Introduction
Despite progress in cancer prevention, early
detection and treatment, cancer remains a major unresolved global
medical challenge (1,2). In 2020, the leading causes of
cancer-related deaths in China included lung cancer, liver cancer,
gastric cancer (GC), breast cancer (BC) and colorectal cancer
(CRC), with liver cancer mortality rising from third place in 2018
to second place in 2020 (3).
Globally, cancer incidence continues to rise, placing increasing
pressure on public health systems and healthcare infrastructure
(4). The initiation and
progression of malignancies are primarily driven by dysregulation
of gene expression, especially within critical regulatory pathways
involving cell proliferation, survival and apoptosis (5). Although there has been some
advancement in understanding these molecular mechanisms, the
complexity and heterogeneity of cancer still pose major challenges
for effective treatment.
In this context, non-coding RNAs (ncRNAs), including
microRNAs (miRNAs/miRs), long non-coding RNAs (lncRNAs) and
circular RNAs (circRNAs), have gained widespread attention due to
their crucial roles in gene expression regulation and modulation of
tumor behavior (6). Among ncRNA
molecules, miRNAs are the most extensively studied. They serve
crucial roles in regulating key biological processes in cancer,
including cell proliferation, apoptosis, migration and invasion
(7,8). Initially considered
'transcriptional noise' or 'junk DNA', ncRNAs, including miRNAs,
have now been proven to be essential for numerous cellular
processes and diseases, particularly cancer (9). Among these miRNAs, miR-100 is
involved in the biological processes of multiple cancer types
(10).
miR-100 (MI0000102), located on human chromosome 11
[chr11:122152229-122152308(-)], is part of the let-7-C gene
cluster. This miRNA has two mature forms, hsa-miR-100-5p and
hsa-miR-100-3p, which are derived from the 5′ and 3′ arms of the
precursor miR-100, respectively (10). miR-100 is frequently dysregulated
in cancer and has been shown to act as a tumor suppressor in
various malignancies, including ovarian cancer (OC), prostate
cancer (PCa), thyroid cancer (TC), bladder cancer and GC (10-14). miR-100 inhibits cancer cell
proliferation, migration and invasion, while promoting apoptosis by
targeting multiple genes involved in these processes (11-15). For example, Zhang et al
(11) showed that miR-100
inhibited the proliferation and cell cycle of FTC-133 cells by
targeting RB protein serine phosphatase from chromosome 3 (RBSP3).
Additionally, miR-100 has been identified as a key regulator of the
cancer cell response to chemotherapy and radiotherapy, enhancing
the chemosensitivity and radiosensitivity of CRC, lung cancer, and
head and neck squamous cell carcinoma (15,16).
miR-100 exerts its effects by targeting the
3′untranslated regions (UTRs) of various key genes involved in
growth signaling and stress responses. For example, miR-100 has
been shown to directly regulate the mTOR pathway, a critical
signaling axis involved in cell metabolism, proliferation and
survival (17). By
downregulating key genes such as insulin-like growth factor 1
receptor (IGF1R), fibroblast growth factor receptor 3 (FGFR3) and
polo-like kinase 1 (PLK1), miR-100 regulates cell cycle
progression, autophagy and chemotherapy resistance (10,17). Furthermore, miR-100 influences
the tumor microenvironment through interactions with lncRNAs and
circRNAs (10). These RNA
molecules act as molecular sponges, regulating multiple cellular
processes, including glycolysis, oxidative stress and tumor
progression (18). Through its
effects on these pathways, miR-100 has emerged as a promising
therapeutic target in cancer treatment, especially for overcoming
chemotherapy resistance and improving the efficacy of traditional
therapies (10).
In addition to its role in malignancies, miR-100 is
also associated with a variety of non-cancerous diseases. The
expression levels of miR-100 are elevated in conditions such as
hypertrophic cardiomyopathy, type 1 and type 2 diabetes,
osteoporosis, and osteoarthritis (19-22). Furthermore, miR-100 has been
shown to regulate apoptosis in different cell types, including
retinal pigment epithelial cells, and serves a crucial role in
normal cellular functions, including embryo implantation and germ
cell proliferation (23-26). These findings further highlight
the diverse biological roles of miR-100 and its potential as a
therapeutic target, with applications not only in cancer but also
in other diseases.
In conclusion, miR-100 represents a promising
biomarker for cancer diagnosis, prognosis and treatment. Its
multifaceted role in regulating critical signaling pathways related
to tumorigenesis, therapeutic resistance and cancer progression
underscores its potential as a target for precision medicine.
Further research into the molecular mechanisms underlying the
functions of miR-100 will provide deeper insights into its
therapeutic applications and may lead to the development of novel
RNA-based cancer therapies, as well as its use in other
diseases.
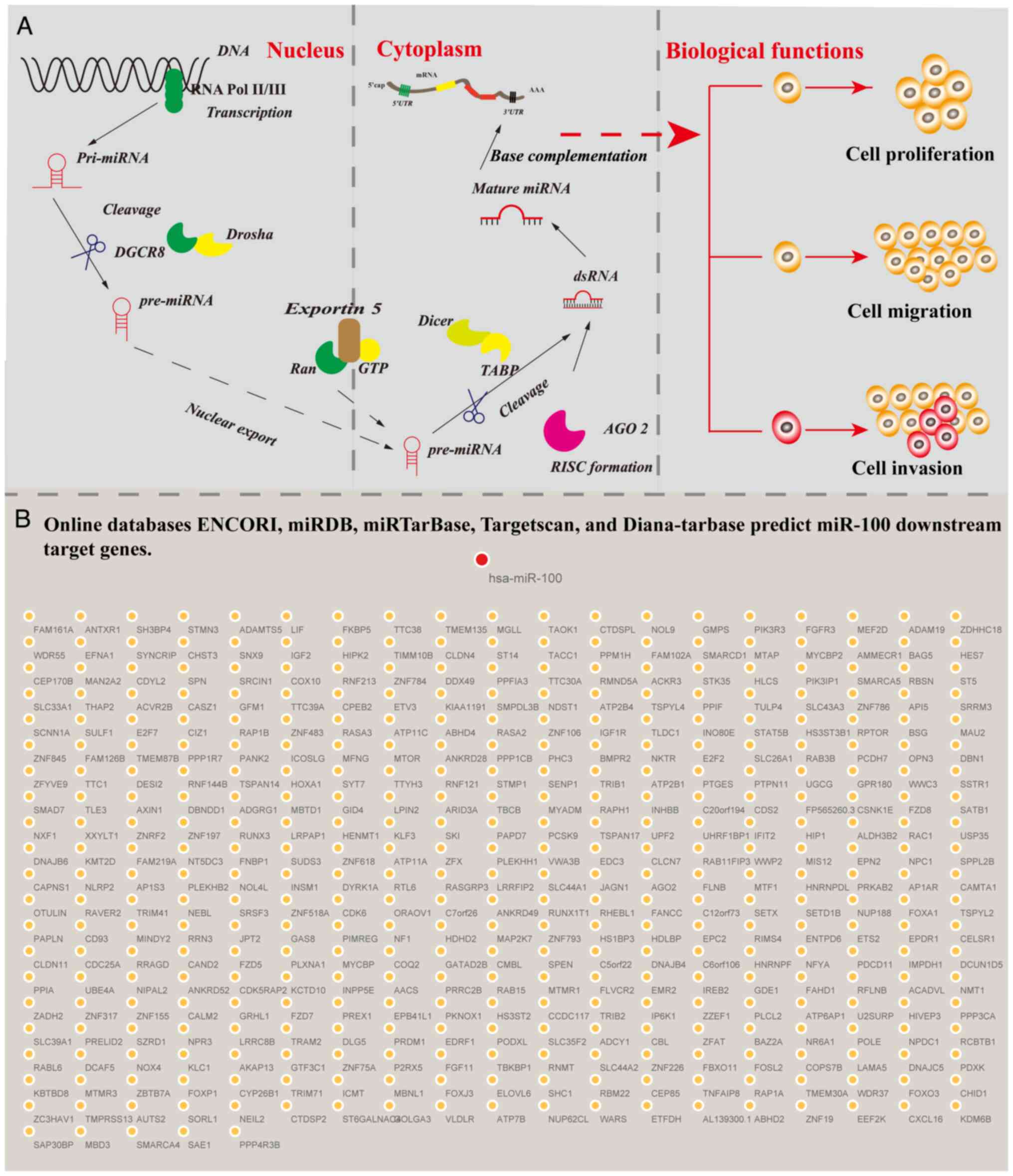
Molecular mechanisms of miR-100 in
cancer
miR-100 was first identified in Drosophila in
2003 and is derived from the let-7-C gene cluster (27). miR-100 is located on the distal
fragile site FRA11F of the 11q13 amplification region on human
chromosome 11 (28). In numerous
malignancies, miR-100 regulates tumor cell proliferation, migration
and invasion by targeting the 3′-UTR of its target genes (29). The specific mechanism by which
miR-100 regulates gene expression involves post-transcriptional
modulation through base pairing of its seed sequence with the
3′-UTR of target mRNAs. miR-100 is initially transcribed in the
nucleus by RNA polymerase II as primary miRNA, which is
subsequently processed by the Drosha and Dicer enzymes into mature
miRNA. These mature miRNAs are incorporated into the RNA-induced
silencing complex (RISC). Within the RISC, miR-100 utilizes its
seed sequence to bind highly complementarily to target mRNAs,
resulting in either mRNA degradation or translational repression.
This regulatory mechanism effectively controls protein expression
levels and influences various cellular processes (Fig. 1A) (29). For instance, miR-100 inhibits the
migration and invasion of renal cancer cells by downregulating
NADPH oxidase 4 (NOX4) (30) and
acts as a tumor suppressor in CRC by suppressing leucine rich
repeat containing G protein-coupled receptor 5 (LGR5) expression
(31). Additionally, the online
databases ENCORI (https://rnasysu.com/encori/), miRDB (https://mirdb.org/), TargetScan (https://www.targetscan.org/vert_80/),
Diana-TarBase (https://diana.imis.athena-innovation.gr/) and
miRTarBase (https://mirtarbase.cuhk.edu.cn/) predicted 385
downstream target genes targeted by miR-100 (Fig. 1B). Accumulating evidence suggests
that miR-100 serves a critical role in the molecular regulation of
various cancer types and serves as an important regulator in tumor
biology (29-32).
miR-100 as a molecular sponge in the
competing endogenous RNA (ceRNA) network
The ceRNA network regulates the interaction of
transcripts post-transcriptionally through the competition of
shared miRNAs. The ceRNA network functionally connects mRNA
encoding proteins with ncRNAs, including miRNAs, lncRNAs,
pseudogenes and circRNAs (32).
Studies have shown that miRNAs often act as molecular sponges for
lncRNAs and circRNAs within the ceRNA network, thereby regulating
the transcription or degradation of protein-coding genes. This
regulation affects various biological processes, including cell
proliferation, migration, invasion, angiogenesis, autophagy and
drug resistance (32-35).
For example, the upregulation of lncRNA HAGLROS
activates the PI3K/AKT/mTOR pathway through the miR-100/autophagy
related 14 axis, promoting the progression of nasopharyngeal
carcinoma (NPC) (36).
Additionally, lncRNA HAGLROS inhibits miR-100 and upregulates SNF2
related chromatin remodeling ATPase 5 (SMARCA5), enhancing the
malignant phenotype of non-small cell lung cancer (NSCLC) cells
(37). Yang et al
(38) found that lncRNA HAGLROS,
as a molecular sponge for miR-100, regulated the expression of mTOR
and zinc and ring finger 2, thereby affecting the mTOR signaling
pathway in OC, making it a potential biomarker for early diagnosis
and prognosis. Shu et al (39) demonstrated that in diffuse large
B-cell lymphoma, lncRNA HAGLROS promoted tumor cell proliferation,
migration and invasion by inhibiting miR-100. Similarly, lncRNA
SDCBP2-AS1 delays the progression of OC through miR-100 targeting
of ependymin related 1 (40).
Chen et al (41) found
that lncRNA HAGLROS, by competitively binding miR-100, activated
the mTORC1 signaling pathway, inhibiting autophagy and promoting
excessive proliferation of GC cells, thereby maintaining their
malignant phenotype. Furthermore, Peng et al (42) reported that lncRNA ZFAS1
regulated the m6A methyltransferase METTL3 through miR-100,
influencing autophagy and tumor progression in NPC.
The targeting of miR-100 by lncRNAs is not limited
to autophagy. miR-100 has also been described as an important
diagnostic marker in cancer. For example, Le et al (43) found that the plasma levels of
lncRNA NCK1-AS1 were elevated in patients with oral squamous cell
carcinoma, and its expression was negatively associated with
miR-100. Shi et al (44)
established the lncRNA DLEU2L-miR-100-5p-TAO kinase 1 ceRNA network
and found that this network was associated with the prognosis of
hepatocellular carcinoma (HCC), suggesting it may serve as a
foundation for clinical prognostic models. Furthermore, Zhou et
al (18) demonstrated that
lncRNA RAET1K, through miR-100, activated hypoxia inducible factor
1 subunit α and regulated glycolysis in HCC cells.
miR-100 is also involved in cancer stem cell-like
properties and therapeutic resistance. For example, Bai et
al (45) found that lncRNA
LINC00589 regulated trastuzumab resistance and multidrug resistance
in BC through the miR-100-discs large MAGUK scaffold protein 5
axis, while miR-100, derived from lncRNA MIR100HG, mediated
resistance to anti-EGFR monoclonal antibody and everolimus by
activating the Wnt/β-catenin signaling pathway (46).
miR-100 also functions as a sponge for circRNAs in
malignant tumors. For example, hsa_circ_0006168 serves as a
molecular sponge for miR-100 and promotes the proliferation,
migration and invasion of esophageal squamous cell carcinoma (ESCC)
by regulating mTOR (47). Yuan
et al (48) found that
hsa_circ_0072309 enhanced autophagy and temozolomide sensitivity in
glioblastoma multiforme (GBM) through miR-100. Additionally,
CircCASC15 regulates radioresistance in cervical cancer (CC)
through the miR-100/mTOR axis (49).
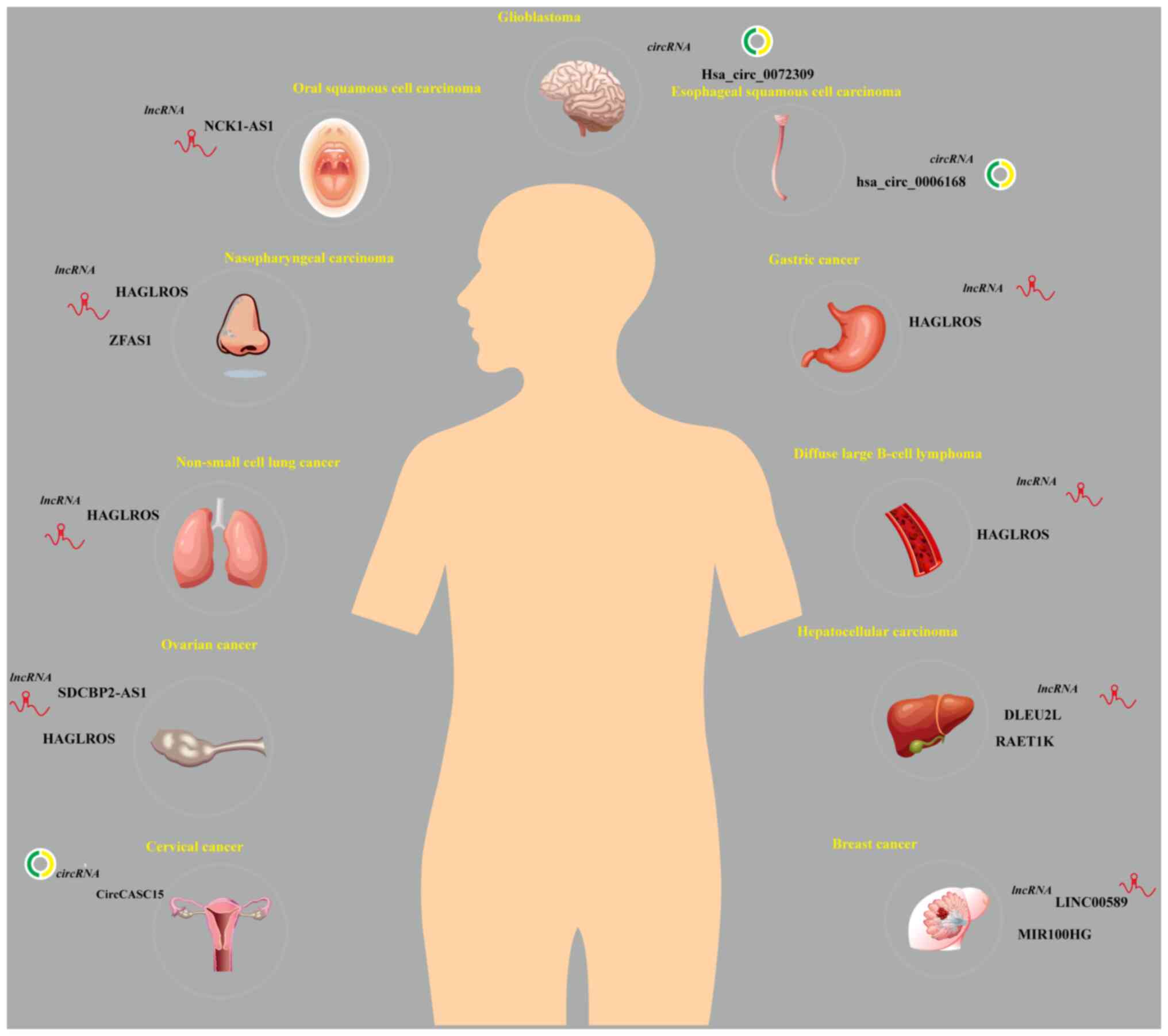
In conclusion, miR-100 acts as a molecular sponge
for both lncRNAs and circRNAs, serving a pivotal role in
tumorigenesis, progression and therapeutic resistance (Fig. 2; Table I).
 | Table ImiR-100 ceRNA network and its role in
cancer. |
Table I
miR-100 ceRNA network and its role in
cancer.
| First author/s,
year | ceRNA | Associated
cancer | Control
mechanism | (Refs.) |
|---|
| Zhang et al,
2020 | HAGLROS | Nasopharyngeal
carcinoma | Activates the
PI3K/AKT/mTOR pathway to promote tumor progression | (36) |
| Li et al,
2021 | | Non-small cell lung
cancer | Inhibits miR-100
and upregulates SMARCA5 to enhance malignancy | (37) |
| Yang et al,
2019 | | Ovarian cancer | Regulates mTOR and
ZNRF2 via miR-100 to affect mTOR signaling | (38) |
| Shu et al,
2022 | | Diffuse large
B-cell lymphoma | Promoting DLBCL
onset and progression by inhibiting miR-100 expression | (39) |
| Chen et al,
2018 | | Gastric cancer | Competes for
miR-100 binding and activates mTORC1, suppressing autophagy and
promoting cell proliferation | (41) |
| Liu et al,
2021 | SDCBP2-AS1 | Ovarian cancer | Regulation of
miR-100 and its target gene EPDR1 delays malignant progression of
ovarian cancer | (40) |
| Peng et al,
2022 | ZFAS1 | Nasopharyngeal
carcinoma | Regulates m6A
methyltransferase METTL3 via miR-100 to affect autophagy and tumor
progression | (42) |
| Le et al,
2020 | NCK1-AS1 | Oral squamous cell
carcinoma | Plasma levels are
inversely associated with miR-100, potentially serving as a
diagnostic biomarker for cancer | (43) |
| Shi et al,
2021 | DLEU2L | Hepatocellular
carcinoma | miR-100 acts as a
sponge and regulates prognosis in patients with hepatocellular
carcinoma | (44) |
| Zhou et al,
2020 | RAET1K | Hepatocellular
carcinoma | Activates HIF1A via
miR-100 to regulate glycolysis | (18) |
| Bai et al,
2022 | LINC00589 | Breast cancer | Regulates drug
resistance via the miR-100-DLG5 axis, involving trastuzumab and
multi-drug resistance | (45) |
| Lu et al,
2017 | MIR100HG | Colorectal
cancer | MIR100HG-derived
miR-100 enhances cetuximab resistance in colorectal cancer cells by
activating the Wnt/β-catenin signaling pathway | (46) |
| Shi et al,
2019 |
hsa_circ_0006168 | Esophageal squamous
cell carcinoma | Regulates mTOR via
miR-100 to promote proliferation, migration and invasion | (47) |
| Yuan et al,
2022 |
hsa_circ_0072309 | Glioblastoma | Enhances autophagy
and temozolomide sensitivity via miR-100 | (48) |
| Yao et al,
2022 | CircCASC15 | Cervical
cancer | Regulates
radioresistance via the miR-100/mTOR axis | (49) |
Role of miR-100 in cancer development
The present review emphasizes the broad role of
miR-100 in the ceRNA network. Beyond acting as a molecular sponge
in the ceRNA axis, modulating cancer-related processes, numerous
studies have indicated that dysregulation of miR-100 is strongly
associated with the onset and progression of various cancer types
(Table II). Specifically,
miR-100 serves a central role in regulating key biological
processes, including cell proliferation, apoptosis, migration,
invasion, metastasis and cell cycle progression, by modulating the
expression of multiple downstream target genes in malignant tumor
cells (10,50). These findings provide compelling
evidence for the molecular mechanisms through which miR-100
influences cancer biology. Related studies are summarized in
Fig. 3 and Table II, laying the foundation for
further exploration of the function of miR-100 in
tumorigenesis.
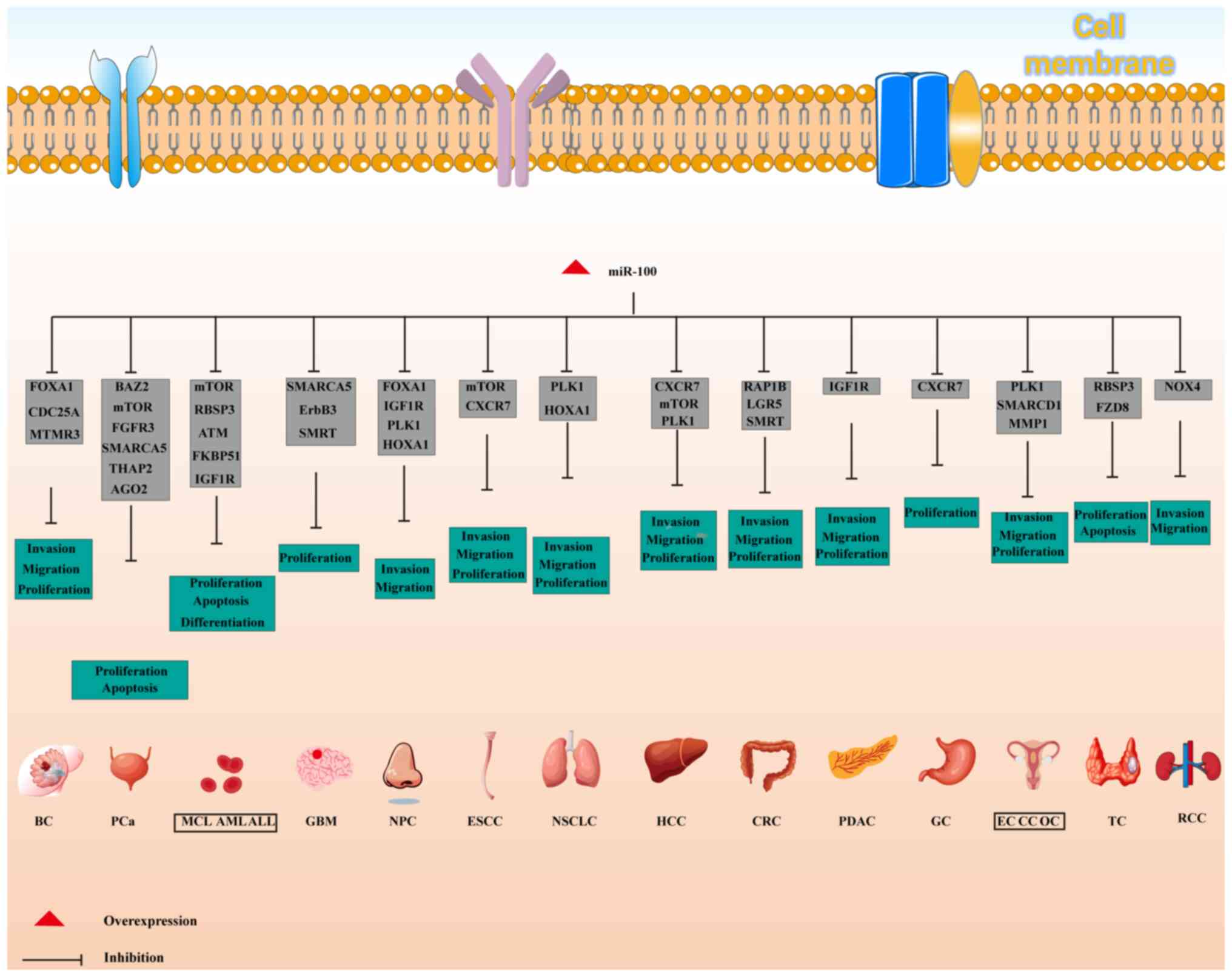 | Figure 3Biological effects and molecular
targets of miR-100 in various cancer types. ALL, acute
lymphoblastic leukemia; AML, acute myeloid leukemia; BC, breast
cancer; CC, cervical cancer; CRC, colorectal cancer; EC,
endometrial cancer; ESCC, esophageal squamous cell carcinoma; GBM,
glioblastoma multiforme; GC, gastric cancer; HCC, hepatocellular
carcinoma; MCL, mantle cell lymphoma; miR, microRNA; NPC,
nasopharyngeal carcinoma; NSCLC, non-small cell lung cancer; OC,
ovarian cancer; PCa, prostate cancer; PDAC, pancreatic ductal
adenocarcinoma; TC, thyroid cancer. |
 | Table IImiR-100 target genes and their
mechanisms in different cancer types. |
Table II
miR-100 target genes and their
mechanisms in different cancer types.
| First author/s,
year | Cancer type | miR-100 target
gene | Mechanism of action
of miR-100 | (Refs.) |
|---|
| Lin et al,
2020 | Mantle cell
lymphoma | mTOR cell cycle
arrest. | Inhibits mTOR
expression to suppress cell proliferation, induce apoptosis and
cause G1 phase | (52) |
| Sun et al,
2020; Zheng et al, 2012 | Acute myeloid
leukemia | RBSP3, ATM,
pRB-E2F1 | Inhibits RBSP3 to
regulate the RB-pRB-E2F1 pathway, and inhibits cell proliferation
and migration. Inhibits ATM to block cell proliferation and induce
apoptosis | (54,55) |
| Li et al,
2013 | Acute lymphoblastic
leukemia | FKBP51, IGF1R,
mTOR | Inhibits FKBP51,
IGF1R and mTOR signaling pathways to suppress cell proliferation
and induce apoptosis | (57) |
| Alrfaei et
al, 2020; Alrfaei et al, 2013 | Glioblastoma | SMARCA5, ErbB3,
SMRT/NCOR2 | Targets SMARCA5 and
ErbB3 to inhibit cell viability and proliferation, extending
survival | (59,60) |
| Peng et al,
2020; He et al, 2020; Sun et al, 2018; Shi et
al, 2010 | Nasopharyngeal
carcinoma | FOXA1, HOXA1,
IGF1R, PLK1 | Regulates FOXA1,
HOXA1 and PLK1 to inhibit cell proliferation and tumor growth | (63-66) |
| Liu et al,
2012; Han et al, 2020 | Non-small cell lung
cancer | PLK1, HOXA1 | Inhibits PLK1 and
HOXA1 expression to suppress tumor progression | (68,69) |
| Zhang et al,
2014; Sun et al, 2013; Zhou et al, 2016; | Esophageal squamous
cell carcinoma | mTOR, CXCR7 | Inhibits mTOR
signaling and CXCR7 to regulate cell proliferation, migration and
invasion, and suppress tumor growth | (71-74) |
| Zhou et al,
2014 Ge et al, 2021; Zhou et al, 2016; Chen et
al, 2013 | Hepatocellular
carcinoma | CXCR7, mTOR,
PLK1 | Targets CXCR7, mTOR
and PLK1 to inhibit tumor growth and cell proliferation | (77-79) |
| Cao et al,
2018; Chen et al, 2015 | Gastric cancer | CXCR7 | Targets CXCR7 to
inhibit cell proliferation and tumor metastasis, potentially
serving as a diagnostic and therapeutic marker | (81,83) |
| Peng et al,
2014; Zhou et al, 2015; Fujino et al, 2017 | CRC | RAP1B, LGR5 | Inhibits RAP1B and
LGR5 to suppress CRC cell proliferation, migration and
invasion | (31,87,88) |
| Huang et al,
2013; Dobre et al, 2021 | Pancreatic ductal
adenocarcinoma | IGF1R | Modulates IGF1R to
regulate pancreatic cancer cell metastasis, potentially serving as
an early diagnostic and therapeutic marker | (91,92) |
| Liu et al,
2022; Chen et al, 2017 | Renal cell
carcinoma | NOX4, mTOR | Inhibits NOX4 and
mTOR expression to suppress cell migration and invasion, promoting
apoptosis | (30,95) |
| Wang et al,
2014; Nabavi et al, 2017; Ye et al, 2020 | Prostate
cancer | BAZ2, mTOR, FGFR3,
SMARCA5, THAP2 | Modulates multiple
genes to inhibit tumor growth, migration and invasion, exerting
tumor-suppressive effects | (99-101) |
| Ma and Han,
2022 | Thyroid cancer | FZD8 | Targets FZD8 to
inhibit papillary thyroid cancer cell proliferation and suppress
cancer progression | (104) |
| Xie et al,
2021; Gebeshuber and Martinez, 2013; Li et al, 2022; Gong
et al, 2015 | Breast cancer | FOXA1, CDC25A,
MTMR3 | Inhibits FOXA1,
CDC25A and MTMR3 to suppress breast cancer cell proliferation,
migration and invasion, and promote apoptosis | (106-109) |
| Schoutrop et
al, 2022 | Ovarian cancer | PLK1 | Inhibits PLK1 to
suppress ovarian cancer cell growth and proliferation, and promote
apoptosis | (112) |
| Huang et al,
2021; Li et al, 2011 | Cervical
cancer | SATB1, AKT/ mTOR,
PLK1 | miR-100 inhibits
cervical cancer progression by targeting SATB1 and PLK1, thereby
inhibiting proliferation, migration and invasion of cervical cancer
cells | (114,115) |
| Takebayashi et
al, 2020 | Endometrial
cancer | SMARCD1 | miR-100-5p promotes
cell invasion by attenuating SMARCD1 expression | (118) |
Mantle cell lymphoma (MCL)
MCL is an aggressive B-cell lymphoma that accounts
for 5-7% of malignant lymphomas in Western Europe, with an annual
incidence of 1-2 cases per 100,000 individuals in 2017 (51). The median age of onset is 65
years, with a male-to-female ratio of ~3:1 (51). Lin et al (52) demonstrated that miR-100 is
downregulated in MCL tissues and cells, suggesting its potential
role as a tumor suppressor. Lin et al (52) revealed that miR-100
overexpression decreased mTOR mRNA and protein levels, thereby
inhibiting cell proliferation, inducing apoptosis. Notably, mTOR
knockdown can reverse these effects, confirming mTOR as a key
downstream target of miR-100 (52). These findings underscore the
crucial role of miR-100 in MCL progression through the modulation
of the mTOR pathway, offering novel insights into the molecular
mechanisms and potential therapeutic strategies for MCL.
Acute myeloid leukemia (AML)
AML is a heterogeneous hematologic malignancy
characterized by the abnormal proliferation of hematopoietic stem
cells in the bone marrow (53).
Approximately 150-200 children aged 0-16 years are diagnosed with
AML annually in Japan (52). Sun
et al (54) demonstrated
that miR-100 was highly expressed in clinical samples and cell
lines of adult AML, where it inhibited cell differentiation and
promoted proliferation by targeting RBSP3. The
miR-100/RBSP3-retinoblastoma protein-E2F transcription factor 1
signaling pathway is involved in the pathogenesis of AML (54). Furthermore, miR-100 expression is
elevated in pediatric AML bone marrow and cell lines, and its
inhibition suppresses cell proliferation and induces apoptosis by
preventing ATM activation (55).
These findings highlight the pathogenic role of miR-100 in AML and
suggest its potential as a diagnostic and therapeutic target for
this malignancy.
Acute lymphoblastic leukemia (ALL)
ALL is the most common pediatric malignancy, and
characterized by abnormal clonal proliferation of lymphocytes
(56). Precursor B-cells account
for 80-85% of cases, with T-cells and mature B-cells less
frequently involved (56,57).
Li et al (57) indicated
that miR-100 was downregulated in clinical tissues of patients with
ALL, and its low expression was associated with poor 5-year overall
survival (OS) rates. Further investigation revealed that
overexpression of miR-100 inhibited ALL cell proliferation and
induced apoptosis by suppressing FKBP prolyl isomerase 5 (FKBP51)
and the IGF1R/mTOR signaling pathway (57). These findings suggest a key role
for miR-100 in ALL and its potential as a therapeutic target to
improve patient prognosis.
GBM
GBM is the most common and aggressive primary brain
tumor in adults (58,59). Despite advances in treatment,
including maximal safe resection, radiotherapy and chemotherapy,
the prognosis remains poor, with a median OS time of 14-20 months
(58). Research has shown that
miR-100 is downregulated in GBM cells. Overexpression of miR-100
reduces cell viability and proliferation, inhibits tumor growth,
and suppresses SMARCA5 and Erb-B3 receptor tyrosine kinase 3
activation (59). Additionally,
Alrfaei et al (60)
demonstrated that miR-100 targeted nuclear receptor corepressor 2,
reducing tumor growth and extending survival in GBM animal models.
These results suggest that miR-100 may serve a critical role in GBM
progression and could be a potential therapeutic target for
improving survival outcomes.
NPC
NPC is a squamous cell carcinoma originating from
the nasopharyngeal epithelium (61). In 2018, ~129,079 new cases of NPC
were diagnosed globally, resulting in 72,987 deaths (62). The development of NPC is
influenced by factors such as dietary habits, lifestyle,
environmental exposure, ethnicity and Epstein-Barr virus infection
(61,62). Peng et al (63) demonstrated that miR-100 was
typically downregulated in NPC tissues, and its expression was
associated with tumor malignancy. Overexpression of miR-100 leads
to the downregulation of forkhead box A1 (FOXA1) and promotes the
malignant invasion of NPC cells (63). Additionally, He et al
(64) confirmed that miR-100
inhibited NPC cell growth and proliferation by targeting homeobox
A1 (HOXA1), and exogenous miR-100 expression suppressed xenograft
tumor growth. Furthermore, Sun et al (65) reported that miR-100 inhibited NPC
cell migration and invasion by targeting IGF1R, while Shi et
al (66) found that high
PLK1 expression in most NPC samples was associated with a higher
risk of recurrence, with miR-100 inhibiting tumor growth through
the regulation of PLK1.
NSCLC
Lung cancer remains the leading cause of
cancer-related deaths in the United States, with ~247,270 new cases
reported in 2020, including 130,340 in men and 116,930 in women
(67). In NSCLC, miR-100 is
widely recognized as a tumor suppressor (68,69). Liu et al (68) indicated that miR-100 was
downregulated in NSCLC tissues, and its reduced expression was
closely associated with advanced clinical stage, higher tumor grade
and lymph node metastasis. Additionally, low miR-100 expression may
serve as a predictive marker for poor prognosis in NSCLC.
Mechanistically, miR-100 exerts its tumor-suppressive effect by
post-transcriptionally regulating PLK1 expression (68). Han et al (69) further confirmed that miR-100
inhibited NSCLC progression both in vitro and in vivo
by targeting HOXA1. The mechanism was that miR-100 inhibited the
activation of the Wnt/β-catenin pathway by targeting HOXA1, thereby
reducing cell migration, invasion and proliferation (69).
ESCC
ESCC is the sixth leading cause of cancer-related
deaths globally, with ~544,000 deaths reported in 2020 (70). miR-100 is downregulated in ESCC
and is closely related to lymph node metastasis and increased
invasiveness (71). Zhang et
al (71) have shown that
miR-100 regulates the migration and invasion of ESCC cells by
targeting mTOR and suppressing the expression of tumor-associated
genes. Sun et al (72)
further confirmed the direct targeting relationship between miR-100
and mTOR. Zhou et al (73) found that miR-100 inhibited the
proliferation and tumor growth of esophageal cancer cells by
targeting chemokine (C-X-C motif) receptor 7 (CXCR7). Additionally,
in 120 ESCC tissue samples, low expression levels of miR-100 were
associated with advanced stage, distant metastasis and deep
invasion (74), highlighting its
potential as a diagnostic marker and therapeutic target for
esophageal cancer progression.
HCC
HCC is the most common type of primary liver cancer,
accounting for ~90% of liver cancer cases (75). The main risk factors for HCC
include chronic hepatitis B virus infection and subsequent liver
cirrhosis (75). Due to the lack
of specific early symptoms and diagnostic biomarkers, the majority
of patients are diagnosed at an advanced stage, with only 10-20%
suitable for curative surgical resection, leading to a generally
poor prognosis (46). Studies
have shown that miR-100 serves an important tumor-suppressive role
in HCC (76-79). Ge et al (77) found that miR-100 inhibited HCC
cell proliferation, invasion and migration by targeting CXCR7. Zhou
et al (78) further
demonstrated that low miR-100 expression was associated with blood
vessel encapsulated tumor clusters (VETC), venous invasion and
microthrombosis in HCC tissues. In VETC-2 and Hepa1-6 mouse models,
miR-100 expression inhibited VETC formation, thereby reducing tumor
metastasis. Furthermore, miR-100 inhibits tumor growth by targeting
mTOR and blocking the mTOR-p70S6K signaling pathway, as well as by
regulating PLK1 to suppress angiopoietin 2 protein levels (79).
GC
GC is a common malignancy globally, with its
complexity arising from the combination of environmental and
genetic factors (80,81). Age is a risk factor for GC, with
a median age at diagnosis of 70 years (80). Due to the asymptomatic and
non-specific nature of early-stage GC, most patients are diagnosed
at an advanced stage, leading to poor prognosis (81). Increasing research has focused on
the molecular mechanisms underlying GC. miR-100 has been found to
be downregulated in GC (81,82). Cao et al (82) found that miR-100 inhibited the
proliferation of GC cells by targeting CXCR7, and its expression
was closely associated with lymph node metastasis, tumor size and
stage. Furthermore, Chen et al (83) confirmed the role of miR-100 as a
tumor suppressor in GC progression, highlighting its potential in
molecular diagnostics and targeted therapy. These studies provide
key insights into the molecular mechanisms of GC and lay the
groundwork for exploring novel therapeutic targets.
CRC
CRC is the third most common cancer worldwide and
the fourth leading cause of cancer-related deaths (84). Dietary and lifestyle factors are
considered major contributors to the rising incidence of CRC
(84). The incidence of newly
diagnosed CRC in China accounts for 49.3% of the global total,
while the related mortality represents 58.3% of global CRC-related
deaths (84,85). The 5-year relative survival rate
stands at 57% (84,85). Approximately 11% of CRC cases
present with metastasis (85).
Preventive colonoscopy remains the most effective strategy for CRC
prevention (86). Previous
studies have highlighted non-invasive biomarkers, with miR-100
emerging as a potential candidate for CRC diagnosis. For example,
Peng et al (87) found
that miR-100 regulated SW620 CRC cell proliferation and invasion by
modulating RAP1B expression. Similarly, Zhou et al (31) demonstrated that miR-100 was
downregulated in colon cancer tissues and suppressed cell
viability, proliferation, migration and invasion by targeting LGR5.
Furthermore, Fujino et al (88) reported that miR-100 was
downregulated in early CRC and was closely associated with lymph
node metastasis. miR-100 inhibited the activation of the
Wnt/β-catenin signaling pathway by targeting HOXA1, thereby
reducing the migratory and invasive capabilities of cancer cells.
These findings underscore the critical role of miR-100 in CRC
pathogenesis and its potential as a diagnostic and therapeutic
target. Further exploration of the mechanisms of miR-100 will
provide deeper insights into the molecular pathology of CRC and
support the development of early detection and personalized
treatment strategies.
Pancreatic ductal adenocarcinoma
(PDAC)
PDAC is a highly fatal malignancy and is expected to
become the leading cause of pancreatic cancer-related deaths in the
U.S. by 2030. Currently, ~90% of patients with PDAC are diagnosed
at advanced stages, with tumors often having spread beyond the
pancreas and >50% presenting with distant metastases (89). Early diagnosis is crucial for
improving prognosis (89). In
the past decade, advances in basic and translational research have
deepened the understanding of the biological processes underlying
pancreatic cancer, which are gradually being applied to improve
diagnostic and therapeutic strategies (89,90). Huang et al (91) found that miR-100 served a key
role in PDAC cell metastasis by regulating IGF1R. Dobre et
al (92) further validated
this mechanism, supporting the involvement of miR-100 in PDAC
molecular pathology. These studies suggest that miR-100 may serve
as a potential biomarker for early diagnosis and targeted therapy
in PDAC, providing valuable insights for clinical application.
Renal cell carcinoma (RCC)
RCC is the third most common cancer of the urinary
system, accounting for 3% of cancers in women and 5% in men,
representing ~90% of all kidney cancers (93). Approximately 400,000 new RCC
cases are reported annually worldwide (30,93,94). RCC is associated with a poor
prognosis, since ~30% of patients present with metastasis at
diagnosis, and another 30% develop metastasis during follow-up
(91,92). Research has shown that miR-100
inhibits RCC cell invasion and migration by downregulating NOX4
expression (30). Additionally,
Chen et al (95) found
that miR-100 was highly expressed in RCC tissues. miR-100 inhibited
cell viability, promoted apoptosis, and inhibited migration and
invasion by inhibiting mTOR. These findings highlight the dual role
of miR-100 in RCC and suggest its potential as a diagnostic,
prognostic and therapeutic target. Further investigation of the
molecular mechanisms of miR-100 will provide valuable insights for
personalized treatment strategies in RCC.
PCa
PCa is one of the most common malignancies worldwide
and a leading cause of cancer-related death (96). Although most PCa cases are
diagnosed as localized or indolent, ~20% of patients present with
high-risk cancer that may progress to fatal disease (97). The role of miR-100 in PCa has
garnered increasing attention. Leite et al (98) first demonstrated that miR-100
expression decreased during the progression of localized PCa to
advanced metastatic stages. Wang et al (99) further revealed that miR-100
affected PCa cell migration and invasion by regulating AGO2.
Additionally, Nabavi et al (100) reported that miR-100 inhibits
PCa cell apoptosis, while Ye et al (101) found that miR-100-5p
downregulation suppressed cell proliferation, migration and
invasion by targeting mTOR. These studies underscore the critical
role of miR-100 in PCa progression and suggest its potential as a
diagnostic, prognostic and therapeutic target. Further research
into the molecular mechanisms of miR-100 will provide essential
support for precision treatment strategies for patients with
high-risk PCa.
TC
TC is one of the fastest-growing malignancies
globally, with its incidence steadily rising over the past 30 years
(102). Although the overall
mortality rate remains stable, some rare subtypes, such as
anaplastic TC, remain a clinical challenge due to their high
invasiveness and limited treatment options (103). The role of miRNAs in TC has
attracted widespread attention. Zhang et al (11) found that overexpression or
knockdown of miR-100 inhibited the translation of RBSP3, thereby
promoting follicular thyroid carcinoma cell proliferation.
Furthermore, Ma and Han (104)
found that miR-100 was downregulated in papillary thyroid carcinoma
(PTC) tissues and cells. Overexpression of miR-100 inhibits the
proliferation and migration, and promotes apoptosis of PTC, which
may be achieved by inhibiting the expression of fragile fission
class receptor 8 (104). These
findings highlight the critical roles of miR-10 and miR-100 in the
progression of different TC subtypes, suggesting their potential as
molecular targets for diagnosis and treatment. Further
investigation into the molecular mechanisms of these miRNAs may
pave the way for the development of precision therapies for TC.
BC
BC is the leading cause of morbidity and mortality
in women worldwide (105). In
2020, BC surpassed lung cancer as the most common malignancy
globally, accounting for 15.5% of all cancer-related deaths in
women (105). Despite the
central role of surgery, radiotherapy and chemotherapy in
treatment, research has increasingly focused on the role of miRNAs
in BC. Xie et al (106)
demonstrated that miR-100 was downregulated in BC, and its
overexpression suppressed the proliferation, migration and invasion
of BC cells by inhibiting FOXA1 expression. Additionally,
Gebeshuber and Martinez (107)
found that stable overexpression of miR-100 reduced insulin like
growth factor 2 expression and inhibited tumor growth. Li et
al (108) also demonstrated
that miR-100 inhibited proliferation, migration and invasion by
targeting cell division cycle 25A (CDC25A), while promoting
apoptosis. Gong et al (109) identified myotubularin related
protein 3 (MTMR3) as another downstream target of miR-100,
mediating apoptosis in BC cells. These studies collectively
highlight the key role of miR-100 in inhibiting BC progression
through the regulation of multiple downstream targets, including
FOXA1, CDC25A and MTMR3. Given its tumor-suppressive effect,
miR-100 offers a potential molecular target for BC diagnosis and
treatment, providing novel directions for precision medicine.
OC
OC is one of the leading causes of cancer-related
death in women and the second most common cancer in women over the
age of 40 years (110). The
high mortality rate is primarily due to most patients being
diagnosed at advanced stages, emphasizing the need for early
diagnostic markers to improve prognosis (111). Studies have indicated that
miR-100 serves an important role in OC, especially in epithelial OC
(EOC) (110,111). For example, Schoutrop et
al (112) found that
miR-100 exerted tumor-suppressive effects in EOC by targeting PLK1,
inhibiting tumor cell growth and proliferation. Nam et al
(113) further confirmed that
miR-100 expression was differential in EOC, suggesting its
involvement in OC development and progression. These findings
suggest that miR-100 may serve as an early diagnostic biomarker and
therapeutic target for OC. Further research into the molecular
mechanisms of miR-100 may provide novel directions for precise
diagnosis and targeted therapy.
CC
CC is the fourth most common malignant tumor among
women worldwide, following BC, CRC and lung cancer (114). Although progress has been made
in CC screening and prevention, its incidence and mortality remain
high in a number of regions, particularly in low-and middle-income
countries (114,115). Huang et al (114) found that miR-100 was
downregulated in CC tissues, with reverse
transcription-quantitative PCR indicating lower expression levels
of miR-100. It was shown that overexpression of miR-100 effectively
inhibited the proliferation, migration and invasion of CC cells
(114). Li et al
(115) confirmed that the
downregulation of miR-100 promoted the progression of CC, at least
partially by losing its inhibitory effect on the target gene PLK1.
These findings suggest that miR-100 acts as a tumor suppressor in
CC, and its mechanism may involve the regulation of key target
genes such as PLK1. The potential diagnostic and therapeutic value
of miR-100 provides a novel direction for personalized medicine in
CC and may offer effective molecular targets for improving patient
prognosis.
Endometrial cancer (EC)
EC is the sixth most common cancer in women
(116). In 2020, there were
417,000 new cases of EC worldwide, with a lifetime risk of ~3% for
women, and the median age at diagnosis is 61 years (116). Although most patients are
diagnosed early and achieve good prognosis through routine surgery,
EC remains the only gynecologic malignancy with an increasing
mortality rate (117). The role
of miRNAs in EC has garnered increasing attention. Takebayashi
et al (118) found that
transfection of hsa-miR-100 enhanced the invasiveness and
proliferative capacity of normal endometrial stromal cells (NESCs).
It was shown that SWI/SNF related BAF chromatin remodeling complex
subunit D1 (SMARCD1) and MMP-1 are key downstream targets of
miR-100. Specifically, miR-100 promoted the invasion and migration
of NESCs by inhibiting SMARCD1 expression and activating MMP-1
(118). These findings suggest
that miR-100 promotes EC invasion and migration via the
SMARCD1/MMP-1 pathway. Further investigation of the molecular
mechanisms of miR-100 will not only aid the understanding of the
progression of EC but may also provide potential diagnostic and
therapeutic targets for the disease.
Diagnostic and prognostic value of
miR-100
The continued rise in global cancer mortality is
not only due to the poor prognosis of cancer itself, but is also
closely related to late diagnosis due to the hidden nature of
different cancer types. Most patients with cancer are diagnosed
when the disease is already in an unresectable stage, which
underscores the importance of finding novel diagnostic methods or
biomarkers (119). Over the
past decade, research on potential cancer biomarkers has grown
exponentially, offering hope for early diagnosis and improving
clinical prognosis (119-121). Early diagnosis and effective
monitoring of treatment responses are crucial for successful cancer
management. However, traditional tumor biopsy methods lack
sufficient sensitivity and specificity for early detection and
ongoing monitoring (121).
Therefore, identifying early diagnostic biomarkers is essential for
improving the early detection and treatment of malignant
tumors.
Studies have highlighted miR-100 as a potential
diagnostic biomarker for various cancer types (122-131). For instance, Wang et al
(122) found that miR-100 was
highly expressed in the plasma of patients with BC, and receiver
operating characteristic (ROC) curve analysis showed that it had
high diagnostic efficiency for early BC. Fuso et al
(123) confirmed this finding.
In HCC, Jin et al (124)
observed upregulation of miR-100, suggesting that it may serve as a
biomarker for HCC. Similarly, Qureshi et al (125) found that miR-100 was
downregulated in the plasma of patients with bladder cancer, with
expression levels associated with clinical features such as
microscopic hematuria, cytological examination, cystoscopy and TNM
staging. Ludwig et al (126) reported that miR-100 was highly
expressed in the serum of patients with Wilms tumor, with ROC
analysis showing a diagnostic sensitivity of 0.90. Blanca et
al (127) found that
miR-100 was a prognostic biomarker for non-invasive bladder cancer.
Furthermore, Yamanaka et al (128) revealed that miR-100 serves an
important role in bladder cancer progression, with reduced
expression associated with shorter progression-free survival and
OS, suggesting its potential in risk stratification for bladder
cancer. In addition to the aforementioned studies, miR-100 has also
been recognized as a potential diagnostic marker for CRC (129,130), GC (131), PCa (132), oral cancer (133) and esophageal cancer (134,135).
In addition to its diagnostic role, miR-100 also
has prognostic implications in cancer. For example, Wang et
al (136) found that high
miR-100 expression in renal clear cell carcinoma (RCC) tissues was
associated with tumor T staging and metastasis, and high miR-100
expression was an independent poor prognostic factor for the OS and
cancer-specific survival of patients with RCC. Liu et al
(137) suggested that miR-100
could serve as a biomarker for predicting lymph node metastasis in
patients with GC. Furthermore, He et al (138) analyzed The Cancer Genome Atlas
(TCGA) and Gene Expression Omnibus datasets, finding that low
miR-100 levels were associated with poor clinical features and
prognosis in patients with HCC. miR-100 was identified as an
independent risk factor for recurrence and OS after liver resection
(138). Zhou et al
(78) further demonstrated that
high miR-100 expression in HCC was associated with tumor grade,
lymph node metastasis, late TNM stage and recurrence, indicating
its negative prognostic significance. In pediatric AML, Hassan
et al (139) reported
that upregulation of miR-100 was associated with poor relapse-free
survival and poor OS, positioning it as a negative prognostic
marker for AML.
While these findings provide strong support for the
potential of miR-100 as an early diagnostic and prognostic
biomarker for cancer, several challenges remain to be addressed.
First, although the high diagnostic value of miR-100 has been
observed in various cancer types, most of the current research is
focused on in vitro experiments and animal models, with a
lack of large-scale clinical trial validation (119-121). Whether these early findings can
be replicated in clinical applications, especially in real-world
environments with large population heterogeneity, still requires
further validation. For example, the differential expression of
miR-100 in different populations (for example, different
ethnicities and sex) may affect its application as a universal
marker (126,127). Secondly, the sensitivity and
specificity of miR-100 as a biomarker remain key issues in current
research, particularly how to ensure diagnostic accuracy in cases
of low early disease burden (10). Additionally, technical challenges
such as improving the stability of miR-100 in blood and
standardizing its detection processes remain bottlenecks for its
broader application.
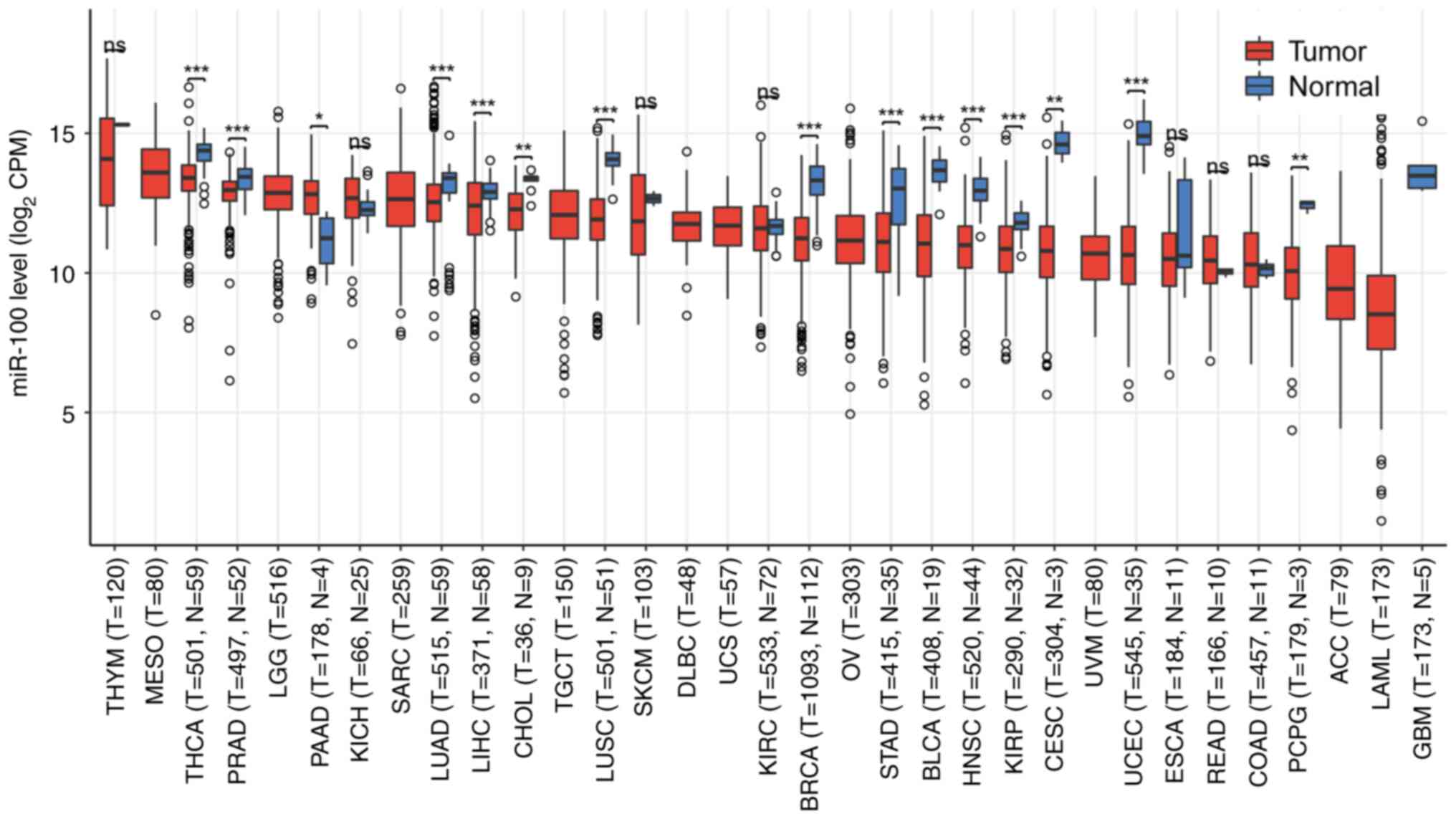
In addition, pan-cancer data from TCGA (https://www.cancer.gov/tcga) were analyzed using R
(version 4.2.1; R Core Team) and trends in miR-100 expression
across various cancer types were identified. As shown in Fig. 4, miR-100 expression differed
significantly in several cancer types, including lung cancer and
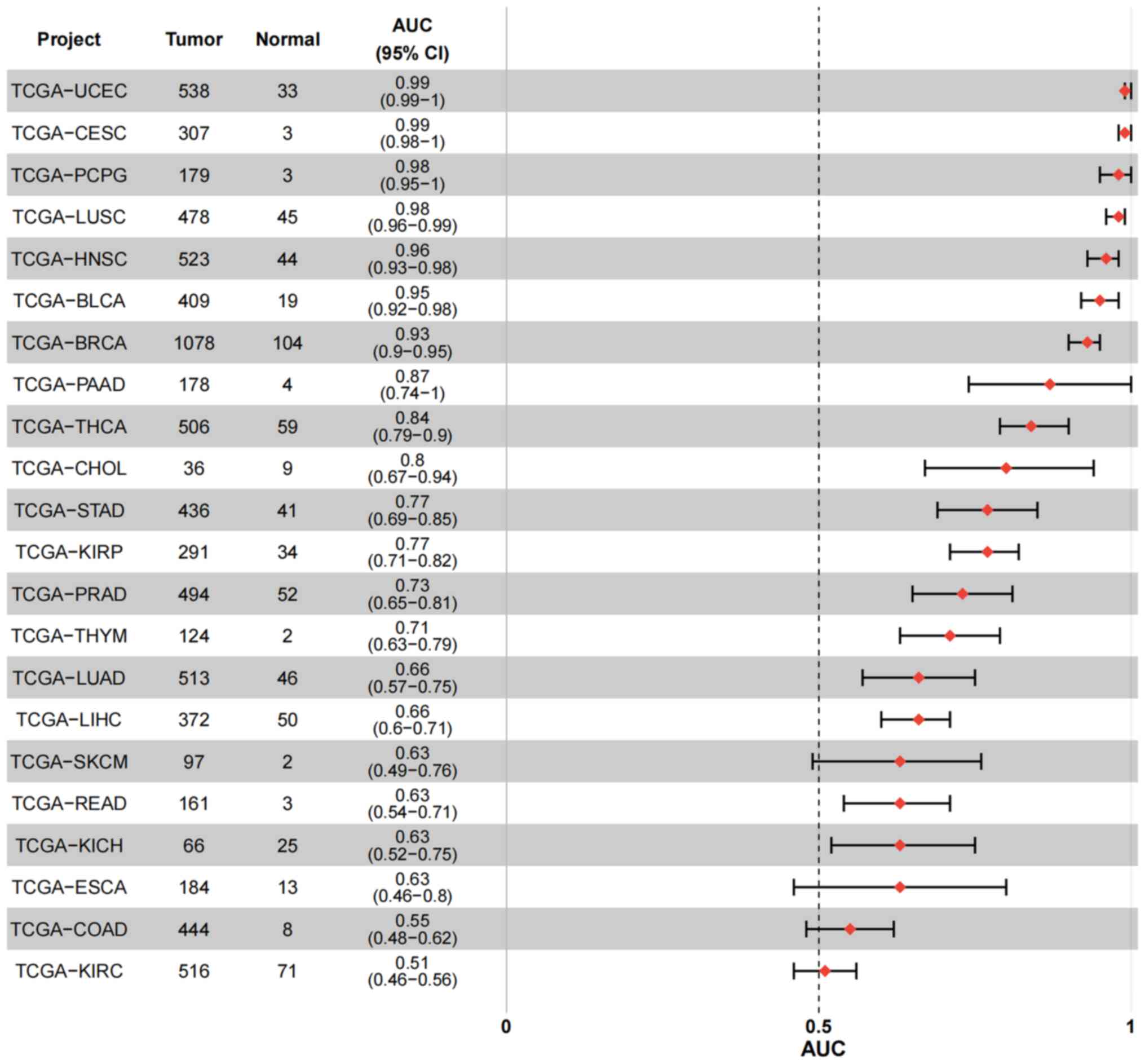
TC. Furthermore, ROC analysis of the TCGA pan-cancer database
revealed that miR-100 exhibited an area under the curve >0.5 in
cancer types such as TC (TCGA-THCA), HCC (TCGA-LIHC), bladder
urothelial carcinoma (TCGA-BLCA), breast invasive carcinoma
(TCGA-BRCA), and head and neck squamous cell carcinoma (TCGA-HNSC)
(Fig. 5), indicating its high
sensitivity and specificity as a potential diagnostic marker.
However, further multi-center, large-scale clinical studies are
required to validate these findings and optimize the use of miR-100
as a clinical biomarker.
In conclusion, miR-100 serves a crucial role in
cancer diagnosis and prognosis, showing clinical potential.
Although current studies provide preliminary evidence for the use
of miR-100 as an early diagnostic and prognostic biomarker, key
issues such as sensitivity, specificity, population applicability
and clinical validation need to be addressed before it can be
widely used in clinical practice. With future clinical trials and
technical optimization, miR-100 is expected to become an important
tool in precision cancer diagnosis and treatment.
miR-100 and chemotherapy/radiotherapy
resistance in cancer
Although progress has been made in cancer
treatment, chemotherapy remains a major treatment modality for
advanced cancer. However, chemotherapy resistance continues to be
one of the key obstacles affecting treatment outcomes (140,141). miRNAs serve an increasingly
important role in regulating cancer cell responses to treatment,
especially in the mechanisms of chemotherapy resistance, where they
influence drug responses by targeting and modulating the expression
of relevant genes (140-142).
miR-100 serves a complex role in regulating the
sensitivity and resistance to chemotherapy in various cancer types.
For example, miR-100 enhances the chemotherapy sensitivity of GBM
cells to cisplatin and temozolomide by targeting FGFR3 (142). In small cell lung cancer
(SCLC), Xiao et al (143) found that the downregulation of
miR-100 was negatively associated with HOXA1 expression, suggesting
that miR-100 serves a key role in regulating SCLC cell survival and
chemotherapy resistance. Similarly, miR-100 restores the
sensitivity of docetaxel-resistant lung adenocarcinoma SPC-A1 cells
to docetaxel by targeting PLK1 (144). In head and neck squamous cell
carcinoma, miR-100 has also been found to be associated with
docetaxel resistance (16). In
cisplatin-resistant OC cells, miR-100 restores sensitivity to
cisplatin by targeting mTOR and PLK1, inhibiting cell proliferation
and inducing apoptosis (145).
Similarly, Liu et al (146) found that miR-100 enhanced the
sensitivity of osteosarcoma cells to cisplatin by targeting IGF1R.
In NSCLC, the downregulation of miR-100 may increase resistance to
ALK inhibitors (such as crizotinib and lorlatinib), presenting a
therapeutic challenge (147).
At the same time, the downregulation of miR-100 promotes paclitaxel
resistance by increasing the expression of β-tubulin V-type,
suggesting its potential as a target for paclitaxel combination
therapy (148). The role of
miR-100 in radiotherapy should also not be overlooked. In CRC,
upregulation of miR-100 enhances cell sensitivity to radiation,
possibly by promoting radiation-induced apoptosis and DNA
double-strand breaks (15). In
childhood acute lymphoblastic leukemia, downregulation of miR-100
contributes to vincristine resistance, and its upregulation
effectively reverses this resistance, restoring the anticancer
efficacy of vincristine (149).
Furthermore, the overexpression or knockout of miR-100 can regulate
ATM expression and alter cell sensitivity to ionizing radiation
(150).
The role of miR-100 in cancer chemotherapy and
radiotherapy underscores its complex regulatory function, which is
influenced by tumor type, therapeutic agents and the specific
characteristics of cancer cells. Specifically, miR-100 expression
in different cancer types may be influenced by various factors,
including the drug resistance characteristics of tumor cells and
changes in the tumor microenvironment. Therefore, the mechanism of
action of miR-100, as a potential therapeutic target, warrants
further investigation. In summary, the role of miR-100 in
chemotherapy and radiotherapy underscores its crucial function in
regulating cancer cell proliferation and drug resistance. In-depth
studies on the mechanisms of miR-100 and its interactions with
chemotherapy drugs and radiotherapy will provide valuable insights
for overcoming resistance in cancer treatment and offer novel
directions for future cancer therapeutic strategies.
miR-100 and signaling pathways
Cellular signal transduction serves a pivotal role
in mediating cellular responses to both internal and external
stimuli. Various intracellular signaling pathways are essential for
regulating biological processes and gene expression. Although these
pathways do not directly engage in transcription, they ultimately
influence gene expression by modulating the activity of
transcription factors (151).
Increasing evidence suggests that miRNAs serve a crucial role in
regulating these signaling pathways in both normal and cancer cells
(10,152,153). Pathways such as the Hippo, Myc,
Notch, TGFβ, p53, epithelial-to-mesenchymal transition (EMT) and
Wnt/β-catenin pathways are closely associated with tumorigenesis
and cancer progression (154).
Tumor-specific alterations in these pathways often serve as
potential targets for developing targeted therapies (155).
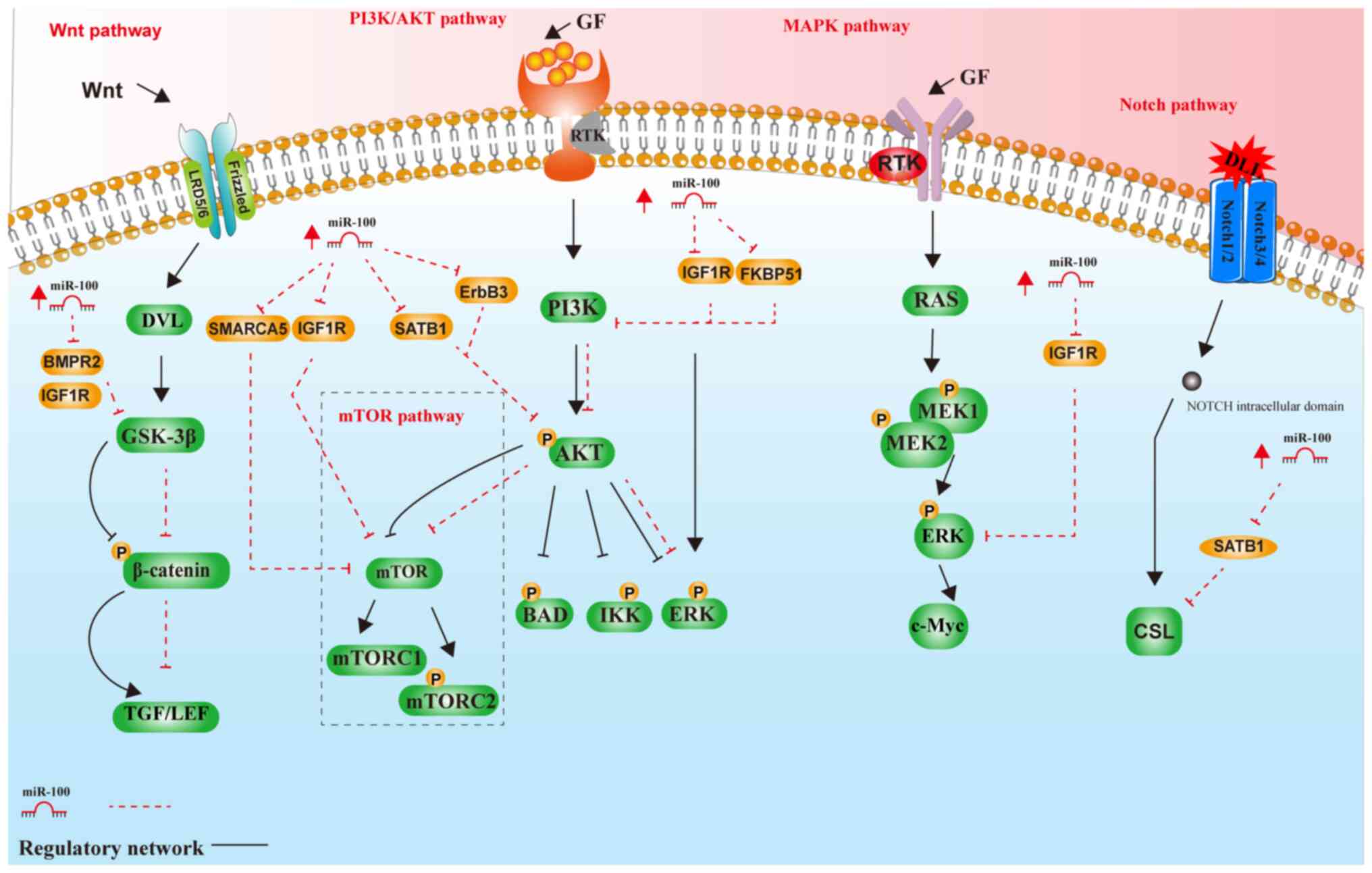
miR-100 and the EMT pathway
miR-100 serves a critical role in regulating EMT, a
process where cells transition from an epithelial to a mesenchymal
phenotype. This transition enhances cancer cell migration, invasion
and metastasis (99,156,157). Studies have shown that miR-100
inhibits EMT by regulating multiple key molecules (99,156,157). For instance, Wang et al
(99) demonstrated that the loss
of miR-100 enhanced the migration and invasiveness in PCa cells,
and promoted the EMT process. This suggests that miR-100 may
suppress EMT by regulating the expression of epithelial markers
such as E-cadherin, and mesenchymal markers such as N-cadherin and
Vimentin (99). In addition, the
well-known EMT-promoting factors zinc finger E-box binding homeobox
(ZEB)1 and ZEB2 induce EMT by repressing E-cadherin expression,
whereas miR-100 inhibits these transcription factors, thereby
suppressing EMT (156).
Specifically, miR-100-mediated inhibition of SMARCA5 suppresses
E-cadherin, thereby inhibiting the EMT process in breast cancer
cells (156). In addition, Yang
et al (157) found that
miR-100 inactivation, in conjunction with arsenic exposure,
activated the EMT process, promoting the malignant transformation
and invasiveness of BEAS-2B lung cells. These findings suggest that
miR-100 inhibits cancer cell migration and invasion by regulating
EMT-related factors however, its loss or reduced expression may
exacerbate malignant transformation.
miR-100 and the AKT/mTOR pathway
The AKT/mTOR signaling pathway serves a vital role
in tumor cell proliferation, survival and metabolism. miR-100
inhibits tumor cell migration and invasion by regulating key
molecules within this pathway (158). Chen et al (95) demonstrated that miR-100 inhibited
mTOR signaling activation, slowing migration and invasion in RCC
cells. The mechanism is that miR-100 reduces cellular metabolic
activity by directly targeting mTORC1 activation, which further
inhibits cell proliferation (95). Additionally, miR-100 suppresses
AKT phosphorylation, which reduces downstream mTOR activity,
thereby inhibiting tumor cell proliferation and migration (95). These findings suggest that
miR-100 not only slows tumor cell proliferation by inhibiting
mTORC1 activity but also limits metabolic activity and
invasiveness, positioning it as a potential target for anticancer
therapy.
miR-100 and the PI3K/AKT pathway
The PI3K/AKT signaling pathway is involved in
various processes, including cell proliferation, survival and
migration (159). miR-100
regulates the PI3K/AKT pathway to inhibit cancer cell proliferation
and promote apoptosis (57). Li
et al (57) demonstrated
that miR-100 targeted IGF1R and FKBP51 to inhibit PI3K/AKT pathway
activation, thereby inhibiting cell proliferation and promoting
apoptosis in ALL cells. These findings provide compelling evidence
for the potential of miR-100 as an anticancer factor.
miR-100 and the Wnt/β-catenin
pathway
The Wnt/β-catenin signaling pathway serves a
critical role in tumor cell proliferation, differentiation and
metastasis. miR-100 regulates key molecules within the
Wnt/β-catenin pathway to suppress tumor cell proliferation and
migration (160). Liu et
al (146) demonstrated that
miR-100 targeted IGF1R to inhibit the Wnt/β-catenin pathway,
slowing the proliferation and migration of osteosarcoma cells.
IGF1R, an upstream activator of the Wnt/β-catenin pathway, is
inhibited by miR-100, which reduces β-catenin activation and
decreases Wnt signaling, thereby slowing tumor progression
(146). Additionally, Peng
et al (161) found that
miR-100 regulated GC cell proliferation and migration by targeting
bone morphogenetic protein receptor type 2. These studies
underscore the essential role of miR-100 in regulating the
Wnt/β-catenin pathway and its potential as a therapeutic target in
cancer.
miR-100 and the Notch signaling
pathway
The Notch signaling pathway is critical for cell
fate determination, proliferation and EMT (161). Yang et al (162) demonstrated that miR-100
regulated the Notch signaling pathway to modulate apoptosis and
proliferation in GC cells. Activation of the Notch pathway is
closely associated with cancer cell proliferation, metastasis and
EMT. Downregulation of miR-100 may lead to abnormal activation of
the Notch pathway, promoting tumor progression. By directly
inhibiting the expression of Notch receptors, miR-100 reduces Notch
signaling, suppressing cancer cell proliferation and metastasis
(162). Huang et al
(163) found that miR-100
downregulated SATB homeobox 1 (SATB1) expression, which in turn
inhibited the AKT/mTOR and Notch signaling pathways, suppressing
the EMT process and tumor invasiveness. SATB1, a transcription
factor involved in several tumor-related signaling pathways, is
directly downregulated by miR-100, inhibiting Notch pathway
activity and reducing tumor cell proliferation and metastasis.
STAT3, a critical transcription factor in tumor progression, is
also downregulated by miR-100, further slowing tumor cell
proliferation and metastasis (163). Further research into the
relationship between miR-100 and the Notch signaling pathway may
provide novel therapeutic strategies for cancer treatment.
miR-100 and the MAPK signaling
pathway
The MAPK pathway in cancer transmits extracellular
signals from the cell membrane to intracellular targets, serving a
pivotal role in regulating various biological processes associated
with tumorigenesis (164). For
example, Liu et al (146) demonstrated that miR-100 was
downregulated in osteosarcoma. miR-100 inhibits osteosarcoma cell
proliferation, migration and invasion by directly targeting IGF1R
and suppressing its expression, thereby modulating the downstream
MAPK signaling pathway (146).
As shown in Figs.
6 and 7, miR-100 regulates
tumor growth, migration and invasion through multiple signaling
pathways, including the EMT, AKT/mTOR, mTOR/STAT1/Notch, PI3K/AKT,
MAPK and Wnt/β-catenin signaling pathways. By targeting key
molecules in these pathways, miR-100 inhibits cancer cell
proliferation, migration and metastasis. However, its role may vary
across different cancer types, suggesting the need for further
investigation of its specific mechanisms in various tumor contexts
(57,99,146,156-163). The multifaceted regulatory role
of miR-100 provides insights into tumorigenesis and offers
potential targets and strategies for cancer therapy. In conclusion,
the role of miR-100 in these key signaling pathways was
illustrated, further emphasizing its therapeutic potential in
oncology.
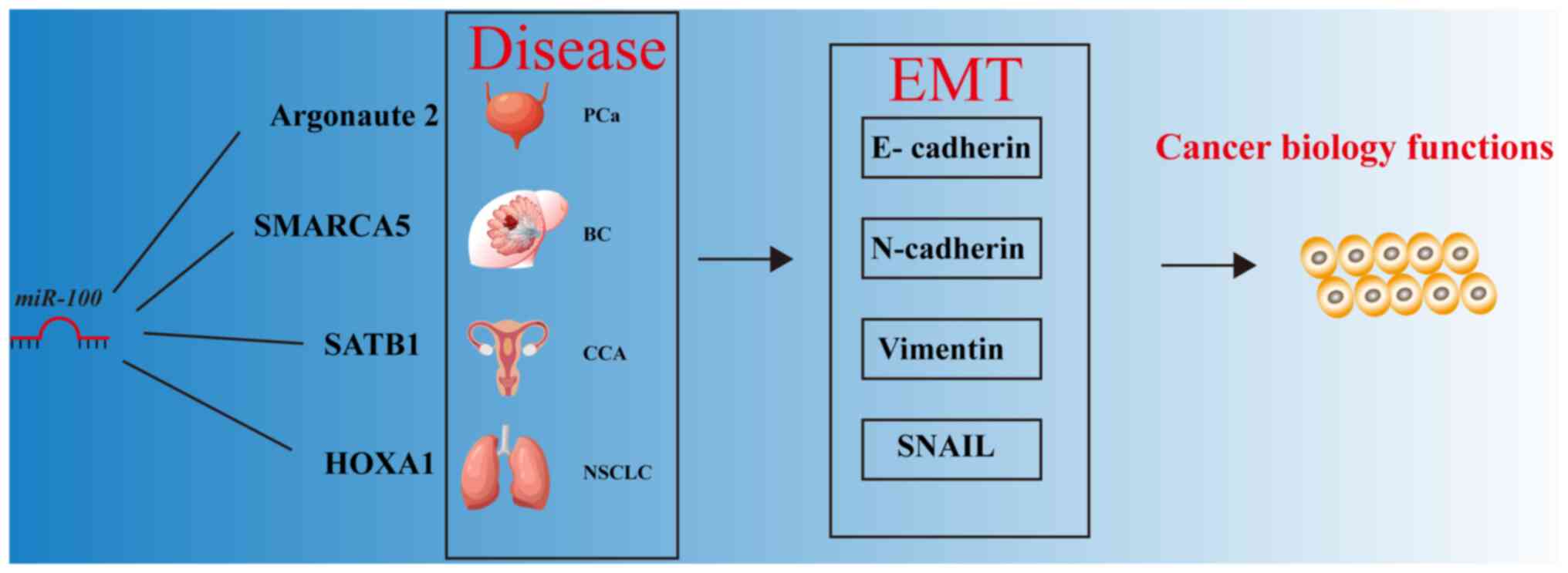 | Figure 6Involvement of miR-100 in the EMT
process in cancer. BC, breast cancer; CCA, cholangiocarcinoma; EMT,
epithelial-to-mesenchymal transition; HOXA1, homeobox A1; miR,
microRNA; NSCLC, non-small cell lung cancer; PCa, prostate cancer;
SATB1, SATB homeobox 1; SMARCA5, SWI/SNF-related, matrix-associated
actin-dependent regulator of chromatin, subfamily A, member 5. |
Role of miR-100 in cancer progression and
its potential as a diagnostic biomarker
The present review highlights the role of miR-100
in cancer progression by targeting multiple protein-coding genes
that regulate cancer cell proliferation, invasion and metastasis.
In addition to directly modulating gene expression, miR-100 also
alters the tumor microenvironment dynamics by influencing specific
signaling pathways (99,156,157). Notably, miR-100 expression
levels in cancer are associated with early diagnosis and prognosis
of patients (78,119-138,165), emphasizing its importance in
understanding cancer biology and its potential as an early
diagnostic biomarker.
Previous studies have shown that miR-100 suppresses
tumor cell proliferation and invasion by downregulating specific
target genes, ultimately reducing tumor metastasis (166,167). The present findings further
emphasize the potential of miR-100 in cancer research and its
clinical applications. However, several key issues remain
unresolved. For instance, the tissue-specific expression patterns
of miR-100 and its potential regulatory mechanisms are not fully
understood (168).
Additionally, although the role of miR-100 in cancer progression is
becoming clearer, its precise function in downstream signaling
pathways requires further investigation. The variability in miR-100
effects across different experimental systems highlights the need
for standardized protocols to ensure the reproducibility and
reliability of results (169,170).
Controversial findings
Despite its tumor-suppressive effects in various
cancer types, some controversial studies suggest that miR-100 may
serve an oncogenic role in certain cancer contexts. For example,
overexpression of miR-100 has been linked to tumor progression and
poor prognosis in NSCLC (171),
contrasting sharply with its tumor-suppressive effects in GC and
peripheral T-cell lymphoma (106-108). This dual role may be attributed
to cancer heterogeneity and differences in the genes targeted by
miR-100 in different cellular environments. Furthermore, research
indicates that the role of miR-100 in various cancer types may be
influenced by differential regulation of its downstream signaling
pathways, such as the PI3K/AKT, mTOR and Wnt/β-catenin pathways,
further complicating its biological functions (172). For comparison, miR-21 and
miR-155 are two other miRNAs that have been extensively studied in
cancer, each exhibiting distinct roles. miR-21 and miR-155 are
commonly upregulated as oncogenic miRNAs in various tumors, where
miR-21 contributes to anti-apoptotic, proliferative and invasive
processes, thereby enhancing drug resistance in tumor cells. By
contrast, miR-155 promotes tumor progression and immune evasion by
modulating the immune microenvironment (173,174). These functional differences
underscore the critical importance of understanding the specific
role of each miRNA within a given cancer context.
Regarding therapeutic strategies, the restoration
of miR-100 is typically achieved by delivering its mimics or
agonists to inhibit tumor growth and enhance the effectiveness of
chemotherapy or targeted therapies. Conversely, therapeutic
approaches for miR-21 and miR-155 primarily focus on restoring
normal cellular functions by inhibiting their expression through
anti-miRNA strategies (175,176).
Therefore, a comparative analysis of miR-100,
miR-21 and miR-155 provides valuable insights into the distinct
mechanisms these miRNAs are associated with in different cancer
contexts. This comparison has implications for optimizing
therapeutic strategies and improving clinical outcomes.
Future directions and research needs
Future research should focus on clarifying the role
of miR-100 as a specific cancer biomarker and evaluating its
therapeutic potential. Comparative studies with other
cancer-related miRNAs will help deepen the understanding of the
unique mechanisms and therapeutic significance of miR-100.
Investigating the tissue-specific roles of miR-100 in different
cancer types is crucial for its clinical application, particularly
in early diagnosis and prognosis. Further exploration of the dual
role of miR-100 and mechanistic insights will contribute to a more
comprehensive understanding of its potential as a therapeutic
target.
Incorporating the latest high-quality studies into
this field will enhance the understanding of the clinical
application of miR-100. This includes refining its role as a
diagnostic or prognostic biomarker in specific cancer types and
exploring its potential as a therapeutic target. In conclusion,
addressing the existing controversies and improving experimental
methodologies will contribute to bridging the gap between
experimental research and clinical practice. These advancements
will pave the way for translating experimental findings into
clinical applications.
Conclusion
As a tumor-suppressive miRNA, miR-100 serves a
critical role in the initiation and progression of various cancer
types. miR-100 inhibits tumor cell proliferation, migration and
invasion by regulating oncogene expression and multiple signaling
pathways, such as the PI3K/AKT and Wnt/β-catenin pathways. miR-100
holds great promise as an early diagnostic biomarker in cancer. Its
stable presence in body fluids such as blood and urine makes it a
potential non-invasive tool for early screening (121-126). The aberrant expression of
miR-100 is closely associated with the development of tumors such
as gastric and lung cancer, making it an ideal candidate for early
detection.
In personalized therapy, the restoration of miR-100
expression can inhibit cancer cell proliferation and invasion,
enhancing the efficacy of chemotherapy or targeted therapies.
However, the clinical application of miR-100 still faces
challenges, including the optimization of delivery systems and
safety assessments. Future research should focus on developing
efficient delivery vectors to improve the stability and
biocompatibility of miR-100 for clinical use.
In conclusion, miR-100 has potential as both a
biomarker and a therapeutic target for early diagnosis and
personalized treatment.
Availability of data and materials
Not applicable.
Authors' contributions
XZ and SL contributed to the literature search and
selected the studies for inclusion. JZ, LZ and SG drafted the
manuscript. XZ, SL and CQ critically revised the content of the
paper. JZ and LZ conceived the topic of review for the study and
revised the manuscript. Data authentication is not applicable. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–148. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao M, Li H, Sun D and Chen W: Cancer
burden of major cancers in China: A need for sustainable actions.
Cancer Commun (Lond). 40:205–210. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao W, Chen HD, Yu YW, Li N and Chen WQ:
Changing profiles of cancer burden worldwide and in China: A
secondary analysis of the global cancer statistics 2020. Chin Med J
(Engl). 134:783–791. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Toden S, Zumwalt TJ and Goel A: Non-coding
RNAs and potential therapeutic targeting in cancer. Biochim Biophys
Acta Rev Cancer. 1875:1884912021. View Article : Google Scholar :
|
|
5
|
Chen B, Dragomir MP, Yang C, Li Q, Horst D
and Calin GA: Targeting non-coding RNAs to overcome cancer therapy
resistance. Signal Transduct Target Ther. 7:1212022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saw PE, Xu X, Chen J and Song EW:
Non-coding RNAs: The new central dogma of cancer biology. Sci China
Life Sci. 64:22–50. 2021. View Article : Google Scholar
|
|
7
|
Calin GA, Dumitru CD, Shimizu M, Bichi R,
Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al:
Frequent deletions and down-regulation of micro-RNA genes miR15 and
miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci
USA. 99:15524–15529. 2002. View Article : Google Scholar
|
|
8
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wightman B, Ha I and Ruvkun G:
Posttranscriptional regulation of the heterochronic gene lin-14 by
lin-4 mediates temporal pattern formation in C. elegans. Cell.
75:855–862. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li C, Gao Y, Zhang K, Chen J, Han S, Feng
B, Wang R and Chen L: Multiple roles of microRNA-100 in human
cancer and its therapeutic potential. Cell Physiol Biochem.
37:2143–2159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang S, Deng B, Zhang Y and Jiang N:
Expression of miR-100 and RBSP3 in FTC-133 cells after exposure to
131I. Nucl Med Commun. 35:932–938. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang S, Xue S, Dai Y, Yang J, Chen Z, Fang
X, Zhou W, Wu W and Li Q: Reduced expression of microRNA-100
confers unfavorable prognosis in patients with bladder cancer.
Diagn Pathol. 7:1592012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang G, Yang L, Hu M, Hu R, Wang Y, Chen
B, Jiang X and Cui R: Comprehensive analysis of the prognostic
significance of Hsa-miR-100-5p and its related gene signature in
stomach adenocarcinoma. Front Cell Dev Biol. 9:7362742021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang XD, Xu XH, Zhang SY, Wu Y, Xing CG,
Ru G, Xu HT and Cao JP: Role of miR-100 in the radioresistance of
colorectal cancer cells. Am J Cancer Res. 5:5452015.PubMed/NCBI
|
|
15
|
Qin X, Yu S, Zhou L, Shi M, Hu Y, Xu X,
Shen B, Liu S, Yan D and Feng J: Cisplatin-resistant lung cancer
cell-derived exosomes increase cisplatin resistance of recipient
cells in exosomal miR-100-5p-dependent manner. Int J Nanomedicine.
12:3721–3733. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dai Y, Xie CH, Neis JP, Fan CY, Vural E
and Spring PM: MicroRNA expression profiles of head and neck
squamous cell carcinoma with docetaxel-induced multidrug
resistance. Head Neck. 33:786–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng J, Wang L, Zhao J, Zheng Z, Peng J,
Zhang W, Wen T, Nie J, Ding L and Yi D: MiR-100-5p regulates
cardiac hypertrophy through activation of autophagy by targeting
mTOR. Hum Cell. 34:1388–1397. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou Y, Huang Y, Hu K, Zhang Z, Yang J and
Wang Z: HIF1A activates the transcription of lncRNA RAET1K to
modulate hypoxia-induced glycolysis in hepatocellular carcinoma
cells via miR-100-5p. Cell Death Dis. 11:1762020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Assmann TS, Recamonde-Mendoza M, De Souza
BM and Crispim D: MicroRNA expression profiles and type 1 diabetes
mellitus:systematic review and bioinformatic analysis. Endocr
Connect. 6:773–790. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pek SL, Sum CF, Lin MX, Cheng AK, Wong MT,
Lim SC and Tavintharan S: Circulating and visceral adipose miR-100
is down-regulated in patients with obesity and Type 2 diabetes. Mol
Cell Endocrinol. 427:112–123. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ai L, Yi W, Chen L, Wang H and Huang Q:
Xian-Ling-Gu-Bao protects osteoporosis through promoting osteoblast
differentiation by targeting miR-100-5p/KDM6B/RUNX2 axis. In Vitro
Cell Dev Biol Anim. 57:3–9. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kelch S, Balmayor ER, Seeliger C, Vester
H, Kirschke JS and van Griensven M: miRNAs in bone tissue correlate
to bone mineral density and circulating miRNAs are gender
independent in osteoporotic patients. Sci Rep. 7:158612017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu J, Kuang L, Chen C, Yang J, Zeng WN, Li
T, Chen H, Huang S, Fu Z, Li J, et al: miR-100-5p-abundant exosomes
derived from infrapatellar fat pad MSCs protect articular cartilage
and ameliorate gait abnormalities via inhibition of mTOR in
osteoarthritis. Biomaterials. 206:87–100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang YS, Chang YC, Chen PH, Li CY, Wu WC
and Kao YH: MicroRNA-100 mediates hydrogen peroxide-induced
apoptosis of human retinal pigment epithelium ARPE-19 cells.
Pharmaceuticals. 14:3142021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tan Q, Shi S, Liang J, Cao D, Wang S and
Wang Z: Endometrial cell-derived small extracellular vesicle
miR-100-5p promotes functions of trophoblast during embryo
implantation. Mol Ther-Nucleic Acids. 23:217–231. 2021. View Article : Google Scholar
|
|
26
|
Huang YL, Huang GY, Lv J, Pan LN, Luo X
and Shen J: miR-100 promotes the proliferation of spermatogonial
stem cells via regulating Stat3. Mol Reprod Dev. 84:693–701. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sempere LF, Sokol NS, Dubrovsky EB, Berger
EM and Ambros V: Temporal regulation of microRNA expression in
Drosophila melanogaster mediated by hormonal signals and
broad-Complex gene activity. Dev Biol. 259:9–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Henson BJ, Bhattacharjee S, O'Dee DM,
Feingold E and Gollin SM: Decreased expression of miR-125b and
miR-100 in oral cancer cells contributes to malignancy. Genes
Chromosomes Cancer. 48:569–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao S, Liu S, Wei W, Qi Y and Meng F:
Advances in targeting of miR-10-associated lncRNAs/circRNAs for the
management of cancer. Oncolo Lett. 25:892023. View Article : Google Scholar
|
|
30
|
Liu X, Zhong L, Li P and Zhao P:
MicroRNA-100 enhances autophagy and suppresses migration and
invasion of renal cell carcinoma cells via disruption of
NOX4-dependent mTOR pathway. Clin Transl Sci. 15:567–575. 2022.
View Article : Google Scholar
|
|
31
|
Zhou MK, Liu XJ, Zhao ZG and Cheng YM:
MicroRNA-100 functions as a tumor suppressor by inhibiting Lgr5
expression in colon cancer cells. Mol Med Rep. 11:2947–2952. 2015.
View Article : Google Scholar
|
|
32
|
Qi X, Zhang DH, Wu N, Xiao JH, Wang X and
Ma W: ceRNA in cancer: Possible functions and clinical
implications. J Med Genet. 52:710–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Basera A, Hull R, Demetriou D, Bates DO,
Kaufmann AM, Dlamini Z and Marima R: Competing Endogenous RNA
(ceRNA) Networks and Splicing Switches in Cervical Cancer: HPV
Oncogenesis, Clinical Significance and Therapeutic Opportunities.
Microorganisms. 10:18522022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang C, Zhou Y, Zhang B, Sheng Z, Sun N,
Yuan B and Wu X: Identification of lncRNA, miRNA and mRNA
expression profiles and ceRNA Networks in small cell lung cancer.
BMC Genomics. 24:2172023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qin L, Li B, Wang S, Tang Y, Fahira A, Kou
Y, Li T, Hu Z and Huang Z: Construction of an Immune-related
prognostic signature and lncRNA-miRNA-mRNA ceRNA network in acute
myeloid leukaemia. J Leukoc Biol. 116:146–165. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang W, Zhang Y and Xi S: Upregulation of
lncRNA HAGLROS enhances the development of nasopharyngeal carcinoma
via modulating miR-100/ATG14 axis-mediated PI3K/AKT/mTOR signals.
Artif Cells Nanomed Biotechnol. 47:3043–3052. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li L, Zhu H, Li X, Ke Y, Yang S and Cheng
Q: Long non-coding RNA HAGLROS facilitates the malignant phenotypes
of NSCLC cells via repressing miR-100 and up-regulating SMARCA5.
Biomed J. 44(6 Suppl 2): S305–S315. 2021. View Article : Google Scholar
|
|
38
|
Yang M, Zhai Z, Zhang Y and Wang Y:
Clinical significance and oncogene function of long noncoding RNA
HAGLROS overexpression in ovarian cancer. Arch Gynecol Obstet.
300:703–710. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shu L, Guo K, Lin ZH and Liu H: Long
non-coding RNA HAGLROS promotes the development of diffuse large
B-cell lymphoma via suppressing miR-100. J Clin Lab Anal.
36:e241682022. View Article : Google Scholar
|
|
40
|
Liu X, Liu C, Zhang A, Wang Q, Ge J, Li Q
and Xiao J: Long non-coding RNA SDCBP2-AS1 delays the progression
of ovarian cancer via microRNA-100-5p-targeted EPDR1. World J Surg
Oncol. 19:1992021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen F, Wu P, Xia R, Yang J, Huo XY, Gu
DY, Tang CJ, De W and Yang F: STAT3-induced lncRNA HAGLROS
overexpression contributes to the malignant progression of gastric
cancer cells via mTOR signal-mediated inhibition of autophagy. Mol
Cancer. 17:62018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Peng J, Zheng H, Liu F, Wu Q and Liu S:
The m6A methyltransferase METTL3 affects autophagy and progression
of nasopharyngeal carcinoma by regulating the stability of lncRNA
ZFAS1. Infect Agent Cancer. 17:12022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Le F, Ou Y, Luo P and Zhong X: LncRNA
NCK1-AS1 in plasma distinguishes oral ulcer from early-stage oral
squamous cell carcinoma. J Biol Res (Thessalon). 27:162020.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shi Y, Zhang DD, Liu JB, Yang XL, Xin R,
Jia CY, Wang HM, Lu GX, Wang PY, Liu Y, et al: Comprehensive
analysis to identify DLEU2L/TAOK1 axis as a prognostic biomarker in
hepatocellular carcinoma. Mol Ther Nucleic Acids. 23:702–718. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bai W, Peng H, Zhang J, Zhao Y, Li Z, Feng
X, Zhang J, Liang F, Wang L, Zhang N, et al: LINC00589-dominated
ceRNA networks regulate multiple chemoresistance and cancer stem
cell-like properties in HER2+breast cancer. NPJ Breast Cancer.
8:1152022. View Article : Google Scholar
|
|
46
|
Lu Y, Zhao X, Liu Q, Li C, Graves-Deal R,
Cao Z, Singh B, Franklin JL, Wang J, Hu H, et al: lncRNA
MIR100HG-derived miR-100 and miR-125b mediate cetuximab resistance
via Wnt/β-catenin signaling. Nat Med. 23:1331–1341. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shi Y, Guo Z, Fang N, Jiang W, Fan Y, He
Y, Ma Z and Chen Y: hsa_circ_0006168 sponges miR-100 and regulates
mTOR to promote the proliferation, migration and invasion of
esophageal squamous cell carcinoma. Biomed Pharmacother.
117:1091512019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yuan F, Zhang S, Sun Q, Ye L, Xu Y, Xu Z,
Deng G, Zhang S, Liu B and Chen Q: Hsa_circ_0072309 enhances
autophagy and TMZ sensitivity in glioblastoma. CNS Neurosci Ther.
28:897–912. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yao T, Yao Y, Chen Z, Peng Y, Zhong G,
Huang C, Li J and Li R: CircCASC15-miR-100-mTOR may influence the
cervical cancer radioresistance. Cancer Cell Int. 22:1652022.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hill M and Tran N: miRNA interplay:
Mechanisms and consequences in cancer. Dis Model Mech.
14:dmm0476622021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Silkenstedt E, Linton K and Dreyling M:
Mantle cell lymphoma-advances in molecular biology, prognostication
and treatment approaches. Br J Haematol. 195:162–173. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lin L, Huang Y, Zhuang W, Lin P and Ma X:
miR-100 inhibits cell proliferation in mantle cell lymphoma by
targeting mTOR. Exp Hematol Oncol. 9:252020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nepstad I, Hatfield KJ, Grønningsæter IS
and Reikvam H: The PI3K-Akt-mTOR signaling pathway in human acute
myeloid leukemia (AML) cells. Int J Mol Sci. 21:29072020.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sun Y, Wang H and Luo C: MiR-100 regulates
cell viability and apoptosis by targeting ATM in pediatric acute
myeloid leukemia. Biochem Biophys Res Commun. 522:855–861. 2020.
View Article : Google Scholar
|
|
55
|
Zheng YS, Zhang H, Zhang XJ, Feng DD, Luo
XQ, Zeng CW, Lin KY, Zhou H, Qu LH, Zhang P and Chen YQ: MiR-100
regulates cell differentiation and survival by targeting RBSP3, a
phosphatase-like tumor suppressor in acute myeloid leukemia.
Oncogene. 31:80–92. 2012. View Article : Google Scholar :
|
|
56
|
Chang JH, Poppe MM, Hua CH, Marcus KJ and
Esiashvili N: Acute lymphoblastic leukemia. Pediatr Blood Cancer.
68(Suppl 2): e283712021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li XJ, Luo XQ, Han BW, Duan FT, Wei PP and
Chen YQ: MicroRNA-100/99a, deregulated in acute lymphoblastic
leukaemia, suppress proliferation and promote apoptosis by
regulating the FKBP51 and IGF1R/mTOR signalling pathways. Br J
Cancer. 109:2189–2198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ou A, Yung WKA and Majd N: Molecular
mechanisms of treatment resistance in glioblastoma. Int J Mol Sci.
22:3512020. View Article : Google Scholar
|
|
59
|
Alrfaei BM, Clark P, Vemuganti R and Kuo
JS: MicroRNA miR-100 decreases glioblastoma growth by targeting
SMARCA5 and ErbB3 in tumor-initiating cells. Technol Cancer Res
Treat. 19:15330338209607482020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Alrfaei BM, Vemuganti R and Kuo JS:
microRNA-100 targets SMRT/NCOR2, reduces proliferation, and
improves survival in glioblastoma animal models. PLoS One.
8:e808652013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Guo R, Mao YP, Tang LL, Chen L, Sun Y and
Ma J: The evolution of nasopharyngeal carcinoma staging. Br J
Radiol. 92:201902442019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lee HM, Okuda KS, González FE and Patel V:
Current perspectives on nasopharyngeal carcinoma. Adv Exp Med Biol.
1164:11–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Peng Q, Zhang L, Li J, Wang W, Cai J, Ban
Y, Zhou Y, Hu M, Mei Y, Zeng Z, et al: FOXA1 suppresses the growth,
migration, and invasion of nasopharyngeal carcinoma cells through
repressing miR-100-5p and miR-125b-5p. J Cancer. 11:2485–2495.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
He W, Huang Y, Jiang CC, Zhu Y, Wang L,
Zhang W, Huang W, Zhou T and Tang S: miR-100 inhibits cell growth
and proliferation by targeting HOXA1 in nasopharyngeal carcinoma.
Onco Targets Ther. 13:593–602. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sun X, Liu X, Wang Y, Yang S, Chen Y and
Yuan T: miR-100 inhibits the migration and invasion of
nasopharyngeal carcinoma by targeting IGF1R. Oncol Lett.
15:8333–8338. 2018.PubMed/NCBI
|
|
66
|
Shi W, Alajez NM, Bastianutto C, Hui AB,
Mocanu JD, Ito E, Busson P, Lo KW, Ng R, Waldron J, et al:
Significance of Plk1 regulation by miR-100 in human nasopharyngeal
cancer. Int J Cancer. 126:2036–2048. 2010. View Article : Google Scholar
|
|
67
|
Alexander M, Kim SY and Cheng H: Update
2020: Management of non-small cell lung cancer. Lung. 198:897–907.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liu J, Lu KH, Liu ZL, Sun M, De W and Wang
ZX: MicroRNA-100 is a potential molecular marker of non-small cell
lung cancer and functions as a tumor suppressor by targeting
polo-like kinase 1. BMC Cancer. 12:5192012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Han W, Ren X, Yang Y, Li H, Zhao L and Lin
Z: microRNA-100 functions as a tumor suppressor in non-small cell
lung cancer via regulating epithelial-mesenchymal transition and
Wnt/β-catenin by targeting HOXA1. Thorac Cancer. 11:1679–1688.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Nagata Y, Yamamoto S and Kato K: Immune
checkpoint inhibitors in esophageal cancer: Clinical development
and perspectives. Hum Vaccin Immunother. 18:21431772022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang N, Fu H, Song L, Ding Y, Wang X,
Zhao C and Zhao Y, Jiao F and Zhao Y: MicroRNA-100 promotes
migration and invasion through mammalian target of rapamycin in
esophageal squamous cell carcinoma. Oncol Rep. 32:1409–1418. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhou F, Tan F, Gao Y, Sun N, Xu X, Shao K
and He J: MicroRNA-99a/100 promotes apoptosis by targeting mTOR in
human esophageal squamous cell carcinoma. Med Oncol. 30:4112013.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhou SM, Zhang F, Chen XB, Jun CM, Jing X,
Wei DX, Xia Y, Zhou YB, Xiao XQ, Jia RQ, et al: miR-100 suppresses
the proliferation and tumor growth of esophageal squamous cancer
cells via targeting CXCR7. Oncol Rep. 35:3453–3459. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhou S, Yang B, Zhao Y, Xu S, Zhang H and
Li Z: Prognostic value of microRNA-100 in esophageal squamous cell
carcinoma. J Surg Res. 192:515–520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nagaraju GP, Dariya B, Kasa P, Peela S and
El-Rayes BF: Epigenetics in hepatocellular carcinoma. Semin Cancer
Biol. 86:622–632. 2022. View Article : Google Scholar
|
|
76
|
Ren Z, Ma X, Duan Z and Chen X: Diagnosis,
therapy, and prognosis for hepatocellular carcinoma. Anal Cell
Pathol (Amst). 2020:81574062020.PubMed/NCBI
|
|
77
|
Ge Y, Shu J, Shi G, Yan F, Li Y and Ding
H: miR-100 suppresses the proliferation, invasion, and migration of
hepatocellular carcinoma cells via targeting CXCR7. J Immunol Res.
2021:99207862021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhou HC, Fang JH, Shang LR, Zhang ZJ, Sang
Y, Xu L, Yuan Y, Chen MS, Zheng L, Zhang Y and Zhuang S: MicroRNAs
miR-125b and miR-100 suppress metastasis of hepatocellular
carcinoma by disrupting the formation of vessels that encapsulate
tumour clusters. J Pathol. 240:450–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chen P, Zhao X and Ma L: Downregulation of
microRNA-100 correlates with tumor progression and poor prognosis
in hepatocellular carcinoma. Mol Cell Biochem. 383:49–58. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Machlowska J, Baj J, Sitarz M, Maciejewski
R and Sitarz R: Gastric cancer: Epidemiology, risk factors,
classification, genomic characteristics and treatment strategies.
Int J Mol Sci. 21:40122020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wei L, Sun J, Zhang N, Zheng Y, Wang X, Lv
L, Liu J, Xu Y, Shen Y and Yang M: Noncoding RNAs in gastric
cancer: Implications for drug resistance. Mol Cancer. 19:622020.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Cao Y, Song J, Ge J, Song Z, Chen J and Wu
C: MicroRNA-100 suppresses human gastric cancer cell proliferation
by targeting CXCR7. Oncol Lett. 15:453–458. 2018.PubMed/NCBI
|
|
83
|
Chen Z, Liu X, Hu Z, Wang Y, Liu M, Liu X,
Li H, Ji R, Guo Q and Zhou Y: Identification and characterization
of tumor suppressor and oncogenic miRNAs in gastric cancer. Oncol
Lett. 10:329–336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang N, Hu X, Du Y and Du J: The role of
miRNAs in colorectal cancer progression and chemoradiotherapy.
Biomed Pharmacother. 134:1110992021. View Article : Google Scholar
|
|
85
|
Zhao W, Dai S, Yue L, Xu F, Gu J, Dai X
and Qian X: Emerging mechanisms progress of colorectal cancer liver
metastasis. Front Endocrinol (Lausanne). 13:10815852022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Czauderna C, Luley K, von Bubnoff N and
Marquardt JU: Tailored systemic therapy for colorectal cancer liver
metastases. Int J Mol Sci. 22:117802021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Peng H, Luo J, Hao H, Hu J, Xie SK, Ren D
and Rao B: MicroRNA-100 regulates SW620 colorectal cancer cell
proliferation and invasion by targeting RAP1B. Oncol Rep.
31:2055–2062. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Fujino Y, Takeishi S, Nishida K, Okamoto
K, Muguruma N, Kimura T, Kitamura S, Miyamoto H, Fujimoto A,
Higashijima J, et al: Downregulation of micro RNA-100/micro
RNA-125b is associated with lymph node metastasis in early
colorectal cancer with submucosal invasion. Cancer Sci.
108:390–397. 2017. View Article : Google Scholar :
|
|
89
|
Wood LD, Canto MI, Jaffee EM and Simeone
DM: Pancreatic cancer: Pathogenesis, screening, diagnosis, and
treatment. Gastroenterology. 163:386–402.e1. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Jiang S, Fagman JB, Ma Y, Liu J, Vihav C,
Engstrom C, Liu B and Chen C: A comprehensive review of pancreatic
cancer and its therapeutic challenges. Aging (Albany NY).
14:7635–7649. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Huang JS, Egger ME, Grizzle WE and McNally
LR: MicroRNA-100 regulates IGF1-receptor expression in metastatic
pancreatic cancer cells. Biotech Histochem. 88:397–402. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Dobre M, Herlea V, Vlăduţ C, Ciocîrlan M,
Balaban VD, Constantinescu G, Diculescu M and Milanesi E:
Dysregulation of miRNAs targeting the IGF-1R pathway in pancreatic
ductal adenocarcinoma. Cells. 10:18562021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Deleuze A, Saout J, Dugay F, Peyronnet B,
Mathieu R, Verhoest G, Bensalah K, Crouzet L, Laguerre B,
Belaud-Rotureau MA, et al: Immunotherapy in renal cell carcinoma:
The future is now. Int J Mol Sci. 21:25322020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Li F, Aljahdali IAM, Zhang R, Nastiuk KL,
Krolewski JJ and Ling X: Kidney cancer biomarkers and targets for
therapeutics: Survivin (BIRC5), XIAP, MCL-1, HIF1α, HIF2α, NRF2,
MDM2, MDM4, p53, KRAS and AKT in renal cell carcinoma. J Exp Clin
Cancer Res. 40:2542021. View Article : Google Scholar
|
|
95
|
Chen P, Lin C, Quan J, Lai Y, He T, Zhou
L, Pan X, Wu X, Wang Y, Ni L, et al: Oncogenic miR-100-5p is
associated with cellular viability, migration and apoptosis in
renal cell carcinoma. Mol Med Rep. 16:5023–5030. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Adamaki M and Zoumpourlis V: Prostate
cancer biomarkers: From diagnosis to prognosis and precision-guided
therapeutics. Pharmacol Ther. 228:1079322021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Leite KR, Tomiyama A, Reis ST,
Sousa-Canavez JM, Sañudo A, Dall'Oglio MF, Camara-Lopes LH and
Srougi M: MicroRNA-100 expression is independently related to
biochemical recurrence of prostate cancer. J Urol. 185:1118–1122.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Leite KR, Morais DR, Reis ST, Viana N,
Moura C, Florez MG, Silva IA, Dip N and Srougi M: MicroRNA 100: A
context dependent miRNA in prostate cancer. Clinics (Sao Paulo).
68:797–802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wang M, Ren D, Guo W, Wang Z, Huang S, Du
H, Song L and Peng X: Loss of miR-100 enhances migration, invasion,
epithelial-mesenchymal transition and stemness properties in
prostate cancer cells through targeting Argonaute 2. Int J Oncol.
45:362–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Nabavi N, Saidy NRN, Venalainen E, Haegert
A, Parolia A, Xue H, Wang Y, Wu R, Dong X, Collins C, et al:
miR-100-5p inhibition induces apoptosis in dormant prostate cancer
cells and prevents the emergence of castration-resistant prostate
cancer. Sci Rep. 7:40792017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Ye Y, Li SL and Wang JJ: miR-100-5p
downregulates mTOR to suppress the proliferation, migration, and
invasion of prostate cancer cells. Front Oncol. 10:5789482020.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chen DW, Lang BHH, McLeod DSA, Newbold K
and Haymart MR: Thyroid cancer. Lancet. 401:1531–1544. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Şah Ünal FT, Gökçay Canpolat A, Elhan AH,
Sevim S, Sak SD, Emral R, Demir Ö, Güllü S, Erdoğan MF, Çorapçıoğlu
D and Şahin M: Cancer rates and characteristics of thyroid nodules
with macrocalcification. Endocrine. 84:1021–1029. 2024. View Article : Google Scholar
|
|
104
|
Ma P and Han J: Overexpression of
miR-100-5p inhibits papillary thyroid cancer progression via
targeting FZD8. Open Med (Wars). 17:1172–1182. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Trapani D, Ginsburg O, Fadelu T, Lin NU,
Hassett M, Ilbawi AM, Anderson BO and Curigliano G: Global
challenges and policy solutions in breast cancer control. Cancer
Treat Rev. 104:1023392022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Xie H, Xiao R, He Y, He L, Xie C, Chen J
and Hong Y: MicroRNA-100 inhibits breast cancer cell proliferation,
invasion and migration by targeting FOXA1. Oncol Lett. 22:8162021.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Gebeshuber CA and Martinez J: miR-100
suppresses IGF2 and inhibits breast tumorigenesis by interfering
with proliferation and survival signaling. Oncogene. 32:3306–3310.
2013. View Article : Google Scholar
|
|
108
|
Li X, Ren Y, Liu D, Yu X and Chen K: Role
of miR-100-5p and CDC25A in breast carcinoma cells. PeerJ.
9:e122632022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Gong Y, He T, Yang L, Yang G, Chen Y and
Zhang X: The role of miR-100 in regulating apoptosis of breast
cancer cells. Sci Rep. 5:116502015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Throwba H PK, Unnikrishnan L, Pangath M,
Vasudevan K, Jayaraman S, Li M, Iyaswamy A, Palaniyandi K and
Gnanasampanthapandian D: The epigenetic correlation among ovarian
cancer, endometriosis and PCOS: A review. Crit Rev Oncol Hematol.
180:1038522022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Volkova LV, Pashov AI and Omelchuk NN:
Cervical carcinoma: Oncobiology and biomarkers. Int J Mol Sci.
22:125712021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Schoutrop E, Moyano-Galceran L, Lheureux
S, Mattsson J, Lehti K, Dahlstrand H and Magalhaes I: Molecular,
cellular and systemic aspects of epithelial ovarian cancer and its
tumor microenvironment. Semin Cancer Biol. 86:207–223. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim
JH, Kim JW and Kim S: MicroRNA expression profiles in serous
ovarian carcinoma. Clin Cancer Res. 14:2690–2695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Huang C, Qin X, Zhao N, Jin H, Zhang S and
Yang H: Erratum: MicroRNA-100 functions as a tumor suppressor in
cervical cancer via downregulating the SATB1 expression and
regulating AKT/mTOR signaling pathway and epithelial-to-mesenchymal
transition. Oncol Lett. 22:7412021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Li BH, Zhou JS, Ye F, Cheng XD, Zhou CY,
Lu WG and Xie X: Reduced miR-100 expression in cervical cancer and
precursors and its carcinogenic effect through targeting PLK1
protein. Eur J Cancer. 47:2166–2174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Crosbie EJ, Kitson SJ, McAlpine JN,
Mukhopadhyay A, Powell ME and Singh N: Endometrial cancer. Lancet.
399:1412–1428. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Chen C, Zhang Q and Kong B: miRNA-576-5p
promotes endometrial cancer cell growth and metastasis by targeting
ZBTB4. Clin Transl Oncol. 25:706–720. 2023. View Article : Google Scholar :
|
|
118
|
Takebayashi K, Nasu K, Okamoto M, Aoyagi
Y, Hirakawa T and Narahara H: hsa-miR-100-5p, an overexpressed
miRNA in human ovarian endometriotic stromal cells, promotes
invasion through attenuation of SMARCD1 expression. Reprod Biol
Endocrinol. 18:312020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Valihrach L, Androvic P and Kubista M:
Circulating miRNA analysis for cancer diagnostics and therapy. Mol
Aspects Med. 72:1008252020. View Article : Google Scholar
|
|
120
|
O'Neill RS and Stoita A: Biomarkers in the
diagnosis of pancreatic cancer: Are we closer to finding the golden
ticket? World J Gastroenterol. 27:4045–40875. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wang H, Meng Q, Qian J, Li M, Gu C and
Yang Y: RNA-based diagnostic markers discovery and therapeutic
targets development in cancer. Pharmacol Ther. 234:1081232022.
View Article : Google Scholar
|
|
122
|
Wang S, Li L, Yang M, Wang X, Zhang H, Wu
N, Jia K, Wang J, Li M, Wei L and Liu J: Identification of three
circulating MicroRNAs in plasma as clinical biomarkers for breast
cancer detection. J Clin Med. 12:3222022. View Article : Google Scholar
|
|
123
|
Fuso P, Di Salvatore M, Santonocito C,
Guarino D, Autilio C, Mulè A, Arciuolo D, Rinninella A, Mignone F,
Ramundo M, et al: Let-7a-5p, miR-100-5p, miR-101-3p, and
miR-199a-3p hyperexpression as potential predictive biomarkers in
early breast cancer patients. J Pers Med. 11:8162021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Jin Y, Wong YS, Goh BKP, Chan CY, Cheow
PC, Chow PKH, Lim TKH, Goh GBB, Krishnamoorthy TL, Kumar R, et al:
Circulating microRNAs as potential diagnostic and prognostic
biomarkers in hepatocellular carcinoma. Sci Rep. 9:104642019.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Qureshi A, Fahim A, Kazi N, Farsi Kazi SA
and Nadeem F: Expression of miR-100 as a novel ancillary
non-invasive biomarker for early detection of bladder carcinoma. J
Pak Med Assoc. 68:759–763. 2018.PubMed/NCBI
|
|
126
|
Ludwig N, Nourkami-Tutdibi N, Backes C,
Lenhof HP, Graf N, Keller A and Meese E: Circulating serum miRNAs
as potential biomarkers for nephroblastoma. Pediatr Blood Cancer.
62:1360–1367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Blanca A, Sanchez-Gonzalez A, Requena MJ,
Carrasco-Valiente J, Gomez-Gomez E, Cheng L, Cimadamore A,
Montironi R and Lopez-Beltran A: Expression of miR-100 and miR-138
as prognostic biomarkers in non-muscle-invasive bladder cancer.
APMIS. 127:545–553. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Yamanaka Z, Sasaki T, Yamanaka A, Kato K
and Nishi H: Circulating and tissue miR-100 acts as a potential
diagnostic biomarker for cervical cancer. Cancer Biomark.
32:551–558. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Bahnassy AA, Salem SE, El-Sayed M,
Khorshid O, Abdellateif MS, Youssef AS, Mohanad M, Hussein M, Zekri
AN and Ali NM: MiRNAs as molecular biomarkers in stage II egyptian
colorectal cancer patients. Exp Mol Pathol. 105:260–271. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Wang X, Chen L, Jin H, Wang S, Zhang Y,
Tang X and Tang G: Screening miRNAs for early diagnosis of
colorectal cancer by small RNA deep sequencing and evaluation in a
Chinese patient population. Onco Targets Ther. 9:1159–1166.
2016.PubMed/NCBI
|
|
131
|
Gong Y, Yang G, Wang Q, Wang Y and Zhang
X: NME2 is a master suppressor of apoptosis in gastric cancer cells
via transcriptional regulation of miR-100 and other survival
factors. Mol Cancer Res. 18:287–299. 2020. View Article : Google Scholar
|
|
132
|
Damodaran M, Chinambedu Dandapani M, Raj
Simon Durai, Sundaram Sandhya, VenkatRamanan S, Ramachandran I and
Venkatesan V: Differentially expressed miR-20, miR-21, miR-100,
miR-125a and miR-146a as a potential biomarker for prostate cancer.
Mol Biol Rep. 48:3349–3356. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Jakob M, Mattes LM, Küffer S, Unger K,
Hess J, Bertlich M, Haubner F, Ihler F, Canis M, Weiss BG and Kitz
J: MicroRNA expression patterns in oral squamous cell carcinoma:
Hsa-mir-99b-3p and hsa-mir-100-5p as novel prognostic markers for
oral cancer. Head Neck. 41:3499–3515. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Zhao JY, Wang F, Li Y, Zhang XB, Yang L,
Wang W, Xu H, Liu DZ and Zhang LY: Five miRNAs considered as
molecular targets for predicting esophageal cancer. Med Sci Monit.
21:32222015. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhang HC and Tang KF: Clinical value of
integrated-signature miRNAs in esophageal cancer. Cancer Med.
6:1893–1903. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Wang G, Chen L, Meng J, Chen M, Zhuang L
and Zhang L: Overexpression of microRNA-100 predicts an unfavorable
prognosis in renal cell carcinoma. Int Urol Nephrol. 45:373–379.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Liu HT, Wang YW, Xing AY, Shi DB, Zhang H,
Guo XY, Xu J and Gao P: Prognostic value of microRNA signature in
patients with gastric cancers. Sci Rep. 7:428062017. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
He QL, Qin SY, Tao L, Ning HJ and Jiang
HX: Prognostic value and prospective molecular mechanism of
miR-100-5p in hepatocellular carcinoma: A comprehensive study based
on 1,258 samples. Oncol Lett. 18:6126–6142. 2019.PubMed/NCBI
|
|
139
|
Hassan NM, Refaat LA, Ismail GN,
Abdellateif M, Fadel SA and AbdelAziz RS: Diagnostic, prognostic
and predictive values of miR-100 and miR-210 in pediatric acute
lymphoblastic Leukemia. Hematology. 25:405–413. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Nussinov R, Tsai CJ and Jang H: Anticancer
drug resistance: An update and perspective. Drug Resist Updat.
59:1007962021. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Bukowski K, Kciuk M and Kontek R:
Mechanisms of multidrug resistance in cancer chemotherapy. Int J
Mol Sci. 21:32332020. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Luan Y, Zhang S, Zuo L and Zhou L:
Overexpression of miR-100 inhibits cell proliferation, migration,
and chemosensitivity in human glioblastoma through FGFR3. Onco
Targets Ther. 8:3391–3400. 2015.PubMed/NCBI
|
|
143
|
Xiao F, Bai Y, Chen Z, Li Y, Luo L, Huang
J, Yang J, Liao H and Guo L: Downregulation of HOXA1 gene affects
small cell lung cancer cell survival and chemoresistance under the
regulation of miR-100. Eur J Cancer. 50:1541–1554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Feng B, Wang R and Chen LB: MiR-100
resensitizes docetaxel-resistant human lung adenocarcinoma cells
(SPC-A1) to docetaxel by targeting Plk1. Cancer Lett. 317:184–191.
2012. View Article : Google Scholar
|
|
145
|
Guo P, Xiong X, Zhang S and Peng D:
miR-100 resensitizes resistant epithelial ovarian cancer to
cisplatin. Oncol Rep. 36:3552–3558. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Liu Y, Zhu ST, Wang X, Deng J, Li WH,
Zhang P and Liu BS: MiR-100 inhibits osteosarcoma cell
proliferation, migration, and invasion and enhances
chemosensitivity by targeting IGFIR. Technol Cancer Res Treat.
15:NP40–NP48. 2016. View Article : Google Scholar
|
|
147
|
Lai Y, Kacal M, Kanony M, Stukan I, Jatta
K, Kis L, Norberg E, Vakifahmetoglu-Norberg H, Lewensohn R,
Hydbring P and Ekman S: miR-100-5p confers resistance to ALK
tyrosine kinase inhibitors Crizotinib and Lorlatinib in EML4-ALK
positive NSCLC. Biochem Biophys Res Commun. 511:260–265. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Lobert S, Jefferson B and Morris K:
Regulation of β-tubulin isotypes by micro-RNA 100 in MCF7 breast
cancer cells. Cytoskeleton. 68:355–362. 2011. View Article : Google Scholar
|
|
149
|
Moqadam FA, Lange-Turenhout EAM, Ariës IM,
Pieters R and den Boer ML: MiR-125b, miR-100 and miR-99a
co-regulate vincristine resistance in childhood acute lymphoblastic
leukemia. Leuk Res. 37:1315–1321. 2013. View Article : Google Scholar
|
|
150
|
Ng WL, Yan D, Zhang X, Mo YY and Wang Y:
Over-expression of miR-100 is responsible for the low-expression of
ATM in the human glioma cell line: M059J. DNA Repair (Amst).
9:1170–1175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Zhou B, Lin W, Long Y, Yang Y, Zhang H, Wu
K and Chu Q: Notch signaling pathway: Architecture, disease, and
therapeutics. Signal Transduct Target Ther. 7:952022. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Lee YJ, Kim WR, Park EG, Lee DH, Kim JM,
Shin HJ, Jeong HS, Roh HY and Kim HS: Exploring the key signaling
pathways and ncRNAs in colorectal cancer. Int J Mol Sci.
25:45482024. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Peng WX, Koirala P and Mo YY:
LncRNA-mediated regulation of cell signaling in cancer. Oncogene.
36:5661–5667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Park JH, Pyun WY and Park HW: Cancer
metabolism: Phenotype, signaling and therapeutic targets. Cells.
9:23082020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Song SK, Jung WY, Park SK, Chung CW and
Park Y: Significantly different expression levels of microRNAs
associated with vascular invasion in hepatocellular carcinoma and
their prognostic significance after surgical resection. PLoS One.
14:e02168472019. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Chen D, Sun Y, Yuan Y, Han Z, Zhang P,
Zhang J, You MJ, Teruya-Feldstein J, Wang M, Gupta S, et al:
miR-100 induces epithelial-mesenchymal transition but suppresses
tumorigenesis, migration and invasion. PLoS Genet. 10:e10041772014.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Yang J, Chen Z, Wang X, Xu M, Fang H, Li
F, Liu Y, Jiang Y, Ding Y, Li J and Wang S: Inactivation of miR-100
combined with arsenic treatment enhances the malignant
transformation of BEAS-2B cells via stimulating
epithelial-mesenchymal transition. Cancer Biol Ther. 18:965–973.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Huang C, Qin X, Zhao N, Jin H, Zhang S and
Yang H: (Corrigendum) MicroRNA-100 functions as a tumor suppressor
in cervical cancer via downregulating the SATB1 expression and
regulating AKT/mTOR signaling pathway and epithelial-to-mesenchymal
transition. Oncol Lett. 22:2021. View Article : Google Scholar
|
|
159
|
Glaviano A, Foo ASC, Lam HY, Yap KCH,
Jacot W, Jones RH, Eng H, Nair MG, Makvandi P, Geoerger B, et al:
PI3K/AKT/mTOR signaling transduction pathway and targeted therapies
in cancer. Mol Cancer. 22:1382023. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Song P, Gao Z, Bao Y, Chen L, Huang Y, Liu
Y, Dong Q and Wei X: Wnt/β-catenin signaling pathway in
carcinogenesis and cancer therapy. J Hematol Oncol. 17:462024.
View Article : Google Scholar
|
|
161
|
Peng CW, Yue LX, Zhou YQ, Tang S, Kan C,
Xia LM, Yang F and Wang SY: miR-100-3p inhibits cell proliferation
and induces apoptosis in human gastric cancer through targeting to
BMPR2. Cancer Cell Int. 19:3542019. View Article : Google Scholar :
|
|
162
|
Yang G, Gong Y, Wang Q, Wang Y and Zhang
X: The role of miR-100-mediated Notch pathway in apoptosis of
gastric tumor cells. Cell Signal. 27:1087–1101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Huang C, Qin X, Zhao N, Jin H, Zhang S and
Yang H: MicroRNA-100 functions as a tumor suppressor in cervical
cancer via downregulating the SATB1 expression and regulating
AKT/mTOR signaling pathway and epithelial-to-mesenchymal
transition. Oncol Lett. 20:1336–1344. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Morgos DT, Stefani C, Miricescu D, Greabu
M, Stanciu S, Nica S, Stanescu-Spinu II, Balan DG,
Balcangiu-Stroescu AE, Coculescu EC, et al: Targeting PI3K/AKT/mTOR
and MAPK signaling pathways in gastric cancer. Int J Mol Sci.
25:18482024. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Qin C, Huang RY and Wang ZX: Potential
role of miR-100 in cancer diagnosis, prognosis, and therapy. Tumour
Biol. 36:1403–1409. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Wei X, Feng Y, Fu Y, Liu F, Chen Q, Zhang
W, Zhao Y, Huang X, Chen Y, Li Q and Zhang Q: miR-100-5p is
upregulated in multiple myeloma and involves in the pathogenesis of
multiple myeloma through targeting MTMR3. Hematology.
28:21968572023. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Eniafe J and Jiang S: MicroRNA-99 family
in cancer and immunity. Wiley Interdiscip Rev RNA. 12:e16352021.
View Article : Google Scholar
|
|
168
|
Gu L, Li H, Chen L, Ma X, Gao Y, Li X,
Zhang Y, Fan Y and Zhang X: MicroRNAs as prognostic molecular
signatures in renal cell carcinoma: A systematic review and
meta-analysis. Oncotarget. 6:32545–32560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Ghafouri-Fard S, Glassy MC, Abak A, Hussen
BM, Niazi V and Taheri M: The interaction between miRNAs/lncRNAs
and Notch pathway in human disorders. Biomed Pharmacother.
138:1114962021. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Servín-González LS, Granados-López AJ and
López JA: Families of microRNAs expressed in clusters regulate cell
signaling in cervical cancer. Int J Mol Sci. 16:12773–12790. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Shemesh R, Laufer-Geva S, Gorzalczany Y,
Anoze A, Sagi-Eisenberg R, Peled N and Roisman LC: The interaction
of mast cells with membranes from lung cancer cells induces the
release of extracellular vesicles with a unique miRNA signature.
Sci Rep. 13:215442023. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Jamali L, Tofigh R, Tutunchi S, Panahi G,
Borhani F, Akhavan S, Nourmohammadi P, Ghaderian SMH, Rasouli M and
Mirzaei H: Circulating microRNAs as diagnostic and therapeutic
biomarkers in gastric and esophageal cancers. J Cell Physiol.
233:8538–8550. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Farasati Far B, Vakili K, Fathi M,
Yaghoobpoor S, Bhia M and Naimi-Jamal MR: The role of microRNA-21
(miR-21) in pathogenesis, diagnosis, and prognosis of
gastrointestinal cancers: A review. Life Sci. 316:1213402023.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Grimaldi AM, Nuzzo S, Condorelli G,
Salvatore M and Incoronato M: Prognostic and clinicopathological
significance of miR-155 in breast cancer: A systematic review. Int
J Mol Sci. 21:58342020. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Seyhan AA: Trials and tribulations of
microRNA therapeutics. Int J Mol Sci. 25:14692024. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Qian H, Maghsoudloo M, Kaboli PJ,
Babaeizad A, Cui Y, Fu J, Wang Q and Imani S: Decoding the promise
and challenges of miRNA-based cancer therapies: An essential update
on miR-21, miR-34, and miR-155. Int J Med Sci. 21:2781–2798. 2024.
View Article : Google Scholar : PubMed/NCBI
|