Introduction
Oral squamous cell carcinoma (OSCC) is the most
prevalent malignant tumour in the oral and maxillofacial region
(1). The high incidence of oral
cancer is attributed to physical, chemical and biological factors.
According to the statistics of American Cancer Association, there
were about 48,000 newly diagnosed cases of OSCC in 2016, which
accounted for 3% of all new malignant tumor cases (2-5). At
present, surgery combined with radiotherapy and chemotherapy is the
primary treatment option for oral cancer. However, the 5-year
survival rate of patients with oral cancer has not significantly
improved over the past decade (6,7). The
invasive and metastatic ability of tumour cells is one of the main
factors affecting the prognosis of patients (8). The growth of tumours is influenced by
the surrounding microenvironment (9). However, the molecular mechanism
underlying the rapid tumour growth, maintenance of invasiveness and
metastatic capability remain unclear.
Epithelial-mesenchymal transformation (EMT) refers
to the biological process in which epithelial cells transform into
cells that exhibit a more mesenchymal phenotype. It has been
previously reported to serve an important role in embryonic
development, wound healing and tumour metastasis (10-14).
The main characteristic of EMT is a reduction in the expression of
cell adhesion molecules such as E-cadherin and the conversion of
expression profiles from keratin to vimentin in the cytoskeleton
(10). It is an important
biological process for the invasion and migration of OSCC cells.
The regulation of EMT involves a complex network of signalling
pathways, including those of the transforming growth factor-β
family, Wnt, Notch, epidermal growth factor (EGF), hepatocyte
growth factor, fibroblast growth factor (FGF) and hypoxia-inducible
factor (HIF) (15-17). Malignant tumour cells mainly meet
their metabolic demands through glycolysis, even under a plentiful
supply of oxygen, in a phenomenon known as the Warburg effect
(18). Glycolysis has been
previously demonstrated to promote the invasion of HeLa cells
(19). A number of transcription
factors, including HIF-1α, c-Myc, NF-κB and p53, have been
previously found to be involved in the regulation of glycolysis in
cancer cells (20-23). In OSCC, pyruvate kinase M1/2
dephosphorylation has been previously demonstrated to promote the
Warburg effect and tumorigenesis, whilst silencing
phosphofructokinase, platelet (PFKP) expression inhibited
starvation-induced autophagy, glycolysis and EMT (24).
PGK participates in the second stage of glycolysis,
where it catalyzes the conversion of 1,3-diphosphoglyceride into
3-phosphoglycerate, consuming a molecule of ADP and produces a
molecule of ATP (25).
Phosphoglycerate kinase (PGK) is an essential enzyme that is
associated with the survival of every organism, where mutations in
PGK results in a number of metabolic disorders, including mental
retardation, neurological disorders and rhabdomyolysis (25). There are PGK two main subtypes of
PGK, namely PGK1 and PGK2, both of which have similar functions and
structures (26). PGK1 serves a
speed limiting role in the second stage of glycolysis during the
regulation of energy production and redox balance (27). Aberrant PGK1 expression has been
previously associated with the occurrence of a number of diseases,
including Parkinson's disease and hereditary non-spherical
hemolytic anemia (28-30). By contrast, the PGK2 gene is only
expressed in spermatogenic cells, where its only known function is
to compensate for the inhibition of PGK1 expression caused by the
inactivation of X chromosomes in spermatocytes (31). Since PGK1 is a mediator of
glycolysis that supplies ATP for tumor cell metabolism under
hypoxic conditions, it has been reported to participate in the
development and progression of numerous cancer types, including
renal, gastric and lung cancer (32-34).
In the present study, hypoxia-induced glycolysis was
established in OSCC tumour cell lines in vitro, where the
effects of PGK1 on the invasive and migratory capabilities of OSCC
cells and the underlying molecular mechanism were investigated.
Results from the present study demonstrated that hypoxia resulted
in the activation of PGK1, in turn promoting glycolysis, increasing
stem cell-like properties and promoting EMT via AKT signalling in
OSCC.
Materials and methods
Patients and tissue samples
In total, 92 OSCC tissue samples and 20 matched
non-cancerous tissue samples were collected from the inpatients
(age range, 34-87 years; sex 58 males and 34 females) at the
Affiliated Stomatological Hospital of Sun Yat-sen University
(Guangzhou, China) from June 2008 to June 2017. Immunohistochemical
assays for PGK1 were performed on all 112 samples. The removal of
tissue samples from patients was approved by the ethical review
committee of the Affiliated Stomatological Hospital of Sun Yat-sen
University.
All patients received radical surgery and none
received any form of pre-surgical adjuvant therapy. The clinical
and pathological characteristics (sex, age, differentiation, T
stage, clinical stage and lymph node metastasis) of the cancer in
each patient were established in accordance with the criteria of
the American Joint Committee on Cancer (35). The experiments were performed with
the understanding and written consent of each subject. The study
methodologies conformed to the standards set by the Declaration of
Helsinki.
Antibodies
Anti-PGK1 (cat. no. ab38007), anti-SRY-box
transcription factor 2 (Sox2; cat. no. ab93689),
anti-octamer-binding transcription factor 4 (Oct4; cat. no.
ab181557), anti-Nanog (cat. no. ab106465), anti-phosphorylated
(p)-AKT (cat. no. ab38449) and anti-AKT (cat. no. ab8805) were
purchased from Abcam (Abcam). Anti-HIF-1α (cat. no. 36169),
anti-E-cadherin (cat. no. 3195), anti-vimentin (cat. no. 5741),
anti-Slug (cat. no. 9585) and anti-β-actin (cat. no. 8457) primary
antibodies were purchased from Cell Signalling Technology, Inc.
Cell lines and treatment
The cell lines HSC3 and HN6 were obtained from the
American Type Culture Collection. Cells were cultured in DMEM
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (Hyclone; GE Healthcare Life Sciences) in 5% CO2
at 37°C under normoxic conditions, where the medium was replaced
every day. For hypoxia treatment, cells were incubated in 1%
O2, 5% CO2 at 37°C for 12 h or 24 h followed
by returning to normoxic conditions for subsequent experimentation.
The AKT activator SC79 (cat. no. HY-18749; MedChemExpress) was
dissolved in DMSO to a stock concentration of 10 mM, where a final
concentration of 10 µM was used at 37°C for 24 h under
normoxic conditions.
RNA interference and lentiviral
transfection
PGK1 and control siRNA were used in this study,
which were synthesised by Guangzhou RiboBio Co., Ltd. After
diluting Lipofectamine® RNAiMAX (Invitrogen; Thermo
Fisher Scientific, Inc.) and PGK1 or control siRNA with Opti-MEM
(Invitrogen; Thermo Fisher Scientific, Inc.), they were mixed and
incubated at room temperature for 5 min. PGK1 siRNA (50 nM) was
then introduced into HSC3 and HN6 cells for 12 h before treatment
according to the manufacturer's protocols. Sequences of the three
PGK1 siRNAs were as follows: PGK1 si-1, 5′-CCAAGT CGGTAGTCCTTAT-3′;
PGK1 si-2 5′-GCTTTCCGAGCT TCACTTT-3′; PGK1 si-3
5′-TGTCACTGCTGACAAGTTT-3′. The negative control duplex used (cat.
no. siN0000001-1-5), which was also provided by Guangzhou RiboBio
Co., Ltd., was not homologous to any mammalian genes.
Lentiviral plasmid pLVX-mCMV-ZsGREEN-Puro was
constructed to overexpress PGK1 whereas the lentiviral plasmid
pLVX-shRNA-tdTomato-Puro was constructed to knock down PGK1
expression in HSC3 cells. The multiplicity of infection of both
PGK1 knockdown and overexpression was 10. The sequence ligated into
the lentiviral plasmid to knock down PGK1 was as follows:
5′-CTGACAAGTTTGATGAGAATGCTCGAGCATTCTCATCAAACTTGTCAGTTTT TT-3′. The
lentivirus packaging process was completed by Guangzhou MingKong
Co., Ltd. The recombinant virus plasmid encoding lentivirus
particles and its three auxiliary packaging vector plasmids
(pGag/Pol, pRev, pVSV-G) were prepared and transfected into 293T
cells with Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). The concentration of all of the plasmids
used per transfection reaction into 293T cells was 300
ng/µl.
Cell viability assay
HSC3 and HN6 cells from the control and hypoxia
groups were seeded at 1,000 per well in 96-well plates and cultured
for 0, 24, 48 and 72 h at 37°C. Cell viability was measured using a
Cell Counting Kit-8 kit assay (CCK-8; Dojindo Molecular
Technologies, Inc.). After adding 10 µl CCK-8 reagent per
well, plates were incubated at 37°C for 1 h. Absorbance in each
well was measured at 450 nm using a microplate reader (Bio-Rad
Laboratories, Inc.) according to the manufacturer protocols.
Immunohistochemistry
The tissues obtained were first fixed in 4%
paraformaldehyde for 24 h at room temperature and embedded in
paraffin to immobilise the tissues, following which
immunohistochemical staining of 3-µm thick paraffin sections
was subsequently performed. The tissue sections were first
deparaffinized with xylene and rehydrated with a descending ethanol
gradient before being boiled in a pressure cooker with sodium
citrate buffer at 99°C for 20 min for antigen retrieval. Endogenous
peroxidase/phosphatase activities within the sections were then
quenched at room temperature for 10 min using 3% hydrogen peroxide,
followed by blocking in 5% goat serum (Wuhan Boster Biological
Technology, Ltd.) at room temperature for 30 min. The sections were
then incubated with the respective PGK1, E-cadherin, Vimentin,
Slug, Sox2, Oct4 and Nanog primary antibodies (1:250 dilution)
overnight at 4°C, following which they were incubated with
horseradish-peroxidase (HRP)-conjugated secondary antibodies
(1:200; cat. no. 7074s; Cell Signaling Technology, Inc.) diluted in
1% BSA (Wuhan Boster Biological Technology, Ltd.) at room
temperature for 30 min. For color development,
3,3′-diaminobenzidine was used. An Axio Imager Z2 light microscope
(magnifications, ×100 and ×400; Zeiss AG) was used for image
acquisition. Immunohistochemical staining was assessed in terms of
staining intensity and the proportion of positively staining cells
using Image-Pro Plus 6.0 software (Media Cybernetics, Inc.). The
scoring system for staining intensity was as follows: i) 0, no
staining; ii) 1, weak staining; iii) 2, medium staining; and iv) 3,
strong staining. The scoring system for the proportion of positive
cells was determined as follows: i) 0, 0%; ii) 1, 25%; iii) 2, 50%;
iv) 3, 75%; and v) 4, 100%. These two scores were then multiplied
together to give the final score, where a score >4 was
classified as high expression whereas a score ≤4 was classified as
low expression.
Western blot analysis
Cells were collected and treated at 4°C for 30 min
with RIPA lysis buffers (Thermo Fisher Scientific, Inc.)
supplemented with protease inhibitors and phosphatase inhibitors
(Thermo Fisher Scientific, Inc.). Cell lysates were collected and
centrifuged at 4°C for 30 min at 14,000 × g. The supernatant was
collected and protein concentration was determined using a
bicinchoninic acid protein assay kit (CoWin Biosciences). Total
protein (25 µg) was subsequently separated by 10% SDS-PAGE
and transferred onto a polyvinylidene fluoride membrane (EMD
Millipore) with 10% SDS-PAGE. After blocking with 10% BSA (CoWin
Biosciences) at room temperature for 1 h, the membranes were
incubated with HIF-1α, PGK1, E-cadherin, Vimentin, Slug, Sox2,
Oct4, Nanog, p-AKT, AKT or β-actin primary antibodies (1:1,000
dilution) overnight at 4°C, followed by incubation with a
HRP-conjugated secondary antibody (1:2,000 dilution; cat. no.
7074s; Cell Signaling Technology, Inc.) at room temperature for 1
h. An enhanced chemiluminescence kit (Cell Signaling Technology,
Inc.) and and the ImageQuant Las4000mini system (GE Healthcare Life
Sciences) were used to visualize the bands. β-actin was used as an
internal control. The signal intensities were quantified using
ImageJ version 1.48u software (National Institutes of Health)
Glucose consumption and lactate
production analysis
Cells were seeded into 6-well plates at the same
density of 2.5×105 cells per well and cultured under
hypoxic conditions for 12 h followed by incubation under normoxic
conditions for 12 h after siRNA transfection. The cell culture
supernatants were obtained from each treatment group, where glucose
consumption and lactic acid production were measured. Glucose
consumption was measured at 37°C for 15 min using glucose detection
kits (cat. no. F006-1-1; Nanjing Jiancheng Bioengineering
Institute) and lactic acid production was measured at 37°C for 5
min using lactic acid detection kits (cat. no. A019-2-1; Nanjing
Jiancheng Bioengineering Institute) in accordance with the
manufacturer's protocol.
Sphere formation
The sphere formation assays were performed in
ultra-low attachment 24-well plates (Corning, Inc.). A total of
1,000 cells were seeded into each well, where DMEM containing 2%
B27 (Invitrogen; Thermo Fisher Scientific, Inc.), 20 ng/ml basic
fibroblast growth factor (bFGF; Invitrogen; Thermo Fisher
Scientific, Inc.) and 20 ng/ml epidermal growth factor (EGF;
Invitrogen; Thermo Fisher Scientific, Inc.) were added. The
experimental group received hypoxia treatment (1% O2, 5%
CO2) at 37°C for 12 h, whilst control cells were
incubated in normoxic (95% air/5% CO2) conditions
without hypoxia treatment. Both treatment groups were then
incubated under normoxic (95% air/5% CO2) conditions at
37°C for 14 days. The resulting parameters of sphere formation,
including the diameter of spheres and the number of spheres per
1,000 cells, were observed using an Axio Imager Z2 light microscope
(magnification, ×100; Carl Zeiss AG).
Migration and invasion assay
For cell migration, 4×104 cells were
seeded into the upper chamber of Transwell assay plates (24-well
insert; pore size, 8 µm; BD Biosciences) suspended in
serum-free medium 12 h following normoxic or hypoxic treatment. The
lower chamber was filled with DMEM supplemented with 10% FBS. For
cell invasion, Matrigel (Corning, Inc.) was added into the upper
chambers at 37°C for 1 h in accordance with the manufacturer's
protocols, following which 8×104 cells were seeded into
the upper chambers with Matrigel-coated membranes 12 h following
normoxic or hypoxic treatment. The Transwell chambers were then
incubated at 37°C under normoxic conditions (5% CO2
atmosphere) for 36 h. After incubation, Transwell membranes was
fixed with 4% paraformaldehyde at room temperature for 30 min,
stained with 0.1% crystal violet at room temperature for 20 min and
observed under the Axio Imager Z2 light microscope (magnification,
×100; Carl Zeiss AG). The number of migrated and invaded cells was
then counted in five randomly selected fields per chamber.
Tumorigenesis in nude mice
A total of 20 female SCID mice (age, 4-6 weeks;
weight, 20-25 g) were purchased from the Laboratory Animal Center
of Sun Yat-sen University (Guangzhou, China) and maintained under
pathogen-free conditions. The temperature of feeding environment is
controlled at 18-26°C under normoxic conditions, the relative
humidity is 40-70% and 12-h light/dark cycle. All mice had free
access to food and water. SCID mice were randomly divided into the
following four groups (n=5 in each group): i) Overexpression
control (Vector); ii) overexpression (PGK1); iii) knockdown control
group (shVector); and iv) knockdown (shPGK1). HSC3 cells were
digested by trypsin for 3 min, neutralized with 10% FBS medium and
collected by centrifugation. Then, HSC3 cells (1×106)
were diluted in 200 µl sterile PBS and injected into the
unilateral abdominal region adjacent to the axilla of the hind
limbs using a 1 ml syringe. Tumour volume was measured weekly
(tumour volume = length x wi dth x width) / 2) and all mice were
euthanised after 1 month after cell injection and the final tumor
measurement, following which the tumour mass was measured. Tissue
specimens were fixed with 4% paraformaldehyde at room temperature
for 24 h and embedded for subsequent immunohistochemical staining.
This present animal study was approved by the Experimental Animal
Ethics Committee of Sun Yat-sen University (Guangzhou, China). The
animal experiment lasted for 2 months. Any mice that were found to
be slow growing, not eating or infected by skin ulceration caused
by mutual fights were euthanized by cervical dislocation. After the
tumor cells are implanted into the mice, if any of the mice were
observed to suffer from anorexia, inability to eat normally,
significant weight loss and other abnormalities, the nutrition
(melon seeds, wheat) will be enhanced immediately. Euthanasia would
be performed in case of rapid weight loss of >20%, weakness,
loss of appetite, inability to drink or eat, skin ulceration and
infection caused by fighting with each other. Animal health and
behaviour were monitored every day and no animal was found dead
during the present study. A combination of criteria was used for
confirming death, including the lack of pulse or breathing and
rigor mortis.
TCGA Data Collection and Processing
The gene expression RNAseq and clinical data of 566
cases of the head and neck squamous cell carcinoma dataset (566
cases) were downloaded from The Cancer Genome Atlas (TCGA) using
the UCSC Xena browser (https://xenabrowser.net) (36). In these cases, the primary tumors
from the oral cavity (oral cavity, oral tongue, floor of mouth,
alveolar ridge, hard palate and buccal mucosa) included 284 oral
squamous cell carcinoma specimens and 30 normal oral epithelial
tissue samples. The gene expression data and survival results of
were analysed using the R software (R version 3.5.3; http://bioconductor.org/biocLite.R) and related R
packages, including 'dplyr', 'tidyr', 'ggplot2', and 'survminer' R
packages (37-40).
Statistical analysis
Unpaired t-test was used to compare two groups of
data and one-way ANOVA followed by Tukey's test was used for
multiple comparisons using SPSS version 19.0 (IBM Corp.). P<0.05
was considered to indicate a statistically significant difference.
χ2 test was used to evaluate the association between
PGK1 expression and the clinicopathological characteristics of
patients with OSCC. Wilcoxon signed rank test was used to compare
the immunostaining scores between adjacent noncancerous tissue
(ANCT) and OSCC. Kruskal-Wallis test followed by Dunn's test was
used to compare the immunostaining scores among the OSCC tissues at
different stages of differentiation. U Mann-Whitney test was used
to compare the immunostaining scores between OSCC tissue specimens
with or without lymph node metastasis. The Kaplan-Meier method and
log-rank test were used to compare the overall survival of patient.
Data were presented as mean ± SD. Each experiment was performed ≤3
times.
Results
Hypoxia promotes the proliferation of
OSCC cells and increases the expression of PGK1
Oral cancer cell lines HSC3 and HN6 were cultured
under hypoxic conditions for 0, 12 and 24 h, where the results
showed that hypoxia for 12 and 24 h significantly increased cell
viability in both oral cancer cell lines (Fig. 1A and B). These findings suggest
that hypoxic conditions at appropriate time periods can promote the
growth of OSCC cells. There were no significant changes in PGK1
expression in HN6 and HSC3 cells under normal culture conditions at
any of the timepoints tested (Fig. 1C
and D). In addition, PGK1 expression in HN6 and HSC cells was
measured following hypoxia treatment. The results showed that the
expression of PGK1 was significantly upregulated 12 and 24 h
following culture under hypoxic conditions (Fig. 1E and F).
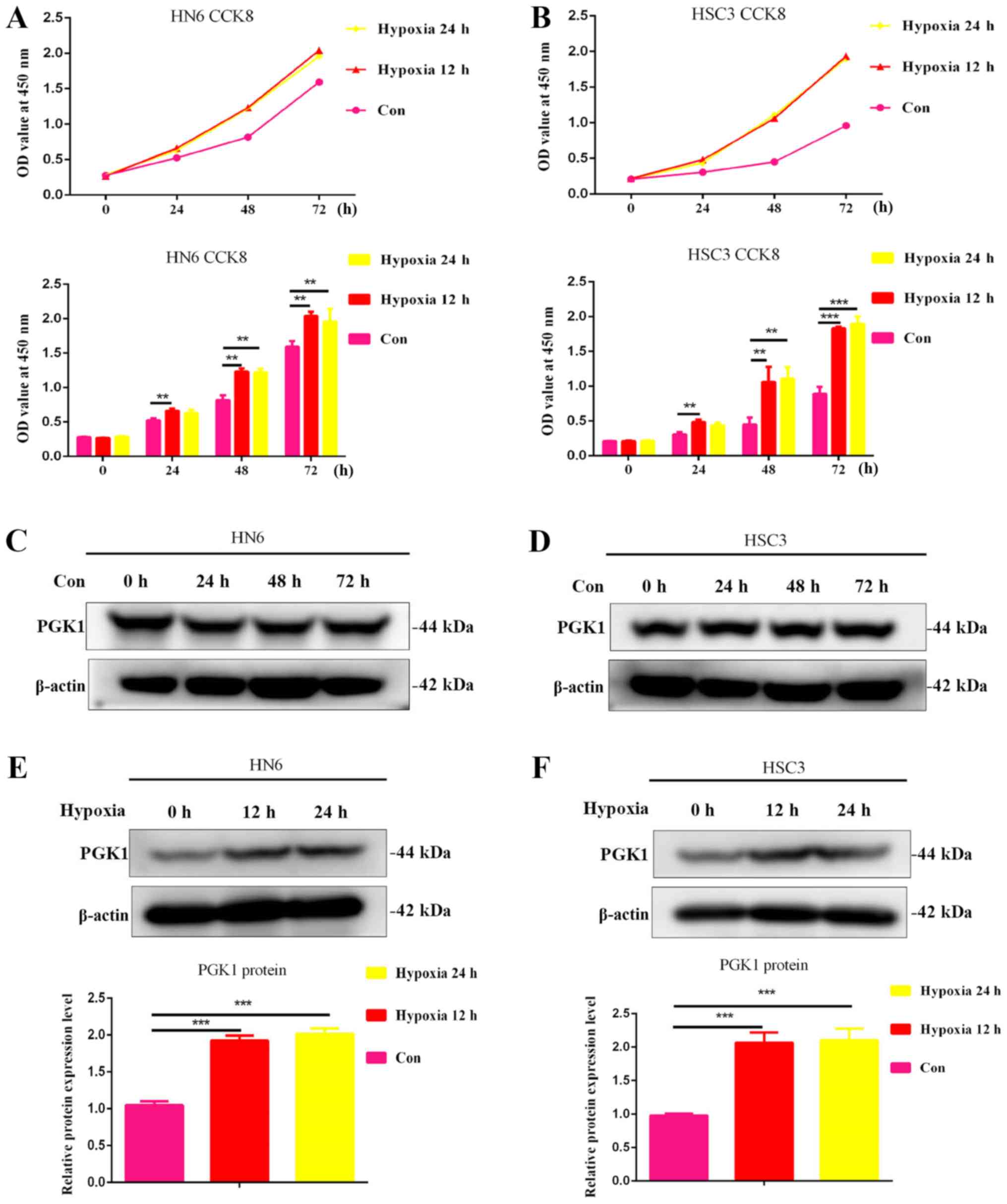 | Figure 1Hypoxia increases oral cancer cell
viability and promotes the expression of PGK1. (A) HN6 and (B) HSC3
cells were cultured under hypoxic conditions for 0, 6, 12, 18 and
24 h, following which cell viability was measured using CCK-8
assays. Western blotting was used to measure changes in PGK1
expression in (C) HN6 and (D) HSC3 cells after normal culture
conditions for 0, 24, 48 and 72 h. Western blotting was used to
measure changes in PGK1 expression in (E) HN6 and (F) HSC3 cells
following 12 and 24 h culturing under hypoxic conditions.
**P<0.01 and ***P<0.001, n=3. Con,
control. PGK1, phosphoglycerate kinase 1; OD, optical density;
CCK8, Cell Counting Kit-8. |
Hypoxia promotes glycolysis, increases
stem cell-like properties and enhances OSCC cells migration and
invasion
To investigate the effect of hypoxia on the glucose
metabolism of OSCC cells, HN6 and HSC3 were cultured under hypoxic
conditions for 12 h followed by incubation under normoxic
conditions for 12 h. Glucose consumption and lactic acid production
were found to be significantly higher in cells cultured under
hypoxic conditions compared with cells cultured under normoxic
conditions (Fig. 2A and B). These
findings suggest that hypoxia may promote glycolysis in OSCC cells.
The effects of hypoxia on OSCC cell invasion and migration, in
addition to the expression of stem cell markers, were subsequently
examined. It was found that the diameter and number of spheroids
formed were significantly higher in cells incubated under hypoxic
conditions compared with cells cultured under normoxic conditions
(Fig. 2C). Hypoxia treatment was
also revealed to upregulate the expression of stem cell markers
Sox2, Oct4 and Nanog in HN6 and HSC3 cells (Fig. 2D). In addition, the expression
levels of PGK1, in addition to those of vimentin and slug, were
found to be upregulated following culture under hypoxic conditions
compared with cells cultured under normoxic conditions (Fig. 2D). By contrast, E-cadherin
expression was revealed to be lower compared with that in normoxic
cells (Fig. 2D). Since EMT is a
key process in potentiating tumour migration and invasion (7), the role of hypoxia in the invasion
and migration of OSCC cells was assessed by Transwell assays.
Hypoxia was found to significantly enhance HN6 and HSC3 cell
migration and invasion compared with normoxic cells (Fig. 2E).
 | Figure 2Effects of hypoxia on glycolysis,
stem-like properties, migration and invasion of oral squamous cell
carcinoma cells. (A) Glucose and (B) lactic acid levels in the
culture supernatant of HN6 and HSC3 cells were measured after
culturing under hypoxic conditions. (C) Both diameters and numbers
of spheres formed by HN6 and HSC3 cells were increased following
culturing under hypoxic conditions. Scale bar, 50 µm. (D)
Hypoxic treatment in HN6 and HSC3 cells upregulated the expression
PGK1, Sox2, Oct4, Nanog, Vimentin and Slug proteins, whilst
reducing the expression of E-cadherin. (E) Transwell assays
revealed that hypoxia treatment enhanced the migration and invasion
of HN6 and HSC3 cells. *P<0.05, n=3. Scale bar, 50
µm. PGK1, phosphoglycerate kinase 1; Sox-2, SRY-box
transcription factor 2; Oct4, octamer-binding transcription factor
4; HIF-1α, hypoxia-inducible factor-1α. |
PGK1 knockdown reverses the effects of
hypoxia on glucose consumption, stem cell-like characteristics and
EMT in OSCC cells
To study the effect of hypoxia treatment on OSCC
cells after PGK1 knockdown, the effect of siRNA transfection on the
PGK1 expression under normoxic conditions was first verified by
western blotting (Fig. 3A). PGK1
knockdown using PGK si-01 was found to partially but significantly
reverse the positive effects of hypoxia on glucose consumption
(Fig. 3B) and lactic acid
production (Fig. 3C). In terms of
EMT, PGK1 knockdown markedly reversed the increases in the
expression of EMT markers vimentin and Slug whilst markedly
preventing the reduction in E-cadherin caused by hypoxia (Fig. 4A). PGK1 knockdown did not
significantly affect HIF-1α expression, which was upregulated by
hypoxia (Fig. 4A). PGK1 knockdown
was found to markedly suppress the expression of stem cell markers
Sox2, Oct4 and Nanog in addition to reversing the hypoxia-induced
upregulation of these stem cell markers (Fig. 4A). Under hypoxic conditions,
silencing PGK1 expression also significantly inhibited cell
migration and invasion (Fig. 4A and
C), whilst also significantly reducing the diameter and number
of spheres formed by HN6 and HSC3 cells compared with those
transfected with control siRNA (Fig.
4A and B).
 | Figure 4Knockdown of PGK1 expression reverses
hypoxia-activated stem-like properties and epithelial-mesenchymal
transition in oral squamous cell carcinoma cells. (A) HN6 and HSC3
cells were treated under hypoxic conditions and/or transfected with
siPGK1, following which the expression of the indicated proteins
was measured by western blotting. (B) Sizes and numbers of
spheroids formed were measured after HN6 and HSC3 cells were
cultured under hypoxic conditions and/or transfected with siPGK1.
Spheroids with diameters >50 μm were displayed. Scale bar, 50
µm. (C) Transwell assays were used to measure HN6 and HSC3
cell migration and invasion following culturing under hypoxic
conditions and/or transfection with siPGK1. *P<0.05,
n=3. Scale bar, 50 µm. PGK1, phosphoglycerate kinase 1;
Sox-2, SRY-box transcription factor 2; Oct4, octamer-binding
transcription factor 4; HIF-1α, hypoxia-inducible factor-1α; si,
small interfering RNA; ConsiRNA, control siRNA. |
PGK1 knockdown inhibits tumour growth
whereas the upregulation of PGK1 expression promotes tumour growth
in vivo
To study the effect of PGK1 on the phenotype of OSCC
tumours in vivo, pre-constructed HSC3 PGK1 knockout (shPGK1)
or overexpression cell lines were injected into the subcutaneous
tissues of SCID mice. Tumour volumes and weights were found to be
significantly reduced in the group injected with cells transfected
shPGK1 with compared with those injected with cells transfected
with the shVector 1 month after injection (Fig. 5A). By contrast, tumour volumes and
weights were demonstrated to be significantly increased in the
group transfected with cells overexpressing PGK1 compared with
those injected with cells transfected with the vector (Fig. 5A). Furthermore, compared with those
in the PGK1 overexpression group, mice in the shPGK1 group also
exhibited markedly lower levels of PGK1, vimentin, Slug, Sox2, Oct4
and Nanog expression, whilst showing higher levels of E-cadherin
expression (Fig. 5B and C).
PGK1 promotes stem cell-like properties
and EMT in OSCC cells through the AKT signalling pathway
The activity of the AKT signalling pathway was next
measured in OSCC cells under normoxic conditions. PGK1 knockdown
was found to reduce p-AKT phosphorylation (Fig. 6A). SC79, an activator of AKT, was
used to investigate the effects of the AKT signalling pathway on
PGK1-mediated stemness and EMT in OSCC cells, also under normoxic
condtions. SC79 treatment was revealed to increase AKT
phosphorylation whilst reversing the inhibitory effects of PGK1
knockdown on vimentin, Slug, Sox2, Oct4 and Nanog expression
(Fig. 6B). In addition, increases
in E-cadherin expression induced by PGK1 knockdown was also found
to be reversed by SC79 treatment (Fig.
6B). SC79 was found to promote significantly potentiate sphere
formation, migration and invasion in HN6 and HSC3 cells following
PGK1 knockdown (Fig. 6C and D).
These findings suggest that PGK1 may promote OSCC stemness and EMT
by activating the AKT signalling pathway.
 | Figure 6PGK1 promotes stem-like properties
and epithelial-mesenchymal transition in oral squamous cell
carcinoma cells through the AKT signalling pathway. (A) Western
blotting was used to measure the phosphorylation of AKT in HN6 and
HSC3 cells after PGK1 knockdown. (B) HN6 and HSC3 cells were
treated with SC79 and/or transfected with siPGK1, following which
the expression levels of the indicated proteins were measured by
western blotting. (C) Sizes and numbers of spheroids formed were
measured after HN6 and HSC3 cells were treated with SC79 and/or
transfected with siPGK1. Spheroids with diameters >50 μm were
displayed. Scale bar, 50 µm. (D) Transwell assays were used
to measure HN6 and HSC3 cell migration and invasion following
treatment with SC79 and/or transfection with siPGK1.
*P<0.05, n=3. Scale bar, 50 µm. PGK1,
phosphoglycerate kinase 1; Sox-2, SRY-box transcription factor 2;
Oct4, octamer-binding transcription factor 4; HIF-1α,
hypoxia-inducible factor-1α; si, small interfering RNA; ConsiRNA,
control siRNA. |
PGK1 expression is correlated with
clinicopathological features in patients with OSCC
To clarify if the levels of PGK1 expression is
associated with the clinicopathological characteristics of patients
with OSCC, PGK1 expression was measured in tissue samples obtained
from 92 patients with OSCC and 20 ANCT specimens by
immunohistochemistry (Fig. 7A).
Semi-quantitative analysis showed that the PGK1 expression in OSCC
specimens was significantly higher compared with that in ANCT
(Fig. 7B). Compared with that in
well-differentiated OSCC tissue samples, PGK1 expression was found
to be significantly higher in moderately and poorly differentiated
OSCC tissues (Fig. 7C). PGK1
expression was also demonstrated to be significantly higher in OSCC
tissue specimens with lymph node metastasis compared with that in
OSCC specimens without lymph node metastasis (Fig. 7D). The relationship between PGK1
expression and the clinicopathological characteristics of patients
with OSCC was next examined. PGK1 expression was found to
significantly associate with tumour differentiation, clinical
staging and lymph node metastasis, but not with age, sex or T stage
(Table I).
 | Table IAssociation between PGK1 expression
and clinicopathological features in patients with OSCC (n=92). |
Table I
Association between PGK1 expression
and clinicopathological features in patients with OSCC (n=92).
| Clinicopathological
features | No. of cases | PGK1 expression
| P-value |
|---|
| High (n) | Low (n) |
|---|
| Sex | | | | 0.052 |
| Male | 58 | 36 | 22 | |
| Female | 34 | 14 | 20 | |
| Age | | | | 0.809 |
| ≥55 | 56 | 31 | 25 | |
| <55 | 36 | 19 | 17 | |
|
Differentiation | | | | 0.001 |
| Well | 16 | 4 | 12 | |
| Moderate +
poor | 76 | 46 | 30 | |
| T stage | | | | 0.107 |
| T1-2 | 77 | 39 | 38 | |
| T3-4 | 15 | 11 | 4 | |
| Clinical stage | | | | 0.008 |
| I-II | 59 | 26 | 33 | |
| III-IV | 33 | 24 | 9 | |
| LN metastasis | | | | 0.018 |
| N− | 68 | 32 | 36 | |
| N+ | 24 | 18 | 6 | |
PGK1 expression levels in patients with
OSCC patients is associated with survival and prognosis
The levels of PGK1 expression was next compared
between 284 OSCC samples and 30 normal oral mucosal epithelial
tissue samples obtained from the TCGA database. PGK1 expression was
revealed to be significantly upregulated in OSCC samples compared
with that in the normal oral mucosal epithelial tissue samples
(Fig. 8A). Survival analysis
showed that the overall survival rate of patients with high
expression of PGK1 was significantly reduced compared with those
with low PGK1 expression (Fig.
8B).
Discussion
Strong invasive and migratory capacities are among
the main causes underlying oral cancer cell metastasis, which is
particularly prone to metastasizing to cervical lymph nodes and
distant organs, significantly reducing the quality of life of
patients (41-43).
Cell division and proliferation are metabolically
active processes within cells. However, energy production within
cancer cells does not particularly depend on the classical
oxidative phosphorylation pathway performed by mitochondria
(44). On the contrary, cancer
cells frequently utilize the glycolytic pathway to produce ATP,
even when the supply of oxygen is sufficient. This is a process
known as the 'Warburg effect' (45). A previous study has found that
glutamine starvation can activate PFKP expression, whilst
downregulation of PFKP can reverse the invasion and migration of
OSCC cells induced by starvation (46). In addition, previous studies have
documented that hypoxia is closely associated with tumorigenesis,
cell development, invasion and migration in oral cancer (47,48).
Hypoxic environments can be readily found in solid
malignant tumors and can lead to alterations in the expression of a
number of molecular biomarkers, including HIF, carbonic anhydrase,
glucose-transporter-1 and vascular endothelial growth factor
(49). HIF-1α, which is activated
under hypoxic conditions, is involved in the regulation of tumor
cell propagation, migration, glucose metabolism and angiogenesis
(50). It allows cells to adapt to
hypoxic environments, contributing to invasion and metastasis. It
was previously found that HIF-1α can promote the malignant
progression of esophageal squamous cell carcinoma by regulating the
expression specificity protein 1 (49). In the present study, it was found
that both HIF-1α and PGK1 expression were upregulated due to
hypoxia, suggesting an association between these two proteins.
PGK1 is a key enzyme in glycolysis, which converts
glyceric acid 1,3-diphosphate to produce glyceric acid 3-phosphate
and ATP (28). A number of studies
previously found that PGK1 expression is significantly associated
with the survival and prognosis of patients with hepatocellular
carcinoma, where PGK1 knockdown can inhibit the proliferation of
hepatocellular carcinoma cell lines (50,51).
PGK1 expression in several tumour tissues was found to be higher
compared with that in normal tissues (26), whilst HIF-1α has been previously
shown to directly regulate PGK1 (52). In liver cancer, the expression
levels of PGK1 and HIF-1α in the hepatocellular carcinoma cell line
HCCLM9 with high metastasis were higher when compared with those in
the hepatocellular carcinoma cell line MHCC97L with low metastasis
(26,33), such that PGK1 has also been
revealed to regulate cell proliferation and metastasis (32). In addition, PGK1 expression was
found to correlate with the survival and prognosis of patients with
breast cancer (53). PGK1
overexpression can promote the development and metastasis of
gastric cancer by activating the C-X-C motif chemokine receptor
4/C-X-C motif chemokine 12/β-catenin signalling pathway (26). The present study found that 12 h of
hypoxia treatment could promote the proliferation of oral cancer
cells. The present study also found that hypoxic conditions
upregulated the expression of PGK1 and stem cell markers in OSCC
cell lines.
EMT is a key mechanism for potentiating invasion and
migration in OSCC cells, where the self -renewing and proliferative
capacities of tumor stem cells is one of the causes of OSCC
recurrence. It has been previously demonstrated that the
differential expression of stem cell markers, including CD44, CD133
and ALDH1, in addition to transcription factors, including Oct4,
Sox2 and Nanog, serve an important role in clonogenesis in oral
squamous cell carcinomas (54,55).
In the present study, hypoxia was shown to upregulate the
expression of EMT markers promote the migration and invasion of
oral cancer cells. Further investigation found that PGK1 knockdown
partially reversed the invasion and migration of oral cancer cells
under hypoxic conditions.
The AKT signalling pathway is involved in many
processes, including proliferation, apoptosis, cancer cell
migration and invasion (56).
Aberrant activation of the AKT signalling pathway has been
previously shown to serve an important role in the malignant
progression of cancer (57,58).
A previous study has demonstrated that the AKT/NFκB signalling
pathway is involved in the invasion and migration of lung cancer
cells, which is closely associated with the maintenance of stem
cell characteristics and EMT (59). Another study has also previously
found that hypoxia promotes glioma cell proliferation, migration
and invasion via PI3K/AKT signalling (58). Results from the present study
suggest that the downregulation of PGK1 inhibits the
phosphorylation of AKT, whilst the AKT activator SC79 reversed the
effects observed following PGK1 knockdown.
Differential observations would have been made if
the present study was performed under hypoxic conditions compared
with cells grown under normoxic conditions. A preliminary study of
the present study found that following hypoxia treatment for >48
h, the cells were in a poor state of health and could not complete
the subsequent proliferation, invasion and migration experiments.
Therefore, the method of hypoxia pre-treatment was applied to study
the effects of hypoxia on the biological behaviour of tumour cells.
The results demonstrated that appropriate hypoxia can promote the
malignant progress of OSCC cells, where further study found that
moderate hypoxia can promote tumour cell proliferation. This was a
limitation of the present study, which remain to be the focus of
future research.
To conclude, data from the present study suggest
that hypoxia can upregulate PGK1 expression and glycolysis whilst
activating the characteristics of oral cancer stem cells and EMT
through the AKT pathway. However, the exact mechanism require
further clarification.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation project of China (grant nos. 81874128
and 81572660) and Sun Yat-Sen University Clinical Research 5010
Program (grant no. 2015018).
Availability of data and materials
All data generated or analyzed during this study are
included in this article.
Authors' contributions
JH developed the concept of the project. YZ designed
and performed the experiments, analysed the results and wrote the
manuscript. HC performed the immunohistochemical staining. YL
collected and analysed clinical specimens and clinical data. FW and
YZ were involved in statistical analysis of the obtained results.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The removal of tissue samples from patients was
approved by the ethical review committee of the Affiliated
Stomatological Hospital of Sun Yat-sen University (Guangzhou,
China). The experiments were performed with the understanding and
written consent of each subject. The study methodologies conformed
to the standards set by the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Massano J, Regateiro FS, Januário G and
Ferreira A: Oral squamous cell carcinoma: Review of prognostic and
predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 102:67–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rajwar YC, Jain N, Bhatia G, Sikka N, Garg
B and Walia E: Expression and Significance of Cadherins and Its
Subtypes in Development and Progression of Oral Cancers: A Review.
J Clin Diagn Res. 9:ZE05–ZE07. 2015.PubMed/NCBI
|
|
3
|
Chang WC, Chang CF, Li YH, Yang CY, Su RY,
Lin CK and Chen YW: A histopathological evaluation and potential
prognostic implications of oral squamous cell carcinoma with
adverse features. Oral Oncol. 95:65–73. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang Z, Xie N, Liu H, Wan Y, Zhu Y, Zhang
M, Tao Y, Zhou H, Liu X, Hou J and Wang C: The prognostic role of
tumour-infiltrating lymphocytes in oral squamous cell carcinoma: A
meta-analysis. J Oral Pathol Med. 48:788–798. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kademani D, Bell RB, Schmidt BL,
Blanchaert R, Fernandes R, Lambert P and Tucker WM; American
Association of Oral and Maxillofacial Surgeons Task Force on Oral
Cancer: Oral and maxil-lofacial surgeons treating oral cancer: a
preliminary report from the American Association of Oral and
Maxillofacial Surgeons Task Force on Oral Cancer. J Oral Maxillofac
Surg. 66:2151–2157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie Y, Zhong L, Duan D and Li T: Casticin
inhibits invasion and proliferation via downregulation of β-catenin
and reversion of EMT in oral squamous cell carcinoma. J Oral Pathol
Med. 48:897–905. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Staton CA, Brown NJ and Reed MW: Current
status and future prospects for anti-angiogenic therapies in
cancer. Expert Opin Drug Discov. 4:961–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klymkowsky MW and Savagner P:
Epithelial-mesenchymal transition: A cancer researcher's conceptual
friend and foe. Am J Pathol. 174:1588–1593. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang C, Liu X, Chen Z, Huang H, Jin Y,
Kolokythas A, Wang A, Dai Y, Wong DT and Zhou X: Polycomb group
protein EZH2-mediated E-cadherin repression promotes metastasis of
oral tongue squamous cell carcinoma. Mol Carcinog. 52:229–236.
2013. View
Article : Google Scholar
|
|
13
|
Dawei H, Honggang D and Qian W: AURKA
contributes to the progression of oral squamous cell carcinoma
(OSCC) through modulating epithelial-to-mesenchymal transition
(EMT) and apoptosis via the regulation of ROS. Biochem Biophys Res
Commun. 507:83–90. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Zheng G, Zhou L, Li P, Yun M, Shi
Q, Wang T and Wu X: Notch signalling induces epithelial mesenchymal
transition to promote metastasis in oral squamous cell carcinoma.
Int J Mol Med. 42:2276–2284. 2018.PubMed/NCBI
|
|
15
|
Krisanaprakornkit S and Iamaroon A:
Epithelial-mesenchymal transition in oral squamous cell carcinoma.
ISRN Oncol. 2012:6814692012.PubMed/NCBI
|
|
16
|
Espinoza I and Miele L: Deadly crosstalk:
Notch signaling at the intersection of EMT and cancer stem cells.
Cancer Lett. 341:41–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Costa LC, Leite CF, Cardoso SV, Loyola AM,
Faria PR, Souza PE and Horta MC: Expression of
epithelial-mesenchymal transition markers at the invasive front of
oral squamous cell carcinoma. J Appl Oral Sci. 23:169–178. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tran Q, Lee H and Park J, Kim SH and Park
J: Targeting Cancer Metabolism - Revisiting the Warburg Effects.
Toxicol Res. 32:177–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu CA, Chao Y, Shiah SG and Lin WW:
Nutrient deprivation induces the Warburg effect through
ROS/AMPK-dependent activation of pyruvate dehydrogenase kinase.
Biochim Biophys Acta. 1833:1147–1156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sasabe E, Tatemoto Y, Li D, Yamamoto T and
Osaki T: Mechanism of HIF-1alpha-dependent suppression of
hypoxia-induced apoptosis in squamous cell carcinoma cells. Cancer
Sci. 96:394–402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng ZX, Sun B, Wang SJ, Gao Y, Zhang YM,
Zhou HX, Jia G, Wang YW, Kong R, Pan SH, et al: Nuclear
factor-κB-dependent epithelial to mesenchymal transition induced by
HIF-1α activation in pancreatic cancer cells under hypoxic
conditions. PLoS One. 6:e237522011. View Article : Google Scholar
|
|
22
|
Joseph JP, Harishankar MK, Pillai AA and
Devi A: Hypoxia induced EMT: A review on the mechanism of tumor
progression and metastasis in OSCC. Oral Oncol. 80:23–32. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng M, Cao MX, Luo XJ, Li L, Wang K,
Wang SS, Wang HF, Tang YJ, Tang YL and Liang XH: EZH2 promotes
invasion and tumour glycolysis by regulating STAT3 and FoxO1
signalling in human OSCC cells. J Cell Mol Med. 23:6942–6954. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang J, Cao R, Zhang Y, Xia Y, Zheng Y,
Li X, Wang L, Yang W and Lu Z: PKM2 dephosphorylation by Cdc25A
promotes the Warburg effect and tumorigenesis. Nat Commun.
7:124312016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Valentin C, Birgens H, Craescu CT,
Brødum-Nielsen K and Cohen-Solal M: A phosphoglycerate kinase
mutant (PGK Herlev; D285V) in a Danish patient with isolated
chronic hemolytic anemia: Mechanism of mutation and
structure-function relationships. Hum Mutat. 12:280–287. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He Y, Luo Y, Zhang D, Wang X, Zhang P, Li
H, Ejaz S and Liang S: PGK1-mediated cancer progression and drug
resistance. Am J Cancer Res. 9:2280–2302. 2019.PubMed/NCBI
|
|
27
|
Beutler E: PGK deficiency. Br J Haematol.
136:3–11. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morales-Briceño H, Ha AD, London K, Farlow
D, Chang FCF and Fung VSC: Parkinsonism in PGK1 deficiency
implicates the glycolytic pathway in nigrostriatal dysfunction.
Parkinsonism Relat Disord. 64:319–323. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hogrel JY, Ledoux I and Béhin A:
Hyperammonaemia following exercise may also reveal PGK1 deficiency.
J Clin Pathol. 72:4522019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Echaniz-Laguna A, Nadjar Y, Béhin A,
Biancalana V, Piraud M, Malfatti E and Laforêt P: Phosphoglycerate
kinase deficiency: A nationwide multicenter retrospective study. J
Inherit Metab Dis. 42:803–808. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McCarrey JR, Kumari M, Aivaliotis MJ, Wang
Z, Zhang P, Marshall F and Vandeberg JL: Analysis of the cDNA and
encoded protein of the human testis-specific PGK-2 gene. Dev Genet.
19:321–332. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie H, Tong G, Zhang Y, Liang S, Tang K
and Yang Q: PGK1 Drives Hepatocellular Carcinoma Metastasis by
Enhancing Metabolic Process. Int J Mol Sci. 18:182017. View Article : Google Scholar
|
|
33
|
Shao F, Yang X, Wang W, Wang J, Guo W,
Feng X, Shi S, Xue Q, Gao S, Gao Y, et al: Associations of PGK1
promoter hypomethylation and PGK1-mediated PDHK1 phosphorylation
with cancer stage and prognosis: A TCGA pan-cancer analysis. Cancer
Commun (Lond). 39:542019. View Article : Google Scholar
|
|
34
|
Zhou JW, Tang JJ, Sun W and Wang H: PGK1
facilities cisplatin chemoresistance by triggering HSP90/ERK
pathway mediated DNA repair and methylation in endometrial
endometrioid adenocarcinoma. Mol Med. 25:112019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, et al: Final version of 2009 AJCC melanoma staging and
classification. J Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goldman M, Craft B, Kamath A, Brooks A,
Zhu J and Haussler D: The UCSC Xena Platform for cancer genomics
data visualization and interpretation. bioRxiv: https://doi.org/10.1101/326470.
2018
|
|
37
|
Wickham H: Tidy Data. J Stat Softw.
59:102014. View Article : Google Scholar
|
|
38
|
Wickham H: ggplot2: Elegant Graphics for
Data Analysis. Springer-Verlag; New York, NY: 2009, View Article : Google Scholar
|
|
39
|
Kassambara A, Kosinski M, Przemyslaw B and
Scheipl F: Drawing survival curves using 'ggplot2'. http://cran.r-project.org/package=survminer.
Accessed May 28, 2020.
|
|
40
|
Wickham H, François R, Henry L and Müller
K; RStudio: dplyr: A Grammar of Data Manipulation. R Package
Version 0.3.0.2. https://cran.r-project.org/web/packages/dplyr/index.html.
Accessed May 29, 2020.
|
|
41
|
Warnakulasuriya S: Living with oral
cancer: Epidemiology with particular reference to prevalence and
life-style changes that influence survival. Oral Oncol. 46:407–410.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Eckert AW, Kappler M, Schubert J and
Taubert H: Correlation of expression of hypoxia-related proteins
with prognosis in oral squamous cell carcinoma patients. Oral
Maxillofac Surg. 16:189–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Adeyemi BF and Kolude B: Clinical
presentation of oral squamous cell carcinoma. Niger Postgrad Med J.
20:108–110. 2013.PubMed/NCBI
|
|
44
|
Hsu PP and Sabatini DM: Cancer cell
metabolism: Warburg and beyond. Cell. 134:703–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Koppenol WH, Bounds PL and Dang CV: Otto
Warburg's contributions to current concepts of cancer metabolism.
Nat Rev Cancer. 11:325–337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen G, Liu H, Zhang Y, Liang J, Zhu Y,
Zhang M, Yu D, Wang C and Hou J: Silencing PFKP inhibits
starvation-induced autophagy, glycolysis, and epithelial
mesenchymal transition in oral squamous cell carcinoma. Exp Cell
Res. 370:46–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen MK, Chiou HL, Su SC, Chung TT, Tseng
HC, Tsai HT and Yang SF: The association between hypoxia inducible
factor-1alpha gene polymorphisms and increased susceptibility to
oral cancer. Oral Oncol. 45:e222–e226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Peerlings J, Van De Voorde L, Mitea C,
Larue R, Yaromina A, Sandeleanu S, Spiegelberg L, Dubois L, Lambin
P and Mottaghy FM: Hypoxia and hypoxia response-associated
molecular markers in esophageal cancer: A systematic review.
Methods. 130:51–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hu X, Lin J, Jiang M, He X, Wang K, Wang
W, Hu C, Shen Z, He Z, Lin H, et al: HIF-1α Promotes the Metastasis
of Esophageal Squamous Cell Carcinoma by Targeting SP1. J Cancer.
11:229–240. 2020. View Article : Google Scholar :
|
|
50
|
Hu H, Zhu W, Qin J, Chen M, Gong L, Li L,
Liu X, Tao Y, Yin H, Zhou H, et al: Acetylation of PGK1 promotes
liver cancer cell proliferation and tumorigenesis. Hepatology.
65:515–528. 2017. View Article : Google Scholar
|
|
51
|
Qian X, Li X and Lu Z: Protein kinase
activity of the glycolytic enzyme PGK1 regulates autophagy to
promote tumorigenesis. Autophagy. 13:1246–1247. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jang CH, Lee IA, Ha YR, Lim J, Sung MK,
Lee SJ and Kim JS: PGK1 induction by a hydrogen peroxide treatment
is suppressed by antioxidants in human colon carcinoma cells.
Biosci Biotechnol Biochem. 72:1799–1808. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fu D, He C, Wei J, Zhang Z, Luo Y, Tan H
and Ren C: PGK1 is a potential survival biomarker and invasion
promoter by regulating the HIF-1α-mediated epithelial-mesenchymal
transition process in breast cancer. Cell Physiol Biochem.
51:2434–2444. 2018. View Article : Google Scholar
|
|
54
|
Patel SS, Shah KA, Shah MJ, Kothari KC and
Rawal RM: Cancer stem cells and stemness markers in oral squamous
cell carcinomas. Asian Pac J Cancer Prev. 15:8549–8556. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mohajertehran F, Sahebkar A, Zare R and
Mohtasham N: The promise of stem cell markers in the diagnosis and
therapy of epithelial dysplasia and oral squamous cell carcinoma. J
Cell Physiol. 233:8499–8507. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Murugan AK, Munirajan AK and Tsuchida N:
Ras oncogenes in oral cancer: The past 20 years. Oral Oncol.
48:383–392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lee MS, Jeong MH, Lee HW, Han HJ, Ko A,
Hewitt SM, Kim JH, Chun KH, Chung JY, Lee C, et al: PI3K/AKT
activation induces PTEN ubiquitination and destabilization
accelerating tumourigenesis. Nat Commun. 6:77692015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Song Y, Zheng S, Wang J, Long H, Fang L,
Wang G, Li Z, Que T, Liu Y, Li Y, et al: Hypoxia-induced PLOD2
promotes proliferation, migration and invasion via PI3K/Akt
signaling in glioma. Oncotarget. 8:41947–41962. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yu J, Luo Y and Wen Q: Nalbuphine
suppresses breast cancer stem-like properties and
epithelial-mesenchymal transition via the AKT-NFκB signaling
pathway. J Exp Clin Cancer Res. 38:1972019. View Article : Google Scholar
|






















