Introduction
Bone is the most common site of metastasis in
prostate cancer (1). Approximately
two-thirds of all bone metastases are located in the spine
(2). A reported one-third of
prostate metastases to the spine become symptomatic as spinal cord
compression or mechanical instability (3–5).
Undoubtedly, spinal metastasis increases the risk of pathological
fracture, spinal cord compression and intractable cancer-induced
bone pain, and decreases survival (6). A previous study reported that the
5-year survival rate approaches 100% in patients with localized
prostate cancer; however, this rate drops to 33% in patients with
distant metastases (7). Therefore,
gaining a better understanding of the mechanism by which prostate
cancer metastasizes to distant organs, including the spine, is
essential to the development of targeted drugs and the improvement
of patient survival.
Chemokines belong to the cytokine family, and are
small secreted proteins composed of 70–100 amino acids (8). Recent studies have demonstrated that
chemokines and their receptors are involved in a variety of
physiological and pathological processes, including cell growth,
differentiation, apoptosis, tumor growth and metastasis (9,10).
C-X3-C motif chemokine ligand 1 (CX3CL1) has been demonstrated to
promote metastases in different types of tumors (11–13).
Unlike other chemokines, CX3CL1 (also termed fractalkine) has a
unique receptor, C-X3-C motif chemokine receptor 1 (CX3CR1). CX3CL1
exists in a membrane-bound and a soluble form. The membrane-bound
form promotes adhesion between tumor cells and endothelial cells,
while the soluble form recruits cells that express CX3CR1 (14). Human prostate cancer cells express
CX3CR1, and the association between CX3CL1 and CX3CR1 regulates
cell adhesion, migration and survival (15). Furthermore, the interaction between
CX3CL1 and its receptor CX3CR1 in the bone marrow serves a crucial
role in directing circulating prostate cancer cells to the bone via
androgen receptors (16). Recent
work using high-throughput microarrays demonstrated that CX3CL1 is
associated with spinal metastasis in different cancer types
(17). However, the underlying
mechanism of CX3CL1 in spinal metastasis remains unclear.
The results of the present study demonstrated that
CX3CL1/CX3CR1 was overexpressed in prostate cancer tissues with
spinal metastasis compared with primary tumors. Overexpression of
CX3CR1 increased cell proliferation, migration and invasion. It was
further observed that the epidermal growth factor receptor
(EGFR)/Src/FAK pathway was activated by CX3CL1/CX3CR1. The
inhibitors of these kinases reversed the cell migration promoted by
CX3CL1/CX3CR1. Notably, overexpression of CX3CR1 induced the spinal
metastasis of prostate cancer in vivo. Thus, the present
data provide a novel signaling pathway that merits further study to
examine the mechanism of CX3CL1/CX3CR1-induced spinal metastasis in
prostate cancer.
Materials and methods
Clinical specimens
A total of 48 clinical specimens were obtained from
the Department of Orthopedic Surgery, Zhongshan Hospital, Fudan
University (Shanghai, China) between December 2014 and December
2017. These included 12 cases of prostate carcinoma with spinal
metastasis (male, 12; age, 67.00±10.43 years), 12 cases of primary
spinal tumor (male/female, 8/4; age, 52.75±16.54 years), 12 samples
of healthy vertebrae (male/female, 7/5; age, 51.92±12.80 years) and
12 samples of healthy limb bone tissue (fractures; male/female,
8/4; age, 53.08±15.81 years). Patients who had received treatment
prior to surgery or for whom the pathological diagnosis was unclear
were excluded. All the clinical samples were approved by the Ethics
Committee of Zhongshan Hospital, Fudan University (no. Y2014-185).
These included 12 cases of prostate carcinoma with spinal
metastasis, 12 cases of primary spinal tumor, 12 samples of healthy
vertebrae and 12 samples of healthy limb bone tissue (fractures).
The diagnoses of the tumors were verified based on postoperative
pathological reports. All the samples that included primary
prostate tumor tissues, prostate tumor tissues with spinal
metastasis and paracancerous tissues were collected during surgery
and placed in a liquid nitrogen tank. The samples were stored at
−80°C until further analysis. Blood samples were collected and
centrifuged for 10 min (1,000 × g at room temperature), and the
supernatant was used for ELISA analysis. Informed consent was
provided by the patients prior to surgery.
Cell lines, lentiviruses and small
interfering (si)RNA
A total of four prostate cancer cell lines (PC-3,
VCaP, LNCaP and 22RV1) and one healthy prostate epithelial cell
line (RWPE-1) were obtained from the Chinese Academy of Sciences
cell bank (Shanghai, China). PC-3 cells were cultured in F-12 (cat.
no. 21127022; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA); VCaP cells were cultured Dulbecco's modified Eagle's medium
(DMEM; cat. no. 10569044; Gibco; Thermo Fisher Scientific, Inc.);
LNCaP and 22RV1 cells were cultured in RPMI-1640 media (cat. no.
61870044; Gibco; Thermo Fisher Scientific, Inc.); and RWPE-1 cells
were cultured in Keratinocyte-SFM medium (cat. no. 10725018;
Invitrogen; Thermo Fisher Scientific, Inc.). These four complete
media all contained 10% fetal bovine serum (FBS; cat. no.
10099-141; Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin
and streptomycin (100 U/ml penicillin and 100 U/ml streptomycin;
cat. no. B557; Invitrogen; Thermo Fisher Scientific, Inc.). Cells
were incubated in a humidified atmosphere containing 5%
CO2 at 37°C. The CX3CR1-overexpressing lentiviruses were
purchased from Shanghai GeneChem Co., Ltd. (Shanghai, China), and
the CX3CR1 and control siRNAs were purchased from Shanghai
GenePharma Co., Ltd. (Shanghai, China). The siRNA sequence
targeting CX3CR1 was: 5′-AAAAATCAACGTGGACTGAGC-3′. The negative
control sequence was: 5′-UUCUCCGAACGUGUCACGUTT-3′.
Treatment with CX3CL1 and
transfection
PC-3 and VCaP cells were treated with CX3CL1 (100
nM; R&D Systems, Inc., Minneapolis, MN, USA) for 30 min at
37°C. For the lentiviral infections, PC-3 and VCaP cells
(2×105) were seeded onto 6-well plates. This was
followed the next day by infection with either control or
CX3CR1-overexpressing lentiviruses in the presence of polybrene
(final concentration, 6 µg/µl). A total of 2–3 days
following infection, cells were subcultured and selected with 5
µg/ml puromycin. For the siRNA transfections, PC-3 and VCaP
cells (2×105) were seeded onto 6-well plates, and 100 nM
of either control siRNA or CX3CR1 siRNA was transfected using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). At 48 h post-transfection, the cells were
recultured for 0–5 days for the different detection procedures. All
the cells were divided into six groups, namely the blank control
(CON), CX3CL1 group, overexpression negative control [NC (OE)],
overexpression (OE), knockdown NC [NC (KD)] and knockdown (KD)
groups, for cell functional analysis.
Cell counting kit-8 (CCK-8) assays
VCaP and PC-3 cell suspensions were added to a
96-well plate at a density of 1×104 cells/ml. A total of
10 µl CCK-8 solution (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was added to each well at the same time every day
for 5 days. Finally, the absorbance at 450 nm was measured using a
microplate reader (Thermo Fisher Scientific, Inc.) following a 2 h
incubation period prior to detection.
Cellular apoptosis assay
A total of 5×105 VCaP and PC-3 cells were
harvested and the cells were suspended in 500 µl binding
buffer [cat. no. 70-AP101-100-BB; Hangzhou Multi Sciences (Lianke)
Biotech Co., Ltd., Hangzhou, China]. Subsequently, 5 µl
Annexin V-fluorescein isothiocyanate and 10 µl propidium
iodide (Invitrogen; Thermo Fisher Scientific, Inc.) was added to
cells and the cells were incubated at 37°C for 15 min in the dark.
Flow cytometry analysis was performed (BD FACSCalibur; BD
Biosciences, Franklin Lakes, NJ, USA) to detect cellular apoptosis.
The results were analyzed using FlowJo software (version 10.0;
FlowJo, LLC, Ashland, OR, USA).
Scratch wound assay
VCaP cells were seeded onto 6-well plates. When
cells had grown over the entire bottom of the well in a monolayer,
a 100 µl pipette tip was used to produce a scratch in the
wells. A total of 2 ml DMEM without FBS was added to maintain the
culture. CX3CL1 (100 nM) was used in all groups except the CON
group. The alteration in distance between the two edges of the
wound was observed through optical microscopy (Olympus-IX51;
Olympus Corporation, Tokyo, Japan) at time points of 0 and 96 h and
×100 magnification.
Transwell assays
A 24-well Transwell plate (8-µm pore size;
Corning Incorporated, Corning, NY, USA) was selected for the
present study. The upper compartment of the polycarbonate filter
was coated with Matrigel (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). The Matrigel matrix (5 mg/ml; 100 µl) formed a
continuous thin layer following drying for 1 h at 37°C. The PC-3
cells (1×105) were seeded onto the upper chamber
containing 100 µl FBS-free F12 culture medium, and 600
µl F12 culture medium containing 20% FBS was added to the
lower chamber. CX3CL1 (100 nM) was used in all groups, except the
CON group. The whole culture plate was incubated for 24 h. Finally,
the lower surface of the upper chamber was observed following 4%
paraformaldehyde fixation for 20 min and 0.1% crystal violet
staining for 15 min at room temperature.
Signaling pathway analysis
The Ingenuity Pathway Analysis (IPA) database
(https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis)
was used to further predict the potential factors involved in the
CX3CL1-associated Src/FAK signaling pathway in prostate cancer.
Western blot analysis
The whole cell lysate of tissues and cells was
harvested in lysis buffer (cat. no. P0013; Beyotime Institute of
Biotechnology, Haimen, China) containing a phosphorylase inhibitor
cocktail (Abcam, Cambridge, MA, USA) and phenylmethanesulfonyl
fluoride (Beyotime Institute of Biotechnology). Additionally, when
EGFR inhibitor afatinib, Src inhibitor bosutinib, and FAK inhibitor
PF-562271 (Selleck Chemicals, Houston, TX, USA) were used, the
cells were pretreated in accordance with previous studies (18–21).
Bromophenol blue 2X (Amresco, LLC, Solon, OH, USA) was added as a
loading buffer. An equal amount (20 µg; bicinchoninic acid
assay) of each sample was electrophoresed on an 8–12% SDS-PAGE gel
and transferred onto polyvinylidene fluoride membranes using an
electrotransfer system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Subsequently, the membranes were incubated with specific
antibodies, following blocking with 5% skimmed milk powder for 2 h
at room temperature. The membranes were incubated with specific
antibodies, including CX3CL1 (1:1,000; cat. no. ab25088), CX3CR1
(1:1,000; cat. no. ab8021; Abcam), Rho-associated protein kinase 1
(ROCK1; 1:2,000; cat. no. ab45171), matrix metalloproteinase-9
(MMP-9; 1:1,000; cat. no. ab73734) (all from Abcam), FAK (1:1,000;
cat. no. 3285), phosphorylated (p)-FAK (1:1,000; cat. no. 3281),
Src (1:1,000; cat. no. 2108), p-Src (1:1,000; cat. no. 6943), EGFR
(1:1,000; cat. no. 5616), p-EGFR (1:1,000; cat. no. 2235) [Cell
Signaling Technology, Inc. (CST), Danvers, MA, USA] and GAPDH
(1:5,000; cat. no. AF1186/AF0006; Beyotime Institute of
Biotechnology) at 4°C overnight. Following washing with TBS three
times, the membranes were incubated with goat anti-rabbit (1:5,000;
cat. no. D111018) or goat anti-mouse (1:5,000; cat. no. D110099)
immunoglobulin G-horseradish peroxidase (BBI Life Sciences,
Shanghai, China) antibodies at 37°C for 2 h. Luminescent liquid
(cat. no. KLS0500; EMD Millipore, Billerica, MA, USA) was added for
color rendering. The film was scanned and the net optical density
value of the strip was analyzed using a gel image processing system
(Image-Pro Plus v7.0; Media Cybernetics, Inc. Rockville, MD, USA).
The relative expression of the target protein was divided by that
of the internal control.
ELISA analysis
The concentration of CX3CL1 in the blood was
detected using Fractalkine ELISA kits (cat. no. DCX310; R&D
Systems, Inc.), in accordance with the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA from prostate carcinoma tissues, healthy
vertebrae tissues, healthy limb bone tissues and prostate cancer
cells was extracted using TRIzol® (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
A total of 2 µg RNA was reverse transcribed (65°C for 5 min,
37°C for 15 min and 98°C for 5 min) to obtain cDNA using a reagent
kit (Invitrogen; Thermo Fisher Scientific, Inc.). Subsequently,
qPCR analyses were conducted using the QuantiNova™ SYBR®
Green PCR kit (Qiagen GmbH, Hilden, Germany) and the qPCR data
collection was performed using a thermocycler (ABI 7500; Thermo
Fisher Scientific, Inc.). PCR conditions were as follows: 95°C for
2 min, 94°C for 20 sec, 58°C for 20 sec and 72°C for 20 sec; 40
cycles. The expression ratio was calculated according to the
2−ΔΔCq method (22) and
data were normalized to GAPDH. The primer sequences are presented
in Table I.
 | Table IPrimer sequences for
CX3CL1/CX3CR1. |
Table I
Primer sequences for
CX3CL1/CX3CR1.
| Name | Sequence
(5′→3′) |
|---|
| CX3CL1 | F:
5′-GACCCCTAAGGCTGAGGAAC-3′ |
| CX3CL1 | R:
5′-AGAAGAGGAGGCCAAGGAAG-3′ |
| CX3CR1 | F:
5′-GACGGTTGCATTTAGCCATT-3′ |
| CX3CR1 | R:
5′-TGCTCAGAACACTTCCATGC-3′ |
| GAPDH | F:
5′-GCGAGATCCCTCCAAAATCAA-3′ |
| GAPDH | R:
5′-GTTCACACCCATGACGAACAT-3′ |
Immunohistochemical and
immunofluorescence staining
Tissue specimens fixed in 4% polyoxymethylene (cat.
no. P1110; Beijing Solarbio Science & Technology Co., Ltd.;
>24 h at room temperature) and embedded in paraffin were
sectioned to 4 µm thick. The sections were sequentially
placed in xylene I for 15 min, xylene II for 15 min, absolute
ethanol I for 5 min, absolute ethanol II for 5 min, 85% alcohol for
5 min, 75% alcohol for 5 min and a distilled water wash. Antigenic
retrieval was performed using sodium citrate (pH 6.0; cat. no.
G1202; Wuhan Servicebio Technology Co., Ltd., Wuhan, China);
samples were placed for 8 min in a microwave oven to boil, followed
by 8 min with the microwave turned off for heat preservation,
followed by a medium heat. The sections were incubated in
H2O2 (3%) for 10 min, and blocked in 5% goat
serum (cat. no. AR1010; Wuhan Boster Biological Technology, Ltd.,
Wuhan, China) for 60 min at room temperature, followed by
anti-CX3CL1 or anti-CX3CR1 or androgen receptor (AR; 1:500; cat.
no. 5153; CST) antibodies at 37°C for 60 min. Following incubation
with the secondary antibody for 45 min at room temperature,
specimens were stained with 3,3′-diaminobenzidine buffer (1:11;
cat. no. K5007; Dako; Agilent Technologies, Inc., Santa Clara, CA,
USA) for 1 min, followed by tap water flushing to stop the staining
at room temperature. The sections were all counterstained with
hematoxylin for 3 min, dehydrated and mounted in neutral balsam
(cat. no. G8590; Beijing Solarbio Science & Technology Co.,
Ltd.). Observation was performed used a microscope (ECLIPSE TI-SR;
Nikon Corporation, Tokyo, Japan) at ×100 magnification.
Immunofluorescence was performed following a procedure described
previously (23).
Immunofluorescence was conducted to detect the expression of
F-actin (1:100; cat. no. ab205; Abcam) in cells. Observation was
performed at ×400 magnification.
Xenograft study
A total of 18 male NOD/SCID mice at 4–6 weeks of age
(14–20 g) were obtained from the Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China). The animal feeding
environment was as follows: 22–27°C; humidity, 40–70%; light/dark
cycle, 12; free access to sterilized feed and water. The animal
studies were approved by the Animal Ethics Committee of Zhongshan
Hospital, Fudan University. These mice were randomly divided into
two equal groups. A total of ~1×106
CX3CR1-overexpressing PC-3 cells or control cells were suspended in
200 µl serum-free medium and injected into the left
ventricle of the mice following sodium pentobarbital anesthesia, as
described previously (24). After
6–8 weeks, the mice received a positron emission tomography (PET)
scan for fluorodeoxyglucose localization. If a suspected spinal
metastasis was found, the lesion underwent a further micro-computed
tomography (CT) scan and pathological examination.
Statistical analyses
The data are expressed as the mean ± standard
deviation. The analyses were performed using SPSS statistical
software version 16.0 (SPSS, Inc., Chicago, IL, USA). The
χ2 test was used for the xenograft study (spinal
tumorigenesis rate). Student's t-tests and one-way analysis of
variance followed by Tukey's post-hoc test were used to make
statistical comparisons between groups (clinical samples and cell
samples). All experiments were repeated at least three times.
P<0.05 was considered to indicate statistical significance.
Results
CX3CL1/CX3CR1 is overexpressed in human
prostate cancer tissue and cell lines
To investigate the potential role of CX3CL1/CX3CR1
in prostate cancer spinal metastasis, 12 cases of prostate
carcinoma with spinal metastasis, 12 cases of primary spinal tumor,
12 samples of healthy vertebrae and 12 samples of healthy limb bone
tissues (fractures) were collected for western blot and RT-qPCR
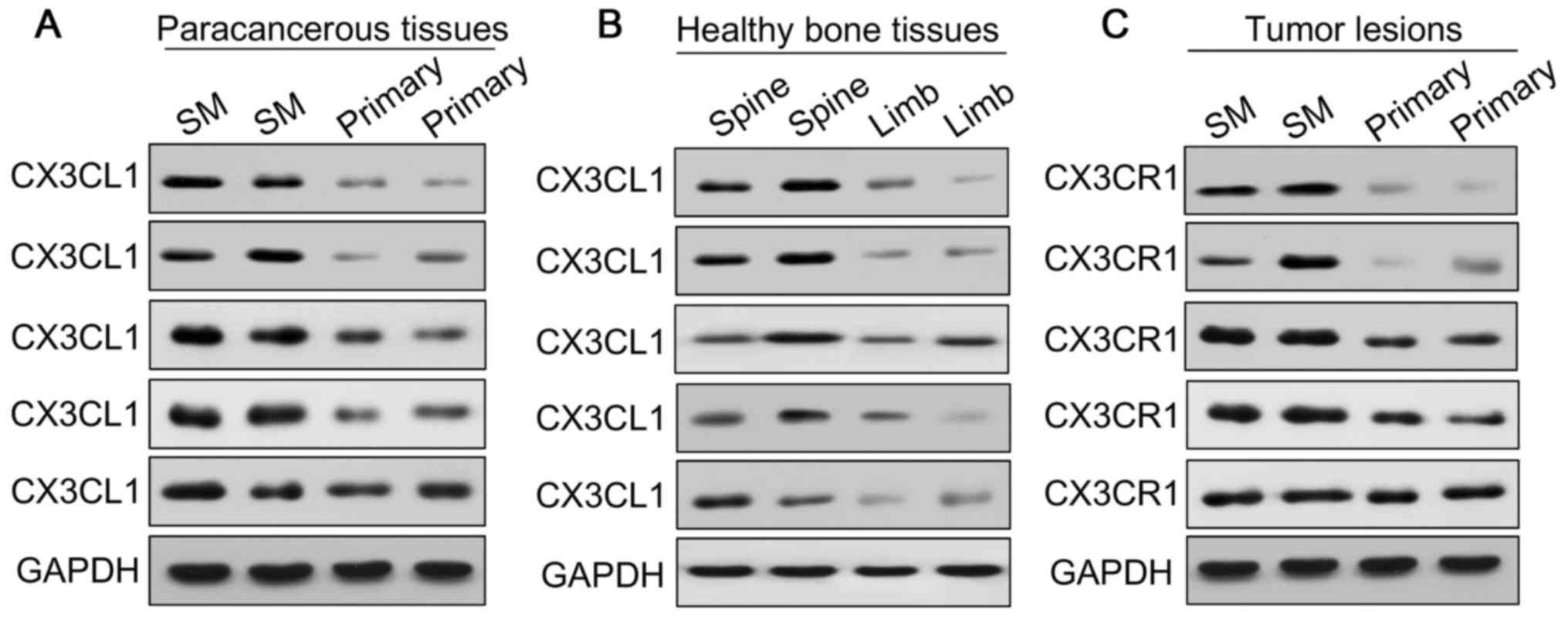
analyses to measure the expression of CX3CL1/CX3CR1 (Figs. 1 and 2). The results demonstrated that the
expression of CX3CL1/CX3CR1 in metastatic spinal lesions from
prostate cancer was higher compared with primary spinal tumors
(P<0.01 and P<0.05; Figs. 1A,
B and 2A). Additionally,
CX3CL1 was highly expressed in healthy spinal osseous tissues
compared with healthy limb osseous tissues (P<0.01 and
P<0.05; Figs. 1A, B and
2B). The ELISA analysis
demonstrated that the average concentration of CX3CL1 in the blood
was 0.31 ng/ml in prostate cancer tissues with spinal metastasis,
which was increased compared with the 0.12 and 0.16 ng/ml observed
in primary tumors and healthy human blood, respectively (P<0.05;
Fig. 1C). Furthermore, it was
identified that the protein expression of CX3CR1 was significantly
increased in prostate carcinomas with spinal metastasis compared
with primary spinal tumors (P<0.05; Figs. 1D, E and 2C).
 | Figure 1CX3CL1/CX3CR1 is overexpressed in
human prostate cancer tissues and cell lines. CX3CL1 was
overexpressed in the paracancerous tissues of spinal metastasis
(SM) and healthy spines, as demonstrated by (A) western blotting,
(B) densitometric analysis of western blotting, and RT-qPCR of the
CX3CL1 mRNA expression level; GAPDH was as a loading control.
*P<0.05, **P<0.01 vs. respective
control. n=12. (C) The concentration of CX3CL1 in the blood of
patients with prostate cancer with SM, primary tumors and healthy
cases was measured by ELISA. **P<0.01 vs. primary;
##P<0.01 vs. healthy. CX3CR1 was overexpressed in
prostate cancer with SM compared with primary cancer tissues as
demonstrated by (D) western blotting, (E) densitometric analysis of
western blotting, and RT-qPCR of the CX3CR1 mRNA expression level;
GAPDH was used as a loading control. *P<0.05 vs.
respective control. n=12. CX3CR1 expression in VCaP cells (derived
from SM) was increased compared with 22RV1 cells (derived from
primary tumors) and RWEP-1 cells (healthy prostate epithelial
cells), as demonstrated by (F) western blotting and (G)
densitometric analysis. **P<0.01 vs. 22RV1;
##P<0.01 vs. RWEP1. (H) The mRNA expression of CX3CR1
in VCaP cells was increased compared with 22RV1 and PC-3 cells.
*P<0.05 vs. 22RV1; ##P<0.01 vs. VCaP.
(I) Clinical samples were collected and examined by
immunohistochemical staining with CX3CL1 and CX3CR1 antibodies.
Representative staining images are presented (×100 magnification).
(J) Immunohistochemical staining of CX3CL1 and CX3CR1 is presented
in healthy human spinal tissue, limb osseous tissues, primary
prostate cancer and healthy prostate tissues (×100 magnification).
SM, spinal metastasis; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; CX3CL1, C-X3-C motif chemokine ligand 1;
CX3CR1, C-X3-C motif chemokine receptor 1. |
In human prostate cancer cells (VCaP, PC-3, LNCap
and 22RV1) and prostate epithelial cells (RWEP-1), it was observed
that the expression of CX3CR1 was higher in prostate cancer cells
compared with normal prostate epithelial cells, as indicated by
western blotting and RT-qPCR (Fig.
1F–H). Furthermore, the expression of CX3CL1 and CX3CR1 was
detected in prostate carcinomas with spinal metastasis and primary
tumor tissues by immunohistochemistry, and it was revealed that the
expression of CX3CL1 and CX3CR1 was increased in prostate
carcinomas with spinal metastasis (Fig. 1I and J). Similar results were
observed in healthy spines and healthy limbs. It was additionally
demonstrated that the expression of CX3CR1 was increased in
prostate cancer tissues compared with healthy prostate tissues
(Fig. 1J).
CX3CR1 promotes cell proliferation and
inhibits cellular apoptosis
To examine the potential role of CX3CR1 in prostate
cancer cell function, a lentivirus was used to construct stable OE
and NC cell lines, in VCaP and PC-3 cells. The cells were
transfected with either the specific siRNA or NC to suppress the
expression of CX3CR1. The expression of CX3CR1 protein and mRNA is
presented in Fig. 3A and B.
Subsequently, the cell proliferation of these cells was measured by
CCK-8 assays. As exhibited in Fig. 3C
and D, overexpression of CX3CR1 promoted cell proliferation and
knockdown of CX3CR1 reduced cell proliferation. In addition,
cellular apoptosis was measured by flow cytometry, and it was
observed that the overexpression of CX3CR1 inhibited cellular
apoptosis, and the repression of CX3CR1 increased cellular
apoptosis (Fig. 3E and F). These
results suggested that CX3CR1 expression may be associated with
prostate cancer cell proliferation and apoptosis.
 | Figure 3CX3CR1 promotes cell proliferation
and inhibits cellular apoptosis. Stably-overexpressing CX3CR1 and
control cell lines (VCaP and PC-3) were constructed by lentivirus
infection. Specific siRNA was used to inhibit the expression of
CX3CR1 (KD) in VCaP and PC-3 cells. The expression of CX3CR1 was
determined by (A) western blotting and reverse
transcription-quantitative polymerase chain reaction analysis (B,
left: VCaP; right: PC-3). Cell counting kit-8 assays was used to
demonstrate VCaP and PC-3 cell proliferation following lentivirus
infection (C, left: VCaP; right: PC-3) or specific siRNA
transfection (D, left: VCaP; right: PC-3). *P<0.05,
**P<0.01 vs. respective NC group. The experiment was
repeated three times. Annexin V-FITC/PI assays were used to detect
the cellular apoptosis of (E) VCaP and (F) PC-3 cells following
lentivirus infection or siRNA transfection. The experiment was
repeated three times. *P<0.05,
**P<0.01. OE, overexpression; NC, negative control;
KD, knockdown; CON, control; CX3CL1, C-X3-C motif chemokine ligand
1; CX3CR1, C-X3-C motif chemokine receptor 1; siRNA, small
interfering RNA; FITC, fluorescein isothiocyanate; PI, propidium
iodide. |
CX3CL1 induces cell migration and
invasion
The effects of CX3CL1/CX3CR1 on cell migration and
invasion were further detected by scratch wound and Transwell
assays. The scratch wound assays revealed that treatment with
CX3CL1 (100 nM) or overexpression of CX3CR1 in VCaP cells increased
the cell migration rates (P<0.05), and knockdown of CX3CR1
decreased VCaP cell migration rates (P<0.01), as presented in
Fig. 4A–C. Additionally, cell
invasion was assessed by Transwell assay, and it was observed that
PC-3 cell invasion was regulated by CX3CR1 expression. In CX3CL1
(100 nM)-treated or CX3CR1-overexpressing PC-3 cells, cell invasion
was increased by 25.95 and 43.63%, respectively (Fig. 4D and E). However, in
CX3CR1-knockdown PC-3 cells, cell invasion decreased by 38.89%
(P<0.05; Fig. 4F).
Additionally, the present study aimed to determine whether the
organization of the actin cytoskeleton was altered in
CX3CR1-overexpressing or knockdown cells. As presented in Fig. 4G, in CX3CL1-treated cells or
CX3CR1-overexpressing cells, F-actin structures formed
lamellipodial protrusions around the periphery of the cells.
However, in CX3CR1-knockdown PC-3 cells, the actin-rich membrane
ruffles were absent. The results of the present study suggested
that CX3CL1 may serve important roles in prostate cancer cell
migration and invasion.
 | Figure 4CX3CL1 induces cell migration and
invasion. Cell migration was measured by scratch wound assay in (A)
CX3CL1-treated, (B) CX3CR1 overex-pression and (C) CX3CR1-knockdown
group at time point of 96 h. Representative images are presented
(left panel; ×100 magnification). The results were summarized from
three independent experiments (right panel). *P<0.05,
**P<0.01 vs. respective control. Cell invasion was
determined by Transwell assay in (D) CX3CL1-treated, (E) CX3CR1
overexpression and (F) CX3CR1-knockdown group. Representative
images are presented (upper panel; ×200 magnification). The results
were summarized from three independent experiments (lower panel).
*P<0.05 vs. respective control. (G) Cells were fixed
and stained with F-actin in CX3CL1 treated, CX3CR1 overexpression
and CX3CR1 knockdown group, the representative images are shown
(magnification, ×400). OE, overex-pression; NC, negative control;
KD, knockdown; CON, control; CX3CL1, C-X3-C motif chemokine ligand
1. |
CX3CL1/CX3CR1 activates the Src/FAK
pathway
Previous work demonstrated that CX3CL1 is associated
with spinal metastasis in different types of cancer (17) and that the Src/FAK signaling
pathway is associated with spinal metastasis (data not shown). The
IPA database predicted that kinases, including Src,
protein-tyrosine kinase 2-β (PTK2B) and FAK were associated with
CX3CL1/CX3CR1 in prostate cancer cells (Fig. 5). Therefore, the activities of
these kinases were examined by detecting their phosphorylated
expression levels. Following treatment with CX3CL1 (100 nM), the
expression of phosphorylated Src (Tyr416) and phosphorylated FAK
(Tyr576/577) increased in a time-dependent manner (Fig. 6A). In addition, the phosphorylation
of epidermal growth factor receptor (EGFR) (Tyr992) was markedly
induced (Fig. 6B). However, the
phosphorylated PTK2B did not alter following treatment with CX3CL1
(Fig. 6B). Additionally,
CX3CR1-overexpressing and knockdown cells were used to detect the
phosphorylation of EGFR, FAK and Src. The results revealed that
similar to treatment with CX3CL1, overexpression of CX3CR1
increased the phosphorylation of EGFR, FAK and Src, while
downregulating CX3CR1 decreased the phosphorylation of EGFR, FAK
and Src in VCaP cells (Fig. 6C and
D).
 | Figure 6CX3CL1/CX3CR1 activates the Src/FAK
pathway. (A) VCaP cells were treated either with or without CX3CL1
(100 nM) for 5, 15, 30, 45 or 60 min, and the expression of Src,
p-Src (Tyr416), FAK and p-FAK (Tyr576/577) was determined by
western blotting. (B) VCaP cells were treated either with or
without CX3CL1 (100 nM) for 5, 15, 30, 45 or 60 min, and the
expression of EGFP, p-EGFR (Tyr992), PTK2B and p-PTK2B (Tyr402) was
measured by western blotting. The expression of EGFR, p-EGFR
(Tyr992), Src, p-Src (Tyr416), FAK and p-FAK (Tyr576/577) was
measured by western blotting in CX3CL1-treated (100 nM), stable
CX3CR1-overexpressing or siRNA-induced CX3CR1-knockdown cells: (C)
VCaP; (D) PC-3. PC-3 cells were pretreated with (E) bosutinib (0.5
nM for 3 h) or with (F) PF-562271 (0.2 nM for 0.5 h), following
which CX3CL1 was added to cells (100 nM for 5, 15, 30, 45 or 60
min), and the expression of p-Src (Tyr416) and p-FAK (Tyr576/577)
was examined. VCaP cells were pretreated with either afatinib (1 or
10 µM for 4 h) or bosutinib (0.25 or 2 µM for 1 h),
following which CX3CL1 (100 nM) was added to cells (100 nM) for 30
min, and the expression of (G) Src, p-Src (Tyr416), (H) EGFR and
p-EGFR (Tyr992) were detected by western blotting. (I and J) The
expression of MMP-9 and ROCK1 was measured by western blotting
CX3CL1-treated (100 nM), stable CX3CR1-overexpressing or
siRNA-induced CX3CR1-knockdown cells: (I) VCaP; (J) PC-3. Src,
proto-oncogene tyrosine-protein kinase Src; FAK, focal adhesion
kinase; EGFR, epidermal growth factor receptor; p, phosphorylated;
OE, overexpression; NC, negative control; KD, knockdown; CON,
control; CX3CL1, C-X3-C motif chemokine ligand 1; PTK2B,
protein-tyrosine kinase 2-β; CX3CR1, C-X3-C motif chemokine
receptor 1; MMP-9, matrix metal-loproteinase-9; ROCK1,
Rho-associated protein kinase 1. |
Subsequently, the Src inhibitor bosutinib, the FAK
inhibitor PF-562271, and the EGFR inhibitor afatinib were used to
further investigate the signaling pathway involved in
CX3CL1/CX3CR1-induced prostate cancer progression. The results
demonstrated that CX3CL1-induced Src and FAK phosphorylation were
blocked by their corresponding inhibitors (Fig. 6E and F). Furthermore, the EGFR
inhibitor afatinib markedly reduced the phosphorylation of Src
(Fig. 6G); however, the Src
inhibitor bosutinib did not inhibit the phosphorylation of EGFR
(Fig. 6H), suggesting that Src is
a downstream effector of EGFR in a CX3CL1/CX3CR1-associated
pathway. Furthermore, the expression of MMP-9 and ROCK1 was
regulated by CX3CR1 expression (Fig.
6I and J). Functionally, the inhibitors of FAK, Src and EGFR
reversed the induction of cell migration caused by treatment with
CX3CL1, as evaluated by scratch wound assay (Fig. 7).
CX3CL1/CX3CR1 facilitates spinal
metastasis of prostate cancer in vivo
Finally, the present study employed xenograft mouse
models to verify the association between CX3CL1 and spinal
metastasis in prostate cancer. PC3 cells were injected into the
left ventricles of the mice, which was the closest way to mimic the
clinical metastasis of the tumor. It was observed that four out of
nine (44.44%) and one out of nine (11.11%) mice in the
CX3CR1-overexpressing and control cell groups, respectively, formed
metastases. All the metastases in the CX3CR1-overexpressing cell
injected group involved the spine, while the control cell injected
group exhibited metastasis to the maxillofacial bones (Fig. 8). PET scans demonstrated that
fluorodeoxyglucose was concentrated in the locality of the spine.
Furthermore, micro-CT scans revealed that lesions in the affected
vertebrae, indicating that they were damaged in
CX3CR1-overexpressing cell-injected mice (Fig. 9A). The diagnosis of spinal
metastasis of prostate cancer was verified by hematoxylin and eosin
staining and positive immunohistochemical staining of AR (Fig. 9B). It was additionally identified
that one case in the CX3CR1 overexpression group exhibited femoral
metastases accompanied by spinal metastases. In addition, CX3CR1
exhibited increased expression in prostate cancer tissues compared
with healthy spines (Fig. 9B and
C). The results of the present study demonstrated that
CX3CL1/CX3CR1 induced spinal metastasis in an in vivo
model.
Discussion
The present study reported that the expression of
CX3CL1/CX3CR1 in prostate cancer tissues with spinal metastasis was
increased compared with primary tumors, and the expression of
CX3CL1 in healthy spines was increased compared with healthy limb
osseous tissues. In addition, in prostate cancer cell lines, the
expression of CX3CR1 was increased in prostate cancer cells lines
that were derived from metastases of the spine, bone and lymph
nodes when compared with cells that were derived from primary
tumors, and healthy human prostate epithelial cells. Furthermore,
overexpression of CX3CL1/CX3CR1 induced cell proliferation,
migration and invasion, while downregulation of CX3CL1/CX3CR1
reduced cell proliferation, migration and invasion and induced
cellular apoptosis. It was also observed that CX3CL1/CX3CR1
increased the phosphorylation of EGFR, FAK and Src. The inhibitors
of these kinases reversed the effects of treatment with CX3CL1 or
overexpression of CX3CR1, and inhibited phosphorylation of these
kinases and cell migration, suggesting that the EGFR, FAK and Src
signaling pathways may be involved in CX3CL1/CX3CR1-induced
prostate cancer progression. Furthermore, overexpression of CX3CR1
induced spinal metastasis in an in vivo prostate cancer
model. Therefore, the present study demonstrated for the first
time, to the best of our knowledge, that CX3CL1/CX3CR1 promotes
prostate cancer spinal metastasis by regulating the Src/FAK
pathway, suggesting that CX3CL1/CX3CR1 may be a potential target in
future studies aimed at preventing prostate cancer progression.
Chemokines serve important roles in the progression
of cancer. Previous studies have demonstrated that the
CX3CL1/CX3CR1 axis is involved in the proliferation, survival and
metastasis of various malignant tumor types (11–13).
In prostate cancer, the CX3CL1/CX3CR1 axis activates the
phosphatidylinositol 3-kinase/RAC-α serine/threonine-protein kinase
pathway and serves an essential role in skeletal metastasis
(16). Additionally, CX3CL1/CX3CR1
increases invasion and metastasis by promoting
epithelial-to-mesenchymal transition through disintegrin and
metalloproteinase domain-containing protein 17/protransforming
growth factor-α/EGFR pathway in hypoxic androgen-independent
prostate cancer cells (13). A
recent study using microarray analysis reported that patients with
spinal metastases from prostate cancer exhibited significantly high
levels of CX3CL1 (17). The
results of the present study demonstrated that CX3CL1 was highly
expressed in healthy spinal osseous tissues compared with healthy
limb osseous tissues, suggesting that prostate cancer was more
prone to metastasis to spinal osseous tissues compared with limb
osseous tissues. Additionally, in prostate cancer tissues with
spine metastasis, the expression of CX3CL1/CX3CR1 was increased
compared with primary prostate cancer, suggesting that
CX3CL1/CX3CR1 promotes the spinal metastasis of prostate cancer. As
the CX3CL1/CX3CR1 axis was involved in the progression of different
types of tumor, the present results suggested that CX3CL1/CX3CR1
may serve essential roles in spine metastasis in other types of
tumor in addition to prostate cancer. The present data demonstrated
that overexpression of CX3CR1 induced cell proliferation, migration
and invasion, while downregulation of CX3CR1 reduced these
processes and increased cellular apoptosis. Recently, a number of
studies reported that CX3CR1 induced apoptosis resistance in
different types of cancer (25–27).
The present results were confirmed by these studies, suggesting
that knockdown of CX3CR1 may serve a suppressive role in tumors,
and CX3CL1/CX3CR1 may serve as a therapeutic target in prostate
cancer. VCaP cells were established from a vertebral metastatic
lesion with lower metastatic potential compared with PC-3 cells;
PC-3 cells were selected to detect the cell motility, and it was
observed that CX3CL1/CX3CR1 promoted prostate cancer cell
migration, invasion and actin organization, while suppression of
CX3CR1 inhibited cell migration and invasion as demonstrated by
scratch wound assay and Transwell assay, consistent with a previous
study (28). As ROCK1 is a key
molecule in the regulation of the cytoskeleton (29,30),
the present study detected the expression of ROCK1 and demonstrated
that CX3CL1/CX3CR1 regulated ROCK1 expression. These results
suggest that CX3CL1/CX3CR1 increases cell migration rates by
regulating cytoskeleton formation.
Src/FAK signaling is known for its important effects
on cell migration through the reorganization of the cytoskeleton
(31). The activation of Src is
achieved by the sequential phosphorylation/autophosphorylation of
tyrosine residues (Tyr527 and Tyr416) (32). FAK is a widely expressed
cytoplasmic protein-tyrosine kinase involved in cell spreading,
migration and survival (33–35).
Recruitment of the Src family kinases leads to the phosphorylation
of Tyr576 and Tyr577 in the catalytic domain of FAK (36). In addition, numerous studies have
demonstrated that aberrant activation of the Src family of kinases
serves important roles in the bone metastasis of prostate cancer
(37). To explain the effects of
CX3CL1/CX3CR1 on cell migration, the phosphorylation of Src and FAK
was detected, and it was observed that treatment with CX3CL1 or
overexpression of CX3CR1 increased the expression of phosphorylated
Src and FAK. Functionally, the inhibitors of EGFR, Src or FAK
reversed cell migration induced by CX3CL1/CX3CR1, suggesting that
the Src/FAK pathway is involved in CX3CL1/CX3CR1-induced cell
migration. Furthermore, we observed that the expression of MMP-9
was increased in CX3CR1 overexpressing cells, which may explain the
role of CX3CL/CX3CR1 in cell invasion.
The predicted results from the IPA database
demonstrated that EGFR and PTK2B interact with Src. The present
data demonstrated that the phosphorylation of EGFR was increased
following treatment with CX3CL1 or overexpression of CX3CR1. In
addition, the EGFR inhibitor afatinib inhibited the phosphorylation
of Src, while the inhibitor of Src did not block the
phosphorylation of EGFR, verifying that Src is a downstream
effector of EGFR in a CX3CL1-associated pathway. These results
revealed that the Src/FAK signaling pathway is activated by CX3CL1
and the phosphorylation of EGFR is required for Src/FAK activation
in prostate cancer cells. The PC-3 cell line was established from a
bone metastasis of a patient with grade IV prostate cancer and is
frequently used as a model of subcutaneous tumors in mice.
Therefore, the PC3 cell line was used to detect the effects of
CX3CL1/CX3CR1 on prostate cancer progression in an in vivo
model, and demonstrated that the final number of spinal metastasis
cases increased in the CX3CR1-overexpressing group compared with
the control group, which is consistent with the clinical specimen
pathological findings. These data further demonstrated that
CX3CL1/CX3CR1 induces spinal metastasis in prostate cancer and
serves important roles in prostate cancer progression.
In conclusion, the CX3CL1/CX3CR1 axis promoted
prostate cancer cell proliferation, migration and invasion through
the Src/FAK pathway in vitro. Future studies are required to
further verify the effects of the CX3CL/CX3CR1 axis on spine
metastasis in other type of solid tumors. The present data suggest
that CX3CL1/CX3CR1 may act as a potential target in metastatic
prostate cancer therapy.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81572629 and
81772855) and the China Postdoctoral Science Foundation (grant no.
2017M621362).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PL and JD conceived and designed this study. PL, YL
and HW conducted the experiments. PL and LJ analyzed and checked
the data. SW collected and pretreated the clinical specimens. PL
wrote the paper. JD supervised the whole experimental works and
revised the manuscript. All authors have read and approved the
final submitted manuscript.
Ethics approval and consent to
participate
All the clinical samples were approved by the Ethics
Committee of Zhongshan Hospital, Fudan University (Shanghai, China;
no. Y2014-185). Informed consent was provided by the patients prior
to surgery. The animal studies were approved by the Animal Ethics
Committee of Zhongshan Hospital, Fudan University.
Patient consent for publication
Informed consent was provided by the patients prior
to surgery.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aaron AD: The management of cancer
metastatic to bone. JAMA. 272:1206–1209. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dominguez DE, Lauper N, Velastegui A and
Reynolds J: Surgical management of the spinal metastases. Rev Med
Suisse. 12:2168–2171. 2016.In French.
|
|
3
|
Wu AS and Fourney DR: Evolution of
treatment for metastatic spine disease. Neurosurg Clin N Am.
15:401–411. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dushyanthen S, Cossigny DA and Quan GM:
The osteoblastic and osteoclastic interactions in spinal metastases
secondary to prostate cancer. Cancer Growth Metastasis. 6:61–80.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choi D, Crockard A, Bunger C, Harms J,
Kawahara N, Mazel C, Melcher R and Tomita K; Global Spine Tumor
Study Group: Review of metastatic spine tumour classification and
indications for surgery: the consensus statement of the Global
Spine Tumour Study Group. Eur Spine J. 19:215–222. 2010. View Article : Google Scholar :
|
|
6
|
Muralidharan A and Smith MT: Pathobiology
and management of prostate cancer-induced bone pain: Recent
insights and future treatments. Inflammopharmacology. 21:339–363.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Przybyla BD, Shafirstein G, Vishal SJ,
Dennis RA and Griffin RJ: Molecular changes in bone marrow, tumor
and serum after conductive ablation of murine 4T1 breast carcinoma.
Int J Oncol. 44:600–608. 2014. View Article : Google Scholar :
|
|
9
|
Salazar N, Castellan M, Shirodkar SS and
Lokeshwar BL: Chemokines and chemokine receptors as promoters of
prostate cancer growth and progression. Crit Rev Eukaryot Gene
Expr. 23:77–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liao YX, Zhou CH, Zeng H, Zuo DQ, Wang ZY,
Yin F, Hua YQ and Cai ZD: The role of the CXCL12-CXCR4/CXCR7 axis
in the progression and metastasis of bone sarcomas (Review). Int J
Mol Med. 32:1239–1246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu JF, Tsao YT and Hou CH:
Fractalkine/CX3CL1 induced intercellular adhesion
molecule-1-dependent tumor metastasis through the
CX3CR1/PI3K/Akt/NF-κB pathway in human osteosarcoma. Oncotarget.
8:54136–54148. 2016.
|
|
12
|
Tardáguila M, Mira E, García-Cabezas MA,
Feijoo AM, Quintela-Fandino M, Azcoitia I, Lira SA and Mañes S:
CX3CL1 promotes breast cancer via transactivation of the EGF
pathway. Cancer Res. 73:4461–4473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang J, Xiao L, Cui R, Li D, Zheng X, Zhu
L, Sun H, Pan Y, Du Y and Yu X: CX3CL1 increases invasiveness and
metastasis by promoting epithelial-to-mesenchymal transition
through the TACE/TGF-α/EGFR pathway in hypoxic androgen-independent
prostate cancer cells. Oncol Rep. 35:1153–1162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferretti E, Pistoia V and Corcione A: Role
of fractalkine/CX3CL1 and its receptor in the pathogenesis of
inflammatory and malignant diseases with emphasis on B cell
malignancies. Mediators Inflamm. 2014:4809412014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shulby SA, Dolloff NG, Stearns ME, Meucci
O and Fatatis A: CX3CR1-fractalkine expression regulates cellular
mechanisms involved in adhesion, migration, and survival of human
prostate cancer cells. Cancer Res. 64:4693–4698. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jamieson WL, Shimizu S, D'Ambrosio JA,
Meucci O and Fatatis A: CX3CR1 is expressed by prostate epithelial
cells and androgens regulate the levels of CX3CL1/fractalkine in
the bone marrow: Potential role in prostate cancer bone tropism.
Cancer Res. 68:1715–1722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu W, Bian C, Liang Y, Jiang L, Qian C
and Dong J: CX3CL1: A potential chemokine widely involved in the
process spinal metastases. Oncotarget. 8:15213–15219.
2017.PubMed/NCBI
|
|
18
|
Wiemer AJ, Wernimont SA, Cung TD, Bennin
DA, Beggs HE and Huttenlocher A: The focal adhesion kinase
inhibitor PF-562,271 impairs primary CD4+ T cell
activation. Biochem Pharmacol. 86:770–781. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu W, He X, Ni Y, Ngeow J and Eng C:
Cowden syndrome-associated germline SDHD variants alter PTEN
nuclear translocation through SRC-induced PTEN oxidation. Hum Mol
Genet. 24:142–153. 2015. View Article : Google Scholar
|
|
20
|
Lee BY, Hochgräfe F, Lin HM, Castillo L,
Wu J, Raftery MJ, Martin Shreeve S, Horvath LG and Daly RJ:
Phosphoproteomic profiling identifies focal adhesion kinase as a
mediator of docetaxel resistance in castrate-resistant prostate
cancer. Mol Cancer Ther. 13:190–201. 2014. View Article : Google Scholar
|
|
21
|
Rabbani SA, Valentino ML, Arakelian A, Ali
S and Boschelli F: SKI-606 (Bosutinib) blocks prostate cancer
invasion, growth, and metastasis in vitro and in vivo through
regulation of genes involved in cancer growth and skeletal
metastasis. Mol Cancer Ther. 9:1147–1157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-DeltaDeltaC(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Li HY, Cui XY, Wu W, Yu FY, Yao HR, Liu Q,
Song EW and Chen JQ: Pyk2 and Src mediate signaling to
CCL18-induced breast cancer metastasis. J Cell Biochem.
115:596–603. 2014. View Article : Google Scholar
|
|
24
|
Sarabia-Estrada R, Zadnik PL, Molina CA,
Jimenez-Estrada I, Groves ML, Gokaslan ZL, Bydon A, Witham TF,
Wolinsky JP and Sciubba DM: A rat model of metastatic spinal cord
compression using human prostate adenocarcinoma: Histopathological
and functional analysis. Spine J. 13:1597–1606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun Y, Wang F, Sun X, Wang X, Zhang L and
Li Y: CX3CR1 regulates osteoarthrosis chondrocyte proliferation and
apoptosis via Wnt/beta-catenin signaling. Biomed Pharmacother.
96:1317–1323. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang H, Cai J, Du S, Guo Z, Xin B, Wang J,
Wei W and Shen X: Fractalkine/CX3CR1 induces apoptosis resistance
and proliferation through the activation of the AKT/NF-κB cascade
in pancreatic cancer cells. Cell Biochem Funct. 35:315–326. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo W, Lin Y, Meng S, Guo Y, Zhang J and
Zhang W: miRNA-296-3p modulates chemosensitivity of lung cancer
cells by targeting CX3CR1. Am J Transl Res. 8:1848–1856.
2016.PubMed/NCBI
|
|
28
|
Yao X, Qi L, Chen X, Du J, Zhang Z and Liu
S: Expression of CX3CR1 associates with cellular migration,
metastasis, and prognosis in human clear cell renal cell carcinoma.
Urol Oncol. 32:162–170. 2014. View Article : Google Scholar
|
|
29
|
Xu Z, Zheng X, Yang L, Liu F, Zhang E,
Duan W, Bai S, Safdar J, Li Z and Sun C: Chemokine receptor 7
promotes tumor migration and invasiveness via the RhoA/ROCK pathway
in metastatic squamous cell carcinoma of the head and neck. Oncol
Rep. 33:849–855. 2015. View Article : Google Scholar
|
|
30
|
Lv Z, Hu M, Ren X, Fan M, Zhen J, Chen L,
Lin J, Ding N, Wang Q and Wang R: Fyn mediates high glucose-induced
actin cytoskeleton reorganization of podocytes via promoting ROCK
activation in vitro. J Diabetes Res. 2016:56718032016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hamaguchi M, Yamagata S, Thant AA, Xiao H,
Iwata H, Mazaki T and Hanafusa H: Augmentation of metalloproteinase
(gelatinase) activity secreted from Rous sarcoma virus-infected
cells correlates with transforming activity of src. Oncogene.
10:1037–1043. 1995.PubMed/NCBI
|
|
32
|
Hunter T: A tail of two src's: Mutatis
mutandis. Cell. 49:1–4. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Parsons JT, Martin KH, Slack JK, Taylor JM
and Weed SA: Focal adhesion kinase: A regulator of focal adhesion
dynamics and cell movement. Oncogene. 19:5606–5613. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Figel S and Gelman IH: Focal adhesion
kinase controls prostate cancer progression via intrinsic kinase
and scaffolding functions. Anticancer Agents Med Chem. 11:607–616.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rentala S, Chintala R, Guda M, Chintala M,
Komarraju AL and Mangamoori LN: Atorvastatin inhibited
Rho-associated kinase 1 (ROCK1) and focal adhesion kinase (FAK)
mediated adhesion and differentiation of
CD133+CD44+ prostate cancer stem cells.
Biochem Biophys Res Commun. 441:586–592. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schlaepfer DD, Hanks SK, Hunter T and van
der Geer P: Integrin-mediated signal transduction linked to Ras
pathway by GRB2 binding to focal adhesion kinase. Nature.
372:786–791. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jin JK, Dayyani F and Gallick GE: Steps in
prostate cancer progression that lead to bone metastasis. Int J
Cancer. 128:2545–2561. 2011. View Article : Google Scholar : PubMed/NCBI
|