Introduction
Of the 20–25% of breast cancers that are HER2
positive approximately 50–60% also express estrogen receptor (ER)
(1). Interaction between HER2 and
ER is well documented since the observation by Benz et al
that transfection of HER2 into ER positive cells results in
tamoxifen resistance (2). It
became widely accepted that HER2 overexpression causes intrinsic
resistance to endocrine therapy and consequently HER2-targeted
therapies combined with chemotherapy are recommended for patients
with HER2 positive/ER positive breast cancer (1). However, response rates in these
patients are lower than in HER2 positive/ER negative tumors
(1). Furthermore, in HER2
positive/ER positive breast cancer patients, the benefit of
trastuzumab progressively decreases as tumor expression of ER
increases (3,4). Thus, crosstalk between ER and HER2
contributes to resistance to both endocrine therapy and HER2
targeted therapy. Studies of HER2 targeted therapies in combination
with hormone therapy have shown clinical benefit in patients with
HER2 positive/ER positive metastatic breast cancer (5,6).
Crosstalk between ER and IGF1R signaling pathways
stimulates proliferation of ER positive breast epithelial cells
in vitro and in vivo (7,8). The
IGF1R ligands, IGF1 and IGF2, can activate un-liganded ER and have
therefore been implicated in the regulation of ER activity.
Estradiol (E2) can regulate IGF action by increasing IGF1R and
insulin-receptor substrate expression in breast cancer cells
resulting in an enhanced response to IGF1. IGF1 and E2 together
synergistically stimulate proliferation of breast cancer cells
(reviewed in ref. 9). Recent
evidence also suggests that E2 and IGF1 can downregulate several
potential tumor suppressors in breast cancer cells and negatively
affect breast cancer outcome (10).
This crosstalk between ER and IGF1R makes dual
inhibition of the receptors an attractive target for the treatment
of ER positive breast cancer and has been evaluated previously in
preclinical models, whereby dual inhibition of ER and IGF1R results
in enhanced apoptosis compared to single agent treatment (11–14).
Anti-ER and anti-IGF1R targeted therapy has also been evaluated
clinically in ER positive breast cancer patients who had progressed
following first-line hormonal therapy (15). This study evaluated the addition of
AMG-479, an anti-IGF1R monoclonal antibody to exemestane or
fulvestrant (aromatase inhibitor/anti-estrogen) compared to single
agent hormonal therapy. However, there was no significant increase
in progression-free survival in the combination arm compared to
single agent treatment. Interestingly, although this trial combined
anti-ER and anti-IGF1R targeted therapy, IGF1R expression was not a
selection criterion (15).
In HER2 positive breast cancer, crosstalk between
IGF1R and HER2 has been extensively investigated in preclinical
studies (16–19) and in a clinical trial. Despite
promising preclinical data for combined HER2/IGF1R inhibition, the
addition of the IGF1R targeted antibody cixutumumab to lapatinib
plus capecitabine failed to show an improvement in progression-free
survival compared to lapatinib plus capetabine (20).
Previous work from our laboratory reported that
phospho-IGF1R/IR staining was detected in 48.8% of HER2 positive
breast tumors and in 44.7% of the HER2/ER positive breast tumors
(19). While dual targeting of
HER2 and ER or HER2 and IGF1R have been evaluated, combined
targeting of ER and IGF1R together with HER2 targeted therapy has
not been investigated in HER2 positive breast cancer. Therefore in
the present study, we evaluated combined targeting of ER and IGF1R
in HER2/ER/IGF1R positive breast cancer cell lines to determine its
potential as a rational therapeutic strategy to improve treatment
response in patients with HER2 positive ER positive tumors.
Materials and methods
Cell lines and culture reagents
Cell lines MCF7, BT474, MDA-MB-361 and HCC1419 cell
lines were obtained from the American Type Culture Collection
(Rockville, MD, USA). The EFM-192A cells were obtained from the
German Tissue Repository DSMZ (Braunschweig, Germany). BT474/Tr
cells were established by continuously culturing BT474 cells in 100
µg of trastuzumab for a period of 9 months, as previously
described (21). All cell lines
except MDA-MB-361 were cultured in RPMI-1640 (Sigma-Aldrich,
Wicklow, Ireland) supplemented with 10% fetal bovine serum (FBS)
with 5% CO2. MDA-MB-361 cells were maintained in L-15
medium (Sigma-Aldrich) supplemented with 15% FBS, without
CO2. All experiments were conducted with cells in the
exponential growth phase and within 10 passages after thawing the
cells. Cell line identity was authenticated by short tandem repeat
(STR) typing (Source BioScience, Nottingham, UK). Stock solutions
of BMS-536924 (Bristol Myers Squibb, Princeton, NJ, USA) and
NVP-AEW541 (Novartis, Basel, Switzerland) (10 mM) were prepared in
dimethyl sulfoxide. Tamoxifen (Sigma-Aldrich) was dissolved in
ethanol as a 10 mM stock solution. Trastuzumab (21 mg/ml) was
purchased from St Vincent's University Hospital (Dublin,
Ireland).
Cell proliferation assays
Proliferation was measured using an acid phosphatase
assay. Cells (3–5×103/well) were seeded in 96-well
plates. Plates were incubated overnight at 37°C, followed by
addition of drug and incubation for 5 days. After washing with PBS,
10 mM paranitrophenol phosphate substrate (Sigma-Aldrich) in 0.1 M
sodium acetate buffer with 0.1% Triton X-100 (Sigma-Aldrich) was
added to each well and incubated at 37°C for 2 h, 50 µl of 1
M NaOH was added and the absorbance was read at 405 nM (with
reference wavelength 620 nM).
Cell cycle assay
Cell cycle assays were performed in 24-well plates
with 2.5×104 cells/well. Following cell adherence, cell
synchronization was achieved by replacing the growth media with
serum-free RPMI for 16 h. Cells were then drug treated at the
indicated concentrations. After 48 h cells were trypsinized and
transferred to a round-bottomed 96-well plate, washed with PBS and
fixed with 70% ethanol overnight. Following fixation, the cells
were washed with PBS and stained with Cell Cycle Reagent (Merck
Millipore, Cork, Ireland), according to the manufacturer's
protocol. Samples were measured on the Guava EasyCyte (Merck
Millipore) and the data were analyzed using Modfit LT software
(Verity Software House, Topsham, ME, USA).
Apoptosis assay
Apoptosis assays were performed in 24-well plates
with 2.5×104 cells/well. After 24 h cells were drug
treated at the indicated concentrations. After 72 h cells were
trypsinized and transferred to a round-bottomed 96-well plate,
washed with PBS and fixed with 70% ethanol at 4°C overnight.
Following fixation the cells were washed with PBS and stained using
the Guava TUNEL Assay kit (Merck Millipore), according to the
protocol for the Guava EasyCyte (Merck Millipore). Apoptotic and
non-apoptotic cell populations were determined and expressed as a
percentage of the total cell population using the Guava TUNEL
Software Module (Merck Millipore).
Immunoblotting
Whole cell lysates were prepared by seeding
approximately 1×106 cells in 100-mm cell culture petri
dishes. Once the cells were 80% confluent the media was removed and
the cell monolayer was washed twice with ice-cold PBS and then
lysed with 500 µl of RIPA buffer, containing 5 µl
100X protease inhibitors, 5 µl 100X PMSF and 5 µl
100X sodium orthovanadate (Sigma-Aldrich). Cells were incubated on
ice for 20 min, sheared with a 21-guage needle and centrifuged at
16,000 x g for 5 min at 4°C. The supernatant was collected and
protein concentration was determined using the BCA assay (Thermo
Fisher Scientific, Hemel Hempstead, UK). Protein lysates (50
µg) were resolved on 10% polyacrylamide gels (Lonza, Slough,
UK), transferred to Hybond ECL nitrocellulose membrane (GE
Healthcare, Cheshire, UK) and blocked using blocking solution [5%
milk powder (Bio-Rad, Hemel Hempstead, UK) in 0.5% PBS-Tween] at
room temperature for 1 h shaking. The membrane was incubated
overnight, shaking, at 4°C with primary antibody: anti-ERα (Santa
Cruz Biotechnology, Heidelberg, Germany) [1:200 in 3% blocking
solution (Bio-Rad)], anti-IGF1Rβ (Santa Cruz Biotechnology) (1:666
in 5% blocking solution), anti-HER2 (Merck Millipore) (1:1000 in 5%
blocking solution) and anti-tubulin (Sigma-Aldrich) (1:1000 in 5%
blocking solution) or anti-GAPDH (R&D Systems, Abingdon, UK)
(1:1000 in 2.5% blocking solution). Three 10 min washes with 0.5%
PBS-Tween were carried out followed by 1 h incubation with
secondary antibody (anti-mouse (Sigma-Aldrich) or anti-rabbit
[Thermo Fisher Scientific) (1:1000)], another three 10 min washes
with 0.5% PBS-Tween and a PBS wash. Protein bands were detected
using Luminol (Santa Cruz Biotechnology).
Enzyme-linked immunosorbant assay
(ELISA)
Total IGF1R was measured using a quantitative ELISA
(R&D Systems) according to the manufacturer's instructions.
Lysates were prepared as described above and 50 µg of
protein was loaded per sample, in triplicate. Total IGF1R levels
are presented as nanogram per milligram of total protein.
Gene expression analysis
BreastMark is an online algorithm which integrates
gene expression and survival data from 26 datasets on 12 different
microarray platforms corresponding to ~17,000 genes in up to 4,738
samples (22). Disease-free
survival (DFS) and overall survival (OS) were analyzed and the 25th
percentile was used to dichotomize IGF1R expression levels in ER
positive breast tumors classified as HER2 subtype based on the gene
classifier ssp2003 (23).
Statistical analysis
Analyses of the differences in response to treatment
in in vitro assays were performed using the Student's t-test
(two-tailed with equal variance). p<0.05 was regarded as
statistically significant. For the Kaplan Meier plots generated by
BreastMark hazard ratios and p-values were calculated using a
log-rank test.
Results
IGF1R expression in HER2/ER positive
tumors
Using publicly available gene expression data made
accessible through BreastMark (22), we examined expression of IGF1R in
tumors classified as HER2 positive using the ssp2003 subtype
classifier (23). High expression
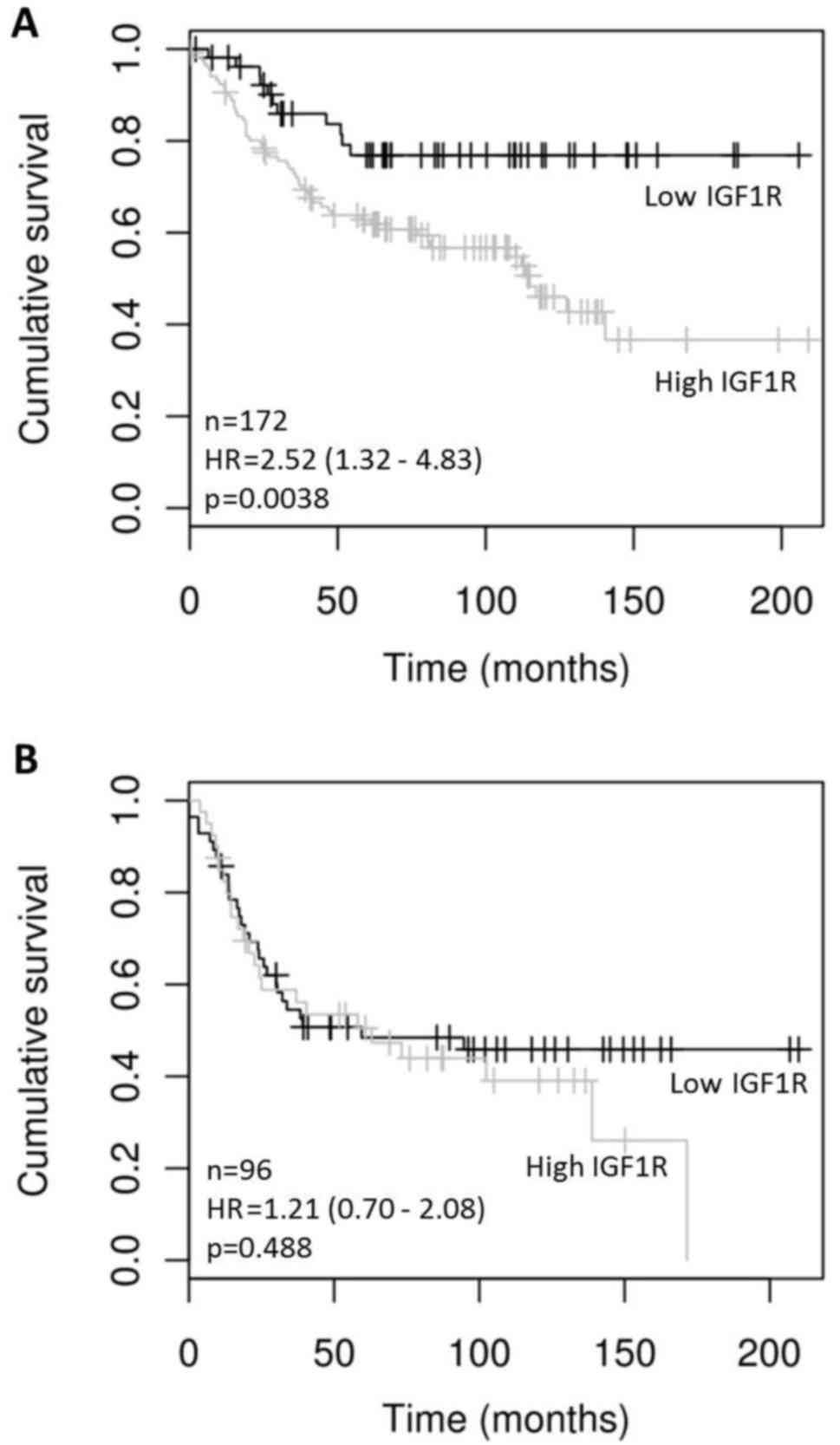
of IGF1R (greater than the 25th percentile) correlated
significantly with DFS in ER positive but not in ER negative HER2
positive tumors (HR=2.52 (1.32–4.83), p=0.0038) (Fig. 1). IGF1R mRNA levels did not
significantly correlate with OS in either HER2 positive ER positive
(p=0.5054) or HER2 positive ER negative (p=0.0675) tumors.
Dual inhibition of IGF1R and ER
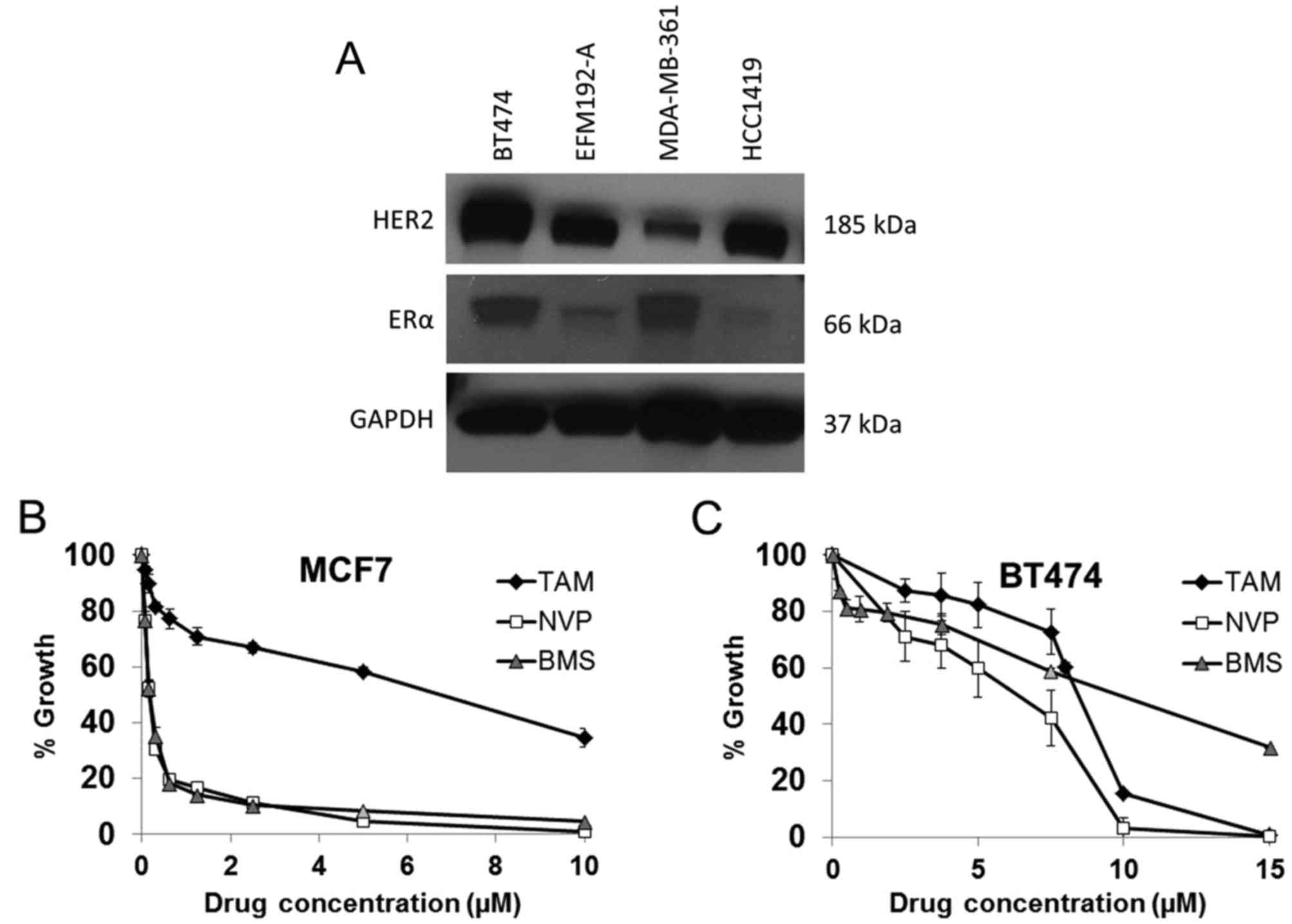
BT474 cells express ER and IGF1R and thus represent
a cell line model of ER and IGF1R positive HER2 amplified breast
cancer (Table I and Fig. 2). MCF7 cells, which are not HER2
amplified, were used as a positive control as they are a model of
ER positive and IGF1R positive breast cancer wherein the two
signaling systems engage in crosstalk resulting in synergistic
growth (24). Tamoxifen inhibited
the growth of both BT474 and MCF7 cells with an IC50 of
5.5±0.3 µM and 5.7±0.5 µM, respectively, indicating
that the cells display a similar sensitivity to ER inhibition
(Fig. 2). IGF1R inhibition was
examined with two anti-IGF1R TKIs, NVP-AEW541 (NVP) and BMS-536924
(BMS). MCF7 cells were equally sensitive to both NVP (0.22±0.01
µM) and BMS (0.19±0.02 µM), while BT474 cells, which
express lower levels of IGF1R, were less sensitive to both NVP
(4.7±0.4 µM) and BMS (11.6±1.1 µM) (Fig. 2).
 | Table ILevels of IGF1R protein (ng/mg)
measured by ELISA in five HER2 positive breast cancer cell
lines. |
Table I
Levels of IGF1R protein (ng/mg)
measured by ELISA in five HER2 positive breast cancer cell
lines.
| Cell line | IGF1R (ng/mg) |
|---|
| BT474 | 3.7±0.5 |
| BT474/Tr | 3.1±0.4 |
| EFM192-A | 0.6±0.1 |
| MDA-MB-361 | 0.7±0.1 |
| HCC1419 | 3.0±0.4 |
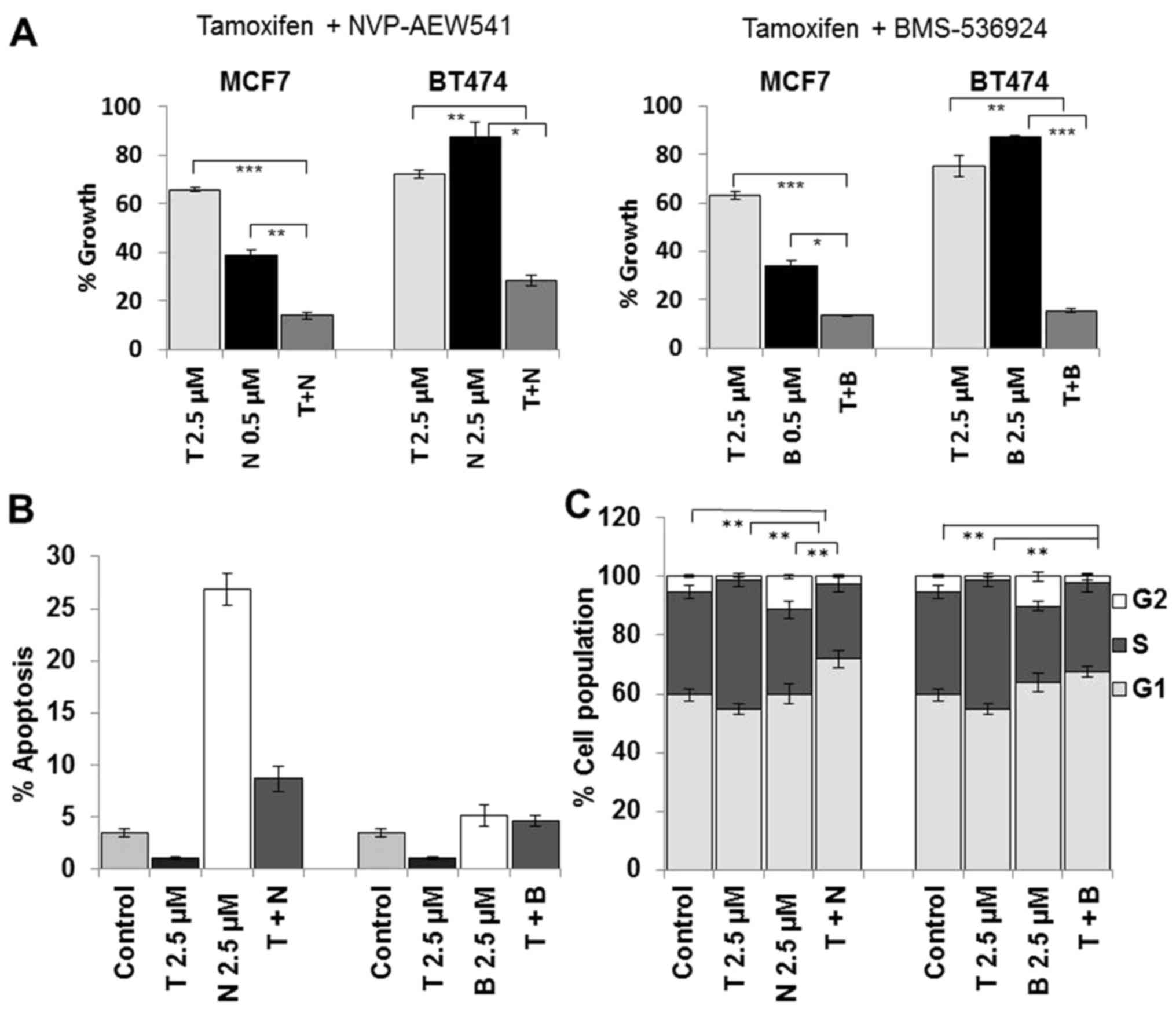
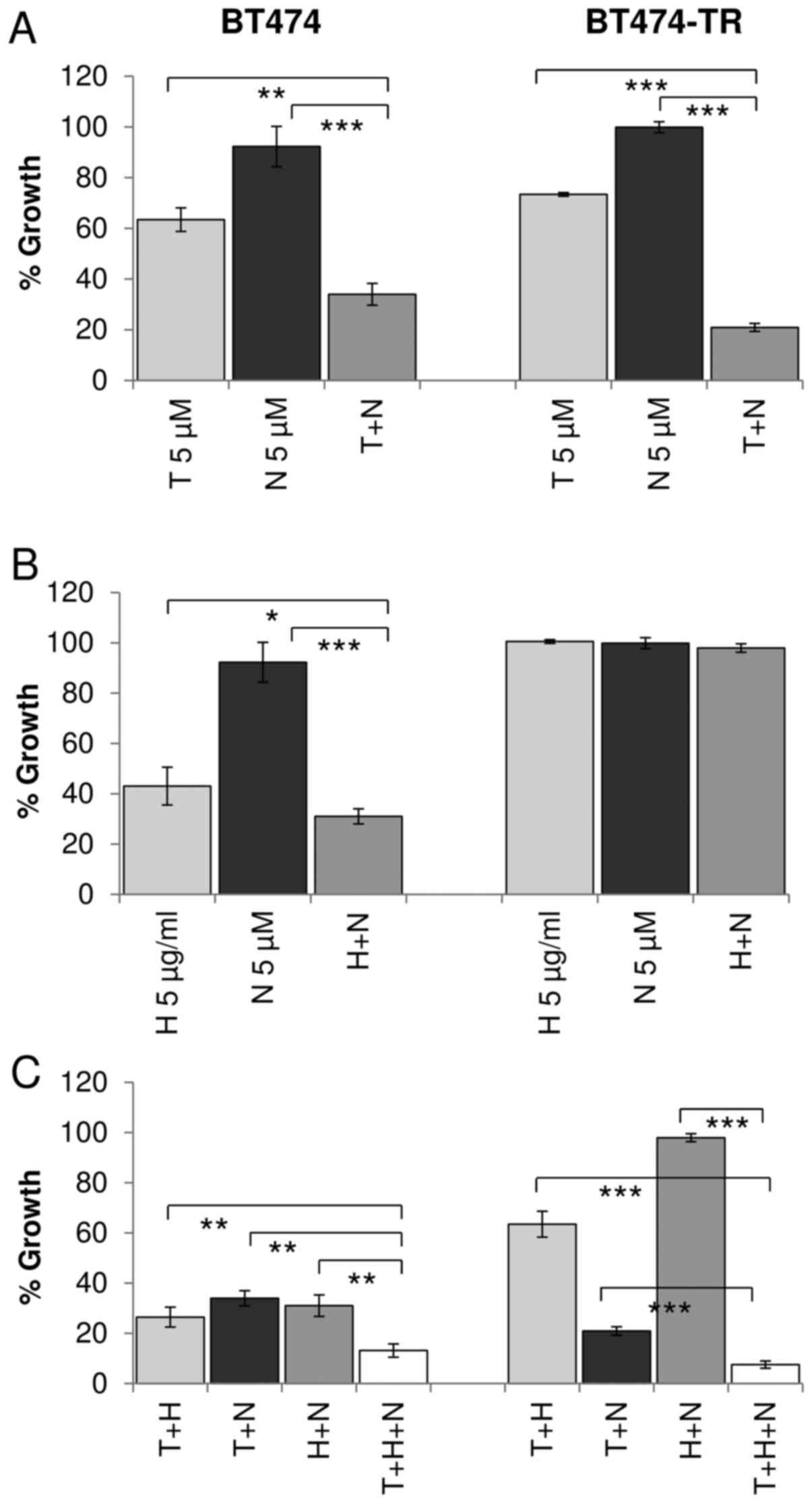
To examine the effects of dual inhibition of ER and
IGF1R, tamoxifen and NVP were administered as single agents and in
combination at a fixed concentration. Despite the observation that
BT474 cells were less sensitive to NVP as a single agent, the
combined treatment with tamoxifen resulted in enhanced growth
inhibition compared to tamoxifen alone (p<0.001) and NVP alone
(p<0.001) (Fig. 3A). To confirm
that the enhanced response to combined tamoxifen and NVP was due to
targeting the ER and not tamoxifen off-target effects we also
tested NVP in combination with fulvestrant and observed a similar
enhanced response to the combination in BT474 cells (data not
shown). The combination treatment also resulted in significantly
greater growth inhibition in MCF7 cells compared to tamoxifen alone
(p<0.0001) and compared to NVP alone (p<0.001). Similar
enhancement of growth inhibition was also seen when tamoxifen was
combined with BMS in both cell lines (Fig. 3A).
Dual inhibition of ER and IGF1R results
in G1 arrest
Single agent tamoxifen treatment did not induce
apoptosis in BT474 cells, compared to control cells (Fig. 3B). Treatment with NVP alone induced
significant apoptosis in BT474 (p<0.001) cells. NVP combined
with tamoxifen did not increase apoptosis compared to treatment
with NVP alone. BMS alone only induced a small increase in
apoptosis in both cell lines and similar to NVP, when combined with
tamoxifen did not induce a significant increase in apoptosis. These
results suggest that the growth inhibitory effects of the
combination treatment are not as a result of enhanced apoptosis.
Neither tamoxifen nor NVP single agent treatment resulted in
increased G1 accumulation (Fig.
3C). However, there was a significant increase in G1
accumulation following combined treatment compared to either
tamoxifen alone (p=0.001) of NVP alone (p=0.010). A similar trend
was also observed when BMS was combined with tamoxifen. These data
suggest that the growth enhancement of the dual combination
treatment is mediated by an increase in cell cycle arrest.
Dual ER and IGF1R inhibition in HER2
positive cell lines
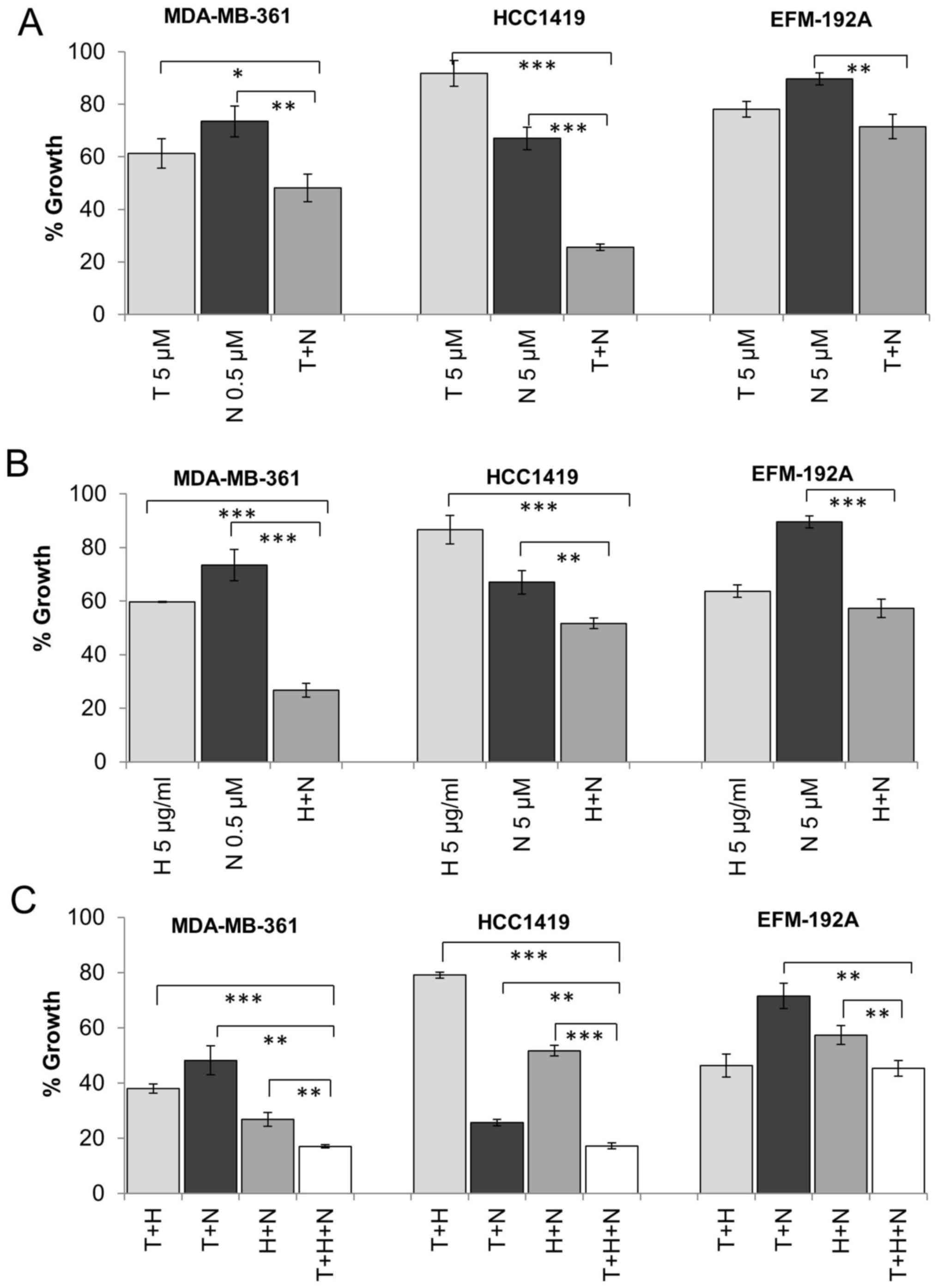
Having established that dual targeting of ER and
IGF1R results in greater inhibition of cell growth in BT474 cells,
we tested the effects of dual therapy in three additional cell line
models of HER2, ER and IGF1R positive breast cancer. MDA-MB-361,
EFM-192A and HCC1419 cells are ER positive as determined by western
blotting (Fig. 2) and expressed
high levels of IGF1R determined by both western blotting and ELISA
with EFM-192A cells exhibiting the lowest levels of IGF1R of the
tested cell lines (Table I).
MDA-MB-361 cells were more sensitive to single agent NVP treatment
and thus were treated with a lower concentration of NVP compared to
EFM-192A cells in these combination experiments. The combination of
tamoxifen and NVP resulted in significantly greater inhibition of
growth compared to tamoxifen or NVP alone in MDA-MB-361 (p=0.041,
p=0.005, respectively) and HCC1419 (p<0.001, p<0.001,
respectively) (Fig. 4A). An
enhanced response was also observed for the combination in EFM-192A
cells (28.5±4.6% growth inhibition), although this did not achieve
statistical significance compared to tamoxifen alone (21.9±3.0%
growth inhibition, p=0.106) (Fig.
4A). Similar results were also achieved when tamoxifen was
combined with BMS (data not shown).
Dual targeting of IGF1R and HER2
We have shown that dual inhibition of IGF1R and ER
is more effective than single agent treatment in four HER2/ER/IGF1R
positive cell lines. We have previously shown that combined
treatment with the HER2 targeted monoclonal antibody trastuzumab
and IGF1R inhibitors produces enhanced response in some HER2
positive breast cancer cell lines (19). Similarly, in this study, combined
trastuzumab and NVP was more effective than trastuzumab or NVP
alone in BT474 (p=0.003, p<0.001, respectively), MDA-MB-361
(p<0.001, p<0.001) and HCC1419 (p<0.001, p=0.005)
(Figs. 4B and 5B). The combination also produced greater
growth inhibition than NVP (p<0.001) alone in the EFM-192A
cells, but the effect of the combination (42.7±3.4% growth
inhibition) compared to trastuzumab alone (36.3±2.3% growth
inhibition) did not achieve statistical significance (p=0.055)
(Fig. 4B). Similar results were
also obtained when trastuzumab was combined with BMS in the three
cell lines (data not shown).
Effect of triple targeted therapy on
ER/HER2/IGF1R positive cells
Next we examined if there may be an additional
benefit when trastuzumab, tamoxifen and IGF1R targeting are
combined compared to dual inhibition. In three of the four cell
lines tested (BT474, MDA-MB-361 and HCC1419), the triple
combination was significantly more effective than dual inhibition
with trastuzumab and NVP, tamoxifen and NVP or tamoxifen and
trastuzumab (Figs. 4C and 5C). In the EFM-192A cells, the addition
of NVP did not significantly enhance response compared to the
double combination of tamoxifen and trastuzumab. Similar results
were obtained for trastuzumab and tamoxifen were combined with BMS
in the four cell lines (data not shown).
Effect of triple targeted therapy on
trastuzumab-resistant cells
Trastuzumab-conditioned BT474/Tr cells were
developed by continuous exposure to trastuzumab and represent a
cell line model of acquired trastuzumab resistance which exhibits a
significantly reduced response to trastuzumab compared to BT474
cells, as previously described (21). Similar to the parental BT474 cells,
combined treatment with tamoxifen and NVP resulted in significantly
greater inhibition of growth compared to either tamoxifen
(p<0.001) or NVP (p<0.001) alone in the BT474/Tr cells
(Fig. 5A). In BT474/Tr cells
trastuzumab alone or in combination with NVP did not inhibit growth
significantly (Fig. 5B). However,
BT474/Tr cells were significantly more sensitive to the
trastuzumab, tamoxifen and NVP triple combination compared to dual
tamoxifen/NVP (p<0.001), dual trastuzumab/NVP (p<0.001) and
dual tamoxifen/trastuzumab (p<0.001) (Fig. 5C). Similar results were obtained
with BMS in BT474/Tr cell line (data not shown).
Discussion
Based on the substantial evidence of crosstalk
between ER and IGF1R signaling pathways, the inferior response
rates to HER2-targeted therapies combined with chemotherapy for
HER2/ER positive tumors, and previous studies from our laboratory
suggesting that 45% of HER2 positive/ER positive breast tumors are
positive for phosphorylated IGF1R/IR (19), we tested combined targeting of ER
and IGF1R in HER2 cell line models of HER2/ER/IGF1R positive breast
cancer. Analysis of publicly available gene expression data
provided further support for IGF1R playing a role in HER2
positive/ER positive breast cancer as higher expression of IGF1R
mRNA is associated with shorter DFS whereas IGF1R expression did
not correlate with DFS in the HER2 positive/ER negative cases.
Combined treatment with tamoxifen and the IGF1R
inhibitor NVP was significantly more effective than single agent
treatment in all cell lines tested. Combined tamoxifen and NVP
treatment resulted in a significant increase in cells arrested in
G1 phase without a significant increase in apoptosis. Two previous
studies reported the enhanced growth inhibitory effects of combined
ER/IGF1R targeted therapy corresponded with significant increases
in apoptosis, but not cell cycle arrest (12,13).
The different effects noted may be due to the different cell
culture conditions, different IGF1R targeted agents (α-IR-3,
AG1024) (13) and/or different ER
targeted agents (letrozole) (12)
used. Furthermore, both NVP-AEW541 and BMS-536924 also target the
insulin receptor (25,26), thus inhibition of IR signaling may
contribute to the induction of cell cycle arrest. Targeting the IR
may also be beneficial in HER2 positive breast cancer as IR
signaling has been implicated in tumor progression in preclinical
models of HER2 positive breast cancer (27). Of note, dual targeting of ER and
IGF1R produced a similar level of growth inhibition in the
trastuzumab resistant BT474 cells, as in the parental cells,
suggesting that this ER/IGF1R targeting strategy may be beneficial
in patients with HER2/ER/IGF1R positive breast cancer following
disease progression on trastuzumab-based treatment.
Similar to our previously published results
(19), trastuzumab combined with
NVP or BMS produced an enhanced response in the HER2 positive
breast cancer cell lines tested. Targeting all three receptors
(HER2, ER and IGF1R) simultaneously produced a significantly
enhanced response compared to targeting two of the receptors in
four of the five HER2 positive breast cancer cell lines tested,
including the trastuzumab resistant BT474/Tr cell line. In EFM-192A
cells, addition of NVP did not significantly improve the
anti-proliferative effect compared to trastuzumab combined with
tamoxifen. The EFM-192A cells have the lowest levels of IGF1R of
the cell lines tested (0.6±0.1 ng/mg), although similar to
MDA-MB-361 (0.7±0.1 ng/mg) (Table
I). While IGF1R levels alone may not account for sensitivity or
resistance to IGF1R inhibition, we have previously reported that
high levels of IGF1R are weakly associated with greater sensitivity
to NVP-AEW541 in a panel of 9 HER2 positive breast cancer cell
lines (p=0.053) (19). Mukohara
et al reported that sensitivity to NVP-AEW541 in MCF7 cells
was due to high expression of both IGF1R and IRS1 (28).
Importantly, in the cell line model of acquired
trastuzumab resistance, BT474/Tr, the triple combination appears to
overcome trastuzumab resistance, and achieved greater than 90%
growth inhibition. Thus the triple combination may be beneficial
for patients with trastuzumab-refractory HER2/ER/IGF1R positive
metastatic breast cancer. The triple targeting strategy may also
represent a promising chemotherapy-free adjuvant treatment strategy
for low risk HER2 positive breast cancer patients. It would be
interesting to test this strategy in a chemotherapy-free study
similar to the perioperative EPHOS-B study of trastuzumab and
lapatinib (29).
To date clinical trials targeting IGF signaling,
using TKIs or monoclonal antibodies targeting the receptor have
produced disappointing results (30,31)
and those results combined with TKI-related toxicities have led to
the termination of several IGF1R targeted therapies, including the
two TKIs tested in this study. The lack of appropriate predictive
biomarkers and the selection of patient subgroups most likely to
respond to IGF1R inhibition is one of the major factors
contributing to the failure of the clinical studies. Optimal
combination strategies also need to be identified using preclinical
studies. Thus targeting IGF1R in combination with hormone therapy
in HER2/ER/IGF1R positive breast cancer may be more successful than
the previous IGF1R breast cancer clinical trials which were
performed in ER positive breast cancer.
As previously mentioned IGF ligands may play a
critical role in determining response/resistance to IGF1R
inhibition (32,33). Targeting IGF ligands may be a
promising therapeutic approach to block IGF1R signaling and in
addition to block IGF2 mediated insulin receptor signaling. Two
monoclonal antibodies, BI 836845 and MEDI-573 which target IGF1 and
IGF2, are currently in clinical development (34,35).
They have shown anti-proliferative activity in a range of cancer
models and are currently in phase I/II studies in ER positive
breast cancer.
In conclusion, our study provides significant
evidence to suggest that targeting HER2, ER and IGF1R could have
clinical benefit in the subgroup of HER2 positive patients whose
tumors co-express ER and IGF1R. Evaluation of the dual (ER/IGF1R)
and/or triple (HER2/ER/IGF1R) targeting combinations in preclinical
in vivo models of breast cancer, in particular patient
derived xenograft models, would be required to further evaluate the
potential clinical benefit of this treatment strategy.
Acknowledgments
This study was supported by funding from the Irish
Research Council (MMcD), the Health Research Board (CSA/2007/11),
the Cancer Clinical Research Trust, Science Foundation Ireland
through Molecular Therapeutics for Cancer, Ireland (08/SRC/B1410)
and the Irish Cancer Society Collaborative Cancer Research Centre
Breast-Predict (CCRC13GAL). 'The opinions, findings and conclusions
or recommendations expressed in this material are those of the
author(s) and do not necessarily reflect the views of the Irish
Cancer Society'.
References
|
1
|
Nahta R and O'Regan RM: Therapeutic
implications of estrogen receptor signaling in HER2-positive breast
cancers. Breast Cancer Res Treat. 135:39–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benz CC, Scott GK, Sarup JC, Johnson RM,
Tripathy D, Coronado E, Shepard HM and Osborne CK:
Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7
cells trans-fected with HER2/neu. Breast Cancer Res Treat.
24:85–95. 1992. View Article : Google Scholar
|
|
3
|
Bhargava R, Dabbs DJ, Beriwal S, Yildiz
IA, Badve P, Soran A, Johnson RR, Brufsky AM, Lembersky BC, McGuire
KP, et al: Semiquantitative hormone receptor level influences
response to trastuzumab-containing neoadjuvant chemotherapy in
HER2-positive breast cancer. Mod Pathol. 24:367–374. 2011.
View Article : Google Scholar
|
|
4
|
Vici P, Pizzuti L, Sperduti I, Frassoldati
A, Natoli C, Gamucci T, Tomao S, Michelotti A, Moscetti L, Gori S,
et al: 'Triple positive' early breast cancer: An observational
multicenter retrospective analysis of outcome. Oncotarget.
7:17932–17944. 2016.PubMed/NCBI
|
|
5
|
Kaufman B, Mackey JR, Clemens MR, Bapsy
PP, Vaid A, Wardley A, Tjulandin S, Jahn M, Lehle M, Feyereislova
A, et al: Trastuzumab plus anastrozole versus anastrozole alone for
the treatment of postmenopausal women with human epidermal growth
factor receptor 2-positive, hormone receptor-positive metastatic
breast cancer: Results from the randomized phase III TAnDEM study.
J Clin Oncol. 27:5529–5537. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnston S, Pippen J Jr, Pivot X,
Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A,
Kennedy MJ, et al: Lapatinib combined with letrozole versus
letrozole and placebo as first-line therapy for postmenopausal
hormone receptor-positive metastatic breast cancer. J Clin Oncol.
27:5538–5546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee AV, Jackson JG, Gooch JL, Hilsenbeck
SG, Coronado-Heinsohn E, Osborne CK and Yee D: Enhancement of
insulin-like growth factor signaling in human breast cancer:
Estrogen regulation of insulin receptor substrate-1 expression in
vitro and in vivo. Mol Endocrinol. 13:787–796. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Molloy CA, May FEB and Westley BR: Insulin
receptor substrate-1 expression is regulated by estrogen in the
MCF-7 human breast cancer cell line. J Biol Chem. 275:12565–12571.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamelers IH and Steenbergh PH:
Interactions between estrogen and insulin-like growth factor
signaling pathways in human breast tumor cells. Endocr Relat
Cancer. 10:331–345. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Casa AJ, Potter AS, Malik S, Lazard Z,
Kuiatse I, Kim HT, Tsimelzon A, Creighton CJ, Hilsenbeck SG, Brown
PH, et al: Estrogen and insulin-like growth factor-I (IGF-I)
independently down-regulate critical repressors of breast cancer
growth. Breast Cancer Res Treat. 132:61–73. 2012. View Article : Google Scholar
|
|
11
|
Ye JJ, Liang SJ, Guo N, Li SL, Wu AM,
Giannini S, Sachdev D, Yee D, Brünner N, Ikle D, et al: Combined
effects of tamoxifen and a chimeric humanized single chain antibody
against the type I IGF receptor on breast tumor growth in vivo.
Horm Metab Res. 35:836–842. 2003. View Article : Google Scholar
|
|
12
|
Lisztwan J, Pornon A, Chen B, Chen S and
Evans DB: The aromatase inhibitor letrozole and inhibitors of
insulin-like growth factor I receptor synergistically induce
apoptosis in in vitro models of estrogen-dependent breast cancer.
Breast Cancer Res. 10:R562008. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chakraborty AK, Welsh A and Digiovanna MP:
Co-targeting the insulin-like growth factor I receptor enhances
growth-inhibitory and pro-apoptotic effects of anti-estrogens in
human breast cancer cell lines. Breast Cancer Res Treat.
120:327–335. 2010. View Article : Google Scholar
|
|
14
|
Hou X, Huang F, Macedo LF, Harrington SC,
Reeves KA, Greer A, Finckenstein FG, Brodie A, Gottardis MM,
Carboni JM, et al: Dual IGF-1R/InsR inhibitor BMS-754807 synergizes
with hormonal agents in treatment of estrogen-dependent breast
cancer. Cancer Res. 71:7597–7607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Robertson JFR, Ferrero JM, Bourgeois H,
Kennecke H, de Boer RH, Jacot W, McGreivy J, Suzuki S, Zhu M,
McCaffery I, et al: Ganitumab with either exemestane or fulvestrant
for postmenopausal women with advanced, hormone-receptor-positive
breast cancer: A randomised, controlled, double-blind, phase 2
trial. Lancet Oncol. 14:228–235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu Y, Zi X, Zhao Y, Mascarenhas D and
Pollak M: Insulin-like growth factor-I receptor signaling and
resistance to trastuzumab (Herceptin). J Natl Cancer Inst.
93:1852–1857. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nahta R, Yuan LXH, Zhang B, Kobayashi R
and Esteva FJ: Insulin-like growth factor-I receptor/human
epidermal growth factor receptor 2 heterodimerization contributes
to trastuzumab resistance of breast cancer cells. Cancer Res.
65:11118–11128. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hartog H, Van Der Graaf WTA, Boezen HM and
Wesseling J: Treatment of breast cancer cells by IGF1R tyrosine
kinase inhibitor combined with conventional systemic drugs.
Anticancer Res. 32:1309–1318. 2012.PubMed/NCBI
|
|
19
|
Browne BC, Eustace AJ, Kennedy S, O'Brien
NA, Pedersen K, McDermott MSJ, Larkin A, Ballot J, Mahgoub T,
Sclafani F, et al: Evaluation of IGF1R and phosphorylated IGF1R as
targets in HER2-positive breast cancer cell lines and tumours.
Breast Cancer Res Treat. 136:717–727. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haluska P, Bernath AM, Ballman KV, Dueck
AC, Linden HM, Goetz MP, Northfelt DW, Hou X, Tenner KS,
Tienchaiananda P, et al: Randomized phase II trial of capecitabine
and lapatinib with or without cixutumumab in patients with HER2
breast cancer previously treated with trastuzumab and an
anthracycline and/or a taxane: NCCTG N0733 (Alliance). J Clin
Oncol. 32(Suppl 5): 6322014.
|
|
21
|
Konecny GE, Pegram MD, Venkatesan N, Finn
R, Yang G, Rahmeh M, Untch M, Rusnak DW, Spehar G, Mullin RJ, et
al: Activity of the dual kinase inhibitor lapatinib (GW572016)
against HER-2-overexpressing and trastuzumab-treated breast cancer
cells. Cancer Res. 66:1630–1639. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Madden SF, Clarke C, Gaule P, Aherne ST,
O'Donovan N, Clynes M, Crown J and Gallagher WM: BreastMark: An
integrated approach to mining publicly available transcriptomic
datasets relating to breast cancer outcome. Breast Cancer Res.
15:R522013. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sorlie T, Tibshirani R, Parker J, Hastie
T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et
al: Repeated observation of breast tumor subtypes in independent
gene expression data sets. Proc Natl Acad Sci USA. 100:8418–8423.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dupont J and Le Roith D: Insulin-like
growth factor 1 and oestradiol promote cell proliferation of MCF-7
breast cancer cells: New insights into their synergistic effects.
Mol Pathol. 54:149–154. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
García-Echeverría C, Pearson MA, Marti A,
Meyer T, Mestan J, Zimmermann J, Gao J, Brueggen J, Capraro HG,
Cozens R, et al: In vivo antitumor activity of NVP-AEW541-A novel,
potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell.
5:231–239. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wittman M, Carboni J, Attar R,
Balasubramanian B, Balimane P, Brassil P, Beaulieu F, Chang C,
Clarke W, Dell J, et al: Discovery of a
(1H-benzoimidazol-2-yl)-1H-pyridin-2-one (BMS-536924) inhibitor of
insulin-like growth factor I receptor kinase with in vivo antitumor
activity. J Med Chem. 48:5639–5643. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferguson RD, Gallagher EJ, Cohen D,
Tobin-Hess A, Alikhani N, Novosyadlyy R, Haddad N, Yakar S and
LeRoith D: Hyperinsulinemia promotes metastasis to the lung in a
mouse model of Her2-mediated breast cancer. Endocr Relat Cancer.
20:391–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mukohara T, Shimada H, Ogasawara N,
Wanikawa R, Shimomura M, Nakatsura T, Ishii G, Park JO, Jänne PA,
Saijo N, et al: Sensitivity of breast cancer cell lines to the
novel insulin-like growth factor-1 receptor (IGF-1R) inhibitor
NVP-AEW541 is dependent on the level of IRS-1 expression. Cancer
Lett. 282:14–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bundred N, Cameron D, Armstrong A, Brunt
A, Cramer A, Dodwell D, Evans A, Hanby A, Hartup S, Hong A, et al:
Effects of perioperative lapatinib and trastuzumab, alone and in
combination, in early HER2+ breast cancer - the UK
EPHOS-B trial (CRUK/08/002). Eur J Cancer. 57(Suppl 2):
LBA62016.
|
|
30
|
Yee D: Insulin-like growth factor receptor
inhibitors: Baby or the bathwater? J Natl Cancer Inst. 104:975–981.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Crudden C, Girnita A and Girnita L:
Targeting the IGF-1R: The tale of the tortoise and the hare. Front
Endocrinol (Lausanne). 6:642015.
|
|
32
|
Javle MM, Shroff RT, Varadhachary GR,
Wolff RA, Fogelman DR, Bhosale P, Wang X, Kar SP, Overman MJ,
Sathyanarayanan S, et al: Tumor IGF-1 expression as a predictive
biomarker for IGF1R-directed therapy in advanced pancreatic cancer
(APC). J Clin Oncol. 30:40542012.
|
|
33
|
McCaffery I, Tudor Y, Deng H, Tang R,
Suzuki S, Badola S, Kindler HL, Fuchs CS, Loh E, Patterson SD, et
al: Putative predictive biomarkers of survival in patients with
metastatic pancreatic adenocarcinoma treated with gemcitabine and
ganitumab, an IGF1R inhibitor. Clin Cancer Res. 19:4282–4289. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao J, Chesebrough JW, Cartlidge SA,
Ricketts SA, Incognito L, Veldman-Jones M, Blakey DC, Tabrizi M,
Jallal B, Trail PA, et al: Dual IGF-I/II-neutralizing antibody
MEDI-573 potently inhibits IGF signaling and tumor growth. Cancer
Res. 71:1029–1040. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Friedbichler K, Hofmann MH, Kroez M,
Ostermann E, Lamche HR, Koessl C, Borges E, Pollak MN, Adolf G and
Adam PJ: Pharmacodynamic and antineoplastic activity of BI 836845,
a fully human IGF ligand-neutralizing antibody, and mechanistic
rationale for combination with rapamycin. Mol Cancer Ther.
13:399–409. 2014. View Article : Google Scholar
|