Colorectal cancer (CRC), which includes cancers of
the colon and rectum, ranks as the third most prevalent cancer
worldwide, constituting 10% of all new cancer cases, and is the
second leading cause of cancer-related mortality, resulting in
>930,000 deaths annually (1,2).
Currently, surgical intervention, chemotherapy and targeted therapy
constitute the cornerstone of CRC treatment. However, at the time
of diagnosis, approximately one-quarter of patients with CRC are
diagnosed with advanced disease, and an additional 20% develop
distant metastases, frequently rendering surgical methods
insufficient for improving prognosis and ineffective for
identifying cancer that has propagated to adjacent organs (3). Thus, improving the prognosis of
patients with locally advanced rectal cancer and metastatic CRC
(mCRC) remains a pivotal and formidable challenge. Survival rates
have demonstrated that 70-75% of patients with mCRC survive beyond
1 year, 30-35% survive >3 years and <20% survive >5 years
(4,5). Furthermore, after neoadjuvant
chemoradiation therapy and total mesorectal excision surgery, 54%
of patients with rectal cancer relapse. Adjuvant chemotherapy or
radiotherapy is required for ~66% of patients with stage II-III
colon cancer and 50% of patients with stage II-III rectal cancer
(6). The challenge of treating
advanced CRC is compounded by resistance to chemotherapy,
radiotherapy, targeted therapy and immunotherapy. Encouragingly,
clinical evidence has demonstrated the effectiveness of neoantigens
(cancer-specific abnormal peptides) in generating immune responses
(7). Therefore, identifying novel
components of the host immune system as relevant biomarkers and
therapeutic targets is crucial. A previous study has emphasized the
role of the tumour microenvironment (TME) in treatment resistance
(8). Components of the TME, such
as tumour-associated macrophages (TAMs), regulatory T cells (Tregs)
and myeloid-derived suppressor cells (MDSCs), have been shown to
dampen the immune response and influence CRC progression (9-11).
As such, the potential roles of these cells in cancer metastasis
and therapy have garnered significant interest.
MDSCs constitute diverse immature cell populations
originating from the bone marrow, all of which exhibit
immunosuppressive activity (12).
As outlined by the dual signalling mode (13), three key processes occur within
MDSCs, namely, migration, expansion and activation, each of which
partially overlap and are facilitated by factors boosting
myelopoiesis and hindering differentiation of mature myeloid cells
and factors promoting the activation of MDSCs. Before these cells
can become functional within the TME, these steps are imperative
(14). Additionally, MDSCs
display several biochemical characteristics crucial for suppressing
immune responses, including enhanced signal transducer and
activator of transcription 3 (STAT3) expression, the induction of
endoplasmic reticulum (ER) stress (which can independently induce
apoptosis and regulate ER chaperones, protecting cells by ensuring
proper protein folding and preventing the accumulation of misfolded
proteins) and the expression of arginase 1 (ARG-1) and S100 calcium
binding protein A8/A9 (S100A8/A9) (15,16).
In healthy individuals, immature myeloid cells
(IMCs) differentiate into granulocytes, macrophages or dendritic
cells (DCs), eventually migrating to specific organs and tissues to
perform regular immune functions (17). However, under pathological
conditions or chronic inflammation, IMCs deviate from their typical
differentiation pathway (18),
evolving into granulocyte-monocyte progenitor cells under the
influence of granulocyte-macrophage colony-stimulating factor
(GM-CSF), granulocyte CSF (G-CSF) and interleukin (IL)-6 (19). These cells then gradually migrate
from the bone marrow into the peripheral blood and spleen where
they are activated by proinflammatory cytokines such as
interferon-γ (IFN-γ), IL-1β and IL-4, consequently forming
relatively immature cells termed MDSCs, which exhibit the
morphology of neutrophils and monocytes and possess
immunosuppressive functions (20-22).
MDSCs are typically divided into two subsets:
Granulocytic or polymorphonuclear-MDSCs (PMN-MDSCs) and
monocytic-MDSCs (M-MDSCs) (12,23,24), with considerably more PMN-MDSCs
than M-MDSCs noted in most tumours (18,25). MDSCs have an important role in the
malignant progression of tumours (26,27), and the presence of MDSCs has been
shown to obstruct the infiltration of CD8+ T cells into
tumours, promote Tregs and modulate natural killer (NK) cell
activity, among other functions (28,29). As such, the suppressive actions of
MDSCs contribute to tumour growth. Furthermore, the concentration
of MDSCs in the peripheral blood is positively associated with the
cancer stage; specifically, elevated levels indicate a more
advanced cancer stage (30).
MDSCs also promote tumour angiogenesis and create premetastatic
niches by suppressing immune cells (26,27). This dual activity encourages the
spread of cancer cells while concurrently diminishing the efficacy
of antitumour drugs and fostering drug resistance, consequently
influencing the inception, progression and prognosis of tumours
(31-33). Research has demonstrated that
distinct MDSC populations, which are involved in CRC (34) metastasis and progression through
multiple mechanisms, are detected in tumour tissues and peripheral
blood of patients with CRC (35).
The present review describes the mechanisms
underlying MDSC aggregation and migration and illustrates the role
of MDSCs in the CRC TME. The present review also surveys current
therapeutic strategies, providing insights that may benefit the
diagnosis, prognosis and treatment of CRC.
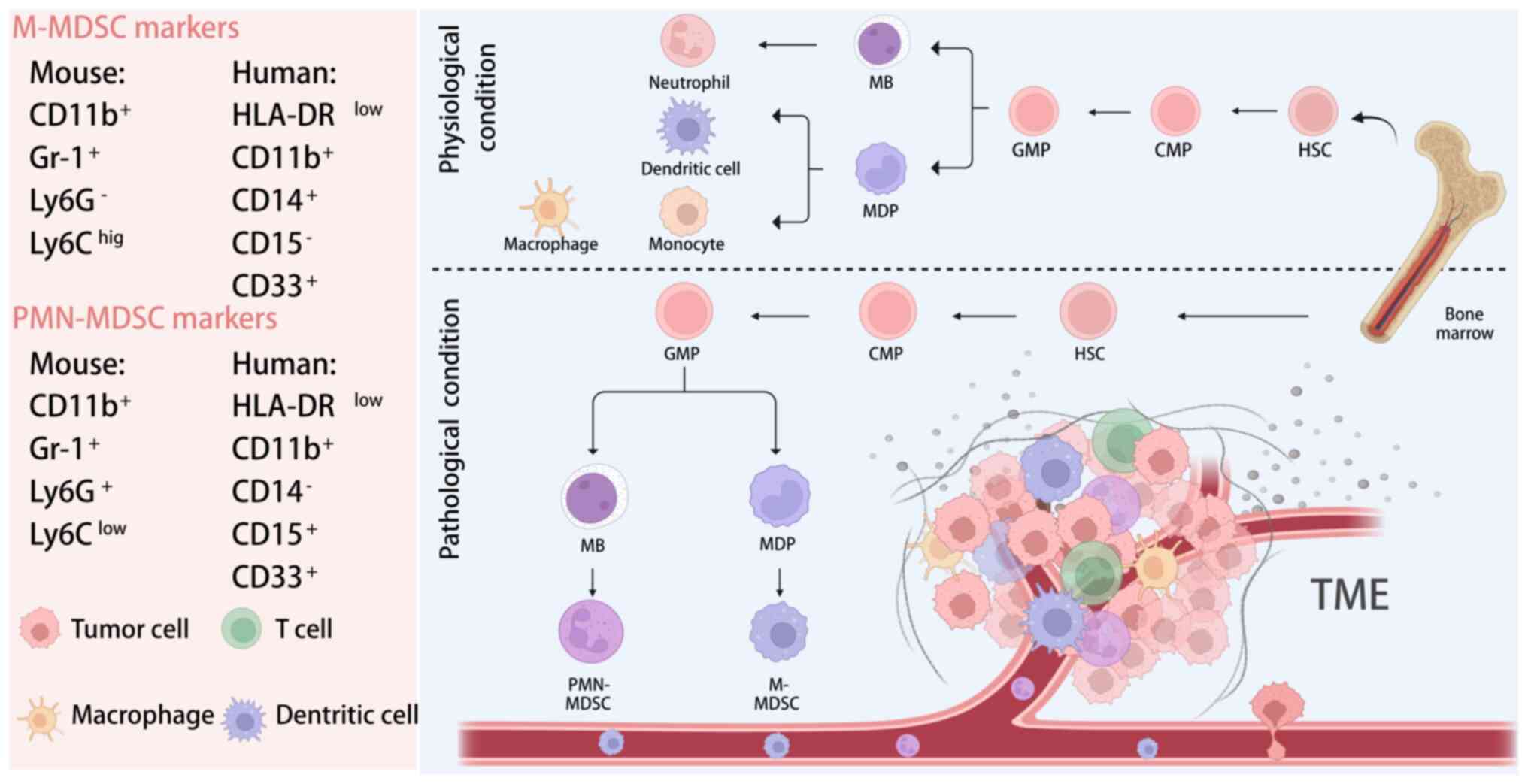
The phenomenon of extramedullary haematopoiesis and
neutrophilia frequently accompanies malignancy (such as CRC), and
these manifestations include cells with suppressive activity that
were initially termed IMCs (17).
With advances in research clarifying their origin and function,
these cells were ultimately designated MDSCs (36) (Fig.
1).
Initially, to distinguish immature MDSCs from normal
cells, researchers detected MDSCs in mice using surface biomarkers,
with the goal of gaining insight into human MDSCs. It was found
that MDSCs in mice are characterized by the markers, CD11b and
Gr-1, and that the Gr-1 antigen complex comprises the components
Ly-6G and Ly-6C. Specifically, mouse PMN-MDSCs are
CD11b+Gr-1+Ly6G+Ly6Clow
and are phenotypically and morphologically similar to neutrophils.
M-MDSCs are
CD11b+Gr-1+Ly6G−Ly6Chigh
and are phenotypically and morphologically similar to monocytes
(37-39). However, a subsequent study has
shown that the two characteristic markers of mouse MDSCs, CD11b and
Gr-1, are not optimal markers for distinguishing different species
of MDSCs in humans (40).
Researchers have identified key surface markers,
such as human leukocyte antigen DR (HLA-DR), CD11b and CD14, which
are intrinsic to monocytes, as pivotal in distinguishing human
MDSCs (41). Another signature of
M-MDSCs is the positive expression of C-C motif chemokine receptor
2 (CCR2) and C-X3-C motif chemokine receptor 1 (CX3CR1) (42,43). A subset of cells bearing the
phenotype of
HLA-DRlow/−CD11b+CD14−CD15+
has been categorized as granulocytic-MDSCs (12,23). Given their morphological and
phenotypic resemblance to neutrophils, they are also aptly referred
to as PMN-MDSCs. Conversely, cells characterized as
HLA-DRlow/−CD11b+CD14+CD15−
are termed M-MDSCs, given their resemblance to monocytes (12).
The aforementioned subtypes also exhibit distinct
T-cell suppressive activities and mechanisms. Additionally, human
M-MDSCs express more CD33 than PMN-MDSCs (44). The distinction among human
monocytes, neutrophils and MDSCs is achieved through the expression
of surface molecules such as HLA-DR and lectin-type oxidized LDL
receptor 1 or CD14, which serve as differentiating markers
(45-48). Previous investigations have
identified early-stage MDSCs (12,49) and fibrocytic MDSCs (50-52), with the former capable of colony
formation and distinguished by a lineage−
(Lin−) (including CD3, CD14, CD15, CD19 and CD56) HLA-DR
CD33+ phenotype. The latter, a subpopulation with
fibrocytic characteristics and immunosuppressive functions, differs
from umbilical cord blood precursors and can be identified by a
CD11blowCD11clowCD33 IL-4Ra+
phenotype. Furthermore, CD49d is a potential marker that
complements CD11b for MDSC categorization (53). A study by Alshetaiwi et al
(54) revealed that high
concentrations of MDSCs in the spleen and primary tumour sites
correspond to significant expression of both CD84 and junction
adhesion molecule-like, suggesting that CD84, which is typically a
lymphoid marker, may represent a novel and specific marker for
MDSCs in CRC.
Chemotaxis and the accumulation of MDSCs in the
colorectum are critical for colorectal tumorigenesis. Furthermore,
gene sequence and epigenetic variations are significant risk
factors for MDSC accumulation (33). In CRC, mutations in the oncogene,
Kirsten rat sarcoma viral oncogene homologue gene (KRAS), are
observed in up to 40% of cases and are associated with increased
tumour aggressiveness and metastasis (55-57). Previous studies have highlighted
the essential role of the SLC25A22 gene in enhancing KRAS
mutation-induced CRC immunosuppression by facilitating asparagine
binding and SRC phosphorylation activation (58,59). This process promotes ERK/ETS
proto-oncogene 2 signalling and drives C-X-C motif chemokine ligand
1 (CXCL1) transcription. The secreted CXCL1 then acts as a
chemoattractant for MDSCs through the chemokine C-X-C motif
receptor 2 (CXCR2), creating an immunosuppressive microenvironment
(60). Furthermore, MDSCs
suppress interferon regulatory factor 2 expression, resulting in
increased CXCL3 binding to CXCR2 on MDSCs and promoting MDSC
migration into the TME, leading to MDSC overaccumulation (58,61). YTH N6-methyladenosine RNA binding
protein 1 (YTHDF1) regulates CXCL1 expression via promoting p65
protein expression to activate NF-κB signal transduction, impacting
MDSC migration through the CXCL1/CXCR2 axis; furthermore, a
reduction in MDSC number is observed upon YTHDF1 gene knockdown
(62).
N6-methyladenosine (m6A) RNA methylation, a
posttranscriptional RNA modification, involves m6A methylase
complexes (writers), demethylases (erasers) and binding proteins
(readers) that regulate their respective axes, promoting normal
biological processes. However, aberrant m6A RNA methylation
promotes MDSC recruitment at the gene expression level, thereby
increasing CRC risk. For instance, the methyltransferase 3
methylase N6-adenosine-methyltransferase complex catalytic subunit
promotes m6A methylation of the transcription factor, basic
helix-loop-helix family member e41, which stimulates CXCL1
expression and mediates MDSC migration and aggregation in the TME
of CRC, forming an immunosuppressive environment and weakening the
immune system, promoting CRC development (63,64). Furthermore, the demethylase, Alk B
homologue 5 (Alkbh5), recruits MDSCs and diminishes NK and
CD8+ T cell populations by decreasing the mRNA stability
and demethylation of axis inhibition protein 2 mRNA, leading to its
dissociation from the m6A reader, insulin like growth factor 2 mRNA
binding protein 1 (65), thereby
resulting in Wnt/β-catenin pathway overactivation. Human Alkbh5
also contributes to gene splicing by placing m6A modifications near
splice sites, influencing MDSC migration and aggregation in the TME
(66-68). In addition, Alkbh5 affects changes
in metabolite content. Alkbh5 modulates monocarboxylate transporter
4 expression (a key enzyme that rapidly transports lactic acid to
plasma membranes) and lactate concentration in the TME (69). Additionally, lactate can increase
the frequency of MDSCs and upregulate expression of FoxP3, an
important transcription factor in Treg development and function
(68-70).
The pathogenesis, progression and metastasis of CRC
are intricately linked to genetic factors within the host as well
as the specific tissue milieu in which the cancer resides. This
local milieu, consisting of immune cells, malignant cells,
fibroblasts, various non-immune cells and connective tissues,
constitutes the TME (71). In
contrast to being a static entity, the TME is dynamic and complex.
Within this setting, common myeloid progenitor cells (CMPs) located
in the bone marrow can differentiate into MDSCs during tumour
progression. In response to inflammatory signals or tumour-released
factors, including growth factors, chemokines and inflammatory
cytokines, CMPs proliferate, promoting the recruitment and
expansion of MDSCs to the tumour locus (72). This process not only facilitates
the influx of IMCs but also inhibits the innate antitumour response
of the host.
CCL2, also known as monocyte chemotactic protein-1,
is a vital component of the CC chemokine family. This protein
significantly impacts the growth, progression and metastasis of
various tumours, including CRC (76-78). CCL2-mediated recruitment of the
CCR2 receptor, which has a high affinity for MDSCs, supports CRC
cell proliferation (79).
Evidence suggests that MDSC accumulation induced by CCL2 enhances
their immunosuppressive capabilities during colorectal
carcinogenesis, with a corresponding increase in CCL2 levels as the
cancer progresses (76,80). Moreover, reactive nitrogen species
produced by MDSCs can modify chemokines such as CCL2 to nitro-CCL2,
which preferentially recruits myeloid cells, in contrast to
unmodified CCL2, which attracts CD8+ T cells (81).
Conversely, MDSCs positive for CXCR2 can engage with
CXCL1/2/3, facilitating the recruitment of MDSC clusters in the
colon and rectum (61,82). The migration and recruitment
orchestrated by members of the CC family, including CCL2, CCL5 and
CSF1, predominantly involve M-MDSCs and monocytes. By contrast, CXC
family members, such as CXCL1, CXCL5, CXCL6, CXCL8 and CXCL12, are
more closely involved with the recruitment of PMN-MDSCs and
granulocytes in CRC. MDSCs express various chemokine receptors,
including CCR2, CCR5 and CXCR2 (21,47). Additionally, CXCL1 and CXCL2 are
crucial in the context of colitis-associated tumours and chronic
colonic inflammation (83).
The regulation of MDSCs extends beyond that of
chemokines to include other inflammatory mediators, such as
prostaglandins and histamine. For instance, histamine enhances the
proliferation of M-MDSCs and increases the expression of enzymes
such as inducible nitric oxide synthase (iNOS) and ARG-1 in the CRC
(84). Conversely, histamine
suppresses the expression of these enzymes in PMN-MDSCs while
promoting the production of the interleukins, IL-13 and IL-4
(85). Hence, histamine
differentially modulates M-MDSC and PMN-MDSC activities (86).
Additionally, cyclooxygenase-2 (COX-2)-synthesized
prostaglandin E2 (PGE2), an inflammatory mediator, is upregulated
in CRC (87). PGE2 not only
induces myeloid cells to secrete procarcinogenic factors, such as
IL-6, CXCL1 and G-CSF, that create a tumour-friendly
microenvironment, but also promotes tumour proliferation and MDSC
activation via STAT3 phosphorylation (87-91). The PGE2 receptor EP1-4, a set of
related G protein-coupled receptors, influences MDSC
differentiation in CRC (92). In
particular, interactions with EP4 promote the differentiation of
immunosuppressive M2 macrophages and MDSCs while diminishing the
proliferation of immunostimulatory M1 macrophages (93).
Leukotriene B4, a 5-lipoxygenase (5LO) derivative of
arachidonic acid, has a chemotactic impact on MDSCs, affecting
their aggregation. Reduced 5LO expression is associated with
decreased MDSC levels in circulation and diminished ARG-1 and iNOS
activity (94,95).
Hypoxia is a common condition within the CRC TME and
plays a crucial role in promoting MDSC proliferation.
Hypoxia-inducible factor 1α (HIF-1α) stimulates the production of
ectonucleoside triphosphate diphosphohydrolase 2 (ENTPD2), and
ENTPD2 functions as an exonuclease on MDSCs, significantly
contributing to their expansion within the CRC TME (17). Furthermore, hypoxia elevates the
levels of vascular endothelial growth factor (VEGF) and its
associated molecules. In addition to their known role in
angiogenesis, these factors also promote MDSC migration from the
bone marrow to the tumour bed (the original tumour site prior to
any treatment) thereby enhancing MDSC proliferation in the CRC TME
(96,97). Additionally, extracellular
vesicles such as exosomes, which are secreted by tumour cells,
directly stimulate the formation of MDSCs while modulating their
activity, a phenomenon observed in various tumour types, including
CRC (98,99).
The presence of gut microorganisms is important for
MDSC amplification and normal metabolism in the gastrointestinal
tract. A recent study has shown that the accumulation of anaerobic
Pseudomonas aeruginosa (Peptostreptococcus
anaerobius) in tumour lesions can mediate MDSC recruitment into
the CRC microenvironment and promote IL-23 secretion by MDSCs,
which leads to epithelial-mesenchymal transition (EMT) and CRC cell
resistance to chemotherapy (100). Fusobacterium nucleatum
plays a role in the early development of inflammatory bowel disease
and colorectal adenomas (101).
Patients with CRC with higher levels of F. nucleatum in the
TME have elevated numbers of both M-MDSCs and PMN-MDSCs and
suppressed NK cell numbers, conditions that promote CRC development
(102-104).
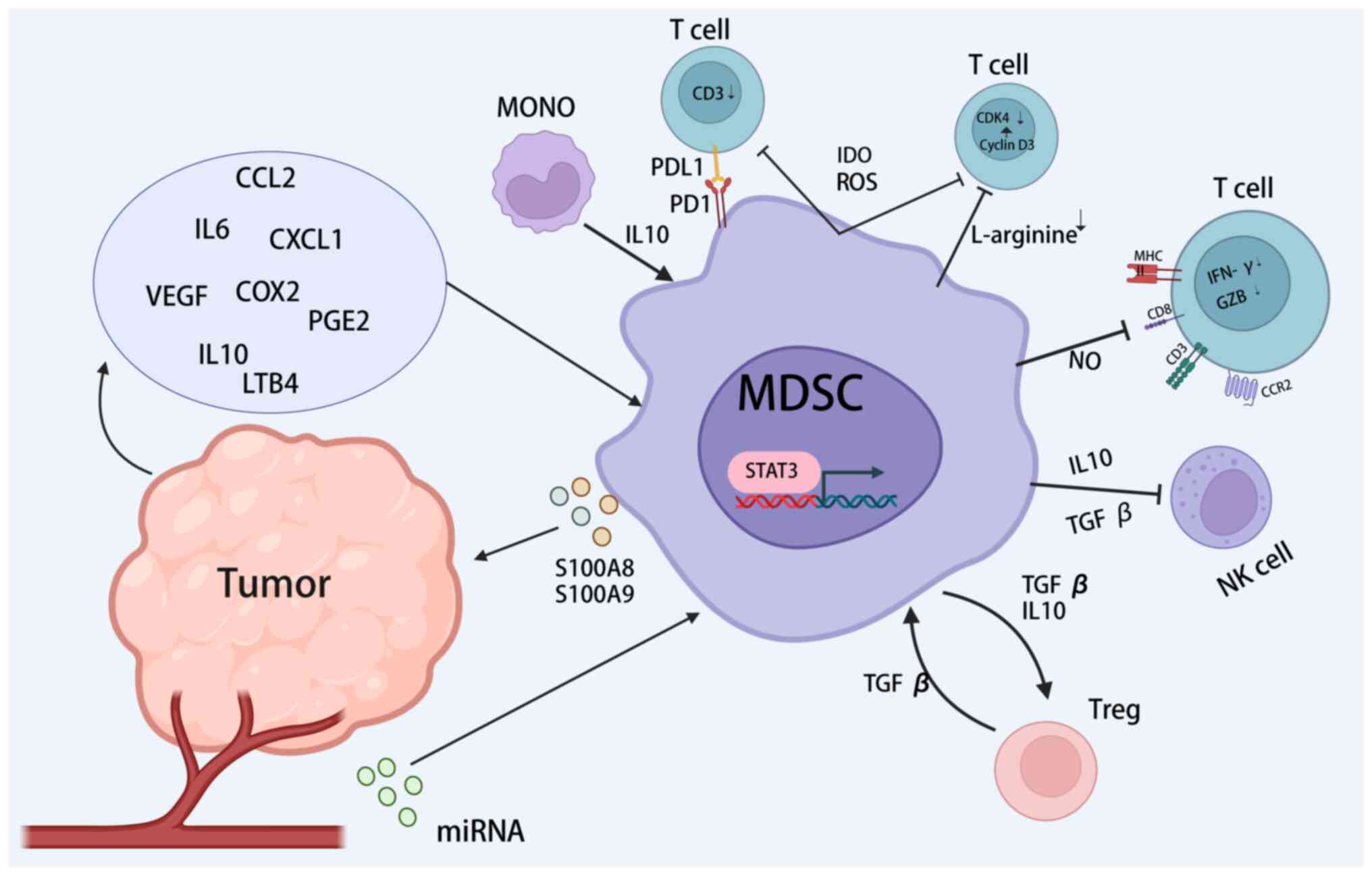
The immunosuppressive functions of activated MDSCs
are mediated by intercellular contacts and paracrine or exosomal
mechanisms. These functions inhibit immune cell activity, including
the degradation of L-arginine, the production of reactive oxygen
and nitrogen species (RONS), engagement of the programmed cell
death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) axis, the
production of immunosuppressive cytokines such as IL-10 and
transforming growth factor-β (TGF-β), the suppression of T cells
and the induction of other immunosuppressive cells (105-107) (Fig. 2). Among the subtypes of MDSCs,
M-MDSCs are characterized by elevated secretion of TGF-β and the
inhibitory cytokines, IL-10, ARG-1 and iNOS, which collectively
suppress non-specific immune responses. By contrast, PMN-MDSCs
predominantly produce high levels of reactive oxygen species (ROS)
and PGE2, and influence antigen-specific immune responses through
direct cell-to-cell interactions (47).
The metabolism of L-arginine within the TME is
fundamentally associated with the immunosuppressive effects exerted
by MDSCs. These cells can metabolize L-arginine through elevated
expression of ARG-1 and iNOS, both of which notably suppress T-cell
function (20,108,109). The depletion of L-arginine by
MDSCs leads to reduced expression of the T cell receptor (TCR)-ζ
chain, diminished production of IFN-γ and decreased proliferation
of activated T cells (110,111). ARG-1, in particular, transforms
environmental arginine into urea and L-ornithine, disrupting T-cell
activation by increasing cell cycle protein D3 and cyclin-dependent
kinase 4 levels and consequently halting the T-cell cycle at the
G0-G1 phase (112). Studies in
colon cancer models have shown that ARG-1 impairs the expansion and
functionality of CD8+ T cells and NK cells within
tumours and hinders the production of inflammatory cytokines and
interferon-inducible genes (113,114). iNOS generates nitric oxide (NO),
which mediates the suppressive impact of MDSCs on immune cell
proliferation and activity (115). Moreover, iNOS-modulated NO
participates in the nitration of the chemokine, CCL2, thereby
reducing CD8+ T-cell infiltration (81). For instance, G-CSF enhances iNOS
levels in MDSCs associated with colitis-linked CRC, thus
facilitating tumour immune evasion. Conversely, inhibiting G-CSF
reduces iNOS expression in MDSCs, which has been identified as a
potential therapeutic target for combating MDSC-induced
immunosuppression in CRC (83,116).
ROS serve as a principal immunosuppressive mechanism
employed by MDSCs. Elevated ROS levels within the CRC
microenvironment not only induce oxidative stress that irreversibly
destroys DNA, proteins and lipids but also results in cell death
(117). ROS also impede
antigen-specific T-cell responses by reducing CD3ζ chain expression
(106). In addition to NADPH
oxidase 2, tumour-derived factors, such as IL-3, IL-6, IL-10,
TGF-β, platelet-derived growth factor and GM-CSF enhance ROS
production in MDSCs through the STAT3 pathway (12,107,118). In an MC38 xenograft model, ROS
and peroxynitrite synthesized by MDSCs facilitated tumour cell
proliferation by causing the nitration of TCRs on CD8+ T
cells and suppressing their proliferation (117,119).
A notable study revealed that MDSCs sourced from
tumour locations exhibited a distinctive upregulation of PD-L1
compared with splenic MDSCs (120). This differential expression was
associated with the selective enhancement of PD-L1 on MDSCs by
HIF-1α under hypoxic conditions and was concurrent with a decrease
in IL-6 and IL-10 levels in MDSCs, correlated with heightened
T-cell activation. Furthermore, the expression of PD-L1 and Fas
ligand (FasL) on the surface of MDSCs is associated with induced
T-cell apoptosis (120-123). Studies utilizing in vitro
culture systems and clinical data have revealed that tumour
cell-secreted factors such as macrophage CSF and VEGF are
responsible for inducing PD-L1 expression in MDSCs (124,125). Notably, the proportion of
PD-L1-expressing MDSCs in patients with CRC has been shown to be
significantly greater than that in healthy donors and posttreatment
patients (126). Additionally,
HIF-1α-driven ENTPD2 expression leads to the conversion of
extracellular ATP into 5′-AMP, which subsequently hinders MDSC
differentiation and perpetuates their immunosuppressive function
(127).
T cells are a primary target for MDSCs within the
TME. MDSCs employ various mechanisms to inhibit T-cell
proliferation and counteract T cell-mediated immune responses,
including direct contact killing, depletion of essential amino
acids crucial for T-cell viability, NO production and reduction of
TCR chain expression (128).
The intercellular contact route of MDSCs functions
as a key link between inflammation and cancer and is largely
facilitated by STAT3 activation. The expression of STAT3 leads to
enhanced expression of proapoptotic agents, such as FasL, perforin
and granzyme A, in MDSCs and macrophages. This arrangement
facilitates the elimination of CD4+ and CD8+
T cells upon direct contact, shifting the balance from tumour
immune surveillance to tumour-promoting inflammation (128).
In addition to affecting L-arginine metabolism, the
impact of MDSCs on T cells involves cysteine and tryptophan, two
additional amino acids vital for T-cell function (111,129). MDSCs express indoleamine
2,3-dioxygenase (IDO), which suppresses T-cell proliferation
through cytotoxic metabolite production and depletion of
L-tryptophan (130). MDSC
proliferation within the TME leads to the exhaustion of cysteine
and tryptophan reserves, which are essential for T-cell activity.
Concurrently, NO emitted by iNOS decreases major histocompatibility
complex class II expression, alters the TCR configuration and
inhibits T-cell expansion (61).
In addition, increased ROS production not only diminishes TCR chain
expression but also hampers the antigen-specific response of
CD8+ T cells. Under hypoxic conditions, MDSCs exhibit
increased PD-L1 expression to initiate 'immunological braking'.
Furthermore, expression of the immunostimulatory receptor, CD40, by
MDSCs halts T-cell proliferation. Consequently, T-cell
proliferation and functionality are compromised, allowing MDSCs to
successfully suppress T-cell immunity (131).
TGF-β and IL-10 are two soluble cytokines secreted
by MDSCs that are involved in T cell and NK cell suppression and
macrophage polarization, respectively. TGF-β influences the
differentiation of CD4 helper T cells into Th1 and Th2 phenotypes
by suppressing the expression of the transcription factors, T-bet
(also termed TBX21) and GATA3 (132-134). In the context of early
gastrointestinal cancer, CD1d-restricted NK T cells induce MDSCs to
produce TGF-β through the IL-13/IL4R/STAT6 signalling pathway. This
mechanism is evident in the CT26 colon tumour model, where the
depletion of this cell group leads to a partial enhancement of the
antitumour immune response (135). Furthermore, MDSCs have been
noted to thwart antigen-specific CD4+ T cells by
secreting TGF-β and IL-10, thereby facilitating the development of
inducible CD4+CD25+Foxp3+ Tregs in
the MCA26 colon tumour model (136). Consequently, immunosuppressive
Tregs augment MDSC function, creating an environment favourable for
tumour proliferation (136).
Additionally, research indicates that IL-10 produced by MDSCs
attenuates the T-cell activation typically mediated by DCs
(137,138).
In patients with CRC, MDSCs play a role in
cultivating the immunosuppressive environment. T cell suppression
by MDSCs is merely one aspect of this. A spectrum of immune cells,
including NK cells, DCs, Tregs, Th17 cells and TAMs, have all been
implicated in interactions with MDSCs, as extensively documented in
the literature (47).
MDSCs diminish the antitumour activity of NK cells
by suppressing NK cell function. This suppression occurs through
the downregulation of the NK cell activation receptors natural
killer group 2, member D and NKp30, or by curtailing the production
of IFN and perforin in a manner dependent on direct cell contact
(139). Furthermore, extended
periods of inflammation intensify the inhibitory impact of MDSCs on
antitumour-activated NK cells through inflammatory mediators, such
as IL-10 and PEG2, or soluble factors, such as iNOS, ROS, ARG-1,
adenosine and IDO (140).
In addition, MDSCs influence macrophage dynamics,
transitioning into M2-type macrophages by downregulating STAT3 and
increasing HIF1α levels or by secreting S100A8/9 proteins. This
secretion promotes the polarization of TAMs from M1-type to
M2-type, with these reoriented M2-type macrophages exhibiting
elevated levels of CD206, CD204, VEGF, CD163 and ARG-1 (141,142).
Accordingly, MDSCs have a substantial domino effect
on diverse immune cell types within the TME, cumulatively fostering
an immunosuppressive milieu conducive to tumour proliferation.
A critical role of MDSCs is their contribution to
cancer metastasis. Liver metastases occur in 20-70% of patients
with CRC, whereas lung metastases are present in 10-20% of patients
(143). The 'seed-soil'
hypothesis suggests that prior to cancer cell dissemination, a
favourable microenvironment (or premetastatic niche) is established
in tissues prone to metastatic disease (144). Emerging research indicates that
MDSC recruitment to the premetastatic niche of future metastatic
target organs is driven by primary tumour cells. For instance,
VEGFα secreted by CRC cells prompts TAMs to produce CXCL1 (143), which subsequently attracts
circulating CXCR2+ neutrophils and MDSCs to target
organs. Additionally, CCL9 from CRC epithelial cells summons IMCs
via the CCR1 receptor (145),
and CCL2 elicits MDSC recruitment via CCR7 (43).
Amplified MDSCs within target organs increase micro
(mi) RNA101 expression in cancer cells, suppressing C-terminal
binding protein-2. This mechanism targets key stem cell genes,
enhancing cancer cell stemness, inherent tumorigenicity, metastatic
potential and drug resistance (146). Moreover, MDSCs directly aid in
the survival of metastatic cancer cells (143) and secrete IL-1 receptor
antagonists to counteract cancer cell senescence (147). MDSCs also facilitate the
establishment of aberrant vascular systems by accumulating matrix
metalloproteinases (148),
significantly decreasing IFN-γ production and fostering a
proinflammatory milieu conducive to EMT (106,149,150). This orchestration remodels the
TME, engenders an inflammatory and proliferative state in the
target organ, compromises immune defence and supplies
preconditioning support for CRC cell colonization, thus supporting
cancer cell survival and expansion within the host organ.
The inhibitory effects of MDSCs on T-cell responses
and multiple cellular functions are critical for antitumour immune
responses. MDSCs are positively correlated with the development and
spread of cancer, and their presence typically decreases the
effectiveness of immunotherapy (151). Given that the accumulation of
MDSCs in the TME is considered to be a major obstacle to tumour
immunotherapy (152), targeting
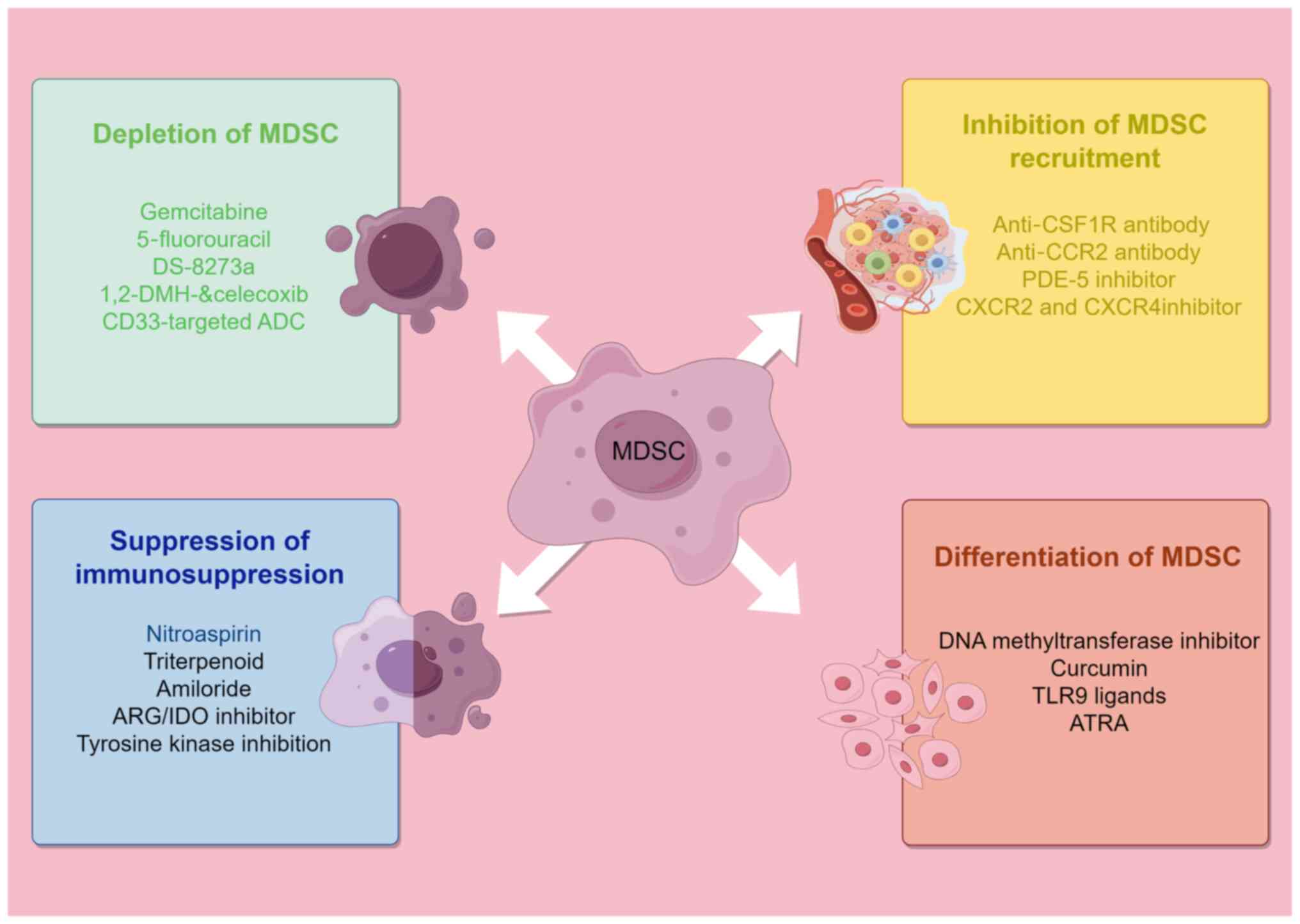
MDSCs has become a new strategy for tumour immunotherapy. Current
clinical studies have focused on four directions (153,154): Depletion of MDSCs, inhibition of
MDSC recruitment, suppression of MDSC immunosuppressive function,
induction of MDSC differentiation (Fig. 3).
The most direct therapeutic strategy for targeted
MDSC therapy is MDSC depletion. The selective MDSC inhibitor,
gemcitabine, decreases the peripheral blood levels of TGF-β1,
PMN-MDSCs and circulating Tregs but has minimal effect on M-MDSCs,
which is beneficial for effector T cell proliferation and the
recovery of antitumour ability (155,156).
Both subtypes of MDSCs are sensitive to
5-fluorouracil (5-FU), whereas other immune cells, such as T cells,
B cells, DCs and NK cells, are not significantly affected (157). In a mouse EL4 (loaded mouse)
model, 5-FU enhanced T cell-dependent antitumour responses by
inducing the apoptosis of MDSCs in the TME and spleen and by
promoting the production of high levels of IFNγ by
tumour-infiltrating T cells (158). Patients with CRC may experience
less immunosuppression and improved clinical results if they follow
the FOLFOX (folinic acid, 5-FU and oxaliplatin) regimen, which may
be linked to a decrease in MDSC counts and the restoration of
antitumour immunity (159). The
administration of celecoxib, a selective COX-2 inhibitor,
significantly reduces the frequency of
Gr1+CD11b+ immature myeloid suppressor cells
during 1,2-dimethylhydrazine diHCl chemotherapy in CRC mice, while
increasing splenic lymphocyte numbers and tumour lymphocyte
infiltration (160). In
addition, the administration of bevacizumab (anti-VEGF) therapy to
patients with CRC results in a decrease in the concentration of
immature progenitor cells, with a moderate increase in the number
of DCs in the peripheral blood (161). Notably, the use of a TNF-related
apoptosis-inducing ligand receptor 2 agonistic antibody (DS-8273a)
decreased the number of MDSCs in the peripheral blood of patients
with CRC without affecting the proportions of myeloid and lymphoid
cell populations. Unfortunately, MDSC numbers were restored to
pretreatment levels by day 42 in most patients (162). Gemtuzumab ozogamicin, an
anti-CD33 immunotoxin agent, was effective in eliminating MDSCs and
reactivating T cells and chimeric antigen receptor T cells in a
wide range of cancer types, including CRC (163).
By preventing MDSCs from responding to chemokines,
the inhibition of MDSC recruitment can effectively reduce the
proportion of MDSCs in the TME and periphery (164,165). Chemokine antagonists help
prevent MDSCs from entering the tumour site, thus modifying the
immunosuppressive microenvironment (166). As an essential chemokine
receptor for MDSC transport (167,168), CXCR2 inhibitors interrupt the
CXCR2/CXCL pathway, effectively reducing MDSC infiltration and
improving cytotoxic T-cell function (58,169). Additionally, the CCR5/CCL5 axis
is essential for tumour progression as it promotes tumour invasion
and MDSC migration to the tumour site (170). Targeting the CCR5/CCL axis
inhibits the progression and invasiveness of a wide range of
tumours (171-174). mCCR5-Ig reduces the migration of
MDSCs and Tregs without affecting effector T-cell recruitment to
the TME (170). The CSF-1
receptor (CSF-1R), a well-defined target of MDSC recruitment,
induces the formation of MDSCs and their transport to the tumour
site (175). CSF-1R inhibitors
interrupt the CSF-1R signalling pathway, resulting in MDSC ablation
or inhibition of their tumour-promoting function and reprogramming
of TAMs (154,176-178). A study demonstrated that in an
azomethane/dextran sulfate sodium-induced model of colon
carcinogenesis, the phosphodiesterase-5 inhibitor, sildenafil,
directly inhibited MDSC infiltration into tumour tissues,
modulating inflammation in the TME (179). In addition, Liang et al
(180) reported that anti-CCR2
antibody treatment reduced radiotherapy-induced M-MDSC infiltration
in colon cancer.
Inhibiting the immunosuppressive mechanisms of
MDSCs is the main therapeutic approach for restoring T-cell
activity and successful immunotherapy. MDSCs suppress the immune
system by affecting L-arginine metabolism through the production of
ARG and NOS (107). In a CRC
mouse model, nitroaspirin modulated the immune status of the tumour
host by increasing the number and function of tumour
antigen-specific T cells through the inhibition of ARG and NOS
activity (181). AT38 (a
reactive nitrogen inhibitor) (182) reduced the iNOS and ARG-1 levels
in a CRC mouse model while effectively reducing the nitration of
MDSC chemokines (81). A study
revealed that tyrosine kinase inhibition by sunitinib reduced
phosphorylated STAT3 and ARG levels in M-MDSCs, inhibited MDSC
function and increased T-cell proliferation (183). Another study showed that methyl
bardoxolone, a synthetic triterpenoid, inhibited MDSC function by
decreasing ROS in mouse MC38 tumour hosts; however, it did not
affect ARG-1 or NO levels (184). Notably, amiloride, a drug used
to treat hypertension, inhibits the formation of tumour-derived
exosomes and reduces the inhibitory function of MDSCs in human CRC
and mouse models (185). The
inhibition of MDSC metabolic processes is also a novel idea, and
the metabolic reprogramming of glycolysis and oxidative
phosphorylation can inhibit the immunosuppressive function of
tumour MDSCs. The most common metastatic target organ for CRC
tumours is the liver (186). A
study of hepatocellular carcinoma showed that IL-37 significantly
affected the expression of genes related to ATP synthesis and
hydrolysis in MDSCs through metabolic reprogramming in
patient-derived tumour xenograft model mice and that the glycolytic
and oxidative phosphorylation processes of MDSCs were promoted and
ATP release was upregulated (187). Thus, the immunosuppressive
ability of MDSCs was weakened and tumour development was
suppressed.
Induction of MDSC differentiation is another
therapeutic approach used to target MDSCs and promoting their
continued differentiation into mature myeloid cells can reduce
their immunosuppressive effects (106). MDSCs are rapidly differentiated
into mature myeloid cells, such as DCs and macrophages, in response
to all-trans retinoic acid (ATRA), a metabolic intermediate of
vitamin A, and ATRA improves T-cell responses in patients with
cancer through specific upregulation of glutathione synthase in
MDSCs and reduced ROS production (25,188-190). In a CRC mouse model, curcumin
administration reduced the number of PMN-MDSCs, activated STAT3 and
NF-κB in MDSCs and induced the differentiation of M-MDSCs into
cells with an M1-like phenotype (191,192). Daurkin et al (193) demonstrated that in the presence
of DNA methyltransferase inhibitors, tumour-infiltrating CD11b
myeloid cells differentiate into mature myeloid cells. In addition,
the activation of Toll-like receptor-9 by CpG promotes MDSC
maturation and differentiation and effectively reduces the
proportion of Ly6Ghigh MDSCs in CRC tumour models
(194).
Immune checkpoint blockade (ICB) therapies
targeting PD-1, PD-L1 and cytotoxic T-lymphocyte associated protein
4 (CTLA-4) have revolutionized gastrointestinal tumour treatment
options in recent years. In preclinical studies, a decrease in the
number of MDSCs was detected in patients who received CTLA4 or
PD-1/PD-L1 treatment (195-197). A recently published study
indicated that the presence of MDSCs is strongly associated with
the transformation of premalignant states, metastasis, recurrence
and resistance to digestive malignancies. MDSCs express
immunosuppressive ligands such as PD-L1 to antagonize the
antitumour response of the immune system; therefore, diminishing
MDSCs in the TME enhances the outcome of anti-PD-1 therapy
(131). However, immune
checkpoint inhibitors demonstrate poor efficacy in patients with
CRC with immune checkpoint resistance and microsatellite stable
(198). Promising melanoma
research indicates that the targeting of the CD300 molecule family
member d (CD300ld), a tumour surface immunosuppressive receptor,
could be combined with anti-PD1 therapy for the treatment of
digestive system cancer. CD300ld is specifically upregulated in
PMN-MDSCs, is a pivotal receptor regulating the recruitment and
immunosuppressive function of PMN-MDSCs and represents the immune
system 'braking site' (199). In
mice, targeting CD300-ld remodels the tumour immune
microenvironment by suppressing the recruitment and function of
PMN-MDSCs, resulting in a broad-spectrum antitumour response. More
concretely, CD300ld upregulates S100A8/A9 expression through STAT3
activation, forming the CD300ld/STAT3/S100A8/A9 axis and thereby
facilitating the immunosuppressive function of PMN-MDSCs (199).
The establishment of an immunosuppressive TME is
one of the key mechanisms by which these CRC cells evade the immune
system. In recent years, MDSCs have been shown to play a crucial
role as major contributors to this process. Studies targeting MDSCs
are mainly focused on the multiple major directions of MDSC
expansion, namely migration, activation, recruitment, action and
metastasis (131). Based on the
studies published thus far, targeting MDSCs has been shown to
reduce the tumour load and represent a promising therapeutic
strategy for CRC (153). In the
present review, the clinicaltrials. gov website was searched and
the therapeutic approaches involving MDSCs and different treatment
regimens for CRC are selectively summarized in Table I.
Although studies targeting MDSCs have reported
notable results, the existence of potential limitations constrains
the continued advancement of strategies targeting MDSCs. First,
MDSCs have a short half-life (200-202), and the strategy of MDSC
depletion in the peripheral blood negatively feeds back to the bone
marrow (203,204), which increases the risk of MDSC
recurrence and accumulation and promotes CRC development. More
effective therapies may aim to block MDSC differentiation in the
bone marrow, inhibit MDSC migration to affected tissues or
manipulate the tissue microenvironment. Second, current targeted
therapies for MDSCs are focused on reducing the number of MDSCs
that have been generated or on inhibiting their function, with
fewer strategies targeting MDSC-induced migration (33). Third, due to the large phenotypic
heterogeneity of MDSCs, complex amplification and functional
networks, different subgroups of MDSCs use different mechanisms to
suppress the immune system, making current therapeutic strategies
targeting MDSCs only partially effective. Therefore, further
investigations into the molecular phenotypes of MDSCs and their
mechanisms in tumour tissues are urgently needed, and the
development of therapeutic strategies targeting MDSC subgroups is
essential for improving the effectiveness of tumour therapy
(12). In addition, based on the
summary provided in the present review, cancer cells and MDSCs in
primary tumours can directly or indirectly promote metastatic
premetastatic niche formation, but the mechanistic similarities and
differences in the interactions between the TME and the
premetastatic niche remain topics worth exploring (143). Moreover, the TME is very complex
and is composed of multiple immune cells and cytokines that form a
complex network. It is therefore difficult to achieve the expected
effect by exclusively targeting MDSCs, and a more desirable
therapeutic effect may be achieved by co-targeting other cells or
targets to enhance the immune system or utilizing special contact
modalities such as exosomes (33).
Iron-mediated death is a unique form of cell death
driven by the accumulation of lipid peroxides. A study on iron
death induction and gastric cancer reported that inhibition of the
Wnt/β-catenin signalling pathway reduced the expression of the
transcription factor, TCF4, inhibited glutathione peroxidase 4 gene
transcription and promoted iron death, resulting in a decrease in
both tumour weight and volume (205), suggesting the feasibility of the
induction of iron-mediated death for the treatment of
gastrointestinal tumours. The triple combination of MDSC blockade
with iron-mediated death induction and ICB therapy greatly restored
tissue immune surveillance and promoted a normal immune response.
This treatment inhibited tumour growth and represented a promising
strategy for targeted therapy of CRC (206).
Exosomes in the TME act as carriers of molecular
delivery exerting indirect effects on MDSC expansion, formation of
an immunosuppressive environment (48), cancer cell growth and invasion
(207,208) and therapy resistance (209), and direct promotion of CRC cell
stemness through S100A9 (210).
Under IL-6 induction, PMN-MDSCs synthesize increased exosomal
miR-93-5p, which promotes the differentiation of M-MDSC into M2
macrophages and increases CRC risk (211). Compared with healthy
individuals, patients with CRC have significantly increased
exosomal miR-19a levels in the serum and have a poorer prognosis
(212,213). This suggests that exosomes in
the TME have the potential to be another potential target in
treating CRC.
Therefore, from the perspective of patients with
CRC, the strategy of targeting MDSCs requires more comprehensive
and refined research and clinical trials, but it remains a
promising class of therapeutic strategy due to its special
immunosuppressive effects in the TME.
In the present review, the nomenclature history of
MDSCs along with the two main subtypes, PMN-MDSCs and M-MDSCs, were
systematically and comprehensively reviewed and the recruitment,
role and metastatic mechanisms of MDSCs in the CRC microenvironment
were revealed. Mutations in genes such as KRAS, adenomatous
polyposis coli, BRAF and phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit α (214,215), epigenetic modifications of m6A,
chemokines in the CC and CXC families, inflammatory mediators,
alterations in intestinal microbes and hypoxic environments may
cause local aggregation of MDSCs and increase the risk of CRC.
The present review proposes that MDSCs possess two
main features: Immunosuppression and metastasis. MDSCs achieve
immunosuppressive functions by generating RONS, degrading
L-arginine, secreting immunosuppressive cytokines via intercellular
contact, paracrine or exocrine mechanisms (216), inhibiting T cell and NK cell
toxicity and inducing other immunosuppressive cells, such as Tregs,
to achieve immunosuppressive functions and provide favourable
conditions for cancer cell survival and growth (217). MDSCs are recruited and expanded
to support immunosuppression. MDSCs inhibit normal T cell
proliferation in patients with CRC, resulting in poor prognosis.
The recruitment of expanded MDSCs enhances cancer stem cell gene
expression, directly promotes metastatic cancer cell survival,
antagonizes cancer cell senescence and acts as a crossroads between
tumour angiogenesis and immunosuppression to provide precursor
support for CRC metastasis; therefore, the presence of MDSCs is
important for CRC cells to colonize, survive and grow in target
organs. Increased MDSC and tumour miRNA101 expression also predicts
poor survival.
In addition, the present review comprehensively
summarized four types of potential therapeutic strategies for CRC:
Depletion of produced MDSCs, inhibition of MDSC recruitment,
suppression of MDSC immunosuppression and induction of MDSC
differentiation. The primary TME can promote premetastatic niche
formation, but the mechanism underlying the interaction between the
primary TME and premetastatic niche formation during the process of
metastasis initiation requires further investigated.
Not applicable.
WZ and SJ wrote the manuscript. HL and YM drew all
the figures and tables of the article. WZ, HY, ZZh and WH revised
the article. HY, ZZh and MW provided many suggestions for the
writing of the article. ZZo designed the study. All authors read
and approved the final version of the manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present review was supported by the National Natural Science
Foundation of China (grant nos. 82103645 and 82260596), The China
Postdoctoral Science Foundation (grant no. 2023M741523) and Science
and Technology Program of Jiangxi Provincial Health and Family
Planning Commission (grant no. 202410246).
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leufkens AM, van den Bosch MAAJ, van
Leeuwen MS and Siersema PD: Diagnostic accuracy of computed
tomography for colon cancer staging: A systematic review. Scand J
Gastroenterol. 46:887–894. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Biller LH and Schrag D: Diagnosis and
treatment of metastatic colorectal cancer: A review. JAMA.
325:669–685. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel RL, Wagle NS, Cercek A, Smith RA
and Jemal A: Colorectal cancer statistics, 2023. CA Cancer J Clin.
73:233–254. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miller KD, Nogueira L, Devasia T, Mariotto
AB, Yabroff KR, Jemal A, Kramer J and Siegel RL: Cancer treatment
and survivorship statistics, 2022. CA Cancer J Clin. 72:409–436.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu YJ, Li X, Chen TT, Wang JX, Zhou YX,
Mu XL, Du Y, Wang JL, Tang J and Liu JY: Personalised
neoantigen-based therapy in colorectal cancer. Clin Transl Med.
13:e14612023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barker HE, Paget JTE, Khan AA and
Harrington KJ: The tumour microenvironment after radiotherapy:
Mechanisms of resistance and recurrence. Nat Rev Cancer.
15:409–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Le DT, Hubbard-Lucey VM, Morse MA, Heery
CR, Dwyer A, Marsilje TH, Brodsky AN, Chan E, Deming DA, Diaz LA
Jr, et al: A blueprint to advance colorectal cancer
immunotherapies. Cancer Immunol Res. 5:942–949. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fletcher R, Wang YJ, Schoen RE, Finn OJ,
Yu J and Zhang L: Colorectal cancer prevention: Immune modulation
taking the stage. Biochim Biophys Acta Rev Cancer. 1869:138–148.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Milette S, Fiset PO, Walsh LA, Spicer JD
and Quail DF: The innate immune architecture of lung tumors and its
implication in disease progression. J Pathol. 247:589–605. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bronte V, Brandau S, Chen SH, Colombo MP,
Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A,
Ostrand-Rosenberg S, et al: Recommendations for myeloid-derived
suppressor cell nomenclature and characterization standards. Nat
Commun. 7:121502016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Condamine T and Gabrilovich DI: Molecular
mechanisms regulating myeloid-derived suppressor cell
differentiation and function. Trends Immunol. 32:19–25. 2011.
View Article : Google Scholar :
|
|
14
|
Condamine T, Mastio J and Gabrilovich DI:
Transcriptional regulation of myeloid-derived suppressor cells. J
Leukoc Biol. 98:913–922. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao F, Hoechst B, Duffy A,
Gamrekelashvili J, Fioravanti S, Manns MP, Greten TF and Korangy F:
S100A9 a new marker for monocytic human myeloid-derived suppressor
cells. Immunology. 136:176–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Veglia F, Sanseviero E and Gabrilovich DI:
Myeloid-derived suppressor cells in the era of increasing myeloid
cell diversity. Nat Rev Immunol. 21:485–498. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Groth C, Hu X, Weber R, Fleming V,
Altevogt P, Utikal J and Umansky V: Immunosuppression mediated by
myeloid-derived suppressor cells (MDSCs) during tumour progression.
Br J Cancer. 120:16–25. 2019. View Article : Google Scholar :
|
|
18
|
Veglia F, Perego M and Gabrilovich D:
Myeloid-derived suppressor cells coming of age. Nat Immunol.
19:108–119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marigo I, Bosio E, Solito S, Mesa C,
Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, et
al: Tumor-induced tolerance and immune suppression depend on the
C/EBPbeta transcription factor. Immunity. 32:790–802. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Consonni FM, Porta C, Marino A, Pandolfo
C, Mola S, Bleve A and Sica A: Myeloid-derived suppressor cells:
Ductile targets in disease. Front Immunol. 10:9492019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lim HX, Kim TS and Poh CL: Understanding
the differentiation, expansion, recruitment and suppressive
activities of myeloid-derived suppressor cells in cancers. Int J
Mol Sci. 21:35992020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma T, Renz BW, Ilmer M, Koch D, Yang Y,
Werner J and Bazhin AV: Myeloid-derived suppressor cells in solid
tumors. Cells. 11:3102022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Talmadge JE and Gabrilovich DI: History of
myeloid-derived suppressor cells. Nat Rev Cancer. 13:739–752. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gabrilovich DI: Myeloid-derived suppressor
cells. Cancer Immunol Res. 5:3–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fleming V, Hu X, Weber R, Nagibin V, Groth
C, Altevogt P, Utikal J and Umansky V: Targeting myeloid-derived
suppressor cells to bypass tumor-induced immunosuppression. Front
Immunol. 9:3982018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Z, Guo J, Weng L, Tang W, Jin S and
Ma W: Myeloid-derived suppressor cells-new and exciting players in
lung cancer. J Hematol Oncol. 13:102020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui C, Lan P and Fu L: The role of
myeloid-derived suppressor cells in gastrointestinal cancer. Cancer
Commun (Lond). 41:442–471. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hess NJ, Kink JA and Hematti P: Exosomes,
MDSCs and tregs: A new frontier for GVHD prevention and treatment.
Front Immunol. 14:11433812023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan PY, Ma G, Weber KJ, Ozao-Choy J, Wang
G, Yin B, Divino CM and Chen SH: Immune stimulatory receptor CD40
is required for T-cell suppression and T regulatory cell activation
mediated by myeloid-derived suppressor cells in cancer. Cancer Res.
70:99–108. 2010. View Article : Google Scholar :
|
|
30
|
Gaißler A, Bochem J, Spreuer J, Ottmann S,
Martens A, Amaral T, Wagner NB, Claassen M, Meier F, Terheyden P,
et al: Early decrease of blood myeloid-derived suppressor cells
during checkpoint inhibition is a favorable biomarker in metastatic
melanoma. J Immunother Cancer. 11:e0068022023. View Article : Google Scholar
|
|
31
|
Kaplan RN, Riba RD, Zacharoulis S, Bramley
AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et
al: VEGFR1-positive haematopoietic bone marrow progenitors initiate
the pre-metastatic niche. Nature. 438:820–827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Condamine T, Ramachandran I, Youn JI and
Gabrilovich DI: Regulation of tumor metastasis by myeloid-derived
suppressor cells. Annu Rev Med. 66:97–110. 2015. View Article : Google Scholar :
|
|
33
|
Li K, Shi H, Zhang B, Ou X, Ma Q, Chen Y,
Shu P, Li D and Wang Y: Myeloid-derived suppressor cells as
immunosuppressive regulators and therapeutic targets in cancer.
Signal Transduct Target Ther. 6:3622021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fědorová L, Pilátová K, Selingerová I,
Bencsiková B, Budinská E, Zwinsová B, Brychtová V, Langrová M, Šefr
R, Valík D and Zdražilová Dubská L: Circulating myeloid-derived
suppressor cell subsets in patients with colorectal
cancer-exploratory analysis of their biomarker potential. Klin
Onkol. 31(Suppl 2): S88–S92. 2018. View Article : Google Scholar
|
|
35
|
Zhang Y, Xu J, Zhang N, Chen M, Wang H and
Zhu D: Targeting the tumour immune microenvironment for cancer
therapy in human gastrointestinal malignancies. Cancer Lett.
458:123–135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gabrilovich DI, Bronte V, Chen SH, Colombo
MP, Ochoa A, Ostrand-Rosenberg S and Schreiber H: The terminology
issue for myeloid-derived suppressor cells. Cancer Res. 67:425–426.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Youn JI, Nagaraj S, Collazo M and
Gabrilovich DI: Subsets of myeloid-derived suppressor cells in
tumor-bearing mice. J Immunol. 181:5791–5802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ueha S, Shand FHW and Matsushima K:
Myeloid cell population dynamics in healthy and tumor-bearing mice.
Int Immunopharmacol. 11:783–788. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mandruzzato S, Brandau S, Britten CM,
Bronte V, Damuzzo V, Gouttefangeas C, Maurer D, Ottensmeier C, van
der Burg SH, Welters MJ and Walter S: Toward harmonized phenotyping
of human myeloid-derived suppressor cells by flow cytometry:
Results from an interim study. Cancer Immunol Immunother.
65:161–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen Z, Yuan R, Hu S, Yuan W and Sun Z:
Roles of the exosomes derived from myeloid-derived suppressor cells
in tumor immunity and cancer progression. Front Immunol.
13:8179422022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cassetta L, Bruderek K,
Skrzeczynska-Moncznik J, Osiecka O, Hu X, Rundgren IM, Lin A,
Santegoets K, Horzum U, Godinho-Santos A, et al: Differential
expansion of circulating human MDSC subsets in patients with
cancer, infection and inflammation. J Immunother Cancer.
8:e0012232020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Flores-Toro JA, Luo D, Gopinath A,
Sarkisian MR, Campbell JJ, Charo IF, Singh R, Schall TJ, Datta M,
Jain RK, et al: CCR2 inhibition reduces tumor myeloid cells and
unmasks a checkpoint inhibitor effect to slow progression of
resistant murine gliomas. Proc Natl Acad Sci USA. 117:1129–1138.
2020. View Article : Google Scholar :
|
|
43
|
Takacs GP, Kreiger CJ, Luo D, Tian G,
Garcia JS, Deleyrolle LP, Mitchell DA and Harrison JK:
Glioma-derived CCL2 and CCL7 mediate migration of immune
suppressive CCR2+/CX3CR1+ M-MDSCs into the
tumor microenvironment in a redundant manner. Front Immunol.
13:9934442023. View Article : Google Scholar
|
|
44
|
Singh L, Muise ES, Bhattacharya A, Grein
J, Javaid S, Stivers P, Zhang J, Qu Y, Joyce-Shaikh B, Loboda A, et
al: ILT3 (LILRB4) promotes the immunosuppressive function of
tumor-educated human monocytic myeloid-derived suppressor cells.
Mol Cancer Res. 19:702–716. 2021. View Article : Google Scholar
|
|
45
|
Veglia F, Hashimoto A, Dweep H, Sanseviero
E, De Leo A, Tcyganov E, Kossenkov A, Mulligan C, Nam B, Masters G,
et al: Analysis of classical neutrophils and polymorphonuclear
myeloid-derived suppressor cells in cancer patients and
tumor-bearing mice. J Exp Med. 218:e202018032021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Condamine T, Dominguez GA, Youn JI,
Kossenkov AV, Mony S, Alicea-Torres K, Tcyganov E, Hashimoto A,
Nefedova Y, Lin C, et al: Lectin-type oxidized LDL receptor-1
distinguishes population of human polymorphonuclear myeloid-derived
suppressor cells in cancer patients. Sci Immunol. 1:aaf89432016.
View Article : Google Scholar
|
|
47
|
Joshi S and Sharabi A: Targeting
myeloid-derived suppressor cells to enhance natural killer
cell-based immunotherapy. Pharmacol Ther. 235:1081142022.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tian X, Shen H, Li Z, Wang T and Wang S:
Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor
microenvironment. J Hematol Oncol. 12:842019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dumitru CA, Moses K, Trellakis S, Lang S
and Brandau S: Neutrophils and granulocytic myeloid-derived
suppressor cells: Immunophenotyping, cell biology and clinical
relevance in human oncology. Cancer Immunol Immunother.
61:1155–1167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gunaydin G, Kesikli SA and Guc D: Cancer
associated fibroblasts have phenotypic and functional
characteristics similar to the fibrocytes that represent a novel
MDSC subset. Oncoimmunology. 4:e10349182015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mazza EM, Zoso A, Mandruzzato S, Bronte V,
Serafini P, Inverardi L and Bicciato S: Gene expression profiling
of human fibrocytic myeloid-derived suppressor cells (f-MDSCs).
Genom Data. 2:389–392. 2014. View Article : Google Scholar
|
|
52
|
Bizymi N, Georgopoulou A, Mastrogamvraki
N, Matheakakis A, Gontika I, Fragiadaki I, Mavroudi I and Papadaki
HA: Myeloid-derived suppressor cells (MDSC) in the umbilical cord
blood: Biological significance and possible therapeutic
applications. J Clin Med. 11:7272022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Haile LA, Gamrekelashvili J, Manns MP,
Korangy F and Greten TF: CD49d is a new marker for distinct
myeloid-derived suppressor cell subpopulations in mice. J Immunol.
185:203–210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Alshetaiwi H, Pervolarakis N, McIntyre LL,
Ma D, Nguyen Q, Rath JA, Nee K, Hernandez G, Evans K, Torosian L,
et al: Defining the emergence of myeloid-derived suppressor cells
in breast cancer using single-cell transcriptomics. Sci Immunol.
5:eaay60172020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dienstmann R, Connor K and Byrne AT;
COLOSSUS Consortium: Precision therapy in RAS mutant colorectal
cancer. Gastroenterology. 158:806–811. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wood LD, Parsons DW, Jones S, Lin J,
Sjöblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, et al: The
genomic landscapes of human breast and colorectal cancers. Science.
318:1108–1113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Vakiani E, Janakiraman M, Shen R, Sinha R,
Zeng Z, Shia J, Cercek A, Kemeny N, D'Angelica M, Viale A, et al:
Comparative genomic analysis of primary versus metastatic
colorectal carcinomas. J Clin Oncol. 30:2956–2962. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liao W, Overman MJ, Boutin AT, Shang X,
Zhao D, Dey P, Li J, Wang G, Lan Z, Li J, et al: KRAS-IRF2 axis
drives immune suppression and immune therapy resistance in
colorectal cancer. Cancer Cell. 35:559–572.e7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wong CC, Xu J, Bian X, Wu JL, Kang W, Qian
Y, Li W, Chen H, Gou H, Liu D, et al: In colorectal cancer cells
with mutant KRAS, SLC25A22-mediated glutaminolysis reduces DNA
demethylation to increase WNT signaling, stemness, and drug
resistance. Gastroenterology. 159:2163–2180.e6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhou Q, Peng Y, Ji F, Chen H, Kang W, Chan
LS, Gou H, Lin Y, Huang P, Chen D, et al: Targeting of SLC25A22
boosts the immunotherapeutic response in KRAS-mutant colorectal
cancer. Nat Commun. 14:46772023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Johnson B: Targeting myeloid-derived
suppressor cell trafficking as a novel immunotherapeutic approach
in microsatellite stable colorectal cancer. Cancers (Basel).
15:54842023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bao Y, Zhai J, Chen H, Wong CC, Liang C,
Ding Y, Huang D, Gou H, Chen D, Pan Y, et al: Targeting
m6A reader YTHDF1 augments antitumour immunity and
boosts anti-PD-1 efficacy in colorectal cancer. Gut. 72:1497–1509.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li Y, He X, Lu X, Gong Z, Li Q, Zhang L,
Yang R, Wu C, Huang J, Ding J, et al: METTL3 acetylation impedes
cancer metastasis via fine-tuning its nuclear and cytosolic
functions. Nat Commun. 13:63502022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chen H, Pan Y, Zhou Q, Liang C, Wong CC,
Zhou Y, Huang D, Liu W, Zhai J, Gou H, et al: METTL3 inhibits
antitumor immunity by targeting m6A-BHLHE41-CXCL1/CXCR2
axis to promote colorectal cancer. Gastroenterology. 163:891–907.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhai J, Chen H, Wong CC, Peng Y, Gou H,
Zhang J, Pan Y, Chen D, Lin Y, Wang S, et al: ALKBH5 drives immune
suppression via targeting AXIN2 to promote colorectal cancer and is
a target for boosting immunotherapy. Gastroenterology. 165:445–462.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhao D, Wu L, Hong M, Zheng S, Wu X, Ye H,
Chen F, Zhang D, Liu X, Meng X, et al: DKK-1 and its influences on
bone destruction: A comparative study in collagen-induced arthritis
mice and rheumatoid arthritis patients. Inflammation. 47:129–144.
2024. View Article : Google Scholar
|
|
67
|
Fujimura T, Kambayashi Y and Aiba S:
Crosstalk between regulatory T cells (Tregs) and myeloid derived
suppressor cells (MDSCs) during melanoma growth. Oncoimmunology.
1:1433–1434. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li N, Kang Y, Wang L, Huff S, Tang R, Hui
H, Agrawal K, Gonzalez GM, Wang Y, Patel SP and Rana TM: ALKBH5
regulates anti-PD-1 therapy response by modulating lactate and
suppressive immune cell accumulation in tumor microenvironment.
Proc Natl Acad Sci USA. 117:20159–20170. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Husain Z, Seth P and Sukhatme VP:
Tumor-derived lactate and myeloid-derived suppressor cells: Linking
metabolism to cancer immunology. Oncoimmunology. 2:e263832013.
View Article : Google Scholar
|
|
70
|
Hayes C, Donohoe CL, Davern M and Donlon
NE: The oncogenic and clinical implications of lactate induced
immunosuppression in the tumour microenvironment. Cancer Lett.
500:75–86. 2021. View Article : Google Scholar
|
|
71
|
Bejarano L, Jordāo MJC and Joyce JA:
Therapeutic targeting of the tumor microenvironment. Cancer Discov.
11:933–959. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hegde S, Leader AM and Merad M: MDSC:
Markers, development, states, and unaddressed complexity. Immunity.
54:875–884. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Walz A, Peveri P, Aschauer H and
Baggiolini M: Purification and amino acid sequencing of NAF, a
novel neutrophil-activating factor produced by monocytes. Biochem
Biophys Res Commun. 149:755–761. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Schulz O, Hammerschmidt SI, Moschovakis GL
and Förster R: Chemokines and chemokine receptors in lymphoid
tissue dynamics. Annu Rev Immunol. 34:203–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Li BH, Garstka MA and Li ZF: Chemokines
and their receptors promoting the recruitment of myeloid-derived
suppressor cells into the tumor. Mol Immunol. 117:201–215. 2020.
View Article : Google Scholar
|
|
76
|
McClellan JL, Davis JM, Steiner JL, Enos
RT, Jung SH, Carson JA, Pena MM, Carnevale KA, Berger FG and Murphy
EA: Linking tumor-associated macrophages, inflammation, and
intestinal tumorigenesis: Role of MCP-1. Am J Physiol Gastrointest
Liver Physiol. 303:G1087–1095. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Qian BZ, Li J, Zhang H, Kitamura T, Zhang
J, Campion LR, Kaiser EA, Snyder LA and Pollard JW: CCL2 recruits
inflammatory monocytes to facilitate breast-tumour metastasis.
Nature. 475:222–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chang YH, Huang YL, Tsai HC, Chang AC, Ko
CY, Fong YC and Tang CH: Chemokine ligand 2 promotes migration in
osteosarcoma by regulating the miR-3659/MMP-3 axis. Biomedicines.
11:27682023. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Behfar S, Hassanshahi G, Nazari A and
Khorramdelazad H: A brief look at the role of monocyte
chemoattractant protein-1 (CCL2) in the pathophysiology of
psoriasis. Cytokine. 110:226–231. 2018. View Article : Google Scholar
|
|
80
|
Chun E, Lavoie S, Michaud M, Gallini CA,
Kim J, Soucy G, Odze R, Glickman JN and Garrett WS: CCL2 promotes
colorectal carcinogenesis by enhancing polymorphonuclear
myeloid-derived suppressor cell population and function. Cell Rep.
12:244–257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Molon B, Ugel S, Del Pozzo F, Soldani C,
Zilio S, Avella D, De Palma A, Mauri P, Monegal A, Rescigno M, et
al: Chemokine nitration prevents intratumoral infiltration of
antigen-specific T cells. J Exp Med. 208:1949–1962. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Katoh H, Wang D, Daikoku T, Sun H, Dey SK
and Dubois RN: CXCR2-expressing myeloid-derived suppressor cells
are essential to promote colitis-associated tumorigenesis. Cancer
Cell. 24:631–644. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang Y, Ding Y, Deng Y, Zheng Y and Wang
S: Role of myeloid-derived suppressor cells in the promotion and
immunotherapy of colitis-associated cancer. J Immunother Cancer.
8:e0006092020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Grauers Wiktorin H, Nilsson MS, Kiffin R,
Sander FE, Lenox B, Rydström A, Hellstrand K and Martner A:
Histamine targets myeloid-derived suppressor cells and improves the
anti-tumor efficacy of PD-1/PD-L1 checkpoint blockade. Cancer
Immunol Immunother. 68:163–174. 2019. View Article : Google Scholar :
|
|
85
|
Martin RK, Saleem SJ, Folgosa L, Zellner
HB, Damle SR, Nguyen GK, Ryan JJ, Bear HD, Irani AM and Conrad DH:
Mast cell histamine promotes the immunoregulatory activity of
myeloid-derived suppressor cells. J Leukoc Biol. 96:151–159. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Sulsenti R and Jachetti E: Frenemies in
the microenvironment: Harnessing mast cells for cancer
immunotherapy. Pharmaceutics. 15:16922023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Obermajer N, Muthuswamy R, Lesnock J,
Edwards RP and Kalinski P: Positive feedback between PGE2 and COX2
redirects the differentiation of human dendritic cells toward
stable myeloid-derived suppressor cells. Blood. 118:5498–5505.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zelenay S, van der Veen AG, Böttcher JP,
Snelgrove KJ, Rogers N, Acton SE, Chakravarty P, Girotti MR, Marais
R, Quezada SA, et al: Cyclooxygenase-dependent tumor growth through
evasion of immunity. Cell. 162:1257–1270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lin Y, He Z, Ye J, Liu Z, She X, Gao X and
Liang R: Progress in understanding the IL-6/STAT3 pathway in
colorectal cancer. Onco Targets Ther. 13:13023–13032. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Su YL, Banerjee S, White SV and
Kortylewski M: STAT3 in tumor-associated myeloid cells:
Multitasking to disrupt immunity. Int J Mol Sci. 19:18032018.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Sinha P, Clements VK, Fulton AM and
Ostrand-Rosenberg S: Prostaglandin E2 promotes tumor progression by
inducing myeloid-derived suppressor cells. Cancer Res.
67:4507–4513. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Tang C, Sun H, Kadoki M, Han W, Ye X,
Makusheva Y, Deng J, Feng B, Qiu D, Tan Y, et al: Blocking Dectin-1
prevents colorectal tumorigenesis by suppressing prostaglandin E2
production in myeloid-derived suppressor cells and enhancing IL-22
binding protein expression. Nat Commun. 14:14932023. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lu W, Yu W, He J, Liu W, Yang J, Lin X,
Zhang Y, Wang X, Jiang W, Luo J, et al: Reprogramming
immunosuppressive myeloid cells facilitates immunotherapy for
colorectal cancer. EMBO Mol Med. 13:e127982021. View Article : Google Scholar :
|
|
94
|
Molfetta R and Paolini R: The
controversial role of intestinal mast cells in colon cancer. Cells.
12:4592023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Cheon EC, Khazaie K, Khan MW, Strouch MJ,
Krantz SB, Phillips J, Blatner NR, Hix LM, Zhang M, Dennis KL, et
al: Mast cell 5-lipoxygenase activity promotes intestinal polyposis
in APCDelta468 mice. Cancer Res. 71:1627–1636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ostrand-Rosenberg S and Fenselau C:
Myeloid-derived suppressor cells: Immune-suppressive cells that
impair antitumor immunity and are sculpted by their environment. J
Immunol. 200:422–431. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Rahma OE and Hodi FS: The Intersection
between tumor angiogenesis and immune suppression. Clin Cancer Res.
25:5449–5457. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Xiang X, Poliakov A, Liu C, Liu Y, Deng
ZB, Wang J, Cheng Z, Shah SV, Wang GJ, Zhang L, et al: Induction of
myeloid-derived suppressor cells by tumor exosomes. Int J Cancer.
124:2621–2633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Fenselau C and Ostrand-Rosenberg S:
Molecular cargo in myeloid-derived suppressor cells and their
exosomes. Cell Immunol. 359:1042582021. View Article : Google Scholar :
|
|
100
|
Gu J, Lv X, Li W, Li G, He X, Zhang Y, Shi
L and Zhang X: Deciphering the mechanism of Peptostreptococcus
anaerobius-induced chemoresistance in colorectal cancer: The
important roles of MDSC recruitment and EMT activation. Front
Immunol. 14:12306812023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Abed J, Emgård JEM, Zamir G, Faroja M,
Almogy G, Grenov A, Sol A, Naor R, Pikarsky E, Atlan KA, et al:
Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma
enrichment by binding to tumor-expressed gal-GalNAc. Cell Host
Microbe. 20:215–225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Han J, Zhang B, Zhang Y, Yin T, Cui Y, Liu
J, Yang Y, Song H and Shang D: Gut microbiome: Decision-makers in
the microenvironment of colorectal cancer. Front Cell Infect
Microbiol. 13:12999772023. View Article : Google Scholar :
|
|
103
|
Kostic AD, Chun E, Robertson L, Glickman
JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold
GL, et al: Fusobacterium nucleatum potentiates intestinal
tumorigenesis and modulates the tumor-immune microenvironment. Cell
Host Microbe. 14:207–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hashemi Goradel N, Heidarzadeh S,
Jahangiri S, Farhood B, Mortezaee K, Khanlarkhani N and Negahdari
B: Fusobacterium nucleatum and colorectal cancer: A mechanistic
overview. J Cell Physiol. 234:2337–2344. 2019. View Article : Google Scholar
|
|
105
|
Cassetta L, Baekkevold ES, Brandau S,
Bujko A, Cassatella MA, Dorhoi A, Krieg C, Lin A, Loré K, Marini O,
et al: Deciphering myeloid-derived suppressor cells: Isolation and
markers in humans, mice and non-human primates. Cancer Immunol
Immunother. 68:687–697. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wu Y, Yi M, Niu M, Mei Q and Wu K:
Myeloid-derived suppressor cells: An emerging target for anticancer
immunotherapy. Mol Cancer. 21:1842022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Gallina G, Dolcetti L, Serafini P, De
Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I,
Zanovello P, et al: Tumors induce a subset of inflammatory
monocytes with immunosuppressive activity on CD8+ T
cells. J Clin Invest. 116:2777–2790. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Bronte V and Zanovello P: Regulation of
immune responses by L-arginine metabolism. Nat Rev Immunol.
5:641–654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Rodriguez PC, Quiceno DG and Ochoa AC:
L-arginine availability regulates T-lymphocyte cell-cycle
progression. Blood. 109:1568–1573. 2007. View Article : Google Scholar
|
|
111
|
Zheng Y, Yao Y, Ge T, Ge S, Jia R, Song X
and Zhuang A: Amino acid metabolism reprogramming: Shedding new
light on T cell anti-tumor immunity. J Exp Clin Cancer Res.
42:2912023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Fujimura T, Mahnke K and Enk AH: Myeloid
derived suppressor cells and their role in tolerance induction in
cancer. J Dermatol Sci. 59:1–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Steggerda SM, Bennett MK, Chen J, Emberley
E, Huang T, Janes JR, Li W, MacKinnon AL, Makkouk A, Marguier G, et
al: Inhibition of arginase by CB-1158 blocks myeloid cell-mediated
immune suppression in the tumor microenvironment. J Immunother
Cancer. 5:1012017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Portale F and Di Mitri D: NK cells in
cancer: Mechanisms of dysfunction and therapeutic potential. Int J
Mol Sci. 24:95212023. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Raber PL, Thevenot P, Sierra R,
Wyczechowska D, Halle D, Ramirez ME, Ochoa AC, Fletcher M, Velasco
C, Wilk A, et al: Subpopulations of myeloid-derived suppressor
cells impair T cell responses through independent nitric
oxide-related pathways. Int J Cancer. 134:2853–2864. 2014.
View Article : Google Scholar
|
|
116
|
Li W, Zhang X, Chen Y, Xie Y, Liu J, Feng
Q, Wang Y, Yuan W and Ma J: G-CSF is a key modulator of MDSC and
could be a potential therapeutic target in colitis-associated
colorectal cancers. Protein Cell. 7:130–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
OuYang LY, Wu XJ, Ye SB, Zhang RX, Li ZL,
Liao W, Pan ZZ, Zheng LM, Zhang XS, Wang Z, et al: Tumor-induced
myeloid-derived suppressor cells promote tumor progression through
oxidative metabolism in human colorectal cancer. J Transl Med.
13:472015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Corzo CA, Cotter MJ, Cheng P, Cheng F,
Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC and
Gabrilovich DI: Mechanism regulating reactive oxygen species in
tumor-induced myeloid-derived suppressor cells. J Immunol.
182:5693–5701. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Nagaraj S, Gupta K, Pisarev V, Kinarsky L,
Sherman S, Kang L, Herber DL, Schneck J and Gabrilovich DI: Altered
recognition of antigen is a mechanism of CD8+ T cell
tolerance in cancer. Nat Med. 13:828–835. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Jachetti E, Sangaletti S, Chiodoni C,
Ferrara R and Colombo MP: Modulation of PD-1/PD-L1 axis in
myeloid-derived suppressor cells by anti-cancer treatments. Cell
Immunol. 362:1043012021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Noman MZ, Desantis G, Janji B, Hasmim M,
Karray S, Dessen P, Bronte V and Chouaib S: PD-L1 is a novel direct
target of HIF-1α, and its blockade under hypoxia enhanced
MDSC-mediated T cell activation. J Exp Med. 211:781–790. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhu J, Powis de Tenbossche CG, Cané S,
Colau D, van Baren N, Lurquin C, Schmitt-Verhulst AM, Liljeström P,
Uyttenhove C and Van den Eynde BJ: Resistance to cancer
immunotherapy mediated by apoptosis of tumor-infiltrating
lymphocytes. Nat Commun. 8:14042017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Trovato R, Canè S, Petrova V, Sartoris S,
Ugel S and De Sanctis F: The engagement between MDSCs and
Metastases: Partners in crime. Front Oncol. 10:1652020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Gabrilovich DI, Ostrand-Rosenberg S and
Bronte V: Coordinated regulation of myeloid cells by tumours. Nat
Rev Immunol. 12:253–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Lasser SA, Ozbay Kurt FG, Arkhypov I,
Utikal J and Umansky V: Myeloid-derived suppressor cells in cancer
and cancer therapy. Nat Rev Clin Oncol. 21:147–164. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Lu C, Redd PS, Lee JR, Savage N and Liu K:
The expression profiles and regulation of PD-L1 in tumor-induced
myeloid-derived suppressor cells. Oncoimmunology. 5:e12471352016.
View Article : Google Scholar
|
|
127
|
Chiu DK, Tse AP, Xu IM, Di Cui J, Lai RK,
Li LL, Koh HY, Tsang FH, Wei LL, Wong CM, et al: Hypoxia inducible
factor HIF-1 promotes myeloid-derived suppressor cells accumulation
through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat Commun.
8:5172017. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zhou J, Qu Z, Sun F, Han L, Li L, Yan S,
Stabile LP, Chen LF, Siegfried JM and Xiao G: Myeloid STAT3
promotes lung tumorigenesis by transforming tumor
immunosurveillance into tumor-promoting inflammation. Cancer
Immunol Res. 5:257–268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Halaby MJ and McGaha TL: Amino acid
transport and metabolism in myeloid function. Front Immunol.
12:6952382021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Prendergast GC, Malachowski WJ, Mondal A,
Scherle P and Muller AJ: Indoleamine 2,3-dioxygenase and its
therapeutic inhibition in cancer. Int Rev Cell Mol Biol.
336:175–203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Arshad J, Rao A, Repp ML, Rao R, Wu C and
Merchant JL: Myeloid-derived suppressor cells: Therapeutic target
for gastrointestinal cancers. Int J Mol Sci. 25:29852024.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Neurath MF, Weigmann B, Finotto S,
Glickman J, Nieuwenhuis E, Iijima H, Mizoguchi A, Mizoguchi E,
Mudter J, Galle PR, et al: The transcription factor T-bet regulates
mucosal T cell activation in experimental colitis and Crohn's
disease. J Exp Med. 195:1129–1143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Lúdvíksson BR, Seegers D, Resnick AS and
Strober W: The effect of TGF-beta1 on immune responses of naïve
versus memory CD4+ Th1/Th2 T cells. Eur J Immunol. 30:2101–2111.
2000. View Article : Google Scholar
|
|
134
|
Singh S, Gouri V and Samant M: TGF-β in
correlation with tumor progression, immunosuppression and targeted
therapy in colorectal cancer. Med Oncol. 40:3352023. View Article : Google Scholar
|
|
135
|
Takaku S, Terabe M, Ambrosino E, Peng J,
Lonning S, McPherson JM and Berzofsky JA: Blockade of TGF-beta
enhances tumor vaccine efficacy mediated by CD8(+) T cells. Int J
Cancer. 126:1666–1674. 2010. View Article : Google Scholar
|
|
136
|
Huang B, Pan PY, Li Q, Sato AI, Levy DE,
Bromberg J, Divino CM and Chen SH: Gr-1+CD115+ immature myeloid
suppressor cells mediate the development of tumor-induced T
regulatory cells and T-cell anergy in tumor-bearing host. Cancer
Res. 66:1123–1131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Han J, Dong L, Wu M and Ma F: Dynamic
polarization of tumor-associated macrophages and their interaction
with intratumoral T cells in an inflamed tumor microenvironment:
From mechanistic insights to therapeutic opportunities. Front
Immunol. 14:11603402023. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Sinha P, Clements VK, Bunt SK, Albelda SM
and Ostrand-Rosenberg S: Cross-talk between myeloid-derived
suppressor cells and macrophages subverts tumor immunity toward a
type 2 response. J Immunol. 179:977–983. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Peng P, Lou Y, Wang S, Wang J, Zhang Z, Du
P, Zheng J, Liu P and Xu LX: Activated NK cells reprogram MDSCs via
NKG2D-NKG2DL and IFN-γ to modulate antitumor T-cell response after
cryo-thermal therapy. J Immunother Cancer. 10:e0057692022.
View Article : Google Scholar
|
|
140
|
Yue J, Li J, Ma J, Zhai Y, Shen L, Zhang
W, Li L and Fu R: Myeloid-derived suppressor cells inhibit natural
killer cells in myelodysplastic syndromes through the TIGIT/CD155
pathway. Hematology. 28:21663332023. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Liu G, Bi Y, Shen B, Yang H, Zhang Y, Wang
X, Liu H, Lu Y, Liao J, Chen X and Chu Y: SIRT1 limits the function
and fate of myeloid-derived suppressor cells in tumors by
orchestrating HIF-1α-dependent glycolysis. Cancer Res. 74:727–737.
2014. View Article : Google Scholar
|
|
142
|
O'Donnell C, Mahmoud A, Keane J, Murphy C,
White D, Carey S, O'Riordain M, Bennett MW, Brint E and Houston A:
An antitumorigenic role for the IL-33 receptor, ST2L, in colon
cancer. Br J Cancer. 114:37–43. 2016. View Article : Google Scholar :
|
|
143
|
Wang D, Sun H, Wei J, Cen B and DuBois RN:
CXCL1 is critical for premetastatic niche formation and metastasis
in colorectal cancer. Cancer Res. 77:3655–3665. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Gao Y, Bado I, Wang H, Zhang W, Rosen JM
and Zhang XHF: Metastasis organotropism: Redefining the congenial
soil. Dev Cell. 49:375–391. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Li B, Zhang S, Huang N, Chen H, Wang P,
Yang J and Li Z: CCL9/CCR1 induces myeloid-derived suppressor cell
recruitment to the spleen in a murine H22 orthotopic hepatoma
model. Oncol Rep. 41:608–618. 2019.
|
|
146
|
Cui TX, Kryczek I, Zhao L, Zhao E, Kuick
R, Roh MH, Vatan L, Szeliga W, Mao Y, Thomas DG, et al:
Myeloid-derived suppressor cells enhance stemness of cancer cells
by inducing microRNA101 and suppressing the corepressor CtBP2.
Immunity. 39:611–621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Di Mitri D, Toso A, Chen JJ, Sarti M,
Pinton S, Jost TR, D'Antuono R, Montani E, Garcia-Escudero R,
Guccini I, et al: Tumour-infiltrating Gr-1+ myeloid cells
antagonize senescence in cancer. Nature. 515:134–137. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Yan HH, Pickup M, Pang Y, Gorska AE, Li Z,
Chytil A, Geng Y, Gray JW, Moses HL and Yang L: Gr-1+CD11b+ myeloid
cells tip the balance of immune protection to tumor promotion in
the premetastatic lung. Cancer Res. 70:6139–6149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Long L, Xiong W, Lin F, Hou J, Chen G,
Peng T, He Y, Wang R, Xu Q and Huang Y: Regulating lactate-related
immunometabolism and EMT reversal for colorectal cancer liver
metastases using shikonin targeted delivery. J Exp Clin Cancer Res.
42:1172023. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Taki M, Abiko K, Ukita M, Murakami R,
Yamanoi K, Yamaguchi K, Hamanishi J, Baba T, Matsumura N and Mandai
M: Tumor immune microenvironment during epithelial-mesenchymal
transition. Clin Cancer Res. 27:4669–4679. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Wang Y, Jia A, Bi Y, Wang Y, Yang Q, Cao
Y, Li Y and Liu G: Targeting myeloid-derived suppressor cells in
cancer immunotherapy. Cancers (Basel). 12:26262020. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Qu P, Wang LZ and Lin PC: Expansion and
functions of myeloid-derived suppressor cells in the tumor
microenvironment. Cancer Lett. 380:253–256. 2016. View Article : Google Scholar
|
|
153
|
De Cicco P, Ercolano G and Ianaro A: The
new era of cancer immunotherapy: Targeting myeloid-derived
suppressor cells to overcome immune evasion. Front Immunol.
11:16802020. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Law AMK, Valdes-Mora F and Gallego-Ortega
D: Myeloid-derived suppressor cells as a therapeutic target for
cancer. Cells. 9:5612020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Li W, Wu K, Zhao E, Shi L, Li R, Zhang P,
Yin Y, Shuai X, Wang G and Tao K: HMGB1 recruits myeloid derived
suppressor cells to promote peritoneal dissemination of colon
cancer after resection. Biochem Biophys Res Commun. 436:156–161.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Eriksson E, Wenthe J, Irenaeus S, Loskog A
and Ullenhag G: Gemcitabine reduces MDSCs, tregs and TGFβ-1 while
restoring the teff/treg ratio in patients with pancreatic cancer. J
Transl Med. 14:2822016. View Article : Google Scholar
|
|
157
|
John David K, Amy VP, Natasha MS, Asha NK
and Kebin L: 5-Fluorouracil regulation of myeloid-derived
suppressor cell differentiation in vitro and in vivo. J Immunol.
198(Suppl 1): S205.52017. View Article : Google Scholar
|
|
158
|
Vincent J, Mignot G, Chalmin F, Ladoire S,
Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C and
Ghiringhelli F: 5-Fluorouracil selectively kills tumor-associated
myeloid-derived suppressor cells resulting in enhanced T
cell-dependent antitumor immunity. Cancer Res. 70:3052–3061. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Kanterman J, Sade-Feldman M, Biton M,
Ish-Shalom E, Lasry A, Goldshtein A, Hubert A and Baniyash M:
Adverse immunoregulatory effects of 5FU and CPT11 chemotherapy on
myeloid-derived suppressor cells and colorectal cancer outcomes.
Cancer Res. 74:6022–6035. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Talmadge JE, Hood KC, Zobel LC, Shafer LR,
Coles M and Toth B: Chemoprevention by cyclooxygenase-2 inhibition
reduces immature myeloid suppressor cell expansion. Int
Immunopharmacol. 7:140–151. 2007. View Article : Google Scholar
|
|
161
|
Osada T, Chong G, Tansik R, Hong T,
Spector N, Kumar R, Hurwitz HI, Dev I, Nixon AB, Lyerly HK, et al:
The effect of anti-VEGF therapy on immature myeloid cell and
dendritic cells in cancer patients. Cancer Immunol Immunother.
57:1115–1124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Dominguez GA, Condamine T, Mony S,
Hashimoto A, Wang F, Liu Q, Forero A, Bendell J, Witt R, Hockstein
N, et al: selective targeting of myeloid-derived suppressor cells
in cancer patients using DS-8273a, an agonistic TRAIL-R2 antibody.
Clin Cancer Res. 23:2942–2950. 2017. View Article : Google Scholar :
|
|
163
|
Fultang L, Panetti S, Ng M, Collins P,
Graef S, Rizkalla N, Booth S, Lenton R, Noyvert B, Shannon-Lowe C,
et al: MDSC targeting with Gemtuzumab ozogamicin restores T cell
immunity and immunotherapy against cancers. EBioMedicine.
47:235–246. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
De Sanctis F, Solito S, Ugel S, Molon B,
Bronte V and Marigo I: MDSCs in cancer: Conceiving new prognostic
and therapeutic targets. Biochim Biophys Acta. 1865:35–48.
2016.
|
|
165
|
Hinshaw DC and Shevde LA: The tumor
microenvironment innately modulates cancer progression. Cancer Res.
79:4557–4566. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Zhou J, Nefedova Y, Lei A and Gabrilovich
D: Neutrophils and PMN-MDSC: Their biological role and interaction
with stromal cells. Semin Immunol. 35:19–28. 2018. View Article : Google Scholar
|
|
167
|
Park SM and Youn JI: Role of
myeloid-derived suppressor cells in immune checkpoint inhibitor
therapy in cancer. Arch Pharm Res. 42:560–566. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Cheng Y, Ma XL, Wei YQ and Wei XW:
Potential roles and targeted therapy of the CXCLs/CXCR2 axis in
cancer and inflammatory diseases. Biochim Biophys Acta Rev Cancer.
1871:289–312. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Yan G, Zhao H, Zhang Q, Zhou Y, Wu L, Lei
J, Wang X, Zhang J, Zhang X, Zheng L, et al: A
RIPK3-PGE2 circuit mediates myeloid-derived suppressor
cell-potentiated colorectal carcinogenesis. Cancer Res.
78:5586–5599. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Umansky V, Blattner C, Gebhardt C and
Utikal J: CCR5 in recruitment and activation of myeloid-derived
suppressor cells in melanoma. Cancer Immunol Immunother.
66:1015–1023. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Tan MCB, Goedegebuure PS, Belt BA,
Flaherty B, Sankpal N, Gillanders WE, Eberlein TJ, Hsieh CS and
Linehan DC: Disruption of CCR5-dependent homing of regulatory T
cells inhibits tumor growth in a murine model of pancreatic cancer.
J Immunol. 182:1746–1755. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Zhang X, Haney KM, Richardson AC, Wilson
E, Gewirtz DA, Ware JL, Zehner ZE and Zhang Y: Anibamine, a natural
product CCR5 antagonist, as a novel lead for the development of
anti-prostate cancer agents. Bioorg Med Chem Lett. 20:4627–4630.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Velasco-Velázquez M, Jiao X, De La Fuente
M, Pestell TG, Ertel A, Lisanti MP and Pestell RG: CCR5 antagonist
blocks metastasis of basal breast cancer cells. Cancer Res.
72:3839–3850. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Deming DA: Advances in immunotherapeutic
strategies for colorectal cancer commentary on: tumoral immune cell
exploitation in colorectal cancer metastases can be targeted
effectively by anti-CCR5 therapy in cancer patients by Halama et
al. J Immunother Cancer. 4:932016. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Cannarile MA, Weisser M, Jacob W, Jegg AM,
Ries CH and Rüttinger D: Colony-stimulating factor 1 receptor
(CSF1R) inhibitors in cancer therapy. J Immunother Cancer.
5:532017. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Holmgaard RB, Zamarin D, Lesokhin A,
Merghoub T and Wolchok JD: Targeting myeloid-derived suppressor
cells with colony stimulating factor-1 receptor blockade can
reverse immune resistance to immunotherapy in indoleamine
2,3-dioxygenase-expressing tumors. EBioMedicine. 6:50–58. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Mitchem JB, Brennan DJ, Knolhoff BL, Belt
BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L,
Piwnica-Worms D, et al: Targeting tumor-infiltrating macrophages
decreases tumor-initiating cells, relieves immunosuppression, and
improves chemotherapeutic responses. Cancer Res. 73:1128–1141.
2013. View Article : Google Scholar
|
|
178
|
Lonardi S, Licini S, Micheletti A, Finotti
G, Vermi W and Cassatella MA: Potential contribution of
tumor-associated slan+ cells as anti-CSF-1R targets in
human carcinoma. J Leukoc Biol. 103:559–564. 2018. View Article : Google Scholar
|
|
179
|
Lin S, Wang J, Wang L, Wen J, Guo Y, Qiao
W, Zhou J, Xu G and Zhi F: Phosphodiesterase-5 inhibition
suppresses colonic inflammation-induced tumorigenesis via blocking
the recruitment of MDSC. Am J Cancer Res. 7:41–52. 2017.PubMed/NCBI
|
|
180
|
Liang H, Deng L, Hou Y, Meng X, Huang X,
Rao E, Zheng W, Mauceri H, Mack M, Xu M, et al: Host
STING-dependent MDSC mobilization drives extrinsic radiation
resistance. Nat Commun. 8:17362017. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
De Santo C, Serafini P, Marigo I, Dolcetti
L, Bolla M, Del Soldato P, Melani C, Guiducci C, Colombo MP, Iezzi
M, et al: Nitroaspirin corrects immune dysfunction in tumor-bearing
hosts and promotes tumor eradication by cancer vaccination. Proc
Natl Acad Sci USA. 102:4185–4190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Molon B, Viola A and Bronte V: Smoothing T
cell roads to the tumor: Chemokine post-translational regulation.
Oncoimmunology. 1:390–392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Chen HM, Ma G, Gildener-Leapman N,
Eisenstein S, Coakley BA, Ozao J, Mandeli J, Divino C, Schwartz M,
Sung M, et al: Myeloid-derived suppressor cells as an immune
parameter in patients with concurrent sunitinib and stereotactic
body radiotherapy. Clin Cancer Res. 21:4073–4085. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Nagaraj S, Youn JI, Weber H, Iclozan C, Lu
L, Cotter MJ, Meyer C, Becerra CR, Fishman M, Antonia S, et al:
Anti-inflammatory triterpenoid blocks immune suppressive function
of MDSCs and improves immune response in cancer. Clin Cancer Res.
16:1812–1823. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Chalmin F, Ladoire S, Mignot G, Vincent J,
Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau
D, et al: Membrane-associated Hsp72 from tumor-derived exosomes
mediates STAT3-dependent immunosuppressive function of mouse and
human myeloid-derived suppressor cells. J Clin Invest. 120:457–471.
2010.PubMed/NCBI
|
|
186
|
Bertocchi A, Carloni S, Ravenda PS,
Bertalot G, Spadoni I, Lo Cascio A, Gandini S, Lizier M, Braga D,
Asnicar F, et al: Gut vascular barrier impairment leads to
intestinal bacteria dissemination and colorectal cancer metastasis
to liver. Cancer Cell. 39:708–724.e11. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Mei Y, Zhu Y, Yong KSM, Hanafi ZB, Gong H,
Liu Y, Teo HY, Hussain M, Song Y, Chen Q and Liu H: IL-37 dampens
immunosuppressive functions of MDSCs via metabolic reprogramming in
the tumor microenvironment. Cell Rep. 43:1138352024. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Hengesbach LM and Hoag KA: Physiological
concentrations of retinoic acid favor myeloid dendritic cell
development over granulocyte development in cultures of bone marrow
cells from mice. J Nutr. 134:2653–2659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Nefedova Y, Fishman M, Sherman S, Wang X,
Beg AA and Gabrilovich DI: Mechanism of all-trans retinoic acid
effect on tumor-associated myeloid-derived suppressor cells. Cancer
Res. 67:11021–11028. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Mirza N, Fishman M, Fricke I, Dunn M,
Neuger AM, Frost TJ, Lush RM, Antonia S and Gabrilovich DI:
All-trans-retinoic acid improves differentiation of myeloid cells
and immune response in cancer patients. Cancer Res. 66:9299–9307.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Tu SP, Jin H, Shi JD, Zhu LM, Suo Y, Lu G,
Liu A, Wang TC and Yang CS: Curcumin induces the differentiation of
myeloid-derived suppressor cells and inhibits their interaction
with cancer cells and related tumor growth. Cancer Prev Res
(Phila). 5:205–215. 2012. View Article : Google Scholar
|
|
192
|
Carroll RE, Benya RV, Turgeon DK, Vareed
S, Neuman M, Rodriguez L, Kakarala M, Carpenter PM, McLaren C,
Meyskens FL Jr and Brenner DE: Phase IIa clinical trial of curcumin
for the prevention of colorectal neoplasia. Cancer Prev Res
(Phila). 4:354–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Daurkin I, Eruslanov E, Vieweg J and
Kusmartsev S: Generation of antigen-presenting cells from
tumor-infiltrated CD11b myeloid cells with DNA demethylating agent
5-aza-2′-deoxycytidine. Cancer Immunol Immunother. 59:697–706.
2010. View Article : Google Scholar
|
|
194
|
Zoglmeier C, Bauer H, Noerenberg D,
Wedekind G, Bittner P, Sandholzer N, Rapp M, Anz D, Endres S and
Bourquin C: CpG blocks immunosuppression by myeloid-derived
suppressor cells in tumor-bearing mice. Clin Cancer Res.
17:1765–1775. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Meyer C, Cagnon L, Costa-Nunes CM,
Baumgaertner P, Montandon N, Leyvraz L, Michielin O, Romano E and
Speiser DE: Frequencies of circulating MDSC correlate with clinical
outcome of melanoma patients treated with ipilimumab. Cancer
Immunol Immunother. 63:247–257. 2014. View Article : Google Scholar
|
|
196
|
Di Giacomo AM, Schenker M, Medioni J,
Mandziuk S, Majem M, Gravis G, Cornfeld M, Ranganathan S, Lou S and
Csoszi T: A phase II study of retifanlimab, a humanized anti-PD-1
monoclonal antibody, in patients with solid tumors (POD1UM-203).
ESMO Open. 9:1023872024. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Kim W, Chu TH, Nienhüser H, Jiang Z, Del
Portillo A, Remotti HE, White RA, Hayakawa Y, Tomita H, Fox JG, et
al: PD-1 signaling promotes tumor-infiltrating myeloid-derived
suppressor cells and gastric tumorigenesis in mice.
Gastroenterology. 160:781–796. 2021. View Article : Google Scholar
|
|
198
|
Kalyan A, Kircher S, Shah H, Mulcahy M and
Benson A: Updates on immunotherapy for colorectal cancer. J
Gastrointest Oncol. 9:160–169. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Wang C, Zheng X, Zhang J, Jiang X, Wang J,
Li Y, Li X, Shen G, Peng J, Zheng P, et al: CD300ld on neutrophils
is required for tumour-driven immune suppression. Nature.
621:830–839. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Kumar V, Cheng P, Condamine T, Mony S,
Languino LR, McCaffrey JC, Hockstein N, Guarino M, Masters G,
Penman E, et al: CD45 phosphatase inhibits STAT3 transcription
factor activity in myeloid cells and promotes tumor-associated
macrophage differentiation. Immunity. 44:303–315. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Su MT, Kumata S, Endo S, Okada Y and Takai
T: LILRB4 promotes tumor metastasis by regulating MDSCs and
inhibiting miR-1 family miRNAs. Oncoimmunology. 11:20609072022.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Ostrand-Rosenberg S, Beury DW, Parker KH
and Horn LA: Survival of the fittest: How myeloid-derived
suppressor cells survive in the inhospitable tumor
microenvironment. Cancer Immunol Immunother. 69:215–221. 2020.
View Article : Google Scholar :
|
|
203
|
Beury DW, Carter KA, Nelson C, Sinha P,
Hanson E, Nyandjo M, Fitzgerald PJ, Majeed A, Wali N and
Ostrand-Rosenberg S: Myeloid-derived suppressor cell survival and
function are regulated by the transcription factor Nrf2. J Immunol.
196:3470–3478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Condamine T, Kumar V, Ramachandran IR,
Youn JI, Celis E, Finnberg N, El-Deiry WS, Winograd R, Vonderheide
RH, English NR, et al: ER stress regulates myeloid-derived
suppressor cell fate through TRAIL-R-mediated apoptosis. J Clin
Invest. 124:2626–2639. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Wang Y, Zheng L, Shang W, Yang Z, Li T,
Liu F, Shao W, Lv L, Chai L, Qu L, et al: Wnt/beta-catenin
signaling confers ferroptosis resistance by targeting GPX4 in
gastric cancer. Cell Death Differ. 29:2190–2202. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Conche C, Finkelmeier F, Pešić M, Nicolas
AM, Böttger TW, Kennel KB, Denk D, Ceteci F, Mohs K, Engel E, et
al: Combining ferroptosis induction with MDSC blockade renders
primary tumours and metastases in liver sensitive to immune
checkpoint blockade. Gut. 72:1774–1782. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Bebelman MP, Smit MJ, Pegtel DM and Baglio
SR: Biogenesis and function of extracellular vesicles in cancer.
Pharmacol Ther. 188:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Hu JL, Wang W, Lan XL, Zeng ZC, Liang YS,
Yan YR, Song FY, Wang FF, Zhu XH, Liao WJ, et al: CAFs secreted
exosomes promote metastasis and chemotherapy resistance by
enhancing cell stemness and epithelial-mesenchymal transition in
colorectal cancer. Mol Cancer. 18:912019. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Xu Z, Zeng S, Gong Z and Yan Y:
Exosome-based immunotherapy: A promising approach for cancer
treatment. Mol Cancer. 19:1602020. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Wang Y, Yin K, Tian J, Xia X, Ma J, Tang
X, Xu H and Wang S: Granulocytic myeloid-derived suppressor cells
promote the stemness of colorectal cancer cells through exosomal
S100A9. Adv Sci (Weinh). 6:19012782019. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Wang Y, Liu H, Zhang Z, Bian D, Shao K,
Wang S and Ding Y: G-MDSC-derived exosomes mediate the
differentiation of M-MDSC into M2 macrophages promoting
colitis-to-cancer transition. J Immunother Cancer. 11:e0061662023.
View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang
G, Song J, Li Z, Zhang Z and Yuan W: Effect of exosomal miRNA on
cancer biology and clinical applications. Mol Cancer. 17:1472018.
View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Matsumura T, Sugimachi K, Iinuma H,
Takahashi Y, Kurashige J, Sawada G, Ueda M, Uchi R, Ueo H, Takano
Y, et al: Exosomal microRNA in serum is a novel biomarker of
recurrence in human colorectal cancer. Br J Cancer. 113:275–281.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Markowitz SD and Bertagnolli MM: Molecular
origins of cancer: Molecular basis of colorectal cancer. N Engl J
Med. 361:2449–2460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Huang D, Sun W, Zhou Y, Li P, Chen F, Chen
H, Xia D, Xu E, Lai M, Wu Y and Zhang H: Mutations of key driver
genes in colorectal cancer progression and metastasis. Cancer
Metastasis Rev. 37:173–187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Jayaraman P, Parikh F, Newton JM, Hanoteau
A, Rivas C, Krupar R, Rajapakshe K, Pathak R, Kanthaswamy K,
MacLaren C, et al: TGF-β1 programmed myeloid-derived suppressor
cells (MDSC) acquire immune-stimulating and tumor killing activity
capable of rejecting established tumors in combination with
radiotherapy. Oncoimmunology. 7:e14908532018. View Article : Google Scholar
|
|
217
|
Li X, Wen D, Li X, Yao C, Chong W and Chen
H: Identification of an immune signature predicting prognosis risk
and lymphocyte infiltration in colon cancer. Front Immunol.
11:16782020. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Javle MM, Bridgewater JA, Gbolahan OB,
Jungels C, Cho MT, Papadopoulos KP, Thistlethwaite FC, Canon JLR,
Cheng L, Ioannidis S, et al: A phase I/II study of safety and
efficacy of the arginase inhibitor INCB001158 plus chemotherapy in
patients (Pts) with advanced biliary tract cancers. J Clin Oncol.
39(Suppl 3): S3112021. View Article : Google Scholar
|
|
219
|
Lorentzen CL, Martinenaite E, Kjeldsen JW,
Holmstroem RB, Mørk SK, Pedersen AW, Ehrnrooth E, Andersen MH and
Svane IM: Arginase-1 targeting peptide vaccine in patients with
metastatic solid tumors-A phase I trial. Front Immunol.
13:10230232022. View Article : Google Scholar
|
|
220
|
Zeng Z, Lan T, Wei Y and Wei X: CCL5/CCR5
axis in human diseases and related treatments. Genes Dis. 9:12–27.
2022. View Article : Google Scholar
|
|
221
|
Snajdauf M, Havlova K, Vachtenheim J Jr,
Ozaniak A, Lischke R, Bartunkova J, Smrz D and Strizova Z: The
TRAIL in the treatment of human cancer: An update on clinical
trials. Front Mol Biosci. 8:6283322021. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Isambert N, Hervieu A, Rébé C, Hennequin
A, Borg C, Zanetta S, Chevriaux A, Richard C, Derangère V, Limagne
E, et al: Fluorouracil and bevacizumab plus anakinra for patients
with metastatic colorectal cancer refractory to standard therapies
(IRAFU): A single-arm phase 2 study. Oncoimmunology.
7:e14743192018. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Schmitz-Winnenthal FH, Hohmann N, Schmidt
T, Podola L, Friedrich T, Lubenau H, Springer M, Wieckowski S,
Breiner KM, Mikus G, et al: A phase 1 trial extension to assess
immunologic efficacy and safety of prime-boost vaccination with
VXM01, an oral T cell vaccine against VEGFR2, in patients with
advanced pancreatic cancer. Oncoimmunology. 7:e13035842018.
View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Johnson B, Kopetz S, Hwang H, Yuan Y,
DePinho RA, Zebala J and Overman MJ: STOPTRAFFIC-1: A phase I/II
trial of SX-682 in combination with nivolumab for refractory
RAS-mutated microsatellite stable (MSS) metastatic colorectal
cancer (mCRC). J Clin Oncol. 40(Suppl 16): TPS36382022. View Article : Google Scholar
|
|
225
|
Hanna CR, O'Cathail SM, Graham J, Adams R
and Roxburgh CSD: Immune checkpoint inhibition as a strategy in the
neoadjuvant treatment of locally advanced rectal cancer. J
Immunother Precis Oncol. 4:86–104. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Lizardo DY, Kuang C, Hao S, Yu J, Huang Y
and Zhang L: Immunotherapy efficacy on mismatch repair-deficient
colorectal cancer: From bench to bedside. Biochim Biophys Acta Rev
Cancer. 1874:1884472020. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Hull MA, Ow PL, Ruddock S, Brend T, Smith
AF, Marshall H, Song M, Chan AT, Garrett WS, Yilmaz O, et al:
Randomised, placebo-controlled, phase 3 trial of the effect of the
omega-3 polyunsaturated fatty acid eicosapentaenoic acid (EPA) on
colorectal cancer recurrence and survival after surgery for
resectable liver metastases: EPA for metastasis trial 2 (EMT2)
study protocol. BMJ Open. 13:e0774272023. View Article : Google Scholar
|