Introduction
Myocardial ischemia-reperfusion (I/R) injury is a
progressive pathological tissue injury that arises as a consequence
of acute ischemia and subsequent reperfusion of myocardial tissue
(1). It has been previously
reported that reperfusion injury can result in myocardial fibrosis,
which contributes to heart failure and ventricular remodeling
(2). Additionally, reperfusion
injury can impact the electrophysiological function of the heart
and myocardial metabolism. This phenomenon accounts for ~50% of
acute myocardial injury in cases of myocardial infarction (1,3).
The imbalance between the oxidant and antioxidant systems leads to
oxidative stress, which is a key contributor to myocardial I/R
injury (4). Reactive oxygen
species (ROS) are byproducts of aerobic metabolism primarily
generated in mitochondria and the cytoplasm. Under physiological
conditions, antioxidant enzymes such as glutathione peroxidase,
superoxide dismutase (SOD) and catalase, along with other small
reducing molecules, scavenge ROS. Upon reperfusion of ischemic
myocardial tissue, there is a surge in ROS levels. Accumulated ROS
target unsaturated fatty acids present in cellular proteins, DNA
and biofilm structures, generating substantial quantities of lipid
peroxides (5). ROS can regulate
the nuclear factor-κB (NF-κB) pathway by influencing upstream
kinases, such as inhibitor of κB kinase (IKK), NF-κB inducing
kinase (NIK), and protein kinase B (AKT), either promoting or
hindering degradation of I-κB or modulating the nuclear
translocation and DNA binding of the transcription factor by
altering NF-κB heterodimers (6).
The NF-κB pathway may serve a protective function in oxidative
stress through various mechanisms. Activation of the NF-κB pathway
may lead to induction of pro-oxidant genes such as the NADPH
oxidase cytochrome B-245 heavy chain subunit gp91phox (6). Moreover, ROS mediates NF-κB/NLR
family pyrin domain containing 3 in angiotensin II-treated
cardiomyocytes by directly binding calcium/calmodulin-dependent
protein kinase II δ (7).
Furthermore, mitochondrial permeability is modified in response to
excess of ROS, leading to the opening of the mitochondrial
permeability transition pore (mPTP) (8). This causes an elevation in
mitochondrial colloid osmotic pressure, resulting in mitochondrial
swelling and further deterioration of mitochondrial functionality
(8).
I/R promotes the mitochondria-mediated apoptosis
pathway and B-cell lymphoma-2 (Bcl-2) and Bcl-2 associated X
protein (Bax) are important regulators of this process (9,10). Decreased Bcl-2/Bax levels trigger
downstream apoptotic signals and the caspase-3 cascade reaction is
activated to initiate apoptosis (11). Bcl-2 family proteins promote
mitochondrial membrane permeability and a large number of
apoptosis-associated proteins are released into the cytoplasm,
whereby cytochrome c forms apoptotic bodies with protein precursors
of apoptotic protease activating factor-1 and caspase-9 after
entering the cytoplasm (11).
This activates caspase-9, which, with the aid of deoxyATP,
initiates the downstream caspase-3 cascade apoptotic response and
promotes the activation of the mitochondrial apoptosis pathway
(11). During I/R injury,
inflammatory factors, including tumor necrosis factor-α (TNF-α) and
interleukin-6 (IL-6), and chemokines are released to recruit
inflammatory cells to the site of injury, where they activate
multiple local inflammatory signaling pathways and the inflammatory
cascade reaction (12). The
NF-κB pathway induces oxidative stress initiation and the
inflammatory response in cardiomyocytes in I/R injury (12). Inactive NF-κB is located in the
cytoplasm; when NF-κB is activated, IκB is phosphorylated by IKK
and then degraded, which may cause transfer of NF-κB subunits from
cytoplasm to the nucleus and complete transcription and translation
of downstream inflammatory factors (13).
Previous studies have reported that preparations
derived from natural product-associated compounds and active
monomers enhance recovery from myocardial injury and cardiovascular
disease (14-16). Liriodendrin is an active
ingredient extracted from Sargentodoxae caulis that has
various biological functions, including antitumor,
anti-inflammatory, antioxidant and antiplatelet aggregation effects
(17,18). By downregulating release of
various inflammatory mediators, liriodendrin produces
anti-inflammatory and antioxidant effects and protects organ
function. Moreover, our previous study reported that liriodendrin
effectively improves ventricular remodeling and cardiac function
following myocardial infarction in rats (19). Liriodendrin regulates
inflammation and I/R injury through multiple signaling pathways,
including the arginine/nitric oxide metabolic, toll-like receptor 4
(TLR4)/NF-κB and the PI3K/Akt autophagy pathway (17,20,21). The potential protective effect of
liriodendrin on myocardial I/R injury in rats may be due to its
ability to inhibit the activity of the ROS and NF-κB signaling
pathway, decrease the production of inflammatory factors and
downregulate cardiomyocyte apoptosis. However, there is a lack of
research on the therapeutic effect of liriodendrin in this context.
Therefore, the aim of the present study was to investigate the
underlying mechanisms by which liriodendrin exerts its protective
effect on cardiac function in a rat model of I/R to provide a novel
potential adjunctive therapy for the treatment of cardiomyocytes
post-I/R. Moreover, the present study investigated the effect of
liriodendrin on mitochondrial function in hypoxic injured
myocardial cells.
Materials and methods
Experimental animals
A total of 30 SPF-class male Wistar rats (age, 8
weeks; weight, 200±10 g) were purchased from Beijing HFK Bioscience
Co., Ltd. The rats were housed (temperature: 22±2°C; humidity:
50±5%) in Tianjin Chest Hospital Cardiovascular Institute (Tianjin,
China) under a constant temperature of 25±1°C and ad libitum
access to food and water, in accordance with the requirements of
the Animal Research: Reporting of In Vivo Experiments
guidelines (version 2.0) (22).
All animal experiments were approved by the Animal Ethics Committee
of Tianjin Chest Hospital (approval no. TJCH-2021-007).
Cell culture
H9C2 cardiomyocytes (cat. no. 3101RATGNR5; National
Collection of Authenticated Cell Cultures) were cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS
(Beyotime Institute of Biotechnology), penicillin (100 U/ml) and
streptomycin (100 µg/ml) at 37°C and 5% CO2. The
culture medium was changed at every 48 h. Cells
(2×105/well) were inoculated into 6-well plates and
pretreated with liriodendrin (20, 50, 100 µM) for 24 h at
37°C. The cells were treated with hypoxia for 4 h at 37°C in
glucose-free DMEM (Gibco; Thermo Fisher Scientific, Inc.) and
placed in an anaerobic incubator (95% N2 and 5%
CO2). Following hypoxia, the cells were reoxygenated in
DMEM containing 4.5 mm glucose and placed in an incubator (95% air
and 5% CO2) at 37 °C for 24 h. Dmethyl sulphoxide (DMSO;
cat. no. ST038; Beyotime Institute of Biotechnology) was used as
control with a final concentration of 0.1%.
Establishment of the in vivo myocardial
infarction model
Wistar rats (matched for age, sex and body weight)
were randomly divided into the sham operation, I/R and liriodendrin
groups (n=10/group). Liriodendrin powder (cat. no. HY-N3377;
MedChemExpress; purity >97%) was diluted with PBS to obtain 10
g/l solution. The liriodendrin group was treated with liriodendrin
solution (10 ml/kg body weight, equivalent to 100 mg/kg body
weight) by intragastric administration daily, from 5 days before
surgery to 3 days after surgery. I/R and sham operation groups were
intragastrically administered normal saline (10 ml/kg/body weight)
daily. After 5 days, animals were anesthetized through inhalation
of 3% sevoflurane (~1.3 minimum alveolar concentration [MAC]) and
endotracheal intubation was used to assist respiration, with a
tidal volume of 28-32 ml/kg, respiratory rate of 75 breaths/min and
an inspiratory to expiratory ratio of 1:2. A thoracotomy was
performed between the 3rd and 4th intercostal space on the left
side of the sternum to expose the heart. The anterior descending
coronary artery was ligated at the lower edge of the left atrial
appendage. Pale myocardium in the anterior wall of the left
ventricle and 0.2 mV ST-elevation in electrocardiograms I, II and
augmented voltage left (aVL) were considered markers of successful
ligation. Following 30 min assisted ventilation, the ligature was
cut to restore the blood supply. In the sham group, the suture was
threaded without ligation. The animals were anesthetized with 3%
sevoflurane (~1.3 MAC) prior to blood collection or
echocardiography. The humane endpoints for animal experiments
followed the guidelines outlined in recommended industry standard
(RB/T) 173-2018 (rbtest. cnca.cn/portal/stdDetail/500828). The
humane endpoints were critical respiratory infections, respiratory
distress and incapacity to autonomously ingest food and water. No
rats have reached any of the humane endpoints. Animals were
euthanized by intravenous administration of pentobarbital sodium
(150 mg/kg).
Detection of myocardial injury
markers
At 2 h after the operation, 1 ml venous blood was
collected from the subclavian vein. Quantitative detection of
creatine kinase isoenzymes (CKMB; cat. no. E-EL-R1327) and
high-sensitivity cardiac troponin (cTn; cat. no. E-EL-R0151) T was
conducted using ELISA kits (both Elabscience Biotechnology Co.
Ltd.).
Echocardiography
Cardiac function was measured by echocardiography
(cat. no. L15-7io; Philips Healthcare) 3 days after surgery.
End-diastolic volume (EDV), left ventricular end-diastolic diameter
(LVIDd), ejection fraction (EF), left ventricular end-systolic
diameter (LVIDs), and end-systolic volume (ESV) were measured in
the short axis of the papillary muscle. A total of three cardiac
cycles was measured for each index and the mean was calculated.
Hematoxylin and eosin (H&E)
staining
After rats were euthanized, the hearts were
harvested and myocardial tissue was fixed in 10% neutral formalin
for 24 h at room temperature. After dehydration, transparency, wax
immersion and embedding, paraffin blocks were produced. Tissues
were sliced in successive sections parallel to the long axis of the
heart at a thickness of 4 µm. H&E staining was performed
at room temperature for 1 min and visualized under a light
microscope (BX53, Olympus Corporation) at a magnification of ×100
and ×400.
TUNEL staining
Apoptosis was detected by TUNEL staining (cat. no.
G1507; Servicebio Technology, Inc.) in three fresh heart tissue
samples from each group of animals and the mean proportion of
TUNEL-positive cells in five randomly selected fields of view was
calculated to determine the apoptosis index of the cardiomyocytes.
In brief, heart tissue samples were fixed in 10% neutral formalin
for 24 h at room temperature. Terminal deoxynucleotidyl transferase
(TdT) incubation buffer (2 µl recombinant TdT enzyme, 5
µl biotin-dUTP labeling mix, 50 µl equilibration
buffer) was added at 37°C for 1 h, and sections were washed 3 times
with PBS. 0.5% streptavidin-HRP buffer was incubated at 37°C for 30
min and then washed 3 times with PBS, 5% DAB chromogenic solution
was added at room temperature for 30 min and then washed 3 times
with PBS, and 0.5% hematoxylin staining solution was stained at
room temperature for 5 min. TUNEL staining was followed by nuclear
staining with DAPI (1 µg/ml; cat. no. D106471-5 mg; Shanghai
Aladdin Biochemical Technology Co., Ltd.) and incubated at room
temperature for 10 min and then washed 3 times with PBS. The
sections use neutral resin for mounting and visualize with light
microscope (BX53, Olympus Corporation). The apoptotic index (AI) of
the myocardial cell was calculated as the proportion of the
apoptotic cells (brown-yellow granules in the nucleus) relative to
the total number of the cells (23).
Immunohistochemical staining. Immunohistochemistry
was used to stain myocardial tissues to determine the expression of
cleaved caspase-3, Bcl-2 and Bax proteins. Myocardial tissue was
fixed in 10% neutral formalin for 24 h at room temperature. After
dehydration with descending alcohol series (95, 85, and 75%),
transparency, wax immersion and embedding, paraffin blocks were
produced. Tissues were sliced in successive sections parallel to
the long axis of the heart at a thickness of 4 µm. The
slices were boiled in an antigen retrieval solution (1.8% 0.1 M
citrate buffer and 8.2% 0.1 M sodium citrate buffer) at 95°C for 10
min. The slices were washed in PBS buffer. Subsequently, slices
were treated with 1% BSA) (cat. no. ST023; Beyotime Institute of
Biotechnology, Haimen) for 15 min at room temperature. Slices were
incubated at 4°C overnight with the following primary antibodies:
cleaved caspase-3 (cat. no. #9664; dilution: 1:500; Cell Signaling
Technology, Inc.), Bcl-2 (cat. no. sc-7382; dilution: 1:200; Santa
Cruz Biotechnology, Inc.) and Bax (cat. no. sc-7480; dilution:
1:200; Santa Cruz Biotechnology, Inc.). Subsequently, the
corresponding secondary antibody, SignalStain® Boost IHC
Detection Reagent (HRP, Rabbit; cat. no. #9664; 1:1,000; Cell
Signaling Technology) for cleaved caspase-3 and m-IgGκ BP-HRP (at.
no: sc-516102; dilution: 1:1,000; Santa Cruz Biotechnology, Inc.)
for Bcl-2 and Bax, with a streptavidin-HRP conjugate 1 h at room
temperature. Moreover, the slices were stained with DAB kit and
Haematoxylin. DAPI (cat. no. D106471-5 mg; Shanghai Aladdin
Biochemical Technology Co., Ltd.) incubation for 10 min at room
temperature was utilized as a nuclear staining. Brown-stained cells
were considered positive cells. Finally, images were captured at
×400 magnification using an opticallight microscope (BX53, Olympus
Corporation) and measured by Motic med 6.0 software (Motic China
Group, Co., Ltd.).
Quantification of inflammatory
factors
After rats were sacrificed, the hearts were
harvested and myocardial tissue from the left ventricular
myocardial injury site was obtained. Tissue was homogenized with
PRO200 hand-held laboratory homogenizer (Bio-Gen PRO200, Genosys
Tech-Trading Co., Ltd.) and centrifuged for 10 min at 3,000 × g and
4°C to obtain supernatant. IL-1β (cat. no. H002-1-1; Nanjing
Jiancheng Bioengineering Institute), TNF-α (cat. no. H052-1-1;
Nanjing Jiancheng Bioengineering Institute), C-C motif chemokine
ligand 2 (MCP-1) (cat. no. H115-1-1; Nanjing Jiancheng
Bioengineering Institute) and ROS (cat. no. E004-1-1; Nanjing
Jiancheng Bioengineering Institute) levels were detected using
ELISA kits according to the manufacturer's instructions. SOD (cat.
no. A001-3-2; Nanjing Jiancheng Bioengineering Institute) levels in
the peripheral venous blood of rats 2 h after the operation were
also measured using an ELISA kit according to the manufacturer's
instructions.
Detection of inflammatory pathway- and
apoptosis-related proteins by western blotting
Total protein was extracted from myocardial tissue
of rats or H9C2 cells and centrifuged at 4°C for 20 min at 10,000 ×
g with lysis buffer (cat. no. P0013; Beyotime Institute of
Biotechnology) containing phenylmethanesulfonyl fluoride (cat. no.
ST506; Beyotime Institute of Biotechnology) on ice for 5 min. The
protein concentration was determined using the BCA method (cat. no.
5000002; Bio-Rad Laboratories, Inc.). The 30 µg protein
samples were then separated using SDS-PAGE with gel (8-12%) and a
5% stacking gel, followed by transfer onto PVDF) membranes. The
membranes were blocked using 5% BSA (cat. no. ST023; Beyotime
Institute of Biotechnology) at room temperature for 2 h. and
subsequently incubated overnight at 4°C with primary antibodies.
Proteins were incubated with antibodies (1:1,000) as follows: NF-κB
(cat. no. ab19870), phosphorylated (p)-NF-κB (cat. no. ab239882),
IKKβ (cat. no. ab178870), IκBα (cat. no. ab178847), p-IκBα (cat.
no. ab133462) (all Abcam), Bcl-2 (cat. no. sc-7382), Bax (cat. no.
sc-7480; both Santa Cruz Biotechnology, Inc.) and cleaved caspase-3
(cat. no. #9664; Cell Signaling Technology). Subsequently, the
corresponding secondary antibody, anti-Rabbit IgG H&L (HRP)
(cat. no. ab205718; 1:10,000; Abcam) for cleaved caspase-3, NF-κB,
p-NF-κB, IKKβ, IκBα, p-IκBα and m-IgGκ BP-HRP (at. no: sc-516102;
dilution: 1:10,000; Santa Cruz Biotechnology, Inc.) for Bcl-2 and
Bax, was incubated for 2 h at room temperature. Protein bands were
visualized using an enhanced chemiluminescence kit (cat. no.
323106; Thermo Fisher Scientific, Inc.). The images were analyzed
using ImageJ software (version 1.54; National Institutes of Health)
and the relative content of the target protein was expressed as the
gray value of the target protein/GAPDH gray value. A total of three
biological replicates was performed for each treatment group.
Certain target proteins and loading controls were screened from
different integral membranes due to the similarities in molecular
weight. Experimental conditions were consistent for protein
detection. Anti-GAPDH (cat. no. sc-32233; 1:2,000; Santa Cruz
Biotechnology, Inc.) was used to as the control antibody.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from rat cardiomyocytes or
H9C2 cells using TRIzol (cat. no. 15596026; Thermo Fisher
Scientific, Inc.) and the TaKaRa-RNA Quantification and Reverse
Transcription kit (Takara Bio, Inc.). RT was performed using the
BeyoRT II M-MLV reverse transcriptase (cat. no. D7160L; Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol. The qPCR procedure was conducted with SYBR Green (cat.
no. SY1020; Beijing Solarbio Science & Technology Co., Ltd.)
and 2×Taq PCR MasterMix (cat. no. PC1150; Beijing Solarbio Science
& Technology Co., Ltd.) using a fluorescent qPCR instrument
(Exicycler 96; Bioneer Corporation). The following thermocycling
conditions were used: 95°C for 5 min, followed by 95°C for 10 sec,
54~62°C (different genes use different annealing temperatures) for
10 sec and 72°C for 15 s. The 2−ΔΔCq method was used to
analyze the target gene expression (24). The primer sequences were as
follows: β-actin forward (F), 5′-TCA GGT CAT CAC TAT CGG CAAT-3′
and reverse (R), 5′-AAA GAA AGG GTG TAA AAC GCA-3′ (annealing
temperature, 54°C); IL-1β F, 5′-CCT CTG TGA CTC GTG GGA TGA-3′ and
R, 5′-CAC GAG CAT TTT TGT TGT TCA-3′ (annealing temperature, 56°C);
TNF-α F, 5′-CTT CTC ATT CCT GCT CGT GG-3′ and R, 5′-TCA GCC ACT CCA
GCT GCT C-3′ (annealing temperature, 55°C); Bcl-2 F, 5′-GGA GCG TCA
ACA GGG AGA TG-3′ and R, 5′-GCA GGT CTG CTG ACC TCA CTTG-3′
(annealing temperature, 59°C); Bax F, 5′-CCA AGA AGC TGA GAG AGT
GTC TC-3′ and R, 5′-AGT TGC CAT CAG CAA ACA TGT CA-3′ (annealing
temperature, 58°C) and cleaved caspase-3 F, 5′-AGC ACT GGA ATG TCA
GCT CGC-3′ and R, 5′-CAG GTC CAC AGG TCC GTTC-3′ (annealing
temperature, 62°C).
Cell morphology by scanning electron
microscope
Cells were harvested and fixed using 2.5%
glutaraldehyde solution for 4 h at 4°C. The supernatant was
discarded after 12 h and cells were rinsed in phosphate buffer (pH
7.0). Cells were fixed with 1% osmium acid solution for 2 h at 4°C
and rinsed with an osmium acid solution. Samples were sliced into
70-90 nm sections after dehydration and embedded. Sections were
stained using lead citrate (0.5%) and uranyl acetate (1%) for 30
min at room temperature and imaged using a scanning electron
microscope with gold as a sputter coating.
Statistical analysis
Data from ≥3 independent experiments are presented
as the mean ± SD. The t-test was used to analyze statistical
differences following Shapiro-Wilk normality test and variance
homogeneity testing using Levene's test. Inter-group differences
were assessed using one-way ANOVA with Tukey's Honestly Significant
Difference post hoc test. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS (version 23.0; IBM Corp.) or GraphPad Prism
software (version 5.02; Dotmatics).
Results
Liriodendrin decreases I/R-induced
myocardial injury
To evaluate the success rate of I/R modeling in rats
and the protective effect of liriodendrin on myocardial I/R injury,
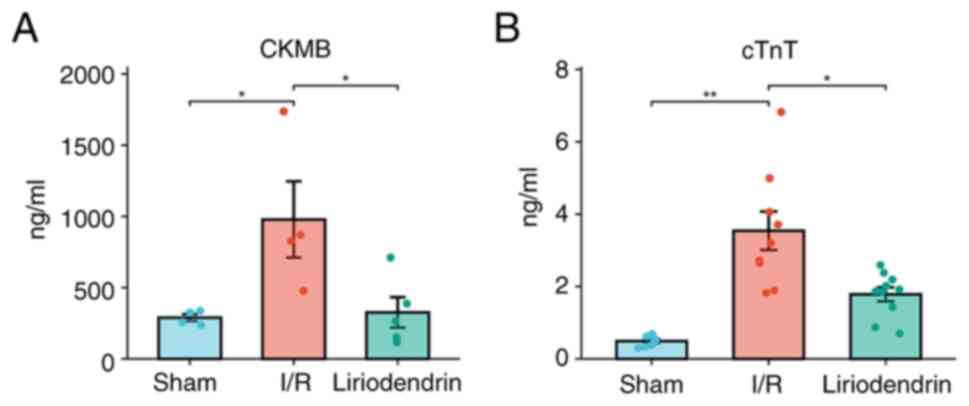
CKMB (Fig. 1A) and cTnT
(Fig. 1B) levels were measured.
Compared with the sham group, the CKMB levels in the I/R group were
significantly increased, indicating successful establishment of the
rat model of myocardial I/R injury. Moreover, the levels of CKMB in
the liriodendrin group were significantly lower compared with those
in I/R group, which indicated that myocardial injury induced by I/R
in rats was alleviated by liriodendrin.
Liriodendrin has a potential protective
effect on ventricular remodeling in I/R rats
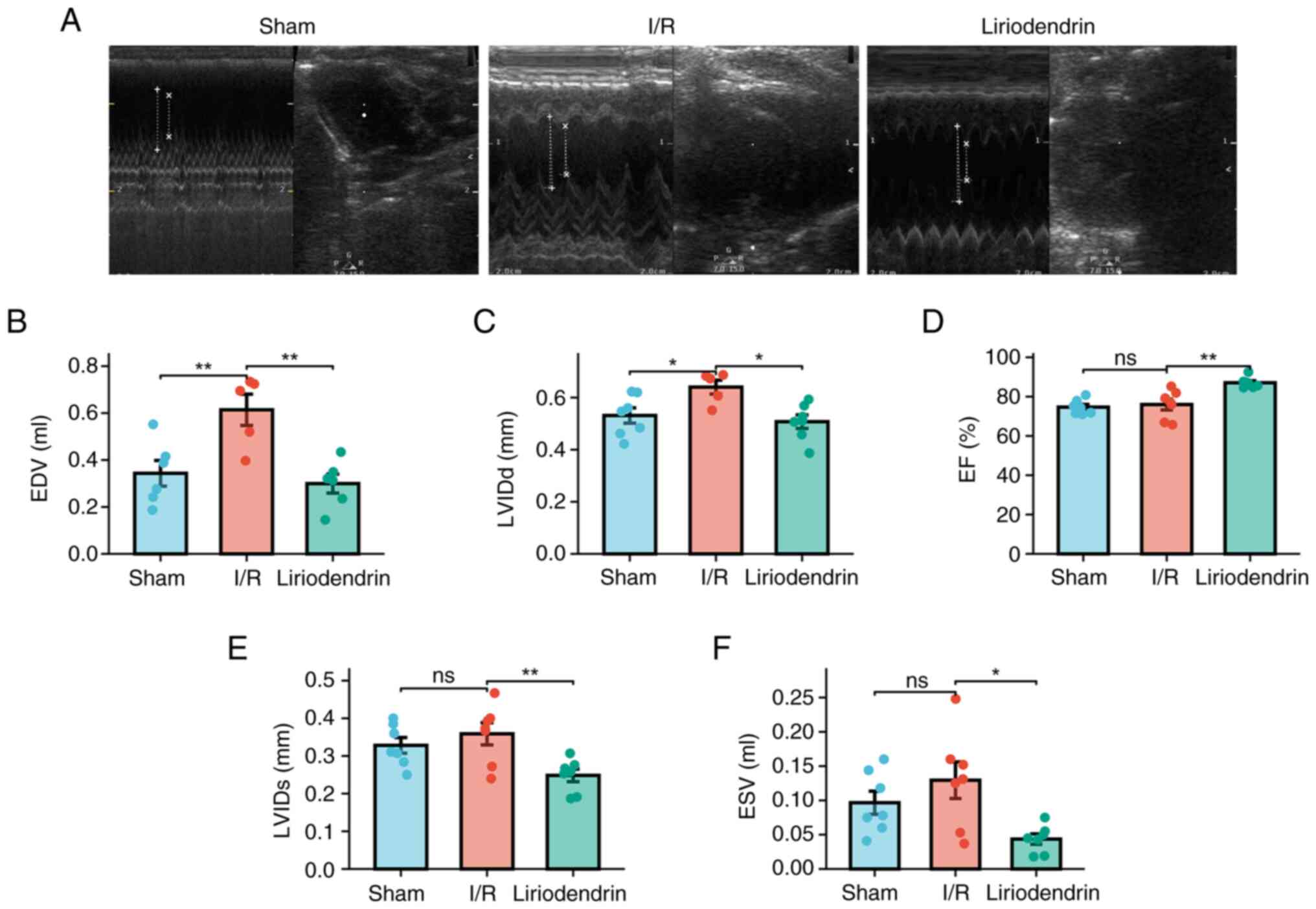
Echocardiography showed significant changes of EDV
(Fig. 2A and B) and LVIDd
(Fig. 2A and C) in I/R compared
to those of the normal rats, while liriodendrin administration
significantly reversed I/R-induced the elevation of EDV and LVIDd
in I/R rats. However, I/R operation had no obvious changes of EF
(Fig. 2A and D), LVIDs (Fig. 2A and E), and ESV (Fig. 2A and F). Interestingly,
liriodendrin administration significantly lowered EF (Fig. 2A and D), LVIDs (Fig. 2A and E), and ESV (Fig. 2A and F) in I/R rats compared with
I/R operation alone.
Myocardial tissue damage and inflammatory
cell infiltration is alleviated by liriodendrin in I/R rats
In the sham group, the left ventricular myocardial
fibers displayed a well-organized and structured arrangement, with
normal morphology of myocardial cells. The H&E staining
demonstrated that in the I/R group, cardiomyocytes exhibited an
indistinct morphology, disorganized arrangement and pyknotic, lysed
or absent nuclei, along with notable infiltration of inflammatory
cells. Liriodendrin group exhibited markedly less severe
pathological alterations compared with the I/R group, including a
notable reduction in the degree of inflammatory cell infiltration
(Fig. 3A).
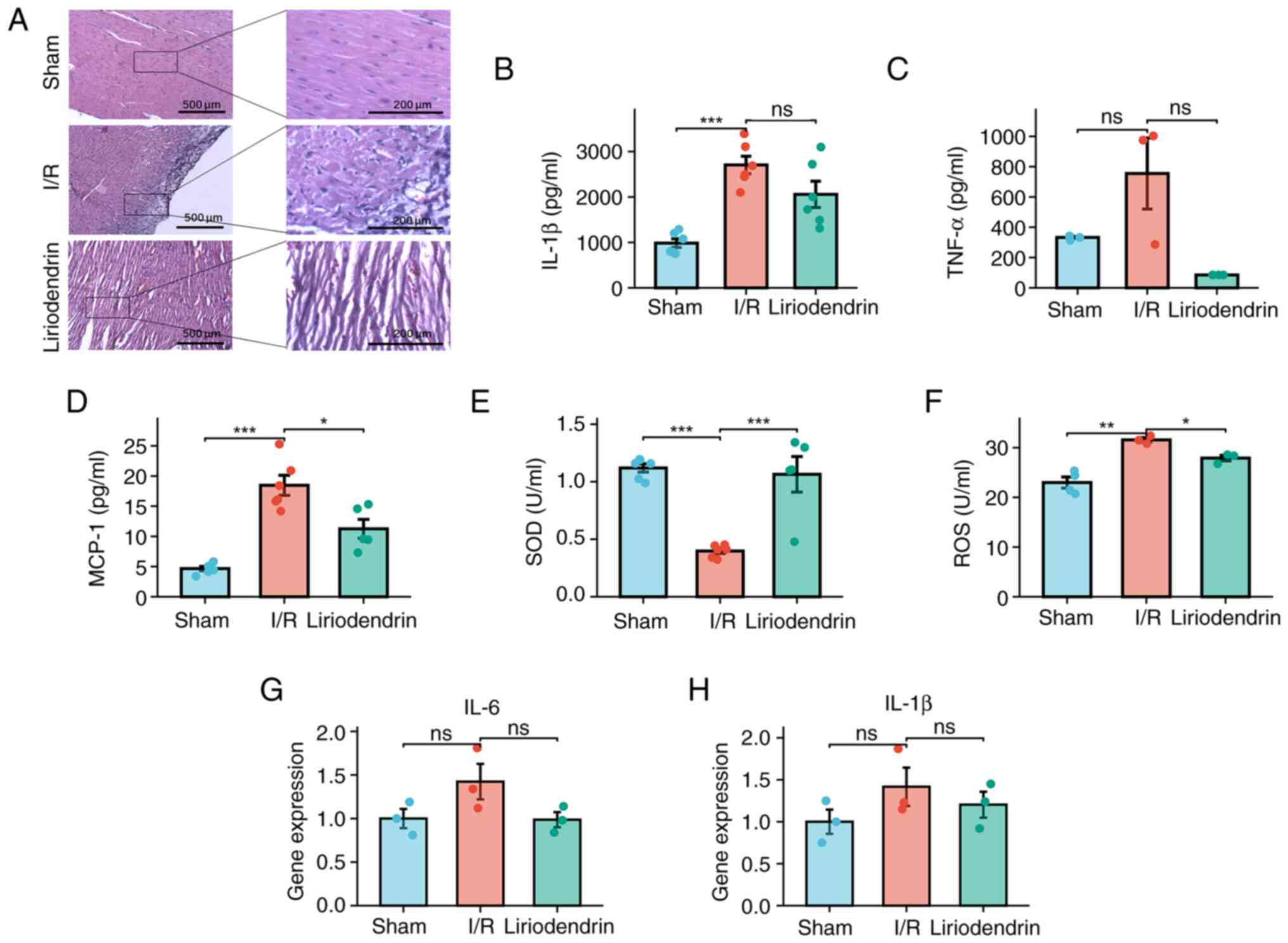 | Figure 3Liriodendrin inhibits I/R-induced
increases in myocardial inflammatory factors and oxidative stress
injury. (A) Hematoxylin and eosin staining (magnification, ×100 and
×400). Protein expression levels of (B) IL-1β, (C) TNF-α, (D)
MCP-1, (E) SOD and (F) ROS in cardiac tissue homogenate. Expression
of (G) IL-6 and (H) IL-1β was evaluated using reverse
transcription-quantitative PCR. *P<0.05;
**P<0.01; ***P<0.001. ns, not
significant; I/R, ischemia/reperfusion; TNF-α, tumor necrosis
factor-α; MCP-1, CC-motif chemokine ligand 2; SOD, superoxide
dismutase; ROS, reactive oxygen species; IL-6, interleukin-6. |
Liriodendrin inhibits I/R-induced
increases in myocardial inflammatory factors and oxidative stress
injury
The inflammatory response cascade is initiated by
IL-1β and TNF-α (25). Increased
IL-1β levels were observed in the heart tissue of the I/R group
when compared with the sham group (Fig. 3B). Conversely, no significant
alteration in TNF-α levels were observed in response to I/R
(Fig. 3C). MCP-1 serves a
crucial role in the migration and infiltration of macrophages,
facilitating their accumulation at the site of inflammation and
exacerbating the inflammatory response (26). Increased expression of MCP-1
within the myocardium of the I/R group was observed compared with
the sham operation group. Conversely, in rats treated with
liriodendrin, there was a significant decrease in MCP-1 expression
(Fig. 3D). SOD serves a crucial
role in the elimination of ROS (27); there was a significant decrease
in SOD levels in the peripheral blood of the I/R group at 2 h
post-injury (Fig. 3E).
Conversely, there was a notable increase in ROS levels in
myocardial tissue of I/R rats, suggesting that I/R injury may
intensify the oxidative stress response in cardiomyocytes (Fig. 3F). Compared with the I/R group,
the liriodendrin group showed a significant increase in SOD
(Fig. 3E) and a significant
decrease in ROS levels (Fig.
3F), suggesting that liriodendrin may suppress tissue
inflammation following I/R injury and counteract oxidative stress
induced by I/R, thereby decreasing cardiomyocyte damage. However,
there were no significant differences in the levels of IL-1β and
IL-6 in the I/R compared with the liriodendrin group (Fig. 3G and H).
Myocardial apoptosis in I/R rats is
decreased by liriodendrin
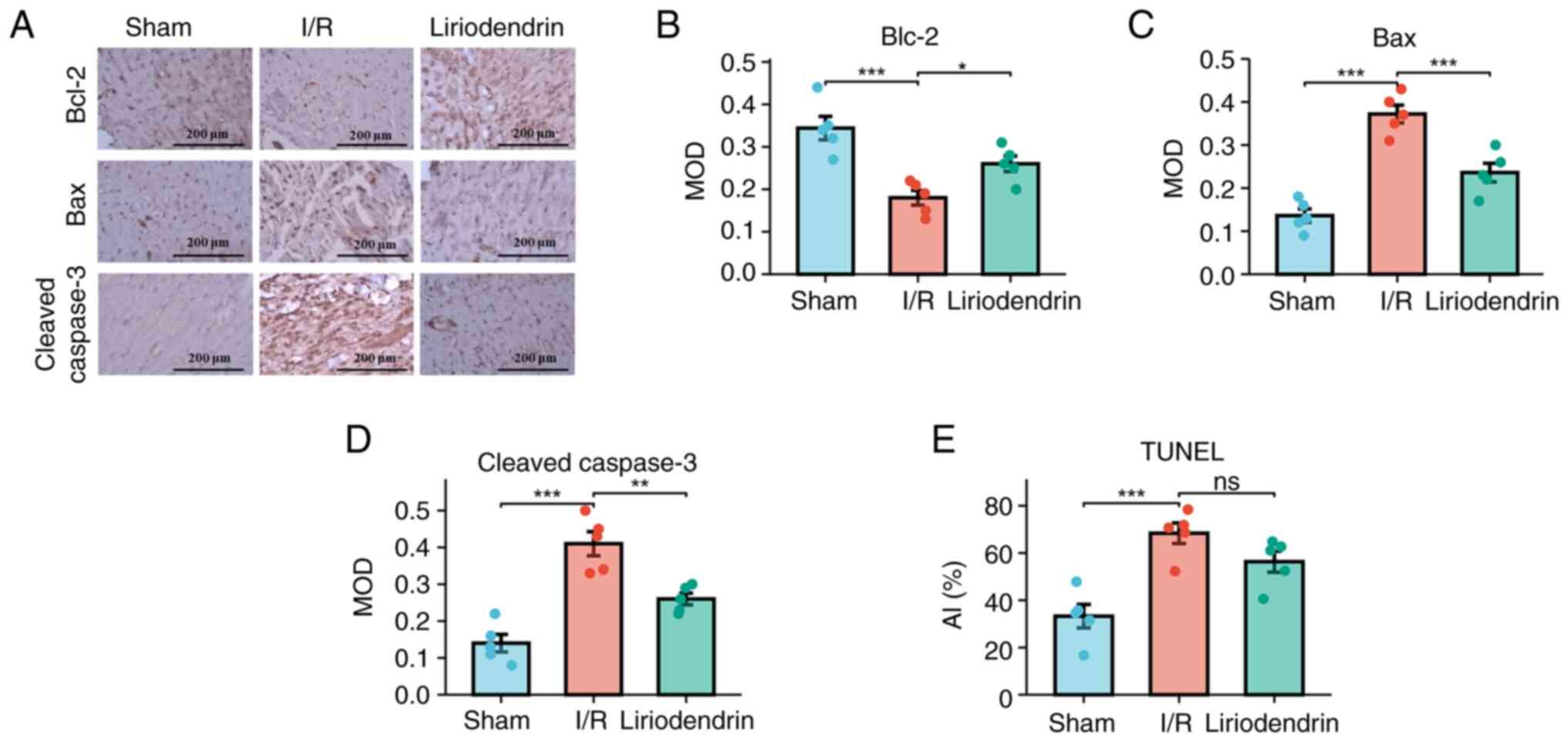
To investigate the protective effect of liriodendrin
on cardiomyocytes, immunohistochemical staining was conducted.
Semi-quantitative analysis of key proteins involved in the
apoptosis pathway was performed and the AI was calculated. Compared
with the sham group, a significant decrease in the anti-apoptotic
protein marker Bcl-2 was observed in the I/R group, suggesting I/R
injury may exert a pro-apoptotic influence on rat cardiomyocytes
(Fig. 4A and B). Furthermore, a
significant increase in Bax (Fig. 4A
and C) and cleaved caspase-3 levels was observed in the I/R
group (Fig. 4A and D). The
liriodendrin group exhibited a significant decrease in expression
levels of Bax and cleaved caspase-3 in cardiomyocytes, along with a
significant increase in expression of the anti-apoptotic protein
Bcl-2. The AI was significantly higher in the I/R compared with the
sham group. The liriodendrin group exhibited a marked decrease in
the apoptosis index but this was not significant (Fig. 4E). These findings suggested that
liriodendrin effectively enhanced the anti-apoptotic capacity of
cardiomyocytes subjected to I/R.
Liriodendrin inhibits the apoptosis
pathway in I/R rats
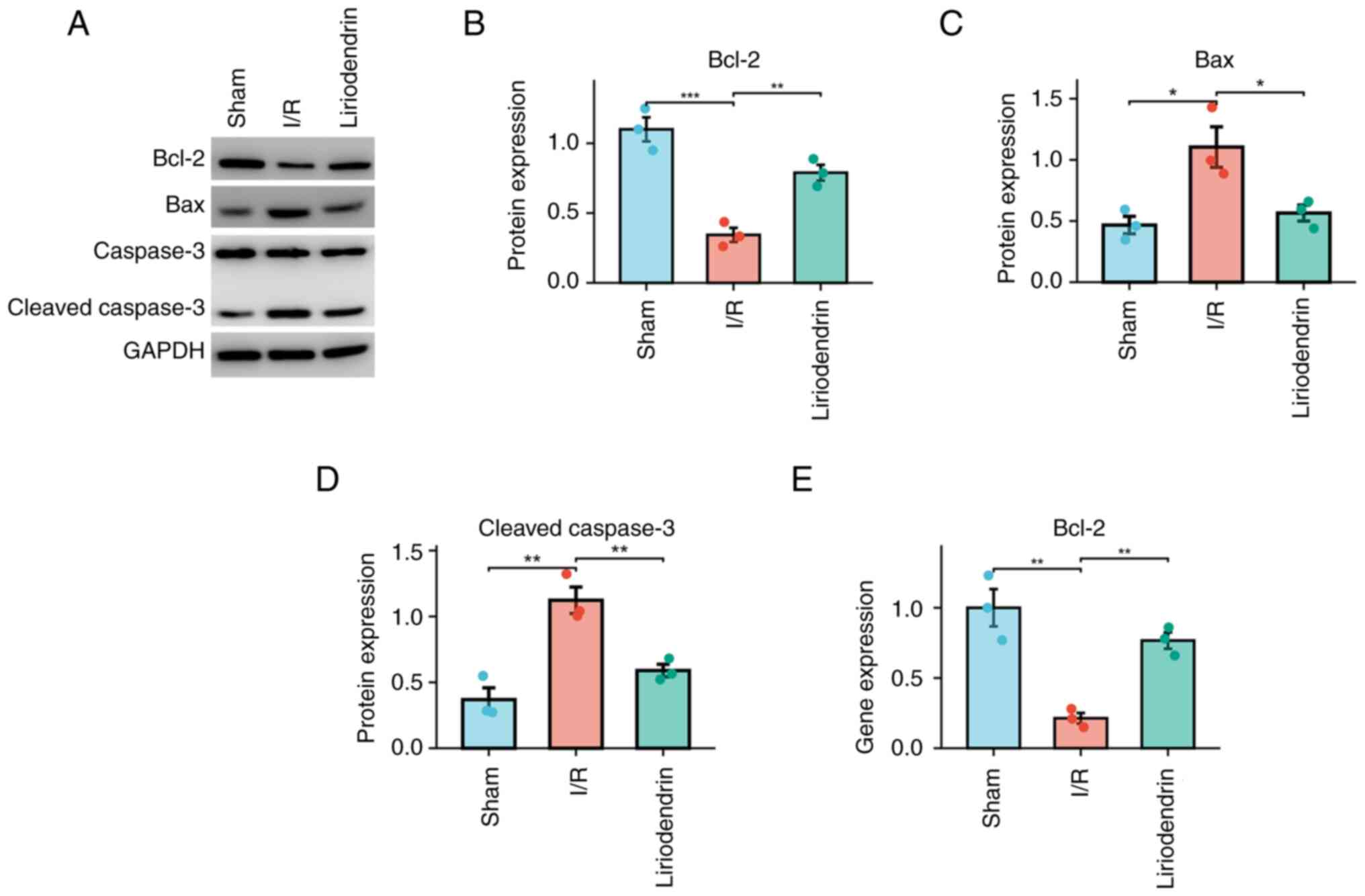
To determine the impact of liriodendrin on the
apoptosis pathway in rats subjected to I/R, western blotting
(Fig. 5A) was used to measure
protein expression levels of Bcl-2 (Fig. 5B), Bax (Fig. 5C) and cleaved caspase-3 (Fig. 5D), which are pivotal proteins
involved in the apoptosis pathway induced by myocardial injury
(28). In accordance with the
findings of immunohistochemical staining, the liriodendrin group
exhibited increased protein expression of Bcl-2 and decreased
protein expression of Bax and cleaved caspase-3 compared with the
I/R group. Furthermore, the liriodendrin group demonstrated a
significant increase in transcriptional activity of Bcl-2 compared
with the I/R group (Fig. 5E).
These results suggested that liriodendrin decreased activation of
the apoptosis pathway of cardiomyocytes in rats subjected to
I/R.
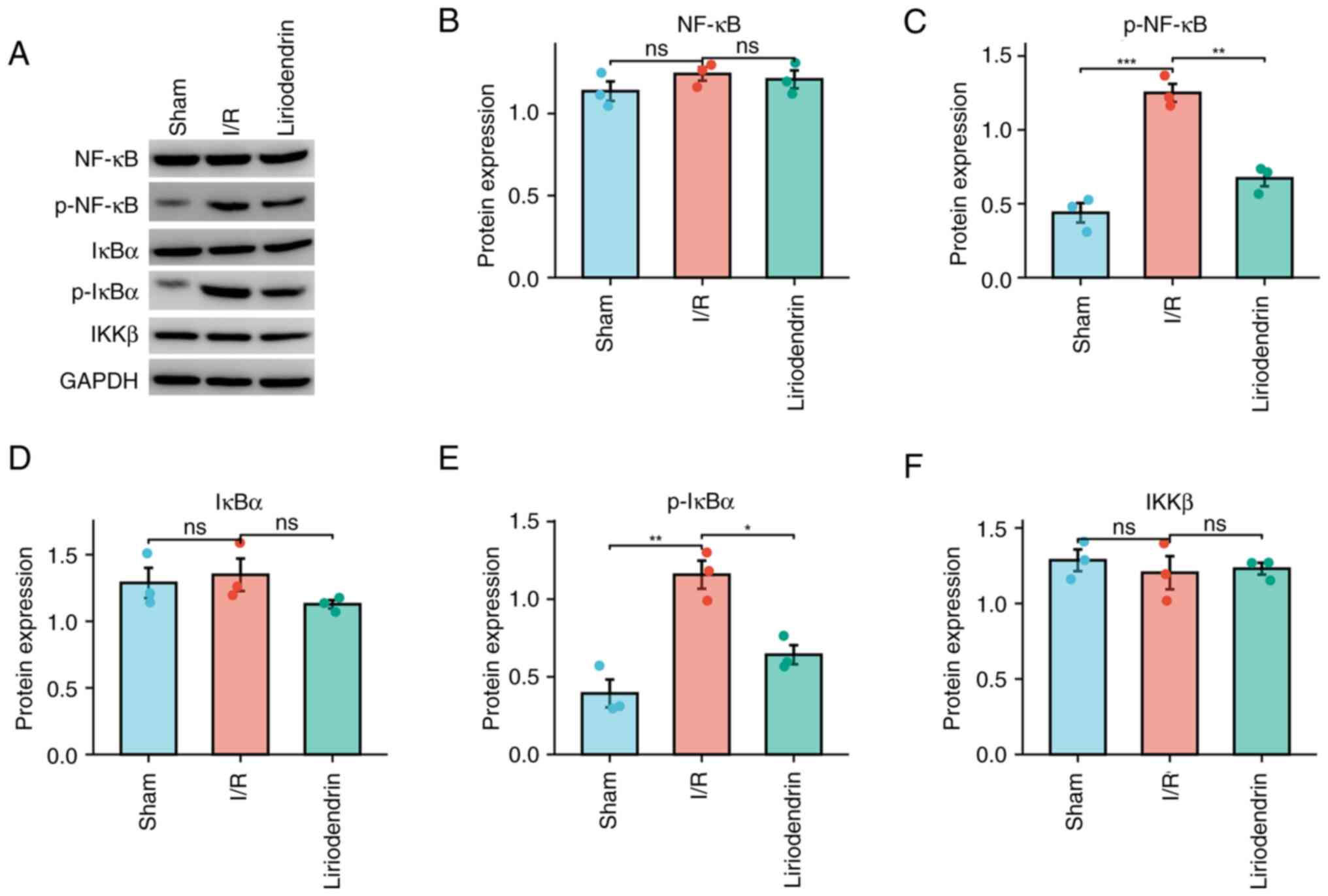
NF-κB signaling pathway-associated
expression in myocardium of I/R rats treated with liriodendrin
Liriodendrin may impede the NF-κB signaling pathway,
which is important in the inflammatory response subsequent to
myocardial infarction (19). In
the context of myocardial injury induced by I/R, liriodendrin
inhibited the release of inflammatory factors. Consequently, the
present study evaluated the protein expression of NF-κB pathway
components, including NF-κB (Fig. 6A
and B), p-NF-κB (Fig. 6C),
IκBα (Fig. 6D), p-IκBα (Fig. 6E) and IKKβ (Fig. 6F). These results demonstrated a
significant decrease in the expression of p-NF-κB and p-IκBα in the
liriodendrin group compared with the I/R group. This suggested that
liriodendrin effectively inhibited the NF-κB signaling pathway in
myocardial I/R injury in rats, mitigating the release of
inflammatory factors.
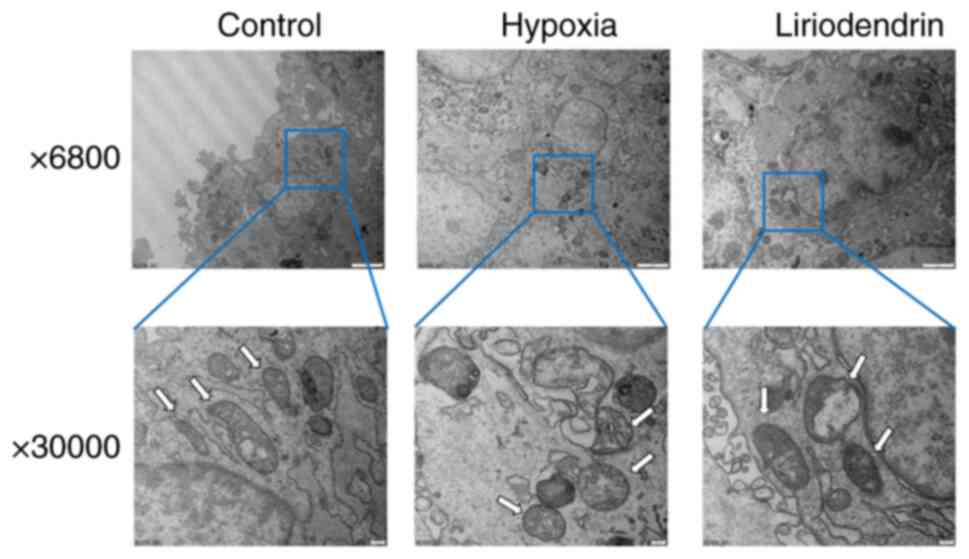
Liriodendrin preserves mitochondrial
morphology in cardiomyocytes
Mitochondria are key target organelles of I/R
injury. ROS induce protein and lipid peroxidation, affect ATP
synthesis, activate phospholipase and damage structure of
mitochondria (29). To clarify
the protective effect of liriodendrin, H9C2 cells were treated with
100 µM liriodendrin for 24 h, hypoxia was induced for 4 h
and cells were cultured with normal medium for 24 h after
reoxygenation to observe the differences in mitochondria. Compared
with normal cells, the mitochondria of cells that underwent
hypoxia/reoxygenation were swollen and demonstrated vacuolar
degeneration, an absence of mitochondrial cristae and an incomplete
outer membrane. A majority of the mitochondria in the
liriodendrin-treated group exhibited normal morphology, with a few
swollen and vacuolated mitochondria (Fig. 7). These results suggested that
liriodendrin may serve a role in preserving the mitochondrial
morphology of cells subjected to I/R.
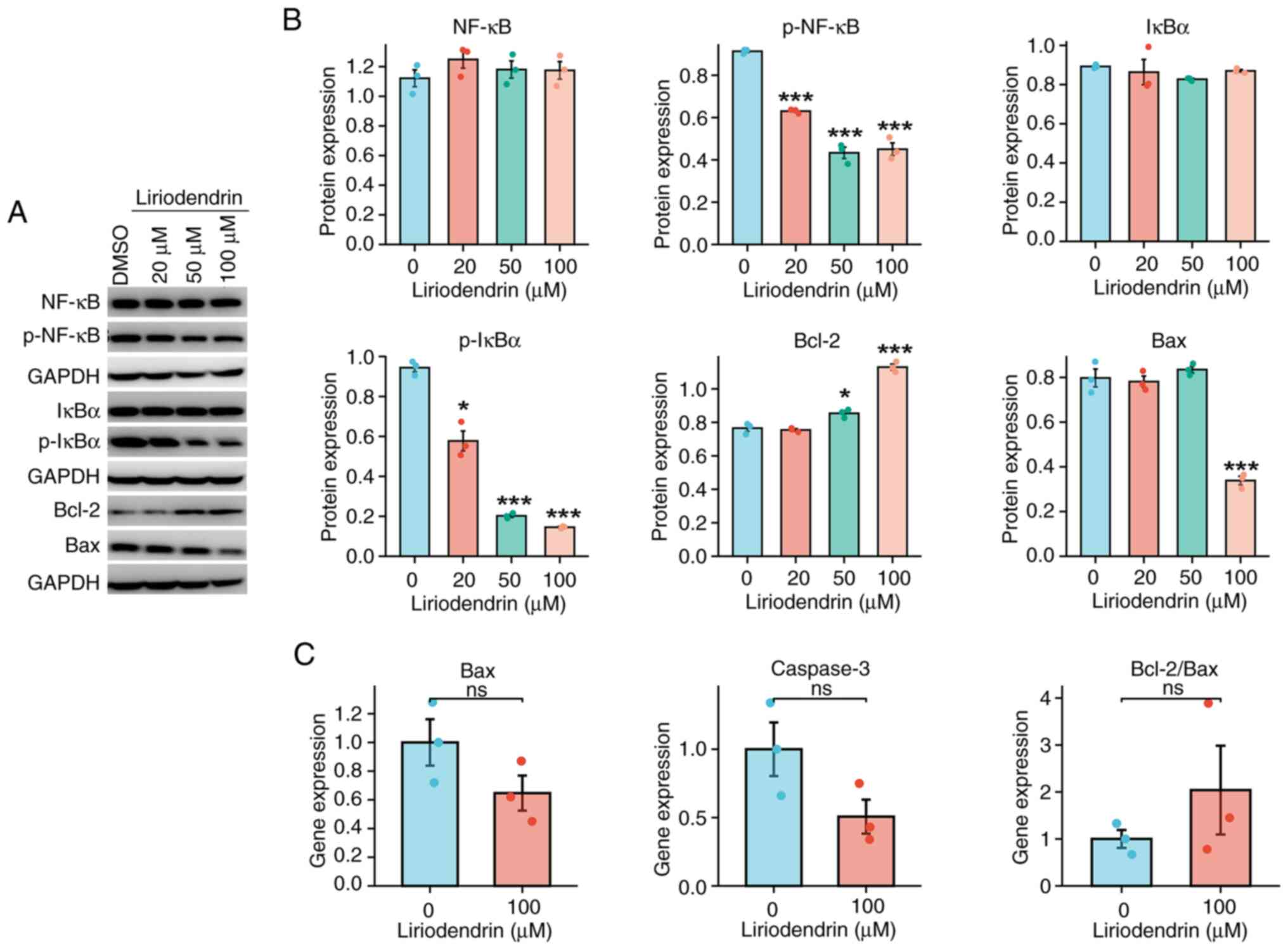
Inhibitory effect of liriodendrin on
NF-κB and apoptotic signaling pathways in hypoxia/reoxygenation
H9C2 cells is dose-dependent
A previous study reported that varying
concentrations of liriodendrin exhibit distinct therapeutic effects
on cardiomyocytes cultured under hypoxic conditions (19). To elucidate the impact of
liriodendrin concentration on the therapeutic efficacy of
hypoxia/reoxygenation in H9C2 cells, pretreatment of H9C2 cells
with three concentrations of liriodendrin was performed. The
protein expression of NF-κB, p-NF-κB, IκBα, p-IκBα, Bax and Bcl-2
was assessed across a range of liriodendrin concentrations (0-100
µM; Fig. 8A and B). All
concentrations of liriodendrin caused a significant decrease in
protein expression levels of p-NF-κB and p-IκBα (Fig. 8A and B). Furthermore, 100
µM liriodendrin caused a significant decrease in Bax protein
expression levels and a significant increase in Bcl-2 protein
expression. The transcriptional activity of genes related to
apoptosis was measured. Transcriptional activity of Bax and
caspase-3 demonstrated a marked decrease, while the ratio of
Bcl-2/Bax showed a marked increase in cells treated with 100
µM liriodendrin; however, these results were not significant
(Fig. 8C).
Discussion
Patients with heart failure are associated with
exaggerated endothelial IR injury, which may contribute to poor
clinical prognosis (30).
However, I/R injury limits the efficacy of interventional therapy
against myocardial injury caused by cardiovascular disease and may
exacerbate the degree of myocardial injury (31). At present, there is a lack of
pharmacotherapy options for treatment of myocardial injury
resulting from I/R. The therapeutic efficacy of liriodendrin in
myocardial infarction has been previously reported (19,21). However, the potential of
liriodendrin for treating myocardial I/R injury remains largely
uncertain. Rat and cell models of I/R injury have been previously
established, showing liriodendrin exhibits ameliorative effects on
I/R injury in a variety of models (19-21). In a myocardial I/R animal model,
pharmacological pretreatment is a frequently employed experimental
approach (32,33). Zhang et al (34) administered liriodendrin (100
mg/kg/day, intragastrically) for 3 days in a dextran sulfate
sodium-induced ulcerative colitis mouse model. Here, liriodendrin
pre-treatment for 5 days significantly improves I/R-induced
apoptosis, inflammation, and mitochondrial damage in rats.
During I/R injury, abnormal metabolism of ROS leads
to injury caused by oxidative stress (35,36). ROS damage cells by reacting with
intracellular proteins, lipids and nucleic acids, resulting in
lipid peroxidation, oxidative DNA damage and protein abnormal
expression (35,36). ROS serve as intracellular
messengers and activate a number of signaling pathways, including
apoptosis pathways, causing cell damage and further ROS production
(35). Mitochondria stimulated
by ROS can generate large amounts of ROS, initiating a cycle of
mitochondrial damage. mPTP increases permeability following I/R
injury, which increases the osmolality of mitochondria (37). This causes mitochondrial
swelling, disappearance of cristae and outer membrane rupture,
releasing apoptosis-associated proteins into the cytoplasm and
activating the apoptotic pathway. Therefore, maintaining integrity
of mitochondrial morphology is key for reducing I/R injury
(38). The present study
demonstrated that ROS in the lesion area was significantly
increased following myocardial I/R in rats. Furthermore, local ROS
generation was significantly decreased in rats following
liriodendrin treatment, while SOD levels in peripheral blood
samples increased. SOD aids maintenance of the oxidative and
antioxidant balance; SOD levels decreased after I/R injury but
significantly increased in the liriodendrin group. This finding
could potentially be attributed to excessive SOD consumption after
reperfusion injury resulting in decreased SOD levels, while
liriodendrin increased the production of SOD. However, increased
SOD and decreased ROS levels may serve a key role in limiting I/R
injury. Bhandary et al (39) performed in vivo and in
vitro experiments, reporting that rutinum, a kind of flavonoid,
could treat I/R injury, accompanied by the activation of SOD and
2,2-Diphenyl-1-picrylhydrazyl. In the present study, in
vitro assays using I/R H9C2 cells showed that mitochondrial
integrity increased in liriodendrin-treated cells, compared with
the I/R group and mitochondrial swelling and fragmentation were
more prevalent in the I/R group, which may be associated with
decreased ROS levels. ROS can cause opening of mPTP channels and
lead to mitochondrial swelling and rupture, while mitochondrial
damage further stimulates production of ROS (40).
TUNEL staining showed that the apoptotic index in
the I/R group was significantly higher compared with that in the
liriodendrin group. Furthermore, in the I/R group, caspase-3 and
Bax expression were significantly higher and Bcl-2 expression
levels were significantly lower in the injured area compared with
the liriodendrin group. These results suggested that liriodendrin
may protect cardiomyocytes of I/R rats by inhibiting the apoptosis
pathway to decrease cardiomyocyte apoptosis. Western blotting
results showed that caspase-3 and Bax protein expression
significantly increased and Bcl-2 protein expression levels
decreased in the I/R group. These changes in protein expression of
caspase-3, Bcl-2 and Bax following liriodendrin treatment may
indicate that liriodendrin inhibited myocardial apoptosis in rats.
The downregulation of apoptosis in reperfused cardiomyocytes
following liriodendrin treatment was also validated at the
transcriptional level using RT-qPCR. It has been previously
reported that the inhibitory effect of liriodendrin on the
apoptosis pathway is determined by its ability to protect
mitochondrial integrity and avoid activation of the
mitochondria-associated apoptosis pathway, although the exact
mechanisms of action remain unclear and warrant further
investigation (41). In the
present study, echocardiographic measurement demonstrated that
there was no significant effect of I/R injury on the cardiac
function in the short term between the I/R and liriodendrin-treated
groups. However, pathological examination showed damaged myocardial
tissue caused by I/R injury and liriodendrin had a therapeutic
effect on these tissues. H&E staining indicated increased
levels of inflammatory cell infiltration around cardiomyocytes in
the I/R group, while there was less inflammatory cell infiltration
in myocardial tissue in the liriodendrin group. Moreover, the
liriodendrin group exhibited a significant decrease in IL-1β, TNF-α
and MCP-1 expression compared with the I/R group. Additionally,
liriodendrin treatment resulted in a reduction in transcriptional
activities of IL-6 and IL-1β, suggesting its potential for the
protection of cardiomyocytes and maintaining viable myocardium in
I/R rats through inhibition of the inflammatory response.
During oxidative stress injury, inflammatory injury
may cause cardiomyocyte death; the NF-κB signaling pathway is
involved in I/R injury (42,43). ROS can activate the NF-κB
signaling pathway by promoting phosphorylation of IKK (44). Under physiological conditions,
NF-κB is stably bound with IκB in the cytoplasm. The IKK complex
can bind to IκB following phosphorylation to promote IκB
phosphorylation and degradation by ubiquitination, which results in
NF-κB dissociation from IκB and activation of the NF-κB signaling
pathway (45). The IKK complex
comprises three subunits: IKKα, IKKβ and IKKγ. IKKβ is the primary
kinase that mediates activation of the NF-κB signaling pathway by
pro-inflammatory factors (13).
Stimulating IL-1 and TNF-α in IKKβ-deficient cells could not
activate the NF-κB signaling pathway (46). Therefore, IKKβ was selected as
the experimental index in the present study. IκBα combined with
NF-κB is a better substrate for IKK enzyme action and its rapid
degradation rate can ensure the swift activation of NF-κB dimers
(13). In the present study,
western blotting demonstrated that liriodendrin decreased protein
expression of p-IKKβ, IKKβ, p-IκBα and IκBα, which indicated that
liriodendrin may exert cardioprotective effects by negatively
regulating the NF-κB signaling pathway to inhibit the inflammatory
response. There was no significant difference in IκBα protein
expression in the liriodendrin compared with the I/R group, which
may be due to rapid degradation rate of IκBα. In theory, the
protein levels of NF-κB, IκBα, and IKKβ should not exhibit
significant discrepancies. Phosphorylation, being a swift
post-translational modification, does not alter the overall protein
quantity, yet variations in activity of phosphorylated localization
as well as the employment of total protein polyclonal antibody
detection result in minor dissimilarities in the total quantity of
these proteins (13,47). Nevertheless, liriodendrin
markedly inhibited expression of p-NF-κB and p-IκBα protein,
indicating liriodendrin possessed partial anti-inflammatory
activity. However, it may not influence all molecules within the
inflammatory signaling pathway, including NF-kB, IκBα, and IKKβ.
These findings suggested that liriodendrin possesses partial
anti-inflammatory activity in I/R rat model.
Liriodendrin mitigates liver I/R injury to prevent
apoptosis of murine hepatocytes, which is accompanied by a decrease
in oxidative stress and production of pro-inflammatory cytokines
(20). Liriodendrin decreases
expression of TLR4 and NF-κB (20) and may be a potent suppressor of
CaCl2-induced arrhythmia (48). Liriodendrin may mitigate fibrosis
induced by myocardial infarction in rats by suppressing excessive
myocardial autophagy, potentially via activation of the
PI3K/Akt/mTOR pathway (21). The
present study aimed to investigate the potential therapeutic effect
of liriodendrin on myocardial I/R and its efficacy in vitro
using H9C2 cells. Here, liriodendrin exerted a
concentration-dependent inhibitory effect on the apoptosis and
NF-κB signaling pathways in cardiomyocytes. The most significant
inhibition of these pathways was observed at a dosage of 100
µM. Protein expression levels of p-NF-κB and p-IκBα were
diminished in a concentration-dependent manner, expression of Bax
was inhibited, while Bcl-2 expression was heightened. Liriodendrin
protected against hypoxia-induced myocardial cell damage through
anti-inflammatory and anti-apoptotic mechanisms. Future experiments
should consider the influence of drug dosage on the efficacy of
treatment. Our previous study also validated this conclusion
(19). The present study
demonstrated that liriodendrin served an important role in
maintaining mitochondrial morphology in hypoxia/reoxygenation
cells.
The present study had certain limitations. To
confirm the disparities between the I/R and liriodendrin groups, it
is imperative to conduct additional studies with increased sample
sizes. Liriodendrin partially mitigated the metabolic disturbance
of ROS by inhibiting the NF-κB and Bax signaling pathways,
preserved mitochondrial equilibrium and diminished cardiomyocyte
apoptosis. Furthermore, quantitative methodology was used to
compare the mitochondrial morphology to determine its contribution
to cardiomyocyte apoptosis. Future studies should address these
issues to enhance the understanding of the therapeutic efficacy of
liriodendrin in myocardial I/R injury. Additionally, the toxicity
of liriodendrin in single and multiple doses was not measured in
the present study. Furthermore, the interplay of ROS-mediated NF-κB
in I/R injury and associated signaling pathways were not examined.
Finally, research into the use of liriodendrin in the clinical
environment is key. Prior to clinical studies, assessment of
toxicology and pharmacokinetics of liriodendrin must be performed
in animal and human pre-clinical trials.
In conclusion, the present study demonstrated
abnormal metabolism of ROS, as well as mitochondrial swelling and
rupture and increased myocardial apoptosis during myocardial I/R
injury. Additionally, activation of the NF-κB signaling pathway and
exacerbation of the inflammatory response were observed, leading to
myocardial injury. However, liriodendrin inhibited ROS metabolism
disorder, preserved mitochondrial homeostasis and mitigated
myocardial apoptosis. Furthermore, NF-κB signaling was suppressed,
leading to decreased inflammatory response, thereby attenuating
severity of the I/R myocardial injury. Future studies should use
network pharmacology to investigate the potential targets and
signaling pathways of liriodendron.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
BL, WY and ZG designed the study. BL, WY, BY, QC,
LZ, YS, NJ and ZG performed the literature review, experiments and
statistical analysis. BL, WY and BY edited the manuscript. All
authors have read and approved the final manuscript. BL and ZG
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Animal experiments were approved by the Ethics
Committee of Tianjin Chest Hospital (approval no. TJCH-2021-007;
Tianjin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by Tianjin Key Laboratory of
Cardiovascular Emergency and Critical Care, Tianjin Municipal
Science and Technology Bureau and Tianjin Key Medical Discipline
(Cardiovascular Surgery) Construction Project (grant no.
TJYXZDXK-042A).
References
|
1
|
Algoet M, Janssens S, Himmelreich U, Gsell
W, Pusovnik M, Van den Eynde J and Oosterlinck W: Myocardial
ischemia-reperfusion injury and the influence of inflammation.
Trends Cardiovasc Med. 33:357–366. 2023. View Article : Google Scholar
|
|
2
|
Liu T, Hao Y, Zhang D, Zhou H, Peng S,
Zhang D, Li K, Chen Y and Chen M: Advanced cardiac patches for the
treatment of myocardial infarction. Circulation. 149:2002–2020.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yap J, Irei J, Lozano-Gerona J, Vanapruks
S, Bishop T and Boisvert WA: Macrophages in cardiac remodelling
after myocardial infarction. Nat Rev Cardiol. 20:373–385. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie S, Xu SC, Deng W and Tang Q: Metabolic
landscape in cardiac aging: insights into molecular biology and
therapeutic implications. Signal Transduct Target Ther. 8:1142023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shadel GS and Horvath TL: Mitochondrial
ROS signaling in organismal homeostasis. Cell. 163:560–569. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lingappan K: NF-κB in oxidative stress.
Curr Opin Toxicol. 7:81–86. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang D, Yu X, Gao K, Li F, Li X, Pu H,
Zhang P, Guo S and Wang W: Sweroside alleviates pressure
overload-induced heart failure through targeting CaMKⅡδ to inhibit
ROS-mediated NF-κB/NLRP3 in cardiomyocytes. Redox Biol.
74:1032232024. View Article : Google Scholar
|
|
8
|
Robichaux DJ, Harata M, Murphy E and Karch
J: Mitochondrial permeability transition pore-dependent necrosis. J
Mol Cell Cardiol. 174:47–55. 2023. View Article : Google Scholar :
|
|
9
|
Zhang X, Sun Y, Yang R, Liu B, Liu Y, Yang
J and Liu W: An injectable mitochondria-targeted nanodrug
loaded-hydrogel for restoring mitochondrial function and
hierarchically attenuating oxidative stress to reduce myocardial
ischemia-reperfusion injury. Biomaterials. 287:1216562022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song JQ, Teng X, Cai Y, Tang CS and Qi YF:
Activation of Akt/GSK-3beta signaling pathway is involved in
intermedin(1-53) protection against myocardial apoptosis induced by
ischemia/reperfusion. Apoptosis. 14:1061–1069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Korotkov SM: Mitochondrial oxidative
stress is the general reason for apoptosis induced by
different-valence heavy metals in cells and mitochondria. Int J Mol
Sci. 24:144592023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Q, Wang L, Wang S, Cheng H, Xu L,
Pei G, Wang Y, Fu C, Jiang Y, He C and Wei Q: Signaling pathways
and targeted therapy for myocardial infarction. Signal Transduct
Target Ther. 7:782022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhuang L, Zong X, Yang Q, Fan Q and Tao R:
Interleukin-34-NF-κB signaling aggravates myocardial
ischemic/reperfusion injury by facilitating macrophage recruitment
and polarization. EBioMedicine. 95:1047442023. View Article : Google Scholar
|
|
14
|
Yuan X, Liu K, Dong P and Han H:
Protective effect and mechanism of different proportions of
'Danggui-Kushen' herb pair on ischemic heart disease. Heliyon.
9:e221502023. View Article : Google Scholar
|
|
15
|
Lin H, Wang W, Peng M, Kong Y, Zhang X,
Wei X and Shang H: Pharmacological properties of Polygonatum and
its active ingredients for the prevention and treatment of
cardiovascular diseases. Chin Med. 19:12024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qi Q, Cai D, Yu X, Shi J, Bai W and Yan N:
Anthocyanins in Subtropical Fruits. CRC Press; Boca Raton, FL: pp.
1–31. 2023
|
|
17
|
Cheng F, Li D, Ma X, Wang Y, Lu L, Hu B
and Cui S: Liriodendrin exerts protective effects against chronic
endometritis in rats by modulating gut microbiota composition and
the arginine/nitric oxide metabolic pathway. Int Immunopharmacol.
126:1112352024. View Article : Google Scholar
|
|
18
|
Zhang S, Hu D, Zhuo Y, Cui L, Li D, Zhang
L, Yang L and Wang X: Protective effect of liriodendrin on IgG
immune complex-induced acute lung injury via inhibiting
SRC/STAT3/MAPK signaling pathway: A network pharmacology research.
Naunyn Schmiedebergs Arch Pharmacol. 396:3269–3283. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li B, Yao BC, Chen QL, Song YQ, Jiang N,
Zhao LL and Guo ZG: The protective role and mechanism of
liriodendrin in rats with myocardial infarction. J Thorac Dis.
14:135–146. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu ZY and Cheng G: Protective effect of
liriodendrin against liver ischaemia/reperfusion injury in mice via
modulating oxidative stress, inflammation and nuclear factor kappa
B/toll-like receptor 4 pathway. Folia Morphol (Warsz). 82:668–676.
2023. View Article : Google Scholar
|
|
21
|
Zhang P, Liu X, Yu X, Zhuo Y, Li D, Yang L
and Lu Y: Protective effects of liriodendrin on myocardial
infarction-induced fibrosis in rats via the PI3K/Akt autophagy
pathway: A network pharmacology study. Comb Chem High Throughput
Screen. 27:1566–1575. 2024. View Article : Google Scholar
|
|
22
|
Percie du Sert N, Hurst V, Ahluwalia A,
Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl
U, et al: The ARRIVE guidelines 2.0: Updated guidelines for
reporting animal research. J Cereb Blood Flow Metab. 40:1769–1777.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rashidi Z, Azadbakht M and Khazaei M:
Hydrostatic pressure improves in-vitro maturation of oocytes
derived from vitrified-warmed mouse ovaries. Iran J Reprod Med.
10:257–264. 2012.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Ge Y, Huang M and Yao YM: Autophagy and
proinflammatory cytokines: Interactions and clinical implications.
Cytokine Growth Factor Rev. 43:38–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wen J, Guan Y, Niu H, Dang Y and Guan J:
Targeting cardiac resident CCR2+ macrophage-secreted MCP-1 to
attenuate inflammation after myocardial infarction. Acta Biomater.
Aug 23–2024.Epub ahead of print. View Article : Google Scholar
|
|
27
|
Jomova K, Alomar SY, Alwasel SH,
Nepovimova E, Kuca K and Valko M: Several lines of antioxidant
defense against oxidative stress: Antioxidant enzymes,
nanomaterials with multiple enzyme-mimicking activities, and
low-molecular-weight antioxidants. Arch Toxicol. 98:1323–1367.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng Y, Yan M, He S, Xie Y, Wei L, Xuan
B, Shang Z, Wu M, Zheng H, Chen Y, et al: Baicalin alleviates
angiotensin II-induced cardiomyocyte apoptosis and autophagy and
modulates the AMPK/mTOR pathway. J Cell Mol Med. 28:e183212024.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Granger DN and Kvietys PR: Reperfusion
injury and reactive oxygen species: The evolution of a concept.
Redox Biol. 6:524–551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Seeger JP, Benda NM, Riksen NP, van Dijk
AP, Bellersen L, Hopman MT, Cable NT and Thijssen DH: Heart failure
is associated with exaggerated endothelial ischaemia-reperfusion
injury and attenuated effect of ischaemic preconditioning. Eur J
Prev Cardiol. 23:33–40. 2016. View Article : Google Scholar
|
|
31
|
Sánchez-Hernández CD, Torres-Alarcón LA,
González-Cortés A and Peón AN: Ischemia/reperfusion injury:
pathophysiology, current clinical management, and potential
preventive approaches. Mediators Inflamm. 2020:84053702020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zeng JJ, Shi HQ, Ren FF, Zhao XS, Chen QY,
Wang DJ, Wu LP, Chu MP, Lai TF and Li L: Notoginsenoside R1
protects against myocardial ischemia/reperfusion injury in mice via
suppressing TAK1-JNK/p38 signaling. Acta Pharmacol Sin.
44:1366–1379. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ding S, Duanmu X, Xu L, Zhu L and Wu Z:
Ozone pretreatment alleviates ischemiareperfusion injury-induced
myocardial ferroptosis by activating the Nrf2/Slc7a11/Gpx4 axis.
Biomed Pharmacother. 165:1151852023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Z, Yang L, Wang B, Zhang L, Zhang Q,
Li D, Zhang S, Gao H and Wang X: Protective role of liriodendrin in
mice with dextran sulphate sodium-induced ulcerative colitis. Int
Immunopharmacol. 52:203–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiang Q, Yi X, Zhu XH, Wei X and Jiang DS:
Regulated cell death in myocardial ischemia-reperfusion injury.
Trends Endocrinol Metab. 35:219–234. 2024. View Article : Google Scholar
|
|
36
|
Lu Y, Chen K, Zhao W, Hua Y, Bao S, Zhang
J, Wu T, Ge G, Yu Y, Sun J and Zhang F: Magnetic vagus nerve
stimulation alleviates myocardial ischemia-reperfusion injury by
the inhibition of pyroptosis through the M(2)AChR/OGDHL/ROS axis in
rats. J Nanobiotechnology. 21:4212023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bou-Teen D, Kaludercic N, Weissman D,
Turan B, Maack C, Di Lisa F and Ruiz-Meana M: Mitochondrial ROS and
mitochondria-targeted antioxidants in the aged heart. Free Radic
Biol Med. 167:109–124. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang L, Yin X, Chen YH, Chen Y, Jiang W,
Zheng H, Huang FQ, Liu B, Zhou W, Qi LW and Li J: Proteomic
analysis reveals ginsenoside Rb1 attenuates myocardial
ischemia/reperfusion injury through inhibiting ROS production from
mitochondrial complex I. Theranostics. 11:1703–1720. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bhandary B, Piao CS, Kim DS, Lee GH, Chae
SW, Kim HR and Chae HJ: The protective effect of rutin against
ischemia/reperfusion-associated hemodynamic alteration through
antioxidant activity. Arch Pharm Res. 35:1091–1097. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Khalifa AR, Abdel-Rahman EA, Mahmoud AM,
Ali MH, Noureldin M, Saber SH, Mohsen M and Ali SS: Sex-specific
differences in mitochondria biogenesis, morphology, respiratory
function, and ROS homeostasis in young mouse heart and brain.
Physiol Rep. 5:e131252017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pomerantz BJ, Reznikov LL, Harken AH and
Dinarello CA: Inhibition of caspase 1 reduces human myocardial
ischemic dysfunction via inhibition of IL-18 and IL-1beta. Proc
Natl Acad Sci USA. 98:2871–2876. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hamid T, Guo SZ, Kingery JR, Xiang X, Dawn
B and Prabhu SD: Cardiomyocyte NF-κB p65 promotes adverse
remodelling, apoptosis, and endoplasmic reticulum stress in heart
failure. Cardiovasc Res. 89:129–138. 2011. View Article : Google Scholar
|
|
43
|
Zhang Z, Liu Y, Ren X, Zhou H, Wang K,
Zhang H and Luo P: Caffeoylquinic acid derivatives extract of
erigeron multiradiatus alleviated acute myocardial ischemia
reperfusion injury in rats through inhibiting NF-KappaB and JNK
activations. Mediators Inflamm. 2016:79619402016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huang Q, Zhan L, Cao H, Li J, Lyu Y, Guo
X, Zhang J, Ji L, Ren T, An J, et al: Increased mitochondrial
fission promotes autophagy and hepatocellular carcinoma cell
survival through the ROS-modulated coordinated regulation of the
NFKB and TP53 pathways. Autophagy. 12:999–1014. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu H, Lin L, Zhang Z, Zhang H and Hu H:
Targeting NF-κB pathway for the therapy of diseases: Mechanism and
clinical study. Signal Transduct Target Ther. 5:2092020. View Article : Google Scholar
|
|
46
|
Sui Y, Park SH, Xu J, Monette S, Helsley
RN, Han SS and Zhou C: IKKβ links vascular inflammation to obesity
and atherosclerosis. J Exp Med. 211:869–886. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dong X, Jiang J, Lin Z, Wen R, Zou L, Luo
T, Guan Z, Li X, Wang L, Lu L, et al: Nuanxinkang protects against
ischemia/reperfusion-induced heart failure through regulating
IKKβ/IκBα/NF-κB-mediated macrophage polarization. Phytomedicine.
101:1540932022. View Article : Google Scholar
|
|
48
|
Feng C, Li BG, Gao XP, Qi HY and Zhang GL:
A new triterpene and an antiarrhythmic liriodendrin from
Pittosporum brevicalyx. Arch Pharm Res. 33:1927–1932. 2010.
View Article : Google Scholar : PubMed/NCBI
|