Introduction
Lung cancer is a malignant tumor that has high
morbidity and mortality rates worldwide; this type of cancer can be
divided into small-cell lung cancer (SCLC) and non-SCLC (NSCLC).
Worldwide, NSCLC accounts for 80-85% of all lung cancer cases and
the 5-year survival rate is generally <30% (1). Lung squamous cell carcinoma (LUSC)
accounts for ~30% of all NSCLC cases worldwide. Owing to the low
mutation rate in its driver genes, there is a lack of effective
targeted drugs for LUSC (2), and
patients with advanced LUSC are treated primarily with
chemotherapy, which has significant toxic side effects (e.g., Bone
marrow suppression and neurotoxicity) and is associated with a poor
prognosis (3). Therefore, there
is an urgent clinical need for the identification of effective
treatments for LUSC with minimal side effects.
In recent years, the potential role and advantages
of flavonoids in lung cancer treatment have attracted considerable
interest. These compounds exhibit antitumor effects through
multiple mechanisms, including cell cycle regulation, apoptosis
induction and inhibition of tumor angiogenesis. Their wide
availability, low toxicity and capacity to synergize with other
therapeutic modalities confer favorable biocompatibility and
significant antitumor efficacy, while minimizing damage to normal
cells (4). Hesperetin (HST) is a
natural flavonoid in citrus, grapefruit and other fruits, which has
diverse pharmacological effects, including anti-inflammatory,
antioxidant, antiviral and antihypertensive activities (5). Notably, the antitumor effects of
HST have garnered attention, and studies have shown that HST has
inhibitory effects on various types of cancer, such as lung
adenocarcinoma, and stomach, esophageal, breast, colon, prostate
and thyroid cancer (6-10). To the best of our knowledge,
there are no studies on HST in LUSC.
To address the urgent clinical need for effective
and low-toxicity treatments for LUSC, the present study is the
first, to the best of our knowledge, to report on the in
vitro and in vivo anti-LUSC effects of HST. The current
study investigated the molecular mechanisms of HST-induced
apoptosis in LUSC cells by assessing apoptosis-related signaling,
focusing on the endoplasmic reticulum stress (ERS) and Norch1
signaling pathways as potential targets.
As precision medicine continues to advance,
therapeutic strategies for LUSC are also being refined. Future
research directions are likely to encompass the development of
novel biomarkers to facilitate personalized treatments. The current
study not only offers novel insights into the treatment of LUSC but
also establishes a foundation for the further exploration of the
clinical potential of HST.
Materials and methods
Cell lines and reagents
Human lung squamous carcinoma cell lines H226
(CRL-5826) and H1703 (CRL-5889) were obtained from the American
Type Culture Collection. H226 and H1703 cells were cultured in RPMI
1640 (cat. no. PM150110; Wuhan Pricella Biotechnology Co., Ltd.)
containing 10% fetal bovine serum (cat. no. A5669701; Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin-streptomycin solution
(cat. no. MA0110; Dalian Meilun Biology Technology Co., Ltd.) at
37°C in a 5% CO2-humidified incubator.
Cell Counting Kit (CCK)-8 was purchased from APeXBIO
Technology LLC (cat. no. K1018). The Cell Cycle and Apoptosis
Analysis Kit (cat. no. C1052) and the mitochondrial membrane
potential assay kit with JC-1 (cat. no. C2006) were obtained from
Beyotime Institute of Biotechnology. The Annexin V-FITC Apoptosis
Detection Kit was obtained from BD Biosciences (cat. no. 556547).
Trypsin/ethylenediaminetetraacetic acid was purchased from Dalian
Meilun Biology Technology Co., Ltd. (cat. no. MA0233). Primary
antibodies against cyclin B1 (1:1,000; cat. no. 12231),
phosphorylated (P-)eIF2α (Ser51) (1:1,000; cat. no. 9721), eIF2α
(1:1,000;cat. no. 5324), CHOP (1:1,000; cat. no. 2895), Bax
(1:1,000;cat. no. 5023) and cleaved caspase-3 (1:1,000; cat. no.
9661), and HRP-conjugated secondary antibodies (1:1,000; goat
anti-rabbit IgG: cat. no. 7074; goat anti-mouse IgG cat. no. 7076)
were obtained from Cell Signaling Technology, Inc. Primary
antibodies against Notch1 (-1:1,000; cat. no. ab52627), Hes1
(1:1,000 cat. no. ab108937), caspase-3 (1:1,000; cat. no. ab32351),
Bcl-2 (1:1,000; cat. no. ab182858), CDK1 (1:1,000; cat. no.
ab133327) and glucose-regulated protein 78 (Grp78; 1:1,000; cat.
no. ab108615) were obtained from Abcam. The GAPDH antibody
(1:5,000; cat. no. 60004-1-Ig) was obtained from Proteintech Group,
Inc.
HST (cat. no. HY-N0168), cisplatin (cat. no.
HY-17394), 4-phenylbutyric acid (4-PBA; cat. no. HY-A0281),
Jagged-1 (cat. no. HY-P1846A) and Ultra High Sensitivity ECL Kit
(cat. no. HY-K1005) were purchased from MedChemExpress. HST was
dissolved in DMSO to 400 mM and diluted with the culture medium to
the desired concentration (the final DMSO concentration in the
culture medium was <0.1%).
CCK-8 assay
To determine cell viability, the H226 and H1703
cells were adjusted to 4.0×104 cells/ml and were
inoculated into 96-well plates. The plates were incubated overnight
in a cell culture incubator at 37°C and 5% CO2. The
experimental groups were treated with HST at concentrations of
18.75, 37.5, 75, 150 300, 600 and 1,200 μM, whereas the
control group was untreated. Incubation was performed at 37°C for
24, 48 and 72 h. Each group comprised five composite wells. At 24,
48 and 72 h, the medium was removed, serum-free medium containing
10% CCK-8 was added, and the cells were incubated at 37°C for 1-2
h. A microplate reader (SpectraMax iD5; Molecular Devices, LLC) was
used to measure absorbance (OD) at a wavelength of 450 nm to
calculate the cell survival rate in each well. The cell survival
rate was calculated as follows: Cell survival rate
(%)=[(ODexperimental well-ODblank
well)/(ODcontrol well-ODblank well)]
×100.
Cell treatment
Based on the results of the CCK-8 assay, the
experimental group was treated with HST at concentrations of 75,
150 and 300 μM for H226 cells, and 37.5, 75 and 150
μM for H1703 cells, whereas the control group was untreated.
Incubation was performed at 37°C for 48 h.
Cell cycle detection
Following HST treatment, the H226 and H1703 cells
were collected, adjusted to 3×105 cells/ml and
precipitated by centrifugation at 1,000 × g for 5 min at 4°C.
Subsequently, 1 ml precooled 70% ethanol was added and the cells
were fixed overnight at 4°C. The cells were then centrifuged at
1,000 × g for 5 min at 4°C and suspended in 0.5 ml PI staining
solution containing RNase before being incubated at 37°C for 30
min. Within 1 h, the cells were detected using flow cytometry (BD
Accuri C6 Plus; Becton, Dickinson and Company) and cell cycle
progression was analyzed using ModFit LT 5.0 software (Verity
Software House, Inc.).
Analysis of mitochondrial membrane
potential
Carbonyl cyanide m-chlorophenylhydrazone (CCCP)
functions as a mitochondrial uncoupler and induces apoptosis
through the dissipation of the mitochondrial membrane potential
(11). The positive control
group was treated with 50 μM CCCP for 30 min at 37°C (data
not shown). Following treatment, the H226 and H1703 cell density
was adjusted to 5×105 cells/ml, and the JC-1 staining
working solution (0.5 ml) was added and mixed according to the
manufacturer's instructions. After incubation at 37°C in the dark
for 20 min, the cells were washed, collected in JC-1 staining
buffer (1X) and examined using a flow cytometer (BD Accuri C6 Plus)
within 30 min. The results were analyzed using FlowJo 10.8.1
software (BD Biosciences).
Apoptosis assay
To quantify the rate of apoptosis, flow cytometry
was performed according to the instructions of the Annexin V-FITC
apoptosis detection kit. After treatment, the H226 and H1703 cells
were harvested promptly by trypsinization, then resuspended in
culture medium. The cells were then collected and adjusted to a
concentration of 3×106 cells/ml. Annexin V-FITC/PI
staining was performed according to the manufacturer's protocol;
the cell suspension and dye were thoroughly mixed, followed by
incubation in the dark at 25°C for 15 min. Detection was carried
out within 1 h using a flow cytometer (BD Accuri C6 Plus). The
results were analyzed using the accompanying CSampler Plus software
v 1.0.34.1 (Becton, Dickinson and Company).
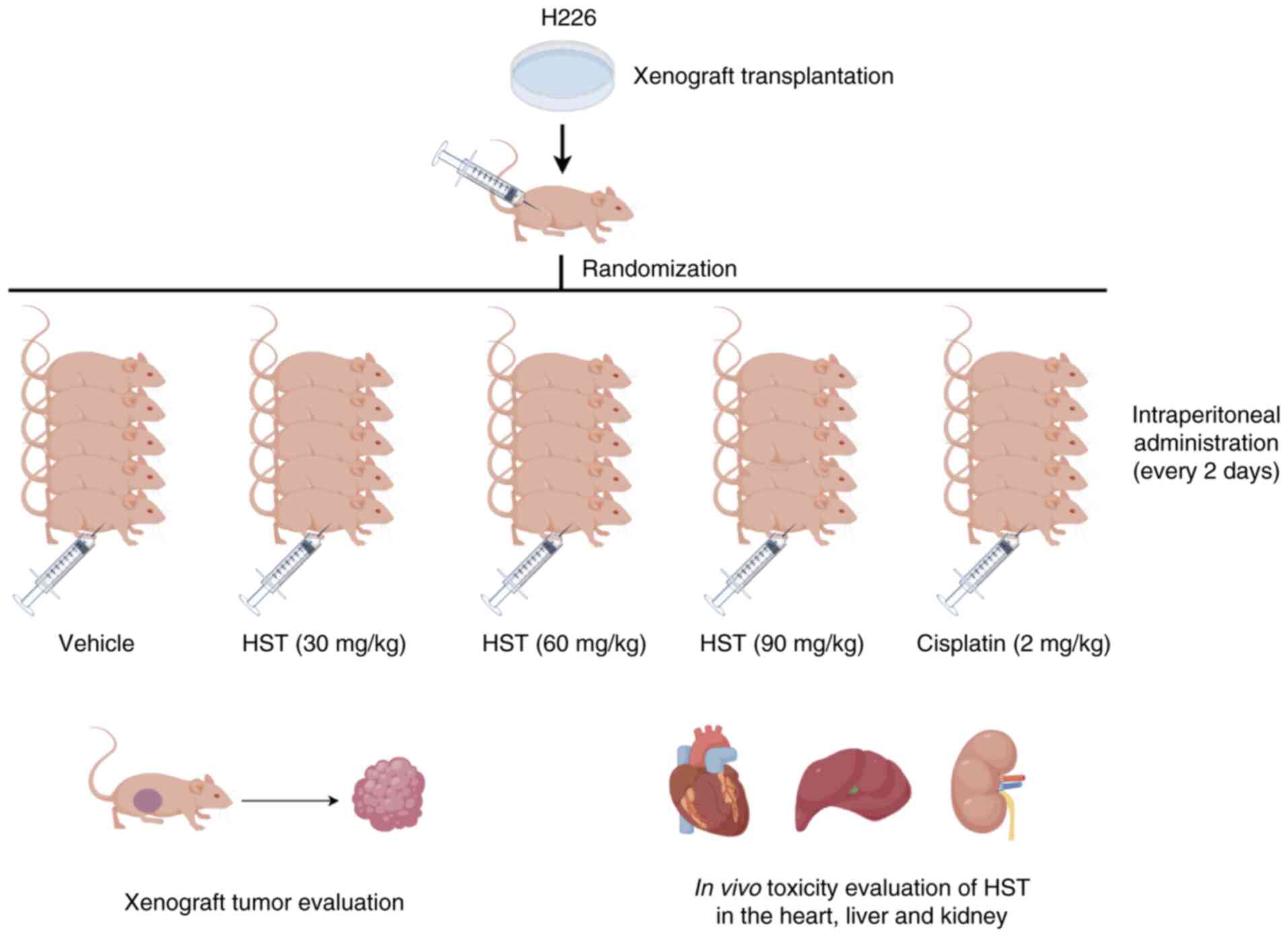
Xenograft model
Animal experiments were reviewed and approved by the
Experimental Animal Welfare Ethics Committee of Fujian Provincial
Hospital [approval no. IACUC-FPH-PZ-20240424(0006); Fuzhou, China].
Notably, Fujian Provincial Hospital was renamed to Fuzhou
University Affiliated Provincial Hospital in 2024. Female BALB/c
nude mice were purchased from Shanghai Lesk Experimental Animal Co.
(cat. no. SDK111083). A total of 30 nude mice (age, 3-5 weeks;
weight, 15-16 g), were housed in a specific pathogen-free barrier
environment throughout the study. The ambient temperature was
maintained at 20-25°C, and the relative humidity was kept at
40-60%. The animals were fed sterilized specialized feed, with
ad libitum access to food and water, and were maintained
under a 12-h light/dark cycle. To establish a xenograft tumor
model, 1×106 H226 cells (100 μl) were injected
subcutaneously into mice. The tumor volume was calculated, and when
it reached 80-100 mm3, the nude mice were divided into
five groups: Mice in the control group were intraperitoneally
injected with 0.2 ml 0.9% normal saline once every 2 days; mice in
the treatment groups were intraperitoneally injected with 30, 60 or
90 mg/kg HST once every 2 days; and mice in the positive control
group were administered cisplatin (2 mg/kg) intraperitoneally every
2 days. After 28 days of intervention, the mice were sacrificed.
For euthanasia, mice were exposed to 60% vol/min CO2 and
subsequently underwent cervical dislocation. Transplanted tumors
and major organs, such as the heart, liver and kidneys, were
subsequently isolated, and inhibition rate of tumor weight was
calculated as follows: Tumor inhibition rate (%)=(mean tumor weight
of the control group-mean tumor weight of the intervention
group)/mean tumor weight of the control group ×100. The following
humane endpoints were adhered to: i) Inability to move or lack of
response to gentle stimulation; ii) difficulty breathing; iii)
diarrhea or urinary incontinence; iv) weight loss of 20% of body
weight before the experiment; v) inability to eat or drink; vi)
obvious anxiety, irritability or tumor weight exceeds 10% of body
weight; vii) skin damage occurred on >30% of body surface area,
or infection and suppuration occur.
The transplanted tumors, heart, liver and kidney
were fixed in 4% paraformaldehyde solution at room temperature for
24 h, then embedded in paraffin and sectioned into 5-μm
slices. The sections were dewaxed and stained with hematoxylin for
5 min and eosin for 4 min at room temperature. Images of the
stained sections were captured using a light microscope (Leica
DM2000 LED; Leica Microsystems, Inc.). The protein expression
levels in the transplanted tumors were detected by western
blotting. A schematic diagram illustrating the animal
experimentation process is presented in Fig. 1.
Western blot analysis
The treated lung cancer cell lines H226 and H1703,
as well as xenograft tumor tissues derived from H226 cells in nude
mice, were utilized for western blot analysis. Total protein was
extracted using lysis buffer (cat. no. R0010; Beijing Solarbio
Science & Technology Co., Ltd.) containing
phenylmethanesulfonyl fluoride (cat. no. P0100; Beijing Solarbio
Science & Technology Co., Ltd.) on ice for 5 min. Protein
concentration was determined using a BCA protein assay kit (cat.
no. ZJ102; Epizyme; Ipsen Pharma). The protein samples (30
μg) were then separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (resolving gel
concentrations, 6, 10 and 12.5%; 5% stacking gel), followed by
transfer onto PVDF membranes (cat. no. ISEQ00005; MilliporeSigma).
After blocking them with 5% skimmed milk powder in TBS-Tween (0.1%)
at room temperature for 1 h, the membranes were incubated with
specific primary antibodies overnight at 4°C. After washing, the
membranes were incubated with the corresponding secondary
antibodies at room temperature for 1 h. The blots were visualized
using the Ultra High Sensitivity ECL Kit (cat. no. HY-K1005;
MedChemExpress) according to the manufacturer's instructions. The
target band levels were analyzed and semi-quantified using ImageJ
image analysis software (version 1.54k; National Institutes of
Health).
Statistical analysis
All quantitative tests were repeated at least three
times and the results are presented as the mean ± SEM. GraphPad
Prism software (version 9.0; Dotmatics) was used for statistical
analyses and image drawing. Comparisons between two groups were
assessed using unpaired Student's t-test and differences between
>2 groups were analyzed using a one-way ANOVA followed by
Tukey's multiple comparisons post hoc test. For non-parametric
data, the Mann-Whitney U test was used to compare two groups,
whereas the Kruskal-Wallis test and Dunn's post hoc test was used
to compared >2 groups. A two-way ANOVA followed by Tukey's
multiple comparisons post hoc test was performed to evaluate the
interactive effects of treatment duration and concentration.
P<0.05 was considered to indicate a statistically significant
difference.
Results
HST reduces the viability of H226 and
H1703 cells
HST inhibited the viability of LUSC in a
concentration- and time-dependent manner (Table I). Compared with in the control
group, the survival rate of H226 cells after 48 h of treatment with
300 μM HST was 49.74±0.59%. The survival rate of H1703 cells
was 65.28±0.71% after treatment with 150 μM HST for 48 h.
These specific concentrations and durations were considered as
optimal for eliciting significant cytotoxic effects while
maintaining experimental feasibility. Notably, H1703 cells were
more sensitive to HST than H226 cells. To avoid an excessive amount
of cell death that would affect the accuracy and reproducibility of
the experiment, subsequent experiments were performed following
treatment with HST for 48 h; with 0, 75, 150 and 300 μM
concentrations used to treat H226 cells and 37.5, 75 and 150
μM for H1703 cells.
 | Table IEffect of HST on the survival rate of
H226 and H1703 cells. |
Table I
Effect of HST on the survival rate of
H226 and H1703 cells.
| HST, μM | H226
| H1703
|
|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h |
|---|
| 0 | 100±1.702 | 100±0.64 | 100±1.07 | 100±2.18 | 100±0.39 | 100±2.25 |
| 18.75 | 98.36±1.13 | 95.42±0.31 | 98.63±3.41 | 93.41±0.93 | 91.74±0.76 | 93.62±0.58 |
| 37.5 | 94.46±0.93 | 95.32±0.98 | 96.99±0.73 | 91.54±0.27 | 86.36±1.59 | 85.51±0.78d |
| 75 | 95.38±1.50 | 94.79±1.77 | 95.44±0.93a | 89.39±0.59 | 79.379±1.35a,d | 72.48±0.36a,e |
| 150 | 86.05±1.51b | 79.00±0.06c,e | 75.33±0.56a,e | 83.60±1.03 | 65.28±0.71c,e | 48.31±0.89c,e |
| 300 | 71.69±0.14a | 49.74±0.59b,e | 36.64±0.50b,e | 66.39±1.72b | 37.26±0.23b,f | 23.25±0.65c,e |
| 600 | 40.56±0.21c | 22.55±0.53b,e | 3.24±0.27b,e | 40.47±0.79c | 9.25±0.07b,e | 1.47±0.24c,e |
| 1200 | 1.89±0.20b | 0.56±0.27b | 0.01±0.03b | 0.85±0.08b | 0.61±0.24b | 0.11±0.05b,f |
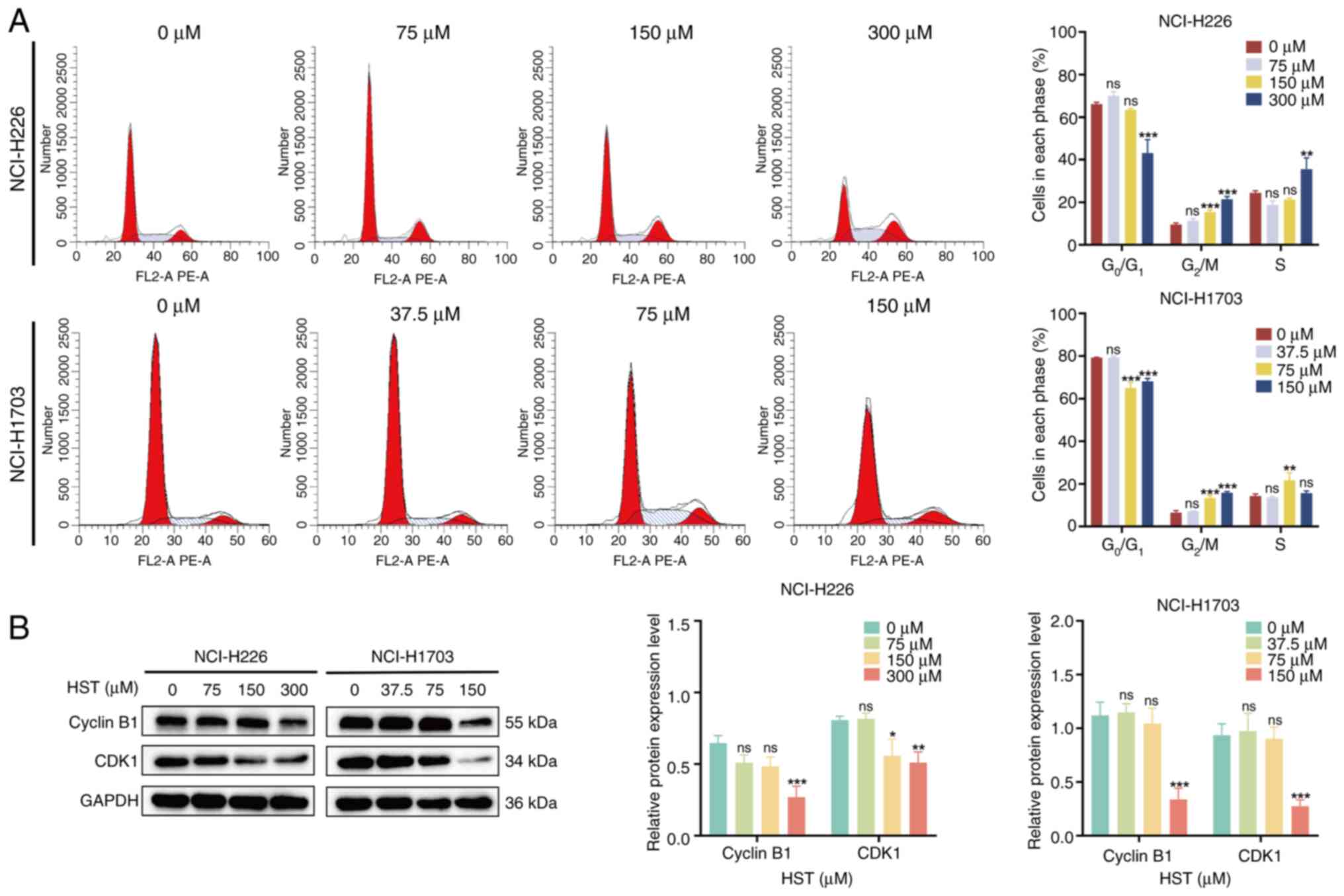
HST induces a G2/M phase cell
cycle block in H226 and H1703 cells
After 48 h of treatment of H226 cells with different
concentrations of HST, there was a significant increase in the
proportion of cells in the G2/M phase (Fig. 2A). Specifically, in the 75, 150
and 300 μM groups, the respective increases were 1.85±0.13,
5.88±0.15 and 12.13±0.24% compared with that in the control group.
Conversely, the proportion of G0/G1 phase
cells in the 150 and 300 μM groups was decreased by
2.72±0.12 and 23.02±3.16%, respectively. Notably, no significant
pattern was observed in the number of S-phase cells. Similarly,
when H1703 cells were treated with 37.5, 75 and 150 μM HST,
the proportion of cells in G2/M phase were increased by
0.67±0.50, 6.89±0.10 and 9.33±0.27%, respectively. Accordingly, the
proportion of cell in the G0/G1 phase was
decreased by 0.02±0.25, 14.26±1.43 and 11.17±0.69%, respectively.
Notably, no significant trend was observed regarding the number of
S-phase cells. Consequently, HST effectively blocked the LUSC cell
cycle in the G2/M phase and suppressed the proliferative
capacity of cells.
To further investigate the mechanism underlying the
effects of HST on cell cycle blockade in H226 and H1703 cells, the
current study examined the expression levels of two proteins
(Cyclin B1 and CDK1) that regulate the G2/M phase.
Compared with those in the control group, the expression levels of
cyclin B1 in H226 cells were not significantly decreased in the 75
and 150 μM HST groups, whereas the levels of CDK1 were
significantly reduced in the 150 μM group, and the protein
expression levels of cyclin B1 and CDK1 were significantly
decreased in the 300 μM group (Fig. 2B). Similarly, 75 μM HST
decreased cyclin B1 and CDK1 protein expression levels in H1703
cells, but this was not statistically significant. However,
treatment with 150 μM HST significantly reduced the protein
expression levels of cyclin B1 and CDK1
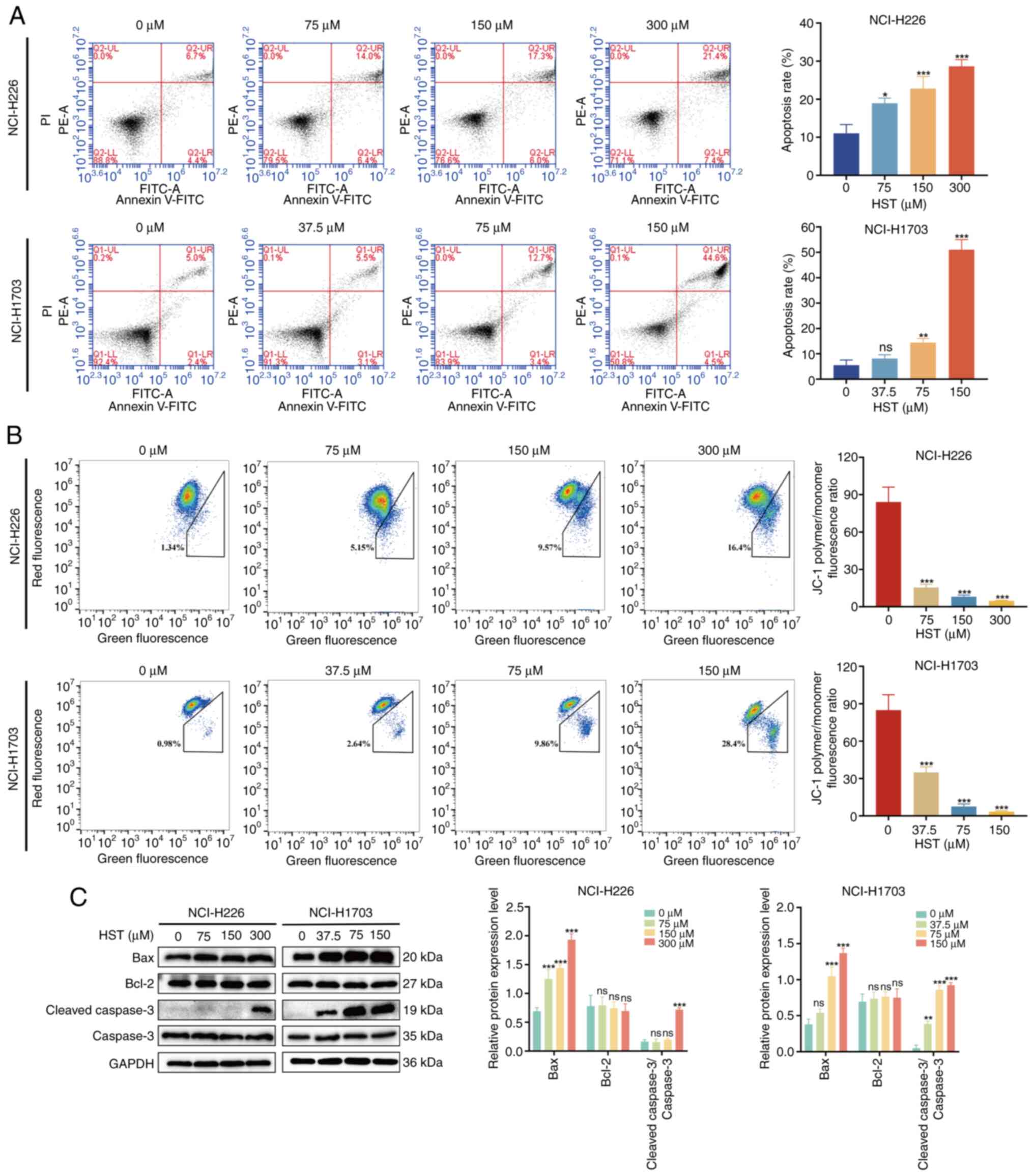
HST induces apoptosis in H226 and H1703
cells
To determine whether the anti-proliferative effects
of HST on H226 and H1703 cells were related to apoptosis, cell
apoptosis was examined using PI and Annexin V-FITC double staining,
and flow cytometry. The rate of apoptosis gradually increased with
increasing HST concentrations (Fig.
3A). After 48 h of treatment with different concentrations of
HST, the apoptosis rate of H226 cells increased from 11.03±1.32 to
28.63±1.01%. In addition, the apoptosis rate of H1703 cells
increased from 7.33±0.52 to 49.73±1.50%, which was statistically
significant compared with in the control group. These findings
indicated that HST may induce the apoptosis of LUSC cells in a
concentration-dependent manner.
Decreased mitochondrial membrane potential levels
are early markers of apoptosis. The JC-1 polymer/monomer
fluorescence ratio reflects mitochondrial membrane potential, with
a decreased ratio indicating membrane potential loss, which is
often associated with apoptosis. Using flow cytometry, the present
study analyzed the mitochondrial membrane potential levels in
HST-treated H226 and H1703 cells. The polymer/monomer fluorescence
ratio of H226 cells treated with HST significantly decreased from
88.10±6.86 to 4.73±0.16% in response to HST (Fig. 3B). In H1703 cells, a
statistically significant decrease in mitochondrial membrane
potential was observed in the HST groups compared with that in the
control group, with a decline from 87.86±7.13 to 3.31±0.44%. The
degree of reduction of mitochondrial membrane potential of H226 and
H1703 cells increased with a gradual increase in HST concentration.
These findings indicated that the apoptosis rate of H226 and H1703
cells gradually increased, whereas the mitochondrial membrane
potential decreased with increasing HST concentrations.
Compared with in the control group, the protein
expression levels of Bax and cleaved caspase-3/caspase-3 ratio in
H226 and H1703 cells gradually increased in response to increasing
HST concentrations (Fig. 3C).
However, there was no significant decrease in the protein
expression levels of Bcl-2, thus suggesting that HST may induce the
apoptosis of H226 and H1703 cells by reducing mitochondrial
membrane potential and increasing the expression of proapoptotic
proteins.
HST induces apoptosis in LUSC cells by
activating ERS
To determine whether HST induced ERS in H226 and
H1703 cells, western blotting was performed to detect the protein
expression levels of Grp78, P-eIF2α, eIF2α and CHOP proteins. The
protein expression levels of Grp78, CHOP and P-eIF2α were
progressively increased in H226 cells in response to increasing HST
concentrations, with significant upregulation observed in the 300
μM group (P<0.001; Fig.
4A). Comparable trends were evident in H1703 cells,
particularly in the 150 μM group, which showed a significant
difference. These results suggested that HST may induce ERS in LUSC
cells, and that H1703 cells have a higher sensitivity than H226
cells. Therefore, H1703 cells were selected for the subsequent
functional reversibility experiments.
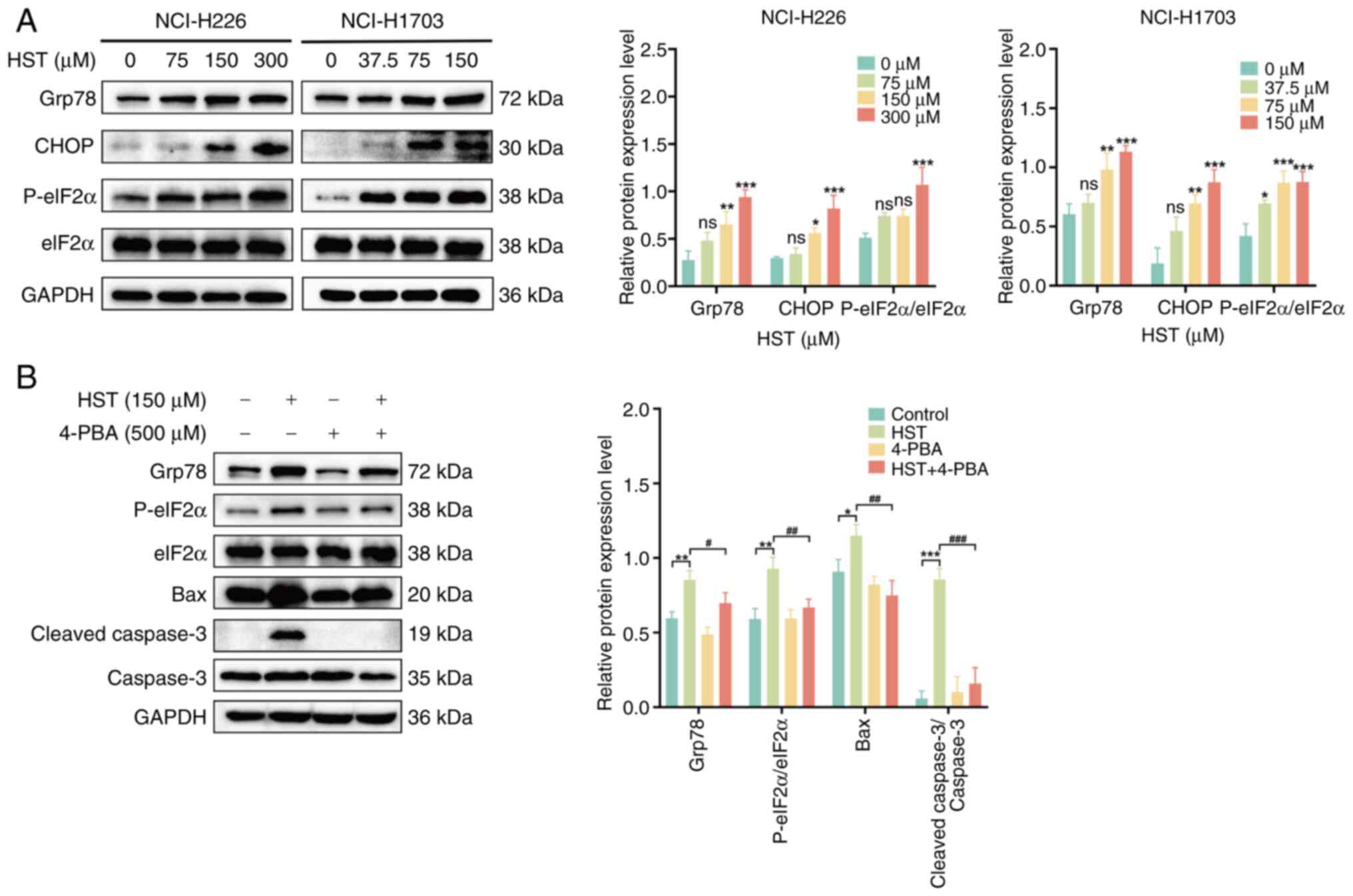 | Figure 4HST induces the apoptosis of lung
squamous cell carcinoma cells by activating the endoplasmic
reticulum stress pathway. (A) Western blotting detected the
expression levels of endoplasmic reticulum stress-related proteins
Grp78, P-eIF2α, eIF2α and CHOP in H226 and H1703 cells treated with
different concentrations of HST for 48 h. (B) H1703 cells were
pretreated with 500 μM 4-PBA for 6 h and were treated with
150 μM HST for 48 h. The protein expression levels of Grp78,
P-eIF2α, eIF2α, Bax, caspase-3 and cleaved caspase-3 were detected
by western blotting. nsP>0.05, *P<0.05,
**P<0.01, ***P<0.001 vs. control group;
#P<0.05, ##P<0.01,
###P<0.001 vs. 150 μM HST group. 4-PBA,
4-phenylbutyric acid; Grp78, glucose-regulated protein 78; HST,
hesperetin; P-, phosphorylated. |
To determine whether HST-induced apoptosis in LUSC
is associated with ERS, H1703 cells were pretreated with 4-PBA (an
ERS inhibitor) for 6 h and the influence of HST on the protein
expression levels of P-eIF2α, eIF2α, Grp78, Bax and cleaved
caspase-3 were observed. Compared with in the 150 μM HST
group, the protein expression levels of P-eIF2α, Grp78, Bax and
cleaved caspase-3 were significantly reduced in the 4-PBA + HST
group (Fig. 4B). These findings
suggested that suppression of ERS may alleviate HST-induced
apoptosis in H703 cells, and that HST could trigger apoptosis in
LUSC cells by activating the ERS signaling pathway.
HST induces apoptosis in LUSC cells by
inhibiting the Notch1 pathway
The Notch1 signaling pathway is also associated with
apoptosis (12). Western
blotting detected Notch1 and Hes-1 protein expression levels in
H226 and H1703 cells. With increasing HST concentration, the
protein expression levels of Notch1 and Hes-1 gradually decreased
in H226 and H1703 cells, particularly in H226 cells treated with
300 μM HST and in H1703 cells treated with 150 μM HST
(Fig. 5A). These findings
indicated that HST inhibited the Notch1 signaling pathway in H226
and H1703 cells. H1703 cells were more sensitive than H226 cells
and were selected for the subsequent functional reversibility
experiments.
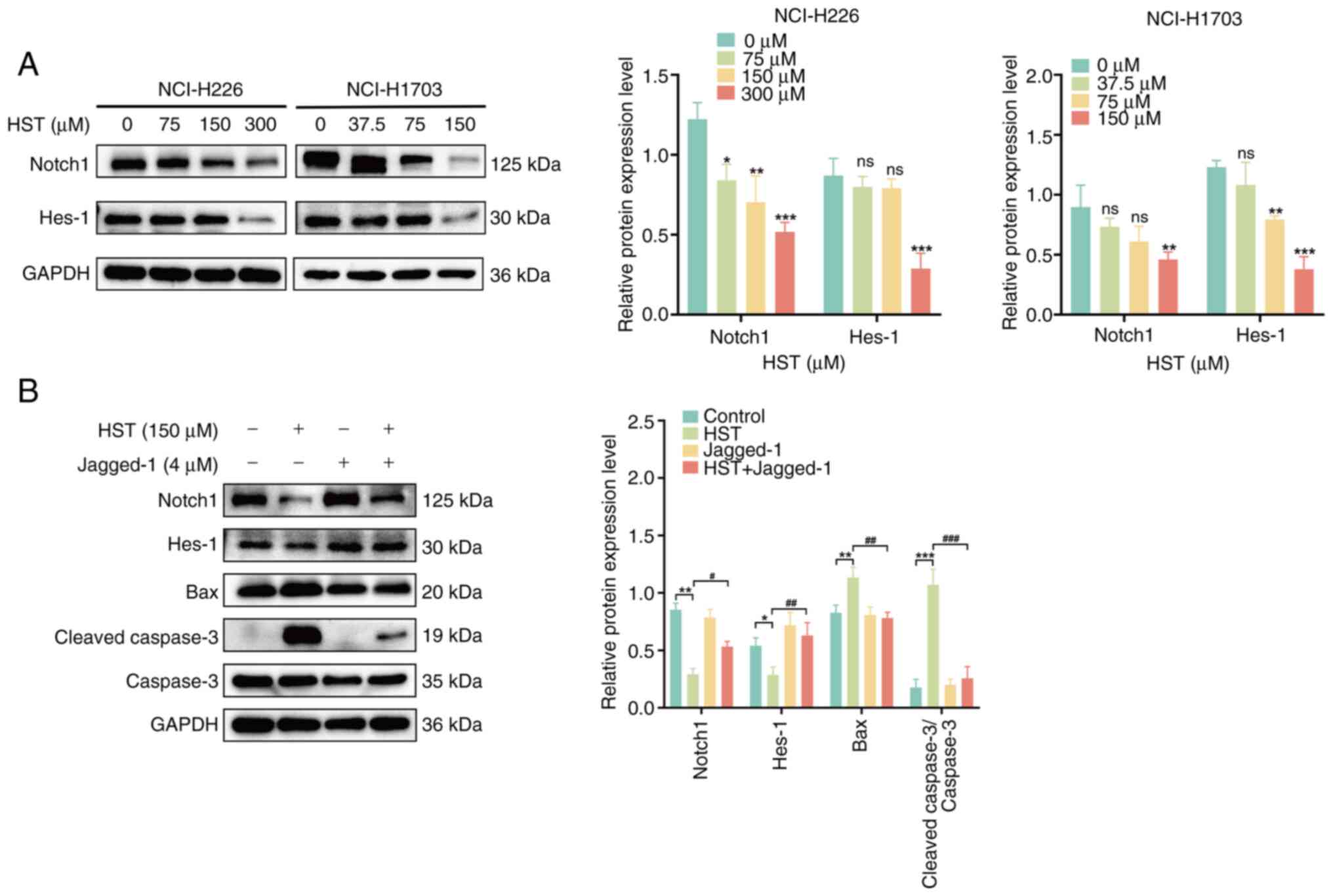 | Figure 5HST induces the apoptosis of lung
squamous cell carcinoma cells by inhibiting the Notch1 signaling
pathway. (A) Western blotting was used to detect the expression
levels of the Notch1 signaling pathway proteins Notch1 and Hes-1 in
H226 and H1703 cells treated with different concentrations of HST
for 48 h. (B) H1703 cells were pretreated with 4 μM Jagged-1
for 8 h, then treated with 150 μM HST for 48 h. The protein
expression levels of Notch1, Hes-1, Bax, caspase-3 and cleaved
caspase-3 were detected by western blotting.
nsP>0.05, *P<0.05,
**P<0.01, ***P<0.001 vs. control group;
#P<0.05, ##P<0.01,
###P<0.001 vs. 150 μM HST group. HST,
hesperetin. |
To confirm whether HST-induced apoptosis in LUSC
cells was associated with the inhibition of Notch1 signaling, H1703
cells were pretreated with the Notch1 receptor activator Jagged-1
for 8 h. A significant increase in Notch1 and Hes-1 protein
expression levels, and a significant decrease in Bax and cleaved
caspase-3 protein expression levels was observed in the
Jagged-1-pretreated group compared with those in the 150-μM
HST group (P<0.05; Fig. 5B).
These findings suggested that HST may promote the apoptosis of LUSC
cells by inhibiting the Notch1 signaling pathway.
HST enhances ERS in LUSC cells through
the Notch1 signaling pathway
Previous results have suggested that HST induces the
apoptosis of LUSC cells by inhibiting the Notch1 signaling pathway
and activating ERS. To investigate the association between
HST-induced inhibition of Notch1 signaling and ERS activation,
H1703 cells were pretreated with 4-PBA and Jagged-1. There was no
significant change in the protein expression levels of Notch1 and
Hes-1 in the 4-PBA pretreatment group compared with those in the
HST group (Fig. 6A). However,
the Jagged-1 pretreatment group exhibited a significant decrease in
P-eIF2α and Grp78 protein expression levels and P-eIF2α/eIF2α ratio
compared with those in the HST group (Fig. 6B), thus implying that the
inhibition of Notch1 signaling may lead to ERS.
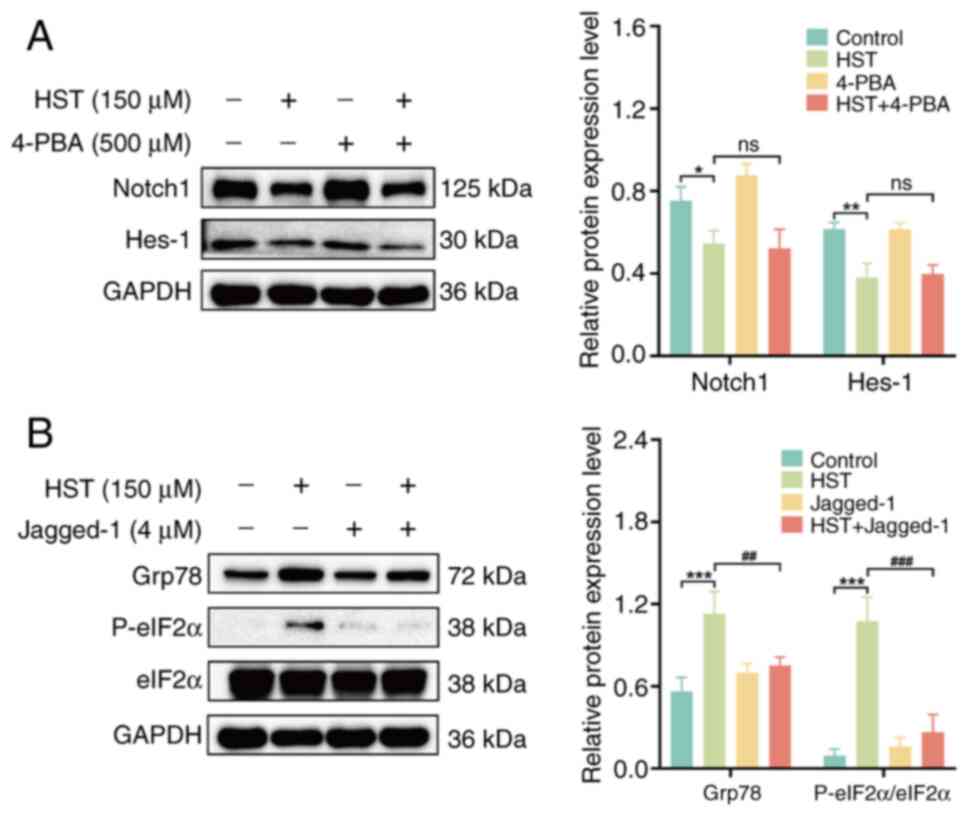 | Figure 6HST induces the apoptosis of lung
squamous cell carcinoma cells by activating endoplasmic reticulum
stress via the Notch1 signaling pathway. (A) H1703 cells were
pretreated with 500 μM 4-PBA for 6 h, then treated with 150
μM HST for 48 h. The protein expression levels of Notch1 and
Hes-1 were detected by western blotting. (B) H1703 cells were
pretreated with 4 μM Jagged-1 for 8 h, then treated with 150
μM HST for 48 h. The protein expression levels of Grp78,
P-eIF2α and eIF2α were detected by western blotting.
nsP>0.05, *P<0.05,
**P<0.01, ***P<0.001 vs. control group;
##P<0.01, ###P<0.001 vs. 150 μM
HST group. 4-PBA, 4-phenylbutyric acid; Grp78, glucose-regulated
protein 78; HST, hesperetin; P-, phosphorylated. |
Effect of HST on lung squamous cells
xenograft tumors in vivo
The present study evaluated the anticancer effects
of HST in vivo using a xenograft tumor model of H1703 cells
in BALB/c male nude mice. No nude mice died during the treatment.
Compared with in the control group, both cisplatin and HST
significantly inhibited tumor growth (Fig. 7A). A dose of 90 mg/kg HST
inhibited tumor growth, with an inhibition rate of 48.22% (Fig. 7B). Histopathological analysis
showed that HST had a specific suppressive effect on transplanted
tumors in nude mice. With increasing HST concentration, necrotic
areas expanded, cell spacing widened, and cellular and nuclear
debris were irregularly distributed (Fig. 7C). Notably, the body weights of
mice treated with HST showed no significant changes compared with
those in the control group; however, noticeable weight loss was
observed in the cisplatin group during treatment (Fig. 7D). Furthermore, no drug-induced
lesions were observed in the vital organs of the mice treated with
90 mg/kg HST (Fig. 7E).
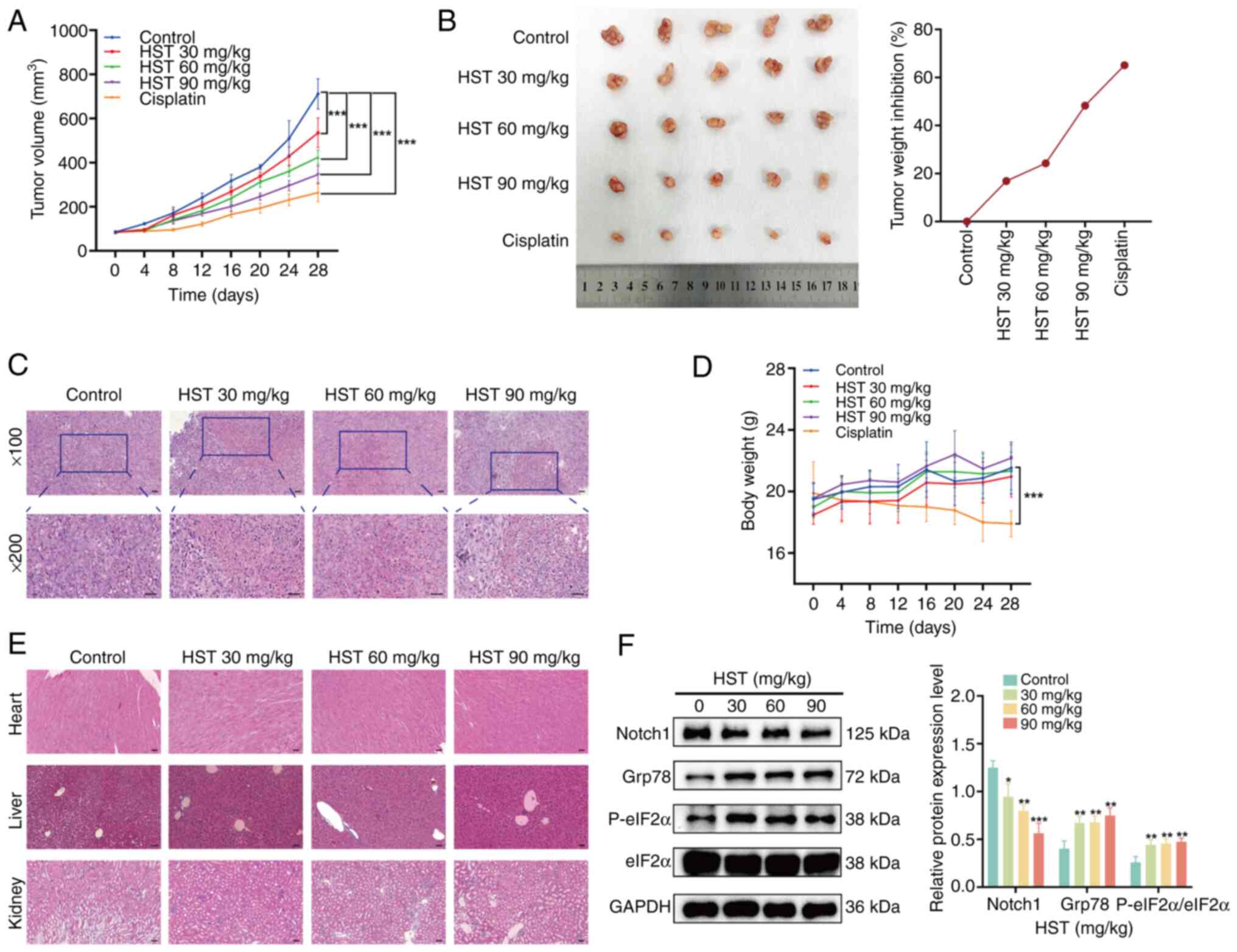 | Figure 7HST inhibits the growth of H226 nude
mouse transplanted tumors in vivo. (A) Volume of
transplanted tumors in nude mice. (B) Weight of the transplanted
tumors in nude mice was recorded, and the inhibition rate of tumor
weight was calculated. (C) Morphological characteristics of
transplanted tumor tissues in nude mice were documented. Scale bar,
300 μm. (D) Body weight of nude mice was determined. (E)
Histopathological features of key organs (heart, liver and kidney)
were determined. Scale bar, 300 μm. (F) Western blotting was
used to detect the expression levels of Notch1, Grp78, eIF2α and
P-eIF2α in transplanted tumor tissues of the nude mice.
*P<0.05, **P<0.01,
***P<0.001 vs. control group. Grp78,
glucose-regulated protein 78; HST, hesperetin; P-,
phosphorylated. |
The current measured the expression levels of
Notch1, Grp78, P-eIF2α and eIF2α in tumors using western blot
analysis. In the HST-treated groups, the levels of Grp78 and
P-eIF2α were significantly increased, whereas the levels of Notch1
were gradually decreased compared with those in the control group
(Fig. 7F). These findings
indicated that HST may suppress tumor growth by triggering ERS and
reducing the activity of the Notch1 pathway.
Discussion
HST is a natural flavonoid found in citrus fruits,
grapefruit and other fruits (13), which has pharmacological effects,
including anti-inflammatory, antioxidant, antiviral and antitumor
properties. The present study revealed that HST can effectively
inhibit the proliferation of H1703 and H226 cells in vitro
and in vivo, as demonstrated by G2/M cell cycle
arrest, apoptosis induction, Notch1 signaling suppression and
activation of ERS.
Cell cycle regulation is closely associated with
cell proliferation, and precise control of cancer cell
proliferation is crucial for cancer prevention and treatment
(14). The prolonged blockade of
tumor cells in the G2/M phase serves a dual purpose: It
inhibits tumor growth, while simultaneously inducing the
accumulation of DNA damage and subsequent apoptosis. Consequently,
targeting the G2/M phase has emerged as a promising
strategy for cancer therapy (15).
Cyclin B and CDK1 are essential regulators of the
G2/M phase transition in the cell cycle. Cyclin B is
synthesized at the end of G1 phase, peaks at the end of
G2 phase and during M phase, and forms the cyclin B-CDK1
complex in the nucleus, thereby promoting the transition from
G2 phase to mitosis. Notably, DNA damage can reduce the
expression of CDK1 and cyclin B1, indicating cell cycle arrest at
the G2/M phase (16).
The present study showed that HST dose-dependently arrested H1703
and H226 cells in the G2/M phase. In addition, HST
significantly reduced the protein levels of cyclin B1 and CDK1. The
effect of HST on the expression of cell cycle-related proteins was
consistent with cellular blockade at G2/M phase.
Therefore, the inhibitory effect of HST on the proliferation of
H226 and H1703 cells may be due to the induction of cellular
blockade in the G2/M phase.
Apoptosis can be divided into the endogenous
mitochondrial pathway, endogenous ERS pathway and exogenous death
receptor pathway (17). Notably,
mitochondria serve a crucial role in the regulation of apoptosis.
Upon receiving apoptotic signals, the proapoptotic protein Bax,
initially located in the cytoplasm, migrates to the mitochondrial
surface, forming a pore penetrating the mitochondrial membrane.
This action reduces the membrane potential and increases membrane
permeability, leading to the release of apoptotic factors. The key
regulatory genes involved in apoptosis, namely Bcl-2 and Bax,
control the downstream activation of caspase-3 proteases and
facilitate apoptotic cell death. A higher Bcl-2/Bax ratio indicates
an increased resistance to apoptosis (18). The present study showed that HST
reduced the mitochondrial membrane potential in a
concentration-dependent manner, increased the rate of apoptosis,
and upregulated the protein expression levels of cleaved caspase-3
while simultaneously decreasing the Bcl-2/Bax ratio in H226 and
H1703 cells. Caspase-3, a death execution protease, is a common
downstream effector in several apoptotic signaling pathways.
It has previously been suggested that ERS is crucial
in tumor progression, metastasis, tumorigenesis and cell survival
(19). ERS not only initiates
cell survival mechanisms but also induces apoptosis. When an ERS
signal is present, PKR-like ER kinase dissociates from Grp78 and
activates CHOP gene transcription via the PERK/eIF2α signaling
pathway, facilitating the expression of the anti-apoptotic gene
Bcl-2 and cell apoptosis (20).
The current study showed that HST upregulated the expression levels
of Grp78, P-eIF2α, CHOP and cleaved caspase-3, which was
significantly attenuated by 4-PBA (an ERS inhibitor). These results
suggested that HST may induce apoptosis in LUSC cells via the ERS
pathway. Previous studies have shown that certain flavonoids,
including luteolin, licochalcone A and wogonoside, promote
ERS-induced apoptosis in lung cancer cells (21-23).
It has also been shown that abnormal activation of
Notch signaling is important in cancer progression through the
maintenance of cancer stem cells (24). The functions of the Notch1
signaling pathway vary among different NSCLC subtypes (25,26). The expression of Notch1 protein
in LUSC has been reported to be significantly higher than that in
normal lung tissue (27), and
the Notch1 expression level is positively associated with disease
progression, metastasis and poor survival rates in patients
(28,29). Inhibition of Notch1 signaling can
induce apoptosis in LUSC cells in both a caspase-dependent and
non-caspase-dependent manner (30). Some flavonoids, such as
dihydromyricetin, rutin and xanthohumol, trigger apoptosis by
inhibiting the Notch1 pathway in cancer cells (31-33). The present study revealed that
HST inhibited the Notch1 signaling pathway in H226 and H1703 cells
in a concentration-dependent manner. Furthermore, Jagged-1 (a
Notch1 receptor activator) weakened the inhibitory effects of HST
on the Notch1 signaling pathway and reduced HST-induced
upregulation of cleaved caspase-3 expression, suggesting that HST
may induce the apoptosis of LUSC cells and inhibit the Notch1
signaling pathway.
Studies have shown that ERS activates the Notch1
signaling pathway (34), whereas
activation of the Notch1 signaling pathway can inhibit the
occurrence of ERS (35,36). Furthermore, Notch1 inhibition may
enhance ERS-induced apoptosis in chronic lymphocytic leukemia
cells, suggesting the involvement of the Notch1 pathway in the
modulation of adaptive or apoptotic responses to ERS (37). The present results showed that
Jagged-1 reduced the HST-induced upregulation of ERS-related
protein expression levels in H1703 cells, whereas 4-PBA did not
alter the inhibitory effects of HST on the Notch1 signaling
pathway, indicating that HST may promote apoptosis in H1703 cells
by activating ERS via inhibition of the Notch1 signaling pathway.
Western blot analysis of transplanted tumors confirmed that HST
inhibited the expression of Notch1, and increased the expression of
Grp78 and P-eIF2α, consistent with the in vitro experimental
results.
The present study identified a link between Notch1
and ERS in the apoptosis of LUSC cells but did not clarify how
Notch1 regulates ERS. Barcelos et al (38) revealed that the downregulation of
the Notch1 pathway may activate ERS and trigger cell death by
inhibiting the expression of Nrf2. Bodduluru et al (39) showed that HST reduces the
expression of Nrf2 in lung cancer cells; therefore, Nrf2 may be a
target of Notch1 and ERS.
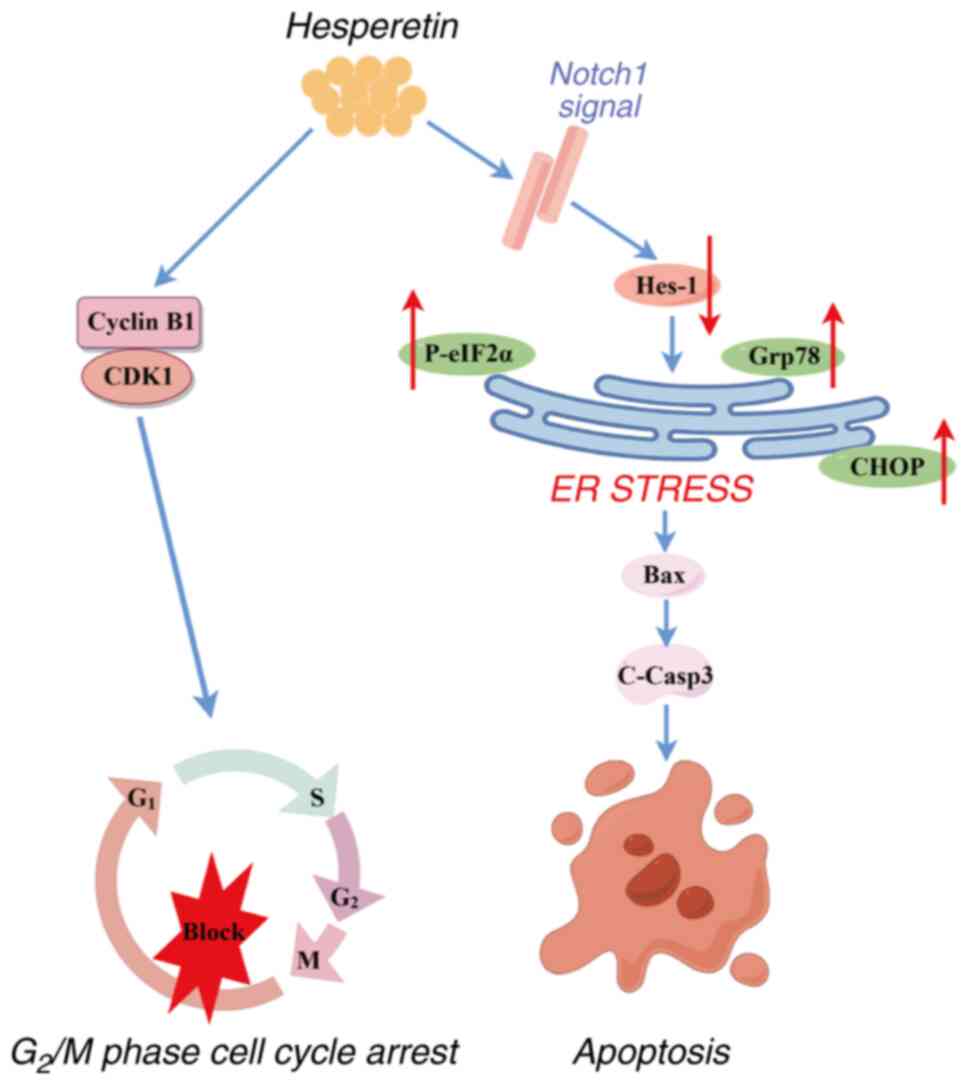
In conclusion, the present study showed that HST
inhibited the proliferation of LUSC cells in vivo and in
vitro, and confirmed the association between the Notch1 and ERS
signaling pathways in LUSC cells (Fig. 8). These findings may expand the
understanding of how HST promotes the apoptosis of lung cancer
cells and provide new ideas for treating lung cancer. These results
demonstrated the potential of HST as a promising therapeutic agent
for LUSC.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
QX was responsible for conducting the experiments,
analyzing data, generating figures and table, and writing the
manuscript. ZH and LT were responsible for generating figures and
table and designing the methodology. ML and MZ were responsible for
designing the methodology. CL was responsible for generating
figures and table, and analyzing data. SC was responsible for
conceptualization. LJ was responsible for conceptualization,
funding acquisition, and writing, reviewing and editing the
manuscript. YS was responsible for conceptualization, supervising
the experiments, funding acquisition, and writing, reviewing and
editing the manuscript. QX and YS confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The animal study was reviewed and approved by the
Experimental Animal Welfare Ethics Committee of Fujian Provincial
Hospital [approval no. IACUC-FPH-PZ-20240424(0006)].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by Fujian Provincial Health
Technology Project (grant no. 2021CXA002), as well as grants from
the High-level Hospital Foster Grants from Fujian Provincial
Hospital of China (grant no. 2019HSJJ25) and the Fujian Provincial
Natural Science Foundation Project of China (grant nos. 2022J01406
and 2022J011013).
References
|
1
|
Li C, Lei S, Ding L, Xu Y, Wu X, Wang H,
Zhang Z, Gao T, Zhang Y and Li L: Global burden and trends of lung
cancer incidence and mortality. Chin Med J (Engl). 136:1583–1590.
2023.PubMed/NCBI
|
|
2
|
Oncology Society of Chinese Medical
Association; Chinese Medical Association Publishing House: Chinese
Medical Association guideline for clinical diagnosis and treatment
of lung cancer (2023 edition). Zhonghua Yi Xue Za Zhi.
103:2037–2074. 2023.In Chinese.
|
|
3
|
Wang J, Shen Q, Shi Q, Yu B, Wang X, Cheng
K, Lu G and Zhou X: Detection of ALK protein expression in lung
squamous cell carcinomas by immunohistochemistry. J Exp Clin Cancer
Res. 33:1092014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arora S, Sheoran S, Baniya B, Subbarao N,
Singh H, Prabhu D, Kumar N, Pawar SC and Vuree S: Hesperidin's role
in the treatment of lung cancer: In-silico and In-vitro findings.
In Silico Pharmacol. 12:1042024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Drilon A, Rekhtman N, Ladanyi M and Paik
P: Squamous-cell carcinomas of the lung: Emerging biology,
controversies, and the promise of targeted therapy. Lancet Oncol.
13:e418–e426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sohel M, Sultana H, Sultana T, Al Amin M,
Aktar S, Ali MC, Rahim ZB, Hossain MA, Al Mamun A, Amin MN and Dash
R: Chemotherapeutic potential of hesperetin for cancer treatment,
with mechanistic insights: A comprehensive review. Heliyon.
8:e088152022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li L, Ren Z, Zhao P and Liu X: Research
progress in antitumor pharmacological activities of hesperidin and
hesperetin. Acta Chin Med. 33:2304–2308. 2018.In Chinese.
|
|
8
|
Elango R, Athinarayanan J, Subbarayan VP,
Lei DKY and Alshatwi AA: Hesperetin induces an apoptosis-triggered
extrinsic pathway and a p53-independent pathway in human lung
cancer H522 cells. J Asian Nat Prod Res. 20:559–569. 2018.
View Article : Google Scholar
|
|
9
|
Wolfram J, Scott B, Boom K, Shen J, Borsoi
C, Suri K, Grande R, Fresta M, Celia C, Zhao Y, et al: Hesperetin
liposomes for cancer therapy. Curr Drug Deliv. 13:711–719. 2016.
View Article : Google Scholar
|
|
10
|
Ramteke P and Umesh CSY: Hesperetin, a
Citrus bioflavonoid, prevents IL-1β-induced inflammation and cell
proliferation in lung epithelial A549 cells. Indian J Exp Biol.
57:7–14. 2019.
|
|
11
|
Chaudhari A, Seol JW, Kim SJ, Lee YJ, Kang
HS, Kim IS, Kim NS and Park SY: Reactive oxygen species regulate
Bax translocation and mitochondrial transmembrane potential, a
possible mechanism for enhanced TRAIL-induced apoptosis by CCCP.
Oncol Rep. 18:71–76. 2007.PubMed/NCBI
|
|
12
|
Shi Q, Xue C, Zeng Y, Yuan X, Chu Q, Jiang
S, Wang J, Zhang Y, Zhu D and Li L: Notch signaling pathway in
cancer: From mechanistic insights to targeted therapies. Signal
Transduct Target Ther. 9:1282024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Babukumar S, Vinothkumar V and
Ramachandhiran D: Modulating effect of hesperetin on the molecular
expression pattern of apoptotic and cell proliferative markers in
7,12-dimethylbenz(a)anthracene-induced oral carcinogenesis. Arch
Physiol Biochem. 126:430–439. 2020. View Article : Google Scholar
|
|
14
|
Canaud G and Bonventre JV: Cell cycle
arrest and the evolution of chronic kidney disease from acute
kidney injury. Nephrol Dial Transplant. 30:575–583. 2015.
View Article : Google Scholar :
|
|
15
|
Jiang L, Liu Y, Tumbath S, Boudreau MW,
Chatkewitz LE, Wang J, Su X, Zahid KR, Li K, Chen Y, et al:
Isopentyl-deoxynboquinone induces mitochondrial dysfunction and
G2/M phase cell cycle arrest to selectively kill NQO1-positive
pancreatic cancer cells. Antioxid Redox Signal. 41:74–92. 2024.
View Article : Google Scholar
|
|
16
|
Zou X, Qu Z, Gao P, Sun S and Ji Y:
Effects of Sulforaphane on G2/M phase arrest in HepG-2 cells and
the expression of Cdk1 and CyclinB1. Acta Chin Med Pharmacol.
38:8–12. 2010.In Chinese.
|
|
17
|
Krueger A, Baumann S, Krammer PH and
Kirchhoff S: FLICE-inhibitory proteins: Regulators of death
receptor-mediated apoptosis. Mol Cell Biol. 21:8247–8254. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Walensky LD: BCL-2 in the crosshairs:
Tipping the balance of life and death. Cell Death Differ.
13:1339–1350. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kato H and Nishitoh H: Stress responses
from the endoplasmic reticulum in cancer. Front Oncol. 5:932015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Faitova J, Krekac D, Hrstka R and Vojtesek
B: Endoplasmic reticulum stress and apoptosis. Cell Mol Biol Lett.
11:488–505. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park SH, Park HS, Lee JH, Chi GY, Kim GY,
Moon SK, Chang YC, Hyun JW, Kim WJ and Choi YH: Induction of
endoplasmic reticulum stress-mediated apoptosis and non-canonical
autophagy by luteolin in NCI-H460 lung carcinoma cells. Food Chem
Toxicol. 56:100–109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qiu C, Zhang T, Zhang W, Zhou L, Yu B,
Wang W, Yang Z, Liu Z, Zou P and Liang G: Licochalcone a inhibits
the proliferation of human lung cancer cell lines A549 and H460 by
inducing G2/M cell cycle arrest and ER stress. Int J Mol Sci.
18:17612017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen S, Wu Z, Ke Y, Shu P, Chen C, Lin R
and Shi Q: Wogonoside inhibits tumor growth and metastasis in
endometrial cancer via ER stress-Hippo signaling axis. Acta Biochim
Biophys Sin (Shanghai). 51:1096–1105. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saran U, Chandrasekaran B, Tyagi A, Shukla
V, Singh A, Sharma AK and Damodaran C: Corrigendum: A small
molecule inhibitor of Notch1 modulates stemness and suppresses
breast cancer cell growth. Front Pharmacol. 14:12075892023.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun J, Dong M, Xiang X, Zhang S and Wen D:
Notch signaling and targeted therapy in non-small cell lung cancer.
Cancer Lett. 585:2166472024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Anusewicz D, Orzechowska M and Bednarek
AK: Lung squamous cell carcinoma and lung adenocarcinoma
differential gene expression regulation through pathways of Notch,
Hedgehog, Wnt, and ErbB signalling. Sci Rep. 10:211282020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zong D, Ouyang R, Li J, Chen Y and Chen P:
Notch signaling in lung diseases: Focus on Notch1 and Notch3. Ther
Adv Respir Dis. 10:468–484. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou L, Wu S, Yu L, Gong X, Song W and
Cheng Z: Expression of CD133 and Notch1 in non-small cell lung
cancer and the clinicopathological significance. Nan Fang Yi Ke Da
Xue Xue Bao. 35:196–201, In Chinese.
|
|
29
|
Yuan X, Wu H, Xu H, Han N, Chu Q, Yu S,
Chen Y and Wu K: Meta-analysis reveals the correlation of Notch
signaling with non-small cell lung cancer progression and
prognosis. Sci Rep. 5:103382015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao H, Hu Y, Wang P, Zhou J, Deng Z and
Wen J: Down-regulation of Notch receptor signaling pathway induces
caspase-dependent and caspase-independent apoptosis in lung
squamous cell carcinoma cells. APMIS. 120:441–450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu CJ, He YF, Yuan WZ, Xiang LJ, Zhang J,
Liang YR, Duan J, He YH and Li MY: Dihydromyricetin-mediated
inhibition of the Notch1 pathway induces apoptosis in QGY7701 and
HepG2 hepatoma cells. World J Gastroenterol. 23:6242–6251. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khan F, Pandey P, Jha NK, Khalid M and
Ojha S: Rutin mediated apoptotic cell death in caski cervical
cancer cells via Notch-1 and Hes-1 downregulation. Life (Basel).
11:7612021.PubMed/NCBI
|
|
33
|
Kunnimalaiyaan S, Sokolowski KM,
Balamurugan M, Gamblin TC and Kunnimalaiyaan M: Xanthohumol
inhibits Notch signaling and induces apoptosis in hepatocellular
carcinoma. PLoS One. 10:e01274642015.PubMed/NCBI
|
|
34
|
Tremblay I, Paré E, Arsenault D, Douziech
M and Boucher MJ: The MEK/ERK pathway promotes NOTCH signalling in
pancreatic cancer cells. PLoS One. 8:e855022013.
|
|
35
|
Zhang M, Yu LM, Zhao H, Zhou XX, Yang Q,
Song F, Yan L, Zhai ME, Li BY, Zhang B, et al:
2,3,5,4′-Tetrahydroxystilbe ne-2-O-β-D-glucoside protects murine
hearts against ischemia/reperfusion injury by activating
Notch1/Hes1 signaling and attenuating endoplasmic reticulum stress.
Acta Pharmacol Sin. 38:317–330. 2017.PubMed/NCBI
|
|
36
|
Gan L, Liu Z, Wu T, Feng F and Sun C: αMSH
promotes preadipocyte proliferation by alleviating ER
stress-induced leptin resistance and by activating Notch1 signal in
mice. Biochim Biophys Acta Mol Basis Dis. 1863:231–238. 2017.
|
|
37
|
Silva Barcelos EC, Rompietti C, Adamo FM,
Dorillo E, De Falco F, Del Papa B, Baldoni S, Nogarotto M, Esposito
A, Capoccia S, et al: NOTCH1-mutated chronic lymphocytic leukemia
displays high endoplasmic reticulum stress response with druggable
potential. Front Oncol. 13:12189892023.PubMed/NCBI
|
|
38
|
Barcelos ECS, Rompietti C, Adamo FM,
Dorillo E, De Falco F, Del Papa B, Baldoni S, Nogarotto M, Esposito
A, Capoccia SJ, et al: NOTCH1-mutated chronic lymphocytic leukemia
displays high endoplasmic reticulum stress response with druggable
potential. Front Oncol. 13:12189892023.
|
|
39
|
Bodduluru LN, Kasala ER, Barua CC, Karnam
KC, Dahiya V and Ellutla M: Antiproliferative and antioxidant
potential of hesperetin against benzo(a)pyrene-induced lung
carcinogenesis in Swiss albino mice. Chem Biol Interact.
242:345–352. 2015.PubMed/NCBI
|