Introduction
Endothelial protein C is an important regulator of
homeostasis in addition to its involvement in the systemic response
to acute inflammation. It is known that circulating protein C
zymogens, secreted by liver, binds to endothelial protein C
receptor (EPCR) with high affinity and stimulates its activation
via the thrombin-thrombomodulin complex. The activated protein C
(aPC), together with its cofactor protein S, degrades factors Va
and VIIIa and thereby interferes with thrombin generation and
inhibits the coagulation cascade (1–3).
EPCR exists as membrane bound as well as free soluble form (sEPCR).
sEPCR, infact, can regulate the quantity of circulating aPC
(4,5).
EPCR is a type 1 transmembrane glycoprotein that
shares considerable homology with the major histocompatibility
complex (6). EPCR is known to be
constitutively released in the plasma in a free soluble form as a
result of proteolytic cleavage. Soluble EPCR has the ability to
trap free aPC, thereby depriving the latter of its anticoagulant
function (within the surrounding environment) (4–6). The
shedding of EPCR is known to be regulated by IL-1β (interleukin),
TNF-α, endotoxin, and via the MAP kinase signaling pathways in
human vascular endothelial cell line (HUVEC) (7) and by the presence of EPCR
A3-haplotype homozygosis (8).
It is known that in severe sepsis aPC is also
involved in preventing thrombosis due to its anticoagulant action
(9). A drop in levels of protein C
in severe sepsis is in fact always associated with poor prognosis
(10). Several studies have
demonstrated the anticoagulant and pro-fibrinolytic nature of aPC
and also its involvement in inflammation (11). The involvement of certain signaling
pathways such as NF-κB (12) and
WNT (13) were also revealed. On
the other hand, the influence of aPC on the immune system is far
from clear today.
With the recent appearance of an increasing number
of publications, the interest in EPCR/aPC, in relation to tumor
biology, is gaining momentum (14–17).
In a previous study, we demonstrated that several solid tumors
express EPCR and that sEPCR in patients with ovarian cancer could
be a biomarker of cancer expansion (18). In addition sEPCR was proposed by us
to be a biomarker of cancer associated hypercoagulability in human
hematologic malignancies (19).
The increase in plasmatic level of sEPCR in cancer
patients leads to increased sequestration/capture and use of
protein C. This could affect or modulate thrombotic events and
immune inflammatory response. As of present, the question of how
sEPCR is involved in tumor immunology, however, remains unclear. On
the basis of our present findings we are inclined to think that
besides being a biomarker of cancer expansion and cancer associated
hypercoagulability (18,19) sEPCR (a regulator of circulating
aPC) could be involved in the innate immune response in ovarian
cancer patients.
Materials and methods
Reagents
Reagents were obtained from following sources:
primary antibody AF2245 against the EPCR (R&D Systems,
Minneapolis, MN, USA); primary antibody ATAP2 against PAR-1
(Invitrogen, Carlsbad, CA, USA); biotinylated anti-rabbit,
anti-mouse and anti-goat IgG, streptavidin-fluorescein conjugate
(Amersham, Buckinghamshire, UK); rabbit anti-goat HRP
(DakoCytomation, Glostrup, Denmark); human recombinant aPC (Lilly,
Suresnes, France).
For phenotyping of immuno-inflammatory cells we used
FITC-anti-CD29, PE-anti-CD30, ECD-anti-CD8, PC5-anti-CD25,
PC7-anti-CD20, APC-anti-CD14, APC AF700-anti-CD45, APC
AF750-anti-CD16, FITC-anti-CD31, PE-anti-CD294, ECD-anti-CD45RA,
PC5-anti-CD146, PC7-anti-CD4, APC-anti-CD56, APC-AF700-anti-CD10,
APC-AF750-anti-CD3 (all from Beckman Coulter, Roissy CDG, France).
FITC-anti-CD94, PE-anti-HLA-G, APC-anti-ROR-G, PercPcy
5.5-anti-CD161. FITC-anti-IL2, PE-anti-IL21, PC5-anti-FOXP3,
PercpCy 5.5-anti-IL17A, APC-anti-TGFβ (transforming growth factor),
PE-anti-CD133, PE-anti-IL10 (all from eBiosciences, Montrouge,
France). The 174 and 100 biotin label-based human antibody array
from RayBioTech (Clinisciences, Montrouge, France) were used.
Cells
Ovarian cancer cell lines (Ovcar-3, Skov-3, ATCC)
were used. Cells were cultured in DMEM medium containing 10% fetal
calf serum, penicillin (50 U/ml), and streptomycin (50
μg/ml) and incubated in a humidified atmosphere containing
5% CO2 at 37°C, as recommended by the supplier (PAA
Laboratories Inc, Etobicoke, ON, USA).
Plasma and blood mononuclear cells
Plasma samples
Patients were aged >18 years, with a histological
proven diagnosis of epithelial cancer of the ovary with
asymptomatic disease in progression (detected by increase of CA125
levels according to Gynecologic Cancer Intergroup criteria).
Ovarian tumors of low malignant potential or non-epithelial ovarian
or mixed epithelial/non-epithelial tumors were excluded. Blood
samples from patients (n=33) were obtained after informed consent,
in accordance with the rules of the revised Helsinki protocol and
with Articles L. Al 1121-1 1, L. 1221-8 and L. 1221-8-1, and
following the Code of Public Health (CSP), the plasmas were
provided to us with a label depicting a code number without the
name of the patient. All participants provide their written consent
to participate in this study and the ethics committees CPP of Ile
de France-1 (regional committee) and National Agency for the Safety
of Drugs and Health Products (ANSM national committee) approved
this consent procedure and this study.
Mononuclear cells
PBMCs were collected from 33 cancer patients at the
Hospital Hôtel-Dieu and mononuclear cells were separated by Ficoll
gradient centrifugation and then stored in 200 μl of DMSO
(10%)/FCS 90% at −80°C.
Phenotyping of immuno-inflammatory cells by
immunocytochemical analysis. Flow cytometry was carried out using
the 30 antibodies as described in Reagents section.
Mononuclear cells were labeled with several
antibodies bound to different fluorescent agents. The controls were
performed using corresponding isotype antibodies. The cytometer
used was a type analyzer LSRII (BD Bioscience, Le Pont de Claix,
France) to 9 colors and 4 lasers.
The antigens, detected on appropriate cells, were
identified and analyzed by ‘DAVID Gene Concept’ which is a
functional annotation tool. It allows ranking functional categories
of sets of genes and unraveling new biological processes associated
with cellular functions. The results are expressed as percentage of
cells for each blood sample. The analysis was done on the area
bound P1 representing lymphocytes. The protocol used in this
project was developed using samples obtained from five healthy
females (aged 25–50 years).
Soluble EPCR-ELISA assay
Soluble EPCR in plasma fluids was measured using
Asserachrom sEPCR immunoassay as recommended by the commercial
supplier (Diagnostic Stago, Asnieres sur Sein, France).
Cytokine array
The effect of APC on the ovarian
cancer cell line
To analyze the pleiotropic role of APC, we examined
the supernatant of Ovcar cells using a protein cytokine array
(RayBio® Human Cytokine Antibody). This technique is
based on the principle of ‘sandwich immunoassay’. It comprises
essentially of screening, in duplicate, 174 different
membrane-coupled anti-cytokines along with appropriate controls
(experiments repeated 3 times).
Ovcar-3 cells (106 cells per ml) were
incubated in presence (or not) of APC (200 ng/ml−1) in
DMEM without fetal calf serum at 37°C in a humidified atmosphere of
5% CO2 for 24 h. Supernatants containing cytokines were
retrieved and the cytokines were allowed to couple with their
specific antibodies previously immobilized on membranes. Membranes
were saturated for 2 h at room temperature with bovine serum
albumin (BSA). Incubation of array membranes with supernatants
(along with controls) was carried out overnight at 4°C using
corresponding antibodies. After several successive washes,
membranes were incubated in the presence of a mixture of antibodies
and anti-cytokines biotinylated at 4°C overnight. Streptavidin,
coupled with HRP, was added on the membranes for 2 h at room
temperature. The presence of antibody coupled proteins was revealed
by applying ECL (enhanced chemiluminescence) to the membranes,
according to the recommendations of the manufacturer. Membranes
were then exposed to photosensitive film (Kodak, X-OMAT, AR,
USA).
The intensity of chemiluminescence captured on the
photosensitive film was measured and recorded. After substracting
the backgroud noise, the results were expressed as a ratio of
chemiluminescence intensity of experimental versus control spots.
The positive control was considered as 1. Less than a −2 ratio
value indicated a reduction of the cytokine and a value greater
than +2 indicated an increase in cytokine expression.
Plasma cytokine array
Plasma cytokine array was performed using different
membranes coupled with 100 anti-cytokines antibodies (RayBio Biotin
Labeled-based Human Antibody Array) as described above for cell
cytokines. Briefly, the membranes were incubated with serum from
ovarian cancer patients (diluted 10 times in PBS and BSA 1%).
Signal intensities were quantified with a Bio-Imaging System
MF-ChemiBis 4.2 (FSVT, Courbevoie, France) and analyzed with Multi
Gauge V3.2 software (Fujifilm). For each spot, the net optical
density level was determined by subtracting the background optical
level from the total raw optical density level.
Statistical analysis
Statistical analyses were performed using Prism 4.0
(GraphicPad) or Cricket Graph III (Computer Associates
International) software. Parametric statistical analysis (mean ±
SEM, linear and exponential regression) was performed using
standard methods.
Spearman’s rank correlation coefficients
(non-parametric measures) were calculated using R software.
Correlation of coefficient significance was determined by a
statistical hypothesis test (α=0.05).
Results
Active protein C induces the secretion
of cytokines in ovarian cancer cell lines
The in vitro cell line, Ovcar-3 and cells of
ovarian tumor, share many biological properties. Both express EPCR
and PAR-1 (18). Both ovarian
cancer cells and ovarian cell lines (Ovcar-3 and Skov cells) could
be targets for circulating aPC or exogenously added aPC. Since
these cells (18) display certain
makers of stem cells (CD117+, CD133+), we
looked for the influence of aPC on the secretion of physiologically
active proteins by the Ovcar and Skov cells and analyzed these
proteins using a RayBioTech protein array method.
Of the 174 proteins proposed in this test only 23
proteins were upregulated (ratio of cells treated by aPC vs.
non-treated cells was >2) while 14 were downregulated (ratio of
cells treated by aPC versus non-treated cells was <2). When
ratio was more than 4 it was considered as a positive response. As
presented in Fig. 1 we observed
that aPC induced the secretion of several cytokines such as TARC
(thymus and activation-regulated chemokine, CCL17), TECK (thymus
expressed chemokine, CCL25), IL-2, TPO (thrombopoietin), BLC (B
lymphocyte chemoattractant, CXCL13), PDGF-BB (platelet-derived
growth factor-BB), CNTF (ciliary neurotrophic factor), I-TAC (T
cell alpha chemoattractant CXCL11, also called interferon-inducible
T cell alpha) and ICAM-3 (intercellular adhesion molecule 3, CD56)
in ovary cell lines at high level (r ≥4). From the data obtained by
gene code analysis of all regulated bioactive proteins using DAVID
v6.7, we found that these proteins may be involved in
cytokine/cytokine-receptor interactions and in immune
responses.
 | Figure 1.The effect of aPC on the ovarian
cancer cell line. In order to analyze the pleiotropic role of
active protein C (after treatments of ovarian cell line with aPC
for 24 h), we examined the cell supernatants using a protein
cytokine array as described in Materials and methods
(RayBio® Human Cytokine Antibody). The ratio of values
between cells treated by aPC and non-treated is shown. The positive
control was considered as 1. Of the 174 proteins proposed in this
test only 23 were upregulated (ratio of cells treated by aPC vs.
non-treated cells was >2). They are TARC, TEK, IL-2, TPO,
ICAM-3, BLC, PDGF-BB, CNTF, I-TAC, LIGHT, SDF-1α, CTACK, TRAIL-R3,
SCF, FLT-3 ligand, ICAM-1, BMP-6, FGF-7, MIP-1α, angiogenin, BDNF
and FGF-6 while 14 proteins such as TGFα, TGFβ, PECAM-1, MIP-3α,
SDF-1β, NGF-R, Siglec-5, BMP-4, prolactin, PARC, MIP-1δ and TNFβ
were downregulated (ratio cells treated by aPC vs. non-treated
cells was <2). The ratio situated beyond 4 was taken into
consideration for the present study. The results indicate that aPC
interacted with tumor cells and activated the secretion of several
cytokines such as thymus and activation-regulated chemokine (TARC,
CCL17), thymus expressed chemokine (TECK, CCL25), interleukin-2
(IL-2), thrombopoietin (TPO), intercellular adhesion molecule-3
(ICAM-3, CD56), B lymphocyte chemoattractant (BLC),
platelet-derived growth factor-BB (PDGF-BB), ciliary neurotrophic
factor (CNTF) and T cell alpha chemoattractant (I-TAC) by ovarian
cell lines. |
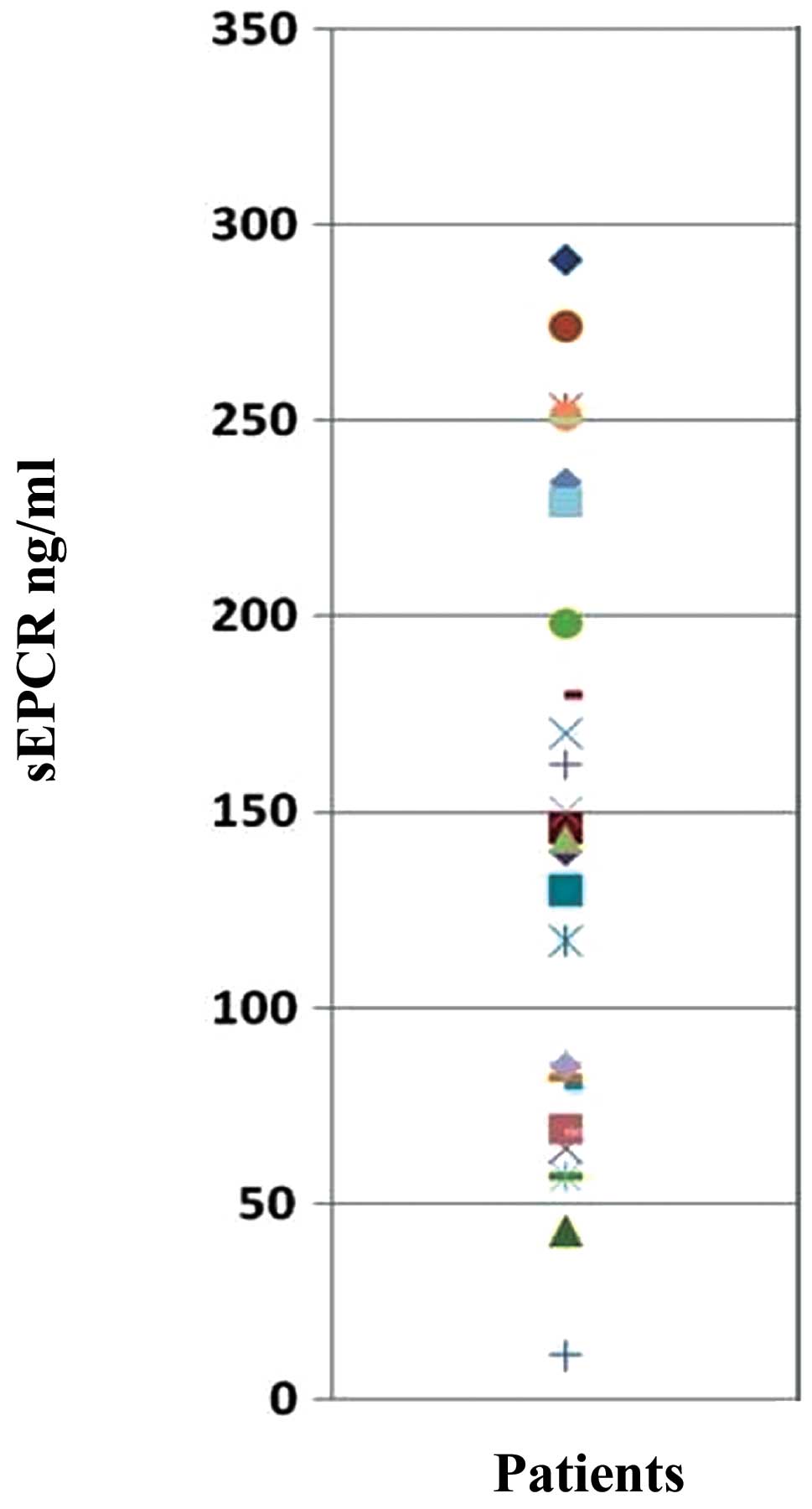
Evaluation of sEPCR in plasma of
ovarian cancer patients
Protein C is involved in septicemia. It can also be
trapped by free sEPCR thus rendering it unavailable for thrombotic
function. We determined by ELISA the quantity of plasmatic sEPCR in
patients diagnosed with ovarian cancer, but asymptomatic. All the
samples tested (n=33) were positive for the presence of sEPCR. The
base-line value of 100±28 ng/ml was considered as normal. Thus, 61%
exhibited a concentration well above the base-line (Fig. 2).
Influence of plasma sEPCR on
circulating immune cells
To underline the significance of sEPCR in tumor
behavior and its influence on circulating immune cells, we analyzed
the correlation of sEPCR level in plasma with that of immune cells
from blood in 33 patients, using antibodies against CD45, CD45ra,
CD3, CD4, CD8, CD25, Foxp3, CD294, CD30, CD56, CD161, CD14, CD16,
CD20, CD31, Cd133, CD31, CD10, CD146, IL-21, IL-2, TGFβ, IL-17a,
IL10, CD94, CD127, ROR-γ, CD127 and CD29.
As indicated in Fig.
3, the patients were divided into 2 groups: group 1 with sEPCR
<100 ng/ml (normal) (Fig. 3A)
and group 2 with sEPCR >100 ng/ml (Fig. 3B). A total of 61% of patients
exhibited a concentration of plasma sEPCR well above the base-line
(normal plasma level, 100±28 ng/ml). Comparing immune cell
phenotypes in patients having a normal level of sEPCR with those
having a high level of sEPCR, it was found that sEPCR level was
correlated with high intensity of cells expressing CD45ra, CD3,
CD8, CD25 and low intensity of cells expressing CD56 (NK cells),
CD294 (TH2 cells), IL-2, IL-10, IL-17a (TH17 cells), IL-21 (TH21
cells) and CD29 markers (r ≥0.60). In addition, when level of sEPCR
was high, a decrease in IL-17a-expressing cells was associated with
decrease of cells expressing CD161 and ROR-γ (RAR-related orphan
receptor) involved in the secretion of IL-17 (Fig. 3).
 | Figure 3.Influence of plasma sEPCR in blood
immune cells. Mononuclear cells from 33 patients were separated by
Ficoll gradient centrifugation and then stored in 200 μl of
DMSO/FCS 50% at −80°C. Phenotyping of immuno-inflammatory cells
were performed by flow cytometry the using 30 antibodies as given
above. (A) When sEPCR is <100 ng/ml (group 1), plasmatic sEPCR
correlated with low intensity of some cell associated markers such
as CD45ra, CD3, CD8 and high intensity of with CD56 (NK cells) and
cell associated IL-2, IL-10, IL-17a, IL-21 and CD29 markers (r
≤0.40). When sEPCR is >100 ng/ml (group 2), plasmatic sEPCR
correlated with high intensity of CD45ra, CD3, CD8, CD25 and low
intensity of CD56, IL-2, IL-10, IL-21 and CD29 markers (r ≥0.60).
The expression of some cell markers such as CD4, FoxP3, CD20 and
CD10 was independent of plasma sEPCR variation (r ≤0.3). A decrease
in IL-17a in group-2 (B) was associated with the diminition of
CD161 and ROR-γ associated cells. These results indicate that
increase of plasma sEPCR was associated with a decrease of effector
immune cells in blood circulation. |
Influence of sEPCR on cytokines and
interleukins in plasma
To understand the influence of sEPCR on plasma
cytokines and interleukins, we analyzed, using protein array test
the correlation between the amount of sEPCR in plasma (groups 1 and
2) and a set of 100 biologically active plasma proteins. We
compared the significance (r ≥0.60) of plasmatic sEPCR in group 1
(sEPCR <100, blue histograms) and in group 2 (sEPCR >100, red
histogramms) (Fig. 4A).
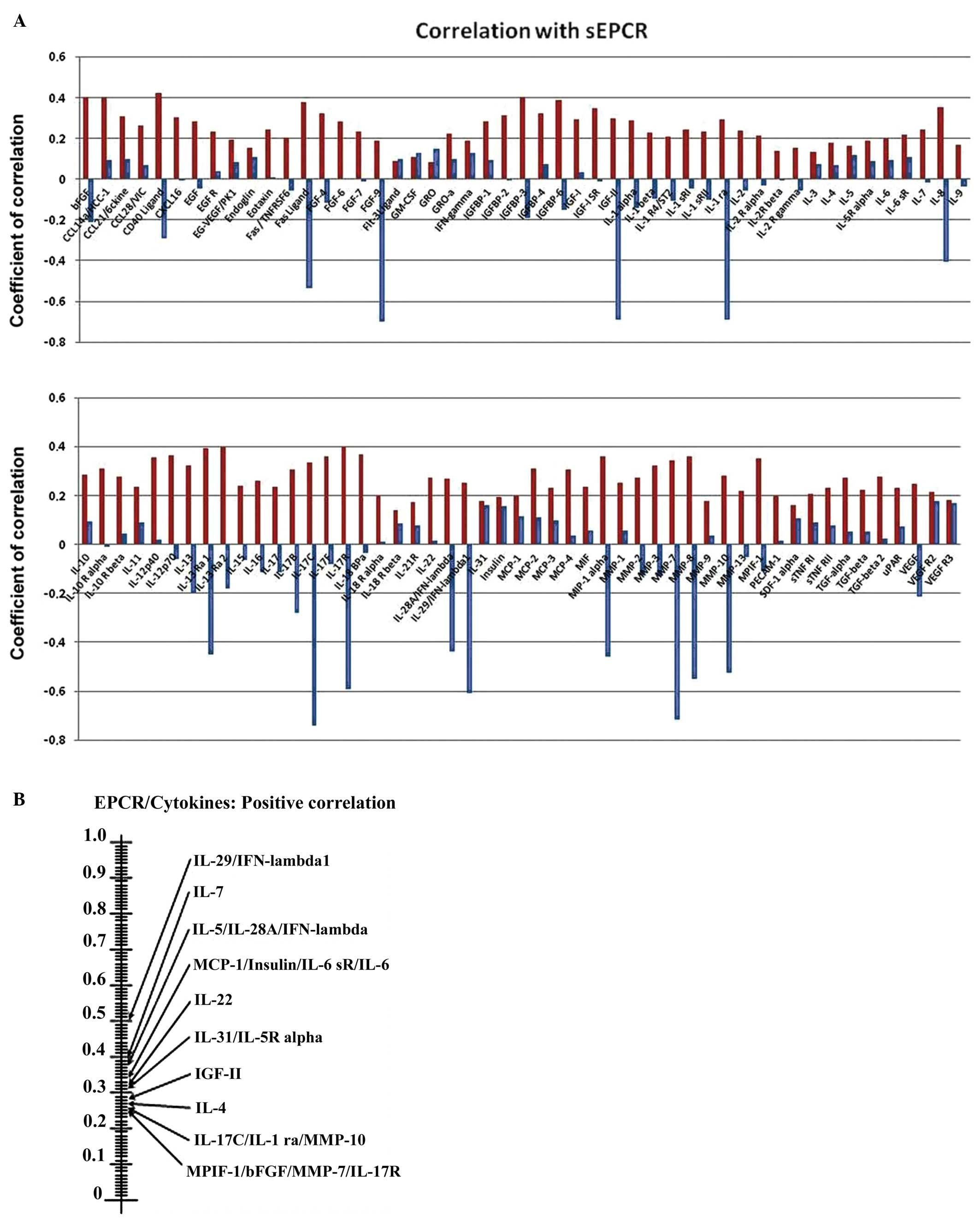 | Figure 4.Influence sEPCR on the level of
cytokines and interleukins in plasma. (A) We analyzed the
correlation between the amount of sEPCR in plasma and a set of 100
biologically active plasma proteins. The membranes were incubated
with serum from ovarian cancer patients (10X in PBS albumin 1%).
Signal intensities were quantified with a Bio-Imaging System
MF-ChemiBis 4.2 and analyzed with Multi Gauge V3.2 software. We
compared the significance (r ≥0.60) of plasmatic sEPCR in group 1
(sEPCR <100, blue histograms) and in group 2 (sEPCR >100, red
histograms). When plasma sEPCR is >100 ng/ml, the expression of
Fas-Ligand, CD 40 ligand, bFGF, FGF-9, IGF-II, IGFbp 3 and 6, IL-ra
(receptor antagonist), IL-8, IL-13ra, IL-17b, IL-17c, IL-17R,
IL-28A, IL-29, MIP-1, MMP-7, MMP-9, MMP-10 (r ≥0.60) and VEGF (r=
0.40) not only reversed but also increased significantly. (B) The
experimental data were further subjected to scrutiny using the
Sprearman statistical test. These results confirmed the results
shown in (A) and also revealed some additional ones between sEPCR
levels and the recently classified type III interferon group. |
The experimental data were further subjected to
scrutiny using the Sprearman statistical test (Fig. 4B). We observed (Fig. 4A) that when plasma sEPCR was higher
than 100 ng/ml; the expression of Fas-Ligand, CD40 ligand, bFGF,
FGF9, IGFII (insulin-like growth factor), IGFbp3 and 6
(insulin-like growth factor binding proteins), ILra (receptor
antagonist), IL-8, IL-13ra, IL-17b, IL-17c, IL-17R, IL-28A, IL-29,
MIP1 (macrophage inflammatory protein), MMP-7 (matrix
metalloproteinase), MMP-9, MMP-10 (r ≥0.60) and VEGF (r=0.40)
increased significantly compared to when sEPCR level was below 100
ng/ml.
Certain proteins such as IGFII, IL-13rα, MIP1α and
MMP7 have already been proposed as biomarkers for ovarian cancer
and particularly those with poor prognosis (20–22).
The Spearman statistical test (Fig.
3B), confirmed the results shown in Fig. 3A and also revealed some additional
ones between sEPCR levels and the recently classified type III
interferon group (IL-28A, and IL-29).
Discussion
In a recently published work we showed that ovarian
cancer cell line Ovcar-3 expressed stem cell markers
CD133+/CD117+(C-Kit) (18). Another ovarian cancer cell line
Skov also expressed C-Kit (80%), a receptor for stromal growth
factor. Similar results were also obtained when ovarian tumors were
screened (results not shown). Ovarian tumors and the ovarian cell
lines also expressed EPCR and PAR-1. In the present study we
analyzed, by a proteins array test, the influence of aPC on the
secretion of biologically active proteins.
Our results indicate that aPC induces the secretion
of cytokines such as TARC (23,24),
TECK (25), IL-2 (26), TPO (27), BLC (28), PDGF-BB (29), CNTF (ciliary neurotrophic factor)
(30), I-TAC (31) and ICAM-3 (32) by tumor cells. These pleiotropic
cytokines and proteins, believed to play a role in the development
of T cells, are in addition chemoattractants for B and T
lymphocytes. In light of these observations, we predict that
ovarian cancer cells could be targets for circulating protein C,
which in turn may be affected by plasma sEPCR.
In the study reported here, we present data on the
significance of soluble endothelial protein C receptor (sEPCR) in
circulating immune cells and the regulation of cytokines and
interleukins in plasma of patients with ovarian cancer.
We investigated the influence of plasma sEPCR on the
immune system by characterizing immune cells from 33 ovarian cancer
patients as described in Materials and methods. Based on the sEPCR
level, group 1 patients, showed high content of CD29, CD31, CD56
and IL-2, IL-10, IL-17a, and IL-21 associated immune cells and low
levels of CD45ra and CD14. Surprisingly, statistical analysis
showed a positive correlation between sEPCR and CD3, CD8 and a
negative correlation between CD56 (NK cells), CD29 (integrin β-1,
present on all blood cells), CD294 (TH2 cells), and the immune
cells containing IL-2, IL-17a, IL-10 and IL-21. IL-2 is one of a
multitude of cytokines produced by lymphocytes and monocytes that
trigger a cascade of immune reactions (33). IL-10 inhibits the synthesis of
pro-inflammatory cytokines such as IFN-γ, IL-2 and TNFα. It has the
ability to suppress the antigen-presentation capacity of antigen
presenting cells (34). The exact
role of T helper (Th) 17 cells in malignancy is currently under
debate. Th17 plays a protective role against extracellular bacteria
and fungi by inducing an inflammatory response (35,36).
Interleukin-21 is a potent immunomodulatory four-α-helical-bundle
type I cytokine and is produced by NKT and CD4+ T cells
(37). CD294 is a prostaglandin D2
receptor that mediates the pro-inflammatory chemotaxis and is
preferentially expressed in CD4+ effectors T helper 2
(Th2) cells (38). These results
indicated that in several ovarian cancer patients with high level
of sEPCR, there was a decrease of immune cells that are otherwise
crucial in innate immune response.
CA125 is currently used and is an important biologic
marker for ovarian cancer (39).
Other plasmatic proteins such as IGFII, IL-13rα, MIP1α and MMP-7
were reported as ovarian cancer biomarkers associated with poor
prognosis (40). In a recent study
we demonstrated a close correlation between increase in sEPCR and
CA125 (18). However, the
correlation between sEPCR and other cytokines, cited above remains
yet to be worked out.
A step in this direction was made by undertaking
measurements of soluble plasma cytokines, chemokines and
interleukins via a proteins array test. All proteins (r ≥0.60)
including Fas-Ligand, CD40 ligand, bFGF, FGF9, IGFII, IGFbp3 and 6,
ILra (receptor antagonist), IL-8, IL-13rα, IL-17b, IL-17c, IL-17R,
IL-28A, IL-29, MIP1α, MMP-7, MMP-9, MMP-10 and VEGF (r= 0.40)
increased when plasma sEPCR was higher than 100 ng/ml (group 2).
Markers such as IGFII, IL-13rα, MIP1α and MMP-7 correlated well
with high amount of plasma sEPCR and CA125 in ovarian cancer.
Results obtained here indicate that sEPCR has an
influence on immune cells and plasma cytokine levels. However, how
this occurs remains to be clarified. Treatment of blood mononuclear
cells (n=5) with aPC (10 μg/ml for 48 h, in vitro)
did not modify significantly the immune cell subpopulation as
judged by presence/absence of different characteristic markers
(results not shown). Further studies are necessary to investigate
the effect of aPC on blood mononuclear cells and their cytokine
profile of antigen presenting or effector cells.
Activated protein C is a key inhibitor of fibrin
formation. Other than hemostastic function, aPC is also known to
exert pleiotropic effects, depending on the cell type expressing
EPCR. Among others, aPC interferes with the endothelial cell p53
pathway (41,42). It also promotes endothelial cell
proliferation through MAPK and PI3K signaling pathways (43,44).
Cancer cells expressing EPCR may therefore benefit from the
cytoprotective effect imparted by APC (45). Scheffer et al reported a
high expression of EPCR in a large panel of tumor cell lines and
have interpreted this in light of the EPCR’s role in coagulation
(16). Beaulieu and Church
(17) claimed that aPC increases
breast cancer cell invasion and chemotaxis through EPCR and PAR-1.
These sound findings are demonstrative of the importance of EPCR
expression and aPC-EPCR signaling pathway in tumor cells.
The overall data suggest that differential
expression patterns of chemokines are involved in the specific
inflammatory microenvironment of ovarian cancers. In a parallel,
but different study (unpublished data) using mRNA-gene array
(n=1200) and DAVID analysis of results, we showed the activation of
innate immune gene family such as Jak-STAT (46) and TOLL-like receptor (47) when the breast cancer MDA-231 cell
line was stimulated by activated protein C. These results suggest
that plasma aPC/sEPCR can influence plasma cytokine levels in a
quantitative and qualitative manner. As a consequence, circulating
or intra-tumoral lymphocytes will respond to variations in plasma
sEPCR. Future investigation by a randomized study needs to analyze
whether all the biomarkers only correlate with severity of the
disease.
The above cited cytokines may have a vast potential
as well as pleiotropic function. A precise understanding of their
involvement in the development of ovarian cancer is critical in
developing effective strategies for disease intervention. This is
particularly important in the light of a downregulation of NK cells
and the immune cells containing interleukin 2, IL-17a (TH17) and
IL-21 which suggests the influence of tumor cells on the innate
immune system.
References
|
1.
|
Dahlbäck B and Villoutreix BO: Molecular
recognition in the protein C anticoagulant pathway. J Thromb
Haemost. 1:1525–1534. 2003.
|
|
2.
|
Li W, Zheng X, Gu J, Ferrell GL, Lupu F,
Esmon NL and Esmon CT: Overexpressing endothelial cell protein C
receptor alters the hemostatic balance and protects mice from
endotoxin. J Thromb Haemost. 3:1351–1359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Taylor FB Jr, Peer GT, Lockhart MS,
Ferrell G and Esmon CT: Endothelial cell protein C receptor plays
an important role in protein C activation in vivo. Blood.
97:1685–1688. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Fukudome K, Kurosawa S, Stearns-Kurosawa
DJ, He X, Rezaie AR and Esmon CT: The endothelial cell protein C
receptor. Cell surface expression and direct ligand binding by the
soluble receptor. J Biol Chem. 271:17491–17498. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Xu J, Qu D, Esmon NL and Esmon CT:
Metalloproteolytic release of endothelial cell protein C receptor.
J Biol Chem. 275:6038–6044. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Fukudome K and Esmon CT: Identification,
cloning, and regulation of a novel endothelial cell protein
C/activated protein C receptor. J Biol Chem. 269:26486–26491.
1994.PubMed/NCBI
|
|
7.
|
Zheng X, Li W, Gu JM, Qu D, Ferrell GL,
Esmon NL and Esmon CT: Effects of membrane and soluble EPCR on the
hemostatic balance and endotoxemia in mice. Blood. 109:1003–1009.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Saposnik B, Lesteven E, Lokajczyk A, Esmon
CT, Aiach M and Gandrille S: Alternative mRNA is favored by the A3
haplotype of the EPCR gene PROCR and generates a novel soluble form
of EPCR in plasma. Blood. 111:3442–3451. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Shorr AF, Janes JM, Artigas A, Tenhunen J,
Wyncoll DL, Mercier E, Francois B, Vincent JL, Vangerow B,
Heiselman D, Leishman AG, Zhu YE and Reinhart K: Randomized trial
evaluating serial protein C levels in severe sepsis patients
treated with variable doses of drotrecogin alfa (activated). Crit
Care. 14:R2292010. View
Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Yan SB, Helterbrand JD, Hartman DL, Wright
TJ and Bernard GR: Protein C levels as a prognostic indicator of
outcome in sepsis and related diseases. Crit Care Med. 28:S49–S56.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Della Valle P, Pavani G and D’Angelo A:
The protein C pathway and sepsis. Thromb Res. 129:296–300.
2012.PubMed/NCBI
|
|
12.
|
Joyce DE, Gelbert L, Ciaccia A, DeHoff B
and Grinnell BW: Gene expression profile of antithrombotic protein
c defines new mechanisms modulating inflammation and apoptosis. J
Biol Chem. 276:11199–11203. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Pereira C, Schaer DJ, Bachli EB, Kurrer MO
and Schoedon G: Wnt5A/CaMKII signaling contributes to the
inflammatory response of macrophages and is a target for the
antiinflammatory action of activated protein C and interleukin-10.
Arterioscler Thromb Vasc Biol. 28:504–510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Tsuneyoshi N, Fukudome K, Horiguchi S, Ye
X, Matsuzaki M, Toi M, Suzuki K and Kimoto M: Expression and
anticoagulant function of the endothelial cell protein C receptor
(EPCR) in cancer cell lines. Thromb Haemost. 85:356–361.
2001.PubMed/NCBI
|
|
15.
|
Wang X, Wang E, Kavanagh JJ and Freedman
RS: Ovarian cancer, the coagulation pathway, and inflammation. J
Transl Med. 3:25–29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Scheffer GL, Flens MJ, Hageman S,
Izquierdo MA, Shoemaker RH and Scheper RJ: Expression of the
vascular endothelial cell protein C receptor in epithelial tumour
cells. Eur J Cancer. 38:1535–1542. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Beaulieu LM and Church FC: Activated
protein C promotes breast cancer cell migration through
interactions with EPCR and PAR-1. Exp Cell Res. 313:677–687. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ducros E, Mirshahi S, Azzazene D,
Camilleri-Broët S, Mery E, Al Farsi H, Althawadi H, Besbes S,
Chidiac J, Pujade-Lauraine E, Therwath A, Soria J and Mirshahi M:
Endothelial protein C receptor expressed by ovarian cancer cells
can be a biomarker of cancer expansion. Int J Oncol. 41:433–440.
2012.
|
|
19.
|
Ducros E, Mirshahi S, Faussat AM, Mirshahi
P, Dimicoli S, Tang R, Pardo J, Ibrahim J, Marie JP, Therwath A,
Soria J and Mirshahi M: Soluble endothelial protein C receptor
(sEPCR) is a likely as a biomarker of cancer associated
hypercoagulability in human hematologic malignancies. Cancer Med.
1:261–267. 2012. View
Article : Google Scholar
|
|
20.
|
Davidson B, Zhang Z, Kleinberg L, Li M,
Flørenes VA, Wang TL and ShihIe M: Gene expression signatures
differentiate ovarian/peritoneal serous carcinoma from diffuse
malignant peritoneal mesothelioma. Clin Cancer Res. 12:5944–5950.
2006. View Article : Google Scholar
|
|
21.
|
Kioi M, Kawakami M, Shimamura T, Husain SR
and Puri RK: Interleukin-13 receptor alpha2 chain: a potential
biomarker and molecular target forovarian cancer therapy. Cancer.
107:1407–1418. 2006.PubMed/NCBI
|
|
22.
|
Amonkar SD, Bertenshaw GP, Chen TH,
Bergstrom KJ, Zhao J, Seshaiah P, Yip P and Mansfield BC:
Development and preliminary evaluation of a multivariate index
assay for ovarian cancer. PLoS One. 4:e45992009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kaushansky K: Lineage-specific
hematopoietic growth factors. N Engl J Med. 354:2034–2045. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Liu YJ, Soumelis V, Watanabe N, Ito T,
Wang YH, Malefyt Rde W, Omori M, Zhou B and Ziegler SF: TSLP: an
epithelial cell cytokine that regulates T cell differentiation by
conditioning dendritic cell maturation. Annu Rev Immunol.
25:193–219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Johnson EL, Singh R, Singh S,
Johnson-Holiday CM, Grizzle WE, Partridge EE and Lillard JW Jr:
CCL25-CCR9 interaction modulates ovarian cancer cell migration,
metalloproteinase expression, and invasion. World J Surg Oncol.
8:622010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Vergara C and Ramirez B: CNTF, a
pleiotropic cytokine: emphasis on its myotrophic role. Brain Res
Brain Res Rev. 47:161–173. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Simmons DL: The role of ICAM expression in
immunity and disease. Cancer Surv. 24:141–155. 1995.PubMed/NCBI
|
|
28.
|
Kunkel EJ and Butcher EC: Chemokines and
the tissue-specific migration of lymphocytes. Immunity. 16:1–4.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Muller YD, Ehirchiou D, Golshayan D,
Buhler LH and Seebach JD: Potential of T-regulatory cells to
protect xenografts. Curr Opin Organ Transplant. 17:155–161. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Dalbeth N and Lee YC: Lymphocytes in
pleural disease. Curr Opin Pulm Med. 11:334–339. 2005. View Article : Google Scholar
|
|
31.
|
Achen MG and Stacker SA: Molecular control
of lymphatic metastasis. Ann NY Acad Sci. 1131:225–234. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Furuya M, Yoneyama T, Miyagi E, Tanaka R,
Nagahama K, Miyagi Y, Nagashima Y, Hirahara F, Inayama Y and Aoki
I: Differential expression patterns of CXCR3 variants and
corresponding CXC chemokines in clear cell ovarian cancers and
endometriosis. Gynecol Oncol. 122:648–655. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Shusterman S, London WB, Gillies SD, Hank
JA, Voss SD, Seeger RC, Reynolds CP, Kimball J, Albertini MR,
Wagner B, Gan J, Eickhoff J, DeSantes KB, Cohn SL, Hecht T, Gadbaw
B, Reisfeld RA, Maris JM and Sondel PM: Antitumor activity of
hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a
Children’s Oncology Group (COG) phase II study. J Clin Oncol.
28:4969–4975. 2010.PubMed/NCBI
|
|
34.
|
Rutz S and Ouyang W: Regulation of
interleukin-10 and interleukin-22 expression in T helper cells.
Curr Opin Immunol. 605–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Wilke CM, I Kryczek I, Wei S, Zhao E, Wu
K, Guobin W and Weiping Z: Th17 cells in cancer: help or hindrance?
Carcinogenesis. 32:643–649. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Romagnani S, Maggi E, Liotta F, Cosmi L
and Annunziato F: Properties and origin of human Th17 cells. Mol
Immunol. 47:3–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Spolski R and Leonard WJ: Interleukin-21:
basic biology and implications for cancer and autoimmunity. Annu
Rev Immunol. 26:57–79. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Saito Y, Kagami S, Kawashima S, Takahashi
K, Ikeda K, Hirose K, Oshitari T, Yamamoto S, Okamoto Y and
Nakajima H: Roles of CRTH2+CD4+T cells in
immunoglobulin G4-related lacrimal gland enlargement. Int Arch
Allergy Immunol. 158(Suppl 1): 42–46. 2012.
|
|
39.
|
Gupta D and Lis CG: Role of CA125 in
predicting ovarian cancer survival - a review of the
epidemiological literature. J Ovarian Res. 2:132009. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Cramer DW, Bast RC Jr, Berg CD, Diamandis
EP, Godwin AK, Hartge P, Lokshin AE, Lu KH, McIntosh MW, Mor G,
Patriotis C, Pinsky PF, Thornquist MD, Scholler N, Skates SJ, Sluss
PM, Srivastava S, Ward DC, Zhang Z, Zhu CS and Urban N: Ovarian
cancer biomarker performance in prostate, lung, colorectal, and
ovarian cancer screening trial specimens. Cancer Prev Res (Phila).
4:365–374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Cheng T, Liu D, Griffin JH, Fernández JA,
Castellino F, Rosen ED, Fukudome K and Zlokovic BV: Activated
protein C blocks p53-mediated apoptosis in ischemic human brain
endothelium and is neuroprotective. Nat Med. 9:338–342. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Riewald M and Ruf W: Protease-activated
receptor-1 signaling by activated protein C in cytokine-perturbed
endothelial cells is distinct from thrombin signaling. J Biol Chem.
280:19808–19814. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Suzuki K and Hayashi T: Protein C and its
inhibitor in malignancy. Semin Thromb Hemost. 33:667–672. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Uchiba M, Okajima K, Oike Y, Ito Y,
Fukudome K, Isobe H and Suda T: Activated protein C induces
endothelial cell proliferation by mitogen-activated protein kinase
activation in vitro and angiogenesis in vivo. Circ Res. 95:34–41.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Kobayashi H, Moniwa N, Gotoh J, Sugimura M
and Terao T: Role of activated protein C in facilitating basement
membrane invasion by tumor cells. Cancer Res. 54:261–267.
1994.PubMed/NCBI
|
|
46.
|
Liu MQ, Zhou DJ, Wang X, Zhou W, Ye L, Li
JL, Wang YZ and Ho WZ: IFN-λ3 inhibits HIV infection of macrophages
through the JAK-STAT pathway. PLoS One. 7:e359022012.
|
|
47.
|
Shatz M, Menendez D and Resnick MA: The
human TLR innate immune gene family is differentially influenced by
DNA stress and p53 status in cancer cells. Cancer Res.
15:3948–3957. 2012. View Article : Google Scholar : PubMed/NCBI
|