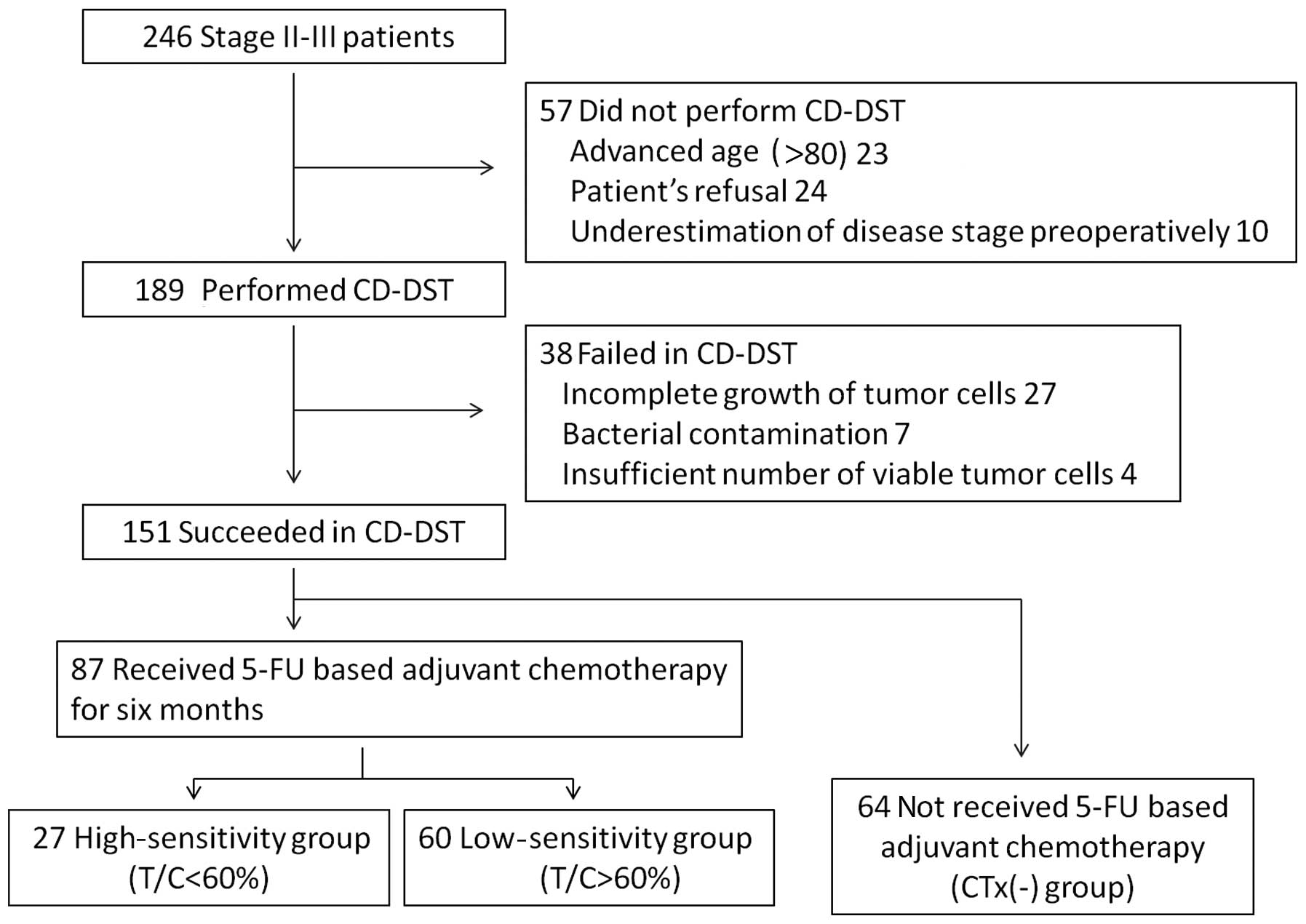
|
1.
|
André T, Boni C, Mounedji-Boudiaf L, et
al: Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment
for colon cancer. N Engl J Med. 350:2343–2351. 2004.
|
|
2.
|
Kuebler JP, Wieand HS, O’Connell MJ, et
al: Oxaliplatin combined with weekly bolus fluorouracil and
leucovorin as surgical adjuvant chemotherapy for stage II and III
colon cancer: results from NSABP C-07. J Clin Oncol. 25:2198–2204.
2007. View Article : Google Scholar
|
|
3.
|
Japanese Society for Cancer of the Colon
and Rectum: JSCCR Guidelines 2010 for the Treatment of Colorectal
Cancer. Kanehara & Co, Ltd.; Tokyo: 2010
|
|
4.
|
Watanabe T, Wu TT, Catalano PJ, et al:
Molecular predictors of survival after adjuvant chemotherapy for
colon cancer. N Engl J Med. 344:1196–1206. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Sargent DJ, Marsoni S, Monges G, et al:
Defective mismatch repair as a predictive marker for lack of
efficacy of fluorouracil-based adjuvant therapy in colon cancer. J
Clin Oncol. 28:3219–3226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Sinicrope FA, Foster NR, Thibodeau SN, et
al: DNA mismatch repair status and colon cancer recurrence and
survival in clinical trials of 5-fluorouracil-based adjuvant
therapy. J Natl Cancer Inst. 103:863–875. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Tsuji S, Midorikawa Y, Takahashi T, Yagi
K, Takayama T, Yoshida K, Sugiyama Y and Aburatani H: Potential
responders to FOLFOX therapy for colorectal cancer by Random
Forests analysis. Br J Cancer. 106:126–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kobayashi H: Collagen gel droplet culture
method to examine in vitro chemosensitivity. Methods Mol Med.
110:59–67. 2005.PubMed/NCBI
|
|
9.
|
Yabushita H, Ohnishi M, Komiyama M, et al:
Usefulness of collagen gel droplet embedded culture drug
sensitivity testing in ovarian cancer. Oncol Rep. 12:307–311.
2004.PubMed/NCBI
|
|
10.
|
Mori S, Kunieda K, Sugiyama Y and Saji S:
Prediction of 5-fluorouracil and cisplatin synergism for advanced
gastrointestinal cancers using a collagen gel droplet embedded
culture. Surg Today. 33:577–583. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Okumura K, Shiomi H, Mekata E, et al:
Correlation between chemosensitivity and mRNA expression level of
5-fluorouracil-related metabolic enzymes during liver metastases of
colorectal cancer. Oncol Rep. 15:875–882. 2006.
|
|
12.
|
Shimizu T, Murata S, Mekata E, et al:
Clinical potential of an antitumor drug sensitivity test and
diffusion-weighted MRI in a patient with a recurrent solid
pseudopapillary tumor of the pancreas. J Gastroenterol. 42:918–922.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Japanese Society for Cancer of the Colon
and Rectum: General Rules for Clinical and Pathological Studies on
Cancer of the Colon, Rectum and Anus. 7th edition. Kanehara &
Co; Tokyo: 2006
|
|
14.
|
Kobayashi H, Higashiyama M, Minamigawa K,
et al: Examination of in vitro chemosensitivity test using collagen
gel droplet culture method with colorimetric endpoint
quantification. Jpn J Cancer Res. 92:203–210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kobayashi H: Development of a new in vitro
chemosensitivity test using collagen gel droplet embedded culture
and image analysis for clinical usefulness. Recent Results Cancer
Res. 161:48–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Higashiyama M, Oda K, Okami J, et al: In
vitro-chemosensitivity test using the collagen gel droplet embedded
culture drug test (CD-DST) for malignant pleural mesothelioma:
possibility of clinical application. Ann Thorac Cardiovasc Surg.
14:355–362. 2008.
|
|
17.
|
Shimada Y, Hamaguchi T, Moriya Y, et al:
Randomized phase III study of adjuvant chemotherapy with oral
uracil and tegafur plus leucovorin versus intravenous fluorouracil
and levofolinate in patients (pts) with stage III colon cancer
(CC): Final results of Japan Clinical Oncology Group study
(JCOG0205). J Clin Oncol. 30:(Suppl; abs. 3524),. 2012.
|
|
18.
|
Benson AB III, Schrag D, Somerfield MR, et
al: American Society of Clinical Oncology recommendations on
adjuvant chemotherapy for stage II colon cancer. J Clin Oncol.
22:3408–3419. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Isogai A, Nagaya M, Matsumoto H, et al: An
anticancer drug sensitivity test to determine the effectiveness of
UFT as postoperative adjuvant chemotherapy for patients with stage
III colorectal cancer. Surgery. 142:741–748. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Hoffman RM: In vitro sensitivity assays in
cancer: a review, analysis, and prognosis. J Clin Lab Anal.
5:133–143. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kondo T, Imamura T and Ichihashi H: In
vitro test for sensitivity of tumor to carcinostatic agents. Gann.
57:113–121. 1966.PubMed/NCBI
|
|
22.
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|