Introduction
Gastric cancer is one of the most common malignant
tumors of the gastrointestinal system and cancer-related causes of
death worldwide. Although screening has reduced the incidence of
advanced disease, gastric cancer remains the most common malignancy
in many countries including Korea (1,2). The
average age at diagnosis of gastric cancer is approximately 60
years; 2 to 9% of patients are younger than 40 years and most are
older than 35 years. Patients younger than 30 years with gastric
cancer are very rare (3,4). Evidence suggests that gastric cancers
occurring early in life are more aggressive and have a worse
prognosis than those occurring later. Such a prognosis may be
attributable to delay in diagnosis, intrinsically aggressive
disease, and a higher frequency of undifferentiated tumor type in
younger patients (4–7); however, these points remain
controversial.
One current theory of cancer origin centers on
cancer stem cells (CSCs) or cancer initiating cells, which are
generally defined as a small population of cells within a tumor
that possesses both the capacity for self-renewal and potential to
generate multiple cell lineages (pluripotency) (8,9).
CSCs are hypothesized to participate in tumor initiation, invasion,
metastasis and drug resistance. Newly discovered stem
cell-associated genes have emerged as diagnostic and prognostic
markers in many types of cancers (8–10).
Musashi-1 (Msi-1) is a highly conserved neural RNA-binding protein,
initially identified in Drosophlia where it is required for
the early asymmetric division of sensory neural precursor cells
(11). In stomach, stem cells
present in the proliferative zone of the neck and isthmus region
give rise to all of the differentiated epithelial cell types
(12). Akasaka et al report
Msi-1 expression predominantly in epithelial cells of the
isthmus/neck region in human antrum, and Msi-1-positive cells in
the proliferative regions do not co-express PCNA or Ki-67,
suggesting that Msi-1 is a marker for cells with progenitor
characteristics before active proliferation in the human stomach
(13). Increasing evidence
supports the activities of Msi-1 in cell proliferation,
differentiation, inhibition of apoptosis and post-translational
modification of proteins, suggesting potential roles for this
protein in tumorigenesis (14,15).
However, the expression and clinical significance of Msi-1 in
gastric cancer, and in particular, the relationship of Msi-1
expression to age at diagnosis of gastric cancer, present questions
that are largely unexplored.
In the present study, we examined Msi-1 expression
in surgical specimens of gastric cancers and analyzed relationships
between Msi-1 expression and clinicopathologic features with
respect to age (age ≤30 versus age ≥60).
Materials and methods
Patients
From 2001 through 2011, radical gastrectomies were
performed in 2,757 patients at Chonbuk National University
Hospital, Korea. Of these, 26 patients (0.9%) were younger than 30
years. For this comparative study of gastric cancer between age
groups, data for 585 patients older than 31 years who were treated
by surgical resection between 2005 and 2007 were selected. Clinical
and pathological information were collected from the hospital
cancer registry database and from individual medical records, and
the data for patients 30 years of age and younger (n=26) were
compared with data for patients 31 years of age and older (n=585).
To evaluate relationships between Msi-1 expression and
clinicopathological data, and to compare these relationships
between younger and older patients, we matched the 26 young
patients with gastric cancer (age ≤30) with 26 older patients (age
≥60, mean age, 68.8 years; 11 male and 15 female). Matching was
conducted for gender, tumor histologic type and tumor stage. The
2010 American Joint Committee on Cancer Staging TNM system was used
for clinical and pathological staging (16). Gastric cancers were classified
according to the WHO classification (17). The Institutional Review Board (IRB)
of Chonbuk National University Hospital approved this study, and
waived the requirement for written informed consent because of the
retrospective design.
Immunohistochemical staining
A representative formalin-fixed, paraffin-embedded,
4-μm section was obtained from the gastric cancer of each
patient. Immunohistochemical staining was performed using a polymer
detection system with the Bond-Max Automatic stainer (Leica,
Bannockburn, IL) in accordance with the manufacturer’s
instructions. Briefly, after antigen retrieval (microwave treatment
for 10 min in 0.01 M EDTA buffer, pH 9), the slide was incubated
with anti-Msi-1 antibody (Abcam, Cambridge, UK) for 30 min. To
determine the relationship between Msi-1 expression and
proliferative activity in the tumor cells, we performed staining
for Ki-67 (Dako, Carpinteria, CA). In addition, we examined
expression profiles of CD 10 (Cell Marque, Hot Springs, AK), Muc2
(Novocastra, Newcastle upon Tyne, UK), Muc5Ac (Novocastra), and
Muc6 (Novocastra) to relate Msi-1 expression to mucin phenotype.
CD10 and Muc2 are markers for the intestinal cells, and Muc5Ac and
Muc6 are markers for gastric foveolar and gastric pyloric cells,
respectively. Peroxidase activity was detected with the enzyme
substrate 3-amino-9-ethyl carbazole. For negative controls,
sections were treated in the same way except that they were
incubated with citrate-buffered saline instead of the primary
antibody.
Immunohistochemical analysis
Msi-1 expression in normal
stomach
Areas with the highest densities of Msi-1 positive
cells in non-malignant tissues were identified on slides by
microscopic examination at ×4 magnification. Msi-1-positive cells
were counted in five areas under ×400 magnification (high power
field; HPF).
Msi-1 expression and mucin phenotype
in gastric cancer
The immunostaining results, positive or negative,
for Msi-1 or mucin phenotype were rated according to a score
calculated by multiplying the area of the stain to the intensity of
the stain. The area of staining was scored as follows: 0 (<5%),
1 (6–30%), 2 (31–60%), and 3 (≥61% of tumor cells). The intensity
of cell staining was scored categorically as follows: 0, no
immunostaining; 1, weak; 2, moderate; and 3, strong. The maximum
combined score was 9 and the minimum score was 0. If the product of
area and intensity scores was equal to or higher than 3,
immunostaining was determined to be positive; otherwise, the tumor
was determined to be negative. The cut-off score for positive Msi-1
expression was determined by receiver-operating characteristic
(ROC) curve analysis. Samples with at least 10% of tumor cell
nuclei stained for Ki-67 were determined to be positive.
Cell lines and culture
We also performed western blot analysis of Msi-1
expression in four different gastric cancer cell lines, MKN-28,
MKN-45, NCI-N87 and KATO III (Korean Cell Line Bank, Seoul, Korea).
All cell lines were cultured in RPMI-1640 medium supplemented with
penicillin and streptomycin (100 U/ml) and 10% fetal bovine serum
(Gibco-BRL, Gaithersburg, MD), at 37°C and with 5% CO2
in a humidified incubator.
Western blot analysis
Cultured cells were extracted with PRO-PREP Protein
Extraction Solution (Intron Biotechnology, Seoul, Korea). Briefly,
proteins were resolved by SDS-PAGE on 12% gels and
electrotransferred to polyvinylidene difluoride (PVDF) membranes
using a semidry transfer method (Bio-Rad, Hercules, CA). The
membrane was then blocked with 5% non-fat dry milk in Tris-buffered
saline (TBS)-0.1% Tween-20 (15 mM NaCl, 100 mM Tris-HCl, pH 7.5)
for 1 h. The membrane was incubated with anti-Msi-1 antibody
(Abcam) overnight at 4°C. Proteins on membranes were visualized
using an enhanced chemiluminescence (ECL) detection system
(Amersham Biosciences, Buckinghamshire, UK) and exposed to a
luminescent image analyzer (LAS-3000, Fuji Film, Tokyo, Japan).
Statistical analysis
Data are expressed as the mean ± SE. Comparisons
between Msi-1 expression and clinicopathological characteristics
were assessed using the χ2 test. The Mann-Whitney Rank
Sum test was used to evaluate differences in Msi-1 expression
between two groups. Survival analyses were performed using the
Kaplan-Meier method, and differences in survival between different
age groups were analyzed by the log-rank test. P-values <0.05
were considered to indicate statistically significant
difference.
Results
Clinicopathological data
Among the 611 patients with gastric cancer studied,
26 patients were younger than age 30 (mean age of patients, 27.8
years; 11 male and 15 female) and 585 were older than age 31 (mean
age of patients, 60.5 years; 392 male and 193 female). Among tumors
from the 26 younger patients, 4 (15.4%) were moderately
differentiated, 11 (42.3%) were poorly differentiated and 11
(42.3%) were signet ring cell carcinomas. According to the Lauren
classification (18), 22 tumors
(84.6%) were of the diffuse type and 4 (15.4%) were of intestinal
type. Sixteen patients (61.5%), were at stage I, four (15.4%) were
stage II, and six (23.1%) were stage III. Among tumors from the 585
patients older than 31 years of age, 100 (17.1%) were
well-differentiated, 215 (36.7%) were moderately differentiated,
148 (25.3%) were poorly differentiated, 93 (15.9%) were signet ring
cell carcinoma and 29 (5.0%) were of other types. Based on the
Lauren classification, 243 (41.5%) tumors were of a diffuse type
and 342 (58.5%) were of intestinal type. Numbers of patients at
stages I, II and III were 386 (66.0%), 93 (15.9%) and 106 (18.1%),
respectively.
Clinicopathological features of younger
patients with gastric cancer
Gastric cancers in the 26 patients age 30 or younger
were significantly associated with female gender (p=0.009) and
diffuse type cancer (poorly differentiated and signet ring cell
types, p=0.001), as compared to gastric cancers in the 585 patients
older than age 31. Clinicopathological features of the younger
gastric cancer patients compared with the older patients are
summarized in Table I.
 | Table I.Clinicopathological data for 611
patients with gastric cancer. |
Table I.
Clinicopathological data for 611
patients with gastric cancer.
| Characteristics | Age ≤30 n=26 (%) | Age >30 n=585
(%) | p-value |
|---|
| Gender | | | 0.009 |
| Female | 15 (57.7) | 193 (33.0) | |
| Male | 11 (42.3) | 392 (67.0) | |
| Differentiation | | | 0.001 |
| Well | 0 (0.0) | 100 (17.1) | |
| Moderate | 4 (15.4) | 215 (36.7) | |
| Poorly | 11 (42.3) | 148 (25.3) | |
| Signet ring
cell | 11 (42.3) | 93 (15.9) | |
| Others | 0 (0.0) | 29 (5.0) | |
| Lauren
classification | | | <0.001 |
| Intestinal | 4 (15.4) | 342 (58.5) | |
| Diffuse | 22 (84.6) | 243 (41.5) | |
| T classification | | | 0.903 |
| T1, T2 | 16 (61.5) | 353 (60.3) | |
| T3, T4 | 10 (38.5) | 232 (39.7) | |
| Node metastasis | | | 0.977 |
| No | 19 (73.1) | 429 (73.3) | |
| Yes | 7 (26.9) | 156 (26.7) | |
| Overall stage | | | 0.814 |
| I | 16 (61.5) | 386 (66.0) | |
| II | 4 (15.4) | 93 (15.9) | |
| III | 6 (23.1) | 106 (18.1) | |
Expression of Msi-1 in gastric cancer
tissue and cells
Similar to a previous study (13), we observed Msi-1 expression
predominantly in epithelial cells of the proliferative zone
(isthmus/neck) in normal stomach, as revealed by nuclear and/or
cytoplasmic immunoreactivity (Fig. 1A
and B). In contrast, the surface foveolar cells and epithelial
cells in the basal regions of the glands did not show Msi-1
immunoreactivity. Chief cells in the base of fundic mucosa showed
weak, non-specific, and mainly cytoplasmic Msi-1 immunoreactivity.
Only minimal or focal staining was present in gastric glands
showing intestinal metaplasia in elderly patients, and this did not
satisfy positive criteria. Non-tumor tissues from the younger
patients showed significantly higher numbers of Msi-1 positive
cells (15.43±1.15/HPF) than those of the older patients
(9.17±0.70/HPF) (p>0.001). In gastric tumor tissues, Msi-1 was
detected predominantly in cytoplasm but also in nuclei of some
tumor cells (Fig. 1C and D). Msi-1
expression was detected more frequently in tumors from the younger
patients (26.9%) than in those from the older patients (7.7%) and
at higher levels [1.79±0.5 and 0.5±0.2, combined scores, in younger
and older patients, respectively (p=0.027)]. Among the 7
Msi-1-positive tumors in younger patients, 3 tumors developed from
pyloric gland mucosa and 4 developed from fundic gland mucosa.
Msi-1 expression was significantly associated with histologic type
(poorly differentiated tubular adenocarcinoma, p=0.029), invasion
depth (p=0.036), lymph node metastasis (p=0.002) and overall stage
(p=0.036) in the younger patients (Table II). However, no significant
correlation was found between Msi-1 expression and
clinicopathologic features in the older patients (Table III). Although Msi-1-positive
carcinomas showed a trend toward higher cell proliferation as
compared with Msi-1-negative carcinomas as revealed by Ki-67
immunostaining, this trend was not statistically significant (p=
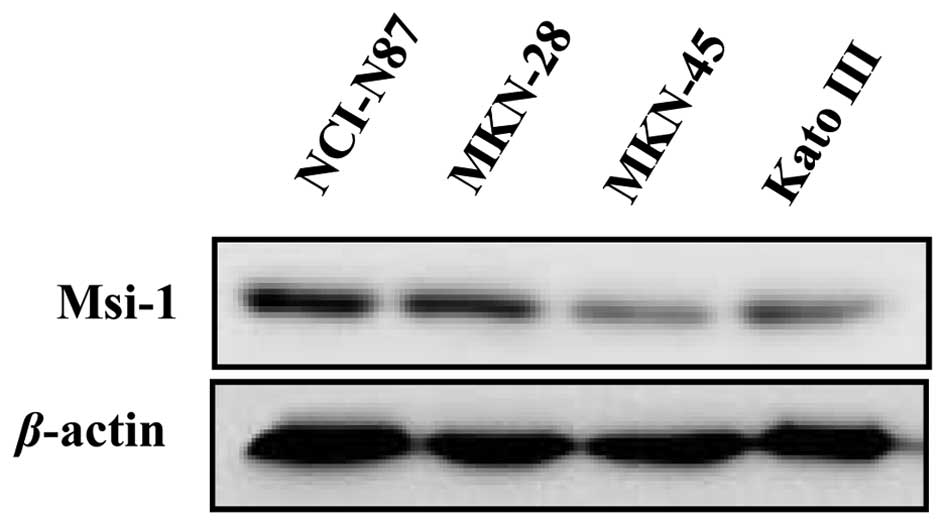
0.079). The level of Msi-1 expression was higher in the NCI-N87 and
MKN-28 cell lines than in the MKN-45 and KATO III cell lines, as
determined by western blot analysis (Fig. 2). This result confirmed the
presence of Msi-1 protein in human gastric cancer.
 | Table II.Correlation of Msi-1 expression with
clinicopathological features of young patients with gastric
cancer. |
Table II.
Correlation of Msi-1 expression with
clinicopathological features of young patients with gastric
cancer.
| Age ≤30 (mean age,
27.8) |
|---|
|
|---|
|
Characteristics | Msi-1(+) n=7
(%) | Msi-1(−) n=19
(%) | Total n=26 (%) | p-value |
|---|
| Gender | | | | 0.353 |
| Female | 3 (42.9) | 12 (63.2) | 15 (57.7) | |
| Male | 4 (57.1) | 7 (36.8) | 11 (42.3) | |
| Histologic
differentiation | | | | 0.029 |
| Moderate | 2 (28.6) | 2 (10.5) | 4 (15.4) | |
| Poor | 5 (71.4) | 6 (31.6) | 11 (42.3) | |
| Signet ring
cell | 0 (0.0) | 11 (57.9) | 11 (42.3) | |
| T
classification | | | | 0.036 |
| T1, T2 | 2 (28.6) | 14 (73.7) | 16 (61.5) | |
| T3, T4 | 5 (71.4) | 5 (26.3) | 10 (38.5) | |
| Lymph node
metastasis | | | | 0.002 |
| No | 2 (28.6) | 17 (89.5) | 19 (73.1) | |
| Yes | 5 (71.4) | 2 (10.5) | 7 (26.9) | |
| Overall stage | | | | 0.036 |
| I | 2 (28.6) | 14 (73.7) | 16 (61.5) | |
| II, III | 5 (71.4) | 5 (26.3) | 10 (38.5) | |
| Ki-67 | | | | 0.079 |
| Negative | 1 (14.3) | 10 (52.6) | 11 (42.3) | |
| Positive | 6 (85.7) | 9 (47.4) | 15 (57.7) | |
| Post-operative
metastasis | | | | 0.463 |
| No | 5 (71.4) | 16 (84.2) | 21 (80.7) | |
| Yes | 2 (28.6) | 3 (15.8) | 5 (19.3) | |
 | Table III.Correlation of Msi-1 expression with
clinicopathological features of elderly gastric cancer
patients. |
Table III.
Correlation of Msi-1 expression with
clinicopathological features of elderly gastric cancer
patients.
| Age ≥60 (mean age,
68.8) |
|---|
|
|---|
|
Characteristics | Msi-1(+) n=2
(%) | Msi-1(−) n=24
(%) | Total n=26 (%) | p-value |
|---|
| Gender | | | | 0.086 |
| Female | 0 (0.0) | 15 (62.5) | 15 (57.7) | |
| Male | 2 (100.0) | 9 (37.5) | 11 (42.3) | |
| Histologic
differentiation | | | | 0.821 |
| Moderate | 0 (0.0) | 4 (16.6) | 4 (15.4) | |
| Poor | 1 (50.0) | 10 (41.7) | 11 (42.3) | |
| Signet ring
cell | 1 (50.0) | 10 (41.7) | 11 (42.3) | |
| T
classification | | | | 0.284 |
| T1, T2 | 2 (100.0) | 15 (62.5) | 17 (65.4) | |
| T3, T4 | 0 (0.0) | 9 (37.5) | 9 (34.6) | |
| Lymph node
metastasis | | | | 0.326 |
| No | 2 (100.0) | 16 (66.7) | 18 (69.2) | |
| Yes | 0 (0.0) | 8 (33.3) | 8 (30.8) | |
| Overall stage | | | | 0.245 |
| I | 2 (100.0) | 14 (58.3) | 16 (61.5) | |
| II, III | 0 (0.0) | 10 (41.7) | 10 (38.5) | |
| Ki-67 | | | | 0.819 |
| Negative | 1 (50.0) | 10 (41.7) | 11 (30.6) | |
| Positive | 1 (50.0) | 14 (58.3) | 25 (69.4) | |
| Post-operative
metastasis | | | | 0.595 |
| No | 2 (100.0) | 21 (87.5) | 23 (88.5) | |
| Yes | 0 (0.0) | 3 (12.5) | 3 (11.5) | |
Mucin phenotypes in Msi-1-positive
gastric cancers
Results of immunostaining for mucin in
Msi-1-positive gastric cancer are summarized in Table IV. In 9 Msi-1 positive gastric
cancers, 6 were Muc5Ac-positive and 4 were Muc1-positive. The
gastric foveolar type, observed in 5 of 9 tumors (56%), was
predominant, whereas no tumor of the gastric pyloric type was
observed. Three of the 9 Msi-1 positive tumors (33%) were of mixed
gastrointestinal types.
 | Table IV.Association between Msi-1 expression
and mucin phenotype in gastric cancer. |
Table IV.
Association between Msi-1 expression
and mucin phenotype in gastric cancer.
| No. | Age/gender | Histopathology | Mucin expression
(area x intensity)
| Stage |
|---|
| Muc1 | Muc2 | Muc5Ac | Muc6 | CD10 |
|---|
| 1 | 30/M | Adenocarcinoma,
PD | 2 × 3 | 0 | 3 × 3 | 0 | 0 | pT3N2 |
| 2 | 30/F | Adenocarcinoma,
PD | 0 | 0 | 1 × 3 | 0 | 0 | pT3N0 |
| 3 | 24/M | Adenocarcinoma,
MD | 1 × 3 | 0 | 2 × 3 | 0 | 1 × 3 | pT4bN3a |
| 4 | 26/F | Adenocarcinoma,
MD | 0 | 0 | 3 × 3 | 0 | 0 | pT1aN1 |
| 5 | 23/F | Adenocarcinoma,
PD | 3 × 3 | 0 | 0 | 0 | 0 | pT3N3a |
| 6 | 28/M | Adenocarcinoma, PD
with SRCF | 1 × 3 | 2 × 3 | 2 × 3 | 0 | 2 × 2 | pT3N2 |
| 7 | 29/M | Adenocarcinoma, PD
with SRCF | 0 | 1 × 3 | 1 × 3 | 0 | 0 | pT1bN0 |
| 8 | 73/M | Signet ring cell
carcinoma | 0 | 0 | 2 × 3 | 0 | 0 | pT1aN0 |
| 9 | 78/M | Adenocarcinoma, PD
with SRCF | 0 | 1 × 3 | 0 | 0 | 0 | pT1bN0 |
Outcome
In 52 patients with gastric cancer (the 26 younger
and 26 older patients matched for comparison), follow-up intervals
ranged from 1.5 to 133.1 months (young patients; 1.5–133.1, older
patients; 6.6–76.4 months). Eight patients died during the
follow-up period. Mean survival (95% confidence interval, CI) of
the younger patients with gastric cancer was 123.6 months (95% CI;
111.1–136.2). Mean survival of older patients was 63.9 months (95%
CI; 54.9–72.9). The 1-, 3- and 5-year survival rates were 96, 91
and 91% in young patients, and 81, 76 and 76% in older patients,
respectively. The two groups did not differ significantly in
survival (p= 0.183). In the 26 young gastric cancer patients, Msi-1
expression was significantly associated with survival (p= 0.014) in
univariate Kaplan-Meier survival analysis. However, Msi-1
expression was not significantly associated with survival in the 26
older patients (p=0.449) (Fig. 3).
Multivariate survival analysis was not applied due to the small
number of patients.
Discussion
Gastric cancer is the fourth most common cancer and
the second leading cause of cancer-related death (1,2).
Gastric cancer occurs predominantly in older age groups and gastric
cancer patients younger than 30 years are very rare (3–7,19).
Studies of gastric cancer in the young typically define patients
30–35 years or less as ‘young’ because intestinal metaplasia, an
aging process affecting the gastric mucosa, is thought to begin at
approximately this time of life (20). However, since actual number of
patients younger than 30 years is very small, most studies of
gastric cancer in young adults include patients up to 35 or 40
years (3–7). Although it has long been believed
that gastric cancer in young patients shows a more aggressive
phenotype and poorer prognosis than that in elderly patients,
supportive data for such beliefs are uncertain (4–7).
Because gastric cancer occurs most frequently in individuals 60
years of age or older, we used a case-control design to compare
features of gastric cancer in the young patients represented in our
database (age ≤30) with those in an older group (age ≥60) matched
for important clinicopathological features, except age.
This study demonstrated for the first time the
following characteristics of Musashi-1 expression in gastric
cancer: i) more frequent detection and higher levels of expression
in younger patients than in older ones; ii) significant
associations with poorly differentiated histologic type, tumor
invasion depth, lymph node metastasis, and overall stage in younger
patients, but no significant correlations with clinicopathological
features in older patients; iii) significant association with
survival in younger patients; and iv) predominance of gastric
foveolar mucin phenotype in Msi-1 positive gastric cancers. The
expression of Msi-1 protein was confirmed directly in gastric
cancer cell lines by western blot analysis. These findings support
an active role for Msi-1 in development of gastric cancer in
younger patients.
Msi-1 represents an evolutionarily conserved family
of RNA-binding proteins with pivotal functions in stem cell
maintenance, and nervous system development (10–13).
Evidence that Msi-1 participates in carcinogenesis and tumor
progression stems from studies of several human cancers (14,15,21–24).
In gastric cancer, relationships between Msi-1 expression and
clinicopathological features have not been fully examined. In this
study, we found that Msi-1 expression correlated with high
histologic grade, tumor invasion depth and lymph node metastasis of
gastric cancer in young patients. Furthermore, a significant
association was found between Msi-1 expression and overall survival
in young patients. Our present findings in young patients with
gastric cancer are consistent with studies of Msi-1 expression in
other tumor types (23–25). In breast cancer patients, Msi-1
overexpression may be related to lymph node metastasis and shorter
survival (23), and in human
glioma, may be related to the degree of malignancy and
proliferative activity (24). In
patients with colon cancer, Msi-1 is associated with advanced
cancer stage, metastasis and poor survival (25). Knockdown of Msi-1 with Msi-1 siRNA
resulted in reduced colon cancer cell proliferation, cancer growth
arrest in xenografts, and increased apoptosis alone and in
combination with radiation therapy in athymic nude mice in
vivo (14). In mouse
intestinal cells, Msi-1 overexpression may induce tumors through
Wnt and Notch activation (26).
Our findings, therefore, together with previous studies support the
contention that Msi-1 plays an important role in gastric cancer
carcinogenesis and progression in young patients.
Our findings of significant associations in young
gastric cancer patients with female gender and with diffuse type
cancer, as compared to the 585 patients older than 31 years, are
supported by previous studies showing higher proportions of female
patients with poorly differentiated histology or diffuse type
cancers in younger age groups (3–7). Our
results, demonstrating the association of Msi-1 expression with
poorly differentiated diffuse types of gastric cancer is in
agreement with previous findings of significant increases in Msi-1
expression in diffuse gastric cancers as compared with the
intestinal type of the cancer (27). In the present study, the expression
of Msi-1 was also associated with gastric foveolar type gastric
cancer showing Muc5Ac immunoreactivity. This is in agreement with
previous studies demonstrating that Msi-1 expression overlapping
with that of Muc5Ac, indicating that Msi-1 positive cells retain
some features of foveolar cell differentiation (13). Previous studies also indicated that
Muc5Ac expression is associated with a diffuse type of gastric
cancer (28). Thus Msi-1 may be
specifically involved in development of diffuse and foveolar types
of gastric cancer.
The level of Msi-1 expression was higher in the
NCI-N87 and MKN-28 cell lines than in the MKN-45 and KATO III cell
lines in this study. The NCI-N87 cells are derived from metastases
of a well-differentiated gastric adenocarcinoma, and MKN-28 cells
are derived from a moderately differentiated gastric adenocarcinoma
that was non-metastatic. Conversely, MKN-45 cells are from a poorly
differentiated gastric adenocarcinoma that was non-metastatic, and
KATO III cells are signet ring cell carcinoma cells derived from
pleural effusion. Therefore, the results obtained in vivo
(i.e., higher Msi-1 expression in a poorly differentiated cell
type) appear to contradict results obtained in vitro, where
the highest level of Msi-1 expression was detected in NCI-N87 cells
derived from a well-differentiated adenocarcinoma. The reasons for
this discrepancy between in vivo and in vitro results
are unclear. Among our young patients with gastric cancer, Msi-1
expression correlated significantly with a poorly differentiated
cancer cell phenotype. However, the surgically resected specimens
from 10 of 11 poorly differentiated carcinomas from elderly
patients did not show positive Msi-1 expression. Moreover, MKN-28,
MKN-45 and KATO III gastric cancer cell lines are derived from 70-,
62- and 55-year-old persons, respectively. The exact donor age was
not reported for the NCI-N87 cell line. We conclude from these
findings that Msi-1 expression may not always match the degree of
cancer cell differentiation and potential for metastasis in gastric
cancer, and that the relationship of Msi-1 expression to biological
properties of the tumor may depend on the patient’s age.
The relationship between patient age and prognosis
in gastric cancer remains controversial. Gastric cancer in young
patients has long been believed to show more aggressive biologic
behavior and poorer prognosis than that in elderly patients
(4–7). In our case-control study with matched
subjects, the survival rates for young patients were better than
those for older patients, although the difference between survival
rates was not significant. These findings are in agreement with a
number of prior reports showing similar survival rates in young and
elderly patients (3,20,29).
Moreover, Eguchi et al reported better prognosis in young
patients with gastric cancer and noted that early diagnosis
improves the prognosis (19).
Taken together, our data suggested that though young patients may
present with advanced stages of cancer and tumors of poorly
differentiated histology, age itself may not independently predict
survival outcome for young patients. Based on the limited number of
our cases and insufficient follow-up periods, a definitive
conclusion on the prognosis of young gastric cancer patients would
be premature. A longer-term follow-up with a larger cohort is
needed to adequately define the clinical and biological behavior of
gastric cancer in young patients.
In conclusion, our study revealed significantly
higher tumor Msi-1 expression in young gastric cancer patients than
in elderly patients, and an association of Msi-1 expression with
aggressive gastric cancer behavior in young patients.
Acknowledgements
This study was supported by a grant
from the National Research Foundation of Korea (NRF) funded by the
Korean Government (MSIP) (No. 2008-0062279). Tissue samples were
provided by the Chonbuk National University Hospital, a member of
the National Biobank of Korea, which is supported by the Ministry
of Health, Welfare and Family Affairs.
References
|
1.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Leung WK, Wu MS, Kakugawa Y, et al:
Screening for gastric cancer in Asia: current evidence and
practice. Lancet Oncol. 9:279–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Bai Y and Li ZS: Endoscopic,
clinicopathological features and prognosis of very young patients
with gastric cancer. J Gastroenterol Hepatol. 26:1626–1629. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Nakamura R, Saikawa Y, Takahashi T, et al:
Retrospective analysis of prognostic outcome of gastric cancer in
young patients. Int J Clin Oncol. 16:328–334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Theuer CP, de Virgilio C, Keese G, et al:
Gastric adenocarcinoma in patients 40 years of age or younger. Am J
Surg. 172:473–476. 1996.PubMed/NCBI
|
|
6.
|
Quijano Orvananos F, Moreno Paquentin E,
Alvarez JJ, Martinez Munive A and Butron Perez L: Gastric carcinoma
in patients under 35 years. Rev Gastroenterol Mex. 64:75–77.
1999.PubMed/NCBI
|
|
7.
|
Smith BR and Stabile BE: Extreme
aggressiveness and lethality of gastric adenocarcinoma in the very
young. Arch Surg. 144:506–551. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Saikawa Y, Fukuda K, Takahashi T, Nakamura
R, Takeuchi H and Kitagawa Y: Gastric carcinogenesis and the cancer
stem cell hypothesis. Gastric Cancer. 13:11–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Clarke MF, Dick JE, Dirks PB, et al:
Cancer stem cells -perspectives on current status and future
directions: AACR Workshop on cancer stem cells. Cancer Res.
66:9339–9344. 2006. View Article : Google Scholar
|
|
10.
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Nakamura M, Okano H, Blendy JA and Montell
C: Musashi, a neural RNA-binding protein required for
Drosophila adult external sensory organ development. Neuron.
13:67–81. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Potten CS, Booth C, Tudor GL, et al:
Identification of a putative intestinal stem cell and early lineage
marker; musashi-1. Differentiation. 71:28–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Akasaka Y, Saikawa Y, Fujita K, et al:
Expression of a candidate marker for progenitor cells, Musashi-1,
in the proliferative regions of human antrum and its decreased
expression in intestinal metaplasia. Histopathology. 47:348–356.
2005. View Article : Google Scholar
|
|
14.
|
Sureban SM, May R, George RJ, et al:
Knockdown of RNA binding protein musashi-1 leads to tumor
regression in vivo. Gastroenterology. 134:1448–1458. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
De Sousa Abreu R, Sanchez-Diaz PC, Vogel
C, et al: Genomic analyses of musashi1 downstream targets show a
strong association with cancer-related processes. J Biol Chem.
284:12125–12135. 2009.PubMed/NCBI
|
|
16.
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer; New York: 2010
|
|
17.
|
Lauwers GY, Carneiro F, Graham DY, et al:
Gastric carcinoma. WHO Classification of Tumours of the Digestive
System. 4th edition. IARC Press; Lyon: pp. 48–58. 2010
|
|
18.
|
Lauren P: The two histological main types
of gastric carcinoma, undifferentiated and so-called
differentiated-type carcinoma. Acta Pathol Microbiol Scand.
64:31–49. 1965.PubMed/NCBI
|
|
19.
|
Eguchi T, Takahashi Y, Yamagata M,
Kasahara M and Fujii M: Gastric cancer in young patients. J Am Coll
Surg. 188:22–26. 1999. View Article : Google Scholar
|
|
20.
|
Katai H, Sasako M, Sano T and Maruyama K:
Gastric carcinoma in young adults. Jpn J Clin Oncol. 26:139–143.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Shu HJ, Saito T, Watanabe H, et al:
Expression of the Musashi1 gene encoding the RNA-binding protein in
human hepatoma cell lines. Biochem Biophys Res Commun. 293:150–154.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Wang T, Ong CW, Shi J, et al: Sequential
expression of putative stem cell markers in gastric carcinogenesis.
Br J Cancer. 105:658–665. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Wang XY, Penalva LO, Yuan H, et al:
Musashi1 regulates breast tumor cell proliferation and is a
prognostic indicator of poor survival. Mol Cancer. 9:2212010.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Toda M, Iizuka Y, Yu W, et al: Expression
of the neural RNA-binding protein Musashi1 in human gliomas. Glia.
34:1–7. 2001. View Article : Google Scholar
|
|
25.
|
Li D, Peng X, Yan D, et al: Msi-1 is a
predictor of survival and a novel therapeutic target in colon
cancer. Ann Surg Oncol. 18:2074–2083. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Rezza A, Skah S, Roche C, Nadjar J,
Samarut J and Plateroti M: The overexpression of the putative gut
stem cell marker Musashi-1 induces tumorigenesis through Wnt and
Notch activation. J Cell Sci. 123:3256–3265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Nikpour P, Emadi-Baygi M, Mohhamad-Hashem
F, Maracy MR and Haghjooy-Javanmard S: MSI1 overexpression in
diffuse type of gastric cancer. Pathol Res Pract. 209:10–13. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Pinto-de-Sousa J, David L, Reis CA, Gomes
R, Silva L and Pimenta A: Mucins MUC1, MUC2, MUC5AC and MUC6
expression in the evaluation of differentiation and
clinicobiological behaviour of gastric carcinoma. Virchows Arch.
440:304–310. 2002. View Article : Google Scholar
|
|
29.
|
Kim DY, Joo JK, Ryu SY, Park YK, Kim YJ
and Kim SK: Clinicopathologic characteristics of gastric carcinoma
in elderly patients: a comparison with young patients. World J
Gastroenterol. 11:22–26. 2005. View Article : Google Scholar
|