Introduction
Lung cancer is the major cause of death in
cancer-related disease in the developed world. It was estimated
that about 1.2 million new cases are diagnosed each year worldwide,
and of those patients with lung cancer, over 1 million die annually
(1). In Taiwan, it is the leading
type of cancer causing death; the reports in 2010 from the
Department of Health (Taiwan) indicated that 36.8 individuals per
100,000 die annually from lung cancer in Taiwan. The treatment of
patients with lung cancer includes surgical resection,
chemotherapy, radiation or the combinations of chemotherapy and
radiation (2,3). However, the outcome remains
unsatisfactory.
The dried body of mylabris (Mylabris
phalerata Pallas) was reported to treat malign sores and to
relieve blood stasis in the Chinese population (2,3).
CTD, a terpenoid, is one of the compounds from mylabris, and has
been shown to induce apoptosis in murine erythroleukemia cells
(4), human hepatocellular
carcinoma cells (5), human
multiple myeloma cells (6), human
pancreatic cancer cell lines (7,8),
human bladder carcinoma cell line (9,10),
breast cancer cells (11) and
human colon cancer cells (12). It
was reported that CTD is a potent and selective inhibitor of
protein phosphatase 2A (PP2A) (8).
In human bladder carcinoma cell line, CTD induced secondary
necrosis and COX-2 overexpression (8) and in rat, CTD induces cystitis
through c-Fos and COX-2 overexpression (13).
Recently, in patients with molluscum contagiosum,
they were treated with CTD and experienced minimal side effects and
provided important prospective safety data (14). Moreover, in primary hepatoma, CTD
and its analogs have shown therapeutic effects in clinical trials,
while these effects include cases at the advanced stage without the
suppression of the bone marrow (15). Although numerous studies have shown
that CTD can induce cytotoxic effects in many cancer cell lines,
however, there is now reports to show that CTD affects human lung
cancer cells, thus, in the present study, we investigated the
cytotoxic effects of CTD on human lung cancer H460 cells and we
found that CTD induced cell death through the induction of
apoptosis in vitro.
Materials and methods
Chemicals and reagents
CTD, dimethyl sulfoxide (DMSO),
4’,6-diamidino-2-phenylindole (DAPI), propidium iodide (PI) and
Trypsin-EDTA were obtained from Sigma Chemical Co. (St. Louis, MO,
USA). Minimum essential medium (MEM), fetal bovine serum (FBS),
L-glutamine and penicillin-streptomycin were purchased from
Gibco®/Invitrogen Life Technologies (Carlsbad, CA, USA).
Primary antibody against caspase-3, cleaved caspase-4, cleaved
caspase-8, AIF, Bax, Bcl-xL, cytochrome c, GRP78, IRE1α,
IRE1β, ATF6α, calpain 1, calpain 2, XBP-1 and peroxidase conjugated
secondary antibodies were purchased from Cell Signaling Technology,
Inc. (Beverly, MA, USA). The enhanced chemiluminescence (ECL)
detection system was obtained from Amersham Life Sciences, Inc.
(Arlington Heights, IL, USA).
Cell culture
The human lung cancer cell line (H460 cells) was
purchased from the Food Industry Research and Development Institute
(Hsinchu, Taiwan). Cells were cultured in RPMI-1640 supplemented
with 10% heat inactivated FBS, 100 U/ml penicillin, 100
μg/ml streptomycin, and 2 mM L-glutamine in a
75-cm2 tissue culture flasks at 37°C under 5%
CO2 in humidified air.
Cell morphology and viability, cell cycle
distribution and sub-G1 assays
H460 cells (2×105 cells/well) were
cultured in 12-well plates for 24 h then treated with CTD at
various concentrations (0, 5, 7.5, 10, 15, 30 μM) or 0.5%
DMSO as a vehicle control for 24 and 48 h. For cell morphological
examinations, cells in the wells were examined and photographed
under contrast phase microscopy. For total viable cell
measurements, cells in each well were harvested, counted and
stained with PI (5 μg/ml) and then were analyzed by using
flow cytometry (FACSCalibur, BD Biosciences, San Jose, CA, USA)
assay as previously described (15). For cell cycle distribution and
sub-G1 phase determinations, all harvested cells from each well
were washed with phosphate buffer solution (PBS), incubated with
RNase A (50 μg/ml) for 30 min, and were stained with PI (50
μg/ml) for 5 min, and analyzed for cell cycle distribution
including sub-G1 phase by using flow cytometry system
(Becton-Dickinson, San Jose, CA, USA). The percentages of cells in
different phases of the cell cycle were analyzed by using the
ModFit LT 3.0 program (Verity Software House, ME, USA), as
previously described (16).
Annexin V-FITC/PI staining for cell
apoptosis
Annexin VFITC/PI staining method was used to confirm
cell apoptosis which were observed in sub-G1 of cell cycle assay.
Briefly, H460 cells (2×105 cells/well) were treated with
CTD for 0, 1, 3 and 6 h. Cells were harvested and washed with PBS,
re-suspended in binding buffer (10 mM HEPES/NaOH pH 7.4, 140 mM
NaCl, 25 mM CaCl2), and stained with FITC-conjugated
Annexin V (Pharmingen, Becton-Dickinson Co., San Diego, CA, USA)
for 15 min in the dark, at room temperature and washed with binding
buffer. All samples were measured for the Annexin V-FITC/PI
fluorescence intensity by flow cytometry as described (17).
DNA fragmentation examination
H460 cells (3×105 cells/well) were placed
in 6-well plates for 24 h and were treated with CTD of various
concentrations (0, 5, 10, 15 μM) for 24 h. DNA samples were
isolated by using DNA isolation kit. The isolated DNA (2 μg)
was investigated by using DNA gel electrophoresis which was carried
out in 0.5% agarose gel in Tris/acetate buffer at 15 V for 2 h as
described previously (17). After
electrophoresis, ethidium bromide was used to stain the DNA in the
gel, and the gel was further examined and photographed by
fluorescence microscopy as previously described (18).
Reactive oxygen species (ROS),
intracellular Ca2+ and mitochondrial membrane potential
(ΔΨm) assays
ROS, Ca2+ and ΔΨm measurements
were performed by flow cytometry. Briefly, H460 cells
(2×105 cells/well) were treated with 10 μM of CTD
for 0, 1, 3, 6, 12 or 24 h. All cells from each treatment and time
point were isolated by centrifugation and then were re-suspended in
500 μl of DCFH-DA (10 μM) for 30 min for further ROS
(H2O2) measurement, re-suspended in 500
μl of Fluo-3/AM (2.5 μg/ml) for 30 min for further
intracellular Ca2+ concentrations measurement and
re-suspended in 500 μl of DiOC6 (4 μmol/l)
for 30 min to further the levels of ΔΨm measurement. All
samples were analyzed by flow cytometry as described (17).
Real-time PCR assay for mRNA levels of
caspase-3 and -8
The mRNA expression of caspase-3 and -8 were
performed by real-time PCR. Briefly, H460 cells (2×105
cells/well) were treated with 10 μM of CTD for 0, 12 and 24
h. Then cells were harvested and total RNA was extracted from each
treatment using the Qiagen RNeasy mini kit (Qiagen, Inc., Valencia,
CA) as described previously (19).
High Capacity cDNA Reverse Transcription Kit (Applied Biosystems,
Foster City, CA) was used to reverse-transcribe all RNA samples at
42°C for 30 min according to the standard protocol of the supplier.
The following conditions were used for quantitative PCR: 2 min at
50°C, 10 min at 95°C, and 40 cycles of 15 sec at 95°C, 1 min at
60°C using 1 μl of the cDNA reverse-transcribed as described
above, 2X SYBR-Green PCR Master mix (Applied Biosystems) and 200 nM
forward and reverse primers: caspase-3 forward,
CAGTGGAGGCCGACTTCTTG and reverse, TGGCACAAA GCGACTGGAT; caspase-8
forward, GGATGGCCACTGTG AATAACTG and reverse, TCGAGGACATCGCTCTCTCA;
GAPDH forward, ACACCCACTCCTCCACCTTT and reverse,
TAGCCAAATTCGTTGTCATACC. All assays were performed by Applied
Biosystems 7300 real-time PCR system in triplicates and expression
fold-changes were derived using the comparative CT method (20,21).
Western blot analysis
H460 cells (1×106 cells/dish) were placed
in 10-cm dish for 24 h then were incubated with 10 μM CTD
for 0, 6, 12, 24 and 48 h. After treatment under each experimental
condition, total cell lysates were denatured with ice-cold lysis
buffer [10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EGTA, 0.3 mM
PMSF, 0.2 mM sodium orthovanadate, 0.1% SDS, 1 mM EDTA, 1% NP-40,
10 mg/ml leupeptin, and 10 mg/ml aprotinin] and then were
centrifuged at 13,000 × g for 10 min at 4°C (16,17).
A Bio-Rad protein assay kit (Hercules, CA, USA) was used for
measuring the total protein of each sample. The clarified protein
lysates (30 μg) were electrophoretically resolved on
denaturing SDS-polyacrylamide gel (12%) followed by transfer onto
nitrocellulose membranes, and then blotted with the relevant
primary antibodies (anti-caspase-3, caspase-4, caspase-8, AIF, Bax,
Bcl-xL, cytochrome c, GRP78, IRE1α, IRE1β, ATF6α, calpain 1,
calpain 2, XBP-1) overnight at 4°C followed by
peroxidase-conjugated secondary antibody for 1 h at room
temperature (25°C). Finally, proteins were visualized by ECL
detection (Amersham Biosciences ECL™) and exposed to X-ray film and
bands obtained were quantified using NIH Image analyzer (NIH,
Bethesda, MD) (17,18).
Confocal laser scanning microscopy
assay
H460 cells (3×105 cells/well) were placed
on 6-well chamber slides and incubated with 10 μM CTD for 24
h. The cells were fixed in 4% formaldehyde in PBS for 15 min
followed by using 0.3% Triton X-100 in PBS for 1 h and by using 2%
BSA for blocking non-specific binding sites. Then cells were
stained by primary antibodies such as anti-cytochrome c,
anti-AIF and anti-Endo G (all in green fluorescence) overnight.
Then cells were washed twice with PBS and were stained with
secondary antibody (FITC-conjugated goat anti-mouse IgG) followed
PI (red fluorescence) staining for nuclein examination as described
previously. Slides with cells were mounted, examined and
photo-micrographed under a Leica TCS SP2 Confocal Spectral
Microscope as described previously (17,18).
Localization of protein in nuclei is demonstrated by the
development of orange color due to red and green overlapped
pixels.
Statistical analysis
All data were performed and expressed as mean ± SD
from triplicate experiments. Statistically significant differences
between the CTD-treated and -untreated (control) groups were
analyzed by Student’s t-test, with values of *p<0.05
considered statistically significant.
Results
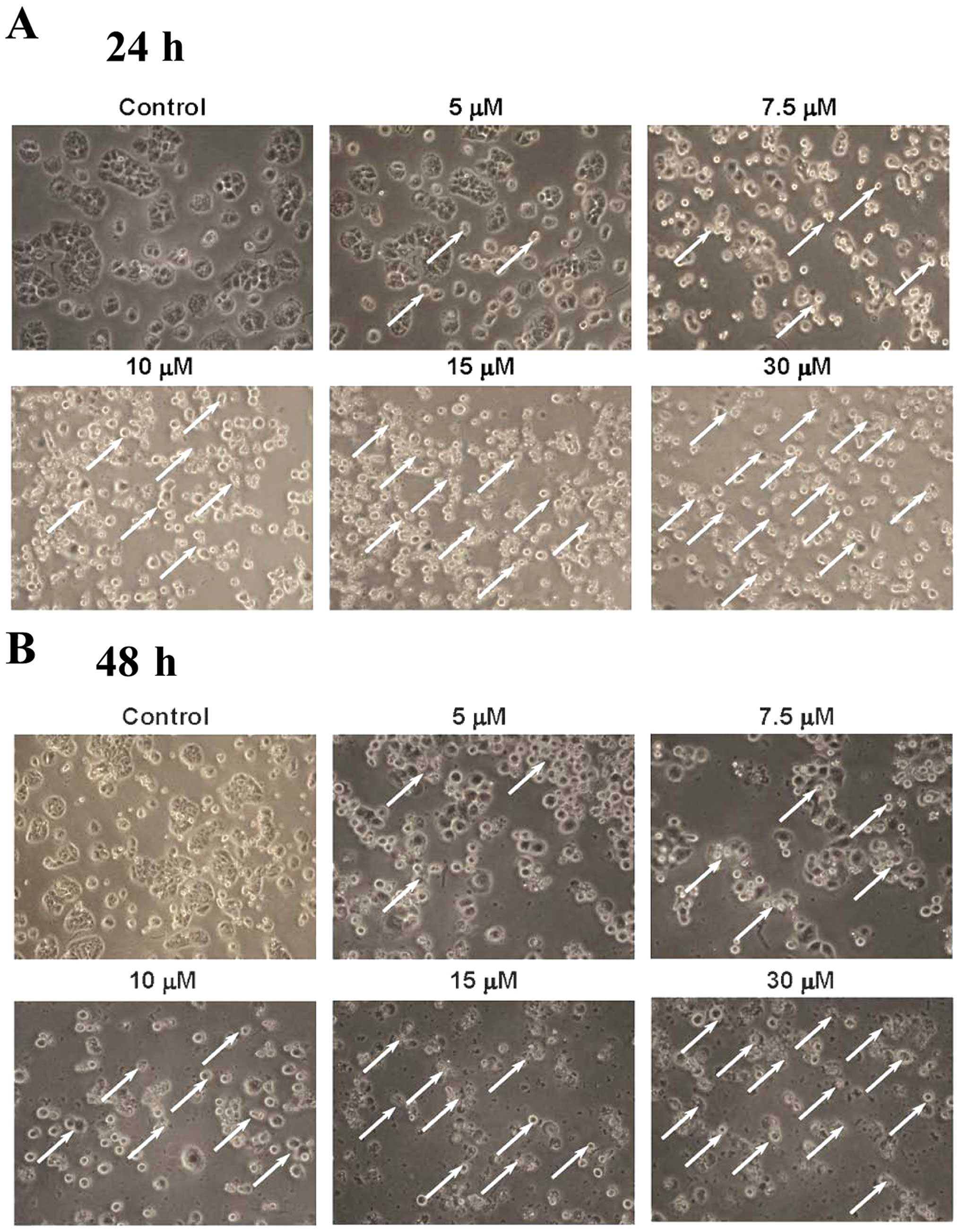
CTD induces cell morphological changes
and decreases the cell viability of H460 cells
H460 cells were treated with 0, 5, 7.5, 10, 15 and
30 μM of CTD for 24 and 48 h before the cells were examined
and photographed for examining the cell morphological changes and
were harvested for the percentage of viable cells and the results
are shown in Fig. 1. Fig. 1A and B indicate that CTD induced
cell morphological changes and led to cell death and debris. CTD
led to enhancement of dead cells following 5–30 μM as
indicated by white arrows. Furthermore, Fig. 1C shows a significant dose-dependent
reduction of living cells with CTD treatment in H460 cells and
these effects are dose-dependent.
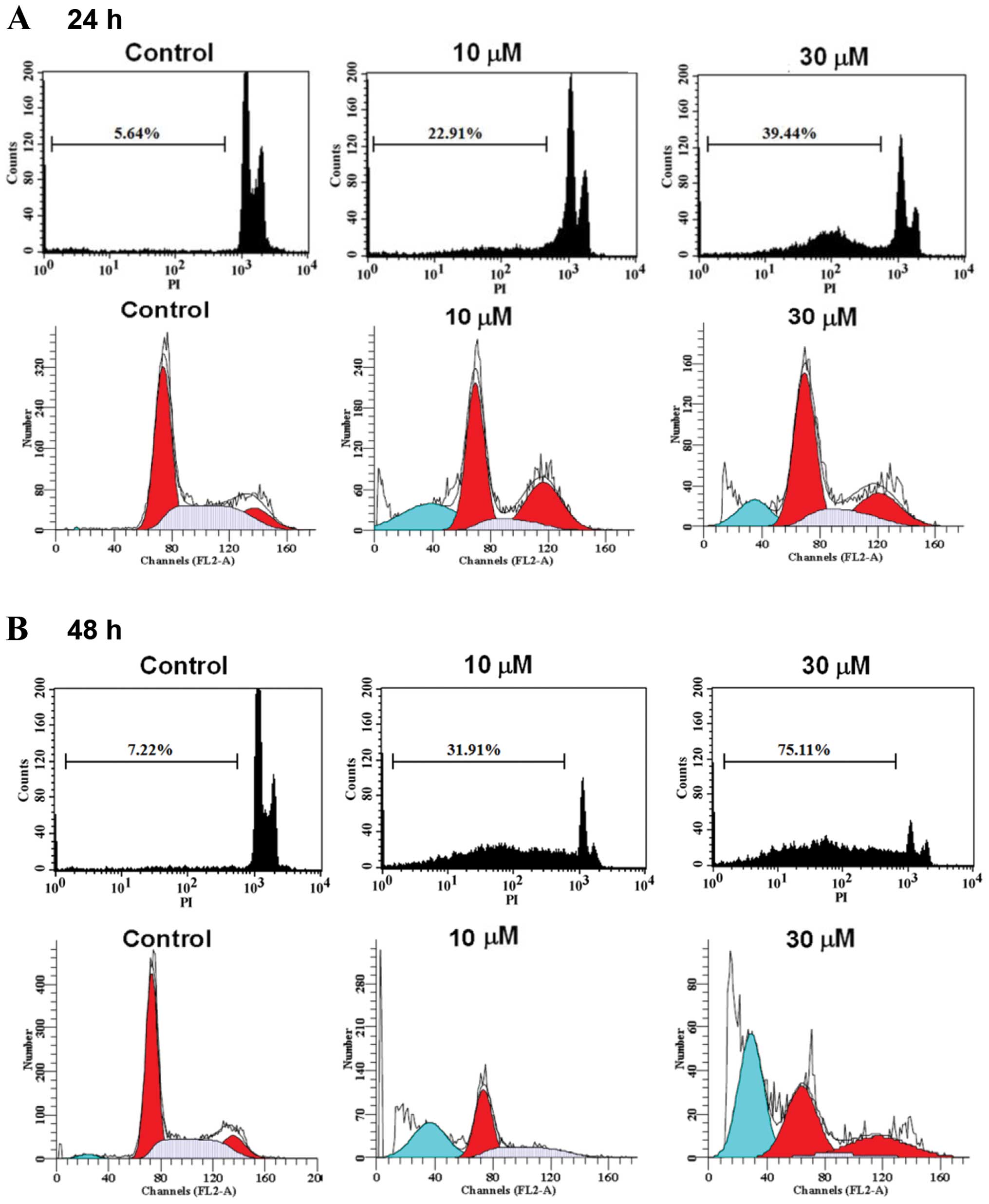
CTD induces sub-G1 phase and apoptosis of
H460 cells
H460 cells were treated with 0, 5, 7.5, 10, 15 and
30 μM of CTD for 24 and 48 h before the cells were examined
for sub-G1 phase in cell cycle assay and the results are shown in
Fig. 2. Data from Fig. 2A–C indicated that CTD induced
sub-G1 phase development, thus CTD induced apoptosis and these
effects are dose-dependent. To determine whether apoptosis mediated
the growth inhibition observed in H460 cells treated with CTD, we
performed an Annexin V-FITC/PI double-staining experiment and
results are shown in Fig. 2D and
E, a considerable increase in apoptotic cells was observed for
H460 cells treated with CTD and these effects are
time-dependent.
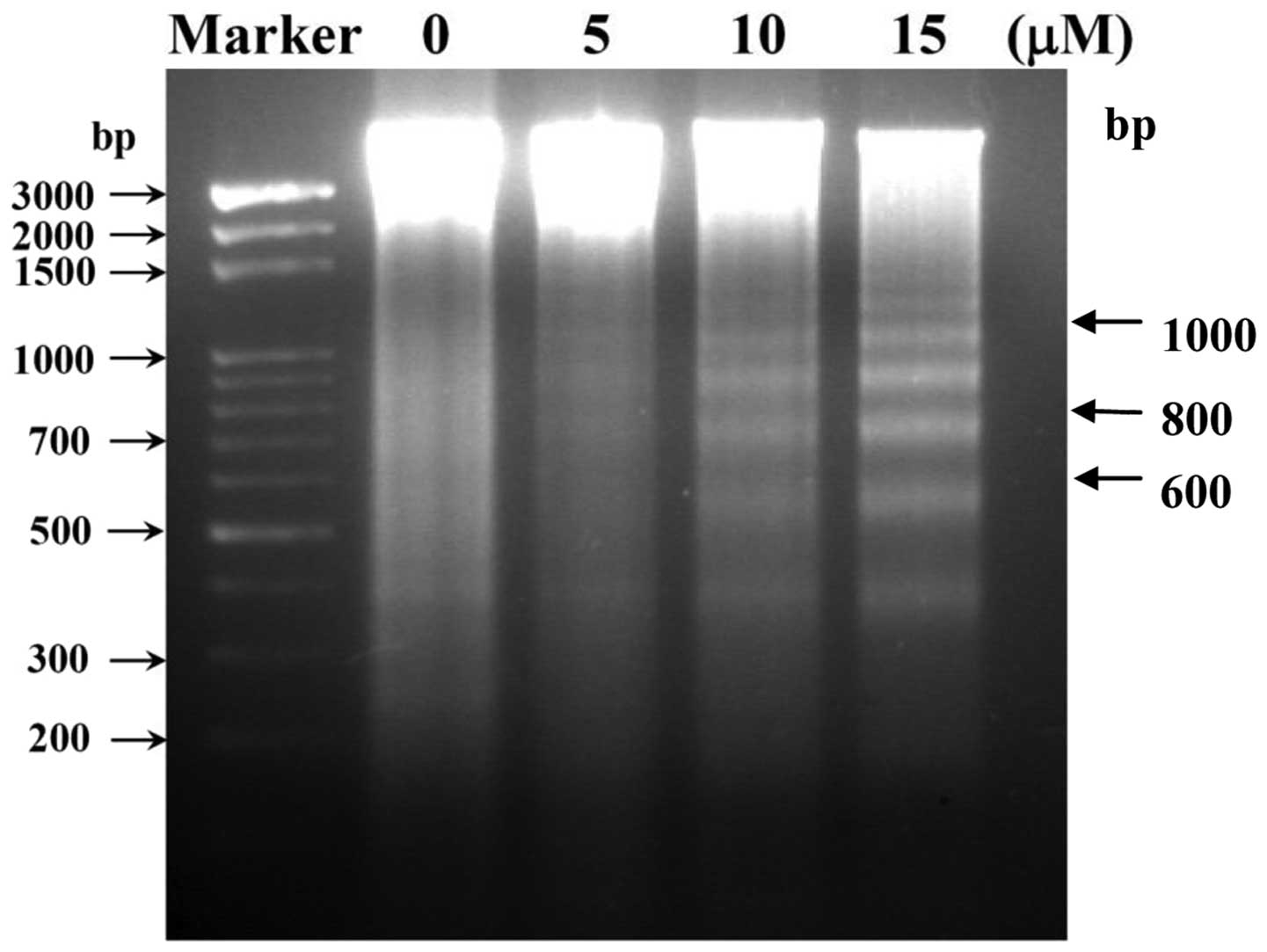
CTD induces DNA fragmentation in H460
cells
In order to delineate the mechanism of cell
apoptosis mediated by CTD, we performed a DNA fragmentation assay,
since DNA fragmentation is the characteristic for apoptosis. H460
cells were treated with 0, 5, 10 and 15 μM of CTD for 24 h
and DNA was then isolated and analyzed by DNA agarose gel
electrophoresis and the results are shown in Fig. 3. Fig.
3 indicates a typical ladder pattern of internucleosomal
fragmentation was observed in cells after 24 h of CTD treatment.
Low-molecular-weight DNA from these cells was resolved in 2.0%
agarose gels (Fig. 3). These
results suggest that CTD is a potent inducer of apoptosis in H460
cells.
CTD induces reactive oxygen species (ROS)
and Ca2+ production and decreases the levels of
mitochondrial membrane potential (ΔΨm) in H460
cells
To further examine the effects of CTD and whether it
induced cell death in H460 cells through the production of
Ca2+ or dysfunction of mitochondrial, the results from
flow cytometric assay are shown in Fig. 4. Fig.
4A demonstrates that CTD decreased ROS from 1–12 h treatment.
However, Fig. 4B indicates that
CTD promoted the production of Ca2+ and these effects
are time-dependent. Fig. 4C
indicated that CTD decreased the levels of ΔΨm and these
effects are time-dependent. Fig.
4B shows that CTD promoted the Ca2+ release from 1 h
up to 24 h treatment, however, the highest levels of
Ca2+ release is in 6 h treatment. Both results indicated
that CTD induced apoptosis of H460 cells is associated with
dysfunction of mitochondria.
CTD promotes the mRNA expressions of
caspase-3 and -8 in H460 cells
To further examine CTD promoting caspase-3 and -8
activities in H460 cells, and whether or not it was through the
expression of mRNA of caspase-3 and -8, the cells were treated with
CTD for 12 and 24 h and then isolated for total RNA followed by
real-time PCR assay and the results are shown in Fig. 5. Data indicated that CTD promoted
the gene expression of mRNA in caspase-3 and -8.
CTD affects apoptosis-associated protein
expression in H460 cells
For further examination on whether CTD induced
apoptosis in H460 cells through the effects of apoptosis-associated
protein, H460 cells were treated with 10 μM of CTD for 0, 6,
12, 24 and 48 h and then total proteins were quantitated and
apoptosis-associated proteins were examined by western blot
analysis and the results are shown in Fig. 6, indicated that CTD significantly
promoted the expression of cleaved caspase-3 and -8, cytochrome
c, Bax and AIF but inhibited the levels of Bcl-xL (Fig. 6A). Furthermore, CTD promoted ER
stress-associated protein expression such as GRP78, IRE1α, IRE1β,
ATF6α and caspase-4 (Fig. 6B). CTD
promoted the expression of calpain 2 and XBP-1, but inhibited
calpain 1 (Fig. 6C) that are
associated with apoptosis pathways. These results indicated that
CTD induced apoptosis in H460 cells through both caspase-dependent
and -independent, ER stress and mitochondria-dependent
pathways.
 | Figure 6.CTD affects apoptosis-associated
protein expression in H460 cells. H460 cells were treated with 10
μM of CTD for 0, 6, 12, 24 and 48 h and then total proteins
were quantitated and apoptosis-associated proteins were examined by
western blot analysis as described in Materials and methods. (A)
Caspase-3 and -8, cytochrome c, Bax, AIF and Bcl-xL. (B)
GRP78, IRE1α, IRE1β, ATF6α and caspase-4. (C) Calpain 1, calpain 2
and XBP-1. |
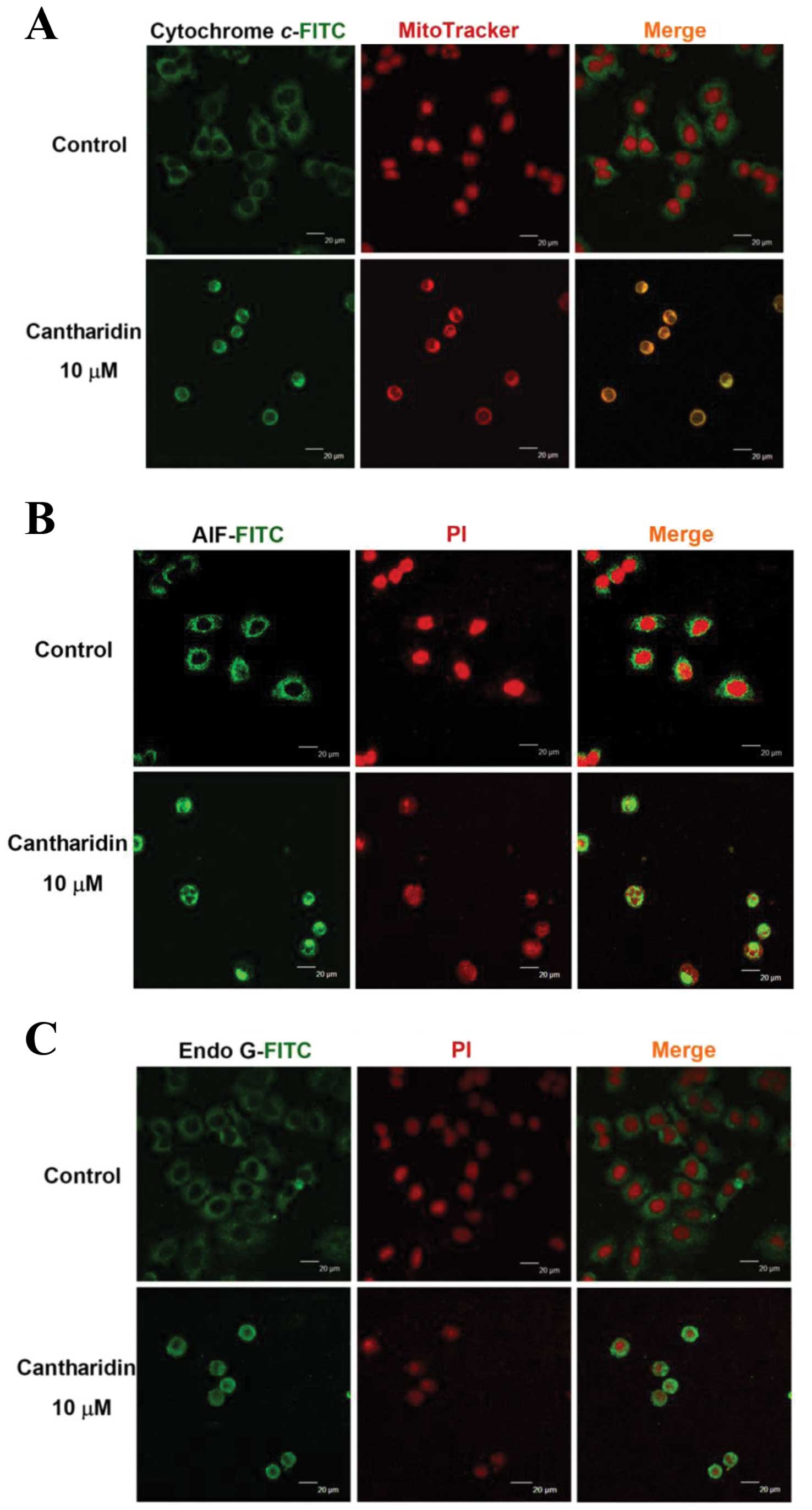
CTD affects the translocation of
apoptotic-associated proteins in H460 cells
In order to confirm that CTD affects the
translocation of cytochrome c, AIF and Endo G involved in
apoptosis in H460 cells, cells were exposed to 10 μM of CTD
for 24 h were then stained by anti-cytochrome c, AIF and
Endo G and then were stained with secondary antibody and examined
and photographed by confocal laser microscopy. The results are
shown in Fig. 7, which indicated
that CTD promoted the cytochrome c (Fig. 7A), AIF (Fig. 7B) and Endo G (Fig. 7C) release from mitochondria in H460
cells when compared to CTD untreated (control) groups.
Discussion
Substantial evidence shows that stimulating or
inducing tumor cell apoptosis has been recognized to be a new
possibility for tumor treatment. Although numerous studies have
shown that CTD induced cell death and induction of apoptosis in
many human cancer cells (5–12),
there are no reports to show that CTD affected human lung cancer
cells. Thus, herein, we investigated the effects of CTD on cell
death of H460 human lung cancer cells in vitro and the
results indicated that CTD induced cell morphological changes
(Fig. 1A) and decreased the
percentage of viable cells (Fig.
1B) via the induction of sub-G1 phase (apoptosis) (Fig. 2), which was examined and measured
by flow cytometric assay. We also used DNA gel electrophoresis and
Annexin V-FITC/PI staining for confirming H460 cell apoptosis which
was induced by CTD. It is well documented that DNA fragmentation is
one of the hallmarks of cell apoptosis (22,23),
and in this study, we also isolated DNA from H460 cells with or
without exposure to CTD, then DNA gel electrophoresis was performed
and the result show increased doses led to increased DNA
fragmentation (Fig. 3) which
indicated that CTD induced apoptosis in H460 cells. We also used
Annexin V-FITC/PI staining for examining the cells and the results
clearly demonstrated that CTD induced apoptosis in H460 cells
(Fig. 2) and these effects are
dose-dependent. Annexin V-FITC/PI staining is well accepted and
widely used for measuring cell apoptosis (24,25).
To investigate the molecular mechanism including the
signal pathway, we found that CTD promoted Ca2+
production and decreased the levels of ΔΨm (Fig. 4B and C), and it also increased the
mRNA expression of caspase-3 and -8 (Fig. 5). It is well known that apoptotic
cell death can be regulated by either the extrinsic or the
intrinsic apoptotic pathway (26–28).
The extrinsic pathway is triggered by an agent connected with CD95
(also named Fas or Apo1) then involving caspase-8 activation
leading to the activation of the downstream effector caspase-3
causing cell apoptosis (29). The
intrinsic pathway involved in the dysfunction of mitochondria then
led to the cytochrome c release, causing caspase-3
activation leading to apoptosis (30,31)
or caused AIF and Endo G release from mitochondria directly
inducing apoptosis (32,33). Based on these observations, we may
suggest that CTD-induced cell apoptosis of H460 cells may be
through the activation of caspase-3 and -8, and this is in
agreement with our earlier report that CTD induced colon cancer
cell apoptosis through caspase-dependent pathways (12). On the other hand it may also be
through dysfunction of mitochondria base on the levels of
ΔΨm decrease and AIF and Endo G, which were increased as
confirmed by western blot analysis (Fig. 6) and confocal laser microscopy
examination (Fig. 7). This is in
agreement with our earlier report that CTD induced bladder cancer
cell apoptosis through mitochondria-dependent pathways (10). Numerous studies and evidence have
demonstrated that agent-induced cancer cell apoptotic cell death
are involved in caspase-dependent and -independent or
mitochondria-dependent and -independent pathways (34,35).
Herein, we may also suggest that CTD-induced apoptosis of H460
cells may be through caspase- and mitochondria-dependent pathways
and also apoptosis through cross-talk between the extrinsic and the
intrinsic pathways.
Based on the results from western blot analysis
(Fig. 6), CTD increased the
protein expression of Bax which is a proapoptotic protein and
decreased the protein expression of Bcl-xL, which is an
anti-apoptotic protein. It is reported that the ratio of Bax/Bcl-xL
is associated with the changes of levels of mitochondria membrane
potential ΔΨm (36,37).
Results in Fig. 4C also show that
CTD decreased the levels of ΔΨm as is obtained from flow
cytometry assay. Based on the results (Fig. 7) from confocal microscopy
examination, that CTD also promoted the release of cytochrome
c, AIF and Endo G in H460 cells. These observations suggest
that CTD induced apoptosis of H460 cells via mitochondria-dependent
pathway. It was reported that CTD is an inhibitor of protein
phosphatase 2A (PP2A) (8) and CTD
also inhibits heat shock factor 1 (HSF1) transcriptional activity
(38). Thus, for further
investigation to identify the direct molecular targets of CTD other
than PP2A, we will further explain its working mechanism.
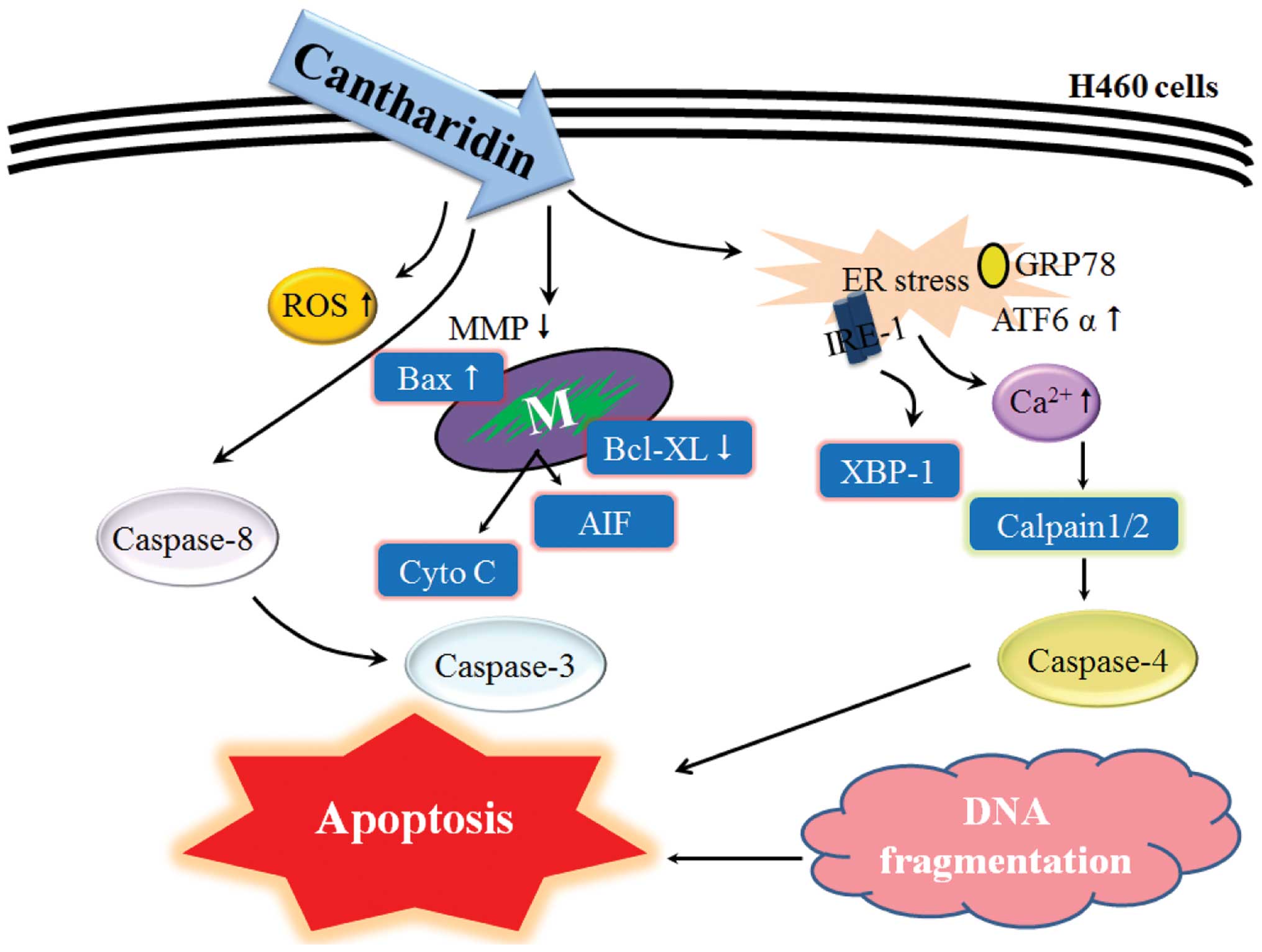
In summary, in the present study, we suggest the
possible significant molecular signal pathways for CTD inducing
apoptosis in H460 cells as shown in Fig. 8. CTD may go through the death
receptor (Fas receptor), activating caspase-8 following the
activation of caspase-3 leading to apoptosis, or to increase the
ratio of Bax/Bcl-xL leading to dysfunction of mitochondria
(decrease the levels of ΔΨm) causing cytochrome
c, AIF and Endo G release then leading to apoptosis.
Acknowledgements
This study was supported by grant CMU
101-AWARD-03(1/2) from China Medical University, Taichung, Taiwan,
R.O.C.
References
|
1.
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2.
|
Parlak C, Mertsoylu H, Guler OC, Onal C
and Topkan E: Definitive chemoradiation therapy following surgical
resection or radiosurgery plus whole-brain radiation therapy in
non-small cell lung cancer patients with synchronous solitary brain
metastasis: a curative approach. Int J Radiat Oncol Biol Phys.
88:885–891. 2014. View Article : Google Scholar
|
|
3.
|
Salama JK, Pang H, Bogart JA, et al:
Predictors of pulmonary toxicity in limited stage small cell lung
cancer patients treated with induction chemotherapy followed by
concurrent platinum-based chemotherapy and 70 Gy daily
radiotherapy: CALGB 30904. Lung Cancer. 82:436–440. 2013.
View Article : Google Scholar
|
|
4.
|
Xu B: The influence of several anticancer
agents on cell proliferation, differentiation and the cell cycle of
murine erythroleukemia cells. Am J Chin Med. 9:268–276. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Wang CC, Wu CH, Hsieh KJ, Yen KY and Yang
LL: Cytotoxic effects of cantharidin on the growth of normal and
carcinoma cells. Toxicology. 147:77–87. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Sagawa M, Nakazato T, Uchida H, Ikeda Y
and Kizaki M: Cantharidin induces apoptosis of human multiple
myeloma cells via inhibition of the JAK/STAT pathway. Cancer Sci.
99:1820–1826. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Li W, Chen Z, Zong Y, et al: PP2A
inhibitors induce apoptosis in pancreatic cancer cell line PANC-1
through persistent phospho rylation of IKKalpha and sustained
activation of the NF-kappaB pathway. Cancer Lett. 304:117–127.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Li W, Xie L, Chen Z, et al: Cantharidin, a
potent and selective PP2A inhibitor, induces an oxidative
stress-independent growth inhibition of pancreatic cancer cells
through G2/M cell-cycle arrest and apoptosis. Cancer Sci.
101:1226–1233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Huan SK, Lee HH, Liu DZ, Wu CC and Wang
CC: Cantharidin-induced cytotoxicity and cyclooxygenase 2
expression in human bladder carcinoma cell line. Toxicology.
223:136–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kuo JH, Chu YL, Yang JS, et al:
Cantharidin induces apoptosis in human bladder cancer TSGH 8301
cells through mitochondria-dependent signal pathways. Int J Oncol.
37:1243–1250. 2010.PubMed/NCBI
|
|
11.
|
Williams LA, Moller W, Merisor E, Kraus W
and Rosner H: In vitro anti-proliferation/cytotoxic activity of
cantharidin (spanish fly) and related derivatives. West Indian Med
J. 52:10–13. 2003.PubMed/NCBI
|
|
12.
|
Huang WW, Ko SW, Tsai HY, et al:
Cantharidin induces G2/M phase arrest and apoptosis in human
colorectal cancer colo 205 cells through inhibition of CDK1
activity and caspase-dependent signaling pathways. Int J Oncol.
38:1067–1073. 2011.PubMed/NCBI
|
|
13.
|
Huan SK, Wang KT, Yeh SD, et al:
Scutellaria baicalensis alleviates cantharidin-induced rat
hemorrhagic cystitis through inhibition of cyclooxygenase-2
overexpression. Molecules. 17:6277–6289. 2012. View Article : Google Scholar
|
|
14.
|
Coloe Dosal J, Stewart PW, Lin JA,
Williams CS and Morrell DS: Cantharidin for the treatment of
Molluscum contagiosum: a prospective, double-blinded,
placebo-controlled trial. Pediatr Dermatol. Aug 16–2012.(Epub ahead
of print).
|
|
15.
|
Yu ZJ: Chinese material medica combined
with cisplatin and lipiodol through transcatheter arterial
embolization in the treatment of primary hepatoma. Zhongguo Zhong
Xi Yi Jie He Za Zhi. 13:327–329. 3231993.(In Chinese).
|
|
16.
|
Chen YH, Hung MC and Shyu WC: Role of
cancer stem cells in brain tumors. BioMedicine. 2:84–91. 2012.
View Article : Google Scholar
|
|
17.
|
Lin YT, Huang AC, Kuo CL, et al: Induction
of cell cycle arrest and apoptosis in human osteosarcoma U-2 OS
cells by Solanum lyratum extracts. Nutr Cancer. 65:469–479.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Chueh FS, Chen YL, Hsu SC, et al:
Triptolide induced DNA damage in A375.S2 human malignant melanoma
cells is mediated via reduction of DNA repair genes. Oncol Rep.
29:613–618. 2013.PubMed/NCBI
|
|
19.
|
Li CC, Lo HY, Hsiang CY and Ho TY: DNA
microarray analysis as a tool to investigate the therapeutic
mechanisms and drug development of Chinese medicinal herbs.
BioMedicine. 2:10–16. 2012. View Article : Google Scholar
|
|
20.
|
Yu FS, Huang AC, Yang JS, et al: Safrole
induces cell death in human tongue squamous cancer SCC-4 cells
through mitochondria-dependent caspase activation cascade apoptotic
signaling pathways. Environ Toxicol. 27:433–444. 2012. View Article : Google Scholar
|
|
21.
|
Ho YT, Lu CC, Yang JS, et al: Berberine
induced apoptosis via promoting the expression of caspase-8, -9 and
-3, apoptosis-inducing factor and endonuclease G in SCC-4 human
tongue squamous carcinoma cancer cells. Anticancer Res.
29:4063–4070. 2009.PubMed/NCBI
|
|
22.
|
Huang CY and Lee SD: Possible
pathophysiology of heart failure in obesity: Cardiac apoptosis.
BioMedicine. 2:36–40. 2012. View Article : Google Scholar
|
|
23.
|
Momtazi-Borojeni AA, Behbahani M and
Sadeghi-Aliabadi H: Antiproliferative activity and apoptosis
induction of crude extract and fractions of avicennia marina. Iran
J Basic Med Sci. 16:1203–1208. 2013.PubMed/NCBI
|
|
24.
|
Ji BC, Yu CC, Yang ST, et al: Induction of
DNA damage by deguelin is mediated through reducing DNA repair
genes in human non-small cell lung cancer NCI-H460 cells. Oncol
Rep. 27:959–964. 2012.PubMed/NCBI
|
|
25.
|
Yin MC: Anti-glycative potential of
triterpenes: A mini-review. BioMedicine. 2:2–9. 2012. View Article : Google Scholar
|
|
26.
|
Igney FH and Krammer PH: Death and
anti-death: tumour resistance to apoptosis. Nat Rev Cancer.
2:277–288. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Kumar S: Caspase function in programmed
cell death. Cell Death Differ. 14:32–43. 2007. View Article : Google Scholar
|
|
28.
|
Xu G and Shi Y: Apoptosis signaling
pathways and lymphocyte homeostasis. Cell Res. 17:759–771. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Wilson MR: Apoptotic signal transduction:
emerging pathways. Biochem Cell Biol. 76:573–582. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Cory S and Adams JM: The Bcl2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Newmeyer DD and Ferguson-Miller S:
Mitochondria: releasing power for life and unleashing the
machineries of death. Cell. 112:481–490. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Liu W, Fan Z, Han Y, Zhang D, Li J and
Wang H: Intranuclear localization of apoptosis-inducing factor and
endonuclease G involves in peroxynitrite-induced apoptosis of
spiral ganglion neurons. Neurol Res. 34:915–922. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Kapoor R, Rizvi F and Kakkar P: Naringenin
prevents high glucose-induced mitochondria-mediated apoptosis
involving AIF, Endo-G and caspases. Apoptosis. 18:9–27. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Chueh FS, Hsiao YT, Chang SJ, et al:
Glycyrrhizic acid induces apoptosis in WEHI-3 mouse leukemia cells
through the caspase- and mitochondria-dependent pathways. Oncol
Rep. 28:2069–2076. 2012.PubMed/NCBI
|
|
35.
|
Lin PC, Liu PY, Lin SZ and Harn HJ:
Angelica sinensis: A Chinese herb for brain cancer therapy.
BioMedicine. 2:30–35. 2012. View Article : Google Scholar
|
|
36.
|
Goloudina AR, Mazur SJ, Appella E, Garrido
C and Demidov ON: Wip1 sensitizes p53-negative tumors to apoptosis
by regulating the Bax/Bcl-xL ratio. Cell Cycle. 11:1883–1887. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Liu FT, Goff LK, Hao JH, Newland AC and
Jia L: Increase in the ratio of mitochondrial Bax/Bcl-XL induces
Bax activation in human leukemic K562 cell line. Apoptosis.
9:377–384. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Kim JA, Kim Y, Kwon BM and Han DC: The
natural compound cantharidin induces cancer cell death through
inhibition of heat shock protein 70 (HSP70) and Bcl-2-associated
athanogene domain 3 (BAG3) expression by blocking heat shock factor
1 (HSF1) binding to promoters. J Biol Chem. 288:28713–28726. 2013.
View Article : Google Scholar
|