1. Introduction
Glutathione (GSH), also known as
γ-glutamylcysteylglycine, is the most common small molecular weight
thiol molecule generated in living cells (1). It is extensively present in all
eukaryotes and is particularly concentrated in the liver. GSH
manifests in two forms, mercaptan reduction (GSH) and disulfide
oxidation (GSSG). GSH is the more common form, with a concentration
>100 times higher than that of GSSG (2). Synthesized from glutamic acid,
cysteine, and glycine, GSH serves a pivotal role in the pathway
using niacinamide adenine dinucleotide to establish a reducing
environment key for cellular function. A previous study (3) underscored the crucial involvement of
GSH in diverse cellular processes, including immunological
function, cell proliferation, differentiation and programmed cell
death. As a regulator agent in key signal transduction pathways,
GSH is involved in maintaining cellular homeostasis. The
dysregulation of GSH expression strongly correlates with onset and
progression of numerous types of disease, including tumors
(4), liver disease (5), diabetes (6) and neurodegenerative disease
(7,8). Tumor cells require elevated GSH
levels to combat reactive oxygen species (ROS) and detoxify
carcinogens. Thus, decreasing intracellular GSH renders tumor cells
more susceptible to oxidative stress and chemotherapeutic drugs
(9).
While existing studies (2,4)
predominantly focused on the anabolic aspects of GSH metabolism,
the catabolic process of GSH has received limited attention
(10,11). Previously, the cytoplasm was
hypothesized to have no role in GSH catabolism. However, the
identification of novel GSH degradation pathways in the cytosol
(12,13) underscores the importance of
exploring GSH degradation. GSH-degrading enzymes are essential for
maintaining GSH homeostasis in cells. Dysregulation of these
enzymes significantly impacts GSH homeostasis, leading to
pathological changes. Such dysregulation is frequently observed in
tumor tissue and has been shown to play an essential role in tumor
development (14,15). Since different GSH-degrading
enzymes are oriented to either intracellular or extracellular GSH
pools, intracellular degrading enzymes directly decrease
intracellular levels of GSH. By contrast, extracellular degrading
enzyme produces cysteine, providing an additional rate-limiting
amino acid for resynthesis of intracellular GSH (16). Therefore, different GSH-degrading
enzymes exhibit different effects on cancer, either promoting or
suppressing it, and their specific functions varies according to
the type of tissue and tumor. In addition, the levels of GSH
degradation products glutamic acid, cysteine and glycine serve as
growth factors, proliferation stimulators and signal transducers of
tumor cells (17,18). Thus, understanding of
GSH-degrading enzymes and their roles in cancer is imperative for
developing more effective therapeutic interventions.
The present review summarizes the crucial role of
enzymes in GSH degradation in tumors, as well as their potential as
biomarkers and targets for tumor therapy and their potential
directions for clinical translation in tumor therapies.
2. Extracellular and intracellular
GSH-degrading enzymes
Initiation steps in the mammalian GSH degradation
pathway fall into two categories, intracellular and extracellular
degradation (Fig. 1) (10). The first category is classical GSH
degradation, which commences with extracellular enzyme γ-glutamyl
transpeptidase (GGT) (11).
Intracellular GSH is released into the extracellular space via
multidrug-resistant protein 1-mediated transporter (19). Once outside the cell, GSH is
hydrolyzed to Cys-Gly and glutamate by plasma membrane-bound GGT,
marking the initial step in extracellular GSH degradation.
Discoveries in the cytoplasmic cation transport regulator homolog
(ChaC) family of γ-glutamylcyclotransferases have expanded the
understanding of GSH degradation (12,13). This family, including ChaC1 and
ChaC2, directly breaks down GSH within the cell into Cys-Gly and
5-oxoproline (11,20).
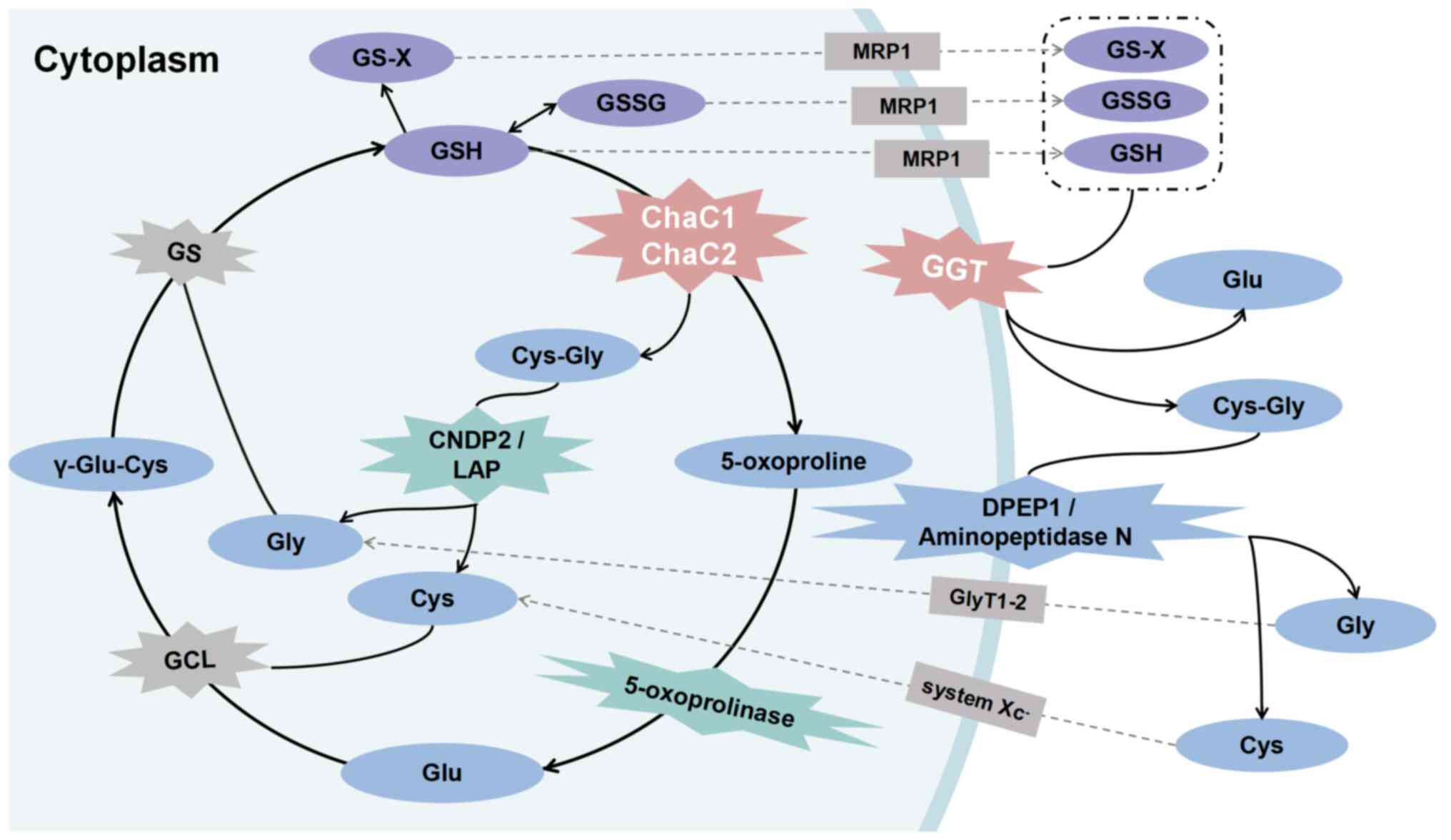 | Figure 1Role of GSH-degrading enzymes in
mammals. The classical GSH degradation pathway occurs
extracellularly. Intracellular GSH, GSSG and GS-X are released from
cells into the extracellular space via MRP1 transporter. GGT,
located on the plasma membrane, hydrolyzes them to Cys-Gly and Glu,
serving as the initial step in extracellular GSH degradation.
Cys-Gly undergoes catalysis to Gly and Cys by DPEP1 or
minopeptidase N. ChaC1 and ChaC2 hydrolyze GSH to Cys-Gly and Glu
or their cyclized form of 5-oxo-proline directly in the cell. Among
these, 5-oxo-proline is hydrolyzed to Glu by 5-oxop-rolinase.
Cys-Gly, induced by cytoplasmic Cys-Gly peptidase LAP or CNDP2, is
hydrolyzed to Gly and Cys, completing the degradation of GSH.
ChaC1, glutathione-specific γ-glutamylcyclotransferase 1; CNDP2,
carnosine dipeptidase 2; DPEP1, M19 metallopeptidase dipeptidase 1;
GCL, glutamate cysteine ligase; GGT, γ-glutamyl transpeptidase;
GlyT1-2, glycine transporter 1 and 2; GS, glutathione synthetase;
LAP, leucyl aminopeptidase; MRP1, multidrug resistance protein
1. |
GGT: Classical perspective
GGT, a core component of the γ-glutamyl cycle
(21), has long been associated
with GSH degradation, predating the discovery of the ChaC family.
Initially considered the sole enzyme capable of degrading GSH, GGT
hydrolyzes the γ-glutamyl bond of extracellular reduced and
oxidized GSH (22). This results
in cleaved glutamate, cysteine and glycine while facilitating the
transfer of γ-glutamyl moiety of GSH to either water (hydrolysis)
or substrates such as peptides (transpeptidation). Consequently,
GGT is classified as a bisubstrate enzyme (23).
GGT is a glycosylated heterodimer protein formed by
the non-covalent combination of a heavy chain subunit (relative
molecular mass, 50,000-62,000) and a light chain subunit (relative
molecular mass 22,000-30,000; Fig.
2) (24). The human GGT
family members are synthesized and cleaved by an autocatalytic
processing reaction. They have a conserved 'sandwich-like'
three-dimensional domain with four layers of αββα folds (23). The catalytic site of GGT consists
of two successive regions: Well-characterized donor site,
specifying the substrates to which donor γ-glutamyl groups bind,
and the acceptor site, about which little is currently known
regarding the involved residues (24).
Anchored in the plasma membrane by the N-terminus of
the heavy chain across the membrane segment, GGT protein, under
physiological conditions, is typically confined to the plasma
membrane. It is distributed on the apical surface of epithelial and
endothelial cells in glands and lumens. A unique characteristic of
GGT is that it is located on the extracellular surface of mammalian
cells with a catalytic active site oriented to the extracellular
environment (11). The kidney
expresses GGT at the highest levels, while notable expression is
also found in bile canaliculi of hepatocytes, ducts within the
pancreas, the apical surface of the intestinal epithelium and the
luminal surface epithelium of many reproductive organs (25).
GGT gene family and proteins
A GGT gene family exists in the human genome
(Table I), suggesting that
regulating GGT activity may be associated with activating different
GGT genes rather than identifying distinct gene loci (26).
 | Table IGGT-homologous sequences. |
Table I
GGT-homologous sequences.
| Genea | Previous names | Functional
protein | Abnormal
expression |
|---|
| GGT1 | Gene 6; GGT type
I | Functional
protein | Dysregulated in
various tumors |
| GGT2 | Clone F15; Gene 3
(L10396); GGT type II | Inactive
propeptide, 94% homologous to part of GGT1 | Low expression in
glioblastoma multiforme |
| GGT3P | Clone F11;
GGT3 | Pseudogene | Not reported |
| GGT4P | Gene 12 (L10398);
clone F30 | Pseudogene | Not reported |
| GGT5 | GGL, γ-glutamyl
leukotrienase; GGTLA1/GGT-rel; GGT5 precursor; GGTLA1 | Functional protein,
40% homologous to GGT1, exhibits <1/46 activity of GGT1 in
hydrolyzing GSH, GSSG and leukotriene C4 | High expression of
GGT5 is beneficial to the prognosis of hepatocellular
carcinoma |
| GGT6 | Rat GGT6
homolog | Not
characterized | Overexpressed in
low-grade glioma |
| GGT7 | GGTL3, GGT4, GGTL5;
GC20M032896 | Not
characterized | Low expression in
gastric cancer; high expression may lead to poor overall survival
in hepatocellular carcinoma; GGT7 polymorphic loci rs6119534 and
rs11546155 are associated with risk of pancreatic disease |
| GGT8P | / | Pseudogene | Not reported |
| GGTLC1 | GGTL6; GGTLA4;
GGTLA4 | Encode only the
light chain part of GGT | Not reported |
| GGTLC2 | Gene 1 (L10394);
GGTL4; GGTL4 | Encode only the
light chain part of GGT | Not reported |
| GGTLC3 | γ-glutamyl
transferase light chain 3; LOC728226 | May encode only the
light chain of GGT | Not reported |
| GGTLC4P | γ-glutamyl
transferase light chain 4 pseudogene | Pseudogene | Not reported |
| GGTLC5P | γ-glutamyl
transferase light chain 5 pseudogene | Pseudogene | Not reported |
The human genome sequence contains 13 GGT homologs,
the most active of which is GGT. The GGT gene was first discovered
on human chromosome 22 at q11.1-q11.2 (27), although it was also subsequently
discovered on additional autosomes (26). In addition, two homologs, GGTLC1
(previously GGTL6, GGTLA4) and GGTLC2, which may only encode the
light-chain portion of GGT, as well as at least three other
homologs, exhibit activity: GGT5 (formerly GGL, GGTLA1/GGT-rel),
GGT6 (formerly rat GGT6 homologous) and GGT7 (formerly GGTL3, GGT4)
(28). While GGT5 and GGT1 share
40% of the amino acid sequence (22), GGT5 is not as active in
hydrolyzing GSH, GSSG and leukotriene C4 as GGT1 is (29). Despite the absence of verified
protein-coding activity, GGT6 and GGT7 exhibit aberrant expression
in conditions such as tumors (30-32) and pancreatic disease (33). Furthermore, proteins expressed by
the human GGT2 gene share 94% of the amino acid sequence encoded by
GGT1 (34), even though GGT2 only
encodes inactive pro-peptides. GGT2 also exhibits abnormal
expression in tumors (30) and
upregulating GGT2 can overcome H2O2-induced
apoptosis (35). These findings
suggest that GGTs play potential roles in disease physiology and
pathology.
Catalytic activity of GGT
As a member of the N-terminal nucleophilic hydrolase
superfamily (Ntn), GGT uses a highly conserved catalytic mechanism.
An N-terminal Thr residue is essential for substrate priming in
human GGT (22), with the
substrate binding site featuring a key Thr side chain. This
facilitates conversion of the γ-glutamyl bond of GSH into an acyl
bond, releasing Cys-Gly and glutamate (11).
GGT gene expression
While the human GGT gene is not fully characterized,
evidence suggests its existence in multiple copies within the human
genome (36). As GGT mRNAs share
a common coding sequence and have 59 untranslated regions (UTRs),
its structural complexity is evident (37). Understanding of the human GGT
promoter remains limited (38).
A number of promoters control human GGT
transcription, and the resulting transcripts undergo selective
splicing in untranslated regions and coding sequences (37). The initiation of GGT mRNA
transcription involves cis-reactive elements, including the
cis-regulatory element (TRE) and the binding element for activator
protein 1 (AP-1) (38). TRE, also
known as 12-O-tetracylacylphobolol 13 acetic acid reactive element,
incorporates binding sites for activating protein 2 (AP-2) and
specific protein 1 (Sp1; Fig.
3).
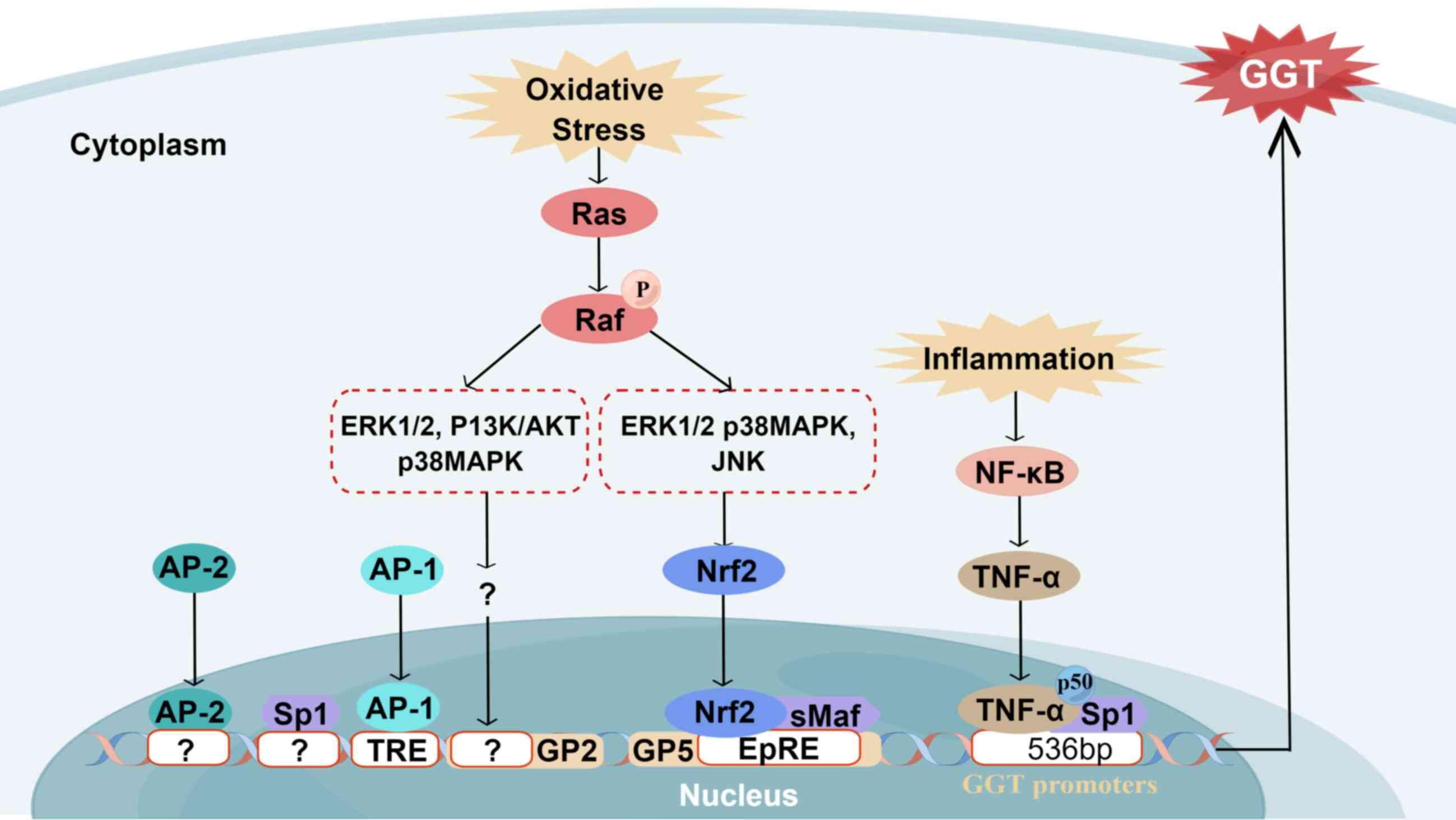 | Figure 3Regulation of GGT. The regulation and
expression of GGT remain incompletely characterized. GGT mRNA
transcription is co-triggered by multiple potential cis-reactive
elements, similar to rat GGT promoters. The proximal region of the
GGT promoter contains the binding sites for TRE (often called AP-1
binding elements), AP-2, and Sp1. Ras protein and its downstream
oxidative stress effectors, such as ERK1/2, p38MAPK, PI3K/AKT and
JNK signaling pathways, serve a key role in upregulating GGT. The
P13K/AKT, ERK1/2 and p38MAPK signaling pathways activate Nrf2 via
EpRE and sMaf. Nrf2 then transfers from cytoplasm to the nucleus,
forming heterodimers with other proteins to bind to EpRE,
participating in the upregulation of GP5 activity. Activated H-Ras
is also implicated in inducing GP2 activation via downstream
ERK1/2, p38MAPK and JNK. Inflammatory conditions activate the NF-κB
pathway, initiating downstream TNF-α transcription and transfer to
the 536 bp site of the nuclear GGT proximal promoter. The site
contains a p50, TNF-α and Sp1 binding site, thereby promoting GGT
expression. AP2, activator protein 2; ERK1/2, extracellular
signal-regulated kinase 1/2; EpRE, electrophile response element;
GGT, γ-glutamyl transpeptidase; GP2, GGT promoter 2; sMaf, small
musculoaponeurotic fibrosarcoma; Sp1, specific protein 1; TRE,
cis-regulatory element. Drawn with Figdraw (figdraw.com). |
A study (39) on
HeLa cells highlighted that phorbol 12-myric acid 13-acetic acid
enhance the expression of human GGT, pinpointing its binding site
at the AP-1 binding site 2,214/2,225 nucleotides upstream from the
transcription start site. More research is necessary to understand
the transcriptional mechanism of the human GGT gene and
promoters.
Although rat and human GGT promoters may have
similar structures, the human promoter is more complex. As the rat
GGT gene is single-copy, replicating unique genes after species
transfer between rats and humans likely results in multiple human
GGT genes (40). Therefore, rat
GGT may provide insight into human GGT expression and
regulation.
In rats, GGT expression is controlled by a tandem
P1-P5 promoter, facilitated by variable splicing. This yields
transcripts sharing the same coding region and diverse 5'-UTRs
(38). These unique promoters
have high tissue stage-specificity (38).
GGT regulation
Upregulation of GGT activity is primarily dependent
on the Ras protein and its downstream effectors, which include
extracellular signal-regulated kinase 1/2 (ERK1/2), p38
mitogen-activated protein kinase (MAPK), phosphatidylinositol
3-kinase (PI3K)/AKT and c-Jun N-terminal kinase (JNK) signaling
pathways (Fig. 3) (41). The Ras protein is a key regulator
of signaling pathways key for normal cell proliferation. Malignant
phenotype of tumor cells is caused by the Ras gene, often mutated
or active in tumor cells, and leads to aberrant tumor cell
proliferation, programmed cell death, invasion and angiogenesis
(42).
Through the electrophile response element (EpRE),
ERK1/2 and p38MAPK signaling pathways actively contribute to
upregulation of GGT promoter 5 (GP5) activity in response to
4-hydroxynonenal (HNE) (43).
Following activation by redox signaling downstream of the MAPK
signal pathway, the EpRE binding protein, also an oxidative
stress-associated transcription factor, induces the dissociation of
Nrf2 from Keap1 via redox modification and/or phosphorylation of
Nrf2. Subsequently, Nrf2 translocates from cytoplasm to the
nucleus, forming heterodimers with other proteins to bind to EpRE.
This leads to the amplification of GGT transcription. In alveolar
type II (L2) cells, EpRE motif in the proximal region of GP5 EpRE
induces the expression of the major GGT transcript mRNA V-2 in the
lung (38). However, pretreatment
with ERK1/2 pathway inhibitor (PD98059) or p38MAPK inhibitor
(SB203580) partly decreases the expression of GGT mRNA V-2 induced
by HNE in L2 cells (38).
Furthermore, activated Ras is also implicated in inducing
activation of GP2 and increasing expression of GGT transcriptional
products and protein in colon cancer cells treated with
naphthoquinone during acute oxidative stress (Fig. 3) (41).
Moreover, inflammatory conditions significantly
enhance GGT levels by activating the NF-κB pathway. NF-κB, the core
transcription factor in the NF-κB signaling pathway, is a dimer
family formed by p50/p105/NF-κB1, p52/p100/NF-κB2, c-Rel, p65/RelA
and RelB (44). It regulates the
expression of chemokines, cytokines, transcription factors and
regulatory proteins, playing a crucial role in inflammation and
immunity (44). Upon cell
stimulation by an external signal, the NF-κB dimer is released from
its inhibitor (IκB) and freely transferred into the nucleus. When
the NF-κB pathway is activated, it triggers production of
pro-inflammatory proteins downstream, such as tumor necrosis
factor-α (TNF-α), which in turn causes inflammatory responses and
pain. Furthermore, by serving as NF-κB activators, these
inflammatory cytokines intensify inflammation by further triggering
the NF-κB pathway (45). In the
536 bp site of the proximal promoter of GGT, a binding site exists
between p50, TNF-α and Sp1, regulated by the activation of the
NF-κB signaling pathway, thereby promoting expression of GGT and
inducing inflammatory response (Fig.
3) (46). Moreover, it has
been reported (46) that
inhibitors of the NF-κB pathway can effectively block the
trans-activation of GGT promoters at different levels. For example,
remicade, a clinically used anti-TNF-α antibody targeting the p50
and p65 NF-κB subtype of small interfering RNA and curcumin, a
well-characterized natural NF-κB inhibitor that is also a dominant
negative inhibitor of κBα (IκBα), can inhibit GGT activation
through distinct mechanisms. This suggests the involvement of the
NF-κB pathway in regulating GGT expression. Therefore, inflammatory
conditions may increase GGT synthesis, potentially acting as a
cellular protective mechanism under increased oxidative stress or
promoting inflammatory progression. Further research is necessary
to explore these possibilities.
ChaC1/ChaC2: Additional perspective
In addition to the well-established extracellular
GSH degradation mechanism, studies have shown an additional
intracellular hydrolysis pathway for GSH degradation (47,48). ChaC protein features a BtrG/γ-GCT
fold and distinctive β-barrels surrounded by α-helices (Fig. 4) (13). Mammals exhibit two isoforms of
ChaC: Mammalian pro-apoptotic factor ChaC1 (formerly MGC4504) and
its homologous counterpart ChaC2. Conversely, only one ChaC member
is present in lower eukaryotes, especially in unicellular
eukaryotes (47).
Gene and protein structure of
ChaC1/ChaC2
The human ChaC1 gene is on chromosome 15q15.1 and
comprises three exons, encoding a protein with 222 amino acid
residues and a molecular weight of ~25 kDa (49). ChaC1, 30% identical to mammalian
and prokaryotic genes, serves a crucial role in basic physiology
(12).
ChaC2 is on chromosome 2p16.2 and encodes a protein
with 184 amino acid residues and a molecular weight of ~20.9 kDa
(20,47,50). A phylogenetic study (47) highlighted that ChaC2 evolved
earlier than ChaC1 and shares key structural similarities with ChaC
proteins of lower eukaryotes. Human ChaC2 and ChaC1 share 50% of
their protein identity (47).
ChaC2 typically exists in dimer crystals with a unique flexible
loop 2 structure, with an open conformation that can facilitate
close contact with crystallographically adjacent ChaC2 molecules.
Additionally, ChaC2 E74Q/E83Q active site mutants exhibit a closed
conformation, regulating the degradation activity of ChaC2 to GSH
(20).
Catalytic activity of ChaC1/ChaC2
ChaC1, an inducible enzyme, can be expressed under
specific stresses or pathological conditions (12,47). It selectively hydrolyzes GSH,
producing Cys-Gly and 5-oxoproline (a cyclized form of glutamate)
(11,51), thereby accelerating formation of
the cellular oxidative environment. The Michaelis constant of ChaC1
for GSH is ~2.2±0.4 mM (47),
comparable with the concentration of intracellular GSH (1-10 mM)
under physiological conditions. ChaC1 often forms dimers or
tetramers, with dimerization being key for regulating enzyme
activity and substrate specificity. Under stress or tumor growth,
there is an increased need for enzyme breakdown, which leads to
formation of dimers or longer oligomers of ChaC1 (20).
ChaC2 is constitutively expressed and exhibits
catalytic efficiency for GSH 10-20 times weaker than that of ChaC1
(47). The lower activity of
ChaC2 may be partly attributed to flexible loop 2, acting as a
gating function to achieve specificity for GSH binding and regulate
a constant GSH degradation rate. In addition, the Glu74 and Glu83
residues of ChaC2 are key for directing the conformation of the
enzyme and regulating enzyme activity (20).
Expression and regulation of
ChaC1/ChaC2
Various signals, including endoplasmic reticulum
(ER) and oxidative stress and viral infection, activate ChaC1
promoters in different cell types, cellular processes and diseases
through the unfolded protein response (UPR) (12,48,52). ChaC1 is downstream of the protein
kinase R-like ER kinase (PERK)/eukaryotic initiation factor-2α
(eIF2α)/activating transcription factor (ATF) 4/ATF3/CCAAT/enhancer
binding protein (C/EBP) homologous protein (CHOP) pathway, serving
as a pro-apoptotic and pro-ferroptosis component downstream of UPR.
ATF4, ATF3, CHOP and C/EBP-β upregulate ChaC1 transcription
(Fig. 5). The activation of ChaC1
by UPR primarily relies on ATF4, while the involvement of CHOP,
C/EBP-β, and ATF3 is indirect (12). A direct relationship between ATF4
and regulatory elements within the ChaC1 promoter has been
identified. A-267 ATF/cAMP response element (CRE) in conjunction
with a novel-248 ATF/CRE modifier (ACM), serving as a binding site
for ATF4 and ATF3 transcription factors, regulates the activity of
the basic ChaC1 promoter. Among these elements, ATF3 predominantly
regulates the basal and stress-induced expression of ChaC1 through
ATF/CRE, while ATF4 primarily regulates stress-induced ChaC1
expression through ATF/CRE and ACM (48). Additionally, conserved-209
CEBP-ATF response element has a limited impact on regulating human
ChaC1 transcription (48,53).
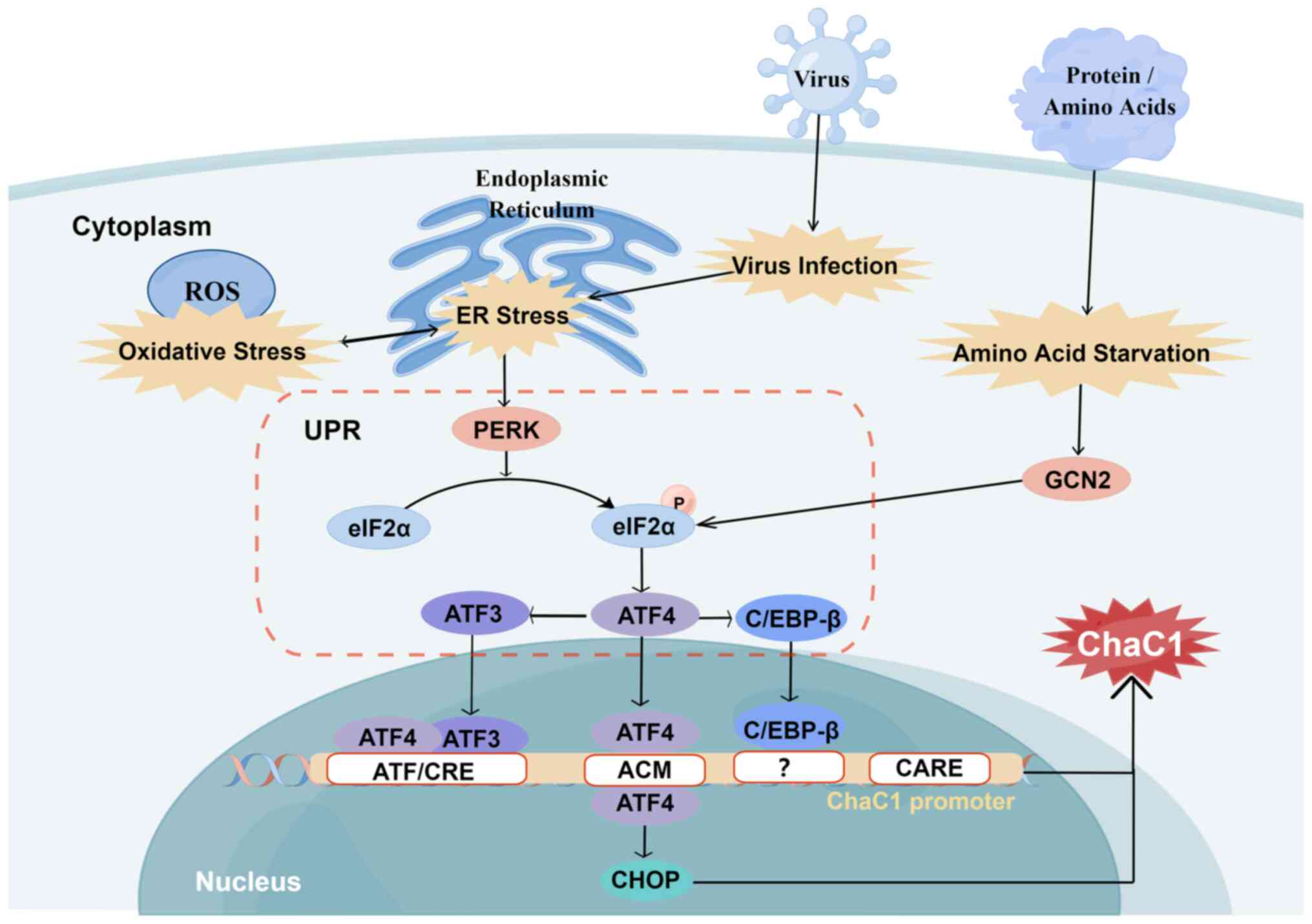 | Figure 5Regulation of ChaC1. Oxidative and ER
stress, and viral infection induce PERK/eIF2α/ATF4/ATF3/CHOP
cascade activation via UPR. ATF3 may primarily regulate basal ChaC1
expression via ATF/CRE, while ATF4 may mainly regulate
stress-induced ChaC1 expression via ATF/CRE and ACM. In response to
ER stress, C/EBP-β recruits ATF4 to the ChaC1 promoter, but the
precise C/EBP-β response element on the ChaC1 promoter remains
unclear. CARE serves a secondary role in regulating human ChaC1
transcription. Amino acid starvation induces ChaC1 expression,
activating ATF4 via the GCN2/eIF2a/ATF4/ATF3 pathway. ACM, a
novel-248 ATF/CRE modifier; ATF/CRE, activating transcription
factor/cAMP response element; CARE, conserved-209 CEBP-ATF response
element; C/EBP-β, CCAAT/enhancer binding protein β; CHOP, C/EBP
homologous protein; eIF2α, eukaryotic initiation factor-2α; ER,
endoplasmic reticulum; GCN2, general control nonderepressible 2;
PERK, protein kinase R-like ER kinase; UPR, unfolded protein
response. Figure constructed using Figdraw (figdraw.com). |
Amino acid starvation can induce the expression of
ChaC1 (Fig. 5). The amino acid
starvation response activates ATF4 via the general control
nonderepressible 2 (GCN2)/eIF2a/ATF4/ATF3 pathway (54). Both ER stress and amino acid
starvation induce stress synergistically by activating ATF4 and
ChaC1 is one of the downstream targets of ATF4. In regulating ChaC1
expression, C/EBP-β has been observed to recruit ATF4 to the ChaC1
promoter in response to ER stress (48). However, the precise C/EBP-β
response element on the ChaC1 promoter remains unclear (48). Further studies are necessary to
elucidate the detailed mechanism of C/EBP-β-mediated ATF4
recruitment and its impact on ChaC1 expression. These findings
highlight the intricate nature of ChaC1 transcriptional regulation
and underscore the importance of maintaining appropriate redox
balance in cells (48). The
mechanism governing ChaC1 protein expression requires further
characterization.
Understanding of the regulation mechanism of ChaC2
is limited. A previous study (47) suggested that ChaC2 is expressed at
higher basal levels under physiological conditions than ChaC1.
However, under cellular stress such as ER stress or amino acid
starvation, ChaC1 is upregulated, while ChaC2 expression remains
unaffected (47). Thus, ChaC2
serves as a constitutively expressed protein for basal hydrolysis
of GSH, acting as a steward for slow and continuous GSH turnover
(47).
3. GSH-degrading enzymes in tumorigenesis
and progression
Tumor cells enhance GGT expression across the entire
cell membrane, facilitating the acquisition of additional cysteine
and cystine from GSH in blood and interstitial fluid to replenish
intracellular GSH levels (22).
Consequently, aberrant GGT expression is observed in various types
of cancer, including ovarian (55), renal cell (56), lung (57), stomach (58) and pancreatic cancer (59). Elevated GGT expression is
generally associated with poor prognosis, as patients with high
levels of GGT in tumors exhibit shorter overall and
progression-free survival (60).
However, some breast tumor tissues exhibit GGT loss (61).
Studies (54,62,63) of the ChaC family have yielded
conflicting findings regarding ChaC expression and its role in
tumor tissues, emphasizing the complexity of GSH regulation and
function. ChaC1, as a tumor-influencing factor, enhances ER stress,
contributing to necroptosis and ferroptosis of multiple cancers,
including metastatic melanoma (64), breast (54), prostate (65) and primary liver cancer (66), Burkitt's Lymphoma (67), head and neck squamous cell
carcinoma (68), glioblastoma
multiforme (GBM) (69), oral
squamous cell carcinoma, T lymphoblastic leukemia Molt4 cells and
colitis-associated carcinogenesis (15). Decreased ChaC1 expression is an
indicator of poor prognosis in kidney renal clear cell carcinoma
(KIRC) (70) and certain types of
gastric cancer (71,72). Conversely, reports suggest that
ChaC1 overexpression may be associated with tumor cell
dedifferentiation, proliferation, invasion and migration, leading
to lower patient survival rates (73,74). ChaC1 serves as a reliable
indicator for poor prognosis of certain types of gastric cancer
(63) and melanoma (75,76), as well as an independent indicator
for elevated risk for female germ line tumors (including breast and
ovarian cancer) (77). Therefore,
different effects of ChaC1 may be linked to the specific functions
of GSH in different types of tumor tissues.
ChaC2 may be implicated in numerous vital
physiological functions, including DNA replication and repair, cell
cycle regulation, RNA and damaged DNA binding, oocyte meiosis and
maturation (50). Its important
physiological role was initially discerned in undifferentiated
human embryonic stem cells (hESCs), where ChaC2 is prominently
expressed and maintains cell self-renewal and pluripotency by
modulating GSH homeostasis. Conversely, downregulation of ChaC2
decelerates the cell cycle progression of hESCs and triggers cell
death (78), underscoring its
pivotal role in regulating human growth and development. In
pathological functions, ChaC2 exhibits a multifaceted role in tumor
tissue. Generally acting as a tumor suppressor, ChaC2 decreases GSH
levels in tumor cells, instigates mitochondrial apoptosis and
autophagy via UPR and hinders tumor cell proliferation and
migration in vitro and in vivo (79). Therefore, ChaC2 expression is
commonly downregulated in tumor tissues, such as gastric and
colorectal cancer (79). ChaC2
may exert a tissue-specific function, promoting the survival of
tumor cells in specific contexts (50,81,82). The expression of ChaC2 increases
with progression of lymph node metastasis and the stage of breast
cancer, aligning with findings of GGT loss (61) and increased ChaC1 expression
(77) in breast cancer cells. The
increased expression of ChaC2 is associated with the high
expression of p53 (50), and
ChaC2 is the upstream regulator of the main antioxidant regulator
Nrf2 (78). In addition mutated
p53 regulates NRF2-dependent antioxidant responses that are
critical for supporting cancer cell survival (83). Therefore, increased ChaC2
expression may be related to regulating the binding of p53 mutants
to Nrf2. When transcription and activity of GSH synthase is
increased, the GSH level of the tissue is increased (80). The GSH overexpression then foster
the survival and proliferation of tumor cells. Additionally,
increased ChaC2 expression targets the Cadherin 1 gene (encoding
E-cadherin) mutation, resulting in E-cadherin loss, increased
epithelial-mesenchymal transformation and lymph node metastasis,
ultimately contributing to the low differentiation of breast cancer
(50). Consequently, overall
ChaC2 expression is associated with lymph node metastasis and stage
progression of breast cancer. ChaC2 promotes lung adenocarcinoma
growth by elevating ROS levels and activating MAPK signaling
pathways (81).
GGT serves as an extracellular degradation enzyme of
GSH, catalyzing enzymatic degradation products to enter cells and
providing an additional cysteine source for intracellular GSH
synthesis. Intracellular degrading enzymes ChaC1 and ChaC2 play a
key role in downregulating intracellular GSH levels, exerting a
counteractive effect to regulate intracellular GSH homeostasis. The
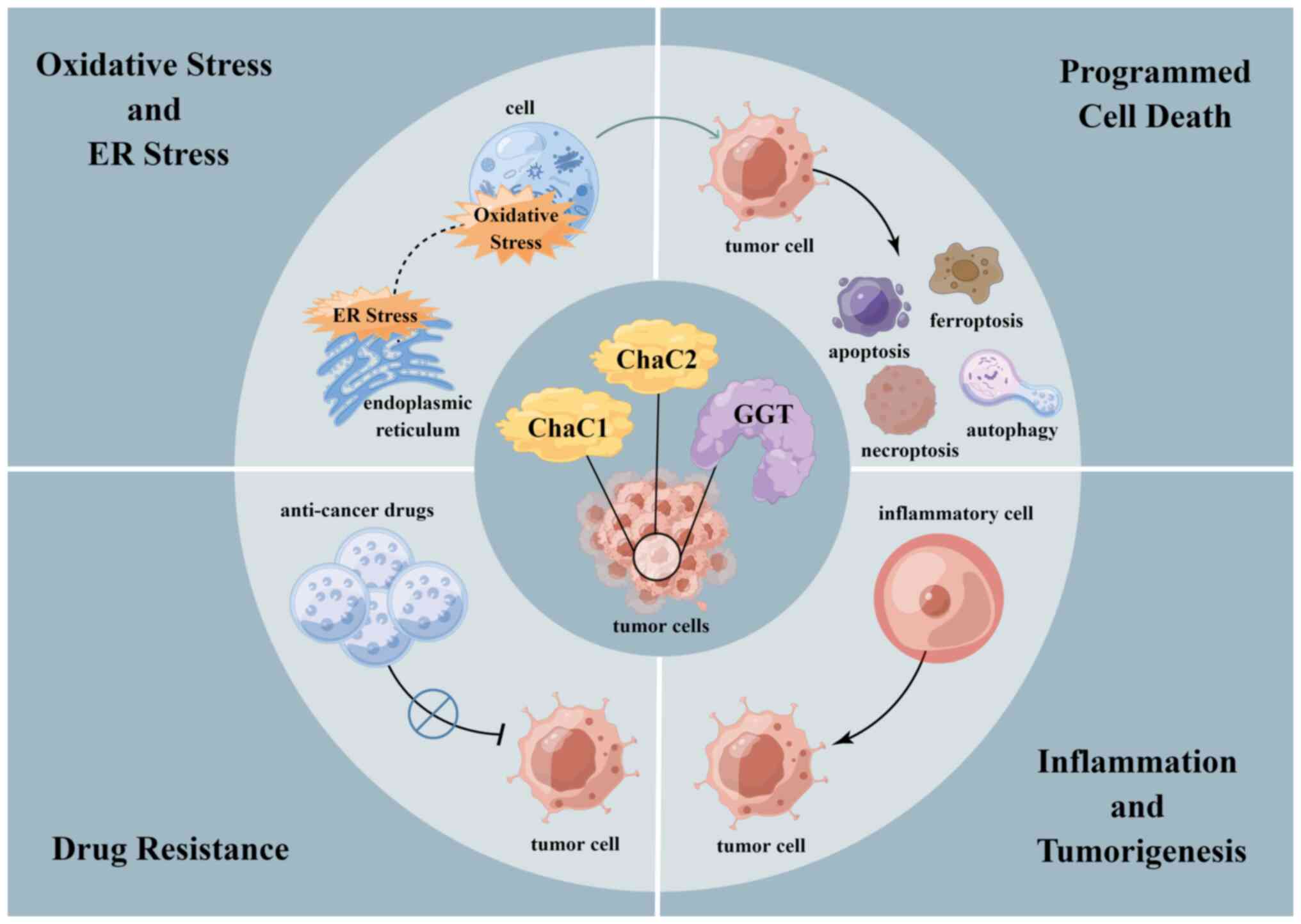
functions of GGT and ChaC1/ChaC2 include regulation of oxidative
and ER stress (38,84), modulation of programmed cell death
(85), promotion of inflammation
and cell drug resistance (24,86) (Fig.
6).
GSH degradation products such as glutamic acid,
glycine and cysteine also play key roles in the metabolic network
of the tumor environment. For example, glutamate regulates
proliferation, migration and survival of neuroprogenitor cells and
immature neurons. While the ability to proliferate and migrate
uncontrollably is characteristic of tumor cells, glutamate has been
shown to serve as a growth factor and signaling medium in certain
types of tumor tissues in both an autocrine and paracrine manner
(17). Glycine is involved in
cell transformation and tumorigenesis via cleavage into one-carbon
metabolism (18). Therefore,
exploring the function of GSH-degrading enzymes in tumors may
clarify the role of the complex metabolic network of tumors.
Regulating oxidative and ER stress
GSH-degrading enzymes key central to coordinating
cellular metabolism by regulating amino acid availability under
physiological conditions (11).
The modulation of the cellular stress environment relies on
regulation of GSH metabolism (4).
Tumor cells, in their quest for survival and proliferation,
generate abnormally high levels of oxidative stress, partly due to
increased cellular redox buffer GSH (87). Therefore, the role of
GSH-degrading enzymes is key to regulate the cell stress
environment.
The GSH degradation pathway initiated by
extracellular GGT effectively controls the intracellular oxidative
stress environment (38). GGT
deficiency leads to oxidative stress and cellular vulnerability to
oxidative damage. Animal studies (88-90) have indicated that GGT knockout
mice exhibit 20% of the plasma cysteine concentration in wild-type
mice. GGT knockout mice experience increased accumulation of DNA
oxidative damage, decreased intracellular GSH levels, elevated
oxidative stress and death by 10 weeks of age due to cysteine
deficiency (88). Patients with
partial GGT homozygous deletions report glutathionuria and
neurodevelopmental disorder (91). Hence, maintaining regular GGT
expression is essential for cellular GSH homeostasis and protecting
cells against oxidative stress.
Cells overexpressing GGT gain an advantage in
environments with physiological and limited cysteine concentrations
by efficiently utilizing extracellular GSH as a source of cysteine
(92). Tumor cells with elevated
intracellular GSH levels often induce overexpression of GGT. It has
been reported (22) that both GSH
depletion and GGT inhibition significantly enhance cytotoxicity
under oxidative stress in tumor cells. Tumor cells with high GGT
expression demonstrate notable oxidative stress tolerance without
DNA damage, while clones with low GGT expression exhibit increased
sensitivity to oxidative stress and apoptosis (93). As a marker of oxidative stress,
GGT expression in advanced tumor cells surpasses that in early
tumor cells. The increased oxidative stress and impaired immune
responses may be key for promoting cancer progression to advanced
stages and may be induced by inflammatory mediators within the
tumor (94). Therefore,
upregulation of GGT in tumor cells provides a potential mechanism
to resist oxidative stress and foster tumor progression.
Simultaneously, changes in the tumor
microenvironment generate persistent ER stress signals in various
types of tumor (95) such as
colorectal (96), pancreatic
cancer (97) and so on. This
state has a dual effect on tumor cells, on one hand controlling
several tumor-promoting features, on the other hand dynamically
reprogramming immune cells and inducing tumor cells autophagy,
apoptosis and ferroptosis (95).
ChaC1, a component of the UPR pathway, is a target of ferroptosis
induced by ER stress signals (12). Thus, ChaC1 is as a key regulator
of tumor development, metastasis and responses to chemotherapy,
targeted therapy and immunotherapy.
Previous research (78) has also revealed a broader range of
functions for ChaC2 than previously understood. ChaC2 inhibits
ChaC1-mediated GSH degradation, indicating competition with ChaC1
to maintain GSH homeostasis (78). ChaC2 directly regulates GSH
production via a ChaC1-independent pathway (78). ChaC2 enhances GSH production by
upregulating Nrf2, a key regulator of antioxidation, and its
downstream glutamate-cysteine ligase (78). These diverse actions underscore
the importance of ChaC2 in maintaining cellular redox homeostasis
and antioxidation mechanisms.
Extensive crosstalk exists between oxidative and ER
stress as oxidative stress can disrupt redox homeostasis in the ER,
triggering ER stress (95). GGT
and ChaC1 may be key factors in this mechanism. A study (98) demonstrated that GGT1 and GGT7
stimulate induction of ER stress-related protein, CHOP-10 and
immunoglobulin heavy chain binding protein BiP, indicating specific
roles for these GGT protein subtypes in ER stress response. This
suggests possible crosstalk between GGTDelta1, GGTDelta7 and ChaC1
via the ER stress/CHOP pathway. A recent study (99) proposed that the ATF4/CHOP/ChaC1
signaling pathway might be vital for apoptosis induced by crosstalk
between oxidative and ER stress. Under extreme heat stress, cells
produce a large amount of ROS, leading to oxidative stress and
protein misfolding in the ER, resulting in ER stress and triggering
ChaC1-associated UPR. Moreover, induction of ChaC1 serves an
essential regulatory role in ER stress-mediated apoptosis of cancer
cells induced by the anticancer monosaccharide xylitol, leading to
secondary induction of oxidative stress in treated cells and
apoptosis (100). This evidence
collectively demonstrates the key role of GGT and ChaC1 in
mediating crosstalk between oxidative and ER stress.
Modulating programmed cell death
Programmed cell death, encompassing apoptosis,
ferroptosis, necrotic apoptosis and autophagy, is instigated by a
series of intracellular processes (101). GGT- and ChaC1/ChaC2-mediated
intracellular GSH depletion can concurrently or sequentially
initiate multiple forms of programmed cell death. Studies (101,102) have indicated that the GSH/GSSG
redox status serves as a vital indicator of tumor programmed cell
death, consistently associating programmed cell death with a
decrease in the GSH/GSSG ratio. Therefore, targeting GGT and
ChaC1/ChaC2 to modulate programmed cell death holds implications
for tumor therapy.
Regulating apoptosis
Apoptosis, the quintessential programmed cell death
process, primarily relies on the caspase family for initiation and
is typically characterized by membrane contraction, chromatin
concentration and apoptotic body formation (103). In tumor cells, the apoptosis
pathway is often impeded by various mechanisms, many of which
contribute to intrinsic resistance to chemotherapy, the most
prevalent anticancer therapy (104). Reducing GSH impairs cellular
antioxidant regulation, increasing ROS production, thereby
accelerating mitochondrial damage and apoptosis induction (101). Consequently, inhibiting GGT1 in
tumor cells facilitates induction of apoptotic phenotypes (105). Simultaneously, research
(106) on another member of the
GGT family has shown that low GGT7 expression may elevate cell ROS
levels, inhibiting apoptosis and fostering tumor proliferation.
This suggests variations in regulation of ROS levels within the GGT
family. ChaC1 overexpression can augment apoptosis by activating
caspase-3/9, degradation of poly (ADP ribose) polymerase, induction
of autophagy, ROS generation, increased intracellular calcium and
loss of mitochondrial membrane potential (69).
Regulating ferroptosis
Ferroptosis, a distinct iron-dependent form of cell
death, arises from lethal accumulation of lipid peroxides (107). In tumor cells, evasion of
ferroptosis mediated by oncogenes or carcinogenic signaling
contributes to tumor onset, progression, metastasis and resistance
to treatment. Simultaneously, some tumor cells, owing to specific
mutations, elevated ROS levels and other unique biological
features, exhibit ferroptosis susceptibility, with their survival
hinging on the ferroptosis defense system (108). For example, ferroptosis
resistance is conferred by frequently occurring PI3K activating
mutations or loss of phosphatase and tensin homolog deleted on
chromosome 10 function in human cancer such as lung adenocarcinoma
(109) and breast cancer
(110) and so on. Conversely,
inhibition of the PI3K/AKT/mTOR signaling axis sensitizes cancer
cells to ferroptosis induction (111). Consequently, targeting
ferroptosis regulation holds implications for cancer immunotherapy
and tumor suppression.
Regarding ferroptosis regulation, GGT-activated
extracellular GSH catabolism produces iron-derived ROS, inducing
lipid peroxidation via NF-κB pathway activation (112). GGT-mediated GSH catabolism via
lipid peroxidation enhances NF-κB DNA binding capacity in tumor
cells (113). GGT increased
intracellular GSH levels (114),
restores the reduced GSH/GSSG ratio and reactivates GSH peroxidase
4, a core ferroptosis regulator. Therefore, elevated GGT expression
increases tumor cell resistance to ferroptosis, safeguarding cells
from ROS and lipid peroxidation, thus driving tumor cell
proliferation, metastasis and chemotherapy drug resistance
(85,107). In addition, GGT activates the
mTORC1 pathway and inhibits integrated stress response (ISR) by
modulating cystine-GSH crosstalk. This inhibition of ferroptosis
promotes cancer development and other cysteine-deficient diseases
(115). Inhibiting GGT impairs
GSH ability to restore mTORC1 signaling and ISR, inducing
ferroptosis. This implies that the role of GGT in inducing capacity
of GSH to release cysteine, rather than GSH itself, modulates the
mTORC1 pathway, ISR and ferroptosis. By contrast, ChaC1 induces
ferroptosis in tumor cells by activating the GCN2/EIF2α/ATF4
pathway to intensify cystine depletion (116). ChaC1 overexpression depletes
GSH, initiating and executing ferroptosis. The deletion of ATF4, an
upstream factor of ChaC1, in embryonic fibroblasts, results in a
ferric oxide-dependent death phenotype, emphasizing the role of
ATF4 as a downstream molecule of the eIF2α/ATF4 pathway (117). Hence, ferroptosis control is
associated with ChaC1 expression.
Regulating necroptosis
Necroptosis, a regulated form of cell death
primarily dependent on receptor-interacting protein kinase 3 and
mixed lineage kinase domain-like, is characterized by widespread
cytoplasm and organelle swelling, plasma membrane rupture and
release of cell components into the microenvironment (118). This pro-inflammatory form of
cell death holds implication for combating pathogen infection,
inflammatory progression (119),
and therefore may also contribute to early prevention of
inflammatory cancer transformation.
Understanding of the impact of GGT and ChaC1/ChaC2
on necroptosis is limited. GGT, one of the virulence factors of
Helicobacter pylori, has been demonstrated to induce
necroptosis in gastric epithelial cells (119). In the early stage of infection,
necroptosis may serve a protective role in the mucosa by triggering
an immune response (119).
However, as the disease progresses, uncontrolled necroptosis
exacerbates mucosal inflammation and contributes to the
transformation of inflammation into cancer (119). Additionally, ChaC1, by
stimulating the GCN2/eIF2α/ATF4 pathway, enhances necroptosis
induced by cystine deprivation (54).
Potential regulation of autophagy
Autophagy, a highly conserved cellular degradation
process, involves breaking down cytoplasmic components and damaged
organelles via lysosomes, recycling resulting macromolecules to
shield cells from diverse stressful conditions. Traditionally
viewed as a cytoprotective mechanism, autophagy, when excessive,
can also instigate cell death and contribute to tumor suppression
(120). GSH is implicated in
inducing autophagy, where low GSH levels serve as a signal
activating autophagy as an adaptive stress response (101). Inhibition of GGT (105) and elevated expression of
ChaC1/ChaC2 (69,79) are associated with autophagy
phenotype. Nevertheless, evidence (79,121) elucidating the precise mechanisms
and interactions between GGT and ChaC1/ChaC2 and initiation and
promotion of autophagy remains limited.
Promoting inflammation
Chronic non-specific inflammation is pivotal in
tumorigenesis (122) and is a
primary environmental factor contributing to the onset and
metastasis of specific types of cancer such as non-small cell lung
cancer and colorectal cancer (122,123). Various blood tests, either
individually or in combination, reflecting local or systemic
inflammation, are valuable prognostic indicators for multiple tumor
types (124).
GGT serves as a well-established inflammatory
marker associated with inflammatory environments and malignancy
(125,126). Combining serum albumin and GGT
levels serves as an inflammatory indicator for assessing the
prognosis of hepatocellular carcinoma (HCC). Patients with elevated
GGT and decreased albumin expression exhibit poorer prognosis,
revealing significant differences in tumor characteristics,
including larger maximum tumor diameters, more tumor nodules and
potential for macroscopic vascular invasion and higher serum tumor
marker levels (124).
Furthermore, although there is limited research on
the effect of human GGT on the colonization of H. pylori, it
is known that H. pylori GGT is a bacterial virulence factor
that contributes to the colonization of H. pylori in human
stomach parietal cells, hence inducing inflammation and gastric
parietal cell carcinogenesis (127). In H. pylori infection,
stimulation of H. pylori GGT accelerates the decrease of GSH
levels in gastric epithelial cells, thereby exacerbating ROS
production, leading to DNA damage and playing a key role in the
emergence of chronic gastritis and gastric cancer (127). H. pylori GGT is also key
for the tolerogenic effect of dendritic cells in H. pylori
infection, ensuring bacterial persistence and cross-protection from
chronic inflammation and autoimmune diseases by promoting H.
pylori to reprogram dendritic cells into tolerogenic phenotypes
(128). More research is needed
to determine whether human GGT functions similarly in H.
pylori-infected gastric parietal cells.
Human ChaC1 has potential ability to promote
inflammation (129). ChaC1 is
highly expressed in gastric cancer associated with H. pylori
infection (129). Infection with
H. pylori triggers ChaC1 overexpression in gastric
epithelial cells, leading to GSH degradation and ROS accumulation,
suppressing nucleotide alterations in TP53 that induce tumor
suppressor gene expression (130). Overexpression of ChaC1 in H.
pylori-infected parietal cells may also lead to H.
pylori-induced somatic mutation, thereby promoting the
development of gastric cancer (131). In summary, high expression of
human ChaC1 is involved in inducing development of gastric
cancer.
ChaC1 expression varies in normal and cystic
fibrosis bronchial epithelial cells, with low ChaC1 expression
hypothesized to play a significant role in regulating the chronic
inflammatory response induced by Pseudomonas aeruginosa (Pa)
(132). When exposed to Pa and
its virulence components, normal bronchial epithelial cells
preferentially produce ChaC1. Conversely, low ChaC1 expression is
associated with increased secretion of inflammatory markers
interleukin-8, interleukin-6 and prostaglandin E2 in the presence
of lipopolysaccharide and flagellin stimulation (132). Low ChaC1 expression also
promotes increased phosphorylation of NF-κB p65, possibly
contributing to the exacerbation of characteristic inflammation in
the lungs of patients with cystic fibrosis following Pa infection
(132). Cystic fibrosis itself
is a risk factor for various cancers, including lung cancer
(133).
Drug resistance
High GGT and low ChaC1 expression in cancer cells
are pivotal factors in developing drug-resistant phenotypes in
tumors (86). GGT expression
provides cells with an additional supply of cysteine, while low
ChaC1 expression hinders degradation of intracellular GSH. Both
factors contribute to GSH consumption in tumor cells during
anticancer chemotherapy, leading to drug resistance. Maintaining
high intracellular GSH expression preserves redox status, allowing
cells to respond to proliferative and differentiation signals in
tissue after toxin injury (22)
and rapidly supplement GSH during pro-oxidant anticancer
therapy.
Chemotherapy-resistant tumors often exhibit high
GGT expression, exemplified by cisplatin resistance (134). Platinum (II) class antitumor
drugs such as cisplatin and oxaliplatin, widely used in cancer
chemotherapy, target DNA damage and overall cytotoxicity in tumor
cells, resulting in cell death (135). GGT-related detoxification of
Pt(II) medication is a key mechanism of drug resistance (136). Cisplatin can strongly bind to
mercaptan metabolites produced by GGT-mediated GSH cleavage,
reducing Pt ion entry into cells and inactivating Pt drugs outside
the cell (136). GGT-transfected
cells show decreased DNA platinization, resulting in decreased
sensitivity to cisplatin and lower susceptibility to DNA damage
(114). GGT-transfected HeLa
cells exposed to cisplatin exhibit a 10-fold increase in cisplatin
resistance (134). Systematic
inhibition of GGT expression is conducive to suppressing
nephrotoxic side effects of cisplatin without diminishing its
intracellular toxicity to tumor cells (137). These findings underscore the key
role of high GGT expression in promoting drug-resistant phenotypes
in tumor cells.
By contrast with GGT, high ChaC1 expression,
combined with drugs such as bortezomib and docetaxel, inhibits
tumor cell viability by blocking cell cycle progression from the G1
phase to the mitotic S phase. Additionally, high ChaC1 expression
increases tumor cell sensitivity to anti-tumor drugs by inducing ER
stress and ferroptosis (65,69,138). GSH depletion triggered by the
Nrf2/ATF3/4/ChaC1 pathway appears to be the primary factor inducing
death in drug-resistant tumor cells (138), positioning ChaC1 as a key target
for certain potential anti-tumor drugs such as busatol (139) and glaucocalyxin A (140). Low expression of ChaC1 appears
in the drug-resistant phenotype of tumor cells (141), which may be related to enhanced
intracellular GSH levels.
4. Role of GSH-degrading enzymes in
medicine
Promising tumor biomarkers
Early tumor screening and diagnosis are key for
effective treatment and favorable prognosis. Reliable and specific
tumor biomarkers are key for accurate screening and diagnosis. The
aberrant expression of GSH-degrading enzymes is associated with the
prognosis of certain cancers such as gastric adenocarcinoma
(72), breast cancer (74) and so on, making them promising
tumor biomarkers.
GGT as a biomarker
GGT has been extensively studied and is widely used
as a biomarker in clinical practice (142-144). Serum GGT activity is a rapid,
reliable and cost-effective method to assess liver function
(145). Therefore, GGT has the
potential to serve as a tumor biomarker. Elevated GGT expression
can indicate the early-stage risk of tumor development, as
suggested by an epidemiological study linking high GGT expression
with increased risk of prostate cancer development (145). Moreover, high GGT expression
indicates poor prognosis in various types of tumors, including
renal cell carcinoma and prostate and urothelial cancer (145). The Cancer antigen 19-9/GGT ratio
serves as an independent prognostic predictor following radical
resection in ampulla carcinoma (146). GGT6 and GGT2 are novel
synergistic prognostic biomarkers for low-grade glioma and GBM,
potentially aiding early detection (30).
GGT probes have been developed to detect GGT
activity accurately for tumor imaging. However, the imaging process
often requires organic solvents, posing risks of damage to the
enzyme and body. A water-soluble fluorescent probe, TCF-GGT
(147), has shown promise by
producing red fluorescence during GGT catalytic hydrolysis without
interference from the background. This water-soluble compound holds
clinical value due to its practical imaging, quick metabolic cycle
and excellent water solubility. Due to shallow tissue penetration
of many GGT-targeted fluorescent probes, their clinical application
is limited. A novel positron emission tomography imaging probe,
([18F]GCPA)2, has been designed to
sensitively and precisely monitor GGT levels in living subjects
because of the high sensitivity and intense tissue penetration of
positron emission tomography (148). In addition, Cy-GSH, a
zero-crosstalk ratio near-infrared GGT fluorescent probe, has also
been designed to visualize deep cancer in vivo. The probe
accurately visualizes tumors and metastases in mice, suggesting
that it could be a convenient tool for fluorescence-guided cancer
surgery (149). More clinically
applicable GGT-targeted probes for visualizing deep cancer need
further studies, which may contribute to the early diagnosis of
clinical tumors.
ChaC1/ChaC2 as biomarkers
ChaC1, a GSH-degrading enzyme, is biomarker for
certain types of tumors. In vitro, ChaC1 induces cell death
in KIRC cell lines, signifying its potential as an effective marker
for poor prognosis in KIRC (70).
High ChaC1 expression also serves as a biomarker for adverse
outcomes in gastric adenocarcinoma (72), corpus endometrial (73) and breast cancer (74) and uveal melanoma (75). Notably, a positive correlation
exists between ChaC1 expression and immune infiltrating cells in
corpus endometrial carcinoma (73). In uveal melanoma (75,150), ChaC1 is associated with poor
overall, progression-free and disease-specific survival and
progression-free interval, making it a promising indicator of
unfavorable tumor prognosis. In aggressive breast tumor subtypes
such as triple-negative breast cancer, ChaC1 exhibits significant
upregulation (151). In
addition, malignant breast cancer tissue with active lymph node
metastases and high proliferation rates demonstrates elevated
levels of ChaC1, supporting its potential for defining tumor
progression and metastasis (Table
II).
 | Table IITumors with altered expression of GGT
and ChaC1/ChaC2. |
Table II
Tumors with altered expression of GGT
and ChaC1/ChaC2.
| First author/s
(year) | Tumor | Expression | (Refs.) |
|---|
| Chen, et al,
2023; Hayashima, et al, 2022; Chen, et al, 2017; Xu,
et al, 2022 | Glioma | Increased ChaC1;
increased GGT | (69,85,154,180) |
| Wen, et al,
2017 | Nasopharyngeal
carcinoma | Increased GGT | (181) |
| Wang, et al,
2022; Mujawar, et al, 2020 | Oral squamous cell
carcinoma | Decreased ChaC1;
increased GGT | (140,182) |
| Mizushima, et
al, 2016 | Head and neck
squamous cell carcinoma | Increased GGT | (183) |
| Lee, et al,
2021 | Laryngeal
cancer | Increased GGT | (184) |
| Gu, et al,
2022 | Thyroid cancer | Increased GGT | (185) |
| Foddis, et
al, 2022 | Malignant pleural
mesothelioma | Increased GGT | (186) |
| Peng, et al,
2023; Lee, et al, 2021 | Lung cancer | Increased GGT and
ChaC2 | (81,184) |
| Huang et al,
2017; Choi, et al, 2017 | Esophagus
cancer | Increased GGT | (187,188) |
| Tian, et al,
2021; Tian, et al, 2022 | Hepatocellular
carcinoma | Increased GGT and
ChaC2 | (32,82) |
| Chen, et al,
2021 | Intrahepatic
cholangiocarcinoma | Increased GGT | (189) |
| Catalano, et
al, 2023; Liao, et al, 2023 | Pancreatic
cancer | Increase GGT | (190,191) |
| Wu, et al,
2021; Zhang, et al, 2022; Tseng, et al, 2021; Liu,
et al, 2017 Ogawa, et al, 2019; Hong, et al,
2021; Yang, et al, 2019 | Gastric cancer | Decreased ChaC1 and
ChaC2; increased GGT and ChaC1 | (62,63,71,79, 131,192,193) |
| Xiao et al,
2022 | Stomach
adenocarcinoma | Decreased
ChaC1 | (72) |
| Liu, et al,
2017; Hong, et al, 2021; Hong, et al, 2020 | Colorectal
cancer | Decreased ChaC2;
increased GGT | (79,192,194) |
| Li et al,
2021; Yang, 2022; Horie, et al, 2020 | Renal clear cell
carcinoma | Decreased ChaC1;
increased GGT | (70,195,196) |
| Nguyen, et
al, 2019; Chand, et al, 2022; Mehta, et al, 2022;
Goebel, et al, 2012; Mehta et al, 2022;
Pankevičiūtė-Bukauskienė, et al, 2023; Seol, et al,
2021 | Breast cancer | Increased GGT and
ChaC1 and 2 | (20,50,74, 77, 151,197,198) |
| Goebel et
al, 2012; Shi1 et al, 2018 | Ovarian cancer | Increased GGT and
ChaC1 | (77,199) |
| Liu, et al,
2022 | Uterine corpus
endometrial carcinoma | Increased
ChaC1 | (73) |
| Schwameis, et
al, 2016 | Uterine
leiomyosarcoma | Increased GGT | (200) |
| Polterauer, et
al, 2011 | Cervical
cancer | Increased GGT | (201) |
| He, et al,
2021; Kawakami, et al, 2017 | Prostate
cancer | Decreased ChaC1;
increased GGT | (65,202) |
| Su et al,
2021 | Bladder cancer | Increased GGT | (203) |
| Takemura, et
al, 2019 | Urothelial
carcinoma | Increased GGT | (204) |
| Liu, et al,
2022 | Cutaneous
melanoma | Increased
ChaC1 | (76) |
| Liu, et al,
2019; Jin, et al, 2021; | Uveal melanoma | Increased
ChaC1 | (75,150) |
| Song et al,
2021 | Acute myeloid
leukemia | Decreased
ChaC1 | (205) |
The abnormal expression of ChaC2 as a tumor
biomarker has garnered recent attention (50,81). As an independent marker of poor
prognosis for certain types of tumors such as breast cancer
(50) and hepatocellular
carcinoma (82), ChaC2 can
monitor early tumor occurrence and treatment effects. Low
expression of ChaC2 independently indicates poor prognosis in
gastrointestinal tumors. ChaC2 induces mitochondrial apoptosis and
autophagy via UPR, serving a pivotal role as a tumor suppressor
gene in the onset, proliferation and metastasis of gastric and
colorectal cancer (79).
Immunohistochemistry and western blot analysis reveal ChaC2
downregulation in most tumor tissue, with ChaC2 expression
positively correlating with 3-year survival rate (79). However, recent findings indicate
that high ChaC2 expression is inversely correlated with overall
survival in patients with breast cancer (50). Elevated ChaC2 expression is also
associated with poor prognosis in HCC (82). This underscores the
tissue-specific effect of ChaC2 on tumors, necessitating further
study (Table II).
Further, the previous study (152) integrates traditional predictors
and ChaC1, a novel biomarker, to develop a prognostic score for
patients with tumors. The score can be used as a reference for
clinical chemotherapy decision-making (152). In evaluating patients with
primary breast cancer, including ChaC1 mRNA expression levels in
the scoring model led to changes in chemotherapy decisions in 16%
of patients (152). In addition,
ChaC1 has been included in prognostic models of renal cell
carcinoma (153) and
glioblastoma (154). Including
ChaC1 in tumor prognosis predictions may guide personalized
treatment options. ChaC1/ChaC2 may be promising targets for
precision medicine.
While studies (50,73,74,151,82) have reported ChaC1/ChaC2 as tumor
biomarkers, the development of ChaC1/ChaC2 tracer fluorescent
probes remains unexplored. Fluorescent probes are the foundation
for tumor-specific imaging in clinical applications. Therefore,
developing optical probes to track ChaC1/ChaC2 in vivo or
in vitro is key for fully realizing the clinical potential
of ChaC1/ChaC2 as tumor markers.
Therapeutic targets for tumors
Drug selection and emerging drug development.
Exploration of GSH-degrading enzyme modulators presents a promising
avenue for impeding cancer progression, overcoming tumor resistance
to pro-oxidative therapy and preserving tumor sensitivity to
chemotherapy (Table III).
 | Table IIIList of the classical and promising
therapeutics affecting GGT or ChaC1/ChaC2. |
Table III
List of the classical and promising
therapeutics affecting GGT or ChaC1/ChaC2.
| First author
(year) | Functional
category | Therapy | Source |
Mechanism/pathways | (Refs.) |
|---|
| Lyons, et
al, 1990 | GGT inhibitor | Glutamine analogues
(acivicin, 6-diazo-5-oxo-norleucine and azaserine) | Fermentation
products of Streptomyces | Glutamine analogs
are competitive inhibitors that directly modify active site
nucleophiles. Due to neurotoxicity, they are no longer used in the
clinic | (161) |
| Han, et al,
2007; Watanabe, et al, 2017 | | GGsTop | Chemical
synthesis | A
phosphonate-basedpotent, non-toxic, highly selective and
irreversible GGT inhibitor. Human GGT recognizes the negative
charge of GGsTop instead of the C-terminal carboxy group of
glutathione by a positively charged critical residue located in the
Cys-Gly binding site | (206,207) |
| King, et al,
2009 | | OU749 | Chemical
synthesis |
Species-specifically non-competitively
inhibiting human GGT. OU749 binds to the covalent E-γ-glutamyl
complex, the F form of the enzyme | (208) |
| Azouz, et
al, 2020 | | Amlodipine | Chemical synthesis;
fully substituted dialkyl 1,4-dihydropyridine-3,5-dicarboxylate
derivative | Currently
unclear | (164) |
| Brancaccio, et
al, 2019 | | Ovothiols | Marine
metazoans | Inhibit
membrane-bound GGT of human cells non-competitively and reduce
proliferation in GGT-positive cell lines with simultaneous
occurrence of a non-protective/cytotoxic form of autophagy,
indicating inhibition of GGT activity is likely involved in the
modulation of autophagic mechanisms | (105) |
| Joo, et al,
2012 | ChaC1
activator | Nisin | Bacterium
Lactococcus lactis | Currently
unclear | (68) |
| Stevens, et
al, 2019 | | Atovaquone | Chemical synthesis
of hydroxynaphthoquinone or analog of ubiquinone | Increased
EIF2α/ATF4/ChaC1 pathway activity | (158) |
| Tomonobu, et
al, 2020 | | Xylitol | Fruits and
vegetables | Induction of CHAC1
by xylitol triggers endoplasmic reticulum stress | (100) |
| Wang, et al,
2019 | | Artesunate | Artemisia
apiacea | Increased
EIF2α/ATF4/ChaC1 pathway activity | (67) |
| Wang, et al,
2021 | |
Dihydroartemisinin | Artemisia
apiacea | Increased
EIF2α/ATF4/ChaC1 pathway activity | (66) |
| Wang, et al,
2022 | | Glaucocalyxin
A | Rabdosia
japonica | Increased
EIF2α/ATF4/ChaC1 pathway activity | (140) |
| Guan, et al,
2020 | | Tanshinone IIA | Salvia
miltiorrhiza Bunge | Currently
unclear | (162) |
| Chen, et al,
2021 | | Omega-3 fatty acids
docosahexaenoic acid or eicosapentaenoic acid | Marine
metazoans | Increased
EIF2α/ATF4/ChaC1 pathway activity | (138) |
| Tseng, et
al, 2021 | Negatively
regulating CHAC1 expression factor | Metformin | Galega
officinalis | Regulating the
Loc100506691-miR-26a-5p-miR-330-5p-ChaC1 axis signaling induces
cell cycle arrest in G2/M phase, inhibiting cancer cell
proliferation | (71) |
| Zhai et al,
2022 | ChaC2
activator | Naringin | Citrus fruit | Upregulating CHAC2
via activation of the Nrf2 signaling pathway | (209) |
Diminishing GGT expression is beneficial for tumor
treatment. Classical GGT inhibitors, including glutamate analogs
such as acivicin, 6-diazo-5-oxo-L-norleucine, and azaserine
(155), have shown clinical
toxicity against embryonic cells (156). γ phosphonoglutamate analogs such
as GGsTop and derivatives of the lead compound
N-[5-(4-methoxybenzyl)-1,3,4-thiadiazol-2-yl]benzenesulfonamide,
OU749, represent another class of GGT inhibitors (22). GGsTop targets GGT, can reduce the
immunosuppressive function of anti-tumor drugs when used in
combination with anti-tumor drugs (157). OU749, a species-specific
uncompetitive GGT inhibitor, exhibits low toxicity and a broad
therapeutic window for humans.
The development of ChaC1 modulators introduces
innovative approaches to cancer treatment. Various potential ChaC1
modulators have been reported, targeting tumor cell cycle arrest
and apoptosis (68,158). Metformin, by regulating the
Loc100506691-miR-26a-5p-miR-330-5p-CHAC1 axis, induces cell cycle
arrest in the G2/M phase, inhibiting cancer cell proliferation
(71). Nisin, an apoptotic
bacteriocin, induces ChaC1 activation, calcium influx and cell
cycle arrest in the G2 phase, leading to increased apoptosis and
decreased cell proliferation in vitro and in vivo
(68). Atovaquone, an
anti-parasitic agent, induces eIF2a phosphorylation at serine 51,
amplifying the eIF2α/ATF/CHOP/ChaC1 signaling pathway under ER
stress (158).
Natural extracts and their bioactive compounds have
recently gained recognition as potential lead molecules in drug
discovery for cancer treatment (159,160). They offer an alternative to
chemical synthetic drugs, potentially minimizing toxic side effects
and holding promise for enhancing clinical anti-tumor therapy. GGT
and ChaC1 modulators derived from natural extracts have been
explored. Ovothiol, a marine-derived 5(N)-methyl-thiohistidine, is
a more effective non-competitive-like GGT inhibitor than
traditional counterparts such as acivicin, 6-diazo-5-oxo-norleucine
and azaserine (105,161). Ovothiol (105) induces apoptosis and autophagy in
GGT-overexpressing cells. The anticancer monosaccharide xylitol
(100) and natural Chinese
herbal extracts, including dihydroartemisinin (66), artesunate (67), glaucocalyxin A (140), and tanshinone IIA (162), upregulate
Prostaglandin-Endoperoxide Synthase 2, p53 and ChaC1 expression.
This amplifies the ATF4/CHOP/ChaC1 cascade under ER stress,
resulting in decreased intracellular GSH and cysteine, increased
intracellular ROS, redox homeostasis disruption, secondary
oxidative stress in cancer cells and selective ferroptosis of
cancer cells (66,100).
In clinical treatment of tumors, a key goal is to
decrease toxicity and resistance of anti-tumor drugs by regulating
low expression of GGT (22) and
high expression of ChaC1 (65).
Blocking nephrotoxicity induced by the anti-tumor drug cisplatin is
achieved by inhibiting GGT (137). The unique renal sulfhydryl acid
metabolic pathway, involving degradation of GSH by GGT, contributes
to cisplatin nephrotoxicity (163). Supramolecular Pt prodrug
nano-assemblies inhibiting c-glutamyl transferase prove beneficial
for overcoming tumor resistance (136). GGT inhibitor amlodipine, through
its anti-inflammatory effect, suppresses p38 MAPK-triggered
pro-inflammatory signaling, decreasing expression of TNF-α and
other downstream targets while upregulating expression of the
transcription factor Nrf2 and the antioxidant protein heme
oxygenase-1 (164). Amlodipine
diminishes the pro-apoptotic effector/anti-apoptotic protein
expression ratio induced by cisplatin, preventing inflammation,
oxidative stress and apoptotic damage (165).
Combining ChaC1 overexpression with chemotherapy is
advantageous in decreasing cell drug resistance. Temozolomide, for
example, induces binding of ChaC1 to the Notch3 protein, inhibiting
activation of Notch3. This weakens the Notch3-mediated downstream
signaling pathway, inducing glioma cell death and indicating that
ChaC1 can influence the cytotoxicity of tumor cells induced by
temozolomide (69). The omega-3
fatty acids docosahexaenoic acid and eicosapentaenoic acid serve a
role in reducing the resistance of tumor cells to bortezomib by
activating the serine synthesis pathway, mitochondrial folate
cycle, methionine cycle-associated GSH synthesis and ChaC1-mediated
GSH degradation in tumor cells, ultimately promoting GSH
degradation (138).
5. Conclusion
Recent years have seen notable advancements in
understanding the role of the intracellular degrading enzyme ChaC
family in regulating cellular GSH levels (165,166). Notably, coordinated actions of
GGT and ChaC1/ChaC2 have been identified in modulating GSH levels
both within and outside the cell (11). These changes in intracellular GSH
levels affect key biological processes, including signal
transduction, cell survival, proliferation (167) and various forms of cell death
(168,169).
The present review underscores the pivotal role of
GSH-degrading enzymes, specifically GGT and ChaC1, in cancer
development. GGT, via degradation of extracellular GSH, provides
cysteine for intracellular GSH synthesis and regeneration,
elevating intracellular GSH levels (170). Consequently, GGT can modulate
the oxidative stress state of cancer cells, inhibiting programmed
cell death (170). GSH
regeneration also counteracts the depletion induced by cancer
chemotherapeutic agents, potentially leading to development of
cancer drug resistance (171).
By contrast, intracellular degradation enzyme ChaC1, differentially
expressed in tumor cells, serves as a pro-apoptotic molecule by
downregulating intracellular GSH levels under amino acid linkage
and ER stress (99). ChaC1
induces various forms of cell death, enhancing ER stress and
influencing the tumor microenvironment (70,151). Moreover, ChaC1 has a mitigating
effect on cell resistance to cancer drugs (69). Together, GGT and ChaC1 regulate
both intracellular and extracellular GSH degradation, emerging as
promising prognostic markers and therapeutic targets for specific
types of tumor such as liver cancer (172,173), breast cancer (74) and so on. Leveraging these enzymes
for targeted anti-tumor therapy holds the advantage of precision
targeting, minimal damage to healthy cells and reduced toxic side
effects, thereby enhancing and reversing tumor drug resistance.
Despite advancements, unanswered questions persist
regarding GSH-degrading enzymes. The determinants of functional and
regulatory changes in GGT and ChaC1/ChaC2 across different
malignancies or settings remain elusive. Although external stimuli
induce ChaC1/ChaC2 gene and GGT expression (15,174,175), the specific regulatory
mechanisms governing these inductions remain unclear. Additionally,
crosstalk effect of different pathways in GGT and ChaC1/ChaC2
regulation requires further investigation. Furthermore, while the
roles of GGT and ChaC1/ChaC2 in tumor tissue are known, these
enzymes represent only the initial steps in GSH degradation. The
interaction between GGT, ChaC1/ChaC2 and downstream enzymes in the
complete degradation of GSH, Cys-Gly degrading enzymes and
5-oxo-proline (176-179) remains unclear. The development
of tumor treatment strategies targeting GSH-degrading enzymes faces
challenges, including different roles of these enzymes in different
types of tumor and their impact on the immune response or tumor
microenvironment.
In conclusion, GGT and the ChaC family of GSH
degrading enzymes, both intracellularly and extracellularly, are
key for maintaining GSH homeostasis and serve key roles in normal
cellular processes and tumor-related stress conditions. A more
comprehensive understanding of these mechanisms may clarify the
potential of GSH-degrading enzymes as targets for tumor diagnosis
and therapeutic interventions.
Availability of data and materials
Not applicable.
Authors' contributions
TZ and CY wrote and revised the manuscript. CY, DH
and WS conceived and designed the review. XZ, SL, LQ, SZ and CZ
performed the literature review. DH and WS revised the manuscript.
Data authentication is not applicable. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
ACM
|
a novel-248 ATF/CRE modifier
|
|
AP-1
|
activator protein 1
|
|
AP-2
|
activating protein 2
|
|
ATF
|
activating transcription factor
|
|
C/EBP-β
|
CCAAT/enhancer binding protein β
|
|
ChaC1
|
cation transport regulator homolog
glutathione specific γ-glutamylcyclotransferase 1
|
|
CHOP
|
C/EBP homologous protein
|
|
eIF2α
|
eukaryotic initiation factor-2α
|
|
EpRE
|
electrophile response element
|
|
ER
|
endoplasmic reticulum
|
|
GBM
|
glioblastoma multiforme
|
|
GCN2
|
general control nonderepressible
2
|
|
GGT
|
γ-glutamyl transpeptidase
|
|
GP5
|
GGT promoter 5
|
|
HCC
|
hepatocellular carcinoma
|
|
hESC
|
human embryonic stem cell
|
|
HNE
|
4-hydroxynonenal
|
|
ISR
|
integrated stress response
|
|
IκB
|
inhibitor of κB
|
|
KIRC
|
kidney renal clear cell carcinoma
|
|
L2
|
alveolar type II
|
|
Pa
|
Pseudomonas aeruginosa
|
|
ROS
|
reactive oxygen species
|
|
Sp1
|
specific protein 1
|
|
UPR
|
unfolded protein response
|
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shanghai Style TCM
Inheritance and Innovation Team Building Project (grant no.
2021LPTD-004), Natural Science Foundation of Shanghai (grant nos.
19ZR1457500, 19ZR1460800 and 18ZR1440300) and Research Project of
Shanghai Health Commission (grant no. 202140348).
References
|
1
|
Lushchak VI: Glutathione homeostasis and
functions: potential targets for medical interventions. J Amino
Acids. 2012:7368372012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu SC: Regulation of glutathione
synthesis. Mol Aspects Med. 30:42–59. 2009. View Article : Google Scholar :
|
|
3
|
Kennedy L, Sandhu JK, Harper ME and
Cuperlovic-Culf M: Role of glutathione in cancer: From mechanisms
to therapies. Biomolecules. 10:14292020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bansal A and Simon MC: Glutathione
metabolism in cancer progression and treatment resistance. J Cell
Biol. 217:2291–2298. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fernández-Checa J, Hirano T, Tsukamoto H
and Kaplowitz N: Mitochondrial glutathione depletion in alcoholic
liver disease. Alcohol. 10:469–475. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guarino MP, Afonso RA, Raimundo N, Raposo
JF and Macedo MP: Hepatic glutathione and nitric oxide are critical
for hepatic insulin-sensitizing substance action. Am J Physiol
Gastrointest Liver Physiol. 284:G588–G594. 2003. View Article : Google Scholar
|
|
7
|
Mandal PK, Roy RG and Samkaria A:
Oxidative stress: Glutathione and its potential to protect
methionine-35 of abeta peptide from oxidation. ACS Omega.
7:27052–27061. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Charisis S, Ntanasi E, Stamelou M,
Xiromerisiou G, Maraki M, Veskoukis AS, Yannakoulia M, Kosmidis MH,
Anastasiou CA, Giagkou N, et al: Plasma glutathione and prodromal
parkinson's disease probability. Mov Disord. 37:200–205. 2022.
View Article : Google Scholar
|
|
9
|
Niu B, Liao K, Zhou Y, Wen T, Quan G, Pan
X and Wu C: Application of glutathione depletion in cancer therapy:
Enhanced ROS-based therapy, ferroptosis, and chemotherapy.
Biomaterials. 277:1211102021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bachhawat AK and Yadav S: The glutathione
cycle: Glutathione metabolism beyond the γ-glutamyl cycle. IUBMB
Life. 70:585–592. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bachhawat AK and Kaur A: Glutathione
degradation. Antioxid Redox Signal. 27:1200–1216. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mungrue I, Pagnon J, Kohannim O,
Gargalovic P and Lusis A: CHAC1/MGC4504 is a novel proapoptotic
component of the unfolded protein response, downstream of the
ATF4-ATF3-CHOP cascade. J Immunol. 182:466–476. 2009. View Article : Google Scholar
|
|
13
|
Kumar A, Tikoo S, Maity S, Sengupta S,
Sengupta S, Kaur A and Bachhawat AK: Mammalian proapoptotic factor
ChaC1 and its homologues function as γ-glutamyl cyclotransferases
acting specifically on glutathione. EMBO Rep. 13:1095–1101. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Ma S, Zhang J, Lou L, Liu W, Gao
C, Miao L, Sun F, Chen W, Cao X and Wei J: MicroRNA-142-3p promotes
renal cell carcinoma progression by targeting RhoBTB3 to regulate
HIF-1 signaling and GGT/GSH pathways. Sci Rep. 13:59352023.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Li W, Ma Y, Zhao X, He L, Sun P
and Wang H: High-fat diet aggravates colitis-associated
carcinogenesis by evading ferroptosis in the ER stress-mediated
pathway. Free Radic Biol Med. 177:156–166. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alborzinia H, Flórez AF, Kreth S, Brückner
LM, Yildiz U, Gartlgruber M, Odoni DI, Poschet G, Garbowicz K, Shao
C, et al: MYCN mediates cysteine addiction and sensitizes
neuroblastoma to ferroptosis. Nat Cancer. 3:471–485. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stepulak A, Rola R, Polberg K and
Ikonomidou C: Glutamate and its receptors in cancer. J Neural
Transm (Vienna). 121:933–944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Locasale JW: Serine, glycine and
one-carbon units: Cancer metabolism in full circle. Nat Rev Cancer.
13:572–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cole SP and Deeley RG: Transport of
glutathione and glutathione conjugates by MRP1. Trends Pharmacol
Sci. 27:438–446. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nguyen YTK, Park JS, Jang JY, Kim KR, Vo
TTL, Kim KW and Han BW: Structural and functional analyses of human
ChaC2 in glutathione metabolism. Biomolecules. 10:312019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Orlowski M and Meister A: The
gamma-glutamyl cycle: A possible transport system for amino acids.
Proc Natl Acad Sci USA. 67:1248–1255. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hanigan MH: Gamma-glutamyl transpeptidase:
Redox regulation and drug resistance. Adv Cancer Res. 122:103–141.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Verma VV, Gupta R and Goel M: Phylogenetic
and evolutionary analysis of functional divergence among gamma
glutamyl transpeptidase (GGT) subfamilies. Biol Direct. 10:492015.
View Article : Google Scholar
|
|
24
|
Mistry D and Stockley RA: Gamma-glutamyl
transferase: The silent partner? COPD. 7:285–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hanigan MH and Frierson HF Jr:
Immunohistochemical detection of gamma-glutamyl transpeptidase in
normal human tissue. J Histochem Cytochem. 44:1101–1108. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Figlewicz DA, Delattre O, Guellaen G,
Krizus A, Thomas G, Zucman J and Rouleau GA: Mapping of human
gamma-glutamyl transpeptidase genes on chromosome 22 and other
human autosomes. Genomics. 17:299–305. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morris C, Courtay C, van Kessel AG, ten
Hoeve J, Heisterkamp N and Groffen J: Localization of a
gamma-glutamyl-transferase-related gene family on chromosome 22.
Hum Genet. 91:31–36. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Heisterkamp N, Groffen J, Warburton D and
Sneddon TP: The human gamma-glutamyltransferase gene family. Hum
Genet. 123:321–232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wickham S, West MB, Cook PF and Hanigan
MH: Gamma-glutamyl compounds: Substrate specificity of
gamma-glutamyl transpeptidase enzymes. Anal Biochem. 414:208–214.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mottaghitalab F, Lanjanian H and
Masoudi-Nejad A: Revealing transcriptional and post-transcriptional
regulatory mechanisms of γ-glutamyl transferase and keratin
isoforms as novel cooperative biomarkers in low-grade glioma and
glioblastoma multiforme. Genomics. 113:2623–2633. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, Zhang L, Chan FKL, Ji J, Yu J and
Liang JQ: Gamma-glutamyltransferase 7 suppresses gastric cancer by
cooperating with RAB7 to induce mitophagy. Oncogene. 41:3485–3497.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tian S, Li J, Guo Y, Dong W and Zheng X:
Expression status and prognostic significance of gamma-glutamyl
transpeptidase family genes in hepatocellular carcinoma. Front
Oncol. 11:7311442021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Samgina TA and Lazarenko VA: The role of
polymorphic variants rs11546155 and rs6119534 of the GGT7 gene and
risk factors in the development of acute pancreatitis. Vopr Pitan.
91:43–50. 2022.In Russian.
|
|
34
|
West MB, Wickham S, Parks EE, Sherry DM
and Hanigan MH: Human GGT2 does not autocleave into a functional
enzyme: A cautionary tale for interpretation of microarray data on
redox signaling. Antioxid Redox Signal. 19:1877–1888. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moon DO, Kim BY, Jang JH, Kim MO,
Jayasooriya RG, Kang CH, Choi YH, Moon SK, Kim WJ, Ahn JS and Kim
GY: K-RAS transformation in prostate epithelial cell overcomes
H2O2-induced apoptosis via upregulation of
gamma-glutamyltransferase-2. Toxicol In Vitro. 26:429–434. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Courtay C, Heisterkamp N, Siest G and
Groffen J: Expression of multiple gamma-glutamyltransferase genes
in man. Biochem J. 297:503–508. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Taniguchi N and Ikeda Y: Gamma-glutamyl
transpeptidase: Catalytic mechanism and gene expression. Adv
Enzymol Relat Areas Mol Biol. 72:239–278. 1998.PubMed/NCBI
|
|
38
|
Zhang H and Forman HJ: Redox regulation of
gamma-glutamyl transpeptidase. Am J Respir Cell Mol Biol.
41:509–515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Daubeuf S, Duvoix A, Wellman-Rousseau M,
Diederich M and Visvikis A: Phorbol ester regulation of the human
gamma-glutamyltransferase gene promoter. Biochem Biophys Res
Commun. 313:300–307. 2004. View Article : Google Scholar
|
|
40
|
Pawlak A, Lahuna O, Bulle F, Suzuki A,
Ferry N, Siegrist S, Chikhi N, Chobert MN, Guellaen G and Laperche
Y: Gamma-glutamyl transpeptidase: A single copy gene in the rat and
a multigene family in the human genome. J Biol Chem. 263:9913–9916.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pandur S, Pankiv S, Johannessen M, Moens U
and Huseby NE: Gamma-glutamyltransferase is upregulated after
oxidative stress through the Ras signal transduction pathway in rat
colon carcinoma cells. Free Radic Res. 41:1376–1384. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Downward J: Targeting RAS signalling
pathways in cancer therapy. Nat Rev Cancer. 3:11–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang H, Liu H, Iles KE, Liu RM,
Postlethwait EM, Laperche Y and Forman HJ: 4-Hydroxynonenal induces
rat gamma-glutamyl transpeptidase through mitogen-activated protein
kinase-mediated electrophile response element/nuclear factor
erythroid 2-related factor 2 signaling. Am J Respir Cell Mol Biol.
34:174–181. 2006. View Article : Google Scholar
|
|
44
|
Mussbacher M, Derler M, Basilio J and
Schmid JA: NF-κB in monocytes and macrophages-an inflammatory
master regulator in multitalented immune cells. Front Immunol.
14:11346612023. View Article : Google Scholar
|
|
45
|
Yao C, Ren J, Huang R, Tang C, Cheng Y, Lv
Z, Kong L, Fang S, Tao J, Fu Y, et al: Transcriptome profiling of
microRNAs reveals potential mechanisms of manual therapy
alleviating neuropathic pain through microRNA-547-3p-mediated
Map4k4/NF-κb signaling pathway. J Neuroinflammation. 19:2112022.
View Article : Google Scholar
|
|
46
|
Reuter S, Schnekenburger M, Cristofanon S,
Buck I, Teiten MH, Daubeuf S, Eifes S, Dicato M, Aggarwal BB,
Visvikis A and Diederich M: Tumor necrosis factor alpha induces
gamma-glutamyltransferase expression via nuclear factor-kappaB in
cooperation with Sp1. Biochem Pharmacol. 77:397–411. 2009.
View Article : Google Scholar
|
|
47
|
Kaur A, Gautam R, Srivastava R, Chandel A,
Kumar A, Karthikeyan S and Bachhawat AK: ChaC2, an enzyme for slow
turnover of cytosolic glutathione. J Biol Chem. 292:638–651. 2017.
View Article : Google Scholar :
|
|
48
|
Crawford RR, Prescott ET, Sylvester CF,
Higdon AN, Shan J, Kilberg MS and Mungrue IN: Human CHAC1 protein
degrades glutathione, and mRNA induction is regulated by the
transcription factors ATF4 and ATF3 and a bipartite ATF/CRE
regulatory element. J Biol Chem. 290:15878–15891. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang B, Jiang M and Zheng M: Biological
characteristics and functions of ChaC1-catalyzed clutathione
degradation in the cytoplasm. Chin J Biochem Mol Biol. 38:284–289.
2022.
|
|
50
|
Chand S, Mehta V, Sharma RK, Anvikar AR
and Chander H: Cancer informatics analysis indicates high CHAC2
associated with unfavorable prognosis in breast cancer. Front
Oncol. 12:10589312022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tsunoda S, Avezov E, Zyryanova A, Konno T,
Mendes-Silva L, Pinho Melo E, Harding HP and Ron D: Intact protein
folding in the glutathione-depleted endoplasmic reticulum
implicates alternative protein thiol reductants. Elife.
3:e034212014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Suyal S, Choudhury C and Bachhawat AK: The
ChaC1 active site: Defining the residues and determining the role
of ChaC1-exclusive residues in the structural and functional
stability. Proteins. 91:567–580. 2023. View Article : Google Scholar
|
|
53
|
Oh-Hashi K, Nomura Y, Shimada K, Koga H,
Hirata Y and Kiuchi K: Transcriptional and post-translational
regulation of mouse cation transport regulator homolog 1. Mol Cell
Biochem. 380:97–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen MS, Wang SF, Hsu CY, Yin PH, Yeh TS,
Lee HC and Tseng LM: CHAC1 degradation of glutathione enhances
cystine-starvation-induced necroptosis and ferroptosis in human
triple negative breast cancer cells via the GCN2-eIF2α-ATF4
pathway. Oncotarget. 8:114588–114602. 2017. View Article : Google Scholar
|
|
55
|
Grimm C, Hofstetter G, Aust S,
Mutz-Dehbalaie I, Bruch M, Heinze G, Rahhal-Schupp J, Reinthaller
A, Concin N and Polterauer S: Association of
gamma-glutamyltransferase with severity of disease at diagnosis and
prognosis of ovarian cancer. Br J Cancer. 109:610–614. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hofbauer SL, Stangl KI, de Martino M,
Lucca I, Haitel A, Shariat SF and Klatte T: Pretherapeutic
gamma-glutamyltransferase is an independent prognostic factor for
patients with renal cell carcinoma. Br J Cancer. 111:1526–1531.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wei JR, Dong J and Li L: Cancer-associated
fibroblasts-derived gamma-glutamyltransferase 5 promotes tumor
growth and drug resistance in lung adenocarcinoma. Aging (Albany
NY). 12:13220–13233. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Huang Y, Zhou H, Zou J and Wang D: GGT5 is
an independent prognostic biomarker in stomach adenocarcinoma. Can
J Gastroenterol Hepatol. 2022:99833512022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xiao Y, Yang H, Lu J, Li D, Xu C and Risch
HA: Serum gamma-glutamyltransferase and the overall survival of
metastatic pancreatic cancer. BMC Cancer. 19:10202019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang Q, Shu X, Dong Y, Zhou J, Teng R,
Shen J, Chen Y, Dong M, Zhang W, Huang Y, et al: Tumor and serum
gamma-glutamyl transpeptidase, new prognostic and molecular
interpretation of an old biomarker in gastric cancer. Oncotarget.
8:361712017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Durham JR, Frierson HF and Hanigan MH:
Gamma-glutamyl transpeptidase immunoreactivity in benign and
malignant breast tissue. Breast Cancer Res Treat. 45:55–62. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wu J, Wang X, Wang N, Ma L, Xie X, Zhang
H, Kang H and Zhou Z: Identification of novel antioxidant gene
signature to predict the prognosis of patients with gastric cancer.
World J Surg Oncol. 19:2192021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang L, Li C, Zhang Y, Zhang J and Yang
X: Ophiopogonin B induces gastric cancer cell death by blocking the
GPX4/xCT-dependent ferroptosis pathway. Oncol Lett. 23:1042022.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gagliardi M, Cotella D, Santoro C, Cora D,
Barlev NA, Piacentini M and Corazzari M: Aldo-keto reductases
protect metastatic melanoma from ER stress-independent ferroptosis.
Cell Death Dis. 10:9022019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
He S, Zhang M, Ye Y, Zhuang J, Ma X, Song
Y and Xia W: ChaC glutathione specific γ-glutamylcyclotransferase 1
inhibits cell viability and increases the sensitivity of prostate
cancer cells to docetaxel by inducing endoplasmic reticulum stress
and ferroptosis. Exp Ther Med. 22:9972021. View Article : Google Scholar
|
|
66
|
Wang Z, Li M, Liu Y, Qiao Z, Bai T, Yang L
and Liu B: Dihydroartemisinin triggers ferroptosis in primary liver
cancer cells by promoting and unfolded protein response-induced
upregulation of CHAC1 expression. Oncol Rep. 46:2402021. View Article : Google Scholar :
|
|
67
|
Wang N, Zeng GZ, Yin JL and Bian ZX:
Artesunate activates the ATF4-CHOP-CHAC1 pathway and affects
ferroptosis in Burkitt's Lymphoma. Biochem Biophys Res Commun.
519:533–539. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Joo NE, Ritchie K, Kamarajan P, Miao D and
Kapila YL: Nisin, an apoptogenic bacteriocin and food preservative,
attenuates HNSCC tumorigenesis via CHAC1. Cancer Med. 1:295–305.
2012. View Article : Google Scholar
|
|
69
|
Chen PH, Shen WL, Shih CM, Ho KH, Cheng
CH, Lin CW, Lee CC, Liu AJ and Chen KC: The CHAC1-inhibited Notch3
pathway is involved in temozolomide-induced glioma cytotoxicity.
Neuropharmacology. 116:300–314. 2017. View Article : Google Scholar
|
|
70
|
Li D, Liu S, Xu J, Chen L, Xu C, Chen F,
Xu Z, Zhang Y, Xia S, Shao Y and Wang Y: Ferroptosis-related gene
CHAC1 is a valid indicator for the poor prognosis of kidney renal
clear cell carcinoma. J Cell Mol Med. 25:3610–3621. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tseng HH, Chen YZ, Chou NH, Chen YC, Wu
CC, Liu LF, Yang YF, Yeh CY, Kung ML, Tu YT and Tsai KW: Metformin
inhibits gastric cancer cell proliferation by regulation of a novel
Loc100506691-CHAC1 axis. Mol Ther Oncolytics. 22:180–194. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Xiao R, Wang S, Guo J, Liu S, Ding A, Wang
G, Li W, Zhang Y, Bian X, Zhao S and Qiu W: Ferroptosis-related
gene NOX4, CHAC1 and HIF1A are valid biomarkers for stomach
adenocarcinoma. J Cell Mol Med. 26:1183–1193. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liu Q, Yu M and Zhang T: Construction of
oxidative stress-related genes risk model predicts the prognosis of
uterine corpus endometrial cancer patients. Cancers (Basel).
14:55722022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mehta V, Suman P and Chander H: High
levels of unfolded protein response component CHAC1 associates with
cancer progression signatures in malignant breast cancer tissues.
Clin Transl Oncol. 24:2351–2365. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Liu Y, Li M, Shi D and Zhu Y: Higher
expression of cation transport regulator-like protein 1 (CHAC1)
predicts of poor outcomes in uveal melanoma (UM) patients. Int
Ophthalmol. 39:2825–2832. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Liu C, Liu Y, Yu Y, Zhao Y and Yu A:
Comprehensive analysis of ferroptosis-related genes and prognosis
of cutaneous melanoma. BMC Med Genomics. 15:392022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Goebel G, Berger R, Strasak AM, Egle D,
Müller-Holzner E, Schmidt S, Rainer J, Presul E, Parson W, Lang S,
et al: Elevated mRNA expression of CHAC1 splicing variants is
associated with poor outcome for breast and ovarian cancer
patients. Br J Cancer. 106:189–198. 2012. View Article : Google Scholar :
|
|
78
|
Wang CK, Yang SC, Hsu SC, Chang FP, Lin
YT, Chen SF, Cheng CL, Hsiao M, Lu FL and Lu J: CHAC2 is essential
for self-renewal and glutathione maintenance in human embryonic
stem cells. Free Radic Biol Med. 113:439–451. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liu S, Fei W, Shi Q, Li Q, Kuang Y, Wang
C, He C and Hu X: CHAC2, downregulated in gastric and colorectal
cancers, acted as a tumor suppressor inducing apoptosis and
autophagy through unfolded protein response. Cell Death Dis.
8:e30092017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Tchantchou F, Graves M, Ashline D, Morin
A, Pimenta A, Ortiz D, Rogers E and Shea TB: Increased
transcription and activity of glutathione synthase in response to
deficiencies in folate, vitamin E, and apolipoprotein E. J Neurosci
Res. 75:508–515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Peng W, Wen L, Jiang R, Deng J and Chen M:
CHAC2 promotes lung adenocarcinoma by regulating ROS-mediated MAPK
pathway activation. J Cancer. 14:1309–1320. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tian Y, Lu J and Qiao Y: A
metabolism-associated gene signature for prognosis prediction of
hepatocellular carcinoma. Front Mol Biosci. 9:9883232022.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Lisek K, Campaner E, Ciani Y, Walerych D
and Del Sal G: Mutant p53 tunes the NRF2-dependent antioxidant
response to support survival of cancer cells. Oncotarget.
9:20508–20523. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Joyce-Brady M, Jean JC and Hughey RP:
Gamma-glutamyltransferase and its isoform mediate an endoplasmic
reticulum stress response. J Biol Chem. 276:9468–9477. 2001.
View Article : Google Scholar
|
|
85
|
Hayashima K and Katoh H: Expression of
gamma-glutamyltransferase 1 in glioblastoma cells confers
resistance to cystine deprivation-induced ferroptosis. J Biol Chem.
298:1017032022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ramsay EE and Dilda PJ: Glutathione
S-conjugates as prodrugs to target drug-resistant tumors. Front
Pharmacol. 5:1812014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liu Y, Hyde AS, Simpson MA and Barycki JJ:
Emerging regulatory paradigms in glutathione metabolism. Adv Cancer
Res. 122:69–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lieberman MW, Wiseman AL, Shi ZZ, Carter
BZ, Barrios R, Ou CN, Chévez-Barrios P, Wang Y, Habib GM, Goodman
JC, et al: Growth retardation and cysteine deficiency in
gamma-glutamyl transpeptidase-deficient mice. Proc Natl Acad Sci
USA. 93:7923–7926. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Barrios R, Shi ZZ, Kala SV, Wiseman AL,
Welty SE, Kala G, Bahler AA, Ou CN and Lieberman MW: Oxygen-induced
pulmonary injury in gamma-glutamyl transpeptidase-deficient mice.
Lung. 179:319–330. 2001. View Article : Google Scholar
|
|
90
|
Rojas E, Valverde M, Kala SV, Kala G and
Lieberman MW: Accumulation of DNA damage in the organs of mice
deficient in gamma-glutamyltranspeptidase. Mutat Res. 447:305–316.
2000. View Article : Google Scholar
|
|
91
|
Darin N, Leckström K, Sikora P, Lindgren
J, Almén G and Asin-Cayuela J: γ-glutamyl transpeptidase deficiency
caused by a large homozygous intragenic deletion in GGT1. Eur J Hum
Genet. 26:808–817. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Alessandro C, Maria F, Aldo P and Alfonso
P: Gamma-glutamyltransferase of cancer cells at the crossroads of
tumor progression, drug resistance and drug targeting. Anticancer
Res. 30:1169–1181. 2010.
|
|
93
|
Giommarelli C, Corti A, Supino R, Favini
E, Paolicchi A, Pompella A and Zunino F: Cellular response to
oxidative stress and ascorbic acid in melanoma cells overexpressing
gamma-glutamyltransferase. Eur J Cancer. 44:750–759. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Panis C, Victorino VJ, Herrera AC, Freitas
LF, De Rossi T, Campos FC, Simão AN, Barbosa DS, Pinge-Filho P,
Cecchini R and Cecchini AL: Differential oxidative status and
immune characterization of the early and advanced stages of human
breast cancer. Breast Cancer Res Treat. 133:881–888. 2012.
View Article : Google Scholar
|
|
95
|
Chen X and Cubillos-Ruiz JR: Endoplasmic
reticulum stress signals in the tumour and its microenvironment.
Nat Rev Cancer. 21:71–88. 2021. View Article : Google Scholar :
|
|
96
|
Shi Y, Jiang B and Zhao J: Induction
mechanisms of autophagy and endoplasmic reticulum stress in
intestinal ischemia-reperfusion injury, inflammatory bowel disease,
and colorectal cancer. Biomed Pharmacother. 170:1159842024.
View Article : Google Scholar
|
|
97
|
Chen H, Xu N, Xu J, Zhang C, Li X, Xu H,
Zhu W, Li J, Liang D and Zhou W: A risk signature based on
endoplasmic reticulum stress-associated genes predicts prognosis
and immunity in pancreatic cancer. Front Mol Biosci.
10:12980772023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Joyce-Brady M, Jean JC and Hughey RP:
Gamma-glutamyltransferase and its isoform mediate an endoplasmic
reticulum stress response. J Biol Chem. 276:9468–9477. 2001.
View Article : Google Scholar
|
|
99
|
Cui Y, Zhou X, Chen L, Tang Z, Mo F, Li
XC, Mao H, Wei X, Wang C and Wang H: Crosstalk between endoplasmic
reticulum stress and oxidative stress in heat exposure-induced
apoptosis is dependent on the ATF4-CHOP-CHAC1 signal pathway in
IPEC-J2 cells. J Agric Food Chem. 69:15495–15511. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Tomonobu N, Komalasari NLGY, Sumardika IW,
Jiang F, Chen Y, Yamamoto KI, Kinoshita R, Murata H, Inoue Y and
Sakaguchi M: Xylitol acts as an anticancer monosaccharide to induce
selective cancer death via regulation of the glutathione level.
Chem Biol Interact. 324:1090852020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lv H, Zhen C, Liu J, Yang P, Hu L and
Shang P: Unraveling the potential role of glutathione in multiple
forms of cell death in cancer therapy. Oxid Med Cell Longev.
2019:31501452019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wen RJ, Dong X, Zhuang HW, Pang FX, Ding
SC, Li N, Mai YX, Zhou ST, Wang JY and Zhang JF: Baicalin induces
ferroptosis in osteosarcomas through a novel Nrf2/xCT/GPX4
regulatory axis. Phytomedicine. 116:1548812023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Pfeffer CM and Singh ATK: Apoptosis: A
target for anticancer therapy. Int J Mol Sci. 19:4482018.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Brancaccio M, Russo M, Masullo M, Palumbo
A, Russo GL and Castellano I: Sulfur-containing histidine compounds
inhibit γ-glutamyl transpeptidase activity in human cancer cells. J
Biol Chem. 294:14603–14614. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Bui TT, Nitta RT, Kahn SA, Razavi SM,
Agarwal M, Aujla P, Gholamin S, Recht L and Li G: γ-Glutamyl
transferase 7 is a novel regulator of glioblastoma growth. BMC
Cancer. 15:2252015. View Article : Google Scholar
|
|
107
|
Hirschhorn T and Stockwell BR: The
development of the concept of ferroptosis. Free Radic Biol Med.
133:130–143. 2019. View Article : Google Scholar :
|
|
108
|
Lei G, Zhuang L and Gan B: Targeting
ferroptosis as a vulnerability in cancer. Nat Rev Cancer.
22:381–396. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wu J, Wen T, Marzio A, Song D, Chen S,
Yang C, Zhao F, Zhang B, Zhao G, Ferri A, et al: FBXO32-mediated
degradation of PTEN promotes lung adenocarcinoma progression. Cell
Death Dis. 15:2822024. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhan CH, Ding DS, Zhang W, Wang HL, Mao ZY
and Liu GJ: The cancer-testis antigen a-kinase anchor protein 3
facilitates breast cancer progression via activation of the
PTEN/PI3K/AKT/mTOR signaling. Bioengineered. 13:8478–8489. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Yi J, Zhu J, Wu J, Thompson CB and Jiang
X: Oncogenic activation of PI3K-AKT-mTOR signaling suppresses
ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci USA.
117:31189–31197. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Accaoui MJ, Enoiu M, Mergny M, Masson C,
Dominici S, Wellman M and Visvikis A:
Gamma-glutamyltranspeptidase-dependent glutathione catabolism
results in activation of NF-kB. Biochem Biophys Res Commun.
276:1062–1067. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Djavaheri-Mergny M, Accaoui MJ, Rouillard
D and Wietzerbin J: Gamma-glutamyl transpeptidase activity mediates
NF-kappaB activation through lipid peroxidation in human leukemia
U937 cells. Mol Cell Biochem. 232:103–111. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Franzini M, Corti A, Lorenzini E,
Paolicchi A, Pompella A, De Cesare M, Perego P, Gatti L, Leone R,
Apostoli P and Zunino F: Modulation of cell growth and cisplatin
sensitivity by membrane gamma-glutamyltransferase in melanoma
cells. Eur J Cancer. 42:2623–2630. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Yu X and Long YC: Crosstalk between
cystine and glutathione is critical for the regulation of amino
acid signaling pathways and ferroptosis. Sci Rep. 6:300332016.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Tang X, Ding CK, Wu J, Sjol J, Wardell S,
Spasojevic I, George D, McDonnell DP, Hsu DS, Chang JT and Chi JT:
Cystine addiction of triple-negative breast cancer associated with
EMT augmented death signaling. Oncogene. 36:4235–4242. 2017.
View Article : Google Scholar
|
|
117
|
Dixon SJ, Patel DN, Welsch M, Skouta R,
Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS
and Stockwell BR: Pharmacological inhibition of cystine-glutamate
exchange induces endoplasmic reticulum stress and ferroptosis.
Elife. 3:e025232014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Galluzzi L, Kepp O, Chan FKM and Kroemer
G: Necroptosis: Mechanisms and relevance to disease. Annu Rev
Pathol. 12:103–130. 2017. View Article : Google Scholar
|
|
119
|
Zhang G, Wang J, Zhao Z, Xin T, Fan X,
Shen Q, Raheem A, Lee CR, Jiang H and Ding J: Regulated necrosis, a
proinflammatory cell death, potentially counteracts pathogenic
infections. Cell Death Dis. 13:6372022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yang Z and Klionsky DJ: An overview of the
molecular mechanism of autophagy. Curr Top Microbiol Immunol.
335:1–32. 2009.PubMed/NCBI
|
|
121
|
Xu G, Wang J, Zhang Y, Chen Z and Deng R:
GGT1 suppresses the development of ferroptosis and autophagy in
mouse retinal ganglion cell through targeting GCLC. Eye Brain.
15:139–151. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Sohrab SS, Raj R, Nagar A, Hawthorne S,
Paiva-Santos AC, Kamal MA, El-Daly MM, Azhar EI and Sharma A:
Chronic inflammation's transformation to cancer: A nanotherapeutic
paradigm. Molecules. 28:44132023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ying HQ, Liao YC, Luo YR, Xiong G, Huang
Y, Nie RW, Xiong CF and Cheng XX: Cancer-elicited inflammation
attenuates response and outcome in tyrosine kinase inhibitor naive
patients with advanced NSCLC. Pharmacol Res. 170:1057342021.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Carr B and Guerra V: Serum inflammation
parameters and survival in hepatocellular carcinoma patients:
Importance of albumin and gamma-glutamyltranspeptidase. Oncology.
101:313–320. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Ozcelik F: Prognostic value of
gamma-glutamyl transpeptidase in liver cirrhosis and hepatocellular
cancer regardless of other parameters. Clin Res Hepatol
Gastroenterol. 45:1017082021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Singh J, Chander J, Singh S, Singh G and
Atal CK: Gamma-glutamyl transpeptidase: A novel biochemical marker
in inflammation. Biochem Pharmacol. 35:3753–3760. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ricci V, Giannouli M, Romano M and
Zarrilli R: Helicobacter pylori gamma-glutamyl transpeptidase and
its pathogenic role. World J Gastroenterol. 20:630–638. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Oertli M, Noben M, Engler DB, Semper RP,
Reuter S, Maxeiner J, Gerhard M, Taube C and Müller A: Helicobacter
pylori γ-glutamyl transpeptidase and vacuolating cytotoxin promote
gastric persistence and immune tolerance. Proc Natl Acad Sci USA.
110:3047–3052. 2013. View Article : Google Scholar
|
|
129
|
Li N, Ouyang Y, Chen S, Peng C, He C, Hong
J, Yang X, Zhu Y and Lu NH: Integrative analysis of differential
lncRNA/mRNA expression profiling in Helicobacter pylori
infection-associated gastric carcinogenesis. Front Microbiol.
11:8802020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Wada Y, Takemura K, Tummala P, Uchida K,
Kitagaki K, Furukawa A, Ishige Y, Ito T, Hara Y, Suzuki T, et al:
Helicobacter pylori induces somatic mutations in TP53 via
overexpression of CHAC1 in infected gastric epithelial cells. FEBS
Open Bio. 8:671–679. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Ogawa T, Wada Y, Takemura K, Board PG,
Uchida K, Kitagaki K, Tamura T, Suzuki T, Tokairin Y, Nakajima Y
and Eishi Y: CHAC1 overexpression in human gastric parietal cells
with Helicobacter pylori infection in the secretory canaliculi.
Helicobacter. 24:e125982019. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Perra L, Balloy V, Foussignière T,
Moissenet D, Petat H, Mungrue IN, Touqui L, Corvol H, Chignard M
and Guillot L: CHAC1 is differentially expressed in normal and
cystic fibrosis bronchial epithelial cells and regulates the
inflammatory response induced by Pseudomonas aeruginosa. Front
Immunol. 9:28232018. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Rousset-Jablonski C, Dalon F, Reynaud Q,
Lemonnier L, Dehillotte C, Jacoud F, Berard M, Viprey M, Van Ganse
E, Durieu I and Belhassen M: Cancer incidence and prevalence in
cystic fibrosis patients with and without a lung transplant in
France. Front Public Health. 10:10436912022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Daubeuf S, Leroy P, Paolicchi A, Pompella
A, Wellman M, Galteau MM and Visvikis A: Enhanced resistance of
HeLa cells to cisplatin by overexpression of
gamma-glutamyltransferase. Biochem Pharmacol. 64:207–216. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhang C, Xu C, Gao X and Yao Q:
Platinum-based drugs for cancer therapy and anti-tumor strategies.
Theranostics. 12:2115–2132. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Wang L, Liu Z, He S, He S and Wang Y:
Fighting against drug-resistant tumors by the inhibition of
γ-glutamyl transferase with supramolecular platinum prodrug
nano-assemblies. J Mater Chem B. 9:4587–4595. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Hanigan MH, Frierson HF Jr, Abeler VM,
Kaern J and Taylor PT Jr: Human germ cell tumours: Expression of
gamma-glutamyl transpeptidase and sensitivity to cisplatin. Br J
Cancer. 81:75–79. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Chen J, Zaal EA, Berkers CR, Ruijtenbeek
R, Garssen J and Redegeld FA: Omega-3 fatty acids DHA and EPA
reduce bortezomib resistance in multiple myeloma cells by promoting
glutathione degradation. Cells. 10:22872021. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Yu X, He Z, Wang Z, Ke S, Wang H, Wang Q
and Li S: Brusatol hinders the progression of bladder cancer by
Chac1/Nrf2/SLC7A11 pathway. Exp Cell Res. 438:1140532024.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Wang X, He MJ, Chen XJ, Bai YT and Zhou G:
Glaucocalyxin A impairs tumor growth via amplification of the
ATF4/CHOP/CHAC1 cascade in human oral squamous cell carcinoma. J
Ethnopharmacol. 290:1151002022. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Zhou H, Shen Q, Fu J, Jiang F, Wang L and
Wang Y: Analysis of lncRNA UCA1-related downstream pathways and
molecules of cisplatin resistance in lung adenocarcinoma. J Clin
Lab Anal. 34:e233122020. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Ha Y, Chon YE, Kim MN, Lee JH and Hwang
SG: Gamma-glutamyl transpeptidase dynamics as a biomarker for
advanced fibrosis in non-alcoholic fatty liver disease. J
Gastroenterol Hepatol. 37:1624–1632. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Zhang S, Xu L and Xu M: Gamma-glutamyl
transpeptidase to albumin ratio holds a prognostic significance
after hepatectomy in patients with hepatocellular carcinoma and
liver cirrhosis. Asian J Surg. 46:1327–1328. 2023. View Article : Google Scholar
|
|
144
|
Yamada Y, Ishizaki M, Kido T, Honda R,
Tsuritani I, Ikai E and Yamaya H: Alcohol, high blood pressure, and
serum gamma-glutamyl transpeptidase level. Hypertension.
18:819–826. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Takemura K, Board PG and Koga F: A
systematic review of serum γ-glutamyltransferase as a prognostic
biomarker in patients with genitourinary cancer. Antioxidants
(Basel). 10:5492021. View Article : Google Scholar
|
|
146
|
Chen RQ, Zhang ZL, Jia YM, Chen RX and
Peng L: Preoperative CA19-9 and GGT ratio as a prognostic indicator
in ampullary carcinoma. BMC Gastroenterol. 23:722023. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Xie H, Gao J, Sun X, Song Y, Zhang Q,
Zhang P and Ding C: A water-soluble fluorescent probe for the
determination of γ-glutamyltransferase activity and its application
in tumor imaging. Talanta. 253:1239432023. View Article : Google Scholar
|
|
148
|
Gao D, Miao Y, Ye S, Lu C, Lv G, Li K, Yu
C, Lin J and Qiu L: A fluorine-18 labeled radiotracer for PET
imaging of γ-glutamyltranspeptidase in living subjects. RSC Adv.
11:18738–18747. 2021. View Article : Google Scholar
|
|
149
|
Ou-Yang J, Li Y, Jiang WL, He SY, Liu HW
and Li CY: Fluorescence-guided cancer diagnosis and surgery by a
zero cross-talk ratiometric near-infrared γ-glutamyltranspeptidase
fluorescent probe. Anal Chem. 91:1056–1063. 2019. View Article : Google Scholar
|
|
150
|
Jin Y, Wang Z, He D, Zhu Y, Gong L, Xiao
M, Chen X and Cao K: Analysis of ferroptosis-mediated modification
patterns and tumor immune microenvironment characterization in
uveal melanoma. Front Cell Dev Biol. 9:6851202021. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Mehta V, Meena J, Kasana H, Munshi A and
Chander H: Prognostic significance of CHAC1 expression in breast
cancer. Mol Biol Rep. 49:8517–8526. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Jahn B, Arvandi M, Rochau U, Fiegl H,
Goebel G, Marth C and Siebert U: Development of a novel prognostic
score for breast cancer patients using mRNA expression of CHAC1. J
Comp Eff Res. 6:563–574. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Lin D, Hu B, Zhu S and Wu Y: Exploring a
ferroptosis and oxidative stress-based prognostic model for clear
cell renal cell carcinoma. Front Oncol. 13:11314732023. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Chen W, Lei C, Wang Y, Guo D, Zhang S,
Wang X, Zhang Z, Wang Y and Ma W: Prognostic prediction model for
glioblastoma: A ferroptosis-related gene prediction model and
independent external validation. J Clin Med. 12:13412023.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Ahluwalia GS, Grem JL, Hao Z and Cooney
DA: Metabolism and action of amino acid analog anti-cancer agents.
Pharmacol Ther. 46:243–271. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Viña JR, Palacin M, Puertes IR, Hernandez
R and Viña J: Role of the gamma-glutamyl cycle in the regulation of
amino acid translocation. Am J Physiol. 257:E916–E922.
1989.PubMed/NCBI
|
|
157
|
Xie Z, Kawasaki T, Zhou H, Okuzaki D,
Okada N and Tachibana M: Targeting GGT1 eliminates the
tumor-promoting effect and enhanced immunosuppressive function of
myeloid-derived suppressor cells caused by G-CSF. Front Pharmacol.
13:8737922022. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Stevens AM, Xiang M, Heppler LN, Tošić I,
Jiang K, Munoz JO, Gaikwad AS, Horton TM, Long X, Narayanan P, et
al: Atovaquone is active against AML by upregulating the integrated
stress pathway and suppressing oxidative phosphorylation. Blood
Adv. 3:4215–4227. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
U Ferreira MJ: Natural product-derived
compounds for targeting multidrug resistance in cancer and
microorganisms. Int J Mol Sci. 24:143212023. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Ashrafizadeh M, Aref AR, Sethi G, Ertas YN
and Wang L: Natural product/diet-based regulation of macrophage
polarization: Implications in treatment of inflammatory-related
diseases and cancer. J Nutr Biochem. 1096472024. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Lyons SD, Sant ME and Christopherson RI:
Cytotoxic mechanisms of glutamine antagonists in mouse L1210
leukemia. J Biol Chem. 265:11377–11381. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Guan Z, Chen J, Li X and Dong N:
Tanshinone IIA induces ferroptosis in gastric cancer cells through
p53-mediated SLC7A11 down-regulation. Biosci Rep.
40:BSR202018072020. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Townsend DM, Deng M, Zhang L, Lapus MG and
Hanigan MH: Metabolism of cisplatin to a nephrotoxin in proximal
tubule cells. J Am Soc Nephrol. 14:1–10. 2003. View Article : Google Scholar
|
|
164
|
Azouz AA, Abdel-Nassir Abdel-Razek E and
Abo-Youssef AM: Amlodipine alleviates cisplatin-induced
nephrotoxicity in rats through gamma-glutamyl transpeptidase (GGT)
enzyme inhibition, associated with regulation of Nrf2/HO-1,
MAPK/NF-κB, and Bax/Bcl-2 signaling. Saudi Pharm J. 28:1317–1325.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Xue Y, Lu F, Chang Z, Li J, Gao Y, Zhou J,
Luo Y, Lai Y, Cao S, Li X, et al: Intermittent dietary methionine
deprivation facilitates tumoral ferroptosis and synergizes with
checkpoint blockade. Nat Commun. 14:47582023. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Sumi D, Taguchi H, Takeuchi K and
Fujishiro H: CHAC1 exacerbates arsenite cytotoxicity by lowering
intracellular glutathione levels. J Toxicol Sci. 48:487–494. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Chen S, Fan J, Xie P, Ahn J, Fernandez M,
Billingham LK, Miska J, Wu JD, Wainwright DA, Fang D, et al: CD8+ T
cells sustain antitumor response by mediating crosstalk between
adenosine A2A receptor and glutathione/GPX4. J Clin Invest.
134:e1700712024. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Xiong Y, Liu T and Chen J: Anisomycin has
the potential to induce human ovarian cancer stem cell ferroptosis
by influencing glutathione metabolism and autophagy signal
transduction pathways. J Cancer. 14:1202–1215. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Liu CX, Gao Y, Xu XF, Jin X, Zhang Y, Xu
Q, Ding HX, Li BJ, Du FK, Li LC, et al: Bile acids inhibit
ferroptosis sensitivity through activating farnesoid X receptor in
gastric cancer cells. World J Gastroenterol. 30:485–498. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Mitrić A and Castellano I: Targeting
gamma-glutamyl transpeptidase: A pleiotropic enzyme involved in
glutathione metabolism and in the control of redox homeostasis.
Free Radic Biol Med. 208:672–683. 2023. View Article : Google Scholar
|
|
171
|
Pompella A, Corti A, Paolicchi A,
Giommarelli C and Zunino F: Gamma-glutamyltransferase, redox
regulation and cancer drug resistance. Curr Opin Pharmacol.
7:360–366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Bai C, Zhang M, Zhang Y, He Y, Dou H, Wang
Z, Wang Z, Li Z and Zhang L: Gamma-glutamyltransferase activity
(GGT) is a long-sought biomarker of redox status in blood
circulation: A retrospective clinical study of 44 types of human
diseases. Oxid Med Cell Longev. 2022:84940762022. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Hamano M, Tomonaga S, Osaki Y, Oda H, Kato
H and Furuya S: Transcriptional activation of Chac1 and other
Atf4-target genes induced by extracellular l-serine depletion is
negated with glycine consumption in Hepa1-6 hepatocarcinoma cells.
Nutrients. 12:30182020. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Ge X, Cai Q, Zhang S, Wu X, Ying P, Ke J
and Yang Z: Treatment with paraquat affects the expression of
ferroptosis-related genes. Hum Exp Toxicol.
42:96032712311675852023. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Dosumu OA, Rotimi SO, Adeleye OO, Akamo
AJ, Osinuga KT, Taiwo OA, Omotosho OO and Sani LO: Vitamin K
protects against 7,12-dimethylbenz(A)anthracene induced
hepatotoxicity in Wistar rats. Environ Toxicol. 36:362–373. 2021.
View Article : Google Scholar
|
|
176
|
Schreiber CL and Smith BD: Molecular
imaging of aminopeptidase N in cancer and angiogenesis. Contrast
Media Mol Imaging. 2018:53151722018. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Amin SA, Adhikari N and Jha T: Design of
aminopeptidase N inhibitors as anti-cancer agents. J Med Chem.
61:6468–6490. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Guzman-Rojas L, Rangel R, Salameh A,
Edwards JK, Dondossola E, Kim YG, Saghatelian A, Giordano RJ,
Kolonin MG, Staquicini FI, et al: Cooperative effects of
aminopeptidase N (CD13) expressed by nonmalignant and cancer cells
within the tumor microenvironment. Proc Natl Acad Sci USA.
109:1637–1642. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Wang X, Shi L, Deng Y, Qu M, Mao S, Xu L,
Xu W and Fang C: Inhibition of leucine aminopeptidase 3 suppresses
invasion of ovarian cancer cells through down-regulation of fascin
and MMP-2/9. Eur J Pharmacol. 768:116–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Xu Y, Zhang N, Chen C, Xu X, Luo A, Yan Y,
Lu Y, Liu J, Ou X, Tan Y, et al: Sevoflurane induces ferroptosis of
glioma cells through activating the ATF4-CHAC1 pathway. Front
Oncol. 12:8596212022. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Wen YF, Yang XZ, Zeng LS, Peng HH, Huang
WJ, Cai LM, Zhou TC and Lin XD: Prognostic impact of pretherapeutic
gamma-glutamyltransferase on patients with nasopharyngeal
carcinoma. PLoS One. 12:e01723452017. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Mujawar SJ, Suchitra G, Kosandal KA,
Choudhari S, Inamdar NA and Ahmed KB: Evaluation of salivary
gamma-glutamyl transpeptidase as a biomarker in oral squamous cell
carcinoma and precancerous lesions. J Oral Maxillofac Pathol.
24:5842020. View Article : Google Scholar
|
|
183
|
Mizushima T, Ohnishi S, Shimizu Y,
Hatanaka Y, Hatanaka KC, Hosono H, Kubota Y, Natsuizaka M, Kamiya
M, Ono S, et al: Fluorescent imaging of superficial head and neck
squamous cell carcinoma using a γ-glutamyltranspeptidase-activated
targeting agent: A pilot study. BMC Cancer. 16:4112016. View Article : Google Scholar
|
|
184
|
Lee YJ, Han KD, Kim DH and Lee CH:
Determining the association between repeatedly elevated serum
gamma-glutamyltransferase levels and risk of respiratory cancer: A
nationwide population-based cohort study. Cancer Med. 10:1366–1376.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Gu J, Xie R, Zhao Y, Zhao Z, Xu D, Ding M,
Lin T, Xu W, Nie Z, Miao E, et al: A machine learning-based
approach to predicting the malignant and metastasis of thyroid
cancer. Front Oncol. 12:9382922022. View Article : Google Scholar
|
|
186
|
Foddis R, Franzini M, Bonotti A, Marino R,
Silvestri R, Fallahi P, Chiappino D, Emdin M, Paolicchi A and
Cristaudo A: Big and free fractions of gamma-glutamyltransferase:
New diagnostic biomarkers for malignant mesothelioma? Diagnostics
(Basel). 12:3112022. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Huang H, Wang XP, Li XH, Chen H, Zheng X,
Lin JH, Kang T, Zhang L and Chen PS: Prognostic value of
pretreatment serum alanine aminotransferase/aspartate
aminotransferase (ALT/AST) ratio and gamma glutamyltransferase
(GGT) in patients with esophageal squamous cell carcinoma. BMC
Cancer. 17:5442017. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Choi YJ, Lee DH, Han KD, Yoon H, Shin CM,
Park YS and Kim N: Elevated serum gamma-glutamyltransferase is
associated with an increased risk of oesophageal carcinoma in a
cohort of 8,388,256 Korean subjects. PLoS One. 12:e01770532017.
View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Chen Y, Liu H, Zhang J, Wu Y, Zhou W,
Cheng Z, Lou J, Zheng S, Bi X, Wang J, et al: Prognostic value and
predication model of microvascular invasion in patients with
intrahepatic cholangiocarcinoma: A multicenter study from China.
BMC Cancer. 21:12992021. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Catalano M, Roviello G, Aprile G, Ramello
M, Conca R, Petrioli R, Perrone G, Ianza A and Mini E: Prognostic
value of alkaline phosphatase and gamma-glutamyl transferase in
patients with metastatic pancreatic cancer. Future Oncol.
19:937–946. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Liao W, Yang Y, Yang H, Qu Y, Song H and
Li Q: Circulating gamma-glutamyl transpeptidase and risk of
pancreatic cancer: A prospective cohort study in the UK Biobank.
Cancer Med. 12:7877–7887. 2023. View Article : Google Scholar
|
|
192
|
Hong SW, Lee HJ, Han K, Moon JM, Park S,
Soh H, Kang EA, Chun J, Im JP and Kim JS: Risk of gastrointestinal
cancer in patients with an elevated level of
gamma-glutamyltransferase: A nationwide population-based study.
PLoS One. 16:e02450522021. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Yang S, He X, Liu Y, Ding X, Jiang H, Tan
Y and Lu H: Prognostic significance of serum uric acid and
gamma-glutamyltransferase in patients with advanced gastric cancer.
Dis Markers. 2019:14154212019. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Hong TC, Yang HC, Chen CL, Kao JH, Liu CJ,
Chen MJ, Wang HY, Kuo YC, Yu LY and Hu KC: Relationship between
serum gamma-glutamyl transferase level and colorectal adenoma. PLoS
One. 15:e02404452020. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Yang LH, Xu LZ, Huang ZJ, Pan HH, Wu M, Wu
QY, Lu T, Zhang YP, Zhu YB, Wu JB, et al: Comprehensive analysis of
immune ferroptosis gene in renal clear cell carcinoma: Prognosis
and influence of tumor microenvironment. Am J Transl Res.
14:5982–6010. 2022.PubMed/NCBI
|
|
196
|
Horie K, Kawakami K, Fujita Y, Matsuda Y,
Arai T, Suzui N, Miyazaki T, Koie T, Mizutani K and Ito M: Serum
exosomal gamma-glutamyltransferase activity increased in patients
with renal cell carcinoma with advanced clinicopathological
features. Oncology. 98:734–742. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Pankevičiūtė-Bukauskienė M, Mikalayeva V,
Žvikas V, Skeberdis VA and Bordel S: Multi-omics analysis revealed
increased de novo synthesis of serine and lower activity of the
methionine cycle in breast cancer cell lines. Molecules.
28:45352023. View Article : Google Scholar
|
|
198
|
Seol A, Wang W, Kim SI, Han Y, Park IS,
Yoo J, Jo H, Han KD and Song YS: Enhanced susceptibility to breast
cancer in Korean women with elevated serum
gamma-glutamyltransferase levels: A nationwide population-based
cohort study. Front Oncol. 11:6686242021. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Shi B, Zhang Z, Jin Q, Wang Z, Tang J, Xu
G, Zhu T, Gong X, Tang X and Zhao C: Selective tracking of
ovarian-cancer-specific γ-glutamyltranspeptidase using a
ratiometric two-photon fluorescent probe. J Mater Chem B.
6:7439–7443. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Schwameis R, Grimm C, Brodowicz T, Petru
E, Hefler-Frischmuth K, Staudigl C, Reinthaller A, Heinze G,
Polterauer S and Polterauer M: Gamma-glutamyltransferase as novel
biomarker in patients with uterine leiomyosarcoma. Sci Rep.
6:337572016. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Polterauer S, Hofstetter G, Grimm C,
Rahhal J, Mailath-Pokorny M, Kohl M, Concin N, Tempfer C, Marth C
and Reinthaller A: Relevance of gamma-glutamyltransferase-a marker
for apoptotic balance-in predicting tumor stage and prognosis in
cervical cancer. Gynecol Oncol. 122:590–594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Kawakami K, Fujita Y, Matsuda Y, Arai T,
Horie K, Kameyama K, Kato T, Masunaga K, Kasuya Y, Tanaka M, et al:
Gamma-glutamyltransferase activity in exosomes as a potential
marker for prostate cancer. BMC Cancer. 17:3162017. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Su S, Liu L, Sun C, Nie Y, Guo H, Hu Y,
Guo S and Pang S: Preoperative serum gamma-glutamyltransferase as a
prognostic biomarker in patients undergoing radical cystectomy for
bladder cancer. Front Oncol. 11:6489042021. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Takemura K, Fukushima H, Ito M, Kataoka M,
Nakanishi Y, Sakamoto K, Suzuki H, Tobisu KI and Koga F: Prognostic
significance of serum γ-glutamyltransferase in patients with
advanced urothelial carcinoma. Urol Oncol. 37:108–115. 2019.
View Article : Google Scholar
|
|
205
|
Song Y, Tian S, Zhang P, Zhang N, Shen Y
and Deng J: Construction and validation of a novel
ferroptosis-related prognostic model for acute myeloid leukemia.
Front Genet. 12:7086992022. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Watanabe B, Tabuchi Y, Wada K and Hiratake
J: Synthesis and evaluation of the inhibitory activity of the four
stereoisomers of the potent and selective human γ-glutamyl
transpeptidase inhibitor GGsTop. Bioorg Med Chem Lett.
27:4920–4924. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Han L, Hiratake J, Kamiyama A and Sakata
K: Design, synthesis, and evaluation of gamma-phosphono diester
analogues of glutamate as highly potent inhibitors and active site
probes of gamma-glutamyl transpeptidase. Biochemistry.
46:1432–1447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
King JB, West MB, Cook PF and Hanigan MH:
A novel, species-specific class of uncompetitive inhibitors of
gamma-glutamyl transpeptidase. J Biol Chem. 284:9059–9065. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Zhai X, Dai T, Chi Z, Zhao Z, Wu G, Yang S
and Dong D: Naringin alleviates acetaminophen-induced acute liver
injury by activating Nrf2 via CHAC2 upregulation. Environ Toxicol.
37:1332–1342. 2022. View Article : Google Scholar : PubMed/NCBI
|