Introduction
In western countries, l-oxaliplatin (l-OHP)-based
adjuvant chemotherapy remains the standard adjuvant chemotherapy
for stage III colorectal cancer (1,2).
Furthermore, 5-fluorouracil (5-FU)-based adjuvant chemotherapy is
one of the standard adjuvant chemotherapy choices for stage III
colorectal cancer in Japan (3).
Predictive markers for adjuvant chemotherapy have also been
evaluated, with several molecular predictors being considered as
satisfactory predictive markers for the efficacy of 5-FU-based
chemotherapy (4–6) and 5-FU/l-OHP combination chemotherapy
(7). However, clinically useful
predictors that determine whether patients should be administered
5-FU-based or l-OHP-based chemotherapy remain to be identified.
Moreover, whether adjuvant chemotherapy is required for patients
with stage II colorectal cancer has not yet been determined.
The collagen gel droplet-embedded culture drug
sensitivity test (CD-DST) is a new in vitro anticancer drug
sensitivity test. One of the advantages of CD-DST, compared to
previous anticancer drug sensitivity tests, is that it favors the
growth of tumor tissues in long-term cultures, due to its use of a
three-dimensional growth assay with an image analysis device that
is able to differentiate between cancer cells and fibroblasts
(8). Previous studies reported
that CD-DST may be useful when devising optimal treatment
strategies for ovarian (9) or
gastrointestinal cancer (10–12).
However, the clinical utility of CD-DST in the prediction of the
response to adjuvant chemotherapy for colorectal cancer remains to
be determined. Therefore, we attempted to investigate the clinical
utility of CD-DST as a predictor of the therapeutic response to
5-FU-based adjuvant chemotherapy in patients with stage II–III
colorectal cancer.
Patients and methods
Patient characteristics
This study included 246 patients with stage II–III
colorectal cancer who underwent curative surgery between January,
2000 and December, 2007 at the Department of Surgery, Shiga
University of Medical Science (Otsu, Japan).
A total of 108 men and 138 women with a median age
at the time of surgery of 66.06 years (range, 35–92 years) were
included in this study. As regards tumor location, 174 patients had
colon and 72 had rectal cancer. Stage II cancer was diagnosed in
119 patients (119/246, 48.4%) and stage III disease in 127 patients
(127/246, 51.6%). Staging was based on the general rules for
clinical and pathological studies on cancer of the colon, rectum
and anus (13).
Generally, stage III patients were younger compared
to stage II patients (P=0.025). In the stage II group, the number
of colon cancer patients was significantly higher compared to that
of rectal cancer patients (P=0.0014). No differences in gender and
histological type were observed between stage II and III
patients.
The number of stage III patients who received
adjuvant chemotherapy was significantly higher compared to that of
stage II patients (P=0.0015).
Study protocol
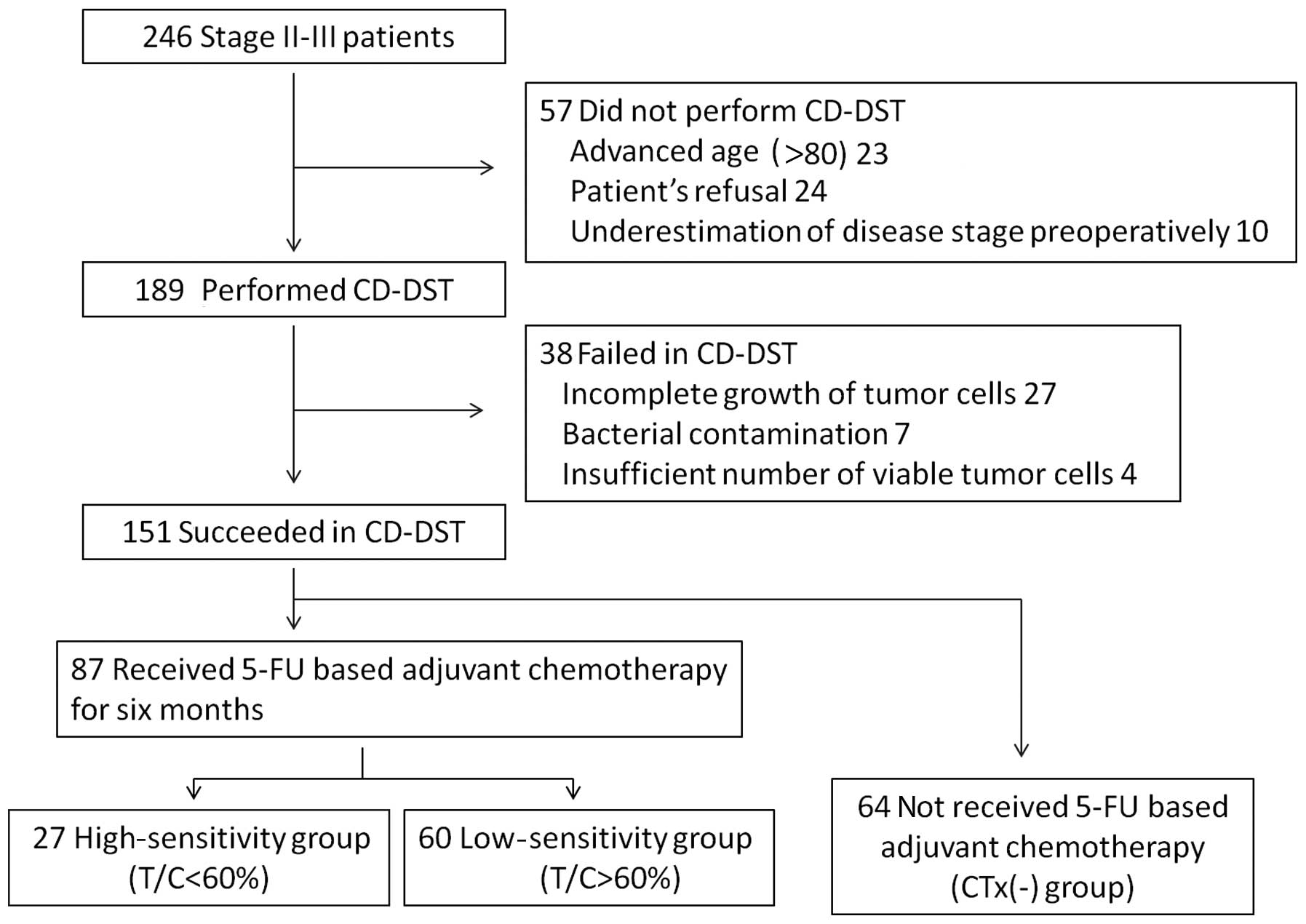
CD-DST was performed in 189 out of the 246 patients
(76.8%) after obtaining written informed consent according to our
institutional guidelines during the study period. CD-DST was not
performed in the remaining 57 patients due to various reasons,
which included advanced age (≥80 years), patient refusal and
underestimation of disease stage preoperatively. Out of the 189
patients, 151 patients (79.9%) underwent successful CD-DST. Failure
of CD-DST in the remaining 38 patients was due to an insufficient
number of viable tumor cells (<1×105 in the initial
assay) in 4 cases, incomplete growth of tumor cells during the
culture period in 27 cases and bacterial contamination in 7 cases
(Fig. 1).
Out of the 151 cases with successful CD-DST, 87
received 5-FU-based adjuvant chemotherapy for 6 months. These
patients were divided into the high-sensitivity (high-group, 27/87,
31.0%) and low-sensitivity (low-group, 60/87, 69.0%) groups. The
remaining 64 patients, who did not receive adjuvant chemotherapy,
were included in the CTx(-) group, in order to verify the clinical
utility of CD-DST (Fig. 1).
This study was a non-randomized retrospective study.
The study protocol conformed to the ethical guidelines established
by the Helsinki Declaration.
CD-DST
5-FU tumor sensitivity was evaluated by CD-DST,
performed as previously described by Kobayashi et al
(8,14,15).
Briefly, tumor tissue was excised from the primary surgical
specimens. Subsequently, the specimens were washed twice with
povidone iodine and twice with antibiotic solution containing 1
mg/ml piperacillin, 0.5 mg/ml kanamycin and 2.5 μg/ml amphotericin
B. The specimens were then digested by dispersion collagenase
enzyme and the dispersed cancer cells were incubated in a collagen
gel-coated flask. Only the viable cells adhering to the collagen
gel layer were then collected and added to reconstructed type I
collagen solution (Cellmatrix Type CDTM; Nitta Gelatin Inc., Yao,
Japan). Three drops of these mixtures were placed in each well of a
6-well multiplate. The plates were incubated in a CO2
incubator at 37°C for 24 h. 5-FU (1.0 μg/ml) was then added to each
well and the plate was incubated for 24 h. Following removal of the
medium containing 5-FU, the well was incubated with PCM-2 medium
(Kurabo Industries Ltd., Osaka, Japan) for 7 days. Neutral red was
added to stain colonies in the collagen gel droplets, which were
then fixed in formalin.
The in vitro chemosensitivity of the tumor
cells to the anticancer agent was expressed as a ratio of the total
colony volume (T) of treated cells to that of untreated cells (C).
Originally, samples with a T/C ratio of ≤50, ≥60 and 51–60% were
defined as in vitro sensitive, resistant and borderline,
respectively. However, in the present study, in accordance to the
results of a previous study (16),
the cut-off ratio was 60%, i.e., samples with a T/C ratio of ≤60%
were considered in vitro sensitive.
Patient follow-up
Cancer recurrence was investigated by chest X-ray
examination, abdominal ultrasonography and/or chest-abdominal CT
every 3 months for 1 year, every 6 months for the following 2 years
and annually thereafter.
Treatment was selected among surgery, systemic
chemotherapy, radiotherapy and microwave coagulation therapy,
according to the guidelines for treatment of colorectal cancer
(3), taking into consideration the
informed consent of the patients.
Statistical analysis
Statistical analyses were performed using the SPSS
software program version 19 (SPSS Inc., Chicago, IL, USA). The
Chi-square test and Fisher’s exact probability test were used to
analyze data. Survival rates were estimated using the Kaplan-Meier
method and the log-rank test was used to compare the curves.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Comparison of clinicopathological
characteristics among groups
In the CTx(-) group, the number of patients with
stage II disease was significantly higher compared to those with
stage III disease (P=0.001). However, no differences were observed
in age, gender, tumor location, histological type, stage, lymphatic
invasion, venous invasion and serum CEA and CA19-9 levels between
patients in the high-, low- and CTx(-) groups. Furthermore, the
chemotherapeutic agents used for adjuvant chemotherapy did not
differ between the high- and low-groups (Table I).
 | Table I.Comparison of the clinicopathological
characteristics among the high-, low- and CTx(-) groups. |
Table I.
Comparison of the clinicopathological
characteristics among the high-, low- and CTx(-) groups.
| Variables | Drug sensitivity
| P-value |
|---|
| High (T/C<60)
n=27 | Low (T/C>60)
n=60 | CTx(-) n=64 |
|---|
| Age (years) | | | | |
| <70 | 17 | 34 | 25 | |
| ≥70 | 10 | 26 | 39 | 0.051 |
| Gender | | | | |
| Male | 18 | 34 | 31 | |
| Female | 9 | 26 | 33 | 0.380 |
| Location | | | | |
| Colon | 22 | 41 | 46 | |
| Rectum | 5 | 19 | 18 | 0.600 |
| Histological
differentiation | | | | |
| High and
moderate | 25 | 57 | 62 | |
| Poor and
others | 2 | 3 | 2 | 0.920 |
| Stage | | | | |
| II | 15 | 18 | 40 | |
| III | 12 | 42 | 24 | 0.001 |
| Lymphatic
invasion | | | | |
| ly 0, 1 | 10 | 19 | 33 | |
| ly 2, 3 | 17 | 41 | 31 | 0.071 |
| Venous invasion | | | | |
| v 0, 1 | 15 | 30 | 40 | |
| v 2, 3 | 12 | 30 | 24 | 0.470 |
| CEA | | | | |
| <5 | 15 | 25 | 28 | |
| ≥5 | 11 | 31 | 28 | 0.950 |
| CA19-9 | | | | |
| <36 | 24 | 41 | 46 | |
| ≥36 | 0 | 10 | 4 | 0.080 |
| Regimen | | | | |
| Oral
fluoropyrimidine | 25 | 47 | - | |
| i.v. 5-FU +
l-LV | 2 | 13 | - | 0.100 |
Overall survival time (OS) and
recurrence-free survival time (RFS) according to the results of
CD-DST
The median duration from operation to follow-up was
53 months (range, 18–107 months). The 5-year OS rate was 96.3% in
the high- and 86.7% in the low-group (P=0.202; Fig. 2). The 5-year RFS rate in the
high-group was significantly higher compared to that in the low-
and CTx(-) groups (92.6 vs. 76.7 and 73.4%, respectively, P=0.040;
Fig. 3). No differences in the
5-year RFS rate were observed between the low- and the CTx(-)
groups (P=0.507). In patients with stage III cancer, the 5-year RFS
rate in the high-group was also significantly higher compared to
that in the low- and CTx(-) groups (92.3 vs. 69.0 and 50.0%,
respectively, P=0.006; Fig. 4).
Furthermore, no differences in the 5-year RFS rate were observed
between stage III patients in the low- and CTx(-) groups
(P=0.069).
Discussion
In Western countries, oxaliplatin (l-OHP)-based
chemotherapy (FOLFOX, FLOX) remains the standard adjuvant
chemotherapy for stage III colon cancer (1,2).
However, Shimada et al (17) reported the favorable outcome
(5-year OS of 87.9%) of 5-FU-based chemotherapy (5-FU +
levofolinate and oral fluoropyrimidine) following curative surgery
in stage III colon cancer patients in Japan. This result was more
favorable compared to the results of previous studies (1,2) with
l-OHP-based adjuvant chemotherapy. Therefore, administration of
5-FU-based chemotherapy for 6 months, regardless of the sensitivity
of individual patients, is one of the standard adjuvant
chemotherapy regimens for the management of stage III colorectal
cancer in Japan. It is generally believed that adjuvant
chemotherapy is unnecessary for stage II colorectal cancer
patients. However, ∼25% of patients with stage II disease, such as
those with penetration of the serosa, perforation, poorly
differentiated histological type, or a yield of <12 lymph nodes,
are considered to bear an accentuated risk of recurrence (18). Therefore, such patients are offered
adjuvant chemotherapy.
It has also been reported that the incidence of
grade 3 neurotoxicity was higher among patients who received
l-OHP-based adjuvant chemotherapy compared to those who received
5-FU-based adjuvant chemotherapy (1,2).
Furthermore, the medical costs of l-OHP-based chemotherapy were
higher compared to those of 5-FU-based chemotherapy. Therefore, we
aim to develop a new method that predicts the therapeutic effect of
adjuvant chemotherapy and enables decision-making regarding whether
patients should be administered 5-FU-based or l-OHP-based
chemotherapy. Several molecular markers have been considered as
satisfactory predictors of the efficacy of 5-FU-based chemotherapy
(4–6), although studies on the effectiveness
of in vitro drug sensitivity tests are limited (19).
Several in vitro chemosensitivity tests for
malignant tumors have been developed and clinically introduced.
Four tests in particular have been widely applied, since they
exhibit a high success rate for primary culture, require a small
number of malignant cells for testing, allow for easy
quantification of anticancer effects without contamination due to
fibroblasts and are cost-effective, rapid and simple. These tests
include CD-DST (8,14,15),
histoculture drug response assay (HDRA) (20), succinate dehydrogenase inhibition
test (21) and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
(22). Among these, CD-DST and
HDRA are commonly used in Japan in clinical practice. The CD-DST is
used in our institute for the selection of potential individualized
chemo-therapy for patients with colorectal cancer, as the HDRA
usually requires high concentrations of anticancer drugs,
approximately 20- to several-hundred fold of the area under the
curve to the observed level in vivo (14,16).
Furthermore, the efficacy of the CD-DST has been clinically
demonstrated in several types of cancer (9–12).
In this study, the 5-year RFS in the high-group was
significantly higher compared to that in the low- and CTx(-)
groups, although the 5-year OS in the high-group was not
significantly higher compared to that in the low-group. These
results may be attributed to the limited case series and the fact
that chemotherapy against colorectal cancer led to prolongation of
patient survival following recurrence. Subgroup analysis
demonstrated that the 5-year RFS rate among stage III patients in
the high-group was significantly higher compared to those in the
low-group. Furthermore, the 5-year RFS rate in the high-group in
this study was comparable, if not superior, to the 5-year RFS rate
of l-OHP regimens reported by previous studies (1,2).
However, no differences were observed among stage II patients (data
not shown). Moreover, no differences in the 5-year RFS in stage II
and III patients were observed between the low- and CTx(-) groups.
These results suggested that patients in the high drug sensitivity
group exhibited a prolonged RFS period compared to patients with
low drug sensitivity. In other words, postoperative 5-FU-based
adjuvant chemotherapy may not have exerted an effect on RFS in
patients who were classified as exhibiting poor drug sensitivity.
These results indicate that l-OHP-based regimens may not be
required in the high-group; however, more potent regimens, such as
l-OHP-based regimens, may be required in the low-group patients.
Although ideally the comparison should have been between high-group
patients who received 5-FU-based chemotherapy and those who did
not, such an analysis was not possible in the present study, due to
the limited number of patients with high sensitivity that did not
receive adjuvant chemotherapy.
Future studies are required to determine whether the
recurrence rate in the low-group may be lowered by l-OHP-based
chemotherapy, whether 5-FU sensitivity testing is able to predict
the therapeutic effect of l-OHP-based chemotherapy and whether
additional l-OHP sensitivity testing is required to predict the
therapeutic effect of l-OHP-based chemotherapy. The limitations of
this study included the limited patient sample and the fact that
this was a non-randomized retrospective study.
In conclusion, we have demonstrated that CD-DST
appears to be useful for the prediction of the therapeutic response
to adjuvant chemotherapy in patients with stage II–III colorectal
cancer. Furthermore, this technology may prove useful for
decision-making with regard to whether patients should be
administered 5-FU-based or l-OHP-based chemotherapy. However, this
study was a small-scale, retrospective analysis conducted at a
single institution. Multi-center prospective randomized trials are
therefore required to gain additional insight into the subject.
References
|
1.
|
André T, Boni C, Mounedji-Boudiaf L, et
al: Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment
for colon cancer. N Engl J Med. 350:2343–2351. 2004.
|
|
2.
|
Kuebler JP, Wieand HS, O’Connell MJ, et
al: Oxaliplatin combined with weekly bolus fluorouracil and
leucovorin as surgical adjuvant chemotherapy for stage II and III
colon cancer: results from NSABP C-07. J Clin Oncol. 25:2198–2204.
2007. View Article : Google Scholar
|
|
3.
|
Japanese Society for Cancer of the Colon
and Rectum: JSCCR Guidelines 2010 for the Treatment of Colorectal
Cancer. Kanehara & Co, Ltd.; Tokyo: 2010
|
|
4.
|
Watanabe T, Wu TT, Catalano PJ, et al:
Molecular predictors of survival after adjuvant chemotherapy for
colon cancer. N Engl J Med. 344:1196–1206. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Sargent DJ, Marsoni S, Monges G, et al:
Defective mismatch repair as a predictive marker for lack of
efficacy of fluorouracil-based adjuvant therapy in colon cancer. J
Clin Oncol. 28:3219–3226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Sinicrope FA, Foster NR, Thibodeau SN, et
al: DNA mismatch repair status and colon cancer recurrence and
survival in clinical trials of 5-fluorouracil-based adjuvant
therapy. J Natl Cancer Inst. 103:863–875. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Tsuji S, Midorikawa Y, Takahashi T, Yagi
K, Takayama T, Yoshida K, Sugiyama Y and Aburatani H: Potential
responders to FOLFOX therapy for colorectal cancer by Random
Forests analysis. Br J Cancer. 106:126–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kobayashi H: Collagen gel droplet culture
method to examine in vitro chemosensitivity. Methods Mol Med.
110:59–67. 2005.PubMed/NCBI
|
|
9.
|
Yabushita H, Ohnishi M, Komiyama M, et al:
Usefulness of collagen gel droplet embedded culture drug
sensitivity testing in ovarian cancer. Oncol Rep. 12:307–311.
2004.PubMed/NCBI
|
|
10.
|
Mori S, Kunieda K, Sugiyama Y and Saji S:
Prediction of 5-fluorouracil and cisplatin synergism for advanced
gastrointestinal cancers using a collagen gel droplet embedded
culture. Surg Today. 33:577–583. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Okumura K, Shiomi H, Mekata E, et al:
Correlation between chemosensitivity and mRNA expression level of
5-fluorouracil-related metabolic enzymes during liver metastases of
colorectal cancer. Oncol Rep. 15:875–882. 2006.
|
|
12.
|
Shimizu T, Murata S, Mekata E, et al:
Clinical potential of an antitumor drug sensitivity test and
diffusion-weighted MRI in a patient with a recurrent solid
pseudopapillary tumor of the pancreas. J Gastroenterol. 42:918–922.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Japanese Society for Cancer of the Colon
and Rectum: General Rules for Clinical and Pathological Studies on
Cancer of the Colon, Rectum and Anus. 7th edition. Kanehara &
Co; Tokyo: 2006
|
|
14.
|
Kobayashi H, Higashiyama M, Minamigawa K,
et al: Examination of in vitro chemosensitivity test using collagen
gel droplet culture method with colorimetric endpoint
quantification. Jpn J Cancer Res. 92:203–210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kobayashi H: Development of a new in vitro
chemosensitivity test using collagen gel droplet embedded culture
and image analysis for clinical usefulness. Recent Results Cancer
Res. 161:48–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Higashiyama M, Oda K, Okami J, et al: In
vitro-chemosensitivity test using the collagen gel droplet embedded
culture drug test (CD-DST) for malignant pleural mesothelioma:
possibility of clinical application. Ann Thorac Cardiovasc Surg.
14:355–362. 2008.
|
|
17.
|
Shimada Y, Hamaguchi T, Moriya Y, et al:
Randomized phase III study of adjuvant chemotherapy with oral
uracil and tegafur plus leucovorin versus intravenous fluorouracil
and levofolinate in patients (pts) with stage III colon cancer
(CC): Final results of Japan Clinical Oncology Group study
(JCOG0205). J Clin Oncol. 30:(Suppl; abs. 3524),. 2012.
|
|
18.
|
Benson AB III, Schrag D, Somerfield MR, et
al: American Society of Clinical Oncology recommendations on
adjuvant chemotherapy for stage II colon cancer. J Clin Oncol.
22:3408–3419. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Isogai A, Nagaya M, Matsumoto H, et al: An
anticancer drug sensitivity test to determine the effectiveness of
UFT as postoperative adjuvant chemotherapy for patients with stage
III colorectal cancer. Surgery. 142:741–748. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Hoffman RM: In vitro sensitivity assays in
cancer: a review, analysis, and prognosis. J Clin Lab Anal.
5:133–143. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kondo T, Imamura T and Ichihashi H: In
vitro test for sensitivity of tumor to carcinostatic agents. Gann.
57:113–121. 1966.PubMed/NCBI
|
|
22.
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|