Introduction
Hepatocellular carcinoma (HCC) is one of the most
common types of malignant tumors worldwide, with high prevalence in
Asia and South Africa (1).
Surgical resection is the most common form of therapy (2), but HCC prognosis remains poor due to
tumor invasiveness, frequent intrahepatic spread and extrahepatic
metastasis (3).
Tumor metastasis is a complex process, and various
factors are involved in each step of the process (4). Recent studies suggest that numerous
genes and proteins play vital roles in the metastasis of cancer
cells. Epithelial-mesenchymal transition (EMT) is the conversion of
epithelial cells to mesenchymal cells; it is critical in the
development of human cancer invasiveness and metastatic potential
(5). During the transition
process, intracellular adhesion between epithelial cells decreases
and they acquire fibroblastoid properties, including downregulation
of E-Cadherin, an epithelial marker, and upregulation of both
N-Cadherin, a mesenchymal marker and Snail, which regulates EMT
(6). EMT assists cancer cells in
entering surrounding tissues by conferring invasive properties and
allowing them to establish a suitable microenvironment for
progression and metastasis (7).
Iwatsuki et al (8) report
that tumor buds, which involve a single cancer cell or small
cluster of cells at the invasive front of tumor tissue, probably
undergo EMT due to down-expression of E-Cadherin.
It has become clear that inflammatory cells present
in the tumor microenvironment play an indispensable role in cancer
progression (9). Tumor-associated
macrophages, characterized as immunosuppressive, pro-angiogenic,
and tumor growth-promoting, are a major component of the
inflammatory cell infiltrate of tumors (10). In a previous study, we found that
high levels of macrophage density, especially at the tumor edge,
and peritumoral macrophage colony-stimulating factor (M-CSF)
predicted high risk of recurrence and metastasis in HCC patients
who had received curative liver resections (11). Jensen et al (12) also found that dense infiltration of
CD68+ macrophages at the tumor border were associated
with poor survival in patients with melanoma. Chen et al
(13)reported that macrophages can
enhance the invasiveness and matrix-degrading activity of small
cell lung cancer cell lines in vitro. Nevertheless, whether
and how tumor-associated macrophages function in HCC progression
and the relationship between tumor-infiltrating macrophages and EMT
in HCC cells remain poorly understood.
By culturing HCC cell lines with macrophages, we
were able to investigate the effect of the latter upon: i) HCC cell
migration and invasion in vitro and ii) EMT in HCC cells. In
addition, antibody microarrays were used to identify the cytokines
mediating the association between paratumoral macrophages and EMT.
To observe the relationship between macrophages and EMT in
vivo, double-staining immunohistochemistry was used to detect
macrophage density and EMT biomarker expression in specimens
obtained from HCC patients.
Materials and methods
Cell preparations
MHCC97L and MHCC97H are hepatitis B virus
(HBV)-positive HCC cell lines with the same genetic background but
different lung metastatic potential. Both were established at our
institute. The human monocyte leukemia cell line THP-1 and two
additional HCC cell lines, HepG2 and Huh7, which are HBV-negative
cell lines with low metastatic potential, were purchased from the
Institute of Biochemistry and Cell Biology, Chinese Academy of
Sciences, Shanghai, China. MHCC97L, MHCC97H, HepG2 and Huh7 were
cultured in Dulbecco’s modified Eagle’s medium (Invitrogen,
Carlsbad, CA, USA) and THP-1 cells were cultured in RPMI-1640
medium (Invitrogen). Media were supplemented with 10%
heat-inactivated fetal bovine serum (FBS) and 1%
penicillin/streptomycin solution (Invitrogen). We cultured the
cells at 37°C in a humidified atmosphere of 5% CO2.
To obtain PMA-treated macrophages, we seeded
1×106 THP-1 cells into the upper insert of a 6-well
Transwell apparatus (0.4 mM pore size; Corning, Lowell, MA, USA)
and treated them with PMA (320 nM) for 24 h.
Patients and specimens
Clinical samples were collected after obtaining
informed consent, according to an established protocol approved by
the Ethics Committee of Fudan University. We collected information
on HCC patients who received curative resections between January
2008 and December 2009 at the Liver Cancer Institute, Zhongshan
Hospital, Fudan University, Shanghai, China. All data collected was
de-identified. All patients included in the study underwent
complete macroscopic removal of their tumors and did not have
distant metastases or any prior anticancer treatment. The
pathologic features of all cases were reviewed by an experienced
pathologist blinded to the original pathology reports. Patients
were divided into two groups, according to whether HCC had recurred
within two years. By using random numbers generated via SPSS
software version 16 (SPSS, Inc., Chicago, IL, USA), 20 patients
from each group were selected for double staining
immunohistochemistry. In total, 40 cases were picked, and 40
paraffin-embedded samples were obtained, including tumoral and
paratumoral tissue, especially from the tumor edge.
Establishment of co-culture system with
macrophages and HCC cells
After a thorough wash, PMA-treated THP-1 macrophages
(upper inserts) were co-cultured with HCC cells (in a 6-well plate,
2×105 cells/well) without direct contact. After 24 h of
co-culture, the upper inserts containing the macrophages were
discarded, and HCC cells were washed and used for subsequent
experiments.
Cell migration and Matrigel invasion
assays: co-cultured HCC cells
A wound-healing assay was used to evaluate the
ability of HCC cells to migrate following culture with PMA-treated
macrophages. Cells were grown to 80–90% confluence in 24-well
plates, and a wound was made by dragging a plastic pipette tip
across the cell surface. The remaining cells were washed three
times to remove cellular debris and incubated at 37°C with
serum-free medium. Migrating cells at the wound front were
photographed after 24 h. All experiments were performed in
triplicate.
Cell invasion assays were performed using 24-well
Trans-wells (8 μm pore size; Corning) pre-coated with Matrigel
(Falcon 354480; BD Biosciences, Franklin Lakes, NJ, USA). In total,
1×105 cells were suspended in 500 μl DMEM containing 1%
FBS and added to the upper chamber, while 750 μl DMEM containing
10% FBS was placed in the lower chamber. After 48 h of incubation,
Matrigel and the cells remaining in the upper chamber were removed
using cotton swabs. Cells on the lower surface of the membrane were
fixed in 4% paraformaldehyde and stained with Giemsa. Cells in 5
microscopic fields (at ×200 magnification) were counted and
photographed. All experiments were performed in triplicate.
Double-staining immunohistochemistry:
CD68, Snail and N-Cadherin in HCC clinical specimens
The double immunohistochemical staining system is
designed for the simultaneous detection of two antigens on one
slide. Five-μm sections of paraffin-embedded clinical HCC samples
were cut using a rotation microtome (Leica RM2125RT). The sections
were deparaffinized in xylene (2×5 min) and rehydrated in graded
alcohols (100–70%, 5 min each) and distilled water. After antigen
retrieval with 0.01% EDTA pH 8.0 (boiled 10 min in a microwave),
slides were processed with the Dako EnVision™ G|2 Double-stain
System (K5361; Dako, Glostrup, Denmark). The primary antibodies
were mouse anti-human CD68 (ready to use; IS61330; Dako), rabbit
anti-human Snail (1:200; BS1853; Bioworld, Visalia, CA, USA), and
rabbit antihuman N-Cadherin (1:1,000; ab18203; Abcam, Burlingame,
CA, USA). The CD68 antibody was applied along with Snail and
N-Cadherin antibodies, respectively, to each specimen. Negative
controls were treated identically except for the omission of the
primary antibodies. Finally, the slides were counterstained with
hematoxylin for 2 min then mounted with water-based mounting
medium. Cells that stained positive for Snail and N-Cadherin were
visualized using DAB+ Chromogen, and CD68 was visualized
using permanent red chromogen. Macrophages were identified by red
stain, while HCC cells undergoing EMT were identified by brown
stain. In each section, staining was captured by Leica QWin Plus
version 3 software (Leica Microsystems, Wetzlar, Germany).
Western blotting: EMT markers and the
JAK2/STAT3 pathway in HCC co-cultures
Protein (30 μg) from the total cell extract of cell
cultures was used to perform sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE), then proteins were transferred onto
polyvinylidene difluoride membranes and incubated with the
corresponding antibodies. The membranes were developed using the
enhanced chemiluminescence method (Pierce, Rockford, IL, USA).
Rabbit anti-human Snail polyclonal antibody (1:1,000; BS1853;
Bioworld), rabbit antihuman E-Cadherin polyclonal antibody
(1:10,000; ab40772; Abcam), and rabbit anti-human N-Cadherin
polyclonal antibody (1:1,000; ab18203; Abcam) were used to detect
the expression of Snail, E-Cadherin and N-Cadherin, respectively.
Rabbit anti-human JAK2/p-JAK2 monoclonal antibody (1:1,000; 3230;
Cell Signaling Technology, Danvers, MA, USA/1:1,000; 4406; Cell
Signaling Technology) and rabbit anti-human STAT3/p-STAT3
monoclonal antibody (1:1,000; 4904; Cell Signaling
Technology/1:2,000; 9145; Cell Signaling Technology) were selected
to detect the JAK2/STAT3 pathway. A monoclonal β-tubulin antibody
(1:1,000; Beyotime Institute of Biotechnology, Haimen, China) was
used as an internal control. Horseradish peroxidase-conjugated
anti-mouse and anti-rabbit antibody (1:5,000; KC-RB-035; KangCheng
Biotechnology, Shanghai, China) were used as secondary antibodies.
The intensity of protein bands was determined by densitometry using
the Bio-Rad system (Bio-Rad Laboratories, Hercules, CA, USA). All
experiments were performed in triplicate.
Immunofluorescence analysis: EMT markers
in HCC co-cultures
Immunofluorescence analysis of tumor cells cultured
on glass coverslips was performed as previously described (14). Cells were cultured for 72 h in the
appropriate medium containing antibodies specific for E-Cadherin
(1:500; ab40772; Abcam), N-Cadherin (1:50; ab18203; Abcam) and
Snail (1:100; BS1853; Bioworld).
Antibody chip assay and ELISA
verification of cytokines produced in HCC co-cultures
Samples of serum-free culture media were obtained
from the supernatant of co-cultured HCC cells (MHCC-97H and Hep-G2)
and macrophages. The levels of 80 human cytokines were determined
using the human cytokine antibody array G-Series 5 (RayBiotech,
Inc., Norcross, GA, USA). Briefly, a panel of antibodies is
immobilized in specific locations on the surface of a glass slide.
Incubating samples with the array results in the capture of
cytokines by the corresponding antibodies. Bound cytokines are then
detected with a cocktail of biotinylated antibodies. Signals are
visualized using streptavidin-HRP conjugate and colorimetry.
Results are expressed in relative units of spot color density,
which reflect the concentration of each chemokine. Color images of
the array were analyzed using the Image Lab 3.0.
Levels of MIP-3α, TNF-α, RANTES, MCP-1, IL-6, IL-8,
IL-1β and GRO-α were measured via ELISA in serum-free supernatant
from HCC cell cultures, following the manufacturer’s instructions
(R&D Systems, Inc., Minneapolis, MN, USA).
Statistical analysis
Statistical analysis was performed using SPSS 16.0
software. Values are expressed as means ± standard deviations. The
normality of the data was assessed by the Kolmogorov-Smirnov test.
For normally distributed variables, the experimental and control
groups were compared using the Student’s t-test. The cut-off for
statistical significance was P≤0.05.
Results
Macrophages enhance HCC cell migration
and invasion in vitro
Phorbol myristyl acetate (PMA)-treated THP-1
macrophages were co-cultured with HCC cells in a non-contact
Transwell system. Matrigel invasion assays showed that the number
of invading HCC cells increased significantly after being
co-cultured with macrophages (Fig.
1C) (MHCC-97H, 44.7±6.5 vs. 234.7±13.3; Hep-G2, 25.0±6.0 vs.
191.7±17.6, P<0.05; Fig. 1D).
In addition, a wound-healing assay, used to measure migration,
demonstrated accelerated wound closure in HCC cells cultured with
macrophages (Fig. 1A and B).
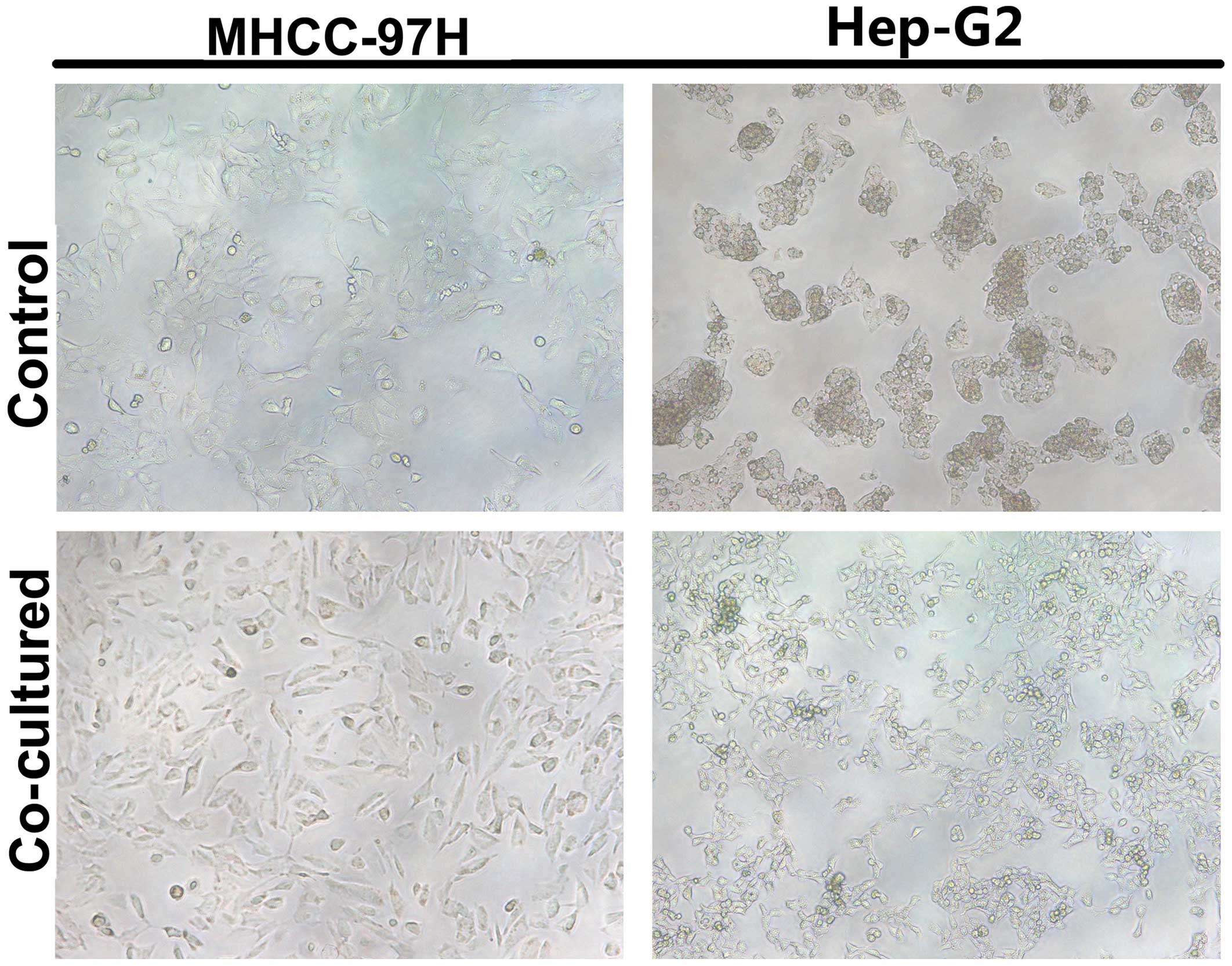
Culturing HCC cells with macrophages is
associated with EMT
Control HCC cells formed clusters in culture and had
tight cellular junctions. By contrast, after being cultured with
macrophages, HCC cells were scattered and spindle shaped, similar
to fibroblast cells, especially the Hep-G2 cell line; originally
growing in a mass, Hep-G2 began to grow in a solitary fashion after
macrophage co-culture. In the co-cultured HCC cells elongated
parapodium could be observed (Fig.
2). To clarify whether EMT had taken place, we assessed the
expression of Snail, E-Cadherin, and N-Cadherin, three key markers
of the EMT process, in HCC/macrophage co-cultures using western
blots. The results showed that Snail and N-Cadherin expression was
significantly enhanced in HCC cells co-cultured with macrophages,
while E-Cadherin expression decreased sharply (Fig. 3).
EMT in clinical HCC specimens
Double-staining immunohistochemistry analysis of HCC
clinical samples showed that macrophages which stained CD68
positive were mainly observed around the edge of the tumor nest
(Fig. 4). Notably, in the area
where macrophages were concentrated, Snail and N-Cadherin
expression was high.
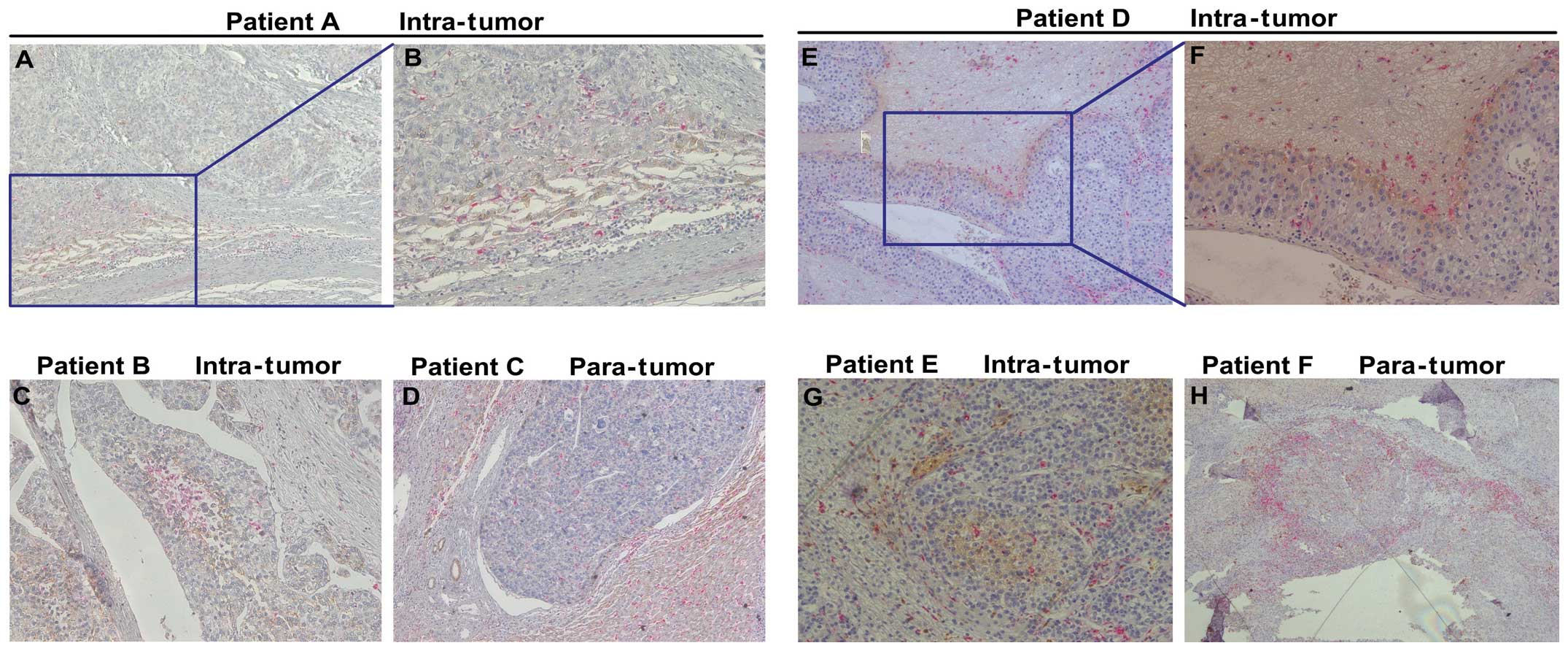 | Figure 4EMT in clinical HCC specimens. The
expression of CD68, Snail and N-Cadhrin was detected by
double-staining immunohistochemistry analysis. (A–D) CD68 (red) and
N-Cadherin (brown) were stained simultaneously on one slide.
CD68-positive macrophages mainly infiltrated the stroma of the
tumor, and N-Cadherin-positive tumor cells were surrounded by
macrophages. (E–H) CD68 (red) and Snail (brown) were stained
simultaneously on one slide. Macrophages were mainly observed
around the edge of the tumor nest and in the area where macrophages
were concentrated. The expression of Snail was also stronger in
such areas. (A, C, D and E, original magnification, ×100; B, F and
G, original magnification, ×200; H, original magnification,
×40). |
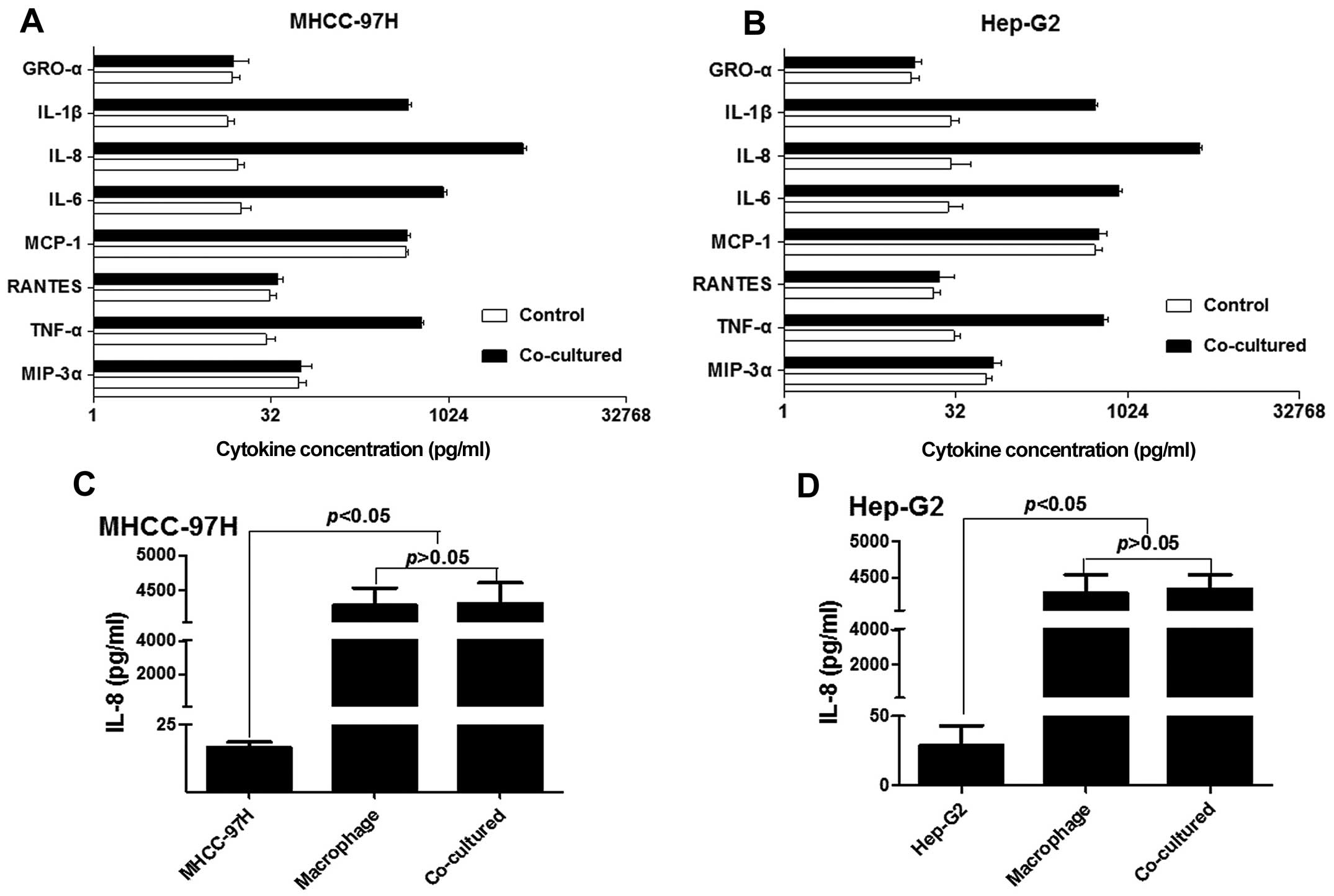
Macrophage-derived IL-8 induces EMT in
cultured HCC cells
We used antibody arrays to investigate which
macrophage- secreted cytokines promoted HCC cell invasion in our
co-culture model. Serum-free culture media was obtained from
cell-normalized cultures; as shown in Fig. 5, levels of macrophage inflammatory
protein-3α (MIP-3α), tumor necrosis factor-α (TNF-α), RANTES,
monocyte chemo-attractant protein (MCP-1), IL-6, IL-8, IL-1β and
growth related oncogene-α (GRO-α) were significantly elevated in
both co-cultured MHCC-97H and Hep-G2 cells.
In order to verify the results obtained from the
array, ELISA was used to assess levels of the cytokines listed
above in the supernatant of HCC/macrophage co-cultures and HCC
control cultures. No significant differences between MIP-3α,
RANTES, MCP-1 and GRO-α levels were found between the co-cultured
and control groups, but the levels of TNF-α, IL-6, IL-8 and IL-1β
increased significantly in the media from co-cultured HCC cells
(Table I; Fig. 6A and B). As shown in Fig. 6C and D, we observed a nearly
100-fold increase in levels of secreted IL-8 in the supernatant of
HCC cells cultured with macrophages relative to control HCC
cells.
 | Table IConcentration of cytokines in the
cell culture medium. |
Table I
Concentration of cytokines in the
cell culture medium.
| MHCC-97H | Hep-G2 |
|---|
|
|
|
|---|
| Control | Co-cultured | Control | Co-cultured |
|---|
| MIP-3α | 54.65±8.87 | 57.42±12.67 | 58.99±6.71 | 68.41±11.48 |
| TNF-α | 28.95±6.03 | 603.89±15.18 | 30.86±4.18 | 625.32±56.43 |
| RANTES | 31.21±4.14 | 36.21±3.96 | 20.34±3.14 | 22.89±8.48 |
| MCP-1 | 442.38±20.95 | 451.20±34.66 | 527.00±77.71 | 571.55±92.29 |
| IL-6 | 17.76±3.55 | 911.68±67.24 | 27.27±9.27 | 848.32±57.29 |
| IL-8 | 16.74±2.09 | 4317.74±291.08 | 29.01±14.13 | 4347.40±192.09 |
| IL-1β | 13.57±2.05 | 461.42±26.73 | 28.69±5.45 | 525.36±34.553 |
| GRO-α | 15.03±2.48 | 15.24±5.57 | 12.95±2.38 | 14.01±2.04 |
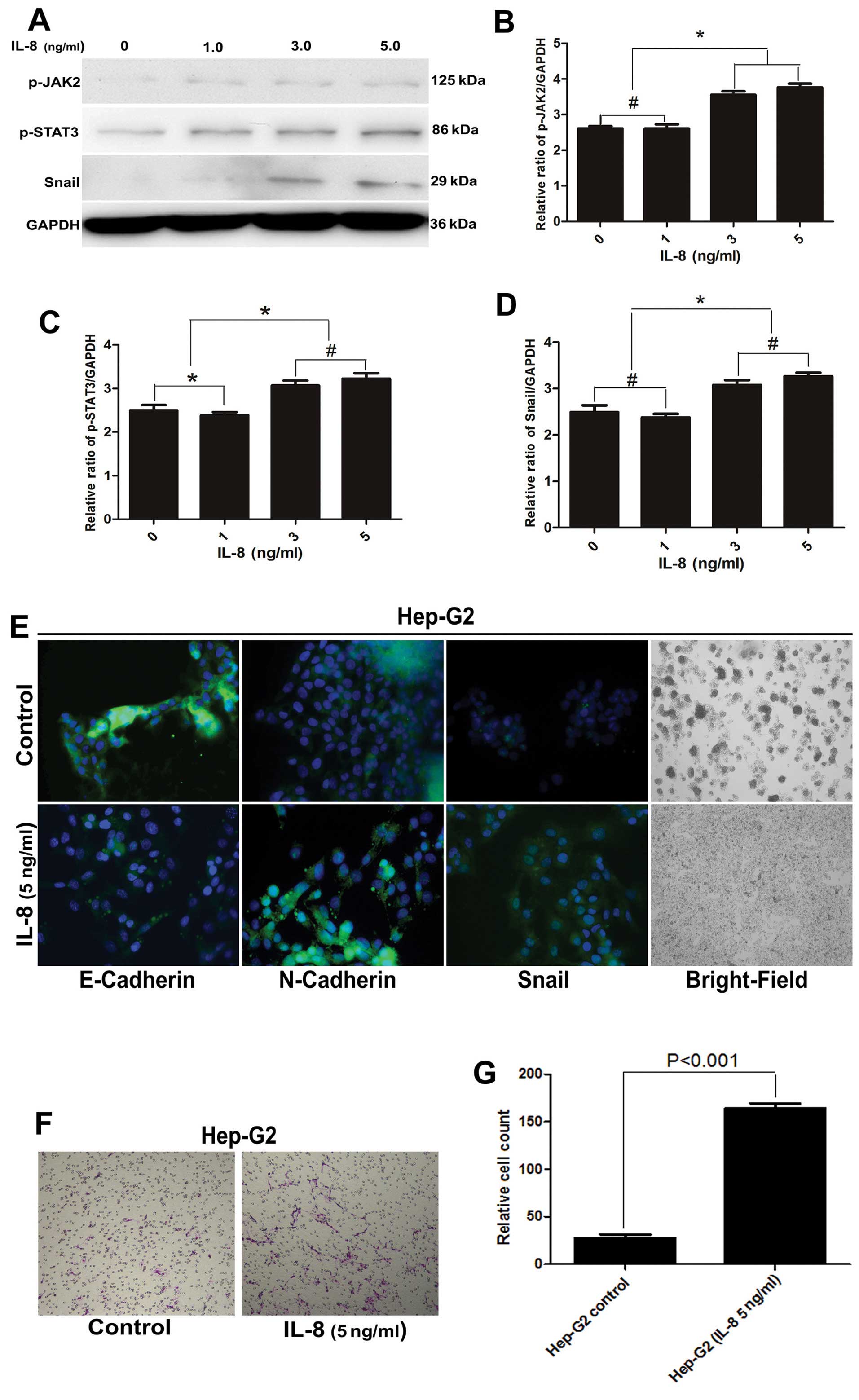
IL-8 induces a mesenchymal-like phenotype
in HCC cells via dose-related activation of the JAK2/STAT3
pathway
To investigate whether IL-8 and JAK2/STAT3
activation plays a critical role in inducing EMT, we cultured
Hep-G2 cells with macrophages in the presence of varying levels of
IL-8 (0, 1, 3 and 5 ng/ml) for 24 h, subsequently testing their
invasive potential in vitro. JAK2/STAT3 activation was
indicated by an increase in phosphor-specific forms of JAK2/STAT3
relative to total forms of JAK2/STAT3. We found the activity of
both JAK2 and STAT3 increased with increasing IL-8 concentrations
(Fig. 7A–D).
In addition, we observed that the ability of Hep-G2
cells to invade the extracellular matrix in vitro increased
in the presence of IL-8 (Fig. 7F and
G). The shape of the cells became fusiform and the conjunctions
between them loose. Immunofluorescence analysis also revealed that
levels of the epithelial marker, E-Cadherin, decreased, while
levels of the mesenchymal markers, N-Cadherin and Snail, increased
(Fig. 7E).
Discussion
Multiple studies on various types of human
carcinomas have demonstrated a positive association between the
activation of an EMT program and poor prognosis or advanced disease
(15–18). This suggests that EMT plays a
critical role in cancer progression. The tumor microenvironment is
composed of an extracellular matrix, fibroblasts, myofibroblasts,
endothelial cells, immune cells and soluble factors. A recent study
revealed that stromal cells in solid tumors represent a dynamic,
flexible asset for tumor progression (19), and it is recognized that the
balance between tumor immunity and tumor progression is important
(20). Macrophages, an important
component of the tumor microenvironment, was observed mainly to
infiltrate the stromal area and it is reported that they can
promote tumor progression and are associated with poor prognosis
(21–25). By using double-staining
immunohistochemistry to investigate clinical samples, we found that
the number of HCC cells that switch from an epithelial to a
mesenchymal-like phenotype may constitute only a small fraction of
the total primary tumor mass; mesenchymal-like cells were
especially common at the edge of tumor nests, while macrophages
were primarily observed in the border areas of the tumor nest and
stroma. This phenomenon indicates that macrophages that have
infiltrated the tumor stroma may play a key role in the EMT process
in HCC. The double-staining immunohistochemistry was applied only
to observe the location of macrophages and tumor cells which were
undergoing EMT in HCC tissues, and no statistical analyses were
performed.
As mentioned above, macrophages were part of tumor’s
adjacent stroma, and their correlations with patient survival have
largely been related to the macrophage secretome which involves
factors that stimulate tumor cell proliferation and survival,
angiogenesis and release of proteases essential for extracellular
matrix remodeling (26–28). In the present study, we
hypothesized that macrophages that infiltrate the stroma could
influence tumor cells in the epithelial state by secretion of
certain cytokines. In order to determine whether the associations
are causal, we used a non-contact system described previously for
co-culture macrophages and HCC cells and THP-1 macrophages were
used as macrophage model because PMA-treated THP-1 macrophages had
an M2 functional profile (13,29).
We demonstrated that culturing two HCC cell lines with macrophages
led to EMT, upexpression of two mesenchymal markers, N-Cadherin and
Snail, and induction of invasiveness. Therefore, it is possible
that soluble factors secreted by macrophages could induce adjacent
epithelial tumor cells to undergo EMT and acquire metastatic
potential.
Although our antibody array results indicated that
the transition of HCC cells from an epithelial to a
mesenchymal-like phenotype was associated with the secretion of
multiple cytokines and chemokines, including MIP-3α, TNF-α, RANTES,
MCP-1, IL-6, IL-8, IL-1β and GRO-α, when ELISA was used to verify
these differences, only levels of TNF-α, IL-6, IL-8 and IL-1β were
found to increase. IL-1β and TNF-α are reported to regulate IL-8
expression in fibroblasts (30),
endothelial cells (31), gastric
carcinoma (32) and prostate
cancer (33). Soria et al
(34) found that coordinated
expression of TNF-α and IL-1β may promote breast cancer recurrence.
Moreover, Akiba et al (35)
discovered that cancerous tissue samples with high IL-8 levels have
a significantly higher frequency of portal vein invasion. Various
studies have also shown that IL-6 is important in the pathogenesis
of HCC, and it has been known for some time that, in humans, IL-6
levels are increased in serum from patients with chronic liver
disease, including cirrhosis and HCC (36,37).
Recent studies have indicated that IL-6 is capable of inducing EMT
in human breast cancer cells (38), so we cannot reject the possibility
that IL-6 contributes to EMT in HCC.
IL-8 expression in cancer has been associated with
tumor growth and survival, increased tumor cell migration and
invasion, and increased neovascularization (39–41).
Therefore, IL-8 was the major focus of the present study. In this
study, IL-8 levels increased in cultures containing both HCC cells
and macrophages, and this increase was much greater than that seen
for IL-6. We hypothesize that the large increase in IL-8 levels was
due to synergy between TNF-α and IL-1β. Our ELISA results showed
that IL-8 concentrations were mainly elevated in the supernatant of
cultured macrophages and co-cultured HCC cells and macrophages,
while the levels of IL-8 in cultured HCC cells were appreciably
lower. This suggests that IL-8 is secreted primarily by the
macrophages and is probably involved in the development of HCC
in vitro.
STAT3 is regarded as a critical transcription
activator for cell cycle or cell survival-related genes, and its
phosphorylation has been linked to HCC tumor progression (42), angiogenesis (43) and tumorigenesis (44). Colomiere et al (45) have reported that activation of the
JAK2/STAT3 pathway may result in EMT-associated phenotypes of
ovarian cancer cells. Moreover, consistent suppression of STAT3
activity may abrogate N-Cadherin and vimentin expression,
consistent with the loss of cell motility in ovarian cancer
(46). In a study of head and neck
tumors, the JAK2/STAT3/Snail signaling pathway was identified as
the major factor in inducing EMT (47), but little attention was paid to the
tumor stroma, which could be the most important trigger for EMT.
Fernando et al (48)
illustrated the essential role of IL-8 signaling in the acquisition
and/or maintenance of the mesenchymal and invasive features of
Brachyury-overexpressing tumor cells; they also showed that IL-8
secreted by HCC cells undergoing EMT could potentiate tumor
progression by inducing adjacent epithelial tumor cells to undergo
EMT (48). These authors, however,
did not investigate pathways downstream of IL-8 that may induce
EMT. Therefore, we investigated the hypothesis that HCC cells may
undergo EMT via the JAK2/STAT3 pathway. Our western blot data from
HCC cultures showed that JAK2/STAT3 activation was positively
associated with IL-8 levels in a dose-dependent fashion. Moreover,
Snail expression also increased with increasing IL-8.
Our data provide novel evidence for the importance
of macrophages in HCC pathogenesis. Moreover, we confirm that the
soluble cytokines secreted by macrophages, such as IL-8, can
trigger a switch in the phenotype of HCC cells. In light of these
results, the development of strategies aimed at interfering with
cytokines appears to be a rational approach for preventing
metastasis, which would improve HCC therapeutic efficacy. In
addition, our results indicate that: i) IL-8 is the most probable
candidate cytokine to induce EMT in HCC cells and ii) the
JAK2/STAT3/Snail signaling pathway may lie downstream of the IL-8
receptor axis. Our research demonstrates, for the first time, the
mechanism by which macrophages that have infiltrated the tumoral
stroma can induce EMT in HCC. They also provide a new perspective
on how IL-8 is linked to the JAK2/STAT3/Snail pathway.
There are several potential limitations of the
present study. It should be noted that this study primarily focused
on macrophages that infiltrated the stroma of the tumor. However,
the tumor immune microenvironment is composed of various kinds of
immune-related cells, such as neutrophils (49), natural killer T cells (50), T lymphocytes (51) and T regulatory cells (52), which can influence the biological
activity of HCC cells. Although the macrophage is widely recognized
as the major type of inflammatory cell that infiltrates tumors, we
cannot be certain that the macrophage is the only cell type
influencing EMT in HCC. In addition, in this study, we focused
primarily on the effect of IL-8 rather than those of the other
cytokines identified by our antibody array. As mentioned above,
IL-6 was another potential trigger of EMT in HCC. Thus, future
studies should investigate the role of additional cytokines in
affecting HCC pathogenesis; moreover, an attempt to characterize
the network of immune-related cytokines in HCC should be
considered.
Acknowledgements
The present study was supported by the funds from
the National Natural Science Fund of China (nos. 81272724 and
81472218), the Major Program of NSFC (no. 81030038), the National
Key Sci-Tech Project, China (2012ZX10002011- 002 and
2012ZX10002013-005) and the Young Investigator Award of Zhongshan
Hospital, Fudan University, China (no. 2013ZSQN16).
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clavien PA, Petrowsky H, DeOliveira ML and
Graf R: Strategies for safer liver surgery and partial liver
transplantation. N Engl J Med. 356:1545–1559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huo TI, Lin HC, Huang YH, et al: The model
for end-stage liver disease-based Japan Integrated Scoring system
may have a better predictive ability for patients with
hepatocellular carcinoma undergoing locoregional therapy. Cancer.
107:141–148. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakakura EK and Choti MA: Management of
hepatocellular carcinoma. Oncology (Williston Park). 14:1085–1102.
2000.
|
|
5
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: at the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwatsuki M, Mimori K, Yokobori T, et al:
Epithelial-mesenchymal transition in cancer development and its
clinical significance. Cancer Sci. 101:293–299. 2010. View Article : Google Scholar
|
|
9
|
Budhu A, Forgues M, Ye QH, et al:
Prediction of venous metastases, recurrence, and prognosis in
hepatocellular carcinoma based on a unique immune response
signature of the liver microenvironment. Cancer Cell. 10:99–111.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sica A, Larghi P, Mancino A, et al:
Macrophage polarization in tumour progression. Semin Cancer Biol.
18:349–355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu XD, Zhang JB, Zhuang PY, et al: High
expression of macrophage colony-stimulating factor in peritumoral
liver tissue is associated with poor survival after curative
resection of hepatocellular carcinoma. J Clin Oncol. 26:2707–2716.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jensen TO, Schmidt H, Moller HJ, et al:
Macrophage markers in serum and tumor have prognostic impact in
American Joint Committee on Cancer stage I/II melanoma. J Clin
Oncol. 27:3330–3337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen JJ, Lin YC, Yao PL, et al:
Tumor-associated macrophages: the double-edged sword in cancer
progression. J Clin Oncol. 23:953–964. 2005. View Article : Google Scholar
|
|
14
|
Ding ZB, Shi YH, Zhou J, et al:
Liver-intestine cadherin predicts microvascular invasion and poor
prognosis of hepatitis B virus-positive hepatocellular carcinoma.
Cancer. 115:4753–4765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fernando RI, Litzinger M, Trono P,
Hamilton DH, Schlom J and Palena C: The T-box transcription factor
Brachyury promotes epithelial-mesenchymal transition in human tumor
cells. J Clin Invest. 120:533–544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blanco MJ, Moreno-Bueno G, Sarrio D, et
al: Correlation of Snail expression with histological grade and
lymph node status in breast carcinomas. Oncogene. 21:3241–3246.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kwok WK, Ling MT, Lee TW, et al:
Up-regulation of TWIST in prostate cancer and its implication as a
therapeutic target. Cancer Res. 65:5153–5162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shih JY, Tsai MF, Chang TH, et al:
Transcription repressor slug promotes carcinoma invasion and
predicts outcome of patients with lung adenocarcinoma. Clin Cancer
Res. 11:8070–8078. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Visser KE, Eichten A and Coussens LM:
Paradoxical roles of the immune system during cancer development.
Nat Rev Cancer. 6:24–37. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Korangy F, Hochst B, Manns MP and Greten
TF: Immune responses in hepatocellular carcinoma. Dig Dis.
28:150–154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bingle L, Brown NJ and Lewis CE: The role
of tumour-associated macrophages in tumour progression:
implications for new anticancer therapies. J Pathol. 196:254–265.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lewis CE and Pollard JW: Distinct role of
macrophages in different tumor microenvironments. Cancer Res.
66:605–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Siveen KS and Kuttan G: Role of
macrophages in tumour progression. Immunol Lett. 123:97–102. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stein M, Keshav S, Harris N and Gordon S:
Interleukin 4 potently enhances murine macrophage mannose receptor
activity: a marker of alternative immunologic macrophage
activation. J Exp Med. 176:287–292. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin EY, Li JF, Gnatovskiy L, et al:
Macrophages regulate the angiogenic switch in a mouse model of
breast cancer. Cancer Res. 66:11238–11246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeisberger SM, Odermatt B, Marty C,
Zehnder-Fjallman AH, Ballmer-Hofer K and Schwendener RA:
Clodronate-liposome-mediated depletion of tumour-associated
macrophages: a new and highly effective antiangiogenic therapy
approach. Br J Cancer. 95:272–281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gocheva V, Wang HW, Gadea BB, et al: IL-4
induces cathepsin protease activity in tumor-associated macrophages
to promote cancer growth and invasion. Genes Dev. 24:241–255. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen JJ, Yao PL, Yuan A, et al:
Up-regulation of tumor interleukin- 8 expression by infiltrating
macrophages: its correlation with tumor angiogenesis and patient
survival in non-small cell lung cancer. Clin Cancer Res. 9:729–737.
2003.PubMed/NCBI
|
|
30
|
Strieter RM, Phan SH, Showell HJ, et al:
Monokine-induced neutrophil chemotactic factor gene expression in
human fibroblasts. J Biol Chem. 264:10621–10626. 1989.PubMed/NCBI
|
|
31
|
Strieter RM, Kunkel SL, Showell HJ, et al:
Endothelial cell gene expression of a neutrophil chemotactic factor
by TNF-alpha, LPS, and IL-1 beta. Science. 243:1467–1469. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kitadai Y, Haruma K, Sumii K, et al:
Expression of interleukin-8 correlates with vascularity in human
gastric carcinomas. Am J Pathol. 152:93–100. 1998.PubMed/NCBI
|
|
33
|
Kooijman R, Himpe E, Potikanond S and
Coppens A: Regulation of interleukin-8 expression in human prostate
cancer cells by insulin-like growth factor-I and inflammatory
cytokines. Growth Horm IGF Res. 17:383–391. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Soria G, Ofri-Shahak M, Haas I, et al:
Inflammatory mediators in breast cancer: coordinated expression of
TNFalpha & IL-1beta with CCL2 & CCL5 and effects on
epithelial-to-mesenchymal transition. BMC Cancer. 11:1302011.
View Article : Google Scholar
|
|
35
|
Akiba J, Yano H, Ogasawara S, Higaki K and
Kojiro M: Expression and function of interleukin-8 in human
hepatocellular carcinoma. Int J Oncol. 18:257–264. 2001.PubMed/NCBI
|
|
36
|
Trikha M, Corringham R, Klein B and Rossi
JF: Targeted anti-interleukin-6 monoclonal antibody therapy for
cancer: a review of the rationale and clinical evidence. Clin
Cancer Res. 9:4653–4665. 2003.PubMed/NCBI
|
|
37
|
Tilg H, Wilmer A, Vogel W, et al: Serum
levels of cytokines in chronic liver diseases. Gastroenterology.
103:264–274. 1992.PubMed/NCBI
|
|
38
|
Sullivan NJ, Sasser AK, Axel AE, et al:
Interleukin-6 induces an epithelial-mesenchymal transition
phenotype in human breast cancer cells. Oncogene. 28:2940–2947.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park SH, Kim JH, Lee DH, et al: Luteolin
8-C-beta-fucopyranoside inhibits invasion and suppresses
TPA-induced MMP-9 and IL-8 via ERK/AP-1 and ERK/NF-kappaB signaling
in MCF-7 breast cancer cells. Biochimie. 95:2082–2090. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lattanzio L, Tonissi F, Torta I, et al:
Role of IL-8 induced angiogenesis in uveal melanoma. Invest New
Drugs. 31:1107–1114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yuan A, Chen JJ, Yao PL and Yang PC: The
role of interleukin-8 in cancer cells and microenvironment
interaction. Front Biosci. 10:853–865. 2005. View Article : Google Scholar
|
|
42
|
Rajendran P, Ong TH, Chen L, et al:
Suppression of signal transducer and activator of transcription 3
activation by butein inhibits growth of human hepatocellular
carcinoma in vivo. Clin Cancer Res. 17:1425–1439. 2011. View Article : Google Scholar
|
|
43
|
Yang SF, Wang SN, Wu CF, et al: Altered
p-STAT3 (tyr705) expression is associated with histological grading
and intratumour microvessel density in hepatocellular carcinoma. J
Clin Pathol. 60:642–648. 2007. View Article : Google Scholar
|
|
44
|
Ogata H, Kobayashi T, Chinen T, et al:
Deletion of the SOCS3 gene in liver parenchymal cells promotes
hepatitis-induced hepatocarcinogenesis. Gastroenterology.
131:179–193. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Colomiere M, Ward AC, Riley C, et al:
Cross talk of signals between EGFR and IL-6R through JAK2/STAT3
mediate epithelial-mesenchymal transition in ovarian carcinomas. Br
J Cancer. 100:134–144. 2009. View Article : Google Scholar :
|
|
46
|
Colomiere M, Findlay J, Ackland L and
Ahmed N: Epidermal growth factor-induced ovarian carcinoma cell
migration is associated with JAK2/STAT3 signals and changes in the
abundance and localization of alpha6beta1 integrin. Int J Biochem
Cell Biol. 41:1034–1045. 2009. View Article : Google Scholar
|
|
47
|
Yadav A, Kumar B, Datta J, Teknos TN and
Kumar P: IL-6 promotes head and neck tumor metastasis by inducing
epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling
pathway. Mol Cancer Res. 9:1658–1667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fernando RI, Castillo MD, Litzinger M,
Hamilton DH and Palena C: IL-8 signaling plays a critical role in
the epithelial-mesenchymal transition of human carcinoma cells.
Cancer Res. 71:5296–5306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhou SL, Dai Z, Zhou ZJ, et al:
Overexpression of CXCL5 mediates neutrophil infiltration and
indicates poor prognosis for hepatocellular carcinoma. Hepatology.
56:2242–2254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xiao YS, Gao Q, Xu XN, et al: Combination
of intratumoral invariant natural killer T cells and
interferon-gamma is associated with prognosis of hepatocellular
carcinoma after curative resection. PLoS One. 8:e703452013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xia YH, Wang ZM, Chen RX, et al: T-cell
apoptosis induced by intratumoral activated hepatic stellate cells
is associated with lung metastasis in hepatocellular carcinoma.
Oncol Rep. 30:1175–1184. 2013.PubMed/NCBI
|
|
52
|
Yang P, Li QJ, Feng Y, et al:
TGF-beta-miR-34a-CCL22 signaling-induced Treg cell recruitment
promotes venous metastases of HBV-positive hepatocellular
carcinoma. Cancer Cell. 22:291–303. 2012. View Article : Google Scholar : PubMed/NCBI
|