Introduction
Cancer remains a major global public health burden,
with incidence and mortality rates rising rapidly. According to
Global Cancer Statistics, nearly 20 million new cases of cancer and
9.7 million cancer-related deaths worldwide were reported in 2022
(1). It is estimated that ~20% of
individuals will develop cancer during their lifetime. Lung cancer
has the highest incidence rate (12.4%), followed by female breast
cancer (BRC) (11.6%), colorectal cancer (CRC) (9.6%), prostate
cancer (7.3%) and gastric cancer (GC) (4.9%) (1). Generally, the initiation and
progression of tumors are caused by genetic or epigenetic
alterations caused by both internal and external factors, which
activate or inhibit specific signaling pathways (2,3).
Therefore, investigating the mechanisms underlying tumorigenesis is
essential for improving the diagnosis, prognosis and the
development of targeted therapies.
The angiopoietin family consists of growth factors
that regulate vascular development, maintenance and remodeling,
playing a pivotal role in angiogenesis. Currently, the angiopoietin
family includes angiopoietin (ANGPT) 1-4 and angiopoietin-like
(ANGPTL) 1-8. ANGPTs act as ligands for the endothelial cell
receptors TIE1 and TIE2 and are crucial in regulating tumor
angiogenesis, inflammation, lymphatic angiogenesis and the
cardiovascular system (4,5). Although ANGPTLs share structural
similarities with ANGPTs, they do not bind to TIE receptors to
mediate their biological functions. The homologous receptors of
ANGPTLs remain unidentified, and they are classified as orphan
ligands (6-9). ANGPTLs, particularly ANGPTL3,
ANGPTL4 and ANGPTL8, have been extensively studied for their roles
in lipid metabolism (10,11). Additionally, ANGPTLs regulate both
acute and chronic inflammation (12) and atherosclerosis (13) through various mechanisms. ANGPTL4,
a member of the angiopoietin family, was initially identified for
its key roles in lipid metabolism (14), inflammatory response (15) and angiogenesis (6,7).
As research has progressed, increasing evidence has demonstrated
that ANGPTL4 is involved in various stages of tumor progression
(8,9).
In the present review, the multifaceted roles of
ANGPTL4 in tumor development and its underlying mechanisms of
action are discussed. While earlier reviews (8,9)
provided a foundational understanding of ANGPTL4, the current
article integrates the latest research and findings, offering a
comprehensive and updated perspective on its functions in cancer
biology.
Biological characteristics of ANGPTL4
In 2000, three research teams successively
identified a new member of the ANGPT family. Kim et al
(16) isolated a new sequence
from human and mouse embryonic cDNA using degeneracy PCR, which was
named hepatic fibrinogen/angiopoietin-related protein (HFARP). Yoon
et al (17) described the
isolation and characterization of a novel target gene induced by
peroxisome proliferator-activated receptor (PPAR) γ ligands, termed
PGAR (for PPAR γ angiopoietin-related); Kersten et al
(18) identified a novel PPARα
target gene called FIAF (fasting-induced adipose factor) through
subtractive hybridization. Subsequently, the gene encoding this
protein was collectively referred to as ANGPTL4 by the HUGO Gene
Nomenclature Committee.
ANGPTL4 is located at 19p13.2, with an mRNA length
of ~2,000 bp and an open reading frame of 1,218 bp. It encodes a
secreted glycoprotein composed of 406 amino acids with a relative
molecular weight of ~45-65 kDa. Structurally similar to other ANGPT
family proteins, ANGPTL4 contains a highly hydrophobic signal
peptide, an N-terminal helical domain with three glycosylation
sites, and a larger C-terminal fibrinogen-like domain, with a small
connecting peptide between these domains. The natural full-length
ANGPTL4 (fANGPTL4) exists as either a dimer or tetramer. It can be
cleaved by furin-like proprotein convertase to yield an N-terminal
coiled-coil fragment (nANGPTL4), containing amino acids 1 to 170,
and a C-terminal fibrinogen-like domain monomer fragment
(cANGPTL4), consisting of amino acids 171 to 406. The cleavage of
fANGPTL4 at the -RXR-site by proprotein convertase depends on the
tissue in which ANGPTL4 is synthesized and the physiological or
pathological conditions (19-21).
In humans, ANGPTL4 exhibits widespread expression,
with notable prominence in the heart, liver, small intestine,
adipose tissue, plasma and placenta, as reported in multiple
studies (22-26). Its expression is regulated by
PPARs (9), glucocorticoid
receptors (27,28), hypoxia-inducible factor-1α
(HIF-1α) (28), transforming
growth factor-β (TGF-β) (29-31) and other regulatory factors.
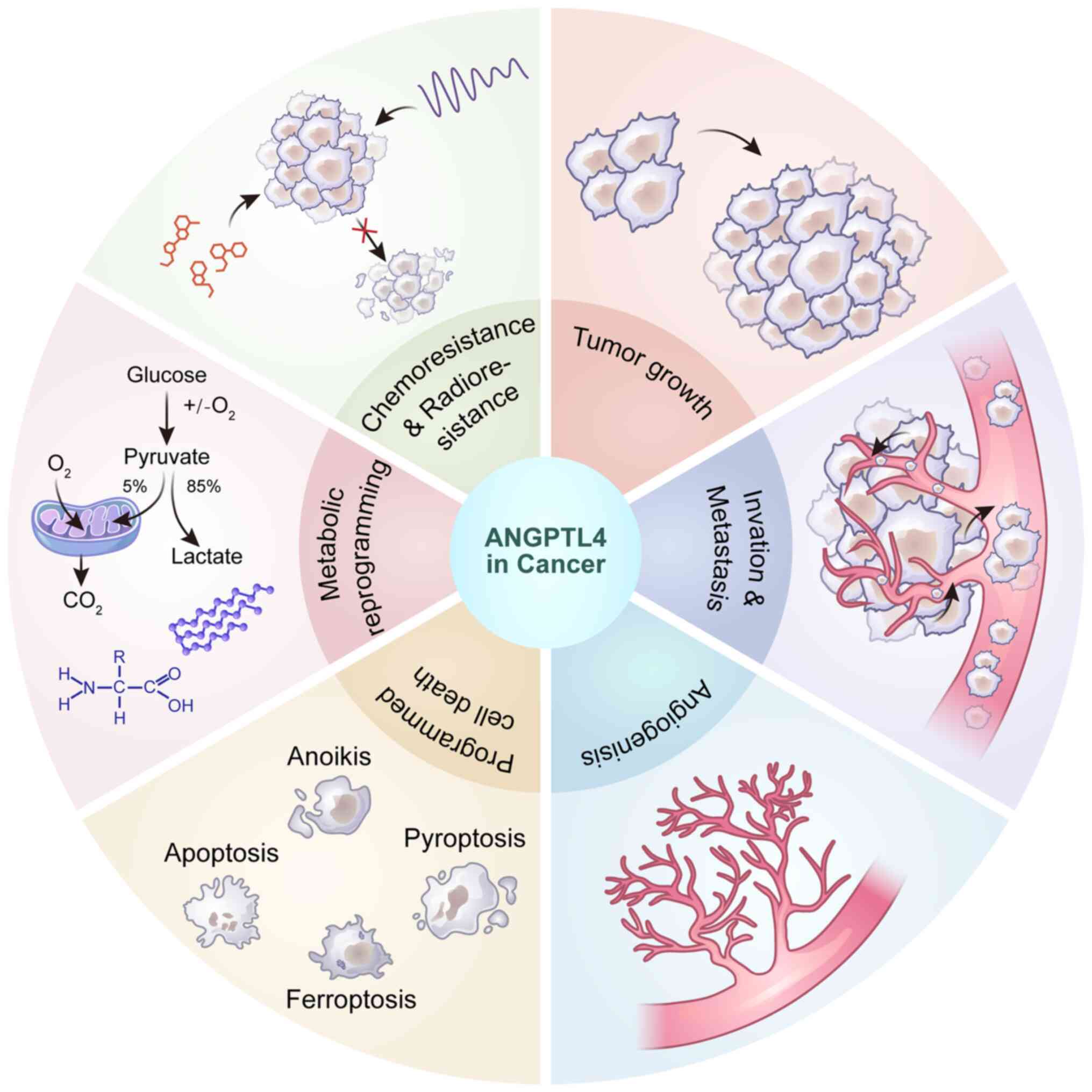
Roles of ANGPTL4 in cancer
Previous findings suggest that ANGPTL4 is commonly
dysregulated in various malignancies, and plays important dual
roles, functioning as either an oncogene or a tumor suppressor
(Fig. 1; Tables I and II). Altered ANGPTL4 expression impacts
diverse cellular phenotypes via multiple signaling pathways,
affecting tumor growth, invasion, metastasis, angiogenesis,
programmed cell death, cell metabolism and treatment resistance
(Fig. 2).
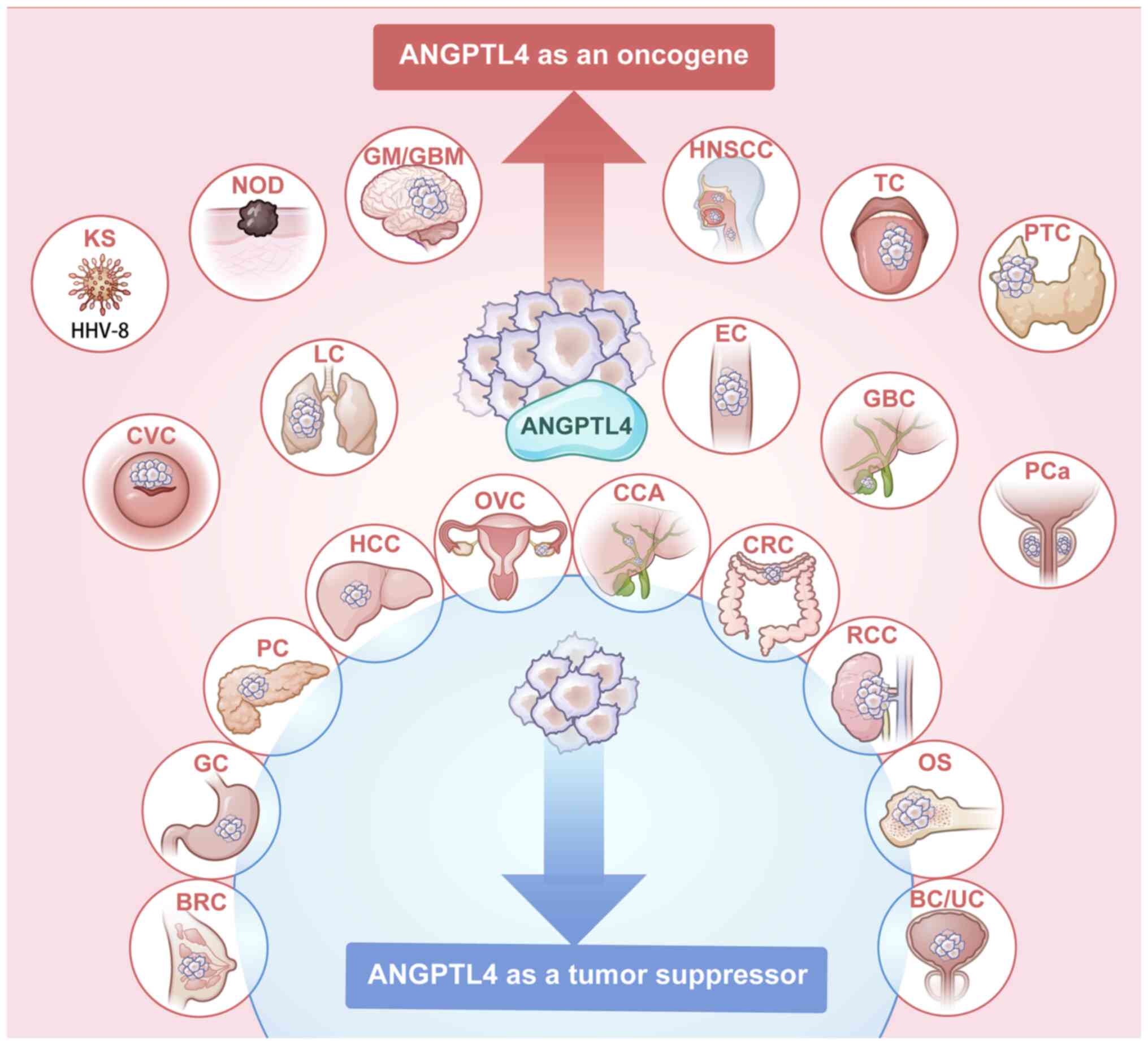 | Figure 1ANGPTL4 plays an oncogenic role in
cancers in red, and plays a tumor-suppressive role in cancers in
blue; ANGPTL4 has controversial roles reported in cancers with both
colors. ANGPTL4, angiopoietin-like 4; KS, Kaposi's sarcoma; NOD,
melanoma; GM, glioma; GBM, glioblastoma; HNSCC, head and neck
squamous cell carcinoma; TC, tongue cancer; PTC, papillary thyroid
cancer; CVC, cervical cancer; RCC, renal cell carcinoma; LC, lung
cancer; EC, esophageal cancer; GBC, gallbladder cancer; PCa,
prostate cancer; BRC, breast cancer; GC, Gastric cancer; PC,
pancreatic cancer; HCC, hepatocellular carcinoma; OVC, ovarian
cancer; CCA, cholangiocarcinoma; CRC, colorectal cancer; OS,
osteosarcoma; BC, bladder cancer; UC, urothelial carcinoma. |
 | Table IANGPTL4 as an oncogene in human
malignancies. |
Table I
ANGPTL4 as an oncogene in human
malignancies.
| First author/s,
year | Cancer type | Upstream
regulators | Downstream
targets | Cellular
phenotypes | (Refs.) |
|---|
| Kolb et al,
2019 | Breast cancer | NLRC4/IL-1β/NF-κB,
MAPK | - | Proliferation,
angiogenesis | (33) |
| Avalle et
al, 2022 | | STAT3 | - | Proliferation,
migration, invasion | (34) |
| Tian et al,
2009 | | PPAR γ | - | Proliferation,
angiogenesis | (145) |
| Zhao et al,
2020 | | - | | Prognosis | (157) |
| Padua et al,
2008 | | TGF-β/Smad | - | Lung
metastasis | (174) |
| Long et al,
2023 | Bladder cancer | - | SDC1 | Prognosis, immune
response | (150) |
| Gong et al,
2019 |
Triple-negative | TGF-β2/Smad | - | Brain
metastasis | (31) |
| Blücher et
al, 2020 | breast cancer | PPARα | FAK | Lipid metabolism,
motility | (133) |
| Adhikary et
al, 2013 | | TGF-β/PPARβ, δ | - | Invasion | (144) |
| Simeon et
al, 2021 | | - | - | Brain, liver
metastases | (175) |
| Zhu et al,
2024 | Colorectal
cancer | TGF-β1/Smad3 | - | Glycolysis, anoikis
resistance, peritoneal metastasis | (30) |
| Ding et al,
2023 | | - |
PKC-LKB1-MAPK-mTOR | Metabolic
reprogramming, proliferation, decreased CD8+ T cell
activation | (32) |
| Wen et al,
2020 | | - | - | Proliferation,
invasion, migration, apoptosis resistance, cisplatin
resistance | (38) |
| Shen et al,
2020 | | - | c-Jun/NOX4 | Metastasis | (55) |
| Li et al,
2015 | | - | BMP7 | Invasion,
migration, apoptosis resistance | (82) |
| Zheng et al,
2021 | | Histone H3 lysine
27 | GLUT-1 | Glycolysis,
Fusobacterium nucleatum colonization | (120) |
| Mizuno et
al, 2022 | | | PI3K/AKT/GLUTs | Glucose
metabolism | (121) |
| Kim et al,
2011 | | PGE2/EP1 | MAPK,
Src/STAT1 | Proliferation | (146) |
| Nakayama et
al, 2011 | | - | - | Venous invasion,
metastasis | (176) |
| Shen et al,
2023 | KRAS/p53 mutant
colorectal cancer | - | IL-8/NOX4 | Metastasis | (56) |
| San et al,
2020 |
Cholangiocarcinoma | - | - | Anoikis
resistance | (91) |
| Aung et al,
2022 | | - | - | Biomarker for
vascular invasion, lymph node metastasis | (159) |
| Nie et al,
2019 | Cervical
cancer | - | - | Prognosis | (158) |
| Shibata et
al, 2010 | Esophageal | - | - | Invasion,
survival | (161) |
| Yi et al,
2013 | cancer | - | - | Prognosis | (177) |
| Chen et al,
2018 | Gastric cancer | - | - | Proliferation,
invasion, apoptosis resistance, cisplatin resistance | (39) |
| Xiao et al,
2024 | | Leptin | LPL/COX-2/PGE2 | Lymph node
metastasis | (60) |
| Nakayama et
al, 2010 | | - | - | Venous
invasion | (178) |
| Baba et al,
2017 | Scirrhous gastric
cancer | HIF-1α | c-Myc, p27;
FAK/Src/PI3K-Akt/ERK | Proliferation, cell
cycle, anoikis resistance, peritoneal metastasis | (90) |
| Katanasaka et
al, 2013 | Glioma |
EGFRvIII/ERK/c-Myc | - | Proliferation,
angiogenesis | (65) |
| Tsai et al,
2019 | Glioblastoma | Sp4 |
EGFR/AKT/4E-BP1 | Temozolomide
resistance | (141) |
| Hefni et al,
2023 | Head and neck
squamous cell carcinoma | - | NRP1/ABL1/PXN | Migration | (58) |
| Chiang et
al, 2020 | |
EGF/COX-2/PGE2/ERK | - | Metastasis | (61) |
| Liao et al,
2017 | | EGF/integrin
β1 | MMP-1 | Anoikis resistance
and metastasis | (87) |
| Shen et al,
2017 | | OA/PPARs | FN/MMP-9 | Anoikis resistance
and metastasis | (88) |
| Bai et al,
2024 | Hepatocellular
carcinoma | - |
BMP7/Smad/MAPK14 | Proliferation,
migration | (40) |
| Bai et al,
2024 | | - | - | Proliferation,
invasion, migration, apoptosis resistance | (41) |
| Bai et al,
2024 | | - | - | Invasion,
migration | (81) |
| Fekir et al,
2019 | | - | PDK4 | Invasion, metabolic
reprogramming, chemoresistance | (138) |
| Li et al,
2011 | | HIF-1α | VCAM1/integrin
β1 | Metastasis | (143) |
| Ma et al,
2010 | Kaposi's
sarcoma | vGPCR | - | Angiogenesis | (179) |
| Izraely et
al, 2018 | Melanoma | TGF-β1 MNK1 | | Brain
metastasis | (46) |
| Yang et al,
2020 | | | MMPs | Invasion, lung
metastasis | (180) |
| Hu et al,
2016 | Uveal melanoma | HIF-1 | - | Angiogenesis | (67) |
| Hu et al,
2023 | Non-small cell lung
cancer | - | - | Proliferation,
invasion, lipid metabolism | (42) |
| Fang et al,
2022 | | - |
NLRP3/ASC/Caspase8 | Pyroptosis and
apoptosis resistance, gefitinib resistance | (43) |
| Hao et al,
2022 | | - | - | Migration, anoikis
resistance | (59) |
| Mo et al,
2020 | | - | - | Migration,
angiogenesis | (68) |
| Zhang et al,
2022 | | - | GPX4, FTH1 | Ferroptosis
resistance, radioresistance | (101) |
| Zhu et al,
2016 | | - | - | Proliferation,
metastasis | (181) |
| Lou et al,
2024 | | - | - | Migration,
apoptosis resistance | (182) |
| Zhang et al,
2018 | Osteosarcoma | HIF-1α | - | Proliferation,
migration | (44) |
| Chen et al,
2020 | | LncRNA
CCAL/miR-29b | - | Angiogenesis | (69) |
| Li et al,
2024 | Ovarian cancer | - | JAK2/STAT3;
ESM1 | Proliferation,
angiogenesis, lipid metabolism | (35) |
| Xu et al,
2024 | | - | ERK1/2 | Proliferation,
migration | (36) |
| Wu et al,
2021 | | - | VEGFR2 | Proliferation,
metastasis, Angiogenesis | (37) |
| Bajwa et al,
2023 | | - | - | Proliferation, cell
adhesion, migration, metastasis, glycolysis | (57) |
| Li et al,
2022 | | TAZ | SOX2 | Cisplatin
resistance | (139) |
| Zhou et al,
2020 | | - | c-Myc/NF-κ B | Carboplatin
resistance | (140) |
| Al-Kadash et
al, 2024 | Pancreatic
cancer | - | ITGB4, APOL1 | Gemcitabine
resistance | (183) |
| Yang et al,
2020 | Papillary thyroid
cancer | - | AKT | Proliferation | (45) |
| Hata et al,
2017 | Prostate
cancer | - | - | Proliferation | (184) |
| Dong et al,
2017 | Renal cell
carcinoma | - | - | Prognosis | (163) |
| Tanaka et
al, 2022 | Tongue cancer | - | - | Prognosis | (162) |
| Tanaka et
al, 2016 | | - | - | Lung
metastasis | (185) |
| Hsieh et al,
2018 | Urothelial
carcinoma | - | ERK/FAK | Proliferation,
migration | (47) |
 | Table IIANGPTL4 as a tumor suppressor in
human malignancies. |
Table II
ANGPTL4 as a tumor suppressor in
human malignancies.
| First author/s,
year | Cancer type | Upstream
regulators | Downstream
targets | Cellular
phenotypes | (Refs.) |
|---|
| Xu et al,
2023 | Colorectal
cancer |
STAT2/linc0223/hnRNPA1 | - | Angiogenesis | (52) |
| Zhang et al,
2021 | | - | ERK | Proliferation,
invasion, migration, metastasis | (63) |
| Yu et al,
2018 |
Cholangiocarcinoma | lncRNA PVT1 | - | Proliferation,
migration | (186) |
| Qian et al,
2019 | Gastric cancer | LMX1A | c-Myc | Tumorigenesis | (50) |
| Kubo et al,
2016 | | - | - | Prognosis | (164) |
| Ng et al,
2014 | Hepatocellular
carcinoma | - | - | Apoptosis,
angiogenesis | (72) |
| Lin et al,
2022 | Osteosarcoma | - | BCAAs/mTOR | Amino acid
metabolism, proliferation | (49) |
| Yang et al,
2020 | Ovarian cancer | TAZ | NOX2 | Ferroptosis,
chemoresistance | (102) |
| Hui et al,
2019 | Pancreatic
cancer | lncRNA
AGAP2-AS1/EZH2 | - | Proliferation,
migration | (48) |
| Jin et al,
2024 | Renal cell
carcinoma | | LAL | Proliferation | (51) |
| Cai et al,
2020 | Triple-negative
breast cancer | - | - | Adhesion, invasion,
migration | (62) |
| Hsieh et al,
2018 | Urothelial
carcinoma | - | - | Proliferation,
invasion, migration | (47) |
ANGPTL4 and tumor growth
ANGPTL4 exhibits varied regulatory effects across
different types of tumors. Ding et al (32) demonstrated that the recombinant
ANGPTL4 protein reduces CD8+ T cell infiltration and
activation through metabolic reprogramming, thereby diminishing
immune surveillance during tumor progression and facilitating tumor
growth in vivo. In breast tumors of obese mice, ANGPTL4
expression has been shown upregulated, and the suppression of
ANGPTL4 leads to a significant reduction in obesity-induced tumor
growth (33). Interleukin-1β
(IL-1β) in primary adipocytes stimulates ANGPTL4 expression through
the activation of nuclear factor-κ B (NF-κB) and mitogen-activated
protein kinase (MAPK) pathways, with hypoxia further enhancing
IL-1β expression (33). This
indicates that ANGPTL4 mediates the crosstalk between
obesity-associated inflammation and BRC progression. Avalle et
al (34) found that
cancer-associated fibroblasts (CAFs) promote tumor growth in BRC,
with signal transducer and activator of transcription 3 (STAT3)
amplifying the effects of CAFs via ANGPTL4. In ovarian cancer
(OVC), ANGPTL4 was significantly upregulated in clinical samples
and correlated with poor prognosis (35). ANGPTL4 promotes OVC progression by
activating the Janus kinase 2 (JAK2)/STAT3 pathway (35). Xu et al (36) further demonstrated that ANGPTL4
enhances ovarian tumor cell proliferation through the extracellular
signal-related kinase (ERK) pathway, and its inhibition can
suppress OVC progression via phosphorylation of vascular
endothelial growth factor receptor 2 (VEGFR2) (37). Additionally, several studies have
shown that ANGPTL4 accelerates the proliferation and progression of
numerous types of malignancies, including CRC (38), GC (39), hepatocellular carcinoma (HCC)
(40,41), lung adenocarcinoma (42,43), osteosarcoma (OS) (44), thyroid cancer (45) and melanoma (46).
However, some studies suggest that ANGPTL4 may
function as a tumor suppressor. Hsieh et al (47) reported the dual roles of ANGPTL4
in urothelial carcinoma (UC), showing that ANGPTL4 mRNA expression
is decreased in UC cells and tumor tissues compared with adjacent
normal bladder epithelial cells. Overexpression of ANGPTL4 inhibits
UC cell proliferation both in vivo and in vitro
(47). Hui et al (48) found that long non-coding RNA
(lncRNA) AGAP2-AS1 downregulates ANGPTL4 expression through its
interaction with the enhancer of zeste homolog 2 (EZH2), thereby
promoting the proliferation and metastasis of pancreatic cancer. A
negative correlation between the expression of ANGPTL4 and OS
progression has also been observed (49). Knocking out ANGPTL4 in OS cells
leads to the accumulation of branched-chain amino acids (BCAAs),
which activates the mechanistic target of rapamycin (mTOR)
signaling pathway and enhances OS cell proliferation (49). In addition, homeobox transcription
factor 1α (LMX1A) has been shown to suppress tumor growth by
activating ANGPTL4 to hinder c-Myc in GC (50). The negative regulatory effects of
ANGPTL4 on tumor growth have also been reported in renal clear cell
carcinoma (51) and CRC (52).
ANGPTL4 and tumor invasion and
metastasis
Metastasis refers to the spread of malignant cells
to distant organs, and it is often the primary cause of mortality
in most cancers, as metastatic cancer cells typically exhibit high
invasiveness and resistance to anticancer therapies. It has been
indicated that ANGPTL4 plays a crucial role in cancer invasion and
metastasis processes.
Hübers et al (53) identified cANGPTL4 and nANGPTL4 as
pro- and antitumor contributors, respectively, in the bidirectional
communication between primary tumors and distant metastases. It was
observed that cANGPTL4 promotes tumor growth and metastasis, while
nANGPTL4 inhibits metastasis and improves overall survival by
suppressing Wnt signaling and reducing vascular distribution at
metastasis sites. These findings suggested that cANGPTL4 could
serve as a potential biomarker for tumor progression and a target
for anti-meta-static therapy.
In BRC, HIF-2 induces the expression of the lncRNA
RAB11B-AS1, which facilitates brain metastasis through the
promotion of ANGPTL4 (54).
Silencing ANGPTL4 in triple-negative BRC (TNBC) cells has been
shown to reduce the metastatic growth of brain tumors in
vivo (31). Tumor cells
secrete IL-1β and tumor necrosis factor-α (TNF-α), which
communicate with astrocytes, leading to increased expression of
transforming growth factor-β2 (TGF-β2) and promoting the brain
metastasis via the TGF-β2/ANGPTL4 axis (31). These findings provide a
theoretical basis for targeting ANGPTL4 in the treatment of BRC
metastasis.
In CRC, ANGPTL4 plays a role in metastasis through
various mechanisms. Shen et al (55) found that NADPH oxidase 4
(NOX4)/reactive oxygen species (ROS) axis is crucial for CRC
metastasis induced by oleic acid (OA). Downregulation of ANGPTL4
leads to the suppression of NOX4, ROS, matrix metalloproteinase-1
(MMP-1) and MMP-9, thus inhibiting OA-induced CRC metastasis
(55). Furthermore, the
metastasis in KRAS/p53 mutant CRC has been shown to depend on the
activation of the ANGPTL4/IL-8/NOX4 axis (56), underscoring the importance of the
ANGPTL4/NOX4 signaling axis in dyslipidemia-related and KRAS/p53
mutant CRC metastasis. Zhu et al (30) focused on the peritoneal metastasis
of CRC. They reported that adipose-derived stem cells (ADSCs)
secrete TGF-β1 to activate Smad3 in CRC cells, which enhances
ANGPTL4 transcription. The upregulation of ANGPTL4 increases the
anoikis resistance, facilitating CRC cell survival in the
peritoneum and promoting metastatic foci formation (30).
Bajwa et al (57) reported that ANGPTL4, derived from
cancer-associated mesothelial cells, promotes the early-stage OVC
metastasis through the interactions between mesothelial cells and
the tumor microenvironment (TME). Additionally, Hefni et al
(58) found that ANGPTL4 induces
the migration of head and neck squamous cell carcinoma (HNSCC)
cells via the neuropilin1/ABL1/paxillin pathway. Similarly, ANGPTL4
has been implicated in the invasion and metastasis of lung cancer
(42,59), with studies showing that modified
Bu Fei decoction inhibits the metastasis of non-small cell lung
cancer (NSCLC) by suppressing ANGPTL4 expression in endothelial
cells (59).
Previous studies have highlighted the pivotal role
of the cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2) pathway in
promoting metastasis through ANGPTL4. In GC, leptin induces the
phosphorylation of ANGPTL4 at the serine 30 residue, reducing its
binding affinity to lipoprotein lipase (LPL), thus enhancing lipid
uptake and intracellular arachidonic acid levels. This accumulation
subsequently activates the COX-2/PGE2 pathway, promoting lymph node
metastasis (60). Moreover,
Chiang et al (61)
demonstrated that the COX-2/PGE2/ANGPTL4 axis, activated by
epidermal growth factor (EGF), enhances metastasis in HNSCC, with
PGE2 promoting ANGPTL4 expression through the ERK pathway.
Despite the general role of ANGPTL4 in promoting
metastasis, some studies have identified its inhibitory effects.
Overexpression of ANGPTL4 has been found to inhibit the adhesion,
migration and invasion of TNBC cells in vitro, with positive
correlations to favorable outcomes in patients with TNBC (62). Moreover, lncRNA AGAP2-AS1
epigenetically inhibits ANKRD1 and ANGPTL4 expression by recruiting
EZH2, promoting pancreatic cancer metastasis (48). In CRC, DNA methylation-mediated
downregulation of ANGPTL4 activates CAFs in the TME, promoting
epithelial-mesenchymal transition (EMT) through the ERK signaling
pathway, which leads to metastasis (63). In vivo experiments also
revealed that overexpression of ANGPTL4 inhibits lung metastasis in
CRC models (63). Additionally,
inhibitory effects of ANGPTL4 on tumor metastasis have been also
observed in OS (64).
ANGPTL4 and tumor angiogenesis
Angiogenesis and increased vascular permeability are
ubiquitous characteristics of all solid tumors. Therefore, research
on the role of ANGPTL4 in cancer angiogenesis holds significant
potential to profoundly enhance our comprehension of cancer
pathogenesis and inform the development of therapeutic
strategies.
Wu et al (37) found that the pro-angiogenic
effects of ANGPTL4 in OVC are mediated via its association with
VEGF2. Similarly, Li et al (35) discovered that ANGPTL4 enhances
angiogenesis in ovarian serous cystadenocarcinoma by activating the
JAK2/STAT3 signaling pathway. The mechanism is potentially due to
the interaction among ANGPTL4, endothelial cell specific molecule 1
and the TME (35). ANGPTL4 has
also been reported to exert pro-angiogenic effects in malignant
glioma cells (65). Knockdown of
ANGPTL4 significantly reduces microvascular density in xenograft
tumors and inhibits tumor growth (65). Additionally, EGF receptor (EGFR)
variant III induces ANGPTL4 expression through the ERK/c-Myc
pathway to regulate angiogenesis (65). In Kaposi's sarcoma, the
upregulation of ANGPTL4 by viral G protein-coupled receptors has
been shown to enhance tumor angiogenesis and vascular permeability
through the Rho/Rho-associated kinase pathway (66). The complementary effect on VEGF, a
potent angiogenic factor, has also been confirmed (66). Furthermore, ANGPTL4 has exhibited
a stimulatory effect on tumor angiogenesis in various cancer types,
including BRC (33), uveal
melanoma (67), NSCLC (68) and OS (69).
The inhibitory effects of ANGPTL4 on angiogenesis
are partially attributed to its suppression of the ERK signaling
pathway and post-translational modifications. Tumor-derived ANGPTL4
has been found to inhibit the angiogenesis and proliferation of
umbilical endothelial cells by suppressing ERK signaling (70). The inactivation of ANGPTL4 through
genetic and epigenetic mechanisms, such as hypermethylation of CpG
islands in the promoter region, causes an increase in tumor growth
and angiogenesis in GC (70).
Yang et al (71) reported
that N-glycosylated cANGPTL4 exerts an inhibitory effect on the
Raf/MEK/ERK signaling cascade in endothelial cells, which
suppresses the angiogenesis induced by alkaline fibroblast growth
factor and VEGF. Furthermore, The inhibitory effects of ANGPTL4 on
angiogenesis have also been observed in CRC (52) and HCC (72), reinforcing its potential as a
modulator of angiogenesis across various cancer types.
ANGPTL4 and programmed cell death
(PCD)
PCD is a widespread mechanism in living organisms,
essential for maintaining cellular homeostasis, development,
immunity and stress response (73). PCD is regulated by numerous
evolutionarily conserved pathways and well-characterized mechanisms
of action (74). Based on
specific morphological, immunological and genetic characteristics,
PCD can be classified into several forms, including apoptosis,
ferroptosis, necroptosis, pyroptosis and autophagy-dependent cell
death (75,76). PCD is crucial in tumor suppression
through its involvement in anticancer therapies. In the present
review, the interactions between various forms of PCD and ANGPTL4
in cancer are discussed.
ANGPTL4 and apoptosis
Apoptosis, the most extensively studied form of PCD,
was first described by Kerr et al in 1972 (77). Apoptosis relies on a cascade of
caspase proteases and can be initiated via extrinsic or intrinsic
pathways (78). As early as the
1970s, studies demonstrated that apoptosis is crucial for
eliminating potentially malignant cells, controlling hyperplasia,
and inhibiting tumor progression (79). The induction of apoptosis is
fundamental in cancer therapy. The dual role of ANGPTL4 in
regulating tumor cell apoptosis has been reported.
Lim et al (80) reported the anti-apoptotic effect
of ANGPTL4 and observed that inhibiting ANGPTL4 leads to the
accumulation of chemotherapy drugs in cells, thereby inducing
apoptosis. The mechanism involves reduced energy production and
accumulation in cancer cells and the weakened drug efflux during
EMT. In HCC (81), ANGPTL4
knockout cell lines exhibited significantly higher levels of
apoptosis. Bai et al (41)
further investigated the role of different ANGPTL4 transcripts and
found a notable increase in ANGPTL4-Transcript 3 expression in HCC
tissues. The overexpression of ANGPTL4-Transcript 3 significantly
confers apoptosis resistance to HCC cells, whereas Transcript 1 has
no such effects (41). Fang et
al (43) conducted a study on
the role of ANGPTL4 in regulating gefitinib resistance in NSCLC
cells. The aforementioned study demonstrated that ANGPTL4 is
crucial in inhibiting cell apoptosis by suppressing the NOD-like
receptor family, pyrin domain containing 3
(NLRP3)/apoptosis-associated Speck-like protein (ASC)/caspase-8
pathway, thereby enhancing resistance to gefitinib (43). ANGPTL4 also enhances the apoptosis
resistance in CRC (38,82). Knocking down bone morphogenetic
protein 7 (BMP7) reverses the anti-apoptotic effect of ANGPTL4
overexpression, suggesting that ANGPTL4 may inhibit apoptosis in
CRC cells by upregulating BMP7 (82).
Most studies to date have highlighted the
anti-apoptotic effect of ANGPTL4 in tumors. However, Hsieh et
al (83) reported the
pro-apoptotic effect of ANGPTL4 in UC. Cyproheptadine was found to
upregulate ANGPTL4 expression and activate apoptosis-related
proteins such as caspase-3 and poly (ADP-ribose) polymerase (PARP),
thereby promoting apoptosis and inhibiting the growth of UC cells.
This process might involve the regulation of glycogen synthase
kinase 3β (GSK3β)/tuberous sclerosis complex subunit 2 (TSC2)/mTOR
and GSK3β/β-catenin signaling pathways.
ANGPTL4 and anoikis
Anoikis is a specialized form of apoptosis that
occurs when cells detach from the surrounding cellular or
extracellular matrix (ECM) (84,85). However, tumor cells can develop
resistance to anoikis, enabling them to evade cell death and
continue proliferating after detachment. Anoikis resistance
facilitates immune evasion, alters the TME, and ultimately
contributes to the invasion and metastasis (85,86).
In HNSCC, EGF induces ANGPTL4 expression,
significantly enhancing anoikis resistance, and promoting migration
and invasion (87). This effect
is mediated through the expression of MMPs, particularly MMP-1, as
regulated by ANGPTL4 (87). Shen
et al (88) discovered
that OA induces ANGPTL4 expression in HNSCC, which significantly
enhances the anoikis resistance by upregulating Ras-related C3
botulinum toxin substrate 1 (Rac1)/cell division control protein 42
(Cdc42) and MMP-9 pathways, thus promoting tumor metastasis.
Zhu et al (89) found that ANGPTL4 secreted by
tumors specifically binds to integrins β1 and β5, leading to the
activation of focal adhesion kinase (FAK) and Rac1. This activation
increases the O2-: H2O2 ratio, subsequently activating the Src,
which triggers the phosphatidylinositol 3-kinase (PI3K)/AKT and
extracellular signal-regulated kinase (ERK) pathways, resulting in
the resistance to anoikis (89).
Similarly, in GC, ANGPTL4 inhibits the occurrence of anoikis by
regulating the focal FAK/Src/PI3K-AKT/ERK pathway and suppressing
caspases-3, -8, and -9 (90).
During the peritoneal metastasis of CRC, ADSCs
enhance the anoikis resistance of CRC cells through the
TGF-β1/Smad3/ANGPTL4 axis (30).
Additionally, Hao et al (59) demonstrated that the downregulation
of ANGPTL4 weakens the endothelial barrier disruption and promotes
anoikis in lung cancer. Furthermore, the overexpression of ANGPTL4
is closely associated with anoikis resistance in cholangiocarcinoma
(91), HCC (92) and uveal melanoma (93).
These findings provide new insights into the
mechanisms of metastasis, suggesting that ANGPTL4 may serve as a
promising therapeutic target for intervening in anoikis and tumor
metastasis. The FAK/Src/PI3K-AKT/ERK pathway, along with MMPs, is
the key mediator of anoikis regulated by ANGPTL4.
ANGPTL4 and ferroptosis
Ferroptosis, an iron-dependent form of cell death,
is characterized by excessive iron accumulation, lipid peroxidation
and cell membrane rupture (94,95). It can be triggered via the
inhibition of intracellular antioxidant enzymes such as glutathione
peroxidase 4 (GPX4) (96,97). Ferroptosis has been identified as
a key mechanism in tumor development and radiation respons
(98-100).
Zhang et al (101) uncovered the molecular function
of ANGPTL4 in hypoxic TME and proposed that hypoxia-induced ANGPTL4
contributes to radiotherapy resistance in NSCLC by regulating
ferroptosis. Under hypoxic conditions, the expression of ANGPTL4 is
significantly upregulated in NSCLC cells and can be enriched in
extracellular vesicles, which can be transferred to adjacent
normoxic cells (101). Both
in vivo and in vitro experiments have confirmed that
ANGPTL4 inhibits ferroptosis by regulating radiation-induced lipid
peroxidation and the expression of hallmark ferroptosis proteins,
such as GPX4 and ferritin heavy chain 1 (101).
By contrast, Yang et al (102) reported the opposite effect in
OVC. The study identified ANGPTL4 as a direct target gene of
transcriptional coactivator with PDZ binding motif (TAZ) through
the integrated genomic analysis (102). The upregulation of ANGPTL4
activated NADPH oxidase 2 (NOX2) in vitro, thereby
increasing the sensitivity to ferroptosis (102).
ANGPTL4 and pyroptosis
Pyroptosis is a previously identified form of PCD
characterized by immune responses and inflammation (103,104). As a critical innate immune
response, pyroptosis induces immune phagocytosis to counter
infections and endogenous threats (105-107). Pyroptosis is triggered by
caspase-1, -4, -5 and -11, and activated by inflammasomes such as
NLRP3 (108-110).
The role of ANGPTL4 in pyroptosis has been reported
in sepsis-induced acute lung injury, where its knockdown was shown
to inhibit macrophage M1 polarization and pyroptosis, thereby
providing a protective effect (111). In a study by Fang et al
(43), ANGPTL4 was found to be
highly expressed in lung adenocarcinoma cells, and its knockdown
leads to increased pyroptosis via the NLRP3/apoptosis-associated
speck-like protein containing a CARD (ASC)/Caspase-8 signaling
pathway (43). Currently, the
research on the regulation of tumor pyroptosis by ANGPTL4 is
limited, and further studies are needed to clarify its precise role
in the pyroptosis of tumor cells.
ANGPTL4 and tumor metabolism
Metabolism is a fundamental biological activity
intrinsic to all organisms, reflecting the processes of matter and
energy transformation. The rapid proliferation and high energy
demand of tumor cells lead to the significant metabolic
alterations, which provide a biochemical basis and directly promote
tumorigenicity and malignancy (112-116). The metabolic reprogramming of
glucose, lipids and amino acids, three major functional
biomolecules, enables tumor cells to acquire energy through various
pathways, supporting their uncontrolled proliferation and survival.
Targeting these metabolic pathways has become a promising
anticancer strategy.
ANGPTL4 and glucose metabolic
reprogramming
Glucose is the most critical energy source for
living organisms, and its metabolic pathways include glycolysis,
the pentose phosphate pathway and oxidative phosphorylation. Even
in the presence of oxygen, tumor cells predominantly produce ATP
through glycolysis, a phenomenon known as aerobic glycolysis or the
Warburg effect (117,118). Aerobic glycolysis is crucial for
the proliferation, growth, invasion and treatment of cancer
(119).
In recent years, an increasing number of studies
have shown that ANGPTL4 plays a significant role in tumor aerobic
glycolysis. Zheng et al (120) found that in Fusobacterium
nucleatum-infected CRC cells, increased acetylation of histone
H3 lysine 27 upregulates the expression of ANGPTL4. ANGPTL4
promotes glucose uptake and aerobic glycolysis in CRC cells both
in vitro and in vivo, which in turn enhances
Fusobacterium nucleatum colonization. This effect is
mediated by ANGPTL4's regulation of glucose transporter-1 (GLUT-1),
thereby promoting the development, metastasis and chemoresistance
of CRC. Similarly, in a study by Mizuno et al (121), it has been shown that ANGPTL4
affects the expression of GLUT-1 and GLUT-3 in CRC, and is
associated with glucose metabolism activity and cancer progression.
These studies indicate that GLUTs, particularly GLUT-1, is a key
molecule in ANGPTL4-mediated regulation of aerobic glycolysis.
Additionally, ADSCs derived from fat tissues have been shown to
promote glycolysis in CRC cells through the TGF-β1/Smad3/ANGPTL4
axis, ultimately facilitating peritoneal metastasis (30). Overall, ANGPTL4 serves as a key
target for regulating aerobic glycolysis and tumor progression in
CRC.
At present, most research on the regulation of
glucose metabolic reprogramming by ANGPTL4 has focused on CRC, and
its regulatory roles and associated molecular mechanisms in other
tumors require further investigations.
A NGPTL 4 and lipid metabolic
reprogramming
Reprogramming of lipid metabolism is a newly
recognized hallmark of malignancy (122,123). Increased lipid uptake, storage
and lipogenesis are observed in various cancers and contribute to
accelerated tumor growth (124-127). ANGPTL4, as a lipid regulatory
factor, has been widely reported for its role in lipid metabolism,
particularly its effects on LPL (7,14,128-131).
Numerous studies have demonstrated the close
association between ANGPTL4 and lipid metabolism in tumors. In GC,
Xiao et al (60)
discovered that leptin induces the phosphorylation of the serine 30
residue of ANGPTL4, thereby reducing its binding affinity with LPL.
This reduction promotes LPL-mediated lipid uptake and increases
intracellular arachidonic acid levels, disrupting cellular lipid
homeostasis, and triggering the COX-2/PGE2 pathway. Consequently,
this process promotes tumor lymphangiogenesis and lymph node
metastasis in GC. Xiao et al (132) reported that ANGPTL4
significantly affects fatty acid oxidation and promotes energy
generation in NSCLC cells, which may be achieved through carnitine
palmitoyl-transferase 1 (CPT1). Hu et al (42) identified ANGPTL4 as a direct
target of miR-133a-3p, with its expression significantly
accelerating lipid metabolism in lung adenocarcinoma.
ANGPTL4 also mediates the metabolic crosstalk
between tumor cells and adipocytes. Blücher et al (133) demonstrated that adipose tissue
secretory factors reprogram tumor lipid metabolism and induce
motility by regulating PPAR α/ANGPTL4 and FAK in TNBC cells, with
ANGPTL4 identified as the key factor in lipid metabolism
regulation. In pancreatic cancer, adipocytes activate the hypoxia
signaling pathway, leading to increased expression of ANGPTL4
(134). This upregulation
enhances β-oxidation in cancer cells and activates the STAT3
signaling pathway, promoting lipid metabolic reprogramming and
metastasis of pancreatic cancer (134).
ANGPTL4 and amino acid metabolic
reprogramming
Amino acids, the building blocks of proteins,
generate metabolites that fuel biosynthetic pathways, produce
energy, and support cancer cell survival. Due to their heightened
proliferative needs, cancer cells often cannot synthesize
sufficient quantities of amino acids. The reprogramming of amino
acid metabolism fulfills this increased demand (135).
Currently, limited research exists on the
relationship between ANGPTL4 and the reprogramming of amino acid
metabolism in cancer. Xiao et al (132) utilized tracer technology and
Seahorse XF technology to explore the metabolic effects of ANGPTL4
in NSCLC. Their findings revealed that ANGPTL4 promotes glutamine
consumption and fatty acid oxidation in NSCLC, both in vitro
and in vivo, while having no significant effect on
glycolysis. ANGPTL4 regulates glutaminase and CPT1, thereby
facilitating glutamine metabolism and fatty acid oxidation, which
in turn supports NSCLC cell proliferation (132). Lin et al (49) identified a negative correlation
between ANGPTL4 and BCAA metabolism in OS samples and cell lines.
The downregulation of ANGPTL4 in OS cells leads to the accumulation
of BCAAs and the activation of mTOR signaling, which promotes OS
cell proliferation (49).
ANGPTL4, chemoresistance and
radioresistance
Chemotherapy and radiotherapy are essential
treatments for unresectable tumors and serve as neoadjuvant
therapies. However, their clinical efficacy is often hindered by
the development of resistance. Chemoresistance and radioresistance
also contribute to tumor recurrence and metastasis, posing major
challenges to patient prognosis (136,137). Research has increasingly focused
on how ANGPTL4, a multifunctional molecule in tumor cell survival,
proliferation and apoptosis, influences the resistance to
chemotherapy and radiotherapy (Table III).
 | Table IIIRoles of ANGPTL4 in drug- or
radio-resistance in human malignancies. |
Table III
Roles of ANGPTL4 in drug- or
radio-resistance in human malignancies.
| First authors/s,
year | Cancer type |
ANGPTL4expression | Upstream
regulators | Downstream
targets | Therapy | (Refs.) |
|---|
| Wen et al,
2020 | Colorectal
cancer | High | - | - | Cisplatin
resistance | (38) |
| Chen et al,
2018 | Gastric cancer | High | | | Cisplatin
resistance | (39) |
| Tsai et al,
2019 | Glioblastoma | High | Sp4 |
EGFR/AKT/4E-BP1 | Temozolomide
resistance | (141) |
| Fekir et al,
2019 | Hepatocellular
carcinoma | High | - | PDK4 | Sorafenib/cisplatin
resistance | (138) |
| Fang et al,
2022 | Non-small cell lung
cancer | High | - |
NLRP3/ASC/Caspase-8 | Gefitinib
resistance | (43) |
| Zhang et al,
2022 | | | - | GPX4, FTH1 |
Radioresistance | (101) |
| Li et al,
2022 | Ovarian cancer | High | TAZ | SOX2 | Cisplatin
resistance | (139) |
| Zhou et al,
2020 | | High | - | c-Myc/NF-κ B | Carboplatin
resistance | (140) |
| Yang et al,
2020 | | High | TAZ | NOX2 | Carboplatin
sensitivity | (102) |
| Cazes et al,
2006 | Pancreatic
cancer | - | - | ITGB4, APOL1 | Gemcitabine
resistance | (172) |
Chemoresistance promoted by ANGPTL4 is closely tied
to its metabolic reprogramming functions. It has been shown that
ANGPTL4 enhances cellular ATP through the c-Myc and NF-κ B
signaling pathways during EMT (80). The increased ATP provides fuel for
multiple ATP-binding cassette (ABC) transporters to upregulate
their expression, ensuring sufficient cellular energy for drug
efflux, thereby conferring chemoresistance in tumors (80). In HCC, ANGPTL4 induces the
upregulation of pyruvate dehydrogenase kinase 4, an inhibitor of
mitochondrial pyruvate dehydrogenase, enhancing the resistance to
sorafenib and cisplatin in stem cells (138). These findings suggest that
targeting the metabolic function of ANGPTL4 and combining the
restoration of mitochondrial metabolic activity with chemotherapy
present attractive therapeutic options in cancer treatment.
In OVC, TAZ has been shown to promote the
resistance to cisplatin via the ANGPTL4/sex-determining region
Y-box2 (SOX2) axis (139).
Similarly, Zhou et al (140) reported that adipocyte-derived
ANGPTL4 induces carboplatin resistance in OVC. ANGPTL4 activates
the c-Myc/NF-κ B pathway, which subsequently stimulates the
expression of the anti-apoptotic proteins and ABC transporter
family members. However, Xu et al (102) came to the opposite conclusion.
They found that TAZ activates the ANGPTL4/NOX2 axis, making OVC
cells sensitive to ferroptosis and chemotherapy. TAZ levels were
lower in chemotherapy-resistant recurrent OVC cells.
In GBM, where temozolomide (TMZ) combined with
radiotherapy is the standard therapy, glioma stem-like cells (GSCs)
are considered to be the primary cause of drug resistance. In the
study of Tsai et al' (141), ANGPTL4 expression was
significantly increased in GSCs. The overexpression of ANGPTL4
activates the PI3K/AKT, EGFR and ERK signaling pathways, enriching
GSCs and resulting in TMZ resistance (141). Additionally, Gordon et al
(142) showed that ANGPTL4
contributes to gemcitabine resistance in pancreatic cancer and
shortens patient survival by regulating the expression of
apolipoprotein L1 and integrin β4. In cholangiocarcinoma, Curcumin
has been shown to enhance chemotherapy efficacy via the inhibition
of ANGPTL4 (91). Furthermore,
ANGPTL4 inhibits pyroptosis and apoptosis of lung adenocarcinoma
cells through the NLRP3/ASC/Caspase-8 signaling pathway,
contributing to the resistance against gefitinib (43).
The role of ANGPTL4 in radioresistance has also
been recently explored. Hypoxia-induced ANGPTL4 expression has been
positively correlated with radiotherapy resistance in NSCLC cells
and xenograft tumors (101).
ANGPTL4 promotes resistance through two mechanisms: intracellular
ANGPTL4 and exosomal ANGPTL4. One pathway involves the upregulation
of GPX4, which inhibits ferroptosis and lipid peroxidation
(101).
The aforementioned studies suggest that ANGPTL4
could serve as a potential therapeutic target to enhance the
efficacy of radiotherapy and chemotherapy. However, research on the
relationship between ANGPTL4 and radioresistance remains limited,
and further investigations are required to improve understanding of
its effects, and underlying mechanisms in chemoresistance and
radioresistance.
Regulation of ANGPTL4 expression and
function in cancer
The expression of ANGPTL4 is quite common in cancer
cells, but the molecular mechanisms of ANGPTL4 in cancer are quite
complex. HIF-1, TGF-β and PPARs are critical upstream regulators of
ANGPTL4 (Table I; Fig. 3). HIF-1, a heterodimeric protein
composed of HIF-1α and HIF-1β subunits, activates the transcription
of numerous genes involved in numerous aspects of cancer biology.
Studies in scirrhous GC (90),
HCC (143), uveal melanoma
(67), and OS (44) have shown that the expression of
ANGPTL4 is directly regulated by HIF-1α, subsequently promoting
malignant processes, including tumor growth and metastasis.
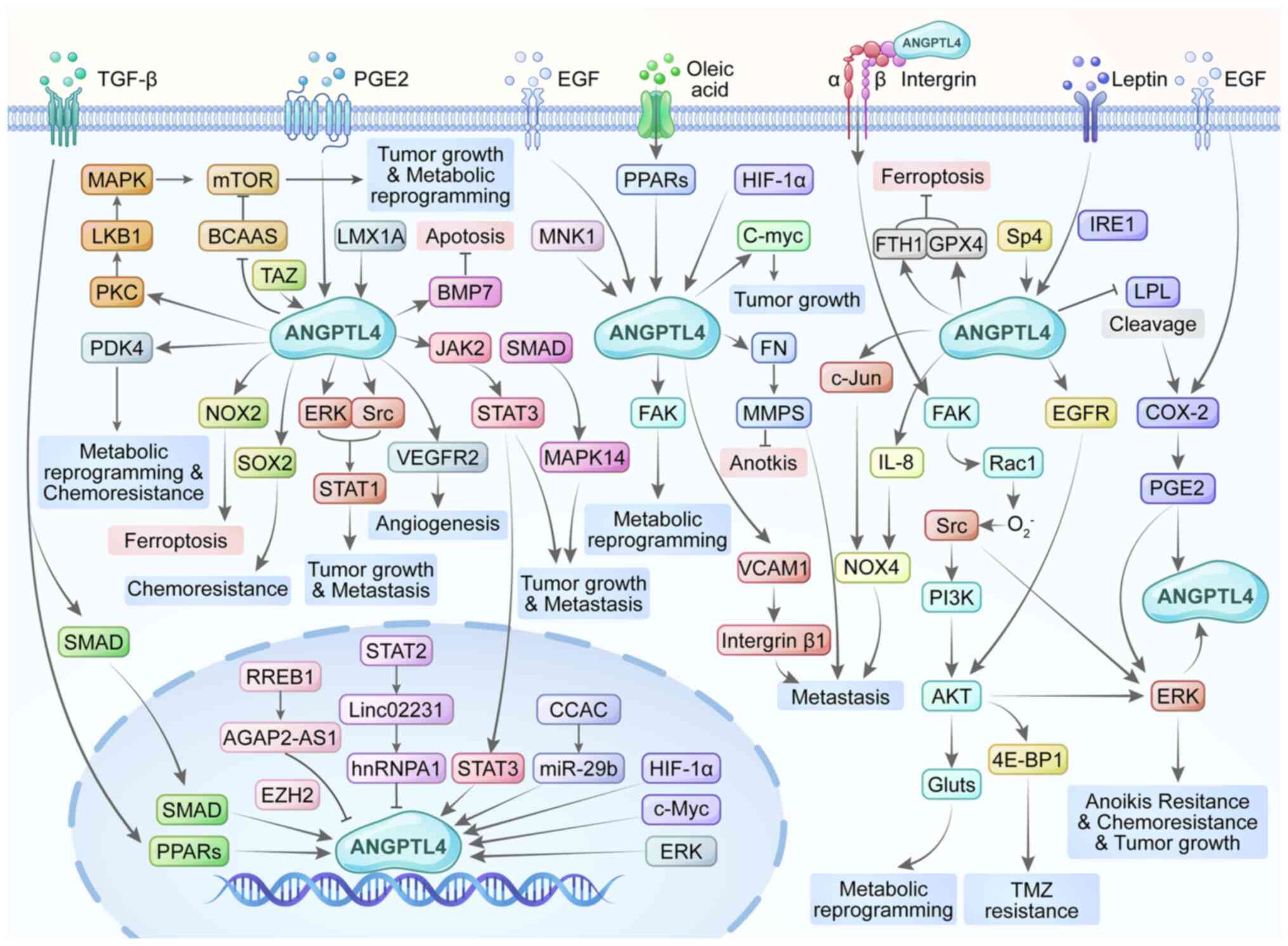 | Figure 3ANGPTL4 regulates the occurrence and
development of tumors through different signaling pathways and
molecular regulation. ANGPTL4, angiopoietin-like 4; 4E-BP1,
eukaryotic translation initiation factor 4E-binding protein 1;
AGAP2-AS1, AGAP2 antisense RNA 1; BCAAs, branched chain amino
acids; BMP7, bone morphogenetic protein 7; COX-2, cyclooxygenase-2;
EGF, epidermal growth factor; ERK, extracellular signal-related
kinase; EZH2, zeste homolog 2; FAK, focal adhesion kinase; FN,
fibronectin; FTH1, ferritin heavy chain 1; Gluts, glucose
transporters; GPX4, glutathione peroxidase 4; HIF-1α,
hypoxia-inducible factor-1α; IL-8, interleukin-8; IRE1,
inositol-requiring enzyme 1; JAK2 Janus kinase 2; LKB1, liver
kinase B1; LMX1A, homeobox transcription factor 1 α; LPL,
lipoprotein lipase; MAPK, mitogen-activated protein kinase; MMPs,
matrix metalloproteinases; MNK1, MAP kinase-interacting
serine/threonine-protein kinase 1; mTOR, mechanistic target of
rapamycin; NOX2, NADPH oxidase 2; NOX4, NADPH oxidase 4; PDK4,
pyruvate dehydrogenase kinase 4; PGE2, prostaglandin E2; PI3K,
phosphoinositide 3-kinase; PKC, protein kinase C; PPARs, peroxisome
proliferator-activated receptors; Rac1, Ras-related C3 botulinum
toxin substrate 1; RREB1, RAS responsive element-binding protein 1;
SOX2, SRY-box transcription factor 2; Sp4, specificity protein 4;
TAZ, transcriptional coactivator with PDZ-binding motif; VCAM1,
vascular cell adhesion molecule 1; miR, microRNA. |
TGF-β is a multifunctional cytokine that plays
important roles in cell proliferation, differentiation, immune
regulation, apoptosis, and tissue repair. The Smad pathway and
PPARs are essential for the expression of ANGPTL4 induced by TGF-β.
In BRC, TGF-β promotes ANGPTL4 expression via the Smad pathway,
mediating the lung metastasis and brain metastasis (31,127). Similarly, TGF-β1 activates
Smad3, which binds to the ANGPTL4 promoter and promotes the
transcription in CRC (30).
Adhikary et al (144)
found that TGF-β can also induce ANGPTL4 expression in a PPAR
β/δ-dependent manner.
PPARs are a class of ligand-dependent transcription
factors in the nuclear receptor superfamily. PPARs regulate the
expression of ANGPTL4 in several cancers, including BRC and HNSCC.
In BRC, PPAR α, PPAR β, and PPAR γ target ANGPTL4 to promote lipid
metabolism, angiogenesis, tumor growth and invasion (133,144,145). In HNSCC, PPARs are induced by OA
and target ANGPTL4, promoting anoikis resistance and metastasis
(88).
Multiple signaling pathways are associated with the
regulation of tumor development by ANGPTL4, with the STATs,
PI3K/AKT, and COX-2/PGE2 pathways being particularly prominent
(Table I; Fig. 3). The STAT family is the
transcription factors, which have been implicated in cancer
development, metastasis and resistance to treatments. To date,
seven STAT genes have been identified: STAT1, STAT2, STAT3, STAT4,
STAT5a, STAT5b and STAT6. Specifically, the functions of ANGPTL4
are closely linked to the STAT1, STAT2 and STAT3 pathways. ANGPTL4
activates the JAK2/STAT3 pathway, which promotes the malignant
progression of OVC (35). In
pancreatic cancer, STAT3 activated by ANGPTL4 also drives metabolic
reprogramming, invasion and metastasis (134). Additionally, ANGPTL4 enhances
CRC cell proliferation through the activation of STAT1 (146). STAT2 induces the
linc02231/hnRNPA1/ANGPTL4 axis, which decreases the expression of
ANGPTL4 and promotes the tumorigenesis and angiogenesis in CRC
(52).
The PI3K/AKT pathway is another critical signaling
cascade in the network of ANGPTL4. PI3K is a lipid kinase
responsible for propagating intracellular signaling cascades and
regulating numerous cellular processes (147). AKT, a major downstream effector
of PI3K, influences multiple vital pathways for tumor growth,
apoptosis and cellular metabolism (147). ANGPTL4 has been found to
activate FAK and Rac1, and subsequently trigger Src. This cascade
leads to the activation of PI3K/AKT and ERK pathways, contributing
to the anoikis resistance (89,90). The PI3K/AKT and ERK signaling
pathways activated by ANGPTL4 also result in GSCs enrichment and
the resistance to TMZ (141).
Moreover, ANGPTL4 regulates the expression of GLUTs via the
PI3K/AKT pathway, which promotes the aerobic glycolysis in CRC
(121).
The COX-2/PGE2 pathway, which plays a significant
role in inflammation, is also implicated in tumor progression. COX
enzymes convert arachidonic acid into five prostaglandins, with
PGE2 being the most abundant. In the present review, the COX-2/PGE2
pathway is highlighted in the ANGPTL4-mediated tumor metastasis and
lipid metabolic reprogramming. This indicates that ANGPTL4 may
facilitate the crosstalk between tumorigenesis and inflammation. In
GC, leptin reduces the binding affinity of ANGPTL4 to LPL,
enhancing LPL-mediated lipid uptake, which increases intracellular
arachidonic acid levels (60).
Arachidonic acid then activates the COX-2/PGE2 pathway, promoting
lymph node metastasis (60).
Additionally, Chiang et al (61) demonstrated that the
COX-2/PGE2/ANGPTL4 axis, activated by EGF, enhances metastasis in
HNSCC.
ANGPTL4 in the TME
The TME consists of various components, including
tumor cells, stromal cells, blood vessels, immune cells and ECM.
These elements affect the biological characteristics of tumors
through complex interactions and are crucial in tumor initiation
and progression (148). The
critical involvement of ANGPTL4 in the TME has been increasingly
highlighted. For example, ANGPTL4 has been shown to activate the
JAK2/STAT3 pathway, enhancing tumorigenesis and angiogenesis in the
TME (35). Zhu et al
(30) emphasized that ADSCs in
the TME promote tumor glycolysis and metastasis via ANGPTL4 in CRC.
Immune cells are an important component of TME, and new evidence
links ANGPTL4 with immune cell dynamics in the TME. In patients
with CRC, ANGPTL4 suppresses the activation of CD8+ T
cells through metabolic reprogramming, leading to diminished immune
surveillance (32). Recombinant
ANGPTL4 has also been reported to induce regulatory T (Treg) cells
and M2 macrophages in mice, which may contribute to the tumor
progression (149). Furthermore,
spatial transcriptomics analyses have revealed that bladder cancer
cells in the stressed or hypoxic state interact with plasma cells
via the ANGPTL4/Syndecan-1 (SDC1) axis, which is associated with
ineffective responses to immunotherapy and poor survival (150).
Sialylation, a common glycosylation modification of
attaching sialic acid to the ends of sugar chains, is widely
present in cell membranes, secreted proteins and serum proteins
(151,152). This process significantly
influences the function, stability, immune recognition and
intercellular communication of cells and proteins in the TME
(151). Recent studies have
identified the distinct roles of ANGPTL4 in podocytopathies due to
varying degrees of sialylation. Hyposialylated ANGPTL4 with a high
isoelectric point (high-pI) is upregulated in patients with minimal
change disease and correlates with increased proteinuria. By
contrast, normal sialylated ANGPTL4 with a neutral isoelectric
point (neutral-pI) reduces proteinuria by binding to β5 integrin
(153,154).
In tumors, high-pI ANGPTL4, with fewer negative
charges, may enhance tumor cell adhesion and migration by binding
more effectively to collagen and fibronectin in the ECM. Its high
affinity for vascular endothelial cells may also enable it to play
a more direct role in angiogenesis. Conversely, neutral-pI ANGPTL4
demonstrates greater stability in body fluids and may support the
sustained regulation of angiogenesis. Moreover, neutral-pI ANGPTL4
can directly bind to sialic acid-binding immunoglobulin-like lectin
(Siglec) receptors, which may inhibit the activity of natural
killer (NK) cells and macrophages, thereby facilitating tumor
immune evasion (155,156). Jin et al (51) found that nANGPTL4 exerts antitumor
effects in clear cell renal cell carcinoma (ccRCC) by regulating
the lysosomal acid lipase activity. However, no studies have
explored whether high-pI and neutral-pI ANGPTL4 exhibit different
impacts in renal and other tumors, as they do in podocytopathies.
Future research could focus on the sialylation of ANGPTL4 in the
TME to clarify its roles and associated mechanisms.
Discussion
A substantial body of research has demonstrated
that ANGPTL4 plays critical biological roles, including the
regulation of tumor growth, metastasis and angiogenesis (Fig. 2). It is also involved in the
regulatory processes of programmed cell death, metabolic
reprogramming and drug resistance (Fig. 2). Taken together, all the evidence
cited in the present review indicates that ANGPTL4 is an important
molecule implicated in various aspects of cancer progression.
ANGPTL4 is overexpressed in various cancers and its
expression is closely linked to clinicopathological features, such
as BRC, cholangiocarcinoma, cervical, esophageal and gallbladder
cancer (157-161). In the majority of cancers, such
as TNBC, GBM and NSCLC, the overexpression of ANGPTL4 significantly
enhances tumor progression (31,42,141). Additionally, ANGPTL4 has been
found to promote the resistance to treatments (Table III).
However, the role of ANGPTL4 in tumors remains
complex and controversial due to its dual effects. ANGPTL4 can both
promote and inhibit tumorigenesis (31,34,62) (Fig.
1). It not only serves as a biomarker for poor prognosis
(157,158,162,163), but also indicates favorable
prognosis (164), and even
exhibits the opposing roles within the same type of cancers
(47). It is hypothesized that
these contradictory effects are related to the structural and
functional characteristics of ANGPTL4. Firstly, ANGPTL4 undergoes
proteolytic cleavage, producing different functional fragments. The
hydrolysis-generated nANGPTL4 and cANGPTL4 fragments may exert
distinct biological effects compared with fANGPTL4. The nANGPTL4
inhibits LPL activity in both blood and adipocytes, leading to
reduced circulating triglyceride levels (165-170). The nANGPTL4 also inhibits
metastasis and improves overall survival by inhibiting WNT
signaling and reducing vascularity at the metastatic site (53). Meanwhile, the cANGPTL4 fragment
plays a role in angiogenesis, increases vascular permeability,
causes endothelial damage, and promotes tumor growth and metastasis
in multiple tumor models (47,53,171,172). Furthermore, fANGPTL4 exists in
tumor tissues, whereas nANGPTL4 is more prevalent in systemic
circulation (53). Secondly, as a
protein capable of entering the nucleus, ANGPTL4 can directly or
indirectly influence the expression of various genes, thereby
performing multiple functions. It may also be secreted into the
extracellular space or transported through exosomes and
extracellular vesicles, potentially contributing to the
intercellular and the inter-organ communication (68,101). This diversity in function may
help explain its complex roles. Finally, differences in the TME and
tissue-specific conditions may contribute to the diverse functions
of ANGPTL4. For instance, while ANGPTL4 expression is low in UC
cell lines and tissue samples, the elevated circulating levels are
observed in patient samples (47).
The strong association between ANGPTL4 and the
tumor progression highlights its potential as a promising biomarker
and therapeutic target. Its expression is closely associated with
prognosis in various cancers, supporting the rationale for
developing ANGPTL4 as a biomarker (157,158,162-164). Additionally, the differential
expression and functions of ANGPTL4 across cancer types suggest
that targeting ANGPTL4 enables offering personalized treatment
strategies tailored to specific tumor characteristics (31,34,62) (Fig.
1). Jin et al (51)
identified a subset of patients with no ANGPTL4 increase in ccRCC,
who had a poorer prognosis than those with high ANGPTL4 expression.
This finding highlights the potential of stratifying patients based
on ANGPTL4 expression levels, which may not only provide the more
accurate predictions of survival outcomes but also optimize
therapeutic interventions. Furthermore, as ANGPTL4 is closely
connected with chemoresistance and radioresistance (Table III), the development of
ANGPTL4-targeted drugs or antibodies for combination therapy holds
significant promise to enhance treatment efficacy and patient
outcomes. ANGPTL4 also acts as a potent pro-angiogenic factor in
certain cancers (35,37,65,66). Targeting ANGPTL4 may therefore
provide a novel anti-angiogenic approach. In addition, as
aforementioned, ANGPTL4 regulates various immune cells in the TME,
including CD8+T cells, NK cells, macrophages and plasma
cells (32,164,165,173). Antibodies against ANGPTL4 may
help restore immune surveillance and enhance antitumor immunity.
Combining ANGPTL4-targeted antibodies with existing
immunotherapies, such as programmed cell death protein 1 and
programmed death-ligand 1 inhibitors, offers new perspectives for
the combination immunotherapy.
Despite current advancements in research, numerous
key gaps remain in fully understanding ANGPTL4. Firstly, ANGPTL4
exhibits both tumor-promoting and tumor-inhibiting effects in the
progression of different cancers, yet its underlying molecular
mechanisms have not been insufficiently elucidated. Secondly,
although ANGPTL4 can be expressed in the nucleus or secreted, the
specific form in which it exists across various organs and diseases
is unclear. Whether the ANGPTL4 protein is modified post-secretion
during this process remains unknown. Thirdly, ANGPTL4 can undergo
glycosylation modifications structurally, but it is unclear how
different modifications such as sialylation affect the role and
mechanism of ANGPTL4 in tumors. Finally, although some inhibitors
targeting the ANGPT family have been explored, including MEDI-3617
and nesvacumab (targeting ANGPT2), and trebananib and AMG780
(targeting both ANGPT1 and ANGPT2), no inhibitors or drugs
targeting ANGPTL4 have been developed to date (173). The clinical application of
ANGPTL4 as a biomarker for cancer and the development of novel
antitumor drugs targeting ANGPTL4 still require extensive
experimental research and exploration.
The present review updates the understanding of
ANGPTL4 in tumors by systematically analyzing its roles in the six
hallmarks of cancer and its interactions with the TME. It also
addresses the dual effects of ANGPTL4 across different cancer types
and highlights its potential in precision medicine. These
contributions provide a comprehensive, in-depth and innovative
perspective on ANGPTL4 in malignancies.
In conclusion, ANGPTL4 predominantly exerts
pro-tumor effects, yet its antitumor functions should not be
overlooked. Further research is necessary to fully understand its
roles, including the functions of different ANGPTL4 fragments, and
the effects and interactions of ANGPTL4 in the TME. Exploring the
diverse mechanisms of ANGPTL4 in human cancers and assessing its
clinical value will be crucial for future studies. With a deeper
understanding of its structure, function and drug response,
supported by comprehensive preclinical analyses, ANGPTL4 holds
significant potential for future application in clinical prediction
and therapy.
Availability of data and materials
Not applicable.
Authors' contributions
ZW and YC conceived the study. RL, MF and PC wrote
the original draft of the manuscript. RL, YL, XS and PZ wrote,
reviewed and edited the manuscript. RL, MF and WH illustrated the
figures. ZW and YC supervised the study. All authors read and
approved the final version of the manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82172664) and the Natural Science
Foundation of Shandong (grant no. ZR2022MH074).
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gilbertson RJ: Mapping cancer origins.
Cell. 145:25–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dhanasekaran R, Deutzmann A,
Mahauad-Fernandez WD, Hansen AS, Gouw AM and Felsher DW: The MYC
oncogene - the grand orchestrator of cancer growth and immune
evasion. Nat Rev Clin Oncol. 19:23–36. 2022. View Article : Google Scholar
|
|
4
|
Huang H, Bhat A, Woodnutt G and Lappe R:
Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer.
10:575–585. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eklund L, Kangas J and Saharinen P:
Angiopoietin-Tie signalling in the cardiovascular and lymphatic
systems. Clin Sci (Lond). 131:87–103. 2017. View Article : Google Scholar
|
|
6
|
Guo L, Li SY, Ji FY, Zhao YF, Zhong Y, Lv
XJ, Wu XL and Qian GS: Role of Angptl4 in vascular permeability and
inflammation. Inflamm Res. 63:13–22. 2014. View Article : Google Scholar
|
|
7
|
Aryal B, Price NL, Suarez Y and
Fernández-Hernando C: ANGPTL4 in Metabolic and Cardiovascular
Disease. Trends Mol Med. 25:723–734. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tan MJ, Teo Z, Sng MK, Zhu P and Tan NS:
Emerging roles of angiopoietin-like 4 in human cancer. Mol Cancer
Res. 10:677–688. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
La Paglia L, Listì A, Caruso S, Amodeo V,
Passiglia F, Bazan V and Fanale D: Potential role of ANGPTL4 in the
cross talk between metabolism and cancer through PPAR signaling
pathway. PPAR Res. 2017:81872352017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kersten S: Angiopoietin-like 3 in
lipoprotein metabolism. Nat Rev Endocrinol. 13:731–739. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sylvers-Davie KL and Davies BSJ:
Regulation of lipoprotein metabolism by ANGPTL3, ANGPTL4, and
ANGPTL8. Am J Physiol Endocrinol Metab. 321:E493–E508. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang J, Song QY, Niu SX, Chen HJ, Petersen
RB, Zhang Y and Huang K: Emerging roles of angiopoietin-like
proteins in inflammation: Mechanisms and potential as
pharmacological targets. J Cell Physiol. 237:98–117. 2022.
View Article : Google Scholar
|
|
13
|
Thorin E, Labbé P, Lambert M, Mury P,
Dagher O, Miquel G and Thorin-Trescases N: Angiopoietin-like
proteins: Cardiovascular biology and therapeutic targeting for the
prevention of cardiovascular diseases. Can J Cardiol. 39:1736–1756.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kersten S: Role and mechanism of the
action of angiopoietin-like protein ANGPTL4 in plasma lipid
metabolism. J Lipid Res. 62:1001502021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zuo Y, He Z, Chen Y and Dai L: Dual role
of ANGPTL4 in inflammation. Inflamm Res. 72:1303–1313. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim I, Kim HG, Kim H, Kim HH, Park SK, Uhm
CS, Lee ZH and Koh GY: Hepatic expression, synthesis and secretion
of a novel fibrinogen/angiopoietin-related protein that prevents
endothelial-cell apoptosis. Biochem J. 346:603–610. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoon JC, Chickering TW, Rosen ED, Dussault
B, Qin Y, Soukas A, Friedman JM, Holmes WE and Spiegelman BM:
Peroxisome proliferator-activated receptor gamma target gene
encoding a novel angiopoietin-related protein associated with
adipose differentiation. Mol Cell Biol. 20:5343–5349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kersten S, Mandard S, Tan NS, Escher P,
Metzger D, Chambon P, Gonzalez FJ, Desvergne B and Wahli W:
Characterization of the fasting-induced adipose factor FIAF, a
novel peroxisome proliferator-activated receptor target gene. J
Biol Chem. 275:28488–28493. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Górecka M, Krzemiński K, Buraczewska M,
Kozacz A, Dąbrowski J and Ziemba AW: Effect of mountain
ultra-marathon running on plasma angiopoietin-like protein 4 and
lipid profile in healthy trained men. Eur J Appl Physiol.
120:117–125. 2020. View Article : Google Scholar :
|
|
20
|
Li L, Foo BJW, Kwok KW, Sakamoto N, Mukae
H, Izumikawa K, Mandard S, Quenot JP, Lagrost L, The WK, et al:
Antibody treatment against angiopoietin-like 4 reduces pulmonary
edema and injury in secondary pneumococcal pneumonia. mBio.
10:e024692019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sodhi A, Ma T, Menon D, Deshpande M, Jee
K, Dinabandhu A, Vancel J, Lu D and Montaner S: Angiopoietin-like 4
binds neuropilins and cooperates with VEGF to induce diabetic
macular edema. J Clin Invest. 129:4593–4608. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kersten S, Lichtenstein L, Steenbergen E,
Mudde K, Hendriks HF, Hesselink MK, Schrauwen P and Müller M:
Caloric restriction and exercise increase plasma ANGPTL4 levels in
humans via elevated free fatty acids. Arterioscler Thromb Vasc
Biol. 29:969–974. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh AK, Aryal B, Chaube B, Rotllan N,
Varela L, Horvath TL, Suárez Y and Fernández-Hernando C: Brown
adipose tissue derived ANGPTL4 controls glucose and lipid
metabolism and regulates thermogenesis. Mol Metab. 11:59–69. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shu L, Wang C, Ding Z, Tang J, Zhu Y, Wu
L, Wang Z, Zhang T, Wang T, Xu Y and Sun L: A novel regulated
network mediated by downregulation HIF1A-AS2 lncRNA impairs
placental angiogenesis by promoting ANGPTL4 expression in
preeclampsia. Front Cell Dev Biol. 10:8370002022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Spitler KM, Shetty SK, Cushing EM,
Sylvers-Davie KL and Davies BSJ: Chronic high-fat feeding and
prolonged fasting in liver-specific ANGPTL4 knockout mice. Am J
Physiol Endocrinol Metab. 321:E464–E478. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alex S, Lichtenstein L, Dijk W, Mensink
RP, Tan NS and Kersten S: ANGPTL4 is produced by entero-endocrine
cells in the human intestinal tract. Histochem Cell Biol.
141:383–391. 2014. View Article : Google Scholar
|
|
27
|
Kuo T, Chen TC, Yan S, Foo F, Ching C,
McQueen A and Wang JC: Repression of glucocorticoid-stimulated
angiopoietin-like 4 gene transcription by insulin. J Lipid Res.
55:919–928. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Inoue T, Kohro T, Tanaka T, Kanki Y, Li G,
Poh HM, Mimura I, Kobayashi M, Taguchi A, Maejima T, et al:
Cross-enhancement of ANGPTL4 transcription by HIF1 alpha and PPAR
beta/delta is the result of the conformational proximity of two
response elements. Genome Biol. 15:R632014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaddatz K, Adhikary T, Finkernagel F,
Meissner W, Müller-Brüsselbach S and Müller R: Transcriptional
profiling identifies functional interactions of TGF β and PPAR β/δ
signaling: Synergistic induction of ANGPTL4 transcription. J Biol
Chem. 285:29469–29479. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu C, Teng L, Lai Y, Yao X, Fang Y, Wang
Z, Lin S, Zhang H, Li Q, Li Y, et al: Adipose-derived stem cells
promote glycolysis and peritoneal metastasis via
TGF-β1/SMAD3/ANGPTL4 axis in colorectal cancer. Cell Mol Life Sci.
81:1892024. View Article : Google Scholar
|
|
31
|
Gong X, Hou Z, Endsley MP, Gronseth EI,
Rarick KR, Jorns JM, Yang Q, Du Z, Yan K, Bordas ML, et al:
Interaction of tumor cells and astrocytes promotes breast cancer
brain metastases through TGF-β2/ANGPTL4 axes. NPJ Precis Oncol.
3:242019. View Article : Google Scholar
|
|
32
|
Ding S, Lin Z, Zhang X, Jia X, Li H, Fu Y,
Wang X, Zhu G, Lu G, Xiao W and Gong W: Deficiency of
angiopoietin-like 4 enhances CD8(+) T cell bioactivity via
metabolic reprogramming for impairing tumour progression.
Immunology. 170:28–46. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kolb R, Kluz P, Tan ZW, Borcherding N,
Bormann N, Vishwakarma A, Balcziak L, Zhu P, Davies BS, Gourronc F,
et al: Obesity-associated inflammation promotes angiogenesis and
breast cancer via angiopoietin-like 4. Oncogene. 38:2351–2363.
2019. View Article : Google Scholar :
|
|
34
|
Avalle L, Raggi L, Monteleone E, Savino A,
Viavattene D, Statello L, Camperi A, Stabile SA, Salemme V, De
Marzo N, et al: STAT3 induces breast cancer growth via ANGPTL4,
MMP13 and STC1 secretion by cancer associated fibroblasts.
Oncogene. 41:1456–1467. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li YK, Gao AB, Zeng T, Liu D, Zhang QF,
Ran XM, Tang ZZ, Li Y, Liu J, Zhang T, et al: ANGPTL4 accelerates
ovarian serous cystadenocarcinoma carcinogenesis and angiogenesis
in the tumor microenvironment by activating the JAK2/STAT3 pathway
and interacting with ESM1. J Transl Med. 22:462024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu J, Wu F, Zhu Y, Wu T, Cao T, Gao W, Liu
M, Qian W, Feng G, Xi X and Hou S: ANGPTL4 regulates ovarian cancer
progression by activating the ERK1/2 pathway. Cancer Cell Int.
24:542024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu Y, Gao J and Liu X: Deregulation of
angiopoietin-like 4 slows ovarian cancer progression through
vascular endothelial growth factor receptor 2 phosphorylation.
Cancer Cell Int. 21:1712021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wen L, Zhang Y, Yang B, Han F, Ebadi AG
and Toughani M: Knockdown of angiopoietin-like protein 4 suppresses
the development of colorectal cancer. Cell Mol Biol
(Noisy-le-grand). 66:117–124. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen JW, Luo YJ, Yang ZF, Wen LQ and Huang
L: Knockdown of angiopoietin-like 4 inhibits the development of
human gastric cancer. Oncol Rep. 39:1739–1746. 2018.PubMed/NCBI
|
|
40
|
Bai Y, Cui G, Sun X, Wei M, Liu Y, Guo J
and Yang Y: ANGPTL4 stabilizes bone morphogenetic protein 7 through
deubiquitination and promotes HCC proliferation via the SMAD/MAPK
pathway. DNA Cell Biol. 43:395–400. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bai Y, Cui G, Sun X, Wei M, Liu Y, Guo J
and Yang Y: Angiopoietin-related protein 4-Transcript 3 increases
the proliferation, invasion, and migration of hepatocellular
carcinoma cells and inhibits apoptosis. DNA Cell Biol. 43:175–184.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hu Q, Chen S, Li Y, Hu T, Hu J, Wang C,
Yang F, Yang X, Zhou F, Liu Z, et al: ANGPTL4, a direct target of
hsa-miR-133a-3p, accelerates lung adenocarcinoma lipid metabolism,
proliferation and invasion. Aging (Albany NY). 16:8348–8360.
2023.
|
|
43
|
Fang Y, Li X, Cheng H, Zhang L and Hao J:
ANGPTL4 regulates lung adenocarcinoma pyroptosis and apoptosis via
NLRP3\ASC\ Caspase 8 signaling pathway to promote resistance to
gefitinib. J Oncol. 2022:36235702022. View Article : Google Scholar
|
|
44
|
Zhang T, Kastrenopoulou A, Larrouture Q,
Athanasou NA and Knowles HJ: Angiopoietin-like 4 promotes
osteosarcoma cell proliferation and migration and stimulates
osteoclastogenesis. BMC Cancer. 18:5362018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang L, Wang Y, Sun R, Zhang Y, Fu Y,
Zheng Z, Ji Z and Zhao D: ANGPTL4 promotes the proliferation of
papillary thyroid cancer via AKT pathway. Onco Targets Ther.
13:2299–2309. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Izraely S, Ben-Menachem S, Sagi-Assif O,
Meshel T, Marzese DM, Ohe S, Zubrilov I, Pasmanik-Chor M, Hoon DSB
and Witz IP: ANGPTL4 promotes the progression of cutaneous melanoma
to brain metastasis. Oncotarget. 8:75778–75796. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hsieh HY, Jou YC, Tung CL, Tsai YS, Wang
YH, Chi CL, Lin RI, Hung SK, Chuang YM, Wu SF, et al: Epigenetic
silencing of the dual-role signal mediator, ANGPTL4 in tumor
tissues and its overexpression in the urothelial carcinoma
microenvironment. Oncogene. 37:673–686. 2018. View Article : Google Scholar
|
|
48
|
Hui B, Ji H, Xu Y, Wang J, Ma Z, Zhang C,
Wang K and Zhou Y: RREB1-induced upregulation of the lncRNA
AGAP2-AS1 regulates the proliferation and migration of pancreatic
cancer partly through suppressing ANKRD1 and ANGPTL4. Cell Death
Dis. 10:2072019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lin S, Miao Y, Zheng X, Dong Y, Yang Q,
Yang Q, Du S, Xu J, Zhou S and Yuan T: ANGPTL4 negatively regulates
the progression of osteosarcoma by remodeling branched-chain amino
acid metabolism. Cell Death Discov. 8:2252022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Qian P, Li J, Zhang X, Li F, Bei S, Li H,
Sun Q and Feng L: LMX1A inhibits C-Myc expression through ANGPTL4
to exert tumor suppressive role in gastric cancer. PLoS One.
14:e02216402019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jin Z, De U, Tithi TI, Kleberg J, Nataraj
A, Jolley E, Carelock ME, Davies BS, Zhang W and Kolb R: ANGPTL4
suppresses clear cell renal cell carcinoma via inhibition of
lysosomal acid lipase. Cancer Res Commun. 4:2242–2254. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu S, Fang Y, Chang L, Bian Y, Wang Y,
Ding J, Wang Y, Zhang Y, Pu J and Wang K: STAT2-induced linc02231
promotes tumorigenesis and angiogenesis through modulation of
hnRNPA1/ANGPTL4 in colorectal cancer. J Gene Med. 25:e35062023.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hübers C, Abdul Pari AA, Grieshober D,
Petkov M, Schmidt A, Messmer T, Heyer CM, Schölch S, Kapel SS,
Gengenbacher N, et al: Primary tumor-derived systemic nANGPTL4
inhibits metastasis. J Exp Med. 220:e202025952023. View Article : Google Scholar
|
|
54
|
Niu Y, Bao L, Chen Y, Wang C, Luo M, Zhang
B, Zhou M, Wang JE, Fang YV, Kumar A, et al: HIF2-induced long
noncoding RNA RAB11B-AS1 promotes hypoxia-mediated angiogenesis and
breast cancer metastasis. Cancer Res. 80:964–975. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shen CJ, Chang KY, Lin BW, Lin WT, Su CM,
Tsai JP, Liao YH, Hung LY, Chang WC and Chen BK: Oleic acid-induced
NOX4 is dependent on ANGPTL4 expression to promote human colorectal
cancer metastasis. Theranostics. 10:7083–7099. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shen CJ, Chan RH, Lin BW, Li NC, Huang YH,
Chang WC and Chen BK: Oleic acid-induced metastasis of
KRAS/p53-mutant colorectal cancer relies on concurrent KRAS
activation and IL-8 expression bypassing EGFR activation.
Theranostics. 13:4650–4666. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bajwa P, Kordylewicz K, Bilecz A, Lastra
RR, Wroblewski K, Rinkevich Y, Lengyel E and Kenny HA:
Cancer-associated mesothelial cell-derived ANGPTL4 and STC1 promote
the early steps of ovarian cancer metastasis. JCI Insight.
8:e1630192023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hefni E, Menon D, Ma T, Asiedu EB, Sultan
A, Meiller T, Schneider A, Sodhi A and Montaner S:
Angiopoietin-like 4 induces head and neck squamous cell carcinoma
cell migration through the NRP1/ABL1/PXN pathway. Cell Signal.
108:1106972023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hao H, Guo Z, Li Z, Li J, Jiang S, Fu J,
Jiao Y, Deng X, Han S and Li P: Modified Bu-Fei decoction inhibits
lung metastasis via suppressing angiopoietin-like 4. Phytomedicine.
106:1544092022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xiao J, Cao S, Wang J, Li P, Cheng Q, Zhou
X, Dong J, Li Y, Zhao X, Xu Z and Yang L: Leptin-mediated
suppression of lipoprotein lipase cleavage enhances lipid uptake
and facilitates lymph node metastasis in gastric cancer. Cancer
Commun (Lond). 44:855–878. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chiang KH, Shieh JM, Shen CJ, Chang TW, Wu
PT, Hsu JY, Tsai JP, Chang WC and Chen BK: Epidermal growth
factor-induced COX-2 regulates metastasis of head and neck squamous
cell carcinoma through upregulation of angiopoietin-like 4. Cancer
Sci. 111:2004–2015. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cai YC, Yang H, Wang KF, Chen TH, Jiang WQ
and Shi YX: ANGPTL4 overexpression inhibits tumor cell adhesion and
migration and predicts favorable prognosis of triple-negative
breast cancer. BMC Cancer. 20:8782020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang K, Zhai Z, Yu S and Tao Y: DNA
methylation mediated down-regulation of ANGPTL4 promotes colorectal
cancer metastasis by activating the ERK pathway. J Cancer.
12:5473–5485. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jiang F, Wang GJ, Huang P, Chen S, Xiao H,
Zhang L and Zou H: Geiparvarin inhibits OS metastasis through
upregulation of ANGPTL4 expression by inhibiting miRNA-3912-3p
expression. Evid Based Complement Alternat Med.
2022:46636842022.PubMed/NCBI
|
|
65
|
Katanasaka Y, Kodera Y, Kitamura Y,
Morimoto T, Tamura T and Koizumi F: Epidermal growth factor
receptor variant type III markedly accelerates angiogenesis and
tumor growth via inducing c-myc mediated angiopoietin-like 4
expression in malignant glioma. Mol Cancer. 12:312013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Xiao W, Tan D, Xiong X, Liu J and Xu J:
Large volume collapse observed in the phase transition in cubic
PbCrO3 perovskite. Proc Natl Acad Sci USA. 107:14026–14029. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hu K, Babapoor-Farrokhran S, Rodrigues M,
Deshpande M, Puchner B, Kashiwabuchi F, Hassan SJ, Asnaghi L, Handa
JT, Merbs S, et al: Hypoxia-inducible factor 1 upregulation of both
VEGF and ANGPTL4 is required to promote the angiogenic phenotype in
uveal melanoma. Oncotarget. 7:7816–7828. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Mo F, Xu Y and Zhang J, Zhu L, Wang C, Chu
X, Pan Y, Bai Y, Shao C and Zhang J: Effects of hypoxia and
radiation-induced exosomes on migration of lung cancer cells and
angiogenesis of umbilical vein endothelial Cells. Radiat Res.
194:71–80. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chen S, Yang M and Chang S: LncRNA CCAL
Promotes Angiogenesis Through Regulating the MiR-29b/ANGPTL4 Axis
in Osteosarcoma. Cancer Manag Res. 12:10521–10530. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Okochi-Takada E, Hattori N, Tsukamoto T,
Miyamoto K, Ando T, Ito S, Yamamura Y, Wakabayashi M, Nobeyama Y
and Ushijima T: ANGPTL4 is a secreted tumor suppressor that
inhibits angiogenesis. Oncogene. 33:2273–2278. 2014. View Article : Google Scholar
|
|
71
|
Yang YH, Wang Y, Lam KS, Yau MH, Cheng KK,
Zhang J, Zhu W, Wu D and Xu A: Suppression of the Raf/MEK/ERK
signaling cascade and inhibition of angiogenesis by the carboxyl
terminus of angiopoietin-like protein 4. Arterioscler Thromb Vasc
Biol. 28:835–840. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ng KT, Xu A, Cheng Q, Guo DY, Lim ZX, Sun
CK, Fung JH, Poon RT, Fan ST, Lo CM and Man K: Clinical relevance
and therapeutic potential of angiopoietin-like protein 4 in
hepatocellular carcinoma. Mol Cancer. 13:1962014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gong L, Huang D, Shi Y, Liang Z and Bu H:
Regulated cell death in cancer: From pathogenesis to treatment.
Chin Med J (Engl). 136:653–665. 2023. View Article : Google Scholar
|
|
74
|
Galluzzi L, Vitale I, Aaronson SA, Abrams
JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews
DW, et al: Molecular mechanisms of cell death: recommendations of
the Nomenclature Committee on Cell Death 2018. Cell Death Differ.
25:486–541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Moujalled D, Strasser A and Liddell JR:
Molecular mechanisms of cell death in neurological diseases. Cell
Death Differ. 28:2029–2044. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Liu Y, Ding W, Wang J, Ao X and Xue J:
Non-coding RNA-mediated modulation of ferroptosis in cardiovascular
diseases. Biomed Pharmacother. 164:1149932023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: a basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Nuñez G, Benedict MA, Hu Y and Inohara N:
Caspases: the proteases of the apoptotic pathway. Oncogene.
17:3237–3245. 1998. View Article : Google Scholar
|
|
79
|
Saraste A and Pulkki K: Morphologic and
biochemical hallmarks of apoptosis. Cardiovasc Res. 45:528–537.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lim MMK, Wee JWK, Soong JC, Chua D, Tan
WR, Lizwan M, Li Y, Teo Z, Goh WWB, Zhu P and Tan NS: Targeting
metabolic flexibility via angiopoietin-like 4 protein sensitizes
metastatic cancer cells to chemotherapy drugs. Mol Cancer.
17:1522018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bai Y, Cui G, Sun X, Wei M, Liu Y, Guo J
and Yang Y: Effect of deletion of ANGPTL4 gene on viability,
migration and invasion ability and apoptosis of hepatocellular
carcinoma cells. Discov Med. 36:173–181. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li X, Chen T, Shi Q, Li J, Cai S, Zhou P,
Zhong Y and Yao L: Angiopoietin-like 4 enhances metastasis and
inhibits apoptosis via inducing bone morphogenetic protein 7 in
colorectal cancer cells. Biochem Biophys Res Commun. 467:128–134.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Hsieh HY, Shen CH, Lin RI, Feng YM, Huang
SY, Wang YH, Wu SF, Hsu CD and Chan MW: Cyproheptadine exhibits
antitumor activity in urothelial carcinoma cells by targeting GSK3β
to suppress mTOR and β-catenin signaling pathways. Cancer Lett.
370:56–65. 2016. View Article : Google Scholar
|
|
84
|
Sattari Fard F, Jalilzadeh N, Mehdizadeh
A, Sajjadian F and Velaei K: Understanding and targeting anoikis in
metastasis for cancer therapies. Cell Biol Int. 47:683–698. 2023.
View Article : Google Scholar
|
|
85
|
Yuan Z, Li Y, Zhang S, Wang X, Dou H, Yu
X, Zhang Z, Yang S and Xiao M: Extracellular matrix remodeling in
tumor progression and immune escape: From mechanisms to treatments.
Mol Cancer. 22:482023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chaojun L, Pengping L, Yanjun L, Fangyuan
Z, Yaning H, Yingbo S, Qi C and Hui L: TJP3 promotes T cell
immunity escape and chemoresistance in breast cancer: A
comprehensive analysis of anoikis-based prognosis prediction and
drug sensitivity stratification. Aging (Albany NY). 15:12890–12906.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liao YH, Chiang KH, Shieh JM, Huang CR,
Shen CJ, Huang WC and Chen BK: Epidermal growth factor-induced
ANGPTL4 enhances anoikis resistance and tumour metastasis in head
and neck squamous cell carcinoma. Oncogene. 36:2228–2242. 2017.
View Article : Google Scholar :
|
|
88
|
Shen CJ, Chan SH, Lee CT, Huang WC, Tsai
JP and Chen BK: Oleic acid-induced ANGPTL4 enhances head and neck
squamous cell carcinoma anoikis resistance and metastasis via
up-regulation of fibronectin. Cancer Lett. 386:110–122. 2017.
View Article : Google Scholar
|
|
89
|
Zhu P, Tan MJ, Huang RL, Tan CK, Chong HC,
Pal M, Lam CR, Boukamp P, Pan JY, Tan SH, et al: Angiopoietin-like
4 protein elevates the prosurvival intracellular O2(-):H2O2 ratio
and confers anoikis resistance to tumors. Cancer Cell. 19:401–415.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Baba K, Kitajima Y, Miyake S, Nakamura J,
Wakiyama K, Sato H, Okuyama K, Kitagawa H, Tanaka T, Hiraki M, et
al: Hypoxia-induced ANGPTL4 sustains tumour growth and anoikis
resistance through different mechanisms in scirrhous gastric cancer
cell lines. Sci Rep. 7:111272017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
San TT, Khaenam P, Prachayasittikul V,
Sripa B, Kunkeaw N and Chan-On W: Curcumin enhances
chemotherapeutic effects and suppresses ANGPTL4 in
anoikis-resistant cholangiocarcinoma cells. Heliyon. 6:e032552020.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhang Z, Cao L, Li J, Liang X, Liu Y, Liu
H, Du J, Qu Z, Cui M, Liu S, et al: Acquisition of anoikis
resistance reveals a synoikis-like survival style in BEL7402
hepatoma cells. Cancer Lett. 267:106–115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ness C, Garred Ø, Eide NA, Kumar T, Olstad
OK, Bærland TP, Petrovski G, Moe MC and Noer A: Multicellular tumor
spheroids of human uveal melanoma induce genes associated with
anoikis resistance, lipogenesis, and SSXs. Mol Vis. 23:680–694.
2017.PubMed/NCBI
|
|
94
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Agmon E, Solon J, Bassereau P and
Stockwell BR: Modeling the effects of lipid peroxidation during
ferroptosis on membrane properties. Sci Rep. 8:51552018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Tang D and Kroemer G: Ferroptosis. Curr
Biol. 30:R1292–R1297. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Tang D, Chen X, Kang R and Kroemer G:
Ferroptosis: Molecular mechanisms and health implications. Cell
Res. 31:107–125. 2021. View Article : Google Scholar :
|
|
98
|
Li D and Li Y: The interaction between
ferroptosis and lipid metabolism in cancer. Signal Transduct Target
Ther. 5:1082020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Pope LE and Dixon SJ: Regulation of
ferroptosis by lipid metabolism. Trends Cell Biol. 33:1077–1087.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhang Y, Liu X, Zeng L, Zhao X, Chen Q,
Pan Y, Bai Y, Shao C and Zhang J: Exosomal protein
angiopoietin-like 4 mediated radioresistance of lung cancer by
inhibiting ferroptosis under hypoxic microenvironment. Br J Cancer.
127:1760–1772. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Yang WH, Huang Z, Wu J, Ding CC, Murphy SK
and Chi JT: A TAZ-ANGPTL4-NOX2 axis regulates ferroptotic cell
death and chemoresistance in epithelial ovarian cancer. Mol Cancer
Res. 18:79–90. 2020. View Article : Google Scholar :
|
|
103
|
Broz P, Pelegrín P and Shao F: The
gasdermins, a protein family executing cell death and inflammation.
Nat Rev Immunol. 20:143–157. 2020. View Article : Google Scholar
|
|
104
|
Wei X, Xie F, Zhou X, Wu Y, Yan H, Liu T,
Huang J, Wang F, Zhou F and Zhang L: Role of pyroptosis in
inflammation and cancer. Cell Mol Immunol. 19:971–992. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Strowig T, Henao-Mejia J, Elinav E and
Flavell R: Inflammasomes in health and disease. Nature.
481:278–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Fang Y, Tian S, Pan Y, Li W, Wang Q, Tang
Y, Yu T, Wu X, Shi Y, Ma P and Shu Y: Pyroptosis: A new frontier in
cancer. Biomed Pharmacother. 121:1095952020. View Article : Google Scholar
|
|
107
|
Man SM, Karki R and Kanneganti TD:
Molecular mechanisms and functions of pyroptosis, inflammatory
caspases and inflammasomes in infectious diseases. Immunol Rev.
277:61–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Huang Y, Xu W and Zhou R: NLRP3
inflammasome activation and cell death. Cell Mol Immunol.
18:2114–2127. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Sharma BR and Kanneganti TD: NLRP3
inflammasome in cancer and metabolic diseases. Nat Immunol.
22:550–559. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Sun B, Bai L, Li Q, Sun Y, Li M, Wang J,
Shi X and Zhao M: Knockdown of angiopoietin-like 4 suppresses
sepsis-induced acute lung injury by blocking the NF-κB pathway
activation and hindering macrophage M1 polarization and pyroptosis.
Toxicol In Vitro. 94:1057092024. View Article : Google Scholar
|
|
112
|
DeBerardinis RJ, Lum JJ, Hatzivassiliou G
and Thompson CB: The biology of cancer: metabolic reprogramming
fuels cell growth and proliferation. Cell Metab. 7:11–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Kaelin WG Jr and Thompson CB: Q&A:
Cancer: Clues from cell metabolism. Nature. 465:562–564. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Martínez-Reyes I and Chandel NS: Cancer
metabolism: Looking forward. Nat Rev Cancer. 21:669–680. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N,
Yi P, Tang L, Pan Q, Rao S, et al: The cancer metabolic
reprogramming and immune response. Mol Cancer. 20:282021.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Pan C, Li B and Simon MC: Moonlighting
functions of metabolic enzymes and metabolites in cancer. Mol Cell.
81:3760–3774. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Hsu PP and Sabatini DM: Cancer cell
metabolism: Warburg and beyond. Cell. 134:703–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Fendt SM: 100 years of the Warburg effect:
A cancer metabolism endeavor. Cell. 187:3824–3828. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Paul S, Ghosh S and Kumar S: Tumor
glycolysis, an essential sweet tooth of tumor cells. Semin Cancer
Biol. 86(Pt 3): 1216–1230. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zheng X, Liu R, Zhou C, Yu H, Luo W, Zhu
J, Liu J, Zhang Z, Xie N, Peng X, et al: ANGPTL4-mediated promotion
of glycolysis facilitates the colonization of fusobacterium
nucleatum in colorectal cancer. Cancer Res. 81:6157–6170. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Mizuno S, Seishima R, Yamasaki J, Hattori
K, Ogiri M, Matsui S, Shigeta K, Okabayashi K, Nagano O, Li L and
Kitagawa Y: Angiopoietin-like 4 promotes glucose metabolism by
regulating glucose transporter expression in colorectal cancer. J
Cancer Res Clin Oncol. 148:1351–1361. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Hanahan D: Hallmarks of cancer: New
dimensions. Cancer Discov. 12:31–46. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Currie E, Schulze A, Zechner R, Walther TC
and Farese RV Jr: Cellular fatty acid metabolism and cancer. Cell
Metab. 18:153–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Jin Z, Chai YD and Hu S: Fatty acid
metabolism and cancer. Adv Exp Med Biol. 1280:231–241. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Lee JY, Nam M, Son HY, Hyun K, Jang SY,
Kim JW, Kim MW, Jung Y, Jang E, Yoon SJ, et al: Polyunsaturated
fatty acid biosynthesis pathway determines ferroptosis sensitivity
in gastric cancer. Proc Natl Acad Sci USA. 117:32433–32442. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Pham DV and Park PH: Adiponectin triggers
breast cancer cell death via fatty acid metabolic reprogramming. J
Exp Clin Cancer Res. 41:92022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Dijk W and Kersten S: Regulation of
lipoprotein lipase by Angptl4. Trends Endocrinol Metab. 25:146–155.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Georgiadi A, Lichtenstein L, Degenhardt T,
Boekschoten MV, van Bilsen M, Desvergne B, Müller M and Kersten S:
Induction of cardiac Angptl4 by dietary fatty acids is mediated by
peroxisome proliferator-activated receptor beta/delta and protects
against fatty acid-induced oxidative stress. Circ Res.
106:1712–1721. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhang R and Zhang K: An updated
ANGPTL3-4-8 model as a mechanism of triglyceride partitioning
between fat and oxidative tissues. Prog Lipid Res. 85:1011402022.
View Article : Google Scholar :
|
|
131
|
Lichtenstein L, Mattijssen F, de Wit NJ,
Georgiadi A, Hooiveld GJ, van der Meer R, He Y, Qi L, Köster A,
Tamsma JT, et al: Angptl4 protects against severe proinflammatory
effects of saturated fat by inhibiting fatty acid uptake into
mesenteric lymph node macrophages. Cell Metab. 12:580–592. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Xiao S, Nai-Dong W, Jin-Xiang Y, Long T,
Xiu-Rong L, Hong G, Jie-Cheng Y and Fei Z: ANGPTL4 regulate
glutamine metabolism and fatty acid oxidation in nonsmall cell lung
cancer cells. J Cell Mol Med. 26:1876–1885. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Blücher C, Iberl S, Schwagarus N, Müller
S, Liebisch G, Höring M, Hidrobo MS, Ecker J, Spindler N, Dietrich
A, et al: Secreted factors from adipose tissue reprogram tumor
lipid metabolism and induce motility by modulating PPARα/ANGPTL4
and FAK. Mol Cancer Res. 18:1849–1862. 2020. View Article : Google Scholar
|
|
134
|
Cai Z, Li Y, Ma M, Wang L, Wang H, Liu M
and Jiang C: Adipocytes promote pancreatic cancer migration and
invasion through fatty acid metabolic reprogramming. Oncol Rep.
50:1412023. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Lieu EL, Nguyen T, Rhyne S and Kim J:
Amino acids in cancer. Exp Mol Med. 52:15–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Lippert TH, Ruoff HJ and Volm M: Intrinsic
and acquired drug resistance in malignant tumors. The main reason
for therapeutic failure. Arzneimittelforschung. 58:261–264.
2008.PubMed/NCBI
|
|
137
|
Olivares-Urbano MA, Griñán-Lisón C,
Marchal JA and Núñez MI: CSC Radioresistance: A therapeutic
challenge to improve radiotherapy effectiveness in cancer. Cells.
9:16512020. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Fekir K, Dubois-Pot-Schneider H, Désert R,
Daniel Y, Glaise D, Rauch C, Morel F, Fromenty B, Musso O, Cabillic
F and Corlu A: Retrodifferentiation of Human Tumor Hepatocytes to
Stem Cells Leads to Metabolic Reprogramming and Chemoresistance.
Cancer Res. 79:1869–1883. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Li C, Wang Q, Luo Y and Xiang J: TAZ
regulates the cisplatin resistance of epithelial ovarian cancer
cells via the ANGPTL4/SOX2 axis. Anal Cell Pathol (Amst).
2022:56321642022.PubMed/NCBI
|
|
140
|
Zhou S, Wang R and Xiao H: Adipocytes
induce the resistance of ovarian cancer to carboplatin through
ANGPTL4. Oncol Rep. 44:927–938. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Tsai YT, Wu AC, Yang WB, Kao TJ, Chuang
JY, Chang WC and Hsu TI: ANGPTL4 induces TMZ resistance of
glioblastoma by promoting cancer stemness enrichment via the
EGFR/AKT/4E-BP1 cascade. Int J Mol Sci. 20:56252019. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Gordon ER, Wright CA, James M and Cooper
SJ: Transcriptomic and functional analysis of ANGPTL4
overexpression in pancreatic cancer nominates targets that reverse
chemoresistance. BMC Cancer. 23:5242023. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Li H, Ge C, Zhao F, Yan M, Hu C, Jia D,
Tian H, Zhu M, Chen T, Jiang G, et al: Hypoxia-inducible factor 1
alpha-activated angiopoietin-like protein 4 contributes to tumor
metastasis via vascular cell adhesion molecule-1/integrin β1
signaling in human hepatocellular carcinoma. Hepatology.
54:910–919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Adhikary T, Brandt DT, Kaddatz K, Stockert
J, Naruhn S, Meissner W, Finkernagel F, Obert J, Lieber S, Scharfe
M, et al: Inverse PPARβ/δ agonists suppress oncogenic signaling to
the ANGPTL4 gene and inhibit cancer cell invasion. Oncogene.
32:5241–5252. 2013. View Article : Google Scholar
|
|
145
|
Tian L, Zhou J, Casimiro MC, Liang B,
Ojeifo JO, Wang M, Hyslop T, Wang C and Pestell RG: Activating
peroxisome proliferator-activated receptor gamma mutant promotes
tumor growth in vivo by enhancing angiogenesis. Cancer Res.
69:9236–9244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Kim SH, Park YY, Kim SW, Lee JS, Wang D
and DuBois RN: ANGPTL4 induction by prostaglandin E2 under hypoxic
conditions promotes colorectal cancer progression. Cancer Res.
71:7010–7020. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Pitt JM, Marabelle A, Eggermont A, Soria
JC, Kroemer G and Zitvogel L: Targeting the tumor microenvironment:
Removing obstruction to anticancer immune responses and
immunotherapy. Ann Oncol. 27:1482–1492. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Zhou S, Tu J, Ding S, Lu G, Lin Z, Ding Y,
Deng B, Zhang Y, Xiao W and Gong W: High expression of
angiopoietin-like protein 4 in advanced colorectal cancer and its
association with regulatory T Cells and M2 macrophages. Pathol
Oncol Res. 26:1269–1278. 2020. View Article : Google Scholar
|
|
150
|
Long F, Wang W, Li S, Wang B, Hu X, Wang
J, Xu Y, Liu M, Zhou J, Si H, et al: The potential crosstalk
between tumor and plasma cells and its association with clinical
outcome and immunotherapy response in bladder cancer. J Transl Med.
21:2982023. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Li F and Ding J: Sialylation is involved
in cell fate decision during development, reprogramming and cancer
progression. Protein Cell. 10:550–565. 2019. View Article : Google Scholar :
|
|
152
|
Ma X, Li M, Wang X, Qi G, Wei L and Zhang
D: Sialylation in the gut: From mucosal protection to disease
pathogenesis. Carbohydr Polym. 343:1224712024. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Calabrese V, Zirino F, Vienna FG, Siligato
R, Cernaro V and Santoro D: Insight into the role of
angiopoietin-like protein 4 in podocypopathies (Review). World Acad
Sci J. 6:292024. View Article : Google Scholar
|
|
154
|
Chugh SS and Clement LC: 'Idiopathic'
minimal change nephrotic syndrome: A podocyte mystery nears the
end. Am J Physiol Renal Physiol. 325:F685–F694. 2023. View Article : Google Scholar
|
|
155
|
Smith BAH, Deutzmann A, Correa KM,
Delaveris CS, Dhanasekaran R, Dove CG, Sullivan DK, Wisnovsky S,
Stark JC, Pluvinage JV, et al: MYC-driven synthesis of siglec
ligands is a glycoimmune checkpoint. Proc Natl Acad Sci USA.
120:e22153761202023. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Stanczak MA and Läubli H: Siglec receptors
as new immune checkpoints in cancer. Mol Aspects Med.
90:1011122023. View Article : Google Scholar
|
|
157
|
Zhao J, Liu J, Wu N, Zhang H, Zhang S, Li
L and Wang M: ANGPTL4 overexpression is associated with progression
and poor prognosis in breast cancer. Oncol Lett. 20:2499–2505.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Nie D, Zheng Q, Liu L, Mao X and Li Z:
Up-regulated of angiopoietin-like protein 4 predicts poor prognosis
in cervical cancer. J Cancer. 10:1896–1901. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Aung TM, Ciin MN, Silsirivanit A, Jusakul
A, Lert-Itthiporn W, Proungvitaya T, Roytrakul S and Proungvitaya
S: Serum angiopoietin-like protein 4: A potential prognostic
biomarker for prediction of vascular invasion and lymph node
metastasis in cholangiocarcinoma patients. Front Public Health.
10:8369852022. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Wang FT, Li XP, Pan MS, Hassan M, Sun W
and Fan YZ: Identification of the prognostic value of elevated
ANGPTL4 expression in gallbladder cancer-associated fibroblasts.
Cancer Med. 10:6035–6047. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Shibata K, Nakayama T, Hirakawa H, Hidaka
S and Nagayasu T: Clinicopathological significance of
angiopoietin-like protein 4 expression in oesophageal squamous cell
carcinoma. J Clin Pathol. 63:1054–1058. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Tanaka T, Imamura T, Irie A, Yoneda M,
Imamura R, Kikuchi K, Kitagawa S, Kubo T, Ogi H and Nakayama H:
Association of high cellular expression and plasma concentration of
angiopoietin-like 4 with tongue cancer lung metastasis and poor
prognosis. Oncol Lett. 24:2992022. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Dong D, Jia L, Zhou Y, Ren L, Li J and
Zhang J: Serum level of ANGPTL4 as a potential biomarker in renal
cell carcinoma. Urol Oncol. 35:279–285. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Kubo H, Kitajima Y, Kai K, Nakamura J,
Miyake S, Yanagihara K, Morito K, Tanaka T, Shida M and Noshiro H:
Regulation and clinical significance of the hypoxia-induced
expression of ANGPTL4 in gastric cancer. Oncol Lett. 11:1026–1034.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Sukonina V, Lookene A, Olivecrona T and
Olivecrona G: Angiopoietin-like protein 4 converts lipoprotein
lipase to inactive monomers and modulates lipase activity in
adipose tissue. Proc Natl Acad Sci USA. 103:17450–17455. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Mysling S, Kristensen KK, Larsson M,
Kovrov O, Bensadouen A, Jørgensen TJ, Olivecrona G, Young SG and
Ploug M: The angiopoietin-like protein ANGPTL4 catalyzes unfolding
of the hydrolase domain in lipoprotein lipase and the endothelial
membrane protein GPIHBP1 counteracts this unfolding. Elife.
5:e209582016. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Yoshida K, Shimizugawa T, Ono M and
Furukawa H: Angiopoietin-like protein 4 is a potent
hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein
lipase. J Lipid Res. 43:1770–1772. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Dijk W, Beigneux AP, Larsson M, Bensadoun
A, Young SG and Kersten S: Angiopoietin-like 4 promotes
intracellular degradation of lipoprotein lipase in adipocytes. J
Lipid Res. 57:1670–1683. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Reimund M, Kovrov O, Olivecrona G and
Lookene A: Lipoprotein lipase activity and interactions studied in
human plasma by isothermal titration calorimetry. J Lipid Res.
58:279–288. 2017. View Article : Google Scholar :
|
|
170
|
Kersten S: New insights into
angiopoietin-like proteins in lipid metabolism and cardiovascular
disease risk. Curr Opin Lipidol. 30:205–211. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Huang RL, Teo Z, Chong HC, Zhu P, Tan MJ,
Tan CK, Lam CR, Sng MK, Leong DT, Tan SM, et al: ANGPTL4 modulates
vascular junction integrity by integrin signaling and disruption of
intercellular VE-cadherin and claudin-5 clusters. Blood.
118:3990–4002. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Cazes A, Galaup A, Chomel C, Bignon M,
Bréchot N, Le Jan S, Weber H, Corvol P, Muller L, Germain S and
Monnot C: Extracellular matrix-bound angiopoietin-like 4 inhibits
endothelial cell adhesion, migration, and sprouting and alters
actin cytoskeleton. Circ Res. 99:1207–1215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Sheridan C: Amgen's angiopoietin blocker
fails in ovarian cancer. Nat Biotechnol. 33:5–6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Padua D, Zhang XH, Wang Q, Nadal C, Gerald
WL, Gomis RR and Massagué J: TGFbeta primes breast tumors for lung
metastasis seeding through angiopoietin-like 4. Cell. 133:66–77.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Simeon J, Thrush J and Bailey TA:
Angiopoietin-like protein 4 is a chromatin-bound protein that
enhances mammosphere formation in vitro and experimental
triple-negative breast cancer brain and liver metastases in vivo. J
Carcinog. 20:82021. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Nakayama T, Hirakawa H, Shibata K, Nazneen
A, Abe K, Nagayasu T and Taguchi T: Expression of angiopoietin-like
4 (ANGPTL4) in human colorectal cancer: ANGPTL4 promotes venous
invasion and distant metastasis. Oncol Rep. 25:929–935. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Yi J, Pan BZ, Xiong L and Song HZ:
Clinical significance of angiopoietin-like protein 4 expression in
tissue and serum of esophageal squamous cell carcinoma patients.
Med Oncol. 30:6802013. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Nakayama T, Hirakawa H, Shibata K, Abe K,
Nagayasu T and Taguchi T: Expression of angiopoietin-like 4 in
human gastric cancer: ANGPTL4 promotes venous invasion. Oncol Rep.
24:599–606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Ma T, Jham BC, Hu J, Friedman ER, Basile
JR, Molinolo A, Sodhi A and Montaner S: Viral G protein-coupled
receptor up-regulates Angiopoietin-like 4 promoting angiogenesis
and vascular permeability in Kaposi's sarcoma. Proc Natl Acad Sci
USA. 107:14363–14368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Yang W, Khoury E, Guo Q, Prabhu SA, Emond
A, Huang F, Gonçalves C, Zhan Y, Plourde D, Nichol JN, et al: MNK1
signaling induces an ANGPTL4-mediated gene signature to drive
melanoma progression. Oncogene. 39:3650–3665. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Zhu X, Guo X, Wu S and Wei L: ANGPTL4
correlates with NSCLC progression and regulates
epithelial-mesenchymal transition via ERK pathway. Lung.
194:637–646. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Lou H, Lin X, Wei G, Wu Z and Xiao Y:
Construction of an Anoikis-related gene prognostic signature and
identification of ANGPTL4 as a key oncogene in lung adenocarcinoma.
Mol Biotechnol. 66:1290–1302. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Al-Kadash A, Alshaer W, Mahmoud IS,
Wehaibi S and Zihlif M: Enhancing chemosensitivity of PANC1
pancreatic cancer cells to gemcitabine using ANGTPL4, Notch1 and
NF-κβ1 siRNAs. Future Sci OA. 10:FSO9182024. View Article : Google Scholar
|
|
184
|
Hata S, Nomura T, Iwasaki K, Sato R,
Yamasaki M, Sato F and Mimata H: Hypoxia-induced angiopoietin-like
protein 4 as a clinical biomarker and treatment target for human
prostate cancer. Oncol Rep. 38:120–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Tanaka T, Imamura T, Yoneda M, Irie A, Ogi
H, Nagata M, Yoshida R, Fukuma D, Kawahara K, Shinohara M and
Nakayama H: Enhancement of active MMP release and invasive activity
of lymph node metastatic tongue cancer cells by elevated signaling
via the TNF-α-TNFR1-NF-κB pathway and a possible involvement of
angiopoietin-like 4 in lung metastasis. Int J Oncol. 49:1377–1384.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Yu Y, Zhang M, Liu J, Xu B, Yang J, Wang
N, Yan S, Wang F, He X, Ji G, et al: Long Non-coding RNA PVT1
promotes cell proliferation and migration by silencing ANGPTL4
expression in cholangiocarcinoma. Mol Ther Nucleic Acids.
13:503–513. 2018. View Article : Google Scholar : PubMed/NCBI
|