Introduction
Non-small cell lung cancer (NSCLC) is the most
common cause of cancer-related mortality. Doublet combination
chemotherapy is currently the first-line therapy for advanced lung
cancer that is not surgically resectable. However, even with
chemotherapy, the prognosis of patients with advanced NSCLC remains
poor, with a 1-year survival rate of 30% (1,2).
Therefore, it is important to develop new treatment regimens in
order to reduce the morbidity and mortality of this fatal
disease.
Telomeres are DNA repeats (TTAGGG)n found
at the end of chromosomes, and play an important role in
maintaining genomic stability (3,4). Due
to the end replication problem, telomeres are progressively lost
with each cell division, eventually leading to cell growth arrest
(replicative senescence) in normal cells (5,6).
Critical telomere shortening may be considered as an initial block
to indefinite cellular proliferation (a hallmark of cancer).
However, telomere shortening may be counteracted by the cellular
ribonucleoprotein reverse-transcriptase telomerase (hTERT), which
uses a part of an internal RNA moiety as a template for the
synthesis of telomeric repeats (7,8).
Telomerase activity is not readily detectable in most quiescent
normal somatic tissues; it is, however, highly expressed in ∼90% of
human tumors. This feature renders this enzyme an attractive,
almost universal, target for cancer therapy. Therefore, various
telomerase inhibitors have been developed over the past few years
(9,10). Among these compounds, a
thio-phosphoramidate oligonucleotide, imetelstat sodium
(GRN163L), is being assessed in clinical trials as a potent human
telomerase inhibitor. This molecule was designed as a competitive
telomerase inhibitor, which binds directly to the active site of
the enzyme, thus inhibiting its activity. The presence of the
covalently conjugated 5′-palmitoyl (C16) lipid group provides more
effective cellular uptake and increased bioavailability of GRN163L
(11,12). This compound is currently in
multiple phase II clinical trials as a potential broad-spectrum
anticancer agent.
Our previous in vitro studies showed that
GRN163L effectively inhibits telomerase activity in A549 lung
cancer cells, reduces their proliferation rate within 3–4 weeks and
progressively shortens telomere length in 5–6 weeks, leading to
apoptotic cell death. Moreover, GRN163L effectively inhibits the
formation and growth of lung metastases in xenograft animal models
in vivo(13). We have also
reported that A549 cells treated with a single dose of GRN163L (1
μM) prior to cell attachment, were relatively weakly
attached to the plate surface substrates and were morphologically
altered (i.e., became rounded), whereas mismatch (MM)
control-treated cells exhibited a typical epithelioid appearance
and normal adhesion properties. These morphological changes were
independent of human telomerase RNA (hTR) subunit expression or
telomerase inhibition and were unrelated to telomere length. We
determined that these effects were due to the molecular structure
of the oligo thio-phosphoramidate and its lipid moiety, the
N3′→P5′-thio-phosphoramidate backbone and the presence of
G-quadruplex-forming triple-G sequences within the GRN163L
(14). However, the exact
mechanism underlying these morphological changes remains
unknown.
Microfilaments, microtubules and intermediate
filaments are fundamental structures of the cytoskeleton, which
play important roles in the determination of cell shape,
proliferation and migration. F-actin filaments are required for
cell shape determination, microtubules are responsible for the
positioning of organelles playing a pivotal role in intracellular
transport and intermediate filaments provide mechanical support and
resistance to stress (15).
Cadherins are Ca2+-dependent adhesion molecules. One of
the most widely investigated is E-cadherin, which influences
cellular shape and cell-cell interactions. The loss of
E-cadherin-mediated adhesion is considered to be characteristic of
the transition from benign lesions to invasive and metastatic
cancer (16). In the present
study, we investigated whether the cytoskeletal and cell adhesion
proteins are associated with the observed rapid morphological
alterations (i.e., ‘rounding effect’) and the loss of adhesion of
A549 lung cancer cells treated with a single dose of GRN163L (1
μM). In addition, since it has been shown that the
overexpression of telomerase in cancer cells increases the level of
matrix metalloproteinase-2 (MMP-2), which is directly involved in
the invasion process (17), we
observed that GRN163L decreased MMP-2 expression, suggesting that
GRN163L exerts some of its anticancer effects in a
telomere-independent manner.
Materials and methods
Cell culture
A549 non-small lung cancer cells were obtained from
the American Type Culture Collection (ATCC; Manassas, VA, USA).
A549 cells were cultured in DMEM containing 10% fetal bovine serum
(FBS; Sigma, St. Louis, MO, USA) and 100 U/ml
penicillin-streptomycin (Sigma). The 13-mer GRN163L (Geron Corp.,
Menlo Park, CA, USA), which complements the template region of
telomerase hTR (also known as hTERC), and the MM control
oligonucleotide, which does not complement the template region of
hTR, were prepared as previously described (11).
Western blot analysis
The A549 cells (1×106) were plated in
6-well plates and immediately treated with MM (1 μM) or
GRN163L (1 μM). The untreated control and treated cells were
collected following a 24-h incubation period and lysed with NP-40
lysis buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1%
NP-40 detergent and 1X protease inhibitor complex (Roche Applied
Science, Indianapolis, IN, USA). Protein concentration was
quantified using the Bradford assay (Sigma), as previously
described (12). A total of 40
μg of protein lysate was subjected to SDS-PAGE, followed by
transfer onto polyvinylidene difluoride membranes. Blocking and
antibody incubation were performed in 5% milk in PBS containing
0.2% Tween-20. Membranes were exposed to X-ray film using the ECL
Plus Detection reagent (Amersham Life Science, Inc., Piscataway,
NJ, USA). E-Cadherin (1:1,000), β-actin (1:400), α-actinin
(1:1,000), pan-cytokeratin (1:1,000), α-tubulin (1:2,000) and
calnexin antibodies (1:10,000) were obtained from Santa Cruz
Biotechnology, Santa Cruz, CA, USA and used for western blot
analysis. Secondary antibody (Sigma) was used in proportion 1:5000.
Densitometry levels for each blot were determined using calnexin as
the loading control.
Immunohistochemistry
A549 cells (1×105) were plated onto glass
coverslips and immediately treated with MM (1 μM) or GRN163L
(1 μM). The cells were fixed in 3% paraformaldehyde for
α-actinin and E-cadherin, and in microtubule stabilization buffer
for F-actin, αβ tubulin and cytokeratin, following a 24-h
incubation period. Fluorescein phalloidin, specific to F-actin, and
mouse monoclonal antibodies against αβ tubulin (Santa Cruz
Biotechnology), pan-cytokeratin (isoforms 1, 4, 5, 6, 8, 10, 13, 18
and 19), α-actinin and E-cadherin (Sigma), were applied.
FITC-conjugated goat anti-rabbit IgG for pan-cadherin and
FITC-conjugated goat anti-mouse IgG (Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) for the other cytoskeletal
proteins were used as secondary antibodies. All antibodies were
diluted 1:100 in PBS and incubated for 90 min at 37°C in a
humidified chamber. Images were examined under a Carl Zeiss LSM 510
META Confocal Laser Scanning microscope (488-nm argon ion, 543-nm
green helium neon, 633-nm red helium-neon laser lines) and
consecutive optical sections were recorded and used for 3D image
reconstruction.
Cell cycle analyses
Real-time PCR was used for cell cycle analyses; the
A549 cells were incubated with GRN163L for 24 h or up to 1 week.
Total RNA was isolated from the control cells and GRN163L-treated
cells using the RNeasy mini kit (Qiagen, Inc., USA, Valencia, CA,
USA), according to the manufacturer’s instructions. cDNA synthesis
was performed using the DyNamo cDNA synthesis kit (Finnzymes,
Espoo, Finland). Primers were designed using Primer Design sofware
(version 2.0; serial number: 52017. Copyright 1990, 91; Scientific
and Educational Software) (Table
I). Real-time PCR was performed using SYBR-Green (Finnzymes).
Samples were heated to 94°C for 5 min as the initial denaturation,
followed by 40 cycles of denaturation at 95°C for 30 sec, 55°C for
30 sec, 72°C for 30 sec and annealing/extension at 75°C for 5 min.
A melt curve stage was added to analyze the PCR product.
Cyclophilin A was used as an internal control gene to normalize for
RNA quantity. The results were analyzed using the Bio-Rad
iCycler-Techgene thermal cycler.
 | Table IPrimer sequences for real-time PCR
analyses of G1 phase genes. |
Table I
Primer sequences for real-time PCR
analyses of G1 phase genes.
| Target | Oligonucleotide
sequences | Base pairs | GenBank accession
no. |
|---|
| Cyclin D1 | F:
5′-ATGAACTACCTGGACCGCTT-3′ | 142 | NM_053056.2 |
| R:
5′-TCGGTGTAGATGCACAGCTT-3′ | | |
| Cdk4 | F:
5′-GACCAGGACCTAAGGACATA-3′ | 146 | NM_000075.2 |
| R:
5′-GTTCTCTGGCTTCAGATCTC-3′ | | |
| Cdk6 | F:
5′-TTCACACCGAGTAGTGCATC-3′ | 122 | NM_001259.5 |
| R:
5′-GAGGTTAGAGCCATCTGGAA-3′ | | |
| Cyclophilin A | F:
5′-AATGGCACTGGTGGCAAGTC-3′ | 219 | NM_021130.3 |
| R:
5′-GCTCCATGGCCTCCACAATA-3′ | | |
Determination of MMP-2 expression
The correlation between telomerase inhibition and
MMP-2 expression was evaluated by real-time PCR. To determine the
effect of GRN163L on MMP-2 expression, GRN163L (1 μM) was
added to the medium 24 h after plating. After an additional 24 h,
RNA was collected for real-time PCR from the control cells and
GRN163L-treated cells using TRIzol reagent (Invitrogen, Carlsbad,
CA, USA). cDNA synthesis was then performed with M-MLV RT
(Invitrogen). MMP-2 expression was assessed by real-time PCR which
was performed using SYBR-Green (Applied Biosystems, Carlsbad, CA,
USA). The primers used for real-time PCR are presented in Table II. Samples were heated to 95°C for
3 min as initial denaturation, followed by 40 cycles of
denaturation at 95°C for 3 sec, 58°C for 20 sec, 72°C for 5 sec and
annealing/extension at 95°C for 1 min, 55°C for 30 sec and 95°C for
30 sec. A melt curve stage was added to analyze the PCR product.
GAPDH was used as the internal control gene to normalize for RNA
quantity.
 | Table IIPrimer sequences for real-time PCR
analyses of matrix metalloproteinase-2 (MMP-2). |
Table II
Primer sequences for real-time PCR
analyses of matrix metalloproteinase-2 (MMP-2).
| Target | Oligonucleotide
sequences | GenBank accession
no. |
|---|
| MMP-2 | F:
5′-GTATCCATCGCCATGCTCC-3′ | NM_004530 |
| R:
5′-AAGAACCAGATCACATACAGGATCA-3′ | |
| GAPDH | F:
5′-GAGTCCACTGGCGTCTTC-3′ | NM_002046.3 |
| R:
5′-GCATTGCTGATGATCTTGAGG-3′ | |
Viral transduction
shRNA (0.5 μg), together with 0.5 μg
of helper plasmids (0.2 μg pMD2G and 0.3 μg psPAX2)
were transfected into 293FT cells with Effectene reagent (Qiagen).
Viral supernatants were collected 48 h after the transfections and
cleared through a 0.45-m filter. The A549 cells were transfected
with viral supernatants containing 2 μg/ml polybrene (Sigma)
and the successfully transfected cells were selected using
puromycin.
Invasion/cell migration assay
A549 cells were treated with 1 μM GRN163L for
24 h. The untreated and treated cells (1×105) were then
plated in Matrigel™-coated invasion chambers (BD Biosciences, San
Jose, CA, USA) and processed according to the manufacturer’s
instructions. shMMP-2 knockdown cells were used as a series of
control cells. Chemoattractant was added to the lower chamber
(below the membrane), and culture medium, containing 10% FBS, was
used for the A549 cells. Cells were incubated for 22 h at 37°C, in
an atmosphere of 5% CO2. Cells were removed from the top
chamber using cotton swabs, washed, then fixed and stained with 6%
glutaraldehyde and 0.5% crystal violet for 30 min. The cells that
migrated through to the bottom of the membrane and stained were
counted by photographing the membrane under a microscope.
Results
GRN163L disrupts the organization of
cytoskeletal elements
In order to investigate whether the rounding effect
observed within 24 h in the GRN163L-treated A549 cells is related
to any changes in the cytoskeleton, the key elements of
cytoskeletal proteins were investigated using western blot analysis
and immunohistochemical staining techniques.
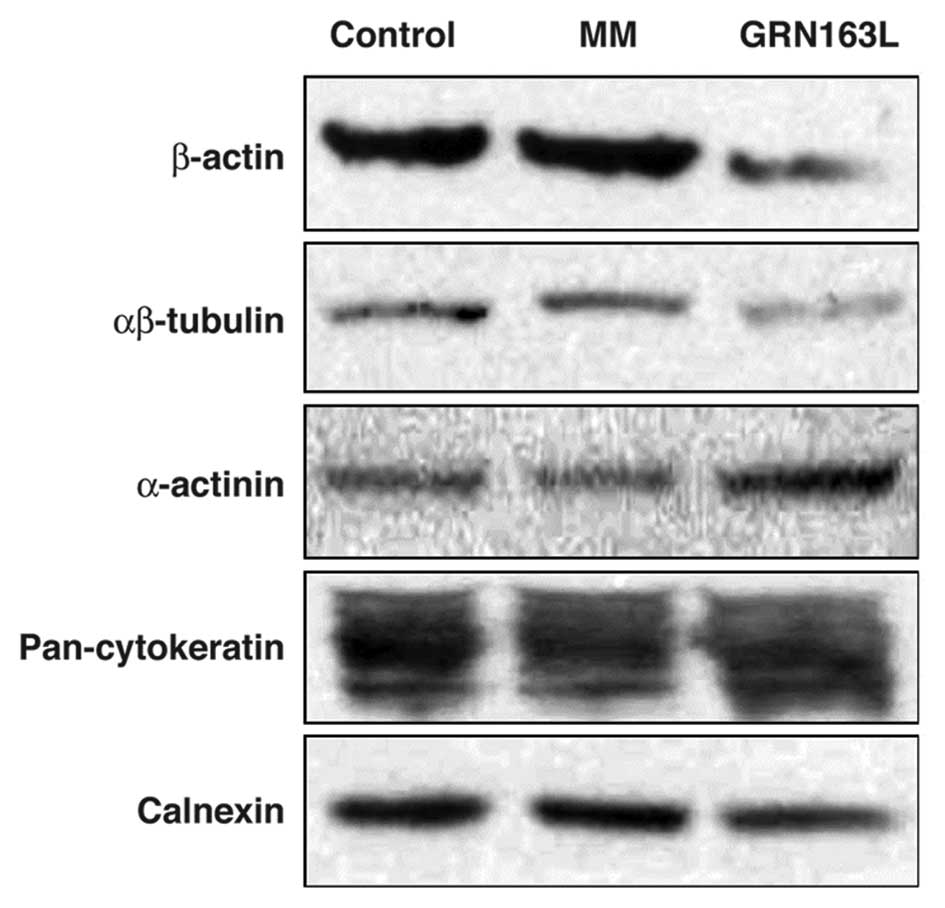
Actin and tubulin are the major proteins of the
cytoskeleton, which determines the shape of the cell. The western
blot analysis results demonstrated that actin and tubulin
expression decreased following a 24-h treatment with GRN163L, when
compared to the control and MM-treated cells (Fig. 1).
As an actin-binding protein, α-actinin plays
multiple roles in different types of cells. In epithelial cells it
is found along actin filament bundles and adherens-type junctions,
where it is involved in the binding of actin to the cell membrane.
We observed an approximate 2- to 3-fold increase in α-actinin
expression in the cells treated with GRN163L for 24 h, compared to
the control and MM-treated cells (Fig.
1).
In the untreated and MM-treated A549 cells, we
observed an organization of dense actin filaments, exhibiting
common cytoplasmic dispersion, as detected by immunohistochemistry
analyses. GRN163L treatment prior to cell attachment disrupted the
cytoplasmic distribution of actin. In the GRN163L-treated cells,
essentially all the actin filaments were displaced and concentrated
along the cell membrane within 24 h. These results demonstrated
that the decrease in actin expression, as well as significant
changes in the morphological distribution of actin in the cell,
were caused by the presence of GRN163L (Fig. 2).
Similar to the intracellular distribution of actin,
extensive bundles of microtubules, which ‘radiate’ throughout the
cytoplasm of A549 cells, was observed in the untreated control
cells. GRN163L treatment altered the perinuclear and radial
organization of the tubulin cytoskeleton and microtubules were
relocated toward the cell membrane, in a pattern similar to that of
actin filaments (Fig. 2). The
immunostaining results demonstrated that α-actinin protein was
localized under the membrane, and was mostly colocalized with actin
filaments (data not shown).
Intermediate filaments form homogeneous polar fibers
within the cells and they are cell-type-dependent. Cytokeratins are
the most common intermediate filaments found in epithelial cells.
Western blot analysis of cytokeratin expression did not demonstrate
any significant difference between the GRN163L-treated and control
cells (Fig. 1). By contrast,
immunohistochemical staining indicated that the cytokeratins were
redistributed evenly throughout the cytoplasm in untreated control
cells, whereas in the GRN163L-treated cells, the cytokeratins were
localized to the cell periphery (Fig.
2).
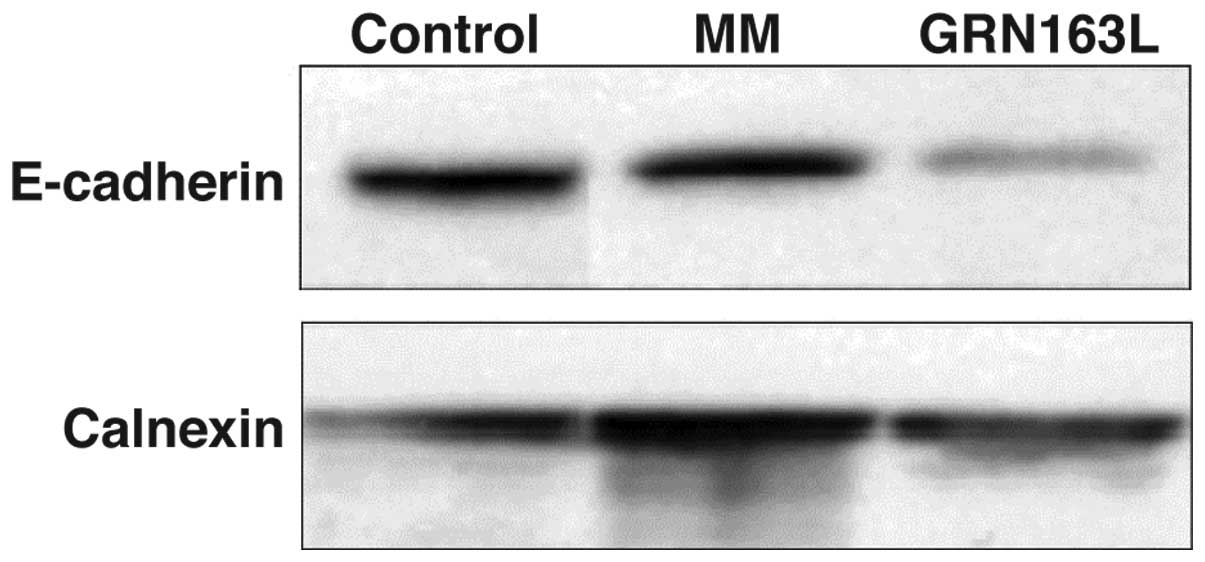
Of note, GRN163L treatment resulted in significant
loss of E-cadherin expression. Western blot analysis results
demonstrated a significant decrease in E-cadherin expression in
A549 cells within 24 h (Fig. 3).
The immunohistochemical analysis results also demonstrated a
decrease in E-cadherin expression at 24 h (Fig. 4).
GRN163L does not cause morphological
changes and loss of cell adhesion in the cytoskeleton after thermal
denaturation (heating for 5 min at 80°C)
After the A549 cells were plated on coverslips,
GRN163L (1 μM) was heated for 5 min at 80°C, then added to
the cell culture medium. Following a 24-h incubation period, the
cells were fixed and stained for actin, tubulin and E-cadherin. The
structures of actin, tubulin and E-cadherin were not markedly
altered in the cells treated with the pre-heated GRN163L.
Approximately 80% of these cells remained attached and exhibited
morphological characteristics (Fig.
5) similar to the untreated control or MM-treated cells (data
not shown). These results demonstrate that morphological changes
and the loss of adhesion are specific to the oligonucleotide and
GRN163L is ‘inactivated’ by heating for 5 min at 80°C.
GRN163L decreases the expression of G1
phase cell cycle control genes
The morphologically altered or ‘rounded’ cells were
unable to proliferate significantly while they were exposed to
GRN163L treatment during the first 72 h. To elucidate the molecular
mechanism behind this initial cell cycle arrest, we evaluated the
mRNA levels of cyclin D1, Cdk4 and Cdk6, which are regulators of
the G1 phase of the cell cycle, by real-time PCR. After plating the
cells, GRN163L was added to the medium and the cells were collected
following 72 h of incubation. No significant change in Cdk4 and
Cdk6 mRNA expression levels was detected following 72 h of
incubation with a single dose of GRN163L (data not shown). When the
second set of cells was treated twice a week and collected for cell
cycle analysis, a significant reduction of cyclin D1, Cdk4 and Cdk6
mRNA expression levels was observed, compared to the untreated
controls (Fig. 6).
GRN163L treatment decreases MMP-2
expression and invasion of A549 lung cancer cells through
Matrigel
When MMP-2 mRNA expression was determined by
real-time PCR, a decrease in MMP-2 mRNA expression of ∼40% was
observed in the A549 cells treated with GRN163L (Fig. 7). To determine whether this
decrease was functional, we examined the
motility/migration/invasive ability of the A549 cells treated with
GRN163L. The cells were exposed to GRN163L for 24 h prior to
plating on Matrigel-coated invasion chambers; the cells were then
allowed to migrate/invade for 22 h. As shown in Fig. 8, GRN163L treatment decreased the
invasive ability of the A549 cells by ∼50%, whereas the untreated
cells were still able to invade. The results for the MM-treated
control cells were similar to those of the untreated cells (data
not shown). The MMP-2 knockdown efficiency was assessed using
quantitative real-time PCR. The results were quantified following
normalization to non-silencing shRNA. The percentage of MMP-2 shRNA
knockdown cells was 28%. shMMP-2 knockdown cells were used as the
negative control in the experiment (Fig. 8) demonstrating the significance of
MMP-2 for the migration through the Matrigel-coated membrane.
Discussion
Unregulated cell proliferation, escape from
apoptosis, increase in tumor neovascularization (angiogenesis),
migration, invasion and metastasis are all common features of
various types of cancer (16).
GRN163L is a telomerase template antagonist, that inhibits
telomerase activity by binding to the template region of hTR. We
discovered that GRN163L exerts additional effects, apart from the
inhibition of telomerase, namely the disruption of cytoskeletal
proteins, such as actin, tubulin, cytokeratin and α-actinin, as
well as E-cadherin organization, and thus impairs cell adhesion and
affects cell morphology. Using western blot analysis, we observed a
significant decrease in actin, tubulin and E-cadherin expression in
the GRN163L-treated cells, compared to the untreated control and MM
oligonucleotide-treated cells (Fig.
2). Immunohistochemical staining also revealed that GRN163L
disrupted the organization of 3 basic elements of the cytoskeleton:
actin, tubulin and intermediate filaments. The altered cell
morphology (i.e., rounding) observed in response to GRN163L
treatment, may be a result of the disorganization of basic elements
of the cytoskeleton, that are the key to sustaining the shape of
the cell and structural scaffold. Of note, the MM control
oligonucleotide (MM oligonucleotide sequence,
5′-Palm-TAGGTGTAAGCAA; GRN163L oligonucleotide sequence,
5′-Palm-TAGGGTTAGACAA) did not have any effect on cell morphology
or cell adhesion (14). Since the
MM control differs from GRN163L only by the lack of 3 contiguous
guanine residues, it is possible that this motif is responsible for
the altered cell morphology and adhesion phenotype upon
treatment.
A similar reduction in migration and metastasis
following treatment with GRN163L has previously been demonstrated
in lung cancer cells using in vivo xenograft animal models
(13,14), although the mechanisms underlying
this effect were not elucidated in these studies. In this study, we
demonstrate that the anti-adhesive effects of GRN163L, which may
also contibute to the antimetastatic properties of this compound,
are related to the disruption of cytoskeletal proteins, resulting
in alterations in cell architecture and the intracellular
relocalization of cytoskeletal elements. Consistent with this
finding, Goldblatt et al(18) demonstrated similar results in
MDA-MB-231 breast cancer cells; when GRN163L was added to the
medium prior to cell attachment, it altered cell morphology, actin
filament organization and focal adhesion formation.
Shin et al(19) demonstrated that actin disruption
induced the phosphorylation of H2AX, a well-known double-strand
break (DSB) marker, leading to G2 phase arrest and consequently
resulting in the apoptosis of MCF-7 cancer cells. Based on these
data, the authors suggested that actin disruption may be a
potential candidate in the development of anticancer therapies for
human cancers. Microtubules are also considered as important
cellular targets for anticancer therapy, due to their key role in
mitosis. Microtubule inhibitors such as taxanes, vinca alkaloids
and epothilones, stabilize or destabilize microtubules, thereby
suppressing microtubule dynamics required for proper mitotic
function, effectively blocking cell cycle progression and resulting
in apoptosis (20).
E-cadherin is critical for epithelial cell-cell
adhesion. It is a well-known fact that, in order to be able to
metastasize, cancer cells require attachment to a solid surface, in
addition to their ability to proliferate and migrate (16). Our western blot analysis and
immunostaining results support the hypothesis that the
GRN163L-induced phenotypic changes may be attributed to alterations
in the structural function of the treated cells. It is also
possible that the downregulation of E-cadherin may reduce the
attachment of cancer cells. In this case, the loss of adhesion may
be due to the change in E-cadherin expression, resulting in the
inability of cancer cells to attach. Additionally, the unattached
‘rounded’ cells lost their proliferative capacity and were
reversibly arrested in the G1 phase of the cell cycle.
It has previously been reported that in NSCLC, the
level of MMP-2 is increased in tumor cells, as well as in the
peritumoral stromal tissues. Furthermore, MMP-2 expression has been
reported to be an indicator of poor prognosis, associated with a
worse overall survival (21). In
this study, we demonstrated that GRN163L treatment led to a
moderate decrease in MMP-2 expression in A549 lung cancer cells.
Additionally, the migration/invasive ability of A549 cells through
Matrigel decreased following a 24-h exposure to 1 μM of
GRN163L. These rapid effects of GRN163L were independent of
telomerase activity and telomere length. In this study, to our
knowledge, we demonstrate for the first time that GRN163L treatment
decreases the migration/invasive capacity of tumor cells, possibly
through the downregulation of MMP-2. These data suggest that
GRN163L treatment following surgery and primary chemo/radiation
therapy may prevent the invasion of residual cancer cells in NSCLC
patients.
In conclusion, in the present study, we demonstrated
that GRN163L altered the cell morphology due to the disruption of
cytoskeletal elements and led to the loss of cell adhesion by
decreasing E-cadherin expression. The cells treated with GRN163L
were arrested in the G1 phase of the cell cycle. Furthermore,
GRN163L inhibited the migration/invasion of A549 lung cancer cells
through the downregulation of MMP-2. Based on these in vitro
data, we hypothesized that residual circulating cancer cells
present in the bloodstream, i.e., after tumor debulking surgery or
chemotherapy, may be unable to attach and proliferate in the
presence of GRN163L, due to the loss of adhesion properties and
proliferative ability. Thus, the addition of a telomerase template
antagonist to the anticancer therapy regimen may not only lead to a
decrease in the growth of the primary tumor mass, but may also
reduce the formation of distant metastases due to its
nontelomerase-related effects.
Acknowledgements
I.M. was supported by TUBITAK
fellowship. This research was supported in part by the Research
Fund of University of Hacettepe, Faculty of Medicine (Project No:
07.01.101.006). This work was also supported in part by NASA Grants
# NNX11AC15G, NNJ05HD36G and NNX09AU95G to J.W.S. This study was
supported by the Scientific and Technological Research Council of
Turkey (TUBITAK) (Project no. 107S232). We thank Adamantia
Papadopoulou (Laboratory of Cell Proliferation and Ageing,
Institute of Biology, National Centre for Scientific Research
‘Demokritos’, Athens, Greece) for technical support.
References
|
1.
|
Dempke WC, Suto T and Reck M: Targeted
therapies for non-small cell lung cancer. Lung Cancer. 67:257–274.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Tassinari D, Scarpi E, Sartori S,
Tamburini E, Santelmo C, Tombesi P and Lazzari-Agli L: Second-line
treatments in non-small cell lung cancer. A systematic review of
literature and metaanalysis of randomized clinical trials. Chest.
135:1596–1609. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Blackburn EH: Switching and signaling at
the telomere. Cell. 106:661–673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Greider CW and Blackburn EH: Telomeres,
telomerase and cancer. Sci Am. 274:92–97. 1996. View Article : Google Scholar
|
|
5.
|
Hiyama E and Hiyama K: Telomere and
telomerase in stem cells. Br J Cancer. 96:1020–1024. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Collins K and Mitchell JR: Telomerase in
the human organism. Oncogene. 21:564–579. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Blasco MA: Telomeres and human disease:
ageing, cancer and beyond. Nat Rev Genet. 6:611–622. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Wai LK: Telomeres, telomerase and
tumorigenesis - a review. Med Gen Med. 6:192004.PubMed/NCBI
|
|
9.
|
Shay JW and Wright WE: Telomerase: a
target for cancer therapeutics. Cancer Cell. 2:257–265. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Harley CB: Telomerase and cancer
therapeutics. Nat Rev Cancer. 8:167–179. 2008. View Article : Google Scholar
|
|
11.
|
Herbert BS, Gellert GC, Hochreiter A, et
al: Lipid modification of GRN163, an N3′→P5′ thio-phosphoramidate
oligonucleotide, enhances the potency of telomerase inhibition.
Oncogene. 24:5262–5268. 2005.PubMed/NCBI
|
|
12.
|
Gryaznov SM: Oligonucleotide N3′→P5′
phosphoramidates and thio-phoshoramidates as potential therapeutic
agents. Chem Biodivers. 7:477–493. 2010.
|
|
13.
|
Dikmen ZG, Gellert GC, Jackson S, et al:
In vivo inhibition of lung cancer by GRN163L: a novel human
telomerase inhibitor. Cancer Res. 65:7866–7873. 2005.PubMed/NCBI
|
|
14.
|
Jackson SR, Zhu CH, Paulson V, et al:
Antiadhesive effects of GRN163L - an oligonucleotide N3′→P5′
thio-phosphoramidate targeting telomerase. Cancer Res.
67:1121–1129. 2007.PubMed/NCBI
|
|
15.
|
Svitkina T: Imaging cytoskeleton
components by electron microscopy. Methods Mol Biol. 586:187–206.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Pećina-Slaus N: Tumor suppressor gene
E-cadherin and its role in normal and malignant cells. Cancer Cell
Int. 3:172003.PubMed/NCBI
|
|
17.
|
Qian Q, Wang Q, Zhan P, Peng L, Wei SZ,
Shi Y and Song Y: The role of matrix metalloproteinase 2 on the
survival of patients with non-small cell lung cancer: a systematic
review with meta-analysis. Cancer Invest. 28:661–669. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Goldblatt EM, Gentry ER, Fox MJ, Gryaznov
SM, Shen C and Herbert BS: The telomerase template antagonist
GRN163L alters MDA-MB-231 breast cancer cell morphology, inhibits
growth, and augments the effects of paclitaxel. Mol Cancer Ther.
8:2027–2035. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Shin IJ, Ahn YT, Kim Y, Kim JM and An WG:
Actin disruption agents induce phosphorylation of histone H2AX in
human breast adenocarcinoma MCF-7 cells. Oncol Rep. 25:1313–1319.
2011.PubMed/NCBI
|
|
20.
|
Perez EA: Microtubule inhibitors:
Differentiating tubulin-inhibiting agents based on mechanisms of
action, clinical activity, and resistance. Mol Cancer Ther.
8:2086–2095. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Hojilla CV, Mohammed FF and Khokha R:
Matrix metalloproteinases and their tissue inhibitors direct cell
fate during cancer development. Br J Cancer. 89:1817–1821. 2003.
View Article : Google Scholar : PubMed/NCBI
|