Introduction
The nasal type of extranodal natural killer
(NK)/T-cell lymphoma (NKTCL) is a rare aggressive lymphoma with a
poor prognosis and is more commonly encountered in East Asia
(1). Nasal NKTCL refers to tumors
arising in the nose, paranasal sinuses and nasopharynx (2,3). It
accounts for 3% of malignant lymphomas in Japan and it is an
Epstein-Barr virus-associated lymphoma (4–6). The
lymphoma cells express P-glycoprotein, which results in multidrug
resistance (MDR) of the tumors (7–10).
The disease primarily affects middle-aged males. Nasal bleeding,
nasal congestion, rhinitis and facial swelling are the predominant
symptoms of this disease. Treatment approaches include radiotherapy
(RT), chemotherapy, or a combination of the two. Complete remission
was observed in ∼72–78% of patients with RT alone; however, 50–60%
of these cases eventually relapsed (11–15).
The reported 5-year overall survival rate for patients with
localized nasal NKTCL treated with cyclophosphamide,
hydroxydaunorubicin, oncovin and prednisone (CHOP) was <50%
(16–18). Treatment results of stage I–II
disease have recently improved; however, a standard treatment has
yet to be established. Dexamethasone, etoposide, ifosfamide and
carboplatin (DeVIC) chemotherapy, which was designed as a salvage
chemotherapeutic regimen for aggressive lymphomas, comprises
non-MDR agents and etoposide and is considered to be effective
against nasal NKTCL (19,20). Currently, we are experimenting with
chemoradiotherapy (CRT) with DeVIC as the concurrent
chemotherapeutic agents for nasal NKTCL. The present study aimed to
evaluate the initial outcomes with this treatment and to assess its
effectiveness and feasibility.
Materials and methods
General
Six patients with nasal NKTCL underwent CRT at
Hirosaki University Hospital (Hirosaki, Japan) and Aomori
Prefectural Hospital (Aomori, Japan) between April, 2004 and
February, 2010. The median follow-up time was 56 months (range,
11–80 months). All 6 patients were staged according to the Ann
Arbor staging criteria. Complete patient evaluation included
physical examination, blood counts, screening blood chemistry tests
(such as hepatic and renal function and lactate dehydrogenase
tests), chest radiographs, whole-body computed tomography (CT)
and/or positron emission tomography (PET)-CT scans and bone marrow
biopsies.
Patient characteristics
The study included 4 males and 2 females with a
median age of 68 years (age range, 29–82 years). In all 6 patients,
the primary tumor originated in the nasal cavity. In 5 patients,
the tumor involved the nasal cavity unilaterally and in 1 patient
it involved the nasal cavities bilaterally and the paranasal
sinuses. Five patients had stage IE disease and 1 patient had stage
IIE disease. The most frequent presenting symptom was nasal
congestion, which was observed in 5 patients. B symptoms were not
observed in any of the patients. Elevated lactate dehydrogenase
levels were observed in 3 patients. The ratio for performance
status (PS) was 0:1 = 3:3 and for International Prognostic Index
score was low:low-intermediate = 3:3 (Table I).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Variables | Value |
|---|
| Age (years) | |
| Range | 29–82 |
| Median | 68 |
| Gender | |
| Male | 4 |
| Female | 2 |
| Primary site | |
| Unilateral nasal
cavity | 5 |
| Bilateral nasal
cavities | 1 |
| Stage | |
| IE | 5 |
| IIE | 1 |
| B symptoms | |
| Yes | 0 |
| No | 6 |
| Lactate dehydrogenase
elevation | |
| Yes | 3 |
| No | 3 |
| PS | |
| 0 | 3 |
| 1 | 3 |
| IPI score | |
| Low | 3 |
|
Low-intermediate | 3 |
Chemotherapy
The patients underwent 3–6 cycles of full-dose DeVIC
regimen. The drug doses and administration schedule were as
follows: dexamethasone (40 mg/day on days 1–3), etoposide (100
mg/m2 on days 1–3), ifosfamide (1.5 mg/m2 on
days 1–3) and carboplatin (300 mg/m2 on day 1). The
chemotherapy was administered concurrently with RT and was repeated
every 3 weeks (Table II).
 | Table IITreatment characteristics. |
Table II
Treatment characteristics.
| Variables | No. of patients |
|---|
| Radiation dose
(Gy) | |
| 50 | 4 |
| 46 | 2 |
| Non-opposing
pair | 2 |
| Multiple field | 4 |
| Chemotherapy
(cycles) | |
| 3 | 3 |
| 4 | 2 |
| 6 | 1 |
Radiotherapy
The patients received RT from a linear accelerator
using 4-MV X-rays. The clinical target volume included the gross
tumor volume and the entire nasal cavity and ipsilateral paranasal
sinus. The patients underwent RT with a conventional fractionation
schedule at a median dose of 50 Gy (range, 46–50 Gy) (Table II). A three-dimensional dose
distribution for a patient with a large tumor is shown in Fig. 1.
Results
The present study included 4 males and 2 females
with a median age of 68 years (age range, 29–82 years) who were
treated with CTR using DeVIC in order to assess its effectiveness.
Following treatment, the patients were followed up for a median of
56 months (range, 11–80 months).
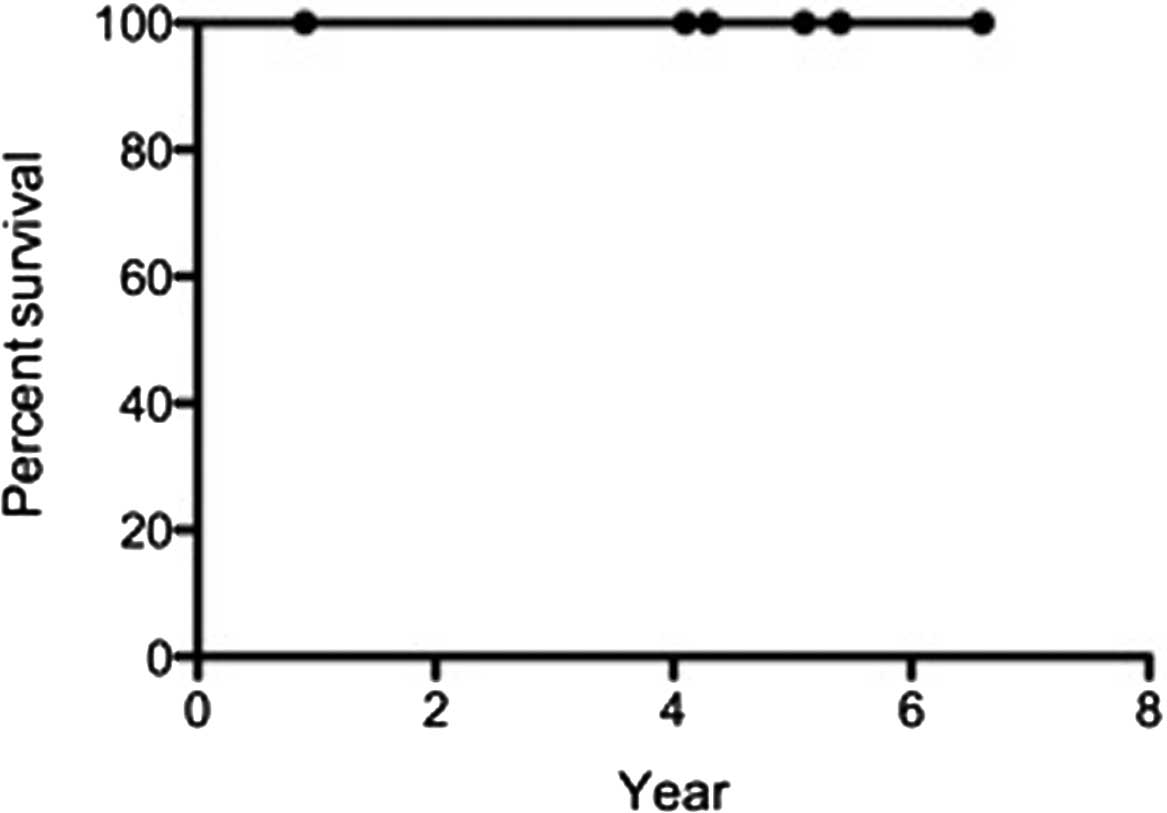
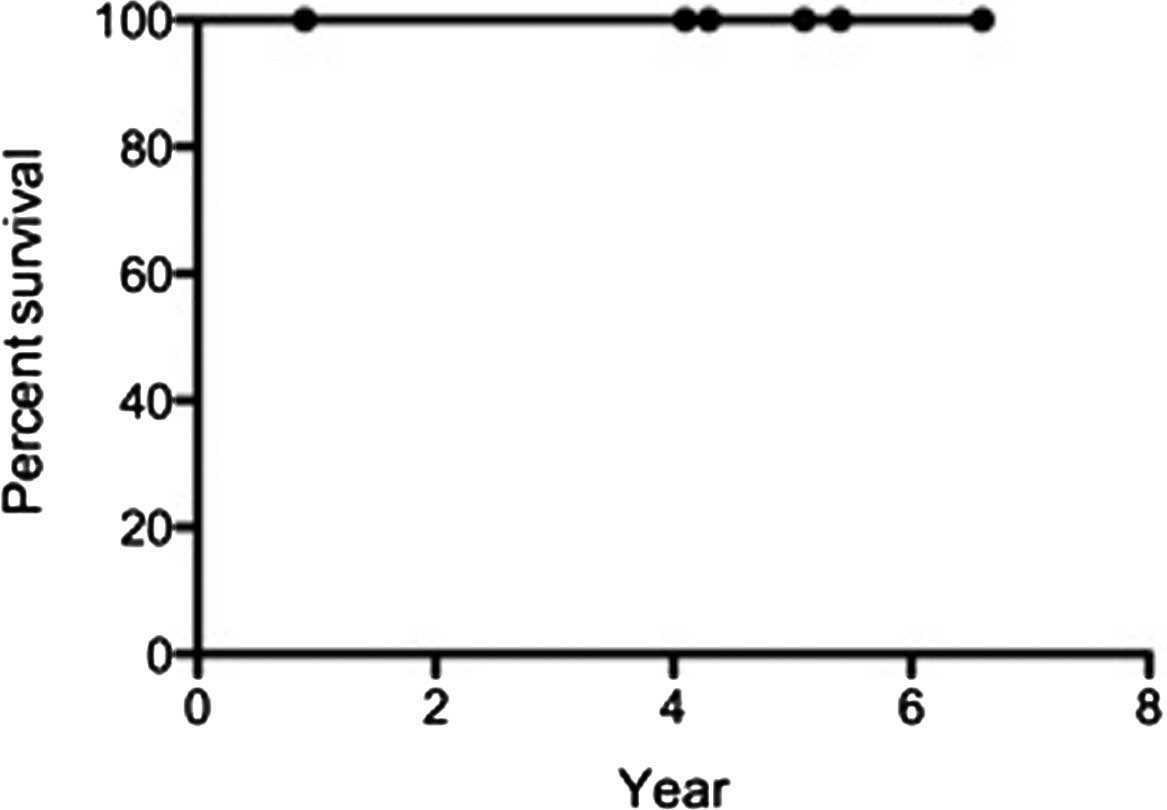
Complete remission was achieved in 5 patients (83%)
after 1 month of treatment. The 5-year overall survival and
disease-free survival rates were 100% (Figs. 2 and 3). Although grade 1–2 radiation-induced
mucositis of the nasal and oral cavities was observed in 3
patients, no severe adverse effects (grade 3) have yet been
reported in any of the patients. Two representative cases are shown
in Figs. 4 and 5. CT scans prior to CRT reveal a
naso-paranasal and a right nasal space-occupying mass of soft
tissue. However, 1 month following the completion of CRT, the mass
of soft tissue completely disappeared.
Discussion
Following treatment with DeVIC and CRT, the results
showed the complete remission rate to be 83%, which was higher
compared to that reported by previous studies on chemotherapy
followed by RT. Sakata et al reported that radiation doses
>52 Gy may be required to obtain local control in patients with
localized nasal NKTCL (21). Koom
et al reported that radiation doses <45 Gy were
significantly associated with local relapse (12). These studies have been cited by
several textbooks. Our median radiation dose was 50 Gy (range,
46–50 Gy). However, Kim et al reported satisfactory local
control with lower doses of ∼40 Gy (22).
DeVIC was added to the regimen for management of
systemic relapse and consolidation. This regimen has been used in
combination with RT since the late 1990s in Japan (20). All the patients received 3–6 cycles
of a full-dose DeVIC regimen and 46–50 Gy of RT.
Severe mucositis and moderate dermatitis are the
side effects of CRT in the nasal cavity; therefore, nutritional
management and pain control are critical. In addition, the
radiation dose to the optic nerve, optic chiasm and eyeball must be
considered in order to avoid visual disorders. All patients
completed the treatment without severe adverse events. To date, no
visual disorders have been reported.
In previous retrospective studies, the local control
and systemic relapse rates of nasal NKTCL were 18.9–47% and
15.1–81%, respectively (12,13,23,24).
In those studies, the patients were either treated with RT alone or
a combination of RT and CHOP-based chemotherapy. The 5-year
survival rate for localized nasal NKTCL is 47–57% (17,18).
Thus far no local recurrence or systemic relapse has
been observed in any of the patients. All 6 patients were still
alive at the time of writing this manuscript. Our initial results
from the present experimental CRT/DeVIC regimen for this aggressive
type of lymphoma were encouraging. Additional investigation is
required on concurrent CRT with 50 Gy/25 fx and 3–6 cycles of DeVIC
comprising non-MDR agents and etoposide for nasal NKTCL.
Our study included patients who were under a short
period of observation and our results suggest that the use of CRT
with DeVIC is effective for the treatment of nasal NKTCL.
References
|
1.
|
No authors listed:. The World Health
Organization classification of malignant lymphomas in Japan:
incidence of recently recognized entities. Lymphoma Study Group of
Japanese Pathologists Pathol Int. 50:696–702. 2000.PubMed/NCBI
|
|
2.
|
Chan JK, Quintanilla-Martinez L, Ferry JA,
et al: Extranodal NK/T-cell lymphoma, nasal type. WHO
Classification of Tumours of Haematopoietic and Lymphoid Tissues.
Swerdlow SH, Campo E, Harris NL, et al: IARC Press; Lyon: pp.
285–288. 2008
|
|
3.
|
Al-Hakeem DA, Fedele S, Carlos R and
Porter S: Extranodal NK/T-cell lymphoma, nasal type. Oral Oncol.
43:4–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Suzuki R, Takeuchi K, Ohshima K and
Nakamura S: Extranodal NK/T-cell lymphoma: diagnosis and treatment
cues. Hematol Oncol. 26:66–72. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Harabuchi Y, Yamanaka N, Kataura A, et al:
Epstein-Barr virus in nasal T-cell lymphomas in patients with
lethal midline granuloma. Lancet. 335:128–130. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Jaffe ES, Chan JK, Su IJ, et al: Report of
the workshop on nasal and related extranodal angiocentric T/Natural
killer cell lymphomas. Definitions, differential diagnosis, and
epidemiology. Am J Surg Pathol. 20:103–111. 1996. View Article : Google Scholar
|
|
7.
|
Jung CK, Lee KY, Kim Y, et al:
Epstein-Barr virus infection, drug resistance and prognosis in
Korean T- and NK-cell lymphomas. Pathol Int. 51:355–363. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yamaguchi M, Kita K, Miwa H, et al:
Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma
cells. Cancer. 76:2351–2356. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Drenou B, Lamy T, Amiot L, et al:
CD3−CD56+non-Hodgkin’s lymphomas with an
aggressive behavior related to multidrug resistance. Blood.
89:2966–2974. 1997.PubMed/NCBI
|
|
10.
|
Egashira M, Kawamata N, Sugimoto K, Kaneko
T and Oshimi K: P-glycoprotein expression on normal and abnormally
expanded natural killer cells and inhibition of P-glycoprotein
function by cyclosporine A and its analogue, PSC833. Blood.
93:599–606. 1999.PubMed/NCBI
|
|
11.
|
Kim GE, Cho JH, Yang WI, et al:
Angiocentric lymphoma of the head and neck: patterns of systemic
failure after radiation treatment. J Clin Oncol. 18:54–63.
2000.PubMed/NCBI
|
|
12.
|
Koom WS, Chung EJ, Yang WI, et al:
Angiocentric T-cell and NK/T-cell lymphomas: radiotherapeutic
viewpoints. Int J Radiat Oncol Biol Phys. 59:1127–1137. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Cheung MM, Chan JK, Lau WH, Ngan RK and
Foo WW: Early stage nasal NK/T-cell lymphoma: clinical outcome,
prognostic factors, and the effect of treatment modality. Int J
Radiat Oncol Biol Phys. 54:182–190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kim WS, Song SY, Ahn YC, et al: CHOP
followed by involved field radiation: is it optimal for localized
nasal natural killer/T-cell lymphoma? Ann Oncol. 12:349–352. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Liang R, Todd D, Chan TK, et al: Treatment
outcome and prognostic factors for primary nasal lymphoma. J Clin
Oncol. 13:666–670. 1995.PubMed/NCBI
|
|
16.
|
Ribrag V, Ell Hajj M, Janot F, et al:
Early locoregional high-dose radiotherapy is associated with
long-term disease control in localized primary angiocentric
lymphoma of the nose and nasopharynx. Leukemia. 15:1123–1126. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Shikama N, Ikeda H, Nakamura S, et al:
Localized aggressive non-Hodgkin’s lymphoma of the nasal cavity: a
survey by the Japan Lymphoma Radiation Therapy Group. Int J Radiat
Biol Phys. 51:1228–1233. 2001.
|
|
18.
|
Isobe K, Uno T, Tamaru J, et al:
Extranodal natural killer/T-cell lymphoma, nasal type: the
significance of radiotherapeutic parameters. Cancer. 106:609–615.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Yamaguchi M, Tobinai M, Oguchi M, et al:
Phase I/II study of concurrent chemoradiotherapy for localized
nasal NK/T-cell lymphoma: Final results of JCOG0211. J Clin Oncol.
27:5594–5600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Yamaguchi M, Ogawa S, Nomoto Y, et al:
Treatment outcome of nasal NK-cell lymphoma: a report of 12
consecutively-diagnosed cases and a review of the literature. J
Clin Exp Hematopathol. 41:93–99. 2001. View Article : Google Scholar
|
|
21.
|
Sakata K, Fuwa N, Kodaira T, et al:
Analyses of dose-response in radiotherapy for patients with mature
T/NK-cell lymphomas according to the WHO classification. Radiother
Oncol. 79:179–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kim SJ, Kim K, Kim BS, et al: Phase II
trial of concurrent radiation and weekly cisplatin followed by VIPD
chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal
NK/T-cell lymphoma: consortium for improving survival of lymphoma
study. J Clin Oncol. 27:6027–6032. 2009. View Article : Google Scholar
|
|
23.
|
Li YX, Yao B, Jin J, et al: Radiotherapy
as primary treatment for stage IE and IIE nasal natural
killer/T-cell lymphoma. J Clin Oncol. 24:181–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Wang B, Lu JJ, Ma X, et al: Combined
chemotherapy and external beam radiation for stage IE and IIE
natural killer T-cell lymphoma of nasal cavity. Leuk Lymphoma.
48:396–402. 2007. View Article : Google Scholar : PubMed/NCBI
|