1. Introduction
Cancer remains a serious threat to human health and
the incidence rates continue to rise. In recent years, the number
of new cases and cancer-associated deaths has shown a significant
upward trend (1-3). The occurrence of cancer is a
multi-factor, multi-step and complex process, and its etiology has
not been fully clarified; however, it is well acknowledged that it
is closely related to various factors, including smoking,
infection, occupational exposures, environmental pollution, an
unhealthy diet and genetic factors (4-8).
With the rapid advances in life sciences and technology, a more
in-depth understanding of tumors has been gained. The search for
more effective treatments to improve the survival rates of patients
with cancer remains a central topic of research in the medical
industry.
mRNA vaccines are a type of nucleic acid preparation
that can transduce gene sequences of exogenous target antigens into
cells via a specific delivery system by means of transcription and
translation, and the transcribed proteins stimulate the body to
produce specific immunological responses, thus enabling the body to
obtain immune protection (9).
mRNA vaccines are the new generation of vaccines after inactivated
vaccines, live attenuated vaccines, subunit vaccines and viral
vector vaccines. With continuous improvements in RNA-based
technologies, this innovative treatment model is predicted to
supplant traditional vaccines and may have the ability to address
the issues associated with small molecules and antibody therapy. In
addition to being used in infectious diseases, mRNA vaccines also
provide more effective and longer-lasting treatment opportunities
for diseases including cancer, rare diseases and nervous system
diseases (10). mRNA vaccines not
only cover a variety of tumor antigens at one time but also
activate a wider range of T-cell responses through the simultaneous
delivery of human leukocyte antigen (HLA)-I and HLA-II molecules,
resulting in a more comprehensive multi-angle attack. Thus, mRNA
vaccines have significant anti-cancer potential. Currently, there
are dozens of clinical trials related to mRNA tumor vaccines around
the world, which include (but are not limited to) melanoma,
pancreatic cancer and colorectal cancer, and in certain trials,
mRNA vaccines are combined with other tumor-immunity drugs
(11-13). The recently developed mRNA-4157
vaccine, which has been declared a 'Breakthrough Therapy
Designation' by the US Food and Drug Administration (FDA) and a
'Priority Medicines' Programme by the European Medicines Agency, is
expected to be the first mRNA tumor vaccine on the market (14).
The present article reviews the current treatment
methods for cancer as well as the development and current status of
mRNA vaccines. On this basis, the value of mRNA vaccines in tumor
treatment is also discussed.
2. Treatment of cancer
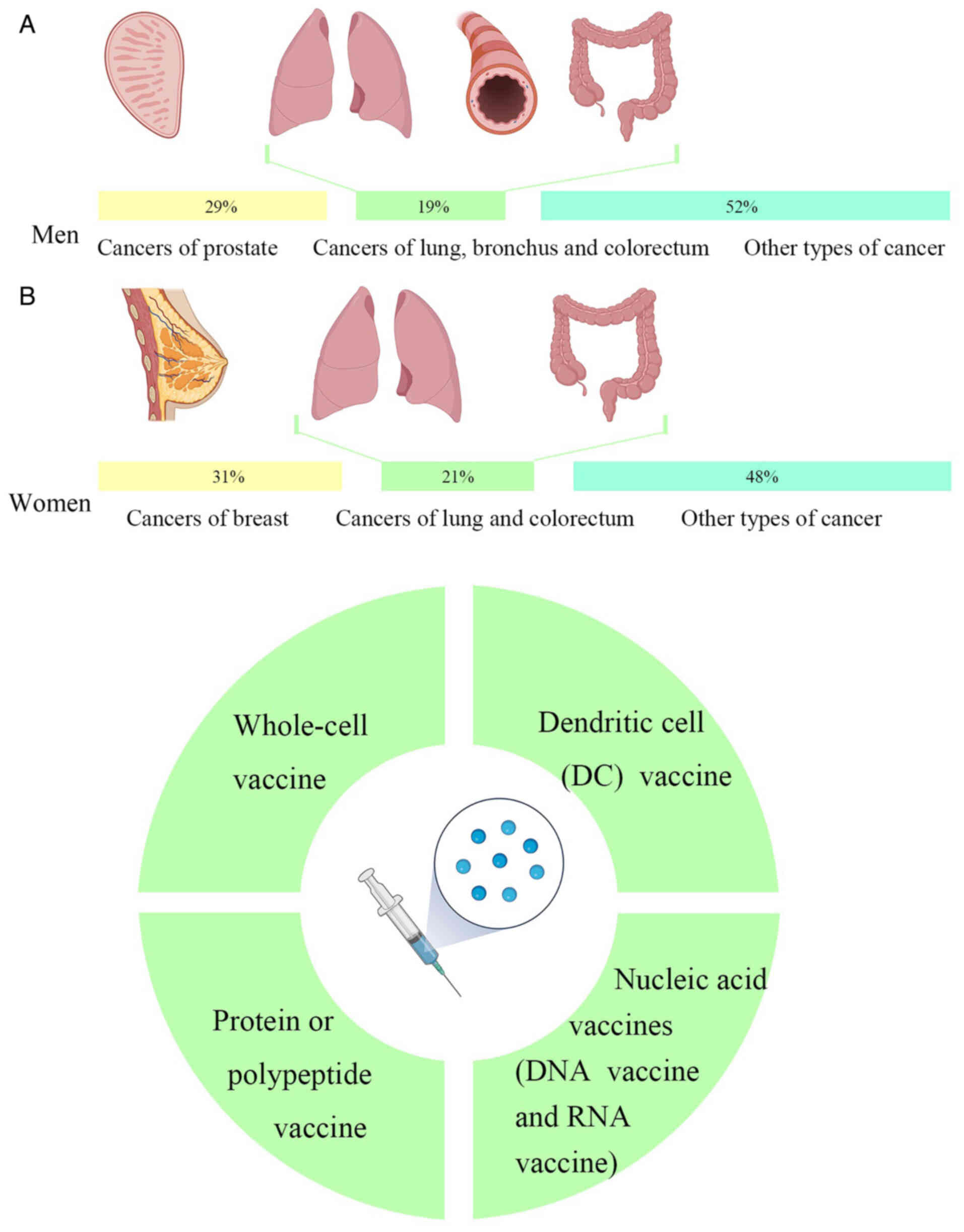
Cancer is a major global public health problem.
According to the latest global cancer data released by the American
Cancer Society, it is estimated that there were 1,958,310 new
cancer cases and 609,820 deaths in the US in 2023. Amongst the
different types of cancer, cancers of the prostate, lung, bronchus
and colorectum account for almost half (48%) of all cases in men,
with prostate cancer alone accounting for 29% of all diagnoses. For
women, breast, lung and colorectal cancers account for 52% of all
newly diagnosed cancers, with breast cancer (BCa) alone accounting
for 31% of cancers in women. Of the projected deaths from cancer,
the highest number of deaths were from lung, prostate and
colorectal cancers in men and from lung, breast and colorectal
cancers in women (Fig. 1A)
(15).
Due to the continuous progress of medical
approaches, there are an ever-increasing number of methods for
treating cancer. According to the type and stage of cancer and the
specific conditions of a patient, choosing the appropriate
treatment can prolong the survival time and improve the quality of
life to a significant extent. The methods of cancer treatment
primarily include surgery, chemotherapy, radiotherapy and
immunotherapy. However, a single treatment is insufficient for the
treatment of cancer and the simple addition of a therapeutic
regimen cannot achieve the goal. Thus, a reasonable and
comprehensive use of a variety of methods is required to better
control and eliminate the tumor; that is, a current comprehensive
multidisciplinary approach should be adopted where possible
(16).
With the development of vaccinations being promoted
by emerging innovations of the digital age, vaccinating a patient
with individual tumor mutations may become the first truly
personalized treatment for cancer (17). All types of vaccines that can
stimulate the body to produce agents that counteract pathogenic
factors for tumorigenesis or activate specific immunity in order to
counteract the immune escape effect of cancers and treat tumors can
be called tumor vaccines (18).
Prophylactic vaccines, which are similar to traditional vaccines,
primarily target pathogenic microorganisms directly related to
cancer, such as Human papillomavirus (HPV) and hepatitis B virus
(HBV). In addition, there are therapeutic vaccines for patients who
already have cancer and these promote the establishment of a
lasting immune memory and a long-term anti-tumor response by
inducing immune responses primarily against 'autoantigens' present
in tumors (19).
At present, the primary types of tumor vaccines are
as follows: i) Whole-cell vaccines; ii) dendritic cell (DC)
vaccines; iii) protein or polypeptide vaccines; and iv) nucleic
acid vaccines, including DNA vaccines and RNA vaccines (Fig. 1B).
3. Overview of mRNA vaccines
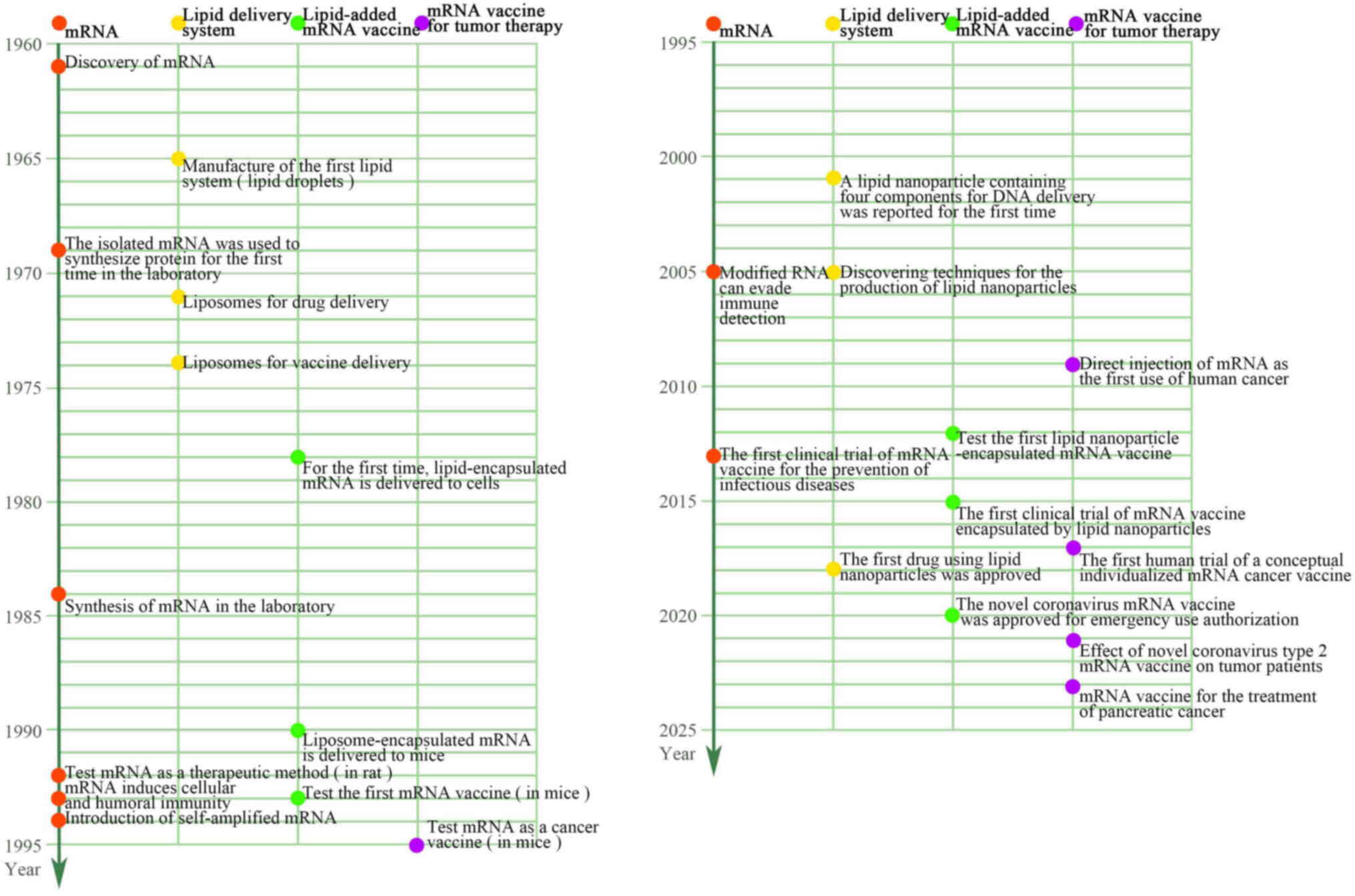
History of mRNA vaccines
During the Coronavirus (CoV) disease 2019 (COVID-19)
pandemic, mRNA vaccines gained attention as a cutting-edge
technology used by several pharmaceutical companies to create
vaccinations (20,21). Of note, the first potential
vaccine to enter phase I clinical trials was an mRNA vaccine.
Brenner et al (22) first
identified mRNAs as an intermediary genetic element in the central
nervous system in 1961. Malone et al (23) in 1989 discovered that mRNAs could
be successfully transfected and expressed in a variety of
eukaryotic cells when encased in a cationic lipid
{N-[1-(2,3-dioleyloxy) propyl]-N,N,N-trimethylammonium chloride},
and this was when the notion of mRNA-based medications was first
conceived. The 1990s saw a boom in mRNA vaccine research, with
preclinical trials being applied to a range of illnesses, including
infectious diseases and cancer. The first successful effort at mRNA
in vivo expression was performed in 1990 when direct
injection of in vitro-transcribed mRNA was sufficient to
introduce the mRNA into mouse skeletal muscle cells (24). This demonstrated the viability of
mRNA vaccines. In 1992, Jirikowski et al (25) discovered that injecting mRNAs
encoding oxytocin and vasopressin into genetically mutated diabetic
insipidus mice cured the condition within a few hours of injection
for a short period of time. An in vitro-generated mRNA
vaccine encoding the influenza virus's nucleoprotein to activate
cytotoxic T-cells of mice was reported by Martinon et al
(26) in 1993. The first
researchers to find that DCs pulsed with mRNA was a viable approach
to trigger T-cell responses were Boczkowski et al (27) in 1996. Zhou et al (28), in 1999, showed the potential of
mRNA vaccines against cancers by directly injecting glycoprotein
100 mRNA encapsulated in hemagglutinin virus of Japan-liposomes
into the spleen, which resulted in tumor growth restriction and a
longer survival time in a mouse model of melanoma. The safety,
effectiveness and industrial manufacturing ability of mRNA vaccines
have improved over the last several decades as research has
advanced and experimental procedures have improved (29). Currently, several mRNA vaccines
are being tested in clinical settings or are readily accessible to
combat cancer and several infectious diseases, including the Zika
virus, cytomegalovirus, influenza virus, metapneumovirus and
parainfluenza virus (30). In
response to the need for a timely and efficient vaccination, there
has been a surge in the research and development of nucleic
acid-based vaccines since the worldwide severe acute respiratory
syndrome (SARS) CoV-2 pandemic (21,31). Moreover, the market value of mRNA
vaccines has grown to tens of billions of dollars, indicating a
promising future for the development of mRNA-based medications,
particularly mRNA vaccines (32)
(Fig. 2).
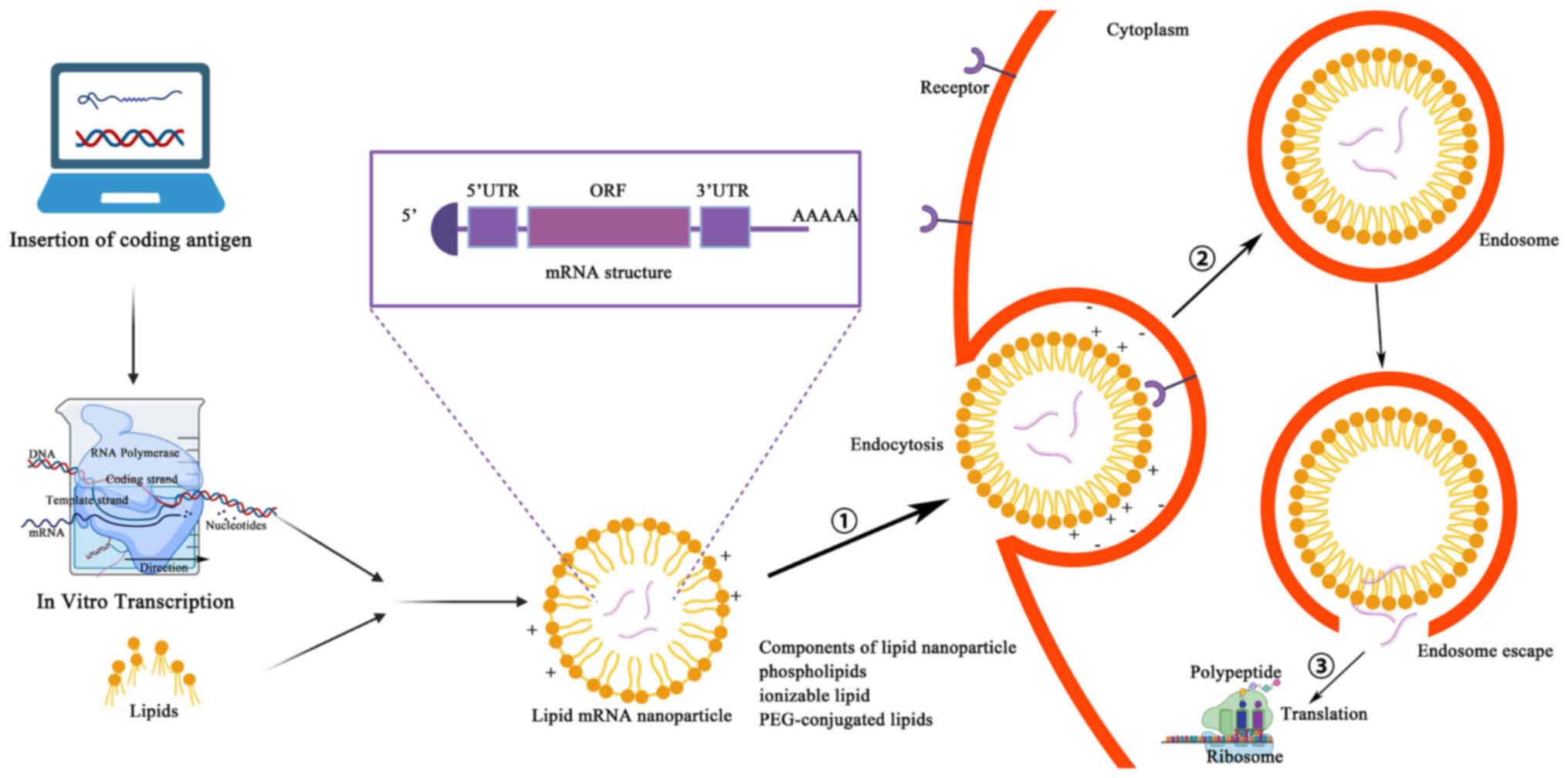
Mechanism of mRNA vaccines
Manufacturing an mRNA vaccine involves several
relatively complex steps. First, the desired mRNA sequence needs to
be designed and synthesized; this sequence usually includes the
nucleotide sequence encoding the target antigen or another
therapeutic protein, and should also contain the appropriate 5' end
cap and 3' end tail sequences to improve mRNA stability and
translation efficiency (33).
Through in vitro transcription, the corresponding mRNA is
synthesized, with the in vitro transcription system
typically consisting of RNA polymerase, buffer solution, nucleoside
triphosphates, zinc ions and cofactors to facilitate transcription.
Mixing the DNA template with the other components of the in
vitro transcription system constitutes a reaction mixture
containing all the necessary components for synthesizing mRNA. This
reaction mixture is then placed into appropriate reaction tubes and
subjected to an in vitro transcription reaction under the
appropriate conditions. During this reaction, the RNA polymerase
identifies the DNA template and uses it to synthesize mRNA
(34). Synthetic mRNAs need to be
purified and modified to remove impurities and improve stability
and certain mRNA vaccines may need to be encapsulated in liposomes
to improve their stability and promote cellular uptake in
vivo. Liposomes are usually composed of constituents such as
phospholipids and cholesterol, which can form tiny lipid vesicles
in the aqueous phase with a lipid bilayer structure. Surfactants
(such as polyethylene glycol) can be added to improve the stability
and cellular uptake efficiency of liposomes. The synthesized mRNA
is mixed with liposomes to form liposome-mRNA complexes. These
complexes can be transfected into target cells by an endocytotic
mechanism, whereby the cell membrane forms invaginations to carry
the liposome-mRNA complexes inside the cell. The endocytosed
vesicles encapsulate the liposome-mRNA complexes in endosomes,
which in turn fuse with other organelles in the cytoplasm and
release their contents into the cytoplasm. After the contents of
the liposome-mRNA complex are released into the cytoplasm, the mRNA
molecules are recognized and translated by intracellular ribosomes
(35,36) (Fig.
3). A fraction of this foreign mRNA eludes destruction by
ubiquitous RNase and is internalized by natural processes unique to
individual cells (such as macropinocytosis in developing DCs) into
the endosomal pathway, where it is released into the cytoplasm by
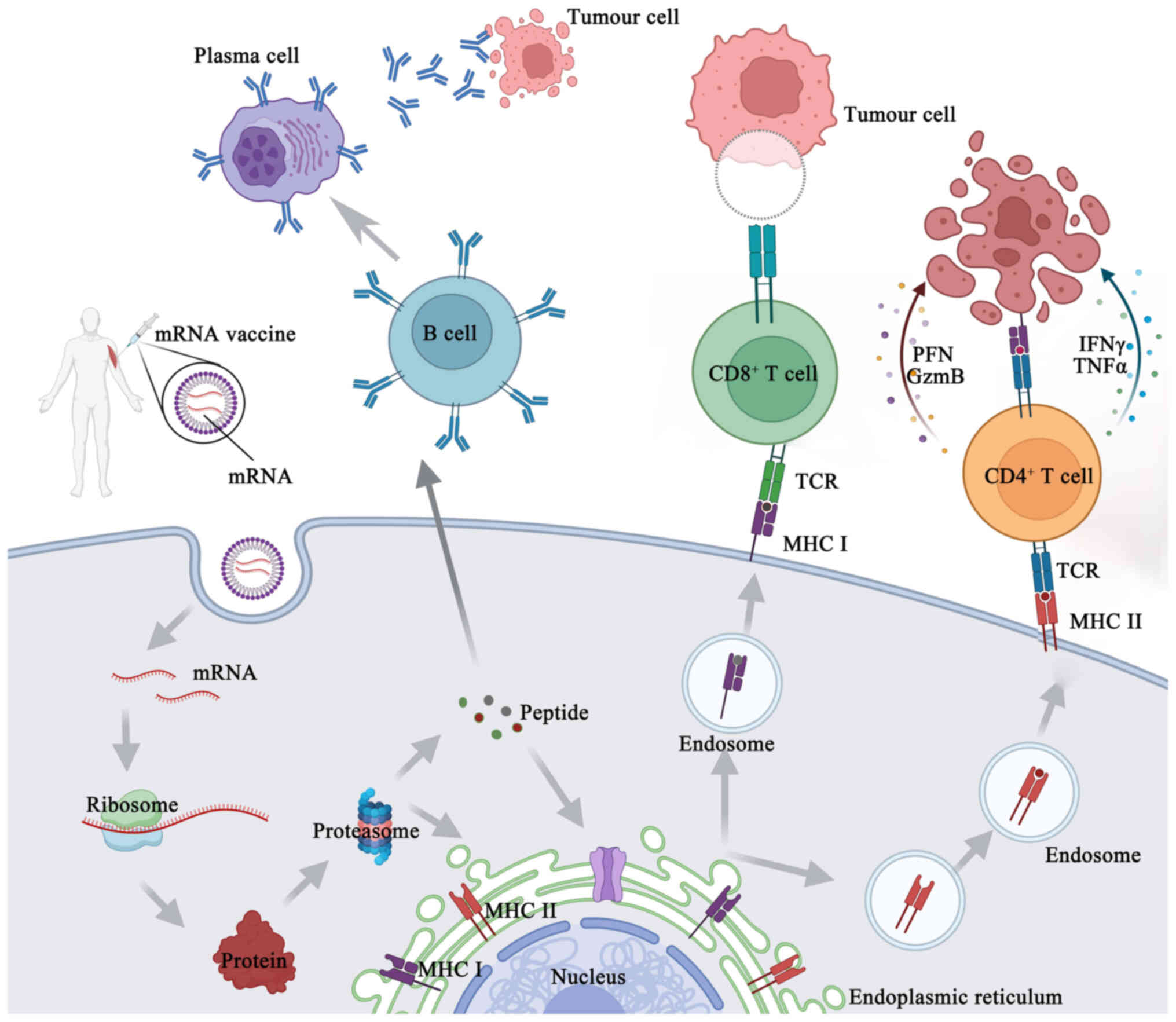
the endosomes (37). Antigenic
proteins are translated from the mRNA by the host cell's
translation machinery. Signal peptides dictate the encoded
protein's final location once it has been produced. These may be
recombinantly created to target the protein to the appropriate
cellular compartment inside the host cell, or they can be inherent
to the original protein's sequence (37). As an alternative, this encoded
protein may affect the function of nearby cells via its secretion,
and it can also affect distant organs if it is released into the
blood. Proteases in the cytoplasm break down antigenic proteins
that are important to cells, creating antigenic peptide epitopes.
The endoplasmic reticulum is where antigenic peptide epitopes are
transferred, loaded onto class I molecules of the major
histocompatibility complex (MHC I) and presented to CD8+
cytotoxic T-cells. The protein product has to be directed to MHC
class II loading compartments in antigen-presenting cells (APCs) in
order to enlist the assistance of cognate T-cells to induce a more
powerful and longer-lasting immune response (10,37). This may be achieved by adding
routing signal-encoding sequences to the mRNA. After the produced
antigen peptide epitope is loaded onto the MHC II molecule, the
antigen-specific CD4+ T-cell response is induced and the
loaded MHC II peptide epitope complex emerges on the cell surface
(33) (Fig. 4). Furthermore, via a process
called cross-priming, foreign antigens that are taken up by DCs may
also be processed and loaded onto MHC class I molecules (38). This allows for the design, in
vitro production and delivery of any desired sequence to any
kind of cell (39).
When mRNA functions as the vector of foreign genes,
it may display certain characteristics similar to those of the mRNA
virus due to its self-adjuvant impact. APCs can identify mRNAs
prior to translation and this identification triggers the
activation of pattern recognition receptors, including Toll-like
receptor (TLR)3, TLR7 and TLR8 (40). Single-stranded and double-stranded
(ds) RNA chains can be detected by TLR3, TLR7 and TLR8, while short
and long filaments of dsRNA can be detected in the cytosol by
retinoic acid-inducible gene-I (RIG-I) and melanoma
differentiation-associated protein 5 (41,42). As a result, the type I interferon
(IFN-I) pathway may be activated, chemokines and proinflammatory
cytokines may be produced and APCs may be activated, all of which
may trigger a robust adaptive response (43).
Advantages of mRNA vaccines
The mRNA tumor vaccine platform offers unique
advantages over other cancer therapies. Different from traditional
radiotherapy and chemotherapy, which are susceptible to drug
resistance, mRNA vaccines allow for the simultaneous coverage of
multiple antigens, thus overcoming vaccine resistance in cancer
treatment. The combination of mRNA vaccines and immune checkpoint
inhibitors (ICIs) can play a role in reversing the pathways that
allow for resistance. Therefore, mRNA vaccines are expected to be a
useful adjunct to ICIs and the combination of the two may allow for
the implementation of precise and individualized treatment for
patients (44). In addition,
conventional cancer treatments can cause significant damage to
normal human cells, whereas mRNA vaccines can target tumor cells
more precisely. Furthermore, the transient nature of mRNA
expression ensures that antigenic stimulation is temporary, which
effectively reduces the possibility of chronic inflammation or
autoimmune reactions (45).
Compared with other tumor vaccine platforms, mRNA vaccines are
highly immunogenic, stimulating a broader immune response in
patients.
While mRNA tumor vaccines show clinical potential,
their economic value is also noteworthy. As a novel therapy, mRNA
vaccines have advantages over other cell and gene therapy products
due to their convenience and low cost of production. Other
gene/cell therapies already on the market, such as chimeric
antibody receptor T-cell immunotherapy (CAR-T), are currently
priced at millions of dollars, which poses a significant financial
burden to patients and society (46,47). This is due to the fact that CAR-T
requires a series of processes such as cell extraction,
modification, in vitro culture and re-infusion for patients,
and it is difficult to scale up the production, thus making it
difficult to reduce the cost of a single product. By contrast, mRNA
vaccines are directly transcribed in vitro. The synthesis
and production steps for mRNA vaccines are standardized, allowing
for the rapid and large-scale production of the product, which
would result in a significant cost advantage of the product.
Therefore, mRNA vaccines are expected to provide a boon to less
economically privileged cancer patients and thus have significant
economic potential in the current medical environment.
Given the several benefits, mRNA technology is a
more appealing option than DNA or even conventional vaccinations.
In vitro transcription (IVT) synthesis eliminates the need
for cells and the regulatory barriers that go along with them,
making mRNA production unquestionably easier, faster and cleaner
than large-scale protein production and purification (48,49), in contrast to conventional
vaccination approaches. The accuracy of antigen design allows for a
quicker response to new risks of pandemics and epidemics in a
timely and efficient manner (50). mRNA is precise because it only
produces certain antigens and triggers a tailored immune response,
in contrast to attenuated or inactivated vaccines. mRNA may trigger
and/or promote an adaptive immune response by binding to pattern
recognition receptors without the need for adjuvants, while subunit
vaccines need adjuvants to elicit an immunological response.
Nucleic acid vaccines are less constrained by HLA types and are
more likely to elicit a wider T-cell response than peptide
vaccines, as they can encode full-length tumor antigens, which
allows APCs to cross-present or present multiple epitopes with both
class I and II patient-specific HLA at the same time (49,51).
The following describes the ways in which mRNA
vaccines are superior to DNA vaccines. The nuclear envelope barrier
that prevents DNA vaccines from working is removed, as only mRNA
has to be transcribed in the cytoplasm, while DNA must be
translated in the nucleus (52).
Consequently, both mitotic and non-mitotic cells may be
successfully transfected with mRNA vaccines (53). Furthermore, mRNA is rarely
integrated into genomic DNA and does not undergo insertion
mutation. Third, since mRNA is rapidly broken down by biological
processes and leaves no trace after 2-3 days, the production of the
coded antigens is only temporary. The mRNA vaccine platform's
flexibility is also useful for manufacturing, since it allows for
standardization of production, as modifications to the encoded
antigen do not alter the physical/chemical properties of the mRNA
backbone. Fourth, there are fewer safety concerns regarding the
presence of viral contaminants and cell-derived impurities than
with other platforms, since production is based on an in
vitro cell-free transcription process (39). Furthermore, RNA vaccinations often
induce stronger immune responses than DNA vaccines. The reasons for
this are not entirely clear, but some theories include low
expression of DNA-sensing machinery, different expression of
nucleic acid sensing pattern recognition receptors, inefficient
delivery of DNA into human cells and the requirement that DNA
crosses both cell and nuclear membranes and is transcribed in the
nucleus in order to successfully transfect a cell (34,54).
Potential limitations of mRNA
vaccines
Although the current success of the SARS-CoV-2 mRNA
vaccine has fueled research into mRNA vaccines for cancer, there
are still limitations to the application of mRNA vaccines in
cancer, which are discussed in the following chapters.
Harsh preservation conditions
mRNA is an unstable molecule that can be easily
cleaved by biological nucleases in the environment due to the
presence of hydroxyl groups on the ribose. Stable activity can be
maintained when it is cryopreserved, but the cost of storage is too
high for economically underdeveloped regions (55). Carrier-based protectants such as
lipid nanoparticles (LNPs) can only preserve mRNA vaccines for a
maximum of 3 months (56).
Cichlidin also protects mRNA from enzymatic degradation but may
make it more difficult to translate, affecting the efficacy of the
mRNA vaccine (57,58).
Relatively low anti-tumor effect when
used alone
It is difficult to qualitatively improve the
anti-tumor effect of mRNA vaccines alone due to their poor
stability, the relatively short production cycle of the proteins,
the heterogeneity of tumor cells and the fact that the immune
microenvironment of advanced tumors is often highly
immunosuppressive. As a result, clinical studies on cancer mRNA
vaccines have shown limited progress (13).
Lack of long-term experimental
findings
Clinical trials for cancer mRNA vaccines are
currently active and generally in the early stages of development.
Clinical trials with reported results, particularly for
personalized cancer mRNA vaccines, are still limited and the
therapeutic efficacy varies widely among patients. There is still a
lack of results from long-term trials of mRNA vaccines in cancer,
so the roadmap to their use as a routine treatment for cancer is
long. It has been reported that mRNA vaccines may be associated
with certain rare and fatal thrombotic events, so the safety of
mRNA vaccine administration is also worth observing long-term
(55). In summary, it is
essential to monitor the long-term efficacy and side effects of
mRNA vaccines in cancer therapy.
Regulatory challenges and ethical
considerations
The development and production of mRNA vaccines for
personalized neoantigens is directed at the genomic data of
individuals, and as a result, the vaccines can pose regulatory and
approval challenges.
Factors influencing mRNA vaccines
Despite all of the benefits of mRNA vaccines, their
fundamental qualities nevertheless place certain restrictions on
their use in the treatment of cancer. For example, the negative
charge of both mRNA and the cell membrane makes mRNA distribution
more challenging (34,37,59,60). Skin and blood contain
extracellular ribonucleases, which readily break down mRNA
(61). Given its inherent
immunogenicity, mRNA may trigger innate immunity by activating a
downstream interferon-related pathway. Paradoxically, this inherent
immunogenicity promotes mRNA degradation, which lowers antigen
expression even though it may be used as an adjuvant-like effect to
increase the immune response (49). dsRNA contaminants are often found
in mRNA in vitro transcription products. Type I IFN
production may be enhanced by dsRNA, a simulant of RNA virus genome
replication intermediates. This further restricts the translation
of mRNA and strengthens the activation of innate immunity (62). Several techniques have been
developed to advance the science of mRNA vaccines in response to
these issues.
Delivery systems for mRNA
vaccines
It is well established that mRNA has a half-life of
~7 h and that its absorption is poor in the absence of a delivery
system (63). In addition, mRNA
is a naturally unstable molecule that is easily broken down by
endonucleases, 3' exonucleases and 5' exonucleases (64). One of the most challenging
applications of mRNA vaccines for cancer is getting the mRNA into a
sufficient number of cells with sufficiently high translation
levels. This requires highly targeted and effective mRNA delivery
mechanisms (65). Thus far, the
identified delivery vectors for cancer mRNA vaccines include LNPs,
polymeric vectors, peptide vectors, DC vectors, extracellular
vesicle vectors and hybrid vectors.
One of the most well-developed, promising and widely
used mRNA non-viral delivery methods is LNP. It is made up of four
components that function together to encase and shield the delicate
mRNA core: Cholesterol, helper phospholipids, cationic or ionizable
lipids and polyethylene glycol (PEG) ylated lipids (66,67). Cholesterol is used as a stabilizer
to increase LNP stability, ionizable cationic lipids can promote
the autonomous aggregation of mRNAs to form a particle of ~100 nm
and release mRNAs in the cytoplasm through ionization, and PEG can
extend the half-life of the LNP complex (34,68). Natural phospholipids support the
nanoparticles to form a lipid bilayer structure. Currently, there
are LNP-mRNA cancer vaccines in clinical trials, e.g.,
mRNA-4157.
Purification of mRNA vaccines
The product of in vitro transcription of mRNA
is primarily a combination of targeted mRNA, non-targeted mRNA,
nucleotides, oligonucleotides and proteins. Chromatographic methods
are often used to separate the target mRNA from other mRNA
impurities in this system, while precipitation and extraction
techniques are utilized to eliminate common impurities from the
mRNA (69,70). Type I IFN production may be
enhanced by dsRNA, a simulant of RNA virus genome replication
intermediates. Consequently, mRNA translation efficiency may be
increased and the type I IFN immune response to mRNA vaccines is
effectively reduced by purifying an mRNA in vitro synthetic
product. During IVT, dsRNA species may be decreased by either
generating RNA at a higher temperature or by lowering the
Mg2+ content (71).
The purification characteristics of dT 25-conjugated
oligonucleotide affinity support resin (dT25-OAS) were examined by
Engel et al (72); due to
its very high binding capacity, dT25-OAS is a desirable substitute
for mRNA purification on a wide scale.
Molecular stabilization of mRNA
vaccines
Increasing protein expression often involves
techniques such as modifying sequences and/or the structure to
improve mRNA stability (increase the half-life) and translation.
Methods include extension of the poly(A) tail, alterations of the
5' cap, engineering untranslated region (UTR) and open reading
frame (ORF) sequence patterns and altering the specific nucleotide
sequence (30,73,74). These changes result in the
production of considerable quantities of the protein for an
extended period of time, ranging from a few minutes to >1 week
(75-77). PERSIST-seq, a platform based on
RNA sequencing, was created recently to comprehensively
characterize the stability of intracellular mRNA of various mRNA
libraries. This platform has enabled and continues to enable
computational trials for mRNA drug enhancement (78).
5' cap modification
The 5' cap is a protective structure that may
trigger mRNA translation, prevent exonuclease cleavage and control
pre-mRNA splicing and nuclear export, amongst other functions
(79,80). The innate immune system uses the
5' cap to distinguish endogenous RNA from exogenous RNA (81). Regarding in vitro mRNA
capping, there are two standard methods. First, mRNA capping and
in vitro transcription may be accomplished by including the
normal cap analog, m7GpppG structure, into the mRNA
transcription system. Second, after the first in vitro
transcription, methyltransferases methylate Cap 0 to Cap 1 for mRNA
capping (82).
The most commonly used capping technique for mRNA
transcription in vitro is capping using cap analogs. Several
cap analogs have now been developed. The anti-reverse cap analogs
(ARCAs), which are altered inside the ribose moiety of the
m7G, are the most often reported cap analogs (83). To enhance the quality of mRNA,
additional alterations to the ARCA structure have been developed
recently. By increasing the affinity of mRNA for eIF4E, phosphorus
modification based on ARCA, for example, may increase mRNA
stability by reducing its sensitivity to decapping enzymes and
improving translation efficiency (84-87). The insertion of a novel chemically
altered cap analog into ARCA increases the mRNA's half-life by
preventing it from being decapped by mRNA decapping enzyme 2
(30). In 2018, 'CleanCap', a
co-transcriptional capping technique, was created as an additional
cap analog. Compared to first-generation cap analogs (such as mCap
and ARCA), which have cap 0 structures at lower efficiencies and
reaction yields, this was far more efficient (88).
Optimization of 5' and 3'-UTRs
The upstream (5'-UTR) and downstream (3'-UTR)
domains of the mRNA coding region include the noncoding portion of
the mRNA sequence or UTR. UTRs have several functions, including
the control of mRNA stability, subcellular localization,
translational efficiency and mRNA export from the nucleus. The mRNA
expression levels in vivo are often increased by UTR
optimization, hence UTR is a key part of cancer mRNA vaccine design
(89-92).
Codon optimization of the ORF
The ORF region is the coding region of mRNA, hence
its translation rate is unquestionably important. Therefore, the
total translation efficiency of mRNA may be maximized by selecting
the right codons in this region (93). To accomplish the goal of
optimizing the sequence, either choosing the optimal codon pair
that is common to the highly expressed protein or maintaining the
same percentage of each codon that naturally exists in the highly
expressed protein in the target cell are the methods used.
Furthermore, to speed up translation, less common codons in the ORF
are often replaced by codons with increased transfer RNA (tRNA)
abundance (94). In such an
instance, it is possible to ensure sufficient levels of tRNA during
the production of foreign mRNA and/or the increased translation of
highly expressed genes utilizing the host's codons (95). To control the translation
elongation rate, the ORF's guanine (G) and cytosine (C) content may
be optimized. Another codon optimization technique that has a
direct correlation to a higher GC content is uridine depletion.
RIG-I can identify areas rich in uridines, and when it is
activated, protein expression may be completely stopped (49).
Poly(A) tail modification
At the 3' end of an mRNA construct is a
polyadenylation region known as the poly(A) tail. It is important
for both the enzymatic stability of mRNA and its translation. To
control translational efficiency, it specifically binds to several
polyadenosyl binding proteins and cooperates with the
7-methylguanosine cap (m7Gppp) on the 5'-end of mRNA
sequences (96). According to
early research, the poly(A) tail is crucial in controlling the
stability and translation efficiency of mRNA. It has been
discovered that longer tails result in increased protein expression
levels in a variety of cell types. Shorter poly(A) sequences, on
the other hand, may encourage this closed-loop shape for effective
translation, according to research by Lima et al (97). In conclusion, to maximize the
translation efficiency of mRNA, the length of the poly(A) sequence
should be modified for various cell types.
Modified nucleotides
Certain mRNA nucleotides undergo
post-transcriptional modifications during mRNA maturation. IVT mRNA
may be synthesized using these naturally occurring modified
nucleotides, such as 5-methylcytidine and pseudouridine. IVT mRNA
exhibits better translation efficiency and stability when it
possesses modified nucleosides such as pseudouridine. This is
hypothesized to be due to the fact that nucleosides prevent TLR,
RIG-I and The RNA-dependent protein kinase R (PKR) from being
activated, making IVT mRNA undetectable to cytoplasmic TLRs such as
TLR3, TLR7 and TLR8, as well as RIG-I and PKR (98). However, it has been suggested that
unmodified mRNA vaccines can assist the immune system in the
recognition of tumor cells, in which case modifications of cancer
mRNA vaccines are not required.
Adjuvants
Adjuvants are substances, either natural or
artificial, that the immune system can easily detect and use to
augment the intended immunological response (99). The injection of its matching
adjuvant may improve the immune response to antigens, even if mRNA
vaccines themselves have a self-adjuvant effect. Adjuvant addition
to mRNAs is a popular topic of research currently.
An mRNA adjuvant called TriMix consists of three
immune-modulatory molecules: CD70, CD40 ligand and active TLR-4.
Patients with stage III or IV melanoma were administered TriMix
mRNA along with additional tumor-antigen mRNAs, and this resulted
in long-lasting clinical relief and an augmented increased immune
response (100,101). The adjuvant pulsed mRNA vaccine
NP technique for c16-r848 was recently established. This technique
significantly improved the amplification of ova-specific
CD8+, which allowed the activated T lymphocytes to
penetrate the tumor bed in vivo, something that was not
observed with the mRNA vaccine NP without adjuvant (100). The RNActive (curevac Ag)
vaccination platform and RNAdjuvant are other popular adjuvants.
Furthermore, certain mRNA delivery vectors, such as protamine and
cationic lipids, may enhance the effect of adjuvants. Pam2Cys, a
synthetic neutral fatty amino acid that can signal through the
TLR2/6 pathway, has been shown to trigger both humoral and cellular
applicative immune responses, which makes it a promising candidate
adjuvant (102). A recent study
integrated Pam2Cys into mRNA-LNP to improve the efficacy of mRNA
vaccines. In prophylactic and therapeutic tumor models using this
vaccine, CD4+ and CD8+ T-cell-dependent anti-tumor responses were
significantly enhanced, while memory anti-tumor responses were also
established. Thus, Pam2Cys is of notable value in the development
of future cancer mRNA vaccines (103).
4. Applications of mRNA vaccines in
infectious diseases
mRNA vaccines are primarily being studied for the
treatment of cancer and infectious disorders. At present, there are
~140 clinical studies assessing the use of mRNA vaccines to treat
various illnesses (39).
Self-amplifying or replicon RNA vaccines and non-replicating mRNA
vaccines are the two primary types of RNA vaccines that have been
assessed. Replicons are self-amplifying mRNAs that are produced
from RNA viruses that have had their structural viral proteins
replaced with mRNA-encoding RNA polymerases and antigens. Thus,
these mRNAs enhance immunogenicity and extend protein expression,
thereby improving efficiency (104,105).
Self-amplifying mRNAs have two ORFs, one encoding
the targeted antigen sequence and the other encoding the viral
replication mechanism, in contrast to the traditional
non-replicating IVT mRNA of 'mature' eukaryotic mRNA. This allows
for long-lasting RNA amplification in cells (49). The delivery mechanism of
non-replicating mRNA vaccines may be further identified, since it
can be administered directly into a range of anatomical areas or by
ex vivo loading of DCs (34). While there are now several
opportunities for the therapeutic use of self-amplifying mRNAs in
the prevention of infectious illnesses, its use as a cancer vaccine
is mostly restricted to preclinical research and has only seen a
small number of clinical studies conducted (13,49).
Diseases caused by viral infection
Nucleic acid-based vaccines induce humoral and
cytotoxic T-cell responses by imitating a viral infection and
expressing vaccine antigens in situ during immunization
(106). This benefit is
essential for eliminating infections or intracellular pathogens,
where strong humoral and cellular immune responses are needed to
provide effective protection (Fig.
5).
Influenza-causing virus
The most effective method to avoid influenza is
vaccination (107). Compared to
traditional vaccines, mRNA vaccines that encode the conserved
regions of the influenza virus's effector protein(s) may induce the
production of specific antibodies, improving prevention and ideally
treatment outcomes (107).
Furthermore, mRNA vaccines are simpler to develop for novel
influenza viruses due to a speedier development process (108). This suggests that mRNA vaccines
may serve as quick and adaptable tools for managing influenza, both
seasonal and pandemic.
Human immunodeficiency virus (HIV)
In the first human clinical trial with naked mRNA
(ihivarna) in combination with a novel HIV immunogen sequence (HTI
immunogen) using a DC activation approach (trimix: CD40 ligand
(CD40L)+ CD70+ encode constitutively active
TLR4 (CATLR4) RNA), and vaccination, the vaccine was safe and well
tolerated. It rapidly increased HIV-1 DNA and RNA in peripheral CD4
T cells and triggered a modest HIV-specific T-cell response
(109). The activating adjuvant
trimix and 16 conserved segments (GAG, pol, Vif and Nef) from the
HIV-1 structural protein were used to make up the HTI trimix. It
encoded a potent activation signal and an efficient HIV recombinant
antigen, making it a promising novel vaccine candidate for
mRNA-based therapy of HIV-1. According to preclinical findings, it
may successfully stimulate T-cells, the release of mature DCs and
the release of antiviral cytokines (particularly IFN-γ). Phase I
and phase IIa clinical studies of HTI-TriMix were completed by the
end of 2019. HIV-1-positive individuals in a phase IIa study were
administered three vaccines at weeks 0-2 and 4, as determined by
ultrasonography guidance using an inguinal lymph node (109,110).
Herpes simplex virus (HSV)
A trivalent vaccination that targets the
glycoproteins C, D and E found on HSV-2 viral particles was
developed recently. After an HSV-2 assault, the vaccination may
shield animals against genital infection and subsequent viral
shedding. In addition, vaccination generated cross-reactive
antibodies, which neutralized HSV-1 and provided defense against
HSV-1 infection. As a result, this vaccine offers a strong defense
against genital HSV-1 and HSV-2 infections and is a suitable
candidate vaccine for human testing (111) (Fig. 5).
Flaviviruses
Numerous flaviviruses, such as Zika virus, Powassan
virus, dengue virus and tick-borne encephalitis virus, have been
treated and infections prevented using mRNA vaccines (112-116). The use of mRNA vaccines against
the Zika virus has garnered significant interest. To assess
immunogenicity and protection in mice, Richner et al
(115) created an
LNP-encapsulated modified mRNA vaccine containing wild-type or
mutant ZIKV structural genes. In a phase I/II human clinical study,
a comparable vaccine called mRNA-1893 resulted in >90%
seroconversion after a prime-boost immunization administered at
dosages of 10 or 30 µg (113)
(Fig. 5).
COVID-19
The use of mRNA vaccines against COVID-19 has
garnered notable interest. To date, several vaccines have been
produced, including mRNA-1273, BNT162, ARCT-021 and the cvncov
vaccine (cv07050101). The near-identical 94 to 95% vaccination
efficacies of the mRNA-1273 and BNT162b2 COVID-19 vaccines, as well
as their rapid development and testing period of a year, are
remarkable achievements in science and medicine (117).
Among the mRNA vaccines under development, only the
mRNA vaccine for COVID-19 is currently on the market. Based on
published clinical data, the COVID-19 mRNA vaccine provides a high
level of protection and safety. With mRNA vaccination, recipients
have significantly higher levels of neutralizing antibodies than
those who have recovered from COVID-19 and who were not vaccinated
and exhibit an enhanced immune response and memory. The mRNA
COVID-19 vaccine provides 95% overall protection against the virus
in individuals aged ≥16 years and can effectively induce a response
to 20+ variants of the virus. Clinically significant safety
concerns have not been identified in the majority of patients
receiving the mRNA COVID-19 vaccine. Myocarditis after neocoronary
mRNA vaccination has been observed in a small percentage of those
vaccinated, with the highest incidence in younger men after a
second dose of the vaccine (118).
Diseases caused by bacterial
infection
Attempts to develop mRNA vaccines against bacterial
and parasitic antigen species are limited, many of which are still
in the preclinical testing stage.
Maruggi et al (119) examined the effectiveness and
immunogenicity of self-amplifying mRNA vaccines that express group
A streptococcal antigen (GAS) and GBS. Self-amplifying mRNA vectors
were able to effectively produce two prototype bacterial antigens:
The GBS pilus 2a backbone protein and the double-mutated GAS
streptolysin-O. In mice infected with GAS and GBS, the antibody
responses produced by self-amplifying mRNA vaccines were able to
consistently provide protection. Jawalagatti et al (120) developed a novel strategy for
creating mRNA vaccines to be taken orally.
5. Applications of mRNA vaccines in various
tumors
Approximately 25 years ago, the viability of an
mRNA-based cancer vaccine was initially shown (121). Since then, the use of mRNA
vaccines in cancer treatment has been investigated in several
preclinical and clinical investigations. A small number of mRNA
cancer vaccine clinical studies have been conducted so far
(122). The majority of trials
have focused on acute myeloid leukemia (AML) (123), multiple myeloma (124), mesothelioma (125), glioblastoma (126), malignant glioma (127) and renal cell carcinoma (128), as well as mRNA vaccines for
pancreatic cancer (129),
melanoma (130), BCa (131), non-small cell lung cancer
(132), prostate cancer
(133), ovarian cancer (134) and colorectal cancer (135) and are still in their early
stages (I and II) (136-140) (Fig. 5). Moderna and Merck Sharp &
Dohme announced that the first combination therapy of an mRNA
personalized cancer vaccine (mRNA-4157) and an anti-programmed cell
death 1 (PD-1) monoclonal antibody (Keytruda) advanced to phase III
clinical trials in July 2023 (14). This was the first mRNA cancer
vaccine to enter phase III clinical trials. Table I summarizes the clinical trials of
mRNA vaccines for the treatment of cancer.
 | Table IRepresentative clinical trials of
mRNA vaccines for different tumors. |
Table I
Representative clinical trials of
mRNA vaccines for different tumors.
| Tumour type | Main ID (NCT
no.) | Status | Phase | Antigen | Sponsor | Investigator | Primary
outcome(s) |
|---|
| Pancreatic
cancer |
ChiCTR2300077339 | Recruiting | 1 | mRNA | Chinese PLA General
Hospital | Chinese PLA General
Hospital | Percentage of
subjects who meet the criteria of DLT in cycle 1; percentage of
subjects with AEs |
| NCT05916261 | Recruiting | 1 | mRNA | Ruijin
Hospital | Ruijin Hospital
Affiliated to Shanghai Jiao Tong University School of Medicine | Disease control
rate; MTD or DLT, If MTD is not reached, biologically effective
dose; objective response rate; reaction of antigen-specific T cells
in peripheral blood |
| Advanced solid
tumors | NCT05198752 | Recruiting | 1 | mRNA | Stemirna
Therapeutics | NA | DLT incidence |
|
ChiCTR1900023000 | Recruiting | 1 | mRNA | Dongfang Hospital
Affiliated to Shanghai Tongji University | Stemirna
Therapeutics, LLC | Specific
CD4+ and CD8+ T lymphocyte responses to
neoantigen |
| Advanced malignant
tumors |
ChiCTR2100050688 | Recruiting | 0 | mRNA | Shanghai East
Hospital | Shanghai East
Hospital | AEs; cytokine
suite |
| Leukaemia | NCT04977024 | Recruiting | 2 | mRNA | GeoVax, Inc. | GeoVax, Inc. | Biological
response |
| Esophageal
cancer; | NCT03908671 | Recruiting | NA | mRNA | Stemirna
Therapeutics | NA | Number of
participants with treatment-related |
| NSCLC | | | | | | | AEs as assessed by
CTCAE v4.03 |
| NCT06077760 | Recruiting | 3 | mRNA | Merck Sharp &
Dohme, LLC | Study Director:
Medical Director, Merck Sharp & Dohme, LLC | Disease-free
survival |
| Melanoma | NCT03897881 | Recruiting | 2 | mRNA | ModernaTX,
Inc. | NA | RFS, assessed using
radiological imaging |
| NCT05933577 | Recruiting | 3 | mRNA | Merck Sharp &
Dohme, LLC | Study Director:
Medical Director, Merck Sharp & Dohme, LLC | RFS |
| Ovarian cancer |
EUCTR2010-020233-56-NO | Authorised | 1, 2 | mRNA | Oslo University
Hospital | Oslo University
Hospital | Immunological
response to the vaccine (induction of specific T-cell response) and
time of disease progression and survival time; clinical treatment
response monitored by clinical examination, CA-125 evaluation and
CT scans every third month |
| Prostatic
cancer |
EUCTR2010-018770-20-NO | Authorised | NA | mRNA | Oslo University
Hospital | NA | Time to treatment
failure defined as two different measurements of prostate-specific
antigen levels >0.5 µg/l with an interval of at least 4 weeks.
At this time the patients are referred to standard radiation
therapy |
| Hepatocellular
carcinoma | NCT05981066 | Recruiting | NA | mRNA | Peking Union
Medical College Hospital | NA | Incidence and
severity of AEs; clinically significant abnormal changes in vital
signs; clinically significant abnormal changes in laboratory
tests |
Pancreatic cancer
Growing data suggest that the intra- and
intertumoral heterogeneity of pancreatic cancer, particularly in
relation to the immune microenvironment and genetic alterations, is
associated with resistance to treatment (141-143). Traditional cancer treatment
approaches often result in the acquisition of resistance,
underscoring the need for targeted, individualized care (140). In patients with pancreatic
ductal carcinoma, a recent phase I clinical study using the
adjuvant tailored mRNA neoantigen vaccine autogene cevumeran, the
vaccination stimulated T-cell activity, which may have been
associated with a delayed recurrence of the illness. In the
clinical trial, 16 of 19 patients were treated with cevumeran after
treatment with Atezolizumab; 8 of the 16 patients who received the
mRNA vaccine produced T-cell translocations that sustained the
production of IFNγ, demonstrating the immune persistence of the
mRNA vaccine. The median relapse-free survival (RFS) of subjects
who responded to the vaccine had not been reached at the median
follow-up time of 18 months. The RFS was significantly higher in
responders than in non-responders. Therefore, it can be concluded
that Atezolizumab (an anti-PD-L1 monoclonal antibody) in
combination with the mRNA vaccine induced extensive T-cell activity
and delayed the recurrence of pancreatic cancer (129). In patients with pancreatic duct
adenocarcinoma (PDAC) undergoing surgical resection and at risk of
delayed recurrence, Kang et al (144) demonstrated that the personalized
mRNA neoantigen vaccine, autogene cevumeran (BNT122), induced
significant T-cell activity when combined with Atezolizumab and
mFOLFIRINOX, a standard adjuvant chemotherapeutic regimen following
surgery for PDAC.
Colon cancer
mRNA vaccines against colon adenocarcinoma are
limited. A significant difficulty is sorting through the several
vaccine candidates to identify possible mRNA vaccines that may be
suitable for a specific type of cancer (145). Thrombospondin 2, follistatin
like 3, troponin T1, biglycan, collagen triple helix repeat
containing 1 and NADPH oxidase 4 are among the targeted antigens
that Liu et al (139)
identified as potential mRNA vaccine candidates. The study also
described aberrant gene expression patterns and the mutational
landscape of CRC. The results may assist in the determination of
patients with CRC who are suitable candidates for immunization and
provide a theoretical foundation for the creation of mRNA cancer
vaccines.
Melanoma
To develop mRNA vaccines and identify appropriate
vaccine populations, Ping et al (146) analyzed potential tumor antigens
in melanoma. Their analysis revealed that protein tyrosine
phosphatase receptor type C, sialic acid binding Ig like lectin 11
(SIGLEC11), cyclic-oligoadenylate-activated single-stranded
ribonuclease and single-stranded deoxyribonuclease 1, leukocyte
immunoglobulin-like receptor subfamily B member 1 and ADAM like
decysin 1 may be antigenic targets for mRNA vaccines for melanoma,
laying the groundwork for the development of mRNA vaccines in
melanoma (146). According to
the findings of Sittplangkoon et al (147), unaltered mRNA vaccines in
melanoma models may result in the production of IFN-I or trigger
downstream signaling cascade responses, both of which are essential
for eliciting strong anti-tumor T-cell responses that regulate
tumor development and metastasis. The experimental vaccine
mRNA-4157/V940 in combination with pembrolizumab may be used as an
adjuvant therapy for the treatment of high-risk melanoma, according
to the results of a recent clinical study. Compared with patients
treated with a PD-1 inhibitor alone, individuals receiving this
combination treatment had a noticeably decreased risk of recurrence
after surgery (130).
BCa
Li et al (148) identified groups of patients with
BCa that may be suitable candidates for mRNA vaccination as well as
putative BCa-associated antigens for the creation of anti-BCa mRNA
vaccines. A total of three tumor-associated antigens were
identified: CD74, interferon regulatory factor 1 and proteasome
activator subunit 2. These antigens were frequently mutated,
amplified or upregulated, and they were associated with immune-cell
infiltration and prognosis. The tumor microenvironment (TME) in
patients with class B immune subgroups may respond well to mRNA
vaccines (148).
Patients in the prostate adenocarcinoma (PRAD)
immune subtypes 2 and 3 groups were more likely to benefit from
immunization, and prospective antigens for the PRAD mRNA vaccine,
including Kelch-like family member 17, carnitine
palmitoyltransferase 1B, IQ motif containing GTPase activating
protein 3, Lck interacting transmembrane adaptor 1, YjeF N-terminal
domain containing 3, coiled-coil domain containing 180, MutS
homolog 5 and cadherin EGF LAG seven-pass G-type receptor 3
(133).
Hepatocellular carcinoma (HCC)
Peking Union Medical College Hospital is conducting
the first human clinical trial of the tumor antigen mRNA
therapeutic vaccine ABOR2014 (IPM511). The vaccine encodes nearly
20 HCC antigens that are frequently upregulated in patients with
HCC. The study aims to evaluate the safety, tolerability and
preliminary efficacy of IPM511 alone and in combination with PD-1
inhibitor therapy in patients with advanced HCC with disease
progression after first-line standard therapy. The study is
currently ongoing and no results have been published, with the
principal investigator stating that IPM511 has been shown to
demonstrate safety and clinical accessibility in the first patient
treated using this approach
Esophageal cancer
A study of a personalized mRNA cancer vaccine
combined with a PD-1 inhibitor for the treatment of advanced
esophageal squamous cell carcinoma was recently reported (149). The patient enrolled in the study
was first screened for neoantigens using whole-transcriptome
sequencing of tissue samples from the lesion. A personalized mRNA
cancer vaccine was developed that was tailored to these
neoantigens. Subsequently, the patient was administered the mRNA
cancer vaccine in combination with a PD-1 inhibitor and the patient
obtained partial remission; the safety of the treatment regimen was
within manageable limits. Thus, mRNA vaccines may be an effective
therapeutic strategy for patients with advanced esophageal
cancer.
6. Functional implementation of mRNA
vaccines in tumor treatment
Immunotherapy of mRNA vaccines
Cancer may now be effectively treated with
immunotherapy, and given the positive early clinical outcomes, the
FDA has authorized an increasing number of immunotherapies. The
foundation of cancer immunotherapy is the immune system's capacity
to identify and eliminate cancer cells (150). With the advent of several
classes of medicines targeted at augmenting immune responses
against malignancies, this area of research has rapidly expanded in
recent years. These consist of various vaccination approaches,
adoptive T-cell treatments, immune checkpoint inhibitors and
cytokines (151-153).
Tumor antigens
Tumor-associated antigens (TAAs) and tumor-specific
antigens (TSAs) are two categories of antigens that are encoded by
mRNAs and have traditionally been the target of immunotherapy.
Given immunologic memory, vaccines that specifically target TAAs or
TSAs may target and kill cancerous cells that exhibit upregulated
expression of antigens and provide a long-lasting therapeutic
response (49).
TAAs
TAA is not unique to tumor cells. While they may
also be found in healthy tissues and cells, particularly in
embryonic tissues, the expression of these antigens is markedly
elevated in tumors. TAAs in immunotherapy are potential targets as
shown by Conry et al (154) who developed the first mRNA
cancer vaccine; this vaccine demonstrated that mice immunized with
mRNA coding for carcinoembryonic antigen (CEA) showed an anti-CEA
antibody response when challenged with CEA expressing tumor cells.
Significant attention has been given to TAA research since this
initial discovery, with positive clinical study outcomes. Three
tumor antigens, including CD247, Fc gamma receptor Ia and
transcription domain associated protein, were identified by Huang
et al (155) in
cholangiocarcinoma. These antigens are associated with an improved
prognosis and the infiltration of antigen-presenting cells. These
antigens may serve as potential candidates for mRNA vaccines
against cholangiocarcinoma (128,155). TAA immunization methods do have
certain drawbacks, however. The TAAs are often not found in
malignant cells. In addition, as not all discovered TAAs induce an
antitumor immune response, selecting TAAs may be challenging and
vaccine development is expensive. There is a possibility that the
tumor itself may downregulate the TAA, allowing for escape when
focusing on a particular antigen (98). Finally, TAAs may also be found in
normal tissues, and vaccines against them may cause peripheral and
central tolerance responses, and this may result in poor
vaccination efficacy or autoimmunity against normal tissues
(156).
TSAs
TSAs, also known as neoantigens, are antigens
unique to tumor cells from somatic random changes and are thus
absent from normal tissues and cells. Tumor-specific mutations are
appealing targets for cancer immunotherapeutics and these mutations
may result in novel antigens, allowing for the development of
customized vaccines, as the mutated antigens are not expressed in
healthy tissues and may thus be identified by T-cells (157,158). The following characteristics of
neoantigen-based vaccinations highlight their benefits over
TAA-based vaccines. Since neoantigens are only produced by tumor
cells, they can only trigger T-cell responses specific to tumors,
limiting harm to non-malignant tissues. Neoantigens offer the
potential to elicit immunological responses against tumors by
evading T-cell central tolerance of self-epitopes. Furthermore,
there may be long-term protection against disease recurrence due to
the capacity of these vaccine-boosted neoantigen-specific T-cell
responses to endure and provide immunological memory beyond therapy
(159). To immunize patients
against metastatic gastric cancer, Cafri et al (160) recently linked the verified and
characterized novel antigens and predicted new epitopes and driver
gene alterations into a single mRNA construct. The developed
vaccine proved safe and produced a T-cell response specific to the
expected new epitopes that had not been discovered before
immunization. The use of neoantigens still faces several challenges
despite the advancements made; these obstacles stem from both
biological and technical factors, including polymorphism of
putative antigens and HLA molecules, gaps in our understanding of
HLA binding motifs for less common HLA alleles and the
heterogeneity of tumors (161-164).
Delivery methods of mRNA vaccines for
tumor antigen
mRNA-transfected DC vaccines
DCs may be transfected with the mRNA expressing
tumor antigen and total tumor mRNA, which can subsequently be
delivered to the host and initiate an immune response. Subcutaneous
application of DCs transfected with TAA mRNA or total mRNA to
tumor-bearing mice has been shown to induce T-cell immunity and
inhibit the growth of established tumors as early as the last
century, which also supports the feasibility of these two distinct
transfection methods (61).
Patients receiving docetaxel for metastatic castration-resistant
prostate cancer showed no adverse effects when Kongsted et
al (165) transfected DCs
expressing numerous TAAs; ~50% of the research participants
exhibited an immune response. A phase 1 clinical trial including
patients with lung cancer is now assessing MIDRIXNEO, a customized
mRNA-loaded dendritic cell vaccine that targets tumor neoantigens
(166).
Direct injection
There are several methods to directly inject the
mRNA encoding the tumor antigen into a host. Local cells, such as
APCs, take up the mRNA and translocate it to the cytoplasm where it
is translated. One of the most common methods of delivering tumor
antigens is via intranodal injection. Phase I research on 29
patients with advanced melanoma started in 2012; unpublished data
from this trial showed that intranodal mRNA injection is safe and
viable (NCT01684241). mRNA encoding tyrosinase, premelanosome
protein, MAGE family member A3 (MAGE-A3), MAGE-C2 and melanoma
antigen preferentially expressed in tumors together with TriMix
mRNA were well tolerated when injected intravenously. In another
phase I clinical study, intranodal delivery of a
neoantigen-specific mRNA vaccine, consisting of 20 mutations
specific to the patient, was studied in patients with stage III and
IV melanoma (NCT02035956), which demonstrated that individual
mutations can be exploited (167).
Administration via the skin
Administration of tumor antigens expressed by an
mRNA may be achievable via the skin. Mice were injected with IVT
mRNA encoding the enhanced green fluorescence protein and the
melanocyte autoantigen TRP2 by blasting their skin with a gene gun.
This successfully elicited cellular immunity specific to antigens,
mediating a protective effect against B16 lung metastases and
inducing vitiligo-like hair decolorization (168). Protamine-condensed naked mRNA
encoding six melanoma-associated antigens was safely and
successfully injected intradermally into two of the four evaluable
patients in a phase I/II clinical trial involving 21 patients with
metastatic melanoma. In addition, 1 of the 7 patients with
measurable disease showed a complete response to the vaccine
(169).
Intranasal administration
The mucosal route, which encodes a tumor antigen
via mRNA, may efficiently elicit both a systemic immune response
and localized mucosal immunity. Nasal-associated lymphoid tissue, a
favorable location for antigen internalization to promote
protective responses against cancer cells, may be reached via
intranasal delivery. In an invasive Lewis lung cancer model, Mai
et al (170) immunized
mice intraperitoneally with a cationic liposome/protamine complex
carrying mRNA expressing cytokeratin 19, and this slowed down the
tumor's development and elicited a robust cellular immune
response.
Intravenous administration
The melanoma fixvac (bnt111) intravenous liposome
RNA (rna-lpx) vaccine, which encodes four non-mutated tumor-related
antigens, was investigated by Sahin et al (171). The rna-lpx vaccination was a
successful method of immunotherapy; robust CD4+ and
CD8+ T-cell immunity was achieved against the vaccine
antigen, which was accompanied by a favorable clinical response. In
certain responders, the antigen-specific cytotoxic T-cell response
was durable and to the degree of often documented adoptive T-cell
treatment.
mRNA-encoded immunomodulators
Tumor immunotherapy, also known as active
nonspecific immunotherapy, was one of the first fields of medicine
to use immune system modulators. Target cell transfection in
vitro, or intravenous or intratumoral delivery, are the primary
methods for producing immunomodulators via mRNA in vivo
(121). The immune system's
cells can communicate across short distances due to cytokines,
which are important regulators of both innate and adaptive
immunity. To stimulate the immune system of patients with cancer,
cytokine therapy has been a significant therapeutic approach and is
a major topic of clinical cancer research at present (172). The goal of mRNA-encoded
cytokine-based immunotherapy is to increase the quantity of
cytokines in the TME with the least amount of toxicity and systemic
exposure possible from the delivery of recombinant proteins
(173).
Given its strong proinflammatory properties, IL-12
is a promising option for cancer immunotherapy. Type 1 T-helper
cell (Th1) differentiation, the acquisition of cytotoxic activities
by CD8+ cells, and the activation of IFN-γ production,
all of which improve phagocytic functions and local inflammation,
are all induced by IL-12 signaling (174). T-cells transfected with mRNA
producing a single-chain IL-12 (scIL-12) made up of the p35 and p40
subunits were the subject of one investigation. In syngeneic and
xenograft mouse models, intratumoral injection of
scIL-12-expressing T-cells led to total rejection of both injected
and remote tumor lesions. The anti-tumor efficaciousness of T-cells
was further enhanced by co-electroporation with 4-1BB ligand
(4-1BBL) mRNA (175). It has
been shown that novel intratumoral IL-12 mRNA treatment can
stimulate Th1 TME transformation and powerful antitumor immunity
(176). Additionally, in mouse
tumor models resistant to checkpoint inhibitors, it was shown that
the combination of immune checkpoint inhibitors with IL-12 mRNA
increased the anticancer response, enhancing overall survival (OS)
and tumor regression (176).
Multiple mRNAs encoding various cytokines have been
validated for their immunotherapeutic effects. The mRNA encoding
four cytokines, IL-12, IFN-α, IL-15 sushi, and
granulocyte-macrophage colony-stimulating factor, was examined by
Hotz et al (177) after
intratumoral administration. These cytokines may promote the
development of immunological memory and have potent anticancer
action. Hewitt et al (178) showed that in a variety of TMEs,
direct intratumoral administration of mRNAs encoding these
cytokines generated strong anticancer responses.
Tumor immunotherapy also involves mRNA-encoded
stimulatory ligands and receptors, in addition to cytokines. The
immune system receives inflammatory signals from stimulatory
ligands and receptors, which may be used in cancer immunotherapy.
By temporarily supplying cells with stimulatory receptors, mRNA may
be used to temporarily activate potent inflammatory signals. While
these immunostimulants are not regarded as cancer vaccines, they
are often administered in conjunction with other immunotherapeutic
treatments, such as checkpoint blockade modulators, to enhance the
humoral and cellular responses. Several studies showed that the
immunostimulatory activity of DCs was significantly increased upon
electroporation with mRNAs encoding co-stimulatory molecules,
including CD83, TNF receptor superfamily member 4 (also known as
OX40) and 4-1BBL. Trimix mRNA, an mRNA-based adjuvant that was
created in 2016, is made up of three mRNA molecules that encode
CATLR4, activating stimulator CD40L, and costimulatory molecule
CD70. Intramuscular injection of tethered IVT mRNA-TLR7 agonists
boosted antigen-specific cell-mediated and humoral responses in
vivo, as shown by Loomis et al (179). Both alone (TriMix mRNA plus TAA
mRNA, or autologous monocyte-derived mRNA co-electroporated
dendritic cells with mRNA encoding CD40 ligand, CD70 and a
constitutively activated TLR4, which is referred as TriMixDC-MEL)
and in combination with ipilimumab checkpoint inhibitor, a
monoclonal antibody that blocks CTLA, the products were able to
elicit a potent immune response, which in turn led to a promising
clinical response and prolonged disease-free survival rates
(NCT01676779 and NCT01302496) (101,180). These phase II studies were
conducted for the treatment of patients with stage III/IV
melanoma.
mRNA-encoded antibodies
Since the first antibody was licensed for use in
cancer therapy, the field of oncology has developed antibody-based
therapeutics at a progressively faster pace. As a result, there are
now several approved antibodies and additional candidates that are
undergoing clinical review (181). The ability of mRNA to produce
various antibodies and antibody types in vitro was shown by
Thran et al (182).
furthermore, the mRNA that encodes tumor antibodies may elicit
robust anti-tumor immunity.
The following methods may be used to categorize the
involvement of antibodies in tumor treatment: i) Antibodies that,
after binding to the target receptor, may facilitate direct death
of tumor cells by inducing apoptosis signals or removing vital
growth signals. ii) Complement dependent cytotoxicity (CDC) or
antibody-dependent cell-mediated cytotoxicity (ADCC) are induced
when immune-mediated cell death is activated by attaching to
antigens specific to cancer cells. iii) Blocking the immunological
checkpoint, which involves preventing the antibody of PD1 or CTLA4,
reactivating the T-cell response specific to the antigen against
cancer cells, and triggering immune-mediated cell death. iv)
Directly engaging T-cells with cancer cells to initiate
immune-mediated cell death (183-188).
Monoclonal antibodies (mAb), mAb fragments and
engineering variations (such as diabodies, triabodies, minibodies
and single-domain antibodies) are the categories of antibodies that
are involved in tumor immunotherapy. Both monoclonal and bispecific
antibodies have been investigated and used extensively (181).
The first mAb licensed for cancer therapy,
rituximab, targets CD20 and induces CDC and, to a lesser degree,
ADCC. It is used to treat chronic lymphocytic leukemia and
non-Hodgkin's lymphoma (189). A
technique that has been beneficial in a preclinical B-cell lymphoma
model is the encoding of this mAb in mRNA (190). Cancer cells starve and
eventually die as a result of trastuzumab's inhibition of kinase
activity, downstream signaling and human EGFR 2 (HER2)/Erb-B2
receptor tyrosine kinase 2 receptor dimerization (186). Direct tumor cell death may also
be induced by antagonistic antibodies binding to apoptosis-inducing
receptors on cancer cells, such as TNF-related apoptosis-inducing
ligand receptors and antibody-drug conjugates that deliver toxic
payloads, particularly to cancer cells (184,188). When intrathecally administered
using a liver-targeting LNP formulation, trastuzumab encoded by
mRNA was retrieved from mouse serum and exhibited ADCC. In a
HER2/neu-positive BCa xenograft model, this approach increased
survival (191). The translation
of anti-programmed cell death ligand 1 (PD-L1) mAb in transfected
cells and the infiltration of CD8+ T-cells into tumors
may be facilitated by injecting antagonistic anti-PD-L1 mAb
expressing self-amplified RNA into mouse tumors (192). Despite the several positive
instances of mAbs in cancer, poor tissue penetration limits the
ability of the complete antibodies to distribute uniformly across
the tumor mass. As a result, efforts have been made to modify the
structure of antibodies, resulting in the creation of novel
antibody fragments with improved tissue penetration (173). The discovery of antibody
fragments has considerably aided in the study of bispecific
antibodies (BsAbs) and these have been employed as building blocks
in the creation of these antibodies. The world's first antibody to
bind two antigens simultaneously was created in the 1960s by
Nisonoff et al (193) by
combining the antigen binding fragments from two rabbit polyclonal
antibody sera using a moderate reoxidation approach (194,195).
One possible benefit of BsAbs over regular mAbs is
their ability to target two distinct sites simultaneously and, to a
certain degree, circumvent tumor drug resistance. However, since
BsAbs have short serum half-lives (just a few hours) they must be
constantly administered to the patient using an infusion pump
(196). One possible method to
overcome this restriction is to employ mRNA to directly generate
therapeutic antibodies in patients.
A recent study highlighted a successful bispecific
targeting method; a clinically authorized LNP encapsulating mRNA
expressing C-C motif chemokine ligand 2 (CCL2) and CCL5
(bisccl2/5I), which can bind and neutralize bispecifically. This
markedly promoted TAM polarization towards the anti-tumor M1
phenotype and lowered immunosuppression in the TME. BisCCL2/5i in
conjunction with PD-1L inhibitor has been shown to result in
long-term survival in a mouse model of pancreatic, colorectal and
liver metastases from primary liver cancer (193). The use of mRNA encoding a BsAb
with ablation Fc immunological impact function was described by Wu
et al (197). The
antibody, also known as XA-1, targets human PD-L1 and PD-1. In
contrast to direct antibody therapy for cancer, this work
demonstrated that treatment with XA-1 mRNA LNPs may efficiently
produce endogenous therapeutic BsAbs via hepatocytes.
mRNA-encoding antigen receptors
By producing transgenic T-cell receptors (TCRs) or
CARs, it has been possible to provide naïve T-cell tumor
selectivity due to the transitory nature of mRNAs (173). mRNAs encoding CARs or TCRs have
shown to be of value; generating transient expression of CARs or
TCRs may prevent the development of cytokine release syndrome, a
negative consequence of chronic T-cell activation (198-201). Considerable interest has been
shown in CAR-T cells as the most promising cancer-adoptive
immunotherapy approach.
With CAR-T treatment, immunological T-cells are
extracted from patients, genetically altered in vitro, and
endowed with a 'chimeric antigen receptor' (CAR) that identifies
the antigen on the surface of cancerous cells. These altered cells
are grown in a lab before being reinfused into the patient
(202). Typically, extracellular
TAA-specific antibody-binding domains are fused to intracellular
T-cell-signaling regions to form chimeric receptors. Virtually
every tumor-associated antigen may be targeted by CAR-T cells
(203). There are several
hazards associated with targeted non-tumor toxicity in CAR-T
treatment. In vitro transcribed mRNA CAR-T cells are
emerging as a safe therapeutic option, since they can avoid focused
anti-tumor toxicity (204). The
majority of studies use mRNA electroporation of CARs, as it is a
practical and scalable method that achieves lymphocyte transfection
rates of >90% without compromising the viability of the cell
product (205-208). CAR expression is observed on the
surface of T cells for ~7 days following mRNA electroporation, and
CARs internalized upon target cell encounter are not restored
(199,207-213). However, the IVT mRNA approach
has drawbacks as well. These include poor tumor infiltration,
manufacturing difficulties when a limited number of T-cells are
available, the risk of side effects when repeated doses of CAR-T
cells are injected and the short lifespan of mRNA-redirected
T-cells, which results in the expression of encoded protein for a
number of days (214). Most
importantly, electroporation has various drawbacks that may
significantly impact the caliber of the CAR-T cells generated
(53). In fact, the integrity of
the cell plasma membrane may be irreparably compromised by the
application of pulsed electric fields. In the transfected cells
that survive, decreased transgene expression, abnormal gene
expression profiles and poor viability may be observed. In fact, it
was demonstrated that when chemically modified 1mΨ mRNA and/or mRNA
that was further purified to remove dsRNA was used, as opposed to
their unmodified and unpurified counterparts, murine T-cells
electroporated with CAR-encoding mRNA showed a markedly reduced
upregulation of checkpoint molecules (PD-1 and lymphocyte
activation gene 3). This gave the immunosilent mRNA-transfected
T-cells a greater ability to kill, which persisted even after the
cells' CAR expression was eliminated. The current results must be
considered in the production of mRNA CAR-T cells to meet the
objectives of the clinical trials, which are now conducting mRNA
CAR studies for both hematologic and solid tumor malignancies
(215).
Currently, mRNA CAR-T cells are receiving
significant interest and showing promising outcomes in the study of
tumor treatment. Treatment of hematological malignancies using mRNA
CAR-T-cell therapy has seen favorable preclinical and clinical
outcomes. Primary targets include CD19, CD37, CD33 and CD123.
Preclinical research on mRNA CAR-T cell treatments for lymphoma and
leukemia has been conducted (204). The approach for Hodgkin's
lymphoma targeting CD19 and the approach for recurrent/refractory
acute myeloid leukemia targeting CD123 are both being tested in
clinical studies. These two studies demonstrate that mRNA CAR-T
treatment is safe (204). A
novel concept for mRNA CAR-T cell treatment was recently presented
by Jetani et al (216),
who identified siglec-6 as a new target of CAR-T cells in AML.
Descartes-08, a novel CD8+ CAR-T cell product, was
created by Lin et al (217) to treat multiple myeloma;
preliminarily sustained responses and a strong treatment index were
shown by the product. Mesothelioma, ovarian cancer, colorectal
cancer, BCa and melanoma are among the solid malignancies for which
mRNA CAR-T treatment has been investigated (204).
Combination therapy-immunotherapy and
mRNA vaccines
mRNA therapy combined with another mode of
treatment may show great promise in the treatment of tumors. The
interaction between tumor antigens and immunomodulators is the
primary focus of current research.
Early research has shown that TAA and IL-12 mRNA
transfection into mature DC boosted TAA-specific CTLs, which may be
exploited to trigger an immune response against tumors that is
mediated by NK effector cells and CTLs (218). The safety and immunogenicity of
cellular immunotherapy using trimixdc Mel were demonstrated by
tests of mRNA encoding fusion proteins of a HLA-class II targeting
signal (DC-LAMP), melanoma-associated antigen encoding the CD40
ligand, CATLR4 and CD70. At the study's IV dosage level, antitumor
efficacy with long-lasting disease control was seen (219).
Sunitinib and rocapuldencel-T were recently
employed in the phase 3 study for metastatic renal cell cancer. OS
of patients receiving combined therapy was not improved by
self-immunization via electroporation with amplified tumor RNA and
CD40L RNA prepared from mature monocyte-derived DCs. However, the
OS-related immune response was enhanced (220).
Protein therapy of mRNA vaccines
Methods of inducing cancer-cell
death
Cancerous cells may be made to produce a deadly
intracellular protein by employing mRNA therapies, which causes the
cells to self-destruct (196).
Van Hoecke et al (221)
delivered mRNA encoding the mixed lineage kinase domain-like
protein intrauterinally, which resulted in arrested tumor growth
and induction of necrotic tumor-cell death.
Protein replacement therapy
The use of IVT mRNA in protein replacement therapy
is predicated on the production of foreign proteins that can either
activate or inhibit cellular pathways, as well as the
supplementation of proteins that are absent or underexpressed. IVT
mRNA injection has been used to target several proteins since its
first preclinical assessment for protein replacement in 1992
(25).
Given their accessibility, the liver, lungs and
heart are the primary targets of protein replacement treatments
based on IVT mRNA. However, other tissues and organs, such as the
skin, the back of the eye or the nasal cavity, have also been
considered potential targets. Rare and hereditary disorders are the
primary targets for this mode of treatment. The disease-causing
down-regulated protein is linked to genetic abnormalities and
encoded by the therapeutic IVT mRNA (222-227).
IVT mRNA as protein replacement therapy is being
studied as a mode of cancer treatment (228). This is very difficult, however,
as it requires recurrent administration, at times even systemic
distribution, and targeted mRNA expression. In the realm of protein
replacement, no clinical trials using IVT mRNA have been started
yet, to the best of our knowledge (229).
Mutations of the oncogene tumor protein (TP53) are
present in 96% of patients with high-grade serous ovarian cancer.
p53 protein expressed by the TP53 gene recognizes and repairs DNA
damage in cells and induces cell death when the damage is not
repairable, thus avoiding the proliferation of aberrant cells to
prevent the onset of cancer. When the TP53 gene is mutated, the
mutant p53 protein that is expressed loses its oncogenic effect. In
a recent study, a team synthesized mRNA encoding the correct p53
protein and loaded it into liposomes for delivery, thus allowing
the mRNA to express functional p53 protein in cancer cells. After
treatment with the above mRNA, patient-derived ovarian organoids
began to shrink and die, and primary tumors and metastases in mouse
models of ovarian cancer almost completely disappeared. These
findings suggest that IVT-mRNA-based protein replacement therapy
may reactivate oncogenic proteins in cancer cells and may thus
serve as a valuable therapeutic option for the treatment of cancer
(230).
Gene editing of mRNA vaccines
mRNA technologies expressing programmable
nucleases, such as zinc-finger nucleases, transcription
activator-like effector nucleases and clustered regularly
interspaced short palindromic repeats (CRISPR)-CRISPR-associated
endonuclease (Cas)9 systems (231), allow for gene editing as a
potential therapeutic approach. By delivering site-specific
alterations into a cell's genomes, such as correcting harmful
mutations or introducing protective changes, these
genome-engineering techniques allow for the replacement or
modification of gene expression (232). CRISPR-Cas9 consists of Cas9
nucleic acid endonuclease and a single guide RNA (sgRNA) and is a
widely used gene editing tool. CRISPR-Cas9 therapy can be used to
permanently abrogate cancer cell survival genes, avoiding the need
for repeat administration and thus improving treatment efficacy
(233). Cancer cells or T-cells
were transfected with Cas9 mRNA and sgRNA encapsulated in LNPs. The
mRNA is translated in the cytoplasm to produce the Cas9 protein,
which then complexes with sgRNA to form ribonucleoproteins with
affinity for specific DNA sequences. Next, ribosomal complexes
translocate to the nucleus to destroy tumor survival genes. When
this happens in T-cells, PD-1 and endogenous TCR genes are knocked
down to induce apoptosis in tumor cells (12). Ling et al (234) treated cervical cancer with Cas9
mRNA and gRNA targeting the E6 or E7 oncogenes. The results showed
that this method not only effectively knocked down the oncogenes
but also reversed the immunosuppressive microenvironment to achieve
anti-tumor effects.
Regenerative medicine
The goal of regenerative medicine is to replace,
regenerate or restore damaged or destroyed tissues, organs or cells
by re-establishing or restoring their normal function (235). Proteins that regulate cellular
biological activity, such as mitosis, migration and
differentiation, and growth factors, cytokines and transcription
factors, are essential to the regeneration process. An appealing
substitute for traditional somatic cell reprogramming and
transdifferentiation is the transfection of somatic cells with IVT
mRNA expressing transcription factors (236). The primary goal of using IVT
mRNA in regenerative medicine is to increase the ability of
patients with type 1 diabetes to secrete insulin. The use of cell
and mesenchymal stem cell engineering for tumor treatment has not
received significant attention. It has been shown that stem cell
exosomes with metastatic stem cell properties have been induced
from stem cells using prostaglandin E2 receptor antagonists and
used in cancer therapy and regenerative medicine. Stem cell
exosomes can reduce tumor resistance (237). Whether it is possible to express
stem cell exosomes with metastatic stem cell properties using mRNA
vaccines for cancer treatment is a point for consideration.
7. Conclusion and future prospects
Patients with cancer have better odds of surviving
thanks to the ongoing development of novel antitumor medications
and therapies; however, a number of issues, including tumor drug
resistance, dose-toxicity and other issues, keep posing new
difficulties. In contrast to standard treatment methods, biotherapy
for the majority of malignancies aims to prevent tumor growth and
incidence by stimulating autoimmunity and obstructing critical
signal transduction. There is some concern regarding the lack of
long-term effects of mRNA-based technologies; researchers are
attempting to challenge how illness is now treated using mRNA
therapy.
mRNA vaccines have shown to be successful in
several applications since Wolff et al (24) showed that mRNA transcribed in
vitro in live cells may create proteins. As a promising novel
platform, mRNA-based vaccines need to overcome the limitations of
cold chain preservation and provide improved adaptability,
efficacy, simplicity and scalability, as well as reduced costs.
mRNA vaccines have shown benefits in infectious illnesses and
cancer is the intended target of mRNA technology. Within the next
2-4 years, several active clinical studies using tailored cancer
vaccines should be finished.
The use of combinatorial mRNA treatment in tumor
therapy seems to have promising prospects. The current methods of
treating tumors, including surgery, radiation therapy and
chemotherapy, work well. As a novel biotherapy, mRNA therapy
requires additional clinical trials to provide a better integration
program before it can be effectively included in current
procedures. In addition, mRNA technology may be utilized in
conjunction with immunotherapy; currently, the majority of research
is focused on immunomodulators. Owing to its distinct benefits,
mRNA is also anticipated to supplant recombinant protein
medications such as ILs, IFNs and cell colony factors. Tumor gene
therapy may progress due to the significant ability of IVT mRNA to
reduce miss effects in gene editing techniques. It is hypothesized
that mRNA treatment may eventually lower the prevalence of
hereditary family malignancies as tumor gene therapy is
accomplished by introducing the target gene into the vector. Thus,
a wider range of patients with cancer may benefit from these novel
solutions.
Future research on mRNA vaccines for cancer should
focus on the development of therapeutic and prophylactic vaccines.
Personalized mRNA vaccines offer novel therapeutic strategies for
tumors, such as pancreatic cancer, that do not respond to current
treatments. In addition, mRNA technology can be used to develop
vaccines for viruses that are important causes of cancer, such as
HPV and HBV. A study indicated that HPV mRNA vaccines are more
effective against tumors than HPV recombinant protein vaccines and
HPV DNA vaccines in animal studies, supporting the future
development of prophylactic vaccines against tumors using mRNA
technology, which will be further evaluated in clinical trials
(238).
Furthermore, the flexibility, adaptability and
rapid production capacity of mRNA vaccines will offer exciting
possibilities for the prevention and treatment of infectious
diseases and cancers in the future, which will contribute to the
reduction of the global disease burden.
Availability of data and materials
Not applicable.
Authors' contributions
BC, YY and XW wrote the manuscript; WY and EZ
created figures and tables; YL, DW, YT and GT revised the
manuscript; SS and JS supervised the research and revised the
manuscript; KG supervised the research, revised the manuscript and
provided financial support. All authors have read and agreed on the
final version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
This study was supported by the Anhui Provincial Key Research
and Development Project (grant no. 202004j07020044, chaired by
KG).
References
|
1
|
Maomao C, He L, Dianqin S, Siyi H, Xinxin
Y, Fan Y, Shaoli Z, Changfa X, Lin L, Ji P and Wanqing C: Current
cancer burden in China: Epidemiology, etiology, and prevention.
Cancer Biol Med. 19:1121–1138. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roy PS and Saikia BJ: Cancer and cure: A
critical analysis. Indian J Cancer. 53:441–442. 2016. View Article : Google Scholar
|
|
3
|
Sobhani N, Scaggiante B, Morris R, Chai D,
Catalano M, Tardiel-Cyril DR, Neeli P, Roviello G, Mondani G and Li
Y: Therapeutic cancer vaccines: From biological mechanisms and
engineering to ongoing clinical trials. Cancer Treat Rev.
109:1024292022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Corbett KS, F lynn B, Foulds K E, Francica
J R, Boyoglu-Barnum S, Werner AP, Flach B, O'Connell S, Bock KW,
Minai M, et al: Evaluation of the mRNA-1273 Vaccine against
SARS-CoV-2 in Nonhuman Primates. N Engl J Med. 383:1544–1555. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scherubl H: Smoking tobacco and cancer
risk. Dtsch Med Wochenschr. 146:412–417. 2021.In German.
|
|
6
|
van Elsland D and Neefjes J: Bacterial
infections and cancer. EMBO Rep. 19:e466322018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schiller JT and Lowy DR: An introduction
to virus infections and human cancer. Recent Results Cancer Res.
217:1–11. 2021. View Article : Google Scholar :
|
|
8
|
Zare Sakhvidi MJ, Lequy E, Goldberg M and
Jacquemin B: Air pollution exposure and bladder, kidney and urinary
tract cancer risk: A systematic review. Environ Pollut.
267:1153282020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan Y, Gao F, Chang Y, Zhao Q and He X:
Advances of mRNA vaccine in tumor: A maze of opportunities and
challenges. Biomark Res. 11:62023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gote V, Bolla PK, Kommineni N, Butreddy A,
Nukala PK, Palakurthi SS and Khan W: A comprehensive review of mRNA
Vaccines. Int J Mol Sci. 24:27002023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deng Z, Tian Y, Song J, An G and Yang P:
mRNA Vaccines: The dawn of a new era of cancer immunotherapy. Front
Immunol. 13:8871252022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu C, Shi Q, Huang X, Koo S, Kong N and
Tao W: mRNA-based cancer therapeutics. Nat Rev Cancer. 23:526–543.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lorentzen CL, Haanen JB, Met Ö and Svane
IM: Clinical advances and ongoing trials on mRNA vaccines for
cancer treatment. Lancet Oncol. 23:e450–e458. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weber JS, Carlino MS, Khattak A, Meniawy
T, Ansstas G, Taylor MH, Kim KB, McKean M, Long GV, Sullivan RJ, et
al: Individualised neoantigen therapy mRNA-4157 (V940) plus
pembrolizumab versus pembrolizumab monotherapy in resected melanoma
(KEYNOTE-942): A randomised, phase 2b study. Lancet. 403:632–644.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaur R, Bhardwaj A and Gupta S: Cancer
treatment therapies: Traditional to modern approaches to combat
cancers. Mol Biol Rep. 50:9663–9676. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sahin U and Tureci O: Personalized
vaccines for cancer immunotherapy. Science. 359:1355–1360. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saxena M, van der Burg SH, Melief CJM and
Bhardwaj N: Therapeutic cancer vaccines. Nat Rev Cancer.
21:360–378. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Wang M, Peng X, Yang Y, Chen Q, Liu
J, She Q, Tan J, Lou C, Liao Z and Li X: mRNA vaccine in cancer
therapy: Current advance and future outlook. Clin Transl Med.
13:e13842023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fang E, Liu X, Li M, Zhang Z, Song L, Zhu
B, Wu X, Liu J, Zhao D and Li Y: Advances in COVID-19 mRNA vaccine
development. Signal Transduct Target Ther. 7:942022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Szabo GT, Mahiny AJ and Vlatkovic I:
COVID-19 mRNA vaccines: Platforms and current developments. Mol
Ther. 30:1850–1868. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brenner S, Jacob F and Meselson M: An
unstable intermediate carrying information from genes to ribosomes
for protein synthesis. Nature. 190:576–581. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Malone RW, Felgner PL and Verma IM:
Cationic liposome-mediated RNA transfection. Proc Natl Acad Sci
USA. 86:6077–6081. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wolff JA, Malone RW, Williams P, Chong W,
Acsadi G, Jani A and Felgner PL: Direct gene transfer into mouse
muscle in vivo. Science. 247(4949 Pt 1): 1465–1468. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jirikowski GF, Sanna PP,
Maciejewski-Lenoir D and Bloom FE: Reversal of diabetes insipidus
in Brattleboro rats: Intrahypothalamic injection of vasopressin
mRNA. Science. 255:996–998. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martinon F, Krishnan S, Lenzen G, Magné R,
Gomard E, Guillet JG, Lévy JP and Meulien P: Induction of
virus-specific cytotoxic T lymphocytes in vivo by
liposome-entrapped mRNA. Eur J Immunol. 23:1719–1722. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boczkowski D, Nair SK, Snyder D and Gilboa
E: Dendritic cells pulsed with RNA are potent antigen-presenting
cells in vitro and in vivo. J Exp Med. 184:465–472. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou WZ, Hoon DS, Huang SK, Fujii S,
Hashimoto K, Morishita R and Kaneda Y: RNA melanoma vaccine:
induction of antitumor immunity by human glycoprotein 100 mRNA
immunization. Hum Gene Ther. 10:2719–2724. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Zhang Z, Luo J, Han X, Wei Y and
Wei X: mRNA vaccine: A potential therapeutic strategy. Mol Cancer.
20:332021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wadhwa A, Aljabbari A, Lokras A, Foged C
and Thakur A: Opportunities and Challenges in the Delivery of
mRNA-based Vaccines. Pharmaceutics. 12:1022020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kackos CM, Surman SL, Jones BG, Sealy RE,
Jeevan T, Davitt CJH, Pustylnikov S, Darling TL, Boon ACM, Hurwitz
JL, et al: mRNA Vaccine Mitigates SARS-CoV-2 Infections and
COVID-19. Microbiol Spectr. 11:e04240222023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park JW, Lagniton PNP, Liu Y and Xu RH:
mRNA vaccines for COVID-19: What, why and how. Int J Biol Sci.
17:1446–1460. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gong H, Wen J, Luo R, Feng Y, Guo J, Fu H
and Zhou X: Integrated mRNA sequence optimization using deep
learning. Brief Bioinform. 24:bbad0012023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pardi N, Hogan MJ, Porter FW and Weissman
D: mRNA vaccines-a new era in vaccinology. Nat Rev Drug Discov.
17:261–279. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Herrera M, Kim J, Eygeris Y, Jozic A and
Sahay G: Illuminating endosomal escape of polymorphic lipid
nanoparticles that boost mRNA delivery. Biomater Sci. 9:4289–4300.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jyotsana N, Sharma A, Chaturvedi A, Budida
R, Scherr M, Kuchenbauer F, Lindner R, Noyan F, Sühs KW, Stangel M,
et al: Lipid nanoparticle-mediated siRNA delivery for safe
targeting of human CML in vivo. Ann Hematol. 98:1905–1918. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sahin U, Kariko K and Tureci O: mRNA-based
therapeutics-developing a new class of drugs. Nat Rev Drug Discov.
13:759–780. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cruz FM, Chan A and Rock KL: Pathways of
MHC I cross-presentation of exogenous antigens. Semin Immunol.
66:1017292023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rosa SS, Prazeres DMF, Azevedo AM and
Marques MPC: mRNA vaccines manufacturing: Challenges and
bottlenecks. Vaccine. 39:2190–2200. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu S, Yang K, Li R and Zhang L: mRNA
Vaccine Era-Mechanisms, drug platform and clinical prospection. Int
J Mol Sci. 21:20202020. View Article : Google Scholar
|
|
41
|
Talotta R: Do COVID-19 RNA-based vaccines
put at risk of immune-mediated diseases? In reply to 'potential
antigenic cross-reactivity between SARS-CoV-2 and human tissue with
a possible link to an increase in autoimmune diseases'. Clin
Immunol. 224:1086652021. View Article : Google Scholar
|
|
42
|
Chen YG and Hur S: Cellular origins of
dsRNA, their recognition and consequences. Nat Rev Mol Cell Biol.
23:286–301. 2022. View Article : Google Scholar :
|
|
43
|
Vaidyanathan S, Azizian KT, Haque AKMA,
Henderson JM, Hendel A, Shore S, Antony JS, Hogrefe RI, Kormann
MSD, Porteus MH and McCaffrey AP: Uridine depletion and chemical
modification increase Cas9 mRNA activity and reduce immunogenicity
without HPLC purification. Mol Ther Nucleic Acids. 12:530–542.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bernardo M, Tolstykh T, Zhang YA, Bangari
DS, Cao H, Heyl KA, Lee JS, Malkova NV, Malley K, Marquez E, et al:
An experimental model of anti-PD-1 resistance exhibits activation
of TGFβ and Notch pathways and is sensitive to local mRNA
immunotherapy. Oncoimmunology. 10:18812682021. View Article : Google Scholar
|
|
45
|
Yao R, Xie C and Xia X: Recent progress in
mRNA cancer vaccines. Hum Vaccin Immunother. 20:23071872024.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wilson RC and Carroll D: The daunting
economics of therapeutic genome editing. CRISPR J. 2:280–284. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Morrison C: $1-million price tag set for
Glybera gene therapy. Nat Biotechnol. 33:217–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jackson NAC, Kester KE, Casimiro D,
Gurunathan S and DeRosa F: The promise of mRNA vaccines: A biotech
and industrial perspective. NPJ Vaccines. 5:112020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Miao L, Zhang Y and Huang L: mRNA vaccine
for cancer immunotherapy. Mol Cancer. 20:412021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ita K: Coronavirus Disease (COVID-19):
Current status and prospects for drug and vaccine development. Arch
Med Res. 52:15–24. 2021. View Article : Google Scholar :
|
|
51
|
Van Nuffel AM, Wilgenhof S, Thielemans K
and Bonehill A: Overcoming HLA restriction in clinical trials:
Immune monitoring of mRNA-loaded DC therapy. Oncoimmunology.
1:1392–1394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ho W, Gao M, Li F, Li Z, Zhang XQ and Xu
X: Next-Generation Vaccines: Nanoparticle-Mediated DNA and mRNA
Delivery. Adv Healthc Mater. 10:e20018122021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Guevara ML, Persano F and Persano S:
Advances in lipid nanoparticles for mRNA-Based cancer
immunotherapy. Front Chem. 8:5899592020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
De Beuckelaer A, Grooten J and De Koker S:
Type I Interferons Modulate CD8(+) T Cell Immunity to mRNA
Vaccines. Trends Mol Med. 23:216–226. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Karam M and Daoud G: mRNA vaccines: Past,
present, future. Asian J Pharm Sci. 17:491–522. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhao P, Hou X, Yan J, Du S, Xue Y, Li W,
Xiang G and Dong Y: Long-term storage of lipid-like nanoparticles
for mRNA delivery. Bioact Mater. 5:358–363. 2020.PubMed/NCBI
|
|
57
|
Stitz L, Vogel A, Schnee M, Voss D, Rauch
S, Mutzke T, Ketterer T, Kramps T and Petsch B: A thermostable
messenger RNA based vaccine against rabies. PLoS Negl Trop Dis.
11:e00061082017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Petsch B, Schnee M, Vogel AB, Lange E,
Hoffmann B, Voss D, Schlake T, Thess A, Kallen KJ, Stitz L and
Kramps T: Protective efficacy of in vitro synthesized, specific
mRNA vaccines against influenza A virus infection. Nat Biotechnol.
30:1210–1216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dowdy SF: Overcoming cellular barriers for
RNA therapeutics. Nat Biotechnol. 35:222–229. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chaudhary N, Weissman D and Whitehead KA:
mRNA vaccines for infectious diseases: principles, delivery and
clinical translation. Nat Rev Drug Discov. 20:817–838. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Heine A, Juranek S and Brossart P:
Clinical and immunological effects of mRNA vaccines in malignant
diseases. Mol Cancer. 20:522021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Schlake T, Thess A, Fotin-Mleczek M and
Kallen KJ: Developing mRNA-vaccine technologies. RNA Biol.
9:1319–1330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Dobrowolski C, Paunovska K, Schrader
Echeverri E, Loughrey D, Da Silva Sanchez AJ, Ni H, Hatit MZC,
Lokugamage MP, Kuzminich Y, Peck HE, et al: Nanoparticle
single-cell multiomic readouts reveal that cell heterogeneity
influences lipid nanoparticle-mediated messenger RNA delivery. Nat
Nanotechnol. 17:871–879. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Solodushko V and Fouty B: Terminal
hairpins improve protein expression in IRES-initiated mRNA in the
absence of a cap and polyadenylated tail. Gene Ther. 30:620–627.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kowalski PS, Rudra A, Miao L and Anderson
DG: Delivering the messenger: Advances in technologies for
therapeutic mRNA delivery. Mol Ther. 27:710–728. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Park KS, Sun X, Aikins ME and Moon JJ:
Non-viral COVID-19 vaccine delivery systems. Adv Drug Deliv Rev.
169:137–151. 2021. View Article : Google Scholar
|
|
67
|
Kim J, Eygeris Y, Gupta M and Sahay G:
Self-assembled mRNA vaccines. Adv Drug Deliv Rev. 170:83–112. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kauffman KJ, Webber MJ and Anderson DG:
Materials for non-viral intracellular delivery of messenger RNA
therapeutics. J Control Release. 240:227–234. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Whitley J, Zwolinski C, Denis C, Maughan
M, Hayles L, Clarke D, Snare M, Liao H, Chiou S, Marmura T, et al:
Development of mRNA manufacturing for vaccines and therapeutics:
mRNA platform requirements and development of a scalable production
process to support early phase clinical trials. Transl Res.
242:38–55. 2022. View Article : Google Scholar
|
|
70
|
Kariko K, Muramatsu H, Ludwig J and
Weissman D: Generating the optimal mRNA for therapy: HPLC
purification eliminates immune activation and improves translation
of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res.
39:e1422011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Linares-Fernandez S, Lacroix C, Exposito
JY and Verrier B: Tailoring mRNA vaccine to balance innate/adaptive
immune response. Trends Mol Med. 26:311–323. 2020. View Article : Google Scholar
|
|
72
|
Engel BJ, Grindel BJ, Gray JP and Millward
SW: Purification of poly-dA oligonucleotides and mRNA-protein
fusions with dT(25)-OAS resin. Bioorg Med Chem Lett. 30:1269342020.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ye Z, Harmon J, Ni W, Li Y, Wich D and Xu
Q: The mRNA Vaccine Revolution: COVID-19 has launched the future of
vaccinology. ACS Nano. 17:15231–15253. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
To KKW and Cho WCS: An overview of
rational design of mRNA-based therapeutics and vaccines. Expert
Opin Drug Discov. 16:1307–1317. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kariko K, Kuo A and Barnathan E:
Overexpression of urokinase receptor in mammalian cells following
administration of the in vitro transcribed encoding mRNA. Gene
Ther. 6:1092–1100. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kallen KJ and Theβ A: A development that
may evolve into a revolution in medicine: mRNA as the basis for
novel, nucleotide-based vaccines and drugs. Ther Adv Vaccines.
2:10–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Holtkamp S, Kreiter S, Selmi A, Simon P,
Koslowski M, Huber C, Türeci O and Sahin U: Modification of
antigen-encoding RNA increases stability, translational efficacy,
and T-cell stimulatory capacity of dendritic cells. Blood.
108:4009–4017. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Leppek K, Byeon GW, Kladwang W,
Wayment-Steele HK, Kerr CH, Xu AF, Kim DS, Topkar VV, Choe C,
Rothschild D, et al: Combinatorial optimization of mRNA structure,
stability, and translation for RNA-based therapeutics. Nat Commun.
13:15362022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ramanathan A, Robb GB and Chan SH: mRNA
capping: Biological functions and applications. Nucleic Acids Res.
44:7511–7526. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li Y and Kiledjian M: Regulation of mRNA
decapping. Wiley Interdiscip Rev RNA. 1:253–265. 2010. View Article : Google Scholar
|
|
81
|
Geall AJ, Verma A, Otten GR, Shaw CA,
Hekele A, Banerjee K, Cu Y, Beard CW, Brito LA, Krucker T, et al:
Nonviral delivery of self-amplifying RNA vaccines. Proc Natl Acad
Sci USA. 109:14604–14609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Mohamad Razif MI, Nizar N, Zainal Abidin
NH, Muhammad Ali SN, Wan Zarimi WNN, Khotib J, Susanti D, Mohd
Jailani MT and Taher M: Emergence of mRNA vaccines in the
management of cancer. Expert Rev Vaccines. 22:629–642. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kim SC, Sekhon SS, Shin WR, Ahn G, Cho BK,
Ahn JY and Kim YH: Modifications of mRNA vaccine structural
elements for improving mRNA stability and translation efficiency.
Mol Cell Toxicol. 18:1–8. 2022. View Article : Google Scholar
|
|
84
|
Warminski M, Kowalska J, Nowak E, Kubacka
D, Tibble R, Kasprzyk R, Sikorski PJ, Gross JD, Nowotny M and
Jemielity J: Structural Insights into the interaction of clinically
relevant phosphorothioate mRNA Cap Analogs with translation
initiation factor 4E Reveal Stabilization via Electrostatic
Thio-Effect. ACS Chem Biol. 16:334–343. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kuhn AN, Diken M, Kreiter S, Selmi A,
Kowalska J, Jemielity J, Darzynkiewicz E, Huber C, Türeci O and
Sahin U: Phosphorothioate cap analogs increase stability and
translational efficiency of RNA vaccines in immature dendritic
cells and induce superior immune responses in vivo. Gene Ther.
17:961–971. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Grudzien-Nogalska E, Jemielity J, Kowalska
J, Darzynkiewicz E and Rhoads RE: Phosphorothioate cap analogs
stabilize mRNA and increase translational efficiency in mammalian
cells. RNA. 13:1745–1755. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Rydzik AM, Kulis M, Lukaszewicz M,
Kowalska J, Zuberek J, Darzynkiewicz ZM, Darzynkiewicz E and
Jemielity J: Synthesis and properties of mRNA cap analogs
containing imidodiphosphate moiety-fairly mimicking natural cap
structure, yet resistant to enzymatic hydrolysis. Bioorg Med Chem.
20:1699–1710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Henderson JM, Ujita A, Hill E,
Yousif-Rosales S, Smith C, Ko N, McReynolds T, Cabral CR,
Escamilla-Powers JR and Houston ME: Cap 1 Messenger RNA Synthesis
with Co-transcriptional CleanCap((R)) Analog by In Vitro
Transcription. Curr Protoc. 1:e392021. View Article : Google Scholar
|
|
89
|
Suknuntha K, Tao L, Brok-Volchanskaya V,
D'Souza SS, Kumar A and Slukvin I: Optimization of Synthetic mRNA
for highly efficient translation and its application in the
generation of endothelial and hematopoietic cells from human and
primate pluripotent stem cells. Stem Cell Rev Rep. 14:525–534.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ryczek N, Lys A and Makalowska I: The
Functional Meaning of 5'UTR in Protein-Coding Genes. Int J Mol Sci.
24:29762023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Creusot RJ, Chang P, Healey DG,
Tcherepanova IY, Nicolette CA and Fathman CG: A short pulse of IL-4
delivered by DCs electroporated with modified mRNA can both prevent
and treat autoimmune diabetes in NOD mice. Mol Ther. 18:2112–2120.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Asrani KH, Farelli JD, Stahley MR, Miller
RL, Cheng CJ, Subramanian RR and Brown JM: Optimization of mRNA
untranslated regions for improved expression of therapeutic mRNA.
RNA Biol. 15:756–762. 2018.PubMed/NCBI
|
|
93
|
Chakraborty C, Sharma AR, Bhattacharya M
and Lee SS: From COVID-19 to Cancer mRNA Vaccines: Moving from
bench to clinic in the vaccine landscape. Front Immunol.
12:6793442021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Beaudoin CA, Bartas M, Volná A, Pečinka P
and Blundell TL: Are There Hidden Genes in DNA/RNA Vaccines? Front
Immunol. 13:8019152022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Gustafsson C, Govindarajan S and Minshull
J: Codon bias and heterologous protein expression. Trends
Biotechnol. 22:346–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Passmore LA and Coller J: Roles of mRNA
poly(A) tails in regulation of eukaryotic gene expression. Nat Rev
Mol Cell Biol. 23:93–106. 2022. View Article : Google Scholar
|
|
97
|
Lima SA, Chipman LB, Nicholson AL, Chen
YH, Yee BA, Yeo GW, Coller J and Pasquinelli AE: Short poly(A)
tails are a conserved feature of highly expressed genes. Nat Struct
Mol Biol. 24:1057–1063. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
McNamara MA, Nair SK and Holl EK:
RNA-Based Vaccines in Cancer Immunotherapy. J Immunol Res.
2015:7945282015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chung JY, Thone MN and Kwon YJ: COVID-19
vaccines: The status and perspectives in delivery points of view.
Adv Drug Deliv Rev. 170:1–25. 2021. View Article : Google Scholar
|
|
100
|
Islam MA, Rice J, Reesor E, Zope H, Tao W,
Lim M, Ding J, Chen Y, Aduluso D, Zetter BR, et al: Adjuvant-pulsed
mRNA vaccine nanoparticle for immunoprophylactic and therapeutic
tumor suppression in mice. Biomaterials. 266:1204312021. View Article : Google Scholar
|
|
101
|
De Keersmaecker B, Claerhout S, Carrasco
J, Bar I, Corthals J, Wilgenhof S, Neyns B and Thielemans K: TriMix
and tumor antigen mRNA electroporated dendritic cell vaccination
plus ipilimumab: link between T-cell activation and clinical
responses in advanced melanoma. J Immunother Cancer. 8:e0003292020.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Franzoni G, Anfossi A, De Ciucis CG,
Mecocci S, Carta T, Dei Giudici S, Fruscione F, Zinellu S, Vito G,
Graham SP, et al: Targeting Toll-Like Receptor 2: Polarization of
Porcine Macrophages by a Mycoplasma-Derived Pam2cys Lipopeptide.
Vaccines (Basel). 9:6922021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Gu Y, Yang J, He C, Zhao T, Lu R, Liu J,
Mo X, Wen F and Shi H: Incorporation of a Toll-like receptor 2/6
agonist potentiates mRNA vaccines against cancer and infectious
diseases. Signal Transduct Target Ther. 8:2732023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ulmer JB, Mason PW, Geall A and Mandl CW:
RNA-based vaccines. Vaccine. 30:4414–4418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
McCullough KC, Milona P, Thomann-Harwood
L, Démoulins T, Englezou P, Suter R and Ruggli N: Self-Amplifying
Replicon RNA Vaccine Delivery to Dendritic Cells by Synthetic
Nanoparticles. Vaccines (Basel). 2:735–754. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Maruggi G, Zhang C, Li J, Ulmer JB and Yu
D: mRNA as a transformative technology for vaccine development to
control infectious diseases. Mol Ther. 27:757–772. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhuang X, Qi Y, Wang M, Yu N, Nan F, Zhang
H, Tian M, Li C, Lu H and Jin N: mRNA Vaccines Encoding the HA
Protein of Influenza A H1N1 virus delivered by cationic lipid
nanoparticles induce protective immune responses in mice. Vaccines
(Basel). 8:1232020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Kallen KJ, Heidenreich R, Schnee M, Petsch
B, Schlake T, Thess A, Baumhof P, Scheel B, Koch SD and
Fotin-Mleczek M: A novel, disruptive vaccination technology:
Self-adjuvanted RNActive((R)) vaccines. Hum Vaccin Immunother.
9:2263–2276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Leal L, Guardo AC, Morón-López S, Salgado
M, Mothe B, Heirman C, Pannus P, Vanham G, van den Ham HJ, Gruters
R, et al: Phase I clinical trial of an intranodally administered
mRNA-based therapeutic vaccine against HIV-1 infection. AIDS.
32:2533–2545. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Jong W, Leal L, Buyze J, Pannus P, Guardo
A, Salgado M, Mothe B, Molto J, Moron-Lopez S, Gálvez C, et al:
Therapeutic Vaccine in Chronically HIV-1-Infected Patients: A
Randomized, Double-Blind, Placebo-Controlled Phase IIa Trial with
HTI-TriMix. Vaccines (Basel). 7:2092019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Egan KP, Hook LM, Naughton A, Pardi N,
Awasthi S, Cohen GH, Weissman D and Friedman HM: An HSV-2
nucleoside-modified mRNA genital herpes vaccine containing
glycoproteins gC, gD, and gE protects mice against HSV-1 genital
lesions and latent infection. PLoS Pathog. 16:e10087952020.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wollner CJ, Richner M, Hassert MA, Pinto
AK, Brien JD and Richner JM: A Dengue Virus Serotype 1 mRNA-LNP
vaccine elicits protective immune responses. J Virol. 95:e02482–20.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wollner CJ and Richner JM: mRNA Vaccines
against Flaviviruses. Vaccines (Basel). 9:1482021. View Article : Google Scholar
|
|
114
|
Rzymski P, Szuster-Ciesielska A,
Dzieciątkowski T, Gwenzi W and Fal A: mRNA vaccines: The future of
prevention of viral infections? J Med Virol. 95:e285722023.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Richner JM, Himansu S, Dowd KA, Butler SL,
Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, et
al: Modified mRNA Vaccines Protect against Zika Virus Infection.
Cell. 169:1762017. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Parums DV: Editorial: mRNA Vaccines and
Future Epidemic, Pandemic, and Endemic Zoonotic Virus Infections.
Med Sci Monit. 27:e9329152021.PubMed/NCBI
|
|
117
|
Haynes BF: A new vaccine to battle
covid-19. N Engl J Med. 384:470–471. 2021. View Article : Google Scholar
|
|
118
|
Fairweather D, Beetler DJ, Di Florio DN,
Musigk N, Heidecker B and Cooper LT Jr: COVID-19, myocarditis and
pericarditis. Circ Res. 132:1302–1319. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Maruggi G, Chiarot E, Giovani C, Buccato
S, Bonacci S, Frigimelica E, Margarit I, Geall A, Bensi G and
Maione D: Immunogenicity and protective efficacy induced by
self-amplifying mRNA vaccines encoding bacterial antigens. Vaccine.
35:361–368. 2017. View Article : Google Scholar
|
|
120
|
Jawalagatti V, Kirthika P and Lee JH: Oral
mRNA Vaccines Against Infectious Diseases-A Bacterial Perspective
(Invited). Front Immunol. 13:8848622022. View Article : Google Scholar
|
|
121
|
Beck JD, Reidenbach D, Salomon N, Sahin U,
Türeci Ö, Vormehr M and Kranz LM: mRNA therapeutics in cancer
immunotherapy. Mol Cancer. 20:692021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Vishweshwaraiah YL and Dokholyan NV: mRNA
vaccines for cancer immunotherapy. Front Immunol. 13:10290692022.
View Article : Google Scholar :
|
|
123
|
Wang F: Identification of tumor antigens
and immune subtypes of acute myeloid leukemia for mRNA vaccine
development. Clin Transl Oncol. 25:2204–2223. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Chung DJ, Sharma S, Rangesa M, DeWolf S,
Elhanati Y, Perica K and Young JW: Langerhans dendritic cell
vaccine bearing mRNA-encoded tumor antigens induces antimyeloma
immunity after autotransplant. Blood Adv. 6:1547–1558. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Wang S, Yang Y, Li L, Ma P, Jiang Y, Ge M,
Yu Y, Huang H, Fang Y, Jiang N, et al: Identification of Tumor
Antigens and Immune Subtypes of Malignant Mesothelioma for mRNA
Vaccine Development. Vaccines (Basel). 10:11682022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Lin H, Wang K, Xiong Y, Zhou L, Yang Y,
Chen S, Xu P, Zhou Y, Mao R, Lv G, et al: Identification of tumor
antigens and immune subtypes of glioblastoma for mRNA vaccine
development. Front Immunol. 13:7732642022. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zhou Q, Yan X, Zhu H, Xin Z, Zhao J, Shen
W, Yin W, Guo Y, Xu H, Zhao M, et al: Identification of three tumor
antigens and immune subtypes for mRNA vaccine development in
diffuse glioma. Theranostics. 11:9775–9790. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Xu H, Zheng X, Zhang S, Yi X, Zhang T, Wei
Q, Li H and Ai J: Tumor antigens and immune subtypes guided mRNA
vaccine development for kidney renal clear cell carcinoma. Mol
Cancer. 20:1592021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Rojas LA, Sethna Z, Soares KC, Olcese C,
Pang N, Patterson E, Lihm J, Ceglia N, Guasp P, Chu A, et al:
Personalized RNA neoantigen vaccines stimulate T cells in
pancreatic cancer. Nature. 618:144–150. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
mRNA Vaccine Slows Melanoma Recurrence.
Cancer Discov. 13:12782023. View Article : Google Scholar
|
|
131
|
Sun Z, Jing C, Zhan H, Guo X, Suo N, Kong
F, Tao W, Xiao C, Hu D, Wang H and Jiang S: Identification of tumor
antigens and immune landscapes for bladder urothelial carcinoma
mRNA vaccine. Front Immunol. 14:10974722023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Kiousi E, Lyraraki V, Mardiki GL, Stachika
N, Damianou AK, Malainou CP, Syrigos N, Gomatou G and Kotteas E:
Progress and Challenges of Messenger RNA Vaccines in the
Therapeutics of NSCLC. Cancers (Basel). 15:55892023. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Zheng X, Xu H, Yi X, Zhang T, Wei Q, Li H
and Ai J: Tumor-antigens and immune landscapes identification for
prostate adenocarcinoma mRNA vaccine. Mol Cancer. 20:1602021.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Wu Y, Li Z, Lin H and Wang H:
Identification of tumor antigens and immune subtypes of high-grade
serous ovarian cancer for mRNA vaccine development. J Cancer.
14:2655–2669. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Song J, Zhang Y, Zhou C, Zhan J, Cheng X,
Huang H, Mao S and Zong Z: The dawn of a new Era: mRNA vaccines in
colorectal cancer immunotherapy. Int Immunopharmacol.
132:1120372024. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Sumi T, Koshino Y, Michimata H, Nagayama
D, Watanabe H, Yamada Y and Chiba H: Cytokine release syndrome in a
patient with non-small cell lung cancer on ipilimumab and nivolumab
maintenance therapy after vaccination with the mRNA-1273 vaccine: A
case report. Transl Lung Cancer Res. 11:1973–1976. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Rausch S, Schwentner C, Stenzl A and Bedke
J: mRNA vaccine CV9103 and CV9104 for the treatment of prostate
cancer. Hum Vaccin Immunother. 10:3146–3152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Liu L, Wang Y, Miao L, Liu Q, Musetti S,
Li J and Huang L: Combination Immunotherapy of MUC1 mRNA
Nano-vaccine and CTLA-4 Blockade Effectively Inhibits Growth of
Triple Negative Breast Cancer. Mol Ther. 26:45–55. 2018. View Article : Google Scholar :
|
|
139
|
Liu C, Papukashvili D, Dong Y, Wang X, Hu
X, Yang N, Cai J, Xie F, Rcheulishvili N and Wang PG:
Identification of Tumor Antigens and Design of mRNA vaccine for
colorectal cancer based on the immune subtype. Front Cell Dev Biol.
9:7835272022. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Huang X, Zhang G, Tang TY, Gao X and Liang
TB: Personalized pancreatic cancer therapy: From the perspective of
mRNA vaccine. Mil Med Res. 9:532022.PubMed/NCBI
|
|
141
|
Samuel N and Hudson TJ: The molecular and
cellular heterogeneity of pancreatic ductal adenocarcinoma. Nat Rev
Gastroenterol Hepatol. 9:77–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Makohon-Moore AP, Zhang M, Reiter JG,
Bozic I, Allen B, Kundu D, Chatterjee K, Wong F, Jiao Y, Kohutek
ZA, et al: Limited heterogeneity of known driver gene mutations
among the metastases of individual patients with pancreatic cancer.
Nat Genet. 49:358–366. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Bear AS, Vonderheide RH and O'Hara MH:
Challenges and opportunities for pancreatic cancer immunotherapy.
Cancer Cell. 38:788–802. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Kang N, Zhang S and Wang Y: A personalized
mRNA vaccine has exhibited potential in the treatment of pancreatic
cancer. Holist Integr Oncol. 2:182023. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Tan H, Yu T, Liu C, Wang Y, Jing F, Ding
Z, Liu J and Shi H: Identifying tumor antigens and immuno-subtyping
in colon adenocarcinoma to facilitate the development of mRNA
vaccine. Cancer Med. 11:4656–4672. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Ping H, Yu W, Gong X, Tong X, Lin C, Chen
Z, Cai C, Guo K and Ke H: Analysis of melanoma tumor antigens and
immune subtypes for the development of mRNA vaccine. Invest New
Drugs. 40:1173–1184. 2022. View Article : Google Scholar :
|
|
147
|
Sittplangkoon C, Alameh MG, Weissman D,
Lin PJC, Tam YK, Prompetchara E and Palaga T: mRNA vaccine with
unmodified uridine induces robust type I interferon-dependent
anti-tumor immunity in a melanoma model. Front Immunol.
13:9830002022. View Article : Google Scholar :
|
|
148
|
Li RQ, Wang W, Yan L, Song LY, Guan X,
Zhang W and Lian J: Identification of tumor antigens and immune
subtypes in breast cancer for mRNA vaccine development. Front
Oncol. 12:9737122022. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Niemi JVL, Sokolov AV and Schioth HB:
Neoantigen vaccines; clinical trials, classes, indications,
adjuvants and combinatorial treatments. Cancers (Basel).
14:51632022. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Oberli MA, Reichmuth AM, Dorkin JR,
Mitchell MJ, Fenton OS, Jaklenec A, Anderson DG, Langer R and
Blankschtein D: Lipid Nanoparticle Assisted mRNA delivery for
potent cancer immunotherapy. Nano Lett. 17:1326–1335. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Mellor AL, Keskin DB, Johnson T, Chandler
P and Munn DH: Cells expressing indoleamine 2,3-dioxygenase inhibit
T cell responses. J Immunol. 168:3771–3776. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Mellman I, Coukos G and Dranoff G: Cancer
immunotherapy comes of age. Nature. 480:480–489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Conry RM, LoBuglio AF, Wright M, Sumerel
L, Pike MJ, Johanning F, Benjamin R, Lu D and Curiel DT:
Characterization of a messenger RNA polynucleotide vaccine vector.
Cancer Res. 55:1397–1400. 1995.PubMed/NCBI
|
|
155
|
Huang X, Tang T, Zhang G and Liang T:
Identification of tumor antigens and immune subtypes of
cholangiocarcinoma for mRNA vaccine development. Mol Cancer.
20:502021. View Article : Google Scholar :
|
|
156
|
Guo Y, Lei K and Tang L: Neoantigen
Vaccine delivery for personalized anticancer immunotherapy. Front
Immunol. 9:14992018. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Grabbe S, Haas H, Diken M, Kranz LM,
Langguth P and Sahin U: Translating nanoparticulate-personalized
cancer vaccines into clinical applications: Case study with
RNA-lipoplexes for the treatment of melanoma. Nanomedicine (Lond).
11:2723–2734. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Esprit A, de Mey W, Bahadur Shahi R,
Thielemans K, Franceschini L and Breckpot K: Neo-Antigen mRNA
Vaccines. Vaccines (Basel). 8:7762020. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Blass E and Ott PA: Advances in the
development of personalized neoantigen-based therapeutic cancer
vaccines. Nat Rev Clin Oncol. 18:215–229. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Cafri G, Gartner JJ, Zaks T, Hopson K,
Levin N, Paria BC, Parkhurst MR, Yossef R, Lowery FJ and Jafferji
MS: mRNA vaccine-induced neoantigen-specific T cell immunity in
patients with gastrointestinal cancer. J Clin Invest.
130:5976–5988. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Roberts ND, Kortschak RD, Parker WT,
Schreiber AW, Branford S, Scott HS, Glonek G and Adelson DL: A
comparative analysis of algorithms for somatic SNV detection in
cancer. Bioinformatics. 29:2223–2230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Karasaki T, Nagayama K, Kuwano H, Nitadori
JI, Sato M, Anraku M, Hosoi A, Matsushita H, Takazawa M, Ohara O,
et al: Prediction and prioritization of neoantigens: integration of
RNA sequencing data with whole-exome sequencing. Cancer Sci.
108:170–177. 2017. View Article : Google Scholar :
|
|
163
|
Horak P, Frohling S and Glimm H:
Integrating next-generation sequencing into clinical oncology:
strategies, promises and pitfalls. ESMO Open. 1:e0000942016.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Bassani-Sternberg M, Bräunlein E, Klar R,
Engleitner T, Sinitcyn P, Audehm S, Straub M, Weber J,
Slotta-Huspenina J, Specht K, et al: Direct identification of
clinically relevant neoepitopes presented on native human melanoma
tissue by mass spectrometry. Nat Commun. 7:134042016. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Kongsted P, Borch TH, Ellebaek E, Iversen
TZ, Andersen R, Met Ö, Hansen M, Lindberg H, Sengeløv L and Svane
IM: Dendritic cell vaccination in combination with docetaxel for
patients with metastatic castration-resistant prostate cancer: A
randomized phase II study. Cytotherapy. 19:500–513. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Ingels J, De Cock L, Mayer RL, Devreker P,
Weening K, Heyns K, Lootens N, De Smet S, Brusseel M, De Munter S,
et al: Small-scale manufacturing of neoantigen-encoding messenger
RNA for early-phase clinical trials. Cytotherapy. 24:213–222. 2022.
View Article : Google Scholar
|
|
167
|
Sahin U, Derhovanessian E, Miller M, Kloke
BP, Simon P, Löwer M, Bukur V, Tadmor AD, Luxemburger U, Schrörs B,
et al: Personalized RNA mutanome vaccines mobilize poly-specific
therapeutic immunity against cancer. Nature. 547:222–226. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Steitz J, Britten CM, Wölfel T and Tüting
T: Effective induction of anti-melanoma immunity following genetic
vaccination with synthetic mRNA coding for the fusion protein
EGFP.TRP2. Cancer Immunol Immunother. 55:246–253. 2006. View Article : Google Scholar
|
|
169
|
Weide B, Pascolo S, Scheel B,
Derhovanessian E, Pflugfelder A, Eigentler TK, Pawelec G, Hoerr I,
Rammensee HG and Garbe C: Direct injection of protamine-protected
mRNA: results of a phase 1/2 vaccination trial in metastatic
melanoma patients. J Immunother. 32:498–507. 2009. View Article : Google Scholar
|
|
170
|
Mai Y, Guo J, Zhao Y, Ma S, Hou Y and Yang
J: Intranasal delivery of cationic liposome-protamine complex mRNA
vaccine elicits effective anti-tumor immunity. Cell Immunol.
354:1041432020. View Article : Google Scholar
|
|
171
|
Sahin U, Oehm P, Derhovanessian E,
Jabulowsky RA, Vormehr M, Gold M, Maurus D, Schwarck-Kokarakis D,
Kuhn AN, Omokoko T, et al: An RNA vaccine drives immunity in
checkpoint-inhibitor-treated melanoma. Nature. 585:107–112. 2020.
View Article : Google Scholar
|
|
172
|
Conlon KC, Miljkovic MD and Waldmann TA:
Cytokines in the Treatment of Cancer. J Interferon Cytokine Res.
39:6–21. 2019. View Article : Google Scholar :
|
|
173
|
Di Trani CA, Fernandez-Sendin M, Cirella
A, Segués A, Olivera I, Bolaños E, Melero I and Berraondo P:
Advances in mRNA-based drug discovery in cancer immunotherapy.
Expert Opin Drug Discov. 17:41–53. 2022. View Article : Google Scholar
|
|
174
|
Komel T, Bosnjak M, Kranjc Brezar S, De
Robertis M, Mastrodonato M, Scillitani G, Pesole G, Signori E,
Sersa G and Cemazar M: Gene electrotransfer of IL-2 and IL-12
plasmids effectively eradicated murine B16.F10 melanoma.
Bioelectrochemistry. 141:1078432021. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Etxeberria I, Bolaños E, Quetglas JI, Gros
A, Villanueva A, Palomero J, Sánchez-Paulete AR, Piulats JM,
Matias-Guiu X, Olivera I, et al: Intratumor Adoptive Transfer of
IL-12 mRNA Transiently Engineered Antitumor CD8(+) T Cells. Cancer
Cell. 36:613–629 e7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Hewitt SL, Bailey D, Zielinski J, Apte A,
Musenge F, Karp R, Burke S, Garcon F, Mishra A, Gurumurthy S, et
al: Intratumoral IL12 mRNA Therapy Promotes TH1 transformation of
the tumor microenvironment. Clin Cancer Res. 26:6284–6298. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Hotz C, Wagenaar TR, Gieseke F, Bangari
DS, Callahan M, Cao H, Diekmann J, Diken M, Grunwitz C, Hebert A,
et al: Local delivery of mRNA-encoded cytokines promotes antitumor
immunity and tumor eradication across multiple preclinical tumor
models. Sci Transl Med. 13:eabc78042021. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Hewitt SL, Bai A, Bailey D, Ichikawa K,
Zielinski J, Karp R, Apte A, Arnold K, Zacharek SJ, Iliou MS, et
al: Durable anticancer immunity from intratumoral administration of
IL-23, IL-36ү, and OX40L mRNAs. Sci Transl Med. 11:eaat91432019.
View Article : Google Scholar
|
|
179
|
Loomis KH, Lindsay KE, Zurla C, Bhosle SM,
Vanover DA, Blanchard EL, Kirschman JL, Bellamkonda RV and
Santangelo PJ: In Vitro Transcribed mRNA vaccines with programmable
stimulation of innate immunity. Bioconjug Chem. 29:3072–3083. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Jansen Y, Kruse V, Corthals J, Schats K,
van Dam PJ, Seremet T, Heirman C, Brochez L, Kockx M, Thielemans K
and Neyns B: A randomized controlled phase II clinical trial on
mRNA electroporated autologous monocyte-derived dendritic cells
(TriMixDC-MEL) as adjuvant treatment for stage III/IV melanoma
patients who are disease-free following the resection of
macrometastases. Cancer Immunol Immunother. 69:2589–2598. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Schlake T, Thran M, Fiedler K, Heidenreich
R, Petsch B and Fotin-Mleczek M: mRNA: A novel avenue to antibody
therapy? Mol Ther. 27:773–784. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Thran M, Mukherjee J, Pönisch M, Fiedler
K, Thess A, Mui BL, Hope MJ, Tam YK, Horscroft N, Heidenreich R, et
al: mRNA mediates passive vaccination against infectious agents,
toxins, and tumors. EMBO Mol Med. 9:1434–1447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Dahlen E, Veitonmaki N and Norlen P:
Bispecific antibodies in cancer immunotherapy. Ther Adv Vaccines
Immunother. 6:3–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Thomas A, Teicher BA and Hassan A:
Antibody-drug conjugates for cancer therapy. Lancet Oncol.
17:e254–e262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Page DB, Postow MA, Callahan MK, Allison
JP and Wolchok JD: Immune modulation in cancer with antibodies.
Annu Rev Med. 65:185–202. 2014. View Article : Google Scholar
|
|
186
|
Hudis CA: Trastuzumab-mechanism of action
and use in clinical practice. N Engl J Med. 357:39–51. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Diamantis N and Banerji U: Antibody-drug
conjugates-an emerging class of cancer treatment. Br J Cancer.
114:362–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
de Miguel D, Lemke J, Anel A, Walczak H
and MartinezLostao L: Onto better TRAILs for cancer treatment. Cell
Death Differ. 23:733–747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Salles G, Barrett M, Foà R, Maurer J,
O'Brien S, Valente N, Wenger M and Maloney DG: Rituximab in B-Cell
Hematologic Malignancies: A review of 20 years of clinical
experience. Adv Ther. 34:2232–2273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Van Hoecke L, Verbeke R, Dewitte H,
Lentacker I, Vermaelen K, Breckpot K and Van Lint S: mRNA in cancer
immunotherapy: Beyond a source of antigen. Mol Cancer. 20:482021.
View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Rybakova Y, Kowalski PS, Huang Y, Gonzalez
JT, Heartlein MW, DeRosa F, Delcassian D and Anderson DG: mRNA
Delivery for Therapeutic Anti-HER2 antibody expression in vivo. Mol
Ther. 27:1415–1423. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Ballesteros-Briones MC, Martisova E,
Casales E, Silva-Pilipich N, Buñuales M, Galindo J, Mancheño U,
Gorraiz M, Lasarte JJ, Kochan G, et al: Short-Term Local Expression
of a PD-L1 Blocking Antibody from a Self-Replicating RNA Vector
induces potent antitumor responses. Mol Ther. 27:1892–1905. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Nisonoff A, Wissler FC and Lipman LN:
Properties of the major component of a peptic digest of rabbit
antibody. Science. 132:1770–1771. 1960. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Wang Y, Tiruthani K, Li S, Hu M, Zhong G,
Tang Y, Roy S, Zhang L, Tan J, Liao C and Liu R: mRNA delivery of a
bispecific single-domain antibody to polarize tumor-associated
macrophages and synergize immunotherapy against liver malignancies.
Adv Mater. 33:e20076032021. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Fudenberg HH, Drews G and Nisonoff A:
Serologic demonstration of dual specificity of rabbit bivalent
hybrid antibody. J Exp Med. 119:151–166. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Garber K: Bispecific antibodies rise
again. Nat Rev Drug Discov. 13:799–801. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Wu L, Wang W, Tian J, Qi C, Cai Z, Yan W,
Xuan S and Shang A: Engineered mRNA-expressed bispecific antibody
prevent intestinal cancer via lipid nanoparticle delivery.
Bioengineered. 12:12383–12393. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Zhao Y, Moon E, Carpenito C, Paulos CM,
Liu X, Brennan AL, Chew A, Carroll RG, Scholler J, Levine BL, et
al: Multiple injections of electroporated autologous T cells
expressing a chimeric antigen receptor mediate regression of human
disseminated tumor. Cancer Res. 70:9053–9061. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Rabinovich PM, Komarovskaya ME, Wrzesinski
SH, Alderman JL, Budak-Alpdogan T, Karpikov A, Guo H, Flavell RA,
Cheung NK, Weissman SM and Bahceci E: Chimeric receptor mRNA
transfection as a tool to generate antineoplastic lymphocytes. Hum
Gene Ther. 20:51–61. 2009. View Article : Google Scholar
|
|
200
|
Morgan RA, Yang JC, Kitano M, Dudley ME,
Laurencot CM and Rosenberg SA: Case report of a serious adverse
event following the administration of T cells transduced with a
chimeric antigen receptor recognizing ERBB2. Mol Ther. 18:843–851.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Brentjens R, Yeh R, Bernal Y, Riviere I
and Sadelain M: Treatment of chronic lymphocytic leukemia with
genetically targeted autologous T cells: Case report of an
unforeseen adverse event in a phase I clinical trial. Mol Ther.
18:666–668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Miliotou AN and Papadopoulou LC: CAR
T-cell Therapy: A new era in cancer immunotherapy. Curr Pharm
Biotechnol. 19:5–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Fiedler K, Lazzaro S, Lutz J, Rauch S and
Heidenreich R: mRNA cancer vaccines. Recent Results Cancer Res.
209:61–85. 2016. View Article : Google Scholar
|
|
204
|
Soundara Rajan T, Gugliandolo A, Bramanti
P and Mazzon E: In Vitro-Transcribed mRNA Chimeric Antigen Receptor
T Cell (IVT mRNA CAR T) therapy in hematologic and solid tumor
management: A preclinical update. Int J Mol Sci. 21:65142020.
View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Zhao Y, Zheng Z, Cohen CJ, Gattinoni L,
Palmer DC, Restifo NP, Rosenberg SA and Morgan RA: High-efficiency
transfection of primary human and mouse T lymphocytes using RNA
electroporation. Mol Ther. 13:151–159. 2006. View Article : Google Scholar
|
|
206
|
Yoon SH, Lee JM, Woo SJ, Park MJ, Park JS,
Kim HS, Park MY, Sohn HJ and Kim TG: Transfer of Her-2/neu
specificity into cytokine-induced killer (CIK) cells with RNA
encoding chimeric immune receptor (CIR). J Clin Immunol.
29:806–814. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Schaft N, Dörrie J, Müller I, Beck V,
Baumann S, Schunder T, Kämpgen E and Schuler G: A new way to
generate cytolytic tumor-specific T cells: Electroporation of RNA
coding for a T cell receptor into T lymphocytes. Cancer Immunol
Immunother. 55:1132–1141. 2006. View Article : Google Scholar
|
|
208
|
Boissel L, Betancur M, Wels WS, Tuncer H
and Klingemann H: Transfection with mRNA for CD19 specific chimeric
antigen receptor restores NK cell mediated killing of CLL cells.
Leuk Res. 33:1255–1259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Kenderian SS, Ruella M, Shestova O,
Klichinsky M, Aikawa V, Morrissette JJ, Scholler J, Song D, Porter
DL, Carroll M, et al: CD33-specific chimeric antigen receptor T
cells exhibit potent preclinical activity against human acute
myeloid leukemia. Leukemia. 29:1637–1647. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Birkholz K, Hombach A, Krug C, Reuter S,
Kershaw M, Kämpgen E, Schuler G, Abken H, Schaft N and Dörrie J:
Transfer of mRNA encoding recombinant immunoreceptors reprograms
CD4+ and CD8+ T cells for use in the adoptive immunotherapy of
cancer. Gene Ther. 16:596–604. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Barrett DM, Zhao Y, Liu X, Jiang S,
Carpenito C, Kalos M, Carroll RG, June CH and Grupp SA: Treatment
of advanced leukemia in mice with mRNA engineered T cells. Hum Gene
Ther. 22:1575–1586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Ang WX, Li Z, Chi Z, Du SH, Chen C, Tay
JC, Toh HC, Connolly JE, Xu XH and Wang S: Intraperitoneal
immunotherapy with T cells stably and transiently expressing
anti-EpCAM CAR in xenograft models of peritoneal carcinomatosis.
Oncotarget. 8:13545–13559. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Almasbak H, Rian E, Hoel HJ, Pulè M,
Wälchli S, Kvalheim G, Gaudernack G and Rasmussen AM: Transiently
redirected T cells for adoptive transfer. Cytotherapy. 13:629–640.
2011. View Article : Google Scholar
|
|
214
|
Miliotou AN and Papadopoulou LC: In
Vitro-Transcribed (IVT)-mRNA CAR Therapy Development. Methods Mol
Biol. 2086:87–117. 2020. View Article : Google Scholar
|
|
215
|
Foster JB, Choudhari N, Perazzelli J,
Storm J, Hofmann TJ, Jain P, Storm PB, Pardi N, Weissman D,
Waanders AJ, et al: Purification of mRNA Encoding Chimeric Antigen
Receptor Is Critical for Generation of a Robust T-Cell Response.
Hum Gene Ther. 30:168–178. 2019. View Article : Google Scholar :
|
|
216
|
Jetani H, Navarro-Bailón A, Maucher M,
Frenz S, Verbruggen C, Yeguas A, Vidriales MB, González M, Rial
Saborido J, Kraus S, et al: Siglec-6 is a novel target for CAR
T-cell therapy in acute myeloid leukemia. Blood. 138:1830–1842.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Lin L, Cho SF, Xing L, Wen K, Li Y, Yu T,
Hsieh PA, Chen H, Kurtoglu M, Zhang Y, et al: Preclinical
evaluation of CD8+ anti-BCMA mRNA CAR T cells for treatment of
multiple myeloma. Leukemia. 35:752–763. 2021. View Article : Google Scholar
|
|
218
|
Bontkes HJ, Kramer D, Ruizendaal JJ,
Meijer CJ and Hooijberg E: Tumor associated antigen and
interleukin-12 mRNA transfected dendritic cells enhance effector
function of natural killer cells and antigen specific T-cells. Clin
Immunol. 127:375–384. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Wilgenhof S, Van Nuffel AMT, Benteyn D,
Corthals J, Aerts C, Heirman C, Van Riet I, Bonehill A, Thielemans
K and Neyns B: A phase IB study on intravenous synthetic mRNA
electroporated dendritic cell immunotherapy in pretreated advanced
melanoma patients. Ann Oncol. 24:2686–2693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Figlin RA, Tannir NM, Uzzo RG, Tykodi SS,
Chen DYT, Master V, Kapoor A, Vaena D, Lowrance W, Bratslavsky G,
et al: Results of the ADAPT Phase 3 Study of Rocapuldencel-T in
combination with sunitinib as first-line therapy in patients with
metastatic renal cell carcinoma. Clin Cancer Res. 26:2327–2336.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Van Hoecke L, Van Lint S, Roose K, Van
Parys A, Vandenabeele P, Grooten J, Tavernier J, De Koker S and
Saelens X: Treatment with mRNA coding for the necroptosis mediator
MLKL induces antitumor immunity directed against neo-epitopes. Nat
Commun. 9:34172018. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Trepotec Z, Lichtenegger E, Plank C, Aneja
MK and Rudolph C: Delivery of mRNA therapeutics for the treatment
of hepatic diseases. Mol Ther. 27:794–802. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Sahu I, Haque AKMA, Weidensee B, Weinmann
P and Kormann MSD: Recent Developments in mRNA-Based protein
supplementation therapy to target lung diseases. Mol Ther.
27:803–823. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Patel S, Ryals RC, Weller KK, Pennesi ME
and Sahay G: Lipid nanoparticles for delivery of messenger RNA to
the back of the eye. J Control Release. 303:91–100. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Magadum A, Kaur K and Zangi L: mRNA-Based
protein replacement therapy for the heart. Mol Ther. 27:785–793.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Lescan M, Perl RM, Golombek S, Pilz M,
Hann L, Yasmin M, Behring A, Keller T, Nolte A, Gruhn F, et al: De
Novo Synthesis of elastin by exogenous delivery of synthetic
modified mRNA into Skin and Elastin-Deficient Cells. Mol Ther
Nucleic Acids. 11:475–484. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Baba M, Itaka K, Kondo K, Yamasoba T and
Kataoka K: Treatment of neurological disorders by introducing mRNA
in vivo using polyplex nanomicelles. J Control Release. 201:41–48.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Kormann MS, Hasenpusch G, Aneja MK, Nica
G, Flemmer AW, Herber-Jonat S, Huppmann M, Mays LE, Illenyi M,
Schams A, et al: Expression of therapeutic proteins after delivery
of chemically modified mRNA in mice. Nat Biotechnol. 29:154–157.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Van Hoecke L and Roose K: How mRNA
therapeutics are entering the monoclonal antibody field. J Transl
Med. 17:542019. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Raab M, Kostova I, Peña-Llopis S, Fietz D,
Kressin M, Aberoumandi SM, Ullrich E, Becker S, Sanhaji M and
Strebhardt K: Rescue of p53 functions by in vitro-transcribed mRNA
impedes the growth of high-grade serous ovarian cancer. Cancer
Commun (Lond). 44:101–126. 2024. View Article : Google Scholar
|
|
231
|
Yin H, Kauffman KJ and Anderson DG:
Delivery technologies for genome editing. Nat Rev Drug Discov.
16:387–399. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Cox DB, Platt RJ and Zhang F: Therapeutic
genome editing: Prospects and challenges. Nat Med. 21:121–131.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Wang SW, Gao C, Zheng YM, Yi L, Lu JC,
Huang XY, Cai JB, Zhang PF, Cui YH and Ke AW: Current applications
and future perspective of CRISPR/Cas9 gene editing in cancer. Mol
Cancer. 21:572022. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Ling K, Dou Y, Yang N, Deng L, Wang Y, Li
Y, Yang L, Chen C, Jiang L, Deng Q, et al: Genome editing mRNA
nanotherapies inhibit cervical cancer progression and regulate the
immunosuppressive microenvironment for adoptive T-cell therapy. J
Control Release. 360:496–513. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Jo JI, Gao JQ and Tabata Y:
Biomaterial-based delivery systems of nucleic acid for regenerative
research and regenerative therapy. Regen Ther. 11:123–130. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Kwon H, Kim M, Seo Y, Moon YS, Lee HJ, Lee
K and Lee H: Emergence of synthetic mRNA: In vitro synthesis of
mRNA and its applications in regenerative medicine. Biomaterials.
156:172–193. 2018. View Article : Google Scholar
|
|
237
|
Lin Z, Wu Y, Xu Y, Li G, Li Z and Liu T:
Mesenchymal stem cell-derived exosomes in cancer therapy
resistance: Recent advances and therapeutic potential. Mol Cancer.
21:1792022. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Ramos da Silva J, Bitencourt Rodrigues K,
Formoso Pelegrin G, Silva Sales N, Muramatsu H, de Oliveira Silva
M, Porchia BFMM, Moreno ACR, Aps LRMM, Venceslau-Carvalho AA, et
al: Single immunizations of self-amplifying or non-replicating
mRNA-LNP vaccines control HPV-associated tumors in mice. Sci Transl
Med. 15:eabn34642023. View Article : Google Scholar : PubMed/NCBI
|