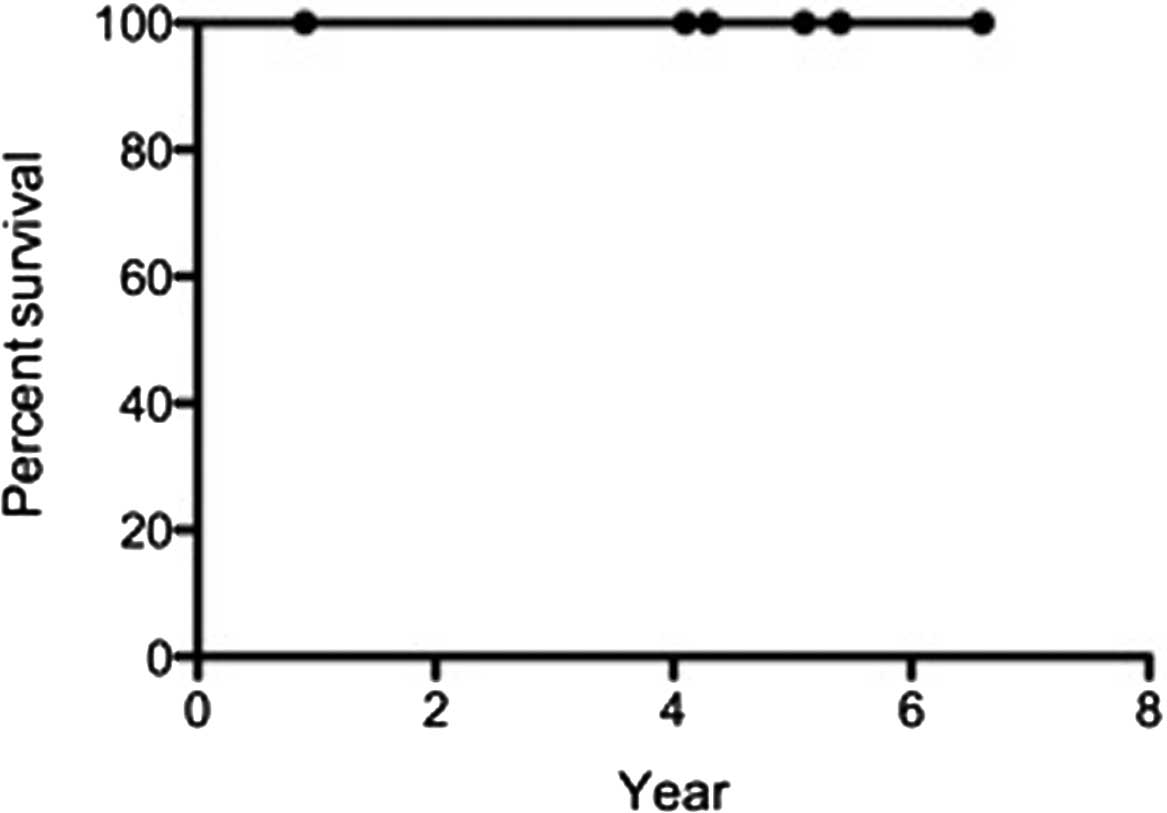
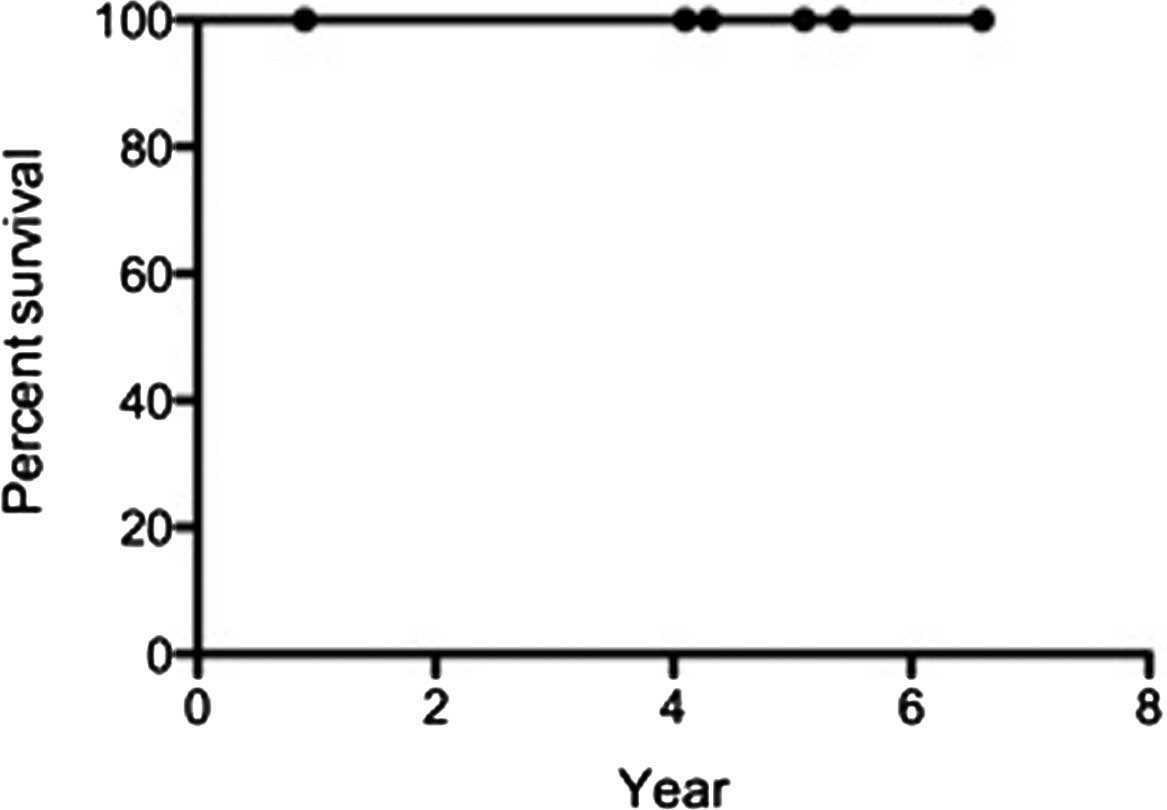
|
1.
|
No authors listed:. The World Health
Organization classification of malignant lymphomas in Japan:
incidence of recently recognized entities. Lymphoma Study Group of
Japanese Pathologists Pathol Int. 50:696–702. 2000.PubMed/NCBI
|
|
2.
|
Chan JK, Quintanilla-Martinez L, Ferry JA,
et al: Extranodal NK/T-cell lymphoma, nasal type. WHO
Classification of Tumours of Haematopoietic and Lymphoid Tissues.
Swerdlow SH, Campo E, Harris NL, et al: IARC Press; Lyon: pp.
285–288. 2008
|
|
3.
|
Al-Hakeem DA, Fedele S, Carlos R and
Porter S: Extranodal NK/T-cell lymphoma, nasal type. Oral Oncol.
43:4–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Suzuki R, Takeuchi K, Ohshima K and
Nakamura S: Extranodal NK/T-cell lymphoma: diagnosis and treatment
cues. Hematol Oncol. 26:66–72. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Harabuchi Y, Yamanaka N, Kataura A, et al:
Epstein-Barr virus in nasal T-cell lymphomas in patients with
lethal midline granuloma. Lancet. 335:128–130. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Jaffe ES, Chan JK, Su IJ, et al: Report of
the workshop on nasal and related extranodal angiocentric T/Natural
killer cell lymphomas. Definitions, differential diagnosis, and
epidemiology. Am J Surg Pathol. 20:103–111. 1996. View Article : Google Scholar
|
|
7.
|
Jung CK, Lee KY, Kim Y, et al:
Epstein-Barr virus infection, drug resistance and prognosis in
Korean T- and NK-cell lymphomas. Pathol Int. 51:355–363. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yamaguchi M, Kita K, Miwa H, et al:
Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma
cells. Cancer. 76:2351–2356. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Drenou B, Lamy T, Amiot L, et al:
CD3−CD56+non-Hodgkin’s lymphomas with an
aggressive behavior related to multidrug resistance. Blood.
89:2966–2974. 1997.PubMed/NCBI
|
|
10.
|
Egashira M, Kawamata N, Sugimoto K, Kaneko
T and Oshimi K: P-glycoprotein expression on normal and abnormally
expanded natural killer cells and inhibition of P-glycoprotein
function by cyclosporine A and its analogue, PSC833. Blood.
93:599–606. 1999.PubMed/NCBI
|
|
11.
|
Kim GE, Cho JH, Yang WI, et al:
Angiocentric lymphoma of the head and neck: patterns of systemic
failure after radiation treatment. J Clin Oncol. 18:54–63.
2000.PubMed/NCBI
|
|
12.
|
Koom WS, Chung EJ, Yang WI, et al:
Angiocentric T-cell and NK/T-cell lymphomas: radiotherapeutic
viewpoints. Int J Radiat Oncol Biol Phys. 59:1127–1137. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Cheung MM, Chan JK, Lau WH, Ngan RK and
Foo WW: Early stage nasal NK/T-cell lymphoma: clinical outcome,
prognostic factors, and the effect of treatment modality. Int J
Radiat Oncol Biol Phys. 54:182–190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kim WS, Song SY, Ahn YC, et al: CHOP
followed by involved field radiation: is it optimal for localized
nasal natural killer/T-cell lymphoma? Ann Oncol. 12:349–352. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Liang R, Todd D, Chan TK, et al: Treatment
outcome and prognostic factors for primary nasal lymphoma. J Clin
Oncol. 13:666–670. 1995.PubMed/NCBI
|
|
16.
|
Ribrag V, Ell Hajj M, Janot F, et al:
Early locoregional high-dose radiotherapy is associated with
long-term disease control in localized primary angiocentric
lymphoma of the nose and nasopharynx. Leukemia. 15:1123–1126. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Shikama N, Ikeda H, Nakamura S, et al:
Localized aggressive non-Hodgkin’s lymphoma of the nasal cavity: a
survey by the Japan Lymphoma Radiation Therapy Group. Int J Radiat
Biol Phys. 51:1228–1233. 2001.
|
|
18.
|
Isobe K, Uno T, Tamaru J, et al:
Extranodal natural killer/T-cell lymphoma, nasal type: the
significance of radiotherapeutic parameters. Cancer. 106:609–615.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Yamaguchi M, Tobinai M, Oguchi M, et al:
Phase I/II study of concurrent chemoradiotherapy for localized
nasal NK/T-cell lymphoma: Final results of JCOG0211. J Clin Oncol.
27:5594–5600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Yamaguchi M, Ogawa S, Nomoto Y, et al:
Treatment outcome of nasal NK-cell lymphoma: a report of 12
consecutively-diagnosed cases and a review of the literature. J
Clin Exp Hematopathol. 41:93–99. 2001. View Article : Google Scholar
|
|
21.
|
Sakata K, Fuwa N, Kodaira T, et al:
Analyses of dose-response in radiotherapy for patients with mature
T/NK-cell lymphomas according to the WHO classification. Radiother
Oncol. 79:179–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kim SJ, Kim K, Kim BS, et al: Phase II
trial of concurrent radiation and weekly cisplatin followed by VIPD
chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal
NK/T-cell lymphoma: consortium for improving survival of lymphoma
study. J Clin Oncol. 27:6027–6032. 2009. View Article : Google Scholar
|
|
23.
|
Li YX, Yao B, Jin J, et al: Radiotherapy
as primary treatment for stage IE and IIE nasal natural
killer/T-cell lymphoma. J Clin Oncol. 24:181–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Wang B, Lu JJ, Ma X, et al: Combined
chemotherapy and external beam radiation for stage IE and IIE
natural killer T-cell lymphoma of nasal cavity. Leuk Lymphoma.
48:396–402. 2007. View Article : Google Scholar : PubMed/NCBI
|