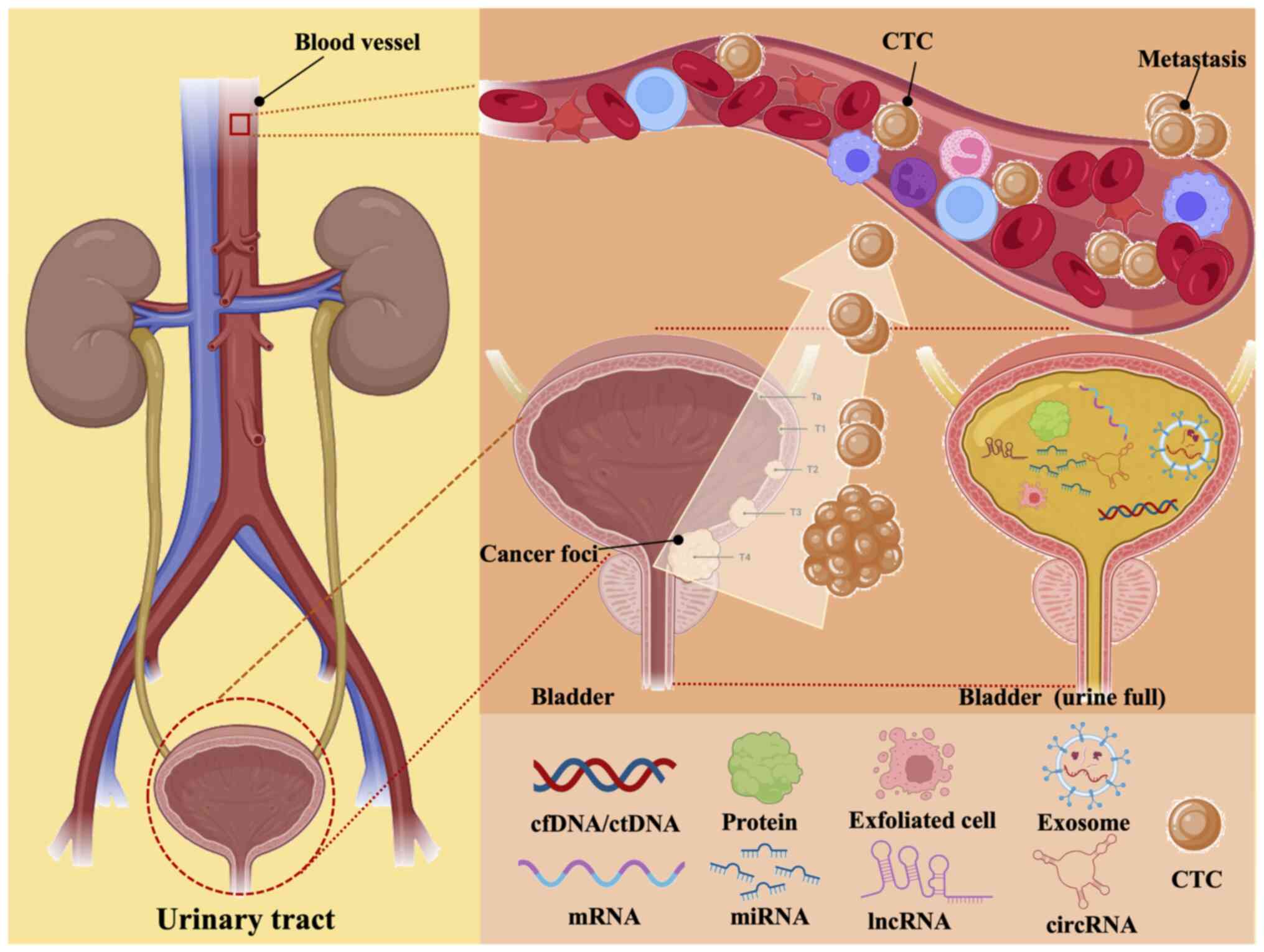
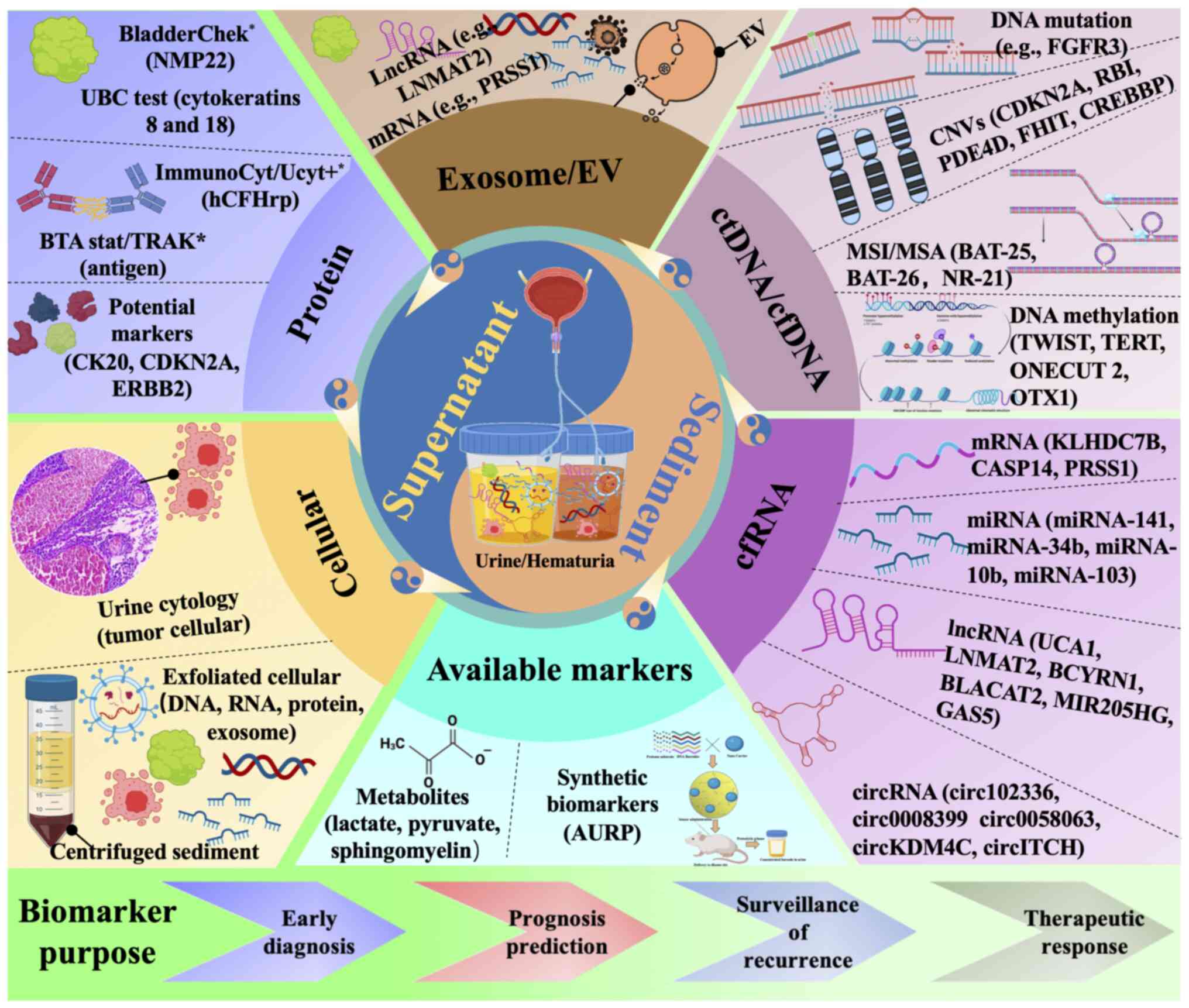
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Richters A, Aben KKH and Kiemeney LALM:
The global burden of urinary bladder cancer: An update. World J
Urol. 38:1895–1904. 2020. View Article : Google Scholar :
|
|
3
|
Tan WS, Rodney S, Lamb B, Feneley M and
Kelly J: Management of non-muscle invasive bladder cancer: A
comprehensive analysis of guidelines from the United States, Europe
and Asia. Cancer Treat Rev. 47:22–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Teoh JY, Kamat AM, Black PC, Grivas P,
Shariat SF and Babjuk M: Recurrence mechanisms of
non-muscle-invasive bladder cancer-a clinical perspective. Nat Rev
Urol. 19:280–294. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Facchini G, Cavaliere C, Romis L, Mordente
S, Facchini S, Iovane G, Capasso M, D'Errico D, Liguori C, Formato
R, et al: Advanced/metastatic bladder cancer: Current status and
future directions. Eur Rev Med Pharmacol Sci. 24:11536–11552.
2020.PubMed/NCBI
|
|
6
|
Devlies W, de Jong JJ, Hofmann F, Bruins
HM, Zuiverloon TCM, Smith EJ, Yuan Y, van Rhijn BWG, Mostafid H,
Santesso N, et al: The diagnostic accuracy of cystoscopy for
detecting bladder cancer in adults presenting with haematuria: A
systematic review from the European Association of Urology
Guidelines Office. Eur Urol. 10:115–122. 2024.
|
|
7
|
Zuiverloon TCM, de Jong FC and Theodorescu
D: Clinical decision making in surveillance of non-muscle-invasive
bladder cancer: The evolving roles of urinary cytology and
molecular markers. Oncology (Williston Park). 31:855–862. 2017.
|
|
8
|
Chamie K, Litwin MS, Bassett JC, Daskivich
TJ, Lai J, Hanley JM, Konety BR and Saigal CS; Urologic Diseases in
America Project: Recurrence of high-risk bladder cancer: A
population-based analysis. Cancer. 119:3219–3227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rezaee ME, Lynch KE, Li Z, MacKenzie TA,
Seigne JD, Robertson DJ, Sirovich B, Goodney PP and Schroeck FR:
The impact of low-versus high-intensity surveillance cystoscopy on
surgical care and cancer outcomes in patients with high-risk
non-muscle-invasive bladder cancer (NMIBC). PLoS One.
15:e02304172020. View Article : Google Scholar
|
|
10
|
Burke DM, Shackley DC and O'Reilly PH: The
community-based morbidity of flexible cystoscopy. BJU Int.
89:347–349. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van Der Aa MN, Steyerberg EW, Sen EF,
Zwarthoff EC, Kirkels WJ, van der Kwast TH and Essink-Bot ML:
Patients' perceived burden of cystoscopic and urinary surveillance
of bladder cancer: A randomized comparison. BJU Int. 101:1106–1110.
2008. View Article : Google Scholar
|
|
12
|
Matulewicz RS, DeLancey JO and Meeks JJ:
Cystoscopy. JAMA. 317:11872017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Talukdar S, Emdad L, Das SK, Sarkar D and
Fisher PB: Noninvasive approaches for detecting and monitoring
bladder cancer. Expert Rev Anticancer Ther. 15:283–294. 2015.
View Article : Google Scholar
|
|
14
|
Yaxley JP: Urinary tract cancers: An
overview for general practice. J Family Med Prim Care. 5:533–538.
2016. View Article : Google Scholar
|
|
15
|
Zhang X and Zhang Y: Bladder cancer and
genetic mutations. Cell Biochem Biophys. 73:65–69. 2015. View Article : Google Scholar
|
|
16
|
Thompson D, Lawrentschuk N and Bolton D:
New approaches to targeting epigenetic regulation in bladder
cancer. Cancers (Basel). 15:18562023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eissa S, Swellam M, Sadek M, Mourad MS, EI
Ahmady O and Khalifa A: Comparative evaluation of the nuclear
matrix protein, fibronectin, urinary bladder cancer antigen and
voided urine cytology in the detection of bladder tumors. J Urol.
168:465–469. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sale MS, Kulkarni VV, Kulkarni PV and
Patil CA: Efficacy of modified cell block cytology compared to fine
needle aspiration cytology for diagnostic oral cytopathology.
Biotech Histochem. 96:197–201. 2021. View Article : Google Scholar
|
|
19
|
Raskin RE and Meyer D: General Categories
of Cytologic Interpretation. Canine and Feline Cytology. A Color
Atlas and Interpretation Guide. 2nd edition. Elsevier; Amsterdam:
pp. 16–33. 2009
|
|
20
|
Sullivan PS, Chan JB, Levin MR and Rao J:
Urine cytology and adjunct markers for detection and surveillance
of bladder cancer. Am J Transl Res. 2:412–440. 2010.PubMed/NCBI
|
|
21
|
Xing J and Reynolds JP: Diagnostic
advances in urine cytology. Surg Pathol Clin. 11:601–610. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feiertag N, Barry E, Abramson M, Park JY,
Kovac E, Aboumohamed A, Watts K and Sankin A: Urine cytology rarely
escalates clinical management in the surveillance of
non-muscle-invasive bladder cancer. Clin Genitourin Cancer.
21:258–264. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lozano F, Raventós CX, Carrion A, Dinarés
C, Hernández J, Trilla E and Morote J: Xpert bladder cancer monitor
for the early detection of non-muscle invasive bladder cancer
recurrences: Could cystoscopy be substituted? Cancers (Basel).
15:36832023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Palou J, Rodríguez-Rubio F, Huguet J,
Segarra J, Ribal MJ, Alcaraz A and Villavicencio H: Multivariate
analysis of clinical parameters of synchronous primary superficial
bladder cancer and upper urinary tract tumor. J Urol. 174:859–861;
discussion 861. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Raitanen MP, Aine R, Rintala E, Kallio J,
Rajala P, Juusela H and Tammela TL; FinnBladder Group: Differences
between local and review urinary cytology in diagnosis of bladder
cancer. An interobserver multicenter analysis. Eur Urol.
41:284–289. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oliveira MC, Caires HR, Oliveira MJ, Fraga
A, Vasconcelos MH and Ribeiro R: Urinary biomarkers in bladder
cancer: Where do we stand and potential role of extracellular
vesicles. Cancers (Basel). 12:14002020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kundal VK, Pandith AA, Hamid A, Shah A,
Kundal R and Wani SM: Role of NMP22 bladder check test in early
detection of bladder cancer with recurrence. Asian Pacific J Cancer
Prev. 11:1279–1282. 2010.
|
|
28
|
Soloway MS, Briggman JV, Carpinito GA,
Chodak GW, Church PA, Lamm DL, Lange P, Messing E, Pasciak RM,
Reservitz GB, et al: Use of a new tumor marker, urinary NMP22, in
the detection of occult or rapidly recurring transitional cell
carcinoma of the urinary tract following surgical treatment. J
Urol. 156:363–367. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miyake M, Goodison S, Giacoia EG, Rizwani
W, Ross S and Rosser CJ: Influencing factors on the NMP-22 urine
assay: An experimental model. BMC Urol. 12:232012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang Q, Zhang G, Li W, Wang J and Sheng
S: Comparison of the diagnostic performance of fluorescence in situ
hybridization (Fish), nuclear matrix protein 22 (nmp22), and their
combination model in bladder carcinoma detection: A systematic
review and meta-analysis. Onco Targets Ther. 12:349–358. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dong Y, Zhang T, Li X, Yu F, Yu H and Shao
S: Urine biomarkers for the diagnosis of bladder cancer: A network
meta-analysis. Urol J. 18:623–632. 2021.PubMed/NCBI
|
|
32
|
Gong YW, Wang YR, Fan GR, Niu Q, Zhao YL,
Wang H, Svatek R, Rodriguez R and Wang ZP: Diagnostic and
prognostic role of BTA, NMP22, survivin and cytology in urothelial
carcinoma. Transl Cancer Res. 10:3192–3205. 2021. View Article : Google Scholar
|
|
33
|
Egawa S and Kuruma H: Search for
biomarkers of aggressiveness in bladder cancer. Eur Urol. 50:20–22.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang X, Cao Y, Liu J, Wang S, Yang Y and
Du P: The diagnostic and prognostic value of nuclear matrix protein
22 in bladder cancer. Transl Cancer Res. 9:7174–7182. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Frantzi M, Makridakis M and Vlahou A:
Biomarkers for bladder cancer aggressiveness. Curr Opin Urol.
22:390–396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Białek Ł, Bilski K, Dobruch J, Krajewski
W, Szydełko T, Kryst P and Poletajew S: Non-invasive biomarkers in
the diagnosis of upper urinary tract urothelial carcinoma-A
systematic review. Cancers (Basel). 14:15202022. View Article : Google Scholar
|
|
37
|
Ecke TH, Scislowski M, Hassan N, Saura M,
Hallmann S, Koch S, Vuolle S, Malakoutikhah M, Kopra K and Härmä H:
Luminophore chemistry for detection of urinary bladder
cancer-comparison to cytology and urinary rapid tests (BTA
stat®, NMP22® BladderChek® and
UBC® Rapid Test). Anticancer Res. 42:5249–5256. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kumar A, Kumar R and Gupta NP: Comparison
of NMP22 BladderChek test and urine cytology for the detection of
recurrent bladder cancer. Jpn J Clin Oncol. 36:172–175. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sajid MT, Zafar MR, Ahmad H, Ullah S,
Mirza ZI and Shahzad K: Diagnostic accuracy of NMP 22 and urine
cytology for detection of transitional cell carcinoma urinary
bladder taking cystoscopy as gold standard. Pak J Med Sci.
36:705–710. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sarosdy MF, Hudson MA, Ellis WJ, Soloway
MS, deVere White R, Sheinfeld J, Jarowenko MV, Schellhammer PF,
Schervish EW, Patel JV, et al: Improved detection of recurrent
bladder cancer using the bard BTA stat test. Urology. 50:349–353.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thomas L, Leyh H, Marberger M, Bombardieri
E, Bassi P, Pagano F, Pansadoro V, Sternberg CN, Boccon-Gibod L,
Ravery V, et al: Multicenter trial of the quantitative BTA TRAK
assay in the detection of bladder cancer. Clin Chem. 45:472–477.
1999.PubMed/NCBI
|
|
42
|
Heicappell R, Wettig IC, Schostak M,
Muller M, Steiner U, Sauter T and Miller K: Quantitative detection
of human complement factor H-related protein in transitional cell
carcinoma of the urinary bladder. Eur Urol. 35:81–87. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Raitanen MP, Marttila T, Kaasinen E,
Rintala E, Aine R and Tammela TL: Sensitivity of human complement
factor H related protein (BTA stat) test and voided urine cytology
in the diagnosis of bladder cancer. J Urol. 163:1689–1692. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Heicappell R, Müller M, Fimmers R and
Miller K: Qualitative determination of urinary human complement
factor H-related protein (hcfHrp) in patients with bladder cancer,
healthy controls, and patients with benign urologic disease. Urol
Int. 65:181–184. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Raitanen MP, Marttila T, Nurmi M, Ala-Opas
M, Nieminen P, Aine R and Tammela TL; Finnbladder Group: Human
complement factor H related protein test for monitoring bladder
cancer. J Urol. 165:374–377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Miyake M, Goodison S, Rizwani W, Ross S,
Bart Grossman H and Rosser CJ: Urinary BTA: Indicator of bladder
cancer or of hematuria. World J Urol. 30:869–873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ecke TH, Arndt C, Stephan C, Hallmann S,
Lux O, Otto T, Ruttloff J and Gerullis H: Preliminary results of a
multicentre study of the UBC rapid test for detection of urinary
bladder cancer. Anticancer Res. 35:2651–2655. 2015.PubMed/NCBI
|
|
48
|
Reid-Nicholson MD, Ramalingam P, Adeagbo
B, Cheng N, Peiper SC and Terris MK: The use of Urovysion
fluorescence in situ hybridization in the diagnosis and
surveillance of non-urothelial carcinoma of the bladder. Mod
Pathol. 22:119–127. 2009. View Article : Google Scholar
|
|
49
|
Dalquen P, Kleiber B, Grilli B, Herzog M,
Bubendorf L and Oberholzer M: DNA image cytometry and fluorescence
in situ hybridization for noninvasive detection of urothelial
tumors in voided urine. Cancer. 96:374–379. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhou H, Yan Y and Song L: The clinical
application of fluorescence in situ hybridization in diagnosing
urothelial carcinoma. Clin Lab. 62:2001–2009. 2016. View Article : Google Scholar
|
|
51
|
Ainthachot S, Sa-ngiamwibool P, Thanee M,
Watcharadetwittaya S, Chamgramol Y, Pairojkul C and Deenonpoe R:
Chromosomal aberrations, visualized using UroVysion®
fluorescence in-situ hybridization assay, can predict poor
prognosis in formalin-fixed paraffin-embedded tissues of
cholangiocarcinoma patients. Hum Pathol. 126:31–44. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Stadler WM and Olopade OI: The 9p21 region
in bladder cancer cell lines: Large homozygous deletions inactivate
the CDKN2, CDKN2B and MTAP genes. Urol Res. 24:239–244. 1996.
View Article : Google Scholar
|
|
53
|
Cairns P, Tokino K, Eby Y and Sidransky2
D: Homozygous deletions of 9p21 in primary human bladder tumors
detected by comparative multiplex polymerase chain reaction. Cancer
Res. 54:1422–1424. 1994.PubMed/NCBI
|
|
54
|
Moonen PM, Merkx GF, Peelen P, Karthaus
HF, Smeets DF and Witjes JA: UroVysion compared with cytology and
quantitative cytology in the surveillance of non-muscle-invasive
bladder cancer. Eur Urol. 51:1275–1280. 2007. View Article : Google Scholar
|
|
55
|
Pycha S, Trenti E, Mian C, Schwienbacher
C, Hanspeter E, Palermo M, Pycha A, Danuser H and D'Elia C:
Diagnostic value of Xpert® BC detection, bladder
epicheck®, Urovysion® FISH and cytology in
the detection of upper urinary tract urothelial carcinoma. World J
Urol. 41:1323–1328. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Halling KC, King W, Sokolova IA, Meyer RG,
Burkhardt HM, Halling AC, Cheville JC, Sebo TJ, Ramakumar S,
Stewart CS, et al: A comparison of cytology and fluorescence in
situ hybridization for the detection of urothelial carcinoma. J
Urol. 164:1768–1775. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Halling KC and Kipp BR: Bladder cancer
detection using FISH (UroVysion assay). Adv Anat Pathol.
15:279–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Laudadio J, Keane TE, Reeves HM, Savage
SJ, Hoda RS, Lage JM and Wolff DJ: Fluorescence in situ
hybridization for detecting transitional cell carcinoma:
Implications for clinical practice. BJU Int. 96:1280–1285. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Youssef RF, Schlomer BJ, Ho R, Sagalowsky
AI, Ashfaq R and Lotan Y: Role of fluorescence in situ
hybridization in bladder cancer surveillance of patients with
negative cytology. Urol Oncol. 30:273–277. 2012. View Article : Google Scholar
|
|
60
|
Messing EM, Teot L, Korman H, Underhill E,
Barker E, Stork B, Qian J and Bostwick DG: Performance of urine
test in patients monitored for recurrence of bladder cancer: A
multicenter study in the United States. J Urol. 174:1238–1241.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Fradet Y and Lockhard C: Performance
characteristics of a new monoclonal antibody test for bladder
cancer: ImmunoCyt trade mark. Can J Urol. 4:400–405. 1997.
|
|
62
|
Yafi FA, Brimo F, Steinberg J, Aprikian
AG, Tanguay S and Kassouf W: Prospective analysis of sensitivity
and specificity of urinary cytology and other urinary biomarkers
for bladder cancer. Urol Oncol. 33:66.e25–e31. 2015. View Article : Google Scholar
|
|
63
|
He H, Han C, Hao L and Zang G: ImmunoCyt
test compared to cytology in the diagnosis of bladder cancer: A
meta-analysis. Oncol Lett. 12:83–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kong T, Qu Y, Zhao T, Niu Z, Lv X, Wang Y,
Ding Q, Wei P, Fu J, Wang L, et al: Identification of novel protein
biomarkers from the blood and urine for the early diagnosis of
bladder cancer via proximity extension analysis. J Transl Med.
22:3142024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Têtu B, Tiguert R, Harel F and Fradet Y:
ImmunoCyt/uCyt+TM improves the sensitivity of urine
cytology in patients followed for urothelial carcinoma. Mod Pathol.
18:83–89. 2005. View Article : Google Scholar
|
|
66
|
Schmitz-Dräger BJ, Beiche B, Tirsar LA,
Schmitz-Dräger C, Bismarck E and Ebert T: Immunocytology in the
assessment of patients with asymptomatic microhaematuria. Eur Urol.
51:1582–1588. 2007. View Article : Google Scholar
|
|
67
|
Alberice JV, Amaral AF, Armitage EG,
Lorente JA, Algaba F, Carrilho E, Márquez M, García A, Malats N and
Barbas C: Searching for urine biomarkers of bladder cancer
recurrence using a liquid chromatography-mass spectrometry and
capillary electrophoresis-mass spectrometry metabolomics approach.
J Chromatogr A. 1318:163–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gómez BB, López-Cortés R, Casas-Nebra FJ,
Vázquez-Estévez S, Pérez-Fentes D, Chantada-Vázquez MDP, Bravo SB
and Núñez C: Detection of circulating serum protein biomarkers of
non-muscle invasive bladder cancer after protein corona-silver
nanoparticles analysis by SWATH-MS. Nanomaterials (Basel).
11:23842021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Schwamborn K, Krieg RC, Grosse J, Reulen
N, Weiskirchen R, Knuechel R, Jakse G and Henkel C: Serum proteomic
profiling in patients with bladder cancer. Eur Urol. 56:989–996.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Nedjadi T, Benabdelkamal H, Albarakati N,
Masood A, Al-Sayyad A, Alfadda AA, Alanazi IO, Al-Ammari A and
Al-Maghrabi J: Circulating proteomic signature for detection of
biomarkers in bladder cancer patients. Sci Rep. 10:109992020.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Thomas S, Hao L, Ricke WA and Li L:
Biomarker discovery in mass spectrometry-based urinary proteomics.
Proteomics Clin Appl. 10:358–370. 2016. View Article : Google Scholar :
|
|
72
|
Ahn JH, Kang CK, Kim EM, Kim AR and Kim A:
Proteomics for early detection of non-muscle-invasive bladder
cancer: Clinically useful urine protein biomarkers. Life (Basel).
12:3952022.PubMed/NCBI
|
|
73
|
Wang YT, Shi T, Srivastava S, Kagan J, Liu
T and Rodland KD: Proteomic analysis of exosomes for discovery of
protein biomarkers for prostate and bladder cancer. Cancers
(Basel). 12:23352020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ren R, Wang H, Xie L, Muthupandian S and
Yang X: Identify potential urine biomarkers for bladder cancer
prognosis using NGS data analysis and experimental validation. Appl
Biochem Biotechnol. 195:2947–2964. 2023. View Article : Google Scholar
|
|
75
|
Jazayeri MH, Aghaie T, Nedaeinia R, Manian
M and Nickho H: Rapid noninvasive detection of bladder cancer using
survivin antibody-conjugated gold nanoparticles (GNPs) based on
localized surface plasmon resonance (LSPR). Cancer Immunol
Immunother. 69:1833–1840. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kumar B, Koul S, Petersen J, Khandrika L,
Hwa JS, Meacham RB, Wilson S and Koul HK: p38 Mitogen-activated
protein kinase-driven MAPKAPK2 regulates invasion of bladder cancer
by modulation of MMP-2 and MMP-9 activity. Cancer Res. 70:832–841.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Koshkin VS, Boyiddle C, Schwartz N, Yu J,
Yu KS, Kang A, Bloudek L, Fang Q, Schafer JM, Baker AF, et al:
Systematic literature review and testing of HER2 status in
urothelial carcinoma (UC). J Clin Oncol. 41:556. 2023. View Article : Google Scholar
|
|
78
|
Zhang HH, Qi F, Zu XB, Cao YH, Miao JG, Xu
L and Qi L: A proteomic study of potential VEGF-C-associated
proteins in bladder cancer T24 cells. Med Sci Monit.
18:BR441–BR449. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang Z, Xu H, Ji J, Shi X, Lyu J, Zhu Y,
Yu H and Wang F: Heterogeneity of PTEN and PPAR-γ in cancer and
their prognostic application to bladder cancer. Exp Ther Med.
18:3177–3183. 2019.PubMed/NCBI
|
|
80
|
Chen Z, Guo Y, Zhao D, Zou Q, Yu F, Zhang
L and Xu L: Comprehensive analysis revealed that CDKN2A is a
biomarker for immune infiltrates in multiple cancers. Front Cell
Dev Biol. 9:8082082021. View Article : Google Scholar
|
|
81
|
Mi Y, Zhao Y, Shi F, Zhang M, Wang C and
Liu X: Diagnostic accuracy of urine cytokeratin 20 for bladder
cancer: A meta-analysis. Asia Pac J Clin Oncol. 15:e11–e19. 2019.
View Article : Google Scholar
|
|
82
|
Chen CJ, Chou CY, Shu KH, Chen HC, Wang
MC, Chang CC, Hsu BG, Wu MS, Yang YL, Liao WL, et al: Discovery of
novel protein biomarkers in urine for diagnosis of urothelial
cancer using iTRAQ proteomics. J Proteome Res. 20:2953–2963. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tsai CH, Chen YT, Chang YH, Hsueh C, Liu
CY, Chang YS, Chen CL and Yu JS: Systematic verification of bladder
cancer-associated tissue protein biomarker candidates in clinical
urine specimens. Oncotarget. 9:30731–30747. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Allione A, Pardini B, Viberti C, Giribaldi
G, Turini S, Di Gaetano C, Guarrera S, Cordero F, Oderda M, Allasia
M, et al: MMP23B expression and protein levels in blood and urine
are associated with bladder cancer. Carcinogenesis. 39:1254–1263.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gobin E, Bagwell K, Wagner J, Mysona D,
Sandirasegarane S, Smith N, Bai S, Sharma A, Schleifer R and She
JX: A pan-cancer perspective of matrix metalloproteases (MMP) gene
expression profile and their diagnostic/prognostic potential. BMC
Cancer. 19:5812019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lokeshwar VB, Schroeder GL, Selzer MG,
Hautmann SH, Posey JT, Duncan RC, Watson R, Rose L, Markowitz S and
Soloway MS: Bladder tumor markers for monitoring recurrence and
screening comparison of hyaluronic acid-hyaluronidase and BTA-stat
tests. Cancer. 95:61–72. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Lokeshwar VB, Habuchi T, Grossman HB,
Murphy WM, Hautmann SH, Hemstreet GP III, Bono AV, Getzenberg RH,
Goebell P, Schmitz-Dräger BJ, et al: Bladder tumor markers beyond
cytology: International consensus panel on bladder tumor markers.
Urology. 66(Suppl 1): S35–S63. 2005. View Article : Google Scholar
|
|
88
|
Friedrich MG, Hellstern A, Toma MI,
Hammerer P and Huland H: Are false-positive urine markers for the
detection of bladder carcinoma really wrong or do they predict
tumor recurrence? Eur Urol. 43:146–151. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Todenhöfer T, Hennenlotter J, Khs U, Tews
V, Gakis G, Aufderklamm S, Stenzl A and Schwentner C: Influence of
urinary tract instrumentation and inflammation on the performance
of urine markers for the detection of bladder cancer. Urology.
79:620–624. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Li A, Zhang T, Zheng M, Liu Y and Chen Z:
Exosomal proteins as potential markers of tumor diagnosis. J
Hematol Oncol. 10:1752017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Song JB, Morrissey JJ, Mobley JM,
Figenshau KG, Vetter JM, Bhayani SB, Kharasch ED and Figenshau RS:
Urinary aquaporin 1 and perilipin 2: Can these novel markers
accurately characterize small renal masses and help guide patient
management? Int J Urol. 26:260–265. 2019. View Article : Google Scholar
|
|
92
|
Benderska-Söder N, Hovanec J, Pesch B,
Goebell PJ, Roghmann F, Noldus J, Rabinovich J, Wichert K,
Gleichenhagen J, Käfferlein HU, et al: Toward noninvasive follow-up
of low-risk bladder cancer-Rationale and concept of the UroFollow
trial. Urol Oncol. 38:886–895. 2020. View Article : Google Scholar
|
|
93
|
Aitekenov S, Gaipov A and Bukasov R:
Review: Detection and quantification of proteins in human urine.
Talanta. 223:1217182021. View Article : Google Scholar
|
|
94
|
Goecks J, Jalili V, Heiser LM and Gray JW:
How machine learning will transform biomedicine. Cell. 181:92–101.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Li Y, Sun L, Guo X, Mo N, Zhang J and Li
C: Frontiers in bladder cancer genomic research. Front Oncol.
11:6707292021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Sarhadi VK and Armengol G: Molecular
biomarkers in cancer. Biomolecules. 12:10212022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Shi R, Wang X, Wu Y, Xu B, Zhao T, Trapp
C, Wang X, Unger K, Zhou C, Lu S, et al: APOBEC-mediated
mutagenesis is a favorable predictor of prognosis and immunotherapy
for bladder cancer patients: evidence from pan-cancer analysis and
multiple databases. Theranostics. 12:4181–4199. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Le NQK, Kha QH, Nguyen VH, Chen YC, Cheng
SJ and Chen CY: Machine learning-based radiomics signatures for
egfr and kras mutations prediction in non-small-cell lung cancer.
Int J Mol Sci. 22:92542021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Gao J, Wu H, Shi X, Huo Z, Zhang J and
Liang Z: Comparison of next-generation sequencing, quantitative
PCR, and sanger sequencing for mutation profiling of EGFR, KRAS,
PIK3CA and BRAF in clinical lung tumors. Clin Lab. 62:689–696.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chauhan PS, Shiang A, Alahi I, Sundby RT,
Feng W, Gungoren B, Nawaf C, Chen K, Babbra RK, Harris PK, et al:
Urine cell-free DNA multi-omics to detect MRD and predict survival
in bladder cancer patients. NPJ Precis Oncol. 7:62023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Sieverink CA, Batista RPM, Prazeres HJM,
Vinagre J, Sampaio C, Leão RR, Máximo V, Witjes JA and Soares P:
Clinical validation of a urine test (uromonitor-V2®) for
the surveillance of non-muscle-invasive bladder cancer patients.
Diagnostics (Basel). 10:7452020. View Article : Google Scholar
|
|
102
|
Hosen MI, Sheikh M, Zvereva M, Scelo G,
Forey N, Durand G, Voegele C, Poustchi H, Khoshnia M, Roshandel G,
et al: Urinary TERT promoter mutations are detectable up to 10
years prior to clinical diagnosis of bladder cancer: Evidence from
the golestan cohort study. EBioMedicine. 53:1026432020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Soputro NA, Gracias DN, Dias BH, Nzenza T,
O'Connell H and Sethi K: Utility of urinary biomarkers in primary
haematuria: Systematic review and meta-analysis. BJUI Compass.
3:334–343. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Springer SU, Chen CH, Rodriguez Pena MDC,
Li L, Douville C, Wang Y, Cohen JD, Taheri D, Silliman N, Schaefer
J, et al: Non-invasive detection of urothelial cancer through the
analysis of driver gene mutations and aneuploidy. Elife.
7:e321432018. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Baffa R, Letko J, McClung C, LeNoir J,
Vecchione A and Gomella LG: Molecular genetics of bladder cancer:
Targets for diagnosis and therapy. J Exp Clin Cancer Res.
25:145–160. 2006.PubMed/NCBI
|
|
106
|
Mertens LS, Claps F, Mayr R, Bostrom PJ,
Shariat SF, Zwarthoff EC, Boormans JL, Abas C, van Leenders GJLH,
Götz S, et al: Prognostic markers in invasive bladder cancer: FGFR3
mutation status versus P53 and KI-67 expression: A multi-center,
multi-laboratory analysis in 1058 radical cystectomy patients. Urol
Oncol. 40:110.e1–110.e9. 2022. View Article : Google Scholar
|
|
107
|
Kim YS, Kim K, Kwon GY, Lee SJ and Park
SH: Fibroblast growth factor receptor 3 (FGFR3) aberrations in
muscle-invasive urothelial carcinoma. BMC Urol. 18:682018.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Calderaro J, Rebouissou S, De Koning L,
Masmoudi A, Hérault A, Dubois T, Maille P, Soyeux P, Sibony M, de
la Taille A, et al: PI3K/AKT pathway activation in bladder
carcinogenesis. Int J Cancer. 134:1776–1784. 2014. View Article : Google Scholar
|
|
109
|
Hao L, Fang J, Xu R, Liu S, Luo G and Wang
X: Significance of the FGFR3 mutation in Chinese patients with
bladder cancer. Transl Androl Urol. 12:761–769. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Sakr SA, Mahran HA, Fahmy AM, El-Kholy MA
and Meawad M: Expression of c-erb-B2 gene in bladder cancer of
Egyptian patients and its correlation with p53 and bcl-2. Biomed
Pharmacother. 76:73–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
George B, Datar RH, Wu L, Cai J, Patten N,
Beil SJ, Groshen S, Stein J, Skinner D, Jones PA and Cote RJ: p53
gene and protein status: The role of p53 alterations in predicting
outcome in patients with bladder cancer. J Clin Oncol.
25:5352–5358. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Landolfi JA and Terio KA: Transitional
cell carcinoma in fishing cats (Prionailurus viverrinus): Pathology
and expression of cyclooxygenase-1, -2, and p53. Vet Pathol.
43:674–681. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Pakmanesh H, Anvari O, Forey N, Weiderpass
E, Malekpourafshar R, Iranpour M, Shahesmaeili A, Ahmadi N,
Bazrafshan A, Zendehdel K, et al: TERT promoter mutations as simple
and non-invasive urinary biomarkers for the detection of urothelial
bladder cancer in a high-risk region. Int J Mol Sci. 23:143192022.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Avogbe PH, Manel A, Vian E, Durand G,
Forey N, Voegele C, Zvereva M, Hosen MI, Meziani S, De Tilly B, et
al: Urinary TERT promoter mutations as non-invasive biomarkers for
the comprehensive detection of urothelial cancer. EBioMedicine.
44:431–438. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Eich ML, Rodriguez Pena MDC, Springer SU,
Taheri D, Tregnago AC, Salles DC, Bezerra SM, Cunha IW, Fujita K,
Ertoy D, et al: Incidence and distribution of UroSEEK gene panel in
a multi-institutional cohort of bladder urothelial carcinoma. Mod
Pathol. 32:1544–1550. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Wang P, Shi Y, Zhang J, Shou J, Zhang M,
Zou D, Liang Y, Li J, Tan Y, Zhang M, et al: UCseek: Ultrasensitive
early detection and recurrence monitoring of urothelial carcinoma
by shallow-depth genome-wide bisulfite sequencing of urinary
sediment DNA. EBioMedicine. 89:1044372023. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Ramos P, Brás JP, Dias C, Bessa-Gonçalves
M, Botelho F, Silva J, Silva C and Pacheco-Figueiredo L:
Uromonitor: Clinical validation and performance assessment of a
urinary biomarker within the surveillance of patients with
non-muscle-invasive bladder cancer patients. J Urol. Nov
19–2024.Epub ahead of print. View Article : Google Scholar
|
|
118
|
Ou Z, Li K, Yang T, Dai Y, Chandra M, Ning
J, Wang Y, Xu R, Gao T, Xie Y, et al: Detection of bladder cancer
using urinary cell-free DNA and cellular DNA. Clin Transl Med.
9:42020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zhang T, Du E, Liu Y, Cheng J, Zhang Z, Xu
Y, Qi S and Chen Y: Anticancer effects of zinc oxide nanoparticles
through altering the methylation status of histone on bladder
cancer cells. Int J Nanomedicine. 15:1457–1468. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Porten SP: Epigenetic alterations in
bladder cancer. Curr Urol Rep. 19:1022018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
van Kessel KEM, Van Neste L, Lurkin I,
Zwarthoff EC and Van Criekinge W: Evaluation of an epigenetic
profile for the detection of bladder cancer in patients with
hematuria. J Urol. 195:601–607. 2016. View Article : Google Scholar
|
|
122
|
Sathianathen NJ, Butaney M, Weight CJ,
Kumar R and Konety BR: Urinary biomarkers in the evaluation of
primary hematuria: A systematic review and meta-analysis. Bladder
Cancer. 4:353–363. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Tripathi K, Goel A, Singhai A and Garg M:
Promoter hypomethylation as potential confounder of Ras gene
overexpression and their clinical significance in subsets of
urothelial carcinoma of bladder. Mol Biol Rep. 48:2183–2199. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Wolff EM, Chihara Y, Pan F, Weisenberger
DJ, Siegmund KD, Sugano K, Kawashima K, Laird PW, Jones PA and
Liang G: Unique DNA methylation patterns distinguish noninvasive
and invasive urothelial cancers and establish an epigenetic field
defect in premalignant tissue. Cancer Res. 70:8169–8178. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Locke WJ, Guanzon D, Ma C, Liew YJ,
Duesing KR, Fung KYC and Ross JP: DNA methylation cancer
biomarkers: Translation to the clinic. Front Genet. 10:11502019.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Xiao Y, Ju L, Qian K, Jin W, Wang G, Zhao
Y, Jiang W, Liu N, Wu K, Peng M, et al: Non-invasive diagnosis and
surveillance of bladder cancer with driver and passenger DNA
methylation in a prospective cohort study. Clin Transl Med.
12:e10082022. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Shindo T, Shimizu T, Nojima M, Niinuma T,
Maruyama R, Kitajima H, Kai M, Itoh N, Suzuki H and Masumori N:
Evaluation of urinary DNA methylation as a marker for recurrent
bladder cancer: A 2-center prospective study. Urology. 113:71–78.
2018. View Article : Google Scholar
|
|
128
|
Ko K, Kananazawa Y, Yamada T, Kakinuma D,
Matsuno K, Ando F, Kuriyama S, Matsuda A and Yoshida H: Methylation
status and long-fragment cell-free DNA are prognostic biomarkers
for gastric cancer. Cancer Med. 10:2003–2012. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Lutfi A, Afghan MK, Swed B and Kasi PM:
False-positive liquid biopsy assays secondary to overlapping
aberrant methylation from non-cancer disease states. Case Rep
Oncol. 16:1536–1541. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Shields MD, Chen K, Dutcher G, Patel I and
Pellini B: Making the rounds: Exploring the role of circulating
tumor DNA (ctDNA) in non-small cell lung cancer. Int J Mol Sci.
23:90062022. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
D'Andrea D, Soria F, Zehetmayer S, Gust
KM, Korn S, Witjes JA and Shariat SF: Diagnostic accuracy, clinical
utility and influence on decision-making of a methylation urine
biomarker test in the surveillance of non-muscle-invasive bladder
cancer. BJU Int. 123:959–967. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Tan WS, Feber A, Sarpong R, Khetrapal P,
Rodney S, Jalil R, Mostafid H, Cresswell J, Hicks J, Rane A, et al:
Who should be investigated for haematuria? Results of a
contemporary prospective observational study of 3556 patients. Eur
Urol. 74:10–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Davalos V and Esteller M: Cancer
epigenetics in clinical practice. CA Cancer J Clin. 73:376–424.
2023. View Article : Google Scholar
|
|
134
|
Wu J, Lin Y, Yang K, Liu X, Wang H, Yu T,
Tao R, Guo J, Chen L, Cheng H, et al: Clinical effectiveness of a
multitarget urine DNA test for urothelial carcinoma detection: A
double-blinded, multicenter, prospective trial. Mol Cancer.
23:572024. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Maas M, Todenhöfer T and Black PC: Urine
biomarkers in bladder cancer-current status and future
perspectives. Nat Rev Urol. 20:597–614. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Iisager L, Ahrenfeldt J, Keller AK,
Nielsen TK, Fristrup N and Lyskjær I: KIDNEY-PAGER: Analysis of
circulating tumor DNA as a biomarker in renal cancer-an
observational trial. Study protocol. Acta Oncol. 63:51–55. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Schardt J, Roth B and Seiler R: Forty
years of cisplatin-based chemotherapy in muscle-invasive bladder
cancer: Are we understanding how, who and when? World J Urol.
37:1759–1765. 2019. View Article : Google Scholar
|
|
138
|
Soave A, Kluwe L, Yu H, Rink M, Gild P,
Vetterlein MW, Marks P, Sauter G, Fisch M, Meyer CP, et al: Copy
number variations in primary tumor, serum and lymph node metastasis
of bladder cancer patients treated with radical cystectomy. Sci
Rep. 10:215622020. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Soave A, Chun FK, Hillebrand T, Rink M,
Weisbach L, Steinbach B, Fisch M, Pantel K and Schwarzenbach H:
Copy number variations of circulating, cell-free DNA in urothelial
carcinoma of the bladder patients treated with radical cystectomy:
A prospective study. Oncotarget. 8:56398–56407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Keck B, Ellmann C, Stoehr R, Weigelt K,
Goebell PJ, Kunath F, Taubert H, Hartmann A, Wullich B and Wach S:
Comparative genomic hybridization shows complex genomic changes of
plasmacytoid urothelial carcinoma. Urol Oncol. 32:1234–1239. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Cancer Genome Atlas Research Network:
Comprehensive molecular characterization of urothelial bladder
carcinoma. Nature. 507:315–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Li R, Du Y, Chen Z, Xu D, Lin T, Jin S,
Wang G, Liu Z, Lu M, Chen X, et al: Macroscopic somatic clonal
expansion in morphologically normal human urothelium. Science.
370:82–89. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Luo T, Yi X and Si W: Identification of
miRNA and genes involving in osteosarcoma by comprehensive analysis
of microRNA and copy number variation data. Oncol Lett.
14:5427–5433. 2017.PubMed/NCBI
|
|
144
|
Wu S, Ou T, Xing N, Lu J, Wan S, Wang C,
Zhang X, Yang F, Huang Y and Cai Z: Whole-genome sequencing
identifies ADGRG6 enhancer mutations and FRS2 duplications as
angiogenesis-related drivers in bladder cancer. Nat Commun.
10:7202019. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zeng S, Ying Y, Xing N, Wang B, Qian Z,
Zhou Z, Zhang Z, Xu W, Wang H, Dai L, et al: Noninvasive detection
of urothelial carcinoma by cost-effective low-coverage whole-genome
sequencing from urine-exfoliated cell DNA. Clin Cancer Res.
26:5646–5654. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Ge G, Peng D, Guan B, Zhou Y, Gong Y, Shi
Y, Hao X, Xu Z, Qi J, Lu H, et al: Urothelial carcinoma detection
based on copy number profiles of urinary cell-free DNA by shallow
whole-genome sequencing. Clin Chem. 66:188–198. 2020. View Article : Google Scholar
|
|
147
|
Ellegren H: Microsatellites: Simple
sequences with complex evolution. Nat Rev Genet. 5:435–445. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Moon C, Gordon M, Moon D and Reynolds T:
Microsatellite instability analysis (MSA) for bladder cancer: Past
history and future directions. Int J Mol Sci. 22:128642021.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Zheng K, Wan H, Zhang J, Shan G, Chai N,
Li D, Fang N, Liu L, Zhang J, Du R, et al: A novel NGS-based
microsatellite instability (MSI) status classifier with 9 loci for
colorectal cancer patients. J Transl Med. 18:2152020. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Zheng J, Miao F, Wang Z, Ma Y, Lin Z, Chen
Y, Kong X, Wang Y, Zhuang A, Wu T and Li W: Identification of MDM2
as a prognostic and immunotherapeutic biomarker in a comprehensive
pan-cancer analysis: A promising target for breast cancer, bladder
cancer and ovarian cancer immunotherapy. Life Sci. 327:1218322023.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Malapelle U, Parente P, Pepe F, De Luca C,
Pisapia P, Sgariglia R, Nacchio M, Gragnano G, Russo G, Conticelli
F, et al: Evaluation of micro satellite instability and mismatch
repair status in different solid tumors: A multicenter analysis in
a real world setting. Cells. 10:18782021. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Kasi PM, Bucheit LA, Liao J, Starr J,
Barata P, Klempner SJ, Gandara D, Shergill A, Madeira da Silva L,
Weipert C, et al: Pan-cancer prevalence of microsatellite
instability-high (MSI-H) identified by circulating tumor DNA and
associated real-world clinical outcomes. JCO Precis Oncol.
7:e23001182023. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Ma YT, Hua F, Zhong XM, Xue YJ, Li J, Nie
YC, Zhang XD, Ma JW, Lin CH, Zhang HZ, et al: Clinicopathological
characteristics, molecular landscape, and biomarker landscape for
predicting the efficacy of PD-1/PD-L1 inhibitors in Chinese
population with mismatch repair deficient urothelial carcinoma: A
real-world study. Front Immunol. 14:12690972023. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Chang L, Chang M, Chang HM and Chang F:
Microsatellite instability: A predictive biomarker for cancer
immunotherapy. Appl Immunohistochem Mol Morphol. 26:e15–e21. 2018.
View Article : Google Scholar
|
|
155
|
Hirotsu Y, Nagakubo Y, Amemiya K, Oyama T,
Mochizuki H and Omata M: Microsatellite instability status is
determined by targeted sequencing with MSIcall in 25 cancer types.
Clin Chim Acta. 502:207–213. 2020. View Article : Google Scholar
|
|
156
|
Zekri AR, Khaled HM, Mohammed MB, Diab FM,
Abdellateif MS, El Deeb S, Badr AM, Mohanad M, Abdallah SO and
Bahnassy AA: Microsatellite instability profiling in Egyptian
bladder cancer patients: A pilot study. Curr Probl Cancer.
43:1004722019. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Awadalla A, Harraz AM, Abol-Enein H,
Laymon M, Ahmed AE, Abdel-Rahim M, Zekri AN and Shokeir AA:
Prognostic influence of microsatellite alterations of
muscle-invasive bladder cancer treated with radical cystectomy.
Urol Oncol. 40:64.e9–64.e15. 2022. View Article : Google Scholar
|
|
158
|
Steiner G, Schoenberg MP, Linn JF, Mao L
and Sidransky D: Detection of bladder cancer recurrence by
microsatellite analysis of urine. Nat Med. 3:621–624. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Wild PJ, Fuchs T, Stoehr R, Zimmermann D,
Frigerio S, Padberg B, Steiner I, Zwarthoff EC, Burger M, Denzinger
S, et al: Detection of urothelial bladder cancer cells in voided
urine can be improved by a combination of cytology and standardized
microsatellite analysis. Cancer Epidemiol Biomarkers Prev.
18:1798–1806. 2009. View Article : Google Scholar
|
|
160
|
Carpagnano GE, Costantino E, Palladino GP,
Lacedonia D, Martinelli D, Orlando S and Foschino-Barbaro MP:
Microsatellite alterations and cell-free dna analysis: Could they
increase the cytology sensitivity in the diagnosis of malignant
pleural effusion? Rejuvenation Res. 15:265–273. 2012. View Article : Google Scholar
|
|
161
|
Wagner SJ, Reisenbüchler D, West NP,
Niehues JM, Zhu J, Foersch S, Veldhuizen GP, Quirke P, Grabsch HI,
van den Brandt PA, et al: Transformer-based biomarker prediction
from colorectal cancer histology: A large-scale multicentric study.
Cancer Cell. 41:1650–1661. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Van Rhijn BW, Van Der Poel HG and Van Der
Kwast TH: Urine markers for bladder cancer surveillance: A
systematic review. Eur Urol. 47:736–748. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Bartoletti R, Dal Canto M, Cai T, Piazzini
M, Travaglini F, Gavazzi A and Rizzo M: Early diagnosis and
monitoring of superficial transitional cell carcinoma by
microsatellite analysis on urine sediment. Oncol Rep. 13:531–537.
2005.PubMed/NCBI
|
|
164
|
Frigerio S, Padberg BC, Strebel RT,
Lenggenhager DM, Messthaler A, Abdou MT, Moch H and Zimmermann DR:
Improved detection of bladder carcinoma cells in voided urine by
standardized microsatellite analysis. Int J Cancer. 121:329–338.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Brisuda A, Pazourkova E, Soukup V, Horinek
A, Hrbáček J, Capoun O, Svobodova I, Pospisilova S, Korabecna M,
Mares J, et al: Urinary cell-free DNA quantification as
non-invasive biomarker in patients with bladder cancer. Urol Int.
96:25–31. 2016. View Article : Google Scholar
|
|
166
|
Bryzgunova OE, Konoshenko MY and Laktionov
PP: Concentration of cell-free DNA in different tumor types. Expert
Rev Mol Diagn. 21:63–75. 2021. View Article : Google Scholar
|
|
167
|
Green EA, Li R, Albiges L, Choueiri TK,
Freedman M, Pal S, Dyrskjøt L and Kamat AM: Clinical utility of
cell-free and circulating tumor DNA in kidney and bladder cancer: A
critical review of current literature. Eur Urol Oncol. 4:893–903.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Ralla B, Stephan C, Meller S, Dietrich D,
Kristiansen G and Jung K: Nucleic acid-based biomarkers in body
fluids of patients with urologic malignancies. Crit Rev Clin Lab
Sci. 51:200–231. 2014. View Article : Google Scholar
|
|
169
|
Li P, Ning J, Luo X, Du H, Zhang Q, Zhou
G, Du Q, Ou Z, Wang L and Wang Y: New method to preserve the
original proportion and integrity of urinary cell-free DNA. J Clin
Lab Anal. 33:e226682019. View Article : Google Scholar
|
|
170
|
Tse RT, Zhao H, Wong CY, Cheng CK, Kong
AW, Peng Q, Chiu PK, Ng CF and Teoh JY: Urinary cell-free DNA in
bladder cancer detection. Diagnostics (Basel). 11:3062021.
View Article : Google Scholar :
|
|
171
|
Augustus E, van Casteren K, Sorber L, van
Dam P, Roeyen G, Peeters M, Vorsters A, Wouters A, Raskin J, Rolfo
C, et al: The art of obtaining a high yield of cell-free DNA from
urine. PLoS One. 15:e02310582020. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Piao XM, Jeong P, Kim YH, Byun YJ, Xu Y,
Kang HW, Ha YS, Kim WT, Lee JY, Woo SH, et al: Urinary cell-free
microRNA biomarker could discriminate bladder cancer from benign
hematuria. Int J Cancer. 144:380–388. 2019. View Article : Google Scholar
|
|
173
|
Zancan M, Galdi F, Di Tonno F, Mazzariol
C, Orlando C, Malentacchi F, Agostini M, Maran M, Del Bianco P,
Fabricio AS, et al: Evaluation of cell-free DNA in urine as a
marker for bladder cancer diagnosis. Int J Biol Markers.
24:147–155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
do Nascimento Alves SIPM, Lavalhegas
Hallack M, Moreira Perez M, da Costa Aguiar Alves B, da Silva LH
and Afonso Fonseca FL: Application of the Z-scan technique for the
detection of CFCDNA (cell-free circulating DNA) and urine DNA
(uDNA) in patients with bladder cancer. Photodiagnosis Photodyn
Ther. 26:131–133. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Nel I, Münch C, Shamkeeva S, Heinemann ML,
Isermann B and Aktas B: The challenge to stabilize, extract and
analyze urinary cell-free DNA (ucfDNA) during clinical routine.
Diagnostics (Basel). 13:36702023. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Markus H, Zhao J, Contente-Cuomo T,
Stephens MD, Raupach E, Odenheimer-Bergman A, Connor S, McDonald
BR, Moore B, Hutchins E, et al: Analysis of recurrently protected
genomic regions in cell-free DNA found in urine. Sci Transl Med.
13:aaz30882021. View Article : Google Scholar
|
|
177
|
Casadio V, Calistri D, Salvi S, Gunelli R,
Carretta E, Amadori D, Silvestrini R and Zoli W: Urine cell-free
DNA integrity as a marker for early prostate cancer diagnosis: A
pilot study. Biomed Res Int. 2013:2704572013. View Article : Google Scholar
|
|
178
|
Casadio V, Calistri D, Tebaldi M,
Bravaccini S, Gunelli R, Martorana G, Bertaccini A, Serra L, Scarpi
E, Amadori D, et al: Urine Cell-Free DNA integrity as a marker for
early bladder cancer diagnosis: Preliminary data. Urol Oncol.
31:1744–1750. 2013. View Article : Google Scholar
|
|
179
|
Nuzzo PV, Berchuck JE, Korthauer K, Spisak
S, Nassar AH, Abou Alaiwi S, Chakravarthy A, Shen SY, Bakouny Z,
Boccardo F, et al: Detection of renal cell carcinoma using plasma
and urine cell-free DNA methylomes. Nat Med. 26:1041–1043. 2020.
View Article : Google Scholar
|
|
180
|
Zhou Q, Liu F, Guo L, Chen R, Yuan X, Li
C, Shu L, Liu H, Zhou Y, Wu Y, et al: A novel urine cell-free DNA
preservation solution and its application in kidney
transplantation. Nephrology (Carlton). 26:684–691. 2021. View Article : Google Scholar
|
|
181
|
Mouliere F, Smith CG, Heider K, Su J, van
der Pol Y, Thompson M, Morris J, Wan JCM, Chandrananda D, Hadfield
J, et al: Fragmentation patterns and personalized sequencing of
cell-free DNA in urine and plasma of glioma patients. EMBO Mol Med.
13:e128812021. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Chang A, Mzava O, Lenz JS, Cheng AP,
Burnham P, Motley ST, Bennett C, Connelly JT, Dadhania DM,
Suthanthiran M, et al: Measurement biases distort cell-free DNA
fragmentation profiles and define the sensitivity of metagenomic
cell-free DNA sequencing assays. Clin Chem. 68:163–171. 2022.
View Article : Google Scholar :
|
|
183
|
Yasuda T, Nadano D, Awazu S and Kishi K:
Human urine deoxyribonuclease II (DNase II) isoenzymes: A novel
immunoaffinity purification, biochemical multiplicity, genetic
heterogeneity and broad distribution among tissues and body fluids.
Biochim Biophys Acta. 1119:185–193. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Nardelli C, Aveta A, Pandolfo SD, Tripodi
L, Russo F, Imbimbo C, Castaldo G and Pastore L: Microbiome
profiling in bladder cancer patients using the first-morning urine
sample. Eur Urol Open Sci. 59:18–26. 2023. View Article : Google Scholar
|
|
185
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar
|
|
186
|
Elballal MS, Sallam AM, Elesawy AE, Shahin
RK, Midan HM, Elrebehy MA, Elazazy O, El-Boghdady RM, Blasy SH,
Amer NM, et al: miRNAs as potential game-changers in renal cell
carcinoma: Future clinical and medicinal uses. Pathol Res Pract.
245:1544392023. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Xie Y, Ma X, Chen L, Li H, Gu L, Gao Y,
Zhang Y, Li X, Fan Y, Chen J and Zhang X: MicroRNAs with prognostic
significance in bladder cancer: A systematic review and
meta-analysis. Sci Rep. 7:56192017. View Article : Google Scholar
|
|
188
|
Pan D, Du Y, Li R, Shen A, Liu X, Li C and
Hu B: miR-29b-3p increases radiosensitivity in stemness cancer
cells via modulating oncogenes axis. Front Cell Dev Biol.
9:7410742021. View Article : Google Scholar :
|
|
189
|
Eissa S, Matboli M, Hegazy MG, Kotb YM and
Essawy NO: Evaluation of urinary microRNA panel in bladder cancer
diagnosis: Relation to bilharziasis. Transl Res. 165:731–739. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Nekoohesh L, Modarressi MH, Mowla SJ,
Sadroddiny E, Etemadian M, Afsharpad M, Zolfaghari F, Barzegari M,
Saffari M, Oskooei VK, et al: Expression profile of miRNAs in urine
samples of bladder cancer patients. Biomark Med. 12:1311–1321.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Suarez-Cabrera C, Estudillo L, Ramón-GilE,
Martínez-Fernández M, Peral J, Rubio C, Lodewijk I, Martínde
Bernardo Á, García-Escudero R, Villacampa F, et al: BlaDimiR: A
urine-based miRNA score for accurate bladder cancer diagnosis and
follow-up. Eur Urol. 82:663–667. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Li HJ, Gong X, Li ZK, Qin W, He CX, Xing
L, Zhou X, Zhao D and Cao HL: Role of long non-coding RNAs on
bladder cancer. Front Cell Dev Biol. 9:6726792021. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Wang X, Song D, Zhu B, Jin Y, Cai C and
Wang Z: Urinary exosomal mRNA as a biomarker for the diagnosis of
bladder cancer. Anticancer Drugs. 35:362–370. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Zhang ZH, Wang Y, Zhang Y, Zheng SF, Feng
T, Tian X, Abudurexiti M, Wang ZD, Zhu WK, Su JQ, et al: The
function and mechanisms of action of circular RNAs in urologic
cancer. Mol Cancer. 22:612023. View Article : Google Scholar
|
|
195
|
Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F
and Liu Y: Long non-coding RNA UCA1 increases chemoresistance of
bladder cancer cells by regulating Wnt signaling. FEBS J.
281:1750–1758. 2014. View Article : Google Scholar
|
|
196
|
Zhao H, Wu W, Li X and Chen W: Long
noncoding RNA UCA1 promotes glutamine-driven anaplerosis of bladder
cancer by interacting with hnRNP I/L to upregulate GPT2 expression.
Transl Oncol. 17:1013402022. View Article : Google Scholar
|
|
197
|
Wang Z, Wang X, Zhang D, Yu Y, Cai L and
Zhang C: Long non-coding RNA urothelial carcinoma-associated 1 as a
tumor biomarker for the diagnosis of urinary bladder cancer. Tumor
Biol. 39:10104283177099902017. View Article : Google Scholar
|
|
198
|
Kong JH, Ha D, Lee J, Kim I, Park M, Im
SH, Shin K and Kim S: Network-based machine learning approach to
predict immunotherapy response in cancer patients. Nat Commun.
13:37032022. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Abbastabar M, Sarfi M, Golestani A, Karimi
A, Pourmand G and Khalili E: Tumor-derived urinary exosomal long
non-coding RNAs as diagnostic biomarkers for bladder cancer. EXCLI
J. 19:301–310. 2020.PubMed/NCBI
|
|
200
|
Bao Z, Zhang W and Dong D: A potential
prognostic lncRNA signature for predicting survival in patients
with bladder urothelial carcinoma. Oncotarget. 8:10485–10497. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Zhao L, Li J, Xue Z and Wang J: Exosomal
noncoding RNAs as noninvasive biomarkers in bladder cancer: A
diagnostic meta-analysis. Clin Transl Oncol. 26:1497–1507. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Bian B, Li L, Ke X, Chen H, Liu Y, Zheng
N, Zheng Y, Ma Y, Zhou Y, Yang J, et al: Urinary exosomal long
non-coding RNAs as noninvasive biomarkers for diagnosis of bladder
cancer by RNA sequencing. Front Oncol. 12:9763292022. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Yu Y, Zhang W, Li A, Chen Y, Ou Q, He Z,
Zhang Y, Liu R, Yao H and Song E: Association of long noncoding RNA
biomarkers with clinical immune subtype and prediction of
immunotherapy response in patients with cancer. JAMA Netw Open.
3:e2021492020. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Lin YH: The effects of intracellular and
exosomal ncRNAs on cancer progression. Cancer Gene Ther.
30:1587–1597. 2023. View Article : Google Scholar
|
|
205
|
Chen C, Luo Y, He W, Zhao Y, Kong Y, Liu
H, Zhong G, Li Y, Li J, Huang J, et al: Exosomal long noncoding RNA
LNMAT2 promotes lymphatic metastasis in bladder cancer. J Clin
Invest. 130:404–421. 2020. View Article : Google Scholar
|
|
206
|
He W, Zhong G, Jiang N, Wang B, Fan X,
Chen C, Chen X, Huang J and Lin T: Long noncoding RNA BLACAT2
promotes bladder cancer-associated lymphangiogenesis and lymphatic
metastasis. J Clin Invest. 128:861–875. 2018. View Article : Google Scholar
|
|
207
|
Huang H, Du J, Jin B, Pang L, Duan N,
Huang C, Hou J, Yu W, Hao H and Li H: Combination of urine exosomal
mRNAs and lncRNAs as novel diagnostic biomarkers for bladder
cancer. Front Oncol. 11:6672122021. View Article : Google Scholar :
|
|
208
|
Laukhtina E, Shim SR, Mori K, D'Andrea D,
Soria F, Rajwa P, Mostafaei H, Compérat E, Cimadamore A, Moschini
M, et al: Diagnostic accuracy of novel urinary biomarker tests in
non-muscle-invasive bladder cancer: A systematic review and network
meta-analysis. Eur Urol Oncol. 4:927–942. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Li KD, Chu CE, Patel M, Meng MV, Morgan TM
and Porten SP: Cxbladder monitor testing to reduce cystoscopy
frequency in patients with bladder cancer. Urol Oncol.
41:326.e1–326.e8. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Fasulo V, Paciotti M, Lazzeri M, Contieri
R, Casale P, Saita A, Lughezzani G, Diana P, Frego N, Avolio PP, et
al: Xpert bladder cancer monitor may avoid cystoscopies in patients
under 'active surveillance' for recurrent bladder cancer (BIAS
Project): Longitudinal cohort study. Front Oncol. 12:8328352022.
View Article : Google Scholar
|
|
211
|
Tortora F, La Civita E, Trivedi P,
Febbraio F, Terracciano D and Cimmino A: Emerging RNA-based
therapeutic and diagnostic options: Recent advances and future
challenges in genitourinary cancers. Int J Mol Sci. 24:46012023.
View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Yang L, Zou X, Zou J and Zhang G:
Functions of circular RNAs in bladder, prostate and renal cell
cancer (Review). Mol Med Rep. 23:3072021. View Article : Google Scholar
|
|
213
|
Jiang YK, Shuai YJ, Ding HM, Zhang H,
Huang C, Wang L, Sun JY, Wei WJ, Xiao XY and Jiang GS: Erratum to:
ARID1A inactivation increases expression of circ0008399 and
promotes cisplatin resistance in bladder cancer. Curr Med Sci.
43:12602023. View Article : Google Scholar
|
|
214
|
Dong W, Bi J, Liu H, Yan D, He Q, Zhou Q,
Wang Q, Xie R, Su Y, Yang M, et al: Circular RNA ACVR2A suppresses
bladder cancer cells proliferation and metastasis through
miR-626/EYA4 axis. Mol Cancer. 18:952019. View Article : Google Scholar
|
|
215
|
Yang C, Yuan W, Yang X, Li P, Wang J, Han
J, Tao J, Li P, Yang H, Lv Q and Zhang W: Circular RNA circ-ITCH
inhibits bladder cancer progression by sponging miR-17/miR-224 and
regulating p21, PTEN expression. Mol Cancer. 17:192018. View Article : Google Scholar
|
|
216
|
Ma X, Ying Y, Sun J, Xie H, Li J, He L,
Wang W, Chen S, Shen H, Yi J, et al: circKDM4C enhances bladder
cancer invasion and metastasis through miR-200bc-3p/ZEB1 axis. Cell
Death Discov. 7:3652021. View Article : Google Scholar
|
|
217
|
Liang Z, Guo W, Fang S, Zhang Y, Lu L, Xu
W and Qian H: CircRNAs: Emerging bladder cancer biomarkers and
targets. Front Oncol. 10:6064852021. View Article : Google Scholar :
|
|
218
|
Chu G, Ji X, Wang Y and Niu H: Integrated
multiomics analysis and machine learning refine molecular subtypes
and prognosis for muscle-invasive urothelial cancer. Mol Ther
Nucleic Acids. 33:110–126. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Harsanyi S, Novakova ZV, Bevizova K,
Danisovic L and Ziaran S: Biomarkers of bladder cancer: Cell-free
DNA, epigenetic modifications and non-coding RNAs. Int J Mol Sci.
23:132062022. View Article : Google Scholar :
|
|
220
|
Martin-Way D, Puche-Sanz I, Cozar JM,
Zafra-Gomez A, Gomez-Regalado MDC, Morales-Alvarez CM, Hernandez
AF, Martinez-Gonzalez LJ and Alvarez-Cubero MJ: Genetic variants of
antioxidant enzymes and environmental exposures as molecular
biomarkers associated with the risk and aggressiveness of bladder
cancer. Sci Total Environ. 843:1569652022. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Babjuk M, Burger M, Capoun O, Cohen D,
Compérat EM, Dominguez Escrig JL, Gontero P, Liedberg F,
Masson-Lecomte A, Mostafid AH, et al: European association of
urology guidelines on non-muscle-invasive bladder cancer (Ta, T1,
and carcinoma in situ). Eur Urol. 81:75–94. 2022. View Article : Google Scholar
|
|
222
|
Chen AA and Grasso M: Is there a role for
FISH in the management and surveillance of patients with upper
tract transitional-cell carcinoma? J Endourol. 22:1371–1374. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Koide K, Sekizawa A, Iwasaki M, Matsuoka
R, Honma S, Farina A, Saito H and Okai T: Fragmentation of
cell-free fetal DNA in plasma and urine of pregnant women. Prenat
Diagn. 25:604–607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Wong FC and Lo YM: Prenatal diagnosis
innovation: Genome sequencing of maternal plasma. Annu Rev Med.
32:419–432. 2016. View Article : Google Scholar
|
|
225
|
Tivey A, Church M, Rothwell D, Dive C and
Cook N: Circulating tumour DNA-looking beyond the blood. Nat Rev
Clin Oncol. 19:600–612. 2022. View Article : Google Scholar
|
|
226
|
Wu Z, Yang Z, Li CS, Zhao W, Liang ZX, Dai
Y, Zhu Q, Miao KL, Cui DH and Chen LA: Differences in the genomic
profiles of cell-free DNA between plasma, sputum, urine, and tumor
tissue in advanced NSCLC. Cancer Med. 8:910–919. 2019. View Article : Google Scholar
|
|
227
|
Lo YM, Chan KC, Sun H, Chen EZ, Jiang P,
Lun FM, Zheng YW, Leung TY, Lau TK, Cantor CR and Chiu RW: Maternal
plasma DNA sequencing reveals the genome-wide genetic and
mutational profile of the fetus. Sci Transl Med. 2:61ra912010.
View Article : Google Scholar
|
|
228
|
Chen G, Jia G, Chao F, Xie F, Zhang Y, Hou
C, Huang Y, Tang H, Yu J, Zhang J, et al: Urine- and blood-based
molecular profiling of human prostate cancer. Front Oncol.
12:7597912022. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Saha MK, Massicotte-Azarniouch D, Reynolds
ML, Mottl AK, Falk RJ, Jennette JC and Derebail VK: Glomerular
hematuria and the utility of urine microscopy: A review. Am J
Kidney Dis. 80:383–392. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
He Y, Pan C, Zhang Y, Lv M and Yang B:
Nomogram for customized recurrence prediction in primary
non-muscle-invasive bladder cancer based on routine blood and urine
parameters. BMC Urol. 24:672024. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Teixeira-Marques A, Lourenço C, Oliveira
MC, Henrique R and Jerónimo C: Extracellular vesicles as potential
bladder cancer biomarkers: Take it or leave it? Int J Mol Sci.
24:67572023. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Latosinska A, Frantzi M, Vlahou A and
Mischak H: Clinical applications of capillary electrophoresis
coupled to mass spectrometry in biomarker discovery: Focus on
bladder cancer. Proteomics Clin Appl. 7:779–793. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Claps F, Rossin G, van Rhijn BWG, Mir MC,
Mertens LS, Ongaro L, Traunero F, Iachimovsky AI, Piasentin A,
Vedovo F, et al: The utility of inflammatory serum markers in the
assessment of perioperative morbidity after radical cystectomy for
bladder cancer. Medicina (Kaunas). 59:9262023. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
López-Cortés R, Gómez BB, Vázquez-Estévez
S, Pérez-Fentes D and Núñez C: Blood-based protein biomarkers in
bladder urothelial tumors. J Proteomics. 247:1043292021. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Lee DH, Yoon H, Park S, Kim JS, Ahn YH,
Kwon K, Lee D and Kim KH: Urinary exosomal and cell-free DNA
detects somatic mutation and copy number alteration in urothelial
carcinoma of bladder. Sci Rep. 8:147072018. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Kobayashi M, Abe H, Arai K, Murakami S and
Kamai T: Circulating tumor cells and cell-free tumor DNA analyses
in urothelial cancer using the LiquidBiopsy platform. Curr Urol.
16:99–106. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Hirotsu Y, Yokoyama H, Amemiya K, Hagimoto
T, Daimon H, Hosaka K, Oyama T, Mochizuki H and Omata M: Genomic
profile of urine has high diagnostic sensitivity compared to
cytology in non-invasive urothelial bladder cancer. Cancer Sci.
110:3235–3243. 2019. View Article : Google Scholar
|
|
238
|
Zhang R, Zang J, Xie F, Zhang Y, Wang Y,
Jing Y, Zhang Y, Chen Z, Shahatiaili A, Cai MC, et al: Urinary
molecular pathology for patients with newly diagnosed urothelial
bladder cancer. J Urol. 206:873–884. 2021. View Article : Google Scholar
|
|
239
|
Payne SR, Serth J, Schostak M, Kamradt J,
Strauss A, Thelen P, Model F, Day JK, Liebenberg V, Morotti A, et
al: DNA methylation biomarkers of prostate cancer: Confirmation of
candidates and evidence urine is the most sensitive body fluid for
non-invasive detection. Prostate. 69:1257–1269. 2009. View Article : Google Scholar
|
|
240
|
Jerónimo C, Usadel H, Henrique R, Silva C,
Oliveira J, Lopes C and Sidransky D: Quantitative GSTP1
hypermethylation in bodily fluids of patients with prostate cancer.
Urology. 60:1131–1135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Goessl C, Müller M, Heicappell R, Krause H
and Miller K: DNA-based detection of prostate cancer in blood,
urine, and ejaculates. Ann N Y Acad Sci. 945:51–58. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Smith CG, Moser T, Mouliere F,
Field-Rayner J, Eldridge M, Riediger AL, Chandrananda D, Heider K,
Wan JCM, Warren AY, et al: Comprehensive characterization of
cell-free tumor DNA in plasma and urine of patients with renal
tumors. Genome Med. 12:232020. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Christensen E, Nordentoft I,
Birkenkamp-Demtröder K, Elbæk SK, Lindskrog SV, Taber A, Andreasen
TG, Strandgaard T, Knudsen M, Lamy P, et al: Cell-free urine and
plasma DNA mutational analysis predicts neoadjuvant chemotherapy
response and outcome in patients with muscle-invasive bladder
cancer. Clin Cancer Res. 29:1582–1591. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Crozier J, Papa N, Perera M, Ngo B, Bolton
D, Sengupta S and Lawrentschuk N: Comparative sensitivity and
specificity of imaging modalities in staging bladder cancer prior
to radical cystectomy: A systematic review and meta-analysis. World
J Urol. 37:667–690. 2019. View Article : Google Scholar
|
|
245
|
Bandyk MG, Gopireddy DR, Lall C, Balaji KC
and Dolz J: MRI and CT bladder segmentation from classical to deep
learning based approaches: Current limitations and lessons. Comput
Biol Med. 134:1044722021. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Humayun-Zakaria N, Ward DG, Arnold R and
Bryan RT: Trends in urine biomarker discovery for urothelial
bladder cancer: DNA, RNA, or protein? Transl Androl Urol.
10:2787–2808. 2021. View Article : Google Scholar
|
|
247
|
Sun T, Hutchinson L, Tomaszewicz K,
Caporelli ML, Meng X, McCauley K, Fischer AH, Cosar EF and Cornejo
KM: Diagnostic value of a comprehensive, urothelial
carcinoma-specific next-generation sequencing panel in urine
cytology and bladder tumor specimens. Cancer Cytopathol.
129:537–547. 2021. View Article : Google Scholar
|
|
248
|
Claps F, Pavan N, Ongaro L, Tierno D,
Grassi G, Trombetta C, Tulone G, Simonato A, Bartoletti R, Mertens
LS, et al: BCG-unresponsive non-muscle-invasive bladder cancer:
Current treatment landscape and novel emerging molecular targets.
Int J Mol Sci. 24:125962023. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Frantzi M, Latosinska A, Flühe L, Hupe MC,
Critselis E, Kramer MW, Merseburger AS, Mischak H and Vlahou A:
Developing proteomic biomarkers for bladder cancer: Towards
clinical application. Nat Rev Urol. 12:317–330. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Xu X, Huang F, Cao M, Chen X, Wang H,
Jiang H, Yu Y, Shen M, Yang Y, Wang B, et al: Cross-platform
comparison of next-generation sequencing and matrix-assisted laser
desorption/ionization time-of-flight mass spectrometry for
detecting KRAS/NRAS/BRAF/PIK3CA mutations in cfDNA from metastatic
colorectal cancer patients. J Clin Lab Anal. 35:e238182021.
View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Raina R, Nada A, Shah R, Aly H, Kadatane
S, Abitbol C, Aggarwal M, Koyner J, Neyra J and Sethi SK:
Artificial intelligence in early detection and prediction of
pediatric/neonatal acute kidney injury: Current status and future
directions. Pediatr Nephrol. 39:2309–2324. 2024. View Article : Google Scholar
|
|
252
|
Lima T, Henrique R, Vitorino R and
Fardilha M: Bioinformatic analysis of dysregulated proteins in
prostate cancer patients reveals putative urinary biomarkers and
key biological pathways. Med Oncol. 38:92021. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Cheng P, Wang R, He S, Yan P, Huang H,
Chen J, Shen J and Pu K: Artificial Urinary biomarkers for early
diagnosis of acute renal allograft rejection. Angew Chem Int Ed
Engl. 62:e2023065392023. View Article : Google Scholar
|
|
254
|
Xu C, Xu M, Hu Y, Liu J, Cheng P, Zeng Z
and Pu K: Ingestible artificial urinary biomarker probes for urine
test of gastrointestinal cancer. Adv Mater. 36:e23140842024.
View Article : Google Scholar
|
|
255
|
Yang Y, Wang J, Huang W, Wan G, Xia M,
Chen D, Zhang Y, Wang Y, Guo F, Tan J, et al: Integrated urinalysis
devices based on interface-engineered field-effect transistor
biosensors incorporated with electronic circuits. Adv Mater.
34:e22032242022. View Article : Google Scholar
|
|
256
|
Hao L, Zhao RT, Welch NL, Tan EKW, Zhong
Q, Harzallah NS, Ngambenjawong C, Ko H, Fleming HE, Sabeti PC and
Bhatia SN: CRISPR-Cas-amplified urinary biomarkers for multiplexed
and portable cancer diagnostics. Nat Nanotechnol. 18:798–807. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Shi H, Li X, Zhang Q, Yang H and Zhang X:
Discovery of urine biomarkers for bladder cancer via global
metabolomics. Biomarkers. 21:578–588. 2016. View Article : Google Scholar : PubMed/NCBI
|