Introduction
Non-small cell lung cancer (NSCLC) is one of the
most common malignant tumors, and the leading cause of
cancer-related death worldwide (1). NSCLC accounts for ~85% of all lung
cancer, including adenocarcinoma, giant cell carcinoma and squamous
cell carcinoma (2). Despite
significant progress in early detection and treatment strategies,
the prognosis of patients with NSCLC remains poor with a 5-year
overall survival rate of ~20% (3). Tumor recurrence, metastasis and drug
resistance lead to treatment failure and are the main cause of
NSCLC-related death (4). Thus,
thorough understanding of the molecular mechanism of NSCLC
progression and metastasis is urgently needed, so as to find novel
therapeutic targets.
Cisplatin is a front line regimen for the treatment
of NSCLC and platinum-based regimens have reported significantly
improved survival outcome in patients with advanced NSCLC (5). Accumulating evidences have
demonstrated that cisplatin in combination with other
chemotherapeutic agents exerted stronger therapeutic efficacy than
single agent alone against NSCLC. Combined treatment with olaparib
(PARP inhibitor) and cisplatin exerted synergistic antitumor
effects on PTEN-deficient NSCLC cells by suppressing DNA repair
(6). Additionally, combinational
treatment with falnidamol (EGFR inhibitor) and cisplatin remarkably
induced G2/M cell cycle arrest, DNA damage and ferroptosis in NSCLC
cells (7). Solamargine, a
steroidal alkaloid glycoside, in combination with cisplatin induced
G0/G1-phase arrest and apoptosis in lung cancer cell lines by
suppressing the hedgehog pathway (8). Combined therapy with arsenic
trioxide and cisplatin exerted synergistic anti-NSCLC effects, and
these effects were partly due to the induction of the
caspase-independent apoptosis pathway (9). Cisplatin has been reported to exert
cytotoxicity by inducing oxidative stress through increasing
production of reactive oxygen species (ROS) (10). Additionally, cisplatin impacted on
mitochondria and induced high level of mitochondrial ROS that led
to ovarian cancer cells' death (11). However, clinical application of
cisplatin in NSCLC has been limited due to its substantial side
effects and drug resistance (12). Therefore, it is important to
develop an effective combined therapeutic strategy that reduces
cytotoxicity and enhances antitumor activity of chemotherapeutic
agents including cisplatin.
Natural products from various traditional Chinese
medicinal herbs have been reported to have a promising antitumor
activity with low cytotoxicity. Jiyuan oridonin A exhibited
antitumor activity by inducing ferroptosis through inhibiting GPX4
and accumulating ROS generation in gastric cancer cells (13). Cardamonin treatment elevated ROS
levels in breast cancer cells by repressing HIF-1α-dependent
metabolic reprogramming (14). A
curcumin derivative, WZ35, induced ROS generation and subsequent
YAP-mediated C-Jun-amino-terminal kinase (JNK) activation in breast
cancer cells (15). Additionally,
dihydroartemisinin potentiated the antitumor activity of
oxaliplatin in colorectal cancer cells by inducing endoplasmic
reticulum (ER) stress through inhibiting expression of PRDX2
(16).
Polydatin
(3,40,5-Trihydroxystibene-3-β-Mono-D-Glucoside; PD) is a
biologically active compound derived from the annual plant species
Polygonum cuspidatum (17). PD has been reported to exert
antitumor effects in various cancers. For instance, PD suppressed
ovarian cancer cell viability by inducing apoptosis (18). In breast cancer, PD induced cell
apoptosis by inhibiting Creb phosphorylation (19). Additionally, PD activated
autophagy and induced apoptosis in human osteosarcoma cells by
inhibiting the STAT3 signaling pathway (20). Combined treatment of osteosarcoma
cells with PD and paclitaxel enhanced antitumor activity when
compared with single treatment alone (21). Moreover, PD in combination with
sunitinib markedly enhanced the antitumor activity by decreasing
pro-inflammatory cytokines and NLRP3 inflammasome expression levels
(22). However, underlying
molecular mechanisms of PD and its effects on anti-NSCLC activity
of cisplatin are largely unknown.
NADPH (nicotinamide adenine dinucleotide phosphate)
oxidase, NOXs, is a family including seven members [NOX1 to NOX5,
dual oxidase 1 (DUOX1) and DUOX2] whose primary function to
generate ROS (23). Accumulating
evidences suggested that NOXs exerted antitumor activities through
generating ROS production in various cancers. Dihydrotanshinone
(DHTS)-mediated activation of NOX5 promoted production of ROS and
inhibited the IL-6/Stat3 pathway, resulting in inhibiting formation
of breast cancer stem cells (CSCs) (24). Anlotinib, a multi-tyrosine kinase
inhibitor, upregulated expression of NOX5 and production of ROS,
and impaired mitochondrial respiration, resulting in promoting
apoptosis in oral squamous cell carcinoma (25). Nevertheless, whether PD activates
NOX5 function to facilitate ROS-mediated downstream signaling
pathways in lung cancer remains largely unknown.
In the present study, the therapeutic efficacy of
combined treatment with PD and cisplatin in NSCLC was
comprehensively investigated. It was identified that PD exerts
anti-NSCLC activity by increasing ROS production through promoting
NOX5 expression. Moreover, PD enhanced anti-NSCLC effects of
cisplatin by activating ROS-mediated ER stress, and the JNK and p38
mitogen-activated protein kinase (MAPK) signaling pathways. Taken
together, the current results provide a valuable foundation for
further clinical development of the combination therapy for the
treatment of NSCLC.
Materials and methods
Cell culture
Human NSCLC cell lines (H1299 and H460) and human
normal lung epithelial cells (BEAS-2B) were purchased from the
Institute of Biochemistry and Cell Biology, Chinese Academy of
Sciences. The cells were cultured routinely in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) in a 5%
CO2 atmosphere at 37°C. Normal human liver cells (MIHA)
were purchased from the Cell Bank of Chinese Academy of Sciences,
and human umbilical vein endothelial cells (HUVEC) were obtained
from the American Type Culture Collection (cat. no. CRL-1730),
which were cultured in F-12K medium supplemented with 10% FBS and
endothelial cell growth supplement at 37°C in a humidified cell
incubator with a supply of 5% CO2.
Chemicals and reagents
Cisplatin was obtained from TargetMol, PD was
purchased from Selleck Chemicals (purity: >97%), carboplatin was
obtained from MedChemExpress and N-Acetyl-l-Cysteine (NAC) was
provided from Sigma Aldrich; Merck KGaA. 3-(4,5-dimethyl
thiazol-2-yl) 2,5-diphenyl tetrazolium bromide (MTT) was purchased
from Beijing Solarbio Science & Technology Co., Ltd. ROS probe
2',7'-dichlorodihydrofluorescin diacetate (DCFH-DA) and H&E
staining kit were supplied from Beyotime Institute of
Biotechnology. Antibodies against activating transcription factor 4
(ATF4; cat. no. 11815S), eukaryotic initiation factor 2 (eIF2α;
cat. no. 9722S), phosphorylated eIF2α (p-eIF2α; cat. no. 3398S),
cleaved-Caspase3 (cat. no. 9664S), Caspase3 (cat. no. 9662S), JNK
(cat. no. 9252T), p-JNK (cat. no. 4668S), p38 MAPK (cat. no.
9212S), p-p38 MAPK (cat. no. 9211S), GAPGH (cat. no. 5174S),
HRP-linked anti-rat IgG antibody (cat. no. 7077S) and HRP-linked
anti-mouse IgG antibody (cat. no. 7076S) were purchased from Cell
Signaling Technology, Inc. Ki-67 (cat. no. ab16667) was purchased
from Abcam. NOX5 (cat. no. 25,350-1-AP), Vinculin (cat. no.
66,305-1-Ig) and Bcl-2 (cat. no. 60,178-1-Ig) antibodies were
purchased from Proteintech Group, Inc.
Cell viability assay
The MTT assay was employed to assess the effects of
PD, cisplatin and their combination on cell viability. In 96-well
plates, H1299 and H460 cells were seeded at a density of
3×103 or 5×103 cells per well, respectively,
and allowed to adhere for 24 h. BEAS-2B, MIHA and HUVEC cells were
seeded at a density of 3×103 per well and incubated for
24 h. The cells were treated with various concentrations of PD (0,
100, 200, 300, 400, 500 and 600 μM) for 72 h. For the
combined treatment, the cells were treated with PD, cisplatin or
their combination at indicated concentrations for 72 h. After the
completion of the incubation period, 25 μl of MTT reagent
was added to each well and allowed to incubate for an additional 3
h. Subsequently, 150 μl of DMSO was added to each well to
dissolve the formazan product. The absorbance of each sample was
measured at a wavelength of 490 nm using a SpectraMax iD3
instrument (Molecular Devices, LLC). The Logit approach was used to
evaluate the IC50 values. The CompuSyn 2.0 software
(http://www.combosyn.com/index.html)
was utilized to compute the combination index (CI) to evaluate drug
interactions. It is noteworthy that a CI value of 1 signifies an
additive effect when two agents are combined, while a CI >1 or
CI <1 indicates an antagonistic or synergistic interaction,
respectively.
Colony formation assay
The cells were seeded at a density of
2×103 cells per well in 6-well plates and were allowed
to adhere in 5% CO2 atmosphere at 37°C overnight.
Subsequently, the cells were subjected to treatment with varying
concentrations of PD (0, 25, 50 and 100 μM for H1299 cells
and 0, 200, 400, 800 μM for H460 cells) for 72 h. For the
combination therapy, the cells were treated with cisplatin (1.5
μM), PD (25 μM for H1299 cells and 500 μM for
H460 cells), or their combination with or without 5 mM NAC
pre-treatment for 2 h. After 7-10 days incubation, the colonies
were subjected to a washing procedure using phosphate-buffered
saline (PBS). Subsequently, a fixative solution consisting of 4%
paraformaldehyde (PFA) was applied for a duration of 15 min at room
temperature. Following fixation, 0.5% Gentian violet staining was
performed for a period of 10 min at room temperature (RT). ImageJ
software (version 1.53t; National Institutes of Health) was used to
quantify colonies (consisting of >50 cells).
Wound healing assay
Wound healing analysis was employed to assess the
capability of cell motility. The H1299 NSCLC cells were seeded into
6-well plates at a density of 3×105, cultured with
RPMI-1640 medium containing 2% FBS, and allowed to reach confluent
monolayers. Then multiple lines were drawn in the cell monolayers
with a bacteria-free 10 μl pipette tip to create wound area.
The scratched cells were then removed from the plates by washing
with PBS. H1299 cells were treated with cisplatin (1.5 μM),
PD (20 μM), or a combination of both, with or without 5 mM
NAC pretreatment for 1.5 h. Following a 48-h incubation period, the
old medium was replaced with fresh RPMI-1640 medium supplemented
with 2% FBS. The evaluation of the combined treatment on cell
migration was performed by capturing images at the point when the
wound in the control group had healed virtually or completely. The
results of this assay were analyzed to determine the effects of the
combined treatment on cell migration.
Measurement of ROS generation
In order to measure intracellular ROS generation,
the cells were treated with the ROS-sensitive dye (DCFH-DA), and
analyzed using flow cytometry. Specifically, the NSCLC cells were
seeded into 6-well plates and cultured overnight in standard
culture medium. The cells were exposed to PD (500 μM) for 3,
6, 9, 12 and 15 h. For combination treatment, the cells were
exposed with cisplatin (60 μM for H1299 and 50 μM for
H460), PD (500 μM), or a combination of both for a duration
of 12 h. In the case of the combination group, NAC pretreatment was
conducted at a concentration of 5 mM for a duration of 1.5 h prior
to treatment. Following treatment, the cells were stained with 10
μM DCFH-DA and incubated at 37°C in the absence of light for
a period of 30 min. The ROS production (DCF fluorescence) was
measured by flow cytometry using FACS Calibur (BD Biosciences), and
data was analyzed using the FlowJo software (Tree Star, Inc.).
Protein extraction and western
blotting
The homogenization of cells or tumor tissues was
accomplished by using protein RIPA lysis buffer (cat. no.
AR0103-100, Boster Biological Technology). Subsequently, lysates
were subjected to centrifugation at 4°C for 15 min at 13,400 × g to
remove any undissolved debris. The Bradford protein assay kit
(Bio-Rad Laboratories, Inc.) was used to measure the concentrations
of total proteins extracted from cells or tumor tissues. The equal
amount of protein (60 μg) and loading buffer were mixed, and
separated by using a 10-12% SDS-PAGE. Then the proteins were
transferred to PVDF membranes, and blocked with 5% fresh non-fat
milk in TBST containing 0.1% Tween 20 for 2 h at RT. The membranes
underwent incubation with the primary antibodies specific to the
experiments overnight at 4°C, which were ATF4 (1:1,000), eIF2α
(1:1,000), p-eIF2α (1:1,000), cleaved-Caspase3 (1:1,000), Caspase3
(1:1,000), JNK (1:1,000), p-JNK (1:1,000), p38 MAPK (1:1,000),
p-p38 MAPK (1:1,000), GAPGH (1:10,000), NOX5 (1:1,000), Vinculin
(1:5,000) and Bcl-2 (1:2,000). Subsequently, the membranes were
subjected to three wash cycles using TBST to remove any unbound
antibodies. After washing, the membranes underwent further
incubation with corresponding horseradish peroxidase-conjugated
secondary antibodies [HRP-linked anti-rat IgG antibody (1:4,000)
and HRP-linked anti-mouse IgG antibody (1:4,000)] for 1 h at RT.
The ECL detection kit was used to detect immunoreactivity (Bio-Rad
Laboratories, Inc.). ImageJ software (version 1.53t; National
Institutes of Health) was used to conduct densitometric
measurements.
In vivo mice xenograft model
Athymic BALB/c female nude mice (age, 5 weeks-old;
weight, 18-20 g; n=25) were purchased from the Vital River
Experimental Animal Center. All animal experiments were carried out
in accordance with the Wenzhou Medical University's Institutional
Animal Care and Use Committee (IACUC) guidelines (approval no.
xmsq2022-0602; Wenzhou, China). The mice were maintained under
specific pathogen-free conditions with stable RT (20-25°C) and
humidity (50-60%), following a 12-h light/dark cycle, and provided
with a standard rodent diet and water ad libitum. The
2.5×106 H460 cells per 100 μl of PBS/Matrigel
mixture (1:1) were subcutaneously injected into the flanks of nude
mice. The mice were divided into five experimental groups (n=5)
when the tumor volumes reached to ~100 mm3. The tumor
bearing mice were intraperitoneally administered 2 mg/kg cisplatin
every three days, 50 mg/kg PD every two days, or their combination
for two weeks. The control group was administered vehicle only, and
ROS inhibitor (NAC) was administered in drinking water (0.5 g/l)
for the combination group. The health and behavior of the mice were
monitored daily, and tumor length (L), width (W) and mouse weight
were measured every 2 days after treatment. The tumor volume was
calculated using the following formula: V=1/2 × L × W2.
When the tumors reached nearly 15 mm in diameter on day 24, all the
mice were sacrificed. No mice reached humane endpoints at the end
of the experiments, defined as either losing 20% of body weight or
exhibiting moribund behavior. At the experimental endpoint, all
mice were euthanized by intraperitoneal injection of pentobarbital
sodium (150 mg/kg) followed by cervical dislocation. The
confirmation of mouse death was further verified by visually
inspecting for cardiac and respiratory arrest. The tumor tissues
were excised and weighed for further investigation. Histological
examination of certain essential organs, including the heart,
liver, kidney and lung were evaluated by using hematoxylin and
eosin (H&E) staining.
Immunohistochemical (IHC) staining
The tumor tissue samples were fixed with 4% PFA for
24 h at room temperature and then embedded in paraffin. The
paraffin blocks were sliced in 5-μm-thick sections and
deparaffinized in xylene (Changshu Chemicals Co., Ltd.) and
dehydrated with an ethanol gradient. Endogenous peroxidase activity
was blocked by incubation with 3% H2O2 in
methanol at room temperature for 15 min. The IHC was carried out
according to the routine procedure. Specifically, the tumor
sections were subjected to overnight incubation at 4°C with a
primary antibody targeting Ki-67 (1:100), followed by subsequent
incubation with HRP-linked anti-rat IgG antibody (1:1,000) for 1 h
at ambient temperature. Detection of the targeted antigen was
achieved through employment of 3,3'-diaminobenzidine (DAB) for
color detection. The histological investigation and potential
toxicity assessment were carried out by H&E staining
(hematoxylin for 5 min and 0.5% eosin for 1-3 min) at room
temperature with heart, liver, kidney and lung tissues. The
immunostained sections were observed using a confocal microscope
(magnification, x200; Leica Microsystems GmbH).
Gene knockdown experiments
H1299 cells (3×105 per well) or H460
cells (5×105 per well) were seeded in 6-well plates and
incubated for 24 h at 37°C. The NOX5 small interfering (si)RNA
(Shanghai GenePharma Co., Ltd.) or non-targeting control siRNA were
transfected into the cells with final concentration of 30 nM using
lipofectamine 3000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), and incubated for 24 h at 37°C. Following incubation, the
cells were treated with various concentrations of PD for different
time-intervals according to experimental requirement. The
effectiveness of knockdown was evaluated via western blotting and
reverse transcription-quantitative PCR (RT-qPCR), and the siRNA
sequences are provided in Table
SI. This methodology is in accordance with established academic
protocols for transfecting cells and evaluating knockdown
efficacy.
RT-qPCR
Total RNAs were extracted by TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. For cDNA synthesis, 1 μg of
total RNA was reverse transcribed using PrimeScript RT Master Mix
(cat. no. RR036A; Takara Biotechnology Co., Ltd.) in accordance
with the manufacturer's protocol. RT-qPCR was performed by using TB
Green Premix Ex Taq II reagent (cat. no. RR820A; Takara) according
to the manufacturer's protocol. Briefly, initial denaturation was
30 sec at 95°C, followed by 40 cycles of denaturation (5 sec at
95°C), annealing (34 sec at 60°C) and extension (10 sec at 95°C).
The relative gene expression was determined using the
2-ΔΔCq method (26),
and GAPDH was used as an internal control. The primer sequences
utilized for the gene amplification are provided in Table SII.
Statistical analysis
All experiments were performed in triplicate (n=3).
The resulting data was presented as the mean ± standard deviation
(SD), and analyzed using GraphPad Prism 8.0 software (Dotmatics).
Statistical analysis was conducted using two-sample t-tests for
comparison between two groups, or one-way ANOVA with Tukey's
multiple comparisons test for comparison between multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
PD induces cytotoxicity in NSCLC
cells
Previous studies have reported that PD exerted
cytotoxicity in various tumor cells with IC50 values
ranging from 200 to 500 μM (27-29). To investigate the function of PD
on NSCLC cell proliferation, H1299 and H460 cells were treated with
varying dosages of PD for 72 h. The chemical structure of PD
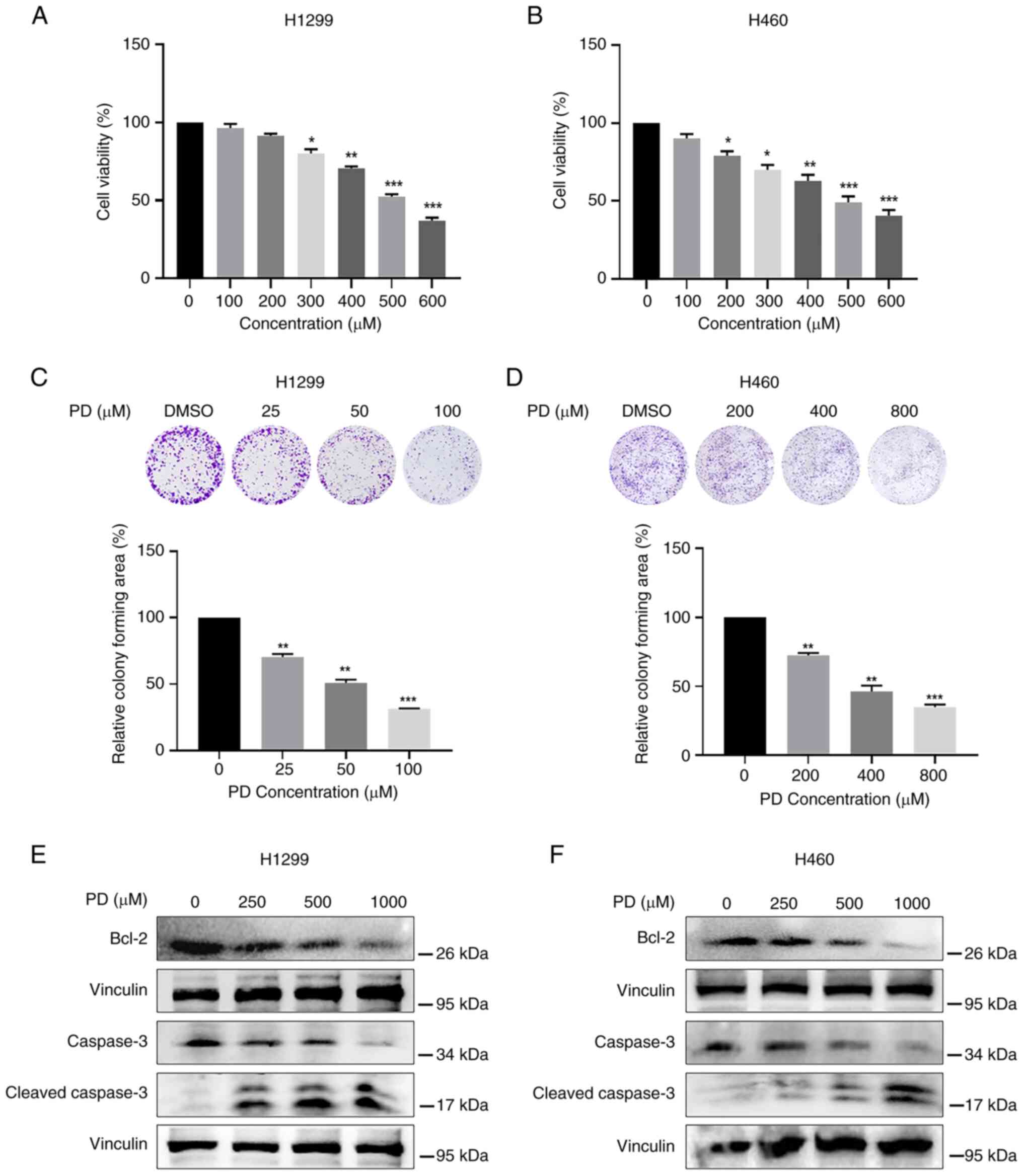
(PubChem CID: 5281718) is demonstrated in Fig. S1A. As revealed in Fig. 1A and B, PD significantly
suppressed both NSCLC cell proliferation in a
concentration-dependent manner with IC50 values of ~500
μM. Additionally, the same experiments were conducted in
normal human cells. As expected, PD showed less cytotoxicity
against BEAS-2B (Fig. S1B), MIHA
(Fig. S1C) and HUVEC (Fig. S1D) cells when compared with NSCLC
cells. In detail, 600 μM PD inhibited normal human cell
proliferation only ~20% when compared with non-PD treatment group.
Colony formation assay further identified that PD significantly
reduced colony forming ability of both H1299 (Fig. 1C) and H460 (Fig. 1D) cells.
The effects of PD on NSCLC cell apoptosis were
further investigated. High doses of PD markedly reduced the
expression level of the anti-apoptotic protein Bcl-2, while
increased the expression of the pro-apoptotic protein
Cleaved-Caspase3 in both H1299 and H460 cells (Fig. 1E and F). These results suggested
that the inhibition of cell proliferation caused by PD might partly
be due to PD-induced apoptotic pathway in NSCLC.
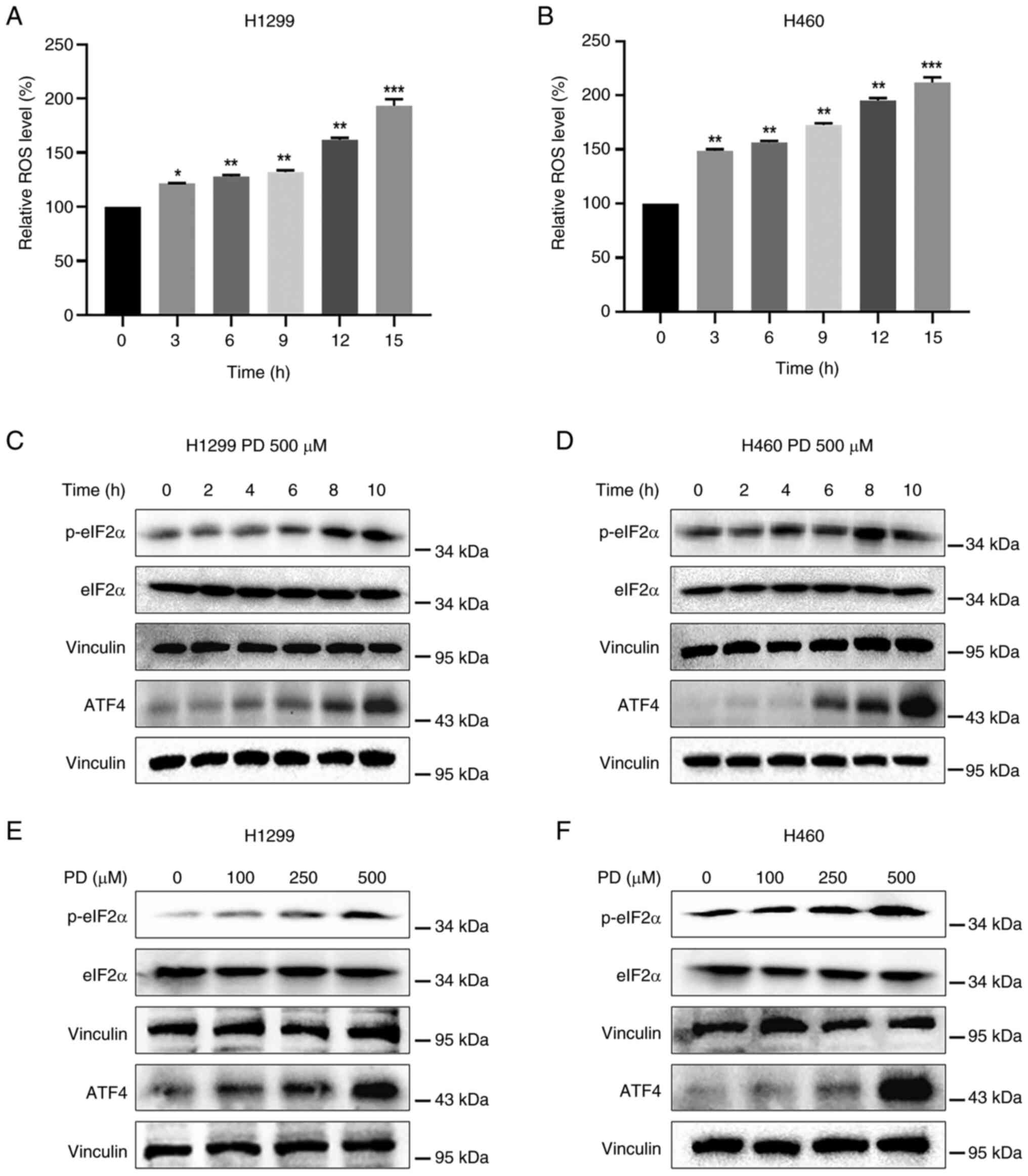
PD activates ROS-dependent ER stress
pathway in NSCLC cells
PD has been revealed to have antitumor properties by
generating ROS (30). Abundant
ROS production stimulates oxidative stress, resulting in cancer
cell death (31). To determine
whether inhibition of NSCLC cell proliferation caused by PD is
associated with the increased ROS generation, H1299 and H460 cells
were treated with PD, followed by incubation with DCFH-DA.
Treatment of both cell lines with PD elevated fluorescence
intensity in a time-dependent manner, suggesting that PD treatment
boosted ROS production (Fig. 2A and
B). Increased ROS production triggers the ER stress response
and promotes the expression levels of p-eIF2α and ATF4 (32,33). Treatment of both NSCLC cell lines
(500 μM) with PD time-dependently elevated p-eIF2α and ATF4
expression levels (Fig. 2C and
D). As expected, PD treatment dose-dependently elevated p-eIF2α
and ATF4 expression levels in NSCLC cells (Fig. 2E and F), indicating that PD exerts
antitumor effects in NSCLC cells by stimulating ROS-mediated ER
stress pathway.
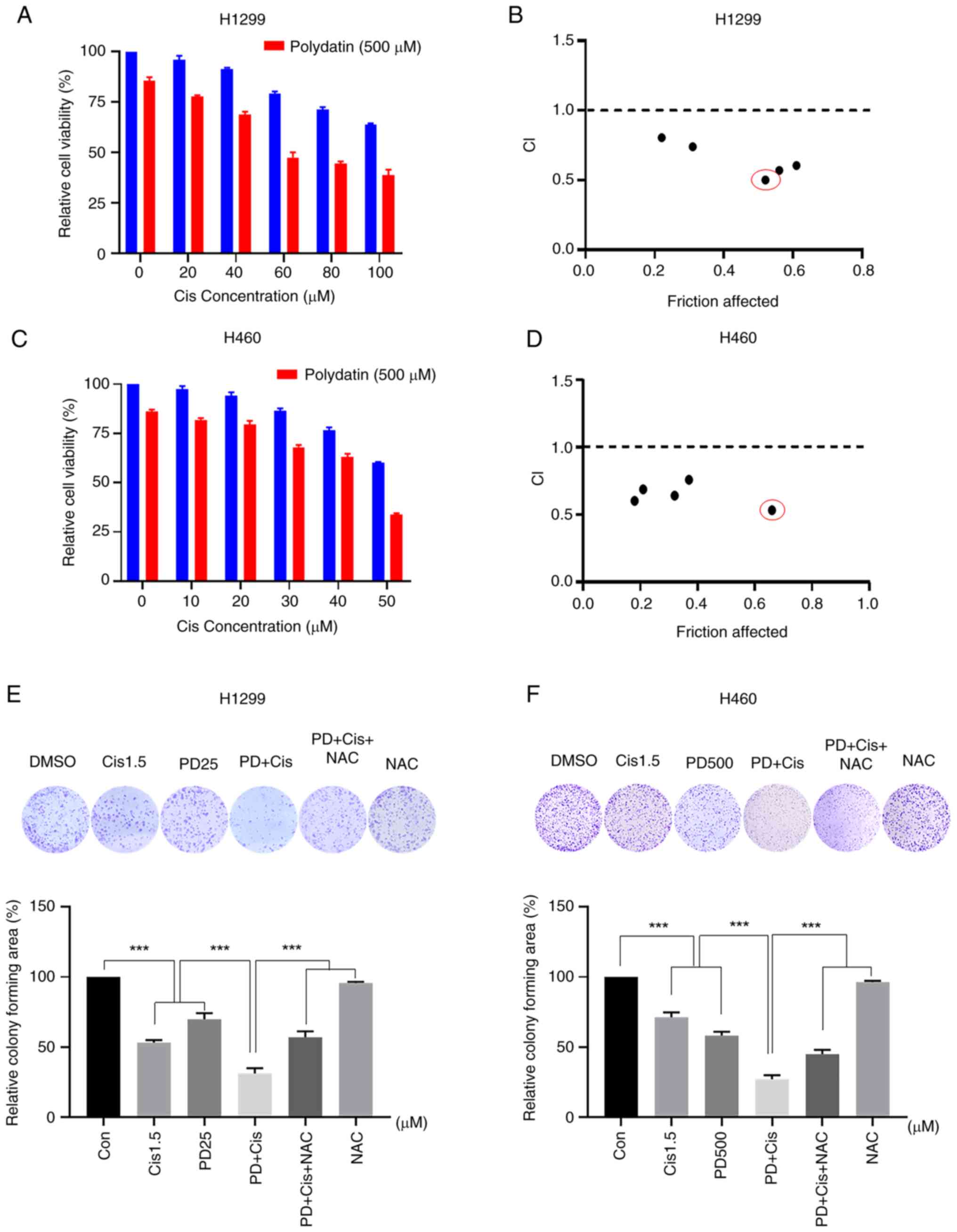
Cisplatin combined with PD exerts
synergistic antitumor activities in NSCLC cells
To evaluate whether the combined treatment has
superior anti-proliferative effects than monotherapy, H1299 and
H460 cells were treated with PD, cisplatin, or their combination.
Treatment of NSCLC with varying doses of cisplatin inhibited cell
viability of both H1299 and H460 cells in a dose-dependent manner.
Additionally, 500 μM PD generally boosted cytotoxicity of
cisplatin against H1299 cells, and robust synergistic inhibitory
impact was observed in combined therapy with 60 μM cisplatin
and 500 μM PD (Fig. 3A).
In the same way, the application of 500 μM PD was found to
augment cisplatin-induced cytotoxicity in H460 cells, and
significant synergistic inhibitory effects were observed with
combined therapy of 50 μM cisplatin and 500 μM PD
(Fig. 3C). CompuSyn 2.0 software
was utilized to calculate CI values assessing the interaction
between PD and cisplatin. Notably, combinations of 60 μM
cisplatin and 500 μM PD in H1299 cells (Fig. 3B) or 50 μM cisplatin and
500 μM PD in H460 cells exhibited the lowest CI values
amongst different combinations (Fig.
3D), indicating synergistic effects between PD and cisplatin in
NSCLC. Furthermore, colony formation assay revealed that combined
treatment of H1299 (25 μM) and H460 (500 μM) cells
with PD and cisplatin (1.5 μM) significantly reduced colony
forming ability of both cell lines when compared with single
treatment alone (Fig. 3E and F).
Importantly, combined treatment-induced inhibitory effects of
colony forming ability were significantly attenuated by the ROS
scavenger, NAC. Wound healing assay also showed similar results as
combination of low dose of PD and cisplatin markedly inhibited the
migration of NSCLC cells, and NAC pre-treatment effectively
reversed this effect (Fig. S2).
Similar with cisplatin, combination therapy with carboplatin and PD
exhibited stronger inhibitory effects on H1299 and H460 cell
viability (Fig. S3A-C) and
colony formation (Fig. S3D-F)
than single treatment alone. These findings indicated that a low
dose of PD could improve antitumor effects of cisplatin and
carboplatin, and that ROS is an important mediator in the
synergistic effects of PD and cisplatin in NSCLC cells.
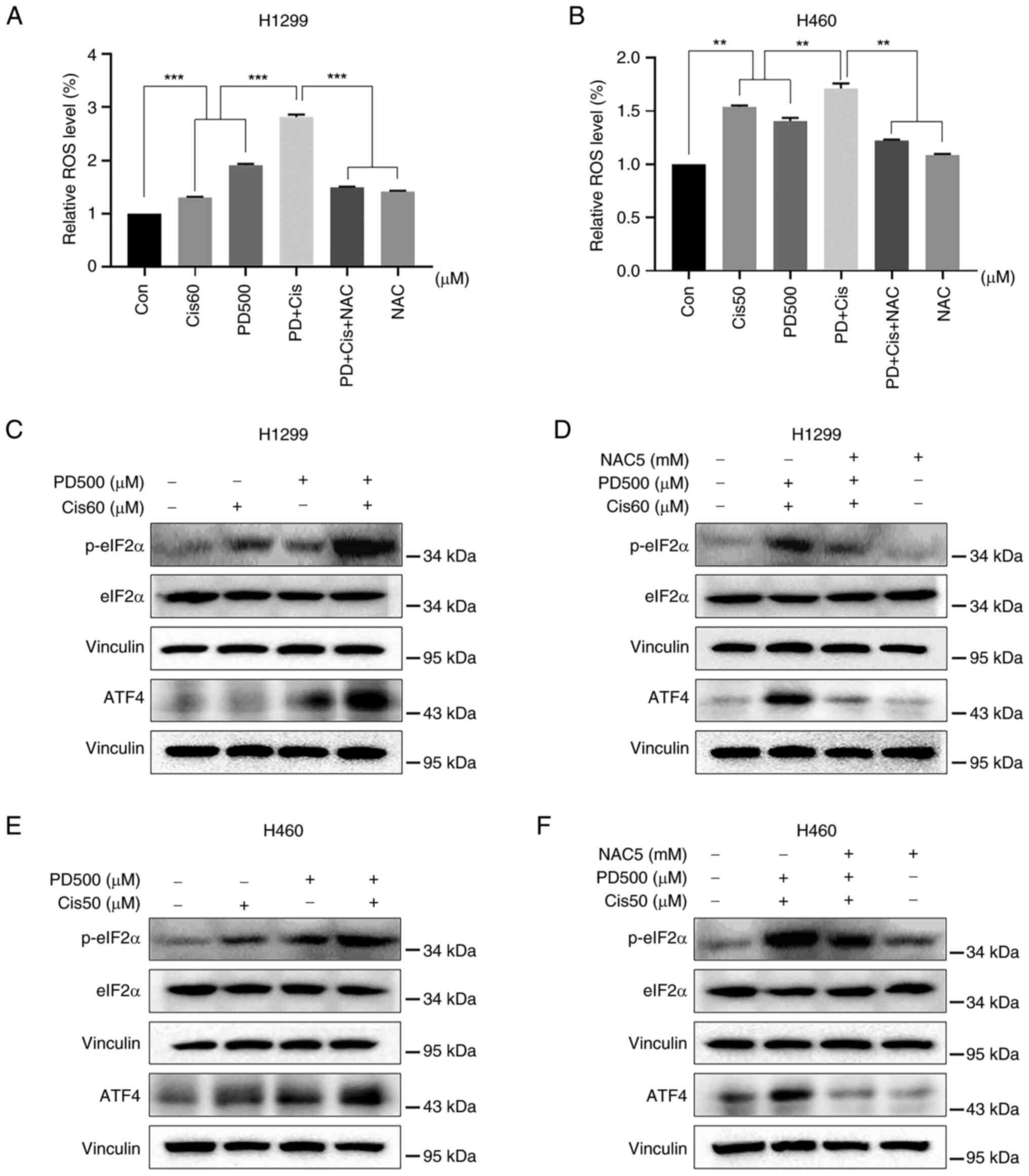
Combined treatment with PD and cisplatin
stimulates ROS-mediated ER stress in NSCLC cells
Cancer cells have been observed to maintain higher
levels of ROS compared with normal cells (34). This aspect provides an interesting
therapeutic window since cancer cells might be more sensitive than
normal cells to agents that induce ROS generation. In the present
study, the intracellular ROS levels were examined in NSCLC cells
after treatment with PD (500 μM), cisplatin (60 μM in
H1299 cells and 50 μM in H460 cells), or combinations
thereof. Combined treatment significantly induced generation of ROS
when compared with PD or cisplatin alone, whilst NAC pre-treatment
effectively prevented generation of ROS induced by combined
treatments (Fig. 4A and B). ER
stress-related proteins in cells were further investigated after
treatment with PD, cisplatin, or their combination. Combined
treatment markedly increased p-eIF2α and ATF4 expression in both
H1299 and H460 cell lines, as compared with treatment with
cisplatin or PD alone (Fig. 4C and
E), whilst pre-treatment with NAC partly attenuated the
expression of these ER stress-related proteins (Fig. 4D and F). These findings suggested
that ROS-mediated ER stress functioned as a key role in the
combined treatment, inducing synergistic antitumor activities in
NSCLC cells.
Combined treatment of NSCLC cells with PD
and cisplatin activates ROS-mediated JNK and p38 MAPK pathways
Apoptosis is tightly linked to the activated JNK and
p38 MAPK pathways (35).
Activation of the JNK and p38 pathways is closely involved in
oxidative stress-induced apoptosis (36). Thus, the p-JNK and p38 protein
levels were investigated in H1299 and H460 cells following
treatment with PD (500 μM), cisplatin (60 μM in H1299
cells or 50 μM in H460 cells), or their combination.
Combined treatment of NSCLC cells with PD and cisplatin
consistently elevated p-JNK and p38 expression (Fig. 5A and C), while pre-treatment with
NAC effectively attenuated p-JNK and p-p38 levels induced by
combined treatment (Fig. 5B and
D). These results indicated that activation of ROS-mediated JNK
and p38 MAPK signaling pathways contributed to the combined
treatment, inducing synergistic antitumor activities.
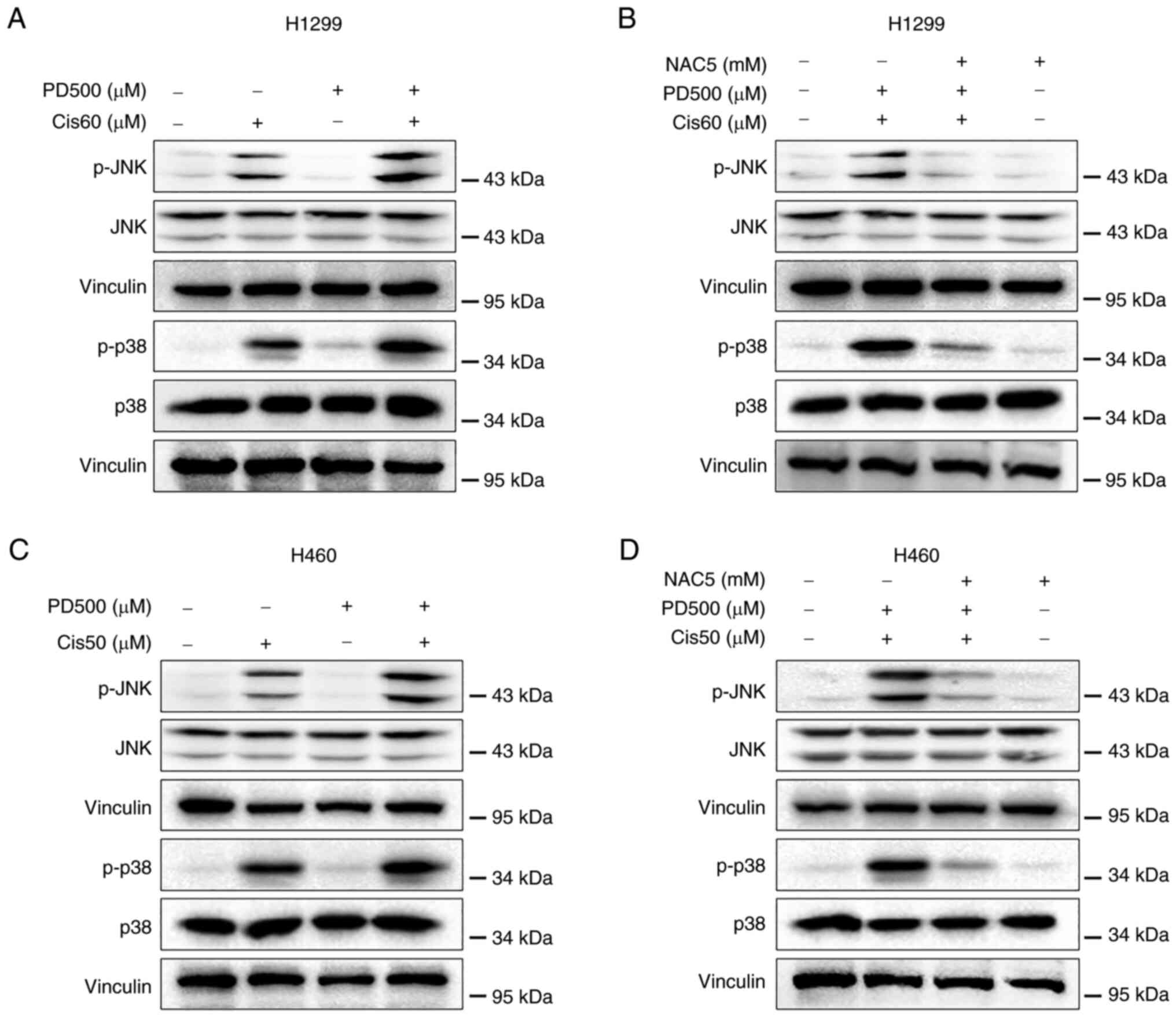 | Figure 5Combined treatment with PD and
cisplatin activates reactive oxygen species-mediated JNK and p38
MAPK pathways in non-small cell lung cancer cells. (A) H1299 and
(C) H460 cells were treated with PD, cisplatin or their combination
at the indicated doses. After 8-h treatment, the protein levels of
p-JNK, JNK, p-p38 and p38 were detected by western blot analysis.
Vinculin was used as the internal control. (B) H1299 and (D) H460
cells were pretreated with NAC (5 mM) for 1.5 h before exposure to
PD and cisplatin combination. After 8-h treatment, the protein
levels of p-JNK, JNK, p-p38 and p38 were detected by western blot
analysis. Vinculin was used as the internal control. PD, polydatin;
NAC, N-Acetyl-l-Cysteine; p-, phosphorylated. |
Combined treatment with PD and cisplatin
exhibits synergistic antitumor activity in mice xenograft
models
To further investigate the synergistic antitumor
effects of combined treatment with PD and cisplatin on NSCLC in
vivo, H460 xenografts were established in nude mice. The mice
were divided into five experimental groups (n=5), and were
administered 2 mg/kg cisplatin every three days, 50 mg/kg PD every
two days, or their combination for two weeks. The combined
treatment group was further divided into two sub-groups, with one
sub-group administered NAC in their drinking water (0.5 g/l). The
results showed that the combined treatment significantly inhibited
tumor growth in vivo compared with cisplatin or PD treatment
alone, while this inhibitory effect was reversed in the NAC
treatment sub-group (Figs. 6A and
B and S4A). There were no
notable differences in body weight changes between the combined
treatment group and the other groups (Fig. S4B). Additionally, H&E
staining demonstrated no histological alterations in vital organs
(heart, lung, kidney and liver) (Fig. S4C), suggesting that the combined
treatment was administered at rational drug doses and did not
induce any significant cytotoxicity in the experimental subjects.
Similar with in vitro results, the expression levels of
p-eIF2α, ATF4, p-JNK and p-p38 in tumor tissues exhibited a marked
increase in the combined treatment group as compared with single
treatment groups. Moreover, pre-treatment with NAC consistently
attenuated the increase of these proteins in tumor tissues from the
combined treatment group (Fig.
6C). As expected, PD treatment increased NOX5 protein
expression, whereas cisplatin did not, suggesting that cisplatin
increases ROS levels through a NOX5-independent pathway.
Accordingly, the combination of cisplatin and PD did not markedly
increase NOX5 protein levels compared with PD treatment alone.
Additionally, NAC pre-treatment did not reverse the combination
treatment-induced NOX5 protein levels, indicating that ROS may not
be upstream of NOX5. Furthermore, the IHC staining analysis
demonstrated that the co-administration of cisplatin and PD
resulted in a significant reduction of Ki-67 positive cells.
Notably, the pre-treatment with NAC reversed the aforementioned
effects induced by the combined treatment (Fig. 6D). These findings suggested that
PD enhanced the antitumor activities of cisplatin in vivo at
least partly by stimulating ROS-mediated ER stress, JNK and p38
MAPK signaling pathways.
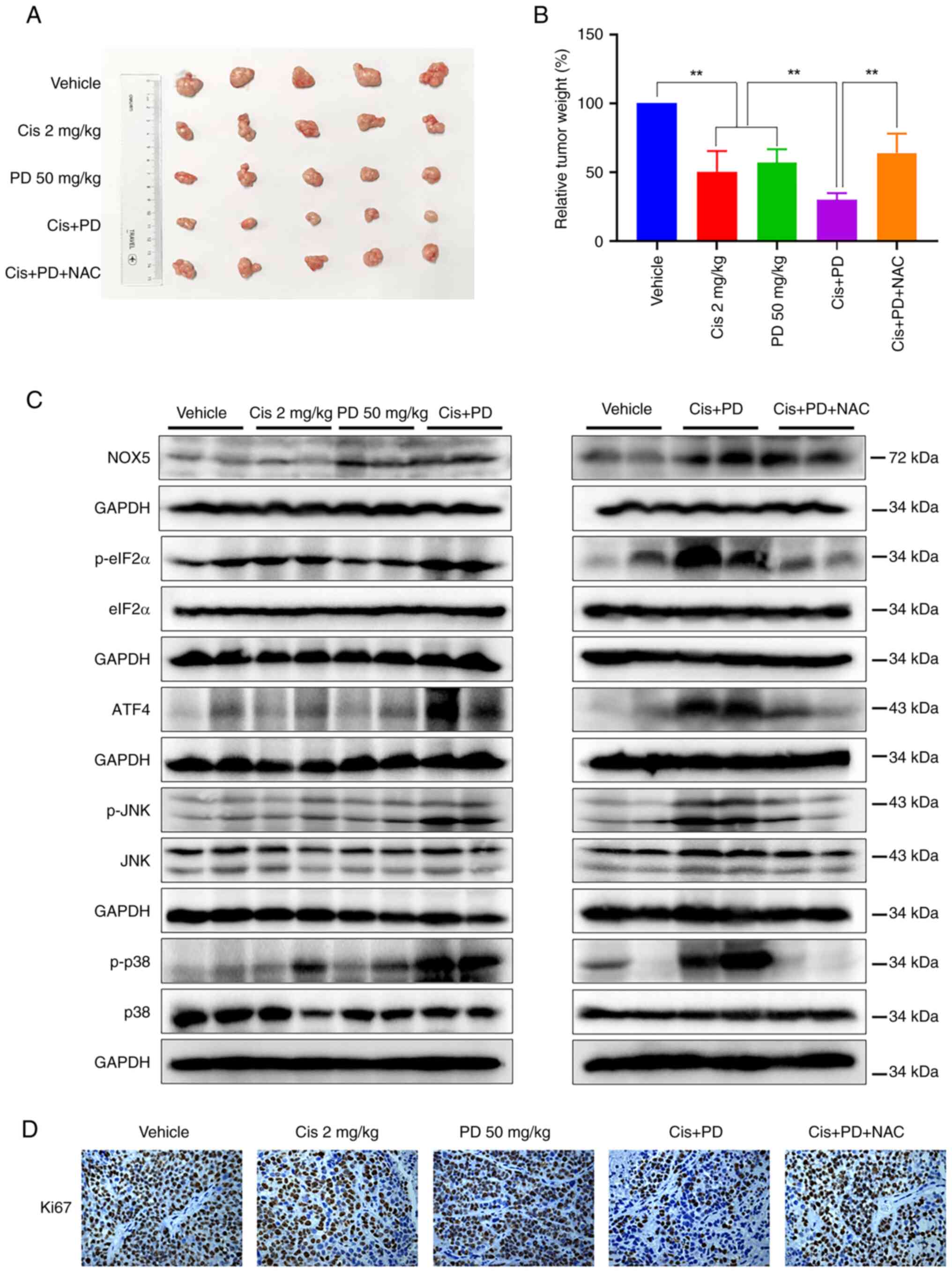 | Figure 6Combined treatment with PD and
cisplatin synergistically inhibits tumor growth in mice xenograft
models. (A and B) The tumor bearing mice were treated with PD (50
mg/kg), cisplatin (2 mg/kg) or their combination. The combined
treatment significantly decreased (A) tumor volume and (B) weight,
and NAC administration (0.5 g/l) reversed these effects. (C) The
protein levels of NOX5, p-eIF2α, ATF4, p-p38, p-JNK and their
corresponding total proteins in mice tumor specimens after
treatment were determined by western blot analysis. GAPDH was used
as the internal control. (D) The Ki-67 expression in tumor tissues
was analyzed by immunohistochemical analysis (magnification, x200;
scale bar, 50 μm). Data were analyzed by using one-way ANOVA
with Tukey's multiple comparisons test. Results are presented as
the mean ± SD from three independent experiments.
**P<0.01. PD, polydatin; NAC, N-Acetyl-l-Cysteine;
NOX5, NADPH oxidase 5; p-, phosphorylated. |
PD exerts antitumor activities by
targeting NOX5 in NSCLC cells
NOX5 activation stimulates production of ROS and
causes subsequent death of tumor cells (24,37,38). It was found that PD treatment
increased generation of ROS in NSCLC cells. Thus, it was
hypothesized that PD might promote NOX5 activity to regulate
generation of ROS. Treatment of NSCLC cells with PD significantly
increased both NOX5 mRNA and protein expression levels (Fig. 7A and B). Similarly, PD treatment
consistently upregulated NOX5 protein expression in mouse xenograft
tumor tissues (Fig. 7C). To
further evaluate the impact of NOX5 in PD-induced generation of
ROS, NOX5 was knocked down by two independent siRNAs. Knocking down
NOX5 consistently reduced both NOX5 mRNA (Fig. 7D and E) and protein expression
levels in H1299 and H460 NSCLC cells (Fig. 7F). Since si-217 exhibited stronger
knockdown effect than si-1112, si-217 was used for further
analyses. Additionally, knocking down NOX5 attenuated the
inhibitory effects of PD on NSCLC colony formation (Figs. 7G and H; S5C and D) and cell viability (Figs. 7I and J; S5A and B). Furthermore, knocking down
NOX5 reduced PD-induced production of ROS in NSCLC cells (Fig. 8A and B). Additionally, knocking
down NOX5 partly diminished PD-induced activation of ER
stress-related proteins (p-eIF2α and ATF4) (Fig. 8C and D), and the JNK and p38 MAPK
pathways (Fig. 8E and F). These
results indicated that NOX5 is a possible target of PD and an
important mediator of PD-induced cell death in NSCLC cells.
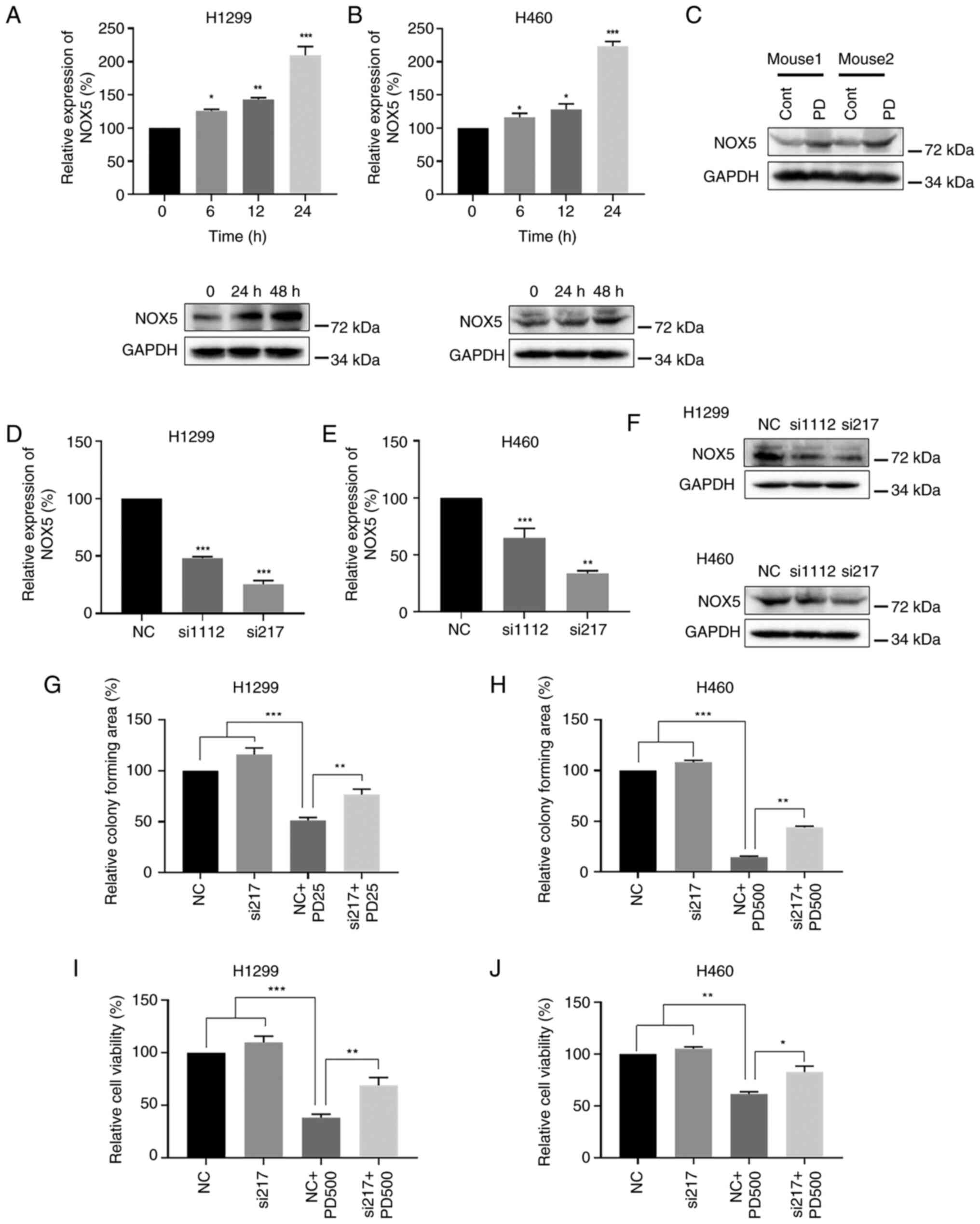 | Figure 7PD treatment increases expression of
NOX5 in non-small cell lung cancer cells. (A) H1299 and (B) H460
cells were treated with PD, and NOX5 mRNA and protein expression
levels were evaluated by RT-qPCR and western blot analyses,
respectively. GAPDH was used as the internal control. (C) Western
blot analysis detecting the expression levels of NOX5 protein in
mice tumor tissues after treatment with PD. GAPDH was used as the
internal control. (D-F) siRNAs against NOX5 were transfected into
H1299 and H460 cells. (D and E) NOX5 mRNA and (F) protein levels
were detected by RT-qPCR and western blot analyses, respectively.
GAPDH was used as the internal control. (G) H1299 and (H) H460
cells were treated with PD (25 μM in H1299 cells and 500
μM in H460 cells), siNOX5 or their combination.
Quantification of colony formation data was calculated by using
ImageJ software. (I) H1299 and (J) H460 cells were treated with PD
(500 μM), siNOX5 or their combination for 48 h. The cell
viability was measured by MTT assay. Data were analyzed by using
one-way ANOVA with Tukey's multiple comparisons test. Results are
presented as the mean ± SD from three independent experiments.
*P<0.05, **P<0.01 and
***P<0.001. PD, polydatin; NOX5, NADPH oxidase 5;
RT-qPCR, reverse transcription-quantitative PCR; si-, small
interfering; NC, negative control. |
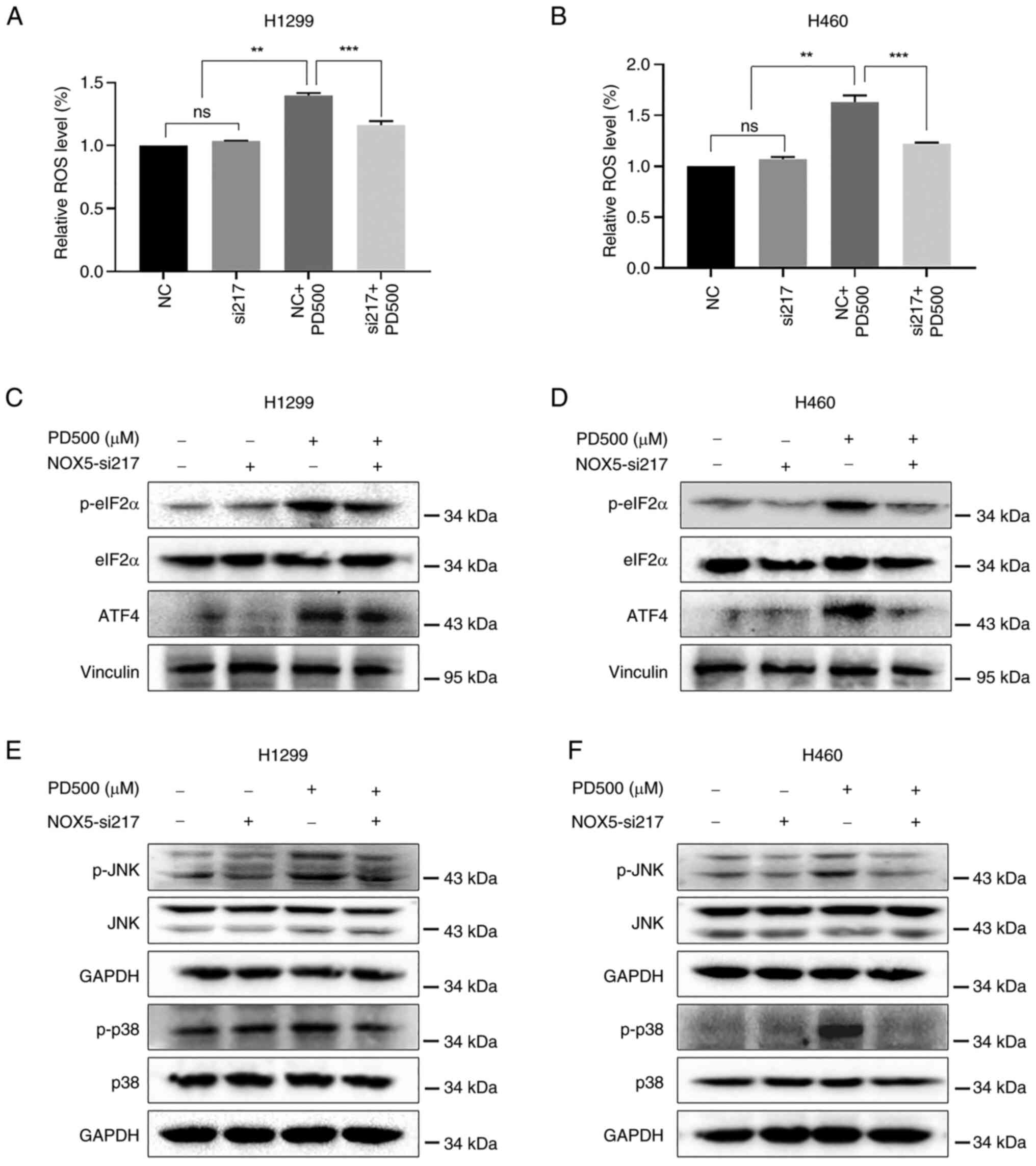 | Figure 8PD exerts antitumor activity in
non-small cell lung cancer cells by stimulating NOX5-ROS-mediated
endoplasmic reticulum-stress, and the JNK and p38 MAPK signaling
pathways. (A) H1299 and (B) H460 cells were treated with siNOX5 for
18 h, and then treated with PD (500 μM) for 12 h. Relative
ROS levels were evaluated by fluorescent DCF-DA probe. Data were
analyzed by using one-way ANOVA with Tukey's multiple comparisons
test. Results are presented as the mean ± SD from three independent
experiments. **P<0.01 and ***P<0.001.
(C-F) H1299 and H460 cells were pretreated with siNOX5 for 18 h
before treatment with 500 μM PD. (C and D) After 6-h
treatment, the protein levels of p-eIF2α, eIF2α, ATF4 were
determined by western blot analysis. Vinculin was used as an
internal control. (E and F) The protein levels of p-JNK, p-p38, JNK
and p38 were detected by western blot analysis after 8-h PD
treatment. GAPDH was used as an internal control. PD, polydatin;
NOX5, NADPH oxidase 5; ROS, reactive oxygen species; si-, small
interfering; p-, phosphorylated; ns, not significant
(P>0.05). |
Discussion
Despite recent advancements in understanding the
molecular, pathological and biological aspect of NSCLC, it remains
a devastating disease with limited options for effective treatments
(39). Additionally, the main
obstacle of chemotherapy and targeted therapy is inevitable drug
resistance (40). Cisplatin-based
chemotherapies were widely recognized as the standard treatment
regimen for numerous types of cancers, including NSCLC. However,
emergence of drug resistance and systemic cytotoxicity limited its
application (41).
A number of studies have reported that natural
products exert prominent antitumor activity by increasing
generation of ROS. Isoalantolactone inhibited prostate cancer cell
proliferation by inducing production of ROS, activating ER stress
and inhibiting the STAT3 signaling pathways (42). Celastrol, a Tripterygium
wilfordii extract, exerted anti-NSCLC activities by increasing
generation of ROS, disrupting mitochondrial membrane potential, and
promoting mitochondrial fission (43). Accumulating evidences have
suggested that combined treatment with natural products and
cisplatin exerted synergetic antitumor activities (44,45). Thus, combined therapeutic
strategies with natural products and chemotherapeutic agents might
overcome drug resistance and minimize cytotoxicity.
In the present study, the unrecognized role of PD in
NSCLC was comprehensively analyzed. It was found for the first
time, to the best of our knowledge, that PD stimulated ROS-mediated
ER stress by targeting NOX5, thereby exerting antitumor activity in
NSCLC. Furthermore, it was revealed that the combination of PD and
cisplatin synergistically enhanced anti-NSCLC activity through the
activation of ROS-mediated ER stress, and the JNK and p38 MAPK
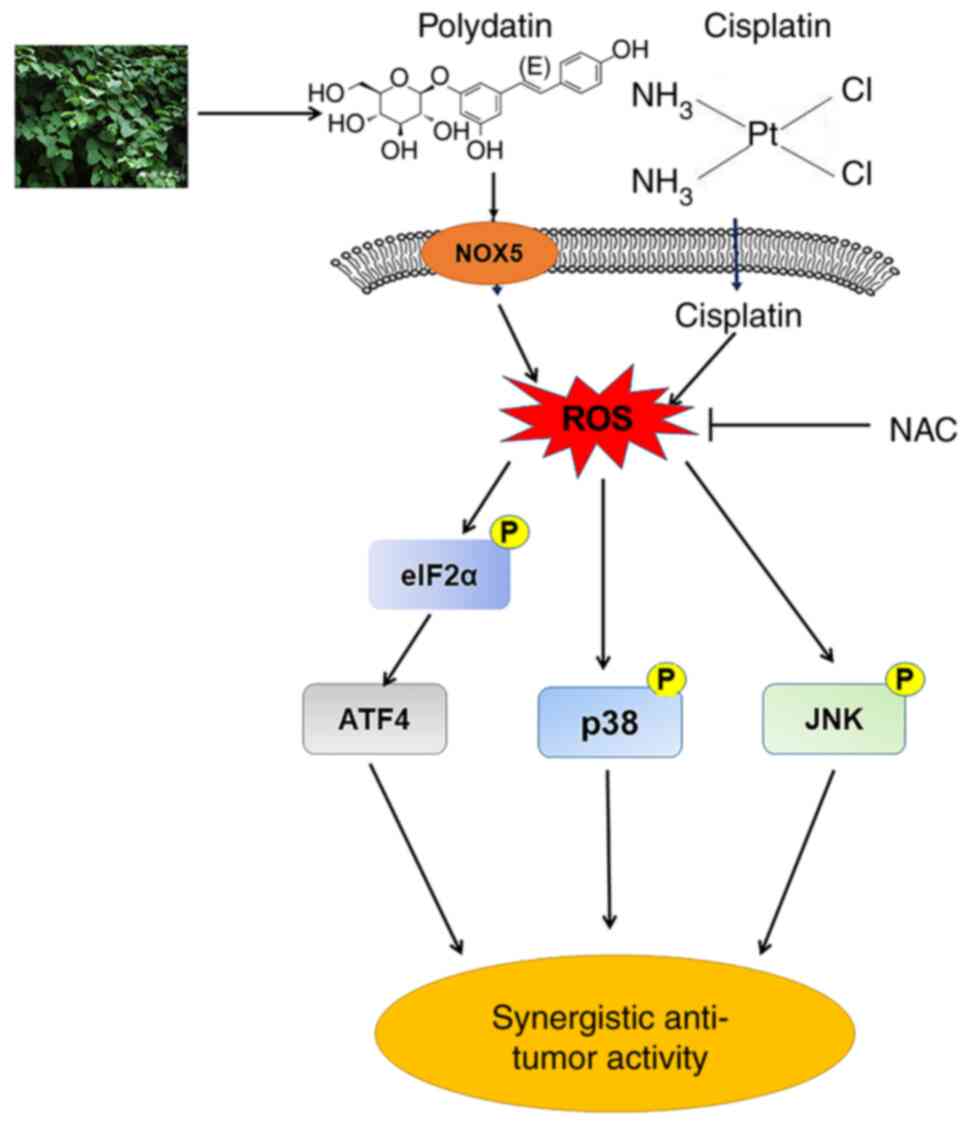
signaling pathways (Fig. 9).
These results highlight the potential therapeutic implications of
PD in NSCLC and provide novel insights suggesting that combination
therapy involving PD and cisplatin may be a promising approach for
the treatment of NSCLC.
PD, a small natural compound from Polygonum
cuspidatum, exerts prominent antitumor activities in various
cancers by inducing apoptosis (27-29). PD inhibited nasopharyngeal
carcinoma cell proliferation by inhibiting ROS-mediated Akt
signaling and triggering ER stress-mediated apoptotic pathways
(30). PD has also been reported
to inhibit Nrf2 protein expression and promote generation of ROS in
triple-negative breast cancer cells (46). Additionally, PD decreased the
mitochondrial membrane potential and increased generation of ROS,
resulting in inducing leukemia cell apoptosis and cell cycle arrest
(47). However, underlying
molecular mechanisms of PD and its effects on cisplatin-mediated
antitumor activity in NSCLC are unclear. In the present study, it
was found that PD synergistically enhanced antitumor activity of
cisplatin in NSCLC by promoting generation of ROS. Importantly,
treatment of normal human cells with high dose of PD showed only
minor cytotoxicity when compared with NSCLC cells, suggesting high
druggable potential of PD in NSCLC. Increased production of ROS
induces abnormal unfolded protein expression in the ER, and
triggers the ER stress response (32). Additionally, Protein kinase R
(PKR)-like ER kinase (PERK)-mediated p-eIF2α-ATF4 pathway
activation was frequently observed under ER stress (33). It was demonstrated that combined
treatment with PD and cisplatin markedly increased the expression
of ER stress-related proteins (ATF4 and p-eIF2α), as compared with
single treatment alone. Moreover, pre-treatment with NAC
effectively attenuated these effects. These results suggested that
PD potentiates antitumor activities of cisplatin by partly
stimulating ROS-mediated ER stress.
Accumulating evidences have shown that ROS-mediated
activation of JNK and p38 MAPK pathways promoted cell apoptosis
(36,48). Nevertheless, association between
the JNK/p38 MAPK signaling pathways and PD induced cell death, and
underlying molecular mechanisms of combined treatment with PD and
cisplatin in NSCLC are largely unknown. The present study
demonstrated that combined treatment consistently increased p-JNK
and p-p38 expression levels by promoting ROS production both in
vitro and in vivo, suggesting that ROS-mediated ER
stress, and the JNK and p38 MAPK pathways contributed to the
synergistic antitumor activity of combined treatment with PD and
cisplatin in NSCLC.
It has been demonstrated that PD treatment
consistently induces generation of ROS in NSCLC cells. However,
direct target of PD and its underlying molecular mechanisms remain
elusive. NOX5, a member of NOXs, plays critical role in regulating
the redox balance in cells. Accumulating evidences suggested that
NOX5 activation is closely involved in production of intracellular
ROS (49,50). NOX5 acts as a either tumor
suppressor or oncogene in a context-dependent manner (24,49). In esophageal squamous cell
carcinoma (ESCC), Src-mediated NOX5 activation was correlated to
the poor prognosis of ESCC (49).
Inversely, DHTS-induced activation of NOX5 inhibited proliferation
of breast CSCs by inhibiting the ROS-mediated STAT3 pathway
(24). However, no studies have
reported the association between PD and NOX5 on production of ROS
in cancer cells. In the present study, it was demonstrated for the
first time to the best of our knowledge, that PD exerted antitumor
activity by promoting expression of NOX5 and subsequent generation
of ROS. Additionally, knockdown of NOX5 significantly attenuated
the antitumor activity of PD and its effects on the activation of
ER stress and the MAPK pathways by inhibiting production of ROS.
These findings suggested a pivotal role of NOX5 in PD-mediated
NSCLC cell death. However, the detailed regulatory mechanisms of PD
on the JNK and p38 MAPK pathways require further elucidation.
In conclusion, PD enhanced the antitumor activities
of cisplatin in NSCLC by activating ROS-mediated ER stress, and the
JNK and p38 MAPK pathways. Mechanistically, PD promoted generation
of ROS in NSCLC cells by inducing expression of NOX5. The present
study indicated that the combined therapy with PD and cisplatin
might be an effective therapeutic strategy for some patients with
NSCLC.
Supplementary Data
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RC conceived the study and designed the research.
SW, QZ, SL, JK, JZ, AO and YS performed the in vitro
experiments. SW, QZ, JW, HS, LN, YY and XT performed mice xenograft
experiments. SW and QZ contributed to the data acquisition. WZ, YZ
and HL analyzed the data. RC, WZ and SW wrote and edited the
manuscript. RC and WZ supervised the research. All authors read and
approved the final manuscript. SW, QZ and WZ confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
All animal experiments were carried out in
accordance with the Wenzhou Medical University's Institutional
Animal Care and Use Committee (IACUC) guidelines (approval no.
xmsq2022-0602; Wenzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 81672305), the Health Commission of
Zhejiang (grant no. 2022RC292) and the Natural Science Foundation
of Zhejiang (grant no. LZ22H160006).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ettinger DS, Akerley W, Borghaei H, Chang
AC, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Ganti AK,
Govindan R, et al: Non-small cell lung cancer. J Natl Compr Canc
Netw. 10:1236–1271. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zarogoulidis K, Zarogoulidis P, Darwiche
K, Boutsikou E, Machairiotis N, Tsakiridis K, Katsikogiannis N,
Kougioumtzi I, Karapantzos I, Huang H and Spyratos D: Treatment of
non-small cell lung cancer (NSCLC). J Thorac Dis. 5(Suppl 4):
S389–S396. 2013.PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bunn PA Jr: The expanding role of
cisplatin in the treatment of non-small-cell lung cancer. Semin
Oncol. 16(4 Suppl 6): S10–S21. 1989.
|
|
6
|
Minami D, Takigawa N, Takeda H, Takata M,
Ochi N, Ichihara E, Hisamoto A, Hotta K, Tanimoto M and Kiura K:
Synergistic effect of olaparib with combination of cisplatin on
PTEN-deficient lung cancer cells. Mol Cancer Res. 11:140–148. 2013.
View Article : Google Scholar
|
|
7
|
Cui Z, Li D, Zhao J and Chen K: Falnidamol
and cisplatin combinational treatment inhibits non-small cell lung
cancer (NSCLC) by targeting DUSP26-mediated signal pathways. Free
Radic Biol Med. 183:106–124. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han Y, Shi J, Xu Z, Zhang Y, Cao X, Yu J,
Li J and Xu S: Identification of solamargine as a cisplatin
sensitizer through phenotypical screening in cisplatin-resistant
NSCLC organoids. Front Pharmacol. 13:8021682022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Zhu X, Zhang Y, Xiang J and Chen H:
Arsenic trioxide exerts synergistic effects with cisplatin on
non-small cell lung cancer cells via apoptosis induction. J Exp
Clin Cancer Res. 28:1102009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xue DF, Pan ST, Huang G and Qiu JX: ROS
enhances the cytotoxicity of cisplatin by inducing apoptosis and
autophagy in tongue squamous cell carcinoma cells. Int J Biochem
Cell Biol. 122:1057322020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kleih M, Böpple K, Dong M, Gaißler A,
Heine S, Olayioye MA, Aulitzky WE and Essmann F: Direct impact of
cisplatin on mitochondria induces ROS production that dictates cell
fate of ovarian cancer cells. Cell Death Dis. 10:8512019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McWhinney SR, Goldberg RM and McLeod HL:
Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 8:10–16.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Song Z, Liu Y, Ma X, Wang W, Ke Y,
Xu Y, Yu D and Liu H: Identification of ferroptosis as a novel
mechanism for antitumor activity of natural product derivative a2
in gastric cancer. Acta Pharm Sin B. 11:1513–1525. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin J, Qiu S, Wang P, Liang X, Huang F, Wu
H, Zhang B, Zhang W, Tian X, Xu R, et al: Cardamonin inhibits
breast cancer growth by repressing HIF-1α-dependent metabolic
reprogramming. J Exp Clin Cancer Res. 38:3772019. View Article : Google Scholar
|
|
15
|
Wang L, Wang C, Tao Z, Zhao L, Zhu Z, Wu
W, He Y, Chen H, Zheng B, Huang X, et al: Curcumin derivative WZ35
inhibits tumor cell growth via ROS-YAP-JNK signaling pathway in
breast cancer. J Exp Clin Cancer Res. 38:4602019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu Y, Chen D, Wu T, Lin H, Ni L, Sui H,
Xiao S, Wang C, Jiang S, Pan H, et al: Dihydroartemisinin enhances
the anti-tumor activity of oxaliplatin in colorectal cancer cells
by altering PRDX2-reactive oxygen species-mediated multiple
signaling pathways. Phytomedicine. 98:1539322022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye J, Piao H, Jiang J, Jin G, Zheng M,
Yang J, Jin X, Sun T, Choi YH, Li L and Yan G: Polydatin inhibits
mast cell-mediated allergic inflammation by targeting PI3K/Akt,
MAPK, NF-κB and Nrf2/HO-1 pathways. Sci Rep. 7:118952017.
View Article : Google Scholar
|
|
18
|
Hogg SJ, Chitcholtan K, Hassan W, Sykes PH
and Garrill A: Resveratrol, acetyl-resveratrol, and polydatin
exhibit antigrowth activity against 3D cell aggregates of the
SKOV-3 and OVCAR-8 ovarian cancer cell lines. Obstet Gynecol Int.
2015:2795912015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen S, Tao J, Zhong F, Jiao Y, Xu J, Shen
Q, Wang H, Fan S and Zhang Y: Polydatin down-regulates the
phosphorylation level of Creb and induces apoptosis in human breast
cancer cell. PLoS One. 12:e01765012017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang CQ, Ma LL, Lv ZD, Feng F, Chen Z and
Liu ZD: Polydatin induces apoptosis and autophagy via STAT3
signaling in human osteosarcoma MG-63 cells. J Nat Med. 74:533–544.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao W, Chen Z and Guan M: Polydatin
enhances the chemosensitivity of osteosarcoma cells to paclitaxel.
J Cell Biochem. 120:17481–17490. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Quagliariello V, Berretta M, Buccolo S,
Iovine M, Paccone A, Cavalcanti E, Taibi R, Montopoli M, Botti G
and Maurea N: Polydatin reduces cardiotoxicity and enhances the
anticancer effects of sunitinib by decreasing pro-oxidative stress,
pro-inflammatory cytokines, and NLRP3 inflammasome expression.
Front Oncol. 11:6807582021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: Physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim SL, Choi HS, Kim JH, Jeong DK, Kim KS
and Lee DS: Dihydrotanshinone-induced NOX5 activation inhibits
breast cancer stem cell through the ROS/Stat3 signaling pathway.
Oxid Med Cell Longev. 2019:92964392019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang Z, Su Q, Li W, Ren H, Huang H and
Wang A: Suppressed mitochondrial respiration via NOX5-mediated
redox imbalance contributes to the antitumor activity of anlotinib
in oral squamous cell carcinoma. J Genet Genomics. 48:582–594.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Bae H, Lee W, Song J, Hong T, Kim MH, Ham
J, Song G and Lim W: Polydatin counteracts 5-fluorouracil
resistance by enhancing apoptosis via calcium influx in colon
cancer. Antioxidants (Basel). 10:14772021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bai L, Ma Y, Wang X, Feng Q, Zhang Z, Wang
S, Zhang H, Lu X, Xu Y, Zhao E and Cui H: Polydatin inhibits cell
viability, migration, and invasion through suppressing the c-Myc
expression in human cervical cancer. Front Cell Dev Biol.
9:5872182021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bang TH, Park BS, Kang HM, Kim JH and Kim
IR: Polydatin, a glycoside of resveratrol, induces apoptosis and
inhibits metastasis oral squamous cell carcinoma cells in vitro.
Pharmaceuticals (Basel). 14:9022021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu H, Zhao S, Zhang Y, Wu J, Peng H, Fan
J and Liao J: Reactive oxygen species-mediated endoplasmic
reticulum stress and mitochondrial dysfunction contribute to
polydatin-induced apoptosis in human nasopharyngeal carcinoma CNE
cells. J Cell Biochem. 112:3695–3703. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64. 2018.
View Article : Google Scholar
|
|
32
|
Verfaillie T, Rubio N, Garg AD, Bultynck
G, Rizzuto R, Decuypere JP, Piette J, Linehan C, Gupta S, Samali A
and Agostinis P: PERK is required at the ER-mitochondrial contact
sites to convey apoptosis after ROS-based ER stress. Cell Death
Differ. 19:1880–1891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Balsa E, Soustek MS, Thomas A, Cogliati S,
García-Poyatos C, Martín-García E, Jedrychowski M, Gygi SP,
Enriquez JA and Puigserver P: ER and nutrient stress promote
assembly of respiratory chain supercomplexes through the PERK-eIF2α
axis. Mol Cell. 74:877–890.e6. 2019. View Article : Google Scholar
|
|
34
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar
|
|
36
|
Kwak AW, Lee MJ, Lee MH, Yoon G, Cho SS,
Chae JI and Shim JH: The 3-deoxysappanchalcone induces ROS-mediated
apoptosis and cell cycle arrest via JNK/p38 MAPKs signaling pathway
in human esophageal cancer cells. Phytomedicine. 86:1535642021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang WC, Li X, Liu J, Lin J and Chung
LWK: Activation of androgen receptor, lipogenesis, and oxidative
stress converged by SREBP-1 is responsible for regulating growth
and progression of prostate cancer cells. Mol Cancer Res.
10:133–142. 2012. View Article : Google Scholar
|
|
38
|
Park S, Oh SS, Lee KW, Lee YK, Kim NY, Kim
JH, Yoo J and Kim KD: NDRG2 contributes to cisplatin sensitivity
through modulation of BAK-to-Mcl-1 ratio. Cell Death Dis. 9:302018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus Docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rotow J and Bivona TG: Understanding and
targeting resistance mechanisms in NSCLC. Nat Rev Cancer.
17:637–658. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fennell DA, Summers Y, Cadranel J, Benepal
T, Christoph DC, Lal R, Das M, Maxwell F, Visseren-Grul C and Ferry
D: Cisplatin in the modern era: The backbone of first-line
chemotherapy for non-small cell lung cancer. Cancer Treat Rev.
44:42–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen W, Li P, Liu Y, Yang Y, Ye X, Zhang F
and Huang H: Isoalantolactone induces apoptosis through
ROS-mediated ER stress and inhibition of STAT3 in prostate cancer
cells. J Exp Clin Cancer Res. 37:3092018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu M, Fan Y, Li D, Han B, Meng Y, Chen F,
Liu T, Song Z, Han Y, Huang L, et al: Ferroptosis inducer erastin
sensitizes NSCLC cells to celastrol through activation of the
ROS-mitochondrial fission-mitophagy axis. Mol Oncol. 15:2084–2105.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sioud F, Amor S, Toumia IB, Lahmar A,
Aires V, Chekir-Ghedira L and Delmas D: A new highlight of Ephedra
alata decne properties as potential adjuvant in combination with
cisplatin to induce cell death of 4t1 breast cancer cells in vitro
and in vivo. Cells. 9:3622020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Araújo RF Jr, Soares LA, da Costa Porto
CR, de Aquino RG, Guedes HG, Petrovick PR, de Souza TP, Araújo AA
and Guerra GC: Growth inhibitory effects of Phyllanthus niruri
extracts in combination with cisplatin on cancer cell lines. World
J Gastroenterol. 18:4162–6168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li J, Zhang J, Zhu Y, Afolabi LO, Chen L
and Feng X: Natural compounds, optimal combination of brusatol and
polydatin promote anti-tumor effect in breast cancer by targeting
Nrf2 signaling pathway. Int J Mol Sci. 24:82652023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cao WJ, Wu K, Wang C and Wan DM:
Polydatin-induced cell apoptosis and cell cycle arrest are
potentiated by Janus kinase 2 inhibition in leukemia cells. Mol Med
Rep. 13:3297–3302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cao X, Fu M, Bi R, Zheng X, Fu B, Tian S,
Liu C, Li Q and Liu J: Cadmium induced BEAS-2B cells apoptosis and
mitochondria damage via MAPK signaling pathway. Chemosphere.
263:1283462021. View Article : Google Scholar
|
|
49
|
Chen J, Wang Y, Zhang W, Zhao D, Zhang L,
Fan J, Li J and Zhan Q: Membranous NOX5-derived ROS oxidizes and
activates local Src to promote malignancy of tumor cells. Signal
Transduct Target Ther. 5:1392020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
da Silva JF, Alves JV, Silva-Neto JA,
Costa RM, Neves KB, Alves-Lopes R, Carmargo LL, Rios FJ, Montezano
AC, Touyz RM and Tostes RC: Lysophosphatidylcholine induces
oxidative stress in human endothelial cells via NOX5
activation-implications in atherosclerosis. Clin Sci (Lond).
135:1845–1858. 2021. View Article : Google Scholar : PubMed/NCBI
|