Cancer is the second leading cause of human
mortality worldwide, with >190,000 new cases diagnosed and
100,000 associated deaths occurring each year, accounting for one
in six global deaths and representing a significant public health
concern (1,2). The current standard of care for
cancer involves a multimodal approach that includes surgical
resection, radiotherapy, chemotherapy, targeted therapy and
immunotherapy (3-5). Advances in surgical techniques and
the development of targeted therapeutic agents have led to
significant progress in cancer treatment (6). However, emerging drug resistance and
metastasis of malignant tumors remain the primary causes of
mortality in patients with cancer, representing a significant
challenge in the field of oncology (7). >90% of cancer-related deaths are
attributed to drug resistance (8,9).
Therefore, exploring drug resistance during cancer treatment and
identifying effective low-toxicity therapeutic targets that can
reverse drug resistance are crucial for reducing adverse effects
and improving overall prognosis.
Although the exact mechanisms underlying drug
resistance in cancer cells remain elusive, current findings
indicate the involvement of numerous genes associated with drug
efflux, DNA repair, apoptosis and diverse cellular signaling
pathways (10,11). In addition to research asserting
that mutations are responsible for tumor development and drug
resistance, recent studies have revealed the tumor microenvironment
(TME) as an integral part of tumorigenesis. The TME, which refers
to the microenvironment surrounding tumor cells, including blood
vessels, immune cells, fibroblasts and extracellular matrix
(12), is strongly associated
with the development of therapeutic resistance in tumor cells
through complex signaling pathways (7,13).
Neutrophils were originally considered as first
responders of the innate immune system against extracellular
pathogens (14). Increasing
evidence suggests that neutrophils also play an important role in
the TME (15-17). Tumor-associated neutrophils (TANs)
are immune cells that infiltrate the TME (18) and act directly or indirectly on
tumor cells by releasing a variety of pro-inflammatory factors,
immunomodulatory factors and angiogenic factors, which either
promote or inhibit tumor occurrence, progression and metastasis
(19). In the current article,
the role of TANs in tumorigenesis and cancer progression in the TME
was summarized, their contribution to therapeutic resistance was
explored and existing TAN-targeted therapeutic strategies were
reviewed.
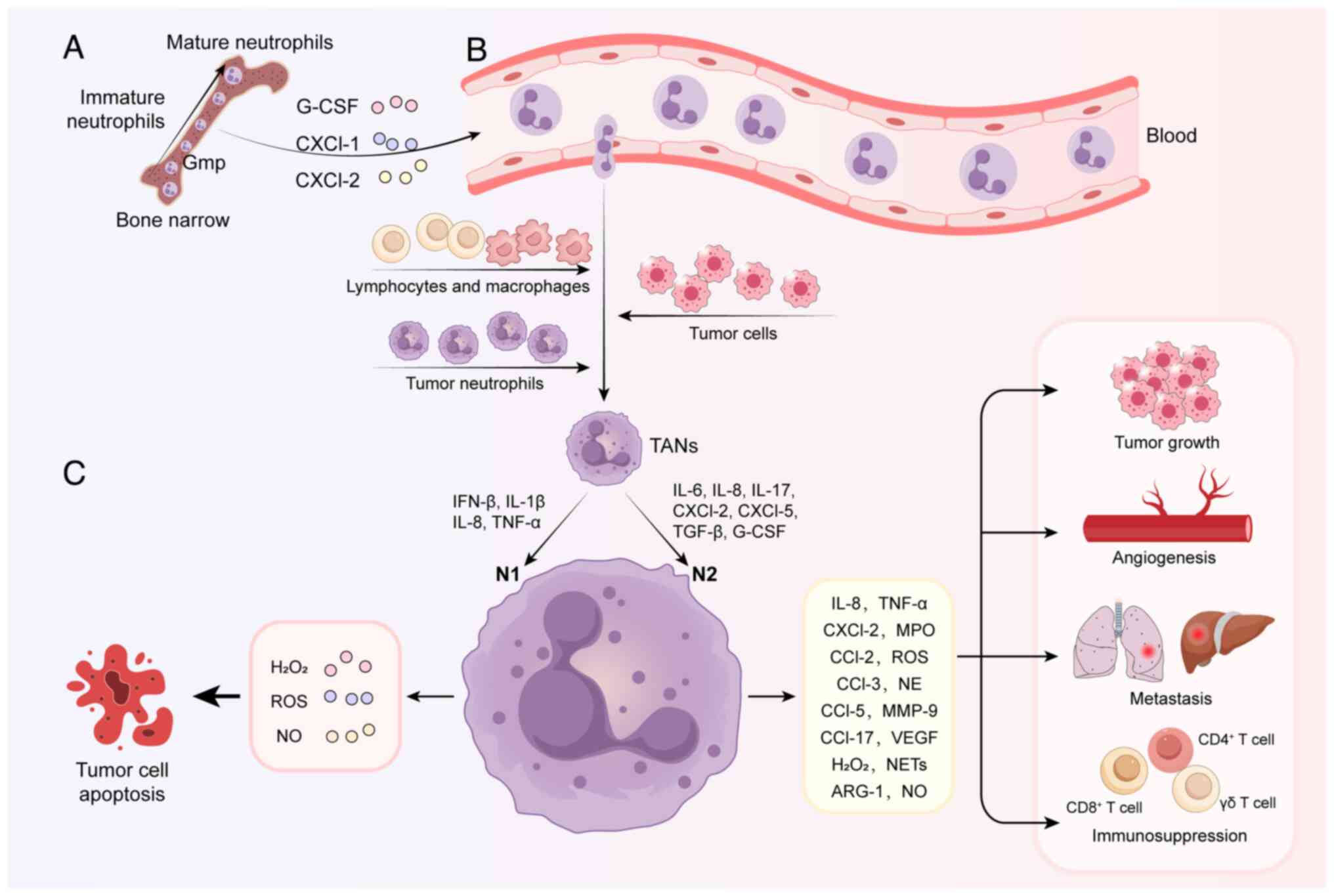
The migration of neutrophils from bone marrow to the
tumor site involves three stages (Fig. 1): Maturation of neutrophils in the
bone marrow, circulation in the blood and chemotaxis to the tumor
site (20). Increasing research
on the body's immune functions have shown that TAN regulation is
highly reprogrammable. Neutrophils can acquire different phenotypes
based on environmental signals (21); these phenotypic differences
determine functional differences among neutrophils and govern
whether the cells are involved in pro- and/or anti-tumor
responses.
Single-cell RNA sequencing (scRNA-seq) provides an
unprecedented view of cellular heterogeneity in the TME. However,
owing to the relatively low RNA levels and high RNase levels of
neutrophils, neutrophil research is challenging. With the
development of scRNA-seq, multiple phenotypes of TANs have been
identified in a variety of cancers (19,24,25). Furthermore, pseudotime analysis
has revealed differentiation trajectories along neutrophil states
(26). In non-small cell lung
cancer, Salcher et al (24) identified four TAN subsets (TAN-1
to TAN-4) and showed that the overall TAN phenotype was
characterized by high expression of oxidized low-density
lipoprotein receptor 1, vascular endothelial growth factor A, CD83,
intercellular adhesion molecule 1 and C-X-C motif ligand receptor
(CXCR)4 but low expression of CXCR1, CXCR2,
prostaglandin-endoperoxide synthase 2, selectin L (CD62L),
colony-stimulating factor (CSF)3R and Fc gamma receptor IIIb
(CD16B). In pancreatic cancer (27), TANs were divided into five
heterogeneous subgroups: A terminally differentiated pro-tumor
subpopulation (TAN-1) associated with poor prognosis, an
inflammatory subpopulation (TAN-2), a transitional population
recently migrated to the TME (TAN-3) and a subpopulation
preferentially expressing interferon-stimulated genes (TAN-4). Xue
et al (19) stratified
patients with liver cancer into five tumor immune microenvironment
subtypes, including immune activation, immune suppression mediated
by myeloid or stromal cells, immune exclusion and immune residence
phenotypes, where differences in the tumor immune microenvironment
steer the development of at least six different types of TAN in
liver cancer. Furthermore, Wu et al (26) generated and integrated single-cell
neutrophil transcriptomes from 17 cancer types and identified 10
distinct states, including inflammation, angiogenesis and antigen
presentation. Notably, non-TANs could differentiate into TANs
(24,25), indicating that the TME can induce
plasticity.
Overall, existing research demonstrates the
remarkable plasticity and re-editable nature of TANs, which is an
important factor to consider when designing anti-tumor therapies.
To date, scRNA-seq has provided reliable support for improved
disease outcome prediction and targeted therapy for specific
differentiation pathways. However, as different clusters of
neutrophils cannot currently be sorted, their functions cannot be
verified. Therefore, isolating these cells will be an important
step forward in neutrophil research.
TANs play a dual role in the TME that depends on the
neutrophil phenotype, the timing and the tumor type. TANs
participate in pro-tumor inflammation by promoting tumor growth,
metastasis and angiogenesis, as well as in remodeling of the
extracellular matrix (15).
Conversely, TANs can also mediate anti-tumor responses by directly
killing tumor cells and participating in cellular networks that
mediate anti-tumor resistance (28,29).
TANs regulate tumor growth and progression. TANs
secrete a variety of molecules that can stimulate tumor growth,
such as TGF-β, neutrophil elastase (NE), interleukin (IL)-17a, C-C
motif chemokine ligand (CCL)2 and IL-8 (20,30-32). Blocking TGF-β shifts TAN
polarization from the N2 to N1 phenotype in the TME, increasing
tumor cell apoptosis and suppressing tumor cell migration (33,34). In pancreatic cancer, a
pro-inflammatory microenvironment can be generated by recruiting
TANs and activating NE release, which contributes to the
progression of pancreatic neoplasms (35). In gastric cancer, TANs induce the
epithelial-to-mesenchymal transition (EMT) of gastric cancer cells
by secreting IL-17a and activating Janus kinase 2 (JAK2)/signal
transducer and activator of transcription 3 (STAT3) signaling in
gastric cancer cells (36).
Furthermore, N2-polarized TANs reportedly promote gastric cancer
metastasis through the exosomal miR-4745-5p/3911-mediated
inhibition of slit guidance ligand 2 expression (37). In a mouse model of lung
adenocarcinoma, TANs exhibit widespread survival and increased
expression of the anti-apoptotic protein Bcl-xL to promote tumor
growth via granulocyte-macrophage (GM)-CSF-induced JAK/ STAT
signaling (38). Another study
found that TANs govern tumor progression in lung cancer through an
IL-10/STAT3/ programmed death-ligand 1 (PD-L1) feedback signaling
loop (39).
Neutrophils are responsible for producing
pro-angiogenic factors, including Bv8 (also known as prokineticin
2), matrix metalloproteinase 9 (MMP-9) and VEGF, which play
important roles in promoting tumor angiogenesis (40-42). Bv8 is a mediator of myeloid
cell-dependent tumor angiogenesis and plays a role in the
angiogenic switch by affecting the neoplastic vasculature and
infiltration of Gr1+ cells (43). MMP-9 is one of the most important
mediators of tumor angiogenesis, with TANs thought to be a major
source of MMP-9 (44,45). VEGF produced during the release of
MMP-9 specifically stimulates the proliferation of vascular
endothelial cells, inhibits the apoptosis of endothelial cells,
promotes angiogenesis and increases vascular permeability (46).
Neutrophils can also induce neutrophil extracellular
traps (NETs), which are highly expressed in a variety of cancers
and promote tumor proliferation, invasion and metastasis (47). Unlike the cell death programs of
necrosis and apoptosis, NETs include MMP-9, NE, myeloperoxidase and
cytoskeletal proteins (48). NETs
contribute to the disruption of normal connections between
endothelial cells, enabling tumor cell extravasation and metastasis
(49-51). Furthermore, NETs encapsulate tumor
cells, protecting them from the cytotoxic effects of surrounding
immune cells (52). Although NETs
promote tumor recurrence and metastasis in a number of ways, tumor
cells, in turn, promote NETs via chemokines (53). In hepatocellular carcinoma (HCC),
acetyl-CoA accumulation induces the H3 acetylation-dependent
upregulation of CXCL1 gene expression, which can lead to TAN
recruitment, NET formation and the promotion of HCC metastasis
(54).
In addition to pro-tumorigenic properties,
neutrophils also exhibit anti-tumor responses. However, few studies
have reported the anti-tumor effects of neutrophils.
Neutrophils can exert anti-proliferative effects.
Direct cell contacts between Fas ligand on human neutrophils and
Fas on tumor cells suppresses tumor cells by causing cell cycle
arrest in vitro (55). In
a mouse model of phosphatase and tensin homolog-deficient uterine
cancer, neutrophils impeded early-stage tumor growth and retarded
malignant progression by inducing tumor cell detachment from the
basement membrane (56).
Neutrophils can kill tumor cells by releasing a
series of effector molecules. For instance, reactive oxygen species
(ROS) are secreted through neutrophil degranulation, mediating cell
killing by opening the transient receptor potential ion channel M2,
which leads to an influx of calcium ions into the target cells
(57,58). In addition to ROS, TANs also
release nitric oxide and tumor necrosis factor (TNF), which are
further involved in tumor suppression (59). Neutrophil-derived TNF-related
apoptosis-inducing ligand reportedly induces apoptosis of leukemic
T cells (60). Furthermore, Cui
et al (61) reported that
neutrophils release catalytically active neutrophil elastase
(ELANE) to kill numerous types of cancer cell both in vitro
and in vivo. ELANE proteolytically liberates the CD95 death
domain, which interacts with histone H1 isoforms to selectively
eradicate cancer cells. ELANE can also attenuate primary tumor
growth and produce a CD8+T cell-mediated abscopal effect
to attack distant metastases. In melanomas, specific neutrophil
subpopulations play an important role in preventing the immune
escape of tumor cells, either through the release of inducible NO
synthase or the direct phagocytosis of antigen-deficient melanoma
cells (62).
TANs engage in complex bidirectional interactions
with macrophages and lymphocytes in the TME through the expression
of multiple cytokines, as well as immunosuppressive and stimulatory
molecules (63,64).
TANs and tumor-associated macrophages (TAMs) are
functional partners in the inflammatory process, hypothesized to
synergize and interact in the TME to promote tumor progression
through similar molecular forms (65). When macrophages take up
TAN-derived factors, they stimulate TAMs toward M1 polarization
(66). NETs induce mononuclear
macrophages to secrete interleukins, such as IL-1β and IL-6, and
recruit progenitor cells of TANs, with activated neutrophils
further releasing IL-8, among other cytokines, to recruit
macrophages (67). In addition,
neutrophils secrete myeloperoxidase, which binds to the mannose
receptor and dominates the secretion of GM-CSF in chronic
inflammatory environments (68).
GM-CSF is an important factor mediating the recruitment and
development of TAMs and critical for TME macrophage recruitment and
polarization (69,70). In early luminal breast cancers,
TAN density correlates with CD163+ M2-like TAM density.
Furthermore, TANs are a negative prognostic factor in tumors with
an elevated M1/M2 TAM ratio, whereas this impact on patient outcome
is lost in tumors with a low M1/M2 ratio (71). Thus, the recruitment and function
of TANs and TAMs are inextricably linked. In intrahepatic
cholangiocarcinoma (ICC), the interaction between TANs and TAMs
produces higher levels of oncostatin M and IL-11, respectively,
which then activate STAT3 signaling in ICC cells. STAT3 knockdown
attenuates the pro-tumorigenic effects of TANs and TAMs in ICC
(63).
TANs can lead to the suppression and depletion of
T-cell function in several ways; this phenomenon will be elaborated
on further below. In addition, activated neutrophils can recruit
type 1 T-helper (Th1) and Th17 cells by releasing multiple
chemokines (72-74). CCL17 released by TANs may also
support tumor growth by promoting the recruitment of regulatory T
(Treg) cells to tumors and inhibiting anti-tumor immune activity
(75). In pancreatic ductal
adenocarcinoma (PDAC), polarized TANs upregulate CCL5 secretion,
which promotes cancer cell migration and invasion and enhances Treg
cell infiltration in the tumor (76). Furthermore, TANs reduce the
cytotoxic and infiltration capacity of natural killer (NK) cells
and regulate the expression of programmed cell death protein-1
(PD-1) and PD-L1 through the G-CSF/STAT3 and IL-18 signaling
pathways, thereby inhibiting the anti-tumor immune activity of NK
cells (77). G-CSF-mobilized
neutrophils inhibit NK-cell activation (78-80). By contrast, NK cells control the
tumor-promoting and angiogenic functions of neutrophils in an
interferon γ-dependent manner by inhibiting VEGF expression
(81).
Other components of the TME, such as
cancer-associated fibroblasts (CAFs), reportedly secrete IL-8,
which further recruit neutrophils into the TME. The infiltrated
neutrophils upregulate Serglycin (SRGN) expression in gastric
cancer cells via the regenerating family member 4. SRGN secreted by
tumor cells then activates the CD44/c-Myc pathway to upregulate
Lysine[K]-specific demethylase 5B expression, thereby promoting
IL-8 production in CAFs. Thus, the SRGN-IL-8-TANs-SRGN loop
facilitates gastric cancer progression (82). In the TME of HCC, CAF-derived
cardiotrophin-like cytokine factor 1 increases CXCL6 and TGF-β
secretion in tumor cells, which subsequently promotes tumor cell
stemness in an autocrine manner, as well as TAN infiltration and
polarization in a paracrine manner (83). Furthermore, fibroblast growth
factor 19 secreted by tumor cells induces the formation of
inflammatory CAFs via the fibroblast growth factor
receptor-JAK2-STAT3 pathway, and the release of C5α and IL-1β from
inflammatory CAFs promotes the formation of NETs, leading to liver
metastases in colorectal cancer (CRC) (84). Other studies have shown that mast
cell-derived TNF promotes the extravasation of neutrophils
(85).
In summary, TANs exhibit dual pro-tumor and
anti-tumor effects. Next, the molecular mechanisms underlying
TAN-mediated resistance to cancer therapy will be explored,
including resistance to immunotherapy, chemotherapy, targeted
therapy and radiotherapy.
Anti-tumor drugs mainly include chemotherapeutic
drugs, molecularly targeted drugs and immune checkpoint inhibitors,
with clinical treatment plans based on a combination of drug
regimens (86). As TANs can
induce resistance to specific drugs through various different
mechanisms, the specific mechanisms of TAN-mediated drug resistance
have been studied in the context of anti-tumor therapy.
Immunotherapy, which predominantly includes immune
checkpoint inhibitors and chimeric antigen receptor (CAR)-T cell
therapy, utilizes the immune system of the patient to attack the
tumor (87). Neutrophils play an
important role in immunotherapy and have a significant impact on
the outcome of tumor treatment.
Anti-PD-1/ PD-L1 therapies have become an important
part of numerous cancer treatments and showed remarkable success.
These therapies effectively inhibit tumor growth and metastasis by
inhibiting the immune checkpoint molecules PD-1/PD-L1 and restoring
the T cell-mediated immune response to the tumor (88-90). However, T-cell depletion, with
which TANs are closely associated, is a major impediment to
immunotherapy (91-93). Numerous studies have revealed that
TANs influence anti-PD-1/PD-L1 therapy primarily by promoting an
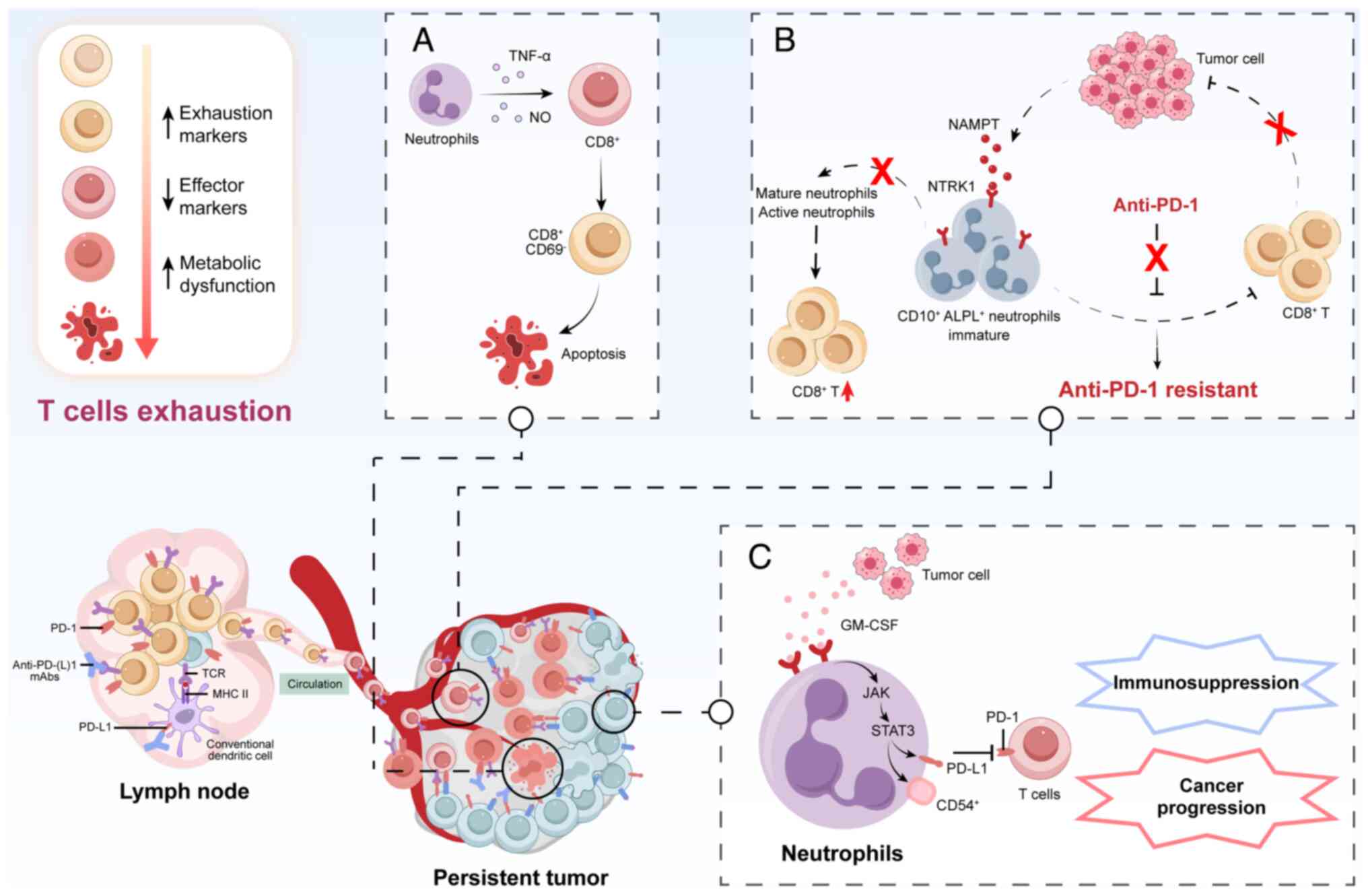
immunosuppressive TME (94,95). Tumor-secreted nicotinamide
phosphoribosyl transferase reprograms CD10+ alkaline
phosphatase, biomineralization associated+ neutrophils
via neurotrophic tropomyosin receptor kinase 1, maintaining their
immaturity and inhibiting their maturation and activation, which
induces apparent 'irreversible' exhaustion of T cells in terms of
their cell number, frequency and gene profile (96). In HCC, overexpression of CT10
regulator of kinase-like (CRKL) shapes the immunosuppressive TME by
recruiting TANs through the upregulation of CRKL/β-catenin/VEGFα
and CXCL1 axes. A decrease in the proportion of activated
CD8+ T cells was accompanied by an increase of depleted
CD8+ T cells in the CRKL overexpression group.
PD-L1+ TANs, a potential subset of TANs regulated by
CRKL, are significantly upregulated in CRKL-overexpressing tumor
tissues, exerting an immune-suppressive effect and resulting in
poor patient prognosis. Studies in mice have verified that
lymphocyte antigen-6 complex, locus G (Ly6G) restores the efficacy
of anti-PD-1 treatment following CRKL overexpression-induced
anti-PD-1 resistance, revealing that CRKL regulates PD-1 resistance
by mediating the infiltration of TANs in HCC (97). Michaeli et al (98) isolated TANs from different mouse
tumor models, which induced apoptosis of CD8+ T cells in
the TME via TNF-α and NO contact-dependent mechanisms. Wang et
al (99) found that tumors
isolated from patients with gastric cancer were infiltrated with
CD54+ TANs, expressing high levels of PD-L1.
Furthermore, GM-CSF secreted by tumor cells activates TANs and
induces PD-L1 expression on TANs through the JAK-STAT3 signaling
pathways (99). These activated
neutrophils contribute to immunosuppression and cancer progression
by inhibiting T-cell immunity in a PD-L1-PD-1-dependent manner. The
multiple mechanisms of T-cell exhaustion induced by neutrophils in
the TME are shown in Fig. 2.
The immunosuppressive potential of NETs is
highlighted by the recent discovery of PD-L1 in NETs. PD-L1 is a
ligand that affects adaptive anti-cancer immune responses and
metastasis combined with hepatic ischemia or reperfusion by
inducing T-cell depletion and dysfunction within the TME in a
murine liver model. In a mouse study, treatment with DNase I to
digest NETs attenuated tumor growth and increased functional T-cell
levels (100). Furthermore,
treatment with an adeno-associated virus gene therapy vector
expressing DNase I in the liver inhibited liver metastases of CRC
by inhibiting neutrophil infiltration and NET formation, as well as
restoring local immune responses at the tumor site by increasing
the percentage of CD8+ T cells (101). NETs have also recently emerged
as powerful modulators of immunotherapy outcomes. In a CRC
xenograft model, although the digestion of NETs with DNase I and
treatment with PD-1 reduced tumor growth, combination of the two
strategies had a synergistic effect. Mechanistically, inhibition of
NETs with DNase I reverses resistance to anti-PD-1 blockade by
increasing CD8+ T-cell infiltration and cytotoxicity
(102).
CAR-T therapy has changed the therapeutic landscape
for hematological malignancies. Current challenges of CAR-T cell
therapy are mainly related to side effects, toxicity, T-cell
depletion and a malignant TME (103-105). The most significant difference
between hematological and solid tumors is the presence of the TME
in solid tumors, with the immunosuppressive nature of the TME being
the likely reason why CAR-T cellular immunotherapy has not been
successful in solid tumors (106,107). The TME includes highly
infiltrating mesenchymal stromal cells, and immunosuppressive cells
such as TANs, myeloid-derived suppressor cells (MDSCs), TAMs, mast
cells and regulatory T cells (108). All of these cellular components
contribute to establishing an immune-suppressive TME capable of
interfering with the efficacy of CAR-T cell therapy (109,110). However, the exact mechanism
requires further investigation.
Chemotherapy is currently the primary clinical
option for tumors, but its efficacy is often limited by drug
resistance. This phenomenon results in the failure of
chemotherapeutic drugs as well as the development of multidrug
resistance, which is the main cause of tumor recurrence, metastasis
and death in most patients (111,112). Specifically, TANs can interact
with other immune cells or modulate the TME to alter the efficacy
of chemotherapy.
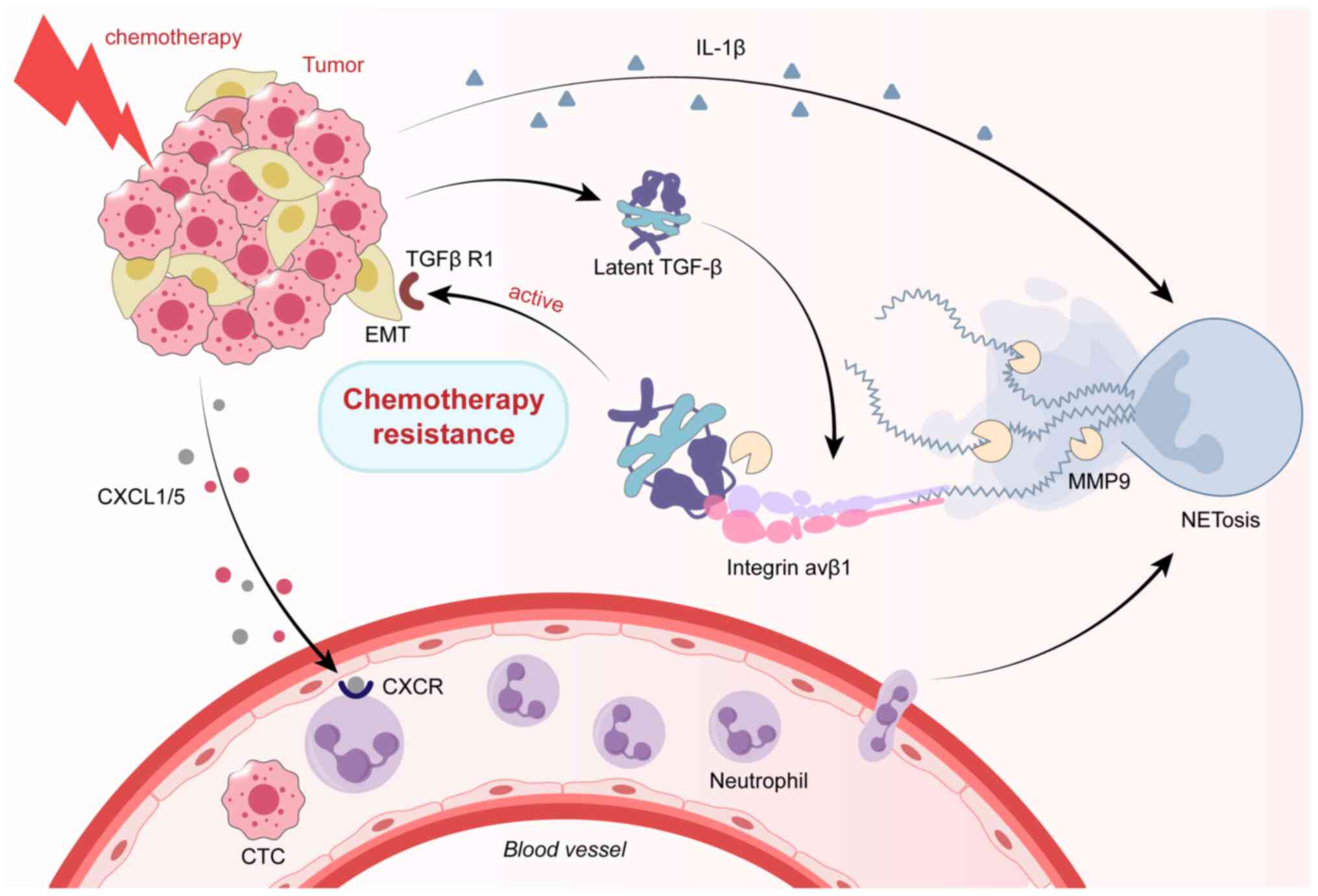
During chemotherapy, neutrophils respond to
chemotherapy by infiltrating the TME and releasing NETs, thereby
promoting chemoresistance (113,114). Mousset et al (113) reported that upregulation of the
CXCL1/5-CXCR2 axis following chemotherapy is involved in the
recruitment of neutrophils to metastatic lungs. Eliminating NETs
through peptidylarginine deiminase 4 inhibitors or DNase I
significantly improved the response to chemotherapy, suggesting a
direct causal relationship between NETs and chemoresistance. In
terms of the mechanism, NETs promote EMT and chemotherapy
resistance by binding and activating TGF-β (a classical inducer of
EMT) (113). In addition,
neutrophils can induce EMT by releasing NETs (115-118). Specifically, neutrophils induce
epithelial stabilization and transcription of zinc finger
e-box-binding homeobox 1 by releasing NETs, thus promoting EMT and
chemotherapy resistance. Furthermore, lung cancer with breast
metastases treated with chemotherapy released CXCL1/5 and IL-1β,
which promoted neutrophil recruitment and NET formation,
respectively, leading to chemoresistance (119). Another study highlighted the
role of neutrophils and NETs in chemotherapy resistance in multiple
myeloma, demonstrating for the first time that neutrophils promote
cancer cell survival by secreting soluble factors in patients
treated with doxorubicin and melphalan (120). Moreover, NETs effectively trap
and inhibit the spread of adriamycin in a two-compartment system,
which may attenuate its ability to induce apoptosis in ovarian
cancer cells (121). The
clinical relevance of NETs in chemotherapy resistance has also been
demonstrated. Plasma NET levels are significantly higher in
patients with metastatic breast cancer exhibiting progressive
disease 15 days after chemotherapy (113). The mechanisms through which
neutrophils induce chemotherapy resistance by infiltrating the TME
and releasing NETs are shown in Fig.
3.
In conclusion, chemotherapy-induced activation of
the NET pathway is a major mechanism of tumor chemoresistance.
Chemokines represent a subset of chemoattractant
cytokines that control the directed migration of immune cells and
play a multifaceted role in tumor cell proliferation, tumor
heterogeneity, stemness, senescence, angiogenesis and tumor
metastasis (122-124). Increasing evidence has revealed
that neutrophil-related chemokines exert a crucial impact on tumor
progression and chemotherapy resistance. In the TME, cancer cells
regulate neutrophil recruitment to tumor sites through the
expression of various chemokine ligands (CXCL1, CXCL2, CXCL5, CXCL6
and CXCL8) for neutrophil receptors CXCR1 and CXCR2 (125,126). Host CXCR2 inhibition by genetic
ablation prevents neutrophil accumulation in pancreatic tumors and
leads to T cell-dependent suppression of tumor growth (127,128). In a zebrafish model of
glioblastoma, CXCR1 mediates the recruitment of neutrophils and
supports the proliferation of tumor-initiating astrocytes (129,130); in melanoma-bearing mice, CXCL1
and CXCL2 chemokines enhance neutrophil recruitment and induce
angiogenesis (131); in HCC,
overexpression of CXCL5 mediates neutrophil infiltration and
indicates poor prognosis (132-134), whereas CXCL6 secretion in tumor
cells promotes TAN infiltration and polarization to accelerate HCC
progression (83); and in
chemotaxis assays and mouse models of thyroid cancer, elevated
concentrations of CXCL8 promote TAN recruitment and cancer
progression (135).
Chemokines play essential roles in TME changes
induced by chemotherapy. Apoptotic CRC cells induced by
chemotherapy release abundant neutrophil-attracting chemokines,
notably CXCL8, thereby attracting neutrophils into the tumor, where
their interaction with neighboring macrophages can promote an
immunologically unfavorable TME (136). CXCL1 and CXCL2 secretion by
metastatic tumor cells recruited neutrophils to the metastatic
liver in mouse models of PDAC. These recruited neutrophils
expressed growth arrest specific 6 (Gas6), which led to AXL
receptor activation on tumor cells, enabling their regrowth.
Furthermore, disruption of neutrophil infiltration or inhibition of
the Gas6/AXL signaling axis in combination with chemotherapy
inhibited metastatic growth (137). To date, growing evidence has
revealed that targeting the chemokine/chemokine receptor axis is a
promising approach to reverse chemoresistance and improve efficacy.
In an orthotopic PDAC model, CXCR2 blockade prevented neutrophil
mobilization from the circulation and augmented chemotherapeutic
efficacy. Targeting both CXCR2+ TANs and
CCR2+ macrophages disrupted myeloid recruitment and
improved the response to FOLFIRINOX chemotherapy in PDAC (138). Overexpression of CXCL1/2 in
breast cancer led to metastasis and resistance to chemotherapy in a
paracrine manner involving the TME. However, CXCR2 blockade
inhibited this vicious cycle, increasing the efficacy of
chemotherapy against breast cancer (139). Reparixin, a small-molecule
inhibitor of the CXCL8-CXCR1/2 axis, offers the possibility of
chemotherapy-induced synergy in breast cancer. For example, a
combination of reparixin with paclitaxel reduced brain metastasis
as well as the population of cancer stem-like cells (140). Sequential treatment with
first-line and second-line chemotherapy and reparixin inhibited
tumor growth, reduced toxicity and prolonged survival in mouse
models of gastric cancer (141).
In addition, a CXCR2-specific small-molecule inhibitor, SB225002,
decreased neutrophil infiltration and reduced tumor growth in lung
cancer (142).
In recent years, advances in molecular biology and
genetics research have shown that malignant tumors exhibit complex
and specific biological defects, including oncogenes, oncogene
mutations and chromatin modifications (143). Targeted therapy utilizes the
specific structural molecules of tumor tissues or cells and drugs
that specifically bind to them to precisely kill tumor cells
(144). However, primary or
acquired resistance limits their clinical use. In this chapter, an
overview of the mechanisms of resistance associated with TANs in
common molecular targeted therapies is presented.
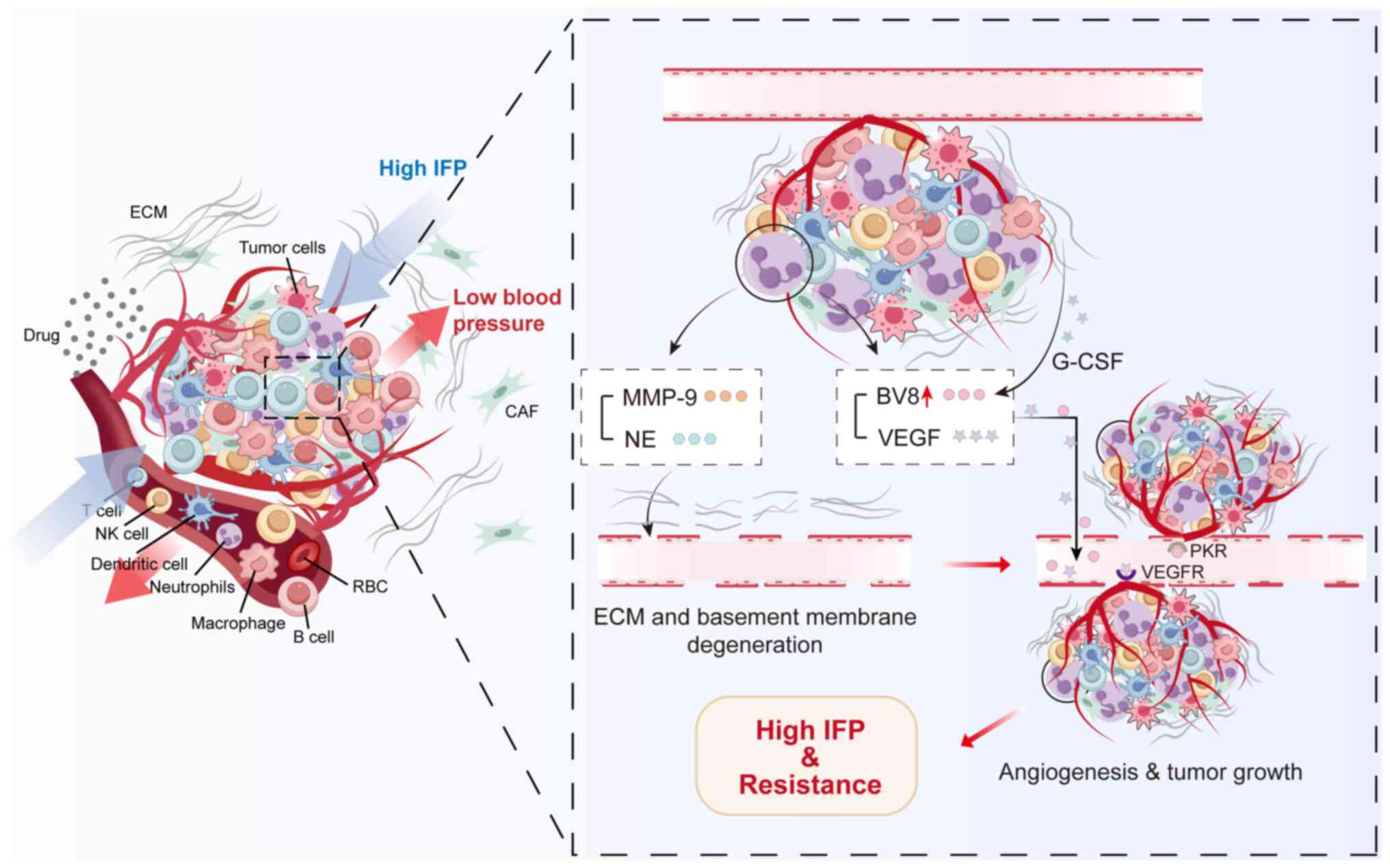
The occurrence and development of tumors depend on
tumor angiogenesis, which provides oxygen and nutrients to tumor
cells, removes metabolic waste and also enables tumor cells to
metastasize (145). Angiogenic
vessels contain irregular branching and form intermittent
arteriovenous shunts, leading to discontinuous perfusion and
disturbed blood flow patterns, resulting in an environment with
abnormally high interstitial fluid pressure (IFP) (146).
VEGF has been identified as a key cytokine involved
in tumor angiogenesis and metastasis (147). TANs release NE and MMP-9 to
degrade the extracellular matrix and later activate VEGF, thus
promoting angiogenesis in the TME (15,20,46). MMP-9 reportedly promotes the
release of VEGF or inhibits the action of anti-angiogenic factors,
exerting a key role in angiogenesis in breast cancer (23,148). In addition to MMP-9, G-CSF
released by tumor and stromal cells upregulated the angiogenic
peptide Bv8 on neutrophils, which promoted angiogenesis by
mediating endothelial cell proliferation (149,150). TANs residing in hypoxic scars
may contribute to distorted blood flow and a high IFP environment
through MMP-9, VEGF and Bv8 (151). High IFP can be a barrier to both
the effective delivery of anti-cancer drugs toward the TME or drug
accumulation within the tumor area, thus promoting tumor resistance
to therapy (152) (Fig. 4).
TANs can also promote angiogenesis through the
direct secretion of IL-17 or indirect activation of the
nucleotide-binding domain, leucine-rich repeat and pyrin
domain-containing protein 3 (NLRP3), leading to increased IL-1β
secretion (153). In mouse tumor
models, treatment with gemcitabine plus 5-fluorouracil induced the
release of proteinase B from TANs and MDSCs, which in turn led to
an IL-1β-dependent increase in IL-17 production and angiogenic
blood vessel formation through the action of NLRP3 (154). Furthermore, neutrophil-derived
Bv8 is associated with resistance to anti-VEGF therapy, whereas
inhibition of G-CSF may increase the efficacy of anti-VEGF therapy
(149,153).
Overall, these studies support the role of
neutrophils in the initial angiogenic switch during tumorigenesis
and reveal the pathways through which TANs may reduce the efficacy
of anti-angiogenic therapy.
EGFR-targeting drugs typically interfere with
activation of the EGFR signaling pathway (155). When EGFR binds to its ligands,
it activates numerous downstream signaling pathways, including
Ras-Raf-MEK-Erk, PI3K-AKT-mTOR and STAT, thus promoting cell
proliferation, growth, angiogenesis and metastasis (156-158). Experiments on A549 cells using
an in vitro co-culture system have shown that elastase
secreted by neutrophils stimulates proteinase-activated receptor 2
and induces EGFR trans-activation to promote drug resistance
(159).
HER-2 is associated with poor prognosis in numerous
cancers but predominantly breast cancer. Drugs targeting HER-2
mainly fall into one of three categories: Monoclonal antibodies,
including trastuzumab and pertuzumab; tyrosine kinase inhibitors,
including lapatinib and eratinib; and antibody-drug conjugates
(160) such as T-DM1. The
results of an autocrine model showed that NE splits cell surface
EGF or TGF-α from the cell membrane to activate signal transduction
(161). TGF-α not only
suppresses HER-2 downregulation by disrupting endocytosis and
lysosome function, but also recruits HER-2 on the cell surface
(162) and may affect the
therapeutic effect of targeting HER-2. Furthermore, combining the
CXCR1/2 inhibitor SCH563705 with lapatinib reduced cancer stem-like
cell activity compared with either treatment alone in HER2-positive
breast cancer via a novel SRC and EGFR/ HER2-dependent mechanism
(163).
KRAS mutations have been implicated in ~40% of CRC
cases, as well as in numerous other types of human cancers, such as
lung cancer, breast cancer and prostate cancer (164-167). Exosomal KRAS mutants exert
stimulatory effects on IL-8 production and NET formation to promote
the growth of CRC cells (168).
Furthermore, when combined with anti-VEGF, neutralizing G-CSF
activity and G-CSF-induced CD11b+Ly6G+
neutrophils is effective in reducing tumor growth and increasing
survival in KRAS-driven PDAC, as indicated in a mouse model with
knockout of pancreatic epithelial-specific TGFβ receptor type II
and activated KRAS (169).
Radiotherapy is one of the most effective approaches
for achieving tumor control (170). Nearly two-thirds of patients
with cancer are treated with radiotherapy, often with the intent to
achieve complete and permanent tumor regression (local control)
(171). However, innate or
acquired radiotherapy resistance remains a significant challenge
that markedly limits the therapeutic effects, leading to cancer
relapse and poor prognosis (172,173). Several crucial aspects
contribute to radiotherapy resistance, including radiation-induced
DNA damage repair, apoptosis escape, cell-cycle arrest, abundance
of cancer stem cells, modification of cancer cells and their
microenvironment, metabolic reprogramming, presence of exosomal and
non-coding RNA and ferroptosis (174-177).
Studies have shown that high neutrophil
infiltration is associated with poor response to radiotherapy
(20,171,178). Neutrophils promote radiotherapy
resistance in various malignant tumors. For instance, in an
irradiated glioblastoma model, Ly6G+ inflammatory cells
promoted the conversion of glioblastoma cells to glioblastoma stem
cells through the NOS2-NO-ID4 regulatory axis. Treatment with
Ly6G-neutralizing antibodies reduced the number of glioblastoma
stem cells and prolonged survival in tumor-bearing mice after
radiotherapy (179). In a model
of local-regional failure for breast cancer after irradiation, high
expression of ectonucleotide pyrophosphatase/phosphodiesterase 1 in
circulating tumor cells enhanced the expression of haptoglobin,
resulting in neutrophil infiltration, NET formation and tumor
relapse (180). Ancey et
al (181) showed that
glucose transporter 1 (GLUT1) expression in TANs promotes lung
cancer growth and resistance to radiotherapy in a mouse model of
lung adenocarcinoma. Loss of GLUT1 accelerates neutrophil turnover
in tumors and reduces a subset of TANs expressing sialic
acid-binding immunoglobulin-like lectin F. In the absence of GLUT1
expression by TANs, tumor growth is diminished and the efficacy of
radiotherapy is augmented (181). Nolan et al (170) found that off-target exposure to
radiation promotes the formation of a premetastatic niche by
neutrophils in a mouse model of breast cancer lung metastasis. By
preventing neutrophil-dependent Notch activation by blocking
degranulation, radiation-enhanced metastases are significantly
offset (170). In bladder
cancer, radiation induces cancer cells to release high mobility
group box 1 (HMGB1), which promotes the formation of NETs through
Toll-like receptor 4 signaling; subsequent inhibition of HMGB1 and
NETs improved the overall radiotherapy response in mouse models
(182). Furthermore, in an
autochthonous mouse model of soft tissue sarcoma, neutrophil
depletion prior to image-guided focal irradiation improved tumor
response to radiotherapy. According to scRNA-seq, tumor
radiosensitization by neutrophil depletion after radiotherapy is
associated with the downregulation of oncogenic transcriptional
programs (171).
Given the impact of TANs on tumors, targeting and
regulating TANs in the TME represents a promising new therapeutic
approach.
Targeted neutrophil therapy focuses on inhibiting
tumor formation, metastasis and angiogenesis by inhibiting the
polarization and recruitment of neutrophils in the TME, preventing
the formation and aggregation of TANs (18,20,184). Several clinical trials of
neutrophil-targeted tumor therapy are currently underway (Table I). In another study,
antibody-dependent cell-mediated cytotoxicity (ADCC) antibody
therapy with monoclonal antibodies (mAbs) was applied to enhance
the ADCC potential of TANs. The results showed that, for two
different tumor targets, EGFR and HER-2, a combination of IgG and
IgA mAbs is more cytotoxic than the antibodies alone (185-187). In addition, as reported by
Kumbhojkar et al (188),
micropatch-loaded neutrophils provide a potent, scalable and
drug-free approach to neutrophil-based cancer immunotherapy.
High infiltration of TANs is associated with poor
prognosis in most human tumors. Neutrophils infiltrate to varying
degrees, as assessed by routine immunohistochemical staining of
neutrophil markers (CD66b in humans and Ly6G in mice) and
neutrophil transcriptional profiles of solid tumors (20). Although TANs are a poor prognostic
indicator of survival in a number of malignant tumors, such as HCC,
cholangiocarcinoma, head and neck cancer and renal cancer (27,189,190), certain studies have found that
TANs can improve the survival rate of patients with colon cancer
(191,192).
The neutrophil-to-lymphocyte ratio (NLR) is
valuable for determining the prognosis of patients with cancer,
with a high NLR associated with poor prognosis in patients with
colorectal, pancreatic, gastric and breast cancers (193-195). A large-scale meta-analysis of
8,500 patients with breast cancer found that a high NLR (1.9 to
5.0) was strongly associated with poor overall survival and reduced
disease-free survival (196). In
patients with CRC and liver metastases who underwent hepatic
lobectomy, an elevated NLR was the only positive predictor of
postoperative recurrence and was positively correlated with tumor
recurrence but negatively correlated with postoperative survival in
patients who underwent in situ liver transplantation for
primary liver cancer (197,198). Furthermore, an elevated NLR
during the postoperative follow-up period was an independent risk
factor for shorter survival in a large number of patients with
gastric cancer who underwent gastric resection (199,200).
Neutrophils have been reported to express
ligand-activated immune checkpoints on T cells. For instance,
PD-L1-expressing neutrophils have prognostic significance in both
HCC and gastric cancer (99).
Zhou et al (134) also
demonstrated that intra-tumor neutrophils express high levels of
CCL2 and CCL17, which correlate with disease progression and
prognosis. The number of CCL2+ and CCL17+
TANs is also positively associated with tumor size, microvascular
invasion, level of tumor differentiation and stage (201). In summary, TANs are potential
biomarkers for tumor therapy.
TANs regulate tumorigenesis and progression by i)
regulating the function of other immune cells, controlling NET
formation and affecting the polarization state, and ii) mediating
resistance to tumor therapy in various ways. However, the existence
and interaction of different immune cell subpopulations in the TME,
the different immune characteristics of the TME in different cancer
types and individual patients, and the depletion of TANs, which can
lead to a reduction of organism immunity, are urgent clinical
problems that must be addressed. Combining existing effective tumor
therapies with neutrophil-targeted therapies may represent a safer
and more effective way to overcome tumor drug resistance. Future
research should further investigate the exact roles, recruitment
pathways and mechanisms of action of TANs to develop therapies that
precisely target TANs and counter drug resistance.
Not applicable.
RH, HJ, HYW, JD and JX conceived the study. RH and
HJ prepared the original draft of the manuscript and drew the
figures. XW, CW, HF, YZ and HCW revised the manuscript. JX and HJ
supervised and approved the final manuscript. Data authentication
is not applicable. All the authors have read and agreed to the
published version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This work was supported by grants from the Cohort and Clinical
Research Program of Wuxi Medical Center, Nanjing Medical University
(grant no. WXKY202302013), 'Taihu Light' Technology Research
Program of Wuxi Science and Technology Bureau (grant no.
K20241014), the General Program of Wuxi Medical Center, Nanjing
Medical University (grant nos. WMCG202319, WMCG202352, WMCG202353
and WMCG202354), the Doctoral Talent Fund of the Affiliated Wuxi
People's Hospital of Nanjing Medical University (grant nos.
BSRC202207, BSRC202303 and BSRC202309) and the Scientific Research
Program of Wuxi Health Commission (grant nos. BJ2023022 and
Q202451).
|
1
|
Jassim A, Rahrmann EP, Simons BD and
Gilbertson RJ: Cancers make their own luck: Theories of cancer
origins. Nat Rev Cancer. 23:710–724. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mattiuzzi C and Lippi G: Cancer
statistics: A comparison between World Health Organization (WHO)
and Global Burden of Disease (GBD). Eur J Public Health.
30:1026–1027. 2020. View Article : Google Scholar
|
|
3
|
Wang J, Yang J, Narang A, He J, Wolfgang
C, Li K and Zheng L: Consensus, debate, and prospective on
pancreatic cancer treatments. J Hematol Oncol. 17:922024.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Long GV, Swetter SM, Menzies AM,
Gershenwald JE and Scolyer RA: Cutaneous melanoma. Lancet.
402:485–502. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Joshi SS and Badgwell BD: Current
treatment and recent progress in gastric cancer. CA Cancer J Clin.
71:264–279. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Visser KE and Joyce JA: The evolving
tumor microenvironment: From cancer initiation to metastatic
outgrowth. Cancer Cell. 41:374–403. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gonçalves AC, Richiardone E, Jorge J,
Polónia B, Xavier CPR, Salaroglio IC, Riganti C, Vasconcelos MH,
Corbet C and Sarmento-Ribeiro AB: Impact of cancer metabolism on
therapy resistance-clinical implications. Drug Resist Updat.
59:1007972021. View Article : Google Scholar
|
|
8
|
Kalli M, Poskus MD, Stylianopoulos T and
Zervantonakis IK: Beyond matrix stiffness: Targeting force-induced
cancer drug resistance. Trends Cancer. 9:937–954. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen D, Gu X, Nurzat Y, Xu L, Li X, Wu L,
Jiao H, Gao P, Zhu X, Yan D, et al: Writers, readers, and erasers
RNA modifications and drug resistance in cancer. Mol Cancer.
23:1782024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He J, Qiu Z, Fan J, Xie X, Sheng Q and Sui
X: Drug tolerant persister cell plasticity in cancer: A
revolutionary strategy for more effective anticancer therapies.
Signal Transduct Target Ther. 9:2092024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nussinov R, Tsai C-J and Jang H:
Anticancer drug resistance: An update and perspective. Drug Resist
Updat. 59:1007962021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Polak R, Zhang ET and Kuo CJ: Cancer
organoids 2.0: Modelling the complexity of the tumour immune
microenvironment. Nat Rev Cancer. 24:523–539. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hessmann E, Buchholz SM, Demir IE, Singh
SK, Gress TM, Ellenrieder V and Neesse A: Microenvironmental
determinants of pancreatic cancer. Physiol Rev. 100:1707–1751.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koenderman L and Vrisekoop N: Neutrophils
in cancer: From biology to therapy. Cell Mol Immunol. 22:4–23.
2025. View Article : Google Scholar :
|
|
15
|
Liu S, Wu W, Du Y, Yin H, Chen Q, Yu W,
Wang W, Yu J, Liu L, Lou W and Pu N: The evolution and
heterogeneity of neutrophils in cancers: Origins, subsets,
functions, orchestrations and clinical applications. Mol Cancer.
22:1482023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Gu J, Wang X, Ji C, Yu D, Wang M,
Pan J, Santos HA, Zhang H and Zhang X: Engineering and targeting
neutrophils for cancer therapy. Adv Mater. 36:e23103182024.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Vlerken-Ysla L, Tyurina YY, Kagan VE
and Gabrilovich DI: Functional states of myeloid cells in cancer.
Cancer Cell. 41:490–504. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Que H, Fu Q, Lan T, Tian X and Wei X:
Tumor-associated neutrophils and neutrophil-targeted cancer
therapies. Biochim Biophys Acta Rev Cancer. 1877:1887622022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xue R, Zhang Q, Cao Q, Kong R, Xiang X,
Liu H, Feng M, Wang F, Cheng J, Li Z, et al: Liver tumour immune
microenvironment subtypes and neutrophil heterogeneity. Nature.
612:141–147. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jaillon S, Ponzetta A, Di Mitri D, Santoni
A, Bonecchi R and Mantovani A: Neutrophil diversity and plasticity
in tumour progression and therapy. Nat Rev Cancer. 20:485–503.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mantovani A, Cassatella MA, Costantini C
and Jaillon S: Neutrophils in the activation and regulation of
innate and adaptive immunity. Nat Rev Immunol. 11:519–531. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fridlender ZG, Sun J, Kim S, Kapoor V,
Cheng G, Ling L, Worthen GS and Albelda SM: Polarization of
tumor-associated neutrophil phenotype by TGF-beta: 'N1' versus 'N2'
TAN. Cancer Cell. 16:183–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shaul ME and Fridlender ZG:
Tumour-associated neutrophils in patients with cancer. Nat Rev Clin
Oncol. 16:601–620. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salcher S, Sturm G, Horvath L, Untergasser
G, Kuempers C, Fotakis G, Panizzolo E, Martowicz A, Trebo M, Pall
G, et al: High-resolution single-cell atlas reveals diversity and
plasticity of tissue-resident neutrophils in non-small cell lung
cancer. Cancer Cell. 40:1503–1520.e8. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ng MSF, Kwok I, Tan L, Shi C,
Cerezo-Wallis D, Tan Y, Leong K, Calvo GF, Yang K, Zhang Y, et al:
Deterministic reprogramming of neutrophils within tumors. Science.
383:eadf64932024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu Y, Ma J, Yang X, Nan F, Zhang T, Ji S,
Rao D, Feng H, Gao K, Gu X, et al: Neutrophil profiling illuminates
anti-tumor antigen-presenting potency. Cell. 187:1422–1439.e24.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang L, Liu Y, Dai Y, Tang X, Yin T, Wang
C, Wang T, Dong L, Shi M, Qin J, et al: Single-cell RNA-seq
analysis reveals BHLHE40-driven pro-tumour neutrophils with
hyperactivated glycolysis in pancreatic tumour microenvironment.
Gut. 72:958–971. 2023. View Article : Google Scholar
|
|
28
|
Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N,
Yi P, Tang L, Pan Q, Rao S, et al: The cancer metabolic
reprogramming and immune response. Mol Cancer. 20:282021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tian S, Chu Y, Hu J, Ding X, Liu Z, Fu D,
Yuan Y, Deng Y, Wang G, Wang L and Wang Z: Tumour-associated
neutrophils secrete AGR2 to promote colorectal cancer metastasis
via its receptor CD98hc-xCT. Gut. 71:2489–2501. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tie Y, Tang F, Wei YQ and Wei XW:
Immunosuppressive cells in cancer: Mechanisms and potential
therapeutic targets. J Hematol Oncol. 15:612022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lianyuan T, Gang L, Ming T, Dianrong X,
Chunhui Y, Zhaolai M and Bin J: Tumor associated neutrophils
promote the metastasis of pancreatic ductal adenocarcinoma. Cancer
Biol Ther. 21:937–945. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amorim C, Docasar CL, Guimarães-Bastos D,
Frony AC, Barja-Fidalgo C, Renovato-Martins M and Moraes JA:
Extracellular vesicles derived from MDA-MB-231 cells trigger
neutrophils to a pro-tumor profile. Cells. 11:18752022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qin F, Liu X, Chen J, Huang S, Wei W, Zou
Y, Liu X, Deng K, Mo S, Chen J, et al: Anti-TGF-β attenuates tumor
growth via polarization of tumor associated neutrophils towards an
anti-tumor phenotype in colorectal cancer. J Cancer. 11:2580–2592.
2020. View Article : Google Scholar :
|
|
34
|
Peng H, Shen J, Long X, Zhou X, Zhang J,
Xu X, Huang T, Xu H, Sun S, Li C, et al: Local release of TGF-β
inhibitor modulates tumor-associated neutrophils and enhances
pancreatic cancer response to combined irreversible electroporation
and immunotherapy. Adv Sci (Weinh). 9:e21052402022. View Article : Google Scholar
|
|
35
|
Tan Q, Ma X, Yang B, Liu Y, Xie Y, Wang X,
Yuan W and Ma J: Periodontitis pathogen Porphyromonas gingivalis
promotes pancreatic tumorigenesis via neutrophil elastase from
tumor-associated neutrophils. Gut Microbes. 14:20737852022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li S, Cong X, Gao H, Lan X, Li Z, Wang W,
Song S, Wang Y, Li C, Zhang H, et al: Tumor-associated neutrophils
induce EMT by IL-17a to promote migration and invasion in gastric
cancer cells. J Exp Clin Cancer Res. 38:62019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J, Yu D, Ji C, Wang M, Fu M, Qian Y
and Zhang X, Ji R, Li C, Gu J and Zhang X: Exosomal
miR-4745-5p/3911 from N2-polarized tumor-associated neutrophils
promotes gastric cancer metastasis by regulating SLIT2. Mol Cancer.
23:1982024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bodac A, Mayet A, Rana S, Pascual J,
Bowler AD, Roh V, Fournier N, Craciun L, Demetter P, Radtke F and
Meylan E: Bcl-xL targeting eliminates ageing tumor-promoting
neutrophils and inhibits lung tumor growth. EMBO Mol Med.
16:158–184. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang S, Sun L, Zuo J and Feng D: Tumor
associated neutrophils governs tumor progression through an
IL-10/STAT3/PD-L1 feedback signaling loop in lung cancer. Transl
Oncol. 40:1018662024. View Article : Google Scholar
|
|
40
|
Huang X, Nepovimova E, Adam V, Sivak L,
Heger Z, Valko M, Wu Q and Kuca K: Neutrophils in cancer
immunotherapy: Friends or foes? Mol Cancer. 23:1072024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bird L: Neutrophils become pro-angiogenic
in tumours. Nat Rev Immunol. 24:1572024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Maas RR, Soukup K, Fournier N, Massara M,
Galland S, Kornete M, Wischnewski V, Lourenco J, Croci D,
Álvarez-Prado ÁF, et al: The local microenvironment drives
activation of neutrophils in human brain tumors. Cell.
186:4546–4566.e27. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qu X, Zhuang G, Yu L, Meng G and Ferrara
N: Induction of Bv8 expression by granulocyte colony-stimulating
factor in CD11b+Gr1+ cells: Key role of Stat3 signaling. J Biol
Chem. 287:19574–19584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fetz AE, Radic MZ and Bowlin GL:
Neutrophils in biomaterial-guided tissue regeneration: Matrix
reprogramming for angiogenesis. Tissue Eng Part B Rev. 27:95–106.
2021. View Article : Google Scholar
|
|
45
|
Vannitamby A, Seow HJ, Anderson G, Vlahos
R, Thompson M, Steinfort D, Irving LB and Bozinovski S:
Tumour-associated neutrophils and loss of epithelial PTEN can
promote corticosteroid-insensitive MMP-9 expression in the
chronically inflamed lung microenvironment. Thorax. 72:1140–1143.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mizuno R, Kawada K, Itatani Y, Ogawa R,
Kiyasu Y and Sakai Y: The role of tumor-associated neutrophils in
colorectal cancer. Int J Mol Sci. 20:5292019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang Y, Liu F, Chen L, Fang C, Li S, Yuan
S, Qian X, Yin Y, Yu B, Fu B, et al: Neutrophil extracellular traps
(NETs) promote non-small cell lung cancer metastasis by suppressing
lncRNA MIR503HG to activate the NF-κB/NLRP3 inflammasome pathway.
Front Immunol. 13:8675162022. View Article : Google Scholar
|
|
48
|
Adrover JM, McDowell SAC, He XY, Quail DF
and Egeblad M: NETworking with cancer: The bidirectional interplay
between cancer and neutrophil extracellular traps. Cancer Cell.
41:505–526. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chu C, Wang X, Yang C, Chen F, Shi L, Xu
W, Wang K, Liu B, Wang C, Sun D and Ding W: Neutrophil
extracellular traps drive intestinal microvascular endothelial
ferroptosis by impairing Fundc1-dependent mitophagy. Redox Biol.
67:1029062023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zheng F, Ma L, Li X, Wang Z, Gao R, Peng
C, Kang B, Wang Y, Luo T, Wu J, et al: Neutrophil extracellular
traps induce glomerular endothelial cell dysfunction and pyroptosis
in diabetic kidney disease. Diabetes. 71:2739–2750. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ngo AT, Skidmore A, Oberg J, Yarovoi I,
Sarkar A, Levine N, Bochenek V, Zhao G, Rauova L, Kowalska MA, et
al: Platelet factor 4 limits neutrophil extracellular trap- and
cell-free DNA-induced thrombogenicity and endothelial injury. JCI
Insight. 8:e1710542023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Teijeira Á, Garasa S, Gato M, Alfaro C,
Migueliz I, Cirella A, de Andrea C, Ochoa MC, Otano I, Etxeberria
I, et al: CXCR1 and CXCR2 chemokine receptor agonists produced by
tumors induce neutrophil extracellular traps that interfere with
immune cytotoxicity. Immunity. 52:856–871.e8. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cristinziano L, Modestino L, Antonelli A,
Marone G, Simon HU, Varricchi G and Galdiero MR: Neutrophil
extracellular traps in cancer. Semin Cancer Biol. 79:91–104. 2022.
View Article : Google Scholar
|
|
54
|
Pan JJ, Xie SZ, Zheng X, Xu JF, Xu H, Yin
RQ, Luo YL, Shen L, Chen ZR, Chen YR, et al: Acetyl-CoA metabolic
accumulation promotes hepatocellular carcinoma metastasis via
enhancing CXCL1-dependent infiltration of tumor-associated
neutrophils. Cancer Lett. 592:2169032024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sun B, Qin W, Song M, Liu L, Yu Y, Qi X
and Sun H: neutrophil suppresses tumor cell proliferation via
fas/fas ligand pathway mediated cell cycle arrested. Int J Biol
Sci. 14:2103–2113. 2018. View Article : Google Scholar :
|
|
56
|
Blaisdell A, Crequer A, Columbus D,
Daikoku T, Mittal K, Dey SK and Erlebacher A: Neutrophils oppose
uterine epithelial carcinogenesis via debridement of hypoxic tumor
cells. Cancer Cell. 28:785–799. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gershkovitz M, Caspi Y, Fainsod-Levi T,
Katz B, Michaeli J, Khawaled S, Lev S, Polyansky L, Shaul ME,
Sionov RV, et al: TRPM2 mediates neutrophil killing of disseminated
tumor cells. Cancer Res. 78:2680–2690. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li Y, Wu S, Zhao Y, Dinh T, Jiang D,
Selfridge JE, Myers G, Wang Y, Zhao X, Tomchuck S, et al:
Neutrophil extracellular traps induced by chemotherapy inhibit
tumor growth in murine models of colorectal cancer. J Clin Invest.
134:e1750312024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Antuamwine BB, Bosnjakovic R, Hofmann-Vega
F, Wang X, Theodosiou T, Iliopoulos I and Brandau S: N1 versus N2
and PMN-MDSC: A critical appraisal of current concepts on
tumor-associated neutrophils and new directions for human oncology.
Immunol Rev. 314:250–279. 2023. View Article : Google Scholar
|
|
60
|
Koga Y, Matsuzaki A, Suminoe A, Hattori H
and Hara T: Neutrophil-derived TNF-related apoptosis-inducing
ligand (TRAIL): A novel mechanism of antitumor effect by
neutrophils. Cancer Res. 64:1037–1043. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cui C, Chakraborty K, Tang XA, Zhou G,
Schoenfelt KQ, Becker KM, Hoffman A, Chang YF, Blank A, Reardon CA,
et al: Neutrophil elastase selectively kills cancer cells and
attenuates tumorigenesis. Cell. 184:3163–3177.e21. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hirschhorn D, Budhu S, Kraehenbuehl L,
Gigoux M, Schröder D, Chow A, Ricca JM, Gasmi B, De Henau O,
Mangarin LMB, et al: T cell immunotherapies engage neutrophils to
eliminate tumor antigen escape variants. Cell. 186:1432–1447.e17.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhou Z, Wang P, Sun R, Li J, Hu Z, Xin H,
Luo C, Zhou J, Fan J and Zhou S: Tumor-associated neutrophils and
macrophages interaction contributes to intrahepatic
cholangiocarcinoma progression by activating STAT3. J Immunother
Cancer. 9:e0019462021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Singhal S, Rao AS, Stadanlick J, Bruns K,
Sullivan NT, Bermudez A, Honig-Frand A, Krouse R, Arambepola S, Guo
E, et al: Human tumor-associated macrophages and neutrophils
regulate antitumor antibody efficacy through lethal and sublethal
trogocytosis. Cancer Res. 84:1029–1047. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wu L and Zhang XH: Tumor-associated
neutrophils and macrophages-heterogenous but not chaotic. Front
Immunol. 11:5539672020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Haider P, Kral-Pointner JB, Mayer J,
Richter M, Kaun C, Brostjan C, Eilenberg W, Fischer MB, Speidl WS,
Hengstenberg C, et al: Neutrophil extracellular trap degradation by
differently polarized macrophage subsets. Arterioscler Thromb Vasc
Biol. 40:2265–2278. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Prame Kumar K, Nicholls AJ and Wong CHY:
Partners in crime: Neutrophils and monocytes/macrophages in
inflammation and disease. Cell Tissue Res. 371:551–565. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Borella R, De Biasi S, Paolini A, Boraldi
F, Lo Tartaro D, Mattioli M, Fidanza L, Neroni A, Caro-Maldonado A,
Meschiari M, et al: Metabolic reprograming shapes neutrophil
functions in severe COVID-19. Eur J Immunol. 52:484–502. 2022.
View Article : Google Scholar
|
|
69
|
Cannarile MA, Weisser M, Jacob W, Jegg AM,
Ries CH and Rüttinger D: Colony-stimulating factor 1 receptor
(CSF1R) inhibitors in cancer therapy. J Immunother Cancer.
5:532017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Cho H, Seo Y, Loke KM, Kim SW, Oh SM, Kim
JH, Soh J, Kim HS, Lee H, Kim J, et al: Cancer-stimulated CAFs
enhance monocyte differentiation and protumoral TAM Activation via
IL6 and GM-CSF Secretion. Clin Cancer Res. 24:5407–5421. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Schmidt E, Distel L, Erber R,
Büttner-Herold M, Rosahl MC, Ott OJ, Strnad V, Hack CC, Hartmann A,
Hecht M, et al: Tumor-associated neutrophils are a negative
prognostic factor in early luminal breast cancers lacking
immunosuppressive macrophage recruitment. Cancers (Basel).
16:31602024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Puerta-Arias JD, Mejía SP and González Á:
The role of the interleukin-17 axis and neutrophils in the
pathogenesis of endemic and systemic mycoses. Front Cell Infect
Microbiol. 10:5953012020. View Article : Google Scholar
|
|
73
|
Murata K, Murao A, Aziz M and Wang P:
Extracellular CIRP induces novel Nectin-2+ (CD112+) neutrophils to
promote Th1 differentiation in sepsis. J Immunol. 210:310–321.
2023. View Article : Google Scholar
|
|
74
|
Parackova Z, Bloomfield M, Klocperk A and
Sediva A: Neutrophils mediate Th17 promotion in COVID-19 patients.
J Leukoc Biol. 109:73–76. 2021. View Article : Google Scholar
|
|
75
|
Mishalian I, Bayuh R, Eruslanov E,
Michaeli J, Levy L, Zolotarov L, Singhal S, Albelda SM, Granot Z
and Fridlender ZG: Neutrophils recruit regulatory T-cells into
tumors via secretion of CCL17-a new mechanism of impaired antitumor
immunity. Int J Cancer. 135:1178–1186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Luo H, Ikenaga N, Nakata K, Higashijima N,
Zhong P, Kubo A, Wu C, Tsutsumi C, Shimada Y, Hayashi M, et al:
Tumor-associated neutrophils upregulate Nectin2 expression,
creating the immunosuppressive microenvironment in pancreatic
ductal adenocarcinoma. J Exp Clin Cancer Res. 43:2582024.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sun R, Xiong Y, Liu H, Gao C, Su L, Weng
J, Yuan X, Zhang D and Feng J: Tumor-associated neutrophils
suppress antitumor immunity of NK cells through the PD-L1/PD-1
axis. Transl Oncol. 13:1008252020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tumino N, Besi F, Di Pace AL, Mariotti FR,
Merli P, Li Pira G, Galaverna F, Pitisci A, Ingegnere T, Pelosi A,
et al: PMN-MDSC are a new target to rescue graft-versus-leukemia
activity of NK cells in haplo-HSC transplantation. Leukemia.
34:932–937. 2020. View Article : Google Scholar :
|
|
79
|
Pelosi A, Besi F, Tumino N, Merli P,
Quatrini L, Li Pira G, Algeri M, Moretta L and Vacca P: NK Cells
and PMN-MDSCs in the graft from G-CSF mobilized haploidentical
donors display distinct gene expression profiles from those of the
non-mobilized counterpart. Front Immunol. 12:6573292021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Mouchemore KA and Anderson RL:
Immunomodulatory effects of G-CSF in cancer: Therapeutic
implications. Semin Immunol. 54:1015122021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ogura K, Sato-Matsushita M, Yamamoto S,
Hori T, Sasahara M, Iwakura Y, Saiki I, Tahara H and Hayakawa Y: NK
cells control tumor-promoting function of neutrophils in mice.
Cancer Immunol Res. 6:348–357. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li X, Xie G, Chen J, Wang Y, Zhai J and
Shen L: Tumour cell-derived serglycin promotes IL-8 secretion of
CAFs in gastric cancer. Br J Cancer. 131:271–282. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Song M, He J, Pan QZ, Yang J, Zhao J,
Zhang YJ, Huang Y, Tang Y, Wang Q, He J, et al: Cancer-associated
fibroblast-mediated cellular crosstalk supports hepatocellular
carcinoma progression. Hepatology. 73:1717–1735. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Li C, Chen T, Liu J, Wang Y, Zhang C, Guo
L, Shi D, Zhang T, Wang X and Li J: FGF19-Induced inflammatory CAF
promoted neutrophil extracellular trap formation in the liver
metastasis of colorectal cancer. Adv Sci (Weinh). 10:e23026132023.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Dudeck J, Kotrba J, Immler R, Hoffmann A,
Voss M, Alexaki VI, Morton L, Jahn SR, Katsoulis-Dimitriou K,
Winzer S, et al: Directional mast cell degranulation of tumor
necrosis factor into blood vessels primes neutrophil extravasation.
Immunity. 54:468–483.e5. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li JY, Chen YP, Li YQ, Liu N and Ma J:
Chemotherapeutic and targeted agents can modulate the tumor
microenvironment and increase the efficacy of immune checkpoint
blockades. Mol Cancer. 20:272021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Oliveira G and Wu CJ: Dynamics and
specificities of T cells in cancer immunotherapy. Nat Rev Cancer.
23:295–316. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yi M, Zheng X, Niu M, Zhu S, Ge H and Wu
K: Combination strategies with PD-1/PD-L1 blockade: Current
advances and future directions. Mol Cancer. 21:282022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Chu X, Tian W, Wang Z, Zhang J and Zhou R:
Co-inhibition of TIGIT and PD-1/PD-L1 in cancer immunotherapy:
Mechanisms and clinical trials. Mol Cancer. 22:932023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wu M, Huang Q, Xie Y, Wu X, Ma H, Zhang Y
and Xia Y: Improvement of the anticancer efficacy of PD-1/PD-L1
blockade via combination therapy and PD-L1 regulation. J Hematol
Oncol. 15:242022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Gjuka D, Adib E, Garrison K, Chen J, Zhang
Y, Li W, Boutz D, Lamb C, Tanno Y, Nassar A, et al: Enzyme-mediated
depletion of methylthioadenosine restores T cell function in
MTAP-deficient tumors and reverses immunotherapy resistance. Cancer
Cell. 41:1774–1787.e9. 2023. View Article : Google Scholar
|
|
92
|
Niederlova V, Tsyklauri O, Kovar M and
Stepanek O: IL-2-driven CD8+ T cell phenotypes: Implications for
immunotherapy. Trends Immunol. 44:890–901. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Si J, Shi X, Sun S, Zou B, Li Y, An D, Lin
X, Gao Y, Long F, Pang B, et al: Hematopoietic progenitor kinase1
(HPK1) mediates T cell dysfunction and is a druggable target for T
cell-based immunotherapies. Cancer Cell. 38:551–566.e11. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Miao S, Rodriguez BL and Gibbons DL: The
multifaceted role of neutrophils in NSCLC in the era of immune
checkpoint inhibitors. Cancers (Basel). 16:25072024. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Xu P, Zhang X, Chen K, Zhu M, Jia R, Zhou
Q, Yang J, Dai J, Jin Y and Shi K: Tumor cell-derived
microparticles induced by methotrexate augment T-cell antitumor
responses by downregulating expression of PD-1 in neutrophils.
Cancer Immunol Res. 11:501–514. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Meng Y, Ye F, Nie P, Zhao Q, An L, Wang W,
Qu S, Shen Z, Cao Z, Zhang X, et al: Immunosuppressive CD10+ALPL+
neutrophils promote resistance to anti-PD-1 therapy in HCC by
mediating irreversible exhaustion of T cells. J Hepatol.
79:1435–1449. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Xie P, Yu M, Zhang B, Yu Q, Zhao Y, Wu M,
Jin L, Yan J, Zhou B, Liu S, et al: CRKL dictates anti-PD-1
resistance by mediating tumor-associated neutrophil infiltration in
hepatocellular carcinoma. J Hepatol. 81:93–107. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Michaeli J, Shaul ME, Mishalian I, Hovav
AH, Levy L, Zolotriov L, Granot Z and Fridlender ZG:
Tumor-associated neutrophils induce apoptosis of non-activated CD8
T-cells in a TNFα and NO-dependent mechanism, promoting a
tumor-supportive environment. Oncoimmunology. 6:e13569652017.
View Article : Google Scholar
|
|
99
|
Wang TT, Zhao YL, Peng LS, Chen N, Chen W,
Lv YP, Mao FY, Zhang JY, Cheng P, Teng YS, et al: Tumour-activated
neutrophils in gastric cancer foster immune suppression and disease
progression through GM-CSF-PD-L1 pathway. Gut. 66:1900–1911. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kaltenmeier C, Yazdani HO, Morder K,
Geller DA, Simmons RL and Tohme S: Neutrophil extracellular traps
promote T cell exhaustion in the tumor microenvironment. Front
Immunol. 12:7852222021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Xia Y, He J, Zhang H, Wang H, Tetz G,
Maguire CA, Wang Y, Onuma A, Genkin D, Tetz V, et al: AAV-mediated
gene transfer of DNase I in the liver of mice with colorectal
cancer reduces liver metastasis and restores local innate and
adaptive immune response. Mol Oncol. 14:2920–2935. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhang H, Wang Y, Onuma A, He J, Wang H,
Xia Y, Lal R, Cheng X, Kasumova G, Hu Z, et al: Neutrophils
extracellular traps inhibition improves PD-1 blockade immunotherapy
in colorectal cancer. Cancers (Basel). 13:53332021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Peng JJ, Wang L, Li Z, Ku CL and Ho PC:
Metabolic challenges and interventions in CAR T cell therapy. Sci
Immunol. 8:eabq30162023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Albelda SM: CAR T cell therapy for
patients with solid tumours: Key lessons to learn and unlearn. Nat
Rev Clin Oncol. 21:47–66. 2024. View Article : Google Scholar
|
|
105
|
Bulliard Y, Andersson BS, Baysal MA,
Damiano J and Tsimberidou AM: Reprogramming T cell differentiation
and exhaustion in CAR-T cell therapy. J Hematol Oncol. 16:1082023.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Pan K, Farrukh H, Chittepu VCSR, Xu H, Pan
CX and Zhu Z: CAR race to cancer immunotherapy: from CAR T, CAR NK
to CAR macrophage therapy. J Exp Clin Cancer Res. 41:1192022.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Hong M, Clubb JD and Chen YY: Engineering
CAR-T cells for next-generation cancer therapy. Cancer Cell.
38:473–488. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhang H, Yu P, Tomar VS, Chen X, Atherton
MJ, Lu Z, Zhang HG, Li S, Ortiz A, Gui J, et al: Targeting PARP11
to avert immunosuppression and improve CAR T therapy in solid
tumors. Nat Cancer. 3:808–820. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
The Lancet Oncology: CAR T-cell therapy
for solid tumours. Lancet Oncol. 22:8932021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Li X, Zhu T, Wang R, Chen J, Tang L, Huo
W, Huang X and Cao Q: Genetically programmable vesicles for
enhancing CAR-T therapy against solid tumors. Adv Mater.
35:e22111382023. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Krishnan SR and Bebawy M: Circulating
biosignatures in multiple myeloma and their role in multidrug
resistance. Mol Cancer. 22:792023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wen X, Huang Z, Yang X, He X, Li L, Chen
H, Wang K, Guo Q and Liu J: Development of an aptamer capable of
multidrug resistance reversal for tumor combination chemotherapy.
Proc Natl Acad Sci USA. 121:e23211161212024. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Mousset A, Lecorgne E, Bourget I, Lopez P,
Jenovai K, Cherfils-Vicini J, Dominici C, Rios G, Girard-Riboulleau
C, Liu B, et al: Neutrophil extracellular traps formed during
chemotherapy confer treatment resistance via TGF-β activation.
Cancer Cell. 41:757–775.e10. 2023. View Article : Google Scholar
|
|
114
|
Saw PE, Chen J and Song E: ChemoNETosis: A
road to tumor therapeutic resistance. Cancer Cell. 41:655–657.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Yang Y, Yu S, Lv C and Tian Y: NETosis in
tumour microenvironment of liver: From primary to metastatic
hepatic carcinoma. Ageing Res Rev. 97:1022972024. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Kong X, Zhang Y, Xiang L, You Y, Duan Y,
Zhao Y, Li S, Wu R, Zhang J, Zhou L and Duan L: Fusobacterium
nucleatum-triggered neutrophil extracellular traps facilitate
colorectal carcinoma progression. J Exp Clin Cancer Res.
42:2362023. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zhang Y, Yang Y, Hu X, Wang Z, Li L and
Chen P: PADs in cancer: Current and future. Biochim Biophys Acta
Rev Cancer. 1875:1884922021. View Article : Google Scholar
|
|
118
|
Zhan X, Wu R, Kong XH, You Y, He K, Sun
XY, Huang Y, Chen WX and Duan L: Elevated neutrophil extracellular
traps by HBV-mediated S100A9-TLR4/RAGE-ROS cascade facilitate the
growth and metastasis of hepatocellular carcinoma. Cancer Commun
(Lond). 43:225–245. 2023. View Article : Google Scholar
|
|
119
|
Mousset A, Bellone L, Gaggioli C and
Albrengues J: NETscape or NEThance: Tailoring anti-cancer therapy.
Trends Cancer. 10:655–667. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ramachandran IR, Condamine T, Lin C,
Herlihy SE, Garfall A, Vogl DT, Gabrilovich DI and Nefedova Y: Bone
marrow PMN-MDSCs and neutrophils are functionally similar in
protection of multiple myeloma from chemotherapy. Cancer Lett.
371:117–124. 2016. View Article : Google Scholar :
|
|
121
|
Tamura K, Miyato H, Kanamaru R, Sadatomo
A, Takahashi K, Ohzawa H, Koyanagi T, Saga Y, Takei Y, Fujiwara H,
et al: Neutrophil extracellular traps (NETs) reduce the diffusion
of doxorubicin which may attenuate its ability to induce apoptosis
of ovarian cancer cells. Heliyon. 8:e097302022. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Goenka A, Khan F, Verma B, Sinha P, Dmello
CC, Jogalekar MP, Gangadaran P and Ahn BC: Tumor microenvironment
signaling and therapeutics in cancer progression. Cancer Commun
(Lond). 43:525–561. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhang R, Dong M, Tu J, Li F, Deng Q, Xu J,
He X, Ding J, Xia J, Sheng D, et al: PMN-MDSCs modulated by CCL20
from cancer cells promoted breast cancer cell stemness through
CXCL2-CXCR2 pathway. Signal Transduct Target Ther. 8:972023.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kang J, La Manna F, Bonollo F, Sampson N,
Alberts IL, Mingels C, Afshar-Oromieh A, Thalmann GN and
Karkampouna S: Tumor microenvironment mechanisms and bone
metastatic disease progression of prostate cancer. Cancer Lett.
530:156–169. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Capucetti A, Albano F and Bonecchi R:
Multiple roles for chemokines in neutrophil biology. Front Immunol.
11:12592020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Rajarathnam K, Schnoor M, Richardson RM
and Rajagopal S: How do chemokines navigate neutrophils to the
target site: Dissecting the structural mechanisms and signaling
pathways. Cell Signal. 54:69–80. 2019. View Article : Google Scholar :
|
|
127
|
Bianchi A, De Castro Silva I, Deshpande
NU, Singh S, Mehra S, Garrido VT, Guo X, Nivelo LA, Kolonias DS,
Saigh SJ, et al: Cell-Autonomous Cxcl1 Sustains Tolerogenic
Circuitries and Stromal Inflammation via Neutrophil-Derived TNF in
Pancreatic Cancer. Cancer Discov. 13:1428–1453. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Chao T, Furth EE and Vonderheide RH:
CXCR2-Dependent accumulation of tumor-associated neutrophils
regulates T-cell immunity in pancreatic ductal adenocarcinoma.
Cancer Immunol Res. 4:968–982. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Corsaro A, Tremonti B, Bajetto A, Barbieri
F, Thellung S and Florio T: Chemokine signaling in tumors:
potential role of CXC chemokines and their receptors as
glioblastoma therapeutic targets. Expert Opin Ther Targets.
28:937–952. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Powell D, Lou M, Barros Becker F and
Huttenlocher A: Cxcr1 mediates recruitment of neutrophils and
supports proliferation of tumor-initiating astrocytes in vivo. Sci
Rep. 8:132852018. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Jablonska J, Wu CF, Andzinski L, Leschner
S and Weiss S: CXCR2-mediated tumor-associated neutrophil
recruitment is regulated by IFN-β. Int J Cancer. 134:1346–1358.
2014. View Article : Google Scholar
|
|
132
|
Haider C, Hnat J, Wagner R, Huber H,
Timelthaler G, Grubinger M, Coulouarn C, Schreiner W, Schlangen K,
Sieghart W, et al: Transforming growth factor-β and Axl induce
CXCL5 and neutrophil recruitment in hepatocellular carcinoma.
Hepatology. 69:222–236. 2019. View Article : Google Scholar
|
|
133
|
Zhou SL, Yin D, Hu ZQ, Luo CB, Zhou ZJ,
Xin HY, Yang XR, Shi YH, Wang Z, Huang XW, et al: A positive
feedback loop between cancer stem-like cells and tumor-associated
neutrophils controls hepatocellular carcinoma progression.
Hepatology. 70:1214–1230. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z,
Chen EB, Fan J, Cao Y, Dai Z and Zhou J: Tumor-associated
neutrophils recruit macrophages and T-regulatory cells to promote
progression of hepatocellular carcinoma and resistance to
sorafenib. Gastroenterology. 150:1646–1658.e17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
He J, Zhou M, Yin J, Wan J, Chu J, Jia J,
Sheng J, Wang C, Yin H and He F: METTL3 restrains papillary thyroid
cancer progression via m6A/c-Rel/IL-8-mediated neutrophil
infiltration. Mol Ther. 29:1821–1837. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Schimek V, Strasser K, Beer A, Göber S,
Walterskirchen N, Brostjan C, Müller C, Bachleitner-Hofmann T,
Bergmann M, Dolznig H and Oehler R: Tumour cell apoptosis modulates
the colorectal cancer immune microenvironment via
interleukin-8-dependent neutrophil recruitment. Cell Death Dis.
13:1132022. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Bellomo G, Rainer C, Quaranta V, Astuti Y,
Raymant M, Boyd E, Stafferton R, Campbell F, Ghaneh P, Halloran CM,
et al: Chemotherapy-induced infiltration of neutrophils promotes
pancreatic cancer metastasis via Gas6/AXL signalling axis. Gut.
71:2284–2299. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Nywening TM, Belt BA, Cullinan DR, Panni
RZ, Han BJ, Sanford DE, Jacobs RC, Ye J, Patel AA, Gillanders WE,
et al: Targeting both tumour-associated CXCR2+ neutrophils and
CCR2+ macrophages disrupts myeloid recruitment and improves
chemotherapeutic responses in pancreatic ductal adenocarcinoma.
Gut. 67:1112–1123. 2018. View Article : Google Scholar
|
|
139
|
Cheng Y, Ma XL, Wei YQ and Wei XW:
Potential roles and targeted therapy of the CXCLs/CXCR2 axis in
cancer and inflammatory diseases. Biochim Biophys Acta Rev Cancer.
1871:289–312. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Schott AF, Goldstein LJ, Cristofanilli M,
Ruffini PA, McCanna S, Reuben JM, Perez RP, Kato G and Wicha M:
Phase Ib pilot study to evaluate reparixin in combination with
weekly paclitaxel in patients with HER-2-negative metastatic breast
cancer. Clin Cancer Res. 23:5358–5365. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Jiang H, Cui J, Chu H, Xu T, Xie M, Jing
X, Xu J, Zhou J and Shu Y: Targeting IL8 as a sequential therapy
strategy to overcome chemotherapy resistance in advanced gastric
cancer. Cell Death Discov. 8:2352022. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Cheng Y, Mo F, Li Q, Han X, Shi H, Chen S,
Wei Y and Wei X: Targeting CXCR2 inhibits the progression of lung
cancer and promotes therapeutic effect of cisplatin. Mol Cancer.
20:622021. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Kiri S and Ryba T: Cancer, metastasis, and
the epigenome. Mol Cancer. 23:1542024. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Fang Y, Wang S, Han S, Zhao Y, Yu C, Liu H
and Li N: Targeted protein degrader development for cancer:
Advances, challenges, and opportunities. Trends Pharmacol Sci.
44:303–317. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Liu ZL, Chen HH, Zheng LL, Sun LP and Shi
L: Angiogenic signaling pathways and anti-angiogenic therapy for
cancer. Signal Transduct Target Ther. 8:1982023. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Zhang T, Jia Y, Yu Y, Zhang B, Xu F and
Guo H: Targeting the tumor biophysical microenvironment to reduce
resistance to immunotherapy. Adv Drug Deliv Rev. 186:1143192022.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Yang M, Mu Y, Yu X, Gao D, Zhang W, Li Y,
Liu J, Sun C and Zhuang J: Survival strategies: How tumor hypoxia
microenvironment orchestrates angiogenesis. Biomed Pharmacother.
176:1167832024. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Li H, Qiu Z, Li F and Wang C: The
relationship between MMP-2 and MMP-9 expression levels with breast
cancer incidence and prognosis. Oncol Lett. 14:5865–5870.
2017.PubMed/NCBI
|
|
149
|
Negri L and Ferrara N: The prokineticins:
Neuromodulators and mediators of inflammation and myeloid
cell-dependent angiogenesis. Physiol Rev. 98:1055–1082. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Shojaei F and Ferrara N: Refractoriness to
antivascular endothelial growth factor treatment: Role of myeloid
cells. Cancer Res. 68:5501–5504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Shojaei F, Wu X, Zhong C, Yu L, Liang XH,
Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N, et al: Bv8
regulates myeloid-cell-dependent tumour angiogenesis. Nature.
450:825–831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Majidpoor J and Mortezaee K: Angiogenesis
as a hallmark of solid tumors-clinical perspectives. Cell Oncol
(Dordr). 44:715–737. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Chung AS, Wu X, Zhuang G, Ngu H, Kasman I,
Zhang J, Vernes JM, Jiang Z, Meng YG, Peale FV, et al: An
interleukin-17-mediated paracrine network promotes tumor resistance
to anti-angiogenic therapy. Nat Med. 19:1114–1123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Li TJ, Jiang YM, Hu YF, Huang L, Yu J,
Zhao LY, Deng HJ, Mou TY, Liu H, Yang Y, et al:
Interleukin-17-producing neutrophils link inflammatory stimuli to
disease progression by promoting angiogenesis in gastric cancer.
Clin Cancer Res. 23:1575–1585. 2017. View Article : Google Scholar
|
|
155
|
Lee JM, McNamee CJ, Toloza E, Negrao MV,
Lin J, Shum E, Cummings AL, Kris MG, Sepesi B, Bara I, et al:
Neoadjuvant targeted therapy in resectable NSCLC: Current and
future perspectives. J Thorac Oncol. 18:1458–1477. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Napolitano S, Martini G, Ciardiello D, Del
Tufo S, Martinelli E, Troiani T and Ciardiello F: Targeting the
EGFR signalling pathway in metastatic colorectal cancer. Lancet
Gastroenterol Hepatol. 9:664–676. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Damare R, Engle K and Kumar G: Targeting
epidermal growth factor receptor and its downstream signaling
pathways by natural products: A mechanistic insight. Phytother Res.
38:2406–2447. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Wang X, Jiang W, Du Y, Zhu D, Zhang J,
Fang C, Yan F and Chen ZS: Targeting feedback activation of
signaling transduction pathways to overcome drug resistance in
cancer. Drug Resist Updat. 65:1008842022. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Kim GT, Hahn KW, Yoon SY, Sohn KY and Kim
JW: PLAG exerts anti-metastatic effects by interfering with
neutrophil elastase/PAR2/EGFR signaling in A549 lung cancer
orthotopic model. Cancers (Basel). 12:5602020. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Swain SM, Shastry M and Hamilton E:
Targeting HER2-positive breast cancer: Advances and future
directions. Nat Rev Drug Discov. 22:101–126. 2023. View Article : Google Scholar
|
|
161
|
Sato T, Takahashi S, Mizumoto T, Harao M,
Akizuki M, Takasugi M, Fukutomi T and Yamashita J: Neutrophil
elastase and cancer. Surg Oncol. 15:217–222. 2006. View Article : Google Scholar
|
|
162
|
Schlessinger J: Common and distinct
elements in cellular signaling via EGF and FGF receptors. Science.
306:1506–1507. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Singh JK, Farnie G, Bundred NJ, Simões BM,
Shergill A, Landberg G, Howell SJ and Clarke RB: Targeting CXCR1/2
significantly reduces breast cancer stem cell activity and
increases the efficacy of inhibiting HER2 via HER2-dependent and
-independent mechanisms. Clin Cancer Res. 19:643–656. 2013.
View Article : Google Scholar
|
|
164
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Singhal A, Li BT and O'Reilly EM:
Targeting KRAS in cancer. Nat Med. 30:969–983. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Biller LH and Schrag D: Diagnosis and
treatment of metastatic colorectal cancer: A review. JAMA.
325:669–685. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Zhu G, Pei L, Xia H, Tang Q and Bi F: Role
of oncogenic KRAS in the prognosis, diagnosis and treatment of
colorectal cancer. Mol Cancer. 20:1432021. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Shang A, Gu C, Zhou C, Yang Y, Chen C,
Zeng B, Wu J, Lu W, Wang W, Sun Z and Li D: Exosomal KRAS mutation
promotes the formation of tumor-associated neutrophil extracellular
traps and causes deterioration of colorectal cancer by inducing
IL-8 expression. Cell Commun Signal. 18:522020. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Pickup MW, Owens P, Gorska AE, Chytil A,
Ye F, Shi C, Weaver VM, Kalluri R, Moses HL and Novitskiy SV:
Development of aggressive pancreatic ductal adenocarcinomas depends
on granulocyte colony stimulating factor secretion in carcinoma
cells. Cancer Immunol Res. 5:718–729. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Nolan E, Bridgeman VL, Ombrato L, Karoutas
A, Rabas N, Sewnath CAN, Vasquez M, Rodrigues FS, Horswell S, Faull
P, et al: Radiation exposure elicits a neutrophil-driven response
in healthy lung tissue that enhances metastatic colonization. Nat
Cancer. 3:173–187. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Wisdom AJ, Hong CS, Lin AJ, Xiang Y,
Cooper DE, Zhang J, Xu ES, Kuo HC, Mowery YM, Carpenter DJ, et al:
Neutrophils promote tumor resistance to radiation therapy. Proc
Natl Acad Sci USA. 116:18584–18589. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Zheng X, Song X, Zhu G, Pan D, Li H, Hu J,
Xiao K, Gong Q, Gu Z, Luo K and Li W: Nanomedicine combats drug
resistance in lung cancer. Adv Mater. 36:e23089772024. View Article : Google Scholar
|
|
173
|
Xu K, Guo H, Xia A, Wang Z, Wang S and
Wang Q: Non-coding RNAs in radiotherapy resistance: Roles and
therapeutic implications in gastrointestinal cancer. Biomed
Pharmacother. 161:1144852023. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Wu Y, Song Y, Wang R and Wang T: Molecular
mechanisms of tumor resistance to radiotherapy. Mol Cancer.
22:962023. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
An L, Li M and Jia Q: Mechanisms of
radiotherapy resistance and radiosensitization strategies for
esophageal squamous cell carcinoma. Mol Cancer. 22:1402023.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Peng J, Yin X, Yun W, Meng X and Huang Z:
Radiotherapyinduced tumor physical microenvironment remodeling to
overcome immunotherapy resistance. Cancer Lett. 559:2161082023.
View Article : Google Scholar
|
|
177
|
Beckers C, Pruschy M and Vetrugno I: Tumor
hypoxia and radiotherapy: A major driver of resistance even for
novel radiotherapy modalities. Semin Cancer Biol. 98:19–30. 2024.
View Article : Google Scholar
|
|
178
|
Wang X, Li X, Wu Y, Hong J and Zhang M:
The prognostic significance of tumor-associated neutrophils and
circulating neutrophils in glioblastoma (WHO CNS5 classification).
BMC Cancer. 23:202023. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Jeon HY, Ham SW, Kim JK, Jin X, Lee SY,
Shin YJ, Choi CY, Sa JK, Kim SH, Chun T, et al: Ly6G+ inflammatory
cells enable the conversion of cancer cells to cancer stem cells in
an irradiated glioblastoma model. Cell Death Differ. 26:2139–2156.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Ruiz-Fernández de Córdoba B, Moreno H,
Valencia K, Perurena N, Ruedas P, Walle T, Pezonaga-Torres A,
Hinojosa J, Guruceaga E, Pineda-Lucena A, et al: Tumor ENPP1
(CD203a)/ haptoglobin axis exploits myeloid-derived suppressor
cells to promote post-radiotherapy local recurrence in breast
cancer. Cancer Discov. 12:1356–1377. 2022. View Article : Google Scholar
|
|
181
|
Ancey PB, Contat C, Boivin G, Sabatino S,
Pascual J, Zangger N, Perentes JY, Peters S, Abel ED, Kirsch DG, et
al: GLUT1 expression in tumor-associated neutrophils promotes lung
cancer growth and resistance to radiotherapy. Cancer Res.
81:2345–2357. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Shinde-Jadhav S, Mansure JJ, Rayes RF,
Marcq G, Ayoub M, Skowronski R, Kool R, Bourdeau F, Brimo F, Spicer
J and Kassouf W: Role of neutrophil extracellular traps in
radiation resistance of invasive bladder cancer. Nat Commun.
12:27762021. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Li H, Zeng J, You Q, Zhang M, Shi Y, Yang
X, Gu W, Liu Y, Hu N, Wang Y, et al: X-ray-activated
nanoscintillators integrated with tumor-associated neutrophils
polarization for improved radiotherapy in metastatic colorectal
cancer. Biomaterials. 316:1230312025. View Article : Google Scholar
|
|
184
|
Rys RN and Calcinotto A: Senescent
neutrophils: A hidden role in cancer progression. Trends Cell Biol.
S0962-8924(24)00187-9. 2024.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Treffers LW, Ten Broeke T, Rösner T,
Jansen JHM, van Houdt M, Kahle S, Schornagel K, Verkuijlen PJJH,
Prins JM, Franke K, et al: IgA-mediated killing of tumor cells by
neutrophils is enhanced by CD47-SIRPα checkpoint inhibition. Cancer
Immunol Res. 8:120–130. 2020. View Article : Google Scholar
|
|
186
|
Brandsma AM, Ten Broeke T, Nederend M,
Meulenbroek LA, van Tetering G, Meyer S, Jansen JH, Beltrán
Buitrago MA, Nagelkerke SQ, Németh I, et al: Simultaneous targeting
of FcγRs and FcαRI enhances tumor cell killing. Cancer Immunol Res.
3:1316–1324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Borrok MJ, Luheshi NM, Beyaz N, Davies GC,
Legg JW, Wu H, Dall'Acqua WF and Tsui P: Enhancement of
antibody-dependent cell-mediated cytotoxicity by endowing IgG with
FcαRI (CD89) binding. MAbs. 7:743–751. 2015. View Article : Google Scholar :
|
|
188
|
Kumbhojkar N, Prakash S, Fukuta T,
Adu-Berchie K, Kapate N, An R, Darko S, Chandran Suja V, Park KS,
Gottlieb AP, et al: Neutrophils bearing adhesive polymer
micropatches as a drug-free cancer immunotherapy. Nat Biomed Eng.
8:579–592. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Quaas A, Pamuk A, Klein S, Quantius J,
Rehkaemper J, Bar utcu AG, Rueschoff J, Zander T, Gebauer F,
Hillmer A, et al: Sex-specific prognostic effect of CD66b-positive
tumor-infiltrating neutrophils (TANs) in gastric and esophageal
adenocarcinoma. Gastric Cancer. 24:1213–1226. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Peng H, Wu X, Liu S, He M, Tang C, Wen Y,
Xie C, Zhong R, Li C, Xiong S, et al: Cellular dynamics in tumour
microenvironment along with lung cancer progression underscore
spatial and evolutionary heterogeneity of neutrophil. Clin Transl
Med. 13:e13402023. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Zhang J, Zhang M, Lou J, Wu L, Zhang S,
Liu X, Ke Y, Zhao S, Song Z, Bai X, et al: Machine learning
integration with single-cell transcriptome sequencing datasets
reveals the impact of tumor-associated neutrophils on the immune
microenvironment and immunotherapy outcomes in gastric cancer. Int
J Mol Sci. 25:127152024. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Ye L, Zhang T, Kang Z, Guo G, Sun Y, Lin
K, Huang Q, Shi X, Ni Z, Ding N, et al: Tumor-infiltrating immune
cells act as a marker for prognosis in colorectal cancer. Front
Immunol. 10:23682019. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Nøst TH, Alcala K, Urbarova I, Byrne KS,
Guida F, Sandanger TM and Johansson M: Systemic inflammation
markers and cancer incidence in the UK Biobank. Eur J Epidemiol.
36:841–848. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Mosca M, Nigro MC, Pagani R, De Giglio A
and Di Federico A: Neutrophil-to-lymphocyte ratio (NLR) in NSCLC,
gastrointestinal, and other solid tumors: Immunotherapy and beyond.
Biomolecules. 13:18032023. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Cupp MA, Cariolou M, Tzoulaki I, Aune D,
Evangelou E and Berlanga-Taylor AJ: Neutrophil to lymphocyte ratio
and cancer prognosis: An umbrella review of systematic reviews and
meta-analyses of observational studies. BMC Med. 18:3602020.
View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Ethier JL, Desautels D, Templeton A, Shah
PS and Amir E: Prognostic role of neutrophil-to-lymphocyte ratio in
breast cancer: A systematic review and meta-analysis. Breast Cancer
Res. 19:22017. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Pecqueux M, Brückner F, Oehme F, Hempel S,
Baenke F, Riediger C, Distler M, Weitz J and Kahlert C:
Preoperative IL-8 levels as prognostic indicators of overall
survival: An extended follow-up in a prospective cohort with
colorectal liver metastases. BMC Cancer. 24:902024. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Hsu YJ, Chern YJ, Wu ZE, Yu YL, Liao CK,
Tsai WS, You JF and Lee CW: The oncologic outcome and prognostic
factors for solitary colorectal liver metastasis after liver
resection. J Gastrointest Surg. 28:267–275. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Huang W, Jiang Y, Xiong W, Sun Z, Chen C,
Yuan Q, Zhou K, Han Z, Feng H, Chen H, et al: Noninvasive imaging
of the tumor immune microenvironment correlates with response to
immunotherapy in gastric cancer. Nat Commun. 13:50952022.
View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Tan S, Zheng Q, Zhang W, Zhou M, Xia C and
Feng W: Prognostic value of inflammatory markers NLR, PLR, and LMR
in gastric cancer patients treated with immune checkpoint
inhibitors: A meta-analysis and systematic review. Front Immunol.
15:14087002024. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
He G, Zhang H, Zhou J, Wang B, Chen Y,
Kong Y, Xie X, Wang X, Fei R, Wei L, et al: Peritumoural
neutrophils negatively regulate adaptive immunity via the
PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J Exp
Clin Cancer Res. 34:1412015. View Article : Google Scholar : PubMed/NCBI
|