Introduction
Malignant mesothelioma (MM) is a rare, but
aggressive cancer which has been associated with asbestos exposure
(1). MM is a malignancy of
mesothelial surfaces, the most common locations being the pleura
(65-70%) and the peritoneum (30%) (2). MM presents with different
histological types, the most common type is epithelioid (~60%),
followed by sarcomatoid (20%), and biphasic (20%), which has
features of both epithelioid and sarcomatoid types (3).
MM often presents clinically with nonspecific
symptoms that may lead to delayed diagnosis (4,5).
In pleural mesothelioma, patients commonly experience dyspnea,
chest pain, and persistent cough, which may mimic other pulmonary
conditions (4). Peritoneal
mesothelioma typically manifests with abdominal pain, ascites,
anorexia, and weight loss (2).
Diagnostic workup includes chest radiography and computed
tomography scans, while definitive diagnosis requires
histopathological examination of biopsy specimens (4,5).
Early detection is challenging, highlighting the need for high
clinical suspicion, especially in individuals with a history of
asbestos exposure (4,6). Due to its insidious onset and
nonspecific symptoms, MM is frequently diagnosed at advanced stages
(6,7). The advanced stage at diagnosis
results in a 5-year survival rate <5%, far lower compared with
the average survival rate of all cancers, which lies at 62.7%
(8,9). The median survival rates also vary
depending on the type, with 19 months for epithelioid, 4 months for
sarcomatoid, and 12 months for biphasic (10). The abysmal prognosis of MM
underlies the imperative for novel approaches.
Telomeres are the nucleoprotein complexes comprised
of repetitive hexameric sequences, that cap eukaryotic chromosomes
ensuring genomic stability by protecting chromosome ends from
degradation and preventing end-to-end fusions (11-13). In human cells telomeres undergo
progressive shortening with each cell division, leading to
senescence or apoptosis once a critical length is reached.
Telomerase activity (TA) maintains telomeres and is physiologically
encountered in stem cells, germline cells, and certain somatic
cells that require extensive proliferative capacity, but in most
somatic cells TA is low or absent (14,15). Telomerase reactivation constitutes
a crucial tumorigenic mechanism as it maintains telomeres,
preventing cellular senescence and apoptosis (16). The relationship between telomere
biology and the tumorigenic process is complex on multiple levels,
and the precise mechanisms have yet to be fully elucidated
(17-21). Clinical application faces several
challenges, including limited cohort sizes, non-standardized
methodologies, and variability in study results. Nevertheless,
manifold aspects of telomere biology have been widely investigated
as potential cancer biomarkers, and corollary to its ubiquitousness
in malignancy it has emerged as a potential therapeutic target
(19,21-23).
To the best of our knowledge, this is the first
comprehensive review with regard to telomere biology in MM
(17,24). The aim of the present review was
to elucidate and contextualize the role of telomere biology in MM
pathophysiology, and thoroughly examine the potential applications
of telomeres and telomerase in MM diagnosis, prognosis and
therapy.
Mechanisms of mesothelioma pathogenesis
It is widely recognized that the most significant
risk factor for the development of MM is asbestos exposure, and
although there have been strict regulations on asbestos use, MM
incidence continues to rise (25). Amphiboles and crocidolite are the
two main types of asbestos, with the former identified as having a
greater role in MM development (26,27). Mesothelioma attributed to asbestos
exposure has an average latency period of 40 years, and in some
cases, it could reach 60-70 years (28). Asbestos exposure may lead to MM in
5-10% or up 25% of the highly exposed cases depending on the
research conducted (28,29). Chronic stress may potentially
increase risk as induced immune dysregulation, and the release of
stress-related hormones such as norepinephrine may further
exacerbate inflammatory responses, promoting tumor progression in
an already inflamed microenvironment (30). Additionally, recent evidence
suggests that tumors may modify not only the local
microenvironment, but create conditions conducive to tumor
progression by influencing systemic homeostasis through
interactions with the neuroendocrine system (31). Other established factors which
increase the risk of MM include exposure to ionizing radiation,
radiotherapy, simian virus 40 (SV40) infection, and genetic
mutations (Fig. 1) (32-35).
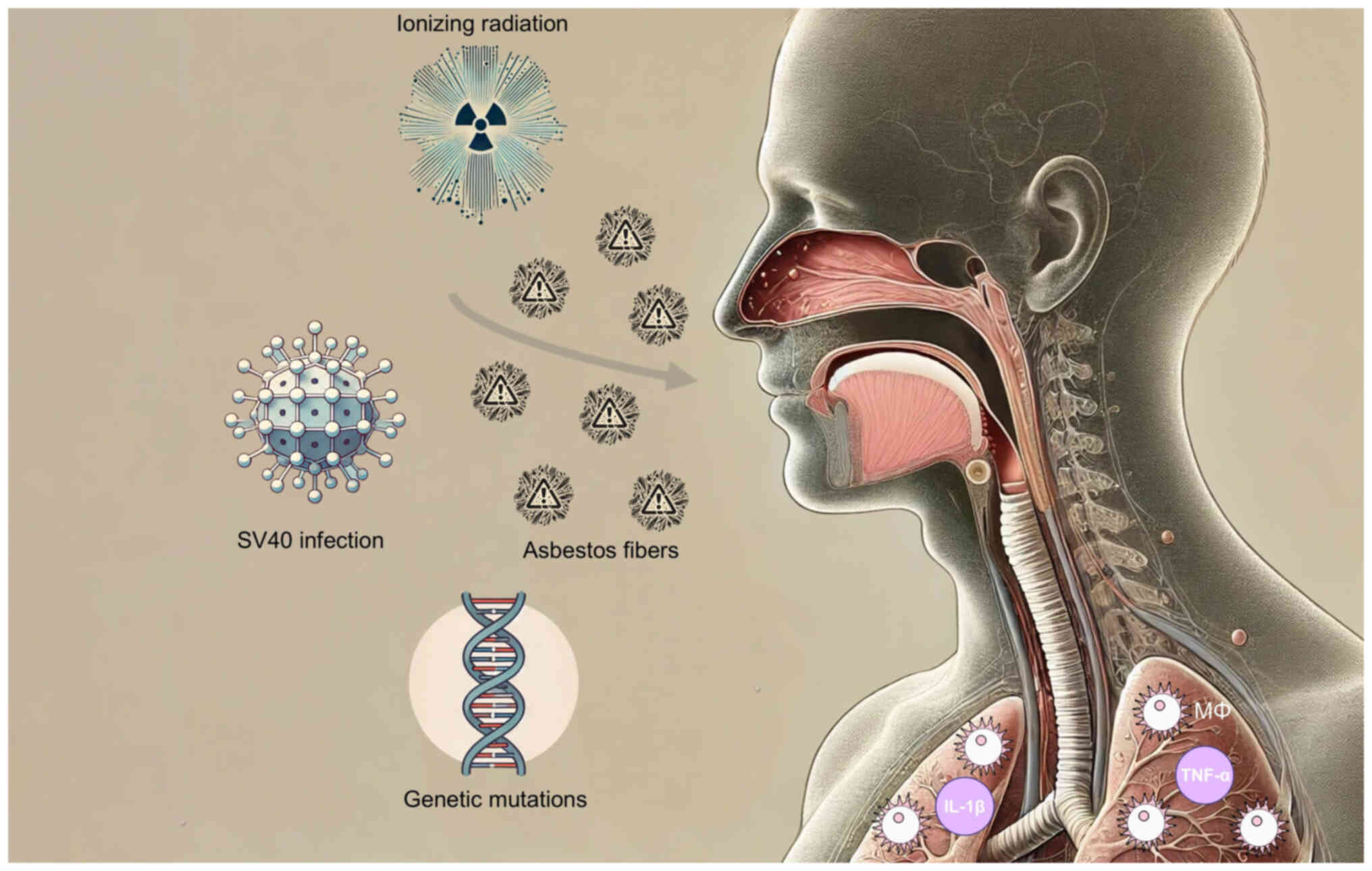 | Figure 1Mesothelioma risk factors. Asbestos
has been established as the primary risk factor for mesothelioma,
and other key risk factors contributing to the risk of mesothelioma
include SV40 infection, ionizing radiation, and genetic mutations.
Asbestos fibers, once inhaled, become lodged in the mesothelial
lining of the lungs, pleura, peritoneum, or pericardium, triggering
a chronic inflammatory response. This inflammatory response
involves the recruitment of macrophages and the release of
pro-inflammatory cytokines, including TNF-α and IL-1β. The inflamed
microenvironment induced by asbestos contributes to tumorigenesis
by activating further signaling pathways that promote mesothelial
cell transformation. |
Asbestos fibers when inhaled lodge in the
mesothelial lining of the lungs, pleura, peritoneum, or
pericardium, inducing a chronic inflammatory response. The response
is characterized by the recruitment of macrophages and the release
of pro-inflammatory cytokines such as tumor necrosis factor-α
(TNF-α) and interleukin-1β (IL-1β) (36). The inflammatory response promotes
the generation of reactive oxygen species (ROS) and reactive
nitrogen species (RNS), resulting in significant DNA damage in
mesothelial cells (37,38).
In the unified theory for the development of cancer
by Spandidos (39), damage to
genes, such as point mutations, deletions, and chromosome
translocations, represent key steps in cancer development, with the
activation of class I (transform the phenotype of the cell
directly) and II (act on the transformed phenotype of the cell
indirectly, through class I and III oncogenes) oncogenes and the
inactivation of class III (tumor suppressor genes) contributing to
cancer progression. DNA damage in class III oncogenes, tumor
suppressor genes is a crucial step is the pathogenesis of MM.
Mutations of the class III oncogene, BRCA1-associated protein 1
(BAP1), are often associated with MM (40), as are mutations of the class III
oncogene neurofibromatosis type 2 (NF2), which encodes merlin, a
protein that regulates contact inhibition and cellular growth
(39,41,42). Rat sarcoma virus (RAS) family
oncogenes, classified as class I oncogenes, are also linked to
tumorigenesis and stimulation of survival pathways in MM (39,43).
Oxidative stress caused by asbestos further
contributes to tumorigenesis through the activation of signaling
pathways related to mesothelial cell transformation. The activation
of the nuclear factor kappa-light-chain-enhancer of activated B
cells (NF-κB) pathway is associated with the transcription of genes
that promote inflammation, cell proliferation, and inhibition of
apoptosis (44,45). Another key signaling pathway is
the Hippo pathway which controls cell proliferation and apoptosis.
In MM, mutations of NF2 and leucyl-tRNA synthetase 1 (LARS1/2) may
lead to Hippo signaling pathway dysregulation (42,46). Furthermore, the activation of the
phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mechanistic
target of rapamycin (mTOR) pathway, which leads to inhibition of
apoptotic signals, has been revealed to be associated with MM
(47), as is the wingless-related
integration site/β-catenin (Wnt/β-catenin) pathway, which causes
increased cell proliferation and invasion (48,49). An essential aspect of contemporary
research in MM pathogenesis involves epigenetic modifications.
Histone modifications alter chromatin structure and gene expression
patterns promoting tumorigenesis in mesothelial cells (50). The hypermethylation of tumor
suppressor genes such as cyclin-dependent kinase inhibitor 2B
(p15INK4B), cyclin-dependent kinase inhibitor 2A
(p16INK4A), Ras association domain family member 1
(RASSF1A) and Ras association domain family 5 (NORE1A) has been
correlated with the development of MM (51). Finally, non-coding RNAs, which
regulate gene expression post-transcriptionally, have been
associated with tumorigenic changes in mesothelial cells (52).
Current and emerging therapeutic strategies
in mesothelioma
The treatment options for MM have evolved over the
years, with the standard treatment modalities being surgery,
chemotherapy, and radiation (53). Surgery is typically used for
patients with overall good health and diagnosis at an early stage,
and is usually combined with other treatment modalities. In the
last decades a shift has been observed from extra-pleural
pneumonectomy (EPP) to pleurectomy decortication (PD) (54). In terms of systematic therapy, the
standard chemotherapeutic regimen as per the EMPHACIS trial
consists of a combination of pemetrexed and cisplatin (55). The evidence for second-line
treatment options is limited (56). Chemotherapy is not curative, and
is often used in a palliative manner (55,56). Radiation therapy is widely used in
MM, although the evidence from clinical trials is limited (57). Newer radiotherapy protocols such
as image guided radiotherapy, proton therapy, and stereotactic
ablative radiotherapy, are still under investigation (58).
The rapid developments in therapeutic modalities
targeting cancer have resulted in manifold emerging treatments for
MM. Immunotherapy has been one of the most revolutionary
developments in cancer treatments, consisting of several treatment
modalities such as adoptive cell transfer (ACT) and immune
checkpoint inhibitors (ICIs), which combat cancer by increasing the
response of the immune system (59). Immunotherapies, including ICIs and
chimeric antigen receptor T-cell therapy (CAR T-cell therapy), have
shown promising results in MM (60,61). Recently the combination of
nivolumab and ipilimumab was approved by the Food and Drug
Administration (FDA) as a first-line treatment option for MM
(62,63). In gene therapy, tools such as
CRISPR-Cas9 are employed to edit genes related to carcinogenesis
and tumor cell proliferation, showing potential for applications in
MM (64-66). Oncolytic virus therapy employs
genetically modified viruses that infect and kill cancer cells
(67). Trials in other
malignancies have demonstrated the efficacy and safety of agents,
such as talimogene laherparepvec (T-VEC) (68). Finally, targeted therapies focus
on the specific molecular profile of the tumor, providing a
personalized medical approach (69). There are numerous trials which
target different aspects of the molecular pathways which induce
carcinogenesis or support tumor growth, such as vascular
endothelial growth factor (VEGF) (70,71), platelet-derived growth factor
(PDGF) (72,73), epidermal growth factor receptor
(EGFR) (74,75), and telomerase (76).
Structure and function of telomeres and
telomerase
Telomeres are complex nucleoprotein structures at
the ends of eukaryotic chromosomes, which protect them from
degradation and end-to-end fusions (77). Telomeres contain a repetitive
hexameric nucleotide sequence (5′-TTAGGG-3′) that forms a
protective cap 10-15 kilobases (kb) long, and the cap prevents
end-to-end fusions and chromosomal degradation (11-13). The shelterin complex protects the
telomeres, regulates their length and structure, and regulates TA.
The complex consists of six core proteins, namely telomeric repeat
binding factor 1 (TRF1), telomeric repeat binding factor 2 (TRF2),
TERF1-interacting nuclear factor 2 (TINF2), repressor activator
protein 1 (RAP1), adrenocortical dysplasia protein homolog (TPP1),
and protection of telomeres 1 (POT1) (78). TRF1 and TRF2 bind directly to the
telomeric DNA, while TINF2, RAP1 and TPP1 stabilize the complex.
POT1 protects the single-stranded 3′ overhang and forms a
heterodimer with TPP1 that is essential for telomere elongation and
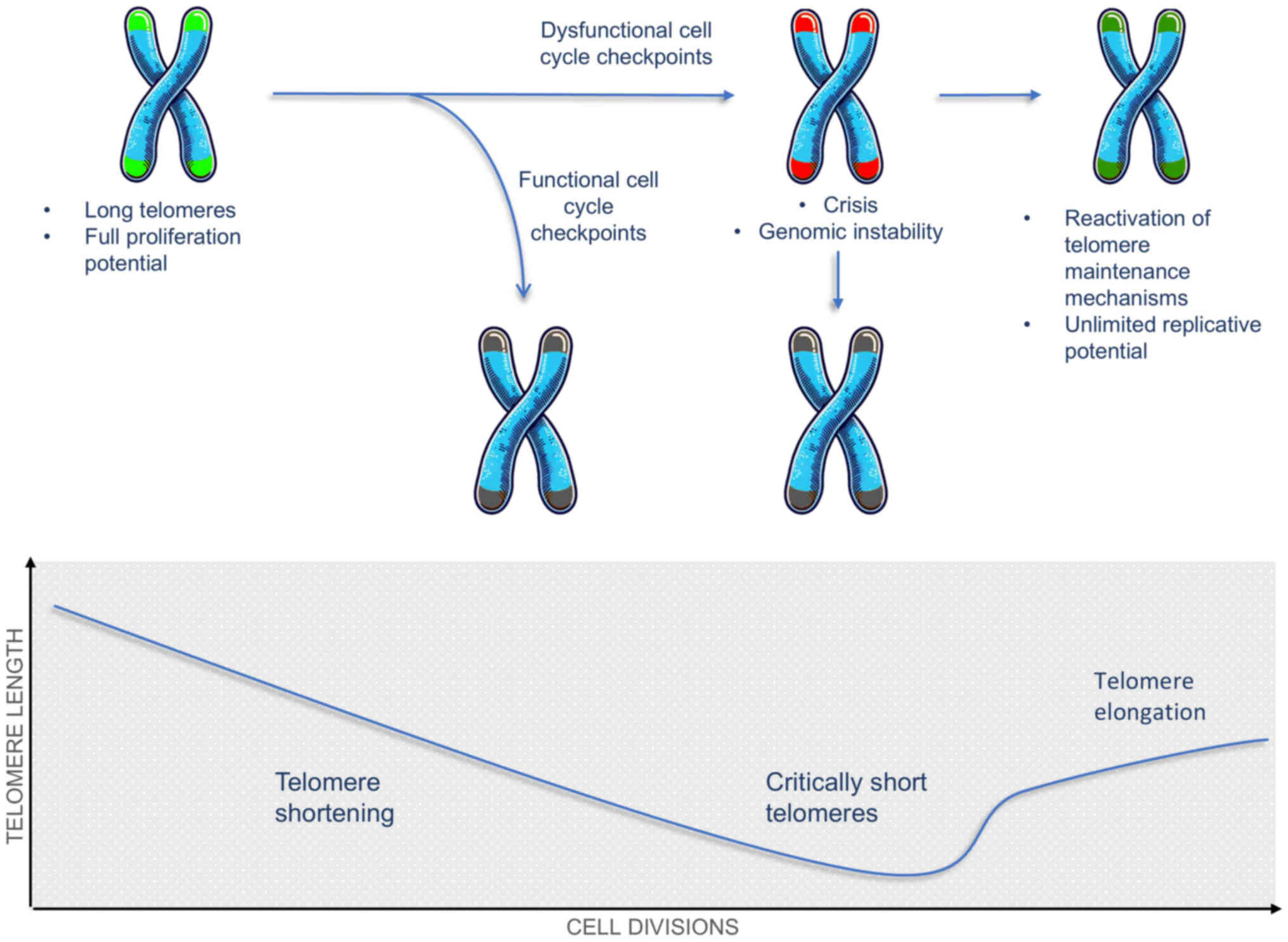
capping (Fig. 2) (79). During cell division telomeres
shorten by 30-200 bp during each cycle, as DNA polymerase cannot
completely replicate the 3′ ends (80). The progressive decrease in length
limits the number of divisions a cell can undergo, and the
phenomenon is known as the Hayflick limit (81). Decrease in telomere length (TL) in
cells with intact cell cycle checkpoints results in senescence, and
in cells with compromised checkpoints telomere crisis is induced
and either telomere maintenance mechanisms (TMMs) are reactivated,
or apoptotic pathways are activated (81,82). The vast majority of cells that
necessitate increased replicative potential activate telomerase. A
minority activates telomerase-independent mechanisms, denoted as
alternative lengthening of telomeres (ALT) (83,84).
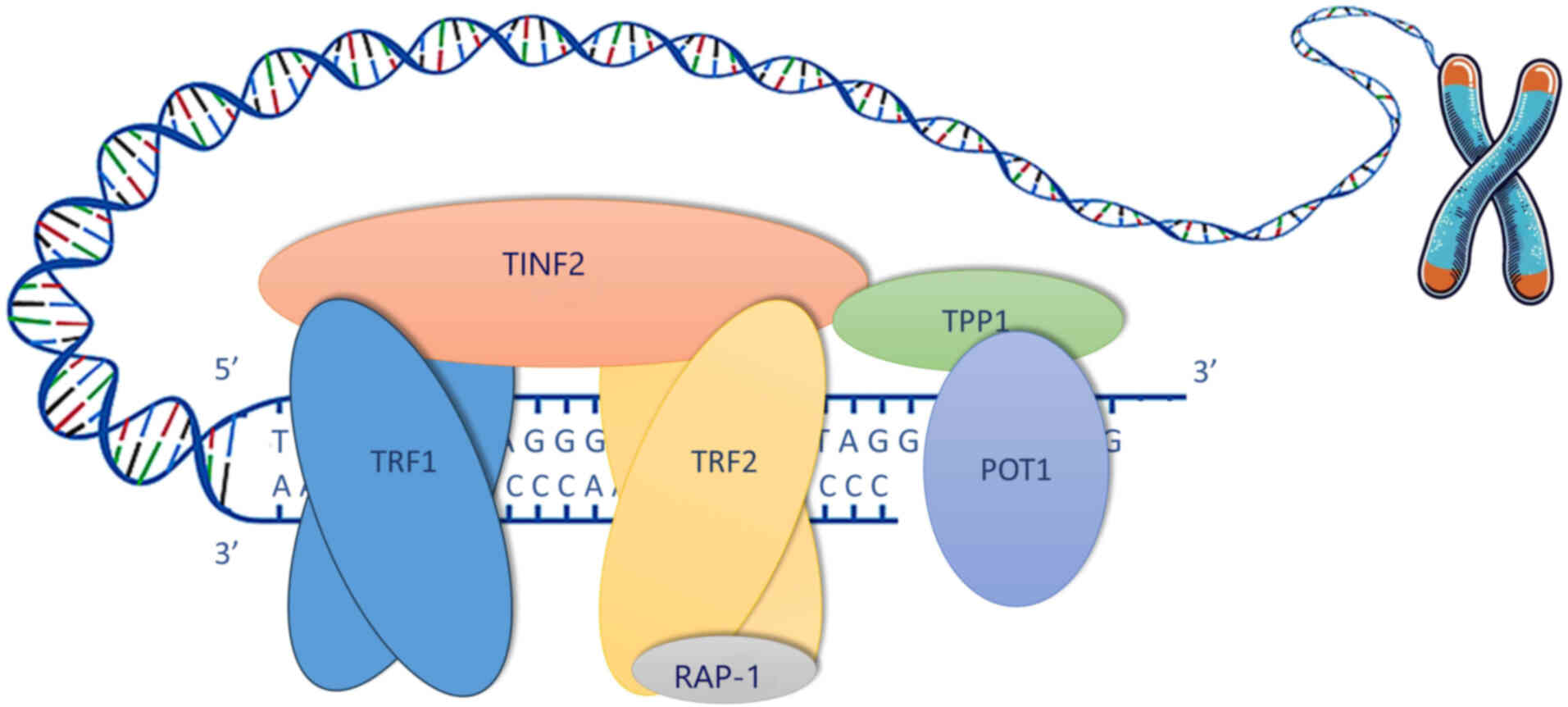 | Figure 2Telomere structure and shelterin
complex. Telomeres are specialized nucleoprotein structures that
cap the ends of eukaryotic chromosomes, Telomeres are composed of
long regions consisting of repetitive hexameric sequences
(5′-TTAGGG-3′) that form a protective cap, typically 10-15
kilobases long in humans. The shelterin complex helps maintain
telomere integrity, and is composed of six core proteins: TRF1,
TRF2, TINF2, RAP1, TPP1, and POT1. TRF1 and TRF2 bind directly to
the double-stranded regions of the telomeric DNA, while TINF2 and
TPP1 contribute to stabilization. RAP1 binds exclusively to TRF2.
The TINF2 subunit links TRF1 and TRF2 while interacting with the
TPP1 subunit. The TPP1 subunit connects to the POT1 subunit forming
a heterodimer which binds the 3′ single-stranded overhang. TRF1,
telomeric repeat binding factor 1; TRF2, telomeric repeat binding
factor 2; TINF2, TERF1-interacting nuclear factor 2; RAP1,
repressor activator protein 1; TPP1, adrenocortical dysplasia
protein homolog; POT1, protection of telomeres 1. |
By adding telomeric repeats to the ends of
chromosomes, telomerase maintains TL thus allowing continued cell
division (85). Telomerase is a
ribonucleoprotein enzyme that is responsible for the maintenance
and lengthening of telomeres (86). Telomerase consists of two main
components: A catalytic subunit, telomerase reverse transcriptase
(TERT), and the RNA component, telomerase RNA component (TERC)
(87). TERT, located on
chromosome 5p15.33, functions as a reverse transcriptase which adds
telomeric repeats based on an 11-nucleotide template region within
TERC (88,89). TA is highly regulated to achieve
proper telomere maintenance (24). TA is often found in stem cells,
germline cells, and certain somatic cells that require extensive
proliferative capacity, however in most somatic cells TA is low or
absent (14,15).
Telomere biology in tumorigenesis
Telomere length and chromosomal
instability
The associations between the elements of telomere
biology and the tumorigenic process are complex and diverse
(17-21). Among the more widely studied
elements is TL, with research investigating the association between
TL and cancer, and indicating that cancer is associated with
shorter telomeres. The proposed mechanism involves short telomeres
leading to genomic instability thus inducing chromosomal
aberrations and carcinogenic mutations, while the reactivation of
telomerase leads to maintenance of TL above the threshold
triggering apoptosis or senescence (17,18,21,90-92). The available clinical research
does not reveal a unanimous conclusion. A significant relationship
between increased cancer risk and short TL was demonstrated in
urological cancers, lung cancer and cancers of the digestive system
(22,23,93). However, in studies of breast and
colorectal cancers the association between risk and short TL was
not significant (94,95) and in melanoma the association was
reversed (96). As TL has been
studied in various cancers and can be readily measured using
peripheral leukocyte TL, it has been proposed as a potential
biomarker for cancer risk and progression (97-99).
Telomerase reactivation in cancer cells
and replicative immortalization
Telomerase elongates short telomeres, preventing
cellular senescence and apoptosis related to multiple cell
divisions. Therefore, TA constitutes a crucial step in the
tumorigenic process (16). Among
key factors in the tightly regulated TA is the TERT promoter.
Mutations in the TERT promoter that result in upregulation of
telomerase expression are frequently identified in cancer (20). TERT promoter mutations may create
new transcription factor binding sites, such as for the
E-twenty-six (ETS) family, leading to an increase in TERT
expression (21). The activation
of telomerase in cancer is regulated at the transcriptional level
by mesenchymal-epithelial transition factor (c-MET), nuclear factor
kappa B p65 subunit (NF-κB p65), myelocytomatosis viral oncogene
homolog (Myc), and specificity protein 1 (Sp1) (100,101). At the post-translational level
phosphorylation, ubiquitination and sumoylation further modulate
telomerase function and stability in cancer cells (101). Epigenetic modifications, such as
TERT promoter methylation may cause telomerase reactivation in
cancer (21). Telomerase
reactivation has been found in ~90% of human cancers (19). It is considered a crucial factor
in tumorigenesis as it can provide the cells with unlimited
replicative potential, enabling cancer cell immortalization and
preventing apoptosis and cellular senescence (Fig. 3) (15,19,102). TA constitutes a potential
diagnostic or prognostic biomarker as it can be measured with high
sensitivity using the telomeric repeat amplification protocol
(TRAP) assay and is present in most cancers (19,103).
TERT promoter mutations in cancer
The mechanism of telomerase reactivation has not yet
been fully elucidated, however according to numerous studies TERT
promoter mutations enhancing TA, are frequently observed in cancer
(20,104). The frequency of TERT promoter
mutations varies between cancer types, with mutations identified in
59% of bladder cancers, 49% of central nervous system (CNS) cancers
and 29% in melanoma (105). TERT
has been found to participate in the maintenance of cancer stem
cells enabling tumor growth and metastasis (106). Additionally, research indicates
that TERT interacts with the oncogenic pathway Wnt/β-catenin
further contributing to tumorigenesis (107). The TERT gene is studied as a
biomarker for cancer diagnosis and prognosis (108). TERT promoter mutations, C228T
and C250T, have been identified as the most common mutations found
in multiple cancers (109,110). TERT expression levels can be
measured in non-invasive ways, including circulating tumor cells
(CTCs) and cell-free DNA (111).
TERT mutations may act as prognostic biomarkers due to their
association with tumor aggressiveness and poor prognosis (104,112), with research linking increased
TERT expression with worse outcomes in patients with cancer
(113).
Telomere biology as a therapeutic
target
Aberrations in telomere biology have been found in
the vast majority of cancers, thereby highlighting the need for
their investigation as potential therapeutic targets (19). The direct inhibition of TA has
been considered as a therapeutic target in oncology research.
Imetelstat, an oligonucleotide that binds to the RNA template of
telomerase, has emerged as a direct inhibitor of telomerase
(114). In clinical trials for
hematologic malignancies, imetelstat showed promising results,
however, serious side effects, including myelosuppression, due to
the inhibition of TA in other cells have been noted (115-117). In cancer telomere maintenance is
achieved by mechanisms of telomerase reactivation, or by another
mechanism called ALT (118).
Ataxia telangiesctasia and Rad-3-related (ATR) inhibitors have been
demonstrated as highly effective against cancer that rely on ALT
for telomerase maintenance. There has been evidence of cancer cells
switching to ALT following telomerase targeted therapies. Thus,
targeting both telomerase and ALT could offer enhanced therapeutic
results (119,120).
Telomere length as a biomarker in
mesothelioma
The utilization of aspects of telomere biology as
potential MM biomarkers has garnered significant attention, with
the most commonly researched biomarkers being TL, TA, and mutations
of TERT and its promoter (22,23,93,99,104,112, 113,121,122). Clinical application faces
several challenges, including limited cohort sizes,
non-standardized methodologies, and variability in study results.
This review critically assesses the potential of TL, TA, and TERT
mutations as biomarkers in MM.
One of the most widely investigated telomere
biology-related biomarkers is TL, yet there are limited studies
exploring the potential of TL as a biomarker in MM (22,23,93,123-127). Telomere shortening has been
significantly associated with the pathogenesis of MM among
individuals with asbestos exposure, with significantly longer
telomeres in the mesothelial cells of pleural effusions of
non-neoplastic disease compared with MM (P<0.001) (124). The result is in concordance with
studies between patients with MM and individuals with pleural
plaques, which found significantly shorter TL in patients with MM
(P<0.001), and is consistent with research in other cancers
demonstrating that telomeres in patients with cancer are shorter
compared with healthy individuals (123,124,128-130). Additionally, a shorter TL was
identified in asbestos-exposed individuals without MM compared with
non-exposed individuals (P=0.047) (124). Aida et al (124) suggest a progressive shortening
of TL, with the longest TL observed in non-exposed individuals,
followed by shorter TL in asbestos-exposed individuals without MM,
and the shortest TL in those diagnosed with MM, which may function
as the basis for a potentially powerful biomarker, but due to the
limited sample size further studies with larger cohorts are
necessary to validate the findings.
The measurement of TL in mesothelial cells could be
utilized as a predictive tool for assessing the risk of MM in
asbestos-exposed individuals. A suggested method, accounting for
the significantly long latency period, may be longitudinal
monitoring of TL in pleural effusions in exposed individuals, which
could facilitate improved risk assessment and early detection of MM
(131). Moreover, assessment of
TL could be utilized to aid in challenging cytological diagnosis,
such as differentiating low grade MM from reactive atypia, where
current methods, such as CDKN2A (p16) deletion analysis via
fluorescence in situ hybridization (FISH) and BAP1
immunocytochemistry, sometimes yield inconclusive results (124,132-135).
In non-MM individuals, an association between
shorter TL and expression of insulin-like growth factor II
mRNA-binding protein 3 (IGF2BP3), cluster of differentiation 146
(CD146), epithelial membrane antigen (EMA), and glucose transporter
1 (GLUT1) has been suggested (124). While not specific to MM, they
are more commonly expressed in MM compared with reactive
mesothelial cells, thus indicating that their association with
telomere shortening should be investigated in MM (124,136).
A notable finding is that older patients with MM had
longer telomeres than younger patients (Spearman's rho=0.370;
P<0.001), which contradicts the general association of telomere
shortening with aging, and highlights the complex dynamics between
aging and cancer in telomere biology (124,128-130,137-139). A potential explanation is that
telomerase reactivation found in MM allows potentially longer TL in
older patients compared with younger patients. The mechanism, of
possibly increased telomerase reactivation in older patients with
MM compared with younger patients with MM, has not yet been
elucidated (124,126,140).
In assessing the relationship between TL and
chemotherapy response, no significant correlation between TL and MM
chemotherapy outcomes was found (123). This is consistent with prior
research in breast cancer, where chemotherapy-induced telomere
shortening was observed to be temporary (141). The impact of TL on
progression-free survival (PFS) in patients with MM was also found
to be not significant (123).
This finding is consistent with studies in other cancers, but
overall the findings regarding TL and survival outcomes are
conflicting, necessitating the need for future cohort studies
(123,142-144). Another limitation is the
variability in measuring methodology across studies, employing
quantitative polymerase chain reaction (qPCR) or quantitative FISH
(Q-FISH) to measure TL (123,124).
Overall, the literature shows the potential of TL as
a biomarker for MM. Studies have revealed shorter TL in patients
with MM, suggesting utility in risk assessment, early detection,
and as a tool in challenging diagnoses. However, the role of TL in
therapeutic response and survival remains inconclusive.
Telomere maintenance mechanisms in
mesothelioma
The majority of cells necessitating increased
replicative potential activate telomerase, while a minority
activates telomerase-independent TMMs, denoted as ALT (83,84). TA is primarily found in stem cells
and germline cells, and is rarely detected in somatic cells, while
telomerase reactivation has been found in 85-90% of human cancers,
including MM, making it a promising cancer biomarker (15,19,145,146). The frequency of TA observed in
MM ranged from 91 to 100%, and the activation of ALT was either
absent or rare (126,145,147,148). Investigation of the basic
mechanisms of telomere maintenance in MM is crucial in ascertaining
the role of TMMs, particularly TA, as a potential biomarker or
therapeutic target in MM. The minimal or absent ALT in MM is in
contrast with research suggesting that ALT is more frequently
observed in mesenchymal tumors (146). Despite ALT being scarcely
detected in pleural MMs, in diffuse malignant peritoneal
mesothelioma (DMPM), ALT was identified in 18% of cases, suggesting
distinct TMMs, which aligns with the differences in their clinical
and molecular profiles (149,150).
To elucidate ALT activation in MM, normal
mesothelial cells were transformed by SV40 resulting in an extended
but finite proliferative capacity subsequently, without TMM
activation until the cells have escaped crisis, suggesting that
these viral oncogenes alone are insufficient to directly activate
TMMs (126). Notably, in other
studies SV40 infection directly induced TMMs in mesothelial cells,
suggesting that differences in experimental approaches may
influence TMM activation (34,151). Following additional genetic
changes to result in immortalization, the study observed that the
immortalization of mesothelial cells could result in either ALT or
TA in pleural mesothelial cells derived from the same individual,
showing activation of either ALT (MeT-4A) or telomerase (MeT-4D)
after escaping crisis (84,126). It is noteworthy that while the
in vitro findings suggest varied TMMs, analysis of TMMs in
tumor samples indicates absence or minimal expression of ALT, and a
high frequency of TA. The reactivation of TA in normal cells with
long telomeres and intact checkpoints only extends cellular
lifespan and additional tumorigenic mutations are required, which
might explain the different TMM profiles, alternatively, these
results may suggest selective pressure in the tumor
microenvironment, potential differences in in vitro
immortalization vs. tumorigenesis in vivo, or differences in
experimental approaches (126,145,147,152,153). The current evidence firmly
indicates that pleural MMs are overwhelmingly telomerase-positive
and ALT-negative, but further studies directly investigating ALT
activation in large cohorts are needed.
Assessing telomerase activity in
mesothelioma
A critical aspect of TA detection, which may
partially explain the variability in results between studies is the
utilization of different measuring methodologies (148). The utilization of TA as a
diagnostic tool necessitates accurate and standardized methodology;
the discrepancies between the accuracy of TRAP in situ, and
cell lysate-based TRAP enzyme-linked immunosorbent assay (ELISA) in
measuring TA in pleural effusions are crucial. In the study by
Hansson et al (148), the
two methods are compared to determine the most reliable method
(148). The TRAP in situ
method found TA in 13 out of 14 malignant cases and 2 out of 2
equivocal cases exhibiting moderate to strong reactivity. Among
benign effusions 5 out of 7 were negative, and in the other cases,
only weak activity was observed, indicating the potential of TA as
a diagnostic tool. However, the use of the cell lysate-based TRAP
ELISA assay incurred significant overlap between malignant and
benign cases. It is suggested that TRAP ELISA may lead to less
accurate results compared with TRAP in situ (148,154).
A potential explanation for the reduced accuracy of
the TRAP ELISA as a diagnostic tool is the presence of
telomerase-positive non-malignant cells, such as lymphocytes which
may express telomerase upon activation (155,156). The study observed that at least
a fraction of the lymphocytes in effusions consistently exhibited
weak or moderate reactivity, which aligns with prior findings that
activated lymphocytes upregulate TA (155). The findings concur with studies
of TA in benign effusions, particularly those rich in lymphocytes,
such as those associated with tuberculosis (157-159). Although TRAP ELISA is easier to
perform, it is less diagnostically reliable than TRAP in
situ. It is suggested that the use of adjustments for
non-malignant cells could potentially enhance TRAP ELISA diagnostic
specificity (148). Currently,
TRAP in situ is suggested as the preferred measurement
technique due to its superior accuracy and ability to more
accurately differentiate malignant cases from benign ones, leading
to a more precise diagnosis (148).
Telomerase activity in mesothelioma
diagnosis
In ascertaining the potential of TA as a biomarker
in MM, a comprehensive review of the available data on TA is
essential to determine its true diagnostic value in MM and is
necessary to elucidate the variability in the published
literature.
In the study by Dhaene et al (145), TA was identified in 91% of MMs
and in both solitary fibrous tumors (SFTs) when examined using
TRAP. TA was detected in all four human MM cell lines examined but
not in normal mesothelial cells. False negatives, which could
impact the assessment as a biomarker, were ruled out through
successful amplification of internal control products in all
samples. The number of MMs which showed no TA could potentially
indicate alternative TMMs, however the study did not explicitly
investigate the activation of ALT (145). The TA in SFTs could have
indicated potentially, reduction in specificity, however the sample
size of SFTs was too small to draw firm conclusions. Furthermore
studies have demonstrated markedly low activity in SFTs with
typical features and increased activity in atypical tumors,
suggesting future studies could investigate TA as a biomarker in
SFT for potential malignant transformation (160,161). As Dhaene et al (145) did not utilize a TRAP in
situ assay, there was an inability to capture the heterogeneity
of TA at the cellular level potentially overlooking intratumoral
heterogeneity, and the accuracy of the result could be affected by
false positives in the benign cases (145).
Further studies detected TA using a TRAP assay in
all MM samples (126,147). Additionally, studies measured
hTERT by employing immunohistochemistry (IHC) to detect TERT
protein levels and in situ hybridization (ISH) to detect
hTERT mRNA. hTERT expression was observed in 98.5% of MM cases by
IHC and in all MM cases tested by ISH (147).
Notably, in the study by Au et al (126), in all MMs examined there was no
detectable c-circle presence, indicating the absence of ALT
activity. ALT activity was not explicitly found in most TA studies
in MM. The absence of ALT in the aforementioned study (126), may be the result of the
relatively small sample size in relation to the frequency, as
studies measuring ALT in MM, have detected it in 3.57% of cases (1
out of 28 cases) (126,152).
The potential of TA as a diagnostic tool is
strongly supported by the literature consistently finding a high
frequency of TA in MM compared with non-malignant cases (126,145,147,148). The use of a TRAP in situ
assay in a cohort of diagnostically refractory effusions reported a
diagnostic sensitivity for malignancy of 91% (162). Additionally, evidence suggests a
strong correlation between TRAP in situ assay and hTERT IHC
(162-164). Cakir et al (163) found that hTERT IHC detected MM
with sensitivity and specificity of 68%.
The TRAP in situ assay demonstrated no
strong nuclear TA in the effusions from patients with benign
diseases (162). This finding
suggests the potential high specificity of strong nuclear TA as a
diagnostic biomarker in effusions. However, the statistical
significance of strong TA as a malignancy marker reached only
P=0.08, potentially due to the small sample size (162). It is suggested that TRAP in
situ may also be used in distinguishing epithelial MMs from
other malignancies, as epithelial MMs exhibited strong TA in all
cases tested, in contrast to the variable TA found in other
malignancies such as adenocarcinoma and squamous cell carcinoma
(145,149,162,165), however future studies are
necessary to examine the validity of this observation. Overall, TA
shows promise as a diagnostic tool for MM, especially when
traditional methods yield inconclusive results, with a sensitivity
of 91%. Due to limited research quantifying the diagnostic capacity
of TA, further studies are warranted to refine the diagnostic
parameters and validate the biomarker clinically in MM.
Research investigating the potential of telomerase
as a biomarker in DMPM has been limited compared with pleural
mesothelioma. It was shown that TA is the predominant TMM in DMPM,
observed in 63.6% of cases, while ALT was identified in 18% of
cases (149). The frequency of
TA was revealed to be significantly lower than that reported in
pleural mesothelioma and ALT frequency was minimal in MM,
indicating a potentially significant variation in telomere biology,
and suggesting differences in application of TA as a biomarker as
well as the need for distinct studies (126,145,147,148). The TA and ALT frequencies in
DMPM also differed comparing the 85-90 and 10-15%, respectively,
found on average in other cancers, indicating a reliance on ALT for
DMPM telomere maintenance (15,146,152). These findings demonstrate the
heterogeneity of TMMs in MM, where DMPM may exhibit a distinct
telomere biology profile compared with pleural mesothelioma and
other cancers (Table I).
 | Table IComparison of TMM frequencies between
MM, DMPM and the average across all cancer types. |
Table I
Comparison of TMM frequencies between
MM, DMPM and the average across all cancer types.
| TMM | MM (refs.) | DMPM (refs.) | Cancer average
(refs.) |
|---|
| Telomerase
activity | 91-100% (126,145,147,148) | 63.6% (149) | 85-90% (15,19,146) |
| Alternative
lengthening of telomeres | 0-3.5% (126,152) | 18% (149) | 10-15% (146,152) |
Studies have yet to investigate the sensitivity and
specificity of TA as a diagnostic biomarker in DMPM. The potential
of TA as a diagnostic tool in DMPM is likely reduced due to the
high frequency of alternative TMMs, however the pattern of TA and
ALT differing from MM and other cancers may allow for the
development of high sensitivity and specificity biomarkers
(145,149,152). Investigation of TMMs as
prognostic biomarkers found that TA+ correlates with a
poorer clinical outcome compared with TA−, with 4-year
relapse hazard ratio (HR) at 3.30 (95% confidence interval,
1.23-8.86; P=0.018) and a cancer-related death HR at 3.56 (95%
confidence interval, 1.03-12.51; P=0.045), which is consistent with
other research (166). ALT
status did not significantly impact clinical outcomes in DMPM,
although a trend towards increased survival is noted in ALT cases.
In other studies, the prognostic value of ALT differed between
cancer types, with improved outcomes in ALT glioblastomas and worse
outcomes in ALT liposarcomas. This may be related to tumor-specific
genetic alterations associated with ALT (167,168). An association between ALT and a
younger age at diagnosis in DMPM is observed, which has also been
indicated in glioblastoma multiforme (168). It is noteworthy that the study
found the coexistence of both TMMs in two DMPM specimens, which has
been reported in other tumor types, including osteosarcoma,
liposarcoma, and glioblastoma multiforme, although it still remains
controversial whether TA and ALT can coexist within the same tumor
cell or if they represent distinct subpopulations within the tumor
(167-172). Furthermore, a subset of DMPM
(~14%) appeared to lack detectable TMMs, a possible interpretation
being the presence of unidentified mechanisms or that an active TMM
may occasionally not be necessary for tumor maintenance. The
observations align with studies in other tumors (168,169,171), and there has been experimental
research that indicates telomere maintenance is not always
necessary for malignant transformation (173).
TMMs represent promising therapeutic targets in
DMPM. Preclinical studies have shown that targeting these
mechanisms can induce tumor cell senescence and apoptosis (174,175). Although no therapies
specifically targeting ALT have been developed in MM, telomerase
inhibitors are currently in clinical trials in MM (76). As TA frequency is lower and ALT is
higher in DMPM compared with MM, potential treatments targeting ALT
are likely to be effective in DMPM; this highlights the importance
of ascertaining TMMs in patients with DMPM, and the need for the
development of therapies targeting ALT (126,176).
TERT gene and TERT promoter mutations in
mesothelioma
The TERT gene, encoding the catalytic subunit of
telomerase, has been the focus of significant research in MM,
especially the mutations of its promoter (123,125,177). TERT promoter mutations enhancing
TA are frequently observed in cancer and have been studied as
biomarkers in cancer (20,104,112).
The TERT gene has also been studied as a biomarker for cancer
diagnosis and prognosis, and it has been suggested that it may
contribute to tumorigenesis beyond its telomere maintenance
function (107,108).
TERT polymorphisms in the risk and
prognosis of mesothelioma
Several single nucleotide polymorphisms (SNPs) have
been investigated as risk and prognostic biomarkers in MM (Table II). A significant relationship
between the rs2736098 T allele and an increased risk of MM has been
observed, with the CT genotype having an odds ratio (OR) of 1.63
(95% CI, 1.20-2.21; P=0.002), and the CT + TT genotype exhibiting
an OR of 1.46 (95% CI, 1.09-1.96; P=0.011) (123). The association of the CT
genotype with increased risk is also demonstrated after adjusting
for age (OR=1.49; 95% CI, 1.06-2.10; P=0.023), the polymorphism has
been linked to lung and bladder cancers (178,179).
 | Table IIAssociation of TERT polymorphisms
with risk, treatment response, and survival in MM. |
Table II
Association of TERT polymorphisms
with risk, treatment response, and survival in MM.
| SNP | Genotype | Risk of developing
MM ORa (95% CI) | Chemotherapy
response rate ORb (95% CI) | Progression-free
survival HRc (95% CI) | Overall survival
HRd (95% CI) |
|---|
|
rs2736098 | CC | Reference | Reference | Reference | Reference |
| CT | 1.49
(1.06-2.10) | 1.20
(0.67-2.16) | 1.31
(0.98-1.76) | 1.02
(0.75-1.39) |
| TT | 0.78
(0.40-1.54) | 1.27
(9.42-3.87) | 1.46
(0.82-2.59) | 0.94
(0.48-1.83) |
| CT + TT | 1.36
(0.98-1.88) | 1.21
(0.69-2.14) | 1.33
(1.00-1.77) | 1.01
(0.75-1.36) |
|
rs2736100 | CC | Reference | Reference | Reference | Reference |
| CA | 0.71
(0.48-1.04) | 0.85
(0.45-1.63) | 0.83
(0.60-1.14) | 0.88
(0.62-1.24) |
| AA | 0.56
(0.35-0.90) | 1.16
(0.53-2.57) | 0.68
(0.45-1.03) | 0.86
(0.56-1.31) |
| CA + AA | 0.66
(0.46-0.95) | 0.94
(0.51-1.71) | 0.78
(0.57-1.06) | 0.87
(0.63-1.21) |
|
rs10069690 | CC | Reference | Reference | Reference | Reference |
| CT | 1.41
(0.99-2.01) | 2.08
(1.13-3.84) | 1.06
(0.79-1.43) | 1.13
(0.82-1.55) |
| TT | 2.22
(1.10-4.48) | 1.85
(0.64-5.31) | 0.81
(0.48-1.38) | 0.74
(0.41-1.35) |
| CT + TT | 1.52
(1.08-2.12) | 2.04
(1.13-3.67) | 1.01
(0.76-1.33) | 1.04
(0.77-1.41) |
The carriers of two rs10069690 T alleles face a
higher risk of MM, with the TT genotype showing an OR of 2.28 (95%
CI, 1.24-4.22; P=0.008). After age adjustment, the OR for the TT
genotype remained significant at 2.22 (95% CI, 1.10-4.48; P=0.026).
This SNP has also been linked to increased risk of malignancy in
numerous other types of cancer, including ovarian, lung, breast and
thyroid cancers (180). By
contrast, the rs2736100 A allele was associated with a decreased
risk of MM, particularly after adjusting for age, with the AA
genotype having an OR of 0.56 (95% CI, 0.35-0.90; P=0.017) and the
CA + AA genotype showing an OR of 0.66 (95% CI, 0.46-0.95;
P=0.026); polymorphisms in this allele have been associated with
increased risk of thyroid cancer, bladder cancer, lung cancer,
myeloproliferative neoplasms, glioma and acute myeloid leukemia
(123,181).
TERT SNPs could potentially be predictive
biomarkers in MM. The rs2736100 A allele was significantly
associated with a lower risk of MM progression, with the AA
genotype exhibiting an HR of 0.68 (95% CI, 0.47-0.98; P=0.038).
However, in studies on other cancers, such as kidney cancer,
multiple myeloma and glioma, it was associated with a poor
prognosis (182-184). The confirmation of TERT SNPs as
potential biomarkers of response to chemotherapy could critically
improve outcomes in MM. The rs10069690 T allele was found to be
associated with a favorable chemotherapy response in MM, with the
CT genotype having an OR of 1.81 (95% CI, 1.03-3.17; P=0.039) and
the CT + TT genotype exhibiting an OR of 1.72 (95% CI, 1.00-2.93;
P=0.048). The associations became stronger after adjustment for
weight loss and Eastern Cooperative Oncology Group (ECOG)
performance status (CT genotype: OR=2.08; 95% CI, 1.13-3.84;
P=0.019; CT + TT genotype: OR=2.04, 95% CI, 1.133.67; P=0.017). By
contrast, in breast cancer and multiple myeloma the rs10069690 T
allele was associated with poor outcomes (184,185).
Caution is suggested in interpreting these results
due to the limited and sometimes contradictory evidence available,
particularly in non-Caucasian populations, and the absence of high
quality meta-analysis on the TERT polymorphisms as prognostic
biomarkers in cancer (123,186). In a previous study there was no
significant association revealed between any TERT polymorphisms and
PFS in patients with MM (all P>0.05) (123).
These findings strongly suggest that TERT SNPs
present valuable biomarkers in MM, with rs2736098 and rs10069690
significantly associated with increased risk, the latter also
associated with improved chemotherapy response, and rs2736100 A
with a decreased risk of disease and low risk of progression. The
validation of the clinical utility of TERT SNPs in MM necessitates
future larger, multi-ethnic cohort studies and meta-analyses to
clarify the roles of these SNPs in risk and prognosis. Mechanistic
studies may elucidate the biological basis of these associations in
MM, allowing for potential targeted therapeutic interventions.
Methylation of the TERT promoter in
mesothelioma
The utilization of real-time methylation-specific
PCR (MSP) has been investigated as a diagnostic biomarker in MM,
showing that DNA methylation is significantly associated with MM
compared with reactive mesothelial proliferations (RM) (187). Methylation of the TERT promoter
results in activating TA, compared with the usual downregulating
function of methylation (188).
While most of the promoter region has been shown to be methylated
in other types of TERT-positive tumors and cell lines, TERT
promoter methylation was infrequent in MM, observed in only one MM
case (4%) and in none of the RM cases (187,189). This may be interpreted as the
result of the specific CpG sites examined not being critical in MM
as they are in other TERT-positive tumors (188,189). Due to the limited evidence,
further research may be necessary to fully ascertain the diagnostic
value of TERT methylation profiling in mesothelioma.
TERT promoter mutations in
mesothelioma
The role of TERT mutations in MM may be critical,
as TA is the predominant TMM (126,145). The frequency of TERT promoter
mutations in MM has exhibited some variation, from 10.4 to 15%, the
most commonly observed frequency being ~12%, and the mean
non-weighted frequency being 12.25%, similar to thyroid follicular
cell-derived carcinoma (10%), and smaller than the frequency
observed in other cancers, 59% of bladder cancers, 49% of CNS
cancers and 29% in melanoma (105,125,177,190,191).
The analysis of a mutational profile of 43 patients
with MM in a Brazilian cohort identified TERT promoter mutations in
11.6% of the cases (191). In a
study of 182 MM samples TERT promoter mutations were detected in
10.4% of MM cases (125),
slightly lower compared with another study (177). In the most comprehensive study
using 266 MM tumors (177), the
TERT promoter was identified with a frequency of 12%, and was the
third most frequently mutated locus in MM, highlighting its
importance in the genetic landscape of this cancer (177,190,192). A study of 61 MM in culture and
71 frozen MM tumor samples, found an overall frequency of 15.2%, a
frequency of 11.3% in MM tumor samples and 19.7% in MM cultures
(190).
A potential limitation of the studies may be
indicated by the lower rate of TERT promoter mutations in MM tumor
samples compared with cell cultures. The observation could be due
to reduced sensitivity in the frozen tumors samples, possibly
caused by the presence of normal cells, however it aligns with TERT
promoter frequencies in other research, which could potentially be
interpreted as an indication of underestimation in other studies
(177,190). Furthermore, the potential
underestimation of mutation frequencies may be due to the inability
of targeted sequencing to accurately detect large exon deletions,
and by the contamination of tumor samples with normal cells, a
common issue in next-generation sequencing studies (177,193). Another interpretation of the
discrepancy between cell line and tumor frequencies could be that
in cell cultures higher TA may confer a selective advantage.
The frequency of TERT promoter mutations has been
revealed to be associated with the subtype of MM, with studies
typically observing a higher frequency in MM of nonepithelioid
histology (125,177,190). A previous study reported TERT
promoter mutation frequency as significantly higher in
nonepithelioid histology (22.2% vs. 5.5% in epithelioid MM;
P<0.001) (125). Quetel et
al (177) also found that
TERT promoter mutations were more common in non-epithelioid MM, and
identified them to be positively associated with the S score
(representing the proportion of sarcomatoid-like molecular features
in a tumor), and with the C2B molecular subtype, suggesting a
potential subtype-specific role in MM pathogenesis (177). By contrast, the study by
Campanella et al (191),
including 43 patients with MM predominantly of the epithelioid
subtype (88.4%), the rest (11.6%) classified as sarcomatoid, found
mutations in only the epithelioid subtype, a potential explanation
being random effects due to the relatively small sample size. There
has yet to be a definitive explanation for the discrepancy, future
studies are needed to further validate the findings of the majority
of the investigations (125,177,191).
In the majority of studies the most frequently
observed mutation in the TERT promoter in the MM population was
C228T, also known as −124C>T, followed by A161C, also known as
−57A>C (125,177,190). C228T was either the most or the
second most commonly identified TERT promoter mutation in MM,
concurring with studies in other cancers revealing it among the
most common mutations (108,125,177). The frequency of the C250T
mutation in the TERT promoter among patients with MM varied across
studies. C250T was identified as the most common mutation in the
study by Campanella et al (191), reported in 7% of all samples,
while other studies have reported it as the third most common
mutation, or, as in the case of Pirker et al (125), not identified it at all, despite
it being among the most frequent TERT promoter mutations in cancer
(108,125,177,191). Further studies may reconcile
these differences. The C158A TERT promoter mutation has been
associated with bladder carcinoma (194). The A161C TERT promoter mutation
has been described as a causal high-penetrance germline mutation in
a melanoma-prone family (104),
and rarely as a somatic event, in melanoma and carcinoma of the
bladder, suggesting the possibility of a high specificity biomarker
for epithelioid MM (195,196).
The distribution of TERT promoter mutations has
been revealed to differ between studies. In the study by Pirker
et al (125), they were
observed as 124C>T (C228T) (68.4%), −57A>C (A161C) (21%) and
−146C>T (C250T) (10.5%), while Quetel et al (177) identified C228T, A161C and C158A
in 81%, 16 and 3% of samples, respectively. The study by Tallet
et al (190) identified
only the C228T TERT promoter mutation, and Campanella et al
(191) identified C250T and
C228T present in 7 and 4% of the cases, respectively. The
distribution of TERT promoter mutations also differed across
histological subtypes, with the −124C>T mutation strongly
prevalent in the non-epithelioid cases, while in epithelioid cases,
the prevalence of the −124C>T mutation was equal with that of
the rare −57A>C mutation (125,195,196). Overall, TERT promoter mutations,
especially C228T and C250T, are significant contributors to
telomere maintenance in MM, driving cancer cell proliferation
through the stabilization of telomeres. The discrepancies in
mutation frequencies across different studies, likely due to
methodological limitations, highlight the need for larger and more
precise studies. The evidence, particularly in non-epithelioid MM,
underscores their potential as biomarkers (Fig. 4).
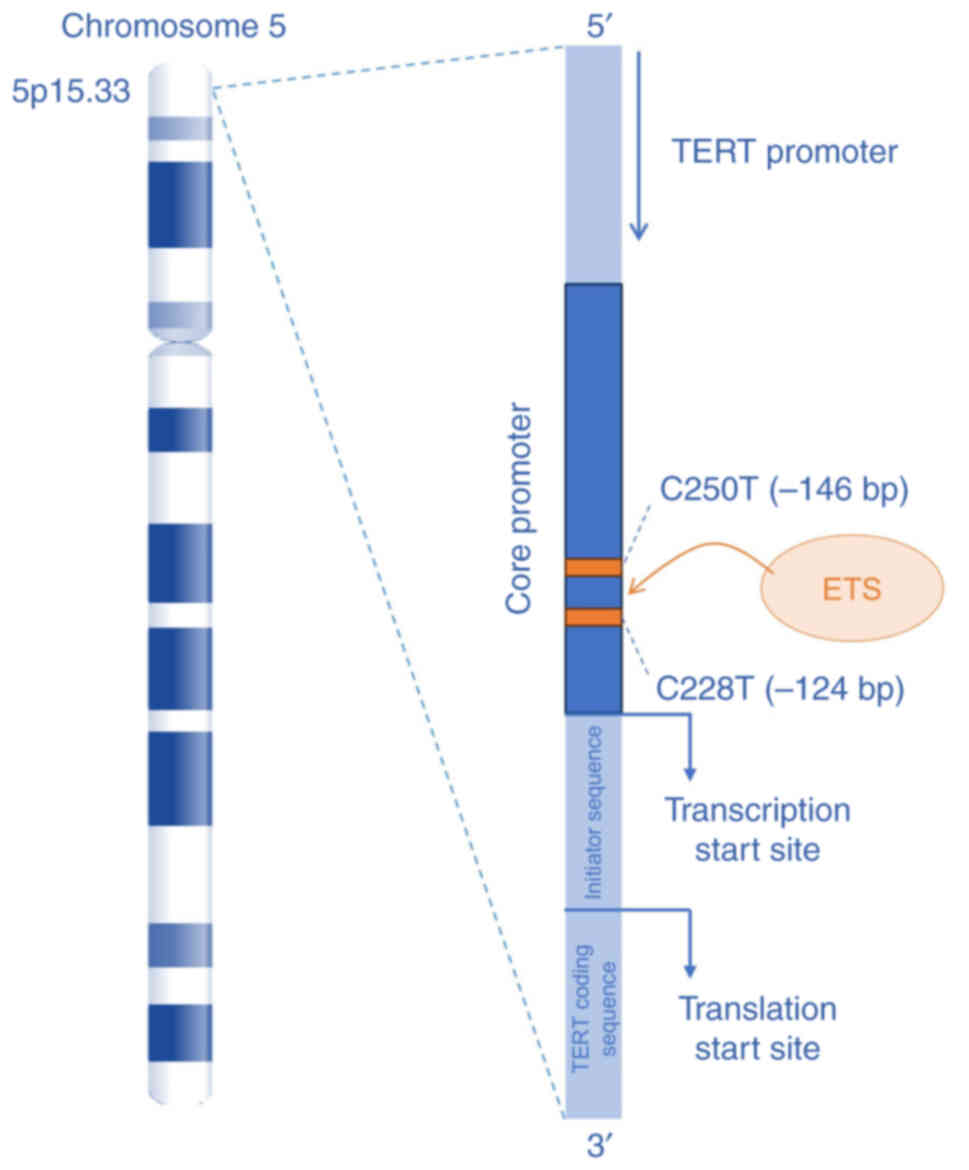 | Figure 4Role of TERT promoter mutations in
mesothelioma. The TERT gene, located on chromosome 5p15.33, is
essential for the proliferation of mesothelioma cells, as TA is the
predominant telomere maintenance mechanism in MM. Mutations in the
TERT promoter region, particularly C228T (−124C>T) and C250T
(−146C>T), have been identified to contribute to telomere
maintenance in MM by increasing TERT expression. These mutations
create de novo binding sites for the ETS family
transcription factors, upregulating TA. The numbering in the
parenthesis reflects the distance from transcription start site of
the TERT. TERT, telomerase reverse transcriptase; TA, telomerase
activity; MM, malignant mesothelioma; ETS, E-twenty-six. |
TERT promoter putations as prognostic
biomarkers in mesothelioma
The current body of evidence indicates that TERT
promoter mutations are associated with overall survival, disease
stage, and response to therapy in MM (125,177). A significant association between
TERT promoter mutations and overall survival was found in 266
patients with MM, with overall survival revealed to be lower in
patients with TERT promoter mutations compared with wild type
(P=0.0004) (177). The
prognostic impact of TERT promoter mutations in MM is also
demonstrated in the study by Pirker et al (125), identifying TERT promoter
mutations as a strong and independent negative prognostic marker
(P<0.0001) in two independent cohorts totaling 184 patients. The
significance of these results persisted across demographic groups
with potentially different therapeutic settings, including both
nonepithelioid and epithelioid MMs, confirming the robustness of
this prognostic marker (125,177). In the TERT promoter-mutated
cases, median overall survival was significantly reduced, 262 vs.
469 days in wild-type cases (P<0.0001), and 353 vs. 459 days for
patients with nonepithelioid MM and epithelioid MM, respectively
(P=0.01) (125). The findings
indicate that TERT promoter mutation status serves as an indicator
of poor survival that may have greater prognostic significance than
histological subtype (125).
These findings highlight the potential value of TERT mutations as a
prognostic biomarker of survival in MM, aligning with studies in
other cancers. However, the number of studies is limited and the
retrospective nature highlights the need for future prospective
studies to confirm the prognostic value of TERT promoter mutations
in MM survival (125,177,197).
The frequency of TERT promoter mutations has been
significantly associated with MM tumor stage. A significantly
higher frequency of TERT promoter mutations was observed in
patients with stage IV tumors (28%) compared with patients with
stage I/III tumors (13%) (P=0.007) (177). The findings align with the
presence of TERT promoter mutations significantly associated with
stage III/IV compared with stage I/II disease (P=0.002) (125). A similar association between
TERT promoter mutation and advanced stage of disease has also been
found in other cancers, denoting the potential of TERT promoter
mutations as prognostic biomarkers (198).
The potential of TERT promoter mutations as
biomarkers of therapeutic response remains underexplored in a
clinical setting. In MM cell lines, TERT promoter mutations were
significantly associated with a greater response to telomerase
inhibition, suggesting a possible higher dependency on TA in MM
with TERT promoter mutations (125). Further large cohort studies are
necessary to validate the observations from MM cell lines.
Mechanisms underlying TERT promoter
mutations in mesothelioma prognosis
The findings discussed in the previous sections
strongly suggest that TERT promoter mutations serve as a useful
prognostic marker in MM. However, while the association between
TERT promoter mutations and poor overall survival is documented,
the underlying mechanisms remain an area of active investigation.
The potential of TERT promoter mutations as biomarkers is usually
interpreted in the context of TERT function in TA. Telomere
maintenance is critical for the proliferation of cancer cells, as
TERT activation leads to TA, as well the stabilization of telomeres
and the avoidance of replicative senescence (145,190). The mutations identified in the
TERT promoter region were shown to create de novo ETS
transcription factor binding sites, indicating that TERT promoter
mutant tumors exhibited higher TERT mRNA expression than wild-type
tumors (P=0.0015) (177,190,194). This upregulation of TA is
crucial in the maintenance of TL, which in turn supports the
unlimited proliferative potential of cancer cells (177). These findings underscore TERT
promoter mutations as potential biomarkers for MM. However, Pirker
et al (125) suggest that
the prognostic value could also be corollary to noncanonical TERT
functions, including effects on cancer cell motility, stemness, and
therapy resistance (125,177,199,200).
TERT promoter mutations have been associated with overexpression of
TERT mRNA, (125). However the
study by Pirker et al (125) identified no significant
correlation between TERT promoter mutation status and TA (P=0.07)
or TL (125), in contrast to
research in other cancers (201).
Additionally, it has been shown that TERT
promoter-mutated MM samples, which are associated with poor
survival, displayed lower chromosomal instability compared with
their wild-type counterparts, a finding contrary to previous
studies linking high chromosomal instability with poor survival in
MM (125,202). The mechanisms underlying this
remain unclear, with one possible explanation being, TERT promoter
mutations resulting in early stabilization of telomeres and
prevention of short telomere-induced chromosomal instability
(203). The low chromosomal
instability may also be attributed to the demonstrated mutual
exclusion of TERT promoter and BAP1 mutations, which have been
associated with high chromosomal instability (177,204-206). Loss of BAP1 has been revealed to
be associated with improved survival outcomes in MM, and the mutual
exclusion of BAP1 and TERT promoter mutations may also partially
explain the poor prognosis observed in patients with TERT promoter
mutations (125,207,208). Furthermore, TERT promoter
mutations have also been significantly associated with deletions of
the RNA binding fox-1 homolog 1 (RBFOX1), glutathione S-transferase
θ1 (GSTT1) genes, and mutations in TNF receptor associated factor 7
(TRAF7), suggesting tumors with TERT promoter mutations may be
prone to gene deletions and specific genomic alterations. The
pattern of alterations may identify a particularly aggressive
subset of MM, elucidating the association with poor prognosis
(125,209). TERT promoter mutations were also
strongly associated with increased in vitro immortalization
potential in MM cells, indicating a link between TERT promoter
mutations and high tumor aggressiveness, aligning with the poor
prognosis (210-212).
Overall, TERT promoter mutations have emerged as
potential independent prognostic indicators of survival in MM. The
mechanisms through which TERT promoter mutations influence
prognosis in MM have yet to be completely elucidated. With regard
to genetic interactions, chromosomal stability, and noncanonical
TERT functions are likely involved in addition to TA. The current
evidence may be limited by the retrospective methodology of the
studies, and sample sizes. Future prospective studies may confirm
these observations, improving the accuracy of MM prognostication
and clinical outcomes.
Therapeutic strategies targeting telomerase
in mesothelioma
MM is characterized by rapid growth and abysmal
prognosis corollary to MM being refractory to conventional
treatment modalities such as chemotherapy, surgery, and
radiotherapy (9,53). Telomere biology offers promising
therapeutic options, which primarily focus on targeting telomerase.
Telomerase is upregulated in >90% of MM cells while being absent
in most non-malignant cells, making it an appealing therapeutic
target, albeit with potential side effects corollary to affecting
cells such as stem cells, activated lymphocytes and germline cells
in which TA is present (126,145,147,148). In MM, oncolytic virotherapy and
cancer vaccines have been investigated as telomerase-specific
therapies that may provide additional therapeutic avenues beyond
conventional chemotherapy and checkpoint inhibitors (76,213,214).
Oncolytic virotherapy targeting
telomerase
Therapeutic modalities based on vectors, including
virotherapy, are promising alternatives to conventional treatments.
Intrapleural viral vector administration is appealing in MM, due to
the anatomical localization of the tumor, which allows for
locoregional delivery (215,216). Research, using
replication-deficient adenoviral vectors to deliver suicide genes
and pro-inflammatory cytokines, demonstrated safety and some
clinical efficacy in generating antitumor immune responses, however
due to the limited distribution of the non-replicative vectors they
failed to achieve broad intratumoral penetration (217). The use of replication-selective,
tumor-specific oncolytic viruses presents a potentially more
efficient alternative (218,219).
Oncolytic virotherapy employs replication-selective
viruses to target cancer cells, thereby inducing oncolysis through
viral replication (220). The
virus selectively infects cancer cells expressing specific
cancer-associated markers, such as telomerase (218,220). The OBP-301 adenovirus, known as
telomelysin, specifically targets telomerase-positive cells, which
may also result in targeting non-malignant cells with TA, by
utilizing the TERT promoter to drive the expression of the critical
for replication viral genes, E1A and E1B (218,219,221). Viral replication leads to the
production of new viral particles within the infected cancer cell,
causing cell lysis and the release of viral material. The newly
produced viruses infect neighbouring cancer cells, amplifying the
oncolytic effect within the tumor microenvironment (222). Thus, OBP-301 may induce cell
lysis of telomerase-positive MM cells, with minimal impact on
normal, telomerase-negative cells, sparing healthy tissue (Fig. 5) (214,219,221,223).
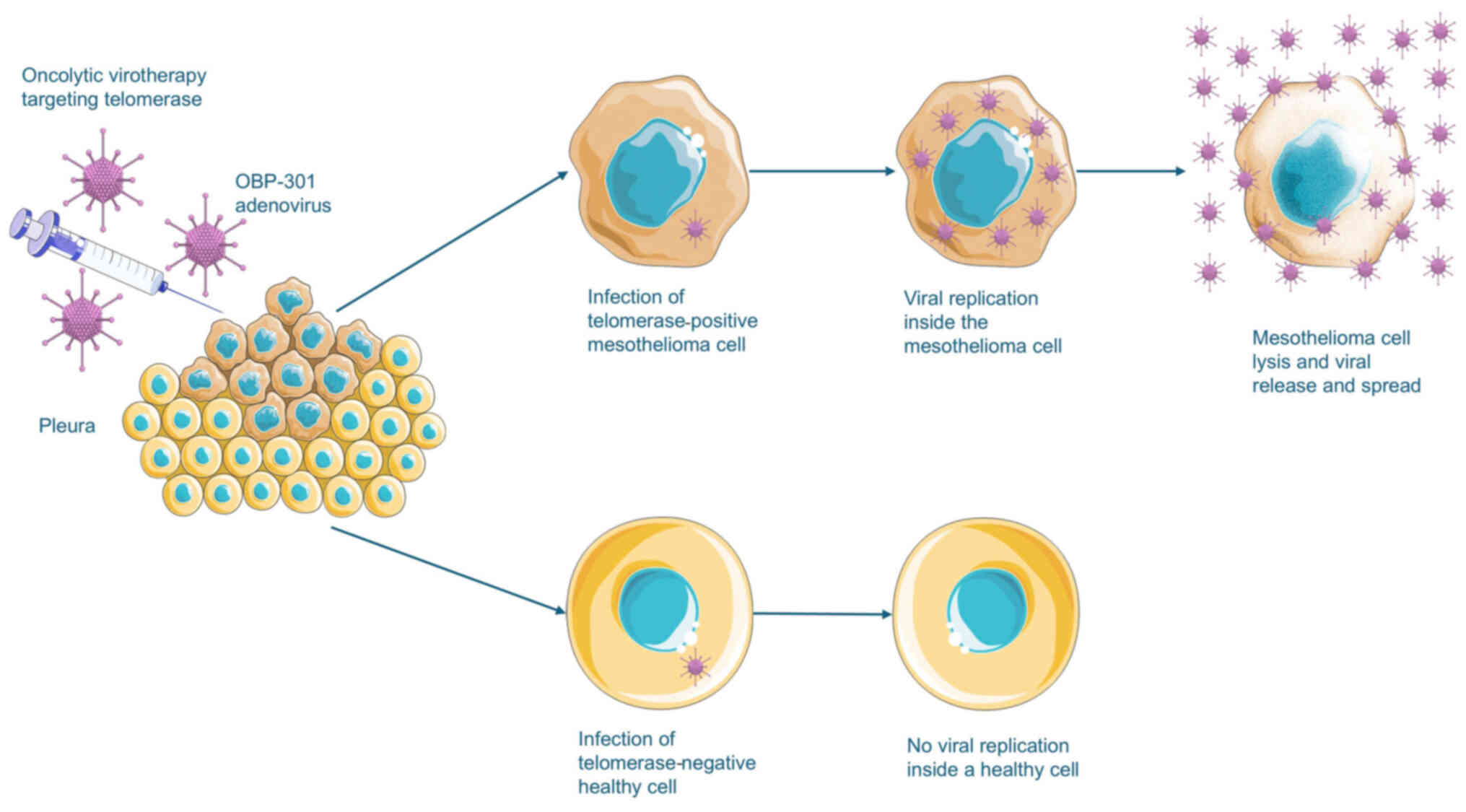 | Figure 5Oncolytic virotherapy targeting
mesothelioma cells. OBP-301 (telomelysin), an oncolytic adenovirus,
targets telomerase-positive cells by utilizing the TERT promoter to
drive the expression of viral genes critical for replication. In
MM, intrapleural administration is appealing as it allows for
effective locoregional delivery. In most healthy tissues, excluding
stem and germline cells, the virus does not replicate leaving these
cells unharmed. In MM cells, viral replication leads to cell lysis
and the release of new viral particles, that may then infect
neighboring MM cells, amplifying the oncolytic effect within the
tumor microenvironment. Parts of the figure were drawn by using
images from Servier Medical Art. Servier Medical Art by Servier is
licensed under a Creative Commons Attribution 4.0 (https://creativecommons.org/licenses/by/4.0/). TERT,
telomerase reverse transcriptase; MM, malignant mesothelioma. |
The therapeutic efficacy of OBP-301 in MM has been
explored in vitro and in vivo (214). OBP-301 was assessed in a panel
of human MM cell lines, each characterized by distinct expression
levels of the coxsackie and adenovirus receptor (CAR) and TERT
(214). It was demonstrated that
OBP-301 induced efficient, dose-dependent cell lysis in MM cell
lines, with significant cytopathic effects observed within 3 days
of infection (214).
Furthermore, the use of a modified version of OBP-301, expressing
green fluorescent protein (GFP) confirmed efficient viral
replication and spread throughout tumor tissues, as evidenced by
persistent fluorescence expression in neighboring tumor cells
(214,218,222).
OBP-301 was also evaluated using an orthotopic
pleural MM model that is based on the inoculation of H2452 cells
into the thoracic cavity of athymic nu/nu mice (214). Intrathoracic delivery of OBP-301
resulted in a significant reduction in tumor dissemination and
tumor burden compared with a replication-defective control
adenovirus or phosphate-buffered saline (214). It was observed that a high dose
of 108 plaque-forming units (PFU) of OBP-301 was
required to achieve a significant antitumor effect, whereas a lower
dose of 107 PFU showed no therapeutic benefit (214). The timing of administration
affected efficacy, with delivery shortly after tumor inoculation
being the most effective in reducing tumor weights and limiting
dissemination. However, it was noted that treatment did not achieve
complete eradication of established tumor nodules, suggesting the
need for combination strategies to improve efficacy (214).
Heparinase-assisted dual virotherapy
targeting telomerase in mesothelioma
A significant limitation to virotherapy efficacy in
solid tumors is the physical barrier posed by the extracellular
matrix (ECM), constituting a significant barrier to the
distribution of viruses (214,224). The addition of a
replication-defective adenovirus expressing the human heparinase
gene (Ad-S/hep) may improve efficacy, as heparan sulphate has a
significant role in limiting the viral spread in ECM, and
heparinase degrades heparan sulphate, increasing the permeability
of the ECM and facilitating deeper viral penetration (225,226).
The co-administration of OBP-301 and Ad-S/hep
resulted in greater viral penetration into three-dimensional tumor
spheroids, with GFP fluorescence detecting penetration in deeper
tumor layers compared with OBP-301 alone (214,223). High-magnification confocal
microscopy images showed that in the OBP-301 monotherapy treatment
group viral distribution was limited to the surface layers of the
spheroids, and in the co-administration with Ad-S/hep group there
was greater uniform penetration and infection throughout the
spheroid (214). In vivo,
dual virotherapy significantly reduced tumor weights in an
orthotopic MM model compared with OBP-301 monotherapy, resulting in
a marked improvement in survival rates, with 71.4% of mice treated
with the combination therapy remaining alive at 12 weeks, compared
with only 14.3% with OBP-301 alone (214).
The findings highlight the potential of a
telomerase-specific oncolytic adenovirus (OBP-301) in the treatment
of MM, especially when combined with agents that enhance ECM
permeability, such as heparinase-expressing adenovirus (214). These results concur with the
findings in other cancers in which it was demonstrated that
oncolytic viral therapy is enhanced by ECM modification (214,224). However, there are risks
associated with ECM degradation, including an increased metastatic
potential due to matrix remodelling. Studies have also found an
association between matrix metalloproteinases (MMPs) and
heparinase, and increased tumor invasion and metastasis (227,228). Additionally, the efficacy of
OBP-301 was revealed to be dose-dependent, and high viral loads
were necessary to achieve significant therapeutic outcomes,
potentially leading to increased toxicity (214). Ongoing clinical trials are
studying the safety and increased dosing of OBP-301 for refractory
advanced liver cancer and evaluating the efficacy of OBP-301
combined with radiotherapy in patients with oesophageal cancer
unfit for standard treatments (229,230). Clinical trials in patients with
MM are needed to confirm and apply the findings of pre-clinical
models with regard to the potential of telomerase-specific
virotherapy both as a standalone treatment and in combination with
other treatment modalities.
Telomerase vaccines in MM
Immunotherapy is a critical addition to the
treatment landscape of MM, with the intra-tumor infiltration of
CD8+ T cells being associated with improved outcomes.
However, numerous MM tumors are considered immunologically 'cold,'
lacking the immune cell infiltration necessary to respond
effectively to immunotherapy (231,232). ICI monotherapy using
pembrolizumab, nivolumab, or avelumab has shown response rates
ranging from 9.3 to 20%, indicating limited efficacy in MM
(60,233,234).
The phase III CheckMate 743 trial showed an
increased median survival for patients treated with a combination
of nivolumab and ipilimumab compared with those treated with
chemotherapy, with the median survival improving from 14.1 to 18.1
months (62). However, the
survival benefit was largely restricted to patients with biphasic
or sarcomatoid histology, while being limited in the epithelioid
subtype. The combination of nivolumab and ipilimumab has been
approved by the FDA as a first-line treatment option for MM
(62,63).
Response rates in MM are still moderate compared
with those observed for ICIs in other cancers, potentially due to
the immunologically 'cold' nature of numerous MM tumors. Thus,
strategies that increase lymphocyte infiltration may be needed to
improve patient outcomes (235-237). One approach is the combination
of ICIs with therapies designed to increase immune cell
infiltration, such as vaccines targeting tumor-associated antigens
(232,238). Telomerase is an attractive
target for therapeutic vaccines in MM, due to its high expression
in MM cells, while being expressed minimally in most normal
tissues, excluding stem cells, germline cells and activated
lymphocytes (126,145,213).
Initial studies of telomerase vaccines have
revealed vaccine-induced tumor-antigen-specific T-cell responses
but did not confer an apparent clinical benefit (7-10,239-241). A potential explanation for this
is that the induced T-cell responses were restricted by immune
checkpoints. The emergence of ICIs has resulted in renewed interest
in cancer vaccines. ICIs act in a complementary manner to vaccines,
resulting in an increase in the induced T-cell response and
suppression of immune checkpoints in the tumor microenvironment
(242,243). Vaccines may generate de
novo antitumor T-cell responses against the tumor of the
patient, which has been identified as a fundamental aspect of
cancer immunotherapy, shown to be necessary to invoke tumor
regression in patients treated with pembrolizumab (244). Thus, cancer vaccines by
complementing ICIs through de novo cancer-specific T-cell
activation may amplify immune responses and enhance the efficacy of
ICIs.
UV1 is a cancer vaccine targeting TERT, the
catalytic subunit of telomerase, aiming to generate a strong immune
response directed against the enzyme (76,245,246). The therapeutic cancer vaccine is
designed to induce an immune response against telomerase, an ideal
as it is nearly universally expressed in MM, and inhibiting it can
selectively disrupt cancer cell proliferation while minimizing
effects on healthy cells (245).
By inducing telomerase-specific immunity, UV1 aims to disrupt MM
cell proliferation without affecting most normal cells (245). The UV1 vaccine consists of long
peptides derived from TERT, aimed at stimulating a CD4+
T-helper type 1 (Th1) immune response. This response is
characterized by the release of pro-inflammatory cytokines such as
interferon (IFN)-γ, TNF-α, and interleukin-2 (IL-2), that lead to
the recruitment and activation of cytotoxic CD8+ T cells
and other immune effector cells, creating an inflammatory tumor
microenvironment, that target and kill telomerase-expressing cancer
cells (cytotoxic CD8+ T cells and other immune effector
cells) (213,245). By inducing a strong
CD4+ response, UV1 aims to overcome the immunological
'cold' environment typically associated with MM, converting it into
an immunologically active microenvironment that is more responsive
to ICIs (236,245).
The programmed death-1 (PD-1) and cytotoxic
T-lymphocyte associated protein 4 (CTLA-4) pathways, that are
targeted by nivolumab and ipilimumab respectively, serve as major
immune checkpoints, differentially inhibiting CD8+ and
CD4+ phenotypes, with research indicating that
anti-CTLA4 and anti-PD-1 immune responses may be mediated through
distinct cellular pathways (247-249). These findings highlight the
mechanistic rationale for combining telomerase vaccines with both
ipilimumab and nivolumab, which target CTLA-4 and PD-1
respectively, in order to maximize the potential therapeutic
benefit (247-249). Furthermore, the evidence from
clinical trials indicates higher and more rapid immune response
rates in patients treated with UV1 combined with immunotherapy
compared with UV1 monotherapy (250-252).
The hTERT peptide vaccine has demonstrated safety
and effectiveness in multiple phase I/II trials in other
malignancies, with common adverse events including pruritus,
fatigue, and gastrointestinal symptoms, and serious adverse events
being rare and primarily allergic in nature. In clinical trials in
other cancers, UV1 induced vaccine-specific immune responses in
67-91% of patients, with survival improvement being correlated to
the patient immunologically responding to the vaccine (250-252).
Clinical trials of telomerase vaccines in
mesothelioma
The NIPU trial, an open-label, randomized, phase II
clinical trial, investigates the efficacy of nivolumab and
ipilimumab with or without UV1 vaccine as a second-line treatment
in inoperable patients with MM, following platinum-based
chemotherapy as first line treatment (76,245). A total of 118 patients were
randomized to receive nivolumab and ipilimumab alone or in
combination with UV1, with PFS as the primary endpoint, and
evaluated using modified RECIST criteria (76,245).
The trial found that median PFS as determined by
blinded independent central review (BICR) was 4.2 months in the
vaccine arm and 4.7 months in the control arm, with an HR of 1.01
(80% CI, 0.751.36) (76).
However, investigator-assessed PFS indicated a potential benefit in
the UV1 arm, with a median PFS of 4.3 months in the vaccine arm and
2.9 months in the control arm leading to an HR of 0.60 (80% CI,
0.450.81); the benefit was particularly strong among patients with
epithelioid tumors (P=0.005). The objective response rate (ORR) was
31% in the vaccine arm and 16% in the control arm. In a follow-up
period of 17.3 months, the median OS was longer in the vaccine arm
with an HR of 0.73 (80% CI, 0.53-1.0) (76). The safety profile of
co-administration of UV1 with ipilimumab and nivolumab was
comparable with that which has been observed in the control arm of
the trial, with the adverse effects potentially specific to the
telomerase vaccine being injection site reactions (14%),
fatigue/asthenia (12%), pruritus (12%), and pyrexia (12%) (76).
The primary endpoint of the clinical trial,
improvement of PFS as assessed by BICR with an 0.6 HR, was not
achieved. However, secondary analysis demonstrated improvement in
PFS in the vaccine arm in all MM histologies and particularly the
epithelioid subtype, a discrepancy that may be corollary to the
diagnostic challenges posed by MM (76,253). This observation suggests that OS
may be considered as a more reliable endpoint for immunotherapy
trials, which concurs with the observations in the CheckMate 743
study, where the HR for PFS was 1.00 but 0.74 for OS (62,76). The improvement in OS of 15.4 vs.
11.1 months (HR 0.73), although not statistically significant,
alongside the BICR-assessed ORR being superior in the vaccine arm,
and the improved PFS in the secondary analysis, collectively
suggest that the UV1 vaccine may still offer clinical benefits.
This being more potentially relevant in patients with the
epithelioid histology, which exhibited a greater favorable response
to the combination therapy, suggesting the potential role of
histological subtype as a factor in predicting treatment efficacy
and the need for more precise patient stratification.
As the UV1 vaccine has shown promising results in
other cancers, and the combination of nivolumab and ipilimumab
despite being approved as first line treatments in MM, lacks
response in most patients, further investigation through more
targeted, histologically-stratified trials is crucial in
ascertaining the potential of telomerase vaccines in MM (62, 76,241,250).
Imaging evaluating telomerase vaccine
efficacy
To ascertain the treatment outcomes of
telomerase-targeted therapies such as the UV1 vaccine,
[18F]-fluorodeoxyglucose positron emission tomography/computed
tomography [18F]-(FDG PET/CT) has emerged as a valuable tool for
detecting tumor metabolic activity (254). The NIPU trial incorporated
[18F]-FDG PET/CT scans at baseline and at week 5 to evaluate
potential predictors of response, including metabolic tumor volume
(MTV), total lesion glycolysis (TLG), and peak-standardized uptake
value (SUVpeak) (254).
Patients who responded to treatment with the UV1
vaccine had significant reductions in TLG, SUVmax, and SUVpeak at
week 5 compared with patients that did not respond to treatment.
This demonstrates the ability of [18F]-FDG PET/CT to detect early
metabolic changes that are related to treatment response,
indicating its potential role as a non-invasive tool in monitoring
telomerase-targeting treatment modalities (254). Studies in other types of cancer
have found significant associations between FDG-PET/CT findings and
overall survival, concurring that FDG-PET/CT may provide indicators
of treatment success (254-256).
Additionally, [18F]-FDG PET/CT revealed insights
into the biological effects of the UV1 vaccine, with significant
reductions in the metabolic parameters, TLG, SUVmax, and SUVpeak,
among responders (254). Metrics
such as SUVmax have often been associated with poor outcomes in
chemotherapy-treated MM, but this relationship did not hold true
for patients treated with immunotherapy and the UV1 vaccine
(254,257). It was found that high baseline
SUV metrics were not indicative of poor outcomes, potentially
reflecting the distinct mechanisms of action involved in
immune-based therapies, where increased immune cell infiltration
and activity may contribute to increased FDG uptake, potentially
indicating the unique characteristics of immunotherapy compared
with the direct cytotoxic effects observed in conventional
chemotherapy (254,258).
The findings indicate the relevance of utilizing
functional imaging to personalize treatment approaches for patients
with MM receiving telomerase-targeted therapies, given its ability
to detect metabolic changes prior to observable anatomical changes,
highlighting its potential as a marker for vaccine efficacy, aiding
in decisions pertaining to continuing or adjusting therapy
(254). The utilization of
[18F]-FDG PET/CT may also allow the exploration of differential
responses across different MM histologies, aiding in identifying
patients who may benefit the most from telomerase-targeting
strategies (76,254).
Conclusion
Telomere biology plays a fundamental role in the
development and progression of MM, holding significant potential
for advancements in diagnosis, prognosis, and therapy. Telomere
shortening is observed in patients with MM and may serve as a
biomarker for early detection and risk assessment, especially in
monitoring disease development in individuals exposed to asbestos.
However, its role as a prognostic biomarker remains inconclusive.
Studies in MM have demonstrated that TA is the predominant TMM,
while being minimally expressed in most normal tissues, albeit with
notable exceptions, thus constituting a promising biomarker for
differentiating malignant from benign mesothelial cells,
particularly when conventional methods yield inconclusive results.
TRAP in situ has been identified as the method of choice for
TA assessment, and has shown high sensitivity and specificity in
distinguishing malignant from benign mesothelial lesions. TERT
promoter mutations have been identified in ~12% of patients with MM
and are more frequently observed in non-epithelioid histologies.
They are associated with poor prognosis and advanced disease stage,
with the most frequently observed mutations being C228T, C250T and
A161C. Furthermore, TERT SNPs have been associated with increased
risk of MM and have potential to act as prognostic biomarkers of
chemotherapy response and disease progression. Telomerase has shown
promise as a therapeutic target in both preclinical and clinical
settings. Oncolytic virotherapy utilizing telomerase-specific
adenoviruses combined with ECM-modifying heparinase-expressing
adenoviruses, have demonstrated antitumoral effects in laboratory
and animal models. Additionally, clinical trials of telomerase
vaccines combined with ICIs have shown encouraging results
particularly in patients with the epithelioid subtype; however,
caution is needed in the interpretation of the findings. Future
research should focus on further elucidating the pathophysiology of
telomere biology in MM. The small cohort sizes in numerous studies
highlight the need for larger prospective cohort studies with
standardized methodologies and subtype-specific research to
validate the potential of telomere biology as a biomarker, and for
randomized clinical trials to examine and develop the therapeutic
applications, leading to improvements in diagnosis, prognosis and
survival.
Availability of data and materials
Not applicable.
Authors' contributions
DAS, DA and VEG conceptualized the study. DA, VEG,
and DAS made a substantial contribution to data interpretation and
analysis. DA wrote and prepared the draft of the manuscript. DAS
and VEG supervised the study and provided critical revisions. All
authors contributed to manuscript revision and approved the final
version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, an artificial
intelligence-powered tool was used to proofread the manuscript and
improve its readability. The manuscript was subsequently revised
and edited. The authors take full responsibility for the final
content of this manuscript. Certain elements within the figures
were sketched using diffusion models.
Acknowledgements
Not applicable.
Funding
Not applicable.
References
|
1
|
Janes SM, Alrifai D and Fennell DA:
Perspectives on the Treatment of malignant pleural mesothelioma. N
Engl J Med. 385:1207–1218. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bridda A, Padoan I, Mencarelli R and Frego
M: Peritoneal mesothelioma: A review. MedGenMed.
9:322007.PubMed/NCBI
|
|
3
|
Attanoos RL and Gibbs AR: Pathology of
malignant mesothelioma. Histopathology. 30:403–418. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scherpereel A, Opitz I, Berghmans T,
Psallidas I, Glatzer M, Rigau D, Astoul P, Bölükbas S, Boyd J,
Coolen J, et al: ERS/ESTS/EACTS/ESTRO guidelines for the management
of malignant pleural mesothelioma. Eur Respir J. 55:19009532020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Husain AN, Colby TV, Ordóñez NG, Allen TC,
Attanoos RL, Beasley MB, Butnor KJ, Chirieac LR, Churg AM, Dacic S,
et al: Guidelines for pathologic diagnosis of malignant
mesothelioma 2017 update of the consensus statement from the
International Mesothelioma Interest Group. Arch Pathol Lab Med.
142:89–108. 2018. View Article : Google Scholar
|
|
6
|
Robinson BW and Lake RA: Advances in
malignant mesothelioma. N Engl J Med. 353:1591–1603. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moore AJ, Parker RJ and Wiggins J:
Malignant mesothelioma. Orphanet J Rare Dis. 3:342008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gloeckler Ries LA, Reichman ME, Lewis DR,
Hankey BF and Edwards BK: Cancer survival and incidence from the
surveillance, epidemiology, and end results (SEER) program.
Oncologist. 8:541–552. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Z, Li VR, Chu FI, Yu V, Lee A, Low D,
Moghanaki D, Lee P and Qi XS: Predicting overall survival for
patients with malignant mesothelioma following radiotherapy via
interpretable machine learning. Cancers (Basel). 15:39162023.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meyerhoff RR, Yang CFJ, Speicher PJ,
Gulack BC, Hartwig MG, D'Amico TA, Harpole DH and Berry MF: Impact
of mesothelioma histologic subtype on outcomes in the surveillance,
epidemiology, and end results database. J Surg Res. 196:23–32.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moon IK and Jarstfer MB: The human
telomere and its relationship to human disease, therapy, and tissue
engineering. Front Biosci. 12:4595–4620. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blackburn EH, Epel ES and Lin J: Human
telomere biology: A contributory and interactive factor in aging,
disease risks, and protection. Science. 350:1193–1198. 2015.
View Article : Google Scholar
|
|
13
|
O'Sullivan RJ and Karlseder J: Telomeres:
protecting chromosomes against genome instability. Nat Rev Mol Cell
Biol. 11:171–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Artandi SE and DePinho RA: Telomeres and
telomerase in cancer. Carcinogenesis. 31:9–18. 2010. View Article : Google Scholar :
|
|
15
|
Shay JW and Bacchetti S: A survey of
telomerase activity in human cancer. Eur J Cancer. 33:787–791.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Robinson NJ and Schiemann WP: Telomerase
in cancer: Function, regulation, and clinical translation. Cancers
(Basel). 14:8082022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maciejowski J and de Lange T: Telomeres in
cancer: Tumour suppression and genome instability. Nat Rev Mol Cell
Biol. 18:175–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan HC, Chang FW, Tsai JD, Lin KM, Chen
CM, Lin SZ, Liu CA and Harn HJ: Telomeres and Cancer. Life (Basel).
11:14052021.PubMed/NCBI
|
|
19
|
Xu Y and Goldkorn A: Telomere and
telomerase therapeutics in cancer. Genes (Basel). 7:222016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dratwa M, Wysoczańska B, Łacina P, Kubik T
and Bogunia-Kubik K: TERT-Regulation and roles in cancer formation.
Front Immunol. 11:5899292020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsatsakis A, Oikonomopoulou T,
Nikolouzakis TK, Vakonaki E, Tzatzarakis M, Flamourakis M, Renieri
E, Fragkiadaki P, Iliaki E, Bachlitzanaki M, et al: Role of
telomere length in human carcinogenesis (Review). Int J Oncol.
63:782023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu X, Han W, Xue W, Zou Y, Xie C, Du J
and Jin G: The association between telomere length and cancer risk
in population studies. Sci Rep. 6:222432016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma H, Zhou Z, Wei S, Liu Z, Pooley KA,
Dunning AM, Svenson U, Roos G, Hosgood HD III, Shen M and Wei Q:
Shortened telomere length is associated with increased risk of
cancer: A meta-analysis. PLoS One. 6:e204662011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shay JW and Wright WE: Role of telomeres
and telomerase in cancer. Semin Cancer Biol. 21:349–353. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bibby AC, Tsim S, Kanellakis N, Ball H,
Talbot DC, Blyth KG, Maskell NA and Psallidas I: Malignant pleural
mesothelioma: An update on investigation, diagnosis and treatment.
Eur Respir Rev. 25:472–486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gilham C, Rake C, Burdett G, Nicholson AG,
Davison L, Franchini A, Carpenter J, Hodgson J, Darnton A and Peto
J: Pleural mesothelioma and lung cancer risks in relation to
occupational history and asbestos lung burden. Occup Environ Med.
73:290–299. 2016. View Article : Google Scholar
|
|
27
|
Nicholson WJ: Comparative dose-response
relationships of asbestos fiber types: Magnitudes and
uncertainties. Ann N Y Acad Sci. 643:74–84. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mott FE: Mesothelioma: A review. Ochsner
J. 12:70–79. 2012.PubMed/NCBI
|
|
29
|
Alpert N, van Gerwen M and Taioli E:
Epidemiology of mesothelioma in the 21st century in Europe and the
United States, 40 years after restricted/banned asbestos use.
Transl Lung Cancer Res. 9(Suppl 1): S28–S38. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lempesis IG, Georgakopoulou VE, Papalexis
P, Chrousos GP and Spandidos DA: Role of stress in the pathogenesis
of cancer (Review). Int J Oncol. 63:1242023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Slominski RM, Raman C, Chen JY and
Slominski AT: How cancer hijacks the body's homeostasis through the
neuroendocrine system. Trends Neurosci. 46:263–275. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Teta MJ, Lau E, Sceurman BK and Wagner ME:
Therapeutic radiation for lymphoma: Risk of malignant mesothelioma.
Cancer. 109:1432–1438. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Galateau-Salle F, Bidet P, Iwatsubo Y,
Gennetay E, Renier A, Letourneux M, Pairon JC, Moritz S, Brochard
P, Jaurand MC and Freymuth F: SV40-like DNA sequences in pleural
mesothelioma, bronchopulmonary carcinoma, and non-malignant
pulmonary diseases. J Pathol. 184:252–257. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carbone M, Gazdar A and Butel JS: SV40 and
human mesothelioma. Transl Lung Cancer Res. 9(Suppl 1): S47–S59.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Betti M, Aspesi A, Sculco M, Matullo G,
Magnani C and Dianzani I: Genetic predisposition for malignant
mesothelioma: A concise review. Mutat Res Rev Mutat Res. 781:1–10.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang H, Testa JR and Carbone M:
Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr
Treat Options in Oncol. 9:147–157. 2008. View Article : Google Scholar
|
|
37
|
Kamp DW and Weitzman SA: The molecular
basis of asbestos induced lung injury. Thorax. 54:638–652. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shukla A, Gulumian M, Hei TK, Kamp D,
Rahman Q and Mossman BT: Multiple roles of oxidants in the
pathogenesis of asbestos-induced diseases. Free Radic Biol Med.
34:1117–1129. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Spandidos DA: A unified theory for the
development of cancer. Biosci Rep. 6:691–708. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Testa JR, Cheung M, Pei J, Below JE, Tan
Y, Sementino E, Cox NJ, Dogan AU, Pass HI, Trusa S, et al: Germline
BAP1 mutations predispose to malignant mesothelioma. Nat Genet.
43:1022–1025. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bianchi AB, Mitsunaga SI, Cheng JQ, Klein
WM, Jhanwar SC, Seizinger B, Kley N, Klein-Szanto AJ and Testa JR:
High frequency of inactivating mutations in the neurofibromatosis
type 2 gene (NF2) in primary malignant mesotheliomas. Proc Natl
Acad Sci USA. 92:10854–10858. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sekido Y and Sato T: NF2 alteration in
mesothelioma. Front Toxicol. 5:11619952023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sekido Y: Molecular pathogenesis of
malignant mesothelioma. Carcinogenesis. 34:1413–1419. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Janssen-Heininger YM, Mossman BT, Heintz
NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG and
van der Vliet A: Redox-based regulation of signal transduction:
Principles, pitfalls, and promises. Free Radic Biol Med. 45:1–17.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yap TA, Aerts JG, Popat S and Fennell DA:
Novel insights into mesothelioma biology and implications for
therapy. Nat Rev Cancer. 17:475–488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang H, Hall SRR, Sun B, Zhao L, Gao Y,
Schmid RA, Tan ST, Peng RW and Yao F: NF2 and Canonical Hippo-YAP
pathway define distinct tumor subsets characterized by different
immune deficiency and treatment implications in human pleural
mesothelioma. Cancers (Basel). 13:15612021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhou S, Liu L, Li H, Eilers G, Kuang Y,
Shi S, Yan Z, Li X, Corson JM, Meng F, et al: Multipoint targeting
of the PI3K/mTOR pathway in mesothelioma. Br J Cancer.
110:2479–2488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Uematsu K, Kanazawa S, You L, He B, Xu Z,
Li K, Peterlin BM, McCormick F and Jablons DM: Wnt pathway
activation in mesothelioma: Evidence of Dishevelled overexpression
and transcriptional activity of beta-catenin. Cancer Res.
63:4547–4551. 2003.PubMed/NCBI
|
|
49
|
Anani W, Bruggeman R and Zander DS:
β-catenin expression in benign and malignant pleural disorders. Int
J Clin Exp Pathol. 4:742–747. 2011.
|
|
50
|
McLoughlin KC, Kaufman AS and Schrump DS:
Targeting the epigenome in malignant pleural mesothelioma. Transl
Lung Cancer Res. 6:350–365. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Destro A, Ceresoli GL, Baryshnikova E,
Garassino I, Zucali PA, De Vincenzo F, Bianchi P, Morenghi E,
Testori A, Alloisio M, et al: Gene methylation in pleural
mesothelioma: Correlations with clinico-pathological features and
patient's follow-up. Lung Cancer. 59:369–376. 2008. View Article : Google Scholar
|
|
52
|
Kubo T, Toyooka S, Tsukuda K, Sakaguchi M,
Fukazawa T, Soh J, Asano H, Ueno T, Muraoka T, Yamamoto H, et al:
Epigenetic silencing of MicroRNA-34b/c plays an important role in
the pathogenesis of malignant pleural mesothelioma. Clin Cancer
Res. 17:4965–4974. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Perera ND and Mansfield AS: The evolving
therapeutic landscape for malignant pleural mesothelioma. Curr
Oncol Rep. 24:1413–1423. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lapidot M and Sattler M: The role of
surgery in pleural mesothelioma. Cancers (Basel). 16:17192024.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S,
Manegold C, et al: Phase III study of pemetrexed in combination
with cisplatin versus cisplatin alone in patients with malignant
pleural mesothelioma. J Clin Oncol. 21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ziółkowska B, Cybulska-Stopa B,
Papantoniou D and Suwiński R: Systemic treatment in patients with
malignant pleural mesothelioma - real life experience. BMC Cancer.
22:4322022. View Article : Google Scholar
|
|
57
|
Price A: What is the role of radiotherapy
in malignant pleural mesothelioma? Oncologist. 16:359–365. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hanna GG, John T and Ball DL:
Controversies in the role of radiotherapy in pleural mesothelioma.
Transl Lung Cancer Res. 10:2079–2087. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang Y and Zhang Z: The history and
advances in cancer immunotherapy: understanding the characteristics
of tumor-infiltrating immune cells and their therapeutic
implications. Cell Mol Immunol. 17:807–821. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cantini L, Hassan R, Sterman DH and Aerts
JGJV: Emerging treatments for malignant pleural mesothelioma: Where
are we heading? Front Oncol. 10:3432022. View Article : Google Scholar
|
|
61
|
Gray SG and Mutti L: Immunotherapy for
mesothelioma: A critical review of current clinical trials and
future perspectives. Transl Lung Cancer Res. 9(Suppl 1): S100–S119.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Baas P, Scherpereel A, Nowak AK, Fujimoto
N, Peters S, Tsao AS, Mansfield AS, Popat S, Jahan T, Antonia S, et
al: First-line nivolumab plus ipilimumab in unresectable malignant
pleural mesothelioma (CheckMate 743): A multicentre, randomised,
open-label, phase 3 trial. Lancet. 397:375–386. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nakajima EC, Vellanki PJ, Larkins E,
Chatterjee S, Mishra-Kalyani PS, Bi Y, Qosa H, Liu J, Zhao H,
Biable M, et al: FDA approval summary: Nivolumab in combination
with ipilimumab for the treatment of unresectable malignant pleural
mesothelioma. Clin Cancer Res. 28:446–451. 2022. View Article : Google Scholar :
|
|
64
|
Uddin F, Rudin CM and Sen T: CRISPR Gene
Therapy: Applications, limitations, and implications for the
future. Front Oncol. 10:13872020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Cross D and Burmester JK: Gene therapy for
cancer treatment: Past, present and future. Clin Med Res.
4:218–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Vachani A, Moon E, Wakeam E and Albelda
SM: Gene therapy for mesothelioma and lung cancer. Am J Respir Cell
Mol Biol. 42:385–393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Pease DF and Kratzke RA: Oncolytic viral
therapy for mesothelioma. Front Oncol. 7:1792017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang T, Jou TH, Hsin J, Wang Z, Huang K,
Ye J, Yin H and Xing Y: Talimogene laherparepvec (T-VEC): A review
of the recent advances in cancer therapy. J Clin Med. 12:10982023.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dagogo-Jack I: Targeted approaches to
treatment of pleural mesothelioma: A review. JCO Precis Oncol.
7:e23003442023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zalcman G, Mazieres J, Margery J,
Greillier L, Audigier-Valette C, Moro-Sibilot D, Molinier O, Corre
R, Monnet I, Gounant V, et al: Bevacizumab for newly diagnosed
pleural mesothelioma in the Mesothelioma Avastin Cisplatin
Pemetrexed Study (MAPS): A randomised, controlled, open-label,
phase 3 trial. Lancet. 387:1405–1414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ceresoli GL, Zucali PA, Mencoboni M, Botta
M, Grossi F, Cortinovis D, Zilembo N, Ripa C, Tiseo M, Favaretto
AG, et al: Phase II study of pemetrexed and carboplatin plus
bevacizumab as first-line therapy in malignant pleural
mesothelioma. Br J Cancer. 109:552–558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zucali PA, Perrino M, De Vincenzo F,
Giordano L, Cordua N, D'Antonio F and Santoro A: A phase II study
of the combination of gemcitabine and imatinib mesylate in
pemetrexed-pretreated patients with malignant pleural mesothelioma.
Lung Cancer. 142:132–137. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tsao AS, Harun N, Lee JJ, Heymach J,
Pisters K, Hong WK, Fujimoto J and Wistuba I: Phase I trial of
cisplatin, pemetrexed, and imatinib mesylate in chemonaive patients
with unresectable malignant pleural mesothelioma. Clin Lung Cancer.
15:197–201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Govindan R, Kratzke RA, Herndon JE II,
Niehans GA, Vollmer R, Watson D, Green MR and Kindler HL; Cancer
and Leukemia Group B (CALGB 30101): Gefitinib in patients with
malignant mesothelioma: A phase II study by the cancer and leukemia
group B. Clin Cancer Res. 11:2300–2304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
De Paepe A, Vermaelen KY, Cornelissen R,
Germonpre PR, Janssens A, Lambrechts M, Bootsma G, Van Meerbeeck J
and Surmont VF: Cetuximab plus platinum-based chemotherapy in
patients with malignant pleural mesothelioma: A single arm phase II
trial. J Chin Oncol. 35(15_suppl): e200302017.
|
|
76
|
Haakensen VD, Öjlert ÅK, Thunold S,
Farooqi S, Nowak AK, Chin WL, Grundberg O, Szejniuk WM, Cedres S,
Sørensen JB, et al: UV1 telomerase vaccine with ipilimumab and
nivolumab as second line treatment for pleural mesothelioma-A phase
II randomised trial. Eur J Cancer. 202:1139732024. View Article : Google Scholar
|
|
77
|
De Lange T: How telomeres solve the
end-protection problem. Science. 326:948–952. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Palm W and de Lange T: How shelterin
protects mammalian telomeres. Annu Rev Genet. 42:301–334. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Martínez P and Blasco MA: Telomere-driven
diseases and telomere-targeting therapies. J Cell Biol.
216:875–887. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Greider CW and Blackburn EH: A telomeric
sequence in the RNA of Tetrahymena telomerase required for telomere
repeat synthesis. Nature. 337:331–337. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Shay JW and Wright WE: Hayflick, his
limit, and cellular ageing. Nat Rev Mol Cell Biol. 1:72–76. 2000.
View Article : Google Scholar
|
|
82
|
Deng Y, Chan SS and Chang S: Telomere
dysfunction and tumour suppression: The senescence connection. Nat
Rev Cancer. 8:450–458. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Bryan TM, Englezou A, Dalla-Pozza L,
Dunham MA and Reddel RR: Evidence for an alternative mechanism for
maintaining telomere length in human tumors and tumor-derived cell
lines. Nat Med. 3:1271–1274. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Bryan TM, Englezou A, Gupta J, Bacchetti S
and Reddel RR: Telomere elongation in immortal human cells without
detectable telomerase activity. EMBO J. 14:4240–4248. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zvereva MI, Shcherbakova DM and Dontsova
OA: Telomerase: Structure, functions, and activity regulation.
Biochemistry (Mosc). 75:1563–1583. 2010. View Article : Google Scholar
|
|
86
|
Nandakumar J and Cech TR: Finding the end:
Recruitment of telomerase to telomeres. Nat Rev Mol Cell Biol.
14:69–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Xin ZT, Beauchamp AD, Calado RT, Bradford
JW, Regal JA, Shenoy A, Liang Y, Lansdorp PM, Young NS and Ly H:
Functional characterization of natural telomerase mutations found
in patients with hematologic disorders. Blood. 109:524–532. 2007.
View Article : Google Scholar
|
|
88
|
de Lange T: Shelterin-mediated telomere
protection. Annu Rev Genet. 52:223–247. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Chen H, Majumdar A, Wang L, Kar S, Brown
KM, Feng H, Turman C, Dennis J, Easton D, Michailidou K, et al:
Large-scale cross-cancer fine-mapping of the 5p15.33 region reveals
multiple independent signals. HGG Adv. 2:1000412021.PubMed/NCBI
|
|
90
|
Okamoto K and Seimiya H: Revisiting
telomere shortening in cancer. Cells. 8:1072019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Blasco MA, Lee HW, Hande MP, Samper E,
Lansdorp PM, DePinho RA and Greider CW: Telomere shortening and
tumor formation by mouse cells lacking telomerase RNA. Cell.
91:25–34. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Karimi B, Yunesian M, Nabizadeh R,
Mehdipour P and Aghaie A: Is leukocyte telomere length related with
lung cancer risk?: A meta-analysis. Iran Biomed J. 21:142–153.
2017. View Article : Google Scholar :
|
|
94
|
Naing C, Aung K, Lai PK and Mak JW:
Association between telomere length and the risk of colorectal
cancer: A meta-analysis of observational studies. BMC Cancer.
17:242017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Benites-Zapata VA, Ulloque-Badaracco JR,
Alarcón-Braga EA, Fernández-Alonso AM, López-Baena MT and
Pérez-López FR: Telomerase activity and telomere length in women
with breast cancer or without malignancy: A systematic review and
meta-analysis. Maturitas. 180:1078822024. View Article : Google Scholar
|
|
96
|
Caini S, Raimondi S, Johansson H, De
Giorgi V, Zanna I, Palli D and Gandini S: Telomere length and the
risk of cutaneous melanoma and non-melanoma skin cancer: A review
of the literature and meta-analysis. J Dermatol Sci. 80:168–174.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Wentzensen IM, Mirabello L, Pfeiffer RM
and Savage SA: The association of telomere length and cancer: A
meta-analysis. Cancer Epidemiol Biomarkers Prev. 20:1238–1250.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Holesova Z, Krasnicanova L, Saade R, Pös
O, Budis J, Gazdarica J, Repiska V and Szemes T: Telomere length
changes in cancer: Insights on carcinogenesis and potential for
non-invasive diagnostic strategies. Genes (Basel). 14:7152023.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chen S, Hu S, Zhou B, Cheng B, Tong H, Su
D, Li X, Chen Y and Zhang G: Telomere-related prognostic biomarkers
for survival assessments in pancreatic cancer. Sci Rep.
13:105862023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang J, Xie LY, Allan S, Beach D and
Hannon GJ: Myc activates telomerase. Genes Dev. 12:1769–1774. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Liu M, Zhang Y, Jian Y, Gu L, Zhang D,
Zhou H, Wang Y and Xu ZX: The regulations of telomerase reverse
transcriptase (TERT) in cancer. Cell Death Dis. 15:902024.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kim NW, Piatyszek MA, Prowse KR, Harley
CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL and Shay
JW: Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Kim NW and Wu F: Advances in
quantification and characterization of telomerase activity by the
telomeric repeat amplification protocol (TRAP). Nucleic Acids Res.
25:2595–2597. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Horn S, Figl A, Rachakonda PS, Fischer C,
Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, et al:
TERT promoter mutations in familial and sporadic melanoma. Science.
339:959–961. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Vinagre J, Almeida A, Pópulo H, Batista R,
Lyra J, Pinto V, Coelho R, Celestino R, Prazeres H, Lima L, et al:
Frequency of TERT promoter mutations in human cancers. Nat Commun.
4:21852013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hiyama E and Hiyama K: Telomere and
telomerase in stem cells. Br J Cancer. 96:1020–1024. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Park JI, Venteicher AS, Hong JY, Choi J,
Jun S, Shkreli M, Chang W, Meng Z, Cheung P, Ji H, et al:
Telomerase modulates Wnt signalling by association with target gene
chromatin. Nature. 460:66–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Colebatch AJ, Dobrovic A and Cooper WA:
TERT gene: Its function and dysregulation in cancer. J Clin Pathol.
72:281–284. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Powter B, Jeffreys SA, Sareen H, Cooper A,
Brungs D, Po J, Roberts T, Koh ES, Scott KF, Sajinovic M, et al:
Human TERT promoter mutations as a prognostic biomarker in glioma.
J Cancer Res Clin Oncol. 147:1007–1017. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Marczyk VR, Maia AL and Goemann IM:
Distinct transcriptional and prognostic impacts of TERT promoter
mutations C228T and C250T in papillary thyroid carcinoma. Endocr
Relat Cancer. 31:e2400582024. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Xu Y, Ren X, Jiang T, Lv S, Gao K, Liu Y
and Yan Y: Circulating tumor cells (CTCs) and hTERT gene expression
in CTCs for radiotherapy effect with lung cancer. BMC Cancer.
23:4752023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Killela PJ, Reitman ZJ, Jiao Y, Bettegowda
C, Agrawal N, Diaz LA Jr, Friedman AH, Friedman H, Gallia GL,
Giovanella BC, et al: TERT promoter mutations occur frequently in
gliomas and a subset of tumors derived from cells with low rates of
self-renewal. Proc Natl Acad Sci USA. 110:6021–6026. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Castelo-Branco P, Leão R, Lipman T,
Campbell B, Lee D, Price A, Zhang C, Heidari A, Stephens D, Boerno
S, et al: A cancer specific hypermethylation signature of the TERT
promoter predicts biochemical relapse in prostate cancer: A
retrospective cohort study. Oncotarget. 7:57726–57736. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Asai A, Oshima Y, Yamamoto Y, Uochi TA,
Kusaka H, Akinaga S, Yamashita Y, Pongracz K, Pruzan R, Wunder E,
et al: A novel telomerase template antagonist (GRN163) as a
potential anticancer agent. Cancer Res. 63:3931–3939.
2003.PubMed/NCBI
|
|
115
|
Tefferi A, Lasho TL, Begna KH, Patnaik MM,
Zblewski DL, Finke CM, Laborde RR, Wassie E, Schimek L, Hanson CA,
et al: A pilot study of the telomerase inhibitor imetelstat for
myelofibrosis. N Engl J Med. 373:908–919. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Wang X, Hu CS, Petersen B, Qiu J, Ye F,
Houldsworth J, Eng K, Huang F and Hoffman R: Imetelstat, a
telomerase inhibitor, is capable of depleting myelofibrosis stem
and progenitor cells. Blood Adv. 2:2378–2388. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Olschok K, Altenburg B, De Toledo MAS,
Maurer A, Abels A, Beier F, Gezer D, Isfort S, Paeschke K,
Brümmendorf TH, et al: The telomerase inhibitor imetelstat
differentially targets JAK2V617F versus CALR mutant
myeloproliferative neoplasm cells and inhibits JAK-STAT signaling.
Front Oncol. 13:12774532023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zhang JM and Zou L: Alternative
lengthening of telomeres: From molecular mechanisms to therapeutic
outlooks. Cell Biosci. 10:302020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
De Vitis M, Berardinelli F and Sgura A:
Telomere length maintenance in cancer: At the crossroad between
telomerase and alternative lengthening of telomeres (ALT). Int J
Mol Sci. 19:6062018. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Flynn RL, Cox KE, Jeitany M, Wakimoto H,
Bryll AR, Ganem NJ, Bersani F, Pineda JR, Suvà ML, Benes CH, et al:
Alternative lengthening of telomeres renders cancer cells
hypersensitive to ATR inhibitors. Science. 347:273–277. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Afshari N, Al-Gazally ME, Rasulova I,
Jalil AT, Matinfar S and Momeninejad M: Sensitive bioanalytical
methods for telomerase activity detection: A cancer biomarker. Anal
Methods. 14:4174–4184. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Kyo S, Takakura M and Inoue M: Telomerase
activity in cancer as a diagnostic and therapeutic target. Histol
Histopathol. 15:813–824. 2000.PubMed/NCBI
|
|
123
|
Mervic A, Goricar K, Blagus T, Franko A,
Trebusak-Podkrajsek K, Fikfak MD, Dolzan V and Kovac V: Telomere
length and TERT polymorphisms as biomarkers in asbestos-related
diseases. Radiol Oncol. 58:87–98. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Aida S, Aida J, Naoi M, Kato M, Tsuura Y,
Natsume I and Takubo K: Measurement of telomere length in cells
from pleural effusion: Asbestos exposure causes telomere shortening
in pleural mesothelial cells. Pathol Int. 68:503–508. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Pirker C, Bilecz A, Grusch M, Mohr T,
Heidenreich B, Laszlo V, Stockhammer P, Lötsch-Gojo D, Gojo J,
Gabler L, et al: Telomerase reverse transcriptase promoter
mutations identify a genomically defined and highly aggressive
human pleural mesothelioma subgroup. Clin Cancer Res. 26:3819–3830.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Au AY, Hackl T, Yeager TR, Cohen SB, Pass
HI, Harris CC and Reddel RR: Telomerase activity in pleural
malignant mesotheliomas. Lung Cancer. 73:283–288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Andreikos D, Kyrodimos E, Kotsinas A,
Chrysovergis A and Papacharalampous GX: The association between
telomere length and head and neck cancer risk: A systematic review
and meta-analysis. Int J Mol Sci. 25:90002024. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Yuan X, Dai M and Xu D: Telomere-related
markers for cancer. Curr Top Med Chem. 20:410–432. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Borges G, Criqui M and Harrington L:
Tieing together loose ends: Telomere instability in cancer and
aging. Mol Oncol. 16:3380–3396. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Cigan SS, Meredith JJ, Kelley AC, Yang T,
Langer EK, Hooten AJ, Lane JA, Cole BR, Krailo M, Frazier AL, et
al: Predicted leukocyte telomere length and risk of germ cell
tumours. Br J Cancer. 127:301–312. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Lagniau S, Lamote K, van Meerbeeck JP and
Vermaelen KY: Biomarkers for early diagnosis of malignant
mesothelioma: Do we need another moonshot? Oncotarget.
8:53751–53762. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Nabeshima K, Matsumoto S, Hamasaki M, Hida
T, Kamei T, Hiroshima K, Tsujimura T and Kawahara K: Use of p16
FISH for differential diagnosis of mesothelioma in smear
preparations. Diagn Cytopathol. 44:774–780. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Hiroshima K, Wu D, Hasegawa M, Koh E,
Sekine Y, Ozaki D, Yusa T, Walts AE, Marchevsky AM, Nabeshima K, et
al: Cytologic differential diagnosis of malignant mesothelioma and
reactive mesothelial cells with FISH analysis of p16. Diagn
Cytopathol. 44:591–598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Jaouen A, Thivolet-Bejui F, Chalabreysse
L, Piaton E, Traverse-Glehen A, Isaac S, Decaussin-Petrucci M,
Depaepe L, Fontaine J, Remy I, et al: BRCA1 associated protein 1
(BAP1) expression in pleural diffuse malignant mesothelioma: A
comparative cytological and histological analyses on 50 patients.
Ann Pathol. 36:111–119. 2016.In French. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Hjerpe A, Ascoli V, Bedrossian C, Boon M,
Creaney J, Davidson B, Dejmek A, Dobra K, Fassina A, Field A, et
al: Guidelines for cytopathologic diagnosis of epithelioid and
mixed type malignant mesothelioma. Complementary statement from the
International Mesothelioma Interest Group, also endorsed by the
International Academy of Cytology and the Papanicolaou Society of
Cytopathology. CytoJournal. 12:262015. View Article : Google Scholar
|
|
136
|
Minato H, Kurose N, Fukushima M, Nojima T,
Usuda K, Sagawa M, Sakuma T, Ooi A, Matsumoto I, Oda M, et al:
Comparative immunohistochemical analysis of IMP3, GLUT1, EMA,
CD146, and desmin for distinguishing malignant mesothelioma from
reactive mesothelial cells. Am J Clin Pathol. 141:85–93. 2014.
View Article : Google Scholar
|
|
137
|
Levstek T, Redenšek S, Trošt M, Dolžan V
and Podkrajšek KT: Assessment of the telomere length and its effect
on the symptomatology of Parkinson's disease. Antioxidants (Basel).
10:1372021. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Lulkiewicz M, Bajsert J, Kopczynski P,
Barczak W and Rubis B: Telomere length: How the length makes a
difference. Mol Biol Rep. 47:7181–7188. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Havas A, Yin S and Adams PD: The role of
aging in cancer. Mol Oncol. 16:3213–3219. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Kusamura S, Baratti D, De Simone M,
Pasqual EM, Ansaloni L, Marrelli D, Robella M, Accarpio F, Valle M,
Scaringi S, et al: Diagnostic and therapeutic pathway in diffuse
malignant peritoneal mesothelioma. Cancers (Basel). 15:6622023.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Benitez-Buelga C, Sanchez-Barroso L,
Gallardo M, Apellániz-Ruiz M, Inglada-Pérez L, Yanowski K, Carrillo
J, Garcia-Estevez L, Calvo I, Perona R, et al: Impact of
chemotherapy on telomere length in sporadic and familial breast
cancer patients. Breast Cancer Res Treat. 149:385–394. 2015.
View Article : Google Scholar
|
|
142
|
Schneider CV, Schneider KM, Teumer A,
Rudolph KL, Hartmann D, Rader DJ and Strnad P: Association of
telomere length with risk of disease and mortality. JAMA Intern
Med. 182:291–300. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Hamada T, Yuan C, Bao Y, Zhang M, Khalaf
N, Babic A, Morales-Oyarvide V, Cochrane BB, Gaziano JM,
Giovannucci EL, et al: Prediagnostic leukocyte telomere length and
pancreatic cancer survival. Cancer Epidemiol Biomarkers Prev.
28:1868–1875. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Pauleck S, Gigic B, Cawthon RM, Ose J,
Peoples AR, Warby CA, Sinnott JA, Lin T, Boehm J, Schrotz-King P,
et al: Association of circulating leukocyte telomere length with
survival in patients with colorectal cancer. J Geriatr Oncol.
13:480–485. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Dhaene K, Hübner R, Kumar-Singh S, Weyn B
and Van Marck E: Telomerase activity in human pleural mesothelioma.
Thorax. 53:915–918. 1998. View Article : Google Scholar
|
|
146
|
Cesare AJ and Reddel RR: Alternative
lengthening of telomeres: Models, mechanisms and implications. Nat
Rev Genet. 11:319–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Kumaki F, Kawai T, Hiroi S, Shinomiya N,
Ozeki Y, Ferrans VJ and Torikata C: Telomerase activity and
expression of human telomerase RNA component and human telomerase
reverse transcriptase in lung carcinomas. Hum Pathol. 32:188–195.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Hansson M, Zendehrokh N, Ohyashiki J,
Ohyashiki K, Westman UB, Roos G and Dejmek A: Telomerase activity
in effusions: A comparison between telomere repeat amplification
protocol in situ and conventional telomere repeat amplification
protocol assay. Arch Pathol Lab Med. 132:1896–1902. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Villa R, Daidone MG, Motta R, Venturini L,
De Marco C, Vannelli A, Kusamura S, Baratti D, Deraco M, Costa A,
et al: Multiple mechanisms of telomere maintenance exist and
differentially affect clinical outcome in diffuse malignant
peritoneal mesothelioma. Clin Cancer Res. 14:4134–4140. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Trupiano JK, Geisinger KR, Willingham MC,
Manders P, Zbieranski N, Case D and Levine EA: Diffuse malignant
mesothelioma of the peritoneum and pleura, analysis of markers. Mod
Pathol. 17:476–481. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Foddis R, De Rienzo A, Broccoli D,
Bocchetta M, Stekala E, Rizzo P, Tosolini A, Grobelny JV, Jhanwar
SC, Pass HI, et al: SV40 infection induces telomerase activity in
human mesothelial cells. Oncogene. 21:1434–1442. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Heaphy CM, Subhawong AP, Hong SM, Goggins
MG, Montgomery EA, Gabrielson E, Netto GJ, Epstein JI, Lotan TL,
Westra WH, et al: Prevalence of the alternative lengthening of
telomeres telomere maintenance mechanism in human cancer subtypes.
Am J Pathol. 179:1608–1615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Hahn WC, Counter CM, Lundberg AS,
Beijersbergen RL, Brooks MW and Weinberg RA: Creation of human
tumour cells with defined genetic elements. Nature. 400:464–468.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Zendehrokh N and Dejmek A: Telomere repeat
amplification protocol (TRAP) in situ reveals telomerase activity
in three cell types in effusions: Malignant cells, proliferative
mesothelial cells, and lymphocytes. Mod Pathol. 18:189–196. 2005.
View Article : Google Scholar
|
|
155
|
Counter CM, Gupta J, Harley CB, Leber B
and Bacchetti S: Telomerase activity in normal leukocytes and in
hematologic malignancies. Blood. 85:2315–2320. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Norrback KF, Dahlenborg K, Carlsson R and
Roos G: Telomerase activation in normal B lymphocytes and
non-Hodgkin's lymphomas. Blood. 88:222–229. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Lee WY: Limitations of detection of
malignancy in pleural effusions using ELISA-based TRAP assay:
comparison with cytological examination. Cytopathology. 16:227–232.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Tangkijvanich P, Tresukosol D,
Sampatanukul P, Sakdikul S, Voravud N, Mahachai V and Mutirangura
A: Telomerase assay for differentiating between malignancy-related
and nonmalignant ascites. Clin Cancer Res. 5:2470–2475.
1999.PubMed/NCBI
|
|
159
|
Braunschweig R, Yan P, Guilleret I,
Delacretaz F, Bosman FT, Mihaescu A and Benhattar J: Detection of
malignant effusions: Comparison of a telomerase assay and cytologic
examination. Diagn Cytopathol. 24:174–180. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Gül I, Dündar O, Bodur S, Tunca Y and
Tütüncü L: The status of telomerase enzyme activity in benign and
malignant gynaecologic pathologies. Balkan Med J. 30:287–292. 2013.
View Article : Google Scholar
|
|
161
|
Miracco C, de Santi MM, Pacenti L,
Schürfeld K, Laurini L, Pirtoli L, Luzi P and Ninfo V: Telomerase
activity, Ki-67, cyclin D1 and A expression, and apoptosis in
solitary fibrous tumors: Additional features of a predictable
course? Pathol Res Pract. 197:475–481. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Adell E and Dejmek A: Telomerase activity
analyzed with TRAP in situ provides additional information in
effusions remaining equivocal after immunocytochemistry and
hyaluronan analysis. Diagn Cytopathol. 42:1051–1057. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Cakir C, Gulluoglu MG and Yilmazbayhan D:
Cell proliferation rate and telomerase activity in the differential
diagnosis. Pathology. 38:10–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Lantuejoul S, Soria JC, Moro-Sibilot D,
Morat L, Veyrenc S, Lorimier P, Brichon PY, Sabatier L, Brambilla C
and Brambilla E: Differential expression of telomerase reverse
transcriptase (hTERT) in lung tumours. Br J Cancer. 90:1222–1229.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Henson JD, Neumann AA, Yeager TR and
Reddel RR: Alternative lengthening of telomeres in mammalian cells.
Oncogene. 21:598–610. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Hiyama E and Hiyama K: Telomerase as tumor
marker. Cancer Lett. 194:221–233. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Montgomery E, Argani P, Hicks JL, DeMarzo
AM and Meeker AK: Telomere lengths of translocation-associated and
nontranslocation-associated sarcomas differ dramatically. Am J
Pathol. 164:1523–1529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Hakin-Smith V, Jellinek DA, Levy D,
Carroll T, Teo M, Timperley WR, McKay MJ, Reddel RR and Royds JA:
Alternative lengthening of telomeres and survival in patients with
glioblastoma multiforme. Lancet. 361:836–838. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Ulaner GA, Huang HY, Otero J, Zhao Z,
Ben-Porat L, Satagopan JM, Gorlick R, Meyers P, Healey JH, Huvos
AG, et al: Absence of a telomere maintenance mechanism as a
favorable prognostic factor in patients with osteosarcoma. Cancer
Res. 63:1759–1763. 2003.PubMed/NCBI
|
|
170
|
Johnson JE, Varkonyi RJ, Schwalm J, Cragle
R, Klein-Szanto A, Patchefsky A, Cukierman E, von Mehren M and
Broccoli D: Multiple mechanisms of telomere maintenance exist in
liposarcomas. Clin Cancer Res. 11:5347–5355. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Costa A, Daidone MG, Daprai L, Villa R,
Cantù S, Pilotti S, Mariani L, Gronchi A, Henson JD, Reddel RR and
Zaffaroni N: Telomere maintenance mechanisms in liposarcomas:
Association with histologic subtypes and disease progression.
Cancer Res. 66:8918–8924. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Claude E and Decottignies A: Telomere
maintenance mechanisms in cancer: Telomerase, ALT or lack thereof.
Curr Opin Genet Dev. 60:1–8. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Seger YR, García-Cao M, Piccinin S,
Cunsolo CL, Doglioni C, Blasco MA, Hannon GJ and Maestro R:
Transformation of normal human cells in the absence of telomerase
activation. Cancer Cell. 2:401–413. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Shay JW and Wright WE: Telomerase: A
target for cancer therapeutics. Cancer Cell. 2:257–265. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Folini M and Zaffaroni N: Targeting
telomerase by antisense-based approaches: Perspectives for new
anti-cancer therapies. Curr Pharm Des. 11:1105–1117. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Jiang WQ, Zhong ZH, Henson JD and Reddel
RR: Identification of candidate alternative lengthening of
telomeres genes by methionine restriction and RNA interference.
Oncogene. 26:4635–4647. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Quetel L, Meiller C, Assié JB, Blum Y,
Imbeaud S, Montagne F, Tranchant R, de Wolf J, Caruso S, Copin MC,
et al: Genetic alterations of malignant pleural mesothelioma:
Association with tumor heterogeneity and overall survival. Mol
Oncol. 14:1207–1223. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Zhou M, Jiang B, Xiong M and Zhu X:
Association between TERT rs2736098 polymorphisms and cancer risk-A
meta-analysis. Front Physiol. 9:3772018. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Wang M and Sun Y: Telomerase reverse
transcriptase rs2736098 polymorphism is associated with lung
cancer: A meta-analysis. J Int Med Res. 48:3000605209361732020.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
He G, Song T, Zhang Y, Chen X, Xiong W,
Chen H, Sun C, Zhao C, Chen Y and Wu H: TERT rs10069690
polymorphism and cancers risk: A meta-analysis. Mol Genet Genomic
Med. 7:e009032019. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Li H, Xu Y, Mei H, Peng L, Li X and Tang
J: The TERT rs2736100 polymorphism increases cancer risk: A
meta-analysis. Oncotarget. 8:38693–38705. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Ma R, Liu C, Lu M, Yuan X, Cheng G, Kong
F, Lu J, Strååt K, Björkholm M, Ma L and Xu D: The TERT locus
genotypes of rs2736100-CC/CA and rs2736098-AA predict shorter
survival in renal cell carcinoma. Urol Oncol. 37:301.e1–301.e10.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Pandith AA, Wani ZA, Qasim I, Afroze D,
Manzoor U, Amin I, Baba SM, Koul A, Anwar I, Mohammad F, et al:
Association of strong risk of hTERT gene polymorphic variants to
malignant glioma and its prognostic implications with respect to
different histological types and survival of glioma cases. J Gene
Med. 22:e32602020. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Dratwa M, Łacina P, Butrym A, Porzuczek D,
Mazur G and Bogunia-Kubik K: Telomere length and hTERT genetic
variants as potential prognostic markers in multiple myeloma. Sci
Rep. 13:157922023. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Zins K, Peka E, Miedl H, Ecker S, Abraham
D and Schreiber M: Association of the telomerase reverse
transcriptase rs10069690 polymorphism with the risk, age at onset
and prognosis of triple negative breast cancer. Int J Mol Sci.
24:18252023. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Nie X, Shang J and Wang W: TERT genetic
polymorphism rs2736100 is associated with an aggressive
manifestation of papillary thyroid carcinoma. Front Surg.
9:10191802023. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Pu RT, Sheng ZM, Michael CW, Rhode MG,
Clark DP and O'Leary TJ: Methylation profiling of mesothelioma
using real-time methylation-specific PCR: A pilot study. Diagn
Cytopathol. 35:498–502. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Guilleret I and Benhattar J: Demethylation
of the human telomerase catalytic subunit (hTERT) gene promoter
reduced hTERT expression and telomerase activity and shortened
telomeres. Exp Cell Res. 289:326–334. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Guilleret I and Benhattar J: Unusual
distribution of DNA methylation within the hTERT CpG island in
tissues and cell lines. Biochem Biophys Res Commun. 325:1037–1043.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Tallet A, Nault JC, Renier A, Hysi I,
Galateau-Sallé F, Cazes A, Copin MC, Hofman P, Andujar P, Le
Pimpec-Barthes F, et al: Overexpression and promoter mutation of
the TERT gene in malignant pleural mesothelioma. Oncogene.
33:3748–3752. 2014. View Article : Google Scholar
|
|
191
|
Campanella NC, Silva EC, Dix G, de Lima
Vazquez F, Escremim de Paula F, Berardinelli GN and Balancin M:
Mutational profiling of driver tumor suppressor and oncogenic genes
in Brazilian malignant pleural mesotheliomas. Pathobiology.
87:208–216. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Pestana A, Vinagre J, Sobrinho-Simões M
and Soares P: TERT biology and function in cancer: Beyond
immortalisation. J Mol Endocrinol. 58:R129–R146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Sato T and Sekido Y: NF2/Merlin
inactivation and potential therapeutic targets in mesothelioma. Int
J Mol Sci. 19:9882018. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Huang DS, Wang Z, He XJ, Diplas BH, Yang
R, Killela PJ, Meng Q, Ye ZY, Wang W, Jiang XT, et al: Recurrent
TERT promoter mutations identified in a large-scale study of
multiple tumour types are associated with increased TERT expression
and telomerase activation. Eur J Cancer. 51:969–976. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Rachakonda PS, Hosen I, de Verdier PJ,
Fallah M, Heidenreich B, Ryk C, Wiklund NP, Steineck G, Schadendorf
D, Hemminki K and Kumar R: TERT promoter mutations in bladder
cancer affect patient survival and disease recurrence through
modification by a common polymorphism. Proc Natl Acad Sci USA.
110:17426–17431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Borah S, Xi L, Zaug AJ, Powell NM, Dancik
GM, Cohen SB, Costello JC, Theodorescu D and Cech TR: Cancer. TERT
promoter mutations and telomerase reactivation in urothelial
cancer. Science. 347:1006–1010. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Lu VM, Goyal A, Lee A, Jentoft M,
Quinones-Hinojosa A and Chaichana KL: The prognostic significance
of TERT promoter mutations in meningioma: A systematic review and
meta-analysis. J Neurooncol. 142:1–10. 2019. View Article : Google Scholar
|
|
198
|
Yang H, Park H, Ryu HJ, Heo J, Kim JS, Oh
YL, Choe JH, Kim JH, Kim JS, Jang HW, et al: Frequency of TERT
promoter mutations in real-world analysis of 2,092 thyroid
carcinoma patients. Endocrinol Metab (Seoul). 37:652–663. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Ramlee MK, Wang J, Toh WX and Li S:
Transcription regulation of the human telomerase reverse
transcriptase (hTERT) Gene. Genes (Basel). 7:502016. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Hannen R and Bartsch JW: Essential roles
of telomerase reverse transcriptase hTERT in cancer stemness and
metastasis. FEBS Lett. 592:2023–2031. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Heidenreich B, Rachakonda PS, Hosen I,
Volz F, Hemminki K, Weyerbrock A and Kumar R: TERT promoter
mutations and telomere length in adult malignant gliomas and
recurrences. Oncotarget. 6:10617–10633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Ivanov SV, Miller J, Lucito R, Tang C,
Ivanova AV, Pei J, Carbone M, Cruz C, Beck A, Webb C, et al:
Genomic events associated with progression of pleural malignant
mesothelioma. Int J Cancer. 124:589–599. 2009. View Article : Google Scholar
|
|
203
|
Chiba K, Lorbeer FK, Shain AH, McSwiggen
DT, Schruf E, Oh A, Ryu J, Darzacq X, Bastian BC and Hockemeyer D:
Mutations in the promoter of the telomerase gene TERT contribute to
tumorigenesis by a two-step mechanism. Science. 357:1416–1420.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Nickerson ML, Dancik GM, Im KM, Edwards
MG, Turan S, Brown J, Ruiz-Rodriguez C, Owens C, Costello JC, Guo
G, et al: Concurrent alterations in TERT, KDM6A, and the BRCA
pathway in bladder cancer. Clin Cancer Res. 20:4935–4948. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Fujimoto A, Furuta M, Shiraishi Y, Gotoh
K, Kawakami Y, Arihiro K, Nakamura T, Ueno M, Ariizumi S, Nguyen
HH, et al: Whole-genome mutational landscape of liver cancers
displaying biliary phenotype reveals hepatitis impact and molecular
diversity. Nat Commun. 6:61202015. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Kwon J, Lee D and Lee SA: BAP1 as a
guardian of genome stability: Implications in human cancer. Exp Mol
Med. 55:745–754. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Carbone M, Adusumilli PS, Alexander HR Jr,
Baas P, Bardelli F, Bononi A, Bueno R, Felley-Bosco E,
Galateau-Salle F, Jablons D, et al: Mesothelioma: Scientific clues
for prevention, diagnosis, and therapy. CA Cancer J Clin.
69:402–429. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Baumann F, Flores E, Napolitano A, Kanodia
S, Taioli E, Pass H, Yang H and Carbone M: Mesothelioma patients
with germline BAP1 mutations have 7-fold improved long-term
survival. Carcinogenesis. 36:76–81. 2015. View Article : Google Scholar :
|
|
209
|
Bueno R, Stawiski EW, Goldstein LD,
Durinck S, De Rienzo A, Modrusan Z, Gnad F, Nguyen TT, Jaiswal BS,
Chirieac LR, et al: Comprehensive genomic analysis of malignant
pleural mesothelioma identifies recurrent mutations, gene fusions
and splicing alterations. Nat Genet. 48:407–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Johanns TM, Fu Y, Kobayashi DK, Mei Y,
Dunn IF, Mao DD, Kim AH and Dunn GP: High incidence of TERT
mutation in brain tumor cell lines. Brain Tumor Pathol. 33:222–227.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Landa I, Ganly I, Chan TA, Mitsutake N,
Matsuse M, Ibrahimpasic T, Ghossein RA and Fagin JA: Frequent
somatic TERT promoter mutations in thyroid cancer: Higher
prevalence in advanced forms of the disease. J Clin Endocrinol
Metab. 98:E1562–E1566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Spiegl-Kreinecker S, Lötsch D, Neumayer K,
Kastler L, Gojo J, Pirker C, Pichler J, Weis S, Kumar R, Webersinke
G, et al: TERT promoter mutations are associated with poor
prognosis and cell immortalization in meningioma. Neuro Oncol.
20:1584–1593. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Zanetti M: A second chance for telomerase
reverse transcriptase in anticancer immunotherapy. Nat Rev Clin
Oncol. 14:115–128. 2017. View Article : Google Scholar
|
|
214
|
Watanabe Y, Kojima T, Kagawa S, Uno F,
Hashimoto Y, Kyo S, Mizuguchi H, Tanaka N, Kawamura H, Ichimaru D,
et al: A novel translational approach for human malignant pleural
mesothelioma: Heparanase-assisted dual virotherapy. Oncogene.
29:1145–1154. 2010. View Article : Google Scholar
|
|
215
|
Sterman DH, Recio A, Vachani A, Sun J,
Cheung L, DeLong P, Amin KM, Litzky LA, Wilson JM, Kaiser LR and
Albelda SM: Long-term follow-up of patients with malignant pleural
mesothelioma receiving high-dose adenovirus herpes simplex
thymidine kinase/ganciclovir suicide gene therapy. Clin Cancer Res.
11:7444–7453. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Molnar-Kimber KL, Sterman DH, Chang M,
Kang EH, ElBash M, Lanuti M, Elshami A, Gelfand K, Wilson JM,
Kaiser LR and Albelda SM: Impact of preexisting and induced humoral
and cellular immune responses in an adenovirus-based gene therapy
phase I clinical trial for localized mesothelioma. Hum Gene Ther.
9:2121–2133. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Sterman DH, Recio A, Carroll RG, Gillespie
CT, Haas A, Vachani A, Kapoor V, Sun J, Hodinka R, Brown JL, et al:
A phase I clinical trial of single-dose intrapleural IFN-beta gene
transfer for malignant pleural mesothelioma and metastatic pleural
effusions: High rate of antitumor immune responses. Clin Cancer
Res. 13(15 Pt 1): 4456–4466. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Kawashima T, Kagawa S, Kobayashi N,
Shirakiya Y, Umeoka T, Teraishi F, Taki M, Kyo S, Tanaka N and
Fujiwara T: Telomerase-specific replication-selective virotherapy
for human cancer. Clin Cancer Res. 10(1 Pt 1): 285–292. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Taki M, Kagawa S, Nishizaki M, Mizuguchi
H, Hayakawa T, Kyo S, Nagai K, Urata Y, Tanaka N and Fujiwara T:
Enhanced oncolysis by a tropism-modified telomerase-specific
replication-selective adenoviral agent OBP-405 ('Telomelysin-RGD').
Oncogene. 24:3130–3140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Lin D, Shen Y and Liang T: Oncolytic
virotherapy: Basic principles, recent advances and future
directions. Sig Transduct Target Ther. 8:1562023. View Article : Google Scholar
|
|
221
|
Nemunaitis J, Tong AW, Nemunaitis M,
Senzer N, Phadke AP, Bedell C, Adams N, Zhang YA, Maples PB, Chen
S, et al: A phase I study of telomerase-specific replication
competent oncolytic adenovirus (telomelysin) for various solid
tumors. Mol Ther. 18:429–434. 2010. View Article : Google Scholar :
|
|
222
|
Kishimoto H, Zhao M, Hayashi K, Urata Y,
Tanaka N, Fujiwara T, Penman S and Hoffman RM: In vivo internal
tumor illumination by telomerase-dependent adenoviral GFP for
precise surgical navigation. Proc Natl Acad Sci USA.
106:14514–14517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Uno F, Fujiwara T, Takata Y, Ohtani S,
Katsuda K, Takaoka M, Ohkawa T, Naomoto Y, Nakajima M and Tanaka N:
Antisense-mediated suppression of human heparanase gene expression
inhibits pleural dissemination of human cancer cells. Cancer Res.
61:7855–7860. 2001.PubMed/NCBI
|
|
224
|
Kiyokawa J, Kawamura Y, Ghouse SM, Acar S,
Barçın E, Martínez-Quintanilla J, Martuza RL, Alemany R, Rabkin SD,
Shah K and Wakimoto H: Modification of extracellular matrix
enhances oncolytic adenovirus immunotherapy in glioblastoma. Clin
Cancer Res. 27:889–902. 2021. View Article : Google Scholar :
|
|
225
|
Eikenes L, Bruland ØS, Brekken C and
Davies Cde L: Collagenase increases the transcapillary pressure
gradient and improves the uptake and distribution of monoclonal
antibodies in human osteosarcoma xenografts. Cancer Res.
64:4768–4773. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
McKenzie EA: Heparanase: A target for drug
discovery in cancer and inflammation. Br J Pharmacol. 151:1–14.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Blackburn JS, Rhodes CH, Coon CI and
Brinckerhoff CE: RNA interference inhibition of matrix
metalloproteinase-1 prevents melanoma metastasis by reducing tumor
collagenase activity and angiogenesis. Cancer Res. 67:10849–10858.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Fitzgerald M, Hayward IP, Thomas AC,
Campbell GR and Campbell JH: Matrix metalloproteinase can
facilitate the heparanase-induced promotion of phenotype change in
vascular smooth muscle cells. Atherosclerosis. 145:97–106. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Shirakawa Y, Tazawa H, Tanabe S, Kanaya N,
Noma K, Koujima T, Kashima H, Kato T, Kuroda S, Kikuchi S, et al:
Phase I dose-escalation study of endoscopic intratumoral injection
of OBP-301 (Telomelysin) with radiotherapy in oesophageal cancer
patients unfit for standard treatments. Eur J Cancer. 153:98–108.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Heo J, Liang JD, Kim CW, Woo HY, Shih IL,
Su TH, Lin ZZ, Yoo SY, Chang S, Urata Y and Chen PJ: Safety and
dose escalation of the targeted oncolytic adenovirus OBP-301 for
refractory advanced liver cancer: Phase I clinical trial. Mol Ther.
31:2077–2088. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Yamada N, Oizumi S, Kikuchi E, Shinagawa
N, Konishi-Sakakibara J, Ishimine A, Aoe K, Gemba K, Kishimoto T,
Torigoe T and Nishimura M: CD8+ tumor-infiltrating lymphocytes
predict favorable prognosis in malignant pleural mesothelioma after
resection. Cancer Immunol Immunother. 59:1543–1549. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Ranki T, Joensuu T, Jäger E, Karbach J,
Wahle C, Kairemo K, Alanko T, Partanen K, Turkki R, Linder N, et
al: Local treatment of a pleural mesothelioma tumor with ONCOS-102
induces a systemic antitumor CD8+ T-cell response,
prominent infiltration of CD8+ lymphocytes and Th1 type
polarization. OncoImmunology. 3:e9589372014. View Article : Google Scholar
|
|
233
|
Alley EW, Lopez J, Santoro A, Morosky A,
Saraf S, Piperdi B and van Brummelen E: Clinical safety and
activity of pembrolizumab in patients with malignant pleural
mesothelioma (KEYNOTE-028): Preliminary results from a
non-randomised, open-label, phase 1b trial. Lancet Oncol.
18:623–630. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Quispel-Janssen J, van der Noort V, de
Vries JF, Zimmerman M, Lalezari F, Thunnissen E, Monkhorst K,
Schouten R, Schunselaar L, Disselhorst M, et al: Programmed death 1
blockade with nivolumab in patients with recurrent malignant
pleural mesothelioma. J Thorac Oncol. 13:1569–1576. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Sharma P, Hu-Lieskovan S, Wargo JA and
Ribas A: Primary, adaptive, and acquired resistance to cancer
immunotherapy. Cell. 168:707–723. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Wang L, Geng H, Liu Y, Liu L, Chen Y, Wu
F, Liu Z, Ling S, Wang Y and Zhou L: Hot and cold tumors:
Immunological features and the therapeutic strategies. MedComm
(2020). 4:e3432023. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Harber J, Kamata T, Pritchard C and
Fennell D: Matter of TIME: The tumor-immune microenvironment of
mesothelioma and implications for checkpoint blockade efficacy. J
Immunother Cancer. 9:e0030322021. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Nasti TH and Eberhardt CS: Vaccination
against cancer or infectious agents during checkpoint inhibitor
therapy. Vaccines (Basel). 9:13962021. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Negrini S, De Palma R and Filaci G:
Anti-cancer immunotherapies targeting telomerase. Cancers (Basel).
12:22602020. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Middleton G, Silcocks P, Cox T, Valle J,
Wadsley J, Propper D, Coxon F, Ross P, Madhusudan S, Roques T, et
al: Gemcitabine and capecitabine with or without telomerase peptide
vaccine GV1001 in patients with locally advanced or metastatic
pancreatic cancer (TeloVac): An open-label, randomised, phase 3
trial. Lancet Oncol. 15:829–840. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Hunger RE, Kernland Lang K, Markowski CJ,
Trachsel S, Møller M, Eriksen JA, Rasmussen AM, Braathen LR and
Gaudernack G: Vaccination of patients with cutaneous melanoma with
telomerase-specific peptides. Cancer Immunol Immunother.
60:1553–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Collins JM, Redman JM and Gulley JL:
Combining vaccines and immune checkpoint inhibitors to prime,
expand, and facilitate effective tumor immunotherapy. Expert Rev
Vaccines. 17:697–705. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Fan T, Zhang M, Yang J, Zhu Z, Cao W and
Dong C: Therapeutic cancer vaccines: Advancements, challenges and
prospects. Sig Transduct Target Ther. 8:4502023. View Article : Google Scholar
|
|
244
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 515:568–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Haakensen VD, Nowak AK, Ellingsen EB,
Farooqi SJ, Bjaanæs MM, Horndalsveen H, Mcculloch T, Grundberg O,
Cedres SM and Helland Å: NIPU: A randomised, open-label, phase II
study evaluating nivolumab and ipilimumab combined with UV1
vaccination as second line treatment in patients with malignant
mesothelioma. J Transl Med. 19:2322021. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Lorigan P, Medina T, Nyakas M, Rutten A,
Feun LG, Cowey CL, Payne M, Hussain I, Kuze T, O'Day S, et al:
Ipilimumab and nivolumab plus UV1, an anticancer vaccination
against telomerase, in advanced melanoma. J Chin Oncol.
42(17_suppl): LBA95192024.
|
|
247
|
Buchbinder EI and Desai A: CTLA-4 and PD-1
pathways: Similarities, differences, and implications of their
inhibition. Am J Clin Oncol. 39:98–106. 2016. View Article : Google Scholar :
|
|
248
|
Wei SC, Sharma R, Anang NAS, Levine JH,
Zhao Y, Mancuso JJ, Setty M, Sharma P, Wang J, Pe'er D and Allison
JP: Negative Co-stimulation constrains T cell differentiation by
imposing boundaries on possible cell states. Immunity.
50:1084–1098.e10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Wei SC, Levine JH, Cogdill AP, Zhao Y,
Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe'er D and
Allison JP: Distinct cellular mechanisms underlie Anti-CTLA-4 and
Anti-PD-1 checkpoint blockade. Cell. 170:1120–1133.e17. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Lilleby W, Gaudernack G, Brunsvig PF,
Vlatkovic L, Schulz M, Mills K, Hole KH and Inderberg EM: Phase
I/IIa clinical trial of a novel hTERT peptide vaccine in men with
metastatic hormone-naive prostate cancer. Cancer Immunol
Immunother. 66:891–901. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Brunsvig PF, Guren TK, Nyakas M,
Steinfeldt-Reisse CH, Rasch W, Kyte JA, Juul HV, Aamdal S,
Gaudernack G and Inderberg EM: Long-term outcomes of a phase I
study with UV1, a second generation telomerase based vaccine, in
patients with advanced non-small cell lung cancer. Front Immunol.
11:5721722020. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Aamdal E, Jacobsen KD, Straume O, Kersten
C, Herlofsen O, Karlsen J, Hussain I, Amundsen A, Dalhaug A, Nyakas
M, et al: Ipilimumab in a real-world population: A prospective
phase IV trial with long-term follow-up. Int J Cancer. 150:100–111.
2022. View Article : Google Scholar
|
|
253
|
Labby ZE, Straus C, Caligiuri P, MacMahon
H, Li P, Funaki A, Kindler HL and Armato SG III: Variability of
tumor area measurements for response assessment in malignant
pleural mesothelioma. Med Phys. 40:0819162013. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Thunold S, Hernes E, Farooqi S, Öjlert ÅK,
Francis RJ, Nowak AK, Szejniuk WM, Nielsen SS, Cedres S, Perdigo
MS, et al: Outcome prediction based on [18F]FDG PET/CT in patients
with pleural mesothelioma treated with ipilimumab and nivolumab +/−
UV1 telomerase vaccine. Eur J Nucl Med Mol Imaging. 52:693–707.
2025. View Article : Google Scholar
|
|
255
|
Creff G, Devillers A, Depeursinge A,
Palard-Novello X, Acosta O, Jegoux F and Castelli J: Evaluation of
the prognostic value of FDG PET/CT parameters for patients with
surgically treated head and neck cancer: A systematic review. JAMA
Otolaryngol Head Neck Surg. 146:471–479. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Im HJ, Oo S, Jung W, Jang JY, Kim SW,
Cheon GJ, Kang KW, Chung JK, Kim EE and Lee DS: Prognostic value of
metabolic and volumetric parameters of preoperative FDG-PET/CT in
patients with resectable pancreatic cancer. Medicine (Baltimore).
95:e36862016. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Yenigün BM, Kahya Y, Soydal Ç, Ata Tutkun
N, Kocaman G, Koçak EM, Özkan E, Dizbay Sak S and Kayı Cangır A:
The prognostic value of 18F-fluorodeoxyglucose positron emission
tomography/computed tomography parameters in patients with
malignant pleural mesothelioma. Turk Gogus Kalp Damar Cerrahisi
Derg. 29:92–100. 2021. View Article : Google Scholar
|
|
258
|
Bagchi S, Yuan R and Engleman EG: Immune
checkpoint inhibitors for the treatment of cancer: Clinical impact
and mechanisms of response and resistance. Annu Rev Pathol.
16:223–249. 2021. View Article : Google Scholar
|