Introduction
Bone fractures are a global public health issue. The
Global Burden of Diseases, Injuries, and Risk Factors Study (GBD)
shows the large burden of fractures worldwide, with 178 million new
fractures, 455 million prevalent fractures, and 25.8 million lived
with disability due to fractures in 2019 (1). Delayed healing or failure of
healing is the most common complication of fractures. Studies have
reported that >10% of patients with postoperative fracture fail
to heal completely (2-4). The tibia is the most susceptible
site in the whole body for delayed healing and non-healing due to
lack of soft tissue coverage and poor blood supply (5,6).
There is a limited number of drugs to promote
fracture healing, and these drugs usually act on only one part of
the bone regeneration process and have a specific optimal
therapeutic window. Accumulating studies have reported that the
clinical efficacy of several existing drugs to promote fracture
healing is unclear and often accompanied by a variety of
complications and side effects, such as heterotopic ossification
and increased risk of infection or cancer (7-11). Thus, it is necessary to develop
more efficient therapeutics for promoting bone formation and
regeneration during bone fracture management.
Growing clinical evidence has shown satisfying
efficacies of traditional Chinese medicine (TCM) in promoting
fracture healing (12-14). Osteoking (Sailing Pharmaceutical
Technology Group Co., Ltd.), a Chinese patent drug, has been
extensively used in the treatment bone conditions, such as bone
fractures, osteoporosis and lumbar disc herniation (15-18). Our previous study indicated that
Osteoking may exert clinical efficacy in relieving the joint pain
and improving life quality of patients with knee osteoarthritis
without any adverse reactions (19). Ling et al (20) identified five chemical components
contained in Osteoking by ultra-performance liquid chromatograph
(UPLC). Our previous study established a rat model of tibial bone
defect to simulate the pathological changes and healing
characteristics of incomplete fracture (21). Following the integration of
transcriptome detection, network analysis and in vivo
experimental validations, our data suggested that Osteoking may
promote bone formation and defect repair by regulating the Z-DNA
binding protein 1-signal transducer and activator of transcription
1-protein kinase R (PKR) axis, leading to inhibition of
receptor-interacting serine threonine-protein kinase 1
(RIPK1)/RIPK3/mixed lineage kinase domain-like protein (MLKL)
activation-mediated necroptosis, and reversing the disturbance of
bone metabolism. However, the therapeutic characteristics and
underlying mechanisms of Osteoking against the complete fracture
remain unclear.
The present study established a rat model of tibial
bone fracture simulating the pathological changes and healing
characteristics of complete fracture to evaluate the therapeutic
effects of Osteoking. Transcriptomics profiling, network analysis
and in vivo experimental validation was performed to
determine the potential targets of Osteoking in promoting bone
fracture healing.
Materials and methods
Animals
Male Sprague-Dawley rats (age, 6 weeks; weight
220±20 g; n=50) were obtained from Beijing Vital River Laboratory
Animal Technology Co., Ltd. (license no. SCXK 2021-0011, Beijing,
China) and were housed (n=5/cage) under specific pathogen-free
conditions at a constant temperature of 24±1°C and relative
humidity of 50-65% on a 12/12-h light/dark cycle and water and food
ad libitum. Prior to the experiments, the rats were allowed
a 1 week acclimatization period. All animal experiments were
approved by Experimental Animal Ethics Committee of Institute of
Chinese Materia Medica, China Academy of Chinese Medical Sciences
(Beijing, China; approval no. IBTCMCACMS21-2304-05).
Tibial bone fracture model
Rat model of tibial bone fracture was induced as
described by Sun et al (22). Rats were anesthetized through
intraperitoneal injection (i.p) of pentobarbital (2%, 2 ml/kg) and
the modeling procedure was completed under sterile conditions.
After removing the hair on the medial side of the right hind leg
and disinfecting with iodine, a 3.0 cm longitudinal incision was
made on the medial aspect of the mid tibia. Following separation of
the surrounding muscles, a high-speed rotary hacksaw cutter was
used to create a complete bone fracture in the middle of the tibia.
A 1.0 mm Kirschner wire (K-wire) was inserted retrograde into the
bone marrow cavity from the fracture ends. When the tip of the
K-wire penetrated below the medial tibial condyle, excess K-wire
was cut and bent against the cortical surface of the bone to allow
removal after sacrifice and the blunt end of the K-wire was passed
through the injured site and fixed in the lower end of the tibial
marrow cavity. The incision was cleaned with 0.9% sodium chloride
solution, sutured and covered with sterile patches. Penicillin
sodium solution (40,000 U/kg, dissolved in sterilized; Hongbao
Veterinary Medicine Co., Ltd.) was injected intramuscularly into
the healthy hind leg for 3 consecutive days postoperatively to
prevent infection.
Grouping and treatment
A total of 50 rats were randomly divided into five
groups (n=10/group): Normal control, bone fracture model and
Osteoking low-(1.3125 ml/kg), medium-(2.625 ml/kg) and high-dose
(5.25 ml/kg), which were equivalent to 0.5, 1.0 and 2.0 times the
daily dosage for patients with fractures in clinics, respectively.
Both normal control and bone fracture model groups received the
same volume of saline. All treatments were performed once/day for 3
weeks via oral administration from the day after surgery. Rats were
sacrificed by overdose of anesthesia (5% pentobarbital, i.p, 2
ml/kg). Death was confirmed by loss of pulse, respiratory arrest
and lack of response to squeezing of toes and tail root. Blood was
collected by abdominal aortic puncture.
X-ray imaging
X-ray imaging was performed as previously described
(21). In brief, X-ray images
were analyzed using the Modified radiographic union score for tibia
fractures scoring scale (23).
Because only lateral radiographs were captured, healing was
determined only in the anterior and posterior cortex; scores for
the cortices were summed to give a final score ranging from 2 (not
healed) to 8 (maximally healed).
Assessment of severity of fracture
General evaluation
The diet, hair color change and general activity of
rats were monitored daily following surgery. The rats were weighed
and the diameters of the right middle tibia were measured using
electronic vernier caliper every 3 days.
Mechanical pain threshold
measurement
Fracture pain levels were assessed by
mechanical-induced hyperalgesia as previously described (24). The experiment was performed every
5 days (three times/rat) and the mean value was calculated.
Inclined plate test
A layer of foam pad was attached to the surface of
the inclined plate. The rats were placed on the inclined plate with
the head at the top end. An angle sensor was attached and the
inclined plate was raised from 0° in increments of 5° until the rat
was able to stay on the inclined plate for ~5 sec. The experiment
was performed every 5 days (three times/rat) and the mean value was
calculated.
Hindlimb weight-bearing test
Rats were placed in a small restrictive container
with forelimbs placed on an inclined plate so that the majority of
weight had to be placed on the hindlimbs. Both hindlimbs were
placed on the left and right test plates of the gauge. Floor
sensors were attached to measure the weight on each hind limb
individually. The greater the difference in weight distribution
across hindlimbs, the worse the static weight-bearing capacity of
the fractured limb. The experiment was performed every 3 days
(three times/rat) and the mean value was calculated.
CatWalk XT gait analysis
At day 20 after the fracture operation, all rats
received track acclimatization training and were placed at the
start of the light-avoidance track (25). A successful gait recording was
defined as the rat was able to reach the end of the track at a
uniform speed without stopping or turning around. The test was
repeated three times/rat and the mean values of the following
parameters were calculated: Stand, swing, swing speed, print area
and mean intensity.
Micro-computed tomography (CT)
examination
K-wire was removed before micro-CT examination and
the region of interest was defined as a total of 7 mm (220 slides)
of bone tissue centered on the fracture line. Micro-CT was
performed as previously described (21), and the following parameters were
calculated: Bone mineral density (BMD), bone volume/tissue volume
ratio (BV/TV), trabecular number (Tb.N), trabecular thickness
(Tb.Th), trabecular separation (Tb.Sp), cortical bone thickness
(Ct.Th), cortical bone area (Ct.Ar), structure model index (SMI)
and connectivity density (Conn.D).
Histopathological evaluation
The right tibia of rats was embedded in paraffin,
cut into 4-μm coronal sections and the pathological changes
of the callus tissue were observed by hematoxylin and eosin
(H&E), safranin O-fast green, Masson and TRAP staining as
previously described (26,27).
Biomechanical test
Three-point bending experiments were performed at
the same position of each specimen using a WDT-20 universal
materials testing machine (Changchun Second Material Testing
Machine Factory; Data S1).
Bone metabolism biochemical indicator
assay
The following indicators were detected by ELISA:
Bone morphogenetic protein-2 (BMP-2), transforming growth factor-β
(TGF-β), bone specific alkaline phosphatase (BALP), type I
procollagen N-terminal peptide (PINP), osteocalcin (OCN), type I
collagen carboxy-terminal peptide (β-CTX) and anti-tartrate acid
phosphatase 5b (TRACP-5b; cat. nos. ml003415, ml038224, ml102832,
ml002856, ml058528, ml003177 and ml038228, respectively; all
Shanghai Enzyme-linked Biotechnology Co., Ltd.) as previously
described (21).
Visceral index
The visceral index was calculated to evaluate the
safety of Osteoking as previously described (27). Briefly, the weight fractions of
the thymus, spleen, liver and kidney relative to the weight of the
brain were calculated.
Data collection and network analysis
Bone fracture-related genes and the Osteoking
targets were identified by transcriptome expression profiling
sequencing and genome-wide expression profiling microarray assay
based on whole blood samples and the affected right tibia tissue of
bone defect rats as previously described (21). Clinical transcriptome microarray
data based on affected bone tissue of patients with fracture were
retrieved from Gene Expression Omnibus (accession no. GSE494,
ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE494).
The differentially expressed genes between fracture and healthy
control groups were screened using the criteria of P<0.05 and
|log2 fold-change|>1. Putative targets of Osteoking
were obtained from ETCM 2.0 database (tcmip.cn/ETCM2/front/#/)
(28) using quantitative
estimate of drug-likeness ≥0.49 and Food and Drug Administration
recommended maximum daily dose <0.7.
Disease-related gene-drug putative target
interaction network was constructed using links between bone
fracture-related genes and Osteoking effective targets collected
from the STRING database (version 12.0; string-db.org/). The topological features of each node
were calculated using CytoHubba plug-in of Cytoscape (version
3.8.2; cytoscape.org), and nodes ranked in the
top 50 for betweenness, closeness and degree were defined as hub
genes. Furthermore, the key network targets were defined as hub
genes with the shortest path value of 1, which is calculated by
Pesca (version 3.0.8) (29) to
evaluate the network association between Osteoking targets and bone
fracture-associated genes. The biological functions of the key
network targets were determined by pathway enrichment analysis
based on the DAVID database (version 2024q2; david.ncifcrf.gov/home.jsp). Finally, the molecular
signaling axis was identified through a literature review on PubMed
(pubmed.ncbi.nlm.nih.gov). The molecular
names were used as the search keywords, and the search results were
limited to literature published within the past 10 years.
Immunohistochemistry and
immunofluorescence assessment
The expression levels of proteins were detected
using immunohistochemistry: the receptor activator of nuclear
factor κB ligand (RANKL), osteoprotegerin (OPG), runt-related
transcription factor 2 (RUNX2), peroxisome proliferator-activated
receptor γ (PPARγ), platelet endothelial cell adhesion molecule
(CD31), and the expression levels of the following proteins were
detected using immunofluorescence: Vascular endothelial growth
factor A (VEGFA) and osterix (OSX) as previously described
(Table SI) (21,26,30). DAPI was used as a fluorescent dye
for labeling and observation of cell nuclei.
Western blotting
The expression levels of proteins in the affected
right tibia tissues of rats, including epidermal growth factor
(EGF), EGF receptor (EGFR), histone deacetylase 1 (HDAC1), Wnt5a,
Catenin Beta 1 (CTNNB1) and β-actin were detected using western
blotting as previously described (Table SI) (31,32).
Biochemical testing
Serum levels of triglyceride (TG), total cholesterol
(TC), low-density lipoprotein cholesterol (LDL-C) and high-density
lipoprotein cholesterol (HDL-C) were detected using a BC-5800 CBC
hematology analyzer (Mindray) according to the manufacturer's
instructions.
Statistical analysis
Statistical differences were analyzed using GraphPad
Prism software (version 8.0.2; Dotmatics). All experiments were
repeated at least three times. Data are presented as the mean ± SD.
For comparisons between multiple groups, one-way ANOVA was
employed, while for comparisons of data between groups at different
time points, repeated measures ANOVA was utilized. ANOVA was
followed by Dunnett's or Bonferroni's post hoc test. Pearson
correlation analysis was used to assess correlation between
indicators. P<0.05 was considered to indicate a statistically
significant difference.
Results
Osteoking promotes recovery of muscle
strength and weight-bearing function of the affected limb in bone
fracture rats
A rat tibial bone fracture model was successfully
established (Fig. 1A). Fracture
rats showed dark hair, poor mental state, lameness, decreased
activity and food intake, slow weight gain, significant swelling of
the affected limb and a significant decrease in mechanical pain
threshold during the first postoperative week (Fig. 1B-D), which decreased in the
second week and disappeared in the third week. Osteoking
effectively accelerated recovery of the general state of the rats
but exerted no significant effects on the improvement of the
swelling degree and pain intensity of the affected limbs in bone
fracture rats (Fig. 1B-D).
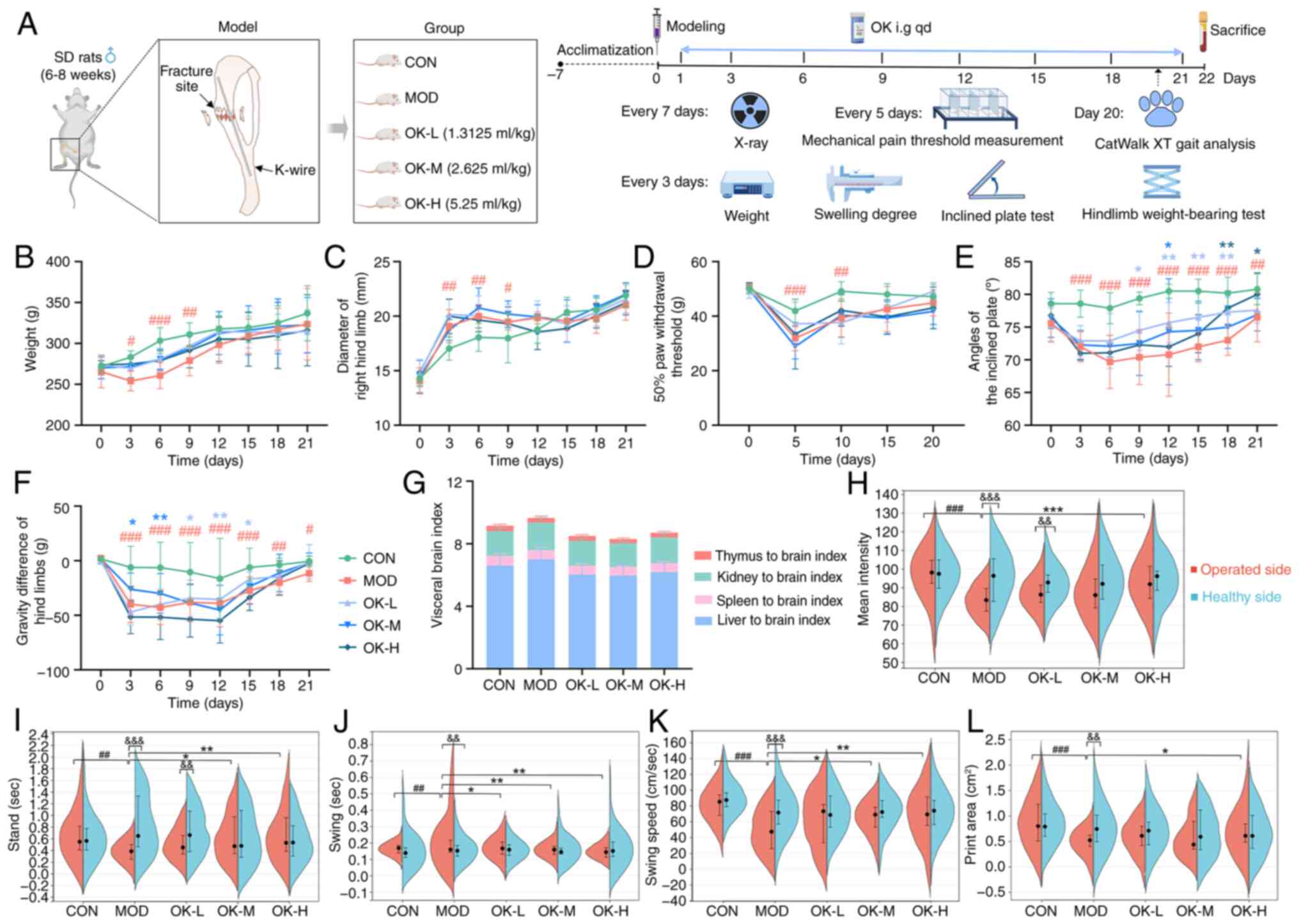 | Figure 1OK promotes recovery of muscle
strength and weight-bearing function of affected limbs in bone
fracture rats. (A) Experimental process and timeline. (B) Body
weight. (C) Right hindlimb diameter. (D) 50% paw withdrawal
threshold. (E) Inclined plate angle. (F) Gravity difference of hind
limbs after fracture modeling. (G) Visceral brain index. CatWalk XT
gait parameters including (H) mean intensity, (I) stand, (J) swing,
(K) swing speed and (L) print area. #P<0.05,
##P<0.01 and ###P<0.001 vs. CON;
*P<0.05, **P<0.01 and
***P<0.001 vs. MOD; &&P<0.01
and &&&P<0.001 vs. healthy side. SD,
Sprague-Dawley; K-wire, Kirschner wire; CON, control; MOD, model;
OK, Osteoking; L, low; M, medium; H, high. |
In addition, the muscle strength and static
weight-bearing capacity of the affected limbs of rats in the
fracture model group were significantly decreased and improved by
Osteoking (Fig. 1E and F).
Compared with the control group, the stand time of the affected
limb in the fracture model group was significantly shortened, the
swing time was significantly prolonged and the swing speed, area
and mean intensity of print were all significantly reduced
(Fig. 1H-L). Moreover, there
were no significant differences in the visceral brain index of the
rats, suggesting that Osteoking may have good safety (Fig. 1G).
Osteoking accelerates bone healing in
bone fracture rats
According to X-ray imaging on days 7, 14 and 21,
healing speed of bone fracture rats was faster in all Osteoking
treatment than in the model group. Notably, the high-dose group had
a blurred fracture line with bridging callus formation visible
around the fracture break on day 14, which was defined as
radiographic healing, and the fracture line disappeared and the
bridging scab was resorbed on day 21, which was defined as healed
(Fig. 2A). The high-dose group
displayed the highest scores on days 7, 14, and 21 (Fig. 2B).
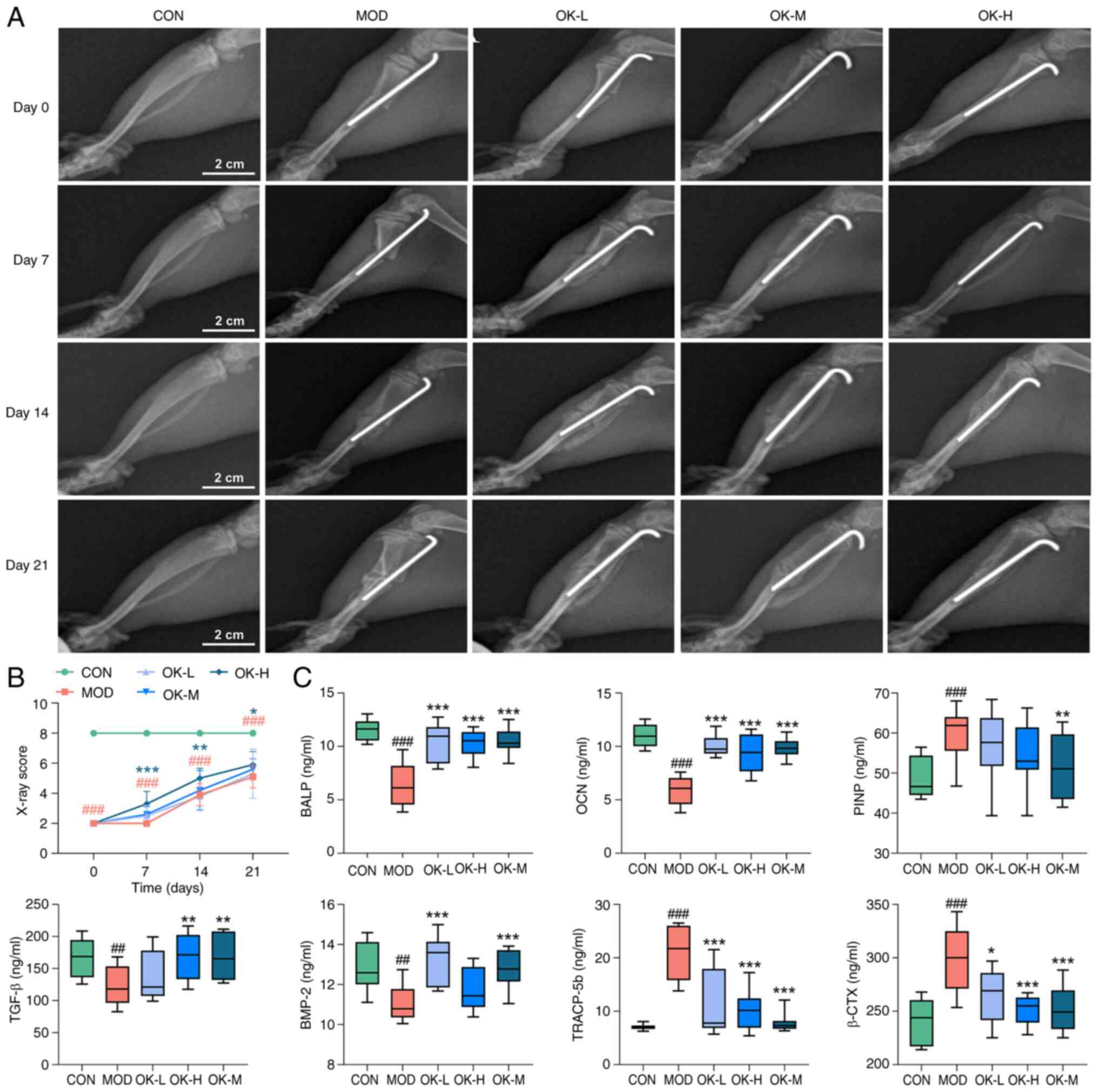 | Figure 2OK accelerates bone healing in bone
fracture rats. (A) X-ray images and (B) scoring (scale bar, 2 cm).
(C) Serum levels of bone growth factors and turnover markers.
##P<0.01 and ###P<0.001 vs. CON;
*P<0.05, **P<0.01 and
***P<0.001 vs. MOD. CON, control; MOD, model; OK,
Osteoking; L, low; M, medium; H, high; BALP, bone specific alkaline
phosphatase; OCN, osteocalcin; PINP, type I procollagen N-terminal
peptide; BMP-2, bone morphogenetic protein-2; TRACP-5b,
anti-tartrate acid phosphatase 5b; β-CTX, type I collagen carboxy
terminal peptide β special sequence. |
Osteoking significantly elevated serum levels of
BMP-2, TGF-β, BALP and OCN in fracture rats, suggesting its
potential to promote bone formation. There were increased levels of
PINP, TRACP-5b and β-CTX in serum obtained from the bone fracture
rats, which were all decreased by the treatment of Osteoking,
suggesting its potential to inhibit bone resorption (Fig. 2C).
Osteoking improves trabecular
microarchitecture and promotes the cortical bone remodeling and
mechanical recovery of the affected limbs in bone fracture
rats
At day 22, stiffness, maximum fracture energy and
maximum load of the callus in bone fracture rats were significantly
decreased comparing with control, and were effectively improved by
Osteoking at medium and high dosages (Fig. 3A and B), suggesting its potential
to promote the recovery of the mechanical properties of the
affected limbs of bone fracture rats.
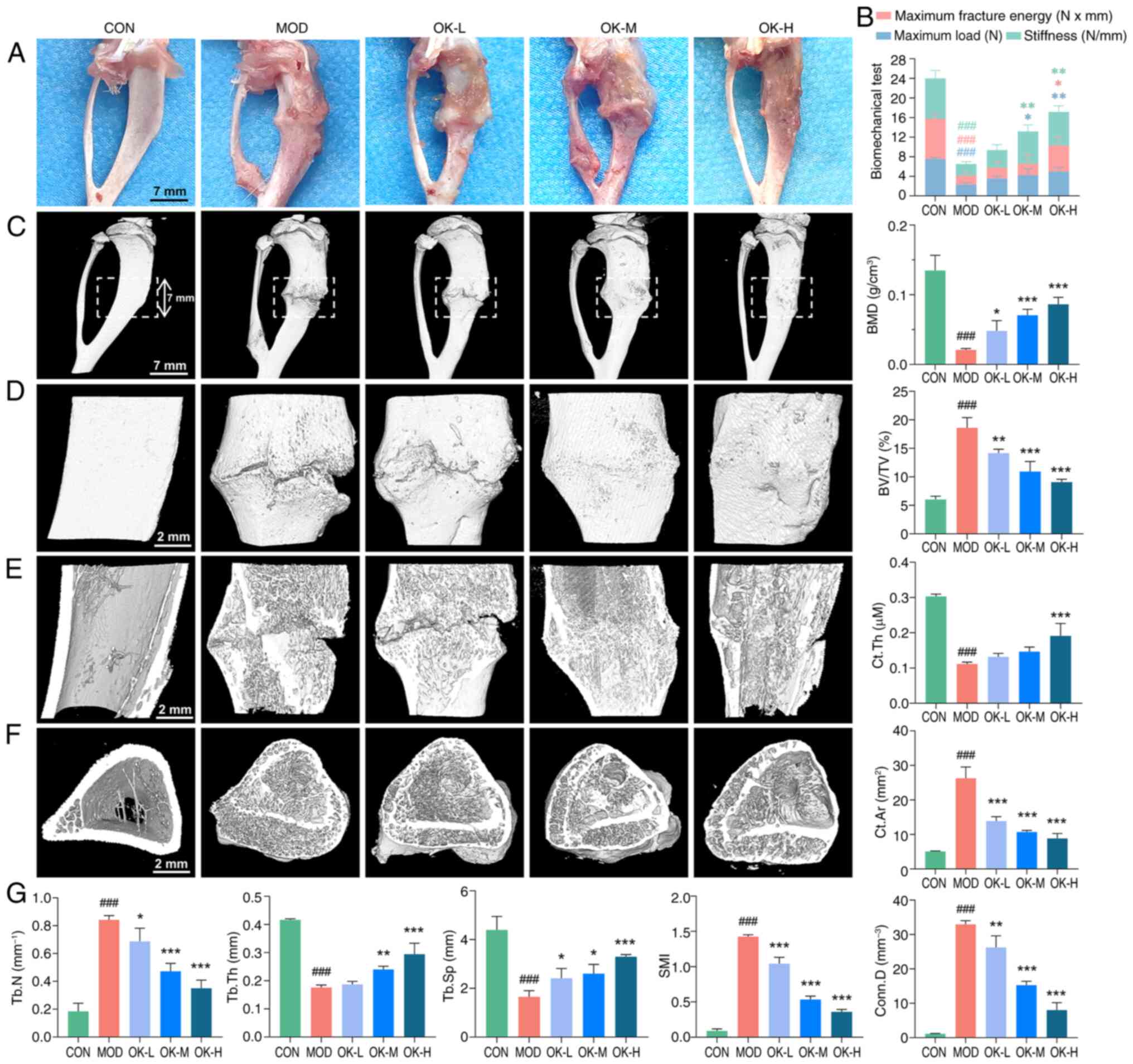 | Figure 3OK improves trabecular
microarchitecture and promotes cortical bone remodeling and
mechanical recovery of affected limbs in bone fracture rats. (A)
Anatomical appearance of the right tibia 3 weeks after the drug
administration (scale bar, 7 mm). (B) Biomechanical test results at
the callus site. Micro-CT three-dimensional reconstruction image of
fracture site including (C) overall surface (scale bar, 7 mm), (D)
local surface (scale bar, 2 mm), (E) local coronal plane (scale
bar, 2 mm), (F) cross-section of tibial marrow cavity (scale bar, 2
mm) and (G) quantitative analysis of bone morphometric parameters.
###P<0.001 vs. CON; *P<0.05,
**P<0.01 and ***P<0.001 vs. MOD. CON,
control; MOD, model; OK, Osteoking; L, low; M, medium; H, high;
BMD, bone mineral density; BV/TV, bone volume/tissue volume ratio;
Ct.Th, cortical bone thickness; Ct.Ar, cortical bone area; Conn.D,
connectivity density; SMI, structure model index; Tb.Sp, trabecular
separation; Tb.Th, trabecular thickness; Tb.N, trabecular
number. |
Micro-CT scanning showed a large fracture gap,
discontinuity of cortical bone, little extracellular callus
formation and abnormal, dense, short and disorganized trabecular
structure in the model group. Following the treatment with
Osteoking, the fracture gap filled with trabeculae, volume of
callus enlarged and connected the cortical bone on both sides of
the fracture and the trabeculae in the medullary cavity were
thickened and fused to the cortical bone. Notably, the callus
tissue in the high-dose group was resorbed and similar to the
morphology and structure of control rats (Fig. 3C-F).
Among bone morphometric parameters based on the
Micro-CT scanning data, BMD, Tb.Th Tb.Sp and Ct.Th were
significantly decreased, while BV/TV, SMI, Tb.N, Ct.Ar and Conn.D
were significantly increased in the model group, which were all
reversed by Osteoking in a dose-dependent manner (Fig. 3G), suggesting its potentials to
improve the microstructure of bone trabeculae and promote
remodeling of callus tissue and cortical bone.
Osteoking promotes endochondral
ossification and callus resorption at the fracture site
H&E staining showed a large area of
fibrocartilaginous callus tissue in the model group, and a small
amount of neovascular tissue in the low-dose group, which invaded
into the internal cartilaginous callus tissue. In medium- and
high-dose groups, extensive remodeling of vascular tissue and the
formation of bony healing tissues were observed; area of bony
tissues was enlarged in a dose-dependent manner (Fig. 4A and B).
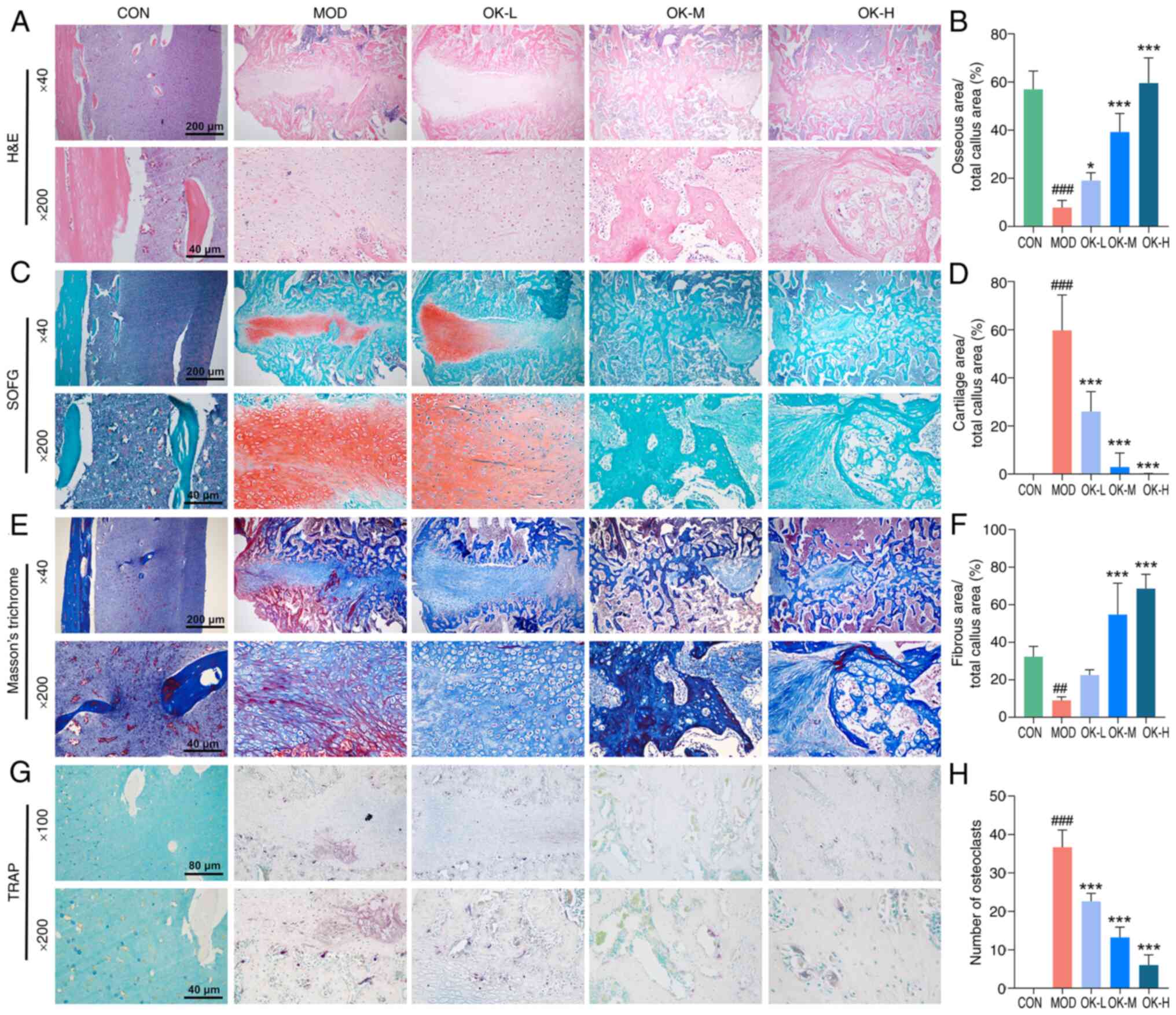 | Figure 4OK promotes endochondral ossification
and callus resorption at the fracture site. (A) Representative
H&E staining and (B) quantitative analysis. (C) Representative
SOFG staining and (D) quantitative analysis. (E) Representative
Masson's Trichrome staining and (F) quantitative analysis.
Magnification, ×40 and ×200; scale bar, 200 and 40 μm. (G)
Representative TRAP staining of osteoclasts and (H) quantitative
analysis. Magnification, ×100 and ×200; scale bar, 80 and 40
μm. ##P<0.01 and ###P<0.001 vs.
CON; *P<0.05 and ***P<0.001 vs. MOD.
CON, control; MOD, model; OK, Osteoking; L, low; M, medium; H,
high; H&E, hematoxylin and eosin; SOFG, safranin O-fast green;
TRAP, tartrate resistant acid phosphatase. |
As chondrogenesis and endochondral ossification are
key steps in the healing process of long bone fractures (33), cartilage area at the fracture
site was compared on day 22 after bone fracture modeling. Control
group showed mature bone tissue; large areas of cartilage tissue
with a large number of neonatal chondrocytes were observed in the
model group (Fig. 4C and D). By
contrast, the area of cartilage tissue was decreased, a small
number of chondrocytes calcified and died, and cartilage matrix was
mineralized in the low-dose group; no cartilage tissue was observed
and mineralization of the bone matrix was increased in the medium-
and high-dose groups (Fig. 4C and
D). Masson's trichrome staining showed significantly increased
collagen fiber staining in the callus tissue of Osteoking treatment
groups (Fig. 4E and F).
To determine whether callus tissues were resorbed by
osteoclasts in the coupled remodeling phase, TRAP staining was
performed to characterize the number and location of osteoclasts.
Osteoclasts were primarily concentrated at the edge of callus
tissue and the number of osteoclasts was increased in the model
group, while number of osteoclasts was significantly decreased by
Osteoking in a dose-dependent manner (Fig. 4G and H).
Osteoking promotes fracture healing by
modulating the adipogenesis-angiogenesis-osteogenesis crosstalk
mediated by the EGF-EGFR-HDAC1-Wnt/β-catenin signaling axis
To investigate the pharmacological mechanisms of
Osteoking against bone fracture, a total of 500 disease-related
genes and 778 drug candidate targets were identified by
transcriptome expression profiling and bioinformatics database
analysis (Fig. 5A). Following
construction of the disease-related gene-drug effective target
interaction network, 31 hub genes with topological importance were
screened, among which 23 key network targets had the shortest path
value of 1, including five bone fracture-related genes and 17
Osteoking targets such as EGFR, HDAC1, and PPARG. Functionally,
these key network targets were significantly associated with
pathways involved in 'bone metabolism', 'angiogenesis', 'lipid
metabolism' and 'immune-inflammation'. Notably, EGFR had the most
interactions with other nodes, and the largest number of the key
network targets was enriched in Wnt signaling (Fig. 5B; Tables SII and SIII). Expression of
EGF, EGFR and HDAC1 mRNA in the model group increased compared with
control group, with a statistically significant difference in
HDAC1, whereas expression levels of Wnt5a and CTNNB1 mRNA showed a
tendency to decrease, with a significant difference in CTNNB1;
these changes were also reversed by Osteoking (Fig. 5C). It was hypothesized that
Osteoking may promote fracture healing through modulating
adipogenesis-angiogenesis-osteogenesis crosstalk mediated by the
EGF-EGFR-HDAC1-Wnt/β-catenin signaling axis (Fig. 5D). Osteoking significantly
decreased expression of EGF, phosphorylated (p-)EGFR and HDAC1 and
enhanced the expression of Wnt5 and β-catenin proteins in the
affected bone fracture tissue (Fig.
6A and B). Immunofluorescence double-labeling results indicated
that Osteoking dose-dependently increased the VEGFA and OSX
expression levels and the positive expression regions of the two
proteins overlapped (Fig. 6C and
D). Immunohistochemical staining showed that medium- and
high-dose Osteoking significantly elevated the expression of CD31
protein in the callus tissues of bone fracture rats (Fig. 7A and B). Osteoking significantly
decreased the serum levels of TC, LDL-C, and HDL-C, which were all
abnormally elevated in the bone fracture rats, but did not
significantly ameliorate abnormal serum levels of TG (Fig. 7C). Expression of VEGFA
(angiogenesis marker) and OSX (osteogenesis marker) were
significantly negatively correlated with the levels of TC and LDL-C
(both adipogenesis markers; Fig. 7D
and E).
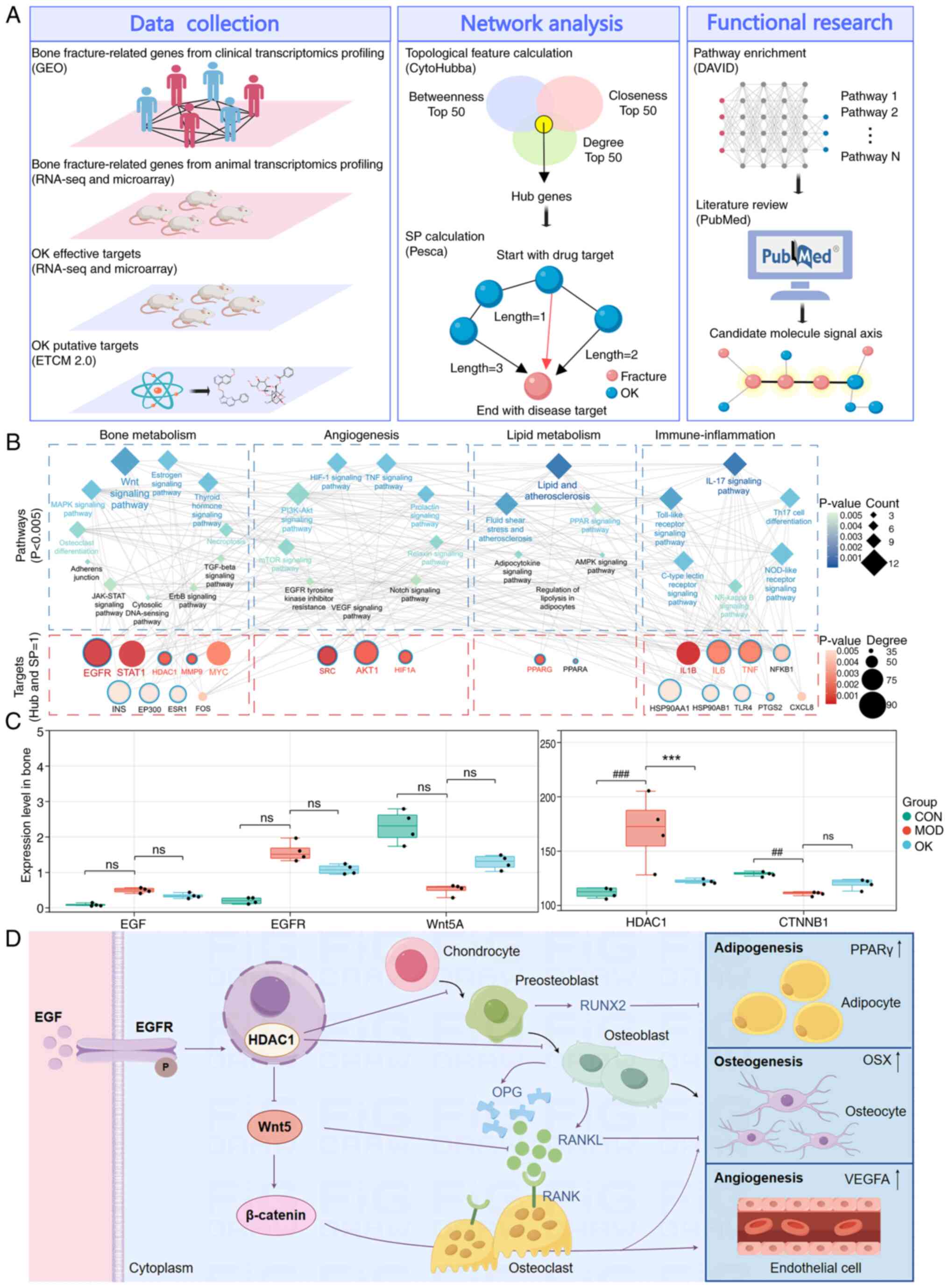 | Figure 5OK promotes fracture healing through
modulating the adipogenesis-angiogenesis-osteogenesis crosstalk
mediated by the EGF-EGFR-HDAC1-Wnt/β-catenin signaling axis. (A)
Flowchart of network analysis. (B) Key network targets and
pathways. Blue border, OK targets. (C) mRNA expression of key
network targets involved in the EGF-EGFR-HDAC1-Wnt/β-catenin
signaling axis based on transcriptome profiling of affected bone
fracture tissues. (D) OK may promote fracture healing through
modulating adipogenesis-angiogenesis-osteogenesis crosstalk
mediated by the EGF-EGFR-HDAC1-Wnt/β-catenin signaling axis.
##P<0.01 and ###P<0.001 vs. CON;
***P<0.001 vs. MOD. CON, control; MOD, model; OK,
Osteoking; L, low; M, medium; H, high; GEO, Gene Expression
Omnibus; seq, sequencing; SP, shortest path; EGFR, epidermal growth
factor receptor; HDAC1, histone deacetylase 1; CTNNB1, catenin β1;
ns, no significance; OPG, osteoprotegerin; RANKL, receptor
activator of nuclear factor κB ligand; OSX, Osterix; VEGFA,
vascular endothelial growth factor A. |
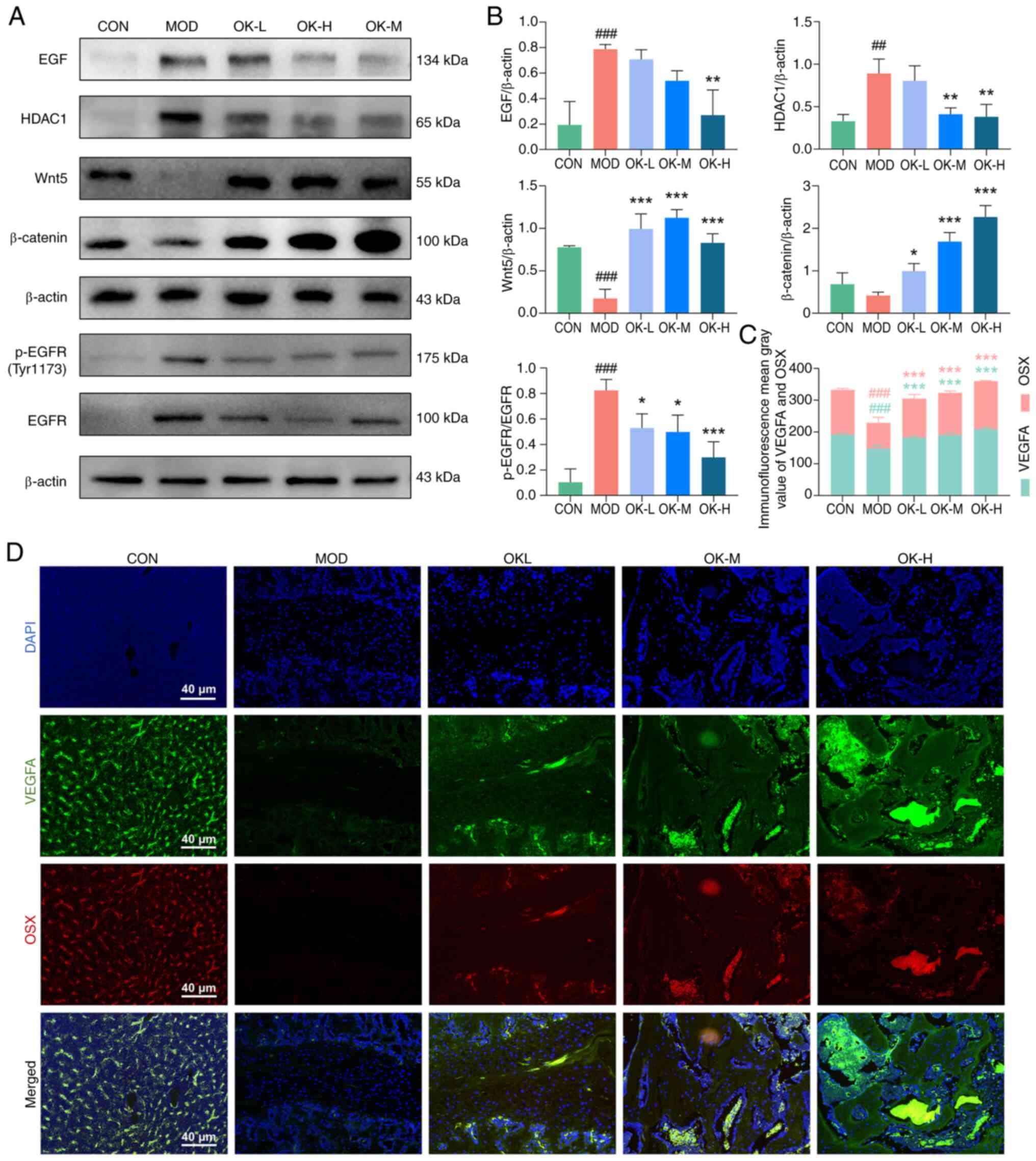 | Figure 6OK modulates the disrupted
EGF-EGFR-HDAC1-Wnt/β-catenin axis and promotes coupling of vascular
and bone tissues during bone fracture healing. (A) Western blot
detection of EGF, p-EGFR, HDAC1, Wnt5 and β-catenin protein
expression and (B) quantitative analyses. (C) VEGFA and OSX
expression detected by (D) immunofluorescence staining (scale bar,
50 μm). ##P<0.01 and ###P<0.001
vs. CON; *P<0.05, **P<0.01 and
***P<0.001 vs. MOD. CON, control; MOD, model; OK,
Osteoking; L, low; M, medium; H, high; EGFR, epidermal growth
factor receptor; HDAC1, histone deacetylase 1; p-, phosphorylated;
VEGFA, vascular endothelial growth factor A; OSX, Osterix. |
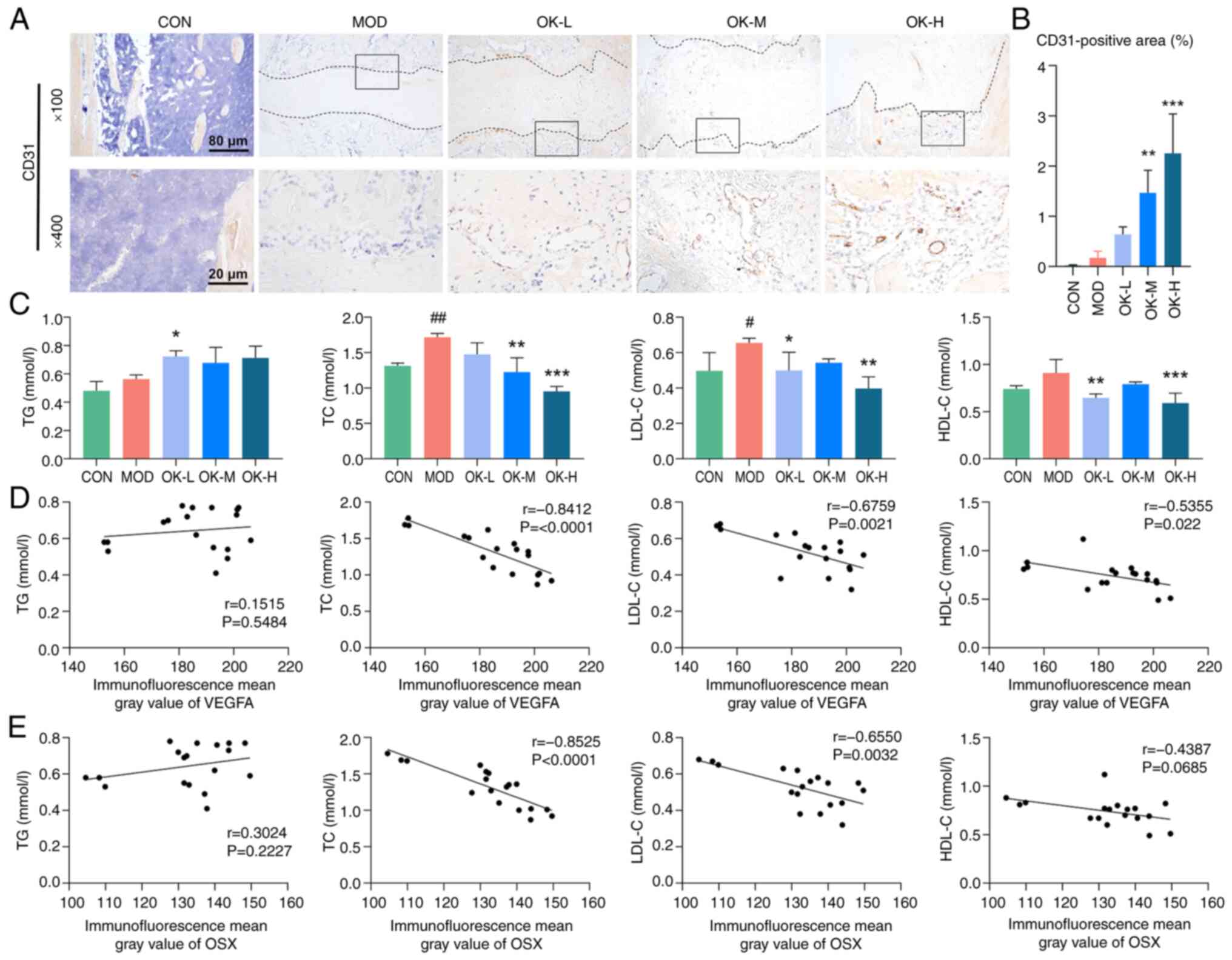 | Figure 7OK promotes angiogenesis and
decreases adipogenesis of bone fracture rats. (A) Representative
immunohistochemical staining of CD31 and (B) quantitative analysis.
Magnification, ×100 and ×400; scale bar, 200 μm and 50
μm; (C) Serum levels of lipid and lipoprotein. Correlation
analysis of (D) VEGFA and (E) OSX expression with lipid and
lipoprotein levels. #P<0.05, ##P<0.01
vs. CON. *P<0.05, **P<0.01 and
***P<0.001 vs. MOD. CON, control; MOD, model; OK,
Osteoking; L, low; M, medium; H, high; TG, triglyceride; TC, total
cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C,
high-density lipoprotein cholesterol; VEGFA, vascular endothelial
growth factor A; OSX, Osterix. |
Osteoking significantly enhanced the expression of
RUNX2 protein, reduced the expression of PPARγ protein and
increased the RUNX2/PPARγ ratio in a dose-dependent manner
(Fig. 8A and B). Osteoking
effectively upregulated OPG and decreased RANKL protein expression
and the ratio of RANKL/OPG in a dose-dependent manner (Fig. 8C and D).
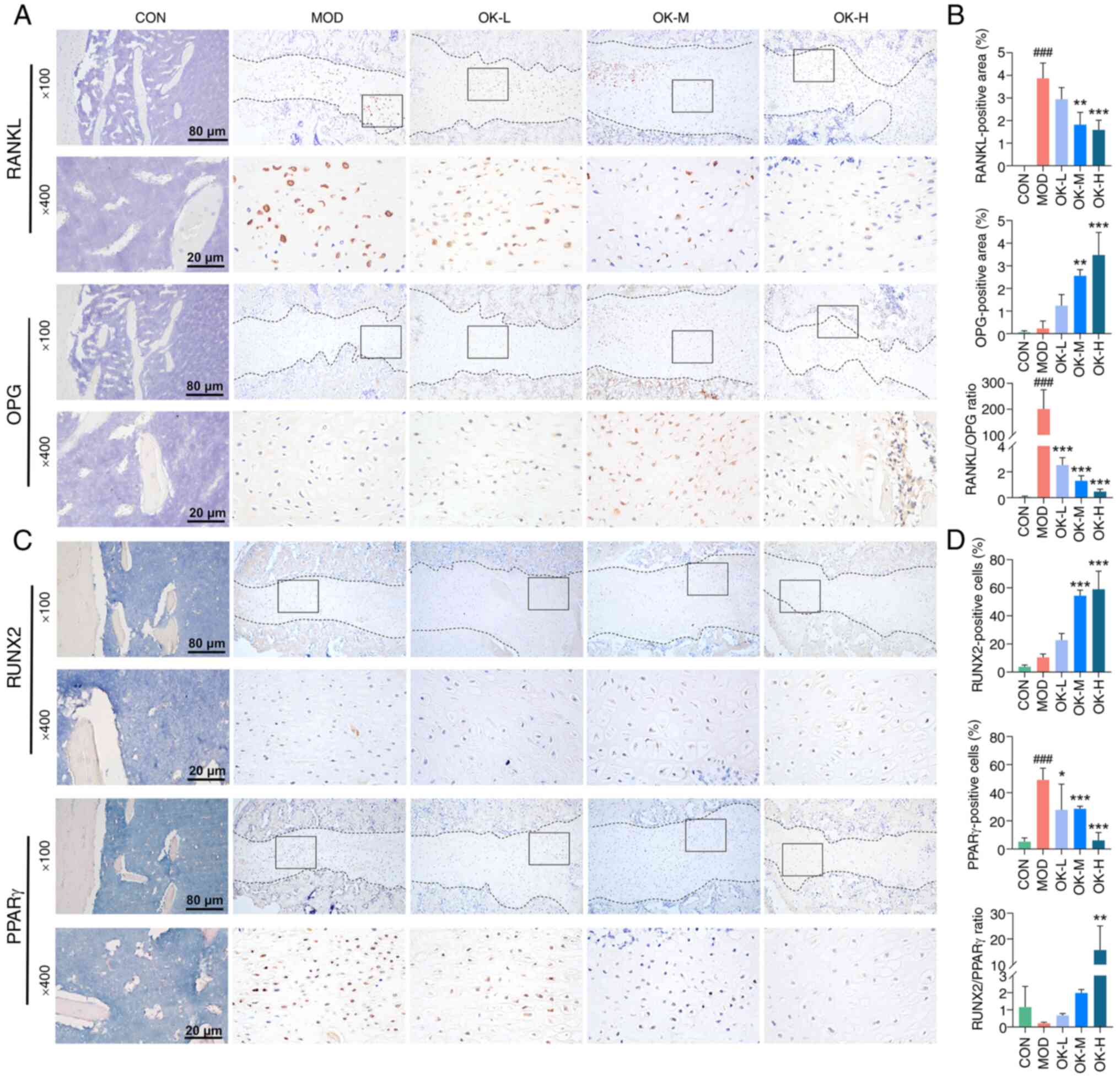 | Figure 8OK inhibits osteoclast and adipocyte
differentiation and promotes osteoblast differentiation of bone
fracture rats. (A) Representative immunohistochemical staining of
RANKL and OPG protein and (B) quantitative analysis. (C)
Representative immunohistochemical staining of RUNX2 and PPARγ
proteins and (D) quantitative analysis. Magnification, ×100 and
×400; scale bar, 200 and 50 μm. ####P<0.001
vs. CON; *P<0.05, **P<0.01 and
***P<0.001 vs. MOD. CON, control; MOD, model; OK,
Osteoking; L, low; M, medium; H, high; RANKL, receptor activator of
nuclear factor κB ligand; OPG, osteoprotegerin. |
Discussion
Growing evidence has shown clinical efficacy of
Osteoking in promoting fracture healing (15,34-36). To determine the promotive
mechanism of Osteoking in bone fractures in terms of
adipogenesis-angiogenesis-osteogenesis crosstalk, the present study
evaluated the therapeutic effects of this Chinese patent drug based
on a rat model of tibial bone fracture through radiological
examination and behavioral experiments. Mechanically,
transcriptomics profiling, network pharmacology and in vivo
experiments demonstrated that the EGF-EGFR-HDAC1-Wnt/β-catenin axis
was a candidate target of Osteoking to promote bone fracture
healing. To the best of our knowledge, the present study is the
first to clarify the pharmacological characteristics of Osteoking
and the underlying molecular mechanisms against bone fracture.
These findings not only provide a theoretical basis for clinical
application of Osteoking in treatment of bone fracture but may also
facilitate development of other effective treatment strategies.
Bone formation and resorption, angiogenesis and
adipogenesis are all essential biological events during fracture
healing (37). Bone is a highly
vascularized tissue and bone formation is associated with blood
vessel growth (38). Vascular
and bone tissue coordinate regeneration and coupling via expression
of common transcriptional and morphogenetic factors during fracture
healing (39,40). Thus, the successful bone
regeneration depends on the ratio and spatial expression of
pro-osteogenic and pro-angiogenic growth factors in callus tissue
(41). VEGFA and OSX are the key
regulator of angiogenesis and specific transcription factor for
bone formation, respectively (42,43). Here, Osteoking effectively
promoted regeneration and coupling of vascular and bone tissue at
the fracture site. Disturbed lipid metabolism affects bone
microenvironmental homeostasis via cross-organ communication,
promotes differentiation of MSCs to adipocytes and osteoclasts and
inhibits commitment to osteogenic lineages, leading to delayed
fracture healing (44). Lipids
impair the bone remodeling process (45). Elevated levels of TG, TC, LDL-C
and HDL-C are associated with increased fracture risk (46-49) and the underlying mechanisms may
be associated with inhibition of osteoblast differentiation leading
to decreased bone formation and inhibition of VEGF-induced
neovascularization (48,50). Accordingly, Osteoking exerted an
inhibitory effect on adipogenesis, which was negatively correlated
with angiogenesis and osteogenesis in bone fracture rats. Notably,
serum levels of TG were elevated in Osteoking groups but not the
model group, implying no association between serum TG levels and
bone fracture severity, consistent with previous reports (51,52); the reasons for this need to be
further explored.
The differentiation of mesenchymal precursors into
vascular, adipocyte, chondrocyte, osteoclast and osteoblast
lineages are controlled by transcription factors, each of which
contributes to the osteogenic response, and combinations of
transcription factors stimulate bone regeneration and repair and
accelerate fracture healing (53). PPARγ is a key regulator and mid-
to late-stage marker of adipogenesis (54), and RUNX2 regulates osteogenesis
(42). The ratio of RUNX2/PPARγ
determines the lipogenic or osteogenic differentiation of bone
marrow mesenchymal stem cells (55). In addition, RANKL is a key
cytokine for osteoclast formation and activation, and its action
can be blocked by OPG, a key transcription factor in late stages of
bone formation and inhibitor of osteoclast differentiation
(56). Thus, the ratio of
RANKL/OPG determines function of osteoclasts (57). Here, immunohistochemistry
demonstrated that Osteoking notably inhibited the adipocyte and
osteoclast differentiation by decreasing expression levels of
PPARγ, RUNX2, RANKL and OPG proteins, as well as their ratios,
suggesting its potential in promoting osteoblast
differentiation.
EGF inhibits osteoblast differentiation and bone
formation by suppressing expression of the osteoblast-specific
transcription factors Runx2 and OSX, as well as the late osteogenic
hallmark gene OPG, in an EGFR-dependent manner (58). The activation of EGFR signaling
leads to decreased bone formation and enhanced bone resorption
(59), promotes osteoclast
differentiation and survival and stimulates bone resorption by
binding RANK and activating RANKL signaling (60). HDAC1 is a typical human histone
deacetylase involved in regulation of bone homeostasis by
interacting with transcription factors controlling gene expression
at specific stages of osteogenesis, angiogenesis and adipogenesis,
and is a key bioactive factor in the fracture healing process
(61,62). As a co-suppressor interacting
with Runx2, HDAC1 decreases transcriptional activity of Runx2
during osteoblast maturation by binding Runx2, thereby inhibiting
osteoblast differentiation and suppressing bone formation (62). HDAC1 is a co-regulator of PPARγ
transcriptional activation, which supports transcriptional
regulation of PPARγ via histone modification, thereby promoting
adipogenesis (61). More
importantly, the roles of Wnt/β-catenin signaling in osteogenesis,
angiogenesis and adipogenesis have been widely reported and its
interactions with various signaling molecules have been well
documented as a potential gene regulatory network coordinating
osteoblast differentiation and bone development (63-68). OPG is a direct target of
β-catenin transcriptional activation and Wnt/β-catenin signaling
activation promotes bone formation by inducing OPG expression,
which may be involved in promoting osteoblastogenesis and
osteoclast function, thereby promoting bone formation (64). Wnt/β-catenin signaling negatively
regulates osteoclast differentiation and decreases bone resorption
(65). In addition,
Wnt/β-catenin signaling inhibits adipogenesis by maintaining
preadipocytes in an undifferentiated state via inhibition of PPARγ
(66). Wnt-5a can induce
osteoblast differentiation by inducing the expression of Runx2, a
key osteogenic transcription factor, while Wnt-5a is also a
transcriptional co-repressor of PPARγ, which can inhibit adipocyte
differentiation by suppressing activation of the promoter of
endogenous PPARγ target genes (67). Wnt is a potent angiogenic factor,
while VEGF is one of the target genes of Wnt; activation of
Wnt/β-catenin signaling promotes transcriptional regulation of VEGF
and angiogenesis (68).
Following bone fracture, a local ischemic hypoxic
microenvironment forms at the end of the fracture, which stimulates
the expression of EGF by releasing mature soluble EGF-like domains
that bind to EGFR. Following autophosphorylation of EGFR, an
intracellular signaling cascade is trigged and nuclear activity of
HDAC1 is activated, leading to downregulation of β-catenin and the
Wnt ligand dickkopf-related protein 1. Inhibition of Wnt activity
leads to increased expression of PPARγ, which induces osteoblasts
to differentiate into adipocytes, and decreased expression of VEGF,
which inhibits angiogenesis. Moreover, inhibition of β-catenin
reduces OPG expression, elevates RANKL expression and enhances
osteoclast activity. Accordingly, dysregulation of Wnt/β-catenin
signaling may promote the adipogenesis and bone resorption and
inhibit the angiogenesis and osteogenesis, leading to the
disturbance of adipogenesis-angiogenesis-osteogenesis axis during
the occurrence and development of bone fracture, which may be
reversed by Osteoking. Consistently, the present data confirmed the
regulatory effects of Osteoking on the EGF-EGFR-HDAC1-Wnt/β-catenin
signaling axis in bone fracture tissues via inhibiting the
expression of EGF, p-EGFR and HDAC1 proteins and enhancing the
expression of Wnt5 and β-catenin protein.
In conclusion, Osteoking may reverse the disturbance
of adipogenesis-angiogenesis-osteogenesis homeostasis caused by
bone fractures and promote fracture healing by regulating the
EGF-EGFR-HDAC1-Wnt/β-catenin axis, paving the way for clinical
application of Osteoking and development of potential therapeutic
agents for treating bone fractures.
Supplementary Data
Availability of data and materials
The data generated in the present study are not
publicly available due to ongoing patent applications but may be
requested from the corresponding author.
Authors' contributions
SZ performed experiments, analyzed data and wrote
the manuscript. LC and CZ performed experiments and analyzed data.
CZ, CG, XH, HZ, ZC and WC performed experiments. CL, NL and YZ
analyzed and interpretated of data. YZ and NL conceived and design
of the study. SZ and YZ confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by Experimental
Animal Ethics Committee of Institute of Chinese Materia Medica,
China Academy of Chinese Medical Sciences, Beijing, China (approval
no. IBTCMCACMS21-2304-05).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by Scientific and Technological
Innovation Project of China Academy of Chinese Medical Sciences
(grant nos. CI2021A03808 and CI2023E001TS02), Sanming Project of
Medicine in Shenzhen (grant no. SZZYSM202311020), Fundamental
Research Funds for the Central Public Welfare Research Institutes
(grant no. ZXKT22025) and Innovation Team and Talents Cultivation
Program of National Administration of Traditional Chinese Medicine
(grant no. ZYYCXTD-C-202002).
References
|
1
|
GBD 2019 Fracture Collaborators: Global,
regional, and national burden of bone fractures in 204 countries
and territories, 1990-2019: A systematic analysis from the global
burden of disease study 2019. Lancet Healthy Longev. 2:e580–e592.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zura R, Xiong Z, Einhorn T, Watson JT,
Ostrum RF, Prayson MJ, Della Rocca GJ, Mehta S, McKinley T, Wang Z
and Steen RG: Epidemiology of fracture nonunion in 18 human bones.
JAMA Surg. 151:e1627752016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ekegren CL, Edwards ER, de Steiger R and
Gabbe BJ: Incidence, costs and predictors of non-union, delayed
union and mal-union following long bone fracture. Int J Environ Res
Public Health. 15:28452018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hendrickx LAM, Virgin J, van den Bekerom
MPJ, Doornberg JN, Kerkhoffs GMMJ and Jaarsma RL: Complications and
subsequent surgery after intra-medullary nailing for tibial shaft
fractures: Review of 8110 patients. Injury. 51:1647–1654. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tian R, Zheng F, Zhao W, Zhang Y, Yuan J,
Zhang B and Li L: Prevalence and influencing factors of nonunion in
patients with tibial fracture: Systematic review and meta-analysis.
J Orthop Surg Res. 15:3772020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Antonova E, Le TK, Burge R and Mershon J:
Tibia shaft fractures: Costly burden of nonunions. BMC
Musculoskelet Disord. 14:422013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davis S, Martyn-St James M, Sanderson J,
Stevens J, Goka E, Rawdin A, Sadler S, Wong R, Campbell F,
Stevenson M, et al: A systematic review and economic evaluation of
bisphosphonates for the prevention of fragility fractures. Health
Technol Assess. 20:1–406. 2016. View
Article : Google Scholar :
|
|
8
|
Hegde V, Jo JE, Andreopoulou P and Lane
JM: Effect of osteoporosis medications on fracture healing.
Osteoporos Int. 27:861–871. 2016. View Article : Google Scholar
|
|
9
|
Davis S, Simpson E, Hamilton J, James MMS,
Rawdin A, Wong R, Goka E, Gittoes N and Selby P: Denosumab,
raloxifene, romosozumab and teriparatide to prevent osteoporotic
fragility fractures: A systematic review and economic evaluation.
Health Technol Assess. 24:1–314. 2020. View
Article : Google Scholar
|
|
10
|
Petrova NL, Donaldson NK, Bates M, Tang W,
Jemmott T, Morris V, Dew T, Meacock L, Elias DA, Moniz CF and
Edmonds ME: Effect of recombinant human parathyroid hormone (1-84)
on resolution of active charcot neuro-osteoarthropathy in diabetes:
A randomized, double-blind, placebo-controlled study. Diabetes
Care. 44:1613–1621. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aro HT, Govender S, Patel AD, Hernigou P,
de Gregorio AP, Popescu GI, Golden JD, Christensen J and Valentin
A: Recombinant human bone morphogenetic protein-2: A randomized
trial in open tibial fractures treated with reamed nail fixation. J
Bone Joint Surg Am. 93:801–808. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ding W, Ji R, Yao S, Ruan P, Ye F, Lou X,
Ni L, Ji Y, Chen J and Ji W: Hu'po Anshen decoction promotes
fracture healing in mice with traumatic brain injury through
BMP2-COX2-ATF4 signaling pathway. FASEB J. 37:e229522023.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Z, Li Y, Liu C, Gu Y and Han G:
Research progress of the mechanisms and applications of
ginsenosides in promoting bone formation. Phytomedicine.
129:1556042024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qian D, Zhang Q, He CX, Guo J, Huang XT,
Zhao J, Zhang H, Xu C and Peng W: Hai-Honghua medicinal liquor is a
reliable remedy for fracture by promotion of osteogenic
differentiation via activation of PI3K/Akt pathway. J
Ethnopharmacol. 330:1182342024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, Kuang H, Zhang Z, Rong K, Yuan Y,
Peng Z, Zhao H, Liu K, Ou L and Kuang J: Efficacy and safety of
Osteoking on fracture healing: A systematic review and
meta-analysis. Front Pharmacol. 15:13634212024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie L, Song X, Lei L, Chen C, Zhao H, Hu
J, Yu Y, Bai X, Wu X, Li X, et al: Exploring the potential
mechanism of Heng-Gu-Gu-Shang-Yu-He-Ji therapy for osteoporosis
based on network pharmacology and transcriptomics. J
Ethnopharmacol. 321:1174802024. View Article : Google Scholar
|
|
17
|
Luo X, Liu J, Wang X, Chen Q, Lei Y, He Z,
Wang X, Ye Y, Na Q, Lao C, et al: Mechanism exploration of
Osteoking in the treatment of lumbar disc herniation based on
network pharmacology and molecular docking. J Orthop Surg Res.
19:882024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun Y, Chen R, Zhu D, Shen ZQ, Zhao HB and
Lee WH: Osteoking improves OP rat by enhancing HSP90-β expression.
Int J Mol Med. 45:1543–1553. 2020.PubMed/NCBI
|
|
19
|
Zhou J, Zheng Z, Luo Y, Dong Y, Yan Y,
Zhang Y, Tang K, Quan R, Lin J, Zhang K, et al: Clinical efficacy
of Osteoking in knee osteoarthritis therapy: A prospective,
multicenter, non-randomized controlled study in China. Front
Pharmacol. 15:13819362024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ling H, Zeng Q, Ge Q, Chen J, Yuan W, Xu
R, Shi Z, Xia H, Hu S, Jin H, et al: Osteoking decelerates
cartilage degeneration in dmm-induced osteoarthritic mice model
through TGF-β/smad-dependent manner. Front Pharmacol.
12:6788102021. View Article : Google Scholar
|
|
21
|
Zhang S, Liu Y, Ma Z, Gao S, Chen L, Zhong
H, Zhang C, Li T, Chen W, Zhang Y and Lin N: Osteoking promotes
bone formation and bone defect repair through
ZBP1-STAT1-PKR-MLKL-mediated necroptosis. Chin Med. 19:132024.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Z, Jin H, Zhou H, Yu L, Wan H and He
Y: Guhong Injection promotes fracture healing by activating
Wnt/beta-catenin signaling pathway in vivo and in vitro. Biomed
Pharmacother. 120:1094362019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alentado VJ, Knox AM, Staut CA, McGuire
AC, Chitwood JR, Mostardo SL, Shaikh MZ, Blosser RJ, Dadwal UC, Chu
TMG, et al: Validation of the modified radiographic union score for
tibia fractures (mRUST) in murine femoral fractures. Front
Endocrinol (Lausanne). 13:9110582022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu X, Mao X, Liu Y, Chen W, Li W, Lin N
and Zhang Y: Preclinical efficacy of TZG in myofascial pain
syndrome by impairing PI3K-RAC2 signaling-mediated neutrophil
extracellular traps. iScience. 26:1080742023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Garrick JM, Costa LG, Cole TB and
Marsillach J: Evaluating gait and locomotion in rodents with the
CatWalk. Curr Protoc. 1:e2202021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Wang X, Ding Z, Lin N and Zhang
Y: Enhanced efficacy with reduced toxicity of tripterygium
glycoside tablet by compatibility with total glucosides of paeony
for rheumatoid arthritis therapy. Biomed Pharmacother.
166:1154172023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li W, Wang K, Liu Y, Wu H, He Y, Li C,
Wang Q, Su X, Yan S, Su W, et al: A novel drug combination of
mangiferin and cinnamic acid alleviates rheumatoid arthritis by
inhibiting TLR4/NFκB/NLRP3 activation-induced pyroptosis. Front
Immunol. 13:9129332022. View Article : Google Scholar
|
|
28
|
Zhang Y, Li X, Shi Y, Chen T, Xu Z, Wang
P, Yu M, Chen W, Li B, Jing Z, et al: ETCM v2.0: An update with
comprehensive resource and rich annotations for traditional Chinese
medicine. Acta Pharm Sin B. 13:2559–2571. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scardoni G, Tosadori G, Pratap S, Spoto F
and Laudanna C: Finding the shortest path with PesCa: A tool for
network reconstruction. F1000Res. 4:4842015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma Z, Chen W, Liu Y, Yu L, Mao X, Guo X,
Jiang F, Guo Q, Lin N and Zhang Y: Artesunate Sensitizes human
hepatocellular carcinoma to sorafenib via exacerbating
AFAP1L2-SRC-FUNDC1 axis-dependent mitophagy. Autophagy. 20:541–556.
2024. View Article : Google Scholar :
|
|
31
|
Li W, Mao X, Wu H, Guo M, Su X, Lu J, Guo
Q, Li T, Wang X, Su W, et al: Deciphering the chemical profile and
pharmacological mechanisms of Baihu-Guizhi decoction using
ultra-fast liquid chromatography-quadrupole-time-of-flight tandem
mass spectrometry coupled with network pharmacology-based
investigation. Phytomedicine. 67:1531562020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mao X, Li W, Chen W, Li Y, Wang Q, Wang X,
Pi Z, Wang D, Xu H, Guo Q, et al: Exploring and characterizing a
novel combination of paeoniflorin and talatizidine for the
treatment of rheumatoid arthritis. Pharmacol Res. 153:1046582020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fu R, Liu C, Yan Y, Li Q and Huang RL:
Bone defect reconstruction via endochondral ossification: A
developmental engineering strategy. J Tissue Eng.
12:204173142110042112021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Min R: Analysis of therapeutic effect of
henggu gushang healing agent combined with PFNA on
intertrochanteric fracture of femur. J JIANGXI Univ CM. 35:43–46.
2023.
|
|
35
|
He P, Yue C, Chen J, Ma M, Yang G, Wang Q
and Liu Y: Observation on the curative effect of Henggu Gushangyu
Mixture in the treatment of femoral intertrochanteric fracture
after internal fixation. Fujian J TCM. 53:60–62. 2022.
|
|
36
|
Liu Z, Zhao X, Luo W and Wang L: The
effect of osteoking on postoperative fracture healing of femoral
neck fracture. Int J Trad Chin Med. 44:1122–1126. 2022.
|
|
37
|
Einhorn TA and Gerstenfeld LC: Fracture
healing: Mechanisms and interventions. Nat Rev Rheumatol. 11:45–54.
2015. View Article : Google Scholar :
|
|
38
|
Menger MM, Laschke MW, Nussler AK, Menger
MD and Histing T: The vascularization paradox of non-union
formation. Angiogenesis. 25:279–290. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bixel MG, Sivaraj KK, Timmen M,
Mohanakrishnan V, Aravamudhan A, Adams S, Koh BI, Jeong HW, Kruse
K, Stange R and Adams RH: Angiogenesis is uncoupled from
osteogenesis during calvarial bone regeneration. Nat Commun.
15:45752024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Maes C, Kobayashi T, Selig MK, Torrekens
S, Roth SI, Mackem S, Carmeliet G and Kronenberg HM: Osteoblast
precursors, but not mature osteoblasts, move into developing and
fractured bones along with invading blood vessels. Dev Cell.
19:329–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang C, Ness VP, Yang X, Chen H, Luo J,
Brown EB and Zhang X: Spatiotemporal analyses of osteogenesis and
angiogenesis via intravital imaging in cranial bone defect repair.
J Bone Miner Res. 30:1217–1230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu S, Chen W, Masson A and Li YP: Cell
signaling and transcriptional regulation of osteoblast lineage
commitment, differentiation, bone formation, and homeostasis. Cell
Discov. 10:712024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Burger MG, Grosso A, Briquez PS, Born GME,
Lunger A, Schrenk F, Todorov A, Sacchi V, Hubbell JA, Schaefer DJ,
et al: Robust coupling of angiogenesis and osteogenesis by
VEGF-decorated matrices for bone regeneration. Acta Biomater.
149:111–125. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rauch A, Haakonsson AK, Madsen JGS, Larsen
M, Forss I, Madsen MR, Van Hauwaert EL, Wiwie C, Jespersen NZ,
Tencerova M, et al: Osteogenesis depends on commissioning of a
network of stem cell transcription factors that act as repressors
of adipogenesis. Nat Genet. 51:716–727. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ambrosi TH, Scialdone A, Graja A, Gohlke
S, Jank AM, Bocian C, Woelk L, Fan H, Logan DW, Schürmann A, et al:
Adipocyte accumulation in the bone marrow during obesity and aging
impairs stem cell-based hematopoietic and bone regeneration. Cell
Stem Cell. 20:771–784.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Schubert J, Lindahl B, Melhus H, Renlund
H, Leosdottir M, Yari A, Ueda P, Jernberg T and Hagström E:
Elevated low-density lipoprotein cholesterol: An inverse marker of
morbidity and mortality in patients with myocardial infarction. J
Intern Med. 294:616–627. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Poiana C, Radoi V, Carsote M and
Bilezikian JP: New clues that may link osteoporosis to the
circulating lipid profile. Bone Res. 1:260–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mandal CC: High Cholesterol deteriorates
bone health: New insights into molecular mechanisms. Front
Endocrinol (Lausanne). 6:1652015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chang PY, Gold EB, Cauley JA, Johnson WO,
Karvonen-Gutierrez C, Jackson EA, Ruppert KM and Lee JS:
Triglyceride levels and fracture risk in midlife women: Study of
women's health across the nation (SWAN). J Clin Endocrinol Metab.
101:3297–3305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fang L, Choi SH, Baek JS, Liu C, Almazan
F, Ulrich F, Wiesner P, Taleb A, Deer E, Pattison J, et al: Control
of angiogenesis by AIBP-mediated cholesterol efflux. Nature.
498:118–122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen H, Shao Z, Gao Y, Yu X, Huang S and
Zeng P: Are blood lipids risk factors for fracture? Integrative
evidence from instrumental variable causal inference and mediation
analysis using genetic data. Bone. 131:1151742020.
|
|
52
|
Barzilay JI, Buzkova P, Kuller LH, Cauley
JA, Fink HA, Sheets K, Robbins JA, Carbone LD, Elam RE and Mukamal
KJ: The association of lipids and lipoproteins with hip fracture
risk: The cardiovascular health study. Am J Med. 135:1101–1108.e1.
2022.PubMed/NCBI
|
|
53
|
Baek WY and Kim JE: Transcriptional
regulation of bone formation. Front Biosci (Schol Ed). 3:126–135.
2011.PubMed/NCBI
|
|
54
|
Kim HY, Jang HJ, Muthamil S, Shin UC, Lyu
JH, Kim SW, Go Y, Park SH, Lee HG and Park JH: Novel insights into
regulators and functional modulators of adipogenesis. Biomed
Pharmacother. 177:1170732024.PubMed/NCBI
|
|
55
|
Huang X, Chen W, Gu C, Liu H, Hou M, Qin
W, Zhu X, Chen X, Liu T, Yang H and He F: Melatonin suppresses bone
marrow adiposity in ovariectomized rats by rescuing the imbalance
between osteogenesis and adipogenesis through SIRT1 activation. J
Orthop Translat. 38:84–97. 2022.PubMed/NCBI
|
|
56
|
Dong Y, Zhou H, Alhaskawi A, Wang Z, Lai
J, Abdullah Ezzi SH, Kota VG, Abdulla Hasan Abdulla MH, Sun Z and
Lu H: Alterations in bone fracture healing associated with TNFRSF
signaling pathways. Front Pharmacol. 13:9055352022.PubMed/NCBI
|
|
57
|
Yu G, Fu X, Gong A, Gu J, Zou H, Yuan Y,
Song R, Ma Y, Bian J, Liu Z and Tong X: Oligomeric
proanthocyanidins ameliorates osteoclastogenesis through reducing
OPG/RANKL ratio in chicken's embryos. Poult Sci.
103:1037062024.PubMed/NCBI
|
|
58
|
Zhu J, Shimizu E, Zhang X, Partridge NC
and Qin L: EGFR signaling suppresses osteoblast differentiation and
inhibits expression of master osteoblastic transcription factors
Runx2 and osterix. J Cell Biochem. 112:1749–1760. 2011.PubMed/NCBI
|
|
59
|
Lees-Shepard JB, Flint K, Fisher M, Omi M,
Richard K, Antony M, Chen PJ, Yadav S, Threadgill D, Maihle NJ and
Dealy CN: Cross-talk between EGFR and BMP signals regulates
chondrocyte maturation during endochondral ossification. Dev Dyn.
251:75–94. 2022.
|
|
60
|
Yi T, Lee HL, Cha JH, Ko SI, Kim HJ, Shin
HI, Woo KM, Ryoo HM, Kim GS and Baek JH: Epidermal growth factor
receptor regulates osteoclast differentiation and survival through
cross-talking with RANK signaling. J Cell Physiol. 217:409–422.
2008.PubMed/NCBI
|
|
61
|
Haberland M, Carrer M, Mokalled MH,
Montgomery RL and Olson EN: Redundant control of adipogenesis by
histone deacetylases 1 and 2. J Biol Chem. 285:14663–14670.
2010.PubMed/NCBI
|
|
62
|
Vishal M, Ajeetha R, Keerthana R and
Selvamurugan N: Regulation of Runx2 by histone deacetylases in
bone. Curr Protein Pept Sci. 17:343–351. 2016.PubMed/NCBI
|
|
63
|
Zhong YT, Liao HB, Ye ZQ, Jiang HS, Li JX,
Ke LM, Hua JY, Wei B, Wu X and Cui L: Eurycomanone stimulates bone
mineralization in zebrafish larvae and promotes osteogenic
differentiation of mesenchymal stem cells by upregulating
AKT/GSK-3β/β-catenin signaling. J Orthop Translat. 40:132–146.
2023.PubMed/NCBI
|
|
64
|
Kramer I, Halleux C, Keller H, Pegurri M,
Gooi JH, Weber PB, Feng JQ, Bonewald LF and Kneissel M: Osteocyte
Wnt/beta-catenin signaling is required for normal bone homeostasis.
Mol Cell Biol. 30:3071–3085. 2010.PubMed/NCBI
|
|
65
|
Albers J, Keller J, Baranowsky A, Beil FT,
Catala-Lehnen P, Schulze J, Amling M and Schinke T: Canonical Wnt
signaling inhibits osteoclastogenesis independent of
osteoprotegerin. J Cell Biol. 200:537–549. 2013.PubMed/NCBI
|
|
66
|
Takada I, Kouzmenko AP and Kato S: Wnt and
PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev
Rheumatol. 5:442–447. 2009.PubMed/NCBI
|
|
67
|
Takada I, Kouzmenko AP and Kato S:
Molecular switching of osteoblastogenesis versus adipogenesis:
Implications for targeted therapies. Expert Opin Ther Targets.
13:593–603. 2009.PubMed/NCBI
|
|
68
|
Olsen JJ, Pohl SÖG, Deshmukh A,
Visweswaran M, Ward NC, Arfuso F, Agostino M and Dharmarajan A: The
role of Wnt signalling in angiogenesis. Clin Biochem Rev.
38:131–142. 2017.
|






















