|
1.
|
Parkin DM, Bray FJ and Pisani P: Global
cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2.
|
DeVita VT, Lawrence TS and Rosenberg SA:
DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of
Oncology. 9th Edition. Lippincott Williams & Wilkins;
Philadelphia: 2009
|
|
3.
|
Montazeri A, Gillis CR and McEwen J:
Quality of life in patients with lung cancer: a review of
literature from 1970 to 1995. Chest. 113:467–481. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Abratt RP and Hart GJ: 10-year update on
chemotherapy for non-small cell lung cancer. Ann Oncol. 17(Suppl
5): v33–v36. 2006.PubMed/NCBI
|
|
5.
|
Pfister DG, Johnson DH, Azzoli CG, et al:
American Society of Clinical Oncology: American Society of Clinical
Oncology treatment of unresectable non-small-cell lung cancer
guideline: update 2003. J Clin Oncol. 22:330–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
No authors listed:. Chemotherapy in
non-small cell lung cancer: a meta-analysis using updated data on
individual patients from 52 randomised clinical trials. Non-small
Cell Lung Cancer Collaborative Group. BMJ. 311:899–909. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Pujol JL, Barlesi F and Daurèsa JP: Should
chemotherapy combinations for advanced non-small cell lung cancer
be platinum-based? A meta-analysis of phase III randomized trials.
Lung Cancer. 51:335–345. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ettinger DS, Bepler G, Bueno R, et al:
Non-small cell lung cancer clinical practice guidelines in
oncology. J Natl Compr Canc Netw. 4:548–582. 2006.PubMed/NCBI
|
|
9.
|
Azzoli CG, Baker S Jr, Temin S, et al:
American Society of Clinical Oncology: American Society of Clinical
Oncology Clinical Practice Guideline update on chemotherapy for
stage IV non-small-cell lung cancer. J Clin Oncol. 27:6251–6266.
2009. View Article : Google Scholar
|
|
10.
|
Le Chevalier T, Brisgand D, Douillard JY,
et al: Randomized study of vinorelbine and cisplatin versus
vindesine and cisplatin versus vinorelbine alone in advanced
non-small-cell lung cancer: results of a European multicenter trial
including 612 patients. J Clin Oncol. 12:360–367. 1994.
|
|
11.
|
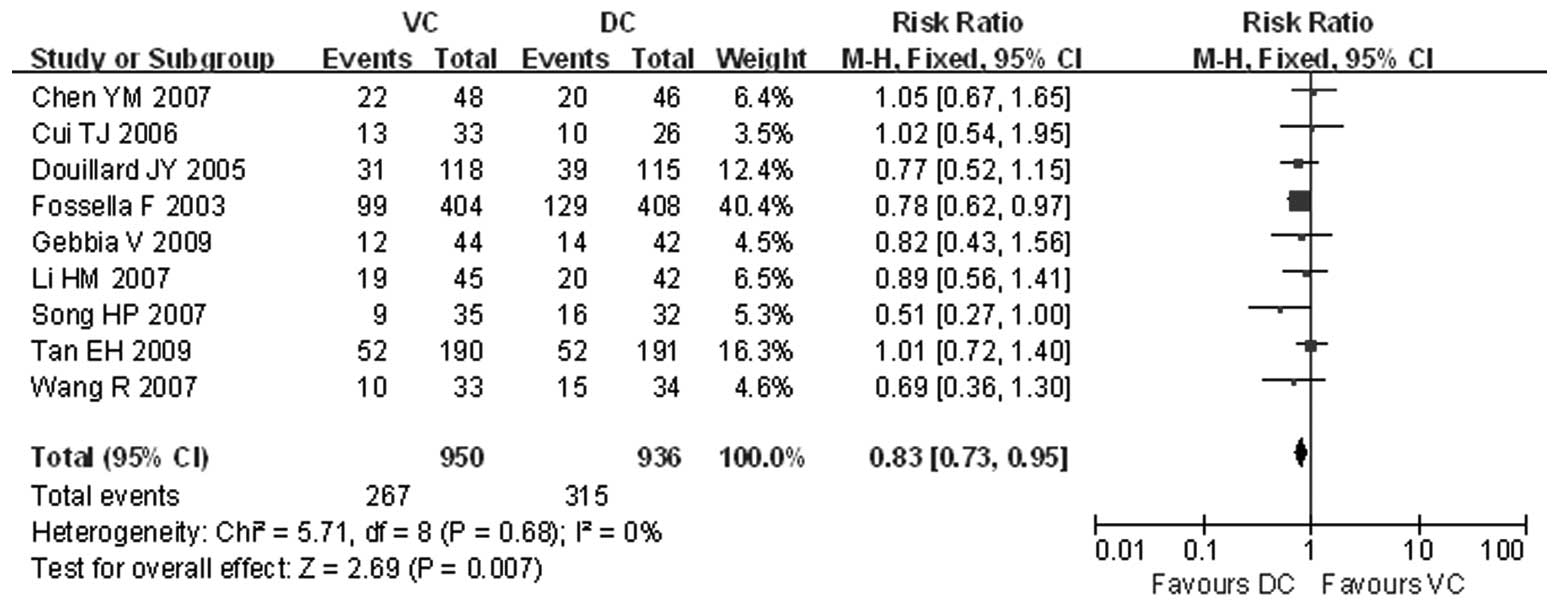
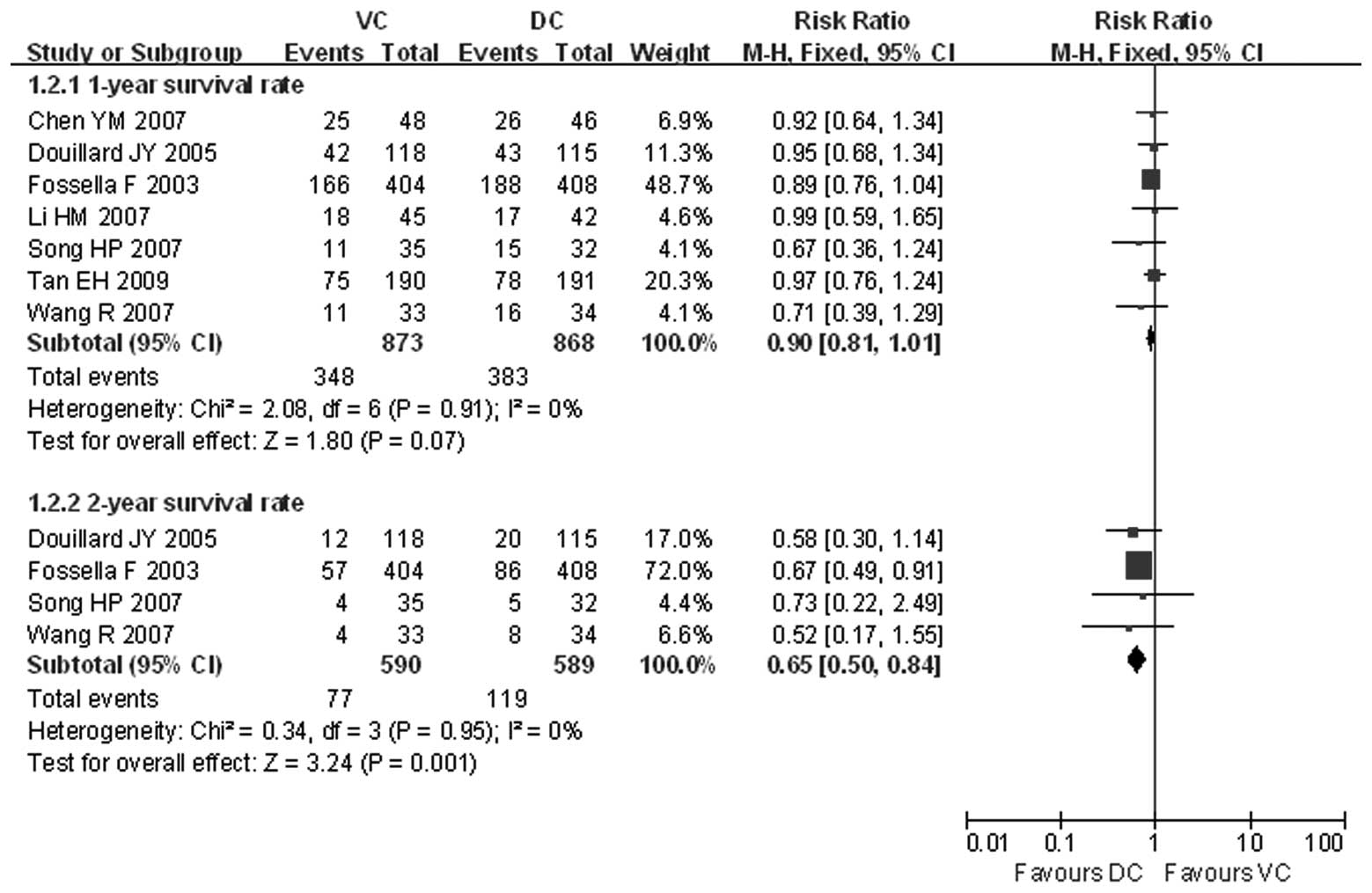
Fossella F, Pereira JR, von Pawel J, et
al: Randomised, multinational phase III study of docetaxel plus
platinum combinations versus vinorelbine plus cisplatin for
advanced non-small-cell lung cancer: the TAX 326 study group. J
Clin Oncol. 21:3016–3024. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Goffin J, Lacchetti C, Ellis PM, et al:
First-line systemic chemotherapy in the treatment of advanced
non-small cell lung cancer: a systematic review. J Thorac Oncol.
5:260–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Jadad AR, Moore RA, Carroll D, et al:
Assessing the quality of reports of randomized clinical trials: is
blinding necessary? Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar
|
|
14.
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
15.
|
Douillard JY, Gervais R, Dabouis G, et al:
Sequential two-line strategy for stage IV non-small-cell lung
cancer: docetaxel-cisplatin versus vinorelbine-cisplatin followed
by cross-over to single-agent docetaxel or vinorelbine at
progression: final results of a randomised phase II study. Ann
Oncol. 16:81–89. 2005. View Article : Google Scholar
|
|
16.
|
Cui TJ, Liu ZH, Chen Z, et al: Comparisons
among four chemo-therapy regimens for advanced non-small cell lung
cancer. J Oncol (Chinese). 12:136–138. 2006.
|
|
17.
|
Chen YM, Perng RP, Shih JF, et al: A
randomized phase II study of docetaxel or vinorelbine in
combination with cisplatin against inoperable, chemo-naïve
non-small-cell lung cancer in Taiwan. Lung Cancer. 56:363–369.
2007.
|
|
18.
|
Li HM, Li HX, Liu KW, et al: Three
chemotherapy regimens including cisplatin for advanced non-small
cell lung cancer. J Shandon Univ China (Health Sci). 45:499–502.
2007.
|
|
19.
|
Wang R, Wang YL and Wang QC: A comparison
of docetaxel plus cisplatin and vinorelbine plus cisplatin in 67
cases with advanced non-small-cell lung cancer. Bulletin Chin
Cancer. 16:476–477. 2007.
|
|
20.
|
Song HP, Qiu WS, Xu JH, et al: First-line
chemotherapy of docetaxel/cisplatin for advanced non-small cell
lung cancer. Chin J Clin Oncol. 34:388–390. 2007.
|
|
21.
|
Tan EH, Rolski J, Grodzki T, et al: Global
Lung Oncology Branch trial 3 (GLOB3): final results of a randomised
multinational phase III study alternating oral and i.v. vinorelbine
plus cisplatin versus docetaxel plus cisplatin as first-line
treatment of advanced non-small-cell lung cancer. Ann Oncol.
20:1249–1256. 2009. View Article : Google Scholar
|
|
22.
|
Gebbia V, Lorusso V, Galetta D, et al:
First-line cisplatin with docetaxel or vinorelbine in patients with
advanced non-small-cell lung cancer: a quality of life directed
phase II randomized trial of Gruppo Oncologico Italia Meridionale.
Lung Cancer. 69:218–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Auperin A, LePechoux C, Pignon JP, et al:
Concomitant radio-chemotherapy based on platin compounds in
patients with locally advanced non-small cell lung cancer (NSCLC):
a meta-analysis of individual data from 1,764 patients. Ann Oncol.
17:473–483. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
D’Addario G, Pintilie M, Leighl NB, et al:
Platinum-based versus non-platinum-based chemotherapy in advanced
non-small-cell lung cancer: a meta-analysis of the published
literature. J Clin Oncol. 23:2926–2936. 2005.PubMed/NCBI
|
|
25.
|
Lynch TJ, Patel T, Dreisbach L, et al:
Cetuximab and first-line taxane/carboplatin chemotherapy in
advanced non-small-cell lung cancer: results of the randomized
multicenter phase III trial BMS099. J Clin Oncol. 28:911–917. 2010.
View Article : Google Scholar : PubMed/NCBI
|