Introduction
Hepatocellular carcinoma (HCC) is the most prevalent
type of liver cancer, accounting for 70-85% of all primary liver
cancer cases worldwide. This severe disease, with high morbidity
and mortality rates, is the third most common cause of
cancer-related death worldwide (1). Despite the continuous development of
therapeutic methods, the rate of recurrence and metastasis in HCC
is also high (2,3). However, the precise mechanism
underlying the onset and progression of HCC remains elusive. A
number of studies have demonstrated the involvement of various
cellular pathways, such as the nuclear factor κB pathway (4,5),
ubiquitin-proteasome system (6)
and autophagy pathway (7), in HCC
pathogenesis. SUMOylation is an essential protein modification
pathway and plays a pivotal role in the regulation of diverse
biological processes, such as cellular localization, protein
activity, protein stability or protein degradation (8). Upregulated expression of SUMOylated
proteins in tumor tissues has been observed, and the activation of
SUMOylation is closely linked to the development of tumors
(9,10).
E2F transcription factor 4 (E2F4), a member of the
E2F family, contributes to tumor progression (11-13). E2F4 influences the expression of
toll-like receptor 8 and CD14, as well as the downstream activation
of the signal transducer and activator of the transcription (STAT1)
pathway (14). Studies have
demonstrated that elevated levels of E2F4 are observed in the
development of various types of cancer, including bladder cancer
(15), Burkitt lymphoma (16), breast cancer (17), gastric cancer (18), cervical cancer (19), colorectal cancer (20) and acute myeloid leukemia (21). Consistently, there is a decrease
in the G1-S phase transition and the proliferation of colon cancer
cells with stable silencing of E2F4 (22). However, the precise regulatory
role of E2F4 in HCC has yet to be elucidated, particularly in terms
of regulating the SUMOylation pathway.
In the present study, the potential role of E2F4 in
HCC was investigated through public database mining. To determine
the changes in target gene expression, western blotting and reverse
transcription-quantitative PCR (RT-qPCR) assays were used.
Furthermore, co-immunoprecipitation (Co-IP), immunofluorescence
co-localization and bimolecular fluorescence complementation (BiFC)
assays were used to observe the interactions between E2F4 and a
copartner. Subsequently, soft agar and Transwell migration assays
were used to detect the effect of E2F4 on the proliferation and
invasion in HCC cells.
Materials and methods
Bioinformatics
Public RNA-sequencing data were obtained from the
Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/; accession no.
GSE112221) (23), which included
10 samples. The data used in the present study were derived from 4
patients. 'N' represents cirrhotic tissue samples, while 'T'
denotes HCC samples. Both the cirrhosis and HCC tissues originated
from the same patients. A comprehensive analysis identified 105
common differentially expressed genes (DEGs) across these 4
patients. Enrichment analysis of these genes was conducted
utilizing Metascape (https://www.metascape.org). Then, ChIP-X (https://www.maayanlab.net/X2K/; version 1.6)
software prediction was used to obtain candidate TFs that regulate
these target genes. Survival analysis and differential expression
were analyzed using the GEPIA2 database (http://gepia2.cancer-pku.cn/#index), which included
gene expression data from The Cancer Genome Atlas (https://portal.gdc.cancer.gov/; TCGA-LIHC)
(24). Correlation analysis was
carried out using the TIMER database (https://cistrome.shinyapps.io/timer/). The protein
E2F4 co-partners were obtained using GENEMANIA (http://genemania.org), HINT (http://hint.yulab.org/) and STRING (https://string-db.org/) databases. E2F4 and DREAM
multi-vulva class B core complex component (LIN9) protein docking
studies were performed using the HDOCK Server (http://hdock.phys.hust.edu.cn/). A cut-off of
P<0.05 was used to define a significant result.
Cell lines and culture
The human liver cancer cell lines, HepG2 (cat. no.
CL-0103), HCC-LM3 (cat. no. CL-0278), Huh7 (cat. no. CL-0120), Huh6
(cat. no. CL-0119), and PLC (cat. no. CL-0415) were obtained from
Procell Life Science & Technology Co., Ltd. MHCC 97H cells
(cat. no. C6585) was purchased from Beyotime Institute of
Biotechnology. The cell lines were cultured in 5% CO2 at
37°C, with DMEM containing 10% fetal bovine serum (Thermo Fisher
Scientific, Inc.; cat. no. 12483020) and 100 U
penicillin/streptomycin (Thermo Fisher Scientific, Inc.; cat. no.
15140122).
Cell transfection
E2F4 and LIN9 overexpression vectors were
constructed using the CV186 lentiviral vector (Shanghai GeneChem
Co., Ltd.). Short hairpin RNA (shRNA) against E2F4 or SUMO2/3
(Table SI) for knockdown were
inserted into the GV298 lentiviral vector (Shanghai GeneChem Co.,
Ltd.; cat. no. GCD0316554). For transfection, the Huh7
(1×106 cells/well) and PLC (1×106 cells/well)
cells were cultured in 6-well plates for 12 h at 37°C, followed by
replacement with fresh medium. Subsequently, 2 μg E2F4-CV186
plasmid was transfected into Huh7 cells or the E2F4-GV298 vector
was transfected into PLC cells using Lipofectamine 3000 (Thermo
Fisher Scientific, Inc.; cat. no. L3000008) in a biological safety
cabinet, based on the kit's instructions. Following transfection,
the cell cultures were maintained at 37°C for an additional 48 h.
Stable cell lines were established with 2 μg/ml puromycin
selection for 2 weeks. Then, the poly-clonal stable cell lines were
cultured in fresh medium with 1 μg/ml puromycin for
maintenance.
RT-qPCR
Total RNA samples from Huh7 or PLC cells were
extracted by the RNeasy Mini Kit (Qiagen China Co., Ltd.; cat. no.
74104). RT was conducted using the Transcriptor First Strand cDNA
Synthesis Kit (Takara Biotechnology Co., Ltd.; cat. no. 6110A)
according to the manufacturer's instructions. In the qPCR analysis,
the SYBR Premix Ex Taq II (Takara Biotechnology Co., Ltd.; cat. no.
RR820A) and primer sets (Table
SII) were applied to determine the transcript levels via the
2−ΔΔCq method (25),
using β-actin (ACTB) as the internal control. The thermocycling
protocol was as follows: Holding stage at 95°C for 30 sec, cycling
stage for 40 cycles at 95°C for 5 sec and 60°C for 30 sec, the
melting curve stage at 95°C for 15 sec, 60°C for 1 min and 95°C for
15 sec.
Western blotting
Protein from the liver cancer cells was isolated by
cell lysis buffer (Beyotime Institute of Biotechnology; cat. no.
P0013). The quantification of protein concentrations was measured
with the BCA Protein Assay Kit (Beyotime Institute of
Biotechnology; cat. no. P0012). Proteins (30 μg) were
subjected to separation on a 10% SDS-polyacrylamide gel, followed
by transfer to PVDF membranes (MilliporeSigma; cat. no. IPVH00010).
The membranes were then blocked with 5% skimmed milk (Beyotime
Institute of Biotechnology; cat. no. P0216) in Tris-buffered saline
containing 0.1% Tween-20 (TBST; Beyotime Institute of
Biotechnology; cat. no. ST671) at room temperature for 1 h and
washed three times with TBST. Then, the membranes were incubated at
4°C overnight with primary antibodies. After washing three times
with TBST, the membranes were incubated with HRP-conjugated Goat
anti-Rabbit IgG (ABclonal Biotech Co., Ltd.; cat. no. AS014,
1:5,000) or HRP-conjugated Goat anti-Mouse IgG (ABclonal Biotech
Co., Ltd.; cat. no. AS003, 1:5,000) at room temperature for 1 h.
Following incubation, the membranes were again washed three times
with TBST. Then, the membranes were incubated with ECL reagent
(MedChemExpress; cat. no. HY-K1005). Band visualization was
performed using a ChemiDoc Imaging System (Bio-Rad Laboratories,
Inc.) for 1 min. Densitometric analysis was conducted using ImageJ
software (National Institutes of Health; version 1.53m). Western
blotting was undertaken using antibodies specific for E2F4, LIN9,
baculoviral IAP repeat containing 5 (BIRC5), cell division cycle
associated 8 (CDCA8), DNA topoisomerase II α (TOP2A), small
ubiquitin like modifier (SUMO)1, SUMO2/3, SUMO1/sentrin specific
peptidase (SENP)1, SENP2, SENP3, SENP5, SENP6, SENP7, SENP8, LIN54,
RBBP4, LIN52, LIN37 and ACTB (Table
SIII).
Co-IP
The Co-IP assay was carried out as previously
reported (26-29). Huh7 and PLC cells were lysed in
300 μl RIPA lysis buffer (Beyotime Institute of
Biotechnology; cat. no. P0013D). Cell lysates were incubated with
10 μg of antibodies specific for E2F4 (Proteintech Group,
Inc.; cat. no. 67812-1-Ig), LIN9 (Proteintech Group, Inc.; cat. no.
17882-1-AP), SUMO2/3 (Proteintech Group, Inc.; cat. no.
11251-1-AP), negative control rabbit IgG (Beyotime Institute of
Biotechnology; cat. no. A7016) or negative control mouse IgG
(Beyotime Institute of Biotechnology; cat. no. A7028) overnight at
4°C. Subsequently, the samples were incubated with 30 μl
Pierce Protein A/G magnetic beads (MedChemExpress; cat. no.
HY-K0202) at 4°C for another 3 h. Following centrifugation at 1,000
× g at 4°C for 10 min, the beads were washed with 1 ml lysis buffer
three times and boiled for 10 min at 100°C. The proteins were
subsequently isolated and identified using western blotting
analysis.
Immunofluorescence co-localization
assay
Huh7 and PLC cells (1×104 cells/well)
were grown on coverslips in 24-well plates at 37°C for 24 h. Cells
were fixed with 4% paraformaldehyde for 30 min at room temperature
(20-25°C) and washed three times with PBS. The glass coverslips
were incubated with 5% milk for 1 h at room temperature, then
treated with antibodies specific for E2F4 (Proteintech Group, Inc.;
cat. no. 67812-1-Ig; 1:100 dilution) and LIN9 (Proteintech Group,
Inc.; cat. no. 17882-1-AP; 1:100 dilution) at 4°C overnight. Next,
the coverslips were treated with Alexa Fluor 488 goat anti-mouse
IgG (Abcam; cat. no. ab150113; 1:1,000 dilution) or Alexa Fluor 594
goat anti-rabbit IgG (Abcam; cat. no. ab150160; 1:1,000 dilution)
at room temperature for 1 h in the dark. Cells were washed and
stained with 4',6-diamidino-2-phenylindole (DAPI; 300 nmol/l) for
10 min at room temperature. The images were imaged under a confocal
microscope. For EdU staining, Huh7 cells were cultured in a 12-well
plate at a density of 1×105 cells/well. Following
corresponding treatment, cell proliferation was detected with the
EdU Cell Proliferation Kit (Beyotime Institute of Biotechnology;
cat. no. C0081S) according to the manufacturer's instructions.
Finally, cells were observed and captured under a fluorescence
microscope.
BiFC assay
Human LIN9 cDNA (1,473 bp) or E2F4 cDNA (1,241 bp)
were respectively subcloned into BiFC vectors pBiFC-VC155 (Addgene,
Inc.; cat. no. 22011) and pBiFC-VN173 (Addgene, Inc.; cat. no.
22010). Co-transfection of the recombinant vectors (1 μg
LIN9-VC155 and 1 μg E2F4-VN173) into Huh7 cells was
performed using Lipofectamine 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 24 h. Next, the cells were fixed with
4% paraformaldehyde at room temperature for 30 min and incubated
with DAPI for 5 min at room temperature. Fluorescence emission was
observed under a confocal microscope, with excitation and emission
wavelengths set at 488 and 500 nm, respectively.
Soft agar assay
Huh7 cells (5×103 per well) were mixed
with a solution of 0.05% Noble agar. The mixture was then incubated
on 6-well plates containing solidified 0.1% Noble agar for 3 weeks.
After the incubation period, the cellular colonies that had formed
were stained using 0.5% crystal violet dye and subsequently
observed and manually counted using a light microscope.
Matrigel invasion assay
The invasiveness of Huh7 cells was assessed using a
12-well Transwell insert with 8.0-μm pores (Corning, Inc.;
cat. no. 3422). The upper wells of the inserts were coated with 100
μl Matrigel (Corning, Inc.; cat. no. 354262; 1 mg/ml) at
37°C for 3 h. Serum-starved cells (1×105 per well) were
seeded into the upper chamber of the Transwell insert with 200
μl serum-free DMEM, and 500 μl DMEM containing 10%
FBS was added to the lower chamber. After cell culture for 24 h,
the invaded cells were fixed with 4% paraformaldehyde at room
temperature for 15 min, stained with 0.1% crystal violet for 30 min
at room temperature, and subsequently quantified manually using a
light microscope.
SUMOylation inhibitor
To assess the impact of SUMOylation on the
proliferation and invasiveness of Huh7 cells, the SUMOylation
inhibitor, TAK-981 (10 nM; MedChemExpress; cat. no. HY-111789), was
added to the cell medium after E2F4-overexpression vector
transfection. Following treatment, the cell cultures were
maintained at 37°C for 24 h, The soft agar assay was then used to
detect cell proliferation and the Matrigel invasion assay was used
to detect invasiveness of Huh7 cells (according to the
aforementioned protocols).
Statistical analysis
In the present study, the data are presented as the
mean ± standard deviation. To compare differences between two
datasets, a two-tailed unpaired Student's t-test was applied. The
differences between multiple groups were calculated using one-way
ANOVA followed by Dunnett's post hoc test. Spearman's correlation
analysis was used to determine the expression correlation. Survival
curves were constructed using the Kaplan-Meier method. High- and
low-expression levels were separated by a suitable adjustable
cut-off value. The log-rank test was utilized to assess differences
in survival. All statistical analyses conducted in the present
study were two-sided. P<0.05 was considered to indicate a
statistically significant difference.
Results
E2F4 is a potential TF in regulating the
progression of HCC
To investigate potential genes involved in the
outcomes of patients with HCC, a comprehensive analysis was
performed using HCC and cirrhosis tissue gene expression profiles
available from the GEO. A total of 105 DEGs in HCC tissues compared
with cirrhosis tissues were identified [fold change (FC) >2;
Fig. 1A]. Furthermore, 76 genes
were upregulated whereas 29 genes were downregulated in patients
with HCC (Fig. 1A). To analyze
the crucial pathways affected by potential regulators, a REACTOME
pathway analysis for 105 DEGs was performed using Metascape
(30). The results revealed that
SUMOylation was a significantly enriched pathway including 5 target
genes (aurora kinase A, BIRC5, chromobox homolog, CDCA8 and TOP2A;
Fig. 1B). To investigate the
crucial TFs that regulate the SUMOylation-associated genes, ChIP-X
software was used to reveal that the top 5 TFs were E2F4, ETS
proto-oncogene 1 (ETS1), interferon regulatory factor 1 (IRF1),
MYCN proto-oncogene and lysine demethylase 6A (KDM6A). Notably,
E2F4 ranked first among the identified TFs by the number of target
genes, including BIRCA, CDCA8 and TOP2A, which were more enriched
in HCC tissues (Fig. 1C and E).
Meanwhile, comprehensive analysis using GEPIA2 (31) showed that upregulation of E2F4
(P=0.016), BIRC5 (P<0.001), CDCA8 (P<0.001) and TOP2A
(P=0.01) were associated with a poorer overall survival (OS) rate
in patients with HCC (Figs. 1D
and S1). A positive correlation
between E2F4 and BIRC5 (ρ=0.395; P<0.001), CDCA8 (ρ=0.58;
P<0.001) or TOP2A (ρ=0.552; P<0.001; Fig. 1F) in HCC specimens was observed
using TIMER (32). The results
indicated that E2F4 may be a potential regulator in the progression
of HCC.
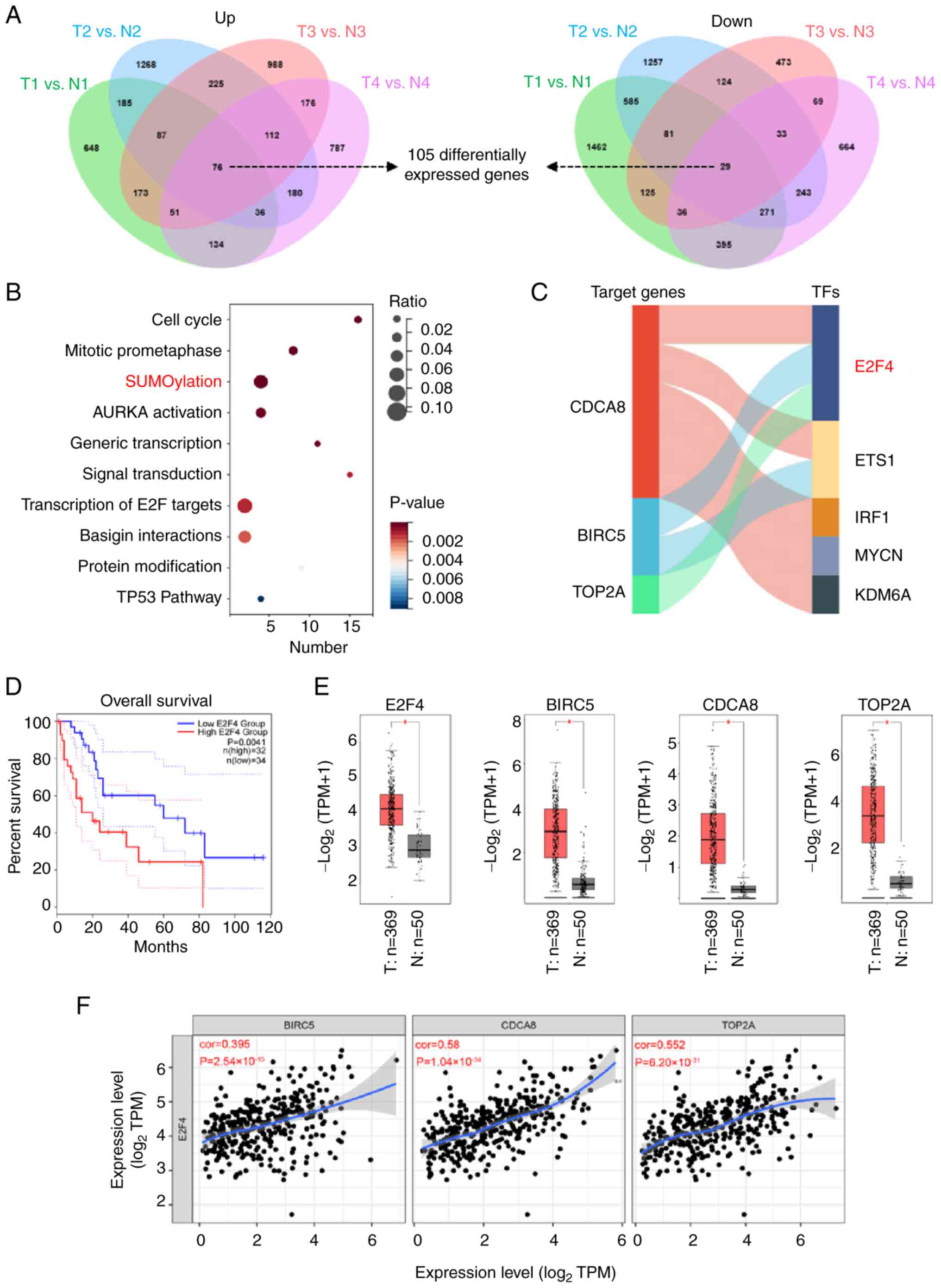 | Figure 1E2F4 is the potential transcription
factor in regulating the progression of HCC. (A) Venn diagram
revealing the DEGs (P<0.05, fold change >2) in the public
dataset (accession no. GSE112221). 'N' represents cirrhotic tissue
samples, while 'T' denotes HCC samples. (B) Gene set enrichment
analysis was conducted on the DEGs obtained from the GSE112221
dataset. (C) The ChIP-X software was used to predict the top 5 TFs
that regulate the expression of SUMOylation-associated genes. (D)
Kaplan-Meier analysis was used to assess the association of E2F4
expression with overall survival, using data from the GEPIA2
database. (E) Relative expression levels of E2F4, BIRC5, CDCA8 and
TOP2A in normal and HCC tissues in TCGA-LIHC dataset. 'N'
represents normal samples, while 'T' represents HCC tissue. (F)
Correlation analysis indicating the relationship between E2F4 and
BIRC5, CDCA8 or TOP2A. *P<0.05. BIRC5, baculoviral IAP repeat
containing 5; CDCA8, cell division cycle associated 8; DEGs,
differentially expressed genes; E2F4, E2F transcription factor 4;
ETS1, ETS proto-oncogene 1; HCC, hepatocellular carcinoma; IRF1,
interferon regulatory factor 1; KDM6A, lysine demethylase 6A; MYCN,
MYCN proto-oncogene; TFs, transcription factors; TOP2A, DNA
topoisomerase II α; TPM, transcripts per million. |
E2F4 promotes the expression of
SUMOylation-associated target genes
To understand the direct effects of E2F4 on BIRC5,
CDCA8 and TOP2A transcription and expression in HCC cell lines,
JASPAR (http://jaspar.genereg.net) was used to
identify the E2F4 binding site in the promotors (Fig. 2A). Moreover, analysis of E2F4
chromatin immunoprecipitation-sequencing datasets (UCSC browser)
also revealed that E2F4 bound at the promoters of BICR5, CDCA8 and
TOP2A in HeLa cells (Fig. 2A).
Western blotting revealed higher E2F4 expression levels in HepG2
and PLC cells and lower E2F4 expression levels in Huh7 cells
(Fig. 2B). Since the present
study focused on HCC, PLC and Huh7 were chosen as the HCC cell line
models. To explore the regulation of SUMOylation-associated target
genes by E2F4, western blotting was performed, which revealed that
stable overexpression of E2F4 increased the protein levels of
BIRC5, CDCA8 and TOP2A in Huh7 cells (Fig. 2C). However, knocking down E2F4
expression led to a decrease in the BIRC5, CDCA5, and TOP2A levels
in PLC cells (Fig. 2C). These
findings demonstrated that E2F4 regulated the expression of
SUMOylation-associated genes in HCC cells.
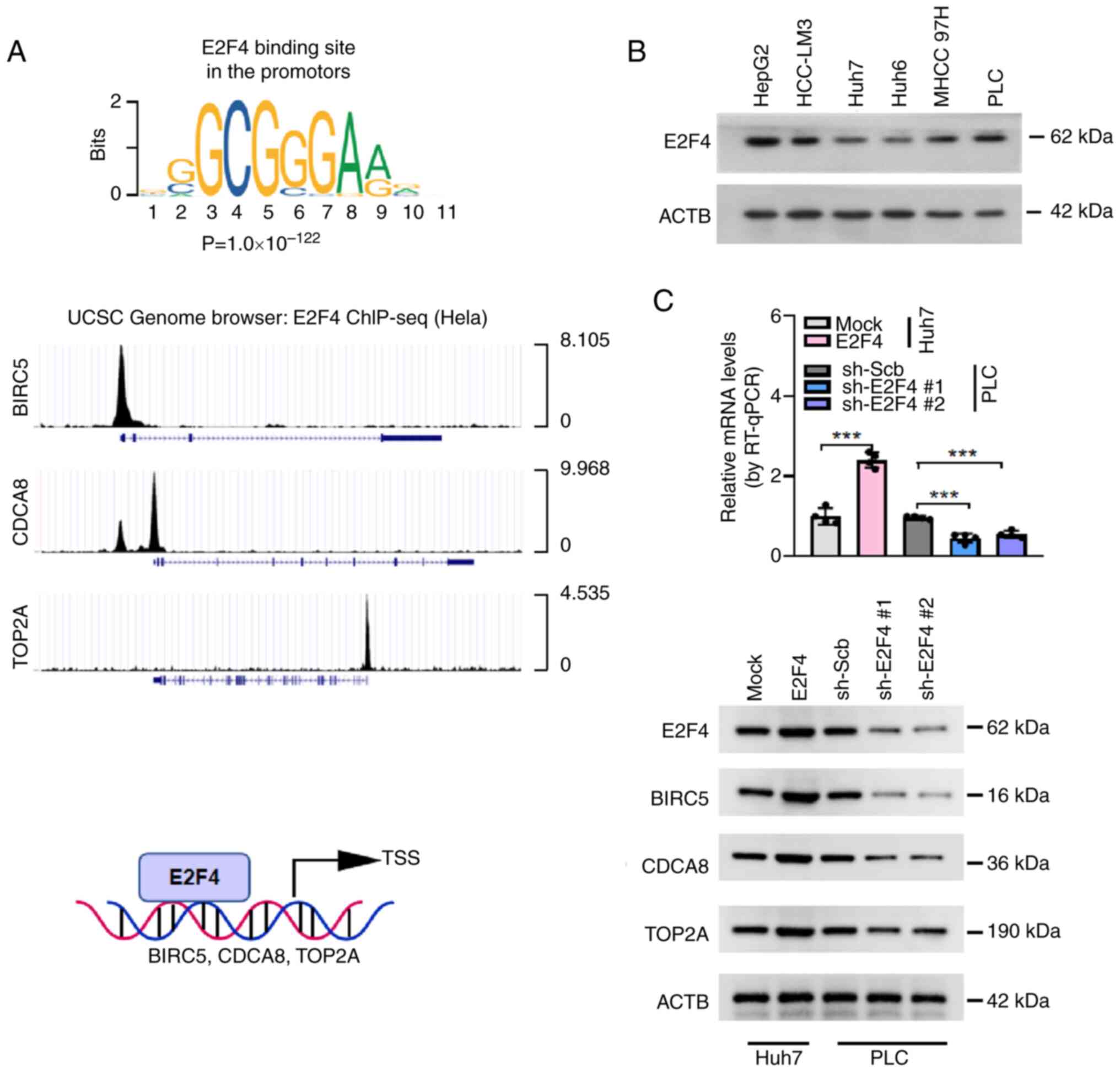 | Figure 2E2F4 promotes the expression of
SUMOylation-associated target genes in HCC cells. (A) E2F4 binding
site in the promotors identified using the JASPAR database, and
publicly available ChIP-seq dataset from the UCSC database was used
to reveal the direct binding of E2F4 to the promoters of BIRC5,
CDCA8 and TOP2A. (B) Western blotting was used to show the relative
protein expression levels of E2F4 in several types of liver cancer
cells. (C) RT-qPCR analysis (n=4 per group) used to show the
transcript levels of E2F4 and western blotting was used to show the
protein expression levels of E2F4, BIRC5, CDCA8 and TOP2A in HCC
cells following empty vector (mock), E2F4 overexpression, sh-Scb,
sh-E2F4 #1 and sh-E2F4 #2 transfections. ***P<0.001.
ACTB, β-actin; BIRC5, baculoviral IAP repeat containing 5; CDCA8,
cell division cycle associated 8; ChIP-seq, chromatin
immunoprecipitation-sequencing; E2F4, E2F transcription factor 4;
HCC, hepatocellular carcinoma; LIN9, lin-9 DREAM MuvB core complex
component; RT-qPCR, reverse transcription-quantitative PCR; Scb,
scramble; sh, short hairpin; TOP2A, DNA topoisomerase II α; TSS,
transcription start site. |
E2F4 facilitates the proliferation of HCC
cells via SUMOylation
The effects of E2F4 on SUMOylation in HCC cells was
further determined by western blotting. In PLC and Huh7 cells,
stable overexpression or knockdown of E2F4 enhanced and reduced the
whole cell SUMOylation levels, respectively (Fig. 3A). The SUMOylation process
involves numerous crucial proteins and specific proteases.
Comprehensive analysis using the GEPIA2 database revealed higher
expression levels of SUMO2/3 and SENP3 in HCC (T) tissues compared
with the normal control (N) group (P<0.05, FC>1.5; Fig. S2A). Consistently, western
blotting analysis following E2F4 overexpression in Huh7 cells
revealed an increase in SUMO2/3 protein levels, but not the levels
of SUMO1 or other SUMO-specific proteases (Fig. S2B). The results of a SUMOylation
assay indicated that the SUMOylation levels of target genes were
elevated in cells overexpressing E2F4 (Fig. S3A and B), which were decreased by
stable knockdown of SUMO2/3 (Fig.
S3B). In addition, the results of an EdU assay revealed that
stable overexpression or knockdown of E2F4 increased and decreased
the number of EdU+ cells compared with the controls,
respectively (Fig. S4A).
Subsequently, soft agar colony formation and Transwell Matrigel
assays were performed to demonstrate that E2F4 significantly
promoted the proliferation and invasion of HCC cells (Fig. S4B and C). To investigate the
involvement of SUMOylation in E2F4-promoted proliferation and
invasion, a SUMOylation inhibitor (TAK981) was applied to HCC cells
stably overexpressing E2F4. Subsequent western blotting indicated
that the SUMOylation levels in Huh7 cells were decreased by TAK-981
treatment (Fig. 3B). The results
of EdU, soft agar colony formation and Transwell Matrigel assays
showed that TAK-981 prevented the increase in proliferation and
invasion levels of HCC cells stably overexpressing E2F4 (Fig. 3C-E). Similarly, the proliferation
and invasion of HCC cells were inhibited by knocking down SUMO2/3
(Fig. S3C). Taken together,
these results suggested that E2F4 elevated the proliferation and
invasion of HCC cells by promoting SUMOylation.
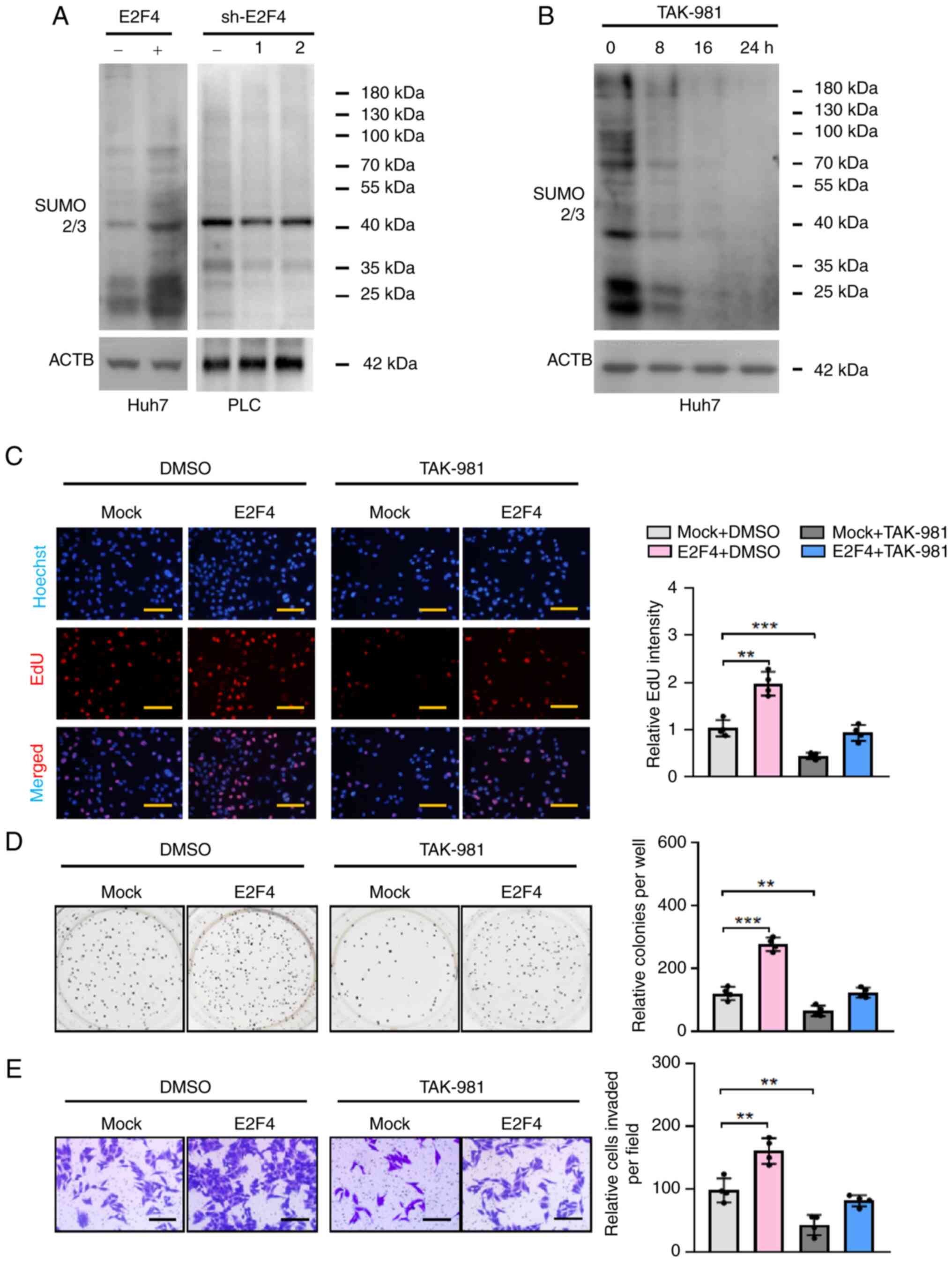 | Figure 3E2F4 facilitates the proliferation of
hepatocellular carcinoma cells via SUMOylation. (A and B) Western
blots revealing the levels of SUMOylation in Huh7 and PLC cells.
(C) EdU staining assays (n=4 per group) showing the proliferation
of Huh7 cells stably transfected with empty vector (mock) or E2F4,
then treated with an inhibitor of SUMOylation (TAK-981, 10 nM). (D)
Representative images (left panel) and quantification (right panel)
of soft agar (n=4 per group) and (E) Transwell Matrigel invasion
(n=4 per group) assays indicating the anchorage-independent
proliferation and invasiveness of Huh7 cells stably transfected
with empty vector (mock) or E2F4, then treated with an inhibitor of
SUMOylation (TAK-981, 10 nM). **P<0.01,
***P<0.001. Scale bars, 100 μm. ACTB, actin-b;
E2F4, E2F transcription factor 4; sh, short hairpin; SUMO2/3, small
ubiquitin like modifier 2/3. |
LIN9 is an E2F4 potential protein
partner
To determine potential protein partners that
interact with E2F4, a E2F4 protein-protein interaction network was
obtained using GENEMANIA (http://genemania.org), HINT (http://hint.yulab.org/) and STRING (https://string-db.org/) databases (Fig. 4A-C). Overlapping the results of
the analyses derived from the aforementioned databases, LIN9, RB
transcriptional corepressor like (RBL) 1, RBL2 and transcription
factor Dp-1 (TFDP1) were identified as potential E2F4 binding
partners (Fig. 4D). Subsequently,
higher LIN9 levels were observed to be associated with a poorer OS
rate in patients with HCC (P=0.0028), whereas the OS curves were
not significantly different between the high and low RBL1 (P=0.17),
RBL2 (P=0.80) and TFDP1 (P=0.22) (Fig. 4E) expression groups. Therefore,
LIN9 was identified as a potential E2F4 co-partner in HCC cells. In
addition, high expression of LIN9 was positively associated with
MKI67 (Ki-67, a biomarker of proliferation) and MCAM (CD136, a
potential biomarker of angiogenesis) in patients with HCC (Fig. 4F). According to the TCGA database,
higher expression levels of E2F4, LIN9, BIRCA5, CDCA8 and TOP2A
were observed in multiple cancer types (Fig. S5). Taken together, these results
indicated that LIN9 likely interacts with E2F4 in HCC cells.
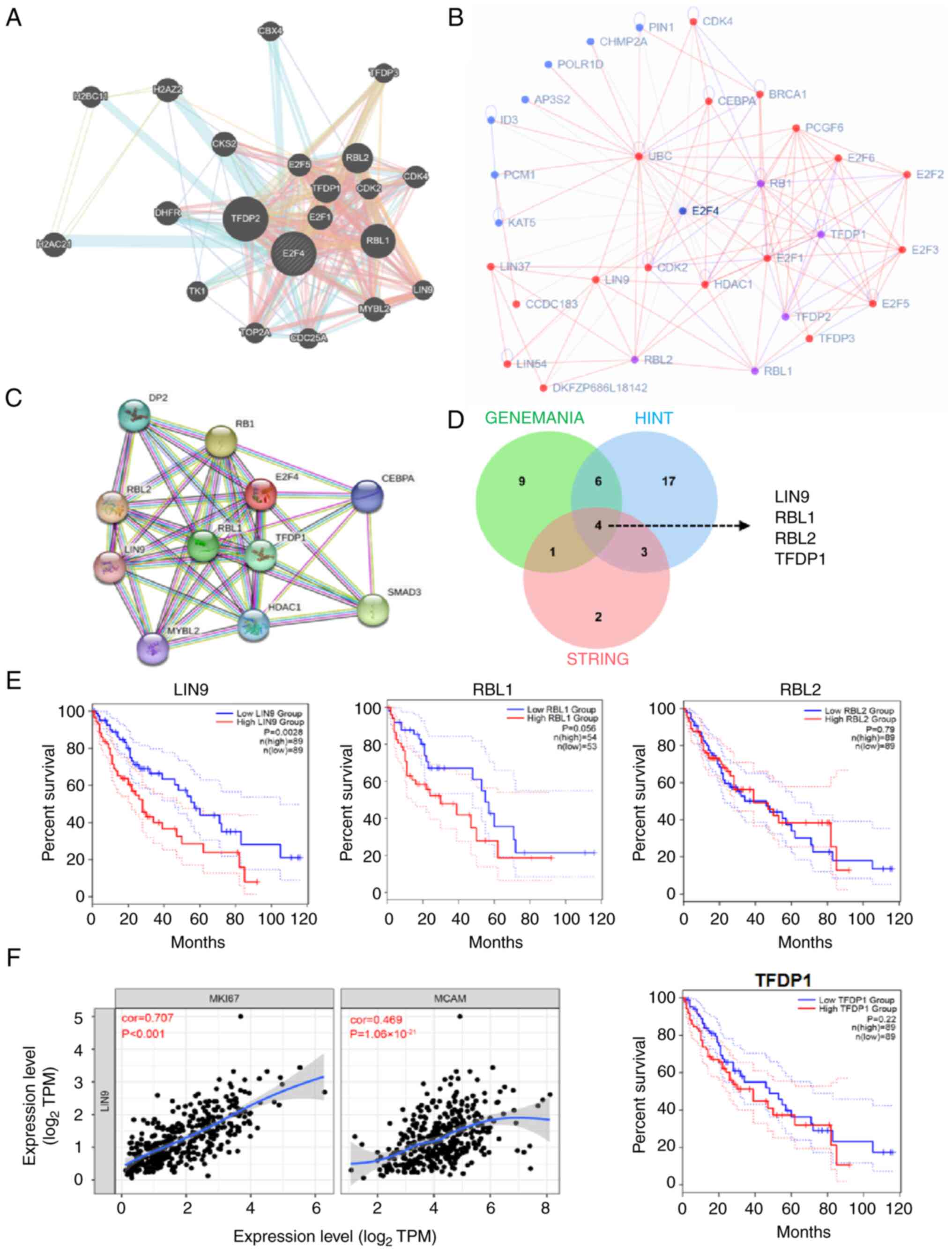 | Figure 4LIN9 is identified as a potential
protein partner of E2F4. Network of E2F4 and E2F4 co-partners in
the public (A) GENEMANIA, (B) HINT and (C) STRING websites. (D)
Venn diagram revealing the four potential proteins that interact
with E2F4, including LIN9, RBL1, RBL2 and TFDP1. (E) Kaplan-Meier
tests showing the overall survival of LIN9, RBL1, RBL2 and TFDP1.
(F) Correlation curves indicating that LIN9 is positively
correlated with proliferation and angiogenesis in hepatocellular
carcinoma. E2F4, E2F transcription factor 4; LIN9, lin-9 DREAM MuvB
core complex component; RBL, RB transcriptional corepressor like;
TFDP1, transcription factor Dp-1; TPM, transcripts per million. |
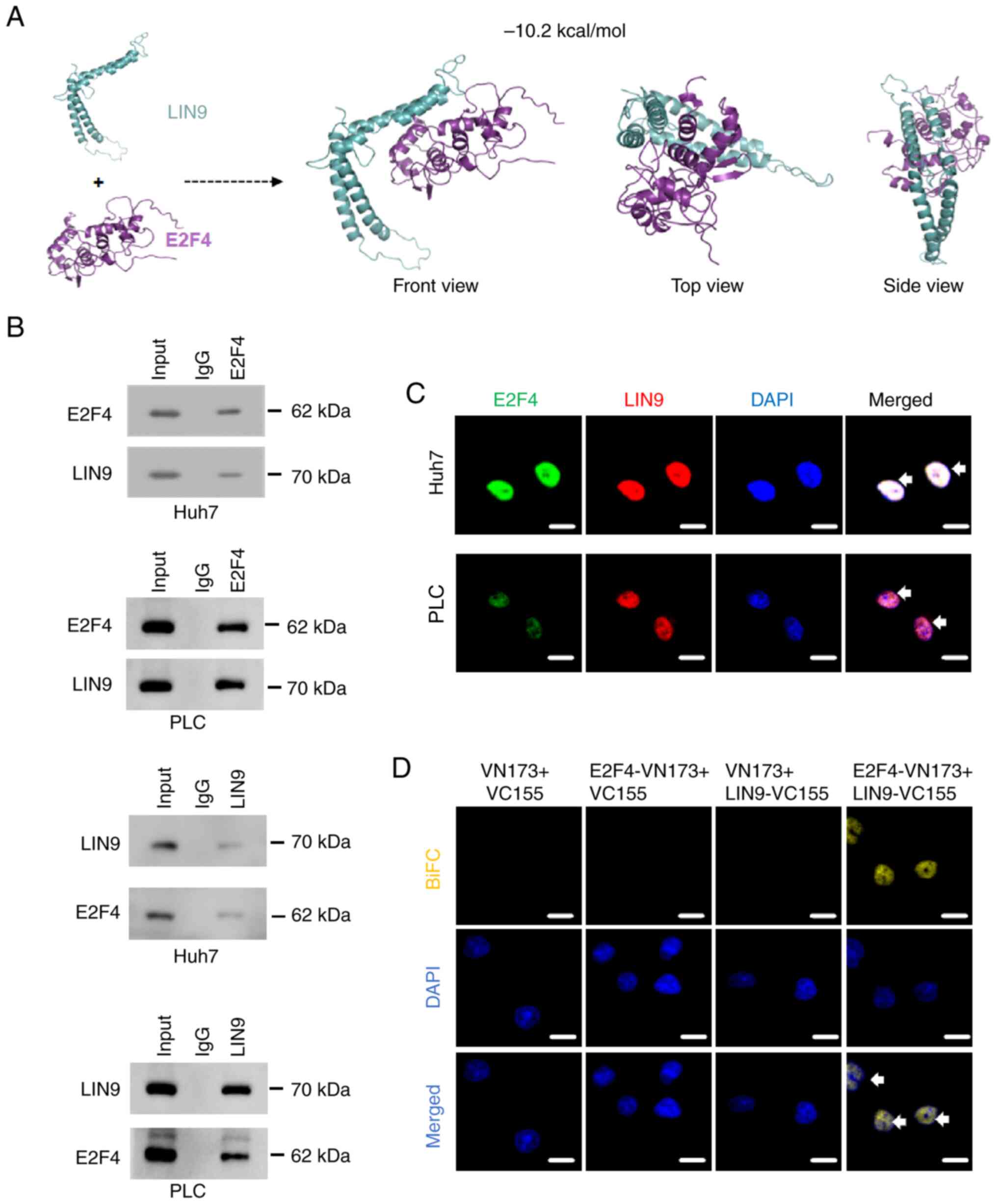
LIN9 physically interacts with E2F4 in
HCC cells
To investigate the interaction between E2F4 with
LIN9, protein docking studies were performed. The binding energy
(-10.2 kcal/mol) revealed that E2F4 directly binds to LIN9
(Fig. 5A). Subsequently, a co-IP
assay was used to demonstrated the endogenous interaction between
E2F4 and LIN9 protein in PLC and Huh7 cells (Fig. 5B). Consistently, the results of an
immunofluorescence staining assay demonstrated that E2F4
co-localized with LIN9 in the nucleus of PLC and Huh7 cells
(Fig. 5C). To further determine
the physical interaction between E2F4 and LIN9, a BiFC assay was
performed to reveal that notable fluorescence occurred in Huh7
cells co-transfected with E2F4 and LIN9 plasmids (Fig. 5D). These findings suggested that
E2F4 physically interacted with LIN9 protein in HCC cells.
LIN9 promotes the proliferation and
invasion of HCC cells via E2F4
The cooperative roles of E2F4 and LIN9 in
SUMOylation in HCC cells were further investigated. The results of
a SUMOylation assay indicated that elevated SUMOylation of LIN9 was
observed in cells stably overexpressing E2F4 (Fig. S3A and B). Unexpectedly, the
interaction between E2F4 and other components (LIN54, LIN52, RBBP4
and LIN37) of the multi-vulva class B (MuvB) complex was prevented
by overexpression of E2F4 (Fig.
S6). The results suggested that E2F4 might inhibit DREAM
complex stability following SUMOylation of LIN9. Western blotting
and RT-qPCR revealed that stable overexpression of LIN9 increased
the transcript and protein expression levels of BIRC5, CDCA8 and
TOP2A, which was prevented by knocking down E2F4 (Fig. 6A and B). Consistently, there was
an increase in the SUMOylation levels and proliferation of Huh7
cells stably overexpressing LIN9, which was rescued by knocking
down E2F4 (Fig. 6C and D). The
results of the soft agar colony formation and Matrigel invasion
assays revealed that overexpression of LIN9 facilitated the
anchor-independent proliferation and invasiveness of Huh7 cells,
which was prevented by knocking down E2F4 (Fig. 6E and F). The aforementioned
findings suggested that E2F4 coordinated with LIN9 to promote
SUMOylation and the progression of HCC. Meanwhile, the identified
SUMOylation-related proteins were significantly highly expressed in
a variety of tumor types, further indicating that SUMOylation plays
a critical role in the progression of cancer (Fig. S5).
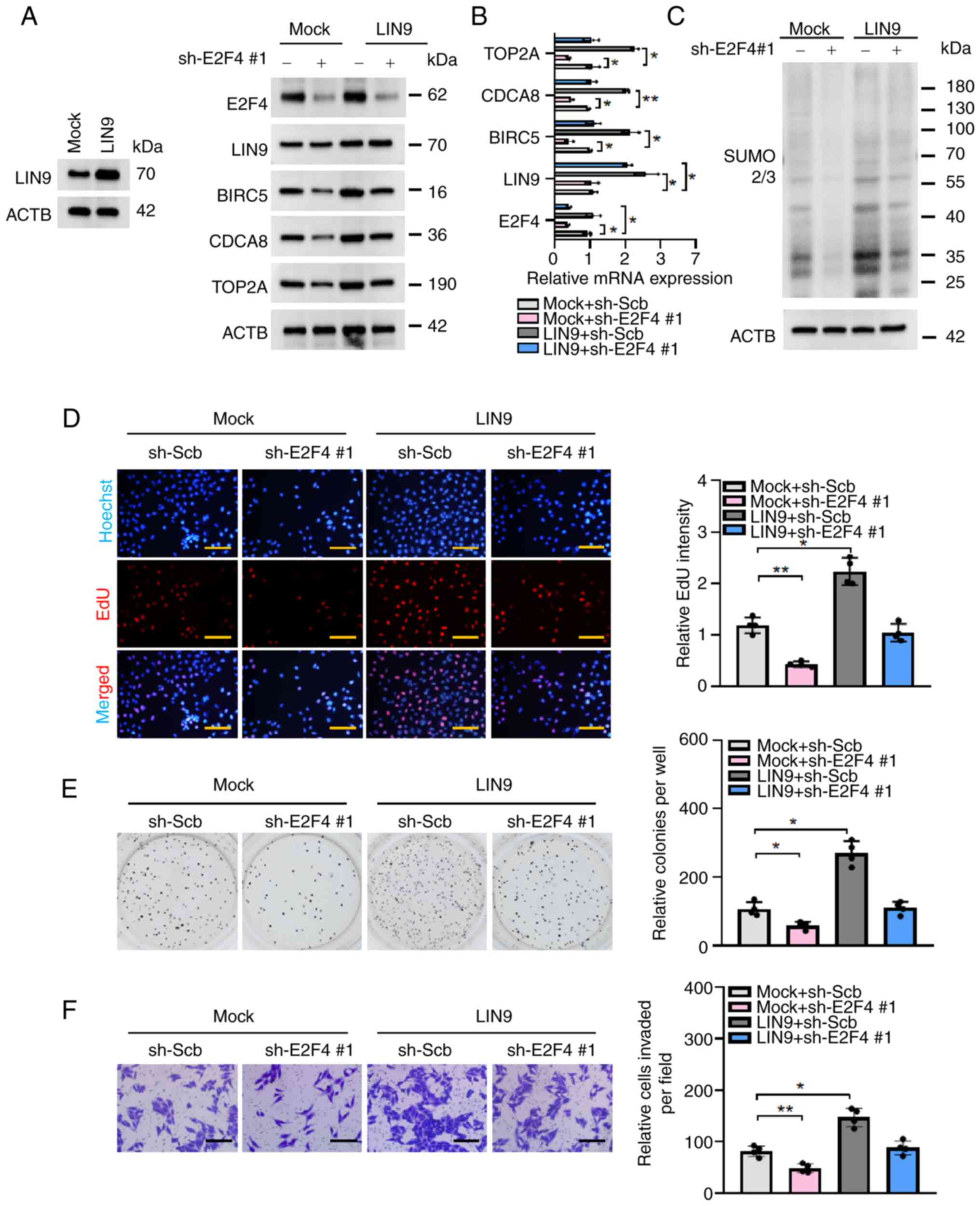 | Figure 6LIN9 promotes the proliferation and
invasiveness of hepatocellular carcinoma cells via E2F4. (A)
Western blotting and (B) reverse transcription-quantitative PCR
(n=3 per group) assays revealing the protein expression and
transcript levels of target genes in Huh7 transfected with sh-Scb
or sh-E2F4 #1 and co-transfected with empty vector (mock) or LIN9.
(C) Western blot revealing the levels of SUMOylation in Huh7
transfected with sh-Scb or sh-E2F4 #1 and co-transfected with empty
vector (mock) or LIN9. (D) EdU staining assays (n=4 per group)
showing the proliferation of Huh7 cells stably transfected with
sh-Scb or sh-E2F4 #1, and co-transfected with mock or LIN9. (E)
Representative images (left panel) and quantification (right panel)
of soft agar (n=4 per group) and (F) Transwell Matrigel invasion
(n=4 per group) assays indicating the anchorage-independent
proliferation and invasiveness of Huh7 cells stably transfected
with sh-Scb or sh-E2F4 #1, and co-transfected with mock or LIN9.
*P<0.05, **P<0.01. Scale bars, 100
μm. ACTB, actin-b; BIRC5, baculoviral IAP repeat containing
5; CDCA8, cell division cycle associated 8; E2F4, E2F transcription
factor 4; LIN9, lin-9 DREAM MuvB core complex component; sh, short
hairpin; Scb, scramble (shRNA); SUMO2/3, small ubiquitin like
modifier 2/3; TOP2A, DNA topoisomerase II α. |
Discussion
HCC ranks as the sixth most prevalent malignancy
globally and emerges as the fourth primary cause of mortality in
cancer-related deaths (1). The
morbidity and mortality of HCC is contingent upon the disease stage
at initial diagnosis. HCC fatalities are primarily caused by
metastasis and post-operative recurrence (3), with an estimated recurrence rate of
60-70% (33). The dysregulation
of gene expression, encompassing inactivation of tumor suppressor
genes and activation of oncogenes, is widely recognized as a
catalyst for tumor metastasis (24,34,35). Therefore, it is crucial to explore
the mechanism of gene expression in patients with HCC. In the
present study, the identified DEGs in HCC were significantly
enriched in the SUMOylation pathway. Several studies have
demonstrated that SUMOylation is closely associated with
tumorigenesis, proliferation (36) and metastasis (37), and higher expression is observed
in the majority of tumor cases (37,38). However, the effects of SUMOylation
in HCC still remain largely unclear.
SUMOylation, a posttranslational modification (PTM)
marked by the attachment of a small ubiquitin-like modifier peptide
to a lysine residue, significantly influences cellular biological
processes and the progression of cancer (39). Comparable to other types of PTMs,
such as phosphorylation, ubiquitination and acetylation,
SUMOylation is a highly dynamic and reversible mechanism that
regulates the translation, subcellular localization, stability and
protein-protein interactions of target proteins (40,41). Proteins such as STAT1, when
modulated via both SUMOylation and de-SUMOylation, have been linked
to the pathogenesis of cancer (42,43). A study has revealed that higher
levels of SUMOylation can be observed in HCC tissues, and
inhibitors of SUMOylation (such as TAK-981 and ML-792) suppress
tumor progression (44). Notably,
the results of the present study further supported these findings
and revealed that TAK-981 prevented the proliferation and invasion
of HCC cells. The higher transcript and protein levels of
SUMOylation-related genes (BIRC5, CDCA8 and TOP2A) in HCC cells was
also observed in the present study. Nevertheless, the specific
function of STAT1 SUMOylation in HCC remains unclear, warranting
future investigative efforts.
Some studies have highlighted the critical role of
BIRC5 in oncogenesis, demonstrating that its overexpression not
only suppresses apoptosis but also endows cells with tumorigenic
capabilities (45,46). Specifically, the dysregulation of
BIRC5-related genes has been tightly linked to the malignant
advancement of HCC (47).
Moreover, BIRC5 has been identified as a downstream target of
microRNA (miR)-497-5p, which notably exhibits diminished
transcription levels in HCC tissues compared with healthy tissues
(48). Circular (circ)ANKRD52
facilitates the proliferation of HCC by sponging miR-497-5p and
increasing BIRC5 expression (49). CDCA8 serves as an essential
component of the chromosomal passenger complex, indispensable for
accurate chromosomal distribution during mitosis (50). Furthermore, abnormal expression of
CDCA8 leads to disruption of cellular homeostasis (51). Targeted suppression of CDCA8
inhibits the MEK/ERK pathway and impedes colony formation by
regulating the CDK1/cyclin B1 signaling axis (52). TOP2A maintains mitotic chromosome
structure and genome stability by resolving DNA topological strains
and controlling genome dynamics (53). Abnormal levels of TOP2A are
related to tumor progression (54), and bioinformatic analyses have
identified TOP2A as a driving factor of HCC progression (55,56). Notably, TOP2A is upregulated and
associated with an unfavorable prognosis in HCC (57), while miR-139-5p inhibits TOP2A
expression, triggering cellular senescence and inhibiting the
proliferation of HCC cells (58).
Furthermore, the interaction between TOP2A and β-catenin
facilitates the detachment of β-catenin from Yes1 associated
transcriptional regulator, which enhances the excessive
proliferation of HCC cells (59).
Taken together, these findings underscore the notable influence of
BIRC5, CDCA8 and TOP2A on the pathogenesis and progression of HCC,
marking them as significant players and potential therapeutic
targets. Therefore, it is critical to identify the key factors that
regulate these target genes.
E2F4, a member of the E2F TF family, markedly
influences tumor progression via the regulation of various gene
expression and cell cycle signaling pathways, such apoptosis,
differentiation and G0 to S phase transition (60-63). The E2F family can be generally
divided into canonical activators (E2F1, E2F2 and E2F3a), canonical
repressors (E2F3b, E2F4, E2F5 and E2F6) and atypical repressors
(E2F7 and E2F8) (64). Notably,
the mRNA expression levels of E2F have been associated with the
progression stage and histopathological grades in HCC, and E2F4 has
emerged as a key regulator of HCC (65,66). E2F4 interacts with the CDCA3
promoter region, leading to increased CDCA3 expression and
facilitating both cell cycle progression and the proliferation of
HCC cells (67). Additionally,
E2F4 has been shown to directly bind to viral covalently closed
circDNA, activating the hepatitis B virus (HBV) core promoter and
upregulating transcription levels (68). Therefore, further exploration of
the epigenetic regulatory roles of E2F4 in HCC is warranted in the
future. Moreover, E2F4 is associated with immune infiltration in
HCC (65). In the present study,
higher expression levels of E2F4 protein compared with the controls
were observed in patients with HCC. Kaplan-Meier analyses also
suggested that E2F4 was associated with poorer survival in patients
with HCC. Therefore, the E2F4 protein may serve as a promising
prognostic indicator for HCC.
The findings of the present study also indicated
that E2F4 directly interacted with LIN9 protein in the nucleus of
HCC cells. In addition, elevated levels of LIN9 were associated
with poorer outcomes in patients with HCC. LIN9 is a part of the
MuvB complex, including LIN9, LIN37, LIN52, RB binding protein 4
and LIN54 (69,70). During the G0 and early G1 phases,
the MuvB core complex engages with E2F4/5, DP1/2 and RB-like
proteins, p130 or p107, to form the DREAM complex, thus inhibiting
gene transcription. In the late phase of G1, the MuvB core is
separated from the DREAM complex and then binds to B-MYB to form
the MMB complex in the S phase, activating cell-cycle genes related
to the S/G2/M phases (71-74).
Deregulation of the DREAM complex has been implicated in multiple
cancer types (such as myeloid leukemia, glioblastoma and prostate,
breast, lung, esophagus, ovarian and pancreas cancer) and plays a
critical role in cell cycle dynamics (75,76). It has been observed that aberrant
expression of the DREAM complex occurs in HCC (77). Similarly, the findings of the
present study suggested that higher expression of LIN9 was related
to a worse OS. Notably, the results of the rescue studies revealed
that overexpression of LIN9 reduced whole cell levels of
SUMOylation and the proliferation and invasion of HCC cells
following stable knockdown of E2F4 expression. An elevation in the
SUMOylation of LIN9 was also noted in Huh7 cells overexpressing
E2F4, which may potentially disrupt the stability of the DREAM
complex. These findings suggested that the LIN9-E2F4 axis promotes
progression in HCC via activation of the SUMOylation pathway.
However, the intricacies of these mechanisms warrant further
in-depth research.
In addition, other major TFs, such as E2F1 (78), ETS1 (79), IRF1 (80) and KDM6A (81), have been identified as prognostic
biomarkers in HCC. E2F1, the classical E2F member, has been
extensively studied. Notably, higher expression of E2F1 is
significantly related to unfavorable prognosis in HCC (78). E2F1 can stimulate the
proliferation of HCC cells by driving the expression of genes such
as B-MYB (82), BRCA1 (83), DNA-binding protein A (84) and stathmin 1 (85), which enhance the development or
progression of HCC. Additionally, mutations in the HBV core
promoter lead to upregulated transcription of E2F1, resulting in
HCC cell proliferation (86).
Notably, nuclear E2F1 expression is positively correlated with the
HCC tumor apoptotic index (87,88). E2F1 may disrupt the regulation of
hepatitis B viral X protein on the p53 promoter and directly
activate the p53 promoter via its specific binding sites, thereby
blocking the HBV life cycle and HBV-associated HCC (89). As such, the intricate functions of
E2F1 in HCC merit further investigation. ETS1, a member of the ETS
family, is a crucial TF in regulating cell proliferation, invasion
and metastasis. The interaction between murine double minute
binding protein and ETS1 activates ETS1, consequently leading to
the progression of HCC cells (90). Moreover, ETS1 also acts as a
co-activator of pregnane X receptor to stimulate drug resistance in
HCC (91,92). miR-338-3p inhibits progression of
HCC through the downregulation of EST1 (93), while small molecule inhibitors
targeting ETS1 have been shown to suppress proliferation or
invasion of HCC cells (94). IRF1
is the main transcription regulator in the interferon-γ signaling
pathway (95). IRF1 promotes
autophagy in HCC cells, thus inhibiting their proliferation
(96). Nevertheless, IRF1
increases the expression of programmed death-ligand 1, which may
facilitate HCC cells in evading the antitumor immune response of
the host (80). KDM6A plays
different functions at different stages of HCC. On one hand, KDM6A
promotes the progression of HCC through the upregulation of FGFR4
expression (97). On the other
hand, KDM6A may hinder the proliferation of HCC cells through the
negative regulation of the TGF-β/SMAD signaling pathway (98). Hence, the complexity of these TFs
underscores the necessity to delve deeper into HCC development and
new molecular therapeutic targets.
In summary, the present study demonstrated that E2F4
protein was highly expressed and related to poorer outcomes in
patients with HCC. Stable expression of E2F4 promoted the whole
cell SUMOylation of proteins by upregulating the transcription and
protein expression levels of SUMOylation-associated genes including
BIRC5, CDCA8 and TOP2A protein. Following treatment with the
inhibitor of SUMOylation, TAK-981, the proliferation and invasion
of HCC cells were downregulated. Notably, it was demonstrated that
E2F4 directly bound to LIN9 protein, leading to the promotion of
E2F4-mediated SUMOylation associated with HCC progression.
Therefore, the results of the present study suggested that E2F4 and
LIN9 play crucial roles in the progression of HCC and indicated
that the E2F4-LIN9-SUMOylation axis may be a valuable potential
therapeutic target in HCC.
Supplementary Data
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZD and QL conceived and designed the present study.
ZM, QL and WW performed most of the experiments, analyzed the data
and wrote the manuscript. ZD and WW supervised the entire project.
ZM, QL, WW and ZD confirm the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Health Commission of
Hubei Province Scientific Research Project (grant no. WJ2021M107),
Hubei Provincial Natural Science Foundation of China (grant no.
2023AFB160) and Scientific Research Project of Tongji Hospital
(grant no. 2023B05).
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Facciorusso A: Drug-eluting beads
transarterial chemoembolization for hepatocellular carcinoma:
Current state of the art. World J Gastroenterol. 24:161–169. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB, Marrero JA, Rudolph L and
Reddy KR: Diagnosis and treatment of hepatocellular carcinoma.
Gastroenterology. 134:1752–1763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen RR, Zhou AY, Kim E, O'Connell JT,
Hagerstrand D, Beroukhim R and Hahn WC: TRAF2 is an
NF-κB-activating oncogene in epithelial cancers. Oncogene.
34:209–216. 2015. View Article : Google Scholar
|
|
5
|
Sunami Y, Ringelhan M, Kokai E, Lu M,
O'Connor T, Lorentzen A, Weber A, Rodewald AK, Müllhaupt B,
Terracciano L, et al: Canonical NF-κB signaling in hepatocytes acts
as a tumor-suppressor in hepatitis B virus surface antigen-driven
hepatocellular carcinoma by controlling the unfolded protein
response. Hepatology. 63:1592–1607. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang X, Yao J, Cui D, Zheng W, Liu Y, Lou
G, Ye B, Shui L, Sun Y, Zhao Y and Zheng M: The TRAF2-p62 axis
promotes proliferation and survival of liver cancer by activating
mTORC1 pathway. Cell Death Differ. 30:1550–1562. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tian Y, Kuo CF, Sir D, Wang L,
Govindarajan S, Petrovic LM and Ou JH: Autophagy inhibits oxidative
stress and tumor suppressors to exert its dual effect on
hepatocarcinogenesis. Cell Death Differ. 22:1025–1034. 2015.
View Article : Google Scholar :
|
|
8
|
Chang HM and Yeh ETHH: SUMO: From bench to
bedside. Physiol Rev. 100:1599–1619. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seeler JS and Dejean A: SUMO and the
robustness of cancer. Nat Rev Cancer. 17:184–197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eifler K and Vertegaal ACO:
SUMOylation-mediated regulation of cell cycle progression and
cancer. Trends Biochem Sci. 40:779–793. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Souza RF, Yin J, Smolinski KN, Zou TT,
Wang S, Shi YQ, Rhyu MG, Cottrell J, Abraham JM, Biden K, et al:
Frequent mutation of the E2F-4 cell cycle gene in primary human
gastrointestinal tumors. Cancer Res. 57:2350–2353. 1997.PubMed/NCBI
|
|
12
|
Wang D, Russell JL and Johnson DG: E2F4
and E2F1 have similar proliferative properties but different
apoptotic and oncogenic properties in vivo. Mol Cell Biol.
20:3417–3424. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwemmle S and Pfeifer GP: Genomic
structure and mutation screening of the E2F4 gene in human tumors.
Int J Cancer. 86:672–677. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zamani-Ahmadmahmudi M, Najafi A and
Nassiri SM: Reconstruction of canine diffuse large B-cell lymphoma
gene regulatory network: Detection of functional modules and hub
genes. J Comp Pathol. 152:119–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng C, Varn FS and Marsit CJ: E2F4
program is predictive of progression and intravesical immunotherapy
efficacy in bladder cancer. Mol Cancer Res. 13:1316–1324. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Molina-Privado I, Jiménez-P R,
Montes-Moreno S, Chiodo Y, Rodríguez-Martínez M, Sánchez-Verde L,
Iglesias T, Piris MA and Campanero MR: E2F4 plays a key role in
Burkitt lymphoma tumorigenesis. Leukemia. 26:2277–2285. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rakha EA, Pinder SE, Paish EC, Robertson
JF and Ellis IO: Expression of E2F-4 in invasive breast carcinomas
is associated with poor prognosis. J Pathol. 203:754–761. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao W, Wang J, Wang X, Cai S, Guo Y, Ye
L, Li D, Hu A, Jin S, Yuan B, et al: Therapeutic targeting of the
USP2-E2F4 axis inhibits autophagic machinery essential for zinc
homeostasis in cancer progression. Autophagy. 18:2615–2635. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong J, Fan H, Deng J and Zhang Q: LncRNA
HAND2-AS1 represses cervical cancer progression by interaction with
transcription factor E2F4 at the promoter of C16orf74. J Cell Mol
Med. 24:6015–6027. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paquin MC, Leblanc C, Lemieux E, Bian B
and Rivard N: Functional impact of colorectal cancer-associated
mutations in the transcription factor E2F4. Int J Oncol.
43:2015–2022. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng Y, Li L, Du Y, Peng X and Chen F:
E2F4 functions as a tumour suppressor in acute myeloid leukaemia
via inhibition of the MAPK signalling pathway by binding to EZH2. J
Cell Mol Med. 24:2157–2168. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garneau H, Paquin MC, Carrier JC and
Rivard N: E2F4 expression is required for cell cycle progression of
normal intestinal crypt cells and colorectal cancer cells. J Cell
Physiol. 221:350–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hlady RA, Sathyanarayan A, Thompson JJ,
Zhou D, Wu Q, Pham K, Lee JH, Liu C and Robertson KD: Integrating
the epigenome to identify drivers of hepatocellular carcinoma.
Hepatology. 69:639–652. 2019. View Article : Google Scholar
|
|
24
|
Cancer Genome Atlas Research Network:
Electronic address: simplewheeler@bcm.edu; Cancer
Genome Atlas Research Network: Comprehensive and integrative
genomic characterization of hepatocellular carcinoma. Cell.
169:1327–1341.e23. 2017. View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Jiang G, Zheng L, Pu J, Mei H, Zhao J,
Huang K, Zeng F and Tong Q: Small RNAs targeting transcription
start site induce heparanase silencing through interference with
transcription initiation in human cancer cells. PLoS One.
7:e313792012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fang E, Wang X, Wang J, Hu A, Song H, Yang
F, Li D, Xiao W, Chen Y, Guo Y, et al: Therapeutic targeting of
YY1/MZF1 axis by MZF1-uPEP inhibits aerobic glycolysis and
neuroblastoma progression. Theranostics. 10:1555–1571. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li H, Yang F, Hu A, Wang X, Fang E, Chen
Y, Li D, Song H, Wang J, Guo Y, et al: Therapeutic targeting of
circ-CUX1/EWSR1/MAZ axis inhibits glycolysis and neuroblastoma
progression. EMBO Mol Med. 11:e108352019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fang E, Wang X, Yang F, Hu A, Wang J, Li
D, Song H, Hong M, Guo Y, Liu Y, et al: Therapeutic targeting of
MZF1-AS1/PARP1/E2F1 axis inhibits proline synthesis and
neuroblastoma progression. Adv Sci (Weinh). 6:19005812019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou Y, Zhou B, Pache L, Chang M,
Khodabakhshi AH, Tanaseichuk O, Benner C and Chanda SK: Metascape
provides a biologist-oriented resource for the analysis of
systems-level datasets. Nat Commun. 10:15232019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang Z, Kang B, Li C, Chen T and Zhang Z:
GEPIA2: An enhanced web server for large-scale expression profiling
and interactive analysis. Nucleic Acids Res. 47(W1): W556–W560.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu
JS, Li B and Liu XS: TIMER: A web server for comprehensive analysis
of tumor-infiltrating immune cells. Cancer Res. 77:e108–e110. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Q, Li F, Zhong C, Zou Y, Li Z, Gao Y,
Zou Q, Xia Y, Wang K and Shen F: Inflammation score system using
preoperative inflammatory markers to predict prognosis for
hepatocellular carcinoma after hepatectomy: A cohort study. J
Cancer. 11:4947–4956. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang Y, Sun A, Zhao Y, Ying W, Sun H,
Yang X, Xing B, Sun W, Ren L, Hu B, et al: Proteomics identifies
new therapeutic targets of early-stage hepatocellular carcinoma.
Nature. 567:257–261. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Clark DJ, Dhanasekaran SM, Petralia F, Pan
J, Song X, Hu Y, da Veiga Leprevost F, Reva B, Lih TSM, Chang HY,
et al: Integrated proteogenomic characterization of clear cell
renal cell carcinoma. Cell. 180:2072020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Du Y, Hou G, Zhang H, Dou J, He J, Guo Y,
Li L, Chen R, Wang Y, Deng R, et al: SUMOylation of the m6A-RNA
methyltransferase METTL3 modulates its function. Nucleic Acids Res.
46:5195–5208. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bogachek MV, Park JM, De Andrade JP,
Lorenzen AW, Kulak MV, White JR, Gu VW, Wu VT and Weigel RJ:
Inhibiting the SUMO pathway represses the cancer stem cell
population in breast and colorectal carcinomas. Stem Cell Reports.
7:1140–1151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He X, Riceberg J, Soucy T, Koenig E,
Minissale J, Gallery M, Bernard H, Yang X, Liao H, Rabino C, et al:
Probing the roles of SUMOylation in cancer cell biology by using a
selective SAE inhibitor. Nat Chem Biol. 13:1164–1171. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hay RT: SUMO: A history of modification.
Mol Cell. 18:1–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gareau JR and Lima CD: The SUMO pathway:
Emerging mechanisms that shape specificity, conjugation and
recognition. Nat Rev Mol Cell Biol. 11:861–871. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Geiss-Friedlander R and Melchior F:
Concepts in sumoylation: A decade on. Nat Rev Mol Cell Biol.
8:947–956. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu B, Swatkoski S, Holly A, Lee LC, Giroux
V, Lee CS, Hsu D, Smith JL, Yuen G, Yue J, et al: Oncogenesis
driven by the Ras/Raf pathway requires the SUMO E2 ligase Ubc9.
Proc Natl Acad Sci USA. 112:E1724–E1733. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang J, Tan GL, Jiang M, Wang TS, Liu GH,
Xiong SS and Qing X: Effects of SENP1-induced deSUMOylation of
STAT1 on proliferation and invasion in nasopharyngeal carcinoma.
Cell Signal. 101:1105302023. View Article : Google Scholar
|
|
44
|
Wang Z, Pan B, Su L, Yu H, Wu X, Yao Y,
Zhang X, Qiu J and Tang N: SUMOylation inhibitors activate
anti-tumor immunity by reshaping the immune microenvironment in a
preclinical model of hepatocellular carcinoma. Cell Oncol (Dordr).
47:513–532. 2024. View Article : Google Scholar
|
|
45
|
Wang B, Li X, Zhao G, Yan H, Dong P,
Watari H, Sims M, Li W, Pfeffer LM, Guo Y and Yue J: miR-203
inhibits ovarian tumor metastasis by targeting BIRC5 and
attenuating the TGFβ pathway. J Exp Clin Cancer Res. 37:2352018.
View Article : Google Scholar
|
|
46
|
Kelly RJ, Lopez-Chavez A, Citrin D, Janik
JE and Morris JC: Impacting tumor cell-fate by targeting the
inhibitor of apoptosis protein survivin. Mol Cancer. 10:352011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu R, Lin L, Zhang B, Wang J, Zhao F, Liu
X and Li Y and Li Y: Identification of prognostic markers for
hepatocellular carcinoma based on the epithelial-mesenchymal
transition-related gene BIRC5. BMC Cancer. 21:6872021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tian LL, Qian B, Jiang XH, Liu YS, Chen T,
Jia CY, Zhou YL, Liu JB, Ma YS, Fu D and Ding ST: MicroRNA-497-5p
is downregulated in hepatocellular carcinoma and associated with
tumorigenesis and poor prognosis in patients. Int J Genomics.
2021:66703902021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang M, Yan X, Wen P, Bai W and Zhang Q:
CircANKRD52 promotes the tumorigenesis of hepatocellular carcinoma
by sponging miR-497-5p and upregulating BIRC5 expression. Cell
Transplant. 30:96368972110088742021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang C, Zhao L, Leng L, Zhou Q, Zhang S,
Gong F, Xie P and Lin G: CDCA8 regulates meiotic spindle assembly
and chromosome segregation during human oocyte meiosis. Gene.
741:1444952020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yamanaka Y, Heike T, Kumada T, Shibata M,
Takaoka Y, Kitano A, Shiraishi K, Kato T, Nagato M, Okawa K, et al:
Loss of Borealin/DasraB leads to defective cell proliferation, p53
accumulation and early embryonic lethality. Mech Dev. 125:441–450.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cui Y and Jiang N: CDCA8 facilitates tumor
proliferation and predicts a poor prognosis in hepatocellular
carcinoma. Appl Biochem Biotechnol. 196:1481–1492. 2024. View Article : Google Scholar
|
|
53
|
Nielsen CF, Zhang T, Barisic M, Kalitsis P
and Hudson DF: Topoisomerase IIα is essential for maintenance of
mitotic chromosome structure. Proc Natl Acad Sci USA.
117:12131–12142. 2020. View Article : Google Scholar
|
|
54
|
Zhong W, Yang Y, Zhang A, Lin W, Liang G,
Ling Y, Zhong J, Yong J, Liu Z, Tian Z, et al: Prognostic and
predictive value of the combination of TOP2A and HER2 in
node-negative tumors 2 cm or smaller (T1N0) breast cancer. Breast
Cancer. 27:1147–1157. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shen S, Kong J, Qiu Y, Yang X, Wang W and
Yan L: Identification of core genes and outcomes in hepatocellular
carcinoma by bioinformatics analysis. J Cell Biochem.
120:10069–10081. 2019. View Article : Google Scholar
|
|
56
|
Gao X, Wang X and Zhang S: Bioinformatics
identification of crucial genes and pathways associated with
hepatocellular carcinoma. Biosci Rep. 38:BSR201814412018.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Meng J, Wei Y, Deng Q, Li L and Li X:
Study on the expression of TOP2A in hepatocellular carcinoma and
its relationship with patient prognosis. Cancer Cell Int.
22:292022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang K, Jiang X, Jiang Y, Liu J, Du Y,
Zhang Z, Li Y, Zhao X, Li J and Zhang R: EZH2-H3K27me3-mediated
silencing of mir-139-5p inhibits cellular senescence in
hepatocellular carcinoma by activating TOP2A. J Exp Clin Cancer
Res. 42:3202023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhao HC, Chen CZ, Tian YZ, Song HQ, Wang
XX, Li YJ, He JF and Zhao HL: CD168+ macrophages promote
hepatocellular carcinoma tumor stemness and progression through
TOP2A/β-catenin/YAP1 axis. iScience. 26:1068622023. View Article : Google Scholar
|
|
60
|
Yang J, Song K, Krebs TL, Jackson MW and
Danielpour D: Rb/E2F4 and Smad2/3 link survivin to TGF-beta-induced
apoptosis and tumor progression. Oncogene. 27:5326–5338. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zwicker J, Lucibello FC, Wolfraim LA,
Gross C, Truss M, Engeland K and Müller R: Cell cycle regulation of
the cyclin A, cdc25C and cdc2 genes is based on a common mechanism
of transcriptional repression. EMBO J. 14:4514–4522. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ikeda MA, Jakoi L and Nevins JR: A unique
role for the Rb protein in controlling E2F accumulation during cell
growth and differentiation. Proc Natl Acad Sci USA. 93:3215–3220.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
van der Sman J, Thomas NS and Lam EW:
Modulation of E2F complexes during G0 to S phase transition in
human primary B-lymphocytes. J Biol Chem. 274:12009–12016. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Aksoy O, Chicas A, Zeng T, Zhao Z,
McCurrach M, Wang X and Lowe SW: The atypical E2F family member
E2F7 couples the p53 and RB pathways during cellular senescence.
Genes Dev. 26:1546–1557. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zheng Q, Fu Q, Xu J, Gu X, Zhou H and Zhi
C: Transcription factor E2F4 is an indicator of poor prognosis and
is related to immune infiltration in hepatocellular carcinoma. J
Cancer. 12:1792–1803. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Huang YL, Ning G, Chen LB, Lian YF, Gu YR,
Wang JL, Chen DM, Wei H and Huang YH: Promising diagnostic and
prognostic value of E2Fs in human hepatocellular carcinoma. Cancer
Manag Res. 11:1725–1740. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu J, Xia L, Wang S, Cai X, Wu X, Zou C,
Shan B, Luo M and Wang D: E2F4 promotes the proliferation of
hepatocellular carcinoma cells through upregulation of CDCA3. J
Cancer. 12:5173–5180. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wei J, Shi Y, Zou C, Zhang H, Peng H, Wang
S, Xia L, Yang Y, Zhang X, Liu J, et al: Cellular Id1 inhibits
hepatitis B virus transcription by interacting with the novel
covalently closed circular DNA-binding protein E2F4. Int J Biol
Sci. 18:65–81. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Korenjak M and Brehm A: E2F-Rb complexes
regulating transcription of genes important for differentiation and
development. Curr Opin Genet Dev. 15:520–527. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lewis PW, Beall EL, Fleischer TC,
Georlette D, Link AJ and Botchan MR: Identification of a Drosophila
Myb-E2F2/RBF transcriptional repressor complex. Genes Dev.
18:2929–2940. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Korenjak M, Taylor-Harding B, Binné UK,
Satterlee JS, Stevaux O, Aasland R, White-Cooper H, Dyson N and
Brehm A: Native E2F/RBF complexes contain Myb-interacting proteins
and repress transcription of developmentally controlled E2F target
genes. Cell. 119:181–193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Litovchick L, Sadasivam S, Florens L, Zhu
X, Swanson SK, Velmurugan S, Chen R, Washburn MP, Liu XS and
DeCaprio JA: Evolutionarily conserved multisubunit RBL2/p130 and
E2F4 protein complex represses human cell cycle-dependent genes in
quiescence. Mol Cell. 26:539–551. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sadasivam S, Duan S and DeCaprio JA: The
MuvB complex sequentially recruits B-Myb and FoxM1 to promote
mitotic gene expression. Genes Dev. 26:474–489. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Fischer M, Grossmann P, Padi M and
DeCaprio JA: Integration of TP53, DREAM, MMB-FOXM1 and RB-E2F
target gene analyses identifies cell cycle gene regulatory
networks. Nucleic Acids Res. 44:6070–6086. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sadasivam S and DeCaprio JA: The DREAM
complex: Master coordinator of cell cycle-dependent gene
expression. Nat Rev Cancer. 13:585–595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
MacDonald J, Ramos-Valdes Y, Perampalam P,
Litovchick L, DiMattia GE and Dick FA: A systematic analysis of
negative growth control implicates the DREAM complex in cancer cell
dormancy. Mol Cancer Res. 15:371–381. 2017. View Article : Google Scholar
|
|
77
|
Wang L and Liu X: Comprehensive analysis
of the expression and prognosis for the DREAM complex in human
cancers. Front Genet. 13:8147252022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tan Z, Chen M, Peng F, Yang P, Peng Z,
Zhang Z, Li X, Zhu X, Zhang L, Zhao Y and Liu Y: E2F1 as a
potential prognostic and therapeutic biomarker by affecting tumor
development and immune microenvironment in hepatocellular
carcinoma. Transl Cancer Res. 11:2713–2732. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ito Y, Miyoshi E, Takeda T, Sakon M, Noda
K, Tsujimoto M, Monden M, Taniguchi N and Matsuura N: Expression
and possible role of ets-1 in hepatocellular carcinoma. Am J Clin
Pathol. 114:719–725. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yan Y, Zheng L, Du Q, Yan B and Geller DA:
Interferon regulatory factor 1 (IRF-1) and IRF-2 regulate PD-L1
expression in hepatocellular carcinoma (HCC) cells. Cancer Immunol
Immunother. 69:1891–1903. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Qu LH, Fang Q, Yin T, Yi HM, Mei GB, Hong
ZZ, Qiu XB, Zhou R and Dong HF: Comprehensive analyses of
prognostic biomarkers and immune infiltrates among histone lysine
demethylases (KDMs) in hepatocellular carcinoma. Cancer Immunol
Immunother. 71:2449–2467. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Nakajima T, Yasui K, Zen K, Inagaki Y,
Fujii H, Minami M, Tanaka S, Taniwaki M, Itoh Y, Arii S, et al:
Activation of B-Myb by E2F1 in hepatocellular carcinoma. Hepatol
Res. 38:886–895. 2008. View Article : Google Scholar
|
|
83
|
Chen Q, Wang L, Jiang M, Huang J, Jiang Z,
Feng H and Ji Z: E2F1 interactive with BRCA1 pathway induces HCC
two different small molecule metabolism or cell cycle regulation
via mitochondrion or CD4+T to cytosol. J Cell Physiol.
233:1213–1221. 2018. View Article : Google Scholar
|
|
84
|
Arakawa Y, Kajino K, Kano S, Tobita H,
Hayashi J, Yasen M, Moriyama M, Arakawa Y and Hino O: Transcription
of dbpA, a Y box binding protein, is positively regulated by E2F1:
Implications in hepatocarcinogenesis. Biochem Biophys Res Commun.
322:297–302. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Chen YL, Uen YH, Li CF, Horng KC, Chen LR,
Wu WR, Tseng HY, Huang HY, Wu LC and Shiue YL: The E2F
transcription factor 1 transactives stathmin 1 in hepatocellular
carcinoma. Ann Surg Oncol. 20:4041–4054. 2013. View Article : Google Scholar
|
|
86
|
Huang Y, Tai AW, Tong S and Lok AS: HBV
core promoter mutations promote cellular proliferation through
E2F1-mediated upregulation of S-phase kinase-associated protein 2
transcription. J Hepatol. 58:1068–1073. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Farra R, Grassi G, Tonon F, Abrami M,
Grassi M, Pozzato G, Fiotti N, Forte G and Dapas B: The role of the
transcription factor E2F1 in hepatocellular carcinoma. Curr Drug
Deliv. 14:272–281. 2017.
|
|
88
|
Sun HX, Xu Y, Yang XR, Wang WM, Bai H, Shi
RY, Nayar SK, Devbhandari RP, He YZ, Zhu QF, et al: Hypoxia
inducible factor 2 alpha inhibits hepatocellular carcinoma growth
through the transcription factor dimerization partner 3/E2F
transcription factor 1-dependent apoptotic pathway. Hepatology.
57:1088–1097. 2013. View Article : Google Scholar
|
|
89
|
Choi M, Lee H and Rho HM: E2F1 activates
the human p53 promoter and overcomes the repressive effect of
hepatitis B viral X protein (Hbx) on the p53 promoter. IUBMB Life.
53:309–317. 2002. View Article : Google Scholar
|
|
90
|
Wang H, Chu F, Zhijie L, Bi Q, Lixin L,
Zhuang Y, Xiaofeng Z, Niu X, Zhang D, Xi H and Li BA: MTBP enhances
the activation of transcription factor ETS-1 and promotes the
proliferation of hepatocellular carcinoma cells. Front Oncol.
12:9850822022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Bhagyaraj E, Ahuja N, Kumar S, Tiwari D,
Gupta S, Nanduri R and Gupta P: TGF-β induced chemoresistance in
liver cancer is modulated by xenobiotic nuclear receptor PXR. Cell
Cycle. 18:3589–3602. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Shao Z, Li Y, Dai W, Jia H, Zhang Y, Jiang
Q, Chai Y, Li X, Sun H, Yang R, et al: ETS-1 induces
Sorafenib-resistance in hepatocellular carcinoma cells via
regulating transcription factor activity of PXR. Pharmacol Res.
135:188–200. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Li YH, Lv MF, Lu MS and Bi JP: Bone marrow
mesenchymal stem cell-derived exosomal MiR-338-3p represses
progression of hepatocellular carcinoma by targeting ETS1. J Biol
Regul Homeost Agents. 35:617–627. 2021.PubMed/NCBI
|
|
94
|
Jie Y, Liu G, E M, Li Y, Xu G, Guo J, Li
Y, Rong G, Li Y and Gu A: Novel small molecule inhibitors of the
transcription factor ETS-1 and their antitumor activity against
hepatocellular carcinoma. Eur J Pharmacol. 906:1742142021.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Tamura T, Yanai H, Savitsky D and
Taniguchi T: The IRF family transcription factors in immunity and
oncogenesis. Annu Rev Immunol. 26:535–584. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Li P, Du Q, Cao Z, Guo Z, Evankovich J,
Yan W, Chang Y, Shao L, Stolz DB, Tsung A and Geller DA:
Interferon-γ induces autophagy with growth inhibition and cell
death in human hepatocellular carcinoma (HCC) cells through
interferon-regulatory factor-1 (IRF-1). Cancer Lett. 314:213–222.
2012. View Article : Google Scholar
|
|
97
|
Guo W, Li S, Qian Y, Li L, Wang F, Tong Y,
Li Q, Zhu Z, Gao WQ and Liu Y: KDM6A promotes hepatocellular
carcinoma progression and dictates lenvatinib efficacy by
upregulating FGFR4 expression. Clin Transl Med. 13:e14522023.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Li Y, Yang J, Zhang X, Liu H and Guo J:
KDM6A suppresses hepatocellular carcinoma cell proliferation by
negatively regulating the TGF-β/SMAD signaling pathway. Exp Ther
Med. 20:2774–2782. 2020.PubMed/NCBI
|