Introduction
The conventional treatment of patients with advanced
breast cancer currently involves three steps: i) neoadjuvant
chemotherapy; ii) surgery of different degrees and iii) adjuvant
chemotherapy. The neoadjuvant and adjuvant chemotherapy comprise
different drug combinations, according to the individual patient
situation. Although the neoadjuvant therapy has not demonstrated
convincing evidence regarding survival benefit (1–6), it
has certain advantages, such as downstaging of an inoperable cancer
to an operable one, increasing the possibility of breast-conserving
surgery and potentially reducing the risk of metastatic disease
(1,7). Although the type of therapy is
important for survival and quality of life after treatment, the
prognosis prior to treatment initiation and the monitoring of the
outcome following treatment are equally important. Therefore,
imaging in combination with biopsy are commonly used techniques and
the benefit of these techniques may be further improved by the use
of serum biomarkers.
Serum thymidine kinase 1 (STK1) levels have been
used for monitoring tumor therapy and for assessing the risk of
recurrence and prognosis of survival in hematological malignancies
as well as in solid tumors (8).
Thymidine kinase 1 (TK1), a marker of cell proliferation, is an
enzyme involved in nucleotide metabolism and is important for the
supply of thymidine monophosphate for DNA synthesis. TK1 is
activated during the late G1 stage of the cell cycle, reaches a
peak during the late S and G2 phase and is degraded during mitosis.
There are two types of thymidine kinase (TK), cytoplasmic (TK1) and
mitochondrial (TK2); however, the expression of the latter is not
associated with cell proliferation (9,10).
TK1 serum levels are determined by its activity (STK1a) or its
concentration (STK1p) (8), which
is determined by specific anti-TK1 antibodies (SSTK Biotech Ltd.,
Shenzhen, China). The STK1a assay is mainly used for patients with
leukaemia and lymphoma, whereas STK1p is useful for hematological
malignancies as well as solid tumors, such as breast carcinoma.
In this study the use of serum thymidine kinase 1
protein (STK1p) concentration for monitoring the results of
treatment (neoadjuvant, surgical and adjuvant) in patients with
locally advanced breast cancer was investigated, including
determination of the risk of metastatic disease following
completion of treatment and prognosis of overall survival.
Patients and methods
Study design
The purpose of this study was to investigate the use
of STK1p in patients with locally advanced breast cancer for the
prognosis of the treatment outcome. The main endpoints were STK1p
in relation to the development of metastasis and to survival.
Patients were administered neoadjuvant treatment for 1 month,
followed by surgery and adjuvant chemotherapy for 6 months. Serum
samples were collected following neoadjuvant treatment and prior to
surgery, and at 1, 3 and 6 months following surgery. Healthy
age-matched females were used as controls. The development of
metastasis and survival were followed up for 44 months. The
variables considered were tumor clinical stage, tumor size, age,
clinical response, metastasis and survival. The number of specimens
(n=51) was selected in order to obtain statistically significant
results. The details are described below.
Patients
A total of 51 female patients with early locally
advanced (n=2), locally advanced (n=47) and advanced (n=2) breast
cancer were recruited in accordance with the NCCN guidelines for
neoadjuvant chemotherapy (11) at
the Department of Breast Surgery, Xiangya Hospital, Changsha,
China, between 2007 and 2011. The breast cancer patients were
diagnosed using cutting needle biopsy and the tumor clinical stage
and size were determined using imaging in combination with vernier
calipers. The majority of the patients (n=49) had invasive ductal
carcinoma, whereas the remaining 2 patients had early locally
infiltrating ductal tumors. The pathological type of the tumors was
assessed after neoadjuvant treatment. All the patients received
standard treatment protocols, i.e., 1 cycle of neoadjuvant
chemotherapy, followed by surgery and 2 cycles of adjuvant
chemotherapy. The surgical treatment was mastectomy in all the
cases.
Information regarding clinical stage, tumor size and
STK1p levels were obtained from all the patients, whereas
information regarding response to treatment [complete response
(CR), partial response (PR), stable disease (SD) and progressive
disease (PD)] was available for 43 patients and survival for 38
patients. The clinical response was assessed after the last cycle
of neoadjuvant treatment, prior to surgery. Staging was performed
according to the International Union Against Cancer TNM staging
system (12). The clinical stage
was based on imaging and cutting needle biopsy techniques and the
tumor size on imaging, all performed prior to neoadjuvant
treatment. According to the AJCC guidelines for breast cancer
staging, the primary tumors were classified into two groups:
<5.0 and ≥5.0 cm, based on the greatest dimension, roughly
corresponding to stage I/II and III/IV disease, respectively.
Estrogen receptor (ER), progesterone receptor (PgR) and human
epidermal growth factor receptor 2 (HER2) were determined after
surgery using immunohistochemistry. Serum samples were collected 1
week after the completion of the neoadjuvant treatment and prior to
surgery, and at 1, 3 and 6 months after surgery. Thus, the serum
samples following surgery were collected during the adjuvant
chemotherapy period. The characteristics of the patients are
presented in Table I. The mean age
of the patients was 45.9±9.3 years (range, 30–67 years).
Age-matched healthy females (n=286), without any tumor, infection,
or other non-tumor diseases or symptoms, were used as controls for
the STK1p values. The mean age of the healthy controls was
46.1±10.5 years (range, 30–67 years).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | No. | P-value |
|---|
| Patients | 51 | |
| Mean age (years)
(range) | 45.7±8.7 (30–67) | |
| Follow-up
(months) | 44 | |
| Pre-neoadjuvant
stage |
| I | 3 | |
| II | 28 | |
| III | 16 | |
| IV | 4 | |
| ER, PgR, HER2
status |
| ER+, PgR+, HER2−,
total | 11 | |
| Death from
metastasis | 0 | |
| ER−, PgR−, HER2−
(TNBC), total | 7 | A vs. B: 0.049 |
| Death from
metastasis | 3 | |
| ER−, PgR−, HER2+,
total | 12 | A vs. C: 0.191 |
| Death from
metastasis | 2 | |
| Other subtypes,
total | 8 | A vs. D: 0.256 |
| Death from
metastasis | 1 | |
| Tumor size (cm) |
| <5.0 | 36 | |
| >5.0 | 15 | |
| Clinical
response |
| CR | 3 | |
| PR | 37 | |
| SD | 2 | |
| PD | 1 | |
Survival
The patients were followed up for 44 months by
tracking medical records, letter and telephone communication.
Information on the survival status of the patients was obtained
until May 5, 2011. No information was available for 13 patients.
The followed-up patients included patients with advanced (n=2),
locally advanced (n=34) and early locally advanced (n=2)
tumors.
Treatments
The neoadjuvant and the adjuvant chemotherapy were
individual treatment programs, depending on the individual
physiological and clinical symptoms. The treatment was designed
according to the NCCN recommendations for breast cancer patients.
The patients were treated with a combination of different types of
drugs. Neoadjuvant therapy was administered for 1–4 cycles and
adjuvant therapy for 1–6 cycles, depending on the clinical
response, which was evaluated by imaging after each cycle. The
types of drugs used for neoadjuvant therapy were as follows: T, 1
patient; TA, 1 patient; TAC, 7 patients; TEC, 2 patients; FAC, 21
patients; FEC, 7 patients; TEC+FEC, 1 patient; TAC+FEC, 1 patient;
and TAC+FAC, 2 patients. Neoadjuvant treatment was not administered
to 8 patients due to a small tumor burden. The drugs used for
adjuvant therapy were as follows: T, 5 patients; TA, 1 patient;
TAC, 5 patients; TEC, 2 patients; TP, 4 patients; FAC, 11 patients;
FEC, 6 patients; T+AC, 4 patients; T+FAC, 10 patients; and T+FEC, 3
patients. The treatment cycle time was 21 days, with a 7-day rest
between the cycles. The concentrations were adjusted according to
the NCCN guidelines. For the drug abbreviations, see the NCCN
guidelines.
STK1 assay
The concentration of STK1 was measured by using a
commercial kit based on an enhanced chemiluminescence (ECL) dot
blot assay (SSTK Biotech Ltd.) (13,14).
Samples comprising 3 μl of serum were directly applied onto
nitrocellulose membranes. The serum samples were probed with
anti-TK1 chicken IgY antibody raised against a peptide (residue
195–225, GQPAG PDNKE NCPVP GKPGE AVAAR KLFAPQ). The TK1 peptide was
dotted at different concentrations (20, 6.6 and 2.2 pM) to obtain
an extrapolation standard curve. The intensities of the spots on
the membrane were determined by a charge-coupled device camera
(CIS-I Imaging System, SSTK Biotech Ltd.). The results were
analysed using a computer program provided by SSTK Biotech Ltd.
Receiver operating characteristic (ROC)
analysis
The ROC analysis of the STK1p results was performed
on preoperative patients with early local breast cancer (n=120)
compared to healthy Chinese female volunteers (n=286). The healthy
controls were free of any known disease. The serum samples from the
breast cancer patients were collected at the Department of
Oncology, Karolinska University Hospital, Sweden. No difference in
the STK1p values was observed between healthy Chinese and Swedish
individuals.
Western blot analysis
The western blot analysis of TK1 in the serum was
performed as previously described (13).
Statistical analysis
The Kaplan-Meier method and the log-rank test were
used for the comparison of survival rates. Cox regression was used
for the univariate and multivariate analyses. The Chi-square and
Student’s t-tests were used for the comparison of TK1 expression
among patients with different pathological stages, ER, PgR and HER2
status and CR to treatment. The ROC analysis was performed by a
computer software program provided by Analyse-It Software, Leeds,
UK. P<0.05 was considered to indicate a statistically
significant difference.
Study approval
The patients provided verbal consent to participate
in this study. The study was approved by the Committee on Research
Ethics at Xiangya Hospital, Zhongnan University, Changsha, China.
This study was conducted in accordance with the Helsinki
Declaration of 1983.
Results
Specificity of anti-TK1 antibody and
sensitivity of STK1p assay
In order to assess the usefulness of the STK1p
assay, the specificity and sensitivity of the ECL dot blot STK1p
assay system were investigated by western blotting and ROC
analysis. In the western blot analysis of native serum TK1 from
breast cancer patients prior to treatment, only one band
corresponding to human TK1 was observed, demonstrating the high
specificity of the anti-TK1 IgY antibody (Fig. 1A). A competing experiment adding an
excess of antigen (a 31-amino acid peptide, 500 nM) revealed no
detectable bands, providing additional evidence for the high
specificity of the IgY anti-TK1 antibody (Fig. 1A).
The ROC-analysis demonstrated that the sensitivity
was high (Fig. 1B, Table II). At an optimized STK1p cut-off
value of 2.00 pM, the sensitivity and specificity were 0.86 and
0.99, respectively. The area under the curve was 0.99 and the
likelihood (+) value was 153.64. The high ROC and likelihood values
demonstrated that the STK1p assay was able to efficiently
distinguish between groups of healthy individuals and breast cancer
patients.
 | Table IIROC analysis of breast cancer
patients (n=120) in relation to healthy individuals (n=286). |
Table II
ROC analysis of breast cancer
patients (n=120) in relation to healthy individuals (n=286).
| Type | ROC value | SE | Z | P-value | Sensitivity | Specificity | Likelihood (+) | n |
|---|
| Breast cancer
patients | 0.99 | 0.004 | 110.95 | <0.0001 | 0.86 | 0.99 | 153.64 | 120 |
Patients
A total of 51 patients were recruited for this
study. The majority of patients had stage II (54.9%) and III
(31.4%) disease (Table I). There
was a statistically significant higher frequency of ER−, PgR− and
HER2-receptor negative patients [triple-negative breast cancer
(TNBC)] that succumbed to the disease within 44 months after
treatment, compared to ER+, PR+ and HER2− patients (Table I). Moreover, 72.5% (37/51) of the
patients exhibited a PR upon treatment, whereas 5.9% (3/51)
achieved a CR and others had SD 3.9 % (2/51) or PD 2% (1/51).
Patients with a tumor size >5.0 cm exhibited a statistically
significant shorter survival, compared to patients with a tumor
size <5.0 cm (P=0.006, data not shown).
The STK1p levels were determined 1 week following
the completion of the neoadjuvant treatment and prior to surgery,
and at 1, 3 and 6 months after surgery. In this group of patients
no pre-treatment STK1p serum samples were obtained due to the
routine clinical procedure. The serum samples at 1 and 3 months
after surgery were collected during the adjuvant chemotherapy and
were handled with caution due to the uncontrolled fluctuations in
the STK1p levels caused by chemotherapy-induced tumor cell
disintegration. By contrast, the STK1p levels at 6 months after
surgery were stabilized and mainly reflected the tumor burden. The
serum samples prior to the initiation of the neoadjuvant treatment
were only obtained from 11 patients due to the routine clinical
procedure and, thus, were not included in the statistical
evaluation of the results. At the end of neoadjuvant treatment, the
average STK1p values of these 11 patients were decreased by 46.9%,
indicating reduced proliferation rates of the breast tumors as a
result of the neoadjuvant treatment (data not shown).
There was no significant difference in the mean
STK1p levels after neoadjuvant treatment and at 1, 3 and 6 months
after surgery (Table III), or in
the number of patients with low or high STK1p values at these time
points (Table IV). This is likely
due to fluctuations in STK1p values caused by the chemotherapy, at
least at 1 and 3 months after surgery. However, there was a
statistically significant difference in the STK1p values between
the low and high STK1p groups at 3 and 6 months after surgery
(Table III).
 | Table IIISTK1p values during treatment in the
low (<2.0 pM) and high (≥2.0 pM) STK1p groups. |
Table III
STK1p values during treatment in the
low (<2.0 pM) and high (≥2.0 pM) STK1p groups.
| Time point | STK1p (pM) | Low | High | P-value |
|---|
| Healthy
controls | 0.5±0.4 | - | - | |
| 1 weeka | 1.8±0.9 | - | - | |
| 1 montha | 1.6±1.1 | - | - | |
| 3 monthsa | 1.8±1.1 | 1.2±0.5 | 3.0±1.0 | |
| 6 monthsa | 1.4±0.9 | 1.1±0.6 | 2.8±0.6 | <0.001 |
 | Table IVNumber of patients with low (<2.0
pM) and high (≥2.0 pM) STK1p values during treatment. |
Table IV
Number of patients with low (<2.0
pM) and high (≥2.0 pM) STK1p values during treatment.
| Time point | Low | High |
|---|
| 1 weeka | 34 | 17 |
| 1 montha | 35 | 16 |
| 3 monthsa | 33 | 18 |
| 6 monthsa | 38 | 13 |
Patients with SD and PD exhibited significantly
higher STK1p values at 6 months after surgery, compared to patients
with CR (Fig. 2). These
differences were not observed in the serum samples from patients at
1 and 3 months after surgery (data not shown).
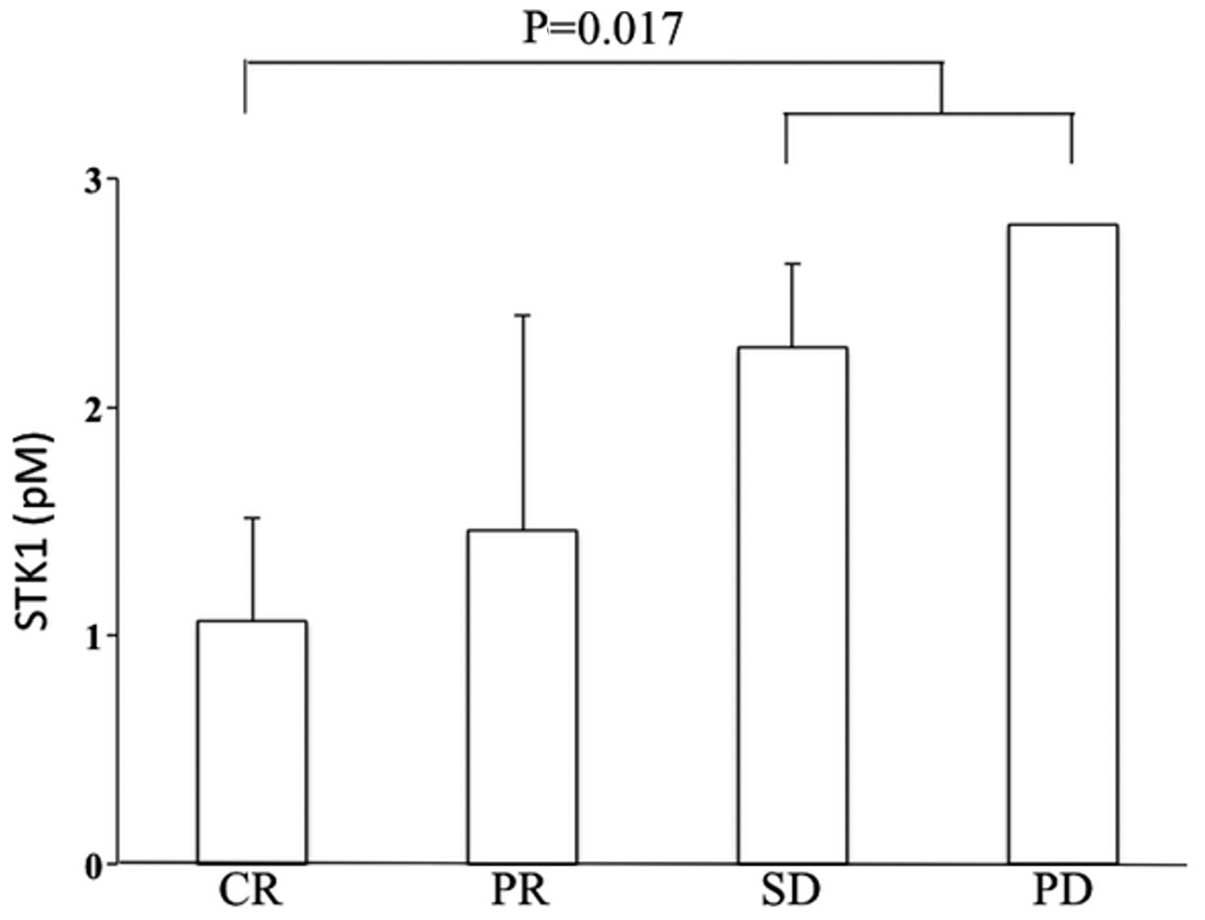 | Figure 2STK1p values in relation to clinical
response at 6 months after surgery. CR, n=3; PR, n=37; SD, n=2; PD,
n=1. STK1p, serum thymidine kinase 1 protein; CR, complete
response; PR, partial response; SD, stable disease, PD, progressive
disease. |
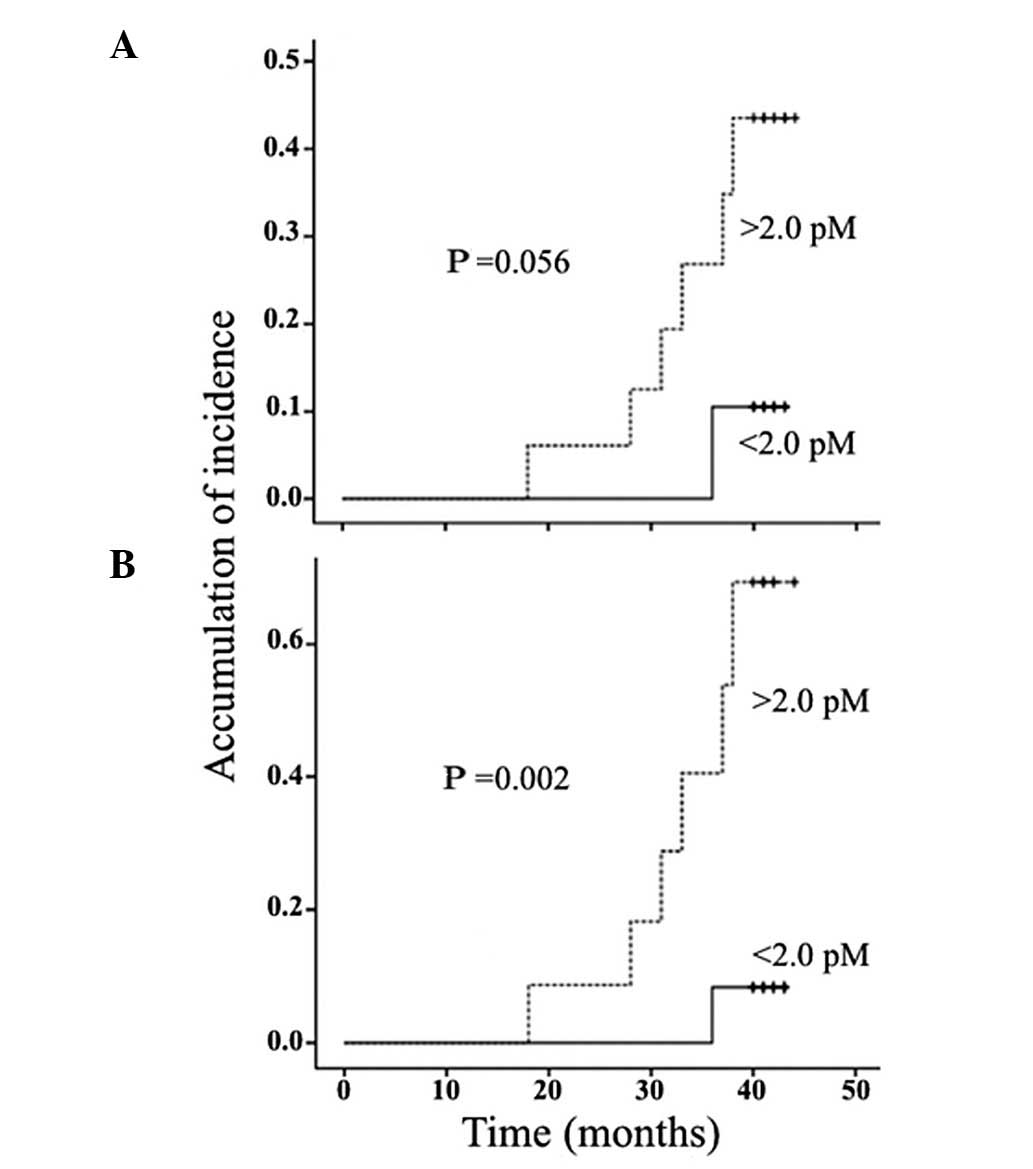
STK1p values and metastatic disease
Patients with high STK1p values (≥2.0 pM) at 3 and 6
months after surgery (Fig. 3A and
B) had a statistically significant higher risk of developing
metastasis, starting at 18 months and continuing up to the end of
the observation period, at 42 months.
A Cox univariate analysis of the variables 6 months
after surgery demonstrated that STK1p levels, clinical stage and
tumor size, but not age, were associated with the development of
metastasis (Table V). A
multivariate analysis at 6 months demonstrated that STK1p was the
only independent prognostic marker for the occurrence of metastatic
disease (Table V). High STK1p
values (≥2.0 pM) 6 months after surgery were associated with an
increased risk of metastasis by 8.2-fold (Table V).
 | Table VCox values of variables in relation
to risk of metastasis at 3 and 6 months following surgery. |
Table V
Cox values of variables in relation
to risk of metastasis at 3 and 6 months following surgery.
| Variables | P-value | Hazard risk | 95% CI |
|---|
| 3 months |
| Univariate |
| STK1p (<2.0
vs. ≥2.0 pM) | 0.057 | - | - |
| Stage (I/II vs.
III/IV) | 0.017 | 7.077 | 1.43–35.16 |
| Size (<5.0 vs.
≥5.0 cm) | 0.017 | 7.077 | 1.43–35.16 |
| Age (<50 vs.
≥51 years) | 0.950 | - | - |
| Multivariate |
| STK1p (<2.0
vs. ≥2.0 pM) | 0.548 | - | - |
| Stage (I/II vs.
III/IV) | 0.017 | 7.077 | 1.43–35.16 |
| Size (<5.0 vs.
≥5.0 cm) | 0.511 | - | - |
| Age (<50 vs.
≥51 years) | 0.898 | - | - |
| 6 months |
| Univariate |
| STK1p (<2.0
vs. ≥2.0 pM) | 0.010 | 8.221 | 1.67–40.87 |
| Stage (I/II vs.
III/IV) | 0.017 | 7.077 | 1.43–35.16 |
| Size (<5.0 vs.
≥5.0 cm) | 0.017 | 7.077 | 1.43–35.16 |
| Age (<50 vs.
≥51 years) | 0.950 | - | - |
| Multivariate |
| STK1p (<2.0
vs. ≥2.0 pM) | 0.010 | 8.221 | 1.67–40.87 |
| Stage (I/II vs.
III/IV) | 0.087 | - | - |
| Size (<5.0 vs.
≥5.0 cm) | 0.087 | - | - |
| Age (<50 vs.
≥51 years) | 0.312 | - | - |
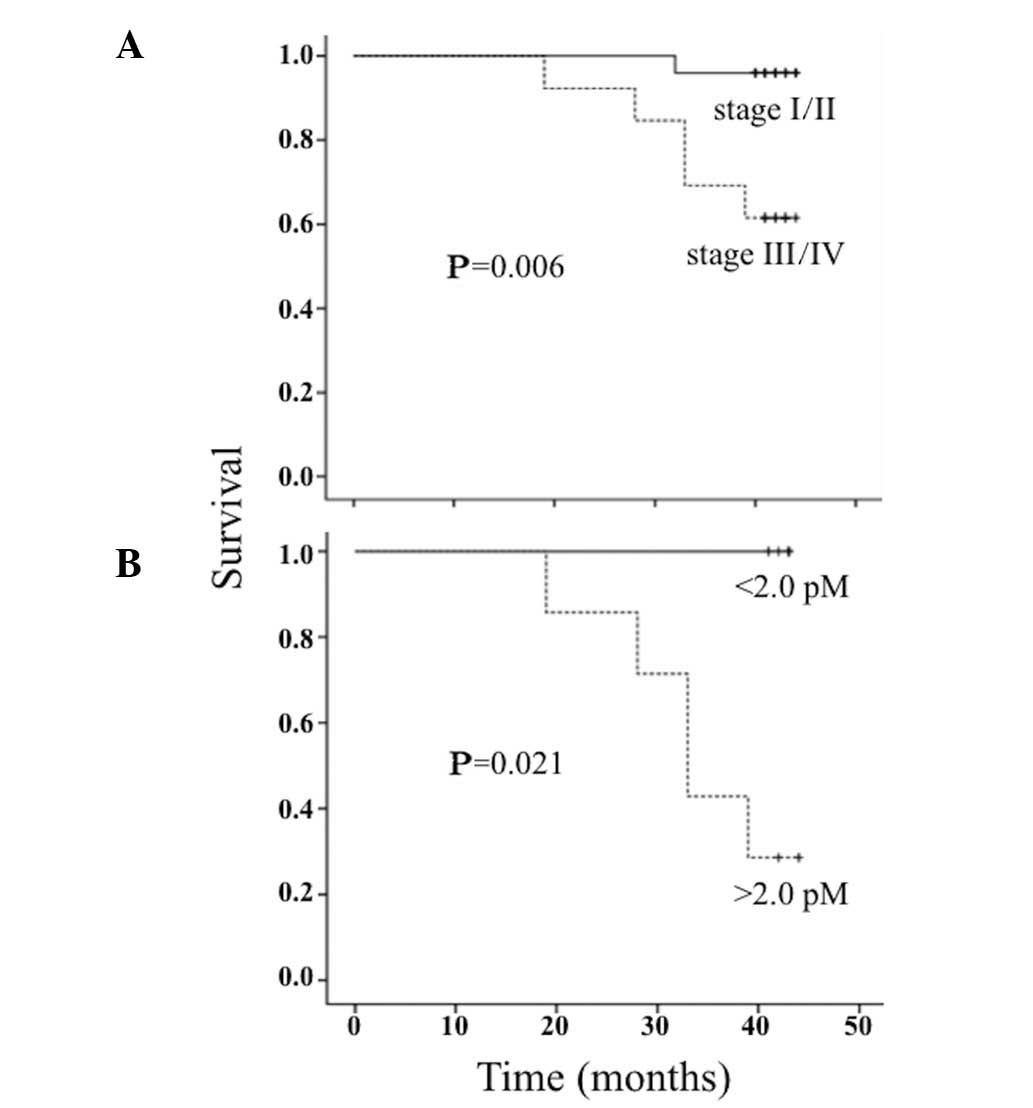
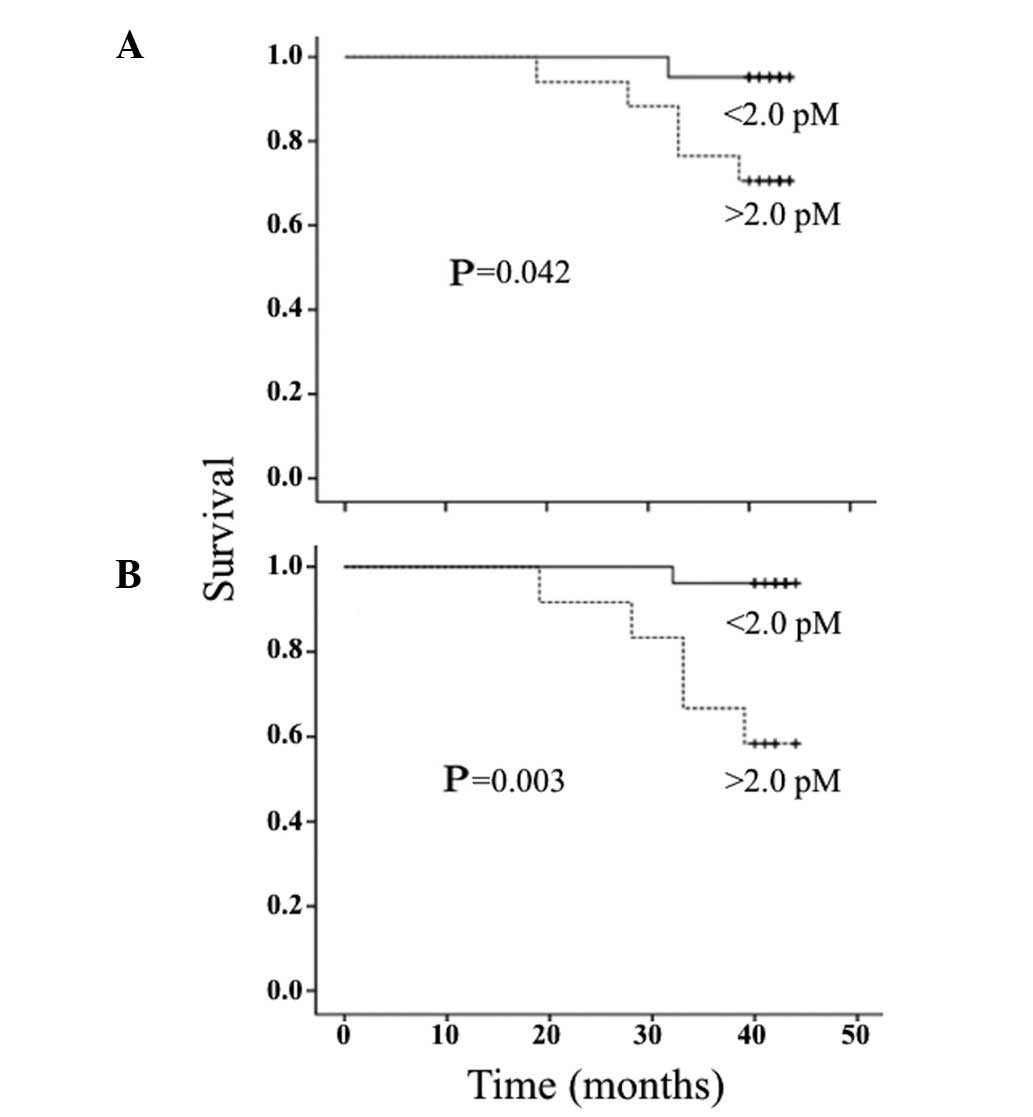
STK1p values and overall survival
There was a statistically significant difference in
survival between patients with stage I/II and stage III/IV disease
(Fig. 4A). Patients with high
STK1p values (≥2.0 pM) exhibited a significantly shorter survival
compared to patients with low STK1p values (<2.0 pM) at 3 and 6
months after surgery (Fig. 5A and
B), but not in the serum samples obtained 1 month after surgery
(data not shown). Of note, patients with stage III/IV disease and
low STK1p values (<2.0 pM, 5/12) exhibited a longer survival
compared to patients with high STK1p values (≥2.0 pM, 7/12)
(Fig. 4B).
In a univariate analysis of patients at 3 and 6
months after surgery, STK1p levels, clinical stage and tumor size,
but not age, were statistically significantly associated with
survival (Table VI). In a
multivariate analysis, STK1p was found to be the only independent
prognostic factor for survival (Table
VI). High STK1p values (≥2.0 pM) at 3 and 6 months after
surgery increased the mortality risk by 11- and 12-fold,
respectively (Table VI).
 | Table VICox values of variables in relation
to survival at 3 and 6 months following surgery. |
Table VI
Cox values of variables in relation
to survival at 3 and 6 months following surgery.
| Variables | P-value | Hazard risk | 95% CI |
|---|
| 3 months |
| Univariate |
| STK1p (<2.0
vs. ≥2.0 pM) | 0.080 | - | - |
| Stage (I/II vs.
III/IV) | 0.028 | 11.071 | 1.29–94.92 |
| Size (<5.0 vs.
≥5.0 cm) | 0.028 | 11.071 | 1.29–94.92 |
| Age (<50 vs.
≥51 years) | 0.891 | - | - |
| Multivariate |
| STK1p (<2.0
vs. ≥2.0 pM) | 0.028 | 11.071 | 1.29–94.92 |
| Stage (I/II vs.
III/IV) | 0.493 | - | - |
| Size (<5.0 vs.
≥5.0 cm) | 0.540 | - | - |
| Age (<50 vs.
≥51 years) | 0.940 | - | - |
| 6 months |
| Univariate |
| STK1p (<2.0
vs. ≥2.0 pM) | 0.021 | 12.67 | 1.48–108.79 |
| Stage (I/II vs.
III/IV) | 0.028 | 11.07 | 1.29–94.92 |
| Size (<5.0 vs.
≥5.0 cm) | 0.028 | 11.07 | 1.29–94.92 |
| Age (<50 vs.
≥51 years) | 0.891 | - | - |
| Multivariate |
| STK1p (<2.0
vs. ≥2.0 pM) | 0.010 | 12.67 | 1.65–40.87 |
| Stage (I/II vs.
III/IV) | 0.497 | - | - |
| Size (<5.0 vs.
≥5.0 cm) | 0.122 | - | - |
| Age (<50 vs.
≥51 years) | 0.122 | - | - |
Discussion
Breast cancer development and progression involves
complex interactions between hormonal receptors and signaling
pathways of growth factors, some of which are evident in the serum.
In patients with locally advanced/advanced breast cancer,
prognostic pathological markers such as nodal disease, presence of
inflammatory breast cancer or poor pathological response and serum
biomarkers such as carcinoembryonic antigen, CA15-3, MMP-2, MMP-9,
tissue polypeptide antigen, tissue polypeptide-specific antigen,
epidermal growth factor receptor and HER2/neu, have been
investigated as predictors of clinical or pathological response to
chemotherapy (15,16). However, the results of these
investigations were not successful in identifying specific
biomarkers for each chemotherapeutic regimen. Recently, the
significance of the HER2 profile for the management and treatment
of primary breast carcinoma was investigated. One study reported
that ER and HER2 immunohistochemistry and HER2 fluorescence in
situ hybridization were not significantly different in primary
breast carcinomas prior to and following neoadjuvant chemotherapy
(17). Another study reported that
decreasing serum HER2/neu values were observed following
neoadjuvant chemotherapy and were correlated with pathological
response (18). However, Mazouni
et al(19) did not observe
any difference in HER2/neu levels between patients with
pathological CR and those with residual disease. Furthermore, a
summary of 65 studies on the association of HER2/neu with prognosis
in breast cancer patients did not lead to any definitive
conclusions (20). Receptor CXCR4
was overexpressed in HER2-negative breast cancer patients and was
correlated with disease-free survival. This was not the case in
HER2-positive breast cancer patients, suggesting that receptor
CXCR4 may be used to distinguish between patients with long- and
short-term survival (21). In a
previous study, we demonstrated that HER2 overexpression was
associated with significantly higher STK1p values prior to
neoadjuvant chemotherapy (22). In
addition, TNBC patients (ER−, PgR− and HER2−) have poor prognostic
characteristics compared to other subtypes, due to lack of common
therapeutic targets (23–26). TNBC patients with residual disease
following neoadjuvant chemotherapy exhibit significantly worse
survival compared to non-TNBC patients, particularly during the
first 3 years after treatment (23). In the present study, despite the
limited patient sample, TNBC was found at a significantly higher
frequency among patients who succumbed to metastatic disease,
compared to patients with ER+, PR+ and HER2−, confirming recent
studies (8,14). Despite the vast availability of
prognostic markers, the results remain confusing and there is need
of alternative biomarkers for monitoring the treatment of breast
cancer patients.
To the best of our knowledge, this study was the
first to demonstrate that the concentration of STK1p is a reliable
monitoring and prognostic marker for the treatment outcome of
patients with advanced breast cancer. The STK1p levels are able to
predict the development of metastasis at 6 months after surgery,
i.e., 12 months prior to the first patients being diagnosed with
metastasis. This was in accordance with findings of a previous
study on low-risk breast cancer patients, in which the STK1p levels
predicted recurrence at 6 months after surgery (27). STK1p is also a more reliable
prognostic factor for overall survival compared to other parameters
such as clinical stage, tumor size and age. Based on a multivariate
Cox analysis, STK1p was found to be an independent predictive
marker for the development of metastasis and a prognostic marker
for survival as early as 3–6 months after surgery. The efficacy of
STK1p as a prognostic marker was even more apparent in the serum
samples collected at 6 months after surgery. Of note, in this study
the STK1p levels were able to identify a group of high-stage
(III/IV) patients with good prognosis, although the follow-up time
was <5 years. Patients with stage III/IV disease are considered
as exhibiting poor survival, which was also demonstrated by this
study (Fig. 5A). Similar results
were reported by a study on pT1 lung carcinoma patients (28), in which TK1 expression, as
determined by immunohistochemistry of lung tumor tissues, was
correlated with survival. Data concerning the expression of TK1 in
tissues obtained from patients with cervical carcinoma also confirm
these results (29)
TK1 levels in the serum may be determined by the
enzyme activity or concentration. STK1p was used in this study,
since it was previously demonstrated that STK1p levels in patients
with solid tumors provide more accurate prognostic information in a
larger proportion of the patients, compared to STK1a (8), as the sensitivity of STK1 activity
detection methods is usually low in patients with solid tumors.
This was also the case in a recent study by Nisman et
al(30) which compared tissues
from primary breast cancer patients with benign breast tissues. The
conclusions of that study were that the Liaison TK assay and the
highly sensitive DiviTum TK activity assay may be used to predict
disease recurrence in the preoperative setting. However, the
sensitivity for the two assays was ~25% and there was no
significant difference in STK1a levels between the benign and
malignant breast disease groups. However, several previous studies
have reported a sensitivity of the STK1p assay of >80% (22,31,32).
In the present study, using an optimized threshold cut-off STK1p
value of 2.0 pM, high ROC value (0.99), high sensitivity (0.86) and
high specificity (0.99) were observed, supporting the use of the
STK1p assay over that of STK1a assays in breast cancer patients.
Thus, STK1p determination provides more accurate predictive and
prognostic information regarding recurrence and survival compared
to enzyme activity measurements. A possible explanation for the
discrepancy between STK1p and STK1a assays is that only a subset of
the TK1 molecules in the serum is enzymatically active.
Inactivation of TK1 may be the result of tumor cell death leading
to denaturation/inactivation of the enzyme.
Depending on the cut-off value, different levels of
sensitivity and specificity were obtained. In our meta-study on
health screening in 2011 (including a total of 35,365 participants)
(30), we optimized the STK1p
cut-off value in order to limit the number of false-positive cases.
At a cut-off value of 2.00 pM, the specificity and sensitivity were
0.99 and 0.80, respectively. The ROC and likelihood values were
high (0.96 and 233.73, respectively). The ROC analysis was
performed on serum samples obtained from 720 cancer patients with
11 different types of tumors and 4,103 sub-healthy individuals
without known malignancies or premalignant conditions. The ROC
values of the different types of tumors were 0.92–1.00. Thus, the
STK1p assay appears to be adequately sensitive for health screening
and for use in clinical oncology.
In conclusion, this study was based on patients
following routine individual treatment and, thus, is not considered
a clinical trial. However, significant differences were observed
between the different groups investigated, suggesting that STK1p is
a useful prognostic and predictive biomarker for monitoring
treatment, the development of metastasis and survival in the
routine clinical setting. Similar studies on STK1p levels in other
types of malignancies support this conclusion (8,14).
In addition, STK1p may be able to identify late-stage (III/IV)
breast cancer patients with better survival expectancy, leading to
improved personalized treatment. However, in order to obtain a more
complete clinical evaluation of the usefulness of STK1p in breast
cancer management, a larger case-control clinical trial is required
and is currently in progress.
Acknowledgements
This study was supported by the Xiangya Hospital,
Central South University, Changsha, China, the Cancer Society of
Stockholm, Faculty of Karolinska Institute, Swedish International
Development Cooperation Agency and by a grant to S.E. from the
Swedish Research Council.
Notes
[1] Conflicts of
interest
Sven Skog and Ellen He serve as consultants for SSTK
Biotech Ltd.; Yuan Li, Ming Zhang and Ji Zhou are employees of SSTK
Biotech Ltd.
References
|
1
|
Fisher B, Bryant J, Wolmark N, et al:
Effect of preoperative chemotherapy on the outcome of women with
operable breast cancer. J Clin Oncol. 16:2672–2685. 1998.PubMed/NCBI
|
|
2
|
Scholl SM, Pierga JY, Asselain B, et al:
Breast tumor response to primary chemotherapy predicts local and
distant control as well as survival. Eur J Cancer. 31A:1969–1975.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Semiglazov VF, Topuzov EE, Bavli JL, et
al: Primary (neoadjuvant) chemotherapy and radiotherapy compared
with primary radiotherapy alone in stage IIb–IIIa breast cancer.
Ann Ocol. 5:591–595. 1994.
|
|
4
|
Makris A, Powles TJ, Dowsett M, et al:
Prediction of response to neoadjuvant chemoendocrine therapy in
primary breast carcinomas. Clin Cancer Res. 3:593–600.
1997.PubMed/NCBI
|
|
5
|
Mauriac L, MacGrogan G, Avril A, et al:
Neoadjuvant chemotherapy for operable breast carcinoma larger than
3 cm: a unicentre randomized trial with a 124-month median
follow-up. Institute Bergonié Bordeaux Groupe Sein (IBBGS). Ann
Oncol. 10:47–52. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van der Hage JA, van de Velde CJ, Julien
JP, et al: Preoperative chemotherapy in primary operable breast
cancer: results from the European Organization for Research and
Treatment of Cancer trial 10902. J Clin Oncol. 19:4224–4237.
2001.
|
|
7
|
Fisher B, Gunduz N and Saffer EA:
Influence of the interval between primary tumor removal and
chemotherapy on kinetics and growth of metastases. Cancer Res.
43:1488–1492. 1983.PubMed/NCBI
|
|
8
|
Zhou J, He E and Skog S: The proliferation
marker thymidine kinase 1 in clinical use (Review). Mol Clin Oncol.
1:18–28. 2013.
|
|
9
|
Sherley JL and Kelly TJ: Regulation of
human thymidine kinase during the cell cycle. J Biol Chem.
263:8350–8358. 1988.PubMed/NCBI
|
|
10
|
Gasparri F, Wang N, Skog S, Galvani A and
Eriksson S: Thymidine kinase 1 expression defines an activated G1
stage of the cell cycle as revealed with site-specific antibodies
and ArrayScan assays. Eur J Cell Biol. 88:779–785. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
NCCN Clinical Practice Guidelines in
Oncology™. Breast Cancer V.2.2007. Available at: http://www.nccn.org.
Accessed May 15, 2007
|
|
12
|
Goldstraw P and Groome P: Lung.
International Union Against Cancer (UICC). TNM Classification of
Malignant Tumours. Sobin LH, Gospodarowicz MK and Wittekind CH: 7th
edition. Wiley-Blackwell; New York, NY: pp. 138–146. 2009
|
|
13
|
Wu C, Yang R, Zhou J, et al: Production
and characterisation of a novel chicken IgY antibody raised against
C-terminal peptide from human thymidine kinase 1. J Immunol
Methods. 277:157–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He E, Xu XH, Guan H, et al: Thymidine
kinase 1 is a potential marker for prognosis and monitoring the
response to treatment of patients with breast, lung and esophageal
cancer and non-Hodgkin’s lymphoma. Nucleosides Nucleotides Nucleic
Acids. 29:352–358. 2010.PubMed/NCBI
|
|
15
|
Coskun U, Yamac D, Gulbahar O, et al:
Locally advanced breast carcinoma treated with neoadjuvant
chemotherapy: are the changes in serum levels of YKL-40, MMP-2 and
MMP-9 correlated with tumor response? Neoplasma. 54:348–352.
2007.PubMed/NCBI
|
|
16
|
Martinez-Trufero J, de Lobera AR, Lao J,
et al: Serum markers and prognosis in locally advanced breast
cancer. Tumori. 91:522–530. 2005.PubMed/NCBI
|
|
17
|
Kinsella MD, Nassar A, Siddiqui MT and
Cohen C: Estrogen receptor (ER), progesterone receptor (PR), and
HER2 expression pre- and post- neoadjuvant chemotherapy in primary
breast carcinoma: a single institutional experience. Int J Clin Exp
Pathol. 5:530–536. 2012.
|
|
18
|
Lee JS, Son BH and Ahn SH: The predictive
value of serum HER2/neu for response to anthracycline-based and
trastuzumab-based neoadjuvant chemotherapy. J Breast Cancer.
15:189–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mazouni C, Hall A, Broglio K, et al:
Kinetics of serum HER-2/neu changes in patients with HER-2-positive
primary breast cancer after initiation of primary chemotherapy.
Cancer. 109:496–501. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leyland-Jones B and Smith BR: Serum HER2
testing in patients with HER2-positive breast cancer: the death
knell tolls. Lancet Oncol. 12:286–295. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Holm NT, Byrnes K, Li BD, et al: Elevated
levels of chemokine receptor CXCR4 in HER-2 negative breast cancer
specimens predict recurrence. J Surg Res. 141:53–59. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen F and Tang L: Significance of S-TK1
detecting in breast cancer patients and its relationship with
prognosis. Can Res Prev Treat. 39:637–641. 2012.(In Chinese).
|
|
23
|
Liedtke C, Mazouni C, Hess KR, et al:
Response to neoadjuvant therapy and long-term survival with
triple-negative breast cancer. J Clin Oncol. 26:1275–1281. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Onitilo AA, Engel JM, Greenlee RT and
Mukesh BN: Breast cancer subtypes based on ER/PR and Her2
expression: comparison of clinicopathologic features and survival.
Clin Med Res. 7:4–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Verma S, Provencher L and Dent R: Emerging
trends in the treatment of triple-negative breast cancer in Canada:
a survey. Curr Oncol. 18:180–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pinhel I, Hills M, Drury S, et al: ER and
HER2 expression are positively correlated in HER2
non-overexpressing breast cancer. Breast Cancer Res. 14:R462012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He Q, Fornander T, Johansson H, et al:
Thymidine kinase 1 in serum predicts increased risk of distant or
loco-regional recurrence following surgery in patients with early
breast cancer. Anticancer Res. 26:4753–4759. 2006.PubMed/NCBI
|
|
28
|
Xu Y, Shi QL, Ma H, et al: High thymidine
kinase 1 (TK1) expression is a predictor of poor survival in
patients with pT1 of lung adenocarcinoma. Tumour Biol. 33:475–483.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen G, He C, Li L, et al: Nuclear TK1
expression is an independent prognostic factor for survival in
pre-malignant and malignant lesions of the cervix. BMC Cancer.
13:249–258. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nisman B, Allweis T, Kadouri L, et al:
Comparison of diagnostic and prognostic performance of two assays
measuring thymidine kinase 1 activity in serum of breast cancer
patients. Clin Chem Lab Med. 51:439–447. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He Q, Zou L, Zhang PA, et al: The clinical
significance of thymidine kinase 1 measurement in serum of breast
cancer patients using anti-TK1 antibody. Int J Biol Markers.
15:139–146. 2000.PubMed/NCBI
|
|
32
|
Chen ZH, Huang SQ, Wang Y, et al:
Serological thymidine kinase 1 is a biomarker for early detection
of tumours - a health screening study on 35,365 people, using a
sensitive chemiluminescent dot blot assay. Sensors (Basel).
11:11064–11080. 2011. View Article : Google Scholar : PubMed/NCBI
|