Introduction
Oral cancers (subtype of head and neck cancer) have
several types and are mostly (~90%) oral squamous cell carcinoma
(OSCC) with complicated biological characteristics and clinical
behavior (1). Commonly, the
well-known risk factors of oral cancer are cigarettes, alcohol
consuption, inflammation, mutation, preneoplasia, UV and HPV
infection (2,3). In spite of advanced cancer diagnosis,
radiotherapy, chemotherapy and surgery, only slight improvement has
been accomplished in the 5-year survival rate of oral cancer
patients over the last few decades (4). Although the visual screening of oral
cavity is an easy examination of oral cancers, cancer lesions are
not readily detected at the early stages (5). Because oral cancer is commonly
diagnosed at late stage, the mortality rate from oral cancer is
~50% (1). OSCC tends to
metastasize or spread as soon as it forms, eventually leading to
high mortality (6). Therefore,
there is a demand for novel molecular targets for the management of
oral cancers.
Recent studies reported that specificity protein 1
(Sp1) played a major role in the proliferation of tissues or organs
as transcription factor and that it was also highly expressed in
many cancer cells (7).
Furthermore, it has been reported that the suppression of Sp1
protein in cancer cells is closely associated with growth
regulation, cell cycle regulation, proliferation, biological
response, differentiation, mortality and cell survival genes
(3,8). For the above reasons, the
downregulation of Sp1 is increasingly attracting attention as a
potential strategy for controlling oral cancer.
In spite of the efforts made to investigate many
natural products to find effective chemicals for oral cancers over
the last several decades (9),
there are currently limited options to treat oral cancer. Even
though the frequency of oral cancer is low, the need for effective
and selective natural products is increasing. Licorice, the root
and rhizome of several Glycyrrhiza species
(Leguminosae), is an important natural sweetening agent and
is widely used as a herbal medicine (3). Licochalcone A (LCA) is a novel
flavonoid isolated from licorice root and is known to possess
several bioactivities such as antioxidant, antibacterial,
antiparasitic, anti-angiogenesis and antitumor effects (10–12).
It reduces significantly TNF-α-induced NF-κB activation,
consequently resulting in decreased inflammatory cytokines
production (13,14). Additionally, LCA not only inhibits
cancer cell proliferation, but also induces apoptosis in prostate
and gastric cancer cells (13,15,16).
Consequently, LCA may be useful as an alternative compound for
traditional anticancer agents.
In this study, therefore, we primarily examined the
OSCC cell’s response to LCA in order to determine the ability of
the LCA to act as a chemotherapy agent. We concluded that LCA
inhibits growth of OSCC cells (HN22 and HSC4) through induction of
apoptotic cell death via suppression of Sp1 and its accompanying
Sp1 regulatory proteins.
Materials and methods
LCA extraction and isolation
The roots of Glycyrrhiza inflata were
purchased from Chonnam Herb Association (Hwasun, Korea). A voucher
specimen (MNUYG-003) was deposited in the College of Pharmacy,
Mokpo National University, Muan, Korea. The air-dried and powdered
G. inflata roots (600 g) were extracted twice with MeOH (4
l) using sonicator for 3 h. After filtration, the MeOH extracts
were evaporated and suspended in distilled water and then defatted
with n-hexane (1 l). The aqueous layer was partitioned with
methylene chloride (3×1 l). The evaporation residues (5 g) were
subjected to flash silica gel chromatography eluting with
n-hexane:EtOAc:MeOH (2:1:0.1-1:1:0.1-100% MeOH) to afford 10
fractions. Fractions 2, 3 and 4 were further subjected to flash
silica gel chromatography, using a chloroform:MeOH (100:1) elution
solution to get LCA (50 mg). LCA was finally purified by column
chromatography using RP18 to an analytically acceptable purity.
Reagents and antibodies
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS), trypsin, penicillin and streptomycin (P/S) and
phospate-buffered saline (PBS) were purchased (Thermo Scientific,
Logan, UT, USA). Antibodies against Sp1, actin, caspase-3, p27, p21
and cyclin D1 were bought from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). Antibodies that can recognize myeloid cell
leukemia-1 (Mcl-1), survivin, Bid, Bax and Bcl-xl were
from Cell Signaling (Danvers, MA, USA). A specific antibody for
poly (ADP-ribose) polymerase (PARP) was obtained from BD Pharmingen
(San Diego, CA, USA). A 4′-6-diamidino-2-phenylindole (DAPI) was
obtained from Sigma-Aldrich, Inc. (St. Louis, MO, USA).
Cell culture
HN22 and HSC4 cells were the human oral squamous
cancer cell lines. HN22 cells and HSC4 cells were, respectively,
provided by Dankook University (Cheonan, Korea) and Hokkaido
University (Hokkaido, Japan). Both cells were cultured in DMEM
containing 10% heat-inactivated FBS and 100 U/ml each of P/S at
37°C with 5% CO2 humidity.
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay
Cell viability of HN22 and HSC4 was determined using
the CellTiter 96® AQueous One Solution Cell
Proliferation Assay kit (Promega, Madison, WI, USA) according to
the manufacturer’s instructions. The cells were seeded in 96-well
plates, grown for 24 h and treated with various concentrations of
LCA. After treatment with LCA for 24 and 48 h, MTS solution was
added to each well and the plates were incubated for 2 h at 37°C.
Changes in absorbance were measured at 490 nm using an Enspire
Multimode Plate reader (Perkin-Elmer, Akron, OH, USA).
DAPI staining
After treatment with LCA, the cells were harvested
by trypsinization. The cells were washed with cold PBS and fixed in
100% methanol at room temperature for 20 min. The cells were
deposited on poly-L-lysine-coated slides, stained with DAPI
solution (2 μg/ml) and observed through a FluoView confocal laser
microscope (Flouview FV10i, Olympus Corp., Tokyo, Japan).
Cell cycle
HN22 (5×105) and HSC4
(7.5×105) cells were seeded and treated with LCA (0, 10,
20 and 40 μM) for 48 h. The harvested cells were washed with 1 ml
PBS and 150 μl of Muse™ Cell cycle reagent (EMD Millipore Corp.
Billerica, MA, USA) was added. Then, cells were incubated at RT for
30 min in the dark. Samples were measured with Muse Cell cycle kit
(Merck Millipore, Billerica, MA, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
To analyze the effect of LCA on OSCC cell lines
(HN22 and HSC4), we performed RT-PCR using total RNAs and primers
designed for the specific gene. Total RNAs were harvested from OSCC
cells treated with or without LCA using the TRIzol®
reagent (Life Technologies, Carlsbad, CA, USA). With 2.5 μg of RNA,
RT-PCR was done using HelixCript™ 1st-strand cDNA synthesis kit
(NanoHelix, Korea) according to the kit instructions. We obtained
cDNA using actin-specific and Sp1-specific primers under following
PCR condition (30 cycles: 1 min at 95°C, 1 min at 56°C and 1 min at
72°C). The actin primers used were as follows; forward: 5′-GTG GGG
CGC CCC AGG CAC CA-3′; and reverse: 5′-CTC CTT AAT GTC ACG CAC GAT
TTC-3′; and the Sp1 primers were; forward: 5′-ATG CCT AAT ATT CAG
TAT CAA GTA-3′; and reverse: 5′-CCC TGA GGT GAC AGG CTG TGA-3′.
Actin was used as an internal control. The RT-PCR products were
visualized with ethidium bromide staining under UV light, after
electrophoresis on a 2% agarose gel.
Western blotting
The lysates of treated cells were prepared using
PRO-PREPTM Protein Extraction Solution (iNtRON
Biotechnology, Korea) and then supernatants were removed by
centrifugation. Proteins were separated by SDS-PAGE gel
electrophoresis and transferred onto a polyvinylidenedifluoride
(PVDF) membrane. After blocking with 5% skim milk in PBST, the
blots were incubated with primary antibody at 4°C overnight with
mild shaking and then followed by its corresponding secondary
antibody. The protein bands were visualized using ECL Plus Western
Blotting Detection system (Santa Cruz Biotechnology).
Annexin V staining
HN22 (5×105) and HSC4
(7.5×105) cells were seeded and allowed to grow for 24
h. At 48 h after treatment with various concentrations of LCA,
cells were harvested by trypsinization and spinning down for
analysis. The cells were analyzed by Muse Cell Analyzer with the
Muse Annexin V & Dead Cell kit (MCH100105, Merck Millipore).
The whole process of analysis was performed following the
instructions of the kit. The percentage of apoptotic and necrotic
cells was calculated from each triplicate sample by statistical
analysis of the dot plot using Muse 1.1.2 analysis software (Merck
Millipore).
Multi-caspase assay
The process was carried out as instructed in the
Muse Multi-Caspase kit (Merck Millipore). Each group, including
negative and positive controls was harvested to measure
quantitatively caspase activation and cell permeability. Cell
samples in 1X caspase buffer with 50 μl of Muse Multi-Caspase
reagent working solution were incubated at 37°C for 30 min. Then,
150 μl of 7-AAD working solution was added to each triplicate
sample and samples were analyzed by Muse Cell Analyzer.
Statistical analysis
Using Student’s t-test, the statistical significance
was assessed. P-value at <0.05, was considered as statistically
significant.
Results
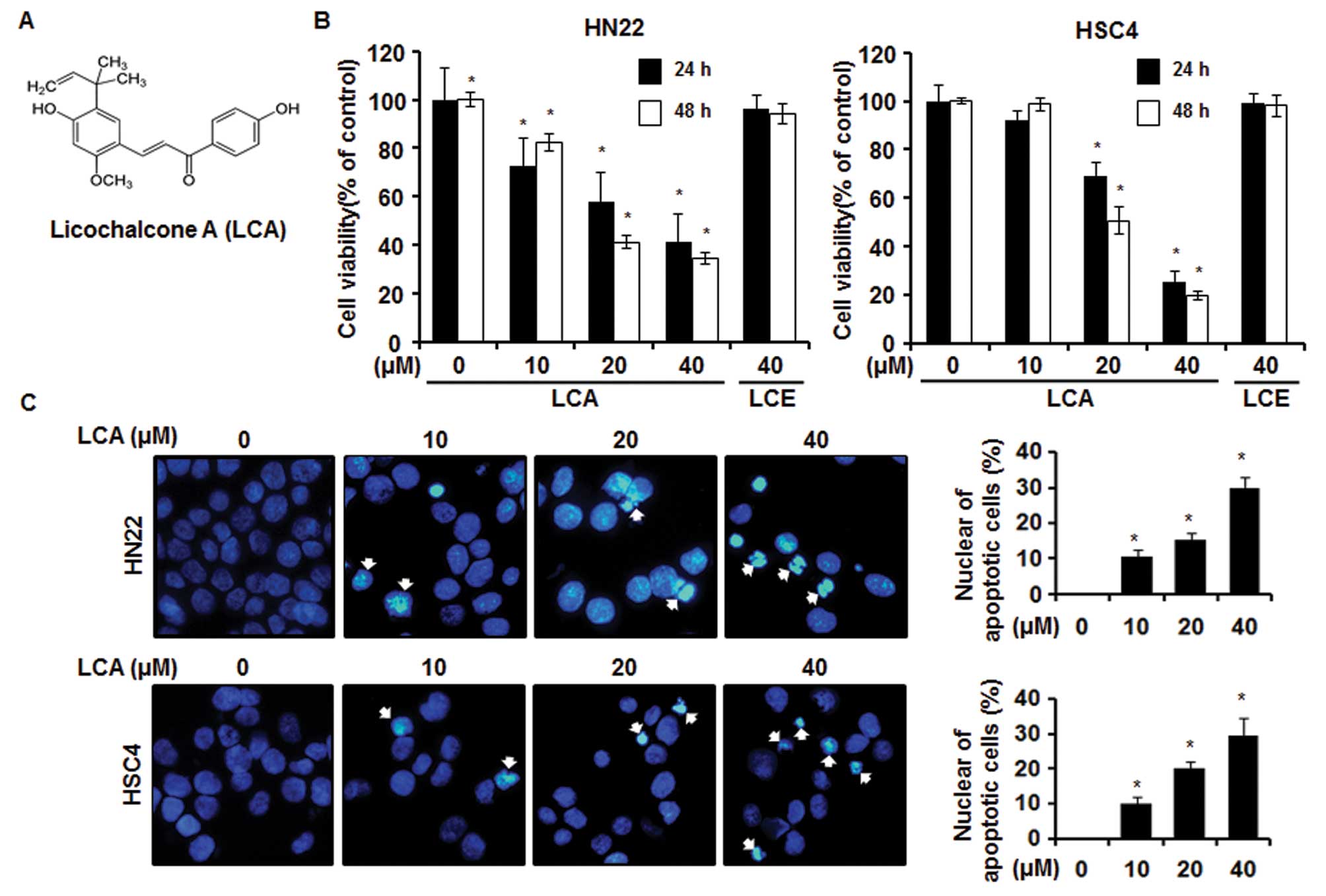
LCA inhibits cell viability of OSCC
We investigated inhibitory effects of LCA (Fig. 1A) and LCE on cell proliferation of
OSCC. LCA and LCE were isolated from the extracts of licorice
(Glycyrrhiza inflata) (17). Both have similar chemical
structures but exert significantly different bioactivities
(18). To examine the anticancer
effects of LCA and LCE in OSCCs, the HN22 and HSC4 cells were
treated with LCA or LCE at various concentrations for different
times (24 and 48 h). In this experiment, we used MTS assay to
quantify cell viability after treatment with natural products.
Fig. 1B showed that cell viability
of HN22 and HSC4 was decreased in a dose- and time-dependent manner
by LCA not LCE. The IC50 of LCA for cytotoxicity of HN22
and HSC4 post 48-h treatment was calculated as 17.87 and 20.42 μM,
respectively. Specifically, cell viabilities of HN22 cells were,
respectively, 82.4±3.4, 41.2±2.7 and 34.4±2.1% of the control group
at 10, 20 and 40 μM of LCA 48 h after treatment. HSC4 had a similar
dose-response relationship to HN22, representing 98.6±2.5, 50.7±5.4
and 19.6±1.7% viability at 10, 20 and 40 μM, respectively.
LCA induces apoptosis in OSCCs
Generally, the proliferation of cancer cells can be
suppressed by apoptosis or induction of cell cycle arrest, or both
(9,19). To investigate if LCA would induce
apoptosis of HN22 and HSC4 cells, DAPI staining, sub-G1
cell cycle analysis and Annexin V staining were conducted. As shown
in Fig. 1C, we found that LCA
induced apoptosis of OSCC cells in dose-dependent manner, as
determined by fragmentation and condensation of DNA. The cell cycle
distribution was analyzed after PI staining by Muse Cell Analyzer.
The treatment of cells with LCA at a dose of 40 μM caused
~43.67±2.66 or 39.8±1.5% induction of sub-G1 cell
population in HN22 (Fig. 1D, left)
and HSC4 (Fig. 1D, right),
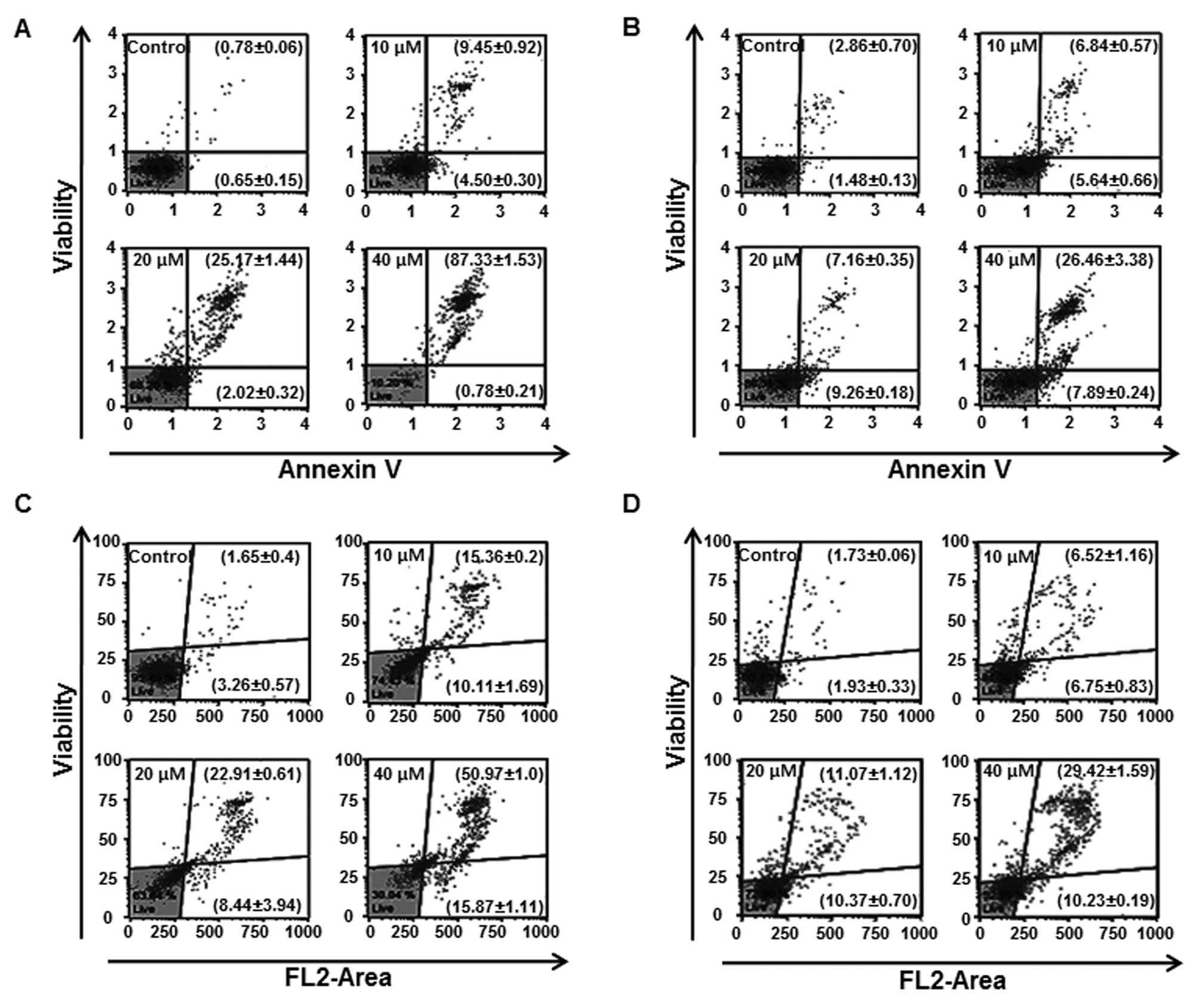
respectively. We quantified apoptotic cells by flow cytometric
analysis of cells with Annexin V/7-AAD double staining. Exposure of
HN22 cells to LCA for 48 h resulted in an increase in the
late-apoptotic cell population (Fig.
5A, upper right quadrant) from 9.45 to 87.33% with LCA 10 and
40 μM, respectively, compared with 0.78% of control cells. In HSC4
cells, the percentage of late-apoptotic cells increased from 2.86%
in control cells to 26.46% after 40 μM treatment with LCA,
respectively (Fig. 5B).
LCA suppresses Sp1 expression in
OSCC
Sp1 protein plays an important role in cell cycle
progression, oncogenesis and apoptotic cell death through
modulation of target gene promoters (20–22).
According to our previous report, Sp1 is an essential transcription
factor for OSCC tumorigenesis (23). Therefore, we reasoned that Sp1
protein might be a target for regulating growth of OSCC. To verify
the correlation of Sp1 expression to apoptosis, Sp1 expression
levels were monitored after cells were exposed to increasing doses
of LCA for given times. LCA significantly downregulated the Sp1
mRNA and protein expression levels in a dose-dependent manner in
the HN22 and HSC4 cells (Fig. 2A and
B). We also compared amounts of Sp1 and caspase-3 in HN22 and
HSC4 cells treated with 40 μM LCA for various times (0, 12, 24, 36
and 48 h). The levels of both Sp1 protein and caspase-3 were
gradually decreased with times by LCA. The Sp1 protein governs the
downstream targets including p27, p21, cyclin D1, Mcl-1 and
survivin, dysregulated expression of which causes apoptosis and
cell cycle arrest in various cancer cells (20,23).
Cell cycle arrest proteins such as p27 and p21 were elevated in
HN22 (Fig. 3A) and HSC4 (Fig. 3B) by LCA treatment while cell
proliferation and survival-related proteins like cyclin D1, Mcl-1
and survivin were diminished (Fig.
3B) in a dose-dependent manner by LCA.
LCA modulates the factors related to
apoptosis of OSCC cells
It has been reported in many previous studies that
the downregulation of Sp1 is associated with apoptosis induction
(24–26). To clarify the link between LCA and
Sp1-mediated apoptosis, we carried out western blot analysis of
apoptosis-regulating proteins with respective specific antibody in
LCA-treated OSCC cells (Fig. 4).
Consequently, as anticipated, a decrease in Bid and
Bcl-xl expression and an increase in Bax were observed
in cells treated with LCA. It is well established that fluctuation
in levels of those proteins is related to apoptotic cell death.
Finally, the cleavage of PARP was enhanced in a dose-dependent
manner by LCA treatment. As shown in Fig. 5C and D, OSCC cells exposed to LCA
showed increase of multi-caspase activity in a dose-dependent
manner. The multi-caspase acitivity of HN22 was (Fig. 5C), respectively, 10.11±1.69,
8.44±3.94 and 15.87±1.11% of early to mid apoptotic cells and
15.36±0.2, 22.91±0.61 and 50.97±1.0% of late apoptotic/dying cells
at 10, 20 and 40 μM of LCA compared with the untreated control
cells when multi-caspase activity was calculated at 48 h
post-treatment. In the case of HSC4 (Fig. 5D), multi-caspase activity was
6.75±0.83, 10.37±0.70 and 10.23±0.19% of early to mid apoptotic
cells and 6.52±1.16, 11.07±1.12 and 29.42±1.59% of late
apoptotic/dying cells at 10, 20 and 40 μM of LCA, respectively. We
conclude that downregulation of Sp1 by LCA leads to apoptotic cell
death.
Discussion
Licorice has been extensively studied because it is
an important natural sweetening agent and widely used as a herbal
medicine. Recent studies have reported that LCA and LCE,
retrochalcones derived from root of Glycyrrhiza inflata,
reduces inflammation, migration, angiogenesis, tumorigenesis, is
antidiabetic, inducing cell cycle arrest and apoptosis in various
cancer cell lines both in vitro and in vivo (10,16,27).
LCA has been reported to induce bladder cancer apoptosis via
modulation of mitochondria dysfunction and endoplasmic reticulum
stress (12). Additionally, LCA
suppresses the migration and invasion of hepatomacellular cell
carcinoma (HCC) by reducing MKK4/JNK via NF-κB-mediated urokinase
plasminogen activator (uPA) expression (10). LCE was found not only to induce
adipocyte differentiation during adipogenesis, but also to have
antidiabetic activity in diabetic mice (27).
Oral squamous cell carcinoma (OSCC) is an aggressive
epithelial malignancy and has a poor prognosis despite
comprehensive understanding of cancer development and advanced
therapy. There is accumulating evidence that LCA and LCE exert an
antitumor effect against a variety of cancers, but neither has been
investigated in OSCC to our knowledge. Therefore, we first sought
to check the biological effects of LCA and LCE on OSCC cell lines
(HN22 and HSC4). In this study, LCA effectively inhibited cell
growth and induced apoptosis so that if could be a suitable
candidate for inhibiting cell growth and inducing apoptosis in OSCC
cells, whereas LCE was not effective in these cell lines. Although
LCA and LCE share very similar structures, they exerted different
biological effects in OSCC cells with respect to cell growth and
death.
The transcription factor Sp1 is known to be
ubiquitously overexpressed in various human cancer cells and
closely associated with tumor activity by cellular processes
(28–30). Many studies have already revealed
the relationship between upregulated Sp1 and biological processes
such as proliferation, differentiation and oncogenesis (31,32).
Therefore, Sp1 has been suggested as a promising target for
molecular therapy against oral cancer. In this study, treatment of
OSCC cells (HN22 and HSC4) with LCA decreased significantly
expression of Sp1 protein in a dose- and time-dependent manner. To
further strengthen the effect of LCA on Sp1, we scrutinized
expression of Sp1 target proteins such as p21, p27, cyclin D1,
Mcl-1 and survivin (9,33,34).
The promoter of Sp1 target proteins contains frequent GC-rich
sequences and can be regulated by Sp1 protein (35–37).
Both p21 and p27, regulators of cell cycle progression (38,39),
were dose-dependently increased when treated with LCA. The
proto-oncogene cyclin D1 governs G1 to S phase
progression and is accordingly involved in the development and
progression of various cancers (40,41).
The member of the Bcl-2 family Mcl-1 regulates mitochondrial
physiology, energy production and anti-apoptotic function (35,42).
It also plays an important role in promoting carcinogenesis
(42,43). The pro-survival protein survivin is
an apoptosis inhibitor and a key regulator of mitosis, closely
associated with carcinogenesis (44). Consistent with its role, it was
demonstrated to be upregulated in many human cancer cells (45). Therefore, cyclin D1, survivin and
Mcl-1 in cancer cells have emerged as potential therapeutic
targets. Following LCA treatment, downregulation of both Sp1 and
Sp1 regulatory proteins (cyclin D1, survivin and Mcl-1) was
observed. Accordingly, it can be summarized that LCA activated
apoptotic signaling pathways in OSCC through regulation of Sp1 and
ensuing Sp1 target proteins. Apoptotic cell death is subclassified
by intrinsic mitochondria-mediated pathway and extrinsic death
receptor-induced pathway. In an intrinsic pathway, mitochondrial
events such as reciprocal expression of anti-apoptotic protein
Bcl-2, Bcl-xl and pro-apoptotic protein Bax are the
prerequisite for the activation of caspases. Consistent with this,
LCA substantially reduced Bid and Bcl-xl expression, but
elevated Bax expression. Subsequently, a cascade of molecular
apoptotic signaling transduction occurred for LCA-mediated
apoptosis.
In conclusion, our results indicate that LCA
mediates its anti-proliferative and apoptotic effects through
suppression of Sp1 and Sp1-mediated signaling pathways. Our study
strongly suggests that LCA is promising for treatment of OSCC that
overexpresses Sp1 through Sp1 regulation and that it is further
applicable as an anticancer drug and/or a conjunction agent.
Acknowledgements
This study was supported by Basic Science Research
Program through the National Research Foundation Korea (NRF) funded
by the Ministry of Education, Science and Technology
(2013R1A1A2A10057695).
Abbreviations:
|
OSCC
|
oral squamous cell carcinoma
|
|
LC
|
licochalcone
|
|
Sp1
|
specificity protein 1
|
|
DMEM
|
Dulbecco’s modified Eagle’s medium
|
|
FBS
|
fetal bovine serum
|
|
PBS
|
phosphate-buffered saline
|
|
Mcl-1
|
myeloid cell leukemia-1
|
|
PARP
|
poly(ADP-ribose) polymerase
|
|
P/S
|
penicillin and streptomycin
|
|
MTS
|
(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)
|
|
DAPI
|
4′-6-diamidino-2-phenylindole
|
|
PI
|
propidium iodide
|
|
LCE
|
licochalcone E
|
|
PI
|
propidium iodide
|
References
|
1
|
Oliveira LR, Castilho-Fernandes A,
Oliveira-Costa JP, et al:
CD44+/CD133+immunophenotype and matrix
metalloproteinase-9 influences on prognosis of early stage oral
squamous cell carcinoma patients. Head Neck. Nov 1–2013.(Epub ahead
of print). View Article : Google Scholar
|
|
2
|
Tanaka T, Tanaka M and Tanaka T: Oral
carcinogenesis and oral cancer chemoprevention: a review. Pathol
Res Int. 2011:4312462011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu HJ, Shin JA, Nam JS, et al: Apoptotic
effect of dibenzylideneacetone on oral cancer cells via modulation
of specificity protein 1 and Bax. Oral Dis. 19:767–774.
2012.PubMed/NCBI
|
|
4
|
Choi ES, Cho SD, Jeon JG, et al: The
apoptotic effect of the hexane extract of Rheum undulatum L.
in oral cancer cells through the down-regulation of specificity
protein 1 and survivin. Lab Anim Res. 27:19–24. 2011.PubMed/NCBI
|
|
5
|
Ariyawardana A and Ekanayake L: Screening
for oral cancer/pre-cancer: knowledge and opinions of dentists
employed in the public sector dental services of Sri Lanka. Asian
Pac J Cancer Prev. 9:615–618. 2008.PubMed/NCBI
|
|
6
|
Yeole BB, Ramanakumar AV and
Sankaranarayanan R: Survival from oral cancer in Mumbai (Bombay),
India. Cancer Causes Control. 14:945–952. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee HE, Choi ES, Shin JA, et al: Apoptotic
effect of methanol extract of Picrasma quassioides by regulating
specificity protein 1 in human cervical cancer cells. Cell Biochem
Funct. 32:229–235. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Firestone GL and Bjeldanes LF:
Indole-3-carbinol and 3-3′-diindolylmethane antiproliferative
signaling pathways control cell-cycle gene transcription in human
breast cancer cells by regulating promoter-Sp1 transcription factor
interactions. J Nutr. 133:S2448–S2455. 2003.
|
|
9
|
Jeon YJ, Ko SM, Cho JH, et al: The HDAC
inhibitor, panobinostat, induces apoptosis by suppressing the
expresssion of specificity protein 1 in oral squamous cell
carcinoma. Int J Mol Med. 32:860–866. 2013.PubMed/NCBI
|
|
10
|
Tsai JP, Hsiao PC, Yang SF, et al:
Licochalcone A suppresses migration and invasion of human
hepatocellular carcinoma cells through downregulation of MKK4/JNK
via NF-κB mediated urokinase plasminogen activator expression. PloS
One. 9:e865372014.PubMed/NCBI
|
|
11
|
Hao H, Hui W, Liu P, et al: Effect of
licochalcone A on growth and properties of Streptococcus
suis. PloS One. 8:e677282013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan X, Li D, Zhao H, et al: Licochalcone
A-induced human bladder cancer T24 cell apoptosis triggered by
mitochondria dysfunction and endoplasmic reticulum stress. Biomed
Res Int. 2013:4742722013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee CS, Kwak SW, Kim YJ, et al: Guanylate
cyclase activator YC-1 potentiates apoptotic effect of licochalcone
A on human epithelial ovarian carcinoma cells via activation of
death receptor and mitochondrial pathways. Eur J Pharmacol.
683:54–62. 2012. View Article : Google Scholar
|
|
14
|
Funakoshi-Tago M, Tanabe S, Tago K, et al:
Licochalcone A potently inhibits tumor necrosis factor
alpha-induced nuclear factor-kappaB activation through the direct
inhibition of IkappaB kinase complex activation. Mol Pharmacol.
76:745–753. 2009. View Article : Google Scholar
|
|
15
|
Fu Y, Hsieh TC, Guo J, et al:
Licochalcone-A, a novel flavonoid isolated from licorice root
(Glycyrrhiza glabra), causes G2 and late-G1 arrests in
androgen-independent PC-3 prostate cancer cells. Biochem Biophys
Res Commun. 322:263–270. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao XY, Hao M, Yang XY, et al:
Licochalcone A inhibits growth of gastric cancer cells by arresting
cell cycle progression and inducing apoptosis. Cancer Lett.
302:69–75. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoon G, Jung YD and Cheon SH: Cytotoxic
allyl retrochalcone from the roots of Glycyrrhiza inflata.
Chem Pharm Bull. 53:694–695. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu Y, Chen J, Li YJ, et al: Antioxidant
and anti-inflammatory activities of six flavonoids separated from
licorice. Food Chem. 141:1063–1071. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang SF, Chang CA, Lee DY, et al: Tumor
cell cycle arrest induced by shear stress: Roles of integrins and
Smad. Proc Natl Acad Sci USA. 105:3927–3932. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee KA, Lee SH, Lee YJ, et al: Hesperidin
induces apoptosis by inhibiting Sp1 and its regulatory protein in
MSTO-211H cells. Biomol Ther. 20:273–279. 2012. View Article : Google Scholar
|
|
21
|
Li L and Davie JR: The role of Sp1 and Sp3
in normal and cancer cell biology. Ann Anat. 192:275–283. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Willoughby JA Sr, Sundar SN, Cheung M, et
al: Artemisinin blocks prostate cancer growth and cell cycle
progression by disrupting Sp1 interactions with the
cyclin-dependent kinase-4 (CDK4) promoter and inhibiting CDK4 gene
expression. J Biol Chem. 284:2203–2213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim DW, Ko SM, Jeon YJ, et al:
Anti-proliferative effect of honokiol in oral squamous cancer
through the regulation of specificity protein 1. Int J Oncol.
43:1103–1110. 2013.PubMed/NCBI
|
|
24
|
Deniaud E, Baguet J, Mathieu AL, et al:
Overexpression of Sp1 transcription factor induces apoptosis.
Oncogene. 25:7096–7105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jutooru I, Chadalapaka G, Sreevalsan S, et
al: Arsenic trioxide downregulates specificity protein (Sp)
transcription factors and inhibits bladder cancer cell and tumor
growth. Exp Cell Res. 316:2174–2188. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shin JA, Kim JJ, Choi ES, et al: In vitro
apoptotic effects of methanol extracts of Dianthus chinensis
and Acalypha australis L. targeting
specificity protein 1 in human oral cancer cells. Head Neck.
35:992–998. 2012.PubMed/NCBI
|
|
27
|
Park HG, Bak EJ, Woo GH, et al:
Licochalcone E has an antidiabetic effect. J Nutr Biochem.
23:759–767. 2012. View Article : Google Scholar
|
|
28
|
Chiefari E, Brunetti A, Arturi F, et al:
Increased expression of AP2 and Sp1 transcription factors in human
thyroid tumors: a role in NIS expression regulation? BMC Cancer.
2:352002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hosoi Y, Watanabe T, Nakagawa K, et al:
Upregulation of DNA-dependent protein kinase activity and Sp1 in
colorectal cancer. Int J Oncol. 25:461–468. 2004.PubMed/NCBI
|
|
30
|
Yao JC, Wang L, Wei D, et al: Association
between expression of transcription factor Sp1 and increased
vascular endothelial growth factor expression, advanced stage, and
poor survival in patients with resected gastric cancer. Clin Cancer
Res. 10:4109–4117. 2004. View Article : Google Scholar
|
|
31
|
Chu S and Ferro TJ: Sp1: regulation of
gene expression by phosphorylation. Gene. 348:1–11. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deniaud E, Baguet J, Chalard R, et al:
Overexpression of transcription factor Sp1 leads to gene expression
perturbations and cell cycle inhibition. PLoS One. 4:e70352009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Blume SW, Snyder RC, Ray R, et al:
Mithramycin inhibits SP1 binding and selectively inhibits
transcriptional activity of the dihydrofolate reductase gene in
vitro and in vivo. J Clin Invest. 88:1613–1621. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pietrzak M and Puzianowska-Kuznicka M:
p53-dependent repression of the human MCL-1 gene encoding an
anti-apoptotic member of the BCL-2 family: the role of Sp1 and of
basic transcription factor binding sites in the MCL-1 promoter.
Biol Chem. 389:383–393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choi KH, Shim JH, Huong LD, et al:
Inhibition of myeloid cell leukemia-1 by tolfenamic acid induces
apoptosis in mucoepidermoid carcinoma. Oral Dis. 17:469–475. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li F and Altieri DC: Transcriptional
analysis of human survivin gene expression. Biochem J. 344(Pt 2):
305–311. 1999. View Article : Google Scholar
|
|
37
|
Li Y, Xie M, Yang J, et al: The expression
of antiapoptotic protein survivin is transcriptionally upregulated
by DEC1 primarily through multiple sp1 binding sites in the
proximal promoter. Oncogene. 25:3296–3306. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sherr CJ and Roberts JM: CDK inhibitors:
positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Murray AW: Recycling the cell cycle:
cyclins revisited. Cell. 116:221–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Weinstein IB: Relevance of cyclin D1 and
other molecular markers to cancer chemoprevention. J Cell Biochem
(Suppl). 25:23–28. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Alao JP: The regulation of cyclin D1
degradation: roles in cancer development and the potential for
therapeutic invention. Mol Cancer. 6:242007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Akgul C: Mcl-1 is a potential therapeutic
target in multiple types of cancer. Cell Mol Life Sci.
66:1326–1336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Andersson Y, Juell S and Fodstad O:
Downregulation of the antiapoptotic MCL-1 protein and apoptosis in
MA-11 breast cancer cells induced by an anti-epidermal growth
factor receptor - Pseudomonas exotoxin a immunotoxin. Int J Cancer.
112:475–483. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chun JY, Hu Y, Pinder E, et al: Selenium
inhibition of survivin expression by preventing Sp1 binding to its
promoter. Mol Cancer Ther. 6:2572–2580. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sun Y, Giacalone NJ and Lu B: Terameprocol
(tetra-O-methyl nordihydroguaiaretic acid), an inhibitor of
Sp1-mediated survivin transcription, induces radiosensitization in
non-small cell lung carcinoma. J Thorac Oncol. 6:8–14. 2011.
View Article : Google Scholar : PubMed/NCBI
|