Introduction
Multidrug resistance (MDR) is a major obstacle to
successful cancer chemotherapy, including breast cancer. Expression
of plasma membrane ATP-binding cassette (ABC) transporter
proteins that act as efflux pumps to actively extrude drug
molecules out of the cell is one of the predominant mechanisms of
MDR (1,2). P-glycoprotein (P-gp),
the first drug resistance ABC transporter to be identified
(3), has been under extensive
investigation for >15 years as a mediator of MDR. Since then,
there has been a rapid increase in the number of identified ABC
transporter proteins. The multidrug resistance-associated protein
was the second ABC transporter protein to be identified (4), followed by several other MRP family
members.
The breast cancer resistance protein (BCRP) is a
recently characterized xenobiotic half-transporter protein
that was first identified in the MCF-7/AdrVp breast cancer cell
line, which has a multidrug-resistant phenotype,
notwithstanding the addition of a P-gp-blocking agent
(verapamil, Vp) (5,6). BCRP is a newly identified member of
the ABC protein family and acts as an energy-dependent
efflux pump (7,8).
BCRP has already been closely investigated. Previous
studies indicated that BCRP confers an atypical MDR phenotype
(9–11). The established transfectant
BCRP-expressing cell lines share a characteristically high
resistance to the anthracenedione mitoxantrone, anthracyclines such
as daunorubicin and doxorubicin, topotecan, bisantrene and
SN-38, the active form of irinotecan, whereas they maintain
sensitivity to cisplatin, paclitaxel and vinca alkaloids such as
vincristine and vinblastine (12).
However, the drug-resistance spectrum and the mechanisms of
action of BCRP have not been fully elucidated.
A transfectant BCRP expression cell model was
established (13) and utilized to
screen clinical anticancer drugs in vitro. Our previous
study results demonstrated that resistance to 5-fluorouracil
(5-FU) may be particularly mediated by conjugation with
BCRP, which acts as a drug extrusion pump in the cell model
(14). Moreover, cell resistance
to 5-FU-induced apoptosis may be reinforced by BCRP
expression (15). 5-FU is
currently one of the most widely used anticancer agents due to its
strong anticancer activity. Our previous study demonstrated
resistance to 5-FU in clinical breast cancer cells: ~2.5% of
clinical breast cancer cells exhibited low-degree
sensitivity and 20% exhibited moderate sensitivity to 5-FU
(16). In addition, BCRP
expression was reported in 20–30% of clinical breast cancer tissue
specimens (17).
Whether BCRP expression is involved in clinical
breast cancer resistance to 5-FU has not been elucidated. It
was hypothesized that BCRP expression is positive in clinical
breast cancer tissue exhibiting low sensitivity to 5-FU. In
the present study, BCRP expression was assessed in clinical breast
cancer tissue specimens using quantitative
reverse-transcriptase polymerase chain reaction
(RT-PCR) by use of the Master SYBR-Green I reagent and
immunohistochemistry (IHC) by use of the BXP-21
anti-BCRP monoclonal antibody. In addition, chemosensitivity
to 5-FU for the BCRP-positive specimens was
colorimetrically assessed with the cytotoxicity assay through
methyl thiazolyl tetrazolium (MTT) reduction. The aim of this study
was to further elucidate the association between BCRP expression
and 5-FU resistance in clinical breast cancer tissue
specimens and optimize breast cancer clinical chemotherapy schemes
in BCRP-positive specimens.
Materials and methods
Tissue specimens
A total of 37 clinical breast cancer tissue
specimens from female patients aged 25–50 years were obtained at
the time of excision at the Deparment of General Surgery of the
Second People’s Hospital of Shenzhen. The placental tissue of a
27-year-old puerperant was obtained from the Department of
Obstetrics of the Second People’s Hospital of Shenzhen and was used
as a positive control for BCRP expression in quantitative
RT-PCR and IHC.
Total RNA isolation and quantitative
RT-PCR
Total RNA was extracted from the clinical breast
cancer tissue specimens and the placental tissue using the TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according
to the manufacturer’s protocol. Staining of the ribosomal bands on
1% agarose gel with ethidium bromide was used to assess the quality
of the isolated RNA. The RNA (1 μg) was reverse transcribed with
reverse transcriptase using random hexamers as primers (Promega,
Madinson, WI, USA). The resulting complementary DNAs (cDNAs) were
diluted by 2.5-fold with DNase/RNase-free water and
the levels of BCRP and the housekeeping gene β-actin were
determined by quantitative PCR on the Mx4000 LightCycler
(Stratagene, La Jolla, CA, USA) using the Master SYBR-Green I
reagent kit under the following conditions: a step of denaturation
at 95°C for 10 min and 40 cycles at 95°C for 5 sec, at 55°C for 30
sec and at 72°C for 30 sec. The endpoint used in PCR quantification
(Ct) was defined as the PCR cycle number that crosses an
arbitrarily placed signal threshold and is a function of the amount
of target cDNA present in the starting material. The BCRP levels
were normalized to those of β-actin in the same samples. The
primers used were 5′-tccact gctgtggcattaaa-3′ and
5′-tgctgaaacactggttggtc-3′ for BCRP and
5′-tccgtggagaagagctacga-3′ and
5′-gtacttgcgctcagaaggag-3′ for β-actin.
IHC
IHC was performed on the frozen sections of clinical
breast cancer and placental tissue that were formalin-fixed and
paraffin-embedded. Briefly, the sections were deparaffinized,
rehydrated and then blocked for endogenous peroxidase. Following
antigen retrieval using a standard microwave method, the sections
were incubated in 10% (v/v) normal goat serum to block non-specific
binding, followed by overnight incubation with the BXP-21
mouse anti-human BCRP monoclonal antibody (Alexis
Biochemicals, San Diego, CA, USA) at 4°C and at a dilution of 1:50.
For the negative control, phosphate-buffered saline was used
instead of the primary antibody. Subsequently, a horseradish
peroxidase-conjugated rabbit anti-mouse secondary IgG
antibody (Dako, Carpinteria, CA, USA) was used at 1:100 dilution
for 1 h at room temperature. The labeling was visualized using
0.05% (w/v) chromogen 3,3′-diaminobenzidine containing 0.01% (v/v)
H2O2 in 0.05 M Tris/HCl buffer (pH 7.6) until
a brown reaction was observed by microscopy. The reaction was
arrested by immersion in distilled water. The sections were
counterstained with haematoxylin and were then dehydrated in
ethanol, cleared in xylene, mounted onto glass slides and examined
under a light microscope. For semiquantification of the
immunostaining, section staining was performed in triplicate sets
to confirm the results. The intensity of immunostaining for BCRP
was semiquantitatively scored as follows: −, absent; +, weak; ++,
moderate; +++, strong.
Cytotoxicity assay
Clinical breast cancer tissue samples were
dissociated into single-cell suspensions, as previously described
(9,18,19).
Cells were placed in 96-well plates (1–3×105 cells/well)
and were exposed to various concentrations of 5-FU and
paclitaxel for 3 days at 37°C in a humidified atmosphere containing
5% CO2. Cell viability was colorimetrically assayed
through MTT reduction. Absorbance (570 nm) was determined.
Cytotoxicity was expressed as the IC50, defined as the
drug concentration leading to 50% growth inhibition. The fold
resistance (FR) to 5-FU was estimated from the ratio of the
IC50 for 5-FU in the cells compared to the
IC50 for paclitaxel.
Data analysis
For each experiment, each concentration was tested
in triplicate. Each experiment was repeated 2–4 times and found to
be reproducible. For statistical analyses, SPSS 13.0 software for
Windows was used (SPSS Inc., Chicago, IL, USA). Error bars are
presented as standard errors of the mean. To identify statistically
significant differences among the means, one-way ANOVA was
performed. P<0.01 was considered to indicate a statistically
significant difference.
Results
BCRP mRNA expression in clinical breast
cancer tissue specimens
To detect BCRP expression at the mRNA level in
clinical breast cancer tissue specimens, high-quality total RNA was
extracted from breast cancer and control placental tissue.
Subsequently, the Ct value of BCRP and the housekeeping gene
β-actin were determined by quantitative RT-PCR by use
of the Master SYBR-Green I reagent. Our results demonstrated that
37 clinical breast cancer tissue specimens expressed BCRP at
different levels. The BCRP levels were normalized to those of
β-actin in the same samples (Table I). The rate of BCRP expression in
clinical breast cancer tissue specimens was 26% (37/140). Our
results were consistent with those previously reported by Kanzaki
et al(20), which suggested
that BCRP expression may contribute to the failure of breast cancer
chemotherapy to a certain extent.
 | Table IBCRP expression measured in clinical
breast cancer and placental tissue specimens with quantitative
RT-PCR. |
Table I
BCRP expression measured in clinical
breast cancer and placental tissue specimens with quantitative
RT-PCR.
| Specimens |
|---|
|
|
|---|
| Variables | Positive BCRP
expression | Negative BCRP
expression | Placentac |
|---|
| Relative Ct
valuea | 0.16±0.07 | - | 0.45±0.05 |
| No. of
specimens | 37 | 103 | 1 |
| % Ratiob | 26 (37/140) | 74 (103/140) | - |
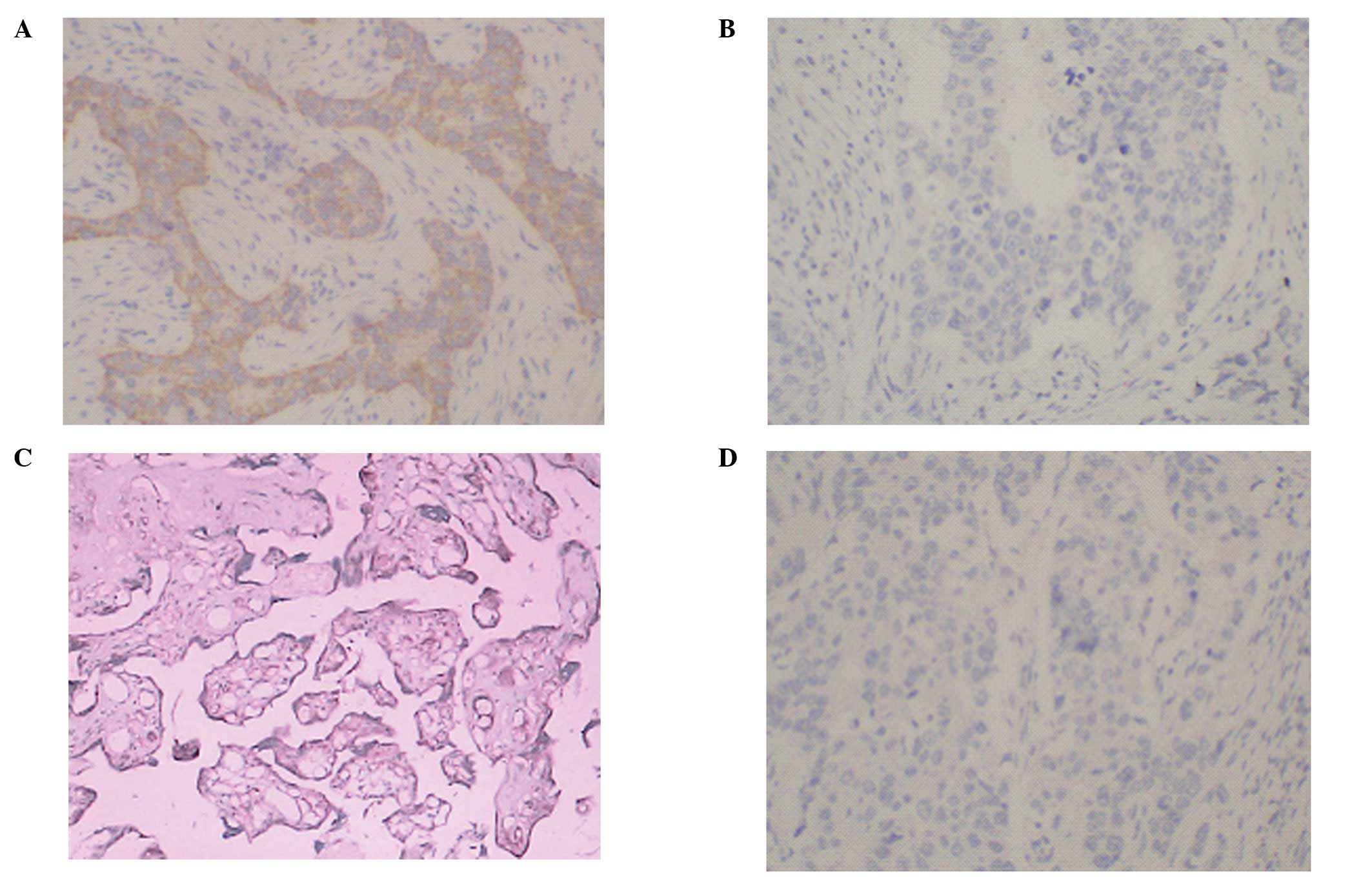
BCRP protein expression in clinical
breast cancer tissue specimens
To confirm the previously observed BCRP mRNA
expression, IHC assay was performed on the frozen sections of
clinical breast cancer and placental tissue by use of the
BXP-21 anti-BCRP monoclonal antibody and the positive
ratio of BCRP expression was 22% (28/140). There was no significant
difference in BCRP expression between the results of PCR and IHC in
the specimens (P>0.05). Our results demonstrated that the
expression of BCRP at the protein level was reflected by a
corresponding increase in the mRNA level for clinical breast cancer
tissue specimens. The representative staining pattern observed in
clinical specimens and control placental tissue are shown in
Fig. 1.
Chemosensitivity of BCRP-positive
clinical breast cancer tissue specimens to 5-FU
To investigate the association of BCRP with
5-FU sensitivity, 37 BCRP-positive clinical breast
cancer tissue specimens were identified with quantitative
RT-PCR and IHC. There was a significant correlation in BCRP
expression between the results of quantitative RT-PCR and
IHC in those specimens. Subsequently, chemosensitivity of the
BCRP-positive clinical specimens to 5-FU was
colorimetrically assessed with the cytotoxicity assay through MTT
reduction. Cytotoxicity was expressed as the IC50,
defined as the drug concentration leading to 50% growth inhibition.
The FR to 5-FU was estimated from the ratio of the
IC50 for 5-FU in the cells compared to the
IC50 for paclitaxel. Our results demonstrated that the
FR to 5-FU was 7–12 compared to the sensitivity to
paclitaxel in those 37 specimens. A linear correlation was observed
between BCRP-positive expression and 5-FU resistance
(Fig. 2).
Discussion
BCRP is a recently described ABC half-transporter
that confers resistance to certain cancer chemotherapeutic drugs.
The drug-resistant spectrum and mechanisms of action of BCRP
are under constant investigation, mainly focusing on its clinical
association with MDR.
Results from previous studies demonstrated that BCRP
may mediate resistance to 5-FU in the cell model (19,20).
5-FU is currently used as a front-line anticancer agent due
to its strong anticancer activity. A previous study reported that
22.5% of clinical breast cancer cells exhibit resistance to
5-FU (21). In addition,
BCRP expression has been reported in 20–30% of clinical breast
cancer tissue using the RT-PCR assay (22). Whether BCRP expression confers
clinical breast cancer resistance to 5-FU has not been
elucidated.
In this study, we investigated whether BCRP
expression in clinical breast cancer is involved in resistance to
5-FU. Quantitative RT-PCR was employed to rapidly
assess BCRP expression in clinical breast cancer tissue specimens.
BCRP mRNA was previously detected by quantitative RT-PCR in
certain drug-resistant cell lines (23). Our results demonstrated that the
rate of BCRP expression was 26% (37/140) in clinical breast cancer
tissue specimens. Our data were consistent with those previously
reported by Kanzaki et al(19). Subsequently, IHC with the
BXP-21 anti-BCRP monoclonal antibody was used to
further confirm BCRP expression in clinical BCRP-positive
breast cancer tissue. The IHC assay demonstrated a BCRP expression
rate of 22% (28/140) in tumor cell membranes, which may be
attributed to the threshold of IHC detection. Our results lead to
the conclusion that the immunostaining of the cell membranes with
BXP-21 monoclonal anti-BCRP antibody is highly associated
with a marked expression of BCRP mRNA and also suggest that
quantitative RT-PCR may be extensively used to detect gene
expression.
There has been extensive investigation focusing on
the failure of 5-FU chemotherapy. Our previous study results
demonstrated that cell resistance to 5-FU-induced
apoptosis is possibly reinforced by BCRP expression in
vitro(17). To confirm the
associaton between BCRP expression and 5-FU resistance in
clinical samples, the chemosensitivity for 37 BCRP-positive
clinical breast cancer tissue specimens to 5-FU was
determined by quantitative RT-PCR and colorimetrically
assessed with the cytotoxicity assay through MTT reduction. The
results demonstrated that the FR to 5-FU was 7–12 in those
37 specimens with different levels of BCRP expression. The BCRP
expression was highly correlated with 5-FU resistance in the
BCRP-positive clinical breast cancer samples. However, the
complete mechanisms that underlie BCRP-mediated 5-FU
resistance, such as the functional BCRP domain that is linked to
5-FU resistance, have not been fully elucidated.
In conclusion, our study results further confirmed
that 5-FU resistance may be mediated by BCRP expression in
clinical breast cancer tissue specimens and may provide evidence
supporting the role of BCRP as a mediator of 5-FU
resistance, which may help optimize breast cancer clinical
chemotherapy schemes in BCRP-positive specimens.
References
|
1
|
Kröger N, Achterrath W, Hegewisch-Becker
S, Mross K and Zander AR: Current options in treatment of
anthracycline-resistant breast cancer. Cancer Treat Rev.
25:279–291. 1999.PubMed/NCBI
|
|
2
|
Cai X, Bikadi Z, Ni Z, et al: Role of
basic residues within or near the predicted transmembrane helix 2
of the human breast cancer resistance protein in drug transport. J
Pharmacol Exp Ther. 333:670–681. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Riordan JR and Ling V: Purification of
P-glycoprotein from plasma membrane vesicles of Chinese hamster
ovary cell mutants with reduced colchicine permeability. J Biol
Chem. 254:12701–12705. 1979.PubMed/NCBI
|
|
4
|
Cole SP, Bhardwaj G, Gerlach JH, Mackie
JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM and
Deeley RG: Overexpression of a transporter gene in a
multidrug-resistant human lung cancer cell line. Science.
258:1650–1654. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Doyle LA, Yang W, Abruzzo LV, Krogmann T,
Gao Y, Rishi AK and Ross DD: A multidrug resistance transporter
from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA.
95:15665–15670. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Noguchi K, Katayama K, Mitsuhashi J and
Sugimoto Y: Functions of the breast cancer resistance protein
(BCRP/ABCG2) in chemotherapy. Adv Drug Deliv Rev. 61:26–33. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Allikmets R, Schriml LM, Hutchinson A,
Romano-Spica V and Dean M: A human placenta-specific ATP-binding
cassette gene (ABCP) on chromosome 4q22 that is involved in
multidrug resistance. Cancer Res. 58:5337–5339. 1998.PubMed/NCBI
|
|
8
|
Natarajan K, Xie Y, Baer MR and Ross DD:
Role of breast cancer resistance protein (BCRP/ABCG2) in cancer
drug resistance. Biochem Pharmacol. 83:1084–1103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan J, Lv H, Peng B, Wang C, Yu Y and He
Z: Role of BCRP as a biomarker for predicting resistance to
5-fluorouracil in breast cancer. Cancer Chemother Pharmacol.
63:1103–1110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lagas JS, van Waterschoot RA, van Tilburg
VA, et al: Brain accumulation of dasatinib is restricted by
P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)
and can be enhanced by elacridar treatment. Clin Cancer Res.
15:2344–2351. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ni Z, Bikadi Z, Rosenberg MF and Mao Q:
Structure and function of the human breast cancer resistance
protein (BCRP/ABCG2). Curr Drug Metab. 11:603–617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Polli JW, Olson KL, Chism JP, et al: An
unexpected synergist role of P-glycoprotein and breast cancer
resistance protein on the central nervous system penetration of the
tyrosine kinase inhibitor lapatinib
(N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine;
GW572016). Drug Metab Dispos. 37:439–442. 2009.PubMed/NCBI
|
|
13
|
Bates SE, Robey R, Miyake K, Rao K, Ross
DD and Litman T: The role of half-transporters in multidrug
resistance. J Bioenerg Biomembr. 33:503–511. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan JH, Zhou JM, Ji N, et al: Epigenetic
regulation mechanism of ABCG2 induced drug-resistant phenotype. J
Med Coll PLA. 26:243–253. 2011. View Article : Google Scholar
|
|
15
|
Zhang W, Ding W, Chen Y, et al:
Up-regulation of breast cancer resistance protein plays a role in
HER2-mediated chemoresistance through PI3K/Akt and nuclear
factor-kappa B signaling pathways in MCF7 breast cancer cells. Acta
Biochim Biophys Sin (Shanghai). 43:647–653. 2011. View Article : Google Scholar
|
|
16
|
Yuan JH, He ZM, Yu YH and Chen ZC:
Expression establishment and functional analysis of breast cancer
resistance protein with doxycycline induced Tet regulating system
in mouse fibroblast cell line PA317. Chin J Cancer. 23:1127–1133.
2004.(In Chinese).
|
|
17
|
Yuan JH, He ZM, Lu H, et al: Breast cancer
resistance protein-mediated 5-fluorouracil resistance and its
mechanism. Chin J Pharmacol Toxicol. 19:357–362. 2005.
|
|
18
|
He ZM, Wei LY and Peng X: Assay of
sensitivity of cancer cell to anticancer drug in glass capillaries.
Chin J Cancer Res. 4:40–45. 1992. View Article : Google Scholar
|
|
19
|
Lemos C, Kathmann I, Giovannetti E, et al:
Cellular folate status modulates the expression of BCRP and MRP
multidrug transporters in cancer cell lines from different origins.
Mol Cancer Ther. 8:655–664. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanzaki A, Toi M, Nakayama K, Bando H,
Mutoh M and Uchida T: Expression of multidrug resistance-related
transporters in human breast carcinoma. Jpn J Cancer Res.
92:452–458. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng G, Peng F, Ding R, Yu Y, et al:
Identification of proteins responsible for the multiple drug
resistance in 5-fluorouracil-induced breast cancer cell using
proteomics analysis. J Cancer Res Clin Oncol. 136:1477–1488. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He Z, Yuan J, Chen Z, Liu S, Shen Z and
Fei H: Chemosensitivity test for 170 human breast carcinoma
samples. Hunan Yi Ke Da Xue Xue Bao. 23:531–534. 1998.(In
Chinese).
|
|
23
|
Volk EL, Farley KM, Wu Y, Li F, Robey RW
and Schneider E: Overexpression of wild-type breast cancer
resistance protein mediates methotrexate resistance. Cancer Res.
62:5035–5040. 2002.PubMed/NCBI
|