Introduction
Lung cancer is the most common cancer in the world
(1). It is the leading cause of
cancer-related mortalities in China, with a rate of about 470,000
people in 2005 (2). The causal
relation between smoking and lung cancer has been unambiguously
established (3). Over 80% of lung
cancer cases are caused by cigarette smoking. In spite of tobacco
control activities and policies, the prevalence of smoking in China
is still high, with 350 million smokers and 740 million passive
smokers (4). Therefore, treatment
of cigarette smoke-induced diseases is essential.
Among the 4,000 identified chemicals in cigarette
smoke, nicotine is the reason why people continue to smoke despite
the obvious side effects on health. Nicotine acts through nicotinic
acetylcholine receptors (nAChRs) that are widely present on
neuronal and non-neuronal cells (5,6). At
the end of last century, Maneckjee and Minna, firstly reported that
lung cancer cell lines also expressed nAChRs (7,8).
Subsequently, studies have shown that nAChRs subunits are expressed
on biopsy specimens of lung cancer (9). The main effects of nicotine on lung
cancer cells involve promotion of cell proliferation and prevention
of drug-induced apoptosis. B-cell lymphoma-2 (Bcl-2) protein family
plays a vital role in regulating apoptosis and activating the
subsequent caspase cascade (10,11).
The process of apoptosis is regulated by a complicated interaction
between proapoptotic (Bcl-2-associated X protein, Bax) and
anti-apoptotic (Bcl-2) proteins. Bax is an essential component in
the nicotine survival signaling pathway, through activating
PI3K/AKT that phosphorylates and inactivates the pro-apoptotic
function of Bax (12). In
addition, nicotine facilitates invasion and metastasis of lung
cancer (13,14). Matrix metalloproteinases (MMPs)
have long been linked with cancer cell invasion and metastasis
(15). Matrix metalloproteinase-2
(MMP-2) and matrix metalloproteinase-9 (MMP-9) are main members of
MMPs and patients with high MMP-2 or MMP-9 expression have poorer
overall survival (16–18). Nicotine increased the activity of
MMP-2 in esophageal squamous carcinoma cells and
epithelial-mesenchymal transition (EMT) in lung cancer cells
(13,19). Thus, though nicotine itself cannot
provoke cancer, it contributes to lung cancer progression already
initiated through diverse processes.
Scutellaria baicalensis, one of the most
popular and multi-purpose herbs used in China, possess potent
anti-cancer activities. The major constituents of
Scutellaria are baicalin, baicalein and wogonin, the
bioactive flavones. These phytochemicals are cytotoxic to various
lung cancer cell lines and suppress tumor growth in vivo
without systemic toxicity (20,21).
The antitumor functions of these flavones are primarily due to
their abilities to induce cell cycle arrest, regulate
apoptosis-related proteins expression, eliminate adhesion and
migration capacities and inhibit metastasis (22,23).
Previous studies from our laboratory have shown that baicalin
attenuated cigarette smoke-induced pulmonary chronic inflammation,
declined inflammatory cells as well as tumor necrosis factor
(TNF)-α, interleukin (IL)-6, IL-8 and MMP-9 production, decreased
nuclear transcription factor-kappaB (NF-κB) p65 expression in the
lung, and inhibited the activation of NF-κB (24,25).
Pulmonary chronic inflammation and lung cancer are closely
correlated. Many common features coexist in both diseases, and the
potential shared biological mechanisms are inflammation, EMT and
others, especially the inflammatory factor (26,27).
Recent reports demonstrated that tobacco smoke promoted lung
tumorigenesis by triggering inflammatory pathways (28).
In view of these observations, we hypothesized that
flavones in Scutellaria may inhibit nicotine-induced lung
cancer progression. The present study was designed to evaluate the
antitumor effects and underlying mechanisms of baicalin, baicalein
and wogonin on nicotine-induced lung cancer development in
vitro. The results of this study may help us designing novel
therapies based on these biosafe agents that target
nicotine-induced proliferation, metastasis and inflammation in lung
cancer cells.
Materials and methods
Materials
Baicalin, baicalein and wogonin were purchased from
Shanghai Ronghe Corp. (Shanghai, China). Nicotine (liquid, N3876)
was purchased from Sigma-Aldrich (St. Louis, MO, USA). CCK-8 kit
was purchased from Dojindo Moleculare Technologies, Inc. (Kumamoto,
Japan). Bcl-2, bax, caspase-3, NF-κB p65 and IκB-α monoclonal
antibodies were purchased from Beyotime Institute of Biotechnology
(Haimen, China). MMP-2 and MMP-9 monoclonal antibodies were
purchased from ABGENT Corp. (San Diego, CA, USA). IL-6 and TNF-α
Human ELISA kits were purchased from Life Technologies Corp. (Grand
Island, NY, USA).
Cell culture and treatments
A549 and H1299 cells were obtained from Chinese
Academy of Sciences (Shanghai, China) and maintained in DMEM high
glucose culture medium supplemented with 10% fetal bovine serum,
100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in
humidified atmosphere with 5% CO2. All cell culture
reagents were purchased from Hyclone Laboratories (Logan, UT,
USA).
A549 and H1299 cells were grown to 70–80% confluency
in 100-mm culture plates in a total volume of 5 ml DMEM medium
containing 10% fetal bovine serum (FBS). Cells were then
serum-starved for 12 h and washed with ice-cold sterile PBS before
being treated with nicotine (10−6 M for A549 cells and
10−8 M for H1299 cells) combined with or without
flavones. Baicalin, baicalein or wogonin, dissolved in dimethyl
sulfoxide (DMSO), were used for the treatment of cells. The final
concentration of DMSO used was <0.1% (v/v). For treatment with
baicalin (50 μM), baicalein (10 μM) or wogonin (1
μM), cells were incubated for 48 h.
Viability assays
Cell viability in A549 and H1299 cells was measured
by the CCK-8 assay kit following the manufacturer’s instructions.
Briefly, A549 and H1299 cells were seeded at a density of 5,000
cells/100 μl/well in 96-well culture plates in serum-free
media and incubated in a humidified incubator at 37°C 12 h prior to
treatment with nicotine with or without baicalin, baicalein or
wogonin as indicated above. After incubation, 10 μl CCK-8
was added to each well for 2 h after which the optical density (OD)
was measured at 490 nm. The percent of viable cells was determined
by the formula: ratio (%)= [OD (baicalin/baicalein/wogonin +
nicotine) − OD (Blank)] / [OD(Control) − OD (Blank)] × 100%. The
cell viability data are averages of 3 independent experiments each
containing 3 replicates.
Flow cytometric analysis
After the treatment of A549 and H1299 cells with
nicotine with or without baicalin, baicalein or wogonin as
mentioned above, 1×106 cells were harvested and washed
once with binding buffer (HEPES buffer: 10 mM HEPES/NaOH, pH 7.4,
150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM
CaCl2). After aspiration of the supernatant, cells were
resuspended in 100 μl binding buffer containing 1 μl
Annexin V-FITC conjugated antibody and 5 μl PI for exactly 5
min in the dark at room temperature. Cells were than analyzed on a
FACSCalibur cytometer (Becton-Dickinson, San Jose, CA, USA). The
data were analyzed using FlowJo software V6.0 (Tree Star, Ashland,
OR, USA).
Wound healing assay
Cells were cultured on 6-well plates
(3×105 cells/well) and treated as indicated above. When
confluent, the monolayers were scratched horizontally with a yellow
pipette tip to obtain a monolayer culture with space without cells.
Media and dislodged cells were aspirated. The cells were incubated
along with nicotine with or without baicalin, baicalein or wogonin
as above. After incubation, cell invasion was observed; three
randomly fields along the scraped line were selected, and images
were photographed on each well using a phase contrast inverted
microscope.
Invasion assay
In vitro cell invasion was performed by the
6.5 mm Transwell® with an 8.0-μm pore
polycarbonate membrane insert (Corning Co., USA). Matrigel was
purchased from BD Biosciences and stored at −20°C. After thawing at
4°C overnight, the matrigel was diluted in serum-free DMEM medium;
50 μl of the diluted matrigel were evenly inoculated into
the upper chamber of the 6.5 mm Transwell membrane and allowed to
form a gel at 37°C. Cells (1×106) suspended in 250
μl of serum-free DMEM were seeded into the upper
compartments of each chamber in the presence of nicotine with or
without baicalin, baicalein or wogonin as previously indicated,
whereas the lower compartments were filled with 500 μl of
DMEM with 10% FBS. After incubation, the noninvasive cells were
removed from the upper surface of the membrane by scrubbing. Cells
on the reverse side were stained with 0.1% crystal violet, and
invasive cells were counted under a microscope at ×400
magnification.
Cytokine analysis
TNF-α and IL-6 levels in A549 and H1299 cell culture
supernatant were measured using ELISA kits in accordance with the
manufacturer’s recommendations.
Real-time quantitative polymerase chain
reaction (PCR)
Total RNA was isolated from A549 and H1299 cells
using TRIzol reagent (Gibco BRL, Gaithersburg, MD, USA). The mRNA
levels were analyzed by real-time quantitative RT-PCR using SYBR
Premix Ex Taq System (Takara, Dalian, China). mRNA was
reverse-transcribed into cDNA by cDNA synthesis kit (Takara).
Specific primers (Table I) were
designed to screen the expression of bcl-2, bax, pro-caspase-3,
MMP-2, MMP-9, phosphor-NF-κB p65 and IκB-α. Real-time PCR was
performed using a SYBR Premix Ex Taq kit (Takara) and run for the
denaturation step at 94°C for 10 sec, the annealing at 58–60°C for
20 sec and the extension at 72°C for 20 sec. The final extension
was at 72°C for 5 min. The annealing temperature was 58°C for bax
and pro-caspase-3 and 60°C for bcl-2, MMP-2, MMP-9, NF-κB p65 and
IκB-α. Each cDNA sample was run in triplicate and the corresponding
non-real-time mRNA sample was included as a negative control. The
primers of β-actin were included in every plate to avoid sample
variations. The mRNA level of each sample for each gene was
normalized to that of β-actin mRNA and gene expression changes
induced by various treatments were determined by the
2−ΔΔCT method (29).
For the validation, real-time PCR was performed 3 times
independently.
 | Table I.Primers used for real-time
quantitative PCR analysis. |
Table I.
Primers used for real-time
quantitative PCR analysis.
| Sequence name | Primers |
|---|
| β-actin-F |
5′-CCTGTACGCCAACACAGTGC-3′ |
| β-actin-R |
5′-ATACTCCTGCTTGCTGATCC-3′ |
| Bcl-2-F |
5′-CCAGGCCGGCGACGACTTCTC-3′ |
| Bcl-2-R |
5′-ATCTCCCGGTTGACGCTCTCCACA-3′ |
| Bax-F |
5′-GGTTGTCGCCCTTTTCTACTT-3′ |
| Bax-R |
5′-TGAGCACTCCCGCCACAA-3′ |
|
Pro-caspase-3-F |
5′-GTGGAATTGATGCGTGATGTT-3′ |
|
Pro-caspase-3-R |
5′-GGCAGGCCTGAATAATGAAA-3′ |
| MMP-2-F |
5′-CACGCTGGGCCCTGTCACTCCT-3′ |
| MMP-2-R |
5′-TGGGGCCTCGTATACCGCATCAAT-3′ |
| MMP-9-F |
5′-TGCCCGGACCAAGGATACAGTTT-3′ |
| MMP-9-R |
5′-AGGCCGTGGCTCAGGTTCAGG-3′ |
| P-p65-F |
5′-CTCCGCGGGCAGCATCC-3′ |
| P-p65-R |
5′-CATCCCGGCAGTCCTTTCCTACAA-3′ |
| IκB-α-F |
5′-CACCCCGCACCTCCACTCCATC-3′ |
| IκB-α-R |
5′-ACATCAGCCCCACACTTCAACAGG′ |
Western blotting
The extraction of cytosolic and nuclear proteins of
the cells was performed according to instructions of protein
extraction kit (Beyotime Biotechnology, Haimen, China). Protein
concentrations were determined by BCA protein assay kit (Beyotime).
Equal amounts of protein were separated by 8% SDS-polyacrylamide
gel electrophoresis, and transferred to polyvinylidene difluoride
(PVDF) membranes. The membranes were then blocked at room
temperature for 1.5 h with 5% (w/v) non-fat milk in TBST buffer and
incubated with primary antibodies in TBST overnight at 4°C with
continuous shaking. After three washes in TBST, membranes were
incubated with secondary antibodies conjugated with horseradish
peroxidase for 1 h and visualized by enhanced chemiluminescence
using Supersignal West Femto Chemiluminescent Substrate (Pierce
Biotechnology Inc., Rockford, IL, USA). Band intensities were
quantified using UN-SCAN-IT gel analysis software (version 6). The
optical density for target protein was shown as a proportion of
β-actin optical density. The western blot data were replicated 3
times.
Statistical analysis
Data are expressed as mean ± SEM. Statistically
significant differences between groups were determined by ANOVA
followed by Bonferroni’s post hoc comparison tests. All
analyses were undertaken using the statistical software SPSS 18.0.
A value of P<0.05 was considered statistically significant.
Results
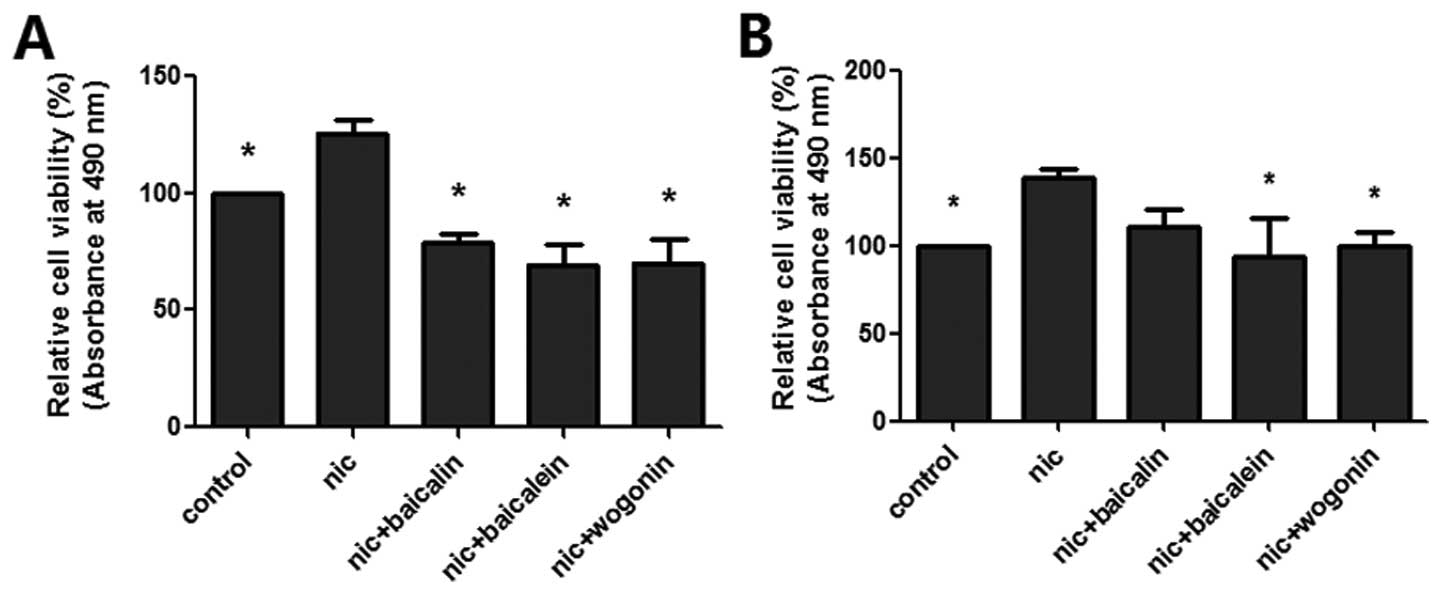
Flavones in Scutellaria inhibit
nicotine-induced cell viability
The cell viability of flavones in Scutellaria
treatment on nicotine-induced lung cancer cells (A549 and H1299)
were determined by CCK-8 assay (Fig.
1). Exposure to nicotine increased cell proliferation in both
lung cancer cell lines; baicalin, baicalein and wogonin all
significantly inhibited nicotine-induced A549 cell proliferation,
while only baicalein and wogonin decreased nicotine-induced H1299
cell proliferation.
Flavones in Scutellaria inhibit
nicotine-induced cell apoptosis
To determine whether the cytotoxicity of flavones in
Scutellaria on nicotine-induced lung cancer cells occurred
by apoptosis, we measured the percentage of Annexin V-positive and
PI-negative cells in each group (Fig.
2). Treatment with baicalin, baicalein or wogonin along with
nicotine for 48 h resulted in an increased number of early-stage
apoptotic A549 cells to 6.90, 19.63 and 22.50% and H1299 cells to
10.67, 10.22 and 10.07% respectively, compared with 3.30% in A549
cells and 2.50% in H1299 cells only treated with nicotine.
Flavones in Scutellaria inhibited
nicotine-induced cell invasion
To examine the effect of flavones in
Scutellaria on nicotine-induced invasion of lung cancer
cells, Transwell membrane coated with Matrigel was utilized. The
results showed that the number of both A549 and H1299 cells invaded
to the lower chamber was significantly increased by a 48 h
treatment of nicotine, while the number of invading cells was
apparently reduced through the Matrigel membrane in groups of
baicalin, baicalein or wogonin along with nicotine (Fig. 3).
Flavones in Scutellaria inhibit
nicotine-induced wound-healing migratory ability
We performed the wound-healing assay to determine
the effect of flavones in Scutellaria on nicotine-induced
migration of lung cancer cell. Compared with the control, an
obvious increase of cells in the denuded zone was observed at the
A549 cells treated with nicotine for 48 h (Fig. 4A). A549 cells exposed to baicalin,
baicalein or wogonin along with nicotine expressed a decreased
ability to migrate as compared to the nicotine group. The
quantitative data revealed that these phytochemicals could inhibit
the nicotine-induced migration of A549 cells (Fig. 4B). The effect of nicotine on H1299
cells for 72 h was similar to that on A549 cells, while data
demonstrated that only baicalein negated nicotine-induced migration
of H1299 cells (Fig. 4C and
D).
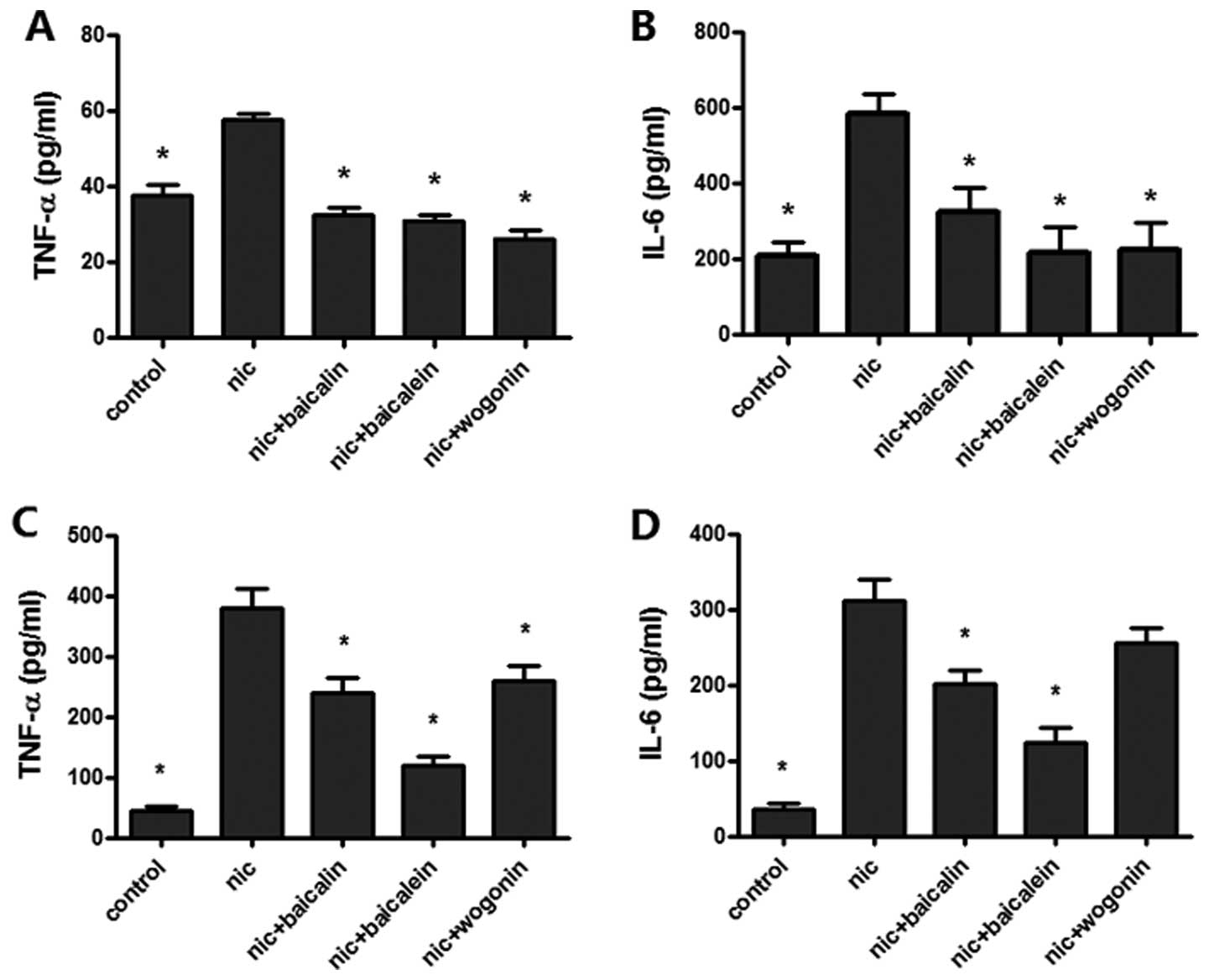
Flavones in Scutellaria inhibit
nicotine-induced TNF-α, IL-6 levels
To test the effect of flavones in Scutellaria
on nicotine-induced lung cancer-associated inflammation, TNF-α and
IL-6 expressions were measured by ELISA for the supernatant of A549
and H1299 cells treated with nicotine combined with or without
various flavones. Treatment with nicotine caused a significant
augment in the levels of TNF-α and IL-6 expression in both A549 and
H1299 cells; nicotine with baicalin, baicalein or wogonin treated
A549 and H1299 cells reduced TNF-α expression and the same treated
A549 cell decreased IL-6 expression, whereas only nicotine plus
baicalin or baicalein showed a decreased expression of IL-6 in
H1299 cells (Fig. 5).
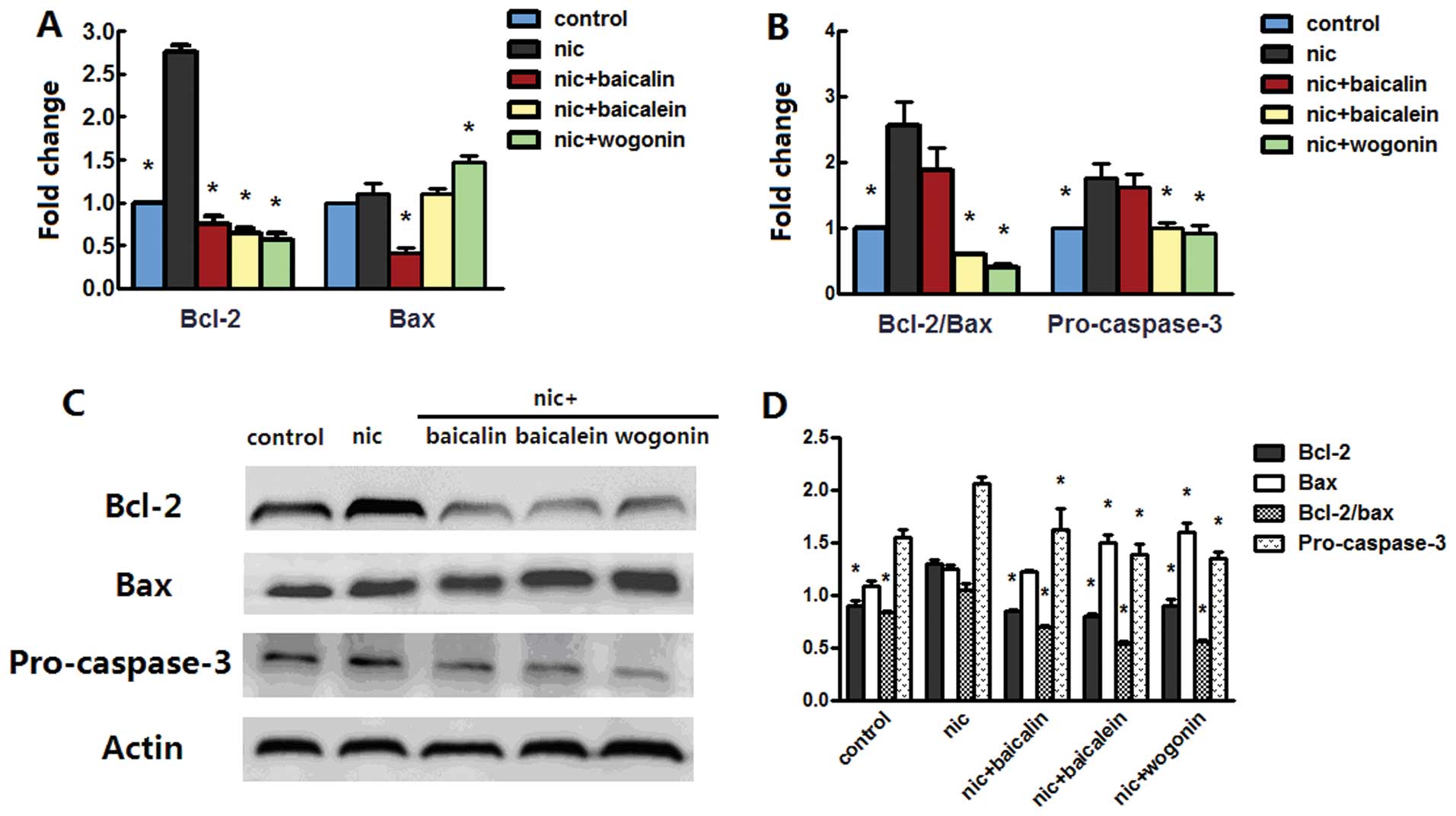
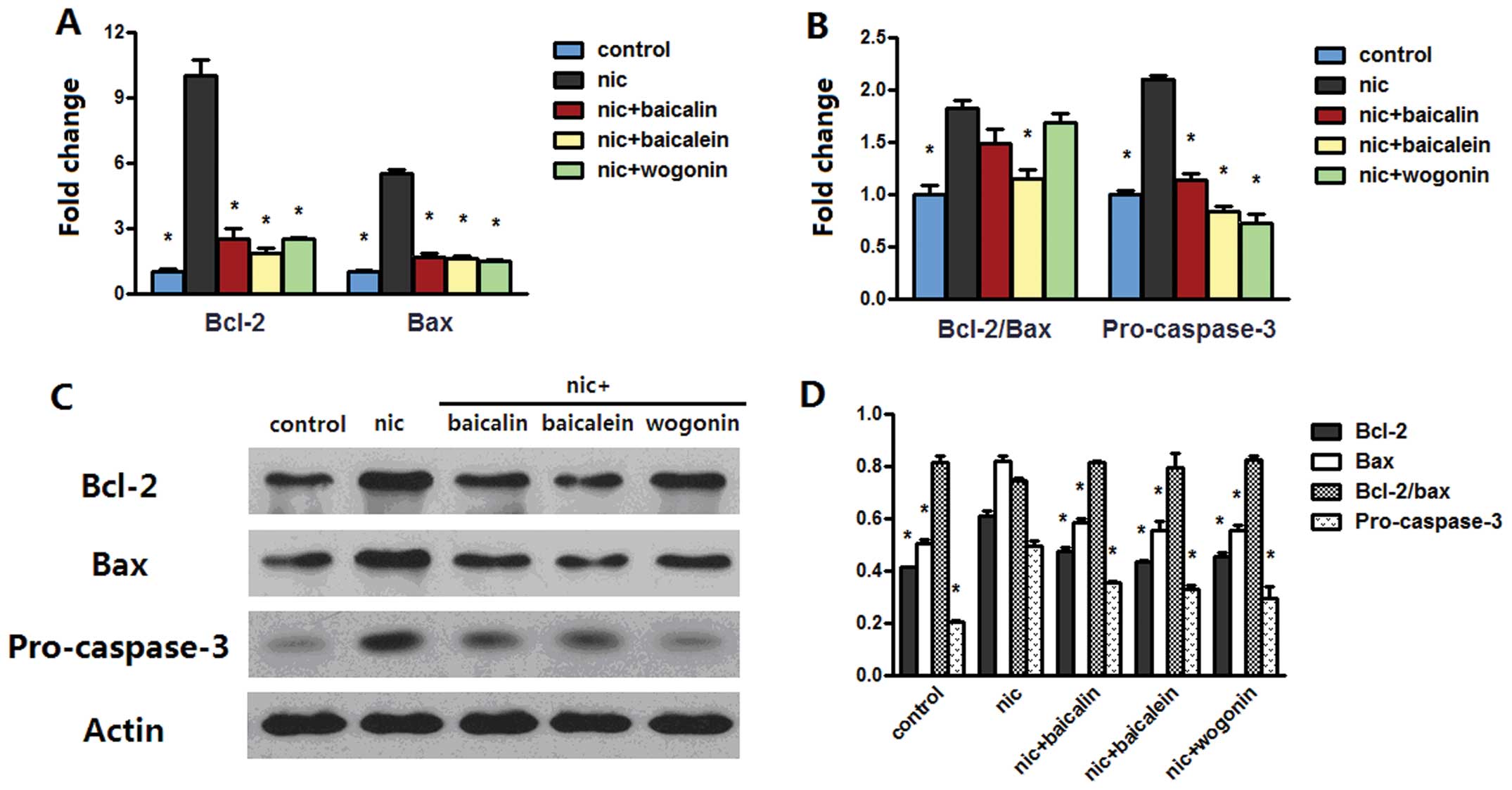
Flavones in Scutellaria modulate
nicotine-induced bcl-2 family and caspase-3
Anti-apoptotic effect of flavones in
Scutellaria on nicotine-induced cells prompted us to examine
whether bcl-2 family and caspase-3 plays any role in the model. The
mRNA level of bcl-2 was increased in both A549 and H1299 cells
treated with nicotine, whereas treatment with nicotine along with
baicalin, baicalein or wogonin sharply decreased the bcl-2 mRNA
level. Nicotine treatment increased bax mRNA level in H1299 cells
but not in A549 cells; treatment with nicotine and baicalin
decreased bax mRNA level in both cell lines, treatment with
nicotine and baicalein only decreased the level of bax mRNA in
H1299 cells, while nicotine and wogonin caused an increased bax
mRNA level in A549 cells but a reduced level in H1299 cells
(Figs. 6A and 7A). The above changes had the effect of
greatly increasing the messenger ratio of the anti-apoptotic bcl-2
to the proapoptotic bax in A549 and H1299 cells treated with
nicotine; the ratio decreased in nicotine along with baicalein or
wogonin treated A549 cells and in nicotine and baicalein treated
H1299 cells. Nicotine treatment greatly increased pro-caspase-3
mRNA level in both cell types, while addition of baicalein or
wogonin in A549 cells or addition of baicalin, baicalein or wogonin
in H1299 cells significantly abrogated this effect (Figs. 6B and 7B). Western blot analysis of bcl-2 and
bax of both cells treated with nicotine reconfirmed the results in
real-time PCR; in groups receiving nicotine with baicalin,
baicalein or wogonin, protein expressions of bcl-2 in A549 and
H1299 cells and the expression of bax in H1299 cells was
dramatically reduced, and in groups receiving nicotine with
baicalein or wogonin, the expression of bax in A549 cells was
increased. These changes caused upregulation of bcl-2/bax protein
expression in nicotine group and downregulation of the ratio in
nicotine along with baicalin, baicalein or wogonin treated A549
cells but no appreciable ratio changes were observed in H1299
cells. Nicotine treatment induced procaspase-3 aggregation in H1299
cells but not in A549 cells, while each of flavones with nicotine
treatment diminished this aggregation in both cell lines (Figs. 6C and D and 7C and D).
Flavones in Scutellaria inhibit
nicotine-induced MMP-2 and MMP-9
To understand the mechanism underlying the
suppression of nicotine-induced migration and invasion by
phytochemicals in Scutellaria, we checked the modulation in
MMP-2 and MMP-9 mRNA and protein levels. Results of real-time PCR
assay depicted upregulation of MMP-2 and MMP-9 mRNA upon nicotine
treatment, whereas these effects were reversed by treatments of
nicotine with baicalin, baicalein or wogonin in A549 and H1299
cells (Fig. 8A and B). Western
blot analysis showed that nicotine-exposed H1299 cells but not A549
cells augmented the MMP-2 and MMP-9 protein expression; nicotine
with baicalein or wogonin inhibited nicotine-induced MMP-2 and
MMP-9 expression in both cell lines, and nicotine and baicalin
treatment only prevented nicotine-induced MMP-2 and MMP-9
expression in H1299 cells (Fig.
8C–F).
Flavones in Scutellaria modulate
nicotine-induced NF-κB p65 and IκB-α
We searched for the mechanisms that may be
responsible for inhibition of nicotine-induced cancer-related
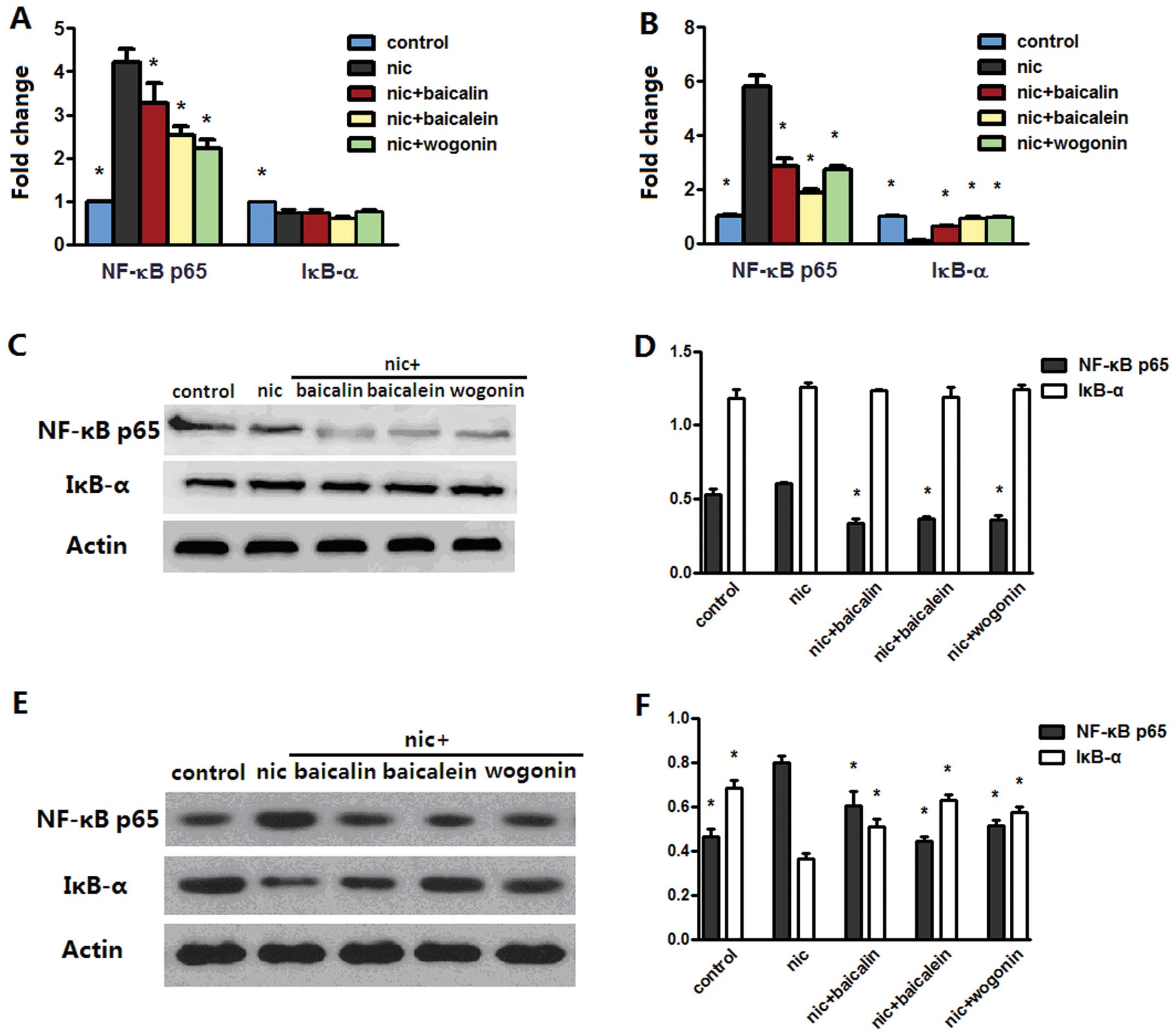
inflammation. Real-time PCR analysis identified that exposure to
nicotine rendered an increased NF-κB p65 and a reduced IκB-α mRNA
levels in both A549 and H1299 cells; nicotine with baicalin,
baicalein or wogonin treatment reverted NF-κB p65 level in both
cell lines and only IκB-α level in H1299 cell lines (Fig. 9A and B). Western blot data
indicated that although nicotine-exposed A549 cells did not induce
apparent NF-κB p65 or IκB-α protein changes, treatment with
nicotine along with baicalin, baicalein or wogonin downregulated
protein expression of NF-κB p65 (Fig.
9C and D). In addition, as expected, NF-κB p65 and IκB-α
protein expression of each group in H1299 cells were consistent
with their mRNA levels.
Discussion
Nicotine has been found to induce proliferation and
metastasis of lung cancer cells. Recently, many anticancer natural
substances, not generating side effects, have elicited considerable
interests. This study furnishes the first evidence that flavonoid
components in Scutellaria baicalensis could inhibit
nicotine-induced proliferation, migration and lung
cancer-associated inflammation.
The imbalance between proliferation and apoptosis
leads to limitless cell proliferation that ultimately develops into
a tumor, making induction of apoptosis the main target of current
chemotherapeutic agents aimed at cancer prevention and treatment
(30). Members of bcl-2 family are
critical regulators of apoptosis; for example, bcl-2, the first
discovered cell death regulator, promotes cell survival, and bax
activates the effector pathways of apoptosis. Our study
demonstrated that baicalin, baicalein and wogonin abrogates
nicotine-induced lung cancer A549 and H1299 cell apoptosis.
Mechanistically, we observed a ratio reduction in bcl-2/bax protein
expression in A549 cells but not in H1299 cells (i.e., increase of
the bax and decrease of bcl-2 levels). Therefore, other factors are
probably involved in the anti-apoptotic process of flavones in
H1299 cells. Caspases, especially caspase-3, plays a pivotal role
in the final common pathway of apoptosis (31). In the present study, a significant
increase in the mRNA expression of pro-caspase-3 was observed after
nicotine treatment in both A549 and H1299 cells, while in protein
expression, nicotine only upregulated pro-caspase-3 in H1299 cells,
but not in A549 cells. This provides evidence that the responses of
different cell lines to nicotine are diverse. Baicalin, baicalein
and wogonin significantly inhibited pro-caspase-3 mRNA and protein
expression triggered by nicotine in both cell types, showing the
involvement of caspase-3 in the flavones-induced apoptosis.
Metastasis is a vital characteristic of malignancy
and primary cause of death for most cancer patients. Exposure of
nicotine promotes the invasive and migratory ability of lung cancer
cells and transplanted tumor in a mouse model (13,14).
In this study, we also found similar effect of nicotine on A549 and
H1299 cells; in addition, our results demonstrated that all
flavones in Scutellaria inhibited nicotine-induced invasion
in both cell lines and migration in A549 cells, and only baicalein
suppressed nicotine-induced migration in H1299 cells. To gain
further knowledge of the mechanism of these flavones on depressing
tumor metastasis, expression of MMP-2 and MMP-9 was detected by
real-time PCR and western blotting. MMPs act as critical mediators
of degrading and remodeling cell extracellular matrix so as to
initiate metastasis (32). It has
been reported that baicalein inhibits pulmonary
carcinogenesis-associated inflammation by interfering with MMP-2
and MMP-9 expression (33). Our
data showed that baicalein and wogonin downregulated the gene and
protein expression of MMP-2 and MMP-9 in both A549 and H1299 cell
lines, indicating a possible role for MMP-2 and MMP-9 in the
process. Interestingly, baicalin only caused some of the gene or
protein changes of MMP-2 or MMP-9 in cancer cells, and therefore
alternative mechanisms may ccause the anti-metastatic effect of
baicalin.
Inflammation plays a key role in lung cancer
promotion and progression. A considerable portion of lung cancer
patients also suffer from chronic obstructive pulmonary disease
(COPD). Inflammatory mediators that are thought to contribute to
the pathogenesis of COPD may also contribute to lung carcinogenesis
(34). Takahashi et al
reported that the inflammatory cells promote lung cancer cell
proliferation to increase IL-6 and TNF-α production by triggering
IKK-β/ NF-κB pathway and subsequently these cytokines increase
proliferation of alveolar epithelial cells (28). Thus, our study investigated TNF-α
and IL-6 levels in cell culture supernatant. Except for wogonin on
nicotine-induced IL-6 expression, all phytochemicals significantly
inhibited TNF-α and IL-6 expression on nicotine-induced A549 and
H1299 cells, which is in line with our previous finding that
baicalin inhibits TNF-α and IL-6 levels in serum and
bronchoalveolar lavage fluid of COPD mice (24). It is indicated that antitumor
effect of these flavones is possibly related to the inhibition of
lung cancer-associated inflammation. NF-κB acts as a critical
mechanistic link between inflammation and cancer. Its activation is
able to upregulate the expression of tumor promoting cytokines,
such as TNF-α or IL-6, and survival genes, such as bcl-2. We have
reported that baicalin attenuates pulmonary inflammation by
inhibiting NF-κB activation in cigarette smoke-induced COPD rat
model (24). In the present study,
expression of NF-κB p65 induced by nicotine in A549 cells differed
from that in H1299 cells, depending on their p53 status (35). Flavones in Scutellaria
dramatically downregulated NF-κB p65 protein expression in both
cell types and elevated IκB-α protein expression in H1299 cells.
These findings suggest baicalin, baicalein and wogonin may play an
anti-inflammatory microenvironment role during nicotine-induced
malignant progression.
Our overall observation proves for the first time
that flavonoid components in Scutellaria baicalensis inhibit
nicotine-induced proliferation, migration and lung
cancer-associated inflammation in vitro. These findings
suggest that baicalin, baicalein and wogonin should be further
explored as promising chemotherapeutic agents against
nicotine-induced tumor growth and metastasis.
Acknowledgements
This study was funded by grants from
National Basic Science Program of China (2009CB523000) and National
Natural Science Foundation of China (81102541).
References
|
1.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Chen W, Zhang S and Zou X: Estimation and
projection of lung cancer incidence and mortality in China.
Zhongguo Fei Ai Za Zhi. 13:488–493. 2010.(In Chinese).
|
|
3.
|
Hecht SS: Cigarette smoking and lung
cancer: chemical mechanisms and approaches to prevention. Lancet
Oncol. 3:461–469. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Zhang J, Ou JX and Bai CX: Tobacco smoking
in China: prevalence, disease burden, challenges and future
strategies. Respirology. 16:1165–1172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Egleton RD, Brown KC and Dasgupta P:
Nicotinic acetylcholine receptors in cancer: multiple roles in
proliferation and inhibition of apoptosis. Trends Pharmacol Sci.
29:151–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Thunnissen FB: Acetylcholine receptor
pathway and lung cancer. J Thorac Oncol. 4:943–946. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Maneckjee R and Minna JD: Opioid and
nicotine receptors affect growth regulation of human lung cancer
cell lines. Proc Natl Acad Sci USA. 87:3294–3298. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Tournier JM and Birembaut P: Nicotinic
acetylcholine receptors and predisposition to lung cancer. Curr
Opin Oncol. 23:83–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Lam DC, Girard L, Ramirez R, et al:
Expression of nicotinic acetylcholine receptor subunit genes in
non-small-cell lung cancer reveals differences between smokers and
nonsmokers. Cancer Res. 67:4638–4647. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kelly PN and Strasser A: The role of Bcl-2
and its pro-survival relatives in tumourigenesis and cancer
therapy. Cell Death Differ. 18:1414–1424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Leber B, Geng F, Kale J and Andrews DW:
Drugs targeting Bcl-2 family members as an emerging strategy in
cancer. Expert Rev Mol Med. 12:e282010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Xin M and Deng X: Nicotine inactivation of
the proapoptotic function of Bax through phosphorylation. J Biol
Chem. 280:10781–10789. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Dasgupta P, Rizwani W, Pillai S, et al:
Nicotine induces cell proliferation, invasion and
epithelial-mesenchymal transition in a variety of human cancer cell
lines. Int J Cancer. 124:36–45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Davis R, Rizwani W, Banerjee S, et al:
Nicotine promotes tumor growth and metastasis in mouse models of
lung cancer. PLoS One. 4:e75242009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Nelson AR, Fingleton B, Rothenberg ML and
Matrisian LM: Matrix metalloproteinases: biologic activity and
clinical implications. J Clin Oncol. 18:1135–1149. 2000.PubMed/NCBI
|
|
16.
|
Bayramoglu A, Gunes HV, Metintas M,
Degirmenci I, Mutlu F and Alatas F: The association of MMP-9 enzyme
activity, MMP-9 C1562T polymorphism, and MMP-2 and -9 and TIMP-1,
-2, -3, and -4 gene expression in lung cancer. Genet Test Mol
Biomarkers. 13:671–678. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Herbst RS, Yano S, Kuniyasu H, et al:
Differential expression of E-cadherin and type IV collagenase genes
predicts outcome in patients with stage I non-small cell lung
carcinoma. Clin Cancer Res. 6:790–797. 2000.PubMed/NCBI
|
|
18.
|
Klein G, Vellenga E, Fraaije MW, Kamps WA
and de Bont ES: The possible role of matrix metalloproteinase
(MMP)-2 and MMP-9 in cancer, e.g. acute leukemia. Crit Rev Oncol
Hematol. 50:87–100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Zong Y, Zhang ST and Zhu ST: Nicotine
enhances migration and invasion of human esophageal squamous
carcinoma cells which is inhibited by nimesulide. World J
Gastroenterol. 15:2500–2505. 2009. View Article : Google Scholar
|
|
20.
|
Lee HZ, Leung HW, Lai MY and Wu CH:
Baicalein induced cell cycle arrest and apoptosis in human lung
squamous carcinoma CH27 cells. Anticancer Res. 25:959–964.
2005.PubMed/NCBI
|
|
21.
|
Leung HW, Yang WH, Lai MY, Lin CJ and Lee
HZ: Inhibition of 12-lipoxygenase during baicalein-induced human
lung nonsmall carcinoma H460 cell apoptosis. Food Chem Toxicol.
45:403–411. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Du G, Han G, Zhang S, et al: Baicalin
suppresses lung carcinoma and lung metastasis by SOD mimic and
HIF-1alpha inhibition. Eur J Pharmacol. 630:121–130. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Li-Weber M: New therapeutic aspects of
flavones: the anti-cancer properties of Scutellaria and its main
active constituents Wogonin, Baicalein and Baicalin. Cancer Treat
Rev. 35:57–68. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Lixuan Z, Jingcheng D, Wenqin Y, Jianhua
H, Baojun L and Xiaotao F: Baicalin attenuates inflammation by
inhibiting NF-kappaB activation in cigarette smoke induced
inflammatory models. Pulm Pharmacol Ther. 23:411–419. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Li L, Bao H, Wu J, et al: Baicalin is
anti-inflammatory in cigarette smoke-induced inflammatory models in
vivo and in vitro: a possible role for HDAC2 activity. Int
Immunopharmacol. 13:15–22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Petty TL: Are COPD and lung cancer two
manifestations of the same disease? Chest. 128:1895–1897. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Yang IA, Relan V, Wright CM, et al: Common
pathogenic mechanisms and pathways in the development of COPD and
lung cancer. Expert Opin Ther Targets. 15:439–456. 2011.PubMed/NCBI
|
|
28.
|
Takahashi H, Ogata H, Nishigaki R, Broide
DH and Karin M: Tobacco smoke promotes lung tumorigenesis by
triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell.
17:89–97. 2010. View Article : Google Scholar
|
|
29.
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
30.
|
Karunagaran D, Joseph J and Kumar TR: Cell
growth regulation. Adv Exp Med Biol. 595:245–268. 2007. View Article : Google Scholar
|
|
31.
|
Fennell DA: Caspase regulation in
non-small cell lung cancer and its potential for therapeutic
exploitation. Clin Cancer Res. 11:2097–2105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Steeg PS: Tumor metastasis: mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Chandrashekar N, Selvamani A, Subramanian
R, Pandi A and Thiruvengadam D: Baicalein inhibits pulmonary
carcinogenesis-associated inflammation and interferes with COX-2,
MMP-2 and MMP-9 expressions in-vivo. Toxicol Appl Pharmacol.
261:10–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Tauler J and Mulshine JL: Lung cancer and
inflammation: interaction of chemokines and hnRNPs. Curr Opin
Pharmacol. 9:384–388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Puliyappadamba VT, Cheriyan VT,
Thulasidasan AK, et al: Nicotine-induced survival signaling in lung
cancer cells is dependent on their p53 status while its
downregulation by curcumin is independent. Mol Cancer. 9:2202010.
View Article : Google Scholar : PubMed/NCBI
|