According to the latest global statistics, 1,926,118
patients were diagnosed with colorectal cancer (CRC) and 903,859
patients died of CRC; CRC is the third most commonly diagnosed
cancer and the second leading cause of cancer-associated death
(1). Notably, nearly half of
patients with CRC are either diagnosed with metastatic CRC at the
outset or develop metastases during the course of the illness. As
the most frequent metastatic site, liver metastasis worsens
prognosis, with <20% of patients with colorectal liver
metastasis surviving >5 years (2). Furthermore, CRC with liver
metastases exhibits decreased antitumor immunity and efficacy of
immunotherapy (3,4). Understanding the regulatory
mechanisms governing each stage of colorectal liver metastasis is
key for developing strategies to disrupt the metastatic
cascade.
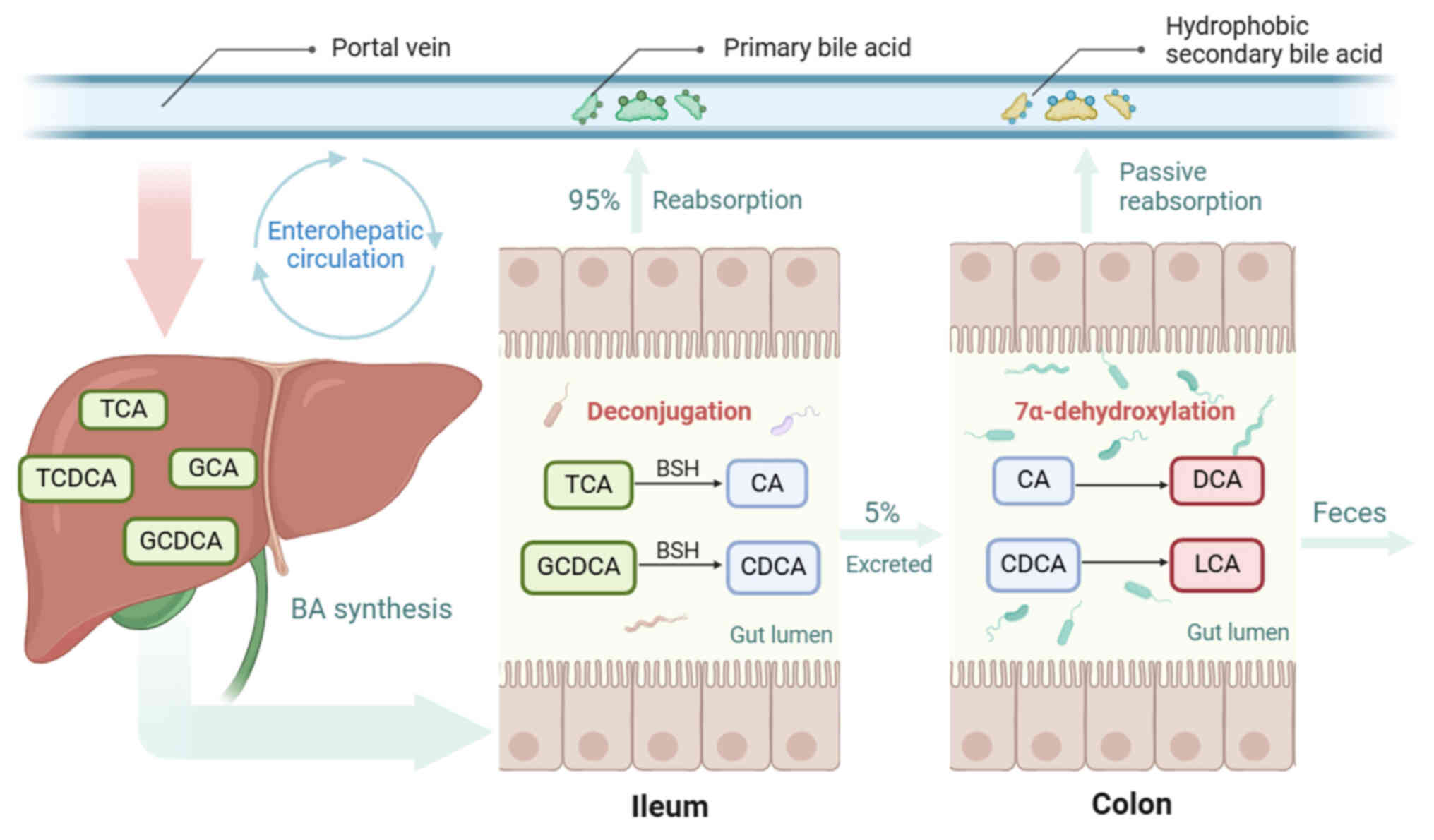
Bile acid (BA) is a cholesterol metabolite that
emulsifies lipids to facilitate digestion and absorption. BA is
classified into primary and secondary BA based on the
dehydroxylation at C7 (Table I).
Primary BAs, including cholic acid (CA) and chenodeoxycholic acid
(CDCA), are initially synthesized in the liver and conjugated with
taurine or glycine. These conjugated primary BAs are stored in the
gallbladder and released into the duodenum upon ingestion. During
their journey through the small intestine, most primary BAs undergo
deconjugation, mediated by bile salt hydrolase, which is harbored
by the gut microbiota Bacteroidetes, Firmicutes and
Actinobacteria (5). The
deconjugated BAs are reabsorbed to replenish the liver BA pool via
enterohepatic circulation. A total of ~5% of primary BAs escape
reabsorption and enter the colon. The gut microbiota, especially
Clostridium and Eubacterium, use 7α-dehydroxylation to
transform CA and CDCA into secondary BAs deoxycholic acid (DCA) and
lithocholic acid (LCA). This endows BAs with hydrophobic
properties, enabling secondary BAs to be passively absorbed into
the portal vein and enter the liver (6,7).
The enterohepatic circulation transports intestinal metabolites
into the liver and constitutes the anatomical basis of the
gut-liver axis (Fig. 1).
Therefore, the intestinal and hepatic BA pools interact
dynamically.
BAs are natural ligands of receptors, including the
farnesoid X receptor (FXR), Takeda G protein-coupled receptor 5
(TGR5), the vitamin D3 receptor (VDR), sphingosine-1-phosphate
receptor 2 (S1PR2) and retinoid-related orphan receptor-γt (RORγt).
Most BA receptors are involved in the regulation of gut-associated
inflammation (8) and glucose and
lipid metabolism (9).
Additionally, by interacting with BA receptors, BAs can modulate
the functional state of immune cells, thereby influencing host
immunity (6). In recent years,
increasing evidence has implicated BAs in tumor development
(10-12). BAs stimulate the pro-inflammatory
signaling in the intestine and facilitate acquisition of a
malignant phenotype (13).
Previous reviews have clarified the association between BA
metabolism and colorectal carcinogenesis (14-16). However, a comprehensive review
summarizing the role of BAs in the progression of CRC remains
lacking. In addition, the immunomodulatory effects of BAs are
primarily discussed in the context of inflammatory bowel disease,
with a focus on intestinal mucosal immunity (17). To the best of our knowledge, few
reviews have concluded the involvement of BAs in regulating the
antitumor immunity (18,19), particularly within the gut-liver
axis. The present review aimed to explore the regulatory role of
BAs at each stage of the colorectal metastatic cascade, from tumor
invasion to impairment of antitumor immunity, to provide a
foundation for development of novel therapeutic strategies for
treating colorectal liver metastasis.
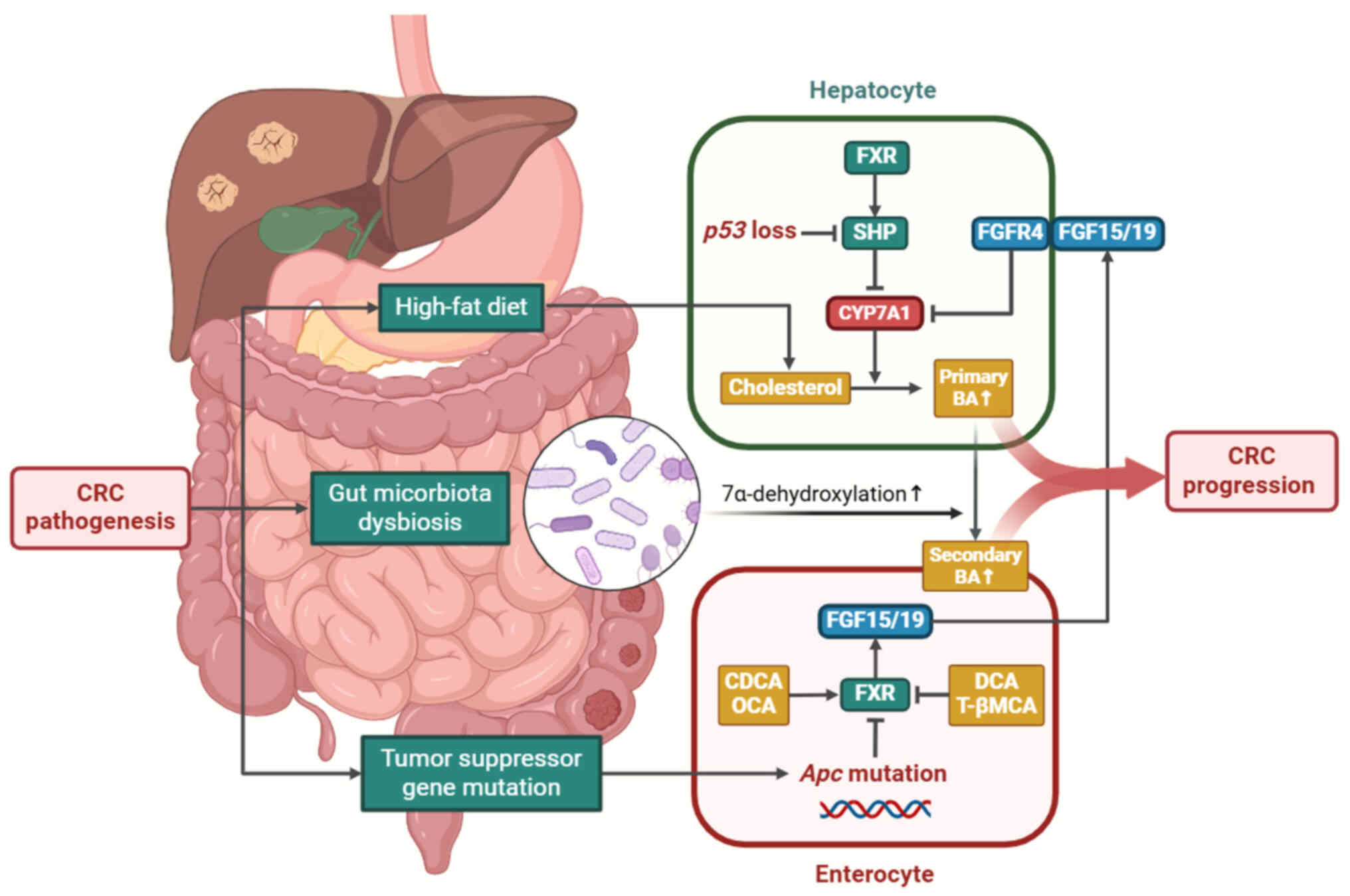
CRC is associated with an augmented BA pool and
increased proportions of secondary BAs in both serum and feces
(20,21). This is likely due to long-term
adoption of a high-fat diet in populations at a high risk of CRC.
Individuals who adopt a high-fat diet acquire ample cholesterol for
BA synthesis, resulting in increased BAs that escape into the
colon, where primary BAs are converted to secondary BAs (22,23). A study confirmed that diets high
in fat and low in fiber lead to higher levels of intestinal BAs in
African Americans than in rural Africans who follow a low-fat diet
(24).
In addition, gut microbiota in patients with CRC
exhibits active catalytic activity for 7α-dehydroxylation, the key
step in producing secondary BAs (25-28). In vitro cultures of
bacteria derived from human CRC tissues have a stronger capacity
for DCA production than those from normal mucosa (25). A meta-analysis of fecal
metagenomes indicated that bacteria containing the BA-inducible
gene operon, which encodes enzymes for 7α-dehydroxylation, are
enriched in patients with CRC (26). Clostridium species catalyze
7α-dehydroxylation (27).
Abundance of Clostridium symbiosum is significantly
increased in the feces of patients with CRC (28), confirming the oncogenic properties
of secondary BAs. A recent study (29) also highlighted the
pro-carcinogenic role of the increased abundance of
Bacteroides in the feces of patients with CRC, which
contains bile salt hydrolase and facilitates BA deconjugation.
Accumulated deconjugated DCA and LCA dampen antitumor immunity in
patients with CRC (29). These
findings suggest that alterations in the intestinal microbiome
during CRC pathogenesis enhance the production of unconjugated
secondary BA.
Furthermore, mutations in tumor suppressor genes are
involved in BA metabolism by regulating the FXR-SHP axis. Under
physiological conditions, FXR activation in enterocytes triggers
fibroblast growth factor (FGF) secretion into enterohepatic
circulation, which inhibits expression of cholesterol
7α-hydroxylase (CYP7A1) by binding to hepatic membrane receptor FGF
receptor (FGFR)4, thereby limiting BA synthesis (30). Specific suppression of FXR in the
intestinal epithelium of mice has been shown to result in increased
DCA excretion in feces (31).
However, FXR expression is downregulated in patients with CRC and
is inversely associated with tumor stage (32). Adenomatous polyposis coli (Apc)
mutations occur in ~85% of patients with CRC, resulting in
intestinal FXR inactivation (33). Knockdown of Apc in mice silences
FXR by stimulating CpG methylation in both tumor tissue and normal
colonic mucosa (34), increasing
total serum BAs (33). In
addition, the tumor suppressor p53, which is mutated in almost half
of patients with CRC, is key for BA homeostasis. Kim and Lee
reported that p53 significantly decreases BA synthesis in mice by
upregulating SHP expression, which directly inhibits the function
of CYP7A1 (35).
Collectively, a high-fat diet, intestinal dysbiosis
and tumor suppressor gene mutations contribute to aberrant BA
metabolism and expanded BA pool (Fig.
2).
Circulating BA levels serve as indicators of CRC
risk. A prospective study reported that high pre-diagnostic
concentrations of most conjugated BAs in serum are positively
associated with an increased risk of colon cancer (36). Unconjugated DCA and
glycolithocholic acid (GLCA) show no significant associations with
colon cancer risk (36). This
contradicts previous reports supporting the toxicity and
carcinogenicity of secondary BAs (37,38). Secondary BAs have
anti-inflammatory effects, and a decreased ratio of fecal secondary
to primary BA may facilitate colitis-associated cancer progression
(39). Therefore, secondary BAs
serve a dual role in colorectal carcinogenesis. Investigating the
optimal secondary BA levels to attenuate systemic inflammation
without triggering cytotoxicity is important in clinical
practice.
Moreover, accumulated BAs in serum and tumor tissues
are associated with poor prognosis and predict early recurrence in
postoperative patients with CRC. A retrospective analysis
demonstrated that increased total serum BA levels are associated
with lower overall survival (OS) and disease-free survival rates in
patients with CRC (40). Notably,
patients with high total BA levels have significantly higher TNM
stages than those with low total BA levels. The proportion of
patients with TNM stage IV CRC in patients with high total BA is
almost twice as large as that in patients with low total BA,
implying a possible role for BAs in distant metastasis of CRC
(40). In addition, the total BA
and systemic immune-inflammation index exhibit good predictive
capacity for early recurrence of CRC after surgical resection
(41). Targeted liquid
chromatography-mass spectrometry enables precise measurement of BA
abundance in tumor tissue. It has been shown that a high ratio of
glycoursodeoxycholic acid (GUDCA) to ursodeoxycholic acid (UDCA) in
colon tumor tissue is positively associated with shorter 5-year OS
and a high ratio of glycochenodeoxycholic acid (GCDCA) to CDCA is
associated with shortened 5-year recurrence-free survival (RFS)
(42). Alterations in fecal BA
composition also warrant attention, given that BAs in feces are in
direct contact with colonic mucosa and can directly regulate the
gut inflammation (43). However,
whether BAs in plasma and feces have different effects on CRC
prognosis requires further investigation.
The distribution of BAs within the colon shows
spatial specificity. A study on a cohort of patients with CRC
(stages I-III) found that the levels of most BA species are
elevated in tumor lesions from right-sided colon cancer (RCC)
compared with those in left-sided colon cancer (LCC) (42). Furthermore, Morris et al
(44) reported that BAs are more
abundant in liver metastasis samples from patients with RCC than
those from patients with LCC. Metastatic liver RCC typically
features more metastatic lesions, a more extensively involved
segment and a poorer 5-year OS than LCC (45,46). However, whether a biased
distribution of BAs contributes to metastatic features of RCC
remains unclear. Lee et al (47) suggested that metastatic tumor
cells may be capable of producing BAs to support malignant
behavior. Further investigating the mechanism by which metastatic
tumor cells generate BAs could provide valuable insight into
targeting the colorectal liver metastasis.
Taken together, serum BAs are implicated in
tumorigenesis and progression of CRC, and the abundance of BAs in
CRC tumor tissue varies between different tumor locations (Table II). Although no prospective
cohort studies have been conducted on the impact of BA levels on
liver metastasis, experiments in mice have demonstrated that
pathologically increased BA levels facilitate liver metastasis
(48,49).
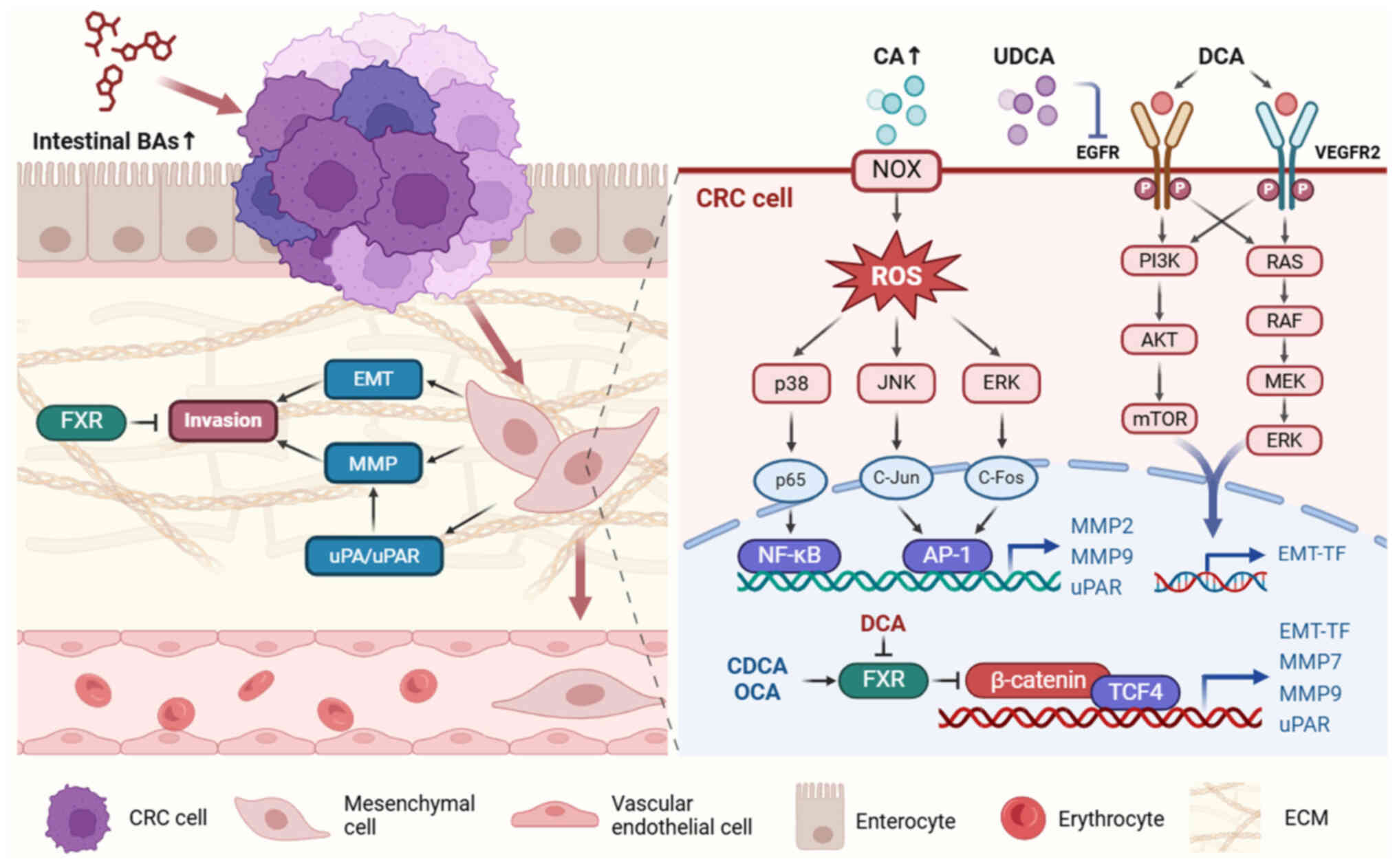
Invasion is a hallmark of tumor malignancy and the
initial step of the metastatic cascade (50). BAs have been shown to stimulate
CRC cell invasion. Treatment with pharmacological inhibitors
indicates that BA-induced invasion involves MAPK, PI3K, PKC, COX2
and Rho/ROCK signaling cascades (51). The invasion and migration of CRC
cells rely on acquisition of a mesenchymal phenotype and
degradation of extracellular matrix (ECM), which is mediated by
MMPs and urokinase-type plasminogen activators (uPAs).
EMT is a cellular program involved in embryonic
development and wound healing and is used by tumor cells to promote
invasion and metastasis (52).
Activation of EMT in tumor cells results in loss of apical-basal
polarity and intercellular adhesion, endowing them with mobility
and dissemination ability (52).
A recent study demonstrated that high-fat diet (HFD)-induced DCA
accumulation facilitates EMT in Apcmin/+ mice, indicated
by downregulation of E-cadherin and upregulation of vimentin and
fibronectin expression in intestinal tumor tissue (53). DCA exposure activates vascular
endothelial growth factor receptor 2 (VEGFR2) in intestinal tumors
of mice, resulting in expression of the EMT-inducing transcription
factors ZEB1 and ZEB2 (53).
Receptor tyrosine kinases, such as VEGFR and epithelial growth
factor receptor (EGFR), serve key roles in proliferation and
migration of CRC cells. The PI3K/AKT/mTOR and RAS/RAF/MEK/ERK
signaling pathways, which are typically downstream of receptor
tyrosine kinases, are implicated in BA-induced CRC invasion
(54,55). DCA activates EGFR in CRC cells,
whereas ursodeoxycholic acid (UDCA) inhibits EGFR (56). UDCA has also been reported to
antagonize EGFR/MAPK signaling and abrogate EGF-induced EMT in bile
duct cancer cells (57); however,
its protective role in regulating EMT in CRC cells remains
unclear.
FXR, a BA receptor predominantly distributed in the
liver and intestine, has been shown to suppress tumor invasion
(58). Decreased FXR expression
in CRC tumor tissues is associated with poor prognosis (59). FXR activation by obeticholic acid
(OCA) suppresses the EMT process, compromising CRC invasion and
migration (60), whereas FXR
inhibition potentiates CRC invasion both in vitro and in
vivo (58). The underlying
mechanism involves FXR interaction with β-catenin to disable the
transcriptional activity of the β-catenin/transcriptional factor 4
complex (58), which mediates the
expression of key EMT transcription factors (52). In addition, DCA suppresses FXR
expression, activating Wnt/β-catenin signaling and promoting the
EMT process (12,58), further demonstrating the role of
secondary BAs in inducing invasiveness of CRC cells.
Tumor cells undergoing EMT generally acquire a
hybrid phenotype between epithelial and mesenchymal states, which
enables them to function as cancer stem cells and increase
metastatic efficacy (52).
Farhana et al (61)
demonstrated that incubating human colonic epithelial cells with
100 μM DCA or LCA for 7 days is sufficient to induce cancer
stem cell transformation, with concomitantly increased expression
of EMT-inducing transcription factors, including SLUG, ZEB2 and
TWIST.
The MMP family of zinc-dependent endopeptidases can
break down ECM components, creating a pathway for tumor cells to
invade underlying stroma. MMPs serve key roles in CRC metastasis by
regulating proteinases, cellular receptors, growth factors and
chemokines (62). MMPs are also
regulated by BAs and, therefore, affect CRC progression. CA
treatment results in an MMP-9-dependent increase in the
invasiveness of human colon SW620 cells (63). Li et al (63) reported that CA-activated NADPH
oxidase generates reactive oxygen species (ROS). p38 MAPK, ERK 1/2
and JNK 1/2, as the downstream signaling pathways of ROS, are
activated and induce NF-κB and AP-1 DNA binding. As a result,
expression of MMP-9 is notably enhanced (63). By contrast, activating FXR with
CDCA or OCA notably suppresses MMP-7 and MMP-9 expression and
inhibits CRC invasion (60,64). Additionally, Halvorsen et
al (65) demonstrated that
LCA, but not sulfated LCA, treatment increases MMP-2 secretion in
CaCo-2 cells in vitro, suggesting intestinal
microbiota-mediated BA sulfation may help regulate CRC
invasion.
The interaction between uPA and uPA receptor (uPAR)
indirectly promotes ECM degradation and tumor invasion via MMP
activation (66). BA-mediated
tumor invasion is achieved by stimulating the expression of uPA and
uPAR. A previous study showed that low DCA concentrations notably
enhance invasiveness of CRC cells by stimulating uPAR expression
(67). Incubating SW480 and LoVo
cell lines with low concentrations of DCA for 30 min facilitates
tyrosine phosphorylation and nuclear translocation of β-catenin,
which directly regulates uPAR expression and is associated with
invasion and migration of CRC cells (67). Baek et al (68) found that LCA facilitates SW620
cell invasiveness by upregulating uPAR in an AP-1-dependent manner.
This may involve MAPK signaling, as ERK 1/2 and p38 inhibitors
block LCA-triggered uPAR upregulation (68). BAs, especially secondary BAs,
promote invasiveness of CRC cells by initiating pro-inflammatory
and oncogenic signaling pathways (Fig. 3).
Tumor-associated angiogenesis is a key step in
hematogenous dissemination, as demonstrated by colorectal liver
metastasis (69).
Neovascularization around the primary tumor renders CRC cells more
likely to enter circulation. Angiogenesis in the pre-metastatic
niche provides CRC cells with nutrients and oxygen to sustain
development (69). Song et
al (53) reported that oral
administration of DCA in Apcmin/+ mice induces more
vasculogenic mimicry channel formation around tumor lesions than in
the control group. This effect is blocked by VEGFR2 inactivation,
suggesting that DCA facilitates angiogenesis via the
VEGFR2-mediated signaling pathway in CRC (53). Previous studies have shown that
LCA stimulates CRC cells to secrete IL-8 in vitro, which is
associated with angiogenesis, and endothelial cells treated with
LCA exhibit an IL-8-dependent increase in tube formation (70,71). By contrast, LCA inhibits
IL-1β-induced IL-8 production via interaction with VDR in colon
cancer cells (72). However, the
exact role of BAs in IL-8 expression in patients with CRC requires
further investigation. In addition, cyclooxygenase-2 and its
product, prostaglandin E2, are implicated in angiogenesis and
associated with VEGF expression and microvessel density in CRC
tissue (73). Studies have shown
that LCA treatment promotes COX-2 expression in CRC cells by
activating peroxisome proliferator-activated receptor α (74), whereas UDCA downregulates
cyclooxygenase-2 expression by inhibiting p38 MAPK activation in
CRC cells (75). These results
suggest the potential of BAs to induce CRC angiogenesis. To the
best of our knowledge, however, evidence concerning the direct
angiogenic effect of BAs is lacking, and the underlying mechanisms
require further investigation.
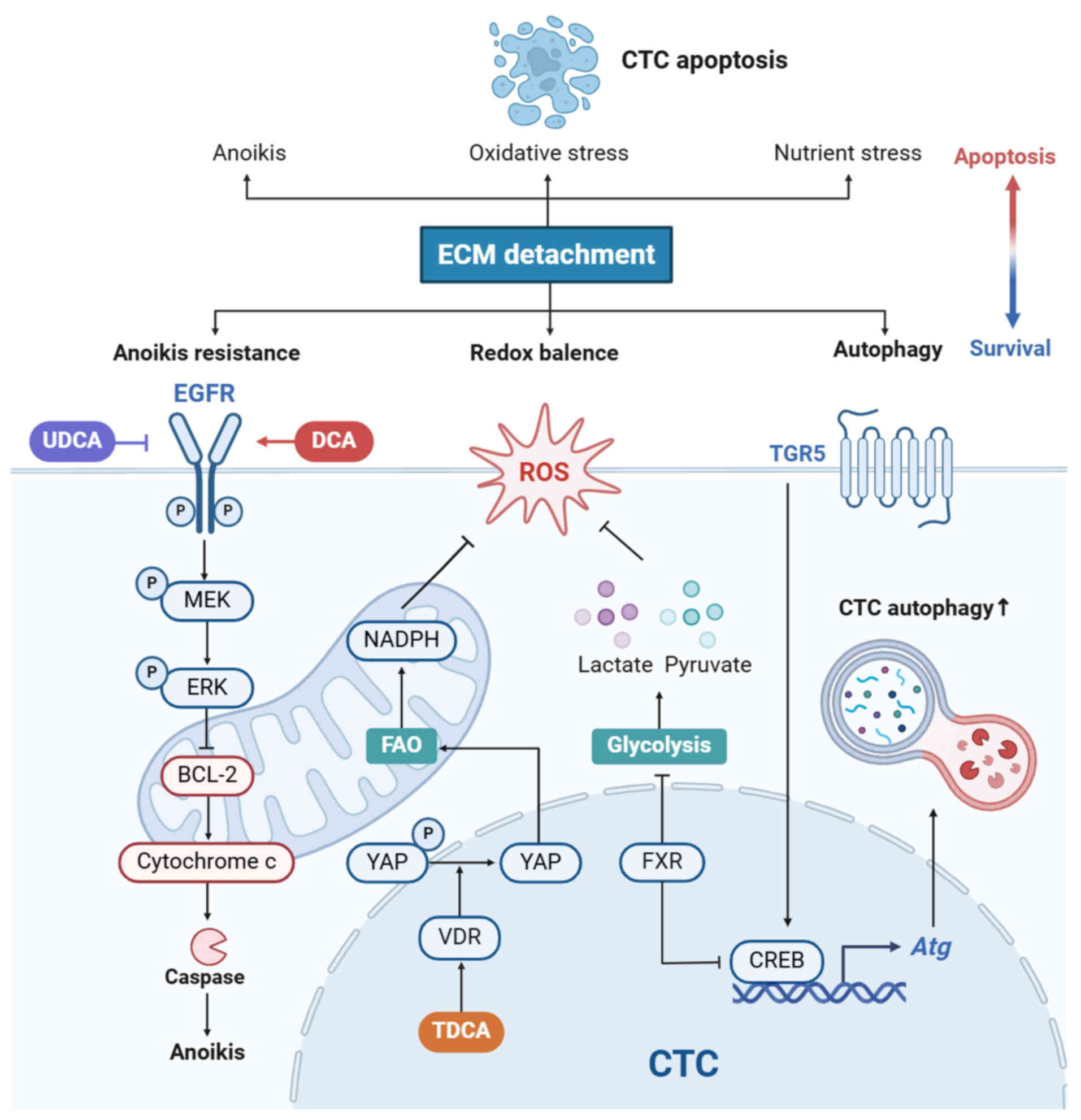
During the metastatic cascade, CTCs that lose
attachment to ECM) are subject to multiple forms of cell death.
Redox imbalance, nutrient stress and anoikis compromise cell
viability and hinder CTC survival (76,77). BAs can rectify defects and shield
CTCs from cell death.
Metabolic characteristics of CTCs influence their
metastatic processes. CTCs remodel metabolism to resist oxidative
stress, adapt to specific demands of different organs and enhance
their survival and metastatic potential (78). Upregulation of fatty acid
oxidation (FAO) promotes CTC survival by increasing NADPH
generation, which enhances defense against ROS and rebalances the
redox state of CTCs (78). The
blockade of FAO aggravates oxidative stress in detached CRC cells
and diminishes distant metastasis (79). BAs trigger tumor metastasis by
affecting FAO. Lee et al (47) demonstrated that in mice,
taurodeoxycholic acid (TDCA) potentiates FAO in tumor cells via YAP
signaling and facilitates lymph node metastasis. Metastatic tumor
cells in lymph nodes can generate TDCA in an autocrine manner,
which then activates VDR to facilitate translocation of YAP into
the nucleus (47). Therefore, it
is hypothesized that high levels of FAO, which are controlled by
BAs, contribute to oxidative stress resistance in CTCs (47). In addition, aerobic glycolysis,
also known as the Warburg effect, blocks pyruvate from the
tricarboxylic acid cycle, bypassing mitochondrial oxidative
phosphorylation-induced ROS generation (80). Lactate and pyruvate production
serve a role in the elimination of ROS (80). A recent study revealed that
silencing FXR expression potentiates aerobic glycolysis in human
colon cancer cells (81), which
may favor survival during detachment from the ECM. Collectively,
BAs promote the metabolic transformation of CTCs towards FAO and
aerobic glycolysis, thereby controlling oxidative stress.
Due to loss of EGFR and β1 integrin during ECM
detachment, CTCs undergo anoikis, a caspase-dependent programmed
cell death. The activation of ERK signaling diminishes BCL-2
activity, which triggers apoptosis by inducing cytochrome c release
(76). Given the considerable
ability of DCA to stimulate EGFR/MAPK signaling, increased
circulating DCA may promote anoikis resistance in CTCs. By
contrast, UDCA facilitates the ubiquitination and degradation of
EGFR in CRC cells, indicating an unfavorable effect on CTC survival
(56). However, these hypotheses
require further evidence. High DCA concentrations trigger ROS
generation and induce cancer cell apoptosis (56), suggesting that both the species
and dose of BAs affect CTC survival and should be considered in
future studies. Collectively, BAs may promote survival of CTCs in
circulation by facilitating metabolic adaptation, autophagy and
anoikis resistance (Fig. 4).
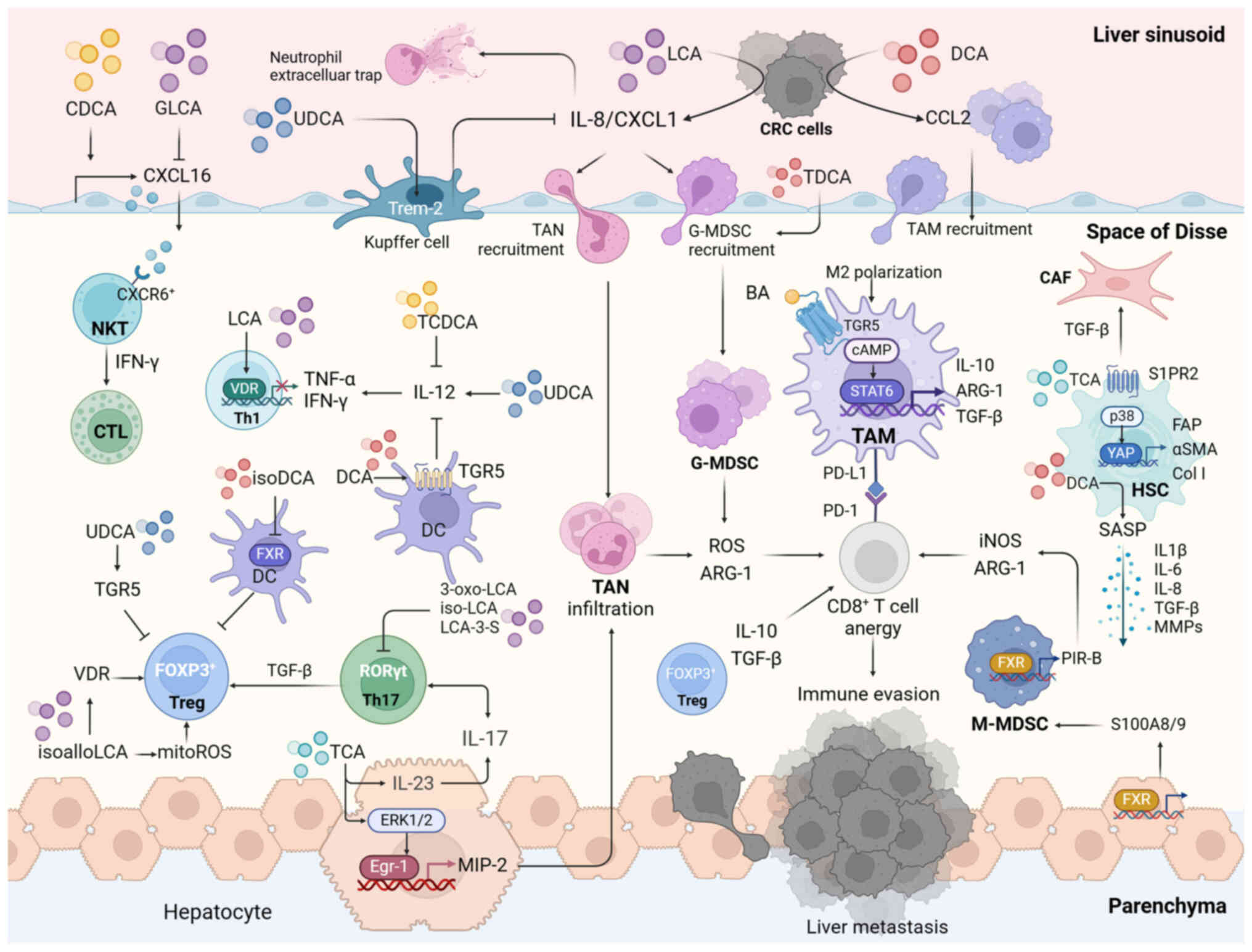
To survive anti-tumor immunity and seed in the
liver, CRC cells downregulate their immunogenicity and recruit
immunosuppressive cells to construct a compatible environment that
favors colonization (85). Many
studies (29,86-89) have focused on the role of BAs in
regulating anti-tumor immunity and reshaping the tumor
microenvironment (TME) (Fig. 5).
Kawarabayashi et al (48)
showed that elevated serum and hepatic BAs levels in bile
duct-ligated (BDL) mice facilitates liver metastasis. A recent
study in mice suggested that taurocholic acid (TCA) expansion
facilitates colorectal liver metastasis by creating a hepatic
immunosuppressive microenvironment (49). These studies provide evidence of
the association between BAs and hepatic pre-metastatic niche
construction. A variety of immune cells serve a role in colorectal
liver metastasis (90). The tumor
microenvironment is complex and it is difficult to pinpoint a
single cell type responsible for metastasis (90,91). It is likely that BAs reshape the
tumor microenvironment by simultaneously regulating the function of
diverse immune cells (Table
III).
TAMs that are extensively present in the TME are
primarily derived from circulating monocytes, which are recruited
by pro-inflammatory signaling molecules, such as CCL2, CCL5 and
colony stimulating factor 1 (CSF1). Based on features of the TME,
macrophages polarize into two subsets: Anti-tumor M1 and pro-tumor
M2. M1 TAMs exert tumoricidal activity by releasing ROS and NO,
whereas M2 TAMs trigger anergy of cytotoxic T lymphocytes (CTLs) by
competitively consuming L-arginine or recruiting immunosuppressive
cells (92). M2 TAMs promote
colorectal liver metastasis by creating a hepatic pre-metastatic
niche (93).
In addition to activating BA receptors, BAs control
phenotypic conversion by regulating macrophage metabolism. Shao
et al (98) demonstrated
that LCA-treated macrophages undergo metabolic transformation from
glycolysis to oxidative phosphorylation, thereby skewing
polarization of macrophages towards the M2 phenotype. This suggests
that accumulated BAs induce M2 TAM polarization by altering the
metabolic pattern in macrophages, in addition to interacting with
BA receptors.
Secondary BAs induce chronic intestinal inflammation
and drive the recruitment of TAMs. Cao et al (99) discovered that BA-induced
alterations in gut microbiota are involved in TAM accumulation.
Feeding DCA changes the proportion of intestinal bacteria in mice,
indicated by increased abundance of Clostridium and a reduced
abundance of butyrate-producing bacteria (99). Transferring fecal microbiota from
DCA-treated to Apcmin/+ mice results in low-grade
intestinal inflammation, leading to an increase in infiltration of
M2 TAMs by amplifying CCL2 signaling (99). This suggests that in addition to
polarization, BAs facilitate monocyte trafficking from the blood
and educate them to immunosuppressive M2 TAMs by regulating the gut
microbiota.
TANs recruited to the TME attain a pro-tumor
phenotype, contributing to colorectal liver metastasis by impairing
anti-tumor immunity, ECM remodulation and angiogenesis (100). In addition, TANs can facilitate
the adhesion and intravasation of circulating CRC cells by
extruding neutrophil extracellular traps (101). A multivariate analysis of
patients with CRC showed that an elevated neutrophil-to-lymphocyte
ratio is associated with liver metastasis and poor overall survival
(102).
CXCL1 is a potent chemoattractant of neutrophils.
UDCA may ameliorate cholestasis-induced neutrophil infiltration by
upregulating expression of Trem-2, which negatively regulates CXCL1
expression in Kupffer cells (104). Pathological accumulation of BAs
in the liver is associated with hepatic inflammation and neutrophil
recruitment in colon cancer cachexia (105). Treatment with cholestyramine or
anti-IL-6 can relieve hepatic neutrophil accumulation (105). These findings indicate an
association between BA-triggered inflammation and increased levels
of hepatic neutrophils and that neutrophil accumulation during
cholestasis may drive colorectal liver metastasis.
MDSCs are a subset of immature monocytes and
granulocytes that express CD11b and Ly-6G, respectively. MDSCs are
recruited by TME-derived pro-inflammatory signaling and contribute
to metastatic CRC (106).
Similarly to TAMs and TANs, MDSCs facilitate tumor evasion, with
granulocytic MDSCs (G-MDSCs) generating ROS and monocytic MDSCs
(M-MDSCs) producing arginase and iNOS (106). Depleting G-MDSCs in transgenic
mice significantly decreases colorectal liver metastasis by
restoring antitumor immunity (107).
Intraperitoneal injection of TCA increases the
proportion of liver-infiltrating MDSCs in mice and facilitates
experimental colorectal liver metastasis (49). This may involve FXR activation, as
the intraperitoneal injection of FXR agonist induces
CD11b+ Ly6Chigh MDSC infiltration by
increasing S100A8 and S100A9 expression in the liver (49). Furthermore, FXR activation
triggers paired immunoglobulin receptor-b expression, which
potentiates immunosuppressive function of MDSCs (108). In mice, the intravenous
injection of TDCA increases levels of splenic MDSCs during sepsis
(109). Following TDCA
treatment, purified splenic MDSCs display an amplified ROS-mediated
immune regulatory capacity similar to that of G-MDSCs (109). Furthermore, IL-8 exerts potent
chemoattractant activity on both neutrophils and MDSCs and induces
neutrophil extracellular trap extrusion by TANs or G-MDSCs to
shield circulating tumor cells from attack (110). A large-scale retrospective
analysis of patients with advanced cancer revealed that IL-8 levels
in the serum are associated with tumor-infiltrating
immunosuppressive myeloid cells and serve as a circulating
biomarker of the therapeutic effects of immune checkpoint blockade
(111). LCA treatment increases
IL-8 expression in CRC cells. This effect is dependent on
suppression of STAT3 phosphorylation, which follows LCA-induced
ERK1/2 activation (71). These
findings suggest that the BA-induced increase in MDSCs could be
attributed to hepatic FXR activation and IL-8 release following
secondary BA-triggered inflammation.
DCs are essential for tumor-adaptive immunity.
Conventional DCs transport and present tumor antigens in draining
lymph nodes to activate T cells via costimulatory signaling
(112). However, treatment with
BAs compromises the antigen-presenting function of DCs. The
secondary BA 3β-hydroxydeoxycholic acid (isoDCA) antagonizes FXR in
DCs and suppresses the transcriptional activity of genes associated
with antigen presentation. Consequently, despite the presence of
DCs, isoDCA exposure inhibits T cell proliferation and increases
the frequency of peripherally induced regulatory T cells (Tregs)
(113). DCA confers an
anti-inflammatory phenotype to DCs via TGR5/cAMP/PKA signaling. By
activating TGR5, DCA treatment decreases the expression of IL-1β,
IL-6, tumor necrosis factor-α (TNF-α), IL-12p70 and co-stimulatory
molecules, such as CD40 and CD86, in bone marrow-derived DCs
(BMDCs) (114). Furthermore,
LCA-treated murine BMDCs undergo TGR5-dependent apoptosis and
autophagy (115). With impaired
glutathione production and increased ROS accumulation, LCA-treated
BMDCs downregulate the expression of proinflammatory cytokines,
resulting in inhibition of T helper (Th)1 and Th17 cell
differentiation and a decrease in IFN-γ and IL-17 secretion
(115). IL-12 participate in Th1
cell polarization and IFN-γ secretion (116). Taurochenodeoxycholic acid
decreases IL-12 secretion in DCs via the TGR5/cAMP signaling
pathway (117), whereas
UDCA-treated BMDCs restore release of IL-12 in a
concentration-dependent manner (118). These results demonstrate that
BAs inhibit adaptive antitumor immunity by impairing the
antigen-presenting capacity of DCs.
By contrast, high expression of Th17 cluster genes
and levels of IL-17A cells in tumor tissue are associated with poor
CRC prognosis (124). Th17 cells
can secrete TWEAK, a cytokine that induces epithelial-mesenchymal
transition of CRC cells, to facilitate colorectal liver metastasis
(125). Isolithocholic acid,
3-oxolithocholic acid and lithocholic acid 3-sulfate have affinity
for the key transcription factor RORγt. They physically interact
with RORγt and inhibit its transcriptional activity, thereby
inhibiting Th17 cell differentiation and IL-17A expression
(126-128). TCAs, which accumulate during
cholestasis, mediate Th17 cell infiltration into the liver.
Additionally, TCA promotes IL-23 production by hepatocytes, which
stimulates IL-17A secretion by Th17 cells (103). IL-17A facilitates IL-23
expression in hepatocytes, thereby forming a positive feedback loop
in which IL-17 and IL-23 are mutually activated (103). IL-17-enriched environment may be
associated with immune suppression as elevated hepatic IL-17A
increases mobilization and recruitment of G-MDSC (129) and Th17 cells can
transdifferentiate into Tregs in a TGF-β-enriched environment
(130). These results indicate
that Th17 cell overload during cholestasis may lead to inflammatory
immunosuppression in the TME and is of predictive value in response
to anti-PD-1 immunotherapy (131,132).
Tregs, the predominant source of IL-10 in the TME,
serve a dual role in CRC progression (133). In the colon, a higher frequency
of tumor-infiltrating FOXP3+ Tregs and IL-10 levels
predict better prognosis in patients with CRC (134), potentially due to suppression of
Th17-mediated inflammation (135). By contrast, Sun et al
(29) showed that
immunosuppressive Tregs recruited to colon tumors promote CRC
progression. In addition, hepatic Treg-derived IL-10 is key in
liver metastasis. It contributes to the systemic upregulation of
PD-L1 in monocytes (133), which
may explain liver metastasis-mediated CD8+ T cell anergy
and immunotherapy resistance (7).
NKT cells exert tumoricidal activity, reeducate the
TME and acquire immunogenic properties (139). BA metabolism, modulated by
intestinal bacteria, affects CXCR6+NKT cells. BAs affect
NKT cell recruitment by regulating expression of CXCL16 in liver
sinusoidal endothelial cells (LSECs), the only known ligands for
CXCR6 (86). In vivo
experiments suggest feeding mice primary BAs facilitates CXCL16
expression and NKT cell accumulation, whereas feeding mice
secondary BAs reverses this effect (86). Consistent with these results, a
study of a liver cancer cohort showed that CDCA is positively
associated with CXCL16 expression in human non-tumor liver tissue,
whereas GLCA is inversely associated (86). Targeting Clostridium, which
facilitates the conversion of primary BAs into secondary BAs,
enhances hepatic NKT cell infiltration as well as their IFN-γ
production (86). Based on these
results and the ability of LSECs to capture nanoparticles in
circulation, Ji et al (140) developed an OCA-nanoemulsion
using an ultrasonic emulsification method for drug delivery. This
nanoemulsion successfully increases expression of CXCL16 in LSECs
and the accumulation of NKT cells, with higher secretion of IFN-γ
in tumors of mice (140). Cheng
et al (141) used a mouse
metastasis model to demonstrate that long-term capsaicin-containing
diet can contribute to the hepatic pre-metastatic niche,
characterized by decreased levels of NKT cells and accumulated
neutrophils and monocytes, by increasing secondary BAs generated by
the gut microbiota. Oral treatment with OCA abolishes this effect
and decreases liver metastasis (141). These results indicate that the
intestinal microbiota serves a key role in regulating antitumor
immunity, with BAs acting as a bridge for communication.
HSCs are perisinusoidal non-parenchymal liver cells
located in the space of Disse. They are the primary precursors of
cancer-associated fibroblasts and facilitate colorectal liver
metastasis (142). Primed HSCs
contribute to tumor immune evasion by stimulating Treg expansion
(142). Additionally, activated
HSCs facilitate angiogenesis and form a fibrotic environment that
promotes CTC adhesion, favoring hepatic disseminated CRC cell
colonization (143,144). Targeting HSCs may enhance
efficacy of antiangiogenic therapy in patients with colorectal
liver metastasis (145).
BAs can stimulate the activation of HSC. Under
cholestatic conditions, mast cells are recruited to the liver to
activate HSCs (146). Incubation
with TCA upregulates the expression of fibroblast activation
protein, α-smooth muscle actin and collagen I in LX2 cells
(human-originated HSCs) in a dose-dependent manner (146). Similar results are observed in
the livers of mice treated with TCA in vivo (49). TCA-induced HSC activation is
controlled by S1PR2/p38/MAPK/YAP signaling; JTE-013, an S1PR2
inhibitor, inhibits this TCA-mediated effect (147). Unconjugated secondary BAs
exhibit greater efficiency in inducing primed HSCs than conjugated
primary BAs, which involve TNF and NF-κB signaling activation
(148). DCA treatment of LX2
cells triggers the secretion of the senescence-associated secretory
phenotype factors and significantly upregulates expression of
IL-1β, IL-6, IL-8, CXCL1, TGF-β, MMP-2, MMP-7 and MMP-9 (149). These findings indicate the
potential role of HSCs in promoting liver metastasis by reshaping
the hepatic pre-metastatic niche; however, evidence regarding the
function of HSCs in CRC is lacking, and the specific regulatory
mechanisms require further exploration.
The NLRP3 inflammasome is a cytoplasmic multimeric
protein complex that amplifies inflammation via caspase-1-dependent
IL-1β and IL-18 secretion (150). According to a recent study,
targeting NLRP3 signaling diminishes PMN-MDSC recruitment, thereby
inhibiting pre-metastatic niche formation (150). BAs, as danger-associated
molecular patterns, participate in NLRP3 inflammasome activation.
Increased CDCA levels during cholestasis activate the NLRP3
inflammasome in macrophages and potentiate cholestasis-associated
liver inflammation (151). By
contrast, FXR may rescue cholestasis-triggered inflammation because
it impairs NLRP3 assembly by physically interacting with its
components (152). DCA activates
the NLRP3 inflammasome via S1PR2-mediated cathepsin B release
independent of FXR or TGR5 (153). The role of BAs in stimulating
NLRP3 is further demonstrated in vivo. NLRP3 was
significantly activated in the liver of mice fed with LCA (154). However, Guo et al
(155) suggested that BAs,
especially LCA and TLCA, suppress NLRP3 activation in macrophages
by facilitating NLRP3 ubiquitination via the TGR5-cAMP-PKA axis and
decreasing IL-1β expression. This discrepancy may be due to the
addition of LPS and NLRP3 agonist nigericin. Given the crucial role
of proinflammatory signaling in immature myeloid cell recruitment,
the effect of NLRP3 on the pre-metastatic niche warrants further
exploration.
Additionally, the FXR agonist GW4064 has been shown
to upregulate PD-L1 expression in CRC cells, thereby potentiating
the efficacy of PD-L1 immune checkpoint blockade in CRC treatment
(159). However, despite
promising preclinical results, concerns regarding systemic use of
FXR agonists persist due to their potential side effects (156). FXR agonists induce hepatocyte
apoptosis by impairing mitochondrial function (160). Therefore, designing FXR agonists
with higher tissue or cell specificity (161) and developing drug delivery
systems to target tumor lesions (140,162) is important.
UDCA improves BA homeostasis by facilitating BA
secretion and excretion and is used for cholestasis treatment
(163). Previous studies have
confirmed that UDCA influences the fecal microbiota composition and
reduces the risk of colorectal adenoma (164,165). In a mouse model of DSS-induced
colitis, UDCA alleviates colonic inflammation and prevents
colitis-associated cancer (165). By stimulating TGR5, UDCA
inhibits the Hippo/YAP signaling pathway in CRC cells, thereby
suppressing malignant progression of CRC (166). UDCA treatment induces anti-tumor
immunity in CRC; this effect is more potent when UDCA is combined
with an anti-PD treatment (87).
Previous studies have elucidated the protective role of UDCA in CRC
carcinogenesis (75,164,167); to the best of our knowledge,
however, few (87) have focused
on the association between UDCA and antitumor immunity in patients
with CRC. The discovery that UDCA enhances the efficacy of
immunotherapy provides new avenues for CRC treatment. As patients
with liver metastasis are more likely to develop cholestasis and
are refractory to immunotherapy, such as anti-PD treatment
(89), the addition of UDCA to
therapeutics may be beneficial.
Recently, the gut microbiota associated with BA
metabolism has gained increasing attention in CRC development
research (6,168,169). Clostridium amplification,
which catalyzes the 7α-dehydroxylation pathway, contributes to
elevated secondary BA levels and facilitates CRC progression
(170). Vancomycin, neomycin,
and primaxin decrease liver metastasis by impeding secondary BA
production (86). Non-absorbable
antibiotic treatment has the same effect of suppressing liver
metastasis of CRC as classic antibiotic cocktail treatment
(171), with fewer systemic side
effects, making it a promising candidate for clinical use.
Moreover, a recent study demonstrated that withdrawal of
broad-spectrum antibiotics increases the abundance of E.
clostridioformis and secondary BA production, compromising the
therapeutic response to PD-1 blockade (172). This suggests that targeting
harmful gut bacteria and maintaining BA homeostasis are conducive
to enhancing the immunotherapy response in treating CRC.
Fecal microbiota transplantation (FMT) is the most
direct method for altering gut microbiome composition to improve
prognosis of patients with CRC (173). Clinical research has confirmed
that FMT protects patients who receive immune checkpoint inhibitor
(ICI) treatment for ICI-associated colitis (174). In addition, the abundance of
Akkermansia municiphila is associated with active response
to ICI therapy (174,175). Transplanting A.
municiphila into unresponsive patients can restore sensitivity
to immunotherapy. However, the safety of FMT remains controversial
(174). Adverse events include
fever, vomiting, infection, relapse of inflammatory bowel disease
and C. difficile infection (176). Further randomized controlled
trials are required before FMT can be widely used in clinical
practice.
The key role of intestinal microbiota in CRC
development is gaining increasing attention (6). As one of the primary metabolites of
gut bacteria, BAs drive CRC metastasis and may be predictive
biomarkers for colorectal liver metastasis (89). The present review summarizes the
specific roles of BAs in regulating tumor invasion, angiogenesis,
anoikis and immune evasion. Mechanistically, BAs serve as signaling
molecules to induce inflammation by activating transcription
factors such as NF-κB, AP-1 and STAT3 (43). In addition, pathologically
accumulated hydrophobic BAs cause cell membrane perturbations and
trigger the release of large amounts of pro-inflammatory mediators
(43). Tumor cells harness these
pro-inflammatory signaling pathways to facilitate malignant
behaviors. Inflammation in the TME triggers trafficking of immune
cells, which are reeducated to acquire immunosuppressive properties
with the help of Bas (18).
Additionally, BA exerts modulatory effects on metabolism of CRC
cells and macrophages by interacting with BA receptors (177). Given the key role of metabolism
in antitumor immunity, the mechanisms by which BAs serve as
metabolic mediators facilitating liver metastasis warrant further
exploration.
BA metabolism disorders, such as cholestasis, are
one of the most common complications in patients with liver
metastasis (178). Accumulated
BAs contribute to the hepatic colonization of tumor cells.
Secondary BAs in feces play an anti-inflammatory role in
colitis-associated CRC (179),
whereas secondary BAs in serum can be transported into the liver
and induce an immunosuppressive pre-metastatic niche (86). Therapeutic agents that can reduce
the reabsorption of secondary BA in the colon may hold promise for
treatment of CRC. Therefore, the prognostic value of fecal and
serum BA levels needs to be clarified in patients with colorectal
liver metastases.
Clinicians should consider BAs when managing
patients with advanced-stage CRC. As aforementioned, elevated serum
levels of total BAs are associated with lower OS and RFS and an
increased incidence of distant metastasis (40). High levels of BAs, particularly
secondary BAs, may impair antitumor immunity and compromise
efficacy of immunotherapy (86).
Therefore, in patients with elevated serum BAs, use of BA synthesis
inhibitors such as OCA and BA secretion promoters such as UDCA may
improve OS and enhance immunotherapeutic outcomes (87,89). Furthermore, expression of BA
receptors in CRC tissue has prognostic implications. Given the
tumor-suppressive role of FXR (159,161,180) and tumor-promoting role of TGR5
(166), immunohistochemical
analysis of these receptors may provide value for CRC
prognosis.
In conclusion, the present review summarizes the
role of BAs in promotion of liver metastasis, as well as promising
therapeutic agents. Future research should focus on the regulatory
mechanisms of BA synthesis and metabolism to develop more
approaches to rebalance BA homeostasis and inhibit colorectal liver
metastasis.
Not applicable.
LS conceived the study and edited the manuscript.
ZL performed the literature review and wrote the manuscript. LD, MC
and XY performed the literature review. NY and ZF revised the
manuscript. Data authentication is not applicable. All authors have
read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by National Natural Science
Foundation of China (grant no. 81972292), Project of Guangzhou
Science and Technology (grant no. 202102020076) and Beijing Natural
Science Foundation (7242139).
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Biller LH and Schrag D: Diagnosis and
treatment of metastatic colorectal cancer: A review. JAMA.
325:669–685. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu J, Green MD, Li S, Sun Y, Journey SN,
Choi JE, Rizvi SM, Qin A, Waninger JJ, Lang X, et al: Liver
metastasis restrains immunotherapy efficacy via macrophage-mediated
T cell elimination. Nat Med. 27:152–164. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JC, Mehdizadeh S, Smith J, Young A,
Mufazalov IA, Mowery CT, Daud A and Bluestone JA: Regulatory T cell
control of systemic immunity and immunotherapy response in liver
metastasis. Sci Immunol. 5:eaba07592020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones BV, Begley M, Hill C, Gahan CG and
Marchesi JR: Functional and comparative metagenomic analysis of
bile salt hydrolase activity in the human gut microbiome. Proc Natl
Acad Sci USA. 105:13580–13585. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ridlon JM and Gaskins HR: Another
renaissance for bile acid gastrointestinal microbiology. Nat Rev
Gastroenterol Hepatol. 21:348–364. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Russell DW: The enzymes, regulation, and
genetics of bile acid synthesis. Annu Rev Biochem. 72:137–174.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thibaut MM and Bindels LB: Crosstalk
between bile acid-activated receptors and microbiome in
entero-hepatic inflammation. Trends Mol Med. 28:223–236. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wahlstrom A, Sayin SI, Marschall HU and
Backhed F: Intestinal crosstalk between bile acids and microbiota
and its impact on host metabolism. Cell Metab. 24:41–50. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hao Z, Liu X, He H, Wei Z, Shu X, Wang J,
Sun B, Zhou H, Wang J, Niu Y, et al: CYP2E1 deficit mediates cholic
acid-induced malignant growth in hepatocellular carcinoma cells.
Mol Med. 30:792024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen W, Ding M, Ji L, Yao J, Guo Y, Yan W,
Yu S, Shen Q, Huang M, Zheng Y, et al: Bile acids promote the
development of HCC by activating inflammasome. Hepatol Commun.
7:e02172023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao Y, Li X, Xu B, Luo L, Guo Q, Wang X,
Sun L, Zhang Z and Li P: Cholecystectomy promotes colon
carcinogenesis by activating the Wnt signaling pathway by
increasing the deoxycholic acid level. Cell Commun Signal.
20:712022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sánchez B: Bile acid-microbiota crosstalk
in gastrointestinal inflammation and carcinogenesis: A role for
bifidobacteria and lactobacilli? Nat Rev Gastroenterol Hepatol.
15:2052018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Režen T, Rozman D, Kovács T, Kovács P,
Sipos A, Bai P and Mikó E: The role of bile acids in
carcinogenesis. Cell Mol Life Sci. 79:2432022. View Article : Google Scholar
|
|
15
|
Liu Y, Zhang S, Zhou W, Hu D, Xu H and Ji
G: secondary bile acids and tumorigenesis in colorectal cancer.
Front Oncol. 12:8137452022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Caliceti C, Punzo A, Silla A, Simoni P,
Roda G and Hrelia S: New insights into bile acids related signaling
pathways in the onset of colorectal cancer. Nutrients. 14:29642022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai J, Sun L and Gonzalez FJ: Gut
microbiota-derived bile acids in intestinal immunity, inflammation,
and tumorigenesis. Cell Host Microbe. 30:289–300. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sipe LM, Chaib M, Pingili AK, Pierre JF
and Makowski L: Microbiome, bile acids, and obesity: How
microbially modified metabolites shape anti-tumor immunity. Immunol
Rev. 295:220–239. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Zhu N, Su X and Yang R: Gut
microbiota: A double-edged sword in immune checkpoint blockade
immunotherapy against tumors. Cancer Lett. 582:2165822024.
View Article : Google Scholar
|
|
20
|
Imray CH, Radley S, Davis A, Barker G,
Hendrickse CW, Donovan IA, Lawson AM, Baker PR and Neoptolemos JP:
Faecal unconjugated bile acids in patients with colorectal cancer
or polyps. Gut. 33:1239–1245. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bayerdörffer E, Mannes GA, Ochsenkühn T,
Dirschedl P, Wiebecke B and Paumgartner G: Unconjugated secondary
bile acids in the serum of patients with colorectal adenomas. Gut.
36:268–273. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dermadi D, Valo S, Ollila S, Soliymani R,
Sipari N, Pussila M, Sarantaus L, Linden J, Baumann M and Nyström
M: Western diet deregulates bile acid homeostasis, cell
proliferation, and tumorigenesis in colon. Cancer Res.
77:3352–3363. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ocvirk S and O'Keefe SJD: Dietary fat,
bile acid metabolism and colorectal cancer. Semin Cancer Biol.
73:347–355. 2021. View Article : Google Scholar
|
|
24
|
O'Keefe SJ, Li JV, Lahti L, Ou J,
Carbonero F, Mohammed K, Posma JM, Kinross J, Wahl E, Ruder E, et
al: Fat, fibre and cancer risk in African Americans and rural
Africans. Nat Commun. 6:63422015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma Y, Zhang Y, Qu R, Zhou X, Sun L, Wang
K, Jiang C, Zhang Z and Fu W: Promotion of deoxycholic acid effect
on colonic cancer cell lines in vitro by altering the mucosal
microbiota. Microorganisms. 10:24862022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wirbel J, Pyl PT, Kartal E, Zych K,
Kashani A, Milanese A, Fleck JS, Voigt AY, Palleja A, Ponnudurai R,
et al: Meta-analysis of fecal metagenomes reveals global microbial
signatures that are specific for colorectal cancer. Nat Med.
25:679–689. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ridlon JM, Harris SC, Bhowmik S, Kang DJ
and Hylemon PB: Consequences of bile salt biotransformations by
intestinal bacteria. Gut Microbes. 7:22–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xie YH, Gao QY, Cai GX, Sun XM, Sun XM,
Zou TH, Chen HM, Yu SY, Qiu YW, Gu WQ, et al: Fecal clostridium
symbiosum for noninvasive detection of early and advanced
colorectal cancer: Test and validation studies. EBioMedicine.
25:32–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun L, Zhang Y, Cai J, Rimal B, Rocha ER,
Coleman JP, Zhang C, Nichols RG, Luo Y, Kim B, et al: Bile salt
hydrolase in non-enterotoxigenic Bacteroides potentiates colorectal
cancer. Nat Commun. 14:7552023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Inagaki T, Choi M, Moschetta A, Peng L,
Cummins CL, McDonald JG, Luo G, Jones SA, Goodwi n B, Richardson
JA, et al: Fibroblast growth factor 15 functions as an
enterohepatic signal to regulate bile acid homeostasis. Cell Metab.
2:217–225. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim I, Ahn SH, Inagaki T, Choi M, Ito S,
Guo GL, Kliewer SA and Gonzalez FJ: Differential regulation of bile
acid homeostasis by the farnesoid X receptor in liver and
intestine. J Lipid Res. 48:2664–2672. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lax S, Schauer G, Prein K, Kapitan M,
Silbert D, Berghold A, Berger A and Trauner M: Expression of the
nuclear bile acid receptor/farnesoid X receptor is reduced in human
colon carcinoma compared to nonneoplastic mucosa independent from
site and may be associated with adverse prognosis. Int J Cancer.
130:2232–2239. 2012. View Article : Google Scholar
|
|
33
|
Fu T, Coulter S, Yoshihara E, Oh TG, Fang
S, Cayabyab F, Zhu Q, Zhang T, Leblanc M, Liu S, et al: FXR
regulates intestinal cancer stem cell proliferation. Cell.
176:1098–1112 e18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Selmin OI, Fang C, Lyon AM, Doetschman TC,
Thompson PA, Martinez JD, Smith JW, Lance PM and Romagnolo DF:
Inactivation of adenomatous polyposis coli reduces bile
acid/farnesoid X receptor expression through Fxr gene CpG
methylation in mouse colon tumors and human colon cancer cells. J
Nutr. 146:236–242. 2016. View Article : Google Scholar
|
|
35
|
Kim DH and Lee JW: Tumor suppressor p53
regulates bile acid homeostasis via small heterodimer partner. Proc
Natl Acad Sci USA. 108:12266–12270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kühn T, Stepien M, López-Nogueroles M,
Damms-Machado A, Sookthai D, Johnson T, Roca M, Hüsing A, Maldonado
SG, Cross AJ, et al: Prediagnostic plasma bile acid levels and
colon cancer risk: A prospective study. J Natl Cancer Inst.
112:516–524. 2020. View Article : Google Scholar :
|
|
37
|
Ajouz H, Mukherji D and Shamseddine A:
Secondary bile acids: An underrecognized cause of colon cancer.
World J Surg Oncol. 12:1642014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zeng H, Claycombe KJ and Reindl KM:
Butyrate and deoxycholic acid play common and distinct roles in
HCT116 human colon cell proliferation. J Nutr Biochem.
26:1022–1028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu L, Yang M, Dong W, Liu T, Song X, Gu
Y, Wang S, Liu Y, Abla Z, Qiao X, et al: Gut dysbiosis and abnormal
bile acid metabolism in colitis-associated cancer. Gastroenterol
Res Pract. 2021:66459702021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cao Y, Deng S, Yan L, Gu J, Yang J, Yang
M, Liu L and Cai K: A nomogram based on pretreatment levels of
serum bilirubin and total bile acid levels predicts survival in
colorectal cancer patients. BMC Cancer. 21:852021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tang S, Chen Y, Tian S and Wang Y:
Predictive nomogram for the prediction of early recurrence of
colorectal cancer. Int J Gen Med. 14:4857–4866. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cai Y, Shen X, Lu L, Yan H, Huang H, Gaule
P, Muca E, Theriot CM, Rattray Z, Rattray NJW, et al: Bile acid
distributions, sex-specificity, and prognosis in colorectal cancer.
Biol Sex Differ. 13:612022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jia W, Xie G and Jia W: Bile
acid-microbiota crosstalk in gastrointestinal inflammation and
carcinogenesis. Nat Rev Gastroenterol Hepatol. 15:111–128. 2018.
View Article : Google Scholar
|
|
44
|
Morris MT, Jain A, Sun B, Kurbatov V, Muca
E, Zeng Z, Jin Y, Roper J, Lu J, Paty PB, et al: Multi-omic
analysis reveals metabolic pathways that characterize right-sided
colon cancer liver metastasis. Cancer Lett. 574:2163842023.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Engstrand J, Nilsson H, Strömberg C, Jonas
E and Freedman J: Colorectal cancer liver metastases-a
population-based study on incidence, management and survival. BMC
Cancer. 18:782018. View Article : Google Scholar
|
|
46
|
Liu W, Wang HW, Wang K and Xing BC: The
primary tumor location impacts survival outcome of colorectal liver
metastases after hepatic resection: A systematic review and
meta-analysis. Eur J Surg Oncol. 45:1349–1356. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee CK, Jeong SH, Jang C, Bae H, Kim YH,
Park I, Kim SK and Koh GY: Tumor metastasis to lymph nodes requires
YAP-dependent metabolic adaptation. Science. 363:644–649. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kawarabayashi N, Seki S, Hatsuse K,
Kinoshita M, Takigawa T, Tsujimoto H, Kawabata T, Nakashima H,
Shono S and Mochizuki H: Immunosuppression in the livers of mice
with obstructive jaundice participates in their susceptibility to
bacterial infection and tumor metastasis. Shock. 33:500–506. 2010.
View Article : Google Scholar
|
|
49
|
Zheng Z, Wei J, Hou X, Jia F, Zhang Z, Guo
H, Yuan F, He F, Ke Z, Wang Y and Zhao L: A high hepatic uptake of
conjugated bile acids promotes colorectal cancer-associated liver
metastasis. Cells. 11:38102022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lambert AW, Pattabiraman DR and Weinberg
RA: Emerging biological principles of metastasis. Cell.
168:670–691. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Debruyne PR, Bruyneel EA, Karaguni IM, Li
X, Flatau G, Müller O, Zimber A, Gespach C and Mareel MM: Bile
acids stimulate invasion and haptotaxis in human colorectal cancer
cells through activation of multiple oncogenic signaling pathways.
Oncogene. 21:6740–6750. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dongre A and Weinberg RA: New insights
into the mechanisms of epithelial-mesenchymal transition and
implications for cancer. Nat Rev Mol Cell Biol. 20:69–84. 2019.
View Article : Google Scholar
|
|
53
|
Song X, An Y, Chen D, Zhang W, Wu X, Li C,
Wang S, Dong W, Wang B, Liu T, et al: Microbial metabolite
deoxycholic acid promotes vasculogenic mimicry formation in
intestinal carcinogenesis. Cancer Sci. 113:459–477. 2022.
View Article : Google Scholar :
|
|
54
|
Stefani C, Miricescu D, Stanescu-Spinu II,
Nica RI, Greabu M, Totan AR and Jinga M: Growth factors,
PI3K/AKT/mTOR and MAPK signaling pathways in colorectal cancer
pathogenesis: Where are we now? Int J Mol Sci. 22:102602021.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Centuori SM, Gomes CJ, Trujillo J, Borg J,
Brownlee J, Putnam CW and Martinez JD: Deoxycholic acid mediates
non-canonical EGFR-MAPK activation through the induction of calcium
signaling in colon cancer cells. Biochim Biophys Acta.
1861:663–670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Centuori SM and Martinez JD: Differential
regulation of EGFR-MAPK signaling by deoxycholic acid (DCA) and
ursodeoxycholic acid (UDCA) in colon cancer. Dig Dis Sci.
59:2367–2380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lee J, Hong EM, Kim JH, Kim JH, Jung JH,
Park SW and Koh DH: Ursodeoxycholic acid inhibits
epithelial-mesenchymal transition, suppressing invasiveness of bile
duct cancer cells: An in vitro study. Oncol Lett. 24:4482022.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yu J, Li S, Guo J, Xu Z, Zheng J and Sun
X: Farnesoid X receptor antagonizes Wnt/β-catenin signaling in
colorectal tumorigenesis. Cell Death Dis. 11:6402020. View Article : Google Scholar
|
|
59
|
Zhang D, Weng S, Cui C, Dong L and Shen X:
Decreased expression of farnesoid X receptor may indicate poor
prognosis in patients with colorectal cancer. Transl Cancer Res.
9:4290–4296. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li S, Xu Z, Guo J, Zheng J, Sun X and Yu
J: Farnesoid X receptor activation induces antitumour activity in
colorectal cancer by suppressing JAK2/STAT3 signalling via
transactivation of SOCS3 gene. J Cell Mol Med. 24:14549–14560.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Farhana L, Nangia-Makker P, Arbit E,
Shango K, Sarkar S, Mahmud H, Hadden T, Yu Y and Majumdar AP: Bile
acid: A potential inducer of colon cancer stem cells. Stem Cell Res
Ther. 7:1812016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Pezeshkian Z, Nobili S, Peyravian N,
Shojaee B, Nazari H, Soleimani H, Asadzadeh-Aghdaei H, Ashrafian
Bonab M, Nazemalhosseini-Mojarad E and Mini E: Insights into the
role of matrix metalloproteinases in precancerous conditions and in
colorectal cancer. Cancers (Basel). 13:62262021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li S, Ung TT, Nguyen TT, Sah DK, Park SY
and Jung YD: Cholic acid stimulates MMP-9 in human colon cancer
cells via activation of MAPK, AP-1, and NF-κB activity. Int J Mol
Sci. 21:34202020. View Article : Google Scholar
|
|
64
|
Peng Z, Chen J, Drachenberg CB, Raufman JP
and Xie G: Farnesoid X receptor represses matrix metalloproteinase
7 expression, revealing this regulatory axis as a promising
therapeutic target in colon cancer. J Biol Chem. 294:8529–8542.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Halvorsen B, Staff AC, Ligaarden S, Prydz
K and Kolset SO: Lithocholic acid and sulphated lithocholic acid
differ in the ability to promote matrix metalloproteinase secretion
in the human colon cancer cell line CaCo-2. Biochem J. 349(Pt 1):
189–193. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dass K, Ahmad A, Azmi AS, Sarkar SH and
Sarkar FH: Evolving role of uPA/uPAR system in human cancers.
Cancer Treat Rev. 34:122–136. 2008. View Article : Google Scholar
|
|
67
|
Pai R, Tarnawski AS and Tran T:
Deoxycholic acid activates beta-catenin signaling pathway and
increases colon cell cancer growth and invasiveness. Mol Biol Cell.
15:2156–2163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Baek MK, Park JS, Park JH, Kim MH, Kim HD,
Bae WK, Chung IJ, Shin BA and Jung YD: Lithocholic acid upregulates
uPAR and cell invasiveness via MAPK and AP-1 signaling in colon
cancer cells. Cancer Lett. 290:123–128. 2010. View Article : Google Scholar
|
|
69
|
Takeda A, Stoeltzing O, Ahmad SA, Reinmuth
N, Liu W, Parikh A, Fan F, Akagi M and Ellis LM: Role of
angiogenesis in the development and growth of liver metastasis. Ann
Surg Oncol. 9:610–616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li S, Nguyen TT, Ung TT, Sah DK, Park SY,
Lakshmanan VK and Jung YD: Piperine attenuates lithocholic
acid-stimulated interleukin-8 by suppressing Src/EGFR and reactive
oxygen species in human colorectal cancer cells. Antioxidants
(Basel). 11:5302022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Nguyen TT, Lian S, Ung TT, Xia Y, Han JY
and Jung YD: Lithocholic acid stimulates IL-8 expression in human
colorectal cancer cells via activation of Erk1/2 MAPK and
suppression of STAT3 activity. J Cell Biochem. 118:2958–2967. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sun J, Mustafi R, Cerda S, Chumsangsri A,
Xia YR, Li YC and Bissonnette M: Lithocholic acid down-regulation
of NF-kappaB activity through vitamin D receptor in colonic cancer
cells. J Steroid Biochem Mol Biol. 111:37–40. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Cianchi F, Cortesini C, Bechi P, Fantappiè
O, Messerini L, Vannacci A, Sardi I, Baroni G, Boddi V, Mazzanti R
and Masini E: Up-regulation of cyclooxygenase 2 gene expression
correlates with tumor angiogenesis in human colorectal cancer.
Gastroenterology. 121:1339–1347. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Oshio H, Abe T, Onogawa T, Ohtsuka H, Sato
T, Ii T, Fukase K, Muto M, Katayose Y, Oikawa M, et al: Peroxisome
proliferator-activated receptor alpha activates cyclooxygenase-2
gene transcription through bile acid transport in human colorectal
cancer cell lines. J Gastroenterol. 43:538–549. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Khare S, Mustafi R, Cerda S, Yuan W,
Jagadeeswaran S, Dougherty U, Tretiakova M, Samarel A, Cohen G,
Wang J, et al: Ursodeoxycholic acid suppresses Cox-2 expression in
colon cancer: Roles of Ras, p38, and CCAAT/enhancer-binding
protein. Nutr Cancer. 60:389–400. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Buchheit CL, Weigel KJ and Schafer ZT:
Cancer cell survival during detachment from the ECM: Multiple
barriers to tumour progression. Nat Rev Cancer. 14:632–641. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hawk MA and Schafer ZT: Mechanisms of
redox metabolism and cancer cell survival during extracellular
matrix detachment. J Biol Chem. 293:7531–7537. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Elia I, Doglioni G and Fendt SM: Metabolic
hallmarks of metastasis formation. Trends Cell Biol. 28:673–684.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang YN, Zeng ZL, Lu J, Wang Y, Liu ZX, He
MM, Zhao Q, Wang ZX, Li T, Lu YX, et al: CPT1A-mediated fatty acid
oxidation promotes colorectal cancer cell metastasis by inhibiting
anoikis. Oncogene. 37:6025–6040. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhong X, He X, Wang Y, Hu Z, Huang H, Zhao
S, Wei P and Li D: Warburg effect in colorectal cancer: The
emerging roles in tumor microenvironment and therapeutic
implications. J Hematol Oncol. 15:1602022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang Z, Pang J, Wang L, Dong Q and Jin D:
CEBPB regulates the bile acid receptor FXR to accelerate colon
cancer progression by modulating aerobic glycolysis. J Clin Lab
Anal. 36:e247032022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Schafer ZT, Grassian AR, Song L, Jiang Z,
Gerhart-Hines Z, Irie HY, Gao S, Puigserver P and Brugge JS:
Antioxidant and oncogene rescue of metabolic defects caused by loss
of matrix attachment. Nature. 461:109–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Carino A, Marchianò S, Biagioli M,
Scarpelli P, Bordoni M, Di Giorgio C, Roselli R, Fiorucci C, Monti
MC, Distrutti E, et al: The bile acid activated receptors GPBAR1
and FXR exert antagonistic effects on autophagy. FASEB J.
35:e212712021. View Article : Google Scholar
|
|
84
|
Seok S, Fu T, Choi SE, Li Y, Zhu R, Kumar
S, Sun X, Yoon G, Kang Y, Zhong W, et al: Transcriptional
regulation of autophagy by an FXR-CREB axis. Nature. 516:108–111.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Shasha T, Gruijs M and van Egmond M:
Mechanisms of colorectal liver metastasis development. Cell Mol
Life Sci. 79:6072022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ma C, Han M, Heinrich B, Fu Q, Zhang Q,
Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, et al: Gut
microbiome-mediated bile acid metabolism regulates liver cancer via
NKT cells. Science. 360:eaan59312018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Shen Y, Lu C, Song Z, Qiao C, Wang J, Chen
J, Zhang C, Zeng X, Ma Z, Chen T, et al: Ursodeoxycholic acid
reduces antitumor immunosuppression by inducing CHIP-mediated TGF-β
degradation. Nat Commun. 13:34192022. View Article : Google Scholar
|
|
88
|
Cong J, Liu P, Han Z, Ying W, Li C, Yang
Y, Wang S, Yang J, Cao F, Shen J, et al: Bile acids modified by the
intestinal microbiota promote colorectal cancer growth by
suppressing CD8(+) T cell effector functions. Immunity.
57:876–889.e811. 2024. View Article : Google Scholar
|
|
89
|
Sun L, Yang N, Liu Z, Ye X, Cheng M, Deng
L, Zhang J, Wu J, Shi M and Liao W: Cholestasis-induced phenotypic
transformation of neutrophils contributes to immune escape of
colorectal cancer liver metastasis. J Biomed Sci. 31:662024.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Liu QL, Zhou H, Zhou ZG and Chen HN:
Colorectal cancer liver metastasis: genomic evolution and crosstalk
with the liver microenvironment. Cancer Metastasis Rev. 42:575–587.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Plundrich D, Chikhladze S, Fichtner-Feigl
S, Feuerstein R and Briquez PS: Molecular mechanisms of tumor
immunomodulation in the microenvironment of colorectal cancer. Int
J Mol Sci. 23:27822022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Pan Y, Yu Y, Wang X and Zhang T:
Tumor-associated macrophages in tumor immunity. Front Immunol.
11:5830842020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu
Y, Zhang Z, Cai S, Xu Y, Li X, et al: Tumor-derived exosomal
miR-934 induces macrophage M2 polarization to promote liver
metastasis of colorectal cancer. J Hematol Oncol. 13:1562020.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Sun R, Zhang Z, Bao R, Guo X, Gu Y, Yang
W, Wei J, Chen X, Tong L, Meng J, et al: Loss of SIRT5 promotes
bile acid-induced immunosuppressive microenvironment and
hepatocarcinogenesis. J Hepatol. 77:453–466. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kawamata Y, Fujii R, Hosoya M, Harada M,
Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, et al:
A G protein-coupled receptor responsive to bile acids. J Biol Chem.
278:9435–9440. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhao L, Zhang H, Liu X, Xue S, Chen D, Zou
J and Jiang H: TGR5 deficiency activates antitumor immunity in
non-small cell lung cancer via restraining M2 macrophage
polarization. Acta Pharm Sin B. 12:787–800. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Rao J, Yang C, Yang S, Lu H, Hu Y, Lu L
and Cheng Fand Wang X: Deficiency of TGR5 exacerbates
immune-mediated cholestatic hepatic injury by stabilizing the
beta-catenin destruction complex. Int Immunol. 32:321–334. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Shao J, Ge T, Tang C, Wang G, Pang L and
Chen Z: Synergistic anti-inflammatory effect of gut microbiota and
lithocholic acid on liver fibrosis. Inflamm Res. 71:1389–1401.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Cao H, Xu M, Dong W, Deng B, Wang S, Zhang
Y, Wang S, Luo S, Wang W, Qi Y, et al: Secondary bile acid-induced
dysbiosis promotes intestinal carcinogenesis. Int J Cancer.
140:2545–2556. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Hedrick CC and Malanchi I: Neutrophils in
cancer: Heterogeneous and multifaceted. Nat Rev Immunol.
22:173–187. 2022. View Article : Google Scholar
|
|
101
|
Zheng W, Wu J, Peng Y, Sun J, Cheng P and
Huang Q: Tumor-associated neutrophils in colorectal cancer
development, progression and immunotherapy. Cancers (Basel).
14:47552022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lin N, Li J, Yao X, Zhang X, Liu G, Zhang
Z and Weng S: Prognostic value of neutrophil-to-lymphocyte ratio in
colorectal cancer liver metastasis: A meta-analysis of results from
multivariate analysis. Int J Surg. 107:1069592022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
O'Brien KM, Allen KM, Rockwell CE, Towery
K, Luyendyk JP and Copple BL: IL-17A synergistically enhances bile
acid-induced inflammation during obstructive cholestasis. Am J
Pathol. 183:1498–1507. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Labiano I, Agirre-Lizaso A, Olaizola P,
Echebarria A, Huici-Izagirre M, Olaizola I, Esparza-Baquer A,
Sharif O, Hijona E, Milkiewicz P, et al: TREM-2 plays a protective
role in cholestasis by acting as a negative regulator of
inflammation. J Hepatol. 77:991–1004. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Thibaut MM, Sboarina M, Roumain M, Pötgens
SA, Neyrinck AM, Destrée F, Gillard J, Leclercq IA, Dachy G,
Demoulin JB, et al: Inflammation-induced cholestasis in cancer
cachexia. J Cachexia Sarcopenia Muscle. 12:70–90. 2021. View Article : Google Scholar
|
|
106
|
Cui C, Lan P and Fu L: The role of
myeloid-derived suppressor cells in gastrointestinal cancer. Cancer
Commun (Lond). 41:442–471. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zeng X, Zhou J, Xiong Z, Sun H, Yang W,
Mok MTS, Wang J, Li J, Liu M, Tang W, et al: Cell cycle-related
kinase reprograms the liver immune microenvironment to promote
cancer metastasis. Cell Mol Immunol. 18:1005–1015. 2021. View Article : Google Scholar :
|
|
108
|
Zhang H, Liu Y, Bian Z, Huang S, Han X,
You Z, Wang Q, Qiu D, Miao Q, Peng Y, et al: The critical role of
myeloid-derived suppressor cells and FXR activation in
immune-mediated liver injury. J Autoimmun. 53:55–66. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Chang S, Kim YH, Kim YJ, Kim YW, Moon S,
Lee YY, Jung JS, Kim Y, Jung HE, Kim TJ, et al: Taurodeoxycholate
increases the number of myeloid-derived suppressor cells that
ameliorate sepsis in mice. Front Immunol. 9:19842018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Alfaro C, Teijeira A, Onate C, Pérez G,
Sanmamed MF, Andueza MP, Alignani D, Labiano S, Azpilikueta A,
Rodriguez-Paulete A, et al: Tumor-Produced interleukin-8 attracts
human myeloid-derived suppressor cells and elicits extrusion of
neutrophil extracellular traps (NETs). Clin Cancer Res.
22:3924–3936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Schalper KA, Carleton M, Zhou M, Chen T,
Feng Y, Huang SP, Walsh AM, Baxi V, Pandya D, Baradet T, et al:
Elevated serum interleukin-8 is associated with enhanced intratumor
neutrophils and reduced clinical benefit of immune-checkpoint
inhibitors. Nat Med. 26:688–692. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Gardner A and Ruffell B: Dendritic cells
and cancer immunity. Trends Immunol. 37:855–865. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Campbell C, McKenney PT, Konstantinovsky
D, Isaeva OI, Schizas M, Verter J, Mai C, Jin WB, Guo CJ, Violante
S, et al: Bacterial metabolism of bile acids promotes generation of
peripheral regulatory T cells. Nature. 581:475–479. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Hu J, Wang C, Huang X, Yi S, Pan S, Zhang
Y, Yuan G, Cao Q, Ye X and Li H: Gut microbiota-mediated secondary
bile acids regulate dendritic cells to attenuate autoimmune uveitis
through TGR5 signaling. Cell Rep. 36:1097262021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Hu J, Zhang Y, Yi S, Wang C, Huang X, Pan
S, Yang J, Yuan G, Tan S and Li H: Lithocholic acid inhibits
dendritic cell activation by reducing intracellular glutathione via
TGR5 signaling. Int J Biol Sci. 18:4545–4559. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Tugues S, Burkhard SH, Ohs I, Vrohlings M,
Nussbaum K, Vom Berg J, Kulig P and Becher B: New insights into
IL-12-mediated tumor suppression. Cell Death Differ. 22:237–246.
2015. View Article : Google Scholar :
|
|
117
|
Ichikawa R, Takayama T, Yoneno K, Kamada
N, Kitazume MT, Higuchi H, Matsuoka K, Watanabe M, Itoh H, Kanai T,
et al: Bile acids induce monocyte differentiation toward
interleukin-12 hypo-producing dendritic cells via a TGR5-dependent
pathway. Immunology. 136:153–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Willart MA, van Nimwegen M, Grefhorst A,
Hammad H, Moons L, Hoogsteden HC, Lambrecht BN and Kleinjan A:
Ursodeoxycholic acid suppresses eosinophilic airway inflammation by
inhibiting the function of dendritic cells through the nuclear
farnesoid X receptor. Allergy. 67:1501–1510. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Kennedy R and Celis E: Multiple roles for
CD4+ T cells in anti-tumor immune responses. Immunol Rev.
222:129–144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Laheurte C, Dosset M, Vernerey D,
Boullerot L, Gaugler B, Gravelin E, Kaulek V, Jacquin M, Cuche L,
Eberst G, et al: Distinct prognostic value of circulating
anti-telomerase CD4(+) Th1 immunity and exhausted PD-1(+)/TIM-3(+)
T cells in lung cancer. Br J Cancer. 121:405–416. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Ho TTB, Nasti A, Seki A, Komura T, Inui H,
Kozaka T, Kitamura Y, Shiba K, Yamashita T, Yamashita T, et al:
Combination of gemcitabine and anti-PD-1 antibody enhances the
anticancer effect of M1 macrophages and the Th1 response in a
murine model of pancreatic cancer liver metastasis. J Immunother
Cancer. 8:e0013672020. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
De M, Ghosh S, Asad M, Banerjee I and Ali
N: Combining doxorubicin with stearylamine-bearing liposomes
elicits Th1 cytokine responses and cures metastasis in a mouse
model. Cancer Immunol Immunother. 69:1725–1735. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Pols TWH, Puchner T, Korkmaz HI, Vos M,
Soeters MR and de Vries CJM: Lithocholic acid controls adaptive
immune responses by inhibition of Th1 activation through the
vitamin D receptor. PLoS One. 12:e01767152017. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Tosolini M, Kirilovsky A, Mlecnik B,
Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH,
Pagès F and Galon J: Clinical impact of different classes of
infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in
patients with colorectal cancer. Cancer Res. 71:1263–1271. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Liu X, Wang X, Yang Q, Luo L, Liu Z, Ren
X, Lei K, Li S, Xie Z, Zheng G, et al: Th17 cells secrete TWEAK to
trigger epithelial-mesenchymal transition and promote colorectal
cancer liver metastasis. Cancer Res. 84:1352–1371. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Hang S, Paik D, Yao L, Kim E, Trinath J,
Lu J, Ha S, Nelson BN, Kelly SP, Wu L, et al: Bile acid metabolites
control T(H)17 and T(reg) cell differentiation. Nature.
576:143–148. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Paik D, Yao L, Zhang Y, Bae S, D'Agostino
GD, Zhang M, Kim E, Franzosa EA, Avila-Pacheco J, Bisanz JE, et al:
Human gut bacteria produce Τ(Η)17-modulating bile acid metabolites.
Nature. 603:907–912. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Xiao R, Lei K, Kuok H, Deng W, Zhuang Y,
Tang Y, Guo Z, Qin H, Bai LP and Li T: Synthesis and identification
of lithocholic acid 3-sulfate as RORγt ligand to inhibit Th17 cell
differentiation. J Leukoc Biol. 112:835–843. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Chung AS, Wu X, Zhuang G, Ngu H, Kasman I,
Zhang J, Vernes JM, Jiang Z, Meng YG, Peale FV, et al: An
interleukin-17-mediated paracrine network promotes tumor resistance
to anti-angiogenic therapy. Nat Med. 19:1114–1123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Downs-Canner S, Berkey S, Delgoffe GM,
Edwards RP, Curiel T, Odunsi K, Bartlett DL and Obermajer N:
Suppressive IL-17A(+)Foxp3(+) and ex-Th17 IL-17A(neg)Foxp3(+)
T(reg) cells are a source of tumour-associated T(reg) cells. Nat
Commun. 8:146492017. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Gopalakrishnan V, Spencer CN, Nezi L,
Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman
K, Wei SC, et al: Gut microbiome modulates response to anti-PD-1
immunotherapy in melanoma patients. Science. 359:97–103. 2018.
View Article : Google Scholar
|
|
132
|
Hurtado CG, Wan F, Housseau F and Sears
CL: Roles for interleukin 17 and adaptive immunity in pathogenesis
of colorectal cancer. Gastroenterology. 155:1706–1715. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Shiri AM, Zhang T, Bedke T, Zazara DE,
Zhao L, Lücke J, Sabihi M, Fazio A, Zhang S, Tauriello DVF, et al:
IL-10 dampens antitumor immunity and promotes liver metastasis via
PD-L1 induction. J Hepatol. 80:634–644. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Ward-Hartstonge KA, McCall JL, McCulloch
TR, Kamps AK, Girardin A, Cretney E, Munro FM and Kemp RA:
Inclusion of BLIMP-1(+) effector regulatory T cells improves the
Immunoscore in a cohort of New Zealand colorectal cancer patients:
A pilot study. Cancer Immunol Immunother. 66:515–522. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Ladoire S, Martin F and Ghiringhelli F:
Prognostic role of FOXP3+ regulatory T cells infiltrating human
carcinomas: the paradox of colorectal cancer. Cancer Immunol
Immunother. 60:909–918. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Song X, Sun X, Oh SF, Wu M, Zhang Y, Zheng
W, Geva-Zatorsky N, Jupp R, Mathis D, Benoist C and Kasper DL:
Microbial bile acid metabolites modulate gut RORγ(+) regulatory T
cell homeostasis. Nature. 577:410–415. 2020. View Article : Google Scholar
|
|
137
|
Pagès F, Berger A, Camus M, Sanchez-Cabo
F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte
D, et al: Effector memory T cells, early metastasis, and survival
in colorectal cancer. N Engl J Med. 353:2654–2666. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Licata LA, Nguyen CT, Burga RA, Falanga V,
Espat NJ, Ayala A, Thorn M, Junghans RP and Katz SC: Biliary
obstruction results in PD-1-dependent liver T cell dysfunction and
acute inflammation mediated by Th17 cells and neutrophils. J Leukoc
Biol. 94:813–823. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Burks J, Olkhanud PB and Berzofsky JA: The
role of NKT cells in gastrointestinal cancers. Oncoimmunology.
11:20096662021. View Article : Google Scholar
|
|
140
|
Ji G, Ma L, Yao H, Ma S, Si X, Wang Y, Bao
X, Ma L, Chen F, Ma C, et al: Precise delivery of obeticholic acid
via nanoapproach for triggering natural killer T cell-mediated
liver cancer immunotherapy. Acta Pharm Sin B. 10:2171–2182. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Cheng P, Wu J, Zong G, Wang F, Deng R, Tao
R, Qian C, Shan Y, Wang A, Zhao Y, et al: Capsaicin shapes gut
microbiota and pre-metastatic niche to facilitate cancer metastasis
to liver. Pharmacol Res. 188:1066432023. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Huang WH, Zhou MW, Zhu YF, Xiang JB, Li
ZY, Wang ZH, Zhou YM, Yang Y, Chen ZY and Gu XD: The role of
hepatic stellate cells in promoting liver metastasis of colorectal
carcinoma. Onco Targets Ther. 12:7573–7580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Eveno C, Hainaud P, Rampanou A, Bonnin P,
Bakhouche S, Dupuy E, Contreres JO and Pocard M: Proof of
prometastatic niche induction by hepatic stellate cells. J Surg
Res. 194:496–504. 2015. View Article : Google Scholar
|
|
144
|
Liu B, Wu T, Lin B, Liu X, Liu Y, Song G,
Fan C and Ouyang G: Periostin-TGF-β feedforward loop contributes to
tumour-stroma crosstalk in liver metastatic outgrowth of colorectal
cancer. Br J Cancer. 130:358–368. 2024. View Article : Google Scholar
|
|
145
|
Qi M, Fan S, Huang M, Pan J, Li Y, Miao Q,
Lyu W, Li X, Deng L, Qiu S, et al: Targeting FAPalpha-expressing
hepatic stellate cells overcomes resistance to antiangiogenics in
colorectal cancer liver metastasis models. J Clin Invest.
132:e1573992022. View Article : Google Scholar
|
|
146
|
Meadows V, Kennedy L, Ekser B, Kyritsi K,
Kundu D, Zhou T, Chen L, Pham L, Wu N, Demieville J, et al: Mast
cells regulate ductular reaction and intestinal inflammation in
cholestasis through farnesoid X receptor signaling. Hepatology.
74:2684–2698. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Yang J, Tang X, Liang Z, Chen M and Sun L:
Taurocholic acid promotes hepatic stellate cell activation via
S1PR2/p38 MAPK/YAP signaling under cholestatic conditions. Clin Mol
Hepatol. 29:465–481. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Saga K, Iwashita Y, Hidano S, Aso Y, Isaka
K, Kido Y, Tada K, Takayama H, Masuda T, Hirashita T, et al:
Secondary unconjugated bile acids induce hepatic stellate cell
activation. Int J Mol Sci. 19:30432018. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Nguyen PT, Kanno K, Pham QT, Kikuchi Y,
Kakimoto M, Kobayashi T, Otani Y, Kishikawa N, Miyauchi M, Arihiro
K, et al: Senescent hepatic stellate cells caused by deoxycholic
acid modulates malignant behavior of hepatocellular carcinoma. J
Cancer Res Clin Oncol. 146:3255–3268. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Theivanthiran B, Yarla N, Haykal T, Nguyen
YV, Cao L, Ferreira M, Holtzhausen A, Al-Rohil R, Salama AKS,
Beasley GM, et al: Tumor-intrinsic NLRP3-HSP70-TLR4 axis drives
premetastatic niche development and hyperprogression during
anti-PD-1 immunotherapy. Sci Transl Med. 14:eabq70192022.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Gong Z, Zhou J, Zhao S, Tian C, Wang P, Xu
C, Chen Y, Cai W and Wu J: Chenodeoxycholic acid activates NLRP3
inflammasome and contributes to cholestatic liver fibrosis.
Oncotarget. 7:83951–83963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Hao H, Cao L, Jiang C, Che Y, Zhang S,
Takahashi S, Wang G and Gonzalez FJ: Farnesoid X receptor
regulation of the NLRP3 inflammasome underlies
cholestasis-associated sepsis. Cell Metab. 25:856–867.e5. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Zhao S, Gong Z, Zhou J, Tian C, Gao Y, Xu
C, Chen Y, Cai W and Wu J: Deoxycholic acid triggers NLRP3
inflammasome activation and aggravates DSS-Induced colitis in mice.
Front Immunol. 7:5362016. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Holtmann TM, Inzaugarat ME, Knorr J,
Geisler L, Schulz M, Bieghs V, Frissen M, Feldstein AE, Tacke F,
Trautwein C and Wree A: Bile acids activate NLRP3 inflammasome,
promoting murine liver inflammation or fibrosis in a cell
type-specific manner. Cells. 10:26182021. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang
L, Zheng M, Zhang X, Xia D, Ke Y, et al: Bile acids control
inflammation and metabolic disorder through inhibition of NLRP3
inflammasome. Immunity. 45:9442016. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Sun L, Cai J and Gonzalez FJ: The role of
farnesoid X receptor in metabolic diseases, and gastrointestinal
and liver cancer. Nat Rev Gastroenterol Hepatol. 18:335–347. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Chapman RW and Lynch KD: Obeticholic
acid-a new therapy in PBC and NASH. Br Med Bull. 133:95–104. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Zhang Y, Jackson JP, StClaire RL III,
Freeman K, Brouwer KR and Edwards JE: Obeticholic acid, a selective
farnesoid X receptor agonist, regulates bile acid homeostasis in
sandwich-cultured human hepatocytes. Pharmacol Res Perspect.
5:e003292017. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Lu L, Jiang YX, Liu XX, Jin JM, Gu WJ,
Luan X, Guan YY and Zhang LJ: FXR agonist GW4064 enhances
anti-PD-L1 immunotherapy in colorectal cancer. Oncoimmunology.
12:22170242023. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Zhang T, Feng S, Li J, Wu Z, Deng Q, Yang
W, Li J and Pan G: Farnesoid X receptor (FXR) agonists induce
hepatocellular apoptosis and impair hepatic functions via FXR/SHP
pathway. Arch Toxicol. 96:1829–1843. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Yin Y, Wang M, Gu W and Chen L:
Intestine-specific FXR agonists as potential therapeutic agents for
colorectal cancer. Biochem Pharmacol. 186:1144302021. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Ji G, Si X, Dong S, Xu Y, Li M, Yang B,
Tang Z, Fang X, Huang L, Song W and Chen X: Manipulating liver bile
acid signaling by nanodelivery of bile acid receptor modulators for
liver cancer immunotherapy. Nano Lett. 21:6781–6791. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Zhang Y, Jiang R, Zheng X, Lei S, Huang F,
Xie G, Kwee S, Yu H, Farrar C, Sun B, et al: Ursodeoxycholic acid
accelerates bile acid enterohepatic circulation. Br J Pharmacol.
176:2848–2863. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Pearson T, Caporaso JG, Yellowhair M,
Bokulich NA, Padi M, Roe DJ, Wertheim BC, Linhart M, Martinez JA,
Bilagody C, et al: Effects of ursodeoxycholic acid on the gut
microbiome and colorectal adenoma development. Cancer Med.
8:617–628. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
He Q, Wu J, Ke J, Zhang Q, Zeng W, Luo Z,
Gong J, Chen Y, He Z and Lan P: Therapeutic role of ursodeoxycholic
acid in colitis-associated cancer via gut microbiota modulation.
Mol Ther. 31:585–598. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Zhang H, Xu H, Zhang C, Tang Q and Bi F:
Ursodeoxycholic acid suppresses the malignant progression of
colorectal cancer through TGR5-YAP axis. Cell Death Discov.
7:2072021. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Shah SA, Volkov Y, Arfin Q, Abdel-Latif MM
and Kelleher D: Ursodeoxycholic acid inhibits interleukin 1 beta
[corrected] and deoxycholic acid-induced activation of NF-kappaB
and AP-1 in human colon cancer cells. Int J Cancer. 118:532–539.
2006. View Article : Google Scholar
|
|
168
|
Zheng Z, Hou X, Bian Z, Jia W and Zhao L:
Gut microbiota and colorectal cancer metastasis. Cancer Lett.
555:2160392023. View Article : Google Scholar
|
|
169
|
Wang X, Zhu B, Hua Y, Sun R, Tan X, Chang
X, Tang D and Gu J: Astragalus mongholicus Bunge and Curcuma
aromatica Salisb. modulate gut microbiome and bile acid metabolism
to inhibit colon cancer progression. Front Microbiol.
15:13956342024. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Liu T, Song X, Khan S, Li Y, Guo Z, Li C,
Wang S, Dong W, Liu W, Wang B and Cao H: The gut microbiota at the
intersection of bile acids and intestinal carcinogenesis: An old
story, yet mesmerizing. Int J Cancer. 146:1780–1790. 2020.
View Article : Google Scholar
|
|
171
|
Deng J, Yuan W, Tan Q, Wei X and Ma J:
Non-absorbable antibiotic treatment inhibits colorectal cancer
liver metastasis by modulating deoxycholic acid metabolism by
intestinal microbes. J Cancer. 13:764–774. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Fidelle M, Rauber C, Alves Costa Silva C,
Tian AL, Lahmar I, de La Varende AM, Zhao L, Thelemaque C, Lebhar
I, Messaoudene M, et al: A microbiota-modulated checkpoint directs
immunosuppressive intestinal T cells into cancers. Science.
380:eabo22962023. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Kaźmierczak-Siedlecka K, Daca A, Fic M,
van de Wetering T, Folwarski M and Makarewicz W: Therapeutic
methods of gut microbiota modification in colorectal cancer
management-fecal microbiota transplantation, prebiotics,
probiotics, and synbiotics. Gut Microbes. 11:1518–1530. 2020.
View Article : Google Scholar
|
|
174
|
Routy B, Le Chatelier E, Derosa L, Duong
CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C,
Roberti MP, et al: Gut microbiome influences efficacy of PD-1-based
immunotherapy against epithelial tumors. Science. 359:91–97. 2018.
View Article : Google Scholar
|
|
175
|
Lan X, Ma J, Huang Z, Xu Y and Hu Y:
Akkermansia muciniphila might improve anti-PD-1 therapy against HCC
by changing host bile acid metabolism. J Gene Med. 26:e36392024.
View Article : Google Scholar
|
|
176
|
Chen D, Wu J, Jin D, Wang B and Cao H:
Fecal microbiota transplantation in cancer management: Current
status and perspectives. Int J Cancer. 145:2021–2031. 2019.
View Article : Google Scholar :
|
|
177
|
Fuchs CD and Trauner M: Role of bile acids
and their receptors in gastrointestinal and hepatic
pathophysiology. Nat Rev Gastroenterol Hepatol. 19:432–450. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Kastelijn JB, van der Loos MA, Welsing PM,
Dhondt E, Koopman M, Moons LM and Vleggaar FP: Clinical outcomes of
biliary drainage of malignant biliary obstruction due to colorectal
cancer metastases: A systematic review. Eur J Intern Med. 88:81–88.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Sinha SR, Haileselassie Y, Nguyen LP,
Tropini C, Wang M, Becker LS, Sim D, Jarr K, Spear ET, Singh G, et
al: Dysbiosis-induced secondary bile acid deficiency promotes
intestinal inflammation. Cell Host Microbe. 27:659–670 e5. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Gadaleta RM, Garcia-Irigoyen O and
Moschetta A: Bile acids and colon cancer: Is FXR the solution of
the conundrum? Mol Aspects Med. 56:66–74. 2017. View Article : Google Scholar : PubMed/NCBI
|