Introduction
Breast cancer is the most common carcinoma among
females, and a major public health issue with >1.6 million
increased cases and 1.2 million deaths each year in China (1,2).
Although surgery, radiotherapy, and chemotherapy could cure a
certain percentage of patients, most patients still die of this
disease (3). Chemotherapy is one
of the predominant strategies in the treatment of breast cancer.
However, with the continuous application of antitumor drugs, the
emergence of multidrug resistance (MDR) seriously restricts
clinical efficacy of chemotherapeutics and results in the failure
of chemotherapy (4). Therefore, it
is urgent to discover effective resistance-reversal agents that may
improve the curative effect of chemotherapy drugs and reverse MDR
in breast cancer.
In our previous study, we established the
methotrexate-resistant breast cancer cells (MCF-7/MTX) and
identified nucleophosmin that is a new drug-resistant target
related to the MDR of breast cancer (5). Nucleophosmin (NPM, also known as B23,
numatrin, or N038) was originally discovered as a non-ribosomal
nucleolar phosphoprotein that was located primarily in the nucleoli
and shuttled between the nucleoli and cytoplasm during the cell
cycle (6). NPM possesses important
roles in multiple cellular activities, not only as an important
player in ribosome biogenesis but also as a potential regulator for
cell growth, proliferation, transformation, and apoptosis (7–9).
However, NPM was obviously overexpressed in MCF-7/MTX cells, and it
could be a critical factor inducing MDR in the breast carcinoma.
There were some studies on the correlation between NPM and MDR. NPM
was upregulated in the adriamycin-resistant human bladder cancer
cells (10). It could increase the
sensitivity of resistant leukemia cells to chemotherapeutic drug
and reverse MDR by suppressing the level of NPM by shRNA (11). Consequently, it is an important way
to decrease MDR of breast cancer by searching for effective
reversal agents to reverse drug resistance.
Several studies have reported that NPM was a member
of histone chaperones which acted a vital role in the dynamic
chromatin organization, gene expression and regulation during
different cellular processes. Importantly, NPM also enhanced the
acetylation-dependent chromatin transcription and affected histone
post-translational modification (12). Certainly, it is well-known that
acetylation and deacetylation of histones represented two important
histone modifications, while histone acetyl transferase (HAT) and
histone deacetylase (HDAC) were two key enzymes to maintain the
dynamic equilibrium of histone acetylation. Once this steady-state
level was interrupted by overexpression of HDAC, it would generate
a malignant tumor and even the occurrence of drug resistance in
different cancers (13,14). Therefore, screening histone
deacetylase inhibitors (HDACi) with efficiency and low toxicity
could suppress tumor cells proliferation caused by histone
deacetylation and reverse drug resistance induced by the activity
of NPM.
NPM as a newfound drug-resistant target, it plays a
vital role in the development and progression of MDR in breast
cancer. In this study, the relationship between abnormal expression
of NPM and activation of PI3K/Akt signaling pathway was addressed.
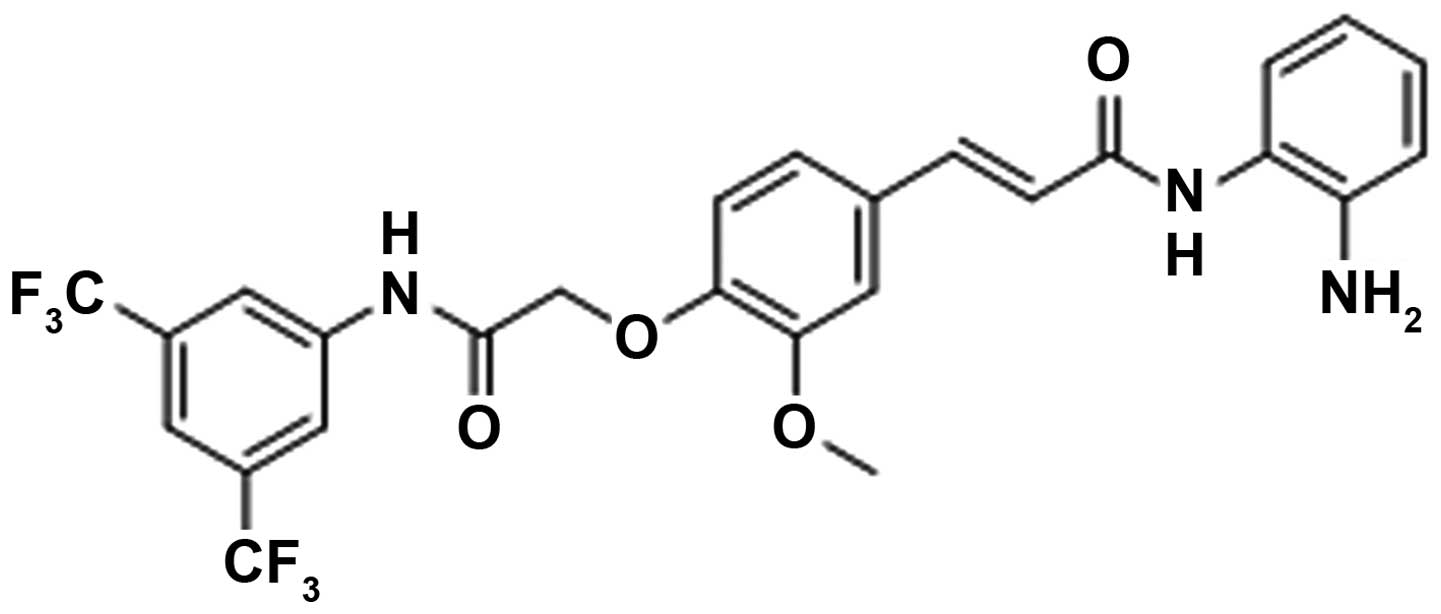
Then, a novel synthetic histone deacetylase inhibitor FA17
(Fig. 1A), possessing tumor
suppressor function (15), was
used to reverse MDR by reducing the activity of NPM in breast
cancer. The reversal effect and molecular mechanism of FA17 were
investigated in vitro and in vivo. Taken together,
our results indicated that the novel histone deacetylase inhibitor
displayed intense reversal activity in the MDR of breast cancer.
FA17 would be expected to become an effective drug candidate for
reversing MDR in clinical trials.
Materials and methods
Chemicals and reagents
Methotrexate was obtained from Pude Pharmaceutical
Company Ltd. (Shanxi, China). The novel synthetic histone
deacetylase inhibitor (FA17) was kindly provided by Professor Jie
Zhang from Health Science Center of Xi'an Jiaotong University.
Verapamil was purchased from China Pharmaceutical Biological
Products Analysis Institute. The Annexin V FLUOS staining kit was
purchased from Invitrogen (USA). The primary antibodies against
NPM, breast cancer resistance protein (BCRP), dihydrofolate
reductase (DHFR), Bcl-2 and Bax were from Epitomics (USA), while
phosphatase and tensin homolog deleted on chromosome 10 (PTEN),
total Akt (t-Akt), phospho-Akt (p-Akt), caspase-9, caspase-3, and
poly(ADP-ribose) polymerase (PARP) antibodies were purchased from
Cell Signaling Technology (USA). P-glycoprotein (P-gp) and reduced
folate carrier (RFC) antibodies were obtained from Abcam (UK), and
multidrug resistance associated protein 1 (MRP1) was from GeneTax
(USA). The β-actin antibody was purchased from Biosynthesis
Biotechnology and the horseradish peroxidase-conjugated goat
anti-rabbit IgG was from Cwbiotech (Beijing, China).
Cell lines and cell culture
The MCF-7/S sensitive human breast cancer cell line
was obtained from Shanghai Institute of Cell Biology in the Chinese
Academy of Sciences (Shanghai, China). The methotrexate-resistant
breast cancer cell line (MCF-7/MTX) was successfully established as
previously described (5) with the
concentration of 220 nM MTX. Both cell types were cultured in
RPMI-1640 medium (Gibco, USA) supplemented with 10% fetal bovine
serum (PAA, Austria) and 1% penicillin/streptomycin in a humidified
atmosphere of 5% CO2 at 37°C.
Western blot analysis
Protein samples were extracted from whole-cell, the
protein concentration was detected by BCA reagent (Beyotime, China)
and then separated by 10 or 12% sodium dodecyl
sulfate-polyacrylamide gels and transferred onto polyvinylidene
difluoride membranes (Millipore, USA). Then the membranes were
blocked with 5% fat-free milk at room temperature for 2 h, and
incubated with diluted antibodies NPM (1:1,000, ab109546), PTEN
(1:1,000, #9188), p-Akt (1:800, #4090), t-Akt (1:1000, #4691),
Bcl-2 (1:2,500, #1017-1), Bax (1:2,500, #1063-1), caspase-9 (1:800,
#9502), caspase-3 (1:800, #9662), PARP (1:800, #9542), P-gp (1:500,
ab98322), MRP1 (1:600, GTX116046), BCRP (1:800, #3765-1), RFC
(1:1,500, ab62302), DHFR (1:20,000, ab124814) and β-actin (1:800,
bs-0061R) overnight at 4°C. The second antibody conjugated to
horseradish peroxidase incubation was developed at room temperature
for 2 h. Protein bands were tested using the SuperSignal West Pico
kit (Pierce, Thermo Scientific). All samples were performed in
triplicates and the protein expression was analyzed using a
quantitative analysis system (Image-Pro Plus).
Small interfering RNA transfection
For in vitro knockdown experiments, the
double-stranded small interfering RNA (siRNA) against human
NPM1 and non-specific siRNA (control siRNA) were purchased
from Shanghai GenePharma Company Ltd. (China). They were used for
transient transfection as previously described (5). MCF-7/MTX cells were seeded at a
density of 5×105 per well into a 6-well plate and
cultured in the medium until cell confluence reached ~40%. The
following day, cells were transfected with 50 nM NPM1 siRNA
and Lipofectamine 2000 reagent (Invitrogen) according to the
manufacturer's instructions.
Quantitative real-time PCR analysis
Total cellular mRNA was extracted using RNAfast2000
kit (Fastagen) and reverse transcription to cDNA was performed
using PrimeScript RT Master Mix Perfect Real-Time kit (Takara).
Quantitative real-time PCR (qRT-PCR) was conducted using SYBR
Premix Ex Taq II (Takara) and the primer sequences and product
lengths are listed in Table I. The
assay was performed on the Bio-Rad CFX96™ Real-time system with the
following procedure: 95°C for 30 sec; 95°C for 5 sec and 60°C for
30 sec (40 cycles); and 95°C for 15 sec, 60°C for 30 sec, 95°C for
15 sec. Each sample was normalized on the basis of β-actin.
Three independent experiments were run to analyze the gene
expressions and each sample was repeated in triplicate.
 | Table IThe primers used in qRT-PCR. |
Table I
The primers used in qRT-PCR.
| Gene | Forward primer
(5′→3′) | Reverse primer
(5′→3′) | Product size
(bp) |
|---|
| NPM |
TGGCAGTGGAGGAAGTCTCT |
ATCAAACACGGTAGGGAAAGTT | 141 |
| MDR1 |
GAGCCCATCCTGTTTGACTG |
GCTGCCCTCACAATCTCTTC | 92 |
| MRP1 |
AAGGTGGACGAGAACCAGAA |
AACAGGGCAGCAAACAGAAC | 110 |
| BCRP |
AGCAGGGACGAACAATCATC |
GCCAATAAGGTGAGGCTATCA | 82 |
| RFC |
TCCTGTCCATCATCTACTTCTTG |
AGTGCCTGTGCTGCCTTCT | 130 |
| DHFR |
TCTCCAAGACCCCAACTGAG |
ATGTGAAAAGCCCGACAAT | 109 |
| β-actin |
TGACGTGGACATCCGCAAAG |
CTGGAAGGTGGACAGCGAGG | 205 |
Drug sensitivity assays
The cell viability under drug treatment was measured
using MTT assay. Cells were seeded at a density of 5×104
per well into 96-well plates and treated with cytotoxic agents at
increasing concentrations for 72-h incubation, then MTT (5 mg/ml)
was added to each well for an additional 4 h. After discarding the
medium, the blue formazan was dissolved in 150 μl of DMSO. The
absorbance was tested at 490 nm on a microplate reader (BioTek,
USA). The resistance factor (RF) was calculated using the equation:
RF = (IC50 value of MCF-7/MTX)/(IC50 value of
MCF-7/S). The data represent three independent experiments.
Reversal effect assays
The reversal effect of FA17 on methotrexate
resistance was evaluated in MCF-7/MTX cells using MTT assay.
MCF-7/MTX cells were seeded at a density of 5×104 per
well into 96-well plates for 24 h, then methotrexate at increasing
concentrations in combination with FA17 were added and cultured for
72 h. The reversal index (RI) was calculated using this equation:
RI = IC50 of methotrexate/IC50 of
methotrexate plus FA17. The data represent three independent
experiments.
Flow cytometry assays
For the cell cycle assay, cells were harvested after
treatment and fixed in 70% cold ethanol at 4°C overnight. After
suspension with PBS, cells were stained with RNase at 37°C for 15
min and with propidium iodide on ice for 30 min away from light.
Then cell cycle distribution was detected immediately using flow
cytometry (BD Bioscience, USA).
Cell apoptosis was measured by Annexin V FLUOS
staining kit according to the manufacturer's instructions. Cells
from different samples were analyzed for live, necrotic, early and
late apoptotic cells by flow cytometry. All experiments were run in
triplicate.
In vivo tumor xenograft model and
immunohistochemistry
Female BALB/c nude mice (18±4 g, 4–6 weeks) were
purchased from Shanghai Laboratory Animal Center of the Chinese
Academy of Sciences. All animal experiments were carried out in
accordance with guidelines and approval of the Institutional Animal
Care and Use Committee of Xi'an Jiaotong University. MCF-7/MTX
cells (200 μl, 1×107 cells) were injected subcutaneously
into mice, and when they all developed tumors with a size ~100
mm3, mice were randomly assigned into four groups (n=6)
and treated intraperitoneally with vehicle, MTX (9 mg/kg), FA17 (10
mg/kg) or in combination every three days for a total of three
weeks. Tumor volume was measured on alternate day and calculated
with the formula a × b2/2 (a, the largest diameter; b,
the smallest diameter), additionally, mouse weight was monitored
three times per week. Upon termination, mice were sacrificed and
tumors were harvested. Protein were exacted from tumors for western
blot analysis. The other tumor tissues were fixed with formalin and
embedded with paraffin, which were analyzed for regular hematoxylin
and eosin (H&E) stain and immunohistochemistry. These tissue
sections were incubated with primary antibody (NPM, 1:200
dilution), and control group with PBS. The immunostaining
intensities or percentages of positive cells were evaluated as
previously described under bright-field microscopy (16). Images from 6 random fields of each
section were used for quantitative analysis (Image-Pro Plus).
Statistical analysis
All data were expressed as mean ± standard deviation
(SD). Statistical analyses were performed using one-way ANOVA, and
P-value <0.05 was considered as statistical significance.
Results
NPM overexpression activates PI3K/Akt
pathway and suppresses cell apoptosis
Our previous study identified that NPM was
significantly overexpressed in the MCF-7/MTX cells, and served as a
novel drug-resistant target in breast cancer using proteomics
technology (5). Similarly, some
reports found upregulation of NPM accounted for MDR phenotype in
various cancers (10,11,17).
In addition, PI3K/Akt signaling pathway played a significant role
in carcinogenesis and development of malignant tumors, so
activation of this pathway emerged in a majority of tumors and even
facilitated the formation of drug-resistance (18–21).
We detected NPM and related factors of PI3K/Akt signaling pathway
both in MCF-7/S and MCF-7/MTX cells by western blotting. As shown
in Fig. 2A and B, NPM was
obviously overexpressed in MCF-7/MTX cells compared with MCF-7/S
cells. The decreased PTEN level and increased p-Akt expression were
both observed in MCF-7/MTX cells, which indicated the PI3K/Akt
pathway was activated in MCF-7/MTX cells. Furthermore, the
downstream pro-survival factor Bcl-2 was upregulated and
pro-apoptotic factor Bax was downregulated, which significantly
suppressed cells apoptosis in MCF-7/MTX cells.
To better understand the effect of drug resistance
on cell apoptosis, the interrelated factors of mitochondrial
apoptosis pathway were also investigated. As Akt augments
phosphorylation, the downstream apoptotic molecules including
cleaved-caspase-9, cleaved-caspase-3 and cleaved-PARP were all
downregulated in MCF-7/MTX cells (Fig.
2C and D). The above demonstrated that NPM-induced resistance
not only activated PI3K/Akt pathway, but also inhibited the
apoptotic function of resistant cells.
Silencing of NPM inhibits the PI3K/Akt
pathway in MCF-7/MTX cells
To further explore the interaction of NPM and
PI3K/Akt pathway, we determined the changes in expression of
related factors after knockdown of NPM. We predicted that
the reduction of NPM could restrain the PI3K/Akt pathway and
accelerate its downstream pro-apoptotic effect. Decreasing
NPM expression by siRNA in MCF-7/MTX cells, mRNA level of
NPM was reduced by >70% compared with the siRNA controls
(Fig. 3A). Additionally,
decreasing NPM expression could accelerate upregulation of PTEN and
attenuate Akt phosphorylation while t-Akt was not apparently
changed in siNPM-resistant cells. Then the expression of downstream
pro-apoptotic factor Bax was elevated, and the pro-survival factor
Bcl-2 was remarkably decreased (Fig.
3B and C). Moreover, mitochondrial apoptosis molecules
cleaved-caspase-9, cleaved-caspase-3 and cleaved-PARP were all
increased (Fig. 3D and E). These
results declared that function of pro-apoptosis was elevated by
devitalizing PI3K/Akt pathway with NPM knockdown in MCF-7/MTX
cells.
The intrinsic cytotoxicity of FA17 on
MCF-7/S and MCF-7/MTX cells
A novel synthetic histone deacetylase inhibitor FA17
was reported to display a promising profile as an anti-tumor
candidate (15), but whether or
not FA17 could reverse breast cancer MDR was not clear. We detected
intrinsic cytotoxicity of FA17 in MCF-7/S and MCF-7/MTX cells by
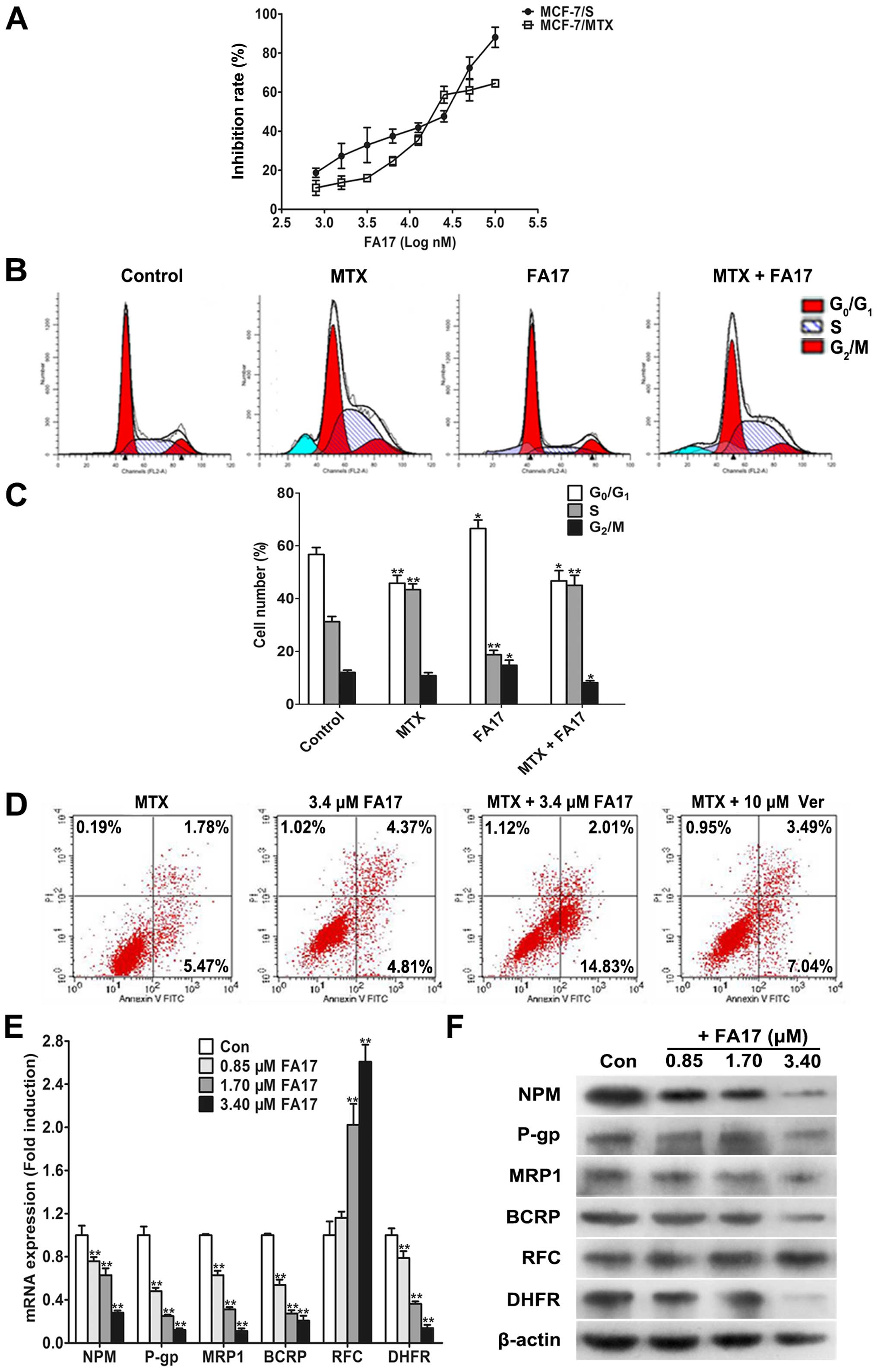
MTT analysis. As shown in Fig. 4A,
FA17 obviously inhibited growth of sensitive and resistant cells in
a dose-dependent manner, and IC50 was 16.6±2.43 and
23.4±2.23 μM, respectively, these data indicated that MCF-7/MTX
cells did not produce resistance to FA17 which may serve as a
candidate chemicals to reverse MDR.
FA17 reverses drug resistance in
MCF-7/MTX cells
The reversal effect of FA17 on the MDR of MCF-7/MTX
cells was analyzed by MTT assay. Three concentrations including
0.85, 1.7 and 3.4 μM under the lower-toxic dose (inhibition rate
<15%) were chosen to evaluate efficacies of FA17 on NPM-mediated
methotrexate resistance in breast cancer cells, and verapamil was
used as a positive control. The results are presented in Table II, RIs of these three doses to
MCF-7/MTX cells was 2.60, 3.80 and 4.38, respectively, thus, the
effect of FA17 higher concentration was close to verapamil and its
dose was markedly less than control. This phenomenon demonstrated
strong reversal activity of FA17 in MCF-7/MTX cells.
 | Table IIEffect of FA17 on IC50
values of methotrexate in MCF-7/MTX cells. |
Table II
Effect of FA17 on IC50
values of methotrexate in MCF-7/MTX cells.
| Group | Concentration
(μM) | IC50 of
MTX (nM) | RI |
|---|
| Control | | 2,818±97.9 | 1.00 |
| FA17 | 0.85 | 1,084±87.5 | 2.60 |
| 1.70 | 741.3±80.2 | 3.80 |
| 3.40 | 642.7±32.6 | 4.38 |
| Verapamila | 10 | 562.3±90.3 | 5.01 |
To further investigate effects of FA17 on cell
cycle, MCF-7/MTX cells were treated with methotrexate (2 μM), FA17
(3.4 μM), and two drug combinations using flow cytometry for
cell-cycle detection. Compared with control, methotrexate and
combination group caused a significant decrease of cells in the
G0/G1 phase and a corresponding increased
proportion of S-phase cells, while FA17 alone treatment apparently
added G0/G1-phase cells and reduced S-phase
cells (Fig. 4B and C). The
experimental results revealed that the role of methotrexate and
combination treatment blocked S-phase cells, but
G0/G1-phase arrest appeared in the FA17
group.
In addition, to assess the effect of FA17 on
methotrexate induced resistance, an apoptosis assay was performed
in MCF-7/MTX cells treated with methotrexate or FA17 alone and
combination group, verapamil was used as a positive control again.
Treatment of cells with FA17 and combination groups significantly
increased cells apoptosis rates compared with methotrexate group,
and FA17 strengthened methotrexate-induced MCF-7/MTX cell
apoptosis, its intensity was apparently greater than the effect of
positive drug verapamil (Fig. 4D).
Thus it was proven that FA17 had the ability to induce resistant
cell apoptosis, while increasing the sensitivity of MCF-7/MTX cells
to methotrexate.
As previous studies have demonstrated that NPM,
membrane transport proteins including P-gp, MRP1, BCRP and
methotrexate resistance-associated RFC and DHFR all had abnormal
expression in resistant cells (5),
we determined if resistant factors were modulated by FA17 in
MCF-7/MTX cells using qRT-PCR and western blot assays. After 48-h
treatment, both mRNA and protein expressions of NPM, P-gp, MRP1,
BCRP, DHFR were downregulated while RFC level was upregulated in
MCF-7/MTX cells (Fig. 4E and F).
The results validated that FA17 levels partially reversed MDR
characteristics of MCF-7/MTX cells at gene and protein levels.
Reversal mechanism of FA17 in MCF-7/MTX
cells
We have discovered that the occurrence of drug
resistance was correlated with the activation of PI3K/Akt pathway,
however, to clarify whether FA17 reversed methotrexate-induced
breast cancer resistance by PI3K/Akt pathway, we determined
expressions of PTEN, Akt and downstream mitochondrial apoptosis
related factors with western blotting. As shown in Fig. 5A and B, Akt phosphorylation was
suppressed along with the intensifying of PTEN level, and it
emerged evidently in a dose-dependent manner, then enervated
relevant factor Bcl-2 level and enhanced Bax expression, which
eventually reduced MDR phenotype of MCF-7/MTX and induced cell
apoptosis.
Next, we investigated the inhibitory effect of FA17
on apoptotic process of resistant cells, the variation of
mitochondrial apoptosis signaling pathway molecule expression was
detected in MCF-7/MTX cells. As expected, caspase-9, caspase-3, and
PARP proteins obvious cleaving belts depended on the dose under
FA17 treatment (Fig. 5C and D),
which predicted appearance of cells apoptosis. Taken together,
these results illuminated that FA17 may reverse drug resistance of
MCF-7/MTX cells through inhibiting the activation of PI3K/Akt and
accelerating mitochondrial apoptosis.
FA17 significantly reverses methotrexate
resistance in vivo
Our research has suggested that FA17 reversed MDR of
MCF-7/MTX cells by PI3K/Akt pathway in vitro. To further
confirm this effect in vivo, we evaluated FA17 alone or
combined with methotrexate treatment in nude mouse xenograft model
when tumors were palpable and continued for 14 days. Animals
bearing MCF-7/MTX cells displayed a tumor growth profile (Fig. 6A and B), comparing with controls,
tumor volumes were significantly decreased after methotrexate and
FA17 combined treatment, and Table
III shows the most effective in attenuating tumor burden (51%
tumor growth inhibition), whereas methotrexate or FA17 alone group
also possessed a certain degree of suppression of tumor growth, and
inhibition rates were 19 and 29%, respectively. In addition, it did
not cause conspicuous reduction in body weight and any other
abnormities under drug therapy.
 | Table IIIThe inhibition of FA17 on nude mouse
xenografted tumors of MCF-7/MTX cells. |
Table III
The inhibition of FA17 on nude mouse
xenografted tumors of MCF-7/MTX cells.
| Body weight
(g) | Tumor volume
(mm3) | | | |
|---|
|
|
| | | |
|---|
| Group | Initial | Final | Initial | Final | Tumor weight
(%) | Growth rate of body
weight (g) | Inhibition rate
(%) |
|---|
| Control | 18.5±0.98 | 21.3±1.28 | 130±30 | 1,320±420 | 1.34±0.29 | 15.14 | |
| MTX | 18.5±1.06 | 20.8±1.02 | 120±20 | 1,020±280 | 0.98±0.31 | 10.85 | 19.40 |
| FA17 | 18.4±1.12 | 20.5±0.91 | 110±20 | 890±240 | 0.79±0.25a | 11.41 | 29.10 |
| MTX + FA17 | 19.6±1.27 | 20.9±0.87 | 130±20 | 590±140a | 0.45±0.21b | 6.63 | 51.49 |
To further explore influence of FA17 and
methotrexate treatment on tumor tissues of nude mice, H&E
staining was performed to observe morphological variations and
immunohistochemical analysis was applied to detect NPM expression.
As shown in Fig. 6C, tumor
necrosis and histomorphology of tumor tissues were not obviously
observed after drug treatment. Whereas, NPM expression was strongly
positive in the control group, and its level was downregulated in
methotrexate or FA17 alone group, but NPM showed almost negative
expression in the combined treatment (Fig. 6D). These results manifested that
methotrexate and FA17 combined therapy significantly inhibited
drug-resistant target NPM and lowered resistance of nude mice.
To clarify the underlying resistance mechanism of
combination where methotrexate and FA17 are more effective than
either drug alone, we next detected expressions of PI3K/Akt pathway
related factors. The immunoblotting revealed that intensity of PTEN
was augmented under drug treatment accompanied by downregulation of
NPM level, especially in the combined group. Furthermore, drug
action inhibited Akt phosphorylation and downstream Bcl-2
expression, and reinforced the role of Bax, which led to resistant
tumor cells apoptosis, eventually blocking the growth of
methotrexate-resistant tumors in nude mice (Fig. 6E and F). These experiments
clarified the molecular mechanism by which FA17 reversed MDR of
breast cancer in vivo.
Discussion
The chemotherapy of breast carcinoma remains a
challenging problem and the reason is drug resistance, due to the
multifarious application of chemotherapeutic agents in clinical
(22). However, breast cancer
resistance is a multistep process involving alterations of certain
relevant genes and proteins and activation of various signaling
pathways (23,24). Consequently, there is an urgent
need to discover new therapeutic targets so that the novel
therapeutic agents can be developed to enhance the effectiveness of
treatment while reducing adverse side reactions (25–27).
Our previous study found that NPM was abnormally overexpressed in
MCF-7/MTX cells compared with sensitive cells. Moreover, recent
studies have shown that NPM, as non-ribosomal nucleolar
phosphoprotein, was high level in a variety of tumors including
gastric cancer (28), colon cancer
(29), thyroid cancer (30), renal cell carcinoma (31) and even expected as a biomolecular
marker for breast cancer (32). In
addition, Wang et al (17)
found that decreased expression of nucleophosmin/B23 could increase
drug sensitivity of adriamycin-resistant Molt-4 leukemia cells.
Upregulation of NPM may take part in mechanism of MDR in bladder
cancer (10) and resistant
leukemic HL-60 cells (11).
NPM was closely correlated with the formation of
MDR, but the molecular mechanism of NPM-induced MDR in breast
cancer is not clear. We know that the genesis and development of
tumor referred to activations of various signaling pathways. There
is some evidence that PI3K/Akt pathway was considered to contribute
to tumorigenesis, progression and even drug resistance in cancers
(19,33–35).
Moreover, the study reported that activity of NPM was closely
associated with activation of PI3K/Akt signaling pathway (36,37).
In this study, we found the effect of negative regulation between
NPM and PTEN in MCF-7/MTX cells, which activated the
phosphorylation of Akt and suppressed cell apoptosis, and then
blocked downstream mitochondrial apoptotic pathway. Similarly,
Pianta et al (30)
demonstrated that upregulation of NPM was related to an increase of
p-Akt level and a decrease of PTEN expression. Further study showed
that knockdown of NPM could attenuate the activation of PI3K/Akt
pathway and promote the reinforcement of downstream pro-apoptotic
effect using siRNA interference. Therefore, these data indicate NPM
as a potential resistant target plays an important role in
occurrence of MDR in breast cancer. Also we could reverse drug
resistance in search of effective reversal agent targeted NPM as a
potent therapeutic strategy for MDR in the clinic.
Following the deepening of research in the resistant
mechanisms of NPM, another considerable issue was addressed.
Investigators discovered that NPM was an important histone
chaperone (38), which impacted
the function of post-translational modification of histones. In
addition, histone deacetylation, as a significant epigenetic
modification, was involved in the occurrence and development of
many malignant tumors, such as lung cancer, ovarian cancer, breast
cancer, colon cancer, pancreatic cancer and leukemia (39–45).
Therefore, the effective histone deacetylase inhibitor offered a
promising strategy for cancer therapy and it may be a potential
reversal agent of tumor resistance (13,46,47).
We screened out a novel synthetic histone deacetylase inhibitor
FA17 that possessed strongly antitumor activity (15), which could reduce the MDR of breast
cancer cells by altering the cycle distribution of MCF-7/MTX,
enhancing methotrexate-induced cell apoptosis and downregulating
related factors of MDR. Furthermore, downregulation of NPM level
and inhibition of PI3K/Akt signaling pathway were both observed in
MCF-7/MTX cells and xenograft tumors after FA17 treatment, which
initiated the downstream apoptotic program. Therefore, it is
apparent that FA17 has potent ability to reverse drug resistance in
breast cancer, and it probably demonstrates the underlying reversal
mechanism by suppressing NPM level to increase PTEN expression and
inactivates PI3K/Akt pathway. These results indicate FA17 could be
an effective candidate compound for reversing breast cancer
resistance.
In conclusion, the potential resistant target NPM
was significantly highly expressed in MCF-7/MTX cells, and its
molecular mechanisms of NPM induced MDR by activation of PI3K/Akt
pathway and suppression of mitochondrial apoptosis pathway in
breast cancer cells. Based on drug-resistant marker NPM, histone
deacetylase inhibitor FA17, as an effective reversal agent, was
screened out in MCF-7/MTX cells. We illuminated reversal mechanisms
of FA17 acting on drug resistance of breast cancer, which could
reverse MDR through decreasing NPM to accelerate PTEM level,
devitalizing PI3K/Akt pathway and enhancing resistant cell
apoptosis in vitro and in vivo. These finding of the
inhibitory effect on NPM might provide therapeutic strategy and
scientific evidence for improving clinical drug resistance, and the
preclinical evaluation of effective reversal agent FA17 offers a
significant value for clinical application in MDR of breast
cancer.
Acknowledgements
We are grateful to Dr Dalin He and Xinyang Wang for
valuable assistance, and Dr Jie Zhang, Hongyan Liu for technical
assistance in this study. This study was supported by National
Natural Science Foundation of China (no s.81502616 and
81473177).
References
|
1
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the Human
Development Index (2008–2030): A population-based study. Lancet
Oncol. 13:790–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fan L, Strasser-Weippl K, Li JJ, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:e279–e289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baguley BC: Multiple drug resistance
mechanisms in cancer. Mol Biotechnol. 46:308–316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen S, Cai J, Zhang W, Zheng X, Hu S, Lu
J, Xing J and Dong Y: Proteomic identification of differentially
expressed proteins associated with the multiple drug resistance in
methotrexate-resistant human breast cancer cells. Int J Oncol.
45:448–458. 2014.PubMed/NCBI
|
|
6
|
Borer RA, Lehner CF, Eppenberger HM and
Nigg EA: Major nucleolar proteins shuttle between nucleus and
cytoplasm. Cell. 56:379–390. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yung BY: Oncogenic role of
nucleophosmin/B23. Chang Gung Med J. 30:285–293. 2007.PubMed/NCBI
|
|
8
|
Lim MJ and Wang XW: Nucleophosmin and
human cancer. Cancer Detect Prev. 30:481–490. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grisendi S, Mecucci C, Falini B and
Pandolfi PP: Nucleophosmin and cancer. Nat Rev Cancer. 6:493–505.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meng Q, Lei T and Zhang M, Zhao J, Zhao XH
and Zhang M: Identification of proteins differentially expressed in
adriamycin-resistant (pumc-91/ADM) and parental (pumc-91) human
bladder cancer cell lines by proteome analysis. J Cancer Res Clin
Oncol. 139:509–519. 2013. View Article : Google Scholar
|
|
11
|
Lin M, Hu J, Liu T, Li J, Chen B and Chen
X: Knockdown of nucleophosmin by RNA interference reverses
multidrug resistance in resistant leukemic HL-60 cells.
Immunobiology. 218:1147–1154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Swaminathan V, Kishore AH, Febitha KK and
Kundu TK: Human histone chaperone nucleophosmin enhances
acetylation-dependent chromatin transcription. Mol Cell Biol.
25:7534–7545. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carafa V, Miceli M, Altucci L and Nebbioso
A: Histone deacetylase inhibitors: A patent review (2009–2011).
Expert Opin Ther Pat. 23:1–17. 2013. View Article : Google Scholar
|
|
14
|
Rao R, Fiskus W, Ganguly S, Kambhampati S
and Bhalla KN: HDAC inhibitors and chaperone function. Adv Cancer
Res. 116:239–262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang F, Lu W, Zhang T, Dong J, Gao H, Li
P, Wang S and Zhang J: Development of novel ferulic acid
derivatives as potent histone deacetylase inhibitors. Bioorg Med
Chem. 21:6973–6980. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang J, Li A, Yang Y and Li X:
Identification of cyclophilin A as a potential prognostic factor
for clear-cell renal cell carcinoma by comparative proteomic
analysis. Cancer Biol Ther. 11:535–546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Chen B, Lin M, Cao Y, Chen Y, Chen
X, Liu T and Hu J: Decreased expression of nucleophosmin/B23
increases drug sensitivity of adriamycin-resistant Molt-4 leukemia
cells through mdr-1 regulation and Akt/mTOR signaling.
Immunobiology. 220:331–340. 2015. View Article : Google Scholar
|
|
18
|
Michl P and Downward J: Mechanisms of
disease: PI3K/AKT signaling in gastrointestinal cancers. Z
Gastroenterol. 43:1133–1139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao AM, Ke ZP, Wang JN, Yang JY, Chen SY
and Chen H: Apigenin sensitizes doxorubicin-resistant
hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin via
inhibiting PI3K/Akt/Nrf2 pathway. Carcinogenesis. 34:1806–1814.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gray MJ, Mhawech-Fauceglia P, Yoo E, Yang
W, Wu E, Lee AS and Lin YG: AKT inhibition mitigates GRP78
(glucose-regulated protein) expression and contribution to
chemoresistance in endometrial cancers. Int J Cancer. 133:21–30.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie X, Tang B, Zhou J, Gao Q and Zhang P:
Inhibition of the PI3K/Akt pathway increases the chemosensitivity
of gastric cancer to vincristine. Oncol Rep. 30:773–782.
2013.PubMed/NCBI
|
|
22
|
Saeki T, Tsuruo T, Sato W and Nishikawsa
K: Drug resistance in chemotherapy for breast cancer. Cancer
Chemother Pharmacol. 56(Suppl 1): 84–89. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pritchard AL and Hayward NK: Molecular
pathways: Mitogen-activated protein kinase pathway mutations and
drug resistance. Clin Cancer Res. 19:2301–2309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cassinelli G, Zuco V, Gatti L, Lanzi C,
Zaffaroni N, Colombo D and Perego P: Targeting the Akt kinase to
modulate survival, invasiveness and drug resistance of cancer
cells. Curr Med Chem. 20:1923–1945. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jayashree BS, Nigam S, Pai A, Patel HK,
Reddy ND, Kumar N and Rao CM: Targets in anticancer research - A
review. Indian J Exp Biol. 53:489–507. 2015.PubMed/NCBI
|
|
26
|
Pan MH, Chiou YS, Chen LH and Ho CT:
Breast cancer chemoprevention by dietary natural phenolic
compounds: Specific epigenetic related molecular targets. Mol Nutr
Food Res. 59:21–35. 2015. View Article : Google Scholar
|
|
27
|
Di Leo A, Curigliano G, Diéras V, Malorni
L, Sotiriou C, Swanton C, Thompson A, Tutt A and Piccart M: New
approaches for improving outcomes in breast cancer in Europe.
Breast. 24:321–330. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding A, Zhao W, Shi X, Yao R, Zhou F, Yue
L, Liu S and Qiu W: Impact of NPM, TFF3 and TACC1 on the prognosis
of patients with primary gastric cancer. PLoS One. 8:e821362013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nozawa Y, Van Belzen N, Van der Made AC,
Dinjens WN and Bosman FT: Expression of nucleophosmin/B23 in normal
and neoplastic colorectal mucosa. J Pathol. 178:48–52. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pianta A, Puppin C, Franzoni A, Fabbro D,
Di Loreto C, Bulotta S, Deganuto M, Paron I, Tell G, Puxeddu E, et
al: Nucleophosmin is overexpressed in thyroid tumors. Biochem
Biophys Res Commun. 397:499–504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sari A, Calli A, Altinboga AA, Pehlivan
FS, Gorgel SN, Balci U, Ermete M, Dincel C and Cakalagaoglu F:
Nucleophosmin expression in renal cell carcinoma and oncocytoma.
APMIS. 120:187–194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nicolini A, Carpi A and Tarro G:
Biomolecular markers of breast cancer. Front Biosci. 11:1818–1843.
2006. View Article : Google Scholar
|
|
33
|
Yap TA, Bjerke L, Clarke PA and Workman P:
Drugging PI3K in cancer: Refining targets and therapeutic
strategies. Curr Opin Pharmacol. 23:98–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hsu HH, Cheng LH, Ho TJ, Kuo WW, Lin YM,
Chen MC, Lee NH, Tsai FJ, Tsai KH and Huang CY: Apicidin-resistant
HA22T hepatocellular carcinoma cells massively promote pro-survival
capability via IGF-IR/PI3K/Akt signaling pathway activation. Tumour
Biol. 35:303–313. 2014. View Article : Google Scholar
|
|
35
|
Burris HA III: Overcoming acquired
resistance to anticancer therapy: Focus on the PI3K/AKT/mTOR
pathway. Cancer Chemother Pharmacol. 71:829–842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Slupianek A, Nieborowska-Skorska M, Hoser
G, Morrione A, Majewski M, Xue L, Morris SW, Wasik MA and Skorski
T: Role of phosphatidylinositol 3-kinase-Akt pathway in
nucleophosmin/anaplastic lymphoma kinase-mediated lymphomagenesis.
Cancer Res. 61:2194–2199. 2001.PubMed/NCBI
|
|
37
|
Bai RY, Ouyang T, Miething C, Morris SW,
Peschel C and Duyster J: Nucleophosmin-anaplastic lymphoma kinase
associated with anaplastic large-cell lymphoma activates the
phosphatidylinositol 3-kinase/Akt antiapoptotic signaling pathway.
Blood. 96:4319–4327. 2000.PubMed/NCBI
|
|
38
|
Eitoku M, Sato L, Senda T and Horikoshi M:
Histone chaperones: 30 years from isolation to elucidation of the
mechanisms of nucleosome assembly and disassembly. Cell Mol Life
Sci. 65:414–444. 2008. View Article : Google Scholar
|
|
39
|
Shi YK, Li ZH, Han XQ, Yi JH, Wang ZH, Hou
JL, Feng CR, Fang QH, Wang HH, Zhang PF, et al: The histone
deacetylase inhibitor suberoylanilide hydroxamic acid induces
growth inhibition and enhances taxol-induced cell death in breast
cancer. Cancer Chemother Pharmacol. 66:1131–1140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Balch C, Naegeli K, Nam S, Ballard B,
Hyslop A, Melki C, Reilly E, Hur MW and Nephew KP: A unique histone
deacetylase inhibitor alters microRNA expression and signal
transduction in chemoresistant ovarian cancer cells. Cancer Biol
Ther. 13:681–693. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Venkannagari S, Fiskus W, Peth K, Atadja
P, Hidalgo M, Maitra A and Bhalla KN: Superior efficacy of
co-treatment with dual PI3K/mTOR inhibitor NVP-BEZ235 and
pan-histone deacetylase inhibitor against human pancreatic cancer.
Oncotarget. 3:1416–1427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kang MR, Lee K, Kang JS, Lee CW, Lee KH,
Kim JH, Yang JW, Kim BG, Han G, Kang JS, et al: KBH-A42, a histone
deacetylase inhibitor, inhibits the growth of doxorubicin-resistant
leukemia cells expressing P-glycoprotein. Oncol Rep. 23:801–809.
2010.PubMed/NCBI
|
|
43
|
Edmond V, Brambilla C, Brambilla E,
Gazzeri S and Eymin B: SRSF2 is required for sodium
butyrate-mediated p21(WAF1) induction and premature senescence in
human lung carcinoma cell lines. Cell Cycle. 10:1968–1977. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bártová E, Pacherník J, Harnicarová A,
Kovarík A, Kovaríková M, Hofmanová J, Skalníková M, Kozubek M and
Kozubek S: Nuclear levels and patterns of histone H3 modification
and HP1 proteins after inhibition of histone deacetylases. J Cell
Sci. 118:5035–5046. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Del Bufalo D, Desideri M, De Luca T, Di
Martile M, Gabellini C, Monica V, Busso S, Eramo A, De Maria R,
Milella M, et al: Histone deacetylase inhibition synergistically
enhances pemetrexed cytotoxicity through induction of apoptosis and
autophagy in non-small cell lung cancer. Mol Cancer. 13:2302014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu Q, Cheng Z, Zhu J, Xu W, Peng X, Chen
C, Li W, Wang F, Cao L, Yi X, et al: Suberoylanilide hydroxamic
acid treatment reveals crosstalks among proteome, ubiquitylome and
acetylome in non-small cell lung cancer A549 cell line. Sci Rep.
5:95202015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Atadja PW: HDAC inhibitors and cancer
therapy. Prog Drug Res. 67:175–195. 2011.
|