Introduction
Minimal deviation adenocarcinoma of the uterine
cervix (MDA), otherwise known as adenoma malignum, is a rare
variant of cervical adenocarcinoma, which represents a diagnostic
challenge in the field of gynecologic oncology. It is a rare
neoplasm with an incidence of 1–3% and was first designated as
‘malignant adenoma of the cervix’ by Gusserow (1). However, Silverberg and Hurt (2) proposed the term ‘minimal deviation
adenocarcinoma’ for this tumor due to its deceptively benign
microscopic appearance. Since that time, only a few cases of MDA
have been reported in the English literature.
In this study, we present two cases of MDA, in order
to demonstrate the characteristics, diagnostic and therapeutic
strategies that distinguish it from ordinary endometrioid
adenocarcinoma.
Case reports
Case 1
A 45-year-old, multiparous woman (gravida 3, para 1,
G3P1) presented with a 5-year history of large amounts of vaginal
discharge. The ThinPrep cytology test revealed moderate
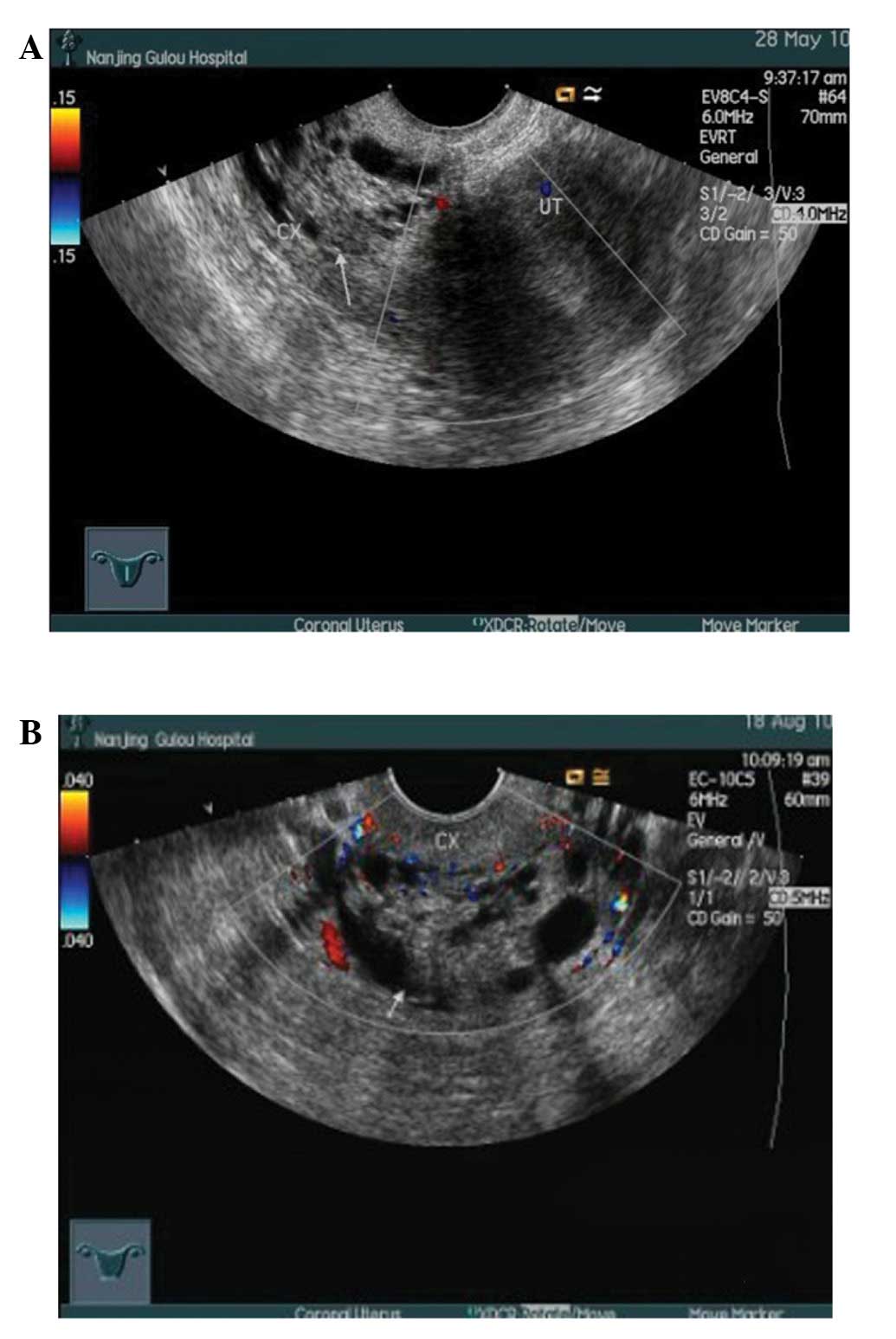
inflammation. Several transvaginal ultrasonography scans revealed
an edematous cervix and multiple cysts with a honeycomb appearance
(Fig. 2B). The inner cervical wall
was not smooth and the tumor marker levels were within the normal
range. Following cervical conization for the cervical cysts, the
biopsies revealed chronic cervical inflammation with the presence
of retention cysts and squamous metaplasia in the fundic portion of
the cervix. Subsequently, the patient underwent laparoscopic
cystectomy and biopsy, hysteroscopy, fractional curettage and
cervical biopsy. The histopathological examination revealed chronic
inflammation of the cervical mucosa. However, the vaginal discharge
did not subside. The patient then underwent a pelvic magnetic
resonance imaging (MRI) examination, which revealed multiple
cervical cysts and hydrops in the pelvic cavity. Medically the
patient was in good condition and her history only revealed an
appendectomy performed in 1983. Following admission to our
department, the gynecologic examination showed large amounts of
vaginal discharge and cervical hypertrophy, with no other abnormal
findings. The patient underwent total abdominal hysterectomy and
the fast-frozen cervical biopsy revealed the presence of
adenocarcinoma; thus, bilateral salpingo-oophorectomy and pelvic
lymphadenectomy was also performed.
Grossly, the cervix was thickened and hard with
multiple retention cysts, with no other abnormal macroscopic
findings (Fig. 1C). The
microscopic examination revealed cervical mucilaginous glands that
were irregular in size and shape with increased apophysis, part of
the glands were surrounded by a loose edematous or desmoplastic
stromal response, the glands typically exhibited deep invasion of
the cervical wall and were adjacent to the cervical adventitia. The
glandular epithelial cells exhibited foci of heteromorphism. The
parametrium and the pelvic lymph nodes showed no evidence of
malignancy. The tumor was staged as Ib2 MDA according to the FIGO
classification. Subsequently, cervical and pelvic radiotherapy was
performed. At the last follow-up the patient was disease-free.
Case 2
A 41-year-old, multiparous woman (G6P1) underwent
myomectomy for a cervical hysteromyoma in 2011 and pathological
examination of the hysteromyoma revealed an MDA. The patient was
medically fit and in good overall condition. Her medical history
revealed myomectomy and oophoritic cystectomy (10 years ago). The
gynecologic examination showed cervical moderate inflammation, with
no other abnormal findings. The patient subsequently underwent
total abdominal hysterectomy, bilateral salpingo-oophorectomy and
pelvic lymphadenectomy.
The gross uterine appearance was normal, apart from
an enlarged corpus. The microscopic examination revealed cervical
chronic inflammation with retention cysts and squamous metaplasia,
adenomyosis and chronic salpingitis. The pelvic lymph nodes
exhibited reactive hyperplasia, with no other abnormalities. The
tumor was stage Ib and there were no high risk factors for the
patient; therefore, adjunctive therapy was not administered. At the
last follow-up the patient exhibited no evidence of tumor
recurrence.
Discussion
To gain more insight into MDA, we performed a review
of the literature, during which 60 cases were identified (Table I). In almost half of these cases,
vaginal bleeding and discharge was the predominant symptom and
pathological examination was used to confirm the diagnosis.
Surgical resection was the first choice for the treatment of
MDA.
 | Table IData of 60 cases of minimal deviation
carcinoma of cervix. |
Table I
Data of 60 cases of minimal deviation
carcinoma of cervix.
| Study (n) | Age (years) | Presenting symptom
(n) | Treatment (n) | Stage (n) | Cytology (n) | Pathology (n) | IHC (n) | Prognosis (n) | Refs. |
|---|
| Chang et
al(5) | 38–59 | Atypical vaginal
discharge (3) | Rad (5) | Ib (2) | Adenoma malignum
(2) | MDA (5) | | Succumbed to the
disease (3) | (3) |
| Radical hysterectomy
with pelvic node dis (3) | Iib (1) | Ordinary
adenocacinoma (1) | CEA+, p53+ (2) |
| IIIb (1) | Unknown (3) | NED (2) |
| AH and BSO (1) | IV (1) | No malignancy
(2) | | |
| Simionescu et
al(1) | 32 | Atypical vaginal
discharge and bleeding | Cx Bx | Unknown | Normal | MDA | CEA+, CA125+,
Ki67+ | Unknown | (4) |
| Steeper et
al(4) | 38–74 | Vaginal bleeding
(3) | Radical hysterectomy
(3) | Ia (1) | Unknown | MDA | CEA+ | Succumbed to the
disease (3) | (5) |
| Vaginal discharge
(1) | Rad (1) | Ib (2) |
| | Iib (1) | NED (1) |
| Du et
al(1) | 27 | Vaginal discharge and
bleeding | Radical hysterectomy,
pelvic node dis | Unknown | Not performed | MDA | CEA+, p53+, Ki67
(10%) | NED | (6) |
| Yang et
al(14) | 31–63 | Vaginal bleeding
(9) | Unknown | I (4) | Adenoma malignum
(1) | MDA | CEA+ (12) | Unknown | (7) |
| Vaginal discharge
(12) | II (7) | Normal (9) | Ki67+ (11) |
| III (2) | Unknown (4) | p53+ (8) |
| IV (1) | | |
| Zhang et
al(9) | 36–50 | Unknown | Unknown | Unknown | Unknown | Unknown | CEA+ (6); α-SMA+
(8) | Unknown | (8) |
| Ki67+ (9) |
| Jiang et
al(1) | 61 | Leucorrhea with blood
streak, menopause | AH, BSO | Unknown | No malignancy | MDA | Not performed | NED | (9) |
| Abiko et
al(1) | 56 | Vaginal
discharge | AH, BSO, pelvic and
para-aortic node dis | Unknown | Not performed | MDA | CEA+; CA19-9+ | Unknown | (10) |
| MUC6+; HIK1083+ |
| Odashiro et
al(3) | 30–45 | Blood-tinted vaginal
discharge | Cx and pelvic rad,
followed by radical hysterectomy | Unknown | Adenocarcinoma in
situ(1) vs. well-differentiated adenocarcinoma (2) | MDA diagnosed by Cx
Bx | Vimentin+ | Succumbed to
metastatic disease (1) | (11) |
| NED (2) |
| Chen (8) | 26–68 | Vaginal discharge
(7) | AH, BSO, pelvic node
dis | Unknown | Unknown | MDA diagnosed by Cx
Bx | CEA+; CK7+; CK19+;
CA19-9+; Vimentin+; SMA+ | Unknown | (12) |
| Vaginal bleeding
(6) |
| Contact bleeding
(2) |
| Menolipsis (1) |
| Kaminski et
al(13) | 31–76 | Vaginal bleeding
(7) | AH (4) | Ib (12) | Not performed
(7) | Endometrioid (7) | Unknown | NED, ≥9 years
(5) | (13) |
| Vaginal discharge
(3) | VH (1) | Iib (1) | Class 1 (4) | Mucinous (5) | Succumbed to the
disease (6)
Lost (2) |
| Cervical stenosis
with associated pyometra (1) | D&C, cone, WH,
pelvic node dis (2) | | Class 3 (2) | Clear-cell (1) |
| Abnormal Pap smear
and bleeding (1) | D&C, cone, WH,
pelvic node dis and postoperative rad (2) | | | | |
| Cervical neoplasm
(2) | | | | |
| D&C, cone, WH,
pelvic node dis and VH (1) | | | | |
| Cx Bx, AH (1) | | | | |
| D&C, cone
(1) | | | | |
| AH, BSO (1) | | | | |
The origin of MDA remains unclear. Previous studies
revealed that the tumor is likely unrelated to human papillomavirus
infection, which distinguishes it from common cervical cancers
(14). Certain studies
demonstrated a close association between MDA and gastric
metaplasia. McGowan et al(15), suggested that the existence of
Peutz-Jeghers syndrome or ovarian tumors may contribute to the
progress of MDA, although no definitive conclusion on this
association was established in our cases. The symptoms and signs of
MDA are not different from those of common cervical adenocarcinoma.
In our first case, the patient presented with profuse watery
discharge and enlarged cervix with retention cysts. The cytological
examination, punch biopsy and cervical conization failed to confirm
a diagnosis of MDA. Therefore, in patients with cervical
hypertrophy presenting with vaginal discharge or irregular
bleeding, MDA should be considered following the elimination of
other possible causes (such as carcinoma tubae)and appropriate
investigations should be conducted, leading to a definitive
diagnosis. The diagnosis of MDA is based on histopathology.
Previous studies demonstrated that the cytological examination of
the cervix as a diagnostic method for MDA is not sufficient.
However, biopsy of the cervix and the cervical canal
(depth >5 mm) and cervical conization contribute to the
definitive diagnosis of MDA (16).
Diagnosis using imaging techniques, such as MRI and
ultrasonography, is often difficult due to the benign appearance of
this tumor; however, they play an important role in evaluating the
dissemination of MDA (17).
T2-weighted MRI, in particular, shows the characteristics of MDA in
detail and exhibits a reliable correlation with histological
findings (18). In our first case,
the T2-weighted MRI revealed a multicystic lesion and fluid
accumulation in the endometrial cavity (Fig. 3B). MDA exhibits a diffusely
infiltrative growth pattern and its differentiation from normal
cervical glands histologically is challenging (19). However, MDA is histologically
characterised by the haphazardous arrangement of endocervical
glands and their deep penetration into the cervical wall, with only
minor cytological atypia. Immunohistochemistry usually serves as an
auxiliary examination of morphology to distinguish MDA from other
cervical diseases. Previous studies revealed that carcinoembryonic
antigen, Ki67, alcian blue-periodic acid-Schiff staining and p53
may play important roles in the disease aetiology (20). Currently, surgery remains the
optimal treatment choice for MDA. The modus operandi for the
patients without a definitive diagnosis should be the same as that
for ordinary adenocarcinoma. However, postoperative adjunctive
therapy may be required for patients with MDA, as the disease is
usually diagnosed at a later stage. From the prognostic point of
view, a firm conclusion cannot be reached, due to the limited
number of reported MDA cases and the limited clinical follow-up. In
our two cases, opportune diagnosis allowed application of the
appropriate treatment, similar to an ordinary well-differentiated
adenocarcinoma. Close follow-up of our two cases was planned in
order to obtain more information about the disease and the efficacy
of the available therapeutic methods.
In conclusion, early diagnosis followed by
appropriate ancillary evaluation and treatment have been a
challenge for gynecologists. Close follow-up of established cases
is essential in gaining more information regarding the disease and
the efficacy of the available therapeutic methods.
References
|
1
|
Gusserow ALS: Ueber Sarcome des Uterus.
Arch Gynecol. 1:240–251. 1870. View Article : Google Scholar
|
|
2
|
Silverberg SG and Hurt WG: Minimal
deviation adenocarcinoma (‘adenoma malignum’) of the cervix: a
reappraisal. Am J Obstet Gynecol. 121:971–975. 1975.
|
|
3
|
Chang J, Zhang S, Zhou H, Liang JX and Lin
ZQ: Clinical analysis of minimal deviation adenocarcinoma of the
cervix: a report of five cases. Chin J Cancer. 27:1310–1314.
2008.(In Chinese).
|
|
4
|
Simionescu C, Georgescu CV, Mărgăritescu C
and Niculescu M: Diagnosis problems in a case of minimal deviation
adenocarcinoma of the cervix. Rom J Morphol Embryol. 47:245–249.
2006.PubMed/NCBI
|
|
5
|
Steeper TA and Wick MR: Minimal deviation
adenocarcinoma of the uterine cervix (‘adenoma malignum’). An
immunohistochemical comparison with microglandular endocervical
hyperplasia and conventional endocervical adenocarcinoma. Cancer.
58:1131–1138. 1986.
|
|
6
|
Du ZS and Zhao Q: Clinicopathological
observation of minimal deviation adenocarcinoma. Mod Med Health.
27:513–514. 2011.
|
|
7
|
Yang Z, Zhu DM and Yang LM: Minimal
deviation adenocarcinoma of uterine cervix: a clinicopathological
and immunohistochemical study of 14 cases. J Clin Res. 24:222–223.
2007.
|
|
8
|
Zhang L and Wang QH: Expression of protein
in cervical minimal deviation adenocarcinoma. Cancer Res Clin.
20:237–240. 2008.
|
|
9
|
Jiang L and Weng SQ: A case report of
minimal deviation adenocarcinoma of uterine cervix. Chin Med
Herald. 8:125–126. 2011.
|
|
10
|
Abiko K, Baba T, Ogawa M, Mikami Y, Koyama
T, Mandai M and Konishi I: Minimal deviation mucinous
adenocarcinoma (‘adenoma malignum’) of the uterine corpus. Pathol
Int. 60:42–47. 2010.
|
|
11
|
Odashiro AN, Odashiro DN and Nguyen GK:
Minimal deviation endometrioid denocarcinoma of the cervix: report
of three cases with exfoliative cytology. Diagn Cytopathol.
34:119–123. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen GQ: The morphologic analysis of 8
cases of minimal deviation adenocarcinoma. China Foreign Med Treat.
20:5–6. 2011.(In Chinese).
|
|
13
|
Kaminski PF and Norris HJ: Minimal
deviation carcinoma (adenoma malignum) of the cervix. Int J Gynecol
Pathol. 2:141–152. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu JY, Hashi A, Kondo T, Yuminamochi T,
Nara M, Hashi K, Murata S, Katoh R and Hoshi K: Absence of human
papillomavirus infection in minimal deviation adenocarcinoma and
lobular endocervical glandular hyperplasia. Int J Gynecol Pathol.
24:296–302. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McGowan L, Young RH and Scully RE:
Peutz-Jeghers syndrome with ‘adenoma malignum’ of the cervix. A
report of two cases. Gynecol Oncol. 10:125–133. 1980.
|
|
16
|
Itoh K, Toki T, Shiohara S, Oguchi O,
Konishi I and Fujii S: A comparative analysis of cross sectional
imaging techniques in minimal deviation adenocarcinoma of the
uterine cervix. BJOG. 107:1158–1163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takatsu A, Shiozawa T, Miyamoto T,
Kurosawa K, Kashima H, Yamada T, Kaku T, et al: Preoperative
differential diagnosis of minimal deviation adenocarcinoma and
lobular endocervical glandular hyperplasia of the uterine cervix: a
multicenter study of clinicopathology and magnetic resonance
imaging findings. Int J Gynecol Cancer. 21:1287–1296. 2011.
|
|
18
|
Oguri H, Maeda N, Izumiya C, Kusume T,
Yamamoto Y and Fukaya T: MRI of endocervical glandular disorders:
three cases of a deep nabothian cyst and three cases of a
minimal-deviation adenocarcinoma. Magn Reson Imaging. 22:1333–1337.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Landry D, Mai KT, Senterman MK, Perkins
DG, Yazdi HM, Veinot JP and Thomas J: Endometrioid adenocarcinoma
of the uterus with a minimal deviation invasive pattern.
Histopathology. 42:77–82. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gong L, Zhang WD, Liu XY, Han XJ, Yao L,
Zhu SJ, Lan M, Li YH and Zhang W: Clonal status and
clinicopathological observation of cervical minimal deviation
adenocarcinoma. Diagn Pathol. 24:252010. View Article : Google Scholar
|