Introduction
Lung cancer is the most common cause of
cancer-related deaths worldwide (1,2). The
overall prognosis is poor with low 5-year survival due to tumor
metastasis and relapse. The cancer stem cells (CSCs) have been
proposed in many types of malignancies in both leukemia and solid
tumors (3–6). Accumulating evidence supports that
CSCs could be responsible for tumor initiation, progression, and
distant metastasis (7–9). Due to the vital role of CSCs in
cancer prognosis, targeted therapies that can eradicate these cells
may eventually lead to cancer cures (10–12).
Although many cancer stem markers have been identified in solid
tumors including melanoma (13),
breast (14), pancreatic (15) and lung cancer (16), few of them can be used as
therapeutic targets owing to the lack of specificity and functional
relevance.
Monoclonal antibody-based treatment of cancer has
been established as one of the most successful therapeutic
strategies for treating patients with malignant tumors in the last
two decades (17–19). Hybridoma technologies, developed by
Köhler and Milstein, for the first time, allowed the generation of
monoclonal antibodies with high specificity (20). Numerous mAbs such as rituximab,
bevacizumab, trastuzumab and emtansine, approved by the USFDA for
the treatment of both hematologic and solid human cancers, have
significantly improved the clinical outcomes of cancer patients
(21). Antibodies can lead to
direct cell killing through receptor blockade or agonist activity
and induction of apoptosis. Several monoclonal antibodies targeting
CSCs have been proposed (22–24).
However, therapeutic antibodies specific for lung cancer stem-like
cells (LCSLCs), have not yet been reported. Thus, we present here a
novel screening approach to identify functional antibodies
targeting LCSLCs.
In a previous study (25), we obtained the multipotent CSC cell
line T3A-A3, derived from a human primary liver cancer tissue,
which possesses high tumorigenic and metastatic potential and
expresses various stem cell-related markers including Sox2, Lin28,
Nanog, c-Myc and Klf4. Interestingly, T3A-A3 cells can
differentiate into corresponding tumor cells when treated with the
tumor cell/tissue-derived conditioned culture medium. In this
study, we constructed a multipotent CSC monoclonal antibody library
containing 2976 mAbs by immunizing the BLAB/c mice with T3A-A3
cells. As a result of assessing the antibody concentration by ELISA
and fixed cell immunofluorescence, 66 monoclonal antibodies that
could recognize lung cancer cells were obtained. Then, we enriched
the LCSLCs using serum-free suspension culture method and screened
the constructed monoclonal antibody library with the objective of
identifying the functional antibodies targeted to LCSLCs. Two
antibodies were selected from the library that could significantly
inhibit the self-renewal and invasion of LCSLCs. Further study
found that these two antibodies recognized two distinct LCSLCs and
their combination effect were notably better than the individual
effect. Thus, our results suggested that this combination might
become a potential novel therapy for lung cancer.
Materials and methods
Monoclonal antibody library
construction
A library of monoclonal antibodies was constructed
according to the standard protocol described previously (26). Briefly, T3A-A3 cells were harvested
in the logarithmic phase of growth and washed twice with
phosphate-buffered saline (PBS). A part of the cells was suspended
in PBS at 1×107/ml and intraperitoneally injected 0.5 ml
into six BALB/C mice (BFK Bioscience, Beijing, China). The rest of
the cells were fixed with 4% paraformaldehyde for 30 min and washed
twice with PBS. Then the cells were suspended in PBS at
1×107/ml and subcutaneously injected 0.5 ml into the
above-mentioned mice. Two weeks later, the same amount of fixed
cells was subcutaneously injected into the mice for booster
immunization per week. Following eighth booster doses, the serum
antibody concentration of the mice was assessed by ELISA.
Subsequently, the splenic cells of the mouse with the highest serum
titer was used to fuse with SP2/0 cells, which were maintained in
HAT medium supplemented with 2.5% methylcellulose (Sigma, St.
Louis, MO, USA) in an atmosphere of 5% CO2 at 37°C.
After culturing for 8–10 days, the monoclonal library containing
2976 clones was established.
The hybridoma cells were maintained in the complete
DMEM growth medium (Invitrogen, Carlsbad, CA, USA). The hybridoma
supernatant collection, antibody production, and purification were
performed using standard protocols. The isotype of the antibody was
determined by commercial isotyping kit (SouthernBiotech,
Birmingham, AL, USA).
Cell culture
Human non-small cell lung carcinoma cell lines
SPCA-1 and A549 were purchased from Chinese Academy of Sciences
Cell Bank (Shanghai, China). Cells were cultured in RPMI-1640 media
containing 10% fetal bovine serum (Gibco, Grand Island, NY, USA),
1% glutamine, and 1% penicillin-streptomycin sulfate (Invitrogen).
All the cell lines were grown at 37°C in 5% CO2
incubator. T3A-A3 cell line was a gift from Professor J. Lou of
Institute of Clinical Medical Sciences, China-Japan Friendship
Hospital. T3A-A3 cell line was maintained in DMEM-F12 supplemented
with 1% FBS, B27 (1:50; Invitrogen), 20 ng/ml human epidermal
growth factor (EGF; Invitrogen), 10 ng/ml basic fibroblast growth
factor (bFGF; Invitrogen), 2 mg/ml heparin (Sigma), 1% glutamine,
and 1% penicillin-streptomycin sulfate (Invitrogen), 5 mg/ml
insulin (Sigma) and 0.5 mg/ml hydrocortisone (Sigma).
Sphere-forming culture and self-renewal
assay
To obtain sphere cultures, cells were plated at a
density of 3×104 cells/T75 ultra-low flask (Corning, NY,
USA) containing 15 ml serum-free medium (SFM) DMEM/F12,
supplemented with B27, 20 ng/ml EGF, 20 ng/ml bFGF, 10 ng/ml LIF
(Invitrogen), 1% glutamine, and 1% penicillin-streptomycin sulfate.
After being cultured for 7 days, the lung cancer spheres were
collected, dissociated into single cell suspension by trypsin-EDTA
solution and cultured to allow the regeneration of spheres.
Third-generation spheres were used for all subsequent
experiments.
To investigate the sphere formation and self-renewal
capacity, the cells were plated in 24-well ultralow attachment
plates (Corning) at a density of 500 cells/well in the SFM with
0.8% methylcellulose (Sigma). The cultures were incubated at 37°C
in 5% CO2. After 11 days, the spheroid colonies were
counted under a microscope. The cloning efficiency was calculated
as the percentage of the original number of seeded cells forming
colonies of >20 cells.
Flow cytometry analysis and
fluorescence-activated cell sorting (FACs)
For antibody library screening, SPCA-1 or A549 cells
(1×106) were incubated with the monoclonal antibody
supernatants followed by Alexa 488 goat anti-mouse (IgG+IgM)
(Jackson, NY, USA). In order to explore if mAbs 12C7 and 9B8
recognized the same cell group, we independently conjugated their
purified antibodies to Alexa 488 (Santa Cruz, CA, USA) and
allophycocyanin (APC) according to the manufacturer's instructions.
Subsequently, the reaction was incubated with the cells in the same
tube for flow cytometry analysis using an LSR II flow cytometer (BD
Bioscience, CA, USA). For fluorescence-activated cell sorting
(FACs), cells were labeled under sterilized conditions and sorted.
The data were analyzed by FlowJo software version 5.7.1 (Tree
star).
Transwell invasion assay
For invasion assay, 2×104 cells were
plated in the top chamber of the Matrigel-coated membrane (24-well
insert; pore size, 8 µm; Corning). Each upper chamber was
precoated with Matrigel (BD Bioscience) according to the
manufacturer's protocol before the invasion assay. Cells were
plated in the medium without serum or growth factors, and the
medium containing 10% FBS was used as a chemoattractant in the
lower chamber. The cells were incubated for 24 h, and those that
did not invade through the pores were removed by a cotton swab. The
cells on the lower surface of the membrane were fixed with methanol
and stained with crystal violet. The number of cells invading
through the membrane was counted under a light microscope (three
random fields/well).
Tumorigenicity assay and antibody
treatment of tumor-bearing mice
For tumorigenicity assay, SPCA-1 or A549 cells were
injected subcutaneously into the back of 4-week-old BALB/C-nude
mice (BFK Bioscience) at a dose of 1×106,
1×105, 1×104 and 5×103 cells,
respectively. The tumor growth was monitored every 7 days after the
inoculation.
For antibody treatment of the tumor-bearing mice,
4-week-old BALB/C-nude mice were randomly divided into nine groups
of five animals each. The weights of mice in each treatment cohort
were similar. SPCA-1 sphere cells (5×105) were injected
subcutaneously into the armpit of the right forelimb of the mice.
Treatment began 3 days after the injection of the tumor cells. The
nine groups received intraperitoneal injections of i) PBS, ii) 40
mg/kg of mouse IgG for negative control, iii) 40 mg/kg of mAb 12C7
for high dose treatment, iv) 10 mg/kg of mAb 12C7 for medium dose
treatment, v) 2.5 mg/kg of mAb 12C7 for low dose treatment, vi) 40
mg/kg of mAb 9B8 for high dose treatment, vii) 10 mg/kg of mAb 9B8
for medium dose treatment, viii) 2.5 mg/kg of mAb 9B8 for low dose
treatment, ix) combination of mAb 12C7 and 9B8 with 20 mg/kg of
each. The respective dosages were administered twice a week for 4
weeks. The animals were sacrificed, and tumors were weighed 33 days
after incubation.
All animal experiments were approved by The Animal
Care and Use Committee of Cancer Hospital, Chinese Academy of
Medical Science, and Peking Union Medical College (Beijing,
China).
Real-time fluorescence quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using Aurum™ total RNA mini
kit (Bio-Rad, CA, USA) and reverse transcribed into cDNA using
TaqMan reverse transcription reagents (Bio-Rad). RT-PCR was
performed with SsoFast™ EvaGreen Supermix with Low ROX (Bio-Rad).
The quantitative PCR reaction was carried out in a 7500 Fast
Real-Time PCR system (Applied Biosystems, Foster City, CA, USA).
The reaction conditions were as follows: 95°C for 30 sec and 40
cycles at 95°C for 10 sec and 60°C for 15 sec. The gene expression
was quantified using the comparative Ct method. The primer
sequences utilized are listed in Table
I. GAPDH expression was used for normalization.
 | Table IPrimers for the real-time PCR
analysis. |
Table I
Primers for the real-time PCR
analysis.
| Gene | Direction | Primer sequences
(5′–3′) |
|---|
| Sox2 | F |
AACCAAGACGCTCATGAAGAAG |
| R |
CTGCGAGTAGGACATGCTGTAG |
| Oct4 | F |
GACAACAATGAAAATCTTCAGGAGA |
| R |
TTCTGGCGCCGGTTACAGAACCA |
| Nanog | F |
GTCCCAAAGGCAAACAACCC |
| R |
GCTGGGTGGAAGAGAACACA |
| GAPDH | F |
TGCACCACCAACTGCTTAGC |
| R |
GGCATGGACTGTGGTCATGAG |
Western blot analysis
For western blot analysis, the whole proteins
extract was prepared from lung cancer cell lines SPC-A1 and A549 by
RIPA buffer (Beyotime, Beijing, China) supplemented with a cocktail
of protease inhibitors (Sigma). The proteins were resolved by 12%
SDS-PAGE, transferred to PVDF membranes, and then probed with
primary antibodies, mAb 12C7 (10 µg/ml) and 9B8 (10
µg/ml). The immunoreaction was visualized by super ECL
detection reagent (Life Technologies, Carlsbad, CA, USA) following
incubation with HRP-conjugated secondary antibodies.
Immunohistochemistry with tissue
microarray
Tissue microarrays were obtained from US Biomax for
IHCs. The tissue microarray contains 160 NSCLC specimens and 32
non-tumor lung tissues. The 160 NSCLC samples consist of 80
squamous carcinoma tissues and 80 adenocarcinomas, whereas the 32
non-tumor lung tissues consist of 19 normal lung tissues and 13
cancer adjacent normal tissues. One tissue was unknown in sex, age,
and TNM, and 15 tissues were unknown in grade. The tissue
microarrays were determined using the UltraSensitive™ S-P
(mouse/rabbit) IHC kit (Maxim, Fuzhou, China) according to the
manufacturer's guidelines. The monoclonal antibodies 12C7 or 9B8
were incubated at 10 µg/ml. The expression levels of the
proteins were scored by malignant/epithelial cells staining
intensity and the percentage of immunoreactive cells. Tissues with
no staining were rated as 0, with faint staining or moderate to
strong staining in 25% of the cells as 1, with moderate staining or
strong staining in 25–50% of the cells as 2, and with strong
staining in 50% of the cells as 3. Lung cancer tissues that
registered levels 0 and 1 were defined as negative for expression,
whereas samples at levels 2 or 3 were defined as positive.
Statistical analysis
Statistical analysis was performed with SPSS 13.0
software (Chicago, IL, USA). All numerical data were expressed as
the average of the values obtained, and the standard deviation (SD)
calculated. Data were analyzed using the two-tailed Student's
t-test or λ2 analysis unless otherwise specified. Data
were considered significant at p<0.05 and p<0.001.
Results
Lung cancer stem-like cells are enriched
in SPCA-1 and A549 cell lines via sphere-forming culture
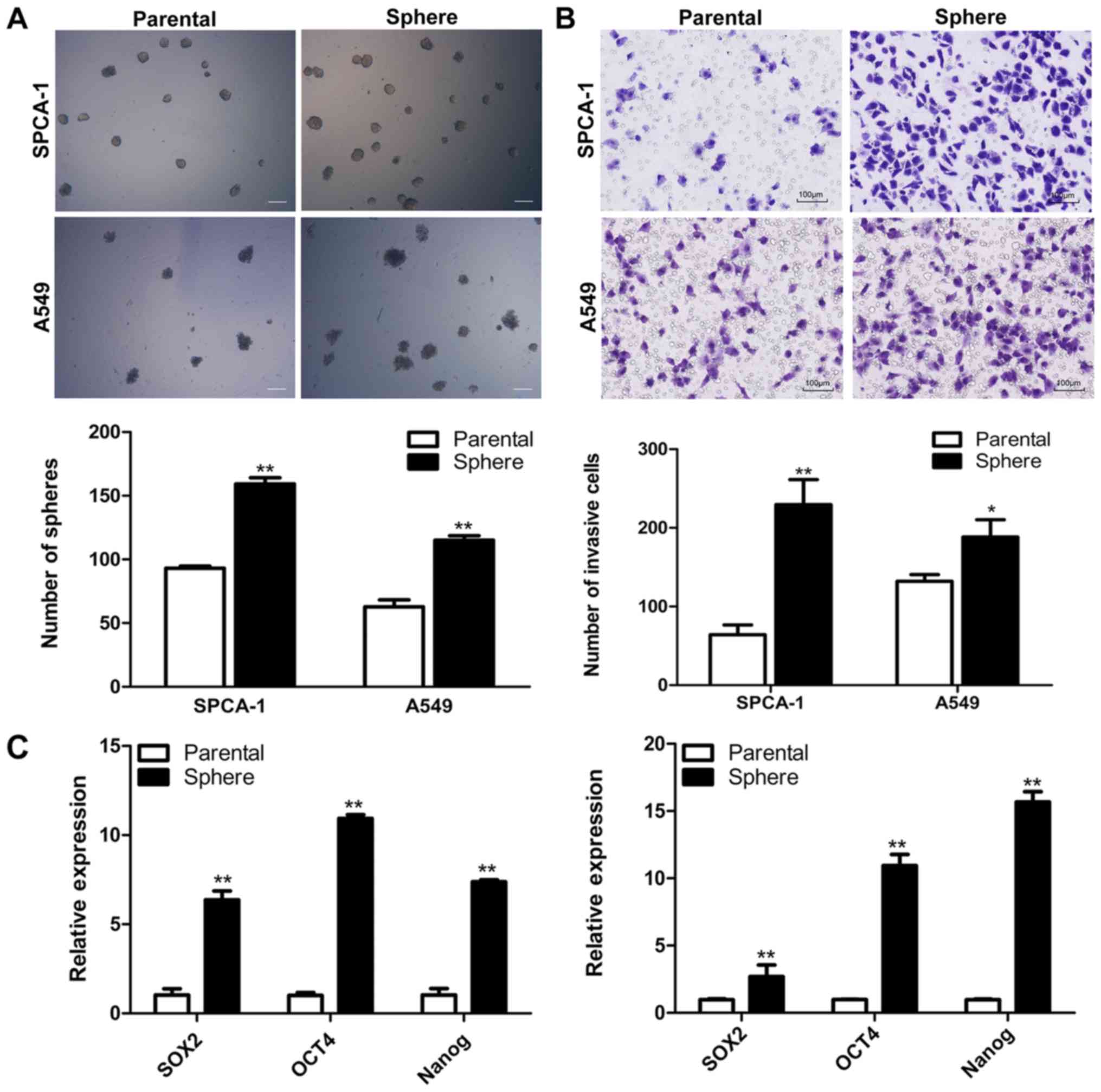
We performed the sphere-forming culture to enrich
LCSCs. Both SPCA-1 and A549 cells could form non-adherent spheres
when cultured in SFM. The sphere cells could be serially passaged
and the third-generation spheres were used for all subsequent
experiment. The self-renewal assay showed that the number of
colonies formed by sphere cells was superior to those formed by
parental cells in both SPCA-1 and A549 cell lines (Fig. 1A). Several studies demonstrated
that LCSLCs might be responsible for cancer metastasis (27–29).
Thus, we performed Matrigel Transwell assay to compare the invasive
potentials of sphere cells with their parental cells. The results
showed that more sphere cells passed through the Matrigel than
their parental cells (Fig. 1B).
RT-qPCR was employed to analyze the stem cell-related gene
expression profiles of parental and sphere cells in both cell
lines. Results indicated that the mRNA level of the stem cell
markers Sox2, Nanog and Oct4 increased in the sphere
cells (Fig. 1C). Then, we
established a xenograft model to assess the tumorigenic capacity of
the sphere cells from SPCA-1 and A549 cell lines as compared to
their parental cells. As shown in Table II, the sphere cells possess much
stronger tumorigenic potentials than their parental cells, since
1×104 sphere cells can initiate tumor formation both in
SPCA-1 and A549 cell lines while there were no nodules found in the
parental cell group with the same cell number inoculated (Table II).
 | Table IITumorigenicity assay of parental and
sphere cells from SPCA-1 and A549 cell lines. |
Table II
Tumorigenicity assay of parental and
sphere cells from SPCA-1 and A549 cell lines.
| Cell type |
1×106 |
1×105 |
1×104 |
5×103 |
|---|
| SPCA-1
parental | 5/5 | 2/5 | 0/5 | 0/5 |
| SPCA-1 sphere | 5/5 | 5/5 | 3/5 | 1/5 |
| A549 parental | 3/5 | 0/5 | 0/5 | 0/5 |
| A549 sphere | 5/5 | 3/5 | 1/5 | 0/5 |
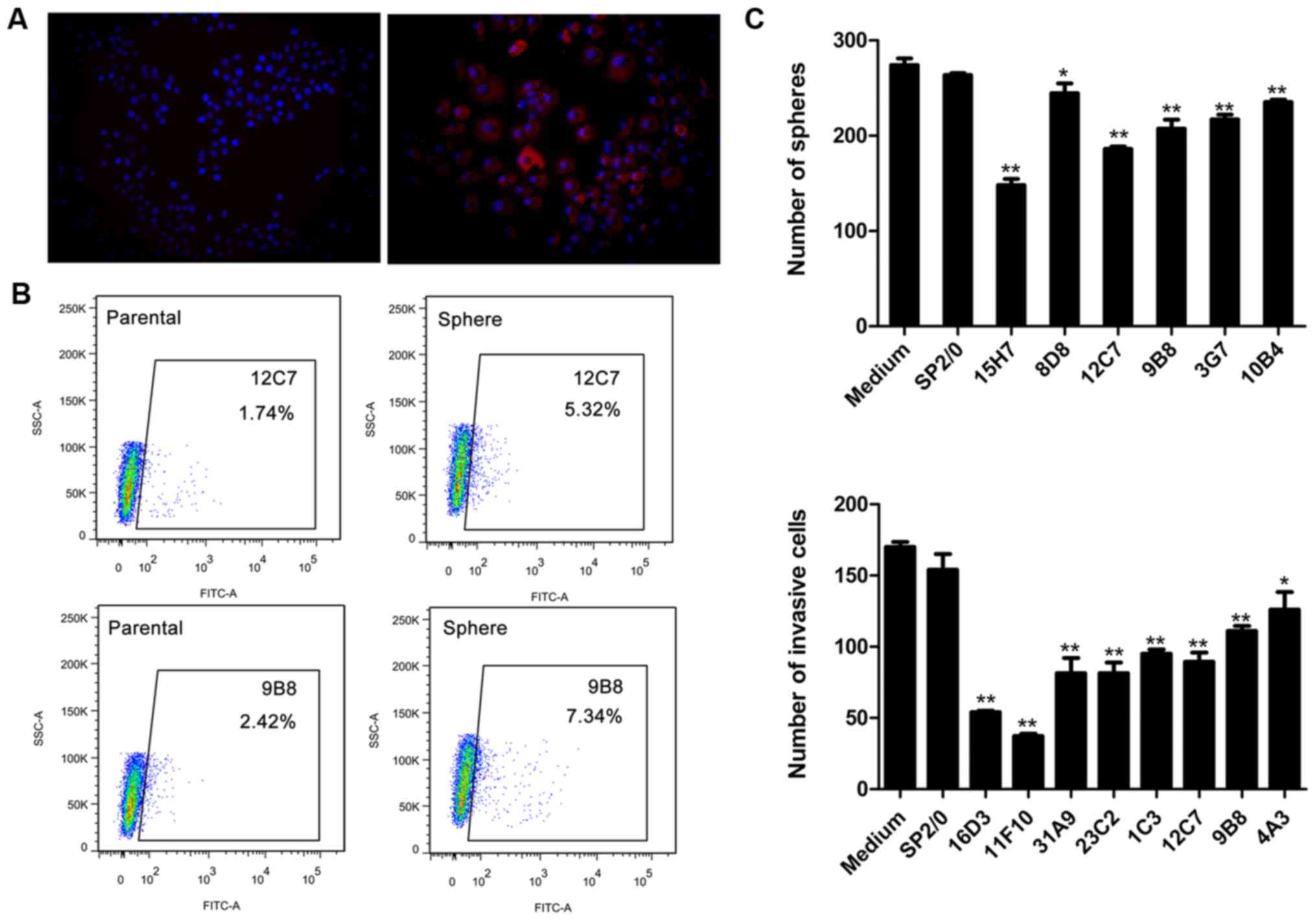
Screening of functional mAbs targeting
LCSCs from multipotent antibody library
We constructed a multipotent CSC monoclonal antibody
library that contained 2,976 mAbs by immunizing BLAB/c mice with
T3A-A3 cells. The hybridoma supernatants were collected using
standard methods, and 1,196 mAbs were reserved with the antibody
concentration assessed >10 µg/ml by ELISA. From the 1,196
mAbs, a total of 66 mAbs showed reactivity to both SPCA-1 and A549
cell lines by fixed cell immunofluorescence (Fig. 2A). Thus, we further screened the
library aiming to identify the monoclonal antibodies that might
bind specifically to LCSLCs and inhibit the self-renewal and
invasion. Firstly, viable cell flow cytometry analysis assay was
performed to detect the expression of antibody targeted antigens in
parental and sphere cells of SPCA-1 and A549 cell lines. As a
result, 33 mAbs targeted antigens could be enriched in sphere cells
compared with the parental cells in both SPCA-1 and A549 cell line
(Fig. 2B). Next, we screened the
above 33 mAbs for their sphere-forming inhibition ability. Six mAbs
showed significant inhibition ability on SPCA-1 and A549 sphere
cells (Fig. 2C). Subsequently, the
invasion inhibition assay revealed that 8 mAbs among 33 mAbs can
suppress the invasion of SPCA-1 and A549 sphere cells (Fig. 2C). Two mAbs, 12C7 and 9B8, showed
both sphere-forming inhibition and invasion inhibition abilities.
Therefore, the two mAbs were chosen to be studied further.
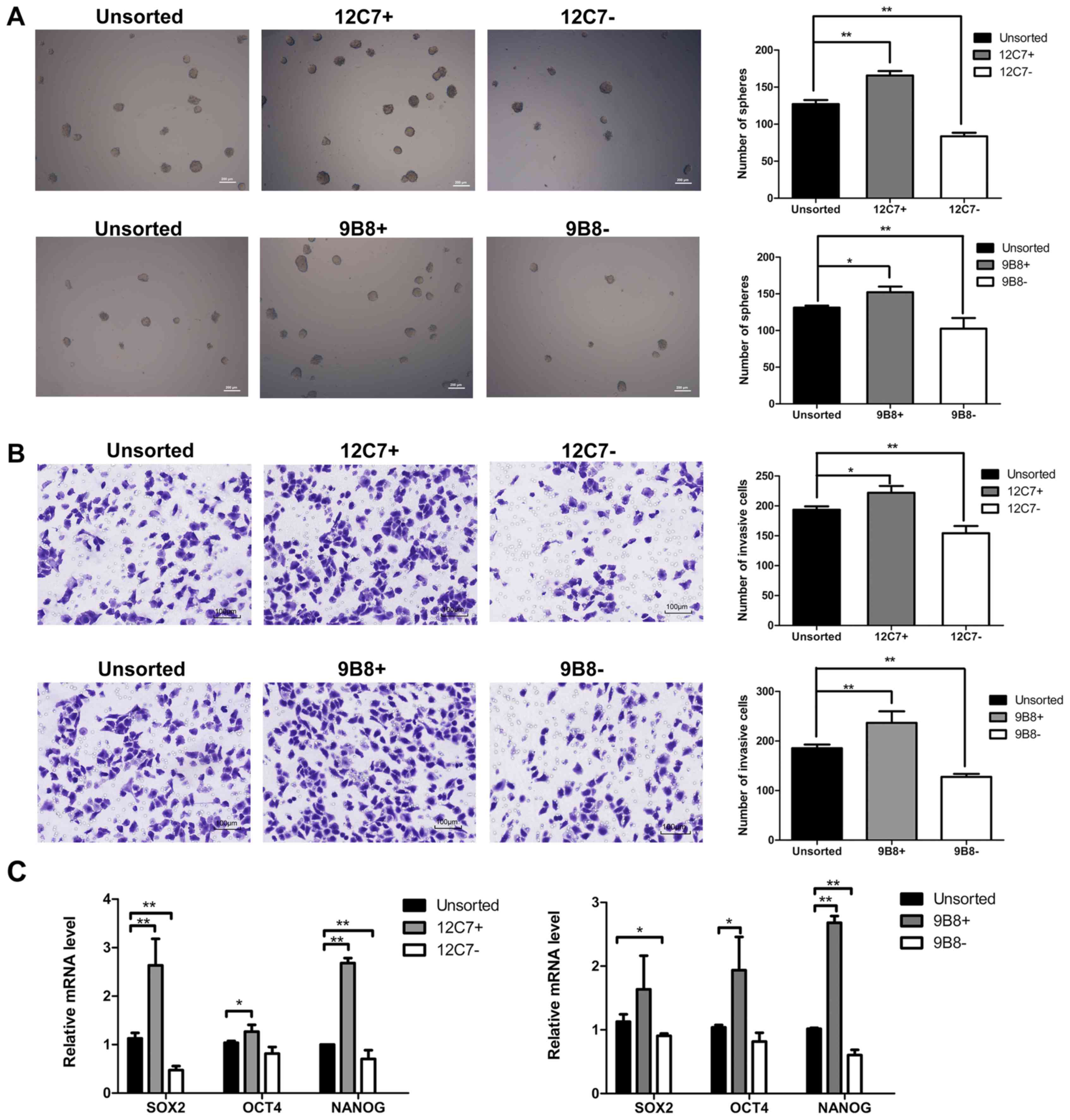
Isolation and characterization of
12C7-positive cells and 9B8-positive cells
To characterize the 12C7-positive cells and
9B8-positive cells, FACS sorting was performed in both SPCA-1 and
A549 sphere cells. We evaluated the self-renewal potential, and the
results showed that the number of spheroid colonies formed by
positive cells was superior to those formed by negative cells and
unsorted cells (Fig. 3A). Then, we
investigated whether the positive cells possess higher invasion
capacity. As shown in the results, the positive cells were
considerably more invasive than the negative and unsorted cells
(Fig. 3B). We further explored the
expression of several 'stemness'-associated genes, including
Sox2, Nanog and Oct4. RT-qPCR showed that both
12C7-positive and 9B8-positive cells expressed higher mRNA levels
of stemness-related markers than the negative and unsorted cells
(Fig. 3C).
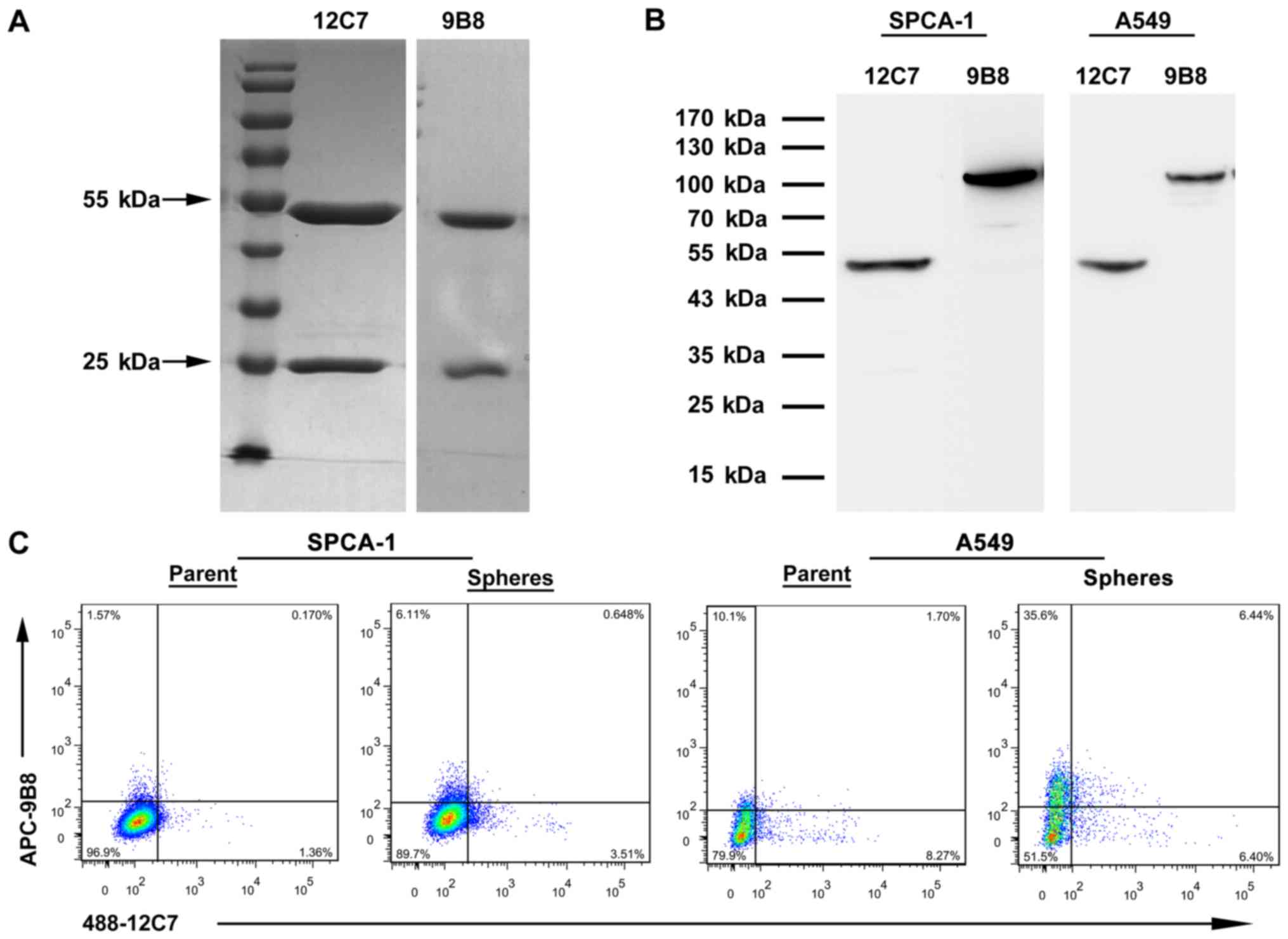
MAb 12C7 and 9B8 identified two distinct
subpopulations of LCSCs
The isotypes of antibody 12C7 and 9B8 were both IgG1
(data not shown) determined by commercial isotyping kit. The two
antibodies were purified by protein g column and the purity
achieved was >90% (Fig. 4A). We
extracted proteins from the lung cancer cell lines SPCA-1 and A549
for western blot analysis. The molecular weight of antigen is 47
kDa recognized by 12C7 and 100 kDa by 9B8 (Fig. 4B). The overlap of 12C7+
cells with 9B8+ cells was estimated by flow cytometry.
mAb 12C7 and 9B8 double-positive cells were only detected in
extremely rare cells which comprised 0.17% of the total cells in
SPCA-1 parent cells and 0.65% in spheres (Fig. 4C). Furthermore, the rare percentage
of double-positive cells was also determined in A549 parent and
sphere cells. The results demonstrated that mAb 12C7 and 9B8 might
identify two distinct subpopulations of LCSCs (Fig. 4C).
Effects of mAbs 12C7 and 9B8 on the
LCSLCs in vitro and in vivo
To investigate the effect of the antibody on
self-renewal and invasion capacity in vitro, we incubated
A549 and SPCA-1 sphere cells with three different concentrations of
mAbs 12C7 and 9B8 for 2 h at 37°C, following which, the
sphere-forming assay and Transwell invasion assay were performed as
previously described. For the sphere forming assay, the same
concentration of fresh mAbs was added every two days. The results
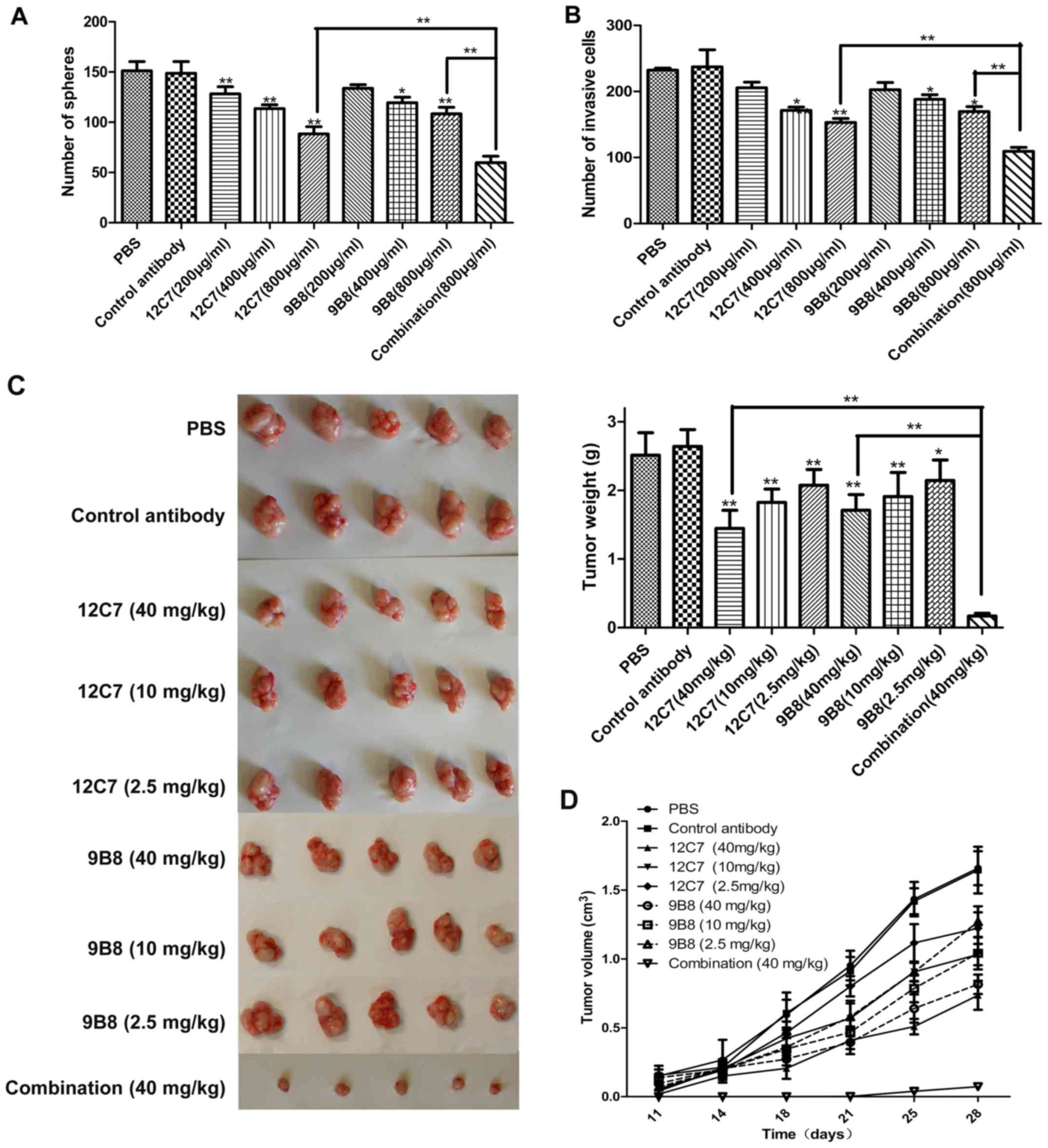
showed that mAbs 12C7 and 9B8 can both suppress the self-renewal
and invasion of sphere cells in a dose-dependent manner (Fig. 5A and B). Then nude mice bearing
SPCA-1 sphere cell xenografts were treated by 12C7 or 9B8 mAbs. The
mean tumor volumes after treatment with mAb 12C7 or 9B8 reduced in
a dose-dependent manner. For mAb 12C7, the tumor weight inhibition
rates for high (40 mg/kg), moderate (10 mg/kg), and low (0.25
mg/kg) dose were 45.27, 30.89 and 21.42%, respectively, whereas for
mAb 9B8, the tumor weight inhibition rates were 35.2, 27.71 and
18.77%, respectively (Fig. 5C and
D). These results indicated that both 12C7 and 9B8 are
functional mAbs, which might be used as potential therapeutic
antibodies for lung cancer treatment.
Combination effects of mAbs 12C7 and 9B8
on the LCSLCs in vitro and in vivo
As mAb 12C7 and 9B8 might identify two distinct
subpopulations of LCSLCs, we explored the combination effects of
mAbs 12C7 and 9B8 on the LCSLCs in vitro and in vivo.
It was found that the group of combination antibodies had a notably
improved effect on suppressing the self-renewal (Fig. 5A) and invasion (Fig. 5B) of LCSLCs than the group of
individual antibodies in vitro. Interestingly, in the in
vivo experiment, the combination group inhibited the tumor
growth at the highest rate of 93%, which is significantly superior
to the same concentration group of the individual antibody
(Fig. 5C and D). This result
strongly suggested that the combination antibody treatment may be
used as a potential novel method for lung cancer therapy.
Clinical significance of antigens
recognized by mAb 12C7 and 9B8 for NSCLC
The antigen expression levels of mAb 12C7 and 9B8 in
human lung cancer tissue were determined by IHC in a tissue
microarray. The results showed that the antigen recognized by mAb
12C7 account for a high proportion in 135 (84.4%) lung cancer
tissues and a low proportion of 7 (21.9%) non-tumor lung tissues
(Table III). Moreover, the
antigen recognized by mAb 9B8 was highly expressed in 132/160
(82.5%) lung cancer tissues and lowly expressed in 8/32 (25%)
normal lung tissues (Table III).
Furthermore, the correlations between mAbs targeted antigens
expression and clinicopathological parameters were evaluated. The
results demonstrated that there was a significant correlation
between the expression of the mAb 12C7 targeted protein and the
pathological grade, indicating a high level of expression in
higher-grade tumors (Table IV).
Moreover, the positive expression of mAb 9B8 targeted protein was
significantly associated with the tumor pathology, which indicated
that the positive expression of mAb 9B8 readily occurred in
adenocarcinoma. The positive expression of mAb 9B8 targeted protein
was also significantly associated with lymph node involvement.
 | Table IIIUpregulation of mAb 12C7 or mAb 9B8
targeted protein in NSCLC specimens compared to non-cancerous lung
specimens. |
Table III
Upregulation of mAb 12C7 or mAb 9B8
targeted protein in NSCLC specimens compared to non-cancerous lung
specimens.
| Group | Cases (n) | 12C7
| 9B8
|
|---|
| Positive (%) | Negative (%) | P-value | Positive (%) | Negative (%) | P-value |
|---|
| NSCLC | 160 | 84.4 | 15.6 | <0.01 | 82.5 | 17.5 | <0.01 |
| Non-tumor | 32 | 21.9 | 78.1 | | 25 | 75 | |
 | Table IVCorrelation between
clinicopathological characteristics with mAb 12C7 or 9B8 targeted
proteins. |
Table IV
Correlation between
clinicopathological characteristics with mAb 12C7 or 9B8 targeted
proteins.
|
Characteristics | Cases (n) | 12C7
| 9B8
|
|---|
| Positive N (%) | λ2 | P-value | Positive N (%) | λ2 | P-value |
|---|
| Age | | | 0.002 | 0.968 | | 1.098 | 0.295 |
| <50 | 44 | 37 (84.0) | | | 34 (77.3) | | |
| ≥50 | 115 | 97 (84.3) | | | 97 (84.3) | | |
| Gender | | | 0.139 | 0.709 | | 0.04 | 0.841 |
| Male | 116 | 97 (83.6) | | | 96 (82.8) | | |
| Female | 43 | 37 (86.0) | | | 35 (81.4) | | |
| Pathology | | | 0.427 | 0.514 | | 6.234 | 0.013 |
| Sequamous | 80 | 66 (82.5) | | | 60 (75) | | |
|
Adenocarcinoma | 80 | 69 (86.25) | | | 72 ((90) | | |
| Grade | | | 13.22 | 0.001 | | 4.92 | 0.085 |
| 1 | 22 | 14 (63.6) | | | 22 (100) | | |
| 2 | 82 | 73 (83.0) | | | 67 (81.7) | | |
| 3 | 41 | 39 (95.1) | | | 33 (80.5) | | |
| Depth of
invasion | | | 0.679 | 0.712 | | 2.87 | 0.239 |
| TX+T1 | 22 | 19 (86.4) | | | 18 (81.8) | | |
| T2 | 108 | 92 (85.2) | | | 86 (76.9) | | |
| T3+T4 | 29 | 23 (79.3) | | | 27 (93.1) | | |
| Lymph node
involvement | | | 0.064 | 0.801 | | 8.29 | 0.004 |
| N0 | 80 | 68 (85.0) | | | 59 (73.8) | | |
| N1 | 79 | 66 (83.5) | | | 72 (91.1) | | |
| Metastasis | | | 0.378 | 0.539 | | 0.43 | 0.511 |
| M0 | 157 | 132 (84.1) | | | 129 (82.2) | | |
| M1 | 2 | 2 (100) | | | 2 (100) | | |
Discussion
In this study, we identified two mAbs, 12C7 and 9B8,
that can specifically bind to the LCSLCs and inhibit the biological
characteristics involving self-renewal and invasiveness.
Interestingly, we also found that these two antibodies separately
targeted the two distinct LCSLCs, and the combination therapeutic
effect was significantly superior to the independent effects both
in vitro and in vivo.
Monoclonal antibody-based cancer therapy is
considered to be more efficient and less toxic than the
conventional therapy. In the last two decades, the mAbs drug is
becoming a vital therapeutic alternative for certain common types
of cancers, including lymphoma, breast, colon, and lung cancers.
The CSC model suggests that tumors are composed of a heterogeneous
group of cells, out of which a small subset of cells, called CSCs,
could be responsible for the tumor initiation and recurrence. Due
to the functional relevance, CSCs can be natural candidates for a
targeted therapy with mAbs. For example, anti-ABCG2 mAbs that
target CD138−CD34− MM cancer stem-like cells
in combination with PTX iron oxide NPs (PTX-NPs), can significantly
suppress the proliferation and invasion of MM cancer cells both
in vitro and in vivo (22).
Multiple subpopulations of LCSLCs have been reported
in lung cancer tissues, such as SP (30), CD133 (16), CD44 (31), and ALDH (32). However, these markers also express
on normal stem cells, and they are mostly uncorrelated with the
stem cell functions. To identify the novel potential therapeutic
antibodies targeting LCSLCs, in this study, we present a screening
approach involving the isolation and selection of mAbs for their
ability to inhibit LCSLCs self-renewal and invasion. We have
constructed a large capacity hybridoma monoclonal antibody library
containing 2976 monoclonals from BLAB/c mice immunized with a
multipotent CSC cell line T3A-A3. This resulted in the isolation of
66 mAbs that could react with lung cancer cells. Through FACs and
function inhibition assay, we obtained 2 mAbs, 12C7 and 9B8. The
stem cell properties of 12C7 positive cells and 9B8 positive cells
were validated by functional experiments and higher stem-cell
related gene expression. Furthermore, they could inhibit
self-renewal and invasion of LCSCs in a dose-dependent manner.
We further analyzed the relationship of the two mAbs
target cells. Western blot analysis confirmed that both the mAbs
recognized different weights of antigens, and FACs proved that
these two antibodies recognized rare double-positive cells. Thus,
it can be concluded that these two antibodies identified two
distinctive subpopulations of LCSCs. According to the CSC
hypothesis, theoretically, tumors can be cured as long as CSCs are
eliminated completely. Moreover, it has been found that the
treatment effect for a particular subpopulation of CSCs is
extremely limited in the study of cancer stem cells. Herein, we
found that the combination therapeutic effect of these two mAbs was
significantly superior to the individual effects both in
vitro and in vivo. Moreover, both 12C7 targeted antigen
and 9B8 targeted antigen were highly expressed in tumor tissues
according to our IHC results in tissue microarray assays, which can
ensure the curative effect once the antibodies were applied in
clinical practice. Our results suggested that the application of
the combination therapy against different subpopulations of cancer
stem cells may significantly improve the prognosis of lung
cancer.
Despite the remarkable development of mAb treatment,
the need for novel therapeutic antibodies persists. The key
challenge is the identification of novel targets that are suitable
for therapeutic antibodies. Here, we report a screening approach
for functional antibodies that target LCSCs from the constructed
multipotent antibody library. However, we should perform a
spectrometric analysis of LC-MS/MS to identify the targets of the
selected antibodies and investigate the underlying mechanism in the
future.
In conclusion, we successfully identified two
functional antibodies that specifically target LCSCs. They can
inhibit cancer self-renewal and invasion both in vitro and
in vivo. Furthermore, we confirmed that these two antibodies
represent two distinct subpopulations of LCSCs, and a combination
of them can significantly improve the therapeutic efficiency,
implying a novel therapeutic approach for clinical strategy.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172033), and
National High-tech R&D Program of China for Young Scholars
(grant no. 2014AA020537).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
3
|
Charafe-Jauffret E, Ginestier C, Iovino F,
Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F,
Dutcher J, et al: Breast cancer cell lines contain functional
cancer stem cells with metastatic capacity and a distinct molecular
signature. Cancer Res. 69:1302–1313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature. 367:645–648. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar
|
|
6
|
Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai
P, Chu PW, Lam CT, Poon RT and Fan ST: Significance of
CD90+ cancer stem cells in human liver cancer. Cancer
Cell. 13:153–166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vermeulen L, Sprick MR, Kemper K, Stassi G
and Medema JP: Cancer stem cells - old concepts, new insights. Cell
Death Differ. 15:947–958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beck B and Blanpain C: Unravelling cancer
stem cell potential. Nat Rev Cancer. 13:727–738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Medema JP: Cancer stem cells: The
challenges ahead. Nat Cell Biol. 15:338–344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takebe N, Harris PJ, Warren RQ and Ivy SP:
Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog
pathways. Nat Rev Clin oncol. 8:97–106. 2011. View Article : Google Scholar
|
|
11
|
Hoey T, Yen WC, Axelrod F, Basi J,
Donigian L, Dylla S, Fitch-Bruhns M, Lazetic S, Park IK, Sato A, et
al: DLL4 blockade inhibits tumor growth and reduces
tumor-initiating cell frequency. Cell Stem Cell. 5:168–177. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okamoto OK and Perez JF: Targeting cancer
stem cells with monoclonal antibodies: A new perspective in cancer
therapy and diagnosis. Expert Rev Mol Diagn. 8:387–393. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sharma BK, Manglik V, O'Connell M,
Weeraratna A, McCarron EC, Broussard JN, Divito KA,
Simbulan-Rosenthal CM, Rosenthal DS and Zapas JL: Clonal dominance
of CD133+ subset population as risk factor in tumor
progression and disease recurrence of human cutaneous melanoma. Int
J Oncol. 41:1570–1576. 2012.PubMed/NCBI
|
|
14
|
Wright MH, Calcagno AM, Salcido CD,
Carlson MD, Ambudkar SV and Varticovski L: Brca1 breast tumors
contain distinct CD44+/CD24− and
CD133+ cells with cancer stem cell characteristics.
Breast Cancer Res. 10:R102008. View
Article : Google Scholar
|
|
15
|
Hermann PC, Huber SL, Herrler T, Aicher A,
Ellwart JW, Guba M, Bruns CJ and Heeschen C: Distinct populations
of cancer stem cells determine tumor growth and metastatic activity
in human pancreatic cancer. Cell Stem Cell. 1:313–323. 2007.
View Article : Google Scholar
|
|
16
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C and De
Maria R: Identification and expansion of the tumorigenic lung
cancer stem cell population. Cell Death Differ. 15:504–514. 2008.
View Article : Google Scholar
|
|
17
|
Scott AM, Allison JP and Wolchok JD:
Monoclonal antibodies in cancer therapy. Cancer Immun.
12:142012.PubMed/NCBI
|
|
18
|
Coulson A, Levy A and Gossell-Williams M:
Monoclonal antibodies in cancer therapy: Mechanisms, successes and
limitations. West Indian Med J. 63:650–654. 2014.
|
|
19
|
Davis TA, Grillo-López AJ, White CA,
McLaughlin P, Czuczman MS, Link BK, Maloney DG, Weaver RL,
Rosenberg J and Levy R: Rituximab anti-CD20 monoclonal antibody
therapy in non-Hodgkin's lymphoma: Safety and efficacy of
re-treatment. J Clin oncol. 18:3135–3143. 2000.PubMed/NCBI
|
|
20
|
Köhler G and Milstein C: Continuous
cultures of fused cells secreting antibody of predefined
specificity. Nature. 256:495–497. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boyiadzis M and Foon KA: Approved
monoclonal antibodies for cancer therapy. Expert Opin Biol Ther.
8:1151–1158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang C, Xiong F, Wang J, Dou J, Chen J,
Chen D, Zhang Y, Luo S and Gu N: Anti-ABCG2 monoclonal antibody in
combination with paclitaxel nanoparticles against cancer stem-like
cell activity in multiple myeloma. Nanomedicine (Lond). 9:45–60.
2014. View Article : Google Scholar
|
|
23
|
Morris MJ, Eisenberger MA, Pili R,
Denmeade SR, Rathkopf D, Slovin SF, Farrelly J, Chudow JJ, Vincent
M, Scher HI, et al: A phase I/IIA study of AGS-PSCA for
castration-resistant prostate cancer. Ann Oncol. 23:2714–2719.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sharkey RM, Hajjar G, Yeldell D, Brenner
A, Burton J, Rubin A and Goldenberg DM: A phase I trial combining
high-dose 90Y-labeled humanized anti-CEA monoclonal antibody with
doxorubicin and peripheral blood stem cell rescue in advanced
medullary thyroid cancer. J Nucl Med. 46:620–633. 2005.PubMed/NCBI
|
|
25
|
Liu H, Zhang W, Jia Y, Yu Q, Grau GE, Peng
L, Ran Y, Yang Z, Deng H and Lou J: Single-cell clones of liver
cancer stem cells have the potential of differentiating into
different types of tumor cells. Cell Death Dis. 4:e8572013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun L, Chen L, Sun L, Pan J, Yu L, Han L,
Yang Z, Luo Y and Ran Y: Functional screen for secreted proteins by
monoclonal antibody library and identification of Mac-2 Binding
protein (Mac-2BP) as a potential therapeutic target and biomarker
for lung cancer. Mol Cell Proteomics. 12:395–406. 2013. View Article : Google Scholar :
|
|
27
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pang R, Law WL, Chu AC, Poon JT, Lam CS,
Chow AK, Ng L, Cheung LW, Lan XR, Lan HY, et al: A subpopulation of
CD26+ cancer stem cells with metastatic capacity in
human colorectal cancer. Cell Stem Cell. 6:603–615. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fan F, Samuel S, Evans KW, Lu J, Xia L,
Zhou Y, Sceusi E, Tozzi F, Ye XC, Mani SA, et al: overexpression of
snail induces epithelial-mesenchymal transition and a cancer stem
cell-like phenotype in human colorectal cancer cells. Cancer Med.
1:5–16. 2012. View
Article : Google Scholar
|
|
30
|
Ho MM, Ng AV, Lam S and Hung JY: Side
population in human lung cancer cell lines and tumors is enriched
with stem-like cancer cells. Cancer Res. 67:4827–4833. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leung EL, Fiscus RR, Tung JW, Tin VP,
Cheng LC, Sihoe AD, Fink LM, Ma Y and Wong MP: Non-small cell lung
cancer cells expressing CD44 are enriched for stem cell-like
properties. PLoS One. 5:e140622010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang D and Shi Y: Aldehyde
dehydrogenase-1 is a specific marker for stem cells in human lung
adenocarcinoma. Med Oncol. 29:633–639. 2012. View Article : Google Scholar
|