW, Lee EJ,
Girard MJA and Mari JM: Morphologic changes in the lamina cribrosa
upon intraocular pressure lowering in patients with normal tension
glaucoma. Invest Ophthalmol Vis Sci. 63:232022. View Article : Google Scholar
|
|
114
|
Strickland RG, Garner MA, Gross AK and
Girkin CA: Remodeling of the lamina Cribrosa: Mechanisms and
potential therapeutic approaches for glaucoma. Int J Mol Sci.
23:80682022. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Yao X, Gao S and Yan N: Structural biology
of voltage-gated calcium channels. Channels (Austin).
18:22908072024. View Article : Google Scholar
|
|
116
|
Fan Gaskin JC, Shah MH and Chan EC:
Oxidative stress and the role of NADPH oxidase in glaucoma.
Antioxidants (Basel). 10:2382021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Irnaten M and O'Brien CJ:
Calcium-Signalling in human glaucoma lamina cribrosa
myofibroblasts. Int J Mol Sci. 24:12872023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Jain R, Watson U, Vasudevan L and Saini
DK: ERK activation pathways downstream of GPCRs. Int Rev Cell Mol
Biol. 338:79–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Woll KA and Van Petegem F: Calcium-release
channels: Structure and function of IP3 receptors and ryanodine
receptors. Physiol Rev. 102:209–268. 2022. View Article : Google Scholar
|
|
120
|
Irnaten M, Duff A, Clark A and O'Brien C:
Intra-cellular calcium signaling pathways (PKC, RAS/RAF/MAPK, PI3K)
in lamina cribrosa cells in glaucoma. J Clin Med. 10:622020.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Gu X, Reagan AM, McClellan ME and Elliott
MH: Caveolins and caveolae in ocular physiology and
pathophysiology. Prog Retin Eye Res. 56:84–106. 2017. View Article : Google Scholar :
|
|
122
|
Aga M, Bradley JM, Wanchu R, Yang YF,
Acott TS and Keller KE: Differential effects of caveolin-1 and -2
knockdown on aqueous outflow and altered extracellular matrix
turnover in caveolin-silenced trabecular meshwork cells. Invest
Ophthalmol Vis Sci. 55:5497–5509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Irnaten M, Zhdanov A, Brennan D, Crotty T,
Clark A, Papkovsky D and O'Brien C: Activation of the NFAT-calcium
signaling pathway in human lamina cribrosa cells in glaucoma.
Invest Ophthalmol Vis Sci. 59:831–842. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Quill B, Irnaten M, Docherty NG, McElnea
EM, Wallace DM, Clark AF and O'Brien CJ: Calcium channel blockade
reduces mechanical strain-induced extracellular matrix gene
response in lamina cribrosa cells. Br J Ophthalmol. 99:1009–1014.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Irnaten M, O'Malley G, Clark AF and
O'Brien CJ: Transient receptor potential channels TRPC1/TRPC6
regulate lamina cribrosa cell extracellular matrix gene
transcription and proliferation. Exp Eye Res. 193:1079802020.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Hu H, Nie D, Fang M, He W, Zhang J, Liu X
and Zhang G: Müller cells under hydrostatic pressure modulate
retinal cell survival via TRPV1/PLCγ1 complex-mediated calcium
influx in experimental glaucoma. FEBS J. 291:2703–2714. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Irnaten M, Barry RC, Wallace DM, Docherty
NG, Quill B, Clark AF and O'Brien CJ: Elevated maxi-K(+) ion
channel current in glaucomatous lamina cribrosa cells. Exp Eye Res.
115:224–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
McElnea EM, Quill B, Docherty NG, Irnaten
M, Siah WF, Clark AF, O'Brien CJ and Wallace DM: Oxidative stress,
mitochondrial dysfunction and calcium overload in human lamina
cribrosa cells from glaucoma donors. Mol Vis. 17:1182–1191.
2011.PubMed/NCBI
|
|
129
|
Wallace DM and O'Brien CJ: The role of
lamina cribrosa cells in optic nerve head fibrosis in glaucoma. Exp
Eye Res. 142:102–109. 2016. View Article : Google Scholar
|
|
130
|
Das A, Kashyap O, Singh A, Shree J, Namdeo
KP and Bodakhe SH: Oxymatrine protects TGFβ1-induced retinal
fibrosis in an animal model of glaucoma. Front Med (Lausanne).
8:7503422021. View Article : Google Scholar
|
|
131
|
Ling C, Zhang D, Zhang J, Sun H, Du Q and
Li X: Updates on the molecular genetics of primary congenital
glaucoma (Review). Exp Ther Med. 20:968–977. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Tsukamoto T, Kajiwara K, Nada S and Okada
M: Src mediates TGF-β-induced intraocular pressure elevation in
glaucoma. J Cell Physiol. 234:1730–1744. 2019. View Article : Google Scholar
|
|
133
|
Zhang YE: Non-smad signaling pathways of
the TGF-β family. Cold Spring Harb Perspect Biol. 9:a0221292017.
View Article : Google Scholar
|
|
134
|
Hachana S and Larrivée B: TGF-β
superfamily signaling in the eye: Implications for ocular
pathologies. Cells. 11:23362022. View Article : Google Scholar
|
|
135
|
Zode GS, Sethi A, Brun-Zinkernagel AM,
Chang IF, Clark AF and Wordinger RJ: Transforming growth factor-β2
increases extracellular matrix proteins in optic nerve head cells
via activation of the Smad signaling pathway. Mol Vis.
17:1745–1758. 2011.
|
|
136
|
Murphy-Ullrich JE and Downs JC: The
Thrombospondin1-TGF-β pathway and glaucoma. J Ocul Pharmacol Ther.
31:371–375. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Lopez NN, Rangan R, Clark AF and
Tovar-Vidales T: Mirna expression in glaucomatous and TGFβ2 treated
lamina cribrosa cells. Int J Mol Sci. 22:2372. 2021. View Article : Google Scholar
|
|
138
|
Zhou L, Wang L, Lu L, Jiang P, Sun H and
Wang H: Inhibition of miR-29 by TGF-beta-Smad3 signaling through
dual mechanisms promotes transdifferentiation of mouse myoblasts
into myofibroblasts. PLoS One. 7:e337662012. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Zhao Y, Zhang F, Pan Z, Luo H, Liu K and
Duan X: LncRNA NR_003923 promotes cell proliferation, migration,
fibrosis, and autophagy via the miR-760/miR-215-3p/IL22RA1 axis in
human Tenon's capsule fibroblasts. Cell Death Dis. 10:5942019.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Hurley DJ, Normile C, Irnaten M and
O'Brien C: The intertwined roles of oxidative stress and
endoplasmic reticulum stress in glaucoma. Antioxidants. 11:8862022.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Baudouin C, Kolko M, Melik-Parsadaniantz S
and Messmer EM: Inflammation in glaucoma: From the back to the
front of the eye, and beyond. Prog Retin Eye Res. 83:1009162021.
View Article : Google Scholar
|
|
142
|
Feng L, Dai S, Zhang C, Zhang W, Zhu W,
Wang C, He Y and Song W: Ripa-56 protects retinal ganglion cells in
glutamate-induced retinal excitotoxic model of glaucoma. Sci Rep.
14:38342024. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Vallée A, Lecarpentier Y and Vallée JN:
Cannabidiol and the canonical WNT/β-catenin pathway in glaucoma.
Int J Mol Sci. 22:37982021. View Article : Google Scholar
|
|
144
|
Boesl F, Drexler K, Müller B, Seitz R,
Weber GR, Priglinger SG, Fuchshofer R, Tamm ER and Ohlmann A:
Endogenous Wnt/β-catenin signaling in Müller cells protects retinal
ganglion cells from excitotoxic damage. Mol Vis. 26:135–149.
2020.
|
|
145
|
Patel AK, Park KK and Hackam AS: Wnt
signaling promotes axonal regeneration following optic nerve injury
in the mouse. Neuroscience. 343:372–383. 2017. View Article : Google Scholar
|
|
146
|
Wang X, Huai G, Wang H, Liu Y, Qi P, Shi
W, Peng J, Yang H, Deng S and Wang Y: Mutual regulation of the
Hippo/Wnt/LPA/TGF-β signaling pathways and their roles in glaucoma
(Review). Int J Mol Med. 41:1201–1212. 2018.
|
|
147
|
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang
X, Zhou Z, Shu G and Yin G: Wnt/β-catenin signalling: Function,
biological mechanisms, and therapeutic opportunities. Signal
Transduct Target Ther. 7:32022. View Article : Google Scholar
|
|
148
|
Wang Z, Li Z and Ji H: Direct targeting of
β-catenin in the Wnt signaling pathway: Current progress and
perspectives. Med Res Rev. 41:2109–2129. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Udeh A, Dvoriantchikova G, Carmy T, Ivanov
D and Hackam AS: Wnt signaling induces neurite outgrowth in mouse
retinal ganglion cells. Exp Eye Res. 182:39–43. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Seitz R, Hackl S, Seibuchner T, Tamm ER
and Ohlmann A: Norrin mediates neuroprotective effects on retinal
ganglion cells via activation of the Wnt/beta-catenin signaling
pathway and the induction of neuroprotective growth factors in
Muller cells. Neurosci. 30:5998–6010. 2010. View Article : Google Scholar
|
|
151
|
Fragoso MA, Patel AK, Nakamura RE, Yi H,
Surapaneni K and Hackam AS: The Wnt/β-catenin pathway cross-talks
with STAT3 signaling to regulate survival of retinal pigment
epithelium cells. PLoS One. 7:e468922012. View Article : Google Scholar
|
|
152
|
Schmitt AM, Shi J, Wolf AM, Lu CC, King LA
and Zou Y: Wnt-Ryk signalling mediates medial-lateral retinotectal
topographic mapping. Nature. 439:31–37. 2006. View Article : Google Scholar
|
|
153
|
Cui J, Shi M, Quan M and Xie K: Regulation
of EMT by KLF4 in gastrointestinal cancer. Curr Cancer Drug
Targets. 13:986–995. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Vallée A and Vallée JN: Warburg effect
hypothesis in autism Spectrum disorders. Mol Brain. 11:12018.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Lee TJ, Kodeboyina SK, Bollinger KE,
Ulrich L, Bogorad D, Estes A, Zhi W, Sharma S and Sharma A: The
abundance of serine protease inhibitors in human aqueous humor and
race and gender-specific alterations in glaucoma patients.
Investigative Ophthalmol Visual Sci. 62:3367. 2021.
|
|
156
|
Basava rajappa D, Galindo-Romero C, Gupta
V, Agudo-Barriuso M, Gupta VB, Graham SL and Chitranshi N:
Signalling pathways and cell death mechanisms in glaucoma: Insights
into the molecular pathophysiology. Mol Aspects Med. 94:1012162023.
View Article : Google Scholar
|
|
157
|
Park HL, Kim JH, Jung Y and Park CK:
Racial differences in the extracellular matrix and histone
acetylation of the lamina cribrosa and peripapillary sclera. Invest
Ophthalmol Vis Sci. 58:4143–4154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Agarwal P and Agarwal R: Trabecular
meshwork ECM remodeling in glaucoma: Could RAS be a target? Expert
Opin Ther Targets. 22:629–638. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Kontoh-Twumasi R, Budkin S, Edupuganti N,
Vashishtha A and Sharma S: Role of serine protease inhibitors A1
and A3 in ocular pathologies. Invest Ophthalmol Vis Sci. 65:162024.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Chitranshi N, Rajput R, Godinez A,
Pushpitha K, Mirzaei M, Basavarajappa D, Gupta V, Sharma S, You Y,
Galliciotti G, et al: Neuroserpin gene therapy inhibits retinal
ganglion cell apoptosis and promotes functional preservation in
glaucoma. Mol Ther. 31:2056–2076. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Gupta V, Mirzaei M, Gupta VB, Chitranshi
N, Dheer Y, Vander Wall R, Abbasi M, You Y, Chung R and Graham S:
Glaucoma is associated with plasmin proteolytic activation mediated
through oxidative inactivation of neuroserpin. Sci Rep. 7:84122017.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Tsuda Y, Nakahara T, Ueda K, Mori A,
Sakamoto K and Ishii K: Effect of nafamostat on
N-methyl-D-aspartate-induced retinal neuronal and capillary
degeneration in rats. Biol Pharm Bull. 35:2209–2213. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Yang X, Zeng Q and Tezel G: Regulation of
distinct caspase-8 functions in retinal ganglion cells and
astroglia in experimental glaucoma. Neurobiol Dis. 150:1052582021.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Choudhury S, Liu Y, Clark AF and Pang IH:
Caspase-7: A critical mediator of optic nerve injury-induced
retinal ganglion cell death. Mol Neurodegener. 10:402015.
View Article : Google Scholar : PubMed/NCBI
|