Introduction
Bladder cancer is a global health problem. Its
incidence ranks ninth worldwide (1). In Egypt, the situation is critical.
Bladder cancer has been and remains one of the most prevalent
malignancies, accounting for 12.22% of the incident cancers and
representing the main bulk of the urinary system malignancy
(2). An aggressive form of this
type of cancer, schistosomiasis-associated bladder cancer, has been
encountered among bladder cancer patients. During the past decade,
certain changes have been observed in the features of bladder
cancer associated with schistosomiasis (bilharziasis) in Egypt,
with a decrease in the frequency of squamous cell carcinoma (SCC)
and an increase in the incidence of transitional cell carcinoma
(TCC) cases (3).
Histopathological evaluation of tissues is the basis
for the grading and pathological staging of urinary bladder cancer,
delineating treatment strategy and predicting subsequent clinical
outcome. However, reliable prognostic information regarding the
biological behavior of these tumors is limited (4).
In addition, bladder cancer has markedly different
behavioral characteristics, however, patients with the same disease
stage may have divergent clinical course and different outcomes
following the same treatment regimen (5).
Therefore, new molecular markers that provide
additional objective information on the biological behavior of
these tumors may allow a more precise assessment and achieve a
better-targeted effective therapy.
Extracellular matrix metalloproteinase inducer
(EMMPRIN), also known as CD147 or basigin, is a multi-functional
cell surface glycoprotein that belongs to the immunoglobulin
superfamily. It is highly expressed on the surface of malignant
cells and acts as an important mediator of tumor cell invasion via
stimulation of matrix metalloproteinases (MMPs) production, tumor
cell-induced angiogenesis via the stimulation of vascular
endothelial growth factor production and multidrug resistance via
hyaluronan-induced signaling (6).
Recent studies have reported its association with poor prognostic
features and aggressive phenotype in several types of tumors,
including prostate cancer (7),
tongue (8), uterine cervix
(9) and esophageal (10) SCCs.
Fascin is a 55-kDa actin-bundling protein that plays
a pivotal role in cell motility, migration and adhesion. There are
three highly related forms of fascin proteins: Fascin 1 (also known
as fascin), expressed by mesenchymal and nervous tissues. The other
two forms, fascin-2 and -3, are expressed in the retinal
photoreceptor cells and testes, respectively (11). Fascin is overexpressed in a wide
variety of tumors including esophageal (12), prostatic (13) and ovarian (14) carcinomas and usually correlates
with aggressive features and poor prognosis. However, it is usually
absent or extremely underexpressed in normal epithelia (11).
A number of studies are available on EMMPRIN
(15–18) and fascin (19–21)
expression in the carcinoma of the urinary bladder. These studies
have reported high EMMPRIN and fascin expression scores in invasive
bladder urothelial carcinoma and suggested that EMMPRIN and fascin
overexpression may be an indicator of aggressive TCC. None of these
studies, however, has assessed the expression of the two proteins
in SCC of the urinary bladder or schistosomiasis-associated bladder
carcinomas.
EMMPRIN has been reported as an inducer of MMPs and
causes tumor invasion and enhances the tumor metastatic potential
(6). Xie et al (22) reported that the effect of fascin on
cell invasion also depended on the activation of MMP-2 and -9.
Although the detailed pathway has yet to be fully established, we
hypothesized the presence of a possible correlation between EMMPRIN
and fascin expression that may have a synergistic effect on bladder
cancer.
In the present study, an immunohistochemical
evaluation of EMMPRIN and fascin proteins in TCC and SCC of the
urinary bladder was conducted in a cohort of Egyptian patients.
Expression of EMMPRIN and fascin was also correlated with the
schistosomal status, as well as with other available
clinicopathological characteristics. Furthermore, we investigated
the presence of possible correlations between EMMPRIN and fascin
expression in this series of urinary bladder carcinomas.
Materials and methods
Patients and tissue specimens
This prospective study included 125 patients (106
males and 19 females) referred to the El-Minia University Hospital
(Minia, Egypt) for the management of bladder tumor between January,
2009 and July, 2011. The patients were subjected to full clinical
evaluations, laboratory investigations and imaging studies. Patient
age ranged from 45 to 70 years (mean, 56.97±6.38 years; median, 56
years). Tumor specimens were obtained by radical cystectomy or
transurethral resection biopsy and were sent to the pathology
department for histopathological examination. Only biopsies
containing muscle tissue were included, in order that muscle
invasion by the tumor could be evaluated.
Histopathology
Hematoxylin and eosin (H&E)-stained sections
were prepared and examined according to standard histopathological
examination (23) to confirm tumor
type, grade and pathological stage. Pathological examination showed
TCC in 86 and SCC in 39 cases. Bilharzial infestation was evaluated
by detection of bilharzial ova in tumor tissue or adjacent
non-neoplastic tissue or from the data already provided in the
patients’ records.
Immunohistochemistry (IHC)
Streptavidin-biotin immunoperoxidase complex
procedure was applied for immuno staining. In brief, 4 μm
sections were deparaffinized with xylene, and rehydrated through
graded alcohol. Endogenous peroxidase activity was blocked by
incubation with 0.3% hydrogen peroxide in methanol for 30 min.
Antigen retrieval was achieved by microwave treatment in sodium
citrate buffer (0.01 M, pH 6.0) for 10 min. Tissue sections were
then incubated with monoclonal antibodies for EMMPRIN (mouse
monoclonal sc-21746 antibody; dilution 1:100; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and fascin (mouse
monoclonal sc-21743 antibody; dilution 1:100; Santa Cruz
Biotechnology, Inc.) for 1 h, followed by biotinylated secondary
antibody for 30 min at room temperature. Visualization of the
reaction was performed with an avidin-biotin complex
immunoperoxidase system using 3,3′ diaminobenzidine (DAB) as a
chromogen. Sections were then counterstained with hematoxylin,
dehydrated, cleared and mounted with distyrene, plasticizer and
xylene (DPX).
Positive and negative control
Each staining batch included positive and negative
control sections. Negative control sections were treated with
phosphate-buffered saline (PBS) instead of primary antibody.
Sections of positive controls used were invasive ductal breast
carcinoma for EMMPRIN and high-grade breast carcinomas for
fascin.
Scoring system
The levels of EMMPRIN and fascin protein expression
were evaluated using a semi-quantitative scoring system, which was
performed as described in previous studies (19,24).
Each slide was evaluated for the intensity of the staining and the
percentage of tumor cells stained positive. Immunoreactivity was
assessed by two pathologists. The correlation of their findings was
high and in case of discrepancies, a consensus was reached by joint
evaluation. The extent of the staining was scored as: <25% of
tumor cells stained, 1; 25–50% of the tumor cells stained positive,
2; 51–75% of the tumor cells stained positive, 3 and >75% of the
tumor cells stained positive, 4. Staining intensity was graded on a
scale of 0–3: no staining, 0; weak, 1; moderate, 2; or intense
staining, 3. The combined score was calculated by multiplying the
intensity and percentage scores yielding an overall score range of
0–12. A final score of ≤2 was considered negative.
Statistical analysis
Statistical analysis was conducted using the SPSS
software version 11.0. Raw data were compiled and used to determine
the means ± standard deviations (SDs), median and ranges of various
variables. Non-parametric statistics were performed to evaluate the
association between EMMPRIN and fascin and various
clinicopathological characteristics. The Kruskal-Wallis test was
carried out to assess the differences of expression in the
clinicopathological variables with >2 groups, and the
Mann-Whitney test was used to evaluate difference of expression in
the dichotomous variables. The Chi-square and Fisher’s exact tests
were used to compare categorical variables. P≤0.05 was considered
to indicate a statistically significant difference.
Results
Clinicopathological data
Table I shows a
summary of the clinicopathological characteristics for the cases
included in this study. A total of 125 patients with primary
bladder cancer were included, 68.8% of whom were TCC, while 31.2%
of were SCC. A significantly lower patient age in SCC cases
compared to TCC cases was observed (P<0.001). The mean patient
age was 52.51±4.26 and 59.00±6.17 years in SCC and TCC,
respectively, with male predominance in the two types. Evidence of
schistosomiasis was noted in 71/125 (56.8%) of patients. A
significantly (P=0.008) lower frequency of associated
schistosomiasis was noted in TCC compared to SCC cases. Regarding
invasiveness, all the SCC and 79.1% of TCC cases were muscle
invasive (T2, T3 and T4) at the time of diagnosis.
 | Table IPatient clinicopathological
characteristics. |
Table I
Patient clinicopathological
characteristics.
| Clinicopathological
characteristics | TCC n=86/125
(68.8%) | SCC n=39/125
(31.2%) |
|---|
| Age (years, %) | | |
| ≤50 | 9 (10.5%) | 15 (38.5%) |
| >50 | 77 (89.5%) | 24 (61.5%) |
| Gender | | |
| Male | 72 (83.7%) | 34 (87.2%) |
| Female | 14 (16.3%) | 5 (12.8%) |
| Grade | | |
| Low | 50 (58.1%) | I 14 (35.9%) |
| High | 36 (41.9%) | II 17
(43.6%)
III 8 (20.5 %) |
| Schistosomal
status | | |
| Negative | 44 (51.2%) | 10 (25.6%) |
| Positive | 42 (48.8%) | 29 (74.4%) |
| Pathological
stage | | |
| T1 | 18 (20.9%) | 0 |
| T2 | 32 (37.2%) | 6 (15.4%) |
| T3 | 25 (29.1%) | 21 (53.8%) |
| T4 | 11 (12.8%) | 12 (30.8%) |
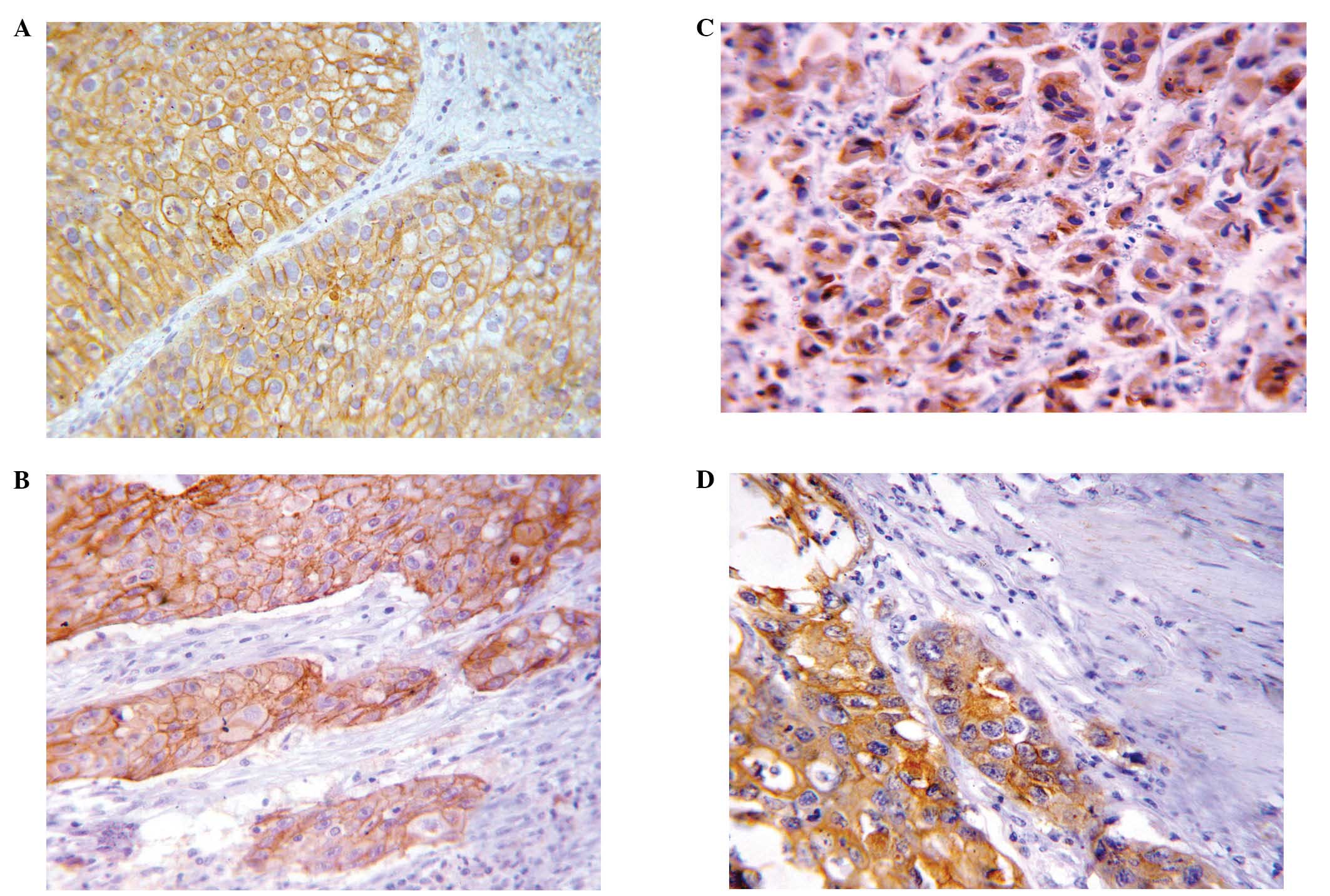
Expression of EMMPRIN and fascin in
normal and neoplastic bladder tissues
EMMPRIN and fascin immuno-reactivity was
undetectable in normal urothelium. However, EMMPRIN and fascin
expression was detectable on the cell membrane (Fig. 1A and B) and cytoplasm (Fig. 1C and D) in 71.2 and 83.2% of
bladder carcinomas, respectively. A significantly higher EMMPRIN
mean of expression was detected in SCC [mean ± SD 6.97±4.21, median
8 (0–12)] compared to TCC [mean ± SD 5.24±4.16, median 6 (0–12)]
cases (P=0.031). Similarly, a higher fascin mean of expression was
noted in SCC [mean ± SD 8.35±3.03, median 8 (2–12)]
compared to TCC [mean± SD 6.77±3.95, median 8 (0–12)] cases,
however, such a difference was not statistically significant
(P=0.053).
Expression of EMMPRIN and fascin in
TCC
Table II summarizes
the immunohistochemical results of ECMMPRIN and fascin expression
in correlation with various clinicopathological parameters in TCC
cases.
 | Table IICorrelation of EMMPRIN and fascin
expression and clinicopathological characteristics of the 86
examined TCC cases. |
Table II
Correlation of EMMPRIN and fascin
expression and clinicopathological characteristics of the 86
examined TCC cases.
| Clinicopathological
characteristics | EMMPRIN Mean ± SD
Median (min-max) | P-value | Fascin Mean ± SD
Median (min-max) | P-value |
|---|
| Age (years) | | | | |
| ≤50 | 5.22±4.46 | 0.690 | 7.66±3.70 | 0.453 |
| 6 (0–12) | | 9 (0–12) | |
| >50 | 5.24±4.15 | | 6.67±3.98 | |
| 6 (0–12) | | 8 (0–12) | |
| Gender | | | | |
| Male | 5.75±4.13 | 0.01 | 7.05±3.90 | 0.127 |
| 6 (0–12) | | 8 (0–12) | |
| Female | 2.64±3.31 | | 5.35±4.03 | |
| 0 (0–9) | | 5 (0–12) | |
| Schistosomiasis
association | | | | |
| Negative | 5.13±4.00 | 0.715 | 6.50±3.86 | 0.549 |
| 6 (0–12) | | 8 (0–12) | |
| Positive | 5.35±4.36 | | 7.07±4.06 | |
| 6 (0–12) | | 8 (0–12) | |
| Grade | | | | |
| Low | 4.04±3.90 | 0.003 | 6.32±3.91 | 0.190 |
| 4 (0–12) | | 6 (0–12) | |
| High | 6.91±3.96 | | 7.41±3.95 | |
| 8 (0–12) | | 8 (0–12) | |
| Pathological
stage | | | | |
| T1 | 1.38±2.45 | <0.001 | 3.22±3.43 | <0.001 |
| 0 (0–9) | | 2 (0–12) | |
| T2 | 4.78±3.76 | | 6.15±3.52 | |
| 5 (0–12) | | 6 (0–12) | |
| T3 | 7.08±3.51 | | 8.64±2.79 | |
| 8 (0–12) | | 9 (0–12) | |
| T4 | 8.72±3.77 | | 10.18±3.12 | |
| 9 (0–12) | | 12 (2–12) | |
Regarding EMMPRIN expression, significant positive
associations between EMMPRIN overexpression and gender, tumor grade
and pathological stage were identified (P=0.01, 0.003 and
<0.001, respectively). EMMPRIN expression was found to be
significantly associated with an increasing invasiveness of the
tumor. Non-muscle invasive urothelial carcinomas (pT1) were
strongly correlated with negative or low EMMPRIN expression scores
compared to muscle invasive tumors (pT2-4) (P<0.001). Similarly,
low-grade tumors were associated with negative or low EMMPRIN
expression scores, whereas high-grade tumors showed higher
expression scores. No significant associations were observed
regarding patient age and schistosomal status. Concerning fascin
expression, statistical analysis demonstrated a significant
positive association between high fascin immunostaining scores and
tumor pathological stage (P<0.001), i.e., the more the increase
of the tumor stage, the higher the fascin expression score. A
highly significant difference was identified between non-muscle
invasive (pT1) and muscle invasive (pT2-4) TCC cases (P<0.001).
No significant associations were detected between fascin expression
and other clinicopathological characteristics.
EMMPRIN and fascin expression in SCC
cases
Table III shows the
immunohistochemical results of EMMPRIN and fascin expression in
correlation with various clinicopathological characteristics in SCC
cases.
 | Table IIICorrelation of EMMPRIN and fascin
expression and clinicopathological characteristics of the 39
examined SCC cases. |
Table III
Correlation of EMMPRIN and fascin
expression and clinicopathological characteristics of the 39
examined SCC cases.
| Clinicopathological
characteristics | EMMPRIN Mean ± SD
Median (min-max) | P-value | Fascin Mean ± SD
Median (min-max) | P-value |
|---|
| Age (years) | | | | |
| ≤50 | 5.66±4.02 | 0.151 | 7.40±3.83 | 0.300 |
| 6 (0–12) | | 8 (2–12) | |
| >50 | 7.79±4.20 | | 8.95±2.31 | |
| 8 (0–12) | | 8.5 (4–12) | |
| Gender | | | | |
| Male | 7.08±4.21 | 0.670 | 8.06±3.12 | 0.477 |
| 8 (0–12) | | 8 (2–12) | |
| Female | 6.20±4.60 | | 9.71±2.36 | |
| 6 (0–12) | | 9 (6–12) | |
| Schistosomiasis
association | | | | |
| Negative | 5.50±4.00 | 0.186 | 7.30±3.33 | 0.182 |
| 5 (0–12) | | 8 (2–12) | |
| Positive | 7.48±4.23 | | 8.72±2.90 | |
| 8 (0–12) | | 9 (2–12) | |
| Grade | | | | |
| I | 4.64±4.25 | 0.002 | 7.28±3.04 | 0.339 |
| 5 (0–12) | | 8 (2–12) | |
| II | 7.05±3.59 | | 9.00±2.80 | |
| 8 (0–12) | | 9 (2–12) | |
| III | 10.87±2.23 | | 8.87±3.35 | |
| 12 (6–12) | | 8.5 (2–12) | |
| Pathological
stage | | | | |
| T1 | - | 0.004 | - | 0.001 |
| - | | - | |
| T2 | 3.50±4.18 | | 4.33±1.96 | |
| 2 (0–9) | | 5 (2–6) | |
| T3 | 6.28±3.63 | | 8.42±2.76 | |
| 6 (0–12) | | 8 (2–12) | |
| T4 | 9.91±3.52 | | 10.25±1.86 | |
| 12 (2–12) | | 10.5 (8–12) | |
Regarding EMMPRIN expression, significant positive
associations between EMMPRIN overexpression and tumor grade and
pathological stage were identified (P=0.002 and 0.004,
respectively). No significant associations were observed regarding
patient age, gender or schistosomal status. Concerning fascin
expression, statistical analysis demonstrated a significant
positive association between high fascin immuno staining scores and
tumor pathological stage (P=0.001), whereas no significant
associations were detected with other clinicopathological
characteristics.
Correlation between EMMPRIN and fascin in
urinary bladder carcinomas
The correlation between EMMPRIN and fascin
immunostaining scores is shown in Fig.
2. Overall, a significant positive correlation was observed
between fascin and EMMPRIN expression in urinary bladder carcinomas
(Spearman’s rho correlation coefficient (r) = 0.500, P<0.001). A
positive correlation was also detected in TCC and SCC cases
(r=0.535, P<0.001 and r=0.372, P=0.020, respectively)
Combined EMMPRIN and fascin
immunophenotype and their correlation with pathological stage
The frequency of varying combined EMMPRIN and fascin
immunoprofiles and their correlation with pathological stage is
shown in Table IV. A highly
significant association was found between various tumor
immunoprofiles and pathological stage (P=<0.001). The
EMMPRIN-/fascin-positive immunophenotype was closely associated
with T3 and T4 tumors, while a great proportion of T1 tumors was
negative for the two markers.
 | Table IVEMMPRIN and fascin immunophenotypes
in correlation with stage in urinary bladder carcinomas
(P=<0.001). |
Table IV
EMMPRIN and fascin immunophenotypes
in correlation with stage in urinary bladder carcinomas
(P=<0.001).
| Pathological stage
|
|---|
|
Immunophenotype | Frequency (%) | T1 n=18 (%) | T2 n=38 (%) | T3 n=46 (%) | T4 n=23 (%) |
|---|
| Both negative | 14 (11.2) | 10 (55.6) | 3 (7.9) | - | 1 (4.3) |
|
EMMPRIN-positive/fascin-negative | 7 (5.6) | 1 (5.6) | 4 (10.5) | 2 (4.3) | - |
|
EMMPRIN-negative/fascin-positive | 22 (17.6) | 4 (22.2) | 10 (26.3) | 7 (15.2) | 1 (4.3) |
| Both positive | 82 (65.6) | 3 (16.7) | 21 (55.3) | 37 (80.4) | 21 (91.3) |
Discussion
During the past decade, certain changes have been
reported in the features of bladder cancer in Egypt. Higher
incidence rates of TCC compared to SCC cases, notable increases in
the patient mean age and a marked decrease in the incidence of
associated schistosomiasis were reported (2,3). In
agreement with those studies, the present study showed a markedly
higher frequency rate of TCC (68.8%) compared to SCC (31.2%) cases
and a relatively higher patient mean age. Evidence of
schistosomiasis was found only in 56.8% of the patients. The
present study also showed a significantly lower frequency of
associated schistosomiasis in TCC compared to SCC cases, as well as
a significantly lower patient age in SCC compared to TCC cases,
with a male to female predominance in the two types of carcinoma,
consistent with the findings of a previous report (2). Notably, the SCC specimens included in
this study were classified as muscle-invasive tumors, indicating
the more aggressive nature of SCC.
Various molecular pathways that have been found to
be associated with bladder cancer are currently available. Recent
studies have already identified the pivotal role of EMMPRIN
(15–18) and fascin (19–21)
proteins in urothelial carcinoma.
Regarding EMMPRIN expression, the present study
showed a positive EMMPRIN expression in 71.2% of bladder
carcinomas, while it was undetectable in normal urothelium. This is
in agreement with previous findings (15–18).
The association between EMMPRIN overexpression and
tumor progression has been documented. EMMPRIN overexpression
induces tumor cell invasion and metastasis via MMPs production
(6), while its silencing
suppressed the proliferation ability of prostate carcinoma cell
line LNCAP and bladder carcinoma cell line J82, suggesting its
involvement in tumor cell proliferatin and growth (16). The present study has demonstrated
significant positive associations between high EMMPRIN expression
scores and advanced pT stage and a high grade in TCC and SCC cases.
In TCC, EMMPRIN was found to be differentially expressed in
muscle-invasive tumors (pT2-4) compared to non-muscle invasive
(pT1) tumors with a progressive increase in its expression from pT1
to pT4. Moreover, high-grade carcinomas, compared to low-grade
tumors, showed significantly higher expression scores. In SCC
cases, the tumors were muscle invasive, however, EMMPRIN expression
was closely associated with tumor stage, with an obvious increase
from pT2 to pT4. Similarly, a strong association between its
expression and SCC grade was identified, where grade III carcinomas
were associated with a significantly increased expression compared
to well-differentiated tumors. These findings are in concordance
with those of previous studies in urothelial carcinomas (15,16,18)
and SCC in other tissues (8,10).
By contrast, in their study Afonso et al
(17) found no significant
association of EMMPRIN with regard to clinicopathological
parameters or patient outcome, which may be attributable to
different case population, different scoring system and cut-off
points used by various studies. However, Afonso et al
(17) found that EMMPRIN
expression added a predictive power of outcome to pathological
stage: patients with pT3/pT4 tumors had a median overall survival
time of 14.7 months, which was significantly reduced to 9.2 months
if the tumors were EMMPRIN-positive.
Regarding fascin expression, 83.2% of the bladder
carcinomas exhibited positive immunoreactivity, while no notable
expression was observed in normal urothelium adjacent to carcinoma.
Similarly, fascin expression was either low or absent in normal
epithelia, while it was upregulated at mRNA and protein levels in
several types of cancers including urothelial carcinoma of the
bladder (19,20).
Invasive and metastatic potential of tumor cells is
often associated with actin cytoskeleton rearrangements involving
several types of actin cross-linking proteins, of which fascin is
the key component. Fascin overexpression is also suggested to
disrupt epithelial junctions and thus to increase the invasive and
metastatic potential of malignant cells (25). In the present study, overexpression
of the fascin protein was significantly correlated with advanced
tumor stage in the TCC and SCC cases. A highly significant
difference was found in fascin expression between non-muscle
invasive tumors and muscle-invasive TCC cases. Additionally,
muscle-invasive tumors (pT2-4) showed a stepwise increase in fascin
expression scores in the two tumor types. Previous studies also
reported a significant association between high fascin expression
and advanced tumor pathological stage with a progressive increase
of its expression from superficial to deeply invasive urothelial
carcinomas of the urinary bladder (19,26).
No significant associations were observed with
regard to tumor grade or other clinicopathological parameters. This
lack of significant association between fascin expression and tumor
grade noted in the present study confirmed the findings of previous
studies (19,20,26).
The present study shows no significant association
between the presence of schistosomiasis and overexpression of
EMMPRIN and fascin. No significant differences regarding EMMPRIN
and fascin expression between schistosomiasis- associated and
non-schistosomiasis-associated tumors was observed, suggesting that
schistosomiasis may not be important in the upregulation of the two
proteins in bladder cancer.
Various subtypes of bladder carcinomas represent
different molecular alterations and biological behaviors (27). Notably, significantly higher
EMMPRIN expression scores were found in SCC compared to TCC cases.
Additionally, there was a tendency for higher fascin expression
scores in SCC compared to TCC cases, a finding that may be
attributable to the more aggressive and muscle-invasive nature of
SCC of the bladder (27,28). The differential expression of these
markers in SCC and TCC also suggest an association between tumor
behavior and their immunoreactivity.
In the literature, a positive correlation between
EMMPRIN and fascin in renal (29)
and colorectal (30) carcinomas
was reported. However, the correlation between EMMPRIN and fascin
proteins in bladder carcinoma has not been previously investigated.
The present study showed a significant positive correlation between
EMMPRIN and fascin expression in urinary bladder carcinomas. This
finding, together with the findings of previous studies suggest a
correlation between the two proteins that may induce a synergistic
effect during tumor progression and invasion.
The main roles of EMMPRIN and fascin in tumor
progression depend on MMPs and fascin-actin interactions, which
cause tumor cell protrusion, invasion and migration (6,11,22).
Therefore, EMMPRIN and fascin were expected to be significantly
correlated with T stages. In this context, we have characterized
the joint expression of EMMPRIN with fascin to identify various
immunoprofiles of EMMPRIN with fascin expression and their
implication on pathological stage. Notably, the majority of T3 and
T4 tumors showed combined positive expression for the two markers.
By contrast, a substantial proportion of T1 tumors were negative
for the two markers. Our finding suggests that the co-localization
of EMMPRIN with fascin in bladder cancer emphasizes the possible
cooperative involvement of these markers in cancer invasion.
In conclusion, the present study demonstrated that
EMMPRIN and fascin are highly expressed in bladder carcinomas,
whereas neither EMMPRIN nor fascin was detectable in the normal
adjacent epithelia. High expression scores were correlated with
bladder cancer progression and aggressive phenotype. The present
study also found no statistically significant differences regarding
EMMPRIN and fascin expression in schistosomiasis-associated and
non-associated bladder carcinomas. A statistically significant
positive correlation between EMMPRIN and fascin expression was
observed in this series. Combined positive expression of the two
markers was strongly associated with advanced stage, suggesting a
correlation between the two proteins that may induce a synergistic
effect during tumor progression and invasion. The correlation
between EMMPRIN with fascin should be clarified on a wider scale of
tumors. Overexpression of EMMPRIN with fascin are potentially
significant biomarkers of urinary bladder cancer aggressiveness
that may help surgeons to identify patients who are likely to
benefit from a personalized therapeutic regimen and more aggressive
treatment strategies.
References
|
1
|
Ploeg M, Aben KK and Kiemeney LA: The
present and future burden of urinary bladder cancer in the world.
World J Urol. 27:289–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Salem HK and Mahfouz S: Changing patterns
(age, incidence, and pathologic types) of schistosoma-associated
bladder cancer in Egypt in the past decade. Urology. 79:379–383.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mokhtar N, Gouda I and Adel I: Cancer
pathology registry 2003–2004 and time trend analysis NCI. El Sheraa
Press; Cairo: 2007
|
|
4
|
Cheng L, Montironi R, Davidson DD and
Lopez-Beltran A: Staging and reporting of urothelial carcinoma of
the urinary bladder. Mod Pathol. 22(Suppl 2): 70–95. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Als AB, Dyrskjøt L, von der Maase H, et
al: Emmprin and survivin predict response and survival following
cisplatin-containing chemotherapy in patients with advanced bladder
cancer. Clin Cancer Res. 13:4407–4414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan L, Zucker S and Toole BP: Roles of the
multifunctional glycoprotein, emmprin (basigin; CD147), in tumour
progression. Thromb Haemost. 93:199–204. 2005.PubMed/NCBI
|
|
7
|
Han ZD, Bi XC, Qin WJ, et al: CD147
expression indicates unfavourable prognosis in prostate cancer.
Pathol Oncol Res. 15:369–374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang Z, Huang H, Li H, et al: EMMPRIN
expression in tongue squamous cell carcinoma. J Oral Pathol Med.
38:518–523. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu W, Liu J, Xiong X, et al: Expression of
MMP9 and CD147 in invasive squamous cell carcinoma of the uterine
cervix and their implication. Pathol Res Pract. 205:709–715. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu S, Li Y, Mi L, et al: Clinical impact
of HAb18G/CD147 expression in esophageal squamous cell carcinoma.
Dig Dis Sci. 56:3569–3576. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hashimoto Y, Skacel M and Adams JC: Roles
of fascin in human carcinoma motility and signaling: prospects for
a novel biomarker? Int J Biochem Cell Biol. 37:1787–1804. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H, Xu L, Xiao D, et al: Fascin is a
potential biomarker for early-stage oesophageal squamous cell
carcinoma. J Clin Pathol. 59:958–964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Darnel AD, Behmoaram E, Vollmer RT, et al:
Fascin regulates prostate cancer cell invasion and is associated
with metastasis and biochemical failure in prostate cancer. Clin
Cancer Res. 15:1376–1383. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin CK, Chao TK, Yu CP, et al: The
expression of six biomarkers in the four most common ovarian
cancers: correlation with clinicopathological parameters. APMIS.
117:162–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhong WD, Chen QB, Ye YK, et al:
Extracellular matrix metalloproteinase inducer expression has an
impact on survival in human bladder cancer. Cancer Epidemiol.
34:478–482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han ZD, He HC, Bi XC, et al: Expression
and clinical significance of CD147 in genitourinary carcinomas. J
Surg Res. 160:260–267. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Afonso J, Longatto-Filho A, Baltazar F, et
al: CD147 overexpression allows an accurate discrimination of
bladder cancer patients’ prognosis. Eur J Surg Oncol. 37:811–817.
2011.PubMed/NCBI
|
|
18
|
Wittschieber D, Stenzinger A, Klauschen F,
et al: Decreased RECK and increased EMMPRIN expression in
urothelial carcinoma of the bladder are associated with tumor
aggressiveness. Pathobiology. 78:123–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karasavvidou F, Barbanis S, Pappa D, et
al: Fascin determination in urothelial carcinomas of the urinary
bladder: a marker of invasiveness. Arch Pathol Lab Med.
132:1912–1915. 2008.PubMed/NCBI
|
|
20
|
Bi J, Chen X, Zhang Y, et al: Fascin is a
predictor for invasiveness and recurrence of urothelial carcinoma
of bladder. Urol Oncol. 30:688–694. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McKnight R, Cohen C and Siddiqui MT:
Fascin stain as a potential marker of invasiveness in carcinomas of
the urinary bladder: a retrospective study with biopsy and cytology
correlation. Diagn Cytopathol. 39:635–640. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie JJ, Xu LY, Zhang HH, et al: Role of
fascin in the proliferation and invasiveness of esophageal
carcinoma cells. Biochem Biophys Res Commun. 337:355–362. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eble JN, Sauter G, Epstein JI and
Sesterhenn IA: World Health Organization classification of tumors.
Pathology and genetics of tumors of the urinary system and male
genital organs. IARC Press; Lyon: pp. 90–109. 2004
|
|
24
|
Takikita M, Hu N, Shou JZ, et al: Fascin
and CK4 as biomarkers for esophageal squamous cell carcinoma.
Anticancer Res. 31:945–952. 2011.PubMed/NCBI
|
|
25
|
Jayo A and Parsons M: Fascin: a key
regulator of cytoskeletal dynamics. Int J Biochem Cell Biology.
42:1614–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tong GX, Yee H, Chiriboga L, et al:
Fascin-1 expression in papillary and invasive urothelial carcinomas
of the urinary bladder. Hum Pathol. 36:741–746. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blaveri E, Simko JP, Korkola JE, et al:
Bladder cancer outcome and subtype classification by gene
expression. Clin Cancer Res. 11:4044–4055. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shokeir AA: Squamous cell carcinoma of the
bladder: pathology, diagnosis and treatment. BJU int. 93:216–220.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsai WC, Sheu LF, Nieh S, et al:
Association of EMMPRIN and fascin expression in renal cell
carcinoma: correlation with clinicopathological parameters. World J
Urol. 25:73–80. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jung EJ, Lee JH, Min BW, et al:
Clinicopathologic significance of fascin, extracellular matrix
metalloproteinase inducer, and ezrin expressions in colorectal
adenocarcinoma. Indian J Pathol Microbiol. 54:32–36. 2011.
View Article : Google Scholar
|