Introduction
Of all malignant tumors, esophageal cancer has the
seventh highest incidence and sixth highest mortality worldwide in
2020 (1). The predominant
pathological classifications of esophageal cancer include
esophageal squamous cell carcinoma (ESCC) and esophageal
adenocarcinoma, with ESCC being the prevailing pathological subtype
(2). China has a high incidence
of esophageal cancer and ESCC. Moreover, China holds the top
position globally in terms of both annual incidence (324,422,
53.7%) and mortality rates (301,135, 55.3%) of esophageal cancer in
2020 (1). Although comprehensive
treatment, which is primarily based on surgery, has been applied in
clinical practice, the poor prognosis, rapid progression and early
metastasis of esophageal cancer lead to a 5-year survival rate of
20% (3), and the primary factor
affecting the prognosis of patients is lymph node metastasis.
Moreover, molecular biomarkers such as PD-L1 have been identified
to predict prognosis and applied as therapeutic targets, which
improves the prognosis of ESCC (4). Therefore, identifying novel
molecular biomarkers associated with lymph node metastasis and the
underlying molecular mechanism are highly important for patients
with ESCC.
The solute carrier subfamily 25 member 1 (SLC25A1),
identified as the mitochondrial citrate/isocitrate carrier or
citrate transport protein, comprises solute carrier proteins
embedded in the inner mitochondrial membrane (5,6).
The SLC25A1 protein facilitates transport of citrate either from
the cytoplasm into the mitochondria to participate in the
tricarboxylic acid cycle reaction as a substrate, producing ATP or,
conversely, from mitochondria into the cytoplasm as precursors for
fatty acid, cholesterol and triglyceride synthesis by exchanging
for malate (7,8). Thus, SLC25A1 serves an important
role in cell energy metabolism and lipid synthesis. Lipid
metabolism reprogramming is one of the hallmarks of malignancy and
can promote cancer progression and metastasis in multiple ways: It
not only supplies the substrates and energy for rapid proliferation
but can also induce epithelial-mesenchymal transition, resistance
to ferroptosis, immune escape and the activation of oncogenic
pathways such as the Hedgehog and mTOR signaling pathways as signal
messengers (9-15). Zhou also confirmed that fatty acid
2-hydroxylase promotes the metastasis of ESCC by regulating lipid
metabolism (16). Upregulation of
SLC25A1 has been discovered in lung and colon cancer and
demonstrates an association with tumor development via the
regulation of lipid metabolism (17,18). Nevertheless, SLC25A1 expression in
ESCC and its role in ESCC development require further
exploration.
It was hypothesized that SLC25A1 may promote the
progression of ESCC by regulating lipid metabolism. Therefore, the
present study aimed to investigate the expression of SLC25A1 in
ESCC and determine whether its expression is correlated with
clinical and pathological attributes. Furthermore, the role of
SLC25A1 in lipid metabolism and oxidative phosphorylation in ESCC
and potential underlying mechanisms were explored to provide
potential novel targets and theoretical foundations for the
treatment of ESCC.
Materials and methods
Patients
A total of 97 cancer tissue samples were obtained
from patients (age, 42-77 years; 75 male, 22 female) with ESCC who
underwent esophageal cancer resection at Shandong Provincial
Hospital affiliated with Shandong First Medical University (Jinan,
China) in January-December 2017, along with corresponding
non-cancerous tissue samples (distance, 5 cm). All patients met the
following criteria: i) Postoperative pathological confirmation of
ESCC; ii) absence of preoperative radiotherapy treatment; iii)
postoperative pathological confirmation of negative cancer tissue
margins; iv) no severe preoperative complications and v) all
patients were followed-up for ≥3 year. The present study was
approved by the Ethics Committee of Shandong Provincial Hospital,
affiliated with Shandong First Medical University (approval no.
SZRJJ: NO.2022-015). All procedures were performed in accordance
with Guidelines for the Work of Ethics Review Committees in China
(19).
Cell lines and culture
Human esophageal cancer cell lines (KYSE150, 30, 450
and 510) and HeLa cervical cancer cells were obtained from the Cell
Resource Center of Shanghai Institutes for Biological Sciences,
Chinese Academy of Sciences. All cells were cultured in RPMI-1640
medium comprising 10% fetal bovine serum (FBS; both Gibco, US) with
L-glutamine and maintained in an incubator with 5% CO2
at 37°C. CTPI-2, a specific blocker of SLC25A1 protein, was used to
inhibit activity of SLC25A1 protein. A stock solution was prepared
by dissolving CTPI-2 (MedChemExpress) powder in DMSO. The stock
solution was diluted with phosphate buffer (Gibco; Thermo Fisher
Scientific, Inc.) to achieve the required concentration (30
μM) before being added into the culture medium for further
use. In the lentivirus transfected group, the cells without
lentivirus transfection were the blank group. In the dosing group,
cells were cultured in the medium without DMSO and CTPI-2 as the
blank group.
Immunohistochemistry
All tissues were fixed with 4% paraformaldehyde for
24 h at 25°C, embedded in paraffin and sectioned into 5-μm
slices. Following deparaffinization, washing with xylene for 45
min) and rehydration with ethanol (75, 85, 95, 100%) for five
minutes each), sections were treated with 0.01 mol/l citrate buffer
for 15 min at 100°C for antigen retrieval. Then the sections were
put into 3% hydrogen peroxide solution for 30 min at 37°C for
quenching. The sections were then treated with 10% goat serum
(G1208-5ML, Servicebio, China) for 10 min at 25°C. The sections
were exposed to anti-SLC25A1 (1:500, 15235-1-AP, Proteintech) or
anti-FGFBP1 (1:500, bs-1768R, Bioss antibodies) antibody at 4°C
overnight. The sections were subsequently incubated for 30 min with
a horseradish peroxidase (HRP)-conjugated secondary antibody from
the Immunohistochemistry kit (1:200, G1215-200T, Servicebio, China)
at 25°C and then stained (25°C, 3 min) with DAB and hematoxylin.
Proportion of positive cells was scored as follows: 0-5, 0; 6-25,
1; 26-50, 2; 51-75, 3 and 76-100%, 4. Positive staining intensity
scoring was as follows: 0, negative, 1 weak, 2 moderate and 3
strong staining. The total immunohistochemistry staining score
(IHS) was calculated by multiplying the proportion of positively
stained cells score by the positive staining intensity score. A
total score of 0-7 represented low expression, whereas a score of
8-12 represented high expression. The samples were observed under a
light microscope (200×) and independently scored by two
pathologists.
Hematoxylin-Eosin staining: All tissues were fixed
in formalin, embedded in paraffin and sectioned into 5-μm
slices. Following deparaffinization and rehydration, the sections
were stained with hematoxylin dye for 3 min, treated with 1%
hydrochloric acid alcohol for 30 sec, stained with eosin dye for 2
min, dehydrated with ethanol (75, 85, 95, 100%), washed with
xylene, and finally sealed with neutral resin. The results were
observed under light microscope.
Lentiviral infection
SLC51A1 RNA-interfering (5′-CCAUCAAGGUGAAGUUCAU-3′)
and negative control lentivirus were obtained from Beijing Tsingke
Biotech. The sequences were negative control: sense
5′-UUCUCCGAACGUGUCACGU-3′. sh)RNA was subcloned into the
pLKO.1-puro vector. The generation system is the second system.
Subsequently, pLKO.1-puro-shRNA plasmid (20 μg) and virus
packaging plasmids (pMD2.G, 5 μg; psPAX2, 10 μg) were
cotransfected into 293T cells (China Center for Type Culture
Collection, Wuhan, China) using Lipofectamine™ 2000 (Thermo Fisher
Scientific, Inc.) at 37°C for 6 h. Medium was replaced with fresh
DMEM (Thermo Fisher Scientific, Inc.) containing 10% FBS and
incubated at 37°C for 48 h. The cell supernatant was collected,
then filtered through a 0.45-μm filter (Pall Life Sciences,
Port Washington, NY, USA). MOI for lentivirus transfection was 20.
KYSE150 and KYSE30 cells in the exponential growth phase were
plated in 6-well plates and cultured for 24 h. SLC51A1-interfering
and negative control lentivirus were inoculated into the cells.
Following 24 h incubation, the cell medium was replaced with
complete medium (RPMI-1640 medium comprising 10% FBS, Gibco, US).
The monoclonal cells were stably and continuously expressed after
screening with purinomycin (5 μg/ml). The time interval
between transduction and follow-up experiment was 10 days. The
purinomycin concentration for maintenance was 0.25
μg/ml.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was isolated from cells using TRIzol
(Thermo Fisher Scientific, US), followed by RT via Evo M-MLV RT Kit
with gDNA Clean for qPCR., Accurate Biology) according to the
manufacturer's instructions. qPCR was performed using SYBR Green
Real-time PCR Master Mix (Takara Biotechnology Co., Ltd.) on a
LightCycler 480 (Roche, Switzerland). Thermocycling conditions:
Initial denaturation: 95°C, 10 sec. Denaturation: 95°C, 5 sec.
Annealing and extension: 60°C, 30 sec, 40 cycles. The relative mRNA
expression was measured by the ∆∆Cq method (20). The internal reference gene was
GAPDH. The primer sequences were as follows: SLC25A1 forward,
5′-CCAUCAAGGUGAAGUUCAU-3′ and reverse, 5′-AUGAACUUCACCUUGAUGG3′;
FGFBP1 (Fibroblast Growth Factor Binding Protein 1) forward,
5′-CTTCACAGCAAAGTGGTCTCA-3′ and reverse,
5′-GACACAGGAAAATTCATGGTCCA-3′ and GAPDH forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse,
5′-TGGTGAAGACGCCAGTGGA-3′.
RNA-seq analysis and bioinformatics
analysis
RNA seq data for human ESCC cells were acquired from
The Cancer Genome Atlas database. (TCGA, ualcan.path.uab.edu/cgi-bin/TCGAExResultNew2.pl?genenam=SLC25A1&ctype=ESCA).
Following transfection of KYSE150 cells with SLC25A1-interfering
lentivirus or nonsense lentivirus, the total RNA was extracted
using TRIzol (Cat. No. 15596026, Thermo Fisher) and treated with
DNase to remove genomic DNA contamination. The NEBNext® Poly (A)
mRNA Magnetic Isolation Module and NEBNext® Ultra™ II mRNA Library
Prep kit (cat. no. NEB #E7770S/L, Cat. No. #E7775S/L, New England
Biolabs, Inc.,) for Illumina® were used for mRNA isolation and
library construction following the manufacturer's protocols. And
then the RNA-seq library was sequenced using an Illumina NovaSeq
6000 PE150 instrument (Illumina, Inc.) by Haplox Genomics Center.
DESeq2 (1.18.1) and edegR (3.209.) were used for Difference
analysis (21,22), and the ClusterProfier (4.8.2) was
used for Reactcome enrichment analysis (23).
Western blot analysis
Tissue and cellular proteins were isolated using
PMSF-containing RIPA lysis buffer (Beijing Solarbio Science &
Technology Co., Ltd.). The protein concentration was evaluated via
a BCA protein concentration assay and the loading quantity of the
samples was calculated. The protein samples were added to 10% PAGE
(20 μg/lane) for electrophoretic separation and
electrotransferred to a PVDF membrane. PVDF membrane at 25°C was
blocked with 10% skimmed milk powder for 2 h to prevent specific
antibody binding. The membrane was incubated with SLC25A1 (1:2,000,
15235-1-AP, Proteintech), AKT (1:1,000, db14689, diagbio),
phosphorylated (p)-AKT (1:1,000, db12718, diagbio), FGFBP1
(1:1,000, bs-1768R, Bioss antibodies), GAPDH (1:1,0000, bs-10900R,
Bioss antibodies) and β-actin (1:1,000, bs-0061R, Bioss antibodies)
primary antibodies in a shaker at 4°C overnight, followed by
rinsing with TBST (Tris Buffered Saline with 0.1% Tween-20). The
membrane was exposed to HRP-conjugated goat anti-rabbit IgG
polyclonal secondary antibody (1:5,000, HA1008, Huabio, China) for
1 h at room temperature on a shaker and rinsing with TBST. Finally,
the PVDF membranes were treated with visualisation reagent
(Immobilon ECL Ultra Western HRP Substrate, Millipore) and detected
with Amersham Imager 680 (GE HealthCare, US). ImageJ (National
Institutes of Health) was used to analyze the gray values of each
blot.
EdU cell proliferation assay
A total of 5,000 transfected or untransfected cells
in the logarithmic growth phase were inoculated into each well of a
96-well plate. After 24 h, the cells were incubated in
CTPI-2-containing or CTPI-2 free medium at 37°C for 2 days. The
cells were fixed with 4% paraformaldehyde at 25°C for 30 min and
stained with an EdU fluorescence staining kit (Cell-Light EdU
Apollo In Vitro Kit; ribobio) according to the manufacturer's
guidelines. Images were captured by inverted fluorescence
microscope (200×) and ZEN 3.3 blue edition software (Zeiss,
Germany).
Colony formation assay
Following 24 h inoculation in 6-well plates, the
untransfected or transfected KYSE150 cells and KYSE30 cells were
cultured with CTPI-2-containing or CTPI-2 free medium. The medium
was changed every 3 days, and the cells were maintained at 37°C
with 5% CO2 for 10 days. The cells were washed with PBS,
fixed with 4% paraformaldehyde at 25°C for 30 min and stained with
0.1% crystal violet at 25°C for 3 min. The number of cells in a
single clone exceeding 50 is called a colony, and the results were
detected by ImageJ software (ImageJ 1.50b, National Institutes of
Health).
Cell Counting Kit (CCK)8 assay
A total of 5,000 cells in the exponential growth
phase were inoculated in 96-well plates. At 24 h post-inoculation,
the medium was changed to CTPI-2-containing or CTPI-2 free medium.
After 24, 48, 72 and 96 h incubation at 37°C, CCK8 reagent
(MedChemExpress, US) was added for 1 h at 37°C. A microplate reader
(Multiskan Go, Thermo) was used to measure the absorbance of each
well at 450 nm.
Wound healing assay
Untransfected KYSE150 cells and KYSE30 cells were
incubated with serum-free medium containing CTPI-2 in 6-well
plates, the transfected KYSE150 cells and KYSE30 cells were
incubated with serum-free medium in 6-well plates. At 90-95%
confluence, a scratch was made in using a pipette tip (200
μl), and the cells were incubated in serum-free medium with
or without CTPI-2 for 24 h. Then, the scratch was imaged under a
light microscope at 0 and 24 h. The wound area at the same location
was subsequently measured via ImageJ. The cell migration rate was
calculated as follows: Cell migration rate (%)=(initial wound
area-wound area after 24 h)/initial wound area ×100%.
Cell migration and invasion assay
Transwell upper chambers coated with Matrigel (BD
Science, US) at 37°C for 1 h were used to determine the invasive
ability of cells, whereas upper chambers lacking Matrigel coating
were used to determine migratory ability. FBS-free RPMI-1640 medium
mixed with 50,000 cells was added to the upper chambers. For
untransfected cells, CTPI-2 reagent (30 μM) was added to the
upper chambers. RPMI-1640 Medium with a 15% FBS concentration was
added to the lower chambers. Following incubation for 24 or 48 h at
37°C in a 5% CO2 incubator, the cells on the lower
surface were fixed with 4% paraformaldehyde at 25°C for 30 min,
stained with crystal violet at 25°C for 10 min and sealed. The
slides were observed under a light microscope (200×) and images
were captured in three randomly selected fields of view.
Apoptosis assay
Apoptosis was detected via flow cytometry using an
Annexin V-PE/7-AAD Apoptosis Detection kit (cat. no. MA0429, Meilun
Biotechnology Co., Ltd.). The transfected or untransfected KYSE150
cells and KYSE30 cells were cultured in 6-well plates until the
cell density reached 85%. The cells were digested with EDTA-free
trypsin and centrifuged (1,000 g, 5 min) to collect the cell
pellets, which was washed with PBS (Gibco, US) solution precooled
at 4°C. Binding buffer working fluid was added to the cell pellets
and the cell concentration was suspended to 1×10^6/ml. 100
μl cell suspension (the total number of cells was 1×10^5)
was absorbed, 5 μl Annexin V-PE and 7-AAD dye were added to
the cell suspension, mixed and incubated at 25°C for 15 min without
light. BD LSRFortessa (BD Biosciences) and BD FACSDiva 7.0 software
(BD Biosciences, US) were used to determine degree of apoptosis.
The apoptosis rate was the sum of early and late apoptotic
cells.
Determination of intracellular lipid
content
The lipid content in the transfected or
untransfected KYSE150 cells and KYSE30 cells was determined via the
Triglyceride, Free Fatty Acid and Total Cholesterol Content Assay
kits (BC0625, BC0595, BC1985) (Beijing Solarbio Science &
Technology Co., Ltd.) according to the manufacturer's
instructions.
BODIPY 493/503 staining of intracellular
lipids
ESCC cells were treated with BODIPY 493/503
fluorescent dye (5 μM) (MedChemExpress) at room temperature
for 30 min and shielded from light, to visualize lipid distribution
within the cells. Images were captured using a fluorescence
microscope.
Measurement of cellular oxygen
consumption rate
A total of 50,000 ESCC cells were seeded in 96-well
plates in the dark. Cells were cultivated at 37°C in a glucose-free
RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) for 24 h. The
oxygen consumption rate was assessed via fluorescence microplate
using Extracellular OCR Plate Assay kit (Dojindo, E297) according
to the manufacturer's instructions.
Measurement of mitochondrial respiratory
chain complex activity
Mitochondrial respiratory chain activity was
evaluated via the Mitochondrial Respiratory Chain Complex I-V
Activity Assay kit (BC0515, BC3230, BC3240, BC0945, BC1445)
(Beijing Solarbio Science & Technology Co., Ltd.), according to
the manufacturer's instructions. The absorbance (340, 605, 550,
550, 660 nm) was measured via a microplate reader (Multiskan Go,
Thermo Fisher Scientific, Inc.) and relative activity was
calculated.
Xenograft model of ESCC
A total of 14 male BALB/c mice (age, 4-6 weeks;
weight, 10-14 g, Charles river) were allocated into negative
control (7 mice) and lentiviral transfection groups (7 mice) and
reared under standard environmental conditions (26-28°C, and the
relative humidity is 40-60%, 12/12-h light/dark cycle, with
commercial rat food and water ad libitum). Subsequently,
1,000,000 lentivirus-transfected or negative control cells were
subcutaneously injected into the right axilla of mice. Tumor size
was measured every 5 days once the xenograft tumors reached a
subcutaneous volume of 100 mm3. After 20 days, the
subcutaneous tumor in nude mice reached its maximum volume of
700-800 mm3, all mice were euthanized, and the xenograft
tumors were surgically excised for volume measurement and tissue
weighing. No nude mice died unexpectedly during the experiment. The
excised tissues were preserved in 4% paraformaldehyde (at 25°C, for
24 h) for immunohistochemical analysis. Tumor volume was calculated
as follows: Volume (mm3)=maximum diameter × minimum
diameter2/2 (24).
Experiments were approved by the Animal Ethics Committee of the
Shandong Provincial Hospital, affiliated with the Shandong First
Medical University (approval no. SDNSFC 2023-0026).
Statistical analysis
SPSS 19.0 (SPSS, Inc.) was used for clinical data
analysis. For continuous variables, unpaired Student's t-test was
performed. The association between SLC25A1 protein expression and
pathological parameters was determined via χ2 or
Fisher's exact probability test (two-tailed). Survival rates were
calculated via the Kaplan-Meier method and analyzed via log-rank
test. Data are presented as the mean ± SD of three independent
experiments. Statistical analysis was conducted using GraphPad
Prism 8 (Dotmatics). Differences between two groups were assessed
via unpaired t-test. One-way ANOVA was used to analyze variations
between >2 groups followed by Least Significance Difference test
was used for the post hoc test. The correlation between SLC25A1 and
FGFBP1 expression was determined by Pearson's correlation analysis.
The outliers were removed or replaced by a median value. P<0.05
was considered to indicate a statistically significant
difference.
Results
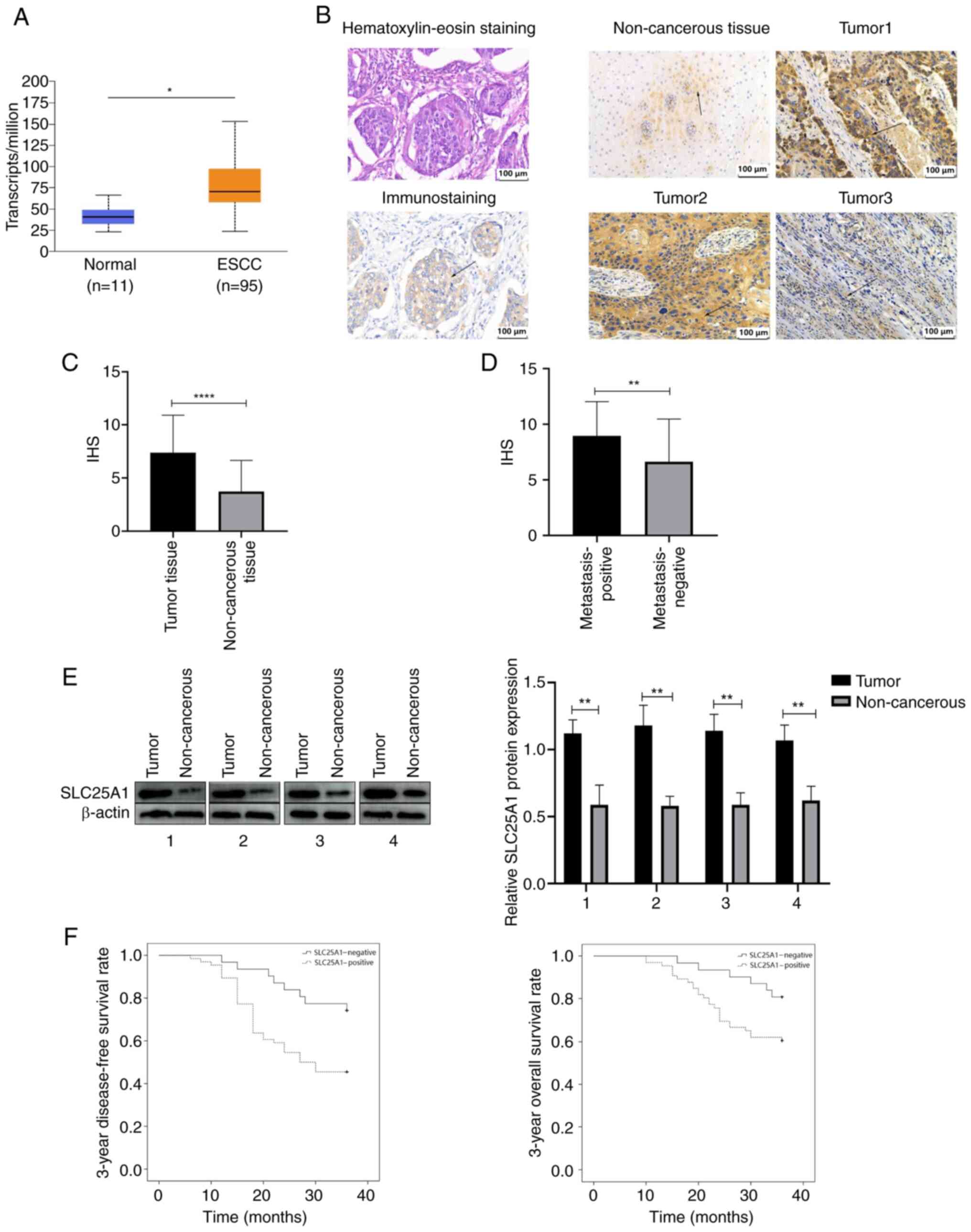
SLC25A1 overexpression is correlated with
TNM stage, recurrence rate and prognosis of ESCC
Expression of SLC25A1 in esophageal squamous cell
carcinoma was significantly greater than that in normal esophageal
mucosa in TCGA (Fig. 1A). Mean
IHS of SLC25A1 in 97 ESCC tissue samples was 7.4±3.5; that of 97
non-cancerous tissue samples was 3.7±2.9. These findings indicate a
significant increase in SLC25A1 expression in ESCC relative to
normal tissues (Fig. 1B and C).
Moreover, the IHS in the lymph node metastasis-positive group was
9.6±1.6, whereas that in the negative group was 4.3±1.9. These
findings suggested significant upregulation of SLC25A1 expression
in the tissues of patients with ESCC metastasis relative to those
without lymph node metastasis (Fig.
1D). Western blot analysis further revealed significant
upregulation of SLC25A1 expression in ESCC tissue (Fig. 1E). χ2-test revealed a
significant association between positive SLC25A1 overexpression and
lymph node metastasis, T stage and postoperative regional lymph
node recurrence in patients with ESCC (Table I). Kaplan-Meier analysis revealed
that patients with positive SLC25A1 expression had a significantly
lower disease-free (45.5 vs. 74.2%) and 3-year overall survival
rate (60.6 vs. 80.6%) than patients with negative SLC25A1
expression (Fig. 1F).
 | Table IAssociation between SLC25A1
expression and clinical characteristics of patients with esophageal
squamous cell carcinoma). |
Table I
Association between SLC25A1
expression and clinical characteristics of patients with esophageal
squamous cell carcinoma).
| Clinical
characteristic | Total cases
(n=97) | SLC25A1-positive
(n=66) | SLC25A1-negative
(n=31) | P-value |
|---|
| Age, years | | | | 0.432 |
| ≥60 | 59 | 30 | 29 | |
| <60 | 38 | 26 | 12 | |
| Sex | | | | 0.591 |
| Male | 75 | 50 | 25 | |
| Female | 22 | 16 | 6 | |
| Pathological T
stage | | | | 0.006 |
| T1 + T2 | 40 | 21 | 19 | |
| T3 + T4 | 57 | 45 | 12 | |
| Lymphatic node
metastasis | | | | 0.021 |
| Positive | 51 | 40 | 11 | |
| Negative | 46 | 26 | 20 | |
| Weight loss | | | | 0.146 |
| Yes | 55 | 35 | 20 | |
| No | 42 | 31 | 11 | |
| Recurrence | | | | 0.008 |
| Yes | 44 | 36 | 8 | |
| No | 53 | 30 | 23 | |
|
Differentiation | | | | 0.199 |
| Well/moderate | 53 | 39 | 14 | |
| Low | 44 | 27 | 17 | |
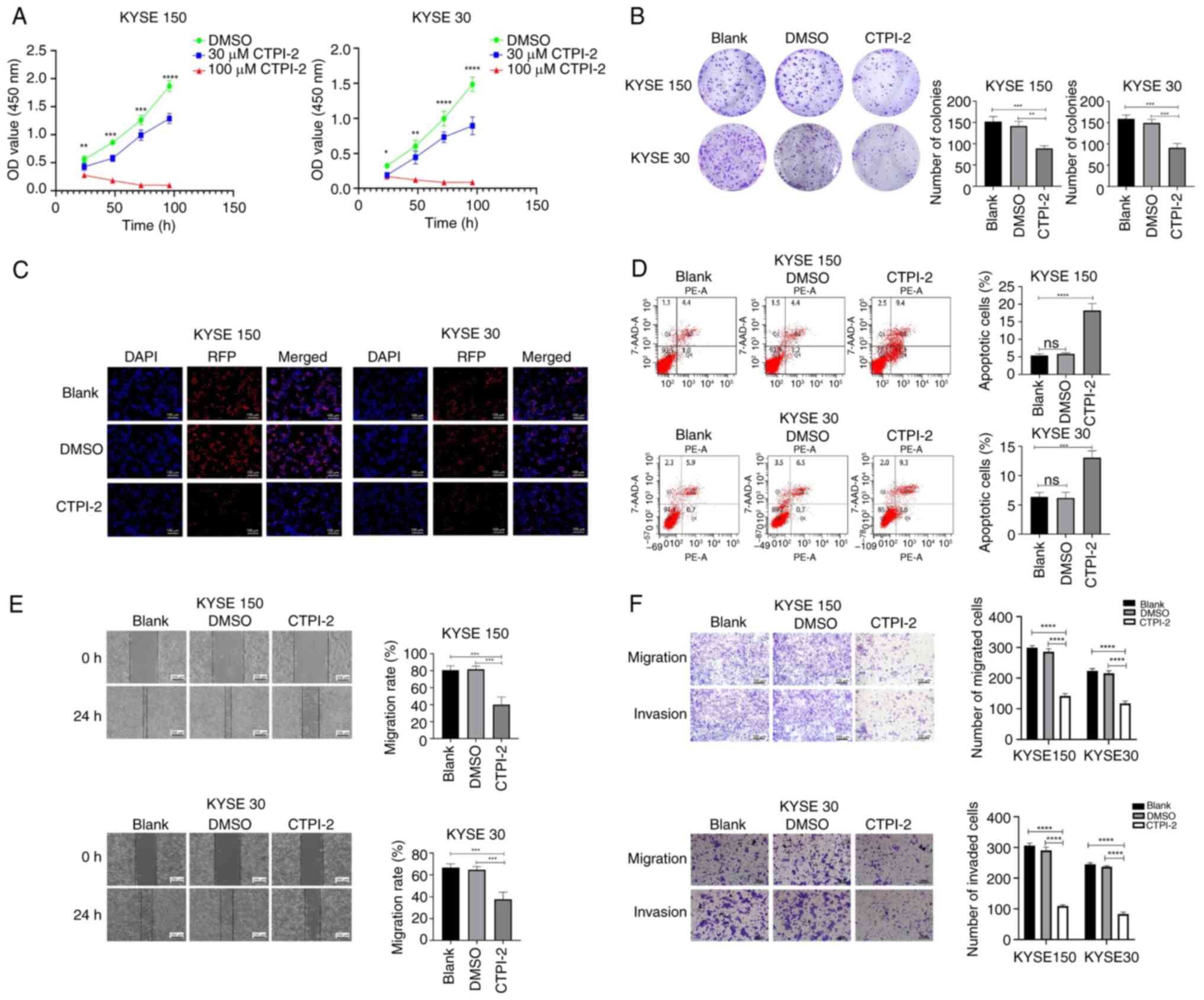
Silencing or blocking SLC21A1 inhibits the
proliferation, invasion and migration of ESCC cells, and promotes
the apoptosis of ESCC cells. Expression of SLC25A1 in ESCC and HeLa
cells was validated via RT-qPCR (Fig.
2A). Compared with KYSE510 cell line, Hela cell line and
KYSE450 cell line, SLC25A1 expression was elevated in KYSE150 and
KYSE30. Given the increased expression of SLC25A1 in ESCC cell
lines, shRNA lentivirus targeting SLC25A1 gene was constructed to
silence SLC25A1 expression in ESCC cells (Fig. 2B). SLC25A1 protein function in
ESCC cells was specifically blocked by CTPI-2. CCK8 assay revealed
that cell proliferation rate was considerably lower in the
shSLC25A1 and CTPI-2 groups than in the blank or DMSO groups
(Figs. 2C and 3A). EdU cell proliferation and colony
formation assays indicated that silencing or blocking SLC25A1
significantly decreased the proliferation and colony formation
abilities of KYSE 150 and 30 cells (Figs. 2D and E and 3B and C).
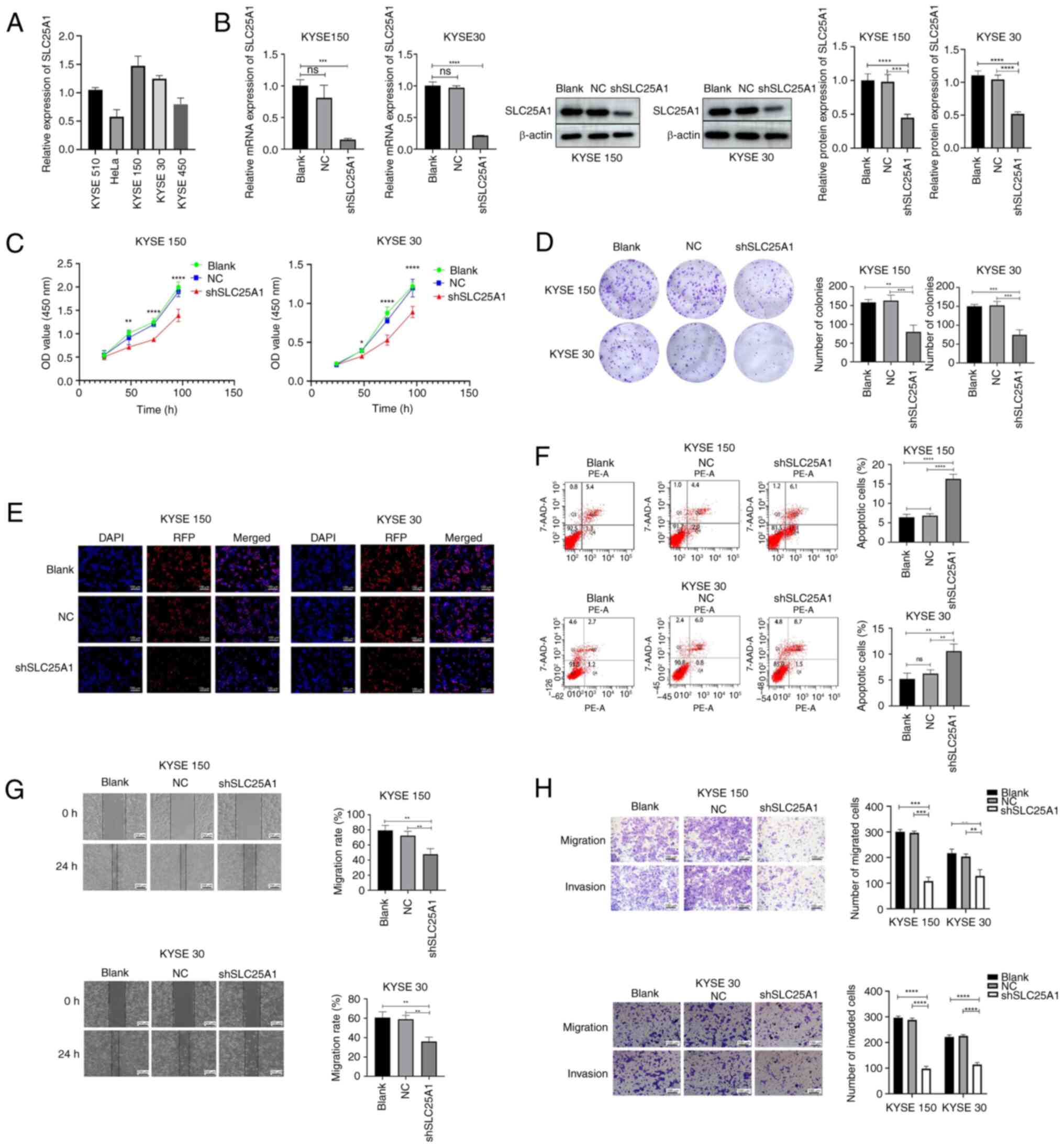 | Figure 2Silencing SLC25A1 inhibits
proliferation, migration and invasion but induces apoptosis of ESCC
cells. (A) SLC25A1 expression in KYSE 30, 150, 450 and 510 and HeLa
cell lines was detected by RT-qPCR. (B) Transfection efficiency of
the SLC25A1-silencing lentivirus was detected via western blotting
and RT-qPCR. (C) KYSE 150 and 30 cell proliferation. (D) Colony
formation assay (1×). (E) EdU staining assay. (F) Apoptosis rates
of KYSE 150 and 30 cells were analyzed via PE-7AAD staining. (G)
Migration ability of ESCC cells was detected via a wound healing
assay. (H) Migration and invasion abilities of ESCC cells were
detected via Transwell assay. **P<0.01,
***P<0.001, ****P<0.0001. SLC25A1,
solute carrier family 25 member 1; ESCC, esophageal squamous cell
carcinoma; RT-q, reverse transcription-quantitative; NC, Negative
control; sh, short hairpin; ns, not significant; RFP, Red
Fluorescent Protein; OD, optical density. |
Flow cytometry assay was conducted to assess whether
silencing SLC25A1 or blocking its protein function promotes
apoptosis in ESCC cells. The proportion of apoptotic KYSE 150 and
30 cells significantly increased when SLC25A1 was silenced or
SLC25A1 protein function was inhibited (Figs. 2F and 3D).
Silencing or inhibiting SLC25A1 significantly
decreased the wound healing ability of ESCC cells in vitro
(Figs. 2G and 3E). Transwell assay demonstrated that
both silencing and inhibiting SLC25A1 could inhibit the in
vitro migration and invasion of KYSE 150 and 30 cells (Figs. 2H and 3F).
SLC5A1 promotes lipid synthesis and
affects oxidative phosphorylation of ESCC cells
Citrate directly provides precursor lipids for
intracellular fatty acid synthesis in the cytoplasm. SLC25A1 is the
exclusive citrate transporter in the mitochondrial membrane
(7). Silencing or specific
blockade of SLC25A1 resulted in a notable decrease in intracellular
fatty acid staining levels (Fig.
4A). Intracellular lipid content assay experiments confirmed
that silencing or inhibition of SLC25A1 protein resulted in
decreased free fatty acid, triglyceride, and cholesterol contents
in KYSE150 and KYSE30 cells (Fig.
4B-D). These findings suggested that SLC25A1 was involved in
lipid synthesis in ESCC cells.
 | Figure 4SLC5A1 promotes lipid synthesis and
affects oxidative phosphorylation of ESCC cells. (A) Intracellular
lipids were stained with fluorescent lipophilic dye BODIPY 493/503.
Levels of (B) free fatty acids, (C) triglycerides, and (D)
cholesterol. Following glucose starvation, (E) oxygen consumption
rate and (F) activity of mitochondrial respiratory chain complexes
in ESCC cells was determined. *P<0.05,
**P<0.01, ***P<0.001,
****P<0.0001. SLC5A1, solute carrier family 25 member
1; ESCC, esophageal squamous cell carcinoma; ns, not significant;
NC, Negative control; sh, short hairpin. |
To determine the effect of SLC25A1 on energy
metabolism of ESCC cells, the oxygen consumption rate was measured
after 24 h starvation. Silencing or inhibiting SLC25A1 led to a
significant decrease in oxygen consumption in KYSE 150 and 30 cells
compared with that in the negative control and blank groups
(Fig. 4E) and activity of the
mitochondrial respiratory chain complex was reduced in cells in
which SLC25A1 was silenced or specifically blocked (Fig. 4F). These findings collectively
suggest that SLC25A1 can facilitate oxidative phosphorylation in
ECSS cells under starvation.
SLC25A1 silencing downregulates FGFBP1
expression and inhibits the AKT signaling pathway in ESCC
cells
The aforementioned results indicated that SLC25A1
may serve a key role in promoting malignant biological behaviors of
ESCC cells, particularly through its regulatory influence on lipid
and energy metabolism processes. High-throughput transcriptome
sequencing was performed on KYSE150 cells to identify the potential
molecular mechanism by which SLC25A1 modulates malignant biological
behavior of ESCC cells. Sequencing and Reactome pathway enrichment
analysis showed that several pathways, including those associated
with 'signaling by interleukins', 'interferon signaling', 'PI3K
cascade: FGFR3', 'FGFR2b ligand binding and activation', 'PI3K
cascade', 'downstream signaling of activated FGFR3' and 'FGFR1
mutant receptor activation', were significantly enriched in the
shSLC25A1 ESCC cells compared with the control cells (Fig. 5A-C). As numerous pathways were
associated with FGFR activation and activation of FGFR could also
activate the PI3K/AKT pathway, the significant downregulation of
FGFBBP1, a key gene in the FGF signaling pathway (25), in shSLC25A1 ESCC cells (Fig. 5D) was investigated. Western
blotting suggested that silencing or inhibiting SLC25A1 led to a
significant decrease in FGFBP1 expression and downstream activation
of the AKT signaling pathway in KYSE 150 and 30 cells (Fig. 5E and F). Furthermore, the
expression FGFBP1 was assessed by immunohistochemistry (Fig. 5G). These results demonstrated
SLC25A1 regulated expression of FGFBP1 and activation of the AKT
signaling pathway in ESCC cells.
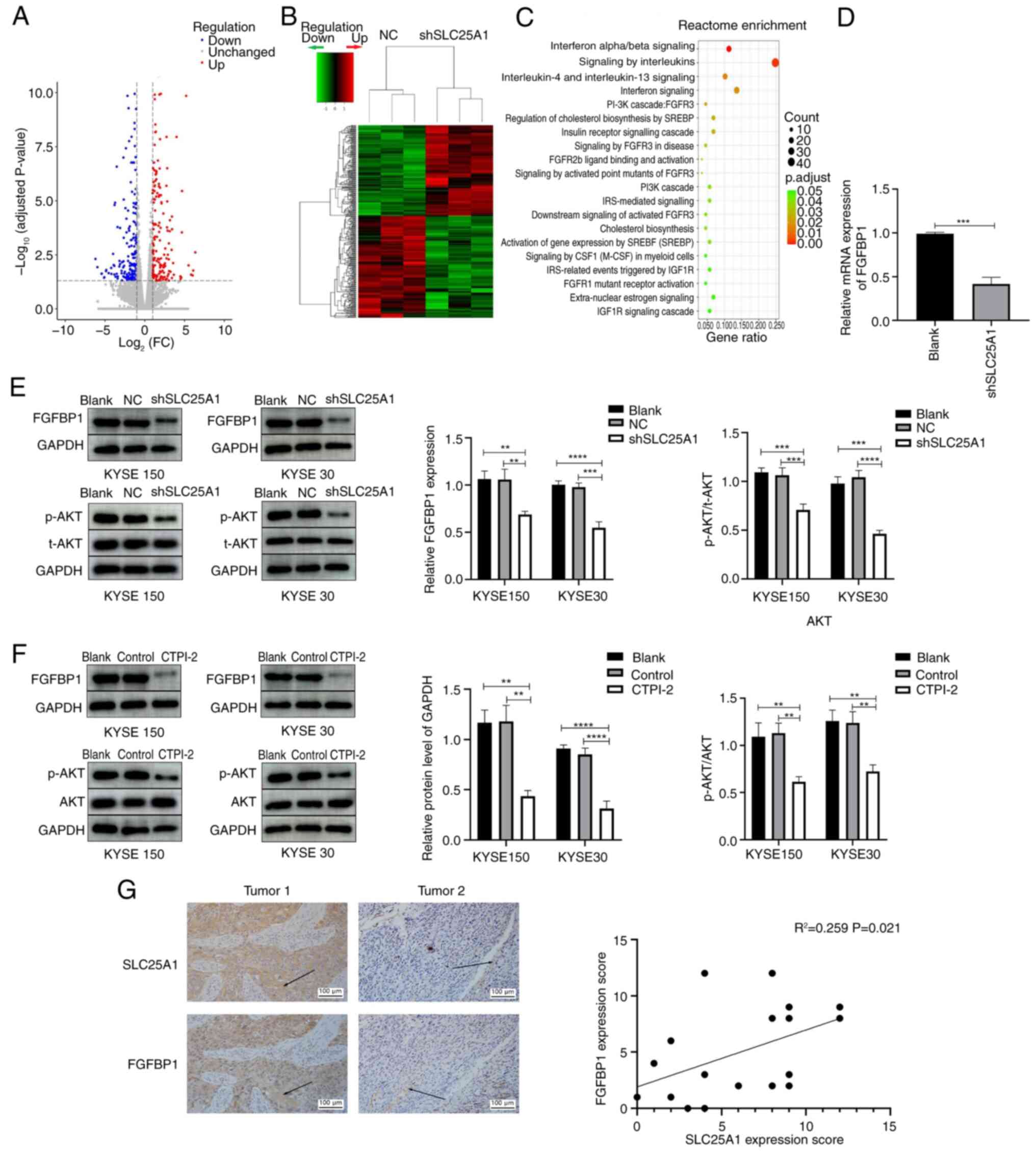 | Figure 5Silencing or inhibiting SLC25A1
downregulates expression of FGFBP1 and suppress the AKT pathway.
(A) Volcano plot and (B) cluster analysis of differential genes in
shSCL25A1 compared with the NC group, red color clusters represent
genes up-regulated while green represents genes down-regulated, and
connecting lines represent clustering result. (C) Reactcome
enrichment analysis result of differential genes in shSCL25A1
compared with the NC group, pathways with number of differential
genes ranking top 20 were showed. (D) Reverse
transcription-quantitative PCR confirmed the downregulation of
FGFBP1 in SLC25A1-silenced ESCC cells. After SLC25A1 was (E)
silenced and (F) inhibited, the expression of FGFBP1 and the
phosphorylation of AKT in KYSE 150 cells and 30 cells were detected
via western blotting. (G) SLC25A1 and FGFBBP1 expression in ESCC
samples was detected via immunohistochemistry. Arrow points to the
representative location for immunohistochemical staining.
**P<0.01, ***P<0.001,
****P<0.0001. SLC25A1, solute carrier family 25
member 1; FGFBP1, fibroblast Growth Factor Binding Protein 1; p-,
phosphorylation; NC, Negative control; sh, short hairpin; FC, Fold
change. |
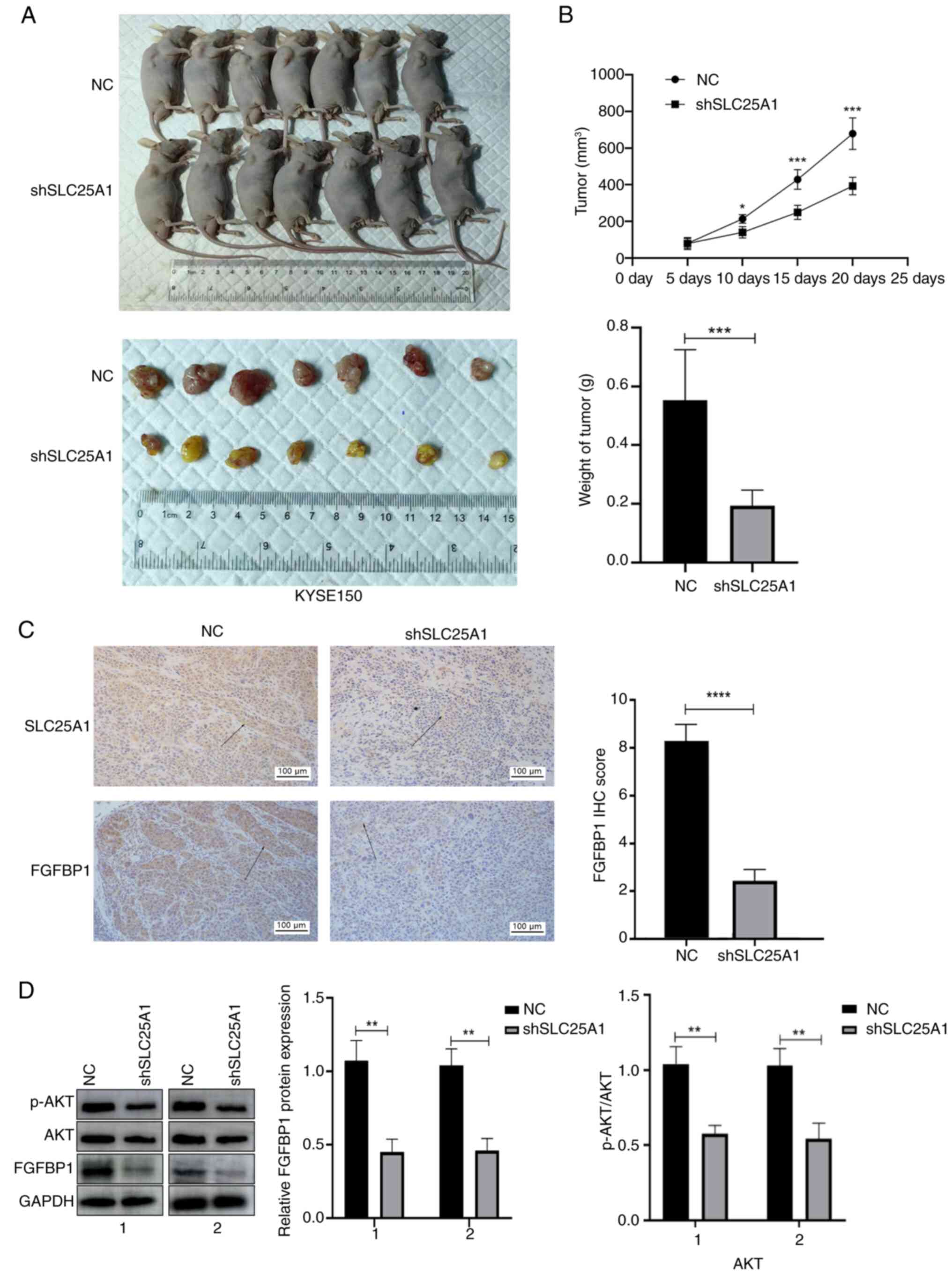
SLC25A1 silencing inhibits tumor growth
in vivo
To ascertain the impact of SLC25A1 silencing on ESCC
cell proliferation in vivo, a tumor xenograft model was
developed by subcutaneously inoculating nude mice with KYSE 150
cells transfected with either SLC25A1-interfering or negative
control lentivirus. Tumors formed by KYSE 150 cells transfected
with SLC25A1-interfering lentivirus exhibited a significantly
decreased size and growth rate (Fig.
6A and B). IHC and western blotting revealed a reduction in the
expression of both SLC25A1 and FGFBP1 in the SLC25A1 silenced group
(Fig. 6C and D). These findings
indicated that silencing SLC25A1 expression effectively inhibited
the proliferation of ESCC cells in vivo.
Discussion
The incidence and mortality of esophageal cancer is
increasing, and China accounts for half of incidence and mortality
of EC: The number of EC cases worldwide increased from 319,969 in
1990 to 534,563 in 2019, while the number of incident cases of EC
in China increased from 173,687 in 1990 to 278,121 in 2019. The
number of EC deaths worldwide increased from 319,332 in 1990 to
498,067 in 2019, and the EC mortality in China increased from
176,602 in 1990 to 257,316 in 2019, with ESCC emerging as the
predominant histological subtype (26). ESCC is highly malignant and prone
to metastasis in the early stage leading to a high mortality : the
5-year relative survival rate is only 20%, which is the second
lowest survival rate after pancreatic cancer (27,28). The limited efficacy of
conventional antitumor drugs and lack of effective molecular
targets and drugs make treatment challenging for patients with
advanced esophageal cancer (1,29,30). The present study explored the
expression of SLC25A1 in ESCC, revealing its impact on malignant
biological behavior of ESCC cells and the underlying
mechanisms.
Citrate is an important substance in the cell. In
mitochondria, citrate, one of the key reaction substrates of the
tricarboxylic acid cycle, generates ATP for cell use through the
mitochondrial electron transport chain (31). Upon transportation into the
cytoplasm facilitated by the mitochondrial citrate carrier SLC25A1,
citrate undergoes cleavage into oxaloacetate and acetyl-CoA, a
process catalyzed by ATP-citrate lyase (ACLY) (28). Acetyl-CoA is a key precursor for
intracellular synthesis of fatty acids and cholesterol. SLC25A1,
which belongs to the ionic protein transporter family, regulates
levels of mitochondrial and cytoplasmic citrate, which is
associated with various physiological metabolic processes such as
lipid metabolism in the liver, cancer and aging (32,33). Abnormal distribution and
regulation of SLC25A1 are associated with various cancers: High
expression of SLC25A1 is observed in non-small cell lung (17) and colon cancer (18). KRAS mutant gene KRASG12D induces
high expression of SLC25A1 in human pancreatic cancer cells via
glioma-associated oncogene homolog 1) and promotes pancreatic
carcinogenesis in mice (34). In
the present study, immunohistochemistry and western blot analysis
revealed upregulation of SLC25A1 expression in ESCC tissues and
cell lines. Moreover, high expression of SLC25A1 in ESCC was
associated with T stage, lymph node metastatic status,
postoperative local lymph node recurrence and poor prognosis. Thus,
upregulation of SLC25A1 expression may be associated with
development of ESCC. Therefore, SLC25A1 may serve as a specific
molecular marker to predict prognosis of patients with ECSS.
To confirm the involvement of SLC25A1 in the onset
and progression of ESCC, lentiviral transfection was executed to
silence expression of the SLC25A1 gene. In addition, CTPI-2, a
specific inhibitor of the SLC25A1 protein, was used to bind to the
functional site of the SLC25A1 protein to inhibit its function.
Inhibition and silencing of SLC25A1 expression suppressed the
proliferation, invasion and migration of the ESCC cell lines KYSE
150 and 30 in vitro and induced apoptosis. Downregulation of
SLC25A1 suppressed the in vivo tumorigenic ability of ESCC
cells. These findings demonstrated the critical role of SLC25A1 in
the malignant biological behavior of ESCC cells.
SLC25A1, a key gene that regulates cellular
metabolism, may be involved in the metabolism of ESCC cells.
Silencing and specific blockade of SLC25A1 resulted in a
significant decrease in lipid synthesis in KYSE 150 and 30 cells,
suggesting the vital role of SLC25A1 in lipid synthesis in ESCC
cells. Lipids are key substances for cell metabolism and survival.
They form crucial components of cell and organelle membranes and
actively participate in formation of cell signaling molecules
(35). As a form of cellular
energy storage, lipids serve a pivotal role in supplying energy for
proliferation of tumor cells (36,37). Owing to the rapid growth and high
metabolic level, the nutritional requirements of tumor tissue often
exceed the supply by the microenviorment (38). Oligotrophic blood vessels in the
early stage of tumor growth cannot provide sufficient nutrition to
tumor tissues. In this case, tumor cells need to regulate their own
metabolic pathways to survive. Lipid metabolism is involved in the
developmental process of numerous types of tumor, such as lung
cancer, colon cancer and breast cancer, especially in metastasis,
and lipid metabolism reprogramming is a hallmark of malignancy
(39,40). Therefore, overexpression of
SLC25A1 may promote the aggressive biological behavior of ESCC
cells via regulation of lipid metabolism. Citrate is involved in
multiple metabolic pathways including lipogenesis, glycolysis and
gluconeogenesis: Citrate is the key substrate of acetyl-CoA for
fatty acid and sterol biosynthesis; it is an allosteric regulator
of enzymes that control glycolysis and gluconeogenesis, such as
1,6-bisphosphatase (41-43). Thus, SLC25A1 may also promote the
progression of ESCC via other metabolic pathways, including
glycometabolism, which needs further exploration.
The SLC25A1 protein transports citrate
bidirectionally between cytoplasm and mitochondria. In addition,
SLC25A1 is involved in energy metabolism in colon cancer during
metabolic stress (37).
Therefore, the present study investigated involvement of SLC25A1 in
the regulation of energy metabolism in ESCC cells. With a
sufficient supply of energy, there was a minimal difference in the
oxygen consumption rate of KYSE 150 and 30 cells between the
treated and untreated groups. However, following starvation, a
substantial reduction in the oxygen consumption rate was observed
in the SLC25A1-silenced and -inhibited groups, concomitant with a
decrease in activity of the mitochondrial respiratory chain
complexes. Compared with normal cells, tumor cells undergo
glycolytic reactions more frequently. Compared with oxidative
phosphorylation, glycolysis can produce ATP at a faster rate for
use by tumor cells (38).
However, when they detach from the extracellular matrix and adapt
to anchorage-independent growth in a low nutrient environment,
tumor cells may undergo oxidative phosphorylation to produce ATP
for survival, which is key for the invasive and metastatic behavior
of tumor cells (6,44). In the absence of glucose, SLC25A1
protein transports cytoplasmic citrate to the mitochondria to
increase oxidative phosphorylation, thereby ensuring cell survival.
The aforementioned results confirm that SLC25A1 is involved in
lipid metabolism and energy metabolism in ESCC and provides
material and energy for the tumor development of ESCC. The
mechanism by which SLC25A1 influences the oxidative phosphorylation
pathway of mitochondria may be complex and is unclear. In addition
to reverse import of cytosolic citrate into mitochondria, the
import of malate into the mitochondria, which leads to an increase
in the tricarboxylic acid cycle flux and generation of reducing
equivalents including NADH/NAD+ for the electron
transport chain also participates this regulation (31).
The present results suggested that SLC25A1 may
promote the malignant biological behavior of ESCC cells by
regulating cellular lipid and energy metabolism. To reveal the
underlying mechanism of SLC25A1 in promoting the onset and
progression of ESCC, high-throughput expression profile sequencing
was performed on SLC25A1-silenced KYSE 150 ESCC and control cells.
The expression of FGFBP1, a key gene in the FGF signaling pathway,
was significantly downregulated. The co-expression of SLC25A1 in
ESCC tissue with FGFBP1 was confirmed via immunohistochemistry.
FGFBP1, belonging to the FGFBP family (45), serves as a secretory chaperone
protein. FGFBP1 releases FGF immobilized in the extracellular
matrix and facilitates binding of FGF to its receptor (46,47). FGF is a crucial molecule
associated with cell proliferation, migration and differentiation
(48). FGFBP1 is highly expressed
in colon and pancreatic cancer and oral squamous cell carcinoma
(49,50). The AKT signaling pathway is a key
signaling pathway during tumor growth and is involved in regulating
the onset and progression of numerous types of tumor, such as ESCC
(51-53). The activation of the AKT pathway
could regulate the downstream genes to directly promote cell
survival, proliferation, migration and angiogenesis; it also serves
an important role in lipid metabolism in the progression of tumors
(54). The AKT pathway may
regulate the expression of key synthetases of lipids, such as fatty
acid synthase, ACLY and acetyl-CoA carboxylase to promote lipid
synthesis (55,56); however, recent studies have
revealed high fat microenvironment promotes the progression of
tumors by activating the AKT pathway (57,58). As a key pathway activated by FGF
binding to its receptor, the AKT pathway may be inhibited upon
downregulation of FGFBP1 expression. In the present study, the
silencing or inhibition of SLC25A1 led to considerable
downregulation of the activation of the AKT signaling pathway in
ESCC cells. These findings suggested that SLC25A1 may activate the
AKT signaling pathway by regulating FGFBP1 expression, facilitating
tumor initiation and progression of ESCC. In addition, FGF receptor
binding could activate not only the AKT pathway, but also the MAPK,
JAK/STAT3 and PLCγ pathways and SLC25A1 could also regulate the TNF
signaling pathway by reducing the expression of TNF-α and IL-6
(59). Therefore, upregulation of
SLC25A1 may promote the progression of ESCC via signaling pathway
network regulation. However, the exact mechanism by which SLC25A1
regulates transcription of FGFBP1 and other signaling pathways
requires further exploration.
In summary, expression of SLC25A1 was elevated in
ESCC and significantly associated with the malignant biological
behavior of ESCC, particularly lymph node metastasis. SLC25A1 may
contribute to the onset and progression of ESCC by regulating ESCC
metabolism. SLC25A1 may promote the development of ESCC by
regulating the FGFBP1-activated AKT signaling pathway.
Consequently, SLC25A1 may serve as a potential novel target for
ESCC treatment and a molecular biological marker for the prediction
of patient prognosis.
Availability of data and materials
The data generated in the present study may be found
in Figshare under accession number (10.6084/m9.figshare.28023275)
or at the following URL: (https://figshare.com/s/a5ed5881f88d63947aa6).
Authors' contributions
GZ conceived the study, designed and performed
experiments and wrote the manuscript. JW and MJ performed the
experiments. XL and MS analyzed the data and edited the manuscript.
All authors have read and approved the final manuscript. GZ and MS
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
All procedures involving human participants were
performed in accordance with the ethical standards of the
Institutional Ethics Committee of Shandong Provincial Hospital
Affiliated to Shandong First Medical University (approval no.
SZRJJ:NO.2022-015). All patients agreed to participate in the
study. All procedures involving animals were in accordance with the
ethical standards of Ethics Committee of Shandong Provincial
Hospital Affiliated to Shandong First Medical University (approval
no. SDNSFC 2023-0026).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from Natural Science
Foundation of Shandong Province (81902418).
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Gouw DJJM, Klarenbeek BR, Driessen M,
Bouwense SAW, van Workum F, Fütterer JJ, Rovers MM, Ten Broek RPG
and Rosman C: Detecting pathological complete response in
esophageal cancer after neoadjuvant therapy based on imaging
techniques: A diagnostic systematic review and meta-analysis. J
Thorac Oncol. 14:1156–1171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen K, Wang X, Yang L and Chen Z: The
Anti-PD-1/PD-L1 immunotherapy for gastric esophageal cancer: A
systematic review and meta-analysis and literature review. Cancer
Control. 28:10732748219974302021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun J, Aluvila S, Kotaria R, Mayor JA,
Walters DE and Kaplan RS: Mitochondrial and plasma membrane citrate
transporters: Discovery of selective inhibitors and application to
structure/function analysis. Mol Cell Pharmacol. 2:101–110.
2010.PubMed/NCBI
|
|
6
|
Rochette L, Meloux A, Zeller M, Malka G,
Cottin Y and Vergely C: Mitochondrial SLC25 carriers: Novel targets
for cancer therapy. Molecules. 25:24172020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Palmieri F: The mitochondrial transporter
family SLC25: Identification, properties and physiopathology. Mol
Aspects Med. 34:465–484. 2013. View Article : Google Scholar
|
|
8
|
Ma C, Gerhard E, Lu D and Yang J: Citrate
chemistry and biology for biomaterials design. Biomaterials.
178:383–400. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rohrig F and Schulze A: The multifaceted
roles of fatty acid synthesis in cancer. Nat Rev Cancer.
16:732–749. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pope ED III, Kimbrough EO, Vemireddy LP,
Surapaneni PK, Copland JA III and Mody K: Aberrant lipid metabolism
as a therapeutic target in liver cancer. Expert Opin therapeutic
targets. 23:473–483. 2019. View Article : Google Scholar
|
|
11
|
Ding X, Zhang W, Li S and Yang H: The role
of cholesterol metabolism in cancer. Am J Cancer Res. 9:219–227.
2019.PubMed/NCBI
|
|
12
|
Kuzu OF, Noory MA and Robertson GP: The
role of cholesterol in cancer. Cancer Res. 76:2063–2070. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beckwitt CH, Brufsky A, Oltvai ZN and
Wells A: Statin drugs to reduce breast cancer recurrence and
mortality 11. Breast Cancer Res. 20:1442018. View Article : Google Scholar
|
|
14
|
Liu W, Chakraborty B, Safi R, Kazmin D,
Chang CY and McDonnell DP: Dysregulated cholesterol homeostasis
results in resistance to ferroptosis increasing tumorigenicity and
metastasis in cancer. Nat Commun. 12:51032021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baek AE, Yu YA, He S, Wardell SE, Chang
CY, Kwon S, Pillai RV, McDowell HB, Thompson JW, Dubois LG, et al:
The cholesterol metabolite 27 hydroxycholesterol facilitates breast
cancer metastasis through its actions on immune cells. Nat Commun.
8:8642017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou X, Huang F, Ma G, Wei W, Wu N and Liu
Z: Dysregulated ceramides metabolism by fatty acid 2-hydroxylase
exposes a metabolic vulnerability to target cancer metastasis.
Signal Transduct Target Ther. 7:3702022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fernandez HR, Gadre SM, Tan M, Graham GT,
Mosaoa R, Ongkeko MS, Kim KA, Riggins RB, Parasido E, Petrini I, et
al: The mitochondrial citrate carrier, SLC25A1, drives stemness and
therapy resistance in non-small cell lung cancer. Cell Death
Differ. 25:1239–1258. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Y, He J, Zhang B, Zhang Z, Jia G, Liu
S, Wu T, He X and Wang N: SLC25A1 promotes tumor growth and
survival by reprogramming energy metabolism in colorectal cancer.
Cell Death Dis. 12:11082021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li EC, Du P, Ji KZ and Wang Z: Chinese
ethics review system and Chinese medicine ethical review: past,
present, and future. Chin J Integr Med. 17:867–872. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar
|
|
23
|
Yu G, Wang LG, Han Y and He QY:
Clusterprofiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tomayko MM and Reynolds CP: Determination
of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother
Pharmacol. 24:148–154. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Z, Qin Y, Ji S, Xu W, Liu M, Hu Q,
Ye Z, Fan G, Yu X, Liu W and Xu X: FGFBP1-mediated crosstalk
between fibroblasts and pancreatic cancer cells via FGF22/FGFR2
promotes invasion and metastasis of pancreatic cancer. Acta Biochim
Biophys Sin (Shanghai). 53:997–1008. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J, Xu J, Zheng Y, Gao Y, He S, Li H,
Zou K, Li N, Tian J, Chen W and He J: Esophageal cancer:
Epidemiology, risk factors and screening. Chin J Cancer Res.
33:535–547. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Merkow RP, Bilimoria KY, Keswani RN, Chung
J, Sherman KL, Knab LM, Posner MC and Bentrem DJ: Treatment trends,
risk of lymph node metastasis, and outcomes for localized
esophageal cancer. J Natl Cancer Inst. 106:dju1332014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lagergren Jand Lagergren P: Oesophageal
cancer. BMJ. 341:c62802010. View Article : Google Scholar
|
|
30
|
Harada K, Rogers JE, Iwatsuki M, Yamashita
K, Baba H and Ajani JA: Recent advances in treating oesophageal
cancer. F1000Res. 9:F1000Faculty Rev-1189. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Peng R, Zhang M, Wang H, Lin J, Wang H,
Wang F, Liu L, Zhao Q and Liu J: Advances into understanding the
vital role of the mitochondrial citrate carrier (CIC) in metabolic
diseases. Pharmacol Res. 161:1051322020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Palmieri F and Monné M: Discoveries,
metabolic roles and diseases of mitochondrial carriers: A review.
Biochim Biophys Acta. 1863:2362–2378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tan M, Mosaoa R, Graham GT,
Kasprzyk-Pawelec A, Gadre S, Parasido E, Catalina-Rodriguez O,
Foley P, Giaccone G, Cheema A, et al: Inhibition of the
mitochondrial citrate carrier, Slc25a1, reverts steatosis, glucose
intolerance, and inflammation in preclinical models of NAFLD/NASH.
Cell Death Differ. 27:2143–2157. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang R, Peng X, Du JX, Boohaker R,
Estevao IL, Grajeda BI, Cox MB, Almeida IC and Lu W: Oncogenic
KRASG12D reprograms lipid metabolism by upregulating SLC25A1 to
drive pancreatic tumorigenesis. Cancer Res. 83:3739–3752. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Morigny P, Boucher J, Arner P and Langin
D: Lipid and glucose metabolism in white adipocytes: Pathways,
dysfunction and therapeutics. Nat Rev Endocrinol. 17:276–295. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cheng C, Geng F, Cheng X and Guo D: Lipid
metabolism reprogramming and its potential targets in cancer.
Cancer Commun (Lond). 38:272018.PubMed/NCBI
|
|
37
|
Currie E, Schulze A, Zechner R, Walther TC
and Farese RV Jr: Cellular fatty acid metabolism and cancer. Cell
Metab. 18:153–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gouirand V, Guillaumond F and Vasseur S:
Influence of the tumor microenvironment on cancer cells metabolic
reprogramming. Front Oncol. 8:1172018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Merino Salvador M, Gómez De Cedrón M,
Moreno Rubio J, Falagán Martínez S, Sánchez Martínez R, Casado E,
Ramírez de Molina A and Sereno M: Lipid metabolism and lung cancer.
Crit Rev Oncol Hematol. 112:31–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Saikia S, Ahmed F, Prajapati BG, Padma VV,
Chorawala MR, Postwala HI and Bhattacharya S: Reprogramming of
lipid metabolism in cancer: New insight into pathogenesis and
therapeutic strategies. Curr Pharm Biotechnol. 24:1847–1858. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Palmieri F, Scarcia P and Monné M:
Diseases caused by mutations in mitochondrial carrier genes SLC25:
A review. Biomolecules. 10:6552020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kunji S, King MS, Ruprecht JJ and
Thangaratnarajah C: The SLC25 carrier family: Important transport
proteins in mitochondrial physiology and pathology. Physiology
(Bethesda). 35:302–327. 2020.PubMed/NCBI
|
|
43
|
Van Schaftingen E and Hers HG: Inhibition
of fructose-1,6-bisphosphatase by fructose 2,6-biphosphate. Proc
Natl Acad Sci USA. 78:2861–2863. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jiang L, Shestov AA, Swain P, Yang C,
Parker SJ, Wang QA, Terada LS, Adams ND, McCabe MT, Pietrak B, et
al: Reductive carboxylation supports redox homeostasis during
anchorage-independent growth. Nat Cell Biol. 532:255–258. 2016.
|
|
45
|
Beer HD, Bittner M, Niklaus G, Munding C,
Max N, Goppelt A and Werner S: The fibroblast growth factor binding
protein is a novel interaction partner of FGF-7, FGF-10 and FGF-22
and regulates FGF activity: Implications for epithelial repair.
Oncogene. 24:5269–5277. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tassi E and Wellstein A: The angiogenic
switch molecule, secreted FGF-binding protein, an indicator of
early stages of pancreatic and colorectal adenocarcinoma. Semin
Oncol. 33(6 Suppl 11): S50–S56. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Taetzsch T, Brayman VL and Valdez G: FGF
binding proteins (FGFBPs): Modulators of FGF signaling in the
developing, adult, and stressed nervous system. Biochim Biophys
Acta Mol Basis Dis. 1864(9 Pt B): 2983–2991. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Abuharbeid S, Czubayko F and Aigner A: The
fibroblast growth factor-binding protein FGF-BP. Int J Biochem Cell
Biol. 38:1463–1468. 2006. View Article : Google Scholar
|
|
49
|
Tassi E, Henke RT, Bowden ET, Swift MR,
Kodack DP, Kuo AH, Maitra A and Wellstein A: Expression of a
fibroblast growth factor-binding protein during the development of
adenocarcinoma of the pancreas and colon. Cancer Res. 66:1191–1198.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rosli SN, Shintani T, Toratani S, Usui E
and Okamoto T: 1α,25 (OH)2D3 inhibits FGF-2
release from oral squamous cell carcinoma cells through
down-regulation of HBp17/FGFBP-1. In Vitro Cell Dev Biol Anim.
50:802–806. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Glaviano A, Foo ASC, Lam HY, Yap KCH,
Jacot W, Jones RH, Eng H, Nair MG, Makvandi P, Geoerger B, et al:
PI3K/AKT/mTOR signaling transduction pathway and targeted therapies
in cancer. Mol Cancer. 22:1382023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: an
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li B, Xu WW, Lam AKY, Wang Y, Hu HF, Guan
XY, Qin YR, Saremi N, Tsao SW, He QY and Cheung ALM: Significance
of PI3K/AKT signaling pathway in metastasis of esophageal squamous
cell carcinoma and its potential as a target for anti-metastasis
therapy. Oncotarget. 8:38755–38766. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Koundouros N and Poulogiannis G:
Reprogramming of fatty acid metabolism in cancer. Br J Cancer.
122:4–22. 2020. View Article : Google Scholar :
|
|
55
|
Tian LY, Smit DJ and Jücker M: The role of
PI3K/AKT/mTOR signaling in hepatocellular carcinoma metabolism. Int
J Mol Sci. 24:26522023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu LZ, Wang B, Zhang R, Wu Z, Huang Y,
Zhang X, Zhou J, Yi J, Shen J, Li MY and Dong M: The activated
CD36-Src axis promotes lung adenocarcinoma cell proliferation and
actin remodeling-involved metastasis in high-fat environment. Cell
Death Dis. 14:5482023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang D, Wei Y, Huang Q, Chen Y, Zeng K,
Yang W and Chen J and Chen J: Important hormones regulating lipid
metabolism. Molecules. 27:70522022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bu L, Zhang Z, Chen J, Fan Y, Guo J, Su Y,
Wang H, Zhang X, Wu X, Jiang Q, et al: High-fat diet promotes liver
tumorigenesis via palmitoylation and activation of AKT. Gut.
73:1156–1168. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xie Y, Su N, Yang J, Tan Q, Huang S, Jin
M, Ni Z, Zhang B, Zhang D, Luo F, et al: FGF/FGFR signaling in
health and disease. Signal Transduct Target Ther. 5:1812020.
View Article : Google Scholar : PubMed/NCBI
|