Introduction
Human glioblastoma is one of the most malignant
brain tumors. Currently, the traditional methods used to treat
glioblastoma include surgery, chemotherapy, radiotherapy and a
combination of these methods (1).
However, the mean survival time of glioblastoma patients is one
year and most patients do not survive more than two years in the
clinical course (1). Therefore,
more useful treatments for glioblastoma patients are required, thus
a number of gene therapies for tumor treatment have been suggested
(2–4).
Human interleukin 15 (hIL15) is a 14–15 kDa
immunostimulatory cytokine that is able to induce cell
proliferation and T-cell differentiation (5–7). A
previous study demonstrated that immunotherapy with
lymphokine-activated killer (LAK) cell cytotoxic activity has been
widely used for breast, lung, ovary and pancreatic tumor treatment
in phase II clinical trials (8).
Due to the short half-life of IL15, repeated daily injection is
required when employing IL15 administration, which is not
convenient for human cancer treatments (9–11).
Therefore, authors of previous studies suggested that replacing
daily administration with gene therapy may be useful for tumor
immunotherapy (12–14).
Type 2 recombinant AAV (rAAV2) infects dividing and
non-dividing cells and maintains long-term gene expression in a
number of tissues, including the liver, brain, retina and muscle
(15,16). Previous studies demonstrated that
rAAVs are non-pathogenic in humans and exhibit low immunogenicity
compared with other viral delivery systems (17,18).
rAAV2 viral vector expressing the glutamic acid decarboxylase (GAD)
gene was successfully used to treat Parkinson’s disease (19,20).
Those results demonstrated that rAAV2 viral vector is safer and
more efficacious for human gene therapy. Therefore, rAAV2 viral
vector is used to carry the human IL15 (hIL15) gene for
glioblastoma therapy in this study.
Gene immunotherapy using rAAV2-bearing human IL15
gene has recently been applied in the treatment of tumors (21–23).
Those studies demonstrated that rAAV2-hIL15 is able to express IL15
proteins and to inhibit cervical tumor and JC breast tumor growth
in mice models. Previously, we determined whether rAAV2-hIL15 was
able to inhibit glioblastoma growth. In the present study,
rAAV2-hIL115 was succesfully produced and purified. Our results
showed that rAAV2-hIL15 possesses bioactivities and is capable of
delaying glioblastoma growth. Our studies may therefore provide a
potential immunotherapy method for glioblastoma treatment in the
future.
Materials and methods
Cell culture
DBTRG (human glioblastoma cells), HEK293 (human
embryonic kidney cells), HT1080 (human fibrosarcoma cells), HT2
(murine IL2/IL15-dependent cells) and YAC-1 (murine cell line
sensitive to LAK cells) cells were obtained from the Bioresource
Collection and Research Center (BCRC, Shinchu, Taiwan). Cells were
cultured in Dulbecco’s modified Eagle’s medium (DMEM) or RPMI-1640
medium supplemented with 10% fetal bovine serum and 100 IU/ml
penicillin/streptomycin. Moreover, since HT2 cells are
IL15-dependent cells, IL15 protein (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) supplementation was required for maintaining
survival. DMEM, RPMI, fetal bovine serum, L-glutamine,
penicillin/streptomycin, sodium pyruvate and non-essential amino
acids were purchased from Invitrogen (Carlsbad, CA, USA).
pAAV-hIL15 construction
The rAAV2 helper-free packing system (pAAV-MCS,
pAAV-RC and pHelper plasmids) was purchased from Strategene (La
Jolla, CA, USA). The human IL15 gene (hIL15) with the IL2 secretory
peptide gene were kindly provided by Dr K.W. Liao (National Chiao
Tung University, Hsin-Chu, Taiwan) and were amplified using the
polymerase chain reaction (PCR) method. Primers containing
EcoRI and BamHI sites were indicated as follows:
sense primer, 5′-GAATTCAAAGAATTCATGTACAGGATGCA ACTCCT and
anti-sense, 3′-GGATCCAAAGGATCCTT AAGAAGTGTTGATGAACATTTGG. The
amplified hIL15 cDNA was cloned into pAAV-MCS to yield the
pAAV-hIL15 plasmid.
Production and purification of rAAV2
The rAAV2 helper-free packing system was used
according to the manufacturer’s instructions in order to produce
rAAV2-hIL15 and rAAV2-vector. Briefly, for the production of
rAAV2-hIL15, HEK293 cells were cultured in fifty 15-cm dishes and
co-transfected with 2 mg pAAV-hIL15, 2 mg pAAV-RC and 2 mg
pAAV-helper plasmids using the CaCl2 method. For
rAAV2-vector production, HEK293 cells were cultured on fifty 15-cm
dishes and co-transfected with 2 mg pAAV-hIL15, 2 mg pAAV-RC and 2
mg pAAV-helper plasmids using the CaCl2 method. After 72
h of transfection, rAAV2-hIL15 and rAAV2-vector were produced in
MEK293 cells. rAAV2-hIL15 and rAAV2-vector were purified with
heparin column using a single-step column purification method and
concentrated with an Amicon Ultra-15 centrifugal filter (Millipore,
Billerica, MA, USA). The titer of rAAV2-hIL15 and rAAV2-vector was
then measured by real-time PCR. rAAV2-hIL15 and rAAV2-vector were
stored at −80°C until further use.
IL15 expression assay
In order to determine IL15 protein expression, the
culture media obtained from 1013 viral particles/ml
rAAV2-hIL15 and rAAV2-vector-infected HT1080 cells
(1.5×105 cells in a 6-well plate) for 3 days were
collected and determined using the ELISA assay (Biosource
International, Camarillo, CA, USA). In brief, the culture media
were added in 96-well plates which were coated with the hIL15
antibody. After a 4-h reaction, the plates were measured under an
ELISA reader at an optical density (OD) of 450 nm.
Bioactivity assay of
rAAV2-hIL15-expressed IL15
The viability of HT2 cells was determined for the
bioactivity assay of rAAV2-hIL15-expressed IL15. HT2 cells are
IL15-dependent cells and IL15 is required for their survival. The
culture media obtained from rAAV2-hIL15- or rAAV2- vector-infected
HT1080 cells were added to HT2 cell culture. After 16 h, the
viability of HT2 cells was determined using the MTS assay (Promega,
Madison, WI, USA). Media contai ning purified recombinant hIL15 (1
μg/ml) (Santa Cruz Biotechnology, Inc.) were added to HT2
cells which served as the positive control.
Cytotoxicity activity assay
Mice were infected with rAAV2-hIL15- or rAAV2-vector
for >4 weeks, respectively. After the experiment mice were
sacrificed, their spleens were collected and LAK cells were
separated from dead cells and red blood cells by using ACK lysis
buffer. YAK-1 cells are the target cells that can be killed by LAK
cells. For the LAK cell cytotoxic activity assay, YAC-1 cells
(target) and LAK cells (effector) were cocultured in a total volume
of 200 μl in RPMI-1640 containing 10% FCS in 96-well plates
at various cell densities to achieve effector-to-target (E/T)
ratios (12.5:1, 25:1 and 50:1) for 4 h at 37°C. Target cell lysis
was determined by using the lactate dehydrogenase (LDH) release
assay using CytoTox 96® Non-Radioactive Cytotoxicity
assay kit (Promega) and was calculated as: (OD490 nm of sample −
OD490 nm with spontaneous release of LDH from target cells − OD490
nm with spontaneous release of LDH from effector cells) ×100/(OD490
nm with maximum release of LDH from target cells − OD490 nm with
spontaneous release of LDH from effector cells).
Animal studies
Female nude mice (4 weeks old) were obtained from
the National Animal Laboratory Center (Taipei, Taiwan). Every 8
nude mouse was infected on one site of the quadriceps muscles with
rAAV2-hIL15 (5×1012 viral particles), rAAV2- vector
(5×1012 viral particles), and PBS (mock), respectively.
After 1 month, 107 DBTRG glioblastoma cells were
transplanted on the lower abdominal region of the mice. Tumor
growth was observed every 4 days, the tumor volume was measured
with the caliper and calculated as 1/2 × length ×
width2. Animals were sacrificed when tumor size was
1,500 mm3.
Statistical analysis
Data are presented as the mean ± SEM. Statistical
significance was analyzed using the Student’s t-test. Survival
analysis was performed using the Kaplan-Meier method. P<0.05 was
considered statistically significant.
Results
rAAV2-hIL15 expresses hIL15 protein with
bioactivity in vitro
HT1080 cells were treated with rAAV2-hIL15,
rAAV2-vector and PBS (mock), respectively. After 72 h, the media
obtained from the above groups were determined for the hIL15 level
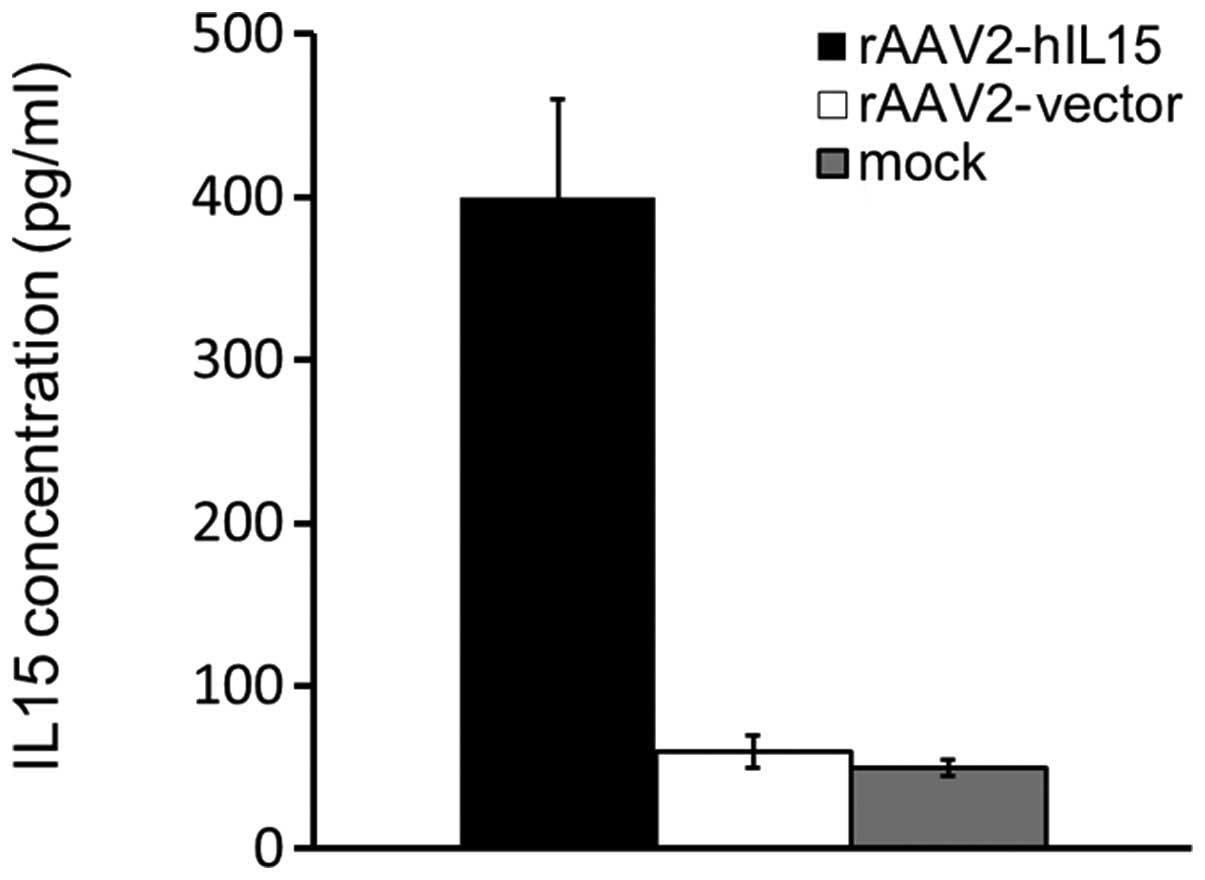
using ELISA. Our data showed that rAAV2-hIL15-treated HT1080 cells
expressed a 4- to 5-fold level of hIL15 protein as compared with
mock or rAAV2-vector groups (Fig.
1). We further determined the bioactivity of
rAAV2-hIL15-expressed hIL15 in vitro using a cell viability
assay. HT2 cells are IL15-dependent cells that survive under IL15
supplementation. In this study, culture media obtained from the
rAAV2-hIL15 and rAAV2-vector were added to HT2 cells for 48 h, and
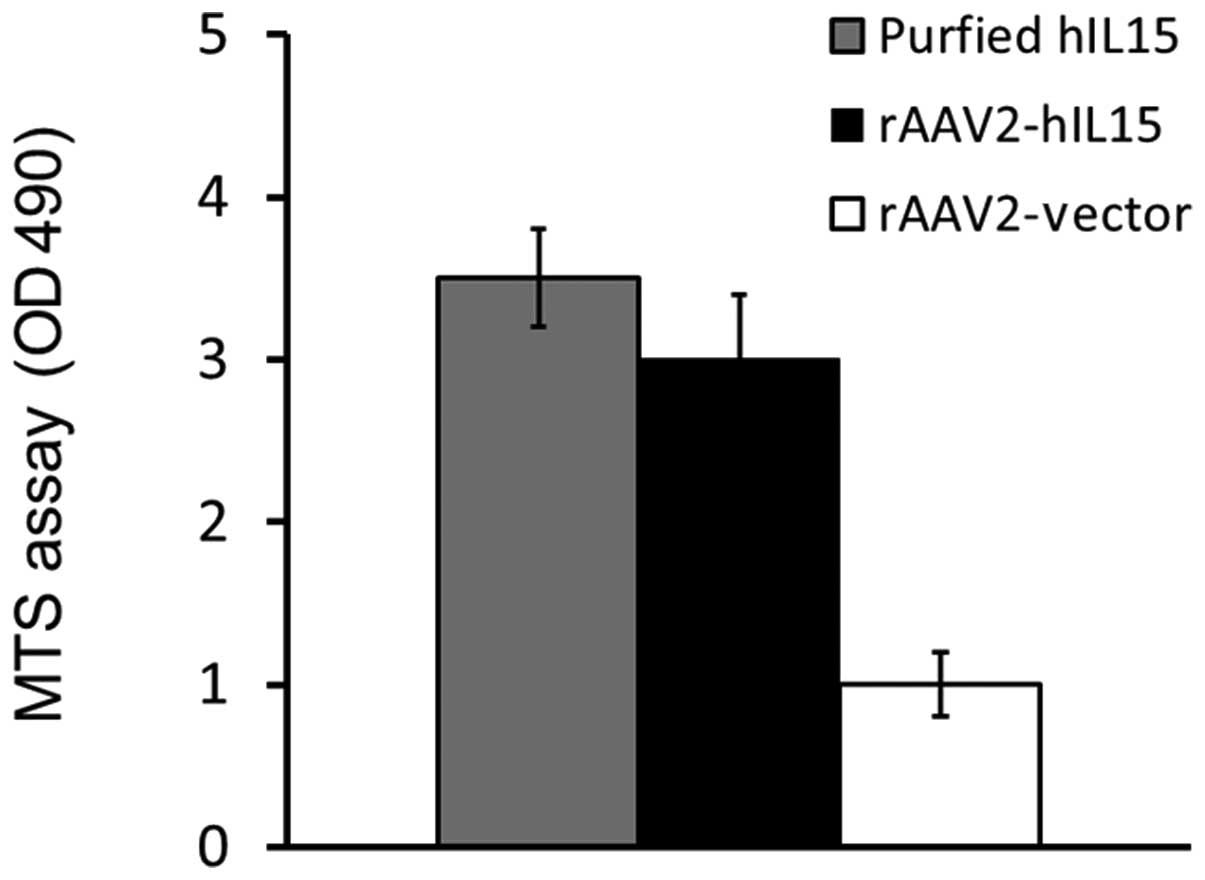
HT2 cell viability was determined by using the MTS assay. Our
results demonstrated that the media obtained from the rAAV2-hIL15
group showed higher HT2 cell viability as compared with that of the
rAAV2-vector group. The purified hIL15 protein served as the
positive control (Fig. 2). Thus,
results showed that rAAV2-hIL15 expressed hIL15 protein with
bioactivity in vitro.
rAAV2-hIL15 induces the production of
IgG1 and IgM in nude mice
Nude mice were injected with rAAV2-hIL15,
rAAV2-vector and mock in the quadriceps muscle of the left thigh,
respectively. After 28 days, the mice sera obtained from the above
three groups were analyzed for immunoglobulin (Ig) production
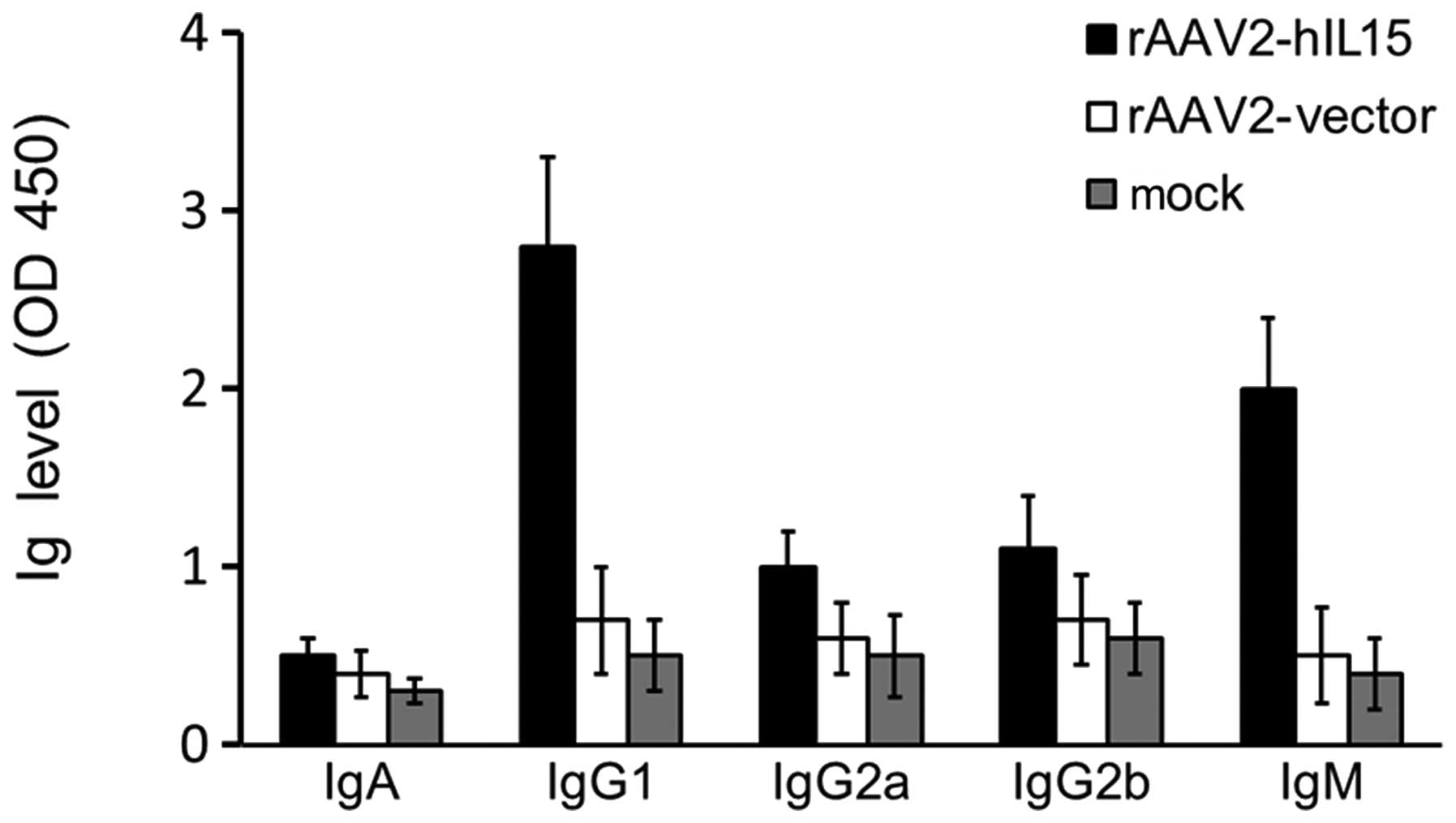
(IgG1, IgG2a, IgG2b, IgM and IgA). IgG1 and IgM levels were higher
in the rAAV2-hIL15 group compared with the rAAV2-vector and mock
groups (Fig. 3). However, the
levels of IgG2a, IgG2b and IgA were not significantly different
among the rAAV2-hIL15, rAAV2-vector and mock groups. Therefore, our
data showed that rAAV2-hIL15 was capable of inducing the production
of IgG1 and IgM in nude mice.
rAAV2-hIL15 activates cytotoxic activity
of LAK cells
Mice were administered an intramuscular injection
over the quadriceps muscle of the left thigh with rAAV2-hIL15 and
rAAV2-vector, respectively, for 28 days. Consequently, the mice
were sacrificed and their spleens collected. LAK cells were then
separated and collected from dead cells and red blood cells. YAK-1
cells, the target cells of LAK cells, were used for the cytotoxic
activity assay of LAK cells. YAK-1 and LAK cells were cocultured
according to different ratios and the cytotoxic activity of LAK
cells was studied using CytoTox 96® Non-Radioactive
Cytotoxicity assay (LDH release assay). The cytotoxic activity of
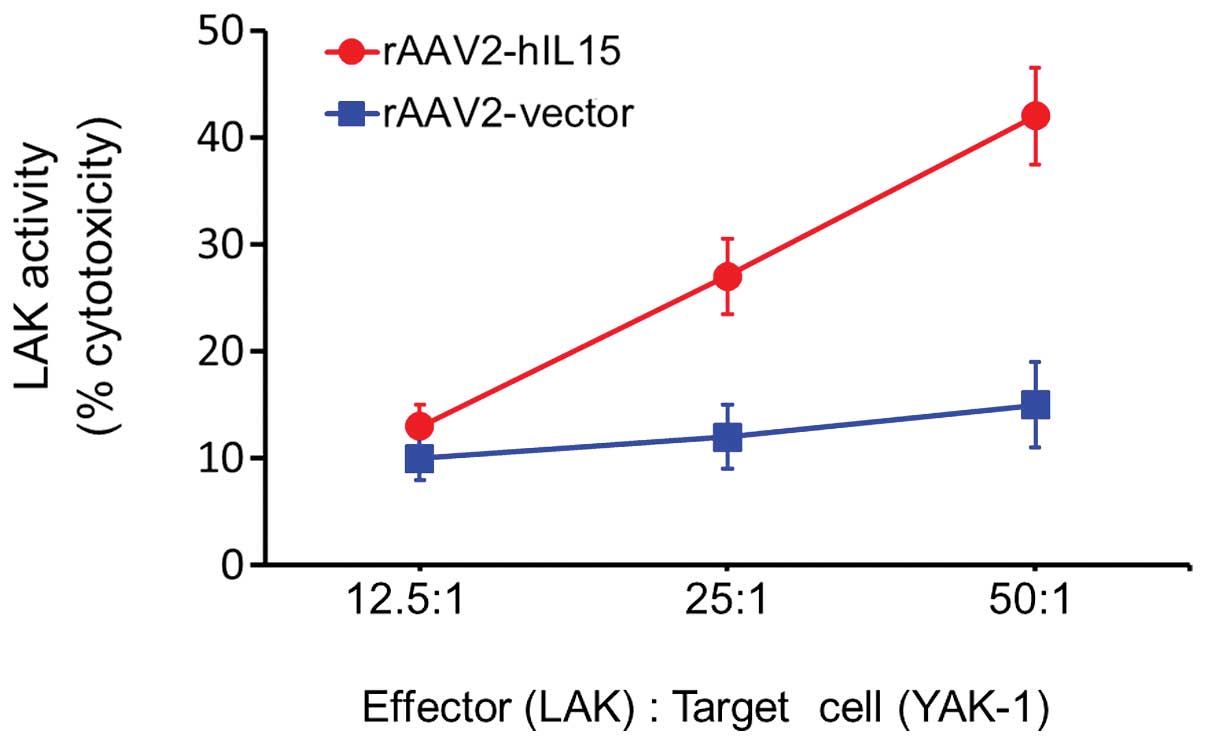
LAK cells was significantly induced in the rAAV2-hIL15 group
(Fig. 4). At an effector/target
cell ratio of 50:1, the cytotoxic activity level of LAK cells was
induced with rAAV2-hIL15 up to 3-fold as compared with the
rAAV2-vector group. This result suggested that rAAV2-hIL15 was able
to activate the cytotoxic activity of LAK cells.
rAAV2-hIL15 delays efficient glioblastoma
growth
After nude mice were intramuscularly injected with
rAAV2-hIL15, rAAV2-vector and mock, respectively, for 28 days, mice
were with human glioblastoma DBTRG cells subcutaneously injected.
Tumor size was determined every 4 days. The tumor growth rate was
slower in the rAAV2-hIL15 group compared with that in the
rAAV2-vector and mock groups (Fig.
5). Although there was still a slow-paced tumor growth in the
rAAV2-hIL15 group, rAAV2-hIL15 delayed tumor growth as compared
with the control groups.
Discussion
Treatment of human malignant brain tumor remains a
challenge and the average survival time of brain tumor patients is
∼12–24 months when traditional therapies are employed (1,24,25).
Thus, new therapies for brain tumor patients have been suggested
and studied (26–28). Currently, gene therapy is also used
in the treatment of brain tumor (28–30).
Adeno-associated virus (AAV), lentivirus, herpes virus and
adenovirus transfer are generally applied in gene therapy (31,32).
Due to the non-pathogenic characteristics and the low
immunogenicity of rAAV2, rAAV2 is considered safer than other viral
delivery systems (33–36). However, rAAV2 is a small virus and
it only carries small genes of ∼3,000 nucleotides (37). Findings of previous studies have
indicated that IL15 has a short half-life and repeated daily
injection is required for immunotherapy in clinical course
(9–11). Therefore, gene therapy is suggested
for IL15 application. Since the human IL15 gene is ∼600-nucleotide
long, it can be carried by rAAV2 viral delivery systems. In this
study, we successfully produced rAAV2-hIL15 and revealed that
rAAV2-hIL15 possesses antitumor bioactivities in a xeno-grafted
brain tumor mice model.
A previous study demonstrated that rAAV2-hIL15 was
able to induce IgG1, IgG2b and IgM production in a nude mice model
(23). However, IgG2b level was
lower than that of IgG1 and IgM. Concerning our results,
rAAV2-hIL15 was able to enhance IgG1 and IgM production, but there
was no significant difference in the IgG2b level between the
rAAV2-hIL15 group and control groups (Fig. 3). A comparison of our results with
those of this previously published study suggests that rAAV2-hIL15
is capable of inducing IgG1 and IgM production although it does not
induce substantial IgG2b production. Recently, it has been
demonstated that rAAV2-hIL15 is able to activate LAK cell cytotoxic
activity (21–23). Our data also showed that
rAAV2-hIL15 induces LAK cell cytotoxic activity in glioblastoma
cells.
It has been suggested that rAAV2-hIL15 inhibits
tumor growth on both human cervical tumors and mouse JC breast
tumors (21–23). Additionally, rAAV2-hIL15 also
inhibits the growth of human glioblastoma cells. Taken together,
these studies suggest that rAAV2-hIL15 inhibits the tumor growth of
various tumor types. Recently, human interleukin 12 (hIL12) was
carried by rAAV2 for the treatment of brain tumor (38). This study has demonstrated that
rAAV2-hIL12 induces Ig production and cytotoxic activity of LAK
cells, and exerts antitumor activity on brain tumor. Ig production,
the cytotoxic activity of LAK cells and the antitumor activity on
human glioblastoma have also been demonstrated in this study by
using rAAV2-hIL15. However, Ig production between rAAV2-hIL12 and
rAAV2-hIL15 is different. IgG1 and IgG2a levels were significantly
increased previously by rAAV2-hIL12 treatment (38). However, IgG1 and IgM levels were
significantly increased by rAAV2-hIL15 treatment in our study.
Although there are different Ig production levels between
rAAV2-hIL12 and rAAV2-hIL15, they are able to inhibit brain tumor
growth effectively. Therefore, we suggest that the rAAV2 gene
transfer system is a useful method for tumor immunotherapy and a
potential therapeutic method that may be combined with hIL12 and
hIL15 for brain tumor immunotherapy in the future.
Acknowledgements
This study was supported by the
National Science Council of Taiwan (nos. NSC99-2320-B-039-030-MY3,
NSC99-2632-B-039-001-MY3 and NSC100-2321-B-039-004 to YLY) and the
University of Texas MD Anderson-China Medical University Hospital
Sister Institution Fund (DMR-101-115 to YLY as well as
NSC101-2311-B-039-001 and CMU100-N2-09 to RHC).
References
|
1
|
Daneyemez M, Gezen F, Canakci Z and
Kahraman S: Radical surgery and reoperation in supratentorial
malignant glial tumors. Minim Invasive Neurosurg. 41:209–213. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stephenson KB, Barra NG, Davies E, Ashkar
AA and Lichty BD: Expressing human interleukin-15 from oncolytic
vesicular stomatitis virus improves survival in a murine metastatic
colon adenocarcinoma model through the enhancement of anti-tumor
immunity. Cancer Gene Ther. 19:238–246. 2012. View Article : Google Scholar
|
|
3
|
McBride WH: Integration of adenovirus
thymidine kinase suicide-gene therapy with surgery and radiation
therapy for malignant glioma. Future Oncol. 8:17–20. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Srivastava D, Joshi G, Somasundaram K and
Mulherkar R: Mode of cell death associated with adenovirus-mediated
suicide gene therapy in HNSCC tumor model. Anticancer Res.
31:3851–3857. 2011.PubMed/NCBI
|
|
5
|
Munger W, DeJoy SQ, Jeyaseelan R Sr, et
al: Studies evaluating the antitumor activity and toxicity of
interleukin-15, a new T cell growth factor: comparison with
interleukin-2. Cell Immunol. 165:289–293. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carson WE, Giri JG, Lindemann MJ, et al:
Interleukin (IL) 15 is a novel cytokine that activates human
natural killer cells via components of the IL-2 receptor. J Exp
Med. 180:1395–1403. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mrozek E, Anderson P and Caligiuri MA:
Role of interleukin-15 in the development of human CD56+
natural killer cells from CD34+ hematopoietic progenitor
cells. Blood. 87:2632–2640. 1996.PubMed/NCBI
|
|
8
|
Sparano JA, Fisher RI, Weiss GR, et al:
Phase II trials of high-dose interleukin-2 and lymphokine-activated
killer cells in advanced breast carcinoma and carcinoma of the
lung, ovary, and pancreas and other tumors. J Immunother Emphasis
Tumor Immunol. 16:216–223. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chapoval AI, Fuller JA, Kremlev SG, Kamdar
SJ and Evans R: Combination chemotherapy and IL15 administration
induce permanent tumor regression in a mouse lung tumor model: NK
and T cell-mediated effects antagonized by B cells. J Immunol.
161:6977–6984. 1998.PubMed/NCBI
|
|
10
|
Lasek W, Basak G, Switaj T, et al:
Complete tumour regressions induced by vaccination with IL-12
gene-transduced tumour cells in combination with IL15 in a melanoma
model in mice. Cancer Immunol Immunother. 53:363–372. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rubinstein MP, Kadima AN, Salem ML, Nguyen
CL, Gillanders WE and Cole DJ: Systemic administration of IL15
augments the antigen-specific primary CD8+ T cell
response following vaccination with peptide-pulsed dendritic cells.
J Immunol. 169:4928–4935. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Croce M, Meazza R, Orengo AM, et al:
Sequential immunogene therapy with interleukin-12- and
interleukin-15-engineered neuroblastoma cells cures metastatic
disease in syngeneic mice. Clin Cancer Res. 11:735–742.
2005.PubMed/NCBI
|
|
13
|
Suzuki K, Nakazato H, Matsui H, et al: NK
cell-mediated anti-tumor immune response to human prostate cancer
cell, PC-3: immunogene therapy using a highly secretable form of
interleukin-15 gene transfer. J Leukoc Biol. 69:531–537.
2001.PubMed/NCBI
|
|
14
|
Kimura K, Nishimura H, Hirose K,
Matsuguchi T, Nimura Y and Yoshikai Y: Immunogene therapy of murine
fibrosarcoma using IL15 gene with high translation efficiency. Eur
J Immunol. 29:1532–1542. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ponnazhagan S, Curiel DT, Shaw DR, Alvarez
RD and Siegal GP: Adeno-associated virus for cancer gene therapy.
Cancer Res. 61:6313–6321. 2001.PubMed/NCBI
|
|
16
|
Xiao X, Li J and Samulski RJ: Efficient
long-term gene transfer into muscle tissue of immunocompetent mice
by adeno-associated virus vector. J Virol. 70:8098–8108.
1996.PubMed/NCBI
|
|
17
|
Jennings K, Miyamae T, Traister R, et al:
Proteasome inhibition enhances AAV-mediated transgene expression in
human synoviocytes in vitro and in vivo. Mol Ther. 11:600–607.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jooss K, Yang Y, Fisher KJ and Wilson JM:
Transduction of dendritic cells by DNA viral vectors directs the
immune response to transgene products in muscle fibers. J Virol.
72:4212–4223. 1998.PubMed/NCBI
|
|
19
|
Emborg ME, Carbon M, Holden JE, et al:
Subthalamic glutamic acid decarboxylase gene therapy: changes in
motor function and cortical metabolism. J Cereb Blood Flow Metab.
27:501–509. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaplitt MG, Feigin A, Tang C, et al:
Safety and tolerability of gene therapy with an adeno-associated
virus (AAV) borne GAD gene for Parkinson’s disease: an open label,
phase I trial. Lancet. 369:2097–2105. 2007.PubMed/NCBI
|
|
21
|
Yu YL, Wei CW, Chen YL, Chen MH and Yiang
GT: Immunotherapy of breast cancer by single delivery with
rAAV2-mediated interleukin-15 expression. Int J Oncol. 36:365–370.
2010.PubMed/NCBI
|
|
22
|
Yiang GT, Chou PL, Tsai HF, et al:
Immunotherapy for SV40 T/t antigen-induced breast cancer by
recombinant adeno-associated virus serotype 2 carrying
interleukin-15 in mice. Int J Mol Med. 29:809–814. 2012.PubMed/NCBI
|
|
23
|
Yiang GT, Harn HJ, Yu YL, et al:
Immunotherapy: rAAV2 expressing interleukin-15 inhibits HeLa cell
tumor growth in mice. J Biomed Sci. 16:472009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kayama T, Kumabe T, Tominaga T and
Yoshimoto T: Prognostic value of complete response after the
initial treatment for malignant astrocytoma. Neurol Res.
18:321–324. 1996.PubMed/NCBI
|
|
25
|
Kowalczuk A, Macdonald RL, Amidei C, et
al: Quantitative imaging study of extent of surgical resection and
prognosis of malignant astrocytomas. Neurosurgery. 41:1028–1038.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li YM and Hall WA: Targeted toxins in
brain tumor therapy. Toxins (Basel). 2:2645–2662. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ahmed AU, Ulasov IV, Mercer RW and Lesniak
MS: Maintaining and loading neural stem cells for delivery of
oncolytic adenovirus to brain tumors. Methods Mol Biol. 797:97–109.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim CY, Park SH, Jeong M, et al:
Preclinical studies for pharmacokinetics and biodistribution of
Ad-stTRAIL, an adenovirus delivering secretable trimeric TRAIL for
gene therapy. Exp Mol Med. 43:580–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee EX, Lam DH, Wu C, et al: Glioma gene
therapy using induced pluripotent stem cell derived neural stem
cells. Mol Pharm. 8:1515–1524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
King GD, Muhammad AK, Larocque D, et al:
Combined Flt3L/TK gene therapy induces immunological surveillance
which mediates an immune response against a surrogate brain tumor
neoantigen. Mol Ther. 19:1793–1801. 2011. View Article : Google Scholar
|
|
31
|
Heilbronn R and Weger S: Viral vectors for
gene transfer: current status of gene therapeutics. Handb Exp
Pharmacol. 143–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lundstrom K: Gene therapy applications of
viral vectors. Technol Cancer Res Treat. 3:467–477. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang L, Calcedo R, Nichols TC, et al:
Sustained correction of disease in naive and AAV2-pretreated
hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy.
Blood. 105:3079–3086. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Flannery JG, Zolotukhin S, Vaquero MI,
LaVail MM, Muzyczka N and Hauswirth WW: Efficient
photoreceptor-targeted gene expression in vivo by recombinant
adeno-associated virus. Proc Natl Acad Sci USA. 94:6916–6921. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Snyder RO and Flotte TR: Production of
clinical-grade recombinant adeno-associated virus vectors. Curr
Opin Biotechnol. 13:418–423. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li SF, Wang RZ, Meng QH, et al:
Intra-ventricular infusion of rAAV1-EGFP resulted in transduction
in multiple regions of adult rat brain: a comparative study with
rAAV2 and rAAV5 vectors. Brain Res. 1122:1–9. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dong JY, Fan PD and Frizzell RA:
Quantitative analysis of the packaging capacity of recombinant
adeno-associated virus. Hum Gene Ther. 7:2101–2112. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chiu TL, Lin SZ, Hsieh WH and Peng CW:
AAV2-mediated interleukin-12 in the treatment of malignant brain
tumors through activation of NK cells. Int J Oncol. 35:1361–1367.
2009.PubMed/NCBI
|