Breast cancer (BC) is a common malignant tumor and
its incidence rate has shown an overall upward trend in the past
decade (1). Despite the progress
in both understanding and treating BC, nearly 30% of patients
suffer from recurrence or metastasis due to the deficiency of
effective treatment or prevention strategies, which is the main
reason for BC-related mortality (2). The extensively used classification
for BC comprises Luminal A, Luminal B, human epidermal growth
factor receptor (EGFR) 2 (HER2) overexpression and triple-negative
BC (TNBC) (3). Studies have
indicated that with early diagnosis and timely treatment, the
overall survival of nonmetastatic BC and de novo metastatic
BC (MBC) has been evidently improved. However, for recurrent MBC
and elderly patients, there has been no improvement in decades
(4). Hence, investigating the
molecular mechanisms underlying the onset and progression of BC,
and enhancing the capacity for monitoring BC treatment efficacy or
identifying promising therapeutic targets, are of immense
importance for precise diagnosis, efficient stratified management
and the development of more refined treatment strategies for
BC.
BC originates from mammary duct epithelial cells,
which is the malignant tumor type with the highest incidence and
mortality rates for women worldwide, accounting for ~30% of cancers
in females (11-13). Clinical manifestations of BC may
include breast lumps, nipple discharge and breast skin changes.
However, early symptoms of part of breast cancer are not obvious or
characteristic, which increases the difficulty of early
identification.
BC exhibits apparent heterogeneity. According to the
status of hormone receptors [estrogen receptor (ER) and
progesterone receptor (PR)] and HER2, BC can be classified into
three primary subtypes: Luminal ER-positive and PR-positive, which
can be further categorized as luminal A and B, HER2-positive BC and
TNBC (14,15). This BC classification based on
biomarkers provides a foundation for further research and more
precise determination of prognosis and selection of personalized
treatments (13). For instance,
the systemic treatment of nonmetastatic BC typically varies based
on the subtype: Hormone receptor-positive tumors generally exhibit
improved outcomes with endocrine therapy, while erb-b2 receptor
tyrosine kinase 2 (ERBB2)-positive tumors typically require
ERBB2-targeted antibodies or small-molecule inhibitors in
combination with chemotherapy. By contrast, patients with
triple-negative tumors tend to display greater sensitivity to
chemotherapy (16).
The prognosis for BC varies among the different
subtypes, which may be the most significant factor. Luminal A is
the molecular subtype with the highest proportion in BC, exhibiting
the lowest malignancy and the highest 5-year survival rate. The
prognosis of patients with luminal B is slightly worse than that of
patients with luminal A according to statistics (17). Compared to other subtypes,
patients who are HER2-positive and those with TNBC often exhibit
greater invasiveness, higher potential for recurrence and
metastasis, and a poorer prognosis. It poses a significant
challenge in the treatment of BC (18-20). In addition, other factors such as
advanced age at diagnosis, later stage of cancer progression,
metastasis, genetic predisposition and even high parity may also
contribute to a worse prognosis (17).
A large portion of BC cases can be attributed to
reproductive and hormonal factors (early menarche, late menopause,
later primiparity age) (21), as
well as lifestyle factors (e.g. overweight, lack of exercise,
alcohol intake and smoking) (22). It has been proved that long-term
contraceptives or menopausal hormone therapy with a combination of
estrogen and progesterone raise the risk of BC as well (23). Familial inheritance is another
universally acknowledged formidable hazard factor for BC. Women
with a family history have a 2-to-4-fold increased probability of
suffering from BC compared to others, with younger diagnosis ages
and even higher mortality rates (24,25). Germline mutations in genes such as
ATM serine/threonine kinase, BRCA1 DNA repair associated (BRCA1),
BRCA2, checkpoint kinase 2 and partner and localizer of BRCA2 are
frequently associated with an increased risk of developing BC
(26,27). However, such mutations are rare in
the general population.
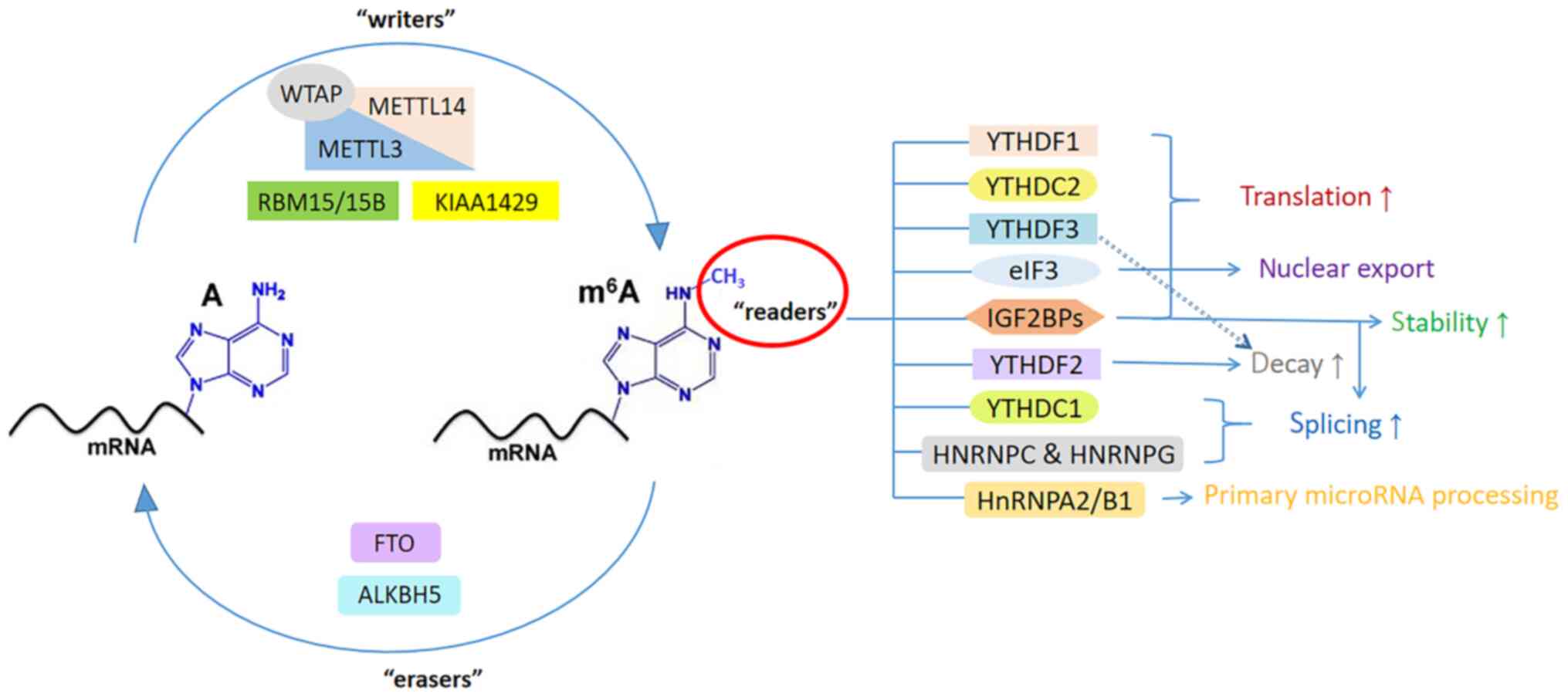
In the HNRNP family, HNRNPA2/B1 contains two
RNA-specific recognition motifs and governs the directional sorting
of miRNAs, thereby promoting primary miRNA processing (55). HNRNPC and HNRNPG can modulate mRNA
abundance and splicing (43).
IGF2BPs have been proven to be a unique and conservative family of
m6A readers, which can enhance translation efficiency in
an m6A-dependent way by regulating alternative splicing
and improving stability (56). In
addition, eIF3 promotes cap (m7GPPPN)-independent and
YTHDF1-dependent mRNA translation (57). The functions of m6A
readers are summarized in Table
III. The functions of m6A regulators are illustrated
in Fig. 1.
Studies have indicated that intricate signal
transduction processes at genetic, transcriptomic and epigenetic
levels influence the occurrence and progression of cancer,
including BC, which is often characterized by genetic and
epigenetic alterations (58).
However, in certain cases, contrary results have
been reported for similar tumors, implying that METTL3 may at times
function as a tumor suppressor (65). For instance, certain researchers
have detected that METTL3 methylation of basic leucine zipper
ATF-like transcription factor (BATF) mRNA inhibits its expression
in gastric cancer (GC), and low expression of BATF mRNA is
significantly associated with postoperative recurrence of GC
(66). In addition, there have
been reports indicating that the knockdown of METTL3 significantly
hastened tumor progression and reduced the lifespan of animals
implanted with glioblastoma stem cells (67). Other studies have demonstrated
that METTL3 expression is decreased in certain cases of renal cell
carcinoma and bladder cancer (68,69). Shi et al (70) found that a low level of METTL3 in
TNBC is indicative of a poor prognosis, suggesting that the reduced
presence of m6A markers contributes to the progression
of TNBC.
To date, certain studies on the mechanism underlying
the role of METTL3 in BC have been published. The present study
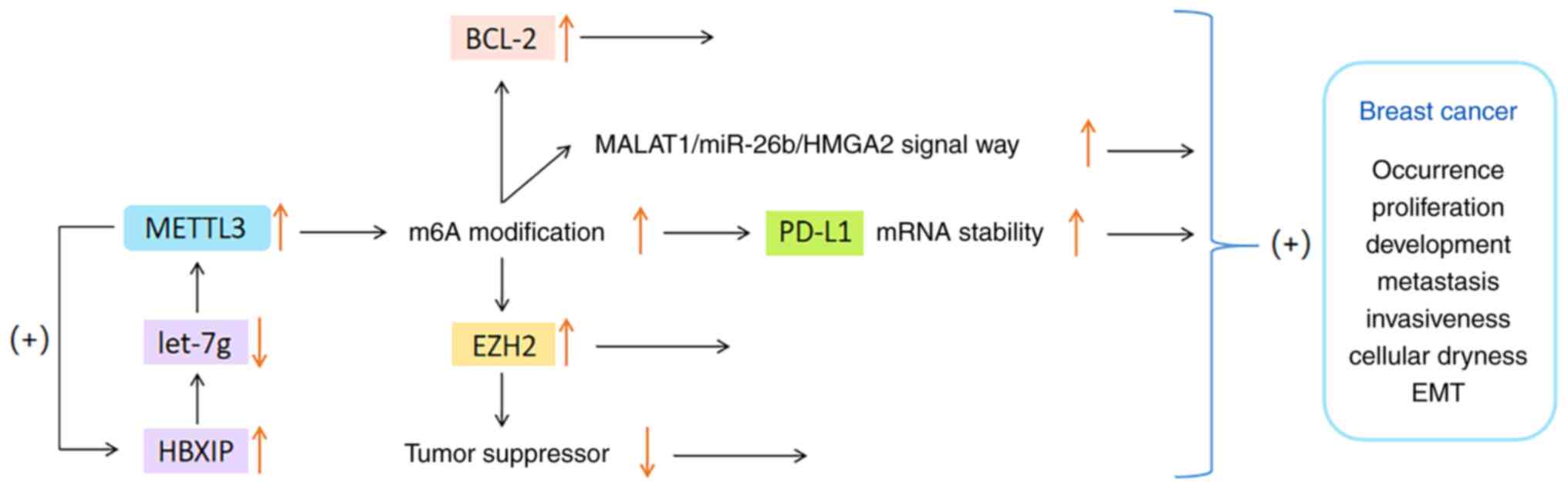
only provides a summary of recent findings. Wan et al
(71) discovered that METTL3
enhances the m6A modification of programmed cell death
ligand 1 (PD-L1) mRNA in BC cells, thereby improving the stability
and expression of PD-L1 mRNA. Knocking down METTL3 can boost
anti-tumor immunity and reduce PD-L1 expression, thus alleviating
the progression of BC. Cai et al (72) have shown that the expression
levels of METTL3 and hepatitis B x-interacting protein (HBXIP) are
very high in BC tissues. HBXIP increases the expression of METTL3
through restraining the expression of tumor suppressor let-7g, and
METTL3 in turn upregulates HBXIP via m6A modification,
thus forming a positive feedback regulatory loop of
HBXIP/let-7g/METTL3/HBXIP, and ultimately causing the malignant
growth of BC cells (72).
It has also been observed that the METTL3 level in
BC is significantly higher than that in surrounding normal tissues,
particularly in patients with T3-T4 BC or lymph node metastasis
(73). Studies revealed that
METTL3 overexpression can upregulate enhancer of zeste homolog 2
through m6A modification. This process results in the
suppression of tumor suppressor genes and promotion of
epithelial-mesenchymal transformation (EMT), which triggers the
occurrence, migration and invasion of BC cells (74,75). In addition, another study
indicated that METTL3 can accelerate the proliferation of BC by
regulating the methylation of BCL-2 or the metastasis associated
lung adenocarcinoma transcript 1 (MALAT1)/miR-26b/high mobility
group AT-hook 2 axis (76).
To sum up, METTL3 has been observed to be
overexpressed in most BC samples, and its expression level appears
to be positively correlated with the malignancy and metastasis of
BC. The specific mechanism of the connection between METTL3 and
BC-cell proliferation may involve multiple signaling pathways, but
the exact mechanism requires to be further studied and clarified.
The functions of METTL3 in BC are shown in Fig. 2.
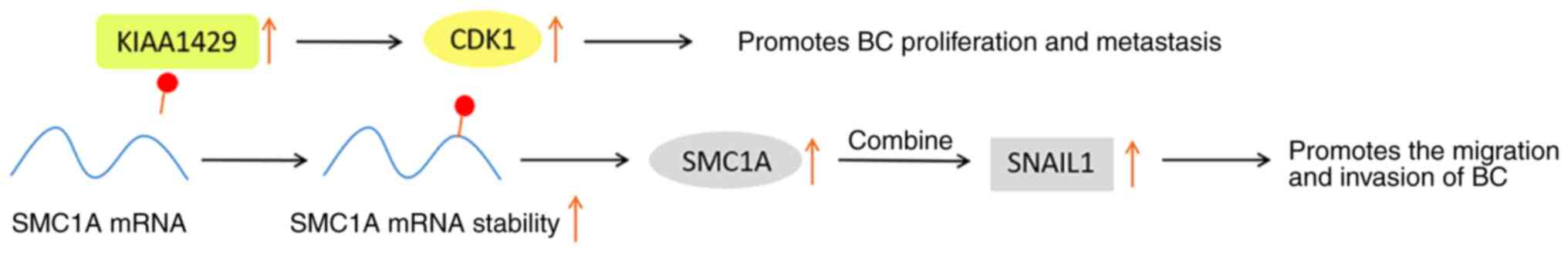
KIAA1429 acts as a scaffold for bridging the core
protein of methyltransferase and it is also involved in the
positive regulation of diverse tumorigenesis. Certain studies have
indicated that KIAA1429 promotes the proliferation and growth of BC
in a way independent of m6A, and the overall survival
period of patients with BC is positively associated with KIAA1429
(77,78).
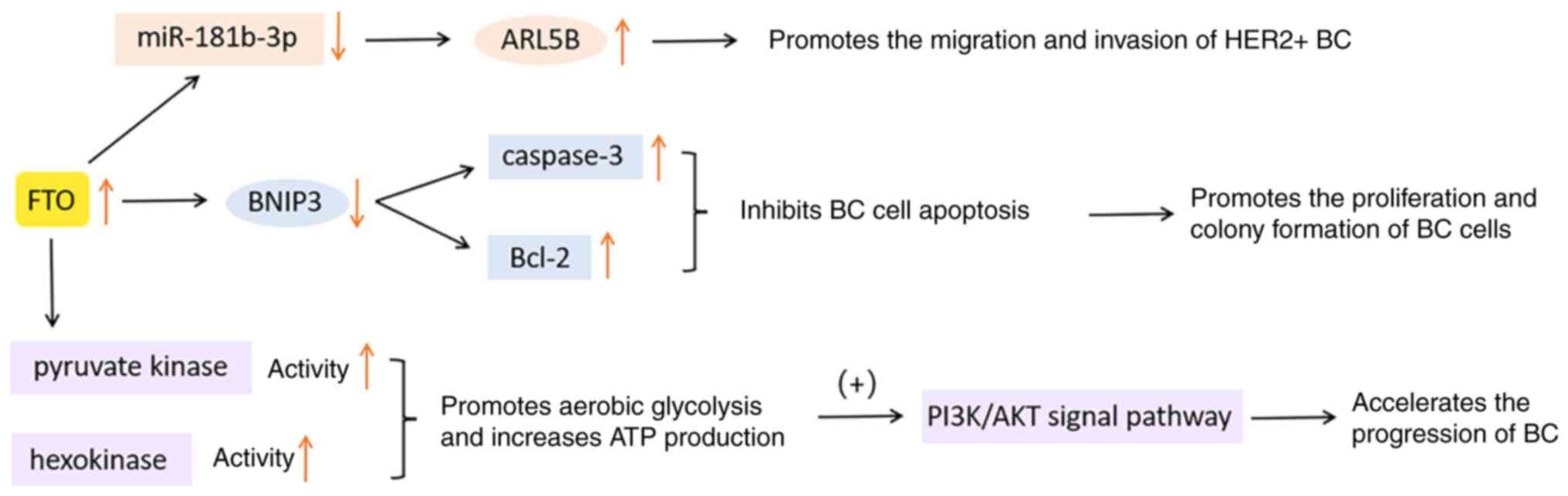
It is known that FTO, as an obesity-related protein,
can catalyze the demethylation of m6A. Numerous studies
have indicated that FTO is significantly upregulated in various
cancerous tissues, including but not limited to cervical squamous
cell carcinoma (79), lung
squamous cell carcinoma (80),
gastric cancer (81) and
pancreatic cancer (82). FTO is
involved in the regulation of tumor progression by decreasing the
abundance of m6A and activating specific signaling
pathways, reducing the overall survival rate of patients afflicted
with malignant tumors (83). In a
significant proportion of BC specimens, an elevated expression of
FTO was observed compared to the adjacent normal breast tissue.
Furthermore, it has been strongly associated with tumor
proliferation, invasion and metastasis (83-85).
In conclusion, deregulation of FTO is a tumorigenic
factor that cannot be ignored. The FTO-m6A axis can be
considered a potential new target for the treatment and diagnosis
of BC. The functions of FTO in BC are presented in Fig. 4.
A growing body of evidence indicates that ALKBH5 is
commonly dysregulated in malignant tumors, which regulates the
expression of multiple oncogenes and contributes to tumor immune
evasion through post-transcriptional mechanisms (89). However, studies indicated that
ALKBH5 has a dual role in cancer, as its expression is not
consistently upregulated or downregulated across all cancer types.
Certain studies have shown a positive association between ALKBH5
levels and BC (90-92).
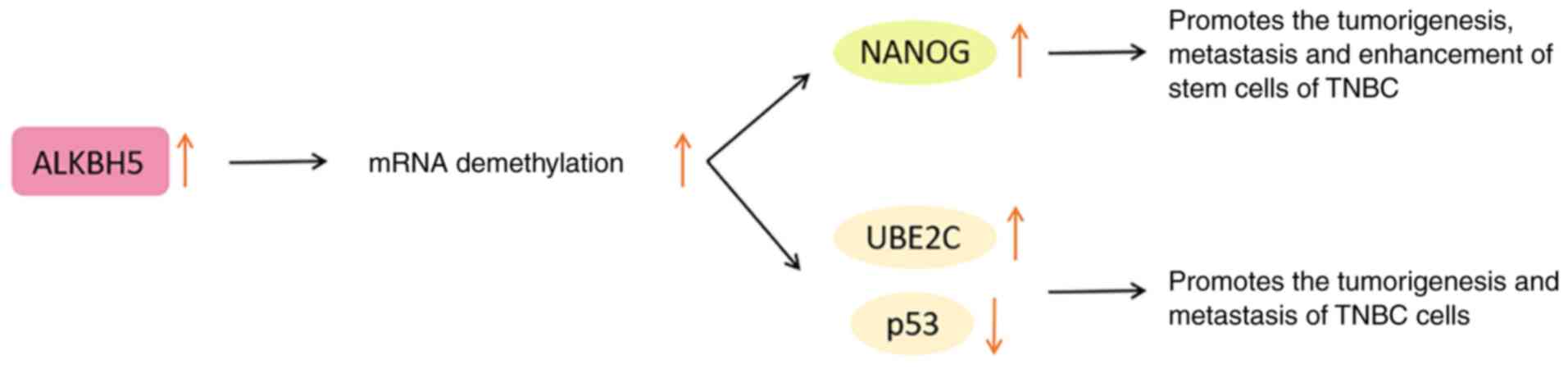
Under anoxic conditions, ALKBH5 mediates the
pluripotency factor Nanog homeobox (NANOG) to regulate the BC stem
cell characteristic specification in a hypoxia-inducible
factor-dependent manner. In other words, ALKBH5 enhances the
demethylation of NANOG mRNA and upregulates NANOG, while knocking
down ALKBH5 inhibits this pluripotency factor (93,94). Therefore, ALKBH5 disorder is
considered to be an important link in the proliferation, metastasis
and enhancement of the stem cell phenotype of BC.
In addition, ALKBH5 upregulates the expression of
ubiquitin conjugating enzyme E2 C (UBE2C) and reduces that of p53
by modifying the m6A of the downstream target gene UBE2C
(91). Among them, UBE2C has been
proven to exert a carcinogenic effect (95). The upregulated p53 is conducive to
decreasing cancer cells and preventing the occurrence of cancer
(96). Therefore, the
ALKBH5/UBE2C/p53 axis is regarded as a potential mechanism for
promoting the tumorigenesis and metastasis of TNBC cells (91).
In general, before ALKBH5 can be utilized as a
therapeutic target for BC, its expression and specific regulatory
mechanism should be further clarified. The functions of ALKBH5 in
BC are presented in Fig. 5.
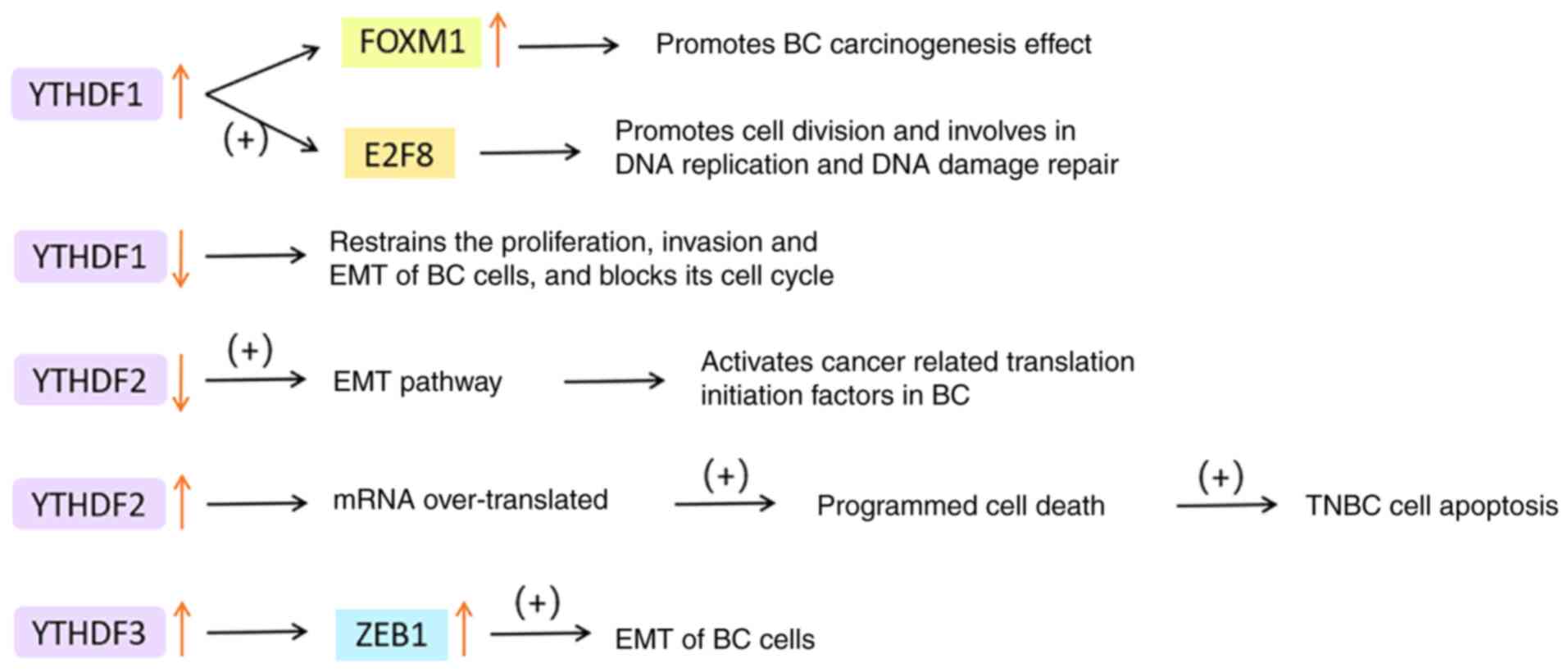
YTHDF3 may boost translation by interacting with
ribosomal protein and significantly raise the translation
efficiency of YTHDF1/3 common target (106). YTHDF3 can enhance the stability
of its target factor zinc finger E-box binding homeobox 1 (ZEB1)
mRNA, which is an EMT transcription factor (107). Chang et al (108)'s study on brain metastasis of BC
indicated that YTHDF3 regulates its own mRNA translation by binding
to m6A residues in its 5'UTR. YTHDF3 also combines with
m6A-modified mRNA to promote the expression of brain
metastasis genes, such as ST6 N-acetylgalactosaminide
α-2,6-sialyltransferase 5, gap junction protein α1 and EGFR. It is
noteworthy that in comparison to primary BC, YTHDF3 expression was
significantly increased in its brain metastases, but not in other
organs such as lung, bone, liver, spleen, lymph nodes and adrenal
glands.
In conclusion, YTHDF disorder is a prevalent
occurrence in cancer tissues. YTHDF1 and YTHDF3 are responsible for
improving the translation efficiency of m6A-modified
mRNA, and they are frequently amplified in BC cells. Their high
levels are closely related to poor prognosis and low survival
rates. Conversely, YTHDF2 promotes mRNA degradation and also acts
as a carcinogen most of the time. It is plausible that the YTH
family proteins work collaboratively to execute their regulatory
role in translation, but their respective roles in cancer cannot be
replaced, providing potential targets for BC treatment. The
functions of YTHDFs in BC are displayed in Fig. 6.
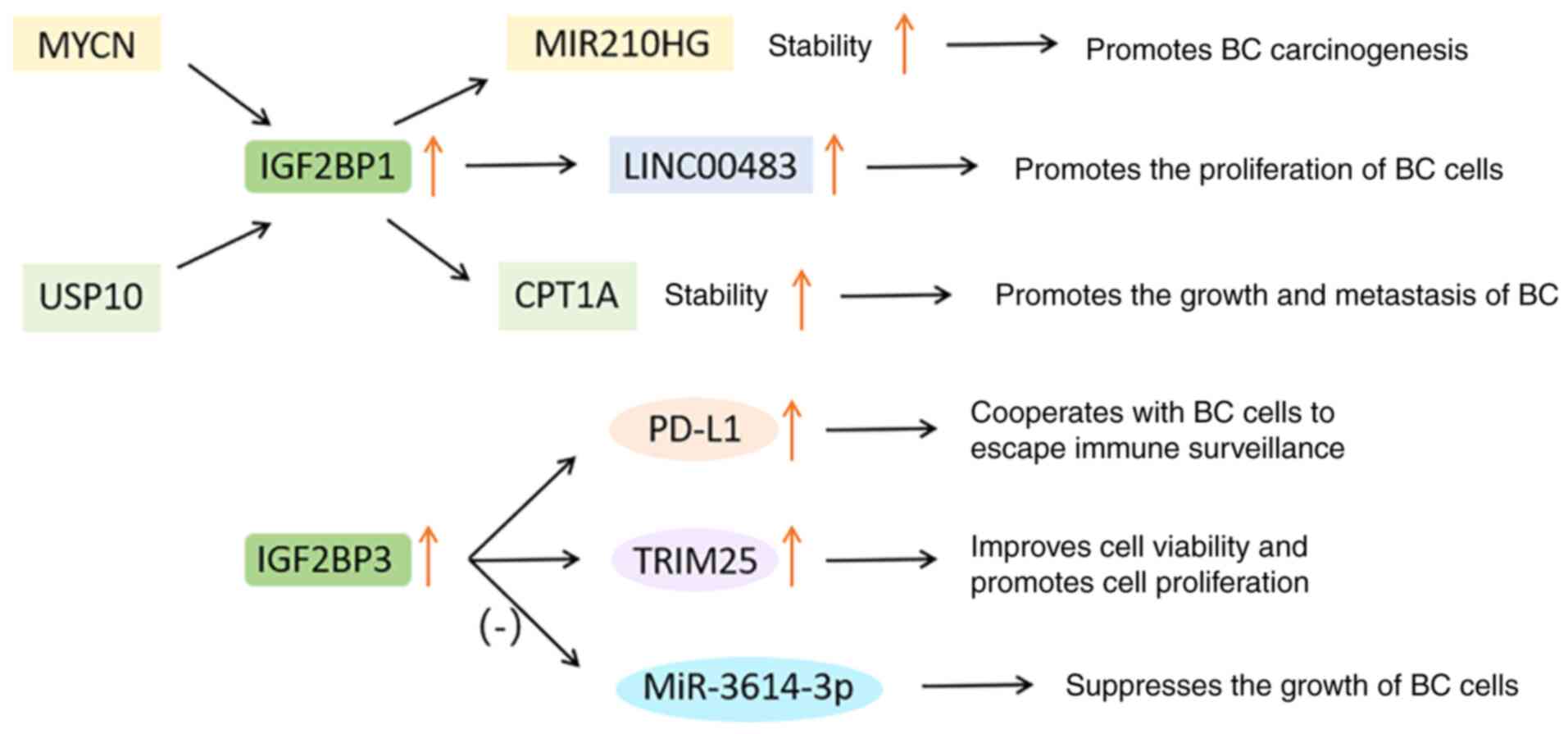
In BC, the level of PD-L1 increases with the
increase of IGF2BP3. Knocking down IGF2BP3 significantly inhibited
the expression of PD-L1, which cooperates with tumor cells to
escape immune surveillance (70).
In addition, the IGF2BP3/tripartite motif containing 25
(TRIM25)/miR-3614 axis represents a new way to regulate tumor cell
proliferation. TRIM25 is mainly expressed in estrogen target
tissues, which can improve cell viability and promote cell
proliferation. MiR-3614-3p can be used as a tumor suppressor to
inhibit the growth of BC cells. IGF2BP3 can induce the expression
of TRIM25 and inhibit the maturation of miR-3614, which conversely
protects TRIM25 mRNA from miR-3614-mediated degradation (116).
In short, the IGF2BP gene and its downstream
targets are generally amplified in BC, thereby resulting in
enhanced proliferation, metastasis and poor prognosis. These
results provide a foundation for evaluating IGF2BP as a potential
target for BC treatment, while the specific mechanism of IGF2BP
should be further studied. The functions of IGF2BPs in BC are shown
in Fig. 7.
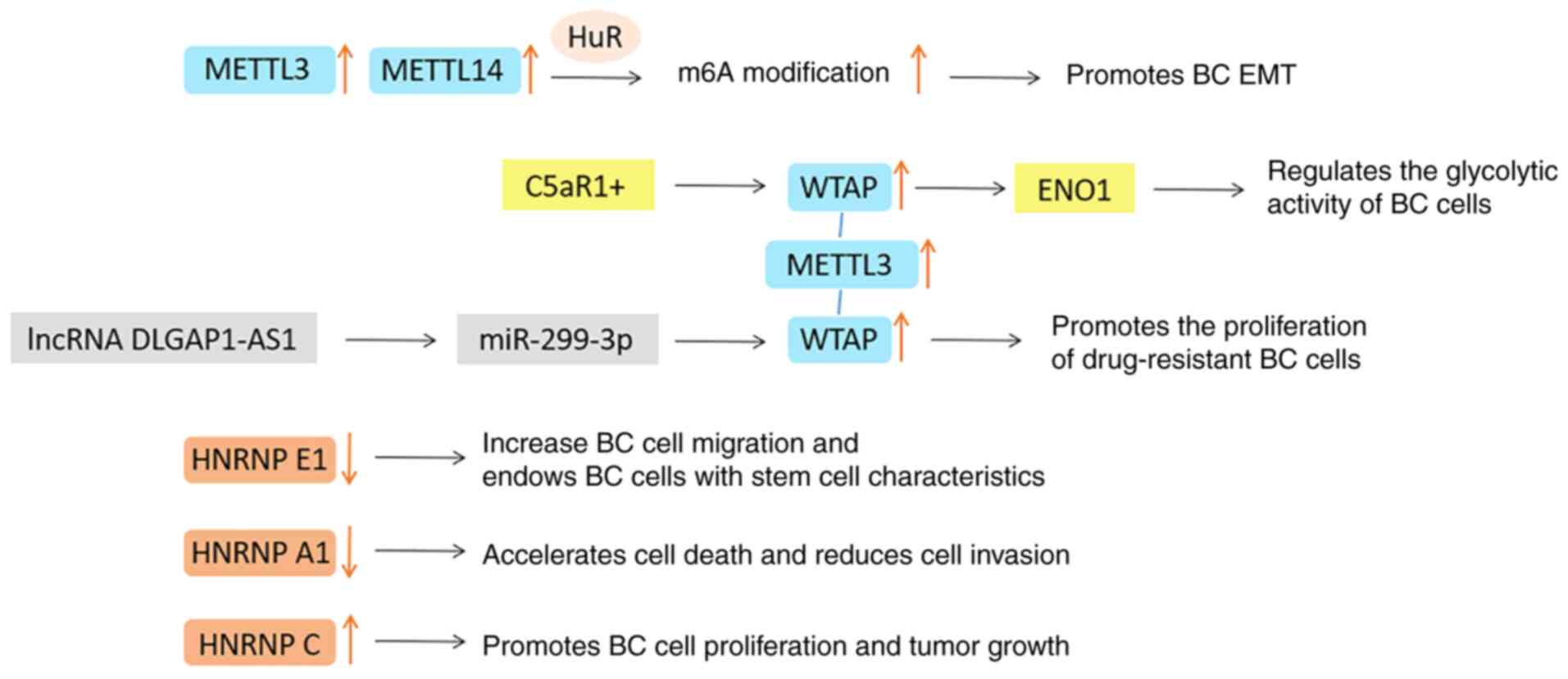
The dysfunction of METTL14, WTAP, RBM15/15B and
ZC3H13 in methyltransferase are also commonly recorded in cancer
databases. METTL14 has been reported as an oncogene in most studies
and its expression is usually positively correlated with the
expression of METTL3 and WTAP. It can improve the stability of
target mRNA through HuR (RNA-binding protein) mediation, involving
in the regulation of cell cycle, EMT and other tumor growth
processes (90). The expression
level of WTAP in BC is higher than that in normal breast tissue,
and it is positively correlated with tumor size and grade (117). Certain scholars have reported
that the complement C5a receptor 1 (C5AR1)+/WTAP/enolase 1 (ENO1)
axis regulates the glycolytic activity of BC cells and the lncRNA
DLG-associated protein 1-antisense 1/miR-299-3p/WTAP axis promotes
the proliferation of drug-resistant BC cells, which is worthy of
further exploration (118,119). However, the function of WTAP in
tumors cannot be separated from the expression of METTL3 (120).
Genomic instability is a hallmark of cancer and
refers to the increased rate at which cells acquire genomic
alterations (125). Certain
regulatory factor-mediated m6A modifications have been
linked to genomic instability, specifically in terms of regulating
the effect of m6A modification on DNA damage and repair
processes (126). While this
relationship has been established and verified in numerous studies
pertaining to tumors, investigations into its role in BC remain
scarce.
METTL3 can be specifically recruited to gene
fragments damaged by ultraviolet radiation and rapidly methylated
RNA; subsequently, m6A-modified RNA starts the DDR
pathway to improve the cell survival rate (126,127). METTL3-mediated m6A
methylation also regulates homologous recombination (HR)-mediated
double-stranded DNA break (DSB) repair (128). Phosphorylated METTL3 can be
localized in the DSB region so that the damaged chromatin region of
the RNA is modified by m6A. The m6A-modified
RNA is then recognized by YTHDC1 and forms a DNA-RNA hybrid with
DSBs, which recruits repair-related proteins and promotes
HR-mediated repair (129). It
has been reported that a low level of METTL3 increases the
sensitivity of cancer cells to the treatment of DNA damage, while
upregulated METTL3 reduces the survival rate of patients with head
and neck squamous cell carcinoma who have received cisplatin or
radiation treatment of DNA damage (126). Knocking down METTL3-mediated and
YTHDC2-mediated m6A modification led to the accumulation
of DNA-RNA hybridization (R loop) and γH2AX (a DSB marker), which
plays a key role in inhibiting cell growth and regulating genome
stability (130).
The pathogenesis of BC primarily entails the
hyperactivation and overexpression of oncogenes, coupled with
deficiencies in DDR gene defects, DDR gene transcription defects
and mitotic defects, among others. The defective repair of damaged
DNA leads to genomic instability, which is closely related to the
malignant progress and poor prognosis of BC.
It has been found that tumor genome subtypes of BC
are related to tumor gene expression, which involves the
methylation gain and loss processes of a large number of loci
(137). The researchers
suggested that extensive aberrations in methylation induce
epigenomic instability, rendering tumors more prone to regulatory
mutation and deterioration. They even linked different methylation
scores with higher epigenetic instability and higher chromosomal
instability in BC, predicting the disease stage and progress
(137).
Based on the above principles and experimental
evidence, it may be reasoned that METTL3-mediated modification of
m6DSB repair may serve as a promising target for cancer
treatment, including BC. Whether targeted inhibition of METTL3 can
reduce the proliferation activity and invasiveness of BC cells by
inhibiting DNA repair or improve the sensitivity of BC cells to DNA
damage therapies (such as chemotherapy or radiotherapy) is also
likely to become a new topic. It may also be true for other
m6A methylases and demethylases.
One of the main reasons for reduced efficacy of
non-surgical treatment for tumors is drug resistance of tumor
cells. Intrinsic resistance is mainly related to gene mutations,
while acquired resistance refers to a weakened response to drugs
after treatment, which may be related to secondary mutations in
drug targets (138). In recent
years, research on the role of m6A regulators in drug
resistance in cancer treatment has made significant progress, which
has also been confirmed in the treatment of BC (139).
Tamoxifen chemotherapy, as a first-line endocrine
therapy option for BC, is facing a major problem of drug
resistance. Research has proved that long-term exposure to
tamoxifen can induce an increase in METTL3 expression, further
resulting in an increase in m6A of the 5'UTR of
adenylate kinase 4 (AK4; a mitochondrial nucleotide kinase) mRNA.
High levels of AK4 inhibit mitochondrial apoptosis and promote ROS
production, activating p38, ultimately leading to increased
resistance of MCF-7 cells to tamoxifen (140). High-expression HNRNPA2B1 in
endocrine-resistant MCF-7 and LCC9 BC cell lines endows cancer
cells with acquired endocrine resistance by activating the Ser/Thr
kinase growth factor signaling pathway that regulates its
downstream target (141). The
increased expression of activating transcription factor 3 (ATF3)
protein caused by low levels of YTHDF2 is also the reason for the
development of tamoxifen-resistant MCF-7 cells (142). Therefore, selective inhibition
of AK4, HNRNPA2B1 and ATF3 may serve as a potential strategy for
preventing BC cells from acquiring endocrine therapy
resistance.
Radiation resistance refers to the adaptability of
tumor cells or tissues to radiation therapy. DDR is one of the main
reasons for tumor cells to develop radiation resistance (150). The transmembrane glycoprotein
neuropilin 1 (NRP1) can enhance the stem cell characteristics of BC
cells, making them resistant to radiation therapy. NRP1 has been
shown to reduce cell apoptosis and enhance radiation resistance by
downregulating Bcl-2 through m6A methyltransferase WTAP
(151).
In addition, a variety of serious problems should
be considered when screening potential targeted drugs. For
instance, the specific ways that m6A-related drugs
affect the methylation level, whether these drugs have
cytotoxicity, whether they are generally applicable to different
subtypes of BC and how to deal with BC resistance should be
determined. In addition, it is worth noting that different subtypes
of BC exhibit varying degrees of different sensitivity to
radiotherapy, chemotherapy, immunotherapy and various drugs, and
they are regulated by m6A modification, which greatly
affects the treatment effect.
Finally, in-depth exploration of cancer epigenomics
and the advancement of high-quality nucleic acid probes facilitate
the precise identification of biomarkers, which is essential for
predicting potential therapeutic targets, individualized treatment
and improvement of prognosis. By resolving these challenges, the
prospect of m6A targeted therapy for BC will expand
significantly.
Not applicable.
YBY was a major contributor in writing the
manuscript. FG and LQR contributed to the information retrieval and
selection. NR and JNP contributed to editing of the figures. QPX
proposed the writing ideas for this article and conducted a final
review. All authors read and approved the final manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This study was funded by the Key Medical Discipline of Hangzhou
City (grant no. 2021-21); Key Medical Discipline of Zhejiang
Province (grant no. 2018-2-3); Key Laboratory of Clinical Cancer
Pharmacology and Toxicology Research of Zhejiang Province (grant
no. 2020E10021); Medical and Health Science and Technology Program
of Zhejiang Province (grant no. 2023KY933); and the Traditional
Chinese Medicine Science and Technology Project of Zhejiang
Province (grant no. 2023ZL565).
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patel S: Breast cancer: Lesser-known
facets and hypotheses. Biomed Pharmacother. 98:499–506. 2018.
View Article : Google Scholar
|
|
3
|
Zhu Z, Albadawy E, Saha A, Zhang J,
Harowicz MR and Mazurowski MA: Deep learning for identifying
radiogenomic associations in breast cancer. Comput Biol Med.
109:85–90. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lord SJ, Bahlmann K, O'Connell DL, Kiely
BE, Daniels B, Pearson SA, Beith J, Bulsara MK and Houssami N: De
novo and recurrent metastatic breast cancer-A systematic review of
population-level changes in survival since 1995. EClinicalMedicine.
44:1012822022. View Article : Google Scholar
|
|
5
|
Dai D, Wang H, Zhu L, Jin H and Wang X:
N6-methyladenosine links RNA metabolism to cancer progression. Cell
Death Dis. 9:1242018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hyun K, Jeon J, Park K and Kim J: Writing,
erasing and reading histone lysine methylations. Exp Mol Med.
49:e3242017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
An Y and Duan H: The role of m6A RNA
methylation in cancer metabolism. Mol Cancer. 21:142022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin H, Wang Y, Wang P, Long F and Wang T:
Mutual regulation between N6-methyladenosine (m6A) modification and
circular RNAs in cancer: Impacts on therapeutic resistance. Mol
Cancer. 21:1482022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Z, Zou H, Dang Q, Xu H, Liu L, Zhang
Y, Lv J, Li H, Zhou Z and Han X: Biological and pharmacological
roles of m6A modifications in cancer drug resistance.
Mol Cancer. 21:2202022. View Article : Google Scholar
|
|
10
|
Deng LJ, Deng WQ, Fan SR, Chen MF, Qi M,
Lyu WY, Qi Q, Tiwari AK, Chen JX, Zhang DM and Chen ZS: m6A
modification: Recent advances, anticancer targeted drug discovery
and beyond. Mol Cancer. 21:522022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
DeSantis CE, Ma J, Gaudet MM, Newman LA,
Miller KD, Goding Sauer A, Jemal A and Siegel RL: Breast cancer
statistics, 2019. CA Cancer J Clin. 69:438–451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Loibl S, Poortmans P, Morrow M, Denkert C
and Curigliano G: Breast cancer. Lancet. 397:1750–1769. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hong R and Xu B: Breast cancer: an
up-to-date review and future perspectives. Cancer Commun (Lond).
42:913–936. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Golshan M, Loibl S, Wong SM, Houber JB,
O'Shaughnessy J, Rugo HS, Wolmark N, McKee MD, Maag D, Sullivan DM,
et al: Breast conservation after neoadjuvant chemotherapy for
triple-negative breast cancer: Surgical results from the brightness
randomized clinical trial. JAMA Surg. 155:e1954102020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Waks AG and Winer EP: Breast cancer
treatment: A review. JAMA. 321:288–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jääskeläinen A, Roininen N, Karihtala P
and Jukkola A: High parity predicts poor outcomes in patients with
luminal B-like (HER2 negative) early breast cancer: A prospective
finnish single-center study. Front Oncol. 10:14702020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choong GM, Cullen GD and O'Sullivan CC:
Evolving standards of care and new challenges in the management of
HER2-positive breast cancer. CA Cancer J Clin. 70:355–374. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu KD, Ye FG, He M, Fan L, Ma D, Mo M, Wu
J, Liu GY, Di GH, Zeng XH, et al: Effect of adjuvant paclitaxel and
carboplatin on survival in women with triple-negative breast
cancer: A phase 3 randomized clinical trial. JAMA Oncol.
6:1390–1396. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garrido-Castro AC, Lin NU and Polyak K:
Insights into molecular classifications of triple-negative breast
cancer: Improving patient selection for treatment. Cancer Discov.
9:176–198. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gaudet MM, Gierach GL, Carter BD, Luo J,
Milne RL, Weiderpass E, Giles GG, Tamimi RM, Eliassen AH, Rosner B,
et al: Pooled analysis of nine cohorts reveals breast cancer risk
factors by tumor molecular subtype. Cancer Res. 78:6011–6021. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nur U, El Reda D, Hashim D and Weiderpass
E: A prospective investigation of oral contraceptive use and breast
cancer mortality: Findings from the Swedish women's lifestyle and
health cohort. BMC Cancer. 19:8072019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Trabert B, Sherman ME, Kannan N and
Stanczyk FZ: Progesterone and breast cancer. Endocr Rev.
41:320–344. 2020. View Article : Google Scholar :
|
|
24
|
Reiner AS, Sisti J, John EM, Lynch CF,
Brooks JD, Mellemkjær L, Boice JD, Knight JA, Concannon P, Capanu
M, et al: Breast cancer family history and contralateral breast
cancer risk in young women: an update from the women's
environmental cancer and radiation epidemiology study. J Clin
Oncol. 36:1513–1520. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ho PJ, Ho WK, Khng AJ, Yeoh YS, Tan BK,
Tan EY, Lim GH, Tan SM, Tan VKM, Yip CH, et al: Overlap of
high-risk individuals predicted by family history, and genetic and
non-genetic breast cancer risk prediction models: Implications for
risk stratification. BMC Med. 20:1502022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu HM, Li S, Black MH, Lee S, Hoiness R,
Wu S, Mu W, Huether R, Chen J, Sridhar S, et al: Association of
breast and ovarian cancers with predisposition genes identified by
large-scale sequencing. JAMA Oncol. 5:51–57. 2019. View Article : Google Scholar :
|
|
27
|
Breast Cancer Association Consortium;
Dorling L, Carvalho S, Allen J, González-Neira A, Luccarini C,
Wahlström C, Pooley KA, Parsons MT, Fortuno C, et al: Breast cancer
risk genes-association analysis in more than 113,000 women. N Engl
J Med. 384:428–439. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ru W, Zhang X, Yue B, Qi A, Shen X, Huang
Y, Lan X, Lei C and Chen H: Insight into m6A methylation
from occurrence to functions. Open Biol. 10:2000912020. View Article : Google Scholar
|
|
29
|
Li Z, Peng Y, Li J, Chen Z, Chen F, Tu J,
Lin S and Wang H: N6-methyladenosine regulates
glycolysis of cancer cells through PDK4. Nat Commun. 11:25782020.
View Article : Google Scholar
|
|
30
|
Huang H, Weng H and Chen J: m6A
modification in coding and non-coding RNAs: Roles and therapeutic
implications in cancer. Cancer Cell. 37:270–288. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Choe J, Lin S, Zhang W, Liu Q, Wang L,
Ramirez-Moya J, Du P, Kim W, Tang S, Sliz P, et al: mRNA
circularization by METTL3-eIF3h enhances translation and promotes
oncogenesis. Nature. 561:556–560. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dominissini D, Moshitch-Moshkovitz S,
Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K,
Jacob-Hirsch J, Amariglio N, Kupiec M, et al: Topology of the human
and mouse m6A RNA methylomes revealed by m6A-seq. Nature.
485:201–206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi B, Liu WW, Yang K, Jiang GM and Wang
H: The role, mechanism, and application of RNA methyltransferase
METTL14 in gastrointestinal cancer. Mol Cancer. 21:1632022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zaccara S, Ries RJ and Jaffrey SR:
Reading, writing and erasing mRNA methylation. Nat Rev Mol Cell
Biol. 20:608–624. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen H, Wang Y, Su H, Zhang X, Chen H and
Yu J: RNA N6-methyladenine modification, cellular
reprogramming, and cancer stemness. Front Cell Dev Biol.
10:9352242022. View Article : Google Scholar
|
|
36
|
Wang X, Feng J, Xue Y, Guan Z, Zhang D,
Liu Z, Gong Z, Wang Q, Huang J, Tang C, et al: Structural basis of
N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature.
534:575–578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zeng C, Huang W, Li Y and Weng H: Roles of
METTL3 in cancer: mechanisms and therapeutic targeting. J Hematol
Oncol. 13:1172020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Y, Peng C, Chen J, Chen D, Yang B, He
B, Hu W, Zhang Y, Liu H, Dai L, et al: WTAP facilitates progression
of hepatocellular carcinoma via m6A-HuR-dependent epigenetic
silencing of ETS1. Mol Cancer. 18:1272019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Su R, Dong L, Li Y, Gao M, He PC, Liu W,
Wei J, Zhao Z, Gao L, Han L, et al: METTL16 exerts an
m6A-independent function to facilitate translation and
tumorigenesis. Nat Cell Biol. 24:205–216. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu Y, Ouyang Z, Sui X, Qi M, Li M, He Y,
Cao Y, Cao Q, Lu Q, Zhou S, et al: Oocyte competence is maintained
by m6A methyltransferase KIAA1429-mediated RNA
metabolism during mouse follicular development. Cell Death Differ.
27:2468–2483. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tan C, Xia P, Zhang H, Xu K, Liu P, Guo D
and Liu Z: YY1-Targeted RBM15B promotes hepatocellular carcinoma
cell proliferation and sorafenib resistance by promoting TRAM2
expression in an m6A-dependent manner. Front Oncol. 12:8730202022.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wen J, Lv R, Ma H, Shen H, He C, Wang J,
Jiao F, Liu H, Yang P, Tan L, et al: Zc3h13 regulates nuclear RNA
m6A methylation and mouse embryonic stem cell
self-renewal. Mol Cell. 69:1028–1038.e6. 2018. View Article : Google Scholar
|
|
43
|
Wang T, Kong S, Tao M and Ju S: The
potential role of RNA N6-methyladenosine in Cancer progression. Mol
Cancer. 19:882020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang L, Song C, Wang N, Li S, Liu Q, Sun
Z, Wang K, Yu SC and Yang Q: NADP modulates RNA m6A
methylation and adipogenesis via enhancing FTO activity. Nat Chem
Biol. 16:1394–1402. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bartosovic M, Molares HC, Gregorova P,
Hrossova D, Kudla G and Vanacova S: N6-methyladenosine demethylase
FTO targets pre-mRNAs and regulates alternative splicing and 3'-end
processing. Nucleic Acids Res. 45:11356–11370. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tang B, Yang Y, Kang M, Wang Y, Wang Y, Bi
Y, He S and Shimamoto F: m6A demethylase ALKBH5 inhibits
pancreatic cancer tumorigenesis by decreasing WIF-1 RNA methylation
and mediating Wnt signaling. Mol Cancer. 19:32020. View Article : Google Scholar
|
|
47
|
Jiang X, Liu B, Nie Z, Duan L, Xiong Q,
Jin Z, Yang C and Chen Y: The role of m6A modification in the
biological functions and diseases. Signal Transduct Target Ther.
6:742021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Du H, Zhao Y, He J, Zhang Y, Xi H, Liu M,
Ma J and Wu L: YTHDF2 destabilizes m(6)A-containing RNA through
direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun.
7:126262016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang X, Zhao BS, Roundtree IA, Lu Z, Han
D, Ma H, Weng X, Chen K, Shi H and He C: N(6)-methyladenosine
modulates messenger RNA translation efficiency. Cell.
161:1388–1399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen Z, Zhong X, Xia M and Zhong J: The
roles and mechanisms of the m6A reader protein YTHDF1 in tumor
biology and human diseases. Mol Ther Nucleic Acids. 26:1270–1279.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zaccara S and Jaffrey SR: A unified model
for the function of YTHDF proteins in regulating
m6A-modified mRNA. Cell. 181:1582–1595.e18. 2020.
View Article : Google Scholar
|
|
52
|
Xiao W, Adhikari S, Dahal U, Chen YS, Hao
YJ, Sun BF, Sun HY, Li A, Ping XL, Lai WY, et al: Nuclear m(6)A
reader YTHDC1 regulates mRNA splicing. Mol Cell. 61:507–519. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Roundtree IA, Luo GZ, Zhang Z, Wang X,
Zhou T, Cui Y, Sha J, Huang X, Guerrero L, Xie P, et al: YTHDC1
mediates nuclear export of N6-methyladenosine methylated
mRNAs. Elife. 6:e313112017. View Article : Google Scholar
|
|
54
|
Mao Y, Dong L, Liu XM, Guo J, Ma H, Shen B
and Qian SB: m6A in mRNA coding regions promotes
translation via the RNA helicase-containing YTHDC2. Nat Commun.
10:53322019. View Article : Google Scholar
|
|
55
|
Wu B, Su S, Patil DP, Liu H, Gan J,
Jaffrey SR and Ma J: Molecular basis for the specific and
multivariant recognitions of RNA substrates by human hnRNP A2/B1.
Nat Commun. 9:4202018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sun CY, Cao D, Du BB, Chen CW and Liu D:
The role of Insulin-like growth factor 2 mRNA-binding proteins
(IGF2BPs) as m6A readers in cancer. Int J Biol Sci.
18:2744–2758. 2022. View Article : Google Scholar :
|
|
57
|
Liu T, Wei Q, Jin J, Luo Q, Liu Y, Yang Y,
Cheng C, Li L, Pi J, Si Y, et al: The m6A reader YTHDF1 promotes
ovarian cancer progression via augmenting EIF3C translation.
Nucleic Acids Res. 48:3816–3831. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang Y, Zhang Y, Du Y, Zhou M, Hu Y and
Zhang S: Emerging roles of N6-methyladenosine (m6A)
modification in breast cancer. Cell Biosci. 10:1362020. View Article : Google Scholar
|
|
59
|
Li Y, Xiao J, Bai J, Tian Y, Qu Y, Chen X,
Wang Q, Li X, Zhang Y and Xu J: Molecular characterization and
clinical relevance of m6A regulators across 33 cancer
types. Mol Cancer. 18:1372019. View Article : Google Scholar
|
|
60
|
Wei M, Bai JW, Niu L, Zhang YQ, Chen HY
and Zhang GJ: The complex roles and therapeutic implications of
m6A modifications in breast cancer. Front Cell Dev Biol.
8:6150712021. View Article : Google Scholar
|
|
61
|
Han H, Yang C, Zhang S, Cheng M, Guo S,
Zhu Y, Ma J, Liang Y, Wang L, Zheng S, et al: METTL3-mediated
m6A mRNA modification promotes esophageal cancer
initiation and progression via Notch signaling pathway. Mol Ther
Nucleic Acids. 26:333–346. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chen M, Wei L, Law CT, Tsang FH, Shen J,
Cheng CL, Tsang LH, Ho DW, Chiu DK, Lee JM, et al: RNA
N6-methyladenosine methyltransferase-like 3 promotes liver cancer
progression through YTHDF2-dependent posttranscriptional silencing
of SOCS2. Hepatology. 67:2254–2270. 2018. View Article : Google Scholar
|
|
63
|
Jin H, Ying X, Que B, Wang X, Chao Y,
Zhang H, Yuan Z, Qi D, Lin S, Min W, et al:
N6-methyladenosine modification of ITGA6 mRNA promotes
the development and progression of bladder cancer. EBioMedicine.
47:195–207. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang J, Bai R, Li M, Ye H, Wu C, Wang C,
Li S, Tan L, Mai D, Li G, et al: Excessive miR-25-3p maturation via
N6-methyladenosine stimulated by cigarette smoke
promotes pancreatic cancer progression. Nat Commun. 10:18582019.
View Article : Google Scholar
|
|
65
|
Ma S, Chen C, Ji X, Liu J, Zhou Q, Wang G,
Yuan W, Kan Q and Sun Z: The interplay between m6A RNA methylation
and noncoding RNA in cancer. J Hematol Oncol. 12:1212019.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Xie JW, Huang XB, Chen QY, Ma YB, Zhao YJ,
Liu LC, Wang JB, Lin JX, Lu J, Cao LL, et al: m6A
modification-mediated BATF2 acts as a tumor suppressor in gastric
cancer through inhibition of ERK signaling. Mol Cancer. 19:1142020.
View Article : Google Scholar
|
|
67
|
Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, Sun
G, Lu Z, Huang Y, Yang CG, et al: m6A RNA methylation
regulates the self-renewal and tumorigenesis of glioblastoma stem
cells. Cell Rep. 18:2622–2634. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zheng W, Dong X, Zhao Y, Wang S, Jiang H,
Zhang M, Zheng X and Gu M: Multiple functions and mechanisms
underlying the role of METTL3 in human cancers. Front Oncol.
9:14032019. View Article : Google Scholar
|
|
69
|
Wang G, Dai Y, Li K, Cheng M, Xiong G,
Wang X, Chen S, Chen Z, Chen J, Xu X, et al: Deficiency of Mettl3
in bladder cancer stem cells inhibits bladder cancer progression
and angiogenesis. Front Cell Dev Biol. 9:6277062021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shi Y, Zheng C, Jin Y, Bao B, Wang D, Hou
K, Feng J, Tang S, Qu X, Liu Y, et al: Reduced expression of METTL3
promotes metastasis of triple-negative breast cancer by m6A
methylation-mediated COL3A1 up-regulation. Front Oncol.
10:11262020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wan W, Ao X, Chen Q, Yu Y, Ao L, Xing W,
Guo W, Wu X, Pu C, Hu X, et al: METTL3/IGF2BP3 axis inhibits tumor
immune surveillance by upregulating N6-methyladenosine
modification of PD-L1 mRNA in breast cancer. Mol Cancer. 21:602022.
View Article : Google Scholar
|
|
72
|
Cai X, Wang X, Cao C, Gao Y, Zhang S, Yang
Z, Liu Y, Zhang X, Zhang W and Ye L: HBXIP-elevated
methyltransferase METTL3 promotes the progression of breast cancer
via inhibiting tumor suppressor let-7g. Cancer Lett. 415:11–19.
2018. View Article : Google Scholar
|
|
73
|
Ma J, Zhang J, Weng YC and Wang JC:
EZH2-mediated microRNA-139-5p regulates epithelial-mesenchymal
transition and lymph node metastasis of pancreatic cancer. Mol
Cells. 41:868–880. 2018.PubMed/NCBI
|
|
74
|
Hu S, Song Y, Zhou Y, Jiao Y and Li G:
METTL3 accelerates breast cancer progression via regulating EZH2
m6A modification. J Healthc Eng. 2022:57944222022.
|
|
75
|
Li W, Xue D, Xue M, Zhao J, Liang H, Liu Y
and Sun T: Fucoidan inhibits epithelial-to-mesenchymal transition
via regulation of the HIF-1α pathway in mammary cancer cells under
hypoxia. Oncol Lett. 18:330–338. 2019.PubMed/NCBI
|
|
76
|
Zhao C, Ling X, Xia Y, Yan B and Guan Q:
The m6A methyltransferase METTL3 controls epithelial-mesenchymal
transition, migration and invasion of breast cancer through the
MALAT1/miR-26b/HMGA2 axis. Cancer Cell Int. 21:4412021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Qian JY, Gao J, Sun X, Cao MD, Shi L, Xia
TS, Zhou WB, Wang S, Ding Q and Wei JF: KIAA1429 acts as an
oncogenic factor in breast cancer by regulating CDK1 in an
N6-methyladenosine-independent manner. Oncogene. 38:6123–6141.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhang X, Dai XY, Qian JY, Xu F, Wang ZW,
Xia T, Zhou XJ, Li XX, Shi L, Wei JF and Ding Q: SMC1A regulated by
KIAA1429 in m6A-independent manner promotes EMT progress in breast
cancer. Mol Ther Nucleic Acids. 27:133–146. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhou S, Bai ZL, Xia D, Zhao ZJ, Zhao R,
Wang YY and Zhe H: FTO regulates the chemo-radiotherapy resistance
of cervical squamous cell carcinoma (CSCC) by targeting β-catenin
through mRNA demethylation. Mol Carcinog. 57:590–597. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liu J, Ren D, Du Z, Wang H, Zhang H and
Jin Y: m6A demethylase FTO facilitates tumor progression
in lung squamous cell carcinoma by regulating MZF1 expression.
Biochem Biophys Res Commun. 502:456–464. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Shimura T, Kandimalla R, Okugawa Y, Ohi M,
Toiyama Y, He C and Goel A: Novel evidence for m6A
methylation regulators as prognostic biomarkers and FTO as a
potential therapeutic target in gastric cancer. Br J Cancer.
126:228–237. 2022. View Article : Google Scholar
|
|
82
|
Azzam SK, Alsafar H and Sajini AA: FTO m6A
demethylase in obesity and cancer: implications and underlying
molecular mechanisms. Int J Mol Sci. 23:38002022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zheng QK, Ma C, Ullah I, Hu K, Ma RJ,
Zhang N and Sun ZG: Roles of N6-methyladenosine demethylase FTO in
malignant tumors progression. Onco Targets Ther. 14:4837–4846.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Niu Y, Lin Z, Wan A, Chen H, Liang H, Sun
L, Wang Y, Li X, Xiong XF, Wei B, et al: RNA N6-methyladenosine
demethylase FTO promotes breast tumor progression through
inhibiting BNIP3. Mol Cancer. 18:462019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Xu Y, Ye S, Zhang N, Zheng S, Liu H, Zhou
K, Wang L, Cao Y, Sun P and Wang T: The FTO/miR-181b-3p/ARL5B
signaling pathway regulates cell migration and invasion in breast
cancer. Cancer Commun (Lond). 40:484–500. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Basu A: The interplay between apoptosis
and cellular senescence: Bcl-2 family proteins as targets for
cancer therapy. Pharmacol Ther. 230:1079432022. View Article : Google Scholar
|
|
87
|
Gao X, Wang Y, Lu F, Chen X, Yang D, Cao
Y, Zhang W, Chen J, Zheng L, Wang G, et al: Extracellular vesicles
derived from oesophageal cancer containing P4HB promote muscle
wasting via regulating PHGDH/Bcl-2/caspase-3 pathway. J Extracell
Vesicles. 10:e120602021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Liu Y, Wang R, Zhang L, Li J, Lou K and
Shi B: The lipid metabolism gene FTO influences breast cancer cell
energy metabolism via the PI3K/AKT signaling pathway. Oncol Lett.
13:4685–4690. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Qu J, Yan H, Hou Y, Cao W, Liu Y, Zhang E,
He J and Cai Z: RNA demethylase ALKBH5 in cancer: From mechanisms
to therapeutic potential. J Hematol Oncol. 15:82022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wu L, Wu D, Ning J, Liu W and Zhang D:
Changes of N6-methyladenosine modulators promote breast cancer
progression. BMC Cancer. 19:3262019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Hu Y, Liu H, Xiao X, Yu Q, Deng R, Hua L,
Wang J and Wang X: Bone marrow mesenchymal stem cell-derived
exosomes inhibit triple-negative breast cancer cell stemness and
metastasis via an ALKBH5-dependent mechanism. Cancers (Basel).
14:60592022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Fry NJ, Law BA, Ilkayeva OR, Carraway KR
and Mansfield KD: N6-methyladenosine contributes to
cellular phenotype in a genetically-defined model of breast cancer
progression. Oncotarget. 9:31231–31243. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang C, Samanta D, Lu H, Bullen JW, Zhang
H, Chen I, He X and Semenza GL: Hypoxia induces the breast cancer
stem cell phenotype by HIF-dependent and ALKBH5-mediated
m6A-demethylation of NANOG mRNA. Proc Natl Acad Sci USA.
113:E2047–E2056. 2016.
|
|
94
|
Zhang C, Zhi WI, Lu H, Samanta D, Chen I,
Gabrielson E and Semenza GL: Hypoxia-inducible factors regulate
pluripotency factor expression by ZNF217- and ALKBH5-mediated
modulation of RNA methylation in breast cancer cells. Oncotarget.
7:64527–64542. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhang S, You X, Zheng Y, Shen Y, Xiong X
and Sun Y: The UBE2C/CDH1/DEPTOR axis is an oncogene and tumor
suppressor cascade in lung cancer cells. J Clin Invest.
133:e1624342023. View Article : Google Scholar :
|
|
96
|
Wang Y, Xie Y, Niu Y, Song P, Liu Y,
Burnett J, Yang Z, Sun D, Ran Y, Li Y and Sun L: Carboxypeptidase
A4 negatively correlates with p53 expression and regulates the
stemness of breast cancer cells. Int J Med Sci. 18:1753–1759. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chen H, Yu Y, Yang M, Huang H, Ma S, Hu J,
Xi Z, Guo H, Yao G, Yang L, et al: YTHDF1 promotes breast cancer
progression by facilitating FOXM1 translation in an m6A-dependent
manner. Cell Biosci. 12:192022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Sun Y, Dong D, Xia Y, Hao L, Wang W and
Zhao C: YTHDF1 promotes breast cancer cell growth, DNA damage
repair and chemoresistance. Cell Death Dis. 13:2302022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Anita R, Paramasivam A, Priyadharsini JV
and Chitra S: The m6A readers YTHDF1 and YTHDF3 aberrations
associated with metastasis and predict poor prognosis in breast
cancer patients. Am J Cancer Res. 10:2546–2554. 2020.PubMed/NCBI
|
|
100
|
Zhong L, Liao D, Zhang M, Zeng C, Li X,
Zhang R, Ma H and Kang T: YTHDF2 suppresses cell proliferation and
growth via destabilizing the EGFR mRNA in hepatocellular carcinoma.
Cancer Lett. 442:252–261. 2019. View Article : Google Scholar
|
|
101
|
Chen YG, Chen R, Ahmad S, Verma R, Kasturi
SP, Amaya L, Broughton JP, Kim J, Cadena C, Pulendran B, et al:
N6-methyladenosine modification controls circular RNA immunity. Mol
Cell. 76:96–109.e9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Paris J, Morgan M, Campos J, Spencer GJ,
Shmakova A, Ivanova I, Mapperley C, Lawson H, Wotherspoon DA,
Sepulveda C, et al: Targeting the RNA m6A reader YTHDF2
selectively compromises cancer stem cells in acute myeloid
leukemia. Cell Stem Cell. 25:137–148.e6. 2019. View Article : Google Scholar
|
|
103
|
Dixit D, Prager BC, Gimple RC, Poh HX,
Wang Y, Wu Q, Qiu Z, Kidwell RL, Kim LJY, Xie Q, et al: The RNA m6A
Reader YTHDF2 maintains oncogene expression and is a targetable
dependency in glioblastoma stem cells. Cancer Discov. 11:480–499.
2021. View Article : Google Scholar :
|
|
104
|
Li J, Xie H, Ying Y, Chen H, Yan H, He L,
Xu M, Xu X, Liang Z, Liu B, et al: YTHDF2 mediates the mRNA
degradation of the tumor suppressors to induce AKT phosphorylation
in N6-methyladenosine-dependent way in prostate cancer. Mol Cancer.
19:1522020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Einstein JM, Perelis M, Chaim IA, Meena
JK, Nussbacher JK, Tankka AT, Yee BA, Li H, Madrigal AA, Neill NJ,
et al: Inhibition of YTHDF2 triggers proteotoxic cell death in
MYC-driven breast cancer. Mol Cell. 81:3048–3064.e9. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Li A, Chen YS, Ping XL, Yang X, Xiao W,
Yang Y, Sun HY, Zhu Q, Baidya P, Wang X, et al: Cytoplasmic
m6A reader YTHDF3 promotes mRNA translation. Cell Res.
27:444–447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lin Y, Jin X, Nie Q, Chen M, Guo W, Chen
L, Li Y, Chen X, Zhang W, Chen H, et al: YTHDF3 facilitates
triple-negative breast cancer progression and metastasis by
stabilizing ZEB1 mRNA in an m6A-dependent manner. Ann
Transl Med. 10:832022. View Article : Google Scholar
|
|
108
|
Chang G, Shi L, Ye Y, Shi H, Zeng L,
Tiwary S, Huse JT, Huo L, Ma L, Ma Y, et al: YTHDF3 induces the
translation of m6A-enriched gene transcripts to promote
breast cancer brain metastasis. Cancer Cell. 38:857–871.e7. 2020.
View Article : Google Scholar
|
|
109
|
Huang H, Weng H, Sun W, Qin X, Shi H, Wu
H, Zhao BS, Mesquita A, Liu C, Yuan CL, et al: Recognition of RNA
N6-methyladenosine by IGF2BP proteins enhances mRNA
stability and translation. Nat Cell Biol. 20:285–295. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Müller S, Glaß M, Singh AK, Haase J, Bley
N, Fuchs T, Lederer M, Dahl A, Huang H, Chen J, et al: IGF2BP1
promotes SRF-dependent transcription in cancer in a m6A- and
miRNA-dependent manner. Nucleic Acids Res. 47:375–390. 2019.
View Article : Google Scholar :
|
|
111
|
Qiao YS, Zhou JH, Jin BH, Wu YQ and Zhao
B: LINC00483 is regulated by IGF2BP1 and participates in the
progression of breast cancer. Eur Rev Med Pharmacol Sci.
25:1379–1386. 2021.PubMed/NCBI
|
|
112
|
Shi W, Tang Y, Lu J, Zhuang Y and Wang J:
MIR210HG promotes breast cancer progression by IGF2BP1 mediated m6A
modification. Cell Biosci. 12:382022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Shi J, Zhang Q, Yin X, Ye J, Gao S, Chen
C, Yang Y, Wu B, Fu Y, Zhang H, et al: Stabilization of IGF2BP1 by
USP10 promotes breast cancer metastasis via CPT1A in an
m6A-dependent manner. Int J Biol Sci. 19:449–464. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zeng F, Yao M, Wang Y, Zheng W, Liu S, Hou
Z, Cheng X, Sun S, Li T, Zhao H, et al: Fatty acid β-oxidation
promotes breast cancer stemness and metastasis via the
miRNA-328-3p-CPT1A pathway. Cancer Gene Ther. 29:383–395. 2022.
View Article : Google Scholar
|
|
115
|
Xiong Y, Liu Z, Li Z, Wang S, Shen N, Xin
Y and Huang T: Long non-coding RNA nuclear paraspeckle assembly
transcript 1 interacts with microRNA-107 to modulate breast cancer
growth and metastasis by targeting carnitine
palmitoyltransferase-1. Int J Oncol. 55:1125–1136. 2019.PubMed/NCBI
|
|
116
|
Wang Z, Tong D, Han C, Zhao Z, Wang X,
Jiang T, Li Q, Liu S, Chen L, Chen Y, et al: Blockade of miR-3614
maturation by IGF2BP3 increases TRIM25 expression and promotes
breast cancer cell proliferation. EBioMedicine. 41:357–369. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wang CQ, Tang CH, Wang Y, Huang BF, Hu GN,
Wang Q and Shao JK: Upregulated WTAP expression appears to both
promote breast cancer growth and inhibit lymph node metastasis. Sci
Rep. 12:10232022. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Ou B, Liu Y, Yang X, Xu X, Yan Y and Zhang
J: C5aR1-positive neutrophils promote breast cancer glycolysis
through WTAP-dependent m6A methylation of ENO1. Cell Death Dis.
12:7372021. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Huang T, Cao L, Feng N, Xu B, Dong Y and
Wang M: N6-methyladenosine (m6A)-mediated
lncRNA DLGAP1-AS1enhances breast canceradriamycin resistance
through miR-299-3p/WTAP feedback loop. Bioengineered.
12:10935–10944. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Fan Y, Li X, Sun H, Gao Z, Zhu Z and Yuan
K: Role of WTAP in cancer: From mechanisms to the therapeutic
potential. Biomolecules. 12:12242022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Howley BV and Howe PH: TGF-beta signaling
in cancer: Post-transcriptional regulation of EMT via hnRNP E1.
Cytokine. 118:19–26. 2019. View Article : Google Scholar
|
|
122
|
Howley BV, Mohanty B, Dalton A, Grelet S,
Karam J, Dincman T and Howe PH: The ubiquitin E3 ligase ARIH1
regulates hnRNP E1 protein stability, EMT and breast cancer
progression. Oncogene. 41:1679–1690. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Loh TJ, Moon H, Cho S, Jang H, Liu YC, Tai
H, Jung DW, Williams DR, Kim HR, Shin MG, et al: CD44 alternative
splicing and hnRNP A1 expression are associated with the metastasis
of breast cancer. Oncol Rep. 34:1231–1238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Wu Y, Zhao W, Liu Y, Tan X, Li X, Zou Q,
Xiao Z, Xu H, Wang Y and Yang X: Function of HNRNPC in breast
cancer cells by controlling the dsRNA-induced interferon response.
EMBO J. 37:e990172018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Duijf PHG, Nanayakkara D, Nones K, Srihari
S, Kalimutho M and Khanna KK: Mechanisms of genomic instability in
breast cancer. Trends Mol Med. 25:595–611. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Hong J, Xu K and Lee JH: Biological roles
of the RNA m6A modification and its implications in
cancer. Exp Mol Med. 54:1822–1832. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Xiang Y, Laurent B, Hsu CH, Nachtergaele
S, Lu Z, Sheng W, Xu C, Chen H, Ouyang J, Wang S, et al: RNA
m6A methylation regulates the ultraviolet-induced DNA
damage response. Nature. 543:573–576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zhang C, Chen L, Peng D, Jiang A, He Y,
Zeng Y, Xie C, Zhou H, Luo X, Liu H, et al: METTL3 and
N6-methyladenosine promote homologous recombination-mediated repair
of DSBs by modulating DNA-RNA hybrid accumulation. Mol Cell.
79:425–442.e7. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
D'Alessandro G, Whelan DR, Howard SM,
Vitelli V, Renaudin X, Adamowicz M, Iannelli F, Jones-Weinert CW,
Lee M, Matti V, et al: BRCA2 controls DNA:RNA hybrid level at DSBs
by mediating RNase H2 recruitment. Nat Commun. 9:53762018.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Abakir A, Giles TC, Cristini A, Foster JM,
Dai N, Starczak M, Rubio-Roldan A, Li M, Eleftheriou M, Crutchley
J, et al: N6-methyladenosine regulates the stability of
RNA: DNA hybrids in human cells. Nat Genet. 52:48–55. 2020.
View Article : Google Scholar
|
|
131
|
Wei J, Yin Y, Zhou J, Chen H, Peng J, Yang
J and Tang Y: METTL3 potentiates resistance to cisplatin through
m6A modification of TFAP2C in seminoma. J Cell Mol Med.
24:11366–11380. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Yang Z, Yang S, Cui YH, Wei J, Shah P,
Park G, Cui X, He C and He YY: METTL14 facilitates global genome
repair and suppresses skin tumorigenesis. Proc Natl Acad Sci USA.
118:e20259481182021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Miranda-Gonçalves V, Lobo J,
Guimarães-Teixeira C, Barros-Silva D, Guimarães R, Cantante M,
Braga I, Maurício J, Oing C, Honecker F, et al: The component of
the m6A writer complex VIRMA is implicated in aggressive
tumor phenotype, DNA damage response and cisplatin resistance in
germ cell tumors. J Exp Clin Cancer Res. 40:2682021. View Article : Google Scholar
|
|
134
|
Qu F, Tsegay PS and Liu Y:
N6-methyladenosine, DNA repair, and genome stability.
Front Mol Biosci. 8:6458232021. View Article : Google Scholar
|
|
135
|
Ji HL, Hong J, Zhang Z, de la Peña Avalos
B, Proietti CJ, Deamicis AR, Guzmán GP, Lam HM, Garcia J, Roudier
MP, et al: Regulation of telomere homeostasis and genomic stability
in cancer by N6-adenosine methylation (m6A).
Sci Adv. 7:eabg70732021. View Article : Google Scholar
|
|
136
|
Maciejowski J and de Lange T: Telomeres in
cancer: Tumour suppression and genome instability. Nat Rev Mol Cell
Biol. 18:175–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Batra RN, Lifshitz A, Vidakovic AT, Chin
SF, Sati-Batra A, Sammut SJ, Provenzano E, Ali HR, Dariush A, Bruna
A, et al: DNA methylation landscapes of 1538 breast cancers reveal
a replication-linked clock, epigenomic instability and
cis-regulation. Nat Commun. 12:54062021. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Lippert TH, Ruoff HJ and Volm M: Intrinsic
and acquired drug resistance in malignant tumors. The main reason
for therapeutic failure. Arzneimittelforschung. 58:261–264.
2008.PubMed/NCBI
|
|
139
|
Taketo K, Konno M, Asai A, Koseki J,
Toratani M, Satoh T, Doki Y, Mori M, Ishii H and Ogawa K: The
epitranscriptome m6A writer METTL3 promotes chemo- and
radioresistance in pancreatic cancer cells. Int J Oncol.
52:621–629. 2018.PubMed/NCBI
|
|
140
|
Liu X, Gonzalez G, Dai X, Miao W, Yuan J,
Huang M, Bade D, Li L, Sun Y and Wang Y: Adenylate kinase 4
modulates the resistance of breast cancer cells to tamoxifen
through an m6A-based epitranscriptomic mechanism. Mol
Ther. 28:2593–2604. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Petri BJ, Piell KM, South Whitt GC, Wilt
AE and Klinge CM, Lehman NL, Clem BF, Nystoriak MA, Wysoczynski M
and Klinge CM: HNRNPA2B1 regulates tamoxifen- and
fulvestrant-sensitivity and hallmarks of endocrine resistance in
breast cancer cells. Cancer Lett. 518:152–168. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Liu X, Yuan J, Zhang X, Li L, Dai X, Chen
Q and Wang Y: ATF3 modulates the resistance of breast cancer cells
to tamoxifen through an N6-methyladenosine-based
epitranscriptomic mechanism. Chem Res Toxicol. 34:1814–1821. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Pan X, Hong X, Li S, Meng P and Xiao F:
METTL3 promotes adriamycin resistance in MCF-7 breast cancer cells
by accelerating pri-microRNA-221-3p maturation in a m6A-dependent
manner. Exp Mol Med. 53:91–102. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Li E, Xia M, Du Y, Long F, Pan F, He L, Hu
Z and Guo Z: METTL3 promotes homologous recombination repair and
modulates chemotherapeutic response by regulating the EGF/Rad51
axis. bioRxiv. 2021.
|
|
145
|
Li S, Jiang F, Chen F, Deng Y and Pan X:
Effect of m6A methyltransferase METTL3-mediated MALAT1/E2F1/AGR2
axis on adriamycin resistance in breast cancer. J Biochem Mol
Toxicol. 36:e229222022. View Article : Google Scholar
|
|
146
|
Wu Y, Wang Z, Han L, Guo Z, Yan B, Guo L,
Zhao H, Wei M, Hou N, Ye J, et al: PRMT5 regulates RNA m6A
demethylation for doxorubicin sensitivity in breast cancer. Mol
Ther. 30:2603–2617. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Wang Y, Cheng Z, Xu J, Lai M, Liu L, Zuo M
and Dang L: Fat mass and obesity-associated protein (FTO) mediates
signal transducer and activator of transcription 3 (STAT3)-drived
resistance of breast cancer to doxorubicin. Bioengineered.
21:1874–1889. 2021. View Article : Google Scholar
|
|
148
|
Liu X, Li P, Huang Y, Li H, Liu X, Du Y,
Lin X, Chen D, Liu H and Zhou Y: M6A demethylase ALKBH5
regulates FOXO1 mRNA stability and chemoresistance in
triple-negative breast cancer. Redox Biol. 69:1029932024.
View Article : Google Scholar
|
|
149
|
Ou B, Liu Y, Gao Z, Xu J, Yan Y, Li Y and
Zhang J: Senescent neutrophils-derived exosomal piRNA-17560
promotes chemoresistance and EMT of breast cancer via FTO-mediated
m6A demethylation. Cell Death Dis. 13:9052022. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Zhuang H, Yu B, Tao D, Xu X, Xu Y, Wang J,
Jiao Y and Wang L: The role of m6A methylation in therapy
resistance in cancer. Mol Cancer. 22:912023. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Wang Y, Zhang L, Sun XL, Lu YC, Chen S,
Pei DS and Zhang LS: NRP1 contributes to stemness and potentiates
radioresistance via WTAP-mediated m6A methylation of Bcl-2 mRNA in
breast cancer. Apoptosis. 28:233–246. 2023. View Article : Google Scholar
|